Introduction
By altering historic environmental conditions, the ongoing climatic and oceanographic change is modifying the structure of biological communities worldwide (Wernberg et al., 2024; Wiens and Zelinka, 2024; Yin and Rudolf, 2024; Dudgeon and Strayer, 2025). A growing number of studies are documenting these changes and trying to understand the causes. While the particular causes vary across ecosystems, they are often related to temperature changes (Menge et al., 2023; White et al., 2023; Pinsky et al., 2025; Wolfe et al., 2025). A general notion posits that the ongoing abiotic changes are destabilizing communities in various ways, decreasing the local abundance of some species while increasing that of others that benefit from the new conditions (Sahade et al., 2015; Zhang et al., 2018; Virta and Teittinen, 2022; Edmunds, 2024a). Many communities, however, frequently experience strong disturbances that leave them switching back and forth between early and intermediate successional stages (Sousa, 1984; Smale, 2007; Sugihara et al., 2018). For such cases, climatic or oceanographic changes that weaken local disturbances might actually increase community stability, at least for some time before the abiotic changes become themselves too strong.
On the Atlantic coast of Nova Scotia (Canada), there are signs suggesting that the above phenomenon is taking place. As done for other shores (Hawkins et al., 2019; Palomo et al., 2019; Menge et al., 2019; Ishida et al., 2021; Thyrring et al., 2021), years ago we conducted annual surveys to understand the latitudinal biogeography of rocky intertidal communities along this coast (Scrosati et al., 2022). In four consecutive years from 2014 to 2017, we measured the summer abundance of seaweeds and invertebrates at mid-to-high intertidal elevations in wave-exposed habitats at nine locations spanning 415 km of the Atlantic coast of mainland Nova Scotia. Based on those surveys, fairly consistent biogeographic patterns emerged. Communities from southern locations generally had more species and a greater coverage of the substrate than communities from northern locations. In turn, southern communities remained relatively stable over the years, while more interannual instability characterized northern communities (Scrosati et al., 2022).
The interannual instability of northern communities was largely driven by the irregular occurrence of intertidal ice scour. Every winter, sea ice develops extensively across the Gulf of St. Lawrence (Saucier et al., 2003), which is a large coastal body of water situated north of Nova Scotia (Figure 1). In late winter and early spring, many ice fragments leave this gulf and drift south following the open Atlantic coast of Nova Scotia until they melt. The southern reach of drift ice on this coast depends on the amount of ice formed in the Gulf of St. Lawrence in winter. When abundant drift ice reaches a location on the Atlantic coast, severe intertidal disturbance occurs as the ice fragments scour the substrate with waves and tides, leaving the substrate barren and triggering a primary succession (Petzold et al., 2014). If drift ice is absent or uncommon in the following year, intertidal communities can advance to a more developed successional stage, only to return to an early stage when drift ice returns in another year. Thus, northern communities on the open Atlantic coast of Nova Scotia have traditionally switched back and forth between early and intermediate successional stages. At southern locations on this coast, drift ice was absent or very rare between 2014-2017, which in part explains the interannual stability and high coverage of the substrate of their intertidal communities (Scrosati et al., 2022).
Figure 1
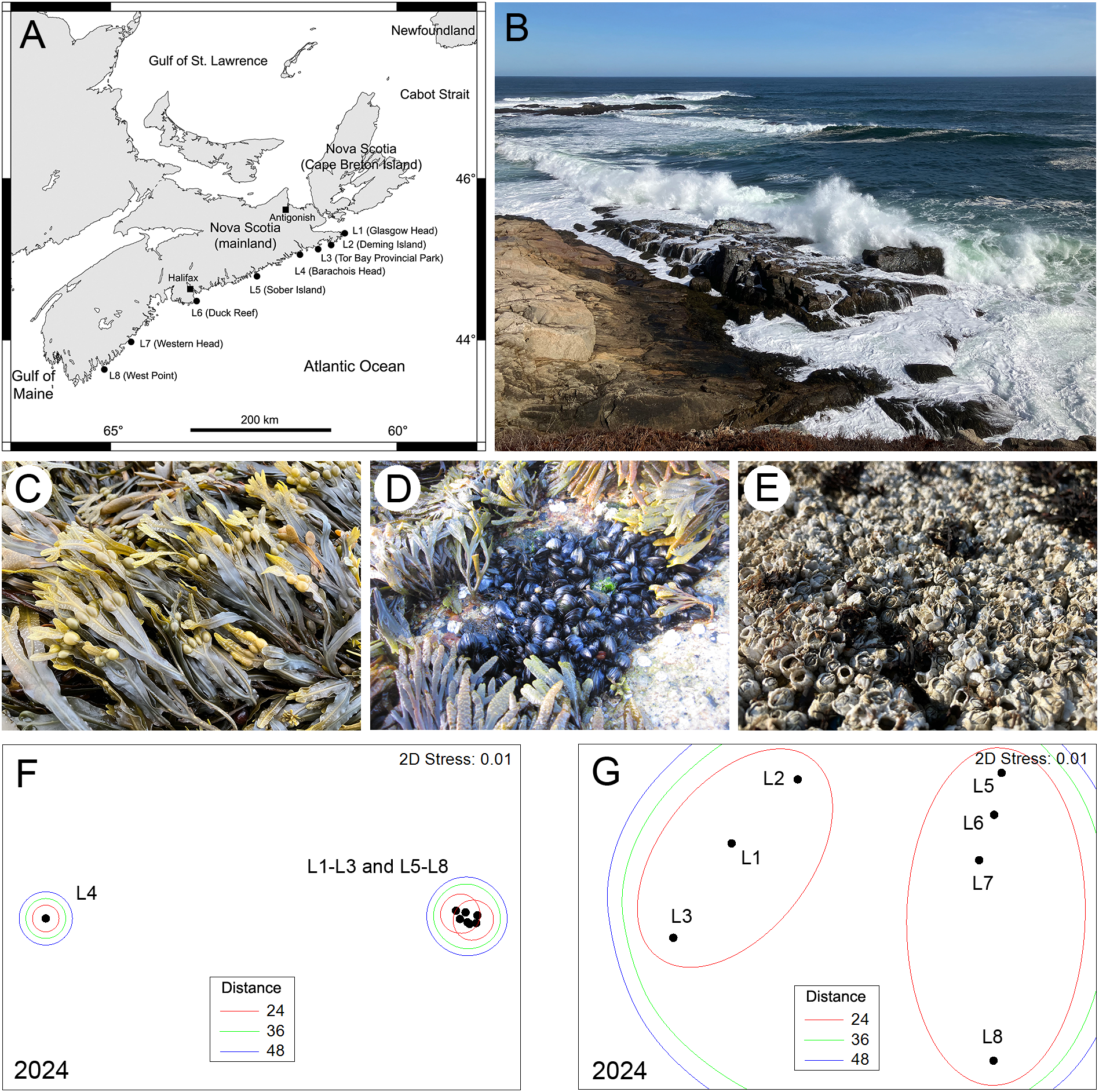
