Abstract
Introduction:
China's rapid economic growth has led to escalating environmental pollution, significantly impacting mangrove ecosystems. The persistence and response to pollution in mangrove ecosystems involve multiple processes, including the accumulation of contaminants in sediments, their transport in plants, and their accumulation in other organisms. However, comprehensive studies on the multidimensional interactions among these processes are limited.
Methods:
This study investigated two mangrove forest areas in Hainan, which were categorized according to the type of mangrove forest cover: planted forests and natural forests. Thirty sampling sites were established to collect data on benthic organisms and their sediment characteristics.
Results:
Elemental As showed moderate, ongoing pollution. The distribution of species in the two regions showed significant population differences. The benthic population density in the natural forest was significantly lower than that in the planted forest, which was mainly due to the prevalence of Batillaria cumingi, and biodiversity indices and habitats in the natural forest were superior to those in the planted forest, which mainly depended on the degree of anthropogenic disturbance. Total phosphorus, nitrogen, dissolved solids, Hg, and sand grains were the most important variables.
Discussion:
Total phosphorus and total nitrogen were the most important environmental factors affecting community composition, while total dissolved solids influenced overall changes in species composition, highlighting the significant influence of the type of mangrove cover on sediment pollution and environmental factors, leading to significant changes in the biomass and density of benthic organisms. This study emphasizes the complex interactions among sediment contamination, mangrove cover, and benthic communities, providing a three-dimensional view of the distribution patterns of mangrove contamination.
1 Introduction
Mangroves are among the most biodiverse and productive wetland ecosystems on Earth (Celis-Hernandez et al., 2020). Mangroves not only provide suitable habitats and abundant food resources for marine organisms but also provide ecological services for the survival and development of humans living in coastal areas (Li et al., 2017). Mangrove sediments, plants, and benthic organisms are the core components of mangrove ecosystems and are closely interconnected (Zhang et al., 2014). The root systems of mangrove plants are a natural habitat for benthic organisms, and the resulting apoplastic material can be decomposed into nutrients that are deposited on the substrate for benthic organisms to feed on (Yamada et al., 2013). Benthic biota are characterized by a long life cycle, strong regionalization, and weak migratory ability, which can play a role in promoting material cycling and energy flows (Ying et al., 2021; Siikamäki et al., 2012). Both mangrove plants and benthic biota depend on the sedimentary environment. Sediment brings together nutrients and toxic elements produced by natural/anthropogenic effects (Bouillon et al., 2008; Mountouris et al., 2002). Some essential nutrients for plants and other organisms are absorbed by plants and other organisms, jeopardizing the human body through the food chain and causing toxicity accumulation (Pan and Wang, 2012), especially heavy metals, which cause contamination because of their toxicity, persistence, non-degradability, and bioaccumulation (Chaudhuri et al., 2014; Nath et al., 2013). Currently, many mangrove areas around the world are declining or disappearing owing to pollution (Yarahmadi and Khorsandi, 2024; Zhu et al., 2021). Because pollution is characterized by interrelatedness and interactions (ELTurk et al., 2019), it is particularly necessary to study pollution in mangrove forests from a three-dimensional perspective, that is, the interrelated roles of sediment elemental contents, plants, and benthic organisms.
Previous research has explored the interactions between sediments, plants, and benthic organisms in mangrove areas, revealing complex interactions. In terms of environmental factors, the diminishing influence of runoff from rivers entering the sea and the increasing influence of the marine water environment can lead to a gradual increase in the diversity of benthic communities (Ma et al., 2012), which generally exhibit higher biodiversity in the frontal zone of the forest belt than in the central zone of the mangrove forest (You et al., 2024). The distribution of benthic communities is closely related to sediment type, with benthic diversity generally being higher in muddy or sandy sediments than in more homogeneous substrate environments such as clayey (Chen et al., 2025). Mangrove vegetation types are closely related to the species distribution of benthic organisms, and mangrove plants with different substrates and physicochemical parameters support different taxa (Parvez Al Usmani, 2018). To obtain more resources, including food or habitat, benthic organisms can use tidal action to migrate to different neighboring microhabitats (Sun et al., 2022). Mangrove vegetation provides habitat for several benthic organisms and simultaneously alters the physicochemical properties of soil sediments, including salinity, acidity, and nutrient content, which in turn can indirectly affect the distribution of benthic organisms (Lee and Shih, 2004; Santos et al., 2020). In terms of spatiotemporal patterns, Muthukumaravel et al. assessed the system functioning of three mangrove forests and noted that the community structural characteristics of benthic organisms reflect the spatiotemporal variability of the abiotic elements of mangrove ecosystems (e.g., sediment elements) (Muthukumaravel et al., 2021; Arbi et al., 2018). In summary, the interactions among sediments, benthic organisms, and mangrove plants show a multidimensional response relationship (Santos et al., 2020; Silva-Camacho et al., 2017); however, most relevant studies focused on two or fewer dimensions, with few studies investigating three or more dimensions (Chen et al., 2013; Hou et al., 2020).
In China, the richest and most diverse mangrove forests are distributed on Hainan Island (Meng et al., 2022). However, the rapid development of aquaculture, tourism, and urban construction has led to a drastic reduction in mangrove area and weakening of ecological functions. This is particularly true for Xincun Harbor and Xinying Bay in Hainan, which are surrounded by residential areas, tourist resorts, fishing harbors, estuaries, and lagoon harbors, which provide a variety of complex pollution input sources. However, the pollution status of mangrove forests in these areas has received little attention to date (Hao et al., 2024; Meng et al., 2022).
This study aimed to combine the relationships among benthic populations/communities, vegetation types, and sediment elements to investigate the regional pollution status and propose strategies to regulate pollution in the mangrove areas of Xincun Harbor and Xinying Bay of Hainan. Based on the vertical distribution pattern in the mangrove ecosystem, this study explores the complex correlation and current pollution status of the area from the perspectives of sediment, plants, and benthic organisms to provide scientifically effective and ecologically significant insights.
2 Materials and methods
2.1 Overview of the study area
The study area is located in the mangrove areas of Xincun Harbor and Xinying Bay on Hainan Island, China (Figure 1). Xincun Harbor is located in Lingshui Li Autonomous County in the southeast of Hainan Province, latitude 18°24′–18°25′ N, longitude 109° E. It is an almost closed natural fishing harbor with a gourd-like appearance, with an average annual temperature of 25.8–30.0°C and a typical tropical marine monsoon climate. The mangrove forests are distributed on both sides of the lagoon, containing 11 native species of true mangroves and 2 non-native species of true mangroves. The non-native species are dominated by Laguncularia racemosa (Gong et al., 2018; Yang et al., 2016). Xinying Bay mangrove forest is located within Xinying Town, Houshui Bay, Lingao County, northern Hainan Province, latitude 19°51′–19°55′ N and longitude 109° E, with an average annual temperature of 23.2°C, and has a tropical oceanic monsoon climate. It contains 15 species of true mangrove, with the main species being Rhizophora stylosa and Bruguiera gymnorhiza (Wen et al., 2023). Although the two areas have the same tropical oceanic monsoon climate, the type of cover and growth environment show significant differences. Specifically, the mangrove forests in Xincun Harbor are more artificially planted and the growth of Laguncularia racemosa is typical, with overall sparseness, whereas Xinying Bay is mainly dominated by natural forests, with a distribution of community aggregation, distinctive levels, and higher density.
Figure 1
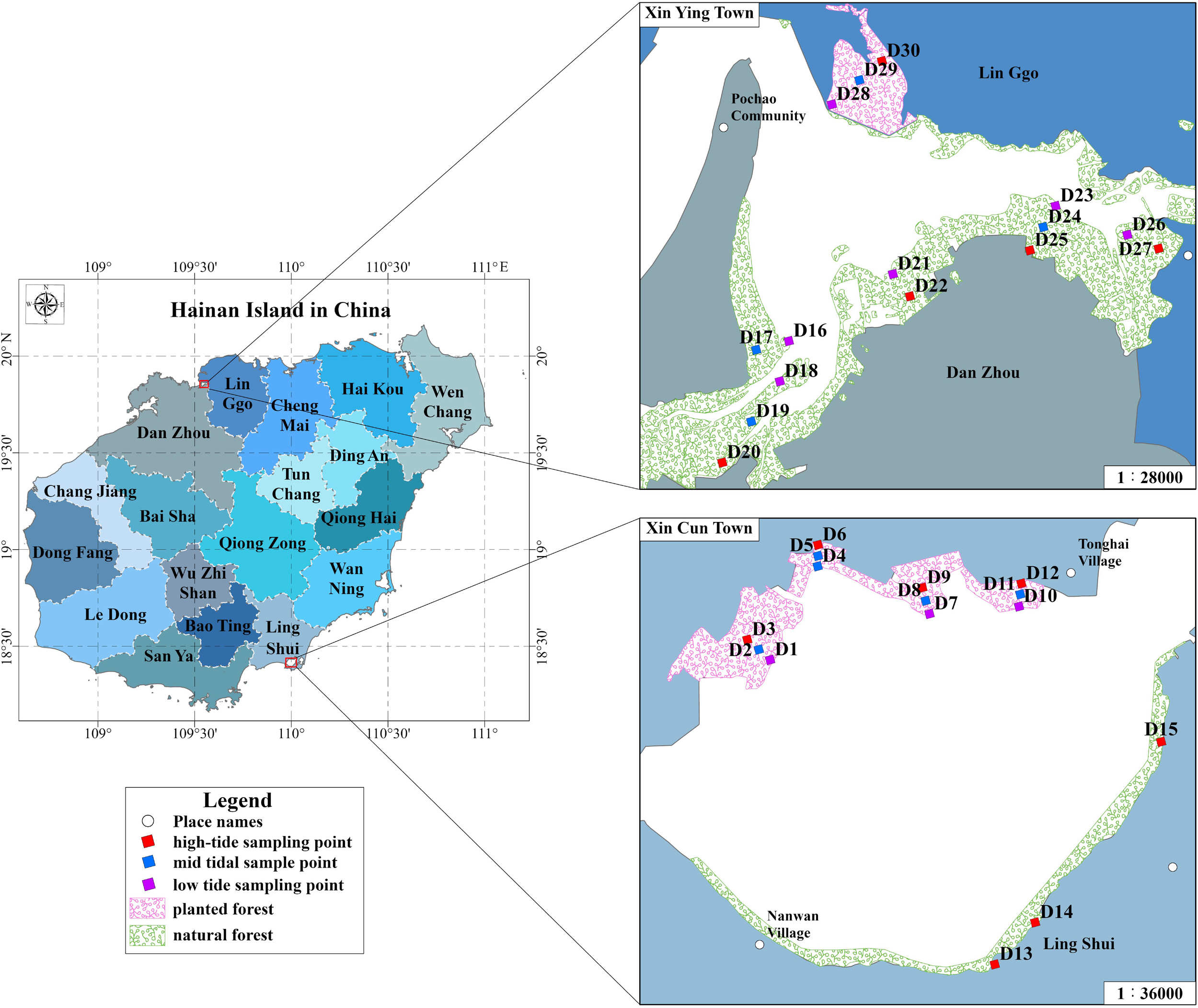
Geographic location of the study area and sampling points. In the figure, the red side indicates the area of planted forests, the green side indicates the area of natural forests, and the distribution of sampling points in the high, middle and low tide zones is indicated by red, blue and purple dots.
