- 1Loro Parque Fundación, Puerto de la Cruz, Spain
- 2Departamento de Ingeniería Industrial, Universidad de La Laguna, La Laguna, Spain
Introduction: Monitoring acoustic activity is a promising approach for assessing animal welfare in cetaceans. In particular, variations in vocal production may reflect changes in arousal and emotional state, which are critical components of welfare.
Methods: We implemented a long-term passive acoustic monitoring system to continuously record the vocal activity of a group of five killer whales (Orcinus orca) under human care at Orca Ocean, Loro Parque. Using a custom detection algorithm and classification criteria, we focused on quantifying the rates of discrete call production throughout the day and across different seasons.
Results: The results revealed strong diel patterns in vocal activity, with increased rates during the day, particularly around feeding and training sessions, and minimal vocal activity overnight. Additionally, we observed seasonal variability, with higher overall call rates during the summer and autumn months. These fluctuations suggest that vocal activity may be influenced by both scheduled events and environmental conditions.
Discussion: Our findings support the use of passive acoustic monitoring as a tool to identify behavioural rhythms and potential indicators of arousal in orcas. This approach may contribute to welfare assessment protocols by providing non-invasive, continuous data on vocal behaviour. Moreover, the system demonstrated robust performance over extended periods, confirming its suitability for long-term implementation in both managed and wild populations.
1 Introduction
Killer whales (Orcinus orca) communicate through a wide range of vocalizations, including pulsed calls, whistles, and echolocation clicks (Ford, 1989, 1991; Thomsen et al., 2002). The ability to produce, perceive, and interpret these sounds is essential for their survival, as it enables critical behaviors such as social interaction, reproduction, and hunting (Ford, 1989; Miller, 2006). Pulsed calls, in particular, likely support intra-group communication, with pods typically sharing between 7 and 17 distinct call types that are structurally unique (Ford, 1989). These discrete calls are highly stereotyped and specific to individual pods, allowing researchers to identify populations and track their movements across oceans (Ford, 1991; Yurk et al., 2002). Pulsed calls and whistles are thought to be communication signals (Ford, 1989; Thomsen et al., 2002) used by killer whales in a variety of behavioural contexts, including social interactions (Ford, 1989; Filatova et al., 2013). These calls are thought to facilitate contact between members of the social group, ensuring cohesion despite physical separation (Ford, 1989, 1991).The characteristics of killer whale vocalizations can also vary depending on ecological circumstances and prey specialization. For instance, Shetland herring-eating killer whales produce a specific call type that matches a vocalization previously identified in Icelandic killer whales (Deecke et al., 2011). This call, thought to function as a herding call, likely aids in concentrating herring during feeding. Additionally, the structure and frequency of discrete calls are influenced by their functional context (Miller, 2006). Analysis of frequency in resident killer whales of the Northeast Pacific, revealed two distinct types: biphonic calls, which include overlapping high-frequency components, and monophonic calls, which do not (Filatova et al., 2009; Filatova, 2020). Biphonic calls exhibit higher source levels, suggesting a larger active space compared to monophonic calls, and likely serve different communicative purposes.
In addition to variations in the structure of vocalizations, sound production patterns also exhibit considerable variability across species and contexts. Studies on primates, for example, highlight the diversity in vocal production across different species, shedding light on its ecological and social drivers (Schneiderová et al., 2020). Among cetaceans, this variability has been explored extensively, particularly in odontocetes, where vocalizations are used to monitor presence and abundance in remote locations through passive acoustic monitoring (PAM) (André et al., 2011; Hanson et al., 2013; Fleishman et al., 2023). Additionally, vocalization patterns often vary with behavioral states, such as activity levels or arousal, which must be accounted for to improve detection probabilities (Bain, 1986; Buckstaff, 2004; Holt et al., 2013; Probert et al., 2021).
For example, in bottlenose dolphins (Tursiops truncatus), whistle emission rates can fluctuate dramatically depending on environmental or social factors. Moore and Ridgway (1996) observed that the number of whistles emitted by two female dolphins over a year ranged from 1,682 to 6,793 per 24-hour period, with sound production negatively correlated with progesterone levels. Similarly, Buckstaff (2004) found that dolphins whistle more frequently during the onset of vessel approaches, possibly reflecting heightened arousal or an adaptive response to signal masking in noisy environments. In contrast, Quick and Janik (2008) noted that whistle rates decreased in larger groups where signal masking by conspecifics might hinder communication. Geographic and ecological factors further contribute to variability in vocal behavior, as highlighted by Oswald et al. (2008), who identified significant differences in whistle rates among small odontocetes in temperate and tropical regions, influenced by predator density, group composition, and environmental conditions.
Killer whales exhibit considerable variability in their vocalization patterns, influenced by behavioral states, social dynamics, and ecological contexts. Early studies (Ford, 1989; van Opzeeland et al., 2005) demonstrated how call sequences, rather than isolated call types, are more strongly associated with group behaviors such as foraging. For example, during carousel feeding, the temporal organization of call sequences appears critical for maintaining group coordination and synchronizing movements. Changes in vocal activity have also been linked to ecological specialization, as shown by (Deecke et al., 2011), who observed distinct patterns of call production in seal-hunting and herring-eating killer whale populations. Seal-hunting groups exhibited lower call rates, possibly to avoid detection by acoustically sensitive prey, whereas herring-eating groups employed higher call rates, including specific herding calls, to concentrate prey during feeding.
Vocalization rates in killer whales also vary depending on environmental and social factors. van Opzeeland et al. (2005) observed fluctuations in call rates linked to activity states, with increased rates during socializing compared to traveling or resting. Additionally, Poupard et al. (2021) noted behavioral responses, such as increased call rates and changes in vocalization patterns, when killer whales encountered external stimuli, including vessels. These findings suggest that vocalization patterns in killer whales are not static but instead dynamically adapt to their ecological and social environments.
Vocalization rates in animals are often linked to levels of arousal, reflecting responses to social and environmental stimuli. In terrestrial mammals such as silver foxes, vocal production rates increase during both positive and negative arousal states, while rhesus monkeys emit higher-frequency calls in negative contexts like aggression (Whitham and Miller, 2024). Among cetaceans, the relationship between vocal activity and arousal has been explored extensively, particularly in bottlenose dolphins (Tursiops truncatus). Whistle production rates have been shown to increase during foraging, human interactions, mother-calf separations, and perceived stress, reflecting heightened arousal in these situations (Eskelinen et al., 2022). In dolphins under human care, peaks in vocal activity are often associated with feeding and training sessions, indicating a connection to anticipatory behaviors and social coordination, while decreases occur during resting periods, particularly at night (Therrien et al., 2012; Probert et al., 2021, 2023). Furthermore, sound production rates during cooperative interactions are higher than during solitary activities, highlighting the role of vocalizations in coordinating social behaviors (Eskelinen et al., 2016).
In killer whales, Weiss et al. (2007); Rehn et al. (2007) explored vocal activity in relation to arousal across different social contexts in the Northern Resident population off Vancouver Island. Their findings revealed significant changes in vocal behavior depending on the presence and identity of accompanying whales. Specifically, high-arousal call types, such as family-specific call subtypes and aberrant calls, were emitted at greater rates during intergroup encounters, while low-arousal calls were more commonly used in less dynamic social settings. These results suggest that killer whale vocalization rates and types are closely tied to social arousal.
Building on these findings (Probert et al., 2021, 2023) emphasized the potential of vocal behavior as a non-invasive tool for monitoring welfare in animals under human care. In their study, vocalization rates and characteristics were associated with scheduled events such as feeding and human interaction, and were thought to reflect underlying emotional states. They proposed that establishing baseline vocalization patterns for individuals provides a valuable index for detecting abnormal changes, which could indicate shifts in welfare state. While increased arousal may not always distinguish between positive and negative emotional states, monitoring vocal behavior offers critical insights into the animals’ experiences and welfare, both in natural settings and under human care.
The use of vocalizations as a proxy for monitoring animal welfare has gained significant attention, beginning with terrestrial mammals. Early studies suggested that vocal behavior can reflect emotional and physiological states, providing a non-invasive means of assessing welfare (Manteuffel et al., 2004; Schön et al., 2004; Hewson, 2004). Advances in automated acoustic analysis have further facilitated the application of vocal monitoring for detecting stress, positive emotions, and even early indicators of disease in animals (Laurijs et al., 2021; Whitham and Miller, 2024; Jones et al., 2024). Notably, studies on bottlenose dolphins have identified the so-called “victory squeal”—a distinct burst-pulse vocalization emitted upon successful task completion or prey capture—as a potential acoustic indicator of positive affect, possibly mediated by dopaminergic activation (Ridgway et al., 2015).
