Abstract
Brominated flame retardants (BFRs) represent the most widely produced and utilized organic flame retardants globally. Compared to terrestrial and freshwater organisms, research on the marine ecotoxicity of BFRs has lagged behind, with no comprehensive review currently synthesizing these studies. Internationally, BFRs have been subjected to regulatory restrictions due to their demonstrated characteristics as persistent organic pollutants. Nevertheless, significant regulatory gaps persist in current BFRs governance frameworks. Addressing this knowledge gap, this paper briefly reviews the distribution of BFRs in the marine environment, while comprehensively reviewing and comparing their toxic effects on marine organisms and summarize toxic mechanisms. Meanwhile, the paper systematically examines global regulatory policies governing BFRs across various nations and proposes recommendations for enhanced regulatory oversight and legislative improvements. Currently, the studies on the marine biological toxicity of three traditional BFRs, namely polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD), and tetrabromobisphenol A, are relatively comprehensive. These BFRs can exert various toxic effects on planktonic, benthic, and nektonic organisms, mainly including growth and development toxicity, reproductive toxicity, immunotoxicity, and neurotoxicity. However, the toxicity studies on novel BFRs, such as decabromodiphenyl ethane, are scarce and urgently need to be initiated. Moreover, researches on the marine biological toxicity mechanisms of BFRs are relatively simplistic, lacking in the characteristics of different BFRs and adverse outcome pathways starting from the molecular level. Within existing global regulatory frameworks, PBDEs, HBCD, and hexabromobiphenyl have been comprehensively prohibited and phased out. However, environmental risk assessments for alternative BFRs remain ongoing, with corresponding legislative actions lagging behind scientific findings.
1 Introduction
Brominated flame retardants (BFRs) are the most-produced and most-used organic flame retardants worldwide, accounting for approximately 21% of the total production of flame retardants (Sharkey et al., 2020). These chemicals are widely used in products requiring flame retardants, such as clothing, electronic products, furniture, motor vehicles, and decoration and building materials, and constitute 5–30% of the products by weight (Rani et al., 2022). Studies have reported that approximately 70% of the flame retardants used in electrical and electronic products worldwide are BFRs (Feiteiro et al., 2021). With developments in the chemical industry, BFRs can be divided into two types: traditional and novel. Traditional BFRs include polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD), and tetrabromobisphenol A (TBBPA) (Xiong et al., 2019). Due to the high ecological risk and human health hazards exhibited by traditional BFRs, a series of novel BFRs (NBFRs) as substitutes have been developed and entered into use. NBFR substitutes for PBDEs include decabromodiphenyl ethane (DBDPE) and bis(2,4,6-tribromophenoxy) ethane (BTBPE), 2,3,4,5,6-pentabromoethylbenzene (PBEB), hexabromobiphenyl (HBB), and 2,3,4,5,6-pentabromotoluene (PBT); the main substitute for HBCD is DBDPE; and NBFR substitute for TBBPA include 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) tetrabromophtalate (TBPH) (Xiong et al., 2019).
If an organic chemical substance can be closely combined with a product and never escape during the production, use, and end-of-life recycling processes of the product, it will not cause potential human health and ecological risks. However, among the most-used BFRs, only TBBPA is chemically reacted with certain materials to form chemical bonds (Liu et al., 2016). When TBBPA or other BFRs are not chemically bonded, they constitute additives that are not tightly combined with the product. Therefore, most BFRs are prone to escape into the environment (Liu et al., 2016). The ocean is a global sink for pollutants. BFRs can enter the marine environment through various channels, including evaporation and escape into the atmosphere followed by sedimentation into the ocean, overflow from the production process and escape during product end of life into the ocean through surface runoff, and the illegal discarding of e-waste and plastic waste directly into the marine environment (Turner, 2022). Due to their high hydrophobicity, BFRs in the marine environment are mainly distributed in sediments rather than dissolved in seawater (Wang et al., 2023a). Research data from the past five years have shown that the BFRs with the highest levels in the marine environment are mainly PBDEs and TBBPA, two traditional BFRs, and DBDPE, an NBFR (Table 1). The maximum content of PBDEs in the sediments of the Bohai Estuary and Xiamen Bay in China are as high as 238 and 276 ng/g, respectively (Liu et al., 2021, 2019), and the maximum content of TBBPA in the coastal area of Guangdong, China, was reported to be 80 ng/g (Chen et al., 2024). The highest concentrations of DBDPE in the highly industrialized Ulsan and Onsan Bays in South Korea and the Pearl River Delta in China were 81.6 and 58.2 ng/g, respectively (Feng et al., 2021; Lee et al., 2020). These data show that marine pollution by traditional BFRs still occurs and that the residues of NBFRs represented by DBDPE are rising, with a tendency to exceed the level of traditional BFRs.
Table 1
| Matrix/Unit | Region | PBDEs | HBCD | TBBPA | DBDPE | BTBPE | PBEB | HBB | PBT | TBB | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seawater (ng/L) |
Ulsan and Onsan Bays, Korea | n.d.–25.7 | n.d.–0.21 | 0.0003–0.32 | Lee et al., 2020 | ||||||
| Bohai, China | n.d.–0.2453 | n.d.–0.578 | n.d.–0.0005 | n.d.–0.01733 | n.d.–0.00197 | n.d.–0.00196 | Liu et al., 2020a | ||||
| Bohai, China | n.d.– 0.089 | n.d.–1.45 | n.d. – 0.052 (sum of BTBPE, PBEB, HBB, and PBT) | Zhen et al., 2021 | |||||||
| Pearl River Delta, China | n.d.–4.28 | n.d.–7.04 | n.d. | n.d.–0.59 | n.d. | n.d. | Feng et al., 2021 | ||||
| Jiaozhou Bay, China | n.d.–7.93 | n.d.–0.31 | Fu et al., 2023 | ||||||||
| East China Sea, China | 0.25–25 | Xie et al., 2022 | |||||||||
| Yellow Sea and Bohai Sea, China | n.d.–0.46 | Gong et al., 2021 | |||||||||
| Sediment (ng/g dw) | Ulsan and Onsan Bays, Korea | 0.12–63.5 | n.d.–81.6 | n.d.–86.9 | n.d.–13.7 | n.d.–62.3 | Lee et al., 2020 | ||||
| Pearl River Estuary and Daya Bay, China | 0.29–43.4 | 0.14–13.0 | n.d.–0.27 | 0.0007–0.06 | n.d.–0.38 | n.d.–0.02 | Hu et al., 2022 | ||||
| Bohai, China | n.d.–17.7 | n.d. – 11.2 | n.d. – 0.23 (sum of BTBPE, PBEB, HBB, and PBT) | Zhen et al., 2021 | |||||||
| 36 rivers estuaries of Bohai Sea and North Yellow Sea, China | 0.0121–238 | n.d.–46.4 | n.d.–0.0228 | n.d.–0.0207 | n.d.–0.0472 | n.d.–0.0345 | n.d.–0.0073 | Liu et al., 2021 | |||
| Yellow Sea and East China Sea, China | 0.0003–0.924 | n.d.– 9.46 | n.d.–0.293 (sum of BTBPE, PBEB, HBB, PBT and TBB) | Li et al., 2019 | |||||||
| 14 Estuaries, South China. | 0.39–81.2 | 0.18–49.9 | n.d.–0.62 | Xie et al., 2021 | |||||||
| Pearl River Delta, China | n.d.–21.55 | 0.74–58.22 | n.d. | n.d.–0.13 | n.d. | n.d. | Feng et al., 2021 | ||||
| Ebro Delta, Spain | n.d.–4.7 | Gil-Solsona et al., 2022 | |||||||||
| East China Sea, China | n.d.–0.27 | Xie et al., 2022 | |||||||||
| Sanmen Bay and Xiamen Bay, China | 2.2–276 | Liu et al., 2019 | |||||||||
| Coastal areas of Guangdong, China | n.d.–18 | n.d.–80 | Chen et al., 2024 | ||||||||
| 24 Fishing ports along the South China coast, China | 1.06–14.1 | 0.02–21.5 | Pan et al., 2022 | ||||||||
| Jiaozhou Bay, China | n.d.–65.76 | n.d.–16.63 | Fu et al., 2023 | ||||||||
| Pearl River Estuary and South China Sea, China | n.d.–0.42 | n.d.–6.14 | Long et al., 2024 | ||||||||
BFRs content in marine environment reported in published papers over the past five years.
n.d., not detected.
BFRs are poorly soluble in seawater but have high lipid solubility. Therefore, BFRs that enter the ocean are likely to accumulate in marine organisms and be gradually amplified along the food chain (Li et al., 2024). Taking PBDEs as an example, the average measured PBDE content in the Bohai Sea, China, where industrial pollution is relatively severe, was 0.07 ng/L (Liu et al., 2020a). The mean PBDE content of zooplankton, invertebrates, and fish in this sea area were 1.1, 1.7, and 4.9 ng/g, respectively (Liu et al., 2021). The mean observed concentration of PBDEs in the body of killer whales, the top oceanic predator, was as high as 1,800 ng/g (Kratofil et al., 2020). BFRs that accumulate in organisms can produce toxic effects. Studies on humans, terrestrial animals, and model organisms have demonstrated that traditional BFRs or NBFRs have different degrees of reproductive, developmental, immune and neurotoxic, endocrine disruptive, and carcinogenic effects, and these studies have been summarized in multiple review papers (Dong et al., 2021; Feiteiro et al., 2021; Gouesse and Plante, 2022; Okeke et al., 2022; Sarkar et al., 2023; Shen et al., 2024; Wu et al., 2020; Xiong et al., 2019). However, to the best of our knowledge, there are few reviews on the toxicity of BFRs in marine organisms.
Given the frequent environmental detection of BFRs and their demonstrated toxicological effects, the international community and major economies have progressively established regulatory frameworks to control BFRs. The Stockholm Convention on Persistent Organic Pollutants (POPs), administered by the United Nations Environment Programme (UNEP), lists multiple BFRs as POPs, mandating signatory states to phase out their production and restrict usage (UNEP, 2017). Regional legislative frameworks, such as those enacted by the key economies in Europe, North America, and East Asian, further reinforce prohibition and restriction regimes targeting BFRs. Nevertheless, the current regulatory system for BFRs faces multifaceted challenges, including a lack of harmonized global restriction criteria, the absence of comprehensive life-cycle supervision, and regulatory gaps concerning NBFRs (Sharkey et al., 2020).
Based on the aforementioned issues, in this review, keywords such as BFRs, PBDEs, HBCD, TBBPA, NBFRs, DBDPE, BTBPE, PBEB, HBB, PBT, TBB, TBPH, marine, toxicity, regulation, and policy were used to retrieve relevant papers from the Web of Science, PubMed, Scopus and ScienceDirect database, aiming to (1) comprehensively summarize toxic effects and mechanism of BFRs on marine organisms; (2) compare the similarities and differences in BFRs toxicity; (3) review the global regulatory policies on BFRs; (4) identify research gaps and provide recommendations for BFRs toxicity studies on marine organisms; (5) provide recommendations for the regulation and legislation of BFRs.
