Abstract
Kelliella miliaris (Philippi, 1844) is a minute bivalve, living on the surface of soft sediments, from the continental shelf to bathyal depths, commonly in the oxygen minimum zones (OMZ) and/or in reducing habitats. The scarcity of data on the biology of Kelliella prompted us to investigate, at ultrastructural level, specimens found in southern Spain. Kelliella miliaris shows several morphological characteristics that would be adaptive for living in the OMZ: (1) presence of numerous muscular fibers in the mantle, mantle edge and gills-visceral mass connection; all of which would allow to actively move the gills and/or enable a better control of the ventral opening in relation to vertical movements of the animal; (2) high number (in relation to body size) and large size of gill filaments, mainly in the large inner demibranch; and (3) long cilia which would provide a large surface for capture of oxygen and a highly effective uptake of oxygen from water. We have observed in all the specimens examined the presence of numerous rod shaped bacteria among the gill cilia. These bacteria show the typical double membrane of Gram-negative bacteria. The analysis of the bacterial DNA revealed that Gammaproteobacteria is the most abundant class, with 53.69% of total reads. The latter, together with the peak of oxygen and the presence of sulfur inside the electron dense granules from the bacteria, determined by TEM-EDX analysis, point to the involvement of these bacteria in the oxidization of the sulfide to thiosulfate. The presence of bacteria in the gills of Kelliella miliaris highlights the importance of the chemosynthetic symbiosis in the OMZs of the oceans that has been probably overlooked up to now. The presence of different microorganisms in the stomach indicates heterotrophy. We have found spermatozoids inside the female gonad, which confirms internal fertilization in K. miliaris. However, the presence of protoconch I and protoconch II, indicates planktotrophic larval development.
1 Introduction
Oxygen minimum zones (OMZs), also called oxygen minimum layers (OMLs), are, in general, pelagic habitats with stable conditions of continuously low oxygen level and low temperature that are found at intermediate depths (400–1000 m depth), over vast areas in most of the world’s oceans. Where the OMZs intersect continental margins, stable low oxygen conditions are also found in the benthic habitat. The OMZs differ from other hypoxic aquatic habitats in that very low oxygen levels are stable over long periods and large areas (Childress and Seibel, 1998). These OMZs develop because the consumption of oxygen relative to replenishment is greater than at shallower and greater depths. The consumption and supply of oxygen at shallower depths is higher than at greater depths where there are strong reductions of biomass (Sewell and Fage, 1948). Those few species that do live in pelagic habitats with very low oxygen concentrations are typically diurnal vertical migrators which enter shallower, more oxygenated waters at night (Childress and Seibel, 1998 and references therein), however, nothing is known about the existence of vertical migrations of the fauna from benthic OMZs.
Persistent O2 deficiency occurs when the amount of dissolved O2 in the water column is consumed faster than it is resupplied through air–sea exchange, photosynthetic O2 production and ventilation. Thus, in profile, OMZs resemble a band of O2-deficient water inserted between two O2-containing water masses. The amount of O2 chosen to define OMZs ranges from <2 μmol O2 per kg water to 90 μmol O2 per kg water (Wright et al., 2012).
Three are the proposed possible physiological adaptations of the organisms to the OMZs: (1) the development of mechanisms for the highly effective capture of oxygen from water; (2) the reduction of metabolic rates or (3) the use of anaerobic metabolism to make up the difference between aerobic capacity and total metabolic demand (Childress and Seibel, 1998). Some groups, such as fishes, crustaceans, mollusks or polychaetes, have specialized circulatory systems and respiratory systems which offer possibilities for adaptations.
To obtain nutrients in poor, oligothrophic conditions, such as those found widely in the deep sea, is considered one of the driving forces for the evolution of chemosymbiosis (Kleiner et al., 2012). Chemosynthetic symbioses represent an adaptation to ecosystems and habitats with reducing conditions, such as cold seeps and hydrothermal vents, but up to now relatively little is known regarding their diversity and functioning apart from a few “model species” on which effort has focused over the last decades (Duperron et al., 2013). Symbioses have often been overlooked in other habitats, in which symbiotic species are not dominant and somewhat smaller. The ability to associate with chemosynthetic bacteria is a recurring feature in the evolution of bivalves, since it has appeared independently in at least five families, Mytilidae, Vesicomyidae, Solemyidae, Lucinidae and Thyasiridae (Southward, 1986; Duperron et al., 2013), and more recently discovered in other two: Nucinellidae and Lasaeidae (Oliver and Taylor, 2012; Oliver, 2012). Four of these families (excluding Mytilidae) are associated with sulfur-oxidizing Gammaproteobacteria. Species of the family Mytilidae are characterized by a broader diversity of associated bacteria. Endosymbiont bacteria found in gill tissues in these families convert otherwise unavailable energy sources (sulfide, methane) to forms readily metabolized by their host, but species of the families Xylophagaidae and Teredinidae, which colonize driftwood or sunken wood, have endosymbiont bacteria for the conversion of terrestrial derived cellulosic carbon from wood into animal biomass in the deep sea (Distel and Roberts, 1997).
Low-O2 marine communities are typically inhabited by a reduced diversity of generally unmineralized, low-density, and small-sized taxa (Sperling et al., 2015), and they are usually opportunistic detritivores (Levin, 2003). Organisms from the OMZs have developed a series of physiological or behavioral adaptations that allow them to maintain aerobic metabolism with reduced energy demand, such as enhancing O2 extraction, transport, and storage (Childress and Seibel, 1998). Some species are facultative anaerobes and they can switch to anaerobic metabolism in order to sustain reduced rates of energy turnover while in hypoxia (Borges et al., 2022 and references therein).
The OMZs are extensive in the eastern Pacific Ocean, the Arabian Sea and off West Africa (Levin et al., 2000 and references therein). The faunal assemblage living at the OMZ has been mainly studied in the Arabian Sea and California slope. Levin et al. (2000) studied the macrobenthic community structure within and beneath the OMZ from the NW Arabian Sea where they found highest macrofaunal densities in the middle of the OMZ, in 700-850 m, intermediate at 400 and 1000 m and lowest at the 1250- and 3400-m stations. In general, the polychaetes accounted for 88,8% of total collected individuals; mollusks and crustacean represented only 3.4 and 5.7% of the specimens respectively. Due to the use of boxcorer, the megafauna was under-represented in this study. Among the 35 taxa of mollusks collected from the NW Arabian OMZ, there were six taxa usually associated with chemosynthetic bacteria (one Lucinoma sp. and five Thyasira spp.) (Dufour, 2005; Taylor and Glover, 2006) and one Kelliella sp. Also in the OMZ of the Arabian Sea, off southern Oman, bacterial symbiosis was reported in two new species of bivalves of the family Nucinellidae (Oliver and Taylor, 2012), in which bacteriocytes with bacteria were observed inside the gills. However, Cook et al. (2000) found in the OMZ on the Oman slope that the abundance of nematodes was correlated with food quality (measured as the hydrogen index) rather than oxygen.
Within the OMZ from the Indian continental margin, changes in abundance of the megafaunal assemblage (composed by Porifera, Cnidaria, Mollusca, Crustacea, Echinodermata, Ascidiacea and Gnatostomata) were correlated to both oxygen availability and sediment organic matter quality (Hunter et al., 2011). Fish dominated the assemblage in the OMZ at 540m, but at 800m high densities of ophiuroids and decapods produced megafaunal abundance peaks, and below this boundary total faunal abundance declined gradually with depth. The latter data support previous evidences that the specific responses of individual taxa to oxygen limitation and organic matter availability determine megafaunal zonation within an OMZ continental margin (Levin et al., 1991; Hunter et al., 2011). Some species were also present in upper, more-oxygenated, ocean layers, but the individuals from the OMZ had physiological adaptations able to survive with low oxygen. In the OMZ of southern end of the California Current, down to oxygen concentrations of 0.2 µml/l, the zooplankton was abundant, with several species that perform nocturnal vertical migration toward upper layers with more oxygen (Longhurst, 1967).
Small-subunit ribosomal RNA (SSU rRNA) gene sequences associated with chemoautotrophic, sulfur-oxidizing gill symbionts of deep-sea clams and mussels were first identified in open-ocean OMZs in the Arabian Sea, the eastern tropical South Pacific and the Namibian upwelling (Fuchs et al., 2005; Stevens and Ulloa, 2008; Lavik et al., 2009). The discovery of potential sulfur oxidizer in non sulfidic water is enigmatic, bringing into question the source of the reducing equivalents that are needed to fix inorganic carbon (Wright et al., 2012).
The physiology of thiotrophic bacteria thriving on the surface of animals (ectosymbionts) is less understood. A longstanding hypothesis proposes that attachment to animals that migrate between reduced and oxic environments would boost sulfur oxidation, as the ectosymbionts would alternatively access sulfide and oxygen, the most favorable electron acceptor (Paredes et al., 2021).
The type species of the bivalve genus Kelliella is K. abyssicola Sars 1870, currently considered junior synonym of Kelliella miliaris (Philippi, 1844) (WoRMS, 2024). Kelliella miliaris is present from off Norway to Canary Islands in the Northeastern Atlantic and in the Mediterranean Sea (Allen, 2001; Krylova et al., 2018; Utrilla et al., 2024). It is a benthic species, living on the surface of soft sediments (Clausen, 1958), from the continental shelf to bathyal depths. The stable δ13 C and δ15 N isotopic composition of K. miliaris was found similar to that of Vesicomya, suggesting heterotrophy (Krylova et al., 2018). Kelliella miliaris was present in high abundance in the low oxygen layer of Cap Breton (southeastern Bay of Biscay) (Jean Claude Sorbe, pers. com.). It is interesting to point that some species of Kelliella, such as K. miliaris, occur in organic carbon enriched habitats (Molina et al., 2019) or even exposed to reduced conditions, and can reach high numbers in sulfidic habitats, where pogonophorans are present (Southward et al., 1981; Krylova et al., 2018). According to the latter authors, the scarcity of data on the biology of Kelliella and in particular the fact that specimens have been observed in organic-rich or even reducing environments warrant further investigations of this group. Therefore, the presence of symbiotic bacteria in the gills of Kelliella miliaris described in this study could be the response to the presence of K. miliaris in low oxygen and/or reducing habitats.
2 Materials and methods
We have examined ultrastructurally five specimens of Kelliella miliaris, two from the Gulf of Cadiz (INDEMARES CHICA, 0211; 36°37.62′ N-07°12.2′ W, 735 m) and three from Bay of Málaga (field work of graduate program Diversidad Biológica y Medio Ambiente, BV57. 36°36.66’ N-4°21.82′ W, 117 m in muddy bottom). Other seven specimens from the Bay of Málaga (DiBiMA, BV68 36°36.83’N, 04°21.70’W; 94-124 m; 09/05/2023 in muddy bottom) were processed: two specimens for scanning electron microscopy (SEM) and five specimens were used for the sequencing of DNA from the bacteria and host. Unfortunately, during both samplings campaigns CTD to analyze oxygen in water were not available.