2.2 Sample collection and identification
Samples collected from 3–6 sample strips established within each of the mangrove forests, perpendicular to the seaward edge of the forest to the landward edge, thereby traversing the three tidal zones, namely the high, middle, and low tidal zones. Then, 1–3 sampling points were identified within each sample strip, and 15 sampling points were deployed in both planted and natural forest areas (Figure 1). At each sampling point, benthic samples were collected using 0.25 × 0.25 m sample squares to a depth of 30 cm, rinsed through a 0.5-mm sieve, fixed in situ with 5–7% neutral buffered formaldehyde, and sent to the laboratory for sample analysis, including species identification, counting, and weighing. Sediment samples (1000 g) were collected at the same sampling points in sealed sampling bags, kept at 0°C, transported to the laboratory for freeze-drying to remove impurities, and divided into two parts after picking out impurities. One part was ground and sieved (160/80 mesh), which was mainly used for the determination of the concentration of heavy metals and nutrient elements (TOC, TN, and TP), and the other was freshly frozen and preserved for the measurements of particle size. Ten sediment elemental indicators were determined, and the methods used for each indicator are listed in Table 1. To ensure the accuracy of the results, each sediment sample was tested three times with an analytical error of <5%, and the average value was considered as the final result.
Table 1
| Sample | Elemental indicators | Analytical test methods/instruments | Reference standard |
|---|---|---|---|
| sediment | Hg | Haikou AFS-9800 Dual Channel Atomic Fluorescence Spectrophotometer | GB/T22105-2008 |
| As | HJ 803-2016 | ||
| Cu | Thermo Fisher ICAP-Q Inductively Coupled Plasma Mass Spectrometer | HJ 803-2016 | |
| Cr | |||
| Pb | |||
| TOC | Potassium dichromate oxidation-spectrophotometry | HJ 615-2011 | |
| TN | Alkaline potassium persulfate digestion-ultraviolet spectrophotometry | GB 17378.5-2007 | |
| TP | Ammonium molybdate spectrophotometry | GB 17378.5-2007 | |
| TDS | Mass method | LY/T1251-1999 | |
| Particle size analysis | Malvern Mastersizer 2000 laser particle sizer | GB/T 12763.9-2007 |
Determination of elemental indicators in sediments.
2.3 Data processing
We analyzed the contamination status of heavy metals in sediments using the geoaccumulation index method (Igeo), which is a method for quantitatively assessing the degree of heavy metal contamination based on the heavy metal content versus geochemical background values (Muller, 1969) using the following formula:
where Igeo is the index of pollution accumulation, Ci is the measured concentration of heavy metal i (mg/kg), and Bi is the geochemical background value of heavy metal i in the sediments (mg/kg). The background values of heavy metals in the surface soil of Hainan Island were used as the geochemical background values, and the background values of As, Hg, Cr, Cu, and Pb were 1.87, 0.02, 22.70, 7.72, and 24.40 mg/kg, respectively (Fu, 2014); the coefficient was taken to be 1.5 to reduce the effect of potential bias in the background concentrations (Al-Kahtany et al., 2023). The geoaccumulation index corresponds to the level of contamination, as shown in Table 2.
Table 2
| Grade | I geo | Pollution degree |
|---|---|---|
| 0 | I geo<0 | Nothing |
| 1 | 0≤Igeo<1 | Lightly |
| 2 | 1≤Igeo<2 | Relatively moderately |
| 3 | 2≤Igeo<3 | Moderately |
| 4 | 3≤Igeo<4 | Relatively heavily |
| 5 | 4≤Igeo<5 | Heavily |
| 6 | I geo≥5 | Severely |
Geological accumulation index pollution classification.
To assess the benthic characteristics of the population and species diversity, we calculated the dominance index (Y), the Shannon-Wiener diversity index (H’), and the Pielou evenness index (J). The dominance index measures the relative importance of a few dominant species within a community. It reflects the degree to which certain species outcompete others in terms of abundance or biomass. A higher dominance index indicates that a few species have a significant advantage over others in the community. The Shannon-Wiener diversity index is based on information theory and combines species richness (number of species) and evenness (uniformity of species distribution) to quantify the overall diversity of a community. The Pielou evenness index assesses the uniformity of species distribution within a community by comparing the actual Shannon-Wiener diversity index to the maximum possible diversity if all species were evenly distributed. Finally, we calculated the Margalef species abundance index (D) based on the characteristics of the benthic community in the study area (van der Linden et al., 2012; Zhu et al., 2012), calculated using Equations 2–5. Benthic community delineation was performed by Bray-Curtis similarity coefficient clustering analysis. The similarity threshold was set at 70% (Gao et al., 2010). To reduce the interference of rare species in the community delineation, data for species with a relative density of less than 1% in the whole study area were not included in the analysis (Hangjun et al., 2020). The analysis was carried out using Past 4.13. Based on the results of the Bray-Curtis cluster analysis, species abundance values were used to identify prominent species in different biomes. Mann-Whitney U was used to test the difference between any two biome indices, and further Bonferroni correction (α=0.0167) was used to control the multiple comparisons error (Bramha et al., 2025), with p < 0.05 indicating a significant difference.
In Equation 2, Y is the dominance index, N is the total number of individuals of all species, Ni is the number of individuals of the ith species, and fi is the frequency of occurrence of the ith species, i.e., the ratio of the number of sampling points in which the species occurs to the total number of sampling points, and the species with Y ≥ 0.01 were designated as dominant species. S is the total number of species.
Based on Equations 1 and 2, several methods were applied to represent the response status of sediment pollution and biological and habitat status. First, principal component analysis (PCA) was used to derive the characteristics of elemental distribution within plantation and natural forests. Data were standardized by the z-score to eliminate the difference in data magnitude before analyses. A correlation coefficient matrix was established to test whether the variables were correlated, i.e., the test principle was that the KMO value should be greater than or equal to 0.6, Bartlett’s test of sphericity significance value should be less than or equal to 0.05, and the principal components with a cumulative contribution rate of 80%-95% were selected (Bramha et al., 2025; Abdi and Williams, 2010). The analyses were performed using SPSS 24.0 software. Second, canonical correspondence analysis (CCA) was used to highlight associations between benthic species and sediment elements. Before analysis, occasional species (i.e., ≤ 2 occurrences of sample points) in the benthos were removed, and biological data were transformed by lg(x+1) to eliminate order of magnitude differences. Using the Monte Carlo permutation test (Monte Carlo test) (Anderson and Braak, 2003), 999 iterations were undertaken to determine the significance of the variables (p<0.05). The analyses and graphing were done in the CANOCO 5.0 software (Bodaghabadi et al., 2011; Hejcmanovā-Nežerková and Hejcman, 2006). Third, due to the accumulation of different sediment elements by different plant species, results in habitat differences (Black and Shimmield, 2003; Pérez et al., 2020) and consequently, we delineated the main flora in the study area, visualized them, and selected the sedimentary elements with significant correlation to the organisms and habitats in the results of the first and second methods of analyses. We then carried out interpolation analysis using ordinary Kriging interpolation to convert the discrete measurement data into a continuous data surface to realize the intuitive expression. Mapping was conducted using ArcMap 10.8.
3 Results
3.1 Characterization of benthic populations and communities
3.1.1 Characterization of benthic populations
A total of 31 species of benthic organisms from three phyla and 19 families were identified in the two regions (Table 3), with 27 species of the phylum Mollusca accounting for 87.09% of the total number of species. The number of biological families was slightly higher in the natural forest area than in the planted forest area, and the number of species types was similar in both areas (Figure 2A). The species with the greatest dominance, as calculated using the dominance index, was Batillaria cumingi, which was only present in the planted forest. The next most dominant species was Assiminea latericea, which was mainly located in the natural forest, and it was also the species with the highest frequency of occurrence. Littoraria melanostoma was the species with the lowest frequency and dominance (Figure 2B). Benthic densities in the study area ranged from 2 to 186 ind./m2 with a mean value of 52.90 ind./m2, and biomasses ranged from 1.28 to 386.54 g/m2 with a mean value of 71.16 g/m2. The total density of organisms in planted forests (1062 ind./m2) was greater than that in natural forests (525 ind./m2). The highest density of benthic organisms was found at sampling point D7 (186 ind/m2), followed by D8 (174 ind/m2), both of which were located in the planted forest. The lowest density was observed at sampling point D15 (7 ind/m2), located in the natural forest. Overall, the differences in biotic densities among sampling sites were high in planted forests but relatively low in natural forests (Figures 2C, D). The total benthic biomass in planted forests (720.04 g/m2) was lower than that in natural forests (1414.9 g/m2), with the highest biomass in sampling site D23 (386 g/m2), followed by D27 (253.77 g/m2), and the lowest in D25 (1.28 g/m2), all three of which were located in natural forests. The benthic biomass in the natural forest was generally richer than that in the planted forest; however, it was also more differentiated (Figures 2E, F).
Table 3
| Phylum | Family | Species (latin scientific name) | Abbreviation in the picture |
|---|---|---|---|
| Mollusca, abbreviated as Ma | Littorinidae; Periwinkleshell | Littoraria melanostoma | sw8 |
| Littorina scabra | |||
| Pharellidae | Pharella acutidens | ||
| Ellobiidae | Ellobium aurismidae | sw11 | |
| Cassidula nucleus | |||
| Allochroa layardi | sw14 | ||
| Ellobium chinensis | |||
| Pythia cecillei | |||
| Muricidae | Thais clavigera | sw7 | |
| Melaniidae | Melanoides tuberculata | ||
| Potamididae | Batillaria cumingi | sw1 | |
| Batillaria zonalis | sw3 | ||
| Cerithidea rhizophorarum | sw10 | ||
| Cerithidea djadjariensis | sw12 | ||
| Terebralia palustris | sw17 | ||
| Veneridae | Anomalodiscus sguamosa | ||
| Tapes platyptycha | |||
| Cryptonema producta | |||
| Marphysa sp. | Lucina scarlatoi | sw15 | |
| Nereididae sp. | Assiminea latericea | sw13 | |
| Triclidae |
Capitellidae sp. Gammaridea sp. |
||
| Neritidae | Clithon oualaniensis | sw6 | |
| Fruticicolidae; Land snail | Acusta tourannensis | ||
| Corbiculidae | Geloina erosa | sw4 | |
| Cerithiidae | Cerithidea microptera | sw2 | |
| Clypeomorus humilis | |||
| Mytilidae; Mussel | Limnoperna fortanei | ||
| Annelidan, abbreviated as An | Eunicidae | Marphysa sp. | sw5 |
| Nereididae | Nereididae sp. | sw9 | |
| Capitellidae | Capitellidae sp. | sw16 | |
| Arthropoda, abbreviated as Aa | Corophiidae | Gammaridea sp. |
List of benthic organisms in the mangrove sediments of Xincun Harbor and Xinying Bay.
