Abstract
Abalone, herbivorous marine mollusks of significant economic and ecological importance, exhibit considerable morphological plasticity. This poses a challenge for accurate species identification, which in turn could undermine the assessment of impacts from harvesting. The present study employed an integrative approach combining geometric morphometrics and DNA barcoding to address potential taxonomic ambiguities in abalone populations from Sabah, Malaysia. Especially in this megadiverse region, it could be expected that multiple species may co-occur. Morphometric analysis of 135 specimens, using 14 shell landmarks, confirmed that all individuals clustered within the Haliotis asinina group when compared with data from Haliotis glabra. This was supported by genetic analyses, which demonstrated 99% sequence similarity among novel CO1 sequences and previously published DNA barcodes from H. asinina. Despite overlapping morphological traits between H. asinina and similar congeners, the integrative approach conclusively identified all specimens as H. asinina. Although there are some limits to shell-based taxonomy, quantitative approaches to both morphological and genetic data can resolve species boundaries. These results underscore the importance of employing integrative methods in biodiversity assessments and conservation strategies for tropical abalone species.
1 Introduction
Abalone (Haliotidae: Haliotis) are marine gastropod mollusk of economic importance in global fisheries and aquaculture (Hernández-Casas et al., 2023; Mamat et al., 2023), and also ecologically important grazers that sustaining crustose coralline algae pavement in rocky, shallow subtidal ecosystems across tropical and temperate regions (Nguyen et al., 2023; Rogers-Bennett, 2023). Abalone are harvested due to high demand for its unique texture and exquisite taste (Cook, 2014; Mamat et al., 2025). The global abalone production has increased substantially in recent decades, rising from negligible quantities in the 1970s to 243,506 metric tons in 2020/2021. Whereas production was once primarily reliant on wild fisheries, aquaculture has now become the dominant source (Hernández-Casas et al., 2023; Cook, 2025). The “donkey’s ear” abalone, Haliotis asinina, in particular has become a lucrative source of income for fish farmers in several Asian countries, particularly Indonesia (Sososutiksno and Gasperz, 2017), the Philippines (Capinpin, 2012), Vietnam (Chieu et al., 2016), and Malaysia (Wood et al., 2015).
Malaysia is recognized as one of the world’s megadiverse countries, with a high level of biodiversity (Tong, 2020). The conservation status of many marine species presumed to occur in the region has yet to be assessed, and is potentially confounded by the presence of cryptic species. Harvesting of abalone is currently unregulated, with no abalone species from Malaysia protected by law, nor are there Species Action Plans or monitoring programs in place. Among the 57 recognized global abalone species, six are distributed in the tropical Indo-Malayan Archipelago region, including Haliotis asinina Linnaeus, 1758, Haliotis diversicolor Reeve, 1846, Haliotis glabra Gmelin, 1791, Haliotis ovina Gmelin, 1791, Haliotis planata G. B. Sowerby II, 1882, and Haliotis varia Linnaeus, 1758 (Geiger and Owen, 2012). There may be more: H. diversicolor for example is divided into subspecies that may represent separate species (e.g. Bachry et al., 2019).
Species discrimination in abalones is primarily based on morphological characters based on shell features such as color pattern, and size, number of open holes, shell sculpture, and also animal body color (Soelistyowati et al., 2013). Identification based on shell morphology has been practiced due to the long-lasting nature of shells, which allows for the examination of both fossils and extant species using similar techniques (Chiappa et al., 2022). Accurate taxonomy and species identification are fundamental for the conservation and management of taxa, as well as for biodiversity studies (Zhou et al., 2016). However, relying solely on shell traits as indicators of evolutionary divergence poses challenges in groups as morphological characters exhibit high plasticity, leading to significant variation in response to local environmental conditions. This makes shell-based delineation often unreliable (Castelin et al., 2017). It is also often difficult to distinguish morphologically similar species, as some may actually represent complexes of cryptic species (Monti et al., 2005). For example, Haliotis tuberculata Linnaeus, 1758 has four different subspecies, each with distinct characteristics (Geiger and Owen, 2012).
Due to the potential unreliability of qualitative morphological characters, morphometric approaches have been implemented. Morphometrics is a quantitative and complimentary approach for studying morphological variation within and between groups (Ruaza et al., 2015; Jackson and Claybourn, 2018). The morphometric truss method is one way to calculate the measuring standard that can aid in proper identification of abalones based on shell morphology (Bachry et al., 2019). The truss method is highly effective and more accurate in capturing information and explain character shape (patterns) by comparing the size of morphological parts between species or populations (Strauss and Bookstein, 1982; Rawat et al., 2017). Morphometric methods have been successful in identifying gastropods through the analysis of shell form (Kirchner et al., 2016). Geometric morphometrics thus offers a way to quantify, analyze, and compare the complex shapes of biological shell structures in an evolutionary context (Mitteroecker and Gunz, 2009).
With more affordable costs for genetic analysis, species identification via barcoding has become faster and more precise (Ali et al., 2014). Molecular markers have proven to be a powerful tool for taxonomic identification of closely related species and for complementing traditional morphology-based taxonomy (An et al., 2013; Barco et al., 2016). Owing to its high evolutionary rates, maternal inheritance, lack of recombination, and rapid base substitution rates, mitochondrial genes have been widely used in phylogeography and molecular identification (Tran et al., 2015; Gu et al., 2016; Kawamura et al., 2017). Although molecular techniques are increasingly employed, external characteristics, such as shell morphology, remain the most straightforward and practical method for identification for scientists in the field, and for use by those in related disciplines like ecology and paleontology, as well as for amateur observers and citizen scientists (Chiappa et al., 2022). Given the ecological and economic importance of Haliotis species, it is essential to clarify species boundaries and ensure accurate identification, as this can influence conservation strategies and aquaculture practices.
In this study, specimens of abalones attributed to H. asinina were collected from five island populations in Sabah, Malaysia. Variation in shell coloration was observed between specimens collected from the western and eastern coasts of Sabah. Western specimens (from Mantanani and Balambangan Islands) exhibited darker shell coloration with a mix of green and brown hues, while eastern specimens (from Labuan Haji, Selakan and Menampilik Islands) displayed lighter, predominantly green shells with hints of brown. Additionally, shell patterning in all populations featured a mixture of blotched shapes, further complicating morphological classification. Given the high potential for cryptic diversity in this region and morphological variation observed in these samples, this study used both morphometric analysis and DNA barcoding to assess the taxonomy and the variation of shell form and barcode fragments among Haliotis specimens from these five populations.
2 Materials and methods
2.1 Sample collections
Specimens of abalone were collected by fishermen from the coastal waters of Sabah, Malaysia (Figure 1): Balambangan Island, Kudat (n = 22), Mantanani Island, Kota Belud (n = 25) and Labuan Haji (n = 29), Selakan (n = 29) and Menampilik (n = 30), Semporna (Table 1; Figure 1). The samples were collected between May 2023 and September 2023 and transported to the International Institute of Aquaculture and Aquatic Sciences (I-AQUAS), in an icebox, with 25 to 30 individuals per box. The samples were collected according to the permits granted by Sabah Biodiversity Council (SaBC) with license reference number of JKM/MBS.1000-2/2 JLD. 16 (146). Photographs of abalone were taken on site and all individuals were labelled to verify identification or for future reference. Individual shells were also taken in order to examine the shell morphology in the laboratory. The size range of 135 specimens in this study varied from 4 cm to 9 cm. Abalone from Sabah waters showing similar shell coloration patterns and shapes from different locations (Figure 2). A small piece of tissue (~ 3 cm3) from each sample were placed in 1.5 mL microcentrifuge tubes in 95% ethanol for preservation and stored at -4°C in the laboratory for further genetics analysis. For comparison, H. glabra specimens (n=10) were used from the collections of the Senckenberg Research Institute and Museum Frankfurt (SMF), which had a shell size range of 2 cm to 5 cm. Samples were analyzed using two approaches: geometric morphometrics and molecular analysis.
