- 1National Research Council (CNR), Institute of Marine Sciences (ISMAR), Naples, Italy
- 2National Research Council (CNR), Institute of Anthropic Impacts and Sustainability in Marine Environment (IAS), Branch of Capo Granitola, Campobello di Mazara, Italy
- 3Dipartimento di Scienze e Tecnologie Ambientali, Biologiche e Farmaceutiche dell’Università degli Studi della Campania “Luigi Vanvitelli”, Caserta, Italy
European sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) are important components of marine food webs, acting as trophic links between primary consumers and higher trophic levels. However, local factors—such as environmental conditions, human activities, coastal pollution, and more generally, climate change have had a significant impact on their trophic interactions, affecting the energy flow, structure, and function of the marine ecosystems in which they live. Although both species are among the most explored in the Mediterranean Sea, due to their commercial and ecological importance, the spatiotemporal variability in their trophic ecology is still poorly addressed, particularly when combining life stage, sampling year, and geographic location. Here, we propose a spatio-temporal analysis of the trophic variability of juvenile and adult anchovy and sardine in two different areas of the central Mediterranean Sea—the Tyrrhenian Sea (TR) and the Strait of Sicily (SS)—over 4 years (2011, 2014, 2015, and 2017). We used a stable isotope (δ13C and δ15N) approach to assess variability in feeding behaviour and isotopic niche metrics (niche width and overlap) across space and time. The two study areas are influenced by different water masses with distinct physical and biological properties, leading to local changes in the marine food web and consequently in the feeding behaviour of both fish species at different life stages. In all sampled years, higher δ15N and δ13C values were found in both anchovy and sardine sampled in the Tyrrhenian Sea (mean values: 8.8‰ and −17.5‰ for anchovy; 9.5‰ and −18.1‰ for sardine) compared with those from the Strait of Sicily (mean values: 6.6‰ and −18.9‰ for anchovy; 6.8‰ and −19.0‰ for sardine). Furthermore, the higher δ¹5N values observed indicate no significant relationship between isotopic values and organism size. Our results support the hypothesis that the higher isotopic signals observed in both juvenile and adult anchovy and sardine in the Tyrrhenian Sea may be linked to higher trophic-level food sources (e.g., predation on large prey such as krill), potentially associated with persistent local features (e.g., submarine canyons). Moreover, the greater number of cases showing wider isotopic niches and higher isotopic overlap between species—and between life stages—in the Tyrrhenian Sea than in the Strait of Sicily suggests higher variability in feeding behaviour and access to more diversified food sources. These data contribute to a better understanding of food web dynamics and may be used to improve ecosystem models, which requires an understanding of the ecological processes that control the interaction between species.
Introduction
Variation in the population dynamics of small pelagic fishes has been widely documented, and progress has been made over the last few decades in understanding the factors driving long-term fluctuations (e.g., Lluch-belda et al., 1992; Chavez et al., 2003; Alheit et al., 2012). However, such changes occur across a variety of scales (e.g., seasonal, inter-annual, decadal), and encompasses fluctuations in both abiotic (temperature, precipitation, light, nutrients) and biotic processes (e.g., migration, growth, reproduction, trophic interactions).
Pelagic food webs can be highly dynamic across space and time, especially at the regional scale and in coastal areas (Hernández-Carrasco et al., 2018), where riverine inputs, upwelling processes, and anthropogenic stressors can deeply modify environmental conditions and the ecological state of the water column (Stenseth et al., 2006; DiBattista et al., 2020). These alterations can have cascading effects on fish populations and pelagic food webs (Cushing, 1995; Russo et al., 2022; Vassallo et al., 2022).
In this context, an important question is whether variability in trophic relationships at the local scale is more strongly linked to spatial or temporal factors in coastal systems. This question has fundamental implications for the ecological study and management of marine ecosystems, including the provision of ecosystem goods and services and the sustainability of economic activities such as fisheries (Lomartire et al., 2021; Nissar et al., 2023).
Small pelagic fish, due to their rapid growth, short life span, and plankton-based diet, are often considered excellent indicators for assessing changes in resource availability associated with rapid, climate-driven environmental shifts in marine systems (e.g., Drinkwater et al., 2010; Peck et al., 2013). In particular, small pelagic fishes such as anchovy (Engraulis encrasicoulus) and sardine (Sardina pilchardus) are key elements both from an economic point of view, representing important species for commercial fishing (FAO, 2022), and for marine ecosystems, linking lower and upper trophic level species (Cury et al., 2000).
Studies on the trophic ecology of these small pelagic fish have largely been conducted based on stomach content analysis (SCA), which provides insight into the most frequently consumed and abundant prey items (e.g., Borme et al., 2009; Costalago and Palomera, 2014; Costalago et al., 2014). In recent decades, scientists have increasingly used alternative techniques, including stable isotope analysis (SIA), to determine the relative contributions of prey types or trophic positions assimilated over longer periods than those captured by SCA (e.g., Pethybridge et al., 2018). This method provides information about the assimilated prey over a specific time window—ranging from a few days to several months—depending on the tissue type analysed (e.g., liver, white muscle, blood) and its isotopic turnover rate (Hesslein et al., 1993; Bearhop et al., 2004). The ability to isotopically analyse muscle tissue from anchovy and sardine at different life stages offers a time-integrated view of dietary shifts, as this tissue has an isotopic turnover rate ranging from several days to one month (Suzuki et al., 2005; Guelinckx et al., 2007; Buchheister and Latour, 2010). Because stable isotope enrichment occurs along the trophic web, nitrogen stable isotope (δ15N) have been utilised as an indicator of the trophic position of organisms along the food web, as well as an indicator of the seasonal and spatial variability in trophic habits (Tanaka et al., 2008; Malzahn and Boersma, 2009). Similarly, as different energy sources often have distinct δ¹³C signatures, carbon stable isotopes can be used to trace the original organic carbon sources at the base of the food web, assess seasonal variation, or detect geographic differences (Vander Zanden and Rasmussen, 1999; 2001; Overman and Parrish, 2001; Castro et al., 2020, and references therein).
In the present study, we compared the isotopic carbon and nitrogen (δ13C and δ15N) signals in white muscles of juvenile and adult individuals of both sardine and anchovy to investigate the spatiotemporal variability in their feeding behaviour across two Mediterranean areas—the Tyrrhenian Sea and the Strait of Sicily —in the years 2011, 2014, 2015, and 2017. These two areas differ markedly in terms of hydrographic conditions, morpho-bathymetry, and productivity (Bonanno et al., 2016; 2018). The SS area is characterised by highly dynamic circulation, a coastal upwelling system (Bonanno et al., 2014), low coastline complexity, minimal human impact, and limited terrigenous input from river runoff. In contrast, the TR area features a large continental shelf, strong riverine influence from several rivers (e.g., Tiber, Garigliano, Volturno, Sarno, and Sele), and high levels of urban, agricultural, and industrial activities as well as a high degree of coastline complexity with the presence of Gulfs.
To our knowledge, only a few studies have evaluated the spatiotemporal variability in the feeding habits of these two small pelagic species in the Mediterranean Sea (e.g., Bachiller et al., 2020; Navarro et al., 2020; Zorica et al., 2021; Fanelli et al., 2023), and these have generally analysed only 1 year of data and/or focused on areas located in close geographic proximity.
Taking all these factors into account, we collected fish samples from both consecutive and non-consecutive years in order to assess the spatiotemporal variability in the feeding habits of anchovy and sardine. We first analysed the inter- and intraspecific differences in the isotopic composition of both species across life stages, areas, and years, focusing on the degree of overlap and the width of the isotopic niches. In a second step, we assessed which factor had the greatest influence on their feeding behaviour (i.e., place or time).
Materials and methods
Study area
This study focuses on anchovy and sardine samples collected in the Strait of Sicily (hereafter SS) and the Tyrrhenian Sea (hereafter TR), specifically along the coasts of Campania, Lazio, and Tuscany up to the island of Elba (Figure 1). The two areas are characterised by differing hydrographic conditions, morpho-bathymetry, and productivity (Figure 1; Bonanno et al., 2016).
From a morphological perspective, the SS area is characterised by the presence of two broad banks in the westernmost and easternmost sectors of the Adventure Bank and the Maltese Bank, respectively—extending nearly 70 nmi offshore. In contrast, the continental shelf between the two banks is relatively narrow (~8 nmi). This distinct topographic pattern also influences the path of the Atlantic Ionian Stream (AIS; Robinson et al., 1999), the most important oceanographic feature in the area. The AIS is the primary driver of productivity during the summer season, as it induces a permanent coastal upwelling along the southern coast of Sicily (Bonanno et al., 2014; Rumolo et al., 2017).
In the SS area, circulation is characterised by a two-layer system flowing in opposite directions: surface Atlantic Water (AW) moves eastward, while Levantine Intermediate Water (LIW) flows westward. The surface flow displays meanders with high spatial and temporal variability (Manzella et al., 1988; Moretti et al., 1993; Bonanno et al., 2014). Relatively intense local phenomena, including cyclonic and anticyclonic gyres, are frequently observed via satellite imagery (Champagne-Philippe et al., 1982). In this area, the coastline is also characterised by a low level of morphological complexity and minimal human pressure. Additionally, the input of terrigenous material from river runoff is of minor importance.
The TR area is characterised by a large continental shelf, with surface circulation mainly influenced by the Middle Tyrrhenian Current (MTC), which, upon approaching the coast near Rome, is strongly affected by the Tiber River (Figure 1)—one of the largest rivers flowing into the Tyrrhenian Sea (Rinaldi et al., 2010; Sammartino et al., 2022). River discharge serves as a major nutrient source in the TR area, where coastline morphology varies between the northern (i.e., Lazio and Tuscany) and southern (i.e., Campania) sectors.
In the northern TR sector, where coastline complexity is low, the Tiber River outflow is responsible for approximately 20% of the total riverine input into the Tyrrhenian Sea (Mikhailova et al., 1999). In contrast, the southern sector (i.e. the Campania coast), is characterised by high coastline complexity, due to the presence of three large gulfs: the Gulf of Gaeta, the Gulf of Naples, and the Gulf of Salerno. Several small and medium-sized rivers discharge into these gulfs—including the Garigliano and Volturno rivers (Gulf of Gaeta), the Sarno River (Gulf of Naples), and the Sele River (Gulf of Salerno). These rivers drain catchment areas that are heavily impacted by urban, agricultural, and industrial activities. In this southern Tyrrhenian sector, the combination of low hydrodynamics and enclosed geomorphological features (i.e., gulfs) promotes the retention of nutrients from river discharge on the continental shelf (Bonanno et al., 2016). The presence of submarine canyons—such as the Cuma Canyon and Dohrn Canyon—also contributes to nutrient retention (Aiello and Caccavale, 2023).
