- 1Aquaculture Division, National Institute of Oceanography and Fisheries (NIOF), Alexandria, Egypt
- 2Fish Production Department, Faculty of Agriculture, Al-Azhar University, Cairo, Egypt
- 3Department of Animal Production, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 4Animal and Fish Production Department, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
- 5Department of Integrative Agriculture, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Abu Dhabi, United Arab Emirates
This work evaluated the effects of dietary supplementation with the seaweed, Pterocladia capillacea, nanoparticles (SN) on the growth, whole-body composition, digestive enzyme activities, feed efficiency, immunological response, antioxidant activity, and gene expression of the whiteleg shrimp, Litopenaeus vannamei. The SN form was conducted using a Planetary Ball Mill PM 400. The particle size of the SN was verified through Dynamic Light Scattering (DLS) analysis. The DLS showed that the mean particle sizes of SN were between 151 nm (13.6%) and 835 nm (64%). Throughout 60 day experimental trial, postlarvae (PLs) of L. vannamei were subjected to one of the following five feeding groups. The first group is a commercially available shrimp feed as a basal diet without any seaweed supplementation, functioning as a negative control (C0%). A second group received the commercial feed supplemented with 2% (20 g/kg) dried seaweed powder (SP2%), functioning as a positive control. The remaining three shrimp groups were fed diets supplemented with seaweed nanoparticles (SN) at concentrations of 0.5% (SN0.5%), 1% (SN1%), and 2% (SN2%), respectively. 750 postlarval (0.053 g/PL) were allocated to five experimental diet groups. Each group consisted of 150 PLs (triplicate). The PLs were fed their corresponding regime three times a day at 10% of their body weight. The results revealed that, compared with those of the positive (C0%) or negative (SP2%) controls, with the increasing dietary supplementation levels of SN, especially SN2%, the growth (FW, WG, and SGR), digestive enzymes (amylase and lipase activities), carcass composition (protein and lipid contents), nutrient efficiency (FI, PI, FER, FCR, and PER), antioxidant activities (SOD and CAT), innate immunity activities (LYS and MDA), and related-immunity gene expressions (p53) of L. vannamei were significantly improved. In conclusion, these findings concluded that applying nanotechnology tools enhances feed additives and significantly maximizes the positive effects of these additives on L. vannamei growth, health, and overall production. Further research is required to understand and explain how seaweed nanoparticles affect these shrimp’s physiological state and upgrade some immune-related gene expressions.
1 Introduction
The aquaculture industry now seems considerably increasing due to the human population’s expansion and increased emphasis on healthy nutrition (Abbas et al., 2023). Farmed seafood is now a vital key diets proposed to boost human wellness (Kwasek et al., 2020). Nowadays, marine shrimp farming has a dramatic rise and is considered a key element in feeding developing nations (Mansour et al., 2022a). Even so, several problems still face aquaculture’s progress. These obstacles include a rise diet prices, diet formulation, low diet efficiency, low water quality efficiency, low survivability, lack of supply of diet ingredients, climate change, harmful environmental impact, farmer practices, increases in water pollution, and maintaining good water quality (Magouz et al., 2021b, a; N’Souvi et al., 2024; Widiasa et al., 2023; Suzuki and Nam, 2023). Among all these obstacles, formulating better feeds with high diet efficiency promises to positively influence shrimp’s development, robustness, and efficient production (Mansour et al., 2022c; Ashour et al., 2024b, a; Ahmed et al., 2019).
Shrimp consumption has risen significantly all over the world. Among all farmed shrimp species worldwide, more than 70% are represented by Litopenaeus vannamei species (Pedrazzani et al., 2023; Chen et al., 2024). To meet the rising global demand for shrimp, several strategies have been developed to improve shrimp diets and nutrition (Emerenciano et al., 2022; Khanjani et al., 2022). The inclusion of natural feed additives into shrimp diets is a one of the most effective and widely strategy can improve and develop the shrimp aquaculture production (Marimuthu et al., 2022). Diet feed additives play a key role in enhancing shrimp’s growth, immunity, antioxidant and gene expression (Ceseña et al., 2021; Mustafa and Al-Faragi, 2021; Ashour et al., 2024a).
Algae (microalgae and seaweed) are extensively utilized in several industries. These applications include algae used as aquafeed additives (Abbas et al., 2023), dietary supplements for human nutrition (Hamid et al., 2025), plant growth enhancers (Mansour et al., 2022b), pharmaceutical ingredients (Leung, 2025), antimicrobial agents (Osman et al., 2010, 2020), bioenergy sources (Elshobary et al., 2021), components in cosmetic products (Kaur and Khattar, 2025), and sustainable tools for phytoremediation (Al-Saeedi et al., 2023).
Among the different families of seaweed, red seaweeds family (Rhodophyceae) stand out due to their rich composition of bioactive compounds. These include polysaccharides, vitamins, alkaloids, flavonoids, phenolic compounds, fatty acid methyl esters, hydrocarbons (Sanjeewa et al., 2018). These biomaterials showed protective effects on aquatic organisms by offering a range of beneficial activities including growth stimulation benefits, anti-inflammatory effects, immune system enhancement, antimicrobial effects, and antioxidant properties (Yazici et al., 2024). In a pioneer study conducted by Zhang et al. (2023), the influence of nine different macroalgae as aquafeed additives on various physiological indicators of L. vannamei over a 28-day feeding trial has been investigated. These macroalgae belonged to three microalgae groups: brown macroalgae (one speices: Sargassum ilicifolium), red seaweeds (three species: Betaphycus gelatinae Acanthophora spicifera, and Gracilaria bailiniae), and green seaweeds (five species: Caulerpa linum, Caulerpa sertularioides Caulerpa lentillifera, Ulva lactuca, and Caulerpa racemosa). Among all the tested species, the red macroalgae A. spicifera showed the most significant enhancement in immune responses, antioxidant potential, and gene expression linked to antioxidation in L. vannamei (Zhang et al., 2023). However, the efficacy of algae feed additives for shrimp and fish is significantly influenced by several factors like the concentration and the form of addition in which they are administered. Thus, it generates recognition that adding appropriate concentration and the form of addition would improve growth rates and feed efficiency while also increasing aquatic animals’ immune systems and significantly boosting their resistance to disease (Sharawy et al., 2022).
In the nanotechnology process, materials are manipulated at the nanoscale, usually ranging from 1 to 100 nanometers (Ashour et al., 2023). By reducing bigger chemical compounds to tiny particles, nanotechnology methodology gives these biomaterials new physicochemical characteristics (Mansour et al., 2022a). Currently, in aquaculture, there’s growing interest in supplementing diets with nanoparticles of macroalgae over traditional additives (powder-particle form, liquid-extract form, or powder-extract form). Although nanotechnology is recently involved in various branches within aquaculture, the use of macroalgae-derived nanoparticles as a supplement in aquaculture feeds remains an underexplored area.
Among red seaweeds species, Pterocladiella capillacea is recognized for its rich content of polysaccharides and a variety of bioactive compounds (Ismail et al., 2023), including phenolics, fatty acids, flavonoids, vitamins, and alkaloids. These constituents contribute to a wide range of beneficial biological activities, such as antioxidant, antimicrobial, anti-inflammatory, immune-enhancing, and growth-promoting effects, which can help protect aquatic organisms from various harmful conditions (Kannan et al., 2024).
This study investigates how supplementing the diet of whiteleg shrimp L. vannamei postlarvae with red macroalgae (Pterocladiella capillacea) nanoparticles influences the shrimp’s growth, innate immunity, antioxidant activities, digestive enzyme activities, nutrient efficiency, and related-immunity gene expressions.
2 Materials and methods
2.1 Seaweeds
2.1.1 Preparation
The Rhodophyceae species, Pterocladiella capillacea, was harvested from the Alexandria coast of Egypt. After collection, the samples were cleaned and air-dried. The dried samples were fine powder grounded and then stored in dark plastic bags at room temperature for further application (Abbas et al., 2023). Subsequently, the seaweed powder form (SP) was prepared to synthesize seaweed nanoparticles (SN) form. This process was conducted at the Egyptian Petroleum Research Institute (EPRI) using a Planetary Ball Mill PM 400, equipped with four grinding stations. The particle size of the SN was verified through Dynamic Light Scattering (DLS) analysis using a Zetasizer Nano Series HT, Nano-25, and Malvern instruments. The DLS showed that the mean particle sizes of SN were between 151 nm (13.6%) and 835 nm (64%) (Supplementary Figure S1A).
2.1.2 Characterization
Based on the work conducted by Ashour et al. (2020), the carcass whole-body composition of SP of carbohydrate, protein, lipid, and ash was reported as presented in Supplementary Table S1. Moreover, SP showed significant content of fatty acids (polyunsaturated fatty acids, monounsaturated fatty acids, and saturated fatty acids) and amino acids (essential and nonessential amino acids). Furthermore, the SP water extract showed a significant amount of phenolic content, carotene content, total antioxidant, flavonoid content, and, as presented in Supplementary Table S1 (Ashour et al., 2020). Based on our previous study, several characterizations of SN were performed as reported by Mansour et al. (2022b). The Scanning Electron Microscopy (SEM) analysis reflected the morphological aspects of SN (Supplementary Figure S1B). The Fourier transform infrared spectroscopy (FTIR) analysis identified several types and numbers of functional groups on the surface of SP, like biomolecules, proteins, polysaccharides, and aromatic compounds, which have been identified to contain functional groups such as hydroxyl, amino, aldehydes, ketones, carboxyl, carboxylates, carbonyl, phosphoryl, esters, and sulfide (Supplementary Figure S1C).
To further validate the bioactive compound and give more insights into the composition of the SN, spectroscopic analysis was conducted on the SN crude extract of SN using ultraviolet (UV) spectra analysis. The UV-visible analysis of SN confirmed that the SN contains several glycosides, alkaloids, pigments, and flavonoid compounds when scanned spectroscopically in the wavelength ranging from 200 nm to 800 nm (Supplementary Figure S1D). Moreover, the Brunauer Emmett Teller (BET) analysis demonstrated accurate measurement of the specific surface area of SN (128 m2/g), besides other related measurements as presented in Supplementary Table S2 and Supplementary Figure S2.
