- 1Chongqing Key Laboratory of Conservation and Utilization of Freshwater Fishes, Animal Biology Key Laboratory of Chongqing Education Commission of China, College of Life Sciences, Chongqing, China
- 2Laboratory of Water Ecological Health and Environmental Safety, Chongqing Normal University, Chongqing, China
Environmental estrogens (EEs) are diverse and widespread in aquatic systems, influencing fish social behaviour. Prior studies have focused on individual EEs, but their combined effects, particularly at environmentally relevant concentrations, remain underexplored. In this study, adult male zebrafish were exposed to EE2-low (5.55 ng/L), EE2-high (11.1 ng/L), and Mix (4-NP, 62.2 ng/L; BPA, 250 ng/L; E1, 4.56 ng/L; E2, 5.53 ng/L; E3, 39.6 ng/L, with an estrogenic potency equal to EE2-low) for 60 days. Post-exposure assays (mirror test and dyadic interaction) revealed that Mix significantly reduced the frequency of approaching mirror, the attack mirror duration, and the frequency of chasing un-exposed fish, indicating a decrease in aggressive behaviour in Mix-fish. However, the ratio of 11-ketotestosterone (11-KT) to 17β-Estradiol (E2) was observed in EE2-exposed fish in addition to Mix-fish, indicating that, in addition to sex hormones, stress hormones and neurotransmitters may also be involved in Mix-altered aggression in zebrafish. In contrast, an elevation in plasma cortisol levels and a reduction in serotonin (5-HT) and dopamine (DA) levels in the brain were found only in Mix-fish, accompanied by altered expression of genes involved in the hypothalamic-pituitary-interrenal (HPI) axis and the 5-HT/DAergic system in the brain. The data suggest that a mixture of EEs may inhibit aggression in male zebrafish by disrupting the HPI/cortisol axis and the 5-HT/DAergic system, thus causing serious ecological consequences. These findings suggest that EEs mixtures may inhibit aggression by disrupting key physiological systems. This, in turn, could undermine the competitive and then survival abilities of zebrafish, and potentially affect their population number and structure.
1 Introduction
Environmental estrogens (EEs) are substances with estrogenic activity present in the environment that can interfere with the synthesis, secretion, and metabolism of endogenous hormones in animals, and can activate or inhibit the function of the endocrine system (Shen et al., 2013; Tan et al., 2024). These diverse and widely distributed substances exhibit various toxicities and are persistent in aquatic environments (Lei et al., 2009; Melo et al., 2021; Huang et al., 2013). Currently, EEs have been detected in major Chinese water systems, including the Yangtze, Yellow, and Jialing Rivers (Lei et al., 2009; Jian et al., 2009; Huang et al., 2013).
Numerous studies have demonstrated that EEs discharged into rivers impair gonadal development, reduced reproductive capacity, and induce androgynism in fish (Shen et al., 2013; Biswas et al., 2020; Gonsioroski et al., 2020; Melo et al., 2021). Additionally, EEs can cause neurological dysfunction, leading to abnormal social behaviours such as reproductive, courtship, and aggressive behaviours, thereby decreasing their mating competitiveness and the ability to defend territories (Larsen et al., 2008; Colman et al., 2009; Filby et al., 2012; Lu et al., 2024; Tan et al., 2024). While most studies have focused on the effects of individual or only a few EEs on the reproductive behaviour of fish (Baatrup and Henriksen, 2015; Melo et al., 2021), fewer have examined their impact on mood regulation (Filby et al., 2012). Given that EEs typically occur as low-concentration mixtures in natural waters, many researchers have believed that their impact on fish emotions may be minimal (Caldwell et al., 2012). For instance, Fenske et al. (2020) discovered that acute or chronic exposure to an environment containing 5 ng/L does not elicit an anxiety response in zebrafish. However, lifelong aquatic exposure suggests that environmentally relevant EEs could disrupt fish emotions over time.
Stress responses are critical emotional reactions in animals encountering competition, predators, or potential threats, including aggression, anxiety, and fear (Zabegalov et al., 2019). Aggressive responses in animals are hostile competitive acts that trigger physiological or psychological harm on conspecific or heterospecific competitors (Brodie et al., 2016). As such, aggressive responses play a significant role in both inter-and intraspecific competition among animals, where they play crucial roles in the reproduction and survival of animal populations (Filby et al., 2012; Liu et al., 2020b).
Aggressive responses are regulated by a combination of hormones, neurotransmitters, and neurons (Filby et al., 2012; Zabegalov et al., 2019). Sex hormones, particularly 11-ketotestosterone (11-KT) in fish, are crucial, with elevated plasma 11-KT levels linked to increased aggression (Filby et al., 2012; De Almeida et al., 2015). Conversely, 17β-estradiol (E2) has been shown to attenuate aggression in male animals (Colman et al., 2009; Filby et al., 2012). In addition, higher brain expression of androgen receptor (AR) mRNA accompanies enhanced aggression in fish (Wacker et al., 2016).
In the brain, sex hormones regulate aggressive responses by modulating the expression of key regulators, including c-fos (FBJ murine osteosarcoma viral oncogene homolog), BTG-2 (B-cell translocation gene 2), Bdnf1 (Brain-Derived Neurotrophic Factor 1, BDNF1), and Dusp1 (Dual Specificity Phosphatase 1) (Karmakar et al., 2010; Allen et al., 2011; Ito et al., 2011; Vaarala et al., 2012; Malki et al., 2016). Androgens stimulate c-Fos and induce aggressive responses by binding to the JUN/AP-1 complex (Haller et al., 2006). Androgens also regulate BDNF1 expression, thereby modulating the release of dopamine (DA) and serotonin (5-HT) to indirectly influence aggression (Tyler and Pozzo-Miller, 2001; Verhovshek et al., 2013). Dusp1, an inducible immediate-early gene regulated by androgens, is implicated in modulating aggressive responses (Vaarala et al., 2012; Malki et al., 2016). Furthermore, Recent studies have shown that BTG-2 (an anti-proliferative factor that regulates the cell cycle) is positively correlated with aggressive behaviour and its synthesis is regulated by E2 (Malki et al., 2016; Karmakar et al., 2010).
Corticosteroids (e.g., cortisol in fish) are important steroid hormones regulating aggressive responses in vertebrates (Zabegalov et al., 2019). In zebrafish, cortisol synthesis is regulated by the hypothalamic-pituitary-interrenal (HPI) axis. Corticotropin-releasing hormone (CRH) stimulates the release of pro-opiomelanocortin (POMC) precursor via G protein-coupled receptors (CRHR1 and CRHR2), which is then processed into adrenocorticotropic hormone (ACTH) by the action of prohormone convertases 1 and 2 (PC1 and PC2) (Herman et al., 2016; Zabegalov et al., 2019). ACTH subsequently acts on the interrenal tissue of fish, stimulating cortisol secretion (Oh et al., 2020). In contrast, CRH-binding protein (CRHBP) can inhibit CRH action by blocking its receptor binding, thus negatively regulating the HPI axis (Herman et al., 2016). Consequently, disruption of the HPI axis and plasma cortisol levels may alter the aggressive responses of fish.
Aggressive responses are not only controlled by the endocrine system, but also precisely modulated and executed by the nervous system. In vertebrates, neurotransmitters such as 5-HT and DA modulate aggressive responses (Chen et al., 2005; Kulikov et al., 2012; Liu et al., 2020a). 5-HT participates in many aspects of animal physiology, while its biosynthesis, metabolism, and activation have been demonstrated to modulate aggressive responses (Kulikov et al., 2012; Manuck et al., 2000). The synthesis of 5-HT is catalysed by tryptophan hydroxylase (TPH, encoded by tph1a and tph1b) and regulated by Pet-1 (Norton et al., 2008; Lillesaar, 2011). After its action, 5-HT is degraded by the monoamine oxidase MAO (Manuck et al., 2000; Fedotova et al., 2014) and the remaining dissociative 5-HT molecules are reabsorbed by the serotonin transporter (SERT) (Norton et al., 2008; Kulikov et al., 2012). DA, which is required for the regulation of reward, mood, and cognition, has also been implicated in aggressive responses and other aberrant behaviours (Chen et al., 2005; Narvaes and Almeida, 2014). DA is synthesized by tyrosine hydroxylase (TH), released at the presynaptic membrane by the vesicular monoamine transporter (VMAT, encoded by SLC18A2), and exerts its effects by binding to its receptors (DRD1 and DRD2) (Chen et al., 2005; Narvaes and Almeida, 2014). DA is inactivated by catechol-O-methyltransferase (COMT), while the remaining dissociative DA molecules are reabsorbed by the DA transporter (DAT, encoded by SLC6A3) on the presynaptic membrane (Kahlig and Galli, 2003; Chen et al., 2005). Disruptions in any process of the 5-HTergic and DAergic pathways may alter the aggressive responses in fish.
Prior studies have shown that EEs such as bisphenol A (BPA), 4-nonylphenol (4-NP), estrone (E1), and estriol (E3) in the Jiangsu section of the Huai River, China, have reached medium-to-high risk levels, posing threats to aquatic life (Huang et al., 2019). To investigate the impact of EEs on the aggressive responses of fish, and considering that the impact of EEs is more pronounced in males, male zebrafish have been used as the experimental animals in this study. Adult male zebrafish were exposed to a mixture of EEs (Mix treatment) at environmentally relevant concentrations (based on the detected concentrations of EEs in the Huai River, Jiangsu Province, China, (4-NP, 62.2 ng/L; BPA, 250 ng/L; E1, 4.56 ng/L; E2, 5.53 ng/L; E3, 39.6 ng/L)) for 60 days (Huang et al., 2019). To compare the toxicity of Mix with that of a single environmental estrogen, adult male zebrafish were further exposed to two concentrations of EE2 (EE2-low (5.55 ng/L, with an estrogenic effect equivalent to Mix) and EE2-high (11.1 ng/L)). Aggressive responses were investigated using the mirror test and male dyadic interaction test. The plasma levels of cortisol, the ratio of 11-KT to E2 (11-KT/E2), and the brain levels of 5-HT and DA were subsequently analysed. Additionally, the mRNA level of genes involved in HPI/cortisol axis (crha, crhb, crhbp, crhr1, crhr2, actha, acthb, pc1, pc2), 5-HTergic (tph1b, tph2, pet1, slc6a4a, slc6a4b, htr1aa, htr1ab, htr2b, mao), and DAergic (th1, comta, comtb, drd1b, drd2a, drd2b, slc6a3, slc18a2) systems, as well as important local factors in the brain (ar, c-fos, btg-2, bdnf1, and dusp1) were analysed by quantitative reverse transcription polymerase chain reaction (qRT-PCR).
2 Materials and methods
2.1 Animals
Adult male zebrafish (AB strain, aged 4 months) sourced from the China Zebrafish Resource Center were acclimated for 14 d in a circulating water system with a constant photoperiod (light/dark: 14/10 h, illumination intensity 500 ± 20 Lux), at 28 ± 0.5°C. During the domestication and treatment period, the water quality parameters were maintained as follows: pH, 7.4 ± 0.2; dissolved oxygen, 6.8 ± 0.5 mg/L. The fish were fed twice daily at 9:00 am. and 4:00 pm. with a flaky diet (Hua Pu Aquatic Development Co., Ltd., Xiamen, China) at a rate of 2% of their body weight per day.
