Abstract
Fish oil and fish meal increase the nutritional value of fish meat for human consumption. The aquafeed industry constantly assess new ingredients to improve flexibility and sustainability of the raw materials. The search for alternative ingredients could be helped by the development of an in vitro platform that could screen promising candidates in a fast and cheap way. Aim of this paper is to determine if a platform using rainbow trout intestinal cell lines can discriminate the functional differences of feed formulations characterized by contrasting properties. We compared a reference diet, rich in fish meal (FM), with diets with high inclusion of either soybean meal (SBM) or feather meal (FTHM). Before exposing the diets to the in vitro platforms, pellets were exposed to gastric and intestinal RT enzymes to extract the bio-accessible fraction (BAF). Rainbow trout cell lines, derived from the proximal (RTpiMI) or distal (RTdiMI) intestine were exposed to BAFs for 21 days. Barrier integrity and functionality were assessed measuring transepithelial electrical resistance, evaluating cell morphology and determining alanine aminopeptidase enzyme activity. Results showed that SBM disrupted the epithelial barrier formed by cells of the proximal but not the distal intestine. However, this effect was reversible, as barrier integrity was fully restored once the SBM was removed. In contrast, FTHM induced a progressive cell proliferation in both proximal and distal intestinal cell line. This milder effect could mimic an inflammatory response, that was induced also by the FM, possibly due to the lack of mucus in vitro, leaving intestinal cells without a physiological protection. In conclusion, in vitro trials showed a variable range of responses to the diets depending on the intestinal region of origin of the cells, providing a functional ranking of the diets. Moreover, the ability of the proximal intestine cells to fully recover from the initial damage caused by SBM suggests that this platform could also be used to identify target molecules that can mitigate the effects of the anti-nutritional factors present in raw materials such as SBM.
Introduction
The high-quality proteins and beneficial omega-3 fatty acids found in fish meat (Raatz et al., 2013), together with the global population growth are the main reasons for the constant increase in fish consumption. Such growing demand cannot be satisfied by wild capture fisheries which reached a plateau some years ago, making the supply of fish from aquaculture an essential industry to meet global demand (Food and Agriculture Organization of the United Nations, 2020). Due to limitations on the further expansion of fish meal, fish oil, and other marine-derived feed ingredients, many alternative raw materials have been utilized to substitute fish meal and fish oil in aquafeeds. However, these alternatives often contain anti-nutritional factors, that may negatively impact fish health and growth (Krogdahl et al., 2022). For this reason, it is increasingly important to search for more sustainable and nutritionally effective alternatives, including algae, yeast, insect meal, and poultry by-products (Barrows and Frost, 2014; Terova et al., 2021; Velichkova et al., 2024). Evaluating new raw materials and functional additives typically requires in vivo feeding trials which are costly, time-consuming, and involve the use of many animals. Therefore, developing an in vitro model able to simulate intestinal functions more ethically and efficiently is gaining increasing interest.
In mammals, in vitro intestinal models offer a cost-effective approach for toxicology and drug absorption studies, reducing the need for animal testing. These models use immortalized cell lines derived from the intestinal epithelium such as Caco-2 (Hidalgo et al., 1989) and IPEC-J2 (Geens and Niewold, 2010). These cells can proliferate indefinitely, providing a tool for conducting repeatable experiments and long-term studies. However, the actively proliferating cells present in the immortalized cell lines often have a lower degree of differentiation, presenting a significant limitation (Maqsood et al., 2013). Indeed, they remain in a less specialized phase, which can distort study results, as poorly differentiated cells might behave differently compared to their more mature counterparts present in the organism. Bicameral culture devices better replicate the intestinal barrier by using a permeable membrane to simulate luminal and vascular sides. This setup provides mechanical cues to the cells which are driven into a further differentiation (Hilgers et al., 1990). The higher similitude allows for more reliable mechanism studies.
The first stable fish intestinal cell line (Kawano et al., 2011), was derived many years ago from the distal intestine in rainbow trout (Oncorhynchus mykiss) and further two have been recently derived from both the proximal and distal intestine of the same species in our laboratory (Pasquariello et al., 2021). These cell lines, named RTpiMI and RTdiMI respectively, have been used in this study. A few stable intestinal cell lines have been derived in other species only very recently: i.e. in grouper (Epinephelus coioides) (Fan et al., 2023), in turbot (Scophthalmus maximus) (Liu et al., 2023), in Japanese flounder (Paralichthys olivaceus) (Su et al., 2024) and in black rockfish (Xue et al., 2024). Our rainbow trout intestinal cell lines preserve many characteristics of the organ from which they originated, notably exhibiting significant cellular heterogeneity, including stem cells, a transient amplifying population, and various cell types at different levels of differentiation (Pasquariello et al., 2021). Moreover, cell lines from the distal intestine have a higher rate of duplication compared to the proximal intestine (Pasquariello et al., 2021) and in general respond differently to the same stimulus indicating that they preserve the memory of their different origin.
The aim of this paper was to determine if an in vitro platform can discriminate the functional differences of feed formulations characterized by contrasting properties. Here we used a reference diet rich in fish meal (FM) well known for its high digestibility and widespread use in the market; a diet with high inclusion of soybean meal (SBM) because of its well-known enteritis inducing effects (Baeverfjord et al., 1996; Bureau et al., 1999); and a third one with high inclusion of feather meal (FTHM), obtained from the processing of poultry slaughterhouse waste, because of its low digestibility (Fanizza et al., 2023).
Materials and methods
In vitro digestion: bio-accessible fraction extraction
Three contrasting diets were formulated according to requirements and produced by extrusion in 3 mm pellet size at Skretting Aquaculture Innovation feed technology plant (Stavanger, Norway): a reference diet, rich in fish meal (FM); a diet with high inclusion of soybean meal (SBM), known to produce intestinal inflammation in vivo, a diet with high inclusion of feather meal (FTHM), obtained from the processing of poultry slaughterhouse waste whose compositions are detailed in Table 1.
Table 1
| Ingredient % | FM | SBM | FTHM |
|---|---|---|---|
| Fish Meal | 35.00 | 5.00 | 5.00 |
| Wheat | 21.73 | 6.07 | 18.19 |
| Wheat Gluten | 9.00 | 23.50 | 13.22 |
| Faba Bean | 5.00 | 4.97 | 5.00 |
| Spc | 10.37 | 8.38 | 15.00 |
| Astaxanthin | 0.05 | 0.05 | 0.05 |
| Fish Oil | 9.43 | 10.48 | 9.97 |
| Rapeseed Oil | 9.50 | 10.56 | 10.05 |
| Vit/Min Premix | 0.75 | 0.75 | 0.75 |
| Phosphate | 0.10 | 1.32 | 1.46 |
| Dl-Methionine | 0.19 | 0.35 | |
| Lysine | 0.57 | 1.12 | |
| Ytrium Premix | 0.10 | 0.10 | 0.10 |
| Soy Bean Meal | 30.00 | ||
| Feather Meal | 20.00 | ||
| Water/Moisture Change | -1.04 | -1.93 | -0.26 |
| Nutrient Volume % | 100.00 | 100.00 | 100.00 |
| Dry Matter | 93.00 | 93.00 | 93.00 |
| Crude Protein | 42.88 | 42.51 | 45.24 |
| Crude Fat | 23.00 | 23.50 | 23.00 |
| Ash | 5.69 | 4.89 | 4.20 |
Feed formulation and proximate composition.
