Abstract
Finfish aquaculture is a key contributor to global seafood production, providing quality protein to consumers across the world, however, the search for regenerative, cost-effective and scalable raw materials continues. Alternatives to wild-caught fish have been explored extensively with limited success. Among the most promising alternatives is autotrophic marine microalgae as they produce essential fatty acids, amino acids and bioactive compounds that support fish growth and immune function. However, their development into aquafeed is limited due to cost. By considering the major benefits of microalgae in aquafeed, as well as biotechnological advancements in the industry, this review aims to consolidate multi-disciplinary findings and highlight research opportunities to facilitate widespread adoption of microalgae into aquafeed.
1 Introduction
With global population growth, food production faces significant challenges including competition for arable land, water use, and sustainable feedstocks (FAO, 2022; Subasinghe et al., 2009). Finfish aquaculture offers solutions by producing high-quality protein without competing for arable land or potable water. However, its sustainability remains under scrutiny due to reliance on wild-caught fish for feed, which is neither scalable nor sustainable (Boyd et al., 2020). Aquaculture production already surpasses that of beef, by weight, and by 2050 is expected to double. Sustainable raw feed products with comparable nutritional composition to wild-caught fish are required to support this intensification. This will require incorporating raw materials that are currently costly, underexplored, or underutilised (FAO, 2022).
Many alternative aquafeed proteins and oils compromise aquaculture’s sustainability and nutritional benefits. For example, soybean production competes for arable land and contains antinutritional factors (Aragão et al., 2022; Pueppke et al., 2020), while insect-based meals lack essential fatty acids (Oosting et al., 2022). In contrast, microalgae cultivation avoids resource competition and supports not only sustainable, but regenerative farming through bioremediation, nutrient cycling, and CO2 bio fixation. Microalgae also provide marine-derived fatty acids and bioactive compounds that are not commonly found in terrestrial-sourced feeds, benefiting both farmed fish health and consumer nutrition (Geada et al., 2021; Neori and Guttman, 2017).
Microalgae are comprised of a diverse range of bioactive compounds that include omega-3 Poly Unsaturated Fatty Acids (ω-3 PUFAs), most importantly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), vitamins, pigments, phenolic compounds, polysaccharides, nucleotides, and peptides. These compounds are essential to human and animal health and have been recognised as: anti-oxidative, anti-inflammatory, immunostimulant, anti-fungal, anti-bacterial, anti-viral and, anti-tumour (Bahi et al., 2023). Of these proteins, carotenoids, like astaxanthin, have stood out as one of the only groups of bioactives that possess all of these health properties, while polyphenols are becoming increasingly of interest due to their high radical scavenging and anti-viral potential (Michalak & Chojnacka, 2015; Pereira & Cotas, 2023) (Figure 1).
Figure 1
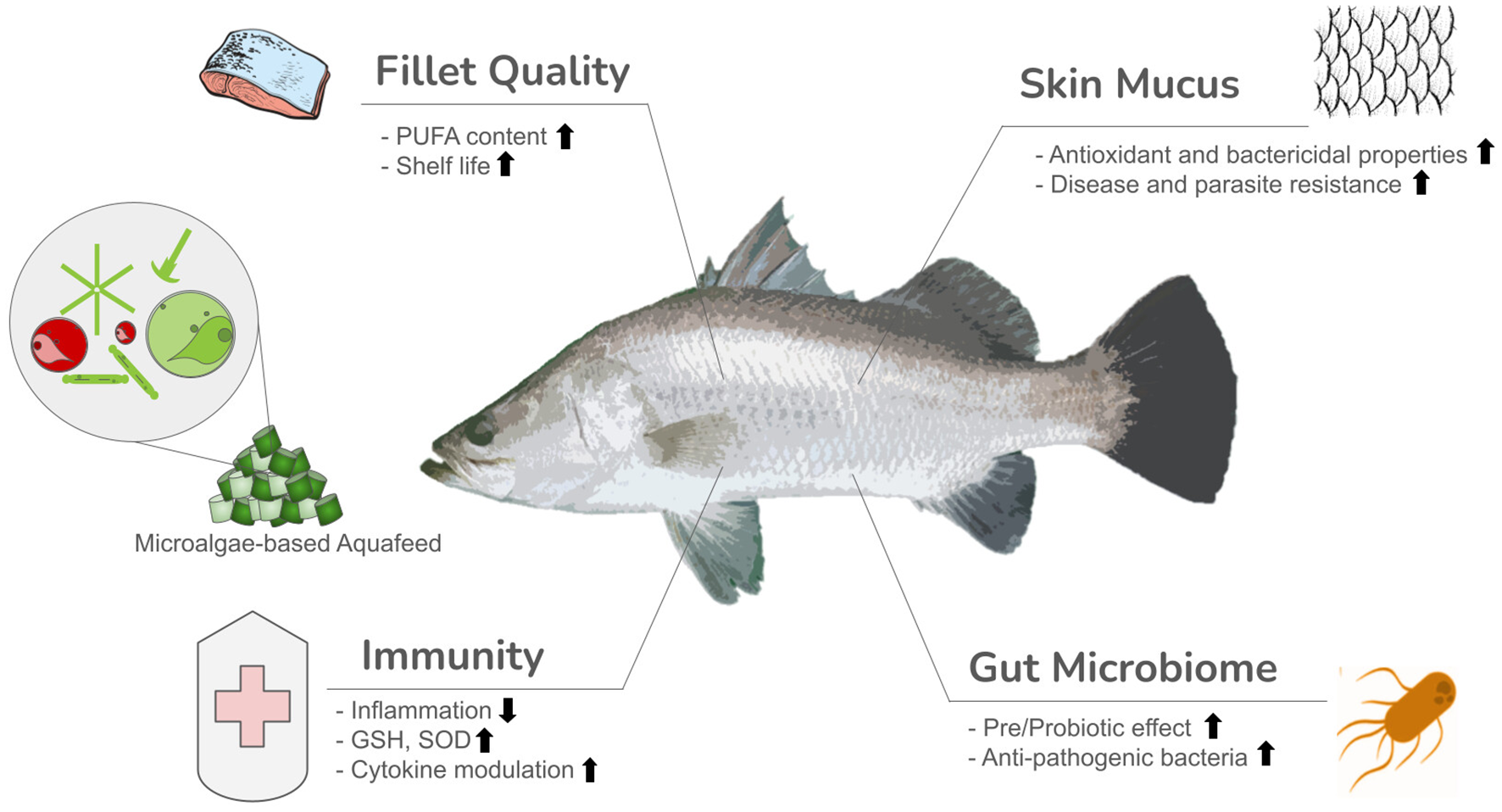
Graphical abstract representing benefits of microalgae supplemented aquafeed. GSH, Glutathione; SOD, Superoxide dismutase.