Abbreviations of species names used in other illustrations in the text are also reflected in this table.
Figure 2
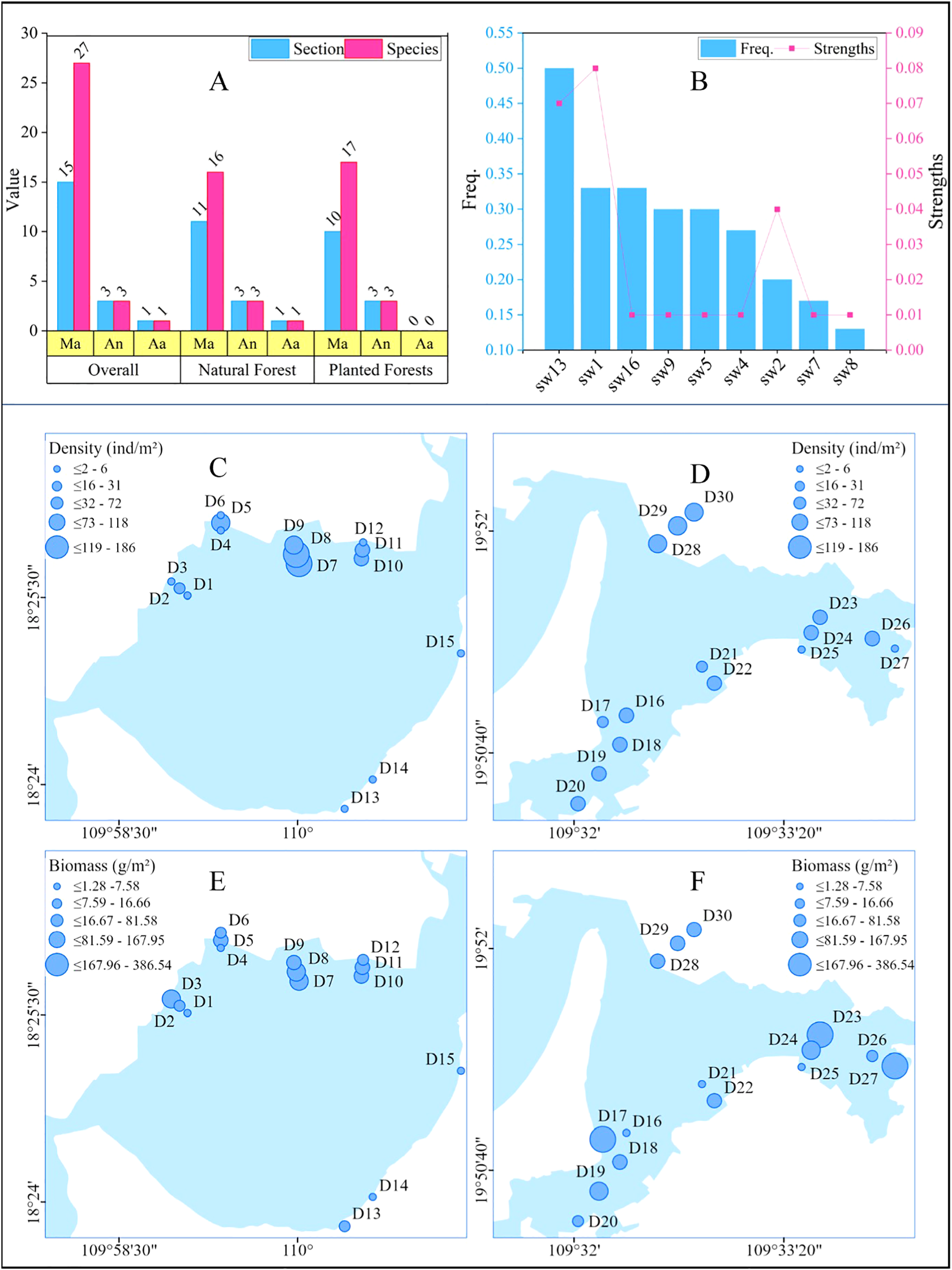
Characterization of benthic populations. (A) shows the distribution status of benthic organisms under the natural forest and plantation forest cover, the pink columns in the figure are the number of species types, the blue columns are the number of families, and the yellow area of the horizontal axis is the abbreviated name of each phylum, and the full name is shown in Table 1; (B) shows the top 9 dominant species, the blue columns are the frequency of occurrence values, and the red folded line is the index value of the dominance degree, and the horizontal axis is the abbreviated name of the species, and the full name is shown in Table 1; (C–F) indicate the density and biomass spatial distribution of benthic organisms at each sampling point, respectively, and the size of the point indicates how many values are present.
3.1.2 Benthic community structure and species diversity
The benthic community structure similarity clustering analysis, species abundance stacking map (Figure 3), and regional map (Figure 1) demonstrated that the distribution of benthic organisms was mainly clustered into three community areas. Clusters I and III were distributed in the plantation forest. Cluster I contained 9 sampling sites, with 9 benthic organisms identified, with Batillaria cumingi accounting for the main proportion, followed by Littoraria melanostoma. Cluster III contained five sampling sites and seven benthic species were identified, with Cerithidea micropterans being dominant. Cluster II was distributed in the natural forest and contained 12 sites, with a total of 12 benthic species identified. Assiminea latericea was dominant, followed by Ellobium aurismidae. The diversity indices of each sampling site are shown in Table 4. The mean values of Shannon’s diversity index (H′) were 0.794 ± 0.404, 1.062 ± 0.361, and 0.264 ± 0.293 for Clusters I, II, and III, respectively, and the mean values of Pielou’s index of species evenness (J) were 0.702 ± 0.311, 0.736 ± 0.173, and 0.184 ± 0.184, respectively. Margalef species abundance index (D) mean values for Clusters I, II, and III were 0.548 ± 0.342, 0.928 ± 0.315 and 0.383 ± 0.342, respectively. The mean values of the three types of indices demonstrate that the ranking of biodiversity of the benthic community was Cluster II > Cluster I > Cluster III. The Mann-Whitney U-test test results demonstrated no significant difference between Clusters I and II except for the Margalef species abundance index (P-values of 0.136, 0.522, and 0.047 for H′, J, and D, respectively). All three indices showed significant differences between clusters II and III (P = 0.004, 0.003, and 0.027 for H, J, and D, respectively). Both indices showed significant differences between Clusters I and III, except for the Margalef species abundance index, which was not significantly different (P-values = 0.071, 0.019, and 0.461 for H, J, and D, respectively).
Figure 3
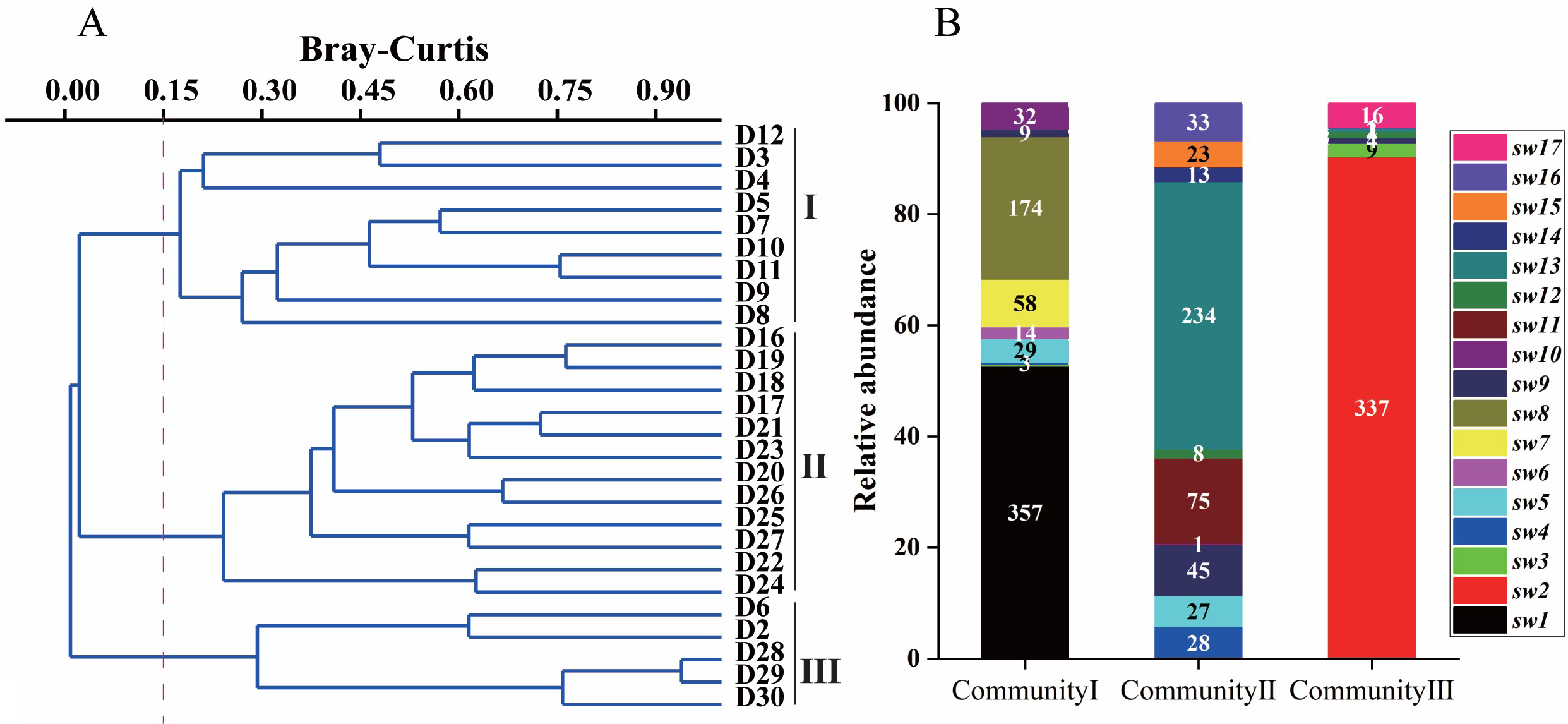
Cluster analysis plot A and species abundance stacking plot B based on cluster analysis results. I, II, and III in (A) correspond to the three biome aggregations, and the point numbers are the sampling points. The three values on the horizontal axis of (B) correspond to the three biomes I, II and III in (A), and the vertical axes are the species abundance values, and the names in the legend are the abbreviated names of the species, and the complete names are detailed in Table 1.
