Abstract
Introduction:
Environmental factors, including light and salinity, influence fish growth. It has been hypothesized that optimizing environmental factors can activate the endocrine system and enhance the growth performance of fish. The aim of the present study was to understand how environmental factors affect the growth performance of juveniles of the Malabar grouper, Epinephelus malabaricus, a valuable species in Asian markets. The combined effects of light wavelength and salinity on growth performance were closely evaluated because each of them had a positive effect on growth stimulation in this species.
Methods:
Juvenile Malabar groupers were reared under combined conditions of blue LED light (BL, 463 nm) and red LED light (RL, 623 nm) at low salinity (LS, 11psu) and high salinity (HS, 34 psu) for two weeks. Biometric parameters were quantified, and related gene expressions were analyzed via qPCR.
Results:
Specific growth rate (SGR) was significantly higher in fish under BL/LS, followed by RL/LS and BL/HS. A higher condition factor (CF) was observed in fish under BL/LS, whereas a lower food conversion rate (FCR) was observed in fish under BL/LS and RL/LS. The transcript levels of acetyl-CoA carboxylase (acaca) and fatty acid synthase (fas) in the liver increased under BL/LS (vs. RL/LS). BL upregulated neuropeptide Y (npy), but not pro-opiomelanocortin (pomc), in the fish brain under both salinities. Transcript levels of growth hormone (gh) in the pituitary gland and insulin-like growth factor-1 (igf-1) in the liver were significantly lower in fish under BL/LS than in those under BL/HS.
Discussion:
The promotion of growth performance in juvenile Malabar groupers were attributable to the additive effects of light wavelength and salinity. Since we demonstrated the combined effects of blue light and isosmotic salinity on lipogenesis and somatic growth, optimization of light and salinity conditions can enhance the aquaculture productivity of Malabar groupers.
1 Introduction
Fish growth is affected by aquatic environmental factors including light (photoperiod, intensity, and wavelength), temperature, and salinity (Brett, 1979). If fish are reared under their optimal conditions, it is expected that growth performance will be maximized in cultured fish and efficient aquaculture will be promoted. The manipulation of a single environmental factor has been extensively studied pertaining to availability of growth induction in certain fish (Boeuf and Le Bail, 1999; Bœuf and Payan, 2001). For example, growth is accelerated by the alteration of light intensity (Tian et al., 2015), photoperiod (Boeuf, 2001), or light wavelength (Ruchin, 2004), although the effect of light conditions on growth differs among species (Boeuf and Le Bail, 1999b). In addition, the availability of isotonic conditions—when internal and external solute concentrations are balanced— for growth induction has also been reported in certain fish species (Bœuf and Payan, 2001). Conversely, there are limited studies evaluating the synergic effect of multiple environmental factors on fish growth. Existing research was carried out in the turbot Scophthalmus maximus (Huang et al., 2014) and the Atlantic salmon Salmo salar (Imsland et al., 2014); the high growth performance in the former case was induced by the combination of temperature (20.88°C) and salinity (24.07‰), while that in the latter case was induced by the combination of temperature (12.7°C) and photoperiod (continuous light). These findings imply that fish concomitantly perceive changes under various environmental conditions and optimize their growth performance. However, to date, relatively few studies have been conducted on the combined effects of light (wavelength) and salinity in fish, although the effectiveness of light wavelengths has been found in many fishes (Sierra-Flores et al., 2016; Zou et al., 2022).
Fish growth is regulated internally by hormones and peptides (Bernier et al., 2009). The endocrine axis plays a crucial role in controlling fish growth, with the GH-IGF-1 axis. Circulating growth hormone (GH) which is secreted in the pituitary gland binds to growth hormone receptors (GHRs) on liver cells, triggering the synthesis and secretion of insulin-like growth factor 1 (IGF-1) (Reinecke et al., 2005). The importance of the endocrine cascade in growth regulation has been demonstrated in several fish species (Moriyama et al., 1994; Pierce et al., 2004). Furthermore, some neuropeptides are involved in appetite in fish species (Delgado et al., 2017). Neuropeptide Y (NPY) is an orexigenic regulator (Kehoe and Volkoff, 2007; Assan et al., 2021) which highly expressed in the brain and stimulates food consumption (Hosomi et al., 2014; Ji et al., 2015). Conversely, pro-opiomelanocortin (POMC), an anorexigenic regulator (Lin et al., 2000), is synthesized in the brain and is suppressed by NPY (Garcia de Yebenes et al., 1995).