2.1 Procedure for transmission electron microscopy
The specimens from the Bay of Malaga were anesthetized for 30 min with isotonic magnesium chloride solution (71 g of MgCl2.6H2O/liter of freshwater) prior to fixation in 2.5% glutaraldehyde buffered with sodium cacodylate (0.1 M, pH 7.4) for at least 48h at 4°C. The specimen from the Gulf of Cadiz was fixed in ethanol 70%. After this, they were completely decalcified by immersion in 2% EDTA (Ethylene Diamine Tetraacetic Acid). They were postfixed in OsO4 (1%) for 60 min at room temperature and dehydrated in acetone series (30%, 50%, 70%, 90%, and 100% twice). Between 50% and 70% steps, the samples were incubated in-bloc in 2% uranyl acetate solution in 50% acetone at 4°C, overnight. Then, the samples were gradually embedded in low-viscosity Spurr’s resin (ref: https://www.emsdiasum.com/docs/technical/datasheet/14300).
Semithin sections (0.5–0.6 μm) were stained with toluidine blue (1%, pH 8.3) and were observed using an Olympus VF120 microscope at the Central Services for Research (SCAI) of the University of Málaga. Ultrathin sections (0.1 μm), from the specimens fixed in glutaraldehyde, were examined with a Thermofisher Scientific Tecnai G2 20 Twin, at the ICTS “NANBIOSIS” U28 unit of the IBIMA Plataform BIONAND, Málaga.
2.2 EDX analysis of the bacteria
We tested the mapping of different elements present in the bacteria together with the spectrum of the elements present inside the electron-lucent and electron-dense granules. For this, ultrathin sections (~100 nm) were observed using a Scanning Transmission Electron Microscopy (TEM-STEM) FEI Talos 200X with Energy Dispersive X-ray analysis (EDX system), from the SCAI of the University of Malaga.
2.3 Procedure for scanning electron microscopy
Specimens for SEM were critical-point CO2-dried and sputter-coated with gold. The specimens were observed with an EM JEOL JSM-840 from the SCAI of the University of Malaga.
2.4 DNA extraction and sequencing by Illumina Miseq technology
Gills were excised from 5 individuals preserved in molecular-grade ethanol. Samples were previously washed in filtered (0.22 mm) and sterilized seawater and total genomic DNA was extracted using the DNeasy Tissue Kit (Qiagen Japan, Tokyo, Japan). Total DNA was resuspended in 100 μL TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0) and stored at 4°C. DNA is quantified by spectrophotometer (NanoDrop ND-1000) and the ratio of absorbances 260:280 and 260:230 is used as a measure of the quality of DNA extraction. For the amplification of host mitochondrial DNA (COI) (Folmer et al., 1994) and bacterial DNA (SSU 16S rRNA), specific primers were used (Klindworth et al., 2013; Abellan-Schneyder et al., 2021).
PCR amplification for COI-DNA barcoding analysis was performed in 50 μl of reaction volume with 1.25 units of GoTaq® G2 Flexi DNA polymerase 5 u/μl (Promega, Wisconsin, USA), 0.2 mM each dNTP, 0.2 μM of primers LCO1490 (5’ GGTCAACAAATCATAAAGATATTGG 3’) and HCO2198 (5’ TAAACTTCAGGGTGACCAAAAAATCA 3’) (Folmer et al., 1994) targeting the COI gene and less than 0.2 μg of template DNA. PCR reactions were heated 3 min. at 95°C, followed by 35 cycles (1 min. at 95°C; 30 secs. at 54°C, 1 min. at 72°C). DNA sequencing was performed bidirectionally by using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and the same primers as used in the amplification reaction in an ABI PRISM 3100 genetic analyzer (Applied Biosystems).
To analyze the microbial communities present on gills, PCR amplification was performed from the purified total DNA sample using the repliQa Hifi ToughMix (Quantabio) and primers IlluAdp_16S_Forward-341F (5’ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG3’) and IlluAdp_16S_Reverse-785R (5’ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC3’) targeting the V3–V4 variable regions of the 16S rRNA gene. The amplification conditions were: 35 cycles (30 secs. at 95°C; 30 secs. at 57°C, 1 min. at 72°C). Agarose gel electrophoresis confirmed a unique band. Quantification was done with Qubit 2.0 fluorometer and Qubit dsDNA BR kit and DNA was purified with Sera-Mag Select ™ (Cytiva). Library was prepared according an in house protocol of Stabvida based on Illumina 16S Metagenomic Sequencing Library preparation protocol (15044223 Rev. B). Library concentration was 21.8 ng/ul. Libraries were further sequenced by NGS in an Illumina MiSeq platform using MiSeq Reagent Kit V3 and 300 bp paired-end at StabVida.
Raw read sequences of the 16S rRNA gene from gills of Kelliella miliaris in this study are publicly available in the NCBI SRA repository within BioProject PRJNA1229195, with BioSample accession number SAMN47125445.
2.5 Bioinformatic analysis
The analysis of the raw sequence data was carried out using QIIME2 2024.10. A total number of 128642 reads were obtained. Reads were denoised using the DADA2 plugin from QIIME2. After denoising a total of 239 unique Operational Taxonomic Units (OTUs) were identified. For taxonomical classification, sequences were subjected to taxonomic assignment against the SILVA 16S database (Release 138.2), with 97% 16S similarity as the cutoff and clustered into OTUs. For classification purposes, only OTUs containing at least 10 sequence reads were considered as significant. Non-specific PCR amplicons were eliminated.
3 Results
3.1 Some relevant morphological characteristics of Kelliella miliaris
Clausen (1958), Allen (2001) and Krylova et al. (2018) provided very good descriptions of the shell and soft parts of Kelliella miliaris. Therefore, here we highlight some morphological characteristics that could be relevant to understand more on its biology.
Kelliella miliaris is a minute bivalve with sub-orbicular valves, with an elongate, finger-shaped foot, able to be extended outside the shell and never concealed by the gills when retracted (contrary to the superficially similar Vesicomya atlantica (Smith, 1885)) (Figure 1A). In the dorsal part of this muscular foot there are large fused pedal ganglia to which two statocysts are attached in its dorsal part (Figure 1B). The mantle edges are much thickened, over all in comparison with the thin mantle epithelium, and show many muscular fibers together with a large blood sinus (Figure 1C). The stomach has a gastric shield and a sacciform, open compartment with secreting cells, which corresponds to the style-sac, not clearly differentiated from the midgut in this species, as already noted by Clausen (1958) (Figure 1D). Inside the stomach it is possible to see different types of organisms, such as bacteria, diatoms, among others, pointing a heterotrophic feeding (Figure 1E). In transversal semithin sections, it was possible to observe female follicles with oocytes at different developing stages at the lateral sides of the visceral mass (Figure 1D). We have seen spermatozoids inside the gonad, which would confirm an internal fertilization in this species (Figure 2A). However, the presence of protoconch I and protoconch II would indicate a larval planctotrophic development (Figure 2B).
Figure 1
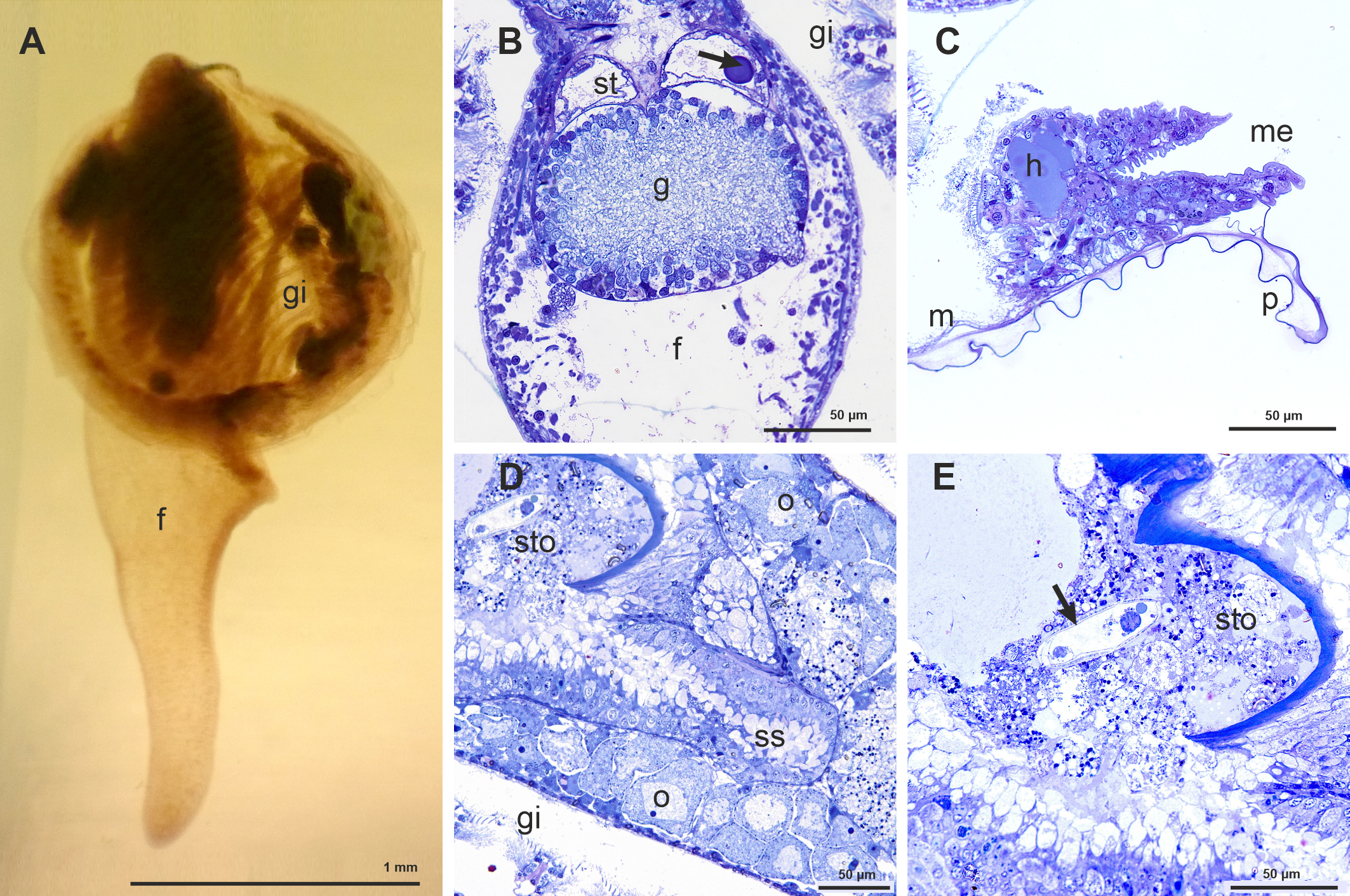
(A) View of one specimen of Kelliella miliaris embedded in spur resin. (B) Semithin transversal section showing part of the foot (f), with the large fused pedal ganglia (g) and the statocists (st); the arrow shows one of the statoliths. (C) Semithin transversal section of the very large mantle edge (me) -in comparison with the mantle (m)- showing a large hemocelic sinus (h) and the periostracum (p) emerging between the outer and middle mantle folds. (D) Semithin transversal section showing part of the stomach (sto) and the style sac (ss). (E) Semithin transversal section showing part of the stomach (sto) with different organisms, among them a possible protozoan (arrow).