All animal experiments were performed in accordance with theGuide for the Care and Use of Laboratory Animals andwereapproved by the Committee of Laboratory Animal Experimentation at Chongqing Normal University (Approval No. CKLCUFF20230803).
2.2 Exposure
After the acclimatisation period, four groups were established: Control, EE2-low (EE2, 5.55 ng/L), EE2-high (EE2, 11.1 ng/L) and Mix (4-NP, 62.2 ng/L; BPA, 250 ng/L; E1, 4.56 ng/L; E2, 5.53 ng/L; E3, 39.6 ng/L) (Huang et al., 2019). Three replicate tanks were established for each group, totalling 12 tanks. Subsequently, 120 acclimated male zebrafish (0.33 ± 0.05 g) were randomly selected and distributed among the individual exposure tanks, with 10 male zebrafish per tank (30 cm × 20 cm × 50 cm, with 20 L water, 20 cm deep). Each group thus comprised 30 male zebrafish. In this study, types and concentrations of EEs utilized in the Mix treatment group were based on the report of Huang et al. (2019) from their investigation in the Huai River Basin, Jiangsu Province, China. Details of the estrogenic potencies and EE2-equivalents of the EES compounds are listed in Supplementary Table S1. The control fish were exposed to the solvent (0.005% DMSO), which was also used for the remaining groups. All treatment groups were processed for 60 days in accordance, following the methods described by Tan et al. (2024). All chemicals were procured from Sigma-Aldrich. The purity of 4-NP (CAS No. 104-40-5), BPA (CAS No. 80-05-7), E1 (CAS No. 53-16-7), E2 (CAS No. 50-28-2), E3 (CAS No. 50-27-1), and EE2 (CAS No. 57-63-6) was ≥98%. Throughout the exposure period, the light exposure, water temperature, and water replacement were in accordance with the methodologies described in our previous study (Tan et al., 2024). The water for the exposed fish was measured weekly, and the relevant measured values are recorded in Supplementary Table S2. Besides, all male zebrafish remained healthy with no abnormalities or mortalities observed in the exposure period.
2.3 Aggressive response test
Following the exposure, aggressive responses were assessed using two methods: the mirror test and dyadic interaction test. Both methods, grounded in fish territoriality and aggressive responses to intruders, are designed as simulated aggressive response tests and have been proven effective (Kalueff and Stewart, 2012; Zabegalov et al., 2019). Besides, during behavioural testing, lighting, temperature, and water quality were maintained consistent with the exposure conditions.
Assessment I: The mirror test was conducted on the exposed-fish following previously methods (Kalueff and Stewart, 2012; Liu et al., 2020b). In the aggressive test tank (15 cm × 20 cm × 25 cm, with a water level of 10 cm), a mirror was positioned on one side of the tank and a parallel line was drawn 5 cm away from mirror surface on the tank floor (Figure 1A). Initially, each experimental fish was individually acclimated in the experimental tank for 18h and kept the mirror obscured by a baffle. An 18 h acclimation period was provided to ensure that the observed aggression could reflect the experimental conditions rather than being influenced by stress responses like anxiety from adapting to a new environment (Kalueff and Stewart, 2012). Subsequently, after the baffle was slowly removed, the fish approached to (entering the area between the parallel line and mirror) the mirror image of itself and attacked it (hitting the mirror). Behaviour was recorded for 20 min using a camera (EOS R50, Canon, Jiangsu, China) mounted above the tank. Thirty fish from each group were tested (N = 30). The parameters of the aggressive response were subsequently analysed: 1) duration approaching mirror, 2) frequency approaching mirror (number of crossing the parallel line but without mirror contact), 3) attack duration, and 4) attack frequency (number of times the fish bite the mirror) (Kalueff and Stewart, 2012; Liu et al., 2020b).
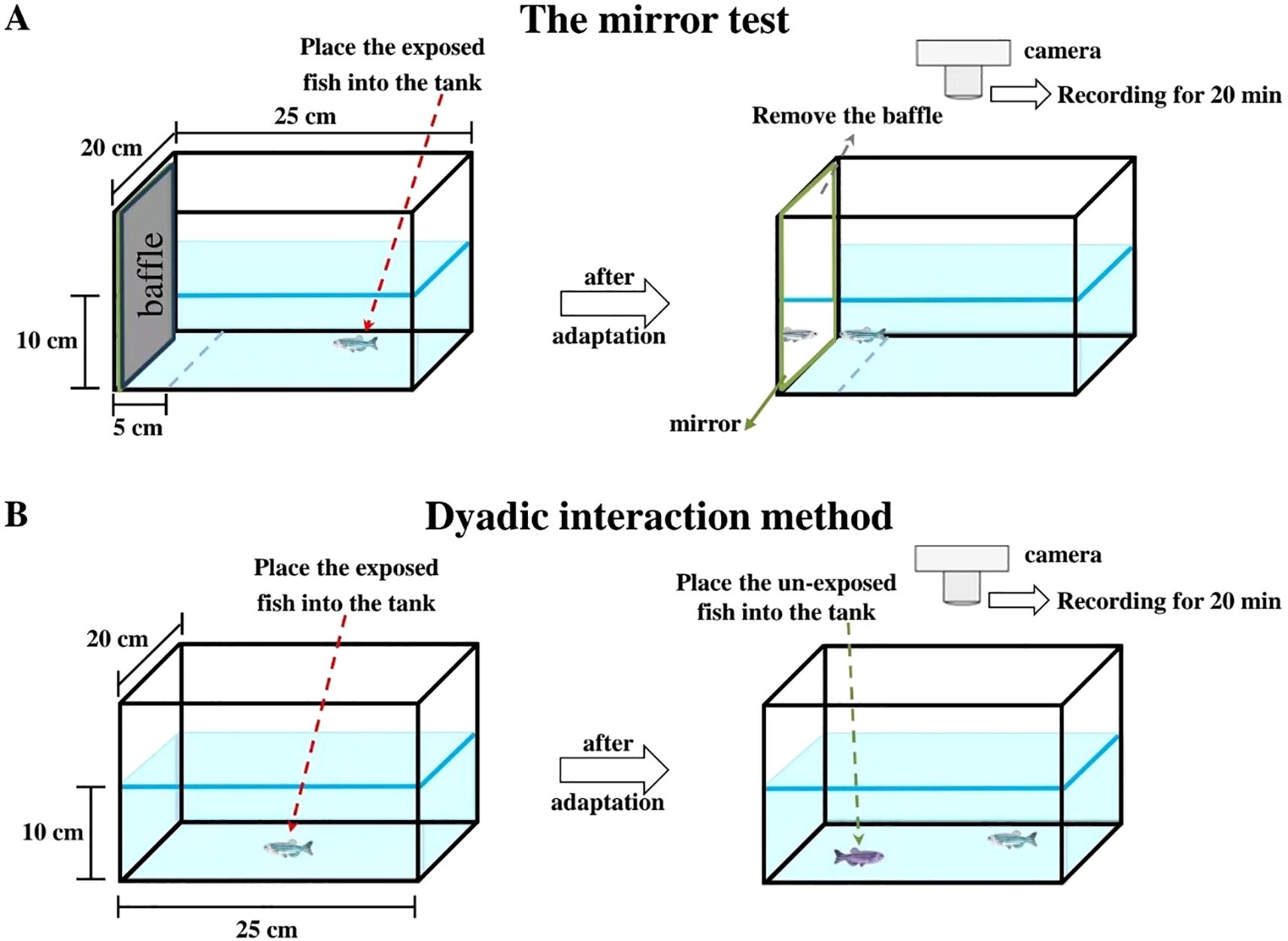
Figure 1. Schematic diagram of the operation for testing the aggressive response of zebrafish. (A) the mirror test; (B) the dyadic interaction method.
Assessment II: In the dyadic interaction test (Figure 1B), exposed-fish were acclimatised individually in the test tank (15 cm × 20 cm × 25 cm, with a water level of 10 cm) for 18 h. Subsequently, an unexposed male zebrafish of similar size (0.32 ± 0.06 g, marked but not affecting conspecific recognition) was introduced into the tank. Behaviour was recorded for 20 min using a camera (EOS R50, Canon, Jiangsu, China) placed above the test tank (N = 30 per group). Generally, the exposed-fish exhibited aggression towards the intruder fish, such as chasing (the exposed fish suddenly accelerates toward against its opponent), pursuing and attempting to bite their opponents until submission was achieved (Zabegalov et al., 2019). Consequently, aggression was assessed by measuring: 1) frequency of chase (the exposed fish chased the unexposed fish) and 2) frequency of attack (the exposed fish bit the unexposed fish) (Liu et al., 2020b). After the experiment,120 unexposed male zebrafish were transferred to a circulating water system to be raised for use in other experiments.
To ensure objectivity and avoid bias, behavioural tests wereconducted in a randomized order. Fish were randomly allocated to experimental conditions using a computer-generated randomization schedule, and tests were performed by researchers blinded to the treatment groups. The same behavioural data for individual fish were collected separately by two trained researchers (with a data discrepancy of less than 15%). The average value of each fish’s same behavioural data was then calculated separately for subsequent data analysis. Following the aggression test, the fish used for behavioural testing were returned to the relevant tanks and allowed to acclimatise for two days before being sampled.
2.4 Sampling
Prior to sampling, zebrafish (10 per tank) were anaesthetised with MS-222 (0.1%) through glass capillary tubes located in the tank corners, in order to avoid additional disturbance. Subsequently, blood samples from four fish per tank were collected using heparinised capillary tubes to make one biological sample (N = 1). The blood samples were transferred to anticoagulant centrifuge tubes and centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was then collected and stored at -20°C to estimate the plasma levels of 11-KT, E2, and cortisol. The brains of three fish per tank were also removed to make one biological sample (N = 1) for subsequent estimation of the 5-HT and DA levels in the brain, and stored at -80°C. The brains from 3 fish per tank were collected as one biological sample (N = 1), and immediately frozen in liquid nitrogen until total RNA extraction.
2.5 Measurement of plasma levels of 11-KT, E2, and cortisol
The plasma 11-KT, E2, and cortisol levels were measured using ELISA kits following the manufacturer’s instructions (Cayman Chemical, USA). As previously described, blood sample was harvested from four fish per tank to prepare one sample (N = 1) and three samples (N = 3) for each group were prepared for analysis. The intra-assay and inter-assay coefficients of variation for hormone measurements were 5.6-6.7% and 4.2-9.4%, respectively. The sensitivity of the 11-KT, E2 and cortisol ELISA kit was 1.3 pg/mL, 20 pg/mL and 35 pg/mL respectively; and the detection ranges were 0.78–100 pg/mL, 0.61 - 10,000 pg/mL and 6.6 - 4,000 pg/mL respectively.
2.6 Measurement of brain level of 5-HT and DA
5-HT and DA levels in the brain were measured using ELISA kits (SINOBESTIO, Shanghai, China). Brains were rinsed with HBSS solution (Beyotime, China) at 4°C, and the weight of each sample (three brains pooled as one sample, N = 1) was accurately determined. Physiological saline was added to each sample at a ratio of 9:1 (mL/g). After thorough homogenization of the tissue, samples were centrifuged at 3000 r.p.m. for 10 minutes at 4°C. The supernatant was harvested and the concentrations of 5-HT and DA were immediately measured according to the manufacturer’s instructions (SINOBESTIO, Shanghai, China). The intra- and inter-assay coefficients of variation for neurotransmitter measurements were 6.6 - 7.5% and 4.7 - 9.5%, respectively. The sensitivity of 5-HT and DA detection was < 0.1 ng/mL and 3.3 pg/mL, respectively. The detection ranges were 1.5–24 ng/mL for 5-HT and 0.5–80 ng/mL for DA, respectively.
