Abstract
Introduction:
This study examines 20 years of killer whale (Orcinus orca) sightings (2002–2023) in the eastern Canadian Arctic, drawing from a comprehensive sighting database spanning 1850–2023. Despite inherent biases favoring data collection near communities and coastal areas, spatiotemporal analyses reveal significant shifts in killer whale distribution linked to changing sea ice conditions.
Methods:
We developed a clustering metric representing the mean distance to the five nearest sightings and included it in a linear model to investigate spatial trends. We investigated temporal trends by modeling the effects of multiple covariates on the ordinal date of killer whale sightings.
Results:
Spatial analysis showed that killer whales are progressively moving away from historically high-use areas and that sighting locations are becoming more dispersed over time. A significant year × sea ice interaction in the temporal analyses indicates observations occur earlier during their arrival period at lower sea ice concentrations over time, suggesting that declining sea ice concentration contributes to earlier arrival. Conversely, for departure periods, killer whales are observed farther south later in the year, likely linked to earlier freeze-up at higher latitudes, and are overall observed later into the year over time. This trend has led to a near doubling of their average presence from 26 days in 2002 to 48 days in 2023 (27 July to 13 September) reflecting an extended open-water season.
Discussion:
These findings underscore the prolonged seasonal use of Arctic regions by killer whales, driven by diminishing sea ice and expanding open-water habitat. Such shifts highlight potential implications for Arctic marine ecosystems as killer whales increasingly overlap with endemic species.
1 Introduction
The expansion of killer whales (Orcinus orca) in Arctic regions driven largely by the ongoing loss of sea ice in response to climate change has significant evolutionary, ecological, and conservation implications (Gilg et al., 2012; Garroway et al., 2024). Historically, seasonal sea ice served as a natural barrier limiting access of killer whales to many Arctic areas, but with the dramatic reduction in summer sea ice cover over recent decades, killer whales have been able to extend their range into previously inaccessible regions (Higdon and Ferguson, 2009; Kovacs et al., 2011). This range expansion in the eastern Canadian Arctic (ECA) may be reshaping the structure of marine ecosystems, as killer whales are apex predators capable of exerting top-down control on prey populations that have previously relied on sea ice to minimize predation risk (Breed et al., 2017; Lennert and Richard, 2017). Their presence poses a potential threat to endemic Arctic marine mammals, including narwhals (Monodon monoceros), belugas (Delphinapterus leucas), and bowhead whales (Balaena mysticetus), which have evolved in predator-sparse environments (Ferguson et al., 2010a, 2012). These shifts in predator-prey dynamics could have cascading ecological effects, potentially altering species distribution, behavior, and survival rates (Baum and Worm, 2009), with long-term implications for Arctic biodiversity and ecosystem function. Furthermore, the increasing presence of killer whales introduces new conservation challenges, as many of the Arctic species they prey upon are already vulnerable to population decline due to the effects of climate change and human activities (Laidre and Heide-Jørgensen, 2005; Moore and Huntington, 2008; Laidre et al., 2015; Orgeret et al., 2022; Kuletz et al., 2024). Understanding the patterns of killer whale sightings in the ECA over the past two decades is crucial for assessing the broader ecological impacts of their range expansion and informing future conservation strategies.
Arctic marine ecosystems are often defined by the presence of sea ice, and ice-associated organisms (Melnikov, 1997). There are three species of endemic Arctic cetaceans: beluga, narwhal, and bowhead whale, as well as many species of marine mammal that migrate into Arctic regions during the ice free/low ice season for feeding (Darnis et al., 2012; McMeans et al., 2013; Kuletz et al., 2024). Killer whales are one such seasonal migrant with a long history of observation in the Arctic, typically observed migrating into the ECA in late spring and departing in the fall as sea ice begins to form (Lefort et al., 2020a).
Sighting data for killer whales in the ECA were collected over time from a diverse network of observers, including Inuit community members, tourists, and researchers. Inuit Traditional Ecological Knowledge (TEK) has been particularly valuable in providing consistent, long-term observations of killer whale presence and behavior (Ferguson et al., 2012; Westdal et al., 2013; Higdon et al., 2014). As human populations and activities in the ECA have increased over the years, sighting effort has expanded, contributing to a more comprehensive dataset. This growing human presence, alongside technological advancements such as larger and faster boats, drones, and aerial surveys, has enhanced the ability to document killer whale occurrence across a broader geographic range (Young et al., 2022).
The sightings dataset was analyzed in conjunction with sea ice metrics, including sea ice coverage, ablation (melting), and formation, to assess how killer whale presence aligns with seasonal changes in ice conditions. As sea ice recedes, larger ice-free areas become accessible to killer whales, enabling them to roam in search of prey. These predators target Arctic endemic species such as narwhals, belugas, bowhead whales, and seals, particularly in regions where these marine mammals congregate for breeding, nursing, and resting during ice-free periods (Laidre and Regehr, 2017). The seasonal availability of concentrated prey in these areas is essential for killer whale energetics (Ferguson et al., 2010a), but also holds critical ecological and cultural importance for Inuit communities, who rely on Arctic marine mammals for both subsistence and cultural continuity via knowledge transfer. Therefore, understanding the timing and geographic patterns of killer whale sightings will provide insight into their ecological role in this rapidly changing environment.
The primary aim of this study is to investigate the spatial and temporal patterns of killer whale sightings in the ECA from 2002 to 2023 and assess how these patterns relate to sea ice dynamics. Clustering analyses, based on the mean distance to the five closest neighboring observations, were used to assess trends in whether sightings occurred in localized hotspots or were more diffusely spread across the region. Additionally, arrival and departure dates were analyzed in relation to sea ice coverage to determine the timing of killer whale presence as it correlates with seasonal ice retreat and formation. By examining general spatial patterns and relationships with environmental variables, we seek to understand the extent and characteristics of killer whale habitat use in the ECA, especially in areas where Arctic endemic marine mammals congregate (Higdon et al., 2012, 2014; Hamilton et al., 2022). These findings are critical for assessing the ecological implications of killer whale range expansion in the Arctic, providing insight into predator-prey interactions, increased pressure on prey species, and informing conservation efforts (Matthews et al., 2020). The relevance of this work extends to both ecological research and the cultural and subsistence needs of Inuit communities that rely on Arctic marine mammals for food security (Huntington et al., 2017).
2 Materials and methods
2.1 Observation data
Killer whale observations in the Canadian Arctic were collected from 1850 to 2023, via community interviews, and searches of Canadian archives and published work (Greely, 1886, 1888; Lee, 1928; Degerbol and Freuchen, 1935; Doan and Douglas, 1953; Stepney and Wooley, 1975; Steltner et al., 1984; Ford et al., 1986; Milani, 1986; Campbell, 1988; Lien et al., 1988; Mitchell and Reeves, 1988; Reeves and Mitchell, 1988; Finley, 1990; Pattie and Webber, 1992; Finley et al., 1993; Kingsley et al., 1994; Gaston and Ouellet, 1997; Richard, 1998; NWMB, 2000; Gonzalez, 2001; Reaney, 2004; Stewart and Lockhart, 2005; Laidre et al., 2006; Stewart, 2008), and word-of-mouth communication to the authors. Location accuracy varies across sightings, and associated coordinates represent best approximations. A prominent objective in our analyses was to investigate the effects of sea ice on killer whale presence in the Canadian Arctic, so observations were subset from 2002 - 2023, to reflect the availability of high-quality sea ice concentration data. Data were also constrained to observations within the ECA, removing all values west of 110°W (n=3). Observations earlier than ordinal date 150 (May 29) (n=4) were removed as they were likely ice entrapped whales (see known entrapment dates in Westdal et al., 2017; Matthews et al., 2019), or questionable identifications.
2.2 Sea ice data
Sea ice concentration data was collected with the Advanced Microwave Scanning Radiometer EOS (AMSR-E) (2002-2011) and AMSR2 (2012-2023) constellation network, and obtained from the Sea Ice Remote Sensing group at the University of Bremen (Spreen et al., 2008; downloaded 20/5/2024). Pixel size for sea ice imagery is 6.25 x 6.25 kilometers, and pixel values either ranged from 0-200 or 0-100 depending on the date; all days were corrected to range from 0-100 to reflect percentage of sea ice cover.
To define the study area sea ice maps were clipped using a 100% minimum convex polygon of the 2002-2023 killer whale observation locations (Figure 1).