Figure 2
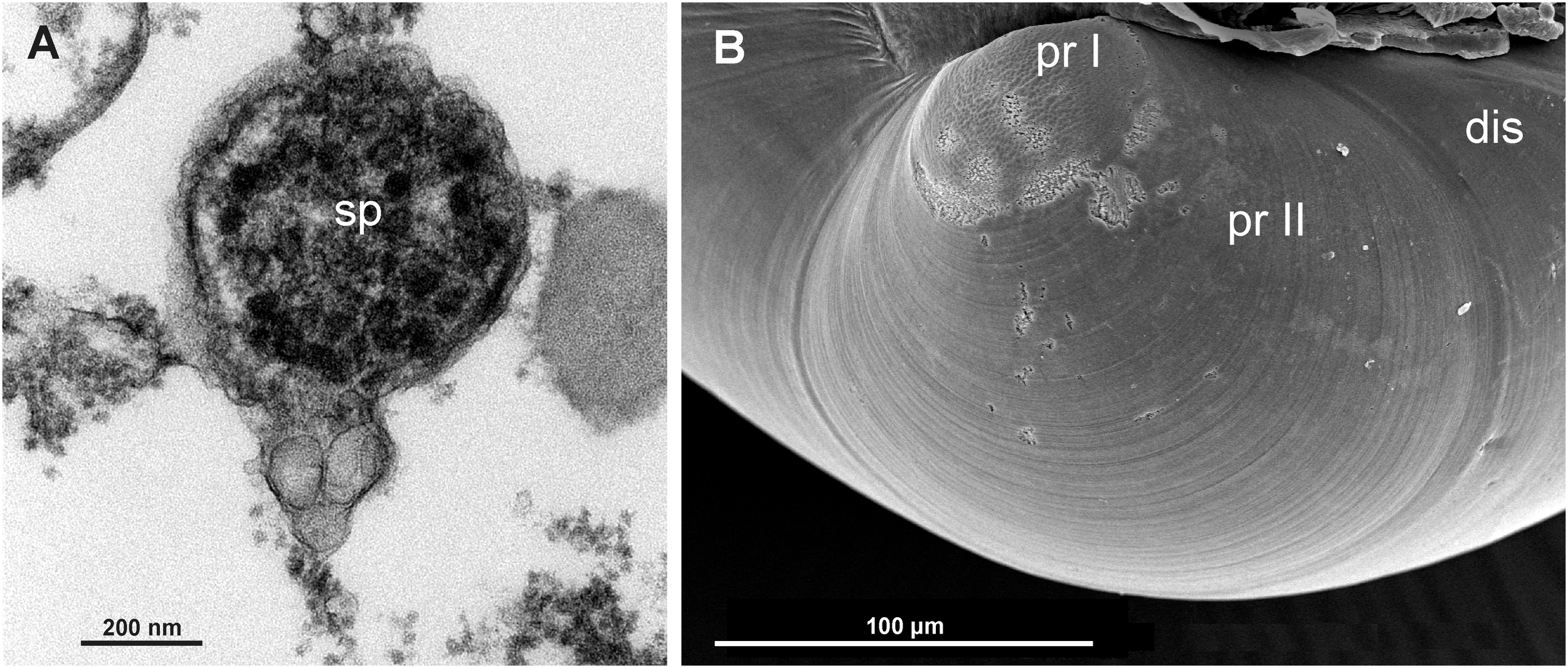
(A) Transmission Electron Microscope (TEM) view of a spermatozoid found inside the female gonad. (B) Scanning Electron Microscope (SEM) view of the protoconch I (pr I), protoconch II (pr II) and disoconch (dis) of Kelliella miliaris, indicative of a planktotrophic development.
The gills have two symmetrical ctenidia that practically cover the visceral mass at both sides (Figure 1A). Each ctenidium consists of a large inner demibranch and a small outer demibranch placed in the posterior third of the inner one. Each demibranch presents a descending and an ascending lamella without interlamellar junctions. These gills are homorhabdic -with gill filaments arranged in a flat, uniform series- like those found in Donax faba, according to the classification of Ridewood (1903).
In relation to body size, gill filaments are large and numerous, mainly in the large inner demibranch. The gill filaments have long cilia (Figure 3A). There are bunches of muscular fibers connecting with the filament at the proximal abfrontal area (Figure 3B). The cilia are present on either side of the filament section, connecting one filament with the next, and together with cirri on the frontal cells facing outside the demibranch (Figure 3A). The cirri in the frontal and lateral-frontal zones are long; the frontal cells present also microvilli (Figure 3C). The lateral cells are cuboidal, but sometimes tends to have a more globular shape, and show numerous mitochondria together with long cilia and microvilli (Figures 3D, E). Between the lateral-frontal cirri and the lateral ciliate cells there are several globular cells devoid of cilia but with long microvilli (Figure 3F).
Figure 3
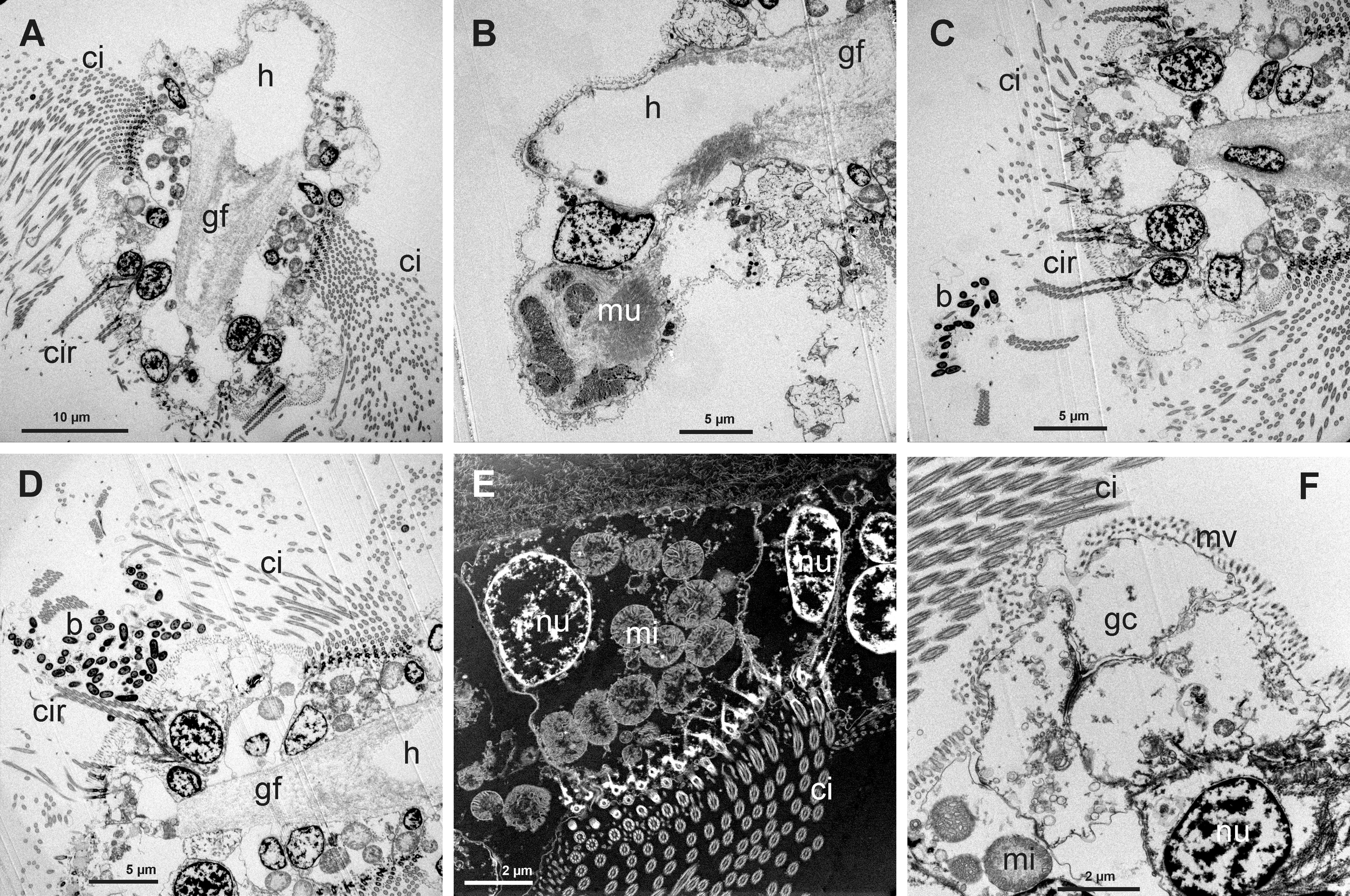
(A) Transmission Electron Microscope (TEM) view of a gill filament (gf) showing the long lateral cilia (ci), frontal cirri (cir) and the large hemocelic sinus (h). (B) TEM view of part of the gill filament (gf) with the hemocelic sinus (h) connected to a bunch of muscular fibers (mu). (C) Frontal TEM view of a gill filament showing a group of bacteria (b) near the frontal cirri (cir); it is possible to see part of the long lateral cilia (ci). (D) TEM view of a group of bacteria (b) located between the frontal cirri (cr) and the long lateral cilia (cir). (E) Scanning Transmission Electron Microscope (TEM-STEM) view of the lateral ciliate cell showing the cilia (ci), the nucleus (nu) and numerous mitochondria (mi); some nuclei from the abfrontal cells are visible. (F) TEM view of the abfrontal and globular cells (gc) located between the lateral ciliate cells and the frontal cells. These globular cells present only microvilli.
3.2 COI-based analysis of host
Sequence analysis of the mitochondrial cytochrome c oxidase subunit I (COI) gene (GenBank: PV147183) from the gill tissue sample revealed 100% identity with previously published sequences of Kelliella miliaris from the North Sea (GenBank accession numbers MF542321, OQ053061, OQ053062 and OQ053063 by Krylova et al., 2018).
3.3 Bacteria in the gills
We have observed in all the specimens examined the presence of rod shaped bacteria among the long lateral cilia, the frontal cilia and cirri and between the frontal-lateral cirri and the lateral cilia (Figures 4A–E). Bacterial symbionts were usually small (ca. 1 µm long and ca. 0.5 µm wide), with the typical double membrane of Gram-negative bacteria (Figures 4F, G).
