Abstract
Green and golden tides caused by Ulva prolifera and Sargassum horneri erupt annually along the coasts of China. During the late stages of algal blooms, massive macroalgal die-offs result in the release of dissolved organic matter (DOM), which can profoundly affect local marine environments and elemental cycles. However, studies addressing these impacts remain insufficient and urgently needed. This study investigates the two primary macroalgal contributors, Ulva prolifera and Sargassum horneri, by simulating their decay and DOM release in seawater using Bacteria-active (BA) and Bacteria-inhibited (BI) groups. The release characteristics of carbon, nitrogen, and phosphorus, as well as their effects on microbial communities, were analyzed. Results show that U. prolifera and S. horneri rapidly release DOM and nutrients during their decay. Significant differences in the quantity and rate of DOM and nutrient release were observed due to structural differences between the two macroalgae. Carbohydrates constitute the major component of the released organic matter and are rapidly utilized by microbes for growth and reproduction. Furthermore, the DOM and nutrients released by macroalgae reshape the composition of microbial communities in the marine microenvironment. This study provides new insights into the potential regional marine ecological impacts of green and golden tide outbreaks from a biogeochemical perspective.
Introduction
The phenomenon of large-scale green tide outbreaks was first recorded in the early 1970s in the Brittany region (Schreyers et al., 2021). Since then, the distribution of green tides has progressively expanded to coastal regions across multiple continents, including Europe, Asia, America, and Australia (Yuan et al., 2022; Song et al., 2022). Moreover, golden tide events caused by Sargassum macroalgae have erupted on a large scale in regions such as West Africa, the Gulf of Mexico, the Brazilian coast, the Caribbean Sea, and the East China Sea, causing severe ecological damage, the main manifestations are nutrient release and increased eutrophication, biotoxicity and disruption of community structure (García-Robledo et al., 2012; Byeon et al., 2019; Gordillo Sierra et al., 2022). Since 2007, green tides dominated by Ulva prolifera have occurred consecutively for 17 years in the South Yellow Sea of China, characterized by their vast scale and severe impacts, significantly damaging the marine ecosystem (Zhou et al., 2015). In some localized areas of the South Yellow Sea, concurrent outbreaks of green and golden tides have been observed, intensifying the severity of these algal blooms. As a result, the outbreaks of green and golden tides have become a prominent marine environmental issue and a global ecological disaster (Xiao et al., 2021).
In recent years, the frequency, geographic extent, and adverse impacts of green and golden tides have shown an increasing trend, driven by rapid industrialization and population growth (Xiao et al., 2021). The outbreak of green tides sharply reduces light penetration into the marine environment, disrupting photosynthesis and leading to the mortality of benthic macroalgae (Van Alstyne et al., 2013; Feng et al., 2024). The outbreak of green tides sharply reduces light penetration into the marine environment, disrupting photosynthesis and leading to the mortality of benthic macroalgae (Van Alstyne et al., 2013; Feng et al., 2024). Additionally, dense algal biomass hinders the movement of plankton, suppressing the growth and reproduction of marine organisms (Du et al., 2023). Furthermore, the visual pollution and unpleasant odors associated with algal blooms adversely affect local tourism. At the later stages of blooms, as macroalgal masses decay, large quantities of organic matter and nutrients are rapidly released from the substantial biomass. This can exert detrimental effects on the regional ecological environment and disrupt carbon and nutrient cycles (Zhang and Wang, 2017; Xiu et al., 2019; Zhang et al., 2021). In addition, microbial utilization and remineralization of DOM is a central part of the marine biogeochemical cycle. Microorganisms remineralize organic carbon, nitrogen, and phosphorus into inorganic forms by degrading algal-derived DOM, affecting regional carbon sink capacity and eutrophication processes (Li et al., 2017; Zhang and Wang, 2017; Wang et al., 2024).
Current research on green and golden tides primarily focuses on the life cycles, reproductive strategies, growth conditions of the causative algae, and the factors driving these macroalgal outbreaks, including environmental variables such as water temperature, light availability, and nutrient concentrations (Gross et al., 2015; Wu et al., 2019, 2024). However, studies on the DOM released by the main macroalgae responsible for these tides (Ulva prolifera and Sargassum horneri) during the late decay stages of blooms, as well as their effects on regional environmental factors and microbial communities, remain relatively limited (Zhao et al., 2018). This study simulates the dynamics of DOM release during the decay of two macroalgae species (Ulva prolifera and Sargassum horneri) and investigates the changes in microbial utilization of DOM, as well as its effects on the composition and abundance of microbial communities. The aim is to explore the potential impacts of green and golden tide outbreaks on regional marine ecosystems and elemental cycling.
Materials and methods
Sample collection and cultivation
The sampling sites’ coordinates are shown in Figure 1. Macroalgae samples were collected during the routine marine environmental monitoring cruise in the South Yellow Sea, off Yancheng, Jiangsu Province, China, in June 2023. The sampling locations were designated as S1 (121°32’52”N; 34°17’53”E), S2 (122°0’55”N; 34°13’52”E), and S3 (122°25’37”N; 34°15’27”E). At each site, no less than 300 g of each of the two algae species was collected, along with 100 L of natural seawater. The collected algae were placed in sealed bags, stored in a cooler (4 °C), and transported back to the laboratory. Upon arrival, the macroalgae were thoroughly rinsed with Milli-Q water to remove attached sediments and other impurities. Excess surface water was removed using absorbent paper (80 × 25, Changde Bkmam Biotechnology Co., Ltd.), with the process repeated three times. For the natural seawater, impurities were removed by filtering the collected seawater through a 0.45 μm mixed cellulose fiber filter membrane (Tianjin Jinteng Experiment Equipment Co., Ltd.) to eliminate autotrophic microorganisms such as algae. Filtration may remove a small amount of bacteria; however, the process of removing particulate matter is still necessary because particulates in seawater could influence subsequent measurements and experimental results. After pretreatment, 20 g of Ulva prolifera and Sargassum horneri were individually placed in 4 L of natural seawater for cultivation as the bacteria-active group (BA). Simultaneously, a bacteria-inhibited group (BI) was established by adding microbial inhibitors (5 mL of saturated mercuric chloride solution), alongside a blank seawater control group without algae. All seawater used in this experiment was in-situ collected during routine monitoring cruises in the South Yellow Sea, and remained unmodified to preserve its natural physicochemical properties. Each treatment (BA: algae-seawater co-culture, BI: algae-seawater with microbial inhibitor, and blank control: seawater only) was established with two independent biological replicates per algal species (U. prolifera and S. horneri), yielding a total of 12 sample replicates (2 species × 3 treatments × 2 replicates) (Zhang and Wang, 2017; Zhang et al., 2020). The macroalgae and pretreated natural seawater were placed in 5 L glass Erlenmeyer flasks, covered with gas-permeable oxygen-sealing polypropylene membranes to prevent contamination while ensuring adequate air exchange. The flasks were incubated in a culture chamber (model LRH-70, Rex (Shanghai) Technology Co., Ltd.) at a constant temperature of 25°C under dark conditions for 150 days. All glassware used for sample handling and cultivation was pre-cleaned by acid washing, rinsed with Milli-Q water, and combusted at 550°C for 5 hours to eliminate organic impurities.
Figure 1
Geographical position of the sampling sites.
Chemical analysis
At specific intervals during the cultivation period (0, 1, 2, 3, 5, 7, 9, 11, 13, 15, 18, 21, 24, 27, 30, 35, 40, 45, 50, 60, 90, 120, and 150 days), 40 mL of culture solution was sampled for analysis. After each sampling, 40 mL of filtered natural seawater was promptly added to maintain the initial volume. A polyethersulfone (PES) syringe filter (Changde Bkmam Biotechnology Co., Ltd.) with a pore size of 0.45 μm was used for filtration. The filter was pre-rinsed with Milli-Q water, and the filtered seawater was subsequently used for chemical analysis. The concentrations of dissolved organic carbon (DOC) and total nitrogen (TN) were determined using a multi-element carbon-nitrogen analyzer (Multi N/C 2100, Analytik Jena AG, Germany). Total sugar concentration was measured according to GB 500.9-2016, nitrite concentration following GB 7493-87, ammonia-nitrogen concentration according to HG 536-2009, nitrate concentration according to GB 7480-87, and phosphate concentration following HJ 670-2013. Ammonium nitrogen (NH4+-N), nitrite nitrogen (NO2–N), and nitrate nitrogen (NO3–N) were the dominant components of the TN released by Ulva prolifera and Sargassum horneri (Lv et al., 2018). During each sampling, the pH and dissolved oxygen levels of the culture solution were monitored in real-time using a pH meter (PHS-25, DELIXI Group Limited Company) and a dissolved oxygen meter (ARB804, INASE Scientific Instrument Co., Ltd., Shanghai). The relevant calculation formula refers to previous study (Weigel and Pfister, 2021).
Microbial community abundance and composition analysis
Samples from both the control and experimental groups were collected on the 150th day of cultivation to analyze microbial diversity in the water after the decomposition and release of Ulva prolifera and Sargassum horneri. The culture solution was first filtered through a 3 μm mixed fiber membrane, followed by filtration through a 0.22 μm mixed fiber membrane to enrich microbial communities. The enriched samples were subjected to high-throughput 16S rRNA sequencing, following protocols outlined in previous studies (Liao et al., 2018).
Data analysis
Statistical analysis and graphical visualization were performed using Origin 2024 and SPSS 27.0. Prior to parametric tests, the normality of all datasets was confirmed by the Shapiro-Wilk test (P> 0.05), and homogeneity of variances was validated via Levene’s test (P> 0.05). One-way analysis of variance (ANOVA) was applied to evaluate differences in microbial community richness among the Ulva prolifera, Sargassum horneri, and blank control groups. The confidence interval for all tests was set at 95%.