(A) Map of the Atlantic Canadian coast in Nova Scotia indicating the name and position of the 8 locations surveyed in 2024. (B) View of typical wave-exposed rocky intertidal habitats surveyed for this study (photo taken at L6 on 9 March 2024). (C) View of a Fucus stand from the surveyed habitats photographed at L5 on 26 July 2024. (D) View of a mussel stand from the surveyed habitats photographed at L6 on 20 June 2015. (E) View of a barnacle stand from the surveyed habitats photographed at L1 on 22 July 2024. (F) NMDS ordination of the centroids of the 8 locations surveyed in 2024 (based on the abundance of sessile species) grouped by threshold Euclidean distances between centroids. (G) Close-up detail of the NMDS ordination of all locations excluding L4. The photographs were taken by RAS at low tide.
Currently, however, environmental conditions on this coast are moving away from what they historically were. For example, the waters of the Gulf of St. Lawrence are warming, which decreases the formation of sea ice in winter (Galbraith et al., 2024) and thus the export of drift ice to the open Atlantic coast. At the same time, pronounced cold snaps following relatively mild conditions in winter (see Figure 1 in Cameron and Scrosati, 2023) can now occur because of the ongoing Arctic amplification (Cohen et al., 2021; You et al., 2021). In addition, cyclones formed in the tropical Atlantic now reach southern Nova Scotia more frequently (Scrosati, 2020, 2023). As these abiotic changes have the potential to alter historic biogeographic patterns along the Nova Scotia coast, we surveyed intertidal communities again in 2024, ten years after our first survey. As repeated monitoring is necessary to detect ecological change (Raimondi et al., 2019; Estes and Vermeij, 2022; Meunier et al., 2024; Sato et al., 2025), the present article makes this new dataset available to facilitate future evaluations of change in addition to evaluating recent changes.
Methods of data collection and analysis
Between 22-28 July 2024, we measured the abundance of seaweeds and sessile invertebrates at eight of the nine locations surveyed annually from 2014 to 2017 (Figure 1). We collected the data in the same habitats surveyed in those four years, which are located at the mid-to-high intertidal zone of wave-exposed areas (those directly facing the open ocean; Figure 1). The geographic coordinates of each location and the surveyed elevations (in meters above chart datum) are available from Scrosati et al. (2022). For simplicity, hereafter we refer to those locations as L1 to L8 from north to south (see their full names in Figure 1). The only location not surveyed in 2024 was the southernmost location surveyed in 2014-2017, which is smaller than all others and thus only allowed for a limited number of sampling units to be examined. In 2024, we followed the same sampling protocol as employed before. At each surveyed location during a low tide, we measured the percent cover of sessile species (seaweeds and sessile invertebrates) and the density of mobile species (snails, etc.) found in 30 random quadrats measuring 20 cm x 20 cm. As done by Scrosati et al. (2022), here we focus on the percent cover of sessile species because the density of mobile species may locally change daily depending on weather conditions at low tide (e.g., cool rainy days vs. warm sunny days), which makes snapshot data less representative of their seasonal abundance. We used field guides (Gibson, 2003; Martínez, 2003) and taxonomic keys (Pollock, 1998; Sears, 1998; Villalard-Bohnsack, 2003) for species identifications. The full dataset on species abundance for 2024, including collection dates, is freely available from the figshare online repository (Scrosati and Cameron, 2025). The 2014-2017 abundance dataset is available from Scrosati et al. (2022). Maps of the specific areas sampled at each location are available from Scrosati and Ellrich (2022).
