Abstract
Feeding microalgae during the larval and post-larval stages of bivalve mollusks is necessary to overcome the high mortality rates that occur during metamorphosis. Proper selection of the microalgae species and strain to be used by considering its nutritional value, cell density, culture time, and type of culture system to improve bivalve productivity is of high importance. In this study a microalgae recirculating culture system was evaluated. The system consisted of an open horizontal photobioreactor inside a hatchery raceway tank (12 m long, 2 m wide, and 0.5 m high) with a central division of 10 m and a driving paddle that generated a continuous flow of water at a speed of 1.25 m/s. The microalgae species cultivated in the photobioreactor were strains of Nannochloris spp. and Phaeodactylum spp., with the former reaching a significantly higher cell density of 19.37 ± 1.31 (106 cell mL-1) than the latter, which obtained a cell density of 1.41± 1.31 (106 cell mL-1). The reproductive cycle for Nannochloris spp. was 59 d, whereas that for Phaeodactylum spp. was 19 d. This study demonstrates that, although both strains of species of microalgae can be cultivated utilizing a horizontal photobioreactor system in hatcheries, Nannochloris spp. strains offer productive advantages including culture duration.
1 Introduction
Aquaculture is an economic activity of great importance in many countries as it provides a source of high-quality animal protein and contributes to food security and economic development (FAO, 2022). The culture of the Peruvian scallop, Argopecten purpuratus, a bivalve mollusk, contributes to the economic, social, and environmental development of countries that engage in this activity, such as Spain, China, Japan, the United States, Panama, Peru, and Chile (Kluger et al., 2019). However, the success of culturing this species, as well as other bivalves, depends on several factors such as cultivation technology, diet, and diseases that can influence the yield and profitability of the crop (FAO, 2016). One of the significant challenges facing aquaculture production of A. purpuratus is the availability and continuous supply of feed during the larval and post-larval stages of this species (FAO, 2016). The culture of A. purpuratus is a complex process that varies between 14 and 18 months, consisting of the production stages: larval (20–35 days), post-larval (40–55 days), pre-fattening (2–3 months), fattening (8–12 months), and harvesting (1–2 months) (Ministerio de la Producción, 2022). The larval stage, when metamorphosis to postlarvae occurs, from 20 to 35 days, is critical because of the physiological and anatomical changes that deplete energy reserves (Crisóstomo et al., 2023). Mortality at this stage can increase between 23% and 77% on the second day after larval attachment and between 20% and 50% on the fifth day after attachment (Farías, 2008; Yupanqui-Ccallata, 2018; Rojas et al., 2023). To control the mass mortality of A. purpuratus larvae by pathogenic Vibrio outbreaks, a diet rich in high-density unsaturated fatty acids (HUFA) is necessary to improve survival from 75-80%.
A. purpuratus individuals filter microalgae to provide the nutrients necessary for normal development (Carreño et al., 2012; Castro-Bustamante, 2018). The type of microalgae chosen for mollusk feeding must comply with a series of characteristics such as high cell density, culture time, adaptability to variable environmental conditions, and nutritional composition (Vivanco et al., 2014). Among the different microalgae strains evaluated, Nannochloris spp. strains present advantages of adaptation to varying salinities (Witt et al., 1981), low sensitivity to light and nutrient changes, and for example, strains KMMCC-119 and 395 can be cultured at temperatures up to 30°C (Bae and Hur, 2011). Nannochloris spp. has been used to feed mollusks such as Pacific oysters, Crassostrea gigas, being suitable for mass culture (Rodríguez-Pesantes, 2020). However, among the disadvantages of its cultivation is contamination with other microalgae species due to temperature variations, such as Phaeodactylum spp. at 25°C and Skeletonema spp. at 18°C (Witt et al., 1981), ciliates (Hue et al., 2020; Zhao et al., 2021), and rotifers (Deruyck et al., 2019; Hong et al., 2024). Nannochloris spp. have been reported to reach an average protein content of 42%, carbohydrate content of 11%, and lipid content of 15% under batch culture conditions in controlled laboratory environments, typically maintained at 20–25°C and at moderate to high light intensities (Witt et al., 1981; Ben-Ammar et al., 2024). Biochemical analysis of strain KMMCC-119 revealed the presence of nervonic acid (C24:1, omega-9 monounsaturated fatty acid), which is essential in medicinal plants and in the biosynthesis of myelin cells for the maintenance and development of the nervous system (Saadaoui et al., 2016), thus favoring the development and health of C. gigas postlarvae. On the other hand, Phaeodactylum tricornutum is a marine diatom whose relevance lies in the production of omega-3 fatty acids, with great emphasis on the production of EPA, whose composition ranges from 14 to 32% (García et al., 2021). Therefore, both Nannochloris spp. and Phaeodactylum spp. cultures have potential as food for bivalve postlarvae; however, there are differences in the growth rate and nutritional composition of microalgae that could affect their value as food, which is a critical factor for their respective use (Zafra-Trelles, 2017). A balanced diet for A. purpuratus includes the supply of different species of microalgae, such as Isochrysis galbana, Diacronema lutheri, Chaetoceros calcitrans, Chaetoceros gracilis Nannochloris maculata, Nannochloris sp, Nannochloropsis oculata, Dunaliella tertiolecta, and Tetraselmis suecica (Ministerio de la Producción, 2022). However, in batch culture conditions, Isochrysis galvana, Chaetoceros gracilis, Thlassiosira pseudonana, and Tetraselmis maculata do not exceed an average density of 4.9 x 106 cel mL-1, which does not meet the high cell density requirement needed for large-scale live feed production (Azaldi and Montoya, 2001) Nannochloris spp. present a growth rate significantly higher than 129 L -1 d -1 (Dogaris et al., 2015). Thus, when deciding which microalgae strain to cultivate, factors such as the high density of the culture and the time at which it will reach the maximum cell density should be taken into account, since the microalgal culture time should coincide with the larval and post-larval culture time of A. purpuratus in the hatchery for up to 90 days (Avendaño et al., 2001; Ministerio de la Producción, 2022), elements that are determinant for continuous and axenic production.
One aspect of microalgae cultivation technology involves the proper selection of the type of photobioreactor for microalgae cultivation, depending on its ease of operation, versatility in automation, low contamination, evaporation rate, fluctuating temperatures, nutrient limitation, culture medium, homogenization efficiency, and mass harvesting properties that can influence microalgal productivity (Brennan and Owende, 2010; Amaro et al., 2011; Handler et al., 2012). That is, superior photobioreactors allow optimal control of the culture conditions (Wang et al., 2012; Tham et al., 2023). Although several studies have demonstrated the feasibility of continuous microalgal production in raceway systems under greenhouse and hatchery conditions — particularly in the southeastern United States (Rusch and Malone, 1998; Theegala et al., 1999; Rusch and Christensen, 2003)— challenges remain in achieving consistently high-density production over extended periods under semi-controlled environments suitable for mollusk hatcheries. These studies have provided valuable insights into the management of environmental variables, but maintaining stable, high biomass levels over larval rearing cycles still requires further optimization. For instance, Bounnit et al. (2020) cultivated Nannochloris spp. QUCCCM31 in parallel 1-L photobioreactors under controlled laboratory conditions, achieving a biomass productivity of 226 mg L-1 d-1, demonstrating low-scale laboratory cultivation. However, to support large scale hatchery production of bivalves, technological advances in mass cultivation of strains under controlled conditions must be developed. These studies suggest that recirculating, open-air, horizontal photobioreactors can be more efficient than open raceways for the cultivation of different species of microalgae. However, low productivity and high costs require improved design and modeling of the technology for mass cultivation of microalgae in recirculating conditions. Therefore, scalability of microalgae cultivation in open raceway tanks within a greenhouse or hatchery is suggested.
The present study aimed to compare the culture of two species of marine microalgae, Phaeodactylum spp. and Nannochloris spp., in a horizontal photobioreactor, located inside a recirculating bivalve culture hatchery, for the feeding of A. purpuratus larvae and postlarvae. The cell density and periodicity of the cultured microalgae were evaluated to determine their viability of mass culture.
2 Materials and methods
2.1 Design and dimensions of the microalgae culture raceway
The raceway photobioreactor was developed at the Centro de Investigaciones Costeras of the University de Atacama (CIC-UDA) in Chile. It consisted of a 12 m long, 2 m wide and 0.75 m high “hippodrome” type raceway (capacity 15 m3), with a central division of 10 m and a microalgal culture storage capacity of 10 m3 operating with an allowable culture height of 0.5 m (Table 1). The raceway culture tank was constructed using 100% reinforced fiberglass, internally coated with a nutritional grade isophthalmic gelcoat barrier. This material was specifically selected to guarantee the safety of microalgae cultures, avoiding the release of potentially contaminating or toxic compounds.
