Abstract
Anaerobic oxidation of methane (AOM) is a key methane sink in marine sediments, driven by electron acceptors such as sulfate, nitrate, and metal oxides. Manganese (Mn) oxides, owing to their high oxidizing capacity and rapid turnover, are also recognized as critical oxidants in AOM. However, Mn-AOM has not been well reviewed although several relevant studies have been published. In this paper, current results about Mn-AOM are summarized by synthesizing its geochemical characteristics, pathways for manganese activity, and microbial communities involved under certain marine environments. Two dominant pathways have been proposed for microbial involvement in Mn-AOM in marine sediments: direct AOM coupled to Mn oxide reduction, and indirect AOM via Mn oxide-mediated sulfate reduction. Mn-AOM activity is observed in methane-rich cold seeps or continental margins with substantial Mn oxides input. Future research efforts are still needed to prioritize quantifying the role of Mn-AOM in global carbon and marine manganese cycles.
1 Introduction
Methane is the second-most important greenhouse gas whose global warming potential is 28 times that of carbon dioxide over 100 years (Stocker, 2013). Methane oxidation occurs through both aerobic and anaerobic ways; however, in marine sediments, anaerobic oxidation predominates (Boetius et al., 2000; Reeburgh, 2007; Knittel and Boetius, 2009). In anoxic marine sediments, annual methane production is estimated at 85–300 Tg, with over 90% of this methane being consumed through anaerobic oxidation (Wefer et al., 2003; Reeburgh, 2007; Knittel and Boetius, 2009). Anaerobic oxidation of methane (AOM) is recognized as a key process in limiting atmospheric methane efflux from the ocean (<2% of the global flux) and in mitigating climate change (Reeburgh, 2007; Knittel and Boetius, 2009). Metabolism of organic matter in marine sediments follows a thermodynamic sequence of electron acceptor utilization, with Gibbs free energy yield decreasing in the order: oxygen, nitrate, manganese oxides, iron (oxyhydr)oxides, sulfate, and organic matter itself. This sequence determines the vertical chemical zonation, leading to oxic, nitrogenous, manganous, ferruginous, sulfidic, and methanogenic zones from the sediment surface downwards. Correspondingly, respiration processes occur sequentially as aerobic respiration, nitrate reduction, manganese reduction, iron reduction, sulfate reduction, and methanogenesis (Jørgensen and Kasten, 2006; Canfield and Thamdrup, 2009; Roberts, 2015). Methane generated in the methanogenic zone diffuses upward and reacts with sulfate, resulting in AOM coupled with sulfate reduction (SR) (Barnes and Goldberg, 1976; Beal et al., 2009).
This reaction (SR-AOM) occurs at a typical narrow zone with steep sulfate and methane gradients, called the sulfate-methane transition zone (SMTZ), and the depth at which sulfate is depleted is termed the sulfate-methane interface (SMI) (Iversen and Jørgensen, 1985; Niewöhner et al., 1998). Microorganisms including anaerobic methanotrophs (ANME) and sulfate-reducing bacteria (SRB) are actively involved in SR-AOM (Boetius et al., 2000; Knittel et al., 2005; Niemann et al., 2006; Wegener et al., 2015). In some marine sediments, sulfate reduction rates are not obligately coupled with AOM rates. Studies have demonstrated that even when sulfate has been significantly depleted or at a very low concentration, methane oxidation still occurs at a considerable rate (Joye et al., 2004; Niemann et al., 2006; Parkes et al., 2007; Bowles et al., 2019). Indeed, when methane diffusion rates or fluxes are elevated, sulfate reduction may be insufficient to oxidize all available methane, suggesting that AOM may become reliant on alternative electron acceptors present in marine sediments (Raghoebarsing et al., 2006; Beal et al., 2009; Ettwig et al., 2010; Haroon et al., 2013). Under such sulfate-limited conditions, the extent to which manganese oxides participate in methane oxidation, their specific geochemical signatures, the detailed reaction pathways of Mn-AOM, and certain natural environments where Mn-AOM occurs remain poorly summarized and systematically reviewed.
Manganese, often associated with iron in marine sediments, while present as a trace element, plays a crucial role in AOM. Mn-AOM is speculated to significantly impact the biogeochemical cycling and sink of elements like phosphorus in marine systems (Slomp et al., 2013; Wu et al., 2020), and to have been environmentally critical for methane consumption in sulfate-poor early Earth conditions (Konhauser et al., 2005; Crowe et al., 2011; Norði et al., 2013; Huang et al., 2023). The sources of manganese in marine sediments are diverse, including terrestrial input, aeolian deposition, hydrothermal venting, and ferromanganese nodules on the seafloor (Martin and Meybeck, 1979; Baturin, 1988; Elderfield and Schultz, 1996; Post, 1999; Poulton and Raiswell, 2000; Mahowald et al., 2005; Blöthe et al., 2015; Uramoto et al., 2019; Zhang et al., 2023). Oxidizing manganese in marine sediments is primarily found as Mn(III) and Mn(IV). Mn(III) predominantly exists in pore waters bound to ligands (Madison et al., 2013). Mn(IV) may exist as colloidal (hydr)oxide or manganese oxide particles. Common manganese oxide minerals include amorphous manganese dioxide (MnO2), lithiophorite, birnessite, todorokite, buserite, pyrolusite (β-MnO2), and so on (Post, 1999; Neaman et al., 2004; Jones et al., 2011).
Manganese exhibits thermodynamically potent oxidizing properties, enabling it to oxidize methane (Hu et al., 2018; Huang et al., 2023). In marine sediments, microorganisms can mediate the reaction between co-located manganese oxides and methane, driving AOM according to [Equation 1] (Beal et al., 2009):
Even when manganese oxides and methane are spatially separated, electric currents in marine sediments, conducted by bacterial nanowires combined with pyrite, soluble electron shuttles and outer-membrane cytochromes, can facilitate electron transport. This conductive network enables the oxidation of organic matter in deeper sediments by utilizing electron acceptors in surface sediments (Nielsen et al., 2010; Pfeffer et al., 2012; Nielsen and Risgaard-Petersen, 2015; Burdorf et al., 2017; Bjerg et al., 2018), suggesting that Mn-AOM is not strictly limited by spatial proximity. Furthermore, manganese oxides exhibit rapid turnover rates and a geological battery effect (Canfield et al., 1993), characterized by their capacity for reversible “charging” (electron uptake from organic donors) and “discharging” (electron transfer to acceptors like oxygen). Birnessite, for example, possesses a substantial electron storage capacity, estimated at approximately 2.7×1023e−/mol Mn (Ye et al., 2025). Electron transfer between birnessite and organic matter proceeds via a two-stage, kinetically discontinuous process, exhibiting rapid manganese reduction followed by slow Mn2+ release. Reduction of birnessite initially results in a series of metastable phases with accumulated low-valent Mn (Mn(III)/Mn(II)) without immediate Mn2+ release; Mn²+ ions are released in significant quantities only upon structural destabilization and mineral dissolution (Ye et al., 2025).Thus, even if there is a very small fraction of manganese in the environment, a significant amount of methane can still be oxidized, contributing to the mitigation of global methane emissions.
Reviews synthesizing Mn-AOM with specific consideration of manganese properties are currently limited, though several reviews on AOM or metal-AOM exist (Riedinger et al., 2014; Oni and Friedrich, 2017; Liang et al., 2019; Vuillemin et al., 2023; Chen et al., 2024; Zhao et al., 2024). This review provides a comprehensive synthesis of the current understanding of Mn-AOM. The main goal is to consolidate existing knowledge, critically discuss inconsistencies and limitations in current findings, and thereby provide researchers with a clear overview of this emerging field. This review achieves this by synthesizing current knowledge on: (i) the geochemical characteristics of Mn-AOM in marine sediments and the pathways of manganese activity; (ii) the associated microbial communities and their interactions with electron acceptors; and (iii) the documented natural environments and the conditions that favor the process. Ultimately, this work seeks to stimulate further research interest and offer enlightening suggestions for future directions about Mn-AOM in marine sediments.
2 Geochemical understanding of Mn-AOM
2.1 Geochemical signatures of Mn-AOM in sediments
Initial research suggesting a microbially mediated reaction between manganese oxides and methane in the natural environment can be traced back to Hein et al.’s (1987) investigation into the formation of 13C-depleted rhodochrosite (MnCO3) deposits in the California Coast Ranges. Beal et al.’s (2009) landmark investigation, involving the introduction of birnessite and ferrihydrite to marine seep sediment from the Eel River Basin, significantly illuminated the potential for metal-dependent AOM (manganese and iron) in marine settings. While their study did not conclusively ascertain the direct reaction of manganese with methane, it prompted numerous investigations into the role of metals (Fe and Mn) in AOM processes.
Among metal-AOM reactions, Fe-AOM has attracted more extensive attention than Mn-AOM (Crowe et al., 2011; Sivan et al., 2011; Egger et al., 2015b; Rooze et al., 2016; Cai et al., 2018; Yang et al., 2023). However, studies have revealed Mn involvement in AOM alongside Fe, with observable geochemical signatures. Dissolved inorganic carbon (DIC) produced by AOM and the authigenic carbonates precipitated from it typically inherit the isotopically light carbon signature of methane (Paull et al., 1992; Kastner et al., 2008; Tong et al., 2013). In porewater profiles, high concentrations of Mn2+ below the SMI, together with increasing δ¹³C-CH4, rising DIC levels, and lower δ¹³C-DIC values at corresponding depths, can be attributed to manganese reduction coupled to AOM (Figure 1) (Riedinger et al., 2014; Xiao et al., 2019, 2023). Decoupling of sulfate reduction and AOM, characterized by high methane-to-sulfate ratios or higher AOM rates relative to sulfate reduction rates, suggests the use of alternative electron acceptors (Segarra et al., 2013; Su et al., 2020). The authigenic precipitation of rhodochrosite, or elevated concentrations of Mn(II) in carbonate phases exhibiting depleted δ¹³C signatures, has been interpreted as diagnostic evidence for manganese-coupled AOM (Xiao et al., 2019). Furthermore, Mn(III/IV) oxides or manganese micronodules in the solid phase have also been documented in hydrate-bearing or cold seep environments, providing suggestive evidence for Mn-AOM (Segarra et al., 2013; Xiao et al., 2019; Yang et al., 2023).
