Abstract
Introduction:
Artificial substrates are increasingly employed in marine ecosystems to support benthic communities by providing habitat and enhancing biodiversity, particularly in areas where natural substrates are limited. Understanding the ecological role of these structures is essential for conservation and management, especially in regions undergoing industrial development, such as offshore oil and gas areas.
Materials and methods:
To investigate the vertical distribution of mobile epifaunal assemblages, stainless-steel settlement plates were deployed across depths ranging from 10 to 44 meters for a duration of 13 months. The study was conducted between the Al Shaheen Oil Platforms and nearby offshore natural reefs within the Qatari Exclusive Economic Zone (EEZ).
Results:
The survey recorded a total of 2,302 individuals from 42 operational taxonomic units (OTUs), including the first documented presence of Galathea sp. in Qatari waters. Distinct patterns of species distribution were observed, with polyclads and sipunculids predominantly occurring in shallow waters, while deeper sections showed reduced abundance, diversity, and biomass. Although no significant horizontal patterns were detected between reef sites, strong ecological connectivity was evident, indicating that the artificial substrates acted as effective "stepping stones" for mobile fauna.
Discussion:
The dominant functional groups included surface deposit feeders (sipunculids), carnivorous flatworms, and polychaetes. Notable depth-related associations were found, such as sipunculids and flatworms with sessile bivalves in shallow areas, and crabs with dead barnacles at deeper depths. These findings provide valuable baseline data on mobile benthic communities inhabiting offshore artificial reefs and highlight their role in promoting biodiversity and connectivity between natural reef habitats. The study also offers important implications for the design, commissioning, and decommissioning of oil and gas infrastructure, with relevance to reef restoration and fisheries enhancement strategies in the region.
Introduction
Artificial structures have been introduced into marine environments for purposes like coastal protection, fisheries enhancement, or oil and gas exploration, with their use dating back to ancient times, such as the Persians deploying artificial reefs to block pirates (Williams, 2006). These structures, such as oil platforms and offshore wind farms, provide hard substrates that support diverse marine communities and contributing to ecosystem restoration and fisheries improvement (Friedlander et al., 2014; Page et al., 2009). The importance of artificial reefs is heightened by the global decline of coral reefs, which, despite covering only 0.2% of the marine environment, host nearly one-third of all marine species and provide critical services (Veron et al., 2009). Coral reefs face significant threats from overfishing (Shantz et al., 2020), pollution (Li et al., 2021), invasive species (Peller and Altermatt, 2024), and climate-related stressors (e.g. Bouwmeester et al., 2020), leading to widespread degradation, as seen in the 2024 Great Barrier Reef bleaching event (Reimer et al., 2024). Meanwhile, artificial reefs increasingly substitute for degraded natural reefs, providing habitat that supports increased biomass, as shown by studies like Polovina and Sakai (1989) in Japan, where artificial reefs boosted the Pacific giant octopus catch. This highlights that fish populations are often habitat-constrained, affecting food availability, reproduction, and predation dynamics (Macreadie et al., 2011). Research on artificial reefs has evolved from focusing on colonization to examining their role in ecosystem restoration, hydrodynamics, and food webs, emphasizing their potential in mitigating reef loss and supporting marine biodiversity (Lee et al., 2018).
Offshore oil and gas platforms, which contribute approximately one-third of global oil and gas production, serve not only industrial purposes but also function as artificial habitats for diverse marine communities (Torquato et al., 2017). With over 8,000 active platforms globally, these structures provide extensive vertical surfaces throughout the water column, supporting fouling organisms and marine species despite occupying a small seafloor footprint. Platforms in regions like the Gulf of Mexico, the Santa Barbara Channel, and the Arabian Gulf have facilitated high marine biodiversity, with recruitment densities surpassing most marine ecosystems (Claisse et al., 2014). These platforms are often located in offshore areas that may experience relatively lower direct coastal human impact compared to nearshore zones, particularly in deeper waters with strong currents, which can facilitate species dispersal and enhance genetic connectivity for corals and associated species (Molen et al., 2018); however, this pattern can vary regionally, as in the Gulf of Mexico, where platforms span a wide depth range from shallow to deep waters. However, they also pose risks, including facilitating the spread of invasive species that can disrupt local ecosystems. Variations in macrofaunal assemblages on platforms are influenced by factors like temperature, food availability, and current-driven larval dispersal, with distinct vertical patterns observed at different depths (Page et al., 2008). Geographical gradients also play a role, with southern platforms supporting more diverse benthic communities, while older northern platforms host higher fish diversity (Friedlander et al., 2014). Despite differences in physical conditions, these platforms resemble natural reefs in their ecological structure, providing valuable insights for conservation, restoration, and climate change studies.
Offshore oil and gas platforms provide structurally complex habitats that support diverse marine communities (e.g., Friedlander et al., 2014; Riera et al., 2023). These artificial structures can serve as important substrates for invertebrate colonization and reef-associated species, contributing to local biodiversity. Studies have documented successful colonization on steel components of platforms, including corals and other sessile fauna (Fitzhardinge and Bailey-Brock, 1989; Bell and Smith, 1999; Torquato et al., 2021). In regions experiencing significant habitat loss, such artificial substrates may offer valuable opportunities for benthic community development and ecosystem enhancement. Our study, conducted near the Al Shaheen oil platforms, focuses on understanding mobile epifaunal assemblages on these artificial structures, providing baseline data crucial for future ecological assessments in the region.
The Arabian Gulf, a shallow and semi-enclosed marine basin, is one of the hottest and saltiest open seas, with summer temperatures exceeding 35°C and salinities over 45 psu due to high evaporation and limited freshwater input. Spanning 1,000 km in length and bordered by deserts, the Gulf connects to the Gulf of Oman through the Strait of Hormuz, driving a unique seasonal circulation. Despite these harsh conditions, the Gulf supports diverse macrofaunal invertebrates crucial to benthic food webs and fisheries. However, over 70% of its coral reefs are degraded due to high temperatures, salinity, and anthropogenic pressures, i.e., overfishing and pollution. Offshore oil platforms, like those in the Al Shaheen Oil Field, have become vital artificial reefs, providing hard substrates for coral and fish communities and enhancing fisheries productivity. These platforms also function as biodiversity hotspots, supporting species new to the region, and help reduce anthropogenic impacts, i.e., fishing. This study aims to investigate the biological connectivity between natural reefs and oil platforms in offshore Qatari waters by examining mobile macrofauna recruited on settlement plates, assessing spatial variations in recruitment, and exploring the relationship between mobile epifauna and sessile assemblages. The results will inform oil platform decommissioning strategies, emphasizing their role in enhancing local productivity and ecological connectivity (Maghsoudlou et al., 2008; Feary et al., 2011; Torquato et al., 2017; Riera et al., 2023).
Materials and methods
Study area
The study focused on the Al Shaheen oil field, located approximately 70–80 km (about 40 miles) off the Qatari coast, where natural coral reefs coexist with oil platforms that also function as artificial reefs (Figure 1). Umm Al Arshan, one of the most diverse reefs in the southern Arabian Gulf, was identified near Block 5 and is relatively less impacted by past coral bleaching events (Burt et al., 2016, Burt et al., 2017). The area, also less affected by anthropogenic activities such as fishing, was selected to examine connectivity between natural and artificial reef structures in offshore deep waters. As this study investigates connectivity between these two habitat types, we agree that reporting spatial relationships is essential. The closest natural reefs lie approximately 50 km west of the Al Shaheen oilfield, and six arrays of settlement plates were deployed along a spatial gradient to capture recruitment patterns across varying distances from both natural and artificial sources. Arrays 1, 2, and 3 were situated nearer to the natural reefs, while arrays 4, 5, and 6 were positioned closer to the oil platforms within the Al Shaheen field. Inter-array distances varied between 10 and 15 km, and all relevant distances can be inferred from the Figure 1. This spatial configuration was explicitly designed to investigate recruitment dynamics and potential larval connectivity between the two reef types.
