- 1Jiangsu Key Laboratory of Marine Genetic Resources and Breeding, Jiangsu Ocean University, Lianyungang, China
- 2Jiangsu Key Laboratory of Marine Bioresources and Environment, Jiangsu Ocean University, Lianyungang, China
- 3Co-Innovation Center of Jiangsu Marine Bio-Industry Technology, Jiangsu Ocean University, Lianyungang, China
- 4Affair Center of Animal Husbandry and Aquaculture in XiangXi Autonomous Prefecture, Jishou, Hunan, China
- 5Jiangsu Key Laboratory of Marine Biotechnology, Jiangsu Ocean University, Lianyungang, China
Chlorine in seawater is toxic to bivalves. This study explored the long-term effects of 20 mg/L, 40 mg/L, and 60 mg/L chlorine on the survival rate, growth, immune, and antioxidant performance of the Cyclina sinensis. After 30 days, neither the 20 mg/L nor 40 mg/L treatment group demonstrated any significant disparity compared with the control group. Nevertheless, significant mortality rates were subsequently observed in all treatment groups. The weight, shell length, and monthly growth rate of C. sinensis in the 60 mg/L group were significantly lower than in the control group (P <0.05). Residual chlorine also triggered the antioxidant stress response mechanism. After 90 days, a significant increase occurred in both the total antioxidant capacity and activity of superoxide dismutase (P <0.05). Chlorine toxicity caused an immune response in the nonspecific immune system of C. sinensis, with alkaline phosphatase and acid phosphatase activities significantly increasing at 30 days and then significantly decreasing (P <0.05). Lysozyme activity also showed a continuous decline after 30 days. These results indicated that C. sinensis was unable to adapt to long-term chlorine toxicity stress but was able to resist chlorine toxicity for a short period (30 days) only when the chlorine concentration was <40 mg/L. Long-term chlorine exposure damaged the immune system of C. sinensis, inhibiting growth and increasing clam mortality. Therefore, in areas where chlorine is used as a disinfectant, the residual chlorine concentration should be tested in the water every 30 days to prevent harm to bivalves caused by long-term chlorine toxicity stress.
1 Introduction
Chlorine-based disinfectants, such as chlorine dioxide and trichloroisocyanuric acid, are widely used in aquaculture (e.g., pathogen control in ponds) and industrial cooling systems (e.g., biofouling prevention in nuclear power plants) due to their potent antimicrobial properties (Chen et al., 2023a; Liu C. et al., 2024; Zou et al., 2003). However, the persistent residual chlorine released into aquatic environments poses critical threats to biodiversity and ecosystem health. While short-term acute exposure to chlorine is known to induce mortality and biochemical stress in aquatic organisms, the long-term impacts of low-concentration chlorine accumulation in water and sediments—especially on benthic species adapted to stable microhabitats—remain poorly understood.
Previous studies have highlighted that shellfish, including the burrowing bivalve Cyclina sinensis, are sensitive to environmental stressors like heavy metals and thermal fluctuations (Chen et al., 2009; Li et al., 2024). Notably, chlorine residues in aquatic sediments, which degrade slowly and can persist for extended periods, may impose chronic stress on these organisms, potentially disrupting growth, immune function, and physiological homeostasis. Despite the ecological and economic importance of Cyclina sinensis in Chinese coastal ecosystems, no research has systematically evaluated the effects of long-term chlorine exposure on its survival, growth performance, and enzymatic responses.
This study addresses this knowledge gap by investigating the impacts of chlorine dioxide—a commonly used chlorinating agent—on Cyclina sinensis, focusing on how chronic, low-concentration exposure influences its growth parameters, antioxidant enzyme activities, and immune responses. By bridging the gap between industrial/aquaculture practices and benthic organism health, our findings aim to provide critical insights into the ecological risks of residual chlorine and inform sustainable management strategies.
Chlorine toxicity arises from its ability to oxidize cellular components, induce oxidative stress, and impair osmoregulation in aquatic organisms (Chen et al., 2023a; Chen et al., 2023b; Liu L. et al., 2024; Venkatnarayanan et al., 2021; Chavan et al., 2016). This sustained oxidative damage and physiological disruption progressively overwhelms antioxidant and osmoregulatory systems, leading to chronic stress responses when organisms cannot fully counteract these perturbations (Pan et al., 2006). Owing to their limited mobility, bivalves are unable to rapidly move to different suitable habitats, rendering them more susceptible to prolonged stress from adverse water conditions. There have been many studies on long-term stress resulting from high temperatures (Liu M. et al., 2024), algal toxins (Benli et al., 2008), heavy metals (Nakanishi and Rosenberg, 2013), and ammonia nitrogen (Ni et al., 2023, 2024), particularly in relation to bivalves, given their aquacultural importance (Liu C. et al., 2024). The burrowing bivalve C. sinensis is an economically important bivalve in China and is widely distributed in coastal tidal flats. Various studies have focused on C. sinensis, investigating its nutritional components (Yan et al., 2024), germplasm resources (Dong et al., 2021), and the effects of high temperature and ammonia nitrogen stress (Liu M. et al., 2024; Ni et al., 2024, 2023; Hongxing et al., 2022), among others. However, less is known about the effects of long-term chlorination stress on C. sinensis.
This study aimed to investigate the long-term effects of chlorine dioxide on the survival, growth, antioxidant capacity, and nonspecific immune responses of C. sinensis under controlled laboratory conditions. By quantifying changes in survival rate, shell length, weight gain, and enzyme activities (total antioxidant capacity [T-AOC], superoxide dismutase [SOD], acid phosphatase [ACP], alkaline phosphatase [AKP], and lysozyme [LZM]), we sought to determine the threshold concentrations and duration of chlorine exposure that trigger detrimental effects. These findings provide critical insights for managing chlorine use in aquaculture and industrial sectors to safeguard C. sinensis populations and maintain coastal ecosystem health.