The effectiveness of light or salinity on the artificial induction of growth performance has been previously reported in the Malabar grouper Epinephelus malabaricus, an important aquaculture species in the countries of East- and South-East Asia (Zhu et al., 2022, 2023). In this study, the juveniles of this species were used to investigate these combined effects. Rearing juveniles of this species under long-day conditions (14-hour light and 10-hour darkness) with blue light (465 nm) enhanced their growth performance and increased the transcript levels of growth-related genes in the endocrine axis including the orexigenic gene npy. Additionally, maintaining the juveniles under lower salinity conditions (11 psu) than seawater (34 psu) resulted in improved growth performance, which was attributed to the reduction in energy consumption and the activation of the GH–IGF-1 system, rather than changes in appetite. If these growth-inducing factors were simultaneously administered to the fish, much higher growth would be expected. Therefore, the present study aimed to explore the combined effects of light wavelength and salinity on growth performance and the transcript levels of genes related to growth, appetite, and lipogenesis in juvenile Malabar groupers. After the fish were reared under a combination of light wavelengths (463 and 623 nm) and salinities (34 and 11 psu), the growth performance was compared among the four groups: blue LED light/high salinity (BL/HS, 463 nm and 34 psu), blue LED light/low salinity (BL/LS, 463 nm and 11 psu), red LED light/high salinity (RL/HS, 623 nm and 34 psu), and red LED light/low salinity (RL/LS, 623 nm and 11 psu). Transcript levels of genes associated with photo perception (rh1, rh2, sws2, and lws), growth (gh, ghr, and igf-1), and appetite regulation (npy and pomc) were evaluated; additionally, acetyl-CoA carboxylase alpha (acaca) and fatty acid synthase (fas) were compared among the experimental groups because they are lipogenesis-related enzymes that are essential for providing the energy necessary for fish growth (Fauconneau et al., 1995) and are pivotal in driving the synthesis of fatty acids from carbohydrates (Batchuluun et al., 2022), respectively.
2 Materials and methods
2.1 Ethics declaration
This study adhered to the guidelines set forth by the Animal Care and Use Committee of the University of the Ryukyus and comply with the regulations governing the care and use of laboratory animals in Japan.
2.2 Experimental design
Juveniles of the Malabar grouper used herein, weighing 102 ± 6 g (body weight; BW) and measuring 192 ± 6 mm (total length; TL), were acquired from the Okinawa Prefectural Sea Farming Center, Okinawa, Japan. Then all experiments were carried out in the Nakagusuku Aquaculture Innovation Center (NAICe), Okinawa, Japan. Ninety-six individuals were acclimated in 500-liter with flowing seawater and continuous aeration for one week, and were fed commercial pellets (Marubeni Nisshin Feed, Tokyo, Japan) daily at 1000 h.
Aquariums (75 liter each) with four different conditions were set in triplicates in the dark room. The experimental conditions were as follows: (1) LED light (463 nm) and seawater (34 psu) (BL/HS), (2) LED light (463 nm) and diluted seawater (11 psu) (BL/LS), (3) LED light (623 nm) and seawater (34 psu) (RL/HS), and (4) LED light (623 nm) and diluted seawater (11 psu) (RL/LS). LED arrays were made by NC LED VINA, Vietnam. The light intensity on the surface of water was set around 24.3 ± 0.2 μmol·m−²·s−¹, which was measured by a spectrometer (Sekonic C-7000 Spectrometer). Seawater and diluted seawater were separately filtered and aerated and one-third of the water was changed daily. Throughout the experiment, the water temperature was maintained at 26 ± 1°C, and the photoperiod was set at LD = 14:10 (light-on at 0530 h and light-off at 1930 h).
Eight fish were maintained in each aquarium and fed pellets daily at 1000 h. Fish were fed to apparent satiation (cessation of feeding activity >1 min) with uneaten feed collected 30 min post-feeding, residual pellets were dried to calculation of actual consumption. The amount of feed consumed was recorded. After two weeks, BW and TL of the fish were measured following anesthesia with 0.01% 2-phenoxyethanol (Kanto Kagaku, Tokyo, Japan) and subsequent decapitation. The brain (diencephalon), pituitary gland, and liver were collected. Tissue samples were frozen in liquid nitrogen after placed in the TriPure Isolation Reagent (Roche Diagnostics, Indianapolis, IN, USA), then preserved at –80°C.