In this article, we provide a basic analysis of the patterns encountered for sessile species in 2024 and highlight the main changes relative to 2014-2017. To test if species composition (a combined measure of species identity and abundance) differed among locations in 2024, we ran a permutational multivariate analysis of variance (PERMANOVA) based on Bray-Curtis distances between quadrats and involving 9999 permutations (Anderson, 2017). As significant differences were detected (see below), we evaluated how species composition varied across locations through a nonmetric multidimensional scaling (NMDS) ordination based on Bray-Curtis scores (Clarke et al., 2014). Because these analyses showed signs of changing latitudinal patterns in communities relative to the 2014-2017 patterns, we examined interannual changes in the abundance of iconic taxa that often define community structure on temperate rocky shores through facilitation or competition (Hawkins et al., 2019; Palomo et al., 2019; Menge et al., 2019; Ishida et al., 2021; Thyrring et al., 2021). In the studied habitats (mid-to-high intertidal zone of wave-exposed areas), these taxa are brown algae of the genus Fucus (F. vesiculosus and F. distichus edentatus), barnacles (Semibalanus balanoides), and mussels of the genus Mytilus (M. edulis and M. trossulus). The species belonging to a same genus were lumped for our analyses because they are sometimes difficult to tell apart in the field (especially the juveniles) and because they have similar ecological effects. Photographs of these organisms are shown in Figure 1. We evaluated interannual changes in the abundance of these taxa at each location through permutational analyses of variance (ANOVA) involving 9999 permutations. We did these analyses with PRIMER 6.1.18 plus PERMANOVA+ 1.0.8 software (Clarke and Gorley, 2006; Anderson et al., 2008).
Since scour by drift ice is a major factor influencing latitudinal patterns in intertidal species composition on this coast (see above), we also evaluated recent changes in coastal ice load to assess their role in the community changes detected in 2024. To measure coastal ice load, we followed the same approach as for the 2014-2017 study (Scrosati et al., 2022). We used daily ice charts (Canadian Ice Service, 2025) to measure the daily concentration of coastal drift ice at our eight locations for each year between 2014 and 2024. Even though drift ice occurs on the open Atlantic coast of mainland Nova Scotia in late winter or early spring, we examined all ice charts available for the November–April periods to ensure the inclusion of all ice data. We then used those daily values to produce an annual score of cumulative ice load for each location, which is a proxy for ice scour intensity (Petzold et al., 2014).
Signs of changing latitudinal community patterns
As for 2014-2017 (Scrosati et al., 2022), species composition in 2024 differed significantly among the surveyed locations (pseudo-F = 33.58, P < 0.001). However, the latitudinal pattern for 2024 showed important differences relative to the patterns found for 2014-2017, as revealed by NMDS ordinations. The ordinations for 2014-2017 can be seen in figure 4 of Scrosati et al. (2022). To facilitate comparisons with those years, here we group locations in the ordination for 2024 using the same threshold distances that were used in the ordinations for 2014-2017.
The ordination for 2024 indicated that, for the first time since we began monitoring this coast, all locations except one (L4) formed a relatively homogeneous group (Figure 1). Differences between the three northernmost locations (L1 to L3) and the four southern locations (L5 to L8) only occurred under the most stringent of the three threshold distances used to group locations (Figure 1). Conversely, for the 2014-2017 period, L1 and L3 were never grouped with the southern locations even under the least stringent threshold distance used, while L2 was grouped with the southern locations in only three of those four years (Scrosati et al., 2022).
The convergence in species composition between northern and southern locations found in 2024 parallels the growing abundance of Fucus seen recently at northern locations (Figure 2). These algae act as foundation species when abundant, as fucoid canopies ameliorate local abiotic conditions and provide safe habitat for small species (Watt and Scrosati, 2013). Fucoid canopies have been consistently extensive at southern locations over the years (Figure 2), which was generally associated with a higher species richness (Scrosati et al., 2022). However, now these canopies are also growing in extent at northern locations, more recently at L1 and L3 than at L2 (Figure 2; ANOVAs’ P < 0.05). This expansion of fucoid canopies is occurring as the frequency of ice scour is decreasing. The irregular occurrence of disturbance by drift ice at northern locations used to keep their intertidal communities fluctuating between early and intermediate successional stages over the years (Petzold et al., 2014; Scrosati et al., 2022). In recent years, however, drift ice along the entire Atlantic coast of mainland Nova Scotia has become rare to inexistent (Figure 2), likely as a result of the decreasing buildup of sea ice in the Gulf of St. Lawrence in winter (Galbraith et al., 2024). Although not as quickly as in recent years, the southern reach of drift ice on the open Atlantic coast of Nova Scotia has been decreasing even before we started our monitoring program. As an example, abundant drift ice reached the coast in the L6 region (central Nova Scotia) in April 1987, causing extensive intertidal disturbance (McCook and Chapman, 1997; Minchinton et al., 1997), but such an intense ice scour has not occurred since then that far south on this coast. In fact, as of 2024, the last occurrence of abundant drift ice causing intense ice scour along this coast was in 2014 and it only reached as far south as L4 (Petzold et al., 2014). This ongoing loss of seasonal drift ice is therefore what may be allowing Fucus to grow at northern locations at levels traditionally seen in southern locations. As expected, these changes are being accompanied by increases in species richness at northern locations. For example, including sessile and mobile species together (see data in Scrosati and Cameron, 2025), species richness has increased by an average of 2.5 times at locations L1 to L3 from 2014 to 2024. Similar increases in species richness and macroalgal cover have been reported for intertidal habitats in Svalbard (Arctic Norway) following recent reductions in sea ice duration and extent (Weslawski et al., 2010).