2 Toxic effects of BFRs on marine organisms
2.1 PBDEs
PBDEs theoretically have 209 homologs due to their different numbers of bromine atoms and substitution positions, but commercial PBDEs are mainly based on tetrabromodiphenyl ether (BDE-47) and decabromodiphenyl ether (BDE-209) homologs (Abbasi et al., 2019). Therefore, studies on the toxic effects on marine organisms have focused mainly on BDE-47 and BDE-209. The toxicity of PBDEs on various marine organisms is the most comprehensively studied among all types of BFRs (Table 2). Marine phytoplankton constitute floating single-celled microalgae, which are primary producers in marine ecosystems. Studies have shown that PBDEs have certain toxicity to marine diatoms, green algae, and dinoflagellates. Three diatoms, Skeletonema costatum, Thalassiosira pseudonana, and Phaeodactylum tricornutum, the dinoflagellate Alexandrium minutum, and the green alga Dunaliella salina have been reported to be inhibited by BDE-47 in terms of population growth and photosynthesis but with different sensitivities. Significant population growth and photosynthesis inhibition were observed in T. pseudonana under BDE-47 exposure at 15 μg/L (Zhao et al., 2019b), while the other four microalgae were inhibited only in the concentration range of 200–800 μg/L BDE-47. Moreover, BDE-47 at 200–800 μg/L can also induce oxidative stress in S. costatum, P. tricornutum, A. minutum, and D. salina (Liu et al., 2020b; Zhang et al., 2022; Zhao et al., 2017). Zhao et al (2020, 2019b). reported that 25 μg/L BDE-47 induced T. pseudonana cell cycle arrest and cell apoptosis, and excessive reactive oxygen species (ROS) were the mediating factors. In addition, Zhao et al. (2019a) specifically focused on the effects of PBDEs on the movement of marine microalgae and found that exposure to three PBDEs, BDE-47, BDE-99, and BDE-153, changed the proportions and swimming speeds of movement cells in Platymonas subcordiformis and changed their swimming mode. The severity of the negative effects of the three PBDEs on the movement of P. subcordiformis were in the order BDE-47>BDE-99>BDE-153. The above studies indicated that the toxic effect of PBDEs on marine phytoplankton was mainly the inhibition of their population growth and photosynthesis.
Table 2
| Biological group | Species name | PBDEs | Exposure concentration | Toxic effect | References |
|---|---|---|---|---|---|
| Phytoplankton | Skeletonema costatum | BDE-47 | 50, 100, 200, 400, 600 μg/L | Population growth inhibition; photosynthesis inhibition; oxidative stress and oxidative damage | Zhang et al., 2022 |
| Thalassiosira pseudonana | BDE-47 | 5, 15, 25 μg/L | Population growth inhibition; photosynthesis inhibition; cell cycle arrest; cell apoptosis | Zhao et al., 2020, 2019b | |
| Phaeodactylum tricornutum | BDE-47 | 800, 4000 μg/L | Population growth inhibition; photosynthesis inhibition; oxidative stress; electron transport obstruction | Liu et al., 2020b | |
| Platymonas subcordiformis | BDE-47 BDE-99 BDE-153 |
0.5, 1, 2, 4, 8, 16, 32 μg/L | Reduced proportion of motor cells; reduced swimming speed and change in swimming pattern | Zhao et al., 2019a | |
|
Alexandrium minutum
Dunaliella salina |
BDE-47 | 100, 500, 1000 μg/L | Population growth inhibition; photosynthesis inhibition; oxidative stress; antioxidant inhibition | Zhao et al., 2017 | |
| Zooplankton | Brachionus Plicatilis | BDE-47 BDE-209 |
8, 80, 800,8000 μg/L | Population growth inhibition; movement inhibition; decreased oviposition and laying; decreased activities of antioxidant enzymes and detoxification enzymes; ROS overproduction; lipid peroxidation; DNA damage; mitochondrial damage; cell apoptosis | Sha et al., 2022, 2015a, 2015b; Jian et al., 2017; Wang et al., 2015, 2021a |
| B. Plicatilis | BDE-47 | 20, 100, 500 μg/L | Decreased amino acid pool levels; disorders of nucleotide synthesis and degradation; decreased expression of glutamine synthetase; increased xanthine oxidase activity; oxidative damage to DNA; activation of p53 signaling pathway | Cao et al., 2023 | |
| B. Plicatilis | BDE-47 | 31.25, 125, 500 μg/L | Food intake and digestive inhibition | Yang et al., 2021 | |
| Tigriopus japonicus | BDE-47 | 0.05, 60, 120 μg/L | Growth inhibition; oxidative stress; changes in the expression of detoxification-, antioxidation-, and apoptosis-related genes | Han et al., 2015 | |
| Brachionus koreanus | BDE-47 | 5, 10, 25, 50 μg/L | Population growth inhibition; shortened life cycle; decreased net reproductive rate; activation of antioxidant system; MAPK activation | Park et al., 2017 | |
| Benthic organisms | Ruditapes philippinarum | BDE-47 | 0.1, 1 μg/L | Decreased testosterone levels; increased expression of vitellogenin and spermatogenesis-related proteins | Liu et al., 2017 |
| R. philippinarum | BDE-47 | 6.25, 12.5, 25, 50, 100 μM In vitro exposure |
Increased blood cell mortality, decreased viability, decreased phagocytic activity, increased lysosomal membrane permeability, destruction of antioxidant system, oxidative stress, and decreased MAPK phosphorylation level | Zhou and Liu, 2022 | |
| Crepidula onyx | BDE-47 | 1, 10 μg/L Dietary exposure |
Reduced fertilization rate; delayed sexual maturity; embryonic development block | Po and Chiu, 2018 | |
| Mytilus edulis | BDE-47 | 0.1, 1, 10 μg/L | Increased blood cell mortality, decreased phagocytic activity, increased lysosomal membrane permeability, changes in antioxidant enzyme activity, oxidative stress, and MAPK activation; impaired immune function; digestive gland damage, obstruction of feeding and digestion; changes in energy supply patterns, changes from aerobic respiration to anaerobic respiration; filaments decrease; adhesion decreases | Gu et al., 2023; Jiang et al., 2017a, 2017b, 2021, 2023 | |
| Mytilus galloprovincialis | BDE-47 | 0.001, 0.01, 0.1 μg/L Dietary exposure |
Digestive gland injury, decreased expression of antioxidant, cell proliferation, drug resistance, decreased xenobiotic detoxification related genes | Messina et al., 2020 | |
| Chlamys farreri | BDE-209 | 0.01, 0.1 μg/L | Decreased phagocytic activity of hemocytes, DNA damage, damage to digestive gland cells | Xia et al., 2020 | |
| Mactra veneriformis | BDE-47 BDE-209 |
0.1, 1, 10 μg/L | Oxidative damage of digestive glands, reduced expression and activity of antioxidant enzymes | Dong et al., 2019; Zhu et al., 2019 | |
| Apostichopus japonicus | BDE-47 | 0.1, 1, 10 μg/L | Steroid hormone balance, nucleotide metabolism and energy metabolism disorders; changes in neurotransmitter levels; impaired neuroprotection | Ding et al., 2023 | |
| Nektonic organism | Oryzias melastigma | BDE-47 | 2500, 5000 μg/kg | Changes in neurotransmitter levels; amino acid metabolism disorders | Lei et al., 2017 |
| O. melastigma | BDE-47 | 0.65, 1.30 μg/g Dietary exposure |
Downregulation of sperm production-related proteins; upregulation of the expression of vitellin and lipoproteins | Fong et al., 2014 | |
| Psetta maxima | BDE-47 BDE-99 |
0.3-300 μg/L | Embryo death and malformation | Mhadhbi et al., 2012 | |
| Sparus aurata | BDE-209 | 0.25, 0.5, 0.75, 1, 2 µM In vitro exposure |
Oxidative stress in fibroblasts; changes in the expression of proteins related to cell cycle, proliferation, energy balance, and oxidative stress | Ruiz et al., 2019 | |
| Diplodus sargus | BDE-209 | 60000 µg/g Dietary exposure |
Lower awareness of a threat situation; increased activity; less time spent within the shoal; reversed lateralization | Dias et al., 2023 | |
| Stenella attenuate | BDE-47 BDE-100 BDE-209 |
0.1-1 μg/mL In vitro exposure |
Upregulation of inflammatory factors and prostaglandins in fibroblasts, oxidative stress, decrease in mitochondrial membrane potential, and mitochondrial damage | Rajput et al., 2021; Ying et al., 2020 | |
| Phoca vitulina | BDE-47 BDE-99 BDE-153 |
1.5, 3, 6, 12 μM In vitro exposure |
Decreased phagocytic activity of peripheral blood leukocytes, oxidative stress, and decreased thiol levels | Frouin et al., 2010 |
Toxic effects of PBDEs on marine organisms.
PBDEs can exert a variety of toxic effects on marine zooplankton rotifers. Multiple studies have reported that BDE-47 exposure inhibits the growth, reproductive ability, and exercise ability of Brachionus plicatilis and Brachionus koreanus, changes the activity of the antioxidant system, and induces oxidative stress. The inhibition of population growth and reproductive ability specifically manifests as a decrease in age-specific survival, the intrinsic rate of increase, the finite rate of increase, life expectancy, generation time, age-specific fecundity, and the net reproductive rate of the two rotifers (Park et al., 2017; Sha et al., 2015b; Wang et al., 2015). The effect on the antioxidant system in B. plicatilis specifically manifests as an increase in the activity of antioxidant enzymes such as glutathione reductase, glutathione peroxidase, superoxide dismutase, catalase, and peroxidase when exposed to lower BDE-47 concentrations (8 and 80 μg/L), whereas activity was inhibited at higher concentrations (800 and 8000 μg/L) (Jian et al., 2017; Sha et al., 2022; Wang et al., 2015). The activities of glutathione S-transferase and glutathione reductase in B. koreanus increased after exposure to BDE-47 at 10, 25, and 50 μg/L (Park et al., 2017). However, none of these changes in the antioxidant system were sufficient to offset BDE-47-induced oxidative stress. In addition, BDE-47 has been observed to inhibit food intake and digestion by B. plicatilis (Yang et al., 2021). Notably, BDE-209 was less toxic than BDE-47 against rotifers at the same concentration (Sha et al., 2022, 2015a, 2015b). Moreover, BDE-47 can also cause growth inhibition and oxidative stress in the copepod zooplankton Tigriopus japonicus (Han et al., 2015). Cao et al. (2023) and Wang et al. (2021a) studied the toxicity effects of PBDEs on marine zooplankton at the molecular level and investigated the related molecular mechanisms. BDE-47 induced disorders of nucleotide synthesis and degradation in B. plicatilis. The changes in the activities of glutamine synthase and xanthine oxidase led to the overproduction of ROS, which in turn caused oxidative DNA damage and activated the p53 signaling pathway to induce apoptosis (Cao et al., 2023). Moreover, excessive ROS damaged the mitochondria of the ovary and directly activated the mitochondrial pathway of cell apoptosis. This may be the mechanism of the reproductive toxicity of BDE-47 to rotifers (Wang et al., 2021a).