The following formulae were used to evaluate growth performance and feed behavior:
where , , t, and are the final body weight (g), initial body weight (g), time (d), and food intake (g), respectively.
2.3 Molecular preparation of genetic material
Tissue samples from the diencephalon, pituitary gland, and liver were first homogenized by tissue grinder (Coyote Bioscience, Jiangsu, China). Total RNA extraction was performed utilizing the TriPure Isolation Reagent (Roche Diagnostics, Indianapolis, IN, USA following the manufacturer’s established protocol. RNA quantification was carried out using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), while RNA quality was evaluated through electrophoresis on 2% agarose gel (TaKaRa Bio, Kusatsu, Japan). The PrimeScript TM RT Reagent Kit with gDNA Eraser (TaKaRa Bio) used to transform the extracted total RNA into cDNA templates. The resulting cDNA was prepared to a concentration of 1000 ng/μL following the manufacturer’s recommended protocol, and then stored at −30°C.
2.4 Real-time quantitative polymerase chain reaction
Gene expression analysis was performed by quantitative real-time PCR (qPCR) using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories) with SYBR Green Premix PCR kit (TaKaRa Bio). The study examined the mRNA levels of opsins (rh1, rh2, sws2, and lws), pomc, and npy in the diencephalon, gh in the pituitary gland, and ghr, igf-1, fas, and acaca in the liver. Primer sets referenced from previous studies by Zhu et al. (2023, 2022) were utilized, as detailed in Table 1. The quantitative PCR (qPCR) reaction mixture, with a total volume of 10 μL, consisted of 5 μL SYBR Premix Ex Taq II (Tli RNase H Plus) from TaKaRa Bio, 0.3 μL each of forward and reverse primers (0.3 M concentration), 2.4 μL nuclease-free water, and 2 μL cDNA template. The thermal cycling protocol included an initial denaturation at 95°C for 2 minutes, followed by 39 cycles of 15-second denaturation at 95°C and 1-minute annealing/extension at 60°C, concluding with a melt-curve analysis ranging from 65°C to 95°C, incrementing 0.5°C every 5 seconds. Technical duplicates were used for each sample, with amplification efficiencies approaching 100% verified through serial dilutions of brain, pituitary, and liver cDNA. Target gene mRNA levels were normalized using two reference genes: Malabar grouper ef1α and β-actin, with primers sourced from Yamashina et al. (2019) and Imamura et al. (2017), respectively (Supplementary Table 1).
Table 1
| Primer | Sequence | Accession no. |
|---|---|---|
| rh1-Forward | 5'-TGAACACCACCGGGATTGTC-3' | KM077949.1 |
| rh1-Reverse | 5'-AGGTGGTGATCATGCAGTGG-3' | |
| rh2-Forward | 5'-GGCACAGAGGGCAAGAACTT-3' | LC726485 |
| rh2-Reverse | 5'-TGGAGAGCATGCAGTTACGG-3' | |
| sws2-Forward | 5'-TCCCTCTGGATACGGACAAC-3' | LC726486 |
| sws2-Reverse | 5'-TGAGCGGAACTGTTTGTTGA-3' | |
| lws-Forward | 5'-CAGGCGGTACAATGAAGAGA-3' | LC726487 |
| lws-Reverse | 5'-AGGAGCCACAGAGGAGACCT-3' | |
| pomc-Forward | 5'-GTCAAGTCTGAGGGCAGCAT-3' | LC726490 |
| pomc-Reverse | 5'-GAGGAACAGGTTGGAGGTGG-3' | |
| npy-Forward | 5'-GGACACACTGGTCTCAGAGC-3' | LC726491 |
| npy-Reverse | 5'-TCACCACAATGATGGGTCGT-3' | |
| gh-Forward | 5'-ACATCTCCACCTGCTTGCTC-3' | LC726488 |
| gh-Reverse | 5'-CAACAGCTTCAACACGGAGC-3' | |
| ghr-Forward | 5'-CTCCTGGATCAGCGTTTCTC-3' | LC768792 |
| ghr-Reverse | 5'-GCTTGGTAGCTTTCCACGTC-3' | |
| igf-1-Forward | 5'-GAATGGACAAATGCCCAGCG-3' | LC726489 |
| igf-1-Reverse | 5'-CGGACCTTTGTCAGCATCCT-3' | |
| fas-Forward | 5'-GGAAAGCAAGCCATTGATGT-3' | LC817412 |
| fas-Reverse | 5'-GACTCTGCTGCTGCTGTCTG-3' | |
| acaca-Forward | 5'-AACGGCTTCTGTCTTCCTGA-3' | LC817416 |
| acaca-Reverse | 5'-GGAGCTCCAGTAGAGGCAAA-3' |
Primers for real-time PCR utilized in this study.