Sampling
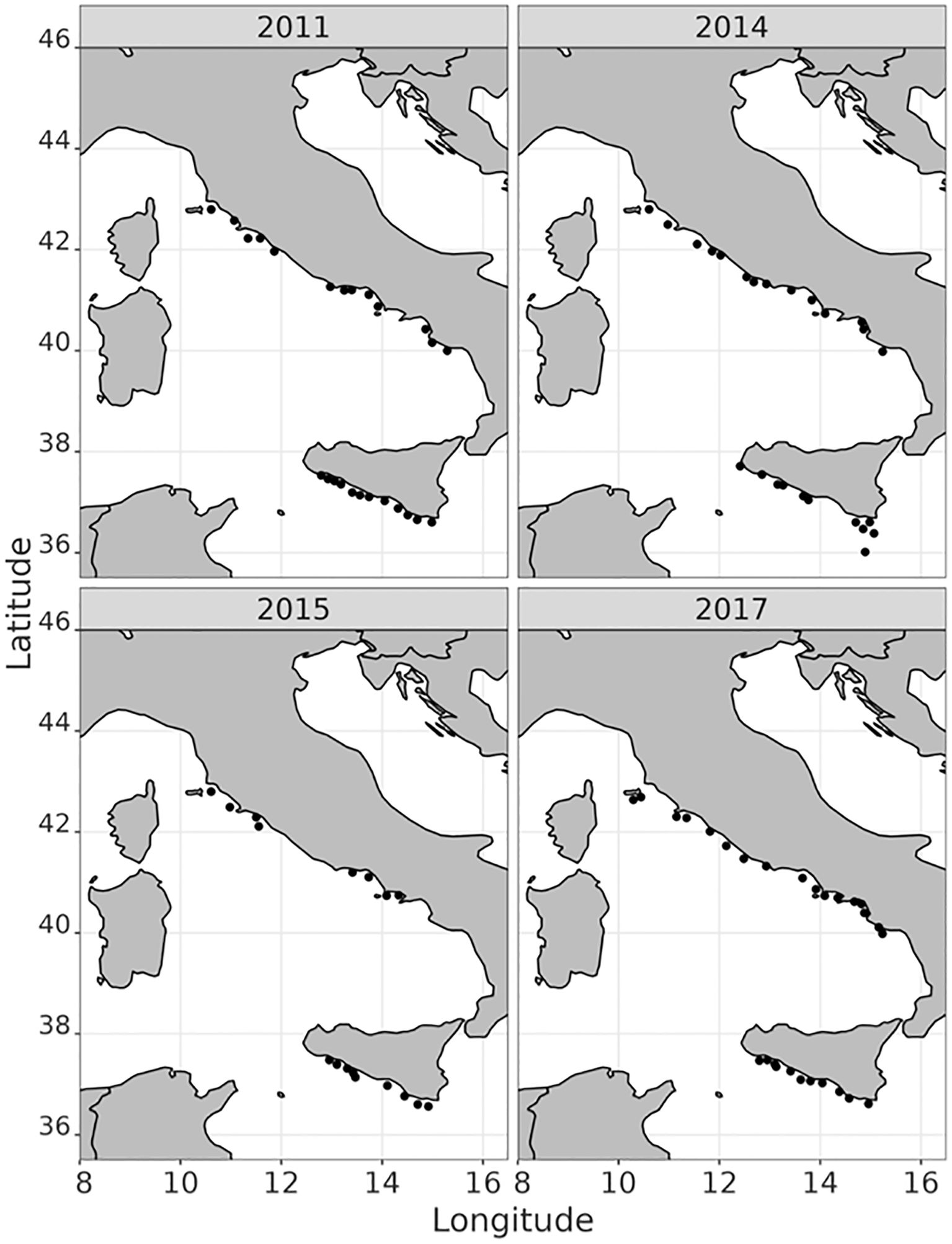
The Mediterranean International Acoustic Surveys (MEDIAS; www.medias-project.eu) programme, established under the EU Fisheries Data Collection Regulation (EC 199/2008), conducts annual acoustic surveys in EU Mediterranean waters to assess the biomass and spatial distribution of anchovy and sardine populations. The biological samples analysed in this study were collected under the MEDIAS umbrella during the “Ancheva” and “Evatir” surveys, which collected acoustic data and biological samples in the Strait of Sicily (including a small portion of the Ionian Sea) and in the Tyrrhenian and Ligurian Seas (Leonori et al., 2021). Anchovy (hereafter ANE) and sardine (hereafter PIL) samples were collected at specific trawl stations during surveys carried out in 2011, 2014, 2015, and 2017 (Figure 2).
Specimens of anchovy and sardine were caught using a midwater pelagic trawl net with the following characteristics: 11 m vertical mouth opening, 18 m horizontal opening, and an 8 mm cod end mesh size. During each haul, the net—equipped with an integrated monitoring system (SIMRAD ITI) to control its position in the water column and monitor catch efficiency—was towed at an average speed of 4 knots for 30 minutes. Once the net was retrieved, a subsample of the total catch was selected. For each species, individuals were measured (total length [TL], in cm), assigned to 1 cm length classes, sorted, counted, and weighed (wet weight [WW], in g). Subsamples included 10 individuals per 1 cm length class for each species (sardine and anchovy), when available. All samples were frozen at −20°C for laboratory analysis.
The length thresholds used to classify individuals as juveniles or adults were 11 cm for anchovy and 13 cm for sardine, following Bachiller et al. (2020). In total, 1,337 fish were collected: 173 anchovies and 452 sardines from the SS area, and 406 anchovies and 306 sardines from the TR area (see Table 1 for details).
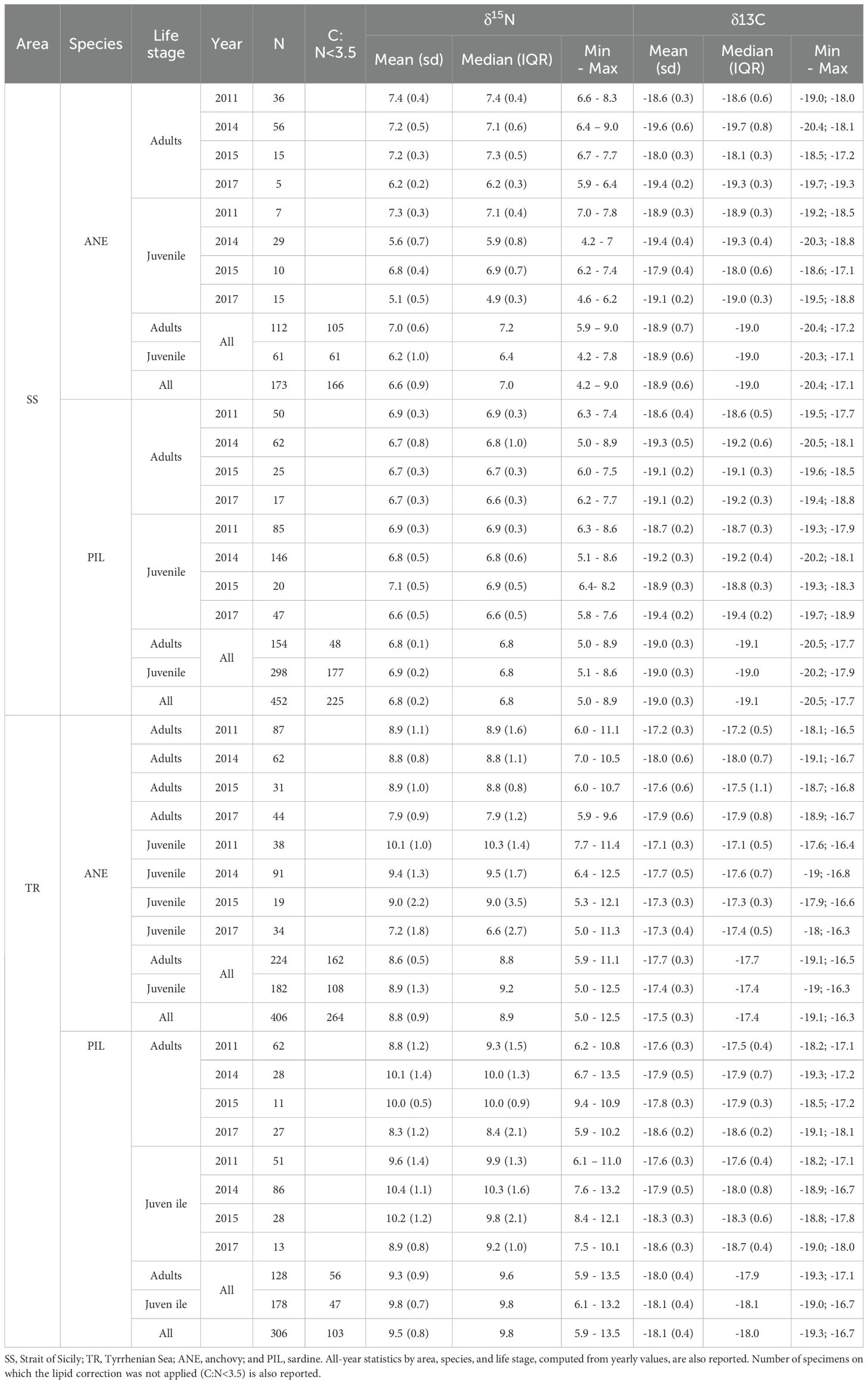
Table 1. Descriptive statistic δ15N and δ13C by area, year, species, and life stage reporting mean, standard deviation (SD), median, inter quartile range (IQR), minimum and maximum values.
Stable isotope analysis
A portion of white muscle near the dorsal fin was extracted from each individual fish, oven-dried at a constant temperature (60°C for 24 h), powdered, and weighed (0.5 mg) into tin capsules.
Although some authors recommend lipid extraction in fish and zooplankton samples prior to stable isotope analysis (Cardona et al., 2012; Tanaka et al., 2008), we did not apply a defatting procedure, consistent with other studies (Logan et al., 2008; Fanelli et al., 2011; Iitembu et al., 2012; Madigan et al., 2012). Instead, we assessed lipid content using the relationship between C:N ratios and δ13C signatures. The method proposed by Logan et al. (2008) to normalise the δ13C values from lipid concentration (Rumolo et al., 2016) was used in order to compare the isotopic values of sardine and anchovy collected in similar areas. In particular, Equation (1a) proposed by Logan et al. (2008) was used for white fish muscle with C:N > 3.5.
where δ13C is the isotope value with lipid content, δ13C’ is the corrected value, a = 7.415, b = -22.732 and c = 0.746.
Stable isotope measurements and C and N concentrations were carried out at the iCONa Laboratory of the University of Campania using a Thermo Fisher Flash EA 1112 elemental analyser coupled to a Thermo Delta V Advantage isotope ratio mass spectrometer (IRMS). Samples were analysed alongside blank tin cups and certified analytical-grade urea of certificated isotopic composition. Three urea capsules were analysed at the beginning of each sequence, and one was analysed every six samples as a quality control measure and to compensate for potential instrument drift. Experimental precision, based on the standard deviation of replicate internal standards, was <0.2‰ for δ15N and<0.1‰ for δ13C. International IAEA reference standards were used to calibrate sample measurements.
The δ15N and δ13C values were obtained in parts per thousand (‰) relative to atmospheric N2 and Vienna Pee Dee Belemnite (VPDB), respectively, using the following formula:
where R = 13C/12C or 15N/14N.