Figure 1
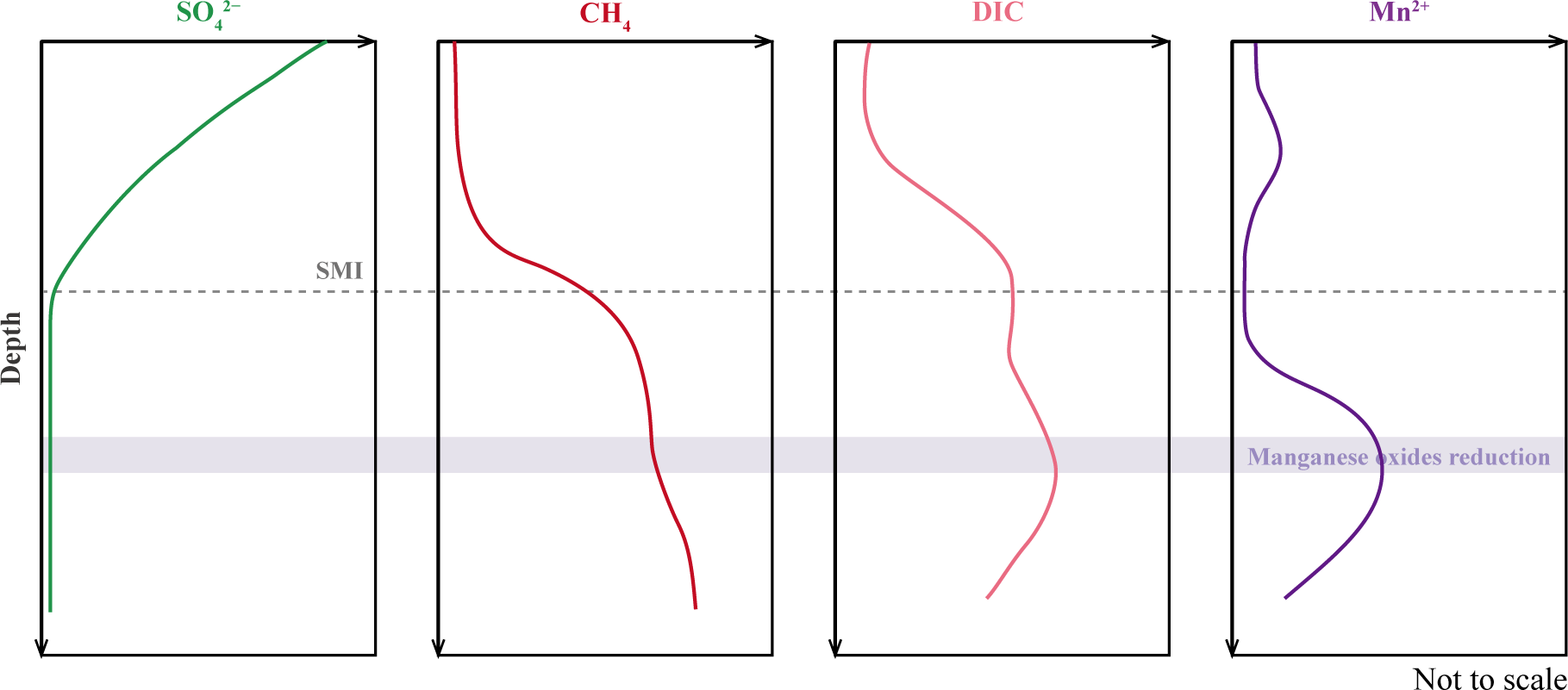
Schematic diagram of pore water geochemical profiles illustrating manganese reduction coupled to AOM.
AOM driven by manganese is thermodynamically favorable and presumed to occur preferentially (Riedinger et al., 2014; Xu et al., 2021). Manganese oxide reduction as an electron acceptor provides substantially more energy (∼20 times) than sulfate reduction, and also more than iron oxide reduction, in AOM process (Table 1) (Beal et al., 2009; Segarra et al., 2013; Ettwig et al., 2016). The high redox potential of manganese oxides enables them to act as terminal electron acceptors in organic matter oxidation, potentially oxidizing methane through HS⁻ and Fe(II) acting as electron shuttles (Wang and Van Cappellen, 1996; Thomsen et al., 2004; De Schamphelaire et al., 2007). Evidence from post-Marinoan Doushantuo cap dolostone formation, where δ34S values of authigenic pyrite correlate positively with carbonate manganese content, suggests manganese reduction influences the HS⁻ pool. This implies manganese oxides can serve as terminal electron acceptors for methane oxidation by oxidizing HS− to SO42− (Cai et al., 2023).
Table 1
| Electron Acceptor | Reaction | ΔG0’ (kJ mol-1 CH4) |
in situ ΔG (kJ mol-1 CH4) | AOM rate (μmol CH4 cm-3 yr-1) | Reference |
|---|---|---|---|---|---|
| Mn(IV): Birnessite | CH4 + 4MnO2 + 7H+ → HCO3− + 4Mn2+ + 5H2O |
N.A. | -383 | N.A. | Leu et al., 2020 |
| -494 | -556 | 14 |
He et al., 2018; Beal et al., 2009 |
||
| -790 | -594 ± 4 /-577 ± 2 |
N.A. | Segarra et al., 2013 | ||
| Fe(III): Ferric Citrate | CH4 + 8Fe3+ + 2H2O → CO2 + 8Fe2+ + 8H+ |
-454 | N.A. | N.A. | Ettwig et al., 2016 |
| Fe(III): Ferrihydrite | CH4 + 8Fe(OH)3 + 15H+ → HCO3− +8Fe2+ + 21H2O | -81.6 (pH = 7) |
-270.3 | 6 |
He et al., 2018; Beal et al., 2009 |
| -571 | -175 ± 8 /-192 ± 8 |
N.A. | Segarra et al., 2013 | ||
| SO42− | CH4 + SO42− → HCO3− + HS− + H2O |
-16.6 | -14 ~ -35 | 52 | Liang et al., 2019; Beal et al., 2009 |
Gibbs free energy changes under standard conditions (ΔG0’) and the calculated in situ ΔG of different electron acceptors.
N.A. means not available.
2.2 Experiments for Mn-AOM pathway
In order to reveal the specific role of manganese in AOM, researchers have conducted controlled incubation experiments to manipulate key variables and subsequently elucidate the underlying pathways of Mn-AOM (Table 2). Laboratory incubations of freshwater and brackish tidal wetland sediments amended with manganese oxides (birnessite) showed a significant increase in the Mn(II) content of the carbonate phase. A greater rate of AOM than SR was also observed, suggesting that AOM may be linked to the reduction of birnessite (Segarra et al., 2013).
Table 2
| Sample location | Incubation condition | Mn-AOM kinetics | Mainly functional microorganism | Pathway | Reference |
|---|---|---|---|---|---|
| Methane-seep sediments from Eel River Basin, California | Artificial sulfate-free seawater, [CH4] = 1.5 mM, [birnessite MnO2] = 10 mM, [Mn2+] = 0.2 mM, [H+] = 10–8 mM, [HCO3−] =11 mM, [CH4] = 1.5 mM, temperature at 10°C | 13CH4: 14 μmol CH4 cm-3 yr-1 | ANME-1 and/or Methanococcoides/ANME-3 with a bacterial partner (e.g. Bacteroides, Proteobacteria including Geobacter, Acidobacteria, and Verrucomicrobia) | MnR-AOM | Beal et al., 2009 |
| Marine sediments from Shenhu area of the northern South China Sea | Anaerobic artificial seawater with sediment (1:1), [birnessite MnO2] = 10 mM, [molybdate] = 30 mM | 14CH4: above 0.45 nmol cm-1 d-1 | ANME-2a/b | MnR-AOM | Xu et al., 2021 |
| Brackish coastal sediments from Dover Bluff salt marsh, Georgia | Sterile-filtered anoxic artificial porewater with sediment (1:3), [birnessite MnO2] = 10 mM, salinity = 21–28 PSU, pH = 7.1, temperature at 20°C | 14CH4: 2.4 CH4 nmol cm-3 slurry d-1 | N.A. | MnR-AOM | Segarra et al., 2013 |
| Marine sediments from north and east of Barrow, Alaskan Beaufort Sea continental margin | Artificial seawater medium with sediment (1:10), Na-birnessite, dark, temperature at 13°C |
14CH4: PC12: ~0.8 nmol cm−3 d−1 (max) PC13: ~2.7 nmol cm−3 d−1 (max) |
N.A. | MnR-S-AOM | Treude et al., 2014 |
| Marine sediments from Haima cold seep, South China Sea | No Incubation | Modeling: 0.32 CH4 μmol cm-2 y-1 | ANME-1,2c; ETH-SRB1 (ethane-dependent sulfate-reducing bacteria) | MnR-AOM | Xiao et al., 2023 |
| Coastal freshwater sediments from Hammersmith Creek River, Georgia | Sterile-filtered anoxic artificial porewater with sediment (1:3), [birnessite MnO2] = 10 mM, salinity = 0–1 PSU, pH = 7.0, temperature at 20°C | 14CH4: 3.6 CH4 nmol cm-3 slurry d-1 | N.A. | MnR-AOM | Segarra et al., 2013 |
| Sediments from a sulfate-rich lake (Lake Cadagno) in the southern Alps of Switzerland | Experiment 1, [vernadite] = 10 mmol L−1; Experiment 2, Slurries from Experiment 1 reamended with either [vernadite or [sulfate], plus [molybdate] = 20 mmol L−1; dark, anoxic, temperature = 25°C, 20 mL 13CH4 | 13CH4: ca. 1.67 μmol CO2 L-1 d-1 | Candidatus Methanoperedens or with potential sulfate-reducing partners Desulfobulbaceae | MnR-S-AOM | Su et al., 2020 |
| Culture of nitrate-AOM enrichment from canal Twentekanaal, Netherlands | At room temperature, pH=7.5 (± 0.15) | 13CH4: 21 nmol CO2 mg(protein)-1 h-1 | ANME-2d and an M. oxyfera-like microorganism | MnR-AOM | Ettwig et al., 2016 |
| Culture of Fe-AOM enrichment from Gold Creek Reservoir in Brisbane, Queensland, Australia | Artificial seawater, CH4 = 0.6–1.2 atm (90% CH4, 5% CO2, 5% N2), birnessite = ~1–2 g dry weight (added per 2–4 months), pH = 6.8–7.5, temperature = 22 ± 2°C | 44.5 μmol CH4 l-1 d-1 | Candidatus Methanoperedens manganicus and Candidatus Methanoperedens manganireducens | MnR-AOM | Leu et al., 2020 |
| Culture from a lab-scale constructed wetland with natural Mn ores | Synthetic anaerobic medium (N2-flushed), [CH4] = pure, [δ-MnO2] = 100–120 mg Mn/L, pH ≈ 7.0 (1 M HCl), temperature = 30 ± 2°C, O2< 0.002% (N2 headspace) | 13CH4: 0.2 to 0.6 pmol CH4 day-1 cell-1 | Candidatus Methanoperedens sp. BLZ1, Luteitalea pratensis | MnR-AOM | Liu et al., 2023 |
Incubation experiments exploring the coupling between manganese oxide reduction and AOM.
N.A. means not available.