Figure 2
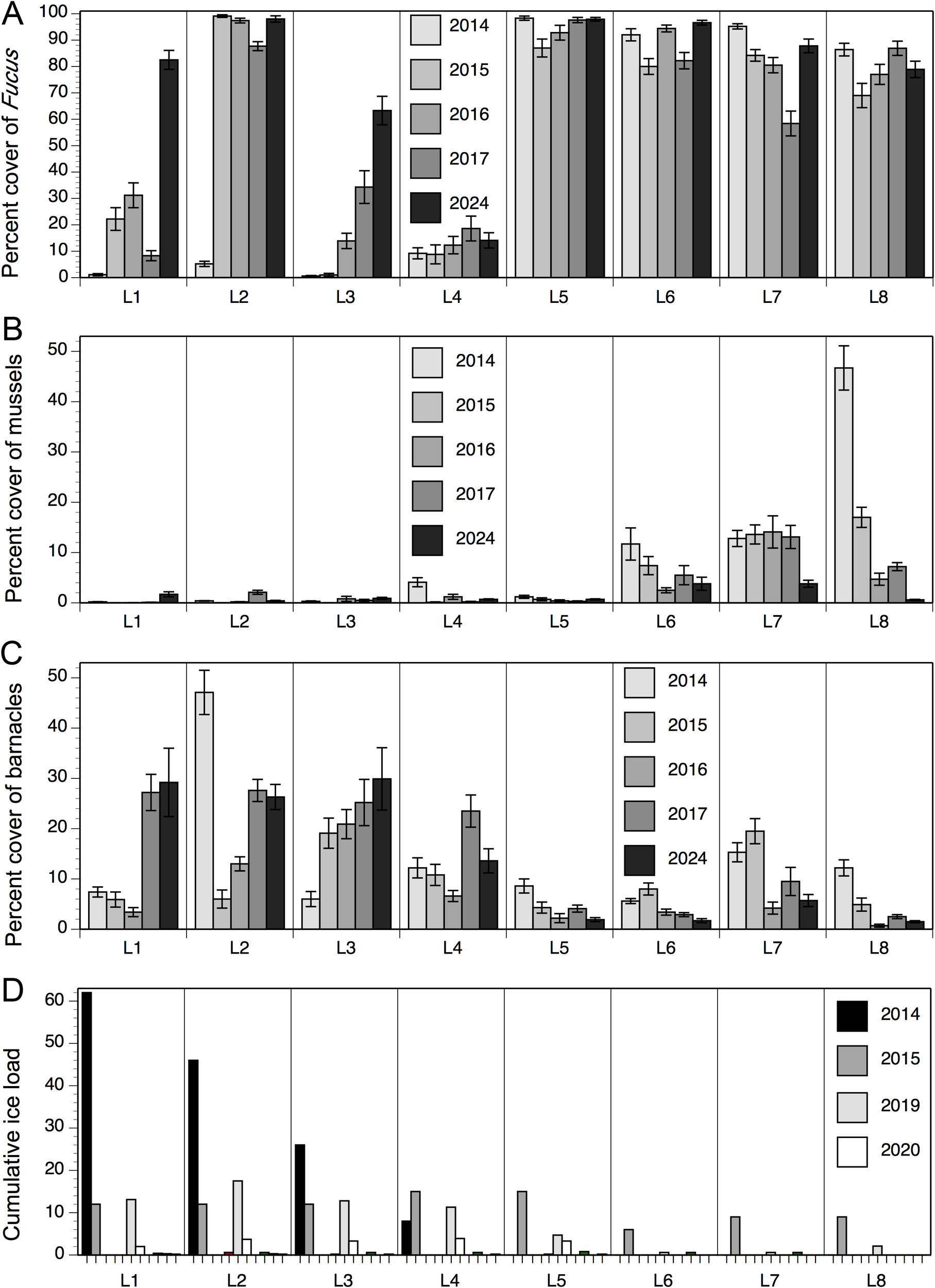
(A) Percent cover of Fucus (mean ± SE) at the mid-to-high intertidal zone of wave-exposed habitats at the 8 studied locations in 2014-2017 (data from Scrosati et al., 2022) and 2024 (data from this article). (B) Percent cover of mussels (mean ± SE) at the mid-to-high intertidal zone of wave-exposed habitats at the 8 studied locations in 2014-2017 (data from Scrosati et al., 2022) and 2024 (data from this article). (C) Percent cover of barnacles (mean ± SE) at the mid-to-high intertidal zone of wave-exposed habitats at the 8 studied locations in 2014-2017 (data from Scrosati et al., 2022) and 2024 (data from this article). (D) Cumulative ice load (in winter to early spring) at the 8 studied locations plotted for each year between 2014 and 2024 (only the codes for 2014, 2015, 2019, and 2020 are indicated in this panel because ice load was null at all locations in 2016, 2017, and 2021 and null at some locations and negligible at others in 2018, 2022, 2023, and 2024).