2 Materials and methods
2.1 Experimental materials
C. sinensis were bred and cultured at a breeding base in Lianyungang, Jiangsu, China. We selected C. sinensis with an average shell length of 1.20 ± 0.10 cm and a body weight of 0.70 ± 0.2g. After cleaning and disinfection, they were acclimatized for 7 days at a salinity of 20–22 ppt, pH 7.9–8.2, water temperature 22°C ± 3°C, light intensity between 1,000 and 8,000 lux, and a light/dark photoperiod of 12:12 h, with continuous microaeration. The clams were fed every morning and evening with a mixed microalgae solution of Isochrysis galbana, Chaetoceros calcitrans, and Platymonas subcordiformis at a feeding concentration of 3 × 105 cells/mL. Any deformed or diseased individuals were removed during the acclimatization period. All clams were treated in strict accordance with the guidelines for the care and use of experimental animals established by the Administration of Affairs Concerning Experimental Animals of the State Council of the People’s Republic of China and approved by the Committee on Experimental Animal Management of the Jiangsu Ocean University.
Chlorine dioxide was sourced from South Ranch effervescent tablets (effective chlorine content: 35%). Tablets were ground into a fine powder, accurately weighed (0.0001 g precision, Mettler Toledo balance), and dissolved in deionized water to prepare a 1000 mg/L stock solution via magnetic stirring for 20 minutes (Wang et al., 2023). Prior to each daily exposure, the stock solution was diluted with filtered seawater (0.45 μm) to target concentrations (0.1, 0.5, 1.0 mg/L ClO2) and mixed for 10 minutes to ensure homogeneity.
Water in exposure tanks was renewed daily at a 30% replacement rate of aged seawater was siphoned from the bottom, and 30% of fresh seawater pre-adjusted to the corresponding chlorine dioxide concentration (using the same dilution method) was gently added to maintain stable salinity and temperature. Chlorine concentrations were monitored hourly during the experiment using a portable chlorine meter (Hach DR900) and adjusted via micropipette titration of stock solution to maintain constant nominal concentrations (coefficient of variation < 5%), ensuring consistent exposure conditions throughout the trial.
2.2 Experimental design
In previous research, we determined that, for C. sinensis, the 96-h median lethal concentration (96 h LC50) of chlorine was 200 mg/L (Wang et al., 2023). Thus, the stress concentration gradient for the current study was set at 20 mg/L (10% LC50), 40 mg/L (20% LC50), and 60 mg/L (30% LC50). Three parallel experiments were conducted for each chlorine dioxide concentration level; a residual chlorine meter (Pocket Colorimeter II, DR 800, HATCH) was used to adjust the chlorine concentration daily (Wang et al., 2023). A control group with no added chlorine was also set up. Each group had three replicates, with 200 clams in each replicate. The culture tanks were filled with 5 cm of sterilized sea mud, and the salinity, dissolved oxygen, pH, daily water exchange volume, light intensity, light/dark photoperiod, microaeration, and feeding of bait were the same as those used for clam acclimatization. During the experiment, dead individuals were promptly removed to avoid affecting the culture water quality. The experimental period was 90 days.
2.3 Sample collection and determination
After the start of the study, the growth of C. sinensis was measured on Days 30, 60, and 90. From each parallel group, 30 C. sinensis were randomly selected, washed, and their surface wiped dry with absorbent paper. The weight of each clam was measured using an electronic balance accurate to 0.001 g, and each shell length was measured using a digital vernier caliper accurate to 0.01 mm. The monthly growth rate of shell length (MGR) and weight gain rate (MWR) of each group were calculated using Equations 1, 2. The number of surviving individuals was counted at each sampling point to calculate the survival rate (Sn) using Equation 3.
where Wn and Wn-1 represent the weight (g) of the nth month and the (n-1)th month respectively; Lnand Ln-1 represent the shell length (cm) of the nth month and the (n-1)th month respectively; F is the number of living clams at each sampling point, and I is the initial number of clams.
On the 30th, 60th, and 90th days of sampling, five clams were taken from each parallel group. Each hepatopancreas was collected, rapidly frozen in liquid nitrogen and stored at -80°C for the determination of enzyme activity indicators.
To prepare the hepatopancreas tissue homogenate, the frozen hepatopancreas tissues were mixed with homogenization medium at a ratio of 1:9 (g:mL). The mixture was homogenized in an ice-water bath using a mechanical homogenizer to obtain a 10% tissue homogenate solution. Then, the homogenate was centrifuged at 2500 rpm for 10 min at 4°C, and the supernatant was collected for the determination of immune enzyme activity. All enzyme activities were analyzed using kits produced by the Nanjing Jiancheng Bioengineering Institute following the manufacturer’s instructions. The total protein content of the tissues was determined by the Coomassie brilliant blue method using the kit from the Nanjing Jiancheng Bioengineering Institute and following the manufacturer’s instructions (Wang et al., 2023).
2.4 Data analysis
SPSS (v.24.0, SPSS Inc., Chicago, IL, USA) was used for all data analysis. One-way ANOVA and Duncan’s analysis were used to compare the differences in the data between groups. All graphs were plotted using Origin 2024 software (Learning edition; OriginLab, Northampton, MA, USA). Differences were considered statistically significant at P <0.05.
3 Results
3.1 Survival rate of C. sinensis after exposure to chlorine stress
There was a consistent decrease in survival rate of C. sinensis at the higher chlorine concentrations (Figure 1A). At 60 mg/L, chlorine, the survival rate of C. sinensis at Day 30 was significantly different from that of the control group (P <0.05) (Figure 1B). On Days 60 and 90, the survival rate across all three experimental groups was significantly lower than in the control group (P <0.05). This effect was particularly pronounced on Day 90, when the survival rates of clams in the 40 mg/L and 60 mg/L groups were both <50%.