2.7 qRT–PCR
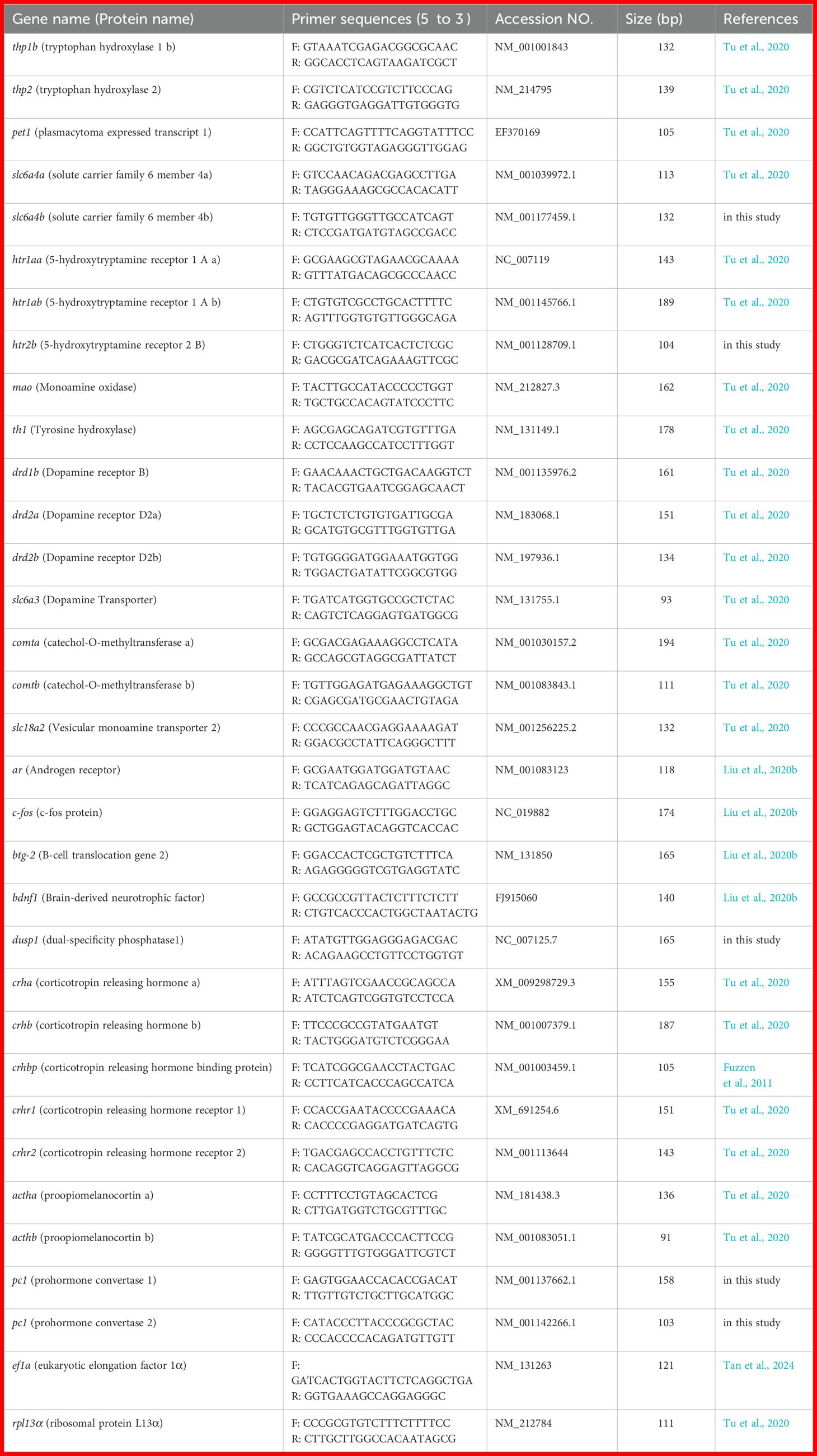
qRT-PCR was performed as previously described (Tu et al., 2020). In summary, total RNA was extracted from samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Three brains were pooled to form one biological sample, with a total of three biological samples (N = 3) per group. RNA quality was verified by agarose gel electrophoresis, with clear 28S and 18S rRNA bands (28S:18S > 1.5) and assessed for concentration and purity (A260/A280 = 1.8–2.1) using a Tnano-800 spectrophotometer (Tuohe Electromechanical Technology Co., Ltd., Shanghai, China). The genomic DNA was removed using DNase I, and cDNA was synthesized thereafter using TakaRa Reverse Transcription Kit (TaKaRa, Dalian, China). The primers sequences for target genes and internal reference genes are listed in Table 1. qRT-PCR reaction was conducted using the SYBR® Premix Ex Taq™ kit (TaKaRa, Dalian, China) on a CFX96TM Real-time PCR Detection system (Bio-Rad, USA) in 96-well plates (Axygen Biosciences). Reactions were run in triplicate under the following conditions: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, 60°C for 30 s, and 72°C for 10 s. To confirm that the reactions were not contaminated, a dissociation curve and non-template controls were performed. Amplification efficiencies (95.7–104.2%) were calculated from Ct values of a serially diluted cDNA. The expression of each target gene was normalized to the geometric mean of ef1α and rpl13a using the 2-ΔΔCT method (Livak and Schmittgen, 2001).
2.8 Statistical analysis
Statistical analysis was conducted using SPSS 27.0 (Chicago, Illinois, USA). The assumptions of normality and homogeneity of variances were assessed by using the Shapiro-Wilk test and the Levene test, respectively. As behavioural data did not meet these assumptions, a generalized linear model (GLM) was applied. Additionally, the GLM suitability was confirmed by the residualplot analysis (the Quantile-Quantile (Q-Q) plots and thestandardized residuals versus predicted values plots (Supplementary Figures S1 and S2). Other data (hormones, neurotransmitters, and gene expression level) satisfied the assumptions, so one-way analysis of variance (ANOVA) was employed, followed by Fisher’s Least Significant Difference (LSD) post hoc tests to determine intergroup differences. Data are presented as mean ± standard error (SE), with P < 0.05 indicating statistical significance. For all analyses, 95% confidence intervals were calculated. The GraphPad Prism 8.0 (San Diego, CA, USA) was used for graphing.
3 Results
3.1 Effect of EEs on aggressive response of zebrafish
The duration of approaching mirror and the frequency of attacking the mirror showed no significant effect between control and treatment groups (P > 0.05, Figures 2A, D). Compared with control, the frequency of approaching the mirror in male zebrafish was significantly reduced in Mix and EE2-high (Pm = 0.043 and Ph = 0.037, Figure 2B). The attack mirror duration of the Mix-fish was significantly decreased compared with the fish in all other groups (P = 0.045, Figure 2C). In dyadic interactions with unexposed males, the frequency of chase of the Mix-treated fish showed a sharp decrease compared with the control (P = 0.042, Figure 2E), but no significant difference compared with EE2-treated fish (P > 0.05, Figure 2E). In contrast, exposure to EE2 and Mix resulted no significant effect on the frequency of attack (P > 0.05, Figure 2F).
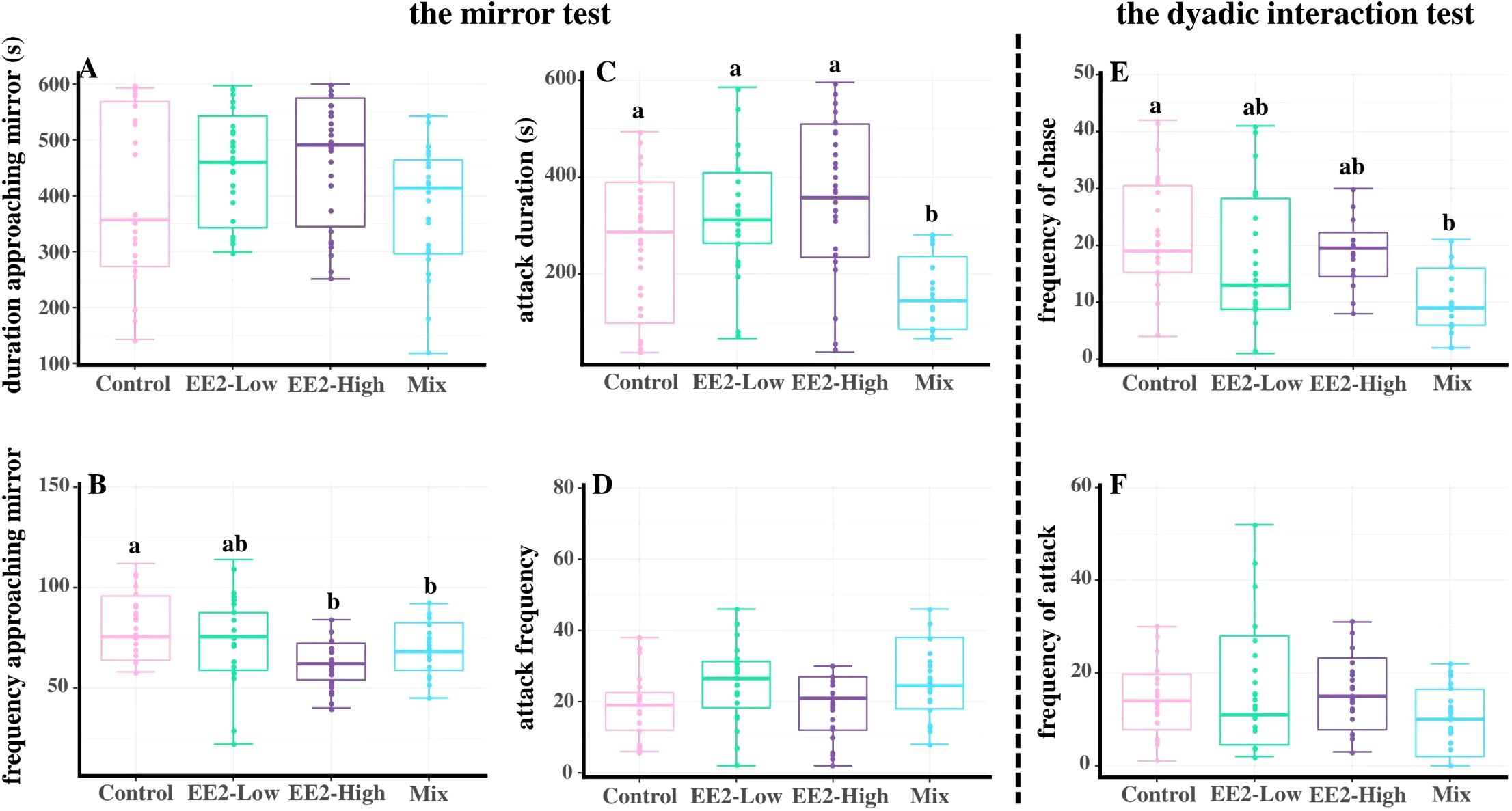
Figure 2. Effect of environmental relevant concentrations of EEs on aggressive response of zebrafish. (A-D) the mirror test; (E, F), dyadic interaction test. (A) duration approaching mirror; (B) frequency approaching mirror; (C) attack duration; (D) attack frequency; (E) frequency of chase; (F) frequency of attack. Different letters on boxplots (a, b) in each figure represent significant differences between groups (P<0.05). Data are presented as mean ± SE, N=30.