2.2 Experimental diets preparation
Throughout 60 day experimental trial, postlarvae (PLs) of L. vannamei were subjected to one of the following five feeding groups. The first group is a commercially available shrimp feed (Aller-Aqua, Egypt; the composition is detailed in Table 1) as a basal diet without any seaweed supplementation, functioning as a negative control (C0%). A second group received the commercial feed supplemented with 2% (20 g/kg) dried seaweed powder (SP2%), functioning as a positive control. The remaining three shrimp groups were fed diets supplemented with seaweed nanoparticles (SN) at concentrations of 0.5% (SN0.5%), 1% (SN1%), and 2% (SN2%), respectively.
The addition of nanoparticle additives into the feed diet was adapted from the procedure reported by (Mabrouk et al., 2022). Briefly, the diet was initially ground into a fine powder, and the designated quantities, either seaweed powder (SP) or seaweed nanoparticles (SN), were carefully incorporated separately into the targeted powdered diets, ensuring that it was completely uniform. The appropriate amount of SP and SN was dissolved in a volume of distilled water (DW) and then sprayed equally onto the surface of the corresponding feed batch. The negative control (C0%) diet had an equivalent spray of DW without any seaweed supplement. Sunflower oil (5 mL/kg diet) was sprayed onto all diets after drying (48 hours/40°C) to achieve a final moisture level of approximately 10%. Finally, the prepared feed pellets were then stored (4°C) until use.
2.3 Experimental procedures
Following a 15-day acclimation period, 750 PLs (0.053 ± 0.002 g per PL), were allocated to five groups. Each group consisted of 150 PLs (triplicate). Along the experiment, each group was housed within a concrete pond with a dimension of 4 m × 2 m × 1 m and subdivided into three net enclosures (hapas), with a dimension of 0.7 m × 0.7 m × 1 m, each containing 50 PLs. Over the subsequent 60 days, the PLs were cultivated while maintaining optimal water quality conditions of salinity, pH, ammonia (NH3), temperature, nitrite (NO2), and nitrate (NO3) levels. Salinity, temperature, and pH were measured daily around midday. Ammonia, nitrite, and nitrate concentrations were weekly assessed. These water quality parameters were assessed following the APHA (2005) standard procedures. In all experimental groups, around 10% of the total water volume was exchanged daily with fresh and clean water. During this study, the measured values of water quality indices indicated that temperature (26.0 ± 1.5°C), salinity (27.5 ± 2 ppt), pH (7.6 ± 0.3), ammonia (0.08 ± 0.04 mg/L), nitrate (0.16 ± 0.04 mg/L), and nitrite (0.08 ± 0.02 mg/L) remained within the acceptable ranges for shrimp farming, as reported by Venkateswarlu et al. (2019)_ENREF_5.
2.4 Shrimp
2.4.1 Procedures
The L. vannamei PLs15 were transported from a private shrimp hatchery located in Kafr El-Sheikh city, Egypt, to the Fish Research Station located in Baltim, Alexandria Branch of NIOF, Egypt. The PLs15 were housed in concrete ponds measuring 1 m × 5 m × 5 m, for a 15-day acclimation period to adjust to the experimental culture conditions. The water parameters during the acclimation period included dissolved oxygen levels above 5 mg/L, a temperature of 27 ± 1°C, and salinity of 26.5 ± 1.0 ppt. To maintain water quality, approximately 10% of the total water volume in the concrete culture pond was replaced daily with fresh and clean water. During acclimatization, the shrimp were fed a control basal diet containing 40.45% protein four times daily until full satiation.
2.4.2 Growth and feed efficiency indicators
To evaluate the effectiveness of the feeding regimen on shrimp survival rate, growth performance, and feed efficiency indicators, the following formulas Equations 1–6 were applied:
2.4.3 Biochemical analysis
For biochemical analysis, at the end of the trial, 15 shrimp were randomly sampled from each group (5 individuals/replicate). The selected shrimp were euthanized, homogenized, dried, ground into powder, and stored at –20°C for later application. The dry matter, crude protein, crude lipid, and ash content of the samples were analyzed according to the AOAC (2003) protocol.
2.4.4 Digestive enzyme activities
Following a 24-hour fasting after the experiment finished, 15 individuals were randomly selected from each group (five individuals per replicate) to analyze the activities of digestive enzymes, including amylase and lipase. These enzymes were measured in homogenized digestive gland tissue using colorimetric assay kits following the manufacturer’s protocol. Shrimp individuals were euthanized by cryoanesthesia, as described by (Becker et al., 2024). Shrimp muscle tissue and internal organs were carefully dissected, weighed, and homogenized in phosphate buffer solution (PBS) with a pH of 7.4. The homogenized samples were then centrifuged for 20 minutes at 3500 rpm, then the supernatants were collected and stored at -20°C until analysis. Enzyme activity was assessed using specific kits for lipase and amylase provided by Biodiagnostic Company, Egypt (catalog Numbers: 281001 and AY1050 respectively). Following the protocols of Moss et al. (1999) and Caraway (1959), the absorbance for lipase activity was determined at 580 nm, while amylase was measured at wavelengths of 660 nm.
2.4.5 Antioxidant enzymes activities
Following a 24-hour fasting after the experiment finished, 15 individuals were randomly selected from each group (five individuals per replicate) to analyze the antioxidants enzymes activities. Shrimp whole body samples were dissected, weighed, homogenized, after adding PBS (pH 7.4), centrifuge (20 min, 2,000–3,000 rpm), and the supernatant was carefully collected and stored at – 20 °C until use. The antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), and lipid peroxidation (malondialdehyde, MDA) were quantified using the spectrophotometry colorimetric method. Specifically, the CAT, SOD, and MDA activities were assessed using commercial kits obtained from Biodiagnostic Company, Egypt. Following the method described previously by (Nishikimi et al., 1972), the CAT activity (Cat Nos. CA2517) was determined at wavelengths of 560 nm. SOD activity (Cat Nos. SD2521) was determined at wavelengths of 510 nm according to (Aebi, 1984). MDA activity (Cat No. 2529) was determined at wavelengths of 534 nm following the protocol described by Ohkawa et al. (1979).
2.4.6 Innate immunity activities
Following a 24-hour fasting after the experiment finished, 15 individuals were randomly selected from each group (five individuals per replicate) to evaluate lysozyme (LYS) activity. Shrimp whole body samples were dissected, weighed, homogenized, after adding PBS (pH 7.4), centrifuge (20 min, 2,000–3,000 rpm), and the supernatant was carefully collected. LYS activity was quantified using an enzyme-linked immunosorbent assay (ELISA) kit (Cat No.: SL0050FI; SunLong Biotech Co., Ltd., China). The assay principle relies on the enzymatic degradation of Micrococcus lysodeikticus cell walls by lysozyme. The LYS activity was measured spectrophotometrically as a decrease in absorbance at 450 nm, according to the manufacturer’s protocol conducted by (Harshbarger et al., 1992).
2.4.7 Immunity-related gene expressions
When the trial ended, for each treatment group, three separate shrimp samples (5 whole-body shrimp/replicate) were applied to prepare the RNA analysis. Initially, samples were subjected to two washes using a phosphate-buffered saline solution (PBS; composition of 1.46 mM KH2PO4, 2.7 mM KCl, 8 mM Na2HPO4, and 137 mM NaCl, pH 7.4). Subsequently, the samples were stabilized for RNA integrity by immersion in RNA later® reagent (Sigma-Aldrich®) at a 1:5 volume ratio and stored at a temperature of -20°C Goncalves et al. (2014). The extraction of total RNA and the subsequent quantitative real-time polymerase chain reaction (qRT-PCR) analysis were conducted following the protocols published by Aguilera-Rivera et al. (2019). Subsequently, for immunity-related gene expressions, the gene-specific primers were detailed in Supplementary Table S3. Using β‐actin as a housekeeping gene (Wang et al., 2007), four immunity-related genes of peroxiredoxin, Prx (Liu et al., 2020), prophenoloxidase, PPO1 (Wang et al., 2007), p53-like protein isoform delta, p53 (Nuñez-Hernandez et al., 2021), and hemocyanin subunit L5, L5H (Wang et al., 2007) were investigated. The prepared sequences underwent quantitative real-time PCR (qRT-PCR) analysis, utilizing a Bio-Rad® iQ5 fluorometric thermocycler. All primers (β‐actin, PPO1, Prx, p53, L5H) were designed based on conserved sequences presented at GenBank, using Primer 5.0 software. Relative gene expression levels were quantified using the 2−ΔΔCt method (Livak and Schmittgen, 2001). All qPCR reactions, inclusive of cDNA synthesis, were conducted in triplicate. To minimize data dispersion and promote a normal distribution, the qPCR data were transformed using a log2 function. Furthermore, the PCR amplification efficiency for each sample was performed using LinRegPCR software (Ramaker et al., 2003).
2.5 Statistical analysis
Earlier, before starting the statistical evaluations, the test of Levene’s was applied to verify the data of normality as well as the assumption of homogeneity. Percentage values were transformed using an arc-sine function (Zar, 1984). The data are expressed as the mean followed by its respective standard deviation (SD). Statistical evaluations were made using SPSS Statistics Software (16.00), using one-way ANOVA, and then followed by Duncan’s (Duncan, 1955) multiple range test under a p < 0.05. All figures were generated using GraphPad Prism 8 software (Swift, 1997). Correlation heatmap with cluster analysis was performed using Julius software.