Using the same approach as previously described (Pasquariello et al., 2023), fish feed pellets were digested in vitro using enzymes extracted from the rainbow trout digestive system. Briefly, following the protocol of Brodkorb et al. (2019) with some adjustments, feed pellets were finely ground before being mixed with water at a ratio of 0.125 g for each ml. Then, feeds were incubated sequentially with stomach (1,500 U/ml pepsin activity, pH 4.0) and intestinal (100 U/ml of trypsin activity, pH 8.0) enzyme extracts at 20°C for six hours each, simulating the stages of gastric and intestinal digestion. The remaining enzymatic activity was inactivated by heating the solutions to 90°C for 10 minutes. The insoluble fraction was then eliminated by centrifuging the samples at 15,000 g for 30 minutes. The supernatant of the in vitro digested feed (BAF) was collected and lyophilized for at least 24 h.
Cell lines routine and seeding of the epithelial cells
We used the RTpiMI (passage 40) and RTdiMI (passage 37) epithelial cell lines, previously isolated from the proximal and distal intestinal tract of rainbow trout (Pasquariello et al., 2021). In both cases, organs were isolated from animals destined for human consumption and, therefore, were not considered as animal experimentation under Directive 2010/63/EU of the European Parliament.
Both cell lines were maintained in complete medium made of Leibovitz’s culture medium (L-15, Thermo Fisher Scientific, Waltham, MA, USA, cat. no. 11415064) supplemented with 2 mM L-glutamine, 10,000 units/mL penicillin, 10.0 mg/mL streptomycin, 25.0 µg/mL amphotericin B (Merck, Darmstadt, Germany, cat. no. A5955), and 5% fetal bovine serum (FBS, Thermo Fisher Scientific Waltham, MA, USA cat. no. 10270106). Both cell lines were cultured in 75 cm2 tissue culture flasks (T75, Sarstedt, Nurmbrecht, Germany, cat. no. 83.3911) at 20°C in air, and medium was replaced twice a week. Every 7 days both lines reached 80% confluency, were washed thrice in PBS, trypsinized, and passaged at 1:2 ratio for regular propagation. Otherwise, cells were counted and seeded at the final density of 2.5×105 cells/cm2, onto ThinCert™ polyethylene terephthalate (PET) translucent permeable supports with 113 mm2 surface and 0.4 µm pore size (Greiner Bio-One, Kremsmunster, Austria, cat. no. 665640) of a bicameral culture device. The medium was replaced twice a week.
Monitoring of the transepithelial electrical resistance
TEER was measured twice a week to determine when the value reached its plateau, as this indicates the establishment of a functional epithelial barrier. Measurements were performed using an EVOM™ Manual machine coupled with STX4 electrodes. Three equidistant points of the insert perimeter were measured, and their average was calculated. A blank insert, without cells, was run as a control, and the TEER value was calculated using the following formula:
Exposure to BAFs
The lyophilized BAF of each diet was resuspended in serum free L-15 medium and mixed by inversion. Samples were centrifuged at 15,000 g for 15 minutes at +4°C to remove any debris and the supernatant was collected and sterilized with a 0.22 µm syringe filter.
To monitor barrier integrity, TEER was measured twice a week at the time of medium replacement during the whole experiment. Once cells reached the TEER plateau value (usually after 14 days of culture), the medium in the apical compartment was replaced with BAFs diluted 50% in L-15 medium (supplemented with 10% FCS, 2% glutamine and 2% antibiotic/antimycotic solution), while in the basolateral compartment we continue to use complete L-15 medium. In each experiment, exposure to BAF was performed on 4 replicates for each feed. Negative controls were cultured in L-15 complete medium without BAF. Exposure was arrested 21 days after TEER reached its plateau.
Recovery test
When a diet caused a significant decrease in the TEER value, half of the samples were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin for histological analysis. In order to assess whether the damage was reversible or not, the others samples were subjected to a recovery test. To this purpose, BAF was removed and substituted with complete L-15 medium. TEER continued to be monitored, and cells were cultured until day 21 as the other samples.
Alanine aminopeptidase activity
At the end of the experiment (21 days), the enzymatic activity of alanine aminopeptidase (AAP) was measured as a marker of intestinal cell differentiation. The in-situ quantification of AAP activity was performed as described by (Ferruzza et al., 2012). Briefly, all inserts were washed three times in PBS supplemented with 1 mM CaCl2 and 1 mM MgCl2 (PBS+) to discourage cell detachment. PBS+ was left in the basolateral compartment, while the enzyme substrate (5 mM L-alanine 4-nitroanilide hydrochloride diluted in 10 mM Tris-HCl and 150 mM NaCl at pH 8.0, in PBS) was added to the apical compartment. After 40 minutes of incubation at room temperature, 100 μl of solution was collected from the apical compartment and transferred to a 96-well plate kept on ice. Absorbance was determined using a microplate reader at an optical density of 405 nm. The enzyme concentration was calculated using a standard curve prepared from a 1 mM p-nitroanilide (p-NA) solution.
Histological analysis
Since the alanine aminopeptidase assay did not damage the epithelial barrier, it was possible to use the same samples for morphological analyses.
Samples were fixed in 4% PFA in PBS, overnight. Then, all inserts were washed three times in PBS, dehydrated, cleared in Histoclear (Histo-Line, UN2319) and embedded in paraffin. For each sample, 3 sections of 5µm were stained with hematoxylin and eosin to assess the general morphology of the cells or with Periodic acid–Schiff (PAS) to detect the presence of PAS-positive vacuoles. A semiquantitative scoring system has been applied to assess the frequency of vacuole distribution in the samples exposed to the different BAFs. The absence of vacuoles has been indicated with “-” while “+”, “++” and “+++” indicated a low (1–3 vacuoles/field), medium (3–5 vacuoles/field) or high frequency (> 5 vacuoles/field) respectively (Figure 1). For each sample, 3 fields were acquired using a Leica DMR microscope equipped with a Nikon DS-Ri2 camera and the NIS-Elements D software, version 5.20.
Figure 1
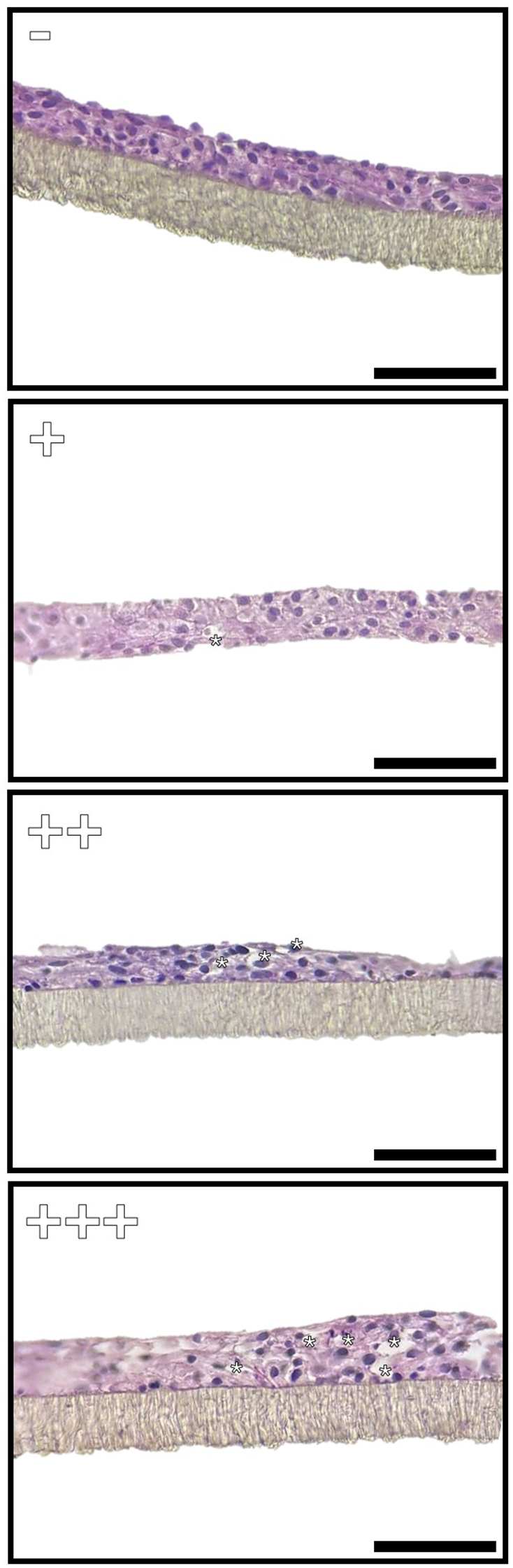
Representative images of the semiquantitative scoring system applied to assess the frequency of vacuole distribution in the samples exposed to the different BAFs. The absence of vacuoles has been indicated with “−” while “+”, “++” and “+++” indicated a low (1–3 vacuoles/field), medium (3–5 vacuoles/field) or high frequency (> 5 vacuoles/field) respectively. Scale bar 40 µm.
