- 1Jiangsu Agri-animal Huasbandry Vocational College, Taizhou, China
- 2Jiangsu Ocean University, Lianyungang, China
- 3Jiangsu Haorun Biological Industry Group Co., Ltd., Taizhou, China
This study investigated the effects of replacing dietary fish meal (FM) with golden apple snail (Pomacea canaliculata) meat meal (PCM) on growth performance, non-specific immunity and intestinal health of juvenile Chinese mitten crab (Eriocheir sinensis). Four hundred juvenile Chinese mitten crabs (2.39 ± 0.03) g were randomly distributed into five groups in quadruplicate (20 crabs per tank), and each group was fed with diets that replaced FM with PCM at 0% (FM-20, containing 200 g/kg FM), 25% (FM-15), 50% (FM-10), 75% (FM-5), and 100% (FM-0) for 8 weeks, respectively. The results showed that the growth, whole body composition, digestive enzyme activities, antioxidant performance, non-specific immunity, intestinal histology and microbiota composition of FM-15 and FM-10 groups all reached the same level as the FM-20 group (P > 0.05). While the replacement ratio reached 75%, the FCR was significantly increased and the peritrophic membrane thickness was significantly decreased (P< 0.05). When 100% FM was replaced, significantly decreased the growth performance, serum antioxidant and immune enzyme activities, mid-intestine folds height, width and the peritrophic membrane thickness (P< 0.05), and significantly increased the abundance of intestinal harmful bacteria Vibrio (P< 0.05). In conclusion, PCM can effectively replace 50% dietary FM (100 g/kg) without negative effects on the growth performance, intestinal health, serum immune, and antioxidant indexes of juvenile E. sinensis.
1 Introduction
Chinese mitten crab (Eriocheir sinensis) is one of the unique aquatic animals in China. It is popular among consumers for the rich nutrition and unique flavor (Chen and Zhang, 2007). With the improvement of aquaculture technology and the increasing market demand, the production of Chinese mitten crab has also been continuously increasing. According to the Liu et al. (2023), the production of Chinese mitten crab has risen from 593,000 tons in 2010 to 815,000 tons in 2022, with a production growth of 37.4%. It has become the second most productive crustacean species in China, only after the Procambarus clarkii. In the traditional culture mode, E. sinensis mainly feeds on chilled fish, cake and other raw materials, which has the problems of water pollution and high cost (Han et al., 2021; Long et al., 2020; Pan et al., 2020). With the promotion of the green and healthy breeding model, the breeding based on formula feed has become the mainstream, and the feed production has increased year by year (Yao et al., 2024a). E. sinensis cultured with formula feed has the advantages of nutrient balance, convenient feeding and less water pollution (Xu et al., 2020). However, fish meal (FM) has become one of the important bottlenecks in the production and promotion of high-quality formula feed due to its shortage of sources and high price.
Over the past few decades, over-exploitation of wild fish stocks has limited the availability and raised the price of FM. Traditionally, fishmeal has been a major protein source in aquaculture feeds due to its high protein content, excellent amino acid profile, and good digestibility. Additionally, the recognition of the reliance on feed fisheries affects the sustainability of aquaculture has intensified the search for alternative protein sources to replace FM (FAO, 2010). For this reason, a significant portion of aquaculture research over the past two decades has focused on finding more accessible, economical and sustainable alternatives to FM. At present, studies have found that soybean meal (Luo et al., 2011; Liu et al., 2021), rapeseed meal (Cai et al., 2014; Luo et al., 2011), cottonseed meal (Jiang et al., 2013), fermented soybean meal (Xu et al., 2020), black soldier fly (Hermetia illucens) larvae meal (Yao et al., 2024b), yellow mealworm (Tenebrio molitor) meal (Yao et al., 2024a) and some mixed protein sources (Zhu et al., 2020) can be used to replace part of the fish meal in the feed of E. sinensis. However, these protein sources also have some defects, livestock and poultry by-products usually have problems such as high ash content, unstable supply and relatively high price (Forster et al., 2003; Yao et al., 2018); Plant protein sources have the disadvantages of unbalanced amino acid composition, anti-nutrient factors and poor palatability (Yao et al., 2020; Xu et al., 2022); The high content of chitin, pesticide residues, toxins, heavy metal ions and some pathogenic microorganisms also limit the use of insect protein in aquatic feed (Abdel-Latif et al., 2021; Zheng et al., 2023; Mohan et al., 2022; Xiang et al., 2019).
Golden apple snail, Pomacea canaliculata (PC), also known as the large bottle snail or apple snail, is a large freshwater food snail of the family Mesogastropoda (Ampullariidae), native to the Amazon River basin in south America. It was introduced in the 1980s as a high-protein food for cultivation in The United States and parts of southeast Asia (Halwart, 1994). It was introduced into Guangdong province, China in 1981, and then popularized in many provinces and cities. Later, due to poor food taste and market failure, it was abandoned in the wild and spread widely (Yang et al., 2018). In 2000, the Expert Committee on Invasive Species of the International Union for Conservation of Nature listed PC as one of the world’s 100 malignant invasive alien species (Joshi, 2005). However, the view of the PC as a pest is one-sided. The fresh meat contains 15.4% protein, 0.3% fat, and a variety of vitamins. In terms of the composition of essential amino acids, it was very close to that of FM (CP 53.5%) (Andriani et al., 2023). Its meat extract has been shown to contain alkaloids, tannins, polyphenols and glycosides (Pertiwi and Saputri, 2020). This shows the feasibility of PC as a feed or feedstock. At the moment, catfish (Pangasius sp.) (Saputria et al., 2020), striped catfish (Pangasianodon hypophthalmus) (Da et al., 2012, 2013), tiger shrimp (Penaeus monodon) (Bombeo-Tuburan et al., 1995), tilapia (Sarotherodon mossambicus) (Catalma et al., 1991) and other aquatic animals have been reported that PC meat meal (PCM) successfully replaced FM in feed without negatively affecting growth performance. However, the application of PCM in E. sinensis feed has not been reported.
In recognition of the above, the main aim of the present study was to evaluate the possibility of replacing dietary FM with PCM in feeds for E. sinensis. PCM was used in this study to replace different proportions of FM in a practical diet containing 200 g/kg FM to evaluate the effects on the growth performance, intestinal health, serum immune, and antioxidant indexes of juvenile E. sinensis. The results would guide the application of DYM in crab diets.
2 Materials and methods
2.1 Ethics statement
In this study, all the operational procedures were granted by ethical rules of Jiangsu Agri-animal Husbandry Vocational College and relevant rules of China.
2.2 Experimental diets and design
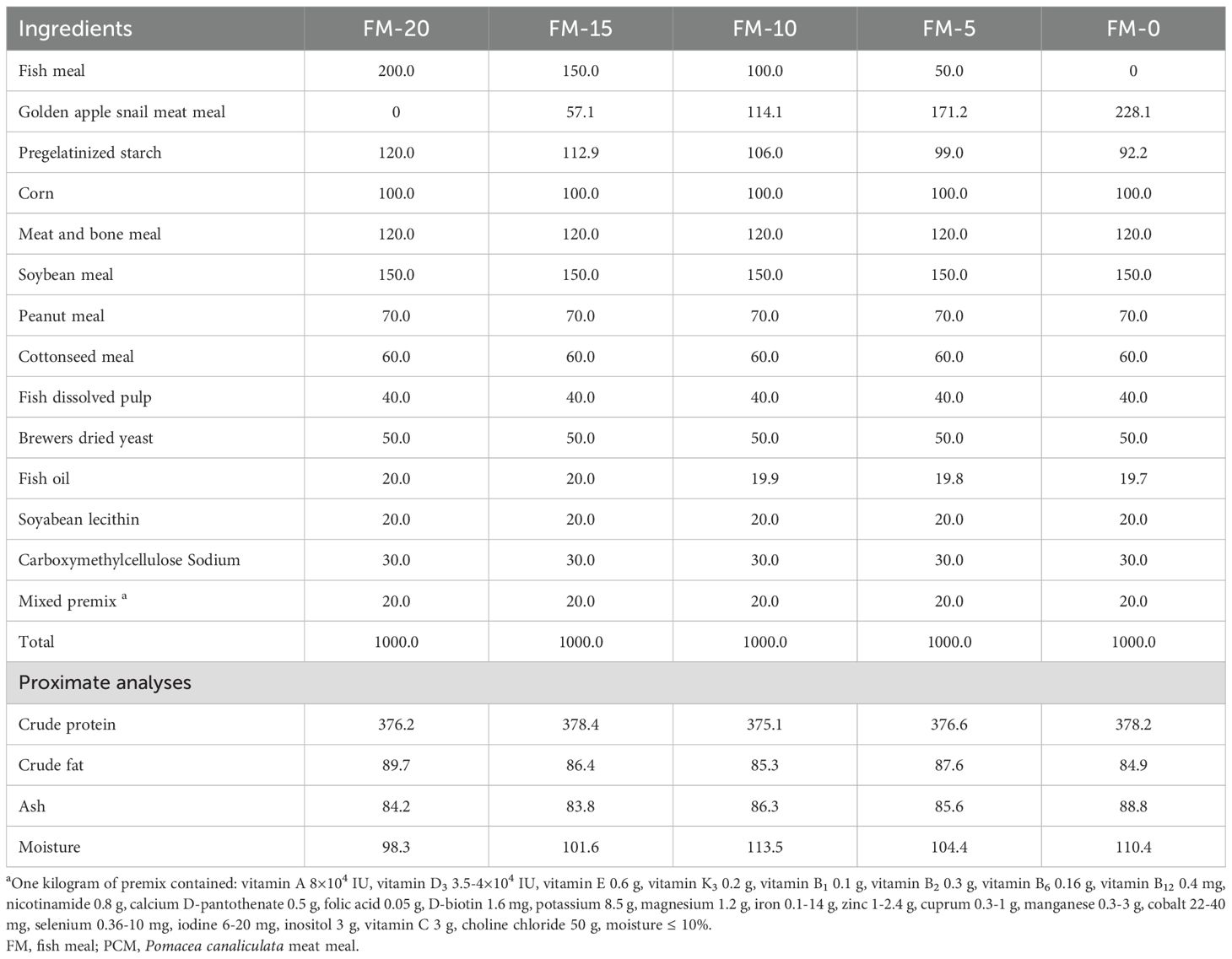
The basal diet (CON, containing 200 g/kg of FM) was formulated with fish meal, soybean meal, meat and bone meal, peanut meal, and cottonseed meal as the main protein sources, pregelatinized starch as the main carbohydrate source, and fish oil and soybean lecithin as the main fat sources. Under the condition of equal protein content in each diet, the amounts of other protein sources were kept constant, and only the amounts of FM and PCM were changed to ensure that the effects on crabs originated solely from the substitution of FM by PCM. On this basis, four isonitrogenous and isolipidic diets (crude protein, 37.5%; crude lipid, 8.9%) were formulated by replacing 25%, 50%, 75%, and 100% of FM with PCM, respectively, and were abbreviated as FM-15, FM-10, FM-5, and FM-0. All ingredients were ground and screened by 60-mesh sieve, and then gradually mixed with fish oil and distilled water (30%) according to the feed formula (Table 1). The mixture was extruded to form sinking pellets (pelleting temperature of 85 ± 5°C) with a single screw extruder (SLP-22# Dantu district Xin Fenghao construction machinery factory). The pellets were post-cooked in oven at 95°C for 20 min, then air-dried, and stored at room temperature store until use.
The FM was purchased from a provender mill in Taizhou, Jiangsu China, and which crude protein and crude lipid contents were 68.0% and 10.1%, respectively. The PCM used in the experiment was collected from local river channels, shelled, and dried in a blast drying oven at 60 °C before being crushed. The crude protein and crude lipid content were 59.6% and 8.9%, respectively.