Statistical analysis
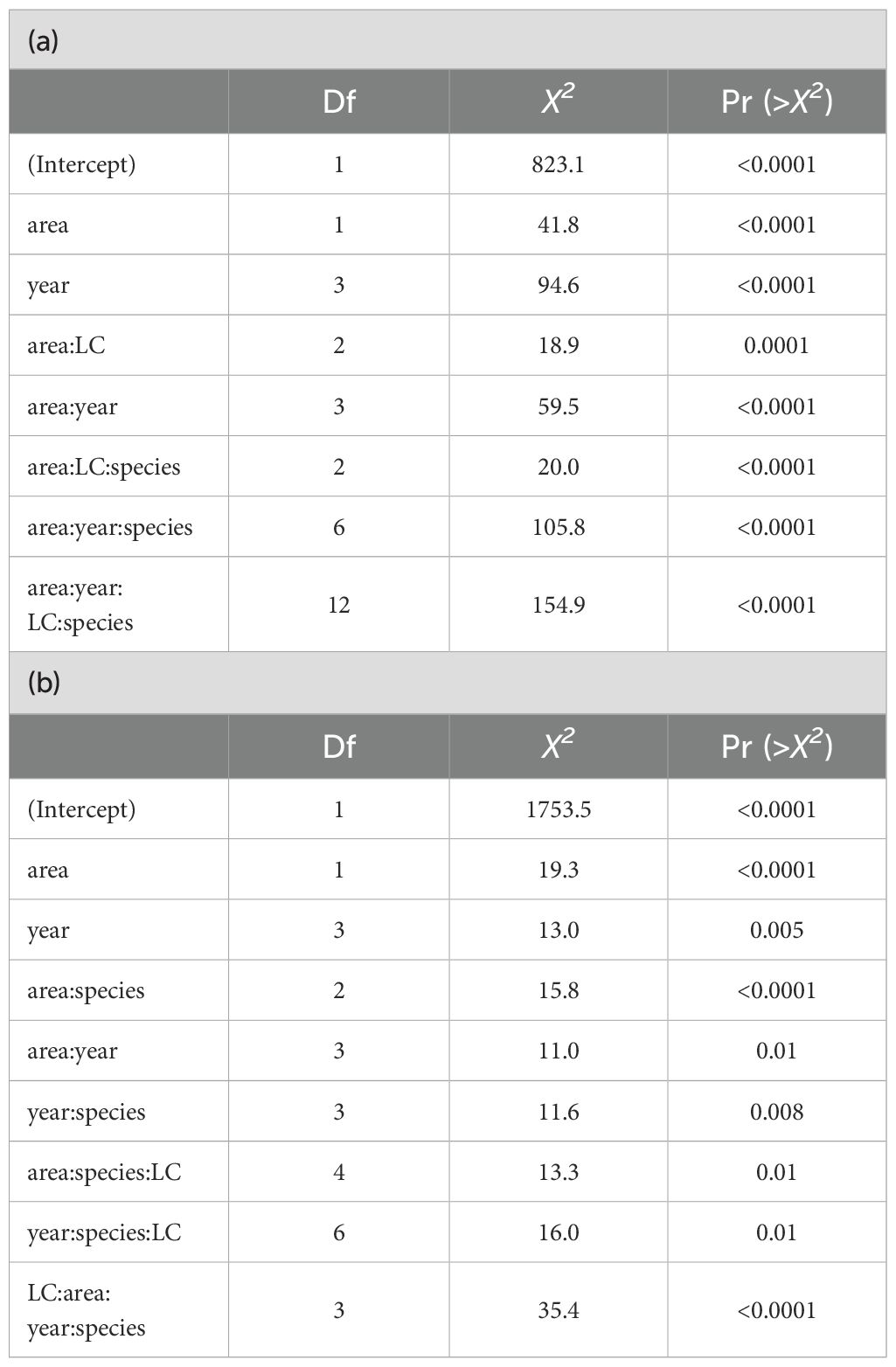
Exploratory analysis of δ13C and δ15N values revealed differences in variance between the two areas, as well as significant relationships between isotope values and fish length in specific cases only (Supplementary Figures S1, S2). Consequently, a generalised least squares (GLS)-based ANOVA approach was used to test for differences in δ15N and δ13C values across areas, years, life stages, and species, explicitly accounting for (i) possible interactions between factors and length class, and (ii) heteroscedasticity by applying variance weighting according to the Area and Year factors.
For both δ13C and δ15N, GLS-based models were constructed to include all possible interactions among predictors. The best-fitting model was selected based on Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) values. Given the expected significant correlation between length class and isotope values—and the unbalanced nature of the dataset—post hoc tests were based on estimated marginal means using the Tukey method.
The standard ellipse area (SEAb; Jackson et al., 2011) was calculated to infer isotopic niche width, a proxy for the trophic diversity (or trophic breadth) exploited by the groups considered. Isotopic niche overlap was assessed by calculating the ratio between the area of the geometric intersection of the ellipses and the area of their union. As Bayesian inference techniques provide robust statistical comparisons for datasets with different sample sizes (Navarro et al., 2020), a Bayesian approach was adopted for both niche width and ellipse overlap, allowing formal testing of the significance of observed differences.
All statistical analyses were performed in R (v.4.1; R Core Team, 2023). GLS models were developed using the ‘nlme’ package (Pinheiro et al., 2023), post hoc tests were conducted with the ‘emmeans’ package (Lenth, 2023), and isotopic niche using the SIBER package (Jackson et al., 2011).
Results
Summary statistics for δ¹5N and δ¹³C values (mean, standard deviation, median, interquartile range, minimum/maximum values, and number of observations) by area, year, species, and life stage are reported in Table 1. Overall, the differences between the mean and median values of δ15N and δ13C were small (maximum absolute differences were 0.6‰ and 0.2‰, respectively), highlighting the rather symmetric distributions for both δ15N and δ13C values in each group (i.e., area, year, species, and life stage).
Pearson correlation analyses between δ15N (and δ13C) fish total length (TL) showed both positive and negative correlations for anchovy and sardine across years and areas, when significant (p < 0.05) (Supplementary Figure S2). For anchovy in the SS area, δ¹5N showed a positive correlation in 2014 and 2017, while δ¹³C showed a negative correlation in 2017 for δ13C; in TR it was positive in 2017 but negative in 2011 and 2014 in terms of δ15N and always negative in 2014, 2015 and 2017 for δ13C. For sardines, when significant, the correlations were always negative in terms of δ15N in both areas (2015 in SS; 2011 and 2017 in TR). For δ¹³C, the correlation was negative in 2014 and 2015 and positive in 2017 in SS, and positive only in 2015 in TR.
Preliminary fits of GLS models highlighted the effect of unequal variance and the lack of independence of residuals for both δ15N and δ13C. Consequently, the within-group heteroscedasticity structure was modelled according to area and year factor, while the within-group correlation structure accounted for the autocorrelation at catch operation level. The predictors selected for δ15N and δ13C best models were area and year factors as well as different interactions involving length class and other factors (Table 2).
δ15N and δ13C differences between areas considering years, species and life stages
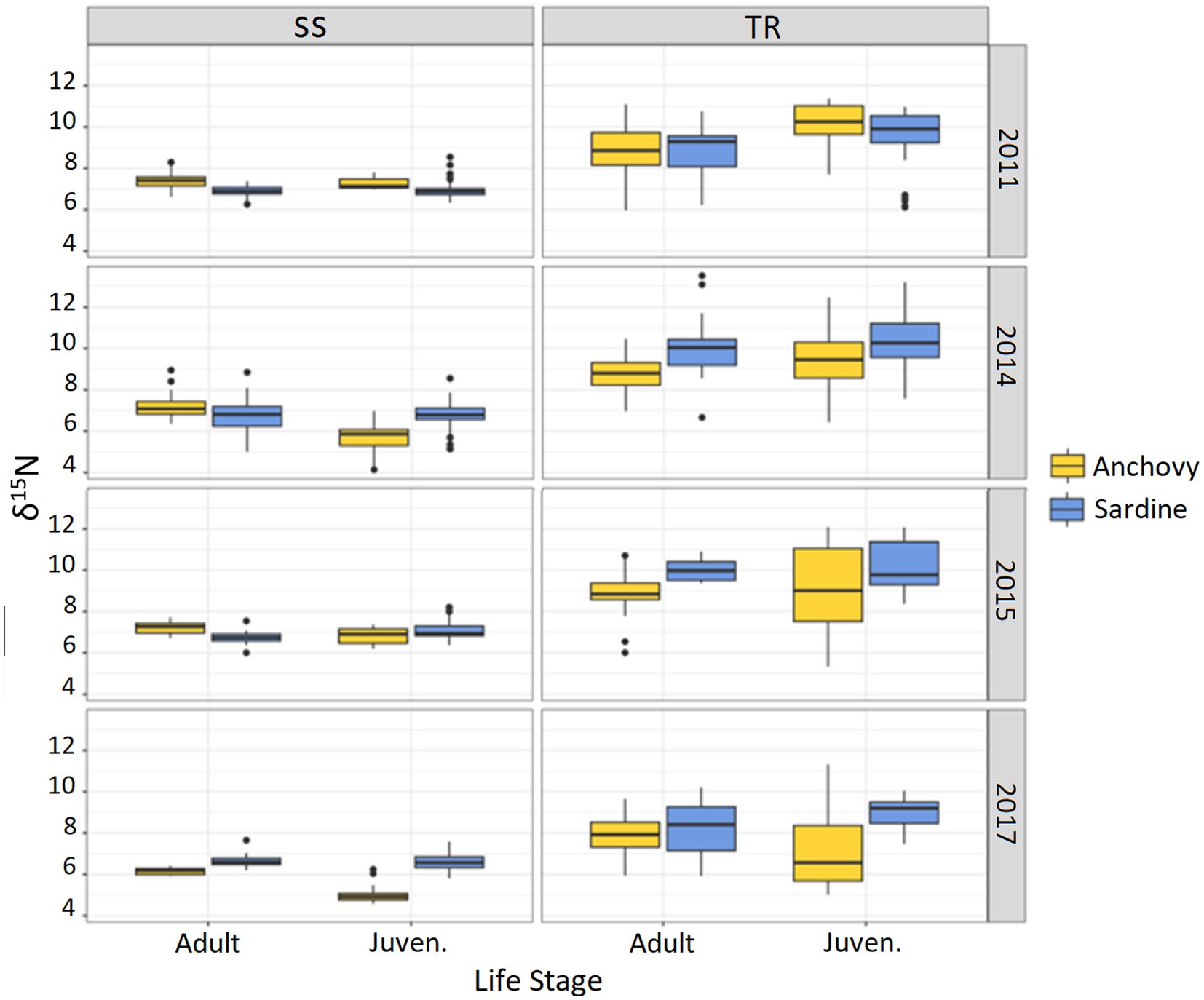
For δ15N, both anchovy and sardine showed lower values in the SS area than in the TR area, with the latter in some cases displaying higher variability (Table 1; Figures 3, 4). In particular, δ15N values for anchovy ranged from 4.2‰ to 9.0‰ in SS and from 5.0‰ to 12.5‰ in TR (mean across all years: 6.6‰ and 8.8‰, respectively). For sardine, δ15N values ranged from 5.0‰ to 8.9‰ in SS and from 5.9‰ to 13.5‰ in TR (mean: 6.8‰ and 9.5‰, respectively).
