- 1Centre for Marine Science and Innovation (CMSI), School of BEES, University of New South Wales, Sydney, NSW, Australia
- 2Department of Climate Change, Energy, Environment and Water, Marine Wildlife, NSW National Parks & Wildlife Service, Sydney, NSW, Australia
- 3Marine Predator Research Group, School of Natural Sciences, Macquarie University, Sydney, NSW, Australia
- 4Marine Science Program, Department of Biodiversity, Conservation and Attractions, Perth, WA, Australia
The spatial distribution of calving habitat of humpback whales (Megaptera novaeangliae) has been understood as confined to warm, low latitude waters. The need for females to reach such habitats to give birth and provide early maternal care underpins the dominant theories on humpback whale migration. In Australia, calving habitat is accepted to be distributed no further south than 23-28°S and is generally presumed not to occur in New Zealand. The aim of this study was to understand if humpback whales birth beyond these limits and, if so, whether cow-calf pairs continue migrating northward. Records of neonate calves were collated from several sources, including government agencies, annual migration surveys and opportunistic citizen science observations, primarily from tourism vessels. Strict inclusion protocols were applied to ensure correct identification of calves as neonates. More than 200 neonates were recorded in all jurisdictions in the study area, to 43°S, approximately 1300 – 1500 km south of the previously reported southern limit of calving grounds in Australasia. These records demonstrate that humpback whales do not confine their calving solely to tropical zones but exhibit a continuum of habitat use throughout their range. The cow-calf pairs were consistently observed to continue northward in Eastern Australia and New Zealand, suggesting that birth does not define the endpoint of the migration. There are management implications of these findings as currently some jurisdictions are not recognized as calving habitats. Therefore, the revision of protection measures in these areas given the new evidence of extended calving habitat would assist in reducing anthropogenic threats to young calves during vulnerable life stages. Additionally, the continuation of northward migration after birth of the calf is an important observation for migration theory. Future studies comparing the outcomes for calves born during migration with those born in the tropical wintering grounds could provide empirical information to evaluate hypotheses on the drivers of migration. The results of this study challenge prevailing notions of calving and migration behavior in this species.
Introduction
Humpback whales (Megaptera novaeangliae) are a highly migratory species that occur in all oceans (Clapham, 1996; Cooke, 2018). The annual migration of thousands of kilometers between high latitude summer habitats and low latitude winter habitats coincides with the reproductive cycle of both sexes (Chittleborough, 1965). Except for a population that is resident in the Arabian Sea year-round (Dakhteh et al., 2017), humpback whales are capital breeders, exploiting the seasonal abundance of food at high latitudes during summer and using the stored energy to meet the energetic demands both of migration and reproduction through the winter (Irvine et al., 2017).
Historically the ‘feeding/breeding’ paradigm has dominated humpback whale ecology, where feeding and reproduction are spatially and temporally separated, with summer ‘feeding grounds’ and winter ‘breeding grounds’ restricted to habitats at the latitudinal extremes of their range (Chittleborough, 1965; Dawbin, 1966). In this paradigm, the summer and winter habitats are connected by a ‘migration corridor’ considered only to be a thoroughfare. Early 20th century whalers reported that very little stomach content was found in whales killed along the migration path and this informed the belief that humpback whales fast through their migration to and from the tropics (Chittleborough, 1965). However, humpback whales have been shown to feed in this migration corridor (Eisenmann et al., 2016; Garrigue et al., 2015; Pirotta et al., 2021, 2022; Barendse et al., 2013; Stockin and Burgess, 2005; Danilewicz et al., 2009; Findlay et al., 2017) along with performing other important behaviors, such as resting (Jones et al., 2023), maintenance of skin health (Meynecke et al., 2023), and song sharing (Warren et al., 2020). In summer, food is abundant in their high latitude habitats and exploiting this rich resource is a key aspect of humpback whale ecology, however feeding is not restricted to these ‘feeding grounds’. This, together with the variety of behaviors observed during migration suggests that the behavior of humpback whales is more complex than the strict spatially and temporally segregated feeding/breeding paradigm. Thirty years ago, Brown and Corkeron (1995) proposed that humpback whales show a behavioral continuum while on migration between summer and wintering grounds, yet this received little attention. This prompted us to question whether breeding and calving are also more spatially distributed than currently understood.
The term ‘breeding grounds’, referring to the low latitude migration terminus, implies an imperative for pregnant females to reach the end point of their migration to give birth. It follows that migration to the tropics is a requirement for calving and breeding, rather than coincident with calving and breeding. Migration is energetically costly. The specific factors driving their need to migrate remain contentious. The putative benefits of low latitude environments to the survivorship and growth of calves underpin the dominant theories of migration (Meynecke et al., 2021) – for example: the thermoregulatory benefit of warm waters allows the calves to devote more energy to growth (Clapham, 2001; Brodie, 1975); the calm waters improve survivorship of calves (Whitehead and Moore, 1982); or wintering grounds offer a refuge from predators that target calves, specifically orca (Orcinus orca) (Corkeron and Connor, 1999; Connor and Corkeron, 2001). Alternative hypotheses suggest benefits unrelated to breeding, for example that warm water is necessary for skin molting (Pitman et al., 2020). Ethical and logistical considerations preclude manipulative experiments that could empirically test some of these hypotheses but comparisons between the outcomes of different migration and/or breeding strategies could offer insight. It is also necessary to disentangle the two assumptions of the feeding/breeding paradigm: 1) that calving and breeding are restricted to discrete ‘breeding grounds’ at the terminus of the migration, that is, calving areas and breeding grounds are mutually inclusive; and 2) calving and/or breeding activities drive migration rather than being coincident with migration. These two aspects of the breeding/feeding paradigm require separate attention and investigation.
The tropical or sub-tropical ‘breeding grounds’, where calves are frequently encountered in winter (hereafter ‘wintering grounds’), are not precisely delineated but it is generally accepted that calving is limited to within 19° – 22°from the equator in both hemispheres (Rasmussen et al., 2007). Many studies have shown that within these latitudes females with newborn calves (neonates) are more frequently found in warm (19°C to 28°C), shallow (15 to 60m), protected waters, which is interpreted as the preferred calving habitat for this species, as seen in the North Pacific (Cartwright et al., 2012; Craig et al., 2014; Currie et al., 2018), Eastern South Pacific (Felix and Haase, 2005; Félix and Botero-Acosta, 2011; Félix and Guzmán, 2014; Guidino et al., 2014; Guzman and Félix, 2017; Oña et al., 2017; Oviedo and Solís, 2008; Rasmussen et al., 2012), Western South Pacific (Smith et al., 2012), Indian Ocean (Trudelle et al., 2016), Western North Atlantic (Whitehead and Moore, 1982) and Western South Atlantic (Bortolotto et al., 2017; Martins et al., 2001; Zerbini et al., 2004). Opportunistic observations of parturition (birth) demonstrate that calving does indeed occur in these low latitude winter habitats, although published accounts of such observations are infrequent (Faria et al., 2013; Ferreira et al., 2011; Paterson and Paterson, 1989; Patton and Lawless, 2021; Ransome et al., 2022a) and the latitudinal limits of calving grounds remain relatively uncertain for all humpback populations.
In Australia, the humpback whales that migrate annually along the western and eastern coasts are recognized as separate populations, respectively the International Whaling Commission (IWC) D stock and E1 stock (hereafter the Western Australia and Eastern Australia populations) (Gales et al., 2011). New Zealand lies in the main migration path of the IWC E2 stock (hereafter New Zealand population), which winters in the waters around New Caledonia, Tonga and Fiji (Gales et al., 2011). The Western Australia population appears to have limited connectivity with other groups, while the Eastern Australia and New Zealand populations are subunits of the broader western South Pacific population (IWC E group), with relatively frequent interchange of individuals (Garrigue et al., 2011; Kaufman et al., 2011; Gales et al., 2011; Valsecchi et al., 2010). Some whales which travel through New Zealand waters have also been documented in the same year within Australian waters (e.g. the distinctive, white male, Migaloo) (Pirotta et al., 2023). Genetic evidence suggests a more complex relationship between these populations than originally thought, as sampled males and females migrating northward together in Eastern Australia were not closely related (Valsecchi et al., 2010). Many whales migrating north along the east coast of New Zealand pass through the Cook Strait, which separates the North and South Islands (Dawbin, 1956). In Eastern Australia, the primary migration path follows the eastern coastline, with some animals intercepting the southern coastline in Victoria, before continuing north (Paterson, 1991). The Western Australia population migrates along the western coastline of Australia, with some individuals intercepting the southern coast (the western Great Australian Bight), then travelling westwards before rounding Cape Leeuwin and continuing north. This appears to be a feature of the northward migration but not the southward migration, as humpbacks are rarely encountered along the Western Australian south coast during the spring months (Chittleborough, 1965; Gales et al., 2011). Humpbacks encountered in South Australia, in the central or eastern Great Australian Bight, are generally considered to be vagrants from either the Eastern Australia or Western Australia populations (Chittleborough, 1965; Kemper, 2005; Ward et al., 2019).
Formerly, calving habitats in Australia were thought to occur exclusively in the tropical wintering grounds, that is, in Eastern Australia north of 21°S, within the Great Barrier Reef Marine Park, and in Western Australia between 15°S and 18°S off the Kimberley coast (Chittleborough, 1965; Jenner et al., 2001; Smith et al., 2012). However, Torre-Williams et al. (2019) showed that calving in Eastern Australia occurs as far south as 28°S, by the presence of neonate calves in Gold Coast Bay, southern Queensland. Similarly, Irvine et al. (2018) recorded neonate calves off North West Cape, Western Australia, extending the range of calving areas in Western Australia by 1000 km to approximately 22.5°S. In both cases, these updated southern boundaries of the calving areas were defined by the scope of the studies, i.e., neither study found a southern limit beyond which neonates were no longer observed, except the limit of the area surveyed.
Birth and early maternal care are vulnerable periods in the life cycle of humpback whales, for both the cow and calf. Anthropogenic threats are increasing and concentrated in waters adjacent to areas with dense human populations, such as found along the east coast of Australia (Seyboth et al., 2023). Understanding the true distribution of calving is important for proper management of risks from both a conservation and animal welfare perspective, as well as informing broader ecological theories on migration.
In Eastern Australia, there have been occasional published accounts of neonate calves in relatively southern locations, including off Byron Bay (29°S) and Sydney (34°S) (Paton, 2016; Pirotta et al., 2020). In Western Australia, a recently published study presented 15 opportunistic observations and four strandings of neonates between 2021 and 2023 from southwestern WA (33-34°S) (Jolliffe et al., 2024). There has been no modern published research on neonate humpback whales in New Zealand. It is unclear whether the few published records of extralimital calves in Australia, listed above, represent outliers or whether they are indicative that calving does occur beyond the currently recognized calving area boundaries. The aim of this study was to determine if humpback whales regularly give birth south of the currently defined limits of calving areas in Australasia and, if so, whether the cow-calf pair continue migrating northward. To answer these questions, we collated observations of recently born (neonate) calves and the direction of travel of the cow-calf pair.