Figure 1
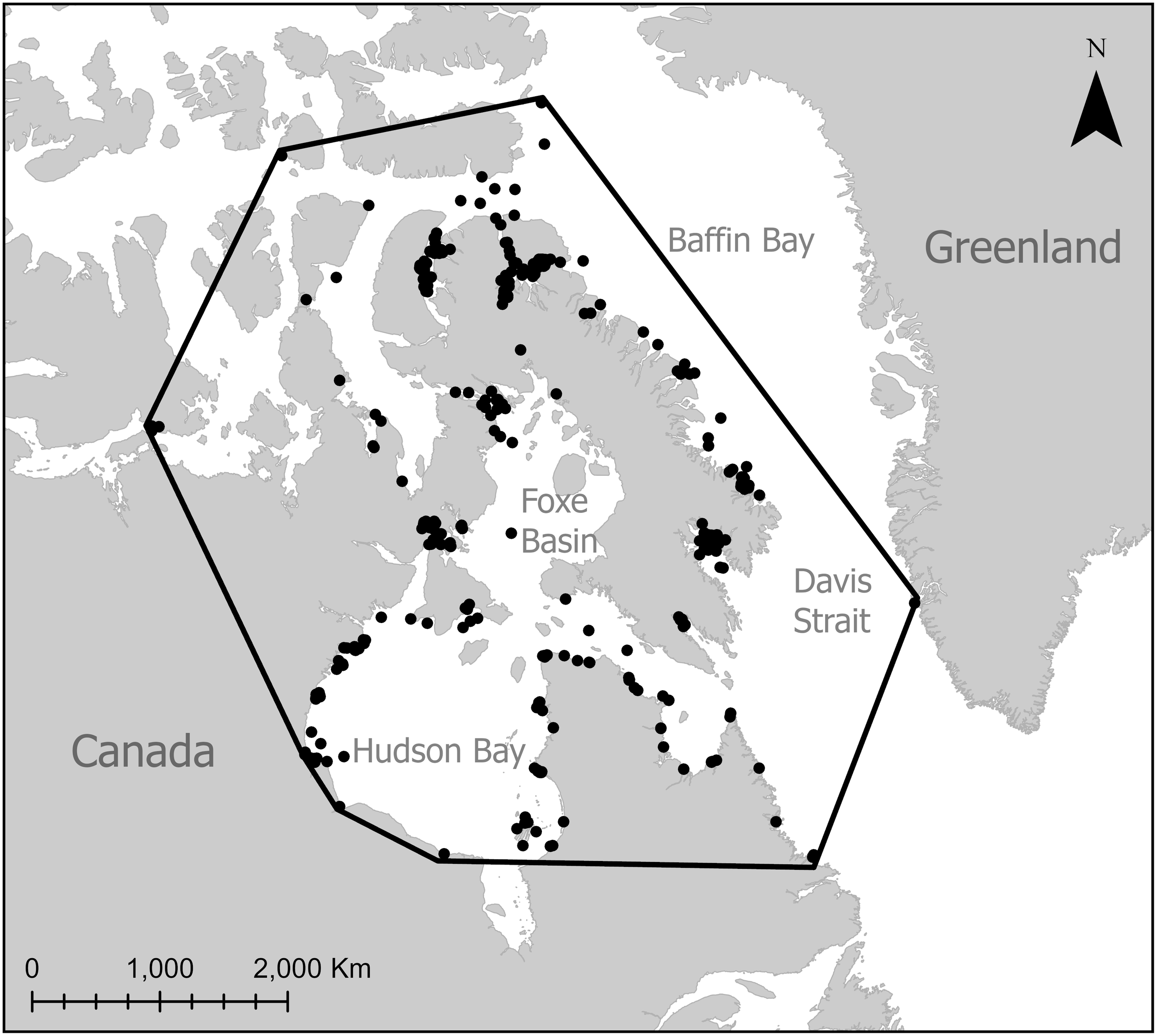
Map showing locations of killer whale sightings in the eastern Canadian Arctic from 2002-2023, the black line shows the study area used to delineate sea ice data.
2.3 Spatial analysis
To investigate spatial patterns in killer whale observations, clustering patterns were determined by developing a clustering metric. The clustering metric for each observation was determined by calculating the mean distance to five nearest sightings with all years combined. The clustering metric was used as the response variable in generalized linear models to determine variables that affect spatial distributions. The explanatory covariates included as potential effects were latitude, longitude, year, and month. No sea ice covariates were included in the clustering model because sea ice covariates are highly temporal, and the clustering metric was calculated for all points across all dates. The top model was chosen using forwards and backwards stepwise model selection based on Akaike Information Criterion (AIC) values.
2.4 Temporal analysis
To investigate temporal patterns, killer whale observations were analyzed in two groups: arrival into and departure out of the study area, as initial data inspection suggested there were two distinct patterns. Observations were separated by median ordinal date of all sightings, 229 (August 17), and all modelling was done for arrival and departure periods separately. Generalized linear models were used to identify variables that influenced the time of year killer whales were being observed. The response variable was the ordinal date of each observation. The explanatory covariates included as potential effects were latitude, longitude, year, sea ice concentration at each observation point (point ice), and mean sea ice concentration in the study area (mean ice). Interaction terms year x mean ice, year x point ice, and point ice x latitude were also included. The top model for arrival and departure was chosen using forwards and backwards stepwise model selection based on AIC values.
All analyses were conducted in RStudio (R Core Team, 2020; version 4.4.0).
3 Results
From 1850-2023 there were 674 documented killer whale observations in the Canadian Arctic. During that period there was an increase in observations (Figure 2). From 2002-2023, killer whales were observed a total of 355 times in the Canadian Arctic, after removing observations outside the study area and before May 29, there were 348 in the ECA. Arrival and departure period were separated by median value, so both periods contained 174 observations. There was no trend in the number of observations across years during the study period (Figure 2), with an average of 16 (range: 3-28) observations per year. Point ice values were missing for 66 of the 348 observations because the resolution of the sea ice data resulted in some marine regions along the coast being treated as land. Due to this, point ice was removed from the models to retain sample size.
Figure 2
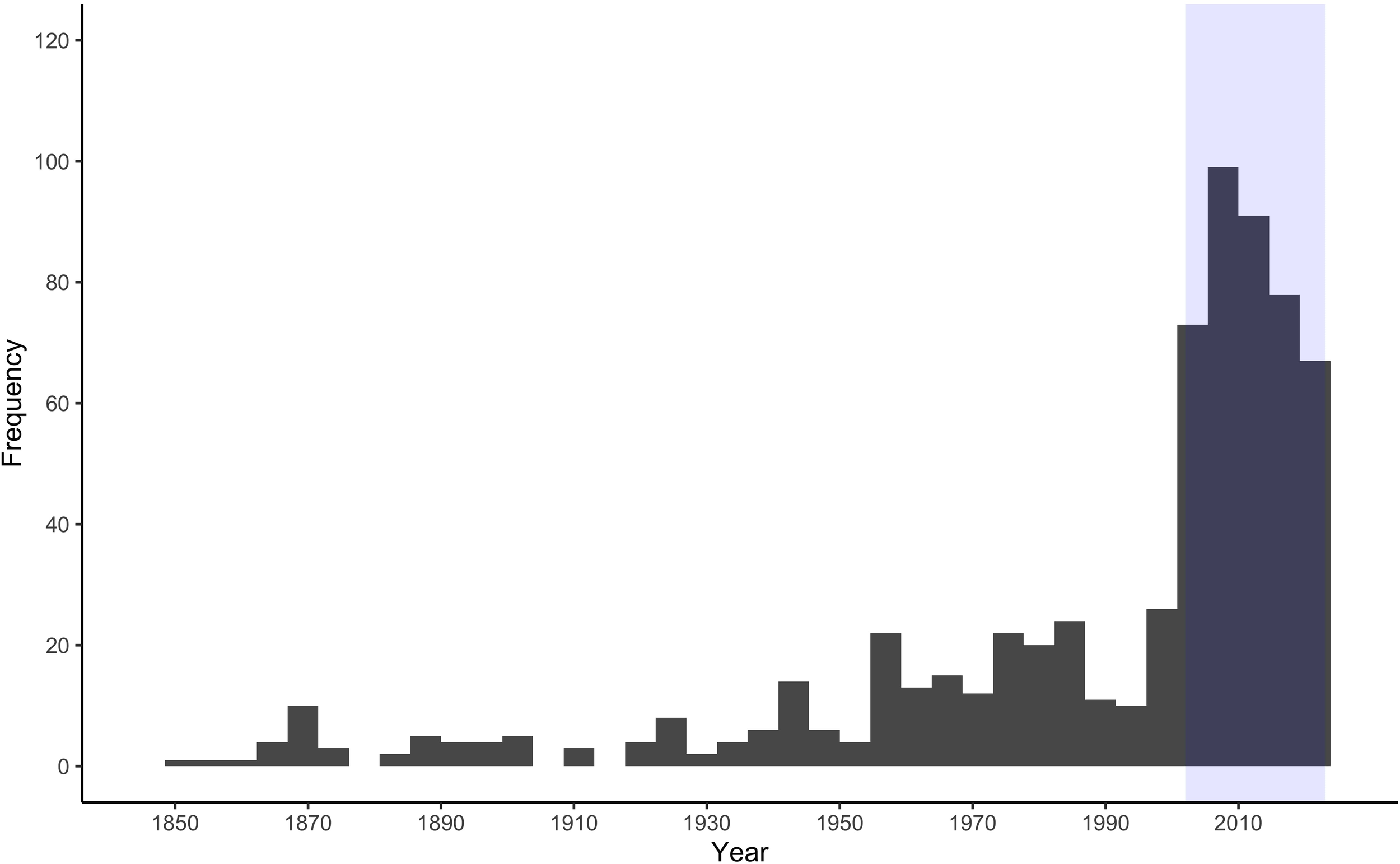
Frequency of killer whale sightings in the eastern Canadian Arctic over time. Blue box shows the study period (2002-2023) for analyses conducted in this study.