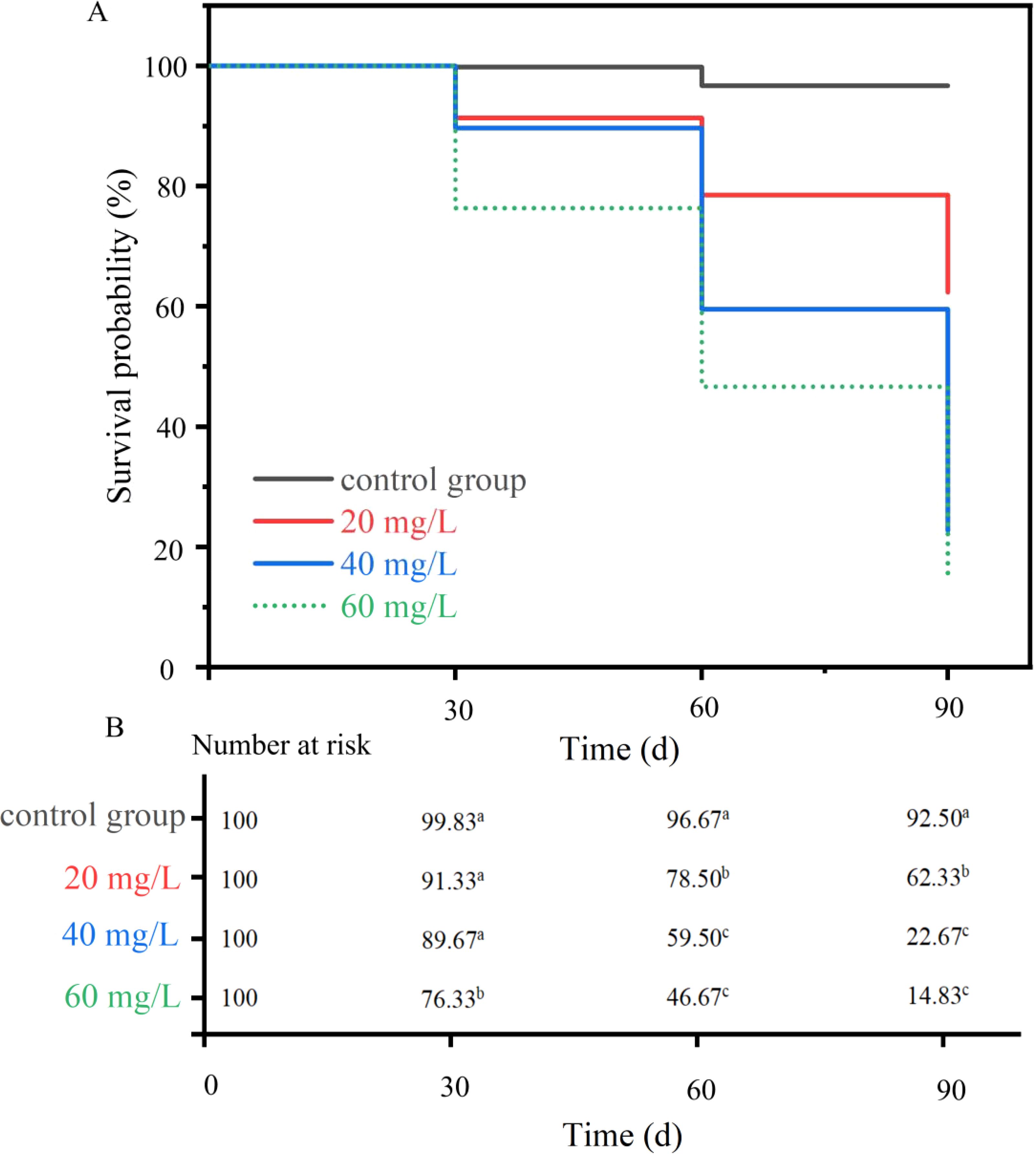
Figure 1. (A, B) Survival probability and risk tables of Cyclina sinensis after chlorine stress. (Significant differences between groups are indicated with different letters, P < 0.05; ns, indicates no significant differences).
3.2 Growth of C. sinensis under chlorination stress
The shell length of C. sinensis differed significantly from that in the control group only in the 40 mg/L and 60 mg/L groups at Day 90 (Table 1). In contrast, body weight was significantly lower than that of the control group only in the 60 mg/L group at Day 90 (Table 2). After 30 days, the MGR of the control group was significantly higher than that of the three treatment groups (P <0.05). After 60 days, the MRG of the 60 mg/L group was significantly lower than that of the other two treatment groups and the control group (Table 3). The MWR in the 60 mg/L group was the lowest observed. On Day 30, the MWR at chlorine concentrations of 40 mg/L and 60 mg/L were significantly different from that of the control group (P <0.05), remaining consistently lower than the control by Day 90. In contrast, the MWR of the 20 mg/L group was significantly different from that of the control group only at Day 60 (Table 4).
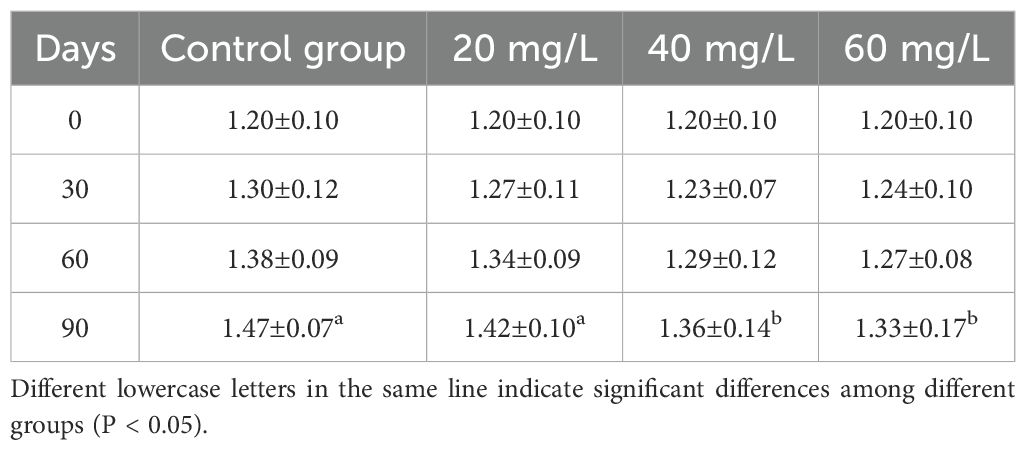
Table 1. Changes of shell length (cm) of Cyclina sinensis under different chlorine concentrations stress.
