Abstract
Explosives released by dumped warfare material pose a threat to the marine environment and can enter the marine food web. 2,4,6-Trinitrotoluene (TNT) is one of the most used explosives in munitions and is, therefore, of special interest. To test the uptake, depuration, and potential biotransformation of TNT, common blue mussels (Mytilus edulis) from the German North Sea were exposed to different TNT concentrations in two laboratory experiments (first experiment, 48-h exposure to TNT concentrations of 0, 0.625, 1.25, and 2.5 mg/L; second experiment, 24-h exposure to 0 and 5 mg/L deuterated TNT) followed by recovery phases in clean artificial seawater (first experiment, 60-h recovery; second experiment, 12-h recovery). Water samples and mussel soft bodies were analyzed for TNT and its metabolites 2-amino-4,6-dinitrotoluene (2-ADNT), 4-amino-2,6-dinitrotoluene (4-ADNT), and 2,4-diamino-6-nitrotoluene (2,4-DANT) using Gas Chromatography – Tandem Mass Spectrometry (GC-MS/MS) techniques. The results showed a continuous uptake of dissolved TNT during exposure and a rapid depuration during the recovery phase, independent of the original TNT exposure concentrations. Furthermore, evidence for the biotransformation of TNT is shown by the presence of labelled ADNTs both in mussel soft bodies analyzed within the recovery phase and in water sampled during the recovery phase. Overall, 57% to 76% of the measured concentration was biotransformed within the first 4 h after the exposure.
1 Introduction
Marine waters worldwide are endangered by dumped warfare material, including munitions, warships, and unexploded ordnances (UXO). The main sources of this material originate from war activities and dumping events after the First and Second World Wars (Beddington and Kinloch, 2005; Böttcher et al., 2011). The problem of munitions at sea is global, but most munition dumpsites are located in coastal waters that were heavily impacted by war activities, including Europe, North America, and several areas of the Pacific (Beck et al., 2018; Beddington and Kinloch, 2005; Beck et al., 2018).
It has been estimated that approximately 1.8 million metric tonnes of conventional munitions were dumped into German waters (Böttcher et al., 2011). After several recovery operations, it is assumed that 1.6 million metric tonnes are still left: 1.3 million metric tonnes in the North Sea and 300,000 metric tonnes in the Baltic Sea (Böttcher et al., 2011). In areas with military activity, munition compounds could be detected in soil, sediment, and ground and surface waters (Jenkins et al., 2001; Talmage et al., 1999). Dumped munitions pose risks in different ways: they still can detonate during underwater construction works or when washed onshore (Appel et al., 2019; Beddington and Kinloch, 2005; Böttcher et al., 2011), and toxic energetic compounds (ECs) can leak out of corroded metal shells (Beck et al., 2018, 2019; Böttcher et al., 2011; Strehse et al., 2017) or get released by blast-in-place detonations (Maser and Strehse, 2020). Thereby, these ECs can get directly in contact with organisms, can disperse in the marine environment (Beddington and Kinloch, 2005; Strehse et al., 2017), and may enter the marine food chain (Böttcher et al., 2011; Ek et al., 2006, 2008). The released ECs, like 2,4,6-trinitrotoluene (TNT), 2-amino-4,6-dinitrotoluene (2-ADNT), and 4-amino-2,6-dinitrotoluene (4-ADNT), are known to be toxic and are suspected to be carcinogenic and mutagenic and to also have genotoxic effects (Bolt et al., 2006; Koske et al., 2019; Sabbioni and Rumler, 2007).
The concentrations of dissolved TNT and metabolites in the environment as a consequence of corrosion and subsequent leaking of munition items depend very much on the shell integrity of the munition item and the distance to the source of dissolved explosives. On a regional scale, background concentrations in seawater of the coastal areas of the North and Baltic Seas are usually low and in the 1- or 2-digit n/L range (Gledhill et al., 2019). When approaching larger dumping grounds, in, e.g., the Baltic Sea, the concentrations of dissolved explosives increase in the water (Frey et al., 2024). Also, in the vicinity of wrecks loaded with munitions, an increase in the concentrations is possible (Maser et al., 2023). When very close to big munition objects or lumps of explosives without shells, concentrations may increase up to mg/L (Beck et al., 2019). Sites with these very high concentrations of dissolved explosives are, of course, rare and cover, in comparison to the marine areas with low background contaminations, only very little space. However, wrecks and also munition items are hard substrates forming rare ecological niches in the North and Baltic Seas. As a consequence, many species can be found at or around these artificial hard substrates, where they get contaminated.
TNT is a nitro-aromatic compound and, until today, one of the most used secondary explosives in munitions (Juhasz and Naidu, 2007; Lotufo et al., 2013). It is chemically and thermally stable, has a low melting point, and is slightly soluble in water with a solubility of 130 mg/L (Juhasz and Naidu, 2007; Lotufo et al., 2013). When it is dissolved, the absorbance to soil or sediment is low (Juhasz and Naidu, 2007). TNT exhibits a stable ring structure due to the presence of three electron-withdrawing nitro groups (–NO2), resulting in steric constraints exhibiting a high electron deficiency of the aromatic ring (Hawari et al., 2000; Heiss and Knackmuss, 2002). Therefore, TNT undergoes reductive rather than oxidative transformation (Heiss and Knackmuss, 2002). Due to the highly oxidative characteristic of the nitro groups, they are first reduced to the nitroso (–NO), hydroxylamino (–NHOH), and amino (–NH2) functional groups. During the transformation processes, the aromatic characteristic is preserved (no ring cleavage), which shows that TNT undergoes (bio)transformation rather than complete mineralization (Hawari et al., 2000). In the marine environment, the main metabolic pathway for TNT is the reduction to 2-ADNT and 4-ADNT via biotic processes, mainly by bacteria (Juhasz and Naidu, 2007; Rosen and Lotufo, 2007b) or metabolic enzymes within tissues of higher biota (Koske et al., 2020). 2-ADNT and 4-ADNT can get both metabolized further to 2,4-diamino-6-nitrotoluene (2,4-DANT) (Juhasz and Naidu, 2007; Serrano-González et al., 2018). An overview of the chemical transformation of TNT is provided by Adomako-Bonsu et al. (2024). The chemical structure of TNT and its derivatives are presented in Figure 1.
Figure 1
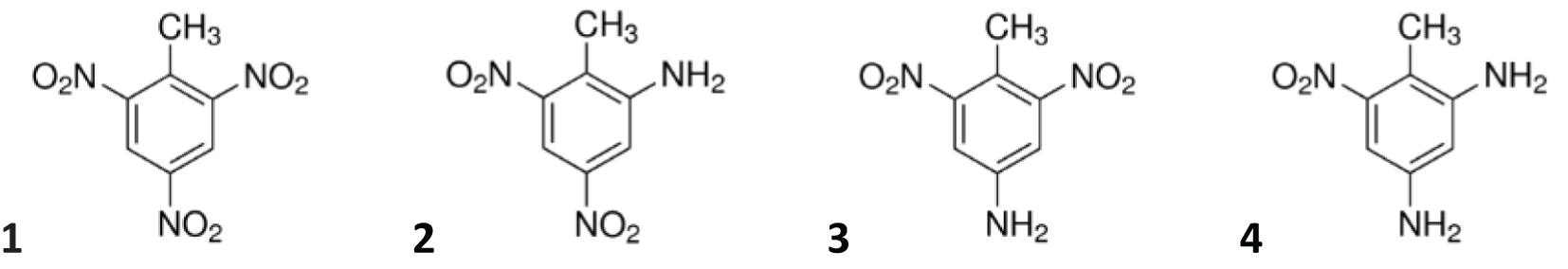
Chemical structures of 2,4,6-trinitrotoluene (TNT; 1), 2-amino-4,6-dinitrotoluene (2-ADNT; 2), 4-amino-2,6-dinitrotoluene (4-ADNT; 3), and 2,4-diamino-6-nitrotoluene (2,4-DANT; 4).
Several studies have shown the toxic effects of TNT and other explosives to marine organisms (like fish, molluscs, polychaetes, echinoderms, arthropods, and algae) with different toxicological endpoints as summarized by Nipper et al. (2009) and Lotufo et al. (2013). For instance, the LC50 values of TNT ranging from 0.98 mg/L (Americamysis bahia) (Nipper et al., 2001) to 19.5 mg/L (Mytilus galloprovincialis) (Rosen and Lotufo, 2007a) had been observed. However, scientists are discordant on whether the parent compound TNT is more or less toxic than its metabolites (Sims and Steevens, 2008). In addition to animals, humans are affected by TNT, displaying severe health effects, such as anemia, liver cancer, toxic hepatitis, and hepatomegaly (Sabbioni and Rumler, 2007; U.S. Department of Health and Human Services: Public Health Service, 1995).
To investigate marine chemical pollution, blue mussels (Mytilus edulis) are common indicator organisms: mussels are sessile filter feeders that can filter a high amount of water per day. They are distributed worldwide, resistant against variable environmental conditions, and are known for accumulating different types of substances from the environment, including contaminants, as reviewed by Strehse and Maser (2020). These characteristics and the fact that mussels are a common prey species make them very suitable for investigating marine pollution. A variety of different studies have successfully used bivalves as indicator organisms (e.g., Brenner et al., 2014; Strehse et al., 2017; Viarengo and Canesi, 1991).
While it is known that blue mussels take up TNT and metabolize it to 2-ADNT and 4-ADNT in their tissues (Schuster et al., 2021; Strehse et al., 2017), it is, however, unclear how long the metabolization of TNT takes and if these metabolites bioconcentrate or accumulate in the tissue of mussels or whether the substances get simply depurated. Therefore, this study was designed with the aim of investigating the kinetics of the potential metabolization and/or depuration of dissolved TNT in blue mussel tissues.
2 Materials and methods
2.1 Origin and handling of mussels
To test the kinetics of the potential metabolization and/or depuration of dissolved TNT in blue mussel soft bodies, two laboratory experiments were conducted. Mussels were collected at the island of Sylt in the North Sea 6 days before the experiments started. In order to reduce the acclimatization stress of the mussels to a minimum, the on-site temperatures [16°C in July (first experiment) and 6°C in February (second experiment)] and salinity (30‰) were recorded, and the laboratories were pre-cooled accordingly. After the collection, the mussels were transported cool and dry in a cool box to the laboratory. For each experiment, the mussels were first evenly distributed across two aquaria containing natural seawater at constant room temperature corresponding to that at the sampling site (16°C for the first experiment and 6°C for the second experiment). Furthermore, a day–night cycle was simulated using artificial room light. The cycle was adjusted according to the on-site conditions at Sylt (first experiment, 7:00 am until 9:00 pm; second experiment, 8:00 am until 5:00 pm). Moreover, each aquarium was aerated using aquarium membrane pumps (M2K3, 350 L/h). Before transferring to artificial seawater, the shell length of the mussels was measured, and individuals between 5 and 6– cm were selected for the experiments, ensuring sufficient tissue material for the planned analysis. Thereafter, the mussels were cleaned thoroughly of epiphytes by scraping with a knife. Subsequently, the mussels were acclimatized to artificial seawater (salinity, 30‰) by replacing 50% of the natural water with artificial seawater every 24 h. In total, acclimatization during the first experiment was conducted over 2 days. During the second experiment, acclimatization was conducted over 9 days. The longer acclimatization time during the second experiment was intended to ensure a better adaptation to the laboratory conditions of the mussels. Artificial seawater was prepared on a daily basis by mixing deionized water and sea salt (Coral Pro, Red Sea) in a 70-L barrel. Salinity, oxygen, and temperature were checked using a portable salinometer WTW LF330 equipped with a WTW TetraCon® 325 testing probe. Before usage, the artificial seawater was cooled down to the temperature of the constant temperature laboratory. The mussels were fed with 4 mL live marine phytoplankton (cell density, 250 million mL−1, Premium Reef Blend, Sustainable Aquatics) per aquarium once before the first exposure started. During the acclimatization for the second experiment, the mussels were fed three times: at the beginning of the acclimatization, at half-time, and 2 days before the start of the exposure phase.