3.1 Spatial analysis
The clustering metric revealed that the mean distance to five nearest observations ranged from 34 - 327 km. The top model included covariates latitude, longitude, and year (Table 1). To explore the effect of year, the strongest effect in the model, year was plotted against the clustering metric, showing increased distance between nearest neighbour observations over time (p < 0.01, R2 = 0.23) (Figure 3).
Table 1
| Covariate | Estimate | Std. Error | t-value | p-value |
|---|---|---|---|---|
| Intercept | -8.673e+03 | 1.099e+03 | -7.894 | <0.001 |
| Long | 2.146 | 0.326 | 6.594 | <0.001 |
| Lat | 2.663 | 0.564 | 4.723 | <0.001 |
| Year | 4.394 | 0.545 | 8.063 | <0.001 |
Model summary for top regression model for covariates affecting spatial clustering in killer whales observed in the eastern Canadian Arctic from 2002-2023.
Figure 3
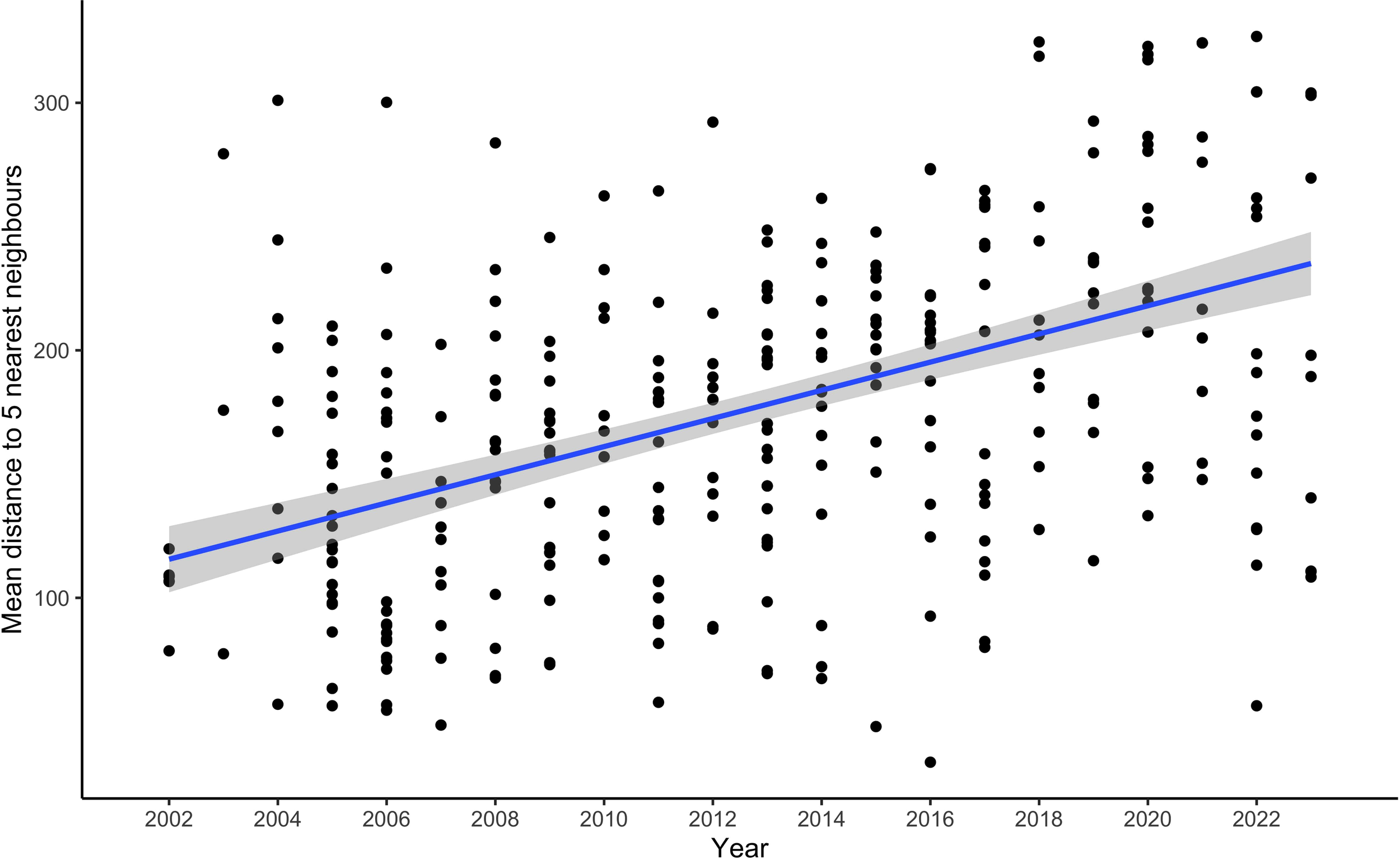
Mean distance to five nearest neighbour observations of killer whale sightings in the eastern Canadian Arctic over time from 2002-2023. Regression line of temporal trend in distance to neighbouring observations shown in blue with 95% confidence interval (CI) in grey (R2 = 0.23).
3.2 Temporal analyses
3.2.1 Arrival model
Arrival observations occurred from June 5 - August 16. The top model included covariates year, mean ice, latitude, and year x mean ice interaction term (Table 2), and all included covariates had significant effects. To further investigate the effects, ordinal date was plotted against year (Figure 4A), which showed a significant downwards trend, suggesting that observations occurred earlier in the year in later years. Killer whales arrived 0.6 days/year earlier over the 21-year study period, resulting in an average arrival 10 days earlier in 2023 (27 July) compared to 2002 (6 August). To visualize the interaction term ordinal date was plotted against sea ice concentration, with a trendline for each year (Figure 5A), which shows that in earlier years killer whales were observed when sea ice concentration was higher, with a tendency towards observations at the same ordinal date having lower associated sea ice concentrations in later years.
Table 2
| Arrival period | ||||
|---|---|---|---|---|
| Covariate | Estimate | Std. Error | t-value | p-value |
| Intercept | 1.448e+03 | 2.693e+02 | 5.378 | <0.001 |
| Year | -0.613 | 0.135 | -4.551 | <0.001 |
| meanice | -51.129 | 23.181 | -2.206 | 0.029 |
| Lat | 0.218 | 0.103 | 2.122 | 0.035 |
| Year:meanice | 0.025 | 0.012 | 2.145 | 0.033 |
| Departure period | ||||
|---|---|---|---|---|
| Covariate | Estimate | Std. Error | t-value | p-value |
| Intercept | 2.331e+03 | 7.493e+02 | -3.111 | 0.002 |
| Year | 1.303 | 0.371 | 3.512 | <0.001 |
| meanice | 350.563 | 112.646 | 3.112 | 0.002 |
| Lon | 0.319 | 0.127 | 2.513 | 0.013 |
| Lat | -0.480 | 0.234 | -2.056 | 0.041 |
| Year:meanice | -0.172 | 0.056 | -3.090 | 0.002 |
Model summary for top regression model for covariates affecting ordinal date of observation of killer whales in the eastern Canadian Arctic from 2002-2023 during the arrival period from June 5- August 16 and departure period from August 17 – December 11.
