- 1Ecosystems, British Antarctic Survey (BAS), Cambridge, United Kingdom
- 2School of Environmental Sciences, Faculty of Science and Engineering, University of Liverpool, Liverpool, United Kingdom
The Southern Ocean is a globally significant site of carbon sequestration with the copepod community exerting a strong influence on the carbon flux. Currently, a holistic understanding of Southern Ocean copepod ecology is limited by a lack of data, particularly during winter. This study analyzed the composition and abundance of copepods caught in a sediment trap (400 m depth) in the Northeast Scotia Sea, providing a view of year-round copepod community dynamics. We found strong seasonal trends in abundance and composition of copepod taxa, with Calanus simillimus and Metridia spp dominating throughout. The capture of Metridinidae copepods likely occurred as they carried out their pronounced diel vertical migrations (DVM). The disproportionate abundance of male specimens of Metridia spp., as well as another member of the Metridinidae, Pleuromamma robusta, indicates sex-specific differences in their DVM ranges, with males remaining deeper. The C5 developmental stage of C. simillimus showed a distinct seasonal pattern, characterized by high abundances in autumn followed by low numbers in winter. We propose that this reflects an autumnal seasonal descent beyond which their fate could be one of three scenarios. Firstly, that these individuals seasonally migrate deeper than the sediment trap depth but remain active and feed on deep particulate matter. Secondly, that they become dormant whilst at this depth and respire their fat reserves. Thirdly, that they become dormant but at shallower depths, at and around the depth of the sediment trap, where they remain static and are not captured. Each of these scenarios has different implications for the seasonal carbon flux generated by C. simillimus. This study highlights the importance of understanding species-specific copepod ecology and emphasizes the need to collect ecological data over full annual cycles.
Introduction
Atmospheric carbon dioxide is fixed as organic matter at the ocean surface and transported to the interior via the biological carbon pump (BCP), isolating it for hundreds of years (Sarmiento, 2006). Copepods play a critical role in the transport of carbon into the deep ocean (Mayor et al., 2014; Jónasdóttir et al., 2015; Steinberg and Landry, 2017); by affecting both the quantity and the depth at which carbon is stored, copepods impact the strength and efficiency of the BCP. However, their influence depends upon the composition and ecology of the species present, with high productivity, high latitude regions acting as globally significant carbon sinks (Barnes and Tarling, 2017; Steinberg and Landry, 2017; Pinti et al., 2023). Copepods contribute to carbon export via fecal pellet production, the carbon-content and sinking rate of fecal pellets being dependent upon species, size and diet (Dagg et al., 2003; Manno et al., 2015; White et al., 2018). Copepods also actively transport organic carbon from the surface to depth through various vertical migrations (Steinberg et al., 2000; Countryman et al., 2022). Diel vertical migration (DVM) involves grazing at the surface during the night and metabolizing food and producing fecal pellets during the day at depth (Hays et al., 1994). Some copepods also migrate seasonally, remaining near the surface during spring and summer and overwintering at depth (e.g. > 500 m). A large portion of seasonal migrators mitigate food scarcity during winter by entering diapause, a physiologically dormant state wherein copepods survive using their lipid reserves (e.g. Calanoides acutus, Atkinson, 1991; Hirche, 1996; Baumgartner and Tarrant, 2017), while others remain active and scavenge throughout winter (e.g. C. propinquus, Bathmann et al., 1993). Diapausing copepods significantly contribute to the active carbon flux by respiring carbon-rich lipids, originating from the surface, directly at depth (Jónasdóttir et al., 2015; Visser et al., 2017; Pinti et al., 2023). This process, termed the lipid pump, is particularly strong at high latitudes, and can store a significant amount of carbon (e.g. 2–6 gC m−2y−1), comparable to the passive/gravitational particulate carbon pump (Jónasdóttir et al., 2015; Visser et al., 2017).
Though the Southern Ocean zooplankton community has long been the subject of research, important questions remain about the life history strategy of multiple copepod species. This is particularly the case for winter when there is a paucity of data (Johnston et al., 2022). Obtaining winter data is difficult due to the considerable financial and logistical considerations of operating in the Southern Ocean throughout the year. An example of this knowledge gap can be characterized by Calanus simillimus, a dominant sub-Antarctic species (Ward et al., 1996a). Though multiple studies have focused on the life-history of C. simillimus (e.g. Atkinson and Peck, 1988; Atkinson, 1989, 1991), its precise overwintering strategy is unclear. It exhibits a bimodal overwintering depth-distribution; the majority of C5 copepodites migrate to deeper water (> 250 m), with adults and younger copepodites remaining closer to the surface (< 250 m) (Atkinson and Peck, 1988; Atkinson, 1989, 1991). However, it is unknown whether C. simillimus undertakes diapause or remains active during winter (Atkinson, 1998).
Copepods can be highly plastic, altering their production cycles, overwintering strategies and vertical distributions with environmental conditions and location (Strand et al., 2020; Schmidt et al., 2025). We are aware of only one study assessing the life cycle of Metridia spp. in South Georgia (Atkinson and Peck, 1988). However, assessment of Metridia gerlachei in other regions have found their reproductive cycle can vary between one generation (Weddell Sea, Kurbjeweit, 1993) and three generations (Bransfield Strait, Huntley and Escritor, 1992; King and LaCasella, 2003). Indeed, genetic analysis of Metridia lucens has found two genetically distinct groups in the Atlantic sector of the Southern Ocean, likely divided by the Polar Front (Stupnikova et al., 2013). Metridia spp. are ecologically significant grazers (Schnack, 1985; Froneman et al., 2000) and perform a pronounced diel vertical migration (DVM) whilst simultaneously remaining winter active (Atkinson, 1989; Osgood and Frost, 1994; Lopez and Huntley, 1995). Consequently, they are an important component of the BCP (King and LaCasella, 2003).
Recently, moored sediment traps have been used to observe and monitor zooplankton communities in remote locations throughout the year (e.g. Kraft et al., 2012, 2013; Ramondenc et al., 2022; Gardner et al., 2023; Atherden et al., 2024). Zooplankton (referred to as ‘swimmers’ in this context) actively swim into the trap where they are preserved in formalin. For diel and seasonal migrating organisms such as copepods, sediment traps offer another, particularly useful function; catching individuals’ descent from the surface to the deep ocean (Ramondenc et al., 2022). In this study, we used a moored sediment trap to characterize seasonal trends in copepod abundance, size and ontogenetic composition, and used these patterns to identify life-cycle strategies. This study is located in the Scotia Sea, a highly productive region in the Southern Ocean characterized by high zooplankton biomass, which substantially contributes to the carbon export of this region (Korb et al., 2005, 2012; Whitehouse et al., 2012; Manno et al., 2015; Liszka et al., 2019; Belcher et al., 2023).
Materials and methods
Sampling
Sampling took place at the P3 observation site (Northeast Scotia Sea, 52.80˚ S, 40.14˚ W, bottom depth 3748 m), as part of the Scotia Open Ocean Observatory programme (SCOOBIES, https://www.bas.ac.uk/project/scoobies/) (Figure 1). A sediment trap was deployed at 378 m during research cruise JR17002, RSS James Clark Ross and recovered by research cruise DY098, RRS Discovery. The sediment trap collected samples between February and December 2018.
Figure 1. Location of the moored sediment trap (400 m) at the P3 observation site (Northeast Scotia Sea, 52.80˚ S, 40.14˚ W, bottom depth 3748 m). Adapted from Atherden et al. (2024), licensed CC-BY-4.0.
The opening of the sediment trap (McLane PARFLUX, 0.5 m2 capture area; McLane labs, Falmouth MA, USA) was fitted with a baffle to prevent larger animals entering the trap. 500 mL bottles were used for automated sample collection. Bottles were filled to the brim with formalin (filtered seawater containing 2% v/v formalin, mixed with sodium tetraborate (BORAX; 0.025% w/v), and 0. 5% w/v sodium chloride). The bottles were placed in a carousel, pre-programmed to rotate and collect material every calendar month, although the collection periods were shorter during the productive period (but collated to 1 month for the present analysis). Once recovered, samples were stored at 4°C until analysis.