Table 1
| Parameter | Value | Notes |
|---|---|---|
| Raceway useful volume | 10 m³ | |
| Raceway dimensions | 10 m (length) × 2 m (width) × 0.5 m (depth) | |
| Paddle wheel blade angle | 45° | Optimized for horizontal flow |
| Paddle wheel speed | 30 rpm | Adjusted to achieve target velocity |
| Surface water velocity | ~1.25 m/s | Verified by CFD modeling |
| Culture temperature | 14.31 ± 1.03°C | Semi-controlled environment |
| Light intensity | 1067.47 ± 443.82 lux | Natural light filtered through hatchery |
| Salinity | 34.53 ± 0.38 PSU | Measured weekly |
| Aeration system | 0.5 HP blower | To enhance mixing and oxygenation |
Operational parameters of the horizontal photobioreactor for marine microalgae culture.
Water movement inside the raceway was generated using a paddle system. The water movement within the raceway was generated using a rotary vane system with a three-phase geared motor. The paddle wheel consisted of 4 flat blades with a 45° inclination, mounted on a horizontal shaft driven by a three-phase geared motor operating at 30 revolutions per minute (rpm). The blade design and rotation speed were determined through Computational Fluid Dynamics (CFD) simulations using Salome v7, an open-source platform for pre- and post-processing in numerical simulations, particularly useful for geometry creation and mesh preparation. Mesh generation was carried out with snappyHexMesh, a meshing utility within the OpenFOAM suite that generates predominantly hexahedral (hex) meshes by iteratively refining and snapping the mesh to complex CAD geometries. This approach ensures high-quality mesh resolution near surfaces and in regions of interest. The resulting mesh enabled accurate modeling of fluid flow and pressure distribution around the blades. These simulations informed the optimization of blade geometry and rotation speed, enhancing hydrodynamic efficiency and achieving a surface circulation velocity of approximately 1.25 m/s. This velocity was higher than that typically reported for open raceways (0.20–0.30 m/s), to ensure sufficient suspension of microalgae cells in a controlled indoor environment while avoiding excessive shear stress. Literature indicates that microalgae such as Nannochloris spp. and Phaeodactylum spp. can tolerate shear rates up to 100–300 s-1 without significant damage (Brennan and Owende, 2010). The system was modeled to maintain shear rates within safe thresholds across most of the raceway volume. Figure 1 illustrates the design and dimensions of the culture system. Theoretical modeling and evaluation of the culture system were conducted to determine the dynamics of the particles in the raceway and the general behavior of the fluid in the system to estimate the correct arrangement of velocities and flows within the raceway.
Figure 1
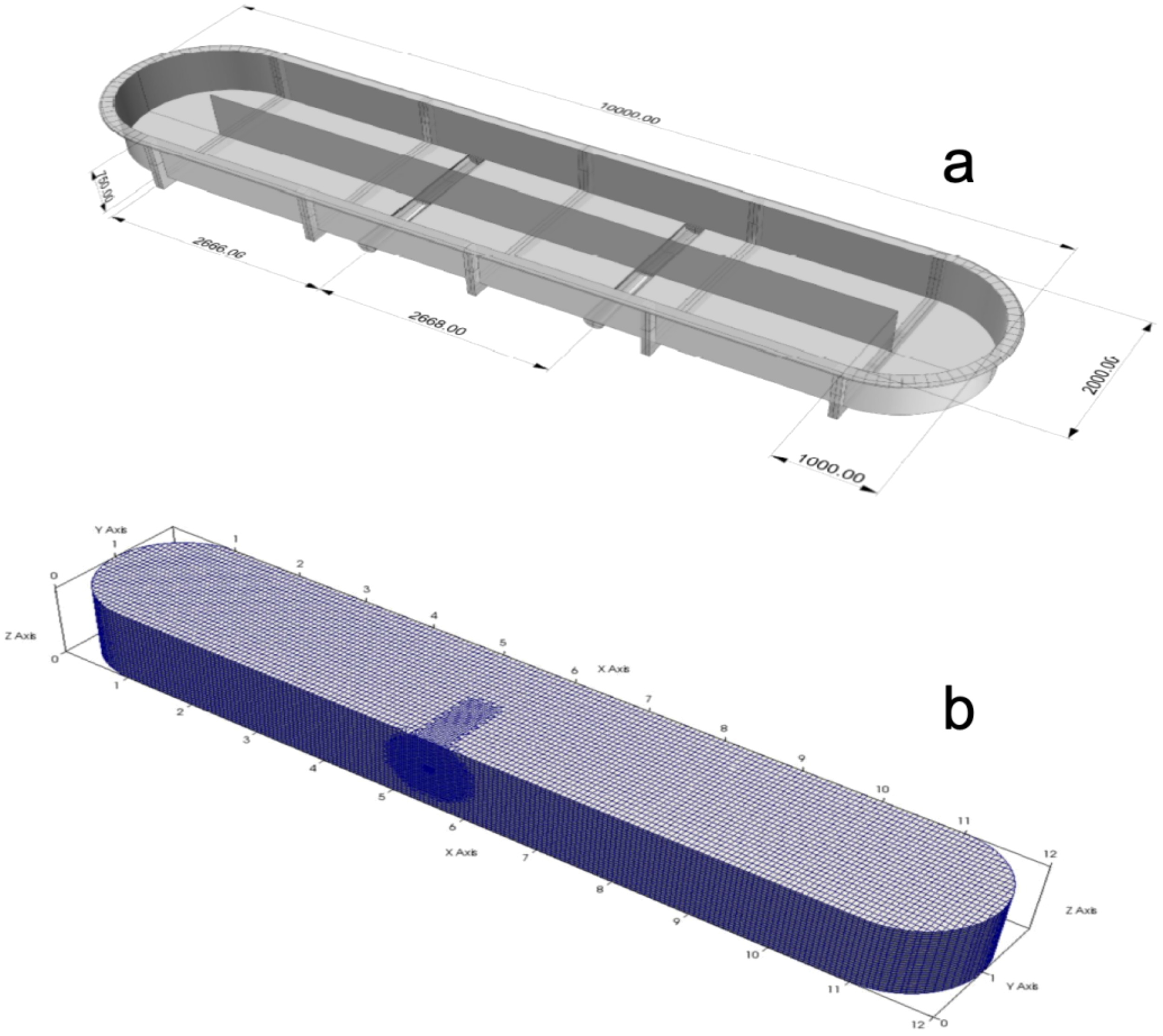
Three-dimensional design and meshing of the horizontal photobioreactor. (a) Top view of the 3D model showing paddle wheel (marked) and central divider. (b) Meshing of the culture raceway generated with 265,468 nodes.
Both were elaborated with the Salome v7.1 software and the snappyHexMesh tool, generating the dynamic geometry of the microalgae culture raceway tank. To validate the computational fluid dynamics (CFD) simulation results, experimental measurements of surface water velocity were conducted using a portable vane anemometer (Model DEF-123). Measurements were taken at five strategic locations along the longitudinal axis of the photobioreactor, near the water surface (0–5 cm depth).
2.2 Microalgae cultivation
The cultured microalgal strains utilized in this study (Phaeodactylum spp. and Nannochloris spp.) were supplied by CIC- UDA. For all culture stages, seawater treated by three filters of 5µm, 2 µm, and 1 µm, irradiated with UV light, disinfected with 0.25 mL L-1 of 2.5% sodium hypochlorite, and neutralized with 0.1 mL L-1 of 24.81% sodium thiosulfate was used. In addition, seawater was enriched at all stages of the culture scale-up with F/2 Guillard medium (Guillard, 1975) at a cell density of 1mL L–1 (Méndez-Ancca et al., 2020). Cultures were inoculated at initial densities of 2.16 × 105, 3.33 × 105, 1.83 × 105, and 7.17 × 105 cells mL-1 for Batches 1, 2, 3, and 4 (grown under static conditions without continuous addition of nutrients), respectively, determined by Neubauer chamber counting. The system operated in batch mode, without recirculation or scheduled daily harvesting.