Primers were designed based on the cloned sequence of the Malabar grouper using bioinformatics methods (Zhu et al., 2022, 2023).
2.5 Statistical analysis
The research results were expressed as means accompanied by standard errors. Statistical evaluations were conducted using Prism 9 (GraphPad Software, San Diego, CA, USA), with statistical significance determined at P < 0.05. Bartlett’s test was employed to examine variance homogeneity, while the Shapiro–Wilk test confirmed the normality of data distribution. Depending on the data’s characteristics, the team utilized either one-way analysis of variance (ANOVA) or the non-parametric Kruskal–Wallis test to identify statistically significant differences. When multiple comparisons were necessary, these initial tests were followed by post-hoc analyses using Tukey’s test or Dunn’s test. For more complex experimental designs involving two or more groups, a two-way ANOVA with Geisser–Greenhouse correction was implemented, with Tukey’s test subsequently applied to conduct multiple comparative analyses.
3 Results
3.1 Biometric parameters
No mortality was observed in fish from any group during the experiment, indicating that this combination of environmental factors was not lethal to juvenile Malabar groupers. Table 2 shows the influence of different light wavelengths and salinity conditions on the biometric parameters of the fish. BW significantly increased under all conditions (P < 0.05), while TL did not show a significant change within two weeks of the study. Comparisons among the experimental groups revealed that BW was significantly higher (P < 0.05) in the BL/LS group than that in the RL/HS group. A significant increase in CF (P < 0.05) was observed in the BL/LS group (vs. the RL/HS and RL/LS groups).
Table 2
| Parameters | Period | Experiment conditions | |||
|---|---|---|---|---|---|
| BL/LS | BL/HS | RL/LS | RL/HS | ||
| Body weight (g) | 0 weeks | 102.7 ± 6.7a | 102.5 ± 7.2a | 102.6 ± 6.1a | 102.5 ± 7.1a |
| 2 weeks | 138.3 ± 15.6c | 122.1 ± 13.4bc | 124.5 ± 9.6bc | 109.0 ± 9.4b | |
| Total length (mm) | 0 weeks | 191.0 ± 7.0a | 193.0 ± 6.9ab | 194.1 ± 4.0ab | 192.0 ± 6.2ab |
| 2 weeks | 201.1 ± 7.5ab | 197.1 ± 7.3ab | 202.9 ± 4.2b | 195.7 ± 5.7ab | |
| Condition factor % | 0 weeks | 1.47 ± 0.08a | 1.42 ± 0.05a | 1.40 ± 0.04a | 1.45 ± 0.08a |
| 2 weeks | 1.69 ± 0.07c | 1.58 ± 0.08bc | 1.48 ± 0.03ab | 1.45 ± 0.07a | |
Body weight, total length, and condition factor of the Malabar grouper juveniles reared under different salinities with LEDs (BL/LS; blue 463 nm - 11 psu, BL/HS; blue 463 nm - 34 psu, RL/LS; red 623 nm - 11 psu, and RL/HS; red 623 nm - 34 psu) for 2weeks.
Different letters represent statistically significant differences at P < 0.05, as determined by a two-way ANOVA with Geisser-Greenhouse correction, with Tukey's test subsequently applied to conduct multiple comparative analyses.
Figure 1 shows the SGR of fish reared under different light wavelengths and salinities for two weeks. The SGR was significantly higher (P < 0.05) in the BL/LS group, followed by the RL/LS and BL/HS groups. The lowest SGR was observed for the RL/HS group. In contrast, the FCR of the BL/LS and RL/LS groups was significantly lower than that of the RL/HS group (P < 0.05) (Figure 2).