Studies on the toxicity of PBDEs to marine benthic organisms have focused mainly on benthic bivalve molluscs. Hemocytes are the main contributors to the immune function of bivalve molluscs, and PBDEs can have a negative effect on the hemocytes of bivalve molluscs, resulting in immunotoxicity. For example, BDE-47 causes increased mortality, a decreased proportion of granulosa cells, decreased phagocytic activity, increased lysosomal membrane permeability, and changes in the activity of the antioxidant system and oxidative stress in Ruditapes philippinarum and Mytilus edulis (Jiang et al., 2017a, 2017b; Zhou and Liu, 2022). BDE-209 caused reduced phagocytic activity and DNA damage in hemocytes of Chlamys farreri (Xia et al., 2020). Second, PBDEs can induce reproductive toxicity in bivalve molluscs. Liu et al. (2017) found that BDE-47 exposure at 0.1 and 1 μg/L led to decreased testosterone levels and increased expression of vitellogenin and spermatogenesis-related proteins in R. philippinarum. Po and Chiu (2018) reported that BDE-47 at 1 and 10 μg/L led to a reduced fertilization rate, delayed sexual maturation, and blocked embryonic development in Crepidula onyx. In addition, PBDEs affect the feeding and digestion functions of bivalve molluscs. BDE-47 can cause structural damage to the digestive glands of M. edulis, Mytilus galloprovincialis, and Mactra veneriformis and reduce digestive enzyme activity and the filter-feeding rate of M. edulis, along with a shift in the energy supply mode from aerobic respiration to anaerobic respiration (Dong et al., 2019; Jiang et al., 2021, 2023; Messina et al., 2020). BDE-209 can also cause gastrointestinal gland damage in C. farreri and M. veneriformis (Xia et al., 2020; Zhu et al., 2019). Gu et al. (2023) innovatively focused on the effects of PBDEs on the fixation ability of bivalve molluscs and found that M. edulis exposed to BDE-47 had fewer foot filaments and reduced adhesion. In addition to bivalve molluscs, PBDEs also have toxic effects on echinoderms. Ding et al. (2023) found that BDE-47 induced disorders of sterol hormone balance, nucleotide metabolism, and energy metabolism in the sea cucumber Apostichopus japonicus, affecting the levels of neurotransmitters and resulting in impaired neuroprotection. The above studies suggested that PBDEs have varying degrees of negative effects on the feeding, metabolism, reproduction, and immunity of marine benthic organisms.
Studies on PBDE toxicity in marine nekton have focused mainly on fish and mammals, organisms with well-developed organs and systems. Lei et al. (2017) and Fong et al. (2014) used metabolomics and proteomics techniques to investigate the toxic effects of BDE-47 on the marine ecotoxicology model organism Oryzias melastigma and found that BDE-47 induced metabolic disorders related to its neurotransmitters and amino acids and altered the expression of the sperm production proteins vitellin and lipoproteins. Mhadhbi et al. (2012) reported that both BDE-47 and BDE-99 had lethal and teratogenic effects on Psetta maxima embryos, but BDE-47 was more toxic than BDE-99 at the same concentration. Through dietary exposure experiments, Dias et al. (2023) found that BDE-209 at 60 ng/g induced behavioral changes in Diplodus sargus, including lower awareness of threat situations, increased activity, less time spent within the shoal, and reversed lateralization. Due to the large size of some marine fish and mammals and the limitation of species protection, in vitro experiments are used for studies of PBDE toxicity in these organisms. Ruiz et al. (2019) found that although in vitro exposure to 0.25–2 μM BDE-209 did not affect the viability of Sparus aurata fibroblasts, it induced oxidative stress and induced changes in the expression of proteins related to the cell cycle, proliferation, energy balance, and oxidative stress. In vitro studies of PBDE toxicity in two marine mammals have been reported. An in vitro toxicology study by Rajput et al. (2021) showed that BDE-47, BDE-100 and BDE-209 induced ROS overproduction in the fibroblasts of Stenella attenuata and further mediated a decrease in the mitochondrial membrane potential and damage to the mitochondrial structure but did not significantly affect cell viability. Among the three PBDEs, only BDE-47 and BDE-209 further upregulated the expression of proapoptotic genes and proteins in S. attenuate fibroblasts. Ying et al. (2020) investigated the toxicity of PBDEs on S. attenuate fibroblasts from the perspective of the immune system and demonstrated that BDE-47 and BDE-209 induce cellular inflammation through the activation of the prostaglandin pathway, thereby impairing the innate immunity of dolphins; however, BDE-100 did not exhibit this effect. These two studies suggested that BDE-100 was less toxic to S. attenuate fibroblasts than were BDE-47 and BDE-209. Additionally, Frouin et al. (2010) found that in vitro exposure to BDE-47, BDE-99, and BDE-153 led to a decrease in the phagocytic activity of peripheral blood leukocytes and decreases in oxidative stress and thiol levels in Phoca vitulina but did not induce cell apoptosis.
2.2 HBCD
HBCD have been banned and phased out worldwide, and few studies have investigated their marine toxicity (Table 3). Two types of marine zooplankton were reported to be sensitive to HBCD. Under exposure to HBCD at 50–600 μg/L, B. plicatilis exhibited toxic phenomena such as slower population growth, prolonged oviposition and larval hatching, reduced body length, shortened survival of individuals, ROS over production, oxidative stress, and cell apoptosis (Lu et al., 2024). After exposure to 8–800 μg/L HBCD in T. japonicus, growth delay and oxidative stress occurred, and the expression of apoptosis-related genes was activated (Hong et al., 2017; Shi et al., 2017). Since industrially produced HBCD are a mixture of three diastereomers (i.e., α-, β-, and γ-HBCD), Hong et al. (2017) examined the toxicity of each HBCD independently and showed that the three HBCD had similar toxicity in T. japonicus, but the ability of α- and β-HBCD to induce oxidative stress was greater than that of γ-HBCD. The toxicity of these HBCD to marine organisms is similar to the toxicity of PBDEs to the same species, but the required concentration of HBCD is generally higher than that of PBDEs.
Table 3
| Biological group | Species name | Exposure concentration | Toxic effect | References |
|---|---|---|---|---|
| Zooplankton | T. japonicus | 8, 30, 80, 300, 800 μg/L | Growth and developmental delay; activation of oxidative stress and apoptosis-related gene expression | Hong et al., 2017; Shi et al., 2017 |
| B. Plicatilis | 50, 100, 300, 500, 600 μg/L | Population growth inhibition; prolonged egg laying and larval hatching time; individual morphological changes with reduced body length; shortened survival time of individuals; ROS overproduction; oxidative stress; cell apoptosis | Lu et al., 2024 | |
| Benthic organisms | Macoma balthica | 100, 250 μg/L | Increased gill cell mortality, nuclear abnormality and micronucleus phenomenon, malfunction of ribosomal genes | Smolarz and Berger, 2009 |
| Venerupis philippinarum | 0.086, 0.86, 8.6 μg/L | Increased antioxidant enzyme activities, oxidative stress and DNA damage in gills and digestive glands | Zhang et al., 2014 | |
| Psammechinus miliaris | 0.009, 0.025, 0.05, 0.1 μM | Abnormal larval morphology and developmental delay | Anselmo et al., 2011 | |
| Macrophthalmus japonicus | 1, 10, 100 μg/L | Increased expression of catalase and p53 genes in the gills and hepatopancreas | Park and Kwak, 2022 | |
| Nektonic organism | Litopenaeus vannamei | 2.1 μg/L | Hepatopancreas tissue damage, changes in antioxidant enzyme levels/activity, oxidative damage; increased liver lesion markers; metabolic disorders; destroyed detoxification function | Yu et al., 2023a |
| O. melastigma | 5, 20, 50, 200 μg/L | Increased heartbeat and increased sinus-arterial bulb distance in the embryo; embryo malformation, DNA oxidative damage, cell apoptosis, inhibition of nucleic acid and protein synthesis | Hong et al., 2014, 2015 |
Toxic effects of HBCD on marine organisms.
For marine benthic organisms, Smolarz and Berger (2009) were the first to show that exposure to HBCD at 100 and 250 μg/L led to increased mortality of gill cells and micronuclei in the nuclei with malfunction of ribosomal genes of the bivalve mollusk Macoma balthica. Zhang et al. (2014) demonstrated that lower doses of HBCD (0.086–8.6 μg/L) also increased antioxidant enzyme activities, oxidative stress, and DNA damage in the gills and digestive glands of Venerupis philippinarum. In addition, Anselmo et al. (2011) showed that HBCD produced teratogenicity and delayed the development of sea urchin Psammechinus miliaris larvae. Park and Kwak (2022) recently showed that HBCD induced an increase in the expression levels of catalase and p53 in the gills and hepatopancreas of the crab Macrophthalmus japonicus.
For marine nektonic organism, Hong et al (2014, 2015). found that HBCD produced toxicity in O. melastigma embryos, which manifested as a rapid heartbeat and increased distance between venous and arterial bulbs in the embryos when exposed to HBCD at 5–200 μg/L, induced DNA oxidative damage, and cell apoptosis in the embryonic myocardium. Molecular studies showed that HBCD inhibited nucleic acid and protein synthesis in O. melastigma embryos. Together, these negative effects lead to embryonic malformations and developmental toxicity in O. melastigma, especially in the cardiovascular system. In addition, Yu et al. (2023a) investigated the toxicity of HBCD to the hepatopancreas using the shrimp Litopenaeus vannamei as the test organism and found that exposure to 2.1 μg/L HBCD caused oxidative damage and structural damage in the hepatopancreas of L. vannamei and that the levels of pathological markers increased, which in turn led to metabolic disorders and destruction of the detoxification function of the hepatopancreas. In general, the toxic effects of HBCD on marine organisms are similar to those of PBDEs, including the induction of oxidative stress, tissue structure damage, and metabolic disorders, resulting in growth inhibition, reproductive inhibition, and organ damage.
2.3 TBBPA
TBBPA is the only traditional BFR still in extensive use, and studies on its toxicity to marine organisms are continuing to be conducted. To date, many reports on the toxicity of TBBPA to marine benthic organisms have been published, but there are few studies on other organisms (Table 4). Hu et al (2015a, 2015b). demonstrated that TBBPA at 400 μg/L produced toxicity to both the digestive glands and gills of the clam C. farreri, including the induction of stress responses in the digestive glands and the inhibition of the expression of genes involved in thyroxine biosynthesis, while inducing the inhibition of the detoxification system, the activation of the antioxidant system, and oxidative stress in both the digestive glands and gills. Organisms of the genus Mytilus are widely used in TBBPA marine organism toxicity assessment. Canesi et al. (2005) were the first to show through in vitro exposure experiments that 1–25 μM TBBPA destabilized the lysosomal membrane of M. galloprovincialis and that the bactericidal activity of hemocytes, release of lysosomal enzymes, phagocytic activity, and extracellular superoxide levels all increased. This finding was essentially consistent with the toxic effects of PBDEs on mussel hemocytes. Ji et al. (2014) subsequently used proteomics approaches to identify changes in protein expression in the hepatopancreas of M. galloprovincialis exposed to 18.4 nM TBBPA. The results suggested that TBBPA induced apoptosis, oxidative stress, immune stress, and energy stress in hepatopancreas cells. Disruption of metabolism leads to disorders of hepatopancreatic cell development and lipid and protein metabolism. Moreover, they found that TBBPA also induced reproductive toxicity and destruction of muscle contraction in M. galloprovincialis. Recent studies have shown that TBBPA has reproductive toxicity and endocrine cardiotoxicity in mussels. Reproductive toxicity includes promoting gametogenesis, altering vertebrate sex hormones, promoting steroid sulfonation and hydrolysis of sulfate steroids, and disrupting steroidogenesis (Wang et al., 2021b, 2023b). Cardiac endocrine toxicity includes heart tissue hemocytes infiltration and myocardial fibrosis, bradyarrhythmia, and arrhythmia; decreased levels of cardiac neurotransmitters; increased levels of acetylcholine; increased acetylcholinesterase activity; imbalanced calcium homeostasis; limited energy supply; and oxidative stress (Yu et al., 2023b). In addition, a recent study by Copeto et al. (2024) suggested that TBBPA induced the activation of detoxification and antioxidant systems and oxidative stress in M. galloprovincialis individuals. In addition to bivalve molluscs, Anselmo et al. (2011) found that TBBPA at 150–1500 nM caused morphological abnormalities and developmental delays in sea urchin P. miliaris larvae; this author observed the same degree of toxicity for HBCD to P. militaris larvae.