Besides the initial addition of 1 mL L-1 F/2 medium (water-soluble mineral medium for the cultivation of marine microalgae), weekly supplementation with 0.5 mL L-1 of concentrated F/2 medium was applied to sustain nutrient levels during the experiment. To prevent and monitor potential contamination during long-term cultivation, several strategies were employed. Seawater used for culture preparation underwent triple-stage filtration (5 µm, 2 µm, 1 µm), ultraviolet (UV) light disinfection, chlorination with 0.25 mL L-1 of 2.5% sodium hypochlorite, and subsequent neutralization with 0.1 mL L-1 of 24.81% sodium thiosulfate. During the cultivation period, weekly microscopic inspections were performed on samples collected from different points of the photobioreactor to detect potential contamination by other microalgae species, bacteria, or zooplankton such as rotifers. No significant contamination events were detected during the 59-day cultivation of Nannochloris spp., ensuring culture viability and stability throughout the experimental period. (Table 2).
Table 2
| Monitoring Method | Description | Frequency |
|---|---|---|
| Triple-stage filtration | 10 µm → 5 µm → 1 µm filtration of seawater | Once (before use) |
| UV disinfection | UV irradiation after filtration | Once (before use) |
| Chemical disinfection | 0.25 mL L-1 sodium hypochlorite, neutralized with 0.1 mL L-1 sodium thiosulfate | Once (before use) |
| Microscopic inspection | Detection of contaminant microalgae, bacteria, or rotifers | Weekly |
Summary of contamination control and monitoring methods applied during microalgal cultivation.
Microalgae cultures were grown under semi-controlled conditions, maintaining a constant temperature, natural illumination, and dissolved oxygen levels, and cultures were aerated with a system powered by one regenerative air blower of 0.5 HP (GEBIAO, Model: GB-370, China). The raceway tank was aerated using a perforated polyethylene tube positioned along the interior side wall of the tank. The tube was perforated with holes spaced every 10 cm and extended along the entire length of the raceway. The aeration configuration ensured uniform air distribution throughout the raceway tank, promoting effective suspension of microalgal cells and maintaining adequate dissolved oxygen levels during algal cultivation. A transparent polycarbonate roof allowed natural solar radiation to penetrate the raceway tank. No artificial lighting or temperature control systems were employed during the experimental period.
Light intensity and temperature were monitored twice daily (at 08:00 h and 16:00 h) using a digital lux meter (Extech Instruments, Model: LT300), and a calibrated digital thermometer (Gain Express, Model: THE-27), respectively.
2.3 Statistical analysis
Due to the logistical constraints associated with operating a single large volume photobioreactor (10,000 L), each batch constituted an independent cultivation event without replication. As such, traditional inferential statistical analyses (e.g., ANOVA or mixed-effects models) were not applied. Growth data were analyzed descriptively, with means and standard deviations calculated for each batch to identify overall trends and variability.
3 Results
3.1 Modeling and construction of the microalgae cultivation raceway tank
The fluid velocities inside the raceway at different levels measured from the bottom of the raceway are presented in Figure 1. The direction of rotation of the paddle is indicated by arrows. It was observed that the greatest water movement occurred in the upper strata (0.50 m depth – velocities up to 1.25 m/s), while water movement decreased approaching a depth of 0.1 m with velocities decreasing to 0.0 m/s. Higher fluid velocity generated greater turbulence in the sector where the rotated vanes rotate. The analysis of water movement in the photobioreactor showed that there were places where eddy-like closed rotating movements were formed, and these occurred mostly at the ends of the raceway. Caution should be taken in monitoring extreme zones, especially zones closest to the bottom of the raceway, because near bottom zones represent an area where particles settle. Therefore, it is advisable to install aeration in these areas to avoid accumulation of particles and dead zones in the raceway (Figure 2).
Figure 2

Particle trajectory inside the raceway photobioreactor (a) isometric view showing water circulation (b) frontal view highlighting paddle wheel position (c) longitudinal view showing the drainage point locations.
After modeling and prediction of the maximum water depth that the experimental raceway could maintain the water mass of the microalgae culture raceway in a moving state, the microalgae culture raceway was built and installed in the premises of CIC-UDA; the horizontal fiberglass culture raceway tank, 10 m long, 2 m wide and 0.5 m useful, contained 10 m3 of microalgae culture. There were also two zones of solid accumulation and flushing drainage (Figures 3, 4). Experimental validation of the fluid dynamic model using surface velocity measurements yielded an average water flow of 1.21 ± 0.08 m/s, confirming the accuracy of the simulated flow pattern and supporting the reliability of the CFD predictions (Figure 3).
Figure 3
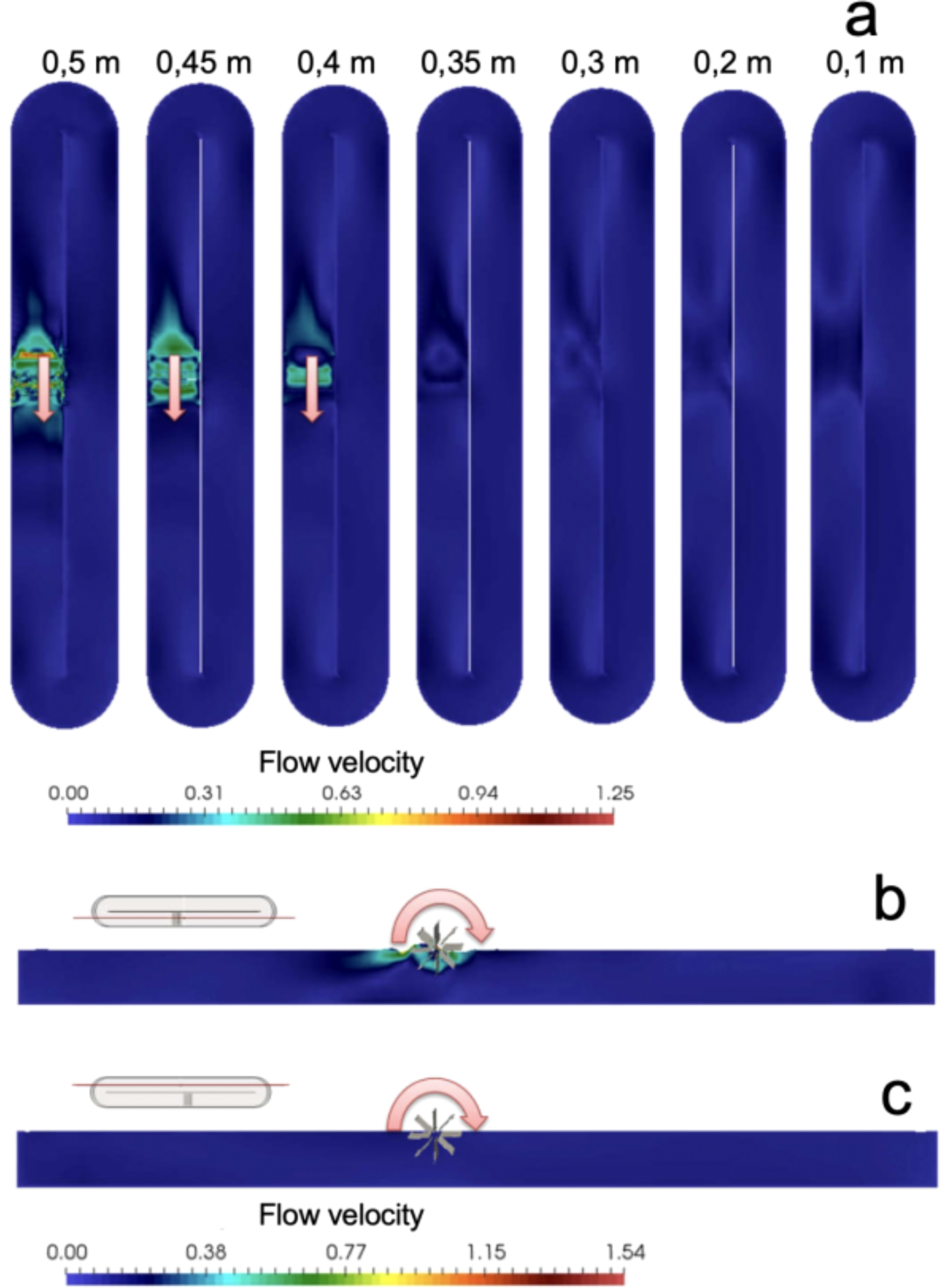
Fluid velocity (m/s) at different depths inside the photobioreactor. (a) Different levels from the bottom of the raceway tank, (b) Side with rotating paddle, (c) Side without rotating paddle.