2.2 Origin and preparation of TNT solution
The TNT used for the first experiment was produced and provided by the German Federal Facility for the Removal and Remediation of Explosives and Chemical Warfare Agents (GEKA, Münster, Germany). For production, the solid TNT of a Second World War grenade was scratched off and dissolved in dichloromethane in a volumetric flask. The extracted explosive aliquots were transferred into 5-L bottles, and the solvent was evaporated at room temperature. Finally, the explosive was re-dissolved in filtered water by stirring at 80°C to get a 100 mg/L stock solution. The concentration was verified by GC–MS/MS analysis before being used in the experiment.
For the second experiment, deuterated TNT (TNT-d5; Figure 2) was used, which was synthesized by a procedure related to that of Kröger and Fels (2000). A mixture of fuming nitric acid (2.96 g, 1.95, 46.9 mmol) and concentrated sulfuric acid (6.27 mL) was prepared under ice cooling. In a 50-mL three-necked flask, toluene-d8 (isotope purity, 99.5%, 940 mg, 1.00 mL, 9.38 mmol) was added to 4.00 mL of concentrated sulfuric acid under ice cooling. The previously prepared ice-cold nitration agent was added slowly. The ice bath was removed, and the solution was stirred for 60 min at room temperature. Stirring was repeated afterwards for 3 h at 95°C. The reaction mixture was then cooled to room temperature and added to ice water, where the product precipitated as a colorless solid. The solid was filtered in vacuo, washed with approx. 300 mL of water, and recrystallized from approx. 10 mL ethanol. Finally, colourless needle-like crystals of deuterated TNT were obtained.
Figure 2
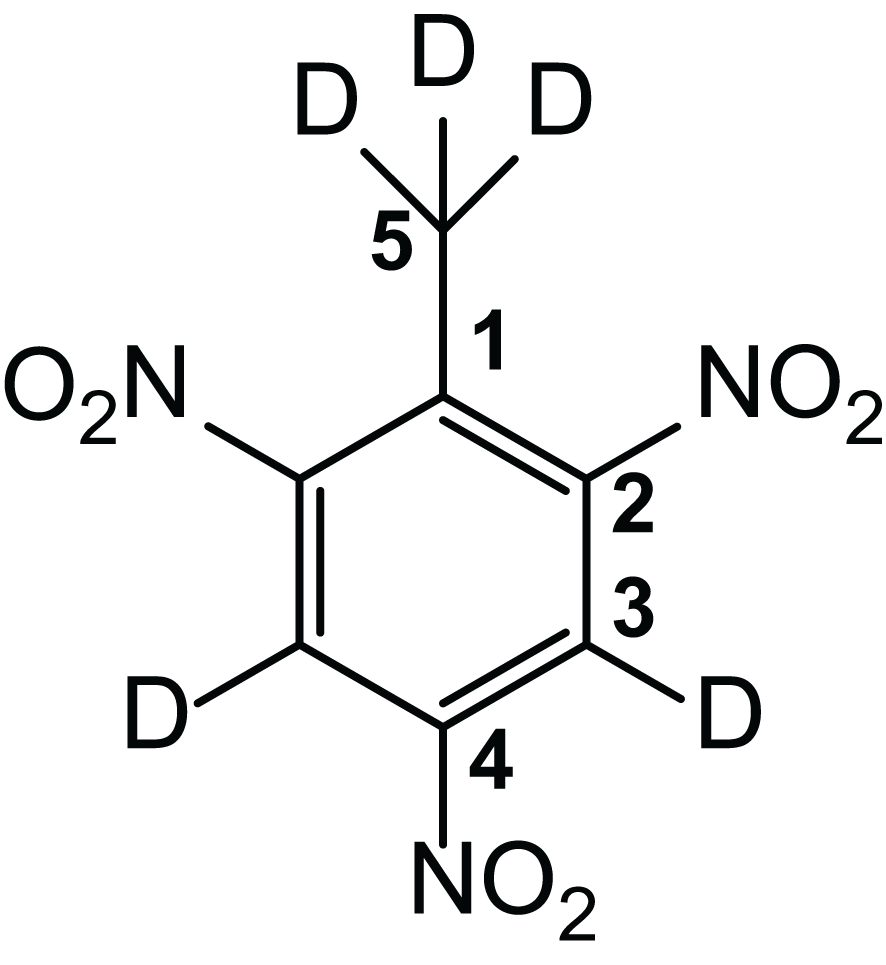
Structural formula of deuterated TNT-d5. Yield: 1.24 g (5.34 mmol, 57%). 13C-NMR (125 MHz, DMSO-d6, 300 K): δ= 150.8 (C-2), 145.6 (C-4), 132.9 (C-1), 122.4 (C-3), 14.3 (C-5) ppm. MS (Electron Ionization (EI), 70 eV): m/z (%) = 214 (100) [M]+. MS (EI, High Resolution (HR), 70 eV): C7D5N3O6 m/z = calc.: 232.04922, found: 232.04936, diff.: 0.63 ppm.
2.3 Experimental setup
To test the rate of the metabolization of TNT in the mussels and the potential of bioaccumulation, two laboratory experiments were conducted. An overview of the experimental setup is provided in Figure 3 and Table 1. During the first experiment, the mussels were exposed for 48 h to different TNT concentrations, followed by 60 h of recovery in clean artificial seawater. According to the on-site conditions at the sampling site at Sylt, the temperature was set to 16°C constantly. The exposure was conducted at artificial room light between 7:00 am and 9:00 pm. The experiment was conducted with three different TNT concentrations and a control without TNT: 0, 0.625, 1.25, and 2.5 mg/L. Treatment concentrations were chosen according to Schuster et al. (2021) to ensure that exposed mussels will still filter normally and individuals are permanently exposed to the selected TNT concentrations. Furthermore, the selected concentrations ensure that the resulting soft body concentrations are within the detection limits of the used analytical method, even under the short exposure times used for this study (Schuster et al., 2021). Furthermore, a TNT stability control containing 2.5 mg/L TNT solution without mussels was installed and tested for TNT degradation. For each TNT concentration, the control treatment, and the TNT stability control, three aquaria were used, resulting in a total of 15 aquaria. The aquaria were filled with 10 L of artificial seawater, including the respective TNT concentration. Except for the TNT stability control aquaria, each aquarium contained 10 mussels. At each sampling time point during the experiments, one individual per aquarium was sampled. In the course of the first experiment, mussel sampling was conducted only during the recovery phase. Sampling took place once at the end of the exposure phase, before the start of the recovery, and then subsequently after 4, 8, 12, 24, 36, 48, and 60 h.
Figure 3
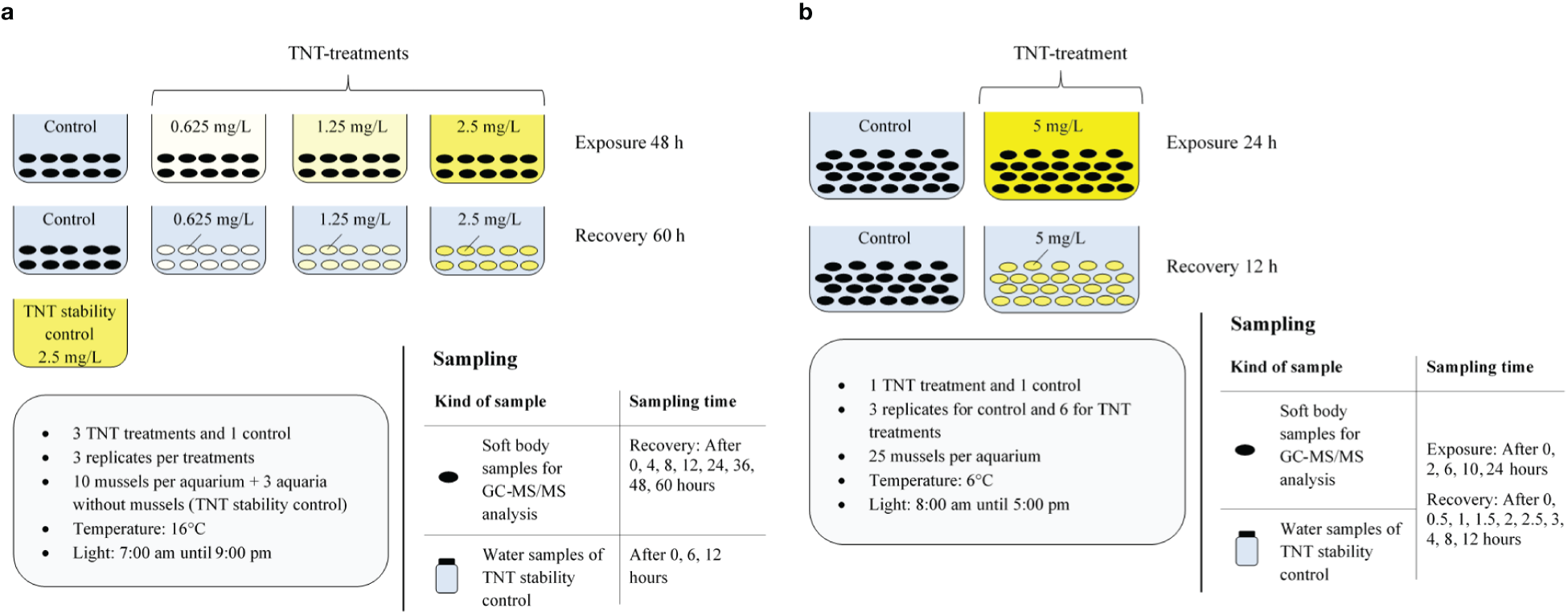
Schematic description of the experimental setup and sampling of the first experiment (A) and the second experiment (B).
Table 1
| First experiment (over 108 h) | Second experiment (over 36 h) | |
|---|---|---|
| Room temperature | 16°C | 6°C |
| Light | 7:00 am to 9:00 pm | 8:00 am to 5:00 pm |
| Salinity | 30‰ | 30‰ |
| Water volume | 10 L | 10 L |
| Number of aquaria | 3 per treatment and control (15 in total) | 6 for TNT treatment and 3 for control (9 in total) |
| Number of mussels in aquaria | 10 | 25 |
| TNT concentrations | 0, 0.625, 1.25, and 2.5 mg/L | 0 and 5 mg/L |
| Used TNT solution | Unmodified | Labelled |
| TNT stability control | Yes (3 aquaria with 2.5 mg/L TNT) | No |
| Exposure | 48 h | 24 h |
| Recovery | 60 h | 12 h |
| TNT re-dosing during exposure | Every 24 h | No |
| Kind of samples | During recovery phase: • Mussel soft body (chem. anal.) • Water only for stability control |
During exposure and recovery phase: • Mussel soft body (chem. anal.) • Water (chem. anal.) |
| Sampling | • 1 soft body during recovery per aquarium and time point: 0, 4, 8, 12, 24, 36, 48, and 60 h • 1 water (stability control) per aquarium and time point: after 0, 6, and 12 h |
1 soft body and 1 water per aquarium and time point: • During exposure: 0, 2, 6, 10, and 24 h • During recovery: 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 8, and 12 h |
Overview of the laboratory conditions and sampling of the two experiments.
TNT, 2,4,6-trinitrotoluene.
