Abstract
Global climate change has decimated historical baseline coral cover in the Caribbean, including wild Acropora cervicornis, now listed as Critically Endangered by the IUCN Red List of Species. Although still at risk to the same environmental conditions that decimated wild populations, propagating A. cervicornis genotypes with increased disease resistance and thermal tolerance could help prevent extinction. Here we document the impacts of the 2023 marine heatwave on the health and survival of nursery reared A. cervicornis corals in Little Cayman that experienced 19.4 Degree Heating Weeks (DHW). In total, 91.6% of the 415 colonies suffered complete mortality with seven out of ten genotypes being eradicated, suggesting a genetic basis for thermotolerance. This accentuates the importance of genetic diversity for survival of A. cervicornis. However, the eradication of over 90% of coral colonies also highlights the futility of putting corals back into the same environment, emphasizing the need for innovative conservation strategies that incorporate novel tools capable of enhancing resilience and ensuring restoration remains viable under future climate conditions.
Introduction
The rapid increase of natural and anthropogenic stressors on Caribbean coral reefs is threatening the capacity of corals to grow, survive and create reef ecosystems (Pandolfi et al., 2003; Hoegh-Guldberg et al., 2007; Hughes et al., 2017; Donovan et al., 2021). Over the past 50 years, the region has undergone dramatic declines resulting in current coral cover less than 50% of historical records (Gardner et al., 2003; Jackson et al., 2014). Scleractinian corals live close to their maximum temperature tolerance, and thus an increase in temperatures of just 1°C can result in expulsion of their photosynthetic algal symbionts, referred to as bleaching (Brown et al., 2002; Suggett and Smith, 2011, 2020). As many corals rely on their algal symbionts to meet most of the energetic needs, this dysbiosis can ultimately lead to starvation and mortality.
The historical loss of corals in the Caribbean and across the globe has led to many initiatives aimed at restoring threatened coral species, such as the staghorn coral, Acropora cervicornis. Previously a dominant coral species on shallow Caribbean reef systems, A. cervicornis experienced significant loss in the early 1980s due to an outbreak of white band disease (Aronson and Precht, 2001, 2006) and continues to decline in abundance with subsequent disease and bleaching events (Gardner et al., 2003) leading to its listing as critically endangered by the IUCN under criteria A2bce (Crabbe et al., 2022). As the main branching species in the region, this decline led to a loss of complexity on the reef, depriving reef biodiversity from hiding spots, feeding, and growing surfaces (Agudo-Adriani et al., 2016). Currently, A. cervicornis is the most common species used in restoration efforts in the Caribbean, representing roughly 30% of restoration projects (Boström-Einarsson et al., 2020). However, a major hurdle to restoration success has been survival post outplanting, primarily driven by continued outbreaks of white band disease and thermal anomaly events (Hughes et al., 2023). This has led many restoration practitioners to explore the potential for rearing disease resistant and temperature tolerant individuals to increase restoration success (Oppen et al., 2015; Quigley, 2024).
Coral thermotolerance, or the ability of corals to withstand higher temperatures, is a crucial factor in their survival in the face of rising ocean temperatures. Through different mechanisms, a colony can prove resilient to heat stress to some extent. First, acclimatization allows corals to adapt to higher temperatures over time enabling them to tolerate heat stress (Sawall et al., 2015; DeCarlo et al., 2019) often achieved by shuffling symbiont species (Baker et al., 2004; Quigley et al., 2019; Karp et al., 2025). However, since A. cervicornis primarily hosts a single species of Symbiodiniaceae and does not experience symbiont shuffling, thermotolerance is likely more strongly driven by the host genome (Muller et al., 2018; Indergard et al., 2022). Second, tolerance may be inherited from parent colonies that experienced heat stress through epigenetics (Eirin-Lopez and Putnam, 2019). Evidence of epigenetics has been shown by several studies on acroporid species reporting changes in DNA methylation patterns associated with heat stress that could underlie the intergenerational transfer of thermal tolerance or phenotypic plasticity to environmental stress (Hazraty-Kari et al., 2022; Hackerott et al., 2023; Guerrero and Bay, 2024). Third, variations in host genetic composition or gene expression response may result in higher temperature tolerances among individuals within a population (Bellantuono et al., 2012; Jin et al., 2016; Kenkel and Matz, 2016; Yetsko et al., 2020; Karp et al., 2025). For example, Jin et al. (2016) identified specific genetic markers in Acropora millepora that were linked to differences in responses to environmental stress, providing evidence for a genetic basis of thermal tolerance. Thus, recent efforts focus on maintaining high genetic diversity and/or manipulating genetic composition of nursery reared corals to achieve higher rates of survival under increased thermal stress (Kiel et al., 2023).
In 2023, the Cayman Islands experienced an unprecedented marine heatwave with water temperatures surpassing 32°C and sustained above 31°C for several weeks. These high temperatures corresponded to an estimated 19.4 Degree Heating Weeks (DHW), a metric representing accumulated heat stress that is calculated based on the rolling sum of the number of weeks for which the sea surface temperature has exceeded the maximum mean monthly temperature accumulated over the preceding 12 weeks by at least 1°C (Gleeson and Strong, 1995; Liu et al., 2013). This excessive heating event led to widescale coral bleaching and mortality across the fore-reef zone in Little Cayman and across the Caribbean (Goreau and Hayes, 2024; Reimer et al., 2024; Doherty et al., 2025; Goodbody-Gringley and Chequer, 2025). Concurrently, this heatwave affected the A. cervicornis colonies growing in a nursery maintained by the Central Caribbean Marine Institute (CCMI), which contained individuals of known genetic identity. This event, therefore, served as an occasion to study thermal tolerance differences among genetically diverse nursery reared individuals in response to a natural heatwave. Using recurrent underwater bleaching and survival surveys along with continuous temperature measurements throughout the thermal stress event, we document critical points in the survival of A. cervicornis and identify specific genotypes with higher thermal tolerance. Understanding the effects of marine heatwaves on a genetically diverse population is the key to successfully restore and repopulate impacted reefs. Moreover, establishing DHW’s thresholds will improve our ability to predict the impacts of future heatwave events on the persistence of coral reefs.
Materials and methods
Study site and data collection
This work took place in CCMI’s A. cervicornis nursery on the north coast of Little Cayman Island that was originally established in 2012 (19.329858, -81.252361; Figure 1A). The nursery is located on the northern side of Little Cayman, roughly 700m from shore at a depth of 16 – 18m (Figure 1B). All corals maintained in the nursery were collected from wild colonies around the island between 2012 and 2018. At the onset of the study the nursery contained 415 A. cervicornis individuals, which represented 10 unique genetic identities (Drury et al., 2017; Brown et al., 2022), referred to hereafter as genotypes B, G, K, LG, O, OB, OR, R, S, and Y, dispersed among 11 vertical frames (Figure 1C). Frames were 3-m wide by 1.5-m high and constructed of PVC with corals hanging from horizontal monofilament lines. The structures were anchored using ropes tied to cinderblocks and held upright by empty plastic jugs partially filled with compressed air. Frames were ~ 1–3 m away from any other frame, and each contained a minimum of 3 different genotypes.