Figure 1
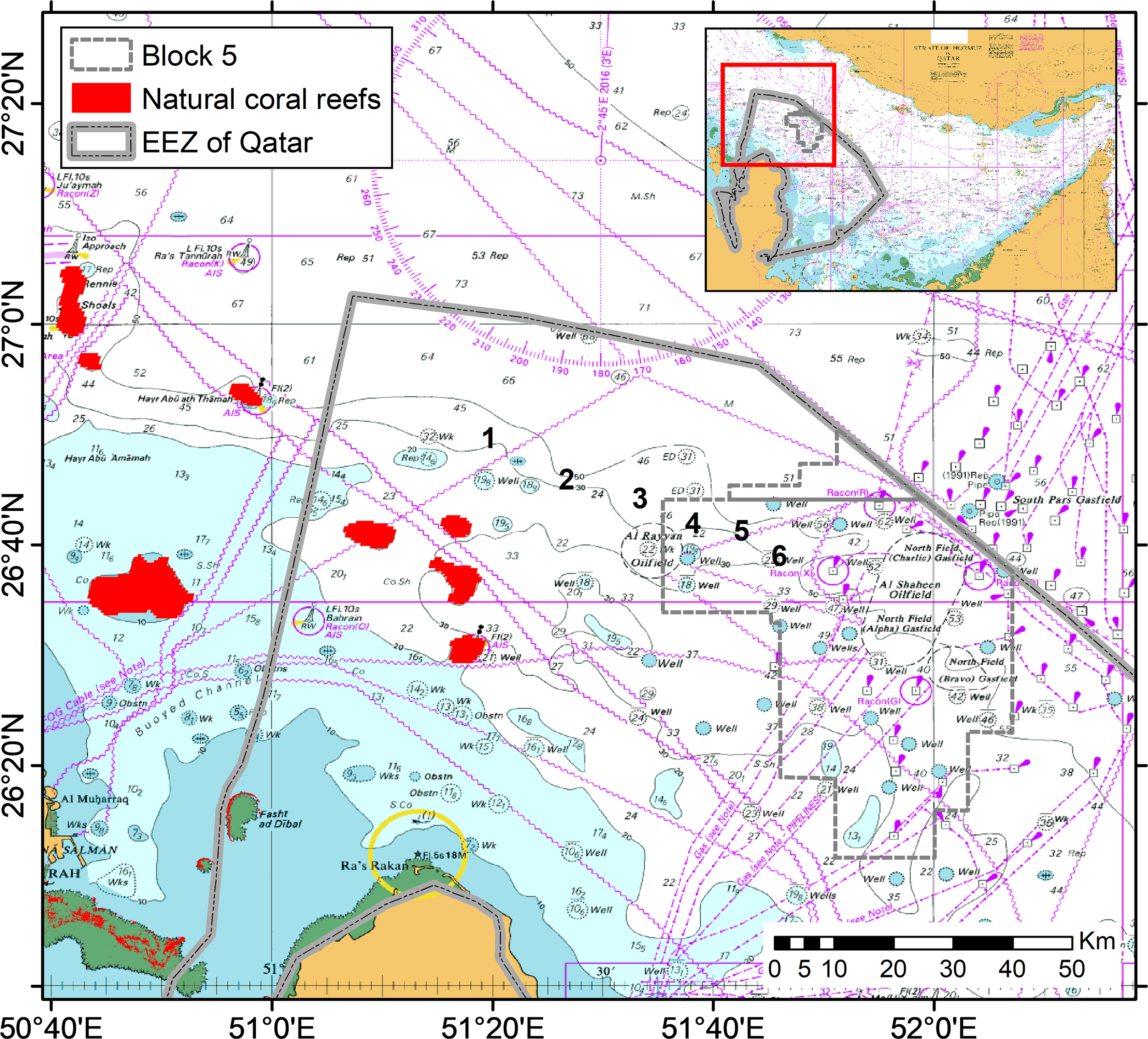
Map of the study area showing the six sites of deployment (numbered 1 to 6), between the Al-Shaheen oil field, within Block 5, and the natural coral reefs in the North of the EEZ of Qatar.
Deployment of settlement plates
Stainless-steel settlement plates (20 × 20 cm) were deployed in October 2016 at six offshore locations situated between the Al Shaheen oil platforms and nearby natural reefs, approximately 70–80 km from the coastline. These six arrays were strategically distributed to create a gradient of distance between the artificial structures and natural reef habitats, allowing for the assessment of spatial patterns in larval recruitment and potential connectivity. At each site, a mooring line was secured to the seafloor using concrete anchors and acoustic release systems, and held 30 plates spaced 1 m apart. The plates were suspended at depths ranging from 10–15 m (top) to 45–48 m (bottom), with the deepest plates positioned approximately 1–2 m above the seafloor (Table 1; Figure 1). This configuration enabled sampling across a depth gradient while maintaining consistency across sites. The plates were constructed to mimic the material and surface texture of oil platform pillars, and were not cleaned prior to deployment to allow for natural biofilm development and realistic settlement conditions.
Table 1
| Site name | Latitude | Longitude | Max depth (m) |
|---|---|---|---|
| 1 | N26 49 40.1 | E51 19 39.8 | 43 |
| 2 | N26 45 54.8 | E51 26 41.8 | 46 |
| 3 | N26 44 09.8 | E51 33 20.8 | 43 |
| 4 | N26 41 56.0 | E51 38 08.5 | 41 |
| 5 | N26 41 15.1 | E51 42 32.5 | 44 |
| 6 | N26 38 59.2 | E51 45 56.4 | 45 |
Sites and their geo-coordinates and maximum depth, as recorded by research vessel janan upon sample retrieval on 3–6 nov 2017.
Sampling and retrieval of substrates
All sampling for this study was conducted during a single survey in November 2017, approximately 13 months after deployment. In November 2017. The plates were retrieved using an acoustic release system during a research cruise aboard the RV Janan. Photographs were taken of the retrieved plates using a Nikon D7100 camera with paired strobes. Half of the plates from each depth were collected for biological analysis, while the remaining plates were redeployed at the same locations for potential future (not included in this study) long-term monitoring.
Upon retrieval, plates were transferred to the vessel’s washing room, where macrofauna was scraped off using a paint scraper, ensuring minimal damage. The collected organisms were washed with seawater and filtered through dual mesh (500 and 50 microns). The macrofauna was then preserved in plastic containers, half in absolute ethanol and the other half in a 5% buffered formalin solution. The samples were labeled, refrigerated at -1°C, and transported to Qatar University’s laboratories for further analysis.
This methodology aimed to assess the biological connectivity between natural reefs and oil platforms by analyzing macrofaunal recruitment and community structure.
Additionally, vertical profiles were obtained at each site using the Vessel CTD (Sea-Bird Electronics SBE911plus). Variables measured included Depth, Temperature, Salinity, Density, Dissolved Oxygen, Fluorescence, Turbidity, PAR/Irradiance, pH, Oxidation Reduction Potential and Pressure.
Sample analysis
Upon arrival at the lab, the macrofauna samples were emptied using funnels and 50-micron sieves. Ethanol samples were processed promptly to prevent distortion, while formalin-fixed samples were processed later in a fume hood. Samples were transferred to plastic trays for sorting under magnifying glasses (MAGNASCOPE R1-89-01). Sorting was done in two stages: first, separating sessile organisms from mobile fauna, and then sorting the mobile fauna into major taxonomic groups (e.g., Annelids, Mollusks, Crustaceans). Organisms were preserved in falcon tubes or eppendorfs, properly labeled for subsequent taxonomic identification.
Taxonomic identification of mobile fauna was done based on morphology, using stereo microscopes (Zeiss SMZ-168 and Olympus SZ2-ST). The identification was cross-checked through taxonomic guides (Jones, 1986; Richmond, 2002; Al Omari, 2011; Alomari, 2016; Naderloo, 2017). Identified taxa were preserved in smaller vials and labeled for dry biomass measurement.
Data analysis
Although plates were deployed at 1 m intervals, for analysis purposes they were grouped into 5 m depth intervals (e.g., 10–14 m, 15–19 m, etc.), resulting in seven depth categories. This grouping allowed for consistent comparisons across depths while reducing potential variability among closely spaced plates. The raw data were organized in Microsoft Excel and analyzed using two fixed factors: Site (6 levels) and Depth (7 levels, grouped in 5 m intervals). The taxa were transformed into operational taxonomic units (OTUs) to standardize identification at varying taxonomic levels. Non-metric multidimensional scaling (nMDS) and Permutational Multivariate Analysis of Variance PERMANOVA (Anderson et al., 2014) were applied to analyze community structure using the Bray-Curtis similarity matrix. SIMPER analysis highlighted the taxa contributing most to observed dissimilarities (Range et al., 2014).
Ecological indices, including species richness, abundance, and diversity (d and J’), were calculated using the DIVERSE routine. Distance-Based Linear Models (DistLM) were used to analyze the relationship between mobile epifauna and sessile fauna. The BEST procedure identified the most significant explanatory variables for variation in mobile fauna. Distance-based redundancy analysis (dbRDA) was used to visualize these relationships, applying non-parametric permutation methods (McArdle and Anderson, 2001).
Abundance-Biomass Comparison (ABC) curves were plotted to assess whether the community structure reflected a disturbed or undisturbed ecosystem. All the multivariate analyses were performed using the PRIMER v6 statistical software with PERMANOVA+ add-on (PRIMER-E, Plymouth Marine Laboratory). Statistically significant difference between the means was chosen at p<0.05. Graphs were plotted using SigmaPlot software (V 11.0, Systat System, Inc.).
Results
Environmental variables
Chlorophyll concentrations, measured through fluorescence, peaked between 10–20 m and were lowest at greater depths (40–50 m) (Figure 2). Temperature records from the data loggers showed clear thermal stratification, with a daily thermocline of 5-6°C on average, and a maximum gradient of 10°C observed in September 2017. Stratification intensified from March to November 2017, with notable temperature fluctuations at shallow (10–15 m) and deep (45–50 m) layers across all sites (Figure 3).