Microalgae have a demonstrated potential to be used in aquaculture, yet large-scale integration into aquafeed remains limited due to gaps in industry-focused research. These include challenges related to cost, but also nutritional profiling, strain selection, and market demand (Lu et al., 2023b; Ma and Hu, 2023). Advancements in upstream biotechnology, production systems, and efficient downstream processing are essential for harnessing the functional and nutritional value of microalgae in aquafeeds.
2 Microalgae diversity and strain selection
Microalgae exhibit broad species diversity with highly variable nutritional profiles. Among the 40 species commonly used in aquaculture, protein, lipid, and carbohydrate contents range from 21–65%, 4–36%, and 1–58%, respectively (Table 1). Traditionally, microalgae have played a vital role as live feed for rotifers, copepods, crustaceans, and shellfish. However, only 19 of these species are currently incorporated into finfish aquafeeds.
Table 1
| Species | Proximate composition % | Animal model | References | |||
|---|---|---|---|---|---|---|
| Protein % | Lipid % | Carbohydrate % | Biological effect | % Inclusion | ||
| Arthrospira platensis | 46-65 | 4-23 | 20-30 | European seabass; ↑Immunity | 5 |
Vieira et al., 2021
Ahmad et al., 2022 Güroy et al., 2022* |
| Chlorella vulgaris | 12-58 | 5-22 | 1-58 | Largemouth bass; ↑FBW, ↑SGR, ↑Liver health | 15 |
Guedes et al., 2015
Ahmad et al., 2022 Xi et al., 2022* |
| Dunaliella salina | 37-57 | 6-18 | 20-32 | No relevant aquaculture-reared fish data | – |
Andriopoulos et al., 2022
Becker, 2007 Lafarga, 2020* |
| Haematococcus pluvialis | 31 | 28 | 27 | Red tilapia; ↑Immunity, ↑FBW, ↑SGR, ↑Antioxidant capacity | 1.5 |
Álvarez et al., 2020
Eldessouki et al., 2024* |
| Nannochloropsis oceanica | 28-43 | 16-36 | 5-25 | European seabass; ↔FCR, ↓HSI | 8 |
Zhang et al., 2023
Cerri et al., 2021 Siddik et al., 2024 Batista et al., 2020* |
| Nannochloropsis oculata | 27-42 | 13-36 | 10-21 | Nile Tilapia; ↓FCR, ↑FBW, ↑Immunity | 5-10 |
Andriopoulos et al., 2022
Andrew et al., 2022 Siddik et al., 2024 Lafarga, 2020 Abdelghany et al., 2020* |
| Pavlova viridis | 27 | 24 | 16 | European seabass; ↓FCR, ↑SGR, | 100 (oil) |
Zhang et al., 2023
Haas et al., 2016* |
| Phaeodactylum tricornutum | 25-44 | 9-24 | 10-25 | Atlantic salmon; ↔FCR, ↔SGR | 6 |
Tibbetts et al., 2015
Cerri et al., 2021 Siddik et al., 2024 Lafarga, 2020 Sørensen et al., 2016* |
| Scenedesmus obliquus | 21-56 | 12-22 | 10-17 | Rainbow trout; ↑Fillet PUFA, ↔FCR, ↔FBW | 5 |
Becker, 2007
Siddik et al., 2024 Skalli et al., 2020* |
| Tisochrysis lutea | 43 | 26 | 14 | European seabass; ↔FCR, ↔SGR | 15 (blend) |
Cerri et al., 2021
Cardinaletti et al., 2018* |
*Study in reference to animal trial.
Final Body Weight (FBW), Feed Conversion Ratio (FCR), Specific Growth Rate (SGR), Hepatosomatic Index (HSI), Polyunsaturated Fatty Acid (PUFA). ↑ Increase compared to control diet, ↓ decrease compared to control diet, ↔ no significant difference observed compared to control diet. Note that the proximate composition of algae species could vary between strains and under different environmental conditions.
In a Web of Science search (2013-2024, keyword: “Microalgae Fish Feed”) Anthrospira sp., Chlorella sp., Scenedesmus sp., Dunaliella sp., and Nannochloropsis sp. make up 52% of research output. This is due to desirable traits like Dunaliella’s lack of a cell wall, which reduce processing costs, Anthrospira’s high protein content and Nannochloropsis’ high EPA content (Table 1). Despite the marine origin of many target species, large-scale production is dominated by freshwater strains such as Chlorella (Siddik et al., 2024), challenging the assumption that microalgae cultivation does not compete with freshwater resources.
Another component of selecting microalgae for aquafeed is the dietary requirements of the species being reared. Carnivorous species like Atlantic Salmon, Barramundi and Sea Bream require high protein and lipid content and have a low carbohydrate tolerance. While popular herbivorous and omnivorous freshwater species in Asia, like Carp, Tilapia and Catfish have a higher tolerance for carbohydrates (Ansari et al., 2021).
There is no standardised method to select microalgae suitable for species-specific aquafeed. Ideally, microalgae would be chosen based on their macronutrient content, aligning with the dietary requirements of specific fish species; however, many selection parameters focus on the cell morphology, environmental resilience, and growth kinetics.
A decision matrix where aquaculture-appropriate microalgae are assessed for these macronutrients and bioactives, and marked accordingly may be of significant use in determining species-specific algae incorporation on a farm-by-farm basis. Mofijur et al. (2022) apply the Preference Ranking Organisation Method for Enrichment Evaluation (PROMETHEE)-GAIA method to select the most suitable microalgae strains for aviation fuel production. Nineteen criteria with equal weighting among biomass production, lipid quality and fatty acid methyl esters were assessed. No decision matrix or defined criteria exist for microalgae species and aquaculture reared fish.
3 Nutritional value of microalgae in aquafeeds
3.1 Protein
The largest cost in aquafeed production is protein, with fishmeal (USD $2,200/tonne FAO., 2024) being the primary source due to its palatability, amino acid composition, lipid profile, and other essential nutrient content. However, factors like cost, scalability, and sustainability persuade companies to substitute protein with poultry meal, blood meal, and soybean meal. While these alternatives contribute to a circular economy and supply some essential amino acids, they are not scalable and often result in poor-quality feed ingredients (Idenyi et al., 2022).
Microalgae contain essential amino acids comparable to or surpassing that of common animal-based proteins like eggs (Wells et al., 2017). Most species relevant to aquaculture provide all essential amino acids, including Arginine, Tyrosine and Taurine, which are important for marine animal development (Zhang et al., 2023). Protein content varies by species (Table 1) but may be increased by cultivating under nitrogen supplementation, low salinity, increased CO2, and by harvesting during the exponential phase (Geada et al., 2021).
