Abstract
The Caspian seal (Pusa caspica), an endemic and endangered obligate piscivore of the Caspian Sea, faces threats to its existence, notably from the reduction of its food resources. Pollution, sea regression, overfishing, and the introduction of non-native species have negatively impacted the seal’s food base. The seals annually gather in haul‐outs during the spring and autumn, a span of approximately 4–6 months. The diet of seals during these critically important periods of life is poorly understood. The fish diet of the P. caspica was assessed by analyzing fish otoliths found in fecal samples collected at haul-out sites in the Northern and Middle Caspian Sea from 2015 to 2022. A total of 8,630 fish otoliths and their fragments were recovered from the fecal samples. The taxonomic status was determined for 94% of the otoliths. Their taxonomic identification was carried out using a reference collection of otoliths obtained directly from 323 individuals representing various fish species. Brief descriptions were made of otoliths from 14 fish species representing Clupeidae, Gobiidae, Cyprinidae, Mugilidae, and Atherinidae. For Cyprinidae, the asteriscus and lapillus were described; for all others, the sagitta. The dietary diversity of seals during their haul-out periods was determined at the species level for golden grey mullet (Chelon auratus) and big-scale sand smelt (Atherina boyeri); at the genus level for shad (Alosa) and tyulka (Clupea); and at the family level for gobies (Gobiidae) and cyprinids (Cyprinidae). Gobies dominate the diet (79.34%), followed by big-scale sand smelt at 15.99%. According to Margalef’s richness index, the diet was more diverse in the deepwater Middle Caspian region. In the future, investigating the seals’ diet in relation to fish distribution at haul-out sites will significantly contribute to understanding their adaptation to rapidly changing conditions and the development of conservation measures for key habitats of this endangered marine mammal of the Caspian Sea. Establishing a comprehensive database of fish otoliths from various regions of the sea is essential for investigating their morphological polymorphism and for applying DNA barcoding in the future to achieve a more accurate assessment of the species composition in the diet of the Caspian seal.
1 Introduction
The Caspian seal (Pusa caspica), a marine mammal endemic to the Caspian Sea, is listed as Endangered on the IUCN Red List (Goodman and Dmitrieva, 2016). This status is consistently recognized across all Caspian countries (Bizikov et al., 2021; Rustamov et al., 2021; Eybatov and Hajiyev, 2022; Baimukanov et al., 2023a).
Given the ongoing decline in the P. caspica population (Baimukanov, 2017), it is crucial to investigate each threat to the species’ survival thoroughly. In addition to sea regression and pollution, habitat degradation, accumulation of toxins in organs and tissues, disease outbreaks, mortality due to shipping and bycatch in fishing nets, and illegal, unreported, and unregulated fishing, particular attention must be paid to the seals’ diet and the adequacy of their food sources (Baimukanov, 2022; Baimukanov et al., 2023b). For instance, the introduction of the comb jelly (Mnemiopsis leidyi Agassiz, 1865) has been linked to a decrease in Caspian tyulka (Clupeonella caspia) stocks (Zaitsev et al., 2003), and overfishing combined with decreased river flow has led to a reduction in Caspian roach (Rutilus caspicus) stocks (Rumyantsev et al., 1975; Ivanov et al., 2023), which are among the seal’s primary prey (Vorozhtsov et al., 1972; Pochtoeva-Zakharova and Khuraskin, 1999). These examples underscore the importance of understanding the current diet of P. caspica.
The initial studies on the diet of P. caspica were conducted in 1905 (Smirnov, 1907). Subsequent research was discontinued but resumed in the late 1920s and continued intermittently throughout the 20th century. The focus of these studies up to the mid-1980s was to gather information on the seal’s impact on commercial fish stocks (Roganov, 1931; Badamshin, 1948, 1966, 1971; Vorozhtsov et al., 1972; Rumyantsev et al., 1975; Krylov, 1983).
It has been demonstrated that the seal’s diet consists of various fish species, mollusks, and crustaceans (such as amphipods, shrimp, mysids, river crayfish, crabs) (Badamshin, 1948; Vorozhtsov et al., 1972; Krylov, 1983); in one case, waterfowl feathers were found in a seal’s stomach (Krylov, 1983), suggesting that birds might occasionally be preyed upon by P. caspica. However, fish dominate the diet, which comprises 98-99% of the food bolus in the animals’ stomachs (Krylov, 1983; Pochtoeva-Zakharova and Khuraskin, 1999). The goby complex and sprat were dominant in the seal’s diet (Badamshin, 1948; Pochtoeva-Zakharova and Khuraskin, 1999; Khuraskin and Zakharova, 2000; Sokolsky and Pankov, 2009). Seasonal variations in feeding were observed, along with differences depending on the location of seal catches and the marine mammals’ health. Experimental feeding studies of seals kept in pools showed that the animals preferred gobies and sprats (Badamshin, 1948; Krylov, 1983).
It is important to note that seals are semi-aquatic animals that form haul-out sites on islands during spring molting and in autumn during anticipation of the ice cover in the Northern Caspian Sea. Seals are most vulnerable during these periods, as they form large aggregations on the islands, which could facilitate the spread of epizootics (Kajiwara et al., 2018) and increase the impact of habitat pollution, poaching, and human disturbance at haul-out sites (Baimukanov, 2017).
How seals survive these critically important yet risky periods of their lives and maintain their energy balance has not yet been fully understood. In 2020, it was established that the largest seal haul-outs occur in the northeastern part of the Caspian Sea, within the Kazakh sector. Notably, in recent years, these periods coinciding with haul-out formation have seen increased incidents of dead seals washing ashore on the coast of the Kazakh part of the Northern Caspian (Baimukanov et al., 2023b).
Whereas all earlier studies of the Caspian seal’s diet were based on the lethal sampling of animals and analysis of stomach contents, the present research, given the seals’ endangered status, relies only on non-lethal methods such as coprological analysis (analysis of hard parts in scat). This method is particularly effective while the seals are on their island haul-outs, allowing for the collection of feces and, through washing and otolith extraction, the study of the taxonomic composition and quantity of fish consumed by the seals (Härkönen, 1986). The ability to estimate the number of fish consumed by seals is a key advantage of the coprological method over DNA barcoding (Trzcinski et al., 2024), which only provides information on the taxonomic richness of the diet. Moreover, this method enables the assessment of the diet of seal aggregations over a defined haul-out period, ranging from several days to up to three months within a given season.
A challenge arises in that there is no definitive guide to the fish of the Caspian Sea by their otoliths. Consequently, starting in 2015, parallel to the coprological study of the seals’ diet, fish were caught in various regions of the Kazakhstani sector of the Caspian Sea, and collections of their otoliths were created. From 2015 to 2022, fecal samples were collected from seals on island haul-outs, followed by laboratory processing and analysis. Comparing the otoliths extracted from the feces with those from the caught fish has enabled an evaluation of the diversity of the P. caspica diet during their haul-out periods on island rookeries.
The objective of this study is to qualitatively (determining the taxonomic status) and quantitatively assess the diversity of fish in the Caspian seal’s diet during their haul-out periods on island rookeries based on a comparative description of the otoliths of the fish that serve as the seals’ prey. To achieve this objective, the following approach was taken: fish were caught and identified in various areas of the Kazakhstani sector of the Caspian Sea; seal feces were collected at haul-out sites in the northeastern part of the sea and in Kendirli Bay; a reference collection of otoliths from different fish species, as well as a collection of otoliths extracted from feces, was compiled and described. Among the fecal samples, otoliths that had not been damaged during passage through the gastrointestinal tract were selected and taxonomically identified. Margalef’s richness index was calculated to compare the diversity of fish consumed by seals across different haul-out sites. Based on the findings, recommendations for future research directions were proposed.
2 Materials and methods
Figure 1 shows a map of fish capture sites and seal fecal sample collection locations. To create a collection of otoliths—representing potential dietary items for the Caspian seal (Pusa caspica) from various areas of the Caspian Sea—fish were caught, with additional specimens provided by the “Kazakhstan Agency of Applied Ecology” and “Tengizchevroil” LLC. Fish were caught using gillnets with 20–60 mm mesh sizes and beam trawls with 10–20 mm mesh sizes. All fish were euthanized and then preserved in 10% formalin. Species identification was performed using the Guide to Fish and Invertebrates of the Caspian Sea (Bohutska et al., 2013) and FishBase (https://www.fishbase.se/search.php). From 2017 to 2023, a total of 323 fish were caught and identified at the species level (Table 1). After measuring linear dimensions and determining sex and gonad maturity stages following standard guidelines (Pravdin, 1966), otoliths were extracted from the fish. The results of the biological analysis were not included in the present article. Extracted left and right otoliths were placed in vials with 95% alcohol for clarification (Härkönen, 1986; Pavlov and Shirokova, 2020), after which they were cleaned from surrounding tissue using a hypochlorite solution (Secor et al., 1991).
Figure 1
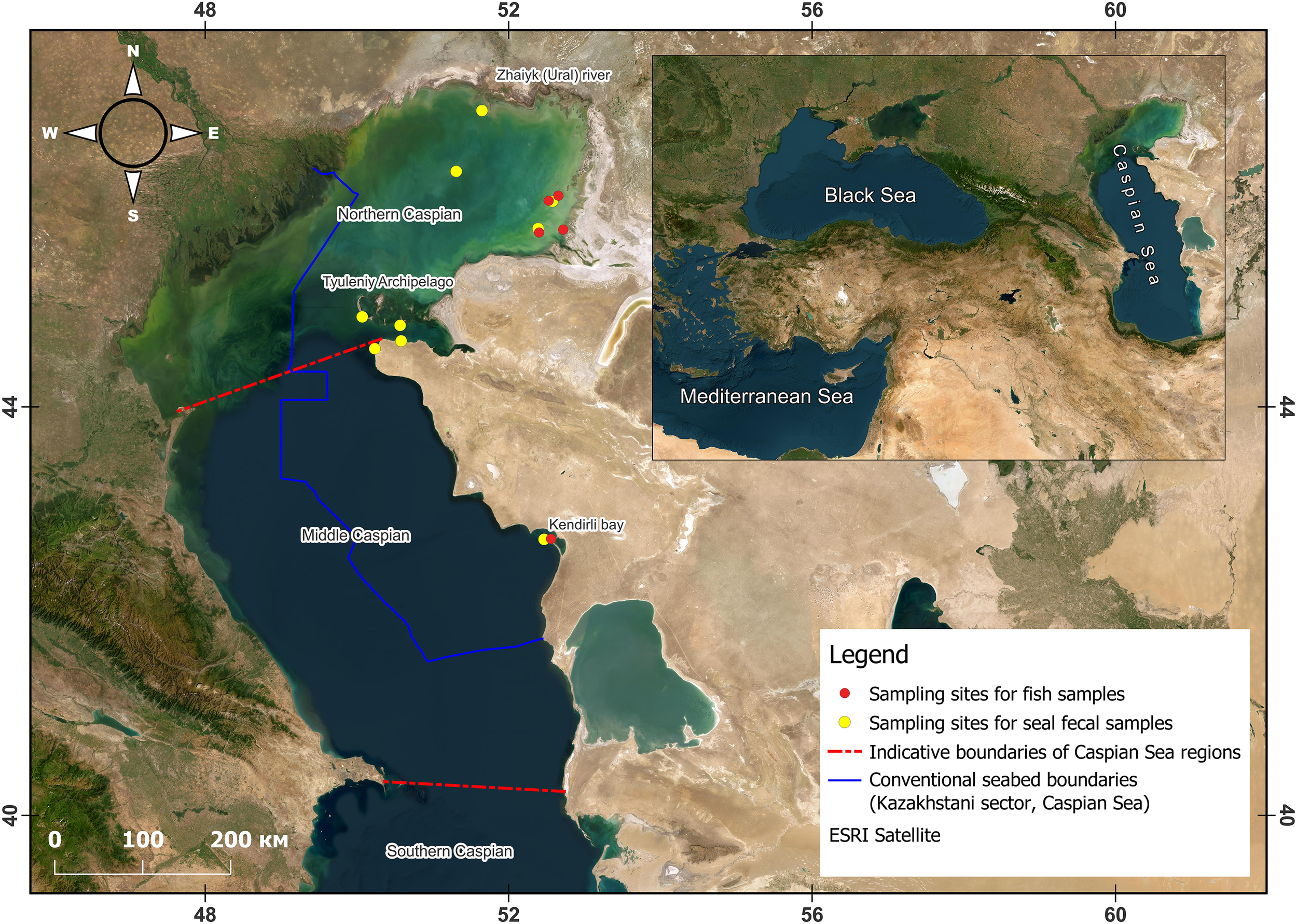
Caspian Sea map of fish catch locations (yellow) and collection of Caspian seal (Pusa caspica) fecal samples (red).