Figure 2
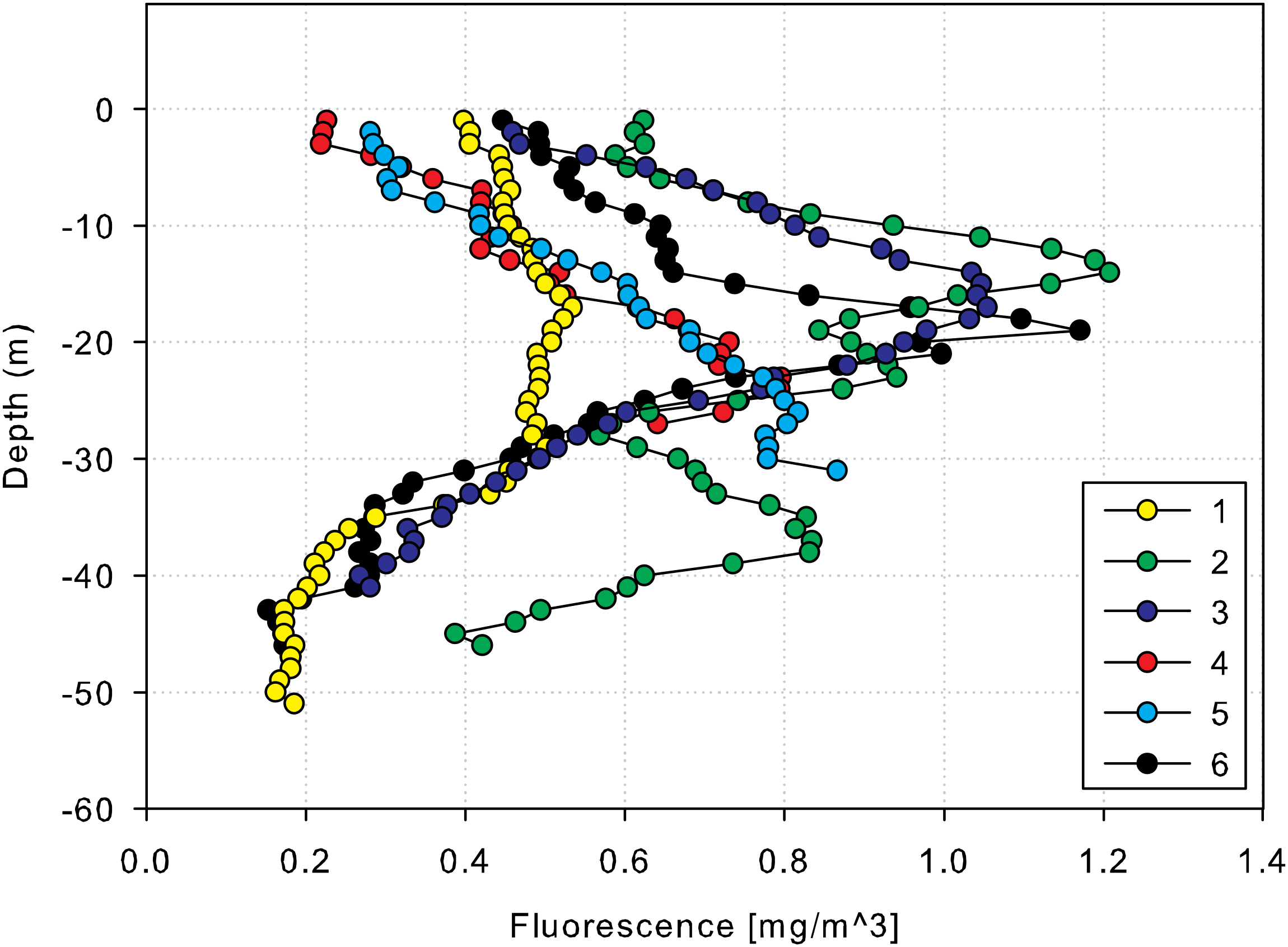
Vertical profiles (0–60 m) for chlorophyll-fluorescence at each of the six deployment sites (1 – 6) obtained using the vessel CTD.
Figure 3
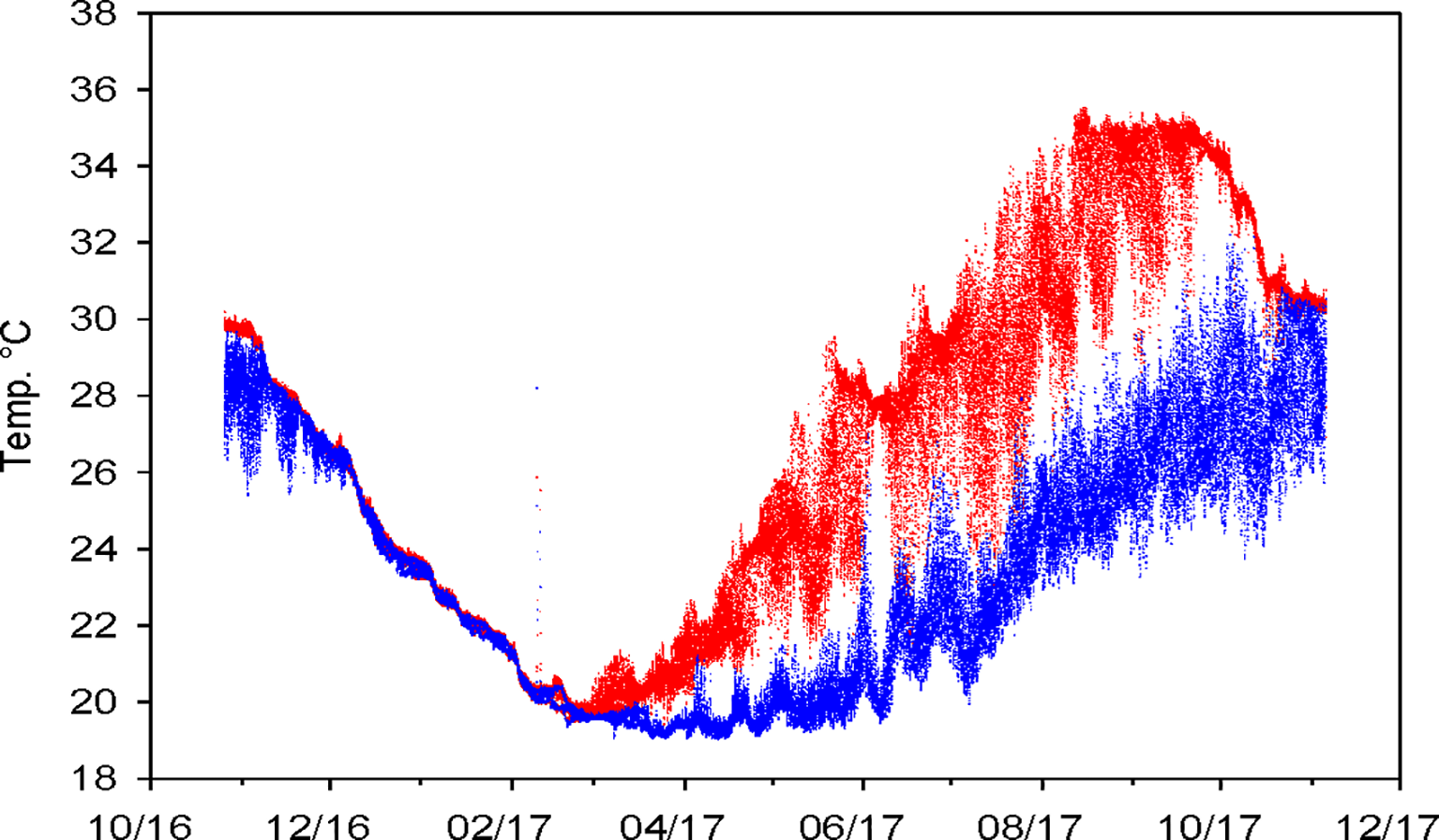
Seawater temperature records from Hobbo© data loggers deployed at 10–15 m (red) and 45–50 m (blue) in each of the 6 vertical structures.
Macrofauna
A total of 42 different operational taxonomic units (OTUs) of macro-benthic recruits were recorded from 2,302 mobile epibenthic individuals collected across six sites at various depths. Of these, 1,553 specimens were identified at the family level, while 663 were identified to genus or species level, and 86 to higher taxonomic levels (class or order), due to incomplete or damaged specimens. The most diverse group was the annelids, represented by 14 families (e.g., Chaetopteridae, Eunicidae, Nereididae, and Terebellidae), followed by arthropods (8 families), echinoderms (5 families), mollusks (2 families), and a few individuals from Sipunculida and Platyhelminthes. The most abundant taxa included the sipunculid Phascolosoma sp., polyclad Stylochidae, and brittle-stars belonging to the families Ophiotrichidae and Ophiocomidae. Among crustaceans, the family Pilumnidae was abundant, while gastropods like the genera Cerithium sp. and Conus sp. were present across sites, although Conus was absent at one site. To our knowledge, the crustacean Galathea sp. was firstly recorded in Qatari waters.
There is a general trend of total abundance decreasing with depth (Figure 4), reflecting the strong influence of depth on macrofaunal community structure. Taxa distribution revealed that sipunculids, polyclads, and polychaetes were most abundant in the upper layers, with polychaetes being particularly diverse at sites 1-AF and 2-DE. Echinoderms were more diverse at site 4-GH, while crustaceans dominated in abundance at this site. Nudibranchs were observed at sites 1-AF and 4-GH, typically within the 20–24 m depth range. PERMANOVA analysis confirmed that site, depth, and their interaction significantly influenced mobile macrofaunal community structure in terms of both abundance and biomass (Tables 2, 3). Notably, depth exhibited the highest Pseudo-F values, reinforcing its role as the key driver of community variation. This depth-related structuring likely reflects the combined effects of abiotic factors, such as the pronounced temperature gradient in the study region, and biotic factors, including species-specific habitat preferences and interactions.