Aeration was ensured by aquarium membrane pumps (M2K3, 350 L/h) using one pump for two aquaria. The exposure to TNT was conducted for 48 h. After 24 h, the water was exchanged completely with new artificial seawater and re-dosed with the respective TNT concentration. Subsequently, a recovery phase of 60 h was conducted by transferring the mussels to new clean aquaria containing 10-L clean artificial seawater each. Also, during the recovery phase, artificial water was exchanged on a daily basis.
Based on the results of the first experiment, a second experiment was planned and conducted. This time, also the uptake of dissolved TNT was recorded in the mussels during the exposure phase (sampling: before the start of exposure and after 2, 6, 10, and 24 h). In addition, the mussels were sampled several times between start and 4 h of the recovery phase to increase the sampling resolution in the first part of the recovery phase (sampling before the start of recovery and after 0.5, 1, 1.5, 2, 2.5, 3, 4, 8, and 12 h). Furthermore, the aquarium water was monitored for dissolved TNT and metabolites in both the exposure and recovery phases. Finally, the TNT used in the second experiment was labelled with deuterium to ensure that the resulting ADNTs are products of metabolization and not a bias due to any contamination. Within the exposure phase, the mussels were exposed for 24 h to one TNT concentration (5 mg/L) and a control treatment (0 mg/L), followed by 12 h of recovery in clean artificial seawater. Three aquaria under control treatment and six aquaria under TNT treatments were used, resulting in a total of nine aquaria. Each aquarium contained 25 mussels in 10-L water (control) or a mix of water and TNT with a concentration of 5 mg/L (TNT treatment). The room temperature was set to 6°C, and artificial room light was set between 8:00 am and 5:00 pm according to the on-site conditions at the island of Sylt at the time of mussel sampling. During the experiment, no TNT re-dosing was conducted.
2.4 Water sampling
During the first experiment, only the water of the TNT stability controls was sampled. Sampling took place at three time points: right after filling the aquaria and adding the TNT solution (0 h), after 6 h, and after 12 h. For each sample, 500 mL of water was filled in Kautex® bottles and stored at −20°C until analysis. TNT concentration in saltwater is considered photochemically stable over several months in the absence of UV light (Luning Prak et al., 2017; Sisco et al., 2015). Furthermore, microbial metabolization in the laboratory setup used was considered unlikely, based on the findings of Harrison and Vane (2010). Therefore, a longer running time of the stability control was not considered necessary.
During the second experiment, water was sampled from all aquaria right before each mussel sampling (Table 1). Sampling was conducted by filling 50 mL water of from each aquarium in 50-mL Falcon tubes. Samples were stored at −20°C until further processing.
2.5 Mussel sampling, dissection, and morphometric measurements
Before starting the exposure phase, individuals were sampled from the acclimatization aquaria to analyze for potential TNT body burdens prior to exposure (three individuals during the first experiment and nine individuals during the second experiment) (Table 1). In both experimental approaches, the mussels were carefully rinsed with fresh artificial seawater before being set into new aquaria for the recovery phase.
To sample the soft body, shells were opened by cutting the adductor muscles. Remaining water between the valves was drained off briefly before total wet weight was determined using a laboratory balance (Sartorius TE412, ± 0.01 g). Afterwards, the complete soft body was transferred into a 15-mL polypropylene conical tube, snap frozen in liquid nitrogen, and stored at −80°C until further processing. For each mussel, the shell length, total wet weight, and shell wet weight were noted. The weight of the shell and soft body (calculated as total wet weight minus shell wet weight) was used for calculating the condition index as described in Equation 1. The condition index was used for subsequent comparison of the physical status of the individual specimen.
2.6 Preparation of mussel samples
For both experiments, the soft bodies of the mussels were defrosted, transferred into a 25-mL Erlenmeyer flask, and homogenized using a T25 Ultra-Turrax (Ika Works Inc., Staufen im Breisgau, Germany). After each sampling, the Ultra-Turrax and Erlenmeyer flask were cleaned with deionized water. For each mussel sample, an approximate weight of 1 g was transferred into 15-mL Falcon tubes and filled with 5 mL gradient grade acetonitrile (Sigma-Aldrich, Steinheim, Germany). The exact weight of mussel soft bodies was documented. Samples were vortexed for 1 min and centrifuged for 5 min at 4,000 rpm at 20°C using an Eppendorf 5810R centrifuge (Eppendorf AG, Hamburg, Germany). Supernatants were made up to 10 mL with acetonitrile, transferred to pre-labelled 15-mL conical tubes, and stored at −20°C until analysis. For analysis, 1-mL solutions were transferred to 1.5-mL brown glass autosampler vials, and 13C15N-TNT (25 ng) was added as an internal standard. All samples were measured twice. Results are displayed as means of the duplicate values. The detection limits of the method used for mussel tissue were 3.5 ng/g w.w. for TNT, 1.5 ng/g w.w. for 4-ADNT, and 1.2 ng/g w.w. for 2-ADNT (Table 2) (Bünning et al., 2021). The influence of matrix effects on the peak area of TNT was corrected using the internal standard throughout the measurements.
Table 2
| Compound | Water | Mussel | ||||
|---|---|---|---|---|---|---|
| LOD (ng/L) | LOQ (ng/L) | R2 | LOD (ng/g w.w.) | LOQ (ng/g w.w.) | R2 | |
| TNT | 90 | 300 | 0.981 | 3.5 | 11.5 | 0.940 |
| 4-ADNT | 60 | 190 | 0.994 | 1.5 | 5.1 | 0.987 |
| 2-ADNT | 60 | 200 | 0.992 | 1.2 | 4.0 | 0.992 |
Limit of detection (LOD), limit of quantification (LOQ), and coefficient of determination (R2) for the GC–MS/MS method.
TNT, 2,4,6-trinitrotoluene; 4-ADNT, 4-amino-2,6-dinitrotoluene; 2-ADNT, 2-amino-4,6-dinitrotoluene.
2.7 GC–MS/MS analysis of mussel soft bodies
For both experiments’ GC–MS/MS analysis, a TRACE 1310 gas chromatograph coupled to a TSQ 8000 EVO Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used. One microliter of sample was injected into a split/splitless injector on a splitless glass wool liner (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 230°C. Separation was carried out on a TG-5MS Amine column (15 m × 0.25 mm × 0.25 μm, Thermo Fisher Scientific Inc., Waltham, MA, USA). Helium was used as carrier gas, with a flow rate of 1.5 mL/min. After 2 min, the injector was purged with a split flow of 20 mL/min. The initial oven temperature (100°C, 0.2 min) was increased to 220°C by 30°C/min (0.3 min) and then with 80°C/min to 280°C (1 min, total run time 6.25 min). The retention times of TNT, 2,4-DANT (2,4-DANT-d5 was not analyzed), 4-ADNT, and 2-ADNT were 3.39, 4.12, 4.21, and 4.41 min, respectively. The mass spectrometer was operated in Multiple Reaction Monitoring (MRM) mode with argon as collision gas and electron ionization (70 eV), according to Bünning et al. (2021). Concentrations were calculated using external standard calibration curves, prepared by diluting a mixture of the explosives in acetonitrile between 0.5 and 1,000 ng/mL. A 10 ng/mL standard mix was measured every 10 measurements, and the values obtained were used to correct the readings. To calculate the chemical concentration per gram of mussel, Equation 2 was used. The detection limits for the water samples were derived from Bünning et al. (2021). They are 90 ng/L for TNT, 60 ng/L for 4-ADNT, and 60 ng/L for 2-ADNT (Table 2).
2.8 Analysis of water and TNT stock solutions
TNT stability controls (2.5 mg/L) and TNT stock solution (100 mg/L) were concentrated using CHROMABOND Easy polystyrene-divinylbenzene copolymer reversed-phase solid-phase extraction columns, 80 µm, 3 mL/200 mg (Macherey-Nagel, Düren, Germany), eluted with acetonitrile, diluted to a concentration of 2.5 µg/mL, and measured by GC–MS/MS using the same method as described above for analyzing the mussel soft bodies. Each sample was measured twice (see Table 3).
Table 3
| Aquaria | Time (h) | TNT (mg/L) | 2-ADNT (mg/L) | 4-ADNT (mg/L) | ||
|---|---|---|---|---|---|---|
| A | B | Mean | ||||
| 1 | 0 | 2.86 | 2.95 | 2.9 | <LOD | <LOD |
| 1 | 6 | 2.90 | 2.91 | 2.9 | <LOD | <LOD |
| 1 | 12 | 2.53 | 2.59 | 2.6 | <LOD | <LOD |
| 2 | 0 | 2.74 | 2.67 | 2.7 | <LOD | <LOD |
| 2 | 6 | 2.60 | 2.62 | 2.6 | <LOD | <LOD |
| 2 | 12 | 2.59 | 2.61 | 2.6 | <LOD | <LOD |
| 3 | 0 | 2.04 | 1.98 | 2.0 | <LOD | <LOD |
| 3 | 6 | 2.18 | 2.19 | 2.2 | <LOD | <LOD |
| 3 | 12 | 1.99 | 2.02 | 2.0 | <LOD | <LOD |
Chemical analysis of water samples from the TNT stability control over time.
Samples were measured twice (A, B) and presented as mean values.
TNT, 2,4,6-trinitrotoluene; 4-ADNT, 4-amino-2,6-dinitrotoluene; 2-ADNT, 2-amino-4,6-dinitrotoluene.
2.9 Toxicokinetic parameter
As toxicokinetic parameters, the bioconcentration factor at steady state (BCFss), the kinetic bioconcentration factor (BCFk), the uptake rate constant (k1), the depuration rate constant (k2), and the half-life (t1/2) were calculated according to the guideline 305 of the Organisation for Economic Co-operation and Development (OECD) (OECD, 2012). BCFss was calculated using Equation 3, where Css is the concentration of TNT in mussel tissue at steady state and Cw is the TNT concentration in water at steady state (steady state is defined as the mean concentration of the last three time points of the exposure phase). The kinetic bioconcentration factor (BCF) was determined using Equation 4.
In accordance with the OECD Test Guidelines (OECD, 2012), to calculate k2, the log-transformed concentration data were first assessed to determine whether the explosives exhibited an exponential decay over time in mussel samples. A clear exponential decline could not be observed. If the exponential decline was only partial, k2 was determined up to the point where the exponential pattern remained approximately valid (Equation 5), with the coefficient of determination (R2) also being calculated to assess the fit. In cases where no exponential decay was observed, k2 was computed using only two time points, representing the most reliable data available (Equation 6), with Ct being the concentration of the substance in the organism at time t and C0 the initial concentration in the organism at the beginning of depuration (t = 0). Half-life t1/2 and k1 were calculated using Equation 7 for half-life and Equation 8 for k1.
In cases of non-detected values, these values were replaced with the limit of quantification (LOQ), which is defined as 3.3 times the limit of detection (Bünning et al., 2021; European Commission Joint Research Centre, 2016). Since only data from mussel samples from the recovery phase were available in the first experiment, only the elimination rate constant k2 was determined.
2.10 Statistics
To reduce potential bias, all treatment groups of both experiments were at least arranged as triplicates. Microsoft Excel 365 was used for data arrangement. The calculation of mean and 95% confidence interval (CI), as well as data analysis and visualization, was performed using R (Version 4.1.0, The R Foundation for Statistical Computing). The 95% CI was calculated using the sample mean (), t-value (t), sample standard deviation (s), and standard error () as described in Equation 9.
The condition index of all mussels per treatment was tested for significant differences by checking for normal distribution and homogeneity of variance using the Shapiro–Wilk test and Levene’s test. Data of the condition index of the first experiment had homogenic variances but were not normally distributed; therefore, the Kruskal–Wallis ANOVA and Dunn’s test were used as non-parametric tests. Data of the condition index of the second experiment had homogenic variances and were normally distributed; therefore, a t-test was performed. Data from chemical analysis were also tested for normal distribution and homogeneity of variance. Afterwards, a one-way ANOVA was performed to test significant changes in concentrations in tissue and water samples. In case of a significant ANOVA test, a subsequent Tukey’s test was performed as a post-hoc test. Significant levels were set to p < 0.05.