Some studies added both manganese oxides and molybdate (a sulfate reduction inhibitor) to determine whether sulfate reduction to HS− occurred under experimental conditions. Xu et al. (2021) observed a significant decrease in the SR rate after the addition of birnessite, but an obvious increase in the AOM rate, and found that the molybdate did not affect the AOM rate. These results suggested that birnessite and sulfate might compete for the electron acceptor of AOM, and that birnessite here can act directly with methane (Xu et al., 2021). However, a similar experiment performed by Su et al. (2020) observed an evident decrease in the AOM rate after the addition of vernadite (δ-MnO2) and molybdate. This observation suggests that SO42− remains a significant factor in AOM, and further implies that manganese oxides may indirectly facilitate AOM by oxidizing reduced sulfur species, potentially linking manganese reduction to SR-AOM (Yao and Millero, 1996; Schippers and Jørgensen, 2001). The discrepancy in experimental results may be attributed to the specific mineral types of the added manganese oxides (birnessite vs. vernadite), which exhibit distinct kinetic and thermodynamic properties that can significantly influence reaction occurrence and rates (Neumann Wallheimer et al., 2025). Additionally, differences in the sediment source environments (marine for Xu et al. (2021); sulfate-rich lake for Su et al. (2020)) likely host different dominant microbial communities, potentially leading to variations in reaction pathways. In marine sediments, a cryptic sulfur cycle beneath the SMTZ, wherein manganese oxides are capable of oxidizing sulfides to sulfate [Equations 2–4], has been previously proposed as a pathway for this indirect involvement in SR-AOM (Jones et al., 2011; Treude et al., 2014):
Beyond sediment enrichments, bioreactor studies have examined Mn-AOM geochemistry (Table 2). A freshwater enrichment culture obtained under anaerobic, nitrate-reducing conditions with a continuous supply of methane showed that the reduction of environmentally relevant forms of Fe(III) and Mn(IV) was coupled with the oxidation of methane (Ettwig et al., 2016). Another freshwater sediment experiment used methane and birnessite to feed the bioreactor, and biochemical profiling of the system revealed Mn(IV)-dependent AOM (Leu et al., 2020). Both studies support the direct coupling of manganese reduction to AOM.
Extant research delineates two dominant pathways of Mn-AOM: (1) direct AOM coupled to Mn oxide reduction (MnR-AOM) (Figure 2A) and (2) indirect AOM via Mn oxide-mediated sulfate reduction (MnR-S-AOM) (Figure 2B).
Figure 2
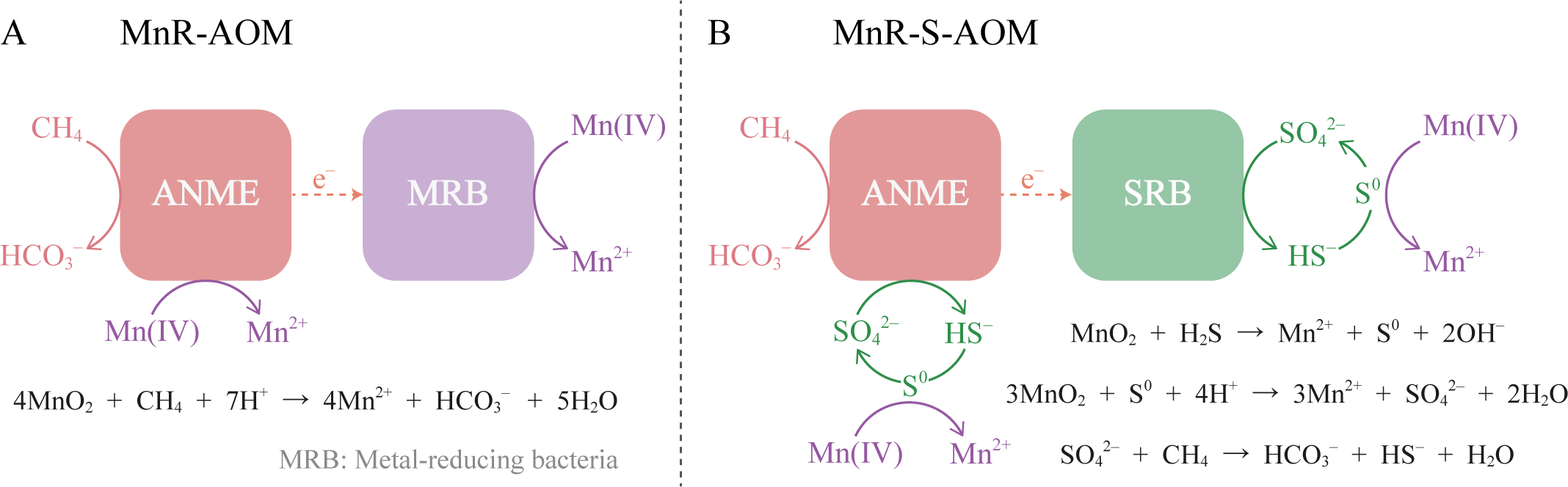
Two pathways of Mn-AOM (modified from He et al., 2018). (A) MnR-AOM, the reduction of manganese oxides directly drives AOM; (B) MnR-S-AOM, where manganese oxides oxidize HS⁻ to produce SO4²⁻, which is then reduced by methane.
MnR-AOM, while thermodynamically advantageous, is kinetically less favorable than SR-AOM. The solid-phase nature of manganese minerals limits the reactive surface area compared to dissolved sulfate, resulting in slower reaction rates with methane than those observed with sulfate (Table 1). This kinetic factor may promote Mn-AOM pathways that utilize sulfur as an intermediate. In addition, 1 mol of methane requires 4 mol of MnO2 [Equation 1], four times the amount of SO42- needed for SR-AOM, which means that MnO2 needs a higher rate of supply.
While both pathways are recognized, the specific environmental and microbial determinants that govern their relative importance and spatial distribution in natural sediments are not yet fully understood. Electron acceptor availability is likely a significant influencing factor, encompassing the supply and bioavailability (mineral form) of manganese oxides (Neumann Wallheimer et al., 2025), as well as the concentrations of sulfate and sulfide (see section 4 for environmental conditions favoring Mn-AOM in marine sediments). The composition and metabolic preferences of microbial communities may also be crucial determinants; their preference for specific electron acceptors or metabolic strategies likely dictates the pathway utilized (see Section 3.2). For instance, microbial consortia adapted to long-term SR-AOM might preferentially utilize sulfur-mediated pathways (MnR-S-AOM) when sulfate is becoming limiting, rather than immediately switching to direct MnR-AOM (e.g., Cai et al., 2023). Additionally, ambient geochemical conditions (e.g., pH, Eh, temperature, salinity), which can significantly impact reaction kinetics and microbial activity, are likely candidates influencing pathway selection (He et al., 2018; Cai et al., 2021b; Zhao et al., 2024). Detailed and systematic studies investigating how these potential determinants influence Mn-AOM pathway selection are needed in future research.
3 Microbial processes in Mn-AOM
3.1 Microbial communities involved in Mn-AOM
The SR-AOM, which is the major pathway of AOM, is typically mediated by syntrophic microbial consortia of ANME and sulfate-reducing bacteria (SRB) in the natural environment (Boetius et al., 2000; Niemann et al., 2006; Wegener et al., 2015). ANME encompass three distinct phylogenetic clades: ANME-1 (subgroups a and b), ANME-2 (subgroups a, b, c, and d), and ANME-3 (Ruff et al., 2015; Cai et al., 2021b). Mn-AOM in natural systems appears to necessitate the involvement of ANME alongside some metal-reducing bacteria (MRB) partners such as Desulfuromonas and Pelobacter members, although thermochemical oxidation of methane by manganese oxides without microbes has also been observed in environments like hydrothermal sediments (Chang et al., 2012; Huang et al., 2023).
Investigation into microbial communities involved in Mn-AOM in marine sediments employs both direct molecular analysis (DNA/RNA extraction and sequencing) of sediment samples and cultivation-based methods aimed at enriching key microorganisms (Beal et al., 2009; Liu et al., 2023; Xiao et al., 2023). Despite the persistent lack of representative pure cultures for Mn-AOM and the resulting unclear identification of the specific microbial populations responsible, shifts in microbial community structure following manganese addition offer valuable insights into potential functional groups (Table 2). For example, a 10-month culture experiment presented by Beal et al. (2009) indicated a substantial increase in manganese reducers within the bacterial community upon birnessite incubation, suggesting a vital role of bacteria in Mn-AOM. These results imply that Mn-AOM may be mediated by ANME-1 and/or Methanococcoides/ANME-3 in association with bacterial partners. House et al. (2011), using FISH and FISH-SIMS, identified that archaea-bacteria mixed aggregates were the most metabolically active entities during Mn-AOM, resembling those involved in SR-AOM. Direct DNA/RNA sequencing and analysis of sediment samples by Xiao et al. (2023) indicated that in methanic sediments, abundant active ANME groups (ANME-1 and ANME-2c) and potential dissimilatory metal reducers (e.g., ETH-SRB1) are potentially involved in metal-AOM in situ. Some studies have illuminated the capacity of ANME to perform the entire process without bacterial partners. Metagenomic sequencing has revealed that ANMEs capable of utilizing manganese solely are ANME-2a/b (Xu et al., 2021), “Ca. M. nitroreducens”-like archaea (Ettwig et al., 2016), “Ca. M. manganireducens” and “Ca. M. manganicus”; the latter three are assigned to ANME-2d (Leu et al., 2020; Cai et al., 2021b). In contrast to Leu et al.’s (2020) research, a recent bioreactor study by Liu et al. (2023) found Mn-AOM to be mediated by an anaerobic consortium comprising Candidatus Methanoperedens sp. BLZ1 and a distinct bacterial partner (Luteitalea pratensis).
Beyond direct Mn-AOM, the microbial functional roles in the MnR-S-AOM pathway are also complex. In the sediments of Cadagno Lake, where manganese is coupled to AOM through the sulfur cycle, the interdependence of ANME and SRB is likely facultative (Su et al., 2020). Analysis of microbial abundance revealed that SRB, specifically members of the Desulfobulbus group, exhibited peak abundance coinciding with only one of the two observed ANME maxima. This observation hints that ANME may engage in collaborative interactions with SRB or, alternatively, possess the capacity to execute AOM independently in MnR-S-AOM (Su et al., 2020).
It is important to note that the utilization of different parental substrates in cultivation experiments contributes to their variable effectiveness in representing in situ marine sediment conditions. Some employ a mixture of sampled marine sediment and artificial seawater, which can relatively better simulate in situ marine conditions (Beal et al., 2009; House et al., 2011; Segarra et al., 2013; Treude et al., 2014). In contrast, studies involving bioreactors primarily focused on the Mn-AOM reaction itself may diverge considerably from natural settings (Ettwig et al., 2016; Leu et al., 2020; Liu et al., 2023). Compared to cultivation experiments, results obtained from direct DNA/RNA extraction and sequencing of sediment samples provide more robust evidence for demonstrating the natural occurrence or ecological significance of microorganisms in marine sediments (Chang et al., 2012; Xu et al., 2021; Xiao et al., 2023). Therefore, future research incorporating in situ analysis of microbial communities and functions in sampled areas is essential for achieving a deeper understanding and stronger evidence of Mn-AOM.
3.2 Interactions between microorganisms and electron acceptors
Manganese oxides occur predominantly in solid phase in natural environments, so Mn-AOM microbes have to possess several strategies for transferring electrons between the cells and the minerals. Existing evidence suggests several potential strategies for this electron transfer process: direct contact between microbial cells and manganese oxide minerals; indirect electron transfer by electron shuttles; and direct electron transfer by nanowires (He et al., 2018; Liang et al., 2019; Leu et al., 2020; Dang et al., 2021) (Figure 3).
