- 1Institute of Agrifood Research and Technology (IRTA), Centre de la Rápita, Aquaculture Program, La Rápita, Spain
- 2Departamento de Biología y Geología, Facultad de Ciencias Experimentales, Universidad de Almería, Almería, Spain
- 3Departament de Biologia Cel·lular, Fisiologia i Immunologia, Facultat de Biologia, Universitat de Barcelona, Barcelona, Spain
- 4Centro Tecnológico de Investigación del Champiñón (CTICH), La Rioja, Spain
- 5IRTA, Centre Mas Bové, Animal Nutrition Program, Constantí, Spain
- 6Facultad de Acuicultura y Ciencias del Mar, Universidad Técnica de Manabí, Portoviejo, Ecuador
The mushroom industry generates by-products often classified as waste, making waste management a significant concern. To address this challenge and meet the growing demand for alternative protein sources in aquafeeds, this study evaluated the in vitro and in vivo digestibility of mushroom meals derived from three commercially cultivated species: Agaricus bisporus (AB), Lentinula edodes (LE), and Pleurotus ostreatus (PO) in rainbow trout (Oncorhynchus mykiss), as well as key performance indicators associated to growth, feed efficiency and body condition. In vitro digestibility was evaluated using semi-permeable membrane bioreactors by measuring liberated amino acids, while in vivo digestibility coefficients (ADCs) and key performance indicator (KPI) analyses were determined by feeding O. mykiss with diets containing AB, LE and PO meals for 42 days. Results showed that AB meal had similar in vitro digestibility to fish meal (p > 0.05), while LE and PO displayed intermediate and lowest digestibility values, respectively. Conversely, the in vivo ADCs of LE and PO meals was similar to the control (p > 0.05), whereas AB meal showed the lowest ADCs. Growth performance, somatic indices, blood biochemistry, whole-body composition, and digestive enzyme activities of O. mykiss were unaffected by AB, LE and PO diets (p > 0.05), but hepatic carbohydrate content and vacuolization increased in LE fed group (p < 0.05). Overall, although the digestibility of the three meals differed in vitro and in vivo, their crude protein ADC values were high (diet: > 90%; ingredient: > 77%) and did not compromise O. mykiss growth or feed efficiency. Thus, mushroom by-products may serve as alternative protein ingredients for aquafeeds.
1 Introduction
The transformation of agrifood systems is widely recognized as a critical strategy for achieving the United Nations Sustainable Development Goal of Zero Hunger (FAO, 2024). Within these systems, aquaculture production is essential for meeting global food demands, as it currently represents the fastest growing sector of agri-food production. However, this rapid expansion involves challenges related to sustainable intensification and sourcing of aquafeed ingredients to meet growing demand. This trend towards diversification in raw materials is accompanied by an effort to explore novel and sustainable alternatives to replace traditional marine-derived ingredients (Naylor et al., 2021; Glencross et al., 2024a), being prioritized those sources that reduce any associated adverse environmental impact (Boyd et al., 2020; Mitra, 2021; Serra et al., 2024). While true, different alternative ingredients are currently being used as nutrient sources for aquafeeds, including plant-based options (e.g., soybeans, cereals), animal by-products (e.g., poultry, rendering, fish trimmings), fishery by-products, insects, algae, biofloc, and single-cell proteins (Ayadi et al., 2012; Glencross et al., 2024a; Serra et al., 2024). Incorporating novel ingredients into current formulations presents several challenges, including the presence of anti-nutritional factors, suboptimal nutrient composition, palatability issues, competition with other sectors, ethical concerns, and fluctuations in availability and price (Gómez et al., 2019; Boyd et al., 2020; Mitra, 2021; Serra et al., 2024). From a holistic point of view and aiming to address these challenges, the integration of by-products or low-value biomass in aquafeed formulations is emerging as a strategy to promote sustainable and environmentally friendly aquaculture practices, as it valorizes resources, thereby reducing the environmental footprint and supporting the promotion of a circular economy (López-Pedrouso et al., 2020; Sandström et al., 2022).
Among the different agri-food production systems, the mushroom production industry for human consumption has shown an almost five-fold increase since the 1990s, with a global production (including truffles) of 48.3 MT in 2022, and an annual growth projection rate of 6.4% until 2026 (Robinson et al., 2019; Shirur et al., 2021). Inevitably, this increase in production has also led to a notable increase in the generation of by-products, often classified as waste, which contribute to ca. 5% to 50% of the total mushroom biomass produced (Antunes et al., 2020; Navarro-Simarro et al., 2024). Specifically, the European Mushroom Growers Group reported that for every metric ton of mushroom grown and processed (sold fresh, canned or frozen), ca. 150 kg of by-products are generated (Infochampi, European Mushroom Growers Group, 2022). These by-products, composed of stems and discarded biomass, are treated as waste in accordance with national and European regulations. In this scenario, waste management is becoming an important challenge for the mushroom industry, as the traditional way of waste management practices are no longer allowed, generating costs (landfill fees and transport) that not only reduce the economic benefits but also create a negative perception from the consumer due to the high carbon footprint associated to these practices. In this context, a new cost-effective and environmentally friendly alternative to traditional strategies for the management of these by-products is required. Therefore, the use of these agricultural residues as alternative sources of nutrients in aquafeeds is emerging as a promising, environmentally and economically sound strategy.
Indeed, mushrooms, as biomass, have an excellent amino acid profile for animal nutrition, with a protein content ranging from 3.9% to 87.1%, depending on the species considered. In addition, mushrooms contain low levels of lipids (0.1-9.23%) and high levels of carbohydrates (12.7-87.1%), minerals (5.27-37.8%), and bioactive compounds with potential health benefits, such as polysaccharides (e.g., trehalose, mannitol, β-glucan, chitin, and chitosan), phenolic compounds (e.g., p-coumaric acid, gallic acid, ferulic acid), and terpenes/terpenoids (Kalač, 2013; You et al., 2022). Several studies have reported the use of mushroom meals as a potential feed ingredient for various marine and freshwater fish species (Katya et al., 2014; Chong et al., 2016; Ahmed et al., 2017; Adejonwo et al., 2020). In general, these studies concluded unaltered or moderate improvements in KPIs, improved survival rates after biotic challenges and a modulation of immune response at dietary inclusion levels no higher than 10%. Evaluating digestibility and its impact on KPIs are crucial when assessing the suitability and optimizing inclusion levels of alternative or novel ingredients in aquafeed formulations (Glencross et al., 2007). Within this context, a key challenge is the development of rapid and reliable methods for accurately predicting the nutritional value of raw ingredients, by-products and processed feeds for specific species to reduce the current reliance on labor-intensive and expensive in vivo nutritional trials. The use of in vitro assays mimicking the conditions and hydrolysis processes taking place in the gut of aquatic species has become a very important tool in this sense, offering a wide array of potential applications (Moyano et al., 2015; Wang et al., 2021; Moyano and Gilannejad, 2025). For this reason, the present study aimed to evaluate the digestibility of mushroom by-products (meals) from three commercially cultivated mushroom species, Agaricus bisporus, Lentinula edodes and Pleurotus ostreatus, both in vitro and in vivo, in rainbow trout (Oncorhynchus mykiss). Although experimental diets formulated for in vivo digestibility trials are not conventionally employed for evaluating key performance indicators (KPIs) related to growth performance, feed efficiency, and fish condition, the researchers extended their investigation to assess the effects of the aforementioned mushroom meals on these parameters as an initial approach to understanding their nutritional impact on fish condition.
2 Materials and method
2.1 Ethics statement
The experimental procedures employed in this study (in vitro and in vivo experiments) complied with EU2010/63 guidelines (Guiding Principles for Biomedical Research Involving Animals) and Spanish laws (32/2007 and RD 1201/2015). In addition, the study was approved by the Ethical Committee of the Institute for Research and Technology in Food and Agriculture (IRTA, Spain) for the use of laboratory animals (E-10/2020) and the Generalitat de Catalunya (CEEA 219/2020).
2.2 In vitro protein hydrolysis
The digestive enzyme extracts used in the assays were obtained from several individuals of O. mykiss (average body weight, BW = 420 g, n = 8) sampled at 4 h post-feeding. Once sacrificed, the digestive tract was removed, stored at -20°C, and later, the stomach and intestine were dissected. Active extracts were prepared from either the stomach or intestinal portions (this latter including pyloric caeca) by mechanical homogenization of tissues and gut contents in distilled water (1:3 w/v) followed by centrifugation (10,000 x g, 30 min, 4°C). The resulting supernatants were then filtered through a dialysis system with a MWCO of 10 kDa (Pellicon XL, Millipore, St. Louis, MO, USA) and after freeze-dried until used in the assays. The activities of acid and alkaline proteases in extracts were measured following the protocols of Anson (1938) and Kunitz (1947), modified by Walter (1984), respectively. Assay conditions were established based on physiologically relevant enzyme:substrate ratios. These ratios were derived by considering the mean total protease production (normalized to live weight) measured in the sampled fish, and the average meal intake for fish of comparable size, obtained from commercial feed tables. These calculated values served as a reference and were scaled to a 50 g “model fish” for the development of the assays (Moyano et al., 2015). All operational parameters are detailed in Table 1.
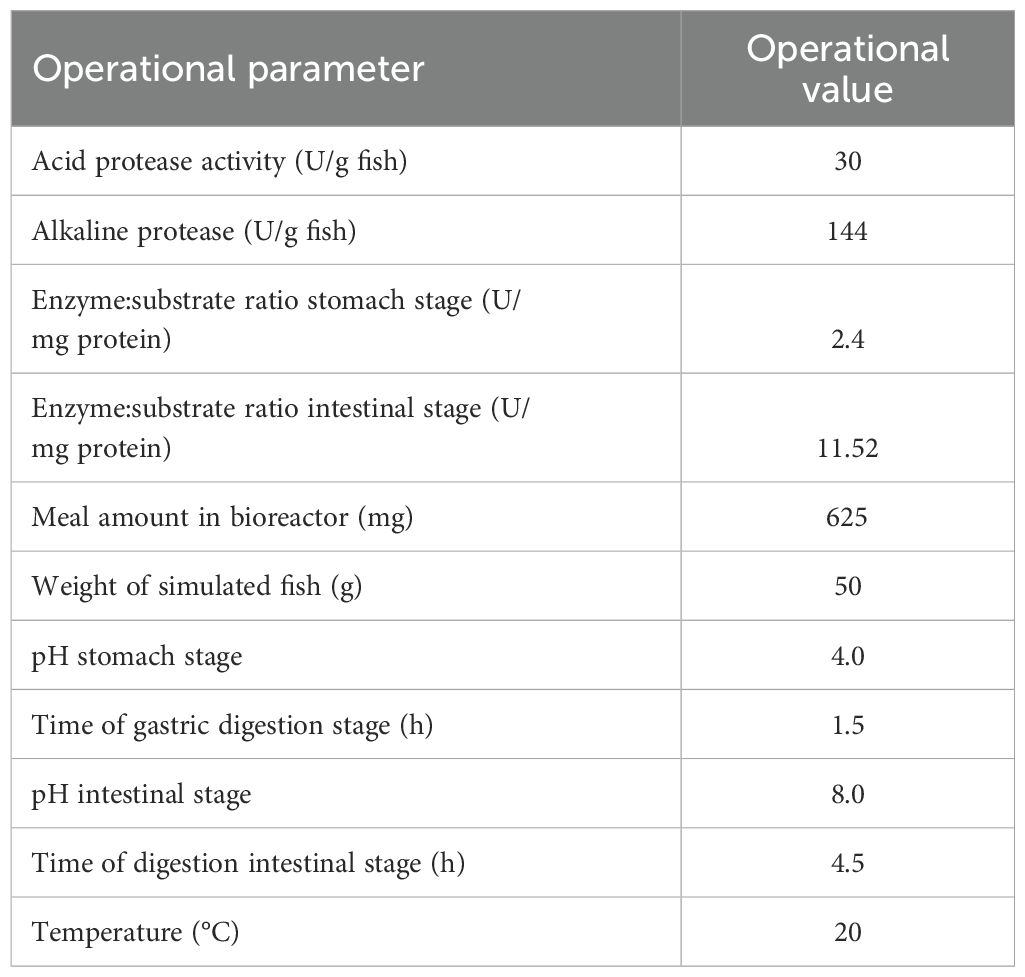
Table 1. Operational parameters used during the in vitro assay for evaluating the in vitro digestibility of mushroom meals.
In vitro assays were conducted to evaluate the digestive bioavailability of proteins from three fungal meals, with fishmeal (NORVIK LT70) serving as a control. Semi-permeable membrane bioreactors, adapted from those described by Morales and Moyano (2010), were employed. Each bioreactor consisted of two chambers separated by a 3.5 kDa MWCO membrane (ZelluTrans, Carl Roth GmbH, Karlsruhe, Germany). The acid digestion phase was simulated by introducing fish enzyme extracts and substrates into the upper chamber, with the pH adjusted accordingly using 0.1 M HCl. Following the acid phase, alkaline hydrolysis was initiated by adjusting the pH to the target value with 0.1 M borate buffer and subsequently adding the intestinal enzyme extract. Amino acids and small peptides released and permeating across the membrane into the lower chamber were collected at designated time intervals via a constant flow of the same alkaline buffer. The release of these products from the substrate was quantified using the orthophthaldehyde (Church et al., 1983). The entire experimental setup, comprising multiple bioreactor units, was maintained within a temperature-controlled chamber at 20°C. Results are presented as cumulative amino acid release over time, as well as the total mass of released amino acids expressed as a percentage of the total protein present in the substrate samples. All assays were performed in triplicate. Additionally, a blank, utilizing heat-inactivated enzyme extracts, was included for each meal to quantify basal free amino acid release not attributable to enzymatic hydrolysis.