3.2 Effects of EEs on the ratio of 11-KT to E2 in plasma and the expression of aggression-related genes in the brain
Compared with the control, after treatment EE2 and Mix, the 11-KT/E2 ratio showed a significant reduction (Pl = 0.001, Ph< 0.001, and PM = 0.001). The 11-KT/E2 ratio had no significant effect between the EE2-low, EE2-high, and Mix groups (P > 0.05, Figure 3A).
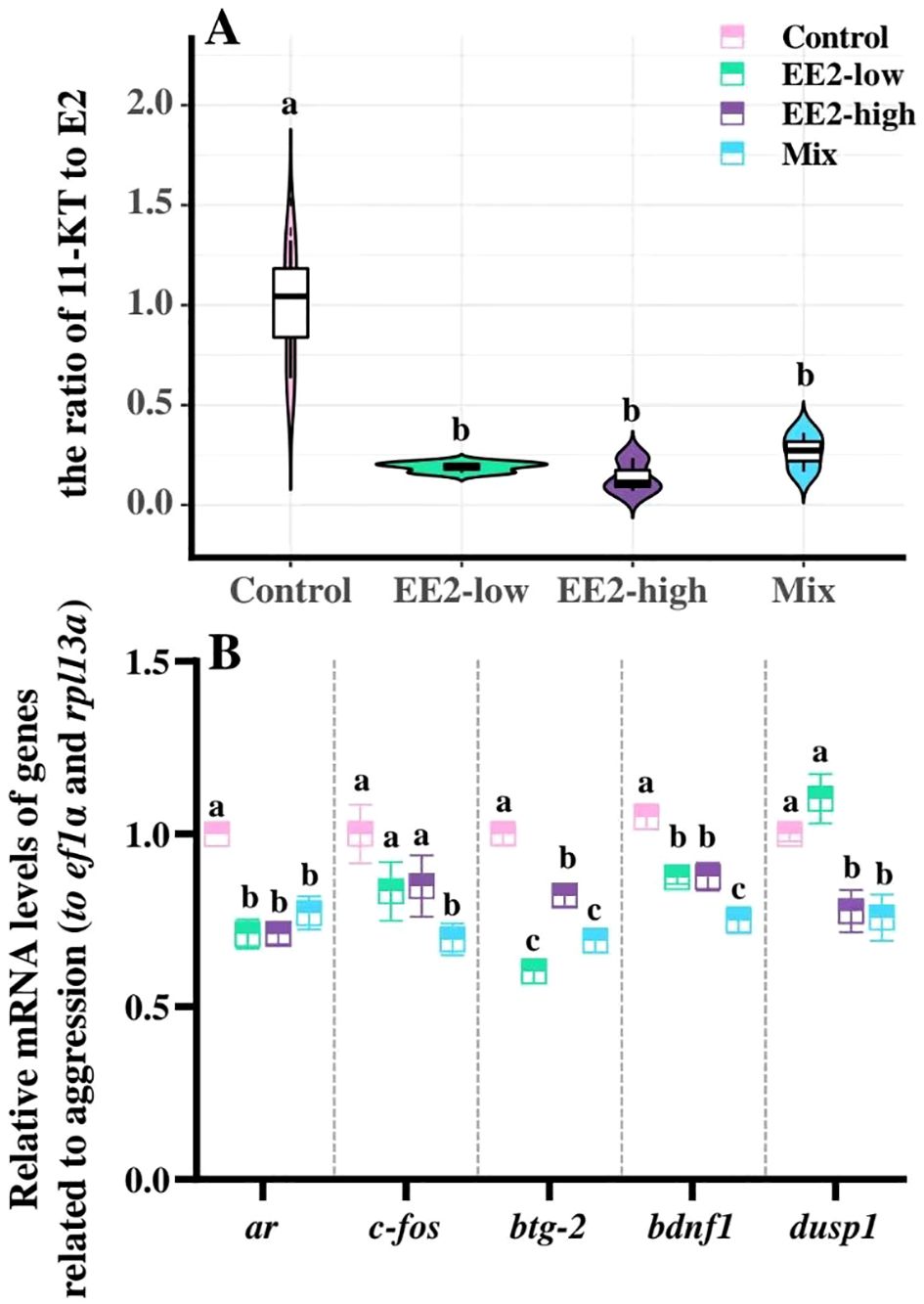
Figure 3. Effect of environmental relevant concentrations of EEs on the ratio of 11-KT to E2 (A) in plasma and expression of aggressive response-related genes (B). Different letters on boxplots (a–c) in each figure represent significant differences between groups (P<0.05). Data are presented as mean ± SE, N=3.
Compared with the control, Mix significantly inhibited the mRNA expression levels of ar, fos, btg-2, bdnf1, and dusp1 (P = 0.002, P = 0.005, P=0.031, P < 0.001, and P = 0.019, respectively). In EE2-low and EE2-high, mRNA expression of ar, btg-2, and bdnf1 was significantly reduced (Pl = 0.001, Pl <0.001, Pl = 0.008, Ph = 0.001, Ph = 0.005, and Ph <0.001, respectively), and dusp1 expression was significantly decreased only in EE2-high (P = 0.027, Figure 3B).
3.3 Effects of EEs on plasma levels of cortisol and expression of genes in HPI axis
Mix significantly elevated plasma cortisol levels in male zebrafish compared with control (P < 0.001), whereas EE2 exposure at neither concentration had no significant effect (P > 0.05, Figure 4A).
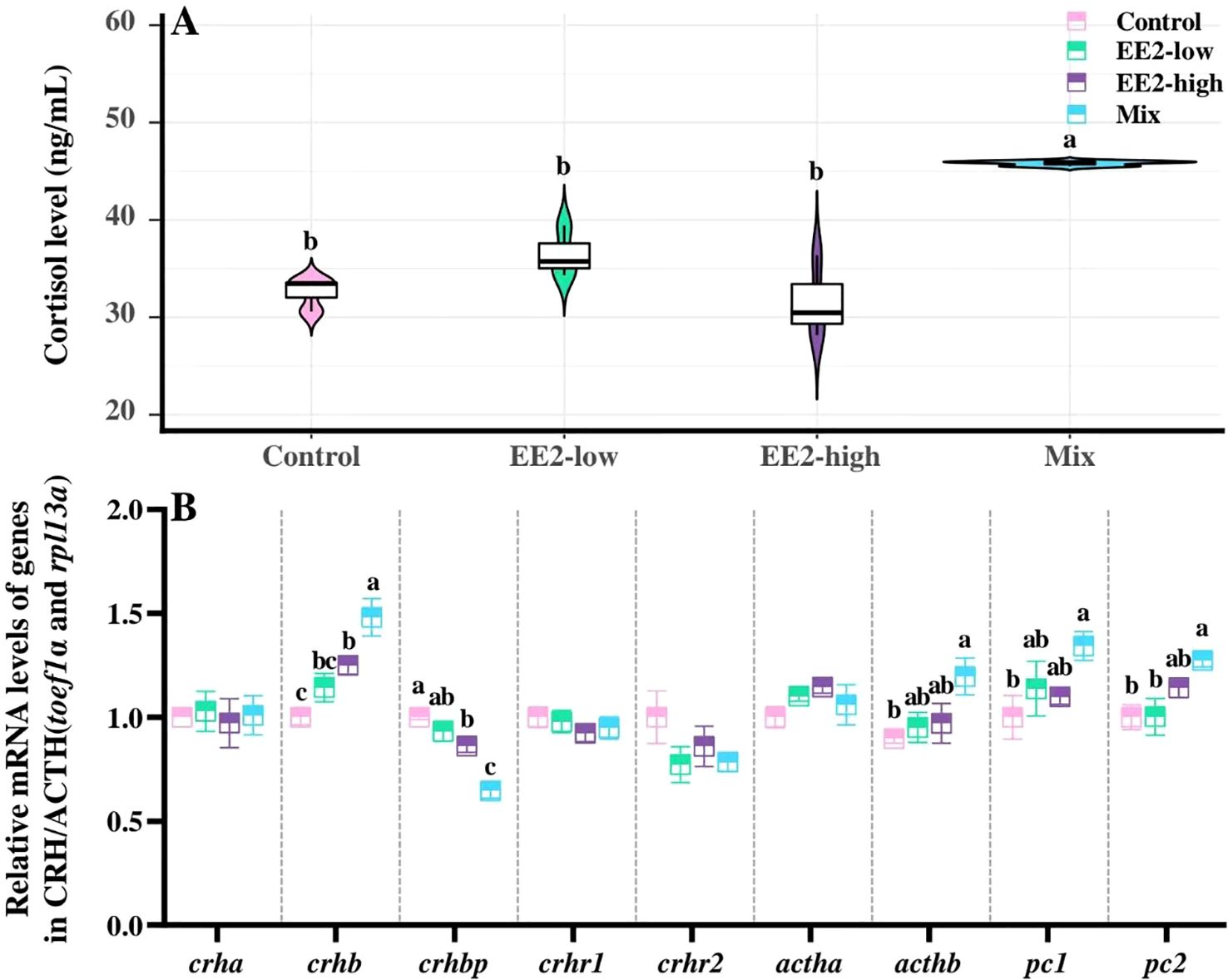
Figure 4. Effect of environmental relevant concentrations of EEs on plasma levels of cortisol (A) and expressions of genes in HPI axis (B). Different letters on violin plots and boxplots (a–c) in each figure represent significant differences between groups (P<0.05). Data are presented as mean ± SE, N=3.
Compared with the control, Mix significantly upregulated the expression of crhb, acthb, pc1, and pc2 (P = 0.001, P = 0.023, P = 0.031, and P = 0.012, respectively), but sharply downregulated crhbp expression (P < 0.001). Besides, the results revealed a significant elevation in crhb expression (P = 0.024) and a significant reduction in crhbp (P = 0.021) in the EE2-high compared with the control. No significant difference in gene expression was observed in EE2-low compared with control (P > 0.05, Figure 4B).
3.4 Effects of EEs on brain levels of 5-HT and expressions of related genes in the brain
Compared with the control, brain 5-HT levels in male fish exhibited a significant decrease after Mix treatment (P = 0.004), while a slight, but not significant, decrease was found in fish treated with EE2 (P > 0.05). Additionally, no significant difference in brain 5-HT levels were observed between Mix- and EE2-treated groups (P > 0.05, Figure 5A).
