Abstract
The yellow-edged lyretail grouper, Variola louti, is ecologically and commercially important in the Red Sea. As a carnivorous species, V. louti plays a vital role in maintaining coral reef ecosystem health. Annually, an estimated 260 tonnes of V. louti are landed along the Saudi Arabian Red Sea coast, contributing approximately 6.5 million USD to the national economy. Given its significance, effective fishery management is crucial for sustainability. This study assessed the growth, maturity, and mortality of V. louti using fishery-dependent samples collected from the eastern Red Sea (February 2022–December 2024). Fork length (FL, n = 7,087) ranged from 10.5 to 64.3 cm, while total weight (W, n = 2,629) varied from 53 to 2,910 g. The FL–W relationship for both sexes combined was W = 0.0117×FL3.1. Von Bertalanffy growth parameters, based on sagittal otolith readings, were FL∞ = 62.28 cm, K = 0.12 year−1, and t0 = −2.26 years. Median FL at first maturity was 24.98 cm, with no significant sex differences. Mean natural mortality (M), derived from three different methods, was 0.243 year−1. Total mortality (Z), estimated using a linearized exponential decay model, was 0.38 year−1. Based on these Z and M estimates, fishing mortality (F) was calculated as 0.137 year−1. Yield-per-recruit and spawning potential ratio analyses indicate that the current F estimate places the stock near the target reference point. The current exploitation rate (E = 0.36) remains well below the limit reference point of 0.5. The V. louti fishery encompasses a broad FL range and multiple age groups, with immature individuals comprising less than 12% of total landings. However, according to the minimum landing size of 33 cm FL set by Saudi Arabian fisheries regulations, 47% of the total catch consisted of mature fish aged 2–3 years but below this threshold (25 cm ≤ FL < 33 cm), while only 41% met the legal-size requirement (FL > 33 cm). The high proportion of legally undersized individuals in landings underscores the need for ongoing monitoring and additional management measures, such as catch quotas, seasonal restrictions, and fishing effort reduction, to ensure long-term stock sustainability.
1 Introduction
Groupers of the family Epinephelidae exhibit complex life history traits, and consequently, many species lack comprehensive life history information necessary for effective management. These species are generally of commercial importanance worldwide, contributing to human diets and supporting commercial, recreational, and subsistence fisheries (Schemmel and Dahl, 2023). Groupers are ubiquitous predators that play a crucial role in regulating the abundance of prey species (Hixon and Carr, 1997; Stewart and Jones, 2001), thereby contributing to the health and stability of coral reef ecosystems. Their ecological importance to reef ecosystems is well recognized (Craig et al., 2013), yet their potential impact on the Red Sea coral reefs from a regional perspective remains largely unexplored. Despite their ecological significance, groupers are particularly vulnerable to fishing pressure due to their life history characteristics, including longevity, late sexual maturation, and spawning aggregations (Rhodes and Tupper, 2007; Craig et al., 2013).
The yellow-edged lyretail grouper Variola louti (Forsskål, 1775) is one of two species in the genus Variola and has a wide Indo-Pacific distribution. It inhabits coral reefs, reef crests, and outer slope habitats at depths ranging from 15 to 250 m (Hiatt and Strasburg, 1960; Heemstra and Randall, 1993; Nair et al., 2018). This non-migratory species primarily feeds on fish, crabs, shrimps, and stomatopods (Froese and Pauly, 2025). V. louti has been reported to reach a maximum fork length (FL) of 81 cm (Craig et al., 2011) and is described as a protogynous hermaphrodite species, undergoing female-to-male sex change at approximately 35 cm FL and 6 years of age (Schemmel and Dahl, 2023).
Globally, traditional fisheries dominate coral reef ecosystems (Salas et al., 2007; Garcia and Rosenberg, 2010), with groupers being a particularly important target species (Schemmel and Dahl, 2023). Despite their commercial importance, few grouper fisheries are regularly monitored, and many are reported to be in decline (Sadovy de Mitcheson et al., 2013; Frisch et al., 2016). In the Western Indian Ocean, groupers are actively targeted by artisanal fisheries, yet information on their populations and fisheries in this region remains limited (Craig et al., 2013). Declines in V. louti populations have been observed in areas where it is frequently targeted, such as in the Maldives and the Mariana Islands (Nair et al., 2018), highlighting the need for improved monitoring and management efforts.
The Red Sea harbors rich coral reef ecosystems. As the nation with the longest coastline along the Red Sea, Saudi Arabia’s small-scale fisheries harvest a significant portion of these resources. Similar to other regions (Rhodes and Tupper, 2007; Huliselan et al., 2017; Giglio et al., 2018), groupers play a crucial role in supporting traditional fisheries along the Saudi Arabian coast of the eastern Red Sea. Groupers account for 47% of the total biomass and 55% of the potential revenue in Saudi markets, and are often sold before reaching sexual maturity (Shellem et al., 2021). Among groupers, V. louti is particularly sought after for its high-quality flesh, making it one of the primary target species in the artisanal fishery of the Saudi Arabian Red Sea coast. According to the Food and Agriculture Organization (FAO, 2024), the average annual catch of V. louti in Saudi Arabia from 2016 to 2020 was 432 tonnes. Of this, an estimated 260 tonnes, approximately 60%, originated from the Red Sea coast (Alshaikhi et al., 2023). The market value of V. louti exceeds 25 USD per kg, and it is one of the most targeted species in the region, alongside Plectropomus marisrubri and P. areolatus (Shellem et al., 2021). However, information on the fishery biology and stock status of this species in the Red Sea is lacking, with the exception of a study by Wahbeh (2005) conducted in the Gulf of Aqaba. This earlier investigation, now over 25 years old, was based on a relatively small sample size of 123 specimens. Due to the absence of a commercial fishery in the Gulf of Aqaba at the time, samples were collected using gillnets and fishing traps. Additionally, scales were used to estimate age and growth, rather than the more widely accepted otolith-based methods. In this context, the present study intended to obtain estimates of population parameters, gather updated information on the fishery biology, and evaluate the stock status of V. louti in the eastern Red Sea.
2 Materials and methods
2.1 Biological data collection
Monthly sampling was conducted at nine major fishing ports along the eastern coast of the Red Sea (Figure 1) from February 2022 to December 2024. These locations were selected based on fisheries statistics provided by the Saudi Arabian Ministry of Environment, Water, and Agriculture (MEWA). During 2022 and 2023, a total of 2,629 fish specimens were collected and examined in the laboratory, with relevant biological data recorded. An additional 4,458 specimens were sampled from fishing ports during 2024; for these specimens, only FL (Sparre and Venema, 1998) was measured. FL was measured to the nearest mm, and total weight (W) was recorded to the nearest g for each specimen. To compare mean FL values between sexes, an independent t-test was initially considered. However, the FL data did not meet the assumptions of normality and homoscedasticity, as assessed using the F-test, Shapiro-Wilk normality test, and normal quantile plots (Sokal and Rohlf, 2012). Consequently, the non-parametric Mann-Whitney test was applied instead (Sokal and Rohlf, 2012).
Figure 1
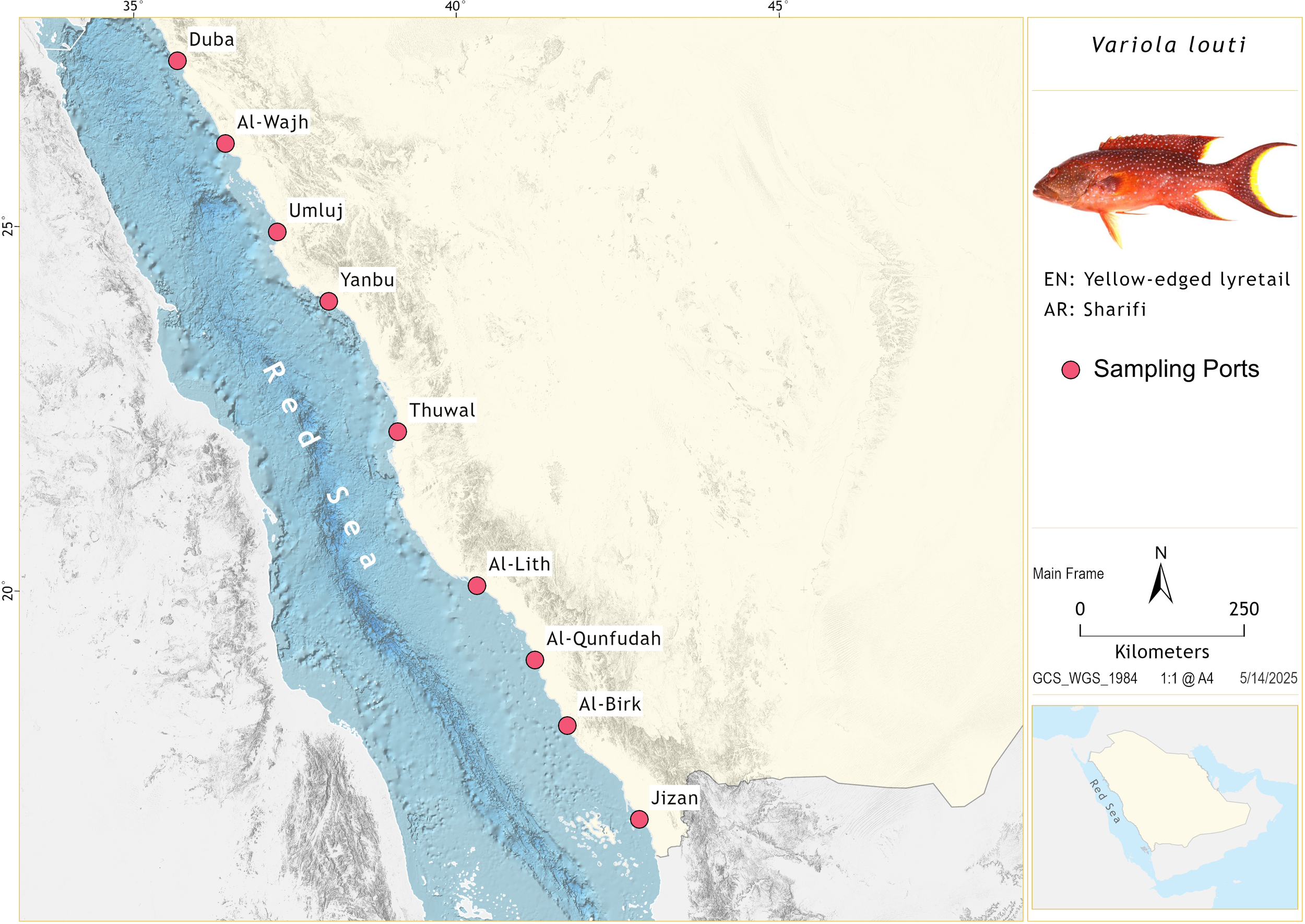
Map showing the ports where V. louti specimens were sampled along the eastern Red Sea coast of Saudi Arabia.
