- Institute of Marine Chemistry and Environment, Ocean College, Zhejiang University, Zhoushan, China
The Zhanjiang Mangrove National Nature Reserve (ZMNNR) is the largest mangrove in China. However, fluxes of lateral exports of dissolved inorganic carbon (DIC), dissolved organic carbon (DOC), and nitrate from the ZMNNR remain unknown, hindering us from evaluating its blue carbon capacity and its resilience to environmental changes. We conducted a comprehensive study of temporal variations in DIC, DOC, nitrate, and sulfate in creek and pore waters, and time-series measurements of 222Rn in creek waters, in response to tidal cycles. Nitrate and sulfate concentrations varied in tidal cycles, forming a tightly negative correlation. DIC and DOC were significantly rich in, but nitrate was substantially depleted in pore waters compared to creek waters. Depleted δ13C values of DIC and DOC in tidal creek waters suggest that both of them were predominantly from organic matter derived from mangroves. Radiocarbon ages of DIC ranged from 149 to 236 years, suggesting minimal or absent mineralization of aged (i.e., centuries-old) organic matter. Time-series measurements of 222Rn in creek waters revealed pore water exchange rate at 14.2 ± 24.5 cm d−1, and the lateral fluxes of DIC and DOC from the mangroves to the neighboring Yingluo Bay, based on an FVCOM model, were 411.6 ± 311.8 and 104.5 ± 145.7 mmol m−2 d−1, respectively. Outwelling fluxes of dissolved carbon were estimated to be equivalent to 7.7% ± 6.8% of annual carbon fixed by mangroves in the study area. The flux of nitrate from the study area to Yingluo Bay was 8.5 ± 7.6 mmol m−2 d−1, making mangroves the sink of nitrate and the source of ammonium.
1 Introduction
As a key “blue carbon” ecosystem, mangroves along subtropical and tropical coastlines account for 10% to 15% of annual coastal carbon sequestration, while only occupying 0.5% of the global coastal area (Alongi, 2014). The carbon sequestration in mangroves is seen as an effective long-term (millennial-scale) carbon sink, due to high primary productivity and sedimentation rates, effective trapping of particles, and reducing conditions that minimize organic carbon oxidation (McLeod et al., 2011; Kelleway et al., 2016). On the other hand, crabs create abundant burrows in most mangrove sediments, facilitating the tidally driven exchange of pore water (Stieglitz et al., 2013; Tait et al., 2016). Tidal movement drives seawater inflow into sediments during flood tides, supplying terminal electron acceptors, such as dissolved oxygen (D.O.), nitrate, and sulfate, into sediments, and the discharge of pore water at ebb tides.
Organic matter in mangrove sediments is partially mineralized, releasing dissolved carbons, with majority in the form of dissolved inorganic carbon (DIC), and approximately 10% as dissolved organic carbon (DOC) (Maher et al., 2013). Both DIC and DOC can be laterally exported into adjacent seas via pore water flushing, a process driven by tidal advection and drainage (Santos et al., 2021; Tamborski et al., 2021). Meanwhile, nutrients such as dissolved inorganic nitrogen and phosphorus in organic matter are also released (Wang et al., 2019) and laterally exported together with DIC and DOC. Such concept of exporting mangrove-derived organic matter and nutrients to surrounding waters in sustaining coastal food webs is referred to as the “outwelling hypothesis” (Odum, 1968; Santos et al., 2021). It was reported that 50% carbon fixed by mangroves could be mineralized and outwelled to coastal seas (Bouillon et al., 2008).
Aerobic microbial decompositions of organic matter in marine sediments are only limited at the top by a few millimeters (Froelich et al., 1979), except where crab burrows and plant roots channel oxygen to deeper sediment layers. Nitrate serves as a thermodynamically favorable electron acceptor to fuel microbial mineralization, through denitrification converting NO3− to N2O or N2 (Hamersley and Howes, 2005) or dissimilatory nitrate reduction to ammonium (DNRA) (Giblin et al., 2013). However, nitrate concentrations in surface oceans are usually very low (from less than 1 to 11.4 μM) (Karl and Letelier, 2008) and deplete quickly during mineralization. On the other hand, sulfate in seawater is abundant, reaching ~29 mM (Millero, 2005), and thus, respiration and sulfate reduction are often considered as the two most dominant mineralization processes of organic carbon in marine sediments (Jørgensen, 1982). However, nitrate concentrations in mangroves could be much higher due to eutrophication from untreated aquaculture effluents, local agricultural runoff, and sewage, among others. For example, nitrate in tidal creeks in Qinglan Bay mangroves, China reached 73.9 to 2,418.1 μM (Zidan et al., 2025), two orders of magnitude higher than those in the surface oceans. As a result, nitrate reduction likely competes with sulfate reduction in mineralization of organic matter in mangrove sediments, which may vary in different sites.
Periodic tidal inundation intermittently replenishes not only electron acceptors, but also marine DIC to sediments, which has δ13C signal from −0.8‰ to 2.4‰ (Mackensen and Schmiedl, 2019). In contrast, DIC generated by mineralization of organic matter has substantially lower δ13C values (Maher et al., 2013), potentially resulting in variations in δ13C signal in tidal creek waters and pore waters through the tidal cycles. For example, in the subtropical southern Moreton Bay, Australia, δ13C values of DIC in creek waters (δ13CDIC-ck) in summer at the lowest tide were about −5.2‰, significantly lower than that at the highest tide (1.5‰) (Maher et al., 2013), reflecting the contribution of DIC derived from organic matter mineralization. δ13CDOC-ck values showed the same tidal variations, but were 18‰ more negative than δ13CDIC-ck (Maher et al., 2013).
Water exchange, including pore water exchange and submarine groundwater discharge (i.e., sediments to creeks), and lateral exchange or “outwelling” (i.e., creeks to ocean) are two different processes for exporting carbon and nutrients from mangroves to adjacent coastal waters (Maher et al., 2013; Faber et al., 2014; Sadat-Noori et al., 2017; Chen et al., 2018a). For measuring the rates of pore water exchange, 222Rn can be used as a conservative tracer as it is chemically unreactive (noble gas) and is produced in easily measurable amounts within sediments as the direct decay product of 226Ra (t1/2 = 1,600 years) in the 238U decay chain (Galhardi and Bonotto, 2017). 222Rn has a short half-life of 3.82 days, and its concentrations in groundwater are typically two to four orders of magnitude higher than in surface waters (Cable et al., 1996; Swarzenski et al., 2001). This provides a way to differentiate surface water from pore water even if they have similar concentrations of other substances (Faber et al., 2014). As a result, 222Rn has been increasingly used to quantify fluxes of dissolved carbon and nutrients in mangrove-dominated creeks and estuaries (e.g., Maher et al., 2013; Tait et al., 2016). Furthermore, the outwelling fluxes can be calculated using the mangrove creek water budget over a daily tidal cycle. Based on a coupled 222Rn and carbon model, Maher et al. (2013) showed that DIC export averaged 250 mmol m−2 d−1 and was an order of magnitude higher than DOC export, and similar to global estimates of the mangrove missing carbon (i.e., 158–225 mmol m−2 d−1) (Alongi, 2009). DIC “outwelling” could act as a long-term carbon sink, with capacity potentially rivaling soil organic carbon stocks (Maher et al., 2018), due to the long residence time of DIC in the oceans (Middelburg et al., 2020). Exported DIN from mangroves to coastal oceans can also support the coastal ecosystem (Santos et al., 2021). As a result, quantifying fluxes of dissolved carbon and nutrients is critical for understanding the broader role of mangroves in carbon and nutrient cycling.
The Zhanjiang Mangrove National Nature Reserve (ZMNNR), the largest and most diverse natural mangrove reserve in China, is located on the northwestern Leizhou Peninsula, the southernmost end of the Chinese mainland (Figure 1a). ZMNNR is a typical tidal estuary, spanning a total area of 20,278 hectares (Zhang and Chen, 2022). The 7,228-hectare mangrove forest (Figure 1b) accounts for 33% of total mangrove area in China. As the first blue carbon project, the Zhanjiang Mangrove Afforestation Project was successfully developed and registered in 2021 on the Verified Carbon Standard platform, the world’s largest voluntary carbon market. However, mechanisms of organic matter mineralization in sediments and annual fluxes of lateral exports of DIC, DOC, and dissolved inorganic nitrogen from the ZMNNR remain largely unknown, hindering us from evaluating its blue carbon capacity and its resilience to environmental changes.
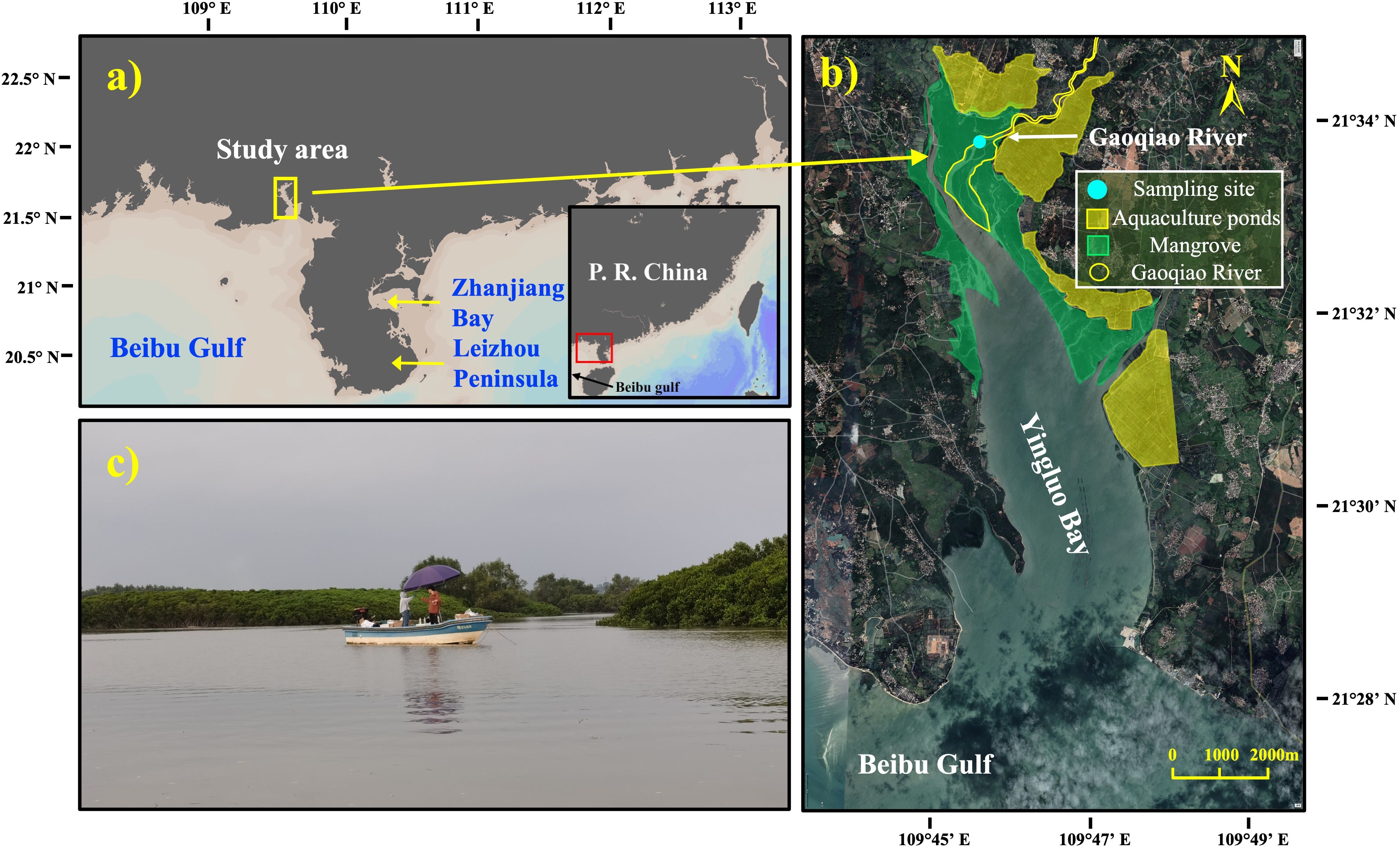
Figure 1. Maps showing the location of Zhanjiang Mangrove National Nature Reserve and sampling sites. (a) A map showing the location of the Zhanjiang Mangrove National Nature Reserve (ZMNNR) in China. (b) A map showing the Gaoqiao mangrove area, the core region of the ZMNNR. (c) A picture showing the sampling site.
Here, we report a comprehensive study of variations in terminal electron acceptors for organic matter mineralization in sediments in response to tidal cycles, and quantification of DIC, DOC, and nitrate fluxes from the ZMNNR to the neighboring Yingluo Bay (Figure 1a). Tidal creek waters and sediment pore waters in situ were collected at hourly intervals for 24 h, and measured for DIC, DOC, nitrate, and sulfate concentrations. Selected creek water samples were measured for δ13CDOC, δ13CDIC, and Δ14CDIC. Time-series measurements of 222Rn in tidal creeks were conducted every hour. An 80-cm-long sediment core was taken and 210Pb chronology was developed in order to estimate sedimentation rates (see Supplementary Materials and Supplementary Figure S1). Our aim is to quantify the pore water exchange rate and fluxes of DIC, DOC, and nitrate from the ZMNNR for the first time and elucidate temporal variability in nitrate, ammonium, and sulfate concentrations in tidal creek and pore waters within the tidal cycle. Our work would provide a framework for evaluating the long-term blue carbon capacity in the ZMNNR and its resilience to climate and environment change.
