- 1Oceanlab, School of Biological Sciences, University of Aberdeen, Cruickshank Building, United Kingdom
- 2Takuvik International Research Laboratory, CNRS/Sorbonne université/Université Laval, Laval, QC, Canada
- 3Biogeochemistry Research Centre, School of Geography, Earth and Environmental Sciences, Plymouth University, Plymouth, United Kingdom
The effects of global warming are most pronounced at high latitudes and are a threat to primary productivity patterns and, in particular, to sea ice algae. Here, we investigated the importance of ice algae in the diet of megabenthic organisms belonging to several feeding guilds across several locations in the Canadian Arctic characterised by different sea ice conditions using two biochemical approaches i.e., stable isotope and highly branched isoprenoid (HBI) lipids analysis. In addition, the short-term ingestion (gut contents) versus mid to long-term assimilation (tissues) of carbon were investigated to depict momentary condition in the present and the recent past. Our results show firstly that, as soon as the ice breaks up, ice algae accounts for a high proportion of the organic matter deposited to the seafloor and can provide a substantial carbon input to benthic communities for a long period of time (up to 79 days after sea ice break up in our case). Overall, organisms responded rapidly and efficiently to this pulse of fresh organic matter but trends in resource utilisation (quality and quantity) were observed based on feeding strategy. Deposit feeders (except those from lasting sea ice cover) and predators/scavengers showed a dominance of ice algae feeding, while suspension feeder showed a stronger reliance on phytoplankton. Finally, the spatial variability in resource utilisation by ophiuroids is likely related to area’s specificities (e.g., primary production, ice break-up timing, grazer abundance) and highlighted their ability to adapt to available food by switching their feeding types. Our data show that sympagic (ice-associated) carbon represents a significant proportion of the carbon ingested by the megabenthic organisms in the Canadian Arctic during spring/summer but appears to be highly variable depending on sea ice conditions and availability (e.g., patchiness, depth) on the seafloor. Overall, the ongoing decline in seasonal sea ice could alter the functioning and dynamic of the benthic food web in the Canadian Arctic if certain feeding types (e.g., deposit feeders) are unable to adapt to a change in primary productivity patterns.
1 Introduction
In the Arctic Ocean, primary production is partitioned between two main sources: phytoplankton living in the water column and sea ice algae growing in and under the sea ice (Leu et al., 2011). Sea ice plays an important ecological role by providing habitat for sea ice algae, and by limiting light availability for phytoplankton. The type (pelagic or sympagic), timing and extent of primary production in the Arctic are strongly controlled by sea ice conditions (e.g. concentration, extent) and snow cover (Ji et al., 2013). For example, ice algae is typically more abundant in landfast sea ice than in pack sea ice, but considerable variation occurs within locations (Leu et al., 2011). On the other hand, polynyas, characterised by areas of open water surrounded by sea ice, are considered to be highly productive areas in terms of primary productivity (Arrigo, 2007).
During spring, when the ice melts and the light penetrates the water column, ungrazed ice algae sink to the seafloor in a tight sympagic-benthic coupling, providing the first substantial carbon input to benthic communities after the food-limited winter (Schollmeier et al., 2018). During this season, ice algae production can increase by orders of magnitude and contribute up to 40% of the total annual primary production in seasonally ice-covered regions (Brown et al., 2011; Forest et al., 2011). Phytoplankton production, on the other hand, occurs later in the season in late spring or early summer when light levels are higher (Schollmeier et al., 2018). Although phytoplankton production is generally higher than ice algae production (10–12 and 5–10 C m-2 y-1, respectively; Gosselin et al., 1997), much of the pelagic production is consumed by pelagic grazers (up to 60% in some regions), which occur in high abundance in late spring/early summer (Campbell et al., 2009; Sherr et al., 2009). Consequently, less organic matter from pelagic production is expected to reach the seafloor at this time of year. However, Olivier et al. (2020) observed a stronger pelagic-benthic coupling in the North Water Polynya since the late 70s, resulting either from changes in sea ice dynamics or due to a mismatch between phytoplankton growth and zooplankton grazing due to phenological change, both allowing more regular transfer of food to the seabed.
Benthic communities rely primarily on these downward export fluxes of food produced in the euphotic zone for their energy needs. Benthic organisms are essential for healthy marine ecosystems, contributing to the global carbon flow through the remineralisation of the organic matter reaching the seafloor and by serving as food for many higher trophic levels such as birds and marine mammals (Bluhm and Gradinger, 2008; Mäkelä et al., 2018; Stirling, 1997).
Ice algae are an essential high-nutritional quality food source for marine organisms due to the presence of long chain omega-3 acids, a subgroup of polyunsaturated fatty acids that are almost exclusively produced by these algae (Amiraux et al., 2021; Søreide et al., 2010). These lipids, which are first ingested by the benthos and then transferred to the higher trophic levels, are necessary for the entire food web as they play a crucial role in the growth and reproduction of marine organisms (GusChina and Harwood, 2009; Jin et al., 2020). However, as a result of climate change and consequently decreasing summer sea ice cover, the open water area (i.e., ice-free) in the Arctic has increased by 27% between 1998 and 2018, with ~59,000 km2 of open water added each year (Lewis et al., 2020). In addition to an increase in the duration of the open-water period (0.78 d/a), the annual net primary production increased by 57% (8.39 Tg C/y) during this period and is primarily attributed to an increase in pelagic production (Arrigo and van Dijken, 2015; Lewis et al., 2020; Li et al., 2019). Current climate models, under different emission scenarios (Moore et al., 2019; Sou and Flato, 2009), suggest that these changes will continue, leading to additional changes in the sea ice dynamics and subsequent primary production patterns. However, the consequences of a shift to a phytoplankton-dominated food web for the benthic fauna are not yet fully understood. In this context, understanding the contribution of sympagic and pelagic organic matter in the Arctic benthic food web is key to understanding current carbon dynamics and to predict how these communities may cope with a change in sea ice dynamics and associated primary production.
Stable isotope analysis (SIA) of carbon and nitrogen is commonly used to trace the organic matter input and transfer in benthic food webs (Layman and Allgeier, 2012). Indeed, nitrogen isotopes ratios (δ15N) can be used to determine the trophic structure of the benthic food web as 15N shows an enrichment of 3.4 ‰ per trophic level (Iken et al., 2001). On the other hand, carbon isotopes ratios (δ13C) can be used to determine the dependence of benthic macrofauna to different food sources as the mean fractionation of 13C between trophic levels is assumed to be from 0‰ to 2‰ (Renaud et al., 2015). Analysis of the stable isotope composition of organic matter from polar sea ice has revealed an enrichment in 13C relative to 12C (Drenzek et al., 2007; Kennedy et al., 2002). Typically, the resultant δ13C values for sea ice-derived organic matter have been found to lie between -13‰ to -22‰, while the related values for phytoplankton-derived organic matter lie between -20‰ and -28‰ (Belt et al., 2008; Brown et al., 2017; Tamelander et al., 2006, 2009). As a result, the stable isotope compositions of ice algae and phytoplankton can be hard to distinguish in many cases as an overlap exist between their isotopic signatures (Bravo et al., 2024; Gradinger, 2009; Lovvorn et al., 2005).
In recent years, a novel proxy method, based on the analysis of highly branched isoprenoid (HBI) lipids has emerged that allows to distinguish more precisely the ice algae and phytoplankton signatures in sediments and marine organisms (Brown and Belt, 2012a; Brown et al., 2014c). HBIs are produced by a narrow range of marine diatoms in both the sympagic and pelagic realms, and have different levels of saturation depending on the synthesising species (Belt, 2018). Among these, IP25 (Ice proxy with 25 carbon atoms; Belt et al., 2007), seems to be produced exclusively by certain Arctic sympagic diatoms belonging to the genera Haslea and Pleurosigma (Brown et al., 2014b). In addition, the di-unsaturated HBI II is also exclusively produced by sympagic diatoms, while the tri-unsaturated HBI III is produced by pelagic algae (Belt et al., 2007; Brown et al., 2011). Due to their high source-specificity and their high persistence in the environment, these HBIs are used to estimate the proportion of assimilated organic matter that originated from sympagic or pelagic source (Brown et al., 2017).
The main advantage of this method is that it is not limited to a qualitative description of the resource utilisation but can provide further quantitative evidence of the accumulation and uptake of the carbon source depending on the body part analysed. In fact, the analysis of tissues allows the study of the medium/long-term diet, while the analysis of the gut contents provides information on short-term ingestion of marine organisms. So far, only a very limited number of studies have investigated the short-term ingestion (Iken et al., 2001; Mäkelä et al., 2018) in Arctic marine organisms and none of them used biochemical tracer methods for this purpose.
Among the benthos, megabenthic communities (usually considered of organisms >1 cm) contribute significantly to the total benthic biomass and can accumulate up to 20-40g WW m-2 in the Canadian Arctic (Piepenburg et al., 1996; Roy et al., 2014, 2015; Stratmann et al., 2020). These organisms show a wide range of feeding strategies, and some of them, such as Ophiuroids, have the ability to change their feeding strategy depending on food availability (Stöhr et al., 2012). Given these characteristics, this makes them an ideal model for studying the differences in resource utilisation among feeding types and the potential adaptability of organisms to the food available in different environments.
In this study, two biochemical approaches (SIA and HBIs) were used to identify the importance of ice-algae as a carbon source (versus phytoplankton), both qualitatively and quantitively, in the diet of Arctic megabenthic organisms belonging to different feeding types and living under different sea ice conditions. Specifically, we ask the following questions: (1) Do ice-algae constitute an important food source for megabenthic organisms? (2) Does the food source ingested differ among feeding guilds? (3) Does the resources utilisation by an ophiuroid change according to sea ice conditions? Also, the short (gut contents) vs mid/long-term (tissues) diet is investigated to study the conditions in the present and recent past. To address that, the Canadian Arctic was chosen as a study site since this area is known to host different geographical areas with different sea ice conditions (polynya, lasting ice, landfast and seasonal ice covers …).
2 Materials and methods
2.1 Study site and sampling
Six stations were sampled in summer 2013 during a ArcticNet cruise aboard the research ice-breaker CCGS Amundsen in three sub-regions with different sea ice conditions in the Canadian Arctic (Baffin Bay and Parry Channel) (Figure 1; Table 1). Two stations (323 and 124) were located within polynyas: Lancaster Sound and North Water Polynya, respectively. Two other stations (600 and 633) with seasonal landfast sea ice coverage were located along the Canadian coast in Northwest Labrador Sea. Then, the remaining two stations (250 and 500), characterised by lasting landfast ice coverage, were located in the Nares Strait and Viscount Melville Sound, respectively. At each station, an USNEL Box corer was deployed at the seafloor to collect the first centimetre of sediment (with 10 mL truncated syringes of an area of 1.5 cm2; n=1) which was immediately frozen on-board for further biochemical analyses. Three stations (one station per sub-region) were selected (i.e., stations 124, 633 and 500) to sample benthic fauna. Megafaunal samples were collected with an Agassiz trawl (effective opening of 1.5m and a net mesh size of 40mm), and the average trawling time and speed were 2–3 minutes and 1.5 knots, respectively. Specimens were identified to the lowest taxonomic level on-board and then frozen at -80°C for HBI and bulk stable isotope analysis. Four species were selected for this study: the echinoderms Ophiopleura borealis (which is a widely spread boreal-arctic species), Psilaster andromeda, Gorgonocephalus sp. and the mollusc Megayoldia thraciaeformis, for their belonging to different feeding types (deposit feeder/scavenger, predator/scavenger, suspension feeder and subsurface deposit feeder, respectively). In addition, the gut contents were removed from the tissues to investigate the short-term ingestion versus the mid/long-term assimilation of carbon, respectively. For the larger-size echinoderms (e.g. Ophiopleura borealis, Psilaster andromeda and Gorgonocephalus sp.), 1 to 2 arms were dissected and processed for stable isotope and HBI analysis, depending on the size of the specimens. For the bivalves (Megayoldia thraciaeformis), the whole animal was used (with exception of the gut and the shell).
