Abstract
Changing ecological factors pose a challenge to many organisms. Global changes and the associated environmental changes have major impacts on marine organisms and threaten the biodiversity of marine ecosystems. It has been shown in previous experimental studies that ocean acidification caused by anthropogenic CO2 release into the atmosphere and subsequent dissolution in seawater will have a significant impact on various marine organisms. Here, we investigated the corrosive effects from acidification on the morphology of isolated shark teeth in an eight-week incubation at a pH of 7.3, the expected seawater pH in the year 2300. The typical littoral blacktip reef shark (Carcharhinus melanopterus), which is often kept in display aquaria under controlled conditions, has been used for this purpose, greatly facilitating minimally invasive sampling for in-situ investigation. The teeth of this typical Requiem Shark species are orthodont teeth, which show strong serration in the teeth of the upper jaw. Using scanning electron microscopy (SEM) we could observe the corrosive effects of acidification on the different tooth structures, such as the root, primary and secondary serrations and the crown of the blacktip reef sharks teeth. Our results show that ocean acidification will have significant effects on the morphological properties of teeth, including visible corrosion on the crown, degradation of root structures, and loss of fine serration details under low pH conditions which could lead to changes in foraging efficiency, energy uptake, and ultimately elasmobranch fitness in future oceans.
Introduction
The impending global changes and the associated environmental alterations pose significant challenges to marine biodiversity (Caldeira and Wickett, 2003; Nagelkerken et al., 2017);. One of the most pressing issues is ocean acidification, a result of increased anthropogenic CO2 emissions. This phenomenon is altering the carbonate chemistry of ocean waters, leading to a decrease in pH levels, with projections suggesting a drop from the current average of 8.1 to as low as 7.3 by the year 2300 (Intergovernmental Panel on Climate Change (IPCC), 2014). Such changes will have profound implications for marine organisms, particularly affecting their physiology and morphology (Fabry et al., 2008; Gobler and Baumann, 2016).
Among the affected marine organisms are sharks, predators crucial to the ecological balance of marine ecosystems and already threatened by overfishing (Heithaus et al., 2008). These predators are facing direct impacts of ocean acidification, which not only alters their immediate environment but also affects their biological functions. For instance, the water conditions projected for the end of this century indicate increased nutritional requirements for sharks and reduced growth rates, suggesting metabolic stress caused by hypercapnia (Pistevos et al., 2015). Furthermore, the heightened CO2 levels combined with increased water temperatures negatively impact the hatching success of some Elasmobranchii species and can diminish the sensitivity of their chemoreceptors, essential for detecting prey and navigating their environment (Dixson et al., 2014; Pistevos et al., 2015). Other recent studies such as Leung et al. (2022) have found no negative effects of ocean acidification on shark teeth, indicating that results may vary depending on species and experimental conditions.
A specific concern is the effect of acidification on calcium carbonate-dependent structures, such as the dermal denticles of sharks. Dermal denticles are vital for hydrodynamics and mobility, as their microstructure reduces drag by controlling boundary layer flow, thereby improving swimming efficiency (Ebert et al., 2021). Their corrosion due to acidification could result in increased energy costs and reduced movement efficiency (Dziergwa et al., 2019). Despite their resilience, sharks face challenges in adapting to rapid environmental changes. While some species, like the Puffadder Shyshark (Haploblepharus edwardsii), can regulate their blood pH in acidified waters, the extent of such adaptive capabilities among different shark species, especially pelagic ones, remains largely unknown (Heinrich et al., 2014; Heuer and Grosell, 2014).
The blacktip reef shark (Carcharhinus melanopterus), a vital predator in tropical coral reefs, is an ideal model for studying the effects of ocean acidification on requiem sharks (Smith and Ohio Biological Survey, 2017). Shark tooth morphology, essential for their predatory lifestyle, has evolved over millions of years, showcasing a wide variety in recent species adapted to their preferred prey (Figure 1) (Whitenack and Motta, 2010). All sharks replace their teeth continuously, but the rate of tooth replacement can vary from species to species, ranging from a few days to several weeks and is unknown for the blacktip reef shark (Luer et al., 1990). Understanding the anatomical structure of shark dentition is critical for interpreting the effects of ocean acidification. The teeth of C. melanopterus are continuously exposed to the seawater (Figure 1A modified after Ebert et al., 2021, B). It has a passive respiratory system and must swim with its mouth permanently open to allow fresh oxygen-rich water to flow through the gills (Ebert et al., 2021). In addition, it can already be seen in the young shark that several rows of teeth are exposed at the same time and that the teeth are not deeply embedded in the jaw tissue (Figure 1B). The shark tooth can be divided into two parts: the root and the crown. Figure 1C (modified after Ebert et al., 2021) provides a detailed view of individual tooth morphology and serration patterns, which are central to our morphometric analysis. The root is in the connective tissue of the jaw and the crown is used to catch and hold the prey (Figure 1A modified after Ebert et al., 2021). The tooth itself consists largely of calcium phosphate or fluorapatite, which is highly mineralized (Chang et al., 2017). The root basically consists of a porous dentin, the osteodentin (Figure 1D modified after Berio, 2021) (Jambura et al., 2020). The crown with an orthodont histology typical of Carcharhiniformes, has a central pulp cavity (Jambura et al., 2020). The pulp cavity is completely enclosed by a layer of orthodentine which is enclosed by an enameloid layer (Figure 1D modified after Berio, 2021) (Jambura et al., 2020).
