Abstract
Obligate freshwater stingrays of the Potamotrygoninae subfamily are endemic to South America and confined to freshwater systems heavily impacted by anthropogenic activities. These pressures often lead to habitat degradation, with unknown impacts on these species’ physiology, behavior, ecology, and survival. Given the increasing pollution of aquatic environments and potential lethal and sublethal effects on exposed biota, this study aimed to review the state of knowledge on the ecotoxicology of species within the Potamotrygoninae subfamily, which includes four genera, namely Heliotrygon, Paratrygon, Plesiotrygon, and Potamotrygon. A systematic review of peer-reviewed articles published in English retrieved seven studies, all focused on Potamotrygon genus stingrays. To date, no investigations have been conducted on species from the other three genera. Five of the reviewed studies focused on the evaluation of metals and metalloids, predominantly assessing mercury in muscle tissue. Additionally, two manually included studies addressed other contaminants, one examined plastic contamination in the intestinal tract of Potamotrygon leopoldi, and the other analyzed morphological deformities in Potamotrygon marquesi, potentially linked to chemical contamination. Some studies focused on human health risk assessments, as freshwater stingrays are routinely consumed by humans in several of their distribution areas. No biomarker assessments have been conducted to date, further restricting evaluations of the species’ health and resilience to environmental stressors. The recovered studies were conducted in four South American countries, with most carried out in Brazil. Knowledge gaps and future research directions are discussed, alongside details of the studies conducted to date.
Introduction
Pollution poses an increasingly severe threat to freshwater ecosystems, driven primarily by human activities such as industrial discharge, agricultural runoff, and urban expansion (Mushtaq et al., 2020). These pollutants, which include a wide range of chemicals such as metals, pesticides, and plastics, are transported through the atmosphere and river runoff, ultimately reaching freshwater systems such as rivers, lakes, and wetlands. Once introduced into these environments, pollutants can accumulate in sediments and water, impacting the health of aquatic organisms (Amoatey and Baawain, 2019; Mushtaq et al., 2020). The effects of chemical pollution in freshwater systems are often more pronounced than in marine environments due to the limited dilution capacity and slower water exchange rates of many freshwater ecosystems (Andričević and Galešić, 2018; Pinheiro et al., 2021). Contaminants such as metals, metalloids, and persistent organic pollutants (POPs) can accumulate in the tissues of aquatic organisms, potentially disrupting crucial biological processes, including alterations in neurological functions, immune response, and reproductive health (Olojo et al., 2005; Vitek et al., 2009; Jezierska et al., 2008). Among the organisms exposed to these contaminants, elasmobranchs (i.e., sharks and rays) are particularly vulnerable to the accumulation and effects of chemical pollutants (Tiktak et al., 2020; Alves et al., 2022). Their susceptibility stems from biological and ecological traits characteristic of K-strategists, such as slow growth, late sexual maturation, and low reproductive rates (Dulvy et al., 2017). However, despite pollution recently being recognized as one of the four primary threats to elasmobranchs (Dulvy et al., 2021), alongside overfishing, habitat loss and degradation and climate change, its impacts remain poorly understood.
Both marine and freshwater rays play an important ecological role through bioturbation during their foraging activities. This behavior is essential for maintaining aquatic ecosystems, as stirring up sediment increases dissolved oxygen concentrations and enhances nutrient availability (D’Andréa et al., 2002; Grew et al., 2024; Nauta et al., 2024). These processes, in turn, promote greater diversity and density of benthic fauna (Thrush, 1991; D’Andréa et al., 2002; Glaspie and Seitz, 2018). Benthic rays are in constant contact with sediment, making them particularly susceptible to bioaccumulation of environmental pollutants as sediments are the ultimate sink for many pollutants, especially chemical ones (de Souza MaChado et al., 2016). This poses a growing threat not only to individual species but also to the overall health of freshwater ecosystems, declining overall biodiversity and disrupting ecological processes such as nutrient cycling and water filtration (Ogidi and Akpan, 2022).
The Potamotrygonidae family comprises a diverse and distinctive lineage of stingrays primarily found in Central and South America (Last et al., 2016). This family is divided into two subfamilies: Styracurinae with one neotropical marine genus (Styracura) and two species (Last et al., 2016), and Potamotrygoninae encompassing a larger group of freshwater stingrays known as potamotrygonins with 38 valid species to date (Silva et al., 2021; Torres et al., 2022). The Potamotrygoninae subfamily is further divided into four genera: Heliotrygon, Paratrygon, Plesiotrygon, and Potamotrygon. These stingrays have evolved specialized physiological adaptations to thrive in freshwater environments (Ballantyne and Robinson, 2010), including a significant reduction in circulating urea levels, a decrease in the size and function of the rectal gland, and renal changes consistent with a more dilute environment (Treberg et al., 2006; Ballantyne and Robinson, 2010).
While numerous studies have explored pollutant contamination in marine elasmobranchs, freshwater species are substantially underrepresented in the literature. This is particularly concerning because freshwater elasmobranchs might be more vulnerable to pollution than their marine counterparts, as the osmoregulatory processes in freshwater environments could potentially enhance the absorption and retention of contaminants (Ikejimba and Sakpa, 2014), among other aspects. In addition, some Potamotrygoninae species are listed as Data Deficient (DD) by the IUCN, indicating lack of sufficient information to make a direct assessment of their risk of extinction, highlighting the need for further in-depth research to effectively implement conservation efforts. Coupled with the more pronounced negative effects of pollutants in freshwater systems, this might increase the risk of adverse impacts on these species. However, the effects of aquatic pollution on this group remain poorly understood and are often underestimated. In this sense, only some contaminant assessments are available for very scarce pollutants for potamotrygonins, and no biochemical or physiological effects have been studied so far.
This review aims to synthesize the current understanding of freshwater pollution (i.e., chemical and plastic) and their potential impacts on potamotrygonins. Additionally, it identifies key knowledge gaps and suggests future research directions to enhance the understanding of the vulnerability of this species group to aquatic pollution. This is important to establish baseline data for future research on this understudied group, highlight critical knowledge gaps that require focus for future research efforts and provide more comprehensive insights on freshwater ecosystem health, relevant for the development of conservation and environmental policies. Furthermore, human health risk assessments are also discussed, as potamotrygonins are heavily consumed in several of their distribution areas.
Methodology
A comprehensive literature search was performed across five major databases: Web of Science, Scielo, PubMed, Scopus, and Google Scholar. The search strategy employed a structured Boolean approach (Greenhalgh and Peacock, 2005) using the terms “POP”, “metal”, “plastic”, “microplastic”, “toxicity”, “freshwater stingray”, “Heliotrygon,” “Paratrygon,” “Plesiotrygon,” and “Potamotrygon”. The search terms were combined and temporal filters were not applied, assessing all available literature with no range of publication year. To expand the scope of the review, the snowball method, as described by Greenhalgh and Peacock (2005), was also applied. This method comprises a convenience sampling method routinely applied when a certain subject is difficult to access or scarce data on a certain subject is available through specific search strings. It consists in analyzing bibliographical references cited in the retrieved reports and searching independently for data on the specific subject of interest, continuing until data saturation (Burns and Grove, 1993). This method helped identify additional relevant publications cited within the initially retrieved studies, including studies that, while not directly focused on pollutant assessments, provided valuable context or supplementary insights related to the topic.
Studies were included if peer-reviewed, concerning Potamotrygoninae species pollution occurrence and levels and published in English. They were excluded if reported in gray literature, did not assess pollutants in Potamotrygoninae species (as some other genera data were retrieved), and published in other languages (e.g., Brazilian Portuguese and Spanish).