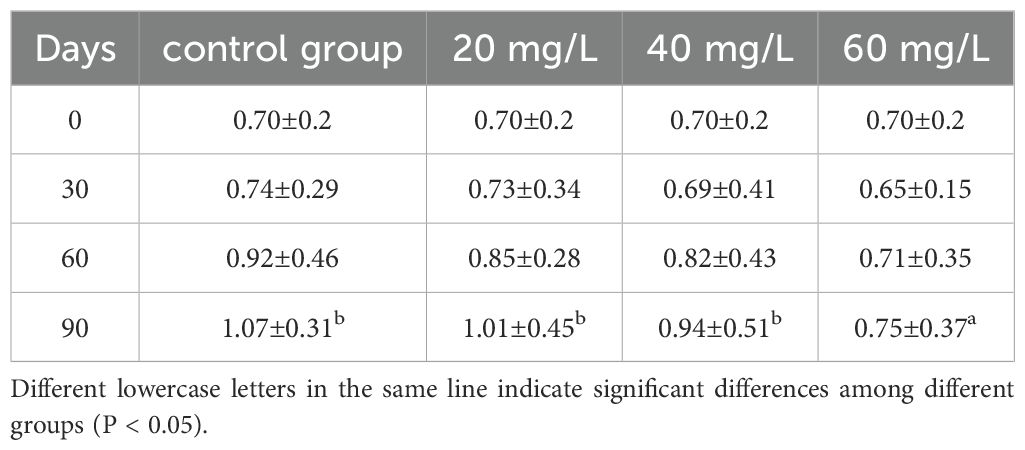
Table 2. Changes of body weight (g) of Cyclina sinensis under different chlorine concentrations stress.

Table 3. Changes of monthly growth rate (%) of Cyclina sinensi under different chlorine concentrations stress.
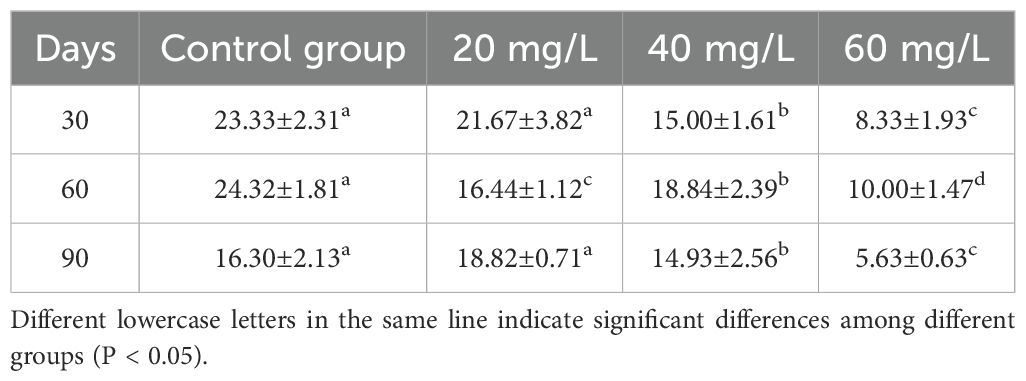
Table 4. Changes of monthly weight gain rate (%) of Cyclina sinensi under stress of different chlorine concentrations.
3.3 Antioxidant status of C. sinensis under chlorine stress
After chronic exposure to different concentrations of chlorine, T-AOC activity in the hepatopancreas of C. sinensis first increased, then decreased, before then increasing again over the study period (Figure 2A). At 60 days, the T-AOC of each treatment group reached the lowest value, with the differences being significant (P <0.05); at 90 days, T-AOC activity in the 40 mg/L and 60 mg/L groups peaked, and were significantly higher than those at other time points (P <0.05).
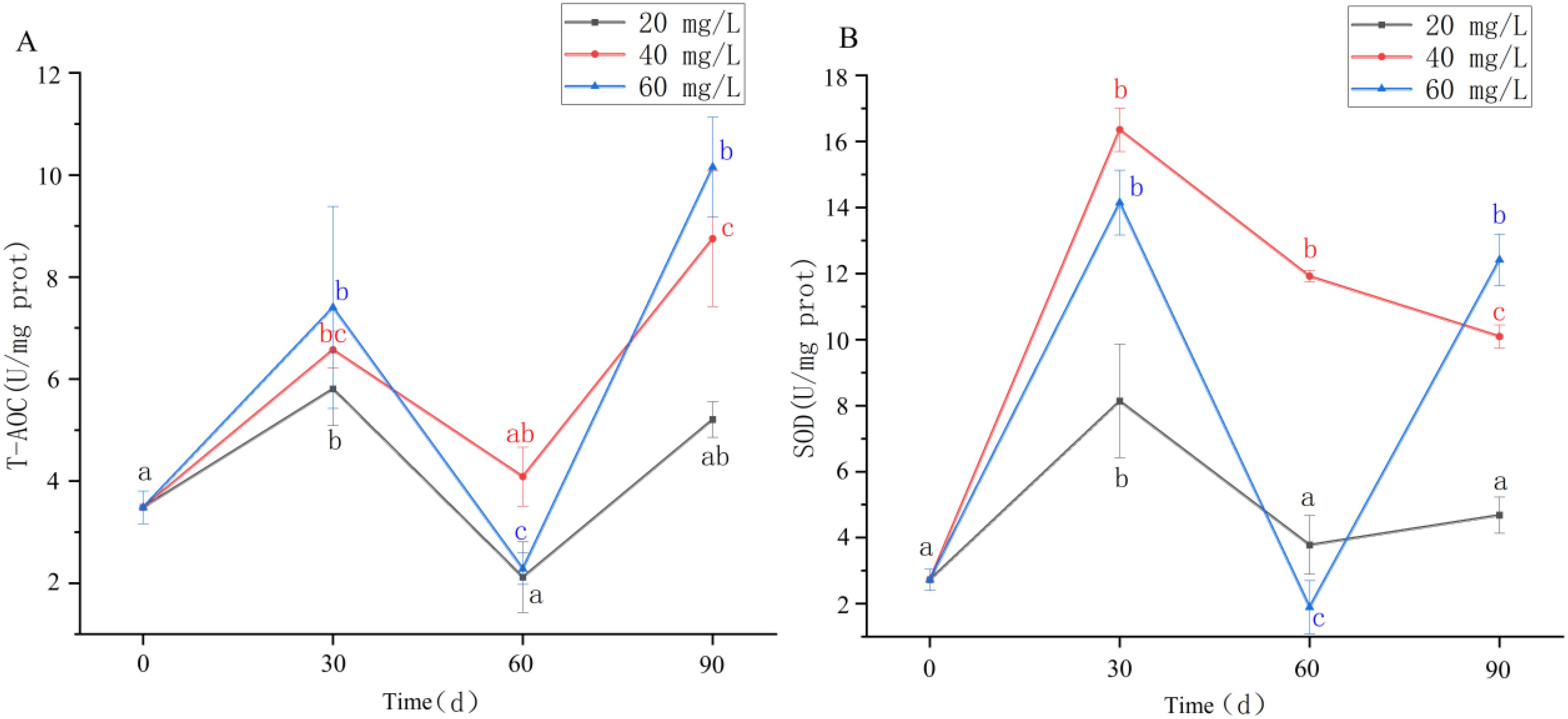
Figure 2. (A, B) T-AOC and SOD enzyme activities in hepatopancreas of Cyclina sinensi after chlorine stress. (Significant differences between different periods are indicated by different letters, P < 0.05).