Figure 4
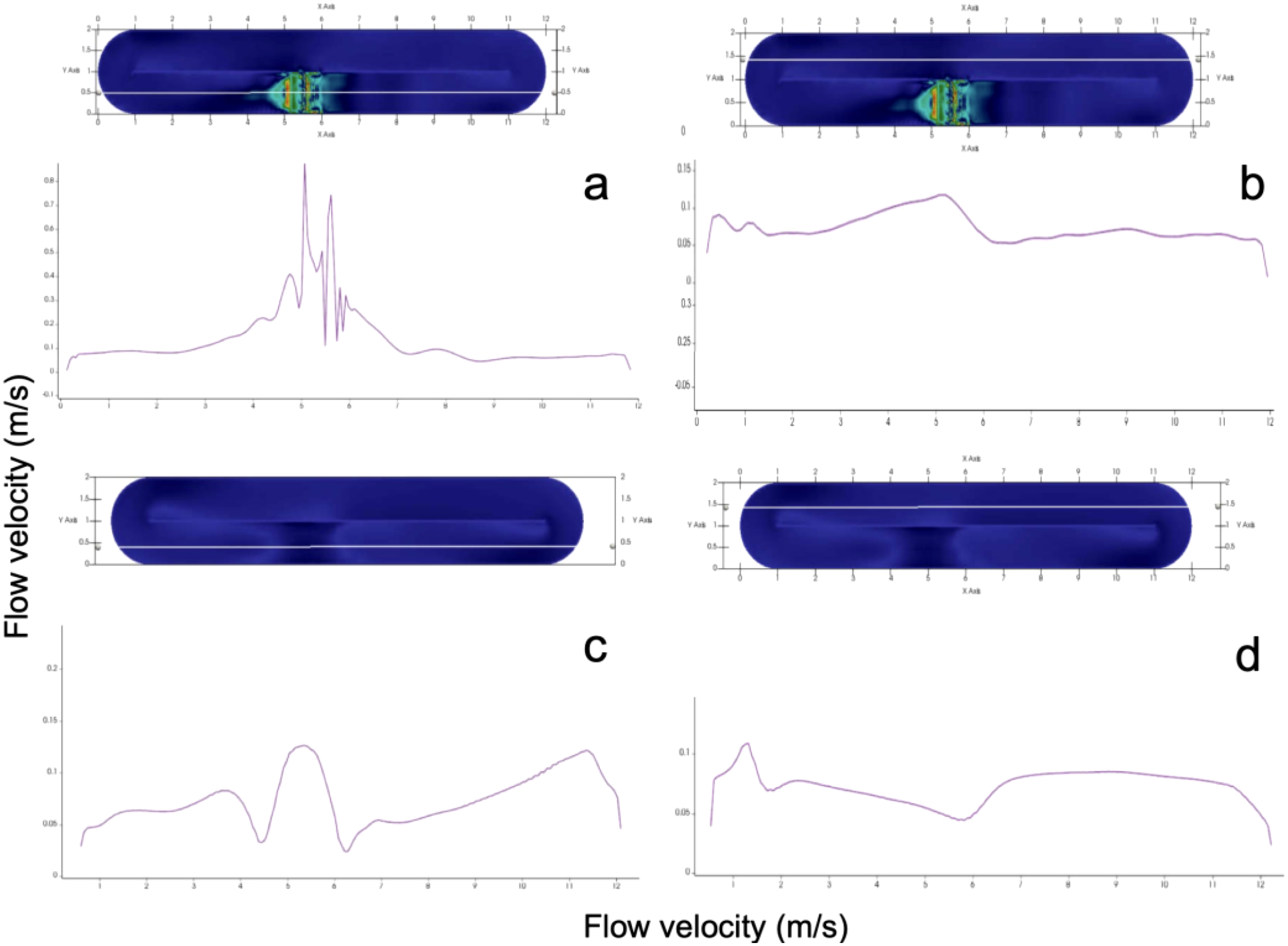
Fluid velocity field (m/s) at different depths inside the photobioreactor (a) Surface layer (0.5 m depth), (b) Intermediate layer (0.4 m depth), (c) Lower layer (0.3 m depth), (d) Bottom layer (0.1 m depth). Color scale indicates velocity magnitude (m/s).
3.2 Microalgae cultivation
The averages of the physical parameters of the culture were: water temperature 14.31 ± 1.03°C, light intensity 1067.47 ± 443.82 lux, salinity 34.53 ± 0.38 PSU, and dissolved oxygen 8.79 ± 0.88 mg L-1. Throughout the experimental period (June–October 2017), temperature and light intensity were monitored daily inside the hatchery where the photobioreactor was located. As shown in Figure 5, temperature fluctuated naturally between approximately 13°C and 16°C, while light intensity varied from around 700 to 1500 lux depending on the month. Lower temperatures and reduced light levels were recorded during July, which corresponded to the austral winter period, whereas higher values were observed in September. Despite these natural fluctuations, both parameters remained within acceptable ranges for the cultivation of Nannochloris spp. And Phaeodactylum spp., supporting stable microalgal growth. When interpreting differences in growth curves among batches, particularly between microalgae species, it is important to consider the potential impact of ambient temperature and light intensity variations alongside the intrinsic growth characteristics of the microalgae. Under these conditions, Phaeodactylum spp. survived in the three batches of cultivation of this species in the months of June, July, and August, and Nannochloris spp. survived in a single batch over 59 days.
Figure 5
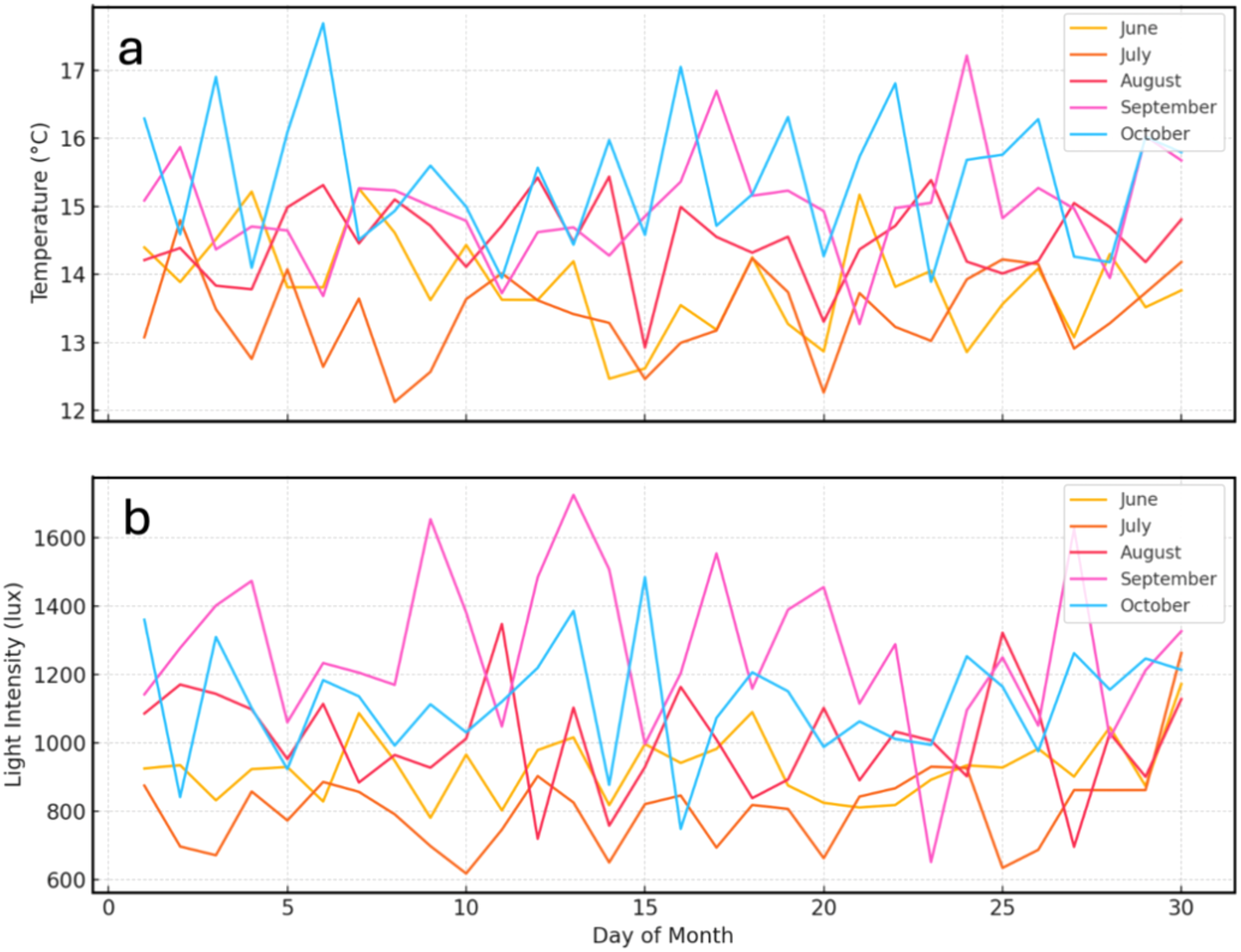
Daily variation of (a) temperature and (b) light intensity inside the hatchery from June to October 2017, recorded under natural light conditions with an approximate photoperiod of 11 hours light and 13 hours dark (11L:13D).