Sample processing and copepod identification
Specimens were identified, measured and counted under an Olympus SZX16 fitted with a Canon EOS 60D DSLR camera. We assumed that copepods with an intact prosome and urosome were active swimmers. Due to their small size, nauplii, Oithona, Microcalanus and Onacea spp. were excluded from any analysis. It is not always possible to discriminate between swimmers and carcasses (i.e., perfectly intact zooplankton that had died shortly before sinking into the sediment trap). However, Ivory et al. (2014) found the number of copepod swimmers to be an order of magnitude higher than sinking carcasses, indicating that carcasses are a relatively minor component. Copepod occurrence was converted to abundance, as follows (Equation 1).
Where possible, copepods were staged and identified to species and genus, however family classifications were sometimes required, particularly where younger developmental stages made precise identification difficult. Copepod length, for each individual, was calculated from images taken with a graduated Petri dish, and subsequently analyzed using ImageJ (Version 1.54g).
Results
Copepod abundance and composition
It was not possible to identify all copepods to species level, however a total of 14 different species were identified as well as 15 different genera. Calanus spp. and Metridia spp. were consistently present and numerically represented a dominant component of copepods throughout sampling, averaging 39.3 ± 13.2% and 22.2 ± 11.4% (mean ± standard deviation) of taxonomic composition, respectively (Figures 2A, B). Calanus spp. abundance reached their maximum of 5 copepods m-2 day-1 in February, decreasing to< 1 copepod m-2 day-1 in May and increasing again thereafter. Metridia spp. abundance followed a similar seasonal pattern with abundance decreasing from March to June (with a minimum of < 1 copepod m-2 day-1 in March) and increasing thereafter into the spring and summer (with a maximum of 2 copepods m-2 day-1 in September). Other taxa only occurred seasonally or occasionally. Pleuromamma spp. and Gaetanus spp. were either present in low abundance or absent from March to August, reaching a maximum of 1 copepod m-2 day-1 in December. Rhincalanus gigas was only present from June to October, and in December (< 1 copepod m-2 day-1). Though the exact timing and magnitude of abundance varied with specific taxa, there was a strong seasonal pattern overall, with the total copepod abundance maximizing at 8 copepods m-2 day-1 in February with a minimum of 1 copepod m-2 day-1 in June.
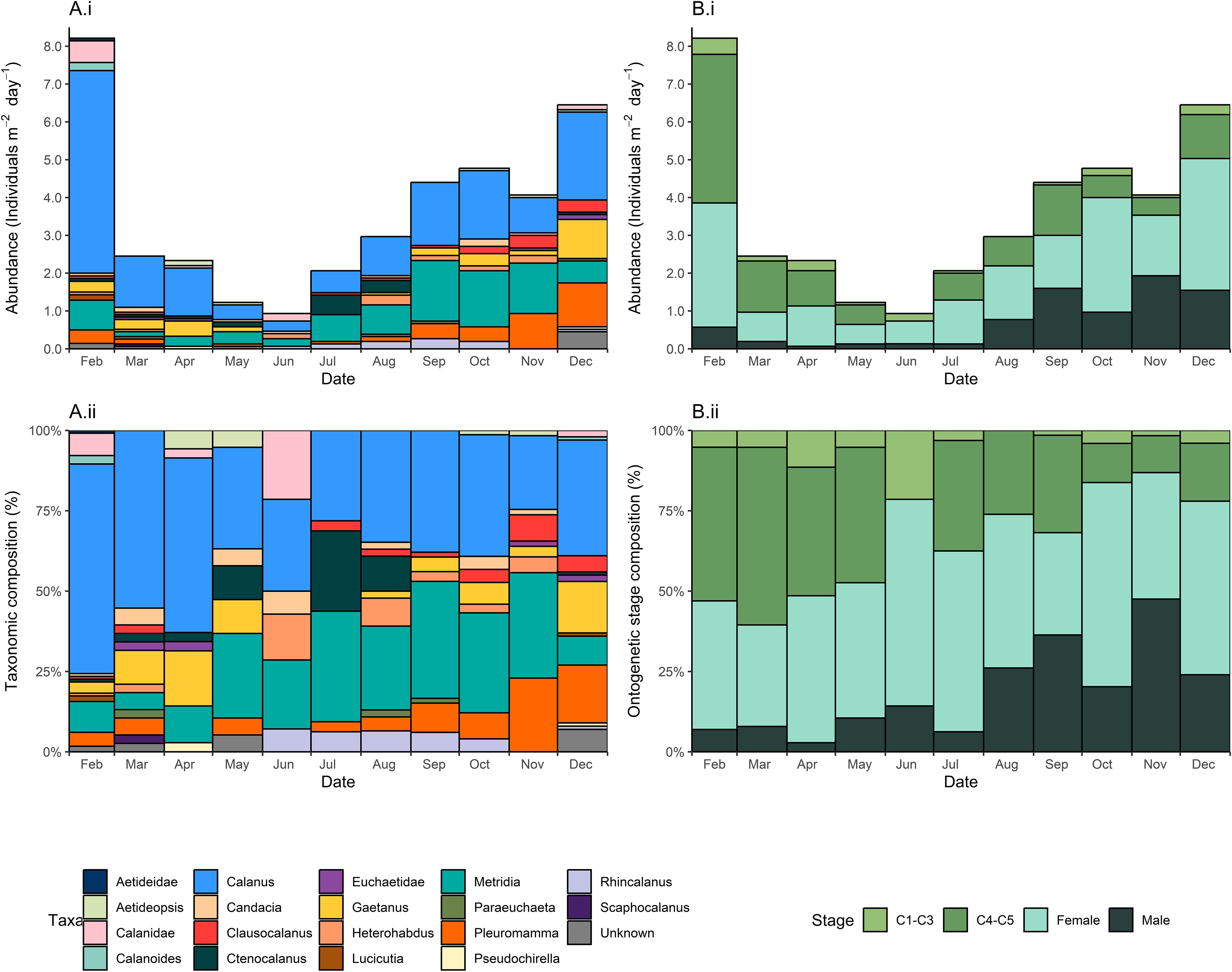
Figure 2. Taxonomic (A.i, A.ii), ontogenetic stage (B.i, B.ii) composition of copepods present in a sediment trap deployed at 400 m in the Northeast Scotia Sea (52.80˚ S, 40.14˚ W). The sediment trap sampled each calendar month from February until December 2018.
Seasonality is also reflected in copepod stage (Figure 2B). Copepodite abundance was greatest in the late spring/summer months (December, February and March). Female copepods had a strong presence throughout the year, however male copepod abundance substantially increased from August onwards, reaching a maximum in November (Figures 2B.i, ii). It should be noted, however, that the presence of certain stages was highly species specific. Metridia spp. and Pleuromamma robusta represented 46.7% and 32.8% of all males, respectively. February, the month with the greatest number of copepodites (<= stage C5), also had the lowest median copepod length (2.24 mm) (Figure 3A). Rhincalanus spp. greatly increased the sampled size range when present, resulting in the greatest copepod size range of 1.33-9.36 mm in August. Median copepod length ranged between 2.24-2.94 mm. From February the median copepod size steadily increases to 2.76 mm in June (wherein a high proportion of adults are present, Figure 2Bii). In July the median copepod size drops to 2.30 mm as smaller taxa such as Ctenocalanus spp. start to appear, there is then a general trend on increase until November and December wherein again a large proportion of adults are present (Figure 2Bii). The distribution of all copepod lengths is bimodal, with peaks at 2.75–3.00 mm and 1.75–2 mm, with these peaks comprising largely of Calanus and Metridia spp. respectively (Figure 3B).
