Abstract
Introduction:
Identifying spawning and nursery areas for exploited fish stocks is crucial for sustainable fisheries management, as these areas support fish reproduction and early development necessary for maintaining healthy fish populations. The mackerel scad, Decapterus macarellus, is a valuable species in Indonesia, playing a crucial role in the livelihoods of coastal communities and the national fishing industry.
Methods:
This study aims to identify the spawning and nursery areas of D. macarellus in the Sulawesi Sea, specifically within Indonesia's Fisheries Management Area (FMA) 716. Field sampling was conducted at 25 sites using bongo net tows to collect D. macarellus larval specimens. Hydro-oceanographic conditions were measured, and larval ages were estimated through otolith analysis. Based on these data, trajectory models were developed to assess the D. macarellus spawning and nursery habitats within the Sulawesi Sea.
Results:
Results indicate that spawning primarily occurs on the northern and western sides of Sangihe and Siau Islands. Nursery areas were identified in the southern Sulawesi Sea and Maluku Sea, where high zooplankton abundance supports juvenile growth.
Discussion:
Our findings highlight environmental conditions and critical habitats for D. macarellus, providing insights into spatial patterns of its spawning and nursery activities. The study underscores the need for pelagic fisheries marine protected area (MPA) to safeguard these critical habitats, ensuring sustainable fisheries amid increasing exploitation pressures. These findings provide a scientific basis for spatial management strategies, supporting Indonesia’s national MPA targets while offering a replicable framework for pelagic habitat conservation in other regions.
1 Introduction
Identification of spawning and nursery sites of exploited fish stocks is crucial for ensuring the sustainability of fisheries management (Sadovy de Mitcheson, 2016). These areas are vital for the reproduction and early development of fish populations, and their protection can help maintain healthy fish stocks and support long-term fishing activities. Understanding where these critical habitats are located allows for the implementation of effective conservation measures and management strategies (Erisman et al., 2017; Campos et al., 2022).
The mackerel scad, Decapterus macarellus, first described by Cuvier in 1833, is widely distributed across the tropical and subtropical regions of the world’s oceans. They inhabit coastal and pelagic zones, often forming large schools in nearshore waters (da Cruz Delgado et al., 2024). D. macarellus is a pelagic fish species, oceanic, fast swimmer, and can be found at depths of 40–200 m (Smith-Vaniz, 1995). D. macarellus has a short lifespan, matures quickly, and preys on plankton (Peck et al., 2013).
D. macarellus plays a significant role in marine ecosystems, serving as both predator and prey, contributing significantly to the marine food web (Le Mézo et al., 2022; Shen et al., 2025). Spawning of the D. macarellus typically occurs during specific temperature ranges, with notable reproductive peaks influenced by environmental conditions, such as salinity and habitat type, impacting both adult and juvenile populations of the species (Hou et al., 2020). Thus, understanding its reproductive behaviors and habitat preferences is essential for ensuring its sustainability and ecological health.
D. macarellus is one of the most economically valuable fish species in Indonesia (MMAF, 2016; Retnoningtyas et al., 2023; Munandar et al., 2024). This pelagic species contributes significantly to the livelihoods of coastal communities and the broader fishing industry at the national level (Baso et al., 2021). In Indonesia, D. macarellus can be found in the Indonesia FMA (Fishery Management Area) 716 which includes the waters of the Sulawesi Sea and the northern part of Halmahera Island. Indonesia FMA 716 is located in North Sulawesi, an area with high coral reef biodiversity, as well as an important area for both small and large pelagic fisheries in Indonesia (Ambo-Rappe and Moore, 2019). Annual fish production in this area reaches 478,765 tons/year (MMAF, 2016). D. macarellus utilizes about 20-30% of those fishery production where most of the fish caught by purse seine net (MMAF, 2016). There was an increase in scads production from 36,630 tons in 2019 to 45,955 tons in 2023 (MMAF, 2025).
D. macarellus is also indicators of ecosystem health, as changes in their population dynamics can reflect shifts in environmental conditions or disturbances. Overfishing, habitat destruction, and climate change can adversely affect their populations, which in turn may lead to cascading effects throughout the food web (Fréon et al., 2005; Zhang et al., 2022). The decline of D. macarellus populations could disrupt the balance of predation and prey relationships, ultimately impacting both lower and higher trophic levels (Ajub et al., 2023; Le Mézo et al., 2022).
Indonesia, as a country with significant pelagic fish production including scads (Simbolon et al., 2019; World Bank and Ministry of Marine Affairs and Fisheries, 2024), needs on development of conservation areas to sustain its pelagic fishery. This is necessary due to high demand and extensive exploitation, which pose a risk of overfishing and threaten the sustainable use of these pelagic resources. Most of critical habitats of pelagic fishes within 12 nautical miles have been designated to conservation areas, most of which fall under the authority of the provincial governments (Soemodinoto et al., 2021). The establishment of pelagic conservation areas has been implemented in several countries, such as in Chagos, United Kingdom, covering an area of approximately 500 million km2. This conservation area was originally aimed at protecting coral reefs, but it also encompasses tuna fishery (Sheppard et al., 2012). Curnick et al. (2020) stated that although there is no evidence of an increase in the stock of bigeye tuna (Thunnus obesus), large pelagic marine protected area (MPA) needs to be harmonized with fisheries management regulations to ensure that conservation benefits are obtained for pelagic fisheries. Indonesia already has 29.9 million hectares of conservation areas (MMAF, 2024), almost all of which are within 12 nautical miles of the provincial government authorities. Based on this, it is necessary to develop conservation areas to protect pelagic fish beyond of the provincial government authorities based on the critical habitats observed, such as the spawning and nursery areas of the pelagic fish.
This research aims to identify the spawning and nursery areas of D. macarellus in the Sulawesi Sea. By identifying these critical habitats, our investigation provides valuable information to develop effective management and conservation strategies for this economically important fish species. Our study highlights identifying key habitats and detailed descriptions of environmental conditions, insights into temporal patterns of spawning and nursery activities, and specific conservation recommendations. These findings are important in informing fisheries management practices and protecting the sustainability of D. macarellus populations within the Sulawesi Sea.
2 Materials and methods
2.1 Study sites
Samples of D. macarellus larvae and juveniles were collected from 25 sites in the Sulawesi Sea, Indonesia, located within the Indonesian Fisheries Management Area 716 (FMA 716) (Figure 1). A research vessel of the Indonesian Ministry of Marine Affairs and Fisheries (KM Bobara, BP3 Bitung) that equipped with the bongo net was used to sample the fish’ larvae and juveniles. The ichthyoplankton sampling was conducted on 23–29 September 2020, at 4 diel periods (i.e., 06:00 - 11:00, 12:00 - 17:00, 18:00 - 23:00, and 00:00 - 05:00). The sampling period follows information from the local fishers experienced that the peak spawning period of the D. macarellus occurred in September, and later this was validated by Retnoningtyas et al. (2024) where D. macarellus spawned throughout the year, peaking in January, March, May, and September. The research vessel cruised from the Bitung fishing Port to Site 1 around Manado Tua Island to Site 25 around Tahuna and Sangihe Islands (Figure 1). The sampling locations were selected based on previous studies of Retnoningtyas et al. (2024).
Figure 1
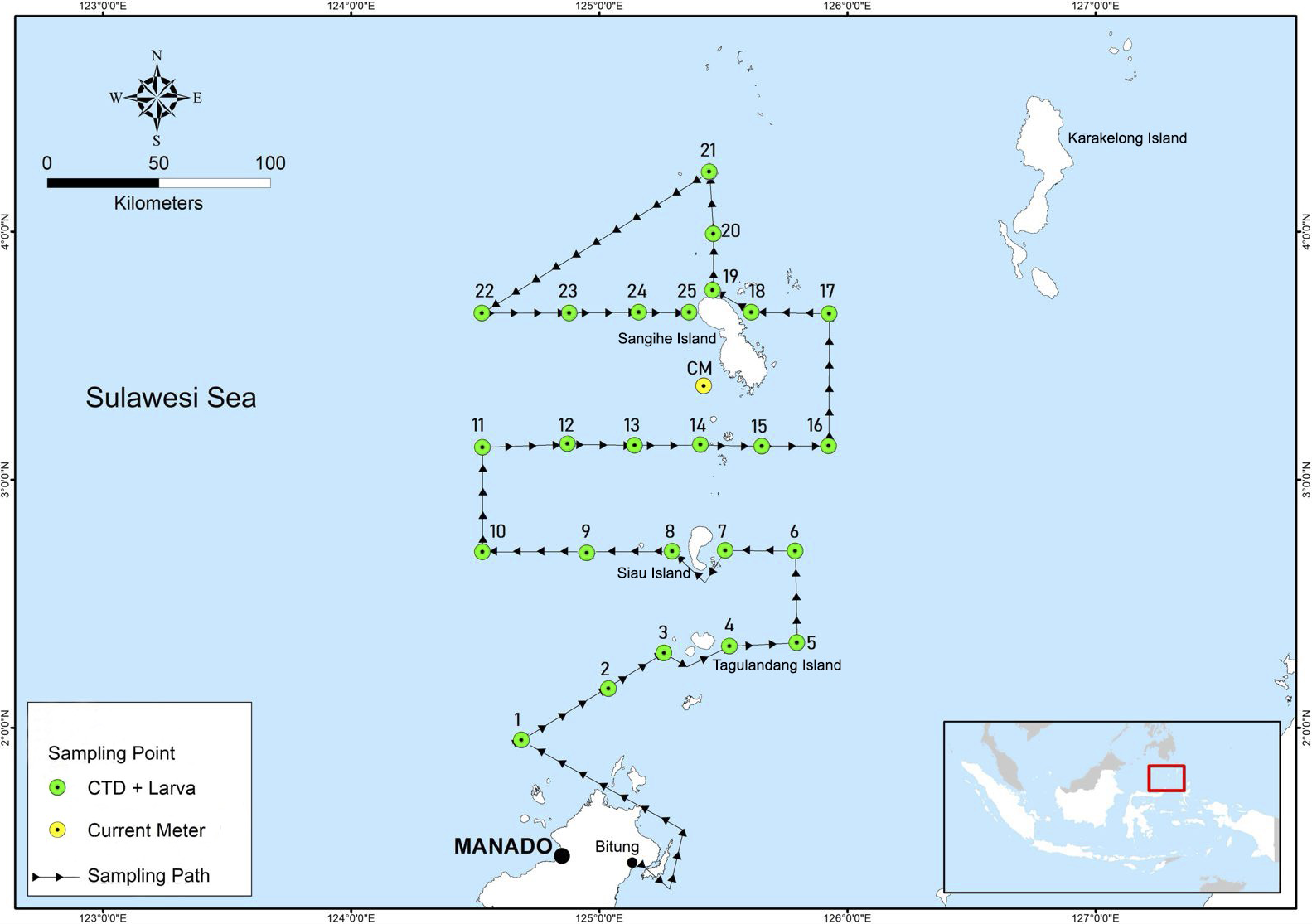
Research vessel tracks and sampling locations of the study. (CTD, Conductivity Temperature Depth; CM, Current Meter).