Throughout the experimental period, natural fluctuations in environmental parameters were recorded (Figure 5), with lower temperatures and light intensities observed during July (Batch 2). These environmental variations coincided with reduced microalgal growth performance during this batch, suggesting that seasonal factors influenced cell density. Therefore, when interpreting differences in growth curves among batches, particularly between species, it is important to consider the potential impact of ambient temperature and light intensity variations alongside the intrinsic growth characteristics of microalgae. It was also observed that the lowest performance in terms of duration and cell density of microalgae were observed in Batch 2 (July), this performance could be mainly explained by the low temperatures of this month and the low light intensity at this time of the year.
Table 3 shows the parameters of the microalgae Phaeodactylum spp. massively cultivated in the periods of June, July, and August, appreciating in each period that the maximum growth was 2.60, 1.80, and 5.51 (106 cell mL-1) correlating to 14 days, 6 days, and 14 days of culture for Phaeodactylum spp.; and 36 days of culture for Nannochloris spp. strains, proportional to the periods mentioned above. For the microalgae Phaeodactylum spp., an increase in cell density was observed in August; however, the average cultivation period was short (14 days), a discontinuous static cultivation of short duration, which is not optimal for the long cultivation period (90 days) of A. purpuratus in hatcheries. From Figure 6, it can be seen why a fourth assay (Batch 4) was carried out with Nannochloris spp., which lasted for two months in September and October (59 days), showing that maximum growth was 36.56 (106 cell mL-1), and the cell density reached at 36 days was very similar to the maximums (4–6 x 107 cell mL-1) reported by Mohammady et al. (2022) for the raceway culture of Nannochloris spp.
Table 3
| Batch | Specie | Temperature (°C) | Dissolved oxygen (mg/mL) | Light intensity (lux) |
Days of culture | Day of max. growth | Cell density (106 cell/mL) | SGR (d-1) |
|---|---|---|---|---|---|---|---|---|
| 1 | Phaeodactylum spp. | 15.11 ± 0.96 | 8.89 ± 1.39 | 1282 ± 399.09 | 17 | 14 | 2.60 | 17.77 |
| 2 | Phaeodactylum spp. | 13.61 ± 0.92 | 8.48 ± 0.65 | 670.75 ± 450.91 | 10 | 4 | 1.80 | 42.16 |
| 3 | Phaeodactylum spp. | 15.24 ± 0.77 | 9.36 ± 0.86 | 711.76 ± 39.33 | 14 | 12 | 5.52 | 28.37 |
| 4 | Nannochloris spp. | 14.89 ± 0.96 | 8.35 ± 0.31 | 1159.41 ± 195.55 | 59 | 34 | 36.56 | 11.56 |
Physical, chemical, and growth parameters of Phaeodactylum spp. and Nannochloris spp. culture.
Figure 6
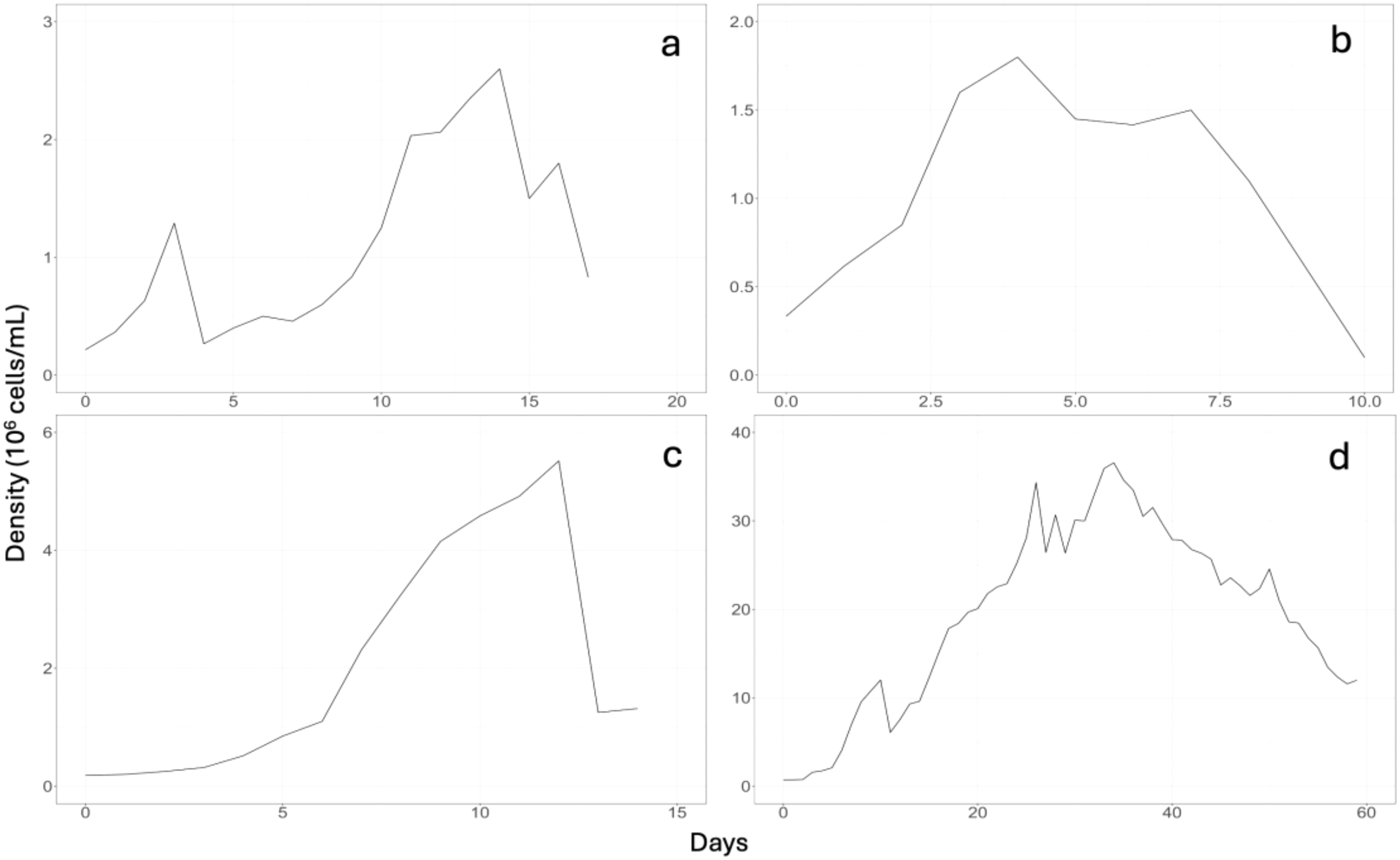
Growth curves for Phaeodactylum spp. and Nannochloris spp. during cultivation in the horizontal photobioreactor. (a) Batch 1, (b) Batch 2, (c) Batch 3 for Phaeodactylum spp. and (d) Batch 4 for Nannochloris spp.
As shown in Figure 3, regarding growth of Phaeodactylum spp., the maximum cell density obtained (Batch 3) was 5 (106 cell mL-1), which is in contrast with the results obtained for Nannochloris spp. (Batch 4), in which the highest cell density was 35 (106 cell mL-1). According to statistical analysis, the mean cell concentration (cell mL-1) of the Nannochloris spp. strain was higher (M= 19.37 x106, SE= 1.31 x106), than that of the Phaeodactylum spp. strain (M=1.41 x106, SE= 1.31x106), specifically 13.5 times higher according to Levene’s test.
4 Discussion
The present investigation demonstrated that the fluid dynamics in the culture raceway, specifically at a water velocity of 1.25 m/s, had a significant impact on the growth and productivity of the microalgae Phaeodactylum spp. and Nannochloris spp. The increased water movement in the upper strata of the raceway enhanced the dispersion of microalgae and their exposure to light and nutrients, which favors growth and productivity (Prieto et al., 2005; Pedruzi et al., 2020). However, specific areas of horizontal raceways, such as the ends and bottom, where eddies form and particles settle, can affect the distribution and density of microalgae (Marcé et al., 2007). Documenting that the pattern of water movement favors microalgae growth in a large-scale horizontal photobioreactor provides valuable insight for optimizing the design and operation of these systems.