In cetaceans, vocal behavior has similarly been used to infer welfare-related states. Bottlenose dolphins (Tursiops truncatus) increase whistle rates during stressful contexts such as capture-release events and medical procedures or boat presence, while decreases in vocal activity have been observed following significant stressors, such as transportation in belugas (Delphinapterus leucas) (Buckstaff, 2004; Castellote and Fossa, 2006; Esch et al., 2009; Jones et al., 2021; Wong et al., 2023). Conversely, elevated vocalization rates have been linked to positive states, such as cooperative activities and anticipatory behaviors (Eskelinen et al., 2016; Probert et al., 2021). These insights have been operationalized at the US Navy Marine Mammal Program in San Diego, where the Welfare Acoustic Monitoring System (WAMS) employs real-time acoustic monitoring to detect deviations in vocal behavior, enabling proactive welfare management (Winship and Jones, 2023).
For killer whales, studies on vocalizations in welfare contexts remain limited. Filatova et al. (2009) found that biphonic calls were more frequent during inter-pod interactions, suggesting a role in cohesion or agonistic signaling. Similarly, Graham and Noonan (2010) identified specific call types associated with rare aggressive chase events in captive orcas, providing a potential acoustic signature for agonistic interactions. However, comprehensive studies linking vocalization rates to arousal or welfare states are lacking, leaving an essential gap in understanding how killer whale vocal behavior may reflect both positive and negative emotional states.
While vocal behavior has been widely recognized as a valuable indicator of affective states in various species, including cetaceans, there is a significant lack of evidence on its applicability as a welfare proxy for killer whales. Existing studies have identified vocalizations associated with agonistic behaviors and negative affective states in orcas, yet comprehensive data linking vocalization rates to welfare remain scarce. This gap highlights the urgent need for systematic research to explore the potential of vocal behavior as an indicator of both positive and negative affective states in this species, as emphasized in the broader field of animal welfare science (Whitham and Miller, 2024).
To address this gap, the goal of this study is to evaluate the potential value of vocalization rate and overall vocal activity, specifically focusing on pulsed calls, as indicators of welfare in killer whales. This research is essential for establishing a robust framework for both welfare monitoring and population assessments. Ultimately, such efforts aim to advance our understanding of welfare in managed care settings while also providing valuable acoustic benchmarks for conservation studies in the wild, enabling more informed and proactive management decisions that prioritize the well-being of killer whales.
2 Materials and methods
2.1 Subjects
Underwater vocalizations of three male (Keto, Tekoa, and Adán) and four female (Kohana, Skyla, Ula, and Morgan) killer whales have been recorded at the Loro Parque facility (Tenerife, Spain) during different periods since early 2007. All individuals, except Morgan, belonged to the second and third generations of killer whales born under human care. These orcas descended from Icelandic (Keto, Tekoa, Kohana, and Skyla), Canadian resident (Keto and Skyla), and transient (Tekoa) lineages. Kohana, Skyla, Keto, and Tekoa were transferred to Loro Parque in February 2006 from SeaWorld parks in the United States (males from San Antonio, Texas; females from Orlando, Florida) (Kremers et al., 2012). Adán was born to Keto and Kohana on October 12, 2010, and Ula was born to Keto and Morgan on September 22, 2018, both at Loro Parque. Morgan was rescued in the Netherlands in 2010 in an emaciated and dehydrated state and was temporarily housed at the Harderwijk Dolphinarium. Several months after her rescue, she began producing vocalizations identified as similar to those of Norwegian herring-eating killer whales, supporting the hypothesis of her origin from this population. Morgan was transferred to Loro Parque in November 2011 following a judicial decision. Shortly after her arrival, she was suspected to be deaf, a condition confirmed through evoked potential measurements (Lucke et al., 2009, 2016). During the analyzed period (2015–2020), six individuals were present in the facility until the birth of Ula on 21 September 2018, after which the group size increased to seven.
2.2 Facilities
The Loro Parque facility (Orca Ocean) comprises four interconnected pools with a combined capacity of 24 million liters (see details in Lüke et al., 2010; Kremers et al., 2012). Trainer interactions occurred eight times daily, with two or three of these sessions, depending on the season, being public presentations. The Orca Ocean facility is equipped with hydrophones (ITC-6050C, International Transducer Corporation, Santa Barbara, CA) embedded in the pool walls. These hydrophones have an open-circuit sensitivity of approximately −157 ± 3 dB re 1 V/µPa and a broad frequency response range of 0.03–70 kHz, making them well-suited for capturing cetacean vocalizations. Initially, these hydrophones were connected to a central server via a multi-channel digitizing card (sample rate: 200 kHz, frequency response: 0.02–75 kHz, resolution: 16 bits). A custom detection software (Lüke et al., 2010) continuously operated on the server, detecting and extracting individual sound events in real time. Each detected event was saved as a separate, time-tagged sound file for subsequent analysis. Over the past two decades, the system has undergone multiple upgrades, resulting in data sets from different time periods with varying characteristics. In 2015, the system was enhanced with individual processing nodes capable of digitizing sound but sound stream was still sent to a central server were a detection software extracted time-tagged sound events from the stream incoming from each hydrophone. In 2020, the nodes were further improved, to implement detection directly on the nodes by extracting sound events exceeding a certain noise threshold. These events were temporarily stored at the nodes before being transferred to a central server for permanent storage via Ethernet. Additionally, since changes in hardware were made, the sampling frequency was set to 192 kHz. In 2022, the nodes were further improved to process sound events using neural networks for advanced analysis. In 2024, the neural network architecture was further improved and was used to conduct the study shown in this paper. To ensure accuracy and prevent event duplication, all recordings used in this research were conducted using a single hydrophone.
2.2.1 Behavioural data collection and welfare scoring
Daily behavioural data for each individual killer whale were collected by animal caretakers at Loro Parque as part of the facility’s established welfare monitoring practices. Observations were recorded using individualized forms during structured training sessions conducted under an operant conditioning regime with positive reinforcement. Requested behaviors encompassed a wide range of contexts, including husbandry and medical procedures, scientific interactions, and public presentations.
Performance in each of the eight daily training sessions was evaluated using a three-point scale reflecting the animal’s level of motivation and engagement. A score of 3 denoted high performance, characterized by a consistent willingness to participate and fulfill behavioural criteria; a score of 2 indicated intermediate performance; and a score of 1 reflected low performance, defined by reluctance to engage, refusal to participate, or failure to meet the expected criteria across repeated requests. The presence of one or more sessions scored as ‘1’ within a day was categorized as an indicator of reduced willingness to participate in training and considered a potential welfare concern in line with published frameworks (Shyne, 2006; Clegg et al., 2017; Delfour et al., 2021). Score 3, considered by caretakers as the expected baseline level of performance, was not systematically recorded; for this reason, only scores of 1 and 2 were included in the analysis, as these represent deviations from normal training engagement and may indicate cognitive bias (Clegg et al., 2017) or welfare-related issues.
In addition to training performance, caretakers recorded social dynamics within the group by documenting specific events such as social displacements, spontaneous separations from the trainer during sessions (splits), and behavioural refusals. Social displacement refers to instances during which one killer whale approaches and causes another individual to vacate its position—such as leaving a station, resource, or trainer’s proximity—due to social pressure, dominance, or competition within the group. Each incident was annotated with the identity of both the initiator and recipient of the interaction. These occurrences were categorized as markers of social instability and included as qualitative welfare indicators following recommendations from C-Well (Clegg et al., 2015), Dolphin WET (Baumgartner et al., 2024), and broader applications of the Five Domains Model (Mellor et al., 2020). Although social displacements, splits, and refusals were recorded at the individual level, they were analyzed as group-level metrics to align with the group-based nature of the vocal activity data. This approach acknowledges the limitations of attributing vocalizations to specific individuals with the available acoustic equipment. However, in some exploratory analyses, individual-level behavioural indicators were compared against group-level vocal metrics to assess whether individual animals might affect collective vocal dynamics.
These behavioural observations formed part of a comprehensive dataset integrated with veterinary and environmental records to support the daily assessment of individual and group welfare. Although some indicators have not yet been fully validated for killer whales, the approach was informed by validated practices in bottlenose dolphins and aligned with emerging standards in cetacean welfare science (Baumgartner et al., 2024).
2.3 Data collection and analysis
To assess the vocal activity of the orcas, we selected a six-year period (2015–2020) from the stored events, explicitly choosing a timeframe during which the recording system (including hydrophone configuration, software version, and detector parameters) remained unchanged. This ensured data consistency and comparability across years by avoiding artifacts introduced by modifications to the detection system. It is important to note that vocalizations could not be attributed to specific individuals, as the monitoring system relied on a single hydrophone and no individual acoustic signatures (e.g., signature calls) were identified or classified. As such, vocal data represent group-level activity rather than individual vocal output. Periods during which the system was non-operational were excluded from the analysis. A classification between örca” and “no orca” events was conducted using a convolutional neural network trained on a dataset comprising 85,158 manually labeled audio segments. The dataset was split into training (80%), validation (10%), and test (10%) subsets. The network achieved a classification accuracy of 95.6%, with a precision of 92.5% and a recall of 90.5% on the validation set. This updated system builds on our previous work (Lüke et al., 2010) but incorporates architectural refinements based on artificial intelligence to enhance discrimination performance. A dedicated manuscript describing the network design and optimization procedures in full detail is currently in preparation. Using that network a data set of about 1.5 million orca calls was extracted from an event database of 4 million events. These classifications, along with their corresponding timestamps, were stored in a database for subsequent statistical analysis. Vocalization rates were quantified both as the percentage of active minutes (defined as minutes with more than one detected vocalization) and as the number of events per orca per minute, the latter serving as a normalization metric to account for group size rather than reflecting individual-level attribution.
