Abstract
Drifting Sargassum horneri (S. horneri) may form "golden tide", in order to analyze the source and occurrence mechanism of drifting S. horneri in the sea area of Gouqi Island, Zhejiang, and to determine whether drifting S. horneri possesses sexual reproductive capability, from September 2022 to May 2023, during the life cycle of the raft S. horneri, the growth, reproduction and morphological differences between raft S. horneri and raft whole、cutting raft S. horneri after simulated drifting life (simulated drifting S. horneri) were studied, and reproduction of natural drifting S. horneri in the sea area of Gouqi Island was observed. The results showed that: (1) Both raft and simulated drifting S. horneri had the ability to reproduce sexually, but the number of receptacles of simulated drifting S. horneri were significantly smaller than that of raft S. horneri in the same month (P<0.05); If fixed S. horneri float long distances after being released from attachment base, growth environment such as temperature and light intensity will change greatly, distribution ratio of growth and reproduction is affected, it may not have the ability to reproduce sexually, and even phenomenon of reproductive alienation will occur. (2) Except for December to February, when the temperature and light intensity were lower, the relative growth rate (RGR) of the simulated drifting S. horneri was significantly higher than that of the raft S. horneri in the same month (P<0.05). This may be attributed to higher sea surface temperatures, greater light intensity, richer nutrient availability, and a resource allocation mechanism favoring growth. (3) The longest primary branch length could be used as an important morphological parameter to distinguish raft and simulated drifting S. horneri. (4) The number of benthic S. horneri on Gouqi Island is decreasing year by year, and a large number of S. horneri are attached to the raft culture facilities, due to their fast growth, long drifting period and strong diffusion ability after drifting life, most of drifting S. horneri in the sea area near Gouqi Island may be derived from raft S. horneri.The above results can provide a scientific basis for the research on the source of drifting S. horneri, ecological restoration and prevention of "golden tide" in the waters near Gouqi Island.
1 Introduction
S. horneri belongs to the Phaeophyta and the Sargassum. It is a dominant large Sargassum species in the shallow sea areas of China’s warm-temperate zones, exhibiting two life history types: attached and drifting (Zeng and Lu, 2000). It primarily reproduces through sexual reproduction, supplemented by vegetative propagation via fragmented branches (Ding et al., 2019). drifting S. horneri is formed when attached S. horneri breaks off under physical or biological conditions and drifts on the sea surface. This drifting group can migrate over long distances driven by wind and ocean currents, accumulating significant biomass. Its life history and ecological functions differ from those of attached S. horneri (Hinojosa et al., 2010). However, the specific differences between the two remain unclear. Therefore, it is essential to conduct research on the relationship between attached and drifting S. horneri.
When environmental conditions are suitable for the growth of drifting S. horneri, its robust vegetative growth capacity may lead to explosive proliferation, potentially triggering “golden tides” (Komatsu, 1985). Golden tides primarily occur in the coastal waters of China, Japan, South Korea, and other countries, causing significant losses to local industries such as aquaculture, tourism, and maritime transportation (Hwang et al., 2016; Liu et al., 2018; Kokubu et al., 2019). Some studies suggest that the drifting S. horneri that invaded Jeju Island, South Korea, in 2017 and caused environmental damage originated from the Zhoushan sea area in China (Lin et al., 2017; Kim et al., 2018). Currently, the mussel farming area in Gouqi Island, Zhoushan, spans approximately 11.2 km2, with a large amount of S. horneri attached to the mussel farming rafts. When mussels are harvested or external factors such as wind and waves disturb the rafts, a significant amount of attached S. horneri detaches from its substrate and begins to float, easily forming large populations of drifting S. horneri. Additionally, benthic S. horneri may also contribute to the formation of drifting populations after detaching from its substrate. However, due to factors such as sediment inhibiting spore attachment and survival (Bi et al., 2018), the number of benthic S. horneri around the island in Zhejiang has been decreasing annually (Sun et al., 2008). The limited quantity of benthic S. horneri may not be sufficient to form large-scale drifting populations. Furthermore, the growth rate of S. horneri after transitioning to a drifting lifestyle also influences the scale of drifting populations. Liu et al. (2021) studied the growth rate differences between benthic and drifting S. horneri on Gouqi Island and found that drifting S. horneri exhibits a significantly higher growth rate, giving it a competitive advantage. Yamauchi (1984) and Choi et al. (2020) also observed extremely rapid biomass growth rates in drifting S. horneri. However, these studies did not analyze the differences in growth rates between raft S. horneri and raft S. horneri after transitioning to a drifting lifestyle. Therefore, to investigate the origins of golden tides in the waters near Zhoushan, it is of great significance to in-depth analyze the growth rate differences between raft S. horneri and raft S. horneri after transitioning to a drifting lifestyle on Gouqi Island.
If drifting Sargassum possesses sexual reproductive capability, it will contribute to the stability and dispersal of its population. When facing environmental changes, natural disasters, or human disturbances, this ability allows Sargassum to form relatively independent populations in different regions, enhancing the overall population’s resilience and survival stability (Álvarez et al., 2021). The reproduction of drifting Sargassum also involves competition with other organisms. In aquatic environments, various plants and plankton compete for limited resources. Through drifting dispersal, Sargassum can better participate in ecological competition, influencing the distribution and population structure of other species and triggering succession processes in ecosystems (Álvarez et al., 2021). Currently, the dominant species of drifting Sargassum in the East China Sea is S. horneri. However, research on the sexual reproductive capability of drifting S. horneri is limited. Some studies suggest that drifting S. horneri does not possess sexual reproductive capability (Pang et al., 2018; Zhu et al., 2019), but due to unclear sample sources, growth environments, and drifting durations of the studied drifting S. horneri, it is necessary to conduct long-term, fixed-point monitoring of the sexual reproductive capability of raft S. horneri after transitioning to a drifting lifestyle on Gouqi Island, as well as to observe the sexual reproduction of naturally drifting S. horneri in the waters around Gouqi Island.
Existing research on the morphological differences between attached and drifting S. horneri has primarily focused on benthic and drifting forms. Zhu et al. (2019) found significant differences in the number of branches per unit length, internode length of branches, number of vesicles, and number of receptacles between benthic and drifting S. horneri in Nanji Island (Pang et al. (2018) demonstrated that the vesicles and receptacles of benthic and drifting S. horneri in the Shandong Peninsula exhibit significant differences. Given that a large amount of S. horneri is attached to the mussel farming rafts on Gouqi Island, it is necessary to analyze the morphological changes between raft S. horneri and drifting S. horneri throughout the lifecycle of the raft S. horneri on Gouqi Island.
In summary, to investigate the origin and occurrence mechanisms of drifting S. horneri in the waters of Gouqi Island, Zhejiang, and to determine whether drifting S. horneri possesses sexual reproductive capability, this study conducts long-term monitoring of raft and simulated drifting S. horneri on Gouqi Island. By examining their growth, reproduction, and morphological differences, as well as clarifying the life history process of simulated drifting S. horneri and observing the reproductive behavior of naturally drifting S. horneri, the research aims to provide scientific insights into the origin of drifting S. horneri in the waters near Gouqi Island, ecological conservation and restoration, and the prevention and control of “golden tides.”
2 Materials and methods
2.1 Overview of the study area and site selection
Gouqi Island is located in the Ma’an Archipelago of Shengsi County, Zhoushan City, Zhejiang Province, with a mussel farming area of approximately 11.2 km2. A large number of mussel farming zones are distributed to the north of the island (Li et al., 2020), and a significant amount of S. horneri is attached to the mussel farming facilities. Based on local environmental conditions and other factors, sites were selected for collecting raft S. horneri, conducting simulated drifting S. horneri experiments, and collecting naturally drifting S. horneri. As shown in Figure 1, the sampling site for raft S. horneri, S1 (30°43′27′′N, 122°45′38′′E), is located on the raft ropes in the mussel farming zone at a depth of about 0.6 meters, where the amount of S. horneri was abundant. The site for the simulated drifting S. horneri experiment, S2 (approximately 200 meters from S1), is situated on the sea surface within the mussel farming zone, which is less affected by human activities such as ship navigation. Naturally drifting S. horneri was collected within a 5-kilometer radius of the S1 and S2 sites.