2.2 Otolith extraction and reading
Sagittal otoliths (n=205) (Figure 2, Supplementary Figure S1) were collected as age-determining hard structures from specimens ranging in FL from 18.5 to 64.3 cm, following Secor et al. (1991). Each otolith was cleaned, marked, and embedded in resin to facilitate precise sectioning. Thin transverse sections, approximately 400–500 µm thick, were prepared using a low-speed saw, with section thickness adjusted based on visual inspection under a microscope to ensure optimal clarity for annuli observation. Final polishing was performed by grinding the sections with an EcoMet™ 30 Twin Manual Grinder Polisher (Buehler). The prepared sections were mounted on glass slides using CrystalBond™ 509 adhesive to ensure stability during analysis. Age determination was conducted under a Leica M205 C stereomicroscope at 40x magnification. Annuli were counted along the ventral margin of the sulcus acousticus, extending from the nucleus to the proximal edge of the otolith. To ensure accuracy, otolith images were enhanced digitally, and readings were performed independently by two experienced readers.
Figure 2
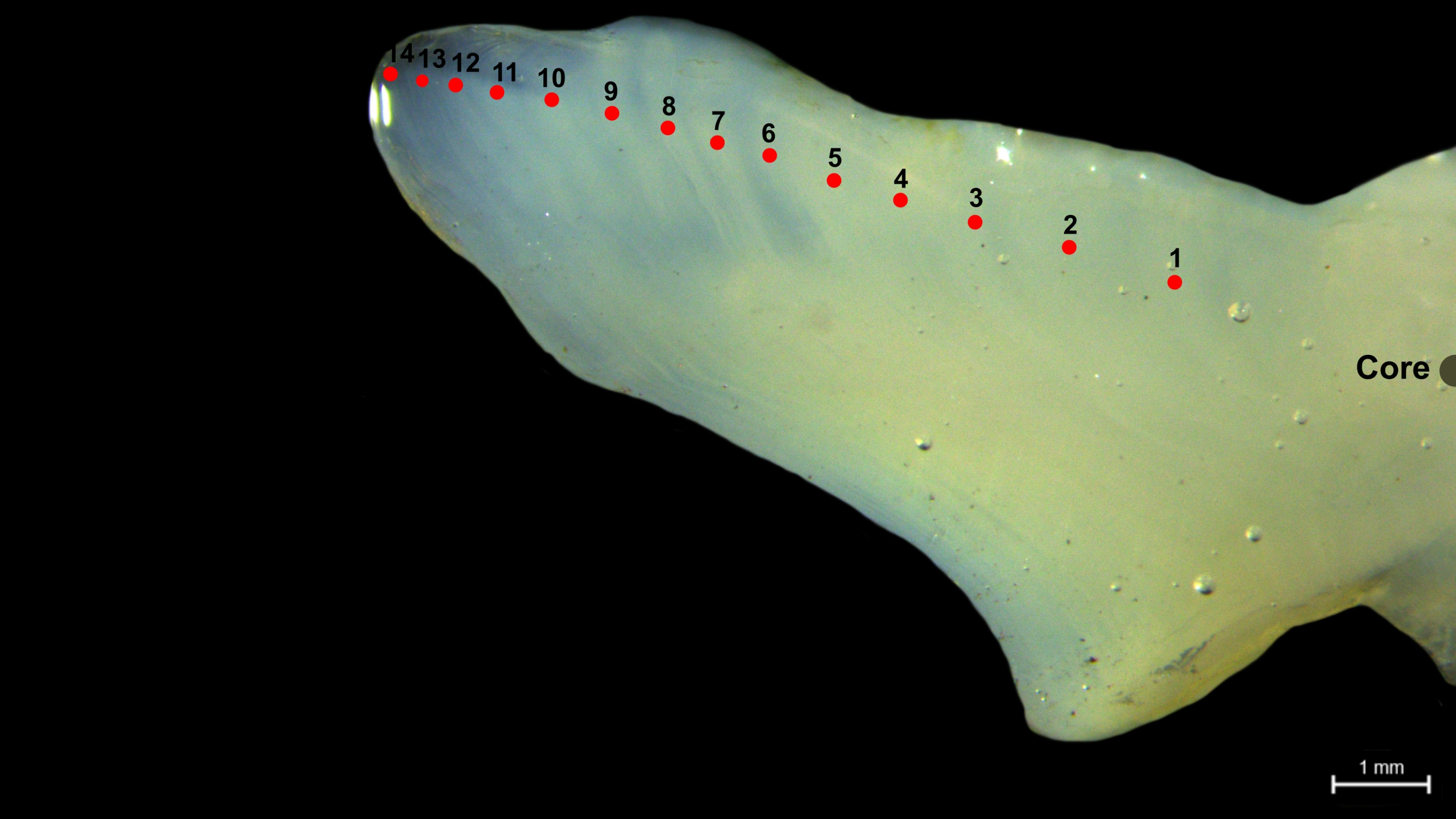
Photomicrograph of transverse sections of a sagittal otolith from a 14-year-old male V. louti specimen from the eastern Red Sea. The red markers highlight the hyaline growth bands representing the annuli.
2.3 Length-weight relationship
The nonlinear relationship between FL and W was analyzed using linear regression after log-transforming all data pairs, assuming a multiplicative error structure (Quinn and Deriso, 1999):
In this relationship, a represents the coefficient describing the proportionality between W and FL, while b is the exponent that characterizes how body mass (W) changes with increasing size (FL). To assess potential differences between sexes, an analysis of covariance (ANCOVA) was performed to compare the a and b values between female and male fish. Departures from isometric growth were evaluated by determining whether the estimated b significantly deviated from the expected value 3. This was assessed by checking whether the confidence interval (CI) for b included the value 3 (Tıraşın, 1993; Quinn and Deriso, 1999).
2.4 Sex and maturity
Sex and gonadal maturity stages of V. louti were determined based on the criteria outlined by Nikolsky (1976), using macroscopic examination of the gonads under standardized lighting conditions to ensure consistency (Supplementary Table S1, Supplementary Figure S2). An exact binomial test was used to compare the observed sex ratio in V. louti samples to the expected 1:1 proportion (Wilson and Hardy, 2002). In addition, a log-likelihood ratio test (G-test) was employed to assess whether the sex ratio varied significantly across years and between seasons (Sokal and Rohlf, 2012). Individuals classified as stage 2 or higher were considered “mature”, while those in stages 0 and 1 were categorized as “immature” (Supplementary Table S1). The median size at first sexual maturity, or the FL at which 50% of fish in the population reach sexual maturity (FL50), was estimated using logistic regression analysis as described by Aydın and Tıraşın (2023). The maturity status of each fish was classified as binary data (immature = 0, mature = 1), and the logistic model was fitted separately to these binomial maturity data for females and males. To evaluate the uncertainty of the FL50 estimates, the bootstrap method (Efron and Tibshirani, 1993; Aydın and Tıraşın, 2023) was applied, generating 5,000 bootstrap samples, from which nonparametric 95% CIs for FL50 were derived using the Bias-Corrected and Accelerated (BCa) method (Efron and Tibshirani, 1993). These CIs were then used to assess whether FL50 differed significantly between females and males by examining whether their ranges overlapped.
2.5 Age and growth
Observed FL-at-age data obtained from otolith readings were used to characterize the growth of V. louti by applying the von Bertalanffy (1938) growth model (Quinn and Deriso, 1999). Originally formulated by Beverton and Holt (1957), this model is expressed as:
where FL∞ represents the asymptotic FL, K is the growth coefficient (also known as the curvature parameter), and t0 is the theoretical age at zero FL. The variables t and FLt denote the age (years) and the expected FL at age t, respectively. The von Bertalanffy growth model was fit to observed FL-at-age data of each sex separately, as well as to combined sex data, using the nonlinear regression method (Ritz and Streibig, 2008), assuming an independent, additive, and normally distributed error structure. The normality of residuals from the fitted models was assessed with normal quantile plots and the Shapiro–Wilk test, while homogeneity of residual variances across sex-specific models was tested via an F-test. Differences in the estimated growth parameters between males and females were evaluated using the likelihood ratio test framework developed by Kimura (1980) and later advocated by Cerrato (1990). The test statistic was compared to the theoretical chi-square distribution with the appropriate degrees of freedom. A large ratio, indicating a significant difference between the reduced model (with combined data and common parameters) and the full model (with sex-specific parameters), can be biologically interpreted as evidence of sexually dimorphic growth. Additional details on the methodology and statistical basis for these comparisons can be found in Kimura (1980); Cerrato (1990), and Quinn and Deriso (1999).
2.6 Mortality rates
Based on the recommendations of Quinn and Deriso (1999); Cope and Hamel (2022), and Maunder et al. (2023), the natural mortality rate (M) was estimated using three empirical methods suggested by Maunder et al. (2023) rather than relying on a single approach. The first two approaches were developed by Hamel and Cope (2022), while the third was proposed by Then et al. (2015). The annual M for V. louti was then determined as the average of the estimates obtained from these three methods:
where tmax represents the maximum age, or longevity, of a species, and K and FL∞ are the von Bertalanffy growth model parameters. Based on the otolith readings, tmax was assumed to be at least 17 years for V. louti (Schemmel and Dahl, 2023). The total mortality rate (Z) was estimated using the classical nonlinear exponential decay model, which assumes a constant rate of decline in the abundance of a fish cohort over time (Sparre and Venema, 1998; Quinn and Deriso, 1999):
Nt represents the number of fish at age t, N0 is the initial population size, and Z is the instantaneous total mortality rate. Assuming a multiplicative error term, the model was linearized through logarithmic transformation for linear regression analysis. The number of fish in each age class t was estimated by scaling (raising) the observed sample numbers (otolith-based age data) to match the annual landings of the species in the Red Sea, based on official statistics from MEWA and FAO (Alshaikhi et al., 2023; FAO, 2024). To account for interannual variability and provide a more stable estimate, the calculations were based on the average of the landings from five consecutive years (2016–2020). Since Z is the sum of M and fishing mortality (F) (i.e., Z = F + M), the F for V. louti was estimated by subtracting the M value from the Z estimate.