Mussels have also become more similar in abundance across the studied locations recently, thereby also contributing to the convergence between northern and southern communities on our coast. However, as opposed to Fucus, mussel cover is now uniformly low along the coast (Figure 2). At northern locations (L1 to L3), mussels used to be rare or absent, but recently they have slightly increased their abundance (Figure 2; ANOVAs’ P < 0.05) possibly as a result of the lack of ice scour and the protection conferred by the newly extensive fucoid canopies. Conversely, at southern locations (L6 to L8), mussels used to be abundant but recently their extent has fallen dramatically (Figure 2; ANOVAs’ P < 0.05). At L8, their abundance had been steadily decreasing since 2014, seemingly as part of a trend also reported farther south in New England (Sorte et al., 2017). However, in February 2023, an unusually severe cold snap was followed by the mass disappearance of mussels at mid-to-high elevations in wave-exposed habitats, which we documented at L6 to L8 (Cameron and Scrosati, 2023). Although some recolonization (probably from populations at lower elevations) took place one year later (Scrosati and Cameron, 2024), their abundance was still untypically low in 2024 (Figure 2).
In contrast to fucoid algae and mussels, barnacles are starting to exhibit differences in abundance between northern and southern locations. In 2014-2017, barnacles occurred in similar abundances along the coast, with relatively moderate values everywhere (except for a marked spike at L2 in 2014 shortly after intense ice scour). However, now their abundance is becoming consistently higher at northern locations than at southern locations, where they are becoming rare (Figure 2; ANOVAs’ P < 0.05). While it is unclear why barnacles are decreasing in abundance in southern communities, their recent gains in northern communities may also respond to the growing extent of fucoid canopies or to warming-induced increases in recruitment (Scrosati and Ellrich, 2024). As barnacle stands facilitate mussel recruitment (Seed, 1969; Navarrete and Castilla, 1990; Menge et al., 2011), mussels might keep increasing in abundance at northern locations and might eventually outcompete barnacles as succession without drift ice proceeds, although extreme abiotic events (such as pronounced cold snaps) might alternatively prevent this from happening.
A separate comment is worth for L4, as this location represents an exception to the changes seen at L1 to L3 in recent years. Between 2014 and 2024, L4 has shown little variation in the abundance of mussels and barnacles (Figure 2) and actually no significant changes in the abundance of fucoid algae (ANOVA’s P = 0.25), which has remained consistently low (Figure 2). While all surveyed locations face open oceanic waters and are thus exposed to strong wave action, the intertidal zone at L4 has a very steep slope that may result in higher hydrodynamic forces that limit species colonization to intertidal substrates more strongly. Either way, L4 remains as an anomaly for the general patterns described above based on the other locations.
Concluding remarks
The historical pattern of latitudinal variation in intertidal community structure along the Nova Scotia coast is changing in connection to ongoing environmental variation. Overall, northern communities seem to be gaining stability as ice scour becomes infrequent to absent, while southern communities are starting to show signs of instability as some of their iconic species, such as mussels, can now show marked interannual changes linked to extreme abiotic events. In broad terms, these changes appear to exemplify trends of poleward movement of community patterns while communities closer to the trailing edge become more unstable, as seen in other systems (Raimondi et al., 2019; Rodman et al., 2022; Pinsky et al., 2020). However, climatic and oceanographic change does not only involve changes in average conditions but also increases in the frequency of extreme events (IPCC, 2023), suggesting that these intertidal systems will change in unexpected ways. For example, it is unknown if mussels will ever regain historical abundances anywhere on the studied coast given their sensitivity to extreme events. Persistently low mussel abundances would likely decrease intertidal diversity through the collapse of the myriad species that mussel stands can host (Arribas et al., 2014), which may end up affecting other levels of the intertidal food web. These unexpected changes might ultimately lead to the establishment of novel community types (Conradi et al., 2024; Kerr et al., 2025) or regime shifts (Kortsch et al., 2012; Meunier et al., 2024). Continued abiotic and biotic monitoring is thus necessary (Edmunds, 2024b) to understand community trajectories and predict future biogeographic changes for this coastal metaecosystem.
Statements
Data availability statement
The new dataset (2024) generated for this article is freely available from the figshare online repository: https://doi.org/10.6084/m9.figshare.28550519.v1.
Author contributions
RS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. NC: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. JE: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing.
Funding
This study was funded by a Discovery Grant awarded to RAS by the Natural Sciences and Engineering Research Council of Canada (NSERC, grant number 311624).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Anderson M. J. (2017). Permutational multivariate analysis of variance (PERMANOVA) (Wiley StatsRef). doi: 10.1002/9781118445112.stat07841
2
Anderson M. J. Gorley R. N. Clarke K. R. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods (Plymouth: PRIMER-E).
3
Arribas L. P. Donnarumma L. Palomo M. G. Scrosati R. A. (2014). Intertidal mussels as ecosystem engineers: Their associated invertebrate biodiversity under contrasting wave exposures. Mar. Biodiv.44, 203–211. doi: 10.1007/s12526-014-0201-z
4
Cameron N. M. Scrosati R. A. (2023). Mass disappearance of intertidal mussels after an unusual winter cold snap in eastern Canada. Ecology104, e4179. doi: 10.1002/ecy.4179
5
Canadian Ice Service (2025). Canadian ice service. Available online at: https://www.Canada.ca/en/environment-climate-change/services/ice-forecasts-observations.html (Accessed March 6, 2025).