3 Results
3.1 Condition index
The condition index of the mussels in both experiments did not show significant differences between the treatments (first experiment, chi2 = 0.55, df = 3, p = 0.908; second experiment, t = 0.76, df = 115, p = 0.45). The mussels of the first experiment had an average condition index of 65 ± 11 (control, 63 ± 11; 0.625 mg/L TNT concentration, 66 ± 12; 1.25 mg/L TNT concentration, 65 ± 10; 2.5 mg/L TNT concentration, 66 ± 10). The mussels used in the second experiment had a condition index of 54 ± 7.
3.2 First experiment
3.2.1 Chemical analysis of water and TNT stock solution
The concentration of the stock solution was determined to be 100 mg/L, and the proportions of 2- and 4-ADNT were less than 1%. The TNT concentrations in the TNT stability control had an average value of 2.5 mg/L ( ± 0.33 mg/L) at the beginning of the experiment (Table 3). During the experimental period of 12 h, the concentration remained constant. In the TNT stability control, no 2- and 4-ADNT were detected.
3.2.2 Chemical analysis of mussel soft bodies
TNT and its metabolites could be detected in ng/g mussel wet weight (Figure 4). In general, the TNT, 2-ADNT, 4-ADNT, and 2,4-DANT concentrations followed the same pattern: all treatments showed the highest concentration right after the exposure phase (0 h), with the exception of the control, containing no energetic compounds. Likewise, the mussels collected and analyzed before the experiment started contained no energetic compounds.
Figure 4
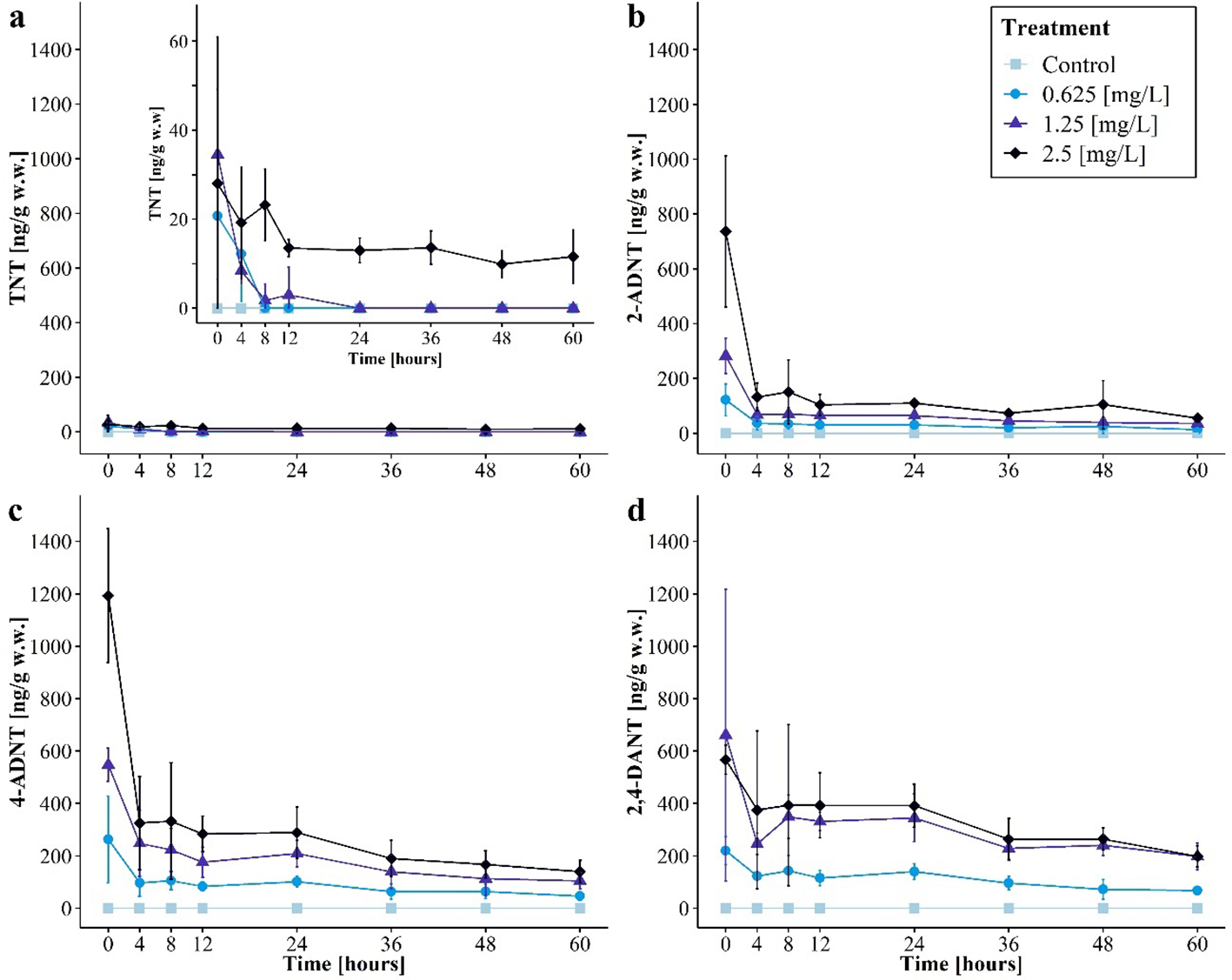
Concentrations of detected 2,4,6-trinitrotoluene (TNT; a), 2-amino-4,6-dinitrotoluene (2-ADNT; b), 4-amino-2,6-dinitrotoluene (4-ADNT; c), and 2,4-diamino-6-nitrotoluene (2,4-DANT; d) (mean of three replicates ± 95% confidence interval) in mussel soft bodies during the recovery phase for the different treatments of the first experiment.
TNT, 2-ADNT, and 4-ADNT soft body concentrations (Figures 4a–c) at time point 0 h were the highest in the 2.5 mg/L treatment (TNT, 28 ng/g, 95% CI: 0–60.9 ng/g; 2-ADNT, 737 ng/g, 95% CI: 460–1,012.9 ng/g; 4-ADNT, 1,193 ng/g, 95% CI: 938.7–1,447.8 ng/g) followed by 1.25 mg/L (TNT, 35 ng/g, 95% CI: 19.9–49.2 ng/g; 2-ADNT, 283 ng/g, 95% CI: 217.6–347.8 ng/g; 4-ADNT, 548 ng/g, 95% CI: 484.0–611.1 ng/g) and 0.625 mg/L as the treatments with the lowest concentrations (TNT, 21 ng/g, 95% CI: 6.5–35.0 ng/g; 2-ADNT, 122 ng/g, 95% CI: 63.8–180.2 ng/g; 4-ADNT, 263 ng/g, 95% CI: 97.7–428.3 ng/g). In contrast, the highest 2,4-DANT soft body concentration (Figure 4d) was detected in the 1.25 mg/L treatment (661 ± 466 ng/g) followed by the 2.5 mg/L (567 ± 47 ng/g) and 0.625 mg/L treatments (219 ng/g, 95% CI: 167.0–272.8 ng/g). However, the detected 2,4-DANT concentration at time point 0 h for the 1.25 mg/L treatment showed high variances. In the course of the experiment, the measured concentrations in the 1.25 mg/L treatment were lower than in the 2.5 mg/L treatment.
Statistical analysis (ANOVA) confirmed significant effects of time on compound concentrations in most treatments: for TNT, significant effects were observed in the 0.625 mg/L and 1.25 mg/L treatments [(F(7, 16) = 6.9, p < 0.001 and F(7, 16) = 17.7, p < 0.001, respectively], while the 2.5 mg/L treatment showed no significant effect (p = 0.467). For 2-ADNT and 4-ADNT, all treatments showed highly significant effects over time (all p < 0.001). Similarly, for 2,4-DANT, a significant effect was found in the 0.625 mg/L treatment [F(7, 16) = 7.6, p < 0.001], but not in the two treatments with higher concentrations (p = 0.087 and p = 0.115, respectively).
Post-hoc comparisons revealed that concentrations at 0 h differed significantly from nearly all later time points for TNT, 2-ADNT, and 4-ADNT, particularly in treatments with higher concentrations. For instance, in the 1.25 mg/L treatment, the difference in 2-ADNT concentration between 0 and 60 h was −246.9 ng/g (95% CI: −311.7 to −182.2, p < 0.001), while in the 1.25 mg/L treatment, differences in TNT concentration remained significant across all time comparisons (e.g., 0 vs. 60 h, −34.56 ng/g, p < 0.00001). Similar patterns of decline were evident for 4-ADNT and 2,4-DANT, with numerous time points differing significantly from 0 h.
Over the first 4 h, the concentrations of energetic compounds in mussel soft bodies decreased rapidly: TNT showed a decrease of 49%, 2-ADNT of 76%, 4-ADNT of 64%, and 2,4-DANT of 47%. In the 0.625 mg/L and 1.25 mg/L treatments, TNT amount within soft body samples was completely depleted within the first 24 h (0.625 mg/L treatment after 8 h and 1.25 mg/L treatment after 24 h), whereas TNT in the 2.5 mg/L treatment was still present until the end of the experiment.
At the end of the experiment, the mussels contained 0% to 42% TNT, 7% to 13% 2-ADNT, 7% to 49% 4-ADNT, and 9% to 35% 2,4-DANT of the starting concentration.
Furthermore, the rapid decrease in tissue concentrations of TNT and its derivatives was also reflected by the high depuration rate constants (k2) as seen in Table 4. The depuration rates and corresponding half-lives (t1/2) of TNT and its derivatives varied with exposure concentrations. At the lowest concentration (0.625 mg/L), TNT showed a moderate depuration rate (k2 = 0.133 h−1) and a half-life of 5.23 h, while the derivatives displayed slightly higher elimination rates and shorter half-lives (e.g., 2-ADNT, k2 = 0.298 h−1, t1/2 = 2.32 h). With increasing exposure concentrations, the patterns differed: for TNT, k2 decreased to 0.094 h−1 at 2.5 mg/L, indicating slower depuration and a prolonged half-life (7.37 h). In contrast, 2-ADNT showed an increasing k2 with concentration (up to 0.430 h−1). 2,4-DANT showed relatively consistent depuration behavior across concentrations with half-lives ranging from 2.81 to 6.72 h.
Table 4
| Toxicokinetic parameter | Treatment | TNT | 2-ADNT | 4-ADNT | 2,4-DANT |
|---|---|---|---|---|---|
| k2 (h−1) | 0.625 mg/L | 0.133 | 0.298 | 0.249 | 0.146 |
| 1.25 mg/L | 0.275 | 0.356 | 0.198 | 0.247 | |
| 2.5 mg/L | 0.094 | 0.430 | 0.324 | 0.103 | |
| t1/2 (h) | 0.625 mg/L | 5.23 | 2.32 | 2.78 | 4.74 |
| 1.25 mg/L | 2.52 | 1.95 | 3.5 | 2.81 | |
| 2.5 mg/L | 7.37 | 1.61 | 2.14 | 6.72 |
Toxicokinetic parameters (depuration rate constant, k2; half-life, t1/2) for TNT, 2-ADNT, 4-ADNT, and 2,4-DANT of the different treatments during the first experiment.
TNT, 2,4,6-trinitrotoluene; 4-ADNT, 4-amino-2,6-dinitrotoluene; 2-ADNT, 2-amino-4,6-dinitrotoluene.