Table 4
| Community | Site | Number of species | H′ | J | D |
|---|---|---|---|---|---|
| I | D3 | 3 | 1.09 | 0.99 | 0.83 |
| D4 | 4 | 1.31 | 0.94 | 1.08 | |
| D5 | 3 | 0.66 | 0.61 | 0.46 | |
| D7 | 5 | 0.78 | 0.49 | 0.77 | |
| D8 | 3 | 0.59 | 0.54 | 0.39 | |
| D9 | 5 | 1.42 | 0.88 | 0.91 | |
| D10 | 2 | 0.60 | 0.87 | 0.23 | |
| D11 | 2 | 0.70 | 1.01 | 0.27 | |
| D12 | 1 | 0.00 | 0.00 | 0.00 | |
| II | D16 | 3 | 0.67 | 0.61 | 0.53 |
| D17 | 3 | 0.95 | 0.86 | 0.61 | |
| D18 | 3 | 0.46 | 0.42 | 0.48 | |
| D19 | 6 | 1.51 | 0.84 | 1.25 | |
| D20 | 4 | 1.11 | 0.80 | 0.81 | |
| D21 | 4 | 0.81 | 0.58 | 0.87 | |
| D22 | 4 | 0.59 | 0.43 | 0.75 | |
| D23 | 6 | 1.51 | 0.84 | 1.32 | |
| D24 | 7 | 1.46 | 0.75 | 1.47 | |
| D25 | 3 | 0.95 | 0.86 | 0.76 | |
| D26 | 5 | 1.41 | 0.88 | 1.08 | |
| D27 | 4 | 1.32 | 0.96 | 1.21 | |
| III | D2 | 1 | 0.00 | 0.00 | 0.00 |
| D6 | 1 | 0.00 | 0.00 | 0.00 | |
| D28 | 4 | 0.27 | 0.19 | 0.63 | |
| D29 | 3 | 0.25 | 0.23 | 0.42 | |
| D30 | 5 | 0.80 | 0.50 | 0.86 |
Diversity indices of macrobenthic communities in the mangrove sediments of Xincun Harbor and Xinying Bay in each community area.
3.2 Analysis of contamination of sedimentary environments
3.2.1 Characterization of heavy metal contamination of sediments
The geoaccumulation indices of heavy metal elements Hg, Cr, Cu, Pb, and As in the sediments under natural and plantation forests were significantly different (Figure 4), and the geoaccumulation indices of the same heavy metal elements in the two forests varied less. The ground accumulation indices of heavy metal elements Hg, Cr, Cu, and Pb in sediments from natural and planted forests were all classified as 0, indicating non-pollution, and the ground accumulation indices of heavy metal element As were all classified as 1-2, indicating relatively moderate pollution. The decreasing order of the average ground accumulation indices of heavy metal elements in the two forests was As > Cu > Cr > Pb > Hg, and the ground accumulation indices of As in both areas were significantly higher than those of Hg, Cr, Cu, and Pb for the moderately polluted element As. Pb > Hg, and the Igeo of As was significantly higher than that of Hg, Cr, Cu, and Pb in both areas. The Igeo of natural forests was slightly higher than that of planted forests for the moderately polluted element As.
Figure 4
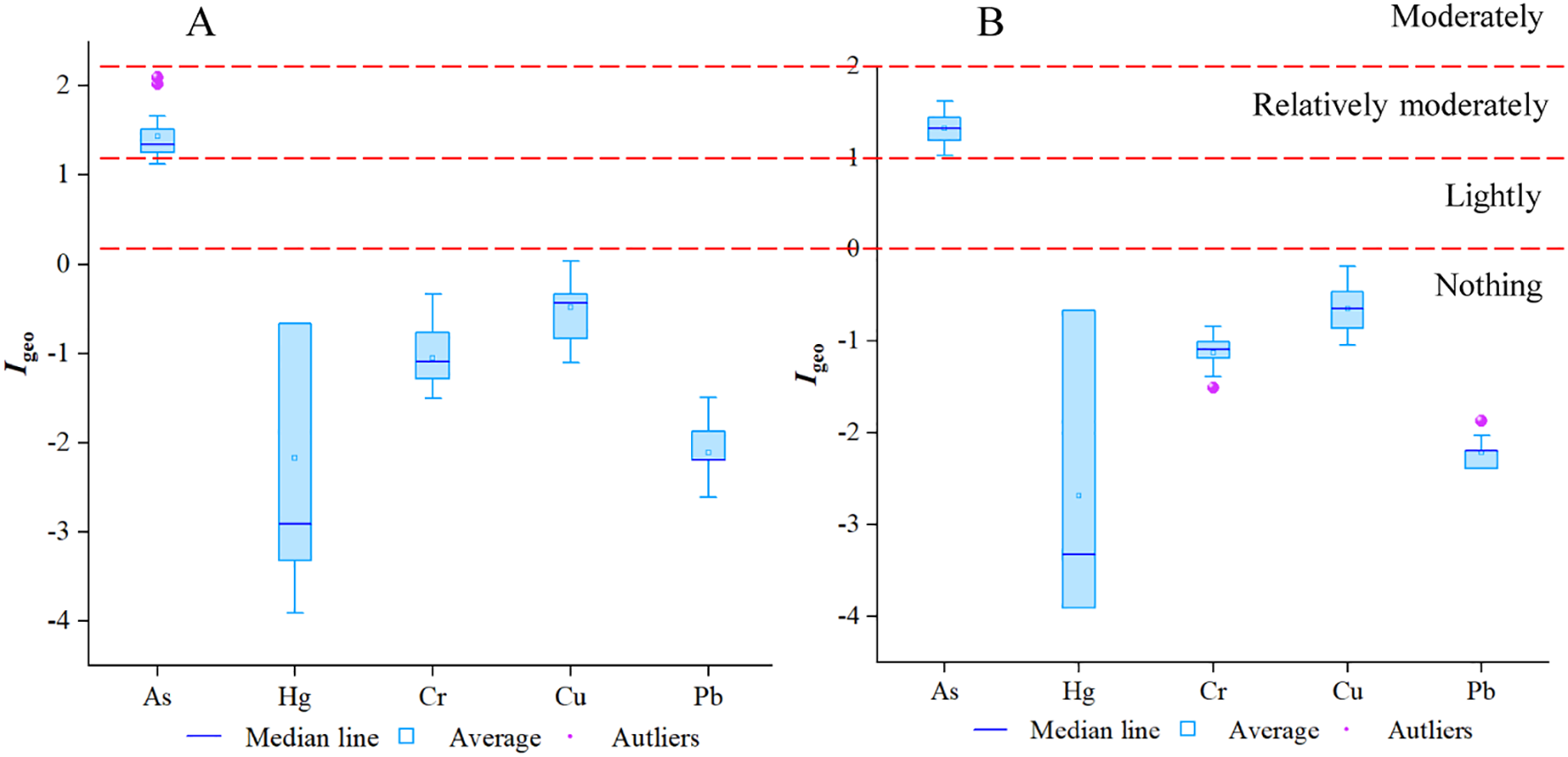
Box plots of heavy metal geoaccumulation indices in sediments, (A, B) are box plots of geoaccumulation indices in planted and natural forests, respectively.
3.2.2 Characterization of sediment element distribution
The elemental contents of the sediments at each sampling point are shown in Table 1. Figure 5 presents a plot of the PCA based on sediment data from the study area, with the first two axes of the PCA explaining 12.4–77.6% of the overall variability. Nutrient elements (TP, TN, and TDS) were more aggregated in the natural forest (Cluster II), sand grains and Hg elements were more aggregated in the plantation forest (Cluster 1), and the heavy metals Cr, Cu, Pb, and As did not show an aggregated distribution in either the plantation or natural forests. The elemental contents in the sampling sites were characterized as follows: The concentrations of TN (0.47%) and Hg (0.019 mg/kg) were higher in sampling sites D25 and D27 in the natural forest than in the other sites (p < 0.05). The concentrations of TDS (8.55 g/kg) and TP (0.354%) were higher in sampling site D18 than the other sites (p < 0.05); and sand grain at D21 (69.7%) was at a lower level (p < 0.05). The concentrations of Hg (0.002 mg/kg), TP (0.027%), TN (0.026%), and TDS (1.74 g/kg) at sampling points D4, D5, D7, and D11 in the plantation forest were lower than those at the other sites (p < 0.05), and the sand grain content (94.7%) at sampling point D12 was higher than at all other sampling points.
Figure 5
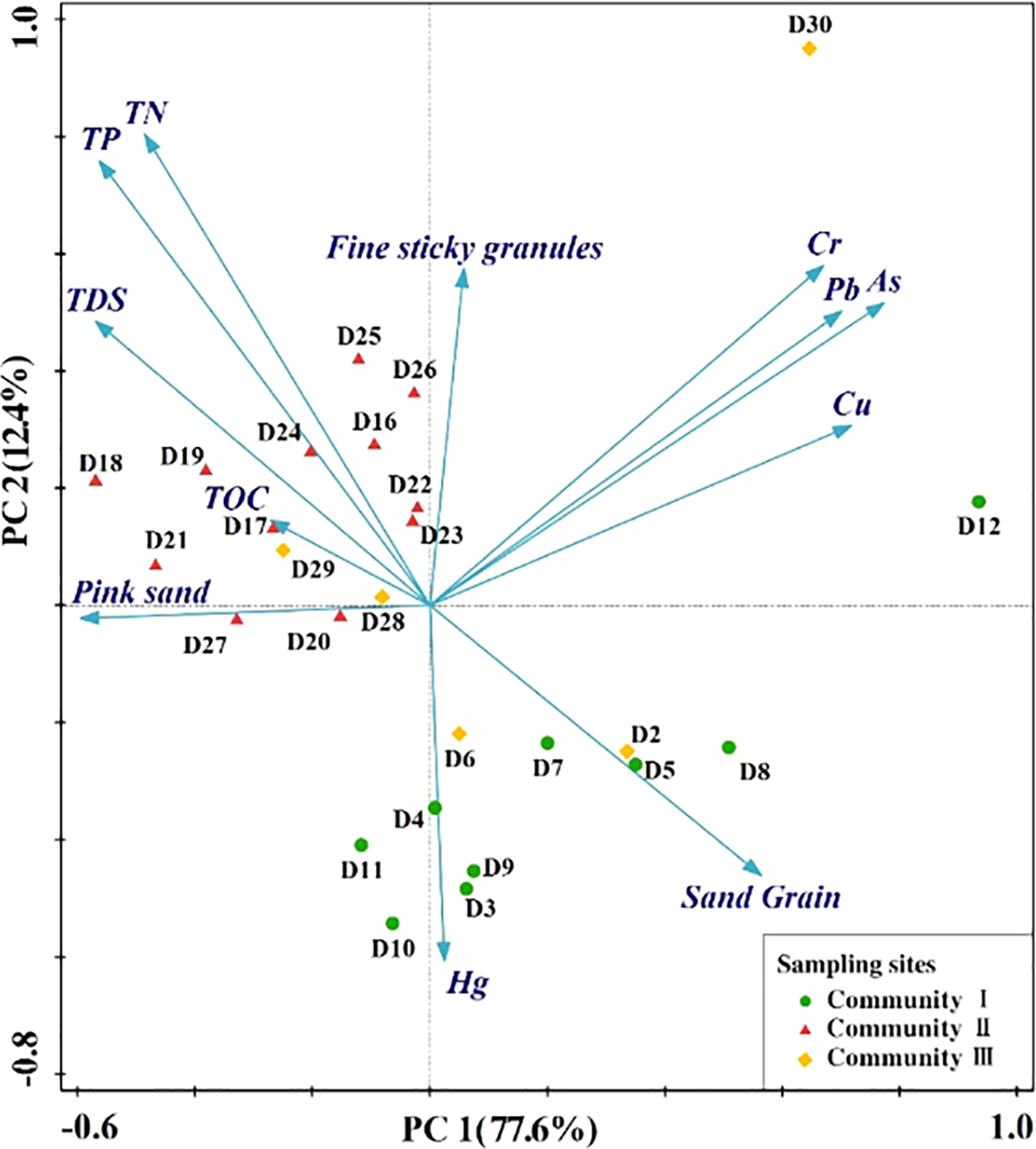
Principal component analysis (PCA) plot of environmental variables.