3 Results
3.1 Water Quality, Growth and Feed Utilization Efficiency
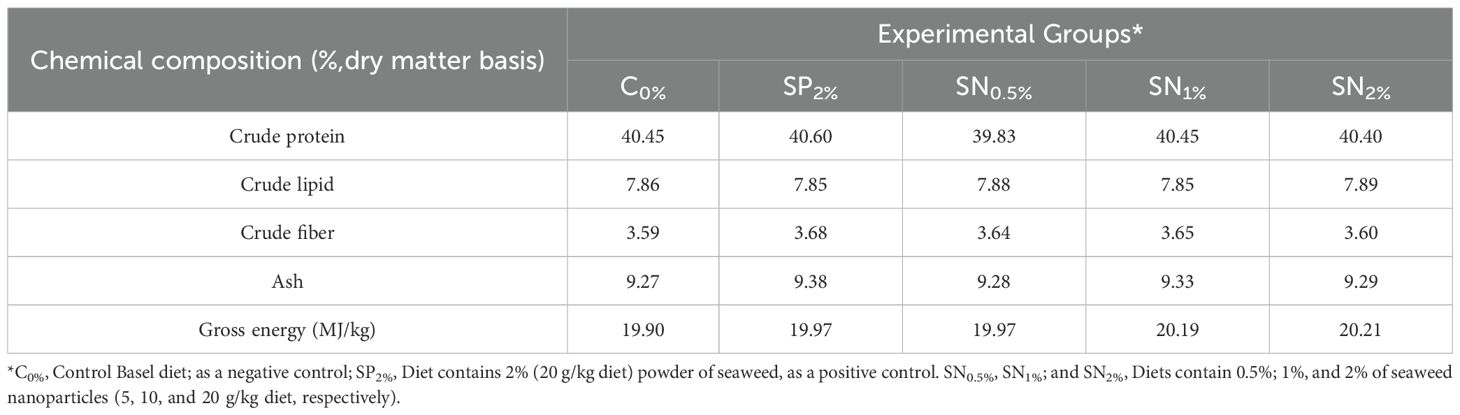
There were no significant differences in water quality parameters between negative control group (C0%), positive control group (SP2%), and seaweed nanoparticle groups (SN0.5%, SN1%, and SN2%). Table 2 presents the survival, nutrient utilization, and growth indicators of shrimp reared in the experimental groups. No significant (p < 0.05) differences were observed in SR among all groups. The data indicated that shrimp reared on diets containing seaweed, whether in powder form (SP) or nanoparticle form (SN), exhibited significantly (p < 0.05) greater growth indices (SGR, WG, and FW) and nutrient efficiency (FI, PI, FER, FCR, and PER) comparing to the control group (C0%). Additionally, increasing the level of nanoparticles tended to enhance the improvement in the measured values (Table 2).
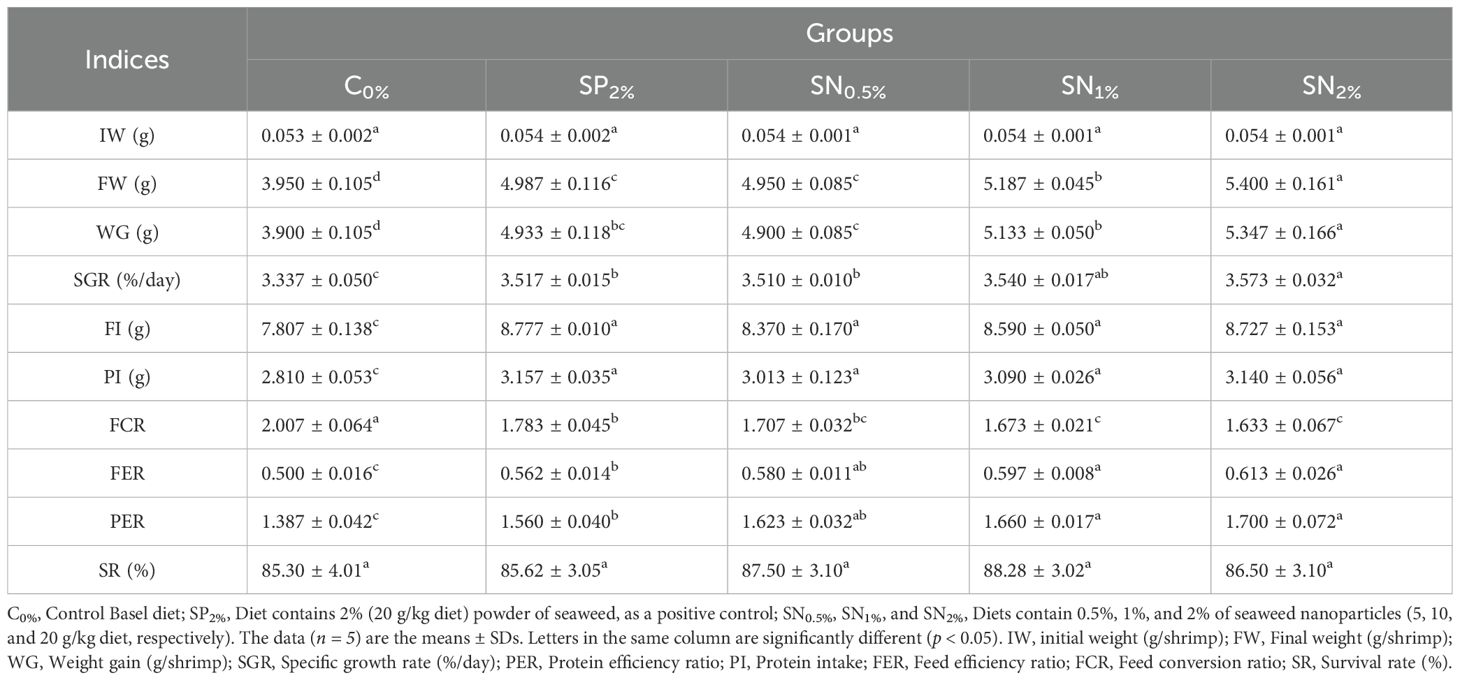
Table 2. Growth and nutrient efficiency indicators of shrimp reared on the experimental dietary supplementation.
Figure 1 shows the polynomial regression chart between WG and FCR. The data presented in Figure 1 concludes that with the increase of SN dietary supplementation level, the polynomial regression of FCR was decreased (r² = 0.9725) and the polynomial regression of WG was increased (r² = 0.8974). From the findings presented in Figure 1, as determined by the polynomial regression model as a machine learning mode, it could be concluded that the optimal range for SN supplementation is found to be between 1.2% and 1.6% (12–16 g/kg diet). This range corresponds to the SN2% treatment group, where WG reached its highest peak and FCR achieved its lowest value.

Figure 1. Polynomial regression chart between feed conversion rate and weight gain of shrimp reared on the experimental dietary supplementations. C0%, Control Basel diet; SP2%, Diet contains 2% (20 g/kg diet) powder of seaweed, as a positive control. SN0.5%, SN1%, and SN2%, Diets contain 0.5%, 1%, and 2% of seaweed nanoparticles (5, 10, and 20 g/kg diet, respectively). The data (n=5) were presented as means (± SD).
3.2 Body carcass compositions
Table 3 reports the body carcass of shrimp reared on experimental diets. Table 3 indicated that shrimp reared on diets containing SN form (SN0.5%, SN1%, and SN2%) exhibited significant (p < 0.05) enhancement in lipid and protein percentages, followed by diets containing SP2%, compared to the control diet (C0%). Shrimp reared in group SN2% showed the greatest significance protein (56.35%), while shrimp reared in group SN1% showed the greatest significance lipid (5.14%). The highest ash (12.67%) and dry matter (29.73%) content was found in the control group (C0%), compared to either the SP or SN groups, as presented in Table 3.

Table 3. Body carcass composition (%) of shrimp reared on the experimental dietary supplementation .
3.3 Digestive enzyme activities
Figure 2 shows the impact of experimental dietary supplementations on shrimp digestive enzyme activities of amylase (Figure 2A) and lipase (Figure 2B).

Figure 2. The activities of digestive enzyme levels of amylase (A) and lipase (B) of shrimp reared on the experimental dietary supplementation. C0%, Control Basel diet; SP2%, Diet contains 2% (20 g/kg diet) powder of seaweed, as a positive control; SN0.5%, SN1%, and SN2%, Diets contain 0.5%, 1%, and 2% of seaweed nanoparticles (5, 10, and 20 g/kg diet, respectively). The data (n=5) were presented as means (± SD), and the capital letters (A > B > C > D) mean significance values at p < 0.05.
From the presented data it could be concluded that the addition of SN to the shrimp diet significantly (p < 0.05) improved the shrimp’s amylase and lipase activities higher than SP and the control diet. Compared to C0% and SP2%, all SN groups (SN0.5%, SN1%, and SN2%) exhibited a higher significant value of amylase (Figure 2A), while SN2% showed a higher significant value of lipase (Figure 2B).
3.4 Antioxidant enzymes activities
Figure 3 exhibits the effect of experimental dietary supplementations on shrimp’s SOD level (Figure 3A), CAT level (Figure 3B), and MDA level (Figure 3C). The addition of SN to shrimp diets significantly (p < 0.05) enhanced the shrimp’s SOD and CAT activities higher than SP and C0%. Shrimp reared in the SN2% group exhibited a higher significant (p < 0.05) value of SOD activity, followed by SN1%, SN0.5%, and SP2%, while the least significant of SOD value was reported in C0% group (Figure 3A). On the other hand, shrimp reared in SN2% and SN1%, groups showed highest values (p < 0.05) of CAT, followed by SN0.5% and SP2%, while the least significant (p < 0.05) CAT value has reported in C0% group (Figure 3B). On the other hand, shrimp reared in either SP or SN groups exhibit significantly (p < 0.05) lower values of MDA, noting that the lowest value was recorded in shrimp treated with the SN2% (Figure 3C).