Figure 1
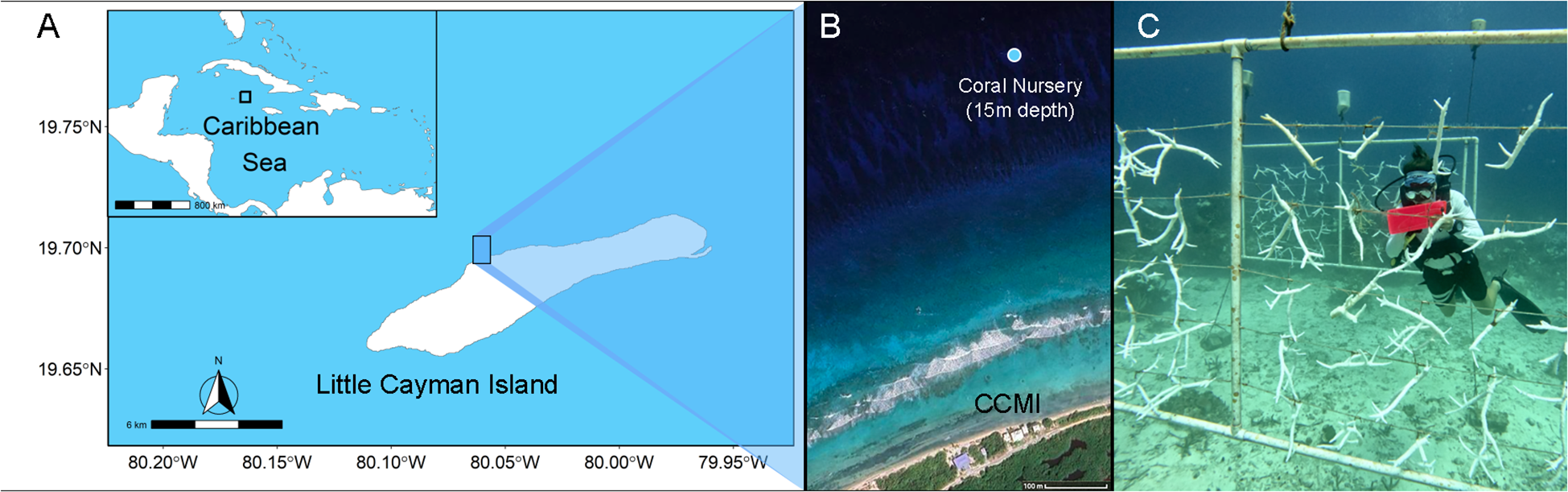
(A) Map of Little Cayman within the Caribbean and position of the nursery on the north shore. (B) The inset displays the position of the coral nursery on Little Cayman. (Imagery (c)2025 Airbus, CNES / Maxar Technologies, Map data (c)2025). (C) Image of the nursery during the peak of the heatwave showing fully bleached colonies.
Health surveys began on June 30, 2023, and were conducted approximately every two weeks thereafter, until January 6, 2024. On each survey, individuals were assigned a visual health status of Diseased, Healthy, Missing, Partially Alive or Dead, and a bleaching status of Bleached, Partially Bleached and Not Bleached. Routine nursery maintenance also occurred on each survey dive.
Seawater temperature was recorded using Onset HOBO Water Temperature Pro v2 Data Loggers secured to the top of a frame at 15m depth, on the mooring line at 10m depth, and at the surface below the mooring buoy. Temperature was recorded every minute from July 18, 2023, through January 6, 2024. Degree Heating Week data was taken from the Coral Reef Watch website (NOAA Coral Reef Watch, 2014, updated daily), which is updated daily by the National Oceanic and Atmospheric Administration (NOAA).
Statistical analysis
Statistical analyses were conducted on R version 4.1.0 (R Core Team, 2023). Temperature was compared among logger deployment depths (satellite surface, in situ surface, 10m, 15m) using a non-parametric Kruskal-Wallis’ tests, as they did not meet the assumption of normality. Post hoc analysis was conducted using a Dunn’s test in the FSA package (Ogle et al., 2025).
The impact of genotype on colony status was assessed using a generalized linear model (GLM) with a Poisson distribution, implemented via the lme4 package in R. The response variable was the count of live colonies per genotype, and the fixed effect was genotype. Sampling date (recorded as day/month) was included as a random effect to account for temporal variation in survivorship. A Poisson distribution was chosen due to the count-based nature of the data, which included no negative values. Prior to model fitting, we assessed assumptions of the Poisson distribution, including the potential for overdispersion, using residual deviance and dispersion tests. No significant overdispersion was detected, justifying the use of the Poisson GLM.
To estimate and visualize survival probabilities over time, Kaplan-Meier survival curves were generated (Kaplan and Meier, 1958) using the survival package (Therneau, 2024). Survival curves were generated separately for each genotype, and differences between curves were evaluated using a Log-rank test with Bonferroni correction for multiple comparisons. Sample sizes per genotype ranged from 10 to 149 and were consistent across analyses.
A dissimilarity matrix was constructed to quantify differences in survival patterns between genotypes. This matrix was derived solely from the pairwise p-values of the Log-rank tests, using the formula: dissimilarity matrix = 1-Log rank test p-value. These values represent inverse similarity in survival between genotypes, such that higher values indicate greater divergence. The matrix was used for hierarchical clustering using the average linkage method, implemented via the hclust function in R. This approach enabled visualization of genotype groupings based on survival divergence across the monitoring period. Only survival data were included in this matrix. All survival data were cumulative across the full monitoring period, rather than separated by date.
Results
Temperature
Seawater temperature differed significantly among the temperature measurements obtained via satellite and in situ loggers at the surface, 10m, and 15m depth (chi-squared = 364, p < 0.001). Surface temperature recorded by the in situ logger was significantly higher than the sea surface temperature registered by NOAA (z =-18.714496, p <0.001), with the highest temperature recorded at the surface by the in situ logger at 33.5 (± 0.01)°C on October 8, 2023 (Figure 2). Because in situ temperature loggers were deployed midway through the heatwave, coral responses were interpreted using NOAA’s DHW values, which is supported by the strong agreement between temperature records in the nursery (15m depth) and satellite-derived sea surface temperatures.