2 Materials and methods
2.1 Study area
The ZMNNR is within the subtropical monsoon climate region, with an annual mean temperature of 23.8°C and an annual mean precipitation of 1,704 mm. The mean relative humidity is 81.8%, but the precipitation is primarily concentrated in the wet season from April to September (Zhang and Chen, 2022). The mean irregular diurnal tidal height is 2.53 m, while the highest tidal height can reach 6.25 m.
The Gaoqiao mangrove area (21°31′–21°35′N, 109°45′–109°48′E) is the core region of the ZMNNR, located along the northeastern coastline of Yingluo Bay, a semi-closed bay. Two small rivers, the Ximi River and the Gaoqiao River, flow into the reserve from the north and then into Yinluo Bay (Figure 1b). The predominant mangrove species in the high tide zone are Aegiceras corniculatum and Bruguiera gymnorhiza, while Avicennia marina, Kandelia obovata, and Rhizophora stylosa are in the low tide zone.
2.2 Collection of creek water during tidal cycles
Creek waters were collected in the main tidal creek in the high tide zone (21°33′58''N, 109°45′34''E) (Figures 1b, c) at 1-h intervals, from April 12, 1 p.m. to April 13, 5 p.m., and from May 16, 10 a.m. to May 17, 4 p.m., 2024 (Tables 1, 2). A submersible pump at a depth of 0.5 m was applied to fill creek water into a 2,500-mL polyethylene bottle using an overflow method. Water samples were measured in situ for D.O., pH, and salinity, using a portable YSI 556 MPS multi-parameter digital water quality meter. Two drops of saturated HgCl2 solution were added to the bottle using a syringe, which can eliminate the influence from the microorganism activities. Bottles were kept in a cooler with ice packs. Once in the lab, water sample was filtered through pre-combusted GF/F filters (Whatman, 47 mm i.d.) into a 60-mL polyethylene bottle without headspace, and kept at 4°C till analyses.
2.3 Collection of sediment pore water in situ during tidal cycles
Boreholes were dug on the creek bank near the site of creek water collection (21°33′52''N, 109°45′32''E, Figure 1c), to a depth of ~40 cm, following the protocol as described in Maher et al. (2013) and Taillardat et al. (2018). Four vacuum-induced soil solution suction cups at the same time were buried in the sediments for extracting pore waters in situ at the same time. Pore waters were purged three times before sampling to ensure reliable collection. The suction cup consisted of a cylindrical porous ceramic cup (SRQ100; Shenzhen Hengjinda Technology, China) sealed with dual stainless tubes. One end of the tube was placed in the sediment to extract pore water, while the other end was connected to Teflon tubing for pore water collection. The ceramic cup is 69.9 mm long, with an outer diameter of 22.2 mm, an inner diameter of 13.5 mm, and a pore size of 100 μm. The cup was inserted into sediment dug at a depth of 40 cm. By connecting the Teflon tubing to a 100-mL pre-combusted glass bottle, which had been vacuum-sealed to approximately −30 kPa, pore water was extracted due to pressure differences.
Pore waters were collected from multiple cups into 100-mL pre-combusted borosilicate glass bottles at 1-h intervals from May 16, 10 a.m. to May 17, 4 p.m., 2024 (Table 3). All pore waters were mixed, and two drops of saturated HgCl2 solution were added to inhibit biological activity. Then, they were transferred in a few 60-mL polyethylene bottles, ensuring that each bottle had no headspace. The bottles were then sealed with Parafilm and stored in a cooler with ice packs. Once in the lab, each sample was immediately filtered through GF/F filters (Whatman; 21 mm i.d.) into a few 35-mL bottles, leaving no headspace, and stored at 4°C till analyses.
2.4 Analysis of 222Rn in creek waters and 226Ra in sediments
Time-series station was deployed on an anchored boat from April 12, 1 p.m. to April 13, 5 p.m., 2024 (Table 1). Surface mangrove creek waters were collected at 1-h intervals by submerging a 1-L plastic bottle to 0.3 m depth, leaving no headspace to minimize atmospheric contamination. Once radon-enriched water is exposed, radon immediately exits the system through atmospheric evasion, radioactive decay, and mixing (Burnett et al., 2010). Collected water samples were analyzed immediately on the boat using a RAD7 radon detector (Durridge Company, USA). The headspace air was sequentially pumped through a Drierite® desiccant to a closed-loop air circulation system and then to an automated radon (222Rn) detector. Each sample was analyzed three times, and the whole run took approximately 45 min.
The 222Rn activity in sediment pore water was obtained from a sediment equilibrium incubation experiment. Approximately 150 g of surface sediment was taken using a grabber, transferred into a 1-L conical bottle, and then mixed with 400 mL of overlying seawater. The bottle was sealed and placed on an oscillator for 1 month so that the activity of 222Rn in the sediment pore water reached a radioactive decay equilibrium with that of the overlying water (Corbett et al., 1998). The overlying water was siphoned into a 250-mL sampling bottle and measured for the activity of 222Rn using a RAD7 radon detector, which was taken as the 222Rn activity in the sediment pore water.
The pronounced radon emanation creates a disequilibrium between 222Rn and its parent isotope 226Ra, with 222Rn concentrations typically exceeding 226Ra levels by several orders of magnitude (Swarzenski, 2007). In order to measure very low concentrations of radium, 40 L of underground water at 0.5 m deep was retrieved and immediately filtered through 20 g of MnO2-impregnated acrylic fibers at a rate of 0.5 L min−1 (Moore, 1976). The fibers were subsequently rinsed with radium-free Milli-Q water to remove salts and particulates. Then, the fiber was combusted in a furnace at 800°C for 8 h. The ash was transferred to a 7-mL plastic vial sealed with epoxy. It was sealed and stored in the lab at room temperature for a month in order to achieve secular equilibrium between 226Ra and its short-lived daughter product before analysis by gamma ray spectrometry. Finally, the ash samples were measured on a gamma spectrometer (ORTEC GWL-120–15-XLB-AWJ) at the Third Institute of Oceanography (TIO), Ministry of Natural Resources of China (MNRC), based on the hypothesis that radon produced by radium decay is quantitatively ejected from the fiber (Peterson et al., 2009).
2.5 Measurements of nitrate, nitrite, ammonium, and sulfate concentrations in tidal creek waters and pore waters
Concentrations of nitrate (NO32-), nitrite (NO22-), ammonium (NH4⁺), and sulfate (SO42−) of water samples were determined shortly after returning to the lab, using spectrophotometric methods. The procedure for nitrate measurement involves the reduction of nitrate to nitrite, using the vanadium reagent (VCl3 solution) reduction method, and its subsequent measurement by colorimetry using the Griess reaction (Moorcroft et al., 2001). Nitrite reacted directly with Griess reagents to produce a pink dye. Those dyes were measured on a Jena SPECORD 50 Plus UV-VIS spectrophotometer, and the detection limit was 0.2 µM. Ammonium was first oxidized to nitrite by sodium hypobromite and then analyzed as nitrite (Laughlin et al., 1997). Concentrations of sulfate were measured using a modified turbidimetric method based on US EPA 9038 guidelines. The standard deviations for those measurements were within 2.5% by repeated analysis (n = 3).
2.6 Measurement of DIC in tidal creek waters and pore waters
Concentrations of DIC in creek and pore waters were analyzed in triplicate using an Apollo SciTech AS-C3 analyzer at the Second Institute of Oceanography, MNRC. An aliquot of water samples was acidified with 10% H3PO4 and the released CO2 was subsequently quantified using a LI-7000 nondispersive infrared detector (LI-COR Environmental, USA). At least three repeated measurements were carried out for each sample, ensuring precision within 0.1% ( ± 2–4 μmol kg−1). Certified reference materials from A.G. Dickson, Scripps Institution of Oceanography (Dickson et al., 2007) were used for daily DIC calibrations. All results were reported as the mean ± standard deviation for triplicate analyses (Tables 1–3).
2.7 Measurements of DOC and δ13C-DOC in creek waters
DOC was measured using a total organic carbon (TOC) analyzer (Multi N/C 3100 TOC/TN, Analytik-Jena, Germany), equipped with a non-dispersive infrared detector, at Nanjing Center, China Geological Survey. Prior to analysis, water samples were filtered through 0.45-μm syringe filters to remove any potential particulate organic matter. The instrument was calibrated using potassium hydrogen phthalate standards (1–10 mg L−1 as C) prepared with ultrapure water, and a calibration curve with an R2 > 0.999 was obtained. DIC was removed by acidification and sparging with the instrument’s built-in system before injecting aliquots (100 μL) into the combustion chamber, where organic carbon was oxidized to CO2 at 800°C. Released CO2 was quantified, and concentrations of DOC were then calculated based on the calibration curve. Procedural blanks, duplicate samples, and certified reference materials were measured repeatedly during the analyses to ensure quality control. All results were reported as the mean ± standard deviation (Tables 1–3).
Six DOC samples, including creek waters at the highest, lowest, and middle tidal heights, were selected for carbon isotope analysis, using an IsoPrime 100 stable isotope ratio mass spectrometer (Elementar, USA) at the TIO, MNRC. The oxidation furnace was set at 850°C and the reduction furnace was set at 600°C to ensure complete conversion of DOC to CO2, which was then trapped and released from the adsorption column at 230°C before being introduced into the mass spectrometer. δ13C values of DOC were reported relative to V-PDB, and standard deviation was ± 0.2‰.
2.8 Radiocarbon (Δ14C) and δ13C analyses of DIC in tidal creek water
Radiocarbon (Δ14C) measurements on water DIC were performed in the Laboratory of Climate and Environmental Change, School of Geographical Sciences, Henan University. The system consists of a CHS2 carbonate handling system, a gas interface system (GIS), and a MICADAS accelerator mass spectrometer (AMS). Immediately before the analysis, 2 mL of water was transferred into a 12-mL Exetainer vial, which was previously sealed and flushed with a stream of helium at 70 mL min−1 for 10 min, using a 2.5-mL gas-tight glass syringe into the septum made of chlorobutyl-C rubber. The syringe was flushed with Milli-Q water and sample water in between samples. Pure phosphoric acid (200 μL; 100% H3PO4) was added into the Exetainer vial using a 1-mL gas-tight glass syringe. The vial was then vigorously shaken to ensure mixing and placed on a heating block at room temperature for 4 h. The CO2-He mixture in the headspace was then sampled with a stream of helium through a two-way needle into a Sicapent water trap before CO2 was concentrated on the zeolite trap of the GIS (Wacker et al., 2013). The zeolite trap was then heated to 450°C, and the concentrated CO2 was desorbed and transferred into the GIS injection syringe by gas expansion with helium. The gas mixture was subsequently fed into the AGE3 for automated graphitization and then measured on the MICADAS AMS (200 kV) for radiocarbon. Radiocarbon data are normalized with Oxalic Acid II standard, and blank was corrected against 14C-free CO2 reference gas analyzed in the same sequence using the BATS software (Wacker et al., 2010). The method accuracy was ±2.5‰ (precision, 1σ). δ13C values of DIC were reported simultaneously.
2.9 222Rn mass balance model and pore water exchange
We adopt a 222Rn mass balance model (Cable et al., 1996; Santos et al., 2015) to estimate the pore water exchange rate and lateral fluxes into Yingluo Bay. The model accounts for spatially integrated pore water exchange processes over timescales aligned with 222Rn’s half-life, taking into account pore water, sediment diffusion, river input, high tide input, and 226Ra support as sources, balanced against sinks from low tide output, atmospheric evasion, radioactive decay, and offshore mixing loss. Following previous studies (Burnett and Dulaiova, 2003; Zhang et al., 2016; Chen et al., 2018a), the mass balance equation is expressed as Equation 1:
Here, Fpw represents pore water-derived 222Rn input flux, Fdiff denotes sediment diffusion flux, Friv indicates river input flux, Fin denotes the input of external seawater at high tide, F226 represents the contribution from dissolved 226Ra, Fout denotes the output of internal seawater at low tide, Fatm indicates the atmosphere diffusion flux at the water–gas interface, Fdec represents the autoradioactive decay, and Fmix denotes mixing loss with low-concentration waters offshore. ΔF represents the difference between 222Rn inventory (I) in two adjacent time periods, defined as the product of 222Rn activity (Bq m−3) and water depth H (m). The unit of fluxes is Bq m−2 h−1.
Fdiff was determined from pore water equilibration experiments and calculated using Equation 2 (Martens et al., 1980; Rodellas et al., 2015).
where Ceq is the activity of 222Rn in sediment pore water, C0 is the activity in the overlying water, and φ is sediment porosity. Molecular diffusion coefficient Dm is temperature-dependent and is calculated using Equation 3 (Lambert and Burnett, 2003):
Riverine input flux (Friv) is calculated by multiplying 222Rn activity at salinity near zero and river runoff data. To ensure dimensional consistency with other terms, the product is divided by the surface area of Yingluo Bay, which can be estimated using a simple Google Maps area calculator, yielding the 222Rn flux per unit area attributable to river input.
The 222Rn flux controlled by tidal migration is calculated using Equation 4 (Zhang et al., 2016):
Here, Δt is the measurement time interval. Ht and Ht + Δt are the water depths at time t and t + Δt, respectively; and Coff represent the average activity of 222Rn in the creek water and 222Rn in offshore water, respectively; Ct is the activity of 222Rn in the creek water at each interval during continuous observation. b is the return flow factor, the portion of the water that leaves the estuary during ebb tide and returns unmixed during the next flood tide. This factor can be approximated as the ratio of average salinity of creek waters to the salinity of the offshore water (salinity of creek water at the highest tide) (Luo, 2018), and we obtain b = 0.73.
F226 from the dissolved 226Ra is calculated using Equation 5 (Wu et al., 2021):
Here, λ is the decay constant of 222Rn (0.181 d−1) and H is the water depth. CRa-226 is the activity of 226Ra.