Figure 4
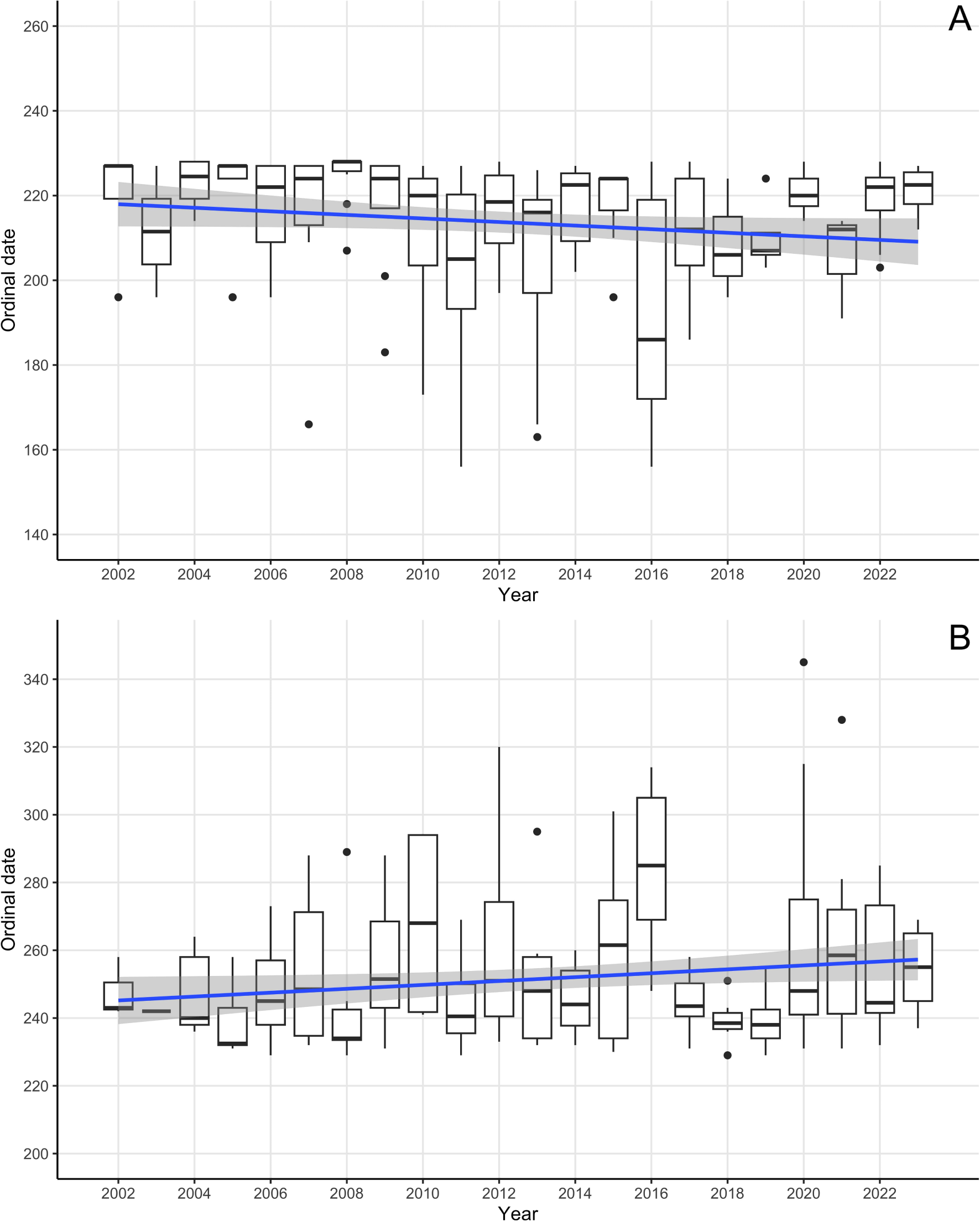
Box plots of ordinal date of killer whale sightings in the eastern Canadian Arctic during the arrival period (A) and departure period (B) from 2002-2023 showing mean, 25th and 75th percentiles, and 95% confidence interval (CI) whiskers. Regression line of temporal trend in ordinal date of observation shown in blue with 95% CI in grey.
Figure 5
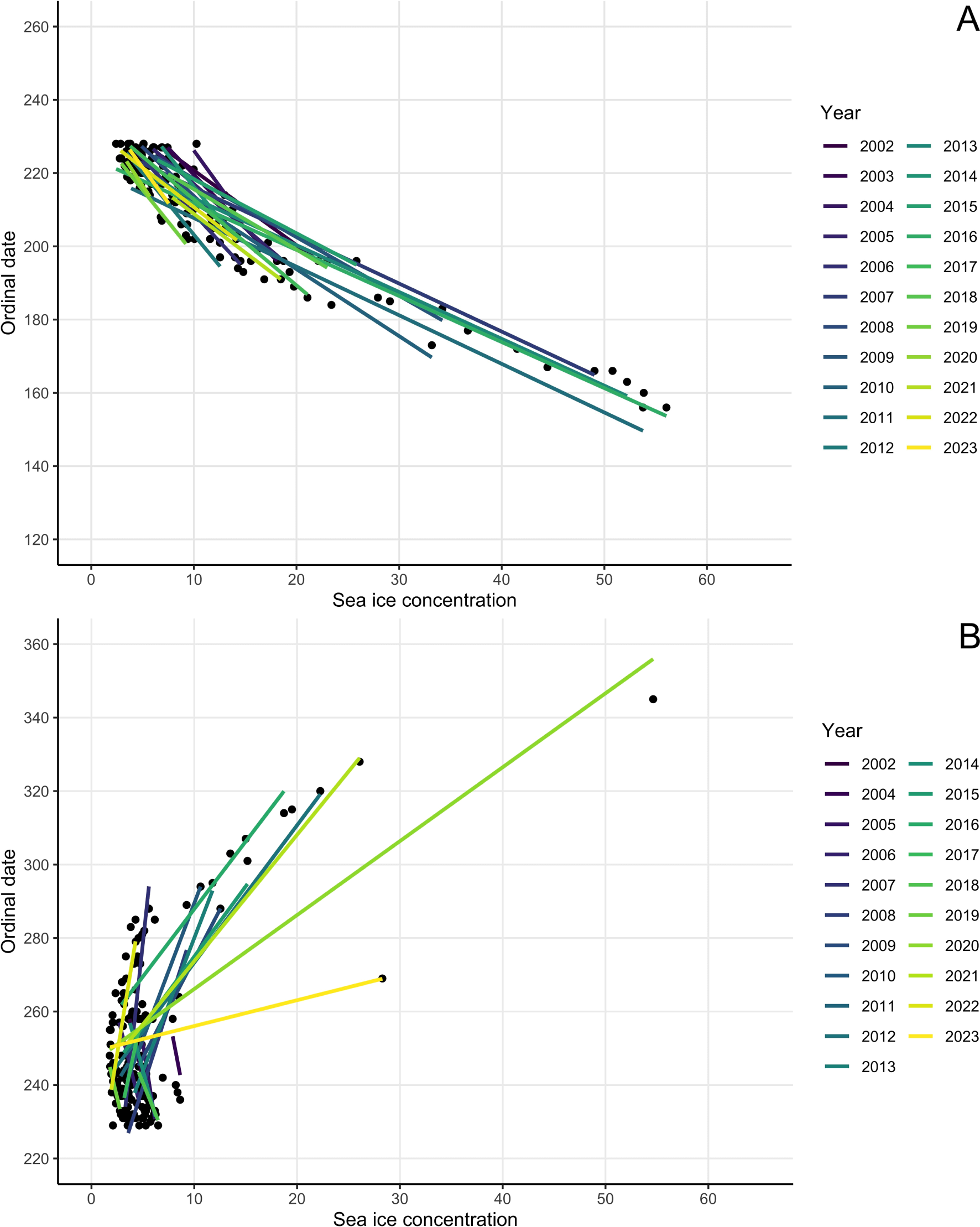
Ordinal dates of killer whale sightings in the eastern Canadian Arctic during the arrival period (A) and departure period (B) from 2002-2023 as a function of regional sea ice cover in the study area on the date of the sighting. Coloured regression lines reflect annual trends.
3.2.2 Departure model
Departure observations occurred from August 17 - December 11. The top model includes covariates year, mean ice, longitude, latitude, and year x mean ice interaction term (Table 2), and all included covariates had significant effects. To further investigate effects, ordinal date was plotted against year (Figure 4B), which showed an upwards trend, suggesting that observations occurred later in the year in later years. Killer whales departed 1.3 days/year later over the 21-year study period, resulting in an average departure 12 days later in 2023 (13 September) compared to 2002 (1 September). To visualize the interaction term, ordinal date was plotted against sea ice concentration, with a regression line for each year (except 2003 which had a single data point) (Figure 5B), which shows that in later years killer whales were observed later during the departure period, at times when sea ice concentration was higher.
4 Discussion
In our spatial model, clustering of observations was significantly affected by latitude and longitude. Higher clustering in southern and western locations in the study area was likely driven by the high density of observations in western Hudson Bay and Foxe Basin. The effect of year on clustering suggests that over time killer whales were being observed further away from higher use areas, or spreading further into other regions in the ECA. This pattern of spatial spreading (increased clustering distance) could be linked to patterns of decreased sea ice coverage in the Arctic over time (Comiso et al., 2008; Kwok, 2018), as well as shifting effort or means of reporting, as our sighting dataset shows a slight increase in tourism related sightings and an increased number of reports collected via social media. Overall, killer whales in the ECA during summer exhibited flexible movement patterns and habitat use, likely influenced by prey availability observed across multiple seasonal visits and varying sea ice conditions. When both temporal models, arrival and departure, are considered together, there is a pattern of an extended period of killer whale observation in the ECA. Over time, arrival observations (June 5 - August 16) occurred earlier and departure observations (August 17 - December 11) occurred later. The ECA Archipelago has shown a slightly greater change in the date of freeze up in the fall versus break up in the spring (Galley et al., 2012; Walsh et al., 2022). Killer whale presence reflects this sea ice trend, as observations extended further into fall versus earlier in the spring over the study period. Previous research has quantified a decline in sea ice cover in the Canadian Arctic that has likely influenced killer whale presence (Higdon and Ferguson, 2009; Higdon et al., 2014; Stern and Laidre, 2016). Generally, the rate of earlier ECA killer whale entry and later departure over the past two decades matches the rate of sea ice decline of 1.2 days/year (Yang et al., 2023).