The caretakers’ daily behavioral records for each individual orca were digitized and compiled into a centralized database for comprehensive analysis. This database cataloged instances where an individual received one or more scores of “1” or “2” in a day, indicating low performance. Additionally, the records detailed occurrences of social displacements and other notable behaviors. Subsequently, the rate of orca pulsed calls per minute was computed using a custom Python script. Whistles were excluded from the analysis, as they represented less than 1% of the total vocal events classified, and the focus was placed on pulsed calls, which constitute the predominant and socially meaningful vocal category in killer whales. The data were then used to determine the number of active minutes per day and across different time periods (Dawn, Day, Dusk, Night), from which the corresponding percentages of vocal activity were derived. These diel periods were dynamically assigned using a custom Python script that calculated astronomical sunrise and sunset times for each date and geographic location. This was achieved using the Astral 3.2 module (Rhodes, 2024), which accounts for seasonal variation in daylight duration. Based on these values, each minute of data was categorized according to its position relative to dawn, daylight, dusk, or night-time.
The statistical analysis was performed using Python 3.10 with libraries NumPy (Harris et al., 2020), SciPy (Virtanen et al., 2020), Pandas, Matplotlib, and Statsmodels (Seabold and Perktold, 2010). Normality of vocalization data was assessed using the Shapiro–Wilk test. Several theoretical probability distributions (Poisson, Negative Binomial, Log-normal, Gamma, and Weibull) were fitted to the data using maximum likelihood estimation to evaluate their suitability for describing vocal activity metrics.
Differences in vocal activity across diel periods (dawn, day, dusk, night), seasons, and hours were evaluated using the non-parametric Kruskal–Wallis H test. Where significant effects were found, post hoc pairwise comparisons were conducted using Mann–Whitney U tests with Bonferroni correction to control for Type I error.
To examine associations between behavioural indicators (e.g., low-performance scores, social splits, displacements) and vocal activity metrics (mean vocalizations per minute, percentage of vocal minutes), we used a Spearman’s rank correlation (for monotonic associations) and Generalised Linear Models (GLMs). GLMs were implemented using Poisson and Negative Binomial distributions, and model selection was guided by Akaike Information Criterion (AIC) comparison and overdispersion statistics.
To address zero-inflation, we evaluated zero-inflated Poisson, zero-inflated Negative Binomial, and hurdle models using the statsmodels discrete count model framework. These models were assessed based on AIC, log-likelihood, and Vuong’s test where appropriate. Since the proportion of zero-valued data was below 20%, Negative Binomial GLMs were retained as the final model form in most cases.
3 Results
3.1 Orca vocalization classification and distribution
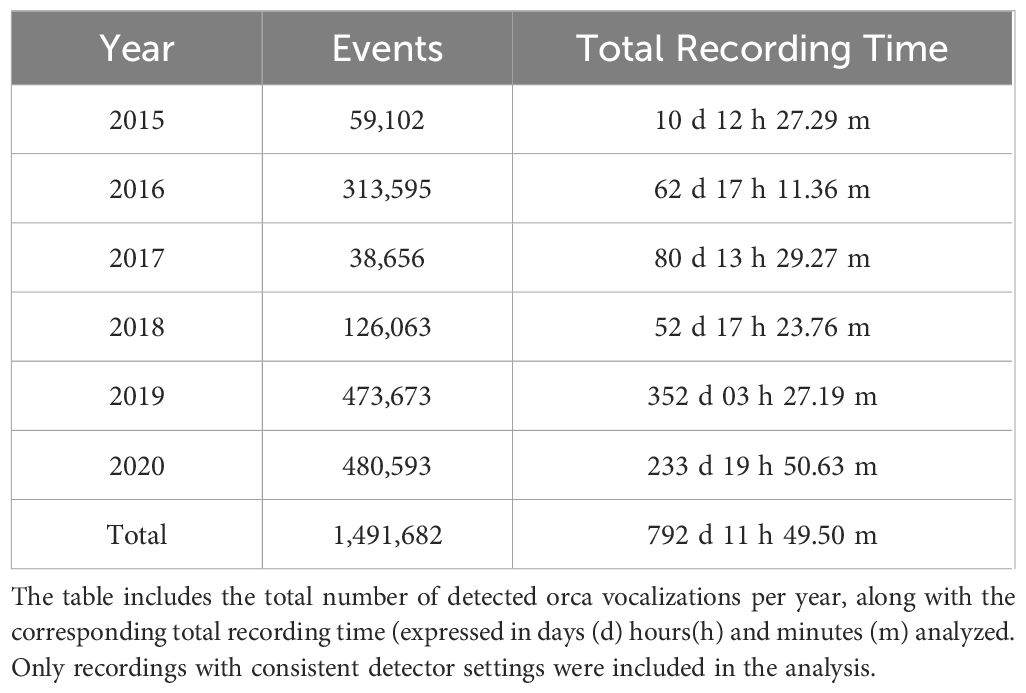
The classification algorithm effectively distinguished between “orca” and “no orca” events, identifying 1,491,682 acoustic events as orca vocalizations from all recorded events between 2015 and 2020 (Table 1). These classified vocalizations were detected over a total of 792.5 days within the study period, with consistent detector parameters maintained throughout.
To evaluate the distribution of vocal events, a histogram of vocalization rates per minute was constructed (See Supplementary Materials Figure S9). A significant percentage of minutes contained zero vocalizations, resulting in a distribution heavily skewed toward zero. The Shapiro-Wilk test confirmed the non-normality of vocal activity (W = 0.672, p < 0.05), and evaluations of theoretical probability models (Poisson, Log-normal, Gamma, Weibull) failed to provide an adequate fit.
Excluding silent periods (i.e., minutes with zero vocalizations), the median number of vocalization events per minute per orca was 0.571, with the 5th and 95th percentiles at 0.143 and 2.500, respectively. Across the entire dataset, vocal activity was detected in 23.9% of all recorded minutes (Table 2).
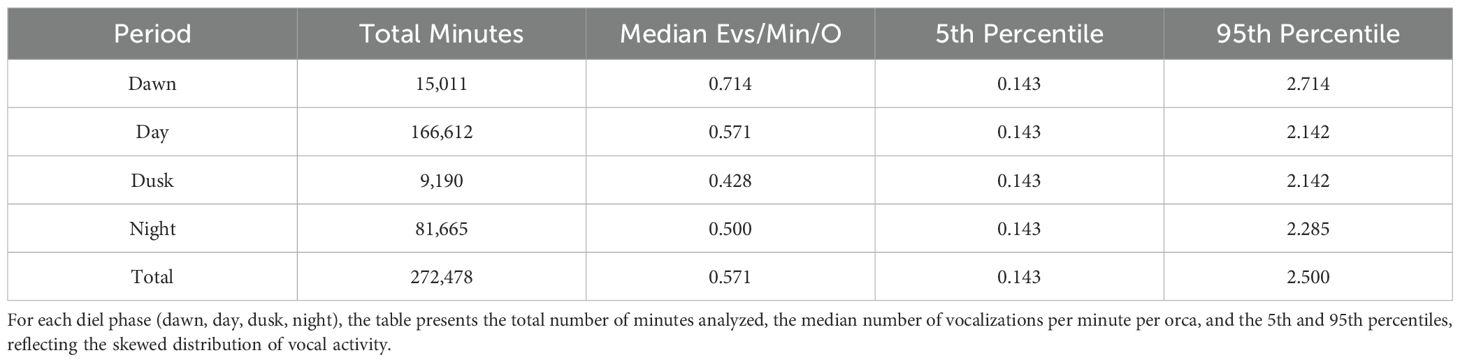
Table 2. Descriptive statistics for the number of vocalization events per minute per orca across diel periods.
3.2 Temporal patterns of vocal activity
Vocal activity levels, normalized per individual, varied significantly across different times of the day. The highest median vocalization rate was recorded at dawn (0.714 events per minute per orca; 5th–95th percentiles: 0.143–2.714), followed by day (0.571; 0.143–2.142), night (0.500; 0.143–2.285), and dusk (0.428; 0.143–2.142). A Kruskal–Wallis test confirmed that these differences were statistically significant (H = 1203.09, p < 0.0001), and Dunn’s post hoc comparisons revealed significant pairwise differences between all diel periods (p < 0.05).