Materials and methods
Study area
The study area included New Zealand and Australia, south of the previously described southern limit of breeding grounds, specifically Gold Coast Bay (approximately 28°S) in Eastern Australia (Torre-Williams et al., 2019) and North West Cape (approximately 22.5°S) in Western Australia (Irvine et al., 2018). This area is comprised of six jurisdictions – those of New Zealand and of the Australian states New South Wales (NSW), Victoria, Tasmania, South Australia (SA) and Western Australia (WA) (Figure 1).
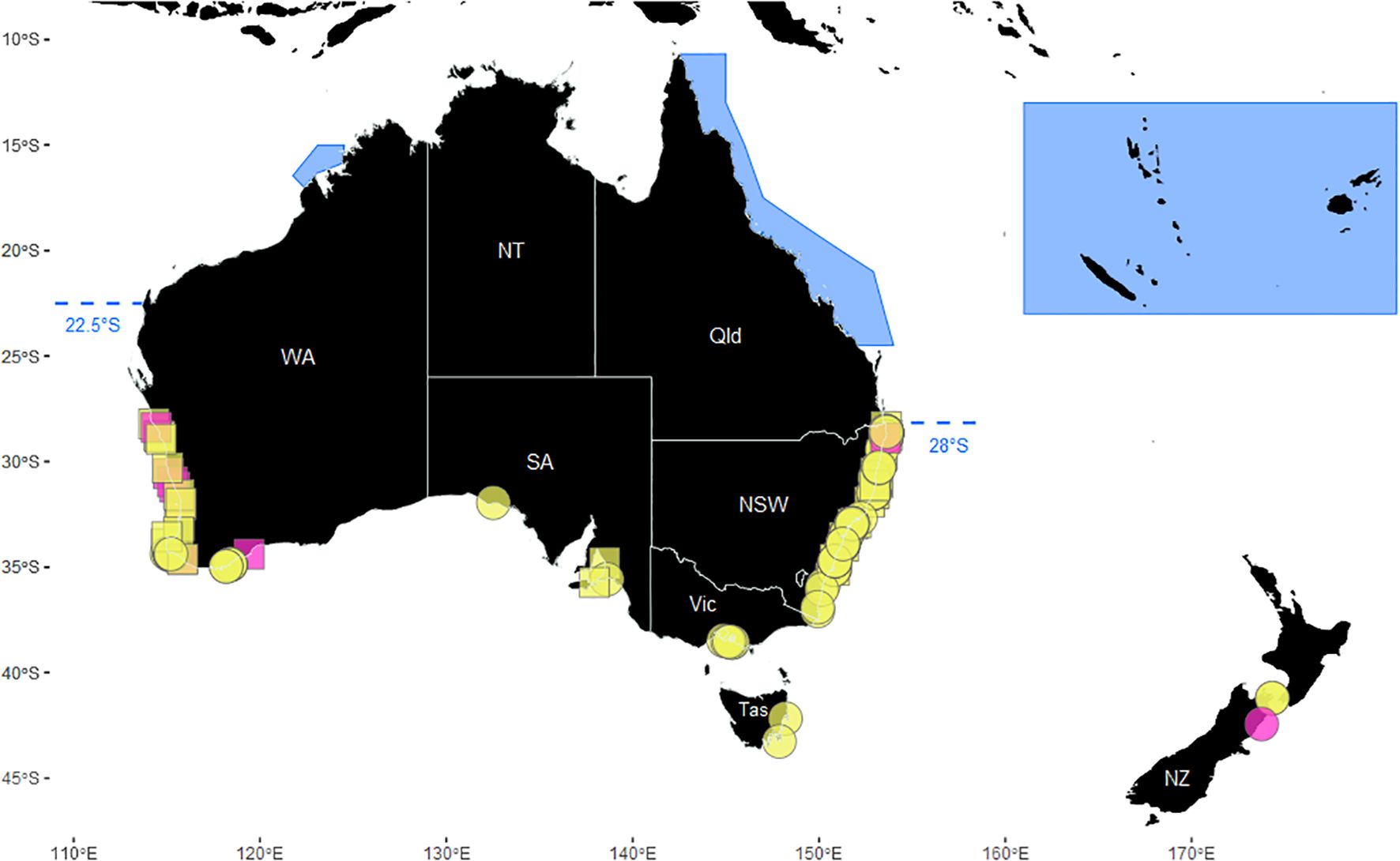
Figure 1. Records of neonate humpback whale calves (n = 209), including opportunistic observations of live cow-calf pairs (circles) and strandings (squares). Records where there was evidence of very recent birth are in pink. The study area is bounded in the north by the currently accepted limits of calving grounds, at approximately 22.5°S (North West Cape) in Western Australia (Irvine et al., 2018) and 28°S (Gold Coast Bay) in Eastern Australia (Torre-Williams et al., 2019), shown by the dashed line. The recognised “breeding grounds” as defined by Gales et al. (2011) for the Western Australia, Eastern Australia and New Zealand populations are indicated by the blue shaded areas. There was no southern limit beyond which neonates were not observed, except the southern limits of geographical locations from which any observations could be made. Neonates were observed in all jurisdictions in the study area, including New Zealand (NZ) and the Australian states of New South Wales (NSW), Victoria (Vic), Tasmania (Tas), South Australia (SA) and Western Australia (WA). The Northern Territory (NT) and Queensland (Qld) are outside of the study area limits.
Data collection
Observations of neonate humpback whale calves within this region were collected from several data sources and were of two types – opportunistic observations of live neonate calves and records of stranded neonates maintained by governmental agencies. Details of all records, including data source, observer, descriptions and location of images are given in the Supplementary Material (Supplementary Table S1).
Opportunistic observations
Opportunistic observations of live neonate calves accompanied by the mother (cow-calf pairs) were primarily citizen science data. The majority of contributions were made by professional staff in the whale-watching tourism industry. Additional data came from a private inshore aerial marine monitoring platform, DroneSharkApp (Pirotta et al., 2022) and from other observers, such as photographers who had published images of neonates in the news media or other public platforms, including social media. Also included are records of neonate calves that were observed during the New Zealand Department of Conservation (DOC) Cook Strait Whale Project (see Bott (2013) for details of the survey methods).
Strandings
In Australia, incidents of marine mammal strandings are managed by state government. These data were derived from records of stranded humpback whale neonate calves from NSW, SA and WA, maintained, respectively, by NSW National Parks and Wildlife Service (NSW NPWS), National Parks and Wildlife Service South Australia (NPWS SA) and the Western Australian Department of Biodiversity, Conservation and Attractions (DBCA). Strandings of neonate calves in South Australia prior to 2005 have previously been published (Kemper, 2005). Records from other Australian state agencies (i.e. Victoria and Tasmania) or New Zealand were not sought, however sightings of live animals contributed by the New Zealand Department of Conservation (DOC) are included.
Observations of neonate strandings in NSW are recorded in a database by NSW NPWS, known as Elements. Strandings are generally observed by the public and reported to NPWS who then ensure detailed and accurate information is added to the database. The database was restricted to humpback whale strandings in NSW with the term “neonate”, “newborn”/”new born”, “calf”, or “birth”. From these results, each event was scrutinized to confirm whether the individual was a neonate. This was based on available body length (animals over 5m excluded), or descriptions and images based on the inclusion criteria (Table 1).
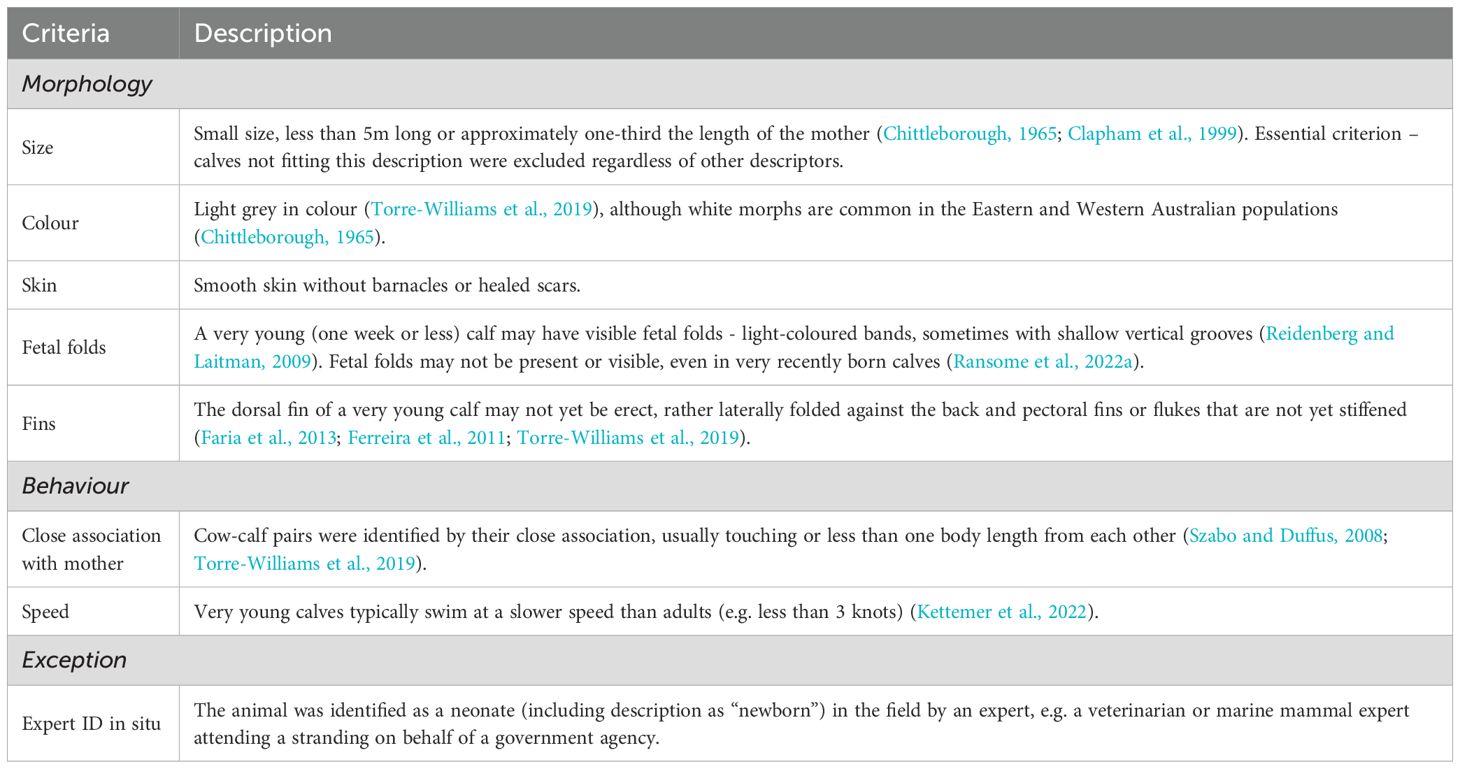
Table 1. Humpback whale calves were considered neonate if they generally fit these inclusion criteria. Size was the only essential criterion.