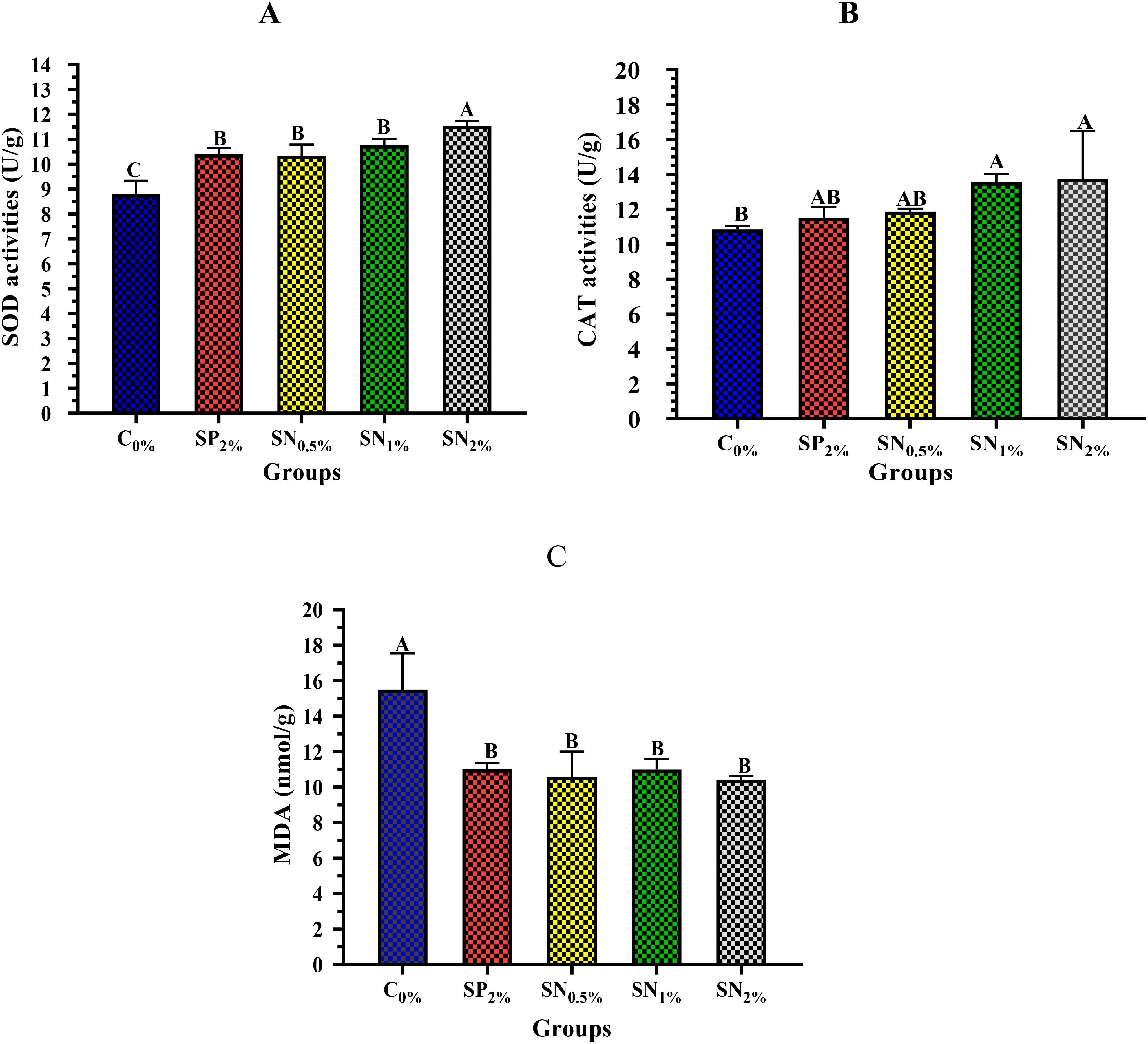
Figure 3. Antioxidant enzyme activities of SOD (A), CAT (B), and MDA (C) of shrimp reared on the experimental dietary supplementations. SOD, Serum superoxide dismutase (IU/g); CAT, Catalase (IU/g); MDA, Malondialdehyde (nmol/g); C0%, Control Basel diet; SP2%, Diet contains 2% (20 g/kg diet) powder of seaweed, as a positive control; SN0.5%, SN1%, and SN2%, Diets contain 0.5%, 1%, and 2% of seaweed nanoparticles (5, 10, and 20 g/kg diet, respectively). The data (n=5) were presented as means (± SD), and the capital letters (A > B > C > D) mean significance values at p <0.05.
3.5 Innate immunity activities
Figure 4 reflects the impact of experimental dietary supplementations on shrimp innate immunity enzyme activities of LYS (Figure 4). The groups containing SN (SN0.5%, SN1%, SN2%) significantly enhanced the shrimp’s LYS activity higher than the SP and C0% groups, noting that the highest value was reported in shrimp reared in the SN2% group (Figure 4).
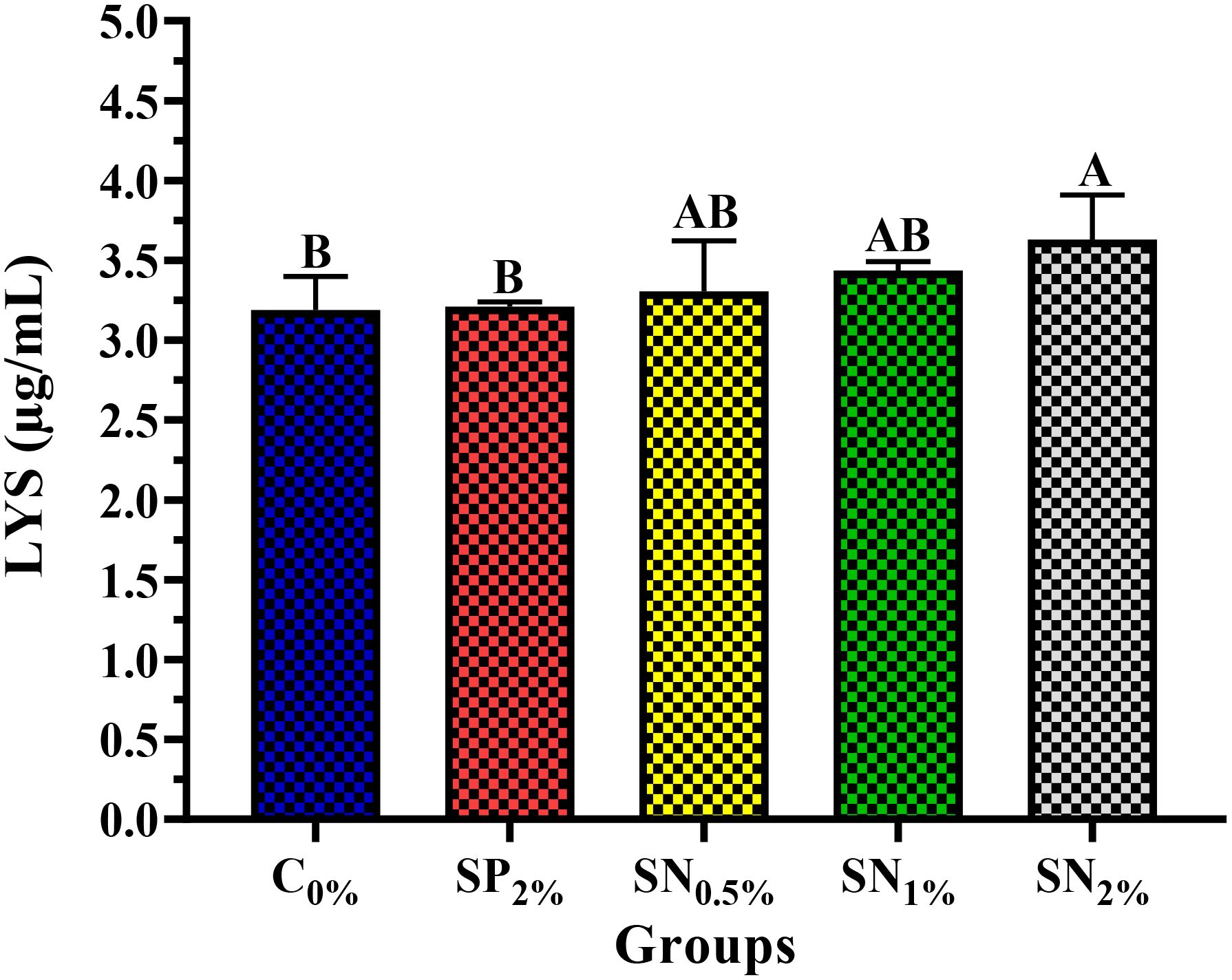
Figure 4. Innate immunity activities of LYS (A) of shrimp reared on the experimental dietary supplementations. LYS, Lysozyme (µg/mL); C0%, Control Basel diet; SP2%, Diet contains 2% (20 g/kg diet) powder of seaweed, as a positive control; SN0.5%, SN1%, and SN2%, Diets contain 0.5%, 1%, and 2% of seaweed nanoparticles (5, 10, and 20 g/kg diet, respectively). The data (n=5) were presented as means (± SD), and the capital letters (A > B > C > D) mean significance values at p <0.05.
3.6 Immunity-related gene expressions
Figure 5 highlights the impact of experimental diets on shrimp’s immunity-related gene expressions of PRX (Figure 5A), PPO1 (Figure 4B), p53 (Figure 4C), and L5H (Figure 4D). Figures 5A-D indicates that there is a significant difference (p < 0.05) in immunity-related gene expressions of shrimp reared in groups containing SP or SN, compared to C0%. Compared to the C0% group, the PRX gene expression was significantly (p < 0.05) upregulated in shrimp reared in group SN1% followed by SN2%, whereas it was not upregulated in other groups (Figure 5A). Figure 5B shows that compared to the C0% group, the gene expression of PPO1 was significantly (p < 0.05) upregulated in shrimp reared in group SN0.5%, followed by SN1%, SN2%, and SP2%.