2.2 Hydro-oceanographic profiles
To support development of the larval trajectory model (see Section 2.6. Determination of spawning and nursery areas using trajectory models), we collected data of hydro-oceanographic profiles (i.e., sea temperature and current) from Copernicus Marine Environment Monitoring Service (CMEMS). We then validated these data with measurement in situ. A CTD was used to collect the in situ vertical sea temperature profiles from 0 to 200 m depth at the 25 sites within the Sulawesi Sea (Figure 1). To validate current data of hydrodynamic modelling, a current meter (INFINITY Compact EM) was deployed at the depth of 5 m with a 10-second recording interval at the western side of Sangihe Island (Figure 1) from 29 September to 15 October 2020.
2.3 Ichthyoplankton sampling
Larvae and juveniles of D. macarellus were collected using a conical bongo net with a diameter of 60 cm, a mesh size of 0.5 mm, and a length of 3 m. The oblique tow method, involving the gradual ascent of the net from a depth of 100 m to the surface, was employed using the research vessel’s winch (González-Quirós et al., 2003). During the towing process, the vessel proceeded at a slow speed of 2–3 knots. The volume of filtered seawater was determined by a TSK flow meter (the Tsurumi-Seiki Co., Ltd.) attached to the net’s opening. Samples retrieved from the net were initially stored in a 1,000 ml mother bottle, then transferred to 16 L ice containers, and sorted immediately on board. The number of larvae and juveniles collected was recorded, and the volume of seawater filtered was utilized to estimate the abundance of D. macarellus larvae and juveniles (individuals per 10,000 m³). At each site, samples from the bongo net were split for molecular and morphological analysis. Specimens for molecular analysis were preserved in absolute ethanol, while those for morphological analysis were first preserved in 10% formalin for 3–4 hours, rinsed with water, and then preserved in 80% ethanol (e.g. Simanjuntak et al., 2015, 2020).
2.4 Fish larval identification and developmental stage
The larva and juvenile of D. macarellus were morphologically identified following the guidelines of Okiyama (2014) and Leis and Carson-Ewart (2000). Genetic identification was performed using DNA barcoding of the mitochondrial cytochrome oxidase C subunit-1 (COI). Fish larvae and juveniles’ samples underwent pretreatment and DNA extraction according to the Qiagen Blood & Tissue Kit protocol. The isolated DNA was replicated using the BIONESIA PCR protocol with BCH/BCL and F1/R1 primers (Ward et al., 2005). The DNA extraction and sequencing process was conducted at BIONESIA Laboratory and Genetica Science Indonesia. The DNA analysis involved comparing the replicated sequences with the GenBank database (NCBI) through phylogenetic tree analysis. This analysis illustrates the evolutionary relationships among the different samples, highlighting the genetic similarities and differences within the Decapterus genus.
The larval development stages of D. macarellus were meticulously determined using morphological criteria outlined by Kendall et al. (1984). Development stages were categorized with precision as follows: preflexion larva with yolk (designated as “A”), preflexion larva without yolk (B), flexion larva (C), post-flexion larva (D), and juveniles (E).
2.5 Age estimation and determination of spawning and nursery habitats
The age of D. macarellus larvae and juveniles were estimated by calculating the daily growth increments on the sagittal otoliths. The age determined from the sagittal otoliths was used to back-calculate the larval hatching and spawning periods, following the method described by Jones (1986) and Simanjuntak (2016). The duration of the incubation period (from egg to larval hatching) and larval body length data from related species, such as D. macrosoma and D. kurra, served as references (Pauly and Pullin, 1988).
2.6 Determination of spawning and nursery areas using trajectory models
Two drift trajectory models were developed using the MOHID water modelling system (MOHID Water Modelling System, 2024) to identify the spawning and nursery areas of D. macarellus. We employed imposed solution from computed hydrodynamics derived from CMEMS data portal (https://marine.copernicus.eu/; product code: GLOBAL_ANALYSISFORECAST_PHY_001_024) as the input to the model. This product is characterized by two-dimensional model output, an hourly temporal resolution, and ~9km of spatial resolution. To check the model, we compared the current frequency generated by the model with measurements from the current meter, and the sea surface temperature output from the model with satellite imagery. The initial condition of the drift model is based on the floating object configuration as the passive tracer and the larvae assume remain the same over the simulation period time. Hence, larval of D. macarellus was assumed as a passive tracer where its movement was highly dependent upon the hydrodynamics in the Sangihe-Talaud Waters. The number of larvae used for simulation dependent on the sampling evidence and releasing it in the ocean’s surface. Due to the low number of larval abundances, the larval abundances were converted to 1000 m3 to allow MOHID to develop the trajectory path. The spawning and nursery phase and age class are determined based on growth calculation of the species, described on the sub chapter age estimation (see section 2.5). Referring to the average larval age from each station, a backward trajectory model was developed ranging from Day-0 up to Day-15 from the sampling day to identify the spawning habitat of D. macarellus. The backward trajectory model was developed using the formula:
Where = (x,y,z,t) represents vector where the larval was sampled; x,y, and z represents the cartesian coordinate; t represents backward time; represents advection velocity of the current velocity; is advection velocity generated by the wind, which can be neglected since wind is already included in the surface current assumption; is velocity resulted diffusion processes; is vertical velocity; and is a vector unit in the vertical axis. The resultant of + was found as the main generator and determined the starting point of larval movement.
Slightly different than the determination of spawning habitat, a combination of backward trajectory and forward trajectory was applied to identify the nursery habitat. Onset the nursery starts at 25 days after hatching, when they attained juvenile stage. A backward trajectory model was developed ranging from Day-0 to Day-15 while the forward trajectory model was developed from D+0 to D+15 to identify the nursery habitat.
3 Results
3.1 Hydro-oceanographic condition of the Sulawesi Sea
The sea surface temperature in the Sulawesi Sea exhibits seasonal variations throughout the year. In the first quarter (January-March), the lowest temperature recorded is 26.7°C and the highest is 32.7°C, with an average temperature of 29°C. In the second quarter (April-June), the lowest sea surface temperature is 26.8°C and the highest is 32.6°C, with an average of 29.5°C. In the third quarter (July-September), the lowest sea surface temperature is 27.3°C and the highest is 32.3°C, with an average of 30.1°C. In the fourth quarter (October-December), the lowest sea surface temperature is 27.8°C and the highest is 32.5°C, with an average of 30°C. Overall, the sea surface temperature in the Sulawesi Sea is cooler in the first and second quarters and becomes warmer in the third and fourth quarters (Figure 2).
Figure 2
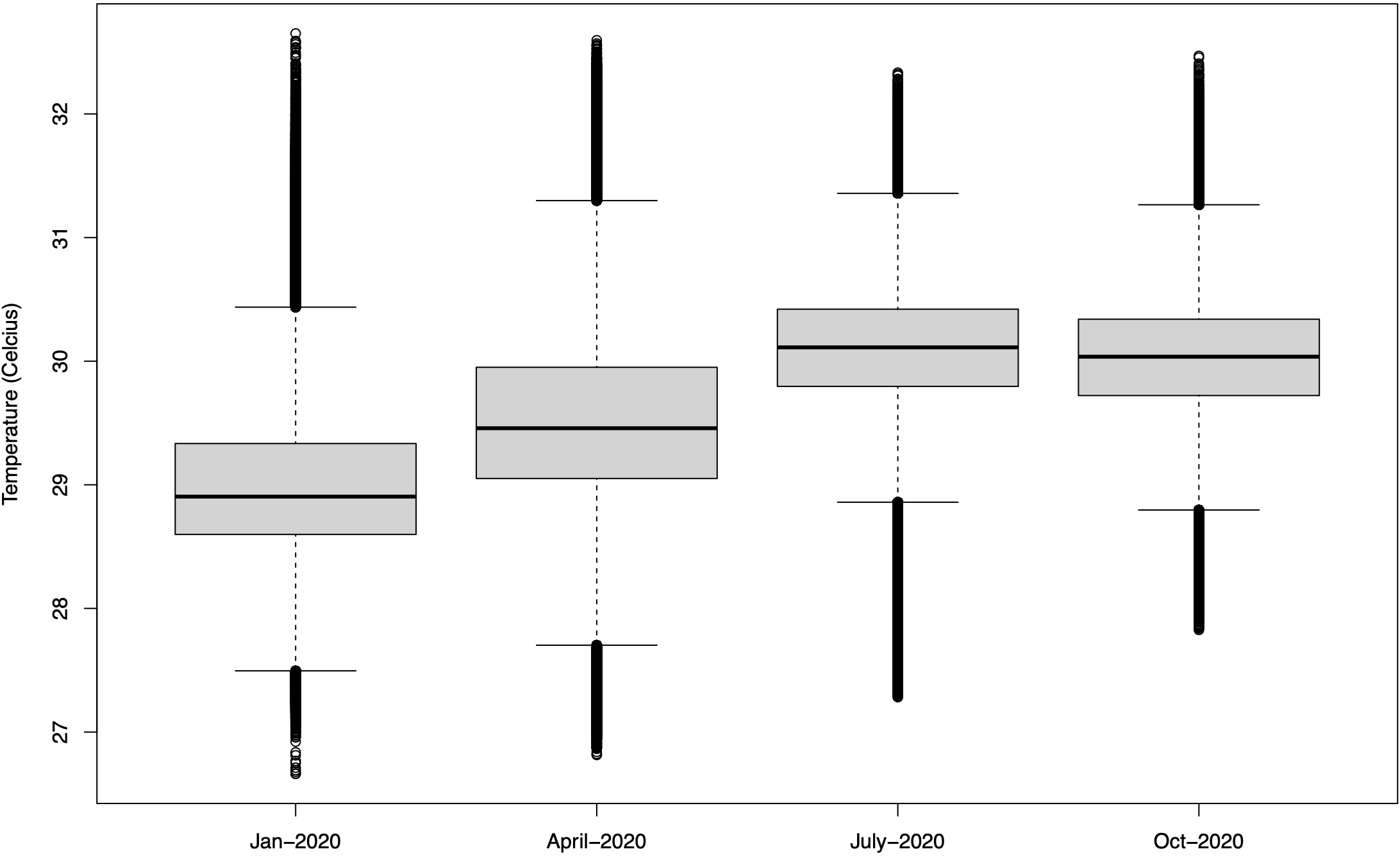
Sea surface temperature variability at the study area in Sulawesi Sea during January, April, July, and October 2020. Data source: Copernicus Marine Environment Monitoring Service (CMEMS).