The Western Australian DBCA established the Western Australian Cetacean Stranding Database in 1982. Cetacean strandings were not recorded in a systematic manner prior to this, with the exception of some specimens archived by the Western Australian Museum on an ad hoc basis. DBCA has maintained this database of cetacean stranding events that includes both live stranded and dead, beach-cast cetaceans. A single data custodian curated the database between 1982 and 2015, and this responsibility has since been shared between two other data custodians within the Department, that are also marine mammal researchers. Reports of stranded cetaceans are usually reported by the public and investigated and validated by DBCA staff before being entered into the database. Information included in the database includes date and location of the stranding, age class, as well as morphometric information, details of the incident and outcome and any photographs or video available. The database was searched for all humpback whale incidents and filtered by location and estimation of whale age class and reported length. Only incidents meeting the inclusion criteria have been included (as per Table 1).
Inclusion criteria
To ensure only reliable records were included, strict inclusion criteria were applied. A calf was considered neonate (i.e. less than one month) if it generally fit the criteria for morphology and behavior (Table 1). Size was considered an essential criterion – calves 5m or larger or estimated more than one third the length of the mother, were excluded regardless of other descriptors. Calves grow rapidly in the first year, so that older calves are noticeably larger than neonates: between 5 to 8m long or at least half the length of the mother at two to three months (Chittleborough, 1965; Clapham et al., 1999; Christiansen et al., 2016). Yearlings (born during the previous season) on their first northbound migration are typically 9 to 10m, approximately three times longer than a neonate (Chittleborough, 1965).
Contributors of opportunistic observations were asked to record the sighting in a form based on the inclusion criteria, or verbally with the authors. Most were experienced whale watching personnel with multiple seasons’ experience observing humpback whales and were familiar with the morphology and behavior of older calves that are encountered during the southern migration. This allowed for the ability to identify calves as neonates by comparison to older calves seen later in the year. The authors confirmed the calf to be a neonate from photographic or video material provided by the observer. If images were not available, observations were included, only if the observer was experienced in the field (e.g. professional marine guide with more than one season of experience), could clearly describe the calf as per the inclusion criteria and details of the encounter were recorded within one week at the most, to ensure details were remembered accurately.
For strandings, an exception to the inclusion criteria was made in instances where the calf was identified as a neonate by an expert in situ, for example, a veterinarian or marine mammal scientist attending a stranding recorded the animal as a neonate even if measurements were not taken.
Location of birth
Timing
To avoid the inclusion of calves born at lower latitudes and already on their southern migration, observations were constrained to the months of the northward migration. Typically, the earliest southbound whales on the Western and Eastern Australia coasts are sighted in August, with cow-calf pairs migrating later, from September onwards (Chittleborough, 1965; Torre-Williams et al., 2019; Burton, 1991; Paterson, 1991). As such, observations later than 31st August were excluded. An exception was made for a small number of stranding records from early September, where the calf fit the above inclusion criteria, and the umbilicus was still attached. No exceptions were made for opportunistic observations of live calves. Based on timing, it was inferred that the calves were born at or further south than the observed location.
Observations of birth
In some cases, there was evidence of very recent parturition (birth). Opportunistic observations were accepted as births either when the mother was clearly seen as solo for a continuous period of twenty minutes or more before the sudden appearance of a calf, or when the appearance of a calf was accompanied by plumes of blood and/or placenta. The placenta may not be expelled immediately after delivery of the calf and so is not always visible to observers present at the time of birth (Ransome et al., 2022a). Stranded animals that had the umbilicus still attached, either recorded in situ or visible in images, were considered to have been born at or near the location they had stranded.
Direction of travel
For opportunistic observations, observers were asked to report the direction of travel of the cow-calf pair to the nearest ordinal direction, or as ‘milling’ if not travelling in any obvious direction. This information could not be determined for all observations and was not available for strandings. It was assumed that the cow-calf pair continued travelling in the observed direction, unless there was evidence to the contrary, e.g. the same, identified cow-calf pair were sighted again in the same location, or the pair were observed in a bay where the geography of the coastline did not allow continued movement in that direction.
Known resightings
Some observers noted that an identifiable cow-calf pair (based on distinctive markings) was observed more than once in an area over multiple days. This applied only to opportunistic observations, not to strandings.
Possible duplicate data
To avoid the inclusion of duplicate data, observations from the same location on the same day were consolidated to one record (e.g. opportunistic observations of a live neonate on the same day by two different observers in Newcastle) unless they were recorded separately by the same observer, or the cows or calves were distinctively visually different and confirmed with images. It cannot be ruled out that some neonates were observed more than once by different observers over multiple days, in different locations. To account for this, observations were identified as possible duplicate observations if they were made within two days of a previous observation and within a distance that the cow-calf pair could reasonably travel in this time. Except where there was clear evidence that the same pair was resighted (e.g. distinctive markings), these potential duplicates were labelled but not consolidated or excluded as it could not be determined whether it was the same or a different animal. Based on a median swim speed of 1.3ms-1 (Kettemer et al., 2022), a cow-calf pair could travel 112km in 24 hours. Adding a conservative buffer of 20%, observations were labelled as potential duplicates if it was within 135km (straight line) on the next calendar day or within 270km in two calendar days. If the cow-calf pair were observed travelling in a particular direction, subsequent observations were labelled as potential duplicates only if observed in that same direction, e.g. an observation would be considered a potential duplicate if it were within 135km to the north of a cow-calf pair observed travelling north the previous day but not if it were to the south of that pair. This was manually checked for each record.
Results
A total of 209 neonate humpback whale calves were recorded in the study area, across all jurisdictions, including 168 opportunistic observations of live calves and 41 strandings (Figure 1; Table 2). Of the observations of live calves, 20 were resights of an identifiable cow-calf pair that had previously been recorded in the same location. This occurred in Jervis Bay, NSW (35°S) in 2023 and Flinders Bay, WA (34°S) in 2016, 2017, 2018 and 2020. A further 20 records were identified as possible duplicates (all observations of live animals, no strandings), giving a conservative minimum of 169 unique records of neonate humpback whale calves in the study area. None of the possible duplicates were strandings, i.e. there were no instances where a live calf was observed prior to a stranding in the same region. The highest latitude observations were from 43°S near Port Arthur, Tasmania in Eastern Australia, 42°S near Kaikoura in New Zealand and 35°S near Albany in Western Australia. The earliest record in the year was a stranding on 19th May 2011 from Bremer Bay, WA. Stranding records were available back as far as 1991. The oldest records of live cow-calf pairs were from 2010 and 2015, made during the New Zealand Government Department of Conservation Cook Strait Whale Project. Some opportunistic observations from 2016 onward were recorded by whale watching tourism operators with sufficient information to be included (in particular, the detailed logs kept by Whale Watch Western Australia operating in Flinders Bay, WA) but most opportunistic observations were made in 2023 and 2024 (both years each accounted for approximately one-third of total opportunistic observations).
Evidence of birth was recorded in 11 instances (three opportunistic observations of live calves and eight strandings) in NSW, WA and New Zealand. The most southerly of these was from 42°S near Kaikoura, New Zealand (Figure 2).

Figure 2. Aerial photographs of birth from near Kaikoura, New Zealand (42°S). Plumes of blood (A) accompanied the appearance of the calf (B). These images were captured during a commercial whale watching flight (Wings Over Whales). Photo credit: South Pacific Helicopters Ltd.
Overall, information about travelling direction was available for 118 and unavailable for 50 of the 168 opportunistic observations of live cow-calf pairs. Most travelling direction data was from NSW, where 100% were moving north (total 94). One additional record from Jervis Bay (travelling northwest) was excluded, as the geography of the bay precluded continued movement in any direction except east to exit the bay. Travelling direction was available for all opportunistic observations in New Zealand (three, all northbound), Tasmania (two – one northbound, one milling) and Victoria (six – four travelling east, one northeast and one southbound) (Figure 3). One of the two pairs observed in South Australia was observed to be milling (Fowlers Bay) and no travelling direction information was available for the other. In Western Australia, travelling direction was available for 13 records, all from south coast locations (Table 3).
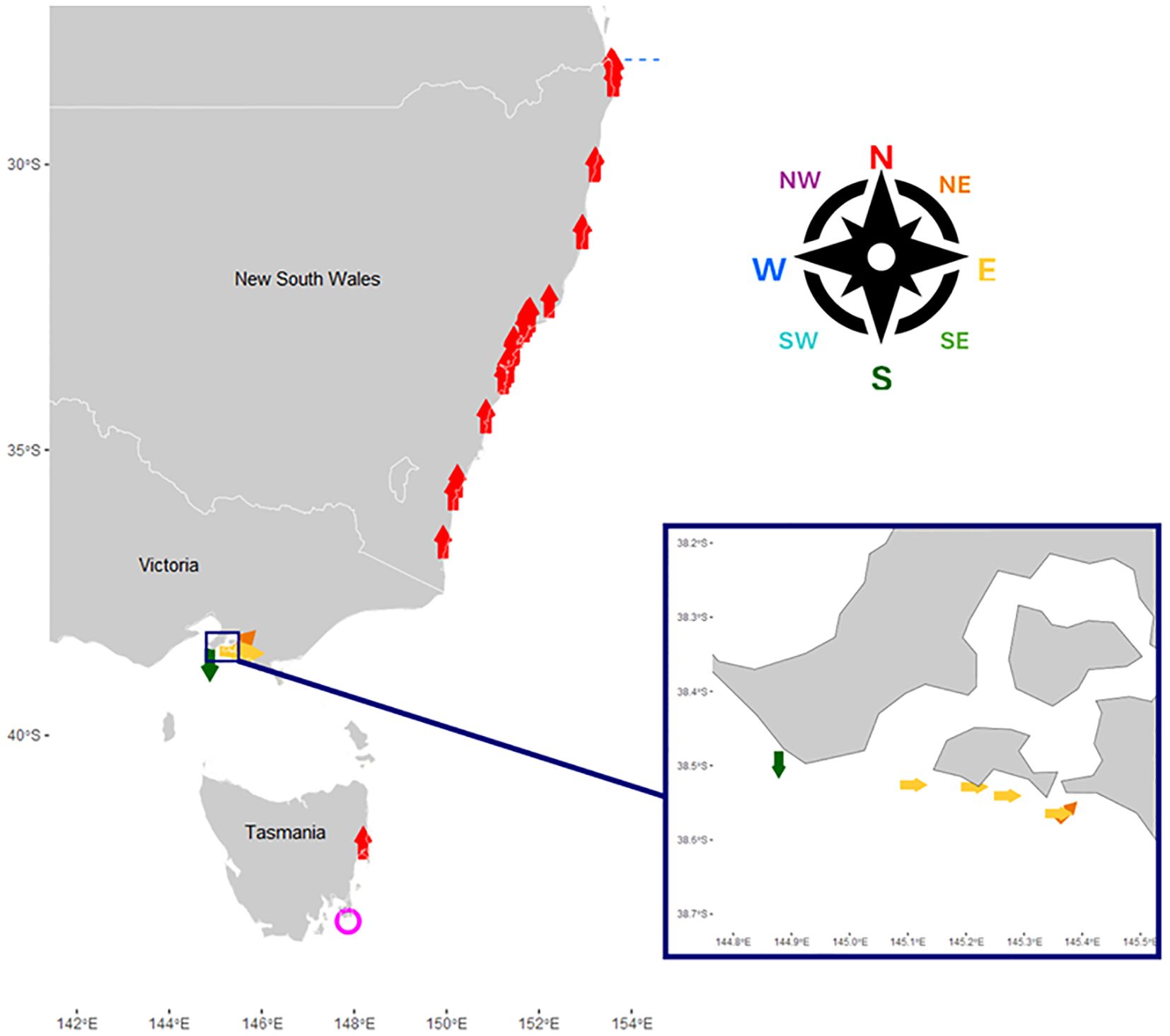
Figure 3. Reported direction of travel of 103 cow-calf pairs opportunistically observed in Eastern Australia, including large-scale inset of Victoria. Direction is indicated by arrows and colors to the nearest ordinal direction. One pair was observed milling, indicated by a pink circle.
