Abstract
Introduction:
The overuse of antibiotics in aquaculture has raised concerns, necessitating the exploration of sustainable alternatives. This study evaluated tea-derived additives as potential substitutes.
Methods:
This study evaluated the effects of black tea powder (BTP) and black tea powder-probiotics mixed fermentation product (TPMFP) on Cherax quadricarinatus growth, antioxidant capacity, and gut microbiota. Cherax quadricarinatus were fed diets supplemented with BTP (1–6%) or TPMFP (2%) for 84 days.
Results:
The addition of BTP (<2%) to the feed enhances the weight, carapace length, and muscle protein content of Cherax quadricarinatus, while higher doses (>3%) showed adverse effects. Moreover, the addition of BTP (1%-6%) can significantly reduce the muscle content of CHO, TG, and HDL (P<0.05). The TPMFP group exhibited the highest muscle protein content and significantly elevated hepatopancreatic SOD, GSH-PX, and AKP activities (P < 0.05), indicating improved antioxidant capacity. Gut microbiota analysis revealed dose-dependent shifts, with Proteobacteria dominating (>70%) and Aeromonas increasing with BTP levels. TPMFP significantly increased the α-diversity indices (richness and evenness) of the gut microbiota. The Mantel test confirmed the relationship between the antioxidant function of the microbial community, with Proteobacteria positively correlated with GSH-PX/SOD, while Fusobacteria and Tensionella negatively correlated with antioxidant enzyme activity.
Discussion:
This study provides a foundation for using tea-derived additives as a sustainable alternative to antibiotics in aquaculture.In the future, metagenomic analysis of key metabolic pathways can be combined to optimize the mechanistic explanation of the application and efficacy of BTP and TPMFP.
1 Introduction
Aquaculture has become a crucial sector in global food production, supplying over 50% of the world’s fish in 2024 and playing a key role in ensuring food security as the global population approaches 10 billion by 2050 (FAO, 2024). Shrimp farming, particularly in Asia, has significantly contributed to economic growth and employment (Vaiyapuri et al., 2021). Species like Cherax quadricarinatus are gaining attention for their rapid growth and adaptability, making them ideal for sustainable aquaculture (Mauro et al., 2024). However, intensified aquaculture has increased disease susceptibility, oxidative stress, and the overuse of antibiotics, leading to antibiotic resistance and environmental pollution (Vaiyapuri et al., 2021; Zhao et al., 2020). This underscores the need for sustainable alternatives, such as plant-derived compounds and probiotics, to improve growth, immunity, and reduce oxidative stress (Bai et al., 2022; Jiang et al., 2023).
Plant phenolic compounds exhibit a wide range of biological activities, including immunostimulatory, anti-inflammatory, antioxidant, and antimicrobial (antibacterial, antifungal, antiviral, and antiprotozoal) properties. These activities can enhance the immune system, stimulate appetite, improve intestinal digestion and function, support mucosal morphology and integrity, and modulate the composition of gut microbiota (Garcia Beltran and Esteban, 2022), making them ideal candidates for inclusion in aquatic feed.These properties. The application of functional feed additives can mitigate issues related to antinutritional factors, low palatability, and low digestibility of sustainable feed (Bai et al., 2022).
Black tea (Camellia sinensis), a widely consumed beverage, is rich in polyphenolic compounds, including theaflavins and thearubigins, which possess significant antioxidant, anti-inflammatory, and antimicrobial properties (Sun et al., 2023). Tea polyphenols (TPs), as natural antioxidants, enhance the endogenous antioxidant defense system, maintain cellular redox balance, significantly improve the immune response, antioxidant capacity, and growth performance of various aquatic species (Qin et al., 2024). At the same time, they can regulate the composition of the gut microbiota, promote the production of beneficial metabolites (Gowd et al., 2019; Liu et al., 2022; Sun and Miao, 2020), and play a role in anti-inflammatory activity, intestinal barrier protection, and bile acid metabolism regulation (Huang et al., 2021; Truong and Jeong, 2022). Dietary supplementation with TPs has been demonstrated to improve growth, feed intake, and immunity in cold-water silver salmon (Yu et al., 2024), as well as enhance growth, feed efficiency, and disease resistance in tilapia (Oreochromis niloticus) and coho salmon (Oncorhynchus kisutch) (Daniel et al., 2024; Yu et al., 2024), highlighting their potential as novel feed additives in aquaculture.
The application of probiotics as functional feed additives in aquaculture has garnered significant attention. Probiotics provide multiple health benefits to cultured species by modulating gut microbiota, enhancing non-specific immune responses, and improving water quality (Rahayu et al., 2024). Studies have shown that the supplementation of probiotics, such as Lactobacillus and bacillus, can significantly improve disease resistance and growth performance in cultured species (Bai et al., 2022; Eissa et al., 2024a). Furthermore, the combined use of probiotics and plant-derived additives has been demonstrated to synergistically enhance immune responses and antioxidant capacity (Roh and Kannimuthu, 2023). Probiotics, prebiotics, metabolic bacteria and medicinal plants are effective feed additives for the control and treatment of aquaculture diseases, providing a healthy management scheme for aquaculture (Bondad-Reantaso et al., 2023; Roh and Kannimuthu, 2023).
This study aims to evaluate the potential effects of black tea powder (BTP) and its fermented product with probiotics as functional feed additives on Cherax quadricarinatus, exploring their comprehensive impacts on growth performance, antioxidant capacity, and gut microbiota. By investigating the mechanisms of these natural additives, this research provides valuable insights into the combined application of natural feed additives and probiotics in aquaculture, offering feasible alternatives to antibiotics and synthetic compounds. Moreover, it enhances the comprehensive utilization of black tea and its by-products, increasing the added value of the tea industry.
2 Materials and methods
2.1 Experimental materials
Cherax quadricarinatus were purchased from Zhangzhou Miaoyuan Aquatic Co., Ltd., with an initial body weight of 4.50 ± 0.28g and carapace length of 1.56 ± 0.10cm. The experimental Cherax quadricarinatus were healthy and of uniform size. The basal diet for the Cherax quadricarinatus was obtained from Guangdong Yuehai Feed Group Fujian Yuehai Feed Co., Ltd. The composition of the basal diet is shown in Table 1.
Table 1
| Component | Crude Protein | Crude Fat | Crude Fiber | Moisture | Ash | Calcium | Total Phosphorus | Sodium Chloride | Lysine Content | Multivitamins | Multiminerals |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | ≥40.0 | ≥4.0 | ≤5.0 | ≤12.0 | ≤16.0 | 0.8-4.0 | 0.5-3.0 | 0.5-3.0 | ≥2.0 | 0.5%-2% | 2%-5% |
Composition of the basal diet for shrimp (unit: %).
The black tea powder (BTP) was purchased from Damin Food (Zhangzhou) Co., Ltd., and was produced by spray-drying Yunnan large-leaf variety black tea. It contains 35% tea polyphenols (TPs), 8.3% caffeine, and 4.5% moisture. The BTP was mixed with Lactobacillus plantarum and Bacillus subtilis (Eissa et al., 2024b), brown sugar, and vitamins according to the proportions outlined in Table 2, and placed into a 10L fermentation tank for fermentation. The temperature was controlled between 25-30°C, and the mixture was stirred every 12 hours. The total fermentation duration was 72 hours. This fermentation method was provided by Xiamen Haiyue Biotechnology Co., Ltd., based on their production experience. After fermentation,the black tea powder-probiotics mixed fermentation product (TPMFP) was stored in a sterile sample tank for future use. All ingredients for the fermentation, except for the black tea powder, were purchased from Xiamen Haiyue Biotechnology Co., Ltd.
Table 2
| Component | Black tea powder | Lactobacillus plantarum (1 × 108 CFU/g) | Bacillus subtilis (6 × 108 CFU/g) | Bacillus coagulans (2 × 108 CFU/g) | Brown sugar | Vitamin B2 (purity 98%) | Vitamin B6 (purity 98%) | Sterilized pure water |
|---|---|---|---|---|---|---|---|---|
| Amount (g,ml) | 100 | 0.25 | 0.5 | 5 | 125 | 0.3 | 0.05 | 4750 |
Composition of the black tea powder-probiotics mixed fermentation product (TPMFP).
(Solid units: g, liquid units: ml).
2.2 Experimental diet formulation and feeding protocol
The control group (CK) was fed a basal diet without any additional substances. The experimental groups (A1, A2, A3, A4, A5, A6) followed the methodology used in the experiment with fermented tea dregs for feeding juvenile largemouth bass (Jiang et al., 2022), with BTP added at 1%, 2%, 3%, 4%, 5%, and 6% of the basal diet weight. The addition method involved weighing the black tea powder according to the proportion of basal diet weight, dissolving the black tea powder in sterile water, with a sterile water volume (ml) to basal diet weight (g) ratio of 1:5. The black tea powder solution was added in layers and mixed into the basal diet, ensuring uniform mixing during the process. The experimental group (CJ) followed the method used in the feeding experiment with dietary yeast for shrimp (Ayiku et al., 2020), adding 2% TPMFP. The addition method involved weighing the TPMFP liquid based on the proportion of basal diet weight and layering it into the basal diet, ensuring uniform mixing during the process. The feed was prepared every 3 days and stored in sterile sealed containers at 4°C for later use.
2.3 Feeding regimen and sample collection
The experiment was conducted in glass aquariums (1.2m×0.6m×0.8 m), with 8 groups, each with 3 replicates, totaling 24 aquariums (Figure 1). Each aquarium housed 30 Cherax quadricarinatus, with a total of 720 Cherax quadricarinatus. The aquaculture system was equipped with internal water circulation and continuous aeration. The water temperature was maintained at room temperature, with a pH of 7.5 ± 0.5, dissolved oxygen levels ranging from 5.5 to 8.7mg/L, and ammonia nitrogen concentration maintained below 0.5mg/L. Based on production experience and studies on the feeding activity of Cherax quadricarinatus in the afternoon (Nie et al., 2024), feeding was done once daily at 16:30, with a feeding amount of 3% of body weight. Uniform feed intake was ensured among groups during the feeding process.The trial duration was 84 days, covering the rapid growth phase of Cherax quadricarinatus. On day 85, 6 randomly selected crayfish from each aquarium were sampled to measure body weight and carapace length. After surface disinfection with 75% ethanol, muscle, hepatopancreas, and intestinal tissues were collected under sterile conditions. The samples were rapidly frozen in liquid nitrogen and stored at -80°C for further analysis.