Figure 1
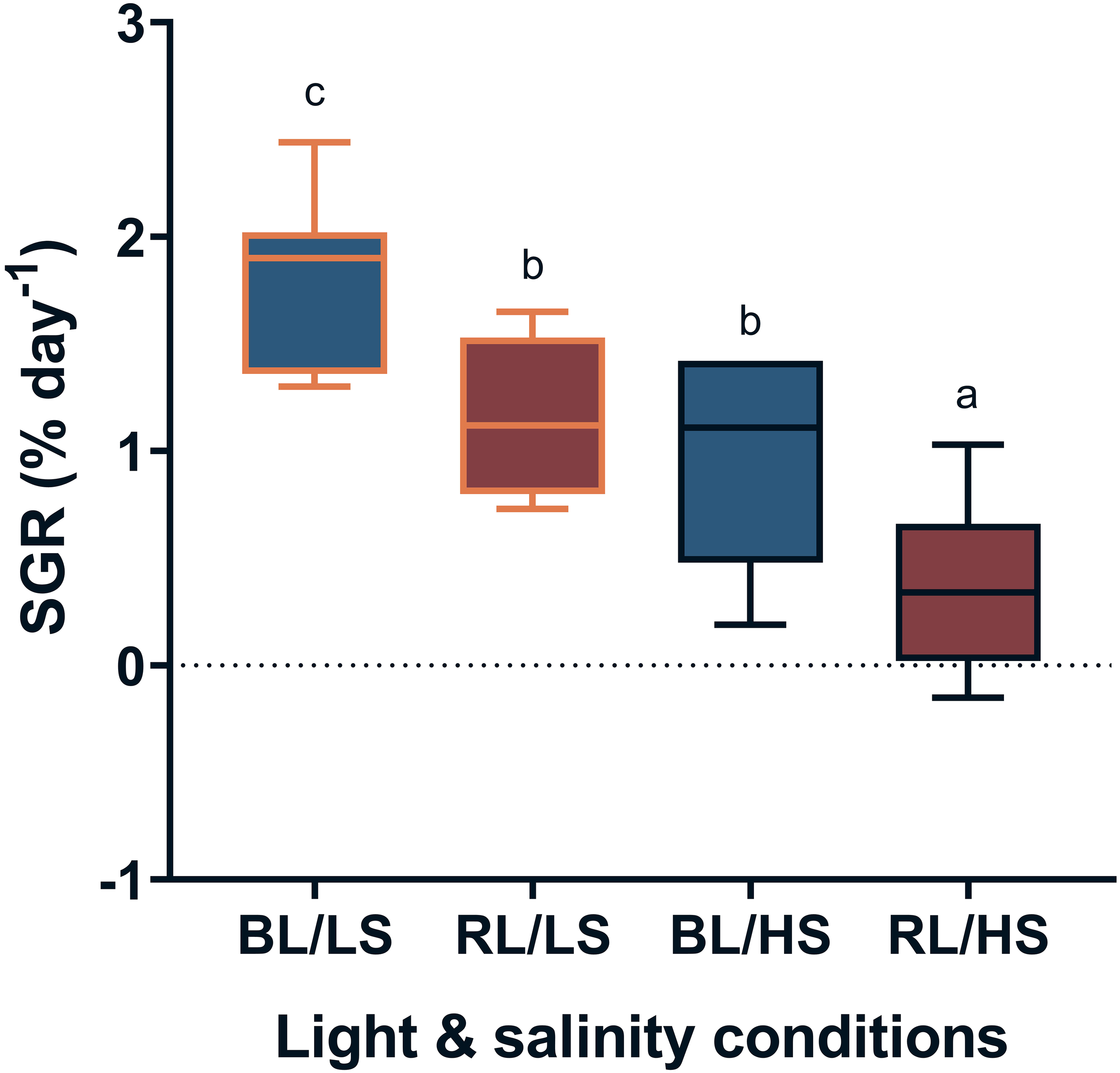
Specific growth rate (SGR) of Malabar grouper juveniles reared under different experimental conditions. Fish were reared under different salinities with LEDs (BL/LS; blue 463 nm - 11 psu, RL/LS; red 623 nm - 11 psu, BL/HS; blue 463 nm - 34 psu, and RL/HS; red 623 nm - 34 psu) for 2weeks. Each value represents mean value ± SEM. Different letters indicate statistically significant differences at P <0.05 by ANOVA/Tukey’s test.