While we acknowledge that these filters may introduce potential bias, we chose to apply them due to the inherent challenges in systematically searching gray literature and publications in other languages. To minimize bias in the selection process, three independent authors examined each record for relevance based on predefined criteria, including the study’s focus, freshwater stingray species, and measurable ecological or toxicological outcomes. For each included study, key data were extracted and documented in a standardized spreadsheet. The spreadsheet recorded essential information such as the full title of the study, authors, year of publication, and the journal or source. It also detailed the geographic location of the study, the specific Potamotrygoninae species investigated, and the analyzed pollutants.
Results
A total of seven studies on stingrays belonging to the Potamotrygon genus were identified, with five obtained through systematic database searches and two additional studies incorporated via the snowball method. An additional review article was retrieved but excluded from the analysis as it did not constitute original research. Among the included studies, five investigated contamination by metals and metalloids, one reported plastic ingestion, and another described morphological deformities in a species, exploring potential associations between these deformities and chemical contamination (Table 1). Notably, no studies addressing persistent organic pollutants (POPs) in this genus were identified.
Table 1
| ID | Reference | Authors | Year | Country |
|---|---|---|---|---|
| 1 | Mercury distribution in fish organs and food regimes: Significant relationships from twelve species collected in French Guiana (Amazonian basin) | Régine et al. | 2006 | French Guiana |
| 2 | Mercury biomagnification in the food web of a neotropical stream | Kwon et al. | 2012 | Venezuela |
| 3 | Morphological deformities in the pelvic fin and clasper in specimens of Potamotrygon marquesi (Chondrichthyes: Myliobatiformes: Potamotrygoninae) | Capretz Batista da Silva and da Silva Casas | 2020 | Brazil |
| 4 | First record of plastic ingestion by a freshwater stingray | Trindade et al. | 2023 | Brazil |
| 5 | Levels of Total Mercury and Health Risk Assessment of Consuming Freshwater Stingrays (Chondrichthyes: Potamotrygoninae) of the Brazilian Amazon | Oliveira et al. | 2023 | Brazil |
| 6 | Human exposure to elements through consumption of raw and cooked fish in an urban region of the central Brazilian Amazon biome: Health risks | Meschede et al. | 2024 | Brazil |
| 7 | Abiotic and biotic factors influencing heavy metals pollution in fisheries of the Western Amazon | Echevarría et al. | 2024 | Ecuador |
Publications on Potamotrygon spp. metal and metalloid contamination and transversally-related articles.
The first toxicological study on the Potamotrygon genus was published in 2006, while the most recent studies (n = 2) were published in 2024. Between these dates, one study was published in 2012, another in 2020, and two in 2023. A total of four or five species were examined across these studies: “Potamotrygon hystrix” (Porcupine Freshwater Stingray), corresponding to either P. orbignyi or P. humerosa, Potamotrygon leopoldi (Xingu Freshwater Stingray), Potamotrygon marquesi (Marques Freshwater Stingray), Potamotrygon motoro (Ocellate Freshwater Stingray), and Potamotrygon orbignyi (Reticulate Freshwater Stingray) (Figure 1). The studies were conducted in four South American countries: four in Brazil (Cassiporé River, and Tapajós-Amazon rivers confluence), one in French Guiana (Maroni River), one in Venezuela (Las Marias River), and one in Ecuador (Pastaza, Napo, and Aguarico rivers).
Figure 1
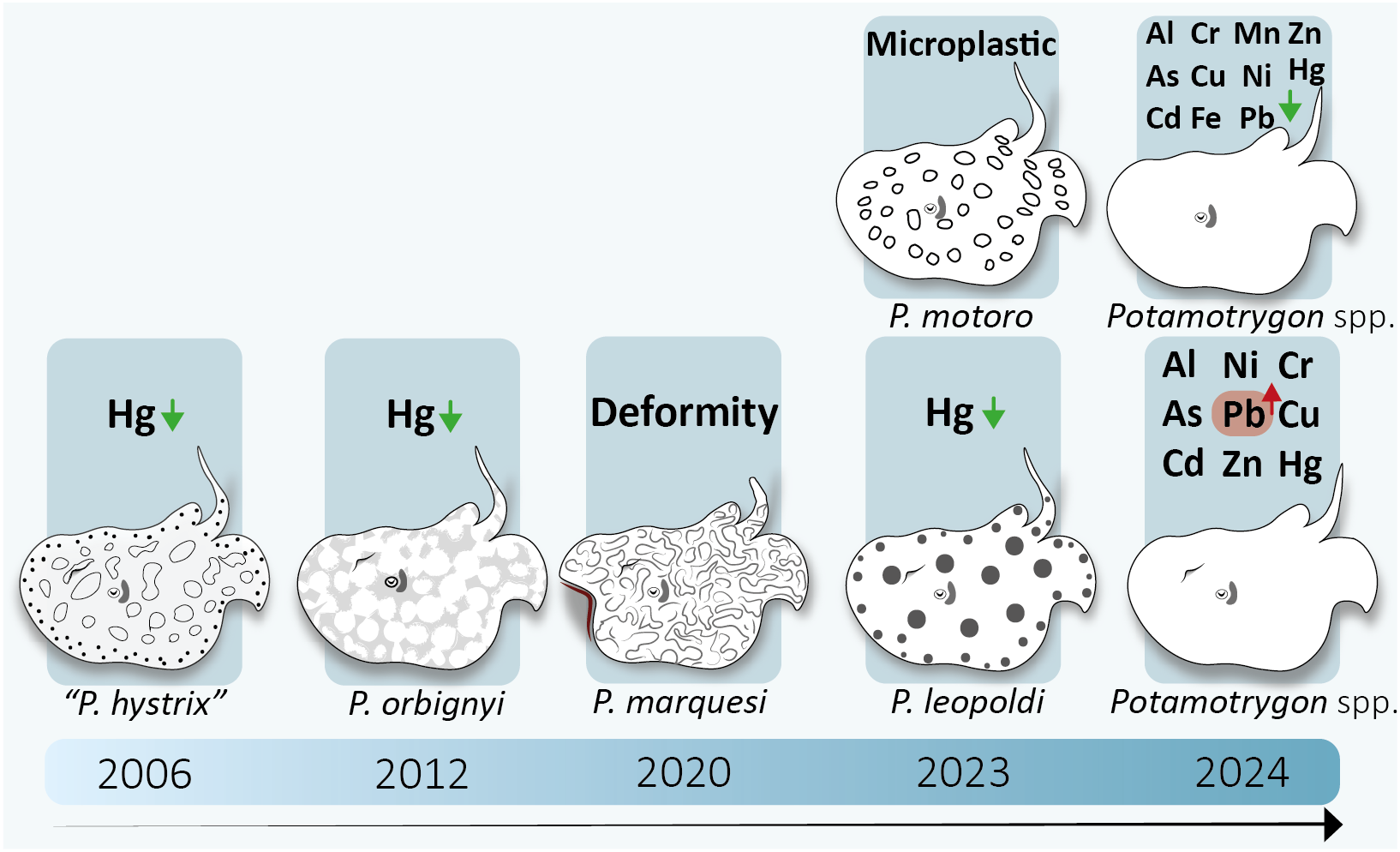
Timeline of the studies with Potamotrygoninae analyzed over 18 years. Each box represents a study. Green arrows indicate values below permissible levels, while red arrows indicate values above permissible levels. Note: Although one paper indicated “P. hystrix”, this species does not occur in the study sampling site (this is discussed ahead).