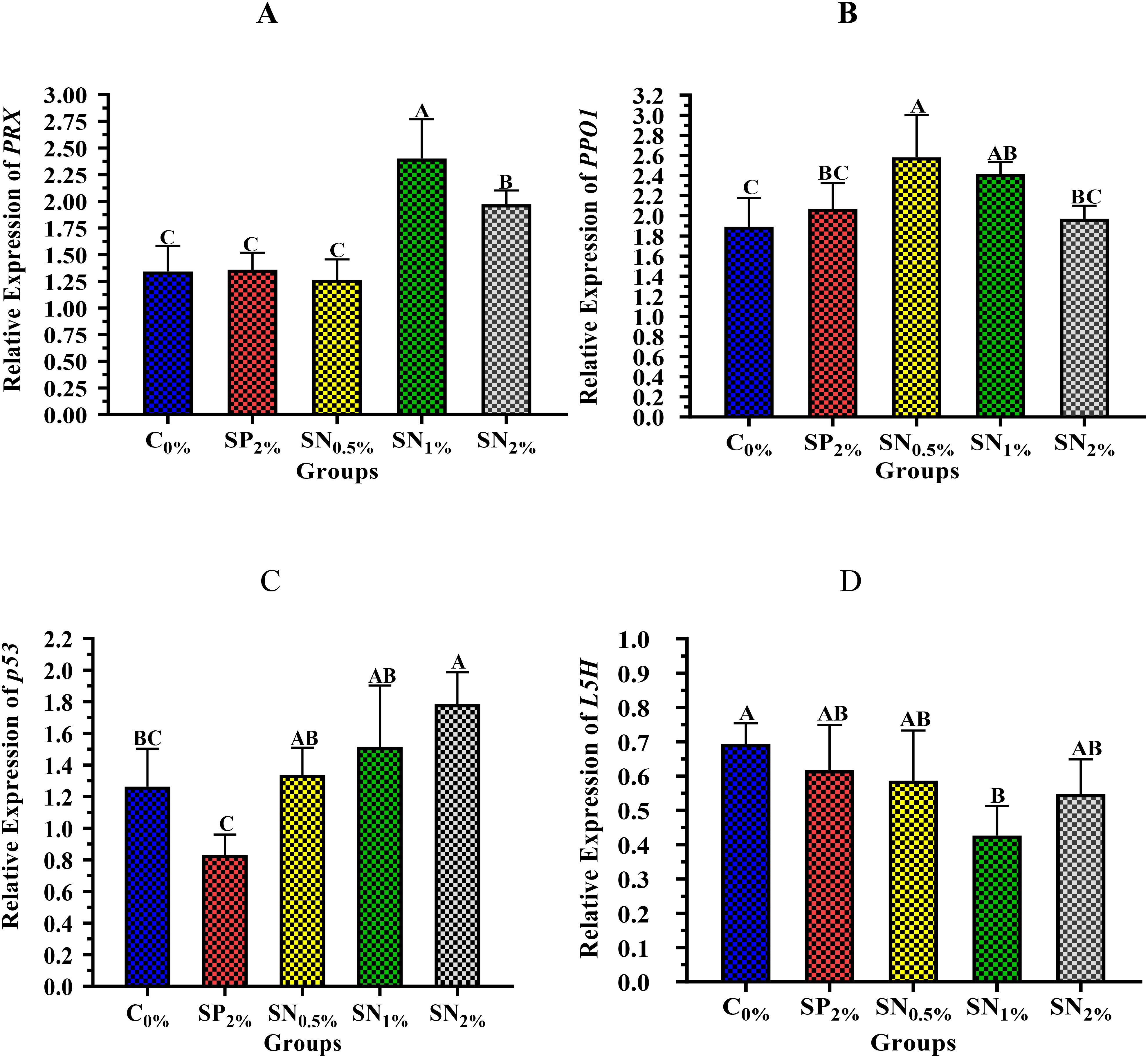
Figure 5. Immunity-related gene expressions of shrimp reared on the experimental dietary supplementations: (A) peroxiredoxin gene, Prx; (B) prophenoloxidase gene, PPO1; (C) p53-like protein isoform delta gene, p53; and (D) hemocyanin subunit L5 gene, L5H. C0%, Control Basel diet; SP2%, Diet contains 2% (20 g/kg diet) powder of seaweed, as a positive control, SN0.5%, SN1%, and SN2%, Diets contain 0.5%, 1%, and 2% of seaweed nanoparticles (5, 10, and 20 g/kg diet, respectively). The data (n =5) were presented as means (± SD), and the capital letters (A > B > C > D) mean significance values at p < 0.05.
Figure 5C shows that the p53 gene expression was significantly (p < 0.05) increased in shrimp reared in group SN2%, followed by SN1%, SN0.5%, and C0%, while the lowest significant (p < 0.05) decrease was achieved by SP2% group. The highest significant (p < 0.05) increase in L5H gene expression was obtained by shrimp reared in the control group C0%, followed by shrimp reared in SP2%, SN0.5%, SN2%, while the lowest significant decrease was achieved by SN1% group (Figure 5D).
3.7 Correlation heatmaps
Figure 6 shows the correlation heatmap analysis with cluster heat map (Figure 6A) and circular heatmap (Figure 6B). Furthermore, association analysis of treatments and estimated variables showed that18 variables out of 23 falls into cluster 1 with a mean association value of 4.96. Cluster 2 contains just 1 variable, but with a much higher means association (86.64). Cluster 3 has 4 variables, with a mean association of 35.33. This also revealed a dose dependent manner of increasing seaweed P. capillacea nanoparticles supplementation.
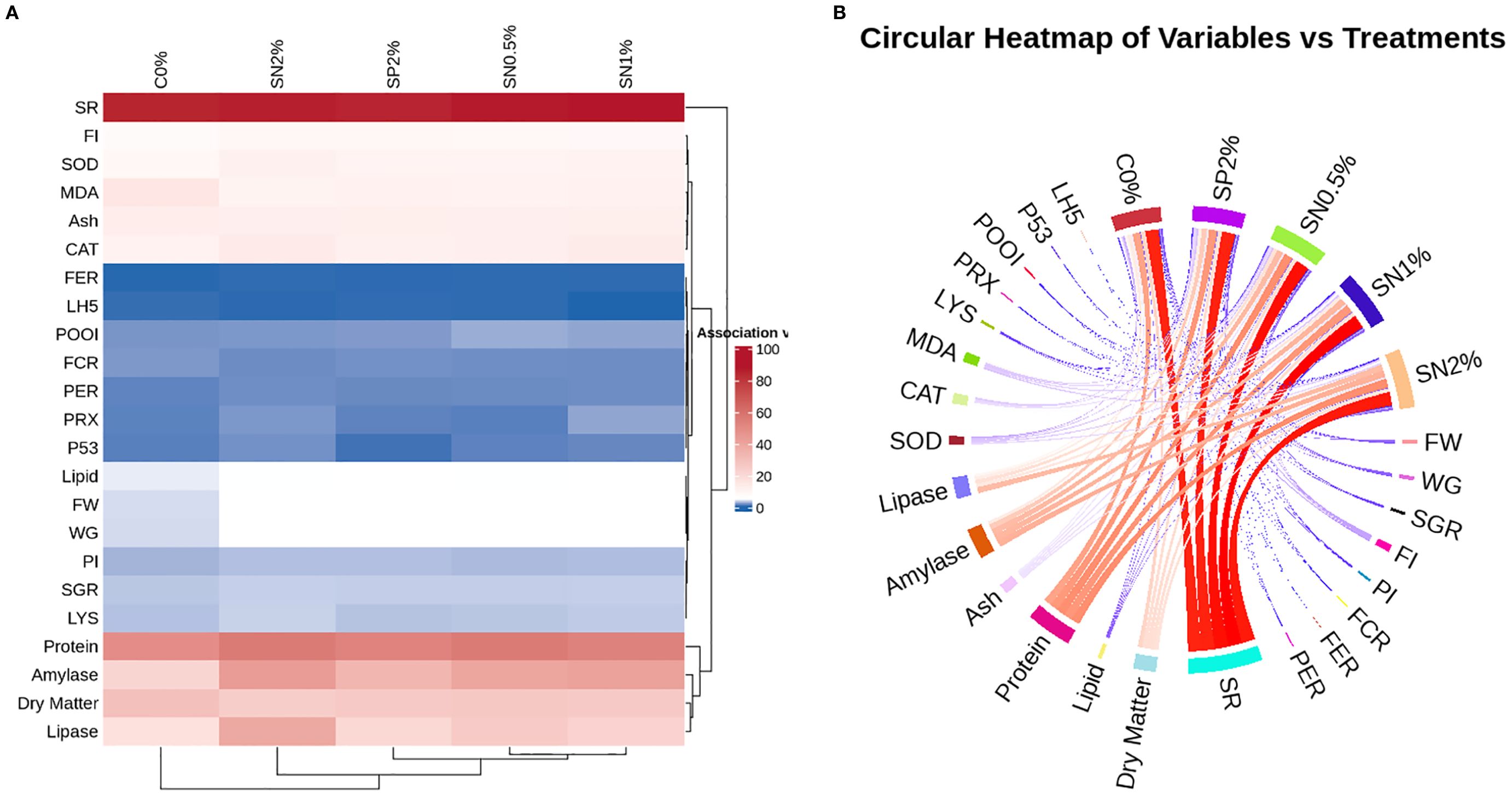
Figure 6. The association of treatments and estimated variables in a clusted heat map (A) or circular heatmap (B). The color scale ranges from blue (low association) through white (medium) to red (high association).