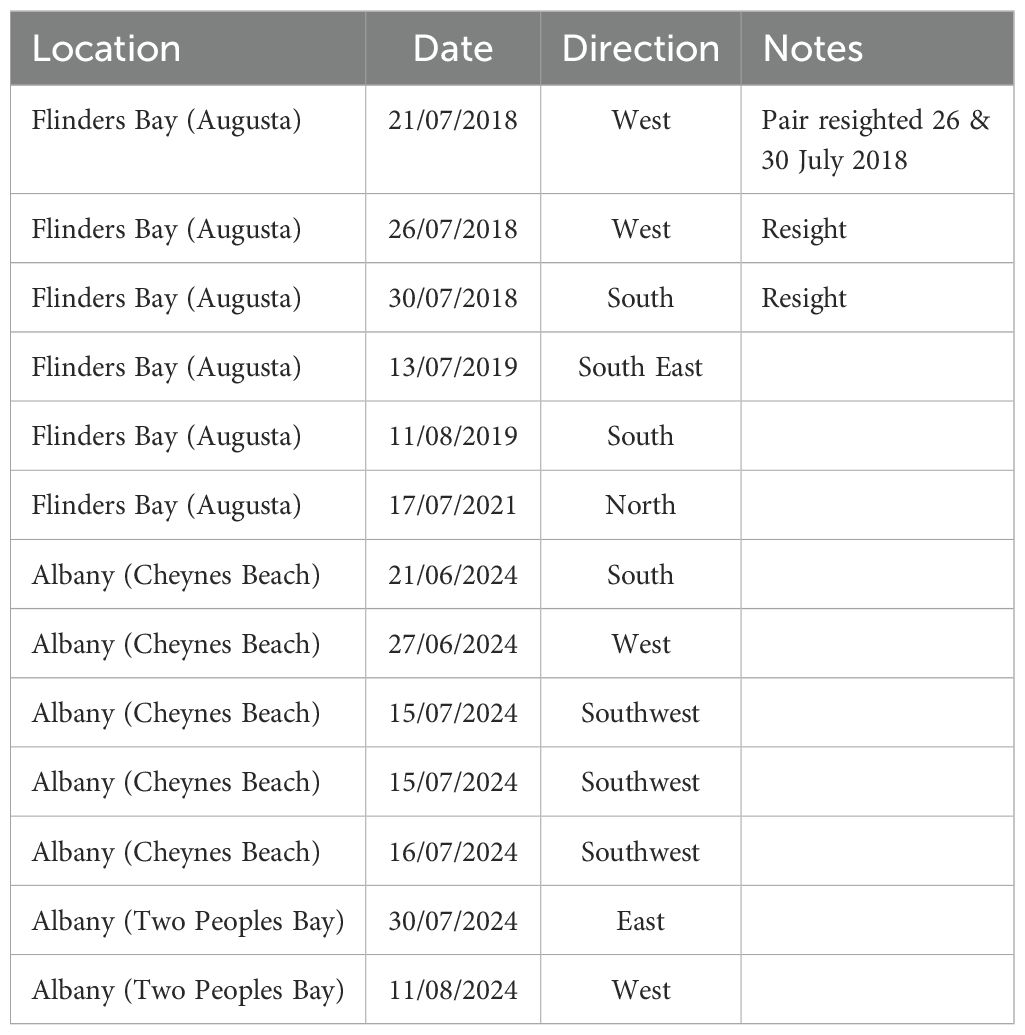
Table 3. Reported direction of travel of opportunistically observed cow-calf pairs in Western Australia.
Discussion
Here we present 209 separate records (conservative minimum of 169 unique records) of neonate humpback whale calves well south of the currently accepted calving limits in Australasia, representing a shift in our understanding of the distribution of calving areas. These numerous observations demonstrate that humpback whales are not confined to calving in wintering grounds. Neonates were observed in all jurisdictions, to the most southerly observable regions in all areas. This represents an extension of calving areas of approximately 12° of latitude/1300 km in Western Australia (from 22.5°S, North West Cape, WA) and approximately 14° of latitude/1500 km in Eastern Australia (from 28°S, Gold Coast Bay, Qld), as well as the inclusion of New Zealand (Irvine et al., 2018; Torre-Williams et al., 2019). No latitudinal limit of neonate calves was found within the study area, except the limits of available coastal or inshore observation sites, so it cannot be excluded that calving occurs offshore at higher latitudes. The calf observed near Port Arthur, Tasmania (43°S) in 2024 appears to be the highest latitude record of a neonate humpback whale from anywhere in the world.
The 11 instances where there was evidence of very recent birth, including the highest latitude example from 42°S near Kaikoura, New Zealand (Figure 2), demonstrate that parturition does indeed occur at these latitudes, supporting the validity of using observations of neonates as a proxy for birth.
Notably, none of the live cow-calf pairs observed in Eastern Australia or New Zealand were travelling in a direction that was inconsistent with continued northward migration. In NSW, 100% of the 94 pairs for which travelling direction information was available were northbound, as were all three observed in New Zealand. Of the two pairs observed in Tasmania, one was travelling north, and the other was milling. Only in Victoria were pairs observed moving in any other direction. Of the six pairs, four were travelling east and one northeast, which is consistent with following a migration route around the South East Corner of Victoria and then northward (Figure 3). One pair was observed travelling south. Precise coordinates were reported for this pair and from their location, any continued northward migration would require first going south to round Cape Schanck. The uniformity of travelling direction and the infrequency of resights suggests that the cow-calf pairs did continue northwards after observation. This agrees with observations made during annual surveys at Stradbroke Island, Qld (27°S), Byron Bay, NSW (29°S) and Sydney, NSW (34°S), and local tracking of cow-calf pairs in Gold Coast Bay, Qld (28°S) (Paterson and Paterson, 1989; Paton, 2016; Pirotta et al., 2020; Torre-Williams et al., 2019).
Opportunistic observations of live cow-calf pairs in WA were available only from south coast locations and as such, no travelling direction information is available from the west coast. Of the 13 WA records for which direction of travel was reported, six were from Flinders Bay, near Augusta (34°S) and of those, three records were of a pair that were seen again in Flinders Bay in subsequent days, confirming that the pair remained in the area. The other three pairs were observed travelling south, southeast and north. The geography of Flinders Bay blocks movement to the north, so the northbound pair cannot reasonably be interpreted as continuing north. The location of the other two pairs was not sufficiently precise to indicate whether their south/southeast movement was a precursor to rounding Cape Leeuwin and subsequently continuing north, although this pathway has previously been described (Chittleborough, 1965; IWC, 2011). A study that deployed satellite tags in Flinders Bay found that all 33 whales tagged in June or July continued north, although females with calves were not targeted (How et al., 2020). The remaining seven records from WA were from near Albany (35°S) (Table 3). Five were travelling west or southwest, which is consistent with continuing migration to the wintering grounds off North West WA, although without observations from other locations along the route, it cannot be concluded that this was the final destination. One pair was travelling south. There was insufficient precision in the location to consider whether the pair might have been navigating around a local obstacle, such as a headland or whether they were moving into open water to the south. Interestingly, one pair was travelling east. This may have been short-term movement, or the pair may have continued east, which raises questions about humpback whale habitat use in the central and eastern Great Australian Bight. Overall, the relatively few and poor spatial coverage of opportunistic observations in WA (especially the paucity of data from the western coastline) does not allow conclusions about the movements of humpback whales following birth of the calf. Future studies, such as photographic mark-recapture or satellite tagging targeting females with calves, may clarify this. However, for Eastern Australia and New Zealand, the uniformity of travelling direction consistent with continued northward migration suggests that parturition does not define the end point of the migration.
Such continuation of migration towards the tropics after birth of the calf has been found elsewhere in the world and so is unlikely to be unique to Australasia. A satellite tracking study in the North Atlantic followed a pregnant female tagged in the Barents Sea through the entire southward migration to the West Indies. Parturition, inferred from timing and speed, occurred in March at approximately 39°N, in deep, offshore water. The cow-calf pair continued the migration (southwest) for approximately 3000 km over 35 days, to the West Indies (19°N), there spending a further 17 days before returning northwards (Kettemer et al., 2022). To date this is the only complete migration track for a humpback whale from this population. This birth in temperate, offshore water could be an unusual occurrence that was coincidentally captured by the satellite track, or it could be a rare insight into the usual distribution of calving for this population. There is at least one other published account of a young calf at a similar date and latitude in the Atlantic, off Newfoundland (42°N), also in March (Williamson, 1961). Unlike off Australia, where humpbacks swim close to the coast, many humpback populations have pelagic migration pathways. This restricts the opportunities to observe and study the behavior of migrating whales. Calves born offshore during migration are unlikely to be observed until arrival in coastal areas close to human habitation. If satellite data had not revealed that the North Atlantic calf was born offshore (Kettemer et al., 2022), it is likely to have been first observed and presumed to have been born off the West Indies. Future satellite tracking studies of pregnant females could establish whether the broader latitudinal calving seen off Australia and New Zealand is common for other populations where migratory corridors are largely pelagic, i.e. the North Pacific (Palacios et al., 2019), South Pacific (Garrigue et al., 2015) and the Southwest Atlantic (Horton et al., 2020; Zerbini et al., 2006).