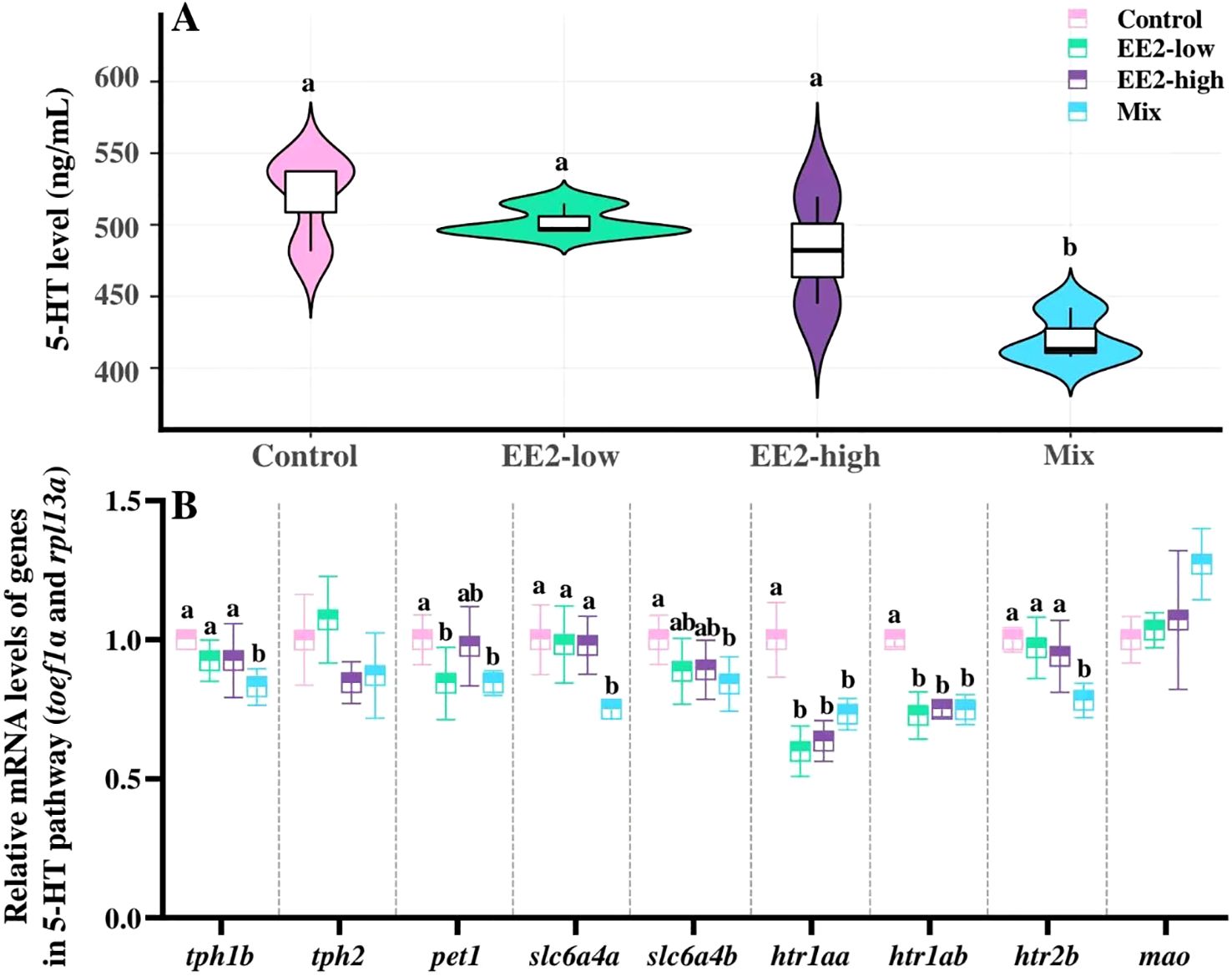
Figure 5. Effect of environmental relevant concentrations of EEs on brain levels of 5-HT (A) and expression of related genes (B). Different letters on violin plots and boxplots (a, b) in each figure represent significant differences between groups (P<0.05). Data are presented as mean ± SE, N=3.
Compared with the control, Mix treatment also significantly downregulated the mRNA expression levels of tph1b, pet1, slc6a4a, slc6a4b, htr1aa, htr1ab, and htr2b (P = 0.003, P = 0.025, P = 0.023, P = 0.021; P = 0.008, P < 0.001, and P = 0.007, respectively). In addition, expression levels of tph1b, slc6a4a, and htr2b were significantly lower in Mix than in both EE2-low and EE2-high (Pl = 0.017, Pl = 0.029, Pl = 0.014, Ph = 0.012, Ph = 0.031, and Ph = 0.048, respectively; Figure 5B).
3.5 Effects of EEs on brain levels of DA and expressions of related genes in the brain
The brain level of DA showed a significant reduction in Mix-fish compared with control (P = 0.039). Besides, the brain DA levels showed no significant effect between the Mix- and EE2-treated fish (P > 0.05, Figure 6A).
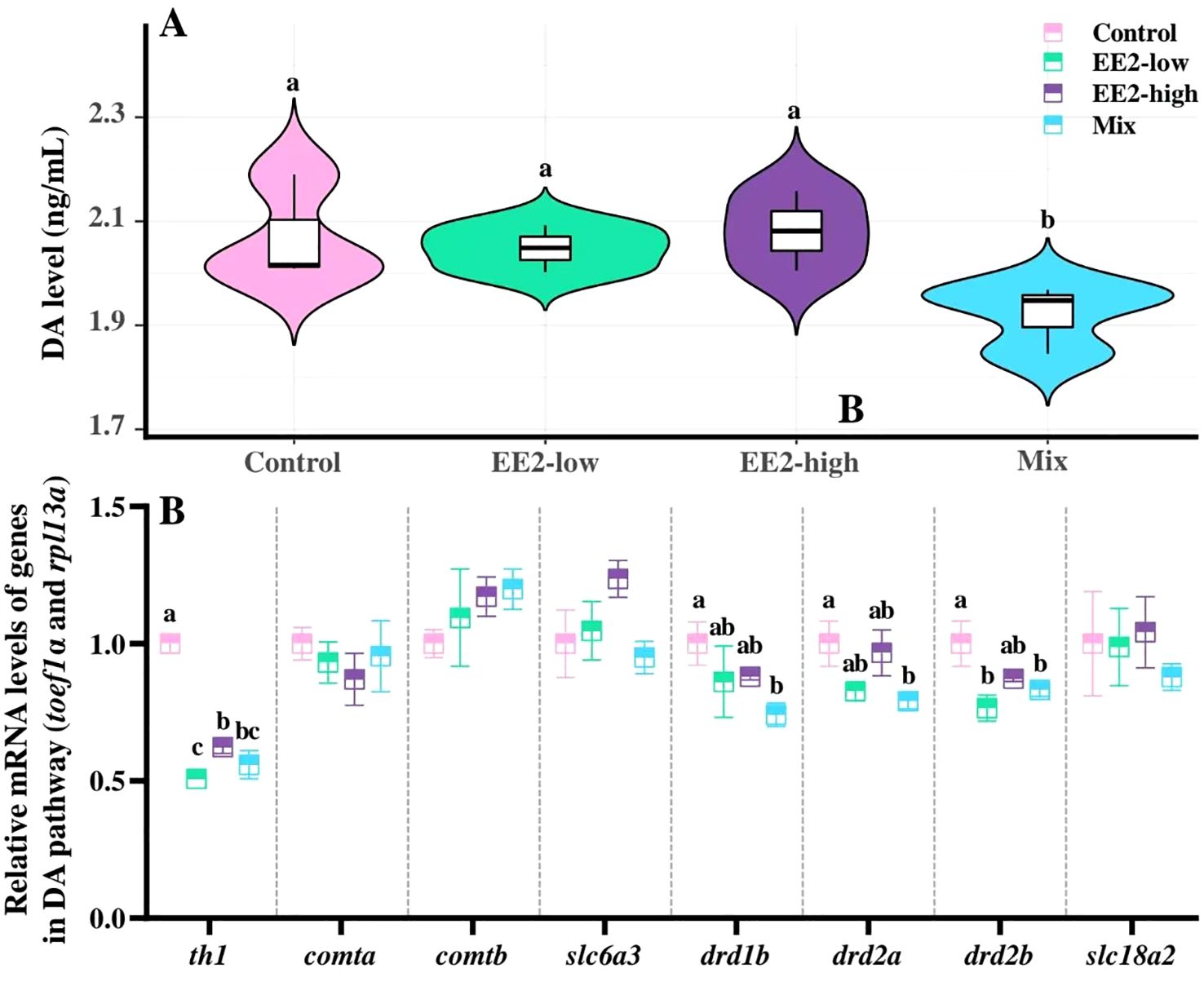
Figure 6. Effect of environmental relevant concentrations of EEs on brain levels of DA (A) and expression of related genes (B). Different letters on violin plots and boxplots (a–c) in each figure represent significant differences between groups (P<0.05). Data are presented as mean ± SE, N=3.
Comparative analysis revealed a significant reduction in the mRNA expression levels of th1, drd1b, drd2a, and drd2b in the brain of Mix-fish compared with control (P < 0.001, P = 0.049, P = 0.045, and P = 0.043, respectively). In both EE2 treatments groups, th1 expression was significantly lower than in control (Pl < 0.001 and Ph < 0.001). Further, a significant decrease in drd2b expression was observed in EE2-low (P = 0.010, Figure 6B).
4 Discussion
4.1 Effect of environmental relevant concentrations of EEs on aggressive response of zebrafish
Although several studies have examined the effects of EEs on the aggressive response of fish, most have focused on the effects of a single high dose of EEs on fish aggression, with limited attention to environmentally relevant concentrations of EEs mixtures (Colman et al., 2009; Filby et al., 2012; Cooper et al., 2021; Melo et al., 2021). In natural aquatic environments, EEs are typically present at low concentrations, potentially causing minimal disruption to fish populations (Larsen et al., 2008; Caldwell et al., 2012). However, EEs are persistent and degrade slowly, thus, behavioural anomalies in fish may emerge following long-term exposure (Huang et al., 2013; Melo et al., 2021; Lu et al., 2024). In this study, we investigated whether long-term exposure to several types of EEs at extremely low (environmentally relevant) concentrations disrupts aggressive responses in male zebrafish.
Our data revealed that only EE2-high fish exhibited a significant reduction in frequency approaching mirror following 60 d of treatment, suggesting that EE2 treatment had minimal impact on male zebrafish aggression. In contrast, three parameters (frequency approaching mirror, attack duration, and frequency of chase) were significantly reduced in Mix-treated fish, indicating attenuated aggression in Mix-exposed fish. Thus, these strongly suggest that although Mix, despite exerting an estrogenic effect similar to EE2-low, has a more pronounced disruptive effect on male zebrafish aggressive response. Moreover, the disruptive effect of Mix was more severe than that of EE2-high, which exerts a 2-fold estrogenic effect. The types and concentrations of EEs used in this study were based on those found in the Huai River Basin (Huang et al., 2019). Prolonged exposure of fish to such an environment could potentially reduce their aggression, thereby decreasing the breeding competitiveness and affecting the reproduction of fish. It could also damage the individual and population competitiveness, survival capabilities, and territorial defence abilities, thereby impacting their population number and ecosystem balance. It is therefore strongly suggested to adopt regulatory and diverse measures to control the release of EEs into aquatic environments.
4.2 Effect of environmental relevant concentrations of EEs on plasma levels of 11-KT, E2 and expression of aggressive response-related genes
11-KT, the most potent androgen regulating spermatogenesis and reproduction in male fish, is known to modulate aggressive responses (Filby et al., 2012; De Almeida et al., 2015). Elevated plasma 11-KT levels are often associated with heightened aggression in fish (Ros et al., 2004; Filby et al., 2012; White et al., 2023). Furthermore, E2 typically mitigate aggression in male fish (Colman et al., 2009; Filby et al., 2012; White et al., 2023). In this study, the 11-KT/E2 ratio was significantly reduced in male zebrafish treated with EE2 and Mix, indicating that EE2 and Mix may attenuate aggression by suppressing 11-KT synthesis and increasing E2 levels, which is consistent with previous research (Tan et al., 2024; Ros et al., 2004; Colman et al., 2009; Filby et al., 2012).