In the first quarter (January-March), the sea current moves from east to southwest with a mean velocity of 0.3554 m/s. In the second quarter (April-June), the current moves from east to west with a mean velocity of 0.38367 m/s. In the third quarter (July-September), the current moves from southeast to southwest with a mean velocity of 0.4545 m/s. In the fourth quarter (October-December), the current moves from northwest to east with a mean velocity of 0.38954 m/s. Information on current patterns in the Sulawesi Sea is important for understanding local climate patterns, marine ecosystem dynamics, and the potential distribution of D. macarellus larval habitat (Figure 3).
Figure 3
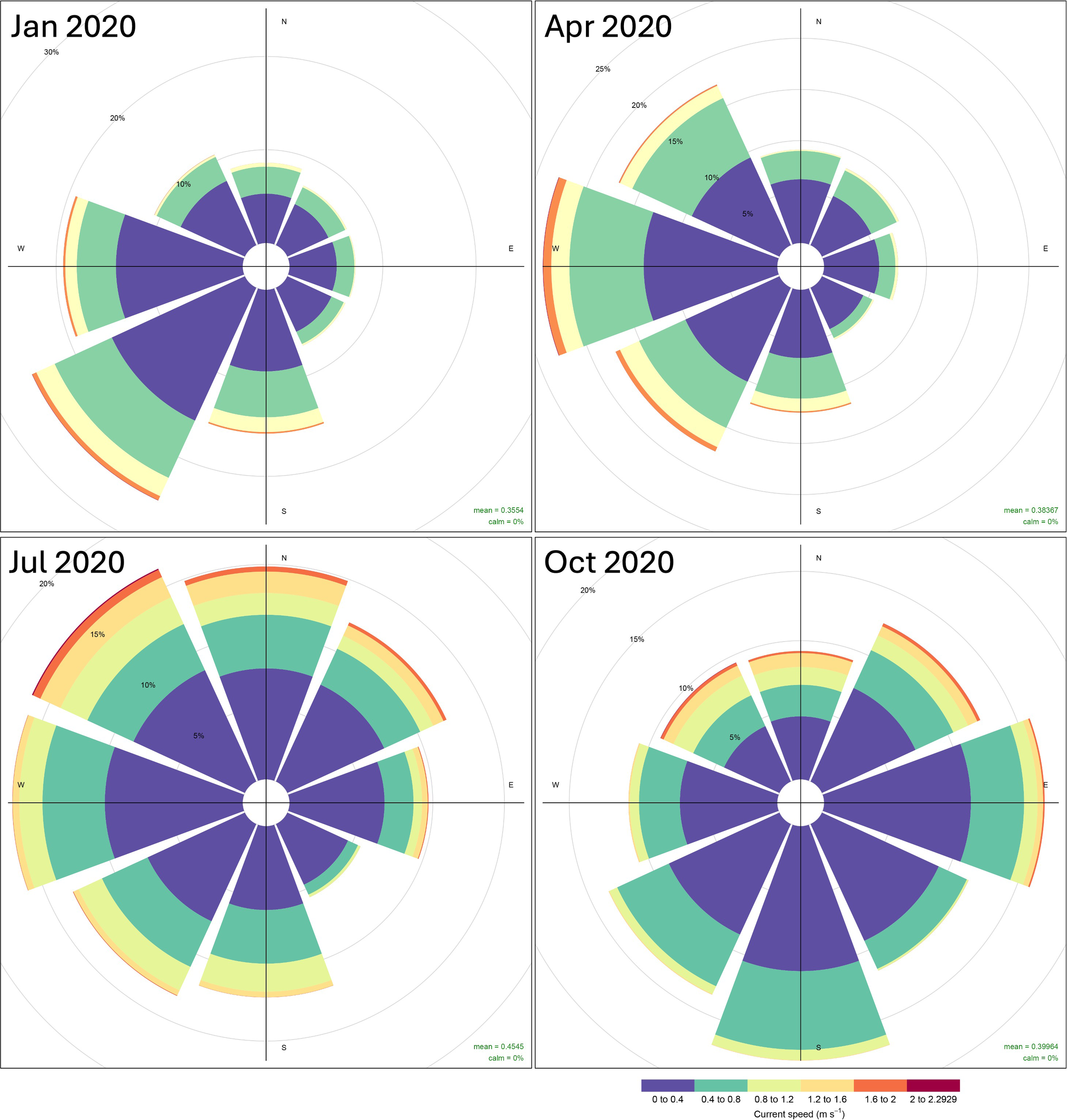
Sea current pattern at the study area in Sulawesi Sea in January, April, July, and October 2020. Colors represent sea current speed ranges; grey circles represent the percentage frequency of sea current coming from each direction. Data source: Copernicus Marine Environment Monitoring Service (CMEMS).
The validation of vertical temperature data from Marine Copernicus (ex-situ) was compared with field observations (in situ) using CTD to assess the suitability and accuracy of both data sets. Vertical temperature validation was conducted at station 11 (representing the west) and station 16 (representing the east validation). The Root Mean Square Error (RMSE) value at station 11 (west validation point) was 1.043. Meanwhile, station 16 (east validation point) has an RMSE value of 0.625. These results show no significant difference between the data from the model results and observations. The low RMSE value indicates that ex-situ measurements are almost accurate or the same as in-situ measurements. The vertical temperature profiles of the two stations are shown in Figure 4.
Figure 4
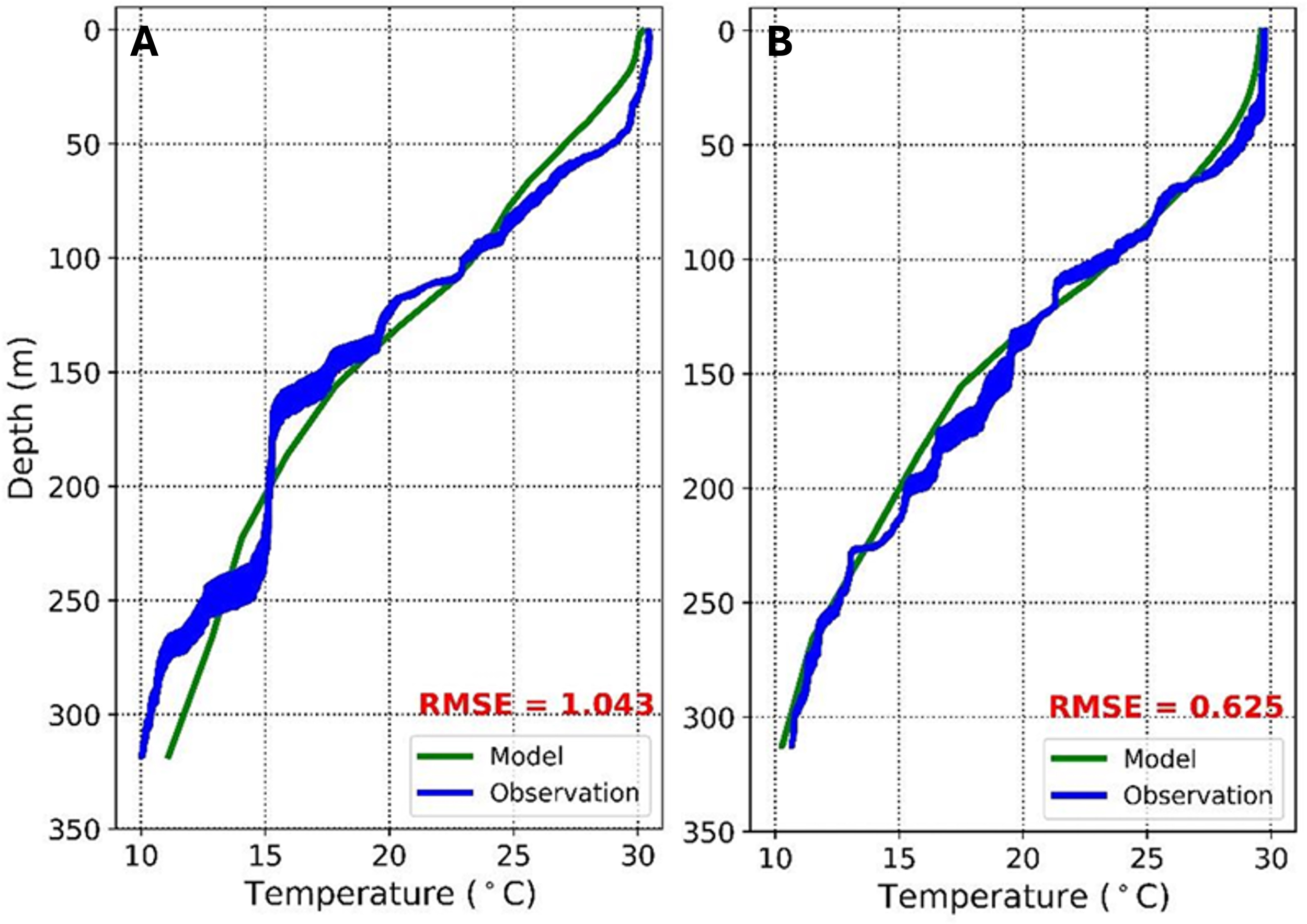
Vertical profiles of sea temperature (°C) relative to depth (meter), from observation (blue lines) and model (green line) at Station 11 (A) and Station 16 (B).
3.2 Species identification of Decapterus macarellus based on morphology and molecular approaches
Larvae and juveniles of D. macarellus were identified according to the criteria set forth by Okiyama (2014) and Leis and Carson-Ewart (2000). D. macarellus larvae exhibit moderate body depth, and the gut is initially straight but begins to coil at a body length of 2.5 mm. The head is moderate to large in preflexion larvae and becomes large in postflexion larvae. The mouth is oblique and of moderate size, while the round eyes are notably large. Two rows of simple preopercular spines develop early in the preflexion stage. Additionally, the larvae display a series of melanophores along the dorsal and ventral midlines of the trunk, with pigmentation observed on the snout, brain, lower jaw, and gas bladder (Figure 5).