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics (Version 28.0.0.1). Normal distribution and homogeneity of variance (homoscedasticity) were tested using Shapiro–Wilk test and Levene’s test, respectively. Results were analyzed using one-way ANOVA followed by Tukey’s post hoc test and are presented as mean ± standard deviation. p-values of less than 0.05 were considered significant.
Results
Functional evaluations
Trans-epithelial electrical resistance
In both cell lines, exposure to FM caused a statistically significant increase of TEER values compared to the control represented by cells cultured in complete L-15 medium only. The exposure to FTHM also resulted in an increase in TEER. However, in the proximal intestine cell line, the increase was significantly lower compared to that induced by FM, while in the distal cell line, the increase was comparable between the two diets. Interestingly, the exposure to SBM caused opposite effects on the two cell lines: in RTpiMI, TEER sharply decreased after just 3 days of exposure while, in RTdiMI, TEER gradually increased having a similar effect as exposure to FM and FTHM (Figures 2A, B).
Figure 2
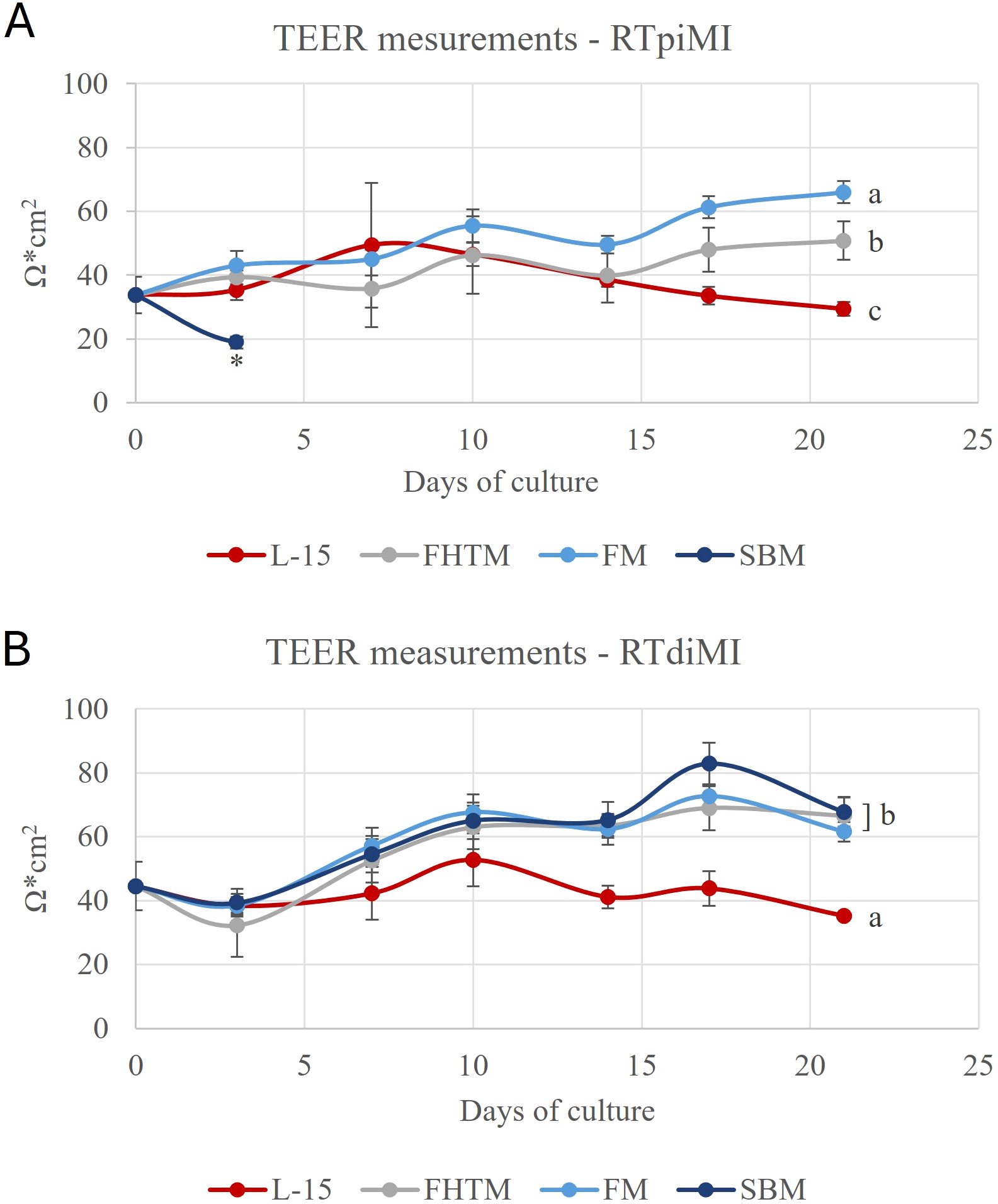
Graphs showing the transepithelial electrical resistance (TEER) values during the chronic exposure to the bioaccessible fractions (BAF) extracted from the three different diets fish meal (FM), feather high meal (FTHM), and soybean meal (SBM) in the RTpiMI (A) and RTdiMI (B) cells. The controls were performed by exposing the platform to L-15 complete medium without BAF. The values are expressed as mean ± standard deviation. Asterisk indicates statistically significant differences (p<0.05) on 3 days of exposure, while different letters refer to day 21.
RTpiMI cells exhibited distinct responses to each diet. SBM caused a sharp reduction in TEER after 3 days, whereas the other two diets led to a significant value increase after 21 days of exposure, with the increase being significantly different between the two. In contrast, TEER values in RTdiMI cells after 21-day exposure to the three diets were statistically similar and all significantly higher than the control (Figures 2A, B).
Following the TEER decrease observed in RTpiMI, cells exposed to SBM for 3 days, a recovery test was performed to assess the extent of the damage. On day 3, SBM was replaced with complete L-15 medium and cultured for a further 18 days until day 21. At the end of the culture the samples reached the same TEER value as the L-15 control (Figure 3).