The atmosphere diffusion flux of 222Rn at the water–gas interface (Fatm) is determined using Equation 6 (MacIntyre et al., 1995).
where k is gas exchange velocity, which varies with wind speed and is calculated using empirical relationships (Pilson, 1998), and the water–air distribution coefficient (α) is temperature-dependent and derived from the Fritz–Weigel equation (Burnett and Dulaiova, 2003). Cw and Cair represent the 222Rn activity in water and air, respectively.
Fdec is calculated using Equation 7 (Chen et al., 2018a; Luo, 2018):
We define the remaining 222Rn after correction for river input, tidal migration, atmospheric evasion, sediment diffusion, 226Ra support, and 222Rn radioactive decay as a net 222Rn flux (Fnet), which are supposed to balance the pore water flux (Fpw) and mixing loss (Fmix). To conservatively estimate the mixing losses, we selected the maximum negative value of the Fnet (the dotted line shown in Figure 2) as the mixed loss flux over the adjacent time interval (Fmix) (Burnett and Dulaiova, 2003; Lambert and Burnett, 2003).
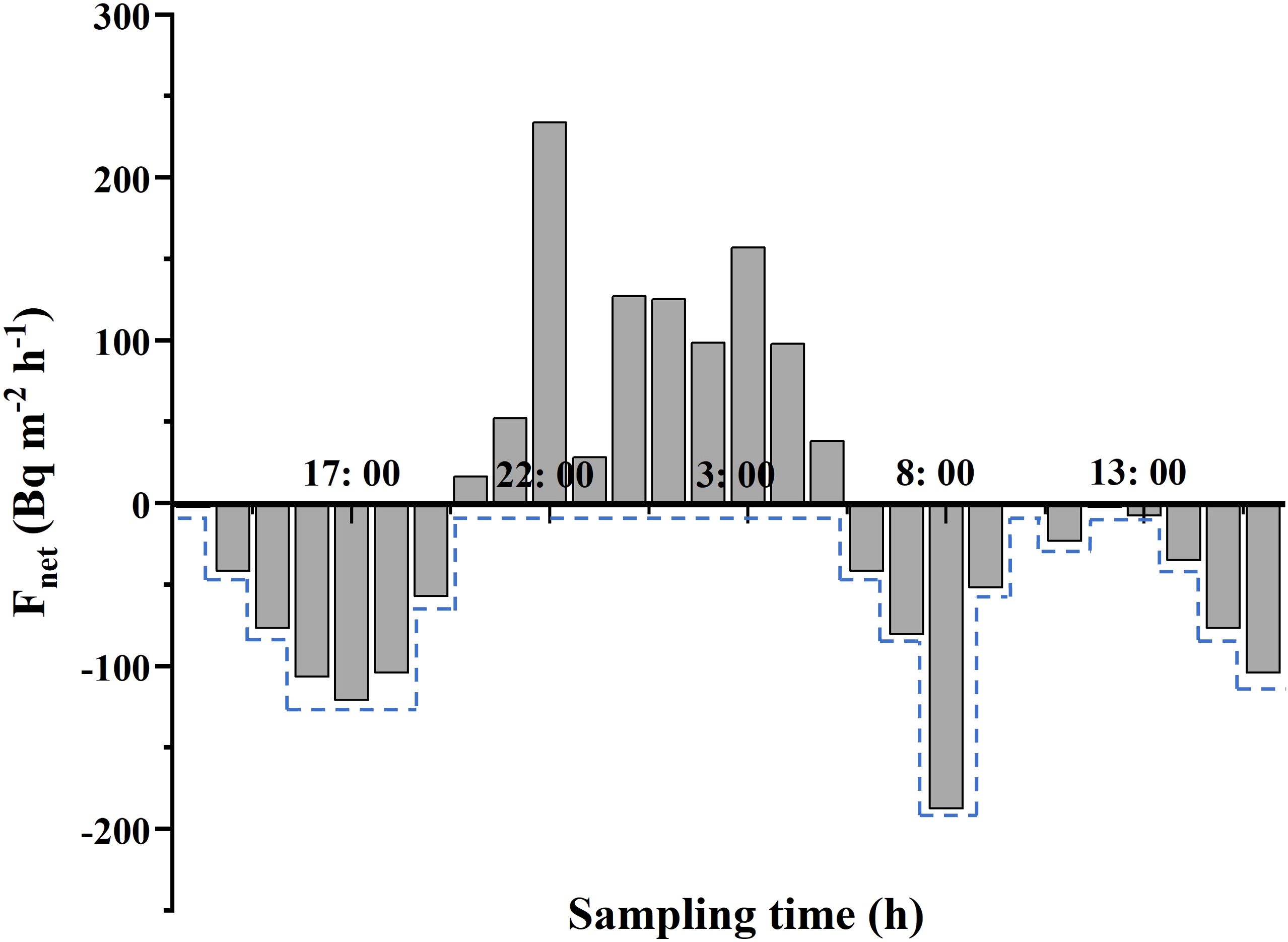
Figure 2. Net 222Rn flux (rectangles) and mixing loss of 222Rn (dotted line) versus time based on continuous 222Rn observation.
2.10 Estimation of lateral fluxes of dissolved carbon and nitrate
Given the symmetrical pattern in variations of tidal heights within a 24-h cycle in April (Figure 3a) and May (Figure 4a) 2024, we can assume that water inflow equals to water outflow over a full 24-h tidal cycle, including both the mangrove creek water in the channel and the overlying water in mangrove sediments during high tide. In this circumstance, the main source of variation is the concentration of dissolved carbon (mol h−1), which can be multiplied by the water discharge (m3 h−1), then integrated over 24 h and divided by the mangrove catchment area (m2), resulting in mmol C m−2 d−1.

Figure 3. Variations in physicochemical parameters in creek waters in a tidal cycle in April 2024. (a) Tidal height; (b) salinity; (c) pH; (d) dissolved oxygen (D.O.); (e) 222Rn; (f) nitrate; (g) sulfate; (h) DIC; (i) DOC.
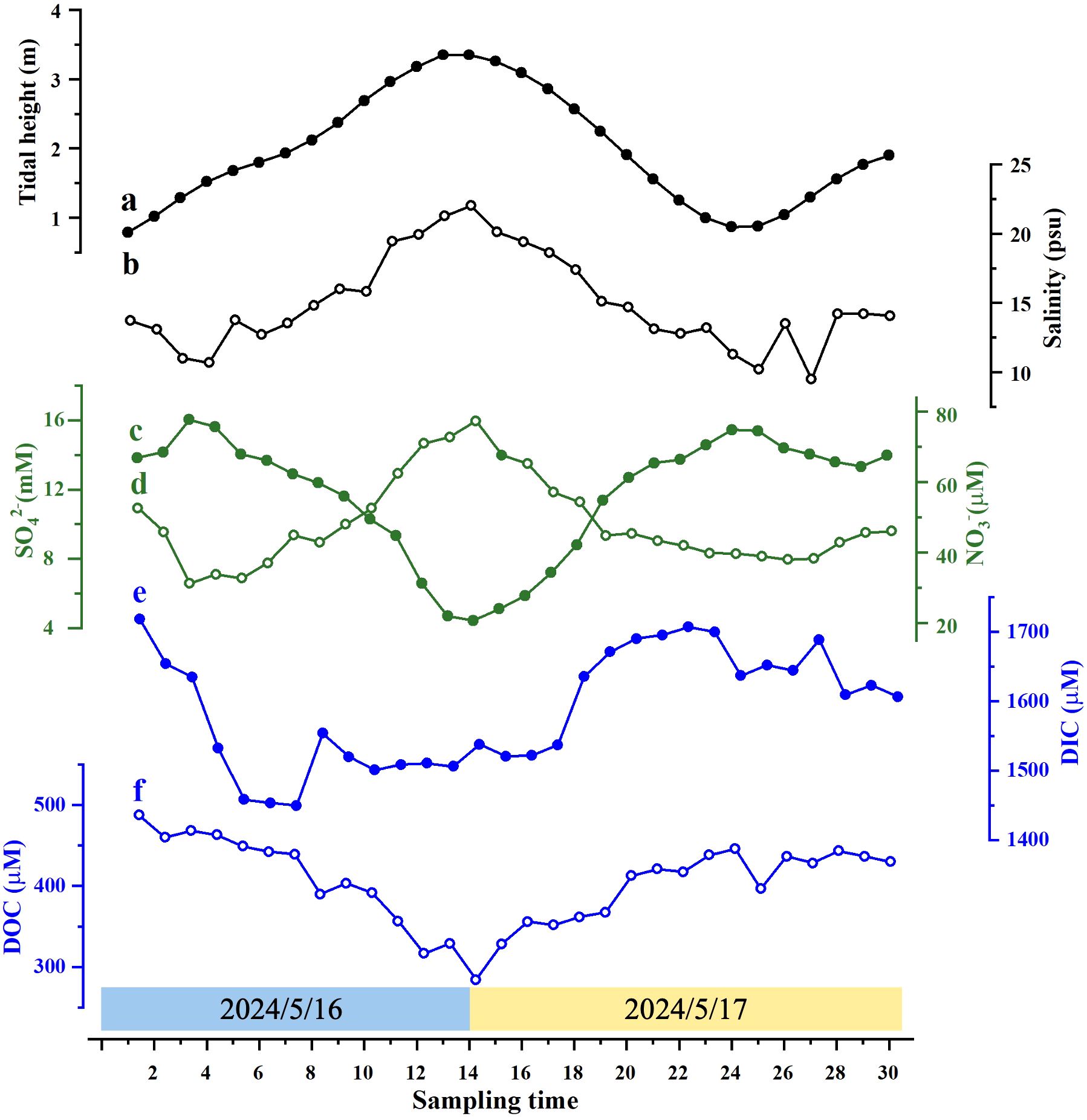
Figure 4. Variations in physicochemical parameters in creek waters in a tidal cycle in May 2024. (a) Tidal height; (b) salinity; (c) nitrate; (d) sulfate; (e) DIC; (f) DOC.
The difference between rising and receding tides over a tidal cycle represents the tidal lateral transport of dissolved carbon and nutrients (Taillardat et al., 2018). The hourly water fluxes across the mangrove–estuary boundary were calculated using an unstructured grid, Finite-Volume Community Ocean Model (FVCOM) (Chen et al., 2006). The water fluxes and time-series concentrations of dissolved carbon and nutrients at the sampling site near the mouth of the tidal creek sampling site were used to estimate fluxes across the mangrove–estuary interface using Equation 8 (Wang et al., 2021):
where F (mol m2 d−1) is net flux over a tidal cycle normalized to a 24-h tidal cycle. Positive values are efflux from mangroves, and negative values are influx to mangroves. Ci is the hourly concentration. Qi (m3 h−1) is the average hourly water discharge across the boundary. A (m2) is the catchment area of mangrove forest.
3 Results
3.1 Variations in physicochemical parameters in creek waters during tidal cycles
3.1.1 Creek waters in April 2024
The tidal height during 12–13 April 2024 ranged from 0.19 to 3.95 m (Table 1; Figure 3a), exhibiting characteristics of a spring tide. The highest salinity (28.8) and pH (7.3) temporally aligned with maximum tidal height (Table 1; Figures 3b, c), while the lowest salinity (15.4) and pH (6.8) coincided with low tide (Pearson correlation r = 0.78, p < 0.01 and r = 0.73, p < 0.01, respectively; Supplementary Figures S2A, B). D.O. displayed close correlation with tidal height (r = 0.90, p < 0.01; Supplementary Figure S2C), reaching a peak of 6.6 mg L−1 near the highest tide and declined to 3.3 mg L−1 during ebb tide (Table 1; Figure 3d). Such tidal synchronizations highlighted the pivotal role of tidal fluctuations in regulating the physicochemical parameters of creek waters.
During the tidal cycle, concentrations of nitrate ranged from 10.6 to 39.4 μM, with an average of 30.0 ± 8.1 μM (n = 28) (Table 1), and were negatively correlated with tidal levels (r = −0.80, p < 0.01; Supplementary Figure S2D), with the highest value at low tides (Figures 3a, f). Nitrite ranged from 0.1 to 3.4 μM, with an average of 1.6 ± 0.9 μM (n = 28), and ammonium from 2.0 to 8.7 μM, with an average of 5.8 ± 1.2 μM (n = 28) (Table 1). Apparently, nitrate was the predominant form of nitrogen in creek waters. Concentrations of sulfate ranged from 10.9 to 23.7 mM, with an average of 15.4 ± 4.1 mM (n = 28) (Table 1; Figure 3g), and were correlated with tide heights (r = 0.82, p < 0.01; Supplementary Figure S2E).
Concentrations of DIC ranged from 1,906 to 2,175 μM with an average of 2,001 ± 62 μM (n = 28) (Table 1). Concentrations of DOC ranged from 174 to 403 μM with an average of 308 ± 62 μM (n = 28) (Table 1), more than an order of magnitude lower than those of DIC. Both DIC and DOC had the lowest values at high tide (Figures 3h, i). Apparently, DIC was the dominant form of dissolved carbon in creek waters, as it was at least five times more abundant than DOC.
3.1.2 Creek waters in May 2024
The second sampling of creek waters took place on 16–17 May 2024, during the neap tide. The tide ranged from 0.79 to 3.35 m (Table 2; Figure 4a), showing a similar pattern to, but significantly smaller than that in April 2024. Salinity ranged from 9.5 to 22.0 (Table 2; Figure 4b) and was correlated with tidal levels (r = 0.91, p < 0.01). pH ranged from 6.8 to 7.4, and D.O. ranged from 3.6 to 7.1 mg L−1 (Table 2).