Figure 4
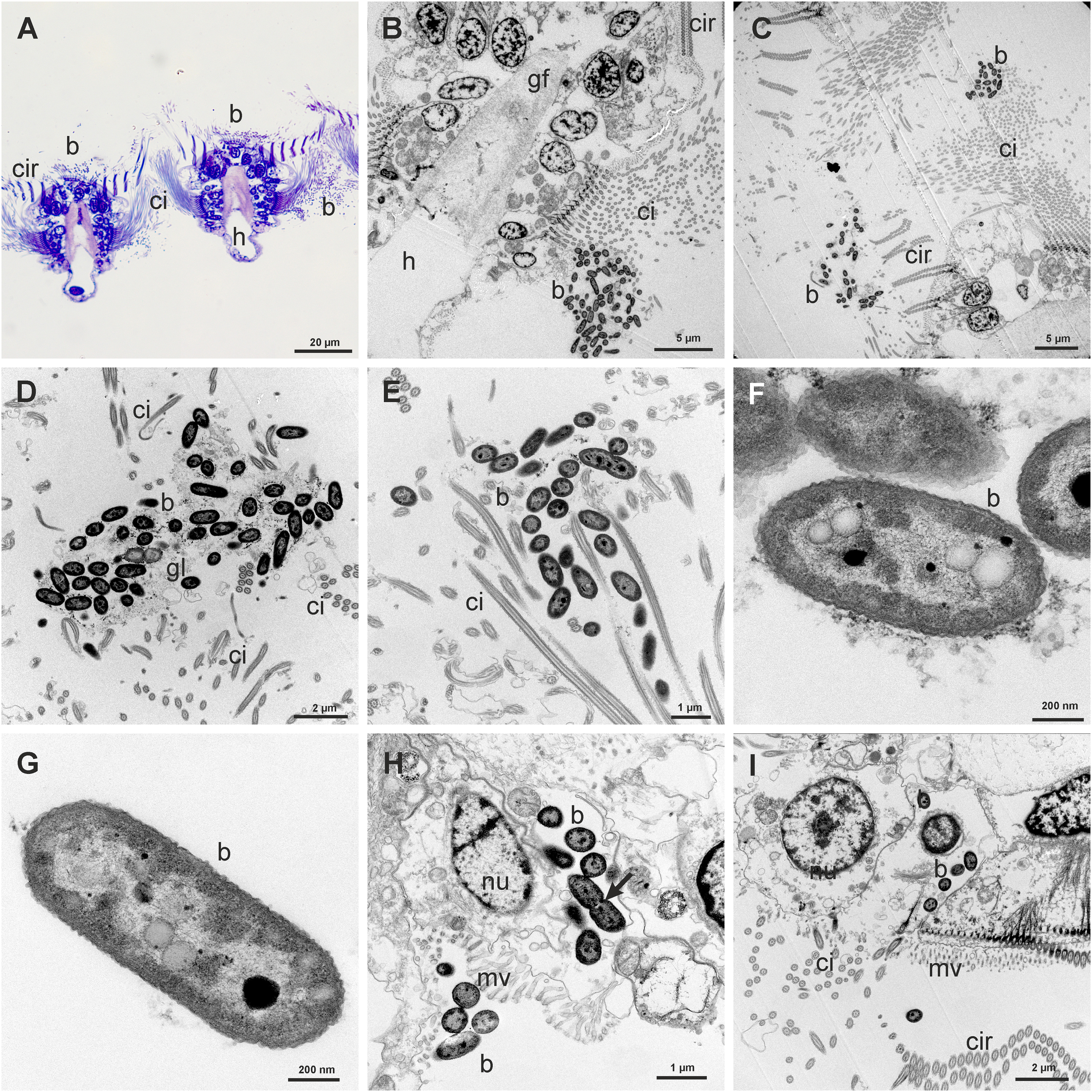
(A) Semithin transversal view of two gill filaments showing groups of bacteria (b) in the frontal and lateral sides. (B) Transmission Electron Microscope (TEM) view of a gill filament (gf) showing a group of bacteria (b) between the long lateral cilia (ci). (C) TEM view of groups of bacteria (b) in the frontal and lateral cilia (ci) of a gill filament. (D, E) Detail of groups of bacteria (b) trapped in a glycocalix network (gl) between the cilia (ci). (F) TEM view of bacteria showing the double membrane and the electron-lucent and electron-dense granules. (G) TEM view of one isolated rod shaped bacterium showing two electron-lucent granules and one electron-dense granule. (H) Group of bacteria inside the cytoplasm of a microvillous (mv) abfrontal cell, one of them in process of division (arrow). There is also a group of bacteria in process of entering in another cell. (I) TEM view of some bacteria (b) inside a microvillous frontal cell.
Bacteria were also found inside the globular abfrontal cell, adjacent to the lateral ciliate cell. The bacteria were free in the cytoplasm and not inside vacuoles or bacteriocyte (Figure 4H). Some bacteria were in a process of division inside this abfrontal cell (Figure 4H: arrow). Isolated bacteria were found also inside the frontal cells (Figure 4I). We have estimated the density of bacteria within the lateral ciliate area and the frontal area of the filaments, taking into account all the ultrathin sections analyzed in TEM. An average density of 58.25 bacteria by µm2 was found in the lateral areas, and of only 6.1 bacteria by µm2 in the frontal area. The number of bacteria inside cells ranges from 3 to 7.
The bacteria appeared in group inside a glycocalix network that is secreted by the microvilli of the lateral and frontal cells (Figures 5A–F). Figure 5G shows the microvilli and Figures 5H, I the secretion of numerous vesicles. The absence of concentric stacks of intracellular membranes in their cytoplasm indicates that these symbionts are not methanotrophic bacteria (Figures 4F, G). All the above data point a symbiotic relationship between Kelliella miliaris and the bacteria. Although there are some bacteria inside the abfrontal and frontal cells, we consider this to be an ectosymbiosis because most of the bacteria were trapped by a glycocalix network among the cilia.
Figure 5
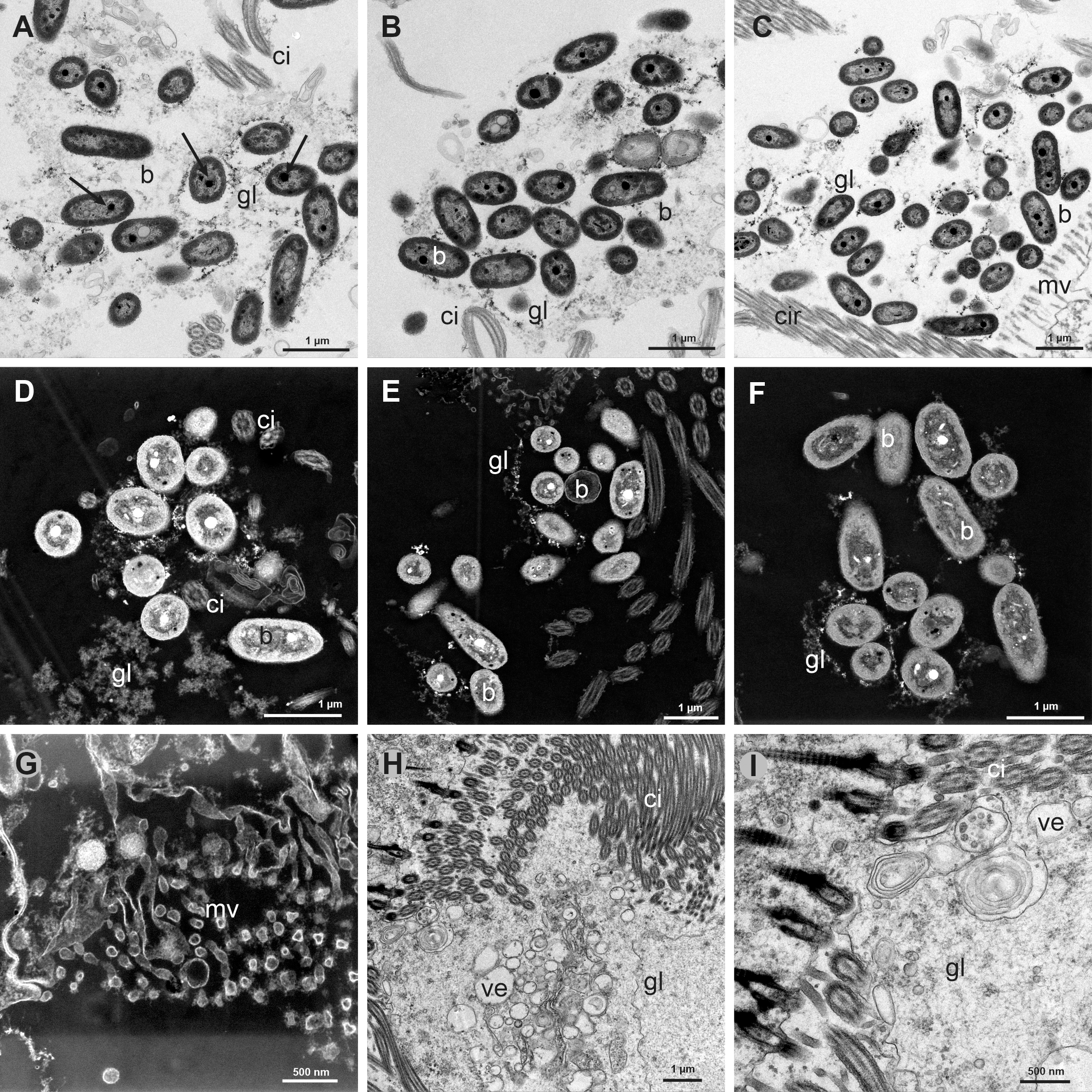
(A-C) Transmission Electron Microscope (TEM) views of groups of bacteria (b) trapped in a glycocalix network (gl) between the cilia (ci) of the gill filaments. Arrows point to some electron-dense granules. (D-F) Scanning Transmission Electron Microscope (TEM-STEM) views of groups of bacteria (b) in the glycocalix network (gl), some of them showing electron-lucent granules, which correspond to the electron-dense granules in TEM. (G) TEM-STEM view of the microvilli (mv) from the abfrontal globular cells. (H) TEM view of the intense secretion of vesicles (ve) and glycocalix (gl) from the microvilli of the gill filament cells. (I) TEM view with intense secretion of vesicles and glycocalix from same section as H.