Figure 3
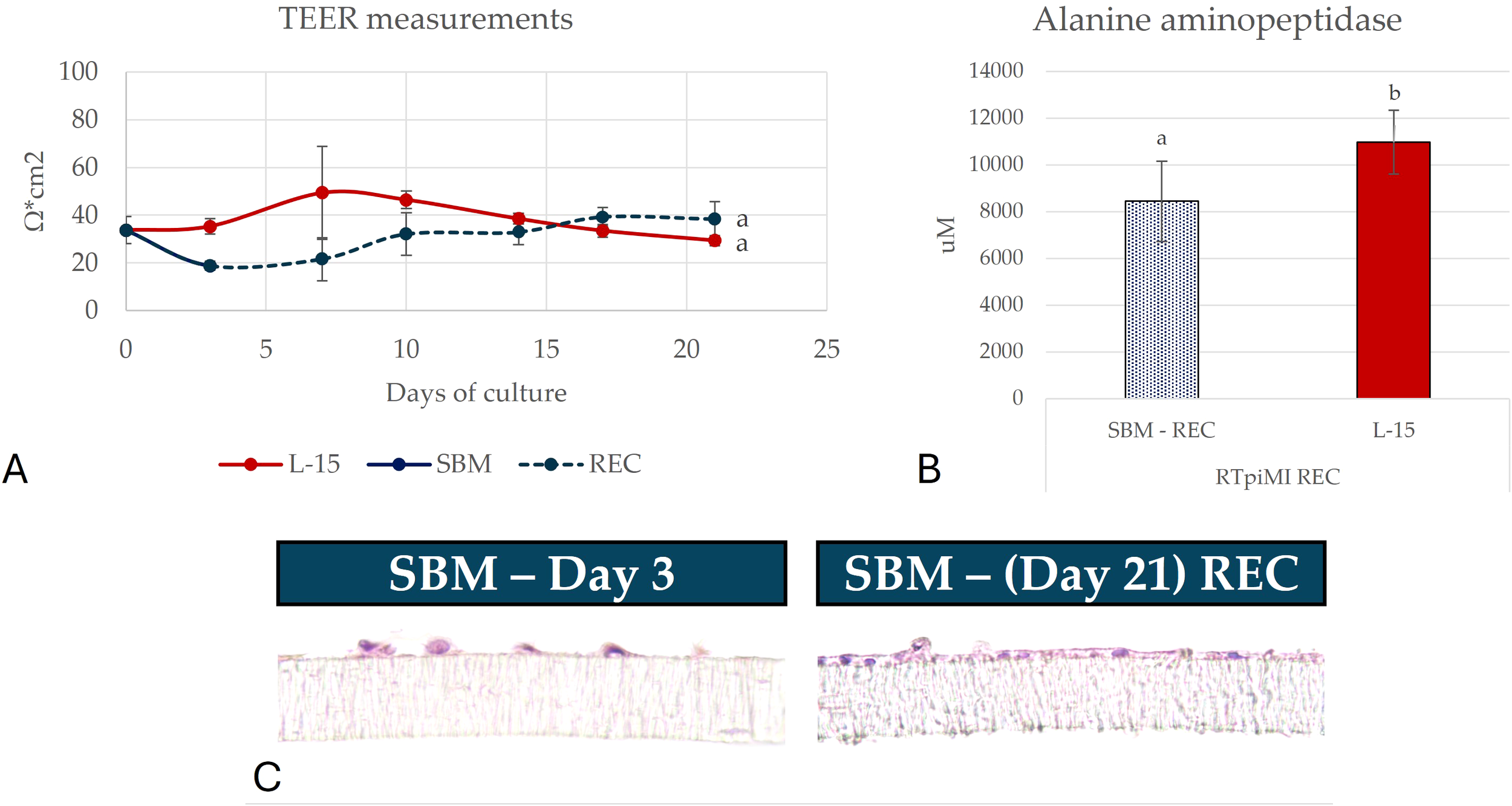
Representative picture showing (A) transepithelial electrical resistance (TEER), (B) alanine aminopeptidase (AAP) activity and (C) the morphology of samples damaged by soybean meal (SBM) and subsequently recovered using L15 medium. The values are expressed as mean ± standard deviation. Different letters indicate statistically significant differences (p<0.05). Scale bar 40 µm.
Alanine aminopeptidase activity
Alanine aminopeptidase activity was quantified at the end of the exposure. In both cell lines, we observed a significant increase of enzymatic activity in cells exposed to all diets.
In RTpiMI cells, the increase was diet dependent: FM increased the enzyme activity, but to a lesser extent than FTHM (Figure 4). Cells exposed to SBM showed a sharp decrease of the enzyme activity following the initial damage. However, after replacing SBM with L-15 complete medium, their AAP activity reached the same level of the control (Figure 3).
Figure 4
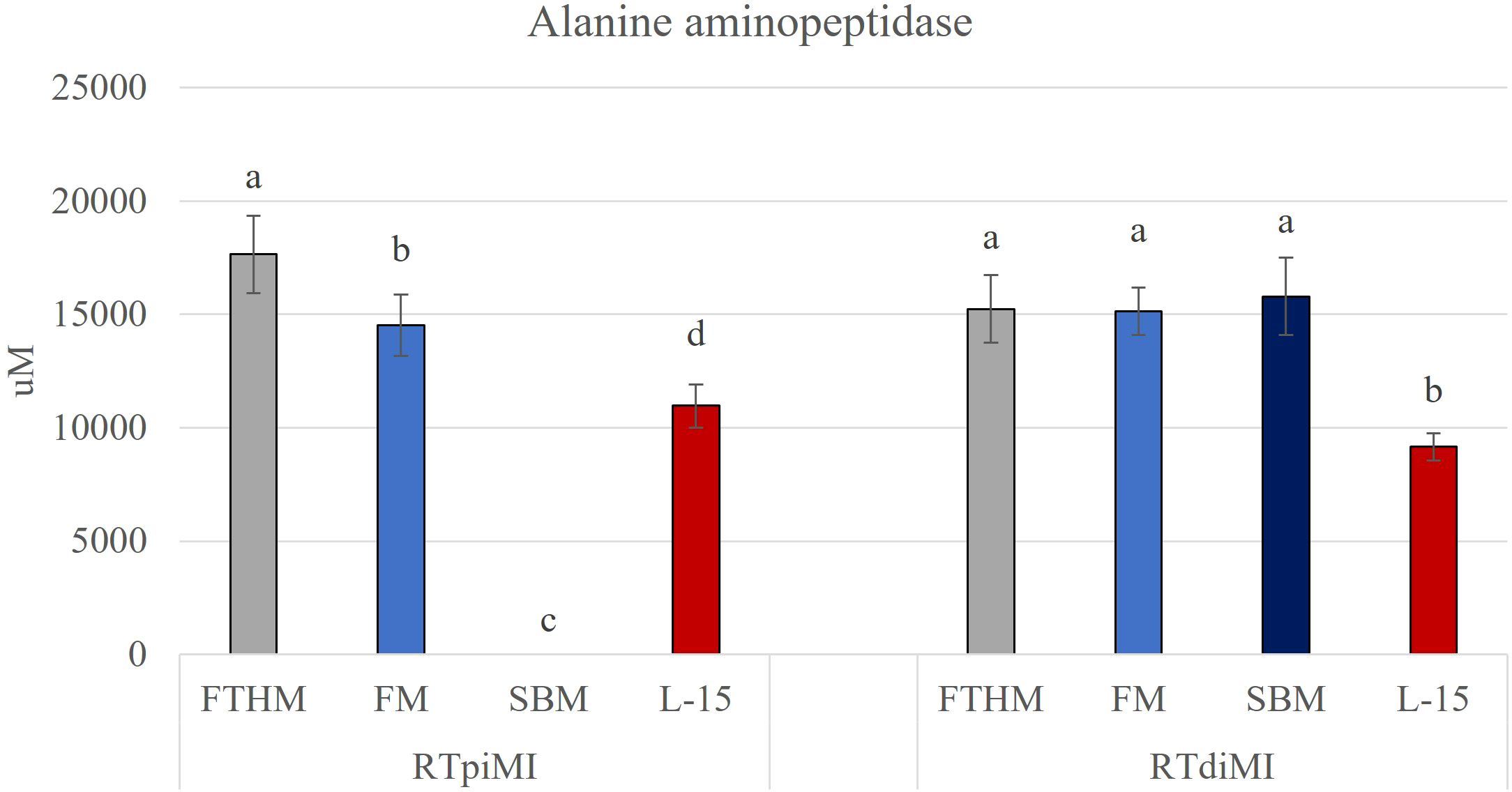
Bar charts show the quantification of alanine aminopeptidase (AAP) activity in the proximal (RTpiMI) and distal (RTdiMI) cell lines cultured on the ThinCert insert after 21 days of exposure to feather high (FTHM), fish meal (FM), and soybean meal (SBM) diets. Values are expressed as mean ± standard deviation. Different letters in the same group indicate statistically significant differences (p<0.05).