2.7 Stock status evaluation
A preliminary assessment of the stock status of V. louti along the eastern Red Sea coast of Saudi Arabia was conducted using yield per recruit (Y/R) analysis, along with estimates of the spawning potential ratio (SPR) and the current exploitation rate (E). SPR is a key metric used to evaluate the reproductive potential of a stock under existing fishing pressure. As defined by Goodyear (1993), SPR represents the equilibrium spawning stock biomass (SSB) per recruit (SSB/R) at a given level of F, divided by the equilibrium SSB/R in an unfished state. This analysis extends traditional predictive models, such as those developed by Thompson and Bell (1934) and Beverton and Holt (1957), which are typically used to estimate Y/R and biomass per recruit (B/R). The computation of SSB/R for a fished stock follows the same principles as B/R estimation; however, it accounts explicitly for the mature portion of the fish capable of spawning (Gabriel et al., 1989; Goodyear, 1993). Like B/R, SPR has a maximum value of 1 when no fishing occurs (F = 0) and decreases progressively toward 0 as F increases. It is also often expressed as a percentage, representing the proportion of the unfished SSB/R that remains at a given F level. For example, when F = 0, % SPR equals 100%.
A Thompson and Bell model (TBM) (Gabriel et al., 1989; Sparre and Venema, 1998) was used to estimate the Y/R and SPR of the V. louti stock under varying levels of F. Input data included the numbers of fish (the same dataset used for Z estimation) and mean weights in each age class, and the previously estimated M value. The same M was applied across all age classes for survivor calculations. The age at first capture (tc) was set at 2 years, and the proportion of mature fish in each age class was determined using logistic regression analysis to compute SSB. The Y/R analysis provides two key reference points for fisheries management. The first, Fmax, represents the F that yields the maximum Y/R and is typically regarded as a limit reference point (LRP) (Caddy and Mahon, 1995). The LRP marks a critical threshold that should not be exceeded to prevent severe stock depletion, indicating unsustainable biomass levels or excessive fishing pressure. The second, F0.1, is a more conservative reference point often considered a biologically precautionary target reference point (TRP). F0.1 is defined as the F at which the slope of the Y/R curve is 10% of the slope at the origin (Quinn and Deriso, 1999). The TRP represents the desired level of stock biomass (SB) or F, aimed at achieving long-term sustainability and optimal yield while maintaining the stock’s health and productivity (Caddy and Mahon, 1995; Caddy and McGarvey, 1996; Prager et al., 2003; Haddon, 2011). While Y/R analysis primarily assesses growth overfishing, it does not account for the impact of fishing on the reproductive capacity of the stock (Clark, 1991; Mace, 1994; Quinn and Deriso, 1999). To address this limitation, SPR analysis provides critical insights into recruitment overfishing by evaluating the effects of fishing on a stock’s ability to sustain itself through adequate SSB (Mace and Sissenwine, 1993; Quinn and Deriso, 1999; Walters and Martell, 2004). A minimum SPR value of 0.4 is widely recommended as a TRP to prevent stock depletion and mitigate the risk of recruitment overfishing (Goodyear, 1993; Mace, 1994; Clark, 2002). The F corresponding to this TRP is commonly denoted as F40%. Walters and Martell (2004) emphasized that historical stock-recruitment data indicate a significantly higher risk of recruitment overfishing when SPR falls below 0.3. Consequently, an SPR of 0.3 is adopted as the LRP in this study.
The value of E was calculated as the ratio of F to Z. In evaluating the current observed E, a value of 0.5 is recognized as an LRP, representing a threshold of sustainable fishing pressure that should ideally be avoided. This threshold is based on the principle that F should not exceed M, expressed as F/M ≤ 1 (Thompson, 1993; Froese et al., 2016).
All statistical analyses and graphical visualizations were performed using R software version 4.3.3 (R Core Team, 2024), with a significance level of 5% applied to all statistical tests.
3 Results
3.1 Length-weight relationship
The FL measurements (n = 7,087) ranged from 10.5 to 64.3 cm, while the W measurements (n = 2,629) varied from 53 to 2,910 g. The non-parametric Mann-Whitney test indicated no significant difference in mean FL between females (31.5 ± 5.78 cm) and males (31.8 ± 7.76 cm). The FL frequency distribution of V. louti from the eastern Red Sea, stratified by sex, is presented in Figure 3.
Figure 3
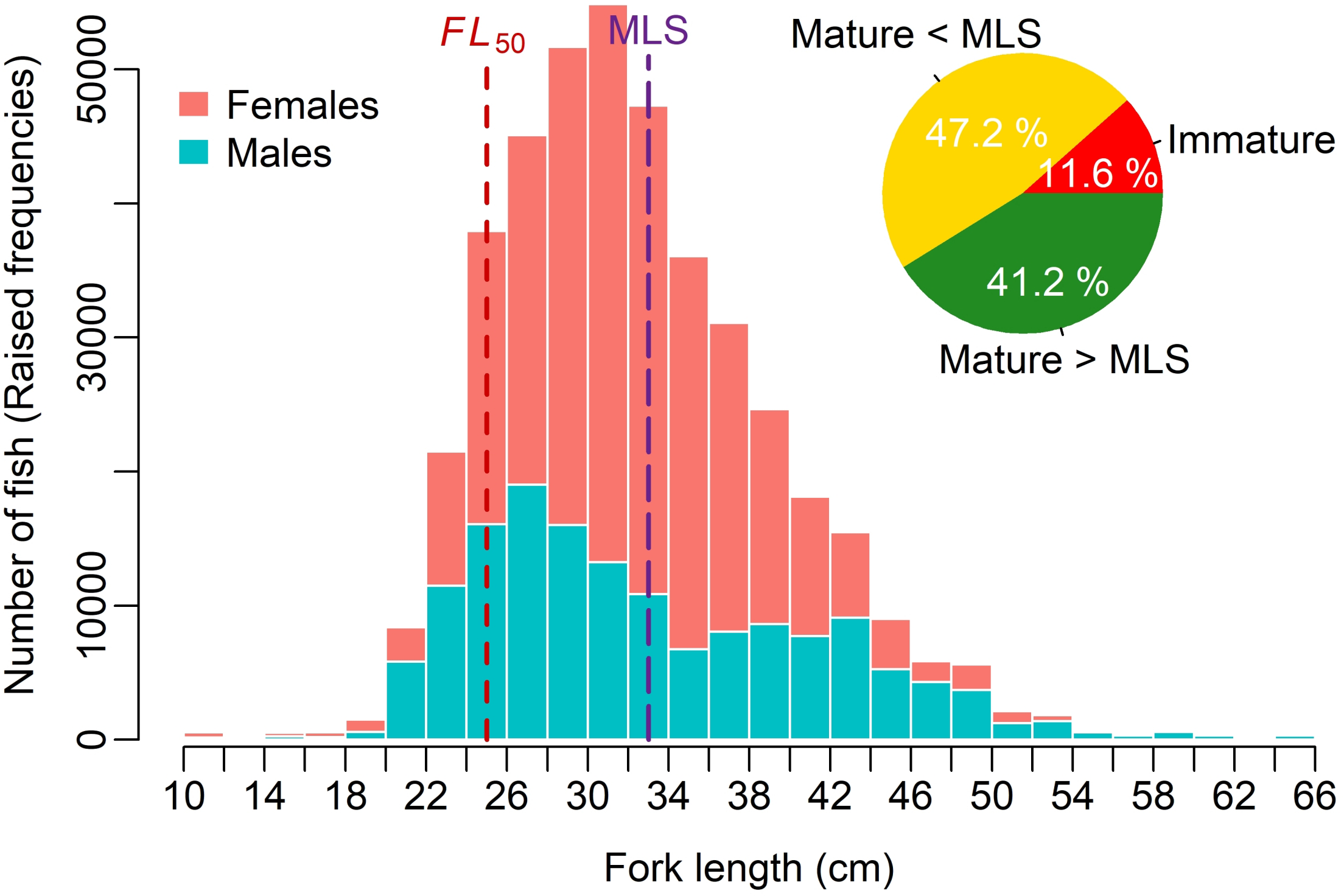
FL frequency distribution (in 2 cm intervals) of V. louti from the eastern Red Sea, stratified by sex. Data were adjusted (raised) based on annual landings reported in MEWA-FAO official statistics (Alshaikhi et al., 2023; FAO, 2024). Estimated proportions of immature fish (FL < 25 cm), mature fish below the minimum landing size (MLS) of 33 cm (25 cm ≤ FL < 33 cm), and mature fish above the MLS (FL ≥ 33 cm) in the overall landings are also shown.
The ANCOVA results indicated no significant differences in the parameters a and b of the FL-W relationship between sexes. Consequently, all data were pooled for linear regression analysis. The estimated values of a (95% CI) and b (95% CI) were 0.012 (0.011–0.013) and 3.10 (3.08–3.12), respectively, with a determination coefficient (r2) of 0.97 (Supplementary Figure S3). V. louti exhibited a positive allometric growth pattern.
3.2 Sex ratio and maturity
The exact binomial test indicated that the observed overall female-to-male sex ratio of 1.7:1 deviated significantly from unity. According to the log-likelihood ratio test (G-statistic), this ratio did not vary significantly between years or between summer and winter seasons. Females were more abundant up to an FL of 41 cm, beyond which males became predominant (Figure 3). The largest female observed measured 52.4 cm FL.
The comparison of nonparametric BCa 95% CIs for FL50, generated separately for each sex using bootstrapping, indicated no significant difference between sexes. The overall FL50 estimated from the logistic model for the combined binomial maturity data was 24.98 cm, with nonparametric BCa 95% CI ranging from 24.56 to 25.54 cm (Figure 4). When the observed FL data were adjusted (raised) based on annual landings of the species from the Red Sea, as reported in MEWA-FAO official statistics (Alshaikhi et al., 2023; FAO, 2024), immature fish (FL < FL50) accounted for approximately 12% of the total annual catch (Figure 3). In contrast, mature individuals (FL ≥ 25 cm) comprised more than 88% of the catch.