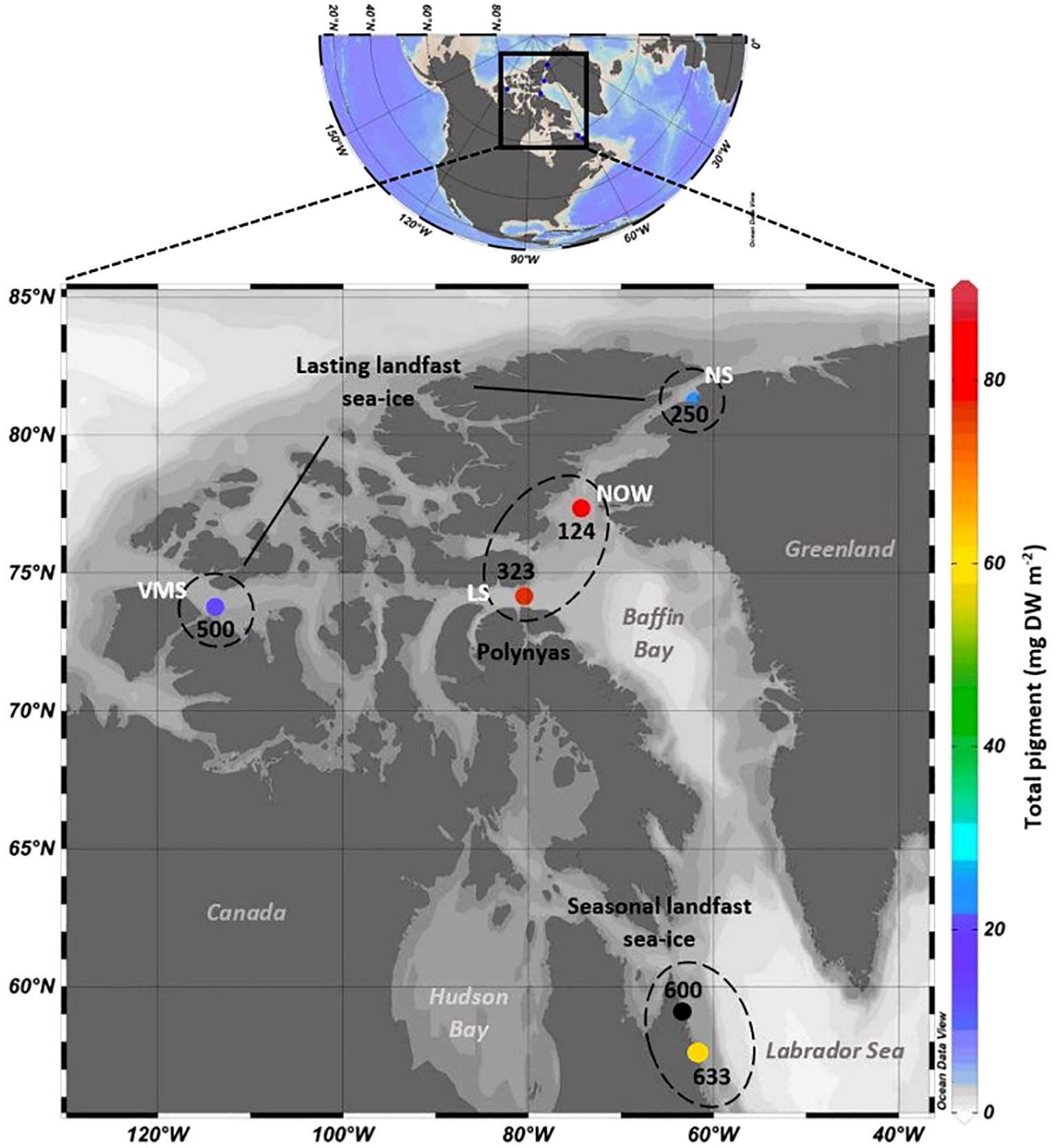
Figure 1. Study region and location of stations sampled in the East Canadian Arctic in 2013. At each station, the total pigment concentration (Chl a + phaeopigment; mg DW m-2) is indicated. No values were obtained for station 600 due to technical issues. NOW, North Water polynya; LS, Lancaster Sound; VMS, Viscount Melville Sound; NS, Nares Strait.
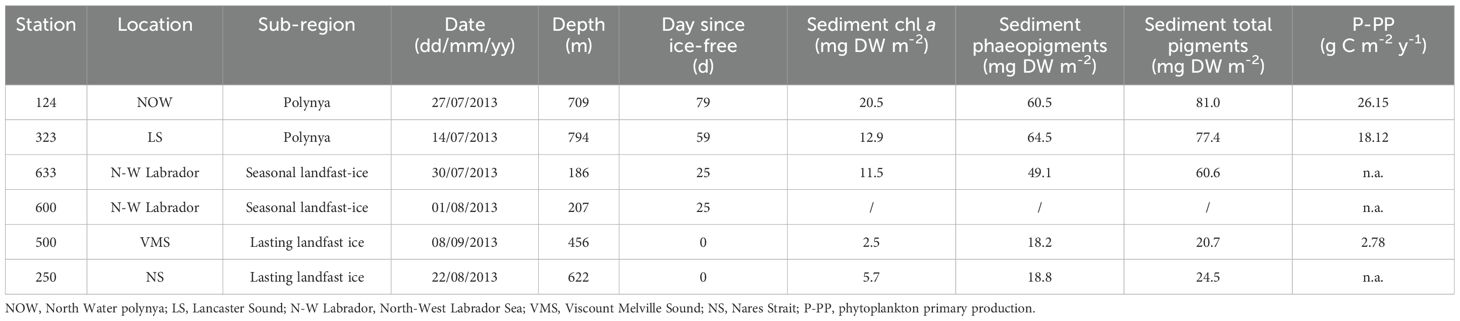
Table 1. Station locations, geographic coordinates, ice conditions, sediment pigment concentrations and satellite-derived phytoplankton primary production from Roy et al. (2015).
The presence and extension of sea ice cover at the sampling sites were determined from the Canadian Ice Service (http://iceweb1.cis.ec.gc.ca/Archive20/page1.xhtml). A window of 2 weeks before the sampling with a concentration of ice less than 20% (i.e., from open water to very open drift) was considered as ice-free.
2.2 Pigment concentrations in sediments
The total pigment concentration in sediments (chlorophyll a and phaeopigment) is commonly used as a proxy to determine the food supply from the water column to the seafloor (Morata et al., 2008, 2011). The determination of pigment contents in sediments was carried out fluorometrically according to a modified version of the protocol by Riaux-Gobin et al. (1993). Approximately two grams of wet sediment were incubated with 10 ml 90% acetone (v/v) for 24 h at 4°C in the dark, and the supernatant was measured in a Turner Design 20 fluorometer before and after acidification (HCl 5% v/v). Quantities are expressed as milligram pigment of dry sediment per square meter (mg DW m-2).
2.3 Stable isotope analysis
Carbon and nitrogen isotope stable isotope ratios (13C:12C and 15N:14N) are powerful tools for the study of the structure and the dynamics of food webs, as they provide insights into trophic relationships and consumer food sources (Michener and Kaufman, 2007). Megafauna specimens were dissected, and gut contents separated from tissues. Fauna and sediment samples were dried at 60°C for 48h. After drying, measurements of their dry weight were carried out and samples were ground into a fine powder using a mortar and pestle. In most cases, three replicates per species and stations were analysed, excepted for Gorgonocephalus sp. where a single specimen was caught. One sediment replicate per station was analysed. Sample preparation for bulk stable isotope analysis (SIA) was performed as described in Kazanidis and Witte, 2016.
Because inorganic carbon is highly enriched in δ13C compared to organic carbon (Jaschinski, 2008), fauna and sediment samples for the δ13C analysis were treated with hydrochloric acid to remove carbonate structures. Since repeated acidification treatment is known to affect the variability of δ15N values (Vafeiadou et al., 2013), each sample of faunal tissue and sediment was divided in two groups. The first group (δ15N) was not acidified and the second group (δ13C) was acidified through the sequential addition of 15 µL of 1 M hydrochloric acid, until the effervescence had stopped indicating the inorganic structures were removed (Kazanidis and Witte, 2016). Due to the limited amount of gut content dry masses, samples could not be divided in two groups to perform both δ13C and δ15N measurements. Since δ15N measurements on gut contents were not relevant in our study, we decided to acidify the samples to obtain δ13C values of gut contents. All samples were then dried at 60°C overnight prior to isotope measurements. The samples were analysed for 13C and 15N isotopes at the James Hutton Institute (Aberdeen, UK) using a Flash EA 1112 Series Elemental Analyser connected via a Conflo III to a DeltaPlus XP isotope ratio mass spectrometer (all Thermo Finnigan, Bremen, Germany). The isotope ratios were traceable to International Atomic Energy Agency reference materials USGS40 and USGS41 (both L-glutamic acid); certified both for δ13C (‰VPDB) and δ15N (‰air N2). Long term precisions for a quality control standard (milled flour) were: δ13C -25.48 ± 0.21 ‰ and δ15N 1.72 ± 0.41 ‰ (mean ± sd, n = 200).