SOD activity in the 20 mg/L and 60 mg/L groups first increased, then decreased, and then increased again (Figure 2B). In contrast, in the 40 mg/L group, SOD activity first increased but then continued to decrease after Day 30, being significantly lower at Day 90 than that at Days 30 and 60 (P <0.05). The lowest value in the 60 mg/L group occurred at Day 60, which was significantly lower than at the other time points across the treatment groups (P <0.05); however, it was significantly higher at Day 90 compared with the other treatment groups (P <0.05). The 20 mg/L group showed less extreme changes in SOD activity.
`After long-term exposure to chlorine stress, ACP activity in the hepatopancreas of the clams in each treatment group first increased and then decreased (Figure 3A). ACP activity in the 20 mg/L concentration group peaked at Day 30 and then began to decline, being significantly lower than the Day 0 value at Day 90 (P <0.05). There was no significant change in ACP activity in the 40 mg/L concentration group compared with Day 0(P > 0.05). By Day 30, the ACP activity in the 60 mg/L concentration group was significantly higher than at Day 0; however, by Day 90, it was significantly lower (P <0.05).
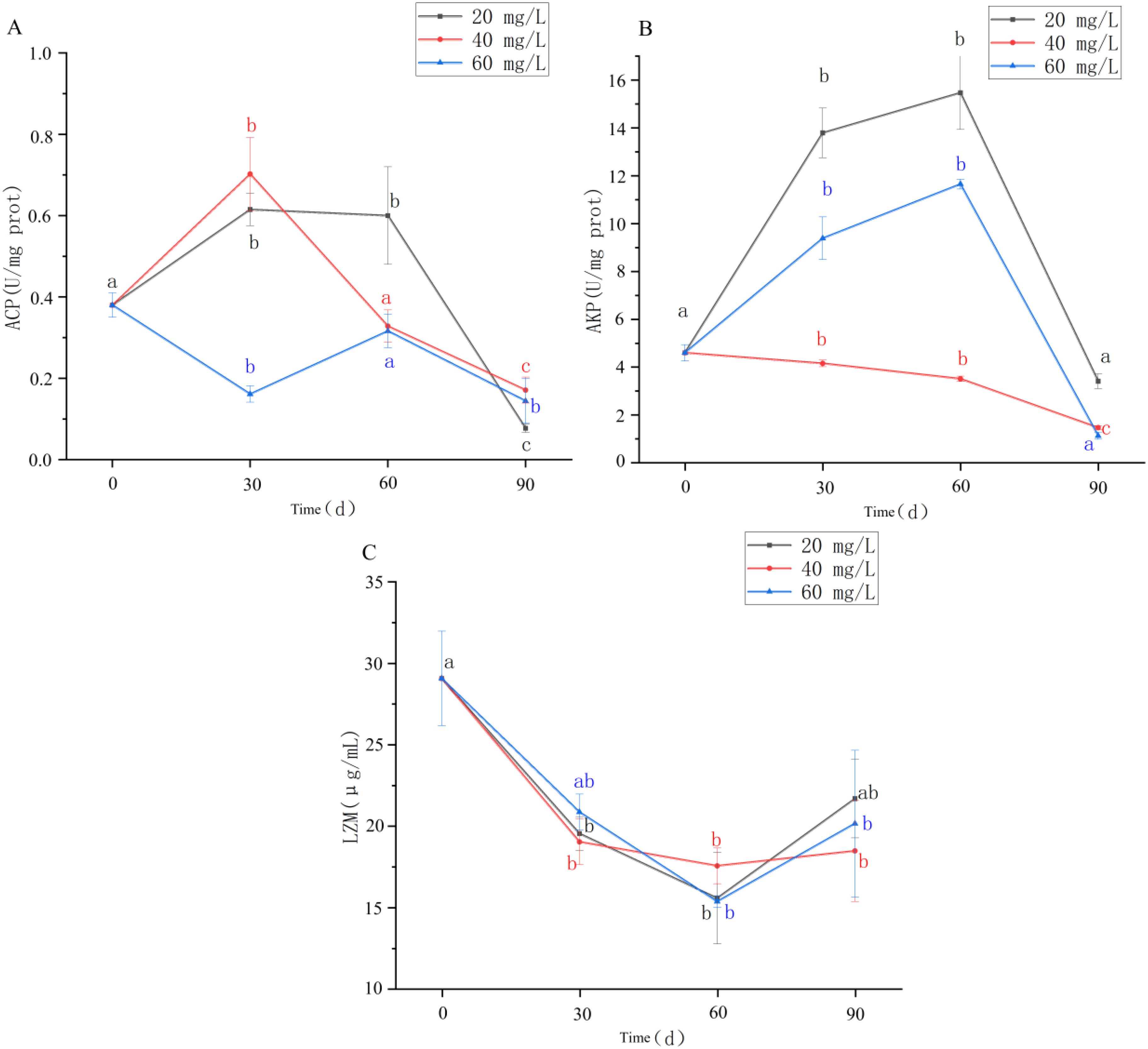
Figure 3. (A-C) ACP, AKP and LZM enzyme activities in hepatopancreas of Cyclina sinensi after chlorine stress. (Significant differences between different periods are indicated by different letters, P < 0.05).