Figure 4
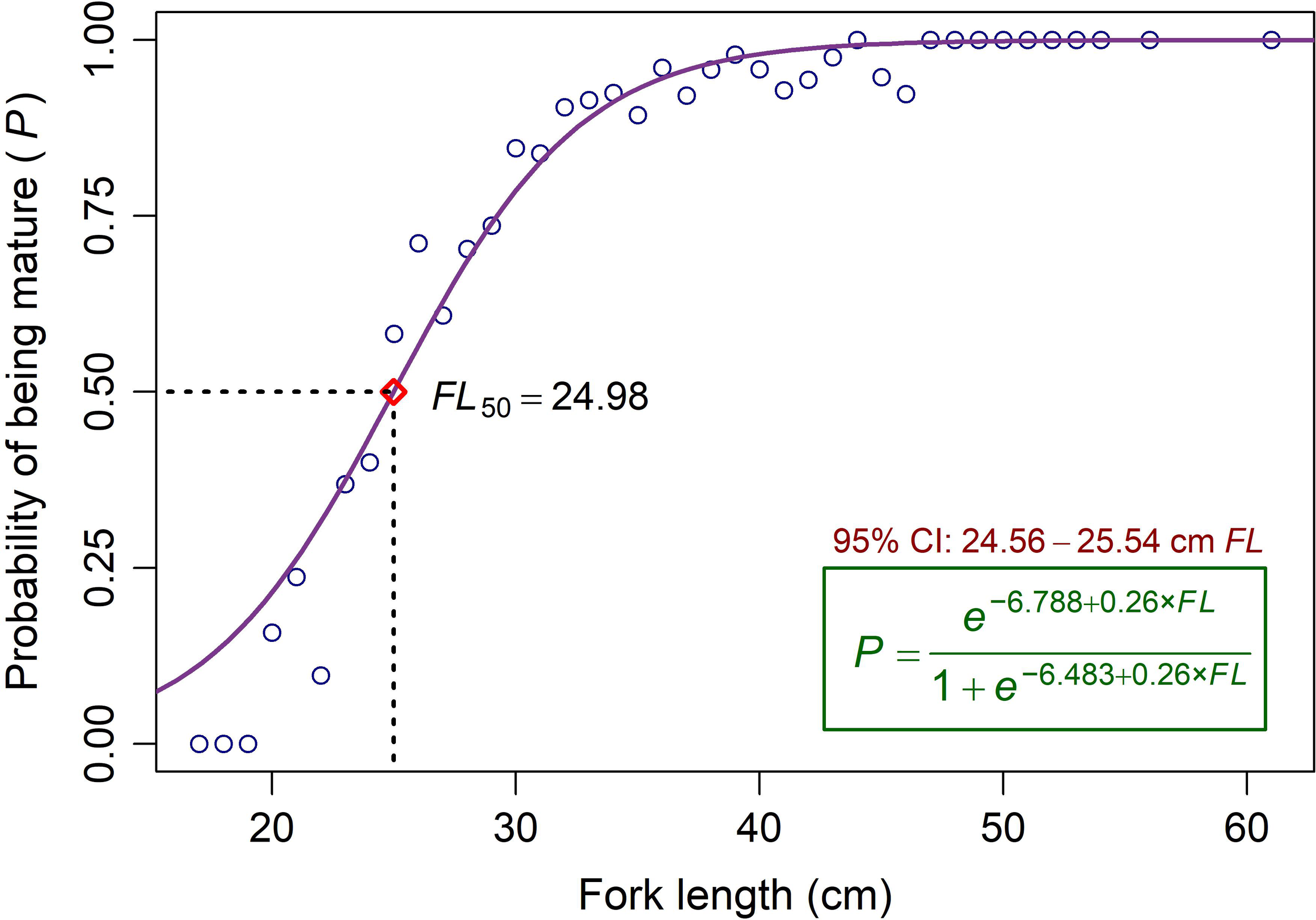
The estimated median FL at first maturity (FL50) of V. louti from the eastern Red Sea. The curve represents the logistic model fitted to the combined binomial maturity data of both sexes. Circles indicate the observed proportions of mature specimens relative to the total sampled fish. The red diamond square marks the estimated FL50 on the logistic curve. CI denotes confidence intervals.
3.3 Age and growth
Otolith readings revealed that the minimum and maximum ages recorded were 1 and 16 years, corresponding to FL of 18 cm and 64 cm, respectively (Table 1). The maximum age was 13 years for females and 16 years for males, with corresponding FLs of 51 cm and 64.3 cm, respectively. Older and larger individuals (> 6 years and > 40 cm) were predominantly male, confirming the presence of protogynous hermaphroditism in the species (Figure 3). The von Bertalanffy growth parameters estimated separately for each sex and for both sexes combined are presented in Table 2. The likelihood ratio test indicated no statistically significant difference between the reduced model (with combined data and shared parameters) and the full model (with sex-specific parameters). Therefore, the observed differences in parameter estimates between sexes were considered insignificant, and modeling the growth of V. louti using a common growth curve was found to be statistically more parsimonious and appropriate than fitting separate curves for each sex (Figure 5).
Table 1
| FL Class (cm) | Age (Years) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| 18 | 1 | |||||||||||||||
| 21 | 3 | |||||||||||||||
| 22 | 8 | |||||||||||||||
| 23 | 8 | |||||||||||||||
| 24 | 14 | |||||||||||||||
| 25 | 5 | 2 | ||||||||||||||
| 26 | 4 | |||||||||||||||
| 27 | 6 | |||||||||||||||
| 28 | 5 | |||||||||||||||
| 29 | 8 | |||||||||||||||
| 30 | 5 | |||||||||||||||
| 31 | 1 | 4 | ||||||||||||||
| 32 | 6 | |||||||||||||||
| 33 | 2 | |||||||||||||||
| 34 | 3 | |||||||||||||||
| 35 | 3 | |||||||||||||||
| 36 | 2 | 6 | ||||||||||||||
| 37 | 2 | 6 | ||||||||||||||
| 38 | 3 | 4 | 3 | 1 | ||||||||||||
| 39 | 1 | 5 | 4 | |||||||||||||
| 40 | 2 | 1 | 2 | 2 | ||||||||||||
| 41 | 2 | 1 | 2 | |||||||||||||
| 42 | 3 | 2 | 2 | |||||||||||||
| 43 | 1 | 2 | 2 | 3 | 1 | |||||||||||
| 44 | 1 | 2 | ||||||||||||||
| 45 | 1 | 1 | 1 | 1 | 1 | |||||||||||
| 46 | 1 | 2 | 2 | |||||||||||||
| 47 | 2 | 2 | 2 | 1 | ||||||||||||
| 48 | 1 | 3 | ||||||||||||||
| 49 | 5 | 2 | 5 | 1 | ||||||||||||
| 51 | 1 | 1 | 1 | 2 | ||||||||||||
| 52 | 2 | 3 | ||||||||||||||
| 53 | 1 | |||||||||||||||
| 56 | 1 | |||||||||||||||
| 61 | 1 | |||||||||||||||
| 63 | 1 | |||||||||||||||
| 64 | 1 | |||||||||||||||
| Total | 39 | 31 | 18 | 9 | 21 | 15 | 16 | 19 | 13 | 15 | 1 | 1 | 2 | 1 | 3 | 1 |
Age length key for V. louti from the eastern Red Sea.
Table 2
| Parameter | Estimate | SE | 95% CI | RSE |
|---|---|---|---|---|
| Females | 2.498 | |||
| FL∞(cm) | 56.24 | 2.819 | 51. 73 – 64.01 | |
| K (year-1) | 0.166 | 0.0257 | 0.116 – 0.220 | |
| t 0 (year) | −1.558 | 0.3543 | −2.398 – −0.939 | |
| Males | 3.077 | |||
| FL∞(cm) | 67.41 | 5.016 | 59.34 – 83.97 | |
| K (year-1) | 0.098 | 0.0182 | 0.060 – 0.139 | |
| t 0 (year) | −2.855 | 0.5496 | −4.264 – −1.844 | |
| Sexes combined | 2.826 | |||
| FL∞(cm) | 62.28 | 2.644 | 57.60 – 69.35 | |
| K (year-1) | 0.122 | 0.0142 | 0.093 – 0.154 | |
| t 0 (year) | −2.262 | 0.312 | −2.999 – −1.665 | |
The von Bertalanffy growth model parameters for V. louti from the eastern Red Sea.
SE, standard error; CI, confidence interval; RSE, residual standard error (square root of the residual mean square).
Figure 5
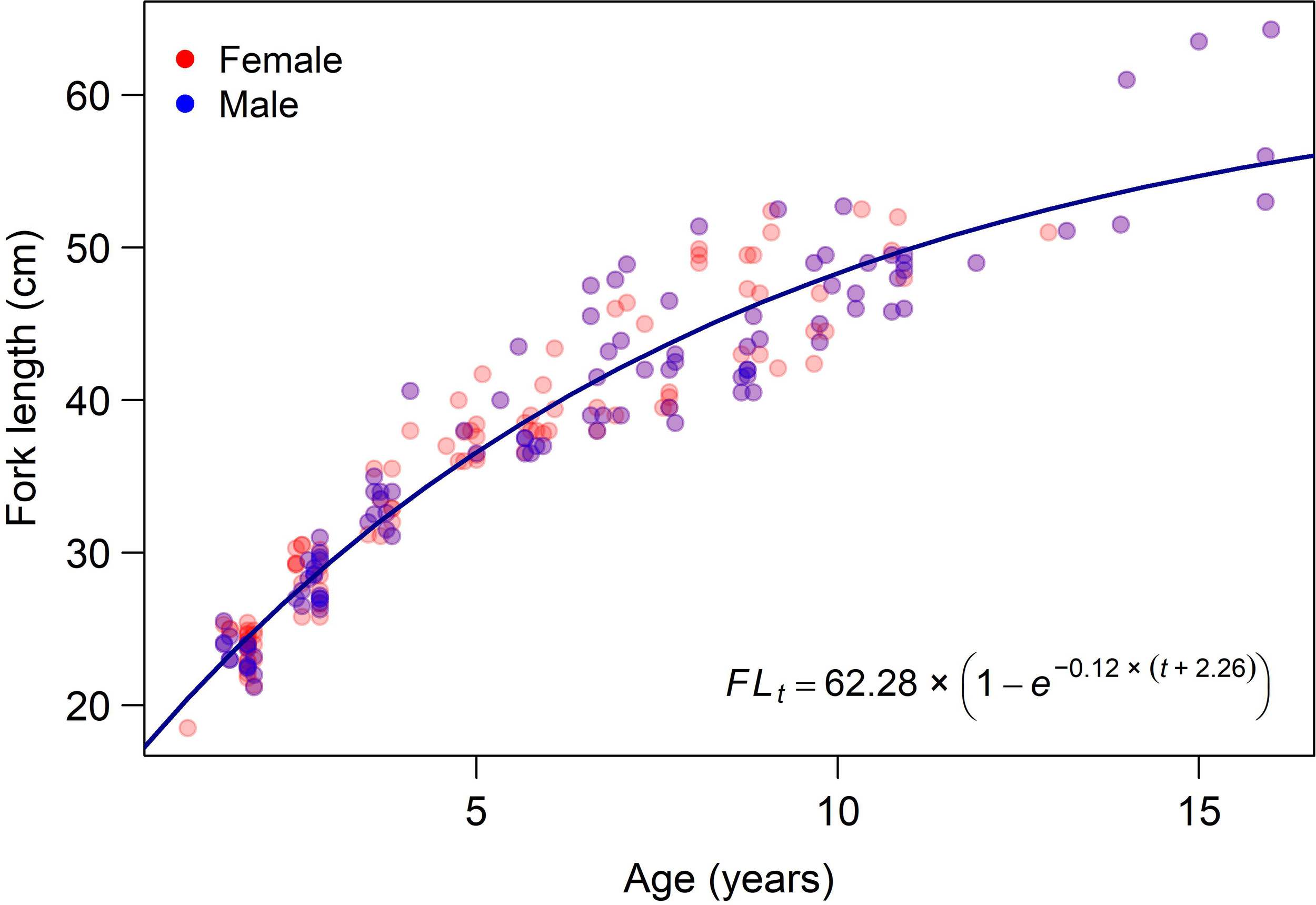
Observed FL-at-age data for V. louti from the eastern Red Sea with the fitted von Bertalanffy growth curve.