Figure 1
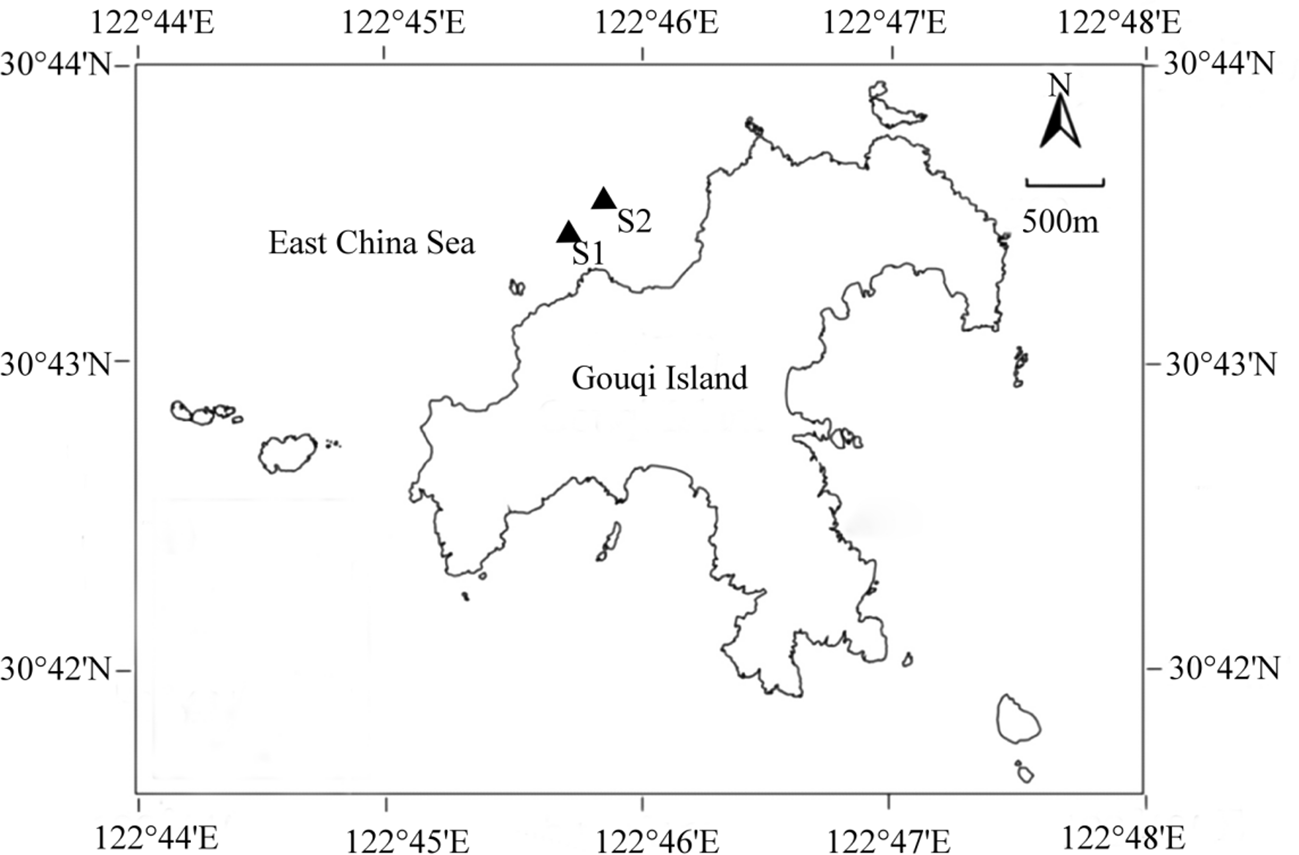
Sampling station.
2.2 Experimental design
Raft S. horneri Biological Parameter Monitoring Experiment: Monthly analysis of the growth, reproduction, and morphological changes of raft S. horneri.
Simulated drifting S. horneri Experiment: Monitoring the growth, reproduction, and morphological changes of whole, cutting raft S. horneri under simulated drifting conditions (referred to as simulated drifting S. horneri) throughout their lifecycle.
Natural drifting S. horneri Reproduction Observation Experiment: Observing the receptacles and reproductive changes of naturally drifting S. horneri in the waters of Gouqi Island.
2.2.1 Raft S. horneri biological parameter monitoring experiment
During the spring tide period at the end of each month from September 2022 to May 2023, within the lifecycle of raft S. horneri, all attached S. horneri (including holdfasts) on a randomly selected 10-meter raft rope at site S1 were collected. Fifteen medium-sized S. horneri individuals were retained each month, and their biological parameters were measured.
2.2.2 Simulated drifting S. horneri experiment
Studies have shown that the growth and reproduction of seaweed after transitioning to a drifting lifestyle vary significantly depending on the part of the plant that breaks off (Umezaki, 1984; Fletcher and Fletcher, 1975). To accurately analyze the reproduction, growth, and morphological changes of fragmented S. horneri on Gouqi Island as a whole, 60 raft S. horneri individuals (including holdfasts) were collected from site S1 at the end of September 2022 (when the raft S. horneri had developed vesicles and acquired the ability to float). Among these, 30 individuals were divided into four equal parts based on plant height (measured from the main growth point to the holdfast). Ten individuals were cut from the top (1/4), middle (1/2), and bottom (3/4) sections, respectively, starting from the main growth point to the holdfast. These three cutting methods were named top cutting, short cutting, and heavy cutting (Ge and Pan, 2002). The cut sections without holdfasts were designated as cutting raft S. horneri samples.
The 30 whole raft S. horneri individuals (including holdfasts) and 30 cutting raft S. horneri individuals collected in September were labeled with serial numbers and tied to ropes using a seedling-clipping method. The ropes were then attached to fixed drifting foam balls in the mussel farming zone at site S2, allowing the S. horneri to float on the sea surface and simulate the light and temperature conditions of a drifting lifestyle (Figure 2). At the end of each month during the spring tide period, the S. horneri were retrieved to measure their biological parameters. During the measurement period, they were temporarily kept in oxygenated water tanks, with water changed daily. After measurement, they were returned to site S2 until the S. horneri gradually deteriorated.
Figure 2

The experimental site of simulated drifting S. horneri.
In March 2023, it was observed that, after a long period of the simulated drifting S. horneri experiment, most of the S. horneri collected in September had detached from the seedling ropes. Therefore, at the end of March and April 2023 (no collection was made in May as the raft S. horneri had severely deteriorated), additional raft S. horneri were collected, and the simulated drifting S. horneri experiment was continued following the aforementioned steps.
2.2.3 Natural drifting S. horneri reproduction observation experiment
In March–April 2023, large-scale natural drifting S. horneri appeared in the waters around Gouqi Island. At the end of each month, 30 naturally drifting S. horneri individuals were randomly collected within a 5-kilometer radius of sites S1 and S2, and their reproductive status was observed.