Figure 2
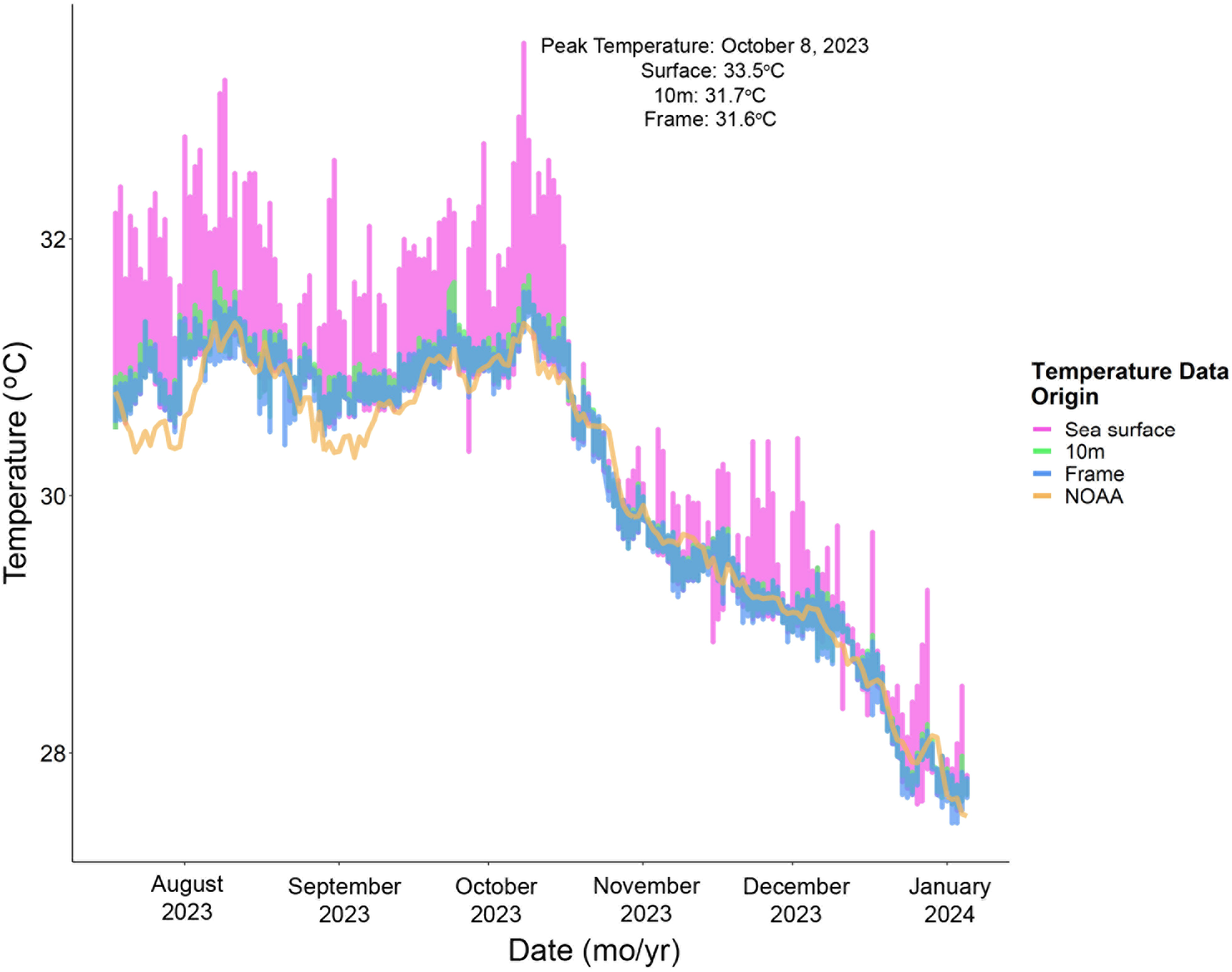
Seawater temperature data recorded from HOBO loggers at the sea surface (pink), 10m depth on the nursery mooring line (green), and 15m depth on a nursery frame (blue). The orange line depicts estimated SST from the NOAA Coral Reef Watch Virtual Stations monitoring. In situ temperature data began on July 18, 2023 and ended on January 6, 2024.
Observational health response
The first signs of bleaching were observed on August 30, 2023, when DHW values rose from 4.5 to 11.6 (Figure 3). At this time, 94.9% (394 colonies) showed signs of bleaching, including 6.9% (29) that were fully bleached, 1.7% (7) dead, and 3.4% (14) remaining healthy. By September 16, 2023, 81.9% (340 colonies) were fully bleached, 6.7% (28) partially bleached, and mortality had increased to 7.7% (32 colonies). The number of healthy colonies remained stable at 3.6% (15). DHW peaked at 19.44 on October 16, 2023. At that point 91.6% (380 colonies) were dead, 2.9% (12) were fully bleached, and 5.5% (23) were partially bleached – no healthy colonies remained. The increase in both bleached (z =-17.332, p = <0.001, GLM) and dead colonies (z =-2.205, p = 0.027, GLM) was significantly associated with rising DHW when accounting for date. As temperatures declined, some recovery was observed. By November 23, 2023, only 3 colonies remained partially bleached (0.7%), while 20 colonies (4.8%) appeared fully recovered. At the final survey on January 6, 2024, 18 colonies (4.3%) were alive and healthy.
Figure 3
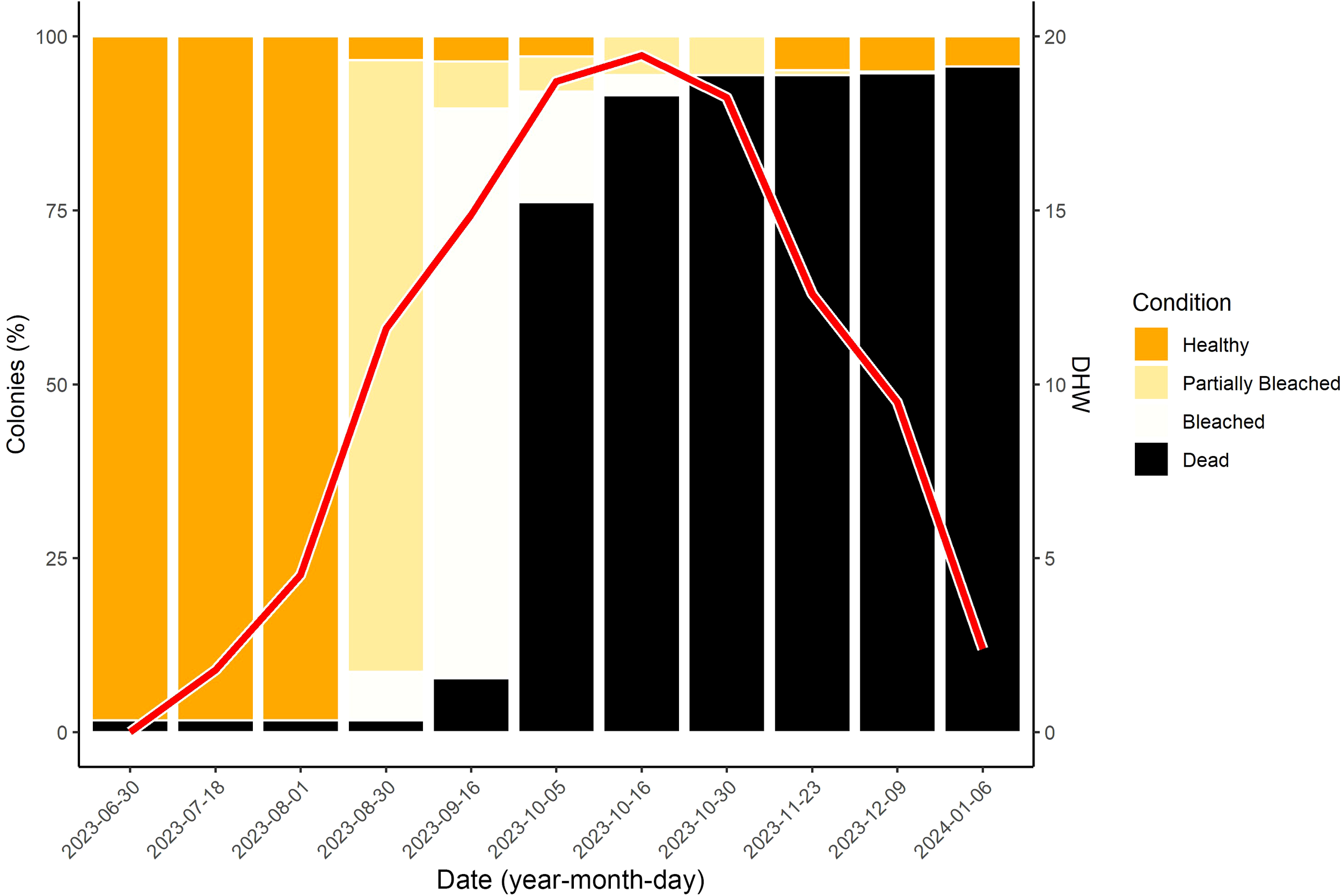
Stacked bar plot of coral conditions (Healthy, Partially Bleached, Bleached, Dead) over the sampling period. The solid red line represents estimated DHW from the NOAA Coral Reef Watch virtual stations during the sample period, with DHW values depicted on the Z-axis.