These findings align with earlier research on killer whale distribution and behavior in polar regions. The arrival model had a significant year x mean ice interaction term with a trend that suggests that for whales observed at a similar time (i.e., ordinal day 220), the mean regional ice cover has declined over time. When the trend of earlier arrival is combined with the year x mean ice effect it can be interpreted that there is lower sea ice concentration during arrival in later years, which contributes to killer whale sightings earlier in the year. The latitude effect suggests a trend where killer whales are observed further north later in the arrival period, which aligns with expected movement of killer whales further into the Arctic as the arrival period progresses and ice retreats (Moore and Huntington, 2008; Higdon et al., 2014; Kimber et al., 2025).
The departure model year x mean ice interaction term suggests that in later years killer whales were present later during the departure period, even when sea ice concentration was higher. The extended presence of killer whales in the Canadian Arctic aligned with the extended ice free season previously quantified (Howell et al., 2009; Rodrigues, 2009), but extended presence as sea ice concentration begins to increase at the end of the open water season could suggest unknown factors affecting extended killer whale presence. Killer whales could potentially be altering their behaviour, engaging in more risky or bold habitat selection as ice freezes (Matthews et al., 2019) or engaging in habitat associated learning. Both latitude and longitude had a significant effect in the departure model. The latitude effect suggests that killer whales are observed further south later in the year, which aligns with expected movement as sea ice freezes up and killer whales depart from the Arctic. The effect of longitude suggests that whales were observed further east later in the departure period, which is likely related to whales exiting the region east through Davis Strait during freeze up (Matthews et al., 2011; Lefort et al., 2020a). Our results emphasize the role of diminishing sea ice as a primary driver of killer whale distribution and prolonged seasonal presence. However, there are other potential drivers, including prey availability, climate variability, and anthropogenic influences.
Overall, killer whales have been arriving in the ECA 0.6 days/year earlier and departing 1.3 days/year later, leading to a near doubling of their average presence from 26 days in 2002 (6 August to 1 September) to 48 days in 2023 (27 July to 13 September), although this trend varies based on sea ice concentration. Killer whales in the Bering Strait present a stronger trend of temporally increased presence aligning with decreased ice coverage although at a smaller spatial scale than our study, with presence increasing by 32 days over only 8 years (Kimber et al., 2025). The extended period of presence provides killer whales with increased opportunities to locate and prey on marine mammals, potentially having ecological impacts on species that seasonally give birth and nurse their calves during the ice-free period (Laidre and Regehr, 2017). Bowhead, beluga, and narwhal, for instance, migrate to specific summering areas to nurse neonates: bowhead whales within icy habitats (Ferguson et al., 2010b), belugas in shallow estuaries (Smith et al., 2017), and narwhals in remote fjords (Laidre et al., 2004). The prolonged open-water season and increased killer whale presence heighten predation risks, particularly for young and inexperienced calves, potentially negatively affecting population dynamics of these vulnerable Arctic species.
There are associated shortcomings with this data set, as observations are biased by both the density and presence of human observers resulting in uneven spatial and temporal coverage. Observations are not random, and thus, results associated with habitat selection cannot be prescriptive of all killer whales in the Canadian Arctic. Sighting data that is not linked to individual or group identification limits our ability to accurately test group arrival and departure times and time spent in the ECA by groups. As a result, our analysis only reflects a general overview of all killer whale groups visiting the region in summer (Lefort et al., 2020a). This restricted the ability to include point ice in the models as it greatly reduced the sample size, which reduced the ability to identify specific individual trends with sea ice and killer whale presence. The mean ice values are potentially more applicable to killer whale presence trends, however, we are investigating arrival and departure trends, which are likely to be impacted by the killer whales’ ability to move throughout the region. The clustering metric and spatiotemporal analyses mitigate some of these data limitations while suggesting areas for improvement in monitoring.
Killer whales fulfill an apex predator trophic role in Arctic ecosystems, with multiple documented predation events on narwhal, beluga whales, bowhead whales, Atlantic walrus (Odobenus rosmarus rosmarus), and multiple species of seal (Steltner et al., 1984; Campbell, 1988; Reeves and Mitchell, 1988; Finley, 1990; Melnikov and Zagrebin, 2005; Laidre et al., 2006; Higdon, 2007; Ferguson et al., 2012; Higdon et al., 2012, 2014; Westdal et al., 2016; Breed et al., 2017). The work presented here suggests that killer whales in the Arctic are both spatially and temporally expanding their presence, moving into regions not previously observed, entering earlier, leaving later, and staying longer. This overall increase in presence has likely put more pressure on Arctic species that were previously less threatened by killer whale predation. Continued declines in sea ice cover will affect prey species by temporally increasing killer whale predation, influencing prey species behaviour, and reducing the availability of sea ice refuge (Breed et al., 2017; Matthews et al., 2020). It is unclear how prey species will respond, but they may shift their summer range further into the Canadian Archipelago or northward into the Last Ice Area (Moore et al., 2019). However, the actual response and demographic success of such changes are likely species-dependent. For example, beluga whales exhibit strong fidelity to their summer areas and show limited adaptability to changes in seasonal habitat use (O’Corry-Crowe et al., 2020; Skovrind et al., 2021). The relationships between killer whales and other species in Arctic ecosystems are expected to be dynamic, as productivity and availability of prey species is predicted to shift as climate change reduces sea ice. For example, the distribution of zooplankton species in the Arctic has changed in response to warming water (Blachowiak-Samolyk et al., 2008; Balazy et al., 2018; Abe et al., 2020), which is predicted to impact bowhead whale abundance and distribution (Laidre et al., 2008; Fortune et al., 2023). Continued documentation of Arctic killer whale sightings will be important for tracking the presence of killer whales in this ecosystem and predicting the effects of increased presence alongside other ecosystem-wide shifts.
Killer whales exhibit remarkable behavioral plasticity, enabling them to exploit newly accessible habitats, including in the Arctic as sea ice recedes (Brakes and Dall, 2016; Bester et al., 2017). This flexibility allows them to adjust their movement patterns and habitat use in response to shifting prey availability and environmental conditions (Tavares et al., 2017). The observed trend of earlier arrivals and later departures reflects an adaptive strategy that maximizes energy intake during extended open-water seasons (Kuletz et al., 2024). These extended periods in the Arctic may also align with reproductive strategies, ensuring access to abundant prey resources, via Arctic associated prey, necessary to support gestation, lactation, and calf growth (Weiss et al., 2023). Such behavioral adaptations underscore killer whales’ ability to thrive in dynamic ecosystems, further influencing predator-prey interactions and Arctic marine food web dynamics.
The increasing presence of killer whales in the ECA underscores the need for monitoring and managing their predation on culturally and economically significant prey species (Stewart, 2008, 2018; Higdon, 2010). Incorporating killer whale distribution shifts into Arctic conservation and policy frameworks is essential for mitigating their economic and cultural impacts and ensuring the sustainability of Arctic endemic species (Garroway et al., 2024). Future research should prioritize understanding prey selection and energy requirements to quantify the potential regulatory effects of killer whale predation on Arctic ecosystems (Lefort et al., 2020b; Breed, 2021). Additionally, continued research on their social structure, acoustic behavior, and genetic and cultural diversity, and similar research on prey species, will provide critical insights into population dynamics and adaptation strategies (Esteban et al., 2016; Jourdain et al., 2019; Sportelli et al., 2022). Modeling future distribution patterns under various climate scenarios will further inform proactive management strategies to address the ecological consequences of continued sea ice loss (van Weelden et al., 2021; Chambault et al., 2022).
Future research could include testing whether increasing killer whale presence is driving measurable changes in the behavior, distribution, and population dynamics of endemic Arctic prey species. For example, satellite telemetry and drone-based behavioural observations could be used to assess changes in habitat use or predator avoidance by seals, as has been explored for narwhals (Breed et al., 2017) and bowhead whales (Matthews et al., 2020). Researchers could examine whether increased killer whale presence correlates with reduced fidelity to calving or moulting sites in ice-associated species, or whether predator-induced stress is altering prey diving behaviour or acoustic signaling—detectable through passive acoustic monitoring (PAM) and biologging technologies. Comparative diet studies of Arctic killer whales and those in other rapidly warming regions (e.g., Southern Ocean, Bering Sea) could help identify convergent ecological responses to climate change.