2.3 Data collection, processing and analysis
The length and diameter of vesicles and receptacles were measured from the largest five samples randomly selected from the main lateral branches. Plant height was measured from the holdfast to the main growth point of the S. horneri. The thickness of the lower main stem was measured as the diameter of the main stem at the morphological lower part of the algae. The internode length and number of primary branches were measured along the main stem at different unit lengths from the morphological lower to upper parts of the algae. For plants with heights of 0–50 cm, a unit length of 10 cm was used; for heights of 50–100 cm, a unit length of 30 cm was used; and for heights over 100 cm, a unit length of 50 cm was used. The internode length of primary branches was measured as the distance between five randomly selected adjacent nodes within the unit length of the main stem. A vernier caliper (accuracy 0.01 cm) was used to measure the length or diameter of vesicles, receptacles, the lower main stem, and primary branch internodes. A ruler (accuracy 0.1 cm) was used to measure plant height and the length of the longest primary branch. Wet weight was measured using an electronic scale (accuracy 0.1 g), and the number of primary branches and receptacles within the unit length of the main stem was recorded manually.
An underwater temperature and light intensity logger (Onset HOBO MX2202, USA) was used to measure and record the temperature and light intensity at the water surface (10 cm below the surface) at site S2 every ten minutes. The relative growth rate (RGR) was calculated using the change in wet weight of S. horneri, with the RGR formula (Liu et al., 2019) as follows:
In the formula, Wt represents the fresh weight of S. horneri after t days, and W0 represents the initial fresh weight of S. horneri.
Descriptive statistics are expressed as (mean ± SE). One-way analysis of variance (one-way ANOVA) was performed using SPSS 25.0.
3 Results
3.1 Reproductive status of raft S. horneri after simulated drifting
In September, 30 whole raft S. horneri individuals and 30 cutting raft S. horneri individuals, which had just developed vesicles, were collected. After undergoing simulated drifting conditions, by March of the following year, only 14 whole raft and 11 cutting raft S. horneri individuals remained. Among these, 9 whole raft and 7 cutting raft individuals had mature female receptacles (Figure 3). The remaining individuals had mature male receptacles, indicating sexual reproductive capability. The average lengths of the receptacles were 2.60 cm and 3.30 cm, respectively, and the average diameters were 0.25 cm and 0.20 cm, respectively. In March, 30 whole raft S. horneri individuals and 10 heavily cutting raft S. horneri individuals, which had not yet developed receptacles, were collected. After simulated drifting, by April, 28 surviving whole raft and 9 heavily cutting raft S. horneri individuals had developed mature male and female receptacles, demonstrating sexual reproductive capability. The average lengths of the receptacles were 3.78 cm and 2.77 cm, respectively, and the average diameters were 0.19 cm and 0.16 cm, respectively (Table 1). In March, 10 top and 10 short cutting raft S. horneri individuals were collected. At this time, receptacles had begun to develop but were not yet mature (Figure 4). After simulated drifting, by April, all 10 surviving top and 9 short cutting raft S. horneri individuals had mature male and female receptacles, demonstrating sexual reproductive capability. The lengths of the receptacles increased significantly (P<0.05), while the diameters slightly decreased (P>0.05) (Table 1). The whole, cutting raft S. horneri individuals collected in March showed signs of deterioration by May after two months of simulated drifting. The whole cutting raft S. horneri individuals collected in April, which had already developed mature male and female receptacles and demonstrated sexual reproductive capability, also showed signs of deterioration by May after simulated drifting. In summary, both raft S. horneri and simulated drifting S. horneri from September to March of the following year exhibited sexual reproductive capability.
Figure 3

Mature female receptacles of simulated drifting S. horneri collected in September.
Table 1
| Month | Rlf | Rdf | Rlt | Rdt | Rls | Rds | Rlh | Rdh |
|---|---|---|---|---|---|---|---|---|
| 3 | / | / | 1.91a | 0.23a | 1.82a | 0.24a | / | / |
| 4 | 3.78 | 0.19 | 3.03b | 0.20a | 3.55b | 0.18a | 2.77 | 0.16 |
| 5 | / | / | / | / | / | / | / | / |
Changes in length and diameter of receptacles of simulated drifting S. horneri collected in March.
(1) Rlf, Receptacle length of full S. horneri; Rdf, Receptacle diameter of full S. horneri; Rlt, Receptacle length of top cut S. horneri; Rdt, Receptacle diameter of top cut S. horneri; Rls, Receptacle length of stub cut S. horneri; Rds, Receptacle diameter of Stub cut S. horneri; Rlh, Receptacle length of heavy shear cut S. horneri; Rdh, Receptacle diameter of heavy shear cut S. horneri; (2) Different superscript letters for the same parameter across different months indicate significant differences between groups (P<0.05).
Figure 4

Immature receptacle of raft S. horneri in March.
As shown in Figure 5, in March, the average number of receptacles per primary branch for raft S. horneri, whole, cutting raft S. horneri collected in September after simulated drifting was 96, 53, and 74, respectively. In April, the average number of receptacles per primary branch for raft S. horneri, whole, cutting raft S. horneri collected in March after simulated drifting was 68, 29, and 46, respectively. The average number of receptacles per primary branch for simulated drifting S. horneri was significantly lower than that of raft S. horneri in the same month (P<0.05). Additionally, in April, it was observed that some naturally drifting S. horneri individuals had receptacles with attached spores, and some receptacles exhibited alienation, such as the regeneration of branches at the tips of the receptacles (Figure 6). In summary, the number of receptacles in simulated drifting S. horneri was significantly lower than that in raft S. horneri in the same month (P<0.05), and naturally drifting S. horneri exhibited alienation in receptacles.
Figure 5
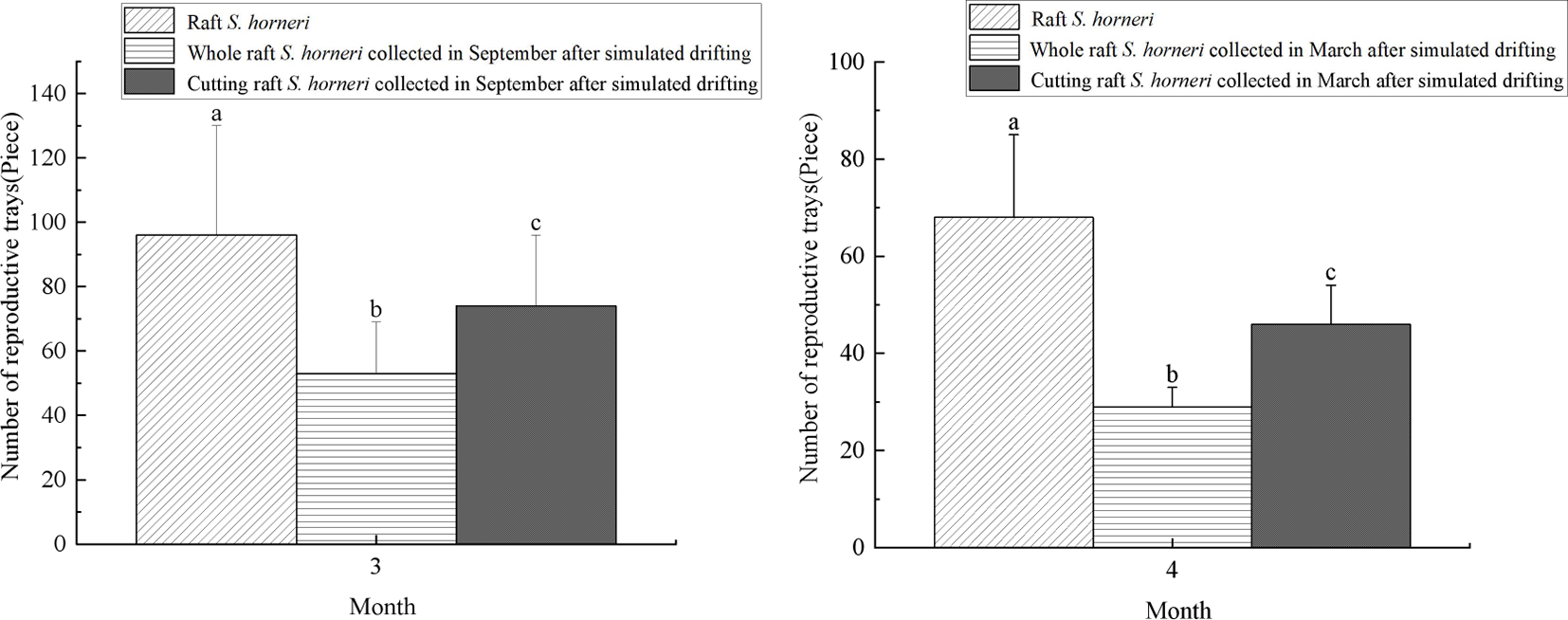
Numbers of receptacles on a single primary branch of S. horneri. Different letters indicate significant differences between groups (P<0.05).