Figure 5
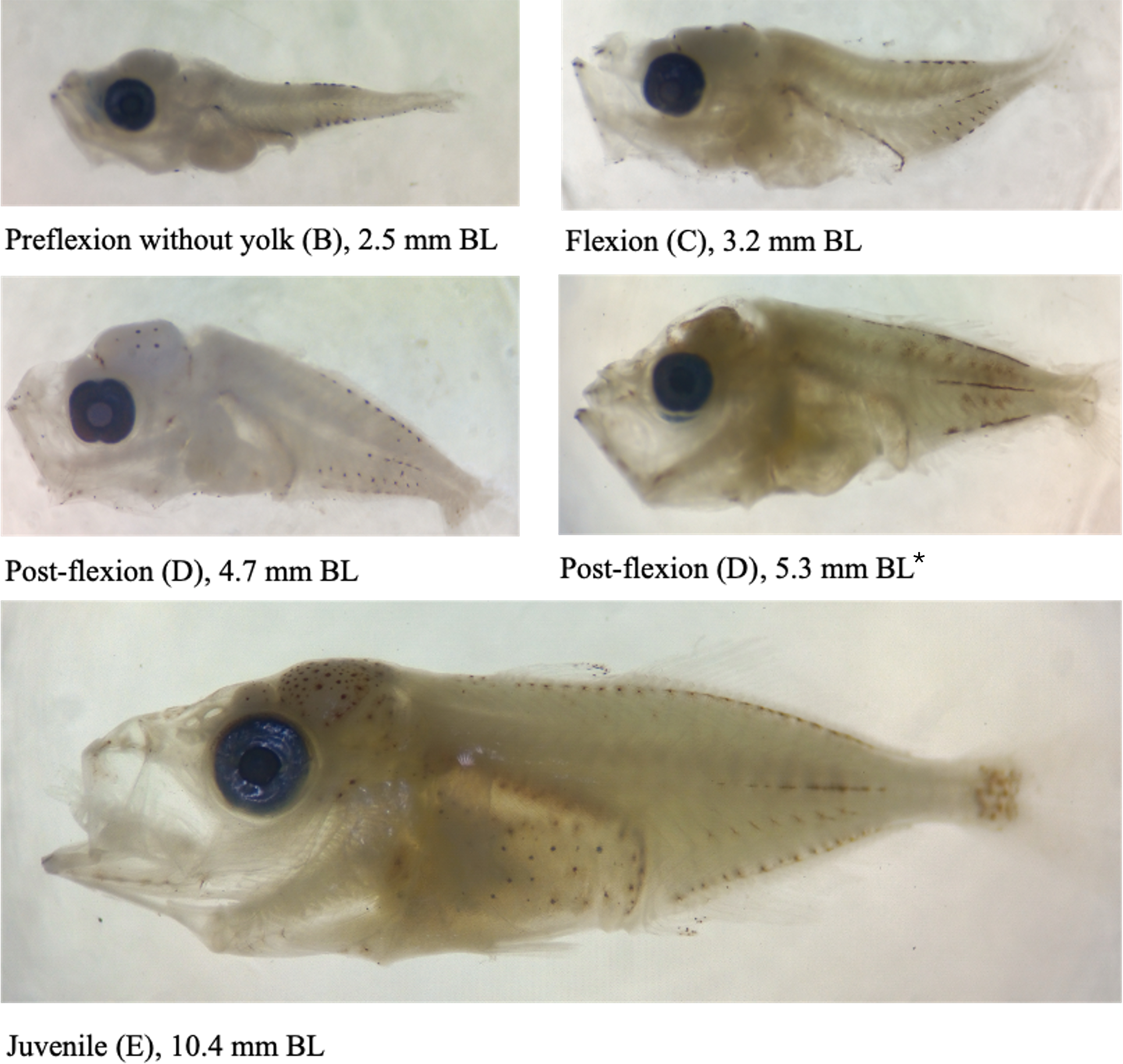
Photos of different Decapterus macarellus larva development stages sampled at the Sulawesi Sea; BL is Body Length. *This specimen was used for the identification using DNA barcoding.
DNA barcoding confirmed the identification of the candidate species as D. macarellus, showing 100% similarity with D. macarellus data available in the NCBI GenBank (Figure 6). The sequence data for D. macarellus larvae and juveniles has been uploaded to NCBI, with the accession number BIOSUB104 002.
Figure 6
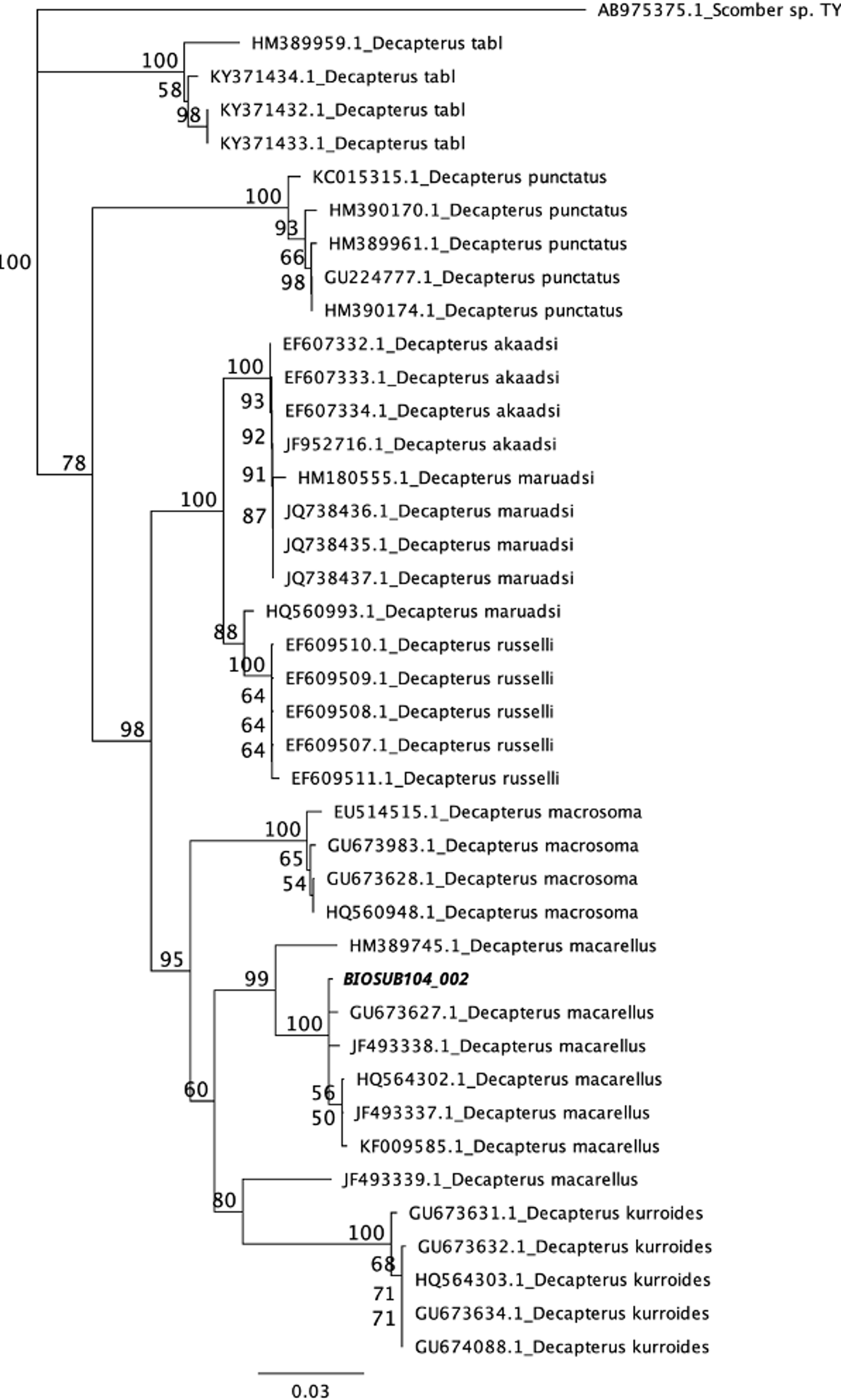
A phylogenetic tree that was constructed for Decapterus macarellus larval samples (BIOSUB104 002) in comparison with other Decapterus species available in the NCBI GenBank.
3.3 Occurrence, abundance, and developmental stages composition of Decapterus macarellus
Larvae and juveniles of D. macarellus were identified in 14 of 25 sampling stations. The highest abundance was recorded at station 7 (east of Sangihe Island), with densities exceeding 200 individuals per 10,000 m³. Station 3 (Talaud Island) followed, with densities ranging from 151 to 200 individuals per 10,000 m³, while station 1 (north of Bunaken National Park) exhibited abundances of 101 to 150 individuals per 10,000 m³. Other stations reported lower abundances, ranging from 1 to 50 individuals per 10,000 m³. The abundance of D. macarellus larvae and juveniles at each station is illustrated in Figure 7.