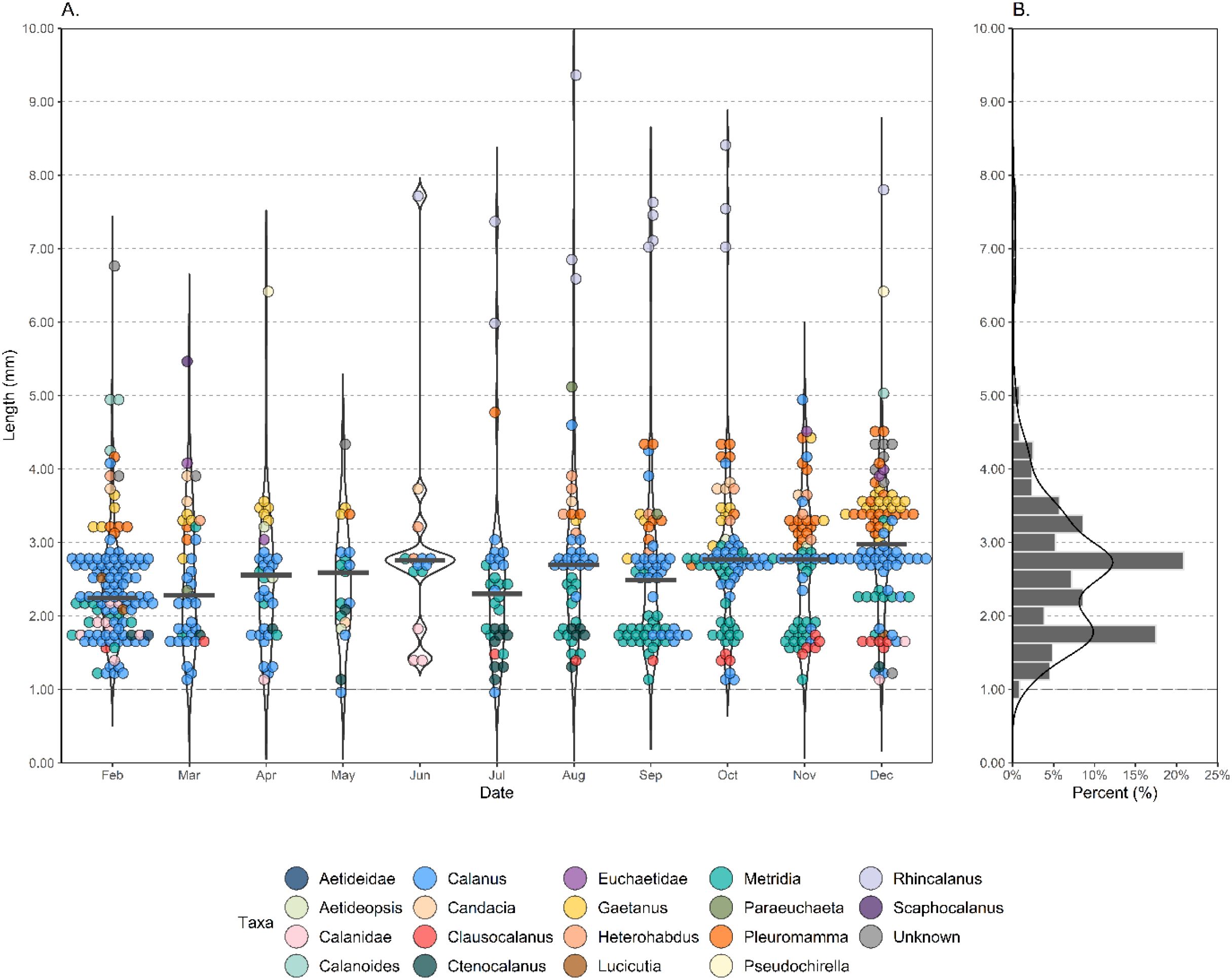
Figure 3. Individual copepod length (A) and total distribution of copepod lengths (B) present in a sediment trap deployed at 400 m in the Northeast Scotia Sea (52.80˚ S, 40.14˚ W). The sediment trap sampled each calendar month from February until December 2018. Size measurements are inclusive of both the prosome and the urosome, with points indicating individual measurements, color indicating taxonomic identity, and curves indicating the density distribution of measurements. The median value per month for individual length measurements (A) is indicated with a grey horizontal bar.
Seasonal variations in size and stage are also apparent for individual species or genera. For Metridia spp. females and copepodites are the majority of stages present from February to July, with males dominating from August onwards (Figure 4B). Metridia spp. median copepod length increases from February to June, reaching a maximum of 2.64 mm where females dominate. Median copepod length then decreases to a minimum of 1.76 mm in September, where the majority of individuals are males. Metridia spp. length then increases in October, as females dominate the sample. For Pleuromamma robusta, males were the dominant stage every month other than July and October (Figure 4C). Consequently, P. robusta median size remained relatively constant (3.13–3.38 mm) with the exception of July and October, where the presence of females increases median copepod length to 4.74 and 4.16 mm, respectively.
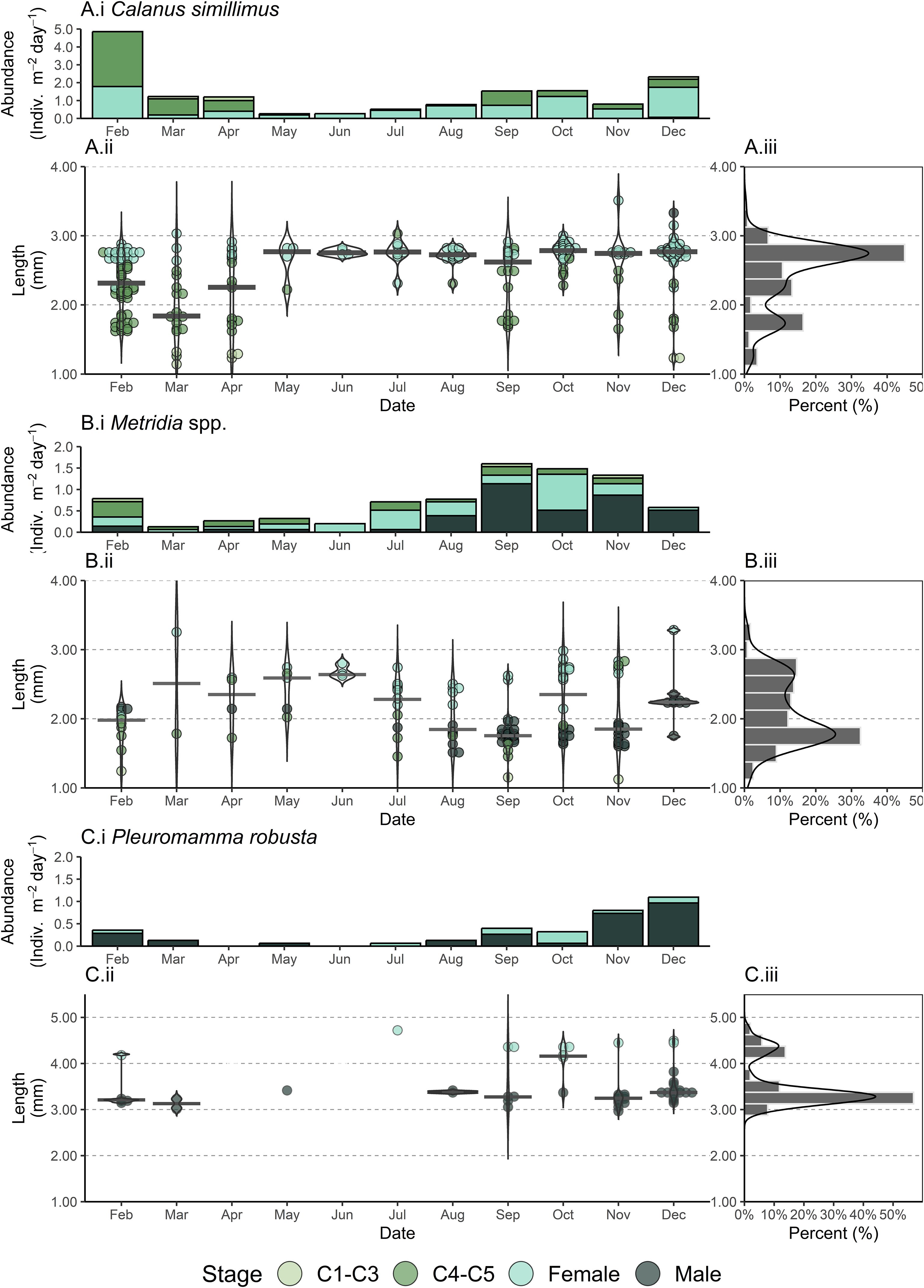
Figure 4. Calanus simillimus (A), Metridia spp (B). and Pleuromamma robusta (C) with regards ontogenetic stage abundance (i), individual copepod length (ii) and total distribution of copepod length (iii) collected from a sediment trap deployed at 400 m in the Northeast Scotia Sea (52.80˚ S, 40.14˚ W). The sediment trap sampled each calendar month from February until December 2018. Size measurements are inclusive of both the prosome and the urosome, with points indicating individual measurements, color ontogenetic stage, and curves indicating the density distribution of measurements. For individual copepod length (ii) the median value per month is indicated with a grey horizontal bar. Note that copepod length scales (ii, iii) start at 1 mm, with taxa specific ranges.
