Abstract
As an important germplasm resource of fish, the cryopreservation of testis and ovary is of great significance to protect endangered species and increase genetic diversity. However, current methods of slow cooling and vitrification in gonad preservation require a specialized cooling equipment or a higher concentration of cryoprotectants to maintain cell viability. The short barbeled velvetchin (Hapalogenys nitens) is an important marine economic fish, and the germplasm resources have been degraded during long-term artificial breeding. Therefore, this study isolated the gonads of mature Hapalogenys nitens and investigated the cryopreservation effect of testis and ovary with three cryoprotectant combinations under four freezing procedures. The results showed that the gonad tissues were cut to blocks of 0.5 cm3, which could effectively cryopreserve the testes or ovaries with the cryoprotectant combinations of 15% ethylene glycol, 10% dimethyl sulfoxide, 0.2 M trehalose or 15% propylene glycol, 0.2 M trehalose, and 15% fetal bovine serum, respectively. The testes with cryoprotectants were only kept 5 cm above liquid nitrogen for 10 min and then immersed in liquid nitrogen, while the ovaries soaked in cryoprotectants were directly stored in the refrigerator at -80°C. After 7 days, the gonads were thawed in a water bath at 10°C for 8 min and analyzed by morphology, and the cell viability was measured by trypan blue or cell viability assay kits, resulting in a high survival rate (>90%). The present study successfully established cryopreservation protocols of gonad tissues in Hapalogenys niten. This was a convenient, rapid, and efficient method for the gonad cryopreservation of Sparidae fishes and provided reference for the preservation of other fish germplasm resources.
1 Introduction
Cryopreservation is a powerful technology that preserve organelles, cells, tissues, or any other biological construction in extremely low temperatures for the long term (Jaiswal and Vagga, 2022). This technology maintains the integrity of cell structure and biological activity by lowering or even pausing cell metabolism by controlling the temperature. In the process of cryopreservation, the cryoprotectants, cooling procedures, and thawing speed are three important factors that affect cell viability (Rakbanjong et al., 2021). There are two types of cryoprotectants. One is the permeating cryoprotectants that permeate cell membranes, such as dimethyl sulfoxide (DMSO), methanol (MeOH), propylene glycol (PG), ethylene glycol (EG), and glycerol (Gly), and the other is non-permeating cryoprotectants which include trehalose, fetal bovine serum (FBS), sucrose, egg yolk powder, and skim milk powder (Yuan et al., 2024; Whaley et al., 2021; Lee et al., 2013). Slow freezing and vitrification are two common cooling procedures (Yong et al., 2020). Generally, slow freezing involves a series of preset temperature gradients to slowly cool the sample to the temperature of liquid nitrogen (-196°C) for long-term storage, while vitrification freezing is a rapid freezing method that usually uses a high concentration of cryoprotectant to rapidly cool the sample, converting liquid into a glass-like amorphous solid without ice crystals (Behl et al., 2023; Amorim et al., 2011). Water baths (10°C or 37°C) have become the primary method of thawing, the appropriate temperature and time of which are chosen according to the characteristics of the tissue or cell (Uhrig et al., 2022; Abdalkarim et al., 2021). The appropriate type and concentration of cryoprotectants, as well as cooling or warming procedures, can reduce the damage caused by ice crystal formation in the cells.
Cryopreservation has shown an important role in human-assisted reproduction and animal artificial breeding (Casciani et al., 2023; Abdalkarim et al., 2021; Xingzhu et al., 2021; Onofre et al., 2016). It is also the most direct way to protect fish germplasm resource. At present, this technology is widely used for the preservation of fish sperm and also involves the storage of germ stem cells, eggs, embryos, and gonadal tissues of a few species (Zhang et al., 2020; Herranz-Jusdado et al., 2019; Martı́nez-Paramo et al., 2017). Cryopreserved gonadal tissues contain the germ stem cells of spermatogonia or oogonia that have been isolated as donor germ cells for transplantation. The combination of cryopreservation and germ cell transplantation could provide an alternative way for the conservation and recovery of endangered, precious, and elite fish resources—for example, the cryopreservation of juvenile gonads and transplantation in California white sturgeon (Acipenser transmontanus) provided an encouraging approach to the management of threatened sturgeon species (Romney et al., 2023). Moreover, the spermatogonia from testes cryopreserved for 5 years still had high capacity to generate functional gametes via interspecies transplantation in salmonids (Lee et al., 2016a). Therefore, the cryopreservation of gonads (ovary and testis) was crucial to fish.
The cryopreservation of fish gonads has been carried out on rainbow trout (Oncorhynchus mykiss) (Lee et al., 2016b, Lee et al., 2013), medaka (Oryzias latipes) (Seki et al., 2017), cyprinid honmoroko (Gnathopogon caerulescens) (Higaki et al., 2017), Murray River rainbowfish (Melanotaenia fluviatilis) (Ivers et al., 2020), Piracanjunba (Brycon orbignyanus) (Santos Marques et al., 2021), Russian sturgeon (Acipenser gueldenstaedtii) (Lujić et al., 2023), and Asian sea bass (Lates calcarifer) (Sreebun et al., 2024). In rainbow trout, the testes and ovaries were successfully cryopreserved with a cryomedium containing 1.3 M or 1.0 M DMSO, 0.1 M trehalose, and 10% egg yolk and 35.2% extender, cooled at -1°C/min for 90 min before immersion in liquid nitrogen, and thawed in a 10°C water bath and rehydrated (Lee et al., 2016b, Lee et al., 2013). Similarly, in zebrafish (Tsai et al., 2009), white sturgeon (Romney et al., 2023), rainbowfish (Ivers et al., 2020), and sea bass (Sreebun et al., 2024), the testes or ovaries with permeating and non-permeating cryoprotectants were cooled slowly in a freezer container for more than 1 h and placed at −80°C before being plunged into liquid nitrogen, while in other fish like medaka (Seki et al., 2017), cyprinid honmoroko (Higaki et al., 2017), Piracanjunba (Santos Marques et al., 2021), and sturgeon (Lujić et al., 2023), the testes or ovaries with permeating cryoprotectants (PG, EG, MeOH and DMSO) and sucrose were cryopreserved for vitrification. The protocol of vitrification was generally balanced or pretreated with a low concentration of permeating cryoprotectants, and then vitrification was carried out with a high concentration of permeating cryoprotectants, in which sucrose dehydration and copper mesh were required for rapid cooling. It should be noted that the tissues preserved by vitrification are small and mostly from immature economic fish or model species, which facilitate the penetration of cryoprotectants and rapid cooling.
The short barbeled velvetchin (Hapalogenys nitens) is an important economic fish mainly distributed in the coastal areas of China, Korea, and Japan (Kang et al., 2015). In China, the low production of Hapalogenys nitens in natural sea areas leads to its relatively limited market supply. In recent years, the experiment of cage culture using natural seedlings of Hapalogenys nitens in Fujian coastal areas has achieved remarkable breeding benefits, but it also causes the problem of germplasm resource degradation. Therefore, the cryopreservation of the germplasm of Hapalogenys nitens is an important task for the sustainable development of fisheries. In this study, we aimed to establish an efficient, rapid, and convenient method for cryopreservation of Hapalogenys nitens testis and ovary and to provide reference for the preservation of marine fish gonads.
2 Materials and methods
2.1 Fish and gonad collection
Mature male and female Hapalogenys niten were purchased from Donghu Market in Ningde City, Fujian Province, China. They were anesthetized by using MS-222 (Sigma-Aldrich, USA), and the basic indicators (body weight, total length, and body length) were measured. Subsequently, the gonads of the fish were removed, weighed, and then placed in L-15 medium on ice for use.