A total of 35 Potamotrygon individuals were analyzed across the reviewed studies. Mercury (Hg) emerged as the most extensively studied element, reported in all five studies (100%). Zinc (Zn) and lead (Pb) were also commonly investigated, alongside chromium (Cr), copper (Cu), and cadmium (Cd), with each analyzed in 40% (n = 2) of the studies. Less frequently analyzed metals and metalloids included nickel (Ni), arsenic (As), and aluminum (Al), each documented in 20% (n = 1) of the studies. In terms of tissue analysis, metals were assessed in muscle tissue in three studies. Of these, two studies identified a broad spectrum of elements, including Al, As, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn, and Hg. Another study specifically focused on Hg concentrations in muscle tissue. Notably, only one study conducted a comprehensive analysis of Hg across multiple tissues, including gills, liver, kidneys, muscle, stomach, and intestine.
One study addressed deformities in the pelvic fin and clasper skeleton of a Potamotrygon marquesi specimen in the State of Acre, Brazil. The authors hypothesized that the cause of these deformations was exposure to chemical contaminants. Another study reported the first record of plastic ingestion by Potamotrygon leopoldi, in which a total of 81 plastic particles were recorded. These consisted of microplastics (< 5 mm, n = 57) and mesoplastics (5–25 mm, n = 24). Eight types of polymers were identified, the most frequently comprised of artificial cellulose fibers.
Discussion
This is the first comprehensive review of the toxicology of potamotrygonins, highlighting a significant knowledge gap both concerning potamotrygonin health and environmental contamination of their habitats and human health risk assessments due to the consumption of these freshwater stingrays. Only studies focused on the genus Potamotrygon were retrieved based on the selected filter criteria, highlighting the limited scope of existing research.
Freshwater stingrays exhibit a high degree of endemism, which, combined with habitat degradation, commercial and incidental fishing (Araújo et al., 2004; Rincón, 2006; Charvet-Almeida, 2006; Almeida et al., 2008), and negative fishing practices, where individuals are captured and mutilated for tail removal without subsequent use of their meat (Compagno and Cook, 1995; Charvet-Almeida, 2001), place these species at considerable risk. Furthermore, some species, particularly within the genus Potamotrygon, are highly sought after by the aquarium trade (Ross and Schäfer, 2000; Rincón and Charvet-Almeida, 2006; Lasso et al., 2016; Tribuzy-Neto et al., 2020), exacerbating their vulnerability.
The first ecotoxicological assessment retrieved herein, carried out by Régine et al. (2006), reported the distribution of Hg in various “Potamotrygon hystrix” (n=4) tissues, along with other freshwater fish species from French Guiana. However, the potamotrygonin specimens identified as “P. hystrix” probably corresponded to P. orbignyi, since P. histrix is endemic to the southern river basins in South America (Rosa, 1985). The study found Hg concentrations of 0.203 mg kg⁻¹ wet weight (w.w.) in muscle tissue, 0.186 mg kg⁻¹ in the intestine, 0.095 mg kg⁻¹ in the stomach, 0.558 mg kg⁻¹ in the liver, and 0.886 mg kg⁻¹ in the kidneys. No mercury concentration data were reported for gills and no human health risk assessments were conducted.
The second study, by Kwon et al. (2012), focused on Hg levels in muscle tissue of P. orbignyi (n=2) as part of a larger assessment of Hg biomagnification in the food chain of the Las Marias River in Venezuela. The researchers reported a Hg concentration of 0.42 mg kg⁻¹ wet weight (w.w.). One study did not meet the research criteria (i.e., published in Brazilian Portuguese) but brought relevant information on Potamotrygon spp. ecotoxicology by reporting findings from areas near mining sites in the State of Amapá, Brazil, where metal concentrations were assessed in the muscle tissue of 55 species of freshwater fish (Lima et al., 2015). Among the species, only one individual, identified as “P. hystrix” (again probably corresponding to P. orbignyi, or even P. humerosa, considering the study area, as P. histrix does not occur in the State of Amapá) specimen was analyzed, and the concentrations of metals were expressed as mg kg⁻¹ wet weight. The results were as follows: Cd - 0.0055 mg kg⁻¹, Cr - 0.0001 mg kg⁻¹, Cu - 0.0197 mg kg⁻¹, Pb - 0.0437 mg kg⁻¹, and Zn - 0.0652 mg kg⁻¹. No human health risk assessment was carried out.
Following the study by Kwon et al. (2012), a notable gap in ecotoxicological research on Potamotrygon spp. is observed, with a new study published only in 2020 by Capretz Batista da Silva and da Silva Casas (2020). These authors reported pelvic fin and clasper skeleton deformities in P. marquesi, analyzing four male specimens (two juveniles and two adults) collected from the Liberdade and Tarauacá rivers in Acre State, Brazil, and deposited in the Ichthyology Center of the Alto Juruá Valley (NIVAJ) and the Zoology Museum of the University of São Paulo (MZUSP). The deformities affected the pelvic girdle, and right clasper skeleton, and led to severe skeletal and muscular abnormalities in the pelvic fin, including the loss of its terminal components, and were suggested as a result exposure to chemical contaminants. However, no metal determinations or human health risk assessments were conducted.
In 2023, the first study on plastic ingestion in freshwater stingrays and the first assessment of human consumption risks for a Potamotrygon was published. Trindade et al. (2023) documented plastic ingestion in P. leopoldi from the Xingu River in Pará State, Brazil. Among 24 specimens analyzed, 16 of them contained plastic particles, totaling 81 particles in all in their gastrointestinal tract, with 70.4% classified as microplastics and 29.6% as mesoplastics. Microplastics ranged from 0.8 to 4.7 mm, while mesoplastics measured 5.2 to 13.7 mm. Most particles were fibers (62.2%), followed by fragments (35.8%). Color analysis revealed a predominance of blue (33.3%), followed by yellow (18.5%), and other colors in smaller proportions. Eight polymer types were identified, with artificial cellulose fiber being the most common. The findings highlight the growing issue of plastic pollution, even in remote freshwater areas, posing direct and indirect risks to freshwater species through ingestion and contaminated prey (Fossi et al., 2017). Again, no human health risk assessments were performed.
Oliveira et al. (2023) conducted the first assessment of mercury-related risks associated with P. motoro meat consumption. Sixteen specimens (four neonates and 12 juveniles) were sampled from Lake Andiroba, Manaquiri, Amazonas State, Brazil. Muscle analyses revealed no differences in total mercury (THg) concentrations between juveniles and neonates. The authors also conducted a human health risk assessment, indicating that mercury values were below the Brazilian consumption limit for non-predatory fish (0.5 mg kg⁻¹) and piscivorous fish (1.0 mg kg⁻¹) as set by the Brazilian Health Regulatory Agency (Agência Nacional de Vigilância Sanitária, ANVISA, 2022). However, they did not report exact Hg concentrations, limiting further interpretations. They did, however, also carry out Estimated Monthly Intake (EMI) assessments, used to estimate the amount of a contaminant that a person would ingest through food consumption over the course of a month, revealing that rural children were exposed to mercury over three times as much than urban children, while young adults and adults showed lower EMI values. Across all groups, EMI values exceeded the provisional tolerated monthly intake (PMI) of 0.017 mg kg⁻¹ month⁻¹, indicating that only urban youth and adults stayed within safe limits for consuming P. motoro, while the hazard quotient was similar across age groups and genders.