In contrast to the Metridinidae, only one male Calanus simillimus was present throughout the sampling period (Figure 4A). For C. simillimus (which represented 90.2% of all Calanus spp. in the sediment trap), the majority of individuals in the sediment trap were females, however younger copepodites dominated from February to April and June. Variation in median C. simillimus size largely matched this trend, as February to April had the lowest monthly median sizes, with a minimum of 1.84 mm in March. For the remainder of the year, C. simillimus monthly median size remained relatively consistent, ranging from 2.62-2.79 mm as females dominated.
Discussion
The copepod assemblage
The sediment trap captured copepods from a range of taxa with a variety of life history strategies. Despite the broad taxonomy and varied copepod ecology present, there was a strong seasonal signal, with abundance peaking in February, reducing until June and increasing thereafter. The seasonal pattern in copepod abundance concurs with previous net-based zooplankton studies in the Scotia Sea (Atkinson and Peck, 1988; Ward et al., 2006, 2012; Atherden et al., 2024). Median copepod length peaked in both winter (June) and spring (November, December) with a minimum in February. Our results highlight taxonomic and ontogenetic composition as important factors in body size structure within zooplankton communities. Seasonal patterns in copepod body size at a community and individual level have been attributed to changes in temperature and food availability and species assemblage (Landry et al., 1994; García-Pámanes and Lara-Lara, 2001; Palomares-García et al., 2013; Horne et al., 2016). The observed seasonal variation in copepod community size at 400 m may have implications for mesopelagic predators. Larger copepods may be more easily detected by predators, or conversely, larger copepods may fall outside some optimal size ranges for certain predators (Cowan, 1996; Saito and Kiørboe, 2001; Jackson and Lenz, 2016). The adult stages in the winter months (June-August) may be either early developers or remnants from the previous growing season. Early developers can disproportionately contribute to recruitment (Richardson et al., 1999; Daase et al., 2013), although their larger body size may then also expose them to elevated predation risks.
Calanus simillimus and Metridia spp. dominated copepod abundance over the year. The relative abundance of Metridia spp. was in good agreement with previous net-based community assessments, with Metridia spp. representing between 5.3–36.4% of individuals in our study and 12.9-38.8% of large (~< 1 mm) copepods in Ward et al (2006, 2012). (Figure 2Aii; Supplementary Table S1). Metridia spp. around South Georgia can perform diel vertical migrations to 400 m (Ward et al., 1995). This behavior may facilitate entrapment rates in the sediment trap (deployed at 400 m) comparable to net abundances. Conversely, Ward et al (2006; 2012, sampling in summer and spring-autumn respectively) reported that C. simillimus represented between 0.3-10.9% of copepods, whereas in our sediment trap, C. simillimus abundance was far higher accounting for between 19.7–63.5% of copepods. Makabe et al. (2016) directly compared sediment trap and net abundances in the Canadian Arctic and found abundances of Metridia longa in sediment trap collections to be directly comparable to those from net catches. Conversely, the same was not found for C. hyperboreus and C. glacialis, which were more abundant in sediment trap samples during winter, coinciding with their reproductive season (Makabe et al., 2016). It is likely that the comparatively high proportion of C. simillimus in our sediment trap is also a consequence of seasonal vertical migration patterns (Atkinson and Sinclair, 2000) which are not resolved as effectively by net-sampling at discrete time points. We found highest seasonal abundances of Metridia spp. in spring (Figure 4Bi), whereas the net-based study by Ward et al. (2012) found the highest abundances in autumn. Atkinson (1998) found that the vertical distribution of Metridia spp. varies seasonally, with pronounced DVM in spring, DVM shallowing in summer and disbursement throughout the water column in autumn. Consequently, differences between peaks in seasonal abundance likely reflect the sediment trap catching active, migrating individuals in spring, while nets sampled through this depth range (0–400 m) would have integrated abundances over 400 m, downscaling overall abundance estimates. We found young Metridia spp. copepodite stages were present in September, November, and February, with spermatophores present on 5 females (from August to October) (Supplementary Table S2). This suggests an extended reproductive season, with the possibility of multiple generations. We are unaware of studies assessing the reproductive phenology of M. lucens in the region. However, our findings are in good agreement of the known reproductive strategy of M. gerlachei in other productive regions (e.g. King and LaCasella, 2003). For C. simillimus, we found females and C5s to be present throughout the year, with their abundance peaking in summer and decreasing in the winter months. Young copepodites were present in March, April, and December. This agrees well with previous work, supporting the hypothesis that C. simillimus is capable of multiple generations within a year (Atkinson, 1991, 1998).
Calanus simillimus overwintering
The precise overwintering strategy of C. simillimus is, as yet, unknown. Atkinson (1989, 1991) demonstrate that C. simillimus has a bi-modal, stage specific distribution. Females and males overwinter at the surface (0–250 m) whereas C5s have been found to overwinter at 500–1000 m (Atkinson, 1991, transect from Falklands to South Georgia) and 250–500 m (Atkinson, 1989, South Georgia). However, it is not clear how C5s migrating to depth sustain themselves and whether they enter diapause like their northern congeners (C. hyperboreus, C. glacialis, C. finmarchicus) (Lee et al., 2006) or are active overwinterers like their southern congener C. propinquus (Bathmann et al., 1993). Atkinson (1998) suggested that C. simillimus may undertake diapause however, to our knowledge, no direct winter observations have been made. In our sediment trap, C5 abundance peaked in February, remaining relatively high until April (autumn) (Figure 4Ai). We suggest the high abundance of C5s in our trap from February to April represents individuals descending to depth to begin overwintering. Other sediment trap studies have caught copepods during their seasonal migration (Makabe et al., 2016; Ramondenc et al., 2022) typified by a peak in sediment trap abundance at the end of summer/autumn followed by a minimum in winter. This is similar to our study, where the autumnal peak in C5 presence is followed by substantial reduction throughout winter, during which time, only three individual C5s were caught from May to August.
Situated at 400m, our sediment trap is within known overwintering depths for C. simillimus C5s (e.g. 250–500+ m). There are multiple explanations for our observed winter minimum in C5 sediment trap abundance (Figure 5). In scenario A, C5s migrate deeper than the sediment trap, but remain active. In scenario B, C5s migrate deeper than the sediment trap, but then enter diapause. Finally, in scenario C, C5s overwinter between 250–500 m and are therefore within the range of the sediment trap, however, they do not enter the trap because they are in diapause and vertically static. We suggest that Scenario A is perhaps the most unlikely, as available food for scavenging tends to decline strongly with depth (Parzanini et al., 2019; Henson et al., 2023). Indeed, the active overwintering congener Calanus propinquus tends to be found at< 250 m depth during winter (Schnack-Schiel and Hagen, 1995; Atkinson and Sinclair, 2000; Pasternak and Schnack-Schiel, 2001). Diapause behavior in C. simillimus (Scenario B and C) may have previously been overlooked as C. simillimus primarily store triacylglycerols (TAGs) rather than wax esters (Ward et al., 1996b). Wax esters are strongly associated with diapause behavior whereas TAGs have been hypothesized to indicate active overwintering (Sargent et al., 1981; Hagen et al., 1993; Kattner et al., 2012). However, Eucalanus bungii, a TAG-storing species, is fully capable of deep, sustained diapause (500–2000 m), suggesting TAG storage does not preclude diapause (Ohtsuka et al., 1993; Yamada et al., 2016).