Results
DOC release and microbial utilization by decomposing macroalgae
The kinetics of dissolved organic carbon (DOC) release by Ulva prolifera and Sargassum horneri varied between the Bacteria-inhibited (BI) and Bacteria-active (BA) groups (Figure 2a). In the BI group, DOC content steadily accumulated, whereas in the BA group, DOC levels increased rapidly at first, followed by a rapid decline and stabilization. Carbohydrates constituted the largest proportion of the algal biomass in both Ulva prolifera and Sargassum horneri, and their total sugar release was measured (Figure 2b). In the BI group, the total sugar concentration from Ulva prolifera increased from 0.32 mg/L on day 1 to 42.38 mg/L on day 35, reaching 54.88 mg/L by the end of the experiment, with a release rate of 5.57 mg/L/day. Similarly, for Sargassum horneri, the total sugar concentration rose to 24.77 mg/L on day 27 and eventually reached 30.82 mg/L, with a release rate of 3.41 mg/L/day. In the BA group, the total sugar concentration for Ulva prolifera and Sargassum horneri peaked on day 11 at 32.71 mg/L and 17.98 mg/L, respectively, before rapidly declining. By day 55, the concentrations stabilized at 5.50 ± 0.5 mg/L and 3.50 ± 0.5 mg/L, respectively.
Figure 2
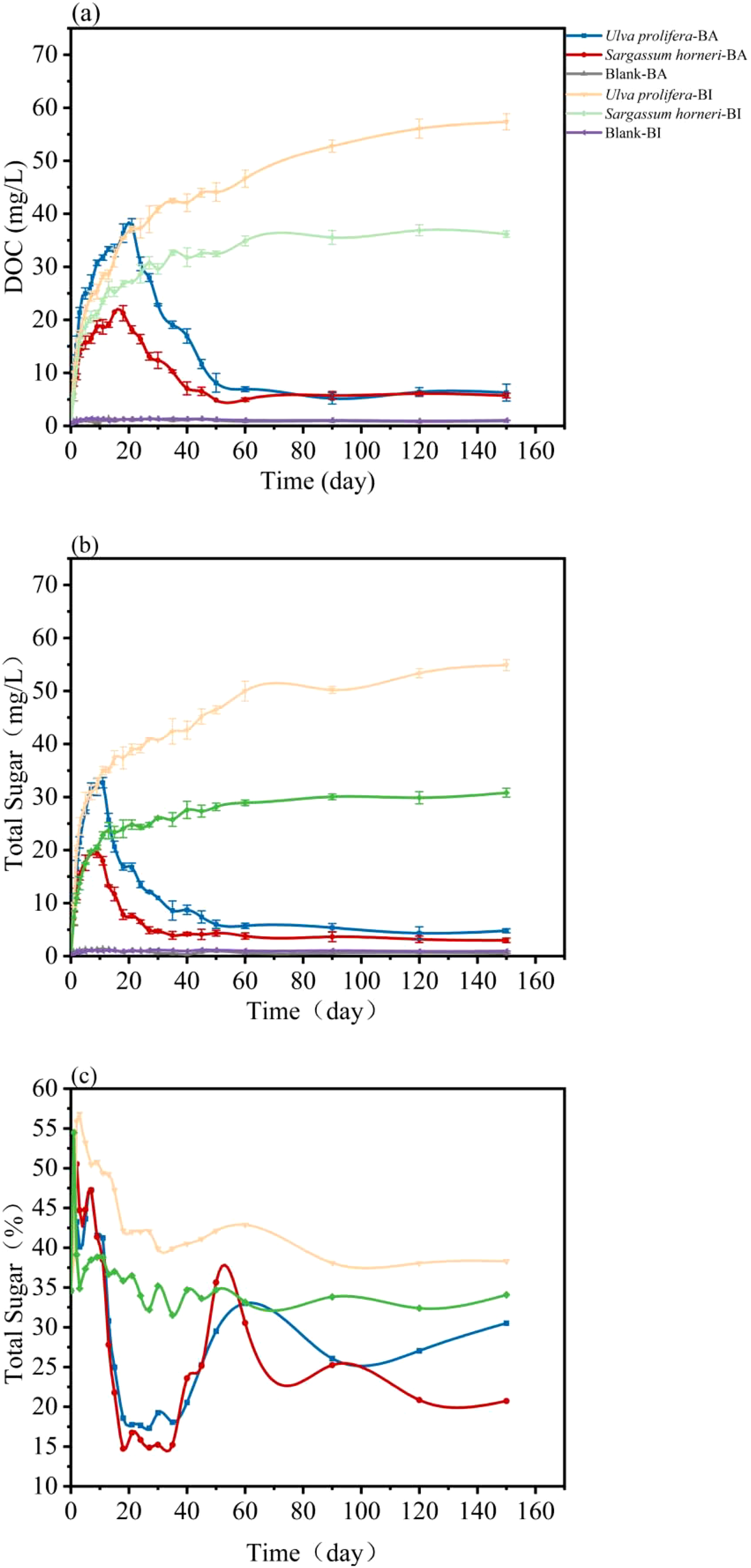
Temporal variation of DOC concentration (a), total sugar concentration (b), and the proportion of total sugar in DOC (c) released by Ulva prolifera and Sargassum horneri in the Bacteria-active and Bacteria-inhibited groups.
The proportion of total sugar within DOC (Figure 2c) exhibited fluctuations between 38.50% and 50.53% during the first eight days in the BA group. After day 18, due to microbial uptake, the total sugar proportion decreased to 18.58% and 14.73% for Ulva prolifera and Sargassum horneri, respectively (Figure 3). In the later stages of the experiment, the total sugar proportion stabilized, with final proportions of 30.51% and 20.74% for Ulva prolifera and Sargassum horneri in the BA group. Microbial utilization rates of DOC released by Ulva prolifera and Sargassum horneri were 89% and 84%, respectively, while the differences in total sugar concentrations were 91% and 90%.
Figure 3
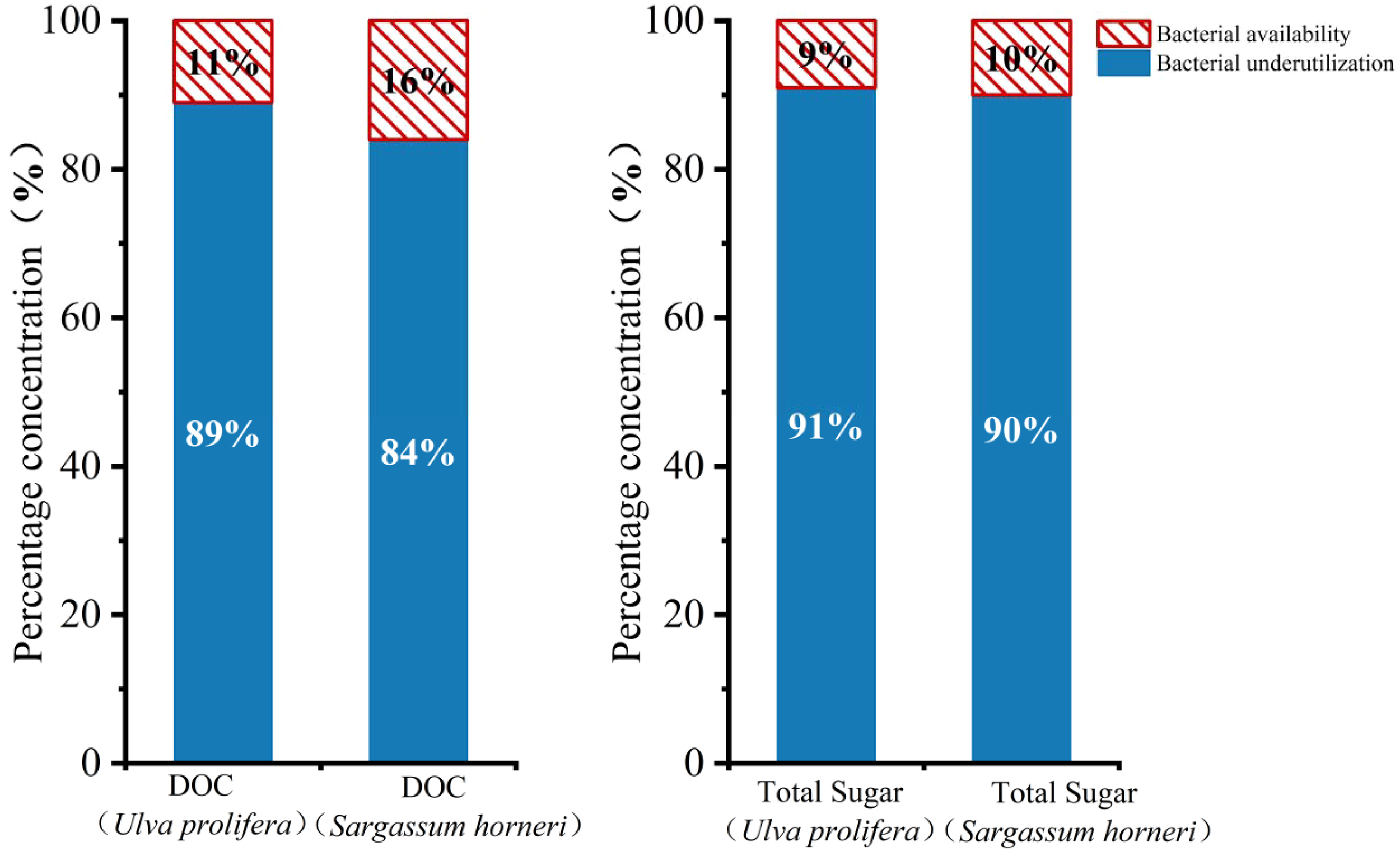
Proportional contributions of DOC and total sugar concentrations released by Ulva prolifera and Sargassum horneri in the Bacteria-active and Bacteria-inhibited groups.