2.3 In vivo digestibility
2.3.1 Experimental diets
To evaluate the digestibility of the three mushroom meals obtained from A. bisporus, P. ostreatus, and L. edodes, three experimental diets (AB, LE, and PO) were formulated based on a basal diet (Control, CTRL) containing 49% crude protein (CP) and 22% crude fat (CF). Mushroom meals were composed of stems and broken, or unmarketable mushrooms sourced from a commercial farm in La Rioja, Spain, and processed at the Centro Tecnológico de Investigación del Champiñón de La Rioja (CTICH, Autol, Spain). Raw materials were processed by firstly cutting them using a sheeter (SAMMIC CK 38V, SAMMIC, Azkoitia, Spain) followed by drying at 35°C (Klarstein Master Jerky 550, Chal-Tec GmbH, Berlin, Germany). The dried material was then grounded using a mill (Retsch ULTRA ZM 200, Retsch GmbH, Haan, Germany), and the resulting mushroom powder (meal) was analyzed and used in the formulation of experimental diets. The proximate composition of the mushroom meals as well as the formulae of experimental diets is shown in Tables 2 and 3, respectively. The inclusion of mushroom meals was based on the design of Cho and Slinger (1979) (30% tested ingredient, mushroom meals; 70% control diet) and the recommendations provided by Glencross et al. (2023), who reported that lower inclusion levels of ingredients for digestibility determination might provide inaccurate values of apparent digestibility coefficients (ADC). Experimental diets were not isoproteic and isolipidic due to the proximate composition of raw materials and the high inclusion level requirement for evaluating digestibility. Yttrium oxide (Y2O3) was used as an inert marker. The experimental diets (pellet size: 4 mm) were formulated and produced by Sparos Lda. (Portugal) as previously described (Ruiz et al., 2024).
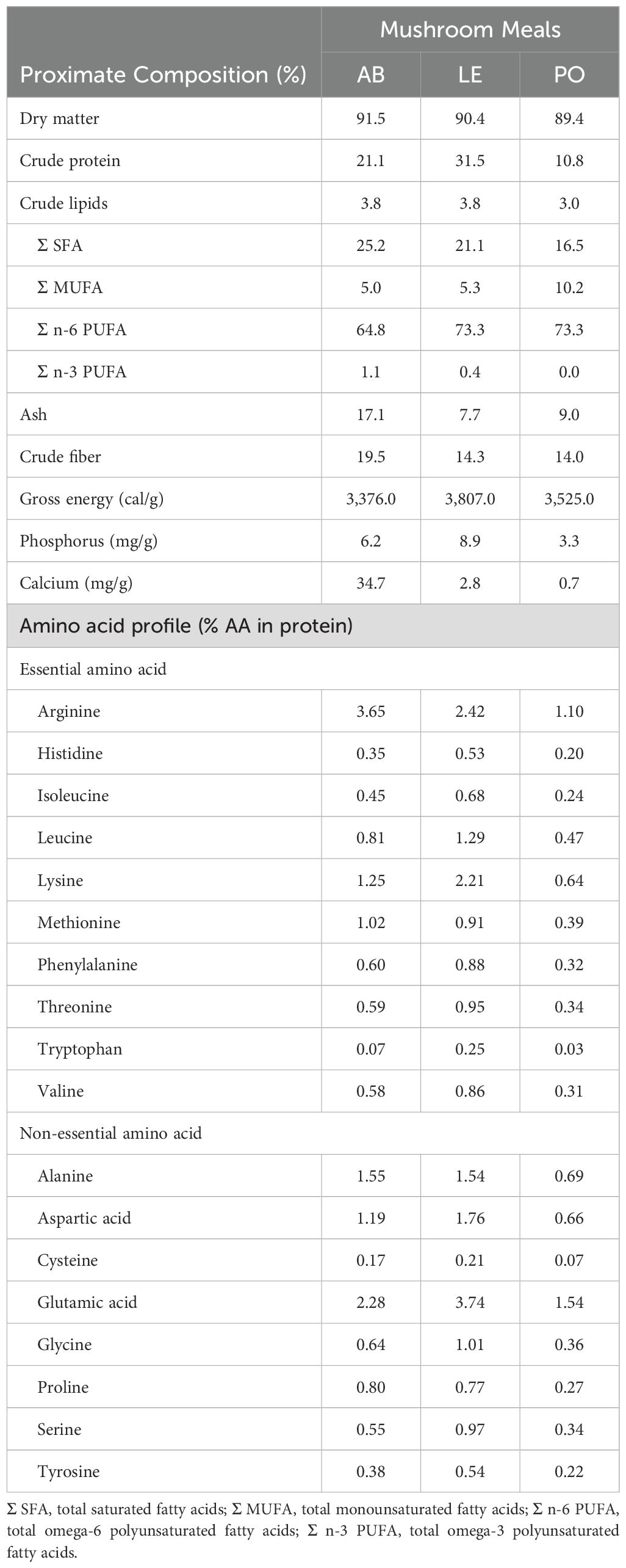
Table 2. Proximate composition and nutritional value of mushroom meals obtained from Agaricus bisporus (AB), Lentinula edodes (LE) and Pleurotus ostreatus (PO)
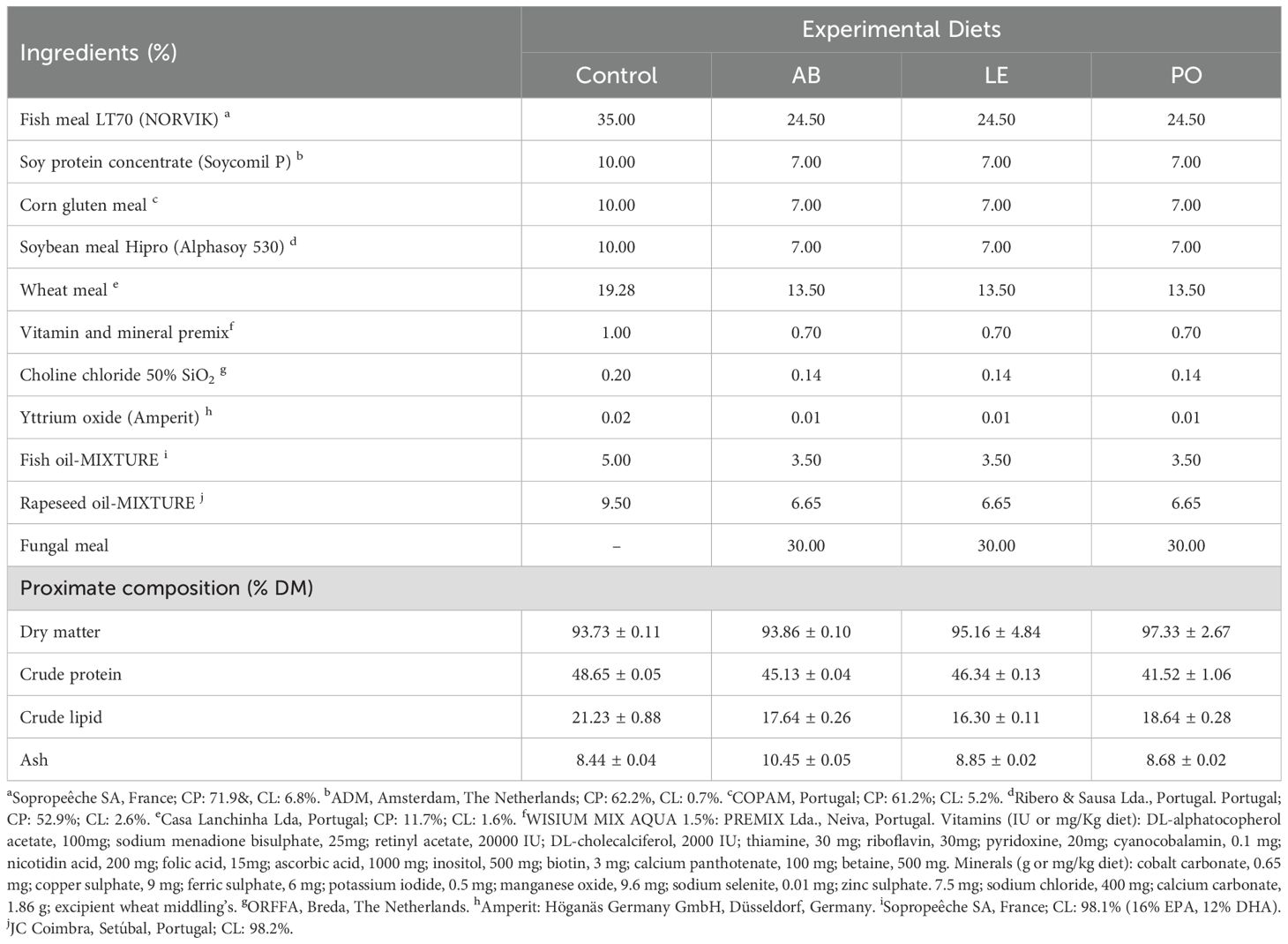
Table 3. Ingredient list and proximate composition (mean ± SD) for experimental diets containing different mushroom meals.
2.3.2 Fish and rearing conditions
Rainbow trout juveniles were obtained from Truchas de Leiza SL (Leiza, Spain) and acclimatized for 7 days in 10 m3 tanks prior to the experiment. After conditioning, fish (initial body weight, BWi: 120.1 ± 0.4 g) were randomly distributed into twelve 2 m3 tanks with an initial stocking density of 45 fish per tank (2.7 kg/m3) in a recirculating system (with mechanical, biological filtration and UV water treatment (IRTAmar™). Each tank had a settling column tank, based on the Guelph system, to collect uneaten feed pellets for accurate feed intake determination. The experimental diets were fed for 42 days at a feeding rate of 1.6-1.8% of tank biomass using automatic feeders (ARVO-TEC T Drum 2000; Arvotec, Huutokosk, Finland) seven days a week. The daily feeding ration was equally divided into two meals, at 08:00 and 13:00 h, and distributed over a period of one hour. Two hours after each meal, uneaten pellets were collected, dried overnight at 100°C, and weighed to calculate daily feed intake. The feed ration was adjusted to maintain 10% – 15% uneaten pellets, ensuring that the fish were fed ad libitum. Environmental conditions were monitored daily and maintained at optimum levels (e.g., temperature: 15.7 ± 0.2°C, dissolved oxygen: 8.8 ± 0.3 ppm). Photoperiod followed natural changes according to the season of the year (February to March at 40.63N–0.66E).
2.3.3 Fish sampling and performance analyses
Fish biometry was conducted on days 24 and 42. Fish were fasted overnight and anesthetized with MS-222 (100 mg L-1) prior to individual measurement of BW (g) and SL (cm). The following key performance indicators associated with somatic growth and feed efficiency were calculated:
After the feeding trial, 16 fish per tank (48 per diet) were fasted overnight and anesthetized with MS-222 (100 mg L-1) for blood sample collection and euthanized with an overdose of MS-222 (350 mg L-1) for tissue collection (gastrointestinal tract, liver and whole fish).
2.3.4 Fecal collection and ADC coefficients calculation
Fecal collection was performed after 30 days of feeding fish with experimental diets, as recommended by Glencross et al. (2023). The collection of excreted feces followed the method described by Tefal et al. (2024) with minor modifications. In brief, the tanks and settling column were cleaned 2 and 4 h after feeding to ensure the absence of uneaten feed in the collection column. Overnight settled feces were collected and centrifuged for 15 min at 5,000 x g (Vandenberg and de la Noüe, 2001). The supernatant was discarded, and the feces were stored at -20°C for further analysis. This process was repeated for three alternate days to obtain a sufficient amount of feces for analysis. The apparent digestibility coefficients (ADCs) for dry matter, protein, lipid, and energy of the control and test diets were calculated using the formula of Cho and Slinger (1979): ; ; where Marker diet and Marker feces are the content of Y2O3 in the diet and feces, respectively; and Nutrient diet and Nutrient feces are the content of the targeted nutrient in diets and feces, respectively. The apparent digestibility coefficient of dry matter, protein, lipid and energy of the test ingredients were calculated according to Bureau and Hua (2006): ; where ADC Test diet is the apparent digestibility coefficient of the test diet and ADC CTRL diet is the apparent digestibility coefficient of the control diet.
2.3.5 Proximate composition of fecal samples, diets liver and whole fish
Proximate composition of experimental diets, fecal samples, liver and whole fish, and marker content of the experimental diets and feces were analyzed according to AOAC (2006). Dry matter content was analyzed by sample drying at 105°C for 14 h (AOAC 925.09), incineration in a muffle furnace for ash content (Nabertherm, Germany 500°C for 5 h, AOAC, 942.05), Dumas procedure for crude protein (Nitrogen analyzer FP-528 Leco, USA, AOAC 968.06) and Büchi Extraction System B-811 (Büchi, Switzerland, AOAC 920.39) for crude fat. The determination of carbohydrates in samples was done using the phenol-sulfuric acid method (Dubois et al., 1956). An adiabatic bomb calorimeter (using the DIN 51900 rule) was used for the analysis of gross energy, and inductively coupled plasma optical emission spectroscopy (ICP-OES Optima 2100DV, Perkin Elmer Life and Analytical Sciences, Shelton, USA) was used for the analysis of yttrium oxide (AOAC 984.27).
2.3.6 Digestive enzymes analyses
Fish sampled after the feeding trial (n = 12 fish per diet) were euthanized, dissected on a glass plate at 0-4°C and their gastrointestinal tract was removed and frozen at -80°C until further analysis. Digestive enzyme activities were analyzed from stomach and intestine samples (including pyloric caeca) following the methods described by Gisbert et al. (2009) and samples were processed and handled following the indications of Solovyev and Gisbert (2016) to avoid their degradation during storage and handling. Stomach samples were homogenized in Milli-Q water, sonicated, and centrifuged. The supernatant was collected and stored at −80°C for pepsin quantification using hemoglobin as substrate in 1 N HCl buffer (pH 3.0) (Nolasco-Soria et al., 2020). Intestinal samples with pyloric caeca were homogenized in Tris-Mannitol buffer with CaCl2 (ph 7.0), sonicated, centrifuged, and the supernatants were collected and stored for pancreatic and brush border enzyme assays. Pancreatic enzymes were quantified as follows: α-amylase using starch as substrate dissolved in Na2HPO4 buffer (pH 7.4) (Métais and Bieth, 1968), trypsin with BAPNA in 50 mM Tris–HCl, 20 mM CaCl2 buffer (pH 8.2) (Holm et al., 1988), chymotrypsin with BTEE as substrate in 80 mM Tris–HCl, 100 mM CaCl2 buffer (pH 7.2) (Worthington Biochemical Corporation, 1991), bile salt-activated lipase with p-nitrophenyl myristate as substrate in 0.25 mM Tris–HCl and 0.25 mM 2-methoxyethanol and 5 mM sodium cholate buffer (pH 9.0) (Iijima et al., 1998), and total alkaline proteases with azo-casein as substrate in 50 mM Tris–HCl buffer (pH 8.0) (García-Carreño and Haard, 1993). Intestinal brush border enzymes activities were measured as follows: alkaline phosphatase with PNPP as substrate dissolved in 50 mM Na2CO3 buffer containing 40 mM MgCl2 (pH 9.8) (Gisbert et al., 2018), aminopeptidase-N with L-leucine p-nitroanilide as substrate in 0.2 M phosphate buffer (pH 7.0) (Maroux et al., 1973), and maltase with d(+)-maltose as substrate in sodium maleate buffer (pH 6.0). Cytosolic leucine-alanine peptidase activity was determined with L-alanine as substrate in 50 mM Tris–HCl (pH 8.0). Soluble protein was quantified using Bradford’s method (Bradford, 1976) with bovine serum albumin as standard. All assays were conducted in triplicate using a spectrophotometer (Tecan™ Infinite M200, Tecan, Switzerland) under standardized conditions (20°C) and expressed as specific activity (U mg protein−1).