Figure 7
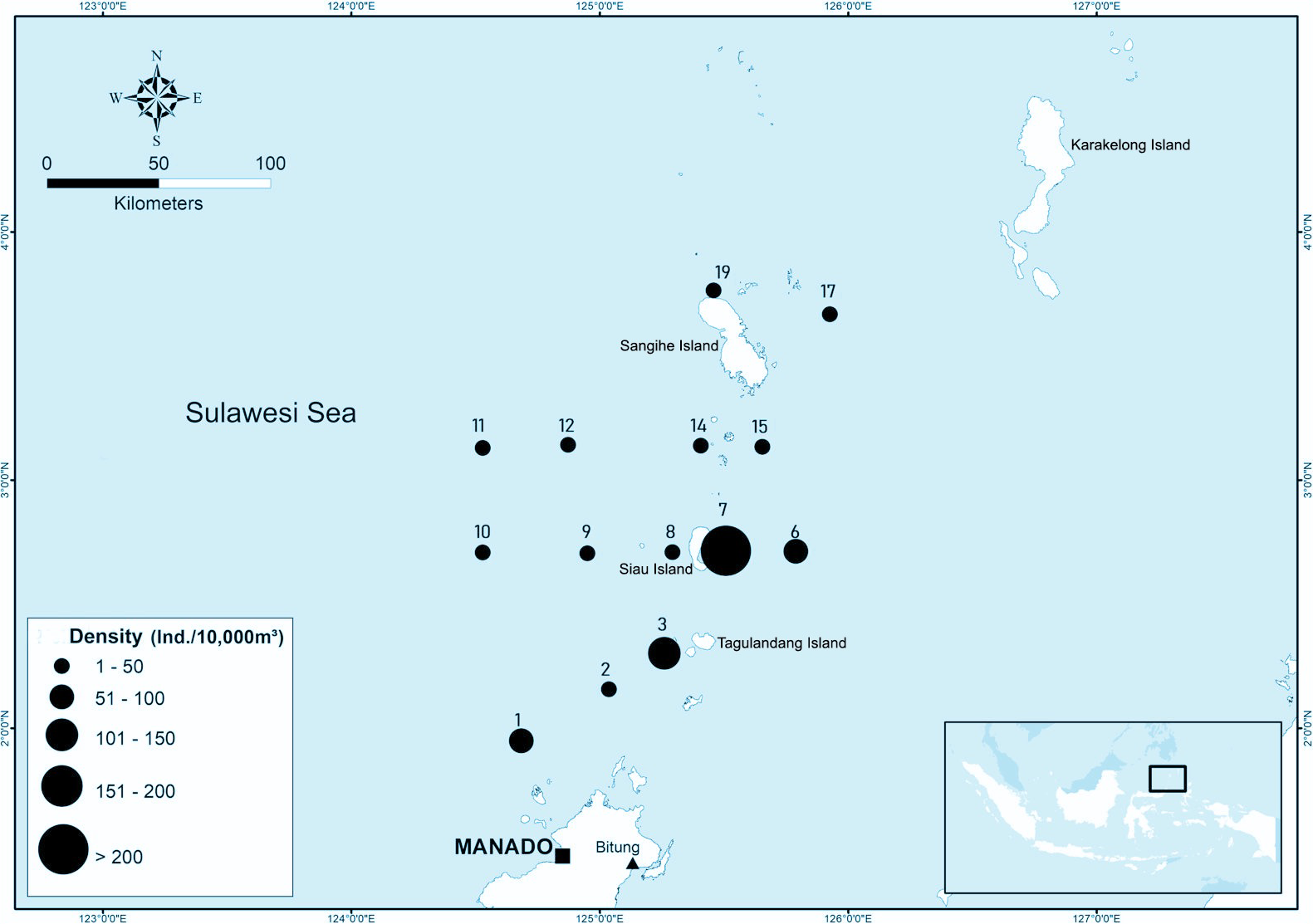
Occurrence and abundance of Decapterus macarellus larvae in each sampling sites in Sulawesi Sea. Different circle sizes represent different density (no.10,000 m-3) ranges.
During the study, we identified four developmental stages of D. macarellus: preflexion larvae without yolk (B), flexion larvae (C), post-flexion larvae, and juveniles (E). No eggs (embryos) or preflexion larvae with yolk (A) were found at any of the study sites. The composition of larval stages of D. macarellus at each station is illustrated in Figure 8.
Figure 8
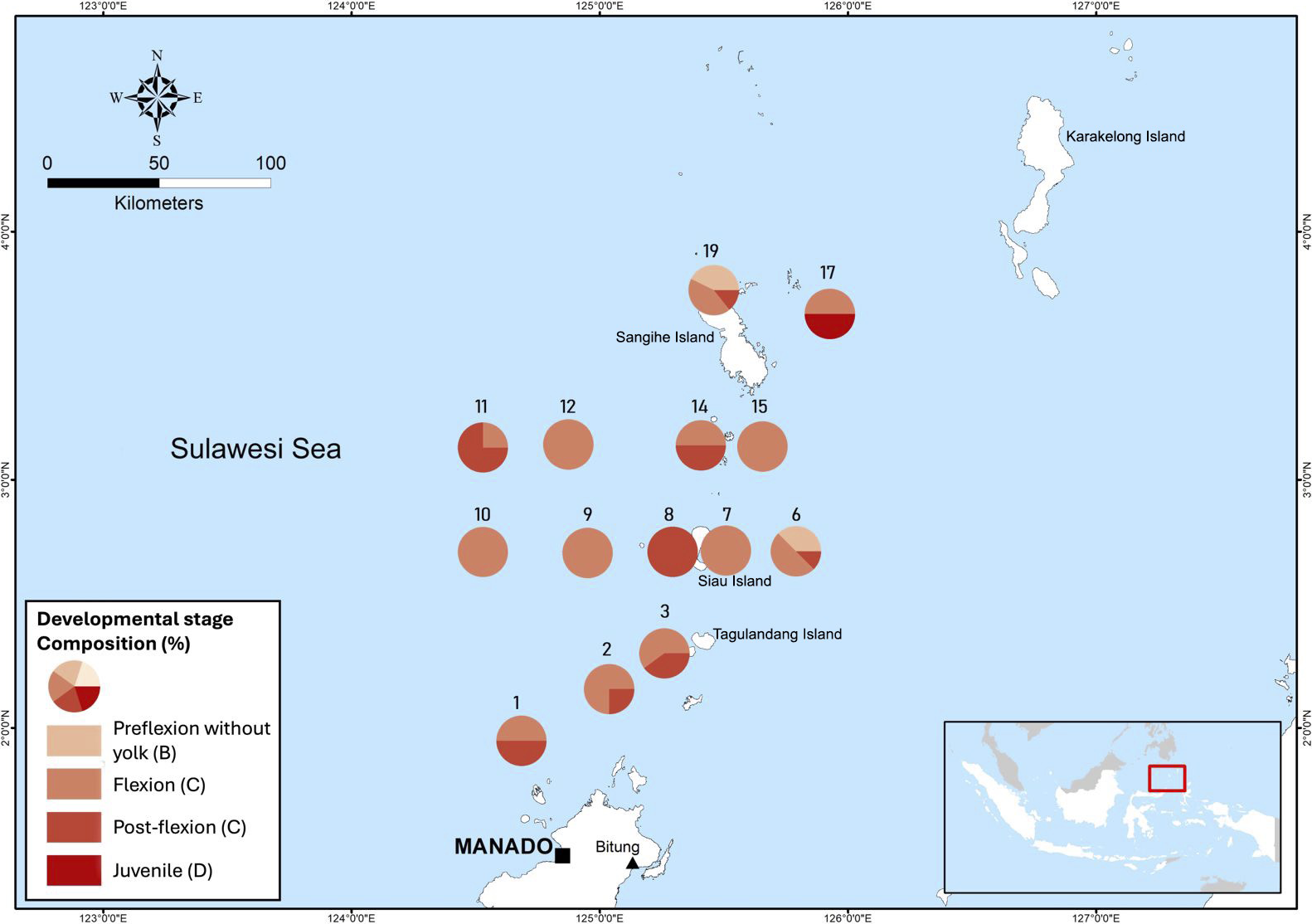
Composition of larval developmental stages (distinguished by color) of the Decapterus macarellus in each sampling site in the Sulawesi Sea.
At station 1, flexion and post-flexion stages were found, each comprising 50% of the total. Station 2 had a composition of 75% flexion larvae and 25% post-flexion larvae. At station 3, flexion stages accounted for 60%, while post-flexion stages made up 40%. Station 6 yielded 40% preflexion larvae without yolk (B), 50% flexion stages, and 10% post-flexion stages. Stations 7, 9, 10, 12, and 15 only contained preflexion larvae without yolk (B), while station 8 exclusively had post-flexion stages. Station 11 reported 75% post-flexion and 25% flexion stages, and station 14 had an equal distribution of flexion (50%) and post-flexion (50%) stages. At station 17, both post-flexion (50%) and juvenile (50%) stages were present. Finally, station 19 recorded preflexion larvae without yolk (45%), flexion stages (45%), and post-flexion stages (10%). The dominant larval developmental stages at each sampling station were used as a basis to develop the larval trajectory model.
3.4 Body length and aging of Decapterus macarellus
The body lengths of D. macarellus larvae and juveniles varied by developmental stage: preflexion without yolk (B) ranged from 2.1 to 3.4 mm, flexion (C) from 2.4 to 6.5 mm, post-flexion (D) from 4.1 to 9.3 mm, and juveniles (E) from 9.2 to 15.4 mm. Otolith analysis revealed that preflexion larvae without yolk were 3–5 days after hatching (DAH), flexion larvae were aged 8–13 DAH, post-flexion larvae were between 14–22 DAH, and juveniles ranged from 23–25 DAH.
The dominant samples of D. macarellus caught were in the flexion stage (age range: 8–13 DAH) and post-flexion stage (14–22 DAH). These fish reach the juvenile stage at 23–25 DAH. To estimate the spawning period, a trajectory model was developed by counting backward 9–14 days from the sampling date, while a forward trajectory model was created by projecting 14–15 days ahead to elucidate the nursery area dynamics of this species.
3.5 Spawning and nursery areas identification of Decapterus macarellus
Identification of spawning and nursery areas of D. macarellus was projected through backtracking and forwardtracking models, respectively. The first transect (stations 1-5) showed the movement of larval from Day-1 to Day-5 towards the west of the Sulawesi Sea, then from Day-6 to Day-15, the movement of larval continued to move westward from the Sulawesi Sea. The second transect (stations 6-10) showed larval movement from Day-1 to Day-5 moving towards the west of the Sulawesi Sea. Then from Day-6 to Day-15 the movement of fish larvae was found to originate from the Philippine waters or north of the Sulawesi Sea which moved south to the waters of Sangihe Island and Tahulandang Island and continued to turn towards the west of the Sulawesi Sea.
Furthermore, in the third transect (11-16), from Day-1 to Day-15, the larva movement is moving from around the southern waters of Sangihe Island towards the western Sulawesi Sea. The fourth transect (stations 17-19) from Day-1 to Day-15 found the larva movement moving from the northern area of the Celebes Sea or Philippine waters towards the south towards the waters around Sangihe Island. Therefore, the spawning ground of D. macarellus is around Sangihe Island and the Sulawesi Sea. The results of the plot of the backtracking model for estimating the spawning ground of D. macarellus are shown in Figure 9.