Figure 6

Catabolic receptacle of natural drifting S. horneri in April.
3.2 Growth of raft S. horneri after simulated drifting
As shown in Figure 7, the RGR were calculated by Equation 1, the maximum RGR of wet weight for whole, cutting raft S. horneri collected in September and subjected to simulated drifting conditions were 6.89%·d-1 and 7.85%·d-1, respectively, while the maximum RGR for raft S. horneri was only 2.15%·d-1. The RGR of simulated drifting S. horneri collected in September was significantly higher than that of raft S. horneri in October, November, and March of the following year (P<0.05). However, in December and February, there was no significant difference in RGR overall (P>0.05), although the RGR still approached or exceeded 1%·d-1. During this period, sea surface temperature and light intensity were relatively low (Figure 8), with temperatures dropping from 15.1°C in December to 9.3°C in February, and light intensity decreasing from 11.1 μmol/m2·s in December to 7.3 μmol/m2·s in February.
Figure 7
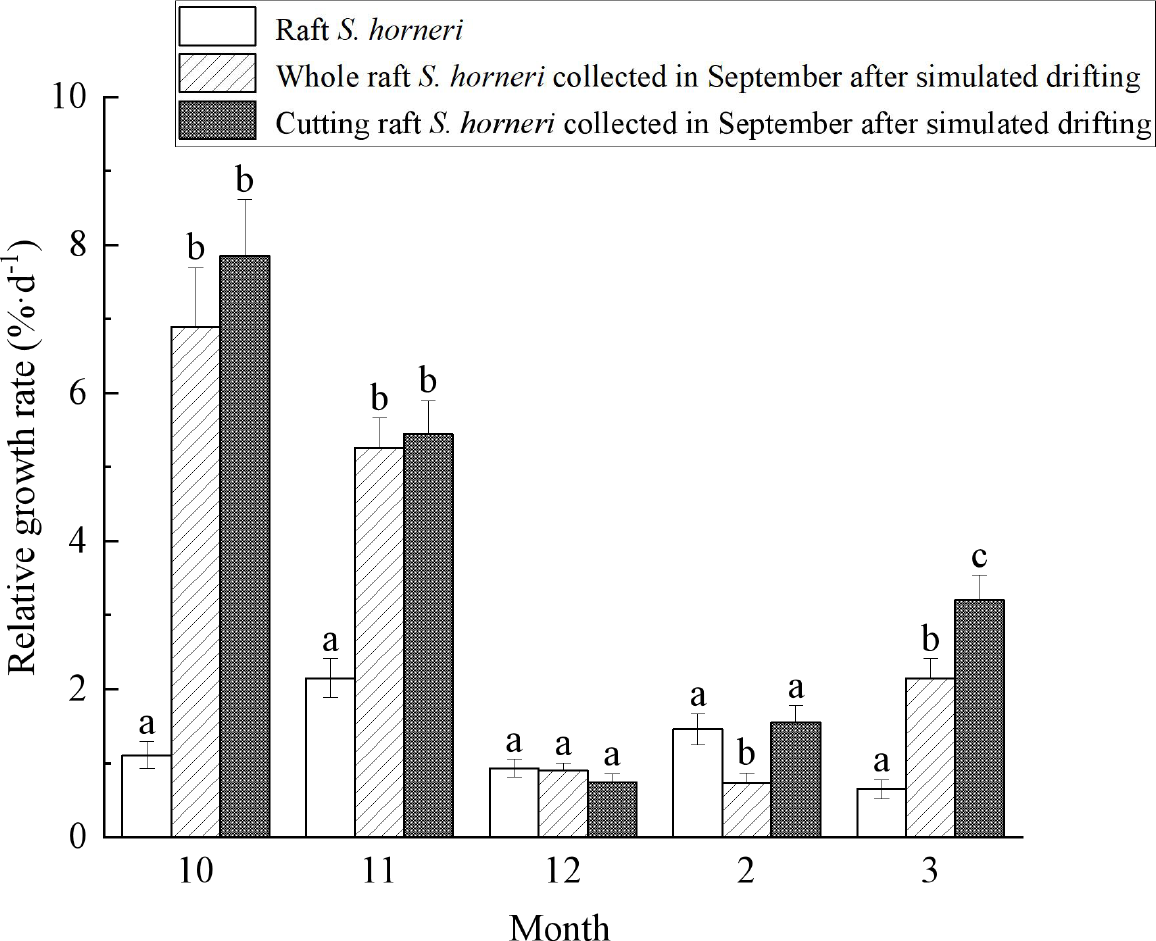
Change of relative growth rate of S. horneri with wet weight. Different letters within the same month indicate significant differences between groups (P<0.05).
Figure 8
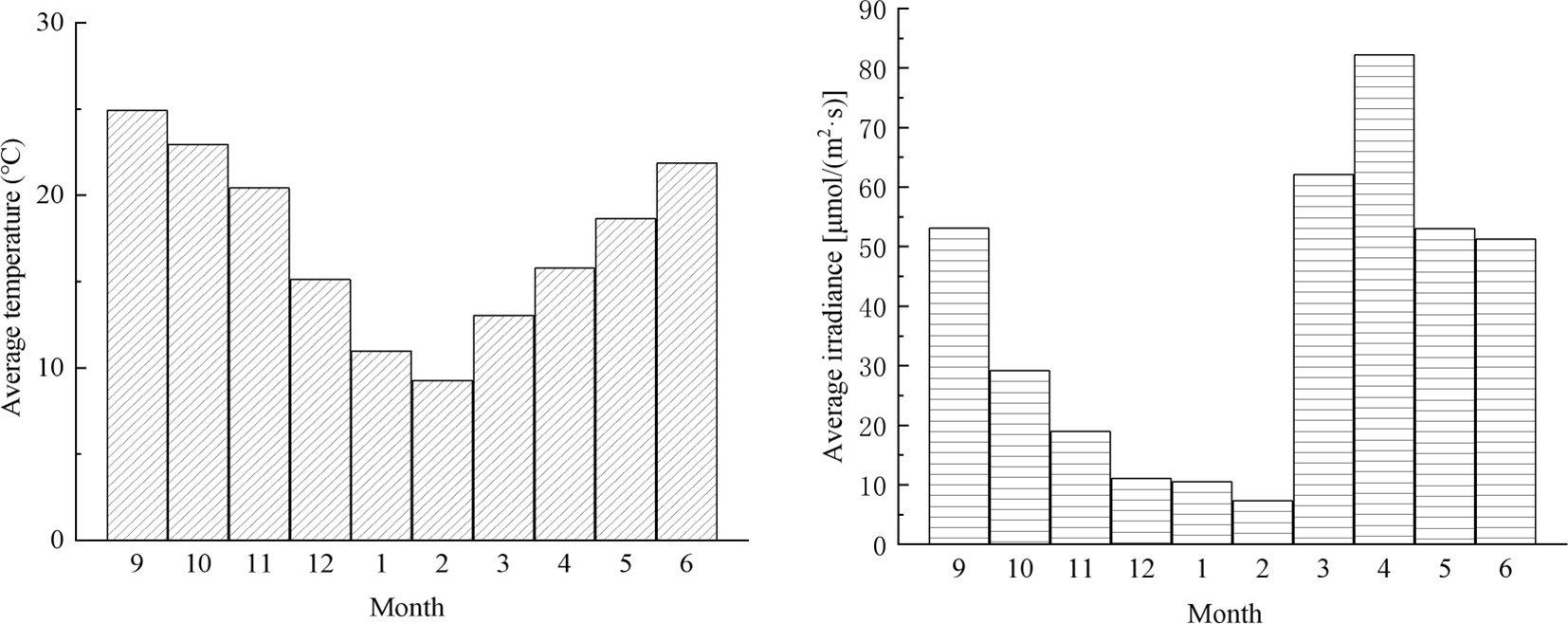
Variation of sea surface temperature and light intensity in Gouqi Island.