Table 4
| Biological group | Species name | Exposure concentration | Toxic effect | References |
|---|---|---|---|---|
| Phytoplankton |
Pseudokirchneriella subcapitata
Nitzschia palea Chlamydomonas reinhardtii |
1.8, 4.8, 9.2, 12.9, 16.5 μM | Reduced growth rate; reduced cell viability | Debenest et al., 2010, 2011 |
| Zooplankton | Pseudodiaptomus inopinus | 0.18, 1.8, 18 μg/L | Delay of the developmental time from nauplius to copepodite; reduced successful rate of development; continuously decreased naupliar developmental rate in two successive generations | Gong et al., 2017 |
| Benthic organisms | C. farreri | 400 μg/L | Changes in gene expression related to digestive gland stress response, detoxification, antioxidation, and innate immunity; inhibition of the expression of genes involved in thyroxine biosynthesis; inhibition of detoxification system of gills and digestive glands, activation of antioxidant system, oxidative stress | Hu et al., 2015a, 2015b |
| P. miliaris | 0.15, 0.5, 0.1, 1.5 μM | Abnormal larval morphology and developmental delay | Anselmo et al., 2011 | |
| R. philippinarum | 62.5, 125, 250, 500, 1000 μg/L | Inhibition of shell growth; reduced filter-feeding rate; increased insulin and thyroid hormone levels | Jiang et al., 2019 | |
| M. galloprovincialis | 1, 10, 100 μg/L | Activation of detoxification system; activation of antioxidant system; oxidative stress | Copeto et al., 2024 | |
| M. galloprovincialis | 0.0184 μM | Hepatopancreatic cell apoptosis, oxidative and immune stress, disruption of energy metabolism, developmental processes, lipid and protein metabolic disorders; reproductive toxicity and destruction of muscle contraction | Ji et al., 2014 | |
| M. galloprovincialis | 0.6, 3, 15, 75, 375 μg/L | Promotion of gametogenesis; alteration in vertebrate sex hormones; promotion of steroid sulfonation and hydrolysis of sulfate steroids; disruption of steroidogenesis | Wang et al., 2021b, 2023b | |
| M. galloprovincialis | 1, 5, 25 μM In vitro exposure |
Lysosomal membrane instability of hemocytes, MAPK phosphorylation; increased blood cell bactericidal activity, lysosomal enzyme release, phagocytic activity, and extracellular superoxide levels | Canesi et al., 2005 | |
| Mytilus coruscus | 1 μg/L | Heart tissue hemocytes infiltration and myocardial fibrosis; bradyarrhythmia and arrhythmia; decreased heart neurotransmitter levels, increased acetylcholine levels, increased acetylcholinesterase activity, imbalance of calcium ion homeostasis, limited energy supply, oxidative stress | Yu et al., 2023b | |
| Nektonic organism | Gadus morhua | 0.001-10 μM In vitro exposure |
Reduced integrity of pituitary cell membranes; reduced metabolic activity; reduced gene expression of gonadotropin subunits and gonadotropin-releasing hormone receptors | von Krogh et al., 2019 |
| O. melastigma | 50, 200, 800 μg/L | Decreased nucleoside, amino acid, and lipid synthesis in the embryo, disruption of the TCA cycle, glycolysis, and lipid metabolism; embryonic developmental inhibition; increased embryonic dopamine levels, decreased inhibitory neurotransmitter levels, enhanced neural activity, lactic acid accumulation, and rapid heart rate | Ye et al., 2016 |
Toxic effects of TBBPA on marine organisms.
In terms of other marine taxa, Debenest et al (2010, 2011). found that TBBPA exposure at 1.8–16.5 μM reduced the growth rate and cell viability of three marine phytoplankton species, Pseudokirchneriella subcapitata, Nitzschia palea, and Chlamydomonas reinhardtii. Gong et al. (2017) found that 0.18–18 μg/L TBBPA delayed the growth of the marine zooplankton Pseudodiaptomus inopinus. The time for development from nauplii to copepods and the developmental success rate were reduced, whereas the developmental rate of nauplii decreased continuously for two consecutive generations. Ye et al. (2016) examined the effects of TBBPA on O. melastigma with respect to embryo toxicity and reported that, under exposure to 50–800 μg/L TBBPA, the synthesis of nucleosides, amino acids, and lipids in embryos was reduced, and the tricarboxylic acid (TCA) cycle, glycolysis, and lipid metabolism were disrupted, resulting in embryonic developmental delay. Moreover, endocrine toxicity in the embryo manifested as increased dopamine levels, decreased levels of inhibitory neurotransmitters, enhanced neural activity, lactic acid accumulation, and increased heart rate. Through an in vitro exposure study, von Krogh et al. (2019) observed that TBBPA at 1–10,000 nM reduced the integrity of the pituitary cell membrane, decreased metabolic activity, and decreased the expression of gonadotropin-related genes in Gadus morhua. These studies on the toxicity of TBBPA to marine benthic organisms are comprehensive: TBBPA exhibits growth and developmental toxicity and produces reproductive and endocrine toxicity in nektonic organism.
2.4 NBFRs
Unlike the understanding of the toxicity of various NBFRs to terrestrial and freshwater organisms, there are few reports on the toxicity of NBFRs to marine organisms.
Taking DBDPE, the novel brominated flame retardant (NBFR) with the most residues in the marine environment, as an example, only one paper has reported its reproductive endocrine disruption effects on the marine bivalve mollusk M. galloprovincialis. Specifically, the study revealed that exposure to 1–500 μg/L DBDPE promoted gametogenesis in mussels, suppressed the expression of cholesterol homeostasis- and transport-related genes, and disrupted nongenomic signaling pathways through dysregulation of associated genes (Wang et al., 2023c). The DBDPE-induced energy metabolism dysfunction in male mussels is considered the cause of the disturbance of the reproductive endocrine system (Wang et al., 2023c). No other reports on the toxic effects of NBFRs on marine organisms were found through searches using the keywords used in this paper.
3 Toxic mechanism of BFRs on marine organisms
Some studies on the toxicity mechanisms of BFRs on marine organisms have been conducted. Studies on the mechanisms are carried out at the tissue and cellular levels, and the results are mainly explained from a biochemical perspective. The existing research indicates that the induction of ROS overproduction, which leads to oxidative stress or oxidative damage, is a universal toxicity mechanism of BFRs on marine organisms (Cao et al., 2023; Lu et al., 2024). This mechanism specifically manifested as BFR exposure induces the overproduction of ROS and changes in the antioxidant systems of marine organisms (Zhang et al., 2022; Yu et al., 2023a). When the antioxidant system cannot remove excessive ROS, intracellular oxidative damage, including damage to DNA and the membrane structure, occurs (Jiang et al., 2017b; Zhu et al., 2019). This injury activates the expression of genes and proteins in signaling pathways such as the tumor suppressor protein p53 and mitogen-activated protein kinase (MAPK), thereby initiating cell apoptosis (Park et al., 2017; Park and Kwak, 2022; Wang et al., 2021a) (Figure 1). Excessive cell apoptosis or necrosis leads to destruction of the structure of biological tissues, thus affecting various life activities (Yu et al., 2023b). In addition, BFRs have been reported many times to induce metabolic disorders of carbohydrate, amino acids, lipids, and nucleosides in marine organisms (Ding et al., 2023; Lei et al., 2017) (Figure 1). These negative effects destroy the production of energy or other substances essential for life activities, resulting in toxic effects (Jiang et al., 2023). However, these mechanism studies lack initiating events at the molecular level to provide a complete evidence chain of the toxicity mechanisms of BFRs on marine organisms (adverse outcome pathway, AOP) and fail to meet the needs of marine ecological risk assessments for BFRs. For example, the specific mechanisms by which BFRs induce ROS overproduction and disrupt energy metabolism remain unclear. Pereira et al (2013, 2014). used a mouse mitochondrial model and found that lipophilic BDE-100 and BDE-154 can insert into the lipophilic gap between the inner and outer membranes of the mitochondria, interfere with the fluidity of the mitochondrial membrane, and lead to mitochondrial membrane permeabilization and mitochondrial membrane potential dissipation. These impacts in turn inhibits oxidative phosphorylation, resulting in reduced ATP production and ROS overproduction. However, whether other BFRs follow this mechanism and whether this mechanism is effective in marine organisms remain unclear.
Figure 1
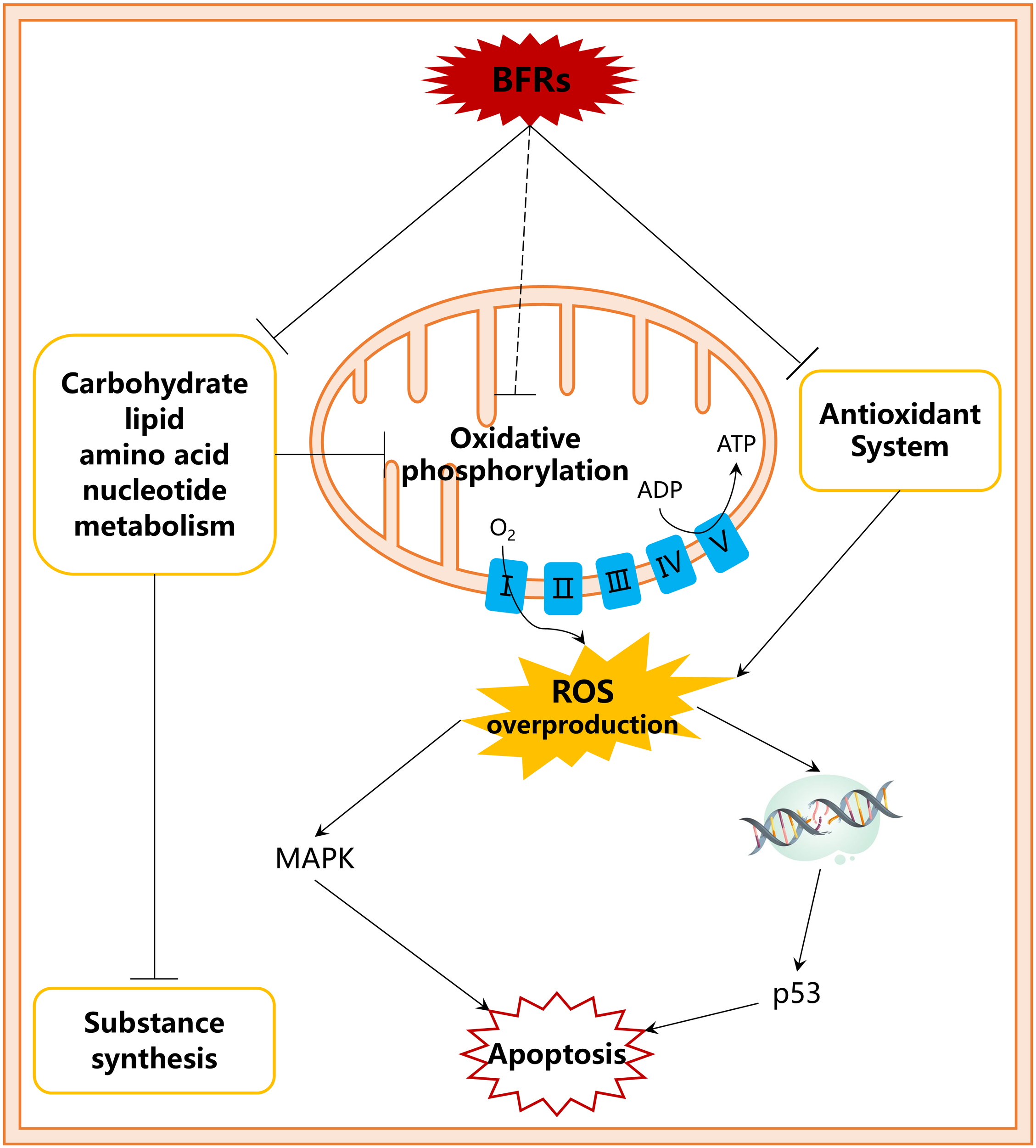
Toxicity mechanism of BFRs in marine organisms. The dashed lines represent presumed toxic effects.