Figure 4
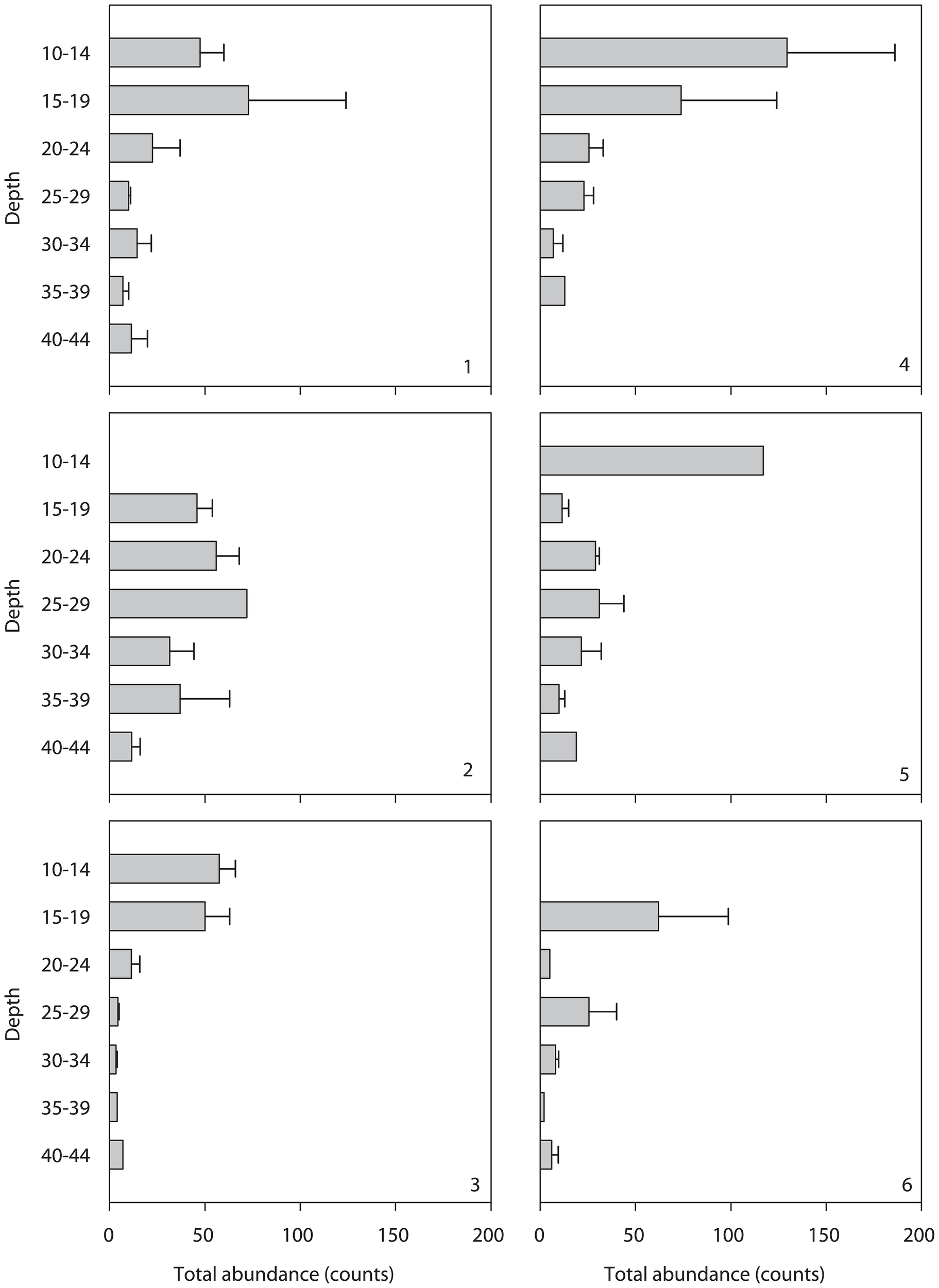
Overall abundance per plate (mean + SE) at each site and depth interval.
Table 2
| PERMANOVA table of results on abundance | ||||||
|---|---|---|---|---|---|---|
| Source | df | SS | MS | Pseudo-F | P (perm) | Unique perms |
| Si | 5 | 29816 | 5963.2 | 3.0483 | 0.001 | 998 |
| De | 6 | 64691 | 10782 | 5.5114 | 0.001 | 998 |
| SixDe** | 27 | 71880 | 2662.2 | 1.3609 | 0.001 | 995 |
| Res | 40 | 78251 | 1956.3 | |||
| Total | 78 | 2.5429E5 | ||||
PERMANOVA on the abundance of all taxa based on a Bray–Curtis resemblance matrix.
Factors: Si, Site; De, Depth; df, degrees of freedom; SS, sum of squares; MS, mean squares. Bold letters denote significant differences (p < 0.05). Type III Sum of Squares was used, and permutations were based on the residuals under a reduced model. ** denotes that not all depths were represented at all sites.
Table 3
| PERMANOVA table of results on biomass | ||||||
|---|---|---|---|---|---|---|
| Source | df | SS | MS | Pseudo-F | P (perm) | Unique perms |
| Si | 5 | 28920 | 5784 | 2.5733 | 0.002 | 998 |
| De | 6 | 66288 | 11048 | 4.9153 | 0.001 | 997 |
| SixDe** | 27 | 86604 | 3207.5 | 1.4271 | 0.009 | 999 |
| Res | 37 | 83164 | 2247.7 | |||
| Total | 75 | 2.7843E5 | ||||
PERMANOVA on the biomass of all taxa based on a Bray–Curtis resemblance matrix.
Factors: Si, Site; De, Depth; df, degrees of freedom; SS, sum of squares; MS, mean squares. Bold letters denote significant differences (p < 0.05). Type III Sum of Squares was used, and permutations were based on the residuals under a reduced model. ** denotes that not all depths were represented at all sites.
The nMDS plots in Figure 5 highlight the taxa contributing most to dissimilarities in abundance among depth intervals at each site. The sipunculid Phascolosoma sp. 1 and the polyclad Stylochidae were dominant at shallower depths (10–19 m) across all sites, displaying strong positive correlations with these layers. Intermediate depths (20–34 m) supported high abundances of polychaetes from families such as Terebellidae, Syllidae, Ophiotrichidae, and Ophiocomidae, while deeper layers (30–44 m) were characterized by increased abundances of Terebellidae, Lumbrineridae, bristle worms (Eunicidae, Nereididae), and decapods (Pilumnidae) (Table 4). Notable horizontal distribution patterns included Polynoidae and Syllidae being abundant at site 1-AF, while the flatworm family Discocelidae was present at sites 1-AF and 5-LJ (Figure 5).
Figure 5
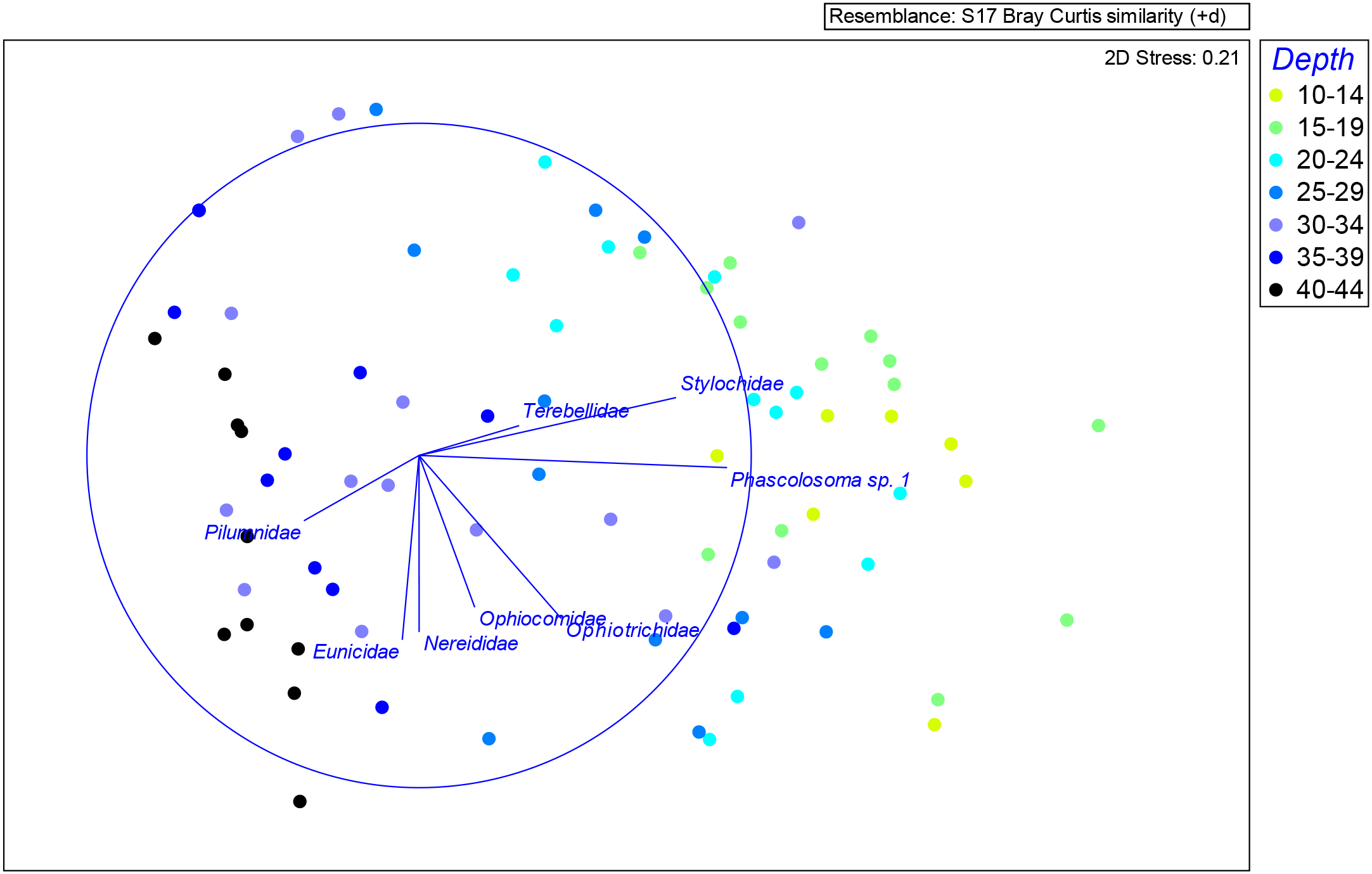
Non-metric multi-dimensional scaling (nMDS) analysis of the entire macrofaunal assemblage based on abundances of all operational taxonomic units (OTUs) across all depth intervals. Each point represents a sample, and the spatial arrangement reflects similarities in community composition. Stress value is provided to indicate the goodness of fit.