Figure 1
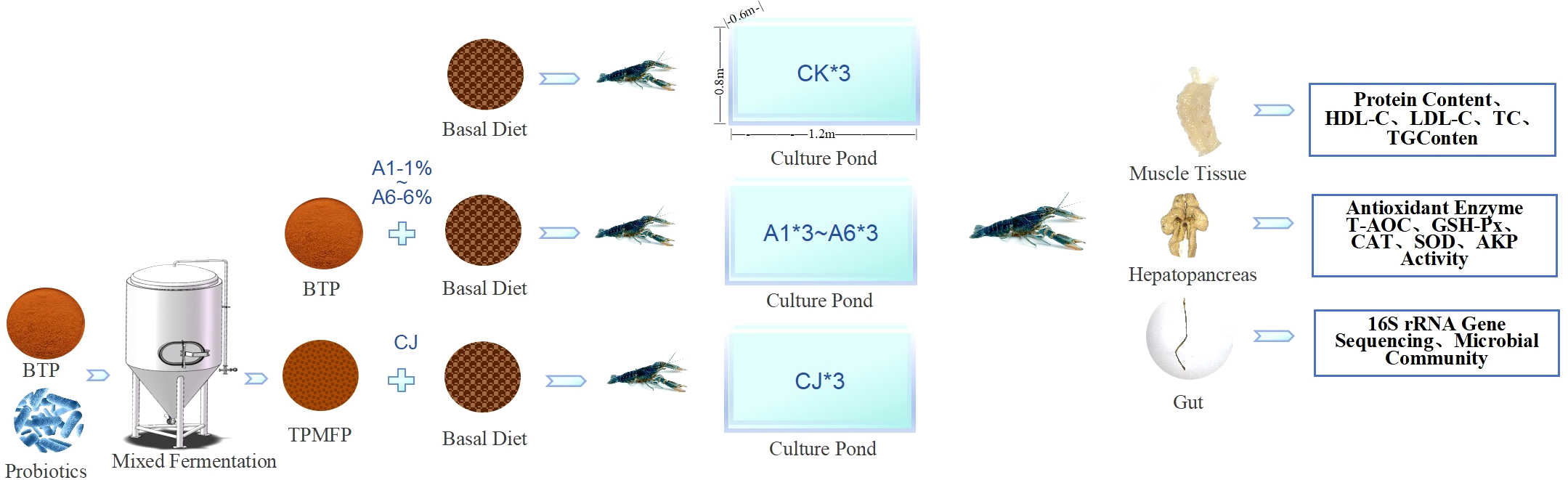
Technical workflow of the experimental methodology.
2.4 Measurement indicators and methods
2.4.1 Determination of total protein, lipids, and enzyme activities
The contents of total protein (TP), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) in muscle tissue, along with TP content and antioxidant enzyme activities in the hepatopancreas—Total Antioxidant Capacity (T-AOC), Glutathione Peroxidase (GSH-Px), Catalase (CAT), Superoxide Dismutase (SOD), and Alkaline Phosphatase (AKP)—were measured using commercial kits from Nanjing Jiancheng Bioengineering Institute. These measurements were conducted with a fully automated microplate reader (Thermo Scientific Skanit 6).
2.4.2 Genomic DNA extraction, 16S rRNA gene sequencing, and library construction
Genomic DNA extraction, 16S rRNA gene sequencing, and library construction were performed following the methodology described by Lin et al. (Lin et al., 2024). Genomic DNA (gDNA) was extracted from Cherax quadricarinatus intestinal samples using the QIAamp DNA Microbiome Kit (Qiagen, Hilden, Germany). The concentration and purity of the extracted gDNA were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The quality of the gDNA was further assessed by electrophoresis, purified, and stored at -20°C for subsequent 16S rRNA gene sequencing analysis.
The V3-V4 region of the 16S rRNA gene was amplified using the KAPA HiFi HotStart Ready Mix PCR Kit. Paired-end sequencing was performed on the Illumina MiSeq platform (Illumina, San Diego, CA, USA). Raw sequencing data were processed and analyzed using the QIIME 2 software package (Bolyen et al., 2019). Sequences were trimmed to remove chimeras, and operational taxonomic units (OTUs) were clustered at a 97% similarity threshold using the RDP database (Cole et al., 2014). To limit taxonomic depth, the confidence threshold was set at 0.7.
2.4.3 Statistical analysis
Growth performance and antioxidant enzyme activity data were analyzed using one-way analysis of variance (ANOVA) followed by LSD post hoc tests in SPSS 18.0. Results were presented graphically,including 95% confidence intervals,with P<0.05 indicating significant differences and P<0.01 indicating highly significant differences between groups.
Microbial community composition, abundance, α-diversity, β-diversity phylogenetic tree construction, and Mantel test correlation analysis were performed using the cnsknowall software (https://www.cnsknowall.com/#/HomePage, last accessed: December 10, 2024). Microbial community composition was analyzed using Venn diagrams (Gao et al., 2021) to illustrate commonalities and specificities among multiple groups. Microbial abundance analysis displayed the top 10 taxa (Yu et al., 2021), highlighting differences and trends across groups, as well as cumulative significance. α-Diversity was assessed using indices including S.obs (Observed richness), Shannon (Shannon diversity index), Simpson (Simpson’s diversity index), and Pielou (Pielou’s evenness index) to evaluate differences among groups. β-Diversity phylogenetic trees were constructed to cluster microbial data and visualize evolutionary relationships. β-Diversity principal coordinate analysis (PCoA) was performed based on the weighted UniFrac metric, and permutational multivariate analysis of variance (PERMANOVA) was conducted using the microeco package (v1.10.0). Results were visualized using ggplot2.
Significant differences in bacterial taxa were detected using linear discriminant analysis effect size (LEfSe) and linear discriminant analysis (LDA) scores, while functional annotation analysis was performed using Lingbo Microclass software (http://www.cloud.biomicroclass.com/CloudPlatform ,last accessed: December 10, 2024). In this study, LEfSe analysis version 2.0 was performed using the following parameters: Kruskal-Wallis rank-sum test for between-group factors with an alpha value of 0.05, paired Wilcoxon rank-sum test for between-subgroup comparisons with an alpha value of 0.05, a threshold of 2 for the LDA log score of discriminative features, and a one-against-one strategy for multi-class classification.Results were visualized using bar plots and cladograms. Functional annotation was performed using FAPROTAX to predict functional profiles based on 16S rRNA gene sequences. Differences in functional categories were visualized using heatmaps, cumulative plots, and bar plots. Mantel tests and global association analyses (using PyCharm 2025.1) were conducted to evaluate the spatial correlations between shrimp gut microbiota (based on Bray-Curtis distance) and enzyme activities (based on Euclidean distance). Data preprocessing included handling outliers and normalization.
3 Results
3.1 Effects of black tea powder and its fermentation products on growth performance, muscle protein, and lipid content in Cherax quadricarinatus
The effects of black tea powder (BTP) supplementation on the growth performance, muscle protein content, and lipid content of Cherax quadricarinatus are illustrated in Figure 2. Group A1 demonstrated significant improvements in body weight, carapace length, and muscle protein content when compared to the control group (CK). However, for groups A2–A6, body weight, carapace length, and muscle protein content decreased as the BTP supplementation level increased. Furthermore, muscle cholesterol (CHO), triglycerides (TG), and LDL content were significantly reduced in groups A1–A6, while HDL levels were elevated. Group CJ exhibited the highest muscle protein content and the lowest TG content. Statistical analysis of the data presented in Table 3 indicates significant differences between groups in terms of metabolic and biochemical markers. The data analysis suggests that the experimental treatments had minimal impact on the weight and carapace length of Cherax quadricarinatus, but significantly affected its biochemical markers.
Figure 2
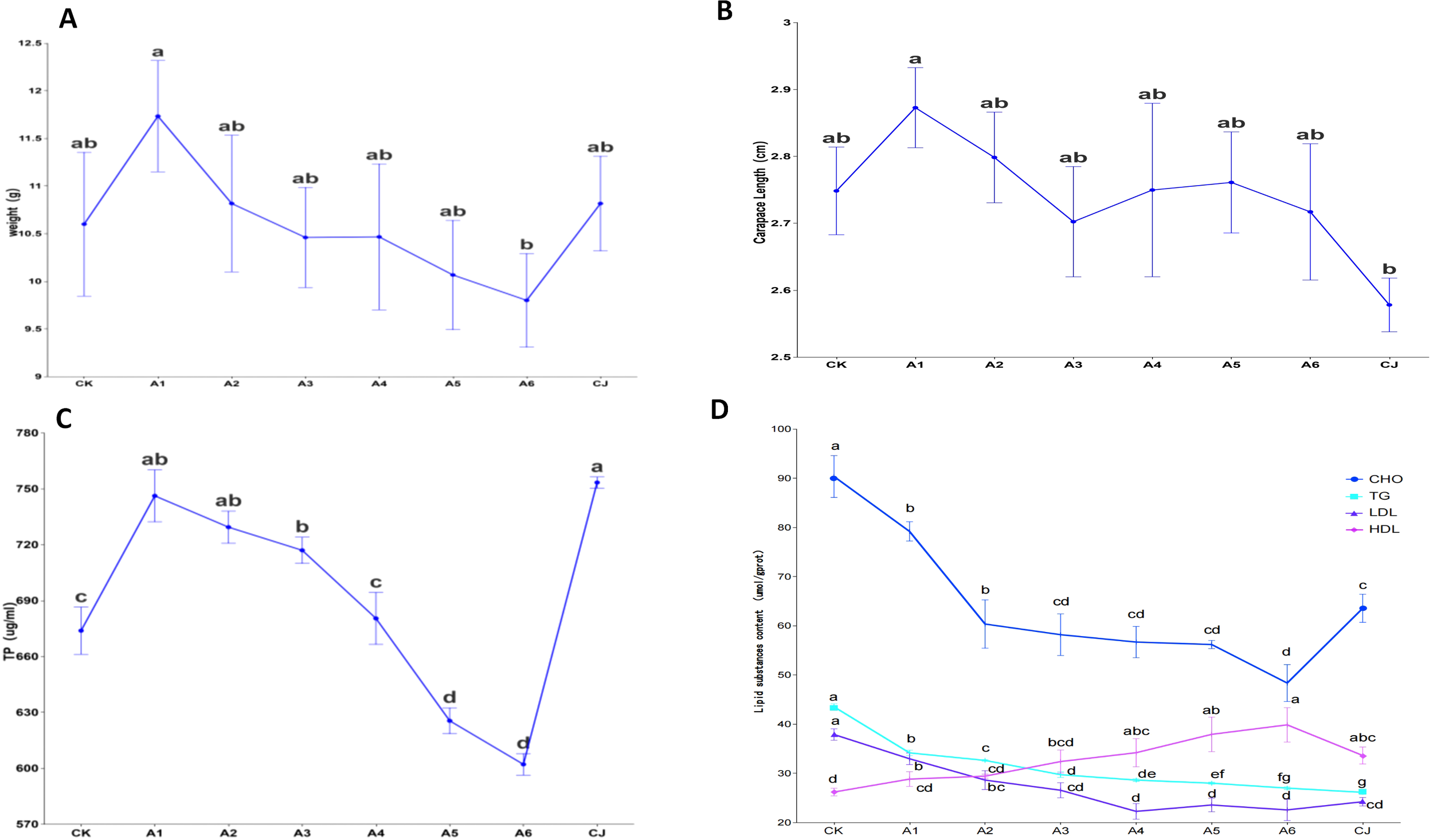
Effects of black tea powder and its fermentation products on body weight (A), carapace length (B), muscle protein content (C), and lipid content (D) in Cherax quadricarinatus. "a-d" letters represent statistically homogeneous subsets, automatically generated by post-hoc tests in ANOVA. Groups that share letters do not have significant differences between them, whereas groups with different letters have significant differences (p<0.05).