Given the high variability in microalgae protein content, it is essential to standardise quantification methods and ensure reported values are directly relevant to practical applications. Since microalgae contain non-protein nitrogen, conversion should use N x 4.78 rather than the traditionally used N x 6.25 (Geada et al., 2021). Additionally, reporting the essential amino acid index (EAAI) would enable cross-species comparisons of protein quality for use in aquaculture.
3.2 Lipids
As fish oil (USD $7,700/tonne (FAO., 2024) is a high-value commodity in aquafeed, many commercial feeds use cheaper terrestrial alternatives such as vegetable oils, e.g. linseed, palm, and soybean. While these can maintain similar food conversion ratios (FCR) and specific growth rates (SGR) in many fish species (Hodar et al., 2020), excessive substitution can lead to deficiencies in essential fatty acids, compromising the health benefits to humans while leading to physiological and immunological disorders in fish (Rahman et al., 2024). Microalgae are among the few primary producers capable of de novo synthesis of ω-3 PUFAs, making them a promising and sustainable alternative to fish oil in aquafeed formulations.
Increased photoautotrophic lipid production has been reported under optimised abiotic conditions for many microalgae species, with dry weight levels reaching up to 70%, and ω-3 PUFA content reaching as high as 50% of total lipids (Sun et al., 2018). The rapid oxidation of PUFAs means their optimal accumulation occurs in environments with low oxidative damage. High light, high temperature, high salinity and late harvesting stage all negatively affect ω-3 PUFA productivity in most microalgae species (Sun et al., 2018). To enhance lipid accumulation in industry-scale production, nitrogen (N) limitation is commonly used in a two-stage cultivation process: the first stage focuses on biomass productivity under N-replete conditions, while the second is N-deplete to enhance lipid production (Liyanaarachchi et al., 2021).
3.3 Bioactives
Bioactives in microalgae include both macronutrients and secondary metabolites such as pigments, phenolic compounds, vitamins, and minerals. Investigating the synergistic effects of these compounds may guide future aquafeed formulation strategies. A key feature of microalgae bioactives is their antioxidative capacity.
3.3.1 Antioxidants
Antioxidants are radical-scavenging molecules that help maintain redox balance within cells. They are vital in fishmeal and aquafeed, stabilising ω-3 PUFAs during storage and providing health benefits to fish. Common synthetic phenolic antioxidants (SPAs) used in aquafeed include ethoxyquin, butylated hydroxytoluene (BHT), and butylated hydroxyanisole (BHA). While ethoxyquin is non-toxic, it is synthesised from p‐phenetidine, a known carcinogen (Bampidis et al., 2022) and was banned in aquafeed by the European Commission in 2022 due to environmental concerns (EU 2022/1375). There are also safety and ecotoxicological concerns of BHT and BHA, with toxic xenobiotic responses observed in Salmo salar (Holaas et al., 2008) and observed thyroid damage, metabolic damage, neurotoxicity and carcinogenesis linked to BHA in fish and humans (Wang et al., 2021). The use of BHA and BHT is still legal in Europe, the USA, Australia and Canada despite some health evaluations that cannot conclude with certainty that these compounds are safe (Australian Industrial Chemicals Introduction Scheme, 2022).
Microalgae provide a natural and safer alternative making antioxidant capacity an important parameter when assessing the suitability of microalgae for aquafeed (Elbahnaswy and Elshopakey, 2024). However, significant variation exists within species, between species, and among extraction methods. For example, antioxidant capacity was often attributed to Total Phenolic Content (Safafar et al., 2015; Hemalatha et al., 2013). This correlation rarely considers that the extraction solvents used to determine the phenolic content by the Folin-Ciocalteu method are also influenced by pigments such as carotenoids and chlorophylls with known radical scavenging properties. Thus, caution must be taken in evaluating microalgae antioxidant capacity, and it is advised to use multiple assays to fully understand radical scavenging potential (Andriopoulos et al., 2022). The most reported, cost-effective, and recommended methods are: DPPH- measuring the donation of electrons or hydrogen atoms; ABTS- measuring electron donation and acceptance and; FRAP- measuring electron donation to reduce ferric ions (Fe3+) to ferrous ions (Fe2+).
3.3.2 Carotenoids
Carotenoids, a type of pigment, are the most researched microalgal antioxidants with uses in aquaculture, cosmetics, pharmaceuticals, and medicine. They accumulate in the thylakoid membrane, where they transfer light energy to chlorophylls, protecting the PSII antenna complexes during high-light via the xanthophyll cycle (Solovchenko, 2013; Coulombier et al., 2021).
Carotenoids are separated into two groups- carotenes which are comprised of only hydrogen and carbon (e.g., α-carotene, β-carotene, and lycopene), and xanthophylls, which also contain oxygen (e.g., astaxanthin, canthaxanthin, fucoxanthin, zeaxanthin, and lutein). Xanthophylls are the most powerful type of carotenoid and of these, astaxanthin provides the most radical scavenging potential due to its unique hydroxyl and ketone functional groups.
In aquaculture, astaxanthin is used as a red-orange pigment to enhance fishes like salmon and trout’s marketability, growth, and immunity (Lu et al., 2021). Synthetic astaxanthin currently dominates the market but is less bioavailable to fish due to its unesterified structure, lacks antioxidative power, and is derived from petrochemicals (Capelli et al., 2013). Natural astaxanthin is preferred in pharmaceutical, cosmetic, and food industries, as further human trials are needed to assess the impacts of synthetic astaxanthin metabolites (Stachowiak and Szulc, 2021).
Haematococcus sp. is the greatest producer of natural astaxanthin (up to 7% dry weight), but its slow growth makes it susceptible to contamination. Haematococcus sp. also competes for freshwater resources and has a tough cell wall matrix complicating astaxanthin extraction. Alternatives such as Chlorella zofingiensis and Scenedesmus obliquus show great potential (Patel et al., 2022).
3.3.3 Polyphenols
Polyphenols are the largest group of secondary metabolites in terrestrial plants and have been shown to alleviate oxidative and inflammatory stress in humans while also improving microbial resistance (Besednova et al., 2020; Del Mondo et al., 2021). They are divided into three main groups: phenolic acids, flavonoids (including flavones, isoflavones, flavanols) and non-flavonoids (including stilbenes, lignans, and tannins).
Polyphenols play many roles in cell defence in microalgae through their antioxidative and antiviral properties, allelopathic signalling, and nutrient uptake regulation (Manzoor et al., 2025). The phenolic content of microalgae (up to 15 mg GAE g−1 DW) is roughly ten times higher than herbs like rosemary sage and mint (0.5–0.6 mg GAE g−1 DW) but lower than agri-food waste (32 mg GAE g−1 DW for dried grape vines) (Andriopoulos et al., 2022; Panzella et al., 2020; Stanciu et al., 2017).