Figure 1
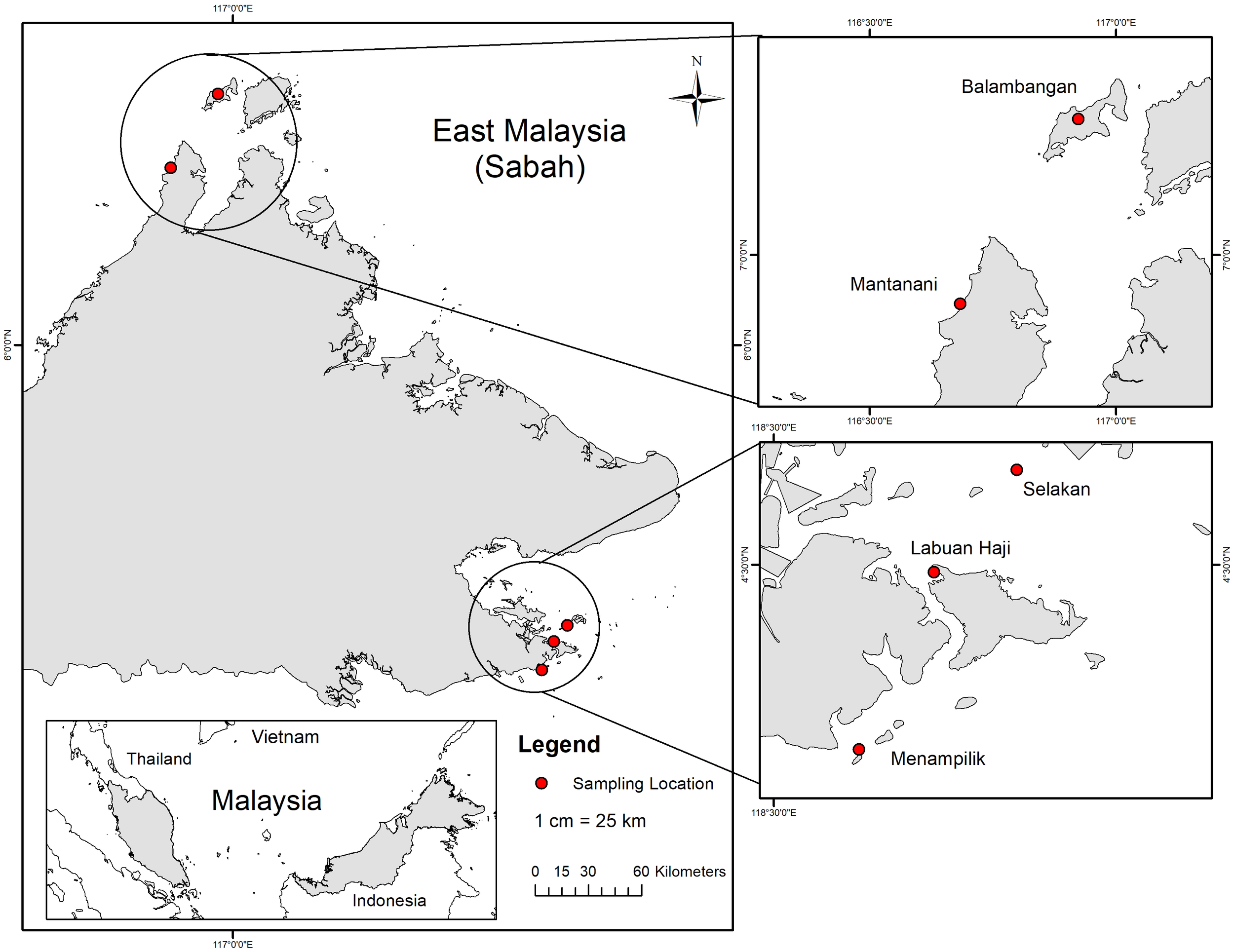
Sampling sites in Sabah, Malaysia.
Table 1
| Province | Location (Islands) | Coordinates | Number of individuals (n) |
|---|---|---|---|
| Kudat | Balambangan | 7°16’35”N 116°55’23”E | 22 |
| Kota Belud | Mantanani | 6°54’04”N 116°41’02”E | 25 |
| Semporna | Labuan Haji | 4°27’53”N 118°46’28”E | 29 |
| Selakan | 4°34’34”N 118°41’51”E | 29 | |
| Menampilik | 4°20’31”N 118°34’08”E | 30 | |
| Total | 135 |
List of sample collections from different island in Sabah waters.
Figure 2
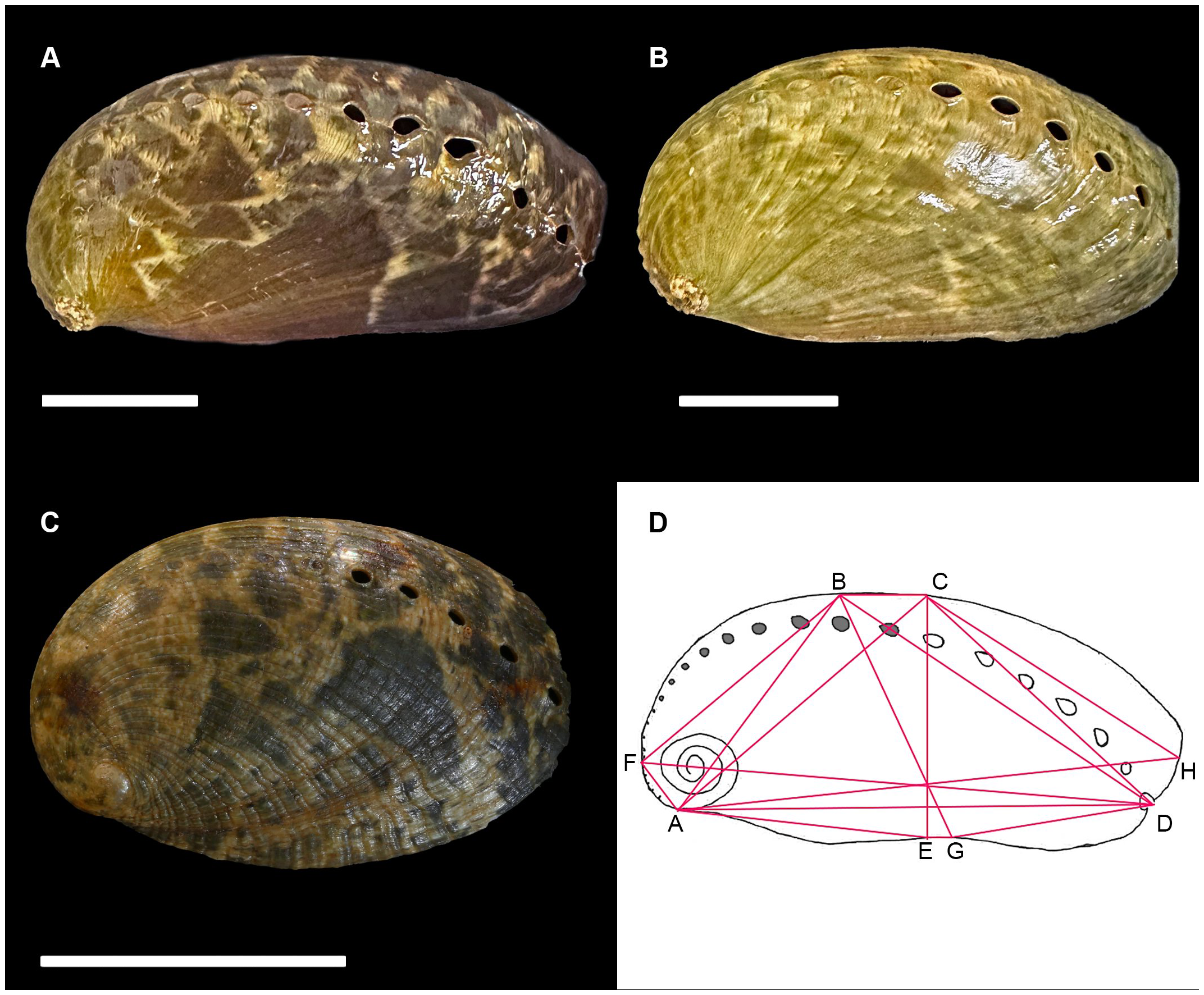
Shell coloration patterns and shapes in Haliotis asinina: (A) Mantanani Island (MT13) from western Sabah, (B) Selakan Island (SE9) from eastern Sabah, and Haliotis glabra(C) specimen SMF 367365 from the Philippines, (D) landmarks for morphometrics parameters from A to H following Bachry et al. (2019). Scale bars 2 cm.