Table 1
| Species | Number of individuals |
|---|---|
| Golden grey mullet (Chelon auratus) | 63 |
| Big-scale sand smelt (Atherina boyeri) | 50 |
| Caspian tyulka (Clupeonella caspia) | 21 |
| Caspian marine shad (Alosa braschnikowi) | 9 |
| Caspian roach (Rutilus caspicus) | 46 |
| Bream (Abramis brama) | 33 |
| Round goby (Neogobius melanostomus) | 22 |
| Caspian sand goby (N. pallasi) | 32 |
| Caspian goby (N. caspius) | 3 |
| Caspian bighead goby (Ponticola gorlap) | 22 |
| Syrman goby (P. syrman) | 14 |
| Volga dwarf goby (Hyrcanogobius bergi) | 4 |
| Eastern tubenose goby (Proterorhinus nasalis) | 2 |
| Caspian tadpole goby (Benthophilus macrocephalus) | 2 |
| Total | 323 |
Fish species caught and the number of individuals sampled in the Caspian Sea.
To analyze the diet of the Caspian seal (Pusa caspica) at haul-out sites located in different areas of the Kazakhstani sector of the Northern and Middle Caspian Sea, a total of 237 fecal samples were collected during the spring and autumn periods between 2015 and 2022 (Table 2). The samples varied in volume and content. When collected immediately after seals had left the haul-out site, feces were soft and moist; in contrast, feces found in areas no longer occupied by seals were dry and hardened. The time elapsed between defecation and collection was estimated to range from several minutes to no more than 2–5 days, based on the frequency of wind shifts and associated wind-driven surges and withdrawals of water typical of the shallow Caspian Sea. Strong winds could either cover (with sand) or destroy the feces, while wind-driven surges might wash them away.
Table 2
| Haul-out site name | Year | Collection date | Number of fecal samples |
|---|---|---|---|
| Kendirli | 2015 | 19 May | 1 |
| 31 October | 8 | ||
| 2016 | 30 August | 4 | |
| 2017 | 30 April | 18 | |
| 11 October | 15 | ||
| 2018 | 14 April | 1 | |
| 2019 | 10–26 April | 22 | |
| Prorva | 2018 | 1–4 November | 78 |
| Remontnye Shalygas | 2022 | 24 April | 6 |
| Durnev Islands | 2016 | 5 May | 7 |
| New Durnev Islands | 2021 | 15 April | 77 |
| Total: | 237 | ||
Dates of collection and number of fecal samples from Caspian Seals (Pusa caspica) at island haul-out sites.
Cylindrical, whole fecal forms were rarely encountered. More often, feces appeared as dispersed fragments or flattened masses likely trampled by the animals, making separation into individual defecations impossible. Samples were typically formed by collecting feces from a localized area of approximately 20–40 cm in diameter. In some cases, larger areas were sampled when feces were densely concentrated and could not be separated into discrete units. As a result, some samples may have included a single fecal mass or fragment, while others represented an undetermined number of defecations. The number of seals contributing to each sample was unknown. Therefore, statistical analysis of otolith content to determine individual feeding patterns was not feasible. Otoliths recovered from the feces represent the diet of an unspecified number of seals at a given haul-out site and point in time.
Each fecal sample was soaked and then sequentially rinsed through sieves with mesh sizes of 1 mm and 0.355 mm, following previously published protocols (Härkönen, 1986; Dellinger and Trillmich, 1988; Baimukanov et al., 2017). After washing, all otoliths and their fragments were transferred from the sieve onto a flat, smooth surface for drying, and subsequently placed in individual zip-lock bags labeled according to the corresponding sample. In addition to otoliths, the samples also contained various fish bones, fragments of mollusk shells, and crustacean chitin; however, these materials are not considered in the present study.
Fecal samples were examined under a binocular microscope, where the largest otoliths of most species—sagittae—were separated by species and fish family. The sagitta is small and indistinct for cyprinid fishes, so the compilation of otolith collections and the determination of their presence in feces were done using asteriscus and lapillus otoliths. The taxonomic identification of fish represented by the otoliths recovered from fecal samples was carried out by comparison with the reference collection and existing otolith atlases (Härkönen, 1986; Smale et al., 1995; Tuset et al., 2008; Lin and Chang, 2012). A total of 8630 otoliths were collected, including unidentified specimens.
Otoliths were classified as unidentified based on the following criteria: broken otoliths and fragments; otoliths that were eroded to the extent that identification, even to the family level, was impossible; and otoliths that were difficult to assign to any taxon with confidence. Otoliths were photographed using a Motic trinocular microscope. They were displayed on their inner side, with the dorsal part oriented upwards and the anterior part directed to the right. The otolith images were processed using ImageJ (for measuring otoliths and setting the scale in figures), Adobe Photoshop (for isolating otoliths and placing them on a black background), and the AFORO website for outlining otoliths (Lombarte et al., 2006). Otolith descriptions were based on schematic drawings, with terminology and labels adopted from various sources (Pierce et al., 1991; Smale et al., 1995; Svetochova and Stasenkova, 2006; Lin and Chang, 2012). Since otoliths can have an ostial sulcus (sulcus open at the front) and a medial sulcus (sulcus not open at either the front or back), they were described using separate figures (Figure 2).
Figure 2
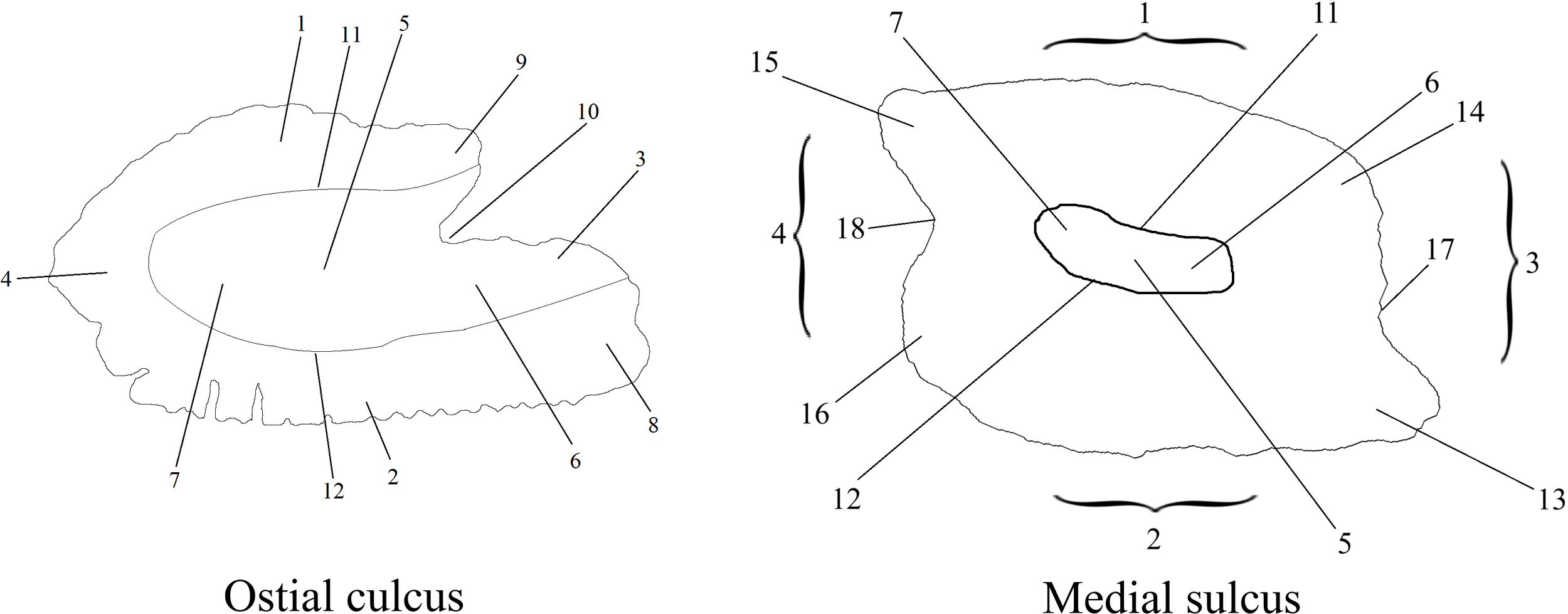
Key Morphological Indicators of Otoliths: 1 – dorsal part, 2 – ventral part, 3 – anterior (rostral) part, 4 – posterior (caudal) part, 5 – sulcus, 6 – ostium, 7 – cauda, 8 – rostrum, 9 – antirostrum, 10 – excisura, 11 – crista superior, 12 – crista inferior, 13 – preventral projection, 14 – predorsal angle, 15 – postdorsal projection, 16 – postventral angle, 17 – anterior notch, 18 – posterior notch.
The current study provides only a general description of the most characteristic features of otoliths that were necessary for comparing the otoliths found in feces with the reference collection and determining their taxonomic status. A more detailed morphological analysis of otolith structure will be the subject of other works, following the approach used in the analysis of Chelon auratus (Baimukanov et al., 2024).
Statistical processing of the data and preparation of graphs illustrating the distribution of fish species in the seal diet were conducted using Excel. The comparison of fish diet diversity across different regions in the seal diet was performed using the Margalef Richness Index* according to the established formula (Margalef, 1958; Magurran, 1988):
where S represents the number of taxa, and N is the total number of otoliths.
* The choice of Margalef’s richness index was due to its straightforward expression of the ratio of the number of species to area or the number of individuals, unlike, for example, the Shannon–Wiener diversity index, which assumes that each sample is a random selection from the community and that species proportions in the sample reflect their actual proportions in nature, or the Gini–Simpson index, which accounts for both inter- and intraspecific relationships. Since the aim of using the index was solely to compare the taxonomic richness of the seals’ diet across different marine areas, Margalef’s index was considered the most appropriate.
3 Results
3.1 Comparative characteristics of otoliths from fish collections and fecal samples
Comparisons of the shapes and key features of otoliths, representing prey items of the Caspian seal (Pusa caspica), are presented separately: the first group includes representatives from the families of Mugilidae, Atherinidae, Clupeidae, and Cyprinidae; the second group comprises the Gobiidae family.
Otoliths collected from fish and fecal samples of the first group are illustrated in Figures 3 and 4 (For each species, samples of both small and large otoliths are presented, except Alosa braschnikowi, for which only relatively large otoliths are shown). In subsequent descriptions of otoliths, emphasis is first placed on those features of fish otoliths that are characteristic of the families. Then, the main morphological parameters crucial for determining the taxonomic status of otoliths from feces are highlighted; these parameters are numbered according to the conventions shown in Figure 2.
Figure 3
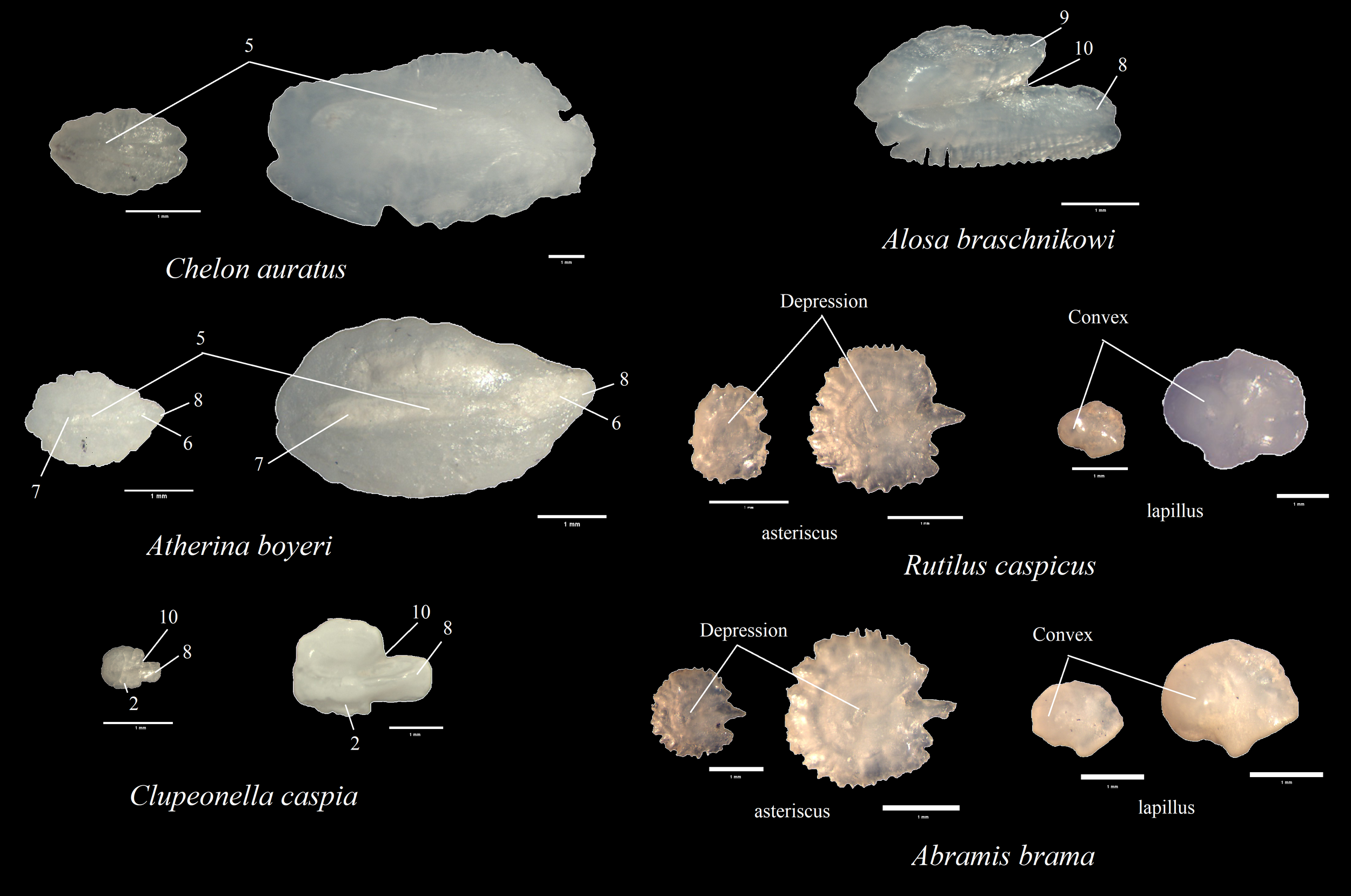
Representative images of otoliths from fish in the reference collection. Each otolith is shown with a 1 mm scale bar below it for size reference.