Interestingly, the anti-viral capacity of polyphenols isn’t solely dependent on their antioxidative potential. Mechanisms by which polyphenols inhibit viruses depend on the type, polyphenol compound, origin, and whether this polyphenol is used as an extract or with other polyphenols (Chojnacka et al., 2021). Polyphenols block virus entry into cells, internalise cell receptors to prevent viral endocytosis, alter virus structure to inhibit replication and enhance immune responses by inhibiting pro-inflammatory cytokines like histamine. The antioxidative capacity of polyphenols supports these mechanisms by also reducing oxidative damage.
While increasing polyphenol levels in microalgae can benefit fish health when fed whole (Manzoor et al., 2025), targeted extraction and polyphenol supplementation into aquafeed is less viable for several reasons. Primarily, microalgae cultivation to produce and extract polyphenols would require more energy than currently overlooked sources like fruit waste, e.g., grape seed extract from the by-product of winemaking (Nirmal et al., 2023). Secondly, polyphenols are also poorly absorbed in the gut of fish, meaning adequate absorption would require alternative delivery methods if isolated from microalgae. Finally, the anti-viral properties of polyphenols are highly specific and inappropriate as broad-spectrum treatments for aquaculture systems- they must be delivered in a targeted manner. Enhancing microalgae’s polyphenol composition remains a promising avenue for promoting fish welfare; however, their use in aquafeed must be aligned with species-specific whole-cell feed.
4 Advances in microalgal biotechnology for aquafeed development
Extending the use of microalgae in finfish aquaculture from hatchery to harvest will require biotechnological advancements across upstream cultivation, production systems, and downstream processing.
4.1 Upstream cultivation
Technological advances in upstream cultivation have great potential, especially across two key fields: metabolic engineering and species-specific strain selection. These advancements will ultimately reduce costs by optimising productivity, improving the accumulation of high-value metabolites, and meeting species-specific nutritional requirements.
Metabolic engineering refers to the enhancement of a targeted metabolic pathway’s efficiency. In microalgae, increased metabolite production, stress resilience, photosynthetic efficiency and even carbon sequestration have been achieved. Robust tools for metabolic engineering include: adaptive laboratory evolution (ALE), genome editing, chemical elicitors (e.g. phytohormones), and co-cultivation strategies (e.g. with bacteria or another microalgae).
Adaptive Laboratory Evolution (ALE) subjects microalgae to a defined stress condition over successive generations. This drives the evolution of genetic variants that can adapt readily, resulting in highly refined strains with enhanced metabolic pathways. For example, stimulating carotenoid production using ALE in microalgae is usually linked to photoprotective pathways. Parkes et al. (2022) enhanced astaxanthin production in three Haematococcus species using blue light, which upregulates the psy, pds, dgat1 and dgat2d gene pathways- precursors for xanthophyll cycle pigments. D. salina and P. tricornutum exposed to a combination of red (75%) and blue (25%) LED light resulted in 3.3-fold higher β-carotene, and 2-fold higher fucoxanthin content, respectively (Fu et al., 2013; Yi et al., 2015). Interestingly, similar increases in astaxanthin production have been observed with ALE under salinity stress, nitrogen deprivation, and glucose supplementation in Haematococcus pluvialis and Chromochloris Zofingiensis (Lu et al., 2021). Recent advancements include the strategic design of multi-factor ALE to enhance microalgae tolerance to multiple stressors for practical applications, along with machine learning models to identify optimal evolutionary endpoints (B. Zhang et al., 2021).
Gene editing is extremely efficient when focused on targeted biosynthesis pathways such as fatty acid production e.g., N. gaditana enhanced from 20% (wild-type) to 40-55% (mutant) in N-replete conditions (Ajjawi et al., 2017). However, ALE might be advantageous over gene editing for enhancing adaptations such as stress tolerance, as these adaptations are typically mediated polygenically. Furthermore, genetically modified microalgae are subject to ethical and regulatory concerns, especially for use as a feedstock. In this respect, biocontainment of mutants can be physically ensured using closed systems such as photobioreactors, as well as biochemical assurances that work under ‘lock-and-key’ methods, such as synthetic auxotrophy and conditional lethality, which can be further explored in Sebesta et al. (2022). Overall, gene editing technology remains highly optimal for the biofuels and bioplastics sectors.
Recent research aimed at enhancing lipid accumulation for aquafeed for large-scale applications focuses on co-cultivation strategies, especially with the phytobiome, the community of bacteria that interact with the extracellular polymeric substances excreted by algae. Co-culturing I. galbana with Marinobacter sp. (Wang et al., 2022; Xu et al., 2024) and Bacillus jeotgali (Xu et al., 2024) enhanced DHA and EPA production, upregulating desaturase genes associated with PUFA biosynthesis. These bacteria create a less oxidative environment through mechanisms such as gas exchange, secretion of phytohormone and quorum signalling compounds, nutrient acquisition, antibiotic production, and degradation of organic matter (Wang et al., 2022).
Use of elicitors such as phytohormones and quorum signalling compounds that influence microbial consortia behaviour also show great potential for regulating axenic cultures, particularly in photobioreactors. Although more research is needed to determine their effects on different algal strains and to identify phytohormone receptors. Preliminary findings are promising with increases in high-value metabolites, specific growth, and stress tolerance reported (Han et al., 2018).
4.2 Strain selection
The genetic diversity of microalgae is expected to expand alongside advances in metabolic engineering. To support the deployment of elite strains across environmental conditions, high-throughput screening technologies are being developed to catalogue complex phenotypic data. Microalgal phenomics aims to emulate existing plant and yeast phenomics databases by constructing a searchable library of phenotypic traits, enabling researchers to identify shared characteristics and distinguish the roles of seemingly redundant gene copies (Fabris et al., 2020). In aquaculture, phenotypic traits such as the nutritional and bioactive content but also digestibility of microalgae could be mapped to specific animal models. This approach would allow strain development to target specific nutritional gaps in key aquaculture species across varying environments.
4.3 Production systems
High production and processing costs have limited the scalability of microalgae-based feeds; however, biotechnological advances in the field of metabolic engineering now allow for greater strain diversification, enabling optimised production in wastewater systems, saline environments, and non-arable land. While recent photobioreactor technologies can improve productivity and reduce biofouling and contamination, downstream costs associated with harvesting and pre-treatment remain significant. As algal growth and metabolite production are intrinsically linked in a complex system, incorporating a biorefinery at the algae production site increases economic feasibility in three key ways. First, it enables full automation from seeding to extraction. Second, it allows for the integration of advanced control systems such as artificial intelligence to dynamically adjust abiotic conditions to optimise metabolite yield. Third, co-locating the biorefinery with the production reduces costs associated with transport and facilitates recycling waste streams such as CO2 emissions generated during production (Lim et al., 2022; Samoraj et al., 2024).