To further investigate intra-day variation in vocal activity, we analyzed the percentage of minutes with vocalizations per hour throughout the 24-hour cycle. As shown in Figure 1, this analysis revealed consistent peaks in vocal activity around 07:00, 11:00, and 16:00. At these peak hours, the median percentage of active minutes reached 32.8% at 07:00, 41.7% at 11:00, and 31.7% at 16:00. The 5th to 95th percentile range for these hours extended from 27.6% to 37.2% (07:00), from 38.3% to 46.7% (11:00), and from 26.7% to 35.0% (16:00). A Kruskal–Wallis test across hours yielded a highly significant result (H = 2027.83, p < 0.001), confirming differences in hourly vocal activity. However, subsequent Mann–Whitney U tests between consecutive hours did not identify statistically significant differences, suggesting that the observed peaks represent broader temporal trends rather than abrupt shifts in vocal behavior.
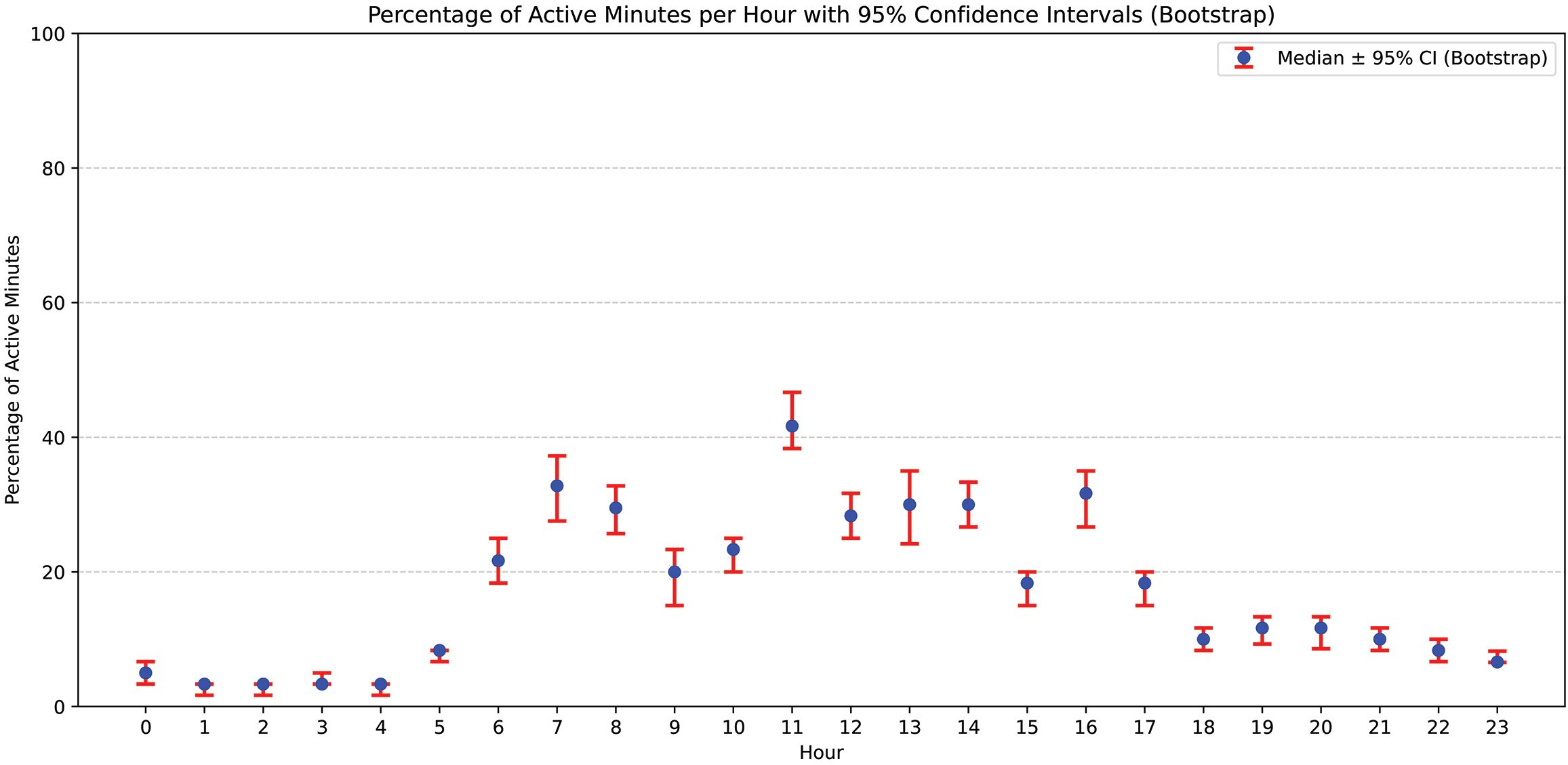
Figure 1. Hourly distribution of vocal activity expressed as the percentage of minutes containing vocalizations. This figure displays the relative percentage of minutes per hour during which vocalizations were detected in the study period (2015–2020). Data are aggregated across all individuals and years.
Seasonal patterns in vocal activity were analyzed using the percentage of active minutes per day as the primary metric, allowing for a standardized comparison across periods with differing recording effort. A Kruskal–Wallis test revealed significant variation in daily vocal activity across seasons (H = 566.95, p < 0.001). The highest median vocal activity was observed in both spring and winter (18.33%), followed by summer (10.00%), and the lowest in autumn (6.67%). Confidence intervals around the median values were narrow, indicating consistent seasonal trends: spring (CI = [16.67%, 18.33%]), winter (CI = [16.67%, 20.00%]), summer (CI = [10.00%, 11.67%]), and autumn (CI = [5.00%, 6.67%]).
Post hoc Mann–Whitney U tests confirmed that vocal activity in autumn was significantly lower than in all other seasons (p < 0.001 for all comparisons). Spring also showed significantly higher vocal activity than summer (p < 0.001), while no significant difference was detected between spring and winter (p = 0.9111). In contrast, winter exhibited significantly greater vocal activity than summer (p < 0.001).
When examining diel trends across seasons, the vocal activity heatmap (Figure 2) revealed a marked increase in vocalization rates during the first months of the year, particularly concentrated during daylight hours. This elevated activity gradually declined toward the latter part of the year, with autumn showing the lowest levels of vocal output. The distribution of vocal activity remained consistently skewed toward daytime hours throughout the year, with reduced activity during nocturnal periods. While these trends align with the seasonal comparisons reported above, it is important to interpret them with caution, as variations in recording effort and sample size across months and seasons may have introduced some degree of observational bias.
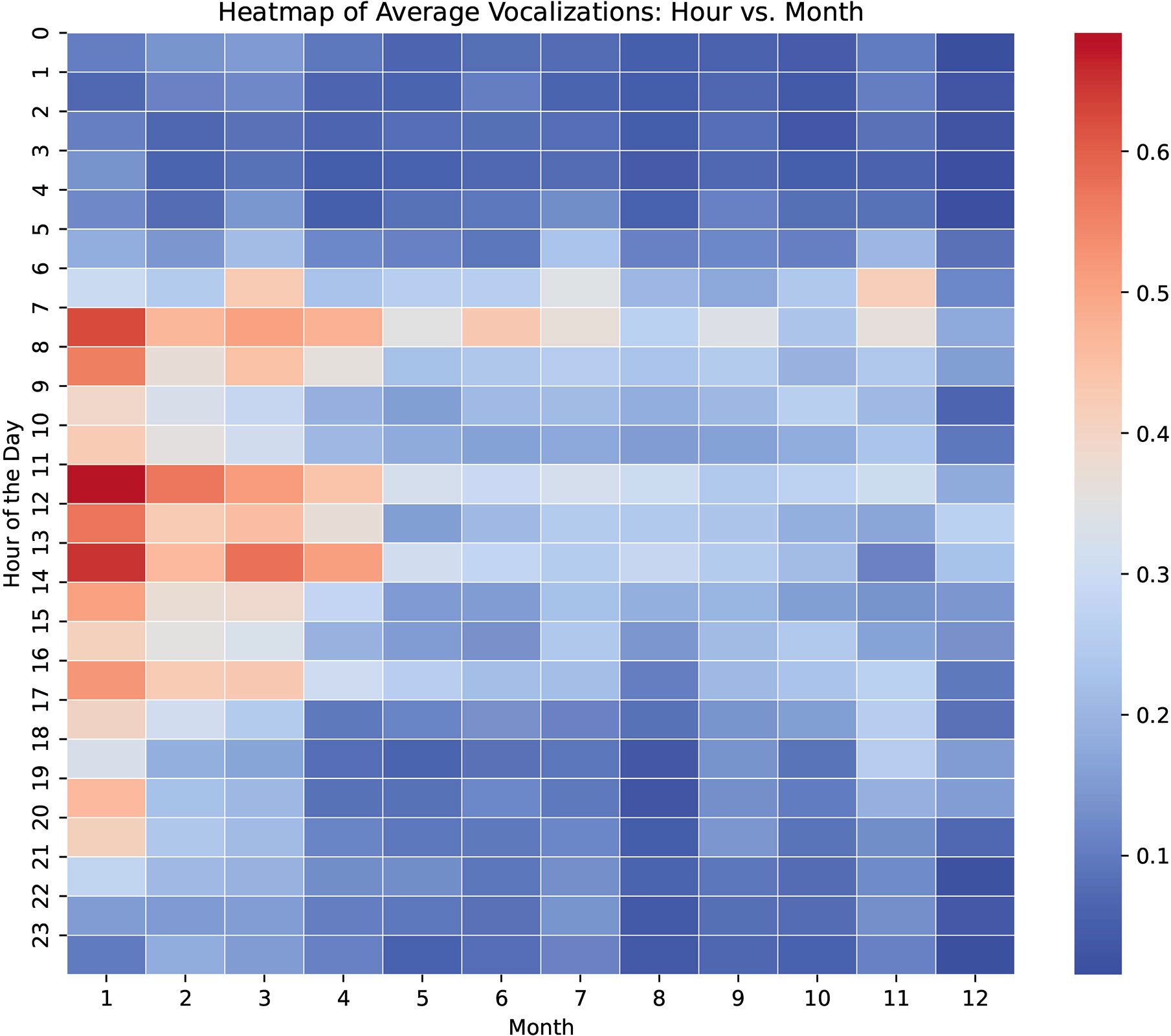
Figure 2. Heatmap of vocal activity across hours of the day and months of the year. Each cell represents the mean percentage of minutes with vocal activity for a given hour and month. Warmer colors indicate higher levels of vocal activity. This visualization highlights diel and seasonal trends in vocalization behavior.