Another promising research direction is to investigate whether migratory connectivity is shifting, for example, whether killer whales are beginning to overwinter farther north. Interannual variability in sea ice phenology may drive year-to-year variation in killer whale migration timing and extent, and continued sea ice loss could be facilitating increased inter-basin connectivity between historically distinct populations (e.g., Baffin Bay and the Beaufort Sea; Heide-Jørgensen et al., 2012). These hypotheses can be tested by combining killer whale telemetry with high-resolution sea ice datasets and applying dynamic habitat models (e.g., species distribution models, step selection functions) that incorporate both ice metrics and prey availability. Finally, future studies could test the hypothesis that killer whale migration timing is more tightly linked to sea ice concentration and freeze-thaw dynamics than to temperature or prey movements alone. This could involve using high-resolution satellite ice data alongside predictive models incorporating the Sea-Ice Model Intercomparison Project (CMIP6) sea ice projections to estimate how arrival and departure windows may shift under continued Arctic warming.
These findings highlight how climate-driven shifts may be reshaping Arctic ecosystems, where diminishing sea ice facilitates extended seasonal use of the ECA region by killer whales, a key marine predator. By adding to knowledge of killer whale demographics, and movement patterns, this study contributes to a broader understanding of predator-prey dynamics in the context of rapid environmental change (Guiden et al., 2019; Arumugam et al., 2020). As Arctic ecosystems face increasing pressure from climate change, this research provides needed insights into the ecological consequences of shifting predator distributions and underscores the importance of integrating these dynamics into conservation and management strategies.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SF: Conceptualization, Data curation, Project administration, Writing – original draft, Writing – review & editing. BB: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. KW: Investigation, Writing – review & editing. SP: Investigation, Writing – review & editing. CW: Investigation, Writing – review & editing. CM: Investigation, Writing – review & editing. JH: Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was provided by Nunavut Wildlife Management Board (3-18-1), Fisheries and Oceans Canada, University of Manitoba, and Natural Sciences and Engineering Research Council (RGPIN/5961).
Acknowledgments
We thank the different individuals and groups that have contributed sighting information and in particular Inuit participants that shared information on Nunavut killer whales. We thank the reviewers for their time and valued insight.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abe Y. Matsuno K. Fujiwara A. Yamaguchi A. (2020). Review of spatial and inter-annual changes in the zooplankton community structure in the western Arctic Ocean during summers of 2008–2017. Prog. Oceanogr.186, 102391. doi: 10.1016/j.pocean.2020.102391
2
Arumugam R. Guichard F. Lutscher F. (2020). Persistence and extinction dynamics driven by the rate of environmental change in a predator–prey metacommunity. Theor. Ecol.13, 629–643. doi: 10.1007/s12080-020-00473-8
3
Balazy K. Trudnowska E. Wichorowski M. Błachowiak-Samołyk K. (2018). Large versus small zooplankton in relation to temperature in the Arctic shelf region. Polar Res.37, 1427409. doi: 10.1080/17518369.2018.1427409
4
Baum J. K. Worm B. (2009). Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol.78, 699–714. doi: 10.1111/j.1365-2656.2009.01531.x
5
Bester M. N. Bornemann H. McIntyre T. (2017). “Antarctic marine mammals and sea ice,” in Sea Ice. Ed. ThomasD. N. (New York: Wiley), 534–555.
6
Blachowiak-Samolyk K. Søreide J. E. Kwasniewski S. Sundfjord A. Hop H. Falk-Petersen S. et al . (2008). Hydrodynamic control of mesozooplankton abundance and biomass in northern Svalbard waters (79–81 N). Deep Sea Res. Part II55, 2210–2224. doi: 10.1016/j.dsr2.2008.05.018
7
Brakes P. Dall S. R. X. (2016). Marine mammal behavior: A review of conservation implications. Front. Marine Sci.3. doi: 10.3389/fmars.2016.00087
8
Breed G. A. (2021). “Chapter 29 - Predators and impacts of predation,” in The Bowhead Whale. Eds. GeorgeJ. C.ThewissenJ. G. M. (Cambridge: Academic Press), 457–470.
9
Breed G. A. Matthews C. J. Marcoux M. Higdon J. W. LeBlanc B. Petersen S. D. et al . (2017). Sustained disruption of narwhal habitat use and behavior in the presence of Arctic killer whales. Proc. Natl. Acad. Sci.114, 2628–2633. doi: 10.1073/pnas.1611707114
10
Campbell R. (1988). Predation on narwhals, Monudon monoceros, by killer whales, Orcinus orca, in the eastern Canadian Arctic. Can. Field-Naturalist102, 689–696. doi: 10.5962/p.356653
11
Chambault P. Kovacs K. M. Lydersen C. Shpak O. Teilmann J. Albertsen C. M. et al . (2022). Future seasonal changes in habitat for Arctic whales during predicted ocean warming. Sci. Adv.8, eabn2422. doi: 10.1126/sciadv.abn2422
12
Comiso J. C. Parkinson C. L. Gersten R. Stock L. (2008). Accelerated decline in the Arctic sea ice cover. Geophys. Res. Lett.35, L01703. doi: 10.1029/2007GL031972
13
Darnis G. Robert D. Pomerleau C. Link H. Archambault P. Nelson R. J. et al . (2012). Current state and trends in Canadian Arctic marine ecosystems: II. Heterotrophic food web, pelagic-benthic coupling, and biodiversity. Climate Change115, 179–205. doi: 10.1007/s10584-012-0483-8
14
Degerbol M. Freuchen P. (1935). Report of the Mammals Collected by the Fifth Thule Expedition to Arctic North America (New York: AMS Press).
15
Doan K. H. Douglas C. (1953). Beluga of the churchill region of hudson bay. Fish. Res. Board Canada Bull.98, 27.
16
Esteban R. Verborgh P. Gauffier P. Giménez J. Foote A. D. de Stephanis R. (2016). Maternal kinship and fisheries interaction influence killer whale social structure. Behav. Ecol. Sociobiol.70, 111–122. doi: 10.1007/s00265-015-2029-3
17
Ferguson S. H. Dueck L. Loseto L. L. Luque S. P. (2010b). Bowhead whale Balaena mysticetus seasonal selection of sea ice. Marine Ecol. Prog. Ser.411, 285–297. doi: 10.3354/meps08652
18
Ferguson S. H. Higdon J. W. Chmelnitsky E. G. (2010a). “The rise of killer whales as a major arctic predator,” in A Little Less Arctic. Eds. FergusonS. H.LosetoL. L.MalloryM. L. (Springer-Verlag Berlin, Berlin), 117–136.
19
Ferguson S. H. Higdon J. W. Westdal K. H. (2012). Prey items and predation behavior of killer whales (Orcinus orca) in Nunavut, Canada based on Inuit hunter interviews. Aquat. Biosyst.8, 1–16. doi: 10.1186/2046-9063-8-3
20
Finley K. (1990). Isabella Bay, Baffin Island: an important historical and present-day concentration area for the endangered bowhead whale (Balaena mysticetus) of the eastern Canadian Arctic. Arctic43, 137–152. doi: 10.14430/arctic1604
21
Finley K. Evans C. Davis R. (1993). “Evaluation of the importance of Isabella Bay, Baffin Island, as summer habitat for the endangered bowhead whale,” in Progress report of 1983 studies (World Wildlife Fund, Canada).
22
Ford J. Nichol L. Canvanagh D. (1986). “Preliminary assessment of the value of underwater vocalization in population studies of narwhals in the Canadian Arctic,” in Whales Beneath the Ice Program (World Wildlife Fund Canada, Vancouver, Canada), 44.
23
Fortune S. M. Trites A. W. LeMay V. Baumgartner M. F. Ferguson S. H. (2023). Year-round foraging across large spatial scales suggest that bowhead whales have the potential to adapt to climate change. Front. Marine Sci.9, 853525. doi: 10.3389/fmars.2022.853525
24
Galley R. J. Else B. G. T. Howell S. E. L. Lukovich J. V. Barber D. G. (2012). Landfast sea ice conditions in the canadian arctic: 1983-2009. Arctic65, 133–144. doi: 10.14430/arctic4195
25
Garroway C. J. de Greef E. Lefort K. J. Thorstensen M. J. Foote A. D. Matthews C. J. D. et al . (2024). Climate change introduces threatened killer whale populations and conservation challenges to the Arctic. Global Change Biol.30, e17352. doi: 10.1111/gcb.17352
26
Gaston A. J. Ouellet H. (1997). Birds and mammals of coats island, NWT. Arctic50, 101–118. doi: 10.14430/arctic1094
27
Gilg O. Kovacs K. M. Aars J. Fort J. Gauthier G. Grémillet D. et al . (2012). Climate change and the ecology and evolution of Arctic vertebrates. Ann. New York Acad. Sci.1249, 166–190. doi: 10.1111/j.1749-6632.2011.06412.x
28
Gonzalez N. (2001). Inuit traditional ecological knowledge of the Hudson Bay narwhal (Tuugaalik) population (Ottawa: Report prepared for Department of Fisheries and Oceans, Iqaluit, Nunavut).