Concentrations of nitrate ranged from 20.7 to 77.7 μM, with an average of 56.7 ± 17.5 μM (n = 30) (Table 2; Figure 4c), and reached the maximum at low tide (r = −0.93, p < 0.01). They were substantially higher than those in April 2024 (Tables 1, 2). Nitrite ranged from 0.8 to 8.8 μM, with an average of 3.4 ± 1.4 μM (n = 30), slightly higher than those in April (Tables 1, 2). Ammonium varied from 0.3 to 3.7 μM, with an average of 1.8 ± 0.8 μM (n = 30), lower than those in April (Tables 1, 2). Concentrations of sulfate ranged from 6.6 to 16.0 mM (Figure 4d) and were correlated with tidal heights (r = 0.84, p < 0.01). The average concentration (10.1 ± 2.5 mM, n = 30) was approximately 34% lower than that in April 2024 (Tables 1, 2).
Concentrations of DIC ranged from 1,450 to 1,719 μM with an average of 1,589 ± 85 μM (n = 30), approximately 21% lower than those in April. However, DOC ranged from 284 to 488 μM with an average of 405 ± 51 μM (n = 30), approximately 31% higher than those in April. Both DIC and DOC reached peaks during low tides (Figures 4e, f).
3.2 Variations in physicochemical parameters in pore waters during tidal cycles
Pore waters were only collected in May 2024. Salinity ranged from 11.2 to 24.8, a much smaller amplitude than that in creek waters (Tables 2 and 3; Figures 4b, 5b).

Figure 5. Variations in physicochemical parameters in pore waters in a tidal cycle in May 2024. (a) Tidal height; (b) salinity; (c) nitrate; (d) sulfate; (e) DIC; (f) DOC.
Concentrations of nitrate in pore waters ranged from 2.0 to 8.3 μM, with an average of 4.2 ± 1.7 μM (n = 30) (Figure 5c), and those from nitrite ranged from 0.0 to 1.6 μM, with an average of 0.3 ± 0.3 μM (n = 30) (Table 3). Both concentrations of nitrate and nitrite in pore waters were less than 10% of those in creek waters collected in the same time (Tables 2, 3; Figure 6a). However, concentrations of ammonium ranged from 0.6 to 5.5 μM with an average of 2.5 ± 0.8 μM (n = 30), approximately 39% higher than those in creek waters (1.8 ± 0.8 μM, n = 30) (Tables 2, 3; Figure 6b). Concentrations of sulfate ranged from 9.8 to 14.1 mM with an average of 12.1 ± 1.0 mM (n = 30), comparable to those in creek waters in the same time (Tables 2, 3), but the amplitude of variations was substantially smaller (Figures 4d, 5d).

Figure 6. Variations in nitrate and ammonium in creek waters and pore water, and their difference in a tidal cycle in May 2024. (a) Nitrate; (b) ammonium.
Concentrations of DIC in pore waters ranged from 3,535 to 5,280 μM, with an average of 4,369 ± 461 μM (n = 30), 2.7-fold higher than those in the creek waters (Tables 2, 3). Concentrations of DOC ranged from 463 to 1,034 μM, with an average of 523 ± 101 μM (n = 30), 1.3-fold higher than those in the creek waters (Tables 2, 3). DIC was the predominant form of dissolved carbon (>80%). Neither DIC nor DOC in pore waters showed any clear correlation with tidal height (Figures 5e, f).
3.3 δ13C-DOC in tidal creek waters
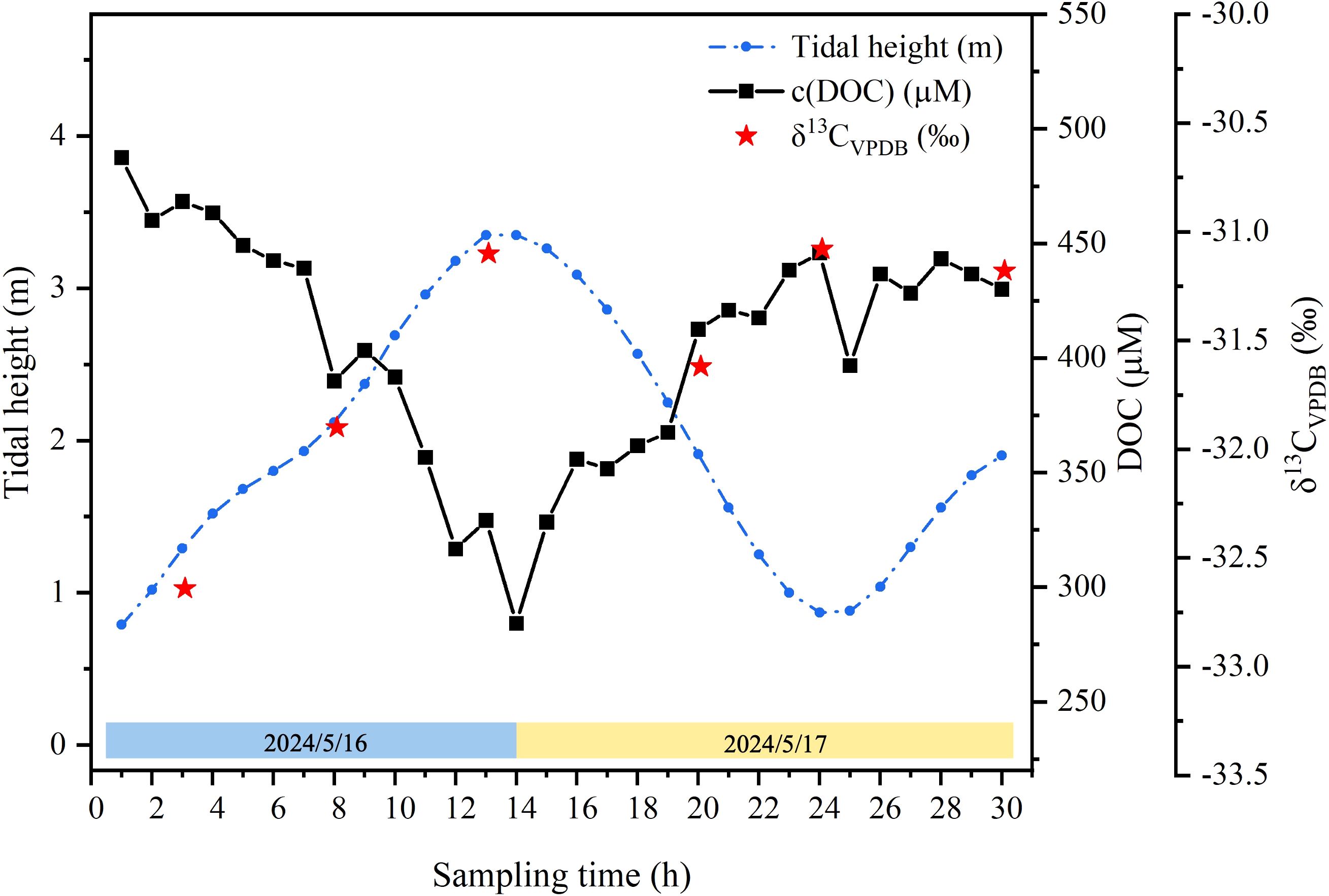
δ13C values of DOC in creek waters collected in April 2024 varied in a small range from −32.6‰ to −31.1‰ (Figure 7). They began with the lowest value −32.6‰ at the lowest tide, rapidly increasing with rising tide, and reached the maximum value −31.1‰ at the highest tide, coincident with the lowest DOC value, and then stayed more or less stable during the receding tide (Figure 7).
3.4 Δ14C and δ13C of DIC in creek waters
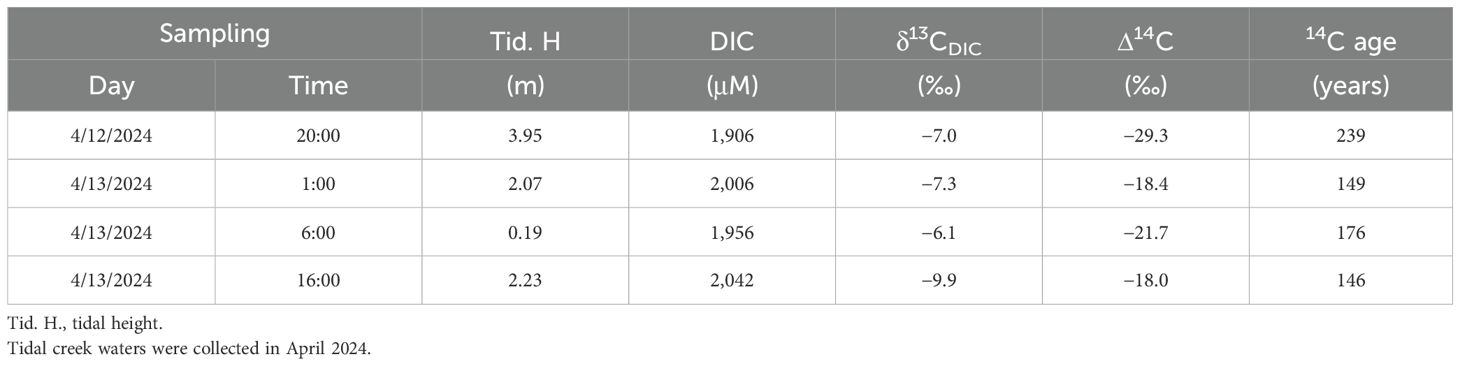
Δ14C of DIC in creek waters ranged from −29.3‰ to −18.4‰, equivalent to 239 to 146 years old (Table 4), and showed no good correlation with tidal heights. δ13C of the corresponding DIC ranged from −9.9‰ to −6.1‰, with the maximum value corresponding to the lowest tide and the minimum value corresponding to the rising tide (Table 4; Figure 8).
3.5 Quantification of pore water exchange
222Rn activities in April 2024 ranged from 100.0 to 683.0 Bq m−3, with an average of 333.7 ± 155.1 Bq m−3 (n = 28) (Table 1), and had no good correlation with tidal height (Supplementary Figure S2F). 222Rn began rising during early ebb tide and then remained more or less the same until the lowest tide at the 16th hour (Figure 3e). Then, it increased rapidly with the rising tide and reached the maximum value of 683 Bq m−3 in the middle of the rising tide, and then declined rapidly (Table 1; Figures 3a, e). The observed hysteresis pattern, i.e., the gradual 222Rn accumulation during ebb tides vs. rapid flushing during floods, is consistent with tidally driven pore water exchange processes, most likely resulting from sediment permeability constraints, as coastal aquifers required multiple tidal cycles to achieve hydraulic equilibrium (Wu et al., 2021).
The measured 226Ra activity was 9.72 ± 0.42 Bq m−3, and F226 derived from Equation 6 was 0.36 ± 0.02 Bq m−2 h−1. After incubating sediments for 1 month to establish radioactive equilibrium, pore water 222Rn activity (2,630 ± 1,198 Bq m−3) exceeded overlying seawater levels (202 ± 103 Bq m−3) by an order of magnitude (Table 5). Combined with temperature-dependent molecular diffusivity (Dm = 1.16 × 10–5 m2 h−1 at 20°C) and porosity (φ = 0.38–0.47), sediment diffusion flux (Fdiff) was calculated as 0.46 ± 0.21 Bq m−2 h−1 (Table 5). The area of Yingluo Bay is estimated approximately 1.32 × 107 m2, using a simple Google Maps area calculator. The riverine discharge data were available from a Chinese agrometeorological service website (https://wheata.cn). We averaged the river’s average runoff data from 1979 to 2024 to get 10.9 m3 s−1. The 222Rn activity at the mouth of the tidal creek was 430 ± 175 Bq m−3, thus yielding riverine input flux (Friv) as 1.28 ± 0.52 Bq m−2 h−1. Fin and Foff ranged from 5.2 to 190.4 with an average of 68.74 ± 49.11 Bq m−2 h−1, and 13.4 to 230.8 with an average of 94.42 ± 62.27 Bq m−2 h−1, respectively. The atmospheric evasion fluxes (Fatm) were calculated using the gas exchange model (MacIntyre et al., 1995), and they ranged from 0.93 to 13.31 Bq m−2 h−1, with an average of 4.68 ± 3.49 Bq m−2 h−1. Decay flux was estimated at 0.23 ± 0.11 Bq m−2 h−1. A conservative estimate was made for the range of Fmix from 2.41 to 187.61 Bq m−2 h−1, with an average value of 65.93 ± 49.11 Bq m−2 h−1 (Figure 2). The sum of Fnet and Fmix constituted the 222Rn flux contributed by pore water, which ranged from 0 to 233.64 Bq m−2 h−1, with an average value of 36.01 ± 62.10 Bq m−2 h−1.
It is worth noting that high tide input (64.3%) and sediment pore water (33.7%) emerged as the predominant 222Rn sources, surpassing inputs from sediment diffusion (0.43%), riverine (1.20%), and decay from 226Ra (0.34%) (Table 5), in agreement with a previous report in Dux Creek on Bribie Island, Moreton Bay, Australia (Davis et al., 2020). Pore water exchange rates were obtained when normalized by pore water endmember 222Rn activity, Fpw/c(Rn) = 14.2 ± 24.5 cm d−1, which was considered as the 222Rn activity of sediment pore water in the ~40-cm borehole.