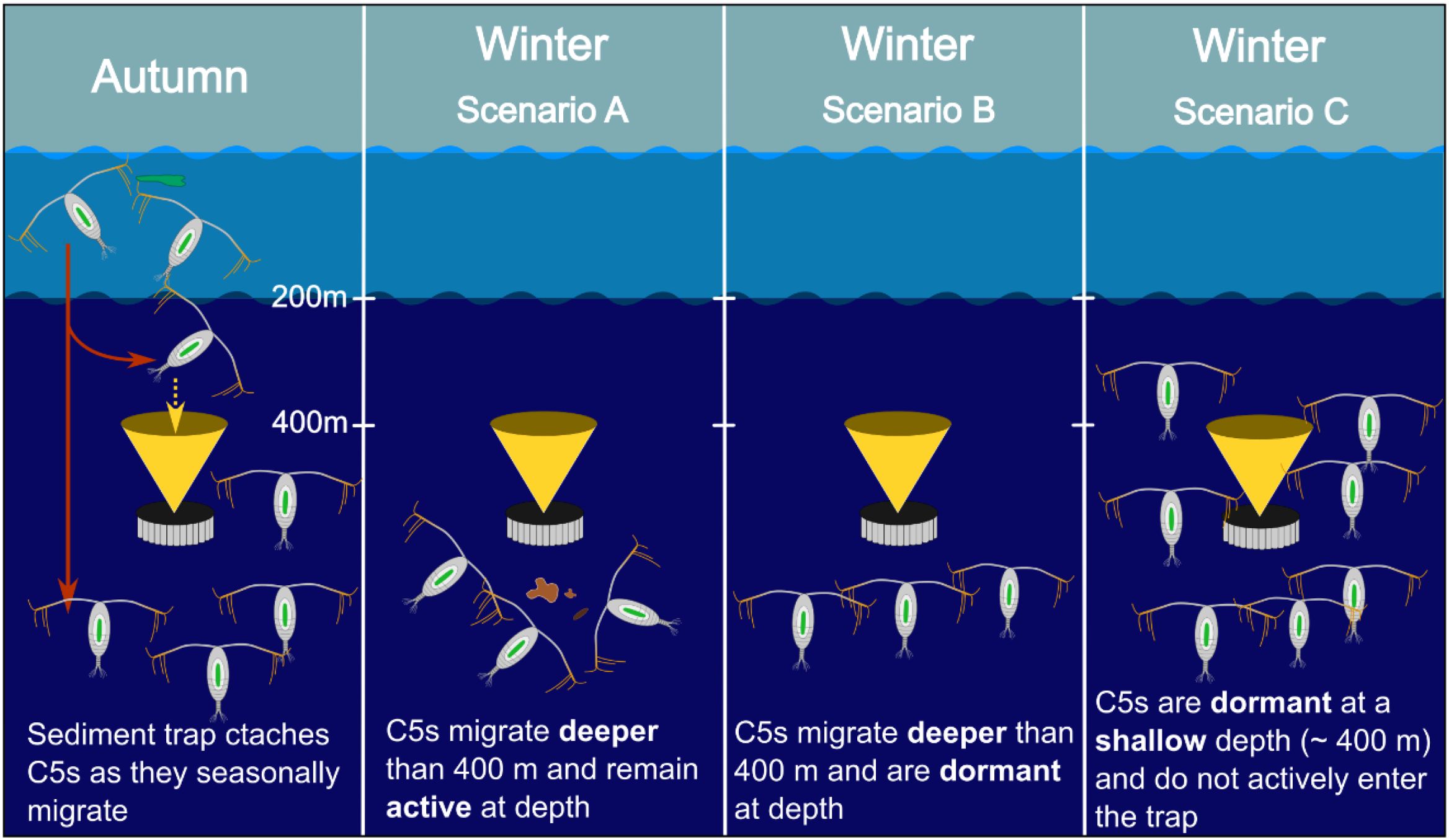
Figure 5. Schematic of potential Calanus simillimus winter ecology (copepodite stage C5) with respect to sediment trap abundance in autumn and winter (moored, 400 m depth). Yellow dotted arrows indicate active individuals entering the sediment trap. Red solid arrows indicate seasonal migration. Copepods with upright orientation indicate individuals in a physiologically dormant state (Diapause).
Though direct observations are required to ascertain if C. simillimus enters diapause, the implication that C. simillimus either is capable of diapause or is active at depths > 400 m, has important consequences for our understanding of their contribution to the biological carbon pump and lipid pump (Jónasdóttir et al., 2015; Boyd et al., 2019). With regards scenario A, active copepods maintain a high respiration rate which may substantially contribute to the lipid pump if fueled by stored reserves. Meanwhile, deep active feeding will, on the one hand, contribute to the fecal pellet flux (Manno et al., 2015) but, on the other, increase particle remineralization through sloppy feeding and particle fragmentation (Mayor et al., 2020). For scenario B, the contribution to the lipid pump will be less than scenario A as respiration decreases in diapause (Hirche, 1996) but at least there is certainty that it is fueled by stored reserves, which is a prerequisite for this carbon flux via the lipid pump. The strength of the lipid pump is affected by diapause depth. Pinti et al. (2023) found that species which diapause deeper, and therefore respire their lipid deeper in the ocean’s interior, sequester carbon for a longer period of time. However, the shallowest diapause depth range investigated was 500–800 m (Pinti et al., 2023). For scenario C, there is uncertainty that diapausing at < 400 m is deep enough for significant carbon sequestration. Determining whether C. simillimus C5s enter diapause, and their winter depth distributions, would require a depth resolved netting campaign in either late autumn or winter. Respiration experiments or estimates (e.g. Electron Transport System activity) and lipid analyses coupled with biomass estimates would help quantify any potential contributions to the carbon flux. Copepods in diapause are distinctively lethargic and unresponsive to physical stimuli, however, transcriptomic signatures for diapause have been established for C. finmarchicus (Lenz et al., 2021), and transcriptomic data could strengthen identification of diapause in C. simillimus. Since the Discovery era (1926–1938), C. simillimus abundance has increased by ~ 55% (Ward et al., 2018) indicating that this species is having an increasing influence on food webs and biogeochemical cycling in an era of Southern Ocean climate change.
Sex specific distributions
We found males to be the dominant stage throughout the year for Pleuromamma robusta and were between August to December for Metridia spp. (Figure 4). In contrast, only one C. simillimus male was captured throughout sampling (December, Figure 4Ai). The high abundance of male P. robusta and Metridia spp. within the sediment trap could reflect sex-specific depth distributions, with males occurring deeper in the water column and therefore entering the sediment trap (400 m) more frequently. Sex-specific depth distributions have been reported for Metridia spp. across multiple environments, including the Southern Ocean. Often male Metridia spp. reside deeper in the water column, with DVM behavior being either weak or not apparent (Hattori, 1989; Osgood and Frost, 1994; King and LaCasella, 2003). Sex-specific distributions of Pleuromamma spp. have been reported in only a small number of studies. In the North Pacific, Ambler and Miller (1987) and Haury (1988) found males to reside deeper than females, with some overlap. In the Iceland Basin, Gislason (2008) found male P. robusta to be deeper than females in both winter (December) and summer (June), but overlapping at other times of the year. To the best of our knowledge, this is the first study to suggest P. robusta males may reside deeper than females in the Southern Ocean.