Figure 9
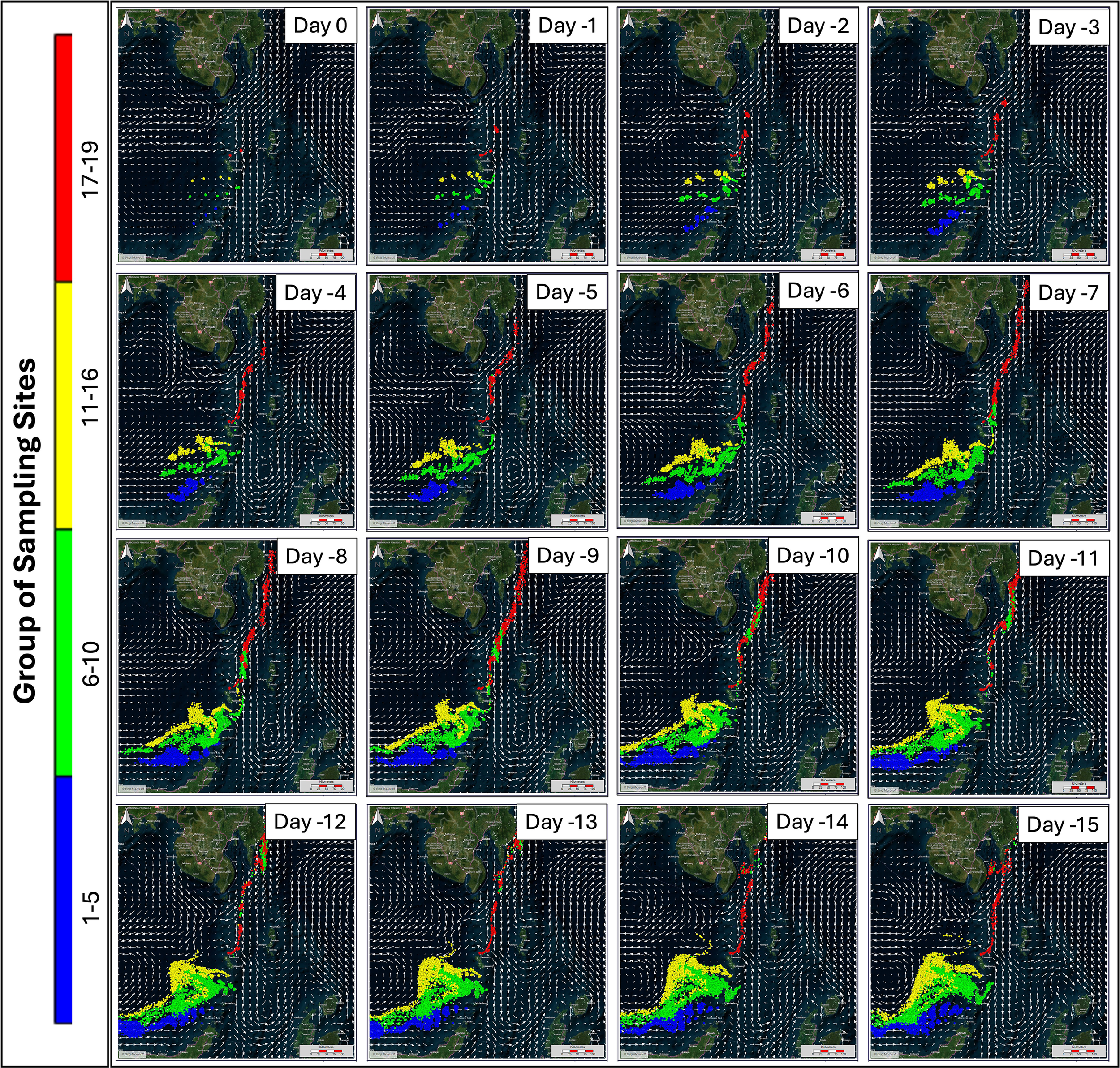
Backtracking model to identify spawning areas of the Decapterus macarellus in the Sulawesi Sea; colors represent different groups of sampling sites.
The results of the forward tracking model in D. macarellus showed that there were different variations in larval movement patterns at each observation station (Figure 10). The estimation of forward tracking in D. macarellus was conducted to estimate the nursery ground based on the larvae movement after being caught at the observation station. In the first transect (stations 1-5) the larvae movement from Day+1 to Day+5 moved from Biaro Island towards Talisei Island and around the northern part of Bunaken National Park. Then from Day+6 to Day+15, the larvae turned back towards the south and gathered between the waters of Talisei Island and Biaro Island. In the second transect (stations 6-10), the larvae from Day+1 to Day+5 moved from the northwest towards the south, and on Day+6 to Day+10, the larvae moved from the south towards the east. From Day+11 to Day+12 the larvae moved from the east towards the northeast approaching North Maluku Island. Then on Day+13 to Day+15, the larvae turned northwest and headed north to the Sulawesi Sea.
Figure 10
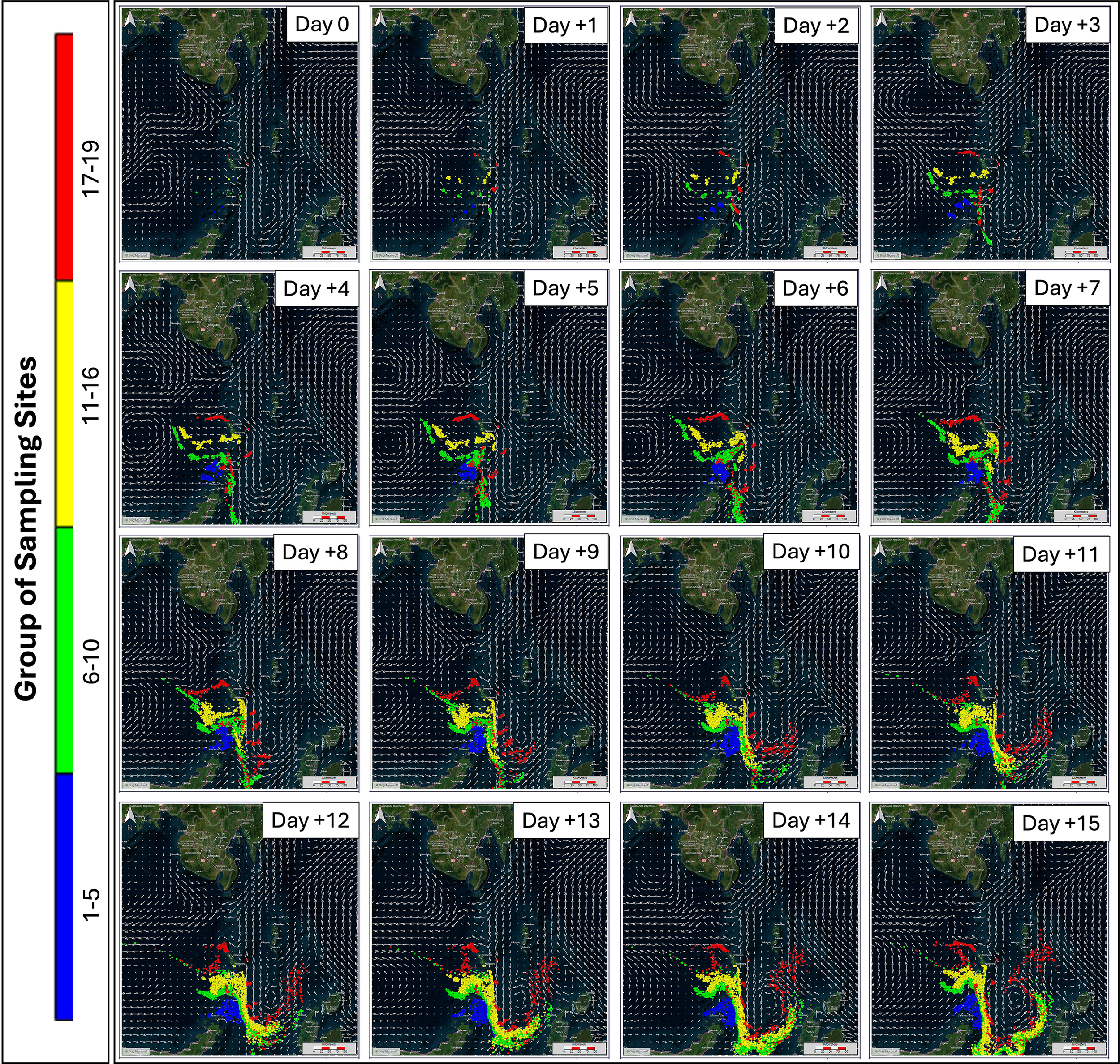
Forward tracking model to identify nursery areas of the Decapterus macarellus in the Sulawesi Sea; colors represent different groups of sampling sites.
Furthermore, the third transect (stations 11-16), the larvae on Day+1 to Day+5 moved westward and turned towards the northwest, then on Day+6 to Day+10 turned southward to the Maluku Sea waters and on Day+11 to Day+15 the larvae moved eastward to the waters around North Maluku and moved back northwestward to the northern waters of the Sulawesi Sea approaching the Philippine waters. The fourth transect (stations 17-19), on Day + 1 to Day + 7 the larvae around Sangihe Island moved westward and turned southward to the waters of Biaro Island and Bitung. On Day+8 to Day+10, the larvae moved eastward to the waters of the North Moluccas Sea, and on Day+11 to Day+15, the larvae moved northward from the Moluccas Sea to the northern waters of the Celebes Sea approaching the Philippine waters. The nursery ground of D. macarellus based on the forward tracking method, is in the Sulawesi Sea, between the waters of Sangihe Island to the waters of Biaro Island, the waters around North Maluku and the north of the Sulawesi Sea bordering Philippine waters. The results of the forward tracking plot for the nursery ground of D. macarellus are shown in Figure 10. The spawning and nursery areas on the map are shown in Figure 11.