In contrast, exposing RTdiMI cells to all diets led to a significant homogeneous increase in this enzyme activity compared to the control (Figure 4).
Morphological evaluations
Cell proliferation
Microphotographs of the morphological appearance of the epithelial samples exposed to the different treatments are presented in Figure 5. At the end of the experiment, the morphological analysis of control samples (not exposed to BAF) revealed a compact monolayer forming a barrier in both cell lines. RTpiMI cells were well-aligned, forming a clear monolayer, while the RTdiMI monolayer was less orderly, and in some areas, cells started to overlap.
Figure 5
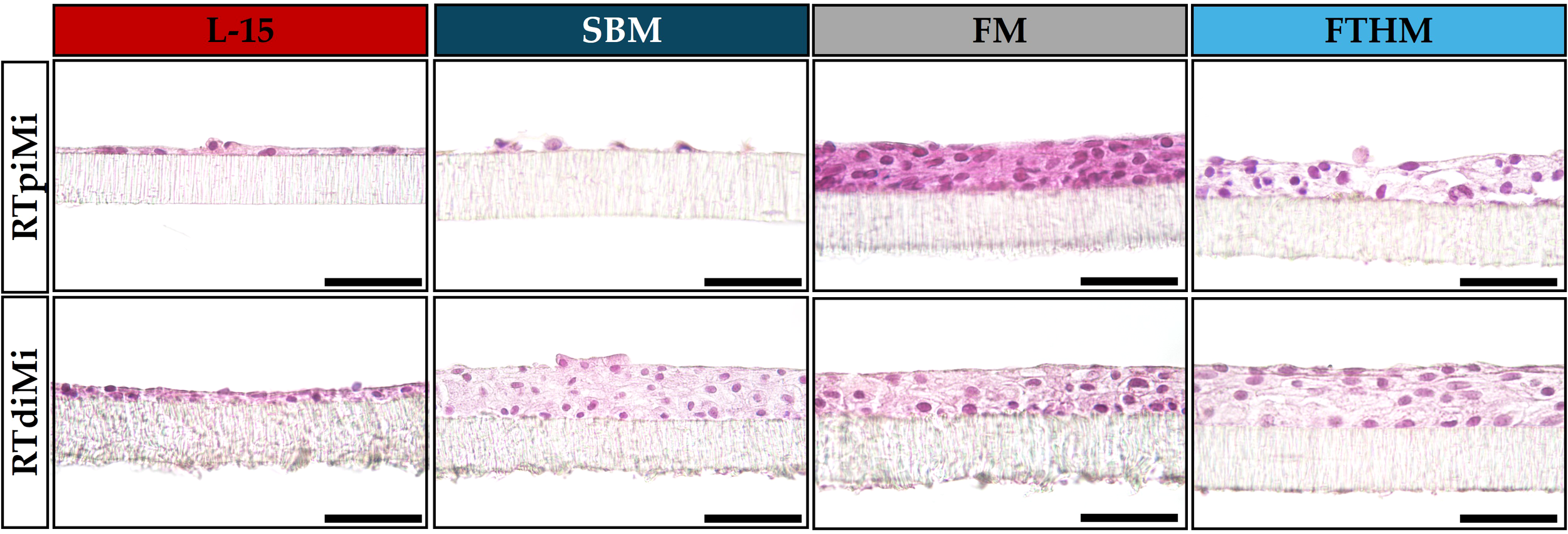
Representative images of sections from the two cell lines (RTpiMI and RTdiMI) exposed to the feather high (FTHM), fish meal (FM), and soybean meal (SBM) diets for 21 days. Controls were performed by exposing the platform to L-15 complete medium without diets bioactive fraction. Sections were stained with hematoxylin and eosin. Scale bar 40 µm.
In samples exposed to FM, both cell lines proliferated, forming a compact stratified epithelium. Cells exposed to this diet exhibited more eosinophilic cytoplasm compared to the respective control, and this difference was more noticeable in RTpiMI cells.
Exposure to FTHM also resulted in epithelium stratification. The numerous layers were more loosely arranged in RTpiMI and more compact in RTdiMI cells. The cytoplasm of cells exposed to this diet showed a less intense staining compared to cells exposed to FM and to control medium (L-15).
The RTpiMI cell monolayer exposed to SBM for 3 days, showed clear disruptions in the cellular barrier in numerous points in parallel with the sharp decrease of TEER and AAP activity described above. At the end of the recovery period, consisting of 18 days of culture with complete L-15 medium, RTpiMI cells were able to recreate a functional monolayer, with even a few areas showing multiple layers (Figure 3). In contrast, the exposure of RTdiMI cells to SBM for 21 days resulted in the formation of a stratified epithelium, with cells showing a lighter cytoplasm compared to those exposed to the other diets and the control.
Presence of vacuolated cells
At the end of the culture period (day 21) morphological analysis showed the presence of vacuoles: round formations within the cell that appeared as empty spaces under the microscope. Since their presence gave the cells a certain resemblance to goblet cells, we investigated whether these vacuoles contained mucins by performing a PAS staining (Figure 6). This revealed the presence of positive vacuoles exclusively in RTpiMI samples, in variable quantities depending on the diet. Specifically, SBM led to the development of the higher number of vacuoles in 3 days, while the other two diets, after 21 days, showed an intermediate number with FTHM, and fewer with FM. Generally, we observed only one vacuole per cell that occupied a large portion of the cytoplasm, pushing the nucleus towards the basal margin. As shown in Table 2, vacuole quantity depended on the diet, with FM inducing the formation of a few vacuoles, FTHM determining an intermediate situation and SBM causing the highest presence of vacuoles already after 3 days. In RTdiMI, only a few diet-induced vacuoles were observed in the SBM, while control medium did not induce vacuoles formation in either cell lines.