AKP activity also showed an initial increase followed by a decrease, although this was less obvious in the 40 mg/L group (Figure 3B). In the 20 and 60 mg/L groups at Day 60, AKP activity was significantly higher than at Day 0 (P < 0.05), being lowest at Day 90, although the difference was not significant compared with that at Day 0 (P >0.05). In the 40 mg/L group, AKP activity continuously decreased from Day 0 and was significantly lower than that of the control group (P <0.05).
LZM activity continuously decreased before Day 60 and then increased, although this increase was less obvious in the 40 mg/L group (Figure 3C). At Day 60, LZM activity in the 20 and 60 mg/L groups was significantly lower than at Day 0 (P <0.05). LZM activity in the 40 mg/L group was significantly lower than at Day 0 across all three time points (P <0.05).
4 Discussion
4.1 Survival and growth: Concentration- and time-dependent toxicity
Chlorine-based disinfectants, widely used in aquaculture and industrial systems (Sorong, 2024; Wang L. et al., 2024), impose pronounced concentration- and time-dependent toxicity on Cyclina sinensis. In this study, survival rates declined significantly after 90 days of exposure to ≥20 mg/L chlorine, with a steep concentration gradient (60 mg/L > 40 mg/L > 20 mg/L), consistent with the concentration-dependent lethality reported in juvenile bamboo clams (Solen strictus) by Wu et al. (2018). This pattern aligns with our prior short-term acute stress study (DOI: 10.3389/fmars.2023.1105065), where the 96-h LC50 of chlorine for C. sinensis was 217.6 mg/L (Wang et al., 2023). Notably, the concentrations tested here (20–60 mg/L) are sub-lethal in acute scenarios but induce chronic toxicity over prolonged exposure, highlighting the critical distinction between short-term high-dose and long-term low-dose stressors.
Time-dependent toxicity was equally evident: survival rates in all treatment groups decreased progressively over 90 days, whereas the control group remained stable. This aligns with Ma et al. (2023), who observed cumulative toxicity in Chlorella vulgaris and koi carp under prolonged chlorine exposure, indicating that C. sinensis lacks adaptive resilience to chronic stress. In contrast to our previous transcriptomic analysis—where acute exposure (50–500 mg/L) disrupted immune signal transduction, apoptosis, and antioxidant gene expression (e.g., GST, HSP) (Wang et al., 2023)—the present study reveals that chronic low-concentration stress (≤60 mg/L) primarily impacts physiological endpoints like enzyme activities and growth. Growth parameters (shell length, body weight) further corroborated this effect: high-concentration treatments (≥40 mg/L) significantly inhibited growth by Day 60, likely due to energy reallocation from anabolic processes to stress adaptation (Bu et al., 2017). Together, these complementary findings—acute molecular perturbations vs. chronic physiological decline—comprehensively characterize chlorine’s dual-threat paradigm: short-term high doses cause immediate cellular damage, while long-term low doses erode fitness through cumulative metabolic strain. This dual framework is essential for establishing safety thresholds in industrial discharge management and aquaculture practices.
4.2 Antioxidant system response: dynamic activation and adaptive limits
The antioxidant enzymes total antioxidant capacity (T-AOC) and superoxide dismutase (SOD) exhibited concentration-dependent activation, peaking at 60 mg/L by Day 90. This upregulation signifies C. sinensis’s attempt to counteract chlorine-induced oxidative stress, a common adaptive mechanism in aquatic invertebrates (Tian et al., 2024). SOD specifically scavenges reactive oxygen species (ROS), preventing lipid peroxidation and DNA damage, while T-AOC reflects the overall scavenging capacity of the organism (Liu L. et al., 2024).
Notably, the delayed peak (Day 90) suggests a gradual escalation of oxidative pressure rather than immediate stress response, implying that low-to-moderate chlorine concentrations (≤40 mg/L) initially induced compensatory mechanisms. However, this adaptive capacity was finite: at 60 mg/L, enzyme activities plateaued after Day 60, potentially indicating saturation of antioxidant defenses and onset of cellular damage, as observed in previous studies on bivalves under heavy metal stress (Wang W. et al., 2024).
4.3 Non-specific immune system changes: Enzymatic responses and ecological implications
Chronic chlorine exposure elicited distinct patterns in non-specific immune enzymes. Acid phosphatase (ACP) and alkaline phosphatase (AKP), key markers of lysosomal activity and immune homeostasis, showed transient activation followed by suppression. ACP increased significantly at all concentrations by Day 30, most pronounced at 60 mg/L, reflecting heightened phagocytic activity or lysosomal membrane permeability (Tian et al., 2024). However, by Day 90, ACP and AKP activities dropped below baseline, indicating immune system exhaustion—a finding consistent with black carp larvae (Mylopharyngodon piceus) under prolonged chemical stress (Wu et al., 2024).
In contrast, lysozyme (LZM), which degrades bacterial cell walls, showed no significant activation across concentrations. Instead, LZM activity was partially inhibited at ≥40 mg/L by Day 60, likely due to energy reallocation toward antioxidant and detoxification pathways (Sokolova, 2013; Sokolova et al., 2012). This trade-off between immune functions highlights the ecological cost of chronic stress: reduced pathogen resistance may increase susceptibility to secondary infections, exacerbating mortality risks in natural populations (Zhang and Wang, 2020).