3.3 Correlation between vocal activity and behavioral variables
Across the six-year study period, trainer-assigned low-performance scores (“1”) represented 4% of all recorded behaviors. Social imbalance events, including separations from trainer control, refusals to perform requested behaviors, and inter-individual displacements, were relatively infrequent, comprising 2%, 1%, and 1% of recorded observations, respectively. The Spearman correlation analysis identified significant but weak relationships between vocalization patterns and behavioral indicators (Figure 3). A significant positive correlation was found between the cumulative count of animals splitting from trainer control and the number of active vocalization minutes per day (r = 0.16,p < 0.001). Similarly, various vocal activity metrics were positively correlated with the frequency of “1” performance scores across individual orcas, regardless of sex (See Table 3 for a detailed explanation on the variables analyzed). It is important to clarify that while vocal activity data were collected at the group level and not attributed to individual animals, some correlations were conducted between group-level vocal indices and individual-level behavioural indicators (e.g., #1sMO1, #2sFO2) in an exploratory manner. The aim was not to infer individual vocal output, but to investigate whether specific individuals—particularly those experiencing cognitive or social challenges—might exert a disproportionate influence on the overall vocal dynamics of the group.
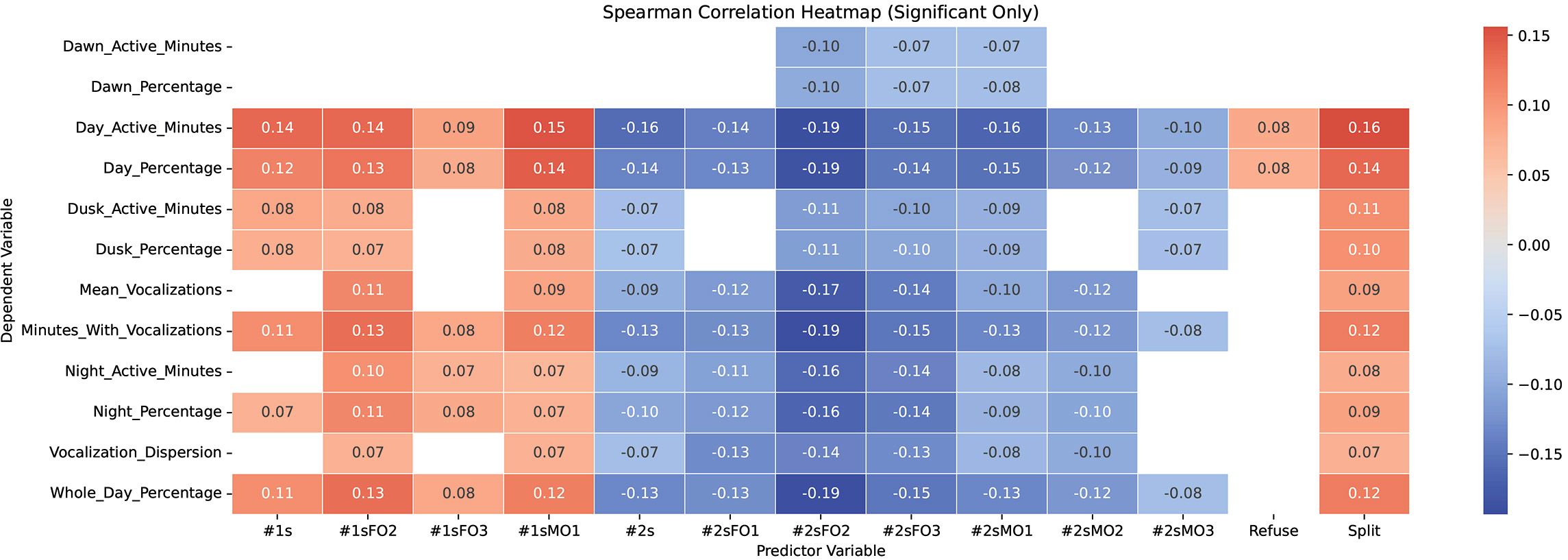
Figure 3. Spearman correlation matrix showing relationships between vocal activity metrics and behavioural indicators. The matrix presents Spearman’s rank correlation coefficients (ρ) between multiple behavioural metrics (e.g., refusals, splits, performance scores) and vocal activity parameters (e.g., percentage of vocal minutes, mean vocalization rate, dispersion). Positive and negative correlations are indicated by blue and red hues, respectively, with intensity reflecting the strength of the correlation.
Conversely, positive behavioral indicators involving both males and females showed a significant negative correlation with vocal activity minutes (r = −0.19,p = 0.001), suggesting that increased vocalization is associated with social instability and cognitive disengagement, while positive social interactions correspond to lower vocal output.
3.4 Generalized linear models for vocal activity
GLMs were used to examine the relationship between social and behavioral factors and vocal activity. Both Poisson and Negative Binomial models were tested, considering interaction terms to assess the influence of various behavioral predictors (See full set of model outputs and parameters in the Supplementary Materials).
The Shapiro–Wilk test confirmed that the distribution of the response variable deviated from normality, supporting the use of Generalized Linear Models (GLMs). Overdispersion was detected in models for Whole Day Percentage, Day Percentage, and Night Percentage, leading to the selection of Negative Binomial models as the best fit based on lower AIC values (See Table 3 for a detailed explanation on the variables analyzed). Zero-inflated and hurdle models were evaluated to account for excess zeros in the dataset, but since the proportion of zero values was low (< 20%), a standard Negative Binomial model was deemed appropriate.
For Mean Vocalizations, no strong predictors were identified, as none of the explanatory variables (#1s, #2s, Split, #1sMO1, #1sFO2, #2sFO2, #2sFO3) reached statistical significance. The low Pseudo R2 (0.02953) suggests that vocalization rates are only weakly related to social behavior.
For Vocalization Dispersion, significant predictors were identified. Low-performance days (#1s) negatively correlated with vocalization dispersion (p = 0.021,β = −0.0622), while one male’s low performance (#1sMO1) showed a significant positive correlation (p = 0.036,β = 0.0942).
For Whole Day Percentage, low-performance days (#1s) and normal-performance days (#2s) negatively impacted vocal activity (p < 0.001). In contrast, social separations (Split) had a significant positive effect (p < 0.001,β = 0.0605). The inclusion of interaction terms, particularly #1s × Split and #2s × Split, improved model fit, suggesting that vocal activity was influenced by the combined effects of cognitive state and social separations.
For Day Percentage, a similar pattern was observed, with low- and normal-performance days negatively correlated with daytime vocal activity (p < 0.001), while social separations increased vocalization (p < 0.001,β = 0.0615).
For Night Percentage, low-performance and normal-performance days correlated with reduced nighttime vocalization (p < 0.001), while specific individuals exhibited increased nighttime vocalization.
The inclusion of interaction terms improved model fit, particularly #1s × Split (p < 0.001,β = 0.0146), indicating that low-performance days and social separations jointly influenced vocalization patterns.
4 Discussion
While animal welfare is inherently an individual-level construct, this study explored the potential for vocal activity measured at the group level to serve as a practical, non-invasive proxy for monitoring collective welfare dynamics in a socially complex species. Given that vocalizations could not be attributed to specific individuals due to the use of a single hydrophone and the absence of distinctive individual acoustic markers, all acoustic analyses were conducted at the group level. We acknowledge this limitation and interpret our findings with appropriate caution. Nonetheless, group-level vocal activity may still reflect meaningful changes in social cohesion, arousal, or stress, particularly in structured social groups where individual states may influence group-wide behavior. As such, this work represents an initial step toward identifying vocal parameters that could inform welfare assessment frameworks, even when individual vocal attribution is not feasible.