Genotypic variability
The full interactive model (date x genotype) revealed a significant effect of genotype on survival (F = 19.15, p < 0.001, GLM), with genotypes O and OB surviving significantly better than genotype G (p < 0.001 for both). The first mortalities due to bleaching occurred in genotypes G, K, OR, and Y by September 6, 2023, with 3, 11, 6, and 5 individuals lost respectively. By the next survey, additional losses occurred across nearly all genotypes except O. By October 30, 2023, all individuals of genotypes B, G, K, LG, R, S, and OR were dead. At the final survey (January 6, 2024), 18 colonies remained: 7 from genotype O (which only suffered losses before the heatwave), 7 from OB (7/15, 47%), 4 from Y (4/35, 11%) (Figure 4). A fitted Random effect model comparing the percent survival by genotype over time confirmed significant differences between OB and all the other genotypes expect O (Tukey post-hoc, Table 1, Figure 4). Genotype O differed significantly only from OR.
Figure 4
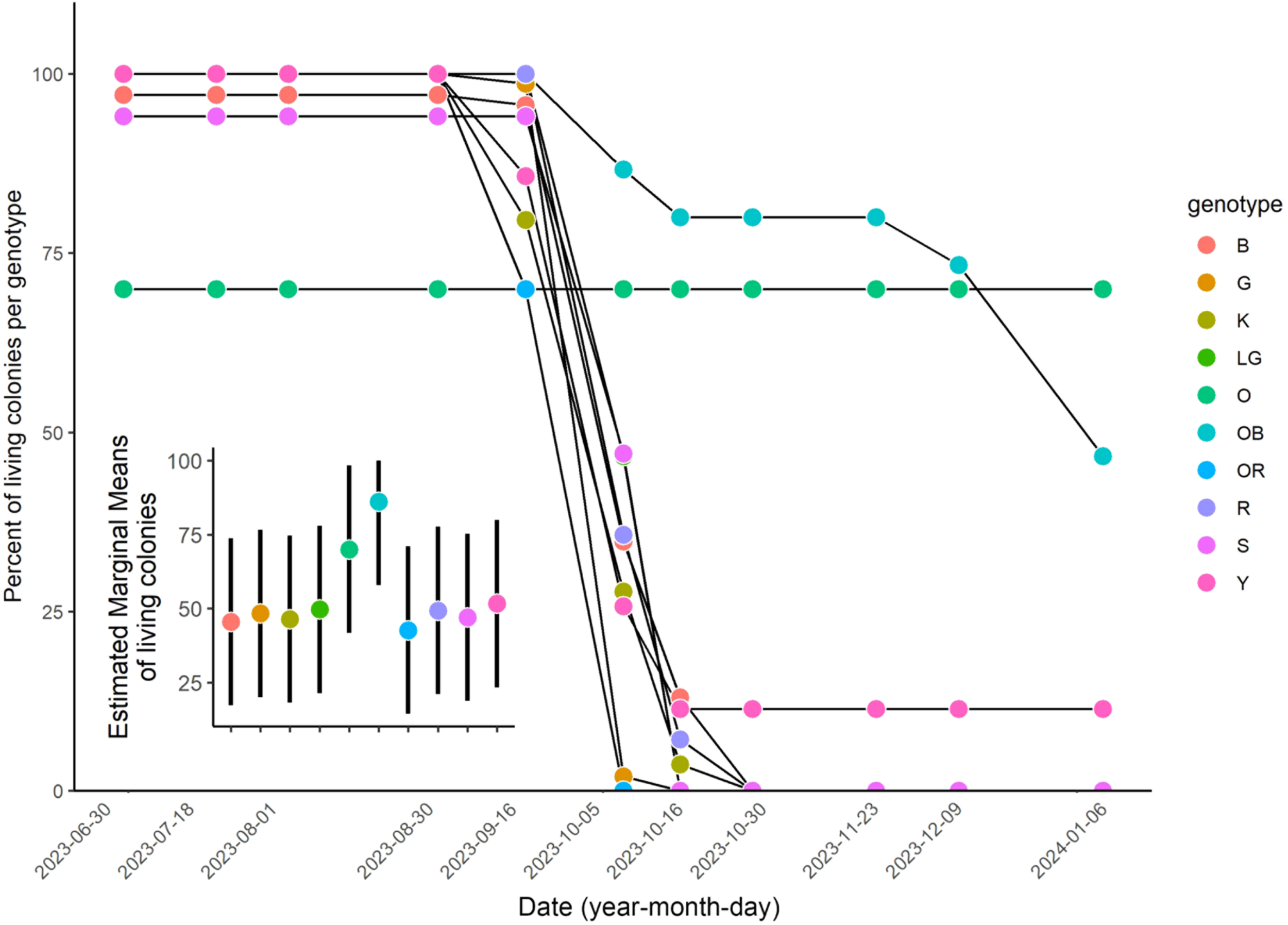
Percent survival of colonies by genotype over the monitoring period from June 30, 2023 to January 6, 2024. Inset: marginal mean survival by genotype estimated by the Generalized Linear Model (GLM).
Table 1
| Genotype | G | B | K | LG | O | OB | OR | R | S |
|---|---|---|---|---|---|---|---|---|---|
| G | |||||||||
| B | 1 | ||||||||
| K | 1 | 1 | |||||||
| LG | 1 | 1 | 1 | ||||||
| O | 0.87 | 0.1967 | 0.1161 | 0.2743 | |||||
| OB | 0.0001 | 0.0004 | 0.0002 | 0.0008 | 0.6079 | ||||
| OR | 1 | 0.9995 | 1 | 0.9972 | 0.0342 | <.0001 | |||
| R | 1 | 1 | 1 | 1 | 0.2526 | 0.0007 | 0.9981 | ||
| S | 1 | 1 | 1 | 1 | 0.1381 | 0.0002 | 0.9999 | 1 | |
| Y | 0.9989 | 1 | 0.9997 | 1 | 0.4189 | 0.002 | 0.9824 | 1 | 0.9999 |
Pairwise comparisons of the estimated marginal mean survival by genotype (Tukey post-hoc method) where light blue represent p <0.05 and purple p <0.01.
Kaplan Meier curves showed that survival probability remained relatively stable for ~100 days into the heatwave. Only genotypes OB and R exhibited any survival beyond 200 days, with no genotype exceeding a 50% survival probability. Genotype OB had a 44.4% survival probability at day 197; genotype R had 6.6% survival probability at day 157. Both genotypes were predicted to reach complete mortality by day 225 (Figure 5).