2.3.7 Blood biochemical and hematological analysis
Blood biochemical parameters (albumin, aspartate aminotransferase, total bilirubin, calcium, chlorides, cholesterol, creatinine, alkaline phosphatase, phosphorus, glucose, iron, lactate dehydrogenase, magnesium, potassium, total protein, sodium, triglycerides, amylase, creatinine kinase, total globulin, lipase, sodium, potassium and chlorides) were analyzed using an UV/Vis spectrophotometer (Vitros® 5600 Integrated System & Analyzer, QuidelOrtho™, San Diego, USA) and hematological parameters (hematocrit, hemoglobin, erythrocytes, mean cell volume, mean cell hemoglobin and mean cell hemoglobin concentration) were measured using a blood count chamber. Both blood biochemical and hemotological analyses (n = 12 fish per diet) were performed by Laboratorios Echevarne, Barcelona (Spain).
2.3.8 Liver morphological analysis
Liver samples were dissected (n= 12 fish per diet) and fixed in 4% neutral-buffered formalin. Fixed samples were embedded in paraffin and cut in sections (4 μm-thick) using a Leica 2055-Autocut microtome (Leica Instruments GmbH, Nussloch, Germany). Sections were stained with hematoxylin and eosin (H&E) and digital images were taken at a final magnification of 200x and 400x by an Olympus VS120 digital scanner (Optic system BX61VS, Tokyo, Japan) coupled with VC50 and VS-XM10 cameras. Digital images were examined using an Olympus VS software (VS-NIS-SQL-V2.6, Tokyo, Japan) by qualitative assessment of the following morphological parameters: hepatocytes pattern, vacuolization level, nuclei alignment around sinusoidal lines, blood congestion and level of hepatic steatosis. A scoring scale ranging from 1 to 4 (1 absent/very low incidence to 4 high incidence) was used to examine morphological differences, and the examinations were performed by two independent observers who were unaware of the experimental conditions. Sections were also stained with Periodic acid–Schiff (PAS) stain (pH = 2.5) for intrahepatic glycogen detection. PAS-stained sections were examined under a light microscope (Motic BA310E; Barcelona, Spain), and PAS-positive scoring was conducted using a previously described scoring scale. Representative images were obtained using a Motic MOTICAMProS5 LiteCamera (Motic, Barcelona, Spain).
2.4 Statistical analyses
Data are expressed as mean ± standard deviation (SD) and was checked for normality and homogeneity of variances. Data expressed as percentages were arcsine transformed prior to statistical analyses. As diets were not isoproteic and isolipidic due to the high level of fiber and ashes in mushroom meals, KPIs were compared between experimental groups using multivariate analysis of covariance (MANCOVA) using dietary crude protein and crude lipid as covariates, followed by Bonferonni posthoc test when significant differences were observed. Results of the in vitro bioavailability assays and ADC were evaluated using one-way ANOVA followed by Tukey’s post hoc test. Morphological scores were analyzed using the Kruskal-Wallis and Mann-Whitney U tests. Pearson correlation analysis was used to evaluate correlation coefficients among in vivo digestibility and KPIs. In all test a confidence interval of 95% was established. Statistical analyses were performed using the SPSS software (Version 21.0, SPSS Inc., Chicago, IL, USA) and the associated Heatmap analysis was performed using R V.4.3.2 software.
3 Results
3.1 In vitro digestibility
The cumulative amino acid release profiles for the hydrolysis of the tested meals, including fishmeal (FM) as a control, are presented in Figure 1. The figure displays the fitted regression lines and corresponding linear equations, enabling a comparison of release rates derived from their slopes (mg AA h-1). Further data, including the time required for 50% protein hydrolysis (calculated from the linear equations) and the percentage of total protein hydrolyzed, are summarized in Table 4. Significant differences were observed in both the rates of amino acid release and the total amount of amino acids liberated from the tested meals. In terms of absolute values, the hydrolysis of the AB meal was not significantly different from that of FM. However, the amino acid release from the PO meal was significantly lower (p < 0.05), while the LE meal exhibited an intermediate value. When amino acid release was normalized to the initial protein content of the samples (625 mg of meal, but with varying crude protein (CP) content, ranging from 10.7% in PO to 68% in FM), a different trend emerged. Under these conditions, all fungal meals demonstrated significantly higher values compared to FM, with the following ranking: PO > AB > LE > FM.
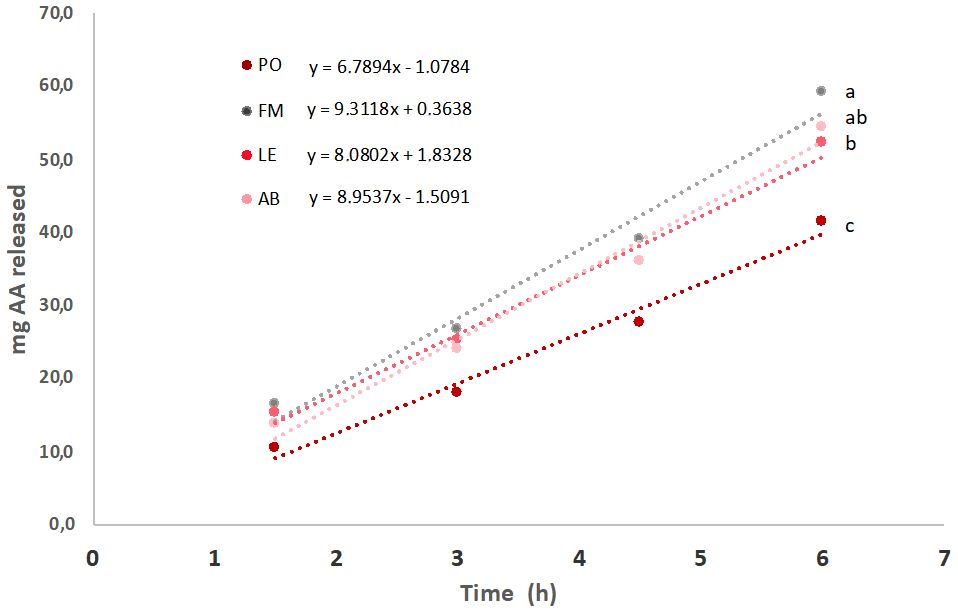
Figure 1. Plot of amino acid release (mg) after hydrolysis of the different tested ingredients under conditions simulating digestion of rainbow trout (Oncorhynchus mykiss). Significant differences in the final values are noted by low-case letters (p < 0.05). Each point is the mean of triplicate assays.
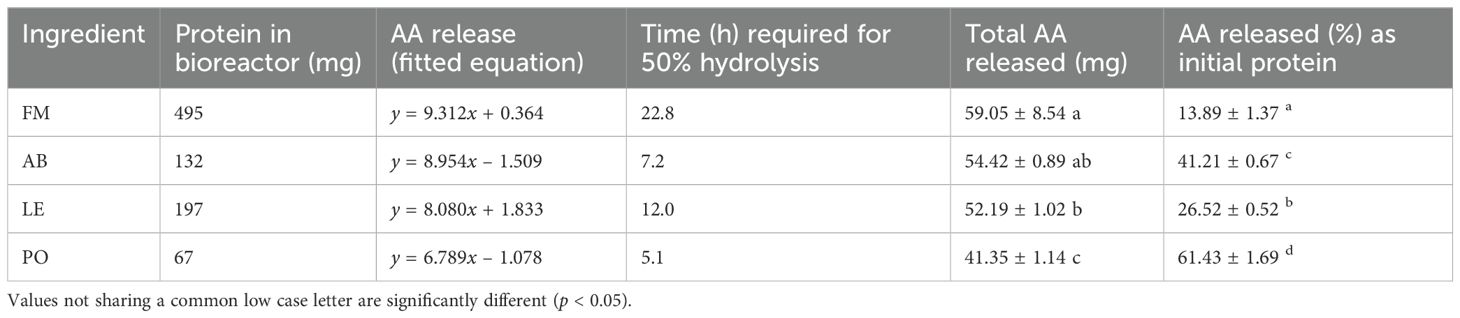
Table 4. Estimated rates of protein hydrolysis and total amount of amino acids hydrolyzed from the different mushroom meals (PO, P. ostreatus; LE, L. edodes; AB, A. bisporus; FM, fishmeal LT70) expressed in absolute values and as % of crude protein.
3.2 In vivo digestibility
3.2.1 Apparent digestibility coefficients
There were no statistically significant differences in the ADCs for dry matter (71-84%), crude lipid (85-93%) and energy (79-87%) between the CTRL and the mushroom meal-based diets (p > 0.05). However, the AB diet presented lower crude protein ADC values (p < 0.05) compared to the CTRL diet but was similar to the LE and PO diets (Figure 2). Regarding ingredient ADCs, the AB diet also showed significantly lower crude protein ADC values (78.55 ± 1.34%) than the PO diet (90.63 ± 6.11%) (p < 0.05), whereas no significant difference was detected between the PO and LE diets (86.96 ± 0.12%) (p > 0.05). Similarly, crude lipid ADC of the AB diet (57.19 ± 4.43%) was significantly lower than in the LE (87.25 ± 4.23%) and PO (62.06 ± 0.21%) diets (p < 0.05). Dry matter and energy ADCs were also lowest for the AB diet, while PO and LE diets displayed intermediate and highest values, respectively.
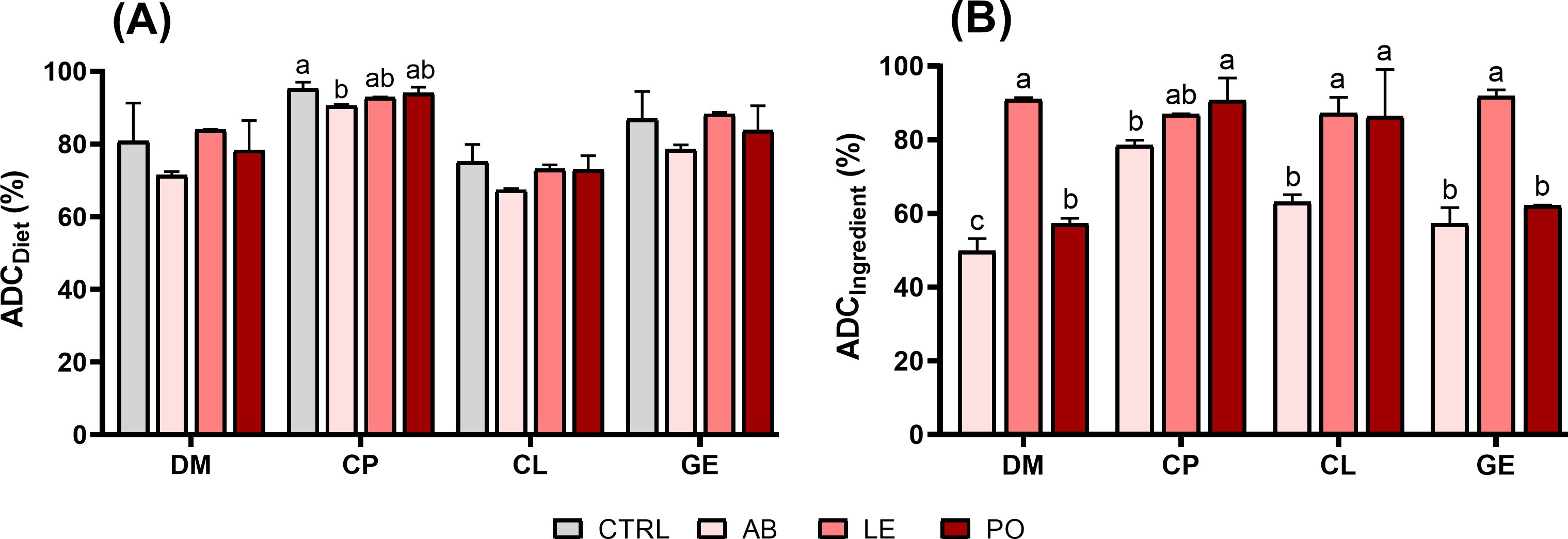
Figure 2. Apparent digestibility coefficients of the experimental diets and ingredients in rainbow trout (O. mykiss): (A) ADCdiet; (B) ADCingredient. DM, dry matter; CP, crude protein; CL, crude lipid; GE, gross energy; CTRL, control diet; AB, A. bisporus diet; LE, L. edodes diet; PO, P. ostreatus diet. Data are expressed as mean ± SD (n = 3). Different superscript letters within each nutrient group indicate significant differences (One-way ANOVA, p < 0.05).
3.2.2 Fish performance
Growth performance and feed utilization parameters of rainbow trout fed the experimental diets are summarized in Table 5. At 24 days, no significant differences in growth performance (BW, WG, and SGR), condition factor (K), or feed efficiency parameters (FI and FCR) were observed among the different groups (MANCOVA, p > 0.05).