Androgens exert their effects via their receptors (AR), and 11-KT may regulate the aggressive response of fish by binding to AR in the brain (Filby et al., 2012; Wacker et al., 2016). Consistent with this, increased aggression in fish is often accompanied by higher ar mRNA expression in brain regions (Wacker et al., 2016; Liu et al., 2020b). The present study found that the expression of ar decreased after all treatments, aligning with the reduced 11-KT/E2 ratio. Notably, both EE2 and Mix treatments inhibited 11-KT synthesis and ar expression, yet only Mix significantly reduced aggression. This discrepancy may be explained by the ability of BPA and NP, components of Mix, to inhibit AR transcriptional and binding activities, thereby disrupting androgen function (Xu et al., 2006; Lerner et al., 2007a). Study has indicated that BPA or 4-NP, acting as androgen receptor antagonists, can bind to AR and thereby disrupt the regulatory function of endogenous androgens on androgen-dependent transcription (Xu et al., 2006). Alternatively, the Mix probably has a stronger estrogenic effect and toxicity than the theoretical value, an effect that is also reflected at the molecular level descried as below. This mechanism may underlie the observed reduction in aggression in Mix-treated fish, which was not seen in EE2-treated fish.
A substantial body of research has identified various local factors within the brain that regulate aggressive responses, including c-fos, Btg-2, Bdnf1, and Dusp1 (Chao et al., 2006; Haller et al., 2006; Malki et al., 2016). Androgens modulate the expression of c-fos, Bdnf1, and Dusp1 via their receptors, thereby influencing aggressive responses (Allen et al., 2011; Ito et al., 2011; Vaarala et al., 2012). In this study, the mRNA levels of btg-2 and bdnf1 were significantly downregulated in the brains of zebrafish following all treatments, while dusp1 expression was inhibited in EE2-high and Mix groups, consistent with decreased the 11-KT/E2 ratio. In contrast, c-fos mRNA levels were only suppressed in Mix-treated fish, correlating with reduced 11-KT levels and aggressive responses. Notably, bdnf1 and dusp1 expression was more sharply suppressed in Mix-treated fish than in EE2-treated fish, suggesting their involvement in regulating aggression. BTG-2, a key effector regulating aggressive responses, is negatively regulated by estrogen via estrogen receptors (Karmakar et al., 2010; Malki et al., 2016). In this study, btg-2 expression in the brain was inhibited in all treatment groups (particularly in Mix-treated fish), indicating that EEs may elevate plasma E2 levels while suppressing btg-2 expression, thereby mitigating aggressive response of zebrafish.
Interestingly, despite similar estrogenic potency, the Mix exerts a more pronounced effect on aggression than EE2. This may be due to BPA in the Mix activating non-genomic pathways like the PI3K/Akt and MAPK signalling pathways via membrane oestrogen receptors (mERs), as well as potential additive effects of the E2, BPA and 4-NP in the Mix (Jalal et al., 2018; Chen et al., 2019). In summary, EEs may mitigate the aggressive response of zebrafish, probably by decreasing the 11-KT/E2 radio, and altering the expression of effectors (especially ar, c-fos, bdnf1 and btg-2) in the brain.
4.3 Effect of environmental relevant concentrations of EEs on plasma levels of cortisol and expressions of genes in HPI axis
Cortisol and corticosterone are key glucocorticoids in fish and tetrapods, known to moderate aggressive responses (Zabegalov et al., 2019; Mehta et al., 2008).
In this study, Mix-treated led to a significant increase in plasma cortisol levels in male zebrafish, concurrently with a reduction in aggressive responses, findings that are in accordance with prior studies (Mehta et al., 2008). This suggests that elevated cortisol levels may be one of the reasons for the diminished aggressive response in zebrafish exposed to Mix. Consistent with this, the mRNA levels of genes involved in cortisol synthesis and secretion, such as crhb, acthb, pc1, and pc2, were significantly upregulated in the brains of Mix-treated fish, while crhbp expression, which is negatively correlated with cortisol synthesis, was significantly downregulated. In contrast, neither plasma cortisol levels nor the expression of most genes in the HPI axis changed significantly in EE2-treated fish, aligning with the unchanged aggressive response. These data suggest that long-term Mix exposure may alter the HPI axis function, elevate plasma cortisol levels, and presumably combine with a combination of other factors, including alterations in E2 levels and neurotransmitter levels and relative genes (as mentioned in the context), which thereby disrupt the aggressive response of zebrafish.
In vertebrates, sex steroids are pivotal in moderating the HPA axis: estrogens stimulate it, whereas androgens attenuate its effects (Pottinger et al., 1996; Fuzzen et al., 2011). In this study, the 11-KT/E2 radio reduced in all treatment groups, while plasma cortisol levels only rose in Mix-treated fish. These data indicate that the increase in plasma cortisol levels is not attributable to direct regulation by sex steroids. Additionally, prior research has indicated that gradient EE2 treatment in roaches (Rutilus rutilus) can suppress 11-KT levels, with no significant alteration in cortisol levels (Flores-valverde et al., 2010). In contrast, direct exposure to BPA, NP, and E2, which are important components of the Mix, has been shown to elevate cortisol levels (Lerner et al., 2007b; Jamei and Sadeghi, 2016). This data suggests that the attenuation of aggressive responses in Mix-treated fish, which contain BPA, NP, and E2, may be achieved through modulation of cortisol levels.
In summary, our findings suggest that environmentally relevant concentrations of EEs could affect the HPI axis and plasma cortisol levels, thereby suppressing aggressive responses in zebrafish.
4.4 Effect of environmental relevant concentrations of EEs on brain levels of 5-HT and DA and expression of related genes
Neurotransmitters, particularly 5-HT and DA, are key modulators of mood and behaviour, and are implicated in the regulation of aggressive responses in vertebrates (Chen et al., 2005; Kulikov et al., 2012; Narvaes and Almeida, 2014).
In this study, a significant decrease in brain 5-HT levels was observed in Mix-fish. This aligns with previous findings that environmental estrogens reduce brain 5-HT levels and aggression (Kulikov et al., 2012). Additionally, expression of genes involved in 5-HT synthesis (tph1b and pet1) and action (htr1aa, htr1ab, and htr2b) were downregulated in Mix-fish, indicating a diminished 5-HTergic effect, aligning with the reduction in aggression. These suggest that Mix exposure may disrupt the aggressive response in zebrafish by disturbing the synthesis and 5-HTergic system. Besides, in this study, brain DA levels were significantly reduced only in Mix-fish, consistent with previous findings that exposure to EEs inhibited DA synthesis in zebrafish (Qi et al., 2024). Moreover, the mRNA expression of genes involved in DA synthesis (th1) and action (drd1b, drd2a, and drd2b) was significantly decreased, suggesting a further decrease in DAergic activity in Mix-exposed fish. These indicate that the DAergic system is also an indispensable effector involved in the modulation of Mix-inhibited aggressive responses in male zebrafish. Besides, previous research has indicated that the 5 - HT/DA system in specific brain regions, such as the raphe nuclei, is implicated in the regulation of aggressive behaviour (Mitsui and Takahashi, 2024). Therefore, whether gene changes are dominated by specific brain regions deserves more in-depth and detailed studies.
Prior research has shown that androgens positively influence the activation and effects of 5-HT and DA systems (Silva et al., 2008). However, in this study, both EE2 and Mix suppressed plasma 11-KT levels, whereas 5-HT and DA levels decreased only in Mix. Our previous work demonstrated that various environmental estrogens can act synergistically or additively (Tan et al., 2024). Concurrently, previous research further indicates that exposure to BPA or E2 can alter levels of 5-HT and DA (Jia and Pittman, 2015). Therefore, the present study suggests that the altered aggressive responses in mixed-exposed fish may be related to the presence of BPA and E2 in Mix, both of which affect the 5-HTergic and DAergic properties, rather than through 11-KT levels.
In fact, numerous studies have reported that the HPA axis negatively modulates 5-HT and DA levels and their activities (Porter et al., 2004; Mcarthur et al., 2010). In this study, plasma cortisol levels were significantly elevated in Mix-fish, while aggressive responses and brain levels of 5-HT and DA were reduced. This suggests that the attenuated aggressive responses in Mix-treated fish may result from alterations in the HPI/cortisol axis and 5-HT/DAergic systems, rather than changes in plasma 11-KT levels. Our findings align with previous studies highlighting the regulatory role of corticosteroids in the 5-HT and DA systems (Porter et al., 2004; Peng et al., 2021).
Previous studies have shown distinct regulatory mechanisms between different types of estrogens (Flores-valverde et al., 2010). As previously mentioned, exposure to EE2 did not alter plasma cortisol levels, whereas exposure to E2, BPA, and NP individually induced significant changes in cortisol levels (Lerner et al., 2007b; Flores-valverde et al., 2010; Jamei and Sadeghi, 2016). In this study, the Mix included E2, BPA, and 4-NP but not EE2, which may explain the differential effects on aggressive responses. While E2, BPA and 4-NP can each independently influence hormone and neurotransmitter levels, in the Mix they may exert synergistic or additive effects, thereby producing more pronounced behavioural changes than EE2-low (Jalal et al., 2018; Chen et al., 2019). The distinct alterations in aggression between EE2 and Mix exposure may thus result from the complex composition of Mix, underscoring the need to investigate the ecological risks posed by EEs in natural waters.
5 Conclusion
The present study indicates that long-term exposure to environmentally relevant concentrations of EEs (Mix) suppresses the aggressive response in male zebrafish. Exposure to Mix altered the plasma levels of cortisol and E2/11-KT ratio, decreased the brain levels of 5-HT and DA, and disrupted the expression of genes involved in the HPI/cortisol axis, the 5-HT/DAergic system, and other response effectors, thereby affecting aggressive responses (Figure 7). In contrast, EE2 treatment did not significantly affect the aggressive response of male zebrafish, nor did plasma cortisol levels and 5-HT/DAergic systems. These results indicate that a mixture of EEs may have a potent impact on aggressive responses in fish, potentially posing significant risks to fish populations and generating potential ecological impacts. However, whether long-term exposure to environmentally relevant concentrations of EEs affect female zebrafish and other fish species remains to be further investigated. Additionally, further studies are required to corroborate the laboratory findings through in situ assessment of the effects of EEs on fish aggressive behaviour. To mitigate the pollution of EEs, it is imperative to reduce the emission of pollutants, actively seek out less toxic alternatives to the relevant pharmaceuticals and develop more effective degradation technologies to accelerate the breakdown of EEs in natural water bodies.