Figure 5
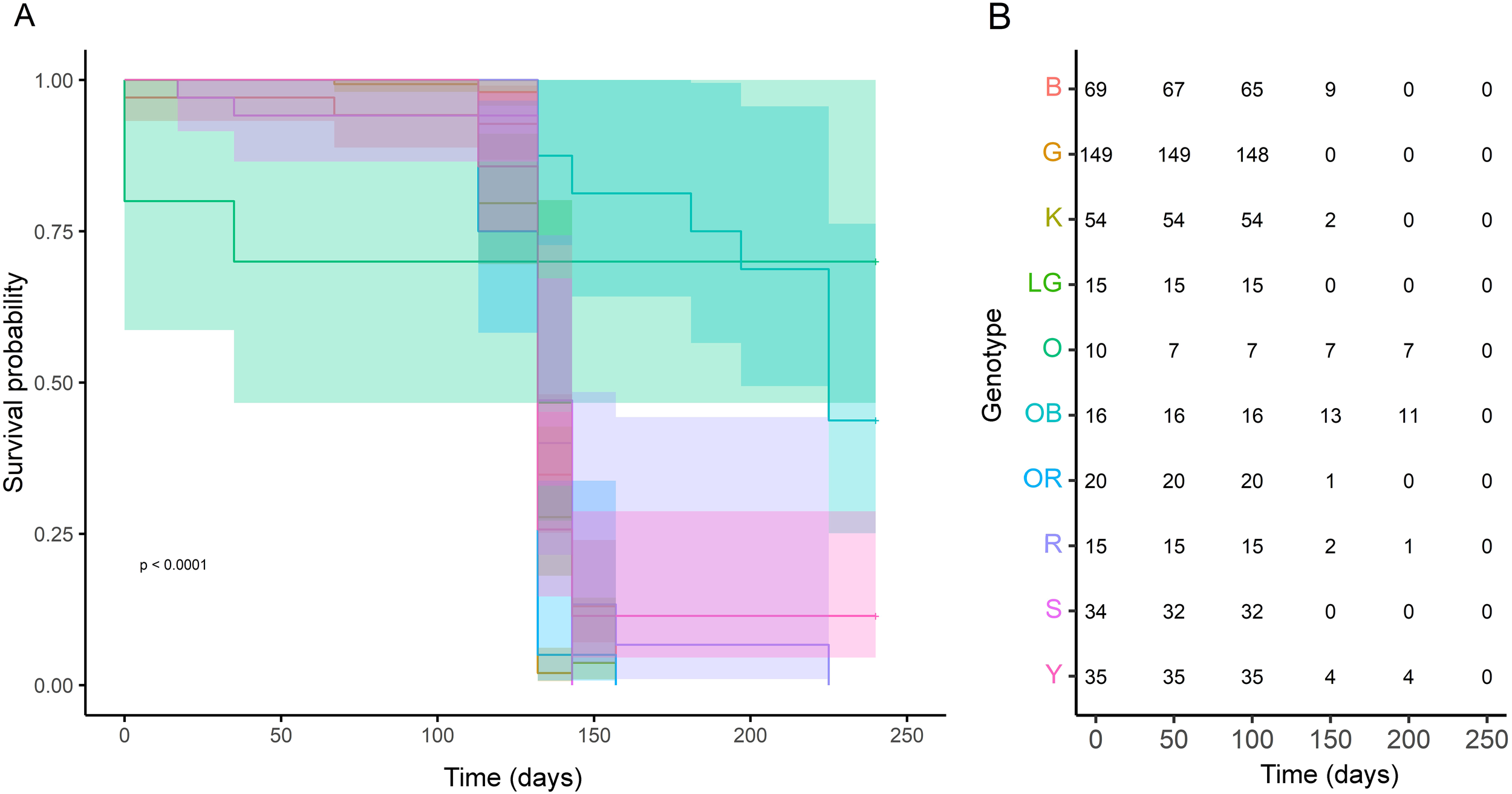
(A) Kaplan Meier survival curve by genotype over time showing significant differences in survival probability by genotype (p < 0.0001). (B) Number of colonies per genotype at risk of mortality over time estimated by the Kaplan Meier curve. Genotypes are represented by unique color identities across both panels.
Hierarchical clustering of genotypes based on survival dissimilarities revealed distinct groupings (Figure 6). Genotype OB, which suffered the lowest mortality, formed its own distinct cluster. The remaining genotypes grouped into two main clusters: one containing Y, O, R and S, and another divided into two sub-clusters of G and K, and LG and OR.
Figure 6
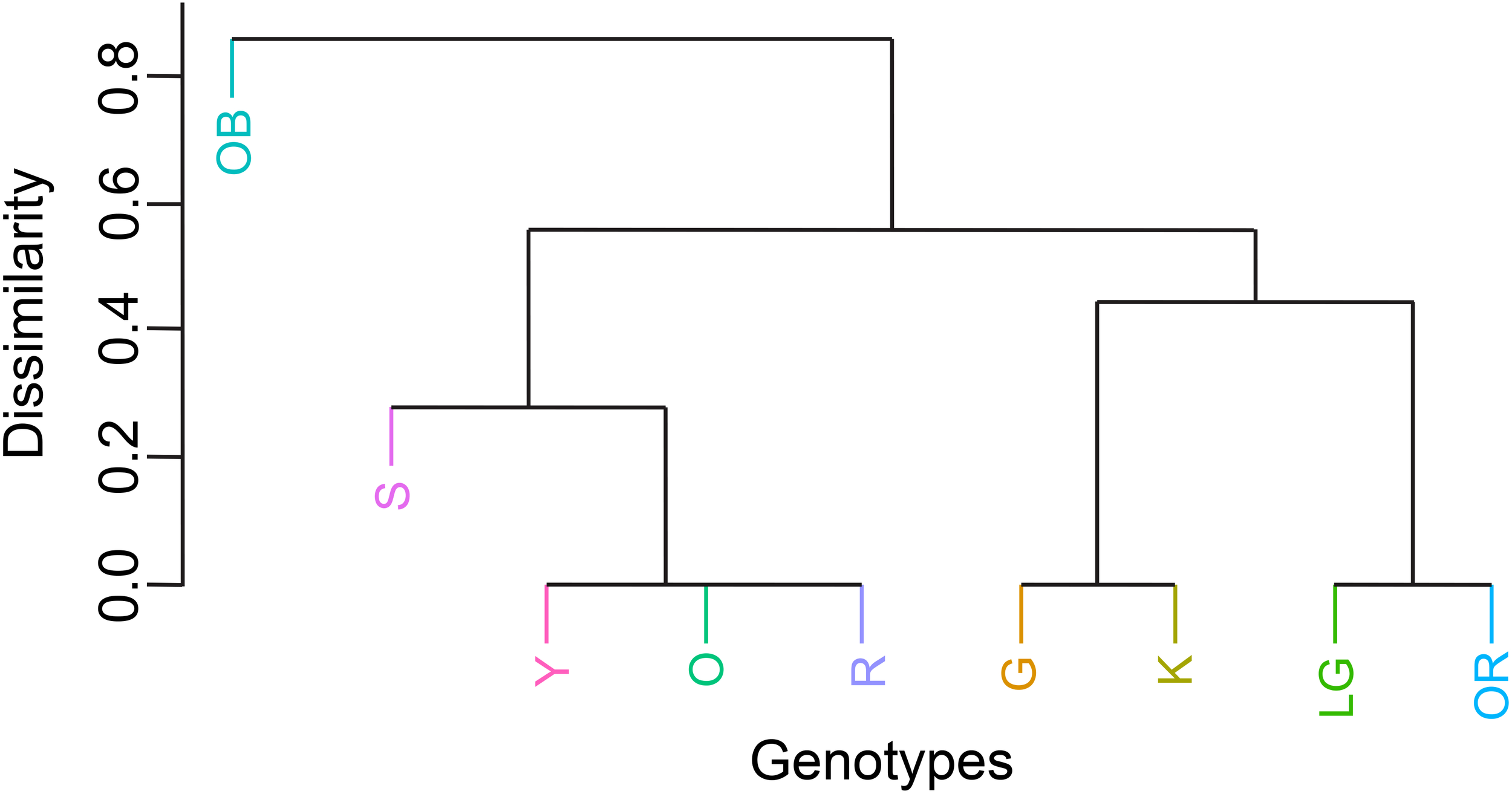
Hierarchical cluster comparing the degree of dissimilarity among genotypes, where genotypes clustering together had similar patterns of survival response to the heat wave. Genotypes on isolated and/or distant branches showed significantly different survival patterns.
Discussion
Our results document the devastating impacts of an extreme heatwave to the potential success of in situ coral propagation for restoration. All the A. cervicornis individuals in our nursery experienced bleaching at the 10 DHW point, and mass mortality occurred by 15 DHWs. At the end of our surveys, 96% of the colonies had suffered mortality after experiencing 19.4 DHWs. Out of the 10 genotypes present at the start of the heatwave, only 3 survived and only a single genotype (O) escaped any heat related casualties. Variations in density of each genotype within the nursery was representative of availability within the overall wild population, and thus these results could have even larger implications on the regional population scale. For example, genotypes B, G, and K, were the most abundant at the start of the heatwave with 69, 149, and 54 fragments in the nursery, representing 66% of the nursery corals, all of which suffered complete mortality. In contrast, genotype (O) was the least abundant at the start of the heatwave, with only 10 fragments, representing 2.4% of the nursery, yet suffered the least mortality.