Submarine groundwater discharge-derived fluxes of dissolved carbon and nitrate could be calculated by multiplying the discharge estimates by their average groundwater concentrations (Davis et al., 2020). However, in all tidally dominated systems, pore water exchange rather than fresh groundwater discharge was the source of radon to surface waters (Webb et al., 2019). Pore water exchange is a recirculation process in the intertidal mangrove sediments. Therefore, to estimate net fluxes of dissolved carbon and nitrate to surface waters, their groundwater endmembers were defined as the differences between concentrations in groundwater and surface waters (Santos et al., 2014). Negative fluxes of nitrate imply that surface water nitrate is consumed within the shallow aquifer during pore water exchange with surface waters. Uncertainties in the radon-derived groundwater exchange rate were likely propagated to fluxes of dissolved carbon and nitrate using the standard deviations of the endmembers. We obtain the net output fluxes of DIC and DOC through pore water exchange as 394.8 ± 681.1 and 16.8 ± 28.9 mmol m−2 d−1, respectively. The input flux of nitrate was estimated at 7.46 ± 12.9 mmol m−2 d−1.
3.6 Estimated boundary fluxes
The fluxes of dissolved carbon and nutrients across the mangrove–estuary boundary were estimated based on the water discharge (Qi) and the hourly concentrations (Ci) according to Equation 8.
The average lateral fluxes of DIC and DOC in April and May 2024 calculated using FVCOM were 411.6 ± 311.8 and 104.5 ± 145.7 mmol m−2 d−1, respectively, and flux of nitrate was 8.5 ± 7.6 mmol m−2 d−1.
4 Discussion
4.1 Carbon dynamics of DIC during tidal cycles
4.1.1 Outflow of DIC from pore water controlled by tidal amplitude
As the bottles were opened after sampling for filtration prior to analysis, a small amount of CO2 could have escaped during the process. However, DIC values in pore waters (3,535–5,280 µM) in May 2024 (Table 3) were generally comparable to those reported in Moreton Bay, Australia (3,767–6,426 µM; Maher et al., 2013), suggesting that such losses were insignificant. DIC values in creek waters collected at the same time (1,450–1,719 µM) (Table 2) were substantially lower than those in pore waters (Tables 2, 3). Our observed DIC values in creek waters were not particularly high, slightly lower than those in mangroves in Moreton Bay (2,000–2,800 µM; Maher et al., 2013) and in the Gulf of Carpentaria, northern Australia (2,050–2,180 µM; Sippo et al., 2016). However, they were within the range in mangrove tidal creeks in the Zhangjiang Estuary (1067–2425 µM in the wet season and 225–325 in the dry season; Yan et al., 2022) and Qinglan Bay, China (1240–3270 µM; Wu et al., 2021), and higher than those in mangrove tidal creeks near Maowei Sea, China (280 to 1280 µM; Chen et al., 2018a). It seems that DIC concentrations in mangrove tidal creeks vary significantly among different sites.
D.O. contents in pore waters (2.5–4.6 mg L−1) were significantly lower than those in creek waters (3.6–7.1 mg L−1) (Tables 2 and 3), suggesting that intense aerobic respiration near the sediment–water interface and DIC, as the product of organic matter mineralization, were enriched in pore waters (Bradbury et al., 2024). Higher concentrations of DIC in tidal creek waters occurred during ebb tide, most likely due to the outflow of pore waters rich in DIC (Tables 2, 3; Figures 4a, e), underscoring the role of pore water as a principal reservoir for mineralized carbon (Koné and Borges, 2008). However, DIC in pore waters showed no correlation with tidal levels (Figures 5a, e), as porewater samples can be highly variable due to effects from crab burrows and mangrove roots (Kristensen et al., 2022), and biogeochemical processes in sediments, such as organic matter decomposition and microbial activity, which may modulate changes in DIC in pore waters (Derrien et al., 2019).
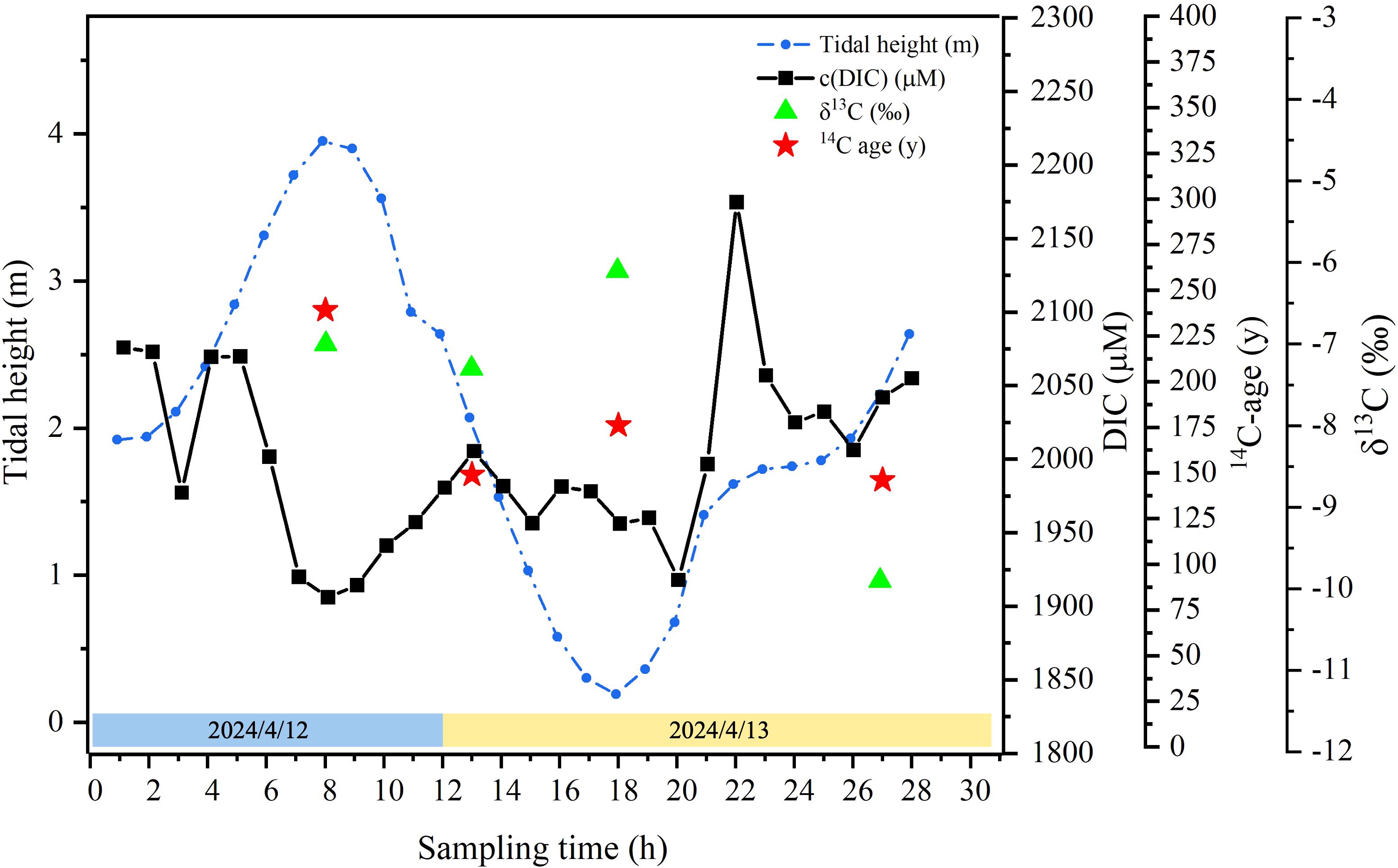
Concentrations of DIC in creek waters and tidal heights in May 2024 formed an inverse correlation (r = −0.63, p < 0.01), indicating the significant contribution of DIC from mangroves during low tides. Ebb tides facilitate the advective transport of pore water-derived DIC into the creek, a phenomenon referred to as “mangrove pump” (Stieglitz et al., 2013). However, DIC concentrations in creek waters in April 2024 varied by only 3% during the tidal cycle, in spite of larger tidal amplitude, and showed no correlation with tidal height (Table 1; Figures 3A, H). In April 2024, we started to collect creek water at a tidal height of 1.92 m. While tidal height increased to the maximum of 3.95 m, DIC actually decreased from 2,076 to 1,914 μM, forming an inverse relationship (r = −0.83, p < 0.01) (Table 1) with tidal heights, suggesting the outwelling of DIC. However, the following decline of tidal heights showed no correlation with DIC concentrations. In fact, the highest DIC concentration in creeks occurred when tidal heights were between 1.5 and 3 m (Table 1). Tidal heights larger than 3 m were accompanied by lower DIC due to the dilution of seawater from the estuary. On the other hand, tides in this region retreat very fast, and nearly all DIC produced through mineralization have already outwelled at a tidal height of 1.5 m. By the time tidal height reached the minimum, there was not much DIC from pore water exchange left in the creek so that DIC concentrations were low.
On the other hand, concentrations of DIC in creek waters in May 2024 were approximately 21% lower than those in April (Tables 1, 2). There may be several reasons for this gap. Enhanced CO2 degassing within the creek due to warmer temperatures in May likely attenuated net DIC exchange, as evidenced by the reduced flux despite comparable mineralization rates (Cabral et al., 2024a). It is also likely that longer inundation periods and elevated tidal amplitudes in April 2024 enhanced anaerobic mineralization in deeper sediment layers, thereby amplifying DIC production in pore waters (Call et al., 2015; Taillardat, et al., 2018), thus resulting in higher DIC level in creek waters.
4.1.2 Terrestrial sources of DIC
δ13C of DIC ranged from −9.9‰ to −6.1‰ during the tide cycle (Figure 8) and had no correlation with tide heights (Table 4). Such phenomenon is in contrast to the positive correlation observed in mangroves in Moreton Bay, Australia (Maher et al., 2013). Pore water exchange is usually considered as a major source of DIC and DOC to the surface waters, accounting for more than 90% of DIC and DOC exports from mangrove ecosystems via tidal pumping (Call et al., 2015). As a result, the lowest δ13C-DIC values were found during the highest DIC concentrations, indicating a negative correlation between δ13C of DIC and concentrations of DIC (r = −0.74, p < 0.05), suggesting that high DIC, enriched with CO2, typically has low δ13C-DIC signatures.
Here, we employ a simple binary δ13C mixing equation (Equation 9) to identify relative contribution from marine sources vs. terrestrial sources:
Here, f is the percentage of DIC contribution from marine sources. The δ13C endmember of terrestrial sources is hard to evaluate, as nearly all DIC measured in the core region of the ZMNNR could have had contributions from marine sources. In mangroves in Moreton Bay, Australia, δ13CDIC values of creek water at the highest tide in summer were 1.8‰ (Maher et al., 2013), exhibiting characteristics of typical marine DIC (−0.8‰ to 2.4‰) (Mackensen and Schmiedl, 2019) as the bay is next to the open ocean. However, in our study, the δ13CDIC value at the highest tide was only −7.0‰ (Table 4), indicating that the incoming seawater already includes the significant component of terrestrial derived DIC, as Yingluo Bay, which connects the ZMNNR, is surrounded by mangroves in both banks (Figure 1b), and far away from an open sea. The same scenario was also found in Can Gio mangroves, Vietnam, where δ13CDIC values were −8.6‰ and −12.6‰ at high and low tides, respectively, as this site exhibited a dense network of rivers and tidal creeks (Taillardat et al., 2018). We did not measure δ13C of DIC in pore waters. A recent study showed that δ13C values of DIC in pore waters and creek waters were −15.6‰ and −5.6‰, respectively, in the Florianópolis mangroves, Brazil (Cabral et al., 2024b). Thus, 13C-depleted DIC in pore waters is an important source that causes negative δ13C values of DIC in our tidal creeks.
We choose 1.8‰ as δ13Cmarine, and the most negative δ13CDIC in tidal creek waters, −9.9‰, as δ13Cterr. Our calculation shows that only 22% to 32% DIC in the creek were from marine sources, and the majority of DIC were from terrestrial sources. We conducted the same calculation for the above-referred mangroves. Terrestrially derived DIC could account for 90% of DIC in creeks during ebb tide in Can Gio, Vietnam (Taillardat et al., 2018), but only less than 40% during ebb tide in Moreton Bay, Australia (Maher et al., 2013). It appears that mangroves influenced by freshwater sources (such as this study and Can Gio, Vietnam) showed a higher percentage of terrestrial DIC, compared to seawater-dominated mangroves where recirculated seawater in intertidal sediments is the main DIC source. The high primary productivity in the ZMNNR produces significant amounts of litter for decomposing into DIC. The Gaoqiao River (Figure 1b) could also carry some terrestrial-derived DIC from the upper stream into mangroves. Furthermore, as the neighboring Yingluo Bay does not connect directly to an open sea and its banks are also dominated by mangroves, even incoming seawater itself also contains the component of litter-derived DIC from the banks, altogether resulting in substantially high proportions of terrestrial-derived DIC in tidal creeks in the ZMNNR.
Δ14C values of DIC in creek waters showed differences in age between high and low tides, with the older DIC at high tide, suggesting that terrestrially derived DIC values at the lowest tide (176 years) were indeed much younger than the incoming seawater’s marine reservoir 14C age in the South China Sea (>300 years; Hirabayashi et al., 2019) or the global average (400 years) (Matsumoto, 2007).
4.2 Carbon dynamics of DOC during tidal cycles
DOC concentrations in pore waters (463–1,034 µM) exceeded those in creek waters (284–488 µM) by a factor of 1.6 to 2.1 (Tables 2, 3). Similar to the case of DIC, there was no obvious correlation between pore water DOC concentrations and tidal heights (Figures 5a, f). On the other hand, DOC in creek waters was closely inversely related to tidal height in April (r = −0.71, p < 0.01) and May 2024 (r = −0.88, p < 0.01), indicating that the predominant source of DOC in creek waters was from sediment pore water.