4 Regulatory policy of BFRs
PBDEs were among the earliest BFRs to be restricted in production and use. Internationally, commercial penta-BDE (a mixture of BDE-47/99/100/153) and octa-BDE (a mixture of BDE-153/183/196/197/207) were listed in Annex A (Elimination) of the Stockholm Convention on POPs in 2009, prohibiting their production and use in state parties (UNEP, 2009). Commercial deca-BDE was added to Stockholm Convention in 2017 but granted exemptions for continued use until 2023 (UNEP, 2017). Earlier regulatory actions of PBDEs emerged in Europe and North America. The Restriction of Hazardous Substances (RoHS) implemented by European Union in 2003 restricted penta-/octa-BDE concentrations to ≤ 0.1% (w/w) in products (EU, 2003), while deca-BDE was subsequently regulated under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in 2017 with identical concentration limits (EU, 2017). In the United States, manufacturers voluntarily discontinued penta-/octa-BDE production through an agreement with the Environmental Protection Agency in 2004 (USEPA, 2004), whereas deca-BDE production formally prohibited in 2021 (USEPA, 2021). Canada’s Polybrominated Diphenyl Ethers Regulations (2008) instituted a comprehensive ban on all PBDE congeners except deca-BDE, which maintains exemptions for specific automotive and aerospace components (Canadian Parliament, 2008). Within the Asia-Pacific region, China fulfills Stockholm Convention obligations by phasing out PBDEs and listing deca-BDE in the List of Key Controlled New Pollutants, prohibiting its production and processing except for limited exemptions expiring December 2023 (State Council of China, 2023). Japan’s Chemical Substances Control Law imposed 0.1% concentration thresholds for penta-/octa-BDE in products in 2014 (MHLW of Japan, 2014), while South Korea established equivalent restrictions for deca-BDE (Ministry of Environment of South Korea, 2015). Contrastingly, penta-/octa-BDE remain exempted in several nations including India, Brazil, and Turkey, whereas deca-BDE retains global exemptions in most nations until 2036 (Sharkey et al., 2020).
HBCD was formally listed in the Stockholm Convention in 2013, with a specific exemption for flame-retardant applications in expanded polystyrene (EPS) and extruded polystyrene (XPS) building materials until 2021 (UNEP, 2013). China implemented pioneering compliance by enacting a comprehensive ban on HBCD production and use in 2016, achieving full phase-out in EPS/XPS industries by 2021 alongside dismantling domestic HBCD production facilities (Jiang et al., 2017c). The European Union similarly regulated HBCD through POPs Regulation (EU) 2016/293 in 2016, establishing a threshold limit of 100 mg/kg in products (0.01% w/w) (EU, 2016). This was subsequently strengthened by amendment (EU) 2024/1555 in 2024, reducing the permissible concentration to 75 mg/kg (0.0075% w/w) (EU, 2024a). Canada prohibited HBCD in building foams in 2017 and implemented a comprehensive ban on manufacturing, sale, use, and import in 2024 (Canadian Parliament, 2017, 2024). In contrast, the United States maintains no federal prohibition under the Toxic Substances Control Act, though selected states (e.g., Maine and Connecticut) designate HBCD as priority-controlled substances restricting its use in children’s products (Negev et al., 2018).
TBBPA has not been listed in the Stockholm Convention and remained excluded from the RoHS following repeated controversies in the European Union. Between 2018 and 2024, the European Commission initiated the RoHS Evaluation Project Pack 15 to assess TBBPA and related substances. In 2022, a proposal to include TBBPA in the RoHS was advanced but subsequently suspended. Ultimately, the European Union officially announced the decision to abandon the inclusion of TBBPA in the RoHS in December 2024 (EU, 2024b). Similarly, major Asian jurisdictions including China, Japan, and South Korea have not incorporated TBBPA into their mandatory restriction lists. Notably, the United States classified TBBPA as an unauthorized food contact material additive under Section 402(a)(2)(C)(i) of the Federal Food, Drug, and Cosmetic Act, prohibiting its application in food packaging, kitchen appliances, and related products (USFDA, 2024). However, the utilization of TBBPA in other application areas remains unrestricted in the United States. Current regulatory frameworks demonstrate that TBBPA remains exempt from comprehensive legal restrictions and continues to be widely utilized in global industrial.
HBB was initially designed as a substitute for PBDEs. However, due to the POPs characteristic shown in the process of application, HBB was formally listed in Annex A (Elimination) of the Stockholm Convention in 2009 for global elimination without any specific exemptions (UNEP, 2009). In contrast, other NBFRs remain under intensive scientific scrutiny and toxicological evaluation, with no comprehensive national or regional regulations currently enacted to restrict their manufacturing or application.
5 Conclusions and further research
Due to the complexity and diversity of marine organisms and their evolutionary status, the toxic effects of BFRs on different marine organisms are somewhat similar but with important differences. BFRs significantly impair marine plankton populations by inhibiting their growth, development, and reproduction. These chemicals disrupt zooplankton mobility and feeding capabilities while suppressing photosynthetic activity in phytoplankton. Marine benthic organisms, particularly bivalve molluscs, experience a range of adverse effects from BFRs exposure, including developmental, reproductive, immunological, and neurotoxic impairments. Similarly, nekton fishes exhibit compromised growth patterns, reproductive dysfunction, and metabolic disturbances when exposed to these persistent pollutants. Though direct evidence from marine mammals remains limited to in vitro studies, experimental data confirm BFRs’ cytotoxic potential in mammalian systems, highlighting ecological risks across marine trophic levels. Notably, this summary involves only three traditional BFRs and does not assess the toxicity of NBFRs. In fact, there are very few studies on the toxicity of NBFRs to marine organisms. The existing research data have shown that the toxicity of the three traditional BFRs to marine organisms is similar. For NBFRs to become suitable substitutes, they need to be demonstrated to be less toxic than traditional BFRs. Therefore, we first suggest that a comprehensive marine organism toxicity study should be carried out as soon as possible on NBFRs present at high levels in the marine environment, such as DBDPE, and that the toxicity level should be comprehensively compared with that of traditional BFRs to provide theoretical support for the production, application, and discharge management of NBFRs. Furthermore, due to the lack of molecular-level understanding of the toxicity mechanisms of BFRs in marine organisms, comprehensive AOPs have yet to be established. We therefore recommend that future studies employ modern molecular biology techniques, such as gene editing (CRISPR-Cas9) and gene silencing (RNAi), to investigate the molecular initiation events of BFR toxicity, focusing on their cellular uptake mechanisms and interactions with DNA or associated proteins. Such holistic, chain-linked investigations into BFR toxicity mechanisms will address current research gaps and facilitate the development of AOPs for marine organism. These works will be critical to meet global demands for ecological risk assessment of BFRs, thereby informing legislative actions to restrict their production and environmental release.
Among traditional BFRs, PBDEs and HBCD have been prohibited from production and use in the vast majority of countries worldwide due to their environmental persistence, bio-accumulative and high toxicity. TBBPA remains under ongoing evaluation regarding its toxicological profile, with no definitive regulations currently imposed on its application. Within the category of NBFRs, HBB stands as the only compound that has been phased out globally, while the environmental and health risks associated with other NBFRs remain under scientific assessment, and none have yet been subjected to prohibition in any national or regional jurisdictions. Although a preliminary global regulatory framework for BFRs has been established, significant deficiencies persist. First, regional discrepancies in restricted BFR lists and threshold standards remain pronounced. For instance, regulatory misalignment exists between the Stockholm Convention and regional standards in the European Union, North America, and certain developing economies. Such inconsistencies may exacerbate risks including regulatory loopholes, regulatory arbitrage, and transboundary pollution. Second, fragmented life-cycle management of BFR-containing products undermines regulatory efficacy. Existing regulations predominantly focus on restricting BFRs production and usage, while oversight of end-of-life disposal and recycling remains inadequate, particularly in developing nations where illegal dismantling, informal recycling, and open incineration practices persist. Third, NBFRs suffer from insufficient data on environmental behavior and toxicological profiles. This knowledge gap has resulted in regulatory frameworks lagging behind industrial advancements, potentially triggering a “substitution-induced pollution” crisis. To address these challenges, the following recommendations are proposed, along with their anticipated benefits:
-
Establish an international collaborative governance system. Benefit: Harmonizing global BFR restriction lists and threshold criteria under platforms like the WHO and UNEP would minimize regulatory arbitrage and transboundary pollution. Unified standards would prevent jurisdictional loopholes, ensuring consistent enforcement across regions and reducing the risk of “pollution havens” in less-regulated economies.
-
Strengthen life-cycle risk management. Benefit: Implementing blockchain-based traceability and IoT monitoring across the BFR product lifecycle (“production-usage-disposal-recycling”) would enhance transparency and accountability. This system would deter illegal disposal practices (e.g., e-waste dumping) and improve compliance in waste treatment, thereby reducing environmental leakage and long-term ecological risks.
-
Accelerate risk assessment of NBFRs. Benefit: Mandating pre-market ecotoxicological screening for NBFRs and establishing early-warning mechanisms would prevent a “substitution-induced pollution” crisis. By proactively identifying high-risk alternatives, policymakers can enforce timely restrictions, avoiding the environmental persistence and toxicity issues observed with traditional BFRs like PBDEs and HBCD.
These recommendations aim to bridge existing regulatory gaps, align scientific advancements with policy actions, and foster sustainable management of flame retardants to safeguard marine ecosystems.
Statements
Author contributions
WZ: Writing – review & editing, Formal analysis, Writing – original draft, Funding acquisition, Project administration. YC: Data curation, Funding acquisition, Writing – original draft. YW: Writing – review & editing. SC: Conceptualization, Writing – review & editing, Funding acquisition, Visualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was financially supported by the Anhui University of Finance & Economics Undergraduate Research Innovation Fund (No. XSKY23220) and China CDC Key Laboratory of Environment and Population Health Open Foundation (2024-CKL-03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abbasi G. Li L. Breivik K. (2019). Global historical stocks and emissions of PBDEs. Environ. Sci. Technol.53, 6330–6340. doi: 10.1021/acs.est.8b07032
2
Anselmo H. M. R. Koerting L. Devito S. van den Berg J. H. J. Dubbeldam M. Kwadijk C. J. A. F. et al . (2011). Early life developmental effects of marine persistent organic pollutants on the sea urchin. Ecotoxicol Environ. Saf.74, 2182–2192. doi: 10.1016/j.ecoenv.2011.07.037
3
Canadian Parliament . (2008). Polybrominated Diphenyl Ethers Regulations Vol. 142 (Ottawa: Canada Gazette, Part II).