3.3 Second experiment
3.3.1 Chemical analysis of water
At the start of the exposure phase, the TNT-d5 concentration in aquaria of the TNT treatment was 5.35 mg/L (95% CI: 5.1–5.6 mg/L) on average (Figure 5a). At the end of the exposure, the TNT-d5 concentration was 4.02 mg/L (95% CI: 3.7–4.4 mg/L). During the exposure phase, a significant decrease of TNT-d5 concentration in water could be detected [F(4, 23) = 8.1, p < 0.001]. The first traces of ADNTs were measured after 10 h of exposure.
Figure 5
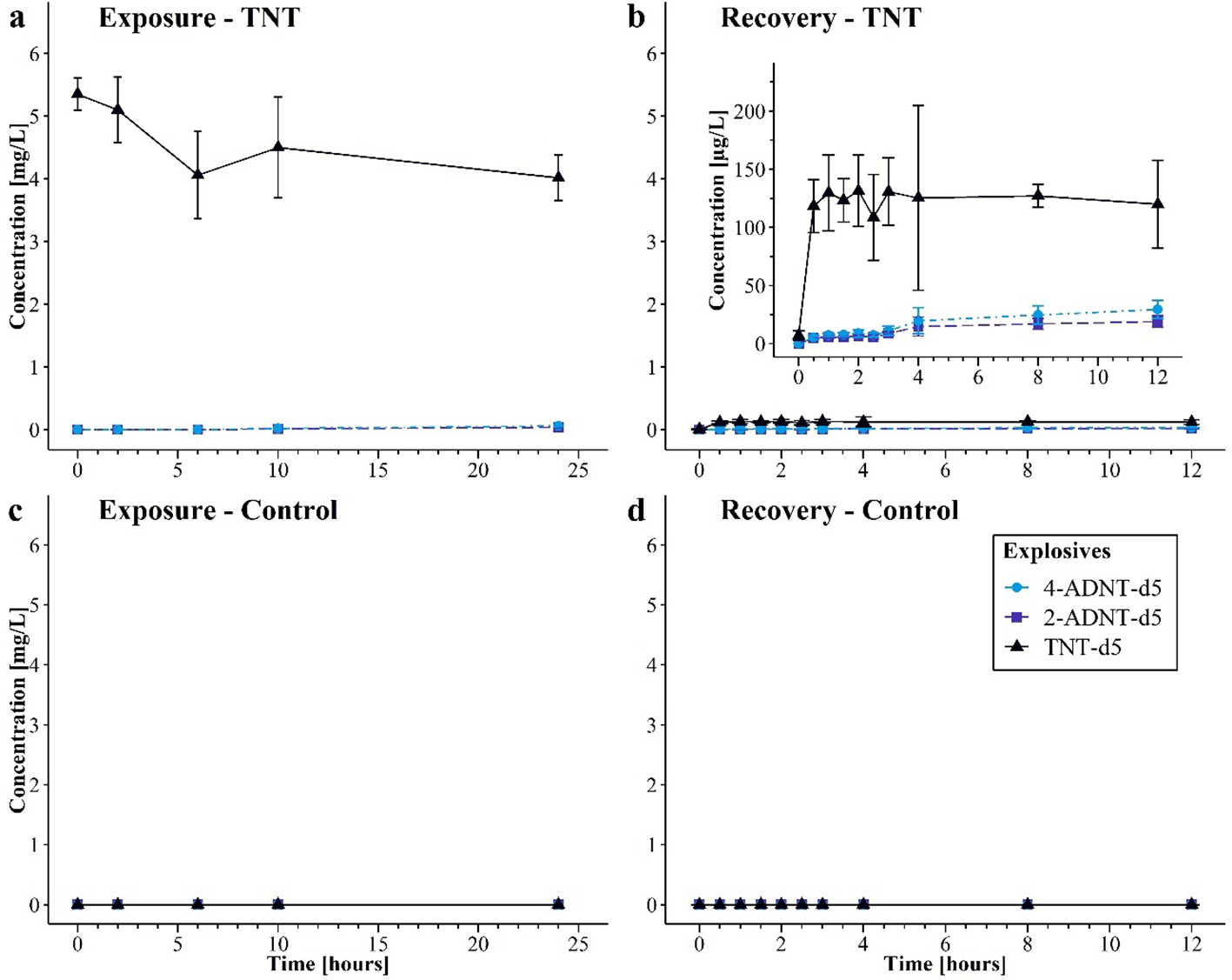
Concentrations of detected 2,4,6-trinitrotoluene (TNT-d5), 2-amino-4,6-dinitrotoluene (2-ADNT-d5), and 4-amino-2,6-dinitrotoluene (4-ADNT-d5) in water samples during the exposure and recovery phase for the second experiment (mean of three replicates for control or six replicates for TNT treatment ± 95% CI). (a) TNT-d5 treatment exposure, (b) TNT-d5 treatment recovery, (c) control exposure, and (d) control recovery.
Water in the control aquaria of the recovery phase contained no TNT nor ADNTs before the mussels were transferred. However, water samples of the recovery aquaria intended for the treated mussels revealed traces of TNT-d5 before the mussels were set into the aquaria (7 µg/L, 95% CI: 2.9–11.0 µg/L). ADNTs were not detected (Figure 5b). Right after the mussels were transferred into the aquaria, the TNT-d5 concentration within water increased significantly (p < 0.001) to 118 µg/L (95% CI: 95.6–141.1 µg/L) in the treated aquaria. At the end of the experiment, 119 µg/L (95% CI: 82.3–157.6 µg/L) TNT-d5 was measured (after 12 h). During the recovery, the concentration showed some variations, but they were not statistically significant. Furthermore, ADNT concentrations started to increase after mussels were present in the aquaria: 30 min after the start of the recovery, 2-ADNT-d5 increased to 5.05 µg/L (95% CI: 3.5–6.6 µg/L), and 4-ADNT-d5 concentration increased to 5.4 µg/L (95% CI: 3.6–7.2 µg/L). After the mussel transfer (0.5 h), the first signs of a statistically significant increase in ADNT-d5 concentrations in water could be detected after 4 h (p < 0.001). After 12 h, 18.98 µg/L (95% CI: 14.1–23.9 µg/L) 2-ADNT-d5 and 29.4 µg/L (95% CI: 21.7–37.1 µg/L) 4-ADNT were measured. This corresponds to an increase in means of 13.9 µg/L compared to 0.5 h of 2-ADNT-d5 (95% CI: 7.2–20.6 µg/L, p < 0.001) and 24 13.93 µg/L of 4-ADNT-d5 (95% CI: 13.8–34.2 µg/L, p < 0.001). In the control treatment, no energetic compounds were detected during exposure and recovery (Figures 5c, d).
3.3.2 Chemical analysis of mussel soft bodies
The results of the second experiment displayed continuous uptake of TNT-d5 in ng/g wet weight by the mussels in the TNT-d5 treatment (before start of exposure, 0 ng/g; end of exposure, 361.7 ng/g, 95% CI: 99.5–623.8 ng/g) with slightly lower concentration after 10 h (Figure 6a). The increase of TNT tissue concentration could be determined as significant [F(4, 25) = 3.09, p = 0.034] with the highest difference after 24 h of exposure (p = 0.019). In the control treatment, no energetic compounds could be detected in soft body samples (Figures 6c, d).
Figure 6
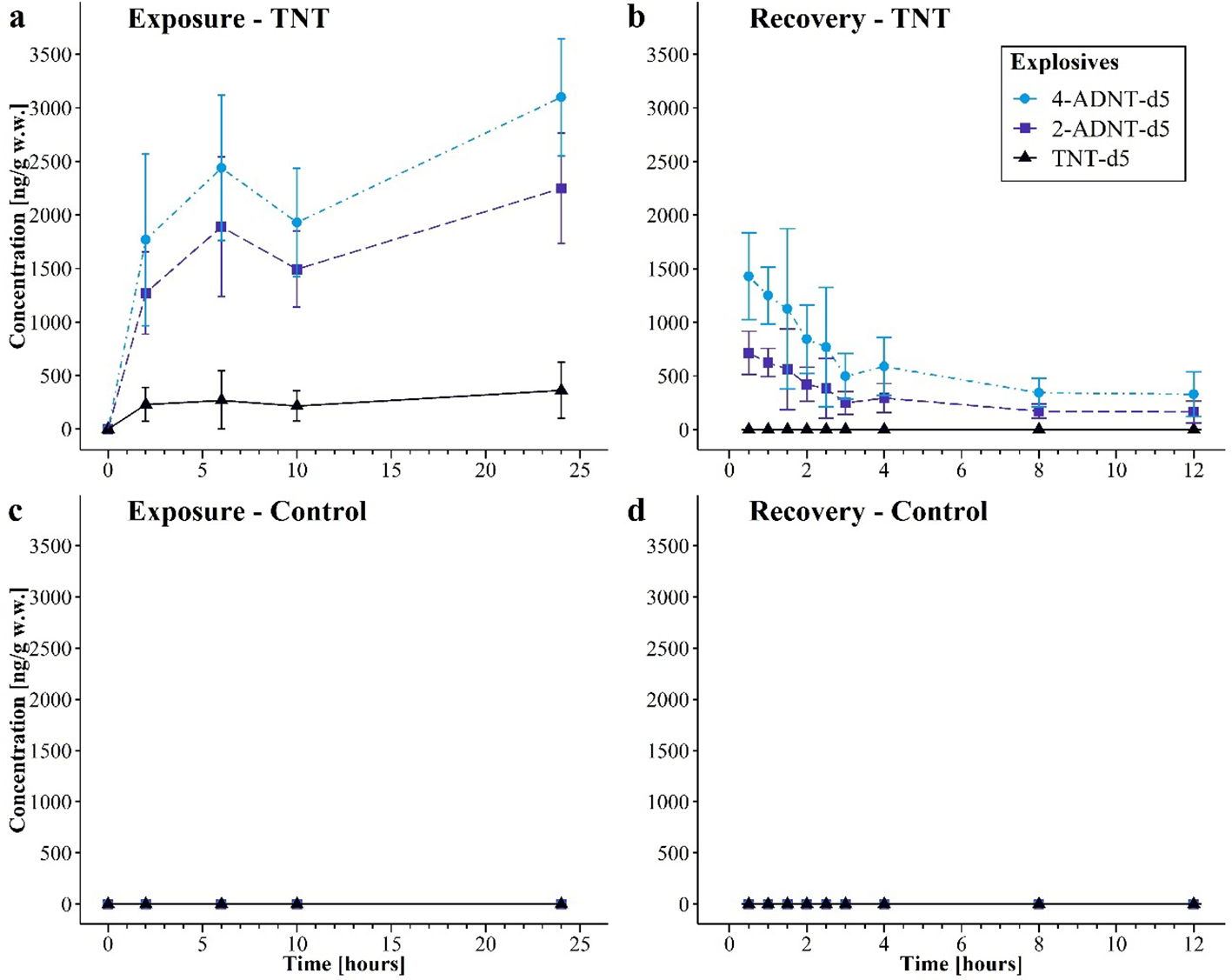
Concentrations of detected 2,4,6-trinitrotoluene (TNT-d5), 2-amino-4,6-dinitrotoluene (2-ADNT-d5), and 4-amino-2,6-dinitrotoluene (4-ADNT-d5) in mussel soft bodies during the exposure and recovery phases for the second experiment (mean ± 95% CI). (a) TNT-d5 treatment exposure, (b) TNT-d5 treatment recovery, (c) control exposure, and (d) control recovery.