2.3.1 Trophic level calculation
The trophic level (TL) of each megafauna was calculated using δ15N values from samples and from a primary consumer as a trophic baseline. M. thraciaeformis was used as trophic baseline since this species displayed the lowest δ15N values and has been shown to belong to the 2nd TL (Table 2). The following equation was used:
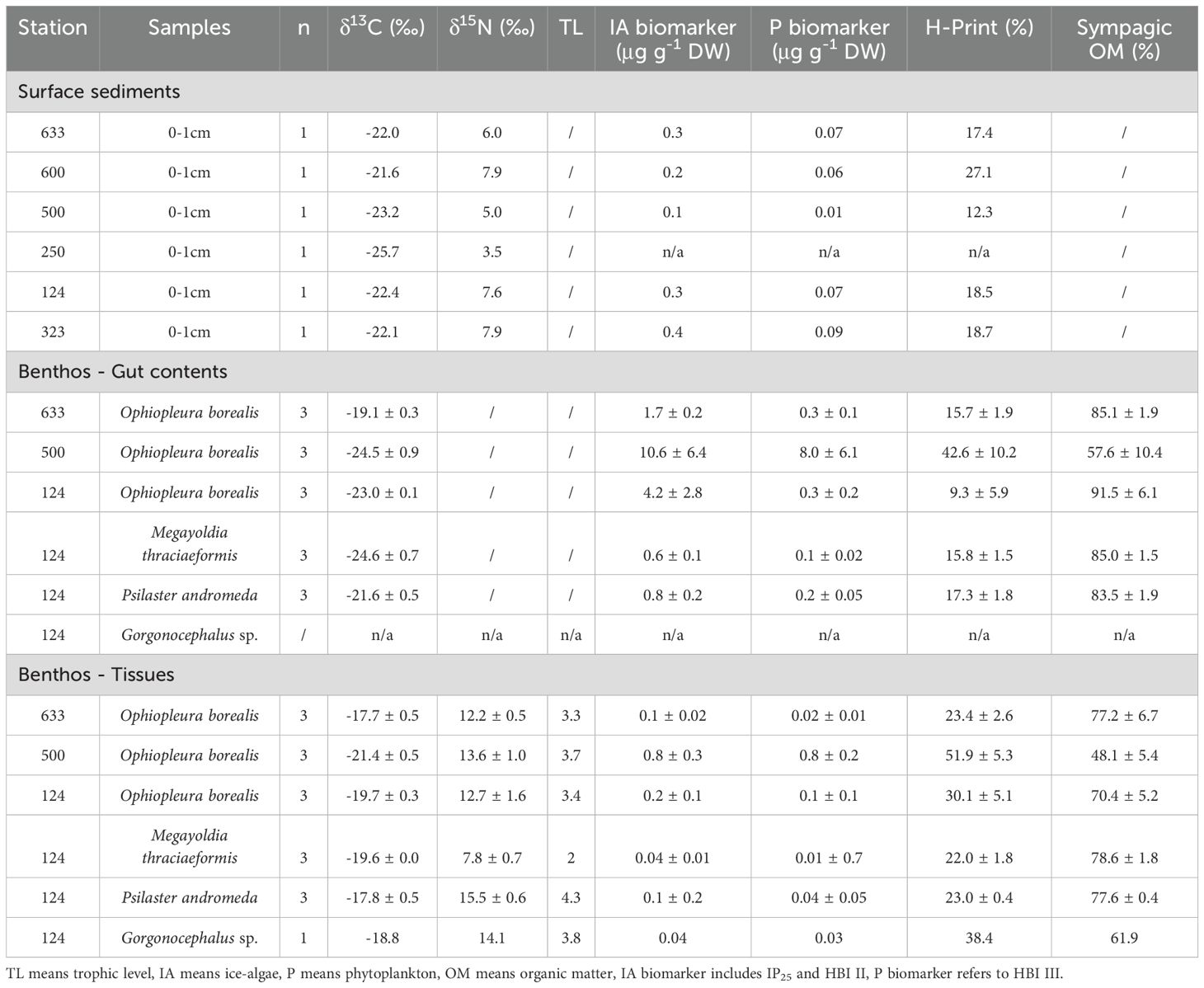
Table 2. Stable isotope and HBI data of surface sediments (0-1cm) and megabenthos from 6 different locations across the Canadian Arctic.
where 3.4‰ is the commonly used trophic shift factor for δ15N between successive trophic levels (Iken et al., 2010).
2.4 Highly branched isoprenoid (HBI) analysis and carbon source quantification
Highly branched isoprenoids (HBIs) in sediments and megafauna specimens were analysed at the School of Geography Earth and Environmental Science (Plymouth, UK) following the protocols by Belt et al. (2012) and Brown and Belt (2012b). Briefly, once the megafauna and sediment samples were freeze-dried (0.2mbar, -45°C, 72h) and homogenized with mortar and pestle, an internal standard (9-octyl-heptadec-9-ene (9-OHD); 0.02 µg) was added to permit the quantification of IP25 and other HBIs according to Belt et al. (2012). Samples were saponified in a methanolic KOH solution (~ 4 mL H2O:MeOH, 1:9; 20% KOH) for 60 min (80°C). HBIs were extracted subsequently with hexane and fractionated using column chromatography with silica gel. HBIs were analyzed by gas chromatography-mass spectrometry and quantified by comparing the mass spectral intensities of molecular ions to that of the internal standard and normalizing for differences in mass spectral fragmentation efficiency and mass sampled (Brown et al., 2017).
To quantify the contribution of sea ice-algae, the “H-Print” index combining both sympagic (IP25, HBI II) and pelagic (HBI III) biomarkers was calculated combining the analytical intensities of three HBI biomarkers IP25 (m/z 350.3), II (m/z 348.3), and III (m/z 346.3), into a single index, according to Brown et al. (2017):
High H-Print values (>50%) are associated with greater contribution of pelagic organic carbon, and low values (<50%) are associated with a greater contribution of sympagic organic carbon. Then, sympagic organic matter proportion was measured, following a previous H-Print calibration (r2 = 0.97, p-value < 0.01; Brown et al., 2017):
In contrast to the H-Print index, this index is used to reflect the sea ice carbon rather than pelagic carbon, with high values indicative of a higher proportion of sea ice derived organic carbon. However, since this index was calculated from feeding experiments, we retained the H-Print index for the sediments.
Biomarker concentrations were calculated following the method used by Müller et al. (2011). Briefly, biomarker concentrations were calculated based on their individual GC-MS responses compared with those of respective internal standards and corrected to the amount of extracted sediments or faunal sample (gut content or tissue). Quantities are expressed in μg g-1 DW. Based on the concentration of the biomarkers IP25, HBI II and HBI III measured, we quantified the relative concentration of ice algae (IP25 + II) and phytoplankton (III) in each sample (Table 2). To better reflect the selective consumption of a specific food source by organisms, we measured the concentration ratios of ice algae and phytoplankton biomarkers found in the gut contents relatively with the sediments (IAGC: IAsed and PGC: Psed;Figure 2), with high values indicative of a strong and selective consumption of organic matter. Ratios were presented on a logarithmic scale due to better visualize the differences (Figure 2).
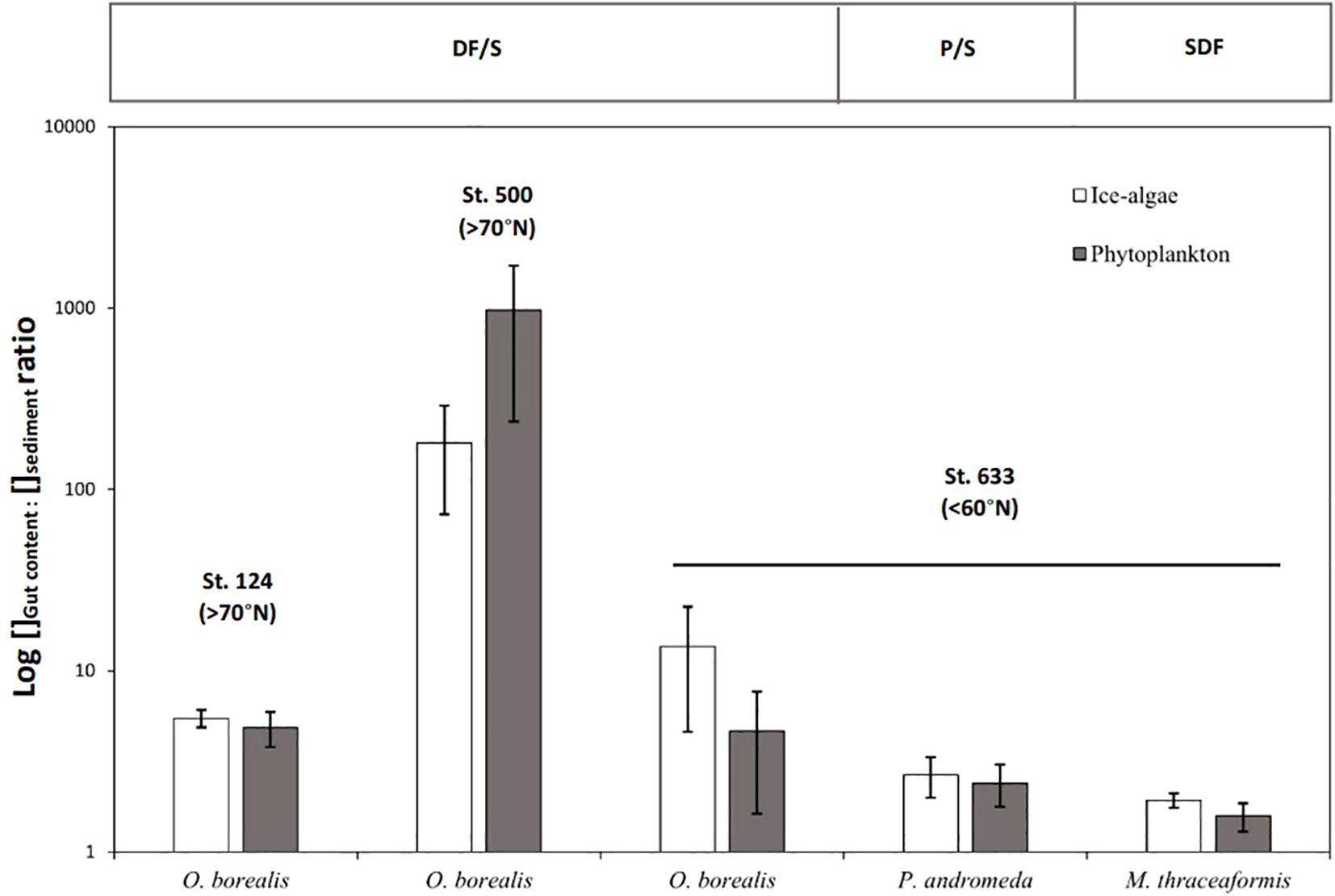
Figure 2. Ice algae and phytoplankton biomarker concentration ratios (w:w) between gut contents and sediments across different station in the Canadian Arctic. DF/S means deposit feeder/suspension feeder, P/S means predator/scavenger and SDF surface deposit feeder.
2.5 Statistical analyses
All statistical analyses were performed on R version 4.2.2 (https://www.r-project.org/). Data were normally distributed with equal variances. A significance level of α = 0.05 was used for all tests. For each analysis, differences in the concentration and relative contribution between ice algae and phytoplankton were tested using an independent sample t-test. The Kolmogorov Smirnov test was used to test whether the H-Prints between the gut contents and the tissues of the megafauna differed from each other. Correlation of pigment concentrations and duration for which the area was ice-free was performed using Pearson’s correlation.
3 Results
3.1 Sediments
Total pigment concentration in the sediments (Chl a + phaeopigments) varied from 20.7 to 81.0 mg DW m-2 (Figure 1; Table 1). The lowest total pigment concentrations were observed for stations 500 and 250 characterized by lasting landfast sea ice, with 20.7 and 24.6 mg DW m-2, respectively, while the highest values were measured at stations 124 and 323, located in the polynyas, with 81.0 and 77.4 mg DW m-2, respectively (Table 1). Total pigment concentrations varied significantly among subregions and were positively correlated with the duration of ice-free periods before the sampling (r2 = 0.95, p-value = 0.013). Carbon stable isotope ratios (δ13C) in the sediments covered a relatively narrow range (from -25.7 and -21.6‰) (Table 2). At stations in the lasting landfast sea ice, δ13C of sediments were more depleted (≤ -23‰) than stations in the polynyas and in the seasonal landfast sea ice (≥-22‰). HBI biomarkers indicated that all surface sediments contained both sympagic (IP25, IIb) and pelagic algae (III) (Table 2). Overall, the relative composition of HBIs in the sediments was strongly dominated by ice algae (72.9% to 87.7%) (Table 2). Sediment from the station 500 exhibited the lowest H-Print value (12.3%) but the lowest ice algae and phytoplankton biomarker concentration (0.06 and 0.01 µg g-1 DW, respectively). At the other stations, ice algae and phytoplankton biomarker concentrations oscillated between 0.2 and 0.4 µg g-1 DW and 0.06 and 0.09 µg g-1 DW, respectively (Table 2).
3.2 Benthos
Carbon stable isotope ratios in megafauna tissues covered a narrow range (from -17.7 ± 0.5 to -21.4 ± 0.5 ‰; Figure 3). Based on δ15N values obtained from the tissues, the subsurface deposit feeder Megayoldia thraciaeformis was part of TL 2 while the deposit feeder Ophiopleura borealis and the suspension feeder Gorgonocephalus sp. were part of the TL 3 (Table 2). The predator/scavenger Psilaster andromeda belonged to TL 4 (Table 2). Overall, δ13C values in the gut contents were depleted by 3.3 ± 1.3 compared to the tissues (Figure 3; Table 2). The mean δ13C values measured in the tissues were significantly lower than those measured in the gut contents (Two sample t-test, p-value < 0.05).