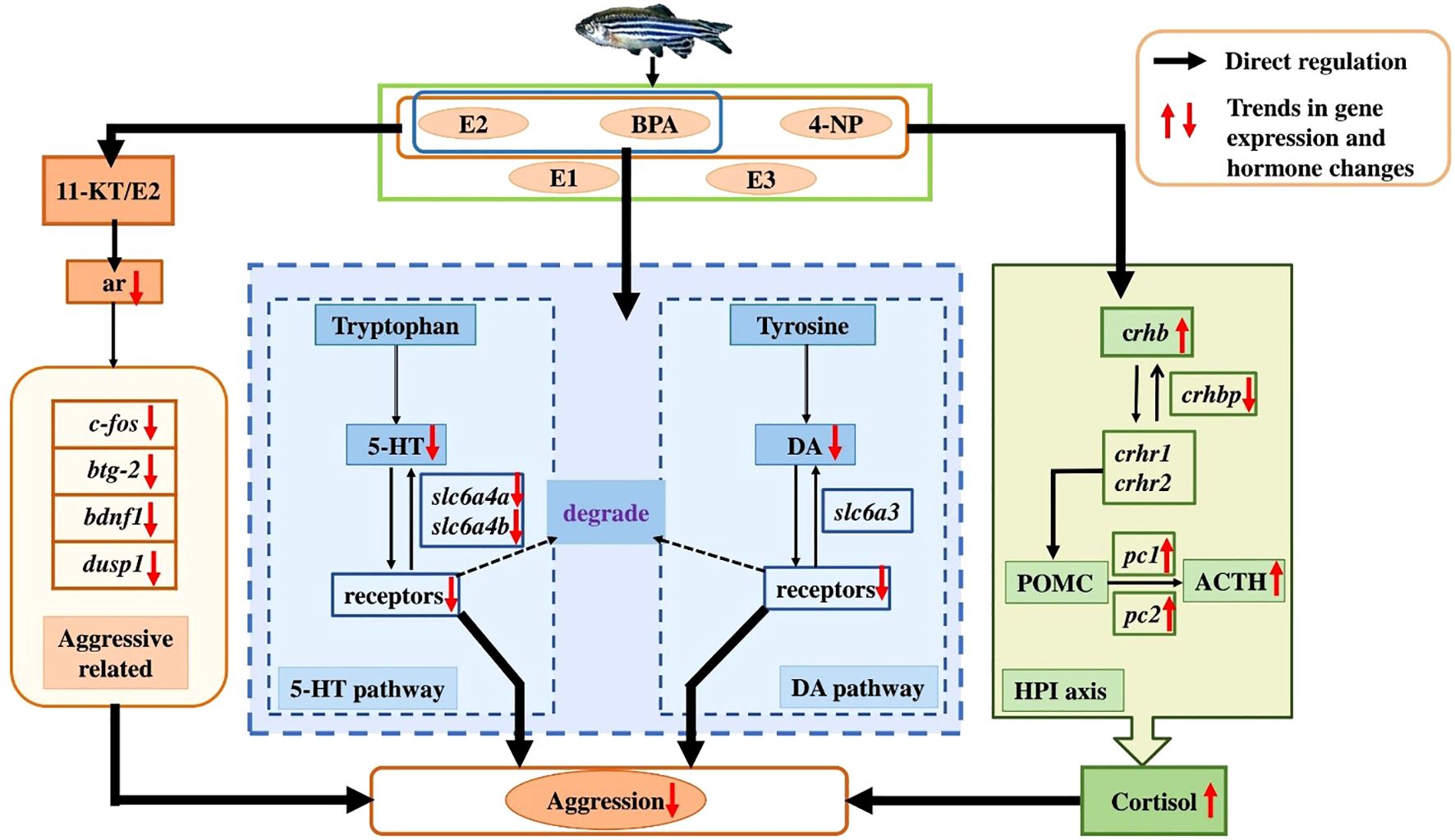
Figure 7. Schematic diagram of EES modulate the aggressive responses in male zebrafish through multiple pathways. Solid black arrows, regulatory pathways that EES directly regulate the HPI axis and the 5-HT/DA system, as well as the 11-KT/E2 ratio, AR and aggressive related pathways, thereby influencing aggressive responses in male zebrafish, as demonstrated in this study; Dashed black arrows, regulatory pathways that the 11-KT/E2 ratio may indirectly regulate the HPI axis and the 5-HT/DA system via AR, thereby modulating aggressive responses; Dashed blue arrows, regulatory pathways that the potential interactions between the HPI axis and the 5-HT/DA system, as well as between BDNF1 and the 5-HT/DA system, as revealed by previous studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Committee of Laboratory Animal Experimentation at Chongqing Normal University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
Y-YZ: Data curation, Investigation, Writing – original draft. HT: Data curation, Investigation, Writing – original draft. Y-JS: Methodology, Writing – review & editing. Q-LC: Formal Analysis, Software, Visualization, Writing – review & editing. Z-HL: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Enhanced Project of scientific research platform in Fuyang Normal University (FSKFKT021D, 2022), the Chongqing Research Program of Basic Research and Frontier Technology (cstc2020jcyj-msxmX0805, cstc2016jcyjA0133), and Innovative research program for graduates of Chongqing Normal University, Chongqing, China (YKC24014).
Acknowledgments
Thanks to Mei-Ling Tan for her help in preliminary work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1578762/full#supplementary-material
References
Allen C. E., Worsley M. A., King A. E., and Boissonade F. M. (2011). Fos expression induced by activation of NMDA and neurokinin-1 receptors in the trigeminal subnucleus cau dalis in vitro: role of protein kinases. Brain Res. 1368, 19–27. doi: 10.1016/j.brainres.2010.10.072
Baatrup E. and Henriksen P. G. (2015). Disrupted reproductive behavior in unexposed female zebrafish (Danio rerio) paired with males exposed to low concentrations of 17α-ethinylestradiol (EE2). Aquat. Toxicol. 160, 197–204. doi: 10.1016/j.aquatox.2015.01.020
Biswas S., Ghosh S., Samanta A., Das S., Mukherjee U., and Maitra S. (2020). Bisphenol A impairs reproductive fitness in zebrafish ovary: Potential involvement of oxidative/nitrosative stress, inflammatory and apoptotic mediators. Environ. pollut. 267, 115692. doi: 10.1016/j.envpol.2020.115692
Brodie M. J., Besag F., Ettinger A. B., Epilepsy, Mula M., Gobbi G., et al. (2016). Antiepileptic drugs, and aggression: an evidence-based review. Pharmacol. Rev. 68, 563–602. doi: 10.1124/pr.115.012021
Caldwell D. J., Mastrocco F., Anderson P. D., Länge R., and Sumpter J. P. (2012). Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environ. Toxicol. Chem. 31, 0–0. doi: 10.1002/etc.1825
Chao M., Rajagopal R., and Lee F. (2006). Neurotrophin signalling in health and disease. Clin. Sci. 110, 167–173. doi: 10.1042/CS20050163
Chen H. L., Zhao L., and Jimmy Yu Q. M. (2019). Determination and reduced life expectancy model and molecular docking analyses of estrogenic potentials of 17β-estradiol, bisphenol A and nonylphenol on expression of vitellogenin gene (vtg1) in zebrafish. Chemosphere 221, 727–734. doi: 10.1016/j.chemosphere.2019.01.093
Chen T. J. H., Blum K., Mathews D., Fisher L., Schnautz N., Schoolfield J., et al. (2005). Are dopaminergic genes involved in a predisposition to pathological aggression?: Hypothesizing the importance of “super normal controls” in psychiatricgenetic research of complex behavioral disorders. Med. Hypotheses 65.4, 703–707. doi: 10.1016/j.mehy.2005.04.037
Colman J. R., Baldwin D., Johnson L. L., and Scholz N. L. (2009). Effects of the synthetic estrogen, 17alpha-ethinylestradiol, on aggression and courtship behavior in male zebrafish (Danio rerio). Aquat. Toxicol. 91, 346–354. doi: 10.1016/j.aquatox.2008.12.001
Cooper R., David A., Lange A., and Tyler C. R. (2021). Health effects and life stage sensitivities in zebrafish exposed to an estrogenic wastewater treatment works effluent. Front. Endocrinol. 12. doi: 10.3389/fendo.2021.666656
De Almeida R. M. M., Cabral J. C. C., and Narvaes R. (2015). Behavioural, hormonal and neurobiological mechanisms of aggressive behaviour in human and nonhuman primates. Physiol. Behav. 143, 121–135. doi: 10.1016/j.physbeh.2015.02.053
Fedotova Y. O., Ordyan N. E., Frolova G. O., and Sobolev V. I. (2014). Involvement of some subtypes of 5-HT2-receptors in affective behavior in female rats. Int. J. Sport Physiol. 5, 41–47. doi: 10.1615/IntJPhysPathophys.v5.i1.40
Fenske L., Concato A. C., Vanin A. P., Tamagno W. A., de Oliveira Sofiatti J. R., Treichel H., et al. (2020). 17-α-Ethinylestradiol modulates endocrine and behavioral responses to stress in zebrafish. Environ. Sci. pollut. Res. 27, 29341–29351. doi: 10.1007/s11356-020-09318-7
Filby A. L., Paull G. C., Searle F., Ortiz-Zarragoitia. M., and Tyler C. R. (2012). Environmental estrogen-induced alterations of male aggression and dominance hierarchies in fish: a mechanistic analysis. Environ. Sci. Technol. 46, 3472–3479. doi: 10.1021/es204023d
Flores-valverde A. M., Horwood J., and Hill E. M. (2010). Disruption of the steroid metabolome in fish caused by exposure to the environmental estrogen 17alpha-ethinylestradiol. Environ. Sci. Technol. 44, 3552. doi: 10.1021/es9039049
Fuzzen M. L. M., Bernier N. J., and van der Kraak G. (2011). Differential effects of 17β-estradiol and 11-ketotestosterone on the endocrine stress response in zebrafish (Danio rerio). Gen. Comp. Endocrinol. 170, 365–373. doi: 10.1016/j.ygcen.2010.10.014
Gonsioroski A., Mourikes V. E., and Flaws J. A. (2020). Endocrine disruptors in water and their effects on the reproductive system. Int. J. Mol. Sci. 21.6, 1929. doi: 10.3390/ijms21061929
Haller J. M., Tóth H. J., Halasz J., and De Boer S. F. (2006). Patterns of violent aggression-induced brain c-fos expression in male mice selected for aggressiveness. Physiol. Behav. 88, 173–182. doi: 10.1016/j.physbeh.2006.03.030
Herman J. P., McKlveen J. M., Ghosal S., Kopp B., and Myers B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 6, 603–621. doi: 10.1002/cphy.c150015
Huang B., Wang B., Ren D., Jin W., Liu J. L., Peng J. H., et al. (2013). Occurrence, removal and bioaccumulation of steroid estrogens in Dianchi Lake catchment, China. Environ. Int. 59, 262–273. doi: 10.1016/j.envint.2013.06.018
Huang Y., Xie X., Zhou L. J., Ji X., Gao B., Xu G. Z., et al. (2019). Multi-phase distribution and risk assessment of endocrine disrupting chemicals in the surface water of the Shaying River, -Huai River Basin, China. Ecotoxicol. Environ. Saf. 173, 45–53. doi: 10.1016/j.ecoenv.2019.02.016
Ito W., Chehab M., and Thakur S. (2011). BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 10, 365–374. doi: 10.1111/j.1601-183X.2010.00676.x
Jalal N., Surendranath R. A., Pathak L. J., Yu S., and Chung C. Y. (2018). Bisphenol A (BPA) the mighty and the mutagenic. Toxicol. Rep. 5, 76–84. doi: 10.1016/j.toxrep.2017.12.013
Jamei M. and Sadeghi A. (2016). Effect of quercetin on cortisol and oxytocin levels, oxytocin receptor gene expression and morphometry of uterus in rats exposed to bisphenol A. Kafkas Univ. Vet. Fak. Derg. 22, 823–828. doi: 10.9775/kvfd.2016.15397
Jia M. and Pittman J. (2015). Deficits in striatal dopamine and hippocampal serotonin following induction of anxiety/depressive-like behaviors by bisphenol A. Arch. Neurosci. 2, e18555. doi: 10.5812/archneurosci.18555
Jian G., Ran Y., Chen D., Yang Y., and Ma X. X. (2009). Occurrence and environmental risk of endocrine-disrupting chemicals in surface waters of the Pearl River, South China. Environ. Monit. Assess 156, 1–4. doi: 10.1007/s10661-008-0474-4
Kahlig K. M. and Galli A. (2003). Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur. J. Pharmacol. 479, 153–158. doi: 10.1016/j.ejphar.2003.08.065
Kalueff A. V. and Stewart A. M. (2012). Zebrafish protocols for neurobehavioral research volume 66 || Assessing social behavior phenotypes in adult zebrafish: shoaling, social preference, and mirror biting tests. Neuromethods 66, 231–246. doi: 10.1007/978-1-61779-597-8_17
Karmakar S., Foster E. A., and Smith C. L. (2010). Estradiol downregulation of the tumor suppressor gene BTG2 requires estrogen receptor-α and the REA corepressor. Int. J. Cancer 124, 1841–1851. doi: 10.1002/ijc.24133
Kulikov A. V., Osipova D. V., Naumenko V. S., Terenina E., Mormède P., and Popova N. K. (2012). A pharmacological evidence of positive association between mouse intermale aggression and brain serotonin metabolism. Behav. Brain Res. 233, 113–119. doi: 10.1016/j.bbr.2012.04.031