Table 4
| Depth range | Species | Average similarity | Contribution (%) | Cumulative (%) |
|---|---|---|---|---|
| 10–14 m | Phascolosoma sp1 | 9.12 | 16.08 | 16.08 |
| Stylochidae | 6.61 | 11.66 | 27.73 | |
| Nereididiae | 6.51 | 11.48 | 39.22 | |
| Polynoidae | 6.51 | 11.48 | 50.70 | |
| Syllidae | 6.30 | 11.11 | 61.81 | |
| 15–19 m | Phascolosoma sp1 | 13.88 | 30.89 | 30.89 |
| Stylochidae | 9.91 | 22.06 | 52.95 | |
| Lumbrineridae | 7.78 | 17.32 | 70.27 | |
| Polynoidae | 3.56 | 7.92 | 78.19 | |
| Syllidae | 3.18 | 7.08 | 85.27 | |
| 20–24 m | Phascolosoma sp1 | 16.02 | 35.87 | 35.87 |
| Stylochidae | 5.84 | 13.08 | 48.95 | |
| Syllidae | 5.71 | 12.79 | 61.75 | |
| Ophiotrichidae | 5.64 | 12.63 | 74.37 | |
| Terebellidae | 2.98 | 6.68 | 81.05 | |
| 25–29 m | Phascolosoma sp1 | 12.58 | 32.47 | 32.47 |
| Ophiocomidae | 9.50 | 24.52 | 56.99 | |
| Ophiotrichidae | 6.58 | 16.98 | 73.97 | |
| Nereididae | 2.93 | 7.56 | 81.53 | |
| 30–34 m | Ophiocomidae | 5.01 | 22.52 | 22.52 |
| Nereididae | 4.54 | 20.41 | 42.93 | |
| Ophiotrichidae | 2.22 | 10 | 52.93 | |
| 35–39 m | Nereididae | 8.66 | 44.75 | 44.75 |
| Eunicidae | 3.42 | 17.69 | 62.45 | |
| Polynoidae | 2.27 | 11.74 | 74.18 | |
| Pilumnidae | 1.48 | 7.66 | 81.85 | |
| 40–44 m | Nereididae | 23.16 | 47.02 | 47.02 |
| Pilumnidae | 19.70 | 39.99 | 87.02 | |
| Eunicidae | 2.98 | 6.04 | 93.06 |
Species similarity percentages across different depth ranges (10–44 m).
Only species contributing significantly to the similarity are included.
“Contributing significantly to the similarity” refers to species identified in SIMPER analysis as having the highest percentage contributions to within-group similarity, typically based on a cumulative contribution threshold of approximately 70%.”
The best DistLM solution identified 8 sessile taxa (bivalves Malleus regula, Modiolus barbatus, Pinctada radiata, Pinna bicolor; the barnacle Amphibalanus amphitrite, dead barnacles; sponges; and tube polychaetes) as explanatory variables. Seven mobile taxa (the sipunculid Phascolosoma sp. 1, the polyclad Stylochidae, and the polychaetes Polynoidae, Syllidae, Lumbrineridae) showed strong positive correlations with these sessile taxa at shallow to moderate depths (Figure 6). Decapods Pilumnidae and Etisus demani were positively correlated with dead barnacles at greater depths. Notably, the sipunculid Phascolosoma sp. 1 showed a strong correlation with Pinna bicolor (Pearson’s r = 0.80), The decapod Pilumnidae with dead barnacles (r = 0.78), and the crustacean Etisus demani with the barnacle Amphibalanus amphitrite (r = 0.73). Crabs, sipunculids, and polyclads were strongly correlated, with increasing counts of dead barnacles correlating with greater crab abundance. Shallow depths with abundant bivalves also supported high numbers of sipunculids and polyclads, whereas deeper depths with lower light penetration showed minimal bivalve presence and fewer mobile taxa (Figure 7).
Figure 6
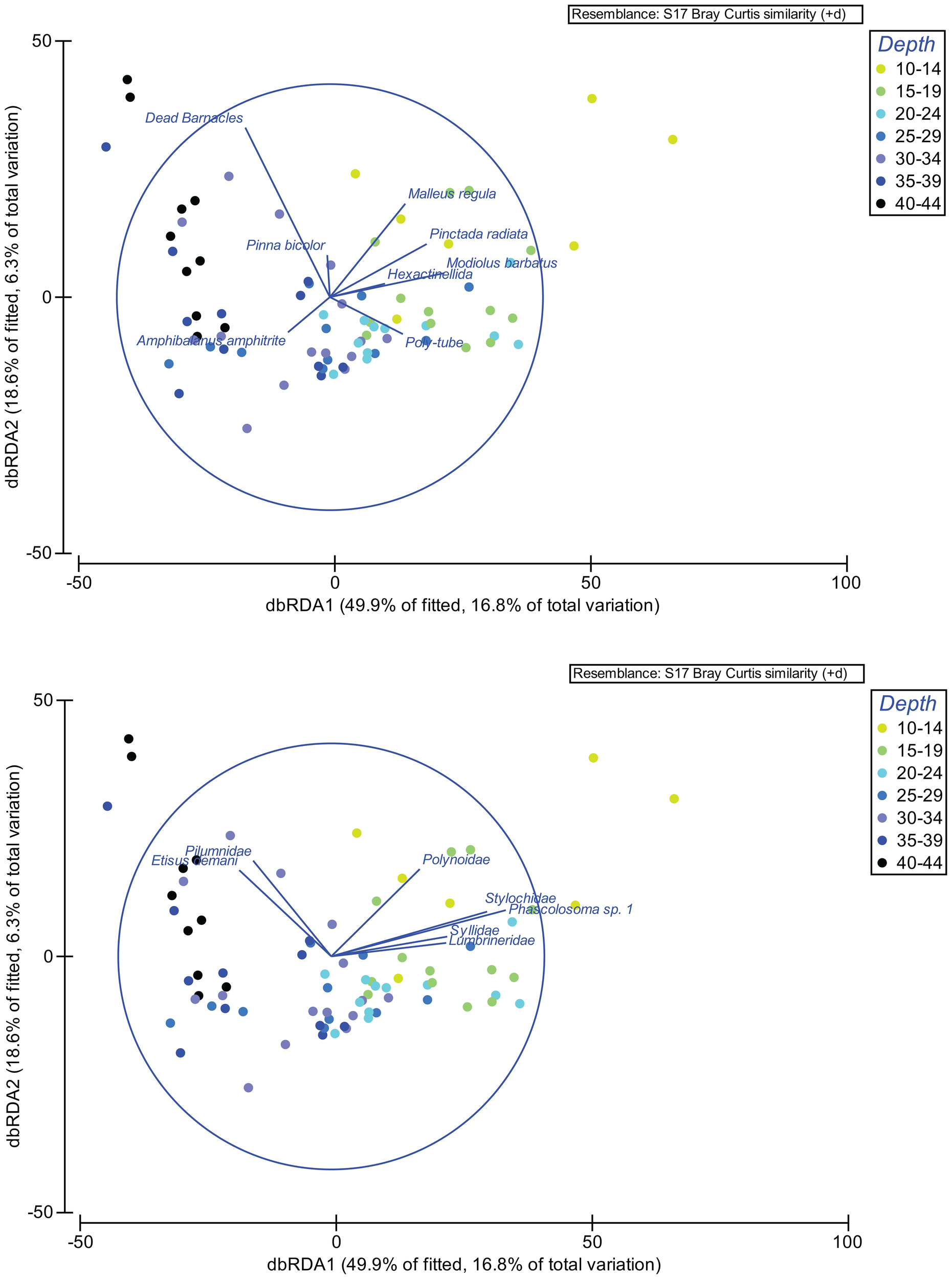
Distance-based redundancy analysis (dbRDA) on untransformed data on the entire macrofaunal assemblage. For mobile fraction (top) and sessile habitat (bottom) vectors represent the correlations between the dbRDA ordination and densities in each depth interval (Count per 20x20 cm Plate) of taxa contributing >10% to overall dissimilarities.