The bacteria showed one large or several smaller and exocentric electron-dense granules, and few electron-lucent non-membrane bound granules in TEM views (Figures 5A-C), which in TEM-STEM views appeared respectively as electron-lucent or electro-dense (Figures 5D-F, 6A). To check if these granules could be sulfur granules, common in some vent and seepage bacteria from reducing sulfidic environment, we have performed by TEM-EDX a mapping and spectrum of the main elements present in them. The EDX analysis of the electron-dense granules (lucent in TEM-STEM views) showed that there is no carbon in these granules (Figures 6B-G, I). The main elements were: oxygen, phosphorus, lead, sulfur and nitrogen (Figure 6I). Oxygen is the most abundant element, showing the greatest peak in the spectrum, followed by phosphorus and lead; the sulfur peak appears inside the large peak of the lead, but the mapping showed abundant sulfur inside the black granules. Finally, nitrogen is the other element present inside the electron-dense granules. In the electron-lucent (electron-dense in TEM-STEM views) small granules (Figure 6H) the spectrum of their content (Figure 6J) showed the nearly absence of sulfur in these granules (Table 1).
Figure 6
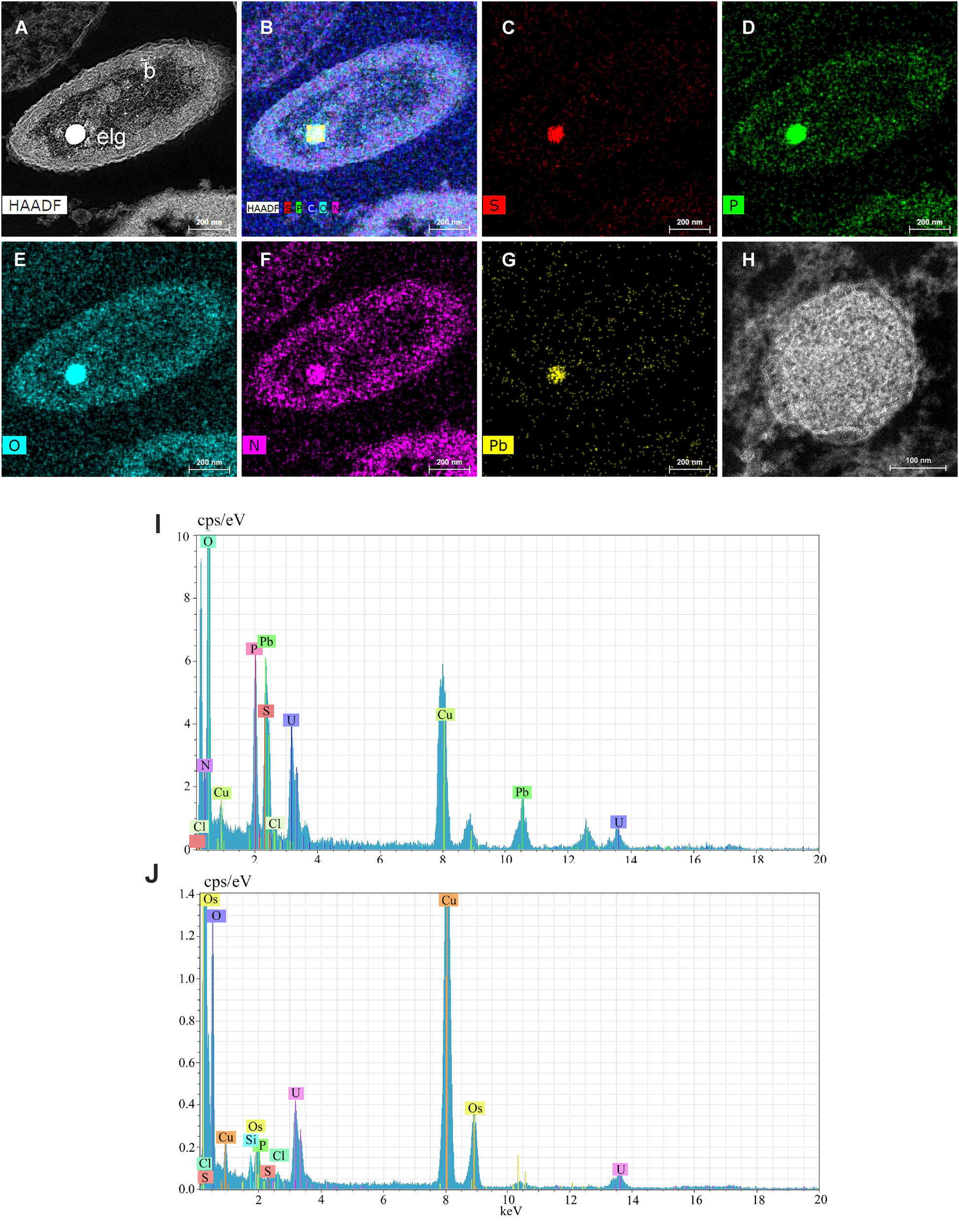
(A-G) Energy Dispersive X-ray analysis on Scanning Transmission Electron Microscope ultrathin sections of bacteria (TEM-STEM-EDX) for mapping the main elements present in the bacteria, particularly in the electron-lucent (electron-dense in TEM view) granule (elg): S, sulfur; P, phosphorus; O, oxygen; N, nitrogen; Pb, lead. (H) Scanning Transmission Electron Microscope (TEM-STEM) view of an electron-lucent granule (electron-dense in TEM). (I) Spectrum of an electron-lucent granule (electron-dense in TEM). (J) Spectrum of an electron-dense granule (electron-lucent in TEM). Most of the conspicuous peaks denote elements used in the fixation process (uranyl U, osmium Os and lead Pb) or the grid (copper Cu).
Table 1
| Element | Electron-lucent granule (TEM_STEM) | Electron-dense granules (TEM-STEM) | ||||
|---|---|---|---|---|---|---|
| AN | wt.% | Err. | AN | wt.% | Err. | |
| Oxygen | 8 | 49.081 | 1.616 | 8 | 36.183 | 1.243 |
| Nitrogen | 7 | 9.544 | 0.439 | 7 | 47.935 | 1.631 |
| Sulfur | 16 | 21.486 | 0.769 | 16 | 1.769 | 0.150 |
| Phosphorus | 15 | 18.603 | 0.686 | 15 | 8.871 | 0.398 |
| Silicon | 14 | 1.286 | 0.114 | 14 | 5.262 | 0.216 |
Comparison of the main elements mapping with electron dispersive X-ray analysis in the electron-lucent and electron-dense granules of the bacteria (from TEM-STEM view).
In comparison with electron-lucent granules, there were a strong increase of nitrogen and reduction of sulfur in the electron-dense granules; also the silicon was higher.
AN, Atomic number; wt.%, percentage (in weight) of the element; Err, error in the percentage in atomic weight.
3.4 Taxonomic composition of bacteria in the gills from Kelliella miliaris
Figure 7 shows the taxonomic composition of the bacteria from the gills of Kelliella miliaris.
Figure 7
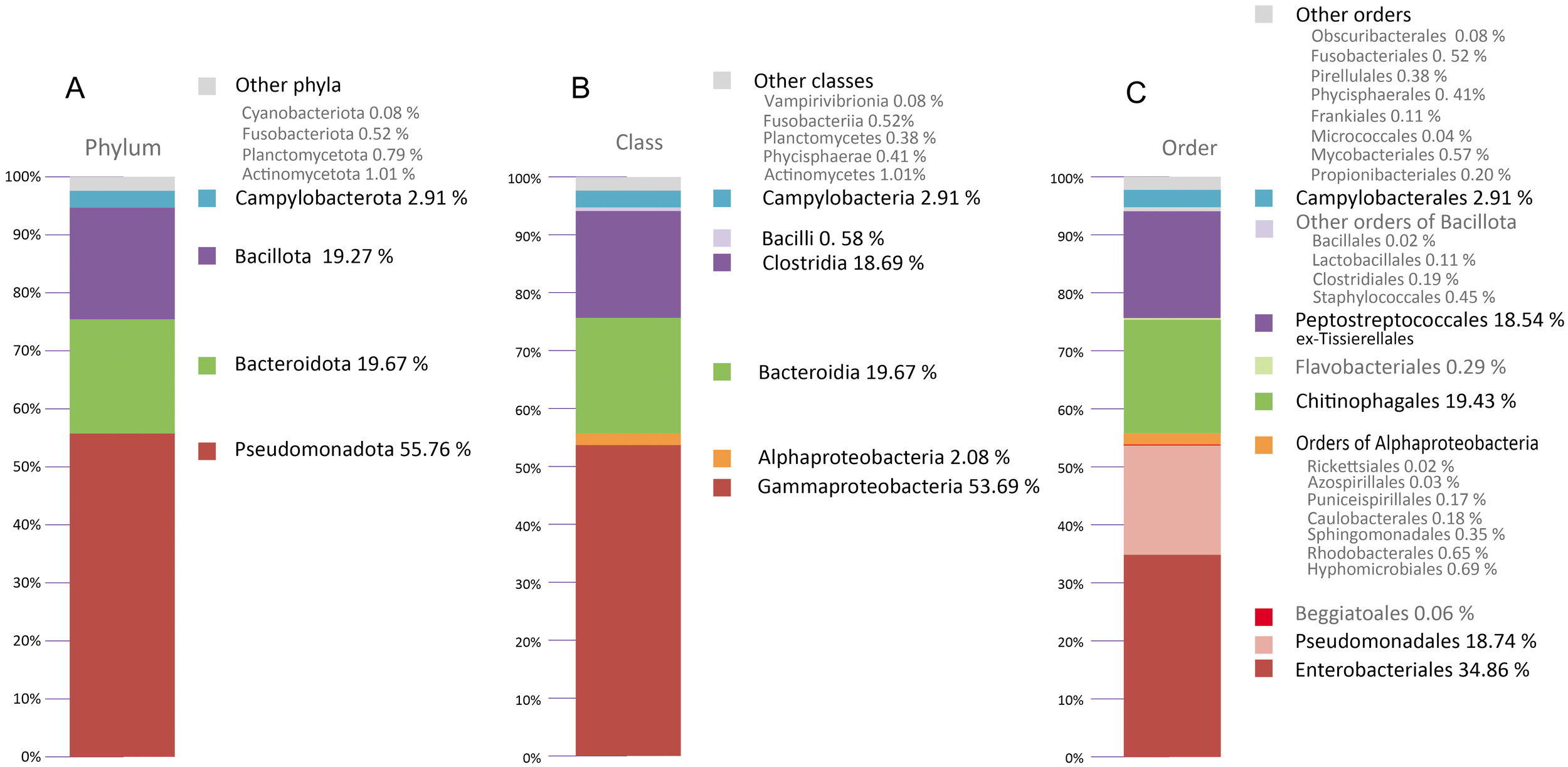
Microbial communities at Phylum(A), Class(B) and Order(C) level identified by sequencing of DNA bacterial from excised gills of Kelliella miliaris.