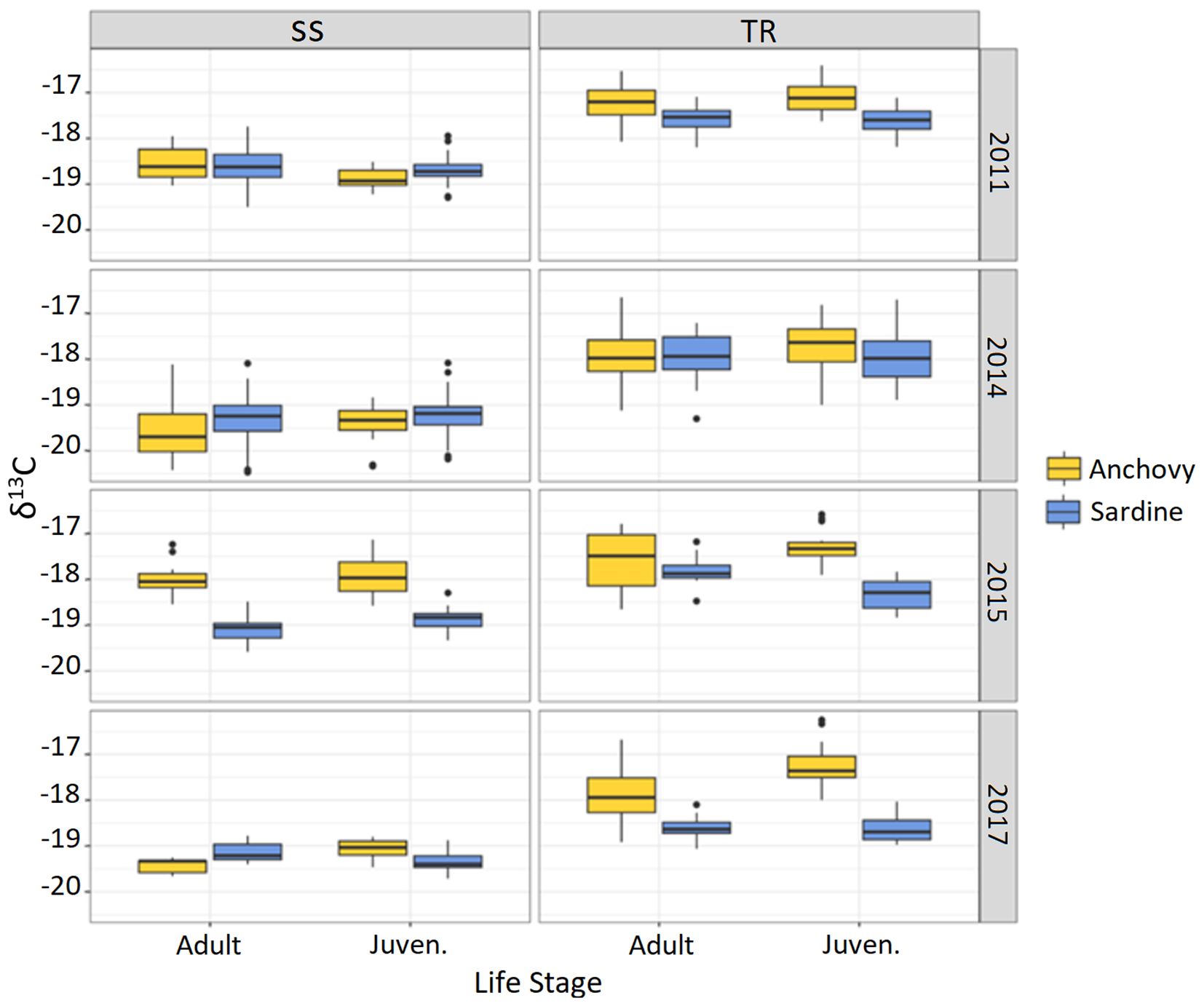
For δ13C, the SS area generally showed less enriched values than TR, although in 2015 the difference appeared smaller than in other years (Table 1; Figure 5). For anchovy, δ¹³C values ranged from −20.4‰ to −17.1‰ in SS and from −19.1‰ to −16.3‰ in TR (mean: −18.9‰ and −17.5‰, respectively). For sardine, δ13C values from −20.5‰ to −17.7‰ in SS and from −19.3‰ to −16.7‰ in TR (mean: −19.0‰ and −18.1‰, respectively).
Post hoc tests revealed significant differences between the two areas in all comparisons (Supplementary Table S2), except for δ15N in 2014 and δ13C in 2015 in adult anchovies. In all cases where differences were significant, the SS area showed lower average values than TR for both isotopes (Supplementary Table S2). In the case of δ15N, for each species, the difference between juveniles in the two areas was generally higher than the one observed for adults.
δ15N and δ13C differences between years considering areas, species, and life stage
Isotopic differences between years were evaluated to highlight the temporal stability of average values at the area, species, and life stage levels (Supplementary Table S3). In the SS area, differences in terms of δ15N values between years were generally not significant for adults of either species. However, significant differences were observed for anchovy juveniles in all pairwise comparisons and for sardine juveniles only in the 2014–2015 comparison. A similar pattern was found in the TR area, though significant differences also emerged for adults of both species.
For δ13C values, significant differences in the SS area were detected for both adults and juveniles of both species. On the contrary, in the TR area, significant differences were less frequent for anchovy (both adults and juveniles) but more consistent for sardine (Supplementary Table S3).
δ15N and δ13C differences between life stages (juveniles and adults) of each species considering years and areas
The comparison between adults and juveniles of both species across years and areas (Supplementary Table S4) showed that, in the TR area, differences in δ15N were consistently significant for anchovy in all years but not for sardine. In the SS area, no clear or consistent patterns were observed. For δ13C, differences between life stages were generally not significant for either species in either area.
δ15N and δ13C differences between species considering year and area
When comparing δ15N values between species at the same life stage (i.e., adult anchovy vs. adult sardine) within the same area (Supplementary Table S5), significant differences were more frequent in the SS area than in the TR where differences were significant in only one case (year 2014). In terms of δ13C, differences were significant in one case only in both areas (SS: 2015; TR: 2011).
δ15N and δ13C differences between life stages of the two species considering areas and years
Comparisons between adults and juveniles of the two species at the area and year level (Supplementary Table S6) revealed that, in the SS area, δ¹5N average values were generally comparable between adult anchovies and juvenile sardines, except in one case (2014), where a significant difference was observed. In contrast, significant differences in δ¹5N were found in three out of 4 years when comparing juvenile anchovies and adult sardines.
In the TR area, the comparison between adult anchovies and juvenile sardines showed significant differences in 2 out of 4 years for both isotopes. Similarly, for δ¹5N, significant differences were also found in 2 out of 4 years when comparing juvenile anchovies and adult sardines, whereas for δ¹³C, the difference was significant in only one case.
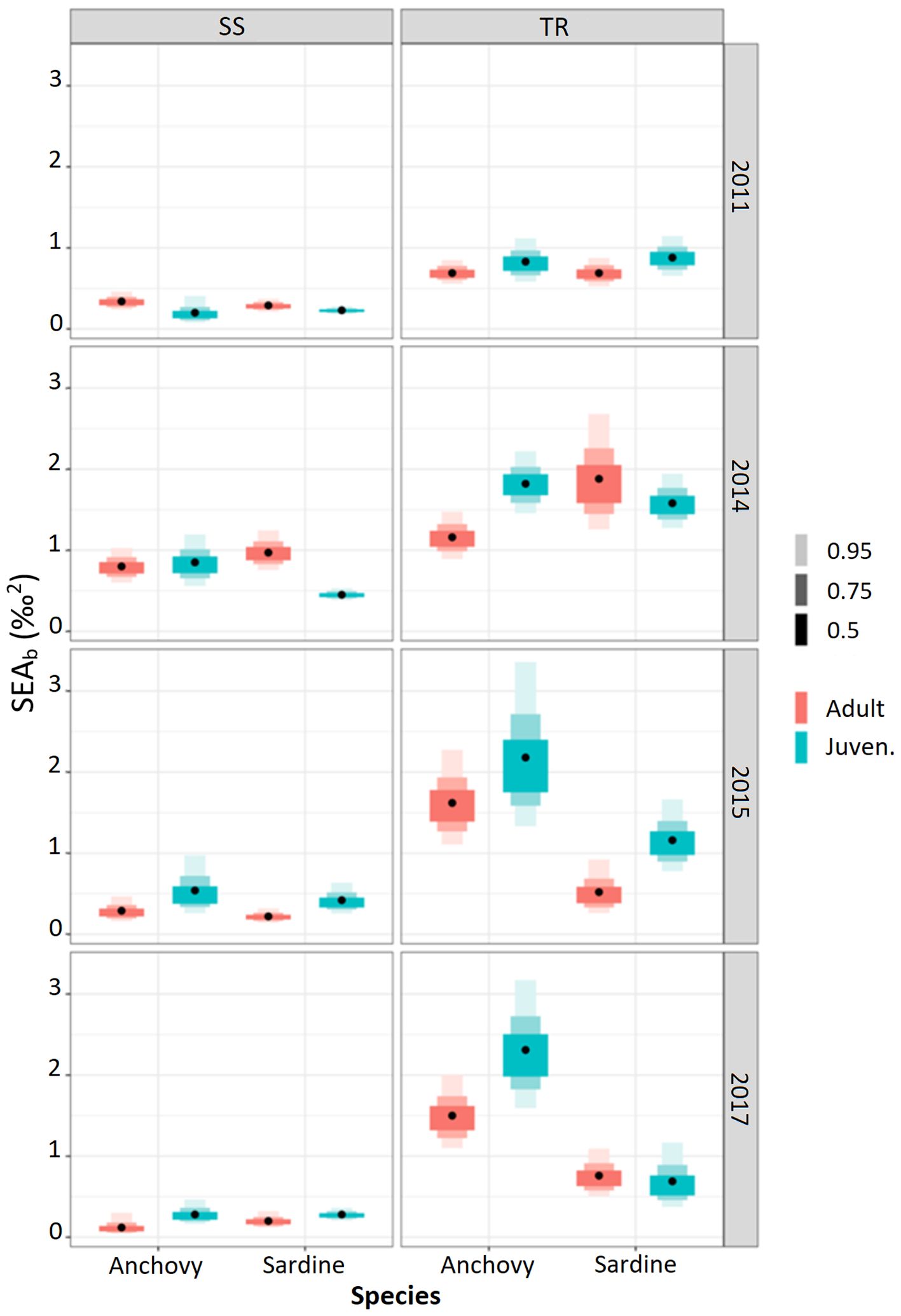
Isotopic niche analysis
The width and overlap of isotopic niches were computed by area, year, species, and life stage (Figures 5, 6; Supplementary Table S1). The comparison of isotopic niche width between areas revealed that niche width in TR was and significantly wider than in the SS area (Supplementary Table S2). In contrast, comparison across years showed significant differences in only a few cases, suggesting a certain degree of temporal stability at the area/species/life stage level (Supplementary Table S3).