Although there have been few modern studies on humpback whale calves outside of the tropics, both the presence of newborn calves and the continuation of migration were often noted in historical papers on this species. In a report on aerial surveys conducted from Point Cloates (23°S) in Western Australia in 1952, Chittleborough (1953) notes that “[i]n June and July almost every humpback … was moving northwards,” including at least 16 cow-calf pairs, of which several were noted as “cow – very young calf”. Further south in Western Australia, commercial whalers had observed that some animals gave birth near Albany (35°S), before continuing northward (Chittleborough, 1965) and, “… cows have been known to give birth to calves in Geographe Bay on the coast of southwest Australia [34°S],” (Kellogg, 1929). As naturalist on Scott’s 1910 Terra Nova expedition, Lillie (1915) reported that in the northern stream of humpbacks migrating past New Zealand “… mothers, with newly born calves, were constantly to be seen off the Bay of Islands [35°S] after the beginning of July.” Kellogg (1929) notes that in the North Atlantic “from March to May females with new-born calves have been observed in the vicinity of the Azores [39 – 40°N]…” and “humpbacks with calves have been observed as early as February in the vicinity of Bermuda [32°N]…” Verrill (1902) extensively described the biology and ecology of humpback whales in Bermuda in the 19th century and states that, “[f]rom the small size of some of the ‘cubs’ [sic] taken with their mothers (15 feet long) [4.6m] it is not improbable that some were born there.” In the Indian Ocean, “females with young have been observed … as early as June and July in the vicinity of Durban [South Africa; 30°S]” (Kellogg, 1929). Conversely, discussions on the latitudinal limits of calving areas are not a feature of these historical texts. Based on information collected from commercial whaling, ‘breeding grounds’ are either discussed very generally, as existing in the tropics or warmer waters [e.g (Kellogg, 1929; Lillie, 1915; Mackintosh, 1942; Verrill, 1902)] or are not discussed at all (e.g (Clark, 1887; Matthews, 1937; Rayner, 1939; Townsend, 1935)). Dawbin (1966), collated data from shore-based whaling stations and concluded that, “… humpbacks require tropical coastal conditions, with water temperature about 25°C, for breeding…” but did not otherwise comment on the spatial distribution of calving and noted that “… [females] accompanied by young calves [are] protected from the catch at nearly all localities.” For this reason, neonate calves are not included in these whaling data. In earlier periods of ship-based whaling, when cow-calf pairs were not protected, humpback whales were not the primary target species but were taken to supplement the catch of other species “between seasons” (Clark, 1887). As such, the whale-ships did not follow humpbacks along their migration route and would purposefully avoid coastal and reef areas due to the navigational hazards presented (Paterson and Paterson, 1989). Therefore, data derived from whaling records generally offers a biased spatial representation of humpback whale calving areas. However, the information contained in these historical accounts does suggest that calving in temperate latitudes is unlikely to be an entirely new occurrence. Calving at higher latitudes may be a return to pre-whaling distribution. The loss of genetic diversity due to whaling has been associated with changes to migratory culture in humpbacks and other mysticetes (Baker et al., 2013; Carroll et al., 2015). Alternatively, this pattern may have been uninterrupted but obscured by the low abundance of humpbacks following their population collapse.
Four neonate calves (two stranded, two live) were recorded in South Australia. Humpbacks are seen regularly in South Australia, although less frequently than other states. These have been assumed to be vagrant individuals from either the Western Australia or Eastern Australia populations (Chittleborough, 1965; Kemper, 2005; Ward et al., 2019). However, records of neonates in South Australia have been published, including at least three strandings (Kemper, 2005; Tomo and Kemper, 2022) and one live newborn calf observed in St Vincent Gulf (near Adelaide, SA, approximately 35°S) in 1961 (Chittleborough, 1965). The movement of the two live pairs is unknown – no travel direction data was available for the pair observed in Encounter Bay (36°S) and the pair observed in Fowlers Bay (32°S) were not moving in any clear direction. The geography of Fowlers Bay prevents further northward travel, and its location is roughly equidistant from the west and east coasts. Early whaling records indicate that cow-calf pairs were present there in the 19th century. Bannister (1986) reported that in Fowlers Bay in 1840, the American ship Amazon took “…8 humpback, incl. 3 cows and calves”. On inspection of the logbook of this vessel (Smith, 1841), we could neither confirm nor contradict whether any of the humpbacks were indeed accompanied by a calf, although the timing of the logged kills, between 11th June and 18th August, does coincide with the peak in births (Chittleborough, 1958; Matthews, 1937). It is unclear whether some or all of the humpback whales in South Australia continue to travel either east or west and then north, or whether the end point of the migration is in the Great Australian Bight. Kemper (2005) did not interpret the presence of stranded calves as an indication that South Australia may contain an undescribed calving area for humpback whales, due to the relatively low water temperature (10 – 20°C). The new evidence of calves being born at higher latitudes and in temperate waters may warrant, generally, reconsideration of temperature as a limiting criterion for humpback whale calving areas and, specifically, more research into the ecology of humpback whales in South Australian waters.
In two locations, observers noted that some cow-calf pairs, identified by distinctive markings, were observed multiple times over the course of several days to weeks. This was recorded in Jervis Bay, NSW in 2023 and in Flinders Bay, WA in 2016, 2017, 2018 and 2020 (Supplementary Table S1). At least one of these calves was born in Flinders Bay, as the visually distinctive female had been clearly observed unaccompanied by a calf the previous day. In Jervis Bay, a birth had been recorded two years previously. It is unclear whether any of the other calves observed in these bays were born there (in total, at least 18 unique calves observed in Flinders Bay and five unique calves in Jervis Bay). Jervis Bay has been identified as a resting habitat for cow-calf pairs during the southward migration (Jones et al., 2023), it may similarly be of importance during the northward migration. Further monitoring and research are needed in both Jervis Bay and Flinders Bay to explore whether these contain regular resting or nursery habitats.
We show that in Australasia calving areas do not entirely overlap with tropical winter ‘breeding grounds’ – some humpback females calve during migration. This extends the distribution of calving areas well into temperate waters. Our findings support Brown and Corkeron’s (1995) proposal that humpback migration is more than just a swim. Breeding encompasses calving, courtship/male competition, copulation and conception. Courtship and male competition behaviors, such as singing, forming competitive pods and escorting mother-calf pairs, are prominent in wintering grounds (Ransome et al., 2022b) but all of these behaviors have been observed in migration corridors (Brown and Corkeron, 1995; Warren et al., 2020). As well as observing competitive pods during northward and southward migrations in Eastern Australia, Brown and Corkeron (1995) recorded many male-female pairs in both directions, suggestive of mating during migration. Although mating is presumed to occur in the wintering grounds, so far, the only published observation of copulation in humpback whales involved two males (Stack et al., 2024). Although unconfirmed, copulation was reported in Antarctica in January 1948 by scientists onboard a Japanese whaling ship (Nishiwaki and Hayashi, 1950). The described surface behaviors, paired lunging with ventral sides together, seem surprisingly conspicuous to go unobserved for the following eighty years but the experienced Japanese whaling crew had prior knowledge of this behavior and it matches very closely with the description given by Lillie (1910), informed by whalers from the Scottish and Irish islands (although there is no indication that the behavior was witnessed in British waters). If this was indeed copulation, conception is unlikely unless the female was not in the expected seasonal anestrus (Chittleborough, 1954). However, Chittleborough (1958) noted that, “…conception rarely occurs in the Antarctic; of 250 foetuses [sic] taken on the west coast of Australia, only one (4 ft 9 in. [1.5m] long when examined on July 9) could have been conceived in the Antarctic”. In the same paper, of hundreds of humpback fetuses reported from Antarctica in the summer months between 1949 and 1956, several are at least three times the average length of the others caught that summer (6 – 11 ft/1.8 – 3.4m compared to 1.9 – 3.7 ft/0.6 – 1.1m), suggesting asynchronous conception. Taken together, no aspect of breeding can be said to occur exclusively within the winter ‘breeding grounds’. Likewise, feeding is not restricted to high latitude summer ‘feeding’ grounds. Feeding has been recorded in many mid and low latitude environments around the world (Pirotta et al., 2021; Owen et al., 2017; Seyboth et al., 2023). Best et al. (1995) and Dawbin (1956) described humpback whales pausing or discontinuing their southward migrations to feed in coastal regions of South Africa and New Zealand, respectively. Rather than total separation of discrete feeding and breeding grounds connected by a thoroughfare, humpback whales use a continuum of habitats across a large latitudinal gradient and breeding may be concentrated towards, but not restricted to, the low latitude end of their range.
An underlying assumption of the feeding/breeding paradigm is that humpback whales must reach the tropical wintering grounds to give birth and as such, requirements for breeding drive the migration (Pitman et al., 2020). We show it is not imperative for the female to reach the wintering grounds to give birth, even if most do. The observation that some humpback whales give birth in temperate water, at least as high as 43°S and then many, if not all, continue to migrate northward, presumably for thousands of kilometers to the wintering grounds, raises an important question with implications for migration theory. If the drive to migrate to the tropics is for the benefit of the calf in its most vulnerable neonatal stage, are those benefits not outweighed by the energetic costs of cold water and migration, as well as the potential predation risk enroute? One explanation is that the wintering habitats offer benefits to adult whales that are sufficient to compel continued migration. If this were true, it would support migration theories that are based on benefits to the adult, not the calf. However, it is worthwhile to consider the ‘cognitive ecology’ of the whale – the internal mechanisms that underlie the animal’s movement (Kashetsky et al., 2021). Does a female that has given birth during migration continue with the intention of reaching some resource for her own benefit, or does she continue because of a genetically encoded instinct or deeply learned spatial behavior, regardless of whether the benefits to her calf are still relevant? As Connor and Corkeron (2001) argue, the cost or benefit of a given migration strategy does not need to be perfect but only relatively favorable to the alternative to be selected for. It is conceivable that a female whale could disadvantage one mid-latitude calf by instinctively continuing the migration but if, during her lifetime, she gave birth to other calves in the wintering grounds that were sufficiently advantaged by that environment, then enough of her offspring and therefore her (presumably heritable or culturally transmitted) strategy of migrating all the way to the tropics would be propagated in the population. As such, in the absence of a greater understanding of the cognitive ecology of humpback whale migration, our findings are neither evidence for nor against any specific theories on migration drivers, nor whether the drivers are related to breeding or some other aspect of their biology.
However, the finding that parturition does not define the endpoint of migration does provide a basis for future investigations that could illuminate the factors driving migration. As a first step, some of the assumptions of this study should be tested. The approach used in this study did not allow for monitoring of cow-calf pairs, except opportunistically (i.e. in Flinders Bay, WA and Jervis Bay, NSW where the pairs were resident for days or weeks) and so the fates of these calves is not known. Similarly, although the uniformity of northward travel in this and previous studies (Paterson and Paterson, 1989; Paton, 2016; Pirotta et al., 2020; Torre-Williams et al., 2019) suggests they continued to the wintering grounds, the ultimate destination cannot be known without satellite telemetry or same-year photo matching studies. Confirming whether the cow-calf pairs do indeed complete the full migration and comparing the survivorship, causes of death and later reproductive success between calves born during migration with those born in the wintering grounds will allow an empirical evaluation of different theories of migration drivers.
Although we demonstrate that calving is not restricted to the wintering grounds, it is likely only a small proportion of calves born during the migration. Our data were collected opportunistically, without controlling for survey effort, so we are cautious to make numerical estimations of the proportion of the total calved population we observed. However, the simple observation that, overall, cow-calf pairs are encountered much more frequently during the southern migration than the northern migration indicates that most calves are born north of the study area.
The findings of significant calving in temperate waters has clear management implications for management authorities. During birth and the early stages of infancy, humpback whales are particularly vulnerable to anthropogenic threats, such as entanglement in fishing gear or shark mitigation devices, vessel strike, pollution and underwater noise (Erbe et al., 2021; Groom and Coughran, 2012; How et al., 2021; Peel et al., 2018; Schilling et al., 2023; Seyboth et al., 2023; Smith et al., 2020; Vanderlaan and Taggart, 2007). Hazards associated with human activity are increasing and concentrated in coastal waters adjacent to urban centers, compounding the broader pressures caused by ecological changes driven by anthropogenic climate change (Seyboth et al., 2023). Compared to a calf born in the tropics, calves born at higher latitudes during migration are potentially more at risk, as they are exposed to a greater spatial extent and wider variety of hazards as they transit through the migratory corridor, as well as increased time exposed to these hazards. For instance, if we consider the termination of migration to be the southernmost point of the Great Barrier Reef Marine Park (approximately 24°S), a calf born in Tasmanian waters at 43°S would need to swim with its mother for several weeks and at least 2300 km during its most vulnerable stage, through waters adjacent to the major urban centers and shipping lanes off southeastern Australia. Injuries were evident in some of the images used to confirm the opportunistic observations of live calves, although their cause is unknown and quantifying such injuries was outside of the scope of this study (e.g. Figure 4).