Table 3
| Metric | Levene’s Test p-value | ANOVA Test p-value | Eta-squared (η²) | Omega-squared (ω²) |
|---|---|---|---|---|
| Weight | 0.9058 | 0.5453 | 0.1309 | 0.0212 |
| Carapace_length | 0.1704 | 0.3305 | 0.1724 | 0.0275 |
| Muscle_protein | 0.8766 | 6.00E-08 | 0.9271 | 0.8952 |
| CHO | 0.8972 | 4.51E-06 | 0.8728 | 0.8172 |
| TG | 0.6681 | 5.27E-08 | 0.9875 | 0.9820 |
| LDL | 0.9668 | 1.29E-05 | 0.8541 | 0.7903 |
| HDL | 0.4651 | 0.0119 | 0.6284 | 0.4659 |
Statistical analysis of growth performance, muscle protein, and lipid content in Cherax quadricarinatus.
3.2 Effects of black tea powder and its fermentation products on hepatopancreatic enzyme activity in Cherax quadricarinatus
Supplementation with BTP and TPMFP significantly enhanced the antioxidant enzyme activity in the hepatopancreas of Cherax quadricarinatus (Figure 3). Group A4 exhibited the highest T-AOC activity, while Group A5 showed the highest CAT activity. Both were very significantly higher than those of the control group (P<0.01). Group CJ demonstrated the highest SOD, GHX-PX, and AKP activities, all of which were extremely higher than those in the control group (P<0.001). Statistical analysis, as presented in Table 4, indicated that the experimental treatments significantly improved the antioxidant enzyme activity in Cherax quadricarinatus, with SOD activities showing the highest statistical significance and a very large effect size, suggesting a strong response to the experimental conditions.
Figure 3
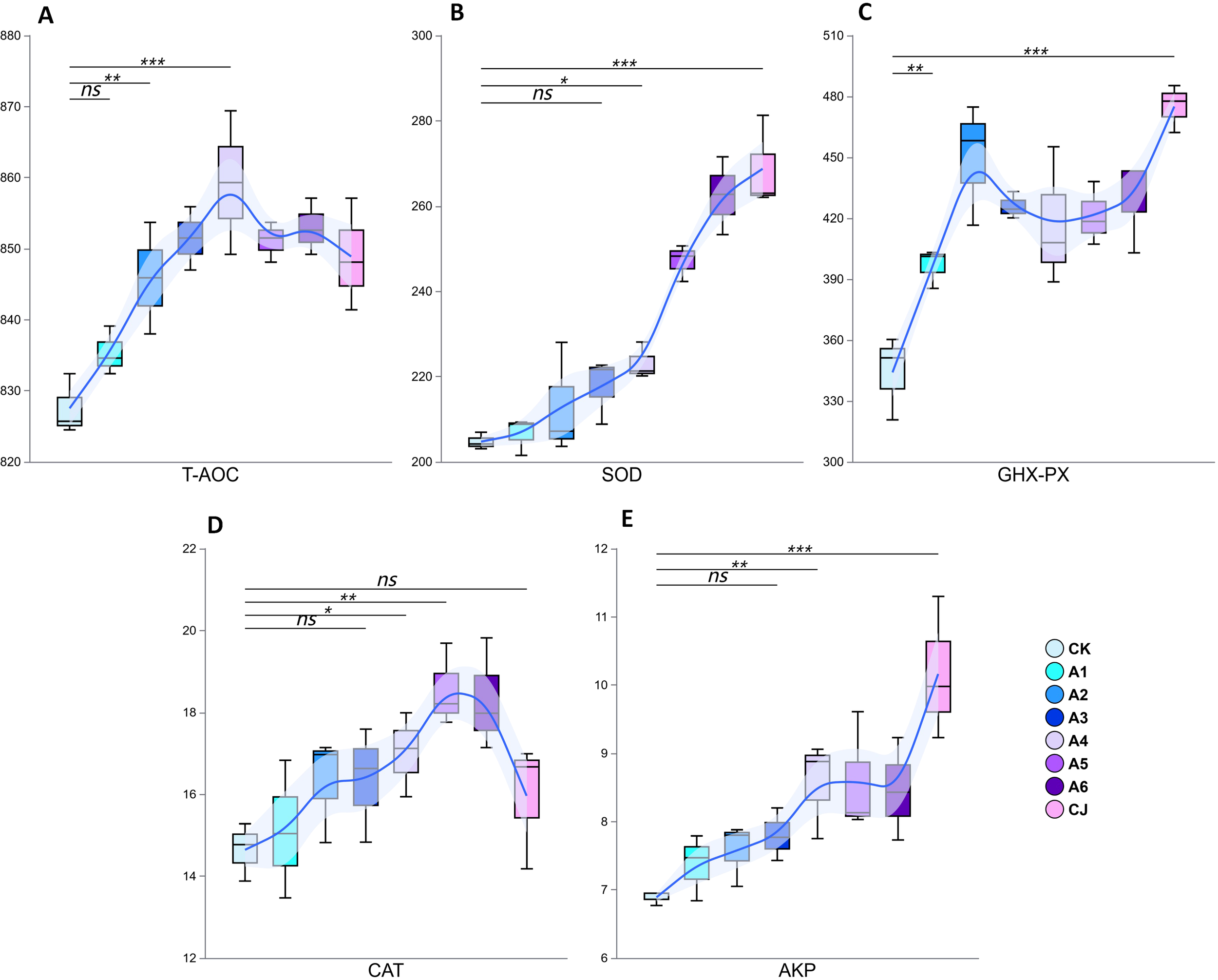
Effects of black tea powder and its fermentation products on hepatopancreatic enzyme activities in Cherax quadricarinatus: T-AOC (A, unit: uM), SOD (B, unit: U/mg prot), GHX-PX (C, unit: IU), CAT (D, unit: U/mg prot), and AKP (E, unit: KU/g prot). *: p<0.05 (significant difference), **: p<0.01 (very significant difference), ***: p<0.001 (extremely significant difference), NS "is" Not Significant "(p>0.05).
Table 4
| Metric | Levene’s Test p-value | ANOVA Test p-value | Eta-squared (η²) | Omega-squared (ω²) |
|---|---|---|---|---|
| T-AOC | 0.6742 | 0.0002 | 0.7881 | 0.6954 |
| SOD | 0.8964 | 3.40E-08 | 0.9322 | 0.9025 |
| GHX-PX | 0.8237 | 8.23E-05 | 0.8136 | 0.7321 |
| CAT | 0.9927 | 0.0183 | 0.6038 | 0.4304 |
| AKP | 0.8564 | 0.0007 | 0.7522 | 0.6438 |
Statistical analysis of antioxidant enzyme activity in the hepatopancreas of Cherax quadricarinatus.
3.3 Effects of black tea powder and its fermentation products on gut microbiota in Cherax quadricarinatus
3.3.1 Composition and abundance of gut microbiota in Cherax quadricarinatus
Venn diagram analysis was conducted to compare the shared and unique microbial taxa among groups CK, A1-A6, and CJ (Figure 4A). The results indicated that 19 taxa were common to all groups, while group CK exhibited 18 unique taxa. The unique taxa counts for groups A1-A6 and CJ were 24, 9, 5, 11, 7, 8, and 69, respectively, with group CJ having the highest number of unique taxa. Notably, as the supplementation level exceeded 2%, the number of unique taxa in groups A2-A6 significantly decreased.
Figure 4
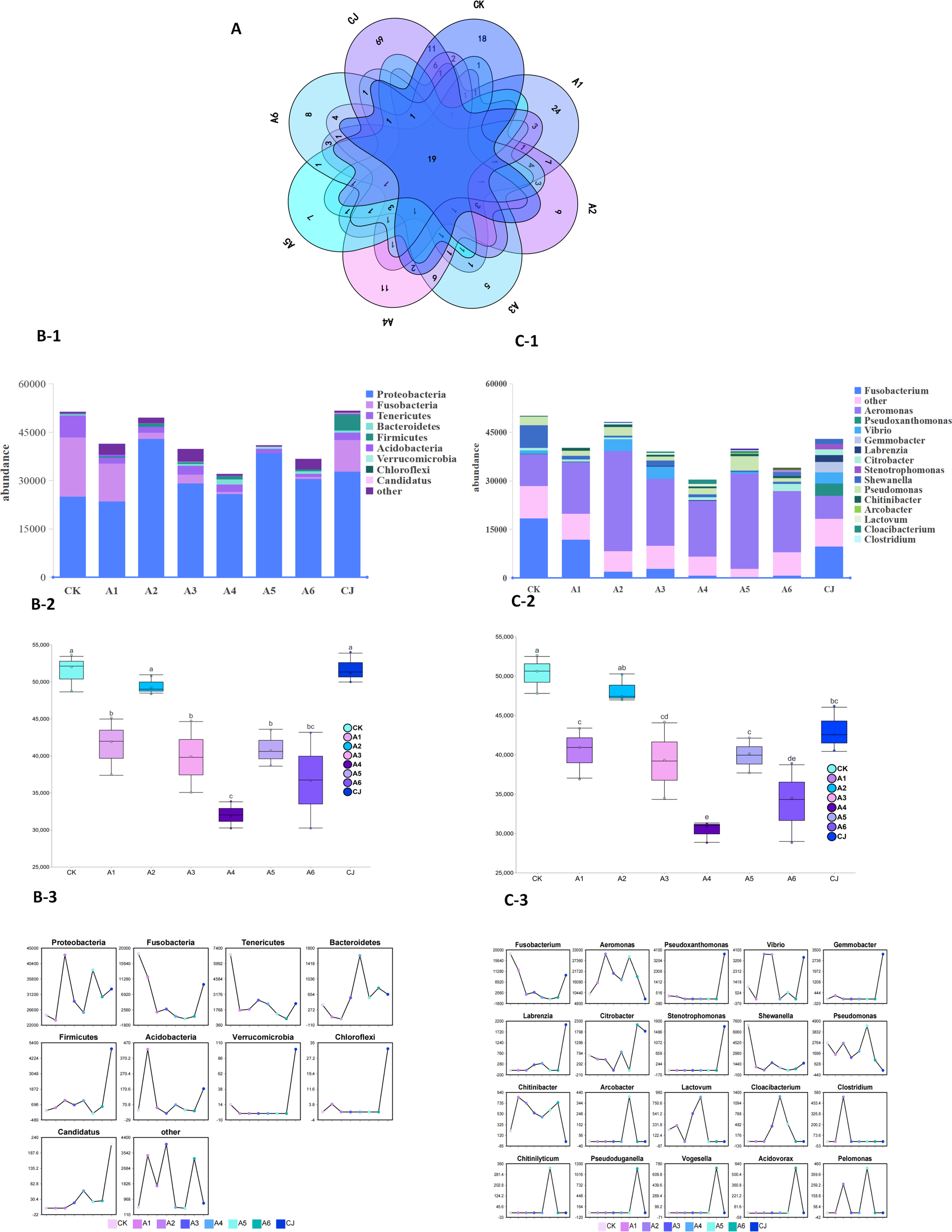
Effects of black tea powder and its fermentation products on gut microbiota composition and abundance in Cherax quadricarinatus. (A) Venn diagram showing shared and unique microbial taxa among groups. (B-1, C-1) Top 10 microbial taxa at the phylum and genus levels. (B-2, C-2) Total abundance of the top 10 taxa at the phylum and genus levels. (B-3, C-3) Changes in the abundance of the top 10 taxa at the phylum and genus levels.