In 2024, two papers were published evaluating metals and metalloids in several freshwater fish species, including Potamotrygon spp. focusing on human health risks. The first was carried out by Meschede et al. (2024), who determined the concentrations of Al, As, Cd, Cr, Cu, Hg, Ni, Pb and Zn in raw and cooked muscle samples of eight fish species consumed in Santarém, Pará State, Brazil, which is located in the confluence of the Tapajós and the Amazon rivers. The potential for non-carcinogenic risks to human health associated with the consumption of cooked fish was evaluated for adults and children. The target species consisted of four carnivorous fish, three omnivores and one detritivore, commercialized and consumed by the population of Santarém, including Potamotrygon spp. Elemental concentrations increased during the cooking process compared to the raw samples but without significant differences. The calculated risk assessment indicated risks to children concerning Hg, for both raw and cooked fish were evaluated. For adults, in one of the scenarios, there was a health risk associated with Hg as a result of the consumption of carnivorous fish. For Potamotrygon spp. (categorized in this study as an omnivorous species), Hg concentrations ranged from 0.1 to 0.3 mg kg-1 w.w. The mean concentrations of elements in raw and cooked fish samples, respectively, in mg kg-1 w.w., were as follows: Al 1.2 and 1.4; Cr: 0.04 and 0.02; Cu 0.1 and 1.4; Hg 0.20 and 0.20; Ni 0.02 and 0.02 and Zn 4.8 and 4.6. Lead presented values above the limit detection, according to the authors, although no specific values were reported. When exposure was combined for all elements, children were at risk when consuming carnivorous and omnivorous fish. For adults, the mixture of elements represented a health risk only for the consumption of carnivorous fish.
The second study carried out in 2024 was conducted by Echevarría et al. (2024), who analyzed the muscle tissue from three Potamotrygon spp. specimens from the Ecuadorian Amazon, reporting the following mean metal and metalloid concentrations, expressed as mg kg-1 w.w.: Al 9.57 ± 7.91; As 0.02 ± 0.02, Cd 0.00 ± 0.00; Cu 0.11 ± 0.05; Fe 5.95 ± 3.19; Mn 0.11 ± 0.05, Ni 0.04 ± 0.06, Pb 0.07 ± 0.02 and Zn 1.59 ± 0.46, while Cr and Hg were not detected. The authors calculated the daily intakes of metals, referring to the estimated amount of a metal that an individual would consume through food on a daily basis. for each specimen. All were below the recommended upper limits for humans by the Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) (USEPA, 1991; FAO, 1997; WHO/FAO, 2009; USEPA, 2023), both based on fish daily intakes for the Ecuadorian Amazon and for the Brazilian Amazon. The Target Hazard Quotient (TQH) or Hazard Index (HI), indices used to estimate non-carcinogenic health risks from long-term exposure to a single contaminant and the sum of THQs for multiple contaminants, respectively, were calculated. All were below 1, indicating no consumer risks.
A preliminary review of the literature on freshwater stingrays reveals a disproportionate emphasis on physiological studies, even before applying exclusion criteria, with ecotoxicological assessments remaining notably scarce. Most physiological research has concentrated on osmoregulatory processes and evolutionary adaptations that enable these species to thrive in freshwater environments (Wood et al., 2002; Duncan et al., 2021). Additionally, studies on reproductive physiology have sought to advance knowledge for captive breeding efforts (Padilha et al., 2021; Ramos et al., 2024), while investigations into stress physiology have aimed to optimize transportation protocols (Brinn et al., 2012; de Lima et al., 2021). Crucially, these studies predominantly focus on stingrays from the genus Potamotrygon, leaving significant gaps in our understanding of other genera within the Potamotrygoninae subfamily. Notably, no studies have examined the physiological impacts of pollutant assimilation and accumulation in these species, creating a knowledge gap in understanding the negative effects of contaminant exposure. Further compounding this knowledge gap is the complete absence of biomarker assessments, essential for evaluating the sub-lethal physiological and cellular effects of environmental contamination. Biomarkers related to oxidative stress, genotoxicity, and enzymatic activity are invaluable early warning indicators of pollution impacts. They could provide key insights into the responses of freshwater stingrays to pollution and habitat degradation.
Freshwater stingrays are frequently subject to species taxonomy and biological studies, yet ecotoxicological studies on this group remain exceedingly limited. Currently, four or five species have been evaluated for pollution, all through in situ studies, although other genera within the Potamotrygoninae subfamily have not yet been evaluated concerning ecotoxicological assessments to date. From a toxicological perspective, species misidentification in freshwater stingray studies can have important implications not only for ecological risk assessments but also for human health. For example, different species may vary significantly in their capacity to bioaccumulate and metabolize pollutants due to differences in diet, trophic level, physiology, and habitat use, leading to different physiological and ecological effects. Misattributing contaminant concentrations to the wrong species can, therefore, obscure true exposure profiles and mask a certain species actual sensitivity or tolerance to contaminants, skewing toxicological thresholds or biomarker data and hinder effective conservation strategies. In addition, mislabeling species can result in inadequate legal protection or misallocated conservation resources, as environmental protections efforts are often species-specific. Furthermore, species misidentification may also lead to inaccurate toxicological risk assessments; if contaminant levels are underestimated in edible tissues due to species misidentification, it may result in an underappreciation of potential human health risks due to pollutants. It may also mask localized risks, as different freshwater stingray species inhabit different river systems or microhabitats, preventing targeted pollutant mitigation efforts.
The unique evolutionary adaptations to freshwater ecosystems make freshwater stingrays (Wood et al., 2002; Duncan et al., 2010b) particularly compelling subjects for further ecotoxicological studies. For instance, they present distinct osmoregulatory strategies compared to marine elasmobranchs. The latter are osmoconformers, retaining high concentrations of urea and trimethylamine N-oxide in their tissues to maintain osmotic balance with seawater (Hammerschlag, 2006; Laxson et al., 2011), while freshwater stingrays are hyperosmotic regulators, continuously excreting water and conserving ions, as their internal osmolarity is much higher than their dilute freshwater surroundings, and they do not retain urea as marine species (Ballantyne and Robinson, 2010). This may lead to different responses to the assimilation and accumulation of toxic compounds. The same is noted for gill permeability and ion transport, as freshwater species have more permeable gills and an increased reliance on active ion transport to maintain homeostasis (Lee et al., 2022), which increase the surface area and activity at the gill epithelium. This, in turn, can enhance metal uptake through cation transporters (Goss et al., 1998), which would make freshwater rays more vulnerable to waterborne contamination compared to marine elasmobranchs.
Other questions arise when comparing freshwater stingrays to marine elasmobranchs. For example, does selenium, an essential metalloid known for its protective effects against mercury and potentially other elements in several marine elasmobranchs (Pantoja-Echevarría et al., 2021; Wang et al., 2023), confer similar benefits to freshwater stingrays? Also, is oxidative stress, a physiological condition that occurs when the production of reactive oxygen species exceeds the body’s antioxidant defense capacity, leading to cellular damage, a consistent response to pollutant exposure in these species, as observed in various marine counterparts, including sedentary species (Vélez-Alavez et al., 2013; Wosnick et al., 2021; Bielmyer-Fraser et al., 2023)? Finally, are the physiological and behavioral effects of pollutants more pronounced in freshwater species due to their unique adaptations? These critical questions remain unanswered, emphasizing the urgent need for research to address these knowledge gaps and to better understand the vulnerability of freshwater stingrays to environmental contaminants.