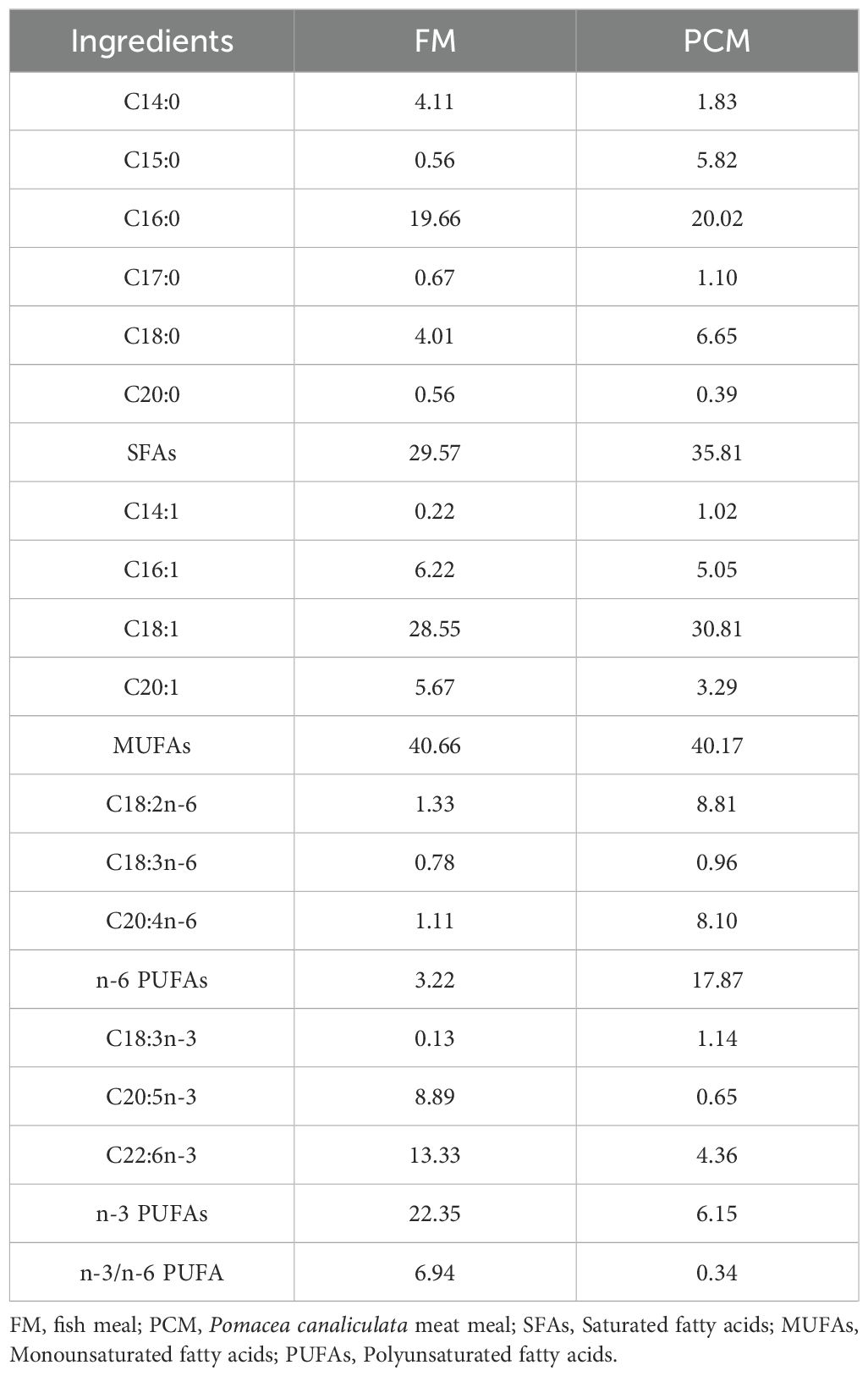
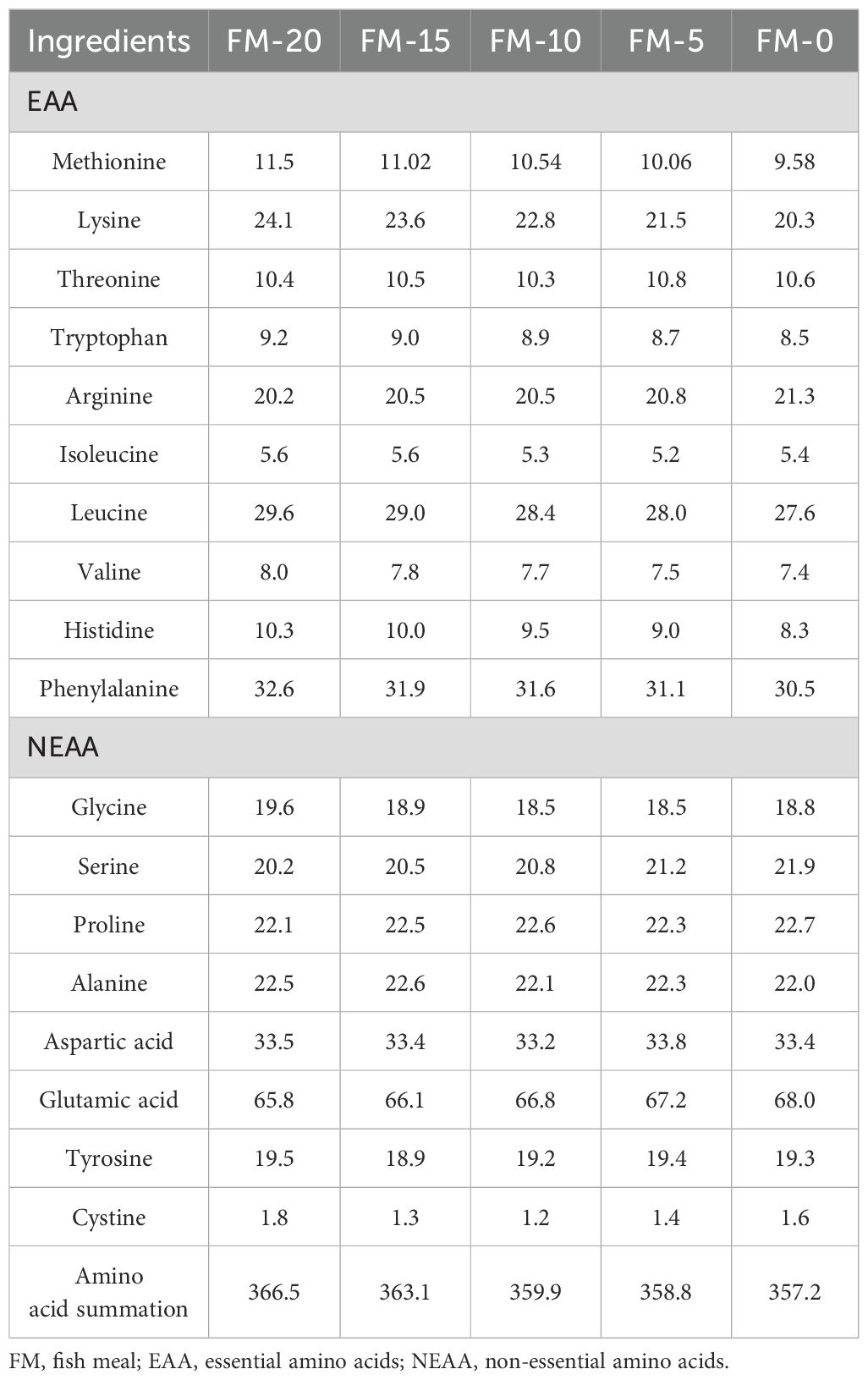
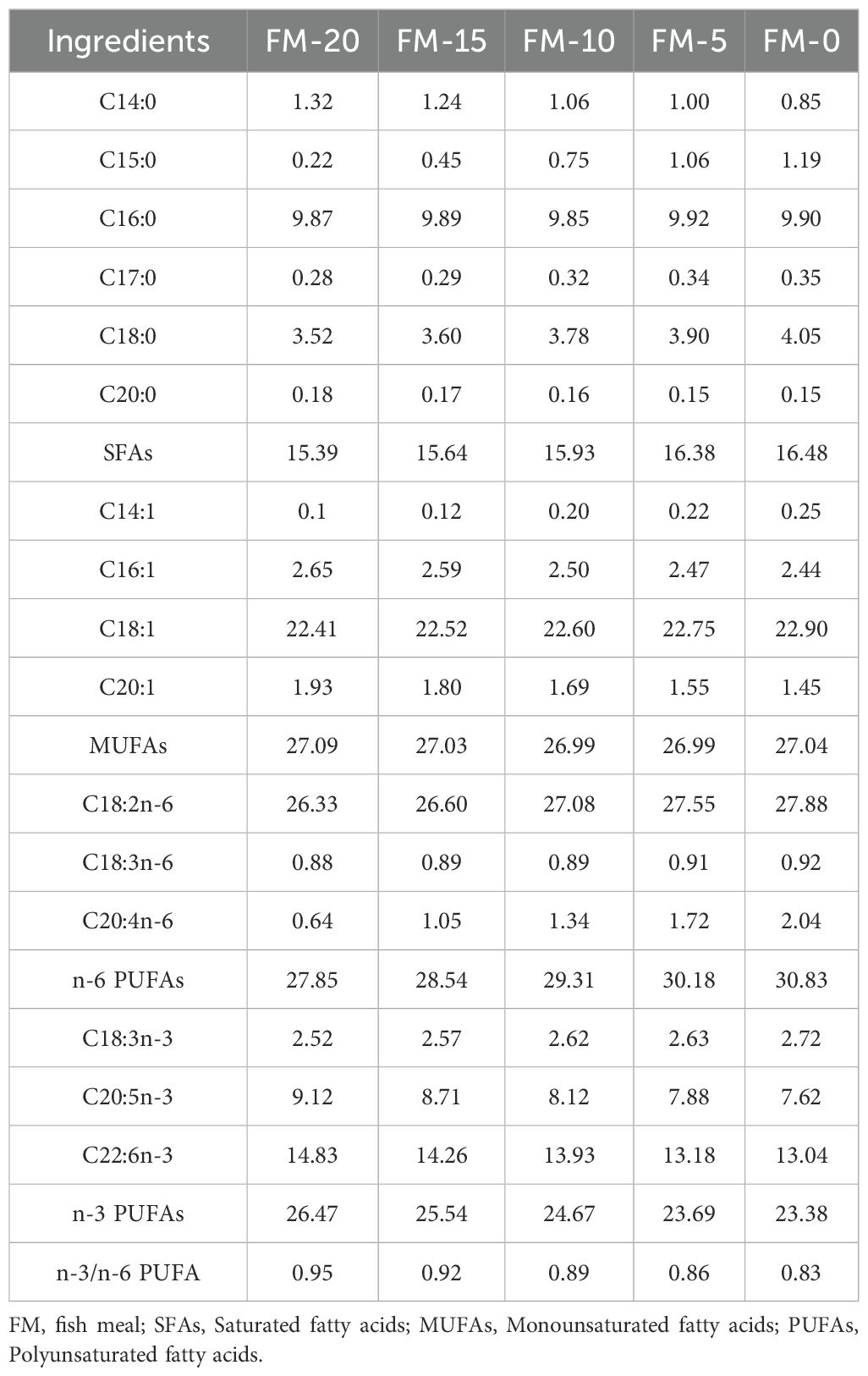
The amino acid composition and the fatty acid composition of feeds, FM and PCM are shown in Tables 2–5.
2.3 Experimental crab and feeding management
E. sinensis was obtained from Jiangsu Haorun Biological Industry Group Co., LTD (Jiangsu, China), and all crabs were temporarily raised for 1 week in a canvas pool (3.0 m × 1.0 m × 1.0 m) and fed with commercial diet. Then, 400 healthy juvenile crabs (2.39 ± 0.03) g in the intermolt period with vigorous appendages were randomly assigned to 20 PVC tanks (1.2 m×1.2 m×0.75 m) with crab nest as a shelter and circulating water (with consistent water quality) after 24 h of starving. There were 5 treatments with 4 replicates (tanks) per treatment and 20 crabs per tank. The groups were arranged in a completely randomized order to minimize the influence of tank position on the experimental results. During the feeding period, crabs were fed twice a day (7:00 and 17:00) for 56 d. The daily feed intake was about 1-3% of the body weight, and was appropriately adjust based on the water temperature, weather and feeding behavior of the crabs to ensure the diets could be eaten up in 2 h after feeding. During the cultivation period, the sediment and faeces were removed by siphoning everyday, and settled river water was supplemented. The water temperature, dissolved oxygen, pH, ammonia nitrogen, and nitrite were kept at 20-26°C, ≥5.0 mgL-1, 7.0-8.5, ≤0.2 mgL-1, and ≤0.05 mgL-1, respectively.
2.4 Sampling
At the end of the feeding experiment, all crabs were deprived of diets for 24 h, and then, the crabs in each tank were counted and weighed. Randomly take 3 crabs from each tank and store them in a -20°C refrigerator for analysis of the entire crab body composition. After four crabs were anesthetized in ice water, 0.5 mL hemolymph was collected using a 1.0 mL sterile syringe, and mixed with the same volume of precooling anticoagulant solution (the formula is sodium citrate 13.2 g/L, citric acid 4.8 g/L, glucose 14.7 g/L). The mixture was immediately centrifuged at 4°C and 12000 rpm for 20 minutes to obtain the supernatant and store in a refrigerator at -80°C. After taking the hemolymph, the hepatopancreas and intestine were dissected aseptically and frozen immediately in liquid nitrogen, then stored at -80°C for the determination of digestive enzyme activity and intestinal microbial study, respectively. Take the intestines of another four crabs from each replicate and load them into a 5 mL centrifuge tube pre filled with 3 mL Bonn’s fixative for the production of intestinal tissue slices. Under sterile conditions, the intestines were taken and placed in a 1.0 mL centrifuge tube for the analysis of intestinal microbial study.
2.4.1 Growth performance
Growth performance indicators including survival, weight gain (WG), feed conversion ratio (FCR), hepatosomatic index (HSI), feed intake (FI) and molting interval (MI):
MI is the interval between the date of second molt and the date of first molt (d).
2.4.2 Proximate composition of feed and crab
Crude lipid, crude protein, ash and moisture of experiment feed and crabs were measured following the method of AOAC (2005). The moisture content and the ash content were analyzed by drying the samples at 105°C to constant weight and incinerating the samples at 550°C for 6 h, respectively. Crude protein was determined using an Auto Kjeldahl System (2300-Auto-analyzer, Foss Tecator, Sweden), and the crude lipid content was analyzed by the chloroform-methanol method.