The top 10 microbial taxa at the phylum (Figure 4B) and genus (Figure 4C) levels were analyzed. At the phylum level (Figures 4B-2), the total abundance was significantly higher in groups CK, A2, and CJ, while group A4 had the lowest total abundance (P<0.05). Significant variations in the top 10 phyla were observed among the groups (Figures 4B-1, B-3). Proteobacteria was the dominant phylum across all groups, with its abundance increasing as the BTP supplementation level rose, reaching over 70% in groups A2-A6.The abundance of Fusobacteria was higher in groups CK, A1, and CJ, but it and Tenericutes significantly decreased with increasing BTP supplementation. Conversely, Bacteroidetes abundance increased with higher BTP supplementation. The abundance of Firmicutes, Verrucomicrobia, Chloroflexi, and Candidatus was relatively higher in group CJ.
At the genus level (Figures 4C-2), the total abundance in group CK was significantly higher than in other groups, while group A4 had the lowest total abundance. The top 10 genera varied significantly among groups (Figures 4C-1, C-3). The abundance of Fusobacterium significantly decreased with increasing BTP supplementation, showing higher abundance in groups CK, A1, and CJ, but it was undetectable in group A5. The abundance of Aeromonas increased with BTP supplementation, whereas Shewanella abundance decreased. Arcobacter and Chitinilyticum were only detected in group A5, Pseudoduganella, Vogesella, and Acidovorax were only detected in group A6, and Stenotrophomonas was only detected in group CJ. The abundance of Pseudoxanthomonas, Gemmobacter, and Labrenzia in group CJ was significantly higher than in other groups.
3.3.2 Analysis of gut microbiota diversity in Cherax quadricarinatus
3.3.2.1 α-diversity analysis of gut microbiota
Alpha diversity analysis was used to assess the diversity within specific regions or ecosystems. The S.obs index reflects the total number of observed species or operational taxonomic units, the Shannon index evaluates species evenness and richness, the Simpson index emphasizes the contribution of rare species to community diversity, and the Pielou index measures the evenness of species abundance within a community. The results (Figure 5) show group CJ exhibited the highest S.obs, Shannon, and Simpson indices, indicating the highest diversity and evenness. Group A6 showed relatively high diversity and evenness, second only to group CJ, while group A3 displayed the lowest diversity and evenness among all groups.
Figure 5
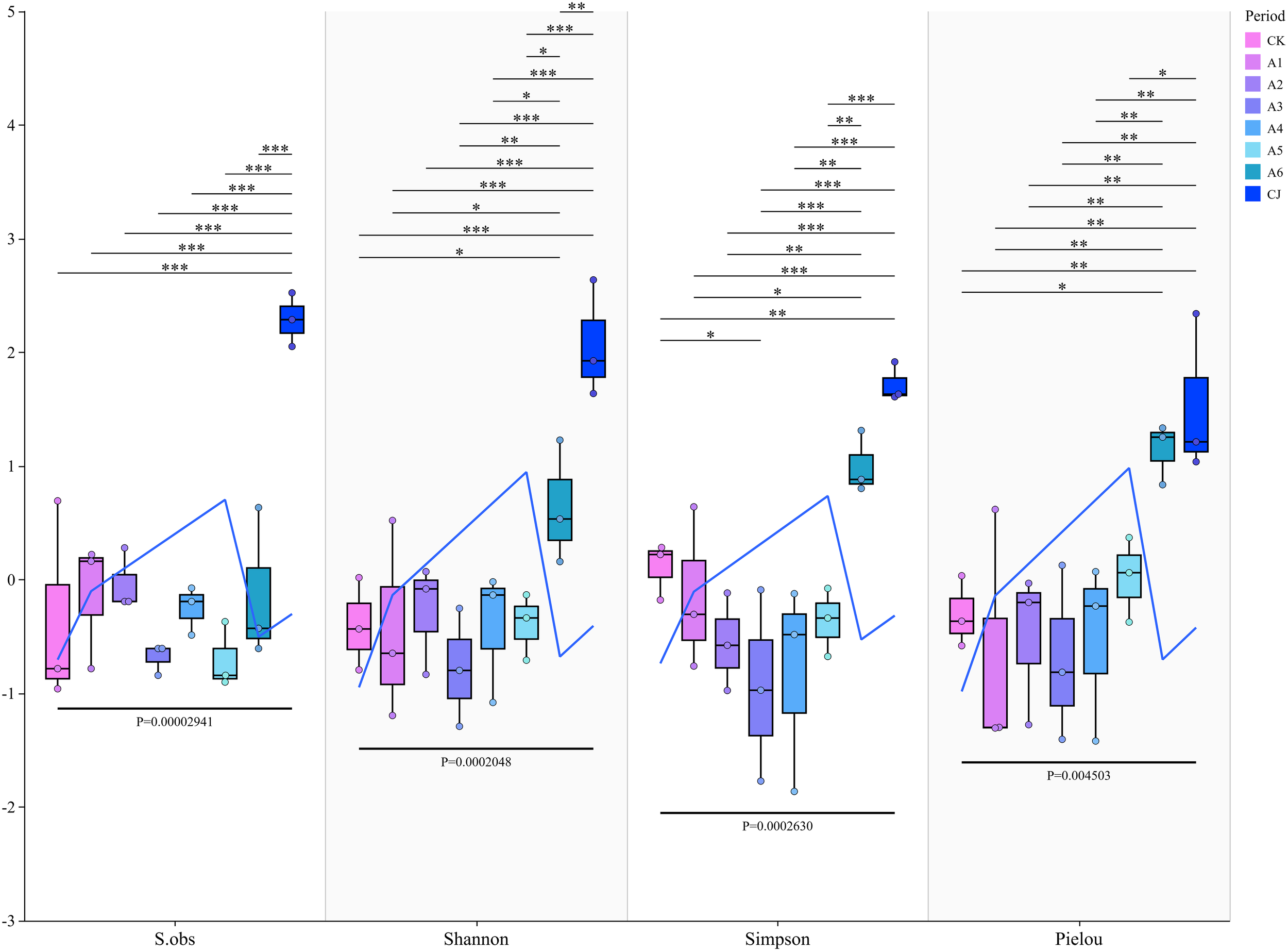
Effects of black tea powder and its fermentation products on α-diversity indices (S.obs, Shannon, Simpson, and Pielou) of gut microbiota in Cherax quadricarinatus. *: p<0.05 (significant difference), **: p<0.01 (very significant difference), ***: p<0.001 (extremely significant difference).
3.3.2.2 β-diversity clustering and PCoA analysis
Hierarchical clustering analysis was employed to construct a phylogenetic tree, illustrating the similarities and groups relationships among the samples (Figure 6A). Principal Coordinate Analysis (PCoA) was conducted to visualize the differences among the samples (Figure 6B). The first principal coordinate (PCo1) accounted for 40.6% of the variation, representing the primary source of variability. The CK, A1, and CJ exhibited PCo1<0, while groups A2 and A3 showed PCo1>0 and PCo2<0, and groups A4, A5, and A6 had PCo1>0 and PCo2>0. Significant differences were observed in PCo1 between groups A1 and A5 and the other groups (P<0.05), and in PCo2 between group A6 and the other groups (P<0.05). These results suggest that the experimental groups can be divided into two main categories and three clusters.