As shown in Figure 9, after whole raft S. horneri collected in March underwent simulated drifting, their wet weight increased significantly (P<0.05), from 35.3 g to 98.1 g in April, with an RGR of 3.41%·d-1. Similarly, cutting raft S. horneri collected in March and subjected to simulated drifting showed a significant increase in wet weight (P<0.05), from 65.9 g to 268.7 g in April, with an RGR of 4.68%·d-1. In contrast, the wet weight of raft S. horneri increased from 115.1 g in March to 147.6 g in April, but this increase was not significant (P>0.05), with an RGR of only 0.83%·d-1, which was significantly lower than that of simulated drifting S. horneri (P<0.05). Simulated drifting S. horneri collected in April showed signs of deterioration by May. In summary, except during the period of low temperature and light intensity from December to February, the RGR of simulated drifting S. horneri was significantly higher than that of raft S. horneri in the same month (P<0.05).
Figure 9
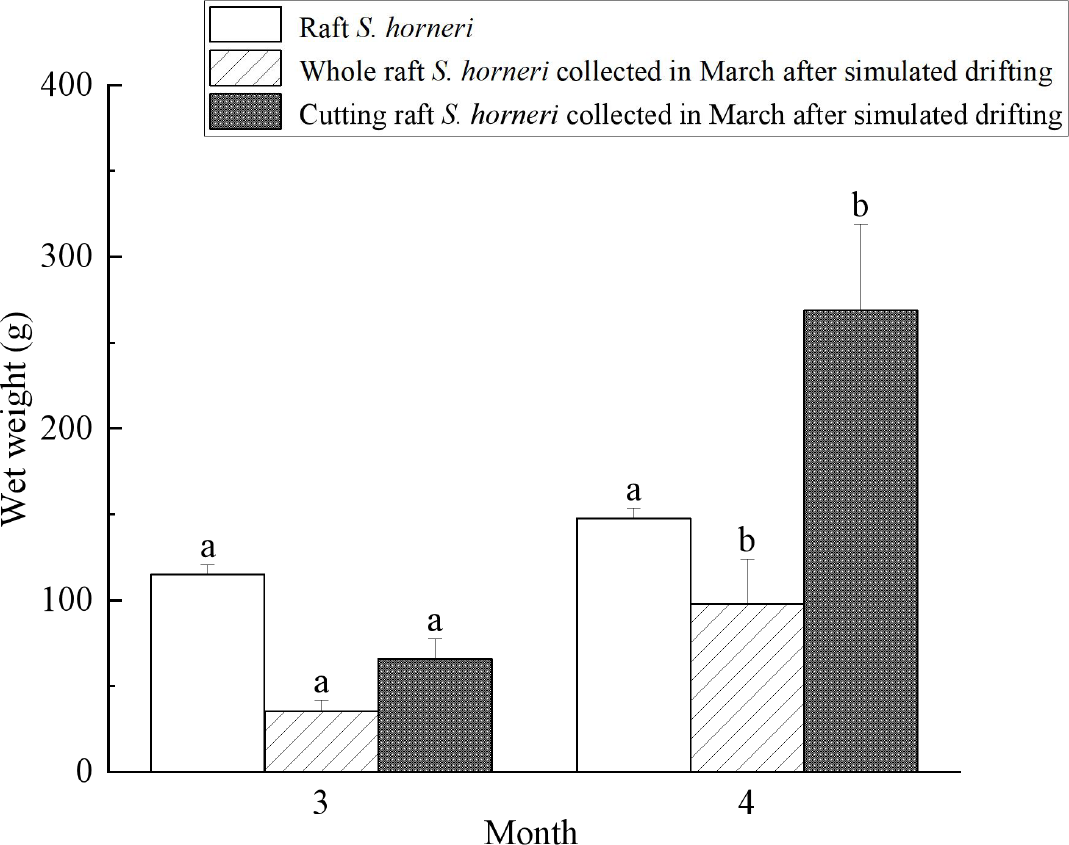
Wet weight change of S. horneri. Different superscript letters for the same parameter across different months indicate significant differences between groups (P<0.05).
3.3 Morphological differences between raft and simulated drifting S. horneri
As shown in Figure 10, the monthly analysis of morphological parameters of raft S. horneri and simulated drifting S. horneri collected in September revealed significant differences overall (P<0.05) in plant height, lower main stem thickness, and primary branch internode length. Parameters with extremely significant differences (P<0.01) included the length of the longest primary branch, while the number of primary branches per unit length, air bladder length, and diameter showed no significant differences (P>0.05).
Figure 10
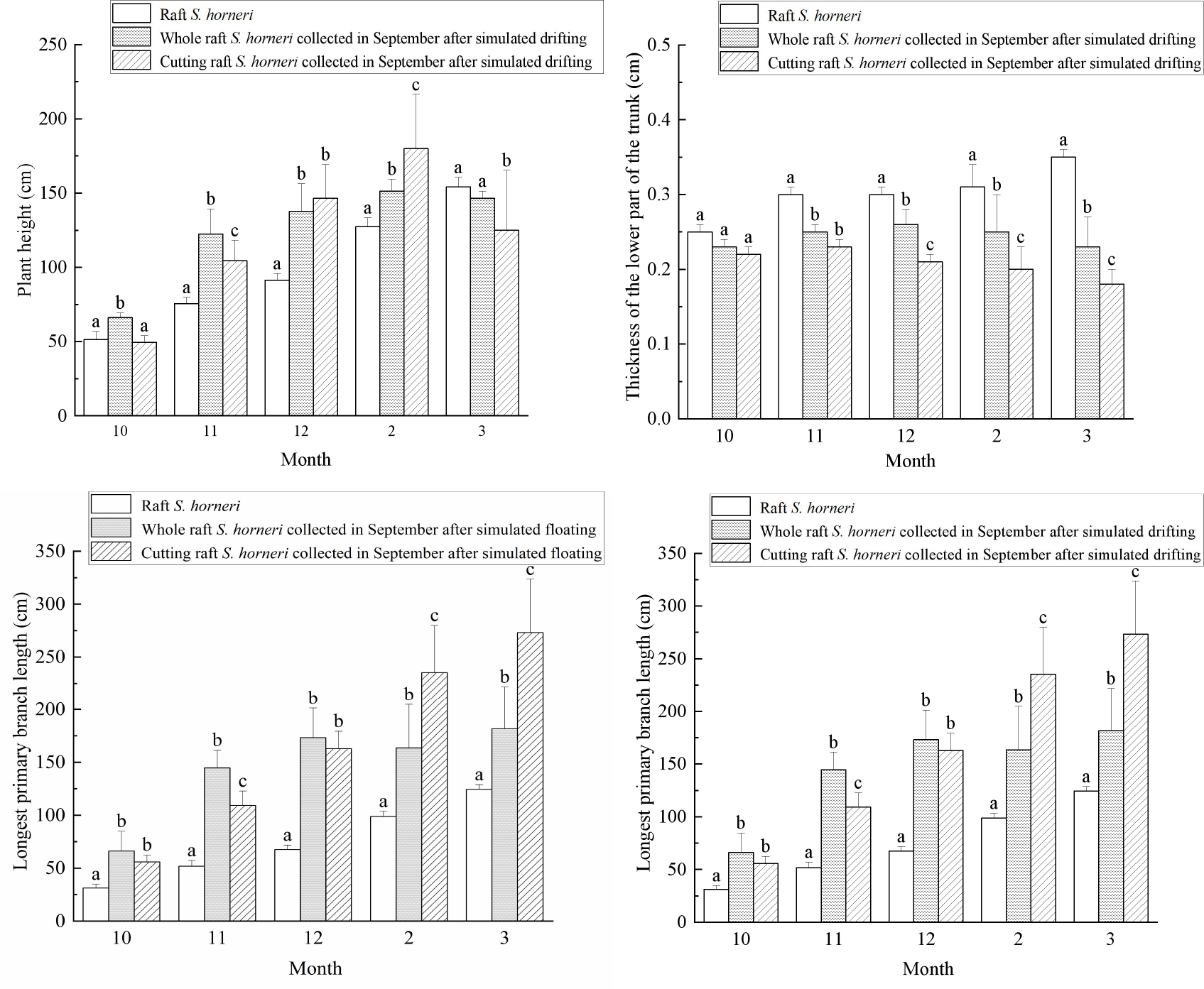
Variation of biological parameters of raft and simulated drifting S. horneri. Different superscript letters within the same month indicate significant differences between groups (P<0.05).