Fishing in South American freshwater basins provides an essential source of affordable, high-quality protein, with fish playing a vital role in the diets of riverside and indigenous communities. In this sense, Potamotrygoninae stingrays are widely consumed in several regions, with reports documenting species such as Potamotrygon orbignyi and P. scobina sold in municipal markets in the State of Pará (Charvet-Almeida, 2001) and P. motoro and Paratrygon aiereba consumed along the Amazon and Rio Negro basins (Duncan et al., 2010a; Araújo, 2005), posing potential health risks related to the intake of contaminated meat. Despite this, few studies have investigated the risks associated with consuming freshwater stingrays, resulting in a critical knowledge gap. This gap is particularly concerning given that many people that consume these stingrays are in socially vulnerable situations, facing increased health risks due to chronic neglect of their communities’ needs. Riparian residents and fishers, in particular, exhibit a strong dependence on fishing activities with a deep cultural and economic connection to river dynamics (Scherer, 2004; Cabral and Almeida, 2006; Fraxe et al., 2007; Isaac and Almeida, 2011). They are, thus, heavily reliant on fish for sustenance, and are among the most at-risk groups, highlighting the urgent need for further research and policy attention concerning contamination of fish resources. Therefore, future studies should prioritize evaluating elemental concentrations in the most commonly consumed freshwater stingray species across different regions.
It is also paramount to incorporate consumption calculations tailored to specific demographic groups, as fish consumption patterns vary across regions, influenced by local culture, regional cuisine, and species availability (Alho et al., 2021). In Northern and Southern South America, for example, where the Amazon and other significant basins like the Orinoco and Paraná-Paraguay provide abundant fish resources, average fish consumption reaches approximately 17.54 kg per capita annually, surpassing the WHO recommended minimum of 12 kg per capita (Food and Agriculture Organization, 2012; Alho et al., 2021). Thus, studies should also include calculations for Target Hazard Quotient (THQ), Total Cancer Risk (TCR), and Hazard Index (HI), considering regional or local consumption rates wherever possible, as significant differences exist between various locations and populations, also including different age groups, i.e., infants, children, adolescents, and adults of both sexes. These studies should also include calculations for Target Hazard Quotient (THQ), Total Cancer Risk (TCR), and Hazard Index (HI), the main human health risk assessment indices employed in these type of assessments, considering regional or local consumption rates wherever possible, as significant differences exist between various locations and populations.
Lastly, integrating pollution- and habitat-degradation driven health impairments into species evaluations such as those considered in the IUCN Red List criteria for assessing species’ risk categories can provide a more comprehensive scenario of extinction risks. This allows the recognition of chronic contaminant exposure as a key threat, improving the reassessment of Data Deficient or Near Threatened species, and informing evidence-based habitat protection and policy interventions. The issue of data deficiency, in fact, comprises a critical barrier to effective conservation planning, as it seems that a DD status may mask a high risk of extinction (Howard and Bickfors, 2014). In this sense, freshwater elasmobranchs often lack the ecological, population, and toxicological data required for IUCN Red List assessments, leading them to remain unassessed or be categorized as DD, despite evidence of habitat degradation, overfishing, and pollution. This hinders the integration of emerging threats such as contaminant exposure, among others, into global conservation frameworks. Some exceptions are noted, such as Potamotrygon boesemani (Suriname Freshwater Stingray) and Potamotrygon magdalenae (Magdalena Freshwater Stingray), both of which have recently been assessed for the first time and reassessed, respectively (Rosa et al., 2024; Mejía-Falla et al., 2024), being classified as Near Threatened (NT) due to population declines linked to habitat degradation. Given this, future ecotoxicological studies are necessary to generate data that can inform assessments of these threats for other species. This need is particularly urgent as, to date, only about 6% of chondrichthyan species have sufficient pollution-related data to confirm its role in population declines and increased extinction risk (Dulvy et al., 2021). The IUCN Shark Specialist Group has just concluded a comprehensive review of Neotropical freshwater stingray species assessments, focusing on those not evaluated in over a decade, species never previously assessed and recently described species, incorporating pollution aspects, among others. This effort is expected to result in more species being classified as at risk of extinction due to habitat degradation and pollution. Furthermore, species currently listed as Data Deficient are likely to be reclassified into higher-risk categories as new data, particularly regarding pollution, become available.
Conclusions
This literature review has made the significant knowledge gap regarding the toxicology of freshwater stingrays extremely evident, particularly for species within the Heliotrygon, Paratrygon, and Plesiotrygon genera, for which no data are currently available. This is especially concerning given the severe threats freshwater ecosystems face due to habitat degradation and pollution. Such conditions may lead to chronic stress in stingray populations caused by contaminant exposure, with unknown effects on their biology, ecology, behavior, and survival. Moreover, riverine community consumption of freshwater stingrays poses additional risks, as contaminant ingestion could exacerbate health issues in these already vulnerable populations. It is also critical to consider the physiological impacts of pollution, which could impair the stress response of animals captured and transported for aquarium purposes, potentially resulting in mortality that remains unexplored. Many freshwater stingray species are at risk of extinction and have just been reassessed or assessed for the first time. For some, declining habitat quality is already a criterion for their categorization into threatened categories, and ecotoxicological assessments could provide additional evidence to further support these classifications. Future studies must aim to generate data on a broader range of species and evaluate a wider variety of pollutants, particularly organic contaminants, which are a well-documented threat in freshwater environments yet remain unstudied in the context of this subfamily. This type of research is paramount for improving conservation strategies and mitigating pollution impacts on these threatened species.
Statements
Author contributions
NDB: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. NW: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. APC: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. EPG: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. RDL: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. PC: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. RAH-D: Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) (RAHD), through a Jovem Cientista do Nosso Estado 2021-2024 grant (process number E26/201.270/2021) and Jovem Pesquisador Fluminense com Vínculo em ICTS do Estado do Rio de Janeiro grant (process number E-26/2010.300/2022), as well as by the Brazilian National Council of Scientific and Technological Development (CNPq), through a productivity grant (process number 308811/2021-6). PC acknowledges a visiting researcher grant (FUNCAP PVS-0215-00123.02.00/23) and a Save Our Seas Foundation Conservation Fellowship (SOSF 588). The implementation of the Projeto Pesquisa Marinha e Pesqueira is a compensatory measure established by the Conduct Adjustment Agreement under the responsibility of the PRIO company, conducted by the Federal Public Ministry-MPF/RJ. This work was also prepared with resources from the Judicial Agreement Term signed between the Federal Public Prosecutor’s Office, the Public Prosecutor’s Office of the State of Paraná and Petrobras, with the intervention of the Brazilian Fund for Biodiversity (Funbio) and the Chico Mendes Institute for Biodiversity Conservation (ICMBio), within the scope of the Sentence Enforcement process No. 5001337-92.2012.4.04.7008, arising from Public Civil Actions 0000041-91.2010.404.7008 (PR) and No. 200270080002601, brought by the IAP -PARANÁ ENVIRONMENTAL INSTITUTE and by the MPPR and MPF, respectively against PETRÓLEO BRASILEIRO S/A -PETROBRÁS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the author(s) used ChatGPT to enhance grammar, improve syntax, and ensure a more fluid and polished text. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Alho T. V. L. Rosa M. Y. de O. de A. P. M. Lobato F. H. S. (2021). Ver-o-freguês”: o perfil socioeconômico do consumidor de peixe do Mercado de Ferro, Ver-O-Peso, Belém (PA). Humanidades Inovação8, 335–343.