Nutrient release and microbial utilization from decomposing macroalgae
The release kinetics of total nitrogen (TN) by Ulva prolifera and Sargassum horneri differed between the BI and BA groups (Figure 4a). In the BI group, TN content steadily accumulated, whereas in the BA group, TN levels rapidly increased initially, followed by a sharp decline and subsequent stabilization. By day 30, TN concentrations in the BI group increased from 0.14 mg/L to 6.72 mg/L for Ulva prolifera and eventually reached 8.01 mg/L, with an overall release rate of 0.88 mg/L/day. The TN concentration for Sargassum horneri in the BI group reached 4.89 mg/L by day 30 and eventually stabilized at 6.13 mg/L, with a release rate of 0.64 mg/L/day. In the BA group, TN concentrations for Ulva prolifera and Sargassum horneri initially increased and then decreased. For Ulva prolifera, the TN concentration peaked at 5.06 mg/L on day 18, while for Sargassum horneri, it peaked at 3.82 mg/L on day 21. By the end of the experiment, the TN concentrations in the BA group were 1.37 mg/L for Ulva prolifera and 1.02 mg/L for Sargassum horneri, with release rates of 0.50 mg/L/day and 0.37 mg/L/day, respectively. The microbial utilization of TN was calculated as 26.56 mg and 20.44 mg for Ulva prolifera and Sargassum horneri, accounting for 82.9% and 83.4% of the total TN released, respectively.
Figure 4
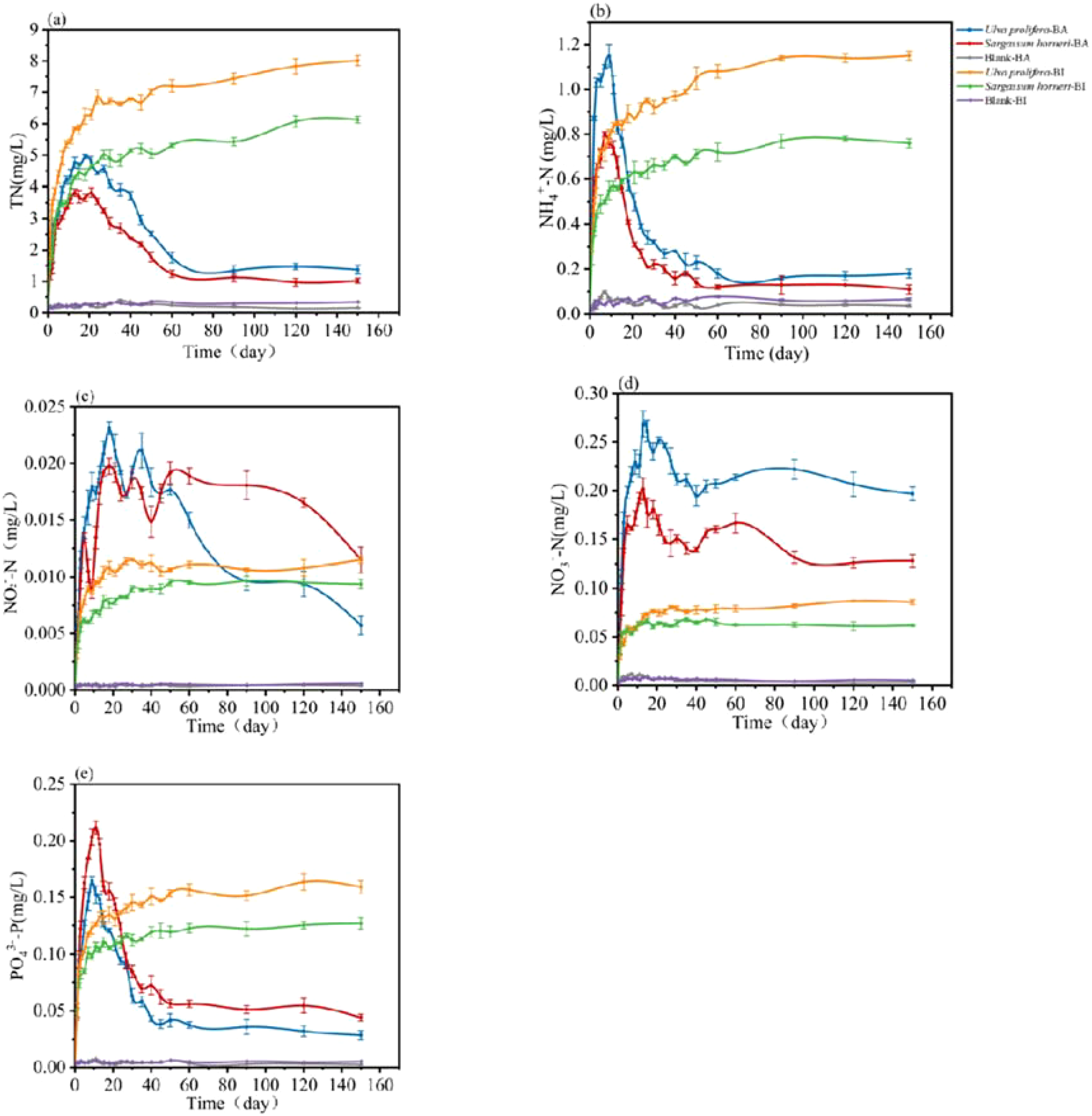
Temporal changes in the concentrations of total nitrogen (TN; a), ammonium nitrogen (NH4+-N; b), nitrite nitrogen (NO2–N; c), nitrate nitrogen (NO3–N; d), and phosphate (PO43–P; e) released by Ulva prolifera, Sargassum horneri, and blank controls in the Bacteria-active and Bacteria-inhibited groups.
Therefore, their concentrations were measured in this study (Figure 4b-d). In the BI group, NH4+-N, NO2–N and NO3–N concentrations steadily accumulated over time. For Ulva prolifera, the NH4+-N concentration increased from 0.01 mg/L on day 1 to 1.03 mg/L on day 3, with a release rate of 0.08 mg/L/day. For Sargassum horneri, the NH4+-N concentration reached 0.49 mg/L on day 5, with a release rate of 0.09 mg/L/day. Similarly, the NO2–N concentration for Ulva prolifera increased from 0.0012 mg/L to 0.0080 mg/L by day 5, while that for Sargassum horneri increased from 0.0013 mg/L to 0.0062 mg/L, with release rates of 0.0014 mg/L/day and 0.0011 mg/L/day, respectively. The NO3–N concentrations released by Ulva prolifera and Sargassum horneri increased from 0.012 mg/L to 0.058 mg/L and from 0.013 mg/L to 0.057 mg/L, respectively, by day 5. The overall release rates were 0.0102 mg/L/day and 0.0089 mg/L/day, respectively. In the BA group, the NH4+-N concentrations released by U. prolifera and S. horneri increased rapidly, reaching their peaks of 0.89 mg/L and 0.8 mg/L on days 18 and 7, respectively, before quickly declining and stabilizing at 1.12 ± 0.02 mg/L and 0.11 ± 0.02 mg/L by day 80. The NO2–Nconcentrations of U. prolifera and S. horneri also rose rapidly, peaking at 0.018 mg/L and 0.013 mg/L on days 9 and 5, respectively, and subsequently decreasing to 0.021 mg/L and 0.019 mg/L by days 35 and 50. For NO3–N, the concentrations of U. prolifera and S. horneri rose sharply, peaking at 0.27 mg/L and 0.20 mg/L on day 13, before stabilizing at 0.196 ± 0.02 mg/L and 0.086 ± 0.02 mg/L, respectively.
The release kinetics of phosphate (PO43–P) by U. prolifera and S. horneri differed significantly between the BI and BA groups (Figure 4e). In the BI group, PO43–P content accumulated gradually, whereas in the BA group, PO43–P concentrations initially increased rapidly, followed by a sharp depletion and subsequent stabilization. By day 2 in the BI group, the PO43–P concentration of U. prolifera increased from 0.0037 mg/L to 0.0725 mg/L, ultimately reaching 0.1268 mg/L with an overall release rate of 0.0127 mg/L/day. The PO43–P concentration released by Sargassum horneri reached 0.0849 mg/L on day 2, eventually stabilizing at 0.1590 mg/L with a release rate of 0.0191 mg/L/day. In the BA group, the PO43–P concentrations of Ulva prolifera and S. horneri initially increased and then decreased, with U. prolifera peaking at 0.1649 mg/L on day 9 and S. horneri peaking at 0.2114 mg/L on day 11. By the end of the experiment, the PO43–P concentrations in the BA group were 0.0285 mg/L and 0.0440 mg/L for U. prolifera and S. horneri, respectively, with release rates of 0.0127 mg/L/day and 0.0164 mg/L/day. The microbial utilization of PO43–P accounted for 0.39 mg and 0.46 mg for U. prolifera and S. horneri, respectively, representing 77.5% and 72.3% of their total release.