Once the mussels had taken up TNT-d5, its metabolites were present. In general, the concentration of ADNTs followed the same pattern as the TNT-d5 concentration (before start of exposure, 0 ng/g; end of exposure, 2,248.3 ng/g, 95% CI: 1,731.4–2,765.3 ng/g 2-ADNT-d5 and 3,098.3 ng/g, 95% CI: 2,551.5–3,645.1 ng/g 4-ADNT-d5). The first peak of ADNT could be detected after 6 h (Figure 6a) (2-ADNT-d5, 1,889.2 ng/g, 95% CI: 1,238.9–2,539.4 ng/g; 4-ADNT-d5, 2,439.2 ng/g, 95% CI: 1,762–3, 115.6 ng/g) followed by a decrease after 10 h (2-ADNT-d5, 1,492.5 ng/g, 95% CI: 1,138.5–1,846.5 ng/g; 4-ADNT-d5, 1,929.2 ng/g, 95% CI: 1,423.0–2,435.3 ng/g). ANOVA indicated a significant increase in 2-ADNT-d5 [F(4, 25) = 25.2, p < 0.001] and 4-ADNT-d5 [F(4, 25) = 26.6, p < 0.001] in mussel tissue. The post-hoc tests indicated those differences, especially between the start of the exposure (0 h) and all later time points (2, 6, 10, and 24 h; all p < 0.001).
After transferring the mussels into clean artificial seawater, TNT-d5 [F(9, 50) = 12.6, p < 0.001], ADNT-d5 [2-ADNT-d5, F(9, 50) = 40.7, p < 0.001], and 4-ADNT [F(9,50) = 26.3, p < 0.001] concentration decreased significantly. Thirty minutes after transfer of the mussels in clean artificial seawater, TNT-d5 and ADNT-d5 concentrations decreased by more than half: TNT could not be detected anymore (p < 0.001), while 2-ADNT-d5 decreased by 58% (concentration after 30 min, 714.3 ng/g, 95% CI: 511.8–916.8 ng/g, p < 0.001) and 4-ADNT-d5 dropped by 54% (concentration after 30 min, 1,428.6 ng/g, 95% CI: 1,023.7–1,833.5 ng/g, p < 0.001) (Figure 6b). During the whole recovery phase, TNT-d5 was not detected, whereas the remaining ADNTs decreased by more than 80% during the first 3 h (concentration after 3 h, 2-ADNT-d5, 250.0 ng/g, 95% CI: 143.4–356.4 ng/g; 4-ADNT-d5, 499.8 ng/g, 95% CI: 286.8–712.7 ng/g). After 12 h of recovery, only 7% 2-ADNT-d5 (164.6 ng/g, 95% CI: 60.3–268.9 ng/g) and 11% 4-ADNT-d5 (329.2 ng/g, 95% CI: 120.6–537.8 ng/g) of the starting concentration were left in mussel soft bodies.
A post-hoc Tukey’s Honestly Significant Difference (HSD) test revealed highly significant differences in concentrations of both derivatives between the end of the exposure phase and all subsequent time points of the recovery (all p < 0.001). The most pronounced decrease was observed between the end of exposure (exposure, 24 h) and the end of the recovery (recovery, 12 h), with a mean reduction in the concentration of 2-ADNT of approximately 2,084 ng/g (95% CI: −2,535 to −1,633 ng/g, p < 0.001) and 4-ADNT of approximately 2,769.1 ng/g (95% CI: −3,516.3 to −2,021.9 ng/g, p < 0.001). Significant differences were also found between adjacent time points during the exposure phase, such as 0.5 and 3 h (2-ADNT, p < 0.05; 4-ADNT, p = 0.005), indicating a continuous depuration process.
The toxicokinetic analysis showed that TNT-d5 exhibited a high depuration rate (k2 = 6.9 h−1) and a very short half-life (t1/2 = 0.1 h), indicating rapid elimination from mussel tissue (Table 5). The uptake rate constant (k1 ) was moderate (0.483 L·kg−1·h−1), resulting in a low bioaccumulation potential, as reflected by both BCFSS and BCFk values of 0.07 L/kg. For the derivatives 2-ADNT-d5 and 4-ADNT-d5, the depuration rates were lower (k2 = 0.439 and 0.389 h−1, respectively), with corresponding half-lives of 1.58 and 1.78 h and acceptable model fits (R2 = 0.714 and 0.811, respectively), suggesting slower elimination compared to TNT-d5.
Table 5
| Toxicokinetic parameter | TNT-d5 | 2-ADNT-d5 | 4-ADNT-d5 |
|---|---|---|---|
| k1 (L·kg−1·h−1) | 0.483 | ||
| k2 (h−1) | 6.9 | 0.439 | 0.389 |
| R2 | 0.714 | 0.811 | |
| t1/2 (h) | 0.1 | 1.58 | 1.78 |
| BCFss (L/kg) | 0.07 | ||
| BCFk (L/kg) | 0.07 |
Toxicokinetic parameters (uptake rate constant, k1; depuration rate constant, k2; half-life, t1/2; model fit, R2; half-life, t1/2; bioconcentration factor at steady state, BCFss; kinetic bioconcentration factor, BCFk) for TNT-d5, 2-ADNT-d5, and 4-ADNT-d5 of the different treatments during the first experiment.
TNT, 2,4,6-trinitrotoluene; 4-ADNT, 4-amino-2,6-dinitrotoluene; 2-ADNT, 2-amino-4,6-dinitrotoluene.
4 Discussion
The temperature, salinity, and light regime used in our laboratories reflected environmental conditions at the time of sampling on the island of Sylt. In that way, potential additional stress due to the laboratory treatment was reduced as much as possible. To verify that the mussels were in a comparable status, only the mussels that were 5–6 cm in length were selected, and the condition index for the mussels was calculated. The results of the condition index showed no significant differences, indicating comparable physiological conditions between the tested mussels. Furthermore, no spawning was observed during the experiments. Spontaneous spawning during the laboratory experiments occurred if mature individuals experienced abrupt temperature, salinity, or pH changes (Dharmaraj et al., 2010) and may be a sign of inadequate acclimatization of the testing animals after the transfer to the laboratory. Therefore, differences in the results of chemical analysis are most likely related to the different exposure scenarios and not to the different physiological status of the individuals. Likewise, there was no mortality observed before and during the experiment. Furthermore, to ensure that no pre-contamination of natural seawater biases the results of the exposure experiment, artificial seawater was mixed based on deionized tap water and special sea salt produced for pollutant-sensitive coral aquaria. This water was then used for treatment and control aquaria.
The results of soft body analysis in this study revealed the uptake of TNT by the mussels as well as the biotransformation of TNT to 2-ADNT, 4-ADNT, and 2,4-DANT in the mussels. This is in accordance with the findings by several studies (e.g., Appel et al., 2018; Ballentine et al., 2015; Ek et al., 2006; Rosen and Lotufo, 2007b; Schuster et al., 2021; Strehse et al., 2017) that detected TNT and its metabolites within transplanted blue mussels as well as in the mussels used in the laboratory. Clearly, TNT uptake in mussels depends on the efficiency of filtering. Several studies have already provided evidence for shell closing of mussels as a defense mechanism against high chemical pollution (Hartmann et al., 2016; Salánki et al., 2003; Schuster et al., 2021; Strehse and Maser, 2020; Viarengo and Canesi, 1991). In the present study, the mussels exposed in the second experiment to relatively high concentrations of dissolved TNT took up the TNT until the end of the experiment, reflecting a continuous uptake over the whole exposure phase rather than a partly interrupted uptake due to longer shell closings. Therefore, the shell closing mechanism is considered to play a minor role in the TNT uptake of the present study. Furthermore, active filtering was observed alongside but not documented. However, data on TNT uptake during the exposure phase of the second experiment revealed a temporary decrease in tissue concentration 10 h after the start of exposure. Interestingly, TNT levels increased again after 24 h. The 10-h sample was taken at 6:30 pm, while the 24-h sample was collected at 8:00 am. This temporary decline in tissue concentration is likely due to the reduced filtration activity of the mussels as they prepared for their rest at night, leading to decreased TNT uptake. However, this fluctuation appears to be part of the mussels’ natural circadian rhythm rather than a response to TNT exposure levels.
Furthermore, several studies have shown the biotransformation of TNT by other invertebrates and marine biota (Ballentine et al., 2015; Rosen and Lotufo, 2007b). In the present study, the biotransformation to 4-ADNT is preferred over 2-ADNT, which results in a slightly higher 4-ADNT concentration. This may be related to the thermodynamically preferred formation of 4-ADNT over 2-ADNT, which was, for instance, detected by McCormick et al. (1976). Similar to these findings, Ballentine et al. (2015) detected a preferred biotransformation of TNT to 4-ADNT over 2-ADNT with a ratio of 3:1 for M. edulis. Of note, the TNT concentration in aquaria without mussels did not change over time, and no biotransformation of TNT took place. In this way, it could be proven for this study that TNT transformation took place exclusively in the presence of mussels.
It is known that M. edulis contains several enzyme systems like cytochrome P450 monooxygenase, which is assumed to play a role in TNT metabolism (Livingstone and Pipe, 1992). Cytochrome P450 contains a variety of different heme proteins that catalyze oxygenase reactions. In organisms, cytochrome P450 is in charge not only of the synthesis and degradation of endogenous substrates (like steroids and fatty acids) but also of the phase I detoxification of xenobiotics, including nitroaromatic compounds (Kovacic and Somanathan, 2014; Stegemann and Hahn, 1994). In general, the phase I detoxification comprises the exposure or addition of functional groups, which describes oxidation, reduction, and hydrolysis (Stegemann and Hahn, 1994). In molluscs, the P450 enzyme systems have been identified in digestive gland tissues as reviewed by Stegemann and Hahn (1994), which is the main detoxification organ in molluscs (e.g., Marigómez et al., 2002; Moore, 1985, 1988). However, overall, there is no direct proof that mussel enzymes metabolize. In fact, it is also possible that this is a microbial metabolization by bacteria present on the soft body of the mussels or within the gut system of the animals.
In the present study, already more than 50% of TNT was transformed in the mussels at the end of the exposure phase to its main derivatives 2-ADNT, 4-ADNT, and 2,4-DANT or depurated within the first 4 h. In the second experiment, even 100% of the TNT got depleted. Likewise, over 76% of the soft body concentration of TNT metabolites got depleted shortly after the start of the recovery phase in both experiments. This fits with the findings of Rosen and Lotufo (2007b), who detected a rapid transformation of TNT to 2-ADNT and 4-ADNT in the phylogenetically related Mediterranean mussel M. galloprovincialis. The fact that 2-ADNT and 4-ADNT soft body concentrations during the recovery phase decreased over time in both experiments indicates the depuration or further biotransformation of those compounds. Correspondingly, water samples of the recovery phase showed a slight increase in ADNT concentration over time, indicating a depuration of those compounds. As shown in the first experiment, in the TNT stability control, TNT biotransformation did not take place in water without mussels. Even though photolysis was shown to be a possible degradation pathway for TNT (Luning Prak et al., 2017), the results of the TNT stability control can exclude this pathway for the conducted experiments of the present study. This clearly reveals that the ADNTs measured in the exposure experiments originate from the mussels.
Surprisingly, the water of the aquaria of the recovery phase for the treated mussels contained TNT before the mussels were transferred into the aquaria. Most likely, an unintended contamination occurred during the experimental setup or the preparation of the samples. After the mussels were set into the aquaria for the recovery phase, the TNT concentration within the water increased rapidly, except for that of the control group. TNT concentrations in water were suddenly in the range of 100 µg, while TNT concentrations in mussel soft bodies were still in the order of ng. The reason for the high concentration within the water of the recovery aquaria was most probably that the mussels, upon their transfer, although rinsed from outside, still contained some of the exposure water within their shells. The exposure water had a concentration of 5 mg/L. Hence, it was likely that the transferred mussels “contaminated” the recovery aquaria when starting their filtering activity again. Even though some TNT contamination took place in this way, the increasing trend of ADNTs over time still can be interpreted as deriving from depuration.