In terms of plant height, raft S. horneri consistently exhibited a growth trend, while simulated drifting S. horneri initially increased and then decreased. From November to February of the following year, the plant height of simulated drifting S. horneri was significantly greater than that of raft S. horneri (P<0.05). For lower main stem thickness, raft S. horneri showed a slow but steady increase, while simulated drifting S. horneri initially increased and then gradually decreased. Overall, the lower main stem thickness of raft S. horneri was significantly greater than that of simulated drifting S. horneri (P<0.05). Regarding primary branch internode length, both raft and simulated drifting S. horneri exhibited a slow growth trend. From November to March of the following year, the primary branch internode length of raft S. horneri was significantly greater than that of simulated drifting S. horneri (P<0.05). For the length of the longest primary branch, both raft and simulated drifting S. horneri showed a consistent growth trend. From October to March of the following year, the length of the longest primary branch of simulated drifting S. horneri was significantly greater than that of raft S. horneri (P<0.01).
4 Discussion
4.1 Sexual reproductive capability of S. horneri in raft mussel farming areas
Raft S. horneri possesses sexual reproductive capability, and the number of receptacles is no less than that of benthic S. horneri (Pang et al., 2018). Simulated drifting S. horneri also exhibits sexual reproductive capability, but the number of receptacles is significantly lower than that of raft S. horneri. In April, the number of receptacles in simulated drifting S. horneri was only 2/5 and 7/10 of that in raft S. horneri. However, Pang et al. (2018) found that naturally drifting S. horneri lacks sexual reproductive capability, with receptacles showing only typical male characteristics and no female characteristics, and the number of receptacles was 1/8 of that in benthic S. horneri. Zhu et al. (2019) also observed that naturally drifting S. horneri primarily relies on vegetative growth to expand its population, with the number of receptacles being 1/5 of that in benthic S. horneri. This discrepancy may be due to the more stable growth environment of simulated drifting S. horneri, which is closer to that of raft S. horneri, with only a 60 cm depth difference, therefore, simulated drifting S. horneri possesses sexual reproductive capability. light intensity plays an important role in the growth and reproduction of S. horneri (Bi et al., 2014), the light intensity of drifting S. horneri is stronger than that of attached S. horneri, the increase in light intensity has a certain negative impact on the reproduction of S. horneri, therefore, the number of receptacles of drifting S. horneri is significantly less than that of attached S. horneri. In contrast, this study found that some naturally drifting S. horneri in the waters of Gouqi Island exhibited sexual reproductive capability, possibly because these individuals had just transitioned to a drifting lifestyle after their receptacles matured. In addition, Zhu et al. (2019) discovered that receptacles of naturally drifting S. horneri had undergone alienation, that is, the receptacles tip regenerated and branched. This study found that some of the receptacles of naturally drifting S. horneri on the Gouqi Island also showed alienation, but no alienation of receptacles was found in the raft and the simulated drifting S. horneri. It might also be due to the significant differences in the growth environment such as temperature and light intensity of the naturally drifting S. horneri.
Studies suggest a trade-off between reproduction and survival in seaweeds (Zhang et al., 2009). Resource allocation theory also indicates that plants have limited resources, and investment in one process often comes at the expense of another (Arizaga and Ezcurrra, 2002). Naturally drifting S. horneri, drifting with ocean currents, may experience significant and prolonged differences in environmental conditions such as temperature and light intensity compared to their original habitat. To cope with these changes and maintain survival, naturally drifting S. horneri may allocate more resources to growth activities, significantly reducing investment in reproduction. In such cases, naturally drifting S. horneri may lack sexual reproductive capability, exhibit fewer receptacles than attached S. horneri, and show alienation in receptacles. However, if the drifting S. horneri remains in an environment similar to its original habitat for an extended period, it may retain sexual reproductive capability, albeit with a reduced number of receptacles, only the number of receptacles may decrease, or the phenomenon of receptacles alienation may not occur.
4.2 Dispersal capability of drifting S. horneri
The drifting period of different Sargassum species varies to some extent. Yatsuya (2008) found that the drifting period of Sargassum is influenced by its density, with lower density leading to a longer drifting period. Due to its low density, S. horneri has a significantly longer drifting period compared to Sargassum fusiforme, Sargassum patens, and Sargassum siliquastrum. This study also found that raft S. horneri can begin simulated drifting as early as the end of September after developing vesicles, with a simulated drifting period lasting up to 6 months; in contrast, Yatsuya (2008) observed that S. horneri naturally drifting in January had a maximum drifting period of only 14 weeks; this discrepancy may be due to the fact that the earlier S. horneri detaches from its substrate after developing vesicles, the longer its drifting period, rising temperatures and the end of the reproductive period can lead to the rapid deterioration of the vesicles. Additionally, studies have shown that the maximum transport speed of drifting seaweed in the Sea of Japan is 17 km/day (Yoshida, 1963). Given its long drifting period, S. horneri can disperse over considerable distances and may retain sexual reproductive capability, significantly expanding its population’s range. Originally native to East Asia, S. horneri was first discovered in the eastern Pacific in 2003 and has since rapidly spread along the southern coast of California, USA (Lindsay et al., 2015), also demonstrating its strong dispersal capability.
4.3 Growth capability of S. horneri in raft mussel farming areas
The biomass of drifting Sargassum can double within days or weeks (Hanisak and Samuel, 1987; Lapointe, 1986). This study also found that the maximum relative growth rates (RGR) of wet weight for whole, cutting raft S. horneri after simulated drifting were as high as 6.89%·d-1 and 7.85%·d-1, respectively. In this study, during December and February, there was no significant difference in RGR between raft and simulated drifting S. horneri, likely due to the lower temperatures and light intensity during this period. Since temperature and light intensity are critical factors influencing the growth of S. horneri (Wang et al., 2023), both attached and simulated drifting S. horneri exhibited slow growth. However, in months with higher temperatures and light intensity, the RGR of raft S. horneri was significantly lower than that of simulated drifting S. horneri. Previous studies have shown that the growth rate of raft S. horneri is significantly higher than that of benthic S. horneri during the same period (Wang et al., 2023). Therefore, the RGR of simulated drifting S. horneri was significantly higher than that of both benthic and raft S. horneri. This may be attributed to higher sea surface temperatures, greater light intensity, richer nutrient availability, and a resource allocation mechanism favoring growth (Arizaga and Ezcurrra, 2002), all of which contribute to the significantly higher RGR of simulated drifting S. horneri compared to attached S. horneri.