3.2.3 Associations between sediment elemental contents and benthic species
CCA was used to study the correlation between benthic species and sediment elemental contents (Figure 6). Based on the CCA ordination results for 17 species and 12 sediment elements, the first axis species-environmental factor correlation was 0.9686, the second axis species-environmental correlation was 0.9025, and the ordination results were reliable. PCA1 and PCA2 explained 44.34% and 18.83% of the variance, respectively, with the two axes explaining 63.17%. The arrows represent the environmental factors and indicate the direction of the factors with the greatest variation. Nutrients (TP, TN, TDS), Hg, and sand grains were the most significant variables for biota aggregation (p < 0.05), and the Monte Carlo permutation test showed that TP and TN were the most significant environmental factors influencing community composition (p < 0.05; F-ratio = 5), followed by TDS (p < 0.05; F-ratio = 4), which influenced overall changes in species composition. These results indicate that TP, TN, and TDS were related to Batillaria zonalis, Clithon oualaniensis, Littoraria melanostoma, Cerithidea rhizophorarum, Cerithidea djadjariensis, Allochroa layardi, Lucina scarlatoi, and Capitellidae sp. Sand grain and Hg were the most effective variables explaining the distribution of Marphysa sp. and Geloina erosa. The distribution of As was significantly and positively correlated with Cluster III and significantly and negatively correlated with Clusters I and II.
Figure 6
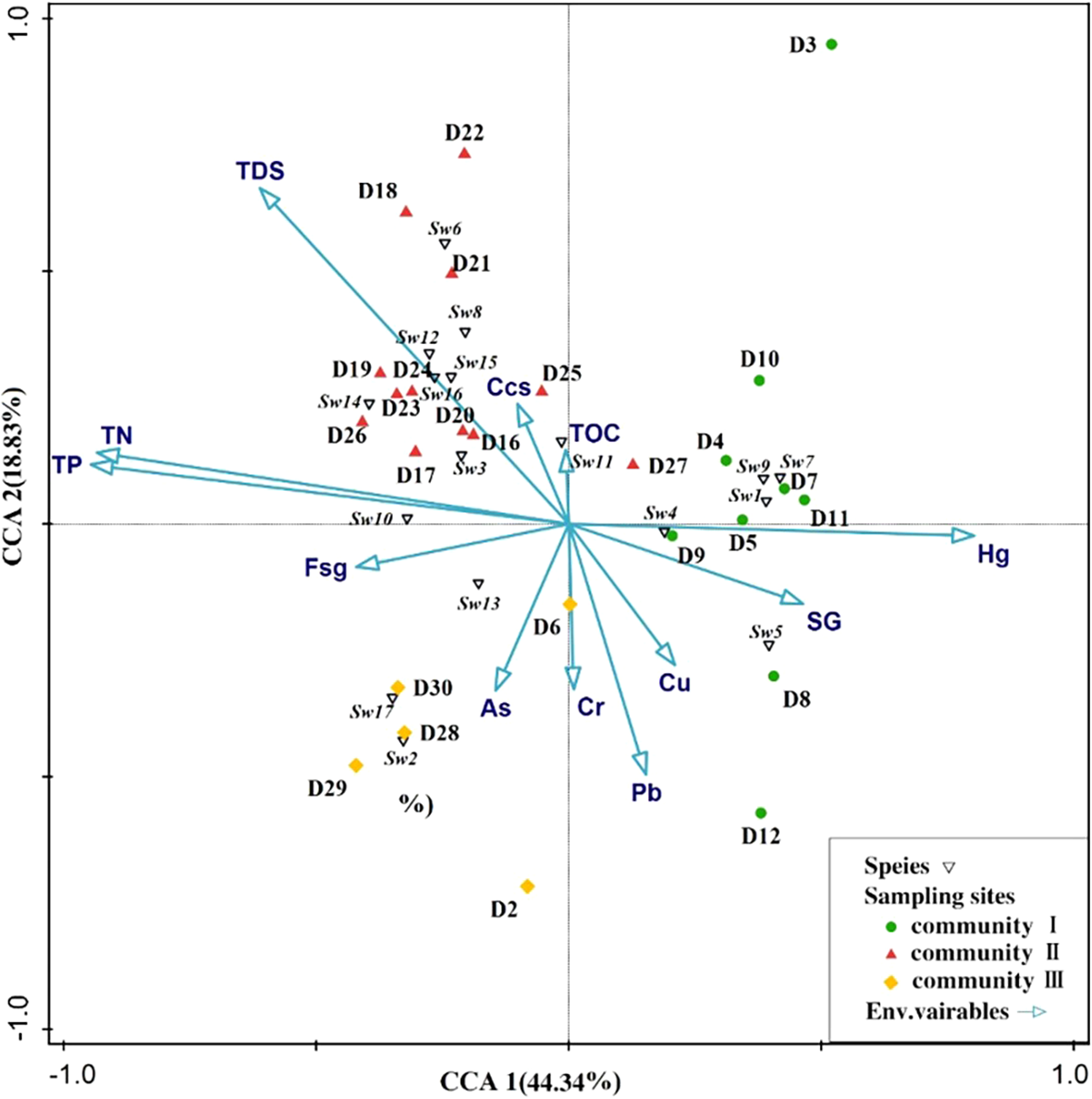
Two-dimensional CCA ordination plot of macrobenthic species versus environmental factors. Fsg denotes fine sticky granules, Ccs denotes pink sand, and SG denotes sand grain. See Table 3 for complete names of abbreviated macrobenthic species names in the figure.
3.3 Correlations between flora and sediment elements.
There was a single type of plant group in the plantation forest, mainly dominated by the Laguncularia racemosa group, covering 12 sampling points such as D1-D12, and the TP values in this area were more aggregated, especially in sampling points D6 and D9 (Figures 7A, C, E). In contrast, the distribution of TN elements was more homogeneous. Figures 7B, D, F show that the natural forests contained four groups, of which Rhizophora stylosa was the main group system, covering 13 sampling points and pooling TN elements in the area, especially at D21–D25, and the distribution of TP elements was more homogeneous. Natural forests were richer than planted forests in terms of community composition, and the TN content of natural forests controlled by Rhizophora stylosa was more concentrated than that of planted forests controlled by Laguncularia racemosa, with multiple areas of higher values. In contrast, the TP values were more concentrated in planted forests.
Figure 7
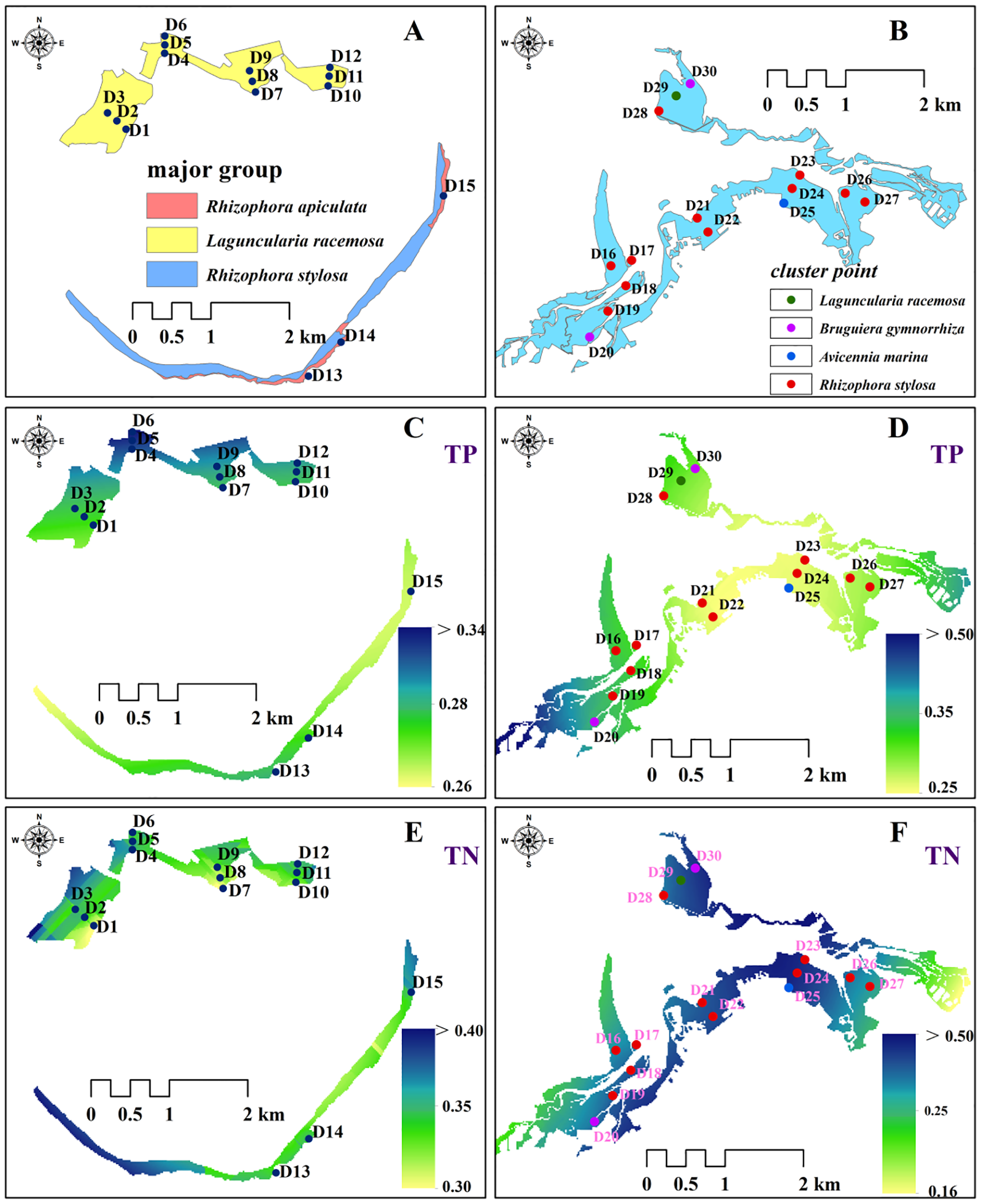
Distribution of communities and interpolation of significant environmental factors. (A, B) show the distribution of the main clusters in the study area; (C, D) and (E, F) are interpolated maps of the content of TP and TN elements, respectively; the main clusters are expressed in the form of points in (B) because of the complex composition of the clusters.