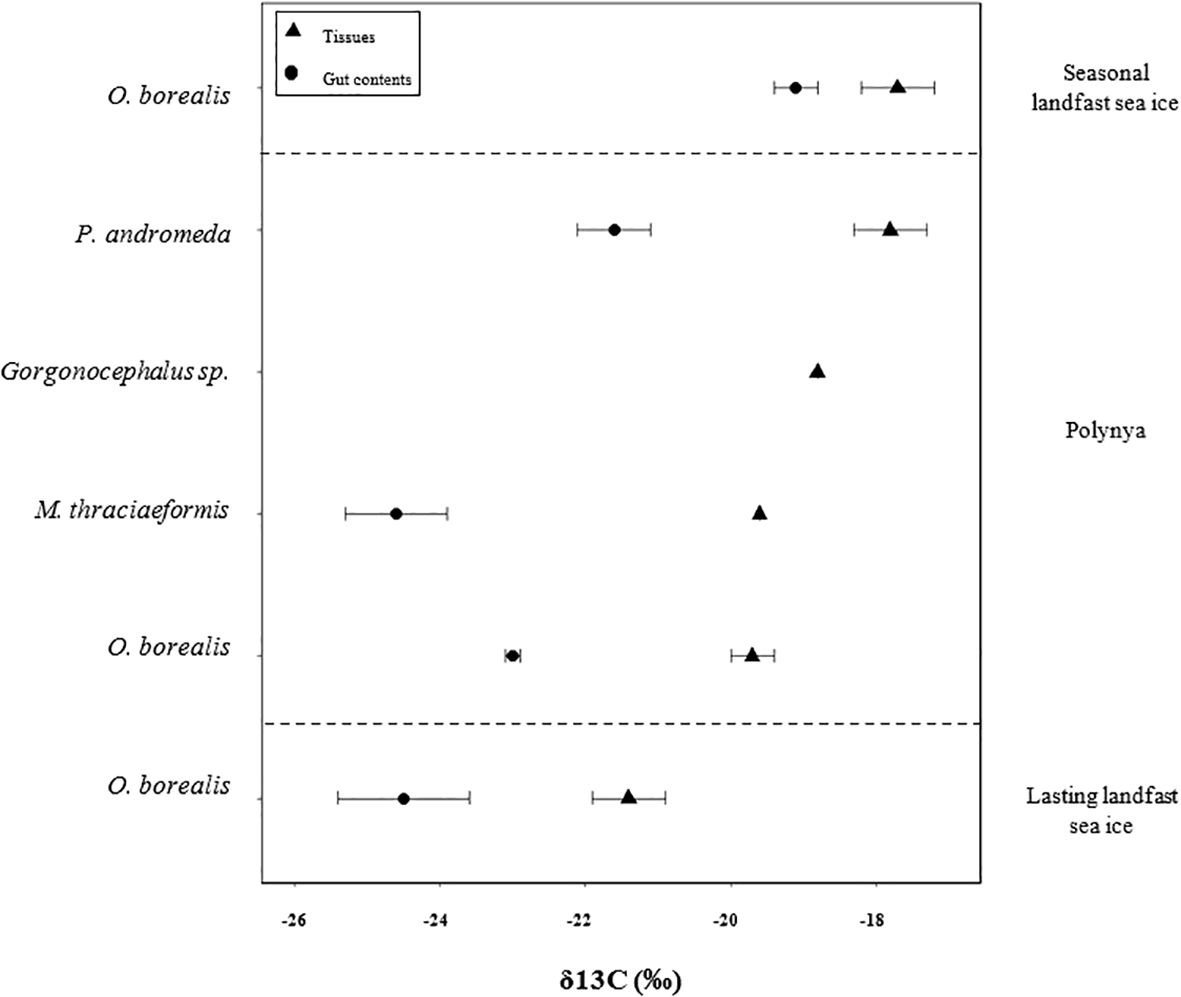
Figure 3. Carbon isotopic ratios of the tissues and the gut contents of the megafauna collected in the Canadian Arctic in 2013 from different sub-regions characterized by different sea ice conditions: lasting landfast sea ice (station 500), polynya (station 124) and seasonal landfast sea ice (station 633). O. borealis, Ophiopleura borealis; P. andromeda, Psilaster andromeda; Gorgonocephalus sp., Gorgonocephalus species; M. thraciaeformis, Megayoldia thraciaeformis.
Similar to the sediments, HBI biomarkers indicated that megafauna contained both ice algae and phytoplankton. Overall, the relative composition of HBIs in the megafauna gut contents and tissues was dominated by ice algae biomarkers (average of 80.5 ± 4.4 and 69.0 ± 3.0%, respectively; Table 2), except for the tissues of O. borealis specimens at station 500 for which phytoplankton biomarker dominated the composition (51.9 ± 5.3%). No significant differences in the relative proportion of each biomarker (IP25, HBI II and HBI III) between the tissues and gut contents were observed (Kolmogorov-Smirnov test, p-value > 0.68 in all five cases), but the H-Prints measured in the gut contents were significantly lower than those measured in the tissues (Two sample t-test, p-value < 0.05 in all five cases).
Overall, whether it was in the gut contents or in the tissues, almost all species exhibited significantly more ice algae than phytoplankton biomarker concentrations (Two sample t-test, p-value < 0.05), except for Ophiopleura borealis at station 500 and Megayoldia thraciaeformis at station 124 (Table 2). In addition, the biomarker concentrations (ice algae and phytoplankton), were significantly higher in the gut contents than in the tissues of all the species (Two sample t-test, p-value < 0.05). The deposit feeder Ophiopleura borealis displayed higher biomarker concentrations of ice algae and phytoplankton in their gut contents (4.2 ± 2.8 and 0.3 ± 0.2 µg g-1 DW, respectively) than the predator Psilaster andromeda (0.8 ± 0.2 and 0.2 ± 0.1 µg g-1 DW) and the subsurface deposit feeder Megayoldia thraceaformis (0.6 ± 0.1 and 0.1 ± 0.1 µg g-1 DW).
For both ice algae and phytoplankton biomarkers, concentration in the sediments were very low compared to those measured in the gut contents of the specimens (Figure 2). Ratios of the ice algae and phytoplankton biomarkers concentrations found in the gut contents compared with the sediments (IAgc: IAsed and Pgc: Psed) were all above 1.5 and reached up to 180 and 975 in Ophiopleura borealis in station 500, respectively (Figure 2). For each species, both ratios did not vary significantly (Two sample t-test, p > 0.10 in all 5 cases).
4 Discussion
4.1 Deposition of ice algae on the seafloor and its subsequent assimilation by arctic megabenthic fauna: importance of ice algae as a carbon source
4.1.1 Availability of ice algae on the seafloor
In marine environments, tracer methods are commonly used to differentiate the sources of organic matter sinking from the water column to the seafloor and their transfer in benthic food webs (Brown and Belt, 2012b; Kelly and Scheibling, 2012; Peterson, 1999). The dual biochemical approach (HBI analysis and SIA), used in this paper, highlighted that ice algae accounted for a relatively high proportion in organic matter deposited on the seafloor (Figure 4; Table 2).
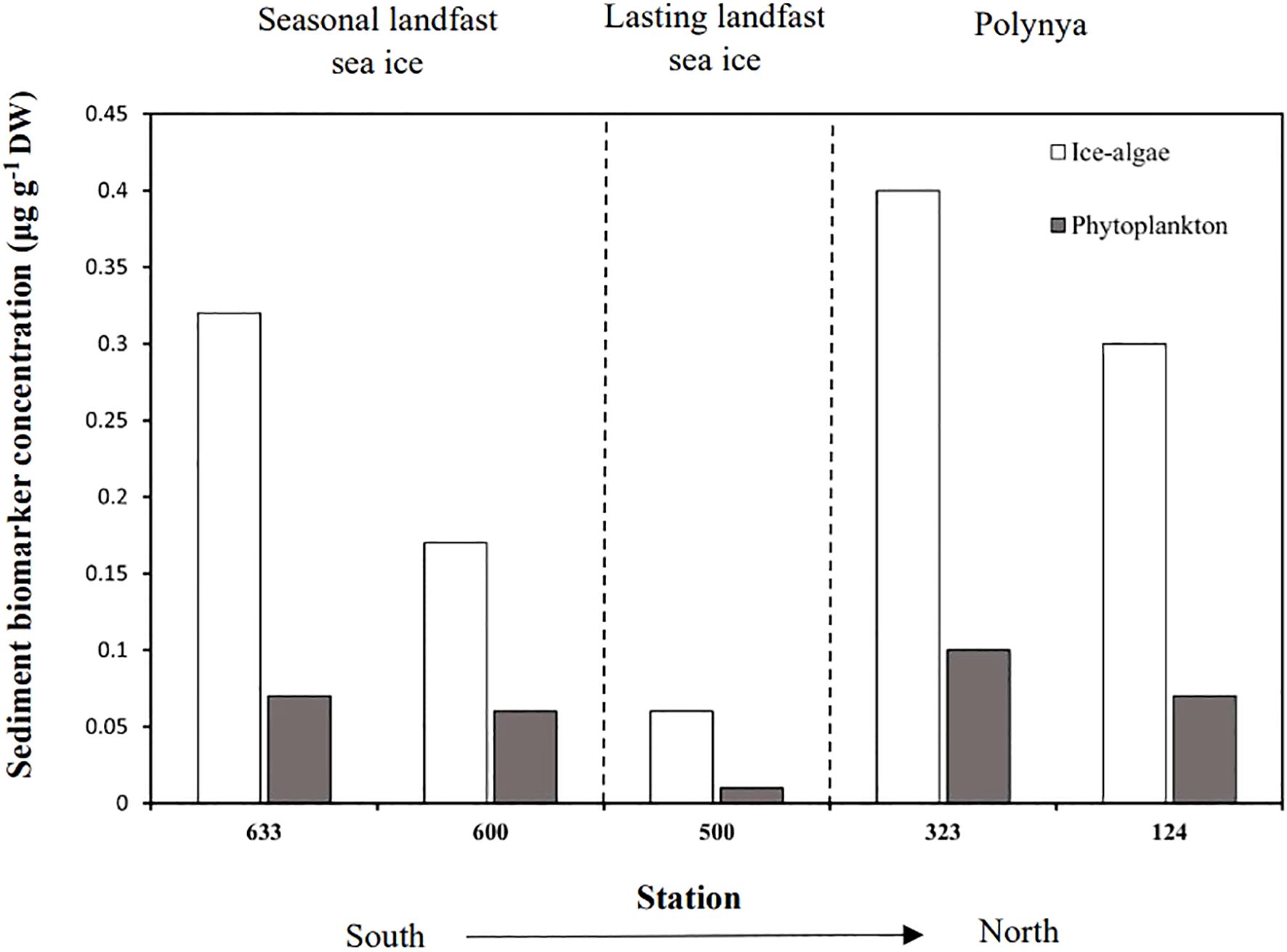
Figure 4. Concentration of sea ice-algae and phytoplankton biomarkers in the sediments (0-1cm) according to sea ice conditions, from south to north.