4.4 Management implications: Mitigating long-term chlorine risks
The concentration- and time-dependent effects observed here underscore the need for cautious chlorine use in aquaculture and industrial settings. First, routine monitoring of residual chlorine in water and sediments is critical, especially in bivalve cultivation areas where C. sinensis serves as an ecological and economic indicator species (Chen et al., 2023a). Second, reducing prolonged high-concentration applications (e.g., >20 mg/L for ≥30 days) and adopting low-dose, intermittent disinfection protocols could minimize cumulative toxicity while maintaining pathogen control.
Industrial systems, such as nuclear power plant cooling systems, should prioritize alternative biofouling control methods (e.g., UV disinfection) in sensitive coastal zones, complemented by regular assessments of benthic community health (Wang W. et al., 2024). These strategies would balance operational efficiency with ecological sustainability, mitigating the long-term threats of chlorine residues to vulnerable species like C. sinensis.
5 Conclusions
In this study, long-term exposure to high concentrations of chlorine increased the mortality rate of C. sinensis. At a chlorine concentration <40 mg/L, C. sinensis was able to resist stress for 30 days, with relatively minor impacts. However, this resistance was not sustained over extended periods and the clams failed to adapt to chronic chlorine exposure. Therefore, when using chlorine as a disinfectant, it was vital to regularly monitor the residual chlorine concentration in the water every 30 days to prevent any long-term adverse effects on these and other aquacultural organisms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Lianyungang Science and Technology Bureau. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CW: Formal analysis, Investigation, Methodology, Software, Writing – original draft. GR: Investigation, Resources, Supervision, Writing – original draft. SY: Investigation, Software, Visualization, Writing – original draft. GZ: Data curation, Investigation, Writing – original draft. ZL: Data curation, Investigation, Writing – original draft. YC: Conceptualization, Methodology, Visualization, Writing – review & editing. ZD: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Key Research and Development Program of China (2023YFD2400800), the National Natural Science Foundation of China (32403059), the Earmarked Fund for Modern Agro-industry Technology Research System (CARS-49), the ‘JBGS’ Project of Seed Industry Revitalization in Jiangsu Province (JBGS [2021] 034), the Jiangsu Marine Resources Development Technology Innovation Center open fund (LWJJ-01), and the Lianyungang Key Research and Development Project (CG2304).
Acknowledgments
We thank International Science Editing (www.internationalscienceediting.com) for editing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Benli A.Ç.K., Köksal G., and Özkul A. (2008). Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L): Effects on gill, liver and kidney histology. Chemosphere 72, 1355–1358. doi: 10.1016/j.chemosphere.2008.04.037
Bu R., Wang P., Zhao C., Bao W., and Qiu L. (2017). Gene characteristics, immune and stress responses of PmPrx1 in black tiger shrimp (Penaeus monodon): Insights from exposure to pathogenic bacteria and toxic environmental stressors. Dev. Comp. Immunol., 771–716. doi: 10.1016/j.dci.2017.07.002
Chavan P., Kumar R., Kirubagaran R., and Venugopalan V. P. (2016). Chlorination-induced genotoxicity in the mussel Perna viridis: assessment by single cell gel electrophoresis (comet) assay. Ecotoxicology Environ. Safety. 130, 295–302. doi: 10.1016/j.ecoenv.2016.04.034
Chen J. H. (2009). Tolerance of broodstock, larvae and juvenile of bay scallop (Argopecten irradians) to chlorine dioxide. Chin. Agric. Sci. Bull. 25, 279–283.
Chen L., Hu G. Y., Cai Y. L., Xiao G. Q., Cai J. B., Zhang X., et al. (2023a). Dynamics of microbial community composition in the intestine of mud clams under coupled conditions of water temperature and residual chlorine. Zhejiang Agric. Sci. 64, 1323–1331. doi: 10.16178/j.issn.0528-9017.20221245
Chen L., Teng S. S., Lu Z., Xiao G. Q., and Cai J. B. (2023b). Acute toxicity of water temperature and residual chlorine to juvenile mud crab scylla paramamosain. Fisheries Sci. 42, 830–838. doi: 10.16378/j.cnki.1003-1111.21131
Dong Z. G., Duan H. B., Zheng H. F., et al. (2021). Research progress in genetic resources assessment, culture technique and exploration utilization of Cyclina sinensis. J. Fisheries China 45, 2083–2098. doi: 10.11964/jfc.20201212545
Hongxing G., Qian N., Jialing L., Dong Z., and Chen S. (2022). Effect of chronic ammonia nitrogen stress on the SOD activity and interferon-induced transmembrane protein 1 expression in the clam Cyclina sinensis. Front. Marine Sci. 9, 104152. doi: 10.3389/FMARS.2022.1034152
Li H. Y., Liang Y. F., Yang Q. H., et al. (2024). Acute toxicity test of four disinfectants on juvenile apostichopus japonicus. J. Fisheries Res., 1–8. doi: 10.14012/j.jfr.2024063
Liu M., Wang Z., Ni H., Zhuo W. Q., Yuan G. Y., and Dong Z. G. (2024). Adaptability of clams to prolonged heatwaves: Influences on gene expression, antioxidant capacity, and histology of Chinese cyclina Cyclina sinensis. Aquaculture Rep., 40102559–102559.
Liu C., Yu S., Song W., and Peng S. J. (2024). Investigation and risk assessment of dioxins and dioxin-like PCBs in main aquatic products in market from Shanghai. Microchemical J., 207111925–111925. doi: 10.1016/j.microc.2024.111925
Liu L. Z., Zhuang H. N., Tian X. L., Zhou Y. J., Wang F. Y., Liu Z. R, et al. (2024). Understanding the probiotic potential of Lactobacillus plantarum: Antioxidant capacity, non-specific immunity and intestinal microbiota improvement effects on Manila clam Ruditapes philippinarum. Fish shellfish Immunol., 154109971. doi: 10.1016/j.fsi.2024.109971
Ma Y. Y., Ding Y. L., Li Z. R., and Zhao S. S. (2023). Toxic effects of residual chlorine on chlorella vulgaris and koi carp. Henan Fisheries 05, 20–22 + 37.