The predominant Phylum detected in the sample was Pseudomonadota (55.76% of total reads) (Figure 7A), of which the class Gammaproteobacteria constituted 53.69% (Figure 7B). The other two more abundant phyla are Bacteroidota (19.67%) (Figure 7A), all of which belong to the class Bacteroidia 19.67% (Figure 7B), and Bacillota (19.27%) (Figure 7A), most of which belong to the class Clostridia (18.74%)(Figure 7B). Regarding the Order, Enterobacterales (34.86%), Pseudomonadales (18.74%) are the most abundant within Gammaproteobacteria; Chitinophagales (19.43%) is almost all the Bacteroidia and Peptostreptococcales (exTissierellales) (18.54%) constituted the main taxonomic group in the class Clostridia (Figure 7C).
4 Discussion
Chemosynthetic symbiosis occurs ubiquitously at oxic-anoxic interfaces in marine environments, although this type of symbioses is mainly related to reducing habitats, such as hydrothermal vents, cold seeps, whale falls o deep sunken wood (Duperron, 2010). There is also symbiosis in other habitats, such as the oxygen minimum zone of the oceans, where the presence of bacteria were found in the protobranchiate bivalves Nucinella owenensisOliver and Taylor, 2012 and Huxleyia haboobaOliver and Taylor, 2012 (Oliver and Taylor, 2012); ectocommensal species, such as the bivalve Syssitomya pourtalesiana, which lives on the spines in the anal zone of the deep-sea echinoid Pourtalesia (Oliver et al., 2013) or shallow-water, low sulfidic environments, where symbiosis was found in the platyhelminths Paracatenula spp., gutless oligochaetes or nematodes (Dubilier et al., 2008; Nussbaumer et al., 2004). Austrogena nerudai Krylova, Sellanes, Valdes, D’Elias, 2014 is a vesicomyid from a restricted area within the benthic realm delimited by the oxygen minimum zone (OMZ) off the south-central Chilean margin (Krylova et al., 2014), where only juveniles were found alive, while all the adults were dead. According to these authors, pliocardiines have not been previously reported from OMZ, however it is quite probable that some species as ‘Vesicomya’ indica Smith, 1904, ‘Vesicomya’ brevis Smith, 1906 and ‘Vesicomya’ cretacea Smith, 1906, described from the bathyal depths of the Arabian Sea and the Bay of Bengal, were actually associated with OMZ. There were no recorded cases of specialized adaptations for different types of reducing environments among pliocardiines, although the authors considered that the metabolism of the species must be related with sulfide cycle, they did not show any images of bacteria inside the gills or sequence documenting their presence.
According to Childress and Girguis (2011), moderate to high rates of chemoautotrophic or methanotrophic metabolism is more demanding for symbiotic hosts in terms of oxygen uptake and proton equivalent elimination, compared to related non-symbiotic annelid, bivalve and gastropod lineages. The high oxygen demand of these symbionts is perhaps the most limiting factor in the pathway to a symbiotic condition. Among the consequences of such demands is the widespread occurrence of circulating and/or tissue haemoglobins in the hosts, to support high metabolic rates in thioautotrophic endosymbiosis. The latter is still more limiting in the oxygen minimum zone, where the oxygen is in short supply.
The microbial symbiotic assemblage has two main constraints in the OMZ, the low level of oxygen with irregular distribution between layers and deeps and along the OMZ and the maintenance of enough input of sulfur. The presence in the NW Arabian Sea OMZ of five species usually associated with chemosymbiotic bacteria (one Lucinoma sp and four Thyasira spp.) (Levin et al., 2000; Dufour, 2005; Taylor and Glover, 2006) would point that both elements are present and with enough amount to maintain a chemosymbiotic relationship. Probably their localization between the cilia of the gill filaments would be adaptive to obtain more easily oxygen and sulfur from the OMZ seawater.
The studied specimens were collected on the edge of the continental shelf of the Bay of Malaga, between 90 and 124 m depth and in the bathyal of Gulf of Cádiz, at 735 m depth, all of them on muddy bottoms. Sequence analysis of the mitochondrial cytochrome c oxidase subunit I (COI) gene (GenBank: PV147183) from the gill tissue sample revealed 100% identity with previously published sequences of Kelliella miliaris from the North Sea (Krylova et al., 2018), which confirms the conclusion based on morphology (Utrilla et al., 2024) that there is only one species of Kelliella (K. miliaris) from Norwegian Sea to the Mediterranean Sea.
Along the littoral of Malaga there are several upwellings of deep Mediterranean water before the strait of Gibraltar. These upwellings carry oxygen and nutrients to the surface, leaving a low oxygen level near the bottom. The specimens of K. miliaris from the Gulf of Cadiz were collected in a deep muddy bottom, in the pathway of the Mediterranean Outflow Water, with usually low level of oxygen near the bottom. The presence of bacteria in the gills of Kelliella miliaris highlights the importance of the symbiosis in oxygen minimum zones of the oceans that has been probably overlooked up to now.
The association of chemosynthetic bacteria with at least seven metazoan phyla (Porifera, Platyhelminthes, Nematoda, Mollusca, Annelida, Arthropoda and Echinodermata) suggests that chemosynthetic symbioses have evolved several times (Dubilier et al., 2008). In Mollusks, chemosynthetic symbiosis have been observed in Solenogastres (Katz et al., 2006), Gastropods (Bates, 2007; Goffredi et al., 2004; Urakawa et al., 2005; Suzuki et al., 2006 and references therein) and Bivalves, where many species contain chemosynthetic bacteria (Dufour, 2005; Dubilier et al., 2008; Taylor and Glover, 2010). Likewise, the ability to associate with chemosynthetic bacteria is a recurring feature in the evolution of bivalves, since it has appeared independently in seven families, Mytilidae, Vesicomyidae, Solemyidae, Lucinidae, Thyasiridae, Nucinellidae and Lasaeidae (Southward, 1986; Dufour, 2005; Dubilier et al., 2008; Duperron et al., 2013; Taylor and Glover, 2010; Oliver and Taylor, 2012; Oliver, 2012) and from different habitats.
The chemosynthetic bacteria can be: (1) epibionts or ectosymbiotic, living outside the host's gills, mouth parts, body surface, among others (Dufour, 2005); (2) endobionts or endosymbiotic, which can be extracellular, such as found in the gutless oligochaetes, in which the bacteria appeared below the cuticle of the body wall, or intracellular, such as found in many bivalves (Dubilier et al., 2008). In most of the studied chemosynthetic species of bivalves, the bacteria live inside the abfrontal gill cells, usually within bacteriocytes, however in Thyasiridae (Dufour, 2005) or some Mytilidae (Gros and Gaill, 2007), the bacteria live among the cilia, near the microvilli of the gill cells, as ectosymbionts. As general rule, there is one type of symbiont by species, usually thiothophs, but in the deep-sea mussels Bathymodiolus azoricus Cosel & Comtet, 1999, Bathymodiolus puteoserpentis Cosel, Métivier & Hashimoto, 1994 and Bathymodiolus brooksi Gustafson, Turner, Lutz & Vrijenhoek, 1998 there is a dual symbiosis (thiotrophs and metanotrophs) in the same bacteriocyte (Fisher et al., 1993; Distel et al., 1995; Duperron et al., 2005, 2006); in Bathymodiolus heckerae Turner, Gustafson, Lutz & Vrijenhoek, 1998 there are four symbionts (two thiotrophs, one metanotroph and one bacterium related with free-living methylotrophs) (Duperron et al., 2007) and in the cold seep mussels Idas sp., six bacterial symbionts coexisting in the same bacteriocyte were found (Duperron et al., 2008).
As expected in filter-feeding and heterotrophic feeding organisms, a wide diversity of bacterial species was found (Figure 7). The most abundant Class in the gill filaments of K. miliaris was Gammaproteobacteria, with 53.69% of total reads, which suggests that a gammaproteobacterium, probably from the order Enterobacterales, with 34.86% of the total reads, would be the dominant gill ectosymbiont of K. miliaris. Most of the previously analyzed chemosynthetic symbionts from bivalves cluster within the single bacterial Class of the Gammaproteobacteria, on the basis of 16S rRNA gene sequences (Distel and Cavanaugh, 1994).
The lack of concentric stacking of intracellular membranes in the cytoplasm of the bacteria trapped between the cilia of the gill filaments reveals they were not methanotrophic, while the presence of sulfur in the electron-dense granules (in TEM views), or electron-lucent (in TEM-STEM view), in these bacteria would indicate some involvement of the bacteria in the sulfur cycling. The peaks of oxygen and phosphorous in theses electron-dense granules could indicate in part polyphosphate compounds as described in the bacteria from Riftia pachyptila tube wall (Lechaire et al., 2002; Gros and Gaill, 2007), but also could point them as autotrophic sulfide-oxidizing bacteria. In the chemosynthetic symbioses, the symbionts need access to a terminal electron acceptor that, in the process of sulfur or methane, may be oxygen (oxic conditions) or a molecule like nitrate (anoxic conditions) (Roeselers and Newton, 2012). The great peak of oxygen of the electron-dense granules points to that part of the oxygen could be electron receptor. Thioautotrophic symbionts are the most common type in Bivalvia and are found in bathymodioline mussels as well as solemyid, thyasirid, lucinid, and vesicomyid clams (Cavanaugh et al., 2006).
The functionality of the chemosynthetic symbioses relies on both the availability of substrates for chemosynthetic metabolism and the existence of particular metabolic pathways in the symbiont to utilize those substrates. There is a link, therefore, between geochemistry and the type of symbiosis that can colonize a particular environment (Roeselers and Newton, 2012). According to these authors, apart from the sequencing, single-cell methods that combine imaging techniques with other type of analysis, such as metabolic ones which allow the visualization of uptake or/and distribution of substrates, would be interesting. In this way, the EDX analysis carried out by the TEM-STEM TALOS microscopy (Figure 6) shows for the first time the distribution of the environmental elements, among them sulfur and oxygen, in the symbiont bacteria as well as their amount.
Childress and Seibel (1998) proposed three possible physiological adaptations of the organisms to the OMZs: (1) the development of mechanisms for highly effective capture of oxygen from water. In relation to this adaptation, increase of the size and/or number of gills has been observed in polychaetes, crustaceans and mollusks; for example the chiton Leptochiton laurae Schwabe & Sellanes, 2010 from the OMZ in Chile exhibits enhanced numbers and size of ctenidia (Gooday et al., 2010). Some groups, such as fishes, crustaceans, mollusks or polychaetes, have specialized circulatory systems in addition to developed respiratory systems which offer possibilities for adaptations to OMZs (Levin, 2003). (2) the reduction of metabolic rates or (3) the use of anaerobic metabolism to make up the difference between aerobic capacity and total metabolic demand. Reduced body size and/or flattened tests, which both lead to an increase in the surface area-to-volume ratio, are also typical feature of some OMZ metazoan macrofauna and hypoxia-tolerant foraminiferal species, respectively (Levin, 2003; Gooday et al., 2010).