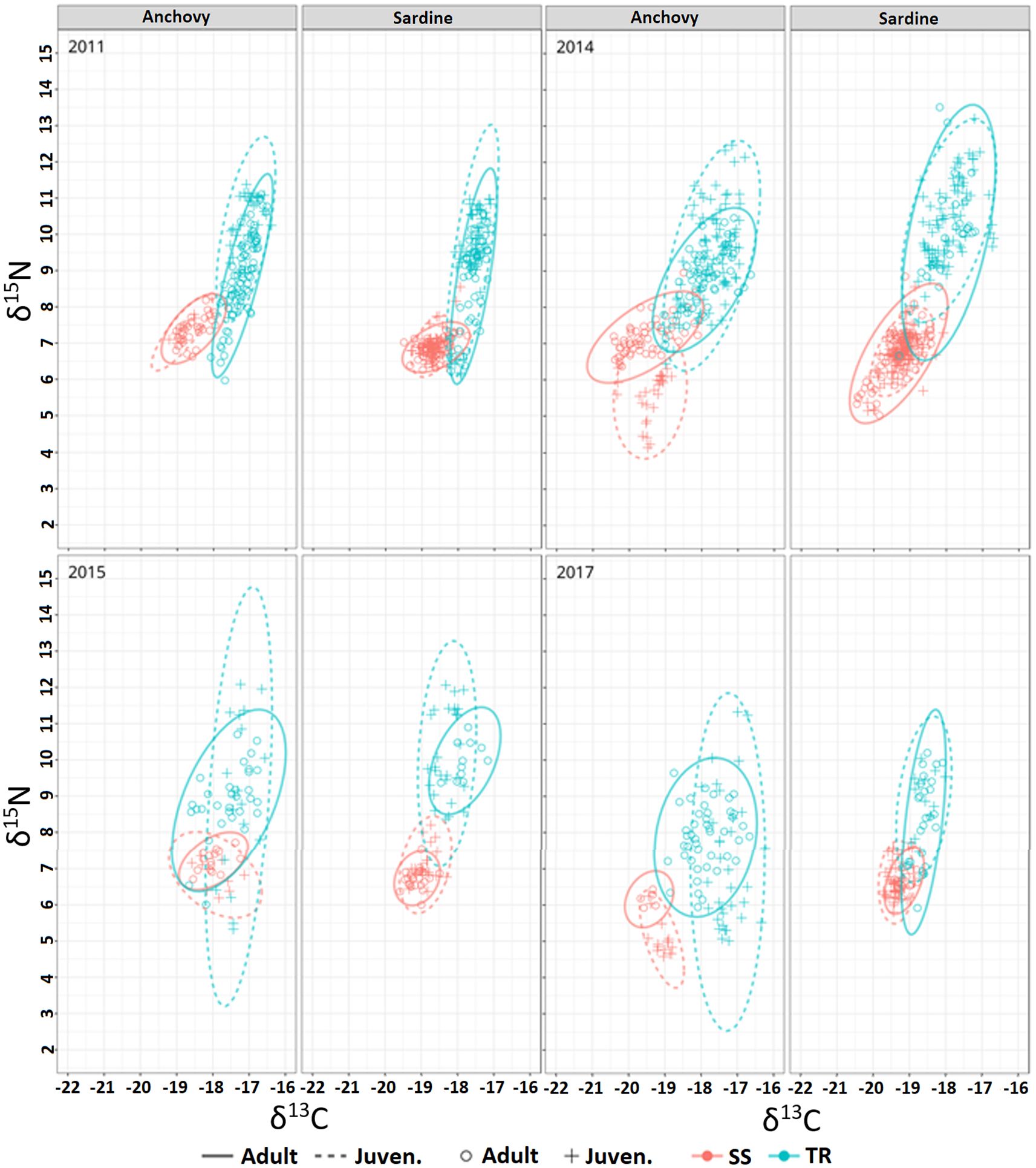
Figure 6. Isotopic ellipses (95%) overlap by area (SS: red lines; TR: blue lines), species, life stage (Adults: continuous lines; Juveniles: dotted lines) and year.
The comparison of niche width between adults and juveniles of the same species and within the same area (Supplementary Table S4) indicated no significant differences for anchovy in SS, whereas significant differences were observed for sardine in two out of four cases. In the TR area, significant differences in niche width were found for both species. In addition, isotopic niche overlap between adults and juveniles of both species was significant in all cases except one (Supplementary Table S4). On average, in the SS area, sardine showed higher overlap than anchovy (30% and 19%, respectively), while in the TR area, overlap was comparable between species (40% for anchovy and 45% for sardine). For both species, overlap was lower in SS than in TR.
When niche width was compared between adult anchovy and adult sardine, no significant differences were observed in SS, while in TR significant differences were found in three out of four cases (Supplementary Table S5). The comparison of isotopic niche overlap between adults of the two species by area and year, evidenced in the SS area significant overlap in two cases out of four, while in the TR area the overlap was found significant in all cases (Supplementary Table S5).
Finally, when comparing niche width across both life stage and species, significant differences were observed in specific cases only, with no consistent pattern (Supplementary Table S6). In terms of niche overlap, in the TR area significant overlap was found in all cases except one, whereas in the SS area overlap was significant in only half of the comparisons (Supplementary Table S6).
Discussion
The obtained results showed that differences in isotopic signals between the two species were greater between areas than within areas across all sampled years—except for δ¹³C in adult anchovy in 2015 (Supplementary Table S2). Specifically, both nitrogen and carbon stable isotopes showed higher values in the Tyrrhenian area (TR) compared with the Strait of Sicily (SS) for both species (Figures 3, 4). It is known that inter-specific variations in the feeding habits of marine fishes are consistent with inter-specific differences associated to local factors that provide variations in the baseline isotopic values in food webs and affect prey presence and abundance (e.g., Costalago and Palomera, 2014; Costalago et al., 2014; Rumolo et al., 2016).
The SS area is less impacted by continental waters, with fewer major cities, effluents, and coastal industries compared to the TR area. Coastal upwelling along the southern coast of Sicily and the presence of offshore banks (Piccioni et al., 1988) generate organic matter during upwelling events, which—after remineralisation—supports primary production (Alvarez-Salgado et al., 2002; Bode et al., 2004). In sectors as the SS area, phytoplankton generally serves as the main foundation of the food web. Productivity is primarily regulated by oceanographic processes such as upwelling and seasonal changes in stratification and mixing, which fertilise the euphotic zone (Bode et al., 2006).
Although phytoplankton in upwelling regions often becomes enriched in 15N (Montoya et al., 1990; Goering et al., 1990; Rolff, 2000), resulting in enriched zooplankton, Rumolo et al. (2016) reported low δ15N values in particulate organic matter collected in the SS area. This may be explained by the discontinuous nature of coastal upwelling along the southern coast of Sicily (Piccioni et al., 1988; Bonanno et al., 2014), where summer phytoplankton growth likely relies on regenerated nitrogen—typically depleted in 15N (Montoya et al., 1990). This mechanism may account for the lower δ15N values found in both anchovy and sardine in the SS area.
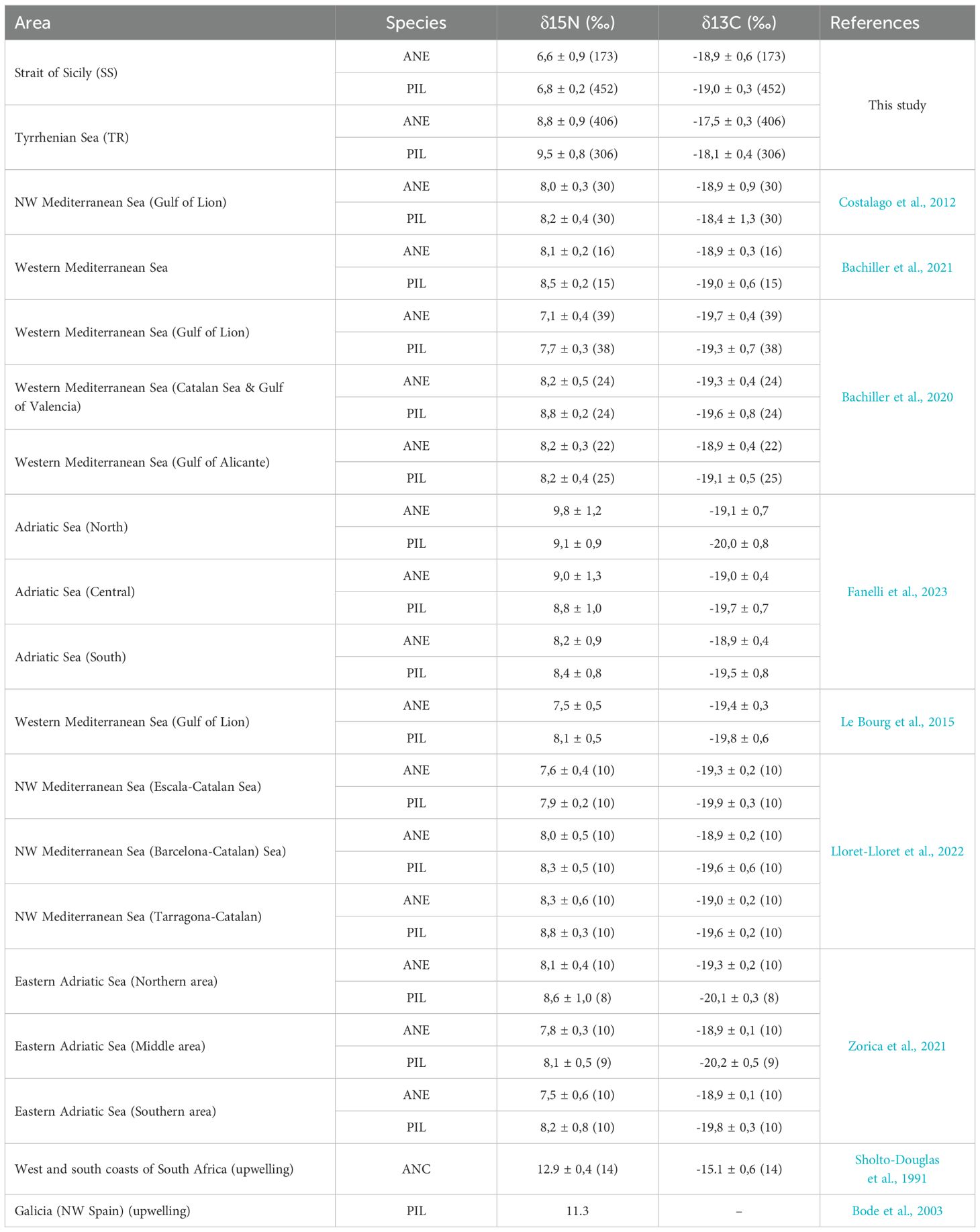
In addition, the mean isotopic signals of anchovy and sardine in the SS area were lower than those reported in the literature (Table 3) for other parts of the Mediterranean Sea or similar upwelling-influenced oceanic systems (Bode et al., 2003; Sholto-Douglas et al., 1991; Costalago et al., 2012; Le Bourg et al., 2015; Bachiller et al., 2020, 2021; Lloret-Lloret et al., 2022; Fanelli et al., 2023). In this context, the absence of a consistent pattern in the correlation (Supplementary Figures S1, S2) between the δ15N and fish total length (positive, null, or negative across years for both species; Supplementary Figures S1, S2), along with the narrow δ15N range, reinforces the hypothesis that anchovy and sardine in the SS area may exploit similar resources—possibly feeding directly on particulate organic matter with low isotopic signatures and/or on prey occupying lower trophic positions depending on resource availability.
However, while juvenile and adult sardines in the SS area exhibited similar feeding behaviour, as indicated by their isotopic niche overlap, anchovies showed greater feeding variability between life stages, with relatively weak isotopic overlap (never exceeding 30%; Supplementary Table S4). These results align with previous studies (e.g., Tudela and Palomera, 1997; Costalago et al., 2012), which describe juvenile and adult sardines as opportunistic filter-feeders with a more heterogeneous diet than anchovy. For anchovy, several authors have reported high variability in feeding between life stages, sometimes involving ontogenetic shifts (Bacha and Amara, 2009; Pepin and Penney, 1997; Scharf et al., 2000; Bachiller and Irigoien, 2013; Palomera et al., 2007; Plounevez and Champalbert, 1999, 2000), while others reported no ontogenetic shift (Bulgakova, 1993; Tudela and Palomera, 1995, 1997; Plounevez and Champalbert, 1999, 2000; Borme et al., 2009; Van der Lingen et al., 2009; Bacha and Amara, 2009; Catalan et al., 2010).