Figure 4. Example of images used to confirm age of calf, in this case with evidence of injuries from unknown cause. Calf was observed in Newcastle, NSW, Eastern Australia in July 2024. The observation was reported as otherwise not unusual and there was no evidence of failure to survive (e.g. corresponding stranding). Photo credit: Dominic May, CoastXP.
There are legislative protections relating to approaching or disturbing humpback mothers with calves at both the state and federal level in Australia and New Zealand, however compliance may be lower due to a lack of awareness in areas where calves are not expected by recreational boaters, commercial operators or management authorities. Cow-calf pairs may also be susceptible to cumulative disturbance throughout their migration (Chou et al., 2020). In light of the documented southward extension of humpback whale calving grounds, it will likely be necessary for jurisdictions to review protection measures to assess, control and evaluate risks spatially and temporally at the local level.
In this study we took a mixed methodological approach, combining complementary opportunistic citizen science data, observations of calves collected opportunistically during annual whale surveys and stranding data held by government agencies. Some limitations are inherent in this approach. Due to the non-random, opportunistic nature of the data, it was not possible to control for survey effort. The quantity, location and recency of records broadly reflects the human population density and hence that of observers. For this reason, the number of neonates has not been presented as a function of time or latitude. For example, more neonates were observed from mid to northern NSW than elsewhere and two-thirds of observations were made in 2023 or 2024, but neither pattern can be interpreted as a true increase as it may be an artifact due to greater data availability. Conversely, areas with no opportunistic observations corresponded to areas of relatively sparse human settlement, such as most of the southern coastline of Australia, the western coast of WA, and most of New Zealand. The stranding data compensated for this to some extent, as an animal stranded on a beach is more likely to be observed than a live calf, although strandings may also go unreported, especially in remote regions. Despite these considerations, this approach allowed much greater spatial and temporal coverage than would have been logistically or financially possible with traditional shore-based or boat-based survey methods. Participation of whale-watching tourism operators was key. Although we relied on voluntary submissions, engagement was high. In many cases, tourism professionals advocated for this project through their networks, leading to submissions from other observers, mostly coastal photographers.
Wide-ranging marine species present unique technological and economic challenges for ecological field studies. Data derived from citizen science initiatives offer new and increasing opportunities to enhance scientific knowledge (Fariello et al., 2024; Fraisl et al., 2022). For projects such as this, whale-watching tourism professionals are ideal citizen science contributors due to their familiarity with humpback whales and the extensive hours spent in the field. As humpback populations globally continue to increase, so too does whale-focused tourism (Meynecke et al., 2017). Simultaneously, technological advancements in photographic equipment and communication platforms mean that opportunities to observe marine mammals throughout their range are increasing, while such opportunistic data also becomes more accessible to researchers (Garcia-Soto et al., 2021). The marketing leverage that can be gained from advertising participation in a research project may be an incentive that researchers can use to encourage data contributions from commercial operators, however it was our experience that the high level of engagement in this project was motivated by an inherent desire to contribute to the knowledge and conservation of humpback whales. Collaborative projects such as this have the potential to improve communication and cooperation between researchers, government agencies and industry. This very low-cost approach may be adapted for use in other locations or on other species, where there is the opportunity to partner with nature-based tourism operators and government agencies.
As discussed above, considerable ecological and biological information was gained by examining historical papers on humpback whales. Some of the information contained in the original works seemed to have been overlooked or misconstrued when cited from subsequent papers, which has likely resulted in an oversimplification of our view of humpback whale ecology. Historical texts and ship logbooks are much more accessible than previously, thanks to digitization, networked cataloguing and open access libraries, and are useful resources for investigating the effects of historical whaling and current environmental change.
The numerous records of neonate humpback whale calves presented here demonstrate that humpback whales regularly give birth well south of the currently recognized calving areas, including in relatively high latitude waters in Western Australia, Eastern Australia and New Zealand. Cow-calf pairs were consistently observed to continue migrating northward in Eastern Australia and New Zealand, suggesting that parturition does not define the end point of the migration. This finding challenges the feeding/breeding paradigm where breeding behaviors are restricted to the ‘breeding grounds’ at the terminus of the migration. Future studies comparing survival and fitness of calves born during migration with those born in the tropics will provide important empirical information to evaluate different hypotheses on migration drivers. More research is required, especially in Western Australia and South Australia, to better understand the distribution of calving habitat and movement following birth in these regions. Overall, the findings of this study have important management implications. Conservation policies and practices reflecting this new information are necessary to ensure females and calves are protected during vulnerable life stages, tailored to local risks. This study highlights a cost-effective, collaborative research approach, with a particular focus on engagement with the whale-watching tourism industry. This approach may be adapted and utilized elsewhere and for different species.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the data included in this study was collated from existing sources and was observational in nature. No data was collected specifically for this study, no animals were approached or impacted. Opportunistic observations were made from shore or tourism vessels. Whale watching tourism operators must adhere to laws and guidelines and observations were reported post hoc. Stranding events were managed and attended by government agencies and other appropriate personnel, in accordance with their own legal, professional and ethical frameworks.
Author contributions
JM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. AD: Data curation, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. VP: Data curation, Project administration, Writing – review & editing, Writing – original draft. AM: Data curation, Writing – original draft, Writing – review & editing. KW: Data curation, Writing – original draft, Writing – review & editing. HR: Data curation, Writing – original draft, Writing – review & editing. TR: Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. Funds were provided by the NSW Government Marine Estate Management Strategy to cover the cost of publication (RG242102) and manuscript preparation (RG234390). No additional funding was provided for this study.
Acknowledgments
The authors would like to acknowledge and thank the following people and organizations: all citizen science contributors for their generosity with time and knowledge, especially the commercial whale watching operators; Doug K Coughran, who curated the stranding database in WA for decades and made sure records were reported; the Organisation for the Rescue and Research of Cetaceans in Australia (ORRCA), whose dedicated members contributed data and facilitate reporting of strandings in NSW; Nadine Bott, leader of the NZ Department of Conservation Cook Strait Whale Project for contribution of data; and Verity Gibbs from National Parks and Wildlife Service SA, for contribution of data.
Conflict of interest
JM was employed as a skipper and whale watching guide from 2022 to 2023 by CoastXP Newcastle, NSW, Australia and received a part-time salary for this work. The research was conducted independently, with no financial relationship to this or any other commercial organisation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1545526/full#supplementary-material
References
Baker C. S., Steel D., Calambokidis J., Falcone E., Gonzalez-Peral U., Barlow J., et al. (2013). Strong maternal fidelity and natal philopatry shape genetic structure in North Pacific humpback whales. Mar. Ecol. Prog. Ser. 494, 291–306. doi: 10.3354/meps10508
Bannister J. (1986). Notes on nineteenth century catches of southern right whales (Eubalaena australis) off the southern coasts of Western Australia. Rep. Int. Whaling Comm. (Special Issue) 10, 255–259.
Barendse J., Best P. B., Carvalho I., Pomilla C. (2013). Mother knows best: occurrence and associations of resighted humpback whales suggest maternally derived fidelity to a southern hemisphere coastal feeding ground. PloS One 8, e81238. doi: 10.1371/journal.pone.0081238
Best P. B., Sekiguchi K., Findlay K. P. (1995). A suspended migration of humpback whales Megaptera novaeangliae on the west coast of South Africa. Mar. Ecol. Prog. Ser. 118, 1–12. doi: 10.3354/meps118001
Bortolotto G. A., Danilewicz D., Hammond P. S., Thomas L., Zerbini A. N. (2017). Whale distribution in a breeding area: spatial models of habitat use and abundance of western South Atlantic humpback whales. Mar. Ecol. Prog. Ser. 585, 213–227. doi: 10.3354/meps12393
Bott N. (2013). Cook Strait Whale Survey Report on Field Results 2013. New Zealand Department of Conservation.
Brodie P. F. (1975). Cetacean energetics, an overview of intraspecific size variation. Ecology 56, 152–161. doi: 10.2307/1935307
Brown M., Corkeron P. (1995). Pod characteristics of migrating humpback whales (Megaptera novaeangliae) off the east Australian coast. Behaviour 132, 163–179. doi: 10.1163/156853995X00676
Burton C. L. (1991). Sighting analysis and photo-identification of humpback whales off Western Australia 1989. Memoirs Queensland Museum 30, 259–270.
Carroll E. L., Baker C. S., Watson M., Alderman R., Bannister J., Gaggiotti O. E., et al. (2015). Cultural traditions across a migratory network shape the genetic structure of southern right whales around Australia and New Zealand. Sci. Rep. 5, 16182. doi: 10.1038/srep16182
Cartwright R., Gillespie B., Labonte K., Mangold T., Venema A., Eden K., et al. (2012). Between a rock and a hard place: habitat selection in female-calf humpback whale (Megaptera novaeangliae) pairs on the Hawaiian breeding grounds. PloS One 7, e38004. doi: 10.1371/journal.pone.0038004
Chittleborough R. (1953). Aerial observations on the humpback whale, Megaptera nodosa (Bonnaterre), with notes on other species. Mar. Freshw. Res. 4, 219–226. doi: 10.1071/MF9530219
Chittleborough R. (1954). Studies on the ovaries of the humback whale, megaptera nodosa (Bonnaterre), on the western Australian coast. Mar. Freshw. Res. 5, 35–63. doi: 10.1071/MF9540035
Chittleborough R. (1958). The breeding cycle of the female humpback whale, Megaptera nodosa (Bonnaterre). Mar. Freshw. Res. 9, 1–18. doi: 10.1071/MF9580001
Chittleborough R. (1965). Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski). Mar. Freshw. Res. 16, 33–128. doi: 10.1071/MF9650033
Chou E., Kershaw F., Maxwell S. M., Collins T., Strindberg S., Rosenbaum H. C. (2020). Distribution of breeding humpback whale habitats and overlap with cumulative anthropogenic impacts in the Eastern Tropical Atlantic. Diversity Distrib. 26, 549–564. doi: 10.1111/ddi.13033
Christiansen F., Dujon A. M., Sprogis K. R., Arnould J. P., Bejder L. (2016). Noninvasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere 7, e01468. doi: 10.1002/ecs2.1468
Clapham P. J. (1996). The social and reproductive biology of humpback whales: an ecological perspective. Mamm. Rev. 26, 27–49. doi: 10.1111/j.1365-2907.1996.tb00145.x
Clapham P. (2001). Why do baleen whales migrate? Mar. Mamm. Sci. 17, 432–436. doi: 10.1111/j.1748-7692.2001.tb01289.x
Clapham P., Wetmore S., Smith T., Mead J. (1999). Length at birth and at independence in humpback whales. J. Cetacean Res. Manage. 1, 141–146.