Figure 7
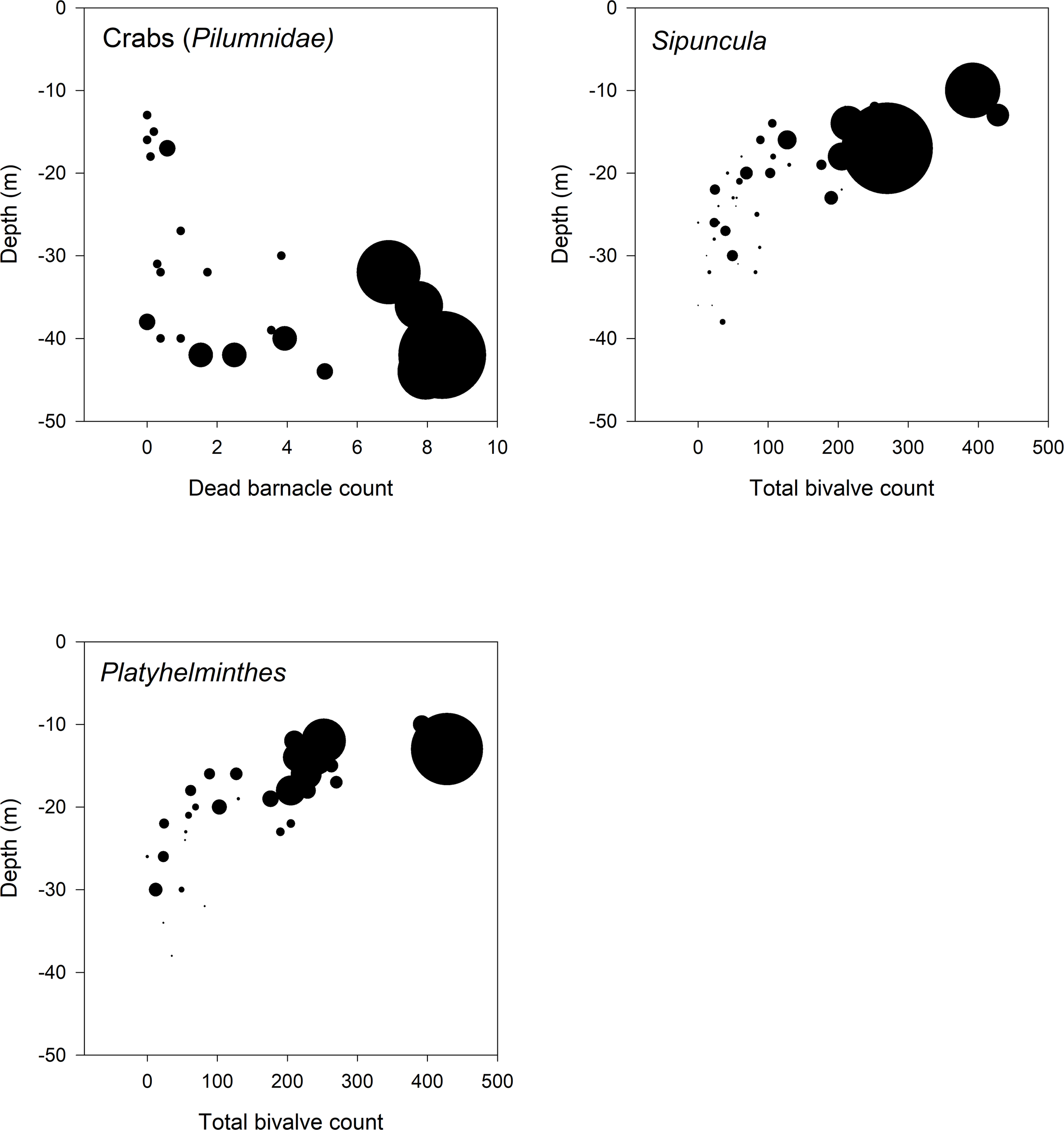
Association of the three most abundant mobile taxa groups with dominant sessile taxa across depth. Each circle represents a sample, with circle size proportional to the abundance of the mobile taxon. Top-left: Crabs (Pilumnidae) abundance in relation to dead barnacle cover.; Top-right: Sipuncula abundance in relation to total bivalve count; Bottom: Platyhelminthes abundance in relation to total bivalve count. Depth (m) is shown on the y-axis (increasing downward), and the x-axis shows sessile group abundance.
Discussion
This study provides important insights into the spatial dynamics of mobile recruits on offshore artificial reefs, specifically examining the role of oil platforms as artificial habitats in Qatari waters. By deploying stainless-steel plates resembling submerged sections of oil platforms across six sites along a transect from natural reefs to oil platform clusters, we documented 32 taxonomic families representative of the local benthic fauna. Our results highlight depth as a primary factor structuring the mobile epifaunal community, significantly influencing both abundance and biomass alongside site location. The strong influence of depth is likely due to associated environmental gradients—such as temperature stratification, light attenuation, hydrostatic pressure, and substrate variability—that change markedly with increasing depth and affect species’ physiological tolerances, habitat preferences, and resource availability. For example, warmer, well-lit shallow waters support higher abundances of taxa such as sipunculids (Phascolosoma sp. 1) and polyclads (Stylochidae), while deeper, cooler, and darker environments favor ophiuroids and decapods. Polychaetes exhibited a broad vertical distribution, reflecting their ecological adaptability and potential dependence on habitat structures provided by sessile organisms. Although significant horizontal variation in community composition occurred, no clear spatial patterns emerged relative to proximity to natural reefs or oil platforms, indicating that vertical environmental gradients associated with depth have a stronger role in shaping these benthic assemblages than horizontal spatial factors. Understanding how depth and related environmental variables drive community structure is essential for interpreting benthic biodiversity patterns and will inform future efforts to assess recruitment dynamics and ecosystem function across natural and artificial reef habitats in this rapidly developing marine region.
The spatial distribution of mobile macrofauna in offshore artificial reefs is influenced by several ecological mechanisms, including feeding guilds and biological competition. For example, bivalves appear to exclude barnacles from colonizing the upper layers of the 20x20 cm plates, likely due to competition (Hardin, 1960). An unpublished study on the sessile communities associated with the Al Shaheen oil field platforms reported that the upper 10 m from the sea surface were dominated by encrusting organisms, particularly from the phyla Bryozoa and Porifera. This biological competition likely explains the scarcity of these organisms deeper in the water column. The vertical distribution of species such as sipunculids (Phascolosoma sp. 1) and polyclads (Stylochidae) can be partly attributed to their feeding behaviors. Sipunculids are deposit feeders, extracting organic matter from surface sediments (Jumars et al., 2015), while polyclads are active predators, sometimes exhibiting parasitic behavior (Cannon, 2003). Bivalves, which are filter-feeders dependent on phytoplankton and suspended organic particulates, were most abundant in the 10–19 m depth range, where chlorophyll levels peak. Phytoplankton are key to the marine food web (Fehling et al., 2012), and higher primary productivity likely drives the occurrence of bivalves at these depths.
The relationship between mobile macrofauna and their sessile precursors is significant. Sipunculids feed on the organic-rich sediments released by bivalves, such as faeces and pseudo-faeces, which form the primary food source for these deposit-feeding worms (Kędra et al., 2018; Murina, 1984). This relationship is particularly evident in the abundance of Phascolosomatids in Qatar’s subtidal reefs, where they are commonly found among pearl oysters (Al-Khayat and Al-Ansi, 2008). Polyclads, in contrast, are carnivores that prey on a variety of sessile organisms, including oysters, which are abundant in the SE Arabian Gulf (Riegl & Purkis, 2012). Their parasitic feeding behavior, specifically targeting oysters, may explain their association with bivalves at deeper depths. A study by Landers and Rhodes (1970) found that some polyclads attack small oysters, reducing their populations significantly. These findings align with the current study’s observations of polyclads’ preference for bivalves, including pearl oysters, as their primary food source.
Feeding strategies and vertical distribution patterns also suggest a connection between certain species and depth-related habitat availability. For instance, mussels like Mytilus edulis are more abundant in shallower depths (0–20 m) due to the high concentration of particulate organic carbon, similar to the abundance of bivalves observed in the 10–19 m range in this study. Sipunculids and polyclads likely depend on these bivalves for food and habitat. Crustaceans, such as shrimps, crabs, and lobsters, are commonly found on reefs in the SE Gulf (George, 2012). The crustacean families Pilumnidae and Xanthidae, for instance, are known to hide in crevices and coral rubble during the day and emerge at night to feed. These crustaceans’ association with barnacles may also be due to the shelter these barnacles provide from predators. Crustacean larvae, which are sensitive to light cycles, may explain the preference for deeper waters where they can seek refuge in barnacle colonies to avoid predation.
Polychaetes, with their diverse feeding strategies, are present across a wide range of depths. Their omnivorous and carnivorous feeding behavior allows them to adapt to varying conditions and exploit different habitats. The distribution of their prey, such as algae, decaying matter, and other invertebrates, influences their vertical distribution. Gastropods, like the carnivorous Conus sp. and the algae-eating Cerithium sp., also exhibit distinct depth preferences. In particular, the cone gastropod Conus sp. feeds on herbivores like bivalves and deposit-feeders like sipunculids, which were abundant in the shallower layers of the study sites. Environmental disturbances can alter community composition, and feeding guilds are often used as indicators of such disturbances (Fauchald and Jumars, 1979; Metcalfe and Glasby, 2008). The high diversity and even distribution of taxa across the study area suggest that the ecosystem remains relatively undisturbed, indicating a healthy, functioning ecosystem in the offshore waters. Polychaetes, in particular, are often used as indicators of environmental health due to their wide range of reproductive and feeding strategies, which allow them to adapt to changing conditions and pollutants like organic matter, heavy metals, and pesticides (Dean, 2008). The diverse polychaete families observed in this study, such as Ampharetidae, Eunicidae, Nereididae, Lumbrineridae, and Polynoidae, reflect the minimal anthropogenic disturbance in the studied area. The deeper waters (35–44 m) were dominated by polychaete families such as Nereididae and Eunicidae, which are common associates of reef platforms in the Gulf waters (George, 2012). The high diversity and wide distribution of these polychaetes suggest a healthy ecosystem. The abundance-biomass comparison curves also support this, showing a consistently higher biomass than abundance, which is indicative of an undisturbed system characterized by slow-growing, “k-strategist” species. In particular, the dominance of the sipunculid Phascolosoma sp. 1 at site 6-IM, where smaller individuals were present, suggests a potential change in population structure, possibly due to delayed recruitment rather than pollution or competition.