6
Clarke K. R. Gorley R. N. (2006). PRIMER v6: User Manual/Tutorial (Plymouth: PRIMER-E).
7
Clarke K. R. Gorley R. N. Somerfield P. J. Warwick R. M. (2014). Change in Marine Communities: An Approach to Statistical Analysis and Interpretation (Plymouth: PRIMER-E).
8
Cohen J. Agel L. Barlow M. Garfinkel C. I. White I. (2021). Linking Arctic variability and change with extreme winter weather in the United States. Science373, 1116–1121. doi: 10.1126/science.abi9167
9
Conradi T. Eggli U. Kreft H. Schweiger A. H. Weigelt P. Higgins S. I. (2024). Reasessment of the risks of climate change for terrestrial ecosystems. Nat. Ecol. Evol.8, 888–900. doi: 10.1038/s41559-024-02333-8
10
Dudgeon D. Strayer D. L. (2025). Bending the curve of global freshwater biodiversity loss: What are the prospects? Biol. Rev.100, 205–226. doi: 10.1111/brv.v100.1
11
Edmunds P. J. (2024a). Decadal-scale time series highlight the role of chronic disturbances in driving ecosystem collapse in the Anthropocene. Ecology105, e4360. doi: 10.1002/ecy.v105.8
12
Edmunds P. J. (2024b). Why keep monitoring coral reefs? BioScience74, biae046. doi: 10.1093/biosci/biae046
13
Estes J. A. Vermeij G. J. (2022). History’s legacy: Why future progress in ecology demands a view of the past. Ecology103, e3788. doi: 10.1002/ecy.v103.11
14
Galbraith P. S. Sévigny C. Bourgault D. Dumont D. (2024). Sea ice interannual variability and sensitivity to fall oceanic conditions and winter air temperature in the Gulf of St. Lawrence, Canada. J. Geophys. Res.: Oceans129, e2023JC020784. doi: 10.1029/2023JC020784
15
Gibson M. (2003). Seashores of the Maritimes (Halifax: Nimbus Publishing).
16
Hawkins S. J. Pack K. E. Firth L. B. Mieszkowska N. Evans A. J. Martins G. M. et al . (2019). “The intertidal zone of the north-east Atlantic region: Pattern and process,” in Interactions in the Marine Benthos: Global Patterns and Processes. Eds. HawkinsS. J.BohnK.FirthL. B.WilliamsG. A. (Cambridge University Press, Cambridge), 7–46.
17
IPCC (2023). Climate change 2023: Synthesis report. Intergovernmental Panel on Climate Change. doi: 10.59327/ipcc/ar6-9789291691647.001 (Accessed March 6, 2025).
18
Ishida K. Tachibana M. Hori M. Okuda T. Yamamoto T. Nakaoka M. et al . (2021). Quantifying the dynamics of rocky intertidal sessile communities along the Pacific coast of Japan: Implications for ecological resilience. Sci. Rep.11, 16073. doi: 10.1038/s41598-021-95348-1
19
Kerr M. R. Ordonez A. Riede F. Atkinson J. Pearce E. A. Sykut M. et al . (2025). Widespread ecological novelty across the terrestrial biosphere. Nat. Ecol. Evol.9, 589–598. doi: 10.1038/s41559-025-02662-2
20
Kortsch S. Primicerio R. Beuchel F. Renaud P. E. Rodrigues J. Lønne O. J. et al . (2012). Climate-driven regime shifts in Arctic marine benthos. Proc. Natl. Acad. Sci. U. S. A.109, 14052–14057. doi: 10.1073/pnas.1207509109
21
Martínez A. J. (2003). Marine Life of the North Atlantic: Canada to New England. (New York: Aqua Quest Publications).
22
McCook L. J. Chapman A. R. O. (1997). Patterns and variations in natural succession following massive ice scour of a rocky intertidal seashore. J. Exp. Mar. Biol. Ecol.214, 121–147. doi: 10.1016/S0022-0981(96)02751-7
23
Menge B. A. Caselle J. E. Milligan K. Gravem S. A. Gouhier T. C. White J. W. et al . (2019). Integrating coastal oceanic and benthic ecological approaches for understanding large-scale meta-ecosystem dynamics. Oceanography32, 38–49. doi: 10.5670/oceanog.2019.309
24
Menge B. A. Hacker S. D. Freidenburg T. Lubchenco J. Craig R. Rilov G. et al . (2011). Potential impact of climate-related changes is buffered by differential responses to recruitment and interactions. Ecol. Monogr.81, 493–509. doi: 10.1890/10-1508.1
25
Menge B. A. Robinson J. W. Poirson B. N. Gravem S. A. (2023). Quantitative biogeography: Decreasing and more variable dynamics of critical species in an iconic meta-ecosystem. Ecol. Monogr.93, e1556. doi: 10.1002/ecm.v93.1
26
Meunier Z. D. Hacker S. D. Menge B. A. (2024). Regime shifts in rocky intertidal communities associated with a marine heatwave and disease outbreak. Nat. Ecol. Evol.8, 1285–1297. doi: 10.1038/s41559-024-02425-5
27
Minchinton T. E. Scheibling R. E. Hunt H. L. (1997). Recovery of an intertidal assemblage following a rare occurrence of scouring by sea ice in Nova Scotia, Canada. Bot. Mar.40, 139–148. doi: 10.1515/botm.1997.40.1-6.139
28
Navarrete S. A. Castilla J. C. (1990). Barnacle walls as mediators of intertidal mussel recruitment: Effects of patch size on the utilization of space. Mar. Ecol. Prog. Ser.68, 113–119. doi: 10.3354/meps068113
29
Palomo M. G. Bagur M. Calla S. Dalton M. C. Soria S. A. Hawkins S. J. (2019). “Biodiversity and interactions on the intertidal rocky shores of Argentina (south-west Atlantic),” in Interactions in the Marine Benthos: Global Patterns and Processes. Eds. HawkinsS. J.BohnK.FirthL. B.WilliamsG. A. (Cambridge University Press, Cambridge), 164–189.