Figure 6
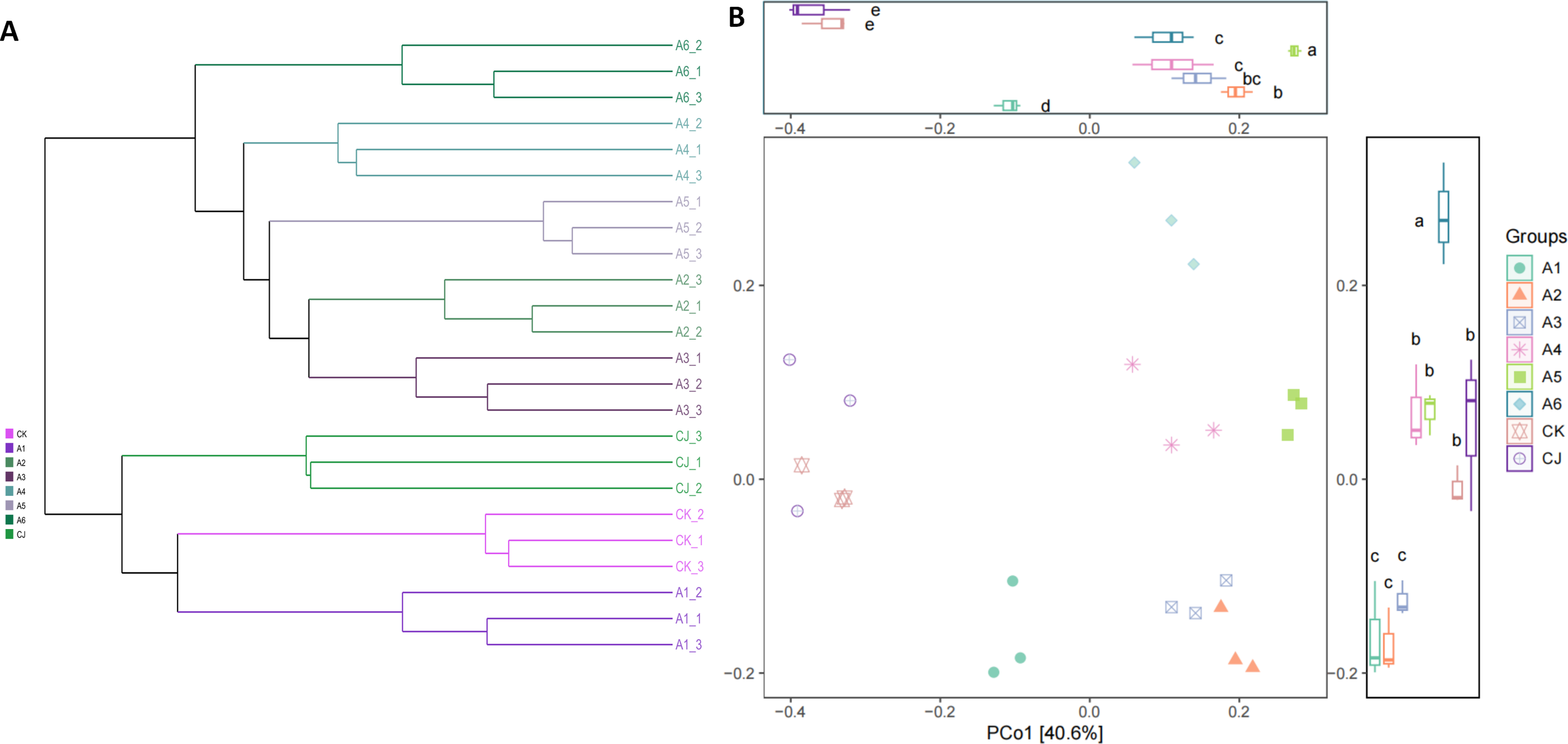
Effects of black tea powder and its fermentation products on β-diversity of gut microbiota in Cherax quadricarinatus. (A) Hierarchical clustering tree. (B) PCoA and significance analysis.
3.3.3 LEfSe analysis of gut microbiota in Cherax quadricarinatus
Linear discriminant analysis effect size (LEfSe) combined with linear discriminant analysis (LDA) scores was used to identify significantly different bacterial taxa among groups (Figure 7A). LEfSe analysis revealed 37 significantly different bacterial taxa between group CK and groups A4–A6 and CJ, while no significant differences were observed between groups A1–A3 and other groups. LDA scores (Figure 7B) showed that group CK had 8 significantly different taxa, primarily Fusobacteriia and Mollicutes. As the BTP supplementation level increased, the number of significantly different taxa in groups A4–A6 decreased. Group A4 was dominated by Flavobacteriia and Bacilli, while groups A5 and A6 were dominated by Betaproteobacteria, Aeromonadales, and Enterobacteriales.Group CJ had the highest number of significantly different taxa, primarily Clostridia, Alphaproteobacteria, and Xanthomonadales.
Figure 7
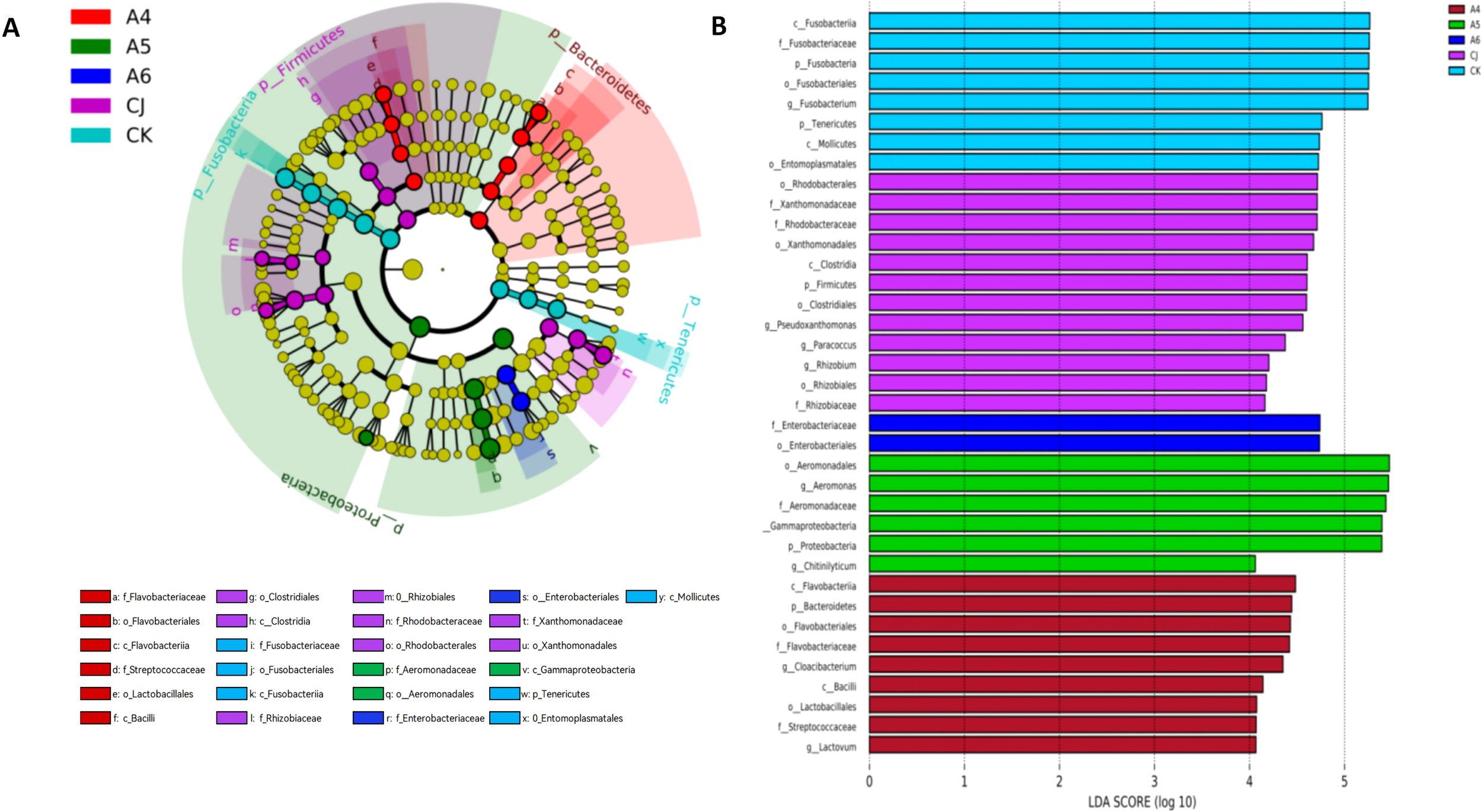
Effects of black tea powder and its fermentation products on significantly different bacterial taxa in gut microbiota of Cherax quadricarinatus. (A) LEfSe analysis results. (B) LDA scores.
3.4 Functional annotation analysis of gut microbiota in Cherax quadricarinatus
Functional annotation of gut microbiota was performed using FAPROTAX (Figure 8). The top 10 functional categories included chemoheterotrophy, fermentation, nitrate reduction, aerobic chemoheterotrophy, nitrogen/nitrate respiration, parasites or symbionts, pathogens, iron respiration, chitinolysis, and mammal gut. The cumulative functional annotation values fluctuated with increasing BTP supplementation, with the highest value in group A2 and the lowest in group A4. The CK and CJ groups showed intermediate values. Chemoheterotrophy, fermentation, and nitrate reduction were the dominant functions, with group A2 having the highest values, followed by group A5. The CK and CJ groups had significantly higher values for aerobic chemoheterotrophy and nitrogen/nitrate respiration than groups A1–A6. Groups A5 and A6 had significantly higher values for parasites or symbionts, pathogens, and iron respiration than other groups.
Figure 8
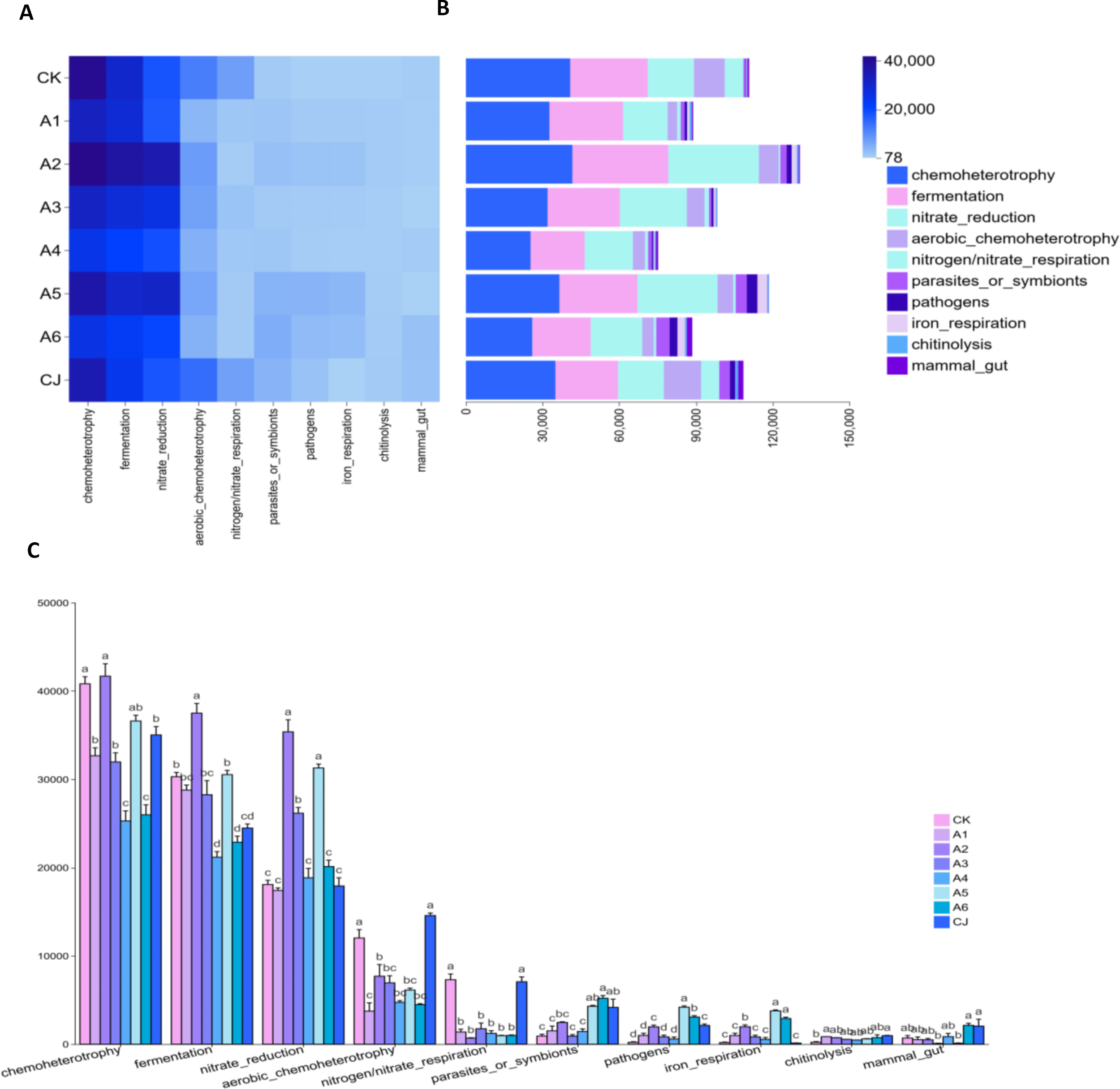
Effects of black tea powder and its fermentation products on functional annotation of gut microbiota in Cherax quadricarinatus. (A) Heatmap of the top 10 functional categories. (B) Cumulative functional annotation values. (C) Significance analysis of the top 10 functional categories.