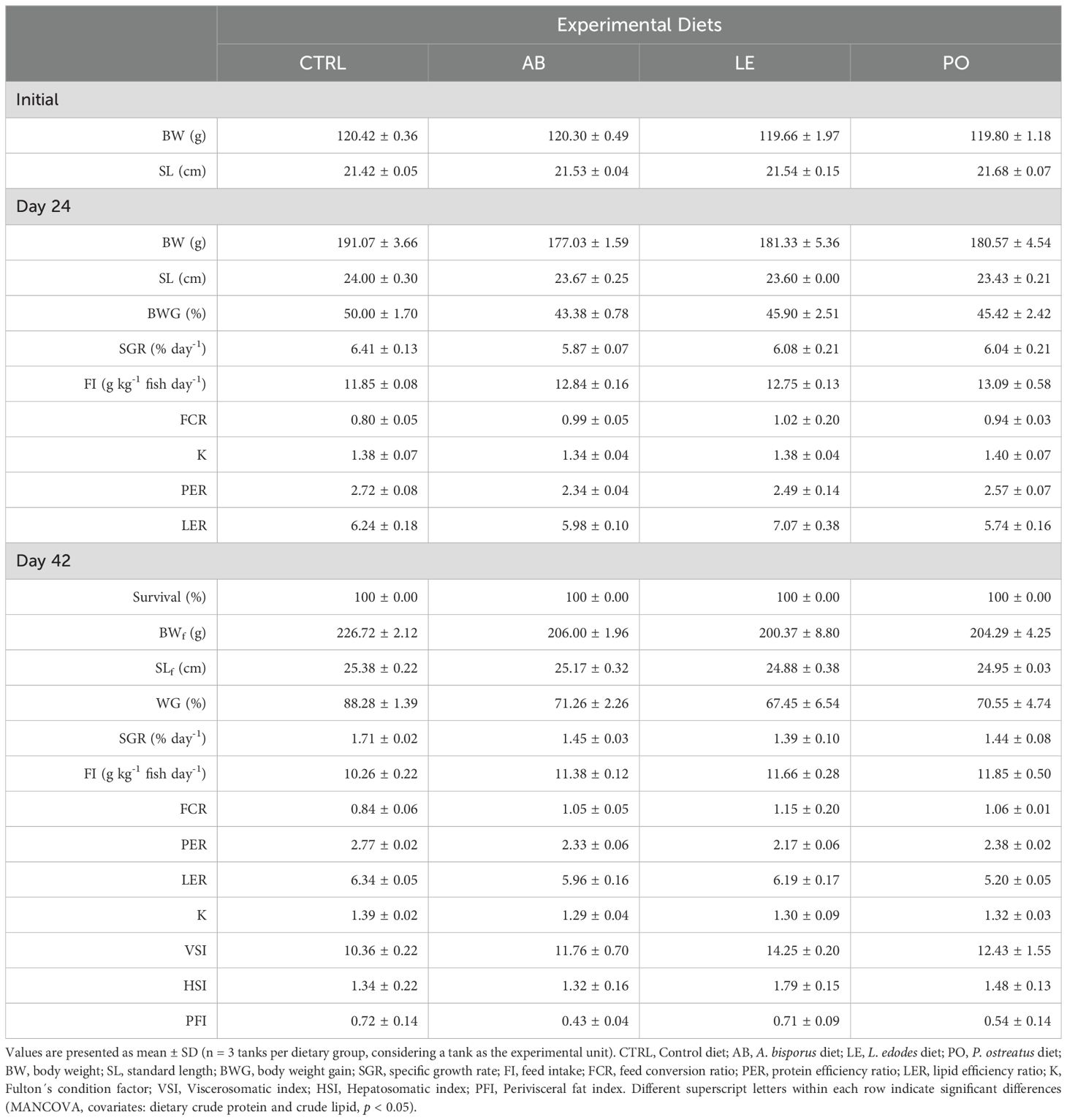
Table 5. Growth performance, feed utilization, nutrient efficiencies and somatic indices of rainbow trout (O. mykiss) fed with experimental diets containing different sources of mushroom meals.
At the end of the feeding trial, although mushroom-fed groups showed numerically lower BW values, ranging from -9% to -11% with respect to the control group, no significant differences in growth performance (BW, BWG, SGR), somatic indices (K, HSI, VSI, PVFI) or feed efficiency parameters (FI and FCR) were observed among the experimental groups (MANCOVA, p > 0.05). Correlation analysis showed that growth performance and feed utilization parameters were correlated with dietary crude protein and lipid ADCs (p < 0.10) (Figures 3A, B). In particular, diet crude lipid ADC was positively correlated to PERD24 (r = 0.54, p = 0.07), whereas dietary crude protein ADC was positively correlated to fish BWD24 (r = 0.55, p = 0.06), BWGD24 (r = 0.52, p = 0.08), SGRD24 (r = 0.52, p = 0.08), and PERD24 and D42 (D24: r = 0.73, p = 0.01; D42: r = 0.55, p = 0.07). Conversely, diet crude protein ADC was negatively correlated to FID24 (r = -0.57, p = 0.05).

Figure 3. Heatmap matrix representing the correlation coefficients (Pearson’s correlation) among apparent digestibility coefficients (ADCs) of the experimental diets and KPIs: (A) ADCs and day 24 and (B) day 42 growth performance, somatic indices and feed utilization; (C) ADCs and liver and whole-body proximate composition; (D) ADCs and digestive enzyme activities; (E) ADCs and blood biochemical and hematological parameters. GE, gross energy ADC; CL, crude lipid ADC; CP, crude protein ADC; DM, dry matter ADC; BW, body weight; BWG, body weight gain; SL, standard length; SGR, specific growth rate; FI, feed intake; FCR, feed conversion ratio; PER, protein efficiency ratio; LER, lipid efficiency ratio; K, condition factor; VSI, viscerosomatic index; HSI, hepatosomatic index; PFI, perivisceral fat index; CHO, carbohydrate; PEP, pepsin; AMY, amylase; CHY, chymotrypsin; LIP, lipase; TRY, trypsin; PRO, total alkaline proteases; ALP, alkaline phosphatase; AP, aminopeptidase-N; MAL, maltase; LAP, leucine-alanine peptidase; Ca, calcium; Cl, chlorides; Mg, magnesium; P, phosphorus; K, potassium; Na, sodium; AST-SGOT, aspartate aminotransferase; CRE, creatinine; LdH, lactate dehydrogenase; ALB, albumin; T. Globulin, total globulin; TP, total protein; CHOL, cholesterol; GLU, glucose; Fe, iron; TBIL, total bilirubin; TGs, triglycerides; ERT, erythrocytes; HCT, hematocrit; HGB, hemoglobin),MC V(mean cell volume; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration. Values inside the plots indicate the correlation coefficients (r) and values in bold and with asterisks (*) indicate significant correlation (p <0.10).
3.2.3 Liver and whole-body proximate composition
Liver and whole-body proximate composition considering dietary crude protein and lipid as covariates revealed significant differences among experimental groups (Table 6, MANCOVA, p < 0.05). Liver carbohydrate content in fish fed the LE diet was higher (p < 0.05) than in fish fed the control diet (p < 0.05), whereas liver protein, lipid and ash content did not vary among experimental groups (p > 0.05). Fish whole body proximate composition was similar in all the experimental treatments (p > 0.05).
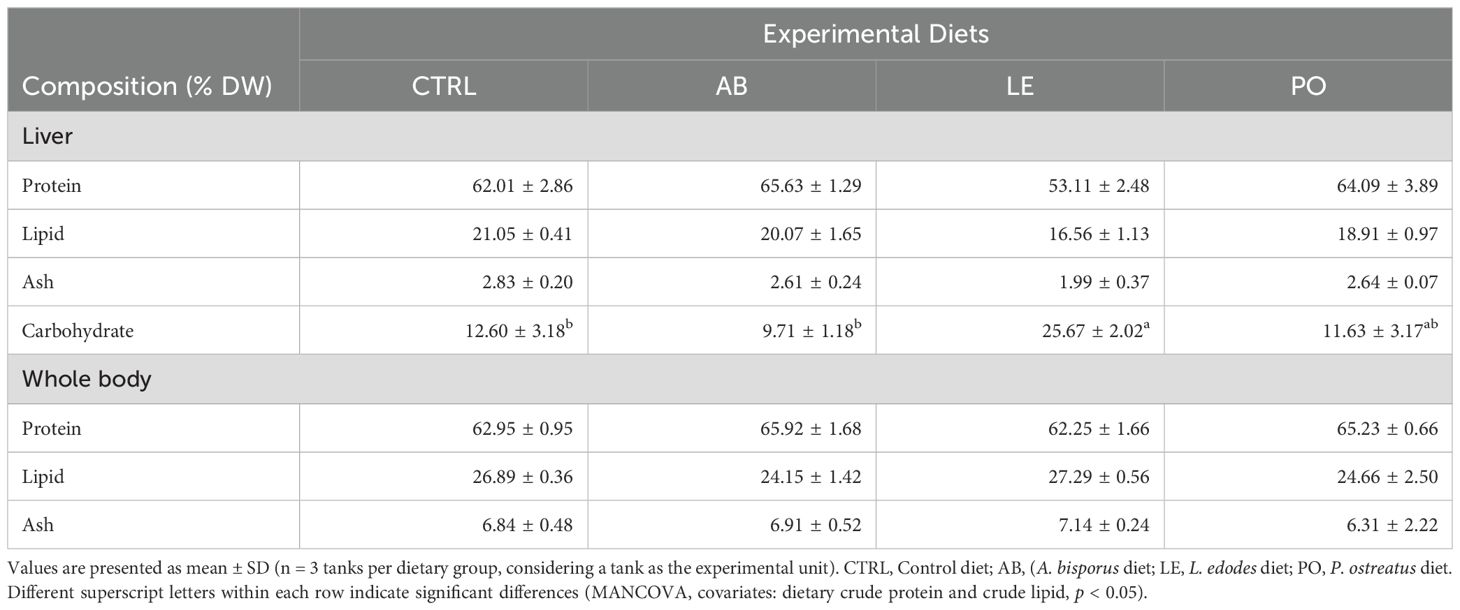
Table 6. Liver and whole-body proximate composition in dry weight (DW, %) of rainbow trout (O. mykiss) fed with the experimental diets containing different sources of mushroom meals for 42 days.
No significant correlation was recorded between liver and whole-body proximate composition with diet crude protein and lipid ADCs (Figure 3C, p > 0.10), whereas a negative correlation was detected between fish whole body crude protein and diet energy (r = -0.54, p = 0.07) and dry matter ADCs (r = -0.52, p = 0.08).
3.2.4 Digestive enzyme activities
The inclusion of mushroom meals in compound diets for rainbow trout did not modify the activity of gastric, pancreatic nor intestinal digestive enzymes (Table 7, MANCOVA, p > 0.05). Correlation analysis showed that the activity of aminopeptidase and maltase negatively correlated to diet crude protein ADC (Figure 3D, aminopeptidase: r = -0.5, p = 0.09; maltase: r = -0.57, p = 0.05).
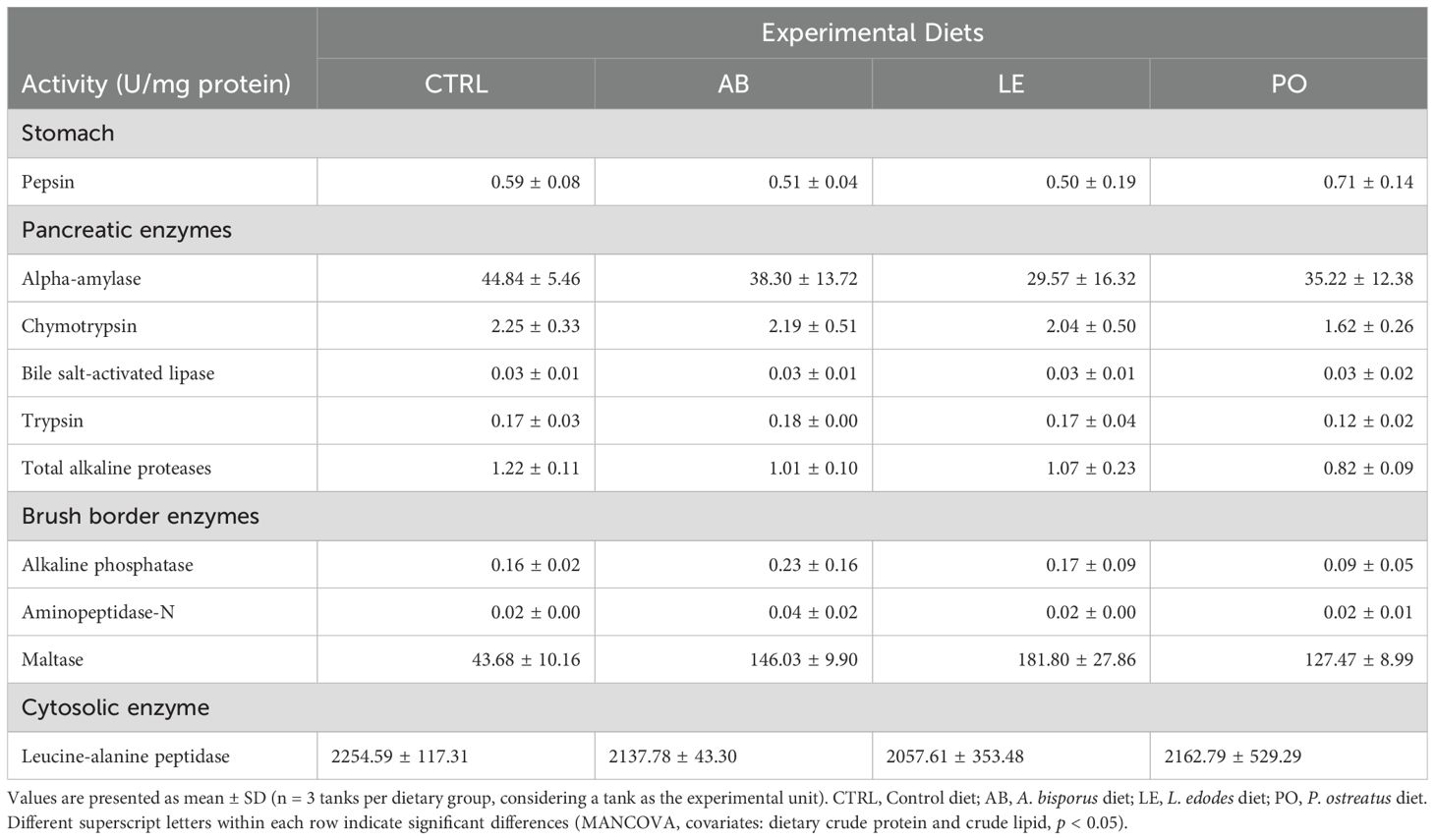
Table 7. Specific activities (U mg protein-1) of gastric (pepsin), pancreatic (α-amylase, chymotrypsin, bile salt-activated lipase, trypsin, total alkaline proteases) and intestinal (alkaline phosphatase, aminopeptidase-N, maltase and leucine-alanine peptidase) digestive enzymes in rainbow trout (O. mykiss) fed with the experimental diets containing different sources of fungal meals for 42 days.