4 Discussion
4.1 Characterization of sediment contamination
Sediment contamination is mainly characterized by heavy metal contamination, which is determined by the toxicity, persistence, non-biodegradability, and bioaccumulation of heavy metals, and as such is attracting widespread attention (Bachirou et al., 2025). Similarly, it is particularly important to focus on pollution from nutrient over-enrichment resulting in coastal eutrophication since mangrove forests have higher total primary production and nutrient accumulation, meaning that mangroves may be sensitive to environmental and anthropogenic impacts on very short time scales (Breithaupt et al., 2018; Atwood et al., 2017). The Igeo results showed that the four heavy metal elements (Hg, Cr, Cu, and Pb) in the sediments of the study area did not pollute the region, with only As showing moderate pollution, which may be due to some anthropogenic activities, with a past survey revealing large areas of fishing row farming, domestic sewage discharges, and corrosion of the hulls of ships in the vicinity of the study area (Hao et al., 2024). This result is consistent with a past study, reflecting ongoing contamination by elemental As. PCA can comprehensively determine the sources of heavy metals in sediments (Hao et al., 2024), and our results demonstrated that Cr, Cu, As, and Pb in the sediments may have similar sources but different sources of Hg. Four elements, Cr, Cu, As, and Pb, may be mainly derived from natural weathering and erosion of continental detrital materials (Zhuang et al., 2018). The As content was higher than the background value, indicating that in addition to natural sources of As, some anthropogenic sources of pollution also existed, including domestic sewage, fish tank farming, and oil discharge from ships (Tang et al., 2023), consistent with the findings of the survey. The results of PCA analysis showed that nutrient elements were more aggregated in natural forests compared to planted forests, particularly for TP, TN, and TDS, which may be because natural forests are richer than planted forests because mixed mangrove forests contain higher elemental accumulations than mangrove forests dominated by a single genus, which would increase the likelihood of higher nutrient accumulations (Atwood et al., 2017; Pérez et al., 2018). TP and total TN are key nutrients in mangrove ecosystems, playing significant roles in maintaining ecosystem health through food chain dynamics. However, excessive inputs of TP and TN can lead to eutrophication, causing algal blooms and oxygen depletion, which disrupt the ecological balance of mangrove systems (Jiang et al., 2025). Ecological restoration, including planting native mangrove species and managing aquaculture activities, can effectively mitigate the impacts of excessive nutrient inputs, thereby protecting the health and biodiversity of mangrove ecosystems. Although mangrove forests are effective biogeochemical barriers that remove large amounts of dissolved and particulate nutrients (mainly nitrogen and phosphorus), they are also susceptible to eutrophication in naturally forested areas (Mack et al., 2024) and therefore require additional attention. There are large areas of mariculture and fisheries around the study area, especially in the Sinchon area (Hao et al., 2024), which experiences localized eutrophication due to the enrichment and accumulation of nutrients caused by mariculture (Zhou et al., 2022). CCA analysis indicated that nutrients (TP, TN, TDS), Hg, and sand grain content were the most significant variables for biota aggregation, indicating that each nutrient and the metal Hg had a significant effect on community structure (Kuk-Dzul et al., 2012). Because Hg is accumulated in organisms and produces toxic effects (Arisekar et al., 2024), it was hypothesized that the biota in the mangrove system of the study area might have been associated with Hg toxicity, which would have adverse consequences. It is assumed that the biota in the mangrove system in the study area may already have experienced toxicity, which will have unfavorable effects on the biological chain, including endangering human health through Hg enrichment in the food chain (Islam et al., 2024). This aspect should be given priority attention and be subject to future monitoring. These results are consistent with the study of Hao et al. (2024). The present study found that As pollution mainly originated from aquaculture and ship activities. Additionally, the monoculture of planted forests leads to hardening of the substrate (Leung, 2015), which limits the diversity of benthic organisms. Meanwhile, high TN enrichment in natural forests may exacerbate the risk of eutrophication (Zhou et al., 2022). Therefore, it is recommended to prioritize the protection of natural forests, limit intensive aquaculture around mangroves, and strengthen monitoring of sediment heavy metal concentrations.
4.2 Habitat analysis of benthic biota
The most dominant species in the study area was Batillaria cumingi, which had an absolute advantage in terms of density. Areas where the organisms were most dense were mostly planted with Laguncularia racemosa, and several beaches in the area were high, with hardened substrate and coral reef debris, which made the depositional environment poor. However, Batillaria cumingi was widely distributed in these areas in large numbers, indicating that it had a strong adaptability and high tolerance to the substrate in the area, This is also closely related to the feeding habits of Batillaria cumingi, which is a Sediment surface feeder, mainly feeding on organic detritus, a behavior that can also lead to direct exposure of Batillaria cumingi to heavy metals (e.g., As). In addition, Assiminea latericea exhibited the highest frequency of occurrence, and it is mostly found in the area dominated by Rhizophora stylosa. The substrate of these areas where the red sea olive grows is softer, and the silt was thicker, Rich in dissolved nutrients (TN, TP). Assiminea latericea is a filter feeder, dependent on suspended particles in the water column, and is significantly influenced by dissolved nutrients (TN, TP), physical properties of the sediment modulate feeding behavior (Scholl et al., 2023). Behera et al. indicated that the loose structure of the sediment can provide higher interstitial space to accommodate a larger number of benthic organisms (Behera et al., 2023), which could be the main reason for the higher frequency of Assiminea latericea in this region. There were significant differences in benthic densities in planted forests, significant differences in benthic biomass in natural forests, and large differences between high- and low-tide sampling sites in the same section (Figure 2) because the small-scale distribution pattern of benthic organisms mainly depends on factors such as tidal state and mangrove vegetation (Chen et al., 2013). Compared with Dongzhai Harbor in Hainan (Li et al., 2017), the lower benthic density in the natural forest of Xinyingwan may be related to the higher level of disturbance from tourism in the present study area. This highlights the negative impacts of anthropogenic pressures on the biological community. Benthic community biodiversity was ranked in the following order: Cluster II > Cluster I > Cluster III. There was no significant difference in the two indices between Cluster I and Cluster II except for the Margalef species abundance index, whereas the opposite was true between Clusters I and III, where there was a complementary relationship, and there was a significant difference in the three indices between Clusters II and III, which suggests that the nature of the habitat exerted an influence on community dynamics (Toumi et al., 2023). The benthic community is an effective ecological indicator for monitoring the status of marine pollution (Mosbahi et al., 2019) and, when combined with Bary-Curtis similarity coefficient clustering analysis, can show the status of environmental quality in the study area. Cluster III was the worst place in terms of environmental quality, followed by Cluster I. Both are in plantation forests, which reflects the frequent anthropogenic disturbances in the area and a high level of human influence. Cluster II in natural forests had the best environmental quality and its exposure to human impacts was relatively weak. These results support those of Murugesan et al., who showed that diversity is higher in natural systems than in artificially developed habitats (Murugesan et al., 2016).
4.3 Relationships between sediments, benthic organisms, and mangrove plants
The relationships between elemental composition in sediments, benthic organisms, and plants are complex and diverse. The association between sediment elemental composition and biological community structure varies from site to site, and regional factors are important in shaping the compositional aspects of benthic communities (Zhang et al., 2021) For example, Langford and Daffern (1975) and Aston and Milner (1980) argued that higher temperatures are thought to affect species phenology in the form of shorter life-cycles and earlier hatching and plumage, while Worthington et al. (2015) argued that most taxa may not have reached their critical thermal tolerance thresholds or could have had their behaviors adapted to higher temperatures (Worthington et al., 2015). A study on macrobenthic invertebrates from a tributary of the Nile River, Egypt, revealed that the most influential environmental variables in structural and functional communities were Na, dissolved oxygen, silicate, pH, Ca, and Cr (Bendary et al., 2023), In the present study, TP, TN, TDS, Hg, and sand grains were the most significant variables affecting the occurrence of taxonomic groups, which then reflected the regional variability output different results. Benthic species, biomass, and diversity were better in natural forests than planted forests because natural/mature mangrove forests generally support higher faunal biodiversity than young or disturbed forests because natural/mature mangrove forests accumulate more nutrients (Chen et al., 2013). This was intuitively demonstrated by the lower benthic biodiversity under planted forest cover compared to that of natural forests in the present study. Different mangrove communities support different benthic biota and different mangrove communities aggregate different sediment elements, which have a significant influence on macrobenthic diversity indices (richness and evenness) (Chen et al., 2015; Leung, 2015). In this study, the Laguncularia racemosa group lineage aggregated TP and the dominant species was Batillaria cumingi and the Rhizophora stylosa group lineage aggregated TN and the dominant species was Assiminea latericea. In addition, the biodiversity under the Laguncularia racemosa cover was lower than that under Rhizophora stylosa (Figures 2, 3, and 7 and Table 3).
This study explored the relationship between sediment contamination and the role of benthos and its habitats. However, the small sample size of the study (30 sampling sites) may limit the generalizability of the results, which did not cover seasonal variations (e.g., the difference in pollutant transport between the rainy and dry seasons) and has not yet been analyzed in terms of microcosmology and microcosmic experiments to validate the toxicity mechanism, etc. To improve the biological prediction of possible environmental contamination in the context of global environmental protection, future research on biological and sediment contamination should focus on less-studied populations and regions and long-term research and monitoring considering seasonal factors, It is necessary to combine the long-term monitoring and multiscale experiments (e.g. plant tissues, biomarkers) to analyze the ecological effects of pollutants in depth.
5 Conclusions
Four heavy metals (Hg, Cr, Cu, and Pb) in the sediments of the study area did not pollute the area, with only As showing moderate pollution, and contamination by As continues. Benthic densities in natural forests were significantly lower than those in planted forests. Species composition differed considerably between natural and planted forests. Biodiversity indices were higher in natural forests than in planted forests. Cluster III had the worst environmental quality, followed by Clusters I and II.
Natural forests contained higher concentrations of nutrients (TP, TN, and TDS) than planted forests and were more susceptible to eutrophication. The overall change in species composition was influenced by TDS, the Laguncularia racemosa group lineage aggregated TP and the biologically dominant species was Batillaria cumingi. The Rhizophora stylosa group system aggregated TN and the dominant species was Assiminea latericea. Biodiversity under Laguncularia racemosa cover was lower than that under Rhizophora stylosa.