4.4 Downstream processing
Technological advances in downstream processing have significant potential for enhancing microalgae utilization in aquaculture, particularly through improved hydrolysis methods that maximize nutrient bioavailability, while reducing costs.
Hydrolysis techniques can significantly improve nutrient bioavailability of aquafeeds for aquaculture species. For example, apparent digestibility coefficients (ADCs) for microalgae proteins in fish can range from 60-85% depending on species and processing method, with mechanical cell disruption typically improving protein ADCs by 10-20%, compared to untreated biomass (Ahmad et al., 2022; Rahman Shah et al., 2018). The use of chemical treatments like acid/alkaline hydrolysis, thermal processing via extrusion, and ultrasonification also demonstrate effectiveness in improving protein digestibility (Sirohi et al., 2021). However, these come at high capital and operational costs. Therefore it is vital that more cost-effective treatments are explored.
Promising “Green methods” show that bacteria can also mediate downstream processing steps including flocculation (Yee et al., 2021) as well as hydrolytic treatment of cell wall during fermentation (Barati et al., 2021), increasing their digestibility in aquafeed (Ali et al., 2024). A better understanding of algae-bacteria interactions could also benefit open raceway farms and waste-water based cultivation systems, where contamination is unavoidable. By providing necessary prebiotics and probiotics to the medium, the enrichment of production-stage bacteria could significantly reduce costs (Úbeda et al., 2017). This synergistic approach may also enhance aquaculture health by inhibiting pathogens during circular cultivation (Smahajcsik et al., 2025).
5 Concluding remarks
Microalgae have a unique potential to future-proof aquaculture by enabling ‘zero-catch’ feeds through the sustainable production of high-quality protein and ω-3 PUFAs. However, the current cost of microalgae-meal ranges from USD $5-10/kg, while fishmeal is approximately USD $2.20/kg (Lu et al., 2023b; FAO, 2022). This significant price gap remains a major barrier to commercial viability.
While microalgae-based feeds are more expensive per unit, they may deliver greater functional value. The aquaculture industry faces many challenges including disease, parasitism, hypoxic conditions, feed preservation, and eutrophication which pose a significant financial burden on farmers, especially during viral and bacterial-induced mass mortalities. Incorporating a functional feed like microalgae may ameliorate this cost burden through improvements in fish immunity and welfare, thus offsetting additional costs associated with microalgae feed. Adopting circular cultivation systems, for example, growing microalgae in aquaculture effluent, may further improve cost-benefit (Ansari et al., 2021).
Encouragingly, studies on fish immunity, stress resilience, and species-specific metabolic pathways are growing, and recent research has focused on the effects of alternative diets on fish health, growth, and well-being. This includes parameters that determine the gut and skin mucus microbiome composition, immune function, and disease resistance through gene expression analysis (Aragão et al., 2022).
Enhancing the antioxidative potential of microalgae through metabolic engineering by targeting xanthophyll pigments, polyphenols, and ω-3 PUFA lipid accumulation is important to supply cost-effective yields. Additionally, to apply these benefits in a practical manner, it is crucial to standardise the reporting of industry-relevant nutritional parameters like total phenolics, antioxidative capacity and protein content. Finally, emphasis should be placed on industry-relevant scale-up studies utilising cost effective processing methods, such as bacterial flocculation and green pre-treatment technologies. Together, these biotechnological advancements will improve the efficiency, quality and scalability of microalgal feedstocks, supporting the transition toward a more sustainable aquaculture industry.
Statements
Author contributions
JT: Writing – original draft, Writing – review & editing. PR: Writing – review & editing. IP: Writing – review & editing. MP: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Open-access publication fees were covered by the state of Bremen.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdelghany M. F. El-Sawy H. B. AbdEl-hameed S. A. A. Khames M. K. Abdel-Latif H. M. R. Naiel M. A. E. (2020). Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish and Shellfish Immunology, 107, 277–288. doi: 10.1016/j.fsi.2020.10.015
2
Ahmad A. Hassan W. Banat F. (2022). An overview of microalgae biomass as a sustainable aquaculture feed ingredient: food security and circular economy. Bioengineered13, 9521–9547. doi: 10.1080/21655979.2022.2061148
3
Ajjawi I. Verruto J. Aqui M. Soriaga L. B. Coppersmith J. Kwok K. et al . (2017). Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat. Biotechnol.35, 647–652. doi: 10.1038/nbt.3865
4
Ali S. Waqas W. Bakky M. A. H. Zada S. Saif U. M. Hasan M. T. et al . (2024). Implications of microalgal–bacterial interactions in modern aquaculture practices: A review of the current knowledge. Rev. Aquaculture. 17, e12980. doi: 10.1111/raq.12980
5
Álvarez C. E. Vardanega R. Salinas-Fuentes F. Ramírez J. P. Muñoz W. B. Jiménez-Rondón D. et al . (2020). Effect of CO2 Flow Rate on the Extraction of Astaxanthin and Fatty Acids from Haematococcus pluvialis Using Supercritical Fluid Technology. Molecules, 25 (24). doi: 10.3390/MOLECULES25246044
6
Andrew A. R. Yong W. T. L. Misson M. Anton A. Chin G. J. W. L . (2022). Selection of Tropical Microalgae Species for Mass Production Based on Lipid and Fatty Acid Profiles. Front. Energy Res.10. doi: 10.3389/fenrg.2022.912904
7
Andriopoulos V. Gkioni M. D. Koutra E. Mastropetros S. G. Lamari F. N. Hatziantoniou S. et al . (2022). Total phenolic content, biomass composition, and antioxidant activity of selected marine microalgal species with potential as aquaculture feed. Antioxidants11 (7), 1320. doi: 10.3390/antiox11071320
8
Ansari F. A. Guldhe A. Gupta S. K. Rawat I. Bux F. (2021). Improving the feasibility of aquaculture feed by using microalgae. Environ. Sci. pollut. Res.28, 43234–43257. doi: 10.1007/s11356-021-14989-x
9