4
Canadian Parliament . (2017). Prohibition of Certain Toxic Substances Regulations 2012 (SOR/2012-285) Amendment Vol. 151 (Ottawa: Canada Gazette Part II).
5
Canadian Parliament . (2024). Order Adding a Toxic Substance to Schedule 1 to the Canadian Environmental Protection Ac Vol. 158 (Ottawa: Canada Gazette Part II).
6
Canesi L. Lorusso L. C. Ciacci C. Betti M. Gallo G. (2005). Effects of the brominated flame retardant tetrabromobisphenol-A (TBBPA) on cell signaling and function of Mytilus hemocytes: Involvement of MAP kinases and protein kinase C. Aquat. Toxicol.75, 277–287. doi: 10.1016/j.aquatox.2005.08.010
7
Cao S. Wang J. Y. You X. Y. Zhou B. Wang Y. Zhou Z. Y. (2023). Purine metabolism and pyrimidine metabolism alteration is a potential mechanism of BDE-47-induced apoptosis in marine rotifer brachionus plicatilis. Int. J. Mol. Sci.24, 12726. doi: 10.3390/ijms241612726
8
Chen X. X. Li X. Fan Y. Q. Hu G. C. Xie H. Chen X. Y. et al . (2024). Inventorization and ecological risk assessment of tetrabromobisphenol A and hexabromocyclododecane in sediments from Guangdong coastal area of South China Sea. Sci. Total Environ.938, 173527. doi: 10.1016/j.scitotenv.2024.173527
9
Copeto S. Ganço S. Ferreira I. J. Silva M. Motta C. Diniz M. (2024). The effects of tetrabromobisphenol A (TBBPA) on the mussel mytilus galloprovincialis: A multi-biomarker approach. Oceans-Switzerland5, 181–195. doi: 10.3390/oceans5020011
10
Debenest T. Gagné F. Petit A. N. Kohli M. Eullafroy P. Blaise C. (2010). Monitoring of a flame retardant (tetrabromobisphenol A) toxicity on different microalgae assessed by flow cytometry. J. Environ. Monit.12, 1918–1923. doi: 10.1039/c0em00105h
11
Debenest T. Petit A. N. Gagné F. Kohli M. Nguyen N. Blaise C. (2011). Comparative toxicity of a brominated flame retardant (tetrabromobisphenol A) on microalgae with single and multi-species bioassays. Chemosphere85, 50–55. doi: 10.1016/j.chemosphere.2011.06.036
12
Dias M. Paula J. R. Pousao-Ferreira P. Casal S. Cruz R. Cunha S. C. et al . (2023). Combined effects of climate change and BDE-209 dietary exposure on the behavioural response of the white seabream. Sci. Total Environ.881, 163400. doi: 10.1016/j.scitotenv.2023.163400
13
Ding K. Xu Q. Z. Zhang X. L. Liu S. L. (2023). Metabolomic insights into neurological effects of BDE-47 exposure in the sea cucumber Apostichopus japonicus. Ecotoxicol Environ. Saf.266, 115558. doi: 10.1016/j.ecoenv.2023.115558
14
Dong L. Y. Wang S. T. Qu J. Z. You H. Liu D. M. (2021). New understanding of novel brominated flame retardants (NBFRs): Neuro (endocrine) toxicity. Ecotoxicol Environ. Saf.208, 111570. doi: 10.1016/j.ecoenv.2020.111570
15
Dong S. Yang Y. Cheng B. Ren C. B. Zhang H. W. Xu H. et al . (2019). Responses of antioxidant defenses in the clam Mactra veneriformis to 2,2’,4,4’−tetrabromodiphenyl ether exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol.217, 98–105. doi: 10.1016/j.cbpc.2018.11.017
16
EU . (2003). Directive 2002/95/EC on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Off. J. Eur. Union L37, 19–23.
17
EU . (2016). Regulation (EU) 2016/293 amending Regulation (EC) No 850/2004 on persistent organic pollutants. Off. J. Eur. Union L58.
18
EU . (2017). Commission Regulation (EU) 2017/227 of 9 February 2017 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards bis(pentabromophenyl)ether. Off. J. Eur. Union L35, 6–9.
19
EU . (2024a). Commission Regulation (EU) 2024/1555 amending Annex I to Regulation (EU) 2019/1021 on persistent organic pollutants. Off. J. Eur. Union L123.
20
EU . (2024b). “Legislative public consultation platform “Have your say”,” in Withdrawal of the initiative to restrict tetrabromobisphenol A (TBBPA) under RoHS Directive 2011/65/EU. Brussels.
21
Feiteiro J. Mariana M. Cairrao E. (2021). Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environ. pollut.285, 117475. doi: 10.1016/j.envpol.2021.117475
22
Feng H. R. Cheng Y. X. Ruan Y. F. Tsui M. M. P. Wang Q. Jin J. et al . (2021). Occurrence and spatial distribution of legacy and novel brominated flame retardants in seawater and sediment of the South China sea. Environ. pollut.271, 116324. doi: 10.1016/j.envpol.2020.116324
23
Fong C. C. Shi Y. F. Yu W. K. Wei F. van de Merwe J. P. Chan A. K. Y. et al . (2014). iTRAQ-based proteomic profiling of the marine medaka (Oryzias melastigma) gonad exposed to BDE-47. Mar. pollut. Bull.85, 471–478. doi: 10.1016/j.marpolbul.2014.04.024
24
Frouin H. Lebeuf M. Hammill M. Masson S. Fournier M . (2010). Effects of individual polybrominated diphenyl ether (PBDE) congeners on harbour seal immune cells in vitro. Mar. pollut. Bull.60, 291–298. doi: 10.1016/j.marpolbul.2009.09.006
25
Fu J. Zhang H. B. Li R. J. Shi T. D. Wang L. S. Cheng G. J. et al . (2023). Spatial distribution, source, and ecological risk of polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane (HBCD) in Jiaozhou Bay, China. Mar. pollut. Bull.192, 114978. doi: 10.1016/j.marpolbul.2023.114978
26
Gil-Solsona R. Castaño-Ortiz J. M. Muñoz-Mas R. Insa S. Farré M. Ospina-Alvarez N. et al . (2022). A holistic assessment of the sources, prevalence, and distribution of bisphenol A and analogues in water, sediments, biota and plastic litter of the Ebro Delta (Spain). Environ. pollut.314, 120310. doi: 10.1016/j.envpol.2022.120310
27
Gong W. J. Wang J. J. Cui W. Zhu L. Y. (2021). Distribution characteristics and risk assessment of TBBPA in seawater and zooplankton in northern sea areas, China. Environ. Geochem Health43, 4759–4769. doi: 10.1007/s10653-021-00948-5
28
Gong W. J. Zhu L. Y. Jiang T. T. Han C. (2017). The occurrence and spatial-temporal distribution of tetrabromobisphenol A in the coastal intertidal zone of Qingdao in China, with a focus on toxicity assessment by biological monitoring. Chemosphere185, 462–467. doi: 10.1016/j.chemosphere.2017.07.033
29
Gouesse R. J. Plante I. (2022). Environmental exposure to brominated flame retardants: unraveling endocrine and mammary gland effects that may increase disease risk. Toxicol Sci.186, 190–207. doi: 10.1093/toxsci/kfac006
30
Gu H. X. Wei S. S. Tu Z. H. Hu M. H. Guo B. Y. Wang Y. J. (2023). Polybrominated diphenyl ether-47 and food shortage impair the byssal attachment and health of marine mussels. Sci. Total Environ.891, 164415. doi: 10.1016/j.scitotenv.2023.164415
31
Han J. Won E. J. Lee M. C. Seo J. S. Lee S. J. Lee J. S. (2015). Developmental retardation, reduced fecundity, and modulated expression of the defensome in the intertidal copepod Tigriopus japonicus exposed to BDE-47 and PFOS. Aquat. Toxicol.165, 136–143. doi: 10.1016/j.aquatox.2015.05.022
32
Hong H. Z. Li D. M. Shen R. Wang X. H. Shi D. L. (2014). Mechanisms of hexabromocyclododecane induced developmental toxicity in marine medaka (Oryzias melastigma) embryos. Aquat. Toxicol.152, 173–185. doi: 10.1016/j.aquatox.2014.04.010
33
Hong H. Z. Lv D. M. Liu W. X. Huang L. M. Chen L. Y. Shen R. et al . (2017). Toxicity and bioaccumulation of three hexabromocyclododecane diastereoisomers in the marine copepod. Aquat. Toxicol.188, 1–9. doi: 10.1016/j.aquatox.2017.04.010
34
Hong H. Z. Shen R. Liu W. X. Li D. M. Huang L. M. Shi D. L. (2015). Developmental toxicity of three hexabromocyclododecane diastereoisomers in embryos of the marine medaka Oryzias melastigma. Mar. pollut. Bull.101, 110–118. doi: 10.1016/j.marpolbul.2015.11.009
35
Hu Y. X. Li Z. R. Xiong J. J. Zhang Z. W. Yuan J. X. Tang Y. et al . (2022). Occurrence and ecological risks of brominated flame retardants and dechlorane plus in sediments from the Pearl River Estuary and Daya Bay, South China. Mar. pollut. Bull.185, 114182. doi: 10.1016/j.marpolbul.2022.114182
36
Hu F. X. Pan L. Q. Cai Y. F. Liu T. Jin Q. (2015a). Deep sequencing of the scallop Chlamys farreri transcriptome response to tetrabromobisphenol A (TBBPA) stress. Mar. Genomics19, 31–38. doi: 10.1016/j.margen.2014.09.004
37
Hu F. X. Pan L. Q. Xiu M. Liu D . (2015b). Dietary accumulation of tetrabromobisphenol A and its effects on the scallop. Comp. Biochem. Physiol. C-Toxicol Pharmacol.167, 7–14. doi: 10.1016/j.cbpc.2014.08.002
38
Ji C. L. Wu H. F. Wei L. Zhao J. M. (2014). iTRAQ-based quantitative proteomic analyses on the gender-specific responses in mussel Mytilus galloprovincialis to tetrabromobisphenol A. Aquat. Toxicol.157, 30–40. doi: 10.1016/j.aquatox.2014.09.008
39
Jian X. Y. Tang X. X. Xu N. N. Sha J. J. Wang Y. (2017). Responses of the rotifer Brachionus plicatilis to flame retardant (BDE-47) stress. Mar. pollut. Bull.116, 298–306. doi: 10.1016/j.marpolbul.2017.01.015
40
Jiang Y. S. Cao S. Zhou B. Cao Q. Y. Xu M. X. Sun T. L. et al . (2023). Hemocytes in blue mussel Mytilus edulis adopt different energy supply modes to cope with different BDE-47 exposures. Sci. Total Environ.885, 163766. doi: 10.1016/j.scitotenv.2023.163766
41
Jiang S. S. Miao J. J. Wang X. Liu P. P. Pan L. Q. (2019). Inhibition of growth in juvenile manila clam Ruditapes philippinarum: Potential adverse outcome pathway of TBBPA. Chemosphere224, 588–596. doi: 10.1016/j.chemosphere.2019.02.157
42
Jiang Y. Tang X. Sun T. Wang Y. (2017a). BDE-47 exposure changed the immune function of haemocytes in Mytilus edulis: An explanation based on ROS-mediated pathway. Aquat Toxicol.182, 58–66. doi: 10.1016/j.aquatox.2016.11.010
43
Jiang Y. Tang X. Zhou B. Sun T. L. Chen H. M. Zhao X. Y. et al . (2017b). The ROS-mediated pathway coupled with the MAPK-p38 signalling pathway and antioxidant system plays roles in the responses of Mytilus edulis haemocytes induced by BDE-47. Aquat Toxicol.187, 55–63. doi: 10.1016/j.aquatox.2017.03.011
44
Jiang C. Tian Y. Zhang C. Sun Y. Ren Z. (2017c). “National strategy and action plan on HBCD control in China to fulfill the obligation of Stockholm Convention,” in 8th International Symposium on Flame Retardants: BFR 2017(York, England).