Unlike conventional open raceway systems, which are exposed to fluctuating environmental conditions and higher contamination risks, the horizontal photobioreactor designed in this study operates within a controlled hatchery environment. This setting allows for greater stability of critical parameters such as temperature, salinity, illumination, and water quality. In addition, prior to the physical construction of the system, hydrodynamic modeling using Salome v7.1 and the snappyHexMesh tool was conducted to optimize internal water flow, minimize dead zones, and improve particle suspension throughout the culture volume. This approach differs substantially from previous raceway designs, which often rely on empirical layouts without detailed fluid dynamics optimization. As a result, the system enabled the continuous culture of Nannochloris sp. for 59 days, covering approximately two-thirds of the hatchery cycle of A. purpuratus larvae and postlarvae. These innovations support the scalability and reliability of microalgae production systems intended for intensive aquaculture hatcheries.
Large-scale outdoor continuous microalgae culture studies are costly and difficult to perform (Novoveská et al., 2023). Therefore, the design of raceways used for mass cultivation in hatcheries can be improved (Costa and de Morais, 2014). Raceways are the most inexpensive method for mass microalgae production (Chisti, 2008), require little energy to move paddles, and are easy to clean (Ugwu et al., 2008). Horizontal photobioreactors inside greenhouses or hatcheries with transparent covers in which sunlight can be used as a stimulant for the growth of microalgae stimulate growth under axenic conditions (Duque-Granda et al., 2019), and accessorily coupled with air conditioning or an automatic heater to avoid temperature fluctuations during the night, allow greater control of cultivation conditions and higher productivity compared to other cultivation systems.
In this study, the maximum cell density result for Nannochloris spp. was 19.37 ± 10.20 (106 cells mL-1), reached on day 36 of culture at a temperature of 14.31 ± 1.03°C and salinity of 34.53 ± 0.38 PSU; maintaining similarity with the density recorded by Nava (2012) who cultivated Nannochloris spp. obtaining the maximum cell density of 19.6 x106 cells mL-1 on day 08 of culture at a temperature of 25 ± 1°C and 25 PSU. Both experiments differed in temperature and salinity at 9.33°C and 10 PSU, respectively. Likewise, Bae and Hur (2011) cultured Nannochloris spp. (KMMCC-395) in 250 mL flasks at 30°C, 30 PSU and 100 µm m-2 s-1 showed a high cell density of 10.733 x 104 cell mL-1 after seven days of culture. In similar studies on a smaller scale (Cho et al., 2007) inoculated microalga Nannochloris oculata placed in 250 mL flasks, maintaining a temperature of 25°C and salinity of 30 PSU, reached a maximum cell density of 308.0 ± 11.51 x 106 cells mL-1, at 21 ± 0.6 days of culture. Similarly, other authors cultivating pilot photobioreactors obtained a biomass of 3.685 g L-1 from the strain Nannochloris spp (Saadaoui et al., 2016). From these studies, it is deduced that the increase in temperature and decrease in salinity favor the rapid growth of Nannochloris spp. However, to achieve a continuous supply of food in the larval and post-larval stages of A. purpuratus, it is necessary to prolong the life of the strain employed.
Regarding the microalga Phaeodactylum spp., in the present experiment a cell density of 1.41 x106 ± 1.31 x106 was obtained at a temperature of 14.31 ± 1.03°C, light intensity 1067.47 ± 443.82 lux and salinity of 34.53 ± 0.38 PSU. Similarly, Jerí (2018) cultured P. tricornutum in a volume of 475 mL, with high CO2 injections (25%) under laboratory conditions at a temperature of 24 ± 1°C and a light intensity of 1500 lx, obtaining a cell density of 1. 67 x106 cell mL-1. In another study Arenas (2023) performed a semi-continuous culture (15 L) of P. tricornutum for 19 days obtaining a higher density than the previous studies of 3.473 x103 ± 375.33 cell mL-1, probably due to the semi-continuous operating conditions. Meiser et al. (2004) managed to harvest from a Phaeodactylum spp strain 1.37 g L-1 biomass (including CO2). Hall et al. (2003), collected up to 1.4 g L-1 d-1 of biomass, growing the same strain in a helical tubular photobioreactor. However, both cell density and biomass of Phaeodactylum spp. were lower than those of Nannochloris spp.
One limitation of the present study is that Phaeodactylum spp. and Nannochloris spp cultures were conducted during different seasonal periods and not under identical environmental conditions. Consequently, observed differences in cell density and culture duration between the two species may partially reflect variations in ambient temperature and light intensity rather than exclusively species-specific responses or photobioreactor performance. Future studies should consider cultivating both species simultaneously under controlled environmental conditions and apply statistical corrections for seasonal effects to better isolate species-specific growth characteristics. The superior performance of Nannochloris spp. observed in this study can be attributed to its well-documented physiological resilience to environmental stress. Previous studies have reported that Nannochloris spp. strains exhibit high tolerance to salinity fluctuations (Witt et al., 1981) and maintain stable growth rates under varying temperature regimes, up to 30°C (Bae and Hur, 2011). Moreover, strains of Nannochloris spp. are characterized by a relatively low sensitivity to light intensity variations, which enables sustained productivity even under suboptimal irradiance conditions (Saadaoui et al., 2016). These physiological traits likely conferred a survival advantage to Nannochloris spp. strains over Phaeodactylum spp. strains in a semi-controlled hatchery environment, where natural seasonal changes in temperature and light were unavoidable. Consequently, the longer culture duration and higher cell density achieved with the Nannochloris spp. strain in this study could be attributed not only to the photobioreactor design but also to the intrinsic robustness of the species. In terms of productivity of the microalgae strains Phaeodactylum spp., the results showed that the maximum growth in the periods of June, July, and August was relatively short (12 days) on average, not optimal for the long culture period (90 days) of A. purpuratus in hatcheries. In contrast, the culture of the Nannochloris spp. stain in this study had a culture period of two months (59 days).
An approximate energy consumption analysis was conducted to assess the preliminary productive viability of the system (Table 4). The combined power consumption of the paddle wheel motor (0.37 kW) and aeration blower (0.37 kW) correspond to the nominal rated power specified by the equipment manufacturers, rather than directly measured consumption. Assuming continuous operation over 59 days, the total estimated energy consumption was 1,047.84 kWh. The estimated dry biomass yield of approximately 5 kg was calculated based on an average biomass concentration of 0.5 g/L– a typical production value for Nannochloris spp. under optimal culture conditions—multiplied by the effective working volume of the photobioreactor (10,000 L). Accordingly, the specific energy consumption was calculated at around 210 kWh per kilogram of biomass produced. Although this value may appear high compared to large-scale open raceway systems, it reflects the improved control of environmental parameters, and the axenic conditions achieved in the hatchery-based photobioreactor. Future optimizations, such as implementing variable-speed drives, intermittent aeration, and real-time energy monitoring, could further reduce energy demands, enhancing the scalability and economic feasibility of the system for industrial aquaculture applications.
Table 4
| Parameter | Value | Notes |
|---|---|---|
| Paddle wheel motor power | 0.37 kW | 0.5 HP motor |
| Aeration blower power | 0.37 kW | 0.5 HP blower |
| Total installed power | 0.74 kW | |
| Operation time | 24 h/day for 59 days | Continuous operation |
| Total energy consumption | 1,047.84 kWh | 0.74 kW × 24 h × 59 days |
| Photobioreactor volume | 10,000 L (10 m³) | |
| Estimated dry biomass yield | 5 kg | 0.5 g/L as reference value |
| Specific energy consumption | 210 kWh/kg dry biomass |
Energy consumption and biomass production efficiency of the hatchery-based horizontal photobioreactor.
It should be noted that in this study, the biochemical composition of the cultivated microalgae was not analyzed, as the primary focus was on validating the photobioreactor’s operational performance under hatchery conditions. Future research should include nutritional analyses of the biomass of microalgae produced and evaluate the feasibility of incorporating algal pastes into the raceway system to optimize larval feeding strategies and enhance operational flexibility. Despite the limitations associated with single-batch large-volume cultivations, the results of this study provide valuable insights for optimizing future operations. Future work will include replicated small-scale photobioreactor trials to statistically validate observed growth dynamics before scaling to full production systems.
5 Conclusions
The duration, density, and productivity of the culture of microalgae strains determined that the ideal species for cultivation in a horizontal photobioreactor are Nannochloris spp. strains, fulfilling the productive requirements of A. purpuratus culture, allowing 67.68% of its productive cycle of 90 days under hatchery conditions., The culture of Phaeodactylum spp. strains in Kallwall and bottles (traditional method) may be included in hatchery operations, however this microlagae species has a short useful life and does not coincide with the productive process of A. purpuratus.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RC: Formal Analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. RP: Data curation, Formal Analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. JH: Data curation, Methodology, Writing – review & editing. SM: Formal Analysis, Resources, Writing – review & editing. OA: Methodology, Supervision, Writing – review & editing. EC: Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Atacama University, Chile, by the FIC: Ostion Tech Project, of the regional government of Atacama (Code: BIP30433072-0) 10.