4 Discussion
Applying nanotechnology tools to aquafeed diets-based algal cells (microalgae and seaweed) significantly maximizes the benefit of their impact on aquatic animals (Mabrouk et al., 2022). However, according to the best of our knowledge, this is the first study in the literature conducted to determine the role of red seaweed P. capillacea nanoparticles on overall improvement in the shrimp L. vannamei postlarvae production. The current investigation revealed that, compared with those of the positive or negative controls, with the increasing dietary supplementation levels of SN, the growth (FW, WG, and SGR), digestive enzymes (amylase and lipase activities), carcass composition (protein and lipid contents), nutrient efficiency (FI, PI, FER, FCR, and PER), antioxidant activities (SOD and CAT), innate immunity activities (LYS and MDA), and related-immunity gene expressions (p53) of L. vannamei were significantly improved. The findings of the current work were confirmed by the findings recorded by Sharawy et al. (2022), who concluded that the implementation of A. platensis nanoparticles as a feed additive in the L. vannamei postlarvae regime significantly led to improvements in growth achievements, enhanced biochemical composition, and promoted growth-related gene (IGF-I and IGF-II) expressions.
In the same line, Mabrouk et al. (2022) highlighted that supplementing Nile tilapia diets, exposed to Aeromonas hydrophila, with the nanoparticles of A. platensis resulted in notable improvements in blood chemistry of total protein, albumin, and globulin levels. In addition, the antioxidant markers (SOD, CAT, GPx, lysozyme activity, and respiratory burst) were substantially elevated in fish fed diets containing these doses compared to both negative and positive controls. Additionally, it significantly decreased serum glucose levels and the enzymatic activities of ALT and AST. However, dietary supplementation with A. platensis nanoparticles effectively mitigated the inflammation in kidney, liver, and spleen tissues that was caused by A. hydrophila (Mabrouk et al., 2022).
In aquaculture, FCR and WG are the most common indicators that can judge the quality of diets. FCR is a ratio of feed input to WG (Wan et al., 2019). It explains how efficiently shrimp can convert feed ingredients into body mass gain. A lower FCR is desirable (Weber et al., 2025). Shrimp aquafeed additives play a vital role in improving both FCR and WG in shrimp diets.
In the current work, the polynomial regression chart between FCR (r² = 0.9725) and WG (r² = 0.8974) demonstrates that when SN dietary supplementation levels rise, the polynomial regression of FCR was decreased while WG was increased. As reported previously by Khanjani et al. (2024), these findings reflect that, by enhancing nutrient absorption and growth promotion, these additives contribute to more efficient and sustainable shrimp farming practices. Our finding are consistent with those published by Ashour et al. (2025), who noted that polynomial regression analysis of FCR was significantly reduced and WG was significantly increased when the inclusion supplementation levels of cyanobacteria D. tharense NIOF17/006 in the diet of shrimp L. vannamei postlarvae were increased.
Regarding the shrimp’s body carcass compositions, the current findings are consistent with those conducted by Abbas et al. (2023), who recorded significant variations in the overall body composition of postlarvae of shrimp L. vannamei when they were fed diets containing varying amounts of polysaccharide derived from S. dentifolium. Furthermore, our results aligned with earlier research by Sharawy et al. (2022) and Mabrouk et al. (2022) that employed A. platensis nanoparticles as feed additives for L. vannamei and O. niloticus diets, respectively.
The activity of digestive and antioxidant enzymes serves as valuable indicators for evaluating feed additive quality (Ashraf et al., 2024). These correlate to enhanced nutrient uptake, efficient digestion, and thus superior health in shrimp (Liu et al., 2024). Our study revealed that with the increasing dietary SN supplementation levels, the digestive enzymes and antioxidant activities significantly improved, resulting in improvement in nutrient absorption, digestion, and overall shrimp health quality. As reported by our previous work conducted by Ashour et al. (2020), P. capillacea has a respectable amount of bioactive compounds (polysaccharide, phenolic, flavonoid, carotene, PUFA, MOFA, EAA, etc., Supplementary Table S1), which showed several biological activities, including antioxidant activities. Our findings were highlighted by Hu et al. (2025), who reported that the utilization of natural antioxidant substrates as feed additives in shrimp aquadiets promotes overall shrimp health quality. The current findings may be due to the content of bioactive compounds and other functional groups present in the utilized red seaweed species. The same findings were supported by several authors when using red seaweed species, in all their forms, as feed additives for shrimp, L. vannamei (Abbas et al., 2023); Oscar, Astronotus ocellatus (Khanzadeh et al., 2024); O. niloticus (Shahabuddin et al., 2024), Northern snakehead, Channa argus (Wang et al., 2025); rainbow trout, Oncorhynchus mykiss (Sotoudeh and Mardani, 2018); rabbit fish, Siganus canaliculatus (Bakky et al., 2023); and hybrid red tilapia (Abdelrhman et al., 2022).
Innate immunity is a valuable key for assessing shrimp’s aquafeed additive efficiency (Chen et al., 2024; Kim et al., 2024). Since shrimp don’t have an adaptive immune system, their innate immunity acts as their first defense mechanism (Rathinam et al., 2024). In shrimp, LYS represent crucial indicators of innate immunity (Saha et al., 2024). MDA, a lipid peroxidation outcome, is an indicator of oxidative stress and the effectiveness of shrimp’s antimicrobial defense system. Lower levels of MDA are generally encouraged (Yan et al., 2025). The ability of the innate immune system to fight off infections is reflected by LYS. Higher lysozyme activity is often encouraged (Chen et al., 2025). To evaluate how aquatic feed additives promote disease resistance and overall shrimp wellness, MDA and LYS work together by providing essential data on the immune system and overall wellness of shrimp (Abdollahzadeh et al., 2025). Our findings showed that with the increasing of SN dietary supplementation, the LYS activities were significantly increased while MDA decreased. These findings reveal that the addition of SN in shrimp diet (as a natural antioxidant substrate) positively improves the innate immunity and, consequently, improves the defense mechanism in shrimp (Hu et al., 2025). These results may be attributed to the detected amount of bioactive compounds in red seaweed P. capillacea (Ashour et al., 2020), in addition to the properties of nanoparticles that are characterized in P. capillacea (Mansour et al., 2022b).
Our investigation showed that incorporating SN into shrimp diets enhanced the expression of immune-related genes, in particular Prx, PPO1, and p53, while no improvement was obtained in L5H. These observations align with the previous study by Zhu et al. (2011), who demonstrated that supplementing L. vannamei diets with P. capillacea led to reduced inflammation, improved immune enzyme activity, and increased expression of immune-related genes. These findings can be the consequence of the bioactive substances (Ashour et al., 2020), as well as the characterization of P. capillacea nanoparticles (Mansour et al., 2022b). Immune-related gene expression in L. vannamei was significantly enhanced by supplementing their diets with P. capillacea polysaccharide (Abbas et al., 2023), a combined seaweed extract (Ashour et al., 2024a), and A. platensis free-lipid (Ashour et al., 2024b).
5 Conclusion
Utilizing nanotechnology tools to enhance shrimp feed additives can significantly maximize the positive effects of these additives, leading to improvements in L. vannamei growth, health, and overall production. Our findings indicate that incorporating nanoparticles of red seaweed P. capillacea at levels up to 2% (20 g/kg of feed) markedly boosts growth, immune function, antioxidant capacity, nutrient efficiency, digestive enzyme activity, and the expression of genes associated with immunity in L. vannamei. Moreover, the polynomial regression model concluded that the optimal range for SN supplementation is found to be between 1.2% and 1.6% (12–16 g/kg diet). This range corresponds to the SN2% treatment group, where WG reached its highest peak and FCR achieved its lowest value. Further research is required to understand and explain how seaweed nanoparticles affect these shrimp’s physiological state and upgrade some immune-related gene expressions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MA: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MM: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft. AIM: Conceptualization, Investigation, Methodology, Validation, Writing – original draft. MN: Software, Writing – original draft. ATM: Data curation, Formal analysis, Funding acquisition, Investigation, Software, Supervision, Validation, Writing – review & editing. EM: Resources, Software, Supervision, Writing – review & editing. AA: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (KFU251722).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1600260/full#supplementary-material
References
Abbas E. M., Al-Souti A. S., Sharawy Z. Z., El-Haroun E., and Ashour M. (2023). Impact of dietary administration of seaweed polysaccharide on growth, microbial abundance, and growth and immune-related genes expression of the pacific whiteleg shrimp (Litopenaeus vannamei). Life 13, 344. doi: 10.3390/life13020344
Abdelrhman A. M., Ashour M., Al-Zahaby M. A., Sharawy Z. Z., Nazmi H., Zaki M. A., et al. (2022). Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquacult. Rep. 25, 101212. doi: 10.1016/j.aqrep.2022.101212
Abdollahzadeh Y., Mazandarani M., Hoseinifar S. H., Lieke T., Van Doan H., and Pourmozaffar S. (2025). Dietary fulvic acid improves immune, digestive and antioxidant parameters in juvenile white-leg shrimp (Litopenaeus vannamei) in a super-intensive system. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 275, 111011.
Aguilera-Rivera D., Rodríguez-Fuentes G., Escalante-Herrera K. S., Guerra-Castro E., Prieto-Davó A., and Gaxiola G. (2019). Differential expression of immune-related genes in Pacific white shrimp, Litopenaeus vannamei, previously reared in biofloc and challenged with Vibrio harveyi. Aquacult. Res. 50, 2039–2046.
Ahmed N., Thompson S., and Glaser M. (2019). Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environ. Manage. 63, 159–172. doi: 10.1007/s00267-018-1117-3
Al-Saeedi S. I., Ashour M., and Alprol A. E. (2023). Adsorption of toxic dye using red seaweeds from synthetic aqueous solution and its application to industrial wastewater effluents. Front. Marine Sci. 10. doi: 10.3389/fmars.2023.1202362
AOAC (2003). Official methods of analysis of the Association of Official Analytical Chemists (Arlington, Virginia: The Association).
APHA (2005). Standard methods for the examination of water and wastewater (Washington, DC, USA: American Public Health Association (APHA).