Aragão C. Gonçalves A. T. Costas B. Azeredo R. Xavier M. J. Engrola S. (2022). Alternative proteins for fish diets: implications beyond growth. Animals12, 1–41. doi: 10.3390/ani12091211
10
Australian Industrial Chemical Introduction Scheme. Australian Department of Health (2022). Butylated hydroxyanisole and related antioxidants (Evaluation statement EVA00029)
11
Bahi A. Ramos-Vega A. Angulo C. Monreal-Escalante E. Guardiola F. A. (2023). Microalgae with immunomodulatory effects on fish. Rev. Aquaculture15, 1522–1539. doi: 10.1111/raq.12792
12
Bampidis V. Azimonti G. Bastos M. de L. Christensen H. Dusemund B. et al . (2022). Safety and efficacy of a feed additive consisting of ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline) for all animal species (FEFANA asbl). EFSA J.20 (3), 7166.44. doi: 10.2903/j.efsa.2022.7166
13
Barati B. Zafar F. F. Rupani P. F. Wang S. (2021). Bacterial pretreatment of microalgae and the potential of novel nature hydrolytic sources. Environ. Technol. Innovation21, 101362. doi: 10.1016/j.eti.2021.101362
14
Batista S. Pereira R. Oliveira B. Baião L. F. Jessen F. Tulli F. et al . (2020). Exploring the potential of seaweed Gracilaria gracilis and microalga Nannochloropsis oceanica, single or blended, as natural dietary ingredients for European seabass Dicentrarchus labrax. J. Appl. Phycology32, 2041–2059. doi: 10.1007/s10811-020-02118-z
15
Becker E. W . (2007). Micro-algae as a source of protein. In Biotechnology Advances25 (2), 207–210. doi: 10.1016/j.biotechadv.2006.11.002
16
Besednova N. N. Andryukov B. G. Zaporozhets T. S. Kryzhanovsky S. P. Kuznetsova T. A. Fedyanina L. N. et al . (2020). Algae polyphenolic compounds and modern antibacterial strategies: Current achievements and immediate prospects. Biomedicines8, 1–19. doi: 10.3390/BIOMEDICINES8090342
17
Boyd C. E. D’Abramo L. R. Glencross B. D. Huyben D. C. Juarez L. M. Lockwood G. S. et al . (2020). Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquaculture Soc.51, 578–633. doi: 10.1111/jwas.12714
18
Capelli B. Bagchi D. Cysewski G. R . (2013) Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement, Nutrafoods12, 145–152. doi: 10.1007/s13749-013-0051-5
19
Cardinaletti G. Messina M. Bruno M. Tulli F. Poli B. M. Giorgi G. et al . (2018). Effects of graded levels of a blend of Tisochrysis lutea and Tetraselmis suecica dried biomass on growth and muscle tissue composition of European sea bass (Dicentrarchus labrax) fed diets low in fish meal and oil. Aquaculture485, 173–182. doi: 10.1016/j.aquaculture.2017.11.049
20
Cerri R. Niccolai A. Cardinaletti G. Tulli F. Mina F. Daniso E. et al . (2021). Chemical composition and apparent digestibility of a panel of dried microalgae and cyanobacteria biomasses in rainbow trout (Oncorhynchus mykiss). Aquaculture, 544. doi: 10.1016/j.aquaculture.2021.737075
21
Chojnacka K. Skrzypczak D. Izydorczyk G. Witek-Krowiak A. Mikula K. Szopa D. (2021). Antiviral properties of polyphenols from plants. Foods10 (10), 2277. doi: 10.3390/foods10102277
22
Coulombier N. Jauffrais T. Lebouvier N. (2021). Antioxidant compounds from microalgae: A review. Mar. Drugs19 (10), 549. doi: 10.3390/md19100549
23
Del Mondo A. Smerilli A. Ambrosino L. Albini A. Noonan D. M. Sansone C. et al . (2021). Insights into phenolic compounds from microalgae: structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol.41, 155–171. doi: 10.1080/07388551.2021.1874284
24
Elbahnaswy S. Elshopakey G. E. (2024). Recent progress in practical applications of a potential carotenoid astaxanthin in aquaculture industry: a review. Fish Physiol. Biochem.50, 97–126. doi: 10.1007/s10695-022-01167-0
25
Eldessouki E. A. A. Elshopakey G. E. Elbahnaswy S. Shakweer M. S. Abdelwarith A. A. Younis E. M. et al . (2024). Influence of astaxanthin-enriched Haematococcus pluvialis microalgae on the growth efficacy, immune response, antioxidant capacity, proinflammatory cytokines, and tissue histomorphology of hybrid red tilapia. Aquacult International. doi: 10.1007/s10499-024-01524-1
26
Fabris M. Abbriano R. M. Pernice M. Sutherland D. L. Commault A. S. Hall C. C. et al . (2020). Emerging technologies in algal biotechnology: toward the establishment of a sustainable, algae-based bioeconomy. Front. Plant Sci.11. doi: 10.3389/fpls.2020.00279
27
FAO (2022). “The state of world fisheries and aquaculture (SOFIA),” in The State of World Fisheries and Aquaculture (SOFIA), vol. 2022. (FAO, Rome).
28
Fu W. Guomundsson Ó. Paglia G. Herjólfsson G. Andrésson Ó.S. Palsson B. O. et al . (2013). Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl. Microbiol. Biotechnol.97, 2395–2403. doi: 10.1007/s00253-012-4502-5
29
Geada P. Moreira C. Silva M. Nunes R. Madureira L. Rocha C. M. R. et al . (2021). Algal proteins: Production strategies and nutritional and functional properties. Bioresource Technol.332, 125125. doi: 10.1016/j.biortech.2021.125125
30
Guedes A. C. Sousa-Pinto I. Malcata F. X . (2015). Application of Microalgae Protein to Aquafeed. In Handbook of Marine Microalgae: Biotechnology Advances. doi: 10.1016/B978-0-12-800776-1.00008-X
31
Güroy B. Güroy D. Bilen S. Kenanoğlu O. N. Şahin I. Terzi E. et al . (2022). Effect of dietary Spirulina (Arthrospira platensis) on the growth performance, immune-related gene expression and resistance to Vibrio anguillarum in European seabass (Dicentrarchus labrax). Aquaculture Research, 53, 2263–2274. doi: 10.1111/are.15745
32
Han X. Zeng H. Bartocci P. Fantozzi F. Yan Y. (2018). Phytohormones and effects on growth and metabolites of microalgae: A review. Fermentation4 (2), 25. doi: 10.3390/fermentation4020025
33
Haas S. Bauer J. L. Adakli A. Meyer S. Lippemeier S. Schwarz K. et al . (2016). Marine microalgae Pavlova viridis and Nannochloropsis sp. as n-3 PUFA source in diets for juvenile European sea bass (Dicentrarchus labrax L.). J. Appl. Phycol.28 (2), 1011–1021. doi: 10.1007/s10811-015-0622-5
34
Hemalatha A. Girija K. Parthiban C. Saranya C. Anantharaman P . (2013). Antioxidant properties and total phenolic content of a marine diatom, Navicula clavata and green microalgae, Chlorella marina and Dunaliella salina. https://www.pelagiaresearchlibrary.com
35
Hodar A. R. Vasava R. Joshi N. H. (2020). Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: a review. J. Exp. Zool. India23 (1), 13–21. Available online at: https://www.researchgate.net/publication/338392541 (Accessed February 15, 2025).