The potential of TNT to bioconcentrate is assumed as low due to its weakly hydrophobic characteristic and the relatively low affinity for lipid-containing tissues (Lotufo et al., 2013; Rosen and Lotufo, 2007b). Former studies already showed depuration and a low BCF of energetic compounds, including TNT and its metabolites, in different aquatic invertebrates and fish (Ballentine et al., 2015; Lotufo et al., 2009, 2013; Rosen and Lotufo, 2007b). For instance, Rosen and Lotufo (2007b) measured a slow uptake (0.8 mL·g−1·h−1) and fast depuration (2.1 h−1) of TNT in the mussel M. galloprovincialis, resulting in a low kinetic BCF (0.3 mL/g) and steady-state BCF (0.23 mL/g). In addition, the authors could also detect the elimination of ADNTs (Rosen and Lotufo, 2007b). Furthermore, Lotufo et al. (2016) found TNT depuration rates between 0.02 and 1.4 h−1. Ballentine et al. (2015) found similar results regarding TNT BCF (1 mL/g) in the blue mussel M. edulis. The toxicokinetic parameters determined in the present study are in the range of the reported studies (e.g., second experiment: k1 = 0.48 L·kg−1·h−1, k2 = 6.9 h−1, BCFss = 0.07 L/kg). However, typically, a substance is only considered bioaccumulative if it has a BCF of above 1,000 (Arnot and Gobas, 2006). Therefore, as indicated by the present study, TNT and its derivatives are not regarded as bioaccumulative. Nevertheless, it has been considered that the lipid content has an influence on the depuration rate of munition compounds (Arnot and Gobas, 2006): the higher the lipid content, the higher the tendency of bioconcentration (Rosen and Lotufo, 2007b). Hence, the potential of bioconcentration within mussel species could slightly differ depending on their lipid content, as discussed by Rosen and Lotufo (2007b; Ballentine et al., 2015; Lotufo et al., 2009, 2013; Rosen and Lotufo, 2007b). The low bioconcentration of energetic compounds in mussel tissue or in marine biota still affects the potential transfer of these compounds within the food web. As organisms being consumed by many other species, mussels are also potentially contributing to the transfer of contaminants via the food web (Beddington and Kinloch, 2005; Farrington et al., 1983). This is especially true due to their efficient uptake of chemicals by intense filtering activity (Strehse and Maser, 2020). In case of energetic compounds, however, the relatively fast depuration shown in the present study and the low tendency for bioaccumulation will not lead to the accumulation of energetic compounds within the mussel itself and thus not in organisms preying on mussels. Since corrosion of the metal casings of munition items continues, more TNT will be dissolved in marine waters over time, thus most probably increasing the uptake of dissolved TNT by mussels and at the same time the uptake of TNT and its metabolites by predators via their food.
Even though TNT and its derivatives got depurated fast in the present study, the literature provides evidence of adverse health effects in a variety of different kinds of organisms including humans (e.g., Koske et al., 2020; Kovacic and Somanathan, 2014; Nipper et al., 2001; Schuster et al., 2021; U.S. Department of Health and Human Services: Public Health Service, 1995). Nipper et al. (2001), for instance, tested the lethal and sublethal effects of munition compounds on sea urchin, algae, polychaetes, opossum shrimp, and redfish. The lowest detected effective concentration ranged from 0.26 to 7.6 mg/L. Exposure to munition compounds harms organisms even in their early stages of development. Rosen and Lotufo (2007a) found a more sensitive reaction of juvenile mussels (M. galloprovincialis) to TNT exposure compared to adults, with a lethal concentration of 0.75 mg/L for juveniles and 19.5 mg/L for adults. This shows that exposure to explosive compounds results in a serious health risk to a variety of organisms, even though the tendency for bioaccumulation seems to be low. As the exposure to munition compounds in the environment proceeds, the impairment of organisms may worsen.
5 Conclusions
The results achieved in the present study revealed TNT uptake, rapid biotransformation, and fast depuration in M. edulis depending on the TNT treatment concentration. Overall, 40% to 75% of the TNT in the mussel soft body was already transformed to 2-ADNT, 4-ADNT, and 2,4-DANT in the first 4 h. The highest TNT treatment (2.5 mg/L in the first experiment and 5 mg/L in the second) had the highest TNT uptake and the highest concentration of derivatives. All derivatives as well as the parent compound TNT decreased over time within mussel soft bodies, indicating further transformation or depuration. Water samples of the recovery phase revealed an increase in ADNTs in the water, supporting the assumption of depuration. Furthermore, the toxicokinetic parameters indicated fast depuration and a low potential for the bioaccumulation of TNT and its derivatives.
However, still, more research is needed for a better understanding of TNT uptake, metabolization, and depuration by mussels to conduct a more reliable risk assessment for TNT in marine biota. Shell closing represents an important mechanism in preventing the uptake of chemicals and, therefore, should also be taken into account in future studies. In addition, the rapid TNT transformation and depuration should be considered in future studies based on wild mussel field sampling strategies.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.pangaea.de/10.1594/PANGAEA.972751.
Ethics statement
The animal study was approved by Dr. Gisela Lannig, Animal Welfare Officer, Alfred Wegener Institute. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
FB: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft. RS: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – review & editing. LB: Formal Analysis, Validation, Writing – original draft. JS: Formal Analysis, Validation, Writing – review & editing. MiB: Formal Analysis, Methodology, Validation, Writing – original draft. RH: Resources, Supervision, Writing – review & editing. EM: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. MaB: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research received funding from the European Union (European Regional Development Fund) under the North Sea Interreg Programme 2014–2020, within the framework of the project “North Sea Wrecks—An Opportunity for Blue Growth: Healthy Environment, Shipping, Energy Production and Transmission (NSW). Further funding was received by the German Federal Ministry of Education and Research (BMBF) within the framework of the project “CONMAR—Concepts for conventional Marine Munition Remediation in the German North and Baltic Sea” (Grant: 03F0912) of the DAM Research Mission sustainMare. The publication costs were financed by the Alfred Wegener Institute’s Open Access Fund.
Acknowledgments
We are very thankful to Dr. Jörn Reinhardt and his team from the German Federal Facility for the Removal and Remediation of Explosives and Chemical Warfare Agents (GEKA, Münster, Germany) for providing the TNT stock solution used for the exposure studies. We also thank our colleague Tim Kress from the AWI-Wattenmeer station on the island of Sylt for providing the blue mussels needed for the experiments. Furthermore, we are also thankful to the three reviewers for providing valuable comments and remarks, helping us to improve the quality of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adomako-Bonsu A. G. Jacobsen J. Maser E. (2024). Metabolic activation of 2,4,6-trinitrotoluene; a case for ROS-induced cell damage. Redox Biol.72, 103082. doi: 10.1016/j.redox.2024.103082
2
Appel D. Beck A. J. Eggert A. Gräwe U. Kampmeier M. Martin H.-J. et al . (2019). Practical Guide for Environmental Monitoring of Conventional Munitions in the Seas - Results from the BMBF funded project UDEMM “Umweltmonitoring für die Delaboration von Munition im Meer” Version 1.1. Ed. GreinertJ. (Kiel, Germany: GEOMAR Helmholtz-Zentrum fur Ozeanforschung). doi: 10.3289/GEOMAR_REP_NS_54_2019
3
Appel D. Strehse J. S. Martin H. J. Maser E. (2018). Bioaccumulation of 2,4,6-trinitrotoluene (TNT) and its metabolites leaking from corroded munition in transplanted blue mussels (M. edulis). Marine Pollution Bull.135, 1072–1078. doi: 10.1016/j.marpolbul.2018.08.028
4
Arnot J. A. Gobas F. A. P. C. (2006). A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev.14, 257–297. doi: 10.1139/a06-005
5
Ballentine M. Tobias C. Vlahos P. Smith R. Cooper C. (2015). Bioconcentration of TNT and RDX in coastal marine biota. Arch. Environ. Contamination Toxicol.68, 718–728. doi: 10.1007/s00244-014-0104-9
6
Beck A. J. Gledhill M. Schlosser C. Stamer B. Böttcher C. Sternheim J. et al . (2018). Spread, behavior, and ecosystem consequences of conventional munitions compounds in coastal marine waters. Front. Marine Sci.5. doi: 10.3389/fmars.2018.00141
7
Beck A. J. van der Lee E. M. Eggert A. Stamer B. Gledhill M. Schlosser C. et al . (2019). In situ measurements of explosive compound dissolution fluxes from exposed munition material in the Baltic sea. Environ. Sci. Technol.53, 5652–5660. doi: 10.1021/acs.est.8b06974
8
Beddington J. Kinloch A. J. (2005). Munitions dumped at sea: A literature review (London: Imperial College London Consultants).
9
Bolt H. M. Degen G. H. Dorn S. B. Plöttner S. Harth V. (2006). Genotoxicity and potential carcinogenicity of 2,4,6-trinitrotoluene: Structural and toxicological considerations. Rev. Environ. Health21, 217–228. doi: 10.1515/REVEH.2006.21.4.217
10
Böttcher C. Knobloch T. Rühl N.-P. Sternheim J. Wichert U. Wöhler J. (2011). Munitionsbelastung der deutschen Meeresgewässer - Bestandsaufnahme und Empfehlungen (Stand 2011) (Hamburg: Bundesamt für Seeschifffahrt und Hydrographie).
11
Brenner M. Broeg K. Frickenhaus S. Buck B. H. Koehler A. (2014). Multi-biomarker approach using the blue mussel (Mytilus edulis L.) to assess the quality of marine environments: season and habitat-related impacts. Marine Environ. Res.95, 13–27. doi: 10.1016/j.marenvres.2013.12.009
12
Bünning T. H. Strehse J. S. Hollmann A. C. Botticher T. Maser E. (2021). A toolbox for the determination of nitroaromatic explosives in marine water, sediment, and biota samples on femtogram levels by GC-MS/MS. Toxics9, 60. doi: 10.3390/toxics9030060
13
Dharmaraj S. Victor A. C. C. Jagadis I. (2010). “Induced Breeding and Seed Production of Molluscs_Coastal and aquaculture mangment,” in Coastal Fisheries and Aquaculture Management. Eds. GopakumarK.DiwanA.D. (New Delhi, India: Narendra Publishing House), 323–350, ISBN: isbn:9789389660227.
14
Ek H. Dave G. Nilsson E. Sturve J. Birgersson G. (2006). Fate and effects of 2,4,6-trinitrotoluene (TNT) from dumped ammunition in a field study with fish and invertebrates. Arch. Environ. Contamination Toxicol.51, 244–252. doi: 10.1007/s00244-005-0117-5
15
Ek H. Nilsson E. Dave G. (2008). Effects of TNT leakage from dumped ammunition on fish and invertebrates in static brackish water systems. Ecotoxicol. Environ. Saf.69, 104–111. doi: 10.1016/j.ecoenv.2006.12.016
16
European Commission Joint Research Centre (2016). Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food (Luxembourg: Publications Office of the EU). doi: 10.2787/8931
17
Farrington J. W. Goldberg E. D. Risebrough R. W. Martin J. H. Bowen V. T. (1983). U.S. “Mussel Watch” 1976-1978: an overview of the trace-metal, DDE, PCB, hydrocarbon and artificial radionuclide data. Environ. Sci. Technol.17, 490–496. doi: 10.1021/es00114a010
18
Frey T. Greiner J. Maser E. Scharsack J. Brenner M. (2024). Munition im Meer – Sachstand und Perspektiven, Section: 3. Umweltbeeinträchtigung Vol. 12 (Germany: Berlin).