It has emerged that sediment HBI composition is similar to that of sea-ice (i.e. IP25 and HBI II dominated the composition), indicating high vertical export of sea ice algae to the seafloor (Brown et al., 2014b). The isotopic values of the sediments support this result, as we found the most enriched δ13C, suggesting a higher contribution of ice algae relatively to phytoplankton, in the stations where the highest amounts ice algae biomarkers were measured (Table 2). Similar patterns in the HBI composition were previously observed in the sediments of Baffin Bay (Yunda-Guarin et al., 2020) and the Amundsen Gulf (Brown and Belt, 2012b).
Moreover, the distribution of ice algae and phytoplankton sediment biomarkers were closely related to the duration for which the areas were ice-free. Sympagic (IP25, HBI II) and pelagic (HBI III) HBIs in the sediments showed highest concentrations at the stations located in the polynyas (in Lancaster Sound and NOW), where sea ice break-up occurred earlier in the season. Our results are in accordance with the high net pelagic and sympagic primary production and vertical fluxes previously observed in these regions (Deming et al., 2002; Harning et al., 2022; Kolling et al., 2020). On the opposite, the stations in the Nares Strait and Viscount Melville, characterized by lasting landfast sea ice, displayed the lowest biomarker concentrations in the sediments. In addition, the spatial distribution of H-Print in surface sediments followed a latitudinal gradient, with sympagic carbon contribution increasing towards the northernmost stations, independently of their depths.
Similar biomarkers studies showed how latitudinal patterns in sea ice conditions influenced the abundance and distribution of HBI lipids in the sediments in different Arctic regions (Harning et al., 2022; Koch et al., 2020b; Navarro-Rodriguez et al., 2013). In Baffin Bay, Stoynova et al. (2013) and Yunda-Guarin et al. (2020) observed diminishing abundances of IP25 in the sediments in areas with less sea ice cover. Our results show similar trends, indicating that sea ice cover has a significant influence on the availability of sympagic carbon on the seafloor.
4.1.2 Resource utilisation by megabenthic fauna
The organic matter produced in the euphotic zone represents a potential food source for the benthos and can be consumed before or after deposition according to the feeding guilds of organisms. By separating the gut contents from the tissues, we were able to depict the amount of food recently ingested by organisms, i.e., the short-term assimilation.
Whether it be ice algae or phytoplankton, biomarker concentrations in the sediments were very low compared to those measured in the gut contents of benthic organisms. IAgc: IAsed and Pgc: Psed ratios were all above 1.5 and reached up to 180 and 975 respectively, suggesting a selective consumption of freshly deposited organic matter (Figure 2). These findings are in agreement with previous observations in the Central Arctic from Boetius et al. (2013), who observed direct grazing on algal deposits and found higher concentrations of pigments in holothuroid guts rather than in bare sediments or even in algal deposits.
Overall, the detection of biomarkers IP25 and HBI II in the gut contents of all the specimens provides evidence for the ingestion of ice algae, directly or through primary consumers. While we could not attribute δ13C values to a specific carbon source, we could still detect the influence of sympagic carbon on these ratios in the same way as for sediments, with highest δ13C in the samples where the highest amounts of ice algae biomarkers were measured. The biomarker concentration as well as relative contribution of ice algae ingested by organisms were higher than those of phytoplankton (excepted for Ophiopleura borealis specimens located at the lasting ice cover station), with sympagic carbon reaching up to 91% (Table 2), highlighting an important consumption of ice algae by the megabenthic fauna. Similarly, the relative contribution of ice algae measured in the tissues, which represents the mid/long-term assimilation, confirms the importance of ice algae as a carbon source in the diet of benthic organisms and corroborated previous findings. Indeed, direct or undirect assimilation (i.e., via feeding on primary consumers) of ice algae-derived carbon by benthic organisms belonging to multiple taxa or feeding guilds were previously revealed (Brown and Belt, 2012b; McMahon et al., 2006). Brown and Belt 2012b showed that the H-Print of benthic organisms is similar to that of ice algae, that confirms the high export of ice algae on the seafloor and that the benthos relies mostly on this carbon source for their energy needs.
Interestingly, the δ13C values measured in the gut contents and in the tissues did not show the same pattern than the HBI analysis (H-Print and biomarker concentrations). While the δ13C values indicated a stronger contribution of ice algae in the tissues relative to the gut contents, the HBI results indicated the opposite (i.e., stronger contribution and concentration of ice algae in the gut contents than in the tissues) (Figure 3, Table 2). Previous studies that used the same biochemical approach (HBI + SIA) have proven similar results between the two methods, indicating that this technique is supposed to be suitable for the study of benthic food web structure (Amiraux et al., 2023; Yunda-Guarin et al., 2020). The discrepancy observed in our results could be due to different turnover rates between the different tracers (HBIs and stable isotopes). Indeed, previous studies that looked at stable isotope turnover rates showed different turnover rates in different animal tissues (up to 64 days) (Heady and Moore, 2013; Kaufman et al., 2008; Tieszen et al., 1983; Zanden et al., 2015). On the other hand, there is very little information on the turnover rates of HBIs. Only one study have investigated it and found a turnover rate of approximately 1 month (Koch et al., 2020a). In addition, short residence time, from days to weeks, of HBIs in various consumer tissues have been suggested from previous studies, independently of their trophic level (Brown and Belt, 2012b, 2012a). Hence, considering that the main food supply occurs at the sea ice break-up (i.e. before ice-free) and assuming the potential difference in turnover rates between HBIs and stable isotopes, our results are coherent with the duration for which the areas were ice-free, at least for the landfast ice and polynya stations.
Based on the amount of ice algae and phytoplankton biomarkers found in the sediments (more ice algae than phytoplankton in all the stations; Figure 4), the fact that ratios (IAgc: IAsed and Pgc: Psed) did not vary significantly for all species and the significant differences observed in the H-Prints/biomarker concentrations between the gut contents and the tissues, our results suggest that the megabenthos do not show any preferential feeding for ice algae, but rather consumes what is most abundant in their close environment.
Our findings are comparable to similar studies conducted in the Pacific Arctic that showed that sympagic production can provide the benthos a high fraction of carbon but is highly variable and mainly dependant on availability and patchiness of sympagic organic matter deposition on the seafloor (Cautain et al., 2022). Therefore, we suggest that the high proportions of sympagic organic material found in the tissues and the gut contents of benthic consumers is mainly due to the time of the sampling, which occurred during or shortly after the sea ice retreat and probably coincided with the maximum availability and uptake of ice algae by benthic consumers.
4.2 Change in the resource utilisation (in terms of quantity and sources) according to the feeding strategies of benthic fauna in polynyas
To acquire food, benthos owns a wide range of feeding strategies adapted to the variety of food sources available. Therefore, differences in the resource utilisation among feeding guilds are expected. A previous study in the Amundsen Gulf by Brown and Belt (2012b) found IP25 in different proportions in different taxa (Arthropoda, Mollusca, Echinoderm, Chordata) exhibiting different feeding strategies. Here, we decided to compare the resource utilisation among various feeding types within polynyas since these areas (i.e. early open water) are very productive, host high biodiversity and might become more common in the future.
Even if ice algae seem to be the dominant source of carbon for the benthos, some differences were perceptible in terms of resource utilisation (quantity and quality) according to feeding types. Inter-species variabilities in HBI concentrations and carbon isotopic ratios are likely linked to the feeding type or the way of life of organisms. Overall, the highest ice algae and phytoplankton biomarker concentrations in the polynya were observed for the deposit feeder Ophiopleura borealis (Table 2). Ophiopleura borealis is a mobile megafauna compared to Megayoldia thraciaeformis which is a burrow dwelling organism, thus the latter had potentially less access to food (Gallagher et al., 1998).
The importance of ice algae to the predator Psilaster andromeda (TL=4) is not clear based on our results. The low biomarker concentrations of ice algae and phytoplankton observed in its gut contents could be attributed to indirect ingestion of ice algae-derived organic matter (i.e., via feeding on primary consumers). Interestingly, this species exhibited similar contribution of ice algae (77.6 ± 0.4%) in tissues compared to the primary consumers (lowest δ15N values) Ophiopleura borealis (77.2 ± 6.7%) and the deposit feeders Megayoldia thraciaeformis (78.6 ± 1.8%) (Table 2). Although this could be due to a potential bioaccumulation of ice algae through the food chain, previous studies concluded that HBI do not bioaccumulate in higher trophic level and thus represent seasonal variations (Brown et al., 2014a, 2017, 2018). Additionally, a previous HBI III depuration experiment made by Koch et al. (2020a) showed that HBI III signal may represent the accumulation over the course of approximately 1 month prior to sampling. Since IP25 specific depuration rates are still unavailable, if we consider that depuration rates for IP25 and HBI III are similar, these findings suggest that the sympagic carbon contribution found in Psilaster Andromeda is due to the indirect-ingestion of ice algae-derived organic matter via feeding on primary consumers, indicating also that this species has a rapid metabolic turnover rate. This would also suggest the study of Psilaster Andromeda gut contents and/or tissues as reliable proxies reflecting current seasonal primary production.
On the other hand, the suspension feeder Gorgonocephalus sp. seems to rely more on open water organic matter (sympagic carbon = 61.89%; δ13C = -18.8‰, Table 2), compared to the other feeding guilds which is coherent with previous studies. Indeed, it has been shown that deposit feeders or grazers assimilate ice algae better than suspension feeders (Koch et al., 2020a; McMahon et al., 2006; Schollmeier et al., 2018). Nonetheless, we assume that the ingestion of phytoplankton was indirect (via primary consumers) since this species is known to feed on zooplankton. This is supported by the fact that zooplankton H-Print can be very close to the one of pelagic organic matter when they feed on it (Brown et al., 2014b) and the trophic position to which Gorgonocephalus sp. belongs to (TL=4). However, our results must interpreted with caution considering the low sample size (n=1).
4.3 Influence of sea ice conditions on the vertical export of organic matter and its subsequent assimilation by the echinoderm Ophiopleura borealis
In the Arctic, especially in shelf regions, the sympagic-pelagic-benthic coupling is particularly tight, so physical and biological properties of sea ice and water column are important factors controlling the benthic ecosystem functioning (Søreide et al., 2013). For instance, significant differences in sediments characteristics were observed among subregions (i.e. polynya, seasonal sea ice cover and lasting sea ice cover areas) (Table 2) and can be related to area’s specificities such as primary production rates, standing stocks, processes occurring in the water column and timing of sea ice break-up. Both stations within the polynyas exhibited the highest pigment as well as IP25 concentrations in sediments (Figure 1; Table 2), that is in accordance with the particularly high net primary production (Tremblay et al., 2006) and vertical fluxes previously measured in the NOW (Smith and Barber, 2007).
Depending on the region, sympagic production contributes differently to total annual net primary production (ice algae + phytoplankton). In Arctic shelves, ice algae represent a variable and relatively low contributor to total annual net primary production on Arctic shelves relatively to phytoplankton (1% - 26%; Legendre et al., 1992; Niemi et al., 2024; Payne et al., 2021), while in the central Arctic Ocean, sympagic primary production can contribute up to 50% of the annual primary production (Fernández-Méndez et al., 2015; Gosselin et al., 1997). However, in Arctic shelves, the contribution of sympagic production to total primary production can be significantly higher when ice algal blooms occur prior to an under-ice or spring phytoplankton bloom (Gradinger, 2009; Hegseth and von Quillfeldt, 2022; Niemi et al., 2024). Most of this production is exported in the water column due to a low grazing pressure and subsequently to the seafloor (Michel et al., 2002) while phytoplankton bloom occurring in June seems mainly consumed in the euphotic zone which results in a lower downward export of phytoplankton (Klein et al., 2002; Tremblay et al., 2006). The high (low) vertical export of ice algae (phytoplankton) is well reflected in our results in the benthic compartment, either within the sediment characteristics or megafauna (Tables 1, 2).