2.2 Morphometric truss method
The truss network system was applied to construct a network on abalone body involving 8 landmarks to generate a total of 16 distance variables following Bachry et al. (2019) (Figure 2). Morphometric parameters in each individual of abalone are divided by the standard length (SL) or FD and are expressed as ratios to remove the influence of absolute size. The package MorphoTools2 version 1.0.2.0 in R-software packages were used to conduct the multivariate analysis. Correlations between characters (parameters) were checked and characters resulting in correlations of r > 0.95 were selectively excluded. Data from the 14 characters retained were used in a principal component analysis (PCA) to examine the differentiation or potential overlap among populations.
2.3 Mitochondrial CO1 gene
DNA was extracted from 25 mg of preserved muscle tissue from each specimen using the Nucleospin Tissue Kit (Macharey Nagel), based on the manufacturer’s instructions. Tissues were PCR-amplified using CO1 gene (forward: AB-CO1F: 5’-TGATCCGGCTTAGTCGGAACTGC-3’) and (reverse: AB-CO1R: 5’-GATGT CTTGAAATTACGGTCGGT-3’) (Metz et al., 1998). PCR amplification was done in 25 μL volumes. Each reaction contains 0.50 µL DNA template, 0.10 µM of each primer; forward and reverse primers, 0.50 µM 10X Easy Taq® Buffer, 0.1 µM of 2.5mM dNTP, 1U Easy Taq® DNA Polymerase (500 U/μl) (Nanogene) and 18.8 µL distilled water (ddH2O). Amplification was carried out in PCR System Thermal Cycler (Biometra Tone, Matrioux). Thermocycling profile is as follows: preheating at 94°C for 30s, 35 cycles of denaturing at 94°C for 30 s, annealing at 50°C for 30 s and extension at 72°C for 1 min, followed by a final extension at 72°C for 1 min. Amplification products were run on a 1.5% agarose gel with TBE buffer at 90 V for 70 min using ladder 100bp (Vivantis). At the end of the run, gels were visualized under UV light using UV transluminator (Maestrogen) with 3 µL GelRed (Nanogene). Successfully amplified products were sent to Apical Scientific Sdn. Bhd. for DNA purification and sequencing analysis.
Sequences were edited using BioEdit Software version 7.2.5 (Hall, 1999) and then contgs manually assembled. CLUSTAL W (Thompson et al., 1994) implemented in MEGA 7.0 (Kumar et al., 2016) was used to align all the edited sequences. The CO1 alignments were manually checked to discard gaps and stop codons by translating the sequences into amino acids. The final aligned sequences obtained from MEGA 7.0 software (Kumar et al., 2016) were used to construct haplotype data files using the DnaSP program version 5.0.1.1 (Rozas et al., 2017). For species identification and confirmation, sequences from this study were compared using the Basic Local Alignment Search Tool (BLAST) of the National Centre of Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov) (Ratnasingham and Hebert, 2007). Phylogenetic trees were constructed based on inclusion of representative haplotypes of abalone sequences, including a previously published H. asinina Genbank CO1 sequence from China (KX233870.1). The relationships between haplotypes and geographic distributions were determined through the Minimum Spanning Network (MSN) using the median-joining method implemented in NETWORK version 5.0.1.1 (Bandelt et al., 1999).
3 Results
From the morphometric analysis, four principal components (PCs) were extracted, with the majority of the total variance explained by the first two components. Principal Component 1 (PC1) exhibited the highest eigenvalue (6.449), accounting for 46.06% of the total variance, followed by PC2 with 18.94% (eigenvalue = 2.652), PC3 with 6.80%, and PC4 with 6.50%. (Table 2). The pattern of variation showed a gradual decrease in explained variance with each subsequent component. The loadings for individual parameters across PCs ranged from 0.022 to 0.568, indicating their respective contributions to the observed variation. The scatter plot based on PC1 and PC2 scores revealed two distinct groups (Figure 3). The first group included all five populations of Haliotis asinina from different islands, which clustered closely together, while the second group consisted of Haliotis glabra, clearly separated from the H. asinina populations (Figure 3). Together, PC1 and PC2 accounted for 65% of the cumulative variance in the dataset (Table 2), highlighting their significance in distinguishing between the two groups.
Table 2
| Parameter | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| AC | 0.321 | 0.052 | 0.146 | 0.022 |
| CE | 0.289 | 0.362 | 0.095 | 0.004 |
| AH | -0.261 | -0.281 | 0.121 | 0.082 |
| AF | 0.311 | 0.314 | 0.101 | -0.078 |
| AD | -0.087 | -0.126 | 0.937 | 0.070 |
| AE | 0.174 | -0.443 | 0.084 | -0.345 |
| CH | -0.328 | 0.174 | 0.040 | -0.054 |
| CD | -0.309 | 0.289 | 0.085 | 0.037 |
| DB | -0.244 | 0.405 | 0.147 | -0.093 |
| BF | 0.277 | -0.093 | 0.025 | 0.568 |
| BA | 0.325 | 0.051 | 0.099 | 0.359 |
| GE | 0.259 | 0.178 | -0.023 | -0.030 |
| DG | -0.213 | 0.323 | -0.016 | 0.313 |
| CB | 0.229 | 0.226 | 0.136 | -0.547 |
| Eigenvalue | 6.449 | 2.652 | 0.958 | 0.905 |
| % Variation | 46.06 | 18.94 | 6.80 | 6.50 |
| % Cumulative Variation | 46.06 | 65.00 | 71.80 | 78.30 |
Coefficient component principal of abalone with Haliotis glabra using Principal Component (PC) analysis.