Ashour M., Al-Souti A. S., Mabrouk M. M., Naiel M. A., Younis E. M., Abdelwarith A. A., et al. (2024a). A commercial seaweed extract increases growth performance, immune responses, and related gene expressions in whiteleg shrimp (Litopenaeus vannamei). Aquacult. Rep. 36, 102154. doi: 10.1016/j.aqrep.2024.102154
Ashour M., Al-Souti A. S., Mamoon A., Ali F. S., Elshobary M. E., Mabrouk M. M., et al. (2025). Cyanobacteria Desertifilum tharense NIOF17/006 as a novel aquafeed additive: effect on the growth, immunity, digestive function, and gene expression. Front. Marine Sci. 12, 1532370. doi: 10.3389/fmars.2025.1532370
Ashour M., El-Shafei A. A., Khairy H. M., Abd-Elkader D. Y., Mattar M. A., Alataway A., et al. (2020). Effect of Pterocladia capillacea seaweed extracts on growth parameters and biochemical constituents of Jew’s Mallow. Agronomy 10, 420. doi: 10.3390/agronomy10030420
Ashour M., Mabrouk M. M., Mansour A. I., Abdelhamid A. F., Kader M. F. A., Elokaby M. A., et al. (2024b). Impact of dietary administration of Arthrospira platensis free-lipid biomass on growth performance, body composition, redox status, immune responses, and some related genes of pacific whiteleg shrimp, Litopenaeus vannamei. PloS One 19, e0300748. doi: 10.1371/journal.pone.0300748
Ashour M., Mansour A. T., Abdelwahab A. M., and Alprol A. E. (2023). Metal oxide nanoparticles’ green synthesis by plants: prospects in phyto-and bioremediation and photocatalytic degradation of organic pollutants. Processes 11, 3356. doi: 10.3390/pr11123356
Ashraf A., Sabu S., Sasidharan A., and Sunooj K. V. (2024). Natural feed supplements from crustacean processing side streams for improved growth of finfishes and crustaceans: a review. J. Anim. Physiol. Anim. Nutri. 109 (2), 376–401.
Bakky M. A. H., Tran N. T., Zhang Y., Hu H., Lin H., Zhang M., et al. (2023). Effects of dietary supplementation of Gracilaria lemaneiformis-derived sulfated polysaccharides on the growth, antioxidant capacity, and innate immunity of rabbitfish (Siganus canaliculatus). Fish Shellf. Immunol. 139, 108933. doi: 10.1016/j.fsi.2023.108933
Becker A. J., Braga A., Magalhães V., Medeiros L. M., Ramos P. B., Monserrat J. M., et al. (2024). Examination of the antioxidant effects of linalool and Lippia alba essential oil in the Pacific whiteleg shrimp, Litopenaeus vannamei, subjected to eyestalk ablation. Aquaculture 592, 741215. doi: 10.1016/j.aquaculture.2024.741215
Caraway W. (1959). α-amylase colorimetric method. Ame. J. Clin. Pathol. 32, 97–99. doi: 10.1093/ajcp/32.1_ts.97
Ceseña C. E., Jacinto E. C., González A. L., Villasante F. V., Castro R. M. M., Ochoa N., et al. (2021). Dietary supplementation of Debaryomyces hansenii enhanced survival, antioxidant and immune response in juvenile shrimp penaeus vannamei challenged with Vibrio Parahaemolyticus. Trop. Subtrop. Agroecosys. 24.
Chen Y., Mitra A., Rahimnejad S., Chi S., Kumar V., Tan B., et al. (2024). Retrospect of fish meal substitution in Pacific white shrimp (Litopenaeus vannamei) feed: Alternatives, limitations and future prospects. Rev. Aquacult. 16, 382–409. doi: 10.1111/raq.12843
Chen Z., Tayyab M., Yao D., Aweya J. J., Zheng Z., Zhao X., et al. (2025). Cell death in crustacean immune defense. Rev. Aquacult. 17, e12976. doi: 10.1111/raq.12976
Elshobary M. E., El-Shenody R. A., and Abomohra A. E. F. (2021). Sequential biofuel production from seaweeds enhances the energy recovery: A case study for biodiesel and bioethanol production. Int. J. Energy Res. 45, 6457–6467. doi: 10.1002/er.v45.4
Emerenciano M. G., Rombenso A. N., Vieira F., d. N., Martins M. A., Coman G. J., et al. (2022). Intensification of penaeid shrimp culture: an applied review of advances in production systems, nutrition and breeding. Animals 12, 236. doi: 10.3390/ani12030236
Goncalves P., Guertler C., Bachère E., De Souza C. R., Rosa R. D., and Perazzolo L. M. (2014). Molecular signatures at imminent death: Hemocyte gene expression profiling of shrimp succumbing to viral and fungal infections. Dev. Comp. Immunol. 42, 294–301. doi: 10.1016/j.dci.2013.09.017
Hamid I., Wanjari R. N., Abass Z., and Abubakr A. (2025). “Algal biotechnology for the production of food,” in Food Security, Nutrition and Sustainability Through Aquaculture Technologies (Springer: Springer).
Harshbarger J. C., Spero P. M., and Wolcott N. M. (1992). Neoplasms in wild fish from the marine ecosystem. Pathobiol. Marine Estuarine Organ. 2, 157.
Hu X., Ma W., Zhang D., Tian Z., Yang Y., Huang Y., et al. (2025). Application of natural antioxidants as feed additives in aquaculture: A review. Biology 14, 87. doi: 10.3390/biology14010087
Ismail M. M., El Zokm G. M., and Miranda Lopez J. M. (2023). Nutritional, bioactive compounds content, and antioxidant activity of brown seaweeds from the Red Sea. Front. Nutr. 10, 1210934. doi: 10.3389/fnut.2023.1210934
Kannan J., Pang K.-L., Ho Y.-N., Hsu P.-H., and Chen L.-L. (2024). A comparison of the antioxidant potential and metabolite analysis of marine fungi associated with the red algae Pterocladiella capillacea from Northern Taiwan. Antioxidants 13, 336. doi: 10.3390/antiox13030336
Kaur S. and Khattar J. I. S. (2025). “Potential of Algal Metabolites in Cosmetics and Personal Care Products,” in Industrial and Biotechnological Applications of Algae (Springer Singapore: Springer).
Khanjani M. H., Mozanzadeh M. T., and Fóes G. K. (2022). Aquamimicry system: a sutiable strategy for shrimp aquaculture–a review. Ann. Anim Sci. 22, 1201–1210. doi: 10.2478/aoas-2022-0044
Khanjani M. H., Mozanzadeh M. T., Gisbert E., and Hoseinifar S. H. (2024). Probiotics, prebiotics, and synbiotics in shrimp aquaculture: Their effects on growth performance, immune responses, and gut microbiome. Aquacult. Rep. 38, 102362. doi: 10.1016/j.aqrep.2024.102362
Khanzadeh M., Hoseinifar S. H., Zargari A., Tabibi H., Van Doan H., and Rabetimarghezar N. (2024). Fucoidan derived from Sargassum ilicifolium affects growth and hemato-immunological parameters and antioxidant defense in Oscar (Astronotus ocellatus). Front. Marine Sci. 11, 1370871. doi: 10.3389/fmars.2024.1370871
Kim B., Jeon H. J., Rhee M. H., Kim J. H., and Han J. E. (2024). The effects of Panax ginseng on growth enhancement, innate immunity, and microbiome profiling in Penaeus vannamei. J. Ginseng Res. 48, 552–558. doi: 10.1016/j.jgr.2024.06.002
Kwasek K., Thorne-Lyman A. L., and Phillips M. (2020). Can human nutrition be improved through better fish feeding practices? A review paper. Crit. Rev. Food Sci. Nutr. 60, 3822–3835. doi: 10.1080/10408398.2019.1708698
Leung E. H. (2025). Current and prospective pharmaceutical use of algal bioproducts. Intechopen. doi: 10.5772/intechopen.1009486
Liu M., Sun C., Zhou Q., Xu P., Wang A., Zheng X., et al. (2024). Supplementation of Yupingfeng polysaccharides in low fishmeal diets enhances intestinal health through influencing the intestinal barrier, immunity, and microflora in Macrobrachium rosenbergii. Front. Immunol. 15, 1480897. doi: 10.3389/fimmu.2024.1480897
Liu W.-C., Zhou S.-H., Balasubramanian B., Zeng F.-Y., Sun C.-B., and Pang H.-Y. (2020). Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish shellf. Immunol. 104, 202–212. doi: 10.1016/j.fsi.2020.05.079
Livak K. J. and Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2– ΔΔCT method. methods 25, 402–408. doi: 10.1006/meth.2001.1262
Mabrouk M. M., Ashour M., Labena A., Zaki M. A., Abdelhamid A. F., Gewaily M. S., et al. (2022). Nanoparticles of Arthrospira platensis improves growth, antioxidative and immunological responses of Nile tilapia (Oreochromis niloticus) and its resistance to Aeromonas hydrophila. Aquacult. Res. 53, 125–135. doi: 10.1111/are.15558
Magouz F. I., Essa M. A., Matter M., Mansour A. T., Gaber A., and Ashour M. (2021a). Effect of different salinity levels on population dynamics and growth of the cyclopoid copepod Oithona nana. Diversity 13, 190. doi: 10.3390/d13050190
Magouz F. I., Essa M. A., Matter M., Tageldein Mansour A., Alkafafy M., and Ashour M. (2021b). Population dynamics, fecundity and fatty acid composition of Oithona nana (Cyclopoida, Copepoda), fed on different diets. Animals 11, 1188. doi: 10.3390/ani11051188
Mansour A. T., Alprol A. E., Abualnaja K. M., El-Beltagi H. S., Ramadan K. M. A., and Ashour M. (2022a). The using of nanoparticles of microalgae in remediation of toxic dye from industrial wastewater: kinetic and isotherm studies. Mater. (Basel) 15. doi: 10.3390/ma15113922
Mansour A. T., Alprol A. E., Ashour M., Ramadan K. M., Alhajji A. H., and Abualnaja K. M. (2022b). Do red seaweed nanoparticles enhance bioremediation capacity of toxic dyes from aqueous solution? Gels 8, 310. doi: 10.3390/gels8050310
Mansour A. T., Ashry O. A., El-Neweshy M. S., Alsaqufi A. S., Dighiesh H. S., Ashour M., et al. (2022c). Effect of agricultural by-products as a carbon source in a biofloc-based system on growth performance, digestive enzyme activities, hepatopancreas histology, and gut bacterial load of Litopenaeus vannamei post larvae. J. Marine Sci. Eng. 10, 1333. doi: 10.3390/jmse10101333
Marimuthu V., Shanmugam S., Sarawagi A. D., Kumar A., Kim I. H., and Balasubramanian B. (2022). A glimpse on influences of feed additives in aquaculture. EFood 3, e6. doi: 10.1002/efd2.v3.1-2
Moss D. M., Rudis M., and Henderson S. O. (1999). Cross-sensitivity and the anticonvulsant hypersensitivity syndrome. J. Emergency Med. 17, 503–506. doi: 10.1016/S0736-4679(99)00042-6
Mustafa S. A. and Al-Faragi J. K. (2021). Supplementation of feed additives on aquaculture feeds: A review. Int. J. Pharma. Res. 13, 561–567.