Figure 11
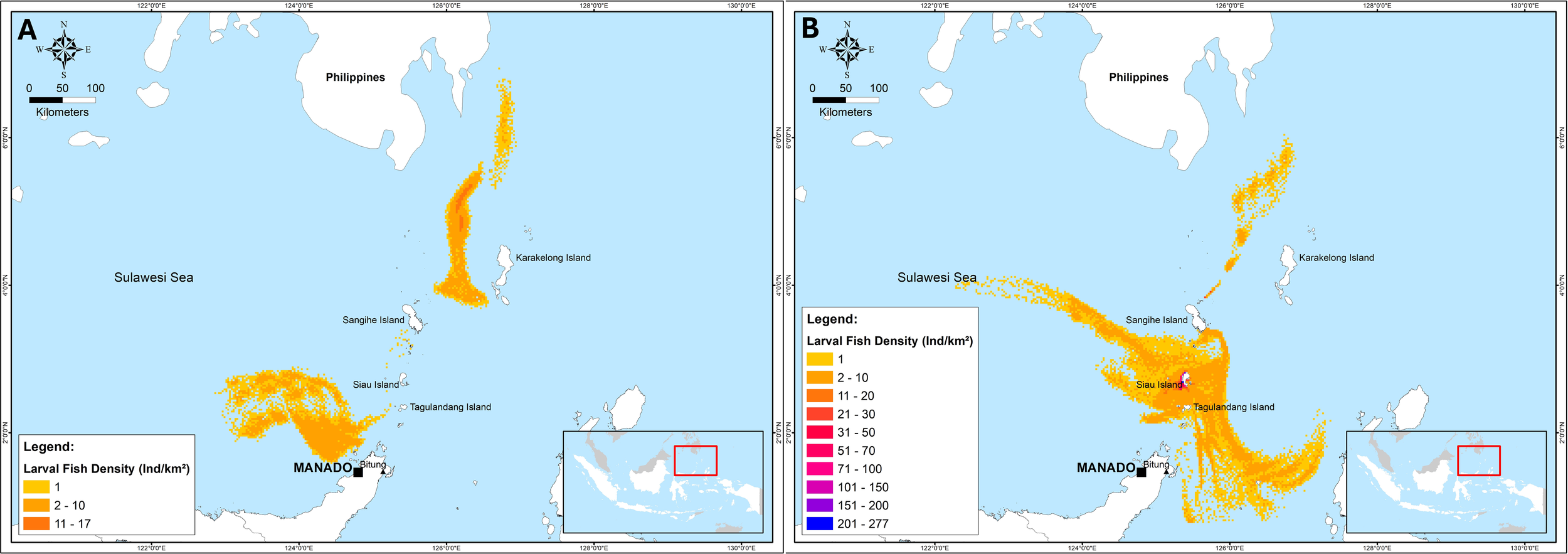
Spawning (A) and nursery (B) areas of the Decapterus macarellus in the Sulawesi Sea based on the trajectory model.
4 Discussion
The identification of spawning and nursery areas of D. macarellus in the Sulawesi Sea was conducted by analyzing the abundance and distribution of the species’ larvae. By utilizing the MOHID water modeling tool, spawning areas were identified through backward trajectory analysis based on the age and location of the larvae. Conversely, forward trajectory analysis was employed to identify nursery ground areas. The findings from this study provide a significant contribution for the siting of pelagic marine protected area (MPA) in the Sulawesi Sea, including potential spatial and temporal management of D. macarellus stocks in the area.
The northern and western sides of Sangihe and Siau Islands are identified as the spawning areas of the D. macarellus in the Sulawesi Sea. According to the age of larvae, we identified the spawning timing of D. macarellus was from the days 8 to 13 days before the fish larval sampling period. This finding validates the findings observed by Retnoningtyas et al. (2024) where mature gonads of the species were also found in these areas. A very high percentage of mature D. macarellus individuals (97-100%) was observed on the northern and western sides of Sangihe and Siau Islands (Retnoningtyas et al., 2024). Additionally, we observed larger areas of spawning at the western side of Sangihe and Siau Islands, which are beyond areas that have been identified by Retnoningtyas et al. (2024). Our study also enriched the methodology used to estimate the spawning areas of the species, using ichthyoplankton research and trajectory model.
The nursery grounds of the D. macarellus in the Sulawesi Sea were observed from the southern side of the Sangihe Islands to the Maluku Sea. These areas are characterized with the high abundance of zooplankton which is the main diet of D. macarellus juveniles. The nursery sites were identified from day 15 onwards post fish larval sampling periods. The correlation between zooplankton abundance and nursery areas highlights the importance of these regions for the early development of the species. Additionally, a high abundance of D. macarellus juveniles was observed at about 90% in lift net catches around these areas (Pers. Observ.). This finding further confirms the importance of these areas as nursery grounds.
Oceanographic condition plays a significant role in the spawning, larval dispersal, and nursery areas of the pelagic fishes. Optimal sea temperatures influence gametogenesis in pelagic fishes (Pankhurst and van der Kraak, 1997). For instance, unfavorable sea temperatures for mature pelagic fishes can indirectly reduce energy investment in growth and reproduction (Jobling et al., 1993). The distribution of seawater temperature strongly contributes to spawning areas of pelagic fishes and is greatly influenced by water mass movement (Steffensmeier et al., 2024). Water mass movement affects other features such as chlorophyll-a and salinity, which impact food availability for juvenile pelagic fish (Eisner et al., 2013). The location of pelagic fish nursery areas can then be identified through water mass movement patterns. It is essential to develop precise water mass movement models that accurately represent field conditions.
Although this research successfully identified key spawning and nursery areas of D. macarellus during the peak reproductive period in September, potential seasonal and interannual variability in habitat use remains unexplored. Future studies incorporating multi-temporal sampling across different reproductive seasons are necessary to assess whether these habitats shift in response to environmental fluctuations. Such insights are critical for informing the spatial and temporal design of marine protected areas (MPAs) to ensure effective protection of essential habitats throughout the species’ life cycle. Moreover, the nursery areas were estimated only for the early juvenile stages, which can last for a specific number of days. The study did not cover the nursery areas for later juvenile stages, which are beyond the research target. This limitation highlights the need for further research to identify and protect nursery areas for all developmental stages of the D. macarellus. Understanding the full range of nursery habitats is essential for comprehensive conservation efforts and for ensuring the long-term sustainability of the species.
The implications of this research are important for the siting of MPA for pelagic fisheries in the Sulawesi Sea, Indonesia. The D. macarellus is the most important fishery resource in Indonesia as this species has becomes the highest production among other fish commodities (MMAF, 2025; Baso et al., 2021), including in the Sulawesi Sea. Ecologically, the scads fish are crucial forage species and targets of major fisheries, acting as a critical link between plankton and higher trophic levels, and supporting predators and fisheries through direct and indirect pathways (Ruzicka et al., 2024). Significant demand for food has been shown for this species which could lead to overfishing if the fishery is not sustainably managed. By identifying the critical spawning and nursery areas of the D. macarellus, the study provides valuable information that can be used to design MPA and their networks that effectively protect these habitats. This approach can help prevent overfishing, support the recovery of fish populations, and ensure the sustainability of the pelagic fisheries in the region. The findings underscore the importance of integrating scientific research into sustainable fisheries management and conservation planning.
The identification of D. macarellus spawning and nursery grounds in this study provided critical ecological evidence that informed the scientific basis for designating the Sulawesi Sea pelagic fisheries MPA in 2024. However, the final MPA boundaries and management measures were determined through a comprehensive, interdisciplinary process that integrated: (1) socioeconomic assessments of fishery dependence and livelihood impacts, (2) governance analyses of enforcement capacity and legal frameworks, and (3) supplementary ecological data on habitat connectivity and ecosystem services. This multidimensional approach ensured the MPA design balanced conservation objectives with sustainable fisheries management. Moreover, Although MPAs represent an effective mechanism for safeguarding critical habitats, their conservation efficacy can be enhanced through complementary seasonal fishery closures aligned with peak spawning periods of the D. macarellus (January, March, May, and September). This integrated management approach addresses urgent exploitation threats while maintaining ecosystem functionality.
MPA for pelagic fisheries is relatively new in Indonesia, necessitating research that integrates fish larvae studies and oceanographic features to identify unique and critical aspects of pelagic fisheries for conservation purposes. This research represents an innovative approach by examining fish larvae and their movements to identify pelagic spawning and nursery areas, which can be recommended for conservation. Furthermore, this method can potentially be replicated in other locations in Indonesia, considering Indonesia’s MPA vision 30x45, where the Government of Indonesia is committed to protecting 30% of its territorial waters (approximately 97.5 million hectares) by 2045 (MMAF, 2024).
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the National Research and Innovation Agency of the Republic of Indonesia. All procedures were conducted in compliance with national regulations and institutional guidelines.
Author contributions
AH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. BW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. AP: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. AT: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. FA: Conceptualization, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. CS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PN: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. RP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. TK: Conceptualization, Funding acquisition, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. NA: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MN: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. SA: Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. HR: Conceptualization, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. N: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. RD: Data curation, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. YH: Conceptualization, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. IY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the KfW Bankengruppe (Grant No. 18_IV_073_IDN_K_Meeresund Küstenschutz). KfW Bankengruppe was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We thank the Ministry of Marine Affairs and Fisheries and the Wildlife Conservation Society Indonesia Program for facilitating the fieldwork. We thank the Provincial Marine and Fisheries Agency of North Sulawesi for their support in data collection. This study has been conducted using E.U. Copernicus Marine Service Information; https://doi.org/10.48670/moi-00016 and https://doi.org/10.48670/moi-00021.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this manuscript, the author(s) used Copilot and DeepSeek to summarize the original abstract. During the review process, the author(s) also used Copilot and DeepSeek for checking English grammar and smoothing some languages in the text. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the published article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ajub P. J. Bertha. M. J. A. Stephan T. J. M. Yuliana N. Hans P. J. (2023). Biological aspects of roundscads (Decapterus spp.) inhabiting the waters of Southeast Maluku, Eastern Indonesia. Fish Aquat Sci.26, 224–233. doi: 10.47853/FAS.2023.e19
2
Ambo-Rappe R. Moore A. M. (2019). “Sulawesi seas, Indonesia,” in World Seas: Environmental Evaluation, The Indian Ocean to the Pacific, 2nd Ed, vol. II . Ed. SheppardC. (Elsevier, United Kingdom), 559–581.
3
Baso A. Najamuddin Firman Hasani M. C. Kasri (2021). Economic analysis of shortfin scads fish (Decapterus spp.) business using purse seine in Bone Regency, Indonesia. IOP Conf. Series: Earth Environ. Sci.860, 12052. doi: 10.1088/1755-1315/860/1/012052
4
Campos W. L. Paraboles L. C. Gulayan S. J. Piloton R. D. R. Handugan E. R. (2022). Establishing a marine protected area network in the Western Danajon Bank Bohol, Central Visayas, Philippines. 黒潮圏科学= Kuroshio Sci.16, 9–14.
5
Curnick D. J. Collen B. Koldewey H. J. Jones K. E. Kemp K. M. Ferretti F. (2020). Interaction between a large marine protected area, pelagic tuna and associated fisheries. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00318
6
da Cruz Delgado K. Osemwegie I. Medina A. D. Nascimento da Luz A. Kubik Z. Kouamelan E. P. (2024). Ex-post evaluation of fishery management policies on wild fisheries production in northern Cabo Verde: An example of mackerel scad (Decapterus macarellus, Carangidae). J. Fish Biol.105, 1212–1226. doi: 10.1111/jfb.15861
7
Eisner L. Hillgruber N. Martinson E. Maselko J. (2013). Pelagic fish and zooplankton species assemblages in relation to water mass characteristics in the northern Bering and southeast Chukchi seas. Polar Biol.36, 87–113. doi: 10.1007/s00300-012-1241-0
8
Erisman B. Heyman W. Kobara S. Ezer T. Pittman S. Aburto-Oropeza O. et al . (2017). Fish spawning aggregations: where well-placed management actions can yield big benefits for fisheries and conservation. Fish Fisheries18, 128–144. doi: 10.1111/faf.12132
9
Fréon P. Cury P. Shannon L. Roy C. (2005). Sustainable exploitation of small pelagic fish stocks challenged by environmental and ecosystem changes: a review. Bull. Mar. Sci.76, 385–462.