2.4.3 Digestive enzyme activity
Frozen hepatopancreas was mixed with 9 times saline (W:V=1:9), then homogenated and centrifuged at 4 °C for 15 min (3000 rpm). The supernatant was taken for the determination of digestive enzyme activity. Digestive enzyme activity was measured according to the instructions of the reagent kit from Nanjing Jiancheng Biotechnology Research Institute, Nanjing, Jiangsu, China.
2.4.4 Serum biochemical indices
The serum frozen in the -80°C refrigerator was take out and thawed at 4°C for the detection of serum biochemical indexes. These indicators include acid phosphatase (ACP), alkaline phosphatase (AKP), lysozyme (LZM), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-PX), catalase (CAT) and malondialdehyde content (MDA). All indicators were measured according to the instructions of the reagent kit from Nanjing Jiancheng Biotechnology Research Institute, Nanjing, Jiangsu, China.
2.4.5 Intestinal histology
When slicing, the mid-gut was dehydrated with ethanol, transparentized with xylene, embedded in paraffin, and sliced with a thickness of 5 μm (Leiken RM2235 slicer, Germany), HE staining, sealing to make permanent preservation slices. The morphological characteristics of intestinal folds were observed under a light microscope and photos were taken (Nikon YS100 micrography system). The intestinal epithelial folds height, width and number were measured using micrometer (the entire midgut cross-section folds number were counted).
2.4.6 Intestinal microbial study
The intestinal samples were sent to Shanghai Majorbio Bio-pharm Technology Co., Ltd. for DNA extraction and PCR amplification by Illumina MiSeq Sequencing platform. The V3–V4 variable regions of 16S rRNA gene was amplified by PCR with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The microbiota community composition and community abundance at phylum and genus levels were analyzed on the online platform of Majorbio Cloud Platform (www.majorbio.com).
2.5 Statistical analysis
The data were analyzed by using SPSS 22.0 statistical software. The results were subjected to Levene’s test to determine homogeneity of variance and no transformation was required. A one-way analysis of variance (ANOVA) was used to determine the effects of the five diets on the various responses including FW, WG, SGR, FCR, etc. for all treatments. Turkey’s multiple range test was used to determine the statistical significance of the differences in means among the treatments. Statistical significance was determined at P< 0.05. In addition, a follow-up trend analysis was performed using orthogonal polynomial contrasts to determine whether the significant effect was linear and/or quadratic. Results means were presented with ± standard error (SE) in tables and/or figures.
3 Results
3.1 Growth performance
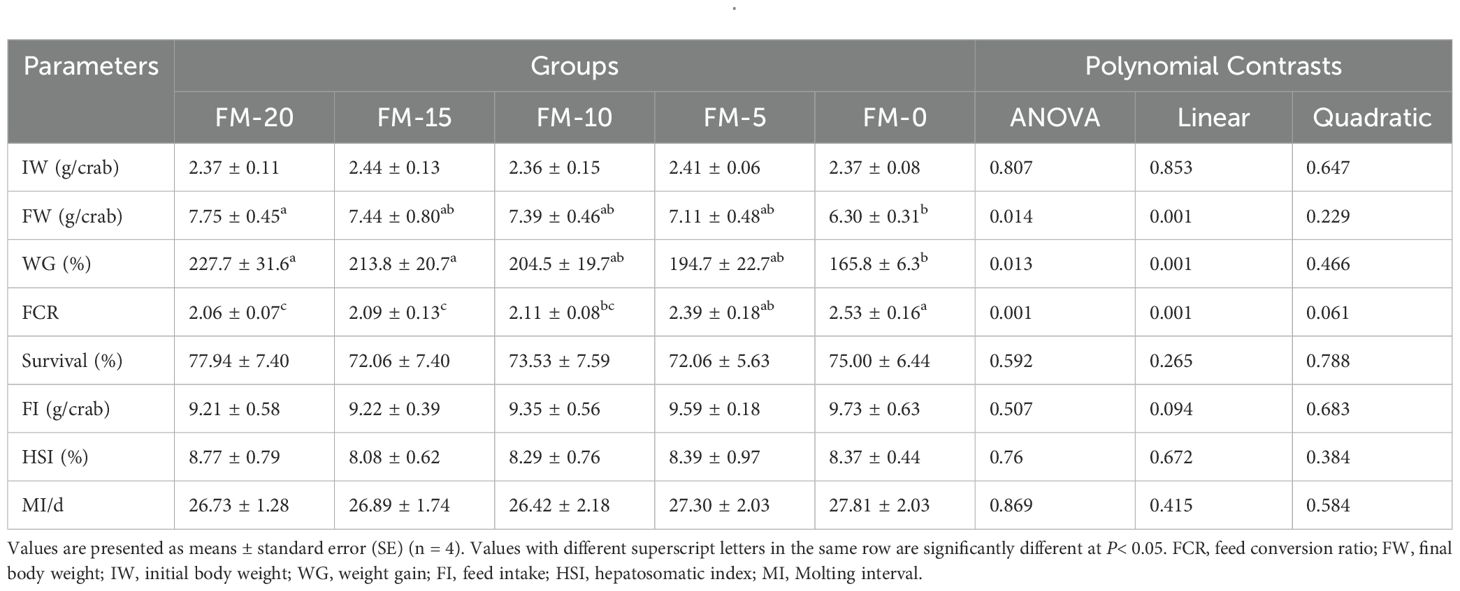
As shown in Table 6, significant linear effects of FM replaced by PCM were observed in FW, WG and FCR (P< 0.05). The FW, WG and FCR shows no significant difference between FM-15, FM-10 and FM-20 groups (P > 0.05). The FW and WG in FM-0 group were significantly lower and FCR was significantly higher than FM-20 group (P< 0.05). There was no significant difference in survival, FI, HSI and MI among all groups (P > 0.05).
3.2 Whole-body proximate composition
No significant effect on whole-body composition was observed by FM replaced with PCM among all groups (P > 0.05) (Table 7).
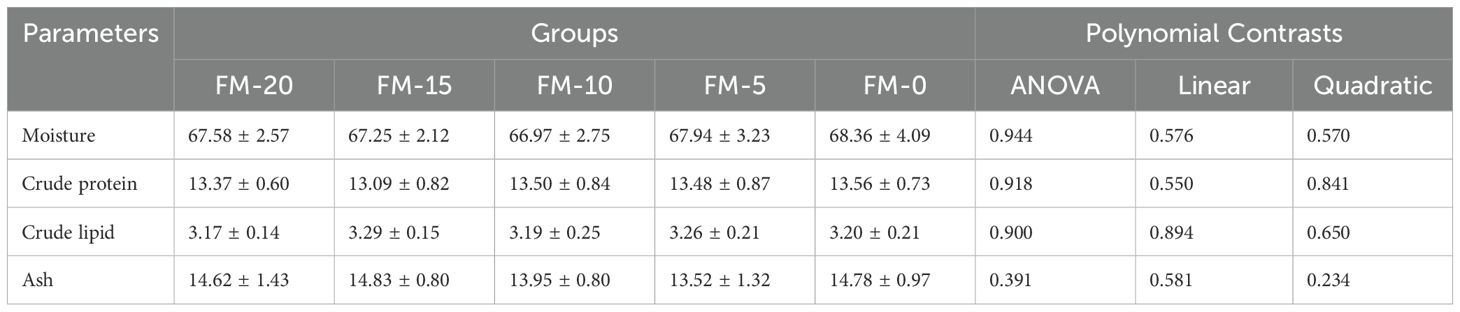
Table 7. Effects of different diets on the proximate composition in whole body of E. sinensis (% in DM).
3.3 Digestive enzymes activities in hepatopancreas
As shown in Table 8, no significant effect on hepatopancreas lipase and amylase activity was observed among groups (P > 0.05). The level of replacing FM with PCM linearly decreased the hepatopancreas protease activity, of which FM-0 group was significantly lower than the FM-20 group (P< 0.05).
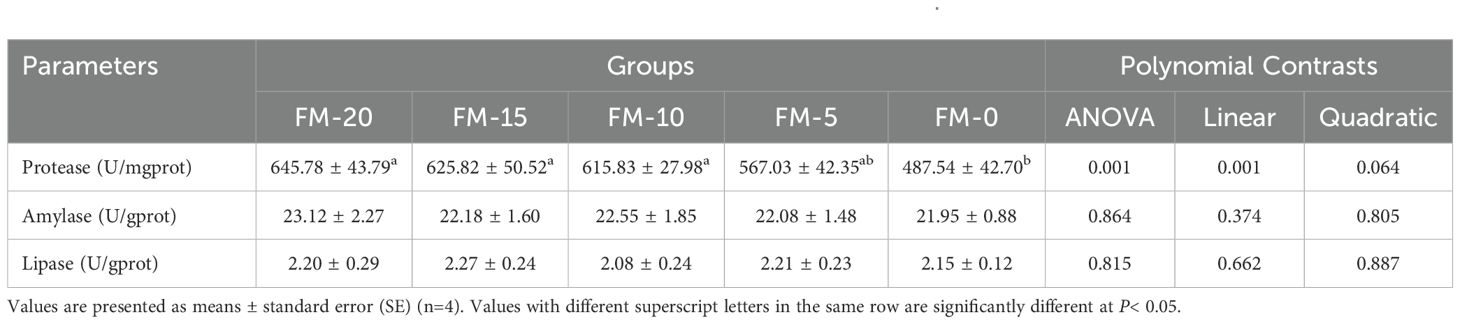
Table 8. Effects of different diets on digestive enzyme activities in hepatopancreas of E. sinensis.
3.4 Serum biochemical indices
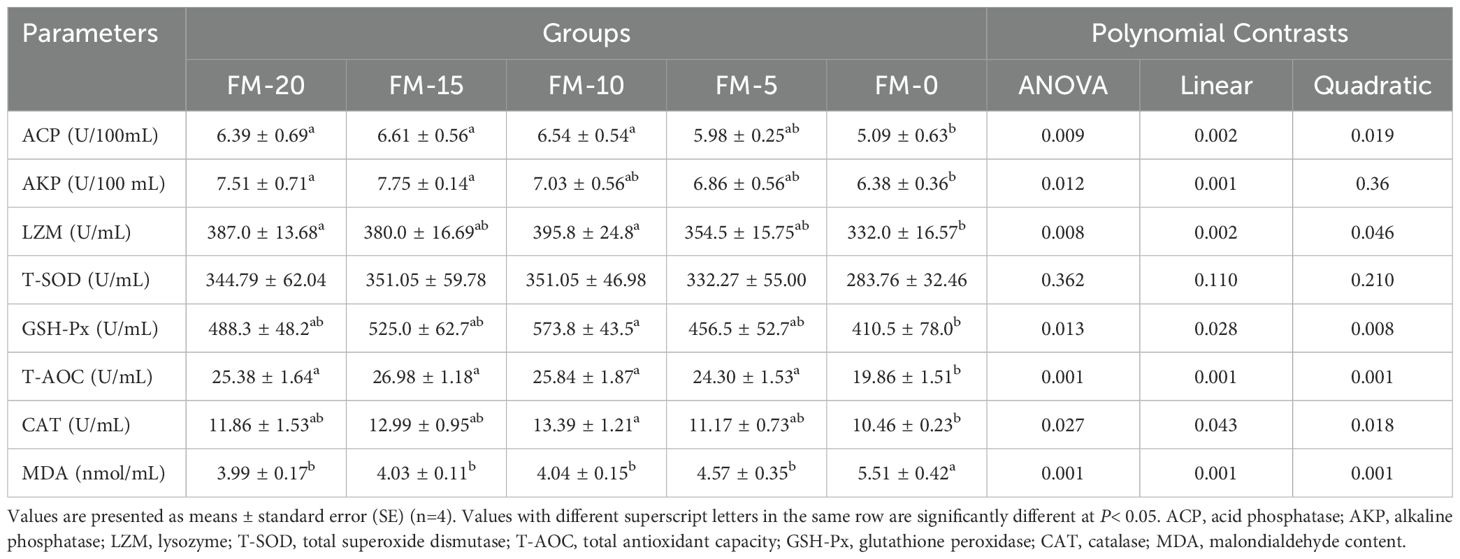
Significant linear and quadratic effects of FM replaced by PCM were observed in serum GSH-Px, T-AOC, CAT, LZM, ACP activities and MDA content (P< 0.05). Significant linear effects were observed in serum AKP activity (P< 0.05). Serum antioxidant oxidase GSH-Px, T-AOC and CAT activities, immune enzymes LZM, ACP, AKP activities and MDA content in FM-15, FM-10 and FM-5 groups all reached the same level as FM-20 group (P > 0.05) (Table 9). At the genus level, the dominant intestinal microflora included Candidatus_Bacilloplasma, Candidatus_Hepatoplasma, Dysgonomonas and Citrobacter etc. (Figure 1).