Kelliella miliaris shows different morphological characteristics that would be adaptive for living in OMZ, such as: (1) presence of numerous muscular fibers in the mantle, mantle edge and gills-visceral mass connection; all of which would allow to actively move the gills and/or enable a better control of the ventral opening in relation to vertical movements of the animal; (2) high number (in relation to body size) and large size of gill filaments, mainly in the large inner demibranch; and (3) long cilia which would provide a large surface for capture of oxygen and a highly effective uptake of oxygen from water. The presence of large blood sinus in the mantle edge could be related to the capture of the oxygen by the blood pigment, which probably was haemoglobin.
We have no data on the putative metabolic reduction or anaerobic metabolism in K. miliaris, but the presence of symbiosis with Gram negative Gammaproteobacteria involving in sulfur cycling would point that there must be a sulfidic environment. According to Brissac et al. (2011) the large electron-lucent periplasmic “vacuole” observed by TEM in the symbionts present in Thyasira sp. would be sulfur granule, like those frequently present in most sulfur-oxidizing bacteria. However, in the electron lucent granules (TEM view) of the bacteria found in K. miliaris, the amount of sulfur was minimum, while the higher amount of sulfur was found in the electron-dense granules (TEM view) or electron-lucent (TEM-STEM view).
The reduced sulfur compound hydrogen sulfide is extremely toxic to animals as it poisons cytochrome c oxidase and arrests aerobic respiration. In the case of the symbiotic mollusks, they typically oxidize sulfide to thiosulfate to reduce toxicity, and their symbionts can use thiosulfate as a reductant (Childress and Girguis, 2011). Our hypothesis is that the peak of oxygen in the electron dense and electron lucent granules of the bacteria would indicate the use of part of this oxygen to oxidize sulfide to thiosulfate. Therefore, the presence of sulfur-oxidizer bacteria in the large gills of K. miliaris would be an adaptation to eliminate the environmental toxic hydrogen sulfide that is usually present in the OMZ. However, future experiments (e.g. metagenomic analysis or enzymatic assays, among others) would be necessary to test this hypothesis.
Kelliella miliaris presents a well-developed stomach with a gastric shield and style sac diverticula, not separate from the mid gut and in which intense secretion by microvilli has been observed by transmission electron microscopy. The presence of different microorganisms inside the stomach, indicates suspension feeding, in agreement with Krylova et al. (2018), who suggested heterotrophy, after analyzing the stable δ13 C and δ15 N isotopic composition of K. miliaris, that they found similar to that of Vesicomya.
All our analyzed specimens were females, with developing oocytes at different degree of maturation. The follicles were located lateral to the digestive tract in the semithin sections. The presence of spermatozoids inside the female gonad, points to an internal fertilization. The latter would be an adaptation to ensure offspring in a minute species with a relatively low number of oocytes, similarly to the small astartid Digitaria digitaria (Marina et al., 2020). The protoconch of K. miliaris, with differentiated protoconch I and protoconch II, indicates planktotrophic larval development. The latter could favor the evasion of the larvae from the bottom reducing environment, which would be dangerous for the development of the protoconch.
Statements
Data availability statement
The datasets presented in this study can be found in the following online repository: https://www.ncbi.nlm.nih.gov/sra/PRJNA1229195.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
OU: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. EV: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. SG: Data curation, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. PM: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. JL: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CS: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by PPRO_RNM141-G-2023 through Junta de Andalucía, Consejería de Univerdidad, Investigación e Innovación and FEDER funds and by the project PID2023-146394NB-I00, of the Spanish Ministry of Science, Innovation and Universities (MCIN/AEI/10.13039/501100011033/”FEDER Una manera de hacer Europa”).
Acknowledgments
We would like to thank our colleague José Luis Rueda (Spanish Institute of Oceanography-CSIC) and the captain and the crew of R/V Cornide Saavedra during the INDEMARES/CHICA 0211 expedition in the Gulf of Cadiz for providing specimens of Kelliella miliaris. We are grateful to the graduate program of Diversidad Biológica y Medio Ambiente (DIBIMA) from the University of Malaga for funding the ship time for collecting benthic fauna from the Bay of Malaga, among them most of the specimens of Kelliella miliaris. We are also grateful to Adolfo Martínez, technician of the University of Malaga of the TEM-STEM FEI Talos 200X and Gregorio Martín Caballero technician of the University of Malaga of the Scanning Electron Microscopic services for their help for EDX analysis and scanning electronic microscopic observations of the samples respectively.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abellan-Schneyder I. MatChado M. S. Reitmeier S. Sommer A. Sewald Z. Baumbach J. et al . (2021). Primer, pipelines, parameters: issues in 16S rRNA gene sequencing. mSphere6, e01202–e01220. doi: 10.1128/mSphere.01202-20
2
Allen J. A. (2001). The family Kelliellidae (Bivalvia: Heterodonta) from the deep Atlantic and its relationship with the family Vesicomyidae. Zool. J. Lin. Soc.131, 199–226. doi: 10.1006/zjls.2000.0233
3
Bates A. E. (2007). Feeding strategy, morphological specialisation and presence of bacterial episymbionts in lepetodrilid gastropods from hydrothermal vents. Mar. Ecol. Progr. Ser.347, 87–99. doi: 10.3354/meps07020
4
Borges F. O. Sampaio E. Santos C. P. Rosa R. (2022). Impacts of low oxygen on marine life: neglected, but a crucial priority for research. Biol. Bull.243, 104–119. doi: 10.1086/721468
5
Brissac T. Rodrigues C. F. Gros O. Duperron S. (2011). Characterization of bacterial symbioses in Myrtea sp. (Bivalvia: Lucinidae) and Thyasira sp.(Bivalvia: Thyasiridae) from a cold seep in the Eastern Mediterranean. Mar. Ecol.32, 198–210. doi: 10.1111/j.1439-0485.2010.00413.x
6
Cavanaugh C. M. McKiness Z. P. Newton I. L. G. Stewart F. J. (2006). “Marine chemosynthetic symbioses,” in The Prokaryotes, 3rd ed. Eds. DworkinM.FalkowS.RosenbergE.SchleiferK. H.StackebrandtE. (Springer, New York), 475–507.
7
Childress J. J. Girguis P. R. (2011). The metabolic demands of endosymbiotic chemoautotrophic metabolism on host physiological capacities. J. Exper. Biol.214, 312–325. doi: 10.1242/jeb.049023
8
Childress J. J. Seibel B. A. (1998). Life at stable low oxygen levels: adaptations of animals to oceanic oxygen minimum layers. J. Exper. Biol.201, 1223–1232. doi: 10.1242/jeb.201.8.1223
9
Clausen C. (1958). On the anatomy and histology of the eulamellibranch Kelliella miliaris (Philippi) with observations on the ciliary mechanisms in the mantle cavity. Nytt Mag. Zool.6, 144–175.
10
Cook A. A. Lambshead P. J. D. Hawkins L. E. Mitchell N. Levin L. A. (2000). Nematode abundance at the oxygen minimum zone in the Arabian Sea. Deep Sea Res. II47, 75–85. doi: 10.1016/S0967-0645(99)00097-1
11
Distel D. L. Lee H. K. W. Cavanaugh C. M. (1995). Intracellular coexistence of methano- and thioautotrophic bacteria in a hydrothermal vent mussel. Proc. Nat. Acad. Sci.92, 9598–9602. doi: 10.1073/pnas.92.21.9598
12
Distel D. L. Roberts S. J. (1997). Bacterial endosymbionts in the gills of the deep-sea wood-boring bivalves Xylophaga atlantica and Xylophaga washingtona. Biol. Bull.192, 253–261. doi: 10.2307/1542719
13
Distel D. L. Cavanaugh C. M. (1994). Independent phylogenetic origins of methanotrophic and chemoautotrophic bacterial endosymbioses in marine bivalves. J. Bacteriol.176 (7), 1932–1938. doi: 10.1128/jb.176.7.1932-1938.1994
14
Dubilier N. Bergin C. Lott C. (2008). Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat. Rev. Microbiol.6, 725–740. doi: 10.1038/nrmicro1992
15
Dufour S. C. (2005). Gill anatomy and relationship to chemoautotrophic symbiont presence in the bivalve family Thyasiridae. Biol. Bull.208, 200–212. doi: 10.2307/3593152
16
Duperron S. (2010). “The diversity of deep-sea mussels and their bacterial symbioses,” in The Vent and Seep Biota. Ed. KielS. (Springer, Dordrecht), 137–167. Topics in Geobiology 33. doi: 10.1007/978-90-481-9572-5_6
17
Duperron S. Bergin C. Zielinski F. McKiness Z. De Chaine E. G. Cavanaugh C. M. et al . (2006). A dual symbiosis shared by two mussel species, Bathymodiolus azoricus and B. puteoserpentis (Bivalvia: Mytilidae), from hydrothemal vents along the northern Mid-Atlantic Ridge. Environ. Microbiol.8, 1441–1447. doi: 10.1111/j.1462-2920.2006.01038.x
18
Duperron S. Gaudron S. M. Rodrigues C. F. Cunha M. R. Decker C. Olu K. (2013). An overview of chemosynthetic symbioses in bivalves from the North Atlantic and Mediterranean Sea. Biogeosciences10, 3241–3267. doi: 10.5194/bg-10-3241-2013
19
Duperron S. Halary S. Lorion J. Sibuet M. Gaill F. (2008). Unexpected co-occurence of 6 bacterial symbionts in the gill of the cold seep mussel Idas sp. (Bivalvia: Mytilidae). Environ. Microbiol.10, 433–445. doi: 10.1111/j.1462-2920.2007.01465.x
20
Duperron S. Nadalig T. Caprais J. C. Sibuet M. Fiala-Medioni A. Amann R. et al . (2005). Dual symbiosis in a Bathymodiolus mussel from a methane seep on the Gabon continental margin (Southeast Atlantic). Appl. Environ. Microbiol.71, 1694–1700. doi: 10.1128/AEM.71.4.1694–1700.2005
21
Duperron S. Sibuet M. MacGregor B. J. Kuypers M. M. Fisher C. R. Dubilier N. (2007). Diversity, relative abundance, and metabolic potential of bacterial endosymbionts in three Bathymodiolus mussels (Bivalvia: Mytilidae) from cold seeps in the Gulf of Mexico. Enviromental Microbiol.9, 1423–1438. doi: 10.1111/j.1462-2920.2007.01259.x
22
Fisher C. R. Brooks J. M. Vodenichar J. S. Zande J. M. Childress J. J. Burke R. A. Jr. (1993). The co-occurrence of methanotrophic and chemoautotrophic sulphur oxydixing bacterial symbionts in a deep-sea mussel. PSZN I: Mar. Ecol.14, 277–289. doi: 10.1111/j.1439-0485.1993.tb00001.x
23
Folmer O. Black M. Hoeh W. Lutz R. Vrijenhoek R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol.3, 294–299.