Ophiopleura borealis H-Print and δ13C measured in the tissues and in the gut contents indicated an important contribution of ice algae in the diet of this species (Table 2). We can assume that the downward export of organic matter must have been substantial and formed a stock of food on the seafloor to benthic organisms since high contents of ice algae and phytoplankton were still detected in the ophiuroid guts, even after 79 ice free days (Table 1). In a similar manner to those from the polynyas, Ophiopleura borealis collected at the seasonally landfast sea ice station exhibited a relatively high contribution of ice algae in their guts and tissues that shows they also rely much on ice algae to cover their energy needs. However, biomarker concentrations of both ice algae and phytoplankton ingested by the ophiuroids were lower (Table 2), the same way as total pigment concentrations in the sediments (60.6 mg DW m2, Table 1), suggesting a moderate vertical export of organic matter and/or a rapid consumption of the fresh settled organic matter by organisms as the ice break-up took place one month ago.
Contrary to the ophiuroids from the two other sea-ice conditions which relied more on ice algae, specimens from Viscount Melville Sound, area with lasting landfast sea ice cover, seemed to show no strong preference for ice algae or phytoplankton. Indeed, measurements of the gut contents (δ13C = -24.5 ± 0.9 ‰; sympagic carbon = 57.6 ± 10.4%, Table 2) and the tissues (δ13C = -21.4 ± 0.5 ‰; sympagic carbon = 48.1 ± 5.4%, Table 2) did not indicate any obvious dependence on ice algae and phytoplankton, compared to the ophiuroids in the polynya and under seasonal sea ice conditions (Table 2). Despite the high ice algae primary productivity rates measured in the landfast ice of the Canadian Archipelago (average of 48 mg m-2 of sympagic chlorophyll a; Leu et al., 2015), this area is known for its weak sympagic-benthic-coupling (i.e. low vertical export) and this is reflected by the very low total pigment and IP25 concentrations in the sediments (Table 2). Similar range of IP25 concentrations were previously measured by Belt et al. (2013) with low values in Viscount Melville Sound (0.02 µg g-1 DW) compared to those in the NOW or Lancaster Sound polynyas (0.1 and 0.15 µg g-1 DW, respectively). This decoupling is likely caused by a pelagic biodiversity particularly rich, resulting an in important grazing of organic matter, and an active transfer of organic matter in the pelagic food webs.
Ophiuroids are able to adapt to food available and to shift their feeding type among a broad range that they hold (suspension-feeding, deposit-feeding, scavenging and predation) all designed for a specific source of organic matter, and some species use more than one feeding strategy (Stöhr et al., 2012). Ophiopleura borealis, which is usually considered as deposit feeder/scavenger, may have changed its feeding strategy to suspension feeding to adapt to the food available, here mainly plankton derived organic matter. Interestingly, despite the apparent low food supply to the seafloor at station 500 (0.06 and 0.01 µg g-1 DW of ice algae and phytoplankton biomarkers, respectively), ice algae and phytoplankton biomarker concentrations in ophiuroids gut contents were particularly high, up to 2.5 and 11.8 times more than concentrations found in those of ophiuroids from polynyas and under seasonal ice cover, respectively (Figure 2). This discrepancy between sediment and gut contents biomarker concentrations could be caused by the patchiness of organic matter on the seafloor, as it has been commonly observed for ice algae underneath the sea ice (Fernández-Méndez et al., 2014; Gosselin et al., 1997) and subsequently on the seafloor after sinking (i.e., ophiuroids are attracted by large sea ice algae patches of 1 to 50cm in diameter) (Boetius et al., 2013; Rybakova et al., 2019) and/or by a rapid response of megafauna to a recent deposit of fresh organic matter.
Our results are in agreement with a previous study conducted by Yunda-Guarin et al. (2022) with three ophiuroid species (including Ophiopleura borealis) in the Canadian Arctic, showing that differences in sea ice cover drove the variability of δ13C of ophiuroids among regions i.e., individuals under greater sea ice concentration were more depleted in δ13C. Along with other studies conducted in East Antarctica (Clark et al., 2015; Michel et al., 2019), our results suggest that ophiuroids are able to adapt their feeding habits in response to a change in sea ice cover and subsequent primary production and highlights the key role of sea ice cover in shaping the food web structure.
In addition, at the time of the sampling, the station was not totally ice-free (Table 1), meaning that the sampling took place during or shortly after the start of the sea ice algae bloom, suggesting that the ophiuroids are able to respond very rapidly and efficiently to a pulse of fresh organic matter.
5 Summary and conclusion
The objective of this study was to examine the importance of both main primary producers in the diet of megabenthic organisms in the Canadian Arctic. First of all, we highlighted different trends of resources utilisation (quality and quantity) by megabenthic organisms from various feeding types and under different sea ice conditions. Overall, deposit/scavenger (excepting those from lasting ice cover station), subsurface deposit feeder and predator showed dominance feeding on ice algae whereas Gorgonocephalus sp. displayed a higher relative contribution of phytoplankton in its tissue, in line with its feeding guild (suspension feeder feeding on zooplankton). Secondly, the high contents of ice algae and phytoplankton biomarkers in guts contents of organisms in comparison to those in sediments indicate a selective feeding and a rapid response of megafauna to a pulse of fresh organic matter, independently of the source. Thirdly, the spatial variabilities between ice cover conditions regarding the quantity of organic matter ingested by brittle stars were likely related to processes occurring in the water column (primary production, grazing) and timing of ice break-up. The area’s specificities were also mirrored in the relative contribution of sources in the diet of benthic organisms. In seasonal ice cover and polynya areas, ice-algae is the predominant carbon source for Ophiuroids while under lasting ice cover, they rely mainly on pelagic derived organic matter as food source. Finally, this study highlighted the ability of benthic organisms (e.g., Ophiopleura borealis) to adapt to the type of food available (shift from deposit/scavenger to suspension feeding on zooplankton).
Therefore, even if ice algae represent a significant proportion of organic matter available to the seafloor over spring/summer, the predicted decline in sea ice cover – and consequent sympagic production – will not necessarily be detrimental to different species in the same way. Despite the low sample size used in this study, we provided the first comparison of the short-term ingestion versus the long-term assimilation of ice algae and phytoplankton using biochemical tracer methods in megabenthic organisms. In view of the predicted alterations in timing, quantity and quality of the organic matter that reach the seafloor in the Arctic, it seems necessary to extend studies on the importance of ice algae and phytoplankton in the diet of benthic organisms to better understand the resilience of the benthic ecosystems.
Data availability statement
The datasets generated for this study are available on request to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because our research was not conducted on cephalopods.
Author contributions
TC: Writing – original draft. UW: Resources, Supervision, Validation, Writing – review & editing. TB: Methodology, Writing – review & editing. PA: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The shiptime in 2013 was funded by the Network of Centres of Excellence of Canada, ArcticNet. Additional funding was also received from the Natural Environment Research Council (NERC) (Grant Number. NE/J023094/1).
Acknowledgments
We are most grateful to the captain, the officers and the crew of the research ice-breaker CCGS Amundsen for their support during the expedition. We thank Solveig Bourgeois for the time she spent on research ice-breaker CCGS Amundsen collecting and analysing the samples. We are also grateful to Rémi Amiraux from Université Laval for the helpful discussions and appreciative reviews. We would also like to thank our reviewers for their helpful comments. This is a contribution to the research programs of Québec-Océan and Takuvik.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amiraux R., Archambault P., Moriceau B., Lemire M., Babin M., Memery L., et al. (2021). Efficiency of sympagic-benthic coupling revealed by analyses of n-3 fatty acids, IP25 and other highly branched isoprenoids in two filter-feeding Arctic benthic molluscs : Mya truncata and Serripes groenlandicus. Organic Geochemistry 151, 104160. doi: 10.1016/j.orggeochem.2020.104160
Amiraux R., Mundy C. J., Pierrejean M., Niemi A., Hedges K. J., Brown T. A., et al. (2023). Tracing carbon flow and trophic structure of a coastal Arctic marine food web using highly branched isoprenoids and carbon, nitrogen and sulfur stable isotopes. Ecol. Indic. 147, 109938. doi: 10.1016/j.ecolind.2023.109938
Arrigo K. R. (2007). Physical control of primary production in Arctic and Antarctic polynyas. In Smith W. O. and Barber D. G.. (eds), Polynyas: Windows to the world, Elsevier Oceanography Series, (Vol. 74, pp. 223–238). Amsterdam, The Netherlands: Elsevier. doi: 10.1016/S0422-9894(06)74007-7
Arrigo K. R. and van Dijken G. L. (2015). Continued increases in Arctic Ocean primary production. Prog. Oceanography 136, 60−70. doi: 10.1016/j.pocean.2015.05.002
Belt S. T. (2018). Source-specific biomarkers as proxies for Arctic and Antarctic sea ice. Organic Geochemistry 125, 277−298. doi: 10.1016/j.orggeochem.2018.10.002
Belt S. T., Brown T. A., Ringrose A. E., Cabedo-Sanz P., Mundy C. J., Gosselin M., et al. (2013). Quantitative measurement of the sea ice diatom biomarker IP25 and sterols in Arctic sea ice and underlying sediments : Further considerations for palaeo sea ice reconstruction. Organic Geochemistry 62, 33−45. doi: 10.1016/j.orggeochem.2013.07.002
Belt S. T., Brown T. A., Rodriguez A. N., Sanz P. C., Tonkin A., and Ingle R. (2012). A reproducible method for the extraction, identification and quantification of the Arctic sea ice proxy IP 25 from marine sediments. Analytical Methods 4, 705−713. doi: 10.1039/c2ay05728j
Belt S. T., Massé G., Rowland S. J., Poulin M., Michel C., and LeBlanc B. (2007). A novel chemical fossil of palaeo sea ice: IP25. Organic Geochemistry 38, 16−27. doi: 10.1016/j.orggeochem.2006.09.013