An additional limitation of this study is the small sample size and the potential influence of social structure on vocal behavior within the group. Although vocalizations were analyzed at the group level, differences in individual vocal output—driven by dominance, age, sex, or social role—may affect the overall acoustic patterns detected. In a socially complex species such as the killer whale, a small number of highly vocal individuals could disproportionately influence group-level acoustic metrics, particularly in the absence of individual call attribution. As such, the interpretation of vocal activity in relation to welfare must consider the social composition and stability of the group under study. This limitation underscores the importance of integrating complementary behavioural and contextual data when using passive acoustic monitoring as a welfare tool.
The analysis of nearly 1.5 million orca vocalizations recorded at Orca Ocean revealed distinct temporal patterns, characterized by a pronounced diurnal rhythm with reduced vocal activity during nocturnal hours (Figure 1). These findings align with previous studies indicating elevated vocal activity associated with group behaviors in wild killer whales (Ford, 1989; van Opzeeland et al., 2005). Similar patterns have also been observed in dolphins under human care, where vocal peaks coincide with feeding and training sessions, suggesting that vocal production may serve roles in anticipatory behaviors and social coordination (Therrien et al., 2012; Probert et al., 2021, 2023). In the present study, vocal peaks were observed at 07:00, corresponding to the start of human activities at the facility, and at 11:00, and 16:00, aligning with training sessions and public presentations. These results further support the hypothesis that vocalization rates are modulated by arousal levels and social engagement (Ford, 1989; van Opzeeland et al., 2005; Graham and Noonan, 2010; Holt et al., 2013; Poupard et al., 2021). However, caution should be exercised in interpreting the elevated vocalization rates observed at dawn, as they may be influenced by the controlled setting and human presence. In contrast, the vocalization rates calculated for day and night more closely align with patterns described for wild populations (Ford, 1989; van Opzeeland et al., 2005).
Although the vocal activity levels reported in this study cannot be directly compared with those of wild populations due to differing contexts and methodologies, the range of call rates observed is broadly consistent with those reported in studies of resident killer whales under naturalistic conditions (Ford, 1989; van Opzeeland et al., 2005; Filatova et al., 2009; Holt et al., 2013; Poupard et al., 2021), suggesting potential functional similarities in vocal behavior. Given that previous research has demonstrated that call rate is not significantly affected by the number of individuals present (Holt et al., 2013), these findings would support the hypothesis that the structure of vocal activity remains relatively stable across different environments. While these values are not suitable for estimating orca abundance using passive acoustic monitoring (PAM) (Holt et al., 2013),provide additional evidence that vocalization rates are influenced by social interactions and arousal rather than external environmental factors. Notably, the median vocal activity per minute during the day (0.571) was not substantially different from that recorded at night (0.500), despite a higher percentage of active minutes occurring during daylight hours. This suggests that variations in vocal activity are primarily driven by fluctuations in the proportion of active minutes rather than substantial changes in call rate per minute across diel phases. Notably, the presence of nighttime vocal activity aligns with previous observations of social and foraging behaviors occurring during nocturnal periods in both wild and managed-care delphinids (Kremers et al., 2014; Deconto and Monteiro-Filho, 2016; Sol et al., 2024).
Analysis of vocal activity throughout the year suggests that the diurnal structure of vocal production persists despite seasonal fluctuations. A notable increase in vocal activity was observed during the initial months of the year, a pattern that remained evident even in months with lower overall activity levels (Figure 2). These results support previous findings that killer whale vocalization rates are closely linked to social arousal (Weiss et al., 2007). However, we acknowledge that in a controlled environment where management routines such as feeding, enrichment, and training are maintained consistently across the year, seasonal or annual shifts in vocal behavior are not necessarily expected, nor are they assumed to be direct indicators of welfare status. The inclusion of seasonal and annual comparisons in this study was not intended to attribute biological significance to temporal variation, but rather to determine whether vocal activity in this managed population is distributed homogeneously or heterogeneously over time. Understanding such distribution patterns provides context for the interpretation of behavioural correlations and helps establish appropriate baselines for welfare monitoring. Nonetheless, the variability in sampling effort across the analyzed period limits the conclusiveness of this trend, and further research with more homogeneous data collection is needed to confirm the annual vocal pattern.
The relationship between vocalization and welfare-related behavioral parameters was explored using performance in trainer-requested tasks as a proxy for cognitive engagement and emotional state (Clegg et al., 2015, 2017). To explore whether vocal activity patterns at different times of day were differentially related to behavioural or cognitive indicators, correlations were conducted using vocal metrics calculated separately for dawn, day, dusk, and night periods. While this approach introduces some redundancy due to the use of overlapping daily data, it was employed in an exploratory framework to assess whether specific temporal windows might offer enhanced sensitivity to welfare-relevant changes. However, to avoid potential issues of pseudo-replication and misinterpretation, only correlations based on daily aggregated vocal indices should be considered in the interpretation of welfare associations. Social imbalance was evaluated based on instances where individuals refused to execute a requested behavior, separated from trainer control, or displaced conspecifics. Low-performance and social imbalance events were relatively infrequent, indicating a high level of behavioral consistency among the animals.
The low frequency of these events raises questions about their suitability as robust indicators of welfare or social balance. However, previous studies have also documented low rates of agonistic interactions in this group, with occurrence rates below 1% (Sánchez–Hernández et al., 2019), a finding further supported by recent activity budget analyses of killer whales under professional care (Hill et al., 2025). This suggests that while these behavioral metrics may not be strong welfare indicators on their own, their low prevalence is consistent with the overall social stability and structured behavioral patterns observed in this managed orca population.
Prior studies have documented substantial modifications in cetacean vocal behavior in response to acute stressors. For example, beluga whales ceased vocalizations entirely following transportation events (Castellote and Fossa, 2006), while bottlenose dolphins increased whistle rates in response to watercraft noise (Buckstaff, 2004) and construction activities (Wong et al., 2023). In orcas, controlled environment studies have demonstrated increased call rates during aggressive events (Graham and Noonan, 2010). In contrast, the present study found only weak correlations between vocal activity and indicators of social imbalance or cognitive bias. This discrepancy may be due to the relatively subtle nature of the social and cognitive challenges assessed, which likely reflect minor fluctuations in welfare rather than significant stressors. Alternatively, it is possible that the orcas in this study did not experience substantial welfare challenges during the observation period. Additionally, the behavioral metrics used to assess cognitive bias and social imbalance may require further refinement to better capture nuances in orca welfare.
The observed variation in vocal activity across different time periods, together with prior findings on orcas (Graham and Noonan, 2010), provided the primary rationale for evaluating vocalization as a potential welfare indicator. Previous studies have demonstrated that reductions in vocal rates are associated with significant stressors in cetaceans (Buckstaff, 2004; Castellote and Fossa, 2006; Esch et al., 2009; Jones et al., 2021; Wong et al., 2023), while increased vocalization rates have been linked to positive welfare states (Eskelinen et al., 2016; Probert et al., 2021). However, the Spearman correlation analysis suggests that, in orcas, this relationship may operate inversely, with low-performance and social imbalance indices positively correlated with vocal production, whereas normal-performance indices were negatively correlated. This finding aligns with previous research indicating that call rates increase during aggressive events (Graham and Noonan, 2010) and that certain vocalizations, such as biphonic calls, occur more frequently during inter-pod interactions (Filatova et al., 2009), suggesting a complex interplay between vocalization patterns and social dynamics.
It is important to note that the observed increase in vocalization rate in this study was calculated using the daily integrated sound rate to ensure comparability with behavioral and cognitive data, which were also assessed at the daily scale. Consequently, these results cannot be directly applied to finer temporal scales, such as diel variations, as the effects were measured within a different timeframe. Future studies should investigate vocal activity and behavior at finer temporal resolutions to determine whether vocal indicators—both qualitative and quantitative—can reliably reflect welfare-related changes over shorter timescales.
Given the weak correlation coefficients (Spearman r < 0.2), these relationships explain only a small proportion of the variance in vocal activity, indicating that additional, unmeasured factors likely exert stronger influences on vocal production in killer whales. Nevertheless, the results suggest a subtle connection between vocalization patterns and welfare, highlighting the potential relevance of vocal parameters as indicators of well-being. Seasonal and monthly variations in vocal activity add further complexity, potentially reflecting external environmental or social influences. Additionally, these fluctuations may be affected by operational constraints of the recording system, leading to nonhomogeneous sampling periods rather than genuine seasonal trends in vocal behavior. Ultimately, the weak statistical associations observed underscore the need for further investigation into the broader ecological, social, and cognitive factors shaping orca vocalization patterns. In particular, future studies should incorporate qualitative acoustic analyses—such as the classification of call types and the identification of nonlinear vocal phenomena—as these structural features may reflect arousal or emotional valence and could offer greater sensitivity for assessing welfare at both the group and individual level (Sportelli et al., 2022).