Figure 4
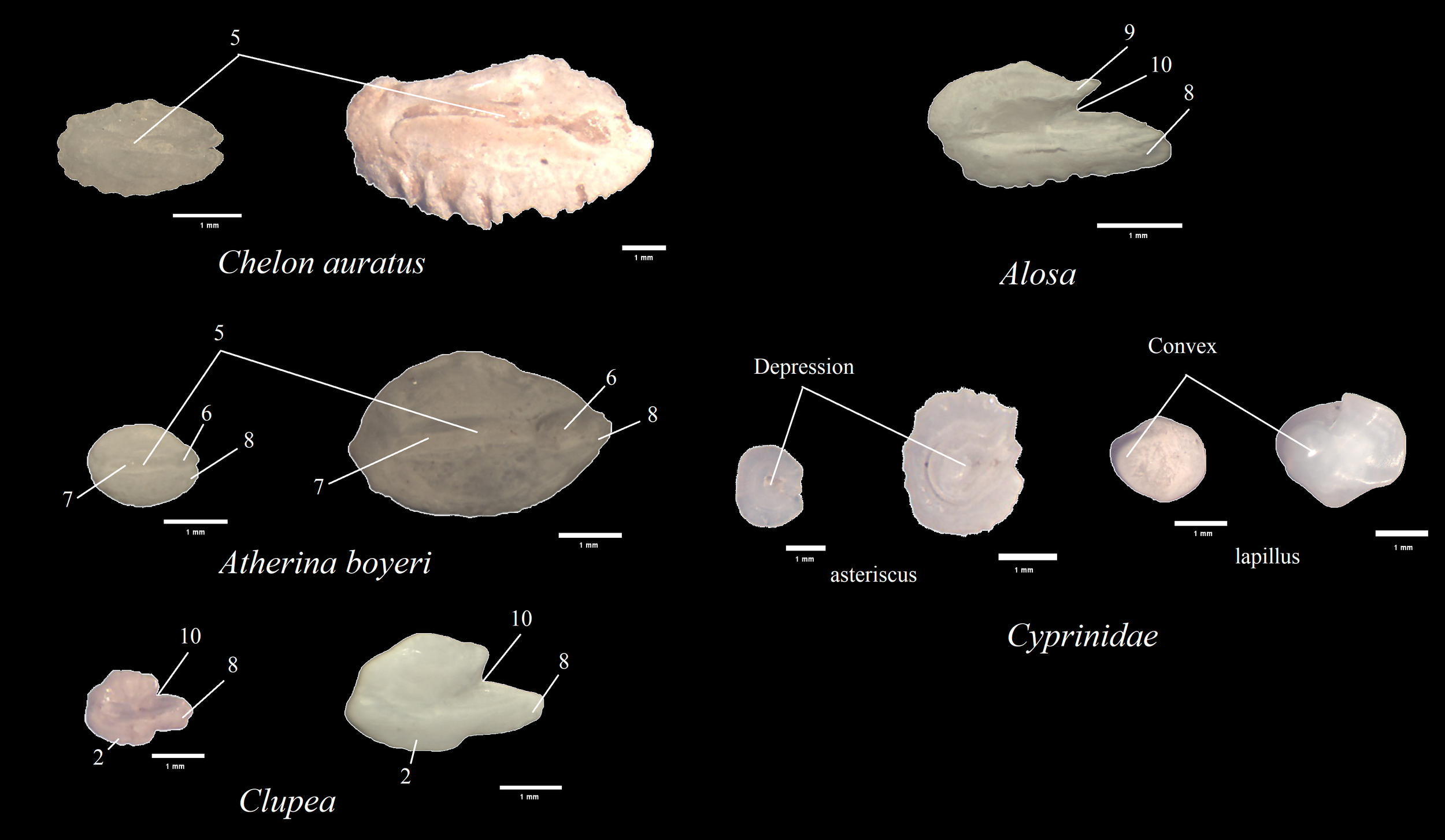
Representative images of otoliths from seal feces in the reference collection. Each otolith is shown with a 1 mm scale bar below it for size reference.
The otolith shape of Chelon auratus (Risso 1810) is elongated and rectangular, consistent in otoliths from feces, and a good distinguishing feature from the otoliths of all other fish in the seal’s diet. A diagnostic feature is also the sulcus that is open at the front, which in mullets is elongated, shifted towards the dorsal side, and has a characteristic bend towards the ventral side at the caudal part. This bend is a reliable species identifier, and it also prevents confusion between mullet otoliths extracted from feces and otoliths of fish from other taxa.
In the ichthyofauna of the Caspian Sea, Atherinidae is represented by a single species - the Big-scale sand smelt (Atherina boyeri Risso, 1810). A. boyeri otoliths have a spindle-shaped, elongated form, sharpened at the front part. The sulcus is elongated, well-defined, and runs along the middle of the otolith. The ostium and cauda are distinguishable. They have a rostrum, but the antirostrum is poorly developed due to the poorly expressed excisura. These features are distinct from the otoliths of all other fish taxa considered dietary items for the seal and preserved in otoliths from feces.
In compiling otolith collections, fish of only one species from each of the two genera of the Clupeidae family were caught: Alosa and Clupeonella, namely, the Caspian marine shad (Alosa braschnikowi Borodin, 1904) and the Caspian tyulka (Clupeonella caspia Svetovidov, 1941). Otoliths of these species have an elongated shape with a prominently large rostrum, a distinguishing feature of the family. However, in tyulkas, the dorsal and ventral sides are smoother and more rounded, while in shads, the edges of the otoliths are more serrated, especially on the ventral side. In tyulkas, the transition from the wing to the rostrum forms an angle of about 90 degrees, which is not present in shads. On the dorsal side, the rostrum is rectangular and transitions into an almost rectangular notch. The antirostrum is poorly expressed. In shads, the antirostrum is well-defined, and the angle between the rostrum and the antirostrum is sharp. In feces, tyulka otoliths retain characteristic features, as do shad otoliths, only due to wear. The rostrum in tyulkas may acquire a slightly pointed shape.
In the Caspian Sea, the Cyprinidae family includes over 30 species (Ivanov et al., 2023). Given that from the entire extensive family of cyprinids in the otolith collections obtained from fish, only the otoliths of two species are present: the Caspian roach, Rutilus caspicus (Jakowlew, 1870) and the bream, Abramis brama (Linnaeus, 1758), we note only the most characteristic features for the family of cyprinid fish, distinguishing them from otoliths of other fish species – dietary items of the seal. For describing otoliths of cyprinid fish, a collection of otoliths – asteriscus and lapillus of roach, as well as asteriscus and lapillus of bream, was compiled.
A comparison shows that the asteriscus and lapillus of cyprinid fish do not resemble the sagittal otoliths of other fish species and generally have more similarities. The asteriscus of cyprinid fish has a disc-shaped, star-like form. The edges are serrated, but in fecal samples, they are smoother. In the otolith’s center is a round depression with a ridged edge opening to the front part. The lapillus generally has a rounded, smoothed form with an uneven surface on the inner side. At the caudal part of all otoliths, there is a large bulge. The caudal side of the otolith has a protrusion. The bulge (convex) on the surface and the protrusion on the caudal part of the otolith of cyprinids serve as reliable identifiers, distinguishing them from other fish otoliths. The same form is observed in otoliths from feces.
The sulcus of Gobiidae otoliths is closed; therefore, there are no rostral projections. The depression (notch) in the anterior and posterior parts of the otolith can be pronounced (Figure 5A), weakly expressed, or absent (Figure 5B), resulting in varying distinctiveness of preventral and postdorsal projections and angles.
Figure 5
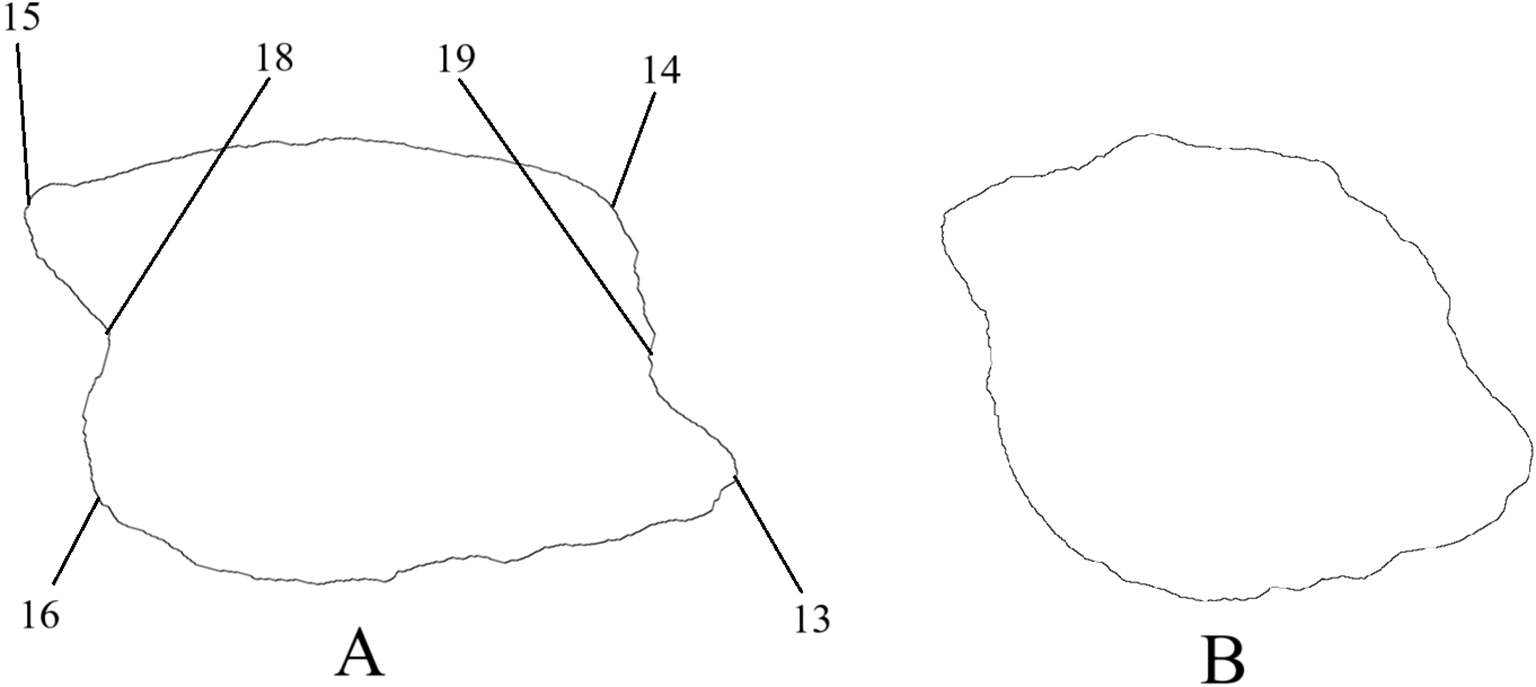
Examples of descriptions of goby fish otoliths: (A) otolith of Ponticola gorlap, with pronounced projections; (B) otolith of Neogobius melanostomus, without pronounced projections.
The otoliths of Gobiidae are diverse, featuring round, elongated-round, rhomboid, and nearly square or rectangular shapes. They have varying degrees of pronounced projections and angles on the anterior and posterior sides. The length and height of the otoliths are very close, or the length is slightly greater than the height. Examples of otolith shapes of fish from the Gobiidae family are shown in Figure 6. For some species, both small and large otoliths are presented (Figures 6A–C), while for others, due to the uniformity of the otolith sample, only a single specimen is shown (Figures 6F–H).