Figure 3
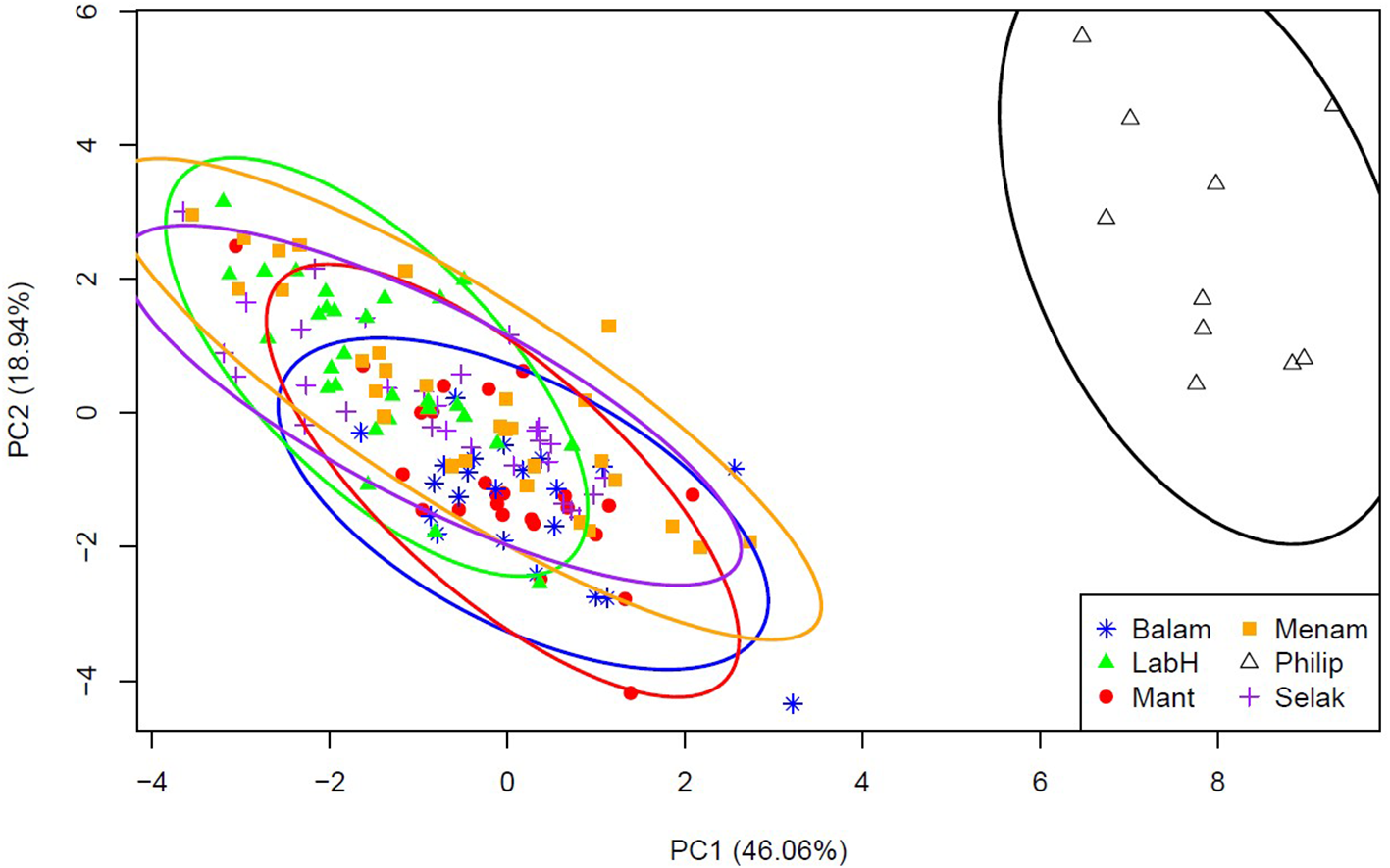
Score plot PC1 against PC2 showed that the five populations of Haliotis asinina clustered together, with only the population of Haliotis glabra being separated. Sabah, Malaysia: Balam, Balambangan; LabH, Labuan Haji; Mant, Mantanani; Menam, Menampilik; Selak, Selakan; Philip, the Philippines.
Molecular barcoding CO1 sequences from the Sabah specimens showed 99% similarity among all samples and previously published sequence data for H. asinina. Sequences of all 37 haplotypes of H. asinina have been deposited in NCBI GenBank with the accession numbers PV545159-PV545195. There are no published CO1 sequence data for H. glabra and H. planata, so interspecific comparisons with the most similar species were not possible. Phylogenetic trees were generated using 37 haplotype sequences derived from 135 samples analyzed in this study, combined with a single Haliotis asinina sequence from China (KX233870.1) and a non-gastropod outgroup, Anadara sativa (GenBank accession KP253075.1). Both Neighbor-Joining (NJ) and Maximum Likelihood (ML) methods were employed for phylogenetic analysis. However, due to identical topologies, only the ML tree was presented (Appendix 1). The phylogenetic tree demonstrated that 99% of the haplotype sequences clustered with Haliotis asinina from China, forming a well-supported monophyletic group distinct from the outgroup. This high degree of similarity with the reference sequence provides strong evidence supporting the identification of the specimens as a single species, H. asinina. Most haplotypes clustered closely, indicating low genetic divergence among the sampled individuals, except Hap14, formed distinct branch, suggesting slightly higher divergence. The reference sequence from China grouped within the main clade, further supporting the close genetic relationship between populations. Notably, haplotypes from the western Sabah populations tended to cluster in the upper part of the tree, while those from eastern Sabah were located in the lower part, suggesting slight geographic structuring within the species (Appendix 1).
This in parallel with the phylogenetic tree analysis, a Minimum Spanning Network (MSN) was constructed using 37 haplotypes identified in this study, along with a reference Haliotis asinina sequence from China (Figure 4). The analysis revealed the formation of two distinct haplogroup clusters, corresponding to the eastern and western populations of Sabah. Haplotype 1, the most prominent haplotype, included individuals from all five populations, but predominantly from the two populations from western Sabah: Balambangan and Mantanani Islands. In contrast, Haplotype 7 was shared by only the three island populations from eastern Sabah: Menampilik, Selakan, and Labuan Haji Islands. The previously published sequence for a single individual of H. asinina from China represents a unique haplotype positioned centrally within the haplotype network (Haplotype 38, Figure 4).