All experiments were performed in accordance with the relevant national and international guidelines and approved by the Institutional Animal Care and Use Committee, Ningde Normal University.
2.2 Experimental design
Referring to the previous studies of Lee et al. (2016b); Rakbanjong et al. (2021), and Lujić et al. (2023), the cryoprotectants EG (Sinopharm, Beijing, China), PG (Sinopharm), DMSO (Solarbio, Beijing, China), FBS (AusgeneX, Shanghai, China), trehalose (Bomei, Anhui, China), and 50% (vol/vol) extender were selected in this study, which form three combinations with which to explore the cryopreservation effect of Hapalogenys nitens gonads under four freezing procedures. The three combinations of cryoprotectants were as follows: group 1: 10% (vol/vol) DMSO + 0.1 M trehalose + 10% (wt/vol) egg yolk, group 2: 15% (vol/vol) EG + 10% (vol/vol) DMSO + 0.2 M trehalose, and group 3: 15% (vol/vol) PG + 0.2 M trehalose + 15% (vol/vol) FBS. The control group was fresh gonadal tissue without cryopreservation, which was used to evaluate the preservation effect of each cryopreservation group. The four freezing procedures were as follows: A—samples were stored directly in a refrigerator at -80°C; B—samples were stored at -80°C in a refrigerator for 1 h and then transferred to liquid nitrogen; C—samples were stored directly in liquid nitrogen; and D—samples were stored 5 cm above liquid nitrogen for 10 min and then transferred into liquid nitrogen.
2.3 Cryopreservation protocol
Considering the permeability of cryoprotectants, the size of germ cells, and the procedure of cryoprotectant removal, the separated gonads were cut into tissue blocks of approximately 1 cm, 1 cm, and 0.5 cm in length, width, and height, respectively, and then placed into a 2-mL cryopreservation tube and covered with the abovementioned 1–1.5 mL cryoprotectants of groups 1–3, respectively. There were four parallels in each group. The cryotubes containing tissue and cryoprotectants were first equilibrated on ice for 60 min, and then the four parallels of each group were cryopreserved corresponding to the four freezing procedures A–D, respectively. In particular, the cryoprotectants need to be removed from the ovaries before undergoing the freezing procedures. After 7 days, the cryotubes were removed and placed in a water bath at 10°C for 8 min. Subsequently, the cryomedium attached to the tissues were wiped off with a paper towel. Finally, the testes were rehydrated in L-15 for 10 min.
2.4 Assessment of cell viability
For testes, about 0.5 g tissue was cut into pieces, washed using L-15 medium twice, and incubated in the digestion solution containing 0.02 g of Collagenase I (Solarbio), 0.015 g of Dispase II (Solarbio), and 2 μL DNase I (Accurate Biology, Hunan, China) in 500 μL L-15 for 1.5 h at 28°C. After digestion and washing, the viability of germ cells in the testes was detected by trypan blue exclusion assay. The cell suspension was mixed with 0.4% trypan blue staining solution (Solarbio) at a ratio of 9:1, and after staining for 2 min, the cells were observed under a microscope (Leica DM7500, Germany). Dead cells were stained blue and could be distinguished from living cells so that the ratio of living cells was counted and cell viability was calculated as follows: cell viability = unstained cells/total cells × 100%.
For ovaries, the tissue blocks were washed with L-15 and digested directly with 0.25% Trypsin-EDTA solution (Pricella, Wuhan, China) for 2 h at 28°C. After digestion and washing, the viability of germ cells in the ovaries was detected by using AO-EB Double Fluorescence Staining Kit (Phygene, Fuzhou, China). According to the instructions of the kit, AO/EB working solution was diluted according to the ratio of reagent (A)/reagent (B)/reagent (C) = 1:1:8, and the cells were stained and observed under a fluorescence microscope (Leica DMI8). According to the instructions of the kit, after treatment with the dye solution, the live cells showed green fluorescence, while the dead cells showed red fluorescence under the fluorescence microscope. Therefore, cell viability can be calculated by counting the number of cells showing green fluorescence and the total number of cells: cell viability = green cells/total cells × 100%.
2.5 Statistical analysis
The software SPSS 26 was used for statistical analyses. In each freezing procedure, the data of three experimental groups (1–3) and the control group were subjected to one-way ANOVA followed by Tukey’s test. All data of the different freezing procedures A–D were presented as mean ± standard error (SE). Differences were considered significant at P < 0.05. The optimal combination of cryoprotectants under each freezing procedure was screened, and the highest values under different procedures were compared to obtain the best freezing preservation scheme.
3 Results
3.1 Mature Hapalogenys niten
The body type of Hapalogenys niten was characterized with high body height and two clear and wide dark-brown-colored lines on the side of the body. However, through breeding and feed blending, the whole body of Hapalogenys niten cultured in artificial culture was black, and the lines became inconspicuous (Figure 1A). The basic indicators of the six mature Hapalogenys niten are shown in Table 1. The mature male fish were heavier and larger than the female fish (Table 1). A dissection revealed that the testes were long strips and milky white (Figure 1B), while the ovaries were rods and orange (Figure 1D). They were all located in the abdominal cavity, above the swim bladder, below the spine, and gathered at the genital pore, forming a “V” shape (Figures 1B,D). Enzymatic digestion showed that the testes were filled with various types of germ cells of spermatogonia, spermatocytes, spermatids, and spermatozoa (Figure 1C), and the ovaries included phases I–V of oocytes (Figure 1D). The gonads were lightly pressed, and there was semen or egg flowing out, indicating that the gonad has developed and matured.