Genus Neogobius
Neogobius melanostomus. The otoliths have a rhomboid shape. The dorsal and ventral edges are rounded, sometimes with irregularities in larger individuals. The anterior part has a pronounced preventral projection, and the posterior part also has a postdorsal projection (Figure 6A).
N. pallasi. The otoliths have a rounded shape. Both the dorsal and ventral edges are rounded. The anterior part is straighter, and the posterior part has a slight depression, with weakly expressed postdorsal projection and postventral angle (Figure 6B). In small otoliths, the postventral angle may be absent.
N. caspius. The otoliths are nearly square in shape. The dorsal edge is rounded, sometimes with bumps. The ventral edge is straight, with minor irregularities. The anterior part is slanted with a pointed preventral projection. The posterior part has a pronounced depression and a strongly expressed postdorsal projection (Figure 6C).
Genus Ponticola
Ponticola gorlap. The otoliths are nearly rectangular-shaped and slightly elongated. The dorsal and ventral edges of the otolith are straight, sometimes with minor irregularities. The posterior part has a pronounced depression. The anterior and posterior parts of the otolith have pointed preventral and postdorsal projections, and predorsal and postventral angles (Figure 6D).
P. syrman. The otoliths remotely resemble a square. However, the anterior part is oblique with a protruding preventral projection. The posterior part features a pronounced depression and a rounded overhanging postdorsal projection, and the postventral angle is significantly smaller than the postdorsal projection. The dorsal edge is rounded, with irregularities. The ventral edge is straight, with irregularities (Figure 6E).
Genus Proterorhinus
Proterorhinus nosalis. The otoliths have a rhomboid shape, with the length and width of the otolith approximately the same. The dorsal and ventral edges are straight. The anterior part of the otolith has a small depression, a preventral projection, and a predorsal angle. The posterior part of the otolith has a postdorsal projection and a postventral angle (Figure 6F).
Figure 6

Examples of otolith shapes from the Gobiidae family: (A)Neogobius melanostomus, (B)N. pallasi, (C)N. caspius, (D)Ponticola gorlap, (E)P. syrman, (F)Proterorhinus nosalis, (G)Hyrcanogobius bergi, (H)Benthophilus macrocephalus. Each otolith is shown with a 1 mm scale bar below it for size reference.
Genus Hyrcanogobius
Hyrcanogobius bergi. The otoliths have a rhomboid, square-like shape, but the dorsal edge is rounded, and the ventral and anterior parts are straight. The posterior part has a pronounced depression approximately in the center, causing the postdorsal projection and the postventral angle to be about equal and well-defined (Figure 6G).
Genus Benthophilus
Benthophilus macrocephalus. The otoliths of this species have an elongated shape. The dorsal part is straight with serrations. The anterior part is also serrated and rounded and gradually transitions into the ventral part, which is straight with small serrations. The posterior area is depressed, with the postdorsal projection being longer than the postventral angle (Figure 6H).
In the feces of P. caspica, otoliths were found that, due to their similarity with otoliths from known species in the collection material, can be identified at the species level (Figure 7). For example, the otoliths in Figure 7A have a rhomboid shape and, due to the pronounced preventral and postdorsal projections, are identified as otoliths of N. melanostomus; the otoliths in Figure 7B, due to their round shape and the presence of a depression in the rear part, are identified as otoliths of N. pallasi; the otoliths in Figure 7C, due to their rectangular shape, the presence of depression in the rear part, and the prominence of the postdorsal projection, are attributed to the otoliths of N. caspius.
Figure 7
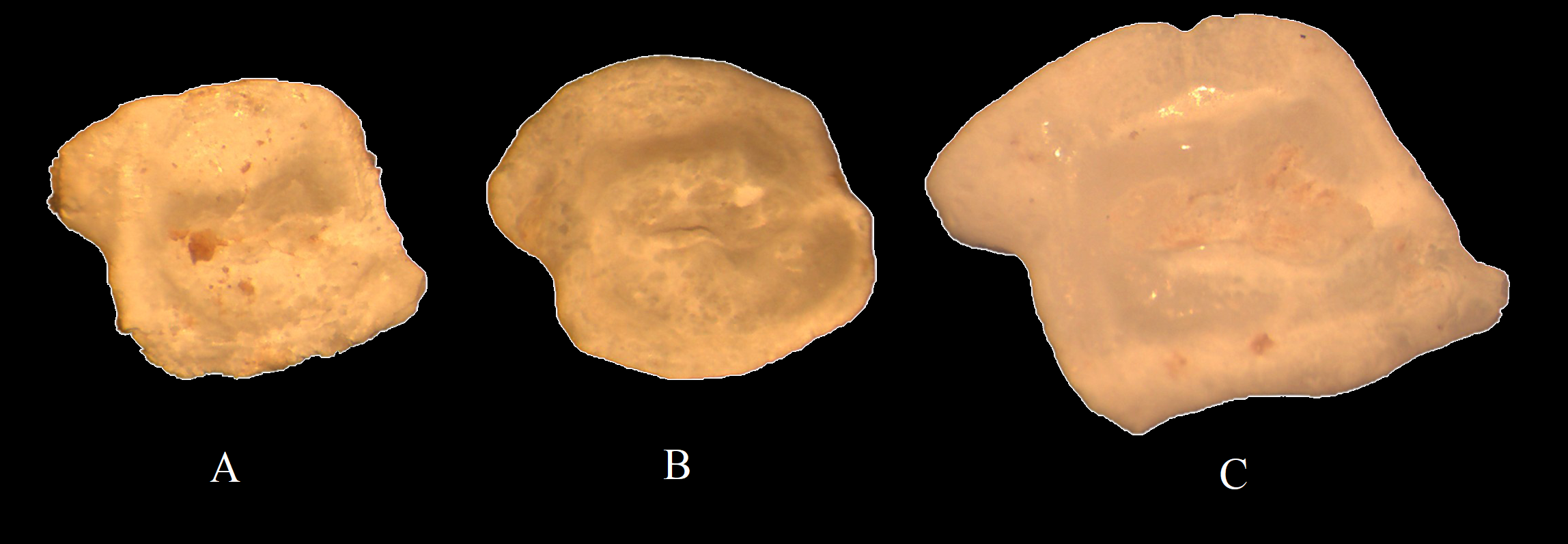
Examples of taxonomic identification of Gobiidae otoliths from feces: (A)Neogobius melanostomus, (B)N. pallasi, (C)N. caspius..
However, there are also otoliths that, due to wear, may exhibit characteristics typical of different species. For instance, Figure 8A depicts an otolith that has a rhomboid elongated shape similar to that of N. melanostomus otolith, yet it has depressions in the anterior and posterior parts typical of P. gorlap; Figure 8B shows an otolith that, with its round shape, resembles N. pallasi otolith, but has a straight ventral edge like those of N. caspius or P. syrman. In Figure 8С, the otolith depicted, which has a shape close to a rhombus and a prominent postdorsal projection, resembles the otolith of N. melanostomus. However, the poor prominence of the preventral projection also provides a certain similarity to the otolith of N. pallasi.
Figure 8
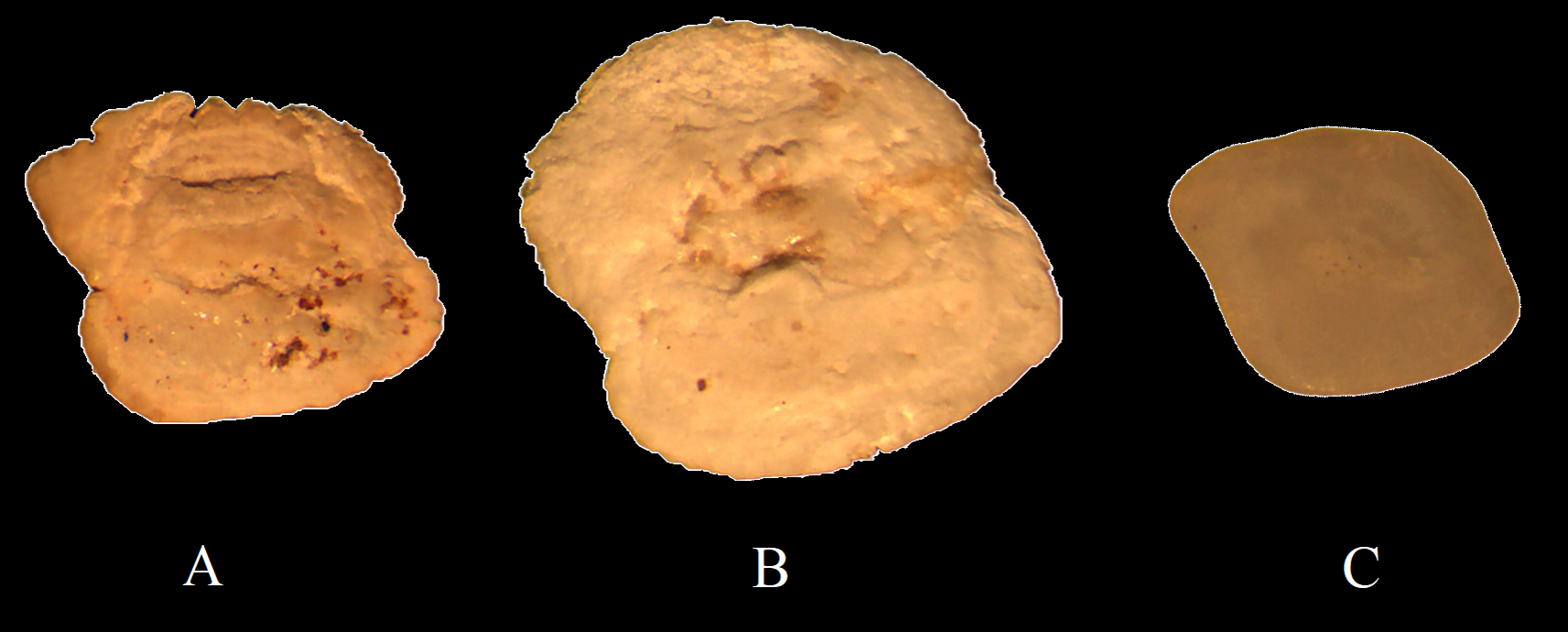
Examples of Gobiidae otoliths from feces, whose identification to species is problematic. (A) Otolith resembling Neogobius melanostomus, with depressions typical of Ponticola gorlap; (B) Round otolith similar to Neogobius pallasi, with a straight ventral edge like Neogobius caspius or Ponticola syrman; (C) Otolith close to Neogobius melanostomus, but with a weak preventral projection as in Neogobius pallasi.
3.2 Determining the diet of the Caspian Seal (Pusa caspica) during haul-out periods
This section presents results from the identification of otoliths from feces based on the description of characteristic taxonomic features of fish otoliths obtained from collected materials.
It should be noted that as otoliths pass through the gastrointestinal tracts of seals, they are subjected to wear and shape alteration (Härkönen, 1986; Biolé et al., 2019; Quigley et al., 2023; Baimukanov et al., 2024), making it difficult or impossible to differentiate between left and right otoliths from a single fish collected from feces. Given this, assuming the statement is true for all identified species-specific otoliths, the count was made of all otoliths, regardless of the body side they belong to. This approach presumably equalizes the chances of otoliths from all fish species being counted in a balanced ratio, whereby the proportion of otoliths from each species should correspond to the share of consumed fish species.
Otoliths and their parts, which were not identified to the level of species, genus, or family, were classified into a separate group of otoliths with an undetermined systematic status. This group of otoliths was also counted during the processing of feces and accounted for 6.2% of all fish otoliths identified in the fecal samples. This group was not included in the subsequent calculations of the relative contribution of fish species to the diet of the Caspian seal. The majority of otoliths found in the fecal samples (8,093 specimens) were identified to a reliably determined taxonomic level, and all further calculations were based on this number (Table 3).
Table 3
| Species | Haul-out site name | Total | ||||
|---|---|---|---|---|---|---|
| Remontnye Shalygas | Prorva | Durnev Islands | New Durnev Islands | Kendirli | ||
| Gobiidae | 4 | 1218 | 258 | 3153 | 1788 | 6421 |
| Clupeidae - Alosa | 3 | 1 | 0 | 101 | 1 | 106 |
| Clupeidae - Clupeonella | 2 | 8 | 0 | 92 | 21 | 123 |
| Atherinidae - Atherina boyeri Risso, 1810 | 2 | 0 | 68 | 325 | 899 | 1294 |
| Mugilidae – Chelon aurata (Risso, 1810) | 0 | 0 | 0 | 0 | 61 | 61 |
| Cyprinidae | 21 | 36 | 0 | 26 | 5 | 88 |
| Otoliths and their parts with an undetermined systematic status | 0 | 49 | 27 | 16 | 445 | 537 |
| Total | 32 | 1312 | 353 | 3713 | 3220 | 8630 |
Number of fish otoliths found in feces collected from various haul-out sites.