3.4 Mortality rates
The annual M estimates obtained from the three methods (in the previously described order) were 0.318, 0.186, and 0.224 for V. louti. The mean M value across these methods was calculated as 0.243 year−1. The value of Z (95% CI), estimated from the linearized exponential decay model using regression analysis, was 0.38 year−1 (0.29–0.47), with an r² of 0.89. Based on these Z and M estimates, the resulting F was calculated as 0.137 year−1.
3.5 Stock status
The results of the TBM Y/R and SPR analyses for this species, based on equilibrium conditions, are presented in Figure 6. In this figure, the left Y-axis represents the SPR, while the right Y-axis displays the total Y/R for a single young fish recruiting to the stock. The figure illustrates the decline in SSB/R relative to the unfished level, as well as the changes in Y/R as a function of F. The estimated Fmax, the LRP, was 0.231 year−1, corresponding to a peak Y/R value of approximately 93 g. Beyond this level, Y/R declined consistently. The more conservative TRP, F0.1, was estimated at 0.161 year−1. The current F value of 0.137 year−1 falls below both Fmax and F0.1. At this F level, the SSB corresponds to approximately 41% of its unexploited level. Additionally, the F associated with the SPR-based TRP (F40%) was computed as 0.144 year−1. As shown in Figure 6, the current F estimate is lower than F40% but still very close, indicating that the stock is being exploited at a level nearly at the TRP, with little margin below it.
Figure 6
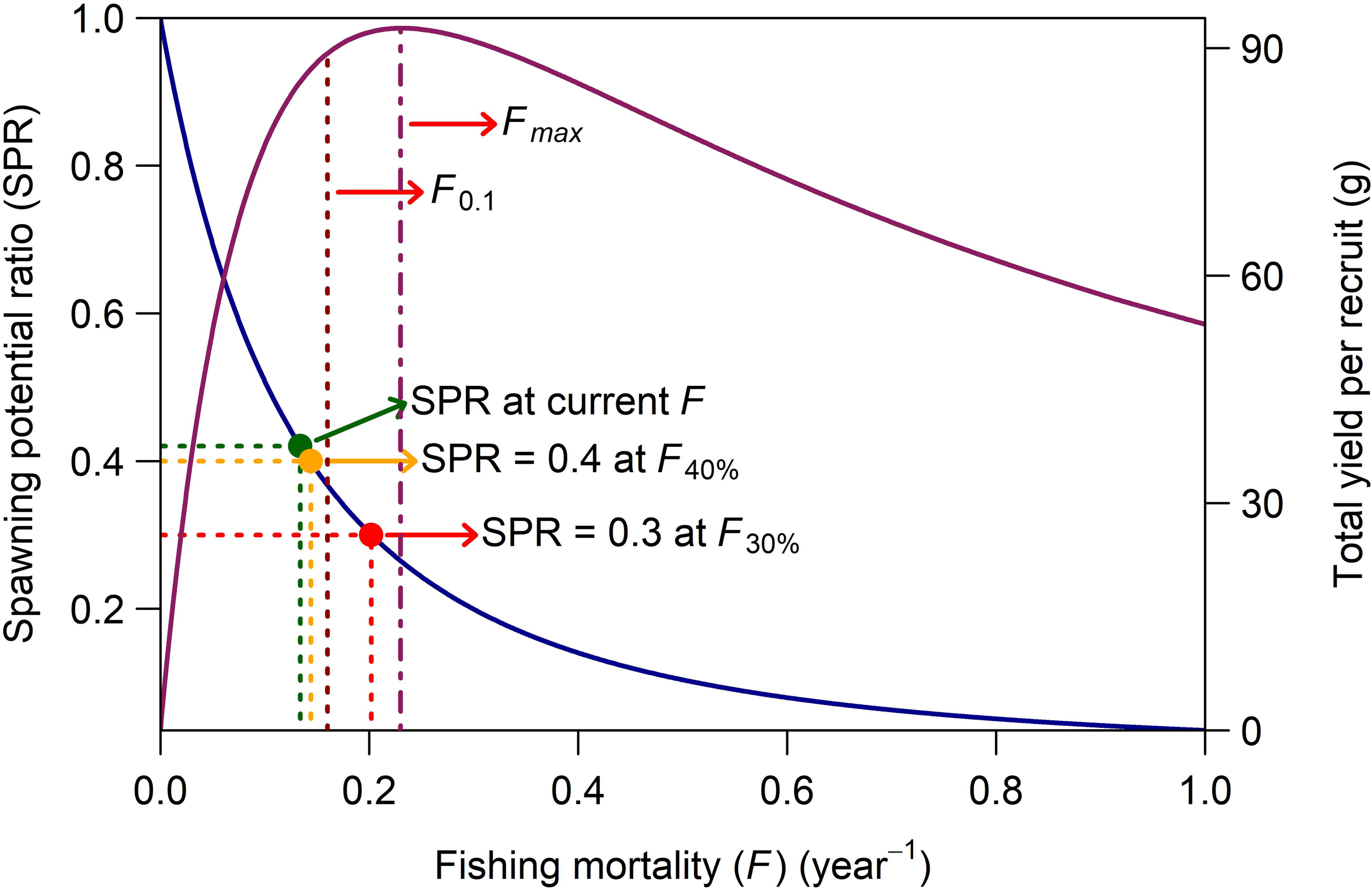
Relationship between SSB/R (dark blue line, corresponding to the left SPR axis) and total Y/R (dark red line, corresponding to the right Y-axis) as a function of F. The SPR and Y/R levels were estimated using the TBM Y/R analysis, assuming an average age of 2 years at first capture. The figure also illustrates the SPR values corresponding to the current F and various biological reference points, including Fmax, F0.1, F40%, and F30%.
The current E derived from the mortality estimates was 0.36 for V. louti in the eastern Red Sea. Consistent with the outcomes of the Y/R and SPR analyses, this E value is substantially below the LRP of 0.5.
3.6 Fishery characteristics
The primary fishing gear used to capture V. louti along the Saudi Arabian Red Sea coast is the handline, with a small proportion (~3%) of the total catch obtained using fishing traps. Handlines captured fish across nearly all FL classes and age groups in the fishery, whereas traps primarily caught individuals between ages 1 and 3 years (Figure 7), corresponding to an FL range of 20–40 cm (Figure 4, Table 1). The age distribution of landings revealed that fish aged 1 to 3 years were the most represented in the Red Sea fishery (Figure 7). All age-1 V. louti specimens were immature. As mentioned, immature fish comprised less than 12% of the total landings, while 88% were mature individuals (Figure 3). However, according to the MEWA regulations, the minimum landing size (MLS) for commercial fisheries targeting this species in Saudi Arabia is 42 cm TL (MEWA, 2021), corresponding to approximately 33 cm FL. Based on this threshold, 47% of the total catch consisted of mature fish below the MLS (25 cm ≤ FL < 33 cm), while only 41% of the landings met the legal size requirement (Figure 3).
Figure 7
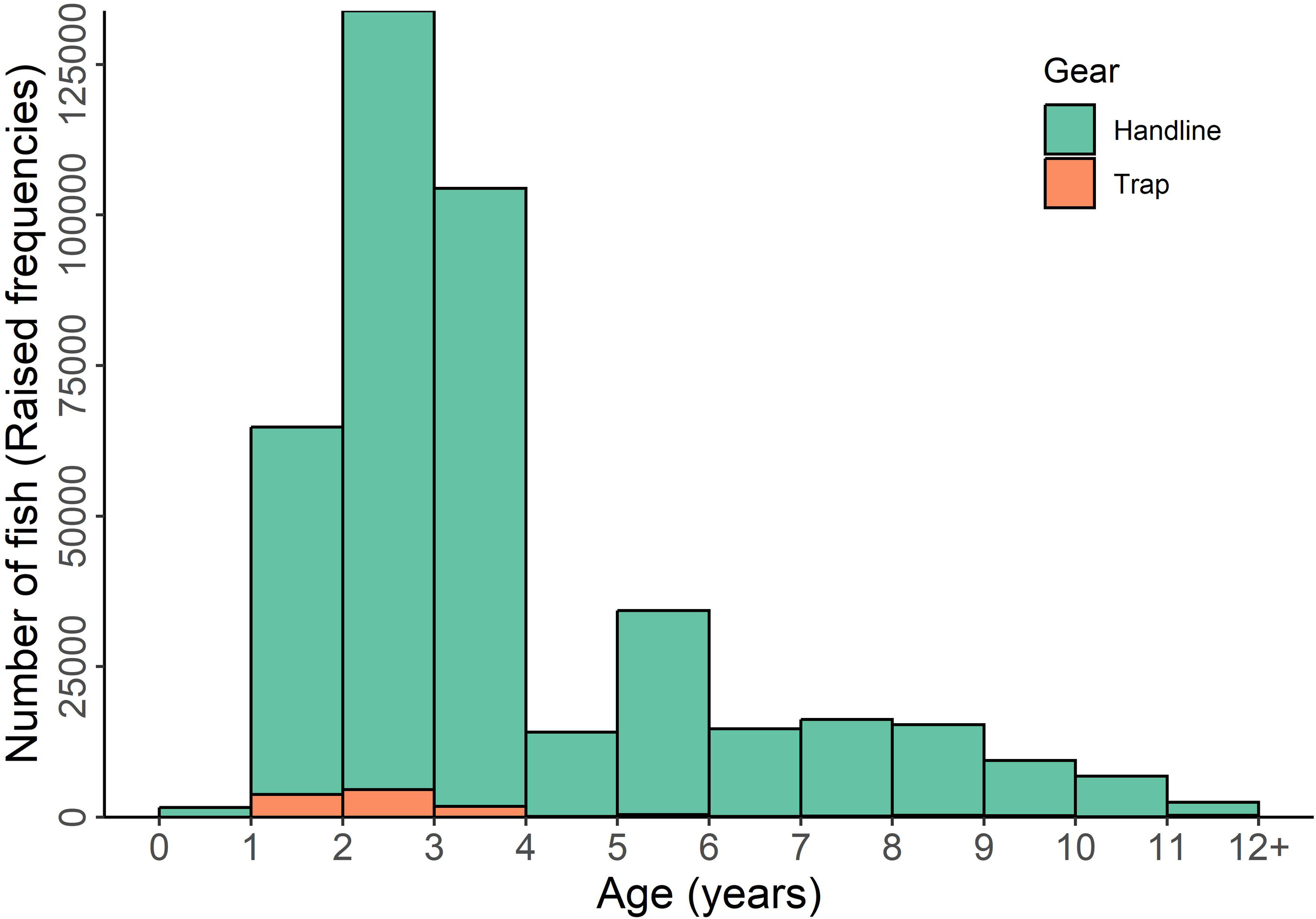
Age distribution of V. louti landings from the eastern Red Sea coast of Saudi Arabia, categorized by gear type. The number of fish in each age class was estimated by scaling the observed otolith-based age data to align with the annual landings of the species in the Red Sea (Alshaikhi et al., 2023; FAO, 2024).