Figure 1
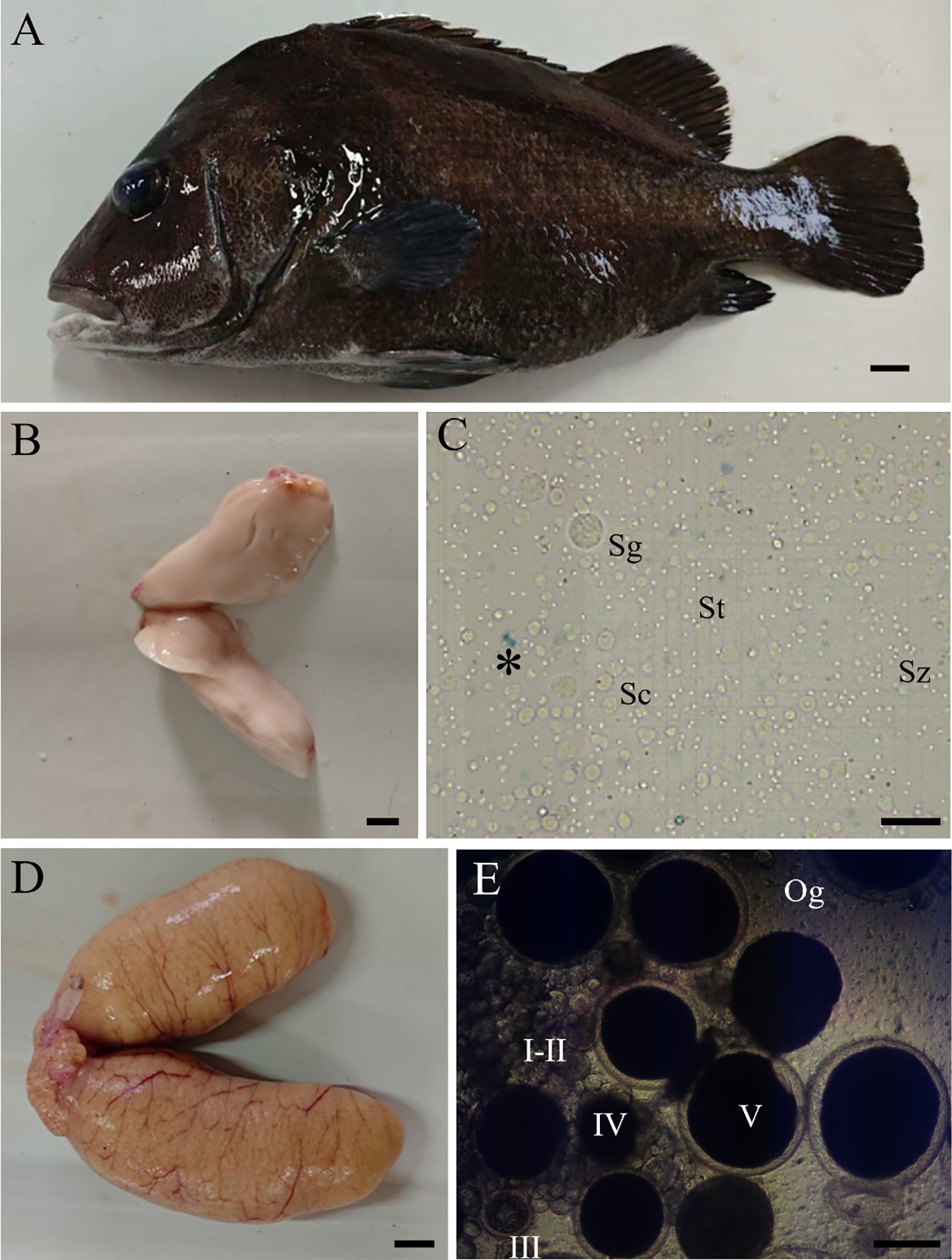
Appearance, gonad morphology, and germ cell of Hapalogenys nitens. (A) Appearance of female fish. (B) Testis morphology. (C) Various germ cells in the testis. (D) Ovary morphology. (E) Various oocytes in the ovary. The asterisk indicates dead cells. Sg, spermatogonia; Sc, spermatocytes; St, spermatids; Sz, spermatozoa; Og, oogonia; I–II, early/late previtellogenic phase; III–IV, early/late vitellogenic phase; V, full-grown stage. Scale bar, 1 cm (A–D), 20 μm (C), and 300 μm (E).
Table 1
| Gender | Number | Total length (cm) | Body length (cm) | Body weight (g) | Gonad weight (g) |
|---|---|---|---|---|---|
| ♂ | 1 | 23.70 | 21.10 | 500.10 | 11.10 |
| 2 | 25.50 | 22.80 | 525.20 | 11.40 | |
| 3 | 24.50 | 21.50 | 535.30 | 17.50 | |
| ♀ | 1 | 20.90 | 18.20 | 404.20 | 32.90 |
| 2 | 21.40 | 19.00 | 425.40 | 33.50 | |
| 3 | 21.60 | 19.10 | 431.10 | 37.60 |
Basic indicators of six mature Hapalogenys niten.
3.2 Germ cell viability detection in testes after cryopreservation
After cryopreservation, the germ cells in the testes of three groups under A–D procedures were digested by an enzyme and determined by trypan blue exclusion. The results showed that in the A–D procedures, the freezing effect of D procedure (testes were stored 5 cm above liquid nitrogen for 10 min and then transferred into liquid nitrogen) was significantly higher than that of the other (A–C) procedures (Figure 2). Moreover, a comprehensive analysis of the combination of cryoprotectants found that group 2 (15% EG + 10% DMSO + 0.2 M trehalose) had better preservation effect than group 1 and group 3 (Figure 2). The testes that were cryopreserved with group 2 under D procedures showed high cell viability, very few cells were stained with trypan blue, suggesting that most of the cells were viable after cryopreservation (Figure 2D2). The testes were filled with spermatogonia, spermatocytes, spermatids, and spermatozoa as with fresh testes (Figure 2D2). In contrast, the testes were cryopreserved with group 2 under other procedures, especially the C procedure. The cells of the testes were broken into tissue fragments and the number was significantly reduced (Figure 2A2–C2). Compared with group 2, for group 1 and group 3 under the four procedures A–D, only a few cells can be observed in the testes, and the preservation effect was markedly inferior (Figures 1A1-D1, A3-D3).
Figure 2
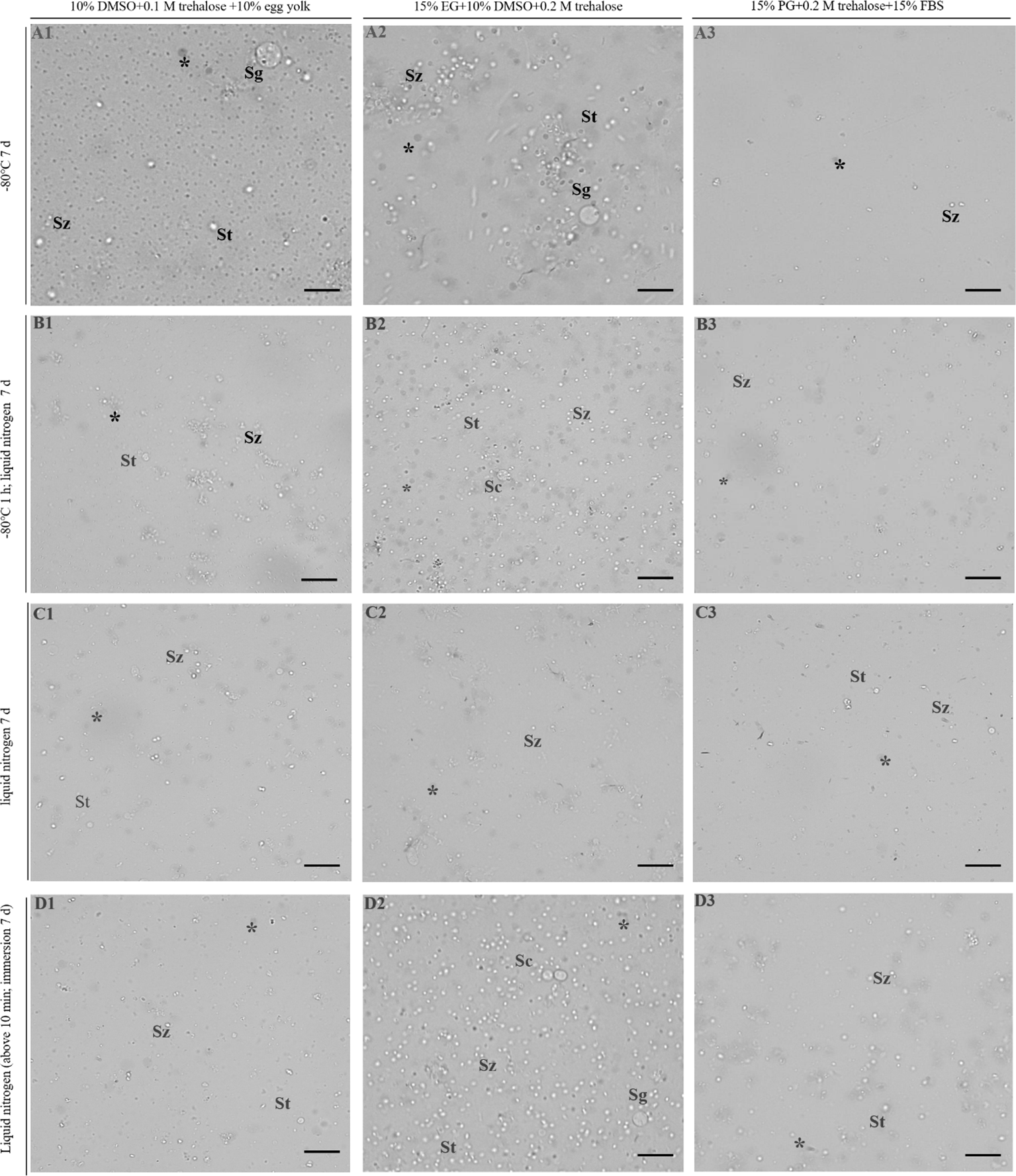
Germ cell viability detection in testes by trypan blue stain. 1–3 represent three combinations of cryoprotectants (groups 1–3), and (A–D) represent four freezing procedures. The dead cells showed an opaque gray color under the grayscale presentation. The asterisk indicates dead cells. Sg, spermatogonia; Sc, spermatocytes; St, spermatids; Sz, spermatozoa. Scale bar = 20 μm.