Figure 1
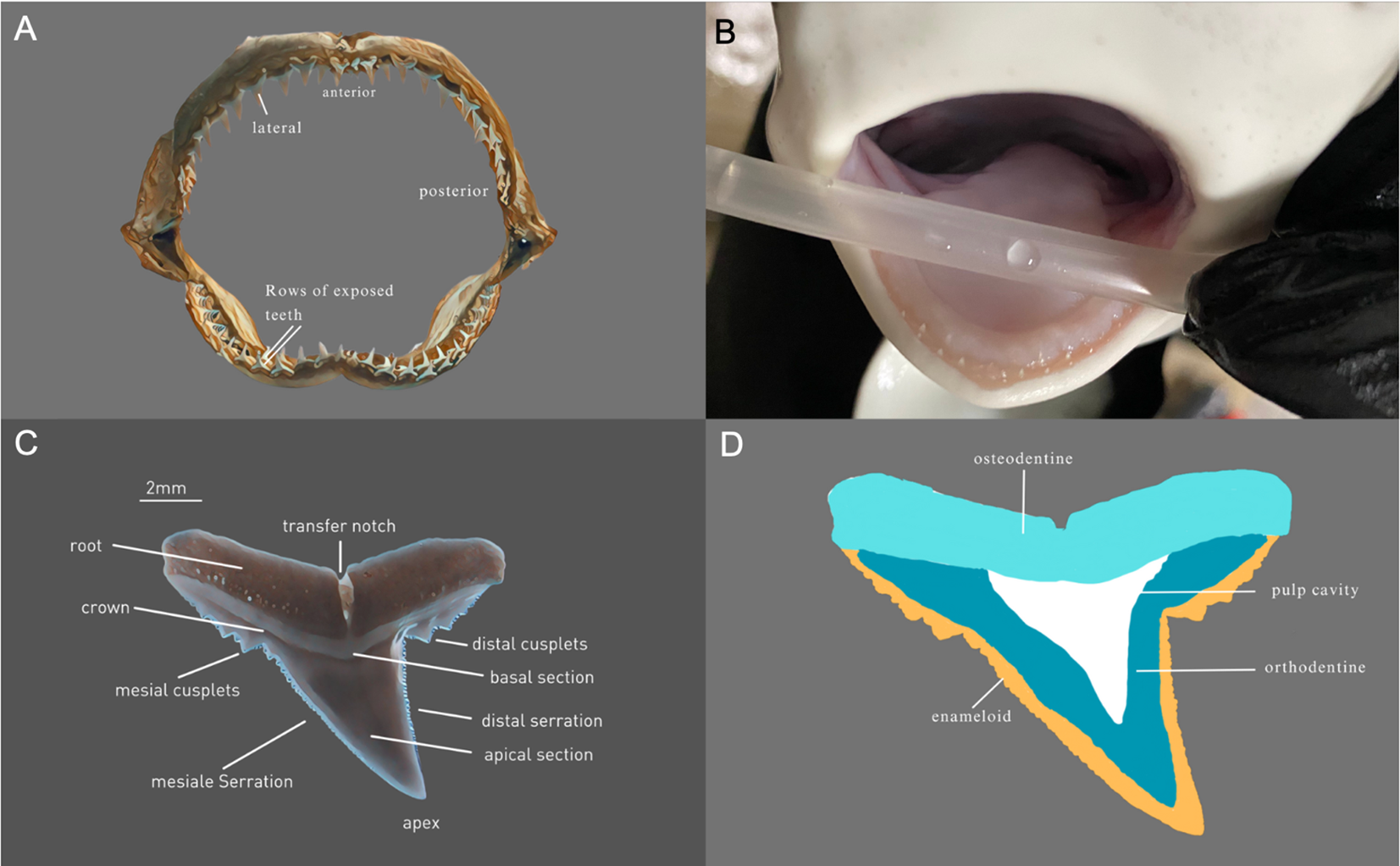
Jaw and teeth morphology of C. melanopterus(A) Dentition C. melanopterus (labeled after Ebert et al., 2021), (B) Jaw of a juvenile C. melanopterus with several rows of exposed teeth that are not deeply embedded in the jaw tissue. (C) Lingual view of a maxillary tooth of C. melanopterus (labeled after Ebert et al., 2021). (D) Schematic representation of tooth histology of C. melanopterus (labeled after Berio, 2021).
Our study focuses on the morphological changes in the teeth of the blacktip reef shark under projected simulated ocean acidification conditions. We investigated the effects of an eight-week incubation of the sharks’ teeth in seawater at a pH of 7.3, a scenario expected by the year 2300. The morphological, histological, and mechanical properties of the teeth were then examined. Using scanning electron microscopy (SEM), we observed the corrosive effects of acidification on different tooth structures, including the root, primary and secondary serrations, and the crown. These findings are crucial in understanding how ocean acidification could impact sharks’ foraging efficiency, energy uptake, and overall fitness in future oceans.
Material and methods
Sample collection
This study was conducted on isolated teeth collected from captive individuals of Carcharhinus melanopterus in the Sealife Oberhausen. No live animals were exposed to acidified conditions. The study sharks, ten specimens of C. melanopterus, were housed in a 1,500,000-liter marine aquarium. The ten blacktip reef sharks, consisted of six males and four females, aged 3–18 years, including seven wild-caught and three Sealife-bred individuals. Artificial seawater in the aquarium was prepared using deionized water and commercially available sea salt mix, adjusted to a salinity of 35 ppt. The water was mixed in aerated storage tanks at least 24 hours prior to use to ensure complete dissolution and stabilization. There was also weekly monitoring of water quality values. A slight reduction in temperature and salinity was noted during the weeks of sample collection. In addition, a drop in pH to 7.9 in the Sealife Aquarium containing the animals from which the teeth were taken for our experiment led to a slight acidification of the water during this period.
Using scuba diving equipment, naturally shed teeth were collected to ensure minimal invasive sampling. Freshly fallen teeth were primarily gathered from a plateau beneath the sharks’ feeding area in the tank. To avoid decomposition by microorganisms, teeth were collected only from sediment-free, smooth surfaces such as under the aquarium glass wall or the plateau itself.