Figure 3
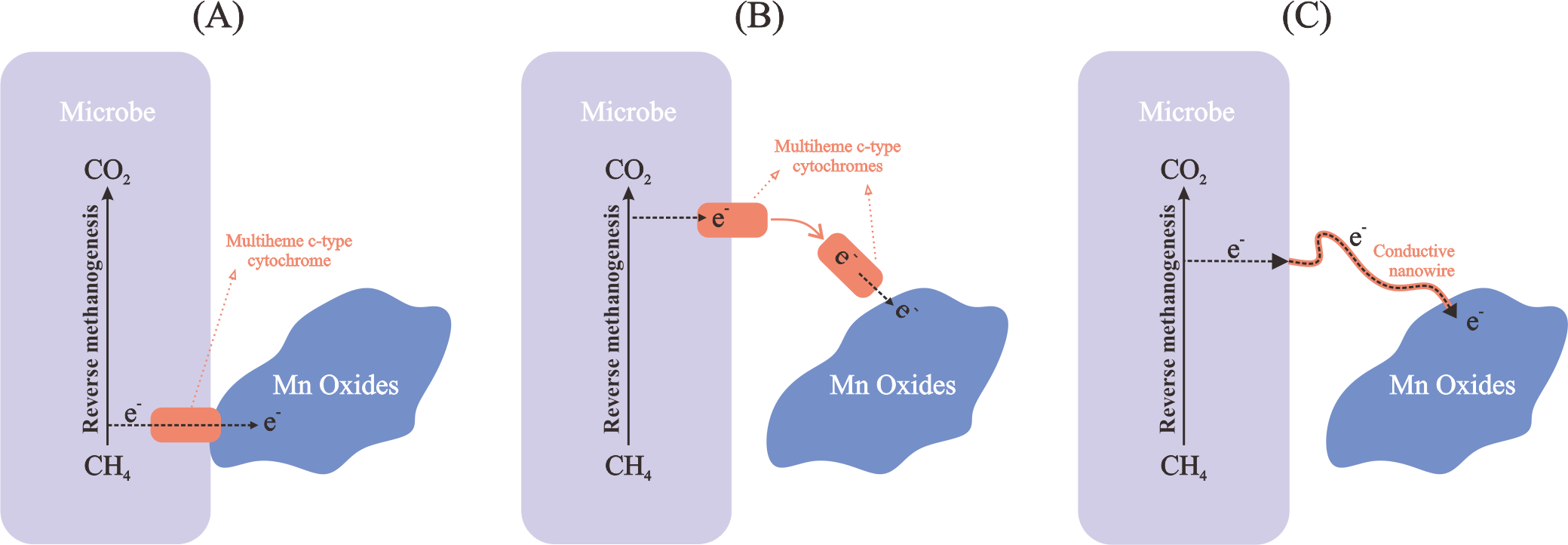
Mechanisms of electron transfer between microbes and Mn oxides. (A) Direct contact with Mn oxides; (B) Indirect electron transfer by electron shuttles; (C) Direct electron transfer by nanowires.
Numerous multiheme c-type cytochrome-encoding genes are found in abundance in ANME-2d members. The genome of “Ca. M. nitroreducens” encodes a great number of multiheme c-type cytochromes that may contribute to electron transfer in Mn-AOM (Haroon et al., 2013; Arshad et al., 2015). However, “Ca. M. nitroreducens” has yet to be directly confirmed to have an intermediate interspecies electron transfer. Besides, 43 and 25 putative multiheme c-type cytochromes were found to be encoded in “Ca. M. manganicus” and “Ca. M. manganireducens,” respectively. In the transcriptomes of “Ca. M. manganicus” and “Ca. M. manganireducens,” 23 and 9 multiheme c-type cytochromes were substantially expressed, respectively (Leu et al., 2020). These c-type cytochromes were previously found to transfer electrons from intracellular to extracellular and therefore facilitate the direct electron transfer between microorganisms and minerals (Cai et al., 2021b). They also have been recognized as shuttles for extracellular electron transfer (Shi et al., 2007; Wang et al., 2014), and it is hypothesized that they perform a similar function in ANME-2d members (Ettwig et al., 2016).
Conductive nanowires constitute another Mn-AOM electron transfer mechanism (Figure 3) (Leu et al., 2020). These conductive structures of “Ca. M. manganicus”, similar to the “Ca. M. nitroreducens” Mnv1 strain (Guerrero-Cruz and Cremers, 2018), show upregulation of archaellum-related genes under oxidative stress. Nanowires are suggested to allow electrons to transfer over greater distances relative to multiheme c-type cytochromes independently (Krukenberg et al., 2018). Mn-AOM-related microbes “Ca. M. manganicus” and “Ca. M. manganireducens” genomes encode for genes of the principal subunit flagellin (flaB), a component of the archaellum. During Mn-AOM experiments in “Ca. M. manganicus,” 2 of the 4 flaB genes were significantly expressed, suggesting the involvement of these conductive appendages in electron transfer (Leu et al., 2020).
In terms of electron acceptor utilization, ANME populations are recognized as versatile methanotrophs, exhibiting metabolic flexibility depending on environmental availability (Wankel et al., 2012; Ettwig et al., 2016; Scheller et al., 2016). While ANME-1, -2, and -3 clades are primarily associated with SR-AOM (Milucka et al., 2012), evidence indicates their involvement in Mn-AOM as well (Beal et al., 2009; Chang et al., 2012; Xu et al., 2021). However, studies suggest a preferential utilization of sulfate over metal oxides by these ANME clades (Riedinger et al., 2014; Egger et al., 2015b). Modeling of the Haima cold seep by Xiao et al. (2023) further supports this preference, indicating a minor contribution of Mn-AOM and Fe-AOM (1.5% each) relative to the dominant SR-AOM (97%). In contrast to these findings, laboratory experiments have demonstrated that some anaerobic methanotrophs prefer metals over sulfate as electron acceptors. Enriched from freshwater sediment, “Ca. M. ferrireducens,” “Ca. M. manganicus,” and “Ca. M. manganireducens” performed Fe(III)- or Mn(IV)-dependent AOM over a prolonged period, even in the presence of sulfate (Cai et al., 2018; Leu et al., 2020). Likewise, “Ca. M. nitroreducens” and “Ca. M. oxyfera” exhibit a preference for nitrate/nitrite reduction, irrespective of sulfate availability in culture media (Haroon et al., 2013; Ettwig et al., 2009).
The versatility of ANME to utilize electron acceptors is likely to be greatly affected by the availability of electron acceptors in the environment. Alterations in external environmental conditions not only induce functional adaptations within existing microbial populations but also contribute to shifts in community composition. For instance, the nitrate-dependent AOM archaeon “Ca. M. nitroreducens” is able to execute Fe- and Mn-AOM when nitrate concentrations are limiting (Haroon et al., 2013; Ettwig et al., 2016). Furthermore, laboratory bioreactor experiments, with conditions modified from initial incubations, further revealed significant shifts in dominant microbial types and the emergence of novel Mn-AOM microbes (e.g., Ca. M. manganicus and Ca. M. manganireducens) (Leu et al., 2020). It is hypothesized that microorganisms in these systems may mutate and/or evolve in response to variations in electron acceptor supply (Guerrero-Cruz et al., 2021). These findings underscore the complexity and under-exploration of microbial diversity and function in Mn-AOM.
4 Environmental conditions for Mn-AOM occurrence
Mn-AOM in natural marine sediments is primarily documented in cold seep and continental margin settings (Table 3). Cold seeps, characterized by exceedingly high methane flux and transport rate, facilitate the interaction of methane with the diverse electron acceptors present in sediments (Liu et al., 2022). High methane flux drives the SMI upwards to near seafloor surface in marine sediments, resulting in shallower redox zones and increased overlap of geochemical zones. This SMI shift enhances the probability of methane encountering manganese oxides above the SMI depth, thus promoting Mn-AOM (Figure 4). Studies in high methane flux regions like the Shenhu area reveal that concentrations of methane can be high up to 15 mM –80 mM and that AOM rates exceeding the SR rate at high methane concentrations (>5 mM), indicating the importance of alternative electron acceptors like manganese oxides (Lapham et al., 2013; Bowles et al., 2019). Incubation experiments further corroborate this, demonstrating simultaneous birnessite and sulfate involvement in AOM (Su et al., 2020). Therefore, in high methane flux settings, SMI upward migration and the resulting increased methane-manganese oxides interaction might drive Mn-AOM reaction. Furthermore, with a shallow SMI, the geological battery effect of manganese minerals may significantly contribute to Mn-AOM, as Mn(II) from manganese oxide reduction can be re-oxidized by oxygen near the surface, potentially establishing a manganese cycle for sustained methane oxidation (Ye et al., 2025). This phenomenon is likely more prevalent in shallow sediments, but further research is needed to fully understand the efficient charge-discharge properties of manganese minerals in natural marine sediments. Moreover, high methane flux environments can exacerbate sulfate depletion, further diminishing sulfate availability and creating conditions conducive to MnR-AOM. Sulfate-depleted conditions are commonly observed in methanogenic zones (beneath the SMI) in marine sediment profiles, where manganese oxide minerals, if present, can react with methane (Figure 4). In addition, vigorous sulfate consumption, fueled by abundant methane, leads to hydrogen sulfide (HS⁻) production. Subsequently, manganese oxides can facilitate the MnR-S-AOM reaction through HS⁻ mediation just below the SMI (Treude et al., 2014; Su et al., 2020). Observations of Mn-AOM in sediments from hydrate-bearing area of the South China Sea may also be linked to methane supply (Xiao et al., 2019). Hydrate formation necessitates methane saturation in porewater, thus ensuring a sustained methane supply for cold seep formation, although cold seep environments and hydrate zones are not always entirely congruent (Zhang et al., 2021b).
Table 3
| Ecosystem | Environment | Sample origin | Pathway | Reference |
|---|---|---|---|---|
| Marine | Cold seep with high methane flux | Methane-seep sediments from Eel River Basin, California | Mn-AOM* | Beal et al., 2009 |
| Marine sediments from Haima cold seep, South China Sea | MnR-AOM | Zhang et al., 2023 | ||
| Marine sediments from Haima cold seep, South China Sea | MnR-AOM | Xiao et al., 2023 | ||
| Continental margin with substantial terrestrial input | Marine sediments from east of the Rio de la Plata, Argentine Basin | Mn-AOM* | Riedinger et al., 2014 | |
| Marine sediments from north and east of Barrow, Alaskan Beaufort Sea continental margin | MnR-S-AOM | Treude et al., 2014 | ||
| Marine sediments from the Shenhu area of the northern South China Sea | MnR-AOM | Xu et al., 2021 | ||
| Marine sediments from Taixinan Basin, Dongsha area, South China Sea | Mn-AOM* | Xiao et al., 2019 | ||
| Coastal zone | Brackish coastal sediments from Dover Bluff salt marsh, Georgia | MnR-AOM | Segarra et al., 2013 | |
| Paleo-marine facies | Post-Marinoan Doushantuo cap dolostone of South China | MnR-S-AOM | Cai et al., 2023 | |
| Freshwater | Coastal freshwater sediments from Hammersmith Creek River, Georgia | MnR-AOM | Segarra et al., 2013 | |
| Sediments from a mud volcano in eastern Taiwan | MnR-AOM | Chang et al., 2012 | ||
| Sediments from a sulfate-rich lake (Lake Cadagno) in the southern Alps of Switzerland | MnR-S-AOM | Su et al., 2020 | ||
| Paleo-lacustrine facies | Triassic sandy conglomerates from the Junggar Basin, northwestern China | MnR-AOM | Cai et al., 2021a |
Documented occurrences of Mn-AOM in marine sediments, freshwater sediments (natural environments), and paleo-environmental settings.