3.5 Correlation analysis between gut microbiota and muscle protein and lipid content and hepatopancreatic enzyme activity in Cherax quadricarinatus
The Mantel test analysis was conducted to evaluate the correlation between gut microbiota and muscle protein and lipid content, as well as hepatopancreatic enzyme activity (Figure 9). The results indicated that the abundance of Fusobacteria and Tenericutes showed a significant positive correlation with muscle protein, lipid CHO, TG, and LDL content (P<0.05), while demonstrating a negative correlation with hepatopancreatic T-AOC and GHX-X activity(P<0.01). The abundance of Proteobacteria was significantly positively correlated with GHX-PX and SOD activity (P<0.01). Notably, the abundance of Bacteroides was significantly positively correlated with T-AOC activity (P<0.01). The Global Mantel test revealed a significant association between gut microbiota and both muscle protein and lipid content (Mantel’s r = 0.542, 95% CI: [0.492, 0.812]), as well as hepatopancreatic metabolic function (Mantel’s r = 0.506, 95% CI: [0.467, 0.778]).
Figure 9
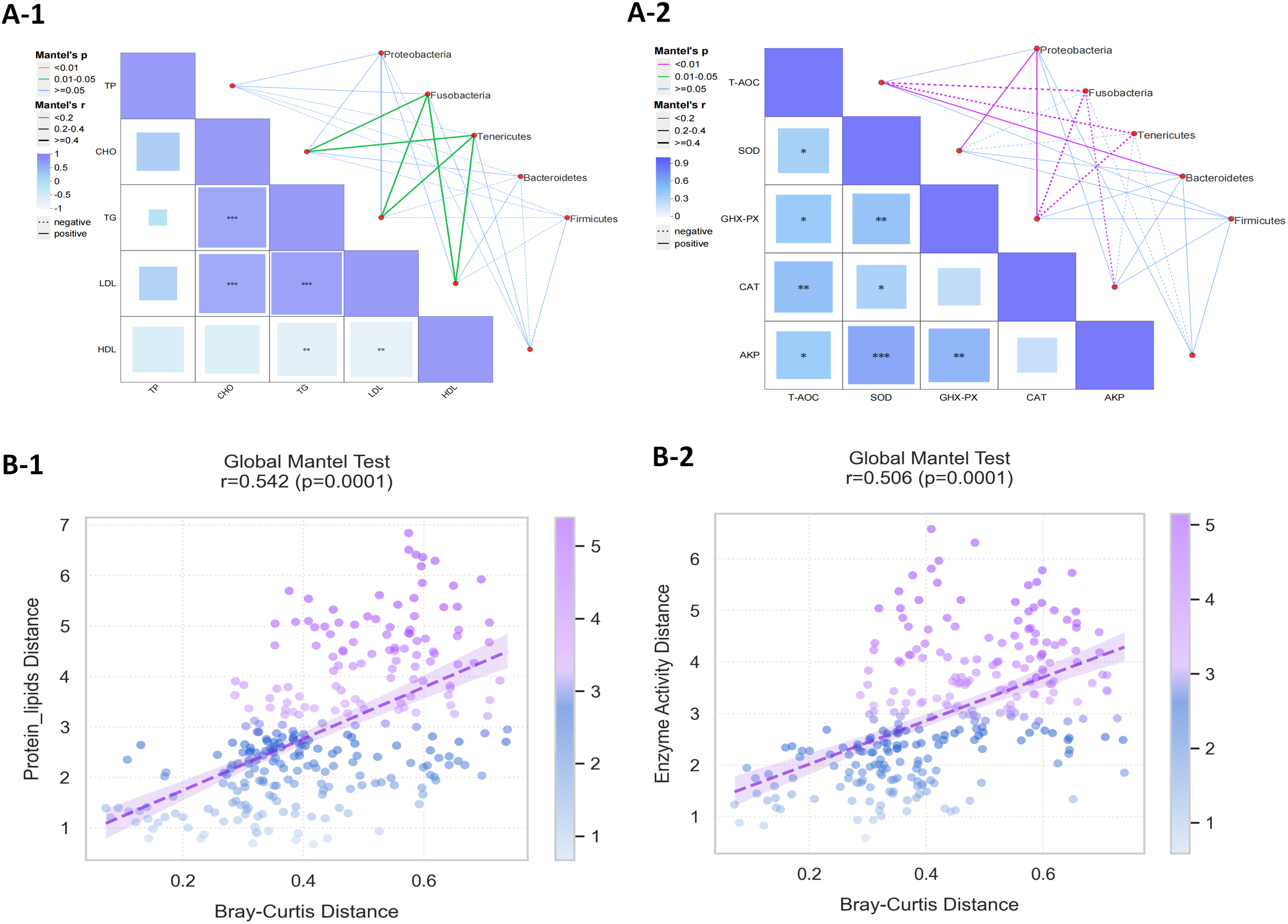
(A) Correlation analysis between gut microbiota and (A-1) muscle protein and lipid content and (A-2) hepatopancreatic enzyme activity in Cherax quadricarinatus. (B) Global bivariate correlation analysis between gut microbial composition (Bray-Curtis distance) and (B-1) muscle protein and lipid content (Protein- Lipids Distance) and (B-2) hepatopancreatic enzyme activity profiles (Enzyme Activity Distance).
4 Results and discussion
4.1 Growth performance and muscle lipid modulation
This study demonstrates that supplementing Cherax quadricarinatus with up to 2% black tea powders (BTP) enhances body weight, carapace length, and muscle protein content, while higher levels (>3%) lead to a decline in these parameters. The black tea powder-probiotics mixed fermentation product (TPMFP) group demonstrated the highest muscle protein content. These results are consistent with studies on juvenile tilapia and largemouth bass (Jiang et al., 2022; Zheng et al., 2017). Additionally, increasing BTP supplementation reduced muscle triglycerides (TG), total cholesterol (TC), and LDL-C, while increasing HDL-C levels. This aligns with previous research showing that black tea reduces fatty acid synthase activity, lowers TG and LDL-C (Du et al., 2005; Zhao et al., 2015), and improves lipid profiles in other species (Zang et al., 2021). In conclusion, low levels of BTP promote growth and enhance lipid metabolism, whereas excessive supplementation has adverse effects. These findings suggest that tea supplementation holds potential benefits for aquaculture.
4.2 Hepatopancreatic antioxidant responses
In our study, the activities of total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), catalase (CAT), and alkaline phosphatase (AKP) in the hepatopancreas showed fluctuating increases with BTP supplementation. Notably, the activities of SOD, GSH-PX, and AKP in the TPMFP-fed group were significantly higher than those in the control group.
Previous studies have reported that in aquatic animals, TPs mitigate drug-induced toxicity (Zhao et al., 2022). TP supplementation, which increases weight gain rate, growth, and antioxidant capacity (CAT, GSH-PX, T-AOC) in grouper, also reduces oxidative stress and improves immunity (Pan et al., 2022). Adding Bacillus subtilis and B. licheniformis(BSL) can significantly enhance the antioxidant status (superoxide dismutase and catalase) of red tilapia (Eissa et al., 2024a). The fermented tea residue enhanced the activities of SOD, GSH-PX, and CAT in juvenile largemouth bass (Jiang et al., 2022). These findings align with our results, suggesting that tea play a positive role in enhancing antioxidant defense and immunity in aquatic species.
4.3 Gut microbiota dynamics and functional shifts
This study demonstrates that the addition of BTP to the diet significantly impacts the gut microbiota of Cherax quadricarinatus. The results showed that as the BTP supplementation level increased, the total amount of intestinal microbes initially rose and then declined.The Proteobacteria abundance increasing as the BTP supplementation level rose, reaching over 70% in groups A2-A6. The abundance of Aeromonas increased with BTP supplementation.The dominant phyla were Proteobacteria, Fusobacteria, and Tenericutes, with the dominant genera being Fusobacterium and Aeromonas, consistent with previous studies (Cheng et al., 2022; Liu et al., 2020). Studies have shown that the fertilized tea residue improves animal intestinal resistance to Aeromonas hydrophila (Jiang et al., 2022).When the BTP addition level reached 5%, Arcobacter and Chitinilyticum were detected; at a 6% addition level, Pseudoduganella, Vogesella, and Acidovorax were identified. Functional annotation analysis of the microbiota revealed that the BTP 2% group showed higher functional annotation values for chemoheterotrophy, fermentation, and nitrate reduction compared to other groups, while the BTP ≥5% group had higher values for parasites/symbionts, pathogens, and iron respiration. This indicates that an appropriate addition of BTP is beneficial for the growth of dominant gut microbiota in Cherax quadricarinatus, but excessive levels lead to microbial imbalance and increased opportunistic pathogens.
These findings are consistent with research showing that theaflavins can increase the diversity of gut microbiota in animals and enhance the abundance of Proteobacteria, Flavonifractor plautii, Bacteroides uniformis, and Eubacterium ramulus (Sun et al., 2023), but excessive levels can decrease their diversity (Liu et al., 2024). In the later stages, detailed research can be conducted on the changes in the abundance of Proteobacteria, Aeromonas, and other bacterial communities due to the addition of high-dose black tea powder. The aim is to distinguish between symbiotic and pathogenic bacterial types, determine whether it causes dysbiosis, and assess its impact on the growth of Cherax quadricarinatus.