Aged teeth, identifiable by more significant root corrosion and algae/bacterial growth, were differentiated from freshly fallen teeth. A total of approximately 600 teeth were collected and screened for morphological integrity and surface condition. Based on visual assessment and grading, only teeth classified as grade 1 (intact and undamaged) were selected for incubation and SEM analysis. Ultimately, 16 teeth (n = 8 per pH treatment) were included in this experiment. The grade 2 group, consisting of 36 teeth (n = 18 per pH treatment), was used for before and after imaging and for measuring changes in tooth circumference. Each treatment condition was run with one replicate aquarium, and teeth were evenly distributed between treatments based on random assignment within the selected quality group. To minimize intra-group variability, teeth were paired according to similar jaw positions, ensuring that each treatment group contained comparable morphological types (Figure 1A modified after Ebert et al., 2021). Each pair comprised one tooth for the acidified seawater group (I = pH 7.3; n=8) and one for the control natural seawater control group (II = pH 8.2; n=8). Control and experimental teeth were air-dried, and then stored dry until the sample was complete. Then all were cleaned in an ultrasonic bath for 5 minutes to remove algae and dirt. The teeth were dried again at 60°C for 3 hours to prepare them for the first image analyses.
Pre-experiment imaging
Initial images of lingual and labial sides were taken using an Olympus SZX2-ILLTQ binocular microscope. Images were quantitatively analyzed using ImageJ to measure and compare tooth circumference pre-(control) and post-incubation (experimental).
Incubation under control and future pH conditions
An eight-week incubation was conducted in two separate 20-liter closed loop aquariums, containing 26 teeth per treatment. The use of a closed system allowed for precise control of seawater parameters and minimized external variability. Although system-level replication was not possible, the design enabled consistent exposure conditions and facilitated morphological comparison between treatment groups. Every system was filled with 20 liters of osmosed water and Red Sea Salt to achieve a salinity of 35 ppt. Weekly water changes of 25% were performed. Aquarium temperatures were maintained at 27°C. Water in both aquariums was circulated and filtered at a rate of 300 l/h using Eheim miniUP Micro Internal Filters. Acidification was achieved by diffusing pre-mixed CO2 gas from Dennerle-brand cylinders, typically used for freshwater aquarium fertilization, directly into the closed-loop seawater systems. The pH was manually regulated through daily measurements using a calibrated pH electrode. Water parameters of temperature and pH-value were recorded and monitored using the IKSAqua Star aquarium control system. pH was measured here with a calibrated IKS pH electrode specifically designed for the Aquastar system. Each tooth was placed in a separate well of a labeled well plate within the aquarium, allowing individual identification throughout the experiment. This setup enabled precise before-and-after comparisons for each sample.
Measurement of tooth total circumference