45
Jiang Y. Wang Y. Sun T. Lu K. Y. Zhao X. Y. Zhang Z. P. et al . (2021). Depicting an energetic chain involved in physiological responses of blue mussel Mytilus edulis coping with BDE-47 exposure. Chemosphere269, 128736. doi: 10.1016/j.chemosphere.2020.128736
46
Kratofil M. A. Ylitalo G. M. Mahaffy S. D. West K. L. Baird R. W. (2020). Life history and social structure as drivers of persistent organic pollutant levels and stable isotopes in Hawaiian false killer whales (Pseudorca crassidens). Sci. Total Environ.733, 138880. doi: 10.1016/j.scitotenv.2020.138880
47
Lee H. K. Lee S. Lim J. E. Moon H. B. (2020). Legacy and novel flame retardants in water and sediment from highly industrialized bays of Korea: Occurrence, source tracking, decadal time trend, and ecological risks. Mar. pollut. Bull.160, 111639. doi: 10.1016/j.marpolbul.2020.111639
48
Lei E. N. Y. Yau M. S. Yeung C. C. Murphy M. B. Wong K. L. Lam M. H. W. (2017). Profiling of selected functional metabolites in the central nervous system of marine medaka (Oryzias melastigma) for environmental neurotoxicological assessments. Arch. Environ. Contamination Toxicol.72, 269–280. doi: 10.1007/s00244-016-0342-0
49
Li M. Gong X. Y. Tan Q. W. Xie Y. H. Tong Y. J. Ma J. Y. et al . (2024). A review of occurrence, bioaccumulation, and fate of novel brominated flame retardants in aquatic environments: A comparison with legacy brominated flame retardants. Sci. Total Environ.939, 173224. doi: 10.1016/j.scitotenv.2024.173224
50
Li Y. N. Zhen X. M. Liu L. Tian C. G. Pan X. H. Tang J. H. (2019). Halogenated flame retardants in the sediments of the Chinese Yellow Sea and East China Sea. Chemosphere234, 365–372. doi: 10.1016/j.chemosphere.2019.06.115
51
Liu K. Li J. Yan S. J. Zhang W. Li Y. J. Han D. (2016). A review of status of tetrabromobisphenol A (TBBPA) in China. Chemosphere148, 8–20. doi: 10.1016/j.chemosphere.2016.01.023
52
Liu P. Miao J. Song Y. Pan L. Q. Yin P. F. (2017). Effects of 2,2’,4,4’-tetrabromodipheny ether (BDE-47) on gonadogenesis of the manila clam Ruditapes philippinarum. Aquat Toxicol.193, 178–186. doi: 10.1016/j.aquatox.2017.10.022
53
Liu K. Y. Qiu Y. Zhou S. S. Lin K. D. Chen D. Qu H. et al . (2019). Spatial distribution and congener profiles of polybrominated diphenyl ethers in surface sediment from sanmen bay and Xiamen Bay, Southeast China. Bull. Environ. Contamination Toxicol.103, 597–603. doi: 10.1007/s00128-019-02681-3
54
Liu Q. Tang X. X. Zhang X. Yang Y. Y. Sun Z. J. Jian X. Y. et al . (2020b). Evaluation of the toxic response induced by BDE-47 in a marine alga, Phaeodactylum tricornutum, based on photosynthesis-related parameters. Aquat. Toxicol.227, 105588. doi: 10.1016/j.aquatox.2020.105588
55
Liu L. Zhen X. M. Wang X. M. Li Y. F. Sun X. Tang J. H. (2020a). Legacy and novel halogenated flame retardants in seawater and atmosphere of the Bohai Sea: Spatial trends, seasonal variations, and influencing factors. Water Res.184, 116117. doi: 10.1016/j.watres.2020.116117
56
Liu L. Zhen X. M. Wang X. M. Zhang D. C. Sun L. T. Tang J. H. (2021). Spatio-temporal variations and input patterns on the legacy and novel brominated flame retardants (BFRs) in coastal rivers of North China. Environ. pollut.283, 117093. doi: 10.1016/j.envpol.2021.117093
57
Long C. Y. Yang W. Y. Lu J. X. Cheng Y. Y. Qiu N. Du S. et al . (2024). Tetrabromobisphenol A and hexabromocyclododecane in sediments from the Zhujiang (Pearl) River Estuary and South China Sea. Acta Oceanol Sin.43, 32–37. doi: 10.1007/s13131-023-2267-6
58
Lu N. Zhang Y. Y. Mu Q. L. Li Y. J. Li Y. Y. Yan Z. Y. et al . (2024). Hexabromocyclododecane-induced reproductive toxicity in Brachionus plicatilis: Impacts and assessment. Aquat. Toxicol.268, 106853. doi: 10.1016/j.aquatox.2024.106853
59
Messina C. M. Ruiz C. E. Regoli F. Manuguerra S. D'Agostino F. Avellone G. et al . (2020). BDE-47 exposure modulates cellular responses, oxidative stress and biotransformation related-genes in Mytilus galloprovincialis. Fish Shellfish Immunol.107, 537–546. doi: 10.1016/j.fsi.2020.11.015
60
Mhadhbi L. Fumega J. Boumaiza M. Manuguerra S. D’Agostino F. Avellone G. (2012). Acute toxicity of polybrominated diphenyl ethers (PBDEs) for turbot (Psetta maxima) early life stages (ELS). Environ. Sci. pollut. Res.19, 708–717. doi: 10.1007/s11356-011-0602-5
61
MHLW of Japan . (2014). Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture, etc. (Revised) (Tokyo: Government of Japan).
62
Ministry of Environment of South Korea . (2015). Act on Registration and Evaluation of Chemicals (No. 13392) (Sejong: Government of South Korea).
63
Negev M. Berman T. Reicher S. Balan S. Soehl A. Goulden S. et al . (2018). Regulation of chemicals in children’s products: How US and EU regulation impacts small markets. Sci. Total Environ.616, 462–471. doi: 10.1016/j.scitotenv.2017.10.198
64
Okeke E. S. Huang B. Mao G. H. Chen Y. Zeng Z. J. Qian X. et al . (2022). Review of the environmental occurrence/analytical techniques, degradation and toxicity of TBBPA and its derivatives. Environ. Res.206, 112594. doi: 10.1016/j.envres.2021.112594
65
Pan Y. F. Liu S. Tian F. Chen H. G. Xu X. R. (2022). Tetrabromobisphenol A and hexabromocyclododecane in sediments from fishing ports along the coast of South China: Occurrence, distribution and ecological risk. Chemosphere302, 134872. doi: 10.1016/j.chemosphere.2022.134872
66
Park J. C. Han J. Lee M. C. Kang H. M. Jeong C. B. Hwang D. S. et al . (2017). Adverse effects of BDE-47 on life cycle parameters, antioxidant system, and activation of MAPK signaling pathway in the rotifer Brachionus koreanus. Aquat. Toxicol.186, 105–112. doi: 10.1016/j.aquatox.2017.02.025
67
Park K. Kwak I. S. (2022). Apoptotic p53 gene expression in the regulation of persistent organic pollutant (POP)-induced oxidative stress in the intertidal crab. Antioxidants11, 771. doi: 10.3390/antiox11040771
68
Pereira L. C. de Souza A. O. Dorta D. J. (2013). Polybrominated diphenyl ether congener (BDE-100) induces mitochondrial impairment. Basic Clin. Pharmacol. Toxicol.112, 418–424. doi: 10.1111/bcpt.12046
69
Pereira L. C. Miranda L. F. de Souza A. O. Dorta D. J. (2014). BDE-154 induces mitochondrial permeability transition and impairs mitochondrial bioenergetics. J. Toxicol. Environ. Health A77, 24–36. doi: 10.1080/15287394.2014.861337
70
Po B. H. K. Chiu J. M. Y. (2018). Transgenerational impairments of reproduction and development of the marine invertebrate Crepidula onyx resulted from long-term dietary exposure of 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47). Environ. pollut.235, 730–738. doi: 10.1016/j.envpol.2017.12.087
71
Rajput I. R. Yaqoob S. Sun Y. J. Edmond S. Liu W. H. (2021). Polybrominated diphenyl ethers exert genotoxic effects in pantropic spotted dolphin fibroblast cell lines. Environ. pollut.271, 116131. doi: 10.1016/j.envpol.2020.116131
72
Rani M. Keshu Meenu Sillanpää M. Shanker U. (2022). An updated review on environmental occurrence, scientific assessment and removal of brominated flame retardants by engineered nanomaterials. J. Environ. Manage.321, 115998. doi: 10.1016/j.jenvman.2022.115998
73
Ruiz C. E. Manuguerra S. Cuesta A. Santulli A. Messina C. M. (2019). Oxidative stress, induced by sub-lethal doses of BDE 209, promotes energy management and cell cycle modulation in the marine fish cell line SAF-1. Int. J. Environ. Res. Public Health16, 474. doi: 10.3390/ijerph16030474
74
Sarkar D. Midha P. Shanti S. S. Singh S. K. (2023). A comprehensive review on the decabromodiphenyl ether (BDE-209)-induced male reproductive toxicity: Evidences from rodent studies. Sci. Total Environ.901, 165938. doi: 10.1016/j.scitotenv.2023.165938
75
Sha J. J. Jian X. Y. Yu Q. Y. Wei M. Li X. Y. Zhao L. D. et al . (2022). Exposure to BDE-47 and BDE-209 impaired antioxidative defense mechanisms in Brachionus plicatilis. Chemosphere303, 135152. doi: 10.1016/j.chemosphere.2022.135152
76
Sha J. J. Wang Y. Chen H. M. Wang M. Wang H. Li X. H. et al . (2015a). Using population demographic parameters to assess impacts of two polybrominated diphenyl ethers (BDE-47, BDE-209) on the rotifer Brachionus plicatilis. Ecotoxicol Environ. Saf.119, 106–115. doi: 10.1016/j.ecoenv.2015.05.009
77
Sha J. J. Wang Y. Lv J. X. Wang H. Chen H. M. Qi L. L. et al . (2015b). Effects of two polybrominated diphenyl ethers (BDE-47, BDE-209) on the swimming behavior, population growth and reproduction of the rotifer Brachionus plicatilis. J. Environ. Sci.28, 54–63. doi: 10.1016/j.jes.2014.07.020
78
Sharkey M. Harrad S. Abdallah M. A. E. Drage D. S. Berresheim H. (2020). Phasing-out of legacy brominated flame retardants: The UNEP Stockholm Convention and other legislative action worldwide. Environ. Int.144, 106041. doi: 10.1016/j.envint.2020.106041
79
Shen C. C. Zhang K. Shi J. Y. Yang J. X. Wang Y. Li Z. et al . (2024). Association between brominated flame retardants and risk of endocrine-related cancer: A systematic review and meta-analysis. Toxicol. Lett.394, 11–22. doi: 10.1016/j.toxlet.2024.02.002
80
Shi D. L. Lv D. M. Liu W. X. Shen R. Li D. M. Hong H. Z. (2017). Accumulation and developmental toxicity of hexabromocyclododecane (HBCD) on the marine copepod. Chemosphere167, 155–162. doi: 10.1016/j.chemosphere.2016.09.160
81
Smolarz K. Berger A. (2009). Long-term toxicity of hexabromocyclododecane (HBCD) to the benthic clam Macoma balthica (L.) from the Baltic Sea. Aquat. Toxicol.95, 239–247. doi: 10.1016/j.aquatox.2009.09.010
82
State Council of China . (2023). List of Key Controlled New Pollutants, (2023 Edition) (Beijing: State Council of the People’s Republic of China).