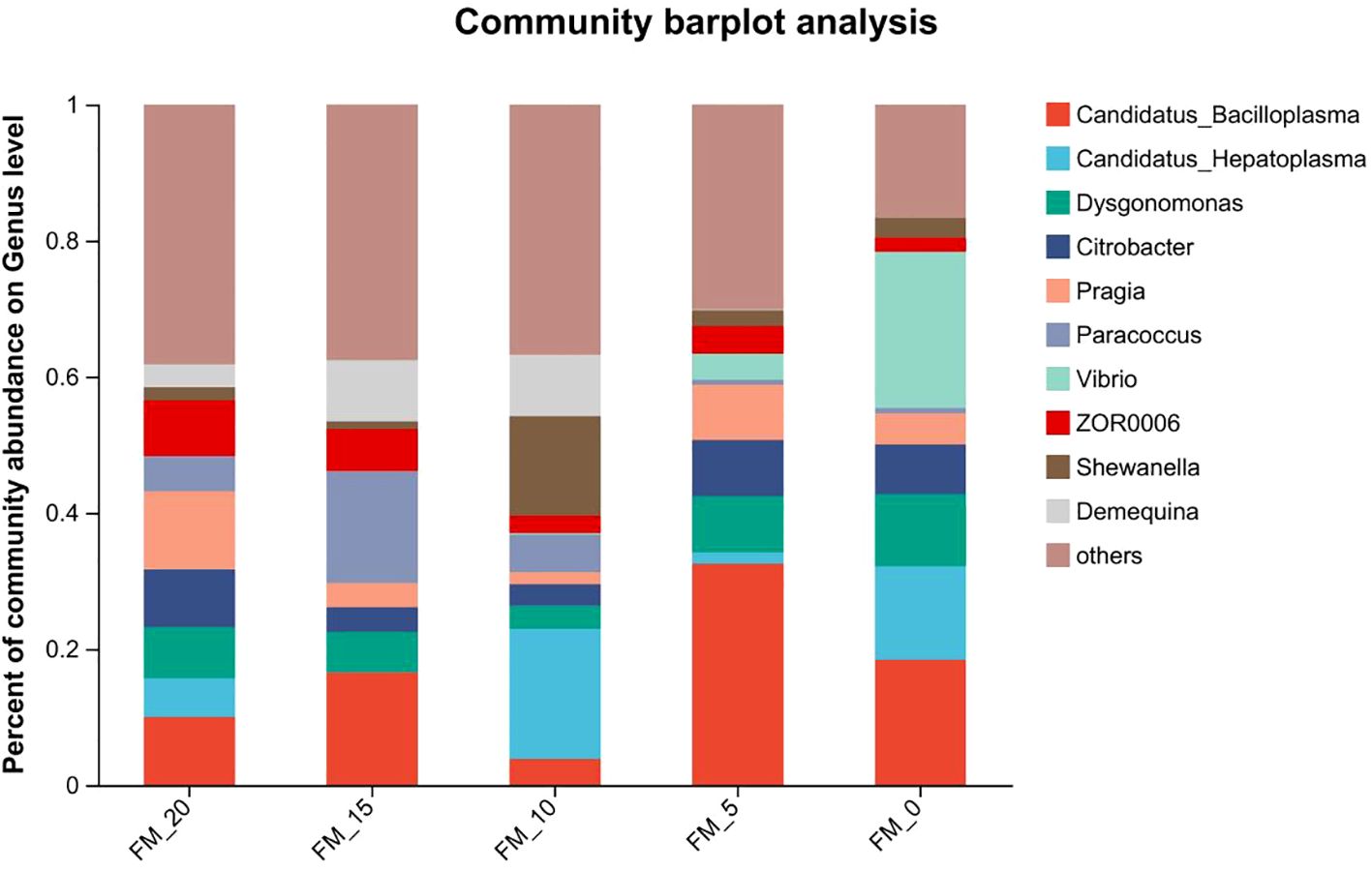
Figure 1. Abundance at the genus level of gut microbiota in E. sinensis feeding with different diets.
3.5 Intestinal histology
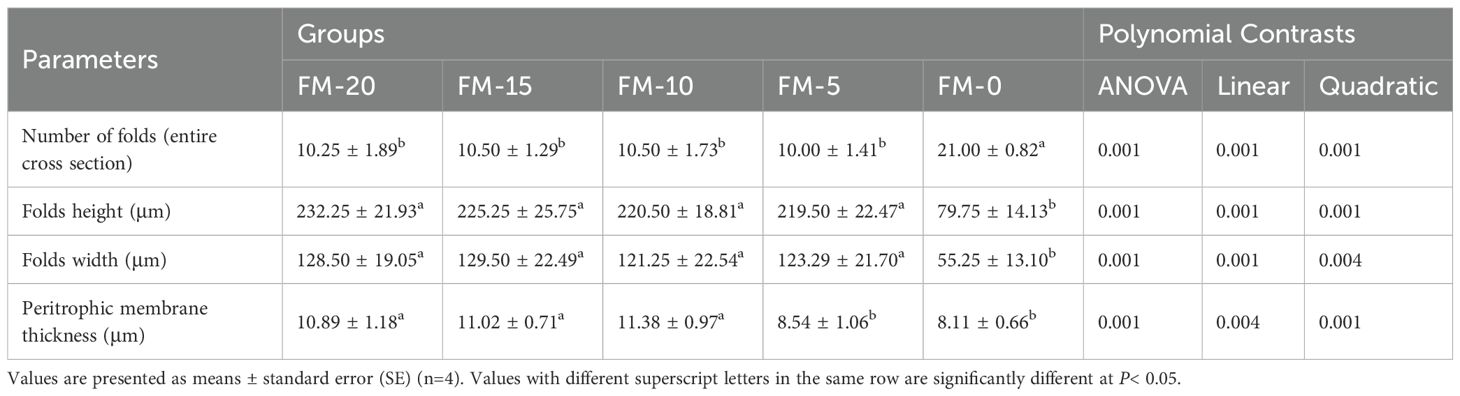
As shown in Table 10, significant linear and quadratic effects of FM replaced by PCM were observed in mid-intestinal folds number, height, width and peritrophic membrane thickness (P< 0.05). Number of folds in FM-0 group was significantly higher and mid-intestinal folds height, width was significantly lower than other groups (P< 0.05). There was no significant difference in intestinal indices between FM-10, FM-15 and FM-20 group (P > 0.05).
The intestinal epithelial cells in groups FM-20, FM-15, FM-10 and FM-5 protruded to form complete folds, while only part of the epithelial cells in FM-0 group protruded to form smaller individual folds, and part of the intestinal epithelial cells were arranged in the basal layer in the form of single columnar cells (Figure 2).
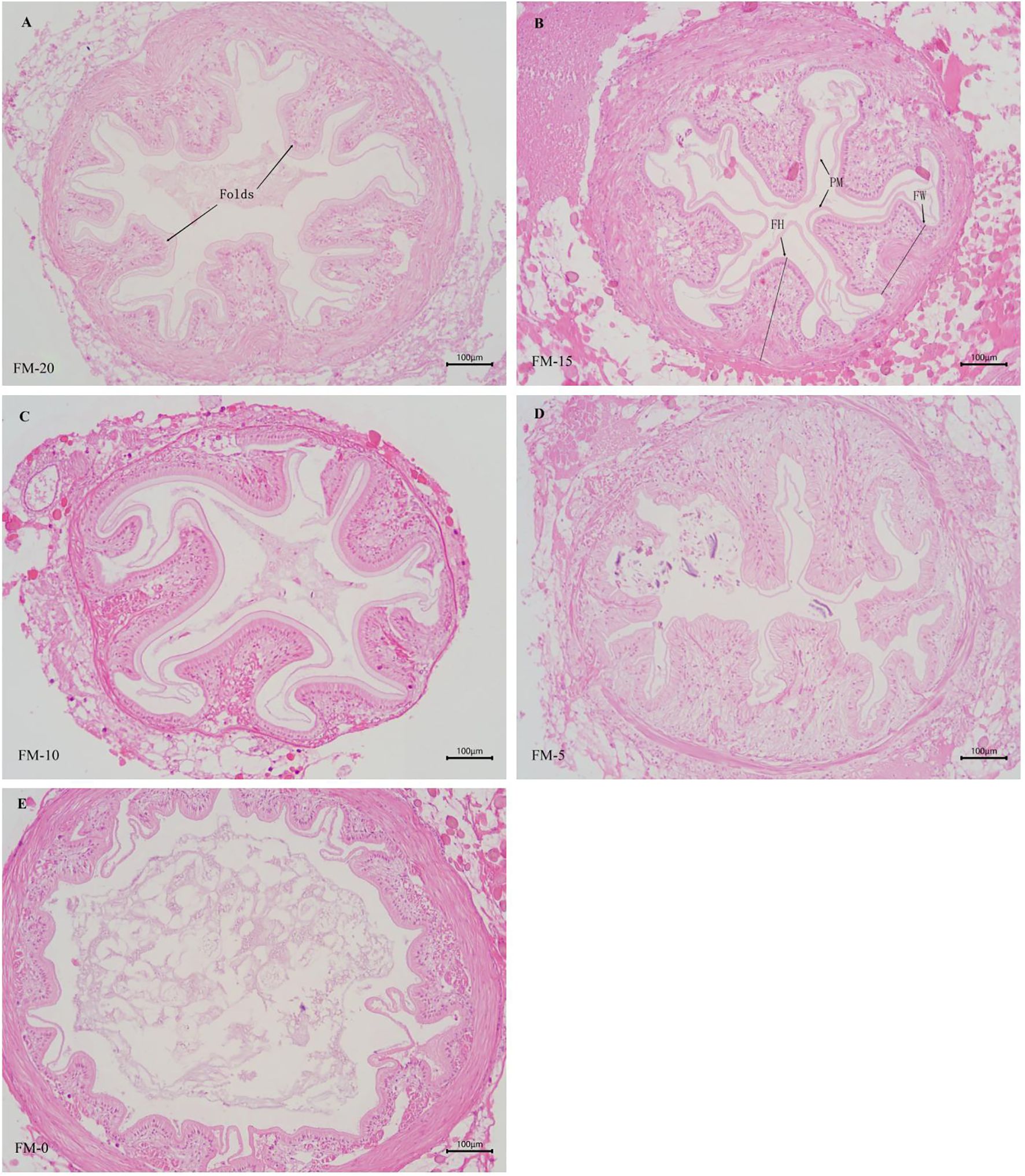
Figure 2. Effects of different diets on intestinal tissue structure of E. sinensis, respectively (100×). (A–E) respectively presented the intestinal morphology of E. sinensis in the FM-20, FM-15, FM-10, FM-5 and FM-0 groups. FH, Folds height; FW, Folds width; PW, Peritrophic membrane.
3.6 Microbial study in intestine using 16 rRNA sequencing
A total of 1,323,128 valid sequences were obtained using 16 rRNA sequencing with sequence lengths ranging from 200 to 521 bp, and the average sequence length was 436 bp. After cluster analysis of the obtained sequences, five groups obtained 663, 548, 527, 278, and 217 representative bacterial communities OTUs, respectively. The OTUs shared by the five treatment groups are 94, while the unique OTUs are 233, 135, 118, 24, and 29, respectively (Figure 3).
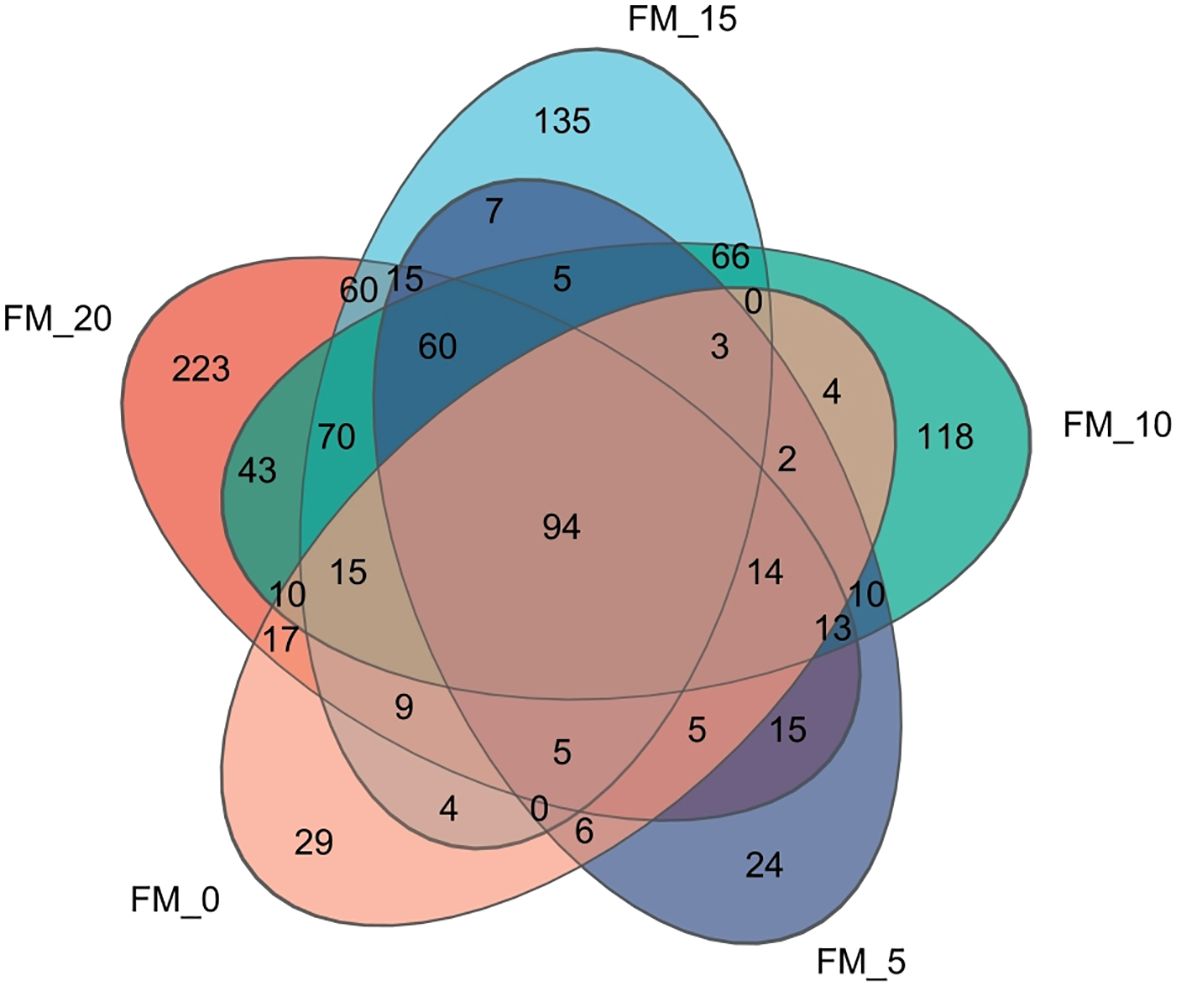
Figure 3. Venn analysis of the OTU numbers of gut microbiota in E. sinensis feeding with different diets.