Feeding Cherax quadricarinatus with TPMFP significantly increased the α-diversity indices (richness and evenness) of the intestinal microbiota compared to other experimental groups. LEfSe analysis identified Clostridia, Alphaproteobacteria, and Xanthomonadales as significantly different microbial taxa in the TPMFP group.These taxa are known to have positive effects on intestinal metabolic absorption (Meng et al., 2021), anti-inflammatory properties (Zhang et al., 2020), antioxidant activity (Kim and Liesack, 2015), and immune regulation (Huang et al., 2024; Liang et al., 2022). Previous studies have shown that post-fermented dark tea extracts increase the proportion of beneficial gut microorganisms and reduce pathogenic bacteria (Gong et al., 2020). Supplementation with Bacillus coagulans and Lactobacillus can enhance the diversity of gut microbiota and increase the abundance of Firmicutes and Bacteroidota in Cherax quadricarinatus (Zhu et al., 2023). Tea polyphenols can prevent inflammation and improve treatment outcomes in patients with inflammatory bowel disease (Truong and Jeong, 2022), and green tea and lactic acid bacteria fermentation products can enhance the immune function of juvenile rainbow trout (Niazi et al., 2023), further supporting the conclusions of this study.
4.4 Microbiota-antioxidant axis
Global Mantel tests confirmed the biological association between gut microbiota composition and hepatopancreatic metabolic function, reinforcing the microbiota-antioxidant axis in Cherax quadricarinatus. Spearman’s correlation analysis revealed a correlation between specific intestinal microbial taxa and hepatopancreatic enzyme activities. Notably, the abundance of Proteobacteria in the gut microbiota was significantly positively correlated with GSH-PX and SOD activities, while Bacteroidetes abundance showed a positive correlation with T-AOC. Conversely, the abundance of Fusobacteria and Tenericutes showed a significant positive correlation with muscle protein, lipid CHO, TG, and LDL content, while demonstrating a negative correlation with hepatopancreatic T-AOC and GHX-X activity. Studies have shown that Fusobacteria including sub-taxa showed high abundance in the hypertriglyceridemia group (Yun et al., 2020). The glutaredoxins, which function as antioxidants by reducing metals and peroxides, are exclusively encoded in Proteobacteria, Bdellovibrionata, and Verrucomicrobia (Johnson and Hug, 2019). The positive correlation between SOD activity and Proteobacteria network connectivity further supports its role as a potential pathological marker (Degli Esposti and Martinez Romero, 2017). T-AOC, a key indicator of antioxidant potential and immune status (Zhu et al., 2019), may be modulated by Bacteroidales, which stimulate intestinal immunity and metabolic pathways via epithelial signaling (Zhou et al., 2022). Additionally, the negative correlations between GSH-PX/T-AOC and genera such as Morganella, Fusobacterium, and Serratia (He et al., 2021) underscore the detrimental effects of these taxa on redox balance. These findings support the results of this study.
5 Conclusions
This study demonstrates that the supplementation of Cherax quadricarinatus with black tea powder (BTP) and black tea powder-probiotics mixed fermentation product (TPMFP) positively affects growth performance, lipid metabolism, antioxidant responses, gut microbiota dynamics and. Specifically, low concentrations (≤2%) of BTP supplementation improved body weight, carapace length, muscle protein content, lipid profiles and antioxidant responses. However, the addition of high concentrations of BTP has a negative effect.The TPMFP group exhibited the highest muscle protein content and improved antioxidant enzyme activity, significantly impacting gut microbiota composition by promoting beneficial taxa and enhancing microbial diversity. Moreover, the study establishes a microbiota-antioxidant axis in Cherax quadricarinatus, with specific microbial taxa, such as Proteobacteria and Bacteroidetes, showing correlations with key hepatopancreatic enzyme activities. These findings suggest that tea plays a positive role in enhancing both antioxidant defenses and immunity in aquatic species as a natural additive to aquatic feed, thereby improving aquaculture productivity.
The current research results are obtained based on a single laboratory environment, but the lack of independent replication by external institutions remains a significant limitation, which is a key direction for future research.Future research should focus on understanding the specific role of beneficial gut bacteria in immune modulation and their contribution to disease resistance in aquaculture. Given that Cherax quadricarinatus exhibits stronger antimicrobial and stress resistance compared to other aquaculture species, further exploration of antimicrobial resistance genes in this species is needed. Investigating the genetic mechanisms underlying these traits could provide valuable insights into enhancing disease resistance through selective breeding or microbial management. Additionally, developing more stable and effective tea-supplemented aquatic feed products, maximizing the retention of active ingredients in tea, could facilitate large-scale application in aquaculture and further improve productivity.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Department of Research and Industry-Education Integration, Xiamen Ocean Vocational College. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
X-JZ: Conceptualization, Data curation, Funding acquisition, Project administration, Software, Validation, Writing – original draft, Writing – review & editing, Visualization. J-FL: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. C-JW: Formal analysis, Investigation, Methodology, Software, Writing – original draft. J-QX: Funding acquisition, Investigation, Methodology, Resources, Writing – review & editing. Y-SC: Investigation, Methodology, Resources, Validation, Writing – review & editing. X-YL: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Education and Scientific Research Project for Middle-aged and Young Teachers in Fujian Province (JAT191306), the Fujian Provincial Team Science and Technology Special Commissioner Project (2023T3506014, 2024T3506017, 2025T3506026), the Special Project of Fujian Collaborative Innovation Center for Applied Technology of Marine Biological Resources (XTZX-HYSW-1804) and Wu-Chengjian Master Craftsman Studio at Xiamen Ocean Vocational College.
Conflict of interest
Author J-QX was employed by Jiuyu Tea Co., Ltd. Author Y-SC was employed by Haiyo Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ayiku S. Shen J. F. Tan B. P. Dong X. H. Liu H. Y. (2020). Effects of dietary yeast culture on shrimp growth, immune response, intestinal health and disease resistance against Vibrio harveyi. Fish Shellfish Immunol.102, 286–295. doi: 10.1016/j.fsi.2020.04.036
2
Bai S. C. Hamidoghli A. Bae J. (2022). “Feed additives: an overview,” in Feed and feeding practices in aquaculture, 195–229. Sawston, Cambridge, UK: Woodhead Publishing Series in Food Science, Technology and Nutrition. doi: 10.1016/b978-0-12-821598-2.00015-1
3
Bolyen E. Rideout J. R. Dillon M. R. Bokulich N. A. Abnet C. C. Al-Ghalith G. A. et al . (2019). Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol.37, 1091. doi: 10.1038/s41587-019-0252-6
4
Bondad-Reantaso M. G. MacKinnon B. Karunasagar I. Fridman S. Alday-Sanz V. Brun E. et al . (2023). Review of alternatives to antibiotic use in aquaculture. Rev. Aquaculture15, 1421–1451. doi: 10.1111/raq.12786
5
Cheng H. Dai Y. Ruan X. Duan X. Zhang C. Li L. et al . (2022). Effects of nanoplastic exposure on the immunity and metabolism of red crayfish (Cherax quadricarinatus) based on high-throughput sequencing. Ecotoxicol Environ. Saf.245, 114114. doi: 10.1016/j.ecoenv.2022.114114
6
Cole J. R. Wang Q. Fish J. A. Chai B. McGarrell D. M. Sun Y. et al . (2014). Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res.42, D633–D642. doi: 10.1093/nar/gkt1244
7
Daniel C. P. Maulu S. Jiang W. Chen S. Miao L. Ge X. (2024). Dietary tea polyphenol supplementation and its impacts on growth performance, plasma parameters, and antioxidant capacity of juvenile genetically improved farmed tilapia (GIFT: Oreochromis niloticus). Cogent Food Agric.10, 1–12. doi: 10.1080/23311932.2024.2378981
8
Degli Esposti M. Martinez Romero E. (2017). The functional microbiome of arthropods. PloS One12, e0176573. doi: 10.1371/journal.pone.0176573
9
Du Y. T. Wang X. Wu X. D. Tian W. X. (2005). Keemun black tea extract contains potent fatty acid synthase inhibitors and reduces food intake and body weight of rats via oral administration. J. Enzyme Inhib Med. Chem.20, 349–356. doi: 10.1080/14756360500148841
10
Eissa E. H. El-Sayed A. M. Hendam B. M. Ghanem S. F. Abd Elnabi H. E. Abd El-Aziz Y. M. et al . (2024a). The regulatory effects of water probiotic supplementation on the blood physiology, reproductive performance, and its related genes in Red Tilapia (Oreochromis niloticus X O. mossambicus). BMC Vet. Res.20, 351. doi: 10.1186/s12917-024-04190-w
11
Eissa E.-S. H. Monier M. N. Abd El-Aziz Y. M. Saadony S. Abu Husein M. S. Abd El Megeed O. H. et al . (2024b). The efficacy of dietary commercial probiotic (Bacillus subtilis) on growth performance, hemato-biochemical response, and histological status of red tilapia (Oreochromis sp.). J. Appl. Aquaculture37, 45–66. doi: 10.1080/10454438.2024.2338595
12
FAO (2024). The state of world fisheries and aquaculture 2024. Available online at: https://www.fao.org (Accessed January 10, 2025).