2
Almeida M. P. de Barthem R. B. da Viana A. S. Charvet-Almeida P. C. (2008). Diversidade de raias de água doce (Chondrichthyes: Potamotrygonidae) no Estuário Amazônico. Arquivo Ciências do Mar.2, 2–89. doi: 10.32360/acmar.v41i2.6067
3
Alves L. M. F. Lemos M. F. L. Cabral H. Novais S. C. (2022). Elasmobranchs as bioindicators of pollution in the marine environment. Mar. pollut. Bull.176, 113418. doi: 10.1016/j.marpolbul.2022.113418
4
Amoatey P. Baawain M. S. (2019). . Effects of pollution on freshwater aquatic organisms. Water Environ. Res.91, 1272–1287. doi: 10.1002/wer.1221
5
Andričević R. Galešić M. (2018). . Contaminant dilution measure for the solute transport in an estuary. Adv. Water Resour.117, 65–74. doi: 10.1016/j.advwatres.2018.05.005
6
ANVISA (2022). Agência nacional de vigilância sanitária do brasil. Normative instruction - in no. 160. Establishes the maximum tolerated limits (MRL) of contaminants in food. Available online at: https://www.in.gov.br/en/web/dou/-/instrucao-normativa-in-n-160-de-1-dejulho-de-2022-413367081 (Accessed 04 november 2024).
7
Araújo M. L. (2005). Plano de Monitoramento de Arraias de Água Doce–Rio Negro, Estado do Amazonas; Relatório Final do IBAMA Vol. 100p (Manaus, Brazil: IBAMA).
8
Araújo M. L. G. Charvet-Almeida P. Almeida M. P. & Pereira H. (2004). “Freshwater stingrays (Potamotrygonidae): status, conservation and management challenges,” in Information document, CITES animals committee, AC20, inf, vol. 8. , 6.
9
Ballantyne J. S. Robinson J. W. (2010). Freshwater elasmobranchs: a review of their physiology and biochemistry. J. Comp. Physiol. B180, 475–493. doi: 10.1007/s00360-010-0447-0
10
Bielmyer-Fraser G. K. Franks B. Somerville R. Hueter R. Newton A. L. Fischer C. (2023). Tissue metal concentrations and antioxidant enzyme activity in western north Atlantic white sharks (Carcharodon carcharias). Aquat Toxicol.261, 106641. doi: 10.1016/j.aquatox.2023.106641
11
Brinn R. P. Marcon J. L. McComb D. M. Gomes L. C. Abreu J. S. Baldisseroto B. (2012). Stress responses of the endemic freshwater cururu stingray (Potamotrygon cf. histrix) during transportation in the Amazon region of the Rio Negro. Comp. Biochem. Physiol. A Mol. Integr. Physiol.162, 139–145. doi: 10.1016/j.cbpa.2011.07.004
12
Burns N. Grove S. K. (1993). The Practice of Nursing Research. Conduct, Critique & Utilization (4th ed.). Saunders.
13
Cabral J. W.C. Almeida O. T. (2006). “Avaliação do mercado da indústria pesqueira na Amazônia,” in A indústria pesqueira na Amazônia. Projeto Manejo dos Recursos Naturais da Várzea. Ed. AlmeidaO. T. (Manaus, IBAMA/ProVárzea), 17–39.
14
Capretz Batista da Silva J. P. da Silva Casas A. L. (2020). Morphological deformities in the pelvic fin and clasper in specimens of Potamotrygon marquesi (Chondrichthyes: Myliobatiformes: Potamotrygoninae). J. Appl. Ichthyology36, 189–196. doi: 10.1111/jai.14005
15
Charvet-Almeida P. (2001). Ocorrência, biologia e uso das raias de água doce na Baía de Marajó (Pará, Brasil), com ênfase na biologia de Plesiotrygon iwamae (Chondrichthyes : Potamotrygonidae) (Universidade Federal do Pará & Museu Paraense Emílio Goeldi: Thesis dissertation. Belém).
16
Charvet-Almeida P. (2006). História natural e conservação das raias de água doce (Chondrichthyes, Potamotrygonidae) no médio Rio Xingu, área de influência do projeto hidrelétrico de Belo Monte (Pará, Brasil) (dissertation, Universidade Federal da Paraíba: Unpubl. PhD thesis).
17
Compagno L. J. V. Cook S. F. (1995). The exploitation and conservation of freshwater elasmobranchs: status of taxa and prospects for the future. In: The Biology of Freshwater Elasmobranchs. Oetinger, M. I. & Zorzi, G. D. J. Aquariculture Aquat. Sci.7, 62–90.
18
D’Andréa A. F. Silva M. L. N. Curi N. Siqueira J. O. Carneiro M. (2002). Atributos biológicos indicadores da qualidade do solo em sistemas de manejo na região do cerrado no sul do estado de Goiás. Rev. Bras. Ciênc. Solo26, 913–923. doi: 10.1590/S0100-06832002000400008
19
de Lima C. L. Morales-Gamba R. D. Malcher Neto T. S. Barcellos J. F. M. Heinzmann B. M. Schmidt D. et al . (2021). Eugenol and Lippia alba essential oils as effective anesthetics for the Amazonian freshwater stingray Potamotrygon wallacei (Chondrichthyes, Potamotrygonidae). Fish Physiol. Biochem.47, 2101–2120. doi: 10.1007/s10695-021-01029-1
20
de Souza MaChado A. A. Spencer K. Kloas W. Toffolon M. Zarfl C. (2016). Metal fate and effects in estuaries: A review and conceptual model for better understanding of toxicity. Sci. Total Environ.541, 268–281. doi: 10.1016/j.scitotenv.2015.09.045
21
Dulvy N. K. Pacoureau N. Rigby C. L. Pollom R. A. Jabado R. W. Ebert D. A. et al . (2021). Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol.31, 4773–4787.e8. doi: 10.1016/j.cub.2021.08.062
22
Dulvy N. K. Simpfendorfer C. A. Davidson L. N. K. Fordham S. V. Bräutigam A. Sant G. et al . (2017). Challenges and priorities in shark and ray conservation. Curr. Biol.27, R565–R572. doi: 10.1016/j.cub.2017.04.038
23
Duncan W. P. da Costa O. T. F. Sakuragui M. M. Fernandes M. N. (2010a). Functional morphology of the gill in amazonian freshwater stingrays (Chondrichthyes: potamotrygonidae): implications for adaptation to freshwater. Physiol. Biochem. Zoology83, 19–32. doi: 10.1086/605458
24
Duncan W. P. Inomata S. O. Fernandes M. N. (2010b). Comércio de raias de água doce na região do médio Rio Negro, estado do Amazonas, Brasil. Rev. Bras. Engenharia Pesca5, 13–22. doi: 10.18817/repesca.v5i2.265
25
Duncan W. P. MaChado R. N. Fernandes M. N. (2021). . Environmentally-induced osmoregulation in Neotropical freshwater stingrays (Myliobatiformes: Potamotrygoninae) after controlling for phylogeny. Comp. Biochem. Physiol. A Mol. Integr. Physiol.262, 111076. doi: 10.1016/j.cbpa.2021.111076
26
Echevarría G. Lujan N. K. Montoya J. Granda-Albuja M. G. Valdiviezo-Rivera J. Sánchez F. et al . (2024). Abiotic and biotic factors influencing heavy metals pollution in fisheries of the Western Amazon. Sci Total Environ.908, 168506. doi: 10.1016/j.scitotenv.2023.168506
27
FAO (1997). “Guidelines for the simple evaluation of dietary exposure to food additives,” in Codex alimentarius commission. Available at: https://www.fao.org/input/download/standards/6/cxg_003e.pdf (Accessed January 1, 2025).
28
Food and Agriculture Organization (2012). The state of world fisheries and aquaculture (Rome, FAO: FAO Fisheries and Aquaculture Department), 209. Available at: http://www.fao.org/docrep/016/i2727e/i2727e.pdf (Accessed January 1, 2025).