Belt S. T., Massé G., Vare L. L., Rowland S. J., Poulin M., Sicre M.-A., et al. (2008). Distinctive 13C isotopic signature distinguishes a novel sea ice biomarker in Arctic sediments and sediment traps. Marine Chem. 112, 158−167. doi: 10.1016/j.marchem.2008.09.002
Bluhm B. A. and Gradinger R. (2008). Regional variability in food availability for arctic marine mammals. Ecol. Appl. 18, S77−S96. doi: 10.1890/06-0562.1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Boetius A., Albrecht S., Bakker K., Bienhold C., Felden J., Fernández-Méndez M., et al. (2013). Export of algal biomass from the melting arctic sea ice. Science 339, 1430−1432. doi: 10.1126/science.1231346
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Bravo G., Archambault P., Witte U., Mäkelä A., Kazanidis G., Ciancio J. E., et al. (2024). Detritus from ice and plankton algae as an important food source for macroinfaunal communities in the canadian arctic. Diversity 16, 10. doi: 10.3390/d16100605
Brown T. A., Alexander C., Yurkowski D. J., Ferguson S. H., and Belt S. T. (2014a). Identifying variable sea ice carbon contributions to the Arctic ecosystem : A case study using highly branched isoprenoid lipid biomarkers in Cumberland Sound ringed seals. Limnology Oceanography 59, 1581−1589. doi: 10.4319/lo.2014.59.5.1581
Brown T. A. and Belt S. T. (2012a). Closely linked sea ice–pelagic coupling in the Amundsen Gulf revealed by the sea ice diatom biomarker IP25. J. Plankton Res. 34, 647−654. doi: 10.1093/plankt/fbs045
Brown T. A. and Belt S. T. (2012b). Identification of the sea ice diatom biomarker IP25 in Arctic benthic macrofauna : Direct evidence for a sea ice diatom diet in Arctic heterotrophs. Polar Biol. 35, 131−137. doi: 10.1007/s00300-011-1045-7
Brown T. A., Belt S. T., Philippe B., Mundy C. J., Massé G., Poulin M., et al. (2011). Temporal and vertical variations of lipid biomarkers during a bottom ice diatom bloom in the Canadian Beaufort Sea : Further evidence for the use of the IP25 biomarker as a proxy for spring Arctic sea ice. Polar Biol. 34, 1857−1868. doi: 10.1007/s00300-010-0942-5
Brown T. A., Belt S., Tatarek A., and Mundy C. (2014b). Source identification of the Arctic sea ice proxy IP 25. Nat. Commun. 5, 4197. doi: 10.1038/ncomms5197
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Brown T. A., Chrystal E., Ferguson S. H., Yurkowski D. J., Watt C., Hussey N. E., et al. (2017). Coupled changes between the H-Print biomarker and δ15N indicates a variable sea ice carbon contribution to the diet of Cumberland Sound beluga whales. Limnology Oceanography 62, 1606−1619. doi: 10.1002/lno.10520
Brown T. A., Galicia M. P., Thiemann G. W., Belt S. T., Yurkowski D. J., and Dyck M. G. (2018). High contributions of sea ice derived carbon in polar bear (Ursus maritimus) tissue. PLoS One 13, e0191631. doi: 10.1371/journal.pone.0191631
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Brown T. A., Yurkowski D. J., Ferguson S. H., Alexander C., and Belt S. T. (2014c). H-Print : A new chemical fingerprinting approach for distinguishing primary production sources in Arctic ecosystems. Environ. Chem. Lett. 12, 387−392. doi: 10.1007/s10311-014-0459-1
Campbell R. G., Sherr E. B., Ashjian C. J., Plourde S., Sherr B. F., Hill V., et al. (2009). Mesozooplankton prey preference and grazing impact in the western Arctic Ocean. Deep Sea Res. Part II 56, 1274−1289. doi: 10.1016/j.dsr2.2008.10.027
Cautain I. J., Last K. S., McKee D., Bluhm B. A., Renaud P. E., Ziegler A. F., et al. (2022). Uptake of sympagic organic carbon by the Barents Sea benthos linked to sea ice seasonality. Front. Marine Sci. 9. doi: 10.3389/fmars.2022.1009303
Clark G. F., Marzinelli E. M., Fogwill C. J., Turney C. S. M., and Johnston E. L. (2015). Effects of sea-ice cover on marine benthic communities : A natural experiment in Commonwealth Bay, East Antarctica. Polar Biol. 38, 1213−1222. doi: 10.1007/s00300-015-1688-x
Deming J. W., Fortier L., and Fukuchi M. (2002). The International North Water Polynya Study (NOW) : A brief overview. Deep Sea Res. Part II 49, 4887−4892. doi: 10.1016/S0967-0645(02)00168-6
Drenzek N. J., Montluçon D. B., Yunker M. B., Macdonald R. W., and Eglinton T. I. (2007). Constraints on the origin of sedimentary organic carbon in the Beaufort Sea from coupled molecular 13C and 14C measurements. Marine Chem. 103, 146–162. doi: 10.1016/j.marchem.2006.06.017
Fernández-Méndez M., Katlein C., Rabe B., Nicolaus M., Peeken I., Bakker K., et al. (2015). synthetic production in the central Arctic Ocean during the record sea-ice minimum in 2012. Biogeosciences 12, 3525−3549. doi: 10.5194/bg-12-3525-2015
Fernández-Méndez M., Wenzhöfer F., Peeken I., Sørensen H. L., Glud R. N., and Boetius A. (2014). Composition, buoyancy regulation and fate of ice algal aggregates in the central arctic ocean. PLoS One 9, e107452. doi: 10.1371/journal.pone.0107452
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Forest A., Tremblay J.-É., Gratton Y., Martin J., Gagnon J., Darnis G., et al. (2011). Biogenic carbon flows through the planktonic food web of the Amundsen Gulf (Arctic Ocean) : A synthesis of field measurements and inverse modeling analyses. Prog. Oceanography 91, 410−436. doi: 10.1016/j.pocean.2011.05.002
Gallagher M. L., Ambrose W. G. Jr., and Renaud P. E. (1998). Comparative studies in biochemical composition of benthic invertebrates (bivalves, ophiuroids) from the Northeast Water (NEW) Polynya. Polar Biol. 19, 167−171. doi: 10.1007/s003000050230
Gosselin M., Levasseur M., Wheeler P. A., Horner R. A., and Booth B. C. (1997). New measurements of phytoplankton and ice algal production in the Arctic Ocean. Deep Sea Res. Part II 44, 1623−1644. doi: 10.1016/S0967-0645(97)00054-4
Gradinger R. (2009). Sea-ice algae : Major contributors to primary production and algal biomass in the Chukchi and Beaufort Seas during May/June 2002. Deep Sea Res. Part II: Topical Stud. Oceanography 56, 1201−1212. doi: 10.1016/j.dsr2.2008.10.016
GusChina I. A. and Harwood J. L. (2009). Algal lipids and effect of the environment on their biochemistry. In Kainz M., Brett M., and Arts M. (eds) Lipids in Aquatic Ecosystems. New York, NY: Springer. 1−24. doi: 10.1007/978-0-387-89366-2_1
Harning D. J., Holman B., Woelders L., Jennings A. E., and Sepúlveda J. (2022). Biomarker characterization of the North Water Polynya, Baffin Bay: implications for local sea ice and temperature proxies. Biogeosciences. 20, 229–249. doi: 10.5194/bg-20-229-2023
Heady W. N. and Moore J. W. (2013). Tissue turnover and stable isotope clocks to quantify resource shifts in anadromous rainbow trout. Oecologia 172, 21−34. doi: 10.1007/s00442-012-2483-9
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Hegseth E. N. and von Quillfeldt C. (2022). The Sub-Ice Algal Communities of the Barents Sea Pack Ice: Temporal and Spatial Distribution of Biomass and Species. Journal of Marine Science and Engineering. 10 (2), 164. doi: 10.3390/jmse10020164
Iken K., Bluhm B., and Dunton K. (2010). Benthic food-web structure under differing water mass properties in the southern Chukchi Sea. Deep Sea Res. Part II: Topical Stud. Oceanogr. 57, 7185. doi: 10.1016/j.dsr2.2009.08.007
Iken K., Brey T., Wand U., Voigt J., and Junghans P. (2001). Food web structure of the benthic community at the Porcupine Abyssal Plain (NE Atlantic) : A stable isotope analysis. Prog. Oceanography 50, 383−405. doi: 10.1016/S0079-6611(01)00062-3
Jaschinski S., Hansen T., and Sommer U. (2008). Effects of acidification in multiple stable isotope analyses. Limnol. Oceanogr. Methods. 5 (1), 12–15. doi: 10.4319/lom.2008.6.12
Ji R., Jin M., and Varpe Ø. (2013). Sea ice phenology and timing of primary production pulses in the Arctic Ocean. Global Change Biol. 19, 734−741. doi: 10.1111/gcb.12074
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Jin P., Hutchins D. A., and Gao K. (2020). The impacts of ocean acidification on marine food quality and its potential food chain consequences. Front. Marine Sci. 7. doi: 10.3389/fmars.2020.543979
Kaufman M. R., Gradinger R. R., Bluhm B. A., and O’Brien D. M. (2008). Using stable isotopes to assess carbon and nitrogen turnover in the Arctic sympagic amphipod Onisimus litoralis. Oecologia 158, 11−22. doi: 10.1007/s00442-008-1122-y
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Kazanidis G. and Witte U. F. M. (2016). The trophic structure of Spongosorites coralliophaga-coral rubble communities at two northeast Atlantic cold water coral reefs. Marine Biol. Res. 12, 932−947. doi: 10.1080/17451000.2016.1216569
Kelly J. R. and Scheibling R. E. (2012). Fatty acids as dietary tracers in benthic food webs. Marine Ecol. Prog. Ser. 446, 1−22. doi: 10.3354/meps09559
Kennedy H., Thomas D., Kattner G., Haas C., and Dieckmann G. (2002). Particulate organic matter in Antarctic summer sea ice : Concentration and stable isotopic composition. Marine Ecol. Prog. Ser. 238, 1−13. doi: 10.3354/meps238001
Klein B., LeBlanc B., Mei Z.-P., Beret R., Michaud J., Mundy C.-J., et al. (2002). Phytoplankton biomass, production and potential export in the North Water. Deep Sea Res. Part II: Topical Stud. Oceanography 49, 4983−5002. doi: 10.1016/S0967-0645(02)00174-1
Koch C. W., Cooper L. W., Grebmeier J. M., Frey K., and Brown T. A. (2020a). Ice algae resource utilization by benthic macro- and megafaunal communities on the Pacific Arctic shelf determined through lipid biomarker analysis. Marine Ecol. Prog. Ser. 651, 23−43. doi: 10.3354/meps13476
Koch C. W., Cooper L. W., Lalande C., Brown T. A., Frey K. E., and Grebmeier J. M. (2020b). Seasonal and latitudinal variations in sea ice algae deposition in the Northern Bering and Chukchi Seas determined by algal biomarkers. PLoS One 15, e0231178. doi: 10.1371/journal.pone.0231178
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Kolling H. M., Stein R., Fahl K., Sadatzki H., de Vernal A., and Xiao X. (2020). Biomarker distributions in (Sub)-arctic surface sediments and their potential for sea ice reconstructions. Geochemistry Geophysics Geosystems 21, e2019GC008629. doi: 10.1029/2019GC008629
Layman C. A. and Allgeier J. E. (2012). Characterizing trophic ecology of generalist consumers : A case study of the invasive lionfish in The Bahamas. Marine Ecol. Prog. Ser. 448, 131−141. doi: 10.3354/meps09511
Legendre L., Ackley S. F., Dieckmann G. S., Gulliksen B., Horner R., Hoshiai T., et al. (1992). Ecology of sea ice biota : 2. Global significance. Polar Biol. 12, 429−444. doi: 10.1007/BF00243114
Leu E., Mundy C. J., Assmy P., Campbell K., Gabrielsen T. M., Gosselin M., et al. (2015). Arctic spring awakening – Steering principles behind the phenology of vernal ice algal blooms. Prog. Oceanography 139, 151−170. doi: 10.1016/j.pocean.2015.07.012
Leu E., Søreide J. E., Hessen D. O., Falk-Petersen S., and Berge J. (2011). Consequences of changing sea-ice cover for primary and secondary producers in the European Arctic shelf seas : Timing, quantity, and quality. Prog. Oceanography 90, 18−32. doi: 10.1016/j.pocean.2011.02.004