The combined analysis of otolith content in feces shows that most of the diet consists of fish from the Gobiidae family; Atherinidae is the second most significant group, almost five times less numerous. Clupeonella ranks third, with ten times fewer numbers than Atherinidae. Other fish play an even more minor role in the diet, ranging from 0.75 to 1.31% (Figure 9).
Figure 9
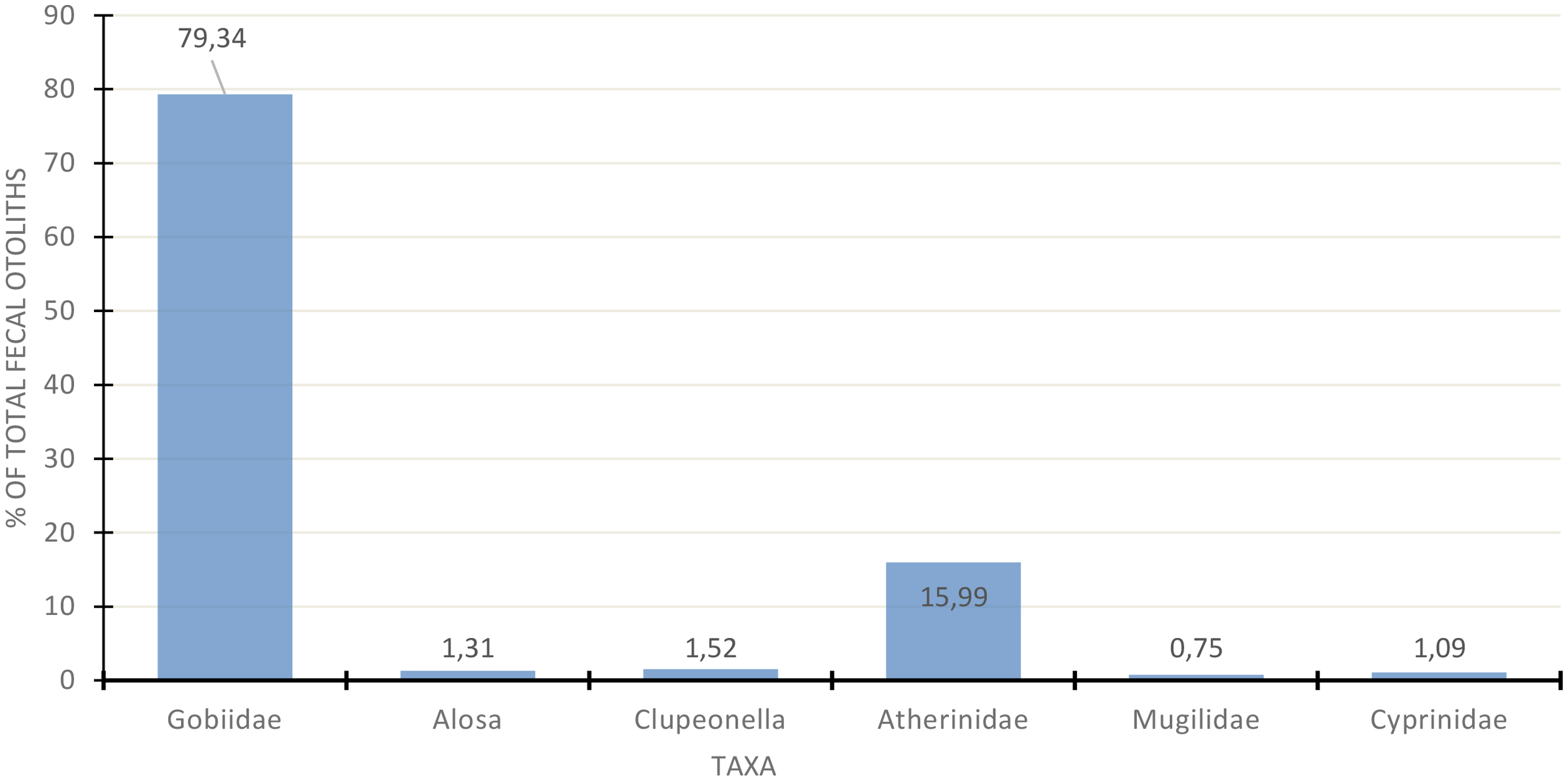
The proportion of fish in the diet of the Caspian seal (Pusa caspica) at island haul-outs in the Northern and Middle Caspian during the period from 2015 to 2022.
3.3 Diet comparison by areas
To identify dietary patterns in relation to the location of haul-out sites, data from different sampling points (Table 3) within the same marine region were combined. Data from the Prorva and Remontnye Shalygas haul-out sites were grouped to describe the diet of seals in the northeastern Caspian Sea; data from the Durnev and New Durnev Islands were combined to represent the Komsomolets Bay area; and data from the Kendirli Bay haul-out site represented the Middle Caspian region.
Figure 10 presents quantitative data from the Caspian seal’s (Pusa caspica) diet analysis based on fecal samples from haul-outs located in different sea areas. In all areas, the overwhelming majority of otoliths in seal feces belong to fish of the Gobiidae family, but it was found that their role in the seal’s diet decreases from north to south. A similar trend is observed in the presence of cyprinid fish in the seal’s diet, albeit in significantly smaller volumes. Mugilidae were not found in the northeast and Komsomolets Bay, which showed a lower Margalef index than the Kendirli Bay area (Table 4).
Figure 10
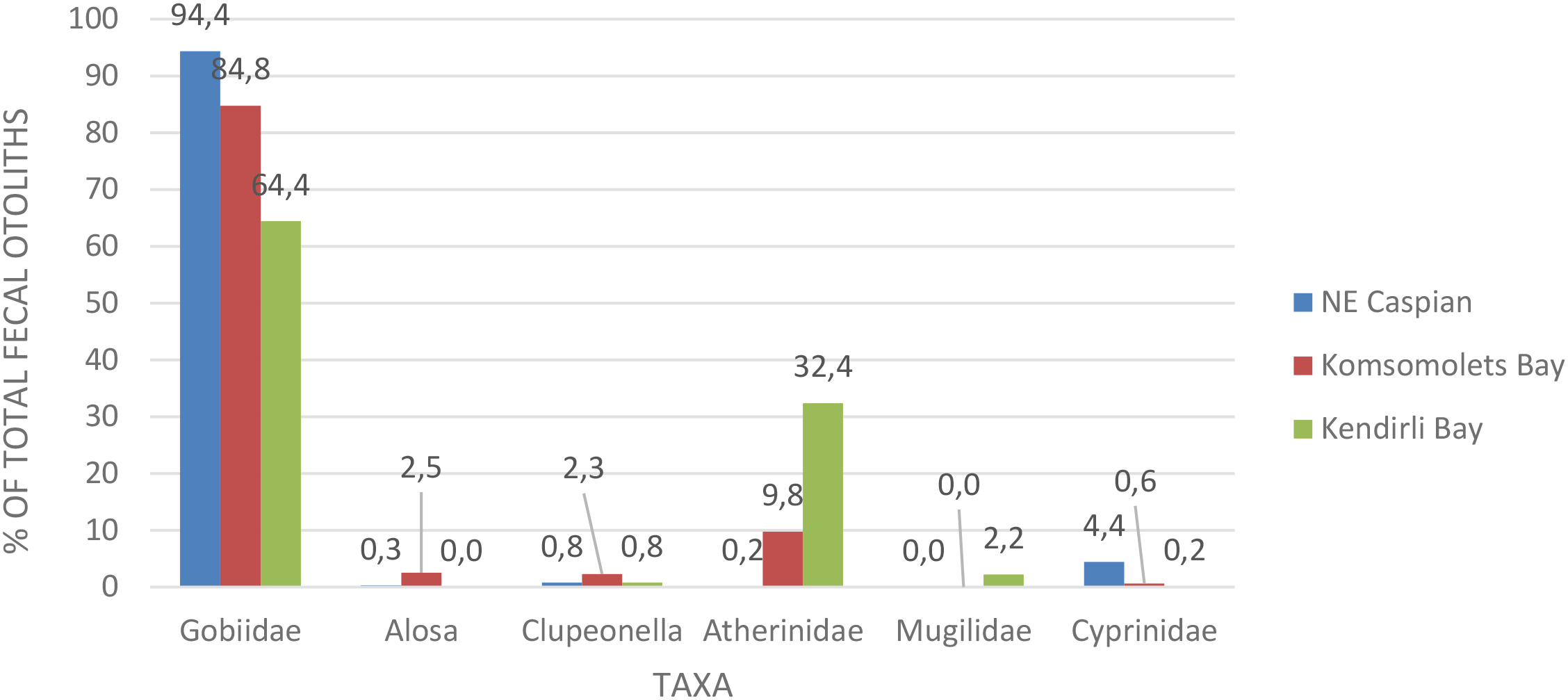
Diversity and quantitative data on the diet of the Caspian seal (Pusa caspica) in various sea regions.
Table 4
| Parameters | Sea areas | Total | ||
|---|---|---|---|---|
| NE Caspian | Komsomolets Bay | Kendirli Bay | ||
| Total otoliths, pieces. | 2775 | 1295 | 4023 | 8093 |
| Number of taxa | 5 | 5 | 6 | 6 |
| Index | 0,56 | 0,48 | 0,63 | 0,56 |
Calculation of the Margalef Index for the diet of seals haul-out in different sea regions.
3.4 Seasonal comparison of diet
When examining the seasonal dynamics of fish diversity in the diet of the Caspian seal (Pusa caspica) across all haul-outs, it is evident that fish from the Gobiidae family dominate (Figure 11). However, it is important to note that summer data were limited to only short-term collections from the Kendirli haul-out in the Middle Caspian, and the presented material also indicates the need for more extensive searches and research on seals’ haul-out during the summer period.
Figure 11
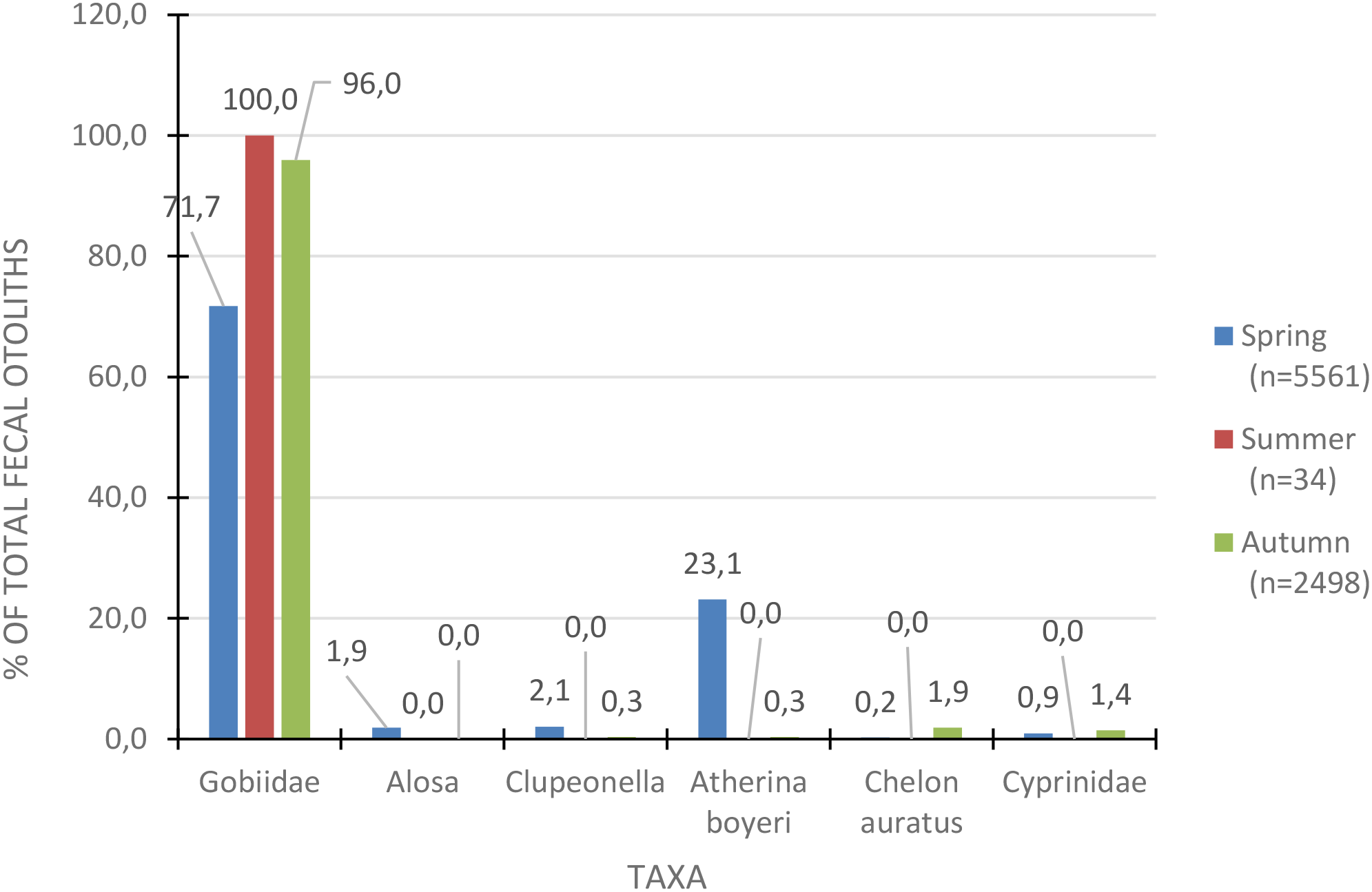
Seasonal diversity of fish species in the seal’s (Pusa caspica) diet across all areas overall years of observation.