Mn-AOM* pathway: study lacks detailed pathway of manganese activity in AOM.
Figure 4
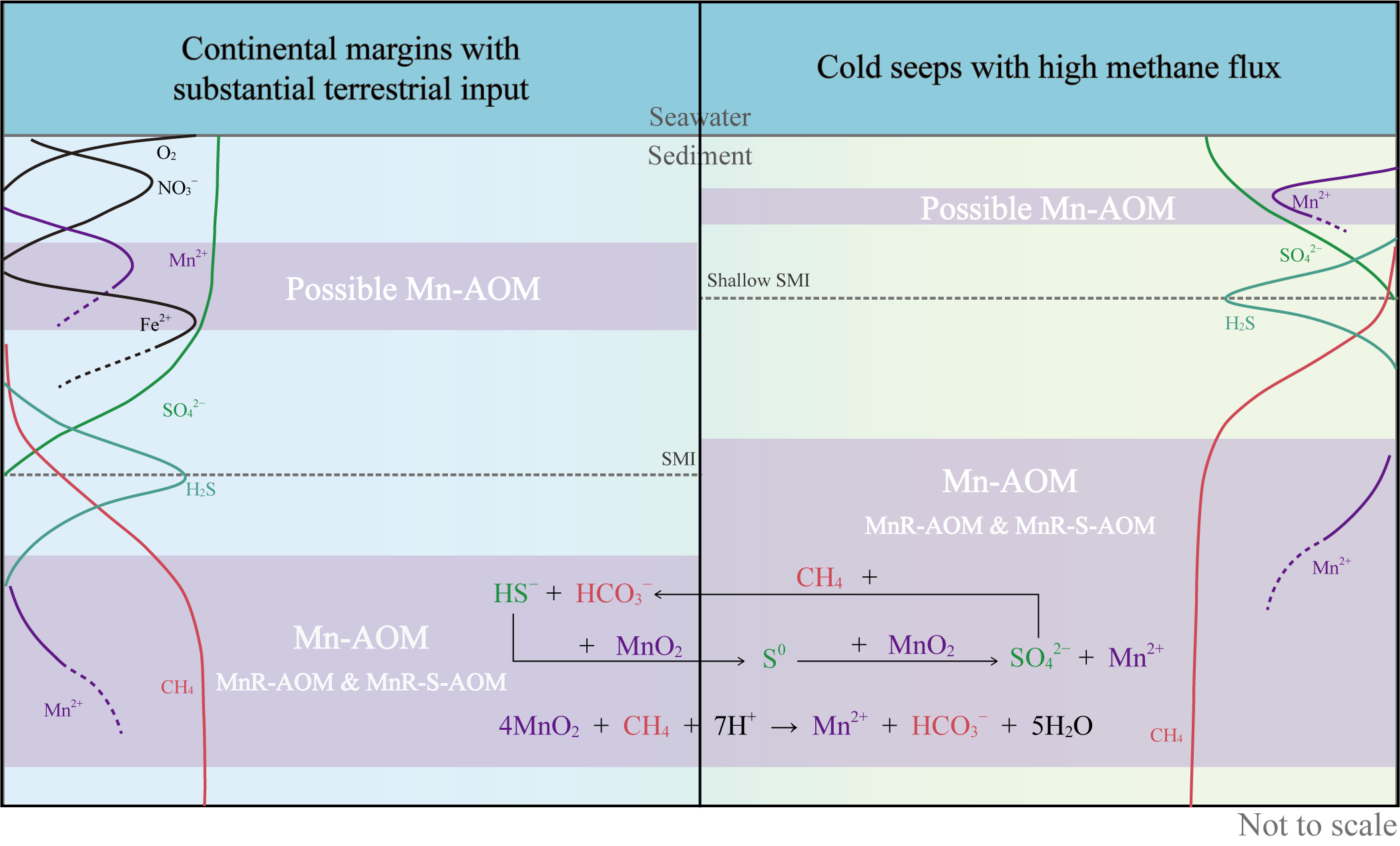
Observed Mn-AOM environments and potential distribution in marine sediment.
Continental margins with substantial terrigenous input and dynamic deposition provide favorable conditions for Mn-AOM (Figure 4). Abundant organic matter, derived from high surface productivity and terrestrial inputs, fuels methanogenesis and accelerates the consumption of dissolved electron acceptors, such as nitrate and sulfate (Chen et al., 2003). Continental margins are significant depositional zones for terrestrial manganese inputs. The continental margin is generally an unsteady marine depositional environment, where frequent mass movements and accompanying turbidity currents can transport large amounts of manganese and reworked organic matter to deep marine sediments (Hensen et al., 2003; Riedinger et al., 2005). High sedimentation rates in these regions can limit the exposure of reactive metal oxides to oxygenated water, thereby preserving significant amounts of manganese oxide phases within methanogenic zones (Riedinger et al., 2014). In these sulfate-depleted zones below the SMI, preserved manganese minerals become available to react with methane. Additionally, continental slopes often host ferromanganese nodules, and common manganese minerals like birnessite and todorokite, occurring as reactive coatings and fine-grained aggregates, are considered potential oxidants for methane (Zhong et al., 2017; Xu et al., 2021; Zhang et al., 2023). Different manganese species exhibit marked variations in chemical reactivity (Neaman et al., 2004), which directly influence reaction prioritization and pathways (Wang et al., 2018; Cai et al., 2023). However, characterization of manganese mineral types and structures in natural marine sediments remains challenging due to manganese’s trace element status and limited mineral formation. Consequently, most studies only rely on selective chemical extraction methods to isolate operationally defined manganese fractions, thereby elucidating manganese speciation and associated geochemical processes (Yang et al., 2018). Sequential extraction analyses reveal four distinct manganese phases in marine sediments: (1) carbonate-associated manganese (e.g., rhodochrosite MnCO3), (2) easily reducible amorphous manganese oxides (e.g., birnessite Na0.7Ca0.3Mn7O14·2.8H2O, vernadite δ-MnO2, nsutite γ-MnO2, and pyrolusite β-MnO2), (3) reducible crystalline manganese oxides (e.g., manganite γ-MnOOH and bixbyite α-Mn2O3), and (4) recalcitrant manganese oxides (e.g., hausmannite Mn3O4) (Brookins, 1988; Glasby and Schulz, 1999; Poulton and Canfield, 2005). Specifically, carbonate-associated manganese represents a reduced form, whereas manganese oxides in groups (2) and (3) exhibit oxidative capacity and are thus likely to be reactive in Mn-AOM processes.
Apart from marine sediments, some coastal areas with severe eutrophication also provide an ideal conditions for Mn-AOM (Table 3) (Segarra et al., 2013; Egger et al., 2015b; Wallenius et al., 2021). Eutrophication exacerbates the depletion of easily reactive dissolved electron acceptors in the upper layers of the sediment, causing the SMI to move upward (Egger et al., 2015b). As a result, manganese oxides in the sediment are exposed to the methanogenic zone, where sulfate, nitrate, and nitrite are depleted. Thus, metal oxides appear to be the only available electron acceptors for these deep methane oxidations. Mn-AOM also occurs in some freshwater sediments (Table 3) (Segarra et al., 2013). The lower sulfate content in the freshwater environment compared to the marine environment and the sufficient terrestrial input of reducible manganese as well as the presence of methane production in the sediments provide favorable conditions for Mn-AOM. Furthermore, direct oxidation of biogenic methane by manganese oxides (MnR-AOM) has been documented in a sulfate-poor paleo-lacustrine facies deposits during early diagenesis in the Triassic sandy conglomerates from the Junggar Basin, northwestern China (Cai et al., 2021a). Redox reactions between manganese oxides and methane have also been observed in the water column [e.g., Lake Matano, Indonesia (Jones et al., 2011)].
No consistent conclusion has been reached regarding the requirement for sulfur mediation in the reaction between manganese oxides and methane in natural environments, nor the conditions that dictate its role. Sulfur-mediated manganese reduction coupled anaerobic oxidation of methane (MnR-S-AOM) has been observed in marine continental margin sediments, sulfate-rich lake sediments, and paleo-marine facies deposits. Direct manganese reduction coupled anaerobic oxidation of methane (MnR-AOM) has been reported in marine cold seep sediments, continental margin sediments, coastal sediments, and paleo-lacustrine facies deposits. Some existing Mn-AOM studies have not definitively elucidated the precise pathways of manganese involvement in the anaerobic oxidation of methane (Table 3).
Overall, Mn-AOM has been identified both in marine and freshwater sediments (Beal et al., 2009; Su et al., 2020; Xu et al., 2021; Xiao et al., 2023), with documented evidence spanning historical periods and contemporary environments (Riedinger et al., 2014; Cai et al., 2021a, 2023). To illustrate the broad scope of Mn-AOM reactions, manganese oxides have been successfully applied for methane removal in constructed wetlands (Liu et al., 2020; Zhang et al., 2021a).
5 Conclusions and outlook
This review synthesizes current research on manganese oxides reduction coupled to anaerobic oxidation of methane (Mn-AOM), covering its geochemical characteristics, reaction pathways, and the microbial species involved. Key points include:
-
Geochemical Signatures: Widespread Mn-AOM is recognized in natural marine sediments by distinctive geochemical profiles, typically showing the co-occurrence of sulfate depletion, methane consumption, and increased dissolved Mn²+ concentrations, and/or the presence of manganese-enriched carbonate phases at corresponding depths.
-
Reaction Pathways: Mn-AOM proceeds via two dominant, microbially mediated pathways involving anaerobic methanotrophic archaea (ANME) and potentially synergistic bacterial partners: (1) direct AOM coupled to Mn oxide reduction (MnR-AOM); and (2) indirect AOM via Mn oxide-mediated sulfate reduction (MnR-S-AOM).
-
Environmental Conditions: Mn-AOM activity is commonly observed in methane-rich cold seeps or/and continental margins with substantial Mn oxides input, typically below the sediment sulfate-methane interface (SMI).
Despite current advancements, significant knowledge gaps necessitate focused future research. Key aspects include:
-
Environmental Controls on Mn-AOM Reactivity: Future research should systematically explore the impact of environmental factors on Mn-AOM, particularly manganese oxide mineral properties (structure, reactivity, transformations) using techniques such as μXRF, EXAFS, XANES, and LA-ICP-MS (Lenz et al., 2014, 2015; Egger et al., 2015a; Hermans et al., 2021; Lenstra et al., 2021), and parameters like salinity, temperature, and pH (Cai et al., 2021b).
-
Microbial Ecology and Mn-AOM Pathway: Future studies should intensify research into the microbial ecology by identifying key microorganisms and their metabolic strategies (especially via in situ detection). Importantly, studies should also aim to differentiate between direct AOM coupled to Mn oxide reduction (MnR-AOM) and indirect AOM via Mn oxide-mediated sulfate reduction (MnR-S-AOM), which are frequently obscured by overlapping geochemical signals. Research must also systematically identify and quantify the environmental, geochemical, and microbial factors controlling their relative importance and spatial distribution.