13
Garcia Beltran J. M. Esteban M. A. (2022). Nature-identical compounds as feed additives in aquaculture. Fish Shellfish Immunol123, 409–416. doi: 10.1016/j.fsi.2022.03.010
14
Gao C. H. Yu G. Cai P. (2021). ggVennDiagram: an intuitive, easy-to-use, and highly customizable R package to generate venn diagram. Front. Genet.12. doi: 10.3389/fgene.2021.706907
15
Gong Z. P. Ouyang J. Wu X. L. Zhou F. Lu D. M. Zhao C. J. et al . (2020). Dark tea extracts: Chemical constituents and modulatory effect on gastrointestinal function. BioMed. Pharmacother.130, 110514. doi: 10.1016/j.biopha.2020.110514
16
Gowd V. Karim N. Shishir M. R. I. Xie L. Chen W. (2019). Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol.93, 81–93. doi: 10.1016/j.tifs.2019.09.005
17
He H. Teng H. Huang Q. He D. An F. Chen L. et al . (2021). Beneficial effects of AOS-iron supplementation on intestinal structure and microbiota in IDA rats. Food Sci. Hum. Wellness10, 23–31. doi: 10.1016/j.fshw.2020.05.009
18
Huang L. Lu T. Lu X. Shi J. Huang Y. Du X. et al . (2024). Comparison of the intestinal microbiota composition and function of red claw crayfish (Cherax quadricarinatus) cultured in ponds and rice fields. Fishes9, 1–15. doi: 10.3390/fishes9090345
19
Huang Z. Sun L. Wang Y. Deng Q. Fang Z. Zhao L. et al . (2021). Protective mechanism of tea polyphenols against muscle quality deterioration of shrimp (Penaeus vannamei) induced by aflatoxin B1. Aquaculture532, 1–10. doi: 10.1016/j.aquaculture.2020.736093
20
Jiang Z. Qian D. Liang Z. Jia Y. Xu C. Li E. (2023). Effects of dietary plant protein sources intake on growth, digestive enzyme activity, edible tissue nutritional status and intestinal health of the omnivorous Redclaw crayfish, Cherax quadricarinatus. Br. J. Nutr.130, 978–995. doi: 10.1017/S0007114522004044
21
Jiang L. Zhou X. Yu J. Bao S. Li J. Wu Q. et al . (2022). Fermented tea residue improved growth performance, liver antioxidant capacity, intestinal morphology and resistance to Aeromonas hydrophila infection in juvenile largemouth bass (Micropterus salmoides). Front. Mar. Sci.9. doi: 10.3389/fmars.2022.999947
22
Johnson L. A. Hug L. A. (2019). Distribution of reactive oxygen species defense mechanisms across domain bacteria. Free Radic. Biol. Med.140, 93–102. doi: 10.1016/j.freeradbiomed.2019.03.032
23
Kim Y. Liesack W. (2015). Differential assemblage of functional units in paddy soil microbiomes. PloS One10, e0122221. doi: 10.1371/journal.pone.0122221
24
Liang X. Luo X. Lin H. Han F. Qin J. G. Chen L. et al . (2022). Growth, health, and gut microbiota of female pacific white shrimp, litopenaeus vannamei broodstock fed different phospholipid sources. Antioxidants (Basel)11, 1–19. doi: 10.3390/antiox11061143
25
Lin X.-Y. Shih Y.-J. Zhang X.-J. Cai Y.-S. Zhou X.-W. Chen J.-S. (2024). Perspective on intestinal microbiota temporal changes of herbal additives treated shrimp in a natural aquaculture setting. Front. Mar. Sci.11. doi: 10.3389/fmars.2024.1332585
26
Liu X. Hu G. Wang A. Long G. Yang Y. Wang D. et al . (2022). Black tea reduces diet-induced obesity in mice via modulation of gut microbiota and gene expression in host tissues. Nutrients14, 1–22. doi: 10.3390/nu14081635
27
Liu S. Qi C. Jia Y. Gu Z. Li E. (2020). Growth and intestinal health of the red claw crayfish, Cherax quadricarinatus, reared under different salinities. Aquaculture524, 1–12. doi: 10.1016/j.aquaculture.2020.735256
28
Liu C. Zeng H. Cui W. Ouyang J. Zhou F. Wen S. et al . (2024). Theaflavins mitigate diabetic symptoms in GK rats by modulating the INSR/PI3K-Akt/GSK-3 pathway and intestinal microbiota. Int. J. Biol. Macromol277, 134331. doi: 10.1016/j.ijbiomac.2024.134331
29
Mauro M. Di Grigoli A. Maniaci G. Hornsby L. B. Badalamenti G. Chirco P. et al . (2024). Cherax destructor (Clar) and Cherax quadricarinatus (von Marten): Biochemical parameters and preliminary analysis of food quality. Aquaculture Rep.36, 1–9. doi: 10.1016/j.aqrep.2024.102162
30
Meng X. Wu S. Hu W. Zhu Z. Yang G. Zhang Y. et al . (2021). Clostridium butyricum improves immune responses and remodels the intestinal microbiota of common carp (Cyprinus carpio L.). Aquaculture530, 1–11. doi: 10.1016/j.aquaculture.2020.735753
31
Niazi A. Shamsaie Mehrgan M. Rajabi Islami H. (2023). Optimizing turmeric and green tea fermentation with Lactobacillus brevis to enhance growth performance, digestive enzymes, and immunity in rainbow trout. Aquaculture577, 1–9. doi: 10.1016/j.aquaculture.2023.739962
32
Nie X. Huang C. Wei J. Wang Y. Hong K. Mu X. et al . (2024). Effects of photoperiod on survival, growth, physiological, and biochemical indices of redclaw crayfish (Cherax quadricarinatus) juveniles. Anim. (Basel)14, 1–17. doi: 10.3390/ani14030411
33
Pan S. Yan X. Li T. Suo X. Liu H. Tan B. et al . (2022). Impacts of tea polyphenols on growth, antioxidant capacity and immunity in juvenile hybrid grouper (Epinephelus fuscoguttatus female symbol x E. lanceolatus male symbol) fed high-lipid diets. Fish Shellfish Immunol.128, 348–359. doi: 10.1016/j.fsi.2022.08.013
34
Qin M. Wang Z. Liang M. Sha Y. Liu M. Liu J. et al . (2024). Effects of dietary supplementation with tea polyphenols and probiotics on laying performance, biochemical parameters intestinal morphology and microflora of laying hens. Int. J. Biol. Macromol256, 128368. doi: 10.1016/j.ijbiomac.2023.128368
35
Rahayu S. Amoah K. Huang Y. Cai J. Wang B. Shija V. M. et al . (2024). Probiotics application in aquaculture: its potential effects, current status in China and future prospects. Front. Mar. Sci.11. doi: 10.3389/fmars.2024.1455905
36
Roh H. Kannimuthu D. (2023). Comparative resistome analysis of Aeromonas species in aquaculture reveals antibiotic resistance patterns and phylogeographic distribution. Environ. Res.239, 117273. doi: 10.1016/j.envres.2023.117273
37
Sun L. Miao M. (2020). Dietary polyphenols modulate starch digestion and glycaemic level: a review. Crit. Rev. Food Sci. Nutr.60, 541–555. doi: 10.1080/10408398.2018.1544883
38
Sun L. Su Y. Hu K. Li D. Guo H. Xie Z. (2023). Microbial-transferred metabolites of black tea theaflavins by human gut microbiota and their impact on antioxidant capacity. Molecules28, 1–17. doi: 10.3390/molecules28155871
39
Truong V.-L. Jeong W.-S. (2022). Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Hum. Wellness11, 502–511. doi: 10.1016/j.fshw.2021.12.008
40
Vaiyapuri M. Pailla S. Rao Badireddy M. Pillai D. Chandragiri Nagarajarao R. Prasad Mothadaka M. (2021). Antimicrobial resistance in Vibrios of shrimp aquaculture: Incidence, identification schemes, drivers and mitigation measures. Aquaculture Res.52, 2923–2941. doi: 10.1111/are.15142
41
Yu C. Li L. Jin J. Zhang B. Wei H. Zhao Y. et al . (2021). Comparative analysis of gut bacterial community composition during a single day cycle in Chinese mitten crab (Eriocheir sinensis). Aquaculture Rep.21, 1–9. doi: 10.1016/j.aqrep.2021.100907
42
Yu H. Sattanathan G. Yu L. Li L. Xiao Y. (2024). Impact of nutritional tea polyphenols on growth, feed efficiency, biochemical traits, antioxidant capacity, haematological parameters and immunity in coho salmon (Oncorhynchus kisutch). Anim. (Basel)14, 1–15. doi: 10.3390/ani14142104
43
Yun K. E. Kim J. Kim M. H. Park E. Kim H. L. Chang Y. et al . (2020). ). Major lipids, apolipoproteins, and alterations of gut microbiota. J. Clin. Med.9, 1–16. doi: 10.3390/jcm9051589
44
Zang L. Shimada Y. Nakayama H. Katsuzaki H. Kim Y. Chu D. C. et al . (2021). Preventive effects of green tea extract against obesity development in zebrafish. . Molecules26, 1–14. doi: 10.3390/molecules26092627
45
Zhang Y. Li Z. Kholodkevich S. Sharov A. Chen C. Feng Y. et al . (2020). Effects of cadmium on intestinal histology and microbiota in freshwater crayfish (Procambarus clarkii). Chemosphere242, 125105. doi: 10.1016/j.chemosphere.2019.125105
46
Zhao Y. Asimi S. Wu K. Zheng J. Li D. (2015). Black tea consumption and serum cholesterol concentration: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr.34, 612–619. doi: 10.1016/j.clnu.2014.06.003
47
Zhao Y. Fang C. Jin C. Bao Z. Yang G. Jin Y. (2022). Catechin from green tea had the potential to decrease the chlorpyrifos induced oxidative stress in larval zebrafish (Danio rerio). Pestic Biochem. Physiol.182, 105028. doi: 10.1016/j.pestbp.2021.105028
48
Zhao Y. Yang Q. E. Zhou X. Wang F.-H. Muurinen J. Virta M. P. et al . (2020). Antibiotic resistome in the livestock and aquaculture industries: Status and solutions. Crit. Rev. Environ. Sci. Technol.51, 2159–2196. doi: 10.1080/10643389.2020.1777815
49
Zheng Q. Han C. Zhong Y. Wen R. Zhong M. (2017). Effects of dietary supplementation with green tea waste on growth, digestive enzyme and lipid metabolism of juvenile hybrid tilapia, Oreochromis niloticus x O. aureus. Fish Physiol. Biochem.43, 361–371. doi: 10.1007/s10695-016-0292-5
50
Zhou Q. Shen B. Huang R. Liu H. Zhang W. Song M. et al . (2022). Bacteroides fragilis strain ZY-312 promotes intestinal barrier integrity via upregulating the STAT3 pathway in a radiation-induced intestinal injury mouse model. Front. Nutr.9. doi: 10.3389/fnut.2022.1063699
51
Zhu L. Kong Y. Chang X. Feng J. Wang X. Hou L. et al . (2023). Effects of two fish-derived probiotics on growth performance, innate immune response, intestinal health, and disease resistance of Procambarus clarkii. Aquaculture562, 1–11. doi: 10.1016/j.aquaculture.2022.738765
52
Zhu K. Zeng X. Tan F. Li W. Li C. Song Y. et al . (2019). Effect of insect tea on D-galactose-induced oxidation in mice and its mechanisms. Food Sci. Nutr.7, 4105–4115. doi: 10.1002/fsn3.1278
Summary
Keywords
Cherax quadricarinatus , black tea, probiotic fermentation, antioxidant enzymes, gut microbiota
Citation
Zhang X-J, Lin J-F, Wu C-J, Xie J-Q, Cai Y-S and Lin X-Y (2025) Response of black tea powder and its fermentation products on growth performance, antioxidant capacity, and gut microbiota of Cherax quadricarinatus. Front. Mar. Sci. 12:1574890. doi: 10.3389/fmars.2025.1574890
Received
11 February 2025
Accepted
08 July 2025
Published
06 August 2025
Volume
12 - 2025
Edited by
Antonella Leone, Istituto di Scienze delle Produzioni Alimentari, Italy
Reviewed by
Zhenlu Wang, Guizhou University, China
Filomena Fonseca, University of Algarve, Portugal
Updates
Copyright
© 2025 Zhang, Lin, Wu, Xie, Cai and Lin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu-Yin Lin, xmhy000@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.