3.2.5 Blood biochemical and hematological parameters
Fish fed mushroom-based diets presented similar blood biochemical and hematological parameters than fed the CTRL diet (Table 8; p > 0.05). The correlation analysis showed a positive correlation between diet crude protein ADC and blood cholesterol levels (Figure 3E, r = 0.55, p = 0.06). Conversely, a negative correlation was detected between diet crude protein ADC and blood lactate dehydrogenase activity (r = -0.55, p = 0.07).
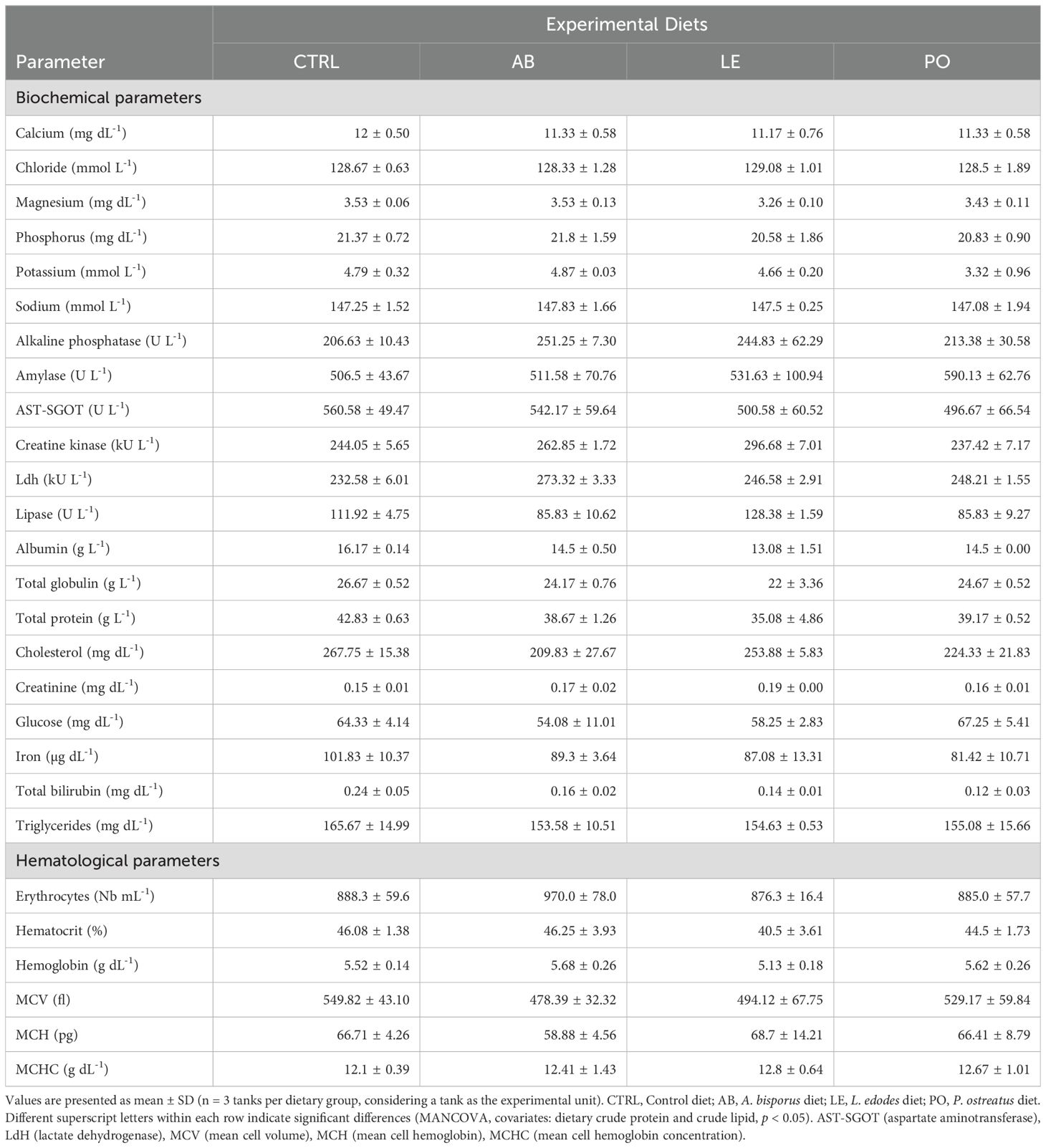
Table 8. Blood biochemical and hematological parameters of rainbow trout (O. mykiss) fed with the experimental diets containing different sources of mushroom meals for 42 days.
3.2.6 Liver morphology
At the end of the feeding trial, semiquantitative histological evaluation of H&E and PAS-stained liver sections revealed a well-organized morphological pattern, and a regular-shaped hepatocyte morphology, and nuclei alignment around sinusoidal spaces in fish fed different experimental diets (Figure 4). However, the incidence patterns for hepatocyte cytoplasmic vacuolization and the presence of PAS-positive granules differed among dietary treatments. Fish fed the LE diet presented a higher incidence (p < 0.05) of cytoplasmic vacuolization due to lipid accumulation compared to fish fed the rest of the dietary treatments (Figures 4, 5C). Similarly, the prevalence of PAS-positive granules (glycogen) within the enterocyte cytoplasm was significantly higher (p < 0.05) in fish fed the LE diet (Figures 4, 5F), suggesting that both lipid and glycogen accumulation contributed to the elevated vacuolization levels observed. Hyperemia was detected indistinctly in fish from all experimental treatments, although the incidence level showed a tendency toward reduction in fish fed the PO diet (Figure 5, p > 0.05).
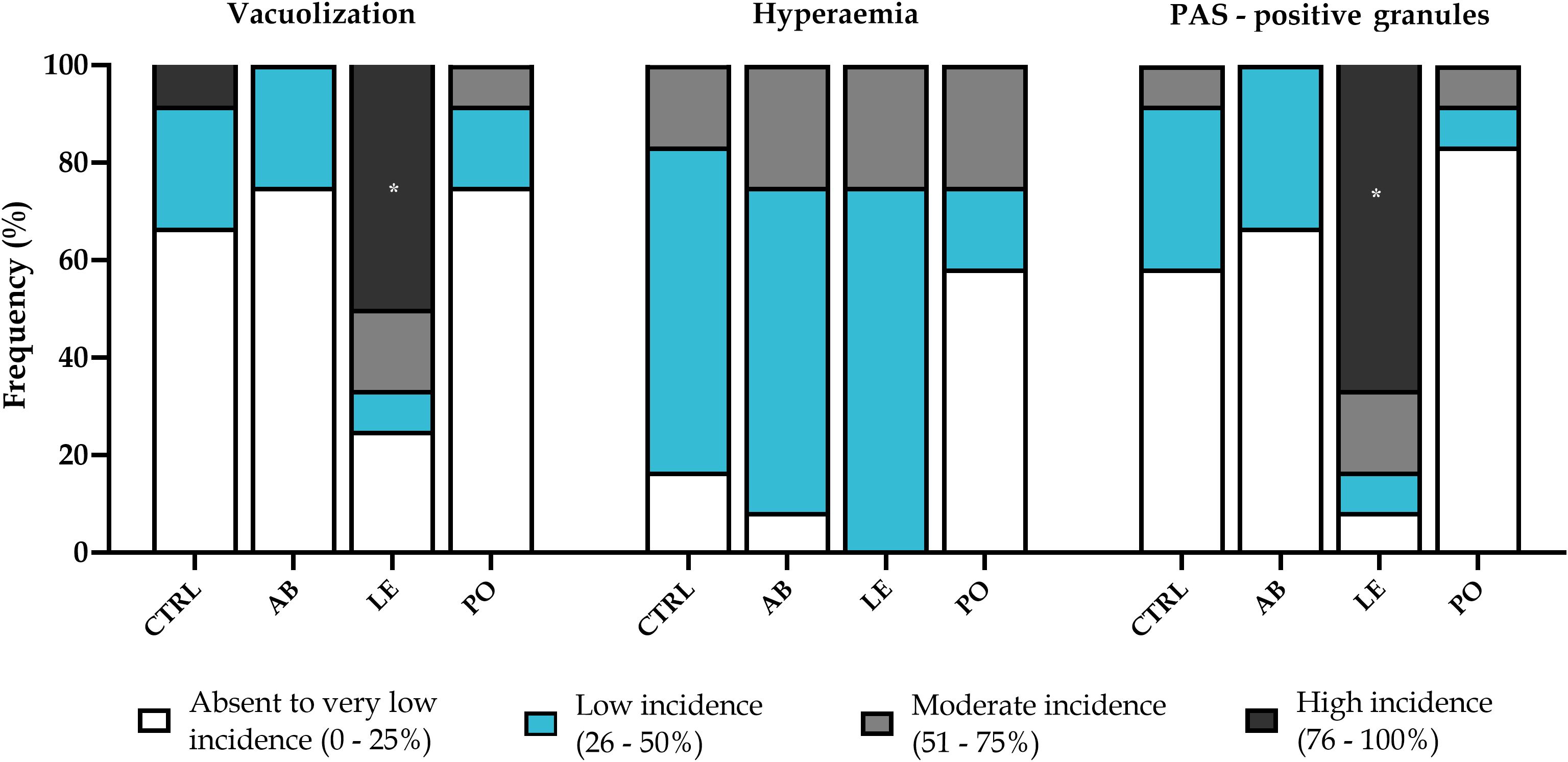
Figure 4. Results (%) of liver scoring from rainbow trout (Oncorhynchus mykiss) fed with the experimental diets containing different sources of mushroom meals for 42 days. Kruskal-Wallis, Mann-Witney U tests, p < 0.05. CTRL, Control diet; AB, A. bisporus diet; LE, L. edodes diet; PO, P. ostreatus diet. The asterisk denotes differences between the LE group and the rest of experimental diets (One-way ANOVA, p < 0.05).
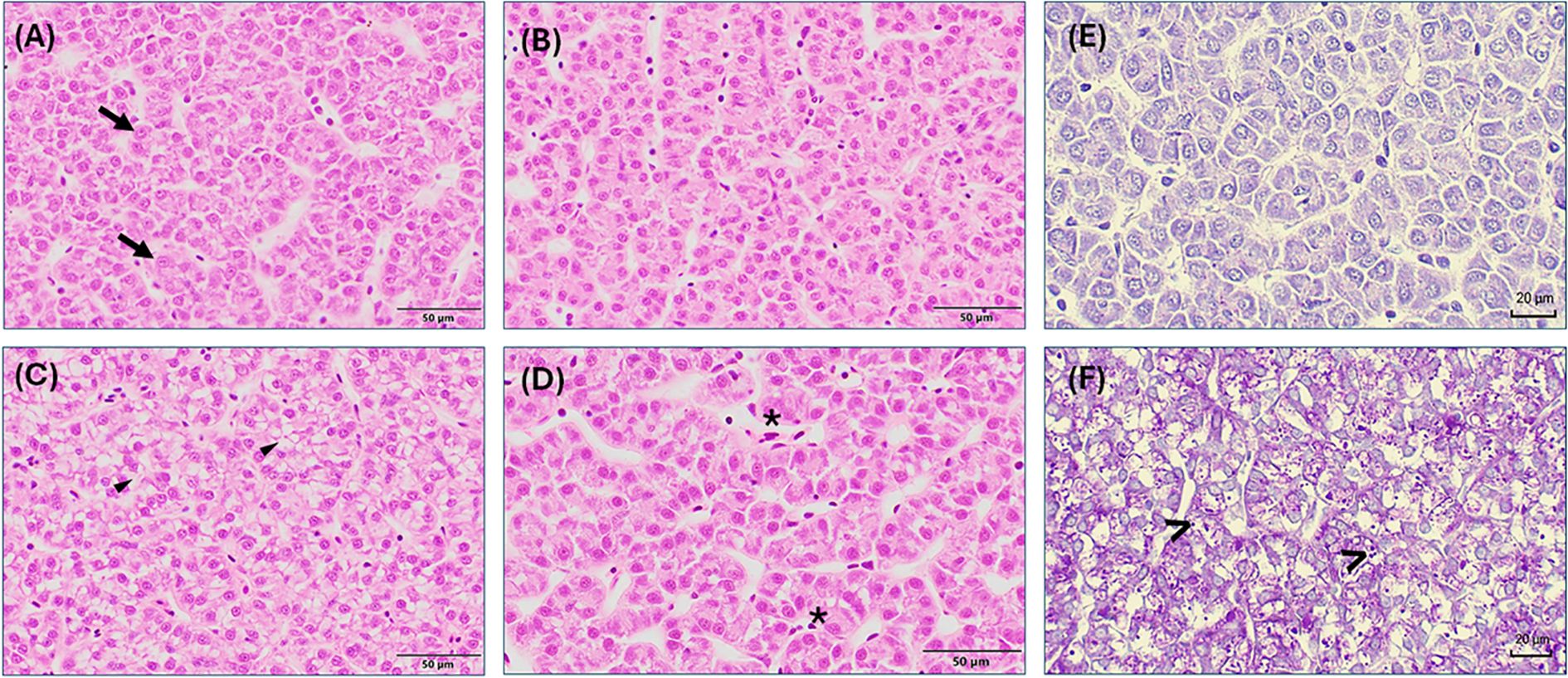
Figure 5. Liver morphology of rainbow trout (Oncorhynchus mykiss) fed with the experimental diets containing different sources of mushroom meals for 42 days. A-D: Hematoxylin and Eosin (H&E) stain, (A) CTRL, (B) AB, (C) LE, (D) PO. (─▶) hepatocytes nucleus, (*) erythrocytes in blood vessels. Observe the vacuolization in the hepatocytes cytoplasm vacuolization ▶) of fish fed with LE diet. Scale bar 50 μm. (E, F) Periodic acid–Schiff (PAS) stain, (E) representative image (CTRL, AB and PO), (F) LE. Observe the high incidence of PAS-positive granules (magenta to bright pink granules) (>) of fish fed with LE diet. Scale bar 20 μm. CTRL, Control diet; AB, A. bisporus diet; LE, L. edodes diet; PO, P. ostreatus diet.