Despite the dramatic bleaching response witnessed across the nursery in 2023, the variation in bleaching and survival found among genotypes suggests that a genetic basis of thermotolerance exists within this population. Specifically, genotypes O and OB proved to have higher thermal tolerance than all other genotypes, while genotypes K, OR, and Y were the first to suffer from bleaching induced mortality, indicating lower thermal tolerance. Likewise, the Kaplan-Meier curve, indicates that genotype plays a significant role in response to increasing DHWs. In a similar in situ study, Ladd et al. (2017) found significant differences in survival among A. cervicornis genotypes in a restored population after exposure to 17 weeks above 30°C in the Florida Keys. Thermal history has been shown to impact thermal tolerance in A. cervicornis, where individuals with prior exposure to heat stress exhibit higher tolerance to a subsequent stress event (DeMerlis et al., 2022; Indergard et al., 2022). However, the corals in this study had been maintained for over five years in a common-garden nursery at a narrow depth range (15–18 m) with uniform temperature conditions, suggesting that thermal history did not contribute to the observed differences in bleaching and survival among genotypes. Genotype-environment interactions are critical to consider for the success of restoration as genotypes of A. cervicornis have been found to differ in disease resistance (Vollmer and Kline, 2008; Brown et al., 2022), growth rates (Griffin et al., 2012; Drury et al., 2017), habitat production (Ladd et al., 2017), and thermal tolerance (Drury et al., 2017; Ladd et al., 2017; Yetsko et al., 2020; this study). For example, Brown et al. (2022) found differences in disease susceptibility among genotypes within the same coral nursery examined here, where genotype G was disease resistant, while genotype K was highly susceptible to disease. Thus, maintaining a high degree of genetic diversity may serve as a vital bet-hedging strategy against environmental stress and climate change-induced losses, making it essential for safeguarding coral restoration efforts. Mechanisms such as assisted evolution to cross-breed thermally tolerant individuals and inoculation with thermally tolerant Symbiodiniaceae species may further enhance coral resilience and survival under future ocean warming scenarios, improving restoration success (Baums et al., 2019; Nitschke et al., 2024; Karp et al., 2025).
As global temperatures continue to rise, so too does the frequency, intensity, and duration of coral bleaching events. The first widespread bleaching event to affect the Caribbean was documented during the 1982-83 ENSO event (Glynn, 1984; Lasker et al., 1984), with the first global bleaching event occurring in 1998 impacting coral cover across all tropical locations (Goreau et al., 2000; Glynn et al., 2001; Aronson et al., 2002; Wilkinson, 2004). A subsequent global bleaching occurred in 2010, followed just four years later by another ENSO driven event spanning 2014 – 2016 that was the most severe and widespread bleaching event ever recoded, resulting in high coral mortality on many reefs (Eakin et al., 2019; Bove et al., 2022; Cetina-Heredia and Allende-Arandía, 2023). Prior to 2023, the Cayman Islands had experienced seven thermal stress events exceeding 4 DHW, reaching 7.4 DHW in 2015 and 6.3 DHW in 2019 (NOAA Coral Reef Watch). However, none of these past thermal stress events exceeded the 8 DHW threshold where mass bleaching and mortality is expected to occur (Donner et al., 2005; Kayanne et al., 2017), emphasizing the severity of the 2023 thermal heatwave event. In the Spring of 2024 as the impacts of the El Nino event began to impact the Southern Hemisphere, the fourth Global Bleaching Event was announced and the forecast for future years suggests temperatures will continue to increase (Reimer et al., 2024). These continued increases will likely be devastating for reef corals, as locally based management strategies cannot mitigate the impacts from the global stressor of climate change (Bruno et al., 2018, 2019; Baumann et al., 2022; Johnson et al., 2022b, a; Doherty et al., 2025). Even the most thermally tolerant genotypes have critical temperature thresholds and survival limitations. Based on our survival models, the thermally tolerant genotypes in our nursery would eventually suffer bleaching induced mortality under prolonged thermal stress. Understanding what those limits are will be imperative for management and restoration decisions that aim to preserve coral reefs in the face of continued global climate change.
Conclusion
This study reveals the physiological response of more than 400 colonies of A. cervicornis as they go through a major thermal stress event. Our study shows that host genotype has a significant impact on survival in the face of extreme weather, with a limited number of genotypes (O and OB) acting as outliers with the capacity to survive prolonged heating events. As the impacts of global bleaching events continue to increase, future studies should incorporate additional physiological and genomic comparisons among representative genotypes to better understand the mechanisms driving temperature tolerance and resilience to mitigate the impacts of future heatwaves on restoration success.
Statements
Data availability statement
The datasets generated and analyzed for this study can be found in the following Zenodo repository https://zenodo.org/records/14392687.
Author contributions
LL: Methodology, Writing – review & editing, Software, Writing – original draft, Investigation, Visualization, Formal analysis, Data curation. JJ: Investigation, Visualization, Writing – original draft, Formal analysis, Writing – review & editing, Methodology, Supervision, Data curation. GG: Conceptualization, Project administration, Validation, Writing – review & editing, Supervision, Funding acquisition, Investigation, Methodology, Formal analysis, Resources, Visualization, Data curation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This project was funded by a RESEMBID grant from the European Union, the Heising-Simons Foundation, The AALL Foundation Trust and The Ernest Kleinwort Charitable Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We would like to express our gratitude to all those who contributed to this article in diving efforts, planning, prepping and as topside safety: Alex Chequer, Balthasar Lloyd von Hoyningen Huene (otherwise known as “Balty”), Caitlin Allison, Hailey Davis, Janna Leigh Randle and Matthew Louis Doherty.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Agudo-Adriani E. A. Cappelletto J. Cavada-Blanco F. Croquer A. (2016). Colony geometry and structural complexity of the endangered species Acropora cervicornis partly explains the structure of their associated fish assemblage. PeerJ4, e1861. doi: 10.7717/peerj.1861
2
Aronson R. B. Precht W. F. (2001). “White-band disease and the changing face of Caribbean coral reefs,” in The ecology and etiology of newly emerging marine diseases, edited by PorterJ. W., 25–38. Dordrecht: Springer Netherlands.