Sex-specific depth distributions likely increase the chances of a reproductive encounter (Titelman et al., 2007). The chances of encountering a mate in a large, three-dimensional space are slim. If males form a ‘deeper stratum’, where there is less light and a reduced risk from visual predators, they can simultaneously increase chance of encountering a female undergoing DVM and increase their chances of survival (i.e. time for an encounter). Indeed, females with spermatophores were present from August until October, coinciding well with increased male presence in the sediment trap. The sex-specific depth distributions of Metridinidae warrant further investigations in high latitude regions. If males remain consistently deep after maturation, they are either surviving using somatic reserves or particle-feeding; both activities can have significant, but potentially opposing, influences on carbon export through either respiration or particle fragmentation at depth (Mayor et al., 2014; Jónasdóttir et al., 2015).
Concluding remarks
This study emphasizes the critical role of data encompassing all seasons in understanding copepod population dynamics in the Southern Ocean and their influence in the carbon cycle. We found that year-round copepod abundance assessments provided ecologically and biogeochemically relevant patterns. Occurrence of individuals in the sediment trap was likely influenced by abundance in the water column as well as taxon-specific ecological traits. Patterns in abundance in C. simillimus C5s suggest they may be dormant over winter, perhaps entering diapause although the possibility that they remain active over wintering cannot be ruled out. This highlights a need for direct observation of C. simillimus during autumn and winter as they may be a previously unaccounted for yet significant contributor to particulate carbon- and lipid pumps. The abundance of male Metridinidae in the sediment trap indicated sex-specific distributions with males residing at greater depths, possibly to enhance mate-encounter rates. This behavior warrants further investigation, particularly to determine how males sustain themselves, through starvation or scavenging. If these deeper-dwelling males rely on somatic reserves, they may contribute to the carbon flux in an analogous manor to the lipid pump. Our findings emphasize the value of assessing zooplankton ecology for understanding and predicting the zooplankton-mediated carbon flux. Incorporation of specific overwintering strategies, sex specific depth distributions and grazing behaviors will improve estimates of zooplankton mediated carbon sequestration, particularly in high latitude regions, where zooplankton population dynamics are poorly constrained.
Data availability statement
The original contributions presented in the study are available from the NERC EDS UK Polar Data Centre https://doi.org/10.5285/0F223636-0DED-4EA3-B300-0C20AF9CD9FA.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
FA: Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. SB: Data curation, Writing – review & editing. PW: Data curation, Writing – review & editing. GW: Data curation, Writing – review & editing. GT: Investigation, Writing – review & editing. CM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. CM was supported by UKRI-FLF project CUPIDO (MR/T020962/1). Work was carried out as part of the Ecosystems programme at the British Antarctic Survey and the Scotia Sea Open Ocean Laboratories (SCOOBIES) sustained observation programme at the British Antarctic Survey in the frame of a WCB-POETS survey cruise (https://www.bas.ac.uk/project/scoobies/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1586990/full#supplementary-material
References
Ambler J. W. and Miller C. B. (1987). Vertical habitat-partitioning by copepodites and adults of subtropical oceanic copepods. Mar. Biol. 94, 561–577. doi: 10.1007/BF00431403
Atherden F., Slomska A., and Manno C. (2024). Sediment trap illustrates taxon-specific seasonal signals in Southern Ocean zooplankton. Mar. Biol. 171, 173. doi: 10.1007/s00227-024-04487-2
Atkinson A. (1989). Distribution of six major copepod species around South Georgia during an austral winter. Polar Biol. 10, 81–88. doi: 10.1007/BF00239152
Atkinson A. (1991). Life cycles of Calanoides acutus, Calanus simillimus and Rhincalanus gigas (Copepoda: Calanoida) within the Scotia Sea. Mar. Biol. 109, 79–91. doi: 10.1007/BF01320234
Atkinson A. (1998). Life cycle strategies of epipelagic copepods in the Southern Ocean. J. Mar. Syst. 15, 289–311. doi: 10.1016/S0924-7963(97)00081-X
Atkinson A. and Peck J. M. (1988). A summer-winter comparison of zooplankton in the oceanic area Around South Georgia. Polar Biol. 8, 463–473. doi: 10.1007/BF00264723
Atkinson A. and Sinclair J. D. (2000). Zonal distribution and seasonal vertical migration of copepod assemblages in the Scotia Sea. Polar Biol. 23, 46–58. doi: 10.1007/s003000050007
Barnes D. K. A. and Tarling G. A. (2017). Polar oceans in a changing climate. Curr. Biol. 27, R454–R460. doi: 10.1016/j.cub.2017.01.045
Bathmann U. V., Makarov R. R., Spiridonov V. A., and Rohardt G. (1993). Winter distribution and overwintering strategies of the Antarctic copepod species Calanoides acutus, Rhincalanus gigas and Calanus propinquus (Crustacea,Calanoida) in the Weddell Sea. Polar Biol. 13, 333–346. doi: 10.1007/BF00238360
Baumgartner M. F. and Tarrant A. M. (2017). The physiology and ecology of diapause in marine copepods. Ann. Rev. Mar. Sci. 9, 387–411. doi: 10.1146/annurev-marine-010816-060505
Belcher A., Henley S. F., Hendry K., Wootton M., Friberg L., Dallman U., et al. (2023). Seasonal cycles of biogeochemical fluxes in the Scotia Sea, Southern Ocean: a stavle isotope approach. Biogeosciences 20, 3573–3591. doi: 10.5194/bg-20-3573-2023
Boyd P. W., Claustre H., Levy M., Siegel D. A., and Weber T. (2019). Multi-faceted particle pumps drive carbon sequestration in the ocean. Nature 568, 327–335. doi: 10.1038/s41586-019-1098-2
Countryman C. E., Steinberg D. K., and Burd A. B. (2022). Modelling the effects of copepod diel vertical migration and community structure on ocean carbon flux using an agent-based model. Ecol. Modell 470, 110003. doi: 10.1016/j.ecolmodel.2022.110003
Cowan J. J. (1996). Size-dependent vulnerability of marine fish larvae to predation: an individual-based numerical experiment. ICES J. Mar. Sci. 53, 23–37. doi: 10.1006/jmsc.1996.0003
Daase M., Falk-Petersen S., Varpe Ø., Darnis G., Søreide J. E., Wold A., et al. (2013). Timing of reproductive events in the marine copepod Calanus glacialis: a pan-Arctic perspective. Can. J. Fish. Aquat. Sci. 70, 871–884. doi: 10.1139/cjfas-2012-0401
Dagg M. J., Urban-Rich J., and Peterson J. O. (2003). The potential contribution of fecal pellets from large copepods to the flux of biogenic silica and particulate organic carbon in the Antarctic Polar Front region near 170°W. Deep Sea Res. Part II: Topical Stud. Oceanography 50, 675–691. doi: 10.1016/S0967-0645(02)00590-8
Froneman P. W., Pakhomov E. A., Perissinotto R., and McQuaid C. D. (2000). Zooplankton structure and grazing in the Atlantic sector of the Southern Ocean in late austral summer 1993. Deep Sea Res. Part I: Oceanographic Res. Papers 47, 1687–1702. doi: 10.1016/S0967-0637(99)00121-1
García-Pámanes J. and Lara-Lara J. (2001). Microzooplankton grazing in the gulf of california. Cienc. Mar. 27, 73–90. doi: 10.7773/cm.v27i1.387
Gardner J., Peck V. L., Bakker D. C. E., Tarling G. A., and Manno C. (2023). Contrasting life cycles of Southern Ocean pteropods alter their vulnerability to climate change. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1118570
Gislason A. (2008). Vertical distribution and seasonal dynamics of mesozooplankton in the Iceland Basin. Mar. Biol. Res. 4, 401–413. doi: 10.1080/17451000802232882
Hagen W., Kattner G., and Graeve M. (1993). Calanoides acutus and Calanus propinquus, Antarctic copepods with different lipid storage modes via wax esters or triacylglycerols. Mar. Ecol. Prog. Ser. 97, 135–142. doi: 10.3354/meps097135
Hattori H. (1989). Bimodal vertical distribution and diel migration of the copepods Metridia pacifica, M. okhotensis and Pleuromamma scutullata in the western North Pacific Ocean. Mar. Biol. 103, 39–50. doi: 10.1007/BF00391063