Effects of macroalgae decomposition on environmental pH and DO
During the decomposition process of U. prolifera and S. horneri, significant temporal changes in pH were observed in both the BI and BA groups (Figure 5a). In both groups, pH initially dropped rapidly, followed by a sharp recovery. By day 2, the pH values in the BI group decreased from 8.11 to 7.84 for Ulva prolifera and from 8.11 to 7.91 for Sargassum horneri, eventually stabilizing at 8.06 and 8.09, respectively. In the BA group, the lowest pH values were recorded on day 9 (6.68) for U. prolifera and on day 7 (7.05) for S. horneri. By the end of the experiment, the pH in the BA group stabilized at 7.73 for U. prolifera and 7.83 for S. horneri.
Figure 5
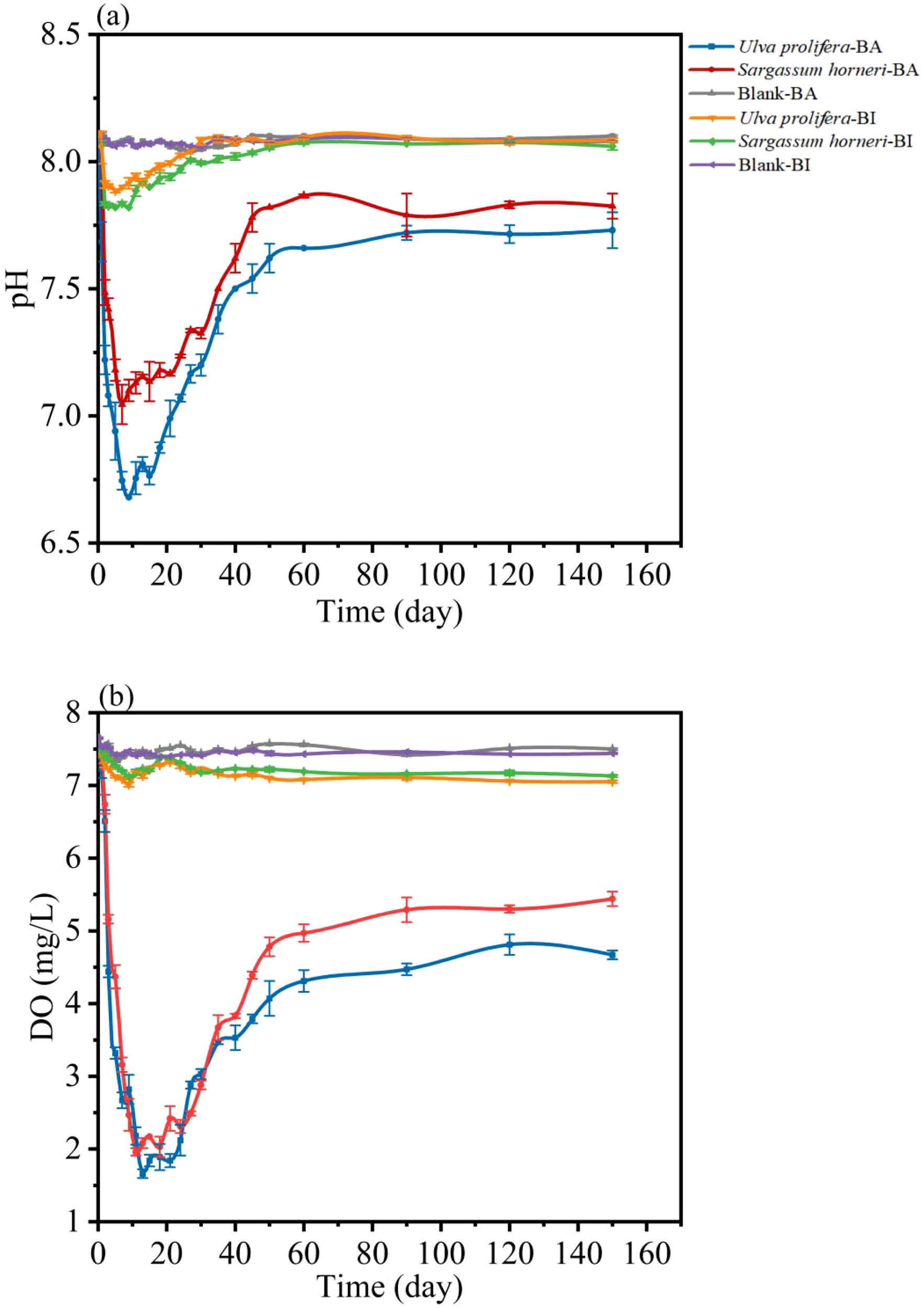
Changes in pH (a) and DO (b) concentrations of Ulva prolifera, Sargassum horneri and blank over culture time in Bacteria-active and Bacteria-inhibited groups.
The dissolved oxygen (DO) release kinetics of U. prolifera and S. horneri also exhibited distinct patterns between the BI and BA groups (Figure 5b). In the BI group, DO levels dropped rapidly, followed by fluctuations and eventual stabilization. In contrast, in the BA group, DO levels first declined sharply, then quickly recovered and stabilized. By day 9, the DO concentration of U. prolifera in the BI group had decreased from 7.65 mg/L to 7.01 mg/L, ultimately stabilizing at 7.05 mg/L. Similarly, S. horneri’s DO concentration in the BI group dropped from 7.65 mg/L to 7.12 mg/L, stabilizing at 7.13 mg/L, with release rates of 1.103 mg/L/day and 1.114 mg/L/day, respectively. In the BA group, the DO concentrations of U. prolifera and S. horneri initially decreased and then rebounded. The minimum DO levels were observed on day 13 (1.66 mg/L) for U. prolifera and on day 11 (1.96 mg/L) for S. horneri. By the end of the experiment, the DO concentrations stabilized at 4.67 mg/L for U. prolifera and 5.44 mg/L for S. horneri, with release rates of 0.57 mg/L/day and 0.62 mg/L/day, respectively. The microbial utilization of DO accounted for 9.52 mg (33.8%) of the total release in U. prolifera and 6.76 mg (23.7%) in S. horneri.
Effects of macroalgae decomposition on environmental microbial community structure
High-throughput sequencing of 16S rRNA was conducted on the microbial communities in the Ulva prolifera, Sargassum horneri, and blank control groups. The results revealed significant differences in microbial community composition at various taxonomic levels across the three experimental groups. Overall, the groups exhibited 23–25 phyla, 35–49 classes, 80–120 orders, 117–187 families, and 150–257 genera. The number of Amplicon Sequence Variants (ASVs)—representing distinct microbial species identified through 16S rRNA sequencing—was 482, 389, and 330 in the U. prolifera, S. horneri, and blank control groups, respectively. At the genus level, 257, 189, and 150 genera were identified in these groups, respectively. Microbial richness followed the order of U. prolifera > S. horneri > blank, and the evenness of microbial communities in the U. prolifera and S. horneri groups was higher than that in the blank control group (Figure 6). At the ASV level, a total of 482, 389, and 330 ASVs were detected in the U. prolifera, S. horneri, and blank groups, respectively. Of these, 42 ASVs were shared among all three groups. There were 88 ASVs shared between U. prolifera and S. horneri, 72 ASVs shared between U. prolifera and the blank group, and 33 ASVs shared between S. horneri and the blank group. The Ulva prolifera group exhibited the highest number of unique ASVs, with 288 species, while the blank control group had the fewest, at 183 species. During the decomposition process, the dominant microbial communities associated with U. prolifera and Sargassum horneri displayed distinct compositions. For U. prolifera, the dominant phyla included Proteobacteria (54.42%), Bacteroidota (13.46%), and Bdellovibrionota (10.73%). In contrast, the dominant phyla for S. horneri were Proteobacteria (74.04%), Bacteroidota (8.89%), and Firmicutes (5.22%). At the genus level, the dominant species in the U. prolifera group were Magnetospiraceae and Pseudohongiella, while Magnetospiraceae dominated the S. horneri group.