30
Petzold W. Willers M. T. Scrosati R. A. (2014). Visual record of intertidal disturbance caused by sea ice in the spring on the Atlantic coast of Nova Scotia. F1000Research3, 112. doi: 10.12688/f1000research.4146.1
31
Pinsky M. L. Hillebrand H. Chase J. M. Antão L. H. Hirt M. R. Brose U. et al . (2025). Warming and cooling catalyse widespread temporal turnover in biodiversity. Nature638, 995–999. doi: 10.1038/s41586-024-08456-z
32
Pinsky M. L. Selden R. L. Kitchel Z. J. (2020). Climate-driven shifts in marine species ranges: Scaling from organisms to communities. Annu. Rev. Mar. Sci.12, 153–179. doi: 10.1146/annurev-marine-010419-010916
33
Pollock L. W. (1998). A Practical Guide to the Marine Animals of Northeastern North America (New Brunswick: Rutgers University Press).
34
Raimondi P. T. Miner C. M. Menge B. A. Blanchette C. A. Lohse D. P. (2019). Quantitative biogeography: Large-scale, long-term change in the rocky intertidal region of the California Current Large Marine Ecosystem. Oceanography32, 26–37. doi: 10.5670/oceanog.2019.308
35
Rodman K. C. Crouse J. E. Donager J. J. Huffman D. W. Sánchez Meador A. J. (2022). Patterns and drivers of recent land cover change on two trailing-edge forest landscapes. For. Ecol. Manage.521, 120449. doi: 10.1016/j.foreco.2022.120449
36
Sahade R. Lagger C. Torre L. Momo F. Monien P. Schloss I. et al . (2015). Climate change and glacier retreat drive shifts in an Antarctic benthic ecosystem. Sci. Adv.1, e1500050. doi: 10.1126/sciadv.1500050
37
Sato H. Ishida K. Noda T. (2025). Temporal trends of community and climate changes in the Anthropocene: 21-year dynamics of four major functional groups in a rocky intertidal habitat along the Pacific coast of Japan. Front. Mar. Sci.11, 1477142. doi: 10.3389/fmars.2024.1477142
38
Saucier F. J. Roy F. Gilbert D. Pellerin P. Ritchie H. (2003). Modeling the formation and circulation processes of water masses and sea ice in the Gulf of St. Lawrence, Canada. J. Geophys. Res.108, 3269. doi: 10.1029/2000JC000686
39
Scrosati R. A. (2020). Cyclone-driven coastal upwelling and cooling depend on location relative to the cyclone’s path: Evidence from Dorian’s arrival to Atlantic Canada. Front. Mar. Sci.7, 651. doi: 10.3389/fmars.2020.00651
40
Scrosati R. A. (2023). Further loss of intertidal mussel stands on the Nova Scotia coast (Canada) after the passage of cyclone Lee. Diversity15, 1150. doi: 10.3390/d15111150
41
Scrosati R. A. Cameron N. M. (2024). Recolonization of intertidal mussels in Nova Scotia (Canada) after their mass disappearance following the severe 2023 winter cold snap. Diversity16, 503. doi: 10.3390/d16080503
42
Scrosati R. A. Cameron N. M. (2025). Abundance of seaweeds and invertebrates in wave-exposed rocky intertidal habitats along the Canadian Atlantic coast (2024). figshare dataset. doi: 10.6084/m9.figshare.28550519.v1
43
Scrosati R. A. Ellrich J. A. (2022). Photos of wave-exposed rocky intertidal locations along the Atlantic coast of Nova Scotia, Canada. figshare online resource. doi: 10.6084/m9.figshare.20740879.v1
44
Scrosati R. A. Ellrich J. A. (2024). Massive barnacle recruitment on the Gulf of St. Lawrence coast of Nova Scotia (Canada) in 2024 linked to increased sea surface temperature. PeerJ12, e18208. doi: 10.7717/peerj.18208
45
Scrosati R. A. Freeman M. J. Ellrich J. A. Petzold W. (2022). Biogeography of algae and invertebrates from wave-exposed rocky intertidal habitats along the Atlantic coast of Nova Scotia (Canada): Latitudinal and interannual patterns and possible underlying drivers. Front. Mar. Sci.9, 987162. doi: 10.3389/fmars.2022.987162
46
Sears J. R. (1998). NEAS Keys to the Benthic Marine Algae of the Northeastern Coast of North America from Long Island Sound to the Strait of Belle Isle (Dartmouth: Northeast Algal Society).