Haury L. R. (1988). Vertical distribution of Pleuromamma (Copepoda: Metridinidae) across the eastern North Pacific Ocean. Hydrobiologia 167, 335–342. doi: 10.1007/BF00026322
Hays G. C., Proctor C. A., John A. W. G., and Warner A. J. (1994). Interspecific differences in the diel vertical migration of marine copepods: The implications of size, color, and morphology. Limnol. Oceanogr. 39, 1621–1629. doi: 10.4319/lo.1994.39.7.1621
Henson S. A., Briggs N., Carvalho F., Manno C., Mignot A., and Thomalla S. (2023). A seasonal transition in biological carbon pump efficiency in the northern Scotia Sea, Southern Ocean. Deep Sea Res. Part II: Topical Stud. Oceanography 208, 105274. doi: 10.1016/j.dsr2.2023.105274
Hirche H. J. (1996). Diapause in the marine copepod, Calanus finmarchicus — A review. Ophelia 44, 129–143. doi: 10.1080/00785326.1995.10429843
Horne C. R., Hirst A. G., Atkinson D., Neves A., and Kiørboe T. (2016). A global synthesis of seasonal temperature–size responses in copepods. Global Ecol. Biogeography 25, 988–999. doi: 10.1111/geb.12460
Huntley M. E. and Escritor F. (1992). Ecology of Metridia gerlachei Giesbrecht in the western Bransfield Strait, Antarctica. Deep Sea Res. Part A. Oceanographic Res. Papers 39, 1027–1055. doi: 10.1016/0198-0149(92)90038-U
Ivory J. A., Tang K. W., and Takahashi K. (2014). Use of neutral red in short-term sediment traps to distinguish between zooplankton swimmers and carcasses. Mar. Ecol. Prog. Ser. 505, 107–117. doi: 10.3354/meps10775
Jackson J. M. and Lenz P. H. (2016). Predator-prey interactions in the plankton: larval fish feeding on evasive copepods. Sci. Rep. 6, 33585. doi: 10.1038/srep33585
Johnston N. M., Murphy E. J., Atkinson A., Constavle A. J., Cotté C., Cox M., et al. (2022). Status, change, and futures of zooplankton in the southern ocean. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.624692
Jónasdóttir S. H., Visser A. W., Richardson K., and Heath M. R. (2015). Seasonal copepod lipid pump promotes carbon sequestration in the deep North Atlantic. Proc. Natl. Acad. Sci. 112, 12122–12126. doi: 10.1073/pnas.1512110112
Kattner G., Graeve M., and Hagen W. (2012). Energy reserves of Southern Ocean copepods: Triacylglycerols with unusually long-chain monounsaturated fatty acids. Mar. Chem. 138–139, 7–12. doi: 10.1016/j.marchem.2012.05.002
King A. and LaCasella E. L. (2003). Seasonal variations in abundance, diel vertical migration, and population structure of Metridia gerlachei at Port Foster, Deception Island, Antarctica. Deep Sea Res. Part II: Topical Stud. Oceanography 50, 1753–1763. doi: 10.1016/S0967-0645(03)00091-2
Korb R. E., Whitehouse M. J., Thorpe S. E., and Gordon M. (2005). Primary production across the Scotia Sea in relation to the physico-chemical environment. J. Mar. Syst. 57, 231–249. doi: 10.1016/j.jmarsys.2005.04.009
Korb R. E., Whitehouse M. J., Ward P., Gordon M., Venables H. J., and Poulton A. J. (2012). Regional and seasonal differences in microplankton biomass, productivity, and structure across the Scotia Sea: Implications for the export of biogenic carbon. Deep Sea Res. Part II: Topical Stud. Oceanography 59–60, 67–77. doi: 10.1016/j.dsr2.2011.06.006
Kraft A., Bauerfeind E., Nöthig E. M., and Bathmann U. V. (2012). Size structure and life cycle patterns of dominant pelagic amphipods collected as swimmers in sediment traps in the eastern Fram Strait. J. Mar. Syst. 95, 1–15. doi: 10.1016/j.jmarsys.2011.12.006
Kraft A., Nöthig E., Bauerfeind E., Wildish D., Pohle G., Bathmann U., et al. (2013). First evidence of reproductive success in a southern invader indicates possible community shifts among Arctic zooplankton. Mar. Ecol. Prog. Ser. 493, 291–296. doi: 10.3354/meps10507
Kurbjeweit F. (1993). Reproduction and life cycles of dominant copepod species from the Weddell Sea, Antarctica. Berichte Zur. Polarforschung 129, 1–237.
Landry M. R., Peterson W. K., and Fagerness V. L. (1994). Mesozooplankton grazing in the Southern California Bight. I. Population abundances and gut pigment contents. Mar. Ecol. Prog. Ser. 115, 55–71. doi: 10.3354/meps115055
Lee R., Hagen W., and Kattner G. (2006). Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser. 307, 273–306. doi: 10.3354/meps307273
Lenz P. H., Roncalli V., Cieslak M. C., et al. (2021). Diapause vs. reproductive programs: transcriptional phenotypes in a keystone copepod. Commun. Biol. 4, 426. doi: 10.1038/s42003-021-01946-0
Liszka C. M., Manno C., Stowasser G., Robinson C., and Tarling G. A. (2019). Mesozooplankton community composition controls fecal pellet flux and remineralization depth in the southern ocean. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00230
Lopez M. D. G. and Huntley M. E. (1995). Feeding and diel vertical migration cycles of Metridia gerlachei (Giesbrecht) in coastal waters of the Antarctic Peninsula. Polar Biol. 15, 21–30. doi: 10.1007/BF00236120
Makabe R., Hattori H., Sampei M., Darnis G., Fortier L., and Sasaki H. (2016). Can sediment trap-collected zooplankton be used for ecological studies? Polar Biol. 39, 2335–2346. doi: 10.1007/s00300-016-1900-7
Manno C., Stowasser G., Enderlein P., Fielding S., and Tarling G. A. (2015). The contribution of zooplankton faecal pellets to deep-carbon transport in the Scotia Sea (Southern Ocean). Biogeosciences 12, 1955–1965. doi: 10.5194/bg-12-1955-2015
Mayor D. J., Gentleman W. C., and Anderson T. R. (2020). Ocean carbon sequestration: Particle fragmentation by copepods as a significant unrecognised factor? BioEssays 42. doi: 10.1002/bies.202000149
Mayor D. J., Sanders R., Giering S. L. C., and Anderson T. R. (2014). Microbial gardening in the ocean’s twilight zone: Detritivorous metazoans benefit from fragmenting, rather than ingesting, sinking detritus. BioEssays 36, 1132–1137. doi: 10.1002/bies.201400100
Ohtsuka S., Ohaye S., Tanimura M., Fukuchi M., Hattori H., Sasaki H., et al. (1993). Feeding ecology of copepodid stages of eucalanus bungii in the chucki and nothern bering seas in october 1988. Proc. NIPR Symposium Polar Biol. 6, 27–37. doi: 10.15094/00005182
Osgood K. E. and Frost B. W. (1994). Ontogenetic diel vertical migration behaviors of the marine planktonic copepods Calanus pacificus and Metridia lucens. Mar. Ecol. Prog. Ser. 104, 13–13. doi: 10.3354/meps104013
Palomares-García R. J., Gómez-Gutiérrez J., and Robinson C. J. (2013). Winter and summer vertical distribution of epipelagic copepods in the Gulf of California. J. Plankton Res. 35, 1009–1026. doi: 10.1093/plankt/fbt052
Parzanini C., Parrish C. C., Hamel J.-F., and Mercier A. (2019). Reviews and syntheses: Insights into deep-sea food webs and global environmental gradients revealed by stavle isotope (δ15 N, δ13 C) and fatty acid trophic biomarkers. Biogeosciences 16, 2837–2856. doi: 10.5194/bg-16-2837-2019
Pasternak A. and Schnack-Schiel S. (2001). Seasonal feeding patterns of the dominant Antarctic copepods Calanus propinquus and Calanoides acutus in the Weddell Sea. Polar Biol. 24, 771–784. doi: 10.1007/s003000100283
Pinti J., Jónasdóttir S. H., Record N. R., and Visser A. W. (2023). The global contribution of seasonally migrating copepods to the biological carbon pump. Limnol. Oceanogr. 68, 1147–1160. doi: 10.1002/lno.12335
Ramondenc S., Nöthig E., Hufnagel L., Bauerfeind E., Busch K., Knüppel N., et al. (2022). Effects of Atlantification and changing sea-ice dynamics on zooplankton community structure and carbon flux between 2000 and 2016 in the eastern Fram Strait. Limnol. Oceanogr. 68, S39–S53. doi: 10.1002/lno.12192
Richardson K., Jónasdóttir S. H., Hay S. J., and Christoffersen A. (1999). Calanus finmarchicus egg production and food availability in the Faroe–Shetland Channel and northern North Sea: October–March. Fish. Oceanogr. 8, 153–162. doi: 10.1046/j.1365-2419.1999.00007.x
Saito H. and Kiørboe T. (2001). Feeding rates in the chaetognath Sagitta elegans: effects of prey size, prey swimming behaviour and small-scale turbulence. J. Plankton Res. 23, 1385–1398. doi: 10.1093/plankt/23.12.1385
Sargent J. R., Gatten R. R., and Henderson. R. J. (1981). Lipid biochemistry of zooplankton from high lattitudes. Oceanis 7, 623–632.