Concentrations of DOC in creek waters were approximately 31% higher than those in April (average 405 ± 51 μM vs. 308 ± 62 μM) (Tables 1, 2), in contrast to that for DIC. The total dissolved carbon (DIC + DOC) in May 2024 was approximately 20% less than those in April 2024. It seems that longer inundation in April resulted in not only stronger mineralization, but also more complete conversion of DOC into DIC, while creek waters in May 2024 had a higher percentage of DOC, as it might be still in the early stage of mineralization (Bouillon et al., 2008).
δ13C values of DOC in selected creek waters revealed a different story from those of DIC. In the first tide cycle, δ13C of DOC increased from −32.6‰ at a tide height of 1.29 m to −31.1‰ at height of 3.35 m (r = 0.995, p < 0.01) (Table 2; Figure 7). However, the value did not change much with receding tide (Figure 7). δ13C values of DOC in tide creeks likely reflect the predominant terrestrial sources. In Moreton Bay, Australia, δ13CDOC values of creek water at the highest and lowest tide in summer were −16.5‰ and −24.3‰, respectively, while δ13C values of particulate organic matter (δ13CPOC) in creek waters varied from −25‰ to −13‰ (Maher et al., 2013), suggesting that δ13CDOC and δ13CPOC of creek waters were in the same range. Such large variations reflected the significant contribution of marine organic matter at a high tidal level, as the site is next to the open ocean. However, δ13CDOC in the Can Gio mangroves, Vietnam varied in a substantially smaller range, −26.7‰ to −25.3‰, almost in the same range as δ13CPOC, −27.1‰ to −26.5‰ (Taillardat et al., 2018). Those values were in agreement with δ13C of sediment organic matter, −27.8‰ ± 0.8‰, but slightly more enriched in 13C than mangrove leaves, −31.6‰ to −27.8‰, indicating a predominantly terrestrial source during the tidal cycle (Taillardat et al., 2018). δ13CDOC values in our study area had a similar small range to, but more depleted in 13C than those in Can Gio mangroves, Vietnam (Taillardat et al., 2018) and mangrove leaves in the study area (−30.5‰ to −27.5‰) (Zhang and Chen, 2022).
Overall, DIC accounted for 80% of the dissolved carbon in the tidal creek and 89% in pore waters in the ZMNNR, in agreement with previous reports (Taillardat et al., 2018). Both DIC and DOC in tidal creek waters were predominantly from organic matter derived from mangroves. It is most likely due to the lack of direct input of seawater to the study area from the South China Sea, as Yingluo Bay is a semi-closed bay connected only to the Beibu Gulf, and both banks are mangroves so that the DIC or DOC in the bay has substantially more components from terrestrial organic matter mineralization.
4.3 Potential main pathway for organic matter mineralization
There was no correlation between tidal levels and nitrate or sulfate concentrations in pore waters (Table 3). However, tidal levels were closely related with sulfate concentrations in creek waters in either April (r = 0.82, p < 0.01; Supplementary Figure S2E) or May 2024 (r = 0.84, p < 0.01), indicating that seawater was the predominant source of sulfate to mangrove sediments. This is also supported by the close correlation between salinity and tidal height in April (r = 0.78, p < 0.01; Supplementary Figure S2A) and May 2024 (r = 0.91, p < 0.01) (Tables 1, 2). On the other hand, tidal levels were negatively correlated with nitrate in creek waters in April (r = −0.80, p < 0.01; Supplementary Figure S2D) and May 2025 (r = −0.93, p < 0.01), indicating that nitrate in tidal creeks was predominantly from the mangroves, largely due to the discharge of untreated aquaculture effluents, local agricultural runoff, and sewage into mangroves. Nitrate concentrations during low tides in April 2024 (35–38 μM, Table 1) were significantly lower, mainly because stronger tide caused dilution by seawater from the Beibu Gulf, as indicated by higher salinities (Table 1). As a result, concentrations of nitrate and sulfate in creek waters were tightly negatively correlated both in April (r = −0.93, p < 0.01) and in May 2024 (r = −0.94, p < 0.01). Nitrate concentrations in the creek waters in May 2024 (56.7 ± 17.5 μM) were significantly higher than those in South China Sea (2.4–11.4 μM) (Lao et al., 2021), but significantly lower than those in Qinglan Bay mangrove, Hainan Island, China, where nitrate in tidal creeks were averaged at 739.6 μM (Zidan et al., 2025).
However, nitrate concentrations were substantially depleted in pore waters (4.2 ± 1.7 µM) relative to those in creek waters (Tables 2, 3; Figure 6a), indicating that 90% of nitrate infiltrated from the creek into sediment were consumed. They were most likely utilized as the electron acceptor for organic matter mineralization, which could take the form of denitrification or DNRA (Hamersley and Howes, 2005; Giblin et al., 2013). Though concentrations of ammonium in pore waters (2.5 ± 0.8 μM) were higher than those in creek waters (1.8 ± 0.8 µM) (Tables 2, 3; Figure 6b), there was less than 10% of total nitrogen in pore waters compared to that in creek waters. It is likely that denitrification caused organic matter mineralization and overall nitrogen loss in sediments in our study area, in agreement with previous reports in mangroves of the Jiulongjiang Estuary (Cao et al., 2017), Dongzhaigang, China (Fu et al., 2025), and Goa, India (Kristensen et al., 1998; Fernandes et al., 2012). However, this inference still needs to be substantiated in conjunction with functional gene or isotope tracer experiments.
Sulfate concentrations in pore waters (9.79–14.12 mM, Table 3) were almost in the same range as those in creek waters (6.58–15.96 mM, Table 2), but the differences between them at the same time (Δsulfate) varied significantly with tide levels. Pore waters were approximately 2% to 100% more enriched in sulfate when tide heights were less than 3.0 m, and depletion in sulfate by 13% to 21% occurred only when tides were close to or higher than 3 m (Tables 2, 3). It seems that consumption of sulfate, as electron acceptor, took place only during high tides, when the supply of sulfate is nearly unlimited. Microbial sulfate reduction is often considered to occur only when more energetically favorable electron acceptors, such as nitrate, have been locally depleted (Froelich et al., 1979). The abundant nitrate in overlying seawater (Tables 1, 2) makes sulfate reduction in the shallow depth of sediment hampered by thermodynamically more favorable nitrate reduction (Canfield et al., 2005). The increase in sulfate in pore waters during some intervals (Tables 2, 3) could be due to the contribution of dissimilatory sulfate reduction in which sulfur-oxidizing bacteria re-oxidize sulfide back to sulfate in pore waters during low tides (Li et al., 2021).
4.4 Lateral water exchange flux of dissolved carbon and nutrients from mangroves
4.4.1 Pore water exchange rate
Pore water exchange rate in mangroves vary substantially at different sites, ranging from 4.9 cm d−1 in Can Gio mangroves, Vietnam (Taillardat et al., 2018) to 179.0 ± 91.9 cm d−1 in a maricultural bay in Hainan Island, China (Liu et al., 2023). Our estimated pore water exchange rate, 14.2 ± 24.5 cm d−1 or 0.59 ± 1.0 cm h−1, in April 2024 in the ZMNNR, is fairly close to the global pore water exchange rate for mangroves, 0.4 cm h−1 (Cabral et al., 2024a). The rates were in accord with those in mangroves in the eastern Australia (16.3 ± 5.1 cm d−1) (Tait et al., 2016), but slightly lower than those in Maowei Sea mangroves (36.0 ± 33.1 cm d−1) (Chen et al., 2018b).
We have to admit the large uncertainty in our model as each item in the balance equation carries assumptions. Future integration of radium isotopes (e.g., 223Ra and 224Ra) to constrain mixing timescales might improve modeling (Rodellas et al., 2015). Nevertheless, our findings reveal a hidden, yet critical vector for carbon and nutrient fluxes in the largest mangrove reserve in China.
4.4.2 Pore water exchange fluxes of dissolved carbon and nitrate
The output fluxes of DIC derived from pore water exchange rate in the ZMNNR were estimated as 394.8 ± 681.1 mmol m−2 d−1, slightly higher than SGD-derived fluxes into a mangrove-dominated tropical bay (Maowei Sea) in the dry season (DIC: 250 ± 240 mmol m−2 d−1; DOC: 250 ± 230 mmol m−2 d−1) but lower than those in the wet season (DIC: 700 ± 820 mmol m−2 d−1; DOC: 310 ± 300 mmol m−2 d−1) (Chen et al., 2018a). They were higher than those in mangroves in Ishigaki Island, Japan (113.3 and 279.2 mmol m−2 d−1 in winter and summer, respectively) (Ohtsuka et al., 2020), and in Moreton Bay, Australia (250 mmol m−2 d−1) (Maher et al., 2013). On the other hand, the output fluxes of DOC in the ZMNRR (16.8 ± 28.9 mmol m−2 d−1) were similar to those in mangroves in Ishigaki Island, Japan (16.7 and 71.7 mmol m−2 d−1 in winter and summer, respectively) (Ohtsuka et al., 2020). However, the fluxes of DIC and DOC in the study area were significantly lower than those in the maricultural bay in Hainan Island, China (12.53 and 0.30 mol m−2 d−1 for DIC and DOC, respectively) (Liu et al., 2023).
Our results indicate that mangroves can be a significant contributor of DIC and DOC to coastal seas, and fluxes of DIC always constitute the predominant component of water exchange, in accord with previous studies (Call et al., 2015).
4.4.3 Outwelling fluxes of dissolved carbon derived from the FVCOM model
Using the FVCOM model, we can obtain the average net export fluxes of DIC and DOC in our study area, which were 411.6 ± 311.8 and 104.5 ± 145.7 mmol m−2 d−1, respectively. This ratio between DIC and DOC fluxes was consistent with previous studies (Maher et al., 2013; Cabral et al., 2021).
The net DIC fluxes were slightly larger than those in mangroves in Moreton Bay mangroves, Australia (~250 mmol m−2 d−1) (Maher et al., 2013). It could be due to the high sedimentation rate and high TOC content in the study area. The sedimentation rate we measured based on the 210Pb model was 0.98 cm year−1 (Supplementary Figure S1), consistent with the sedimentation rate of 0.65–1.10 cm year−1 in Yingluo Bay (Zhu et al., 2016) and was in the range of 0.93 to 1.37 cm year−1 in the northern Beibu Gulf (Gan et al., 2013). Such a high sediment rate also explains the young Δ14C age of DIC in tidal creek waters.
We did not analyze the TOC in sediments. A previous study near the study area showed that the highest TOC content in the sediment could reach as high as 98.1 g kg−1 (Liu et al., 2024). The average organic carbon content in the 0- to 50-cm soil (28.4 ± 8.8 g kg−1) was about the same range as in the sediments of Moreton Bay, Australia (Hayes et al., 2017). If we adopt 28.4 g kg−1 as the representative TOC value, and the average density of soil (2.0 g cm−3) (Lin et al., 2024), we estimate that annual burial of organic carbon in the sediment in the ZMNNR is approximately 556.8 g C m−2 year−1. This number is consistent with the global accumulated rate (>200 g C m−2 year−1) (Alongi, 2014). The total net export flux of DIC and DOC was 516.1 mmol m−2 d−1. The catchment of the Gaoqiao River within the core region mangrove is approximately 5 × 106 m2, estimated using a simple Google Maps area calculator. Accordingly, the annual total net fluxes of DIC and DOC from the core region to Yingluo Bay can be estimated at (1.13 ± 1.00) × 1010 g C year−1. The core region area of the Gaoqiao mangrove is 7,228 hectares (Zhang and Chen, 2022); therefore, the annual burial carbon is 4.02 × 1010 g C year−1. Our initial estimate would be that 28.1% ± 24.9% of total organic matter buried in the sediment in the ZMNNR was mineralized and outwelled. However, that would likely overestimate the rate of mineralization, as organic matter buried in the sediments are relatively refractory, after those labile components such as carbohydrates have been already decomposed.
An alternative way is to use the ratio of mineralized organic carbon vs. gross primary productivity (GPP). The globally annual averaged GPP of mangrove ecosystems varied between 1,971 and 2,100 g C m−2 year−1 (Sun et al., 2024). If we use the average value, 2,035 g C m−2 year−1, and core region area of 7,228 hectares (Zhang and Chen, 2022), we can get the average annual GPP at 1.47 × 1011 g C year−1. Finally, we estimate that 7.7% ± 6.8% of the GPP in the study area was mineralized and outwelled.
4.4.4 Outwelling fluxes of dissolved nitrogen derived from the FVCOM model
The flux of nitrate derived from FVCOM was 8.5 ± 7.6 mmol m−2 d−1, comparable to those in mangroves in the Oyster Channel of the Clarence River in Yamba, New South Wales, Australia (8.4 mmol m−2 d−1) (Wadnerkar et al., 2021). The nitrate concentrations in creek waters (43.8 ± 19.2 μM; Tables 1, 2) were substantially higher than those in Yingluo Bay seawater (11.4 μM for wet season), but the ammonium concentrations (3.7 ± 2.3 μM; Tables 1, 2) were slightly lower than that of seawater (6.6 μM for wet season) (Lao et al., 2021). It is most likely that the mangrove-fringed Yingluo Bay served as a source of ammonium-N to the mangroves, but as a sink of nitrate.
4.5 The role of freshwater sources
The positive correlations between salinity or sulfate concentration and tidal height (Supplementary Figures S2A, E) suggest that incoming seawater controls the large variations in physiochemical parameters, evidenced in high salinity, high pH, high D.O., and low DOC at high tide level (Figure 2). However, neither 222Rn (Supplementary Figure S2F) nor DIC (Supplementary Figure S2G) in tidal creeks in April 2024 had correlations with tidal height or salinity, suggesting that freshwater sources might play an important role in this system.