29
Greely A. W. (1886). Three years of Arctic service: an account of the Lady Franklin Bay expedition of 1881-84, and the attainment of the farthest north (London: C. Scribner’s sons).
30
Greely A. W. (1888). Report on the Proceedings of the United States Expedition to Lady Franklin Bay, Grinnell Land (Washington: US Government Printing Office).
31
Guiden P. W. Bartel S. L. Byer N. W. Shipley A. A. Orrock J. L. (2019). Predator: prey interactions in the anthropocene: reconciling multiple aspects of novelty. Trends Ecol. Evol.34, 616–627. doi: 10.1016/j.tree.2019.02.017
32
Hamilton C. D. Lydersen C. Aars J. Acquarone M. Atwood T. Baylis A. et al . (2022). Marine mammal hotspots across the circumpolar Arctic. Diversity Distribut.28, 2729–2753. doi: 10.1111/ddi.13543
33
Heide-Jørgensen M. P. Laidre K. L. Quakenbush L. T. Citta J. J. (2012). The Northwest Passage opens for bowhead whales. Biol. Lett.8, 270–273. doi: 10.1098/rsbl.2011.0731
34
Higdon J. (2007). “Status of knowledge on killer whales (Orcinus orca) in the Canadian Arctic,” in Fisheries and Oceans Canada, Canadian Science Advisory Secretariat Research Document 2007/048. (Ottawa: Canadian Science Advisory Secretariat). Available at: http://www.dfo-mpo.gc.ca/csas/Csas/Home-Accueil_e.htm (Accessed January 24, 2025).
35
Higdon J. W. (2010). Commercial and subsistence harvests of bowhead whales (Balaena mysticetus) in eastern Canada and West Greenland. J. Cetacean Res. Manage.11, 185–216. doi: 10.47536/jcrm.v11i2.623
36
Higdon J. W. Ferguson S. H. (2009). Loss of Arctic sea ice causing punctuated change in sightings of killer whales (Orcinus orca) over the past century. Ecol. Appl.19, 1365–1375. doi: 10.1890/07-1941.1
37
Higdon J. W. Hauser D. D. Ferguson S. H. (2012). Killer whales (Orcinus orca) in the Canadian Arctic: distribution, prey items, group sizes, and seasonality. Marine Mammal Sci.28, E93–E109. doi: 10.1111/j.1748-7692.2011.00489.x
38
Higdon J. W. Westdal K. H. Ferguson S. H. (2014). Distribution and abundance of killer whales (Orcinus orca) in Nunavut, Canada—an Inuit knowledge survey. J. Marine Biol. Assoc. U. K.94, 1293–1304. doi: 10.1017/S0025315413000921
39
Howell S. E. L. Duguay C. R. Markus T. (2009). Sea ice conditions and melt season duration variability within the Canadian Arctic Archipelago: 1979–2008. Geophys. Res. Lett.36, L10502. doi: 10.1029/2009GL037681
40
Huntington H. P. Quakenbush L. T. Nelson M. (2017). Evaluating the effects of climate change on indigenous marine mammal hunting in northern and western alaska using traditional knowledge. Front. Marine Sci.4. doi: 10.3389/fmars.2017.00319
41
Jourdain E. Ugarte F. Víkingsson G. A. Samarra F. I. P. Ferguson S. H. Lawson J. et al . (2019). North Atlantic killer whale Orcinus orca populations: a review of current knowledge and threats to conservation. Mammal Rev.49, 384–400. doi: 10.1111/mam.12168
42
Kimber B. M. Braen E. K. Wright D. L. Harlacher J. M. Crance J. L. Berchok C. L. (2025). Less ice, more predators: passive acoustic monitoring shows variation in killer whale (Orcinus orca) presence in the U.S. Arctic with declining sea ice. Polar Biol.48, 21. doi: 10.1007/s00300-024-03332-y
43
Kingsley M. C. Cleator H. J. Ramsey M. A. (1994). Summer distribution and movements of narwhals (Monodon monoceros) in Eclipse Sound and adjacent waters, North Baffin Island, NWT. Meddelelser om Grønland Biosci.39, 163–174. doi: 10.7146/mogbiosci.v39.142544
44
Kovacs K. M. Lydersen C. Overland J. E. Moore S. E. (2011). Impacts of changing sea-ice conditions on Arctic marine mammals. Marine Biodivers.41, 181–194. doi: 10.1007/s12526-010-0061-0
45
Kuletz K. J. Ferguson S. H. Frederiksen M. Gallagher C. P. Hauser D. D. W. Hop H. et al . (2024). A review of climate change impacts on migration patterns of marine vertebrates in Arctic and Subarctic ecosystems. Front. Environ. Sci.12. doi: 10.3389/fenvs.2024.1434549
46
Kwok R. (2018). Arctic sea ice thickness, volume, and multiyear ice coverage: losses and coupled variability, (1958–2018). Environ. Res. Lett.13, 105005. doi: 10.1088/1748-9326/aae3ec
47
Laidre K. L. Heide-Jørgensen M. P. (2005). Arctic sea ice trends and narwhal vulnerability. Biol. Conserv.121, 509–517. doi: 10.1016/j.biocon.2004.06.003
48
Laidre K. L. Heide-Jørgensen M. P. Logdson M. L. Hobbs R. C. Heagerty P. Dietz R. et al . (2004). Seasonal narwhal habitat associations in the high Arctic. Marine Biol.145, 821–831. doi: 10.1007/s00227-004-1371-1
49
Laidre K. L. Heide-Jørgensen M. P. Orr J. R. (2006). Reactions of narwhals, Monodon monoceros, to killer whale, Orcinus orca, attacks in the eastern Canadian Arctic. Can. Field-Naturalist120, 457–465. doi: 10.22621/cfn.v120i4.355
50
Laidre K. L. Regehr E. V. (2017). “Arctic marine mammals and sea ice,” in Sea Ice. Ed. ThomasD. N. (New York: Wiley), 516–533.
51
Laidre K. L. Stern H. Kovacs K. M. Lowry L. Moore S. E. Regehr E. V. et al . (2015). Arctic marine mammal population status, sea ice habitat loss, and conservation recommendations for the 21st century. Conserv. Biol.29, 724–737. doi: 10.1111/cobi.12474
52
Laidre K. L. Stirling I. Lowry L. F. Wiig O. Heide-Jorgensen M. P. Ferguson S. H. (2008). Quantifying the sensitivity of arctic marine mammals to climate-induced habitat change. Ecol. Appl.18, S97–S125. doi: 10.1890/06-0546.1
53
Lee H. P. (1928). Policing the top of the world (London: John Lane the Bodley Head Limited).
54
Lefort K. J. Garroway C. J. Ferguson S. H. (2020b). Killer whale abundance and predicted narwhal consumption in the Canadian Arctic. Global Change Biol.26, 4276–4283. doi: 10.1111/gcb.15152
55
Lefort K. J. Matthews C. J. D. Higdon J. W. Petersen S. D. Westdal K. H. Garroway C. J. et al . (2020a). A review of Canadian Arctic killer whale (Orcinus orca) ecology. Can. J. Zool.98, 245–253. doi: 10.1139/cjz-2019-0207
56
Lennert A. E. Richard G. (2017). At the cutting edge of the future: Unravelling depredation, behaviour and movement of killer whales in the act of flexible management regimes in Arctic Greenland. Ocean Coastal Manage.148, 272–281. doi: 10.1016/j.ocecoaman.2017.08.016
57
Lien J. Stenson G. B. Jones P. W. (1988). Killer whales (Orcinus orca) in waters off Newfoundland and Labrador 1978-1986. Rit Fiskideildar11, 194–201.
58
Matthews C. J. Breed G. A. LeBlanc B. Ferguson S. H. (2020). Killer whale presence drives bowhead whale selection for sea ice in Arctic seascapes of fear. Proc. Natl. Acad. Sci.117, 6590–6598. doi: 10.1073/pnas.1911761117
59
Matthews C. J. D. Luque S. P. Petersen S. D. Andrews R. D. Ferguson S. H. (2011). Satellite tracking of a killer whale (Orcinus orca) in the eastern Canadian Arctic documents ice avoidance and rapid, long-distance movement into the North Atlantic. Polar Biol.34, 1091–1096. doi: 10.1007/s00300-010-0958-x
60
Matthews C. J. D. Raverty S. A. Noren D. P. Arragutainaq L. Ferguson S. H. (2019). Ice entrapment mortality may slow expanding presence of Arctic killer whales. Polar Biol.42, 639–644. doi: 10.1007/s00300-018-02447-3
61
McMeans B. C. Rooney N. Arts M. T. Fisk A. T. (2013). Food web structure of a coastal Arctic marine ecosystem and implications for stability. MEPS482, 17–28. doi: 10.3354/meps10278
62
Melnikov A. (1997). Arctic Sea Ice Ecosystem (Florida: CRC Press).