Atherinidae occupies the second highest proportion of the diet (23.1%) only during spring. In autumn, the dominant species are gobies, which account for 96% of the diet. When examining the diversity of fish in the seal’s diet in spring, it is apparent that different fish dominate in different areas of the sea – in the northeastern part, it is Cyprinidae, near the Komsomolets Bay – Gobiidae, and in the Kendirli Bay – Atherinidae (Figure 12). The composition of fish in the summer period is characterized by limited collections only at the Kendirli haul-out, represented only by gobies and reflected in Figure 11. In autumn, gobies dominate the diet of seals both in the northeast of the Caspian and in the Middle Caspian at Kendirli Bay, making up over 95% of each area (Figure 13).
Figure 12
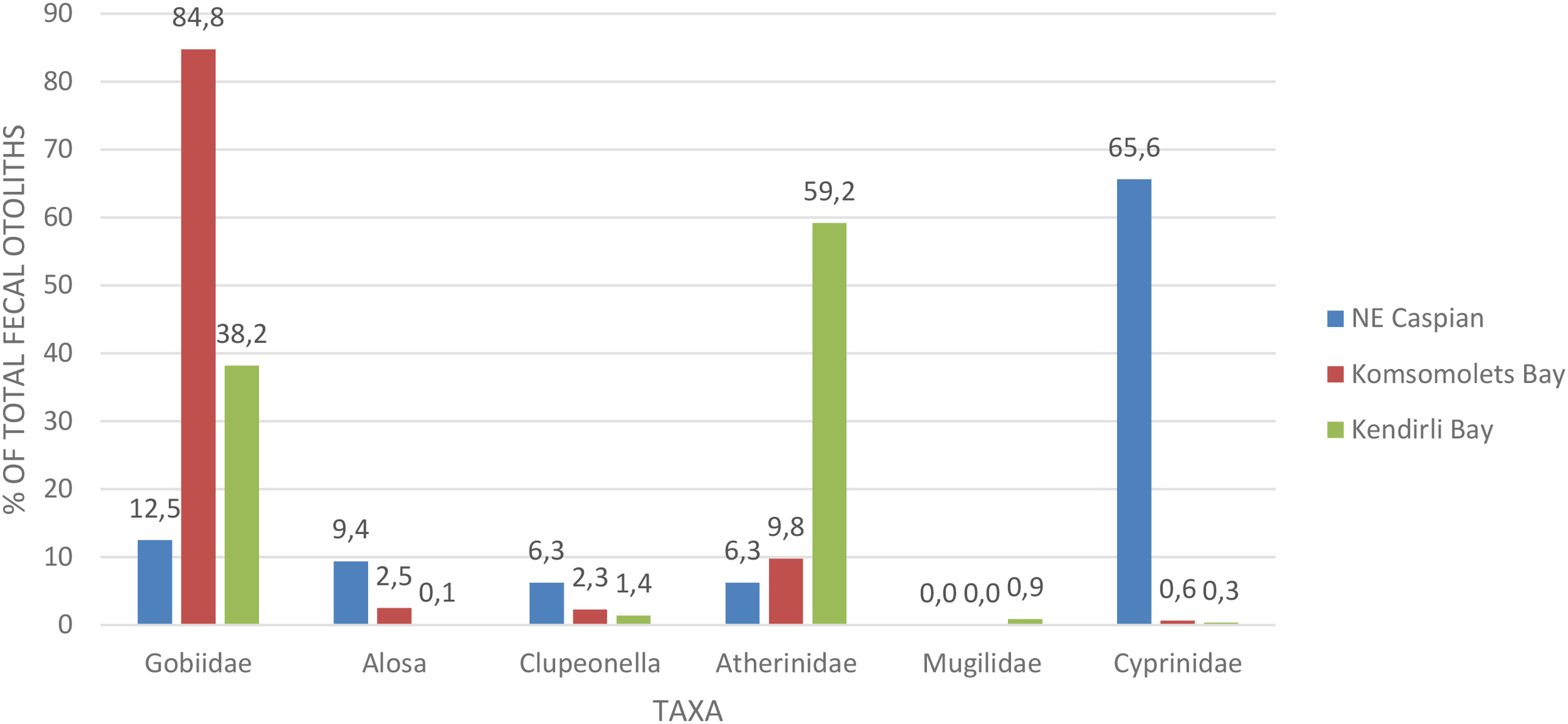
The Caspian seal (Pusa caspica) diet during the spring period while hauling out on island rookeries in various regions of the sea.
Figure 13
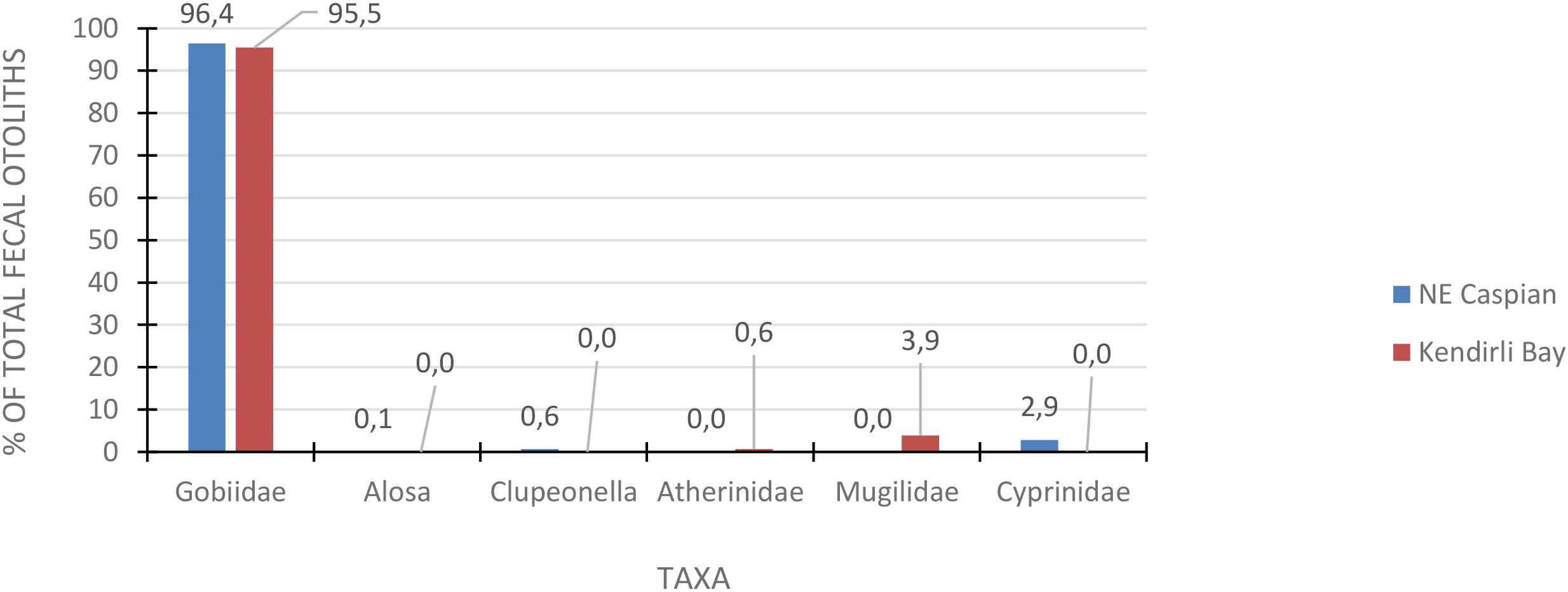
The Caspian seal’s diet (Pusa caspica) during the autumn period while hauling out on island rookeries in various sea regions.
4 Discussion
In the annual cycle of the Caspian seal (Pusa caspica), haul-outs on island rookeries are prolonged during the spring molting period (March-May) and in the autumn before the transition to ice (from September to November). Research shows that in recent years (2015-2022), seals predominantly congregate on islands and shoals in the northeastern Caspian during these periods, with smaller haul-outs forming in the Middle Caspian (Baimukanov et al., 2023b).
A retrospective analysis indicates a lack and inconsistency of information about the seals’ diet during haul-out periods on island rookeries. Previous assertions claimed that seals do not feed during molting (Smirnov, 1907). Stomach dissections on the shoals showed that in the spring, 92% of gastrointestinal tracts (GIT) were empty, while in autumn, 34.5% were empty (Badamshin, 1948). In spring, occurrences of tyulkas and gobies were balanced at 6% each, needlefish (Syngnathus caspius Eichwald, 1831) were present at 2%, with no roaches found, while in autumn, gobies dominated the diet at 31.1%, with no tyulkas found and a small number of roaches present at 6.9%. Another study reported that all 12 stomachs of seals hunted on shoals were empty (Vorozhtsov et al., 1972). Another paper merely states that gobies and crustaceans dominate the diet of island rookeries without providing quantitative data (Pochtoeva-Zakharova and Khuraskin, 1999). Hence, all the above data do not fully describe the range of the seals’ diet during the haul-out periods on the islands. Moreover, the method of slaughtering animals for research shows the individual diet of only some seals over a very short haul-out period. Unlike the coprological method applied in our study, it does not reveal the diet of the entire seal aggregation.
Collecting seal feces on island rookeries during haul-out periods, extracting fish otoliths from them, and creating collections for researching the diet of P. caspica, an obligate piscivore, has opened up new opportunities for studying the dietary diversity of this marine mammal. Alongside creating a collection of otoliths obtained from feces, a collection of otoliths from directly caught fish of various species was also created. Comparing otoliths from the two mentioned collections allows us to reliably determine the fish diet of this endangered species, both qualitatively (to a specific taxonomic status) and quantitatively (to determine the proportion of consumed fish of different taxonomic statuses). In this context, it is important to standardize the description of otoliths for some fish groups. As demonstrated in this paper, when describing otoliths of the Gobiidae family, some researchers describe the presence of a rostrum and other rostral structures, while others do not. Since the criterion for the presence or absence of a rostrum is the type of otolith sulcus—open or closed, depending on whether the sulcus reaches the edge of the otolith (Smale et al., 1995)—our descriptions followed the second approach, denying rostral structures in gobies due to the closed sulcus of fish in this family.
When determining the diet of P. caspica, it is necessary to consider the species richness and endemism of fish that are potential prey for this marine mammal. Additionally, passing through the seals’ GIT and the resulting changes in otoliths, at the current level of knowledge, do not allow us to determine the entire fish diet of P. caspica to the species level. Species identification of otoliths from feces was only conducted for big-scale sand smelt (Atherina boyeri) and golden grey mullet (Chelon auratus). Confidence in the accuracy of identifying these species is linked to the fact that Atherina boyeri is the only representative of the Atherinidae family in the Caspian Sea, and that the identification of Chelon auratus was conducted with great care (Baimukanov et al., 2024). Additionally, one representative of this family, Chelon saliens, predominantly inhabits the Southern Caspian, while another, Mugil cephalus, has not naturalized and is no longer found in the natural environment of the Caspian Sea (Ivanov et al., 2023). Notably, all three species of mullets in the Caspian were introduced for acclimatization. The Caspian Sea hosts 34 species of fish belonging to the family Gobiidae, divided into 2 subfamilies: Gobiinae and Benthophilinae, and 12 genera. Many of these species are Caspian endemics (Ivanov et al., 2023). This work provides descriptions of the forms of only eight species from 5 genera, also showing that the shapes of otoliths can change due to wear as they pass through the seals’ GIT, which also presents challenges for identifying otoliths of goby fish to genera and species.
Literature sources provide little information describing large otoliths of Cyprinidae—asteriscus and lapillus—that can be used to identify otoliths in seal feces (Smale et al., 1995; Lin and Chang, 2012). We briefly described and compared the otoliths of only two species—Rutilus caspicus (Jakowlew, 1870) and Abramis brama (Linnaeus, 1758). As a result, common features were identified, which we accept as characteristic of the Cyprinidae family. Since the Cyprinidae family in the Caspian Sea is represented by more than 30 species, based on the obtained description, we identified the taxonomic affiliation of cyprinid fish otoliths to the family level with greater certainty. Comparing the shapes of otoliths obtained from fish and feces confirms this possibility, allowing their identification in feces.