Clark A. H. (1887). The American whale-fishery 1877-1886. Science 9 (217), 321–324. doi: 10.1126/science.ns-9.217S.321
Connor R. C., Corkeron P. J. (2001). Predation past and present: killer whales and baleen whale migration. Mar. Mamm. Sci. 17, 436–439. doi: 10.1111/j.1748-7692.2001.tb01290.x
Corkeron P. J., Connor R. C. (1999). Why do baleen whales migrate? 1. Mar. Mamm. Sci. 15, 1228–1245. doi: 10.1111/j.1748-7692.1999.tb00887.x
Craig A. S., Herman L. M., Pack A. A., Waterman J. O. (2014). Habitat segregation by female humpback whales in Hawaiian waters: avoidance of males? Behaviour 151, 613–631. doi: 10.1163/1568539X-00003151
Currie J. J., Stack S. H., Mccordic J., Roberts J. (2018). Utilizing occupancy models and platforms-of-opportunity to assess area use of mother-calf humpback whales. Open J. Mar. Sci. 8, 276–292. doi: 10.4236/ojms.2018.82014
Dakhteh S., Ranjbar S., Moazeni M., Mohsenian N., Delshab H. (2017). The Persian Gulf is part of the habitual range of the Arabian Sea Humpback whale population. J. Mar. Biol. Oceanogr. 3, 2. doi: 10.4172/2324-8661.1000178
Danilewicz D., Tavares M., Moreno I. B., Ott P. H., Trigo C. C. (2009). Evidence of feeding by the humpback whale (Megaptera novaeangliae) in mid-latitude waters of the western South Atlantic. Mar. Biodivers. Records 2, e88. doi: 10.1017/S1755267209000943
Dawbin W. H. (1956). The migrations of humpback whales which pass the New Zealand coast. Trans. R. Soc. New Z. 84 (1), 147–196.
Dawbin W. H. (1966). The seasonal migratory cycle of humpback whales. Whales dolphins porpoises, 145–170. doi: 10.1525/9780520321373-011
Eisenmann P., Fry B., Holyoake C., Coughran D., Nicol S., Bengtson Nash S. (2016). Isotopic evidence of a wide spectrum of feeding strategies in Southern Hemisphere humpback whale baleen records. PloS One 11, e0156698. doi: 10.1371/journal.pone.0156698
Erbe C., Schoeman R. P., Peel D., Smith J. N. (2021). It often howls more than it chugs: wind versus ship noise under water in Australia’s maritime regions. J. Mar. Sci. Eng. 9, 472. doi: 10.3390/jmse9050472
Faria M.-A., Deweerdt J., Pace F., Mayer F.-X. (2013). Observation of a humpback whale (Megaptera novaeangliae) birth in the coastal waters of Sainte Marie Island, Madagascar. Aquat. Mamm. 39, 296. doi: 10.1578/AM.39.3.2013.296
Fariello C. M., Meynecke J.-O., De Bie J. (2024). Defining humpback whale (Megaptera novaeangliae) potential distribution in the Great Barrier Reef Marine Park: a two-way approach. Pacific Conserv. Biol. 30, 1–15. doi: 10.1071/PC23032
Félix F., Botero-Acosta N. (2011). Distribution and behaviour of humpback whale mother–calf pairs during the breeding season off Ecuador. Mar. Ecol. Prog. Ser. 426, 277–287. doi: 10.3354/meps08984
Félix F., Guzmán H. M. (2014). Satellite tracking and sighting data analyses of Southeast Pacific humpback whales (Megaptera novaeangliae): is the migratory route coastal or oceanic? Aquat. Mamm. 40 (4), 329–340. doi: 10.1578/AM.40.4.2014.329
Felix F., Haase B. (2005). Distribution of humpback whales along the coast of Ecuador and management implications. J. Cetacean Res. Manage. 7, 21–31. doi: 10.47536/jcrm.v7i1.753
Ferreira M. C. E., Maia-Nogueira R., De Jesus A. (2011). Surface observation of a birth of a humpback whale (Megaptera novaeangliae) on the northeast coast of Brazil. Latin Am. J. Aquat. Mamm. 9, 160–163. doi: 10.5597/lajam00182
Findlay K. P., Seakamela S. M., Meÿer M. A., Kirkman S. P., Barendse J., Cade D. E., et al. (2017). Humpback whale “super-groups” – A novel low-latitude feeding behaviour of Southern Hemisphere humpback whales (Megaptera novaeangliae) in the Benguela Upwelling System. PloS One 12, e0172002. doi: 10.1371/journal.pone.0172002
Fraisl D., Hager G., Bedessem B., Gold M., Hsing P.-Y., Danielsen F., et al. (2022). Citizen science in environmental and ecological sciences. Nat. Rev. Methods Primers 2, 64. doi: 10.1038/s43586-022-00144-4
Gales N., Bannister J., Findlay K., Zerbini A., Donovan G. (2011) Report of the Workshop on the Comprehensive Assessment of Southern Hemisphere humpback whales. J. Cetacean Res. Manage. 3, 1–50. doi: 10.47536/jcrm.vi3
Garcia-Soto C., Seys J. J. C., Zielinski O., Busch J. A., Luna S. I., Baez J. C., et al. (2021). Marine citizen science: current state in Europe and new technological developments. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.621472
Garrigue C., Clapham P. J., Geyer Y., Kennedy A. S., Zerbini A. N. (2015). Satellite tracking reveals novel migratory patterns and the importance of seamounts for endangered South Pacific humpback whales. R. Soc. Open Sci. 2, 150489. doi: 10.1098/rsos.150489
Garrigue C., Franklin T., Constantine R., Russell K., Burns D., Poole M., et al. (2011). First assessment of interchange of humpback whales between Oceania and the east coast of Australia. J. Cetacean Res. Manage., 269–274. doi: 10.47536/jcrm.vi.314
Groom C., Coughran D. (2012). Entanglements of baleen whales off the coast of Western Australia between 1982 and 2010: patterns of occurrence, outcomes and management responses. Pacific Conserv. Biol. 18, 203. doi: 10.1071/PC130203
Guidino C., Llapapasca M. A., Silva S., Alcorta B., Pacheco A. S. (2014). Patterns of spatial and temporal distribution of humpback whales at the southern limit of the southeast pacific breeding area. PloS One 9, e112627. doi: 10.1371/journal.pone.0112627
Guzman H. M., Félix F. (2017). Movements and habitat use by Southeast Pacific humpback whales (Megaptera novaeangliae) satellite tracked at two breeding sites. Aquat. Mamm. 43, 139. doi: 10.1578/AM.43.2.2017.139
Horton T. W., Zerbini A. N., Andriolo A., Danilewicz D., Sucunza F. (2020). Multi-decadal humpback whale migratory route fidelity despite oceanographic and geomagnetic change. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00414
How J., Coughran D., Double M., Rushworth K., Hebiton B., Smith J., et al. (2020). Mitigation measures to reduce entanglements of migrating whales with commercial fishing gear.
How J. R., de la Mare W. K., Coughran D. K., Double M. C., De Lestang S. (2021). Gear modifications reduced humpback whale entanglements in a commercial rock lobster fishery. Mar. Mamm. Sci. 37, 782–806. doi: 10.1111/mms.12774
Irvine L. G., Thums M., Hanson C. E., Mcmahon C. R., Hindell M. A. (2017). Quantifying the energy stores of capital breeding humpback whales and income breeding sperm whales using historical whaling records. R. Soc. Open Sci. 4, 160290. doi: 10.1098/rsos.160290
Irvine L. G., Thums M., Hanson C. E., Mcmahon C. R., Hindell M. A. (2018). Evidence for a widely expanded humpback whale calving range along the Western Australian coast. Mar. Mamm. Sci. 34, 294–310. doi: 10.1111/mms.2018.34.issue-2
Jenner K. C. S., Jenner M.-N. M., Mccabe K. A. (2001). Geographical and temporal movements of humpback whales in western Australian waters. APPEA J. 41, 749–765. doi: 10.1071/AJ00044
Jolliffe C., Russell G., Mcpherson C., Elsdon B. (2024). Evidence of humpback whale calving in south-west Western Australia. Discover Anim. 1, 14. doi: 10.1007/s44338-024-00012-3
Jones A., Bruce E., Cato D. H. (2023). Characterising resting patterns of mother-calf humpback whale groups in a semi-enclosed embayment along the Australian east coast migration pathway. Sci. Rep. 13, 14702. doi: 10.1038/s41598-023-41856-1
Kashetsky T., Avgar T., Dukas R. (2021). The cognitive ecology of animal movement: evidence from birds and mammals. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.724887
Kaufman G., Coughran D., Allen J. M., Burns D., Burton C., Castro C., et al. (2011). Photographic evidence of interchange between east Australia (BS E-1) and west Australia (BS–D) humpback whale breeding populations. Sci. Committ. Int. Whaling Comm.
Kellogg R. (1929). What is known of the migrations of some of the whalebone whales. Annual Report of the Smithsonian Institute 1928 (Washington D.C: USGPO).
Kemper C. M. (2005). Records of humpback whales Megaptera novaeangliae in South Australia. Trans. R. Soc. South Aust. 129, 53–58.
Kettemer L. E., Rikardsen A. H., Biuw M., Broms F., Mul E., Blanchet M.-A. (2022). Round-trip migration and energy budget of a breeding female humpback whale in the Northeast Atlantic. PloS One 17, e0268355. doi: 10.1371/journal.pone.0268355
Lillie D. G. (1910). Observations on the anatomy and general biology of some members of the larger cetacea. Proc. Zool. Soc. London 1910, 769–792. doi: 10.1111/j.1096-3642.1910.tb01916.x
Lillie D. G. (1915). “Cetacea,” in British Antarctic (‘Terra Nova’) Expedition 1910 (London: British Museum Natural History). https://www.biodiversitylibrary.org/page/29517685.
Martins C. C. A., Morete M. E., Coitinho M. H. E., Freitas A. C., Secchi E. R., Kinas P. G. (2001). Aspects of habitat use patterns of humpback whales in the Abrolhos Bank, Brazil, breeding ground. Memoirs of the Queensland Museum. 47 (2), 563–70.
Matthews L. H. (1937). The Humpback Whale, Megaptera nodosa. Discovery Reports, Vol. XVII. 7–92. Cambridge University Press, London.
Meynecke J.-O., De Bie J., Barraqueta J.-L. M., Seyboth E., Dey S. P., Lee S. B., et al. (2021). The role of environmental drivers in humpback whale distribution, movement and behavior: A review. Front. Mar. Sci. 8, 720774. doi: 10.3389/fmars.2021.720774
Meynecke J.-O., Gustafon J., Cade D. E. (2023). Exfoliating whales–sandy bottom contact behaviour of humpback whales. J. Mar. Sci. Eng. 11, 600. doi: 10.3390/jmse11030600
Meynecke J.-O., Richards R., Sahin O. (2017). Whale watch or no watch: the Australian whale watching tourism industry and climate change. Region. Environ. Change 17, 477–488. doi: 10.1007/s10113-016-1034-z
Nishiwaki M., Hayashi K. (1950). Biological survey of fin and blue whales taken in the Antarctic season 1947–48 by the Japanese fleet. Sci. Rep. Whales Res. Inst. Tokyo 3, 132–190.
Oña J., Garland E. C., Denkinger J. (2017). Southeastern Pacific humpback whales (Megaptera novaeangliae) and their breeding grounds: distribution and habitat preference of singers and social groups off the coast of Ecuador. Mar. Mamm. Sci. 33, 219–235. doi: 10.1111/mms.12365
Oviedo L., Solís M. (2008). Underwater topography determines critical breeding habitat for humpback whales near Osa Peninsula, Costa Rica: implications for Marine Protected Areas. Rev. Biol. Trop. 56, 591–602.