4 Discussion
The evaluation of digestibility and the analysis of the effects on KPIs are important considerations when assessing the suitability and dosing optimal levels of inclusion of alternative/novel ingredients in feed formulations (Glencross et al., 2007). In this context, a key challenge for the global feed industry is to identify a rapid and reliable method to accurately predict the nutritional value of raw ingredients, by-products, and processed feeds for specific species, thereby reducing the reliance on labor-intensive and costly in vivo nutritional trials (Moyano et al., 2015; Wang et al., 2021). As digesta moves through the digestive tract, changing from chyme in the stomach to feces in the posterior intestine, in vitro digestibility models, such as the one used in the present study, that simulate the different digestive phases of this process, may provide a good correlation with in vivo digestive dynamics (Moyano et al., 2015).
In the present study, the in vitro assays demonstrated that the protein fraction of all tested mushroom meals was readily hydrolyzed by rainbow trout digestive enzymes, and to a greater extent than the protein present in fishmeal. Furthermore, significant differences were observed among the mushroom meals themselves. Notably, the protein fraction of the PO meal showed to be highly hydrolysable, considering its relatively low concentration of crude protein. The bioavailability of the protein fraction of mushroom meals was likely influenced by their crude fiber content, which is primarily composed of complex polysaccharides and chitin. While fungi are recognized for their excellent nutritional profiles, their composition varies both among and within species (Kalač, 2013). For example, variations in nutrient composition among Ganoderma species (G. lingzhi and G. lucidum) have been shown to affect protein digestibility (Fraile-Fabero et al., 2021). Similarly, Colosimo et al. (2021) reported that the digestibility of fungi can be somewhat limited by their chitin-rich cell walls. Consequently, these compositional differences may affect protease accessibility to substrates, influencing the rate of peptide bond cleavage, and subsequent amino acid release (Peña et al., 2017; Ayimbila and Keawsompong, 2023; Prakash et al., 2023).
Regarding the estimated ADC values of the protein fraction of the mushroom meals, a significantly higher value was estimated for the PO diet (90.63 ± 6.11%) than for the AB diet (78.55 ± 1.34%), whereas the LE diet showed an intermediate value (86.96 ± 0.12%). These results differ from those obtained with the in vitro assay. These discrepancies may be explained by considering that the two assays measure different parameters and are influenced by distinct factors (Moyano et al., 2015). One key factor that likely affected the digestibility differences between the in vivo and in vitro conditions is the food matrix complexity. In in vivo studies, mushroom meals were incorporated into a complex feed matrix with other components, whereas in vitro experiments tested the ingredients in their pure form. The interactions between ingredients, further enhanced during pellet manufacturing, may alter how fish digestive proteases access the protein fraction in mixed feeds compared to when meals are evaluated individually (Booth et al., 2013; Xing et al., 2023). In addition, the net efficiency of the digestion process evaluated in vivo may be also affected by other factors, like the protein solubility and buffering capacity of the digesta, which do not respond in the same manner in the in vitro tests. For example, the addition of vegetable protein sources into a diet has been shown to significantly impact the solubility and buffering capacity of other feed ingredients (Márquez et al., 2013; Xing et al., 2024). Besides the aforementioned, it is also important to consider other aspects that may limit the possible correlation between both types of assays performed in vitro and in vivo. In this sense, the in vitro experiments estimate the potential amino acids available for intestinal absorption after enzymatic protein hydrolysis (i.e., mushroom meal in the current study). In contrast, the in vivo digestibility measurements express results as a percentage of ingested amount of feed, considering not only the intestinal absorption of nutrients, but also modifications from fish metabolism and intestinal microbiota interactions (Fuller and Tomé, 2005).
The ADC of the whole protein fraction of the experimental diets (not specifically of the mushroom meals) revealed a great similarity among them, with the only exception of the significantly lower value measured in diet including AB in spite this meal showed comparable in vitro digestibility to that of FM. Similar results have been reported in other ingredients with high levels of ash like poultry by-product meal (PBM), as it was demonstrated in yellowtail snapper (Ocyurus chrysurus) when comparing in vitro versus in vivo digestibility methods for PBM (Barreto et al., 2024). The former authors attributed the reduced digestibility of PBM in vivo to its elevated ash content despite showing a high level of protein hydrolysis in vitro. In the present study, also a negative correlation was observed between ash content in mushroom meals and diet crude protein ADC values. These results indicated that the high ash levels of mushroom meals may be responsible for the lower protein digestibility under practical feeding conditions. Nonetheless, the in vivo ADC values of the three mushroom meals (diet protein ADCs: > 90%, ingredient protein ADCs > 77%) were found comparable to FM- and wheat flour-based dry ingredients (Sørensen, 2012), lupin and soybean protein products (Glencross et al., 2024b), grain distillers dried yeast (Hauptman et al., 2014) and even higher than those of other alternative protein sources such as brewery by-products (Cheng et al., 2004; Nazzaro et al., 2021). Those results may be explained not only by the good quality of the protein fractions but also by a sort of digestive compensation promoted by the host and the intestinal microbiota. Gut bacterial communities are known to produce carbohydrate-active enzymes, which play a role in the metabolism and utilization of dietary fibers (Holscher, 2017). Although, as previously mentioned, fiber was hypothesized as one of the factors that might affect the action of digestive proteases in vitro, this was not the case for the in vivo assays and ADC of the PO and LE diets that were comparable to that of CTRL diet. This suggests that gut microbiota may have been involved in the utilization of dietary fiber, thereby potentially contributing to the observed improvement in the in vivo digestibility of the mushroom meals. In this sense, studies evaluating the effect of tested diets on gut microbiota are currently on-going to decipher the role of mushroom meals as prebiotics and their capacity to modulate host’s microbiome.
As already pointed out in the introduction, regardless of the fact that studies focused on the assessment of the in vivo digestibility of diets do not generally extend their analyses to KPIs associated with growth performance, feed efficiency and fish condition, authors decided to evaluate such parameters as proxies of the nutritional effects of high levels of dietary mushroom meals in aquafeeds, prior to conducting nutritional dose-response studies to determine their optimal levels of inclusion. In this sense, regarding the in vivo effects of the inclusion of mushroom meals in the diets of rainbow trout, the inclusion of 30% of A. bisporus, P. ostreatus and L. edodes meals did not compromise rainbow trout performance. Indeed, the slightly lower performance observed in fish fed diets containing mushroom meals was mainly attributed to their lower dietary content of crude proteins and lipids rather than to the quality of the tested mushroom meals as confirmed by the MANCOVA analyses. The high fiber content (31-48%) of mushroom meals, coupled with their high inclusion levels, made it impossible to formulate isoproteic and isolipidic diets. This fact also affected the PER and LER values of fish fed the AB, PO, and LE diets, which were slightly lower but not significantly different from those fed the control diet. A similar approach may be applied when dealing with feed efficiency parameters. Thus, although the MANCOVA did not detect differences in FCR and FI among experimental diets, FI values in fish fed mushroom meal-based diets tended to be higher than those observed in fish fed the control diet, which may be related to the fact that fish regulated their feed intake to meet their energy requirements for growth and physiological maintenance (Kaushik and Médale, 1994).
Nutritional trials with mushroom meals in rainbow trout have been limited to their use as a feed supplement, with inclusion ranging up to 2%, rather than as an alternative ingredient. These studies reported no effects or minor improvements in KPIs related to growth performance and feed utilization (Baba et al., 2015; Manayi et al., 2016; Uluköy et al., 2016; Baba and Uluköy, 2022). In other fish species, the optimal inclusion levels of fermented P. ostreatus by-products were reported to be up to 6.3% in compound feeds for Amur catfish (Silurus asotus), and 2.15 to 2.75% for A. bisporus and 6.7% for P. pulmonarius in the diets for Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus), respectively (Adejonwo et al., 2020; Dawood et al., 2020). Although the current findings are not directly comparable to those of the aforementioned studies since a higher inclusion level of mushroom meal was used to obtain reliable results from the in vivo digestibility trial, which are not generally applicable for practical diets (Glencross et al., 2023), it is interesting to note that rainbow trout growth performance and feed utilization remained unaltered when fish were fed with high dietary inclusion levels of mushroom meals. Similarly, the present study found no significant changes in whole body proximate composition among fish fed the control and mushroom based diets, which is in agreement with the findings observed in red tilapia (Oreochromis sp.) fed with up to 1.5% of P. sajorcaju (Suplementasi et al., 2012). These findings suggest that mushroom meals, even at high inclusion levels, do not negatively impact growth performance and feed utilization, and they are able to maintain the nutritional value of fish, which reinforce the potential of these meals as novel aquafeed ingredients. Under current experimental conditions, these comparable effects may be explained by the high in vivo digestibility of the three meals (diet crude protein ADC: >90%; ingredient crude protein ADC: >77%), even though the ADC values of the AB diet were significantly lower compared to the rest of the treatments due to its higher ash content. This was further confirmed by the correlation analyses wherein there was a strong relationship between diet ADCs and fish growth performance. The increased protein ADC of the experimental diets corresponded to reduced feed intake and improved protein utilization and growth performance. This is consistent with the findings of other studies where the ADCs of protein, lipid and energy positively correlated with fish growth and feed efficiency (Mundheim et al., 2004; Rasmussen and Jokumsen, 2009).
Regarding digestive enzyme activities, the activity of gastric, pancreatic or intestinal digestive enzymes was not affected by the dietary inclusion of mushroom meals in compound diets for rainbow trout. These results are of relevance since unaltered digestive enzyme activities suggest that the rate of digestion and nutrient availability in the circulatory system is maintained, supporting the growth of the entire organism (Gisbert et al., 2018). However, in absolute terms, and particularly for enzymes involved in carbohydrate metabolism, the activity of the enzyme maltase was increased by 3 to 4.2 times in fish fed diets rich in mushroom meals compared to fish fed the basal diet, which may indicate an effect of dietary carbohydrate content and composition on sugar metabolism, which in turn may be influencing their reserve storage. In this context, there is scarce information on the effect of mushroom meals on the digestive processes in fish. Safari and Sarkheil (2018) showed higher trypsin, α-amylase and lipase activities in Koi carp fingerlings (Cyprinus carpio) fed diets supplemented with P. eryngii (1 to 2%). Although these studies demonstrated an improvement in digestive enzyme activities with the dietary inclusion of mushroom meals, the present study differs as mushroom meals were included at a high dietary level. Despite this, our results showed that the high inclusion of mushroom meals did not affect protein and lipid related digestive enzyme activities, which is of special relevance as it indicates that the digestive system capacity of the fish, subsequently nutrients available for growth was not compromised.
In addition, no significant differences in somatic indices were found among different experimental groups, neither on the liver crude protein and lipid levels. However, a higher level of carbohydrate was found in the liver of fish fed the LE diet compared to those fed the control diet, whereas fish fed the AB and PO diets showed intermediate levels. This result was further supported by the liver morphological patterns observed where fish fed the LE diet presented a higher glycogen deposition within hepatocytes. This is of special relevance since the liver is a key metabolic organ responsible for a wide array of functions, including carbohydrate, protein and fat metabolism (Bruslé, 1996). Indeed, the dietary carbohydrate content of the mushroom meals maybe inducing slight glycogen and hepatic lipid deposition due to an excess of glucose storing either as glycogen through glucogenesis, or as lipids via lipogenesis (Li et al., 2022). Carnivorous fish species rely on gluconeogenesis to produce glucose from other precursors than carbohydrates to meet their energy demands. This process is upregulated in response to a high carbohydrate dietary content combined with low immediate energy demand, which in turn will facilitate glycogen deposition (Panserat et al., 2019). In fact, as mentioned before, the observed maltase activity values and higher liver carbohydrate content point in the same line, since in absolute terms, fish fed the alternative diets showed a 3 to 4.2-fold increase in maltase activity compared to that observed in fish fed the control diet. In addition, fish fed the LE diet presented a larger hepatocyte cytoplasm vacuolization than fish fed the AB and PO diets, which showed an intermediate level in relation to animals fed the control diet. In this particular case, it appears that the accumulation of lipids and glycogen in the liver was not exclusively related to the carbohydrate content and nature of raw materials supplied but also might be influenced by other bioactive compounds associated with a particular species of mushroom. In line with the morphological patterns observed, in other animal models, even with a greater capacity for glucose regulation via the insulin-glucagon axis than fish, prolonged periods of dietary supplementation with LE increased hepatic steatosis in mice, whereas this pattern was not observed in mice fed a diet containing an AB meal (Chandra et al., 2011). These results were attributed to the higher content of eritadenine in the LE meal, since this bioactive compound has been shown to have comparable activity with insulin, modulating metabolic pathways involved in glucose metabolism and uptake, glucose transporter-4 translocation and activation of several nutrient signaling pathways (Kaur et al., 2024). This might be also related to the increased glycogen deposition inferred in the liver of fish fed with the LE diet by increasing carbohydrate utilization or the metabolic fate of protein and lipids towards glycogenesis (Soares et al., 2013; Li et al., 2022). On the contrary, Xu et al. (2023) reported an improvement in the hepatic condition of largemouth bass (Micropterus salmoides) fed a diet containing fermented LE meal at 5%, even though these results are not directly comparable to the current study due to the different levels of inclusion and the nature of the tested LE meal. This fact highlights the importance of the use of pre-processing or pre-treatments to facilitate the use of certain novel or alternative raw materials with nutritional potential and an adequate production volume to be introduced into the basket of raw materials available for aquafeed production, as occurred with some plant protein sources (Drew et al., 2007; Siddik et al., 2024).
On the other hand, no significant alterations were observed in blood biochemical and hematological parameters among the experimental groups, with values within the normal range for this fish species (Manera and Britti, 2006; Kopp et al., 2011; Coşkun et al., 2016). These results indicated that a high inclusion of mushroom meals up to 30% in the diets for rainbow trout did not compromise the nutritional and metabolic condition of fish as indicated by plasmatic values of glucose, protein and triglycerides. In addition, plasmatic electrolytes were stable among dietary groups with no effects on plasma homeostasis (Mozanzadeh et al., 2016; Parma et al., 2020). In this sense, levels of plasma electrolytes have also been used as indicators of the secondary stress response in fish (Guardiola et al., 2018; Hrubec et al., 2000), and under the current experimental conditions, the similar values found among groups might support their similar physiological conditions regardless of the tested diet. Furthermore, the lack of differences in the levels of non-specific plasmatic enzymes, which are generally considered useful health biomarkers since their elevated levels may indicate tissue damage or metabolic disorders (Peres et al., 2013; Klein et al., 2020), suggested that the high levels of dietary inclusion of mushroom meals were safe for rainbow trout.