Ocean currents play a key role in larval dispersal, as invertebrate larvae are generally poor swimmers and rely on current patterns for horizontal movement (Daigle and Metaxas, 2011). Differences in recruitment across depths—specifically, the greater taxa diversity observed in shallower waters (10–19 m) compared to deeper ones (35–44 m)—may be influenced by depth-related hydrodynamic conditions, although water current data were not directly measured in this study. Recruitment likely occurred in the winter months, when significant mixing of the water column occurred, allowing for the transport of planktonic larvae to the recruitment sites (Verspagen et al., 2004). This mixing, coupled with the lack of thermocline during winter, facilitated the recruitment process. However, the onset of thermal stratification in March 2017 may have limited the migration of species to different depths, except for those species with a wider temperature tolerance.
The findings from this study, while largely consistent with known patterns of epifaunal recruitment in the Arabian Gulf (Torquato et al., 2021), also highlight several important distinctions and raise additional questions. When compared to previous studies on artificial reefs such as oil and gas platforms in the region, the community assemblages observed on the stainless-steel settlement plates showed comparable taxonomic diversity, especially in terms of bivalves, polychaetes, and sipunculids (Lozano-Cortés et al., 2019; Torquato et al., 2021). However, notable differences were observed in relative abundance and biomass distribution, likely influenced by the orientation and limited surface area of the plates compared to complex reef structures. The long incubation period (~13 months) likely favored the establishment of slow-growing, k-strategist species over opportunistic r-strategists, which tend to dominate short-term colonization studies (Schoener and Schoener, 1981; García and Salzwedel, 1995; Langhamer et al., 2009, among others). This prolonged exposure may also explain the high frequency of dead barnacles recorded, especially at deeper sites. The presence of numerous empty barnacle tests could indicate post-settlement mortality, potentially driven by competition with bivalves for space or changes in environmental conditions such as hypoxia, sedimentation, or predation. Barnacle mortality may also be attributed to overgrowth by fouling organisms or the physiological stress of prolonged submersion without access to light and primary productivity in deeper zones.
Conclusions
The results revealed clear patterns in species distribution, with taxa such as polyclads and sipunculids being dominant at shallower depths and scarce in deeper water columns. Abundance, diversity, and biomass of the assemblages showed a negative correlation with depth, indicating a decline in these metrics with increasing depth. However, no distinct horizontal gradient patterns were observed between reefs, suggesting that further investigation into population structure and recruitment dynamics is warranted. Importantly, the limited size and orientation of the stainless-steel plates—deployed independently and not fully aligned with the platform structures—may have influenced the accuracy of settlement patterns, potentially underrepresenting the effects of local current energy and spatial variability.
The study suggests that the mobile epifaunal assemblages colonizing the plates are characteristic of an undisturbed offshore environment. Our findings also support the idea that offshore artificial substrates, such as oil platforms, may function as ecological “stepping stones,” facilitating connectivity among spatially separated habitats (Bram et al., 2005; Adams et al., 2014). This concept aligns with the Stepping Stones Hypothesis, which posits that artificial structures can enhance dispersal and gene flow by providing intermittent habitat patches in otherwise unsuitable environments. The observed similarity in species composition, vertical biomass patterns, and abundance across the six structures suggests a high degree of connectivity, potentially supporting this hypothesis—though further studies integrating genetic and larval dispersal data are needed to test this explicitly.
Dominant functional groups included surface deposit feeders, carnivorous flatworms, and taxa such as polychaetes and ophiuroids, which reflect a range of feeding strategies and indicate the ecological versatility of colonizing assemblages. The study provides valuable baseline data for understanding macro-benthic community recruitment on artificial substrates in offshore environments. These insights will be important for comparing spatial distribution patterns, investigating competition for limited substrate, and guiding best practices for the commissioning and decommissioning of oil and gas platforms. Additionally, this work lays important groundwork for future research on the ecological roles of artificial reefs in Qatari waters, with implications for reef restoration strategies and fisheries management.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
NA: Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft. RR: Conceptualization, Investigation, Supervision, Validation, Writing – review & editing. MA: Data curation, Formal Analysis, Methodology, Software, Writing – review & editing. RB-H: Funding acquisition, Investigation, Project administration, Resources, Validation, Writing – review & editing. PR: Conceptualization, Funding acquisition, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was done under the framework of the NPRP project ‘Connectivity, diversity and genetic flow between offshore natural coral reefs and oil platforms – NPRP n° 7-1129-1-201’, funded by the Qatar National Research Fund (a member of The Qatar Foundation). Additional funding was provided by QU student grant n° QUST-1-CAS-2018-40.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adams T. P. Miller R. G. Aleynik D. Burrows M. T. (2014). Offshore marine renewable energy devices as stepping stones across biogeographical boundaries. J. Applied Ecol.51 (2), 330–338.
2
Al-Khayat J. A. Al-Ansi M. A. (2008). Ecological features of oyster beds distribution in Qatari waters, Arabian Gulf. Asian J. Sci. Res.1, 544–561. doi: 10.3923/ajsr.2008.544.561
3
Alomari N. (2016). Guide to polychaetes annelida in Qatar marine sediments. (Doha (Qatar)).
4
Al Omari K. (2011). Protected areas in the Arabian Peninsula. Zoology in the Middle East54 (sup3), 21–26. Available online at: https://www.tandfonline.com/doi/abs/10.1080/09397140.2011.10648897.
5
Anderson M. J. (2014). Permutational multivariate analysis of variance (PERMANOVA). Wiley statsref: Statistics reference online. 1–15.
6
(2018). WoRMS - world register of marine species. Available online at: http://www.marinespecies.org/index.php. (Accessed March 24, 2025).
7
Bell N. Smith J. (1999). Coral growing on North Sea oil rigs. Nature402, 601. doi: 10.1038/45127
8
Bouwmeester J. Riera R. Range P. Ben-Hamadou R. Samimi-Namin K. Burt J. A. (2020). Coral and reef fish communities in the thermally extreme Persian/Arabian Gulf: insights into potential climate change effects. Perspect. Mar. Anim. forests World, 63–86.
9
Bram J. B. Page H. M. Dugan J. E. (2005). Spatial and temporal variability in early successional patterns of an invertebrate assemblage at an offshore oil platform. J. Exp. Mar. Biol. Ecol.317, 223–237. doi: 10.1016/j.jembe.2004.12.003
10
Burt J. A. Ben-Hamadou R. Abdel-Moati M. A. R. Fanning L. Kaitibie S. Al-Jamali F. et al . (2017). Improving management of future coastal development in Qatar through ecosystem-based management approaches. Ocean Coast. Manage.148, 171–181. doi: 10.1016/j.ocecoaman.2017.08.006
11
Burt J. A. Smith E. G. Warren C. Dupont J. (2016). An assessment of Qatar’s coral communities in a regional context. Mar. pollut. Bull.105, 473–479. doi: 10.1016/j.marpolbul.2015.09.025
12
Cannon L. R. G. (2003). Marine flatworms: the world of polyclads (Victoria (Australia): Csiro Publishing).
13
Claisse J. T. Pondella D. J. Love M. Zahn L. A. Williams C. M. Williams J. P. et al . (2014). Oil platforms off California are among the most productive marine fish habitats globally. Proc. Natl. Acad. Sci. United States America111, 15462–15467.
14
Daigle R. M. Metaxas A. (2011). Vertical distribution of marine invertebrate larvae in response to thermal stratification in the laboratory. J. Exp. Mar. Biol. Ecol.409, 89–98. doi: 10.1016/j.jembe.2011.08.008
15
Dean H. (2008). The use of polychaetes (Annelida) as indicator species of marine pollution: a review. Rev. Biol. Trop.56, 11–38.
16
Fauchald K. Jumars P. A. (1979). The diet of worms: a study of polychaete feeding guilds. Oceanography Mar. Biol. Annu. Review.17, 193–284.
17
Feary D. A. Burt J. A. Bartholomew A. (2011). Artificial marine habitats in the Arabian Gulf: Review of current use, benefits and management implications. Ocean Coast. Manage.54, 742–749. doi: 10.1016/j.ocecoaman.2011.07.008
18
Fehling J. Davidson K. Bolch C. J. Brand T. D. Narayanaswamy B. E. (2012). The relationship between phytoplankton distribution and water column characteristics in North West European shelf sea waters. PloS One7 (3), e34098.
19
Fitzhardinge R. C. Bailey-Brock J. H. (1989). Colonization of artificial reef materials by corals and other sessile organisms. Available online at: https://www.ingentaconnect.com/content/umrsmas/bullmar/1989/00000044/00000002/art00004. (Accessed February 20, 2025).