Figure 6
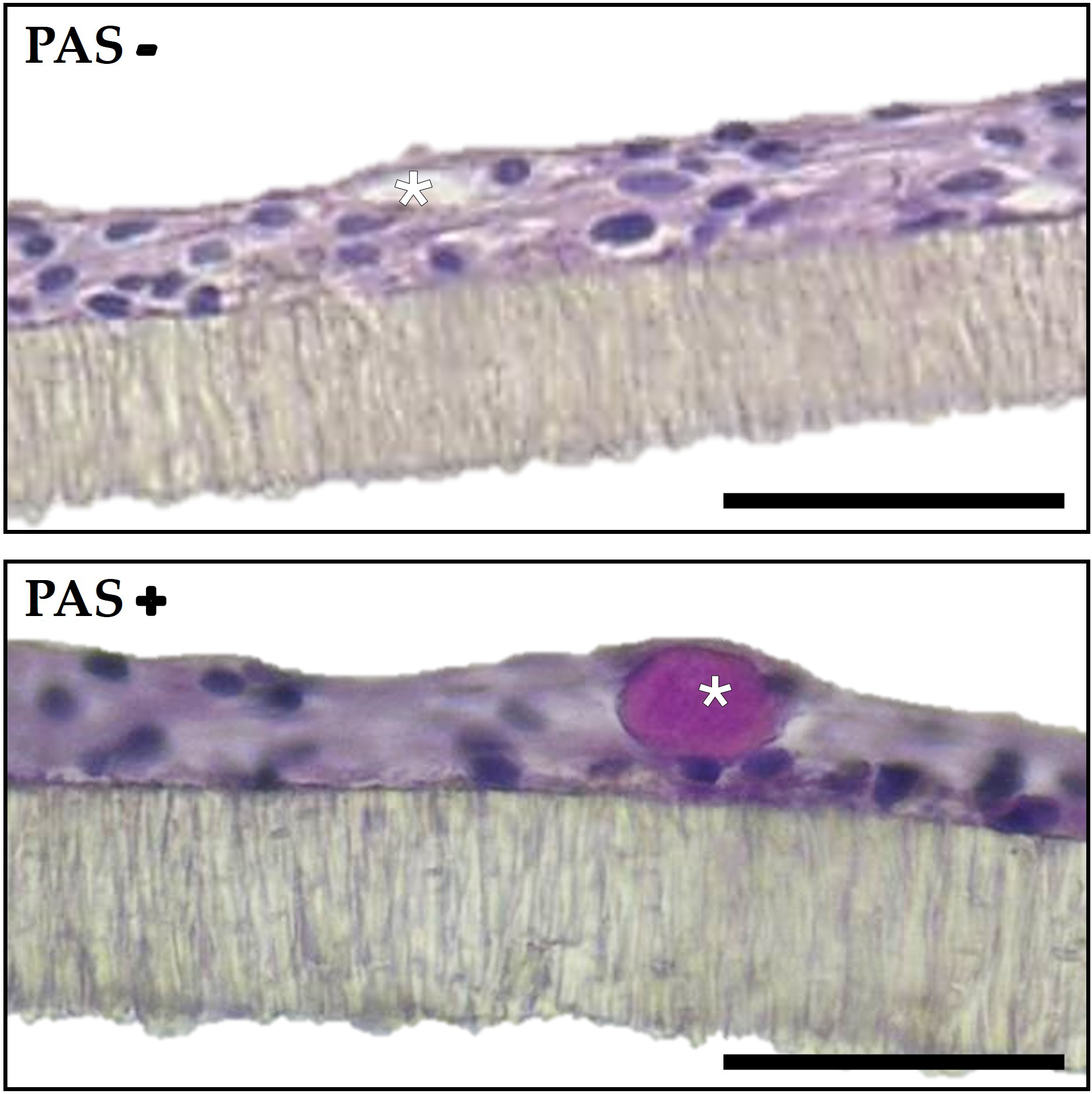
Representative image of ThinCert membrane sections with RTpiMI cells exposed to the fish meal diet (FM) for 21 days, showing a PAS-negative and a PAS-positive vacuole. (asterisks). Scale bar 40 µm.
Table 2
| Vacuoles rate | ||
|---|---|---|
| RTpiMI | RTdiMI | |
| L-15 | – | – |
| FTHM | ++ | – |
| FM | + | – |
| SBM | +++ | + |
| Recovery | – | |
Frequency of vacuole observation in ThinCert insert sections from various samples of two cell lines (proximal and distal) subjected to 21 days of three different pre-digested diets, their respective controls, and the recovery test.
Sections were stained using PAS staining. Evaluations are based on a semi-quantitative scoring system as follows: absence of vacuoles has been indicated with “−” while “+”, “++” and “+++” indicate a low (1–2 vacuoles/field), medium (3–4 vacuoles/field) or high frequency (> 5 vacuoles/field) respectively.
Discussion
In this study we evaluated the ability of rainbow trout intestinal epithelial cells to provide reliable and meaningful information on the effects of three contrasting diets on the health and function of the intestinal wall. At the end of the 3-week in vitro trial, both proximal and distal cell line cultured in control medium, remained in a monolayer with constant TEER values, confirming previous observations showing that these cells when grown in a dual chamber system slow down their proliferation upon reaching confluency, and increase their differentiation (Pasquariello et al., 2021; Verdile et al., 2023b). On the contrary, exposure to FM resulted in a gradual TEER increase in both cell lines which remained stable throughout the experimental period. This observation also confirms the results obtained previously by our research group (Verdile et al., 2023a), but a gradual increase in TEER values was observed also following the exposure to FTHM. Statistical analyses, however, revealed a difference between the two cell lines: in RTdiMI, the increase with FM and FTHM diets was comparable, whereas statistically significantly different TEER values were observed when the two diets were administered to RTpiMI. This difference suggests a higher sensitivity of RTpiMI to different diet components and, unlike RTdiMI, they responded more distinctly to the different formulations.
Morphological analyses showed that all the samples with increased TEER values developed a multilayered epithelium. Epithelial proliferation following exposure to FM was accompanied by a TEER increase also in a previous study (Verdile et al., 2023a). The current study confirms cellular proliferation in response to a reference diet indicating the stability and repeatability of the system. Indeed, it was found that FTHM also induced the formation of a multilayer barrier, making it possible to compare responses to diets containing both plant- or animal-based proteins. As with the TEER values, differences were observed between proximal and distal cell lines. The epithelial barrier formed by RTpiMI cells exposed to FTHM appeared looser compared to the FM-exposed cells, while the RTdiMI epithelial layer after exposure to FTHM was compact and well-organized, like that exposed to the FM diet. The same level of compactness observed in the RTdiMI cells exposed to FM and FTHM diets reflects the similarity of TEER values observed after exposure to all diets. On the contrary, the looser barrier formed by RTpiMI exposed to FTHM corresponded to a lower TEER compared to the FM-exposed cells. It is noteworthy that SBM administration to RTdiMI also resulted in cell proliferation, comparable to FM and FTHM diets in the same cell line. This result suggests that RTdiMI cells are characterized by a greater proliferation propension and a lower susceptibility to diet variation at the inclusion level of 50% tested in this study. Since control samples, not exposed to any diet, remained monolayered, it is possible that increased proliferation is a cellular response to diet exposure. In this context, it is important to note that, unlike in vivo intestinal wall, this model lacks the mucus layer, which would normally provide physiological protection (Sharba et al., 2022). This may in part explain why cells trigger adaptive mechanisms even when exposed to the FM that is considered the reference diet.
Exposure of RTpiMI cells to SBM caused clear damage to the cellular layer within 3 days, demonstrated by TEER reduction, lower AAP activity and morphological alterations, while RTdiMI cells proliferated forming a multilayered epithelium. This is in contrast with what is commonly observed in vivo, where standard soybean meals in the diet for salmonids are known to damage mostly the distal intestine (van den Ingh et al., 1991). Two distinct but non-mutually exclusive causes may explain what happened in vitro. On one hand, RTpiMI, derived from the more absorptive intestinal segment (Verdile et al., 2020b), might be more adversely affected by the high SBM diet. On the other hand, RTpiMI cell, characterized by a lower proliferation rate compared to RTdiMI (Pasquariello et al., 2021), might have been unable to counteract the stressful condition by replacing the damaged cells, leading to the loss of barrier integrity.