4.4 Investigation of morphological differences between raft and simulated drifting S. horneri
Studies have shown significant differences in internode length and the number of branches per unit length between benthic and drifting S. horneri (Zhu et al., 2019). This study found significant differences in plant height, lower main stem thickness, and primary branch internode length between raft and simulated drifting S. horneri, with extremely significant differences in the length of the longest primary branch. Therefore, the length of the longest primary branch can serve as an important morphological parameter for distinguishing between raft and simulated drifting S. horneri. The observed morphological differences between raft and simulated drifting S. horneri may be attributed to genetic, external environmental, and internal physiological differences (Hou, 2008). drifting S. horneri, having detached from its attached lifestyle, often exhibits the ability to adjust its physiological traits to adapt to surface light and temperature conditions, demonstrating physiological plasticity (Cheang et al., 2008; Koch et al., 2016). It is speculated that this physiological plasticity, driven by the need to adapt to external environments, may lead to significant morphological differences between raft and simulated drifting S. horneri.
4.5 Analysis of the origin of drifting S. horneri in the waters near Gouqi Island
drifting S. horneri possesses unparalleled advantages over other drifting Sargassum species, including rapid growth, a long drifting period, and potential sexual reproductive capability, leading to the continuous expansion of its population. drifting S. horneri likely originates from attached S. horneri (Yoshida, 1963). When the algal body breaks, the fragments use the buoyancy of their vesicles to float on the sea surface with ocean currents, transitioning to a drifting lifestyle (Qian, 2013). Studies have found that both benthic and raft S. horneri in the waters south of Gouqi Island, Zhejiang, are annual (Yang et al., 2024). In this region, drifting S. horneri also experiences blade and air bladder decay due to rising seawater temperatures, with only the stems sinking to the seabed and decomposing, making drifting S. horneri annual as well, with a lifecycle starting from September when vesicles form and ending in June of the following year. Therefore, the drifting S. horneri in the waters near Gouqi Island may originate from attached S. horneri that detaches from its substrate and undergoes rapid, explosive growth each year.
Before the large-scale artificial farming of mussels, increased seabed sediment and reduced seawater transparency (Sun et al., 2008) caused the distribution of benthic S. horneri to shift upward, making it more susceptible to wind and waves. As a result, large amounts of benthic S. horneri detached from their substrates, making benthic S. horneri the primary source of drifting S. horneri in the waters near Gouqi Island. However, the distribution area and population of benthic S. horneri have been declining annually (Sun et al., 2008), while the rapid increase in mussel farming rafts has provided an ideal habitat for the extensive attachment of S. horneri. Given the massive scale of drifting S. horneri populations in the waters near Gouqi Island (Ding et al., 2019) it is highly likely that the majority of drifting S. horneri originates from raft S. horneri on Gouqi Island. Additionally, this study found that simulated drifting S. horneri exhibits a high RGR, consistent with the rapid expansion capability observed during “golden tide” events. Therefore, the majority of drifting S. horneri in the waters near Gouqi Island likely originates from raft S. horneri.
5 Conclusions and prospects
In terms of sexual reproduction, raft S. horneri possesses sexual reproductive capability, and the number of receptacles is no less than that of benthic S. horneri. If attached S. horneri detaches from its substrate and floats in an environment similar to its original habitat for an extended period, it may retain sexual reproductive capability, albeit with a reduced number of receptacles. However, if it undergoes long-distance drifting, significant changes in environmental conditions such as temperature and light intensity may result in the loss of sexual reproductive capability and even lead to alienation in receptacles. In terms of growth rate, the maximum relative growth rate (RGR) of raft S. horneri after transitioning to a simulated drifting lifestyle is approximately three times that of raft S. horneri. Morphologically, the length of the longest primary branch is a critical parameter for distinguishing between raft and drifting S. horneri. Regarding the origin of drifting S. horneri, the population of benthic S. horneri around Gouqi Island has been declining annually. In contrast, the extensive attachment of S. horneri to raft farming facilities means that once detached, its rapid growth, long drifting period, and strong dispersal capability suggest that the majority of drifting S. horneri in the waters near Gouqi Island likely originates from raft S. horneri. These findings provide a scientific basis for understanding the origin of drifting S. horneri in the waters near Gouqi Island and for research on preventing and controlling “golden tides.” Additionally, further investigation is needed to explore the growth, reproduction, and morphological changes of whole and fragmented S. horneri in the intertidal zone after transitioning to a drifting lifestyle, as well as the allocation ratio of growth and reproduction in naturally drifting S. horneri in the East China Sea in response to environmental changes.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Author contributions
HW: Formal analysis, Data curation, Methodology, Writing – review & editing, Writing – original draft, Investigation. QY: Writing – review & editing. WZ: Writing – review & editing. HM: Writing – review & editing. FL: Writing – review & editing, Software. YB: Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the project titled “Zhejiang Provincial Basic Public Welfare Project” (LTGS24C030002), “Zhejiang Key R&D Program” (2023C03120) and Zhejiang Marine Fisheries Research Institute.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Álvarez C. D. Sangil C. Sansón M. (2021). Fertile drifting individuals of the invasive alien Sargassum muticum (Fucales, Phaeophyceae) reach the coasts of the Canary Islands (eastern Atlantic Ocean). Aquat. Bot.168, 103322. doi: 10.1016/j.aquabot.2020.103322
2
Arizaga S. A. Ezcurrra E. (2002). Propagation mechanisms in Agave macroacantha (Agavaceae), a tropical arid-land succulent rosette. Am. J. Bot.89, 632–641. doi: 10.3732/ajb.89.4.632
3
Bi Y. X. Feng M. P. Zhang Y. Z. Liang J. Wang W. D. (2018). Spatial distribution patterns of S. horneri in the coastal waters of the Ma’an Archipelago. Acta Ecol. Sinica.38, 309–315. doi: 10.1016/j.chnaes.2018.02.006
4
Bi Y. X. Zhang S. Y. Wang W. D. Wu Z. L. (2014). Vertical distribution pattern of Sargassum horneri and its relationship with environmental factors around Gouqi Island. Acta Ecol. Sinica.34, 4931–4937. doi: 10.5846/stxb201301030013
5
Cheang C. C. Chu K. H. Ang P. J. (2008). Morphological and genetic variation in the populations of Sargassum hemi-phyllum (phaeophyceae) in the northwestern pacific. J. phycol.44, 855–865. doi: 10.1111/j.1529-8817.2008.00532.x
6
Choi S. K. Oh H. J. Yun S. H. Lee H. J. Lee K. Han Y. S. et al . (2020). Population dynamics of the ‘Golden tides’ Seaweed, S. horneri, on the Southwestern coast of Korea: The extent and formation of golden tides. Sustainability12, 2903–2903. doi: 10.3390/su12072903
7
Ding X. W. Zhang J. H. Zhuang M. M. Kang X. Y. Zhao X. H. He P. M. et al . (2019). Growth of Sargassum horneri distribution properties of golden tides in the Yangtze Estuary and adjacent waters. Marine Fish.41, 188–196. doi: 10.13233/j.cnki.mar.fish.2019.02.007
8
Fletcher R. L. Fletcher S. M. (1975). Studies on the recently introduced brown Alga Sargassum muticum (yendo) fensholt. II. regenerative ability. Botanica Marina.18, 157–162. doi: 10.1515/botm.1975.18.3.157
9
Ge G. H. Pan Y. X. (2002). Pruning is key to flower cultivation. Hunan Forest.2), 25–25.