20
Friedlander A. M. Ballesteros E. Fay M. Sala E. (2014). Marine communities on oil platforms in Gabon, west africa: high biodiversity oases in a low biodiversity environment. PloS One9, e103709. doi: 10.1371/journal.pone.0103709
21
García C. B. Salzwedel H. (1995). Successional patterns on fouling plates in the bay of Santa Marta, Colombian Caribbean. Boletín Investigaciones Marinas y Costeras-INVEMAR24, 95–121.
22
George J. D. (2012). “Reef-associated macroinvertebrates of the SE gulf,” in Coral reefs of the gulf: adaptation to climatic extremes, coral reefs of the world. Eds. RieglB. M.PurkisS. J. (Springer, Dordrecht), 253–308. doi: 10.1007/978-94-007-3008-3_13
23
Hardin G. (1960). The competitive exclusion principle. Science131, 1292–1297. doi: 10.1126/science.131.3409.1292
24
Jones D. A. (1986). A field guide to the sea shores of Kuwait and the Arabian Gulf (Kuwait: University of Kuwait).
25
Jumars P. A. Dorgan K. M. Lindsay S. M. (2015). Diet of worms emended: an update of polychaete feeding guilds. Annu. Rev. Mar. Sci.7, 497–520. doi: 10.1146/annurev-marine-010814-020007
26
Kędra M. Grebmeier J. M. Cooper L. W. (2018). Sipunculan fauna in the Pacific Arctic region: a significant component of benthic infaunal communities. Polar Biol.41, 163–174. doi: 10.1007/s00300-017-2179-z
27
Landers W. S. Rhodes E. W. (1970). Some factors influencing predation by the flatworm Stylochus ellipticus (Girard) on oysters. Chesapeake Sci.11, 55–60. doi: 10.2307/1351343
28
Langhamer O. Wilhelmsson D. Engström J. (2009). Artificial reef effect and fouling impacts on offshore wave power foundations and buoys–a pilot study. Estuarine Coast. Shelf Sci.82, 426–432. doi: 10.1016/j.ecss.2009.02.009
29
Lee M. O. Otake S. Kim J. K. (2018). Transition of artificial reefs (ARs) research and its prospects. Ocean Coast. Manage.154, 55–65. doi: 10.1016/j.ocecoaman.2018.01.010
30
Li Y. Zou X. Zou S. Li P. Yang Y. Wang J. (2021). Pollution status and trophic transfer of polycyclic aromatic hydrocarbons in coral reef ecosystems of the South China Sea. ICES J. Mar. Sci.78, 2053–2064. doi: 10.1093/icesjms/fsab081
31
Lozano-Cortés D. Joydas T. V. Abdulkader K. Krishnakumar P. K. Qurban M. A. (2019). Marine invertebrates colonizing a causeway in the Manifa offshore oilfield, Saudi Arabia. Mar. Biodiversity49, 2473–2483. doi: 10.1007/s12526-019-00969-5
32
Macreadie P. I. Fowler A. M. Booth D. J. (2011). Rigs-to-reefs: will the deep sea benefit from artificial habitat? Front. Ecol. Environ.9, 455–461. doi: 10.1890/100112
33
Maghsoudlou A. Eghtesadi Araghi P. Wilson S. Taylor O. (2008). Status of coral reefs in the ROPME sea area (The persian gulf, gulf of Oman and arabian sea). (Townswille, Australia: ROPME). 79–90.
34
McArdle B. H. Anderson M. J. (2001). Fitting multivariate models to community data: a comment on distance‐based redundancy analysis. Ecology82 (1), 290–297.
35
Metcalfe K. N. Glasby C. J. (2008). Diversity of Polychaeta (Annelida) and other worm taxa in mangrove habitats of Darwin Harbour, northern Australia. J. Sea Res.59, 70–82. doi: 10.1016/j.seares.2007.06.002
36
Molen J. van der, García-García L. M. Whomersley P. Callaway A. Posen P. E. Hyder K. (2018). Connectivity of larval stages of sedentary marine communities between hard substrates and offshore structures in the North Sea. Sci. Rep.8 (1), 14772. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6170480/.
37
Murina G. V. V. (1984). Ecology of sipuncula. Mar. Ecol. Prog. series. Oldendorf17, 1–7. doi: 10.3354/meps017001
38
Naderloo R. (2017). Atlas of crabs of the persian gulf (Cham, Switzerland: Springer International Publishing). Available online at: https://www.springer.com/br/book/9783319493725. (Accessed January 28, 2025).
39
Page H. M. Culver C. S. Dugan J. E. Mardian B. (2008). Oceanographic gradients and patterns in invertebrate assemblages on offshore oil platforms. ICES J. Mar. Sci.65, 851–861. doi: 10.1093/icesjms/fsn060
40
Page H. M. Dugan J. E. Piltz F. (2009). “Fouling and antifouling in oil and other offshore industries,” in Biofouling. Eds. DürrS.ThomasonJ. C. (London, UK: Wiley-Blackwell), 252–266. doi: 10.1002/9781444315462.ch18/summary
41
Peller T. Altermatt F. (2024). Invasive species drive cross-ecosystem effects worldwide. Nat. Ecol. Evol.8, 1087–1097. doi: 10.1038/s41559-024-02380-1
42
Polovina J. J. Sakai I. (1989). Impacts of artificial reefs on fishery production in shimamaki, Japan. Available online at: https://www.ingentaconnect.com/content/umrsmas/bullmar/1989/00000044/00000002/art00043. (Accessed March 15, 2025).
43
Range P. Martins M. Cabral S. Piló D. Ben-Hamadou R. Teodósio Chícharo M. et al . (2014). Relative sensitivity of soft-bottom intertidal macrofauna to increased CO2 and experimental stress. Mar. Ecol. Prog. Ser.509, 153–170. doi: 10.3354/meps10861
44
Reimer J. D. Peixoto R. S. Davies S. W. Traylor-Knowles N. Short M. L. Cabral-Tena R. A. et al . (2024). The Fourth Global Coral Bleaching Event: Where do we go from here? Coral Reefs43, 1121–1125. doi: 10.1007/s00338-024-02504-w
45
Richmond M. D. (2002). Field guide to the seashores of eastern africa and the western Indian ocean islands. 2nd edition (Dar es Salaam: Sida: The Swedish International Development Co-operation).
46
Riegl B. M. Purkis S. J. (2012). Coral reefs of the Gulf: adaptation to climatic extremes in the world’s hottest sea. In Coral reefs of the Gulf: Adaptation to climatic extremes. (Dordrecht: Springer Netherlands), pp. 1–4.
47
Riera R. Torquato F. Range P. Ben-Hamadou R. Møller P. R. Tuset V. M. . (2023). Are offshore platforms a good candidate to restore functional diversity of reef fish communities in the Arabian Gulf? Regional Stud. Mar. Sci.66, 103171.
48
Schoener A. Schoener T. W. (1981). The dynamics of the species-area relation in marine fouling systems: 1. Biological correlates of changes in the species-area slope. Am. Nat.118, 339–360. doi: 10.1086/283827
49
Shantz A. A. Ladd M. C. Burkepile D. E. (2020). Overfishing and the ecological impacts of extirpating large parrotfish from Caribbean coral reefs. Ecol. Monogr.90, e01403. doi: 10.1002/ecm.1403
50
Torquato F. Jensen H. M. Range P. Bach S. S. Ben-Hamadou R. Sigsgaard E. E. et al . (2017). Vertical zonation and functional diversity of fish assemblages revealed by ROV videos at oil platforms in The Gulf. J. Fish Biol.91, 947–967. doi: 10.1111/jfb.13394
51
Torquato F. Omerspahic M. H. Range P. Bach S. S. Riera R. Ben-Hamadou R. (2021). Epibenthic communities from offshore platforms in the Arabian Gulf are structured by platform age and depth. Mar. pollut. Bull.173, 112935. doi: 10.1016/j.marpolbul.2021.112935
52
Veron J. E. N. Hoegh-Guldberg O. Lenton T. M. Lough J. M. Obura D. O. Pearce-Kelly P. et al . (2009). The coral reef crisis: The critical importance of < 350ppm CO 2. Mar. pollut. Bull.58, 1428–1436.
53
Verspagen J. M. H. Snelder E. O. F. M. Visser P. M. Huisman J. Mur L. R. Ibelings B. W. (2004). Recruitment of benthic microcystis (cyanophyceae) to the water column: internal buoyancy changes or resuspension?1. J. Phycology40, 260–270. doi: 10.1111/j.1529-8817.2004.03174.x
54
Williams T. (2006). Sinking poor decision-making with best practices: A case study of artificial reef decision-making in the florida keys. Theses and dissertations. Available online at: https://scholarscompass.vcu.edu/etd/838. (Accessed March 20, 2025).
Summary
Keywords
Offshore reefs, benthic communities, ecological distribution, oil platforms, invertebrates
Citation
Aziz NA, Riera R, AlDibis M, Ben-Hamadou R and Range P (2025) Linking oil platforms and natural reefs: insights into mobile epifauna dynamics. Front. Mar. Sci. 12:1590577. doi: 10.3389/fmars.2025.1590577
Received
09 March 2025
Accepted
11 July 2025
Published
06 August 2025
Volume
12 - 2025
Edited by
Bernardo Antonio Perez Da Gama, Fluminense Federal University, Brazil
Reviewed by
Rachel L. Mugge, Naval Research Laboratory, United States
Nadav Shashar, Ben-Gurion University of the Negev, Israel
Updates
Copyright
© 2025 Aziz, Riera, AlDibis, Ben-Hamadou and Range.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro Range, prange@qu.edu.qa
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.