Figure 6
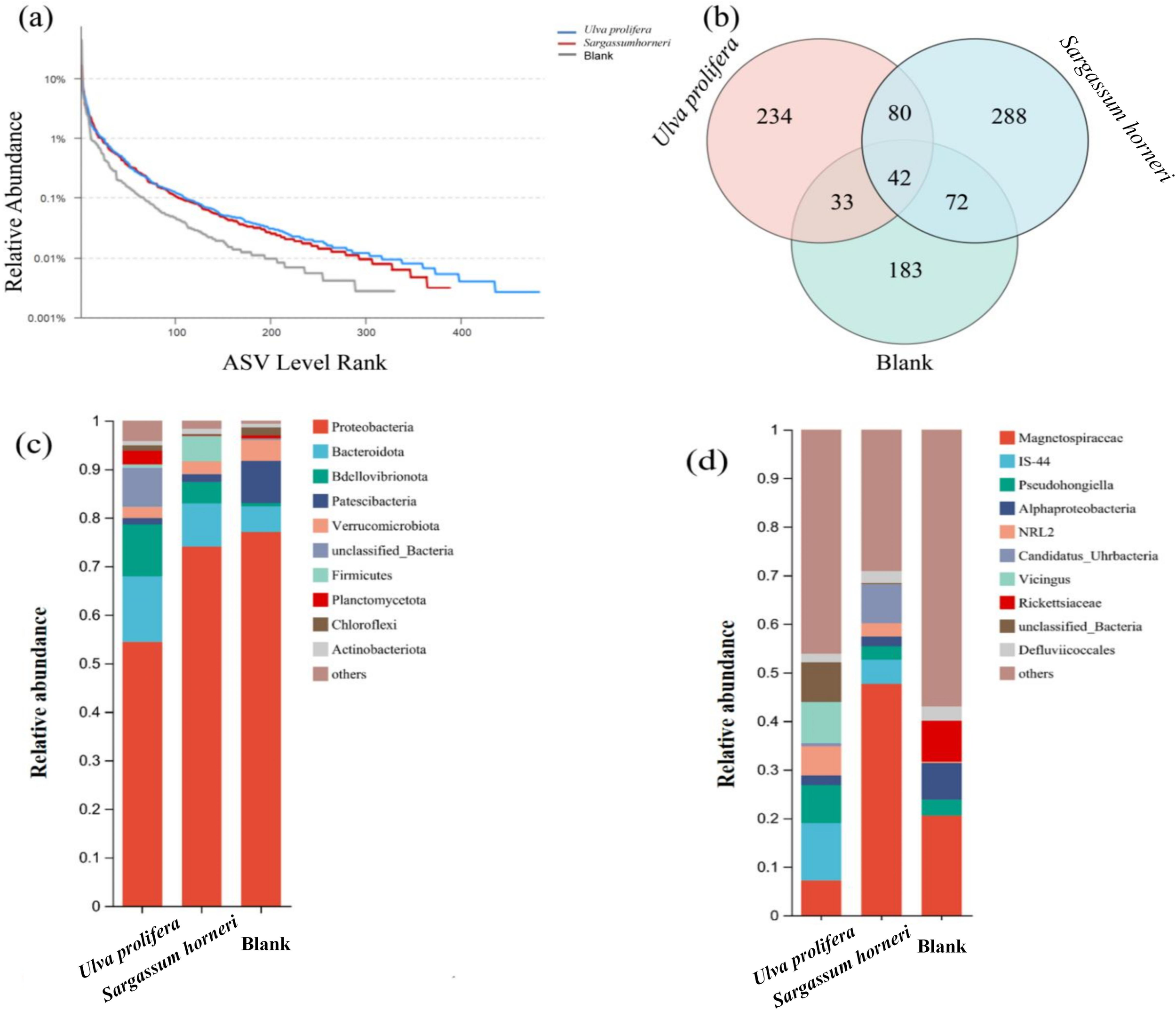
(a) Rank-abundance curves of microbial diversity in seawater during the decomposition of Ulva prolifera, Sargassum horneri, and the blank control. (b) Venn diagram showing ASV overlap between U. prolifera, S. horneri, and blank seawater. (c) Composition of microbial communities at the phylum level during the decomposition of U. prolifera and S. horneri. (d) Composition of microbial communities at the genus level during the decomposition of U. prolifera and S. horneri.
Discussions
In this study, both macroalgae exhibited rapid release of DOC during the initial stage, typically within a few hours, releasing substantial amounts of DOC. This phase was potentially controlled by physical and chemical processes, such as hydrolysis and organic matter dissolution effects (Zhang and Wang, 2017; Zhang et al., 2020). Following the initial stage, DOC release controlled by chemical processes gradually slowed down, while microbial growth and reproduction played a dominant role in the degradation of algal biomass, resulting in a gradual decrease in DOC concentration in the culture medium. Total sugars were identified as the main components of the organic matter released by both macroalgae species, which has also been described in other studies (Dave et al., 2021), and differences were observed in the rates and amounts of DOC and total sugar release between the two species. These differences may be attributed to the higher pectin and cellulose content in the cellular structure of Sargassum horneri compared to Ulva prolifera (Dave et al., 2021). These high-molecular-weight substances are more resistant to microbial degradation and utilization (Mei et al., 2019; Sun et al., 2024). In contrast, the hollow tubular structure of U. prolifera provides a larger specific surface area, enhancing physical and chemical processes as well as microbial contact, thereby accelerating the degradation of algal biomass (Pan et al., 2022). Moreover, these structural differences further influence the growth and reproduction of environmental microbial communities associated with the two macroalgae (Pan et al., 2022; Chen et al., 2023; Xie et al., 2024).On the other hand, the total sugar concentration exhibited a strong positive correlation with the organic carbon concentration for Ulva prolifera (R² = 0.97, p < 0.01) and Sargassum horneri (R² = 0.98, p < 0.01). In contrast, the data for the BA groups of U. prolifera (R² = 0.55, p < 0.01) and S. horneri (R² = 0.47, p < 0.01) were more dispersed and showed weaker correlations (Figure 7), indicating that bacteria utilized sugar components more rapidly. Previous studies have found that sugars serve not only as critical sources of energy and structural components in microbial growth but also play key roles in metabolic regulation and microbial community dynamics (Pan et al., 2022; Chen et al., 2023).
Figure 7
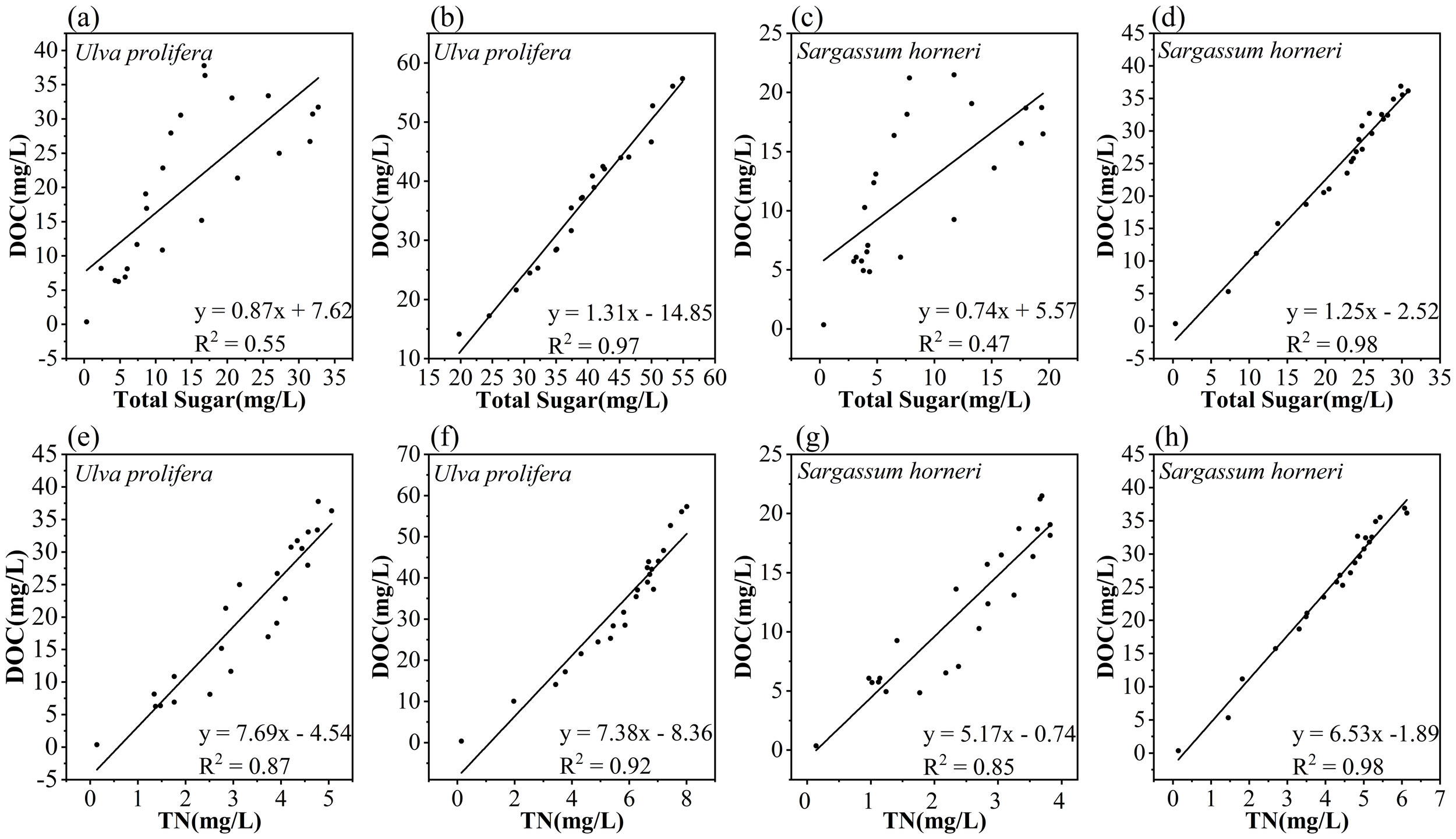
Correlation between DOC and total sugar concentrations released by Ulva prolifera and Sargassum horneri in the Bacteria-active (a, c) and Bacteria-inhibited (b, d) groups; Correlation between DOC and TN released by Ulva prolifera and Sargassum horneri in the Bacteria-active (e, g) and Bacteria-inhibited (f, h) groups.
It has been demonstrated that during the decomposition and senescence of plants, nutrients such as N and P from their tissues are released into the surrounding water (Zhang et al., 2021). The processes of DOC release during the senescence of U. prolifera and S. horneri are accompanied by the dissolution of large amounts of nutrients, complicating the changes in nutrient concentrations and their effects in the aquatic environment (Chen et al., 2020). The relationship between DOC released by U. prolifera and S. horneri and TN during their cultivation period is shown in Figure 7. The BA group of Ulva prolifera (R² = 0.87, p < 0.01) exhibited a significant correlation with its BI group (R² = 0.92, p < 0.01). Similarly, the BA group of Sargassum horneri (R² = 0.85, p < 0.01) showed a strong linear correlation with its BI group (R² = 0.98, p < 0.01). These findings suggest that carbon and nitrogen are released in an inherent proportion during the senescence of U. prolifera and S. horneri, indicating that TN release is predominantly in the form of organic nitrogen (Zhang and Wang, 2017). Microbial nitrification and denitrification play critical roles in altering nitrogen dynamics during plant senescence (Zhao et al., 2023). Additionally, the deposition of dead algal biomass and other organic matter through physical settling may influence the concentration and distribution of nitrogen in the water column (Li et al., 2017). Through ammonification and nitrification processes, microorganisms can convert organic nitrogen into inorganic nitrogen ions such as NH4+-N, NO2–N and NO3–N, integrating these into the aquatic environment (Raut et al., 2018). As microbial growth and reproduction progress, microorganisms not only consume DOC and TN as nutritional sources but also alter their concentrations and compositions through metabolic processes (Yang et al., 2021; Zhang et al., 2020). Consequently, this microbially mediated transformation may increase variability in the relationship between DOC and TN release, reducing their correlation (Li et al., 2017). A similar phenomenon is observed in the release of P, as shown in Figure 7.