29
Fossi M. Panti C. (2017). Sentinel species of marine ecosystems. Oxford Res Encycl Environ Sci.
30
Fraxe T. J. P. Pereira H. S. Witkoski A. C. (2007). Comunidades ribeirinhas amazônicas: modos de vida e uso dos recursos naturais (Manaus: EDUA).
31
Glaspie C. N. Seitz R. D. (2018). Habitat complexity mediates benthic predator-prey interactions in Chesapeake Bay. PLoS ONE13 (10), e0205162. doi: 10.1371/journal.pone.0205162
32
Goss G. G. Perry S. F. Fryer J. N. Laurent P. (1998). Gill morphology and acid-base regulation in freshwater fishes. Comp. Biochem. Physiol. A.119, 107–115. doi: 10.1016/S1095-6433(97)00401-7
33
Greenhalgh T. Peacock R. (2005). Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. Bmj331, 1064–1065. doi: 10.1136/bmj.38636.593461.68
34
Grew M. Gaston T. F. Griffin A. S. Duce S. J. Raoult V. (2024). Ray bioturbation rates suggest they shape estuary processes. Remote Sens. Ecol. Conserv. doi: 10.1002/rse2.411
35
Hammerschlag N. (2006). Osmoregulation in elasmobranchs: a review for fish biologists, behaviourists and ecologists. Mar. Freshw. Behav. Physiol.39, 209–228. doi: 10.1080/10236240600815820
36
Howard S. D. Bickfors D. P. (2014). Amphibians over the edge: silent extinction risk of Data Deficient species Vol. 20 (DP Bickford. Diversity and distributions: SD Howard), 837–846.
37
Ikejimba C. C. Sakpa S. O. (2014). Comparative study of some heavy metals’ concentrations in water and Tympanotonus fuscatus var radula samples of Egbokodo River, Warri, Nigeria. Int J Modern Biological Res2014, 7–15.
38
Isaac V. J. Almeida M. C. (2011). El consumo de pescado en la Amazonía Brasileña. Copescaalc Documento Ocasional Vol. 13 (Roma: FAO), 43.
39
Jezierska B. Lugowska K. Witeska M. (2008). The effects of heavy metals on embryonic development of fish. Fish Physiol. Biochem.35, 625–640. doi: 10.1007/s10695-008-9284-4
40
Kwon S. Y. McIntyre P. Flecker A. Campbell L. (2012). Mercury biomagnification in the food web of a neotropical stream. Sci. Total Environ.417–418, 92–97. doi: 10.1016/j.scitotenv.2011.11.060
41
Lasso C. A. Rosa R. S. Morales-Betancourt M. A. Garrone-Neto y Carvalho D.M. (2016). “XV. Rayas de agua dulce (Potamotrygonidae) de Suramérica. Parte II: Colombia, Brasil, Perú, Bolivia, Paraguay, Uruguay y Argentina. Serie Editorial Recursos Hidrobiológicos y Pesqueros Continentales de Colombia,” in Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH) (Bogotá, D. C., Colombia), 435.
42
Last P. White W. T. De M. R. Séret B. Stehmann M. F. W. et al . (2016). Rays of the world (Australia: CSIRO publishing), 790.
43
Laxson C. J. Condon N. E. Drazen J. C. Yancey P. H. (2011). Decreasing urea:trimethylamine N-oxide ratios with depth in chondrichthyes: a physiological depth limit? Physiol. Biochem. Zool84, 494–505. doi: 10.1086/661774
44
Lee C. E. Charmantier G. Lorin-Nebel C. (2022). Mechanisms of Na⁺ uptake from freshwater habitats in animals. Front. Physiol.13. doi: 10.3389/fphys.2022.1006113
45
Lima D. P. Santos C. de S. R. Yoshioka E. T. O. Bezerra R. M. (2015). Contaminação por metais pesados em peixes e água da bacia do rio Cassiporé, Estado do Amapá, Brasil. Acta Amaz.45, 405–414. doi: 10.1590/1809-4392201403995
46
Mejía-Falla P. A. Márquez-Velázquez V. Navia A. F. (2024). Potamotrygon magdalenae. The IUCN Red List of Threatened Species 2024: e.T161385A124475262. doi: 10.2305/IUCN.UK.2024-1.RLTS.T161385A124475262.en
47
Meschede M. S. C. Zagui G. S. Celere B. S. MaChado G. P. Gomes-Silva G. Santos D. V. et al . (2024). Human exposure to elements through consumption of raw and cooked fish in an urban region of the central Brazilian Amazon biome: Health risks. Environ. pollut.347, 123728. doi: 10.1016/j.envpol.2024.123728
48
Mushtaq N. Singh D. V. Bhat R. A. Dervash M. A. Hameed O. (2020). “Freshwater contamination: sources and hazards to aquatic biota,” in Freshwater pollution dynamics and remediation. Eds. QadriH.BhatR. A.MehmoodM. A.DarG. H. (Springer, Singapore), 27–50. doi: 10.1007/978-981-13-8277-2_3
49
Nauta J. Leurs G. Nieuwenhuis B. O. Mathijssen D. R. A. H. Olff H. Bouma T. J. et al . (2024). Bioturbation by benthic stingrays alters the biogeomorphology of tidal flats. Ecosystems27, 493–507. doi: 10.1007/s10021-024-00901-4
50
Ogidi O. I. Akpan U. M. (2022). “Aquatic biodiversity loss: impacts of pollution and anthropogenic activities and strategies for conservation,” in Biodiversity in Africa: potentials, threats and conservation. Ed. Chibueze IzahS. (Springer Nature, Singapore), 421–448. doi: 10.1007/978-981-19-3326-4_16
51
Oliveira A. T. de Rodrigues P.de A. Ramos Filho A. M. da S. M. F. da S. A. R. et al . (2023). Levels of total mercury and health risk assessment of consuming freshwater stingrays (Chondrichthyes: potamotrygoninae) of the Brazilian Amazon. Int. J. Environ. Res. Public Health20, 6990. doi: 10.3390/ijerph20216990
52
Olojo E. Olurin K. B. Mbaka G. Oluwemimo A. D. (2005). Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead. Afr. J. Biotechnol.4, 117–122.
53
Padilha F. L. A. Colbachini H. Ramos S. D. Reisfeld L. C. Henrique P. C. Leite R. F. et al . (2021). Validation of manual semen collection methods and sperm evaluation in living freshwater stingrays (Potamotrygon falkneri) kept in ex-situ conditions. Environ. Biol. Fish104, 463–469. doi: 10.1007/s10641-021-01086-8
54
Pantoja-Echevarría L. M. Marmolejo-Rodríguez A. J. Galván-Magaña F. Elorriaga-Verplancken F. R. Tripp-Valdez A. Tamburin E. et al . (2021). Mercury and selenium concentrations in different tissues of brown smooth-hound shark (Mustelus henlei) from the western coast of Baja California Sur, Mexico. Mar. pollut. Bull.170, 112609. doi: 10.1016/j.marpolbul.2021.112609
55
Pinheiro J. P. S. Windsor F. M. Wilson R. W. Tyler C. R. (2021). Global variation in freshwater physico-chemistry and its influence on chemical toxicity in aquatic wildlife. Biol. Rev. Camb Philos. Soc.96, 1528–1546. doi: 10.1111/brv.12711
56
Ramos S. D. Jorge-Neto P. N. Colbachini H. Gricio E. A. de Moraes Francisco F. Padilha F. L. A. et al . (2024). Cryopreservation of potamotrygon stingrays’ Semen: enhancing one conservation effort. J. Zoological Botanical Gardens5, 305–315. doi: 10.3390/jzbg5020021
57
Régine M.-B. Gilles D. Yannick D. Alain B. (2006). Mercury distribution in fish organs and food regimes: Significant relationships from twelve species collected in French Guiana (Amazonian basin). Sci. Total Environ.368, 262–270. doi: 10.1016/j.scitotenv.2005.09.077
58
Rincón Filho G. (2006). Aspectos taxonômicos, alimentação e reprodução da raia de água doce Potamotrygon orbignyi (Castelnau) (Elasmobranchii: Potamotrygonidae) no Rio Paranã, Tocantins 2006. iv, 132 f. Thesis (doctorate) - Universidade Estadual Paulista, Instituto de Biociências.