3.3 Germ cell viability detection in ovaries after cryopreservation
The morphology and structure of germ cells from frozen ovaries were observed under a microscope. The results showed that group 1 under A–D procedures had the worst preservation effect, and almost all of the germ cells in the ovary had an abnormal morphology (Figure 3). It is manifested as yolk granulation, shrinkage, and even rupture of egg membrane, resulting in the content flowing out (Figures 3A1-D1). Only a few oogonia and primary oocytes had normal morphology (Figures 3A1-D1). Compared with group 1, group 2 and 3 have better preservation results under the A–D procedure (Figures 3A2-D2, A3-D3). Among them, the preservation effect of the two groups under A procedure was better than that of other procedures, especially group 3 which showed the best cell morphology (Figures 3A2-D2, A3-D3). The oocytes of group 3 (15% PG + 0.2 M trehalose + 15% FBS) under A procedure (ovaries were stored directly in a refrigerator at -80°C) were of normal size, with a full, regular shape and smooth surface and did not show cell rupture, atrophy, depression, granulation, or cavitation, which was most similar to the cell morphology of the control group (Figure 3A3). In addition, the preservation effect of groups 2 and 3, respectively, under B–D procedures was slightly inferior to that of A procedure, and the cells showed a similar morphology after preservation (Figures 3A2-D2, A3-D3). A few oocytes showed an irregular shape, slight granulation and shrinkage, and almost no rupture of egg membrane (Figures 3B2-D2, B2-D3).
Figure 3
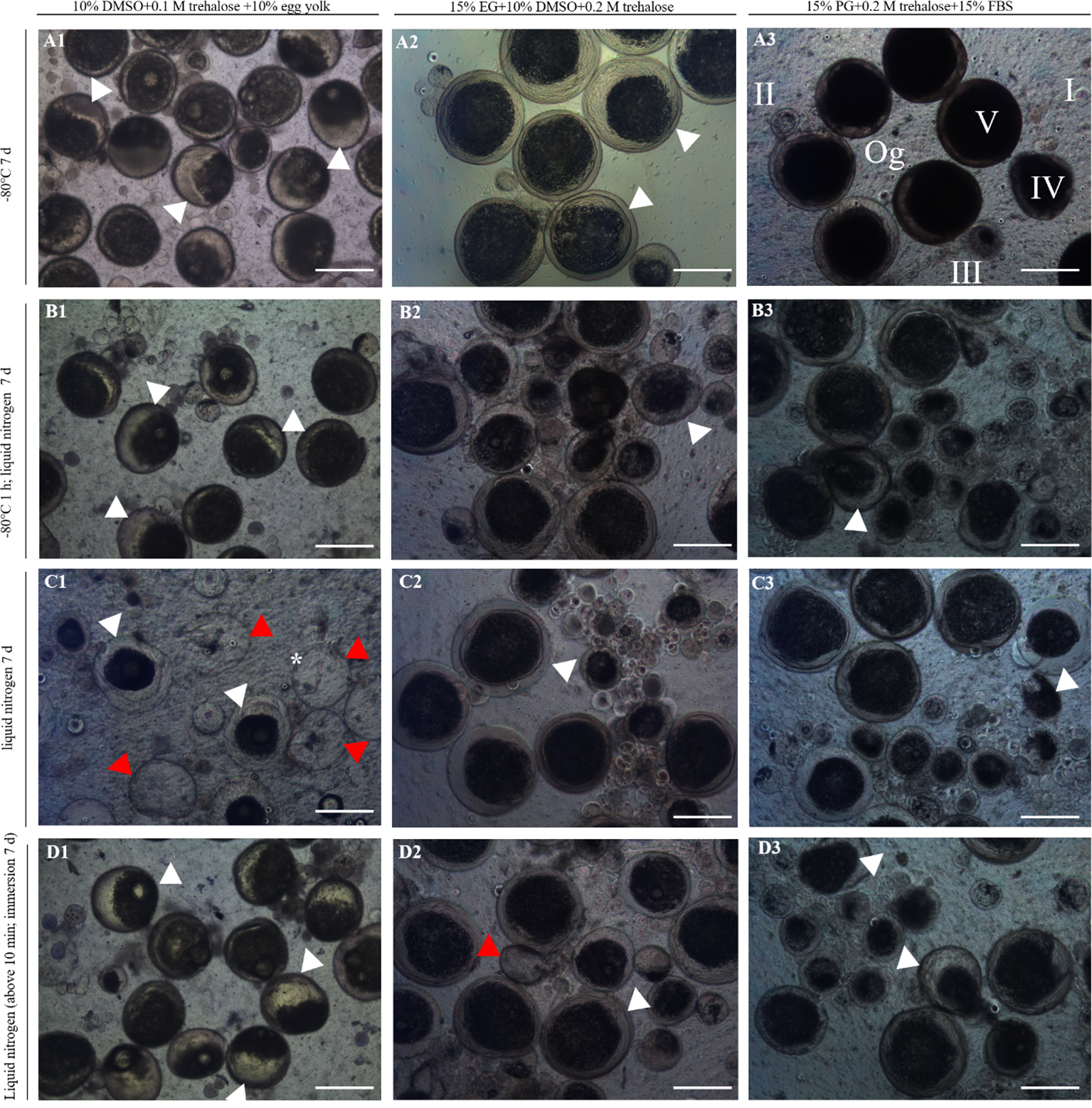
Morphology and structure of ovaries after cryopreservation. 1–3 represent three combinations of cryoprotectants (groups 1–3), and (A–D) represent four freezing procedures. The red arrows indicate oocyte rupture and the black arrows indicate shrinkage or granulation of the yolk. Og, oogonia; I–II, early/late previtellogenic phase; III–IV, early/late vitellogenic phase; V, full-grown stage. Scale bar = 300 μm. The asterisk indicates dead cells.
Based on the abovementioned morphology and structure observations, the cell viability was further verified by using AO-EB Double Fluorescence Staining Kit. The results showed that almost all of the germ cells from the cryopreserved ovaries with group 3 under A procedure showed a green color, which was the same as that of the control, indicating that almost all of the cells were living cells, and the cryopreservation effect was the best (Figures 4A1-A3, B1-B3). On the contrary, the germ cells from the cryopreserved ovaries with group 1 under C procedure were almost all red except the very small cells, indicating that almost all oocytes except oogonia had died (Figures 4C1-C3). The dyeing effect of the other groups and procedures are between the above, and the dyeing pictures were omitted. In conclusion, the results of cell viability identification were consistent with morphology and structure.