The Clupeidae family in the Caspian Sea is represented by 2 genera: Alosa (shads) and Clupeonella (tyulkas). Caspian shads include seven species with several subspecies. Caspian tyulkas are represented by three species (Ivanov et al., 2023). Since the current otolith reference collection consists only of two species: Alosa braschnikowi braschnikowi (Borodin, 1904)—a subspecies of Alosa braschnikowi—and Clupeonella caspia (Svetovidov, 1941), only the diagnostic features of the family and the distinguishing features of the genera have been identified and described. It was found that these features are preserved when otoliths pass through the seals’ GI tract.
Thus, the study of otoliths of various fish species—potential dietary items of P. caspica—allows us to differentially assess the diversity of the seals’ diet during haul-out periods on island rookeries—at the species level: mullet and big-scale sand smelt; at the genus level: shads and tyulkas; at the family level: gobies and cyprinids. It is worth noting that the authors have initiated and are continuing studies aimed at providing a more comprehensive description of otolith morphology, calculating various shape indices, and conducting comparative analyses between otoliths extracted from fish and those recovered from fecal samples. These efforts are focused on assessing the effects of erosion as otoliths pass through the gastrointestinal tract of seals. Based on these studies, it will become possible to establish otolith growth equations in relation to fish size, estimate erosion coefficients, and reconstruct the original length of fish consumed by seals for each species. Each of these tasks is extensive and requires dedicated articles, as demonstrated in the study of golden grey mullet (Chelon auratus, Risso 1810) otoliths (Baimukanov et al., 2024). Consequently, the number of unidentified otoliths can be significantly reduced in the future. However, at the current stage of research, given the alarming conservation status of the Caspian seal—Endangered (Goodman and Dmitrieva, 2016)—it was essential to assess the taxonomic composition of the seal’s diet and to identify key challenges both in diet composition and in the study of feeding during haul-out periods on island rookeries amid the sea level regression.
Fecal findings on haul-outs unequivocally indicate that seals feed during this period. Of course, one cannot exclude that some feces with fish otoliths on the rookeries were from those seals that had just come from the sea to haul out and defecate. In turn, observations of the behavior of seals showed that seals actively molting rarely go into the water, preferring to conserve energy and, by warming up well, shed their old fur on land. During the period when rookeries were set up on islands with stable conditions, i.e., not completely flooded by the sea, conditions favored the prolonged presence of seals on them and a better molt. However, some seals did leave the islands, apparently searching for food, and returned to the rookery in the evening and at night, when many fecal findings were made on the rookeries.
Since 2020, the regression of the sea’s coastline has been progressing (Baimukanov, 2019; Baimukanov et al., 2023b). “Stable” islands do not have time to form, and seals haul out on temporary islands—shoals—that are subject to surge phenomena and can be flooded with water during prolonged surges. Consequently, seals, one way or another, go to sea, and the likelihood of them consuming food becomes even higher.
Interestingly, in the northeast of the Caspian in the spring, cyprinid fish dominate the diet of seals (Figure 12). Such species as roach and bream are semi-migratory and, inhabiting coastal areas, go to spawn in the estuaries of nearby rivers in the spring. The rookeries in the northeast of the Caspian are located relatively close to a major tributary of the Caspian—the Ural River (Figure 1)—and therefore the dominance of these fish in the diet of seals in the spring is quite understandable - an increase in the abundance of migratory fish provides favorable feeding opportunities for seals.
The presence of big-scale sand smelt (Atherina boyeri) in the diet of seals hauling out at the Kendirli rookery in significant numbers—up to 23% in the spring period—is also quite logical given that the paths of this fish species’ spawning migrations run through the deep-water Middle Caspian near the rookery (Ivanov et al., 2023).
Unfortunately, the absence of data on the summer and autumn diet of seals hauling out on the northeastern Caspian and Komsomolets Bay islands does not allow for a comparison across different areas during these seasons. It is only noted that in the Middle Caspian (Kendirli rookery), gobies play a dominant role in the diet of seals in the summer and autumn. The impoverishment of the seals’ summer diet can be attributed to the small number of samples collected at this time, but it is still interesting that only gobies were present in the diet in the summer. As is known, during the summer fattening period, seals prefer to eat tyulkas (Khuraskin and Zakharova, 2000). Perhaps the absence of tyulka otoliths in feces in the summer reflects that during the summer fattening period, some of the seals are on the rookery, switching to a diet of gobies inhabiting Kendirli Bay (Baimukanov et al., 2020). In autumn, the diversity is higher; besides gobies, tyulkas, big-scale sand smelt, mullet, and cyprinid fish are encountered, but in very small numbers. The intensity of feeding possibly decreases after migration from the south to the north, and the seals hauling out prefer not to search far for food but to “snack” on local species of gobies. Therefore, the diversity of gobies in the diet of seals in the autumn is higher, to the detriment of the diversity of other fish species. In general, it can be noted that during the spring-summer and autumn periods, when P. caspica hauls out on island rookeries, the dominant group of fish is gobies. Significantly less numerous, but still the second most important species in the Komsomolets and Kendirli Bays are A. boyeri. All other fish species, including tyulkas, shads, mullet, and cyprinids, occupy an insignificant place in the diet. According to the Margalef index, the diet is more diverse in the deep-water Middle Caspian.
It is noteworthy that tyulkas, despite their increased numbers in the sea in recent years (Ivanov et al., 2023), are encountered in very small quantities in the diet of hauling-out seals. This can also serve as indirect evidence that during molting and pre-winter aggregation, the seals’ feeding activity is low and directed towards searching for food near the location of the rookeries, where tyulkas, as pelagic fish, are rarely encountered. It should be noted that the caloric content of tyulkas is 2.7 times higher than that of a representative of the Gobiidae family—Neogobius pallasi, which also has 1.2 times less protein and 16.4 times less fat content (Skurikhina, 1987). Therefore, if after the summer fattening period in autumn, seals, having accumulated subcutaneous fat, can be satisfied with a sparse goby diet. In the spring, emaciated after winter and reproduction, seals experience an acute food shortage. This may be reflected in the decreased body condition of the Caspian seal during spring haul-out periods. According to Krylov, a shortage of tyulka in the diet between 1978 and 1986 led to an 11–16% reduction in blubber thickness in seals, which is considered an indicator of poor population health (Glebych et al., 2008). Thus, the analysis of the diet of seals during the spring-summer and autumn periods while hauling out on island rookeries shows a low diversity of fish in the diet: the main dietary component consists of representatives of the Gobiidae family, with cyprinids being the second most important species in the northeastern part of the sea, and A. boyeri in the Komsomolets and Kendirli Bays. The limited understanding of fish stock distribution in the shallow waters of the northeastern Caspian Sea and the foraging behavior of P. caspica hinders accurate interpretation of the findings presented above. In the future, comprehensive research focused on assessing the composition of ichthyofauna in coastal waters near haul-out sites, identifying local movements of P. caspica during island haul-out periods through satellite tagging, and analyzing dietary composition will help clarify the ecological drivers behind the selective consumption of certain fish species. Such studies are essential for informing effective conservation strategies for this Endangered species.
The results also indicate that species identification by otoliths from feces requires an assessment of diversity, variability, and comparison with otoliths from collections gathered from fish living in different conditions and areas of the sea. For this, an additional collection of materials from different areas of the sea is required to study the polymorphism of otoliths. Particular attention should be given to the application of DNA barcoding for more accurate species-level identification of P. caspica fish diet (Conwell et al., 2024; Trzcinski et al., 2024). Within the framework of such research, it is essential to conduct morpho-genetic studies of Caspian fish populations and to identify genetic markers in their DNA to expand the reference library of DNA barcodes for the fish species of the Caspian Sea.
Studying the diet of P. caspica at various haul-out sites across the sea, together with data on fish distribution and local foraging migrations, will provide a foundation for the establishment of protected marine areas in the Caspian Sea. This will support the delineation and conservation of the seal’s critical habitats during the spring and autumn haul-out periods, when the species is most vulnerable.
5 Conclusion and future directions
The comparison of otoliths collected from fish with those that have passed through the gastrointestinal tract (GIT) of Caspian seals (Pusa caspica) has provided a way to assess the diet of marine animals during a critically important period of their annual cycle—their time on island rookeries. This analysis, based on a coprological in vivo method, was conducted for the first time and shows that, firstly, seals do not cease feeding during the spring, summer, and autumn haul-out periods; secondly, the diet varies in terms of the presence of certain fish at the family, genus, or species level depending on the location of the rookeries and the season. However, it indicates that seals predominantly feed on goby fish species. The low nutritional value of gobies during the spring molting period could indicate a deficiency in the seals’ diet.
On the other hand, the materials from the conducted studies require a more systematic study of seal feeding behavior during haul-out periods. Previous research on seal migrations lamented the absence of contemporary data on seals’ diet, which complicates analyzing the relationship between seal distribution and their prey (Dmitrieva et al., 2016). Now, this gap can be avoided with data on the content of fish otoliths in seal feces. However, comprehensive studies are needed on the species diversity and distribution of fish, migrations, feeding behavior, and diet of seals. Undoubtedly, in addition to studying seal feces in the future, other in vivo methods for studying the diet of seals, such as molecular-genetic analysis for a more accurate assessment of the diet (Brown et al., 2012; Bowen and Iverson, 2013; Krause et al., 2020), and studying the GIT of dead seals washed ashore at different times, should also be applied.
As shown in this work, we are at the beginning of the path. With rare exceptions (for example, Chelon auratus and Atherina boyeri), species identification of otoliths from seal feces presents difficulties. A detailed study of otoliths from gobies, cyprinids, and other fish families is necessary to determine interspecific and population-level variability, as well as the changes that occur in otoliths during their passage through the gastrointestinal tract of the seal. The first work in this direction has been conducted to assess the content of golden grey mullet in the diet of seals (Baimukanov et al., 2024). Since P. caspica is a transboundary species and its feeding occurs in different areas of the sea, specialists from other Caspian countries may join in clarifying the diet of this species.
Creating a unified database of Caspian Sea fish otoliths will allow for studying their polymorphism and conducting identification of otoliths from feces or GIT of seals to the species level and assessing the sufficiency of fish food for seals. In addition to studying seal feces, in the future, other in vivo methods of dietary studies, such as molecular-genetic analysis, should also be applied for a more accurate assessment of the species composition of fish in the diet. In addition to fecal analysis, future studies of P. caspica diet should incorporate other in vivo methods, such as DNA barcoding, to enable more accurate identification of fish species in the diet. Examination of the gastrointestinal tracts of deceased seals washed ashore at different times of the year is also essential.
In conjunction with these studies, it is important to assess local fish stocks near haul-out sites and to investigate the foraging behavior and local movements of P. caspica. Thus, future studies examining seal diet in relation to fish distribution near haul-outs will help identify critical marine areas for seals. This will significantly contribute to research on seal adaptation to rapidly changing conditions and aid in developing measures to conserve the key habitats of disappearing endemics.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the local ethics committee of the Institution “Institute of Hydrobiology and Ecology”. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MB: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. AS: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. AI: Investigation, Methodology, Resources, Writing – review & editing. AB: Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research has been funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP14871482).
Acknowledgments
The authors are grateful to the staff of Kazakhstan Agency of Applied Ecology LLP and Tengizchevroil LLP for providing fish specimens used to create the reference otolith collection. The authors express their special thanks to Irina Suvorova for providing copies of rare scientific publications on the diet of the Caspian seal.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. While preparing this work, the authors used Grammarly to improve language translation, overall clarity, and readability. After using this tool/service, the authors reviewed and edited the content as needed and took full responsibility for the publication’s content.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Badamshin B. I. (1948). On the feeding of the Caspian seal. Proc. Volga-Caspian Sci. Fisheries Station10, 129–134.
2
Badamshin B. I. (1966). Biology and fishing of the Caspian seal. Fish Resour. Kazakhstan’s Water Bodies Their Use5, 94–123.
3
Badamshin B. I. (1971). On the mass death of the Caspian seal. Proc. Caspian Sci. Res. Institute Fisheries26, 261–264.
4
Baimukanov M. T. (2017). How to save the Caspian seal (Pusa caspica) (Almaty, Kazakhstan: News of the National Academy of Sciences of the Republic of Kazakhstan, Institute of Plant Biology and Biotechnology) 324, 100–111. Available online at: https://ihe.kz/images/publication/mirgaliy/2017_7.pdf.
5
Baimukanov M. T. (2019). On the prospects for developing the accounting of population size, tagging, and associated in vivo studies of Caspian seals (Pusa caspica) (Almaty, Kazakhstan: Collection of Scientific Works of the Scientific and Production Center of Fisheries), 188–197. Available online at: https://ihe.kz/images/publication/mirgaliy/ok2019.pdf (Accessed July 27, 2025).
6
Baimukanov M. T. (2022). On the impact of climate change and regression of the Caspian Sea on the distribution and population size of the Caspian seal (Pusa caspica). In Proceedings of the International Scientific Conference “Climate Change in the Caspian Sea Region. OstrovskayaE. V.DegtyarevaL. V. (Astrakhan: Sorokin R.V), 172–174. Available at: https://ihe.kz/images/publication/mirgaliy/ok2019.pdf (Accessed July 27, 2025).