Larsen M. G., Hansen K. B., Henriksen P. G., and Baatrup E. (2008). Male zebrafish (Danio rerio) courtship behaviour resists the feminising effects of 17alpha-ethinyloestradiol–morphological sexual characteristics do not. Aquat. Toxicol. 87, 234–244. doi: 10.1016/j.aquatox.2008.02.003
Lei B. L., Huang S. B., Zhou Y. Q., Wang D. H., and Wang Z. J. (2009). Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. Chemosphere 76, 36–42. doi: 10.1016/j.chemosphere.2009.02.035
Lerner D. T., Björnsson B. T., and Mccormick S. D. (2007a). Larval exposure to 4-nonylphenol and 17β-estradiol affects physiological and behavioral development of seawater adaptation in atlantic salmon smolts. Environ. Sci. Technol. 41, 4479–4485. doi: 10.1021/es070202w
Lerner D. T., Björnsson B. T., and McCormick S. D. (2007b). Aqueous exposure to 4-nonylphenol and 17β-estradiol increases stress sensitivity and disrupts ion regulatory ability of juvenile Atlantic salmon. Environ. Toxicol. Chem. 26, 1433–1440. doi: 10.1897/06-451R1.1
Lillesaar C. (2011). The serotonergic system in fish. J. Chem. Neuroanat. 41, 294–308. doi: 10.1016/j.jchemneu.2011.05.009
Liu Z. H., Li Y. W., Hu W., Chen Q. L., and Shen Y. J. (2020b). Mechanisms involved in tributyltin-enhanced aggressive behaviors and fear responses in male zebrafish. Aquat. Toxicol. 220, 105408. doi: 10.1016/j.aquatox.2020.105408
Liu S., Yu M., Xie X., Ru Y., and Ru S. (2020a). Carbofuran induces increased anxiety-like behaviors in female zebrafish (Danio rerio) through disturbing dopaminergic/norepinephrinergic system. Chemosphere 253, 126635. doi: 10.1016/j.chemosphere.2020.126635
Livak K. J. and Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lu C., Lv Y., Meng X., Yang T., Liu Y., Kou G., et al. (2024). The potential toxic effects of estrogen exposure on neural and vascular development in zebrafish. Ecotoxicol. Environ. Saf. 283, 116862. doi: 10.1016/J.ECOENV.2024.116862
Malki K., Du Rietz E., Crusio W. E., Pain O., Paya-Cano J., Karadaghi R. L., et al. (2016). Transcriptome analysis of genes and gene networks involved in aggressive behavior in mouse and zebrafish. Am. J. Med. Genet. B. 171, 827–838. doi: 10.1002/ajmg.b.32451
Manuck S. B., Flory J. D., Ferrell R. E., and Muldoon M. F. (2000). A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 95, 9–23. doi: 10.1016/S0165-1781(00)00162-1
Mcarthur S., Mchale E., Dalley J. W., Buckingham J. C., and Gillies G. E. (2010). Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J. Neuroendocrinol. 17, 475–482. doi: 10.1111/j.1365-2826.2005.01331.x
Mehta P. H., Jones A. C., and Josephs R. A. (2008). The social endocrinology of dominance: basal testosterone predicts cortisol changes and behavior following victory and defeat. Peng. J. Pers. Soc Psychol. 94, 1078–1093. doi: 10.1037/0022-3514.94.6.1078
Melo L. E., de Paulo D. V., Montagner C. C., and Carvalho P. S. M. (2021). Behavioral and reproductive effects in Poecilia vivipara males from a tropical estuary affected by estrogenic contaminants. Mar. pollut. Bull. 169, 112543. doi: 10.1016/j.marpolbul.2021.112543
Mitsui K. and Takahashi A. (2024). Aggression modulator: Understanding the multifaceted role of the dorsal raphe nucleus. Bioessays 46.4, 2300213. doi: 10.1002/bies.202300213
Narvaes R. and Almeida R. M. M. D. (2014). Aggressive behavior and three neurotransmitters: dopamine, GABA, and serotonin—a review of the last 10 years. Psychol. Neurosci. 7, 601–607. doi: 10.3922/j.psns.2014.4.20
Norton W. H., Folchert A., and Bally-Cuif L. (2008). Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J. Comp. Neurol. 511, 521–542. doi: 10.1002/cne.21831
Oh J., Kim D. H., Kim G. Y., Park E. J., Ryu J. H., Jung J. W., et al. (2020). Hydrangeae dulcis folium attenuates physical stress by supressing ACTH-induced cortisol in zebrafish. Chin. J. Integr. Med. 26, 130–137. doi: 10.1007/s11655-019-3204-6
Peng B. B., Xu Q., Liu J., Guo S., Borgland S. L., and Liu S. (2021). Corticosterone attenuates reward-seeking behavior and increases anxiety via D2 receptor signaling in ventral tegmental area dopamine neurons. J. Neurosci. 41.7, 1566–1581. doi: 10.1523/JNEUROSCI.2533-20.2020
Porter R. J., Gallagher P., Watson S., and Young A. H. (2004). Corticosteroid-serotonin interactions in depression: a review of the human evidence. Psychopharmacology 173, 1–17. doi: 10.1007/s00213-004-1774-1
Pottinger T. G., Carrick T. R., Hughes S. E., and Balm P. H. M. (1996). Testosterone, 11-ketotestosterone, and estradiol-17β modify baseline and stress-induced interrenal and corticotropic activity in trout. Gen. Comp. Endocrinol. 104.3, 284–295. doi: 10.1006/gcen.1996.0173
Qi Z. P., Zhai Y., Han Y., Li K. Y., Wang T. C., Li P., et al. (2024). Genetic evidence for estrogenic effects of benzophenone-2 on zebrafish neurodevelopment and its signaling mechanism. Environ. Sci. Technol. 58.49, 21433–21449. doi: 10.1021/acs.est.4c06892
Ros A. F. H., Bruintjes R., Santos R. S., Canario A. V. M., and Oliveira R. F. (2004). The role of androgens in the trade-off between territorial and parental behavior in the Azorean rock-pool blenny, Parablennius parvicornis. Horm. Behav. 46, 491–497. doi: 10.1016/j.yhbeh.2004.04.007
Shen Z. G., Fan Q. X., Yang W., Zhang Y. L., Hu P. P., and Xie C. X. (2013). Effects of non-steroidal aromatase inhibitor letrozole on sex inversion and spermatogenesis in yellow catfish Pelteobagrus fulvidraco. Biol. Bull. 225, 18–23. doi: 10.1086/BBLv225n1p18
Silva M. A. D. S., Mattern C., Topic B., Buddenberg T. E., and Huston J. P. (2008). Dopaminergic and serotonergic activity in neostriatum and nucleus accumbens enhanced by intranasal administration of testosterone. Eur. Neuropsychopharmacol. 19, 53–63. doi: 10.1016/j.euroneuro.2008.08.003
Tan M. L., Shen J. Y., Chen L. Q., Wu F. R., and Liu Z. H. (2024). Environmentally relevant estrogens impaired spermatogenesis and sexual behaviors in male and female zebrafish. Aquat Toxicol. 273, 107008–107008. doi: 10.1016/J.AQUATOX.2024.107008
Tu X., Li Y. W., Chen Q. L., Shen Y. J., and Liu Z. H. (2020). Tributyltin enhanced anxiety of adult male zebrafish through elevating cortisol level and disruption in serotonin, dopamine and gamma-aminobutyric acid neurotransmitter pathways. Ecotox. Environ. Saf. 203, 111014. doi: 10.1016/j.ecoenv.2020.111014
Tyler W. J. and Pozzo-Miller L. D. (2001). BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J. Neurosci. 21, 4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001
Vaarala M. H., Hirvikoski P., Kauppila S., and Paavonen T. K. (2012). Identification of androgen-regulated genes in human prostate. Mol. Med. Rep. 6.3, 466–472. doi: 10.3892/mmr.2012.956
Verhovshek T., Rudolph L. M., and Sengelaub D. R. (2013). Brain-derived neurotrophic factor and androgen interactions in spinal neuromuscular systems. Neuroscience 239, 103–114. doi: 10.1016/j.neuroscience.2012.10.028
Wacker D. W., Khalaj S., Jones L. J., Champion T. L., Davis J. E., Meddle S. L., et al. (2016). Dehydroepiandrosterone (DHEA) heightens aggression and increases androgen receptor and aromatase mRNA expression in the brain of a male songbird. J. Neuroendocrinol. 28, 12443. doi: 10.1111/jne.12443
White K. J., Rivas M. G., and Pradhan D. S. (2023). Sex differences in aggressive intensities and brain steroids during status resolution in a sex changing fish, Lythrypnus dalli. Hormones Behav. 153, 105373. doi: 10.1016/j.yhbeh.2023.105373
Xu L. C., Sun H., Chen J. F., Bian Q., and Wang X. R. (2006). Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicol 216, 197–203. doi: 10.1016/j.tox.2005.08.006
Keywords: environmental estrogens, zebrafish, aggression, HPI/cortisol axis, serotonin, dopamine
Citation: Zhang Y–Y, Tao H, Shen Y–J, Chen Q–L and Liu Z–H (2025) Effect of environmentally relevant concentrations of estrogens on the aggressive response of male zebrafish. Front. Mar. Sci. 12:1578762. doi: 10.3389/fmars.2025.1578762
Received: 18 February 2025; Accepted: 04 July 2025;
Published: 24 July 2025.
Edited by:
Vikash Kumar, Central Inland Fisheries Research Institute (ICAR), IndiaReviewed by:
Mohamed Hamed, Louisiana State University, United StatesJia Jing Yi, Ocean University of China, China
Heloysa Araujo-Silva, Federal University of Rio Grande do Norte, Brazil
Ramasamy Vasantharekha, SRM University, India
Copyright © 2025 Zhang, Tao, Shen, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi–Hao Liu, bWluZW51dEAxNjMuY29t
†These authors have contributed equally to this work
 Ying–Ying Zhang1,2†
Ying–Ying Zhang1,2† Yan–Jun Shen
Yan–Jun Shen Zhi–Hao Liu
Zhi–Hao Liu