24
Fuchs B. M. Woebken D. Zubkov M. V. Burkill P. Amann R. (2005). Molecular identification of picoplankton populations in contrasting waters of the Arabian Sea. Aquat. Microb. Ecol.39, 145–157. doi: 10.3354/ame039145
25
Goffredi S. K. Warén A. Orphan V. J. Van Dover C. L. Vrijenhoek R. C. (2004). Novel forms of structural integration between microbes and a hydrothermal vent gastropod from the Indian Ocean. Appl. Environ. Microbiol.70, 3082–3090. doi: 10.1128/AEM.70.5.3082-3090.2004
26
Gooday A. J. Bett B. J. Escobar E. Ingole B. Levin L. A. Neira C. et al . (2010). Habitat heterogeneity and its influence on benthic biodiversity in oxygen minimum zones. PSZNI. Mar. Ecol.31, 125–147. doi: 10.1111/j.1439-0485.2009.00348.x
27
Gros O. Gaill F. (2007). Extracellular bacterial association in gills of ‘wood mussels’. Cah. Biol. Mar.48, 103–109.
28
Hunter W. R. Oguri K. Kitazato H. Ansari Z. A. Witte U. (2011). Epi-benthic megafaunal zonation across an oxygen minimum zone at the Indian continental margin. Deep Sea Res. I58, 699–710. doi: 10.1016/j.dsr.2011.04.004
29
Katz S. Cavanaugh C. M. Bright M. (2006). Symbiosis of epi-and endocuticular bacteria with Helicoradomenia spp. (Mollusca, Aplacophora, Solenogastres) from deep-sea hydrothermal vents. Mar. Ecol. Progr. Ser.320, 89–99. doi: 10.3354/meps320089
30
Kleiner M. Wentrup C. Lott C. Teeling H. Wetzel S. Young J. et al . (2012). Metaproteomics of a gutless marine worm and its symbiotic microbial community reveal unusual pathways for carbon and energy use. Proc. Nat. Acad. Sci.109, e1173–E1182. doi: 10.1073/pnas.112119810
31
Klindworth A. Pruesse E. Schweer T. Peplies J. Quast C. Horn M. et al . (2013). Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucl. Acids Res.41, e1. doi: 10.1093/nar/gks808
32
Krylova E. M. Sahling H. Borowski C. (2018). Resolving the status of the families Vesicomyidae and Kelliellidae (Bivalvia: Venerida), with notes on their ecology. J. Moll. Stud.84, 69–91. doi: 10.1093/mollus/eyx050
33
Krylova E. M. Sellanes J. Valdés F. D’Elía G. (2014). Austrogena: a new genus of chemosymbiotic bivalves (Bivalvia; Vesicomyidae; Pliocardiinae) from the oxygen minimum zone off central Chile described through morphological and molecular analyses. Syst. Biodiv.12, 225–246. doi: 10.1080/14772000.2014.900133
34
Lavik G. Stührmann T. Brüchert V. van der Plas A. Mohrholz V. Lam P. et al . (2009). Detoxification of sulphidic African shelf waters by blooming chemolithotrophs. Nature457, 581–584. doi: 10.1038/nature07588
35
Lechaire J. P. Shillito B. Frébourg G. Gaill F. (2002). Elemental characterization of microorganism granules by EFTEM in the tube wall of a deep-sea vent invertebrate. Biol. Cell94, 243–249. doi: 10.1016/S0248-4900(02)01199-1
36
Levin L. A. (2003). Oxygen minimum zone benthos_adptation and community response to hypoxia. Oceanogr. Mar. Biol. Ann. Rev.41, 1–45.
37
Levin L. A. Gage J. D. Martin C. Lamont P. A. (2000). Macrobenthic community structure within and beneath the oxygen minimum zone, NW Arabian Sea. Deep Sea Res. II47, 189–226. doi: 10.1016/S0967-0645(99)00103-4
38
Levin L. A. Huggett C. L. Wishner K. F. (1991). Control of deep-sea benthic community structure by oxygen and organic-matter gradients in the eastern Pacific Ocean. J. Mar. Res.49, 763–800. doi: 10.1357/002224091784995756
39
Longhurst A. R. (1967). Vertical distribution of zooplankton in relation to the eastern Pacific oxygen minimum. Deep Sea Res.14, 51–63. doi: 10.1016/0011-7471(67)90028-9
40
Marina P. Urra J. Bueno J. D. D. Rueda J. L. Gofas S. Salas C. (2020). Spermcast mating with release of zygotes in the small dioecious bivalve Digitaria digitaria. Sci. Rep.10, 12605. doi: 10.1038/s41598-020-69457-2
41
Molina E. J. Silberberger M. J. Kokarev V. Reiss H. (2019). Environmental drivers of benthic community structure in a deep sub-arctic fjord system. Est. Coast. Shelf Sci.225, 106239. doi: 10.1016/j.ecss.2019.05.021
42
Nussbaumer A. D. Bright M. Baranyi C. Beisser C. J. Ott J. A. (2004). Attachment mechanism in a highly specific association between ectosymbiotic bacteria and marine nematodes. Aquat. Microb. Ecol.34, 239–246. doi: 10.3354/ame034239
43
Oliver P. G. (2012). Taxonomy of some Galeommatoidea (Mollusca, Bivalvia) associated with deep-sea echinoids: A reassessment of the bivalve genera Axinodon Verrill & Bush 1898 and Kelliola Dall 1899 with descriptions of new genera Syssitomya gen. nov. and Ptilomyax gen. nov. Eur. J. Taxonomy12, 1–24. doi: 10.5852/ejt.2012.12
44
Oliver P. G. Taylor J. D. (2012). Bacterial symbiosis in the Nucinellidae (Bivalvia: Solemyida) with descriptions of two new species. J. Moll. Stud.78, 81–91. doi: 10.1093/mollus/eyr045
45
Oliver P. G. Southward E. C. Dando P. R. (2013). Bacterial symbiosis in Syssitomya pourtalesiana Oliver, 2012 (Galeommatoidea: Montacutidae), a bivalve commensal with the deep-sea echinoid Pourtalesia. J. Moll. Stud.79 (1), 30–41.
46
Paredes G. F. Viehboeck T. Lee R. Palatinszky M. Mausz M. A. Reipert S. et al . (2021). Anaerobic sulphur oxidation underlies adaptation of a chemosynthetic symbiont to oxic-anoxic interfaces. mSystems6, e01186–e01120. doi: 10.1128/msystems.01186-20
47
Ridewood W. G. (1903). On the structure of the gills in the Lamellibranchia. Phil. Trans. R. Soc London B195, 147–284.
48
Roeselers G. Newton I. L. (2012). On the evolutionary ecology of symbioses between chemosynthetic bacteria and bivalves. Appl. Microbiol. Biotechnol.94, 1–10. doi: 10.1007/s00253-011-3819-9
49
Sewell R. B. S. Fage L. (1948). Minimum oxygen layer in the ocean. Nature162, 949–951. doi: 10.1038/162949a0
50
Southward A. J. Southward E. C. Dando P. R. Rau G. H. Felbeck H. Flügel H. (1981). Bacterial symbionts and low 13C/12C ratios in tissues of Pogonophora indicate unusual nutrition and metabolism. Nature293, 616–619. doi: 10.1038/293616a0
51
Southward E. C. (1986). Gill symbionts in thyasirids and other bivalve molluscs. J. Mar. Biol. Ass. U.K.66, 889–914. doi: 10.1017/S0025315400048517
52
Sperling E. A. Knoll A. H. Girguis P. R. (2015). The ecological physiology of earth’s second oxygen revolution. Annu. Rev. Ecol. Evol. Syst.46, 215–235. doi: 10.1146/annurev-ecolsys-110512-135808
53
Stevens H. Ulloa O. (2008). Bacterial diversity in the oxygen minimum zone of the eastern tropical South Pacific. Environ. Microbiol.10, 1244–1259. doi: 10.1111/j.1462-2920.2007.01539.x
54
Suzuki Y. Kojima S. Watanabe H. Suzuki M. Tsuchida S. Nunoura T. et al . (2006). Single host and symbiont lineages of hydrothermal-vent gastropods Ifremeria nautilei (Provannidae): biogeography and evolution. Mar. Ecol. Progr. Ser.315, 167–175. doi: 10.1017/S0025315400048517
55
Taylor J. D. Glover E. A. (2006). Lucinidae (Bivalvia)–the most diverse group of chemosymbiotic molluscs. Zool. J. Lin. Soc.148, 421–438. doi: 10.1111/j.1096-3642.2006.00261.x
56
Taylor J. D. Glover E. A. (2010). “Chemosymbiotic bivalves,” in The Vent and Seep Biota. Ed. KielS. (Springer, Dordrecht), 107–135. Topics in Geobiology 33. doi: 10.1007/978-90-481-9572-5_5
57
Urakawa H. Dubilier N. Fujiwara Y. Cunningham D. E. Kojima S. Stahl D. A. (2005). Hydrothermal vent gastropods from the same family (Provannidae) harbour ε-and γ-proteobacterial endosymbionts. Environ. Microbiol.7, 750–754. doi: 10.1111/j.1462-2920.2005.00753.x
58
Utrilla O. Gofas S. Salas C. (2024). The genus Kelliella (Bivalvia) in the Atlantic Ocean. J. Moll. Stud.90, eyae035. doi: 10.1093/mollus/eyae035
59
WoRMS Editorial Board . (2024). World Register of Marine Species. VLIZ. Available at https://www.marinespecies.org (Accessed December 10, 2024). doi: 10.14284/170
60
Wright J. J. Konwar K. M. Hallam S. J. (2012). Microbial ecology of expanding oxygen minimum zones. Nat. Rev. Microbiol.10, 381–394. doi: 10.1038/nrmicro2778
Summary
Keywords
Kelliella miliaris , oxygen minimum layer, chemosymbiosis, gammaproteobacteria, internal fertilization, planctotrophic protoconch
Citation
Utrilla O, Viguera E, Gofas S, Marina P, López-Téllez J-F and Salas C (2025) Life at oxygen minimum zone: bacterial symbiosis in the gills of the bivalve Kelliella miliaris. Front. Mar. Sci. 12:1587729. doi: 10.3389/fmars.2025.1587729
Received
04 March 2025
Accepted
30 May 2025
Published
20 June 2025
Volume
12 - 2025
Edited by
Laura Núñez Pons, Anton Dohrn Zoological Station Naples, Italy
Reviewed by
Maria Costantini, Anton Dohrn Zoological Station, Italy
Mirko Mutalipassi, Stazione Zoologica Anton Dohrn, Italy
Updates
Copyright
© 2025 Utrilla, Viguera, Gofas, Marina, López-Téllez and Salas.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carmen Salas, casanova@uma.es
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.