4 Discussion
Groupers face a major threat from overexploitation and inadequate management due to their high commercial and food value (Sadovy de Mitcheson et al., 2013). Understanding the biological characteristics of each grouper species at a regional level is essential for developing accurate and effective fisheries management strategies that ensure their long-term sustainability (Shellem et al., 2021). However, such species-specific data for the eastern Red Sea remain scarce, despite the extensive coral reef habitats in the region that support thousands of artisanal fishers. Despite the great demand for V. louti in the Red Sea and its importance to the fishery, information on its life history and fishery biology in the region has been lacking. This study provides the first comprehensive information on V. louti in the eastern Red Sea, presenting key population parameters, stock assessment, and biological insights that enable comparisons with similar studies conducted elsewhere.
The range of FL measurements observed in the present study is comparable to those reported in a previous study from the Gulf of Aqaba, a gulf at the northern tip of the Red Sea (Wahbeh, 2005), as well as in other regions worldwide (Ignatius and Faizah, 2021; Schemmel and Dahl, 2023). The b value estimated from the FL-W relationship in this study indicates a positive allometric growth pattern for V. louti in the eastern Red Sea. This b estimate is significantly higher than all other b values reported from the Gulf of Aqaba (Wahbeh, 2005), the Indo-West Pacific (Ignatius and Faizah, 2021), and the western Indian Ocean (Mahé et al., 2022) (Table 3). These findings suggest regional differences in the allometric growth pattern for the species. It is important to note that, unlike the present study, all other referenced studies used total length (TL) measurements, which may account for the discrepancies in these comparisons.
Table 3
| Study Area | Size range (cm) | Age range (years) | Sex ratio | b | L50(cm) | L ∞ (cm) | K (year-1) | t 0 (years) | Z (year-1) | M (year-1) | F (year-1) | E | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present study (eastern Red Sea) | 10.5–64.3 | 1–16 | 1.7:1 | 3.1 | 24.98 | 62.28 | 0.12 | −2.26 | 0.38 | 0.24 | 0.14 | 0.36 | Present study |
| Gulf of Aqaba (northern Red Sea) | 15–51.6 (TL) |
1–12 | 1:01 | 3.02 (♀) 2.93 (♂) |
29 (♀) 17.6 (♂) |
64.6 (♀) 65.4 (♂) |
0.100 (♀) 0.102 (♂) |
−1.41 (♀) −1.37 (♂) |
Wahbeh (2005) | ||||
| La Réunion Island (western Indian Ocean) |
29–75 (TL) | 2.95 | 34 | 76.57 | 0.21 | Mahé et al. (2022) | |||||||
| Sibolga (Indo-West Pacific) | 24–41 (TL) |
2.35 | 48.55 | 0.13 | −0.78 | 0.92 | 0.42 | 0.50 | 0.55 | Ignatius and Faizah (2021) | |||
| Palau (South Pacific) | 28.5 | 48.3 | 1.4 | Prince et al. (2015) | |||||||||
| Guam (Indo-West Pacific) | 19–49.7 | 2–17 | 1.5:1 | 26 | 43.7 | 0.28 | −0.2 | 0.37 | Schemmel and Dahl (2023) | ||||
| Seychelles (western Indian Ocean) | 2.35 | 51 | 0.48 | 0.28 | Grandcourt (2005) |
Summary of the parameter estimates for the length-weight relationship, size at first maturity, von Bertalanffy growth model, mortality, and exploitation rates of V. louti reported from different regions of the world.
Studies that are based on TL measurements, in contrast to FL used in the present study, are indicated with “(TL)” in the “Size range” column. ♀ and ♂ denote female and male, respectively.
The observed female-to-male sex ratio deviated from unity, with a ratio of 1.7:1. In contrast, a previous study from the Gulf of Aqaba reported a balanced 1:1 sex ratio, with male predominance observed in August (Wahbeh, 2005). The protogynous hermaphroditic nature of V. louti, characterized by the presence of older males, was evident in this study. However, the younger population comprised both sexes. This pattern may be attributed to several factors, including the failure of some females to transition to males due to the absence of genetic or environmental cues, the possibility of sex change occurring before sexual maturation in some individuals, and variations in female size at sex reversal among populations of the same species, as suggested by Sadvoy and Shapiro (1987). The current findings are consistent with those of Schemmel and Dahl (2023) from Guam, where older and larger males were observed compared to females.
The FL50 estimated from combined-sex data in the present study is comparable to the FL50 reported from Guam (Schemmel and Dahl, 2023). However, higher TL50 values have been reported for females from the Gulf of Aqaba (Wahbeh, 2005) and for combined-sex from La Réunion Island in the western Indian Ocean (Mahé et al., 2022) and Palau in the South Pacific (Prince et al., 2015). In contrast, a comparatively lower TL50 estimate was documented for males from the Gulf of Aqaba (Wahbeh, 2005) (Table 3). These observed differences may be attributed to spatial and temporal variations, including environmental factors, varying levels of fishing pressure across different localities, or sampling periods. It is also important to note that, unlike the present study and that of Schemmel and Dahl (2023), which utilized FL measurements, the estimates reported from the Gulf of Aqaba (Wahbeh, 2005) and the La Réunion Island (Mahé et al., 2022) were based on TL measurements (Table 3). This methodological difference may have contributed to the discrepancies observed in the comparisons, as conversions between FL and TL can introduce variability, particularly if not standardized across studies.
This study estimated that V. louti attains an FL∞ of 62.28 cm with a maximum observed age of 16 years in the eastern Red Sea. The von Bertalanffy growth parameters reported for this species in different parts of the world, along with the estimates from this study, are presented in Table 3. From a regional perspective, Wahbeh (2005) recorded a maximum age of 12 years, with a TL∞ estimates of 64.7 cm for females and 65.7 cm for males from the Gulf of Aqaba. These minor differences may be attributed to the discrepancy in measurement standards, specifically, the use of TL in the Gulf of Aqaba study compared to FL in the present analysis, as well as spatial and temporal variations between the stwo studies. Across different tropical regions, asymptotic size estimates for V. louti show considerable variation. Reported FL∞ values include 43.7 cm off Guam in the Indo-West Pacific (Schemmel and Dahl, 2023), 48.3 cm off Palau in the South Pacific (Prince et al., 2015), and 51 cm in the Seychelles in the western Indian Ocean (Grandcourt, 2005). Estimates based on TL∞ comprise 48.55 cm off Sibolga in the Indo-West Pacific (Ignatius and Faizah, 2021) and 76.57 cm off La Réunion Island in the western Indian Ocean (Mahé et al., 2022). A comparison of present findings with all previous studies suggests that V. louti in the eastern Red Sea reaches a relatively large asymptotic size (Table 3). These variations in growth potential are likely influenced by environmental conditions, genetic variability, and fishing pressure (Haddon, 2011). The parameter K also varies considerably across regions, with comparatively higher values like 0.48 year−1 off Seychelles (Grandcourt, 2005), 0.28 year−1 off Guam (Schemmel and Dahl, 2023), and 0.13 year-1 off Sibolga (Ignatius and Faizah, 2021). Regionally, a similar K value of 0.1 year−1 has been reported from the Gulf of Aqaba (Wahbeh, 2005).
The Red Sea spans a considerable latitudinal gradient, leading to variation in ambient sea temperature that could influence growth and body size in V. louti, as observed in many fish species (Ahti et al., 2020). In this study, samples were collected from nine major fishing ports covering a wide geographic range (Figure 1) as part of a fisheries assessment initiative by MEWA, aimed at generating baseline data for V. louti and other species along the eastern Red Sea coast. The sampling was not based on a balanced design stratified by latitude or port. Otoliths were collected and processed without detailed spatial or temporal consideration, and ageing samples were selected randomly to cover a broad size range rather than specific locations. As a result, the number of otoliths per site is insufficient for robust spatial or latitudinal comparisons. It is recommended that future studies be designed with stratified spatial and temporal sampling frameworks to better investigate environmental drivers of growth variation in this species.
The estimated Z of 0.38 year−1 in the present study is consistent with the findings of Schemmel and Dahl (2023) off Guam. In contrast, a higher Z value of 0.92 year−1 was reported in Sibolga (Indo-West Pacific) by Ignatius and Faizah (2021). This substantial difference may be attributed to environmental variations and differences in fishing pressure (Table 3).
Based on the yield-per-recruit (Y/R) estimates derived from the TBM analysis for V. louti in the eastern Red Sea (Figure 6), the current F of 0.137 year−1 is substantially lower than both F01 and Fmax. This finding suggests that growth overfishing is not occurring, as the stock is being harvested at a rate that allows individuals to reach their maximum growth potential. Consequently, the fishery remains within safe biological limits, preserving long-term stock productivity. However, any significant increase in future fishing effort, and consequently in F, could elevate the risk of growth overfishing.
The SPR analysis provides no evidence of recruitment overfishing for the V. louti stock in the eastern Red Sea. At the current F level, the SSB is approximately 41% of its unexploited level, remaining above the SPR-based TRP and within safe biological limits (Figure 6). This indicates that the stock retains sufficient reproductive capacity to sustain the fishery. However, while the current F estimate is below F40%, it is still very close, indicating that the stock is being exploited at a level near its upper sustainable limit (Figure 6). Therefore, any future increase in F, even if incremental, could significantly heighten the risk of overexploitation. This concern is especially critical if increased fishing pressure coincides with the compounding effects of climate change, which could further stress the stock and undermine its long-term sustainability.