As mentioned above, RT intestinal cell lines lack a distinct goblet cells population. However, after exposure to different diets, some vacuoles appeared especially in the RTpiMI cells, giving them a certain resemblance to goblet cells. Since both cell lines express the muc 1 gene (Pasquariello et al., 2021), we investigated whether these vacuoles contained mucins by performing PAS staining. Indeed PAS-positive cells were observed in different amounts depending on the diet as described above and summarized in Table 2. In a few cases, RTpiMI exposed to FM, vacuoles were positive for this staining, confirming the presence of mucins and supporting the hypothesis that they might be goblet cells. Goblet cells in vivo produce mucus that protects the epithelial cell layer (Sharba et al., 2022). Changes in mucus composition have been observed as part of response to stress (Hamidian et al., 2018), with cells losing their PAS positivity and showing an affinity for Alcian blue staining instead (Verdile et al., 2023c, 2020b). In our model, it is possible that the PAS-negative cells altered their mucin production profile as a stress response, resulting in their loss of reactivity to PAS staining. Since vacuoles were present only in conjunction with diet administration and absent in control samples, it is plausible that cells were induced to differentiate into mucus secreting cells as a protective response to the dietary stress condition. Notably, the number of goblet cells in the proximal intestine is significantly higher compared to the distal tract in vivo (Verdile et al., 2020a). Assuming that epithelial cells in the proximal intestine are more prone to differentiating into a secretory lineage than those in the distal segment, we can infer that the cell lines used in this experiment retained characteristics distinctive of their in vivo counterparts (Verdile et al., 2020b). This may also explain why only a few vacuoles were observed in the RTdiMI.
We measured alanine aminopeptidase (AAP) activity to assess whether exposure to different diets induced functional modifications. This enzyme, typically present in the enterocytes brush border, is involved in peptide digestion, which can then be absorbed (Hooton et al., 2015). Its function makes it a marker for evaluating cell differentiation and the degree of similarity of the in vitro model to its in vivo counterpart. All samples analyzed after 21 days of exposure exhibited higher AAP activity compared to control samples. In RTpiMI cells, AAP activity decreased sharply after a 3-day exposure to SBM, whereas a 21-day exposure to FTHM increased its activity significantly more than the exposure to FM. AAP increase can be a feedback mechanism caused by low digestibility of the feather meal, as observed in salmon (Hartviksen et al., 2014). Conversely, in RTdiMI cells, the three diets elicited the same level of AAP increase.
Overall, the results obtained with RTpiMI cells indicate that it was possible to identify differences among diets with the SBM giving the most severe outcomes, the FTHM inducing mild stress responses, while the FM diet was the least disruptive. At the same time no significant effects were noted among diets when administered to RTdiMI cells. Recently, Lokka et al. (submitted) used the same formulations tested in this study to conduct an in vivo feeding trial with rainbow trouts. Numerous parameters were evaluated and depending on which one was considered, diets could be ranked in different ways. For instance, SBM resulted in the lowest growth rate and had the most detrimental impact on gut health, FTHM exhibited the lowest digestibility, and FM, as expected, showed the overall best performance. Therefore, it is difficult to draw a close correlation between the in vitro and in vivo results. Since we mostly used morphological parameters, our results better replicated the health outcome of the in vivo trialIn a way this was confirmed also by the recovery experiment following the damage induced by the SBM, resulting in RTpiMI cells cultured in medium alone to re-establish the barrier integrity and its functions within 18 days. This is consistent with SBM-induced intestinal inflammation in vivo that is also reversible as described by Baeverfjord et al. (1996), who observed that a functional epithelium is restored a few weeks after removing the SBM diet and substituting it with a reference diet. This suggests that the in vitro platform could be used for studying and understanding the recovery mechanisms providing valuable insights into the cellular and molecular processes that regulate intestinal repair and renewal.
Conclusions
Overall, the response of both cell lines to the exposure to different diets somehow mimics a stress state (e.g. enterocytes proliferation, differentiation towards a secretory lineage), which may have been amplified by the lack of a protective mucous layer. Additionally, the proximal intestine cell line appears to be more sensitive to the specific effects of the formulations used, whereas the distal cells response was more homogeneous among the different diets. Grading complete diets is a complex process even in vivo, where different outcomes are obtained depending on the parameters considered. The in vitro platform provides a simpler outcome that may become more elaborated by developing a wider range of functional tests.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The requirement of ethical approval was waived by Comitato etico dell’Università degli Studi di Milano for the studies involving animals because organs were isolated from animals destined for human consumption and, therefore, were not considered as animal experimentation under Directive 2010/63/EU of the European Parliament. The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
FC: Writing – original draft, Writing – review & editing, Data curation, Formal Analysis, Investigation. NV: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. MC: Writing – review & editing, Methodology, Resources. GL: Writing – review & editing. DP: Writing – review & editing, Resources. AT: Writing – review & editing, Supervision. AB: Writing – review & editing, Supervision. TK: Investigation, Supervision, Writing – review & editing. RF: Resources, Supervision, Writing – review & editing. TB: Conceptualization, Supervision, Writing – review & editing. FG: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the European Union’s Horizon 2020 research and innovation program under grant agreement No 828835.
Acknowledgments
The authors thank Denise Tirloni for her help with cell maintenance and the morphological analysis.
Conflict of interest
Authors DP and RF were employed by Skretting Aquaculture Innovation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Baeverfjord G. Krogdahl A. Baeverfjord G. Krogdahl A. (1996). Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: a comparison with the intestines of fasted fish. JFDis19, 375–387. doi: 10.1046/J.1365-2761.1996.D01-92.X
2
Barrows F. T. Frost J. B. (2014). Evaluation of the nutritional quality of co-products from the nut industry, algae and an invertebrate meal for rainbow trout, Oncorhynchus mykiss. Aquaculture434, 315–324. doi: 10.1016/J.AQUACULTURE.2014.08.037
3
Brodkorb A. Egger L. Alminger M. Alvito P. Assunção R. Ballance S. et al . (2019). INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc.14, 991–1014. doi: 10.1038/s41596-018-0119-1
4
Bureau D. P. Harris A. M. Cho C. Y. (1999). Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture180, 345–358. doi: 10.1016/S0044-8486(99)00210-0
5
Fan J. Zheng J. Lai W. Liu S. Liang X. Wang Q. et al . (2023). Establishment of a new intestinal cell line from the grouper (Epinephelus coioides) for aquatic virus pathogenesis. Aquaculture562 (5), 738771. doi: 10.1016/J.AQUACULTURE.2022.738771
6
Fanizza C. Trocino A. Stejskal V. Prokešová M. D. Zare M. Tran H. Q. et al . (2023). Practical low-fishmeal diets for rainbow trout (Oncorhynchus mykiss) reared in RAS: Effects of protein meals on fish growth, nutrient digestibility, feed physical quality, and faecal particle size. Aquac. Rep.28, 101435. doi: 10.1016/J.AQREP.2022.101435
7
Ferruzza S. Rossi C. Scarino M. L. Sambuy Y. (2012). A protocol for in situ enzyme assays to assess the differentiation of human intestinal Caco-2 cells. Toxicol. Vitro26, 1247–1251. doi: 10.1016/j.tiv.2011.11.007
8
Food and Agriculture Organization of the United Nations (2020). World Fisheries And Aquaculture. Sustainability in action Rome. doi: 10.4060/ca9229en
9
Geens M. M. Niewold T. A. (2010). Preliminary Characterization of the Transcriptional Response of the Porcine Intestinal Cell Line IPEC-J2 to Enterotoxigenic Escherichia coli, Escherichia coli and E. coli Lipopolysaccharide. Comp. Funct. Genomics2010, 469583. doi: 10.1155/2010/469583
10
Hamidian G. Zirak K. Sheikhzadeh N. Khani Oushani A. Shabanzadeh S. Divband B. (2018). Intestinal histology and stereology in rainbow trout (Oncorhynchus mykiss) administrated with nanochitosan/zeolite and chitosan/zeolite composites. Aquac. Res.49, 1803–1815. doi: 10.1111/are.13634
11
Hartviksen M. Bakke A. M. Vecino J. G. Ringø E. Krogdahl Å. (2014). Evaluation of the effect of commercially available plant and animal protein sources in diets for Atlantic salmon (Salmo salar L.): digestive and metabolic investigations. Fish. Physiol. Biochem.40, 1621–1637. doi: 10.1007/S10695-014-9953-4/TABLES/5
12
Hidalgo I. J. Raub T. J. Borchardt R. T. (1989). Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology96, 736–749. doi: 10.1016/S0016-5085(89)80072-1
13
Hilgers A. R. Conradi R. A. Burton P. S. (1990). Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharmaceutical Res.7, 902–910. doi: 10.1023/A:1015937605100/METRICS
14
Hooton D. Lentle R. Monro J. Wickham M. Simpson R. (2015). The secretion and action of brush border enzymes in the mammalian small intestine. Rev. Physiol. Biochem. Pharmacol.168, 59–118. doi: 10.1007/112_2015_24
15
Kawano A. Haiduk C. Schirmer K. Hanner R. Lee L. E. J. Dixon B. et al . (2011). Development of a rainbow trout intestinal epithelial cell line and its response to lipopolysaccharide. Aquac. Nutr.17, e241–e252. doi: 10.1111/j.1365-2095.2010.00757.x
16
Krogdahl Å. Kortner T. M. Hardy R. W. (2022). Antinutrients and adventitious toxins. Fish. Nutr. (Cambridge: Academic Press), 775–821. doi: 10.1016/B978-0-12-819587-1.00001-X
17
Liu Y. Ge X. Li C. Xue T. (2023). Derivation and characterization of new cell line from intestine of turbot (Scophthalmus maximus). In Vitro Cell Dev. Biol. Anim.59, 153–162. doi: 10.1007/S11626-022-00746-Y/FIGURES/7
18
Løkka G. Kortner T. M. Chikwati E. Valen E. Peggs D. Fontanillas R. et al . Single cell protein as feed ingredient for rainbow trout (oncorhynchus mykiss) compared to feeds based on fish meal, soybean meal and feather meal. In TorstensenB E. (Ed.), Aquaculture Europe23 (pp. 803–804). European Aquaculture Society.