-
Environmental Distribution, Prevalence, and Significance: Investigations should be broadened to assess the environmental distribution and prevalence of Mn-AOM across diverse natural systems. Quantifying its contribution to global carbon and marine manganese cycles is a key priority, which can be achieved through methodologies such as regional flux estimations (e.g., Crowe et al., 2011; Slomp et al., 2013), model simulations (e.g. Xiao et al., 2023; Neumann Wallheimer et al., 2025), incubation experiments (e.g., Bowles et al., 2019), and analysis of geological records (e.g., Hu et al., 2018).
In conclusion, future research efforts directed towards these areas will be crucial for a comprehensive understanding of Mn-AOM and its significance in global methane dynamics and biogeochemical cycles.
Statements
Author contributions
YX: Conceptualization, Writing – review & editing, Writing – original draft, Visualization, Methodology. HL: Supervision, Conceptualization, Writing – review & editing, Funding acquisition. YL: Writing – review & editing. HY: Funding acquisition, Writing – review & editing, Methodology. YT: Writing – review & editing. YHL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is funded by DD20221703, DD20230063 from the China Geological Survey to HL, and National Natural Science Foundation of China (No.42303075) to HY.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AOM, anaerobic oxidation of methane; SR, sulfate reduction; SR-AOM, sulfate reduction coupled with anaerobic oxidation of methane; Metal-AOM, metal reduction coupled with anaerobic oxidation of methane; Fe-AOM, iron oxide reduction coupled with anaerobic oxidation of methane; Mn-AOM, manganese oxide reduction coupled with anaerobic oxidation of methane; MnR-AOM, direct AOM coupled to Mn oxide reduction; MnR-S-AOM, indirect AOM via Mn oxide-mediated sulfate reduction; ANME, anaerobic methanotrophs; SRB, sulfate-reducing bacteria; MRB, metal-reducing bacteria.
References
1
Arshad A. Speth D. R. de Graaf R. M. Op den Camp H. J. M. Jetten M. S. M. Welte C. U. (2015). A metagenomics-based metabolic model of nitrate-dependent anaerobic oxidation of methane by Methanoperedens-like archaea. Front. Microbiol.6. doi: 10.3389/fmicb.2015.01423
2
Barnes R. O. Goldberg E. D. (1976). Methane production and consumption in anoxic marine sediments. Geology4, 297. doi: 10.1130/0091-7613(1976)4<297:MPACIA>2.0.CO;2
3
Baturin G. N. (1988). The Geochemistry of Manganese and Manganese Nodules in the Ocean (Dordrecht: Springer Netherlands). doi: 10.1007/978-94-009-3731-4
4
Beal E. J. House C. H. Orphan V. J. (2009). Manganese- and iron-dependent marine methane oxidation. Science325, 184–187. doi: 10.1126/science.1169984
5
Bjerg J. T. Boschker H. T. S. Larsen S. Berry D. Schmid M. Millo D. et al . (2018). Long-distance electron transport in individual, living cable bacteria. Proc. Natl. Acad. Sci.115, 5786–5791. doi: 10.1073/pnas.1800367115
6
Blöthe M. Wegorzewski A. Müller C. Simon F. Kuhn T. Schippers A. (2015). Manganese-cycling microbial communities inside deep-sea manganese nodules. Environ. Sci. Technol.49, 7692–7700. doi: 10.1021/es504930v
7
Boetius A. Ravenschlag K. Schubert C. J. Rickert D. Widdel F. Gieseke A. et al . (2000). A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature407, 623–626. doi: 10.1038/35036572
8
Bowles M. W. Samarkin V. A. Hunter K. S. Finke N. Teske A. P. Girguis P. R. et al . (2019). Remarkable capacity for anaerobic oxidation of methane at high methane concentration. Geophys. Res. Lett.46, 12192–12201. doi: 10.1029/2019GL084375
9
Brookins D. G. (1988). Eh-pH Diagrams for Geochemistry (Berlin, Heidelberg: Springer Berlin Heidelberg). doi: 10.1007/978-3-642-73093-1
10
Burdorf L. D. W. Tramper A. Seitaj D. Meire L. Hidalgo-Martinez S. Zetsche E.-M. et al . (2017). Long-distance electron transport occurs globally in marine sediments. Biogeosciences14, 683–701. doi: 10.5194/bg-14-683-2017
11
Cai C. Leu A. O. Xie G.-J. Guo J. Feng Y. Zhao J.-X. et al . (2018). A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction. ISME J.12, 1929–1939. doi: 10.1038/s41396-018-0109-x
12
Cai C. Li K. Liu D. John C. M. Wang D. Fu B. et al . (2021a). Anaerobic oxidation of methane by Mn oxides in sulfate-poor environments. Geology49, 761–766. doi: 10.1130/G48553.1
13
Cai C. Liu D. Hu Y. Huang T. Jiang Z. Xu C. (2023). Interlinked marine cycles of methane, manganese, and sulfate in the post-Marinoan Doushantuo cap dolostone. Geochim. Cosmochim. Acta346, 245–258. doi: 10.1016/j.gca.2023.02.014
14
Cai C. Zhang X. Wu M. Liu T. Lai C.-Y. Frank J. et al . (2021b). Roles and opportunities for microbial anaerobic oxidation of methane in natural and engineered systems. Energy Environ. Sci.14, 4803–4830. doi: 10.1039/D1EE00708D
15
Canfield D. E. Thamdrup B. (2009). Towards a consistent classification scheme for geochemical environments, or, why we wish the term ‘suboxic’ would go away. Geobiology7, 385–392. doi: 10.1111/j.1472-4669.2009.00214.x
16
Canfield D. E. Thamdrup B. Hansen J. W. (1993). The anaerobic degradation of organic matter in Danish coastal sediments: iron reduction, manganese reduction, and sulfate reduction. Geochim. Cosmochim. Acta57, 3867–3883. doi: 10.1016/0016-7037(93)90340-3
17
Chang Y.-H. Cheng T.-W. Lai W.-J. Tsai W.-Y. Sun C.-H. Lin L.-H. et al . (2012). Microbial methane cycling in a terrestrial mud volcano in eastern Taiwan. Environ. Microbiol.14, 895–908. doi: 10.1111/j.1462-2920.2011.02658.x
18
Chen C.-T. A. Liu K.-K. Macdonald R. (2003). “Continental margin exchanges,” in Ocean Biogeochemistry: The Role of the Ocean Carbon Cycle in Global Change. Ed. FashamM. J. R. (Springer, Berlin, Heidelberg), 53–97. doi: 10.1007/978-3-642-55844-3_4
19
Chen C. Wang J. Algeo T. J. Zhu J.-M. Poulton S. W. Wang Z. et al . (2024). Application of pyrite trace-metal and S and Ni isotope signatures to distinguish sulfate- versus iron-driven anaerobic oxidation of methane. Chem. Geol.662, 122211. doi: 10.1016/j.chemgeo.2024.122211
20
Crowe S. A. Katsev S. Leslie K. Sturm A. Magen C. Nomosatryo S. et al . (2011). The methane cycle in ferruginous Lake Matano: Methane cycle in ferruginous Lake Matano. Geobiology9, 61–78. doi: 10.1111/j.1472-4669.2010.00257.x
21
Dang C.-C. Xie G.-J. Liu B.-F. Xing D.-F. Ding J. Ren N.-Q. (2021). Heavy metal reduction coupled to methane oxidation:Mechanisms, recent advances and future perspectives. J. Hazard. Mater.405, 124076. doi: 10.1016/j.jhazmat.2020.124076
22
De Schamphelaire L. Rabaey K. Boon N. Verstraete W. Boeckx P. (2007). Minireview: The potential of enhanced manganese redox cycling for sediment oxidation. Geomicrobiol. J.24, 547–558. doi: 10.1080/01490450701670137
23
Egger M. Jilbert T. Behrends T. Rivard C. Slomp C. P. (2015a). Vivianite is a major sink for phosphorus in methanogenic coastal surface sediments. Geochim. Cosmochim. Acta169, 217–235. doi: 10.1016/j.gca.2015.09.012
24
Egger M. Rasigraf O. Sapart C. J. Jilbert T. Jetten M. S. M. Röckmann T. et al . (2015b). Iron-mediated anaerobic oxidation of methane in brackish coastal sediments. Environ. Sci. Technol.49, 277–283. doi: 10.1021/es503663z
25
Elderfield H. Schultz A. (1996). Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu. Rev. Earth Planet. Sci.24, 191–224. doi: 10.1146/annurev.earth.24.1.191
26
Ettwig K. F. Butler M. K. Le Paslier D. Pelletier E. Mangenot S. Kuypers M. M. M. et al . (2010). Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature464, 543–548. doi: 10.1038/nature08883
27
Ettwig K. F. van Alen T. van de Pas-Schoonen K. T. Jetten M. S. M. Strous M. (2009). Enrichment and molecular detection of denitrifying methanotrophic bacteria of the NC10 phylum. Appl. Environ. Microbiol.75, 3656–3662. doi: 10.1128/AEM.00067-09
28
Ettwig K. F. Zhu B. Speth D. Keltjens J. T. Jetten M. S. M. Kartal B. (2016). Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc. Natl. Acad. Sci.113, 12792–12796. doi: 10.1073/pnas.1609534113
29
Glasby G. P. Schulz H. D. (1999). Eh ph Diagrams for mn, fe, co, ni, cu and as Under Seawater Conditions: Application of two new Types of eh ph Diagrams to the Study of Specific Problems in Marine Geochemistry. Aquat. Geochem.5, 227–248. doi: 10.1023/A:1009663322718
30
Guerrero-Cruz S. Cremers G. (2018). Response of the anaerobic methanotroph “Candidatus methanoperedens nitroreducens” to oxygen stress. Appl. Environ. Microbiol.84, e01832-18. doi: 10.1128/AEM.01832-18
31
Guerrero-Cruz S. Vaksmaa A. Horn M. A. Niemann H. Pijuan M. Ho A. (2021). Methanotrophs: Discoveries, environmental relevance, and a perspective on current and future applications. Front. Microbiol.12. doi: 10.3389/fmicb.2021.678057
32
Haroon M. F. Hu S. Shi Y. Imelfort M. Keller J. Hugenholtz P. et al . (2013). Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature500, 567–570. doi: 10.1038/nature12375
33
He Z. Zhang Q. Feng Y. Luo H. Pan X. Gadd G. M. (2018). Microbiological and environmental significance of metal-dependent anaerobic oxidation of methane. Sci. Total Environ.610–611, 759–768. doi: 10.1016/j.scitotenv.2017.08.140
34
Hein J. Koski R. Yeh H.-W. (1987). “Chert-hosted manganese deposits in sedimentary sequences of the Franciscan Complex, Diablo Range, California,” in Siliceous Sedimentary Rock-Hosted Ores and Petroleum (United States: Van Nostrand Reinhold Co. Inc., New York, NY), 206–230.