While this study was conducted on killer whales under managed care, some behavioural patterns observed—such as the diel modulation of vocal activity or correlations with social dynamics—may reflect more general features of orca acoustic behavior. Nonetheless, caution is warranted when drawing parallels between captive and wild populations. Animals in zoological settings are subject to different ecological constraints, social structures, and acoustic environments than their wild counterparts, and these differences can influence both the rate and function of vocal output. In particular, the absence of natural foraging and predation and the presenceo of human interaction routines may modify vocal behavior. However, controlled conditions also provide opportunities for long-term, fine-scale monitoring and individual-level analysis that are difficult to achieve in the wild. Therefore, while findings from managed-care studies are not directly generalizable, they can offer hypotheses and methodological frameworks—such as vocal monitoring as a welfare proxy—that may be adapted for wild populations using passive acoustic monitoring, especially in resident groups with known vocal dialects and social cohesion patterns.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. A histogram of adjusted event counts, along with the full set of model outputs and parameters from the Generalised Linear Models GLMs, has been included in the Supplementary Materials to enhance transparency and facilitate interpretation.
Ethics statement
The animal study was approved by Research Ethics and Animal Welfare Committee of the University of La Laguna (CEIBA2024-3515). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JA: Project administration, Formal Analysis, Conceptualization, Writing – review & editing, Methodology, Writing – original draft, Investigation, Visualization, Funding acquisition. JL: Software, Writing – review & editing, Supervision, Writing – original draft, Data curation. FR: Software, Supervision, Data curation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The development of the hardware and software tools used for the sound detection and classification was funded by Loro Parque Fundación.
Acknowledgments
The authors thanks Chira Ivaldi, Charlotte Kirschner, Marta Ortega, and Renée Van Reeuwijk for their assistance in call classification.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to improve the English language in the manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1595113/full#supplementary-material
References
André M., van der Schaar M., Zaugg S., Houégnigan L., Sánchez A. M., and Castell J. V. (2011). Listening to the deep: Live monitoring of ocean noise and cetacean acoustic signals. Mar. pollut. Bull. 63, 18–26. doi: 10.1016/j.marpolbul.2011.04.038
Bain D. E. (1986). Acoustic behavior of Orcinus: Sequences, periodicity, behavioral correlates and an automated technique for call classification. In Kirkevold B. C. and Lockard J. S. (Eds.), Springer Behav. Biol. killer whales, 335–371.
Baumgartner K., Hüttner T., Clegg I. L. K., Hartmann M. G., Garcia-Párraga D., Manteca X., et al. (2024). Dolphin-wet—development of a welfare evaluation tool for bottlenose dolphins (Tursiops truncatus) under human care. Animals 14, 701. doi: 10.3390/ani14050701
Buckstaff K. C. (2004). Effects of watercraft noise on the acoustic behavior of bottlenose dolphins, Tursiops truncatus, in sarasota bay, florida. Mar. Mammal Sci. 20, 709–725. doi: 10.1111/j.1748-7692.2004.tb01189.x
Castellote M. and Fossa F. (2006). Measuring acoustic activity as a method to evaluate welfare in captive beluga whales (Delphinapterus leucas). Aquat. Mammals 32, 325–333. doi: 10.1578/AM.32.3.2006.325
Clegg I. L., Borger-Turner J. L., and Eskelinen H. C. (2015). C-well: The development of a welfare assessment index for captive bottlenose dolphins (Tursiops truncatus). Anim. Welfare 24, 267–282. doi: 10.7120/09627286.24.3.267
Clegg I. L., Rödel H. G., and Delfour F. (2017). Bottlenose dolphins engaging in more social affiliative behaviour judge ambiguous cues more optimistically. Behav. Brain Res. 322, 115–122. doi: 10.1016/j.bbr.2017.01.026
Deconto L. S. and Monteiro-Filho E. L. (2016). Day and night sounds of the guiana dolphin, sotalia guianensis (Cetacea: Delphinidae) in southeastern Brazil. Acta Ethologica 19, 61–68. doi: 10.1007/s10211-015-0223-y
Deecke V. B., Nykänen M., Foote A. D., and Janik V. M. (2011). Vocal behaviour and feeding ecology of killer whales orcinus orca around shetland, uk. Aquat. Biol. 13, 79–88. doi: 10.3354/ab00353
Delfour F., Vaicekauskaite R., García-Párraga D., Pilenga C., Serres A., Brasseur I., et al. (2021). Behavioural diversity study in bottlenose dolphin (Tursiops truncatus) groups and its implications for welfare assessments. Animals 11, 1715. doi: 10.3390/ani11061715
Esch H. C., Sayigh L. S., Blum J. E., and Wells R. S. (2009). Whistles as potential indicators of stress in bottlenose dolphins (Tursiops truncatus). J. Mammalogy 90, 638–650. doi: 10.1644/08-MAMM-A-069R.1
Eskelinen H. C., Richardson J. L., and Tufano S. (2022). Stress, whistle rate, and cortisol. Mar. Mammal Sci. 38, 765–777. doi: 10.1111/mms.12883
Eskelinen H. C., Winship K. A., Jones B. L., Ames A. E. M., and Kuczaj S. A. (2016). Acoustic behavior associated with cooperative task success in bottlenose dolphins (Tursiops truncatus). Anim. Cogn. 19, 789–797. doi: 10.1007/s10071-016-0978-1
Filatova O. A. (2020). Independent acoustic variation of the higher- and lower-frequency components of biphonic calls can facilitate call recognition and social affiliation in killer whales. PloS One 15, e0236749. doi: 10.1371/journal.pone.0236749
Filatova O. A., Fedutin I. D., Nagaylik M. M., Burdin A. M., and Hoyt E. (2009). Usage of monophonic and biphonic calls by free-ranging resident killer whales (Orcinus orca) in kamchatka, Russian far east. Acta Ethologica 12, 37–44. doi: 10.1007/s10211-009-0056-7
Filatova O. A., Guzeev M. A., Fedutin I. D., Burdin A. M., and Hoyt E. (2013). Dependence of killer whale (Orcinus orca) acoustic signals on the type of activity and social context. Biol. Bull. 40, 790–796. doi: 10.1134/S1062359013090045
Fleishman E., Cholewiak D., Gillespie D., Helble T., Klinck H., Nosal E., et al. (2023). Ecological inferences about marine mammals from passive acoustic data. Biol. Rev. 98, 1633–1647. doi: 10.1111/brv.12969
Ford J. K. B. (1989). Acoustic behaviour of resident killer whales (Orcinus orca) off vancouver island, british columbia. Can. J. Zoology 67, 727–745. doi: 10.1139/z89-105
Ford J. K. B. (1991). Vocal traditions among resident killer whales (Orcinus orca) in coastal waters of british columbia. Can. J. Zoology 69, 1454–1483. doi: 10.1139/z91-206
Graham M. A. and Noonan M. (2010). Call types and acoustic features associated with aggressive chase in the killer whale (Orcinus orca). Aquat. Mammals 36, 9–18. doi: 10.1578/AM.36.1.2010.9
Hanson M. B., Emmons C. K., Ward E. J., Nystuen J. A., and Lammers M. O. (2013). Assessing the coastal occurrence of endangered killer whales using autonomous passive acoustic recorders. J. Acoustical Soc. America 134, 3486–3495. doi: 10.1121/1.4821206
Harris C. R., Millman K. J., van der Walt S. J., Gommers R., Virtanen P., Cournapeau D., et al. (2020). Array programming with numpy. Nature 585, 357–362. doi: 10.1038/s41586-020-2649-2
Hewson C. J. (2004). Do vocalizations tell us anything about animal welfare? les vocalisations nous communiquent-elles un message sur le bien-etreˆ des animaux? Can. Veterinary J. 621, 621–624.