Figure 4
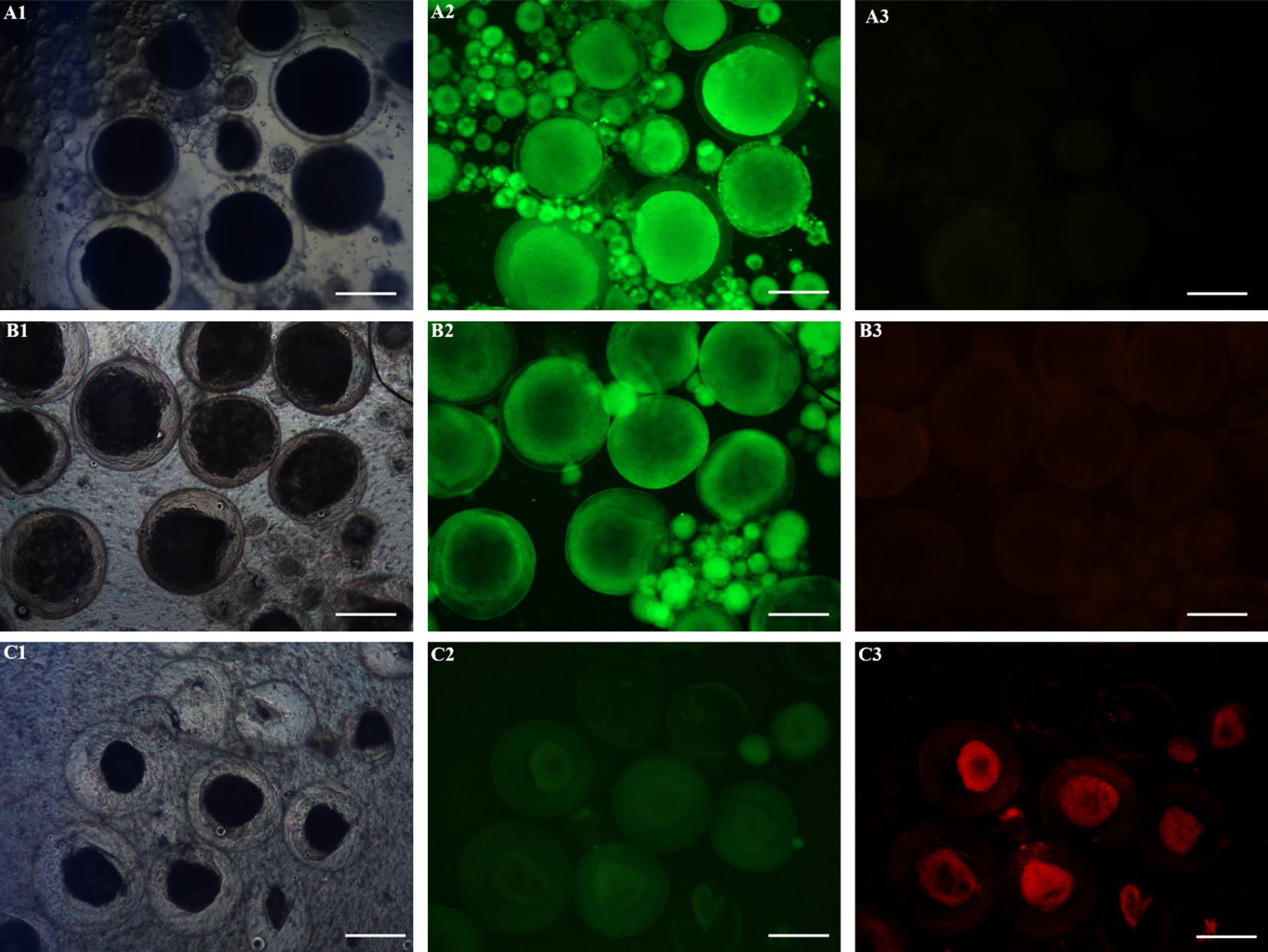
Germ cell viability detection in ovaries by using AO-EB Double Fluorescence Staining Kit. The live cells showed green fluorescence, while the dead cells showed red fluorescence. Scale bar = 300 μm. (A1–A3) control; (B1–B3) The ovaries with the cryoprotectant combination of 15% PG, 0.2 M trehalose, and 15% FBS were stored directly at -80°C in a refrigerator for 7 days. (C1–C3) The ovaries with the cryoprotectant combination of 10% DMSO, 0.1 M trehalose, and 10% egg yolk were stored directly in liquid nitrogen for 7 days. Scale bar = 300 μm.
3.4 Gonad germ cell viability statistics
For testes, the cell viability was calculated according to the results of trypan blue staining (Figure 5). The results showed that the germ cells from cryopreserved testes with group 2 were significantly different from the other groups in all three groups (P < 0.05), except for the C procedure, which was better than the other groups under A, B, and D procedures. The cell survival rate of groups 1 and 3 was mostly below 40%, which was not suitable for the cryopreservation of testes. For group 2, the freezing effect produced by different procedures varied greatly, with procedure D being the best, followed by procedures B and A and procedure C being the worst. The group 2 under D procedure was not significantly different from the fresh control (P > 0.05). Therefore, the conditions of group 2 (15% EG + 10% DMSO+ 0.2 M trehalose) and D procedure (testes were stored 5 cm above liquid nitrogen for 10 min and then transferred into liquid nitrogen) were the most suitable for the testis cryopreservation of Hapalogenys niten.