35
Hensen C. Zabel M. Pfeifer K. Schwenk T. Kasten S. Riedinger N. et al . (2003). Control of sulfate pore-water profiles by sedimentary events and the significance of anaerobic oxidation of methane for the burial of sulfur in marine sediments. Geochim. Cosmochim. Acta67, 2631–2647. doi: 10.1016/S0016-7037(03)00199-6
36
Hermans M. Astudillo Pascual M. Behrends T. Lenstra W. K. Conley D. J. Slomp C. P. (2021). Coupled dynamics of iron, manganese, and phosphorus in brackish coastal sediments populated by cable bacteria. Limnol. Oceanogr.66, 2611–2631. doi: 10.1002/lno.11776
37
House C. H. Beal E. J. Orphan V. J. (2011). The apparent involvement of ANMEs in mineral dependent methane oxidation, as an analog for possible Martian methanotrophy. Life1, 19–33. doi: 10.3390/life1010019
38
Hu W.-X. Kang X. Cao J. Wang X.-L. Fu B. Wu H.-G. (2018). Thermochemical oxidation of methane induced by high-valence metal oxides in a sedimentary basin. Nat. Commun.9, 5131. doi: 10.1038/s41467-018-07267-x
39
Huang Q. Jiang S.-Y. Pi D.-H. Konhauser K. O. Wen X.-P. Lu L.-Y. et al . (2023). Thermochemical oxidation of methane by manganese oxides in hydrothermal sediments. Commun. Earth Environ.4, 224. doi: 10.1038/s43247-023-00891-6
40
Iversen N. Jørgensen B. B. (1985). Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark)1. Limnol. Oceanogr.30, 944–955. doi: 10.4319/lo.1985.30.5.0944
41
Jones C. Crowe S. A. Sturm A. Leslie K. L. MacLean L. C. W. Katsev S. et al . (2011). Biogeochemistry of manganese in ferruginous Lake Matano, Indonesia. Biogeosciences8, 2977–2991. doi: 10.5194/bg-8-2977-2011
42
Jørgensen B. B. Kasten S. (2006). “Sulfur cycling and methane oxidation,” in Marine Geochemistry. Eds. SchulzH. D.ZabelM. (Springer, Berlin, Heidelberg), 271–309. doi: 10.1007/3-540-32144-6_8
43
Joye S. B. Boetius A. Orcutt B. N. Montoya J. P. Schulz H. N. Erickson M. J. et al . (2004). The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chem. Geol.205, 219–238. doi: 10.1016/j.chemgeo.2003.12.019
44
Kastner M. Torres M. Solomon E. Spivack A. J. (2008). Marine pore fluid profiles of dissolved sulfate: Do they reflect in situ methane fluxes? Fire in the Ice. 8. 8(3). Available at: https://netl.doe.gov/sites/default/files/publication/HMNewsSummer08.pdf.
45
Knittel K. Boetius A. (2009). Anaerobic oxidation of methane: Progress with an unknown process. Annu. Rev. Microbiol.63, 311–334. doi: 10.1146/annurev.micro.61.080706.093130
46
Knittel K. Lösekann T. Boetius A. Kort R. Amann R. (2005). Diversity and distribution of methanotrophic archaea at cold seeps. Appl. Environ. Microbiol.71, 467–479. doi: 10.1128/AEM.71.1.467-479.2005
47
Konhauser K. O. Newman D. K. Kappler A. (2005). The potential significance of microbial Fe(III) reduction during deposition of Precambrian banded iron formations. Geobiology3, 167–177. doi: 10.1111/j.1472-4669.2005.00055.x
48
Krukenberg V. Riedel D. Gruber-Vodicka H. R. Buttigieg P. L. Tegetmeyer H. E. Boetius A. et al . (2018). Gene expression and ultrastructure of meso- and thermophilic methanotrophic consortia. Environ. Microbiol.20, 1651–1666. doi: 10.1111/1462-2920.14077
49
Lapham L. Wilson R. Riedel M. Paull C. K. Holmes M. E. (2013). Temporal variability of in situ methane concentrations in gas hydrate-bearing sediments near Bullseye Vent, Northern Cascadia Margin. Geochem. Geophys. Geosystems14, 2445–2459. doi: 10.1002/ggge.20167
50
Lenstra W. K. Klomp R. Molema F. Behrends T. Slomp C. P. (2021). A sequential extraction procedure for particulate manganese and its application to coastal marine sediments. Chem. Geol.584, 120538. doi: 10.1016/j.chemgeo.2021.120538
51
Lenz C. Behrends T. Jilbert T. Silveira M. Slomp C. P. (2014). Redox-dependent changes in manganese speciation in Baltic Sea sediments from the Holocene Thermal Maximum: An EXAFS, XANES and LA-ICP-MS study. Chem. Geol.370, 49–57. doi: 10.1016/j.chemgeo.2014.01.013
52
Lenz C. Jilbert T. Conley D. J. Slomp C. P. (2015). Hypoxia-driven variations in iron and manganese shuttling in the Baltic Sea over the past 8 kyr. Geochem. Geophys. Geosystems16, 3754–3766. doi: 10.1002/2015GC005960
53
Leu A. O. Cai C. McIlroy S. J. Southam G. Orphan V. J. Yuan Z. et al . (2020). Anaerobic methane oxidation coupled to manganese reduction by members of the Methanoperedenaceae. ISME J.14, 1030–1041. doi: 10.1038/s41396-020-0590-x
54
Liang L. Wang Y. Sivan O. Wang F. (2019). Metal-dependent anaerobic methane oxidation in marine sediment: Insights from marine settings and other systems. Sci. China Life Sci.62, 1287–1295. doi: 10.1007/s11427-018-9554-5
55
Liu W. Xiao H. Ma H. Li Y. Adyel T. M. Zhai J. (2020). Reduction of methane emissions from manganese-rich constructed wetlands: Role of manganese-dependent anaerobic methane oxidation. Chem. Eng. J.387, 123402. doi: 10.1016/j.cej.2019.123402
56
Liu W. Xu S. Ma H. Li Y. Mąkinia J. Zhai J. (2023). Anaerobic consortia mediate Mn(IV)-dependent anaerobic oxidation of methane. Chem. Eng. J.468, 143478. doi: 10.1016/j.cej.2023.143478
57
Liu H. Zhan L. Lu H. (2022). Mechanisms for upward migration of methane in marine sediments. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.1031096
58
Madison A. S. Tebo B. M. Mucci A. Sundby B. Luther G. W. (2013). Abundant porewater Mn(III) is a major component of the sedimentary redox system. Science341, 875–878. doi: 10.1126/science.1241396
59
Mahowald N. M. Baker A. R. Bergametti G. Brooks N. Duce R. A. Jickells T. D. et al . (2005). Atmospheric global dust cycle and iron inputs to the ocean. Glob. Biogeochem. Cycles19, GB4025. doi: 10.1029/2004GB002402
60
Martin J.-M. Meybeck M. (1979). Elemental mass-balance of material carried by major world rivers. Mar. Chem.7, 173–206. doi: 10.1016/0304-4203(79)90039-2
61
Milucka J. Ferdelman T. G. Polerecky L. Franzke D. Wegener G. Schmid M. et al . (2012). Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature491, 541–546. doi: 10.1038/nature11656
62
Neaman A. Waller B. Mouélé F. Trolard F. Bourrié G. (2004). Improved methods for selective dissolution of manganese oxides from soils and rocks. Eur. J. Soil Sci.55, 47–54. doi: 10.1046/j.1351-0754.2003.0545.x
63
Neumann Wallheimer R. Halevy I. Sivan O. (2025). Modeling the controls on microbial iron and manganese reduction in methanic sediments. Geochim. Cosmochim. Acta400, 32–50. doi: 10.1016/j.gca.2025.05.026
64
Nielsen L. P. Risgaard-Petersen N. (2015). Rethinking sediment biogeochemistry after the discovery of electric currents. Annu. Rev. Mar. Sci.7, 425–442. doi: 10.1146/annurev-marine-010814-015708
65
Nielsen L. P. Risgaard-Petersen N. Fossing H. Christensen P. B. Sayama M. (2010). Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature463, 1071–1074. doi: 10.1038/nature08790
66
Niemann H. Lösekann T. de Beer D. Elvert M. Nadalig T. Knittel K. et al . (2006). Novel microbial communities of the Haakon Mosby mud volcano and their role as a methane sink. Nature443, 854–858. doi: 10.1038/nature05227
67
Niewöhner C. Hensen C. Kasten S. Zabel M. Schulz H. D. (1998). Deep sulfate reduction completely mediated by anaerobic methane oxidation in sediments of the upwelling area off Namibia. Geochim. Cosmochim. Acta62, 455–464. doi: 10.1016/S0016-7037(98)00055-6
68
Norði K.À. Thamdrup B. Schubert C. J. (2013). Anaerobic oxidation of methane in an iron-rich Danish freshwater lake sediment. Limnol. Oceanogr.58, 546–554. doi: 10.4319/lo.2013.58.2.0546
69
Oni O. E. Friedrich M. W. (2017). Metal oxide reduction linked to anaerobic methane oxidation. Trends Microbiol.25, 88–90. doi: 10.1016/j.tim.2016.12.001
70
Parkes R. J. Cragg B. A. Banning N. Brock F. Webster G. Fry J. C. et al . (2007). Biogeochemistry and biodiversity of methane cycling in subsurface marine sediments (Skagerrak, Denmark). Environ. Microbiol.9, 1146–1161. doi: 10.1111/j.1462-2920.2006.01237.x
71
Paull C. K. Chanton J. P. Neumann A. C. Coston J. A. Martens C. S. Showers W. (1992). Indicators of methane-derived carbonates and chemosynthetic organic carbon deposits: Examples from the Florida Escarpment. PALAIOS7, 361–375. doi: 10.2307/3514822
72
Pfeffer C. Larsen S. Song J. Dong M. Besenbacher F. Meyer R. L. et al . (2012). Filamentous bacteria transport electrons over centimetre distances. Nature491, 218–221. doi: 10.1038/nature11586
73
Post J. E. (1999). Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci.96, 3447–3454. doi: 10.1073/pnas.96.7.3447
74
Poulton S. W. Canfield D. E. (2005). Development of a sequential extraction procedure for iron: implications for iron partitioning in continentally derived particulates. Chem. Geol.214, 209–221. doi: 10.1016/j.chemgeo.2004.09.003
75
Poulton S. W. Raiswell R. (2000). Solid phase associations, oceanic fluxes and the anthropogenic perturbation of transition metals in world river particulates. Mar. Chem.72, 17–31. doi: 10.1016/S0304-4203(00)00060-8
76
Raghoebarsing A. A. Pol A. van de Pas-Schoonen K. T. Smolders A. J. P. Ettwig K. F. Rijpstra W. I. C. et al . (2006). A microbial consortium couples anaerobic methane oxidation to denitrification. Nature440, 918–921. doi: 10.1038/nature04617
77
Reeburgh W. S. (2007). Oceanic methane biogeochemistry. Chem. Rev.107, 486–513. doi: 10.1021/cr050362v
78
Riedinger N. Formolo M. J. Lyons T. W. Henkel S. Beck A. Kasten S. (2014). An inorganic geochemical argument for coupled anaerobic oxidation of methane and iron reduction in marine sediments. Geobiology12, 172–181. doi: 10.1111/gbi.12077
79
Riedinger N. Pfeifer K. Kasten S. Garming J. F. L. Vogt C. Hensen C. (2005). Diagenetic alteration of magnetic signals by anaerobic oxidation of methane related to a change in sedimentation rate. Geochim. Cosmochim. Acta69, 4117–4126. doi: 10.1016/j.gca.2005.02.004
80
Roberts A. P. (2015). Magnetic mineral diagenesis. Earth-Sci. Rev.151, 1–47. doi: 10.1016/j.earscirev.2015.09.010
81
Rooze J. Egger M. Tsandev I. Slomp C. P. (2016). Iron-dependent anaerobic oxidation of methane in coastal surface sediments: Potential controls and impact: Iron-dependent oxidation of methane. Limnol. Oceanogr.61, S267–S282. doi: 10.1002/lno.10275
82
Ruff S. E. Biddle J. F. Teske A. P. Knittel K. Boetius A. Ramette A. (2015). Global dispersion and local diversification of the methane seep microbiome. Proc. Natl. Acad. Sci.112, 4015–4020. doi: 10.1073/pnas.1421865112
83
Scheller S. Yu H. Chadwick G. L. McGlynn S. E. Orphan V. J. (2016). Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science351, 703–707. doi: 10.1126/science.aad7154
84
Schippers A. Jørgensen B. B. (2001). Oxidation of pyrite and iron sulfide by manganese dioxide in marine sediments. Geochim. Cosmochim. Acta65, 915–922. doi: 10.1016/S0016-7037(00)00589-5
85
Segarra K. E. A. Comerford C. Slaughter J. Joye S. B. (2013). Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochim. Cosmochim. Acta115, 15–30. doi: 10.1016/j.gca.2013.03.029
86
Shi L. Squier T. C. Zachara J. M. Fredrickson J. K. (2007). Respiration of metal (hydr)oxides by Shewanella and Geobacter : a key role for multihaem c -type cytochromes. Mol. Microbiol.65, 12–20. doi: 10.1111/j.1365-2958.2007.05783.x
87
Sivan O. Adler M. Pearson A. Gelman F. Bar-Or I. John S. G. et al . (2011). Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol. Oceanogr.56, 1536–1544. doi: 10.4319/lo.2011.56.4.1536
88
Slomp C. P. Mort H. P. Jilbert T. Reed D. C. Gustafsson B. G. Wolthers M. (2013). Coupled dynamics of iron and phosphorus in sediments of an oligotrophic coastal basin and the impact of anaerobic oxidation of methane. PLoS One8, e62386. doi: 10.1371/journal.pone.0062386
89
Stocker T. (Ed.) (2013). Climate change 2013: the physical science basis: Working Group I contribution to the Fifth assessment report of the Intergovernmental Panel on Climate Change (New York: Cambridge University Press).