83
Turner A. (2022). PBDEs in the marine environment: Sources, pathways and the role of microplastics. Environ. pollut.301, 118943. doi: 10.1016/j.envpol.2022.118943
84
UNEP . (2009) in Stockholm Convention on Persistent Organic Pollutants (as amended 2009), Stockholm, Sweden, Secretariat of the Stockholm Convention.
85
UNEP . (2013) in An amendment to Annex A adopted by the Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants at its sixth meeting. SC-6/13, Stockholm, Sweden, Secretariat of the Stockholm Convention.
86
UNEP . (2017). COP-8/32: Report on the Conference of Parties to the Stockholm Convention on persistent Organic Pollutants on the works of its eigth meeting. Ed. UNEP (United Nations Environment Programme, Geneva, Switzerland).
87
USEPA . (2004). Voluntary Phase-out of PentaBDE and OctaBDE by U.S. Manufacturers [Industry Agreement].
88
USEPA . (2021). Regulation of Persistent, Bioaccumulative, and Toxic Chemicals Under TSCA Section 6(h); Phenol, Isopropylated Phosphate (3:1); Decabromodiphenyl Ether; and 2,4,6-Tris(tert-butyl)phenol. Federal Register, Vol. 86. 880–948.
89
USFDA . (2024). Federal Food, Drug, and Cosmetic Act §402(a)(2)(C)(i) (U.S. Food and Drug Administration (FDA).
90
von Krogh K. Ropstad E. Nourizadeh-Lillabadi R. Haug T. M. Weltzien F. A. (2019). In vitro effects of bisphenol A and tetrabromobisphenol A on cell viability and reproduction-related gene expression in pituitaries from sexually maturing atlantic cod (Gadus morhua L.). Fishes4, 48. doi: 10.3390/fishes4030048
91
Wang B. Y. Zhou B. Zhang H. Guo Y. Cui X. Y. Sun Y. et al . (2021a). A possible speculation on the involvement of ROS and lysosomes mediated mitochondrial pathway in apoptosis of rotifer Brachionus plicatilis with BDE-47 exposure. Sci. Total Environ.787, 147315. doi: 10.1016/j.scitotenv.2021.147315
92
Wang S. Ji C. L. Li F. Zhan J. F. Sun T. Tang J. H. et al . (2021b). Tetrabromobisphenol A induced reproductive endocrine-disrupting effects in mussel Mytilus galloprovincialis. J. Hazardous Mater416, 126228. doi: 10.1016/j.jhazmat.2021.126228
93
Wang N. Lai C. Xu F. H. Huang D. L. Zhang M. M. Zhou X. R. et al . (2023a). A review of polybrominated diphenyl ethers and novel brominated flame retardants in Chinese aquatic environment: Source, occurrence, distribution, and ecological risk assessment. Sci. Total Environ.904, 166180. doi: 10.1016/j.scitotenv.2023.166180
94
Wang S. Sun Z. D. Ren C. B. Li F. Xu Y. J. Wu H. F. et al . (2023b). Time- and dose-dependent detoxification and reproductive endocrine disruption induced by tetrabromobisphenol A (TBBPA) in mussel Mytilus galloprovincialis. Mar. Environ. Res.183, 105839. doi: 10.1016/j.marenvres.2022.105839
95
Wang H. Tang X. Sha J. Chen H. M. Sun T. L. Wang Y. (2015). The reproductive toxicity on the rotifer Brachionus plicatilis induced by BDE-47 and studies on the effective mechanism based on antioxidant defense system changes. Chemosphere135, 129–137. doi: 10.1016/j.chemosphere.2015.03.090
96
Wang S. Wang Z. Y. Wang X. M. Qu J. Y. Li F. Ji C. L. et al . (2023c). Histopathological and transcriptomic analyses reveal the reproductive endocrine-disrupting effects of decabromodiphenyl ethane (DBDPE) inmussel. Sci. Total Environ.862, 160724. doi: 10.1016/j.scitotenv.2022.160724
97
Wu Z. N. He C. Han W. Song J. Li H. J. Zhang Y. D. et al . (2020). Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ. Res.187, 109531. doi: 10.1016/j.envres.2020.109531
98
Xia B. Zhang J. Zhao X. G. Feng J. Teng Y. Chen B. J. et al . (2020). Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ. pollut.258, 113657. doi: 10.1016/j.envpol.2019.113657
99
Xie J. L. Sun Y. X. Cheng Y. Y. Chen Y. S. Chen L. G. Xie C. M. et al . (2021). Halogenated flame retardants in surface sediments from fourteen estuaries, South China. Mar. pollut. Bull.164, 112099. doi: 10.1016/j.marpolbul.2021.112099
100
Xie J. H. Zhao N. Zhang Y. Y. Feng J. Teng Y. Chen B. J. (2022). Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in seawater and sediment from East China Sea. Chemosphere287, 132218. doi: 10.1016/j.chemosphere.2021.132218
101
Xiong P. Yan X. T. Zhu Q. Q. Qu G. B. Shi J. B. Liao C. Y. et al . (2019). A review of environmental occurrence, fate, and toxicity of novel brominated flame retardants. Environ. Sci. Technol.53, 13551–13569. doi: 10.1021/acs.est.9b03159
102
Yang Y. Y. Jian X. Y. Tang X. X. Ma W. Q. Sun Z. J. Zhang X. et al . (2021). Feeding behavior toxicity in the marine rotifer Brachionus plicatilis caused by 2,2 ‘,4,4 ‘-tetrabromodiphenyl ether (BDE-47): Characteristics and mechanisms. Chemosphere271, 129512. doi: 10.1016/j.chemosphere.2020.129512
103
Ye G. Z. Chen Y. J. Wang H. O. Ye T. Huang Q. S. Chi Y. L. et al . (2016). Metabolomics approach reveals metabolic disorders and potential biomarkers associated with the developmental toxicity of tetrabromobisphenol A and tetrachlorobisphenol A. Sci. Rep.6, 35257. doi: 10.1038/srep35257
104
Ying H. Rajput I. R. Sanganyado E. Sun Y. J. Yu F. Liang B. et al . (2020). Immune stimulation effect of PBDEs via prostaglandin pathway in pantropical spotted dolphin: An in vitro study. Chemosphere254, 126717. doi: 10.1016/j.chemosphere.2020.126717
105
Yu Y. H. Hu L. H. Tian D. D. Yu Y. Y. Lu L. Z. Zhang J. M. et al . (2023a). Toxicities of polystyrene microplastics (MPs) and hexabromocyclododecane (HBCD), alone or in combination, to the hepatopancreas of the whiteleg shrimp, Litopenaeus vannamei. Environ. pollut.329, 121646. doi: 10.1016/j.envpol.2023.121646
106
Yu Y. Y. Tong D. F. Yu Y. H. Tian D. D. Zhou W. S. Zhang X. Y. et al . (2023b). Toxic effects of four emerging pollutants on cardiac performance and associated physiological parameters of the thick-shell mussel (Mytilus coruscus). Environ. pollut.334, 122244. doi: 10.1016/j.envpol.2023.122244
107
Zhang L. Jiang F. H. Kong X. F. Wang Y. Wang J. R. Zhang T. P. et al . (2022). Toxic effect of BDE-47 on the marine alga Skeletonema costatum: Population dynamics, photosynthesis, antioxidation and morphological changes. Chemosphere286, 131674. doi: 10.1016/j.chemosphere.2021.131674
108
Zhang H. Pan L. Q. Tao Y. X. (2014). Antioxidant responses in clam Venerupis philippinarum exposed to environmental pollutant hexabromocyclododecane. Environ. Sci. pollut. Res.21, 8206–8215. doi: 10.1007/s11356-014-2801-3
109
Zhao X. Lin X. Qu K. Xia B. Sun X. M. Chen B. J. (2019a). Toxicity of BDE-47, BDE-99 and BDE-153 on swimming behavior of the unicellular marine microalgae Platymonas subcordiformis and implications for seawater quality assessment. Ecotoxicol Environ. Saf.174, 408–416. doi: 10.1016/j.ecoenv.2019.02.050
110
Zhao Y. Tang X. Qu F. Lv M. C. Liu Q. Li J. et al . (2020). ROS-mediated programmed cell death (PCD) of Thalassiosira pseudonana under the stress of BDE-47. Environ. pollut.262, 114342. doi: 10.1016/j.envpol.2020.114342
111
Zhao Y. R. Tang X. X. Quigg A. Lv M. C. Zhao Y. (2019b). The toxic mechanisms of BDE-47 to the marine diatom Thalassiosira pseudonana-a study based on multiple physiological processes. Aquat. Toxicol.212, 20–27. doi: 10.1016/j.aquatox.2019.04.010
112
Zhao Y. Wang Y. Li Y. Santschi P. H. Quigg A. (2017). Response of photosynthesis and the antioxidant defense system of two microalgal species (Alexandrium minutum and Dunaliella salina) to the toxicity of BDE-47. Mar. pollut. Bull.124, 459–469. doi: 10.1016/j.marpolbul.2017.07.038
113
Zhen X. M. Li Y. F. Tang J. H. Wang X. M. Liu L. Zhong M. Y. et al . (2021). Decabromodiphenyl ether versus decabromodiphenyl ethane: source, fate, and influencing factors in a coastal sea nearing source region. Environ. Sci. Technol.55, 7376–7385. doi: 10.1021/acs.est.0c08528
114
Zhou S. Liu J. (2022). In vitro immunotoxicity and possible mechanisms of 2,2 ‘,4,4 ‘-tetrabromodiphenyl ether (BDE-47) on Ruditapes philippinarum hemocytes. Fish Shellfish Immunol.127, 386–395. doi: 10.1016/j.fsi.2022.06.039
115
Zhu N. Yang Y. Y. Xu H. Wang Q. Wei Y. Y. Li M. Z. et al . (2019). Bioaccumulation of decabromodiphenyl ether affects the antioxidant system in the clam Mactra veneriformis. Environ. Toxicol. Pharmacol.68, 19–26. doi: 10.1016/j.etap.2019.03.004
Summary
Keywords
brominated flame retardants, toxic effects, toxic mechanism, marine organisms, regulatory policy
Citation
Zhang W, Chen Y, Wang Y and Cao S (2025) The marine organism toxicity and regulatory policy of brominated flame retardants: a review. Front. Mar. Sci. 12:1609843. doi: 10.3389/fmars.2025.1609843
Received
11 April 2025
Accepted
30 July 2025
Published
20 August 2025
Volume
12 - 2025
Edited by
Juan José Alava, University of British Columbia, Canada
Reviewed by
Xiaoshan Zhu, Hainan University, China
Maria Luisa Vannuccini, University of Tuscia, Italy
Qinglin Mu, Zhejiang Provincial Zhoushan Marine Ecological Environmental Monitoring Station, China
Updates
Copyright
© 2025 Zhang, Chen, Wang and Cao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sai Cao, caosai@ouc.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.