Hill H. M. M., Themelin M., Dudzinski K. M., Felice M., and Robeck T. (2025). Individual variation in activity budgets of a stable population of killer whales in managed care across a year. Behav. Processes 224, 105135. doi: 10.1016/j.beproc.2024.105135
Holt M. M., Noren D. P., and Emmons C. K. (2013). An investigation of sound use and behavior in a killer whale (Orcinus orca) population to inform passive acoustic monitoring studies. Mar. Mammal Sci. 29, 193–202. doi: 10.1111/j.1748-7692.2012.00599.x
Jones B. L., Oswald M., Tufano S., Baird M., Mulsow J., and Ridgway S. H. (2021). A system for monitoring acoustics to supplement an animal welfare plan for bottlenose dolphins. J. Zoological Botanical Gardens 2, 222–233. doi: 10.3390/jzbg2020015
Jones B., Sportelli J., Karnowski J., McClain A., Cardoso D., and Du M. (2024). Dolphin health classifications from whistle features. J. Mar. Sci. Eng. 12, 2158. doi: 10.3390/jmse12122158
Kremers D., Jaramillo M. B., Boye M., Lemasson A., and Hausberger M. (2014). Nocturnal vocal activity in captive bottlenose dolphins (Tursiops truncatus): Could dolphins have presleep choruses? Anim. Behav. Cogn. 1, 464–469. doi: 10.12966/abc.11.04.2014
Kremers D., Lemasson A., Almunia J., and Wanker R. (2012). Vocal sharing and individual acoustic distinctiveness within a group of captive orcas (Orcinus orca). J. Comp. Psychol. 126, 433–445. doi: 10.1037/a0028858
Laurijs K. A., Briefer E. F., Reimert I., and Webb L. E. (2021). Vocalisations in farm animals: A step towards positive welfare assessment. Appl. Anim. Behav. Sci. 236, 105264. doi: 10.1016/j.applanim.2021.105264
Lucke K., Finneran J. J., Almunia J., and Houser D. (2016). Variability in click-evoked potentials in killer whales (Orcinus orca) and determination of a hearing impairment in a rehabilitated killer whale. Aquat. Mammals 42, 184–192. doi: 10.1578/AM.42.2.2016.184
Lucke K., Siebert U., a Lepper P., and Blanchet M.-A. (2009). Temporary shift in masked hearing thresholds in a harbor porpoise (Phocoena phocoena) after exposure to seismic airgun stimuli. J. Acoustical Soc. America 125, 4060–4070. doi: 10.1121/1.3117443
Lüke J. P., Marichal-hernández J. G., Rosa F., Almunia J., Philipp J., Marichal-hernández J. G., et al. (2010). Real time automatic detection of Orcinus orca vocalizations in a controlled environment. Appl. Acoustics 71, 771–776. doi: 10.1016/j.apacoust.2010.04.003
Manteuffel G., Puppe B., and Schön P. C. (2004). Vocalization of farm animals as a measure of welfare. Appl. Anim. Behav. Sci. 88, 163–182. doi: 10.1016/j.applanim.2004.02.012
Mellor D. J., Beausoleil N. J., Littlewood K. E., McLean A. N., McGreevy P. D., Jones B., et al. (2020). The 2020 five domains model: Including human–animal interactions in assessments of animal welfare. Animals 10, 1870. doi: 10.3390/ani10101870
Miller P. J. (2006). Diversity in sound pressure levels and estimated active space of resident killer whale vocalizations. J. Comp. Physiol. A: Neuroethology Sensory Neural Behav. Physiol. 192, 449–459. doi: 10.1007/s00359-005-0085-2
Moore S. E. and Ridgway S. H. (1996). Patterns of dolphin sound production and ovulation. Aquat. Mammals 22, 175–184.
Oswald J. N., Rankin S., and Barlow J. (2008). To whistle or not to whistle? geographic variation in the whistling behavior of small odontocetes. Aquat. Mammals 34, 288–302. doi: 10.1578/AM.34.3.2008
Poupard M., Symonds H., Spong P., and Glotin H. (2021). Intra-group orca call rate modulation estimation using compact four hydrophones array. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.681036
Probert R., Bastian A., Elwen S. H., James B. S., and Gridley T. (2021). Vocal correlates of arousal in bottlenose dolphins (Tursiops spp.) in human care. PloS One 16, e0250913. doi: 10.1371/journal.pone.0250913
Probert R., James B. S., Elwen S. H., and Gridley T. (2023). Vocal cues to assess arousal state of bottlenose dolphins (Tursiops spp.) involved in public presentations. J. Zoological Botanical Gardens 4, 711–727. doi: 10.3390/jzbg4040050
Quick N. J. and Janik V. M. (2008). Whistle rates of wild bottlenose dolphins (Tursiops truncatus): Influences of group size and behavior. J. Comp. Psychol. 122, 305–311. doi: 10.1037/0735-7036.122.3.305
Rehn N., Teichert S., and Thomsen F. (2007). Structural and temporal emission patterns of variable pulsed calls in free-ranging killer whales (Orcinus orca). Behaviour 144, 307–329. doi: 10.1163/156853907780425703
Rhodes B. (2024). Astral: An astronomy calculations library for Python (3.2). Available online at: https://sffjunkie.github.io/astral/.
Ridgway S., Dibble D. S., Alstyne K. V., and Price D. (2015). On doing two things at once: dolphin brain and nose coordinate sonar clicks, buzzes and emotional squeals with social sounds during fish capture. J. Exp. Biol. 218, 3987–3995. doi: 10.1242/jeb.130559
Sánchez–Hernández P., Krasheninnikova A., Almunia J., and Molina–Borja M. (2019). Social interaction analysis in captive orcas (Orcinus orca). Zoo Biol. 38, 323–333. doi: 10.1002/zoo.21502
Schneiderová I., Singh N. J., Baklová A., Smetanová M., Gomis N. B., and Lhota S. (2020). Northern lesser galagos (Galago Senegalensis) increase the production of loud calls before and at dawn. Primates 61, 331–338. doi: 10.1007/s10329-019-00784-3
Schön P. C., Puppe B., and Manteuffel G. (2004). Automated recording of stress vocalisations as a tool to document impaired welfare in pigs. Anim. Welfare 13, 105–110. doi: 10.1017/S096272860002683X
Seabold S. and Perktold J. (2010). statsmodels: Econometric and statistical modeling with python. In. 9th Python Sci. Conf., 92–96. doi: 10.25080/issn.2575-9752
Shyne A. (2006). Meta-analytic review of the effects of enrichment on stereotypic behavior in zoo mammals. Zoo Biol. 25, 317–337. doi: 10.1002/zoo.20091
Sol M., Ollier C., Boisseau O., Ridoux V., and Virgili A. (2024). Temporal patterns in dolphin foraging activity in the mediterranean sea: insights from vocalisations recorded during the accobams survey initiative. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1378524
Sportelli J. J., Jones B. L., and Ridgway S. H. (2022). Non-linear phenomena: a common acoustic feature of bottlenose dolphin (Tursiops truncatus) signature whistles. Bioacoustics 00, 1–20. doi: 10.1080/09524622.2022.2106306
Therrien S. C., Thomas J. A., Therrien R. E., and Stacey R. (2012). Time of day and social change affect underwater sound production by bottlenose dolphins (Tursiops truncatus) at the brookfield zoo. Aquat. Mammals 38, 65–75. doi: 10.1578/AM.38.1.2012.65
Thomsen F., Franck D., and Ford J. K. B. (2002). On the communicative significance of whistles in wild killer whales (Orcinus orca). Naturwissenschaften 89, 404–407. doi: 10.1007/s00114-002-0351-x
van Opzeeland I. C., Corkeron P. J., Leyssen T., Similä T., and Parijs S. M. V. (2005). Acoustic behaviour of norwegian killer whales, Orcinus orca, during carousel and seiner foraging on spring-spawning herring. Aquat. Mammals 31, 110–119. doi: 10.1578/AM.31.1.2005.110
Virtanen P., Gommers R., Oliphant T. E., Haberland M., Reddy T., Cournapeau D., et al. (2020). Scipy 1.0: fundamental algorithms for scientific computing in python. Nat. Methods 17, 261–272. doi: 10.1038/s41592-019-0686-2
Weiss B. M., Symonds H., Spong P., and Ladich F. (2007). Intra- and intergroup vocal behavior in resident killer whales, Orcinus orca. J. Acoustical Soc. America 122, 3710–3716. doi: 10.1121/1.2799907
Whitham J. C. and Miller L. J. (2024). Utilizing vocalizations to gain insight into the affective states of non-human mammals. Front. Veterinary Sci. 11. doi: 10.3389/fvets.2024.1366933
Winship K. A. and Jones B. L. (2023). Acoustic monitoring of professionally managed marine mammals for health and welfare insights. Animals 13, 2124. doi: 10.3390/ani13132124
Wong C.-H., Tsai M.-A., Ko F.-C., Wang J.-H., Xue Y.-J., and Yang W.-C. (2023). Skin cortisol and acoustic activity: Potential tools to evaluate stress and welfare in captive cetaceans. Animals 13, 1521. doi: 10.3390/ani13091521
Keywords: bioacoustics, killer whale welfare, vocal activity monitoring, cetacean well-being assessment, passive acoustic analysis
Citation: Almunia J, Lüke JP and Rosa F (2025) Vocal activity as a welfare indicator in killer whales in managed care. Front. Mar. Sci. 12:1595113. doi: 10.3389/fmars.2025.1595113
Received: 18 March 2025; Accepted: 22 July 2025;
Published: 13 August 2025.
Edited by:
Fannie W. Shabangu, Department of Forestry, Fisheries and the Environment, South AfricaReviewed by:
Heather M. Hill, St. Mary’s University, United StatesRachel Probert, Stellenbosch University, South Africa
Copyright © 2025 Almunia, Lüke and Rosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Javier Almunia, ZGlyQGxvcm9wYXJxdWUtZnVuZGFjaW9uLm9yZw==
 Javier Almunia
Javier Almunia Jonas Philipp Lüke2
Jonas Philipp Lüke2 Fernando Rosa
Fernando Rosa