Figure 5
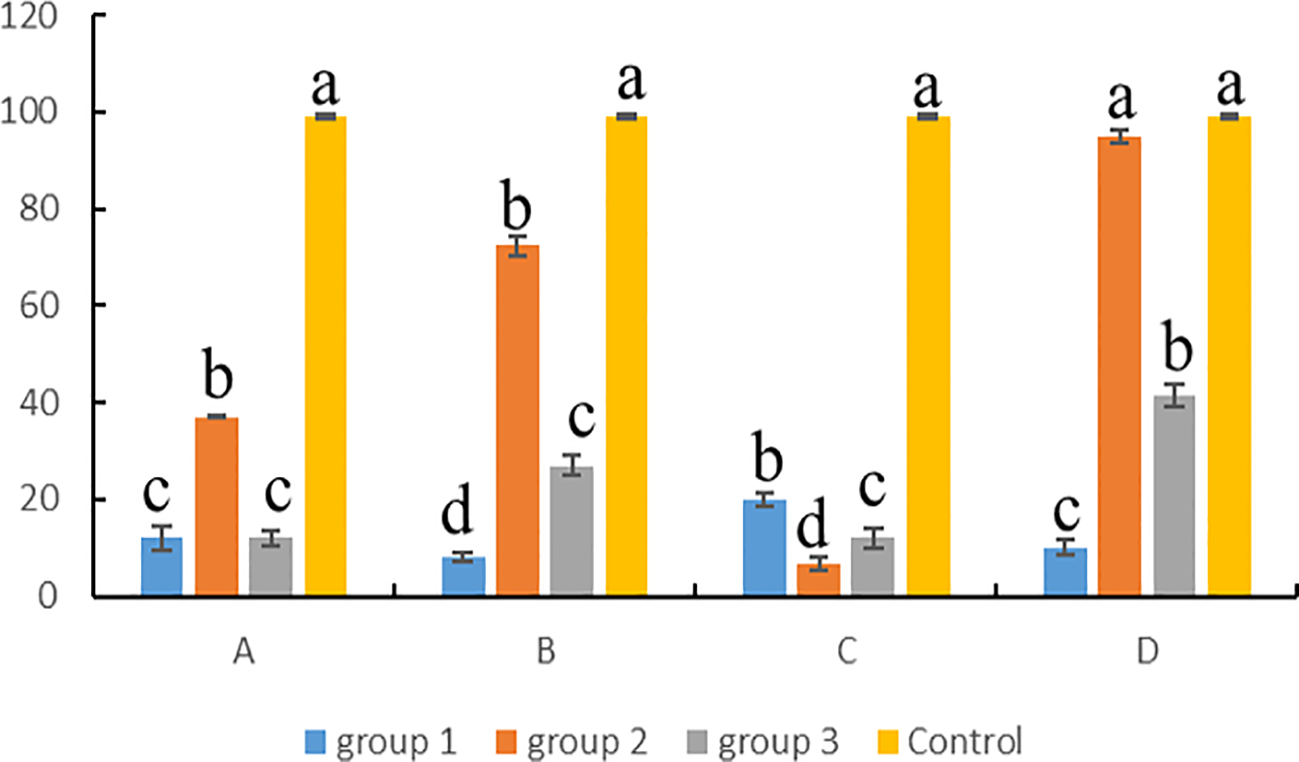
Statistics of germ cell viability in testes after cryopreservation. Group 1: 10% DMSO + 0.1 M trehalose + 10% egg yolk; group 2: 15% EG + 10% DMSO + 0.2 M trehalose; group 3: 15% PG + 0.2 M trehalose + 15% FBS. (A) -80°C for 7 days, (B) -80°C for 1 h and liquid nitrogen for 7 days, (C) liquid nitrogen for 7 days, (D) above liquid nitrogen for 10 min and immersion in liquid nitrogen for 7 days. Differences among groups were evaluated by one-way ANOVA followed by Tukey’s test. Values with different superscripts are significantly different among groups (P < 0.05). Lowercase letters (a, b, c, etc.) correspond to the significance level 0.05 in the statistical test, that is, the threshold for significance of the difference is 5%.
For ovaries, cell viability was counted according to the results of AO-EB Double Fluorescence Staining kit (Figure 6). The results showed that the germ cells from cryopreserved ovaries with group 1 under A–D procedures had the worst cell viability, and there were significant differences with the others (P < 0.05). Except for group 1, the cell survival rate of groups 2 and 3 under A–D procedures was above 80%, showing a good preservation effect. Among them, group 3 was better than group 2, except for C procedures; under the other three procedures, there were significant differences between the two groups (P < 0.05). In group 3, the A procedure showed the highest cell viability and the best preservation effect compared with other procedures, and there was no significant difference with the fresh control (P > 0.05). Therefore, the conditions of group 3 (15% PG + 0.2 M trehalose + 15% FBS) and A procedures (ovaries were stored directly in a refrigerator at -80°C) were the most suitable for the cryopreservation of Hapalogenys niten ovaries.
Figure 6
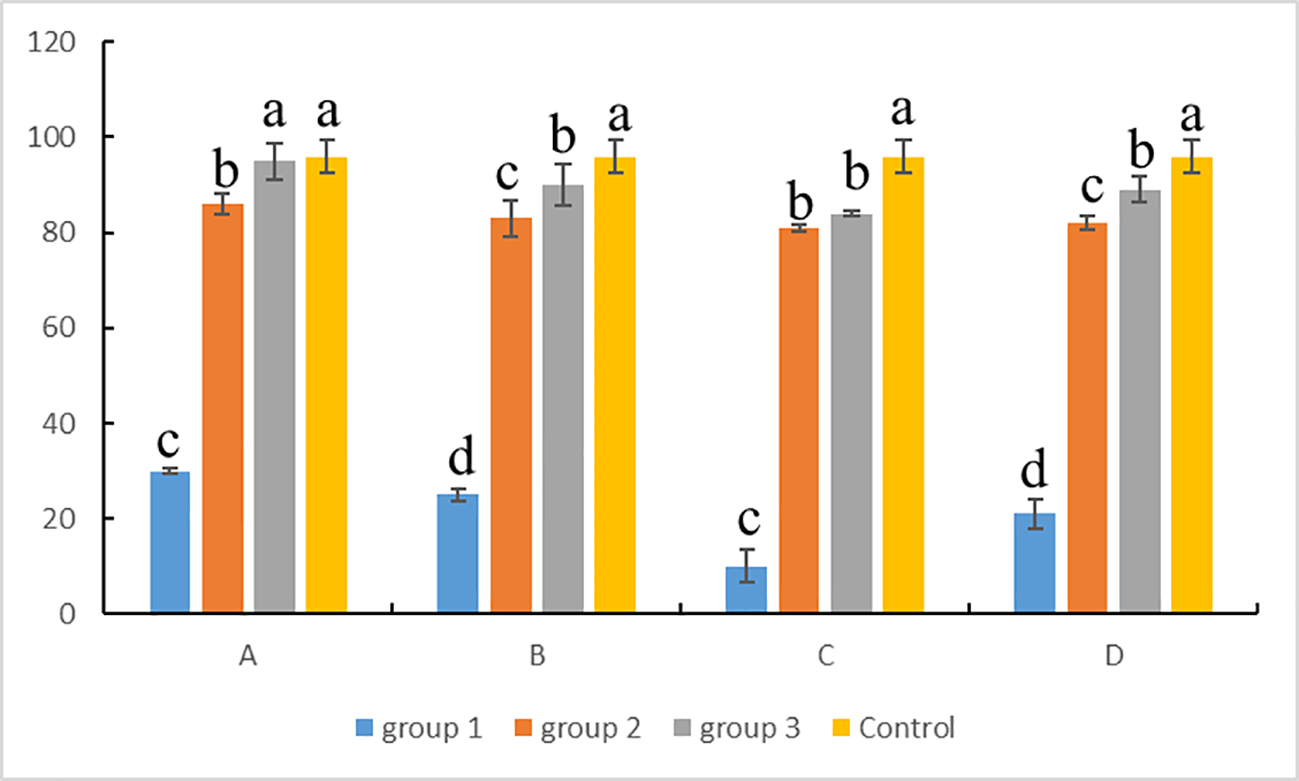
Statistics of germ cell viability in ovaries after cryopreservation. Group 1: 10% DMSO + 0.1 M trehalose + 10% egg yolk; group 2: 15% EG + 10% DMSO + 0.2 M trehalose; group 3: 15% PG + 0.2 M trehalose + 15% FBS. (A) -80°C for 7 days, (B) -80°C for 1 h and liquid nitrogen for 7 days, (C) liquid nitrogen for 7 days, (D) above liquid nitrogen for 10 min and immersion in liquid nitrogen for 7 days. Differences among groups were evaluated by one-way ANOVA followed by Tukey’s test. Values with different superscripts are significantly different among groups (P < 0.05). Lowercase letters (a, b, c, etc.) correspond to the significance level 0.05 in the statistical test, that is, the threshold for significance of the difference is 5%.
4 Discussion
The present study successfully established the cryopreservation protocols of gonad tissues in Hapalogenys niten. The testes and ovaries were isolated from mature individuals and cryopreserved with a specific cryoprotectant combination and freezing procedure, resulting in cell viability of over 90% after thawing. This further proved that the cryoprotectants of PG, EG, DMSO, FBS, and trehalose could be used to preserve the gonads of fish under the simple procedure of cooling and thawing. The study demonstrated a convenient, rapid, and efficient method for the gonad cryopreservation of Sparidae fishes and provided reference for the preservation of other fish germplasm resources.