19
Maqsood M. I. Matin M. M. Bahrami A. R. Ghasroldasht M. M. (2013). Immortality of cell lines: Challenges and advantages of establishment. Cell Biol. Int. 37 (10), 1038–1045. doi: 10.1002/cbin.10137
20
Pasquariello R. Pavlovic R. Chacon M. A. Camin F. Verdile N. Løkka G. et al . (2023). Development of a rainbow trout (Oncorhynchus mykiss) intestinal in vitro platform for profiling amino acid digestion and absorption of a complete diet. Animals13, 2278. doi: 10.3390/ani13142278
21
Pasquariello R. Verdile N. Pavlovic R. Panseri S. Schirmer K. Brevini T. A. L. et al . (2021). New stable cell lines derived from the proximal and distal intestine of rainbow trout (Oncorhynchus mykiss) retain several properties observed in vivo. Cells10, 1555. doi: 10.3390/cells10061555
22
Raatz S. K. Silverstein J. T. Jahns L. Picklo M. J. (2013). Issues of fish consumption for cardiovascular disease risk reduction. Nutrients. doi: 10.3390/nu5041081
23
Sharba S. Sundh H. Sundell K. Benktander J. Santos L. Birchenough G. et al . (2022). Rainbow trout gastrointestinal mucus, mucin production, mucin glycosylation and response to lipopolysaccharide. Fish. Shellfish Immunol.122, 181–190. doi: 10.1016/J.FSI.2022.01.031
24
Su L. Guo B. Feng R. Pang Y. Song J. Zhou S. et al . (2024). Establishment and characterization of an intestinal epithelial cell line from Japanese flounder (Paralichthys olivaceus). Aquaculture580, 740325. doi: 10.1016/J.AQUACULTURE.2023.740325
25
Terova G. Gini E. Gasco L. Moroni F. Antonini M. Rimoldi S. (2021). Effects of full replacement of dietary fishmeal with insect meal from Tenebrio molitor on rainbow trout gut and skin microbiota. J. Anim. Sci. Biotechnol.12 (1), 30. doi: 10.1186/s40104-021-00551-9
26
van den Ingh T.S.G.A.M. Krogdahl Å. Olli J. J. Hendriks H.G.C.J.M. Koninkx J. G. J. F. (1991). Effects of soybean-containing diets on the proximal and distal intestine in Atlantic salmon (Salmo salar): a morphological study. Aquaculture94, 297–305. doi: 10.1016/0044-8486(91)90174-6
27
Velichkova K. Sirakov I. Stoyanova S. Simitchiev A. Yovchev D. Stamatova-Yovcheva K. (2024). Effect of replacing fishmeal with algal meal on growth parameters and meat composition in rainbow trout (Oncorhynchus mykiss W.). Fishes9, 249. doi: 10.3390/fishes9070249
28
Verdile N. Camin F. Chacon M. A. Pasquariello R. Pavlovic R. Peggs D. et al . (2023a). Evaluation of rainbow trout (Oncorhynchus mykiss) organotypic intestinal platforms: cellular responses after long-term exposure to in vitro digested feed. Front. Mar. Sci.10 (4), 745. doi: 10.3389/fmars.2023.1239682
29
Verdile N. Camin F. Pavlovic R. Pasquariello R. Stuknytė M. De Noni I. et al . (2023b). Distinct organotypic platforms modulate rainbow trout (Oncorhynchus mykiss) intestinal cell differentiation in vitro. Cells12, 1843. doi: 10.3390/CELLS12141843/S1
30
Verdile N. Cardinaletti G. Faccenda F. Brevini T. A. L. Gandolfi F. Tibaldi E. (2023c). Ectopic stem cell niches sustain rainbow trout (Oncorhynchus mykiss) intestine absorptive capacity when challenged with a plant protein-rich diet. Aquaculture564, 739031. doi: 10.1016/j.aquaculture.2022.739031
31
Verdile N. Pasquariello R. Brevini T. A. L. Gandolfi F. (2020a). The 3D pattern of the rainbow trout (Oncorhynchus mykiss) enterocytes and intestinal stem cells. Int J Mol Sci. (2020) 21 (23), 9192. doi: 10.3390/ijms21239192
32
Verdile N. Pasquariello R. Scolari M. Scirè G. Brevini T. A. L. Gandolfi F. (2020b). A detailed study of rainbow trout (Onchorhynchus mykiss) intestine revealed that digestive and absorptive functions are not linearly distributed along its length. Animals10. doi: 10.3390/ani10040745
33
Xue T. Wang Y. Liu Y. Liu Y. Li C. (2024). Establishment and characterization of SSI cell line from Sebastes schlegelii intestine for investigating the immune response to Pathogenic Bacteria. Fish. Shellfish Immunol.154, 109993. doi: 10.1016/J.FSI.2024.109993
Summary
Keywords
intestinal cell lines, in vitro platform, rainbow trout, in vitro feeding trial, soybean meal, feather meal
Citation
Camin F, Verdile N, Chacon MA, Løkka G, Peggs D, Tandler A, Bitan A, Kortner TM, Fontanillas R, Brevini TAL and Gandolfi F (2025) Use of a rainbow trout (Oncorhynchus mykiss) intestinal in vitro platform to evaluate different diets. Front. Mar. Sci. 12:1576618. doi: 10.3389/fmars.2025.1576618
Received
14 February 2025
Accepted
24 April 2025
Published
15 May 2025
Volume
12 - 2025
Edited by
Gladstone Sagada, Victory Farms, Kenya
Reviewed by
Laura Braga Ribeiro, University of Algarve, Portugal
Yuwen Dong, University of Pennsylvania, United States
Updates
Copyright
© 2025 Camin, Verdile, Chacon, Løkka, Peggs, Tandler, Bitan, Kortner, Fontanillas, Brevini and Gandolfi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. Gandolfi, fulvio.gandolfi@unimi.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.