Nakanishi M. and Rosenberg D. W. (2013). Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathology 35, 123–137. doi: 10.1007/s00281-012-0342-8
Ni Q., Liang X. F., Yang S. Y., Ge H. X., and Dong Z. G. (2024). Molecular and physiological responses in the ammonia transport pathways in clam Cyclina sinensis exposed to chronic ammonia nitrogen. Aquaculture Rep., 35101952. doi: 10.1016/j.aqrep.2024.101952
Ni Q., Liu J. L., Huang X., Ge H. X., Dong Z. G., and Peng Y. X. (2023). Effects of chronic ammonia nitrogen stress on hydrolases and interleukin 17–3 (IL-17–3) in clam Cyclina sinensis. Aquaculture Int. 4, 2339–2354. doi: 10.1007/s10499-023-01090-y
Pan L. Q., Ren J., and Liu J. (2006). Responses of antioxidant systems and LPO level to benzo (a) pyrene and benzo (k) fluoranthene in the haemolymph of the scallop Chlamys ferrari. Environ. Pollution 141, 443–451. doi: 10.1016/j.envpol.2005.08.069
Sokolova I. M. (2013). Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr. Comp. Biol. 53, 597–608. doi: 10.1093/icb/ict028
Sokolova I. M., Frederich M., Bagwe R., Lannig G., and Sukhotin A. A. (2012). Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Marine Environ. Res. 79, 1–15. doi: 10.1016/j.marenvres.2012.04.003
Sorong Z. X. (2024). Diagnosis and control measures of mycoplasma bovis disease. Anim. Husbandry Veterinary Sci. Inf. 10), 135–137. doi: 10.3969/J.ISSN.1671-6027.2024.10.046
Tian H. J., Ren S. J., Yang Z. G., and Gu X. Z. (2024). Effect of compound chinese herb medicine on resistance to white spot syndrome infection in red swamp crayfish procambarus clarkii. Fisheries Sci. 43, 129–135. doi: 10.16378/j.cnki.1003-1111.22043
Venkatnarayanan S., Murthy P. S., Kirubagaran R., Veeramani P., and Venugopalan V. P. (2021). Response of green mussels (Perna viridis) subjected to chlorination: investigations by valve movement monitoring. Environ. Monitoring Assess. 193, 1–2. doi: 10.1007/s10661-021-09008-y
Wang W., He G. P., Guan X. J., Chen Z. L., Li D. P., Sun L. Y., et al. (2024). Research on the water intake and drainage methods of the coastal nuclear power plants in China and the associated impacts on the Marine ecological environment. J. Zhejiang Univ. (Science Edition) 51, 769–780. doi: 10.3785/j.issn.1008-9497.2024.06.013
Wang L. W., Li X. N., Xie J. P., Feng Q. F., Wang C. Y., and Dai X. L. (2024). Effects of Salinity, Temperature, Ammonia, and Nitrite Stress on Growth and Physiological Biochemical Indices of Litopenaeus vannamei. J. Of Shanghai Ocean Univ., 1–17. Available at: http://kns.cnki.net/kcms/detail/31.2024.S.20240514.1352.002.htmL.
Wang S., Reng G., Li D., Fan S., Yan S. J., Liu M., et al. (2023). Transcriptome reveals the immune and antioxidant effects of residual chlorine stress on Cyclina sinensis. Front. Marine Sci. 10, 1105065. doi: 10.3389/fmars.2023.1105065
Wu Y. P., Chen A. H., Zhang Y., Cao Y., Chen S. H., and Zhang Z. D. (2018). Study on the toxicity of residual chlorine to juvenile bamboo clam and its median lethal concentration. Fishery Sci. Technol. Inf. 45, 158–161. doi: 10.16446/j.cnki.1001-1994.2018.03.009
Wu H., Yuan X., He Y., Gao J. W., Xie M., Xie Z. G, et al. (2024). Niclosamide subacute exposure alters the immune response and microbiota of the gill and gut in black carp larvae, Mylopharyngodon piceus. Ecotoxicology Environ. Saf., 279116512–116512. doi: 10.1016/j.ecoenv.2024.116512
Yan S. Y., Xia Q., Cui Z. Q., Ren G. L., Li X. Y., Ge H. X., et al. (2024). Environmental adaptation, growth performance and nutrient content of the clam Cyclina sinensis from different geographic locations. Front. Marine Sci. 11. doi: 10.3389/fmars.2024.1397324
Zhang J. and Wang G. (2020). Molecular cloning and expression analysis of C-type lysozyme gene in haliotis diversicolor. J. Jimei Univ. (Natural Science) 25, 328–335. doi: 10.19715/j.jmuzr.2020.05.02
Keywords: Cyclina sinensis, chlorine, long-term stress, antioxidant performance, nonspecific immunity
Citation: Wang C, Ren G, Yan S, Zhou G, Li Z, Chen Y and Dong Z (2025) Long-term effects of chlorine stress on the growth and biochemical indices of Cyclina sinensis. Front. Mar. Sci. 12:1603510. doi: 10.3389/fmars.2025.1603510
Received: 31 March 2025; Accepted: 16 May 2025;
Published: 05 June 2025.
Edited by:
Ana Varela Coelho, Universidade Nova de Lisboa, PortugalReviewed by:
Jaime Eugenio Figueroa, Austral University of Chile, ChileYuwen Dong, University of Pennsylvania, United States
Copyright © 2025 Wang, Ren, Yan, Zhou, Li, Chen and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yihua Chen, Y3lpaHVhQDE2My5jb20=; Zhiguo Dong, ZHpnNzcxMkAxNjMuY29t
†These authors have contributed equally to this work
 Chaohua Wang1,2,3†
Chaohua Wang1,2,3† Zhiguo Dong
Zhiguo Dong