Regarding δ13C, which is generally considered a conservative tracer of the primary producers at the base of the food web and thus a useful indicator of foraging habitat (France, 1995; Hobson, 1999), the values presented in the SS area for both species were comparable to those reported from other Mediterranean regions (see Table 3). This suggests that anchovy and sardine in SS likely explored similar habitats and shared food sources.
In contrast to the SS area, the higher δ15N and δ13C values observed in anchovy and sardine in the TR area may be attributed either to a strong feeding preference for larger prey items or to feeding within an environment characterised by a more isotopically enriched baseline. Although particulate organic matter (POM) and zooplankton samples were not collected during the 2014, 2015, and 2017 surveys, this study focused on isotopic niche width and overlap in consumers (anchovy and sardine), which revealed greater differences between areas than among years. These area-based differences were also confirmed by Rumolo et al. (2016), who, analysing isotopic data from a single year in the Strait of Sicily and the Tyrrhenian Sea, found higher δ15N values of POM in the Tyrrhenian Sea. This was attributed to riverine input supplying high levels of inorganic nutrients, which can lead to 15N enrichment in POM (e.g., McClelland et al., 1997), and in turn to enriched δ15N in zooplankton and fish.
Moreover, Rumolo et al. (2016) reported higher isotopic values in adult anchovies and sardines from the Tyrrhenian Sea compared to other Mediterranean regions, linking these values to a higher dietary contribution of mesozooplankton species, particularly chaetognaths and euphausiids. Although the presence of chaetognaths and euphausiaceans such as Meganyctiphanes norvegica in the TR area has not been extensively studied, Wiebe and D’Abramo (1972) found Euphausia kcrohnii, Nematoscelis megalops, Meganyctiphanes norvegica, and Stylocheiron abbreviatum to be abundant in the western Tyrrhenian basin, especially in May and June. Moreover, Mussi et al. (2004) reported the presence of crustacean exoskeletons—belonging to Meganyctiphanes norvegica—in fin whale faecal material collected near Ischia Island (Figure 1), close to the Cuma submarine canyon, where marine mammals have been repeatedly observed (Mussi et al., 1999; 2014).
The co-occurrence of large predators and small pelagic fish in the TR area (Leonori et al., 2021) is likely linked to the abundance of large zooplankton species, potentially triggered by the high load of nutrients provided by different mechanisms such as submarine canyon advection and river discharge. Isotopic values of anchovy and sardine in the TR area—particularly δ15N—were consistent with those reported in the northern Adriatic Sea (Table 3), a region with comparable environmental characteristics, including the presence of large rivers and densely populated coastlines (Fanelli et al., 2023; Micheli et al., 2013). In the TR area, anchovy showed no consistent correlation between δ¹5N and total length (TL); in some cases, higher δ¹5N values were found in juveniles than in adults (Supplementary Table S4). For sardine, results indicated a negative correlation between δ15N and TL, though not always statistically significant. This trend is difficult to interpret due to the limited availability of isotopic and stomach content data for juvenile small pelagic fish in the Mediterranean (Bachiller et al., 2020). However, some authors (Fanelli et al., 2023; Bode et al., 2003 and references therein) found this negative trend in small pelagic fish interpreting such relationship as the consequence of the increasing consumption of phytoplankton by adults, as most clupeids are known to employ both particle-feeding and filter-feeding (James, 1988).
In the TR area, δ13C values were, on average, more enriched than in other areas of the Mediterranean Sea, suggesting that both species may explore more coastal areas and carbon sources (Table 3). However, our results, which examined dietary variations based on assimilated food (in about one month), did not allow us to estimate individual specialisation linked to habitat use. Such an assessment would require considering the temporal and spatial variability in the δ13C of both prey and consumers.
Taking into account the investigated inter- and intraspecific differences in isotopic composition—by comparing area, life stage, and year—the patterns of isotopic niche overlap and width for the two species in each area and between areas (Supplementary Tables S2–S6) showed the presence of different feeding systems explored by anchovy and sardine. Although the greater niche overlap observed in TR compared to SS may indicate high interspecific competition, the broader isotopic niche widths indicate dietary plasticity. This plasticity may enable the development of alternative feeding strategies (e.g. division of the niche between juveniles and adults) in response to competition (Andrades et al., 2019; Cathcart et al., 2019).
Moreover, the higher isotopic values and trophic overlap observed in sardine and anchovy in the TR area could also be related to increased consumption of larger prey (e.g., decapods and euphausiids), as previously reported by Rumolo et al. (2016), and/or driven by the presence of other high-trophic-level prey such as siphonophores and jellyfish, as noted by Bachiller et al. (2020) in the western Mediterranean Sea. In contrast, in the SS area, the low isotopic values together with smaller isotopic niche width, suggested the presence of lower trophic levels organisms and less food resources explored by the fish species. Furthermore, the variability observed in isotopic niche overlap may reflect differences in the spatial distribution of the two fish species (Barra et al., 2015).
In addition to the clear difference evidenced between the SS and TR areas, it is important to highlight that observed differences are temporally stable, indicating a certain degree of stability in feeding behaviour. This stability likely reflects underlying ecosystem characteristics rather than interannual variability in environmental conditions. Nonetheless, the high variability observed in the SS area in terms of niche overlap, along with the lower variability in isotopic values compared to TR—suggests that the SS area may be a more sensitive ecosystem, more vulnerable to environmental disturbances, including those induced by climate change.
Given the key role of small pelagic fish in energy transfer along the marine food web, careful management of these resources is should be carefully considered. On the contrary, the TR area could appear to be a more robust ecosystem, capable of handling natural or anthropogenic disturbances. To support this hypothesis, future studies should include isotopic analyses of POM and zooplankton assemblages, using a more targeted sampling design spanning multiple years.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
PR: Resources, Data curation, Writing – original draft, Visualization, Conceptualization, Writing – review & editing, Investigation, Validation, Methodology, Supervision, Formal analysis. MB: Software, Formal analysis, Writing – review & editing, Conceptualization, Data curation, Writing – original draft, Methodology, Resources, Visualization, Investigation, Validation. AB: Visualization, Data curation, Resources, Writing – original draft, Conceptualization, Investigation, Validation, Writing – review & editing, Methodology, Supervision. SA: Visualization, Data curation, Formal analysis, Methodology, Conceptualization, Writing – review & editing, Investigation, Validation, Supervision. RF: Writing – review & editing, Visualization, Formal analysis, Writing – original draft, Methodology, Supervision, Validation, Data curation, Investigation. AG: Methodology, Data curation, Investigation, Writing – original draft, Formal analysis, Visualization. GB: Writing – original draft, Methodology, Visualization, Data curation, Validation, Investigation, Conceptualization, Writing – review & editing. SGh: Formal analysis, Data curation, Methodology, Writing – original draft, Visualization, Investigation, Supervision. SGe: Formal analysis, Writing – original draft, Methodology, Data curation, Investigation, Supervision. MD: Software, Writing – original draft, Supervision, Writing – review & editing, Investigation, Formal analysis, Conceptualization, Methodology, Validation, Data curation. CL: Writing – original draft, Investigation, Visualization, Formal analysis, Software, Validation, Data curation, Conceptualization, Supervision, Writing – review & editing, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 – Call for Tender No. 3138 of 16 December 2021, rectified by Decree No. 3175 of 18 December 2021, issued by the Italian Ministry of University and Research and funded by the European Union - NextGenerationEU. Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022, adopted by the Italian Ministry of University and Research, CUP B83C22002930006. Project title: “National Biodiversity Future Center – NBFC.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1602042/full#supplementary-material
References
Aiello G. and Caccavale M. (2023). The coastal areas of the Bay of Naples: the sedimentary dynamic and geological evolution of the Naples Canyons. Geosciences 13, 226. doi: 10.3390/geosciences13080226
Alheit J., Pohlmann T., Casini M., Greve W., Hinrichs R., Mathis M., et al. (2012). Climate variability drives anchovies and sardines into the North and Baltic Seas. Prog. Oceanogr. 96, 128–139. doi: 10.1016/j.pocean.2011.11.015
Alvarez-Salgado X. A., Beloso S., Joint I., Nogueira E., Chou L., Perez F. F., et al. (2002). New production of the NW Iberian shelf during the upwelling season over the period 1982–1999. Deep Sea Res. 49, 1725–1739. doi: 10.1016/S0967-0637(02)00094-8
Andrades R., Jackson A. L., Macieira R. M., Reis-Filho J. A., Bernardino A. F., Joyeux J. C., et al. (2019). Niche-related processes in island intertidal communities inferred from stable isotopes data. Ecol. Indic. 104, 648–658. doi: 10.1016/j.ecolind.2019.05.039
Bacha M. and Amara R. (2009). Spatial, temporal and ontogenetic variation in diet of anchovy (Engraulis encrasicolus) on the Algerian coast (SW Mediterranean). Estuar. Coast. Shelf Sci. 85, 257–264. doi: 10.1016/j.ecss.2009.08.009
Bachiller E., Albo-Puigserver M., Giménez J., Pennino M. G., Marí-Mena N., Esteban A., et al. (2020). A trophic atitudinal gradient revealed in anchovy and sardine from the Western Mediterranean Sea using a multi-proxy approach. Sci. Rep. 10, 17598. doi: 10.1038/s41598-020-74602-y
Bachiller E., Gimenez J., Albo-Puigserver M., Pennino M. G., Marí-Mena N., Esteban A., et al. (2021). Trophic niche overlap between round sardinella (Sardinella aurita) and sympatric pelagic fish species in the Western Mediterranean. Ecol. Evol. 11, 16126–16142. doi: 10.1002/ece3.8293
Bachiller E. and Irigoien X. (2013). Allometric relations and consequences or feeding in small pelagic fish in the Bay of Biscay. ICES J. Mar. Sci. 70, 232–243. doi: 10.1093/icesjms/fss17
Barra M., Petitgas P., Bonanno A., Somarakis S., Woillez M., Machias A., et al. (2015). Interannual Changes in biomass affect the spatial aggregations of anchovy and sardine as evidenced by geostatistical and spatial indicators. PloS One 10, e0135808. doi: 10.1371/journal.pone.0135808
Bearhop S., Adams C. E., Waldron S., Fuller R. A., and Macleod H. (2004). Determining trophic niche width: a novel approach using stable isotope analysis. J. Anim. Ecol. 73, 1007–1012. doi: 10.1111/j.0021-8790.2004.00861.x
Bode A., Alvarez-Ossorio M. T., and Varela M. (2006). Phytoplankton andmacrophyte contributions to littoral food webs in the Galician upwelling estimatedfrom stable isotopes. Mar. Ecol. Prog. Ser. 318, 89–102. doi: 10.3354/meps318089
Bode A., Barquero S., González N., Alvarez-Ossorio M. T., and Varela M. (2004). Contribution of heterotrophic plankton to nitrogen regeneration in the upwelling ecosystem of A Coruña (NW Spain). J. Plankton Res. 26, 1–18. doi: 10.1093/plankt/fbh003
Bode A., Carrera P., and Lens S. (2003). The pelagic food web in the upwelling ecosystem of Galicia (NW Spain) during spring: natural abundance of stable carbon and nitrogen isotopes. ICES J. Mar. Sci. 60, 11–22. doi: 10.1006/jmsc.2002.1326
Bonanno A., Barra M., Basilone G., Genovese S., Rumolo P., Goncharov S., et al. (2016). Environmental processes driving anchovy and sardine distribution in a highly variable environment: The role of the coastal structure and riverine input. Fisheries Oceanogr. 25, 471–490. doi: 10.1111/fog.12166
Bonanno A., Barra M., Mifsud R., Basilone G., Genovese S., Di Bitetto M., et al. (2018). Space utilization by key species of the pelagic fish community in an upwelling ecosystem of the Mediterranean Sea. Hydrobiologia 821, 173–190. doi: 10.1007/s10750-017-3350-9
Bonanno A., Placenti F., Basilone G., Mifsud R., Genovese S., Patti B., et al. (2014). Variability of water mass properties in the Strait of Sicily in summer period of 1998–2013. Ocean Sci. 10, 759–770. doi: 10.5194/os-10-759-2014
Borme D., Tirelli V., Brandt S., Umani S. F., and Arneri E. (2009). Diet of Engraulis encrasicolus in the northern Adriatic Sea (Mediterranean): ontogenetic changes and feeding selectivity. Mar. Ecol. Prog. Ser. 392, 193–209. doi: 10.3354/meps08214
Buchheister A. and Latour R. J. (2010). Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Can. J. Fish Aquat. Sci. 67, 445–461. doi: 10.1139/F09-196
Bulgakova Y. V. (1993). Daily feeding dynamics of the Black Sea anchovy, Engraulis encrasicolus. J. Ichthyol. 33, 78–88.