10
Hanisak M. D. Samuel M. A. (1987). Growth rates in culture of several species of Sargassum from Florida, USA. Hydrobiologia151-152, 399–404. doi: 10.1007/BF00046159
11
Hinojosa I. A. Pizarro M. Ramos M. Thiel M. (2010). Spatial and temporal distribution of drifting kelp in the channels and fjords of southern Chile Estuarine. Coast. Shelf Sci.87, 367–377. doi: 10.1016/j.ecss.2009.12.010
12
Hou L. F. (2008). Research on plant growth simulation under the regulation of plant hormones Vol. 12-13 (Chongqing: Chongqing University).
13
Hwang E. K. Lee S. J. Ha D. S. Chan S. P. (2016). Sargassum golden tides in the Shinan-Gun and Jeju Island, Korea. Korean J. Fish. Aquat. Sci.49, 689–693. doi: 10.5657/KFAS.2016.0689
14
Kim H. S. Sanjeewa K. K. A. Fernando I. P. S. Ryu B. Yang H. W. Ahn G. et al . (2018). A comparative study of S. horneri Korea and China strains collected along the coast of Jeju Island South Korea: its components and bioactive properties. Algae33, 341–349. doi: 10.4490/algae.2018.33.11.15
15
Koch K. Thiel M. Hagen W. Graeve M. Gómez I. Jofre D. et al . (2016). Short-and long-term acclimation patterns of the giant kelp Macrocystis (Laminariales, phaeophyceae) along a depth gradient. J. phycol.52, 260–273. doi: 10.1111/jpy.12394
16
Kokubu Y. Rothäusler E. Filippi J. B. Durieux E. D. H. Komatsu T. (2019). Revealing the deposition of macrophytes transported offshore: evidence of their long-distance dispersal and seasonal aggregation to the deep sea. Sci. Reports.9, 1–11. doi: 10.1038/s41598-019-39982-w
17
Komatsu T. (1985). Temporal fluctuations of water temperature in a Sargassum forest. J. Oceanograph. Soc. Japan41, 235–243. doi: 10.1007/BF02109273
18
Lapointe B. E. (1986). Phosphorus-limited photosynthesis and growth of Sargassum natans and Sargassum fluitans (phaeophyceae) in the western North Atlantic. Deep-Sea Res.33, 391–399. doi: 10.1016/0198-0149(86)90099-3
19
Li X. M. Zhang S. Y. Wang K. Chen L. R. Chen T. H. (2020). Effects of annual temperature variation on growth of Sargassum horneri in Gouqi Island. Oceanol. Limnol. Sinica.51, 1136–1143. doi: 10.11693/hyhz20191000197
20
Lin S. M. Huang R. Ogawa H. Liu L. C. Wang Y. C. Chiou Y. (2017). Assessment of germling ability of the introduced marine brown alga, S. horneri, in northern Taiwan. J. Appl. Phycol.29, 2641–2649. doi: 10.1007/s10811-017-1088-4
21
Lindsay M. Paulina R. S. Daniel R. Sally H. Carolynn C. John E. et al . (2015). Range expansion of a non-native, invasive macroalga S. horneri (Turner) C. Agardh 1820 in the eastern Pacific. BioInvasions Records4, 243–248. doi: 10.3391/bir.2015.4.4.02
22
Liu F. Liu X. F. Wang Y. Jin Z. Moejes F. W. Sun S. (2018). Insights on the S. horneri golden tides in the Yellow Sea inferred from morphological and molecular data. Limnol. Oceanogr.63, 1762–1773. doi: 10.1002/lno.10806
23
Liu T. Ma Z. L. Li H. Xu Z. G. (2019). Effects of light intensity and nitrate levels on growth and photosynthetic characteristics of Sargassum horneri. Chin. J. Ecol.38, 762–769. doi: 10.13292/j.1000-4890.201903.026
24
Liu Z. Y. Sun P. Qin S. Li J. J. Zhuang L. C. Song W. L. et al . (2021). Comparative analysis of growth and photosynthetic characteristics in benthic and floating Sargassum horneri. Chin. J. Ecol.40, 76–83. doi: 10.13292/j.1000-4890.202101.028
25
Pang Y. L. Liu Z. Y. Ding L. P. Fu W. T. Yu S. X. Sun Z. D. et al . (2018). Morphological comparison and analysis of the vesicle and receptacle of floating and attached Sargassum horneri in Shan dong peninsula. Marine Sci.42, 84–91. doi: 10.11759/hykx20171210001
26
Qian S. B. (2013). Phycology Vol. 675-676 (Qingdao: China Ocean University Press).
27
Sun J. Z. Chen W. D. Zhuang D. G. Zheng H. Y. Lin L. Pang S. J. (2008). In situ ecological studies of the subtidal brown alga Sargasssum horneri at Nanji Island of China. South China Fish. Sci.4, 58–63.
28
Umezaki I. (1984). Ecological studies of S. horneri (Turner) C. Agardh in Obama Bay, Japan Sea. Bull. Japan. Soc. Sci. Fish.50, 1193–1200. doi: 10.2331/suisan.50.1193
29
Wang H. J. Yang Q. F. Zhu W. D. Miao H. Bi Y. X. (2023). Comparative growth analysis of intertidal zone and raft Sargassum horneri in Gouqi Island. J. Fish. Sci. China30, 1364–1373. doi: 10.12264/JFSC2023-0271
30
Yamauchi K. (1984). The formation of Sargassum beds on artificial substrata by transplanting seedlings of S.horneri (Turner) C.Agardh and S.muticum (Yendo) Fensholt. Bull. Japan. Soc. Sci. Fish.50, 1115–1123. doi: 10.2331/suisan.50.1115
31
Yang Q. F. Wang H. J. Zhu W. D. Miao H. Liu F. Y. Bi Y. X. et al . (2024). Regenerative capacity of Sargassum horneri in the waters of Gouqi Island, Zhejiang Province. Acta Ecol. Sinica.44, 9274–9284. doi: 10.20103/j.stxb.202308101723
32
Yatsuya K. (2008). Drifting period of Sargassacean thalli estimated by the change in density. J. Appl. Phycol.20, 797–800. doi: 10.1007/s10811-007-9293-1
33
Yoshida T. (1963). Studies on the distribution and drift of the drifting seaweeds. Bull. Jap. Sea Reg. Fish Res. Lab.23, 141–186.
34
Zeng C. K. Lu B. R. (2000). Chinese Academy of Sciences (Volume 3: Phaeophyta, Book 2: Fucusales) Vol. 43-44 (Beijing: Science Press).
35
Zhang S. Q. Li W. Liu S. Pan H. J. (2009). Size-dependence of reproductive allocation of Sargassum thunbergii (Sargassaceae, Phaeophyta) in Bohai Bay, China. Aquat. Bot.91, 194–198. doi: 10.1016/j.aquabot.2009.06.003
36
Zhu Q. Ren J. R. Chen J. J. Yang R. (2019). The morphological and ultrastructural profiling between floating and benthic Sargassum horneri. J. Biol.36, 51–54. doi: 10.3969/j.issn.2095-1736.2019.03.051
Summary
Keywords
raft S. horneri, simulated drifting S. horneri, growth, reproduction, morphology
Citation
Wang H, Yang Q, Zhu W, Miao H, Liu F and Bi Y (2025) Study on the growth, reproduction and morphology of raft and drifting S. horneri in Gouqi Island. Front. Mar. Sci. 12:1596071. doi: 10.3389/fmars.2025.1596071
Received
19 March 2025
Accepted
24 April 2025
Published
14 May 2025
Volume
12 - 2025
Edited by
Yang Liu, Ocean University of China, China
Reviewed by
Zhenhua Wang, Shanghai Ocean University, China
Wei Liu, Shanghai University, China
Updates
Copyright
© 2025 Wang, Yang, Zhu, Miao, Liu and Bi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanxin Bi, byx369@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.