Post-incubation, teeth were again cleaned in an ultrasonic bath and dried for 3 hours at 60°C in an oven. Following this procedure imaging was conducted similarly to pre-experiment procedures. The circumference of each tooth was measured using ImageJ (Schneider et al., 2012) before and after the treatment. Images were converted to black and white. Thresholds were manually adjusted to segment specific intensity ranges within the images, allowing isolation of relevant regions (e.g., darker or lighter areas). These regions were then selected using ImageJ’s ‘Magic Wand’ tool to enable automated and reproducible measurements.
SEM analysis
The oven-dried teeth were mounted on aluminum pin sample plates (manufacturerPlano) for scanning electron microscopy using conductive carbon adhesive pads. They were then sputtered with gold in a sputter coater for 120 seconds to achieve a uniform gold layer to improve conductivity. The measurements were carried out on the Zeiss Supra 55 VP scanning electron microscope at a cathode voltage of 10 kV and a working distance of 30 to 40 mm, using 11x and 50x magnifications. The image data were recorded with the SE2 detector.
Measurement of root corrosion
Root corrosion was quantified using ImageJ (Schneider et al., 2012) on 11x magnified lingual side images. Randomized images were analyzed for black areas indicative of root holes, calculating an index representing relative corrosion per area. For the determination of a corrosion per area index with ImageJ, a root section of 1 mm2 or 60105px square was used to calculate the proportion of black area using the Threshold function (Schneider et al., 2012).
Analysis of basal section damage
Damage to the basal section was evaluated randomized by trained personnel to assign the overall condition to the most appropriate group from 1–4 based on sample images with no (1), minor (2), medium (3), and heavy damage (4) of the basal section (see Figure 2). Randomized evaluation of the images was conducted to avoid a biased interpretation.
Figure 2
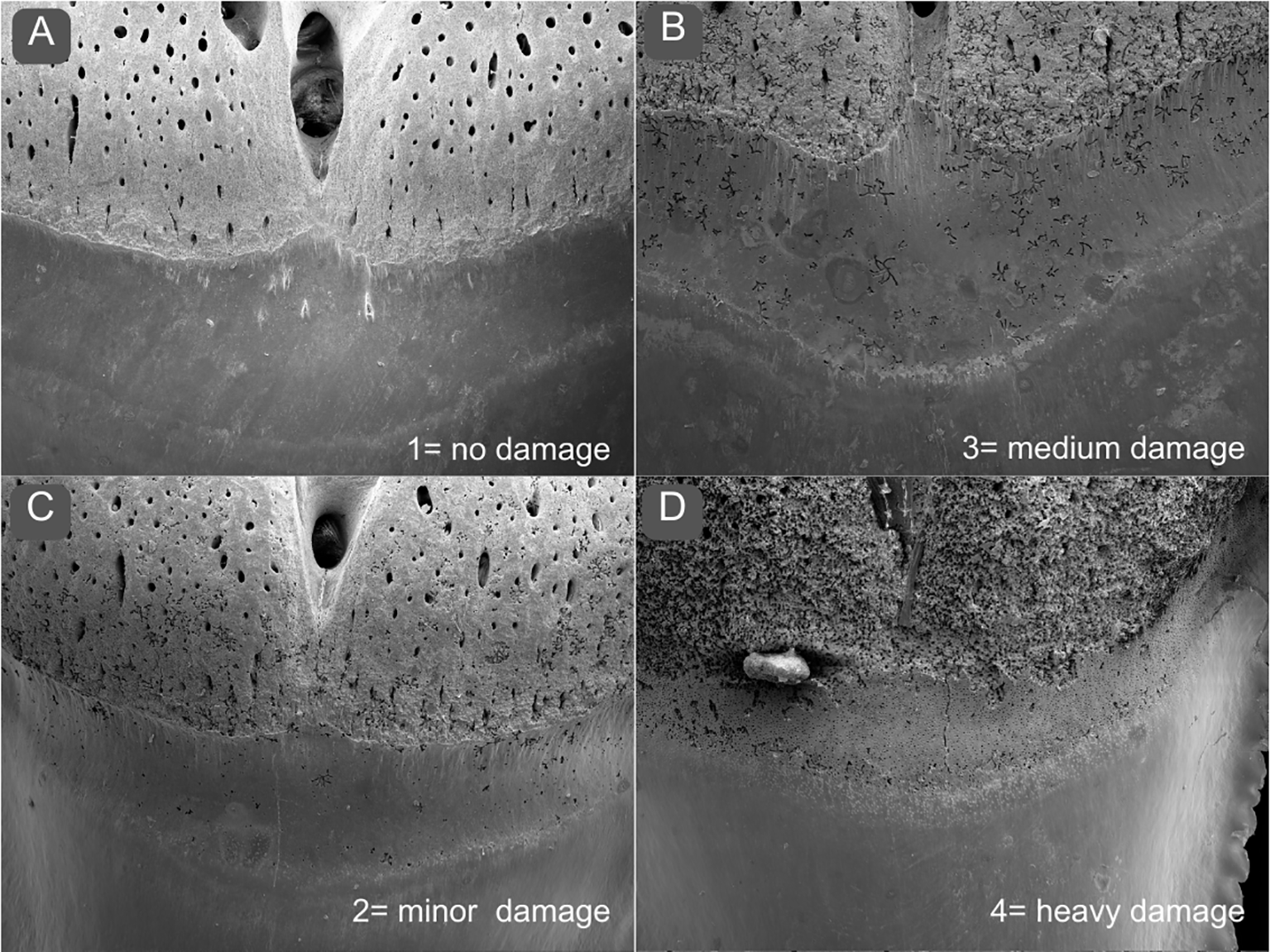
Scoring of teeth basal section damage. To assess the overall condition of the basal section, the teeth were graded between (A) 1= no damage, (B) 2= minor damage, (C) 3= medium damage, (D) 4= heavy damage.
Statistics
In order to analyze potential differences in tooth morphology between the two treatment groups after the experimental period, data sets were first tested for normal distribution using the Shapiro–Wilk test. Upon confirmation of normality, unpaired two-tailed t-tests were conducted to compare group means for measured variables of crown damage and change in circumference. The significance threshold was set at p < 0.05. Data are presented as means ± standard deviation unless otherwise noted.
Results
Despite the small water volume of the experimental tank system, the pH was consistently maintained with an average pH of 8.19 for the control tank. In the acidification tank showed some daily variation with an average of pH 7.37 (Figure 3A). The average temperature was 27.0°C for the control tank, and 26.8°C for the acidification tank (Figure 3B). Salinity was measured daily using a refractometer and maintained at 35 ppt.
Figure 3
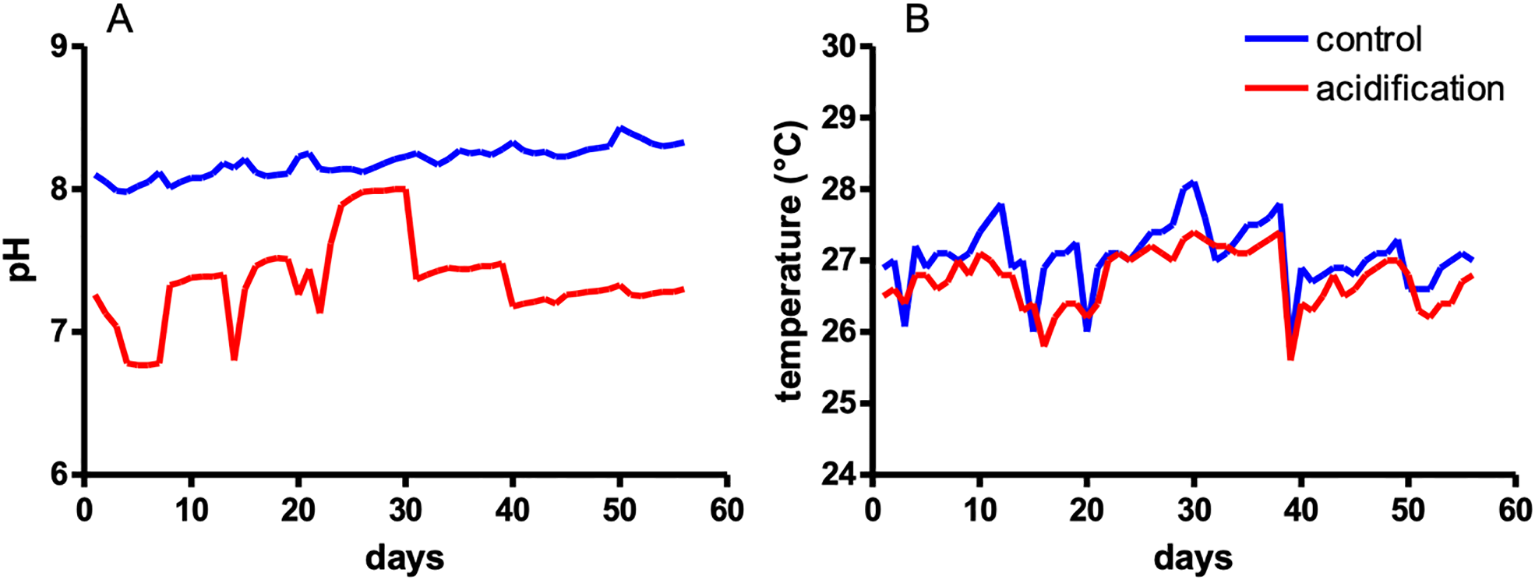
Seawater pH and temperature conditions during the experiment. Constantly measured pH (A) and temperature (B).