Lewis K. M., van Dijken G. L., and Arrigo K. R. (2020). Changes in phytoplankton concentration now drive increased Arctic Ocean primary production. Science 369, 198−202. doi: 10.1126/science.aay8380
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Li H., Ke C., Zhu Q., and Shu S. (2019). Spatial-temporal variations in net primary productivity in the Arctic from 2003 to 2016. Acta Oceanologica Sin. 38, 111−121. doi: 10.1007/s13131-018-1274-5
Lovvorn J. R., Cooper L. W., Brooks M. L., Ruyck C. C. D., Bump J. K., and Grebmeier J. M. (2005). Organic matter pathways to zooplankton and benthos under pack ice in late winter and open water in late summer in the north-central Bering Sea. Marine Ecol. Prog. Ser. 291, 135−150. doi: 10.3354/meps291135
Mäkelä A., Witte U., and Archambault P. (2018). Short-term processing of ice algal- and phytoplankton-derived carbon by Arctic benthic communities revealed through isotope labelling experiments. Marine Ecol. Prog. Ser. 600, 21−39. doi: 10.3354/meps12663
McMahon K. W., Ambrose W. G., Johnson B. J., Sun M.-Y., Lopez G. R., Clough L. M., et al. (2006). Benthic community response to ice algae and phytoplankton in Ny Ålesund, Svalbard. Marine Ecol. Prog. Ser. 310, 1−14. doi: 10.3354/meps310001
Michel L. N., Danis B., Dubois P., Eleaume M., Fournier J., Gallut C., et al. (2019). Increased sea ice cover alters food web structure in East Antarctica. Sci. Rep. 9 (1), 8062. doi: 10.1038/s41598-019-44605-5
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Michel C., Gosselin M., and Nozais C. (2002). Preferential sinking export of biogenic silica during the spring and summer in the North Water Polynya (northern Baffin Bay) : Temperature or biological control? J. Geophysical Res. 107, 1–1-1−14. doi: 10.1029/2000JC000408
Michener R. H. and Kaufman L. (2007). Stable isotope ratios as tracers in marine food webs: An update. Stable Isotopes in Ecology and Environmental Science. 2, 238−282. doi: 10.1002/9780470691854.ch9
Moore G. W. K., Schweiger A., Zhang J., and Steele M. (2019). Spatiotemporal variability of sea ice in the arctic’s last ice area. Geophysical Res. Lett. 46, 11237−11243. doi: 10.1029/2019GL083722
Morata N., Poulin M., and Renaud P. E. (2011). A multiple biomarker approach to tracking the fate of an ice algal bloom to the sea floor. Polar Biol. 34, 101−112. doi: 10.1007/s00300-010-0863-3
Morata N., Renaud P. E., Brugel S., Hobson K. A., and Johnson B. J. (2008). Spatial and seasonal variations in the pelagic–benthic coupling of the southeastern Beaufort Sea revealed by sedimentary biomarkers. Marine Ecol. Prog. Ser. 371, 47−63. doi: 10.3354/meps07677
Müller J., Wagner A., Fahl K., Stein R., Prange M., and Lohmann G. (2011). Towards quantitative sea ice reconstructions in the northern North Atlantic: A combined biomarker and numerical modelling approach. Earth Planetary Sci. Lett. 306, 137148. doi: 10.1016/j.epsl.2011.04.011
Navarro-Rodriguez A., Belt S. T., Knies J., and Brown T. A. (2013). Mapping recent sea ice conditions in the Barents Sea using the proxy biomarker IP25 : Implications for palaeo sea ice reconstructions. Quaternary Sci. Rev. 79, 26−39. doi: 10.1016/j.quascirev.2012.11.025
Niemi A., Bluhm B. A., Juul-Pedersen T., Kohlbach D., Reigstad M., Søgaard D. H., et al. (2024). Ice algae contributions to the benthos during a time of sea ice change : A review of supply, coupling, and fate. Front. Environ. Sci. 12. doi: 10.3389/fenvs.2024.1432761
Olivier F., Gaillard B., Thébault J., Meziane T., Tremblay R., Dumont D., et al. (2020). Shells of the bivalve Astarte moerchi give new evidence of a strong pelagic-benthic coupling shift occurring since the late 1970s in the North Water polynya. Philos. Trans. R. Soc. A: Mathematical Phys. Eng. Sci. 378, 20190353. doi: 10.1098/rsta.2019.0353
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Payne C. M., Bianucci L., van Dijken G. L., and Arrigo K. R. (2021). Changes in under-ice primary production in the chukchi sea from 1988 to 2018. J. Geophysical Research: Oceans 126, e2021JC017483. doi: 10.1029/2021JC017483
Peterson B. J. (1999). Stable isotopes as tracers of organic matter input and transfer in benthic food webs : A review. Acta Oecologica 20, 479−487. doi: 10.1016/S1146-609X(99)00120-4
Piepenburg D., Chernova N. V., von Dorrien C. F., Gutt J., Neyelov A. V., Rachor E., et al. (1996). Megabenthic commmunities in the waters around Svalbard. Polar Biol. 16, 431−446. doi: 10.1007/s003000050074
Renaud P. E., Løkken T. S., Jørgensen L. L., Berge J., and Johnson B. J. (2015). Macroalgal detritus and food-web subsidies along an Arctic fjord depth-gradient. Front. Marine Sci. 2. doi: 10.3389/fmars.2015.00031
Riaux-Gobin C., Wafar M. V. M., and Klein B. (1993). Production primaire potentielle microphytobenthique d’une slikke de nord Bretagne: Stratification verticale. J. Exp. Marine Biol. Ecol. 169, 215231. doi: 10.1016/0022-0981(93)90194-S
Roy V., Iken K., and Archambault P. (2014). Environmental drivers of the canadian arctic megabenthic communities. PLoS One 9, e100900. doi: 10.1371/journal.pone.0100900
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Roy V., Iken K., and Archambault P. (2015). Regional variability of megabenthic community structure across the canadian arctic. Arctic 68, 180−192. doi: 10.14430/arctic4486
Rybakova E., Kremenetskaia A., Vedenin A., Boetius A., and Gebruk A. (2019). Deep-sea megabenthos communities of the Eurasian Central Arctic are influenced by ice-cover and sea-ice algal falls. PLoS One 14, e0211009. doi: 10.1371/journal.pone.0211009
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Schollmeier T., Oliveira A. C. M., Wooller M. J., and Iken K. (2018). Tracing sea ice algae into various benthic feeding types on the Chukchi Sea shelf. Polar Biol. 41, 207−224. doi: 10.1007/s00300-017-2182-4
Sherr E. B., Sherr B. F., and Hartz A. J. (2009). Microzooplankton grazing impact in the Western Arctic Ocean. Deep Sea Res. Part II 56, 1264−1273. doi: 10.1016/j.dsr2.2008.10.036
Smith W. O. and Barber D. G. (2007). “Chapter 13 polynyas and climate change : A view to the future,” in Elsevier Oceanography Series. (Elsevier) 74, 411−419. doi: 10.1016/S0422-9894(06)74013-2
Søreide J. E., Carroll M. L., Hop H., Ambrose W. G., Hegseth E. N., and Falk-Petersen S. (2013). Sympagic-pelagic-benthic coupling in Arctic and Atlantic waters around Svalbard revealed by stable isotopic and fatty acid tracers. Marine Biol. Res. 9, 831−850. doi: 10.1080/17451000.2013.775457
Søreide J. E., Leu E., Berge J., Graeve M., and Falk-Petersen S. (2010). Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Global Change Biol. 16, 3154−3163. doi: 10.1111/j.1365-2486.2010.02175.x
Sou T. and Flato G. (2009). Sea ice in the canadian arctic archipelago : modeling the past, (1950-2004) and the future, (2041-60). J. Climate 22, 2181−2198. doi: 10.1175/2008JCLI2335.1
Stirling I. (1997). The importance of polynyas, ice edges, and leads to marine mammals and birds. J. Marine Syst. 10, 9−21. doi: 10.1016/S0924-7963(96)00054-1
Stöhr S., O’Hara T. D., and Thuy B. (2012). Global diversity of brittle stars (Echinodermata : ophiuroidea). PLoS One 7, e31940. doi: 10.1371/journal.pone.0031940
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Stoynova V., Shanahan T. M., Hughen K. A., and de Vernal A. (2013). Insights into Circum-Arctic sea ice variability from molecular geochemistry. Quaternary Sci. Rev. 79, 63−73. doi: 10.1016/j.quascirev.2012.10.006
Stratmann T., van Oevelen D., Martínez Arbizu P., Wei C.-L., Liao J.-X., Cusson M., et al. (2020). The BenBioDen database, a global database for meio-, macro- and megabenthic biomass and densities. Sci. Data 7. doi: 10.1038/s41597-020-0551-2
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Tamelander T., Kivimäe C., Bellerby R. G. J., Renaud P. E., and Kristiansen S. (2009). Base-line variations in stable isotope values in an Arctic marine ecosystem : Effects of carbon and nitrogen uptake by phytoplankton. Hydrobiologia 630, 63−73. doi: 10.1007/s10750-009-9780-2
Tamelander T., Renaud P. E., Hop H., Carroll M. L., Ambrose W. G., and Hobson K. A. (2006). Trophic relationships and pelagic–benthic coupling during summer in the Barents Sea Marginal Ice Zone, revealed by stable carbon and nitrogen isotope measurements. Marine Ecol. Prog. Ser. 310, 33−46. doi: 10.3354/meps310033
Tieszen L. L., Boutton T. W., Tesdahl K. G., and Slade N. A. (1983). Fractionation and turnover of stable carbon isotopes in animal tissues : Implications for δ13C analysis of diet. Oecologia 57, 32−37. doi: 10.1007/BF00379558
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Tremblay J.-É., Hattori H., Michel C., Ringuette M., Mei Z.-P., Lovejoy C., et al. (2006). Trophic structure and pathways of biogenic carbon flow in the eastern North Water Polynya. Prog. Oceanography 71, 402−425. doi: 10.1016/j.pocean.2006.10.006
Vafeiadou A.-M., Adão H., Troch M. D., and Moens T. (2013). Sample acidification effects on carbon and nitrogen stable isotope ratios of macrofauna from a Zostera noltii bed. Marine Freshwater Res. 64, 741−745. doi: 10.1071/MF12169
Yunda-Guarin G., Brown T. A., Michel L. N., Saint-Béat B., Amiraux R., Nozais C., et al. (2020). Reliance of deep-sea benthic macrofauna on ice-derived organic matter highlighted by multiple trophic markers during spring in Baffin Bay, Canadian Arctic. Elementa: Sci. Anthropocene 8, 47. doi: 10.1525/elementa.2020.047
Yunda-Guarin G., Michel L. N., Nozais C., and Archambault P. (2022). Interspecific differences in feeding selectivity shape isotopic niche structure of three ophiuroids in the Arctic Ocean. Marine Ecol. Prog. Ser. 683, 81−95. doi: 10.3354/meps13965
Zanden M. J. V., Clayton M. K., Moody E. K., Solomon C. T., and Weidel B. C. (2015). Stable isotope turnover and half-life in animal tissues : A literature synthesis. PLoS One 10, e0116182. doi: 10.1371/journal.pone.0116182
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: sea ice algae, phytoplankton, HBIs, stable isotopes, sympagic-benthic coupling, Canadian Arctic, Arctic megabenthos
Citation: Combaz T, Witte U, Brown TA and Archambault P (2025) Importance of ice algae versus phytoplankton in the diet of megabenthic organisms under contrasting sea ice conditions (Canadian Arctic): a dual biochemical approach (SIA and HBIs). Front. Mar. Sci. 12:1574292. doi: 10.3389/fmars.2025.1574292
Received: 10 February 2025; Accepted: 12 May 2025;
Published: 02 June 2025.
Edited by:
Tilmann Harder, University of Bremen, GermanyReviewed by:
Doreen Kohlbach, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI), GermanyPaloma Carvalho, Fisheries and Oceans Canada (DFO), Canada
Copyright © 2025 Combaz, Witte, Brown and Archambault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thibaud Combaz, Y29tYmF6LnRoaWJhdWRAZ21haWwuY29t
 Thibaud Combaz
Thibaud Combaz Ursula Witte
Ursula Witte Thomas A. Brown3
Thomas A. Brown3 Philippe Archambault
Philippe Archambault