Figure 4
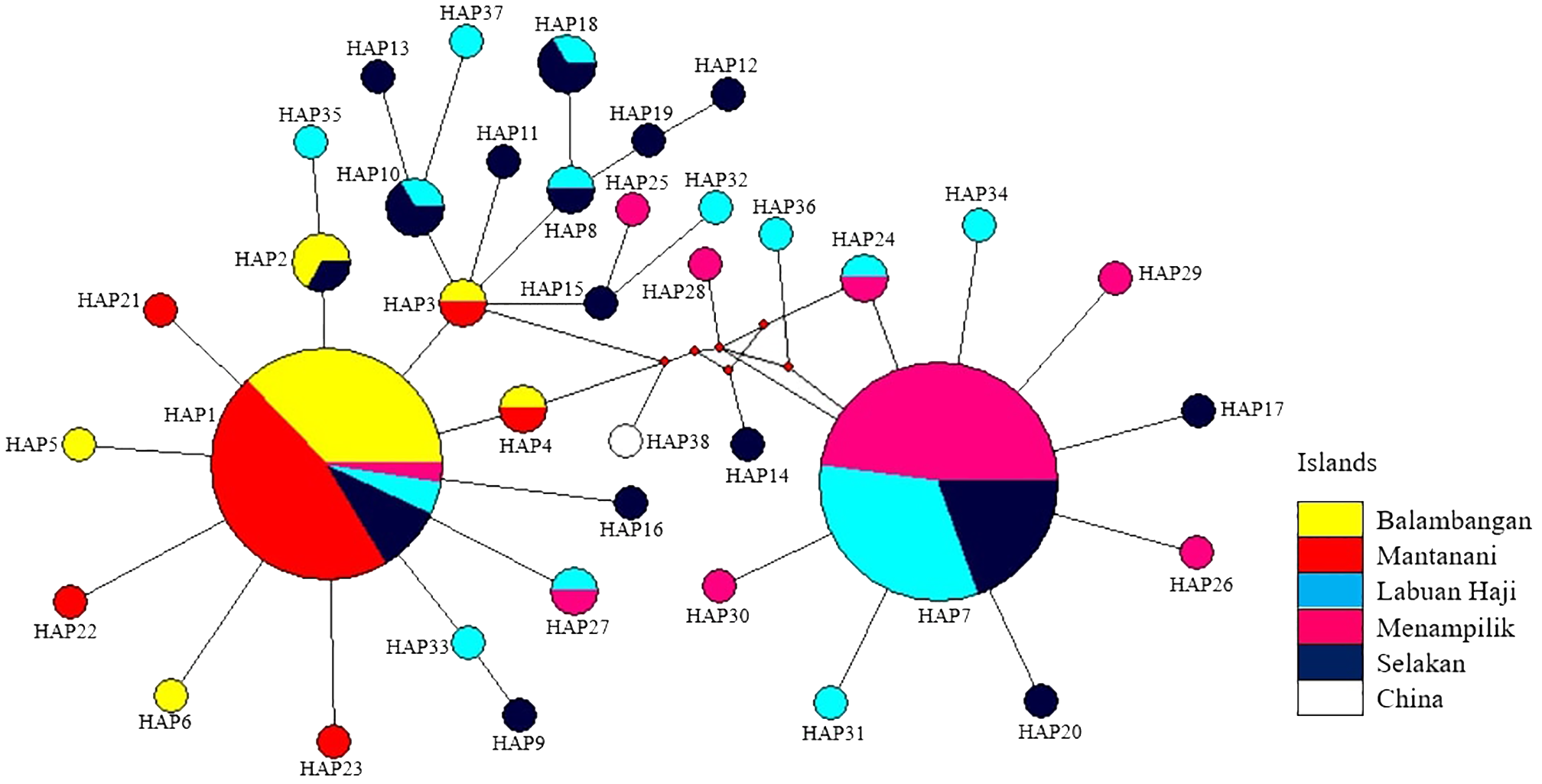
Minimum Spanning Network (MSN) of 37 haplotypes, including one reference sequence from China, inferred from the mitochondrial DNA cytochrome c oxidase subunit 1 (mtDNA CO1) gene. Node sizes are proportional to haplotype frequencies, with the smallest nodes representing a single individual. Red dots represent median vector.
4 Discussion
Effective species differentiation requires organisms to exhibit consistent and distinguishable characteristics, allowing for their classification into distinct groups, whether the taxonomic diagnosis relies on morphological, anatomical, molecular, or other traits (Pfenninger et al., 2006). The current study integrates shell morphology, geometric morphometrics and molecular DNA barcoding to address potential taxonomic ambiguity of abalone in Malaysia. Despite regional variation in shell color patterns, both morphometric analyses and DNA barcoding consistently confirmed that all collected specimens belong to Haliotis asinina. The integration of morphometric and molecular approaches provides an accurate framework for species identification in Haliotis and other taxa (Chiappa et al., 2022; Bachry et al., 2019).
Haliotis asinina, like many marine organisms, has a planktonic larval stage that allows for wide dispersal. A population genetics comparison of H. asinina across Thailand found minimal genetic differentiation, attributed to the wide dispersal of larvae, allowing populations to remain genetically connected despite geographic separation (Klinbunga et al., 2003). The absence of significant genetic divergence in H. asinina samples across Sabah waters suggests a lack of cryptic species in the region. The presence of geographically distinct haplotypes, especially two distinct groups for western and eastern Sabah populations, reflects population structuring and limited gene flow between some regions. This reflects the known oceanographic conditions in the Coral Triangle region that leads to a source-sink dynamic, with larvae flowing from the South China Sea to the Sulu Sea and Celebes Sea (e.g. Kool et al., 2011). H. asinina likewise shows inflow from western toward eastern Sabah, with both of the major haplotypes occurring in the populations sampled in eastern Sabah. The analysis here underscores the intricate relationship between genetic connectivity and population differentiation across geographic regions, reflecting the influence of both historical and ecological factors on genetic structure.
The connectivity of H. asinina over a very broad distribution is not in conflict with the distinct environmental characteristics of the sampling locations. Dietary composition is well documented to significantly influence abalone shell pigmentation (Gallardo et al., 2003; Liu et al., 2009), and the role of diet in determining shell coloration has been demonstrated in multiple species of abalone, including Haliotis asinina (Gallardo et al., 2003), Haliotis discus hannai (Liu et al., 2009; Ju et al., 2016), and Haliotis laevigata (Hoang et al., 2016; Purvis et al., 2025). Notably, seaweed coloration differs between the western and eastern coasts of Sabah, with brown seaweed predominating in the west and green seaweed in the east. Seaweed plays a critical role in abalone productivity by providing habitat and supplying essential nutrients and energy necessary for growth and reproduction (Britton et al., 2023). Most abalone species exhibit preferences for specific algal types, which depend on the local availability of macroalgal species (Serviere-Zaragoza et al., 2003). Wild abalone are primarily herbivorous, grazing on both microalgal and macroalgal species (Bautista-Teruel et al., 2011; Stone et al., 2023). Semporna in eastern Sabah is widely recognized for its suitability as a natural habitat and as a center for seaweed cultivation (Hussin and Khoso, 2017). The majority of households in Semporna rely on fishing as their primary source of income, with most full-time fishers, including abalone harvesters and those who cultivate seaweed belonging to the Bajau Laut ethnic group (Wood et al., 2015). The presence of seaweed beds is strongly associated with a higher likelihood of abalone occurrence. Previous studies have also confirmed that abalone tend to favor areas with dense seaweed beds (Cook, 2023).
Cryptic species, which are morphologically similar but genetically distinct, are increasingly recognized in marine environments. For instance, RAPD markers distinguished Haliotis rufescens Swainson, 1822 from Haliotis discus hannai, revealing that individuals with ambiguous morphology were actually variants of H. rufescens (Marín et al., 2007). Similarly, in nudibranchs, the species Dendronotus europaeus was discovered within the D. frondosus, an algae complex, exhibiting significant external variation but conserved internal features (Korshunova et al., 2017). Another example includes Atrina pectinata, which was found to comprise six potential cryptic species based on mtCO1 analysis, with evidence of hybridization between lineages (Liu et al., 2011). These studies highlight the importance of integrating multiple lines of evidence to uncover cryptic diversity in marine mollusks.