As local bedrock is mainly composed of volcanic rocks and there is no carbonate rock, freshwater from the Gaoqiao River contains insignificant DIC to tidal creeks, leading to the predominance of seawater. As a result, DIC in tidal creeks in April 2024 varied by only 3% through the tidal cycle (Table 1). However, decomposition of litter in the upper streams of the river, local agriculture, and aquaculture effluents provide a significant source of DOC to the tidal creeks, in addition to organic matter mineralization in mangrove sediments. That caused ±20% variations in DOC concentrations in tidal creeks thorough the tidal cycle (Table 2).
4.6 Ecological significance and broader implications
We have to admit that we did not employ any specific methods to verify denitrification as the predominant pathway for organic carbon mineralization in the study area. Future work would need to incorporate microbial community analysis through DNA sequencing or isotope tracing. In the study area, untreated discharge of aquaculture effluents, agriculture runoff, and sewage into mangroves result in substantially higher nitrate load to the sediments. On the one hand, mangroves sieve and remove excess nitrate (NO32-) from the system (Pang and Wang, 2021), reducing the output of nitrate to coastal habitats (Gonneea et al., 2004), and thus playing an important role in mitigating eutrophication (Fan et al., 2024; Xiao et al., 2018). On the other hand, nitrate addition significantly enhances mineralization of organic matter in sediments, even at sediment depths typically considered resistant to decomposition, largely driven by stimulated denitrification (Bulseco et al., 2019). As a result, long-term blue carbon capacity is likely further reduced with increasing nitrate load from anthropogenic activities. We call for improved management of discharges into mangroves and integrated strategies that account for the interplay between nitrogen loading and carbon dynamics in coastal ecosystems.
5 Conclusion
We conducted a comprehensive study on temporal variations in DIC, DOC, nitrate, and sulfate in creek and pore waters in response to tidal cycles, and quantification of the exchange fluxes of DIC, DOC, and nitrate in the largest mangroves in China. Nitrate concentrations in tidal creek waters are inversely correlated with tidal heights, but nitrate in pore waters is always depleted, suggesting its source from eutrophication in mangroves. Mineralized organic matter were mainly from terrestrial sources, evidenced in depleted 13C in both DIC and DOC in tidal creeks, but no aged organic matter was involved with mineralization. Based on an FVCOM model, the “outwelling” fluxes of DIC and DOC from the core region of the ZMNNR to the neighboring Yingluo Bay were 411.6 ± 311.8 and 104.5 ± 145.7 mmol m−2 d−1, respectively, equivalent to 7.7% ± 6.8% of annual gross primary productivity in the ZMNNR.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YX: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. ZZ: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China under Grant No. 42376042 (ZZ).
Acknowledgments
We are indebted to three reviewers for their insightful comments and constructive criticisms, which significantly helped improve our manuscript. We are heartily obliged to Dr. Xiaodong Miao and Yafei Wei for providing radiocarbon analyses, and Bin Wang for the assistance in analyzing dissolved inorganic carbon. We thank the Administration of the Zhanjiang Mangrove National Nature Reserve and Shunxi Liu and Yongtian Chen for assistance in fieldwork.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1590259/full#supplementary-material
References
Alongi D. (2009). The energetics of mangrove forests (Dordrecht: Springer Science & Business Media). 1–85.doi: 10.1007/978-1-4020-4271-3
Alongi D. M. (2014). Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 6, 195–219. doi: 10.1146/annurev-marine-010213-135020
Bouillon S., Borges A. V., Castañeda-Moya E., Diele K., Dittmar T., Duke N. C., et al. (2008). Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochem. Cycles. 22, GB2013. doi: 10.1029/2007GB003052
Bradbury H. J., Thomas N. C., Mleneck-Vautravers M., Hodell D. A. (2024). Revisiting the relationship between the pore water carbon isotope gradient and bottom water oxygen concentrations. Geochim. Cosmochim. Acta 366, 84–94. doi: 10.1016/j.gca.2023.12.011
Bulseco A. N., Giblin A. E., Tucker J., Murphy A. E., Sanderman J., Hiller-Bittrolff K., et al. (2019). Nitrate addition stimulates microbial decomposition of organic matter in salt marsh sediments. Global. Change Biol. 25, 10. doi: 10.1111/gcb.14726
Burnett W. C., Dulaiova H. (2003). Estimating the dynamics of groundwater input into the coastal zone via continuous radon-222 measurements. J. Environ. Radioact. 69, 21–35. doi: 10.1016/S0265-931X(03)00084-5
Burnett W. C., Peterson R. N., Santos I. R., Hicks R. W. (2010). Use of automated radon measurements for rapid assessment of groundwater flow into Florida streams. J. Hydrol. 380, 298–304. doi: 10.1016/j.jhydrol.2009.11.005
Cable J. E., Burnett W. C., Chanton J. P., Weatherly G. L. (1996). Estimating groundwater discharge into the northeastern Gulf of Mexico using radon-222. Earth Planet. Sci. Lett. 144, 591–604. doi: 10.1016/S0012-821X(96)00173-2
Cabral A., Dittmar T., Call M., Scholten J., de Rezende C. E., Asp N., et al. (2021). Carbon and alkalinity outwelling across the groundwater-creek-shelf continuum off Amazonian mangroves. Limnol. Oceanogr. Lett. 6, 369–378. doi: 10.1002/lol2.10210
Cabral A., Reithmaier G. M. S., Yau Y. Y. Y., Cotovicz L. C. Jr., Barreira J., Viana B., et al. (2024b). Large porewater-derived carbon outwelling across mangrove seascapes revealed by radium isotopes. J. Geophys. Res. Oceans 129, e2024JC021319. doi: 10.1029/2024JC021319
Cabral A., Yau Y. Y. Y., Reithmaier G. M. S., Cotovicz L. C., Barreira J., Broström G., et al. (2024a). Tidally driven porewater exchange and diel cycles control CO2 fluxes in mangroves on local and global scales. Geochim. Cosmochim. Acta 374, 121–135. doi: 10.1016/j.gca.2024.04.020
Call M., Maher D. T., Santos I. R., Ruiz-Halpern S., Mangion P., Sanders C. J., et al. (2015). Spatial and temporal variability of carbon dioxide and methane fluxes over semi-diurnal and spring-neap-spring timescales in a mangrove creek. Geochim. Cosmochim. Acta 150, 211–225. doi: 10.1016/j.gca.2014.11.023
Canfield D. E., Kristensen E., Thamdrup B. (2005). Aquatic geomicrobiology. Adv. Mar. Biol. 48, 1–599. doi: 10.1016/s0065-2881(05)48017-7
Cao W., Guan Q., Li Y., Wang M., Liu B. (2017). The contribution of denitrification and anaerobic ammonium oxidation to N2 production in mangrove sediments in Southeast China. J. Soils Sediment. 17, 1767–1776. doi: 10.1007/s11368-017-1653-0
Chen C., Beardsley R., Cowles G. (2006). An unstructured grid, finite-Volume coastal ocean model (FVCOM) system. Oceanography 19, 78–89. doi: 10.5670/OCEANOG.2006.92
Chen X., Lao Y., Wang J., Du J., Liang M., Yang B. (2018b). Submarine groundwater-borne nutrients in a tropical bay (Maowei Sea, China) and their impacts on the oyster aquaculture. Geochem. Geophy. Geosy. 19, 932–951. doi: 10.1002/2017GC007330
Chen X., Zhang F., Lao Y., Wang X., Du J., Santos I. R. (2018a). Submarine groundwater discharge-derived carbon fluxes in mangroves: an important component of blue carbon budgets? J. Geophys. Res. Oceans 123, 6962–6979. doi: 10.1029/2018JC014448
Corbett D., Burnett W., Cable J. E., Clark S. B. (1998). A multiple approach to the determination of radon fluxes from sediments. J. Radioanal. Nucl. Chem. 236, 247–252. doi: 10.1007/bf02386351
Davis K., Santos I. R., Perkins A. K., Webb J. R., Gleeson J. (2020). Altered groundwater discharge and associated carbon fluxes in a wetland-drained coastal canal. Estuar. Coast. Shelf Sci. 235, 106567. doi: 10.1016/j.ecss.2019.106567
Derrien M., Shin K.-H., Hur J. (2019). Biodegradation-induced signatures in sediment pore water dissolved organic matter: Implications from artificial sediments composed of two contrasting sources. Sci. Total Environ. 694, 133714. doi: 10.1016/j.scitotenv.2019.133714
Dickson A. G., Sabine C., Christian J. (2007). Guide to best practices for ocean CO2 measurements. PICES Special Publ. 3, 191. doi: 10.25607/OBP-1342
Faber P. A., Evrard V., Woodland R. J., Cartwright I. C., Cook P. L. (2014). Porewater exchange driven by tidal pumping causes alkalinity export in two intertidal inlets. Limnol. Oceanogr. 59, 1749–1763. doi: 10.4319/lo.2014.59.5.1749
Fan Y., Zhou Z., Liu F., Qian L., Yu X., Huang F., et al. (2024). The vertical partitioning between denitrification and dissimilatory nitrate reduction to ammonium of coastal mangrove sediment microbiomes. Water. Res. 262, 122113. doi: 10.1016/j.watres.2024.122113
Fernandes S. O., Michotey V. D., Guasco S., Bonin P. C., Bharathi P. A. L. (2012). Denitrification prevails over anammox in tropical mangrove sediments (Goa, India). Mar. Environ. Res. 74, 9–19. doi: 10.1016/j.marenvres.2011.11.008
Froelich P. N., Klinkhammer G. P., Bender L. L., Luedtke M. L., Heath G. R., Cullen D., et al. (1979). Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: sub-oxic diagenesis. Geochim. Cosmochim. Acta 43, 1075–1090. doi: 10.1016/0016-7037(79)90095-4
Fu C., Shen Z., Tang S., Li F., Quan X., Wang Y., et al. (2025). Tidal-driven N2O emission is a stronger resister than CH4 to offset annual carbon sequestration in mangrove ecosystems. Sci. Total Environ. 964, 178568. doi: 10.1016/j.scitotenv.2025.178568
Galhardi J. A., Bonotto D. M. (2017). Radionuclides (222Rn, 226Ra, 234U, and 238U) release in natural waters affected by coal mining activities in southern Brazil. Water Air Soil pollut. 228, 207. doi: 10.1007/s11270-017-3381-x
Gan H., Lin J., Liang K., Xia Z. (2013). Selected trace metals (As, Cd and Hg) distribution and contamination in the coastal wetland sediment of the northern Beibu gulf, South China Sea. Mar. pollut. Bull. 66, 252–258. doi: 10.1016/j.marpolbul.2012.09.020
Giblin A. E., Tobias C. R., Song B., Weston N., Banta G. T., Rivera-Monroy V. (2013). The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26, 124–131. doi: 10.5670/oceanog.2013.54
Gonneea M. E., Paytan A., Herrera-Silveira J. A. (2004). Tracing organic matter sources and carbon burial in mangrove sediments over the past 160 years. Estuar. Coast. Shelf Sci. 61, 211–227. doi: 10.1016/j.ecss.2004.04.015
Hamersley M. R., Howes B. L. (2005). Coupled nitrification-denitrification measured in situ in a Spartina alterniflora marsh with a 15NH4+ tracer. Mar. Ecol. Prog. Ser. 299, 123–135. doi: 10.3354/meps299123
Hayes M. A., Jesse A., Hawke B., Baldock J., Tabet B., Lockington D., et al. (2017). Dynamics of sediment carbon stocks across intertidal wetland habitats of Moreton Bay, Australia. Global Change Biol. 23, 4222–4234. doi: 10.1111/gcb.13722
Hirabayashi S., Yokoyama Y., Suzuki A., Esat T., Miyairi Y., Aze T., et al. (2019). Local marine reservoir age variability at Luzon Strait in the South China Sea during the Holocene. Nucl. Instrum. Meth. B. 455, 171–177. doi: 10.1016/j.nimb.2018.12.001
Jørgensen B. B. (1982). Ecology of the bacteria of the sulphur cycle with special reference to anoxic-oxic interface environments. Philos. Trans. R. Soc Lond. B 298, 543–561. doi: 10.1098/rstb.1982.0096
Karl D. M., Letelier R. M. (2008). Nitrogen fixation-enhanced carbon sequestration in low nitrate, low chlorophyll seascapes. Mar. Ecol. Prog. Ser. 364, 257–268. doi: 10.3354/meps07547
Kelleway J. J., Saintilan N., Macreadie P. I., Ralph P. J. (2016). Sedimentary factors are key predictors of carbon storage in SE Australian saltmarshes. Ecosystems 19, 865–880. doi: 10.1007/s10021-016-9972-3
Koné Y. J., Borges A. V. (2008). Dissolved inorganic carbon dynamics in the waters surrounding forested mangroves of the Ca Mau Province (Vietnam). Estuar. Coast. Shelf Sci. 77, 409–421. doi: 10.1016/j.ecss.2007.10.001
Kristensen E., Jensen M. H., Banta G. T., Hansen K., Holmer M., King G. M. (1998). Transformation and transport of inorganic nitrogen in sediments of a southeast Asian mangrove forest. Aquat. Microb. Ecol. 15, 165–175. doi: 10.3354/ame015165
Kristensen E., Valdemarsen T., Moraes P. C., Güth A. Z., Sumida P. Y. G., Quintana C. O. (2022). Pneumatophores and crab burrows increase CO2 and CH4 emission from sediments in two Brazilian fringe mangrove forests. Mar. Ecol. Prog. Ser. 698, 29–39. doi: 10.3354/meps14153
Lambert M. J., Burnett W. C. (2003). Submarine groundwater discharge estimates at a Florida coastal site based on continuous radon measurements. Biogeochemistry 66, 55–73. doi: 10.1023/B:BIOG.0000006057.63478.fa
Lao Q., Liu G., Shen Y., Su Q., Lei X. (2021). Biogeochemical processes and eutrophication status of nutrients in the northern Beibu Gulf, South China. J. Earth Syst. Sci. 130, 199. doi: 10.1007/s12040-021-01706-y
Laughlin R. J., Stevens R. J., Zhuo S. (1997). Determining nitrogen-15 in ammonium by producing nitrous oxide. Soil Sci. Soc Am. J. 61, 462–465. doi: 10.2136/sssaj1997.03615995006100020013x
Li M., Fang A., Yu X., Zhang K., He Z., Wang C., et al. (2021). Microbially-driven sulfur cycling microbial communities in different mangrove sediments. Chemosphere 273, 128597. doi: 10.1016/j.chemosphere.2020.128597
Lin J., Tian Y., Zhang Y., Bai X., Zhang Q., Tao J., et al. (2024). Characteristics of spatial distribution of soil organic carbon in mangrove wetlands at the estuary of Maoling River, Beibu Gulf. Mar. Environ. Sci. 43, 339–347. doi: 10.12111/j.mes.2023-x-0117
Liu T., Bao K., Chen M., Neupane B., Gao C., Zaccone C. (2024). Human activity has increasingly affected recent carbon accumulation in Zhanjiang mangrove wetland, South China. iScience 27, 109038. doi: 10.1016/j.isci.2024.109038
Liu J., Chen Y., Wang Y., Du M., Wu Z. (2023). Greenhouse gases emissions and dissolved carbon export affected by submarine groundwater discharge in a maricultural bay, Hainan Island, China. Sci. Total Environ. 857, 159665. doi: 10.1016/j.scitotenv.2022.159665
Luo H. (2018). Study of submarine groundwater discharge by Ra and its associated nutrient fluxes into the Qinzhou Bay, China (East China Normal University). Available at: https://kns.cnki.net/kcms2/article/abstract?v=6h6U53PWxNRfvU1x2mA28hePy8DzW8cOQnZzYNX_jhSXCmEMkwwBFH_6r9dRD7WVU9U4nEGSiOc86Bi4Clk1Myp00P8xzsu7qiPtUAjqnpxY3yHxc0I0FYBcbNYAeHXVRHfWjGqZPcTzK7gEAjL2f77hH4Xhm4X68M9c6HABsaBsUArASz3fgsBGWE1EhDTx1l5cRcSTWxU=&unip (Accessed December 16, 2018).