63
Melnikov V. Zagrebin I. (2005). Killer whale predation in coastal waters of the Chukotka Peninsula. Marine Mammal Sci.21, 550–556. doi: 10.1111/j.1748-7692.2005.tb01248.x
64
Milani D. (1986). Wildlife Observation Program, Hudson Bay 1985 (Alberta, Canada: Canterra Energy Limited. Calgary).
65
Mitchell E. Reeves R. (1988). Records of killer whales in the western North Atlantic, with emphasis on Canadian waters. Rit Fiskideildar11, 161–193.
66
Moore S. E. Huntington H. P. (2008). Arctic marine mammals and climate change: impacts and resilience. Ecol. Appl.18, S157–S165. doi: 10.1890/06-0571.1
67
Moore G. W. K. Schweiger A. Zhang J. Steele M. (2019). Spatiotemporal variability of sea ice in the arctic’s last ice area. Geophys. Res. Lett.46, 11237–11243. doi: 10.1029/2019GL083722
68
NWMB (2000). “Final report of the Inuit bowhead knowledge study,” in Nunavut Wildlife Management Board (Iqaluit, Nunavut: Nunavut Wildlife Management Board).
69
O’Corry-Crowe G. Suydam R. Quakenbush L. Smith T. G. Lydersen C. Kovacs K. M. et al . (2020). Group structure and kinship in beluga whale societies. Sci. Rep.10, 11462. doi: 10.1038/s41598-020-67314-w
70
Orgeret F. Thiebault A. Kovacs K. M. Lydersen C. Hindell M. A. Thompson S. A. et al . (2022). Climate change impacts on seabirds and marine mammals: The importance of study duration, thermal tolerance and generation time. Ecol. Lett.25, 218–239. doi: 10.1111/ele.13920
71
Pattie D. Webber M. (1992). 1st record of the Minke Whale, Balaenoptera-acutorostrata, in Manitoba waters. Can. Field-Naturalist106, 266–267. doi: 10.5962/p.356944
72
R Core Team (2020). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
73
Reaney B. (2004). “Bowhead meat wasted,” in Northern News Services online, September 13, 2004. (Yellowknife) Available at: http://www.nnsl.com/frames/newspapers/2004-09/sep13_04bow.html.
74
Reeves R. Mitchell E. (1988). Distribution and seasonality of killer whales in the eastern Canadian Arctic. Rit Fiskideildar11, 136–160.
75
Richard P. (1998). Hudson Bay Narwhal (Fisheries and Oceans Canada, Winnipeg, MB: DFO Science Stock Status Report E5-44. Central and Arctic Region).
76
Rodrigues J. (2009). The increase in the length of the ice-free season in the Arctic. Cold Regions Sci. Technol.59, 78–101. doi: 10.1016/j.coldregions.2009.05.006
77
Skovrind M. Louis M. Westbury M. V. Garilao C. Kaschner K. Castruita J. A. S. et al . (2021). Circumpolar phylogeography and demographic history of beluga whales reflect past climatic fluctuations. Mol. Ecol.30, 2543–2559. doi: 10.1111/mec.15915
78
Smith A. J. Higdon J. W. Richard P. Orr J. Bernhardt W. Ferguson S. H. (2017). Beluga whale summer habitat associations in the Nelson River estuary, western Hudson Bay, Canada. PloS One12, e0181045. doi: 10.1371/journal.pone.0181045
79
Sportelli J. J. Jones J. M. Frasier K. E. Westdal K. H. Ootoowak A. J. Higdon J. W. et al . (2022). Killer whale (Orcinus orca) pulsed calls in the eastern canadian arctic. Arctic75, 344–363. doi: 10.14430/arctic75350
80
Spreen G. Kaleschke L. Heygster G. (2008). Sea ice remote sensing using AMSR-E 89-GHz channels. J. Geophys. Res.: Oceans113, C02S03. doi: 10.1029/2005JC003384
81
Steltner H. Steltner S. Sergeant D. (1984). Killer whales, Orcinus-orca, prey on narwhals, Monodon-monoceros-an eyewitness account. Can. Field-Naturalist98, 458–462. doi: 10.5962/p.355190
82
Stepney P. H. R. Wooley R. L. (1975). Survey of marine mammals in Lancaster Sound, October 1975 (Sidney, BC: Prepared for Norlands Petroleum by Renewable Resources Consulting Services, Ltd).
83
Stern H. L. Laidre K. L. (2016). Sea-ice indicators of polar bear habitat. Cryosphere10, 2027–2041. doi: 10.5194/tc-10-2027-2016
84
Stewart D. (2008). Commercial and subsistence harvests of narwhals (Monodon monoceros) from the Canadian eastern Arctic (Winnipeg, MB: Arctic Biological Consultants, Winnipeg, MB for Canada Department of Fisheries and Oceans), ii + 97.
85
Stewart D. B. (2018). Commercial and subsistence catches of beluga whales (Delphinapterus leucas) from Cumberland Sound, Nunavut 1840-2016. Can. Tech. Rep. Fish. Aquat. Sci.3250, viii + 89.
86
Stewart D. B. Lockhart W. (2005). “An overview of the Hudson Bay marine ecosystem,” in Canadian Technical Report of Fisheries and Aquatic Sciences no. 2856. (Winnipeg)
87
Tavares S. B. Samarra F. I. P. Miller P. J. O. (2017). A multilevel society of herring-eating killer whales indicates adaptation to prey characteristics. Behav. Ecol.28, 500–514. doi: 10.1093/beheco/arw179
88
van Weelden C. Towers J. R. Bosker T. (2021). Impacts of climate change on cetacean distribution, habitat and migration. Climate Change Ecol.1, 100009. doi: 10.1016/j.ecochg.2021.100009
89
Walsh J. E. Eicken H. Redilla K. Johnson M. (2022). Sea ice breakup and freeze-up indicators for users of the Arctic coastal environment. Cryosphere16, 4617–4635. doi: 10.5194/tc-16-4617-2022
90
Weiss M. N. Ellis S. Franks D. W. Nielsen M. L. K. Cant M. A. Johnstone R. A. et al . (2023). Costly lifetime maternal investment in killer whales. Curr. Biol.33, 744–748.e743. doi: 10.1016/j.cub.2022.12.057
91
Westdal K. H. Davies J. McPherson A. Orr J. Ferguson S. H. (2016). Behavioural changes in belugas (Delphinapterus leucas) during a killer whale (Orcinus orca) attack in southwest Hudson Bay. Can. Field-Naturalist130, 315–319. doi: 10.22621/cfn.v130i4.1925
92
Westdal K. H. Higdon J. W. Ferguson S. H. (2013). Attitudes of Nunavut Inuit toward Killer Whales (Orcinus orca). Arctic66, 279–290. doi: 10.14430/arctic4307
93
Westdal K. H. Higdon J. W. Ferguson S. H. (2017). Review of killer whale (Orcinus orca) ice entrapments and ice-related mortality events in the Northern Hemisphere. Polar Biol.40, 1467–1473. doi: 10.1007/s00300-016-2019-6
94
Yang M. Qiu Y. Huang L. Cheng M. Chen J. Cheng B. et al . (2023). Changes in sea surface temperature and sea ice concentration in the arctic ocean over the past two decades. Remote Sens.15, 1095. doi: 10.3390/rs15041095
95
Young B. G. Koski W. R. Kilabuk R. Watt C. A. Ryan K. P. Ferguson S. H. (2022). Collaborative field research using drones for whale photo-identification studies in Cumberland Sound, Nunavut. Drone Syst. Appl.10, 256–265. doi: 10.1139/dsa-2021-0026
Summary
Keywords
killer whale, Arctic, sea ice, distribution, eastern Canadian Arctic, sightings
Citation
Ferguson SH, Biddlecombe BA, Westdal K, Petersen SD, Watt C, Matthews CJD and Higdon JW (2025) Killer whale range expansion and extended seasonal presence in the eastern Canadian Arctic, 2002-2023. Front. Mar. Sci. 12:1595960. doi: 10.3389/fmars.2025.1595960
Received
18 March 2025
Accepted
05 May 2025
Published
03 June 2025
Volume
12 - 2025
Edited by
Xuelei Zhang, Ministry of Natural Resources, China
Reviewed by
Mridula Srinivasan, NMFS, United States
Jaime Bolaños-Jiménez, Universidad Veracruzana, Mexico
Keith Mullin, U.S. National Marine Fisheries Service, United States
Updates
Copyright
© 2025 Ferguson, Biddlecombe, Westdal, Petersen, Watt, Matthews and Higdon.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brooke A. Biddlecombe, brooke.biddlecombe@ec.gc.ca
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.