36
Holaas E. Bohne V. B. Hamre K. Arukwe A. (2008). Hepatic retention and toxicological responses during feeding and depuration periods in Atlantic salmon (Salmo salar) fed graded levels of the synthetic antioxidant, butylated hydroxytoluene. J. Agric. Food Chem.56, 11540–11549. doi: 10.1021/jf8025524
37
Idenyi J. N. Eya J. C. Nwankwegu A. S. Nwoba E. G. (2022). Aquaculture sustainability through alternative dietary ingredients: Microalgal value-added products. Eng. Microbiol.2, 100049. doi: 10.1016/j.engmic.2022.100049
38
Lafarga T . (2020). Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Taylor and Francis Inc. In Food Reviews International36 (6), 559–583. doi: 10.1080/87559129.2019.1655572
39
Lim H. R. Khoo K. S. Chia W. Y. Chew K. W. Ho S. H. Show P. L. (2022). Smart microalgae farming with internet-of-things for sustainable agriculture. Biotechnol. Adv.57, 107931. doi: 10.1016/j.bioteChadv.2022.107931
40
Liyanaarachchi V. C. Premaratne M. Ariyadasa T. U. Nimarshana P. H. V. Malik A. (2021). Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review. Algal Res.57, 102353. doi: 10.1016/j.algal.2021.102353
41
Lu Q. Li H. Zou Y. Liu H. Yang L. (2021). Astaxanthin as a microalgal metabolite for aquaculture: A review on the synthetic mechanisms, production techniques, and practical application. Algal Res.54, 102178. doi: 10.1016/j.algal.2020.102178
42
Lu Q. Lu Y. Yang L. (2023b). Challenging problems of applying microalgae for aquaculture environment protection and nutrition supplementation: A long road traveled and still a far way to go. Front. Bioengineering Biotechnol.11. doi: 10.3389/fbioe.2023.1151440
43
Ma M. Hu Q. (2023). Microalgae as feed sources and feed additives for sustainable aquaculture: Prospects and challenges. Rev. Aquaculture2023, 818–835. doi: 10.1111/raq.12869
44
Manzoor Z. Sajad A. Qadiri S. S. N. Shah F. A. Dar S. A. Mandu S. M. (2025). Polyphenols as antiviral agents: Assessing their potential usage and benefits in aquaculture. Aquaculture Int.33. doi: 10.1007/s10499-024-01778-9
45
Michalak I. Chojnacka K . (2015). Algae as production systems of bioactive compounds. Engineering in Life Sciences, 15 (2), 160–176. doi: 10.1002/elsc.201400191
46
Mofijur M. Ashrafur Rahman S. M. Nguyen L. N. Mahlia T. M. I. Nghiem L. D. (2022). Selection of microalgae strains for sustainable production of aviation biofuel. Bioresource Technol.345, 126408. doi: 10.1016/j.biortech.2021.126408
47
Neori A. Guttman L. (2017). Thoughts on algae cultivation toward an expansion of aquaculture to the scale of agriculture. In ButuA.LakatosA.BulsuraP. (Eds.), Proceedings of the 7th International Conference on Innovation in Chemical, Agricultural, Biological and Environmental Sciences (pp. 38–43). HEAIG Group. doi: 10.15242/heaig.h1217234
48
Nirmal N. P. Khanashyam A. C. Mundanat A. S. Shah K. Babu K. S. Thorakkattu P. et al . (2023). Valorization of fruit waste for bioactive compounds and their applications in the food industry. Foods12 (3), 556. doi: 10.3390/foods12030556
49
Oosting S. van der Lee J. Verdegem M. de Vries M. Vernooij A. Bonilla-Cedrez C. et al . (2022). Farmed animal production in tropical circular food systems. Food Secur.14, 273–292. doi: 10.1007/s12571-021-01205-4
50
Panzella L. Moccia F. Nasti R. Marzorati S. Verotta L. Napolitano A. et al . (2020). Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. In Frontiers in NutritionFrontiers Media S.A. 7. doi: 10.3389/fnut.2020.00060
51
Parkes R. Barone M. E. Herbert H. Gillespie E. Touzet N. (2022). Antioxidant activity and carotenoid content responses of three haematococcus sp. (Chlorophyta) strains exposed to multiple stressors. Appl. Biochem. Biotechnol.194, 4492–4510. doi: 10.1007/s12010-022-03926-4
52
Patel A. K. Tambat V. S. Chen C. W. Chauhan A. S. Kumar P. Vadrale A. P. et al . (2022). Recent advancements in astaxanthin production from microalgae: A review. Bioresource Technol.364, 128030. doi: 10.1016/j.biortech.2022.128030
53
Pereira L. Cotas J . (2023). Therapeutic Potential of Polyphenols and Other Micronutrients Marine Origin. In Marine Drugs. MDPI. 21 (6). doi: 10.3390/md21060323
54
Pueppke S. G. Nurtazin S. Ou W. (2020). Water and land as shared resources for agriculture and aquaculture: Insights from asia. Water (Switzerland)12. doi: 10.3390/w12102787
55
Rahman M. A. Tantikitti C. Suanyuk N. U-taynapun K. Chirapongsatonkul N. Forster I. et al . (2024). Use of mixtures of algal and vegetable oils as fish oil replacers in Asian seabass (Lates calcarifer) feeds and their effects on growth, digestive enzymes, immune biomarkers, fatty acid profiles, and expression of genes involved in fatty acid biosynthesis. Aquaculture Int.32, 2427–2453. doi: 10.1007/s10499-023-01278-2
56
Rahman Shah M. Antonio Lutzu G. Alam A. Sarker P. Kabir Chowdhury M. A. Parsaeimehr A. et al . (2018). Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol30, 197–213. doi: 10.1007/s10811-017-1234-z/Published
57
Safafar H. Wagenen J. Van, Møller P. Jacobsen C. (2015). Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs13, 7339–7356. doi: 10.3390/md13127069
58
Samoraj M. Çalış D. Trzaska K. Mironiuk M. Chojnacka K. (2024). Advancements in algal biorefineries for sustainable agriculture: Biofuels, high-value products, and environmental solutions. Biocatalysis Agric. Biotechnol.58, 103224. doi: 10.1016/j.bcab.2024.103224
59
Sebesta J. Xiong W. Guarnieri M. T. Yu J. (2022). Biocontainment of genetically engineered algae. Front. Plant Sci.13. doi: 10.3389/fpls.2022.839446
60
Siddik M. A. B. Sørensen M. Islam S. M. M. Saha N. Rahman M. A. Francis D. S. (2024). Expanded utilisation of microalgae in global aquafeeds. In Rev. Aquaculture16, 6–33. doi: 10.1111/raq.12818