At the phylum level, Firmicutes, Proteobacteria, Actinobacteriota, and Bacteroidota are the most abundant bacteria in the intestinal microflora of E. sinensis, accounting for 97.07%, 93.75%, 95.75%, 97.89%, and 99.16%, respectively (Figure 4).
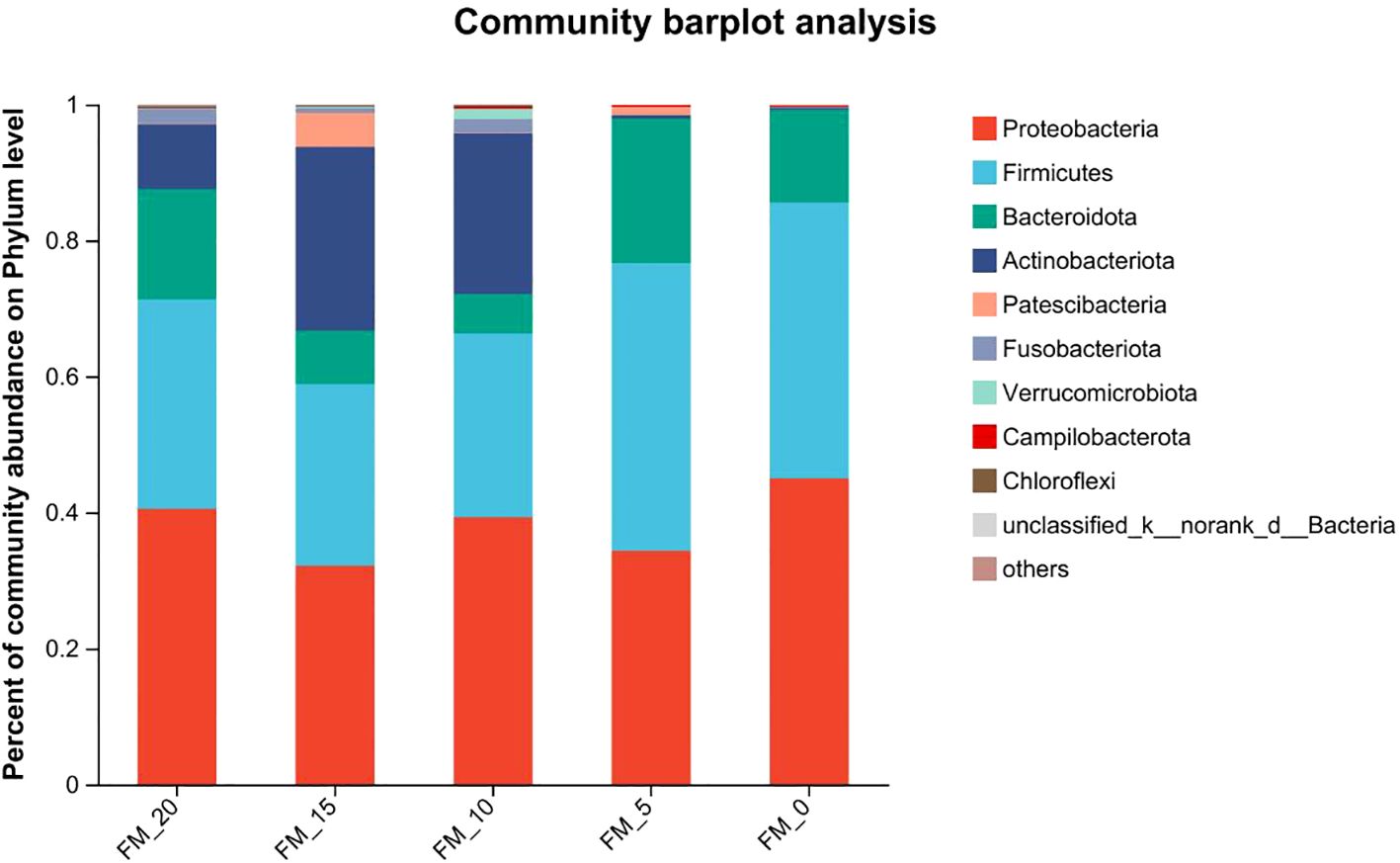
Figure 4. Abundance at the phylum level of gut microbiota in E. sinensis feeding with different diets.
At the phylum level, there were significant differences in the abundance of Actinobacteriota and Verrucomicrobiota in the microbial composition of each treatment group (P< 0.05) (Figure 5).

Figure 5. Comparison of difference at the phylum level of gut microbiota in E. sinensis feeding with different diets.
At the gene level, there were significant differences in the abundance of Pragia, Vibrio, Nocardioides, Pseudomonas, Aeromonas, Chitinibacter, Pseudoxanthomonas in the microbial composition of each treatment group (P< 0.05). The abundance of vibrio increased with the increase of fishmeal substitution level (Figure 6).
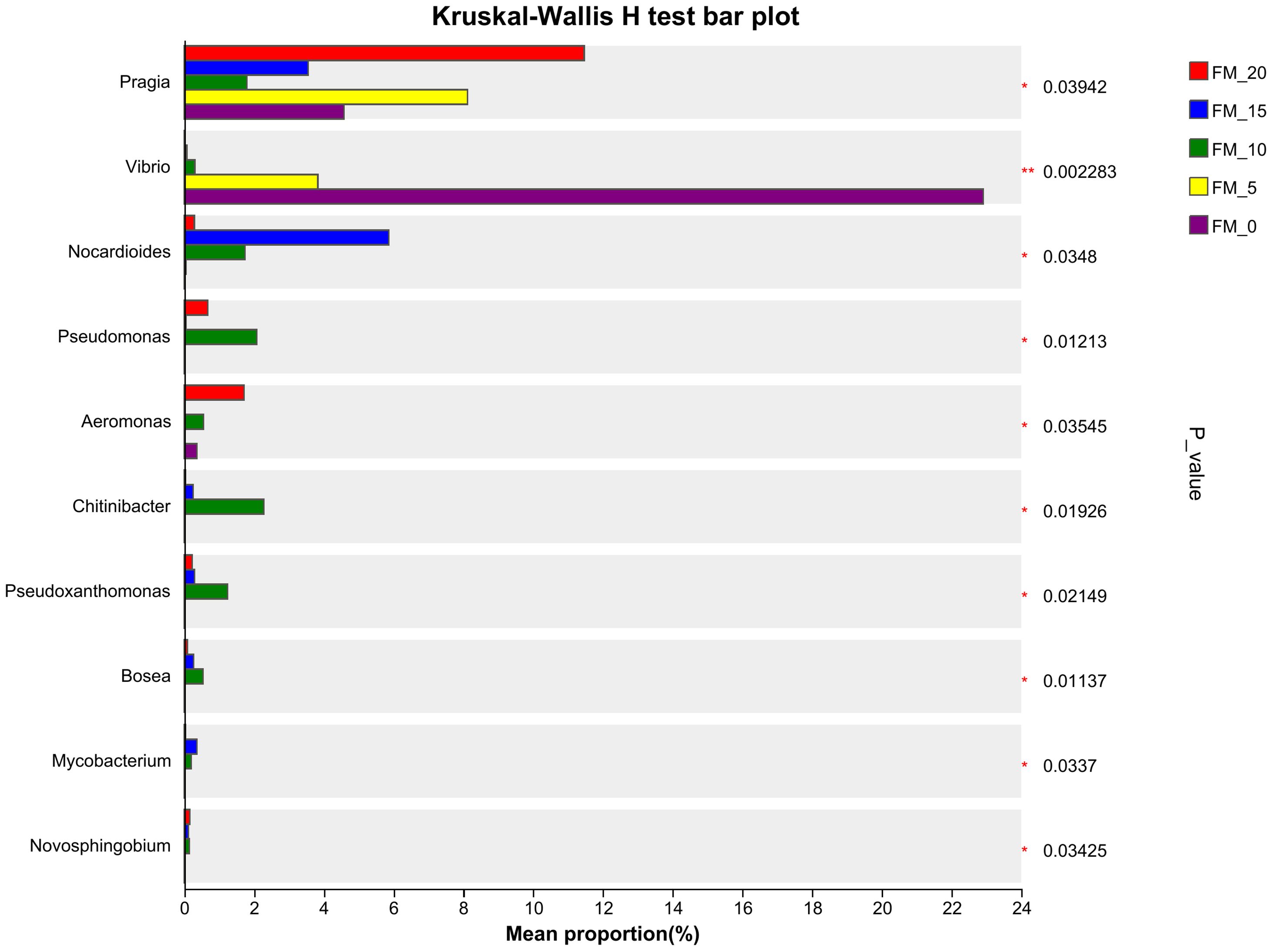
Figure 6. Comparison of difference at the genus level of gut microbiota in E. sinensis feeding with different diets.
4 Discussion
4.1 Growth performance
Owing to the poor taste and flavor, as well as the presence of the parasitic Angiostrongylus cantonensis (Castillo-Ruiz et al., 2018), golden apple snail was not suitable for human consumption. Nevertheless, it is regarded as a high-quality food source for certain livestock and poultry due to its high-quality nutritional composition (Dharmawati et al., 2022). With the increasing adoption of biological control techniques for golden apple snails, their feasibility as a source of protein for aquatic animal feed has received widespread attention (Visca and Palla, 2018; Ghosh et al., 2017; Yanti et al., 2022; Da et al., 2012). The research results of Da et al. (2012) on striped catfish (Pangasianodon hypophthalmus) and Jintasataporn et al. (2004) on giant freshwater prawn (Macrobrachium rosenbergii) showed that PCM resoundingly replaced 100% dietary FM without significant impact on growth performance. However, in this experiment, PCM effectively replaced 50% FM without significant effect on growth performance of the E. sinensis. The FCR significantly increased when the replacement ratio reached 75%, it was also accompanied by a significant decrease in WGR and FW when the replacement ratio reached 100% (Table 6), which is different from the above research results. The research results of Kiriratnikom et al. (2010) on Pacific white shrimp (Litopenaeus vannamei) showed that the partial replacement of FM with PCM did not affect the growth performance of the cultured animals. However, a higher proportion of FM replacement significantly reduced the growth performance. Similar results also shown in the research of Pertiwi and Saputri (2020) on Pasupati catfish (Pangasius sp.) The optimal replacement level of PCM for FM in feed varies significantly among different aquatic animals, which may be related to their distinct feed composition and nutritional requirements. Different species of aquatic animals have varying basic nutritional requirements for feed, and the nutritional level of the feed after substitution must meet these fundamental needs. As the highest quality animal protein source, FM has the advantages of balanced amino acids and rich fatty acid content. The replacement of FM with PCM will inevitably lead to changes in some nutritional components of the diet. Therefore, the optimal replacement level depends on the changes in the nutritional level after replacement. Considering amino acid nutrition, various farmed animals have distinct requirements for essential amino acids (Oliveira et al., 2021). Ye et al. (2010) found that the optimal requirements for lysine and methionine in the feed of juvenile E. sinensis are 2.34% and 1.12%, respectively. In this experiment, the amino acids composition of the FM-20 group feed aligned with the requirements of juvenile crabs. However, the contents of methionine and lysine in PCM are 1.07% and 3.26%, respectively, which are much lower than those in FM (2.03% and 5.21%). With the increase of FM substitution, the levels of methionine and lysine in the feed decreased significantly. When the replacement level reaches 75%, the contents of methionine and lysine in the feed are far below the requirements of juvenile crabs, thus leading to a decrease in growth performance (Table 4, Table 6).