Sarmiento J. L. (2006). Ocean Biogeochemical Dynamics (Princeton, New Jersey, USA: Princeton University Press). doi: 10.1515/9781400849079
Schmidt K., Niehoff B., Cornils A., Hagen W., Flores H., Heuzé C., et al. (2025). Seasonal vertical migration of large polar copepods reinterpreted as a dispersal mechanism throughout the water column. Commun. Earth Environ. 6, 431. doi: 10.1038/s43247-025-02389-9
Schnack S. B. (1985). “Feeding by euphausia superba and copepod species in response to varying concentrations of phytoplankton,” in Antarctic Nutrient Cycles and Food Webs (Springer Berlin Heidelberg, Berlin, Heidelberg), 311–323. doi: 10.1007/978-3-642-82275-9_45
Schnack-Schiel S. and Hagen W. (1995). Life-cycle strategies of Calanoides acutus, Calanus propinquus, and Metridia gerlachei (Copepoda: Calanoida) in the eastern Weddell Sea, Antarctica. ICES J. Mar. Sci. 52, 541–548. doi: 10.1016/1054-3139(95)80068-9
Steinberg D. K., Carlson C. A., Bates N. R., Goldthwait S. A., Madin L. P., and Michaels A. F. (2000). Zooplankton vertical migration and the active transport of dissolved organic and inorganic carbon in the Sargasso Sea. Deep Sea Res. Part I: Oceanographic Res. Papers 47, 137–158. doi: 10.1016/S0967-0637(99)00052-7
Steinberg D. K. and Landry M. R. (2017). Zooplankton and the ocean carbon cycle. Ann. Rev. Mar. Sci. 9, 413–444. doi: 10.1146/annurev-marine-010814-015924
Strand E., Bagøien E., Edwards M., Broms C., and Klevjer T. (2020). Spatial distributions and seasonality of four Calanus species in the Northeast Atlantic. Prog. Oceanogr. 185, 102344. doi: 10.1016/j.pocean.2020.102344
Stupnikova A. N., Molodtsova T. N., Mugue N. S., and Neretina T. V. (2013). Genetic variability of the Metridia lucens complex (Copepoda) in the Southern Ocean. J. Mar. Syst. 128, 175–184. doi: 10.1016/j.jmarsys.2013.04.016
Titelman J., Varpe O., Eliassen S., and Fiksen O. (2007). Copepod mating: chance or choice? J. Plankton Res. 29, 1023–1030. doi: 10.1093/plankt/fbm076
Visser A. W., Grønning J., and Jónasdóttir S. H. (2017). Calanus hyperboreus and the lipid pump. Limnol. Oceanogr. 62, 1155–1165. doi: 10.1002/lno.10492
Ward P., Atkinson A., Murray A. W. A., Wood A. G., Williams R., and Poulet S. A. (1995). The summer zooplankton community at South Georgia: biomass, vertical migration and grazing. Polar Biol. 15, 195–208. doi: 10.1007/BF00239059
Ward P., Atkinson A., and Tarling G. (2012). Mesozooplankton community structure and variability in the Scotia Sea: A seasonal comparison. Deep Sea Res. Part II: Topical Stud. Oceanography 59–60, 78–92. doi: 10.1016/j.dsr2.2011.07.004
Ward P., Shreeve R., Atkinson A., Korb B., Whitehouse M., Thorpe S., et al. (2006). Plankton community structure and variability in the Scotia Sea: austral summer 2003. Mar. Ecol. Prog. Ser. 309, 75–91. doi: 10.3354/meps309075
Ward P., Shreeve R. S., and Cripps G. C. (1996b). Rhincalanus gigas and Calanus simillimus: lipid storage patterns of two species of copepod in the seasonally ice-free zone of the Southern Ocean. J. Plankton Res. 18, 1439–1454. doi: 10.1093/plankt/18.8.1439
Ward P., Shreeve R., Cripps G., and Trathan P. (1996a). Mesoscale distribution and population dynamics of Rhincalanus gigas and Calanus simillimus in the Antarctic Polar Open Ocean and Polar Frontal Zone during summer. Mar. Ecol. Prog. Ser. 140, 21–32. doi: 10.3354/meps140021
Ward P., Tarling G. A., and Thorpe S. E. (2018). Temporal changes in abundances of large calanoid copepods in the Scotia Sea: comparing the 1930s with contemporary times. Polar Biol. 41, 2297–2310. doi: 10.1007/s00300-018-2369-3
White M. M., Waller J. D., Lubelczyk L. C., Drapeau D. T., Bowler B. C., Balch W. M., et al. (2018). Coccolith dissolution within copepod guts affects fecal pellet density and sinking rate. Sci. Rep. 8, 9758. doi: 10.1038/s41598-018-28073-x
Whitehouse M. J., Atkinson A., Korb R. E., Venables H. J., Pond D. W., and Gordon M. (2012). Substantial primary production in the land-remote region of the central and northern Scotia Sea. Deep Sea Res. Part II: Topical Stud. Oceanography 59–60, 47–56. doi: 10.1016/j.dsr2.2011.05.010
Yamada Y., Nishida S., Graeve M., and Kattner G. (2016). Lipid and fatty acid/alcohol compositions of the subarctic copepods Neocalanus cristatus and Eucalanus bungii from various depths in the Oyashio region, western North Pacific. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 198, 57–65. doi: 10.1016/j.cbpb.2016.04.003
Keywords: sediment trap, copepod, seasonality, winter, Calanus simillimus
Citation: Atherden F, Ward P, Blackbird S, Wolff G, Tarling GA and Manno C (2025) Seasonal analysis of Southern Ocean copepod ecology using a moored sediment trap. Front. Mar. Sci. 12:1586990. doi: 10.3389/fmars.2025.1586990
Received: 03 March 2025; Accepted: 06 October 2025;
Published: 24 October 2025.
Edited by:
James J. Pierson, University of Maryland, United StatesReviewed by:
Kim Bernard, Oregon State University, United StatesHillary Glandon, University of Illinois at Urbana-Champaign, United States
Copyright © 2025 Atherden, Ward, Blackbird, Wolff, Tarling and Manno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florence Atherden, ZmxyZGVuMTlAYmFzLmFjLnVr; Clara Manno, Y2xhbm5vQGJhcy5hYy51aw==
 Florence Atherden
Florence Atherden Peter Ward
Peter Ward Sabena Blackbird
Sabena Blackbird George Wolff
George Wolff Geraint A. Tarling
Geraint A. Tarling Clara Manno
Clara Manno