In bacterial-active environments, the DOC released by the BA group of Ulva prolifera (R² = 0.86, p < 0.01) and Sargassum horneri (R² = 0.89, p < 0.01) exhibited significant negative correlations with environmental pH (Figure 7). Studies have shown that higher rates of plant detritus addition lead to lower pH levels, likely due to the release of organic matter from plants into the water, which is subsequently decomposed by microorganisms into acidic substances, such as carbon dioxide and humic acids, thereby reducing the pH of the aquatic environment (Xu et al., 2022; Zhang et al., 2024). Similarly, significant negative correlations were observed between DO and DOC in the BA group for both U. prolifera (R² = 0.67, p < 0.01) and S. horneri (R² = 0.67, p < 0.01) (Figure 8). This phenomenon may result from oxygen consumption by microbial respiration in the water, leading to a decrease in DO concentration. The rapid proliferation of bacteria accelerates oxygen consumption beyond the reoxygenation rate, causing the aquatic environment to enter an anaerobic state within a short period (Wang et al., 2014). As the release rate of organic carbon from the plants decreased and microorganisms continuously consumed the existing organic matter, the concentration of DOC gradually declined. This reduction in DOC concentration slowed the microbial oxidation rate, eventually allowing atmospheric reoxygenation to exceed oxygen consumption. Consequently, during the later stages of the experiment, the DO concentration in the water began to recover gradually (Wang et al., 2024). These findings indicate that microorganisms rapidly impact the environment by utilizing organic matter released from macroalgae, which may pose potential short-term adverse effects on nearby marine organisms.
Figure 8
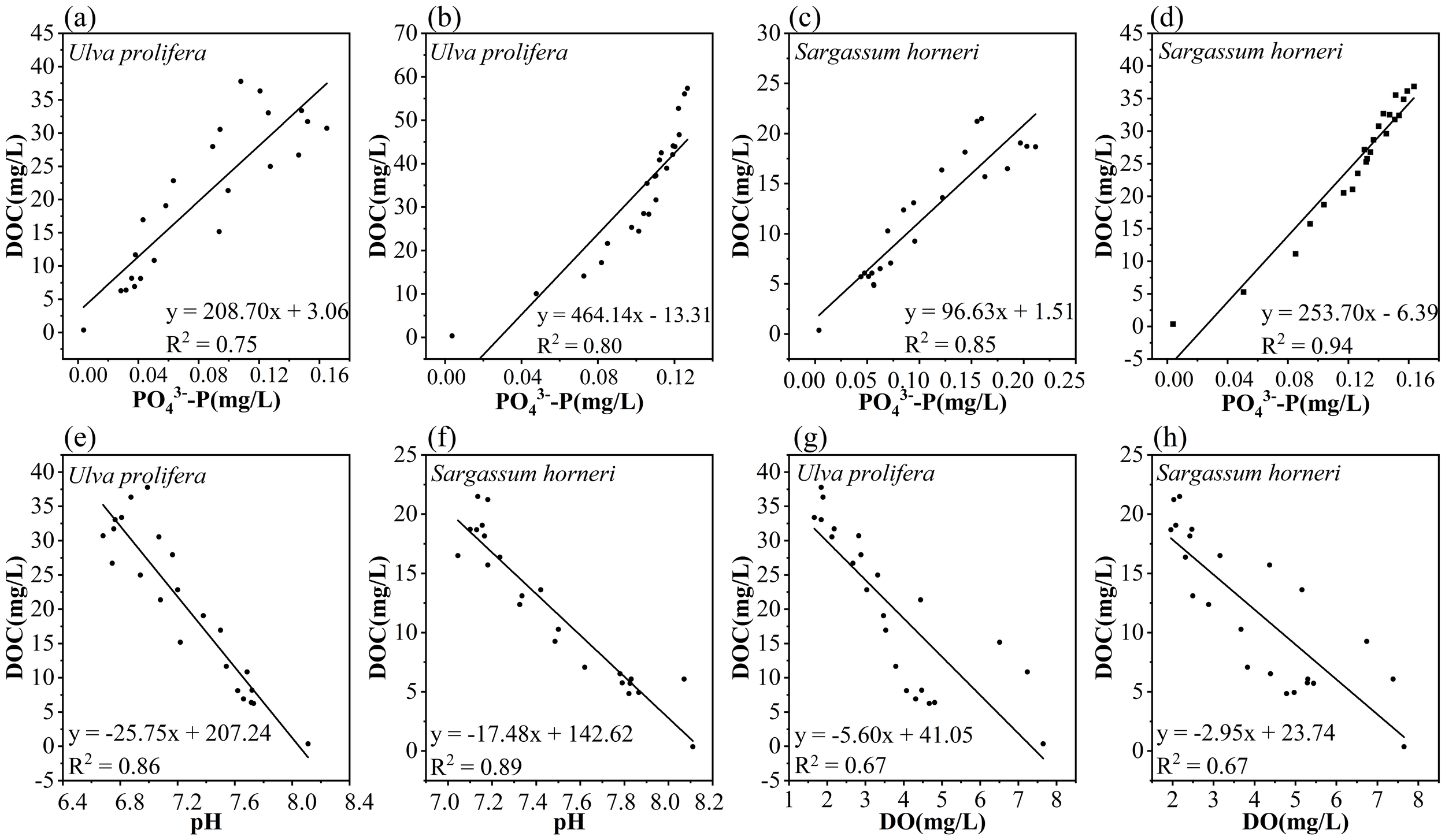
Correlation between DOC and PO43–Preleased by Ulva prolifera and Sargassum horneri in the Bacteria-active (a, c) and Bacteria-inhibited (b, d) groups; Correlation between DOC and pH (e, f) and DO concentration (g, h) in the Bacteria-active group of Ulva prolifera (left) and Sargassum horneri (right).
The Alpha diversity of microbial communities was evaluated using the Sobs index, Chao index, Shannon index, and Simpson index (Figure 9). The Coverage index for all samples was 100%, indicating the reliability of the sequencing results. The Sobs and Chao indices of U. prolifera were higher than those of S. horneri, indicating that the substantial organic matter released by U. prolifera more effectively promoted microbial proliferation. The Shannon and Simpson indices revealed that microbial communities in the senescent environments of both U. prolifera and S. horneri exhibited higher richness and evenness. Therefore, the apoptosis process of Ulva prolifera and Sargassum horneri may significantly affect the structure and function of microbial communities, further emphasizing the potential impacts of macroalgal apoptosis on aquatic ecosystems (García-Robledo et al., 2012; Zhu et al., 2022). Based on the analysis of the relative abundance of microbial phyla, the Proteobacteria phylum accounted for the highest proportion in the aquatic environment of this study, followed by the Bacteroidota phylum (Figure 6). Research indicates that the Proteobacteria and Bacteroidota phyla play crucial roles in organic matter degradation and nitrogen cycling. Specifically, Alphaproteobacteria can utilize DOM and decompose carbon and nitrogen compounds such as ammonium and methane, thereby facilitating carbon and nitrogen cycling. Betaproteobacteria primarily function as heterotrophs, participating in the decomposition of organic matter and the cycling of nutrients in water bodies, thus contributing to the maintenance of ecological balance (Bi et al., 2019). Additionally, Gammaproteobacteria include key ammonia-oxidizing bacteria, which are considered major contributors to the nitrogen cycle (Supty et al., 2024). Meanwhile, Deltaproteobacteria have been shown to promote the cycling of nitrogen, phosphorus, and sulfur in wetland ecosystems (Mehdizadeh Allaf and Peerhossaini, 2022). In this study, bacteria from the Proteobacteria and Bacteroidota phyla facilitated the cycling of elements such as carbon and nitrogen through the decomposition of organic matter, thereby influencing the material cycling and energy flow within the entire ecosystem (Ballot et al., 2005; Zhu et al., 2022; Zhao et al., 2022).