3
Aronson R. B. Precht W. F. (2006). Conservation, precaution, and Caribbean reefs. Coral Reefs25, 441–450. doi: 10.1007/s00338-006-0122-9
4
Aronson R. Precht W. Toscano M. Koltes K. (2002). The 1998 bleaching event and its aftermath on a coral reef in Belize. Mar. Biol.141, 435–447. doi: 10.1007/s00227-002-0842-5
5
Baker A. C. Starger C. J. McClanahan T. R. Glynn P. W. (2004). Corals' adaptive response to climate change. Nature430(7001), 741–741. doi: 10.1038/430741a
6
Baumann J. H. Zhao L. Z. Stier A. C. Bruno J. F. (2022). Remoteness does not enhance coral reef resilience. Global Change Biol.28, 417–428. doi: 10.1111/gcb.15904
7
Baums I. B. Baker A. C. Davies S. W. Grottoli A. G. Kenkel C. D. Kitchen S. A. et al . (2019). Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecol. Appl.29, e01978. doi: 10.1002/eap.1978
8
Bellantuono A. J. Granados-Cifuentes C. Miller D. J. Hoegh-Guldberg O. Rodriguez-Lanetty M. (2012). Coral thermal tolerance: tuning gene expression to resist thermal stress. PloS One7, e50685. doi: 10.1371/journal.pone.0050685
9
Boström-Einarsson L. Babcock R. C. Bayraktarov E. Ceccarelli D. Cook N. Ferse S. C. A. et al . (2020). Coral restoration – A systematic review of current methods, successes, failures and future directions. PloS One15, e0226631. doi: 10.1371/journal.pone.0226631
10
Bove C. B. Mudge L. Bruno J. F. (2022). A century of warming on Caribbean reefs. PloS Climate1, e0000002. doi: 10.1371/journal.pclm.0000002
11
Brown A. L. Anastasiou D.-E. Schul M. MacVittie S. Spiers L. J. Meyer J. L. et al . (2022). Mixtures of genotypes increase disease resistance in a coral nursery. Sci. Rep.12, 19286. doi: 10.1038/s41598-022-23457-6
12
Brown B. E. Downs C. A. Dunne R. P. Gibb S. W. (2002). Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Mar. Ecol. Prog. Ser.242, 119–129. doi: 10.3354/meps242119
13
Bruno J. F. Bates A. E. Cacciapaglia C. Pike E. P. Amstrup S. C. van Hooidonk R. et al . (2018). Climate change threatens the world’s marine protected areas. Nat. Climate Change8, 499–503. doi: 10.1038/s41558-018-0149-2
14
Bruno J. F. Côté I. M. Toth L. T. (2019). Climate change, coral loss, and the curious case of the parrotfish paradigm: why don’t marine protected areas improve reef resilience? Annu. Rev. Mar. Sci.11, 307–334. doi: 10.1146/annurev-marine-010318-095300
15
Cetina-Heredia P. Allende-Arandía M. E. (2023). Caribbean marine heatwaves, marine cold spells, and co-occurrence of bleaching events. J. Geophysical Research: Oceans128, e2023JC020147. doi: 10.1029/2023JC020147
16
Crabbe J. Rodríguez-Martínez R. Villamizar E. Goergen L. Croquer A. Banaszak A. (2022). “Acropora cervicornis (Lamarck 1816),” in The IUCN Red List of Threatened Species2022, e.T133381A165860142. (Accessed August 26, 2025).
17
DeCarlo T. M. Harrison H. B. Gajdzik L. Alaguarda D. Rodolfo-Metalpa R. D’Olivo J. et al . (2019). Acclimatization of massive reef-building corals to consecutive heatwaves. Proc. R. Soc. B: Biol. Sci.286, 20190235. doi: 10.1098/rspb.2019.0235
18
DeMerlis A. Kirkland A. Kaufman M. L. Mayfield A. B. Formel N. Kolodziej G. et al . (2022). Pre-exposure to a variable temperature treatment improves the response of Acropora cervicornis to acute thermal stress. Coral Reefs41, 435–445. doi: 10.1007/s00338-022-02232-z
19
Doherty M. L. Johnson J. V. Goodbody-Gringley G. (2025). Widespread coral bleaching and mass mortality during the 2023–2024 marine heatwave in Little Cayman. PloS One20, e0322636. doi: 10.1371/journal.pone.0322636
20
Donner S. D. Skirving W. J. Little C. M. Oppenheimer M. Hoegh-Guldberg O. (2005). Global assessment of coral bleaching and required rates of adaptation under climate change. Global Change Biol.11, 2251–2265. doi: 10.1111/j.1365-2486.2005.01073.x
21
Donovan M. K. Burkepile D. E. Kratochwill C. Shlesinger T. Sully S. Oliver T. A. et al . (2021). Local conditions magnify coral loss after marine heatwaves. Science372, 977–980. doi: 10.1126/science.abd9464
22
Drury C. Manzello D. Lirman D. (2017). Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis. PLoS One12, e0174000. doi: 10.1371/journal.pone.0174000
23
Eakin C. M. Sweatman H. P. A. Brainard R. E. (2019). The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs38, 539–545. doi: 10.1007/s00338-019-01844-2
24
Eirin-Lopez J. M. Putnam H. M. (2019). Marine environmental epigenetics. Annu. Rev. Mar. Sci.11, 335–368. doi: 10.1146/annurev-marine-010318-095114
25
Gardner T. A. Côté I. M. Gill J. A. Grant A. Watkinson A. R. (2003). Long-term region-wide declines in Caribbean corals. Science301, 958–960. doi: 10.1126/science.1086050
26
Gleeson M. W. Strong A. E. (1995). Applying MCSST to coral reef bleaching. Adv. Space Res.16, 151–154. doi: 10.1016/0273-1177(95)00396-V
27
Glynn P. W. (1984). Widespread coral mortality and the 1982–83 El Niño warming event. Environ. Conserv.11, 133–146. doi: 10.1017/S0376892900013825
28
Glynn P. W. Maté J. L. Baker A. C. Calderón M. O. (2001). Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niño–Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull. Mar. Sci.69, 79–109.
29
Goodbody-Gringley G. Chequer A. D. (2025). Mesophotic reefs offer thermal refuge to the 2023 Caribbean mass bleaching event in the Cayman Islands. Sci. Rep.15, 16496. doi: 10.1038/s41598-025-01813-6
30
Goreau T. J. F. Hayes R. L. (2024). 2023 Record marine heat waves: coral reef bleaching HotSpot maps reveal global sea surface temperature extremes, coral mortality, and ocean circulation changes. Oxford Open Climate Change4, kgae005. doi: 10.1093/oxfclm/kgae005
31
Goreau T. McClanahan T. Hayes R. Strong A. (2000). Conservation of coral reefs after the 1998 global bleaching event. Conserv. Biol.14, 5–15. doi: 10.1046/j.1523-1739.2000.00011.x
32
Griffin S. Spathias H. Moore T. Baums I. Griffin B. (2012). “Scaling up Acropora nurseries in the Caribbean and improving techniques,” in YellowleesD.HughesT. P. (Eds) Proceedings of the 12th International Coral Reef Symposium. (Townsville: ARC Centre of Excellence for Coral Reef Studies). 9–13.
33
Guerrero L. Bay R. (2024). Patterns of methylation and transcriptional plasticity during thermal acclimation in a reef‐building coral. Evolutionary Applications17 (7), e13757. doi: 10.1111/eva.13757
34
Hackerott S. Virdis F. Flood P. J. Souto D. G. Paez W. Eirin-Lopez J. M. (2023). Relationships between phenotypic plasticity and epigenetic variation in two Caribbean Acropora corals. Mol. Ecol.32, 4814–4828. doi: 10.1111/mec.17072
35
Hazraty-Kari S. Tavakoli-Kolour P. Kitanobo S. Nakamura T. Morita M. (2022). Adaptations by the coral Acropora tenuis confer resilience to future thermal stress. Commun. Biol.5, 1371. doi: 10.1038/s42003-022-04309-5
36
Hoegh-Guldberg O. Mumby P. J. Hooten A. J. Steneck R. S. Greenfield P. Gomez E. et al . (2007). Coral reefs under rapid climate change and ocean acidification. Science318, 1737–1742. doi: 10.1126/science.1152509
37
Hughes T. P. Kerry J. Álvarez-Noriega M. Alvarez-Romero J. G. Anderson K. D. Baird A. H. et al . (2017). Global warming and recurrent mass bleaching of corals. Nature543, 373–377. doi: 10.1038/nature21707
38
Hughes T. P. Baird A. H. Morrison T. H. Torda G. (2023). Principles for coral reef restoratin in the anthropocene. One Earth6, 656-665. doi: 10.1016/j.oneear.2023.04.008
39
R Core Team (2023). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing).