5 Conclusions
Results from the present study indicated that for total AAs released in vitro, the AB meal shows similar values to FM. However, when expressing the total AAs released relative to mushroom meal content in % CP, the PO meal presents the higher values, since it contains only 10.8% CP versus 21.1% and 31.5% of AB and LE meals, respectively. Regarding their in vivo digestibility, the overall ADCs of the three meals were high, with the PO and LE diets showing comparable ADC values to the CTRL diet, whereas the AB diet displayed the lowest digestibility values. In terms of the effect of the three mushroom meals on KPIs, the dietary inclusion of mushroom meals (30% of the diet) did not compromise rainbow trout growth performance, feed utilization, somatic indices, blood parameters, plasmatic biomarkers, digestive enzyme activities or the whole-body proximate composition. However, the inclusion of the LE meal in the diet increased the hepatic glycogen and lipid deposition, which might be attributed to the changes in glucose metabolism in the liver. In conclusion, mushroom by-products represent potential ingredients for rainbow trout diets, that may contribute significantly to the current strategy for waste reduction within the mushroom sector and promote a sustainable and circular industry.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by IRTA ethics committee and Generalitat de Catalunya. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CS: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. ST: Investigation, Writing – review & editing. FM: Methodology, Writing – review & editing. IS: Methodology, Writing – review & editing. SO: Investigation, Methodology, Writing – review & editing. MT: Funding acquisition, Investigation, Methodology, Resources, Writing – review & editing. NT: Funding acquisition, Methodology, Writing – review & editing. JT: Methodology, Writing – review & editing. YC-Q: Investigation, Methodology, Writing – review & editing. EG: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was conducted within the GreenBlueCircle project (TED2021-132054B-C21/C22) funded by the Ministerio de Ciencia e Innovación (Spain). Carl John Saromines was supported by a predoctoral grant from IRTA. Silvia Torrecillas is financed by a Ramón y Cajal fellowship (RYC2021-031414-I) funded by MICIU/AEI/10.13039/501100011033 and, as appropriate, by “ESF Investing in your future”, by “ESF+” or by “European Union NextGenerationEU/PRTR.
Acknowledgments
Authors would like to express their gratitude to the project team and staff and visiting researchers of IRTA, assistance provided during the conduct of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adejonwo O. A., Omitoyin B. O., Ajani E. K., Ogunwole O. A., and Omitoyin S. A. (2020). Growth, gut morphology and microflora of African catfish Clarias gariepinus fed mushroom (Pleurotus pulmonarius) stalk meal supplemented diets. Ribarstvo Croat. J. Fish. 78, 79–90. doi: 10.2478/cjf-2020-0008
Ahmed M., Abdullah N., Yusof H. M., Shuib A. S., and Razak S. A. (2017). Improvement of growth and antioxidant status in Nile tilapia, Oreochromis niloticus, fed diets supplemented with mushroom stalk waste hot water extract. Aquac. Res. 48, 1146–1157. doi: 10.1111/are.12956
Anson M. L. (1938). The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol. 22, 79–89. doi: 10.1085/jgp.22.1.79
Antunes F., Marçal S., Taofiq O., Morais A. M. M. B., Freitas A. C., Ferreira I. C. F. R., et al. (2020). Valorization of mushroom by-products as a source of value-added compounds and potential applications. Molecules 25, 2818. doi: 10.3390/molecules25112672
AOAC (2006). Official Methods of Analysis of the Association of Official Analytical Chemists, Eighteenth ed (Gaithersburg, MD, USA: Association of Official Analytical Chemists).
Ayadi F. Y., Rosentrate K. A., and Muthukumar K. (2012). Alternative protein sources for aquaculture feeds. J. Aquac. Feed Sci. Nutr. 4, 1–26. doi: 10.3923/joafsnu.2012.1.26
Ayimbila F. and Keawsompong S. (2023). Nutritional quality and biological application of mushroom protein as a novel protein alternative. Curr. Nutr. Rep. 12, 290–307. doi: 10.1007/s13668-023-00468-x
Baba E. and Uluköy G. (2022). Effects of dietary supplemented shiitake mushroom extract on growth, non-specific immune parameters and in-vitro resistance against Aeromonas hydrophila in rainbow trout (Oncorhynchus mykiss). J. Limnol. Freshw. Fish. Res. 8, 28–36. doi: 10.17216/limnofish.898170
Baba E., Uluköy G., and Öntaş C. (2015). Effects of feed supplemented with Lentinula edodes mushroom extract on the immune response of rainbow trout, Oncorhynchus mykiss, and disease resistance against Lactococcus garvieae. Aquaculture 448, 476–482. doi: 10.1016/j.aquaculture.2015.04.031
Barreto A., Arenas M., Álvarez‐González A., and Suárez‐Bautista J. (2024). Evaluation of in vitro and in vivo digestibility of potential feed ingredients for juvenile Yellowtail Snapper. North Am. J. of Aquac. 86, 179–192. doi: 10.1002/naaq.10316
Booth M. A., Allan G. L., and Smullen R. P. (2013). Digestibility of common feed ingredients by juvenile mulloway Argyrosomus japonicus. Aquaculture 414–415, 140–148. doi: 10.1016/j.aquaculture.2013.07.045
Boyd C. E., D’Abramo L. R., Glencross B. D., Huyben D. C., Juarez L. M., Lockwood G. S., et al. (2020). Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc 51, 578–633. doi: 10.1111/jwas.12714
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Bruslé J. (1996). “The structure and function of fish liver,” in Fish Morphology: Horizon of New Research. Eds. Dutta H. M. and Munsi J. S. D. (CRC Press, Balkelma, Rotterdam, Netherlands), pp.7–pp93.
Bureau D. P. and Hua K. (2006). Letter to the editor of aquaculture. Aquaculture 252, 103–105. doi: 10.1016/j.aquaculture.2006.01.028
Chandra L. C., Smith B. J., Clarke S. L., Marlow D., D’Offay J. M., and Kuvibidila S. R. (2011). Differential effects of shiitake- and white button mushroom-supplemented diets on hepatic steatosis in C57BL/6 mice. Food Chem. Toxicol. 49, 3074–3080. doi: 10.1016/j.fct.2011.09.001
Cheng Z. J., Hardy R. W., and Huige N. J. (2004). Apparent digestibility coefficients of nutrients in brewer’s and rendered animal by-products for rainbow trout (Oncorhynchus mykiss (Walbaum)). Aquac. Res. 35, 1–9. doi: 10.1111/j.1365-2109.2004.00941.x
Cho E. Y. and Slinger S. L. (1979). “Apparent digestibility measurement in foodstuff for rainbow trout,” in World Symposium on Fin Fish Nutrition and Fish Feed Technology, vol. 2. (Hamburg, Germany: Heenemann GmbH), 239–247.
Chong V., Al-Azad S., and Shapawi R. (2016). Comparison of two edible mushroom extract as aquaculture feed additive to enhance immune response of Asian seabass. Trans. Sci. Technol. 3, 427–432.
Church F. C., Swaisgood H. E., Porter D. H., and Catignani G. L. (1983). Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 66, 1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2
Colosimo R., Warren F. J., Edwards C. H., Ryden P., Dyer P. S., Finnigan T. J., et al. (2021). Comparison of the behavior of fungal and plant cell wall during gastrointestinal digestion and resulting health effects: A review. Trends. Food Sci. Technol. 110, 132–141. doi: 10.1016/j.tifs.2021.02.001
Coşkun O. F., Aydın D., and Duman F. (2016). Comparison of some blood parameters of rainbow trout (Oncorhynchus mykiss) living in running and still water. Iran. J. Fish. 15, 497–507.
Dawood M. A. O., Eweedah N. M., El-Sharawy M. E., Awad S. S., Van Doan H., and Paray B. A. (2020). Dietary white button mushroom improved the growth, immunity, antioxidative status and resistance against heat stress in Nile tilapia (Oreochromis niloticus). Aquaculture 523, 735229. doi: 10.1016/j.aquaculture.2020.735229
Drew M. D., Borgeson T. L., and Thiessen D. L. (2007). A review of processing of feed ingredients to enhance diet digestibility in finfish. Anim. Feed Sci. Technol. 138, 118–136. doi: 10.1016/j.anifeedsci.2007.06.019
Dubois M., Gilles K. A., Hamilton J. K., Rebers P. T., and Smith F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
FAO (2024). The State of World Fisheries and Aquaculture 2024 – Blue Transformation in action (Rome). doi: 10.4060/cd0683en
Fraile-Fabero R., Ozcariz-Fermoselle M. V., Oria-De-rueda-salgueiro J. A., Garcia-Recio V., Cordoba-Diaz D., Jiménez-López M. D. P., et al. (2021). Differences in antioxidants, polyphenols, protein digestibility and nutritional profile between Ganoderma lingzhi from industrial crops in Asia and Ganoderma lucidum from cultivation and Iberian origin. Foods 10, 1750. doi: 10.3390/foods10081750
Fuller M. F. and Tomé D. (2005). In vivo determination of amino acid bioavailability in humans and model animals. J. AOAC Int. 88, 923–934. doi: 10.1093/jaoac/88.3.923
García-Carreño F. L. and Haard N. F. (1993). Characterization of proteinase classes in langostilla (Pleuroncodes planipes) and crayfish (Pacifastacus astacus) extracts. J. Food Biochem. 17, 97–113. doi: 10.1111/j.1745-4514.1993.tb00864.x
Gisbert E., Giménez G., Fernández I., Kotzamanis Y., and Estévez A. (2009). Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture 287, 381–387. doi: 10.1016/j.aquaculture.2008.10.039
Gisbert E., Nolasco H., and Solovyev M. (2018). Towards the standardization of brush border purification and intestinal alkaline phosphatase quantification in fish with notes on other digestive enzymes. Aquaculture 487, 102–108. doi: 10.1016/j.aquaculture.2018.01.004
Glencross B. D., Booth M., and Allan G. L. (2007). A feed is only as good as its ingredients - A review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 13, 17–34. doi: 10.1111/j.1365-2095.2007.00450.x
Glencross B., Ling X., Gatlin D., Kaushik S., Øverland M., Newton R., et al. (2024a). A SWOT analysis of the use of marine, grain, terrestrial-animal and novel protein ingredients in aquaculture feeds. Rev. Fish. Sci. Aquac. 32, 396–434. doi: 10.1080/23308249.2024.2315049
Glencross B. D., Carter C. G., Duijster N., Evans D. R., Dods K., McCafferty P., et al. (2024b). A comparison of the digestibility of a range of lupin and soybean protein products when fed to either Atlantic salmon (Salmo salar) or rainbow trout (Oncorhynchus mykiss). Aquaculture 237 (1–4), 333–346. doi: 10.1016/j.aquaculture.2004.03.023
Glencross B., Muñoz-Lopez P., Matthew C., MacKenzie S., Powell A., Longshaw M., et al. (2023). Digestibility of bacterial protein by Atlantic salmon (Salmo salar) is affected by both inclusion level and acclimation time. Aquaculture 565, 739137. doi: 10.1016/j.aquaculture.2022.739137
Gómez B., Munekata P. E. S., Zhu Z., Barba F. J., Toldrá F., Putnik P., et al. (2019). Challenges and opportunities regarding the use of alternative protein sources: Aquaculture and insects. Adv. Food Nutr. Res. 89, 259–295. doi: 10.1016/bs.afnr.2019.03.003
Guardiola F. A., Saraiva-Fraga M., Cuesta A., and Esteban M. A. (2018). Changes in natural haemolytic complement activity induced by stress in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 78, 317–321. doi: 10.1016/j.fsi.2018.04.056
Hauptman B. S., Barrows F. T., Block S. S., Gaylord T. G., Paterson J. A., Rawles S. D., et al. (2014). Evaluation of grain distillers dried yeast as a fish meal substitute in practical-type diets of juvenile rainbow trout, Oncorhynchus mykiss. Aquaculture 432, 7–14. doi: 10.1016/j.aquaculture.2014.03.026
Holm H., Hanssen L. E., Krogdahl A., and Florholmen J. (1988). High and low inhibitor soybean meals affect human duodenal proteinase activity differently: In vivo comparison with bovine serum albumin. J. Nutr. 118, 515–520. doi: 10.1093/jn/118.4.515
Holscher H. D. (2017). Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8, 172–184. doi: 10.1080/19490976.2017.1290756
Hrubec T. C., Cardinale J. L., and Smith S. A. (2000). Hematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis hybrid). Vet. Clin. Pathol. 29, 7–12. doi: 10.1111/j.1939-165X.2000.tb00389.x
Iijima N., Tanaka S., and Ota Y. (1998). Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiol. Biochem. 18, 59–69. doi: 10.1023/A:1007725513389
Infochampi, European Mushroom Growers Group (2022). Information retrieved on 10th December 2024. Available online at: https://www.infochampi.eu/.