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Conflict of interest
Author JH was employed by Finfish Aquaculture Sociedad Anónima Cerrada.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Amaro H. M. Guedes A. C. Malcata F. X. (2011). Advances and perspectives in using microalgae to produce biodiesel. Appl. Energy88, 3402–3410. doi: 10.1016/j.apenergy.2010.12.014
2
Arenas Y. A. (2023). Composición bioquímica de las diatomeas Phaeodactylum tricornutum y Thalassiosira weissflogii en cultivo estático y semicontinuo (Baja California: Centro de Investigación Científica y de Educación Superior de Ensenada).
3
Avendaño R. E. Riquelme C. E. Escribano R. Reyes N. (2001). Sobrevivencia y crecimiento de post-larvas de Argopecten purpuratus (Lamarck 1819) en Bahía Inglesa, Chile: efectos del origen, distribución en la bahía y bacterioflora larval. Rev. Chil. Hist. Natural74, 669–679. doi: 10.4067/S0716-078X2001000300012
4
Azaldi E. Montoya N. (2001). Evaluación de la composición bioquímica nutricional de microalgas en diferentes fases de crecimiento y condiciones de cultivo. Fundación CENAIM-ESPOL. Available at: http://www.dspace.espol.edu.ec/handle/123456789/8534 (Accessed December 20, 2024).
5
Bae J.-H. Hur S.-B. (2011). Selection of suitable species of chlorella, nannochloris, and nannochloropsis in high- and low-temperature seasons for mass culture of the rotifer Brachionus plicatilis. Fish Aquat. Sci.14, 323–332. doi: 10.5657/FAS.2011.0323
6
Ben-Ammar F. E. Saidane F. Messaoud C. Hamdi M. (2024). Screening of efficient microalgae strains isolated from Tunisian ecosystems: Assessment of algal growth rate and added-value bioproducts for biorefinery applications. Biocatal Agric. Biotechnol.58, 103140. doi: 10.1016/j.bcab.2024.103140
7
Bounnit T. Saadaoui I. Rasheed R. Schipper K. Al Muraikhi M. Al Jabri H. (2020). Sustainable production of nannochloris atomus biomass towards biodiesel production. Sustainability12, 2008. doi: 10.3390/su12052008
8
Brennan L. Owende P. (2010). Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renewable Sustain. Energy Rev.14, 557–577. doi: 10.1016/j.rser.2009.10.009
9
Carreño Y. K. Acosta E. Gómez-León J. (2012). Crecimiento y supervivencia de larvas de Argopecten nucleus alimentadas con diferentes dietas microalgales. Boletín Investigaciones Marinas y Costeras-INVEMAR1, 103–120.
10
Castro-Bustamante J. (2018). Diseño de una planta piloto para el crecimiento de microalgas heterótrofas87 (Valladolid: Universidad de Valladolid). Available at: https://uvadoc.uva.es/handle/10324/32691 (Accessed December 20, 2024).
11
Chisti Y. (2008). Biodiesel from microalgae beats bioethanol. Trends Biotechnol.26, 126–131. doi: 10.1016/j.tibtech.2007.12.002
12
Cho S. H. Ji S.-C. Hur S. B. Bae J. Park I.-S. Song Y.-C. (2007). Optimum temperature and salinity conditions for growth of green algae Chlorella ellipsoidea and Nannochloris oculata. Fisheries Sci.73, 1050–1056. doi: 10.1111/j.1444-2906.2007.01435.x
13
Costa J. A. V. de Morais M. G. (2014). “An open pond system for microalgal cultivation,” in Biofuels from Algae, eds. PandeyA.LeeD.-J.ChistiY.SoccolC. R. (Burlington, MA: Elsevier), 1–22. doi: 10.1016/B978-0-444-59558-4.00001-2
14
Crisóstomo R. O. Pepe-Victoriano R. Méndez-Ancca S. Zambrano-Cabanillas A. W. Marín-Machuca O. Perez H. M. et al . (2023). Reproductive conditioning of the Peruvian scallop Argopecten purpuratus in different environments. Fishes9, 9. doi: 10.3390/fishes9010009
15
Deruyck B. Thi Nguyen K. H. Decaestecker E. Muylaert K. (2019). Modeling the impact of rotifer contamination on microalgal production in open pond, photobioreactor and thin layer cultivation systems. Algal Res.38, 101398. doi: 10.1016/j.algal.2018.101398
16
Dogaris I. Welch M. Meiser A. Walmsley L. Philippidis G. (2015). A novel horizontal photobioreactor for high-density cultivation of microalgae. Bioresour Technol.198, 316–324. doi: 10.1016/j.biortech.2015.09.030
17
Duque-Granda D. Montoya-Vallejo C. Botero-Botero L. R. (2019). Cadmium (Cd) tolerance evaluation of three strains of microalgae of the genus Ankistrodesmus, Chlorella and Scenedesmus. Rev. Facultad Ingeniería Universidad Antioquia92, 60–69. doi: 10.17533/udea.redin.20190523
18
FAO . (2016).El cultivo de moluscos bivalvos: una guía para la producción sostenible en aguas tropicales y subtropicales. Available online at: https://www.fao.org/fishery/docs/DOCUMENT/aquaculture (Accessed January 28, 2025).
19
FAO . (2022).El estado mundial de la pesca y la acuicultura 2022. Hacia la transformación azul. Roma, Italia. Available online at: https://openknowledge.fao.org/items/92319005-6232-450f-8c75-4d4fcf24720d (Accessed January 28, 2025).
20
Farías A. (2008). Nutrición y alimentación en moluscos bivalvos, in Estado actual del cultivo y manejo de moluscos bivalvos y su proyección futura: factores que afectan su sustentabilidad en América Latina. Taller Técnico Regional de la FAO, eds. LovatelliA.FaríasA.UriarteI. (Puerto Montt, Chile: FAO), 20–24. Available at: https://www.fao.org/fishery/docs/DOCUMENT/aquaculture/aq2008_09/root/i0444s.pdf (Accessed May 31, 2025)
21
García T. C. T. M. A. G. E. (2021). Phaeodactylum tricornutum, una fuente potencial de ácido eicosapentaenoico. Rev. BioTecnología25, 66–77.
22
Guillard R. R. L. (1975). “Culture of phytoplankton for feeding marine invertebrates,” in Culture of Marine Invertebrate Animals (Springer US, Boston, MA), 29–60. doi: 10.1007/978-1-4615-8714-9_3
23
Hall D. O. Acién Fernández F. G. Guerrero E. C. Rao K. K. Grima E. M. (2003). Outdoor helical tubular photobioreactors for microalgal production: Modeling of fluid-dynamics and mass transfer and assessment of biomass productivity. Biotechnol. Bioeng82, 62–73. doi: 10.1002/bit.10543
24
Handler R. M. Canter C. E. Kalnes T. N. Lupton F. S. Kholiqov O. Shonnard D. R. et al . (2012). Evaluation of environmental impacts from microalgae cultivation in open-air raceway ponds: Analysis of the prior literature and investigation of wide variance in predicted impacts. Algal Res.1, 83–92. doi: 10.1016/j.algal.2012.02.003
25
Hong J. Sobhi M. Zheng H. Hu X. Cui Y. Yu Z. et al . (2024). Effective removing of rotifer contamination in microalgal lab-scale raceway ponds by light-induced phototaxis coupled with high-voltage pulse electroshock. Bioresour Technol.394, 130241. doi: 10.1016/j.biortech.2023.130241
26
Hue N. T. K. Khang D. T. Men T. T. Vanoverberghe I. Callens M. Muylaert K. (2020). Isolation and identification of herbivorous ciliates from contaminated microalgal cultures. Eur. J. Protistol76, 125743. doi: 10.1016/j.ejop.2020.125743
27
Jerí N. J. (2018). Efecto de la concentración de dióxido de carbono y agitación del cultivo sobre la fijación de carbono por la microalga Phaeodactylum tricornutum 2018 (Lima, Peru: Universidad Cesar Vallejo). Available online at: https://hdl.handle.net/20.500.12692/20878 (Accessed January 28, 2025).