To detect changes in teeth morphologies in response to a change in pH, the circumferences of the teeth were measured pre- and post-incubation. Interestingly, a significantly larger change in teeth circumference could be detected in teeth incubated at pH 7.3 compared to teeth incubated at pH 8.2 (Figure 4). The average increase in tooth circumference was 0.73 mm for teeth at pH 7.3, compared to 0.38 mm for those at pH 8.2. This indicates a greater increase in secondary serration at pH 7.3, resulting in a change in the total circumference. This was measured via 2D image analysis and may reflect increased surface irregularities or structural edge degradation rather than true dimensional growth.
Figure 4
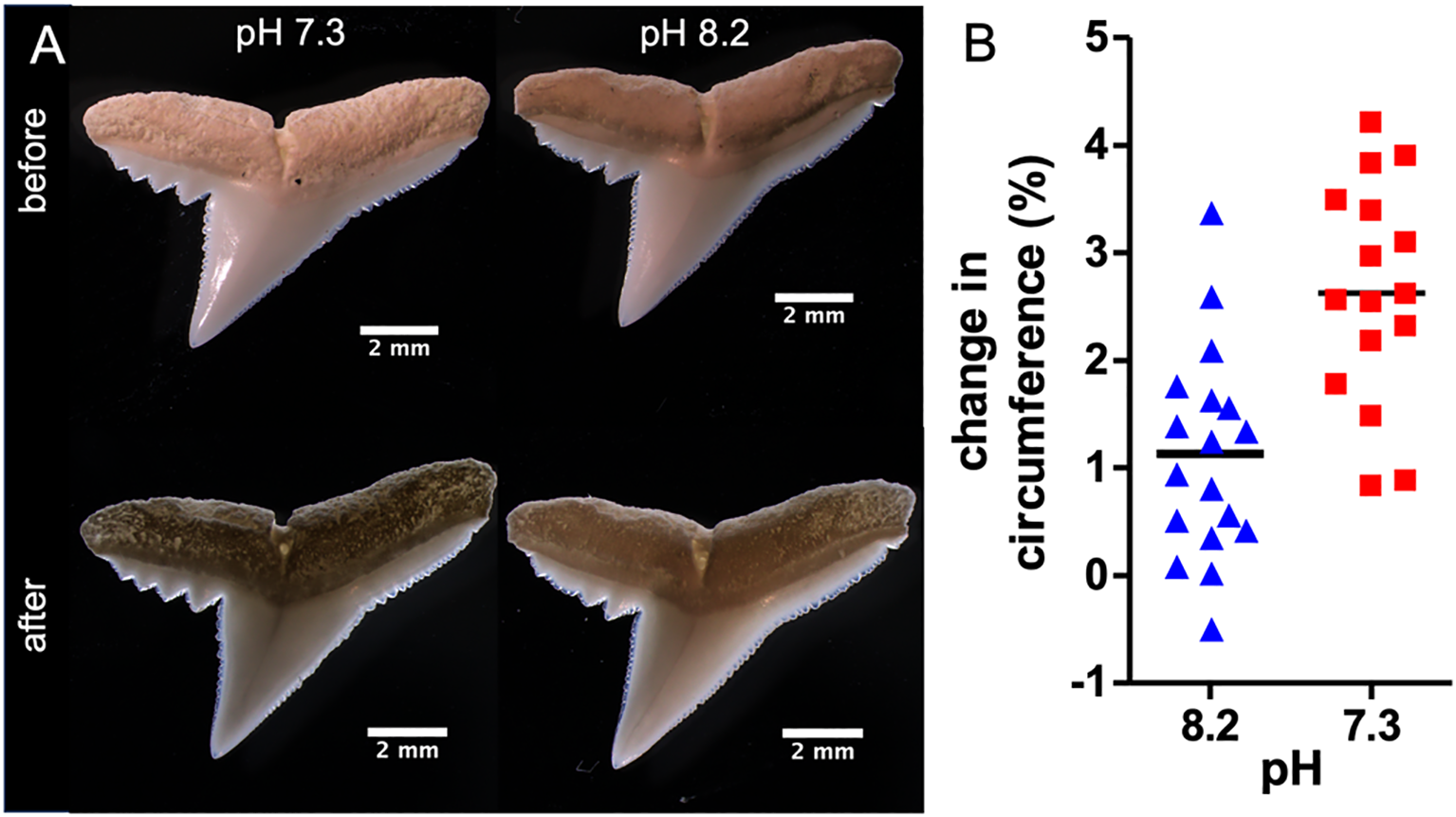
Relative changes in circumference of teeth. (A) Representative images of teeth before and after the eight-week incubation at pH 7.3 and pH 8.2 respectively. (B) Relative changes in the circumference of C. melanopterus teeth after eight weeks of incubation, pH 7.3 (n=16) and pH 8.2 (n=18), t-test *** p< 0.001.
To reveal changes in teeth morphology on the microscale scanning electron microscopy (SEM) was performed (Figures 5A-D). In order to assess teeth conditions, a scoring system was developed to evaluate the condition of the teeth at the basal section (Figure 2). The evaluation revealed a significant difference in the overall condition of the teeth at the basal section. While the teeth incubated at pH 8.2 were in relatively good condition, the condition of the teeth incubated at pH 7.3 had significantly deteriorated (Figures 5A, B, E).