47
Seed R. (1969). The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores I. Breeding and settlement. Oecologia3, 277–316. doi: 10.1007/BF00390380
48
Smale D. A. (2007). Ice disturbance intensity structures benthic communities in nearshore Antarctic waters. Mar. Ecol. Prog. Ser.349, 89–102. doi: 10.3354/meps07104
49
Sorte C. J. B. Davidson V. E. Franklin M. C. Benes K. M. Doellman M. M. Etter R. J. et al . (2017). Long-term declines in an intertidal foundation species parallel shifts in community composition. Glob. Change Biol.23, 341–352. doi: 10.1111/gcb.13425
50
Sousa W. P. (1984). The role of disturbance in natural communities. Ann. Rev. Ecol. Syst.15, 353–391. doi: 10.1146/annurev.es.15.110184.002033
51
Sugihara N. G. van Wagtendonk J. W. Fites-Kaufman J. (2018). “Fire as an ecological process,” in Fire in California’s Ecosystems. Ed. van WagtendonkJ. W. (University of California Press, Berkeley), 58–74.
52
Thyrring J. Wegeberg S. Blicher M. E. Krause-Jensen D. Høgslund S. Olesen B. et al . (2021). Latitudinal patterns in intertidal ecosystem structure in West Greenland suggest resilience to climate change. Ecography44, 1156–1168. doi: 10.1111/ecog.05381
53
Villalard-Bohnsack M. (2003). Illustrated Key to the Seaweeds of New England (Kingston: The Rhode Island Natural History Survey).
54
Virta L. Teittinen A. (2022). Threshold effects of climate change on benthic diatom communities: Evaluating impacts of salinity and wind disturbance on functional traits and benthic biomass. Sci. Total Environ.826, 154130. doi: 10.1016/j.scitotenv.2022.154130
55
Watt C. A. Scrosati R. A. (2013). Bioengineer effects on understory species richness, diversity, and composition change along an environmental stress gradient: Experimental and mensurative evidence. Estuar Coast. Shelf Sci.123, 10–18. doi: 10.1016/j.ecss.2013.02.006
56
Wernberg T. Thomsen M. S. Baum J. K. Bishop M. J. Bruno J. F. Coleman M. A. et al . (2024). Impacts of climate change on marine foundation species. Annu. Rev. Mar. Sci.16, 247–282. doi: 10.1146/annurev-marine-042023-093037
57
Weslawski J. M. Wiktor J. Kotwicki L. (2010). Increase in biodiversity in the Arctic rocky littoral, Sorkappland, Svalbard, after 20 years of climate warming. Mar. Biodiv.40, 123–130. doi: 10.1007/s12526-010-0038-z
58
White R. H. Anderson S. Booth J. F. Braich G. Draeger C. Fei C. et al . (2023). The unprecedented Pacific Northwest heatwave of June 2021. Nat. Commun.14, 727. doi: 10.1038/s41467-023-36289-3
59
Wiens J. J. Zelinka J. (2024). How many species will Earth lose to climate change? Glob. Change Biol.30, e17125. doi: 10.1111/gcb.17125
60
Wolfe J. D. Luther D. A. Jirinec V. Collings J. Johnson E. I. Bierregaard R. O. et al . (2025). Climate changes aggravates bird mortality in pristine tropical forests. Sci. Adv.11, eadq8086. doi: 10.1126/sciadv.adq8086
61
Yin H. Rudolf V. H. W. (2024). Time is of the essence: A general framework for uncovering temporal structures of communities. Ecol. Lett.27, e14481. doi: 10.1111/ele.14481
62
You Q. Cai Z. Pepin N. Chen D. Ahrens B. Jiang Z. et al . (2021). Warming amplification over the Arctic Pole and Third Pole: Trends, mechanisms, and consequences. Earth-Sci. Rev.217, 103625. doi: 10.1016/j.earscirev.2021.103625
63
Zhang Y. Loreau M. He N. Wang J. Pan Q. Bai Y. et al . (2018). Climate variability decreases species richness and community stability in a temperate grassland. Oecologia188, 183–192. doi: 10.1007/s00442-018-4208-1
Summary
Keywords
alga, barnacle, intertidal, mussel, seaweed, sea ice
Citation
Scrosati RA, Cameron NM and Ellrich JA (2025) Signs of latitudinal changes in the stability of rocky intertidal communities from Atlantic Canada in relation to ongoing environmental variation. Front. Mar. Sci. 12:1590589. doi: 10.3389/fmars.2025.1590589
Received
09 March 2025
Accepted
05 May 2025
Published
04 June 2025
Volume
12 - 2025
Edited by
Sérgio P. Ávila, University of the Azores, Portugal
Reviewed by
Maria Gabriela Palomo, Independent researcher, Ciudad de Buenos Aires, Argentina
Madelyn Moawad, National Institute of Oceanography and Fisheries (NIOF), Egypt
Updates
Copyright
© 2025 Scrosati, Cameron and Ellrich.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ricardo A. Scrosati, rscrosat@stfx.ca
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.