28
Kluger L. C. Scotti M. Vivar I. Wolff M. (2019). Specialization of fishers leads to greater impact of external disturbance: Evidence from a social-ecological network modelling exercise for Sechura Bay, northern Peru. Ocean Coast. Manag179, 1–15. doi: 10.1016/j.ocecoaman.2019.104861
29
Marcé R. Feijoó C. Navarro E. Ordoñez J. Gomà J. Armengol J. (2007). Interaction between wind-induced seiches and convective cooling governs algal distribution in a canyon-shaped reservoir. Freshw. Biol.52, 1336–1352. doi: 10.1111/j.1365-2427.2007.01771.x
30
Meiser A. Schmid-Staiger U. Trösch W. (2004). Optimization of eicosapentaenoic acid production by Phaeodactylum tricornutum in the flat panel airlift (FPA) reactor. J. Appl. Phycol16, 215–225. doi: 10.1023/B:JAPH.0000048507.95878.b5
31
Méndez-Ancca S. Alvarez Y. Sosa L. E. Vizcarra Yhordan. G. (2020). Concentración celular y biomasa seca en tres especies de microalgas marinas: Chlorella vulgaris, Nannochloropsis oculata y Tetraselmis striata. Rev. Investigaciones Altoandinas - J. High Andean Res.22, 155–160. doi: 10.18271/ria.2020.603
32
Ministerio de la Producción (2022). Anuario Estadístico Pesquero y Acuícola 2022 (Lima, Perú: Ministerio de la Producción). Available online at: https://ogeiee.produce.gob.pe/index.php/en/shortcode/oee-documentos-publicaciones/publicaciones-anuales/item/1116-anuario-estadistico-pesquero-y-acuicola-2022.
33
Mohammady N. G.-E. El-Khatib K. M. El-Galad M. I. Abo El-Enin S. A. Attia N. K. El-Araby R. et al . (2022). Preliminary study on the economic assessment of culturing Nannochloropsis sp. in Egypt for the production of biodiesel and high-value biochemicals. Biomass Convers Biorefin12, 3319–3331. doi: 10.1007/s13399-020-00878-9
34
Nava G. (2012). Efecto de la salinidad en el crecimiento de la Microalga Nannochloris sp (Lima: Universidad Nacional Agraria La Molina).
35
Novoveská L. Nielsen S. L. Eroldoğan O. T. Haznedaroglu B. Z. Rinkevich B. Fazi S. et al . (2023). Overview and challenges of large-scale cultivation of photosynthetic microalgae and cyanobacteria. Mar. Drugs21, 445. doi: 10.3390/md21080445
36
Pedruzi G. O. L. Amorim M. L. Santos R. R. Martins M. A. Vaz M. G. M. V. (2020). Biomass accumulation-influencing factors in microalgae farms. Rev. Bras. Engenharia Agrícola e Ambiental24, 134–139. doi: 10.1590/1807-1929/agriambi.v24n2p134-139
37
Prieto M. J. Mogollon M. J. Castro A. L. Sierra L. A. (2005). Efecto del medio Y condiciones de cultivo en la productividad de tres diatomeas marinas con potencial acuícola. Rev. MVZ Cordoba10, 544–554. doi: 10.21897/rmvz.476
38
Rodríguez-Pesantes D. F. (2020). Descripción del ciclo de vida temprano, evaluación de dietas monoalgales, bialgales y selección de sustratos para la fijación de la ostra de roca Striostrea prismatica (Gray 1825) (Guayaquil: Centro Nacional de Acuicultura e Investigaciones Marinas). Available online at: https://dspace.espol.edu.ec/bitstream/123456789/51495/1/T-76773.pdf.
39
Rojas I. Cárcamo C. B. Defranchi Y. Jeno K. Rengel J. Araya M. et al . (2023). A diet rich in HUFAs enhances the energetic and immune response capacities of larvae of the scallop Argopecten purpuratus. Animals13, 1416. doi: 10.3390/ani13081416
40
Rusch K. A. Christensen J. M. (2003). The hydraulically integrated serial turbidostat algal reactor (HISTAR) for microalgal production. Aquac Eng.27, 249–264. doi: 10.1016/S0144-8609(02)00086-9
41
Rusch K. A. Malone R. F. (1998). Microalgal production using a hydraulically integrated serial turbidostat algal reactor (HISTAR): a conceptual model. Aquac Eng.18, 251–264. doi: 10.1016/S0144-8609(98)00033-8
42
Saadaoui I. Al Ghazal G. Bounnit T. Al Khulaifi F. Al Jabri H. Potts M. (2016). Evidence of thermo and halotolerant Nannochloris isolate suitable for biodiesel production in Qatar Culture Collection of Cyanobacteria and Microalgae. Algal Res.14, 39–47. doi: 10.1016/j.algal.2015.12.019
43
Tham P. E. Lim H. R. Khoo K. S. Chew K. W. Yap Y. J. Munawaroh H. S. H. et al . (2023). Insights of microalgae-based aquaculture feed: A review on circular bioeconomy and perspectives. Algal Res.74, 103186. doi: 10.1016/j.algal.2023.103186
44
Theegala C. S. Malone R. F. Rusch K. A. (1999). Contaminant washout in a hydraulically integrated serial turbidostat algal reactor (HISTAR). Aquac Eng.19, 223–241. doi: 10.1016/S0144-8609(98)00051-X
45
Ugwu C. U. Aoyagi H. Uchiyama H. (2008). Photobioreactors for mass cultivation of algae. Bioresour Technol.99, 4021–4028. doi: 10.1016/j.biortech.2007.01.046
46
Vivanco G. Oliva D. Abarca A. (2014). Efecto de dietas en base a microalgas tradicionales, nativas y dietas artificiales sobre el crecimiento y supervivencia en larvas velígeras de la almeja taquilla, Mulinia edulis. Rev. Biol. Mar. Oceanogr49, 339–349. doi: 10.4067/S0718-19572014000200012
47
Wang B. Lan C. Q. Horsman M. (2012). Closed photobioreactors for production of microalgal biomasses. Biotechnol. Adv.30, 904–912. doi: 10.1016/j.bioteChadv.2012.01.019
48
Witt U. Koske P. H. Kuhlmann D. Lenz J. Nellen W. (1981). Production of Nannochloris spec. (Chlorophyceae) in large-scale outdoor tanks and its use as a food organism in marine aquaculture. Aquaculture23, 171–181. doi: 10.1016/0044-8486(81)90012-0
49
Yupanqui-Ccallata E. R. (2018). Efecto de tres diferentes densidades de cultivo en la tasa de asentamiento larval y supervivencia post larval en un hatchery de concha de abanico, Argopecten Purpuratus (Lamarck 1819) (Arequipa: Universidad Nacional de San Agustín de Arequipa). Available online at: https://repositorio.unsa.edu.pe/items/a6857250-19c8-4ffe-b843-bf73a07cc881.
50
Zafra-Trelles A. M. (2017). Cultivo de microalgas marinas potenciales para la acuicultura del litoral entre Puerto Salaverry y Puerto Chicama, La Libertad, Perú. Arnaldoa24, 567–582. doi: 10.22497/arnaldoa.242.24209
51
Zhao L. Zhang Y. Geng X. Hu X. Zhang X. Xu H. et al . (2021). Potential to resist biological contamination in marine microalgae culture: Effect of extracellular substances of Nannochloropsis oceanica on population growth of Euplotes vannus and other protozoa. Mar. pollut. Bull.172, 112868. doi: 10.1016/j.marpolbul.2021.112868
Summary
Keywords
aquaculture nutrition, live feed, marine aquaculture, microalgal growth, raceway photobioreactor
Citation
Crisóstomo-Gamboa RO, Pepe-Victoriano R, Huanacuni JI, Méndez-Ancca S, Acosta-Angulo O and Cruces E (2025) Preliminary evaluation of a raceway photobioreactor for mass culture of microalgae for Peruvian scallop, Argopecten purpuratus postlarvae. Front. Mar. Sci. 12:1580581. doi: 10.3389/fmars.2025.1580581
Received
20 February 2025
Accepted
06 June 2025
Published
30 June 2025
Volume
12 - 2025
Edited by
Charles Weirich, NOAA National Sea Grant Office, United States
Reviewed by
Biji Xavier, Central Marine Fisheries Research Institute (ICAR), India
Minghao Chen, Harvard University, United States
Tim Pfeiffer, Agricultural Research Service (USDA), United States
Updates
Copyright
© 2025 Crisóstomo-Gamboa, Pepe-Victoriano, Huanacuni, Méndez-Ancca, Acosta-Angulo and Cruces.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renzo Pepe-Victoriano, rpepev@unap.cl; Jordan I. Huanacuni, jordan.92ihp@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.