10
González-Quirós R. Cabal J. Álvarez-Marqués F. Isla A. (2003). Ichthyoplankton distribution and plankton production related to the shelf break front at the Avilés Canyon. ICES J. Mar. Sci.60, 198–210. doi: 10.1016/S1054-3139(03)00009-2
11
Hou G. Wang J. Chen Z. Zhou J. Huang W. Zhang H. (2020). Molecular and morphological identification and seasonal distribution of eggs of four Decapterus fish species in the Northern South China Sea: a key to conservation of spawning ground. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.590564
12
Jobling M. Baardvik B. M. Christiansen J. S. Jørgensen E. H. (1993). The effects of prolonged exercise training on growth performance and production parameters in fish. Aquaculture Int.1, 95–111. doi: 10.1007/BF00692614
13
Jones C. (1986). Determining age of larval fish with the otolith increment technique. U.S. Fishery Bull.84, 91–103.
14
Kendall A. W. Jr. Ahlstrom E. H. Moser H. G. (1984). Early life history stages of fishes and their characters. Special Publ. Am. Soc. Ichthyologists Herpetologists1, 11–22.
15
Leis J. M. Carson-Ewart B. M. (2000). The Larvae of Indo-Pacific Coastal Fishes: An Identification Guide to Marine Fish Larvae (Fauna Malesiana Handbook 2. E.J.) Leiden: Brill.
16
Le Mézo P. Guiet J. Scherrer K. Bianchi D. Galbraith E. (2022). Global nutrient cycling by commercially targeted marine fish. Biogeosciences19, 2537–2555. doi: 10.5194/bg-19-2537-2022
17
MMAF (Ministry of Marine Affairs and Fisheries) . (2016). Marine and fisheries resource potential in Indonesian fisheries management area716. Jakarta: Amafrad Press, 190 pp. [in Bahasa Indonesia]
18
MMAF (Ministry of Marine Affairs and Fisheries) (2024). Spatial planning for the expansion of conservation area coverage to achieve the 30x45 conservation vision. Technical Report. Directorate General of Marine Spatial Management, Ministry of Marine Affairs and Fisheries. 64 pp. [in Bahasa Indonesia]
19
MMAF (Ministry of Marine Affairs and Fisheries) (2025). Data statistik (Jakarta: Kementerian Kelautan dan Perikanan). Available online at: https://portaldata.kkp.go.id/portals/data-statistik/layer1.
20
MOHID Water Modelling System (2024). Available online at: http://www.mohid.com/pages/models/mohidwater/mohid_water_quality.shtml(Accessed July 28, 2024).
21
Munandar A. Simanjuntak C. P. H. Taryono Domdoni T. A. Lisamy S. E. A. Nurfaiqah S. et al . (2024). Reproductive biology of the mackerel scad Decapterus macarellus (Cuvier 1833) from the southern waters of western Java, Indonesia. Bio Web Conferences112, 4002. doi: 10.1051/bioconf/202411204002
22
Okiyama M. (2014). An Atlas of Early Stage Fishes in Japan. 2nd ed (Tokyo, Japan: Tokai University Press).
23
Pankhurst N. W. van der Kraak G. (1997). “Effects of stress on reproduction and growth of fish,” in Fish Stress and Health in Aquaculture. Eds. IwamaG. K.PickeringA. D.SumpterJ. P.SchreckC. B. (Cambridge University Press, Cambridge), 73–93.
24
Pauly D. Pullin R. S. V. (1988). Hatching time in spherical, pelagic, marine fish eggs in response to temperature and egg size. Environ. Biol. Fishes22, 261–271. doi: 10.1007/BF00004892
25
Peck M. A. Reglero P. Takahashi M. Catalán I. A. (2013). Life cycle ecophysiology of small pelagic fish and climate-driven changes in populations. Prog. Oceanography116, 220–245. doi: 10.1016/j.pocean.2013.05.012
26
Retnoningtyas H. Agustina S. Natsir M. Ningtias P. Hakim A. Dhani A. K. et al . (2024). Reproductive biology of the mackerel scad, Decapterus macarellus (Cuvier 1833), in the Sulawesi Sea, Indonesia. Regional Stud. Mar. Sci.69, 103300. doi: 10.1016/j.rsma.2024.103300
27
Retnoningtyas H. Yulianto I. Wiryawan B. Kleinertz S. Palm H. W. (2023). Stock discrimination of mackerel scad Decapterus macarellus (Cuvier 1833) in the eastern Indonesia based on metazoan fish parasite composition. Regional Stud. Mar. Sci.61, 102840. doi: 10.1016/j.rsma.2023.102840
28
Ruzicka J. Chiaverano L. Coll M. Garrido S. Tam J. Murase H. et al . (2024). The role of small pelagic fish in diverse ecosystems: knowledge gleaned from food-web models. Mar. Ecol. Prog. Ser.741, 7–27. doi: 10.3354/meps14513
29
Sadovy de Mitcheson Y. (2016). Mainstreaming fish spawning aggregations into fishery management calls for a precautionary approach. BioScience66, 295–306. doi: 10.1093/biosci/biw013
30
Shen Q. Zhang P. Yu W. Xiong P. Cai Y. Li J. et al . (2025). Impact of climate change on the habitat distribution of Decapterus macarellus in the South China Sea. J. Mar. Sci. Eng.13, 156. doi: 10.3390/jmse13010156
31
Sheppard C. R. C. Ateweberhan M. Bowen B. W. Carr P. Chen C. A. Clubbe C. et al . (2012). Reefs and islands of the Chagos Archipelago, Indian Ocean: why it is the world’s largest no-take marine protected area. Aquat. Conservation: Mar. Freshw. Ecosyst.22, 232–261. doi: 10.1002/aqc.1248
32
Simanjuntak C. P. H. (2016). Early life history of the endemic engraulid, Coilia nasus (Japan: Ariake Bay. Ph.D. Thesis, Kochi University).
33
Simanjuntak C. P. H. Kinoshita I. Fujita S. Takeuchi K. (2015). Reproduction of the endemic engraulid, Coilia nasus, in freshwaters inside a reclamation dike of Ariake Bay, western Japan. Ichthyological Res.62, 374–378. doi: 10.1007/s10228-014-0448-1
34
Simanjuntak C. P. H. Noviana Putri A. K. Rahardjo M. F. Djumanto Syafei L. S. et al . (2020). Species composition and abundance of small fishes in seagrass beds of Karang Congkak Island, Kepulauan Seribu National Park, Indonesia. IOP Conf. Series: Earth Environ. Sci.404, 012063. doi: 10.1088/1755-1315/404/1/012063
35
Simbolon D. Nababan B. La Elson (2019). Fish characteristics and distribution of potential fishing zones in the malacca strait using hydroacoustic assessment during the southwest monsoon season. Malaysian Appl. Biol.48, 63–75.
36
Smith-Vaniz W. F. (1995). “Carangidae. Jureles, pámpanos, cojinúas, zapateros, cocineros, casabes, macarelas, chicharros, jorobados, medregales, pez pilota,” in Guia FAO para Identification de Especies para lo Fines de la Pesca, Pacifico Centro-Oriental. Eds. FischerW.KruppF.SchneiderW.SommerC.CarpenterK. E.NiemV. (FAO, Rome), 940–986. Available at: http://www.fao.org/docrep/010/v6250s/v6250s00.htm(Accessed August 18, 2024).
37
Soemodinoto A. Rusandi A. Hakim A. (2021). Tinjauan orientasi efektivitas rencana pengelolaan program kawasan konservasi kementerian kelautan dan perikanan. Bappenas Working Papers.4, 106–138. doi: 10.47266/bwp.v4i1.81
38
Steffensmeier Z. D. Mayes K. B. Perkin J. S. (2024). Linking short-term movement rate of pelagic-broadcast spawning fishes to river fragment length and conservation status. Biol. Conserv.293, 110585. doi: 10.1016/j.biocon.2024.110585
39
Ward R. D. Zemlak T. S. Innes B. H. Last P. R. Hebert P. D. N. (2005). DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B: Biol. Sci.360, 1847–1857. doi: 10.1098/rstb.2005.1716
40
World Bank and Ministry of Marine Affairs and Fisheries (2024). Towards Higher Performing Fisheries: Options for Sustainable and Productive Fisheries in Indonesia (Jakarta, Indonesia: The World Bank).
41
Zhang K. Li M. Li J. Sun M. Xu Y. Cai Y. et al . (2022). Climate-induced small pelagic fish blooms in an overexploited marine ecosystem of the South China Sea. Ecol. Indic.145, 109598. doi: 10.1016/j.ecolind.2022.109598
Summary
Keywords
ichthyoplankton, larval trajectory model, mackerel scads, Decapterus macarellus , pelagic fisheries MPA, Sulawesi Sea
Citation
Hakim A, Wiryawan B, Purbayanto A, Taurusman AA, Agung F, Simanjuntak CPH, Ningtias P, Prasetia R, Kartawijaya T, Andayani N, Natsir M, Agustina S, Retnoningtyas H, Nabil, Darmawan R, Herdiana Y and Yulianto I (2025) Spawning and nursery areas of Decapterus macarellus: siting pelagic fisheries marine protected area (MPA) in the Sulawesi Sea, Indonesia. Front. Mar. Sci. 12:1606963. doi: 10.3389/fmars.2025.1606963
Received
06 April 2025
Accepted
16 June 2025
Published
03 July 2025
Volume
12 - 2025
Edited by
Çetin Keskin, Istanbul University, Türkiye
Reviewed by
Shengjie Ren, Queensland University of Technology, Australia
Sinan Mavruk, Çukurova University, Türkiye
Updates
Copyright
© 2025 Hakim, Wiryawan, Purbayanto, Taurusman, Agung, Simanjuntak, Ningtias, Prasetia, Kartawijaya, Andayani, Natsir, Agustina, Retnoningtyas, Nabil, Darmawan, Herdiana and Yulianto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Budy Wiryawan, budywi@apps.ipb.ac.id
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.