The results provide new insights into the impacts of human activities on marine pollution and organisms, particularly in mangrove areas. To prevent the continuation of As pollution and the potential harm caused by Hg to living organisms, a series of measures focusing on emission source management should be considered, including banning intensive mariculture patterns based on fertilizer application, strengthening pollution source control of heavy metals and nutrients, and enhancing hydrodynamics to reduce the accumulation of fine particulate matter.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
FZ: Conceptualization, Investigation, Writing – original draft. ZH: Data curation, Funding acquisition, Investigation, Project administration, Resources, Writing – review & editing. ZC: Data curation, Investigation, Methodology, Writing – original draft. JL: Formal analysis, Writing – original draft, Writing – review & editing. MZ: Data curation, Methodology, Writing – original draft. WD: Project administration, Resources, Writing – review & editing. XG: Investigation, Visualization, Writing – original draft. LC: Data curation, Methodology, Writing – original draft. ZW: Data curation, Methodology, Writing – original draft. YX: Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Geological Survey Project of China Geological Survey (DD20220993; DD20251040; DD20242618) and Hainan Provincial Natural Science Foundation of China (424MS116).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1542864/full#supplementary-material
References
1
Abdi H. Williams L. J. (2010). Principal component analysis. Wiley Interdiscip. Reviews: Comput. Stat2, 433–459. doi: 10.1016/b978-0-12-815739-8.00012-2
2
Al-Kahtany K. Nour H. E. El-Sorogy A. S. Alharbi T. (2023). Ecological and health risk assessment of heavy metals contamination in mangrove sediments, Red Sea coast. Mar. pollut. Bull.192, 115000. doi: 10.1016/j.marpolbul.2023.115000
3
Anderson M. Braak C. T. (2003). Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Sim73, 85–113. doi: 10.1080/00949650215733
4
Arbi I. Liu S. Zhang J. Wu Y. Huang X. (2018). Detection of terrigenous and marine organic matter flow into a eutrophic semi-enclosed bay by δ13C and δ15N of intertidal macrobenthos and basal food sources. Sci. Total Environ.613, 847–860. doi: 10.1016/j.scitotenv.2017.09.143
5
Arisekar U. Shalini R. Shakila R. J. Iburahim S. A. Anantharaja K. Rathinam R. B. et al . (2024). Selenium and mercury concentration, Se/Hg molar ratio and risk–benefit assessment of marine fish consumption: Human health risks and protective role of Se against Hg toxicity. Food Res. Int.180, 114086. doi: 10.1016/j.foodres.2024.114086
6
Aston R. J. Milner A. G. P. (1980). A comparison of populations of the isopod Asellus aquaticus above and below power stations in organically polluted reaches of the River Trent. Freshw. Biol.10, 1–14. doi: 10.1111/j.1365-2427.1980.tb01175.x
7
Atwood T. B. Connolly R. M. Almahasheer H. Carnell P. E. Duarte C. M. Ewers Lewis C. J. et al . (2017). Global patterns in mangrove soil carbon stocks and losses. Nat. Climate Change7, 523–528. doi: 10.1038/nclimate3326
8
Bachirou D. Marie N. B. B. L. Paul-Désiré N. (2025). Assessment of pollution and ecological risk associated with heavy metals in sediments from the rivers of Batouri gold mining area (East Cameroon): geochemical and statistical approaches. J. Sediment Environ.10, 159–171. doi: 10.1007/s43217-025-00216-x
9
Behera R. Mishra S. Sharma S. D. Mahapatro D. Pati S. S. Raut D. et al . (2023). Influence of water quality and sediment nature on macrobenthic community structure along Paradeep, an industrial and port influenced tropical coastal stretch of North East coast of India, Bay of Bengal. Reg. Stud. Mar. Sc62, 102970. doi: 10.1016/j.rsma.2023.102970
10
Bendary R. E. Ibrahim S. M. Goher M. E. Elsaied H. E. El Shabrawy G. M. El Mordy M. A. et al . (2023). Taxonomic and functional structure of macrobenthic invertebrate communities and their response to environmental variables along the subbranches of the Nile River (rayahs), Egypt. Environ. Sci. pollut. R30, 28803–28817. doi: 10.1007/s11356-022-24140-z
11
Black K. D. Shimmield G. B. (Eds.) (2003). Biogeochemistry of Marine Systems (1st ed.). (CRC Press). doi: 10.1201/9780367812423
12
Bodaghabadi M. B. Salehi M. H. Martínez-Casasnovas J. A. Mohammadi J. Toomanian N. Borujeni I. E. (2011). Using Canonical Correspondence Analysis (CCA) to identify the most important DEM attributes for digital soil mapping applications. Catena86, 66–74. doi: 10.1016/j.catena.2011.02.009
13
Bouillon S. Borges A. V. Castañeda-Moya E. Diele K. Dittmar T. Duke N. C. et al . (2008). Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochem. Cycles22 (2), 1–12. doi: 10.1029/2007GB003052
14
Bramha S. Pradhan U. Sarangapani R. Chandrasekaran S. Krishnaveni M. (2025). Distribution of natural radionuclides and associated geological properties in shelf sediment of southwest (SW) Bay of Bengal: a multivariate statistical approach. Reg. Stud. Mar. Sc82, 104035. doi: 10.1016/j.rsma.2025.104035
15
Breithaupt J. L. Smoak J. M. Byrne R. H. Waters M. N. Moyer R. P. Sanders C. J. (2018). Avoiding timescale bias in assessments of coastal wetland vertical change. Limnol. Oceanogr.63, S477–S495. doi: 10.1002/lno.v63.S1
16
Celis-Hernandez O. Giron-Garcia M. P. Ontiveros-Cuadras J. F. Canales-Delgadillo J. C. Pérez-Ceballos R. Y. Ward R. D. et al . (2020). Environmental risk of trace elements in mangrove ecosystems: An assessment of natural vs oil and urban inputs. Sci. Total Environ.730, 138643. doi: 10.1016/j.scitotenv.2020.138643
17
Chaudhuri P. Nath B. Birch G. (2014). Accumulation of trace metals in grey mangrove Avicennia marina fine nutritive roots: The role of rhizosphere processes. Mar. pollut. Bull.79, 284–292. doi: 10.1016/j.marpolbul.2013.11.024
18
Chen M. Li J. Huang G. Lin Y. Zeng S. Xu Y. et al . (2025). Distribution characteristics and influencing factors of benthic diatoms on several typical beaches along the southern coast of China. Mar. Environ. Res.204, 106854. doi: 10.1016/j.marenvres.2024.106854
19
Chen Q. Li J. Zhang L. Lu H. Ren H. Jian S. (2015). Changes in the macrobenthic faunal community during succession of a mangrove forest at Zhanjiang, South China. J. Coastal. Res.31, 315–325. doi: 10.2112/JCOASTRES-D-13-00019.1
20
Chen G. Yu D. Ye Y. Chen B. (2013). Impacts of mangrove vegetation on macro-benthic faunal communities. Shengtai Xuebao/Acta Ecol. Sin.33, 327–336. doi: 10.5846/stxb201111091699
21
ELTurk M. Abdullah R. Zakaria R. M. Bakar N. K. A. (2019). Heavy metal contamination in mangrove sediments in Klang estuary, Malaysia: Implication of risk assessment. Estuar. Coast. Shelf Sci.226, 106266. doi: 10.1016/j.ecss.2019.106266
22
Fu Y. (2014). Studies on Soil Geochemistry and High-Quality Agriculture in Hainan Island. (Doctoral dissertation) (WuHan: China University of Geosciences, Wuhan).
23
Gao F. Yin H. B. Hu W. P. Deng J. C. Gao J. F. (2011). Ecological characteristics of macrobenthic communities in the Chaohu basin basin and their relationship with environmental factors. Journal of Animal & Veterinary Advances10(5), 627–634. doi: 10.3923/javaa.2011.627.634
24
Gong H. M. Liu Y. Xiao Y. Y. Li C. H. (2018). Assessment on heavy metals pollution and potential ecological risk of seawater and surface sediments in coastal waters: a case study in Xincun Lagoon. Res. Agric. Modernization39, 700–708. doi: 10.13872/j.1000-0275.2018.0039
25
Hangjun W. Weimin Y. Yi L. Qing Z. Yalin L. (2020). Macrobenthic community and its relationship with environmental factors in the Yueqing Bay, Zhejiang Province,China. Haiyang Xuebao42, 75–86. doi: 10.3969/j.issn.0253-4193.2020.02.008
26
Hao Z. Qian J. Zheng F. Lin B. Xu M. Feng W. et al . (2024). Human-influenced changes in pollution status and potential risk of sediment heavy metals in Xincun Bay, a typical lagoon of Hainan, China. Mar. pollut. Bull.199, 115949. doi: 10.1016/j.marpolbul.2023.115949
27
Hejcmanovā-Nežerková P. Hejcman M. (2006). A canonical correspondence analysis (CCA) of the vegetation–environment relationships in Sudanese savannah, Senegal. S Afr. J. Bot.72, 256–262. doi: 10.1016/j.sajb.2005.09.002
28
Hou Y. Kong F. Li Y. Xi M. Yu Z. (2020). Key factors of the studies on benthic macroinvertebrate in coastal wetlands: Methods and biodiversity. Ecohydrol. Hydrobiol.20, 424–436. doi: 10.1016/j.ecohyd.2020.02.004
29
Islam M. A. Kabir S. Lubis A. A. Sugiharto U. Islam M. M. Hossen M. B. (2024). 210Pb dating and neutron activation analysis of the Sundarban mangrove sediments: sedimentation rate and metal contamination history. Radiochimica Acta112, 273–287. doi: 10.1515/ract-2023-0245
30
Jiang Y. Zhang Z. Friess D. A. Li Y. Zhang Z. Xin R. et al . (2025). Restoring mangroves lost by aquaculture offers large blue carbon benefits. One Earth8 (1). doi: 10.1016/j.oneear.2024.11.003
31
Kuk-Dzul J. G. Gold-Bouchot G. Ardisson P. L. (2012). Benthic infauna variability in relation to environmental factors and organic pollutants in tropical coastal lagoons from the northern Yucatan Peninsula. Mar. pollut. Bull.64, 2725–2733. doi: 10.1016/j.marpolbul.2012.09.022
32
Langford T. E. Daffern J. R. (1975). The emergence of insects from a British River warmed by power station cooling-water: Part I—The use and performance of insect emergence traps in a large, spate-river and the effects of various factors on total catches, upstream and downstream of the cooling-water outfalls. Hydrobiologia46, 71–114. doi: 10.1007/BF00038727
33
Lee H. Y. Shih S. S. (2004). Impacts of vegetation changes on the hydraulic and sediment transport characteristics in Guandu mangrove wetland. Ecol. Eng.23, 85–94. doi: 10.1016/j.ecoleng.2004.07.003
34
Leung J. Y. (2015). Habitat heterogeneity affects ecological functions of macrobenthic communities in a mangrove: Implication for the impact of restoration and afforestation. Glob. Ecol. Conserv.4, 423–433. doi: 10.1016/j.gecco.2015.08.005
35
Li Y. F. Du F. Y. Gu Y. G. Ning J. J. Wang L. G. (2017). Changes of the macrobenthic faunal community with stand age of a non-native mangrove species in Futian Mangrove National Nature Reserve, Guangdong, China. Zool. Stud.56 (19). doi: 10.6620/ZS.2017.56-19
36
Ma K. Huang B. Liu F. X. (2012). Biodiversity of macro zoobenthos in mangrove forest around Dongzhai Harbor. J. Ecol. Rural Environ.28, 675–680.