Cardona L., Álvarez De Quevedo I., Borrell A., and Aguilar A. (2012). Massive consumption of gelatinous plankton by Mediterranean apex predators. PloS One 7, e31329. doi: 10.1371/journal.pone.0031329
Castro L. R., González V., Claramunt G., Barrientos P., and Soto S. (2020). Stable isotopes (δ13C, δ15N) seasonal changes in particulate organic matter and in different life stages of anchoveta (Engraulis ringens) in response to local and large scale oceanographic variations in north and central Chile. Prog. Oceanogr. 186, 102342. doi: 10.1016/j.pocean.2020.102342
Catalan I. A., Folkvord A., Palomera I., Quílez-Badía G., Kallianoti F., Tselepides A., et al. (2010). Growth and feeding patterns of European anchovy (Engraulis encrasicolus) early life stages in the Aegean Sea (NE Mediterranean). Estuar. Coast. Shelf Sci. 86, 299–312. doi: 10.1016/j.ecss.2009.11.033
Cathcart C. N., Dunker K. J., Quinn T. P., Sepulveda A. J., von Hippel F. A., Wizik A., et al. (2019). Trophic plasticity and the invasion of a renowned piscivore: a diet synthesis of northern pike (Esox lucius) from the native and introduced ranges in Alaska, USA. Biol. Invasions 21, 1379–1392. doi: 10.1007/s10530-018-1909-7
Champagne-Philippe M., Harang L., and Le Vourch J. (1982). Remote sensing of thermal fronts. Societe Francaise Photogrammetrie Teledetection Bull. 86, 5–23.
Chavez F. P., Ryan J., Lluch-Cota S. E., and Ñiquen. M. (2003). From anchovies to sardines and back: Multidecadal change in the Pacific Ocean. Science 299, 217–221. doi: 10.1126/science.1075880
Costalago D., Navarro J., Alvarez-Calleja I., and Palomera I. (2012). Ontogenetic and seasonal changes in the feeding habits and trophic levels of two small pelagic fish species. Mar. Ecol. Prog. Ser. 460, 169e181. doi: 10.3354/meps09751
Costalago D. and Palomera I. (2014). Feeding of European pilchard (Sardina pilchardus) in the northwestern Mediterranean: from late larvae to adults. Scientia Marina 78, 41–54. doi: 10.3989/scimar.03898.06D
Costalago D., Palomera I., and Tirelli V. (2014). Seasonal comparison of the diets of juvenileEuropean anchovy Engraulis encrasicolus and sardine Sardina pilchardus in the Gulf of Lions. J. Sea Res. 89, 64–72. doi: 10.1016/j.seares.2014.02.008
Cury P., Bakun A., Crawford R. J. M., Jarre A., Quinones R. A., Shannon L. J., et al. (2000). Small pelagics in upwelling systems: Patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 57, 603–618. doi: 10.1006/jmsc.2000.0712
Cushing D. H. (1995). The long-term relationship between zooplankton and fish: IV. spatial/temporal variability and prediction. ICES J. Mar. Sci. 52, 611–626. doi: 10.1016/1054-3139(95)80076-X
DiBattista J. D., Reimer J. D., Stat M., Masucci G. D., Biondi P., De Brauwer M., et al. (2020). Environmental DNA can act as a biodiversity barometer of anthropogenic pressures in coastal ecosystems. Sci. Rep. 10, 8365. doi: 10.1038/s41598-020-64858-9
Drinkwater K. F., Beaugrand G., Kaeriyama M., Kim S., Ottersen G., Perry R. I., et al. (2010). On the processes linking climate to ecosystem changes. J. Mar. Syst. 79, 374–388. doi: 10.1016/j.jmarsys.2008.12.014
Fanelli E., Cartes J. E., and Papiol V. (2011). Trophodynamics of zooplankton fauna on the Catalan slope (NW Mediterranean): insight from δ13C and δ15 N analysis. J. Mar. Syst. 87, 79–89. doi: 10.1016/j.jmarsys.2011.03.003
Fanelli E., Da Ros Z., Menicucci S., Malavolti S., Biagiotti I., Canduci G., et al. (2023). The pelagic food web of the Western Adriatic Sea: a focus on the role of small pelagics. Sci. Rep. 13, 14554. doi: 10.1038/s41598-023-40665-w
FAO (2022). The State of World Fisheries and Aquaculture, (2022). Towards Blue Transformation (FAO: Rome). doi: 10.4060/cc0461en
France R. L. (1995). Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar. Ecol. Prog. Ser. 124, 307–312. doi: 10.3354/meps124307
Goering J., Alexander V., and Haubenstock N. (1990). Seasonal variation of stable carbon and nitrogen isotope ratios of organisms in a North Pacific Bay. Estuar. Coast. Shelf Sci. 30, 239–260. doi: 10.1016/0272-7714(90)90050-2
Guelinckx J., Maes J., Van Den Driessche P., Geysen B., Dehairs F., and Ollevier F. (2007). Changes in δ13C and δ15N in different tissues of juvenile sand goby Pomatoschistus minutus: a laboratory diet-switch experiment. Mar. Ecol. Prog. Ser. 341, 205–215. doi: 10.3354/meps341205
Hernández-Carrasco I., Orfila A., Rossi V., and Garçon V. (2018). Effect of small scale transport processes on phytoplankton distribution in coastal seas. Sci. Rep. 8, 8613. doi: 10.1038/s41598-018-26857-9
Hesslein R. H., Hallard K. A., and Ramlal P. (1993). Replacement of sulfur, carbon, and nitrogen in tissue of growing broad Whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can. J. Fish. Aquat. Sci. 50, 2071–2076. doi: 10.1139/f93-230
Hobson K. A. (1999). Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120, 314–326. doi: 10.1007/s004420050865
Iitembu J. A., Miller T. W., Ohmori K., Kanime A., and Wells S. (2012). Comparison of ontogenetic trophic shift in two hake species, Merluccius capensis and Merluccius paradoxus, from the Northern Benguela Current ecosystem (Namibia) using stable isotope analysis. Fish. Oceanogr. 21, 215–225. doi: 10.1111/j.1365-2419.2012.00614.x
Jackson A. L., Inger R., Parnell A. C., and Bearhop S. (2011). Comparing isotopic niche widths among and within communities: SIBER - Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602. doi: 10.1111/j.1365-2656.2011.01806.x
James A. G. (1988). Are clupeoid microphagists herbivorous or omnivorous? A review of the diets of some commercially important clupeids. S Afr. J. Mar. Sci. 7, 161–177. doi: 10.2989/025776188784379017
Le Bourg B., Bănaru D., Saraux C., Nowaczyk A., Le Luherne E., Jadaud A., et al. (2015). Trophic niche overlap of sprat and commercial small pelagic teleosts in the Gulf of Lions (NW Mediterranean Sea). J. Sea. Res. 103, 138–146. doi: 10.1016/j.seares.2015.06.011
Lenth R. (2023). Emmeans: Estimated Marginal Means, aka Least-Squares Means_. R package version 1.8.9. Available online at: https://CRAN.R-project.org/package=emmeans (Accessed February 10, 2025).
Leonori I., Tičina V., Giannoulaki M., Hattab T., Iglesias M., Bonanno A., et al. (2021). History of hydroacoustic surveys of small pelagic fish species in the European Mediterranean Sea. Mediterr. Mar. Sci. 22, 751–768. doi: 10.12681/mms.26001
Lloret-Lloret E., Albo-Puigserver M., Gimenez J., Navarro J., Pennino M. G., Steenbeek J., et al. (2022). Small pelagic fish fitness relates to local environmental con-ditions and trophic variables. Prog. Oceanogr. 202, Article 102745. doi: 10.1016/j.pocean.2022.102745
Lluch-belda D., Schwartzlose R. A., Serra R., Parrish R., Kawasaki T., Hedgecock D., et al. (1992). Sardine and anchovy regime fluctuations of abundance in four regions of the world oceans: a workshop report. Fisheries Oceanogr. 114, 339–347. doi: 10.1111/j.1365-2419.1992.tb00006.x
Logan J. M., Jardine T. D., Miller T. J., Bunn S. E., Cunjak R. A., and Lutcavage M. E. (2008). Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. J. Anim. Ecol. 77, 838–846. doi: 10.1111/j.1365-2656.2008.01394.x
Lomartire S., Marques J. C., and Gonḉalves A. M. M. (2021). The key role of zooplankton in ecosystem services: a perspective of interaction between zooplankton and fish recruitment. Ecol. Indic. 129, 107867. doi: 10.1016/j.ecolind.2021.107867
Madigan D. J., Litvin S. Y., Popp B. N., Carlisle A. B., Farwell C. J., and Block B. A. (2012). Tissue turnover rates and isotopic trophic discrimination factors in the endothermic teleost, pacific bluefin tuna (Thunnus orientalis). PloS One 7, e49220. doi: 10.1371/journal.pone.0049220
Malzahn A. M. and Boersma M. (2009). Trophic flexibility in larvae of two fish species (lesser sandeel, Ammodytes marinus and dab, Limanda limanda). Scientia Marina. 73, S1, 131–139. doi: 10.3989/scimar.2009.73s1131
Manzella G. M. R., Gasparini G. P., and Astraldi M. (1988). Water exchange through the eastern and western Mediterranean through the Strait of Sicily. Deep Sea Res. I 35, 1021–1035. doi: 10.1016/0198-0149(88)90074-X
McClelland J. W., Valiela I., and Michener R. H. (1997). Nitrogen-stable isotope signatures in estuarine food webs: a record of increasing urbanization in coastal watersheds. Limnol. Oceanogr. 42, 930–937. doi: 10.4319/lo.1997.42.5.0930
Micheli F., Halpern B. S., Walbridge S., Ciriaco S., Ferretti F., Fraschetti S., et al. (2013). Cumulative human impacts on Mediterranean and Black Sea marine ecosystems: Assessing current pressures and opportunities. PloS One 8, e79889. doi: 10.1371/journal.pone.0079889
Mikhailova M. V., Bellotti P., Valeri P., and Tortora P. (1999). Intrusion of seawater into the river part of the Tiber mouth. Water Resour. 26, 679–686.