7
Baimukanov M. T. Baimukanova A. M. Baimukanov T. T. Isbekov K. B. Dauenov E. S. Ryskulov S. E. (2020). Results of surveys of Caspian seal (Pusa caspica) abundance on the island haulout sites in the Kazakhstan zone of the Caspian Sea, 2015–2018. In Proceedings of the X International Conference “Marine Mammals of the Holarctic, dedicated to the memory of A.V. Yablokov (Arkhangelsk), 48–59 (Arkhangelsk, Russia: Conference Organizing Committee). Available at: https://ihe.kz/images/publication/pdf/pdf2021_1.pdf (Accessed July 27, 2025).
8
Baimukanov M. Shagilbayev A. Iskakov A. (2024). Determination of body length regression formulas for the golden grey mullet (Chelon auratus, Risso 1810) based on otoliths found in the feces of the Caspian Seal (Pusa caspica Gmelin 1788). Reg. Stud. Mar. Sci.80, 103888. doi: 10.1016/j.rsma.2024.103888
9
Baimukanov M. T. Shagilbayev A. U. Ryskulov S. E. Iskakov A. A. Sydykova Z. A. Kuznetsova T. V. et al . (2023b). Main results and prospects for studies of the population of the Caspian seal (Pusa caspica Gmelin 1788) during the haul-out periods in the Kazakhstani part of the Caspian Sea. In Zoological Research in Kazakhstan in the 21st Century: Results, Problems, and Prospects. Collection of articles from the international scientific conference. 715–722 (Almaty, Kazakhstan: Institute of Zoology). Available at: https://ihe.kz/images/publication/mirgaliy/2023_1r.pdf (Accessed July 27, 2025).
10
Baimukanov M. T. Sydykova Z. A. Ryskulov S. E. Sirazhitdinova M. K. Seitkozhina D. A. Baimukanova A. M. (2023a). On the action plan for the conservation of the Caspian seal (Pusa caspica gmelin 1788) in the republic of Kazakhstan. In Proceedings of the XII International Scientific and Practical Conference “Marine Research and Education (MARESEDU-2023). 640–647 (Moscow, Russia: Lomonosov Moscow State University). Available at: https://ihe.kz/images/publication/mirgaliy/2023_10.pdf (Accessed July 27, 2025).
11
Baimukanov M. T. Zhdanko L. A. Sydykova Z. A. (2017). Developing the method for collecting and primary processing of feces of Caspian seals (Pusa caspica) to study their diet. (Almaty, Kazakhstan) Available online at: https://ihe.kz/images/publication/mirgaliy/2017_1.pdf. (Accessed July 27, 2025).
12
Biolé F. G. Callicó Fortunato R. Thompson G. A. Volpedo A. V. (2019). Application of otolith morphometry for the study of ontogenetic variations of Odontesthes argentinensis. Environ. Biol. Fishes102, 1301–1310. doi: 10.1007/s10641-019-00908-0
13
Bizikov V. A. Chernook V. I. Sidorov V. K. (2021). Estimation of the population size of the Caspian seal based on instrumental aerial surveys on the ice in the northern part of the Caspian Sea in 2012, 2020, and 2021. Use Conserv. Natural Resour.168, 81–92.
14
Bohutska N. G. Kiyashko P. V. Naseka A. M. Orlova M. I. (2013). Identifier of fish and invertebrates of the Caspian Sea (St. Petersburg, Moscow: Scientific Publications KMK). Available online at: https://www.zin.ru/labs/marine/papers/caspian_sea_v1.pdf. (Accessed July 27, 2025).
15
Bowen W. D. Iverson S. J. (2013). Methods of estimating marine mammal diets: A review of validation experiments and sources of bias and uncertainty. Mar. Mammal Sci.29, 719–754. doi: 10.1111/j.1748-7692.2012.00604.x
16
Brown S. L. Bearhop S. Harrod C. McDonald R. A. (2012). A review of spatial and temporal variation in grey and common seal diet in the United Kingdom and Ireland. J. Mar. Biol. Assoc. U. K.92, 1711–1722. doi: 10.1017/S0025315411002050
17
Conwell H. C. Lewis Z. K. Thomas A. Acevedo-Gutiérrez A. Schwarz D. (2024). Sex-specific diet differences in harbor seals (Phoca vitulina) via spatial assortment. Ecol. Evol.14, e11417. doi: 10.1002/ece3.11417
18
Dellinger T. Trillmich F. (1988). Estimating diet composition from scat analysis in otariid seals (Otariidae): is it reliable? Can. J. Zool.66, 1865–1870. doi: 10.1139/z88-269
19
Dmitrieva L. Jüssi M. Jüssi I. Kasymbekov Y. Verevkin M. Baimukanov M. et al . (2016). Individual variation in seasonal movements and foraging strategies of a land-locked, ice-breeding pinniped/Mar. Ecol. Prog. Ser.554, 241–256. doi: 10.3354/meps11804
20
Eybatov T. M. Hajiyev D. V. (2022). Fossils and modern pinnipeds of Azerbaijan. ANAS Transactions on Earth Sciences, 106–118. Baku, Azerbaijan. Available online at: https://journalesgia.com/storage/806/Eybatov_ANAS_Transactions_2022_1.pdf. (Accessed July 27, 2025).
21
Glebych A. I. Sokolsky A. F. Abdurakhmanov G. M. Umerbaeva R. I. Tatarintseva T. A. Terletskaya O. V. et al . (2008). Current state of bioproductivity of the caspian sea and causes of seal population degradation over the last 300 years (Astrakhan: KPC “Polygraphcom), 176 pp.
22
Goodman S. Dmitrieva L. (2016). Pusa caspica. IUCN Red List Threat. Species22. doi: 10.2305/IUCN.UK.2016-1.RLTS.T41669A45230700.en
23
Härkönen T. (1986). Guide to the otoliths of the bony fishes of the Northeast Atlantic (Denmark: Danbiu).
24
Ivanov V. P. Paltsev V. N. Shipulin S. V. (2023). Fish resources of the Caspian Sea (Moscow: VNIRO Publishing). Available online at: https://elibrary.ru/item.asp?id=54473747.
25
Kajiwara N. Watanabe M. Wilson S. Eybatov T. Mitrofanov I. V. Aubrey D. G. et al . (2018). Persistent organic pollutants (POPs) in Caspian seals of unusual mortality event during 2000 and 2001/Environ. Pollut.152, 431–442. doi: 10.1016/j.envpol.2007.06.075
26
Khuraskin L. S. Zakharova N. A. (2000). “Contemporary conditions for the formation of the Caspian seal population resources,” in Marine Mammals of the Holarctic, Proceedings of the International Conference. 414–417 (AArkhangelsk, Russia: Conference Organizing Committee).
27
Krause D. J. Goebel M. E. Kurle C. M. (2020). Leopard seal diets in a rapidly warming polar region vary by year, season, sex, and body size. BMC Ecol.20, 32. doi: 10.1186/s12898-020-00300-y
28
Krylov V. I. (1983). On the impact of the Caspian seal (Pusa caspica) on fish populations. Zoological J. LXII6, 930–936.
29
Lin C.-H. Chang C.-W. (2012). Otolith atlas of Taiwan fishes (Taiwan: National Museum of Marine Biology and Aquarium).
30
Lombarte A. Chic O. García–Ladona E. Parisi-Baradad V. Olivella R. Piera J. et al . (2006). A web-based environment for shape analysis of fish otoliths. The AFORO database. Sci. Mar.70, 147–152.
31
Magurran A. E. (1988). Ecological diversity and its measurement (Dordrecht: Springer Netherlands). doi: 10.1007/978-94-015-7358-0
32
Margalef R. (1958). Information theory in ecology. Gen. Syst.3, 36–71. Available at: https://www.vliz.be/en/imis?module=ref&refid=126440&printversion=1&dropIMIStitle=1.
33
Pavlov D. A. Shirokova E. A. (2020). Variability in otolith structure in populations of the Amur sleeper (Perccottus glenii, Odontobutidae) in Central Russia. Questions Ichthyology60, 52–62. doi: 10.31857/S0042875219060146
34
Pierce G. T. Boyle P. R. Diack S. W. (1991). Identification of fish otoliths and bones in faeces and digestive tracts of seals. J. Zool.224, 320–328. doi: 10.1111/j.1469-7998.1991.tb04810.x
35
Pochtoeva-Zakharova N. Khuraskin L. S. (1999). On the diet of the Caspian seal. In: Proceedings of the Atlantic Scientific Research Institute of Fisheries and Oceanography (AtlantNIRO), pp. 43–44. Kaliningrad, Russia: AtlantNIRO Publishing.
36
Pravdin I. F. (1966). Guide to studying fish (Primarily freshwater) (Moscow: Food Industry Publishing).
37
Quigley L. A. Caiger P. E. Govindarajan A. F. McMonagle H. Jech J. M. Lavery A. C. et al . (2023). Otolith characterization and integrative species identification of adult mesopelagic fishes from the western North Atlantic Ocean. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1217779
38
Roganov A. N. (1931). The Caspian seal and its fishery. Proc. V-K Sci. Fisheries Station7, 1–28.
39
Rumyantsev V. D. Vorozhtsov G. A. Khuraskin L. S. Yusupov M. K. (1975). The state of Caspian seal stocks and prospects for their utilization. Proc. All-Union Sci. Res. Institute Mar. Fisheries Oceanography108, 185–189.
40
Rustamov E. A. Shcherbina A. A. Belousova A. V. Mammedov S. B. (2021). Status of the Caspian seal in the Turkmen sector of the Caspian 2012-2021. Curr. Issues Zoology Ecol.3, 133–138.
41
Secor D. H. Dean J. M. Laban E. H. (1991). Manual for otolith removal and preparation for microstructural examination. Available online at: https://agris.fao.org/search/en/providers/122621/records/64739670ce9437aa76003f8e. (Accessed July 27, 2025).
42
Skurikhina I. M. (1987). Chemical composition of food products: book 1: reference tables of the main nutrients and energy value of food products. 2nd Ed (Moscow, USSR: Agropromizdat).
43
Smale M. J. Watson G. Hecht T. (1995). Otolith atlas of southern African marine fishes (Grahamstown, South Africa: J.L.B. Smith Institute of Ichthyology). doi: 10.5962/bhl.title.141860
44
Smirnov N. A. (1907). On the winter seal fishery in the Caspian Sea (St. Petersburg, Russian Empire).
45
Sokolsky A. F. Pankov A. G. (2009). Current status and causes of population decline of the Caspian seal. South Russia: Ecology Dev.1, 49–52.
46
Svetochova O. N. Stasenkova N. I. (2006). On the structure of the otolith of the lesser herring (Clupea pallasii suworovi) and its use as a marker (Archangelsk: Archangelsk Regional Museum), 273–280.
47
Trzcinski M. K. Majewski S. Nordstrom C. A. Schulze A. D. Miller K. M. Tucker S. (2024). DNA analysis of scats reveals spatial and temporal structure in the diversity of harbour seal diet from local haulouts to oceanographic bioregions. Mar. Ecol. Prog. Ser.743, 113–138. doi: 10.3354/meps14655
48
Tuset V. M. Lombarte A. Assis C. A. (2008). Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Scientia Marina. 72, 7–198. doi: 10.3989/scimar.2008.72s17
49
Vorozhtsov G. A. Rumyantsev V. D. Sklyarova G. A. Khuraskin L. S. (1972). Diet of seals in the northern caspian. Proc. All-Union Sci. Res. Institute Mar. Fisheries Oceanography89, 19–29.
50
Zaitsev V. F. Melyakina E. I. Sokolsky A. F. (2003). Ecological consequences of the introduction of the comb jelly Mnemiopsis in the seas of the Ponto-Caspian complex. Successes Modern Natural Sci.10, 36–38. Available at: https://s.natural-sciences.ru/pdf/2003/10/10.pdf.
Summary
Keywords
Caspian seal, otoliths, diet, nutrition, feces, fish, haul out
Citation
Baimukanov M, Shagilbayev A, Iskakov A and Baimukanova A (2025) Assessment of the fish diet of the Caspian seal (Pusa caspica Gmelin, 1788) during haul out periods on island sites: results, challenges, and perspectives. Front. Mar. Sci. 12:1565902. doi: 10.3389/fmars.2025.1565902
Received
23 January 2025
Accepted
17 July 2025
Published
05 August 2025
Volume
12 - 2025
Edited by
Peter H. Dutton, National Oceanic and Atmospheric Administration, United States
Reviewed by
Raül Triay-Portella, University of Las Palmas de Gran Canaria, Spain
Kelly Flanders, University of California, San Diego, United States
Updates
Copyright
© 2025 Baimukanov, Shagilbayev, Iskakov and Baimukanova.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anuar Shagilbayev, a_shagilbayev@ihe.kz
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.