37
Mack M. R. Langley J. A. Feller I. C. Chapman S. K. (2024). The ecological consequences of nutrient enrichment in mangroves. Estuar. Estuar. Coast. Shelf S300, 108690. doi: 10.1016/j.ecss.2024.108690
38
Meng Y. Gou R. Bai J. Moreno-Mateos D. Davis C. C. Wan L. et al . (2022). Spatial patterns and driving factors of carbon stocks in mangrove forests on Hainan Island, China. Global Ecol. Biogeogr.31, 1692–1706. doi: 10.1111/geb.13549
39
Mosbahi N. Serbaji M. M. Pezy J. P. Neifar L. Dauvin J. C. (2019). Response of benthic macrofauna to multiple anthropogenic pressures in the shallow coastal zone south of Sfax (Tunisia, central Mediterranean Sea). Environ. pollut.253, 474–487. doi: 10.1016/j.envpol.2019.06.080
40
Mountouris A. Voutsas E. Tassios D. (2002). Bioconcentration of heavy metals in aquatic environments: the importance of bioavailability. Mar. pollut. Bull.44, 1136–1141. doi: 10.1016/S0025-326X(02)00168-6
41
Muller G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal2 (3), 109–118.
42
Murugesan P. Pravinkumar M. Muthuvelu S. Ravichandran S. Vijayalakshmi S. Balasubramanian T. (2016). Benthic biodiversity in natural vis-a-vis artificially developed mangroves of south east coast of India. Indian journal of marine sciences45 (8), 1049–1058.
43
Muthukumaravel K. Pradhoshini K. P. Vasanthi N. Raja T. Jaleel M. A. Arunachalam K. D. et al . (2021). Assessment of seasonal variation in distribution and abundance of plankton and ichthyofaunal diversity in relation to environmental indices of Karankadu Mangrove, South East Coast of India. Mar. pollut. Bull.173, 113142. doi: 10.1016/j.marpolbul.2021.113142
44
Nath B. Birch G. Chaudhuri P. (2013). Trace metal biogeochemistry in mangrove ecosystems: a comparative assessment of acidified (by acid sulfate soils) and non-acidified sites. Sci. Total Environ.463, 667–674. doi: 10.1016/j.scitotenv.2013.06.024
45
Pan K. Wang W. X. (2012). Trace metal contamination in estuarine and coastal environments in China. Sci. Total Environ.421, 3–16. doi: 10.1016/j.scitotenv.2011.03.013
46
Parvez Al Usmani S. M. (2018). Habitat heterogeneity and spatio-temporal distribution of macrobenthos in in a tropical estuarine mangrove ecosystem. J. Ecophysiol. Occup. Health18 (3–4), 80–85. doi: 10.18311/jeoh/2018/21374
47
Pérez A. Libardoni B. G. Sanders C. J. (2018). Factors influencing organic carbon accumulation in mangrove ecosystems. Biol. Lett.14, 20180237. doi: 10.1098/rsbl.2018.0237
48
Pérez A. MaChado W. Gutierrez D. Smoak J. M. Breithaupt J. L. Saldarriaga M. S. et al . (2020). Carbon and nutrient accumulation in mangrove sediments affected by multiple environmental changes. J. Soils Sediments20, 2504–2509. doi: 10.1007/s11368-020-02612-4
49
Santos T. M. T. Rabelo D. M. L. Beasley C. R. Braga C. F. (2020). Vertical distribution of macrobenthic community of tropical saltmarshes on the Amazon coast. Reg. Stud. Mar. Sci.40, 101536. doi: 10.1016/j.rsma.2020.101536
50
Scholl E. A. Cross W. F. Guy C. S. Dutton A. J. Junker J. R. (2023). Landscape diversity promotes stable food-web architectures in large rivers. Ecol. Lett.26, 1740–1751. doi: 10.1111/ele.14289
51
Siikamäki J. Sanchirico J. N. Jardine S. L. (2012). Global economic potential for reducing carbon dioxide emissions from mangrove loss. P. Natl. Acad. Sci. U. S. A109, 14369–14374. doi: 10.1073/pnas.1200519109
52
Silva-Camacho D. D. S. Gomes R. D. S. Santos J. N. Araújo F. G. (2017). Distribution of benthic fauna in sediment grains and prop roots of a mangrove channel in south-eastern Brazil. J. Mar. Biol. Assoc. U. K97, 377–385. doi: 10.1017/S0025315416000485
53
Sun Y. X. Li X. X. Tan Y. Wang J. Dong Y. W. (2022). Microhabitat thermal environment controls community structure of macrobenthos on coastal infrastructures. Estuar. Coast. Shelf S277, 108060. doi: 10.1016/j.ecss.2022.108060
54
Tang D. Liu X. Xia Z. Hou J. Yang X. Li P. et al . (2023). Sources of organic matter and carbon stocks in two mangrove sediment cores and surface sediment samples from Qinglan Bay, China. Sci. Total Environ.893, 164897. doi: 10.1016/j.scitotenv.2023.164897
55
Toumi C. De Cáceres M. Grall J. Boyé A. Thiébaut É. Maguer M. et al . (2023). Long-term coastal macrobenthic Community Trajectory Analysis reveals habitat-dependent stability patterns. Ecography2023, e06489. doi: 10.1111/ecog.06489
56
van der Linden P. Patrício J. Marchini A. Cid N. Neto J. M. Marques J. C. (2012). A biological trait approach to assess the functional composition of subtidal benthic communities in an estuarine ecosystem. Ecol. Indic.20, 121–133. doi: 10.1016/j.ecolind.2012.02.004
57
Wen D. Hong M. Wang H. Cao Q. Zhou W. Wang X. et al . (2023). Spatiotemporal dynamics and potential restoration of mangroves in Circum-Xinying-Bay region, Hainan Province, China. J. Sea Res.193, 102368. doi: 10.1016/j.seares.2023.102368
58
Worthington T. A. Shaw P. J. Daffern J. R. Langford T. E. L. (2015). The effects of a thermal discharge on the macroinvertebrate community of a large British river: implications for climate change. Hydrobiologia753, 81–95. doi: 10.1007/s10750-015-2197-1
59
Yamada K. Maegawa S. Toyohara H. (2013). Benthic animal contribution to cellulose breakdown in sediments of mangrove estuaries in the southwestern islands of Japan. Plankton Benthos Res.8, 96–101. doi: 10.3800/pbr.8.96
60
Yang Y. Shu G. Liang Z. Yunwei W. Gaocong L. Yaping W. et al . (2016). Grain size distribution of surface sediments and sedimentary environment in the lagoon of Xincun, Hainan Island. Haiyang Xuebao38, 94–105. doi: 10.3969/j.issn.0253-4193.2016.01.009
61
Yarahmadi H. Khorsandi Z. (2024). Mangrove forest decline on Iran’s Gulf coast. Science383, 1067–1067. doi: 10.1126/science.ado0376
62
Ying T. Xunhao Z. Ci C. Yue L. Lu W. Zhaoying G. et al . (2021). Temporal and spatial distributions of macroinvertebrates and their influencing environmental factors. Shengtai Xuebao41, 747–760. doi: 10.5846/stxb201912202752
63
You Q. Deng W. Tang X. Liu Y. Lei P. Chen J. et al . (2024). Monitoring of mangrove dynamic change in Beibu Gulf of Guangxi based on reconstructed time series images. Sci. Total Environ.917, 170395. doi: 10.1016/j.scitotenv.2024.170395
64
Zhang Z. W. Xu X. R. Sun Y. X. Yu S. Chen Y. S. Peng J. X. (2014). Heavy metal and organic contaminants in mangrove ecosystems of China: a review. Environ. Sci. pollut. Res.21, 11938–11950. doi: 10.1007/s11356-014-3100-8
65
Zhang Q. Yang T. Wan X. Wang Y. Wang W. (2021). Community characteristics of benthic macroinvertebrates and identification of environmental driving factors in rivers in semi-arid areas–a case study of Wei River Basin, China. Ecol. Indic.121, 107153. doi: 10.1016/j.ecolind.2020.107153
66
Zhou W. Wu H. Huang J. Wang J. Zhen W. Wang J. et al . (2022). Elevated-CO2 and nutrient limitation synergistically reduce the growth and photosynthetic performances of a commercial macroalga Gracilariopsis lemaneiformis. Aquaculture550, 737878. doi: 10.1016/j.aquaculture.2021.737878
67
Zhu B. Liao J. Shen G. (2021). Combining time series and land cover data for analyzing spatio-temporal changes in mangrove forests: A case study of Qinglangang Nature Reserve, Hainan, China. Ecol. Indic.131, 108135. doi: 10.1016/j.ecolind.2021.108135
68
Zhu H. N. Yuan X. Z. Zeng G. M. Jiang M. Liang J. Zhang C. et al . (2012). Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index. T. Nonferr. Metal. Soc.22, 1470–1477. doi: 10.1016/S1003-6326(11)61343-5
69
Zhuang Q. Li G. Liu Z. (2018). Distribution, source and pollution level of heavy metals in river sediments from South China. Catena170, 386–396. doi: 10.1016/j.catena.2018.06.037
Summary
Keywords
mangrove forests, sediment contamination characteristics, benthic habitats, mangrove wetlands, role relationships
Citation
Zheng F, Huang Z, Chen Z, Liu J, Zhou M, Ding W, Guo X, Chen L, Wang Z and Xu Y (2025) Characterization of sediment contamination and benthic habitat response in mangrove ecosystems of Hainan Province. Front. Mar. Sci. 12:1542864. doi: 10.3389/fmars.2025.1542864
Received
10 December 2024
Accepted
12 May 2025
Published
29 May 2025
Volume
12 - 2025
Edited by
Xinchen Gu, China Institute of Water Resources and Hydropower Research, China
Reviewed by
Satyanarayan Bramha, Indira Gandhi Centre for Atomic Research (IGCAR), India
Shiquan Chen, Hainan Academy of Ocean and Fisheries Sciences, China
Mohammed O. Aljahdali, King Abdulaziz University, Saudi Arabia
Updates
Copyright
© 2025 Zheng, Huang, Chen, Liu, Zhou, Ding, Guo, Chen, Wang and Xu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zanhui Huang, 540177626@qq.com; Yan Xu, grawain007@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.