Owen K., Kavanagh A. S., Warren J. D., Noad M. J., Donnelly D., Goldizen A. W., et al. (2017). Potential energy gain by whales outside of the Antarctic: prey preferences and consumption rates of migrating humpback whales (Megaptera novaeangliae). Polar Biol. 40, 277–289. doi: 10.1007/s00300-016-1951-9
Palacios D. M., Mate B. R., Baker C. S., Hayslip C. E., Follett T. M., Steel D., et al. (2019). Tracking North Pacific Humpback Whales To Unravel Their Basin-Wide Movements (Newport, Oregon, USA: Marine Mammal Institute).
Paterson R. (1991). The migration of humpback whales Megaptera novaeangliae in east Australian waters. Mem. QLD Museum 30, 333–341.
Paterson R., Paterson P. (1989). The status of the recovering stock of humpback whales Megaptera novaeangliae in East Australian waters. Biol. Conserv. 47, 33–48. doi: 10.1016/0006-3207(89)90018-9
Paton D. (2016). Conservation management and population recovery of East Australian Humpback Whales (Southern Cross University).
Patton D., Lawless S. (2021). Surface and Underwater Observation of a Humpback Whale (Megaptera novaeangliae) Birth in Progress off Lahaina, Maui, and Subsequent Encounter of the Female with a Healthy Calf. Aquat. Mamm. 47, 550–558. doi: 10.1578/AM.47.6.2021.550
Peel D., Smith J. N., Childerhouse S. (2018). Vessel strike of whales in Australia: the challenges of analysis of historical incident data. Front. Mar. Sci. 5, 69. doi: 10.3389/fmars.2018.00069
Pirotta V., Franklin W., Mansfield L., Lowe J., Peterson O. (2023). Sighting records of “Migaloo” the white humpback whale provide evidence of Australian site fidelity and use of New Zealand waters as a migratory route. Aust. Zool. 42, 1014–1028. doi: 10.7882/AZ.2022.043
Pirotta V., Hocking D. P., Iggleden J., Harcourt R. (2022). Drone observations of marine life and human–wildlife interactions off Sydney, Australia. Drones 6, 75. doi: 10.3390/drones6030075
Pirotta V., Owen K., Donnelly D., Brasier M. J., Harcourt R. (2021). First evidence of bubble-net feeding and the formation of ‘super-groups’ by the east Australian population of humpback whales during their southward migration. Aquat. Conserv.: Mar. Freshw. Ecosyst. 31, 2412–2419. doi: 10.1002/aqc.v31.9
Pirotta V., Reynolds W., Ross G., Jonsen I., Grech A., Slip D., et al. (2020). A citizen science approach to long-term monitoring of humpback whales (Megaptera novaeangliae) off Sydney, Australia. Mar. Mamm. Sci. 36, 472–485. doi: 10.1111/mms.12651
Pitman R. L., Durban J. W., Joyce T., Fearnbach H., Panigada S., Lauriano G. (2020). Skin in the game: Epidermal molt as a driver of long-distance migration in whales. Mar. Mamm. Sci. 36, 565–594. doi: 10.1111/mms.12661
Ransome N., Bejder L., Jenner M., Penfold G., Brosig V. J., Kitson C., et al. (2022a). Observations of parturition in humpback whales (Megaptera novaeangliae) and occurrence of escorting and competitive behavior around birthing females. Mar. Mamm. Sci. 38, 408–432. doi: 10.1111/mms.12864
Ransome N., Kew A., Duque E., Morais M., Wright W., Smith J. N. (2022b). Escorting of a mother humpback whale (Megaptera novaeangliae) and the death of her calf during aggressive mating behavior. Mar. Mamm. Sci. 38, 1643–1653. doi: 10.1111/mms.12922
Rasmussen K., Calambokidis J., Steiger G. H. (2012). Distribution and migratory destinations of humpback whales off the Pacific coast of Central America during the boreal winters of 1996–2003. Mar. Mamm. Sci. 28, E267–E279. doi: 10.1111/j.1748-7692.2011.00529.x
Rasmussen K., Palacios D. M., Calambokidis J., Saborío M. T., Dalla Rosa L., Secchi E. R., et al. (2007). Southern Hemisphere humpback whales wintering off Central America: insights from water temperature into the longest mammalian migration. Biol. Lett. 3, 302–305. doi: 10.1098/rsbl.2007.0067
Rayner G. W. (1939). Preliminary results of the marking of whales by the discovery committee. Nature 144, 999–1002. doi: 10.1038/144999a0
Reidenberg J. S., Laitman J. T. (2009). “Cetacean Prenatal Development,” in Encyclopedia of Marine Mammals, 2nd ed. Eds. Perrin W. F., Würsig B., Thewissen, J. G. M. (Academic Press, London).
Schilling H. T., Dedden A. V., Crocetti S., Liggins G., Lorigan S., Marshall A., et al. (2023). Regional oceanography affects humpback whale entanglements in set fishing gear. Conserv. Sci. Pract. 5, e13034. doi: 10.1111/csp2.13034
Seyboth E., Meynecke J.-O., De Bie J., Roychoudhury A., Findlay K. (2023). A review of post-whaling abundance, trends, changes in distribution and migration patterns, and supplementary feeding of Southern Hemisphere humpback whales. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.997491
Smith R. G. (1841). Logbook of the Amazon (Ship) out of Fairhaven, MA, mastered by Robert G. Smith, on a whaling voyage between 1839 and 1841.
Smith J. N., Grantham H. S., Gales N., Double M. C., Noad M. J., Paton D. (2012). Identification of humpback whale breeding and calving habitat in the Great Barrier Reef. Mar. Ecol. Prog. Ser. 447, 259–272. doi: 10.3354/meps09462
Smith J. N., Kelly N., Childerhouse S., Redfern J. V., Moore T. J., Peel D. (2020). Quantifying ship strike risk to breeding whales in a multiple-use marine park: the Great Barrier Reef. Front. Mar. Sci. 7, 67. doi: 10.3389/fmars.2020.00067
Stack S. H., Krannichfeld L., Romano B. (2024). An observation of sexual behavior between two male humpback whales. Mar. Mamm. Sci. 40, e13119. doi: 10.1111/mms.13119
Stockin K. A., Burgess E. A. (2005). Opportunistic feeding of an adult humpback whale megaptera novaeangliae) migrating along the coast of Southeastern Queensland, Australia. Aquat. Mamm. 31, 120. doi: 10.1578/AM.31.1.2005.120
Szabo A., Duffus D. (2008). Mother–offspring association in the humpback whale, Megaptera novaeangliae: following behaviour in an aquatic mammal. Anim. Behav. 75, 1085–1092. doi: 10.1016/j.anbehav.2007.08.019
Tomo I., Kemper C. M. (2022). Strandings in St Vincent gulf bioregion, South Australia: 12-year study monitors biology and pathology of cetaceans. Oceans 3 (4), 439–463. doi: 10.3390/oceans3040030
Torre-Williams L., Martinez E., Meynecke J., Reinke J., Stockin K. (2019). Presence of newborn humpback whale (Megaptera novaeangliae) calves in Gold Coast Bay, Australia. Mar. Freshw. Behav. Physiol. 52, 199–216. doi: 10.1080/10236244.2019.1671769
Townsend C. H. (1935). The distribution of certain whales as shown by log book records of American whaleships. Zoologica 19, 1–50. doi: 10.5962/p.203715
Trudelle L., Cerchio S., Zerbini A. N., Geyer Y., Mayer F.-X., Jung J.-L., et al. (2016). Influence of environmental parameters on movements and habitat utilization of humpback whales (Megaptera novaeangliae) in the Madagascar breeding ground. R. Soc. Open Sci. 3, 160616. doi: 10.1098/rsos.160616
Valsecchi E., Corkeron P. J., Galli P., Sherwin W., Bertorelle G. (2010). Genetic evidence for sex-specific migratory behaviour in western South Pacific humpback whales. Mar. Ecol. Prog. Ser. 398, 275–286. doi: 10.3354/meps08280
Vanderlaan A. S., Taggart C. T. (2007). Vessel collisions with whales: the probability of lethal injury based on vessel speed. Mar. mammal Sci. 23, 144–156. doi: 10.1111/j.1748-7692.2006.00098.x
Verrill A. E. (1902). The Bermuda islands: an account of their scenery, climate, productions, physiography, natural history and geology, with sketches of their discovery and early history, and the changes in their flora and fauna due to man (New Haven, Conn). https://www.biodiversitylibrary.org/page/33067294.
Ward R., Mccauley R. D., Gavrilov A. N., Charlton C. M. (2019). Underwater sound sources and ambient noise in Fowlers bay, South Australia, during the austral winter. Acoust. Aust. 47, 21–32. doi: 10.1007/s40857-019-00150-9
Warren V. E., Constantine R., Noad M., Garrigue C., Garland E. C. (2020). Migratory insights from singing humpback whales recorded around central New Zealand. R. Soc. Open Sci. 7, 201084. doi: 10.1098/rsos.201084
Whitehead H., Moore M. J. (1982). Distribution and movements of West Indian humpback whales in winter. Can. J. Zool. 60, 2203–2211. doi: 10.1139/z82-282
Williamson G. (1961). Winter sighting of a humpback suckling its calf on the Grand Bank of Newfoundland. Norwegian Whaling Gazette 8, 335–341.
Zerbini A. N., Andriolo A., Da Rocha J. M., Simoes-Lopes P. C., Siciliano S., Pizzorno J. L., et al. (2004). Winter distribution and abundance of humpback whales (Megaptera novaeangliae) off Northeastern Brazil. J. Cetacean Res. Manage. 6, 101–107. doi: 10.47536/jcrm.v6i1.796
Keywords: Humpback whale (Megaptera novaeangliae), calving area, breeding grounds, neonate calves, migration, citizen science, industry engagement
Citation: McPhee-Frew J, Dedden AV, Pirotta V, Marshall A, Waples K, Raudino HC and Rogers TL (2025) Humpback whales (Megaptera novaeangliae) continue migration after giving birth in temperate waters in Australia and New Zealand. Front. Mar. Sci. 12:1545526. doi: 10.3389/fmars.2025.1545526
Received: 15 December 2024; Accepted: 24 March 2025;
Published: 20 May 2025.
Edited by:
Peter H. Dutton, National Oceanic and Atmospheric Administration, United StatesReviewed by:
Peter Corkeron, Griffith University, AustraliaWally Alfred Chapman Franklin, Southern Cross University, Australia
Copyright © 2025 McPhee-Frew, Dedden, Pirotta, Marshall, Waples, Raudino and Rogers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jane McPhee-Frew, ai5tY3BoZWVmcmV3QHVuc3cuZWR1LmF1
 Jane McPhee-Frew
Jane McPhee-Frew Adelaide V. Dedden
Adelaide V. Dedden Vanessa Pirotta
Vanessa Pirotta Andrew Marshall2
Andrew Marshall2 Kelly Waples
Kelly Waples Holly C. Raudino
Holly C. Raudino Tracey L. Rogers
Tracey L. Rogers