90
Su G. Zopfi J. Yao H. Steinle L. Niemann H. Lehmann M. F. (2020). Manganese/iron-supported sulfate-dependent anaerobic oxidation of methane by archaea in lake sediments. Limnol. Oceanogr.65, 863–875. doi: 10.1002/lno.11354
91
Thomsen U. Thamdrup B. Stahl D. A. Canfield D. E. (2004). Pathways of organic carbon oxidation in a deep lacustrine sediment, Lake Michigan. Limnol. Oceanogr.49, 2046–2057. doi: 10.4319/lo.2004.49.6.2046
92
Tong H. Feng D. Cheng H. Yang S. Wang H. Min A. G. et al . (2013). Authigenic carbonates from seeps on the northern continental slope of the South China Sea: New insights into fluid sources and geochronology. Mar. Pet. Geol.43, 260–271. doi: 10.1016/j.marpetgeo.2013.01.011
93
Treude T. Krause S. Maltby J. Dale A. W. Coffin R. Hamdan L. J. (2014). Sulfate reduction and methane oxidation activity below the sulfate-methane transition zone in Alaskan Beaufort Sea continental margin sediments: Implications for deep sulfur cycling. Geochim. Cosmochim. Acta144, 217–237. doi: 10.1016/j.gca.2014.08.018
94
Uramoto G.-I. Morono Y. Tomioka N. Wakaki S. Nakada R. Wagai R. et al . (2019). Significant contribution of subseafloor microparticles to the global manganese budget. Nat. Commun.10, 400. doi: 10.1038/s41467-019-08347-2
95
Vuillemin A. Morlock M. Paskin A. Benning L. G. Henny C. Kallmeyer J. et al . (2023). Authigenic minerals reflect microbial control on pore waters in a ferruginous analogue. Geochem. Perspect. Lett.28, 20–26. doi: 10.7185/geochemlet.2339
96
Wallenius A. J. Dalcin Martins P. Slomp C. P. Jetten M. S. M. (2021). Anthropogenic and environmental constraints on the microbial methane cycle in coastal sediments. Front. Microbiol.12. doi: 10.3389/fmicb.2021.631621
97
Wang X. Liu Y. Zhang Y. Zhang T. Chang H. Zhang Y. et al . (2018). Structural requirements of manganese oxides for methane oxidation: XAS spectroscopy and transition-state studies. Appl. Catal. B Environ.229, 52–62. doi: 10.1016/j.apcatb.2018.02.007
98
Wang Y. Van Cappellen P. (1996). A multicomponent reactive transport model of early diagenesis: Application to redox cycling in coastal marine sediments. Geochim. Cosmochim. Acta60, 2993–3014. doi: 10.1016/0016-7037(96)00140-8
99
Wang F.-P. Zhang Y. Chen Y. He Y. Qi J. Hinrichs K.-U. et al . (2014). Methanotrophic archaea possessing diverging methane-oxidizing and electron-transporting pathways. ISME J.8, 1069–1078. doi: 10.1038/ismej.2013.212
100
Wankel S. D. Adams M. M. Johnston D. T. Hansel C. M. Joye S. B. Girguis P. R. (2012). Anaerobic methane oxidation in metalliferous hydrothermal sediments: influence on carbon flux and decoupling from sulfate reduction. Environ. Microbiol.14, 2726–2740. doi: 10.1111/j.1462-2920.2012.02825.x
101
Wefer G. Billett D. Hebbeln D. Jørgensen B. B. Schlüter M. van Weering T. C. E. (Eds.) (2003). Ocean Margin Systems. (Berlin, Heidelberg: Springer Berlin Heidelberg). doi: 10.1007/978-3-662-05127-6
102
Wegener G. Krukenberg V. Riedel D. Tegetmeyer H. E. Boetius A. (2015). Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature526, 587–590. doi: 10.1038/nature15733
103
Wu D. Xie R. Liu J. Yang F. Sun T. Liu L. et al . (2020). Zone of metal-driven anaerobic oxidation of methane is an important sink for phosphorus in the Taixinan Basin, South China Sea. Mar. Geol.427, 106268. doi: 10.1016/j.margeo.2020.106268
104
Xiao X. Luo M. Zhang C. Zhang T. Yin X. Wu X. et al . (2023). Metal-driven anaerobic oxidation of methane as an important methane sink in methanic cold seep sediments. Microbiol. Spectr.11, e05337–e05322. doi: 10.1128/spectrum.05337-22
105
Xiao X. Zhou Q.-Z. Fu S.-Y. Liang Q.-Y. Xu X.-P. Li Y. et al . (2019). Petrographical and geochemical signatures linked to Fe/Mn reduction in subsurface marine sediments from the hydrate-bearing area, Dongsha, the South China Sea. Minerals9, 624. doi: 10.3390/min9100624
106
Xu L. Zhuang G.-C. Montgomery A. Liang Q. Joye S. B. Wang F. (2021). Methyl-compounds driven benthic carbon cycling in the sulfate-reducing sediments of South China Sea. Environ. Microbiol.23, 641–651. doi: 10.1111/1462-2920.15110
107
Yang H. Lu H. Ruffine L. (2018). Geochemical characteristics of iron in sediments from the Sea of Marmara. Deep Sea Res. Part II Top. Stud. Oceanogr.153, 121–130. doi: 10.1016/j.dsr2.2018.01.010
108
Yang H. Zhang P. Lu H. Shi M. Li J. Lu Y. et al . (2023). Magnetic properties of gas hydrate-bearing sediments and their association with iron geochemistry in the Sea of Marmara, Turkey. Chem. Geol.620, 121339. doi: 10.1016/j.chemgeo.2023.121339
109
Yao W. Millero F. J. (1996). Oxidation of hydrogen sulfide by hydrous Fe(III) oxides in seawater. Mar. Chem.52, 1–16. doi: 10.1016/0304-4203(95)00072-0
110
Ye H. Hu Z. Yin R. Boyko T. D. Liu Y. Li Y. et al . (2025). Electron transfer at birnessite/organic compound interfaces: mechanism, regulation, and two-stage kinetic discrepancy in structural rearrangement and decomposition. Geochim. Cosmochim. Acta388, 253–267. doi: 10.1016/j.gca.2024.10.009
111
Zhang W. Liang J. Liang Q. Wei J. Wan Z. Feng J. et al . (2021b). Gas hydrate accumulation and occurrence associated with cold seep systems in the northern South China Sea: An overview. Geofluids2021, 1–24. doi: 10.1155/2021/5571150
112
Zhang K. Wu X. Chen J. Wang W. Luo H. Chen W. et al . (2021a). The role and related microbial processes of Mn-dependent anaerobic methane oxidation in reducing methane emissions from constructed wetland-microbial fuel cell. J. Environ. Manage.294, 112935. doi: 10.1016/j.jenvman.2021.112935
113
Zhang H. Zhou J. Yuan P. Dong Y. Fan W. Yu X. et al . (2023). Implication from mineralogical and geochemical characteristics of authigenic micronodules in the Haima Cold Seeps for understanding the manganese geochemistry in active seepage. J. Geophys. Res. Oceans128, e2022JC019194. doi: 10.1029/2022JC019194
114
Zhao Y. Liu Y. Cao S. Hao Q. Liu C. Li Y. (2024). Anaerobic oxidation of methane driven by different electron acceptors: A review. Sci. Total Environ.946, 174287. doi: 10.1016/j.scitotenv.2024.174287
115
Zhong Y. Chen Z. González F. J. Hein J. R. Zheng X. Li G. et al . (2017). Composition and genesis of ferromanganese deposits from the northern South China Sea. J. Asian Earth Sci.138, 110–128. doi: 10.1016/j.jseaes.2017.02.015
Summary
Keywords
manganese oxide, AOM (anaerobic oxidation of methane), Mn-AOM, microbial communities, marine sediments
Citation
Xue Y, Lu H, Li Y, Yang H, Tang Y and Lu Y (2025) Anaerobic oxidation of methane by manganese oxides in marine sediments: a review. Front. Mar. Sci. 12:1609892. doi: 10.3389/fmars.2025.1609892
Received
11 April 2025
Accepted
16 June 2025
Published
27 June 2025
Volume
12 - 2025
Edited by
Khan M. G. Mostofa, Tianjin University, China
Reviewed by
Sai Xu, Nanjing University of Science and Technology, China
Mohammad Mohinuzzaman, Noakhali Science and Technology University, Bangladesh
Yinan Deng, Guangzhou Marine Geological Survey, China
Updates
Copyright
© 2025 Xue, Lu, Li, Yang, Tang and Lu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hailong Lu, hlu@pku.edu.cn; Hailin Yang, hyang@pku.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.