Figure 5
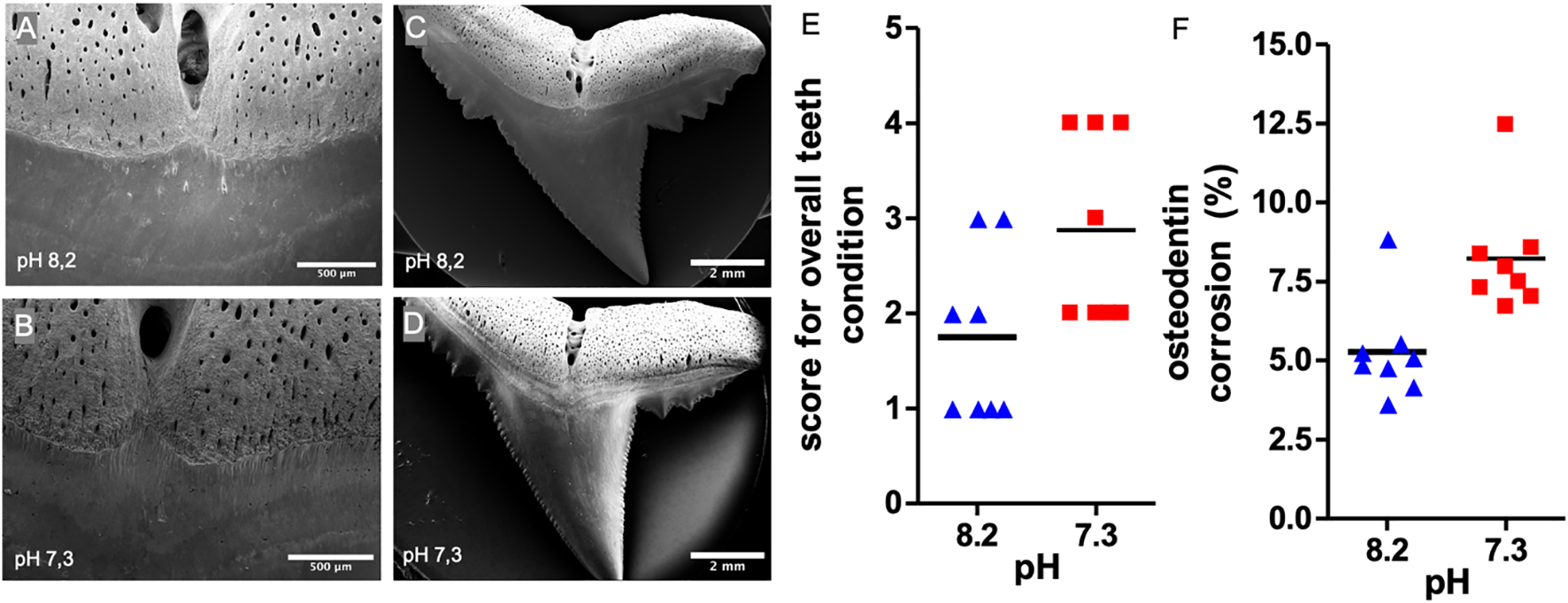
SEM analyses of osteodentin corrosion at C. melanopterus teeth. (A-D) Representative SEM images of teeth and basal sections incubated at pH 7.3 and pH 8.2, respectively (E) Randomized rating of teeth condition (Mann Whitney test, n=8, *p<0,05) (F) Relative root corrosion per area osteodentin at the root after eight weeks of incubation at pH 7.3 and pH 8.2 respectively, t-test, n=8, **p<0.01.
In addition, root and osteodentin corrosion were examined from the lingual side of the teeth. Teeth incubated at pH 7.3 showed a significantly higher level of root corrosion (8.2% of the root surface) compared to those at pH 8.2 (5.3% of the root surface) (Figures 5C, D, F).
A detailed examination of the transition from root to crown revealed damages in teeth incubated at pH 7.3. Randomized analysis showed a significant difference in the occurrence of cracks or holes in the teeth between the two groups (Figure 6).
Figure 6
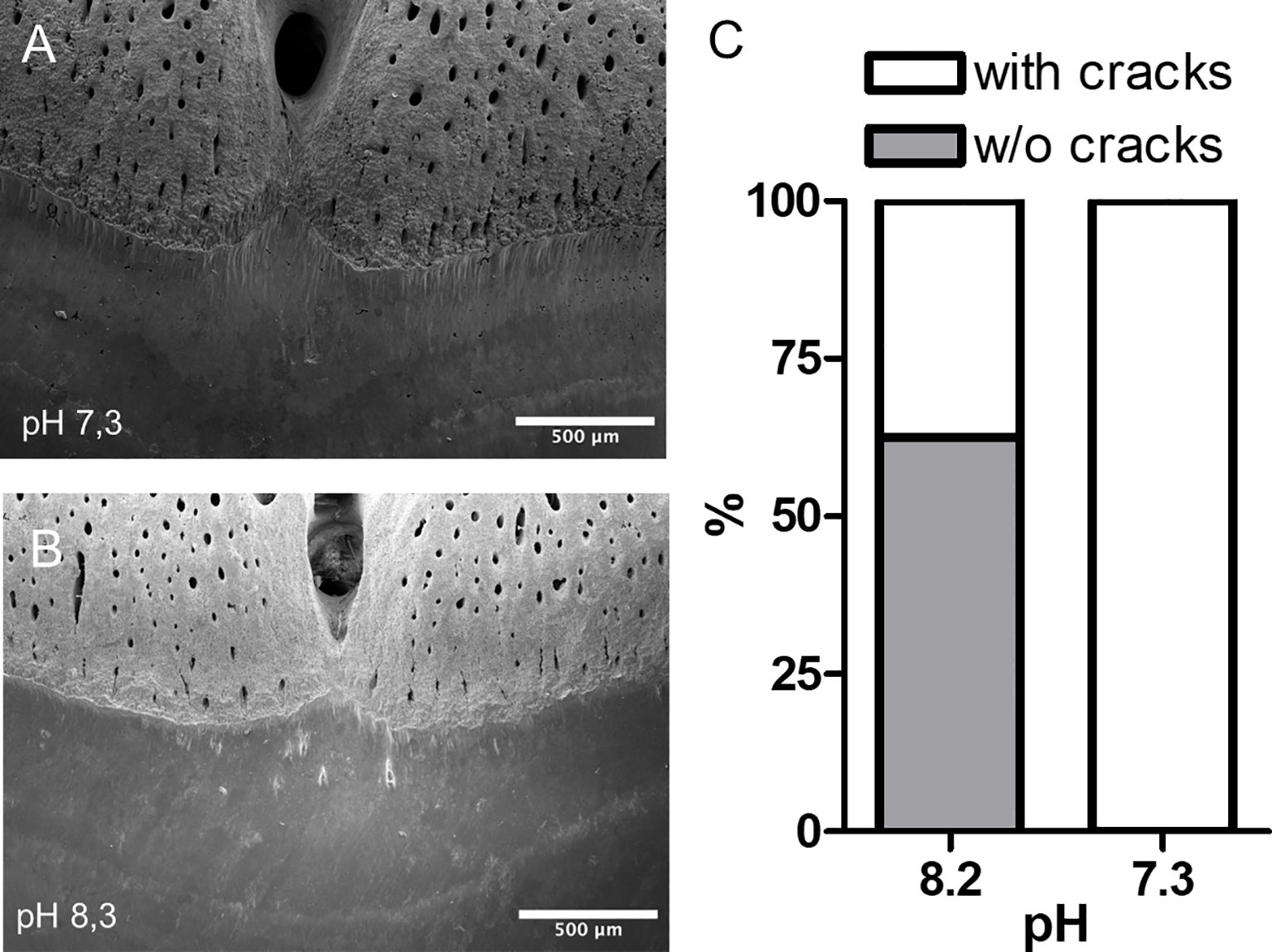
SEM analyses of the basal section of shark tooth crowns. Representative images of tooth incubated at pH 7.3 (A) and pH 8.2 (B). The image highlights the presence of holes and cracks in the tooth enamel at pH 7.3. (C) Evaluation of the occurrence of cracks in shark teeth (Fisher’s exact test, n=8, p=0.026).