19
Gledhill M. Beck A. J. Stamer B. Schlosser C. Achterberg E. P. (2019). Quantification of munition compounds in the marine environment by solid phase extraction - ultra high performance liquid chromatography with detection by electrospray ionisation - mass spectrometry. Talanta200, 366–372. doi: 10.1016/j.talanta.2019.03.050
20
Harrison I. Vane C. H. (2010). Attenuation of TNT in seawater microcosms. Water Sci. Technol.61, 2531–2538. doi: 10.2166/wst.2010.171
21
Hartmann J. T. Beggel S. Auerswald K. Stoeckle B. C. Geist J. (2016). Establishing mussel behavior as a biomarker in ecotoxicology. Aquat. Toxicol.170, 279–288. doi: 10.1016/j.aquatox.2015.06.014
22
Hawari J. Beaudet S. Halasz A. Thiboutot S. Ampleman G. (2000). Microbial degradation of explosives: biotransformation versus mineralization. Appl. Microbiol. Biotechnol.54, 605–618. doi: 10.1007/s002530000445
23
Heiss G. Knackmuss H.-J. (2002). Bioelimination of trinitroaromatic compounds: immobilization versus mineralization. Curr. Opin. Microbiol.5, 282–287. doi: 10.1016/s1369-5274(02)00316-8
24
Jenkins T. F. Pennington J. C. Ranney T. A. Berry T. E. Miyares P. H. Walsh M. E. et al . (2001). Characterization of Explosives Contamination at Military Firing Ranges *ERDC TR-01–5 Final/Technical Report* Vol. 36 (Hanover: NH: US Army Engineer Research and Development Center).
25
Juhasz A. L. Naidu R. (2007). Explosives: Fate, dynamics, and ecological impact in terrestrial and marine environments. Rev. Environ. Contamination Toxicol.191, 163–215. doi: 10.1007/978-0-387-69163-3_6
26
Koske D. Goldenstein N. I. Kammann U. (2019). Nitroaromatic compounds damage the DNA of zebrafish embryos (Danio rerio). Aquat. Toxicol.217, 105345. doi: 10.1016/j.aquatox.2019.105345
27
Koske D. Straumer K. Goldenstein N. I. Hanel R. Lang T. Kammann U. (2020). First evidence of explosives and their degradation products in dab (Limanda limanda L.) from a munition dumpsite in the Baltic Sea. Marine Pollution Bull.155, 111131. doi: 10.1016/j.marpolbul.2020.111131
28
Kovacic P. Somanathan R. (2014). Nitroaromatic compounds: Environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J. Appl. Toxicol.34, 810–824. doi: 10.1002/jat.2980
29
Kröger M. Fels G. (2000). 14C synthesis revisited. J. Labelled Compound Radiopharmaceuticals43, 217–227. doi: 10.1002/(SICI)1099-1344(20000315)43:3<217::AID-JLCR305>3.0.CO;2-U
30
Livingstone D. R. Pipe R. K. (1992). “Mussels and environmental contaminants: molecular and cellular aspects,” in The mussel Mytilus; ecology, physiology, genetics, and culture. Ed. GoslingE. G. (Elsevier, Amsterdam), 425–464, ISBN: isbn:978-0444887528.
31
Lotufo G. R. Belden J. B. Fisher J. C. Chen S. F. Mowery R. A. Chambliss C. K. et al . (2016). Accumulation and depuration of trinitrotoluene and related extractable and nonextractable (bound) residues in marine fish and mussels. Environ. Pollut.210, 129–136. doi: 10.1016/j.envpol.2015.11.049
32
Lotufo G. R. Lydy M. Rorrer G. L. Cruz-Uribe O. Cheney D. P. (2009). “Bioconcentration, Bioaccumulation, and Biotransformation of Explosives and Related Compounds in Aquatic Organisms,” in Ecotoxicology of Explosives. Eds. SunaharaG. I.LotufoG.KupermanR. G.HawariJ. (CRC Press, Boca Raton, London, New York), 135–155. doi: 10.1201/9781420004342.ch6
33
Lotufo G. R. Rosen G. Wild W. Carton G. (2013). Summary Review of the Aquatic Toxicology of Munitions Constituents (Dordrecht, Netherlands: Springer Science+Business Media), 1–124.
34
Luning Prak D. J. Breuer J. E. Rios E. A. Jedlicka E. E. O’Sullivan D. W. (2017). Photolysis of 2,4,6-trinitrotoluene in seawater and estuary water: Impact of pH, temperature, salinity, and dissolved organic matter. Marine Pollution Bull.114, 977–986. doi: 10.1016/j.marpolbul.2016.10.073
35
Marigómez I. Soto M. Cajaraville M. P. Angulo E. Giamberini L. (2002). Cellular and subcellular distribution of metals in molluscs. Microscopy Res. Technique56, 358–392. doi: 10.1002/jemt.10040
36
Maser E. Bünning T. H. Brenner M. Van Haelst S. De Rijcke M. Müller P. et al . (2023). Warship wrecks and their munition cargos as a threat to the marine environment and humans: The V 1302 “JOHN MAHN” from World War II. Sci. Total Environ.857, 159324. doi: 10.1016/j.scitotenv.2022.159324
37
Maser E. Strehse J. S. (2020). Don’t Blast”: blast-in-place (BiP) operations of dumped World War munitions in the oceans significantly increase hazards to the environment and the human seafood consumer. Arch. Toxicol.94, 1941–1953. doi: 10.1007/s00204-020-02743-0
38
McCormick N. G. Feeherry F. E. Levinson H. S. (1976). Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl. Environ. Microbiol.31, 949–958. doi: 10.1128/aem.31.6.949-958.1976
39
Moore M. N. (1985). Cellular responses to pollutants. Marine Pollution Bull.16, 134–139. doi: 10.1016/0025-326X(85)90003-7
40
Moore M. N. (1988). Cytochemical responses of the lysosomal system and NADPH-ferrihemoprotein reductase in molluscan digestive cells to environmental and experimental exposure to xenobiotics. Marine Ecol. Prog. Ser.46, 81–89. doi: 10.3354/meps046081
41
Nipper M. Carr R. S. Biedenbach J. M. Hooten R. L. Miller K. Saepoff S. (2001). Development of marine toxicity data for ordnance compounds. Arch. Environ. Contamination Toxicol.41, 308–318. doi: 10.1007/s002440010253
42
Nipper M. Carr R. S. Lotufo G. R. (2009). “Aquatic toxicology of explosives,” in Ecotoxicology of Explosives. Eds. SunaharaG. I.LotufoG.KupermanR. G.HawariJ. (CRC Press, Boca Raton, London, New York), 77–115. doi: 10.1201/9781420004342.ch4
43
OECD (2012). Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure *OECD Guidelines for the Testing of Chemicals, Section 3*, (305) (Paris: OECD Publishing). doi: 10.1787/9789264185296-en
44
Rosen G. Lotufo G. R. (2007a). Toxicity of explosive compounds to the marine mussel, Mytilus galloprovincialis, in aqueous exposures. Ecotoxicol. Environ. Saf.68, 228–236. doi: 10.1016/j.ecoenv.2007.03.006
45
Rosen G. Lotufo G. R. (2007b). Bioaccumulation of explosive compounds in the marine mussel, Mytilus galloprovincialis. Ecotoxicol. Environ. Saf.68, 237–245. doi: 10.1016/j.ecoenv.2007.04.009
46
Sabbioni G. Rumler R. (2007). Biomonitoring of workers cleaning up ammunition waste sites. Biomarkers12, 559–573. doi: 10.1080/13547500701456206
47
Salánki J. Farkas A. Kamardina T. Rózsa K. S. (2003). Molluscs in biological monitoring of water quality. Toxicol. Lett.140-141, 403–410. doi: 10.1016/s0378-4274(03)00036-5
48
Schuster R. Strehse J. S. Ahvo A. Turja R. Maser E. Bickmeyer U. et al . (2021). Exposure to dissolved TNT causes multilevel biological effects in Baltic mussels (Mytilus spp.). Marine Environ. Res.167, 105264. doi: 10.1016/j.marenvres.2021.105264
49
Serrano-González M. Y. Chandra R. Castillo-Zacarias C. Robledo-Padilla F. Rostro-Alanis M. d. J. et al . (2018). Biotransformation and degradation of 2,4,6-trinitrotoluene by microbial metabolism and their interaction. Defence Technol.14, 151–164. doi: 10.1016/j.dt.2018.01.004
50
Sims J. G. Steevens J. A. (2008). The role of metabolism in the toxicity of 2,4,6-trinitrotoluene and its degradation products to the aquatic amphipod Hyalella azteca. Ecotoxicol. Environ. Saf.70, 38–46. doi: 10.1016/j.ecoenv.2007.08.019
51
Sisco E. Najarro M. Bridge C. Aranda R. (2015). Quantifying the degradation of TNT and RDX in a saline environment with and without UV-exposure. Forensic Sci. Int.251, 124–131. doi: 10.1016/j.forsciint.2015.04.0
52
Stegemann J. J. Hahn M. E. (1994). “Biochemistry and molecular biology of monooxygenases: current perspectives on forms, functions and regulation of cytochrome P450 in aquatic species,” in Aquatic Toxicology: Molecular, Biochemical, and Cellular Perspectives. Eds. MalinsD. C.OstranderG. K. (Lewis Publisher Inc, Boca Raton, FL, USA), 87–204, ISBN: isbn:9781351069878.
53
Strehse J. S. Appel D. Geist C. Martin H. J. Maser E. (2017). Biomonitoring of 2,4,6-trinitrotoluene and degradation products in the marine environment with transplanted blue mussels (M. edulis). Toxicology390, 117–123. doi: 10.1016/j.tox.2017.09.004
54
Strehse J. S. Maser E. (2020). Marine bivalves as bioindicators for environmental pollutants with focus on dumped munitions in the sea: A review. Marine Environ. Res.158, 105006. doi: 10.1016/j.marenvres.2020.105006
55
Talmage S. S. Opresko D. M. Maxwell C. J. Welsh C. J. Cretella F. M. Reno P. H. et al . (1999). Nitroaromatic munition compounds: environmental effects and screening values. Rev. Environ. Contamination Toxicol.161, 1–156. doi: 10.1007/978-1-4757-6427-7_1
56
U.S. Department of Health and Human Services: Public Health Service (1995). Toxicological profile for 2,4,6-Trinitroluene. Atlanta, GEORGIA: Agency of Toxic Substances and Diseases Registry (Atlanta, Georgia: Agency of Toxic Substances and Diseases Registry).
57
Viarengo A. Canesi L. (1991). Mussels as bioindicators of pollution. Aquaculture94, 225–243. doi: 10.1016/0044-8486(91)90120-V
Summary
Keywords
TNT, derivatives, 2-ADNT, 4-ADNT, metabolic rate, bioaccumulation
Citation
Binder FI, Schuster RM, Bünning LTH, Strehse JS, Brockmann M, Herges R, Maser E and Brenner M (2025) Depuration kinetics of trinitrotoluene (TNT) and its metabolites in exposed blue mussels (Mytilus edulis L.). Front. Mar. Sci. 12:1582441. doi: 10.3389/fmars.2025.1582441
Received
24 February 2025
Accepted
16 May 2025
Published
17 June 2025
Volume
12 - 2025
Edited by
Camilla Della Torre, University of Milan, Italy
Reviewed by
Tomasz Brzeziński, University of Warsaw, Poland
Stefano Tasselli, National Research Council (CNR), Italy
Updates
Copyright
© 2025 Binder, Schuster, Bünning, Strehse, Brockmann, Herges, Maser and Brenner.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Brenner, matthias.brenner@awi.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.