61
Sirohi R. Ummalyma S. B. Sagar N. A. Sharma P. Awasthi M. K. Badgujar P. C. et al . (2021). Strategies and advances in the pretreatment of microalgal biomass. J. Biotechnol.341, 63–75. doi: 10.1016/j.jbiotec.2021.09.010
62
Skalli A. Firmino J. P. Andree K. B. Salomón R. Estévez A. Puig P. et al . (2020). The inclusion of the microalga scenedesmus sp. In diets for rainbow trout, onchorhynchus mykiss, juveniles. Animals, 10 (9), 1–22. doi: 10.3390/ani10091656
63
Smahajcsik D. Roager L. Strube M. L. Zhang S.-D. Gram L. (2025). Stronger together: harnessing natural algal communities as potential probiotics for inhibition of aquaculture pathogens. Microbiol. Spectr. 21, e0042125 doi: 10.1128/spectrum.00421-25
64
Solovchenko A. E . (2013). Physiology and adaptive significance of secondary carotenogenesis green microalgae. In Russian Journal of Plant Physiology60 (1), 1–13. doi: 10.1134/S1021443713010081
65
Sørensen M. Berge G. M. Reitan K. I. Ruyter B . (2016). Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar) -Effect on nutrient digestibility, growth and utilization of feed. Aquaculture, 460, 116–123. doi: 10.1016/j.aquaculture.2016.04.010
66
Stachowiak B. Szulc P. (2021). Astaxanthin for the food industry. Molecules26. doi: 10.3390/molecules26092666
67
Stanciu G. Cristache N. Lupsor S. Dobrinas S . (2017). Evaluation of Antioxidant Activity and Total Phenols Content in Selected Spices. Rev. Chim.68, 1429–1434
68
Subasinghe R. Soto D. Jia J. (2009). Global aquaculture and its role in sustainable development. Rev. Aquaculture1, 2–9. doi: 10.1111/j.1753-5131.2008.01002.x
69
Sun X. M. Ren L. J. Zhao Q. Y. Ji X. J. Huang H. (2018). Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels11, 272. doi: 10.1186/s13068-018-1275-9
70
The State of World Fisheries and Aquaculture 2024 . (2024). In The State of World Fisheries and Aquaculture 2024. FAO. doi: 10.4060/cd0683en
71
Tibbetts S. M. Milley J. E. Lall S. P . (2015). Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol.27 (3), 1109–1119. doi: 10.1007/s10811-014-0428-x
72
Úbeda B. Gálvez J.Á. Michel M. Bartual A. (2017). Microalgae cultivation in urban wastewater: Coelastrum cf. pseudomicroporum as a novel carotenoid source and a potential microalgae harvesting tool. Bioresource Technol.228, 210–217. doi: 10.1016/j.biortech.2016.12.095
73
Vieira M. V. Turkiewicz I. P. Tkacz K. Fuentes-Grünewald C. Pastrana L. M. Fuciños P. et al . (2021). Microalgae as a potential functional ingredient: Evaluation of the phytochemical profile, antioxidant activity and in-vitro enzymatic inhibitory effect of different species. Molecules, 26 (24). doi: 10.3390/molecules26247593
74
Wang W. Xiong P. Zhang H. Zhu Q. Liao C. Jiang G. (2021). Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res.201, 111531. doi: 10.1016/j.envres.2021.111531
75
Wang Y. Y. Xu S. M. Cao J. Y. Wu M. N. Lin J. H. Zhou C. X. et al . (2022). Co-cultivation of Isochrysis galbana and Marinobacter sp. can enhance algal growth and docosahexaenoic acid production. Aquaculture556. doi: 10.1016/j.aquaculture.2022.738248
76
Wells M. L. Potin P. Craigie J. S. Raven J. A. Merchant S. S. Helliwell K. E. et al . (2017). Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycology29, 949–982. doi: 10.1007/s10811-016-0974-5
77
Xi L. Lu Q. Liu Y. Su J. Chen W. Gong Y. et al . (2022). Effects of fish meal replacement with Chlorella meal on growth performance, pigmentation, and liver health of largemouth bass (Micropterus salmoides). Animal Nutrition, 10, 26–40. doi: 10.1016/j.aninu.2022.03.003
78
Xu Y. Wu M. Cao J. Wang Y. Zhang L. Yan X. et al . (2024). Enhancement of Docosahexaenoic Acid and Eicosapentaenoic Acid Biosynthesis in Isochrysis galbana by Bacillus jeotgali. Mar. Biotechnol. 26, 991–999. doi: 10.1007/s10126-024-10337-5
79
Yee C. S. Okomoda V. T. Hashim F. Waiho K. Abdullah S. R. S. Alamanjo C. et al . (2021). Marine microalgae co-cultured with floc-forming bacterium: Insight into growth and lipid productivity. PeerJ9, e11217. doi: 10.7717/peerj.11217
80
Yi Z. Xu M. Magnusdottir M. Zhang Y. Brynjolfsson S. Fu W. et al . (2015). Photo-oxidative stress-driven mutagenesis and adaptive evolution on the marine diatom phaeodactylum tricornutum for enhanced carotenoid accumulation. Mar. Drugs13, 6138–6151. doi: 10.3390/md13106138
81
Zhang J. X. Ran Z. S. Xie H. X. Kong F. Zhang M. Q. Zhou Y. et al . (2023). A systematic analysis and evaluation of nutritional composition of 23 strains of marine microalgae commonly used in aquaculture. Algal Res.72, 103122. doi: 10.1016/j.algal.2023.103122
82
Zhang B. Wu J. Meng F. (2021). Adaptive laboratory evolution of microalgae: A review of the regulation of growth, stress resistance, metabolic processes, and biodegradation of pollutants. Front. Microbiol.12. doi: 10.3389/fmicb.2021.737248
Summary
Keywords
aquaculture, nutrition, microalgae, biotechnology, aquafeed
Citation
Tierney J, Ralph PJ, Pirozzi I and Pernice M (2025) Harnessing microalgae for finfish nutrition: advances in biotechnology and aquafeed development. Front. Mar. Sci. 12:1611271. doi: 10.3389/fmars.2025.1611271
Received
14 April 2025
Accepted
23 June 2025
Published
14 July 2025
Volume
12 - 2025
Edited by
Luca Parma, University of Bologna, Italy
Reviewed by
Luis Poersch, Federal University of Rio Grande, Brazil
Updates
Copyright
© 2025 Tierney, Ralph, Pirozzi and Pernice.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justin Tierney, Justin.m.tierney@student.uts.edu.au
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.