MacIntyre S., Wanninkhof R., Chanton J. P. (1995). “Trace gas exchange across the air-sea interface in freshwater and coastal marine environments,” in Biogenic trace gases: measuring emissions from soil and water. Eds. Matson P. A., Harriss R. C. (Oxford, UK: Blackwell Science Ltd), 52–97.
Mackensen A., Schmiedl G. (2019). Stable carbon isotopes in paleoceanography: atmosphere, oceans, and sediments. Earth Sci. Rev. 197, 102893. doi: 10.1016/j.earscirev.2019.102893
Maher D. T., Call M., Santos I. R., Sanders C. J. (2018). Beyond burial: Lateral exchange is a significant atmospheric carbon sink in mangrove forests. Biol. Lett. 14, 20180200. doi: 10.1098/rsbl.2018.0200
Maher D. T., Santos I. R., Golsby-Smith L., Gleeson J., Eyre B. D. (2013). Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnol. Oceanogr. 58, 475–488. doi: 10.4319/lo.2013.58.2.0475
Martens C. S., Kipphut G., Klump J. V. (1980). Sediment-water chemical exchange in the coastal zone traced by in situ Radon-222 flux measurements. Science 208, 285–288. doi: 10.1126/science.208.4441.285
Matsumoto K. (2007). Radiocarbon-based circulation age of the world oceans. J. Geophys. Res. 112, C09004. doi: 10.1029/2007JC004095
McLeod E., Chmura G. L., Bouillon S., Salm R., Bjork M., Duarte C. M., et al. (2011). A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560. doi: 10.1890/110004
Middelburg J. J., Soetaert K., Hagens M. (2020). Ocean alkalinity, buffering and biogeochemical processes. Rev. Geophys. 58, e2019RG000681. doi: 10.1029/2019RG000681
Moorcroft M. J., Davis J., Compton R. G. (2001). Detection and determination of nitrate and nitrite: a review. Talanta 54, 785–803. doi: 10.1016/S0039-9140(01)00323-X
Moore W. (1976). Sampling 228Ra in the deep ocean. Deep Sea Res. Oceanogr. Abstr. 23, 647–651. doi: 10.1016/0011-7471(76)90007-3
Odum E. P. (1968). A research challenge: evaluating the productivity of coastal and estuarine water. In: Proceedings of the Second Sea Grant Conference, 63–64.
Ohtsuka T., Onishi T., Yoshitake S., Tomotsune M., Kida M., Iimura Y., et al. (2020). Lateral export of dissolved inorganic and organic carbon from a small mangrove estuary with tidal fluctuation. Forests 11, 1041. doi: 10.3390/f11101041
Pang Y., Wang J. (2021). Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 794, 148699. doi: 10.1016/j.scitotenv.2021.148699
Peterson R. N., Burnett W. C., Dimova N., Santos I. R. (2009). Comparison of measurement methods for radium-226 on manganese-fiber. Limnol. Oceanogr. 54, 890–904. doi: 10.4319/lom.2009.7.196
Pilson M. E. Q. (1998). An introduction to the chemistry of the sea (New Jersey: Prentice Hall). doi: 10.1017/CBO9781139047203
Rodellas V., Garcia-Orellana J., Masqué P., Feldman M., Weinstein Y. (2015). Submarine groundwater discharge as a major source of nutrients to the Mediterranean Sea. Proc. Natl. Acad. Sci. 112, 3926–3930. doi: 10.1073/pnas.1419049112
Sadat-Noori M., Santos I. R., Tait D. R., Reading M. J., Sanders C. J. (2017). High porewater exchange in a mangrove-dominated estuary revealed from short-lived radium isotopes. J. Hydrol. 553, 188–198. doi: 10.1016/j.jhydrol.2017.07.058
Santos I. R., Beck M., Brumsack H. J., Maher D. T., Dittmar T., Waska H., et al. (2015). Porewater exchange as a driver of carbon dynamics across a terrestrial-marine transect: Insights from coupled 222Rn and pCO2 observations in the German Wadden Sea. Mar. Chem. 171, 10–20. doi: 10.1016/j.marchem.2015.02.005
Santos I. R., Bryan K. R., Pilditch C. A., Tait D. R. (2014). Influence of porewater exchange on nutrient dynamics in two New Zealand estuarine intertidal flats. Mar. Chem. 167, 57–70. doi: 10.1016/j.marchem.2014.04.006
Santos I. R., Burdige D. J., Jennerjahn T. C., Bouillon S., Cabral A., Serrano O., et al. (2021). The renaissance of Odum’s outwelling hypothesis in ‘Blue Carbon’ science. Estuar. Coast. Shelf Sci. 255, 107361. doi: 10.1016/j.ecss.2021.107361
Sippo J. Z., Maher D. T., Tait D. R., Holloway C., Santos I. R. (2016). Are mangroves drivers or buffers of coastal acidification? Insights from alkalinity and dissolved inorganic carbon export estimates across a latitudinal transect. Glob. Biogeochem. Cycles 30, 753–766. doi: 10.1002/2015GB005324
Stieglitz T. C., Clark J. F., Hancock G. J. (2013). The mangrove pump: The tidal flushing of animal burrows in a tropical mangrove forest determined from radionuclide budgets. Geochim. Cosmochim. Acta 102, 12–22. doi: 10.1016/j.gca.2012.10.033
Sun Z., An Y., Kong J., Zhao J., Cui W., Zhang T., et al. (2024). Exploring the spatio-temporal patterns of global mangrove gross primary production and quantifying the factors affecting its estimation. Sci. Total Environ. 908, 168262. doi: 10.1016/j.scitotenv.2023.168262
Swarzenski P. W. (2007). U/Th series radionuclides as coastal groundwater tracers. Chem. Rev. 107, 663–674. doi: 10.1021/cr0503761
Swarzenski P. W., Reich C. D., Spechler R. M., Kindinger J. L., Moore W. S. (2001). Using multiple geochemical tracers to characterize the hydrogeology of the submarine spring off Crescent Beach, Florida. Chem. Geol. 179, 187–202. doi: 10.1016/S0009-2541(01)00322-9
Taillardat P., Ziegler A. D., Friess D. A., Widory D., Van V. T., David F., Nguyen T. (2018). Carbon dynamics and inconstant porewater input in a mangrove tidal creek over contrasting seasons and tidal amplitudes. N. Marchand C. Geochim. Cosmochim. Acta 237, 32–48. doi: 10.1016/j.gca.2018.06.012
Tait D. R., Santos I. R., Maher D. T., Macklin P. A. (2016). Mangrove pore water exchange across a latitudinal gradient. Geophys. Res. Lett. 43, 3334–3341. doi: 10.1002/2016GL068289
Tamborski J. J., Eagle M., Kurylyk B. L., Kroeger K. D., Wang Z. A., Henderson P., et al. (2021). Porewater exchange-driven inorganic carbon export from intertidal salt marshes. Limnol. Oceanogr. 66, 1774–1792. doi: 10.1002/lno.11721
Wacker L., Christl M., Synal H. A. (2010). Bats: a new tool for AMS data reduction. Nucl. Instrum. Meth. B. 268, 976–979. doi: 10.1016/j.nimb.2009.10.078
Wacker L., Fahrni S. M., Hajdas I., Molnar M., Synal H. A., Szidat S., et al. (2013). A versatile gas interface for routine radiocarbon analysis with a gas ion source. Nucl. Instrum. Meth. B. 294, 315–319. doi: 10.1016/j.nimb.2012.02.009
Wadnerkar P. D., Batsaikhan B., Conrad S. R., Davis K., Correa R. E., Holloway C., et al. (2021). Contrasting radium-derived groundwater exchange and nutrient lateral fluxes in a natural mangrove versus an artificial canal. Estuar. Coast. 44, 123–136. doi: 10.1007/s12237-020-00778-1
Wang J., Beusen A., Liu X., Bouwman A. F. (2019). Aquaculture production is a large, spatially concentrated source of nutrients in Chinese freshwater and coastal seas. Environ. Sci. Technol. 54, 1464–1474. doi: 10.1021/acs.est.9b03340
Wang F., Cheng P., Chen N., Kuo Y. M. (2021). Tidal driven nutrient exchange between mangroves and estuary reveals a dynamic source-sink pattern. Chemosphere 270, 128665. doi: 10.1016/j.chemosphere.2020.128665
Webb J. R., Santos I. R., Maher D. T., Tait D. R., Cyronak T., Sadat-Noori M., et al. (2019). Groundwater as a source of dissolved organic matter to coastal waters: Insights from radon and CDOM observations in 12 shallow coastal systems. Limnol Oceanogr. 64, 182–196. doi: 10.1002/lno.11028
Wu Z. J., Zhu H. N., Tang D. H., Wang Y. Q., Zidan A., Cui Z. G. (2021). Submarine groundwater discharge as a significant export of dissolved inorganic carbon from a mangrove tidal creek to Qinglan Bay (Hainan Island, China). Cont. Shelf Res. 223, 104451. doi: 10.1016/j.csr.2021.104451
Xiao K., Wu J., Li H., Hong Y., Wilson A. M., Jiao J. J., et al. (2018). Nitrogen fate in a subtropical mangrove swamp: Potential association with seawater-groundwater exchange. Sci. Total Environ. 635, 586–597. doi: 10.1016/13j.scitotenv.2018.04.143
Yan R., Feng J., Wang Y., Fu L., Luo X., Niu L., et al. (2022). Distribution, sources, and biogeochemistry of carbon pools (DIC, DOC, and POC) in the mangrove-fringed zhangjiang estuary, China. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.909839
Zhang Z., Chen S. (2022). Carbon sources and trophic levels in the food web of the largest mangrove reserve of China. Cont. Shelf Res. 248, 104657. doi: 10.1016/j.csr.2022.104854
Zhang Y., Li H., Wang X., Wang X., Zheng C., Wang C., et al. (2016). Estimation of submarine groundwater discharge and associated nutrient fluxes in eastern Laizhou Bay, China using 222Rn. J. Hydrol. 533, 103–113. doi: 10.1016/j.jhydrol.2015.11.027
Zhu Y., Zhao F., Guo J., Wu G., Lin G. (2016). Below-ground organic carbon distribution and burial characteristics of the Gaoqiao mangrove area in Zhanjiang, Guangdong, Southern China. Acta Ecologica Sin. 36, 7841–7849. doi: 10.5846/stxb201511102276
Keywords: mangrove sediments, organic matter mineralization, dissolved inorganic carbon, pore water exchange, outwelling fluxes, nitrate reduction
Citation: Xu Y and Zhang Z (2025) Tidally driven outwelling of dissolved carbon and nitrate from the largest mangroves of China. Front. Mar. Sci. 12:1590259. doi: 10.3389/fmars.2025.1590259
Received: 09 March 2025; Accepted: 17 April 2025;
Published: 14 May 2025.
Edited by:
Yunping Xu, Shanghai Ocean University, ChinaReviewed by:
Alex Cabral, Trinity College Dublin, IrelandLei Xing, Ocean University of China, China
Qiangqiang Zhong, State Oceanic Administration, China
Copyright © 2025 Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaohui Zhang, emhhb2h1aV96aGFuZ0B6anUuZWR1LmNu
‡ORCID: Zhaohui Zhang, orcid.org/0000-0001-6316-326X
 Yifei Xu
Yifei Xu Zhaohui Zhang
Zhaohui Zhang