Several guiding principles have emerged in response to the collapse of numerous fisheries worldwide in recent decades to reduce the risk of management failure. Foremost among these is the principle that F should not exceed M, expressed as F/M ≤ 1 (Thompson, 1993; Williams and Shertzer, 2003; Zhou et al., 2012; Froese et al., 2016). This condition implies that the E should be ≤ 0.5. As Froese et al. (2016) emphasized, fisheries must harvest at a rate that nature can sustainably support. Consequently, an E value of 0.5 is often used as an LRP, a threshold indicating sustainable fishing pressure that, ideally, should not be reached or exceeded. More risk-averse management strategies have also been proposed, recommending smaller F/M ratios to further reduce the risk of overfishing. Examples include ratios of 0.87 (Zhou et al., 2012), 0.8 (Thompson, 1993), 0.75 (Williams and Shertzer, 2003), and 0.67 (Patterson, 1992), corresponding to E values of 0.47, 0.44, 0.43, and 0.4, respectively. These more conservative levels offer additional safeguards against overexploitation by accounting for uncertainties in stock assessments and environmental variability. As described by Schemmel and Dahl (2023), V. louti in the eastern Red Sea appears to be less vulnerable to exploitation compared to other grouper species, as well as to V. louti populations in other studied areas, such as Sibolga (Ignatius and Faizah, 2021) and Palau (Prince et al., 2015). Higher E values observed in Sibolga and Palau (Table 3) indicate that significant portions of those stocks are being harvested, which, if not effectively managed, could lead to recruitment overfishing and population decline.
Although the Y/R and SPR analyses (Figure 6), the current E (Table 3), and the low proportion of immature fish in the overall catch (Figure 3) suggest that the V. louti stock in the eastern Red Sea remains within safe biological limits, it is important to note that only 41% of the landings met the minimum legal size requirement (≥ 33 cm FL) set by MEWA for commercial fisheries in Saudi Arabia. Nearly half of the landings consisted of mature fish below this legal limit, primarily 2- to 3-year-old individuals (Table 1, Figure 5). This finding aligns with the observations of Shellem et al. (2021), who reported a higher prevalence of smaller groupers in regional markets. A similar trend has been observed in grouper fisheries worldwide, as To and Sadovy (2009) documented a shift from large-bodied individuals to smaller, often sexually immature fish in fish markets.
Given that the exploitation of V. louti in the eastern Red Sea begins at the juvenile stages, additional management measures are essential to ensure this species’ long-term sustainability. Small-scale artisanal fisheries account for nearly 70% of the seafood supply in the Saudi Arabian Red Sea (Jin et al., 2012; Shellem et al., 2021). Although handline is a traditional and relatively environmentally friendly fishing gear, its efficiency in capturing groupers remains high (Huliselan et al., 2017). Hook size regulations are critical for selective fishing, helping to prevent the capture of immature fish and mature individuals below the MLS. However, enforcing MLS requirements in small-scale, multispecies artisanal reef fisheries presents significant challenges, potentially leading to overfishing, particularly among groupers. These observations align with the concept of “fishing down the marine food web” in the Red Sea, as described by Pauly et al. (1998). In addition to MLS regulation, further management strategies, such as catch quotas, seasonal restrictions, fishing effort reduction (e.g., limiting fishing days), and stock monitoring, could help promote sustainable fisheries management (Nair et al., 2018; Shellem et al., 2021).
5 Conclusions and recommendations
This study provides the first comprehensive assessment of V. louti in the eastern Red Sea, encompassing key population parameters and stock status evaluation. The assessment of the V. louti stock suggests that current fishing activity in the Saudi Arabian Red Sea is being conducted at a sustainable level, provided that the present fishing effort remains unchanged. However, key concerns persist regarding stock structure and long-term sustainability. Specifically, almost 12% of the total catch consists of immature fish, while nearly 48% comprises mature but legally undersized individuals. This highlights the need for continued vigilance and the implementation of management measures to maintain a healthy and balanced age structure within the population. The commercial significance of this species is further underscored by an estimated annual catch of 260 tonnes, contributing approximately 6.5 million USD to the national economy. Given its economic importance, effective management strategies are essential to safeguard the long-term viability of this valuable fishery.
To enhance sustainability, more selective hook sizes should be implemented to reduce the capture of immature and legally undersized fish. Additionally, strict enforcement of existing MLS regulation, coupled with the complete elimination of trap fishing, would further contribute to the preservation of the stock. Future efforts should also explore adaptive management strategies, such as seasonal closures, catch quotas, and fishing effort reductions, to mitigate the risk of overexploitation and ensure the resilience of V. louti stock in the eastern Red Sea.
The findings presented in this study are based on the assumption that all V. louti specimens caught originate from a single stock and that all reported landings along the Saudi Arabian Red Sea coast belong to this stock. To validate this assumption, future genetic studies are recommended to determine whether V. louti in the region represents a single, homogeneous population or comprises multiple distinct populations.
Looking ahead, it is imperative to maintain robust data collection efforts for continuous monitoring and assessment. Additionally, integrating environmental variables into the modeling framework will be essential for a comprehensive understanding of the population dynamics of the species. Given the potential impacts of climate change on fish stocks, future research should also incorporate climate-related factors into management strategies to ensure the long-term sustainability of the fishery.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the samples were obtained from commercial fish markets.
Author contributions
SK: Data curation, Formal Analysis, Investigation, Writing – original draft. ZO: Data curation, Formal Analysis, Visualization, Writing – review & editing. GM: Investigation, Validation, Writing – review & editing. SC: Investigation, Validation, Writing – review & editing. AA: Investigation, Validation, Writing – review & editing. NB: Investigation, Validation, Writing – review & editing. ET: Conceptualization, Methodology, Resources, Supervision, Writing – original draft. MD: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Ministry of Environment, Water and Agriculture, Saudi Arabia (Funding number: 83092).
Acknowledgments
We extend our gratitude to all team members of the KAUST-KBD Fisheries Program for their invaluable assistance and support during both field and laboratory work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1582582/full#supplementary-material
References
1
Ahti P. A. Kuparinen A. Uusi-Heikkilä S. (2020). Size does matter - the eco-evolutionary effects of changing body size in fish. Environ. Rev.28, 311–324. doi: 10.1139/er-2019-0076
2
Alshaikhi A. Alshaye K. Ageely O. Samarali R. Alharbi B. Alhafedh Y. et al . (2023). Fisheries Statistics Saudi Arabia 2016-2021 (Riyadh: Technical report by General Fisheries Directorate Ministry of Environment, Water and Agriculture & FAO Saudi Arabia).
3
Aydın C. M. Tıraşın E. M. (2023). Information on the deep-water giant red shrimp Aristaeomorpha foliacea (Risso 1827) (Crustacea, Decapoda, Aristeidae) population in Antalya Bay (Eastern Mediterranean Sea, South of Turkey) based on the MEDITS protocol. Reg. Stud. Mar. Sci.60, 102885. doi: 10.1016/j.rsma.2023.102885
4
Beverton R. J. H. Holt S. J. (1957). On the Dynamics of Exploited Fish Populations Vol. 2 (London: Fisheries Investigations Series).
5
Caddy J. F. Mahon R. (1995). Reference points for fisheries management Vol. 347 (Rome: FAO Fisheries Technical PaperFAO).
6
Caddy J. F. McGarvey R. (1996). Targets or limits for management of fisheries? N. Am. J. Fish. Manage.16, 479–487. doi: 10.1577/1548-8675(1996)016<0479:TOLFMO>2.3.CO;2
7
Cerrato R. M. (1990). Interpretable statistical tests for growth comparisons using parameters in the von Bertalanffy equation. Can. J. Fish. Aquat. Sci.47, 1416–1426. doi: 10.1139/f90-160
8
Clark W. G. (1991). Groundfish exploitation rates based on life history parameters. Can. J. Fish. Aquat. Sci.48, 734–750. doi: 10.1139/f91-088
9
Clark W. G. (2002). F35% revisited ten years later. N. Am. J. Fish. Manage.22, 251–257. doi: 10.1577/1548-8675(2002)022<0251:FRTYL>2.0.CO;2
10
Cope J. M. Hamel O. S. (2022). Upgrading from M version 0.2: An application-based method for practical estimation, evaluation, and uncertainty characterization of natural mortality. Fish. Res.256, 106493. doi: 10.1016/j.fishres.2022.106493
11
Craig M. T. Bertoncini A. A. Carpenter K. E. Cheung W. W. Choat J. H. Cornish A. S. et al . (2013). Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish.14, 119–136. doi: 10.1111/j.1467-2979.2011.00455.x
12
Craig M. T. Sadovy de Mitcheson Y. Heemstra P. C. (2011). Groupers of the world. A field and market guide (Florida: CRC Press, Boca Raton).
13
Efron B. Tibshirani R. J. (1993). An Introduction to the Bootstrap (New York: Chapman and Hall).
14
FAO (2024). FAO Fisheries and Aquaculture - FishStatJ - Software for Fishery and Aquaculture Statistical Time Series (Rome: FAO Fisheries and Aquaculture Division).
15
Frisch A. J. Cameron D. S. Pratchett M. S. Williamson D. H. Williams A. S. Reynolds A. D. (2016). Key aspects of the biology, fisheries and management of coral grouper. Rev. Fish Biol. Fisheries26, 303–325. doi: 10.1007/s11160-016-9427-0
16
Froese R. Pauly D. (2025). FishBase (World Wide Web electronic publication). Available at: www.fishbase.org (Accessed on January 29, 2025).
17
Froese R. Winker H. Gascuel D. Sumaila U. R. Pauly D. (2016). Minimizing the impact of fishing. Fish Fish.17, 785–802. doi: 10.1111/faf.12146
18
Gabriel W. L. Sissenwine M. P. Overholtz W. J. (1989). Analysis of spawning stock biomass per recruit: An example for Georges Bank haddock. N. Am. J. Fish. Manage.9, 383–391. doi: 10.1577/1548-8675(1989)009<0383:AOSSBP>2.3.CO;2
19
Garcia S. M. Rosenberg A. A. (2010). Food security and marine capture fisheries: characteristics, trends, drivers and future perspectives. Phil. Trans. R. Soc B365, 2869–2880. doi: 10.1098/rstb.2010.0171
20
Giglio V. J. Ternes M. L. F. Luiz O. J. Zapelini C. Freitas M. O. (2018). Human consumption and popular knowledge on the conservation status of groupers and sharks caught by small-scale fisheries on Abrolhos Bank, SW Atlantic. Mar. Policy.89, 142–146. doi: 10.1016/j.marpol.2017.12.020
21
Goodyear C. P. (1993). Spawning stock biomass per recruit in fisheries management: Foundation and current use. Can. Spec. Pub. Fish. Aquat. Sci.120, 67–82.