Relying solely on morphology for species identification in abalone can lead to misidentification, as evidenced by case of H. sorenseni Bartsch, 1940, which was initially misidentified as white abalone but later revealed to be H. kamtschatkana Jonas, 1845 (Gruenthal and Burton, 2005). Molecular techniques have proven to be important in differentiating closely related Haliotis species, and molecular studies of tropical species with subtle morphological differences often reveal or confirm cryptic lineages. For example, analysis of CO1 barcode data for H. diversicolor squamata Reeve, 1846 found a molecular separation as well as the known morphological differences, suggesting this lineage should be recognized as a distinct species (Bachry et al., 2019). By contrast, our study found that the difference in shell colors in eastern and western Sabah were reflected neither shape (morphometric) nor molecular separation but rather a single H. asinina species group with some population structure following oceanographic patterns.
Abalone shell morphology exhibits significant intraspecific variation, driven by a complex interplay of genetic and environmental factors, which can be a challenge for species delimitation. Variations in shell color, size, and shape are influenced by environmental factors such as reef topography, water movement, diet, habitat, and temperature (Saunders et al., 2009; Bachry et al., 2019). For example, shell color in Haliotis discus hannai Ino, 1953 is genetically controlled, with dominant alleles for green shells and recessive alleles resulting in orange and bluish shells, following Mendelian inheritance patterns. However, diet can modify shell color within genotypes, potentially providing camouflage advantages (Liu et al., 2009; Kobayashi et al., 2004).
Similarly, epipodial coloration in Haliotis rufescens may represent phenotypic variants within a species, rather than hybridization, resulting from genetic polymorphism or phenotypic plasticity (Marín et al., 2007). Morphological variations in Haliotis rubra Leach, 1814, such as growth rate and size at maturity, also exhibit significant differences between ‘stunted’ and ‘non-stunted’ populations over fine spatial scales. These differences are hypothesized to be influenced by resource availability, where low algal cover and simplified topography are associated with stunted populations, while high algal abundance and complex topography correspond to non-stunted populations (Saunders et al., 2009). Together, these findings underscore the intricate interactions between genetic and environmental factors in shaping abalone morphology and complicating species identification. Therefore, although visually distinct, these variations do not always reflect genetic differentiation.
This study highlights the necessity of integrating morphometrics and genetic data into conservation strategies to ensure the accurate identification of abalone species. Different management approaches may be required to preserve the genetic integrity of abalone populations, for example that over-harvesting could have a greater impact on genetic diversity in western Sabah, which acts as a source rather than a sink population. The absence of regulatory frameworks for abalone harvesting in this region exacerbates the risk of overexploitation. In contrast to countries where abalone fisheries are tightly regulated (Smallwood et al., 2023), Malaysia’s unregulated abalone fishery threatens the long-term sustainability of these species. Establishing species action plans, monitoring programs, and legal protections is essential for safeguarding these valuable marine resources.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by INSTITUTIONAL ANIMAL CARE AND USE COMMITEE (IACUC), UNIVERSITI PUTRA MALAYSIA. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft. YE: Data curation, Methodology, Resources, Supervision, Validation, Writing – review & editing. JS: Formal analysis, Methodology, Software, Visualization, Writing – review & editing. SN: Conceptualization, Data curation, Writing – review & editing. NW: Funding acquisition, Project administration, Writing – review & editing. NK: Investigation, Resources, Writing – review & editing. FM: Conceptualization, Writing – review & editing. SA: Methodology, Writing – review & editing. AA: Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Research Partnership for Sustainable Development (SATREPS) Program entitled ‘Development of Advanced Hybrid Ocean Thermal Energy Conversion (OTEC) Technology for Low Carbon Society and Sustainable Energy System: First Experimental OTEC Plant of Malaysia’ funded by Japan Science and Technology Agency (JST) and Japan International Cooperation Agency (JICA) and Ministry of Higher Education Malaysia (MoHE) and led by the Institute of Ocean Energy Saga University (IOES) of Japan and UTM Ocean Thermal Energy Centre (UTM OTEC), Universiti Teknologi Malaysia (UTM). Registered Program Cost Centre: #R.K120000.78094L887, Project (Cost center: #6300235). This project was also funded by Government of Canada’s International Climate Finance Initiative dan International Development Research Centre (IDRC) Ottawa, Canada under subproject of Incorporating Climate Resilience Species in Integrated Multi-Trophic Aquaculture (IMTA) Systems Through Spatial-Temporal Configurations for Sustainable Aquaculture System (No. Vot: 6380188-10201). N-SM was supported by a Senckenberg Global Fellowship 2024. This is contribution number 75 of the Senckenberg Ocean Species Alliance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1577263/full#supplementary-material
References
1
Ali M. A. Gyulai G. Hidvégi N. Kerti B. Hemaid F. M. A. A. Pandey A. K. et al . (2014). The changing epitome of species identification - DNA barcoding. Saudi J. Biol. Sci.21, 204–2231. doi: 10.1016/j.sjbs.2014.03.003
2
An H. S. Lee J. W. Hong S. W. (2013). Population genetic structure of the Korean Pacific abalone Haliotis diversicolor supertexta inferred from microsatellite marker analysis. Biochem. Syst. Ecol.48, 76–84. doi: 10.1016/j.bse.2012.11.010
3
Bachry S. Solihin D. D. Gustiano R. Soewardi K. Butet N. A. (2019). Morphometric character and morphology of abalone Haliotis squamata Reeve 1864 in coastal southern Java and Bali. J. Ilmu Teknol. Kelaut. Tropis.11, 273–284. doi: 10.29244/jitkt.v11i1.24672
4
Bandelt H. J. Forster P. Röhl A. (1999). Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol.16, 37–48. doi: 10.1093/oxfordjournals.molbev.a026036
5
Barco A. Raupach M. J. Laakmann S. Neumann H. Knebelsberger T. (2016). Identification of North Sea mollusks with DNA barcoding. Mol. Ecol. Resour.16, 288–297. doi: 10.1111/1755-0998.12440
6
Bautista-Teruel M. N. Koshio S. S. Ishikawa M. (2011). Diet development and evaluation for juvenile abalone, Haliotis asinina Linne: Lipid and essential fatty acid levels. Aquac312, 172–179. doi: 10.1016/j.aquaculture.2011.01.004
7
Britton D. Mundy N. M. James C. McAllister J. (2023). Nutritional quality of kelp as a key driver of commercial abalone productivity (Australia: Institute for Marine and Antarctic Studies, University of Tasmania). Available at: https://fishing.tas.gov.au/Documents/Final%20report%20AIRF%202020_47.PDF. (Accessed December 20, 2024)
8
Capinpin Jr E. C (2012). Using local ecological knowledge and environmental education in resource management of abalone in Carot, Anda, Pangasinan. Sci. Diliman24, 43–55.
9
Castelin M. Williams S. T. Buge B. Maestrati P. Lambourdière J. Ozawa T. et al . (2017). Untangling species identity in gastropods with polymorphic shells in the genus Bolma Risso 1826 (Mollusca, vetigastropoda). Eur. J. Taxon.288, 1–21. doi: 10.5852/ejt.2017.288
10
Chiappa G. Fassio G. Corso A. Crocetta F. Modica M. V. Oliverio M. (2022). How many abalone species live in the Mediterranean sea? Diversity14, 1–16. doi: 10.3390/d14121107
11
Chieu H. D. Phuong L. D. Duy D. A. Tuan B. M. Thoa N. K. (2016). “Aquaculture-based enhancement and restoration of many-colored abalone resources (Haliotis diversicolor Reeve 1846) in Bach Long VI national marine protected area, Vietnam,” in Consolidating the strategies for fishery resources enhancement in Southeast Asia. Eds. KawamuraH.IwataT.TheparoonratY.ManajitN.SulitV. T. (Vietnam: SEAFDEC Publications). Available at: http://repository.seafdec.or.th/handle/20.500.12067/718 (Accessed December 14, 2024).