N’Souvi K., Sun C., Che B., and Vodounon A. (2024). Shrimp industry in China: overview of the trends in the production, imports and exports during the last two decades, challenges, and outlook. Front. Sustain. Food Syst. 7, 1287034. doi: 10.3389/fsufs.2023.1287034
Nishikimi M., Rao N. A., and Yagi K. (1972). The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854. doi: 10.1016/S0006-291X(72)80218-3
Nuñez-Hernandez D., Camacho-Jiménez L., Leyva-Carrillo L., Peregrino-Uriarte A. B., Valenzuela-Soto E. M., Gómez-Jiménez S., et al. (2021). Expression of p53 isoforms in response to two levels of hypoxia and effects of p53 knock-down in glyceraldehyde-3-phosphate dehydrogenase in the white shrimp Litopenaeus vannamei. Aquacult. Res. 52, 2313–2323.
Ohkawa H., Ohishi N., and Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. doi: 10.1016/0003-2697(79)90738-3
Osman M. E. H., Abo-Shady A. M., and Elshobary M. E. (2010). In vitro screening of antimicrobial activity of extracts of some macroalgae collected from Abu-Qir bay Alexandria, Egypt. Afr. J. Biotechnol. 9, 7203–7208.
Osman M. E. H., Abo-Shady A. M., Elshobary M. E., Abd El-Ghafar M. O., and Abomohra A. E.-F. (2020). Screening of seaweeds for sustainable biofuel recovery through sequential biodiesel and bioethanol production. Environ. Sci. Pollut. Res. Int. 27 (26):32481–32493. doi: 10.1007/s11356-020-09534-1
Pedrazzani A. S., Cozer N., Quintiliano M. H., Tavares C. P., d. S., Da Silva U., et al. (2023). Non-invasive methods for assessing the welfare of farmed white-leg shrimp (Penaeus vannamei). Animals 13, 807. doi: 10.3390/ani13050807
Ramaker H.-J., Van Sprang E. N., Westerhuis J. A., and Smilde A. K. (2003). Dynamic time warping of spectroscopic BATCH data. Anal. Chim. Acta 498, 133–153. doi: 10.1016/j.aca.2003.08.045
Rathinam R. B., Acharya A., Robina A. J., Banu H., and Tripathi G. (2024). The immune system of marine invertebrates: Earliest adaptation of animals. Comp. Immunol. Rep. 7, 200163. doi: 10.1016/j.cirep.2024.200163
Saha S., Chukwuka A. V., Saha N. C., Faggio C., and Sabet H. M. (2024). Natural and synthetic immunomodulators: inferences for stress responses in aquaculture fish. Immunomodul. Aquacult. Fish Health, 18–28.
Sanjeewa K., Lee W., and Jeon Y.-J. (2018). Nutrients and bioactive potentials of edible green and red seaweed in Korea. Fish. Aquat. Sci. 21, 1–11. doi: 10.1186/s41240-018-0095-y
Shahabuddin A., Hannan M. A., Hossain M. F., Hemal S., Khanam R., Afroz T., et al. (2024). Evaluation of Ethanolic Extract of Red Seaweed (Gracilariopsis lemaneiformis) on Growth and Haematological Parameters of Nile Tilapia (Oreochromis niloticus). Aquacult. Fish Fish. 4, e70011. doi: 10.1002/aff2.70011
Sharawy Z. Z., Ashour M., Labena A., Alsaqufi A. S., Mansour A. T., and Abbas E. M. (2022). Effects of dietary Arthrospira platensis nanoparticles on growth performance, feed utilization, and growth-related gene expression of Pacific white shrimp, Litopenaeus vannamei. Aquaculture 551, 737905. doi: 10.1016/j.aquaculture.2022.737905
Sotoudeh E. and Mardani F. (2018). Antioxidant-related parameters, digestive enzyme activity and intestinal morphology in rainbow trout (Oncorhynchus mykiss) fry fed graded levels of red seaweed, Gracilaria pygmaea. Aquacult. Nutr. 24, 777–785. doi: 10.1111/anu.2018.24.issue-2
Suzuki A. and Nam V. H. (2023). Blue revolution in Asia: the rise of the shrimp sector in Vietnam and the challenges of disease control. Agric. Dev. Asia Afr. 289.
Swift M. L. (1997). GraphPad prism, data analysis, and scientific graphing. J. Chem. Inf. Comput. Sci. 37, 411–412. doi: 10.1021/ci960402j
Venkateswarlu V., Seshaiah P., Arun P., and Behra P. (2019). A study on water quality parameters in shrimp L. vannamei semi-intensive grow out culture farms in coastal districts of Andhra Pradesh, India. Int. J. Fish. Aquat. Stud. 7, 394–399.
Wan A. H., Davies S. J., Soler-Vila A., Fitzgerald R., and Johnson M. P. (2019). Macroalgae as a sustainable aquafeed ingredient. Rev. Aquacult. 11, 458–492. doi: 10.1111/raq.2019.11.issue-3
Wang M., Wang W., Guo L., Tan S., Xue H., Wang Y., et al. (2025). Seaweed residue hydrolysate enhances the intestinal health, immunity and disease resistance in northern snakehead (Channa argus). Fish Shellf. Immunol. 157, 110115. doi: 10.1016/j.fsi.2025.110115
Wang Y.-C., Chang P.-S., and Chen H.-Y. (2007). Tissue expressions of nine genes important to immune defence of the Pacific white shrimp Litopenaeus vannamei. Fish shellf. Immunol. 23, 1161–1177. doi: 10.1016/j.fsi.2007.04.004
Weber P., Wigraiboon S., Sutthi N., Kasamesiri P., and Thaimuangphol W. (2025). The Physiological Benefits and Economic Value of Using Fairy Shrimp as Fish Meal for Flowerhorn Cichlids; Amphilophus citrinellus (Günther 1864)× Cichlasoma trimaculatum (Günther 1867). Fishes 10, 132.
Widiasa I., Susanto H., Ting Y., Suantika G., Steven S., Khoiruddin K., et al. (2023). Membrane-based recirculating aquaculture system: Opportunities and challenges shrimp farming. Aquaculture, 740224.
Yan H., Wang Y., Liang H., Duan Y., Wang J., Zhou C., et al. (2025). Effects of lysophospholipids on the antioxidant capacity, digestive performance, and intestinal microbiota of litopenaeus vannamei. Biology 14, 90. doi: 10.3390/biology14010090
Yazici M., Zavvar F., Hoseinifar S. H., Nedaei S., and Doan H. V. (2024). Administration of Red Macroalgae (Galaxaura oblongata) in the Diet of the Rainbow Trout (Oncorhynchus mykiss) Improved Immunity and Hepatic Gene Expression. Fishes 9, 48. doi: 10.3390/fishes9020048
Zar J. H. (1984). Statistical significance of mutation frequencies, and the power of statistical testing, using the Poisson distribution. Biometr. J. 26, 83–88. doi: 10.1002/bimj.4710260116
Zhang Z., Shi X., Wu Z., Wu W., Zhao Q., and Li E. (2023). Macroalgae improve the growth and physiological health of white shrimp (Litopenaeus vannamei). Aquacult. Nutr. 2023, 8829291. doi: 10.1155/2023/8829291
Keywords: amylase, FcR, lipase, macroalgae, nanotechnology tools, seaweed, shrimp dietary supplementation, whiteleg shrimp
Citation: Ashour M, Mabrouk MM, Mansour AIA, Naiel MAE, Mansour AT, Mohamed E and Abdelhamid AF (2025) Seaweed (Pterocladia capillacea) nanoparticles improves growth performances, digestive enzymes, antioxidant activities, innate immunity, and related-immunity gene expressions of Litopenaeus vannamei. Front. Mar. Sci. 12:1600260. doi: 10.3389/fmars.2025.1600260
Received: 26 March 2025; Accepted: 12 May 2025;
Published: 03 June 2025.
Edited by:
Kumbukani Mzengereza, Mzuzu University, MalawiReviewed by:
Zhenlu Wang, Guizhou University, ChinaAmit Ranjan, Tamil Nadu Fisheries University, India
Copyright © 2025 Ashour, Mabrouk, Mansour, Naiel, Mansour, Mohamed and Abdelhamid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Ashour, bWljcm9hbGdhZV9lZ3lwdEB5YWhvby5jb20=; Abdallah Tageldein Mansour, YW1hbnNvdXJAa2Z1LmVkdS5zYQ==; Ehab Mohamed, ZWhhYi5yZWRhQHVhZXUuYWMuYWU=
 Mohamed Ashour
Mohamed Ashour Mohamed M. Mabrouk2
Mohamed M. Mabrouk2 Abdallah Tageldein Mansour
Abdallah Tageldein Mansour