In previous studies, slow cooling and vitrification methods had been used to cryopreserve fish gonad tissues (Lujić et al., 2023; Ivers et al., 2020; Higaki et al., 2017). The two methods differ in the rate of cooling. For slow cooling, most testes or ovaries were cooled at a rate of -1°C/min in a freezer container for more than 1 h before being plunged into liquid nitrogen. Although slow cooling resulted in high cell viability after cryopreservation in rainbowfish (63.5%) (Rivers et al., 2022), rainbow trout (72.9%) (Franek et al., 2019), and carp (65%) (Lee et al., 2016b), it required the use of special temperature control equipment such as a freezer container that greatly increases the cost of preservation, and it is not suitable for the timely preservation of gonad in the sea, field, laboratory, or farm without equipment. Unlike slow cooling, vitrification allowed the rapid freezing of the gonad, requiring only high concentrations of cryoprotectants, especially permeating agents. In medaka, the vitrification medium which included 30% (vol/vol) EG (Seki et al., 2017) was used in the cryopreservation of whole testes. Moreover, 5 M PG and 5 M DMSO were shown to result in the best survival of gonial cells in the vitrification of cyprinid honmoroko (Higaki et al., 2018). However, the high concentrations of permeating cryoprotectants, such as DMSO, can be toxic to germ cells.
In contrast, this study explored the gonad preservation effect of three groups of cryoprotectant combinations under four freezing procedures. The freezing procedure adopted two-step or direct freezing, without a special freezer container. In order to reduce the toxicity, the cryoprotectant concentration was lower in this study compared with vitrification. At the same time, in order to prevent poor protection caused by the low concentration of cryoprotectants, the whole gonad was cut into tissue blocks of approximately 1 cm, 1 cm, and 0.5 cm in length, width, and height, respectively. The testes of Hapalogenys niten could be efficiently cryopreserved with 15% EG, 10% DMSO, and 0.2 M trehalose, which were stored 5 cm above liquid nitrogen for 10 min and then transferred into liquid nitrogen in this study. The other cryoprotectant combinations such as 10% DMSO, 0.1 M trehalose, and 10% egg yolk did not show a good preservation effect in this study, although it could protect testes of rainbow trout better with slow cooling by using a Bicell plastic freezing container (Lee et al., 2013). This implied that lower concentrations of EG and DMSO could present a better protective effect and were suitable for the simple preservation of testes with liquid nitrogen. This function of EG and DMSO was consistent with previous research in sperm cryopreservation (Lomda et al., 2024; Li et al., 2013). Compared to vitrification, the lower concentration of cryoprotectants not only reduces the cytotoxicity but also eliminates the need to configure two solution systems of equilibration and vitrification. Similarly, the ovaries of Hapalogenys niten were successfully cryopreserved with 15% PG, 0.2 M trehalose, and 15% FBS in a refrigerator at -80°C. However, the temperature of -80°C might not be suitable for long-term storage due to its inability to completely terminate metabolism. The other freezing procedures of ovaries required storing at -80°C in a refrigerator for 1 h and then transferring to liquid nitrogen, and the ovaries were stored 5 cm above liquid nitrogen for 10 min and then transferred into liquid nitrogen, which also showed a relatively high cell viability (>80%) and could be used for long-term storage.
In addition, in this study, cell survival was identified by morphology and trypan blue or cell viability assay kits, and a high rate was obtained. Subsequently, the preservation effect can be further evaluated by isolating germ stem cells and performing germ cell transplantation, or a comprehensive decision can be made by molecular means such as PCR and in situ hybridization, histology, and flow cytometry.
5 Conclusion
We first realized the gonad cryopreservation of Hapalogenys nitens and the cell viability of over 90% after thawing. The results showed that the cryoprotectant combination of 15% EG, 10% DMSO, 0.2 M trehalose and 15% PG, 0.2 M trehalose, and 15% FBS could effectively protect the testes and ovaries, respectively. The testes or ovaries with cryoprotectants were cryopreserved in liquid nitrogen or in a refrigerator at -80°C. The freezing procedure was relatively simple and required no specialized equipment compared to slow cooling. Moreover, unlike vitrification methods, the lower concentration of cryoprotectants reduced the cytotoxicity of germ cells. This study provided a simple, rapid, and efficient method for gonad cryopreservation, which was of great significance for germplasm preservation and artificial breeding of fish.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
All experiments were performed in accordance with the relevant national and international guidelines and approved by the Institutional Animal Care and Use Committee, Ningde Normal University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LZ: Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization, Funding acquisition. FL: Investigation, Methodology, Conceptualization, Writing – original draft. KH: Investigation, Formal analysis, Writing – review & editing. ZS: Investigation, Writing – review & editing. JC: Investigation, Writing – review & editing. SZ: Writing–review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This present study received financial support from the Scientific Research Program of Ningde Normal University (2022Y06 and 2022ZDK02), Natural Science Foundation of Fujian (2022J05270).
Conflict of interest
Authors JC and ZS were employed by Ningde Yiye Marine Industry Development Company Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdalkarim S. Daghigh-Kia H. Mehdipour M. Najafi A. (2021). Does ergothioneine and thawing temperatures improve rooster semen post-thawed quality? Poult. Sci.100, 101405. doi: 10.1016/j.psj.2021.101405
2
Amorim C. A. David A. Van Langendonckt A. Dolmans M. M. Donnez. J. (2011). Vitrification of human ovarian tissue: effect of different solutions and procedures. Fertil. Steril.95, 1094–1097. doi: 10.1016/j.fertnstert.2010.11.046
3
Behl S. Joshi V. B. Larson N. B. Young M. C. Bilal M. Walker D. L. et al . (2023). Vitrification versus slow freezing of human ovarian tissue: a systematic review and meta-analysis of histological outcomes. J. Assist. Reprod. Genet.40, 455–464. doi: 10.1007/s10815-022-02692-w
4
Casciani V. Monseur B. Cimadomo D. Alvero R. Rienzi L. (2023). Oocyte and embryo cryopreservation in assisted reproductive technology: past achievements and current challenges. Fertil. Steril.120, 506–520. doi: 10.1016/j.fertnstert.2023.06.005
5
Franek R. Tichopad T. Steinbach C. Xie X. Lujic J. Marinovic Z. et al . (2019). Preservation of female genetic resources of common carp through oogonial stem cell manipulation. Cryobiology.87, 78–85. doi: 10.1016/j.cryobiol.2019.01.016