The fatty acid composition of feed is also one of the important factors affecting growth. The research of Zhang et al. (2014) on swimming crab (Portunus trituberculatus) and Chen et al. (2011) on gibel carp (Carassius auratus gibelio) reported that an appropriate level of n-3 PUFAs lead to optimal growth performance, while low levels of n-3 PUFAs significantly reduced the growth performance of aquatic animals. Similar results have also been obtained in experiments on aquatic animals such as black sea bream (Acanthopagrus schlegeli) (Ma et al., 2013), grouper (Epinephlus Coioides) (Zhu et al., 2012), and sea cucumber (Apostichopus japonicus) (Liao et al., 2015). It was determined that the content of n-3 PUFAs in FM was 22.35%, while that in PCM was only 6.15% (Table 3). With the increasing proportion of PCM replacing FM, the content of n-3 PUFAs in the feed significantly declined, eventually manifesting as growth inhibition. The reason for this growth inhibition may be the deficiency of essential fatty acids in the feed, leading to immune imbalance in E. sinensis, which in turn triggers a series of inflammatory reactions, thereby affecting normal growth. There are multiple factors that affect the growth performance of E. sinensis. In addition to the imbalance of feed amino acids and fatty acids, fish meal also contains unknown growth factors (NRC, 2015), which are not present in PCM. Therefore, the use of PCM as a substitute for FM should be comprehensively considered.
4.2 Digestive enzyme activity
The digestive and absorptive abilities of animals for nutrients are one of the main factors determining their growth and development. The activity of digestive enzymes can reflect the strength of intestinal digestive capacity to a certain extent (Yao et al., 2024a). At present, a large number of studies have proven that the digestive enzyme activity is regulated by the nutritional composition of the feed (Fountoulaki et al., 2005; Santigosa et al., 2008; Castro et al., 2013; Sivaramakrishnan et al., 2017; Fuentes-Quesada and Lazo, 2018). Huang et al. (2020) found that the content of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) in the stomach and intestine of rainbow trout were positively correlated with the levels of DHA and EPA in their feed. Low levels of DHA and EPA in the feed inhibited the protease activity in the gastrointestinal tract of rainbow trout. Similar findings were also reported by You et al. (2019) and Bowyer et al. (2012) in golden pompano (Trachinotus ovatus) and yellowtail kingfish (Seriola lalandi). In this experiment, PCM as an alternative protein source was used to replace FM, with DHA and EPA contents of 4.36% and 0.65% respectively, which were much lower than the 13.33% and 8.89% in FM (Table 3). As the proportion of PCM replacing FM increased, the contents of DHA and EPA in the feed decreased significantly. When the substitution reached 100%, the trypsin activity of the crab was significantly reduced (Table 8), which was consistent with the above research results. Low levels of DHA and EPA can reduce the fluidity of cell membranes and alter the microenvironment of cell membranes, thus further affecting the activity of attached enzymes (Calder, 2015). Therefore, the content of DHA and EPA in feed can affect the activity of digestive enzymes in aquatic animals by influencing the content of DHA and EPA in the body. In addition, the imbalance of amino acids in PCM is also one of the reasons for the decrease in trypsin activity of the crab. Trypsin is a type of serine protease with typical characteristics of serine proteases, which can specifically degrade the carboxyl terminal peptide bonds of arginine or lysine in polypeptide chains (Amino et al., 2001). As shown in Table 2, the arginine content in PCM is close to that in FM, while the lysine content is only 32.6 g/kg, much lower than the 52.1 g/kg in fish meal. The decrease in the content of specifically degraded amino acids will inversely affect the secretion of trypsin.
4.3 Antioxidative capacity and non-specific immunity
During the metabolic process in aquatic animals, a large amount of reactive oxygen species (ROS) was produced, including superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and singlet oxygen (O2). Excessive ROS can cause severe cellular damage and exert toxic effects on biological molecules such as DNA, proteins, and lipids (Yang et al., 2015; Kuang et al., 2012). To avoid the toxicity of excessive ROS, the body has developed its own antioxidant defense system, in which antioxidant enzyme systems such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) work together with endogenous antioxidants to exert antioxidant functions (Ferrante et al., 2008). Numerous studies have demonstrated that an appropriate content of polyunsaturated fatty acids (PUFAs) in feed can promote the antioxidant enzyme system of fish (Zhang et al., 2017; Li et al., 2017; Huang et al., 2018). Ji et al. (2025) found that, under the condition of the same dietary fat content, the total antioxidant capacity of grass carp in the DHA-deficient group was significantly lower than that in the normal group. Zuo et al. (2012) found that the SOD activity of large yellow croaker with a lower intake of EPA and DHA was significantly lower than that of the group with a higher content. Similar results also shown in the research of Yu et al. (2016) on sea cucumber. In this experiment, as the proportion of PCM replacing dietary FM increased, the content of DHA and EPA in the feed decreased significantly. When the replacement amount of FM reached 100%, the crab serum T-AOC activities was significantly reduced, and the MDA content was significantly increased, which is consistent with the above research results. An appropriate content of dietary n-3 HUFA can protect aquatic animals from cellular and tissue damage by inhibiting the excessive production of ROS or scavenging the generated ROS. When the dietary content of n-3 HUFA is too low, excessive ROS will exert toxic effects on the body.
Humoral immune factors, such as Lysozyme (LZM), alkaline phosphatase (AKP), and acid phosphatase (ACP) in serum are important parameters for the non-specific immune ability of crustaceans. LZM can hydrolyze the β-1,4-glycosidic bond between N-acetylglucosamine and N-acetylmuramic acid in the peptidoglycan of bacterial cell walls, thereby causing bacterial lysis and effectively killing Gram-positive bacteria (Li et al., 2015). ACP and AKP can inhibit the growth and reproduction of pathogens in the body, and the level of their activity can be used to evaluate the strength of the body’s non-specific immune function (Amoah et al., 2019; Yao et al., 2021). In this study, the substitution of 75% dietary FM (150 g/kg) with PCM had no significant effect on the activities of ACP, AKP, and LZM in the serum of E. sinensis (Table 9). However, when the replacement ratio reached 100%, the activities of LZM, ACP, and AKP were all significantly lower than those of the control group. The negative impact of PCM substituting for FM on the serum immunity of crabs may be related to the changes in the content of dietary n-3 polyunsaturated fatty acids (PUFAs). PUFAs are important components of the cell membrane, playing a crucial role in maintaining membrane fluidity, surface enzyme activity, hormone receptor binding, and signal transduction (Mai, 2011). Therefore, changes in the unsaturated fatty acids in the phospholipids of immune cell membranes will affect the immune function of aquatic animals. Zuo et al. (2012) investigated the effects of different n-3 PUFA contents on the non-specific immune function of large yellow croaker. The results showed that the expression levels of immune genes TLR22 and MyD88 in the liver and kidney of large yellow croaker fed with 0.15% n-3 PUFAs were significantly lower than those in the 0.98% group, and the non-specific immune capacity was significantly reduced. Yu et al. (2016) also found that the LZM activity in the coelomic fluid of sea cucumber fed with 0.46% n-3 PUFA was significantly lower than that in the 0.85% group, and the expression of LZM mRNA in the intestine was significantly down-regulated. Similar findings were reported by Lin and Shiau (2003) and Thompson et al. (1996) on juvenile Epinephelus malabaricus and Atlantic salmon. The results of this experiment are basically consistent with the above studies, which may be due to the high content of n-6 PUFA, which can increase the expression of inflammatory factors in the body, causing oxidative stress and leading to long-term “sub-health” status. It can be seen that the moderate substitution of dietary FM with PCM does not affect the serum immune enzyme activity of E. sinensis. However, when the substitution rate exceeds 75%, it will cause significant negative effects.
4.4 Intestinal health
Intestinal microbiota plays a key role in regulating the host’s digestion, absorption, metabolism, and immune response, which together with the intestinal tissues and contents to form a specific dynamic equilibrium system. Disruption of this balance can increase the susceptibility to intestinal pathogens, thereby triggering intestinal infections (Wang et al., 2018; Ray et al., 2010). Similar to other animals, the composition of aquatic animal intestinal microbiota is affected by factors such as the breeding environment, dietary composition and species, with dietary composition being the main factor affecting intestinal microbiota composition (Ingerslev et al., 2014; Mansfield et al., 2010). In this experiment, the predominant gut microbiota of crabs were Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, which is consistent with the findings of Yang et al. (2024) and Jiang et al. (2023). Among them, Actinobacteria with significant intergroup differences were observed. Actinobacteria contains beneficial bacteria such as Bifidobacterium, crucial for intestinal health, as well as the largest genus Streptomyces, which mediates the degradation of polymers such as chitin and cellulose. Actinobacteria also includes various pathogenic bacteria that can increase the risk of intestinal diseases in aquatic animals. However, we are more concerned with the beneficial or pathogenic bacterial genera within the gut of animals. In this study, the replacement of FM with PCM at 100%, the abundance of Vibrio in the intestine significantly increased (P<0.05) (Figure 6). Vibrio is a large family belonging to the Gram-negative γ-Proteobacteria phylum, which is often classified as a pathogenic bacterium (Loo et al., 2020). It is a common pathogenic bacterium in aquaculture, which has a strong pathogenic effect on fish, shrimp, crabs, shellfish, etc. An increased abundance of Vibrio in the intestine may lead to intestinal flora imbalance and endanger the health of the host (Nursyam, 2017). The substitution of FM with PCM in the diet led to an increase in the abundance of potentially pathogenic bacterial genera in the gut of crabs, which may be related to the changes in polyunsaturated fatty acids after the substitution. Currently, several studies have shown that the content and types of polyunsaturated fatty acids in feed have a direct impact on the intestinal health of animals. Zhang et al. (2015) reported that mice fed diet rich in n-6 PUFA, the number of potential pathogenic bacteria in the Enterobacteriaceae family increased significantly, the generation of pro-inflammatory gut microbiota was promoted. Dong et al. (2023) discovered that as the ratio of dietary n-3/n-6 PUFA decreased, the relative abundance of Enterococcus, a pathogenic bacterium in the intestine of spotted seabass (Lateolabrax maculatus), gradually increased, causing an imbalance in the intestinal microbiota, triggering inflammation, and thus reducing the immunity of spotted seabass. You et al. (2019) replaced fish oil with soybean oil in the diet for golden pompano (Trachinotus ovatus), the ratio of n-3/n-6 PUFA decreased significantly. The diversity of intestinal microbiota in the group fed with soybean oil feed was significantly reduced, and the abundance of pathogenic bacteria such as Mycoplasma and Vibrio was significantly increased, and the number and height of intestinal villi were significantly reduced. Dong et al. (2023) and Tan et al. (2022) suggest that a high proportion of n-6 PUFA can inhibit the gene expression of anti-inflammatory factors by promoting intestinal inflammation, thereby affecting the intestinal health of aquatic animals. In this study, the replacement of FM with PCM at 100%, the content of n-3 PUFA in the feed decreased, the content of n-6 PUFA increased, and the ratio of n-3/n-6 PUFA decreased. In the intestinal histology of E. sinensis, the height, width, and peritrophic membrane thickness of the intestine folds in the 100% group were significantly lower than those in the FM-20 group (P<0.05) (Table 10), and the abundance of Vibrio in the intestine significantly increased (P<0.05) (Figure 6). It can be seen that replacing FM with moderate amount of PCM does not affect the intestinal health of E. sinensis. When the substitution amount exceeds 75%, it has a negative impact on the intestinal tissue structure and intestinal microbiota.
5 Conclusion
In this experiment, in the diet of E. sinensis with a FM content of 200g/kg, PCM can effectively replace 50% FM, and has no significant effect on the growth performance, non-specific immunity and intestinal health of juvenile E. sinensis; Adverse effects on growth performance and intestinal health were happened when the replacement level at 75%; growth performance, non-specific immunity and intestinal health deteriorated when the replacement level at 100%. In summary, the substitution level of FM with PCM was suggested to be 50% (100 g/kg).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal studies were approved by Jiangsu Agri-animal Husbandry Vocational College. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
WY: Funding acquisition, Project administration, Supervision, Writing – review & editing. CZ: Investigation, Methodology, Writing – original draft. RJ: Formal analysis, Writing – original draft. STZ: Data curation, Investigation, Writing – original draft. WJ: Formal analysis, Resources, Software, Writing – original draft. GH: Funding acquisition, Methodology, Writing – original draft. SZZ: Investigation, Methodology, Writing – original draft. AL: Data curation, Resources, Writing – original draft. JW: Formal analysis, Funding acquisition, Writing – original draft. QW: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the Jiangsu Agri-animal Husbandry Vocational College School level research project (NSF2025ZR08); Jiangsu Agricultural Industry Technology System-Eriocheir sinensis (JATS(2023) 280); Taizhou agricultural science and technology support project: study on ecological rotation culture of E. sinensis and P. clarkii (TN202205).
Acknowledgments
We are grateful to all the laboratory members for their technical advice and helpful discussions.