Discussion
While previous studies have suggested that shark teeth, due to their fluorapatite-rich composition, are relatively resistant to ocean acidification (Leung et al., 2022), our findings indicate that isolated, shark teeth are nonetheless susceptible to visible corrosion under experimentally acidified conditions. This notable corrosion damage in teeth incubated in acidified seawater underscore the potential vulnerability of shark dentition particularly in Carcharhinus melanopterus to future ocean acidification scenarios.
As shark tooth roots are not protected by soft tissue, unlike in mammals, they are naturally exposed to surrounding water. This exposure makes them especially susceptible to pH-induced degradation, notably in the osteodentine-rich regions. The results revealed significant morphological changes affecting both crown and root structures. These included visible corrosion as well as changes in tooth circumference. However, the observed increase in average tooth circumference under high CO2 conditions should be interpreted with caution. Given that measurements were based on 2D images, this effect may reflect increased edge irregularities or serration degradation rather than actual tissue expansion. Additionally, teeth used in this study were naturally shed, air-dried, and additionally dried at 60°C prior to incubation. While this approach ensured standardization and measurement consistency, it may have affected structural integrity before exposure to experimental conditions.
Thus, our findings represent the purely chemical effects of ocean acidification on non-living, mineralized tissue, excluding physiological processes such as remineralization or internal buffering. In contrast to in vivo studies such as Leung et al. (2022), which acclimated juvenile Port Jackson sharks to acidified conditions and analyzed in-situ effects on mineralization, our setup did not involve live animals. Leung et al. used whole organisms exposed to a pH of 7.7 over a 30-day period and found minimal effects on tooth calcium content. However, their results also noted increased damage at tooth tips. Our study differs in that it isolates chemical corrosion under more extreme scenario (pH 7.3). This is intended as a first step in assessing whether changes can be detected under worst-case conditions.
The damage we observed to enameloid and dentin layers underlines that even the typically more crystalline components of shark teeth are not immune to acidification. Degradation of the crown structure could compromise the mechanical properties of teeth, especially in regions involved in prey capture and processing. Increased serration, while potentially beneficial for cutting efficiency, might lead to structural weakness and higher susceptibility to breakage.
Given that the water conditions at Sealife Oberhausen differ from natural reef habitats (e.g., lower salinity, higher temperatures), it is also possible that the baseline condition of collected teeth was already affected, further complicating interpretation. Future studies should extend these findings by examining effects on tooth synthesis, chemical structure, and mechanical resilience in vivo. Additionally, it remains unclear how ocean acidification may influence the process of biomineralization itself. Studies have shown that acidification can interfere with calcium uptake and fixation, potentially increasing energetic costs for organisms that rely on carbonate-based structures (Byrne and Przeslawski, 2013). In elasmobranchs, calcium is absorbed primarily through the gills and gastrointestinal tract, and the regulation of calcium transporters may be affected under acidified conditions. Notably, juvenile blacktip reef sharks have demonstrated the ability to maintain blood pH levels under low-pH conditions, indicating some level of physiological compensation for acidosis without major increases in metabolic expenditure (Rummer et al., 2020). Investigating adaptation mechanisms such as enhanced fluoride transport or ion regulation may reveal whether species like C. melanopterus can compensate for acidification effects, as suggested for other elasmobranchs (Leung et al., 2022). Understanding these capacities is critical to assess future resilience and potential impacts on predator-prey dynamics and ecosystem stability (Myers et al., 2007; Creel and Christianson, 2008).
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because the study only includes examinations of shark teeth from animals kept in the Sealife Oberhausen aquarium. The teeth were collected in the aquarium with the permission of Sealife Oberhausen. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MB: Formal Analysis, Visualization, Data curation, Writing – review & editing, Writing – original draft, Investigation, Conceptualization. TH: Writing – review & editing, Resources. OW: Resources, Writing – review & editing. SK: Writing – review & editing, Investigation, Visualization, Methodology. CB: Supervision, Conceptualization, Writing – review & editing. SF: Data curation, Validation, Resources, Conceptualization, Project administration, Writing – review & editing, Methodology, Writing – original draft, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Heinrich-Heine University Düsseldorf.