6
Herranz-Jusdado J. G. Gallego V. Morini M. Rozenfeld C. Pérez L. Müller T. et al . (2019). Eel sperm cryopreservation: An overview. Theriogenology133, 210–215. doi: 10.1016/j.theriogenology.2019.03.033
7
Higaki S. Kuwata N. Tanaka K. Tooyama I. Fujioka Y. Sakai N. et al . (2017). Successful vitrification of whole juvenile testis in the critically endangered cyprinid honmoroko (Gnathopogon caerulescens). Zygote.25, 652–661. doi: 10.1017/S0967199417000430
8
Higaki S. Todo T. Teshima R. Tooyama I. Fujioka Y. Sakai N. et al . (2018). Cryopreservation of male and female gonial cells by vitrification in the critically endangered cyprinid honmoroko Gnathopogon caerulescens. Fish Physiol. Biochem.44, 503–513. doi: 10.1007/s10695-017-0449-x
9
Ivers N. Daly J. Jones R. Temple-Smith P. (2020). Cryopreservation of testicular tissue from Murray River Rainbowfish, Melanotaenia fluviatilis. Sci. Rep.10, 19355. doi: 10.1038/s41598-020-76378-7
10
Jaiswal A. N. Vagga A. (2022). Cryopreservation: A review article. Cureus.14, e31564. doi: 10.7759/cureus.31564
11
Kang H. W. Cho J. K. Son M. H. Park J. Y. Hong C. G. Chung J. S. et al . (2015). Gonadal development, spawning and plasma sex steroid levels of the indoor cultured grunt, hapalogenys nitens. Dev. Reprod.19, 33–41. doi: 10.12717/devrep.2015.19.1.033
12
Lee S. Iwasaki Y. Shikina S. Yoshizaki G. (2013). Generation of functional eggs and sperm from cryopreserved whole testes. Proc. Natl. Acad. Sci. U S A.110, 1640–1645. doi: 10.1073/pnas.1218468110
13
Lee S. Iwasaki Y. Yoshizaki G. (2016a). Long-term (5 years) cryopreserved spermatogonia have high capacity to generate functional gametes via interspecies transplantation in salmonids. Cryobiology.73, 286–290. doi: 10.1016/j.cryobiol.2016.08.001
14
Lee S. Katayama N. Yoshizaki G. (2016b). Generation of juvenile rainbow trout derived from cryopreserved whole ovaries by intraperitoneal transplantation of ovarian germ cells. Biochem. Biophys. Res. Commun.478, 1478–1483. doi: 10.1016/j.bbrc.2016.08.156
15
Li P. Hulak M. Li Z. H. Sulc M. Psenicka M. Rodina M. et al . (2013). Cryopreservation of common carp (Cyprinus carpio L.) sperm induces protein phosphorylation in tyrosine and threonine residues. Theriogenology.80, 84–89. doi: 10.1016/j.theriogenology.2013.03.021
16
Lomda A. M. Mehaisen G. M. K. Stino F. K. R. Saad M. F. Ghaly M. M. Partyka A. et al . (2024). The characteristics of frozen-thawed rooster sperm using various intracellular cryoprotectants. Poult. Sci.103, 104190. doi: 10.1016/j.psj.2024.104190
17
Lujić J. Franěk R. Marinović Z. Kašpar V. Xie X. Horváth Á. et al . (2023). Vitrification of the ovarian tissue in sturgeons. Theriogenology.196, 18–24. doi: 10.1016/j.theriogenology.2022.11.009
18
Martínez-Páramo S. Horváth Á. Labbé C. Zhang T. Robles V. Herráez P. et al . (2017). Cryobanking of aquatic species. Aquaculture.472, 156–177. doi: 10.1016/j.aquaculture.2016.05.042
19
Onofre J. Baert Y. Faes K. Goossens E. (2016). Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum. Reprod. Update.22, 744–761. doi: 10.1093/humupd/dmw029
20
Rakbanjong N. Okutsu T. Chotigeat W. Songnui A. Wonglapsuwan M. (2021). Cryopreservation of Germ Cells of Banana Shrimp (Fenneropenaeus merguiensis) and Black Tiger Shrimp (Penaeus monodon). Mar. Biotechnol. (NY).23, 590–601. doi: 10.1007/s10126-021-10048-1
21
Rivers N. Daly J. Jones R. Currie P. D. Temple-Smith P. (2022). Cryopreservation and flow cytometric analysis of ovarian tissue in murray river rainbowfish, melanotaenia fluviatilis. Anim. (Basel).12, 794. doi: 10.3390/ani12060794
22
Romney A. L. T. Myers D. M. Martin F. R. Scanlan T. N. Meyers S. A. (2023). Germ cell recovery, cryopreservation and transplantation in the California white sturgeon, Acipenser transmontanus. Sci. Rep.13, 16905. doi: 10.1038/s41598-023-44079-6
23
Santos Marques L. Rodrigues de Freitas T. Batista Rodrigues R. Dos Santos Teixeira N. Pérez-Atehortúa M. Rosa-Silva H. T. et al . (2021). Vitrification protocol for immature Brycon orbignyanus ovarian tissue as an extinction escape strategy. Cryobiology.103, 116–122. doi: 10.1016/j.cryobiol.2021.08.004
24
Seki S. Kusano K. Lee S. Iwasaki Y. Yagisawa M. Ishida M. et al . (2017). Production of the medaka derived from vitrified whole testes by germ cell transplantation. Sci. Rep.7, 43185. doi: 10.1038/srep43185
25
Sreebun S. Booncherd K. Thongchaitriwat S. Ichida K. Pasomboon P. Yazawa R. et al . (2024). Cryopreservation of the whole testes of Asian sea bass (Lates calcarifer) and its effects on apoptosis, germ cell-specific gene expression, germ cell transplantability, and DNA methylation. Theriogenology.229, 178–190. doi: 10.1016/j.theriogenology.2024.08.025
26
Tsai S. Rawson D. M. Zhang T. (2009). Development of cryopreservation protocols for early stage zebrafish (Danio rerio) ovarian follicles using controlled slow cooling. Theriogenology.71, 1226–1233. doi: 10.1016/j.theriogenology.2009.01.014
27
Uhrig M. Ezquer F. Ezquer M. (2022). Improving cell recovery: freezing and thawing optimization of induced pluripotent stem cells. Cells.11, 799. doi: 10.3390/cells11050799
28
Whaley D. Damyar K. Witek R. P. Mendoza A. Alexander M. Lakey J. R. (2021). Cryopreservation: an overview of principles and cell-specific considerations. Cell. Transplant.30, 963689721999617. doi: 10.1177/0963689721999617
29
Xingzhu D. Qingrui Z. Keren C. Yuxi L. Yunpeng H. Shien Z. et al . (2021). Cryopreservation of porcine embryos: recent updates and progress. Biopreserv. Biobank.19, 210–218. doi: 10.1089/bio.2020.0074
30
Yong K. W. Laouar L. Elliott J. A. W. Jomha N. M. (2020). Review of non-permeating cryoprotectants as supplements for vitrification of mammalian tissues. Cryobiology.96, 1–11. doi: 10.1016/j.cryobiol.2020.08.012
31
Yuan L. Chen B. Zhu K. Ren L. Yuan X. (2024). Development of macromolecular cryoprotectants for cryopreservation of cells. Macromol. Rapid. Commun.45, e2400309. doi: 10.1002/marc.202400309
32
Zhang J. Tian Y. Li Z. Wu Y. Li Z. Cheng M. et al . (2020). Optimization of vitrification factors for embryo cryopreservation of kelp grouper (Epinephelus moara). Theriogenology.142, 390–399. doi: 10.1016/j.theriogenology.2019.10.002
Summary
Keywords
Hapalogenys niten , fish, testis, ovary, cryopreservation
Citation
Zhou L, Li F, Han K, Sun Z, Chen J and Zheng S (2025) Successful cryopreservation of matured testis and ovary for the short barbeled velvetchin (Hapalogenys nitens). Front. Mar. Sci. 12:1597747. doi: 10.3389/fmars.2025.1597747
Received
24 March 2025
Accepted
01 May 2025
Published
23 May 2025
Volume
12 - 2025
Edited by
Chun Liu, Chinese Academy of Fishery Sciences (CAFS), China
Reviewed by
Shengjie Ren, Queensland University of Technology, Australia
Yifan Liu, Zhejiang Ocean University, China
Updates
Copyright
© 2025 Zhou, Li, Han, Sun, Chen and Zheng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhou, zhoulisdta@163.com; Shizhong Zheng, zhengshizhong@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.