12
Cook P. A. (2014). The worldwide abalone industry. J. Mod. Econ.5, 1181–1186. doi: 10.4236/me.2014.513110
13
Cook P. A. (2023). Introduction, taxonomy, and general biology of abalone. Developments Aquaculture Fisheries Sci.42, 1–8. doi: 10.1016/B978-0-12-814938-6.00001-4
14
Cook P. A. (2025). Worldwide abalone production: an update. New. Zeal. J. Mar. Fresh.59, 4–10. doi: 10.1080/00288330.2023.2261869
15
Gallardo W. G. Bautista-Teruel M. N. Fermin A. C. Marte C. L. (2003). Shell marking by artificial feeding of the tropical abalone Haliotis asinina Linne juveniles for sea ranching and stock enhancement. Aquac Res.34, 839–842. doi: 10.1046/j.1365-2109.2003.00890.x
16
Geiger D. L. Owen B. (2012). Abalone: World-Wide Haltiotidae (Germany: ConchBooks, Hackenheim).
17
Gruenthal K. M. Burton R. S. (2005). Genetic diversity and species identification in the endangered white abalone (Haliotis sorenseni). Conserv. Genet.6, 929–939. doi: 10.1007/s10592-005-9079-4
18
Gu D. E. Mu X. D. Xu M. Luo D. Wei H. Li Y. Y. et al . (2016). Identification of wild tilapia species in the main rivers of south China using mitochondrial control region sequence and morphology. Biochem. Syst. Ecol.65, 100–107. doi: 10.1016/j.bse.2016.02.007
19
Hall T. A. (1999). BIOEDIT: A user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser.41, 95–98.
20
Hernández-Casas S. Seijo J. C. Beltrán-Morales L. F. Hernández-Flores Á. Arreguín-Sánchez F. Ponce-Díaz G. (2023). Analysis of supply and demand in the international market of major abalone fisheries and aquaculture production. Mar. Policy148, 1–9. doi: 10.1016/j.marpol.2022.105405
21
Hoang T. H. Qin J. G. Stone D. A. Harris J. O. Duong D. N. Bansemer M. S. (2016). Color changes of greenlip abalone (Haliotis laevigata Donovan) fed fresh macroalgae and dried algal supplement. Aquac456, 16–23. doi: 10.1016/j.aquaculture.2016.01.022
22
Hussin H. Khoso A. (2017). Seaweed cultivation and coastal communities in Malaysia: An overview. Asian Fish. Sci.30, 87–100. doi: 10.33997/j.afs.2017.30.2.003
23
Jackson I. S. Claybourn T. M. (2018). Morphometric analysis of inter-and intraspecific variation in the Cambrian helcionelloid mollusk Mackinnonia. Palaeontol61, 761–773. doi: 10.1111/pala.12368
24
Ju Z. Y. Viljoen C. Hutchinson P. Reinicke J. Horgen F. D. Howard L. et al . (2016). Effects of diets on the growth performance and shell pigmentation of Pacific abalone. Aquac. Res.47, 4004–4014. doi: 10.1111/are.12851
25
Kawamura K. Miyake T. Obata M. Aoki H. Komaru A. (2017). Population demography and genetic characteristics of the Pacific Oyster Crassostrea gigas in Japan. Biochem. Syst. Ecol.70, 211–221. doi: 10.1016/j.bse.2016.12.006
26
Kirchner S. Harl J. Kruckenhauser L. Duda M. Sattmann H. Haring E. (2016). Phylogeography and systematics of Pyramidula (Pulmonata: Pyramidulidae) in the eastern Alps: still a taxonomic challenge. J. Molluscan Stud.82, 110–121. doi: 10.1093/mollus/eyv047
27
Klinbunga S. Pripue P. Khamnamtong N. Puanglarp N. Tassanakajon A. Jarayabhand P. et al . (2003). Genetic diversity and molecular markers of the tropical abalone (Haliotis asinina) in Thailand. Mar. Biotechnol.5, 505–517. doi: 10.1007/s10126-002-0108-8
28
Kobayashi T. Kawahara I. Hasekura O. Kijima A. (2004). Genetic control of bluish shell color variation in the Pacific abalone, Haliotis discus hannai. J. Shellfish Res.23, 1153–1157.
29
Kool J. T. Paris C. B. Barber P. H. Cowen R. K. (2011). Connectivity and the development of population genetic structure in Indo-West Pacific coral reef communities. Glob. Ecol. Biogeogr.20, 695–706. doi: 10.1111/j.1466-8238.2010.00637.x
30
Korshunova T. Martynov A. Bakken T. Picton B. (2017). External diversity is restrained by internal conservatism: new nudibranch mollusk contributes to the cryptic species problem. Zool. Scr.46, 683–692. doi: 10.1111/zsc.12253
31
Kumar S. Stecher G. Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol.33, 1870–1874. doi: 10.1093/molbev/msw054
32
Liu J. Li Q. Kong L. Zheng X. (2011). Cryptic diversity in the pen shell Atrina pectinata (Bivalvia: Pinnidae): high divergence and hybridization revealed by molecular and morphological data. Mol. Ecol.20, 4332–4345. doi: 10.1111/j.1365-294X.2011.05275.x
33
Liu X. Wu F. Zhao H. Zhang G. Guo X. (2009). A novel shell color variant of the Pacific abalone Haliotis discus hannai Ino subject to genetic control and dietary influence. J. Shellfish Res.28, 419–424. doi: 10.2983/035.028.0226
34
Mamat N.-S. Esa Y. Manan H. Wong N. L. W. Sigwart J. D. Nor S. A. M. et al . (2025). Prevailing knowledge on aquaculture of abalone in Southeast Asia: A review. Pertanika J. Trop. Agric.48, 137–158. doi: 10.47836/pjtas.48.1.08
35
Mamat N.-S. Esa Y. WS Wong N. L. Mohd Nor S. A. Abdul Kadar N. Sigwart J. D. et al . (2023). The complete mitogenome of Haliotis asinina (Gastropoda, Haliotidae) from Malaysian waters provides further insights into the phylogeny of the abalone. N. Z. J. Mar. Freshw. Res.59, 79–87. doi: 10.1080/00288330.2023.2278731
36
Marín S. A. Haye P. A. Marchant S. Winkler F. M. (2007). Molecular markers used to analyze species-specific status in abalones with ambiguous morphology. J. Shellfish Res.26, 833–837. doi: 10.2983/0730-8000(2007)26[833:MMUTAS]2.0.CO;2
37
Metz E. C. Robles-Sikisaka R. Vacquier V. D. (1998). Nonsynonymous substitution in abalone sperm fertilization genes exceeds substitution in introns and mitochondrial DNA. Proc. Natl. Acad. Sci.95, 10676–10681. doi: 10.1073/pnas.95.18.10676
38
Mitteroecker P. Gunz P. (2009). Advances in geometric morphometrics. Evol. Biol.36, 235–247. doi: 10.1007/s11692-009-9055-x
39
Monti M. M. Nappo A. G. Giorgini M. (2005). Molecular characterization of closely related species in the parasitic genus Encarsia (Hymenoptera: Aphelinidae) based on the mitochondrial cytochrome oxidase subunit I gene. Bull. Entomol. Res.95, 401–408. doi: 10.1079/BER2005371
40
Nguyen T. V. Alfaro A. C. Venter L. Ericson J. A. Ragg N. L. McCowan T. et al . (2023). Metabolomics approach reveals size-specific variations of blackfoot abalone (Haliotis iris) in Chatham Islands, New Zealand. Fish. Res.262, 1–8. doi: 10.1016/j.fishres.2023.106645
41
Pfenninger M. Cordellier M. Streit B. (2006). Comparing the efficacy of morphologic and DNA-based taxonomy in the freshwater gastropod genus Radix (Basommatophora, Pulmonata). BMC Evol. Biol.6, 1–14. doi: 10.1186/1471-2148-6-100
42
Purvis M. Currie K. L. Bates A. L. Bansemer M. S. Qin J. G. Harris J. O. et al . (2025). Color change of greenlip abalone, Haliotis laevigata Donovan, fed dried macroalgae meals in concurrent laboratory and on-farm trials. New Zeal. J. Mar. Freshw. Res.59, 101–129. doi: 10.1080/00288330.2023.2293734
43
Ratnasingham S. Hebert P. D. N. (2007). BOLD: the barcode of life data system (www.barcodinglife.org). Mol. Ecol. Notes.7, 355–364. doi: 10.1111/j.1471-8286.2007.01678.x
44
Rawat S. Benakappa S. Kumar J. Naik K. Pandey G. Pema C. W. (2017). Identification of fish stocks based on Truss Morphometric: A review. J. Fish. Life Sci.2, 9–14.