Conflict of interest
Authors GH and SZ7th author were employed by Jiangsu Haorun Biological Industry Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Latif H. M. R., Abdel-Tawwab M., Khalil R. H., Metwally A. A., Shakweer M. S., Ghetas H. A., et al. (2021). Black soldier fly (Hermetia illucens) larvae meal in diets of European seabass: Effects on antioxidative capacity, non-specific immunity, transcriptomic responses, and resistance to the challenge with Vibrio alginolyticus. Fish. Shellf. Immunol. 111, 111–118. doi: 10.1016/j.fsi.2021.01.013
Amino R., Tanaka A. S., Schenkman S. (2001). Triapsin, an unusual activatable serine protease from the saliva of the hematophagous vector of Chagas’ disease Triatoma infestans (Hemiptera: Reduviidae). Insect Biochem. Molec. 31, 465–472. doi: 10.1016/S0965-1748(00)00151-X
Amoah K., Huang Q. C., Tan B. P., Zhang S., Chi S. Y., Yang Q. H., et al. (2019). Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish. Shellf. Immunol. 87, 796–808. doi: 10.1016/j.fsi.2019.02.029
Andriani Y., Pratama R. I., Rostini I. (2023). A review of the potential of golden apple snail (Pomacea Canaliculata) as a raw material for aquaculture feed. Int. J. Sci. Multidiscip. Res. 1, 655–664. doi: 10.55927/ijsmr.v1i6.5257
AOAC (Association of Official Analytical Chemists) (2005). Official Methods of Official Analytical Chemists International. 16th ed (Arlington, VA: Association of Official Analytical Chemists).
Bombeo-Tuburan I., Fukumoto S., Rodriguez E. M. (1995). Use of the golden apple snail, cassava, and maize as feeds for the tiger shrimp, Penaeus monodon, in ponds. Aquaculture 131, 91–100. doi: 10.1016/0044-8486(94)00329-M
Bowyer J. N., Qin J. G., Adams L. R., Thomson M. J. S., Stone D. A. J. (2012). The response of digestive enzyme activities and gut histology in yellow tail kingfish (Seriola lalandi) to dietary fish oil substitution at different temperatures. Aquaculture 368, 19–28. doi: 10.1016/j.aquaculture.2012.09.012
Cai C. F., Wu P., Ye Y. T., Song L., Hooft J., Yang C. G., et al. (2014). Assessment of the feasibility of including high levels of oilseed meals in the diets of juvenile Chinese mitten crabs (Eriocheir sinensis): Effects on growth, non-specific immunity, hepatopancreatic function, and intestinal morphology. Anim. feed Sci. tech. 196, 117–127. doi: 10.1016/j.anifeedsci.2014.07.002
Calder P. C. (2015). Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 1851, 469–484. doi: 10.1016/j.bbalip.2014.08.010
Castillo-Ruiz M., Cañon-Jones H., Schlotterbeck T., Lopez M. A., Tomas Á., Martín R. S. (2018). Safety and efficacy of quinoa (Chenopodium quinoa) saponins derived molluscicide to control of Pomacea maculata in rice fields in the Ebro Delta, Spain. Crop Prot. 111, 42–49. doi: 10.1016/j.cropro.2018.04.016
Castro C., Pérez-Jiménez A., Coutinho F., Pousão-Ferreira P., Brandão T. M., Oliva-Teles A., et al. (2013). Digestive enzymes of meagre (Argyrosomus regius) and white seabream (Diplodus sargus). Effects of dietary brewer’s spent yeast supplementation. Aquaculture 416, 322–327. doi: 10.1016/j.aquaculture.2013.09.042
Catalma M. T., Capili D. T., Antalan R. A., Serra A. B., Barroga A. J., Orden E. (1991). Golden snail (Pomacea sp.) use in animal feeds. Int. Rice Res. Instit. Newslett. 16, 26–27.
Chen D. W., Zhang M. (2007). Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 104, 1200e5. doi: 10.1016/j.foodchem.2007.01.042
Chen J., Zhu X., Han D., Yang Y., Lei W., Xie S. (2011). Effect of dietary n-3 HUFA on growth performance and tissue fatty acid composition of gibel carp Carassius auratus gibelio. Aquacult. Nutr. 17, e476–e485. doi: 10.1111/j.1365-2095.2010.00784.x
Da C. T., Lundh T., Lindberg J. E. (2012). Evaluation of local feed resources as alternatives to fish meal in terms of growth performance, feed utilisation and biological indices of striped catfish (Pangasianodon hypophthalmus) fingerlings. Aquaculture 364, 150–156. doi: 10.1016/j.aquaculture.2012.08.010
Da C. T., Lundh T., Lindberg J. E. (2013). Digestibility of dietary components and amino acids in animal and plant protein feed ingredients in striped catfish (Pangasianodon hypophthalmus) fingerlings. Aquacult. Nutr. 19, 741–750. doi: 10.1111/anu.12021
Dharmawati S., Natsir M. H., Sjofjan O., Hartutik H. (2022). Effects of the replacing of fish meal with freshwater snail flesh (Pomacea paludosa) treated with bromelain enzyme on growth performance, relatively organ weight, and digestibility of Alabio ducks (Anas plathyrhyncos Borneo). Adv. Anim. Vet. Sci. 11, 159–165. doi: 10.17582/journal.aavs/2023/11.1.159.165
Dong Y., Wei Y., Wang L., Song K., Zhang C., Lu K., et al. (2023). Dietary n-3/n-6 polyunsaturated fatty acid ratio modulates growth performance in spotted seabass (Lateolabrax maculatus) through regulating lipid metabolism, hepatic antioxidant capacity and intestinal health. Anim. Nutr. 14, 20–31. doi: 10.1016/j.aninu.2023.04.005
Ferrante I., Ricci R., Aleo E., Passi S., Cataudella S. (2008). Can enzymatic antioxidant defences in liver discriminate between wild and sea cage-reared Bluefin Tuna quality? Aquaculture 279, 182–187. doi: 10.1016/j.aquaculture.2018.01.018
Food and Agriculture Organization (FAO) (2010). Fisheries and Aquaculture (Food and Agriculture Organization_(FAO). Available online at: http://www.eoearth.org/article/ (Accessed June 19, 2011).
Forster I. P., Dominy W., Obaldo L., Tacon A. G. J. (2003). Rendered meat and bone meals as ingredients of diets for shrimp Litopenaeus vannamei (Boone 1931). Aquaculture 219, 655–670. doi: 10.1016/S0044-8486(02)00457-X
Fountoulaki E., Alexis M. N., Nengas I., Venou B. (2005). Effect of diet composition on nutrient digestibility and digestive enzyme levels of gilthead sea bream (Sparus aurata L.). Aquacult. Res. 36, 1243–1251. doi: 10.1111/j.1365-2109.2005.01232.x
Fuentes-Quesada J. P., Lazo J. P. (2018). The effect of lipid type on lipid digestion enzymes during larval development of the California halibut, Paralichthys californicus. Aquaculture 488, 49–60. doi: 10.1016/j.aquaculture.2018.01.018
Ghosh S., Jung C., Meyer-Rochow V. B. (2017). Snail as mini-livestock: Nutritional potential of farmed Pomacea canaliculata (Ampullariidae). Agr. Nat. Resour. 51, 504–511. doi: 10.1016/j.anres.2017.12.007
Halwart M. (1994). The golden apple snail Pomacea canaliculata in Asian rice farming systems: present impact and future threat. Int. J. Pest Manage. 40, 199–206. doi: 10.1080/09670879409371882
Han W. F., Sun Y. F., Liu J., Zhang Y. W., Lu Z. Z., Cheng Y. X. (2021). Effect of different feeding modes on the growth, biochemical composition, and living environment of the juvenile Chinese mitten crab Eriocheir sinensis. Aquaculture 541, 736687. doi: 10.1016/j.aquaculture.2021.736687
Huang Y. S., Lin Z. D., Rong H., Hao M. L., Zhu D. S., Li S. K., et al. (2018). Effects of conjugated linoleic acid on growth, body composition, antioxidant status, lipid metabolism and immunity parameters of juvenile Chu’s croaker, Nibea coibor. Aquacul. Res. 49, 546–556. doi: 10.1111/are.13486
Huang M., Zhou Y. E., Ge J., Agustsson T., Li L., Gao Q., et al. (2020). Fatty acid composition and digestive enzyme activities of rainbow trout in response to dietary docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) during salinity acclimation. J. Ocean Univ. China 19, 1430–1440. doi: 10.1007/s11802-020-4470-9
Ingerslev H. C., Jørgensen L. V. G., Strube M. L., Larsen N., Dalsgaard I., Boye M., et al. (2014). The development of the gut microbiota in rainbow trout (Oncorhynchus mykiss) is affected by first feeding and diet type. Aquaculture 424, 24–34. doi: 10.1016/j.aquaculture.2013.12.032
Ji S. H., Xue R. R., Zhou L., Sun J., Ji H. (2025). The individual and combined effect of DHA and high-fat diet on flesh quality, antioxidant capacity and myofiber characteristics of grass carp (Ctenopharyngodon idellus). Aquaculture 595, 741487. doi: 10.1016/j.aquaculture.2024.741487
Jiang H. B., Chen L. Q., Qin J. G., Gao L. J., Jiang X. Q. (2013). Partial or complete substitution of fish meal with soybean meal and cottonseed meal in Chinese mitten crab Eriocheir sinensis diets. Aquacult. Int. 21, 617–628. doi: 10.1007/s10499-012-9594-5
Jiang W. B., Jia X. Y., Xie N. J., Wen C., Ma S., Jiang G. Z., et al. (2023). Aquafeed fermentation improves dietary nutritional quality and benefits feeding behavior, meat flavor, and intestinal microbiota of Chinese mitten crab (Eriocheir sinensis). Anim. Nutr. 14, 1–19. doi: 10.1016/j.aninu.2023.04.002
Jintasataporn O., Tabthipwon P., Yenmark S. (2004). Substitution of golden apple snail meal for fishmeal in giant freshwater prawn, Macrobrachium rosenbergii (de man) diets. Kasetsart J. 38, 66–71.
Kiriratnikom A., Kiriratnikom S., Choksawatdikorn P., Ruengklay K. (2010). “Applications of apple snail meal as alternative protein source in white shrimp (Penaeus vannamei) feed,” in Program & Abstracts of the International Symposium on Fish Nutrition & Feeding. Qingdao, China.
Kuang S. Y., Xiao W. W., Feng L., Liu Y., Jiang J., Jiang W. D., et al. (2012). Effects of graded levels of dietary methionine hydroxy analogue on immune response and antioxidant status of immune organs in juvenile Jian carp (Cyprinus carpio var. Jian). Fish shellf. Immunol. 32, 629–636. doi: 10.1016/j.fsi.2011.12.012
Li J. G., Xu Y. P., Jin L. J., Li X. Y. (2015). Effects of a probiotic mixture (Bacillus subtilis YB-1 and Bacillus cereus YB-2) on disease resistance and non-specific immunity of sea cucumber, A postichopus japonicus (S elenka). Aquacul. Res. 46, 1–12. doi: 10.1111/are.12453
Li Q., Zhu H. Y., Wei J. J., Zhang F., Li E. C., Du Z. Y., et al. (2017). Effects of dietary lipid sources on growth performance, lipid metabolism and antioxidant status of juvenile Russian sturgeon A cipenser gueldenstaedtii. Aquacul. Nutr. 23, 500–510. doi: 10.1111/anu.12418
Liao M. L., Ren T. J., Chen W., Jiang Z. Q., Yang H., Han Y. Z. (2015). Optimum Level of Dietary n-3 Highly Unsaturated Fatty Acids for Juvenile Sea Cucumber, Apostichopus japonicus. J. World Aquacult. Soc 46, 642–649. doi: 10.1111/jwas.12231
Lin Y. H., Shiau S. Y. (2003). Dietary lipid requirement of grouper, Epinephelus malabaricus, and effects on immune responses. Aquaculture 225, 243–250. doi: 10.1016/S0044-8486(03)00293-X
Liu Q., Wen B., Man D., Jiang Y. S., Li X. D., Zuo R. (2021). Substitution of fish meal with soybean meal for juvenile Chinese mitten crab (Eriocheir sinensis) reared at three salinities: Effects on survival, growth, antioxidant capacity and body composition. Aquacult. Res. 52, 3723–3735. doi: 10.1111/are.15217
Liu X. Z., Cui L. F., Wang X. T., Yuan X. C., Sun H. W., Chen J. Y., et al. (2023). China fishery statistical yearbook (Beijing, China: China Agriculture Press).
Long X. W., Guo Q., Wang X. C., Francis D. S., Cheng Y. X., Wu X. G. (2020). Effects of fattening period on ovarian development and nutritional quality of adult female Chinese mitten crab Eriocheir sinensis. Aquacult 519, 734748. doi: 10.1016/j.aquaculture.2019.734748
Loo K. Y., Letchumanan V., Law J. W. F., Pusparajah P., Goh B. H., Mutalib N. S. A., et al. (2020). Incidence of antibiotic resistance in Vibrio spp. Rev. Aquacult. 12, 2590–2608. doi: 10.1111/raq.12460
Luo Z., Li X. D., Wang W. M., Tan X. Y., Liu X. (2011). Partial replacement of fish meal by a mixture of soybean meal and rapeseed meal in practical diets for juvenile Chinese mitten crab Eriocheir sinensis: effects on growth performance and in vivo digestibility. Aquacult. Res. 42, 1615–1622. doi: 10.1111/j.1365-2109.2010.02751.x
Ma J. J., Shao Q. J., Xu Z. R., Zhou F. (2013). Effect of dietary n-3 highly unsaturated fatty acids on growth, body composition and fatty acid profiles of juvenile black seabream, Acanthopagrus schlegeli (Bleeker). J. World Aquacult. Soc 44, 311–325. doi: 10.1111/jwas.12032
Mansfield G. S., Desai A. R., Nilson S. A., Van Kessel A. G., Drew M. D., Hill J. E. (2010). Characterization of rainbow trout (Oncorhynchus mykiss) intestinal microbiota and inflammatory marker gene expression in a recirculating aquaculture system. Aquacult 307, 95–104. doi: 10.1016/j.aquaculture.2010.07.014
Mohan K., Rajan D. K., Muralisankar T., Ganesan A. R., Sathishkumar P., Revathi N. (2022). Use of black soldier fly (Hermetia illucens L.) larvae meal in aquafeeds for a sustainable aquaculture industry: A review of past and future needs. Aquaculture 553, 738095. doi: 10.1016/j.aquaculture.2022.738095
National Research Council (NRC) (2015). Nutrient requirements of fish and shrimp (Washington: National Academy Press).