59
Rincón G. Charvet-Almeida P. (2006). O monitoramento da pesca ornamental de raias de água doce está sendo efetivo? Problemas e possíveis soluções nas esferas envolvidas. Soc Bras. para o Estud. Elasmobrânquios - SBEEL16.
60
Rosa R. S. (1985). A systematic revision of the south american freshwater stingrays (Chondrichthyes: potamotrygonidae). Ph.D. Dissertation (Williamsburg: College of William and Mary), 523.
61
Rosa R. Torres Y. T. P. Charvet P (2024). Potamotrygon boesemani. The IUCN Red List of Threatened Species 2024: e.T188065333A188067184. doi: 10.2305/IUCN.UK.2024-1.RLTS.T188065333A188067184.e
62
Ross R. A. Schäfer F. (2000). Aqualog süSswasser rochen - freshwater rays (Germany: Mörfelden-Walldorf).
63
Scherer E. F. (2004). “Mosaico terra-água: a vulnerabilidade social ribeirinha na Amazônia - Brasil,” in VIII Congresso Luso-brasileiro de Ciências Sociais (Universidade de Coimbra, Portugal. Disponível em). Available at: https://www.ces.uc.pt/lab2004/pdfs/EliseScherer.pdf (accessed January 1, 2025).
64
Silva J. P. C. B. Rosa R. S. Loboda T. S. Lasso C. A. (2021). Taxonomy needs rigor: a response to Roberts’ (2020). Zootaxa5052, 597–600. doi: 10.11646/zootaxa.5052.4.10
65
Thrush S. F. (1991). Spatial patterns in soft-bottom communities. Trends Ecol. Evol.6, 75–79. doi: 10.1016/0169-5347(91)90178-Z
66
Tiktak G. P. Butcher D. Lawrence P. J. Norrey J. Bradley L. Shaw K. et al . (2020). Are concentrations of pollutants in sharks, rays and skates (Elasmobranchii) a cause for concern? A systematic review. Mar Pollut Bull.160, 111701. doi: 10.1016/j.marpolbul.2020.111701
67
Torres Y. Faria V. V. Charvet P. (2022). Current status and future perspectives of Neotropical freshwater stingrays (Potamotrygoninae, Myliobatiformes) genetics. Environ. Biol. Fish105, 1111–1127. doi: 10.1007/s10641-022-01320-x
68
Treberg J. R. Speers-Roesch B. Piermarini P. M. Ip Y. K. Ballantyne J. S. Driedzic W. R. (2006). The accumulation of methylamine counteracting solutes in elasmobranchs with differing levels of urea: a comparison of marine and freshwater species. J. Exp. Biol.209, 860–870. doi: 10.1242/jeb.02055
69
Tribuzy-Neto I. A. Beltrão H. Benzaken Z. S. Yamamoto K. C. (2020). Analysis of the ornamental fish exports from the amazon state, Brazil. Boletim do Instituto de Pesca46 (4), e554. doi: 10.20950/1678-2305.2020.46.4.554
70
Trindade P. A. A. Brabo L. D. M. Andrades R. Azevedo-Santos V. M. Andrade M. C. Candore L. et al . (2023). First record of plastic ingestion by a freshwater stingray. Sci. Total Environ.880, 163199. doi: 10.1016/j.scitotenv.2023.163199
71
USEPA (1991). “U.S. Environmental protection agency (EPA),” in Risk assessment guidance for superfund (RAGS), volume I: human health evaluation manual (Part B). Available at: https://rais.ornl.gov/documents/HHEMB.pdf (Accessed January 1, 2025).
72
USEPA (2023). “U.S. Environmental protection agency (EPA),” in Regional screening levels (RSLs) – user’s guide. Available at: https://www.epa.gov/risk/regional-screening-levels-rsls-users-guide (Accessed January 1, 2025).
73
Vélez-Alavez M. Labrada-Martagón V. Méndez-Rodriguez L. C. Galván-Magaña F. Zenteno-Savín T. (2013). Oxidative stress indicators and trace element concentrations in tissues of mako shark (Isurus oxyrinchus). Comp. Biochem. Physiol. A.165, 508–514. doi: 10.1016/j.cbpa.2013.03.006
74
Vitek M. P. Brown C. M. Colton C. A. (2009). APOE genotype-specific differences in the innate immune response. Neurobiol Aging.30(9):1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014
75
Wang M. H. Chen C. F. Albarico F. P. J. B. Tsai W. P. Chen C. W. Dong C. D. (2023). Mercury and selenium concentrations and their toxicological implications in silky sharks (Carcharhinus falciformis) in the northwestern Indian Ocean. Reg. Stud. Mar. Sci.66, 103165. doi: 10.1016/j.rsma.2023.103165
76
WHO/FAO (2009). “World health organization (WHO), & Food and agriculture organization (FAO),” in Principles and methods for the risk assessment of chemicals in food (Environmental Health Criteria 240). Available at: https://apps.who.int/iris/bitstream/handle/10665/44027/9789241597470_eng.pdf (Accessed January 1, 2025).
77
Wood C. M. Matsuo A. Y. O. Gonzalez R. J. Wilson R. W. Patrick M. L. Val A. L. (2002). Mechanisms of ion transport in Potamotrygon, a stenohaline freshwater elasmobranch native to the ion-poor blackwaters of the Rio Negro. J. Exp. Biol.205, 3039–3054. doi: 10.1242/jeb.205.19.3039
78
Wosnick N. Chaves A. P. Leite R. D. Nunes J. L. S. Saint’Pierre T. D. Willmer I. Q. et al . (2021). Nurse sharks, space rockets and cargo ships: metals and oxidative stress in a benthic, resident and large-sized mesopredator, Ginglymostoma cirratum. Environ. pollut.288, 117784. doi: 10.1016/j.envpol.2021.117784
Summary
Keywords
contamination, ecotoxicology, freshwater batoids, human health risks, metals and metalloids, potamotrygonins
Citation
Barbosa ND, Wosnick N, Chaves AP, Giareta EP, Leite RD, Charvet P and Hauser-Davis RA (2025) Ecotoxicology of Potamotrygoninae freshwater stingrays: bioaccumulation, toxicological risks, and conservation implications. Front. Mar. Sci. 12:1582093. doi: 10.3389/fmars.2025.1582093
Received
23 February 2025
Accepted
07 May 2025
Published
03 June 2025
Volume
12 - 2025
Edited by
Matteo Oliva, Centro Interuniversitario di Biologia Marina ed Ecologia Applicata “G. Bacci” di Livorno (CIBM), Italy
Reviewed by
Joanna Giannessi, University of Pisa, Italy
Jairo Zocche, University of the Extreme South of Santa Catarina, Brazil
Updates
Copyright
© 2025 Barbosa, Wosnick, Chaves, Giareta, Leite, Charvet and Hauser-Davis.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Ann Hauser-Davis, rachel.hauser.davis@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.