Figure 9
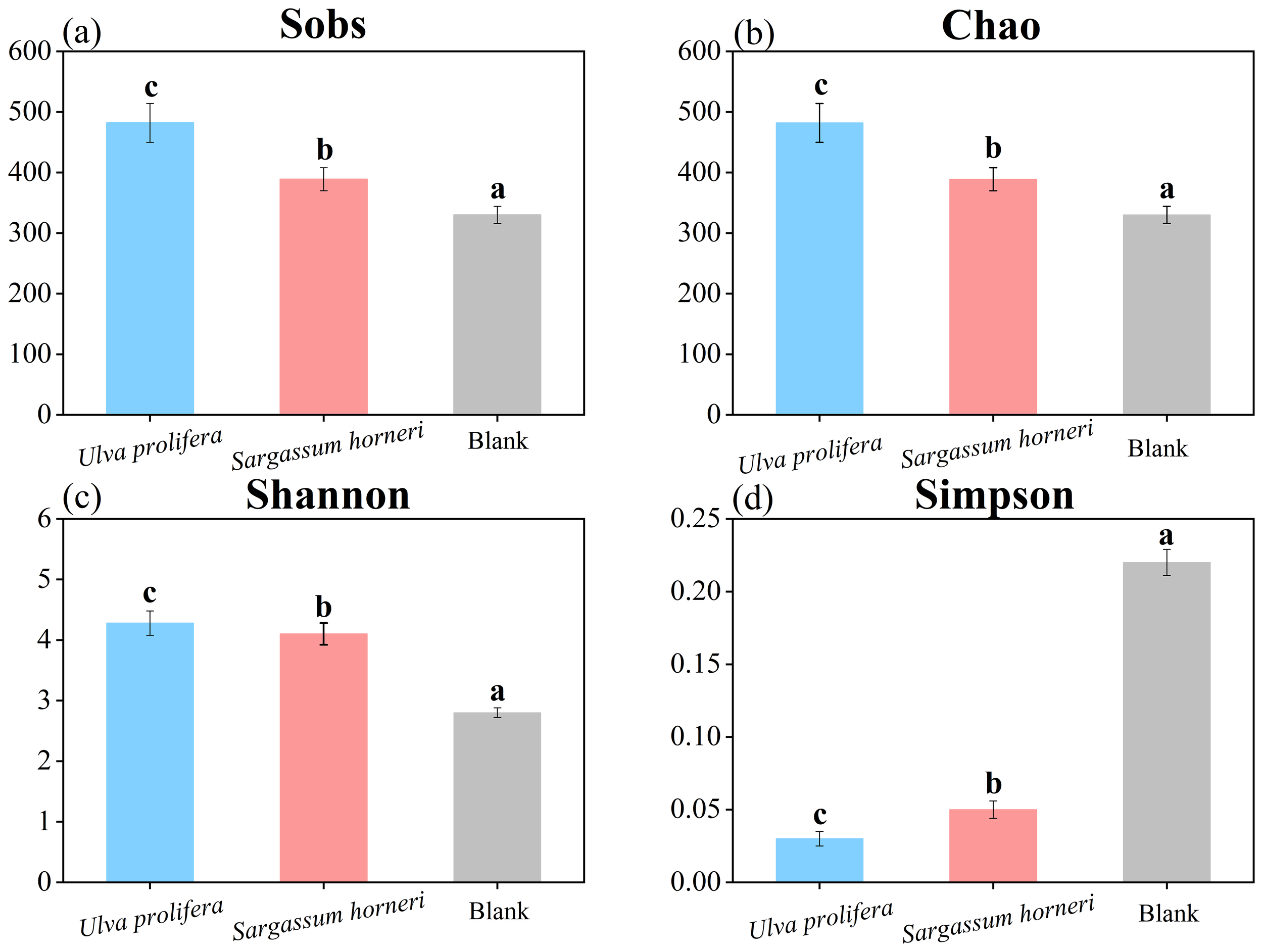
Alpha diversity indices (Sobs, Chao, Shannon, and Simpson) of microbial communities in the Ulva prolifera, Sargassum horneri, and blank control groups.
The findings revealed that each gram of Ulva prolifera released 11.466 mg of organic carbon, 1.602 mg of nitrogen, and 0.025 mg of phosphorus into the coastal waters, while each gram of Sargassum horneri released 7.234 mg of organic carbon, 1.226 mg of nitrogen, and 0.032 mg of phosphorus. Based on estimations, the outbreak biomass of U. prolifera and S. horneri in 2023 was approximately 5.70×105 tons and 5.25×105 tons, respectively (Yu et al., 2023; Zhang et al., 2023). Through calculations, the total release of organic carbon by U. prolifera and S. horneri was estimated to be 6535.62 tons and 3797.85 tons, respectively, resulting in a combined release of 10,333.47 tons. Similarly, U. prolifera and S. horneri released 913.14 tons and 643.65 tons of nitrogen, respectively, with a combined total of 1556.79 tons. For phosphorus, the respective releases were 16.80 tons and 14.25 tons, summing to 31.05 tons. The short-term influx of these organic substances and nutrient salt into coastal waters may significantly alter the aquatic environment, leading to changes in the composition and abundance of microbial communities, as well as impacting carbon and nutrient cycling. Such disruptions of balance are likely to have profound implications for the coastal marine ecosystem (Zhang and Wang, 2017). These ecological disruptions highlight the importance of enhancing monitoring and management strategies to mitigate the negative impacts on marine biodiversity and ecosystem health. Therefore, this study recommends implementing necessary removal measures during the early stages of algal blooms to reduce subsequent adverse ecological effects.
Conclusion
In this study, the two main causative macroalgae of green and golden tides, Ulva prolifera and Sargassum horneri, by simulating their decay and DOM release in seawater using Bacteria-active (BA) and Bacteria-inhibited (BI) groups were investigated. The results show that U. prolifera and S. horneri rapidly release DOM and nutrients during their decay. Significant differences in the quantity and rate of DOM and nutrient release were observed due to structural differences between the two macroalgae. Carbohydrates constitute the major component of the released organic matter and are rapidly utilized by microbes for growth and reproduction. Furthermore, the DOM and nutrients released by macroalgae reshape the composition of microbial communities in the marine microenvironment.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
TZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JS: Conceptualization, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. XH: Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft. BW: Formal Analysis, Investigation, Methodology, Project administration, Validation, Writing – review & editing. JR: Investigation, Methodology, Validation, Writing – original draft. SH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. XC: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This work was supported by: National Natural Science Foundation of China (No. 42206139); Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. SJCX23_1834). We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service. We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ballot A. Krienitz L. Kotut K. Wiegand C. Pflugmacher S. (2005). Cyanobacteria and cyanobacterial toxins in the alkaline crater lakes Sonachi and Simbi, Kenya. Harmful Algae.4, 139–150. doi: 10.1016/J.HAL.2004.01.001
2
Bi Y. Wang F. Zhang W. (2019). Omics analysis for dinoflagellates biology research. Microorganisme7, 288. doi: 10.3390/MICROORGANISMS7090288
3
Byeon S. Y. Oh H. J. Kim S. Yun S. H. Kang J. H. Park S. R. et al . (2019). The origin and population genetic structure of the ‘golden tide’ seaweeds, Sargassum horneri, in Korean waters. Sci. Rep.9, 7757. doi: 10.1038/s41598-019-44170-x
4
Chen Y. Song D. Li K. Gu L. Wei A. Wang X. (2020). Hydro-biogeochemical modeling of the early-stage outbreak of green tide (Ulva prolifera) driven by land-based nutrient loads in the Jiangsu coast. Mar. pollut. Bull.153, 111028. doi: 10.1016/J.MARPOLBUL.2020.111028
5
Chen Y. Zheng M. Jiang J. Hu W. Xu N. Li Y. (2023). Enhancement of growth in Ulva prolifera by diurnal temperature difference combined with nitrogen enrichment. Mar. Environ. Res.186, 105905. doi: 10.1016/J.MARENVRES.2023.105905
6
Dave N. Varadavenkatesan T. Singh R. S. Giri B. S. Selvaraj R. Vinayagam R. (2021). Evaluation of seasonal variation and the optimization of reducing sugar extraction from Ulva prolifera biomass using thermochemical method. Environ. Sci. pollut. R.28, 58857–58871. doi: 10.1007/S11356-021-12609-2/FIGURES/6
7
Du X. Li X. Cheng K. Zhao W. Cai Z. Chen G. et al . (2023). Virome reveals effect of Ulva prolifera green tide on the structural and functional profiles of virus communities in coastal environments. Sci. Total. Environ.883, 163609. doi: 10.1016/J.SCITOTENV.2023.163609
8
Feng G. Zeng Y. Wang J. Dai W. Bi F. He P. et al . (2024). A bibliometric review of Green Tide research between 1995-2023. Mar. pollut. Bull.208, 116941. doi: 10.1016/J.MARPOLBUL.2024.116941
9
García-Robledo E. Corzo A. Papaspyrou S. Morris E. P. (2012). Photosynthetic activity and community shifts of microphytobenthos covered by green macroalgae. Environ. Microbiol. Rep.4, 316–325. doi: 10.1111/J.1758-2229.2012.00335.X
10
Gordillo Sierra A. R. Amador-Castro L. F. Ramírez-Partida A. E. García-Cayuela T. Carrillo-Nieves D. Alper H. S. (2022). Valorization of Caribbean Sargassum biomass as a source of alginate and sugars for de novo biodiesel production. J. Environ. Manage.324, 116364. doi: 10.1016/J.JENVMAN.2022.116364
11
Gross M. Jarboe D. Wen Z. (2015). Biofilm-based algal cultivation systems. Appl. Microbiol. Biot.99, 5781–5789. doi: 10.1007/S00253-015-6736-5/METRICS
12
Li H. Zhang Y. Tang H. Shi X. Rivkin R. B. Legendre L. (2017). Spatiotemporal variations of inorganic nutrients along the Jiangsu coast, China, and the occurrence of macroalgal blooms (green tides) in the southern Yellow Sea. Harmful Algae.63, 164–172. doi: 10.1016/J.HAL.2017.02.006
13
Liao M. Xie Y. Mao Y. Lu Z. Tan A. Wu C. et al . (2018). Comparative analyses of fecal microbiota in Chinese isolated Yao population, minority Zhuang and rural Han by 16sRNA sequencing. Sci. Rep.8, 1–10. doi: 10.1038/s41598-017-17851-8
14
Lv J. Wang X. Feng J. Liu Q. Nan F. Jiao X. et al . (2018). Comparison of growth characteristics and nitrogen removal capacity of five species of green algae. J. Appl. Phycol.31, 409–421. doi: 10.1007/S10811-018-1542-Y/METRICS
15
Mehdizadeh Allaf M. Peerhossaini H. (2022). Cyanobacteria: model microorganisms and beyond. Microorganisms10, 696. doi: 10.3390/microorganisms10040696
16
Mei X. Wu C. Zhao J. Yan T. Jiang P. (2019). Community structure of bacteria associated with drifting sargassum horneri, the causative species of golden tide in the yellow sea. Front. Microbiol.10. doi: 10.3389/fmicb.2019.01192
17
Pan Z. Yu Y. Chen Y. Yu C. Xu N. Li Y. (2022). Combined effects of biomass density and low-nighttime temperature on the competition for growth and physiological performance of Gracilariopsis lemaneiformis and Ulva prolifera. Algal. Res.62, 102638. doi: 10.1016/J.ALGAL.2022.102638