Montoya J. P., Horrigan S. G., and McCarthy J. J. (1990). Natural abundance of 15N in particulate nitrogen and zooplankton in the Chesapeake Bay. Mar. Ecol. Prog. Ser. 65, 35–61. doi: 10.3354/meps065035
Moretti M., Seasonne E., Spezie G., and De Maio A. (1993). Results of investigations in the Sicily Channel, (1986–1990). Deep-Sea Res. II 40, 1181–1192. doi: 10.1016/0967-0645(93)90066-V
Mussi B., Miragliuolo A., De Pippo T., Gambi M. C., and Chiota D. (2004). The submarine canyon of Cuma (southern Tyrrhenian Sea, Italy), a cetacean key area to protect. Eur. Res. Cetaceans 15.
Mussi B., Miragliuolo A., Monzini E., Diaz Lopez B., and Battaglia M. (1999). Fin whale (Balaenoptera physalus) feeding ground in the coastal waters of Ischia (Archipelago Campano). Eur. Res. Cetaceans 13, 330–335.
Mussi B., Miragliuolo A., Zucchini A., and Pace D. S. (2014). Occurrence and spatio-temporal distribution of sperm whale (Physeter macrocephalus) in the submarine canyon of Cuma (Tyrrhenian Sea, Italy). Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 59–70. doi: 10.1002/aqc.2460
Navarro J., Albo-Puigserver M., Serra P. E., Sáez-Liante R., and Coll M. (2020). Trophic strategies of three predatory pelagic fish coexisting in the north-western Mediterranean Sea over different time spans. Estuar. Coast. Shelf Sci. 246, 107040. doi: 10.1016/j.ecss.2020.107040
Nissar S., Bakhtiyar Y., Arafat M. Y., Andrabi S., Bhat A. A., and Yousuf T. (2023). A review of the ecosystem services provided by the marine forage fish. Hydrobiologia 850, 2871–2902. doi: 10.1007/s10750-022-05033-1
Overman N. C. and Parrish D. L. (2001). Stable isotope composition of walleye: 15N accumulation with age and area-specific differences in δ13C. Can. J. Fisheries Aquat. Sci. 58, 1253–1260. doi: 10.1139/f01-072
Palomera I., Olivar M. P., Salat J., Sabatés A., Coll M., García A., et al. (2007). Small pelagic fish in the NW Mediterranean Sea: an ecological review. Prog. Oceanogr. 74, 377–396. doi: 10.1016/j.pocean.2007.04.012
Peck M. A., Reglero P., Takahashi M., and Catal´an I. A. (2013). Life cycle ecophysiology of small pelagic fish and climate-driven changes in populations. Prog. Oceanogr. 116, 220–245. doi: 10.1016/j.pocean.2013.05.012
Pepin P. and Penney R. W. (1997). Patterns of prey size and taxonomic composition in larval fish: are there general size-dependent models? J. Fish Biol. 51, 84–100. doi: 10.1111/j.1095-8649.1997.tb06094.x
Pethybridge H. R., Choy C. A., Polovina J. J., and Fulton E. A. (2018). Improving marine ecosystem models with biochemical tracers. Ann. Rev. Mar. Sci. 10, 199–228. doi: 10.1146/annurev-marine-121916-063256
Piccioni A., Gabriele M., Salusti E., and Zambianchi E. (1988). Wind-induced upwellings off the southern coast of Sicily. Oceanol. Acta 11, 309–314.
Pinheiro J., Bates D., and R Core Team (2023). nlme: Linear and Nonlinear Mixed Effects Models_. R package version 3.1-163. Available online at: https://CRAN.R-project.org/package=nlme (Accessed February 10, 2025).
Plounevez S. and Champalbert G. (1999). Feeding behaviour and trophic environment of Engraulis encrasicolus (L.) in the Bay of Biscay. Estuar. Coast. Shelf Sci. 49, 177–191. doi: 10.1006/ecss.1999.0497
Plounevez S. and Champalbert G. (2000). Diet, feeding behaviour and trophic activity of the anchovy (Engraulis encrasicolus L.) in the Gulf of Lions (Mediterranean Sea). Oceanol. Acta 23, 175–192. doi: 10.1016/S0399-1784(00)00120-1
R Core Team (2023). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/ (Accessed February 10, 2025).
Rinaldi E., Buongiorno Nardelli B., Zambianchi E., Santoleri R., and Poulain P. M. (2010). Lagrangian and Eulerian observations of the surface circulation in the Tyrrhenian Sea. J. Geophys. Res. 115, C04024. doi: 10.1029/2009JC005535
Robinson A. R., Sellschopp J., Warn-Varnas A., Leslie W. G., Lozano C. J., Haley P. J., et al. (1999). The Atlantic Ionian stream. J. Mar. Syst. 20, 129–156. doi: 10.1016/S0924-7963(98)00079-7
Rolff C. (2000). Seasonal variation in δ13C and δ15N of size-fractionated plankton at a coastal station in the northern Baltic proper. Mar. Ecol. Prog. Ser. 203, 47–65. doi: 10.3354/meps203047
Rumolo P., Basilone G., Fanelli E., Barra M., Calabrò M., Genovese S., et al. (2017). Linking spatial distribution and feeding behavior of Atlantic horse mackerel (Trachurus trachurus) in the Strait of Sicily (Central Mediterranean Sea). J. Sea Res. 121, 47–58. doi: 10.1016/j.seares.2017.01.002
Rumolo P., Bonanno A., Barra M., Fanelli E., Calabrò M., Genovese S., et al. (2016). Spatial variations in feeding habits and trophic levels of two small pelagic fish species in the central Mediterranean Sea. Mar. Environ. Res. 115, 65–77. doi: 10.1016/j.marenvres.2016.02.004
Russo L., Casella V., Marabotti A., Jord.n F., Congestri R., and D’Alelio D. (2022). Trophic hierarchy in a marine community revealed by network analysis on co-occurrence data. Food Webs 32, e00246. doi: 10.1016/j.fooweb.2022.e00246
Sammartino M., Aronica S., Santoleri R., and Buongiorno Nardelli B. (2022). Retrieving Mediterranean Sea surface salinity distribution and interannual trends from multi-sensor satellite and in situ data. Remote Sens. 14, 2502. doi: 10.3390/rs14102502
Scharf F. S., Juanes F., and Rountree R. A. (2000). Predator size-prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar. Ecol. Prog. Ser. 208, 229–248. doi: 10.3354/meps208229
Sholto-Douglas A. D., Field J. G., James A. G., and van der Merwe N. J. (1991). 13C/12C and 15N/14N isotope ratios in the Southern Benguela Ecosystem: indicators of food web relationships among different size-classes of plankton and pelagic fish; differences between fish muscle and bone collagen tissues. Mar. Ecol. Prog. Ser. 78, 23–31. doi: 10.3354/meps078023
Stenseth N. C., Llope M., Anad.n R., Ciannelli L., Chan K. S., Hjermann D., et al. (2006). Seasonal plankton dynamics along a cross-shelf gradient. Proc. R. Soc B 273, 2831–2838. doi: 10.1098/rspb.2006.3658
Suzuki K. W., Kasai A., Nakayama K., and Tanaka M. (2005). Differential isotopic enrichment and half-life among tissues in Japanese temperate bass (Lateolabrax japonicus) juveniles: implications for analysing migration. Can. J. Fish Aquat. Sci. 62, 671–678. doi: 10.1139/f04-231
Tanaka H., Takasuka A., Aoki I., and Ohshimo S. (2008). Geographical variations in the trophic ecology of Japanese anchovy, Engraulis japonicus, inferred from carbon and nitrogen stable isotope ratios. Mar. Biol. 154, 557e568. doi: 10.1007/s00227-008-0949-4
Tudela S. and Palomera I. (1995). Diel feeding intensity and daily ration in the anchovy Engraulis encrasicolus in the north- west Mediterranean Sea during the spawnlng penod. Mar. Ecol. Prog. Ser. 129, 55–61. doi: 10.3354/meps129055
Tudela S. and Palomera I. (1997). Trophic ecology of the European anchovy Engraulis encrasicolus in the Catalan Sea (northwest Mediterranean). Mar. Ecol. Prog. Ser. 160, 121–134. doi: 10.3354/meps160121
Van der Lingen C., Africa F. S., Bertrand A., Bode A., Brodeur R. D., and Oceanic N. (2009). “Trophic dynamics,” in Alheit Climate Change and Small Pelagic Fish. Eds. Y. O. Checkley D. M. and C. Roy J. (Cambridge University Press), 112–157. Available online at: https://www.researchgate.net/publication/215739118_Chapter_7_Trophic_dynamics.
Vander Zanden M. J. and Rasmussen J. B. (1999). Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80, 1395–1404. doi: 10.1890/0012-9658(1999)080[1395:PCCANA]2.0.CO;2
Vander Zanden M. J. and Rasmussen J. B. (2001). Variation in δ15N and δ13C trophic fractionation: Implications for aquatic food web studies. Limnol. Oceanogr. 46(8), 2061–2066. doi: 10.4319/lo.2001.46.8.2061
Vassallo P., Bellardini D., Castellano M., Dapueto G., and Povero P. (2022). Structure and functionality of the mesozooplankton community in a coastal marine environment: Portofino marine protected area (Liguria). Diversity 14, 19. doi: 10.3390/d14010019
Wiebe P. H. and D’Abramo L. (1972). Distribution of euphausiid assemblages in the Mediterranean Sea. Mar. Biol. 15, 139–149. doi: 10.1007/BF00353642
Keywords: stable isotopes, Engraulis encrasicolus, Sardina pilchardus, trophic ecology, spatiotemporal variability, feeding habits
Citation: Rumolo P, Barra M, Bonanno A, Altieri S, Ferreri R, Gargano A, Basilone G, Gherardi S, Genovese S, Di Cicco MR and Lubritto C (2025) Spatiotemporal variability in the feeding habits of anchovy and sardine: a comparison of upwelling and river-runoff driven ecosystems. Front. Mar. Sci. 12:1602042. doi: 10.3389/fmars.2025.1602042
Received: 28 March 2025; Accepted: 21 July 2025;
Published: 25 August 2025.
Edited by:
Chiara Piroddi, Joint Research Centre, ItalyReviewed by:
Arturo Tripp, National Polytechnic Institute (IPN), MexicoSule Gurkan, Ege University, Türkiye
Copyright © 2025 Rumolo, Barra, Bonanno, Altieri, Ferreri, Gargano, Basilone, Gherardi, Genovese, Di Cicco and Lubritto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Barra, bWFyY28uYmFycmFAY25yLml0; Angelo Bonanno, YW5nZWxvLmJvbmFubm9AY25yLml0
 Paola Rumolo
Paola Rumolo Marco Barra
Marco Barra Angelo Bonanno
Angelo Bonanno Simona Altieri
Simona Altieri Rosalia Ferreri2
Rosalia Ferreri2 Gualtiero Basilone
Gualtiero Basilone Simona Genovese
Simona Genovese