Nursyam H. (2017). Antibacterial activity of metabolites products of Vibrio alginolyticus isolated from sponge Haliclona sp. against Staphylococcus aureus. Ital. J. Food Saf. 6, 18–22. doi: 10.4081/ijfs.2017.6237
Oliveira T. S., Khan K. U., Boaratti A. Z., Rodrigues A. T., Reis M. P., Sakomura N. K., et al. (2021). Evaluation of the optimum dietary essential amino acid pattern for adult pacu (Piaractus mesopotamicus). Aquaculture 540, 736686. doi: 10.1016/j.aquaculture.2021.736686
Pan J., Wu R. F., Wu X. G., Long X. W., Wang X. C., Cheng Y. X. (2020). Impacts of different feeding modes on the gonadal development, total edible yield, and nutritional composition of male Chinese mitten crab (Eriocheir sinensis). Aquacult. Fish. 5, 300–307. doi: 10.1016/j.aaf.2020.01.004
Pertiwi M. P., Saputri D. D. (2020). Golden apple snail (Pomacea canaliculata) as an alternative protein source in Pasupati catfish (Pangasius sp.) fish feed. Nusantara Biosci. 12, 162–167. doi: 10.13057/NUSBIOSCI/N120212
Ray A. K., Roy T., Mondal S., Ringø E. (2010). Identification of gut-associated amylase, cellulase and protease-producing bacteria in three species of Indian major carps. Aquacult. Res. 41, 1462–1469. doi: 10.1111/j.1365-2109.2009.02437.x
Santigosa E., Sánchez J., Médale F., Kaushik S., Pérez-Sánchez J., Gallardo M. A. (2008). Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 282, 68–74. doi: 10.1016/j.aquaculture.2008.06.007
Saputria D. D., Pertiwia M. P., Kurniasih S. (2020). “Utilization of golden apple snail (Pomacea canaliculata) as pasupati catfish (Pangasius sp.) fish feed alternative,” in International Conference on Fisheries and Marine Research (ICoFMR 2020).
Sivaramakrishnan T., Sahu N. P., Jain K. K., Muralidhar A. P., Saravanan K., Ferosekhan S., et al. (2017). Optimum dietary lipid requirement of Pangasianodon hypophthalmus juveniles in relation to growth, fatty acid profile, body indices and digestive enzyme activity. Aquacult. Int. 25, 941–954. doi: 10.1007/s10499-016-0090-1
Tan P., Ding Y., Li X. S., Dong X. J., Mai K. S., Ai Q. H. (2022). Nrf2 pathway in vegetable oil-induced inflammation of large yellow croaker (Larimichthys crocea). Fish. Shellf. Immunol. 127, 778–787. doi: 10.1016/j.fsi.2022.05.046
Thompson K. D., Tatner M. F., Henderson R. J. (1996). Effects of dietary (n-3) and (n-6) polyunsaturated fatty acid ratio on the immune response of Atlantic salmon, Salmo salar L. Aquacult. Nutr. 2, 21–31. doi: 10.1111/j.1365-2095.1996.tb00004.x
Visca M. D. Jr., Palla S. Q. (2018). Golden apple snail, Pomacea canaliculata meal as protein source for rabbitfish, Siganus guttatus culture. AACL. Bioflux 11, 533–542.
Wang A. R., Ran C., Ringø E., Zhou Z. G. (2018). Progress in fish gastrointestinal microbiota research. Rev. Aquacult 10, 626–640. doi: 10.1111/raq.12191
Xiang J. H., Qin L., Zhao D., Xiong F., Wang G. T., Zou H., et al. (2019). Growth performance, immunity and intestinal microbiota of swamp eel (Monopterus albus) fed a diet supplemented with house fly larvae (Musca domestica). Aquacult. Nutr. 26, 693–704. doi: 10.1111/anu.13029
Xu C. Y., Liu W. B., Zhang D. D., Liu J. D., Zheng X. C., Zhang C. Y., et al. (2020). Effects of partial fish meal replacement with two fermented soybean meals on the growth of and protein metabolism in the Chinese mitten crab (Eriocheir sinensis). Aquacul. Rep. 17, 100328. doi: 10.1016/j.aqrep.2020.100328
Xu X. Y., Yang H., Zhang C. Y., Bian Y. H., Yao W. X., Xu Z., et al. (2022). Effects of replacing fishmeal with cottonseed protein concentrate on growth performance, flesh quality and gossypol deposition of largemouth bass (Micropterus salmoides). Aquaculture 548, 737551. doi: 10.1016/j.aquaculture.2021.737551
Yang Q. Q., Liu S. W., He C., Yu X. P. (2018). Distribution and the origin of invasive apple snails, Pomacea canaliculata and P. maculata (Gastropoda: Ampullariidae) in China. Sci. Rep. 8, 1185.
Yang S. P., Liu H. L., Wang C. G., Yang P., Sun C. B., Chan S. M. (2015). Effect of oxidized fish oil on growth performance and oxidative stress of Litopenaeus vannamei. Aquacult. Nutr. 21, 121–127. doi: 10.1111/anu.12143
Yang M., Pan T. S., Li T., Duan G. Q., Jiang H., Ling J. (2024). The effects of dietary L-theanine on the growth performance, non-specific immunity, antioxidant status, and intestinal microflora of female Chinese mitten crabs (Eriocheir sinensis). Aquacult. Rep. 34, 101923. doi: 10.1016/j.aqrep.2024.101923
Yanti R. Z., Muchlisin Z. A., Nur F. M., Irham M., Batubara A. S., Muhammadar A. A., et al. (2022). Effect of natural feeds on the growth performance, survival rate and feed utilization of the tropical shortfin eel Anguilla bicolor McClelland 1844 (Pisces: Anguillidae) Larvae. Polish J. Natural Sci. 37, 381. doi: 10.31648/pjns.7922
Yao W. X., Li X. Q., Zhang C. Y., Wang J., Cai Y. W., Leng X. (2021). Effects of dietary synbiotics supplementation methods on growth, intestinal health, non-specific immunity and disease resistance of Pacific white shrimp, Litopenaeus vannamei. Fish. Shellf. Immunol. 112, 46–55. doi: 10.1016/j.fsi.2021.02.011
Yao W., Wu X. Y., Gao Y. J., Wu M. J., Lu S. D., Li X. J., et al. (2018). Effects of replacing fishmeal protein by hemoglobin powder protein on growth performance, food intake, feeding-related gene expression and gut histology of hybrid grouper (Epinephelus fuscoguttatus× Epinephelus lanceolatus) juveniles. Aquaculture 488, 235–243. doi: 10.1111/are.14583
Yao W. X., Zhang C. Y., Li X. Q., He M., Wang J., Leng X. J. (2020). The replacement of fish meal with fermented soya bean meal or soya bean meal in the diet of Pacific white shrimp (Litopenaeus vannamei). Aquacul. Res. 51, 2400–2409. doi: 10.1111/are.14583
Yao W. X., Zhang C. Y., Mao H. X., Hua G. A., Liu Q., Zhao S. T., et al. (2024b). Effects of dietary defatted black soldier fly (Hermetia illucens) larvae meal substituting fish meal on growth, antioxidative capacity, immunity, intestinal histology and microbiota of juvenile Chinese mitten crab (Eriocheir sinensis). Aquacul. Rep. 38, 102302. doi: 10.1016/j.aqrep.2024.102302
Yao W. X., Zhang C. Y., Zhang S., Hua G. A., Zhao S. T., Shuang H. Y., et al. (2024a). The potential of defatted yellow mealworm (Tenebrio molitor) meal as an alternative protein source for juvenile Chinese Mitten Crab (Eriocheir sinensis). Aquacul. Nutt. 2024, 8782924. doi: 10.1155/2024/8782924
Ye J. Y., Wang Y. H., Guo J. L., Chen J. M., Pan Q., Shen B. Q. (2010). Lysine, methionine and arginine requirements of juvenile Chinese mitten crab (Eriocheir sinensis). J. Fish. China Chin. 34, 1541–1548. doi: 10.3724/SP.J.1231.2010.06944
You C. H., Chen B. J., Wang M., Wang S. Q., Zhang M., Sun Z. J., et al. (2019). Effects of dietary lipid sources on the intestinal microbiome and health of golden pompano (Trachinotus ovatus). Fish. Shellf. Immunol. 89, 187–197. doi: 10.1016/j.fsi.2019.03.060
Yu H. B., Gao Q. F., Dong S. L., Zhou J. S., Ye Z., Lan Y. (2016). Effects of dietary n-3 highly unsaturated fatty acids (HUFAs) on growth, fatty acid profiles, antioxidant capacity and immunity of sea cucumber Apostichopus japonicus (Selenka). Fish. Shellf. Immunol. 54, 211–219. doi: 10.1016/j.fsi.2016.04.013
Zhang W. X., Sun S. M., Ge X., Xia S. L., Zhu J., Miao L., et al. (2017). Effects of dietary linolenic acid on growth, fatty acid composition, immune function and antioxidant status of juvenile blunt snout bream, Megalobrama amblycephala. Aquacult. Res. 48, 5430–5438. doi: 10.1111/are.13358
Zhang X., Xiang R., Li X. X., Lin Y., Liu S. S., Gong S. J. (2015). High ratio of dietary n-6/n-3 polyunsaturated fatty acids affects gut microbiota in mice. Prog. Modern Biomed. 15, 4824–4833.
Zhang W., Xie F. J., Jin M., Zhou Q. C., Li M., Han C. M. (2014). Effects of dietary n-3 highly unsaturated fatty acid content on growth performance and fatty acid composition of juvenile swimming crab (Portunus trituberculatus). Chin. J. Anim. Nutr. 26, 1254–1264.
Zheng Y. D., Hou C. H., Chen J., Wang H. M., Yuan H., Hu N. J., et al. (2023). Integrating microbiome and transcriptome analyses to understand the effect of replacing fishmeal with Tenebrio molitor meal in Pacific white shrimp (Litopenaeus vannamei) diets. Aquaculture 575, 739818. doi: 10.1016/j.aquaculture.2023.739818
Zhu Q. G., Lin J. B., Huang Z. C., Chen D. H., Liang P., Qin Z. Q. (2012). Effects of dietary n-3 highly unsaturated fatty acids on growth and muscle fatty acid composition of juvenile grouper (Epinephlus Coioides). J. Guangdong Ocean Univ. 96, 86–96.
Zhu S. C., Long X. W., Turchini G. M., Francis D. S., Deng D., Cheng Y. X., et al. (2020). Dietary fishmeal replacement with a mixed-blend protein evokes sex-specific differences on culture performance and physiological effects on Chinese mitten crab. Aquacult. Nutr. 00, 1–16. doi: 10.1111/anu.13146
Zuo R. T., Ai Q. H., Mai K. S., Xu W., Wang J., Xu H. G., et al. (2012). Effects of dietary n-3 highly unsaturated fatty acids on growth, nonspecific immunity, expression of some immune related genes and disease resistance of large yellow croaker (Larmichthys crocea) following natural infestation of parasites (Cryptocaryon irritans). Fish. Shellf. Immunol. 32, 249–258. doi: 10.1016/j.fsi.2011.11.005
Keywords: Eriocheir sinensis, Pomacea canaliculata, growth performance, non-specific immunity, antioxidant, intestinal health
Citation: Yao W, Zhang C, Jia R, Zhao S, Jiang W, Hua G, Zhao S, Lin A, Wang J and Wang Q (2025) The potential of golden apple snail (Pomacea canaliculata) meat meal as an alternative protein source for juvenile Chinese mitten crab (Eriocheir sinensis). Front. Mar. Sci. 12:1577956. doi: 10.3389/fmars.2025.1577956
Received: 17 February 2025; Accepted: 09 April 2025;
Published: 30 April 2025.
Edited by:
Songlin Li, Shanghai Ocean University, ChinaReviewed by:
Sofia Priyadarsani Das, National Taiwan Ocean University, TaiwanD. K. Meena, Central Inland Fisheries Research Institute (ICAR), India
Copyright © 2025 Yao, Zhang, Jia, Zhao, Jiang, Hua, Zhao, Lin, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxiang Yao, MjY0MjA2NjgwN0BxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Wenxiang Yao
Wenxiang Yao Chunyan Zhang1†
Chunyan Zhang1†