40
Indergard M. O. Bellantuono A. Rodriguez-Lanetty M. Heng F. Gilg M. R. (2022). Acclimation to elevated temperatures in Acropora cervicornis: effects of host genotype and symbiont shuffling. Mar. Ecol. Prog. Ser.701, 41–65. doi: 10.3354/meps14192
41
Jackson J. Donovan M. Cramer K. Lam V. (2014). Status and trends of Caribbean coral reefs (Gland, Switzerland: Global Coral Reef Monitoring Network, IUCN), 1970–2012.
42
Jin Y. K. Lundgren P. Lutz A. Raina J. B. Howells E. J. Paley A. S. (2016). Genetic markers for antioxidant capacity in a reef-building coral. Science Advances. 2(5), e1500842. doi: 10.1126/sciadv.1500842
43
Johnson J. V. Dick J. T. A. Pincheira-Donoso D. (2022a). Local anthropogenic stress does not exacerbate coral bleaching under global climate change. Global Ecol. Biogeography31, 1228–1236. doi: 10.1111/geb.13506
44
Johnson J. V. Dick J. T. A. Pincheira-Donoso D. (2022b). Marine protected areas do not buffer corals from bleaching under global warming. BMC Ecol. Evol.22, 58. doi: 10.1186/s12862-022-02011-y
45
Kaplan E. L. Meier P. (1958). Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc.53, 457–481. doi: 10.1080/01621459.1958.10501452
46
Karp R. F. Lepiz-Conejo F. Matsuda S. B. Corbett B. Wen A. D. Unsworth J. D. et al . (2025). Heat-tolerant algal symbionts may prevent extirpation of the threatened elkhorn coral, Acropora palmata, in Florida during intensifying marine heatwaves. Coral Reefs44, 953–965. doi: 10.1007/s00338-025-02652-7
47
Kayanne H. Suzuki R. Liu G. (2017). Bleaching in the Ryukyu Islands in 2016 and associated degree heating week threshold. Galaxea J. Coral Reef Stud.19, 17–18. doi: 10.3755/galaxea.19.1_17
48
Kenkel C. D. Matz M. V. (2016). Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nat. Ecol. Evol.1, 1–6. doi: 10.1038/s41559-016-0014
49
Kiel P. M. Formel N. Jankulak M. Baker A. C. Cunning R. Gilliam D. S. et al . (2023). Acropora cervicornis data coordination hub, an open access database for evaluating genet performance. Bull. Mar. Sci.99, 119–136. doi: 10.5343/bms.2022.0064
50
Ladd M. C. Shantz A. A. Bartels E. Burkepile D. E. (2017). Thermal stress reveals a genotype-specific tradeoff between growth and tissue loss in restored Acropora cervicornis. Mar. Ecol. Prog. Ser.572, 129–139. doi: 10.3354/meps12169
51
Lasker H. R. Peters E. C. Coffroth M. A. (1984). Bleaching of reef coelenterates in the San Blas Islands, Panama. Coral Reefs3, 183–190. doi: 10.1007/BF00288253
52
Liu G. Rauenzahn J. L. Heron S. F. Eakin C. M. Skirving W. J. Christensen T. R. L. et al . (2013). NOAA coral reef watch 50 km satellite sea surface temperature-based decision support system for coral bleaching management. NOAA Tech. Rep. NESDIS143.
53
Muller E. M. Bartels E. Baums I. B. (2018). Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis. eLife7, e35066. doi: 10.7554/eLife.35066
54
Nitschke M. R. Abrego D. Allen C. E. Alvarez-Roa C. Boulotte N. M. Buerger P. et al . (2024). The use of experimentally evolved coral photosymbionts for reef restoration. Trends Microbiol.32, 1241–1252. doi: 10.1016/j.tim.2024.05.008
55
Ogle D. H. Doll J. C. Wheeler A. P. Dinno A. (2025). FSA: Simple Fisheries Stock Assessment Methods. R package version 0.10.0. Available online at: https://fishr-core-team.github.io/FSA/.
56
Oppen M. J. H. Oliver J. K. Putnam H. M. Gates R. D. (2015). Building coral reef resilience through assisted evolution. PNAS112, 2307–2313. doi: 10.1073/pnas.1422301112
57
Pandolfi J. M. Bradbury R. H. Sala E. Hughes T. P. Bjorndal K. A. Cooke R. G. et al . (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science301, 955–958. doi: 10.1126/science.1085706
58
Quigley K. M. (2024). Breeding and selecting corals resilient to global warming. Annu. Rev. Anim. Biosci.12, 209–332. doi: 10.1146/annurev-animal-021122-093315
59
Quigley K. M. Willis B. L. Kenkel C. D. (2019). Transgenerational inheritance of shuffled symbiont communities in the coral Montipora digitata. Sci. Rep.9, 13328. doi: 10.1038/s41598-019-50045-y
60
Reimer J. D. Peixoto R. S. Davies S. W. Traylor-Knowles N. Short M. L. Cabral-Tena R. A. et al . (2024). The Fourth Global Coral Bleaching Event: Where do we go from here? Coral Reefs43, 1121–1125. doi: 10.1007/s00338-024-02504-w
61
Sawall Y. Al-Sofyani A. Hohn S. Banguera-Hinestroza E. Voolstra C. R. Wahl M. (2015). Extensive phenotypic plasticity of a Red Sea coral over a strong latitudinal temperature gradient suggests limited acclimatization potential to warming. Sci. Rep.5, 8940. doi: 10.1038/srep08940
62
Suggett D. J. Smith D. J. (2011). Interpreting the sign of coral bleaching as friend vs. foe. Global Change Biol.17, 45–55. doi: 10.1111/j.1365-2486.2009.02155.x
63
Suggett D. J. Smith D. J. (2020). Coral bleaching patterns are the outcome of complex biological and environmental networking. Global Change Biol.26, 68–79. doi: 10.1111/gcb.14871
64
Therneau T. (2024). A Package for Survival Analysis in R. R package version 3.8-3. Available online at: https://CRAN.R-project.org/package=survival.
65
Vollmer S. V. Kline D. I. (2008). Natural disease resistance in threatened staghorn corals. PloS One3, e3718. doi: 10.1371/journal.pone.0003718
66
Wilkinson C. C. (2004). Status of Coral Reefs of the World. Global Coral Reef Monitoring Network and Australian Institute of Marine Science, Townsville, Australia.
67
Yetsko K. Ross M. Bellantuono A. Merselis D. Rodriguez-Lanetty M. Gilg M. R. (2020). Genetic differences in thermal tolerance among colonies of threatened coral Acropora cervicornis: potential for adaptation to increasing temperature. Mar. Ecol. Prog. Ser.646, 45–68. doi: 10.3354/meps13407
Summary
Keywords
coral bleaching, survival, restoration, climate change, global warming, thermal tolerance, Caribbean, Little Cayman
Citation
Le Gall L, Johnson JV and Goodbody-Gringley G (2025) Extirpation of Acropora cervicornis genotypes from a coral nursery during the 2023 marine heatwave undermines conservation efforts. Front. Mar. Sci. 12:1599155. doi: 10.3389/fmars.2025.1599155
Received
24 March 2025
Accepted
20 August 2025
Published
08 September 2025
Volume
12 - 2025
Edited by
Jesús Ernesto Arias González, National Polytechnic Institute of Mexico (CINVESTAV), Mexico
Reviewed by
Esti Kramarsky-Winter, Ben-Gurion University of the Negev, Israel
Viridiana Alvarado, Center for Research and Advanced Studies - Mérida Unit, Mexico
Updates
Copyright
© 2025 Le Gall, Johnson and Goodbody-Gringley.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gretchen Goodbody-Gringley, ggoodbody@reefresearch.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.