45
Rogers-Bennett L. (2023). Ecology of eastern pacific abalone. Developments Aquaculture Fisheries Sci.42, 251–289. doi: 10.1016/B978-0-12-814938-6.00008-7
46
Rozas J. Ferrer-Mata A. Sánchez-DelBarrio J. C. Guirao-Rico S. Librado P. Ramos-Onsins S. E. et al . (2017). DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol.34, 3299–3302. doi: 10.1093/molbev/msx248
47
Ruaza F. C. Herrera M. L. Seronay R. A. (2015). Morphological Polymorphism of Canarium urceus urceus (linnaues), 1758 (Mollusca: Gastropoda) in marine areas of Caraga Region, Philippines. SDSSU J. Multidiscip. Res.3, 89–94.
48
Saunders T. M. Connell S. D. Mayfield S. (2009). Differences in abalone growth and morphology between locations with high and low food availability: morphologically fixed or plastic traits? Mar. Biol.156, 1255–1263. doi: 10.1007/s00227-009-1167-4
49
Serviere-Zaragoza E. García-Hernández V. C. Siqueiros-Beltrones D. A. (2003). Diversity and distribution of macroalgae associated with abalone (Haliotis spp.) habitats in Baja California Sur, Mexico. Bull. Mar. Sci.72, 725–739.
50
Smallwood C. B. Ryan K. L. Lai E. K. M. Rudd L. J. Strain L. W. S. (2023). Recreational fishing for Abalone in Western Australia in 2021/22: estimates of participation, effort and catch. Fisheries Research Report No. 333 (Western Australia: Department of Primary Industries and Regional Development).
51
Soelistyowati D. T. Kusumawardhani A. Junior M. Z. (2013). Phenotype seeds characterization on interspecific hybridization of abalone Haliotis asinina and Haliotis squamata. J. Akua. Indo.12, 26–32. doi: 10.19027/jai.12.25-30
52
Sososutiksno C. Gasperz J. (2017). Economic and financial feasibility of abalone culture development in Hulaliu village, District of Maluku Tengah, Maluku Province. AACL Bioflux.10, 1492–1498.
53
Stone D. A. Bansemer M. S. Currie K. L. (2023). Abalone nutrition. Developments Aquaculture Fisheries Sci.42, 9–44. doi: 10.1016/B978-0-12-814938-6.00002-6
54
Strauss R. E. Bookstein F. L. (1982). The truss: body form reconstructions in morphometrics. Syst. Zool.31, 113–135. doi: 10.1093/sysbio/31.2.113
55
Thompson J. D. Higgins D. G. Gibson T. J. (1994). CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res.22, 4673–4680. doi: 10.1093/nar/22.22.4673
56
Tong P. S. (2020). More policies and laws is it better for biodiversity conservation in Malaysia? Conserv. Sci. Pract.2, 1–11. doi: 10.1111/csp2.235
57
Tran T. N. Niu D. H. Nguyen H. D. Xie S. M. Li J. L. (2015). Populations genetic structure of the razor clam Sinonovacula constricta from China, Korea and Vietnam. Biochem. Syst. Ecol.61, 429–436. doi: 10.1016/j.bse.2015.07.020
58
Wood E. M. Mapait J. B. Bavoh E. M. Ng J. V. Yusah H. M. (2015). Abalone culture and farming in Tun Sakaran Marine Park, Sabah. Mar. Conserv. Soc. 1–24 Available at: https://lighthouse-foundation.org/Binaries/Binary1063/Abalone-culture-and-farming-in-TSMP-2015.pdf (Accessed December 16, 2024).
59
Zhou Z. M. Newman C. Buesching C. D. Meng X. Macdonald D. W. Zhou Y. (2016). Revised taxonomic binomials jeopardize protective wildlife legislation. Conserv. Lett.9, 313–315. doi: 10.1111/conl.12289
Appendix 1
Phylogenetics relationship (Maximum Likelihood) between haplotypes of Haliotis asinina with one sequence from China and one outgroup (Anadara sativa). Haplotypes from western Sabah populations are primarily clustered in the upper part of the tree, while those from eastern Sabah are found in the lower part. The bootstrap probability values are presented at the nodes. Scale showed rate of nucleotide substitution.
Summary
Keywords
abalone, marine gastropods, mollusk, integrative taxonomy, morphometrics, genetic analysis
Citation
Mamat N-S, Esa Y, Sigwart JD, Nor S-AM, Wong NLWS, Kadar NA, Machado FM, Abdul-Halim SAA and Aminarrashid AAA (2025) Combining shells and sequences to untangle taxonomy of abalone in Sabah, Malaysia. Front. Mar. Sci. 12:1577263. doi: 10.3389/fmars.2025.1577263
Received
15 February 2025
Accepted
22 May 2025
Published
09 June 2025
Volume
12 - 2025
Edited by
Ivana Veneza, Federal University of Western Pará, Brazil
Reviewed by
Mbaye Tine, Gaston Berger University, Senegal
Farhad Badshah, Chinese Academy of Agricultural Sciences (CAAS), China
Updates
Copyright
© 2025 Mamat, Esa, Sigwart, Nor, Wong, Kadar, Machado, Abdul-Halim and Aminarrashid.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzine Esa, yuzine@upm.edu.my
‡ORCID: Nur Leena W. S. Wong, orcid.org/0000-0002-8008-6400; Nazia Abdul Kadar, orcid.org/0000-0003-4362-3059; Ahmad Ammar Akhyar Aminarrashid, orcid.org/0009-0008-4733-6931
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.