22
Grandcourt E. (2005). Demographic characteristics of selected Epinepheline groupers (Family: Serranidae; subfamily: Epinephelinae) from Aldabra atoll, Seychelles. Atoll Res. Bull.539. 199–216. doi: 10.5479/si.00775630.539.199
23
Haddon M. (2011). Modelling and Quantitative Methods in Fisheries. 2nd Ed (London: CRC Press, Taylor & Francis).
24
Hamel O. S. Cope J. M. (2022). Development and considerations for application of a longevity-based prior for the natural mortality rate. Fish. Res.256, 106477. doi: 10.1016/j.fishres.2022.106477
25
Heemstra P. C. Randall J. E. (1993). FAO Species Catalogue. Vol. 16. Groupers of the world (family Serranidae, subfamily Epinephelinae). An annotated and illustrated catalogue of the grouper, rockcod, hind, coral grouper and lyretail species known to date. Rome: FAO. FAO Fish. Synop.125, 382.
26
Hiatt R. W. Strasburg D. W. (1960). Ecological relationships of the fish fauna on coral reefs of the Marshall Islands. Ecol. Monogr.30, 65–127. doi: 10.2307/1942181
27
Hixon M. A. Carr M. H. (1997). Synergistic predation, density dependence, and population regulation in marine fish. Science277, 946–949. doi: 10.1126/science.277.5328.946
28
Huliselan N. V. Wawo M. Tuapattinaja M. A. Sahetapy D. (2017). Present status of grouper fisheries at waters of Kotania Bay, Western Seram District Maluku Province. IOP Conf. Series: Earth Environ. Sci. 89/1, 1–9. doi: 10.1088/1755-1315/89/1/012002
29
Ignatius T. H. Faizah R. (2021). Population parameters of the yellow-edged lyretail (Variola louti, Forsskål 1775) in Sibolga Waters. E3S Web Conferences322, 1015. doi: 10.1051/e3sconf/202132201015
30
Jin D. I. Kite-Powell H. Hoagland P. Solow A. (2012). A bioeconomic analysis of traditional fisheries in the Red Sea. Mar. Resour. Econ.27, 137–148. doi: 10.5950/0738-1360-27.2.137
31
Kimura D. K. (1980). Likelihood methods for the von Bertalanffy growth curve. Fish. Bull.77, 765–776.
32
Mace P. M. (1994). Relationships between common biological reference points used as thresholds and targets of fisheries management strategies. Can. J. Fish. Aquat. Sci.51, 110–122. doi: 10.1139/f94-013
33
Mace P. M. Sissenwine M. P. (1993). How much spawning per recruit is enough? Can. Spec. Publ. Fish. Aquat. Sci.120, 110–118.
34
Mahé K. Gentil C. Brisset B. Evano H. Lepetit C. Boymond-Morales R. et al . (2022). Biology of exploited groupers (Epinephelidae family) around La Réunion Island (Indian Ocean). Front. Mar. Sci.9. doi: 10.3389/fmars.2022.935285
35
Maunder M. N. Hamel O. S. Lee H. H. Piner K. R. Cope J. M. Punt A. E. et al . (2023). A review of estimation methods for natural mortality and their performance in the context of fishery stock assessment. Fish. Res.261, 106489. doi: 10.1016/j.fishres.2022.106489
36
MEWA (2021). Ministry of Environment, Water and Agriculture (Riyadh: Implementing Regulation of the Agriculture Law issued by Royal Decree No (M/64) dated 10/8/1442 H for the Department of Marine Fisheries). May 20, 2021.
37
Nair R. Samoilys M. Cabanban A. S. (2018). Variola louti (The IUCN Red List of Threatened Species 2018), e.T132738A100572909. doi: 10.2305/IUCN.UK.2018-2.RLTS.T132738A100572909.en
38
Nikolsky G. V. (1976). The Ecology of Fishes. 6th Ed (New York: Academic Press).
39
Patterson K. (1992). Fisheries for small pelagic species: an empirical approach to management targets. Rev. Fish Biol. Fish.2, 321–338. doi: 10.1007/BF00043521
40
Pauly D. Christensen V. Dalsgaard J. Froese R. Torres F. J. (1998). Fishing down marine food webs. Science279, 860–863. doi: 10.1126/science.279.5352.860
41
Prager M. H. Porch C. E. Shertzer K. W. Caddy J. F. (2003). Targets and limits for management of fisheries: a simple probability-based approach. N. Am. J. Fish. Manage.23, 349–361. doi: 10.1577/1548-8675(2003)023<0349:TALFMO>2.0.CO;2
42
Prince J. Steven V. Valentino K. Adrian H. (2015). Length-based SPR assessment of eleven Indo-Pacific coral reef fish populations in Palau. Fish. Res.171, 42–58. doi: 10.1016/j.fishres.2015.06.008
43
Quinn T. J. Deriso R. B. (1999). Quantitative Fish Dynamics (New York: Oxford University Press).
44
R Core Team (2024). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing, Version 4.3.3). Available at: https://www.R-project.org/ (Accessed April 1, 2025).
45
Rhodes K. L. Tupper M. H. (2007). A preliminary market-based analysis of the Pohnpei, Micronesia, grouper (Serranidae: Epinephelinae) fishery reveals unsustainable fishing practices. Coral Reefs.26, 335–344. doi: 10.1007/s00338-007-0202-5
46
Ritz C. Streibig J. C. (2008). Nonlinear Regression with R (New York: Springer).
47
Sadovy de Mitcheson Y. S. Craig M. T. Bertoncini A. A. Carpenter K. E. Cheung W. W. L. Choat J. H. (2013). Fishing groupers towards extinction: a global assessment of threats and extinction risks in a billion dollar fishery. Fish. Fish.14, 119–136. doi: 10.1111/j.1467-2979.2011.00455.x
48
Sadvoy Y. Shapiro D. Y. (1987). Criteria for the diagnosis of hermaphroditism in fishes. Copeia1987, 136–156. doi: 10.2307/1446046
49
Salas S. Chuenpagdee R. Seijo J. C. Charles A. (2007). Challenges in the assessment and management of small-scale fisheries in Latin America and the Caribbean. Fish. Res.87, 5–16. doi: 10.1016/j.fishres.2007.06.015
50
Schemmel E. Dahl K. (2023). Age, growth, and reproduction of the yellow−edged lyretail Variola louti (Forsskal 1775). Environ. Biol. Fish.106, 1247–1263. doi: 10.1007/s10641-023-01411-3
51
Secor D. Dean J. Laban E. (1991). Manual for otolith removal and preparation for microstructural examination. Ed. BelleW. (Sydney: Baruch Institute for Marine Biology and Coastal Research, Technical Publication Number, 1991-01), 84 pp.
52
Shellem C. T. Ellis J. I. Coker D. J. Berumen M. L. (2021). Red Sea fish market assessments indicate high species diversity and potential overexploitation. Fish. Res.239, 105922. doi: 10.1016/j.fishres.2021.105922
53
Sokal R. R. Rohlf F. J. (2012). Biometry. The Principles and Practice of Statistics in Biological Research. 4th Ed (New York: W. H. Freeman).
54
Sparre P. Venema S. C. (1998). Introduction to tropical fish stock assessment (Rome: FAO Fisheries Technical Papers, No. 306/1, Rev. 2).
55
Stewart B. D. Jones G. P. (2001). Associations between the abundance of piscivorous fishes and their prey on coral reefs: implications for prey-fish mortality. Mar. Biol.138, 383–397. doi: 10.1007/s002270000468
56
Then A. Y. Hoenig J. M. Hall N. G. Hewitt D. A. (2015). Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci.72, 82–92. doi: 10.1093/icesjms/fsx199
57
Thompson G. (1993). A proposal for a threshold stock size and maximum fishing mortality rate. Can. Spec. Publ. Fish. Aquat. Sci.120, 303–320.
58
Thompson W. F. Bell H. (1934). Biological statistics of the Pacific halibut fishery. 2. Effect of changes in intensity upon total yield, and yield per unit gear Vol. 8 (Seattle: Report of the International Fisheries Commission), 48 pp.
59
Tıraşın E. M. (1993). Balık populasyonlarının büyüme parametrelerinin araştırılması. Turk. J. Zool.17, 29–82.
60
To A. W. L. Sadovy de M. Y. (2009). Shrinking baseline: The growth in juvenile fisheries, with the Hong Kong grouper fishery as a case study. Fish Fish.10, 396–407. doi: 10.1111/j.1467-2979.2009.00326.x
61
von Bertalanffy L. (1938). A quantitative theory of organic growth (inquiries on growth laws. II). Hum. Biol.10, 181–213.
62
Wahbeh M. I. (2005). Some aspects of reproduction and growth of the grouper, Cephalopholis miniata (Forsskål), the blacktip grouper, Epinephelus fasciatus (Forsskål), and the lunartail grouper, Variola louti (Forsskål) from the North-eastern coast of the Gulf of Aqaba (Red Sea), Jordan. Dirasat-Pure Sci. Volume32, 172–181.
63
Walters C. Martell S. J. D. (2004). Fisheries Ecology and Management (Princeton: Princeton University Press).
64
Williams E. H. Shertzer K. W. (2003). Implications of life-history invariants for biological reference points used in fishery management. Can. J. Fish. Aquat. Sci.60, 710–720. doi: 10.1139/f03-059
65
Wilson K. Hardy I. C. W. (2002). “Statistical analysis of sex ratios: An introduction,” in Sex Ratios, Concepts and Research Methods. Ed. HardyI. C. W. (Cambridge University Press, Cambridge), 48–92.
66
Zhou S. Yin S. Thorson J. T. Smith A. D. M. Fuller M. (2012). Linking fishing mortality reference points to life history traits: an empirical study. Can. J. Fish. Aquat. Sci.69, 1292–1301. doi: 10.1139/f2012-060
Summary
Keywords
life history, age determination, stock assessment, reference points, fisheries management, Epinephelidae
Citation
Kadengal ST, Okba Z, Muthu Palani GB, Charles S, Adam AMS, Boulila N, Tıraşın EM and Dimech M (2025) Population dynamics and fishery biology of the yellow-edged lyretail grouper Variola louti (Forsskål, 1775) along the eastern coast of the Red Sea. Front. Mar. Sci. 12:1582582. doi: 10.3389/fmars.2025.1582582
Received
24 February 2025
Accepted
28 May 2025
Published
17 June 2025
Volume
12 - 2025
Edited by
Stephen J. Newman, Western Australian Fisheries and Marine Research Laboratories, Australia
Reviewed by
Yiping Luo, Southwest University, China
Martina Scanu, University of Bologna, Italy
Updates
Copyright
© 2025 Kadengal, Okba, Muthu Palani, Charles, Adam, Boulila, Tıraşın and Dimech.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sirajudheen Thayyil Kadengal, sirajudheen.kadengal@kaust.edu.sa
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.