Conflict of interest
Authors TH and OW were employed by Sealife Oberhausen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Berio F. (2021). Multiscale variation of 3D tooth forms in selachians and developmental and evolutionary inferences: Odyssey of a scyliorhinid tooth. Populations and Evolution [q-bio.PE], Université de Lyon. NNT, 2021LYSEN010. https://theses.hal.science/tel-03279588
2
Byrne M. Przeslawski R. (2013). Multistressor impacts of warmingand acidification of the ocean on marine invertebrates’ Life histories. Integr. Comp. Biol.53, 582–596. doi: 10.1093/icb/ict049 , PMID:
3
Caldeira K. Wickett M. E. (2003). Anthropogenic carbon and ocean pH. Nature425, 365–365. doi: 10.1038/425365a , PMID:
4
Chang H. Chien M. Kao C. Chao Y. Yu P. Chang C. et al . (2017). Structural characterization of fluoride species in shark teeth. Chem. Commun.53, 3838–3841. doi: 10.1039/c6cc10114c , PMID:
5
Creel S. Christianson D. W. (2008). Relationships between direct predation and risk effects. Trends Ecol. Evol.23, 194–201. doi: 10.1016/j.tree.2007.12.004 , PMID:
6
Dixson D. L. Jennings A. R. Atema J. Munday P. L. (2014). Odor tracking in sharks is reduced under future ocean acidification conditions. Glob. Change Biol.21, 1–9 52. doi: 10.1111/gcb.12678 , PMID:
7
Dziergwa J. Singh S. Bridges C. Kerwath S. E. Enax J. Auerswald L. (2019). Acid-base adjustments and first evidence of denticle corrosion caused by ocean acidification conditions in a demersal shark species. Sci. Rep.9, 1–10. doi: 10.1038/s41598-019-54795-7 , PMID:
8
Ebert D. A. Dando M. Fowler S. (2021). Sharks of the world: a complete guide (Vol. 19) (Princeton, NJ: Princeton University Press).
9
Fabry V. J. Seibel B. A. Feely R. A. Orr J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci.65, 414–432. doi: 10.1093/icesjms/fsn048
10
Gobler C. J. Baumann H. (2016). Hypoxia and acidification in ocean ecosystems: coupled dynamics and effects on marine life. Biol. Lett.12, 20150976. doi: 10.1098/rsbl.2015.0976 , PMID:
11
Heinrich D. Rummer J. L. Morash A. J. Watson S. Simpfendorfer C. A. Heupel M. R. et al . (2014). A product of ist environment: the epaulette shark (Hemiscyllium ocellatum) exhibits physiological tolerance to elevated environmental CO2. Conserv. Physiol.2, cou047. doi: 10.1093/conphys/cou047 , PMID:
12
Heithaus M. R. Frid A. Wirsing A. J. Worm B. (2008). Predicting ecological consequences of marine top predator declines. Trends 53 Ecol. Evol.23, 202–210. doi: 10.1016/j.tree.2008.01.003 , PMID:
13
Jambura P. L. Türtscher J. Kindlimann R. Metscher B. D. Pfaff C. Stumpf S. et al . (2020). Evolutionary trajectories of tooth histology patterns in modern sharks (Chondrichthyes, Elasmobranchii). J. Anat.236, 753–771. doi: 10.1111/joa.13145 , PMID:
14
Leung J. Y. Nagelkerken I. Pistevos J. C. A. Xie Z. Zhang S. Connell S. D. (2022). Shark teeth can resist ocean acidification. Global Change Biol.28, 2286–2295. doi: 10.1111/gcb.16052 , PMID:
15
Luer C. A. Blum P. Gilbert P. W. (1990). Rate of tooth replacement in the nurse shark, ginglymostoma cirratum. Copeia1990, 182. doi: 10.2307/1445834
16
Myers R. A. Baum J. K. Shepherd T. D. Powers S. P. Peterson C. H. (2007). Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science315, 1846–1850. doi: 10.1126/science.1138657 , PMID:
17
Nagelkerken I. Goldenberg S. U. Ferreira C. M. Russell B. D. Connell S. D. (2017). Species interactions drive fish biodiversity loss in a high-CO2 world. Curr. Biol.27, 2177–2184.e4. doi: 10.1016/j.cub.2017.06.023 , PMID:
18
Rummer J. L. Bouyoucos I. A. Mourier J. Nakamura N. Planes S. (2020). Responses of a coral reef shark acutely exposed to ocean acidification conditions. Coral Reefs39, 1215–1220. doi: 10.1007/s00338-020-01972-0
19
Schneider C. C. Rasband W. Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods9, 671–675. doi: 10.1038/nmeth.2089 , PMID:
20
Smith M. F. L. Ohio Biological Survey (2017). The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and Their Relatives (Columbus, OH: Ohio Biological Survey), 433–450.
21
Whitenack L. B. Motta P. J. (2010). Performance of shark teeth during puncture and draw: implications for the mechanics of cutting. Biol. J. Linn. Soc.100, 271–286. doi: 10.1111/j.1095-8312.2010.01421.x
22
Intergovernmental Panel on Climate Change (IPCC). (2014). Climate Change 2013 – The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press). doi: 10.1017/CBO9781107415324 , PMID:
23
Pistevos J. Nagelkerken I. Rossi T. Olmos M. Connell S. D. (2015). Ocean acidification and global warming impair shark hunting behaviour and growth. Sci. Rep.5, 16293. doi: 10.1038/srep16293 , PMID:
24
Heuer R. M. Grosell M. (2014). Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol.307, R1061–84.doi: 10.1152/ajpregu.00064.2014 , PMID:
Summary
Keywords
shark jaw, shark morphology, Elasmobranchii, Chondrichthyes, ocean acidification (OA)
Citation
Baum M, Haussecker T, Walenciak O, Köhler S, Bridges CR and Fraune S (2025) Simulated ocean acidification affects shark tooth morphology. Front. Mar. Sci. 12:1597592. doi: 10.3389/fmars.2025.1597592
Received
21 March 2025
Accepted
16 July 2025
Published
27 August 2025
Volume
12 - 2025
Edited by
Lu Cai, Ministry of Water Resources and Chinese Academy of Sciences, China
Reviewed by
Laura Sordo, Portuguese Institute for Sea and Atmosphere (IPMA), Portugal
Francisco Márquez-Borrás, The University of Auckland, New Zealand
Updates
Copyright
© 2025 Baum, Haussecker, Walenciak, Köhler, Bridges and Fraune.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian Fraune, fraune@hhu.de; Maximilian Baum, baum-maxi@web.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.