Kalač P. (2013). A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 93, 209–218. doi: 10.1002/jsfa.5960
Katya K., Yun Y. H., Park G., Lee J. Y., Yoo G., and Bai S. C. (2014). Evaluation of the efficacy of fermented by-product of mushroom, Pleurotus ostreatus, as a fish meal replacer in juvenile amur catfish, Silurus asotus: Effects on growth, serological characteristics and immune responses. Asian-Australas. J. Anim. Sci. 27, 1478–1486. doi: 10.5713/ajas.2014.14038
Kaur J., Azad F., Khan A. M. A., Farzaan M., Ahmad J., Farooqi H., et al. (2024). In-vitro modulation of glucose and lipid metabolism by Lentinula edodes extracts in obesity and type 2 diabetes models. Pharmacol. Res. - Mod. Chin. Med. 13, 100540. doi: 10.1016/j.prmcm.2024.100540
Kaushik S. J. and Médale F. (1994). Energy requirements, utilization and dietary supply to salmonids. Aquaculture 124, 81–97. doi: 10.1016/0044-8486(94)90364-6
Klein R., Nagy O., Tóthová C., and Chovanová F. (2020). Clinical and diagnostic significance of lactate dehydrogenase and its isoenzymes in animals. Vet. Med. Int. 2020, 5346483. doi: 10.1155/2020/5346483
Kopp R., Mareš J., Lang Š., Brabec T., and Ziková A. (2011). Assessment of ranges plasma indices in rainbow trout (Oncorhynchus mykiss) reared under conditions of intensive aquaculture. Acta Univ. Agric. Silvic. Mendelianae Brun 59, 181–188. doi: 10.11118/actaun201159060181
Kunitz M. (1947). Crystalline soybean trypsin inhibitor II. Gen. Prop. J. Gen. Physiol. 30, 291–310. doi: 10.1085/jgp.30.4.291
Li X., Han T., Zheng S., and Wu G. (2022). “Hepatic glucose metabolism and its disorders in fish,” in Recent Advances in Animal Nutrition and Metabolism. Advances in Experimental Medicine and Biology, vol. 1354 . Ed. Wu G. (Springer, Cham), 203–217. doi: 10.1007/978-3-030-85686-1_11
López-Pedrouso M., Lorenzo J. M., Cantalapiedra J., Zapata C., Franco J. M., and Franco D. (2020). Aquaculture and by-products: Challenges and opportunities in the use of alternative protein sources and bioactive compounds. Adv. Food Nutr. Res. 92, 127–185. doi: 10.1016/bs.afnr.2019.11.001
Manayi A., Vazirian M., Zade F. H., and Tehranifard A. (2016). Immunomodulation effect of aqueous extract of the artist’s conk medicinal mushroom, Ganoderma applanatum (Agaricomycetes), on the rainbow trout (Oncorhynchus mykiss). J. Med. Mushrooms 18, 927–933. doi: 10.1615/IntJMedMushrooms.v18.i10.80
Manera M. and Britti D. (2006). Assessment of blood chemistry normal ranges in rainbow trout. J. Fish Biol. 69, 1427–1434. doi: 10.1111/j.1095-8649.2006.01205.x
Maroux S., Louvard D., and Baratti J. (1973). The aminopeptidase from hog-intestinal brush border. Biochim. Biophys. Acta 321, 282–295. doi: 10.1016/0005-2744(73)90083-1
Márquez L., Øverland M., Martínez-Llorens S., Morken T., and Moyano F. J. (2013). Use of a gastrointestinal model to assess potential amino acid bioavailability in diets for rainbow trout (Oncorrhynchus mykiss). Aquaculture 384–387, 46–55. doi: 10.1016/j.aquaculture.2012.12.008
Métais P. and Bieth J. (1968). Détermination de l’amylase par une microtechnique. Ann. Biol. Clin. 26, 133–142.
Mitra A. (2021). Thought of alternate aquafeed: conundrum in aquaculture sustainability? Proc. Zool. Soc 74, 1–18. doi: 10.1007/s12595-020-00352-4
Morales G. A. and Moyano F. J. (2010). Application of an in vitro gastrointestinal model to evaluate nitrogen and phosphorus bioaccessibility and bioavailability in fish feed ingredients. Aquaculture 306, 244–251. doi: 10.1016/j.aquaculture.2010.05.014
Moyano F. J. and Gilannejad N. (2025). “Applications of in vitro digestive simulations,” in Feed and Feeding for Fish and Shellfish. Ed. Kumar V. (London: Academic Press), 249–278. doi: 10.1016/B978-0-443-21556-8.00007-7
Moyano F. J., Saénz de Rodrigáñez M. A., Díaz M., and Tacon A. G. J. (2015). Application of in vitro digestibility methods in aquaculture: Constraints and perspectives. Rev. Aquacult. 7, 223–242. doi: 10.1111/raq.12065
Mozanzadeh M. T., Yavari V., Marammazi J. G., Agh N., Mohammadian T., Yaghoubi M., et al. (2016). Dietary docosahexaenoic acid to eicosapentaenoic acid ratios effects on hemato-immunological and plasma biochemical parameters in silvery-black porgy (Sparidentex hasta) juveniles. Comp. Clin. 25, 1107–1114. doi: 10.1007/s00580-016-2307-0
Mundheim H., Aksnes A., and Hope B. (2004). Growth, feed efficiency and digestibility in salmon (Salmo salar L.) fed different dietary proportions of vegetable protein sources in combination with two fish meal qualities. Aquaculture 237, 315–331. doi: 10.1016/j.aquaculture.2004.03.011
Navarro-Simarro P., Gómez-Gómez L., Ahrazem O., and Rubio-Moraga Á. (2024). Food and human health applications of edible mushroom by-products. New Biotechnol. 81, 43–56. doi: 10.1016/j.nbt.2024.03.003
Naylor R. L., Hardy R. W., Buschmann A. H., Bush S. R., Cao L., Klinger D. H., et al. (2021). A 20-year retrospective review of global aquaculture. Nature 591, 551–563. doi: 10.1038/s41586-021-03308-6
Nazzaro J., Martin D. S., Perez-Vendrell A. M., Padrell L., Iñarra B., Orive M., et al. (2021). Apparent digestibility coefficients of brewer’s by-products used in feeds for rainbow trout (Oncorhynchus mykiss) and gilthead seabream (Sparus aurata). Aquaculture 530, 735796. doi: 10.1016/j.aquaculture.2020.735796
Nolasco-Soria H., Nolasco-Alzaga H. R., and Gisbert E. (2020). The importance of pepsin-like acid protease quantification in aquaculture studies: a revision of available procedures and presentation of a new protocol for its assessment. Rev. Aquac. 12, 1928–1943. doi: 10.1111/raq.12417
Panserat S., Marandel L., Seiliez I., and Skiba-Cassy S. (2019). New insights on intermediary metabolism for a better understanding of nutrition in teleosts. Annu. Rev. Anim. Biosci. 7, 195–220. doi: 10.1146/annurev-animal-020518-115250
Parma L., Pelusio N. F., Gisbert E., Esteban M. A., D’Amico F., Soverini M., et al. (2020). Effects of rearing density on growth, digestive conditions, welfare indicators and gut bacterial community of gilthead sea bream (Sparus aurata, L. 1758) fed different fishmeal and fish oil dietary levels. Aquaculture 518, 734854. doi: 10.1016/j.aquaculture.2019.734854
Peña E., Hernández C., Ibarra-Castro L., and Álvarez-González C. A. (2017). In vitro protein digestibility of different grow-out stages of spotted rose snapper (Lutjanus guttatus, Steindachner 1869). Aquac. Nutr. 23, 1204–1215. doi: 10.1111/anu.12489
Peres H., Santos S., and Oliva-Teles A. (2013). Selected plasma biochemistry parameters in gilthead seabream (Sparus aurata) juveniles. J. Appl. Ichthyol. 29, 630–636. doi: 10.1111/j.1439-0426.2012.02049.x
Prakash S., Maas R. M., Fransen P. M. M. M., Kokou F., Schrama J. W., and Philip A. J. P. (2023). Effect of feed ingredients on nutrient digestibility, waste production and physical characteristics of rainbow trout (Oncorhynchus mykiss) faeces. Aquaculture 574, 739621. doi: 10.1016/j.aquaculture.2023.739621
Rasmussen R. S. and Jokumsen A. (2009). Digestibility in selected rainbow trout families and relation to growth and feed utilisation. Aquacult. Int. 17, 187–197. doi: 10.1007/s10499-008-9191-9
Robinson B., Winans K., Kendall A., Dlott J., and Dlott F. (2019). A life cycle assessment of Agaricus bisporus mushroom production in the USA. Int. J. Life Cycle Assess. 24, 456–467. doi: 10.1007/s11367-018-1456-6
Ruiz A., Gisbert E., Estévez A., Reyes-López F. E., Vallejos-Vidal E., Tort L., et al. (2024). Strategy combining mammalian fats with supplementation of pungent spices in aquafeeds, to mitigate negative impacts of fish oil replacement in fish performance, fillet quality and hepatic condition of gilthead seabream (Sparus aurata). Aquaculture 593, 741284. doi: 10.1016/j.aquaculture.2024.741284
Safari O. and Sarkheil M. (2018). Dietary administration of eryngii mushroom (Pleurotus eryngii) powder on haemato-immunological responses, bactericidal activity of skin mucus and growth performance of koi carp fingerlings (Cyprinus carpio koi). Fish Shellfish Immunol. 80, 505–513. doi: 10.1016/j.fsi.2018.06.046
Sandström V., Chrysafi A., Lamminen M., Troell M., Jalava M., Piipponen J., et al. (2022). Food system by-products upcycled in livestock and aquaculture feeds can increase global food supply. Nat. Food. 3, 729–740. doi: 10.1038/s43016-022-00589-6
Serra V., Pastorelli G., Tedesco D. E. A., Turin L., and Guerrini A. (2024). Alternative protein sources in aquafeed: Current scenario and future perspectives. Vet. Anim. Sci. 25, 100381. doi: 10.1016/j.vas.2024.100381
Shirur M., Barh A., and Annepu S. K. (2021). “Sustainable production of edible and medicinal mushrooms: implications on mushroom consumption,” in Climate Change and Resilient Food Systems. Eds. Hebsale Mallappa V. K. and Shirur M. (Springer, Singapore), 1–14. doi: 10.1007/978-981-33-4538-6_12
Siddik M. A., Julien B. B., Islam S. M., and Francis D. S. (2024). Fermentation in aquafeed processing: Achieving sustainability in feeds for global aquaculture production. Rev. Aquac. 16, 1244–1265. doi: 10.1111/raq.12894
Soares A. A., De Sá-Nakanishi A. B., Bracht A., Da Costa S. M. G., Koehnlein E. A., De Souza C. G. M., et al. (2013). Hepatoprotective effects of mushrooms. Molecules 18, 7609–7630. doi: 10.3390/molecules18077609
Solovyev M. and Gisbert E. (2016). Influence of time, storage temperature and freeze/thaw cycles on the activity of digestive enzymes from gilthead sea bream (Sparus aurata). Fish Physiol. Biochem. 42, 1383–1394. doi: 10.1007/s10695-016-0226-2
Sørensen M. (2012). A review of the effects of ingredient composition and processing conditions on the physical qualities of extruded high-energy fish feed as measured by prevailing methods. Aquac. Nutr. 18, 233–248. doi: 10.1111/j.1365-2095.2011.00924.x
Suplementasi K., Sebagai C., Prebiotik B., Berasaskan M., Roti Terhadap Kadar U., Ikan P., et al. (2012). Effect of mushroom supplementation as a prebiotic compound in super worm-based diet on growth performance of red tilapia fingerlings. Sains Malaysiana 41, 1197–1203.
Tefal E., Peñaranda D. S., Martínez-Llorens S., Tomás-Vidal A., Jauralde I., Lagos L., et al. (2024). Feeding of rainbow trout (Oncorhynchus mykiss) with organic ingredients replacing fish meal. Aquaculture 592, 7415257. doi: 10.1016/j.aquaculture.2024.741257
Uluköy G., Baba E., and Öntaş C. (2016). Effect of Oyster Mushroom, Pleurotus ostreatus, Extract on Hemato-Immunological Parameters of Rainbow Trout, Oncorhynchus mykiss. J. World Aquac. Soc 47, 676–684. doi: 10.1111/jwas.12318
Vandenberg G. W. and de la Noüe J. (2001). Apparent digestibility comparison in rainbow trout (Oncorhynchus mykiss) assessed using three methods of faeces collection and three digestibility markers. Aquac. Nutr. 7, 237–245. doi: 10.1046/j.1365-2095.2001.00181.x
Walter H. E. (1984). “Proteinases: Methods with hemoglobin, casein and azocoll as substrates,” in Methods of enzymatic analysis. Ed. Bergmeyern H. J. (Verlag Chemie, Weinham), 270–277.
Wang R., Mohammadi M., Mahboubi A., and Taherzadeh M. J. (2021). In-vitro digestion models: a critical review for human and fish and a protocol for in-vitro digestion in fish. Bioengineered 12, 3040–3064. doi: 10.1080/21655979.2021.1940769
Worthington Biochemical Corporation (1991). Worthington Enzyme Manual: Enzymes, Enzyme Reagents, Related Biochemical (Freehold, New Jersey: Worthington Biochemical Corp.).
Xing S., Liang X., Wang H., Xie X., Wierenga P. A., Schrama J. W., et al. (2023). The impacts of physical properties of extruded feed on the digestion kinetics, gastrointestinal emptying and stomach water fluxes of spotted seabass (Lateolabrax maculatus). Aquaculture 570, 739442. doi: 10.1016/j.aquaculture.2023.739442
Xing S., Liang X., Wierenga P. A., Wang H., Hou A., Wang J., et al. (2024). Effects of protein sources and extrusion processing conditions via pellet physical quality on the feed intake, gastrointestinal emptying and digestion of spotted seabass (Lateolabrax maculatus). Aquac. Rep. 37, 102243. doi: 10.1016/j.aqrep.2024.102243
Xu Z., Hu J., Zhang Y., and Bai L. (2023). Evaluation of largemouth bass (Micropterus Salmoide) fed selenium yeast diets: Growth, histopathology, antioxidant ability, and apoptosis. Aquac. Rep. 29, 101505. doi: 10.1016/j.aqrep.2023.101505
Keywords: circular economy, feed protein, in vitro digestibility, in vivo digestibility novel ingredient, mushroom meal, rainbow trout, sustainability
Citation: Saromines CJ, Torrecillas S, Moyano FJ, Sanahuja I, Ojeda S, Tello Martín ML, Tous N, Tarradas J, Cruz-Quintana Y and Gisbert E (2025) Evaluation of the digestibility and effects on key performance indicators of mushroom-derived alternative protein sources in aquafeeds for rainbow trout (Oncorhynchus mykiss). Front. Mar. Sci. 12:1606812. doi: 10.3389/fmars.2025.1606812
Received: 06 April 2025; Accepted: 12 May 2025;
Published: 02 June 2025.
Edited by:
Kumbukani Mzengereza, Mzuzu University, MalawiReviewed by:
Gabriel Cardenete, University of Granada, SpainYuwen Dong, University of Pennsylvania, United States
Copyright © 2025 Saromines, Torrecillas, Moyano, Sanahuja, Ojeda, Tello Martín, Tous, Tarradas, Cruz-Quintana and Gisbert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enric Gisbert, ZW5yaWMuZ2lzYmVydEBpcnRhLmNhdA==
 Carl John Saromines
Carl John Saromines Silvia Torrecillas
Silvia Torrecillas Francisco Javier Moyano
Francisco Javier Moyano Ignasi Sanahuja
Ignasi Sanahuja Sonia Ojeda4
Sonia Ojeda4 Maria Luisa Tello Martín
Maria Luisa Tello Martín Núria Tous
Núria Tous Joan Tarradas
Joan Tarradas Yanis Cruz-Quintana
Yanis Cruz-Quintana Enric Gisbert
Enric Gisbert