18
Raut Y. Morando M. Capone D. G. (2018). Diazotrophic macroalgal associations with living and decomposing sargassum. Front. Microbiol.9. doi: 10.3389/FMICB.2018.03127/BIBTEX
19
Schreyers L. van Emmerik T. Biermann L. Le Lay Y.-F. (2021). Spotting green tides over brittany from space: three decades of monitoring with landsat imagery. Remote Sens.13, 1408. doi: 10.3390/rs13081408
20
Song M. Kong F. Li Y. Zhao J. Yu R. Zhou M. et al . (2022). A massive green tide in the yellow sea in 2021: field investigation and analysis. Int. J. Env. Res. Pub. He.19, 11753. doi: 10.3390/ijerph191811753
21
Sun B. Zhao X. Qu T. Zhong Y. Guan C. Hou C. et al . (2024). The causal link between nitrogen structure and physiological processes of Ulva prolifera as the causative species of green tides. Sci. Total. Environ.953, 176170. doi: 10.1016/j.scitotenv.2024.176170
22
Supty M. S. A. Jahan K. Lee J. S. Choi K. H. (2024). Epiphytic bacterial community analysis of ulva prolifera in garorim and muan bays, Republic of Korea. Microorganisms12, 1142. doi: 10.3390/MICROORGANISMS12061142/S1
23
Van Alstyne K. L. Anderson K. J. Van Hees D. H. Gifford S. A. (2013). Dopamine release by Ulvaria obscura (Chlorophyta): environmental triggers and impacts on photosynthesis, growth, and survival of the releaser. J. Phycol.49, 719–727. doi: 10.1111/JPY.12081
24
Wang R. Wang S. Cao R. Han J. Huang T. Wen G. (2024). The apoptosis of Chlorella vulgaris and the release of intracellular organic matter under metalimnetic oxygen minimum conditions. Sci. Total. Environ.907, 168001. doi: 10.1016/J.SCITOTENV.2023.168001
25
Wang X. Chen R. F. Cable J. E. Cherrier J. (2014). Leaching and microbial degradation of dissolved organic matter from salt marsh plants and seagrasses. Aquat. Sci.76, 595–609. doi: 10.1007/s00027-014-0357-4
26
Weigel B. L. Pfister C. A. (2021). The dynamics and stoichiometry of dissolved organic carbon release by kelp. Ecology102, e03221. doi: 10.1002/ecy.3221
27
Wu H. Cheng F. Chen J. Li H. Xu J. He P. et al . (2024). Species-specific responses of bloom-forming algae to the ocean warming and acidification. Plants13, 2433. doi: 10.3390/PLANTS13172433/S1
28
Wu H. Feng J. Li X. Zhao C. Liu Y. Yu J. et al . (2019). Effects of increased CO2 and temperature on the physiological characteristics of the golden tide blooming macroalgae Sargassum horneri in the Yellow Sea, China. Mar. pollut. Bull.146, 639–644. doi: 10.1016/J.MARPOLBUL.2019.07.025
29
Xiao J. Wang Z. Liu D. Fu M. Yuan C. Yan T. (2021). Harmful macroalgal blooms (HMBs) in China’s coastal water: Green and golden tides. Harmful Algae.107, 102061. doi: 10.1016/J.HAL.2021.102061
30
Xie X. Chen L. Shao S. Zhou Y. Wu J. Zhou Q. et al . (2024). Growth inhibition and toxic effects of microplastics on Chlorella vulgaris. Algal. Res.78, 103378. doi: 10.1016/J.ALGAL.2023.103378
31
Xiu B. Liang S. K. He X. L. Wang X. L. Cui Z. G. Jiang Z. J. (2019). Bioavailability of dissolved organic nitrogen and its uptake by Ulva prolifera: Implications in the outbreak of a green bloom off the coast of Qingdao, China. Mar. pollut. Bull.140, 563–572. doi: 10.1016/J.MARPOLBUL.2019.01.057
32
Xu C. Yu S. Hu J. Effiong K. Ge Z. Tang T. et al . (2022). Programmed cell death process in freshwater Microcystis aeruginosa and marine Phaeocystis globosa induced by a plant derived allelochemical. Sci. Total. Environ.838, 156055. doi: 10.1016/J.SCITOTENV.2022.156055
33
Yang X. Lin K. Tan L. Wang J. (2021). Utilization and release of biogenic elements by macroalgae Ulva prolifera: A mesocosm experiment off the coast of Qingdao, China. Mar. pollut. Bull.170, 112612. doi: 10.1016/J.MARPOLBUL.2021.112612
34
Yu S. Sun J. Wang Q. Wu J. Liu J. (2023). Extraction of bioactive polysaccharide from Ulva prolifera biomass waste toward potential biomedical application. Int. J. Biol. Macromol.235, 123852. doi: 10.1016/j.ijbiomac.2023.123852
35
Yuan Y. Luo B. Li Z. He Y. Xia L. Qin Y. et al . (2022). Effects of green tide on microbial communities in waters of the Jiangsu coastal area, China. Water. Environ. Res.94, e10797. doi: 10.1002/WER.10797
36
Zhang T. Feng Z. H. Luo C. L. Sun Y. X. Li J. Z. Xu J. T. et al . (2020). Fluorescence characterization and microbial degradation of dissolved organic matter leached from salt marsh plants in the Yellow River Delta. J. Plant Ecol.13, 525–537. doi: 10.1093/jpe/rtaa040
37
Zhang P. Shen M. C. Zhang X. Y. Wang H. Y. Wang Z. P. (2023). Valorization of the pelagic Sargassum horneri for co-production of erythritol and alginate oligosaccharides. Biores. Technol.379, 128984. doi: 10.1016/j.biortech.2023.128984
38
Zhang T. Wang X. (2017). Release and microbial degradation of dissolved organic matter (DOM) from the macroalgae Ulva prolifera. Mar. pollut. Bull.125, 192–198. doi: 10.1016/j.marpolbul.2017.08.029
39
Zhang J. Wang N. Zhang Z. Gao Y. Dong J. Gao X. et al . (2024). Combined effects of toxic Microcystis aeruginosa and high pH on antioxidant responses, immune responses, and apoptosis of the edible freshwater bivalve Corbicula fluminea. Ecotoxicol. Environ. Saf.280, 116568. doi: 10.1016/J.ECOENV.2024.116568
40
Zhang P. Xin Y. Zhong X. Yan Z. Jin Y. Yan M. et al . (2021). Integrated effects of Ulva prolifera bloom and decay on nutrients inventory and cycling in marginal sea of China. Chemosphere264, 128389. doi: 10.1016/J.CHEMOSPHERE.2020.128389
41
Zhao G. He H. Wang H. Liang Y. Guo C. Shao H. et al . (2022). Variations in Marine Bacterial and Archaeal Communities during an Ulva prolifera Green Tide in Coastal Qingdao Areas. Microorganisms10, 1204. doi: 10.3390/MICROORGANISMS10061204
42
Zhao J. Jiang P. Qiu R. Ma Y. Wu C. Fu H. et al . (2018). The Yellow Sea green tide: A risk of macroalgae invasion. Harmful Algae.77, 11–17. doi: 10.1016/J.HAL.2018.05.007
43
Zhao C. Sun J. Shen Y. Xia Z. Hu M. Wu T. et al . (2023). Removable carbon and storage carbon of golden tides. Mar. pollut. Bull.191, 114974. doi: 10.1016/J.MARPOLBUL.2023.114974
44
Zhou Y. Tan L. Pang Q. Li F. Wang J. (2015). Influence of nutrients pollution on the growth and organic matter output of Ulva prolifera in the southern Yellow Sea, China. Mar. pollut. Bull.95, 107–114. doi: 10.1016/J.MARPOLBUL.2015.04.034
45
Zhu J. Chen G. Zhou J. Zeng Y. Cheng K. Cai Z. (2022). Dynamic patterns of quorum sensing signals in phycospheric microbes during a marine algal bloom. Environ. Res.212, 113443. doi: 10.1016/J.ENVRES.2022.113443
Summary
Keywords
macroalgae, dissolved organic matter, nutrients, microbial communities, algal blooms
Citation
Zhang T, Sui J, Huo X, Wen B, Ren J, Li S and Chen X (2025) Characteristics and ecological effects of dissolved organic matter released by the main causative macroalgae of green and golden tides in the yellow sea. Front. Mar. Sci. 12:1563239. doi: 10.3389/fmars.2025.1563239
Received
20 January 2025
Accepted
05 June 2025
Published
26 June 2025
Volume
12 - 2025
Edited by
Linda Wegley Kelly, University of California, United States
Reviewed by
Lais F O Lima, San Diego State University, United States
Zeming Zhang, Ningbo University, China
Updates
Copyright
© 2025 Zhang, Sui, Huo, Wen, Ren, Li and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihu Li, 1997000041@jou.edu.cn; Xiaocong Chen, chenxiaocong@jou.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.