Figure 2
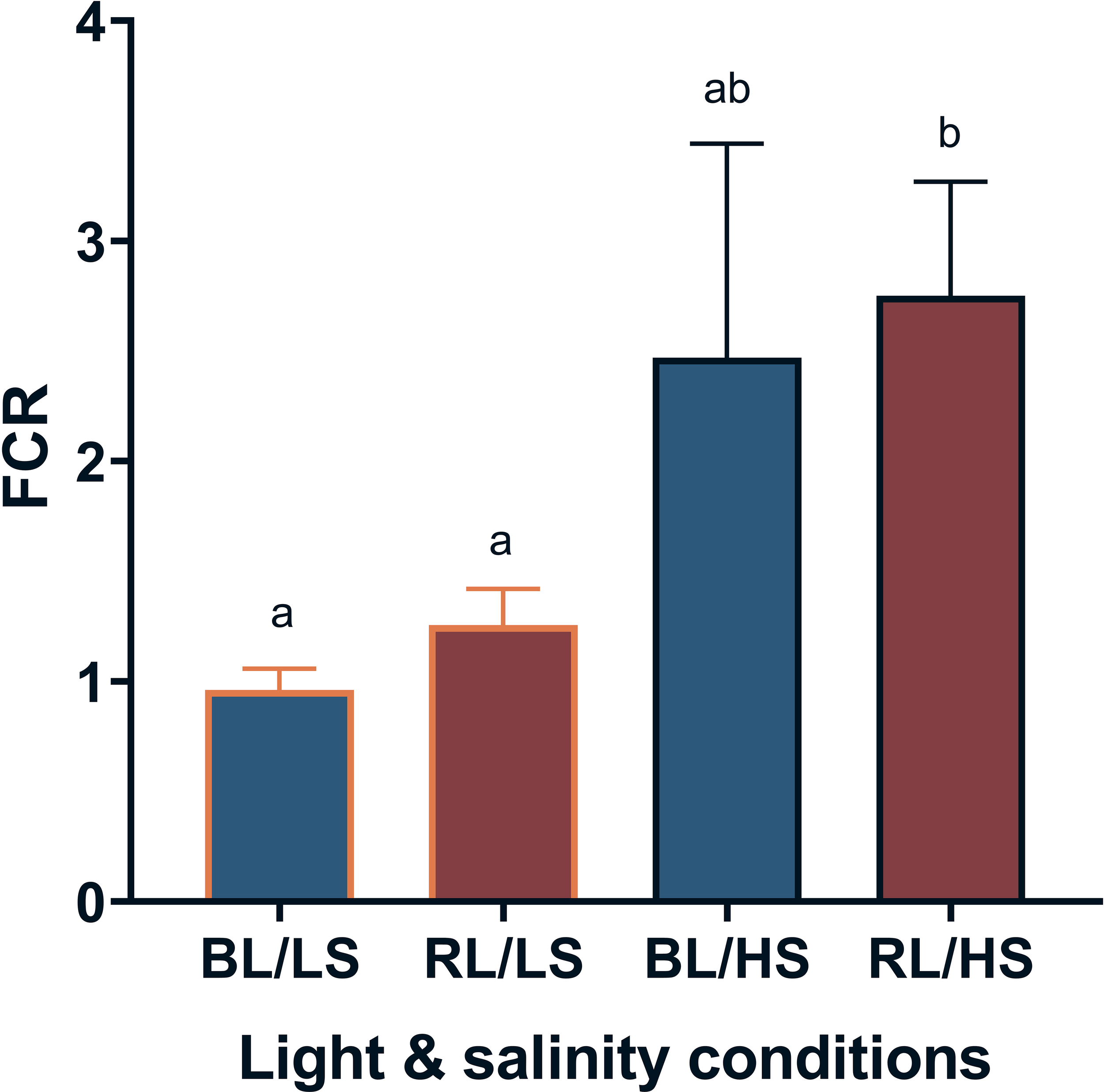
Feed conversion ratio (FCR) of Malabar grouper juveniles reared under different salinities. Fish were reared under different salinities with LEDs (BL/LS; blue 463 nm - 11 psu, RL/LS; red 623 nm - 11 psu, BL/HS; blue 463 nm - 34 psu, and RL/HS; red 623 nm - 34 psu) for 2weeks. Each value represents mean value ± SEM. Different letters indicate statistically significant differences at P <0.05 by Kruskal-Wallis/Dunn’s test.
3.2 Gene expression
The mRNA levels of visual opsins in the diencephalon were compared in fish under experimental conditions (Figure 3). The transcript levels of rh1 and rh2 exhibited no significant difference among the four groups (Figures 3a, b). The BL/HS group showed significantly higher transcription level of sws2 compared to the BL/LS and RL/LS groups (P < 0.05; Figure 3c). Additionally, in the RL/LS group, lws expression was elevated relative to the BL/LS group (P < 0.05; Figure 3d).
Figure 3
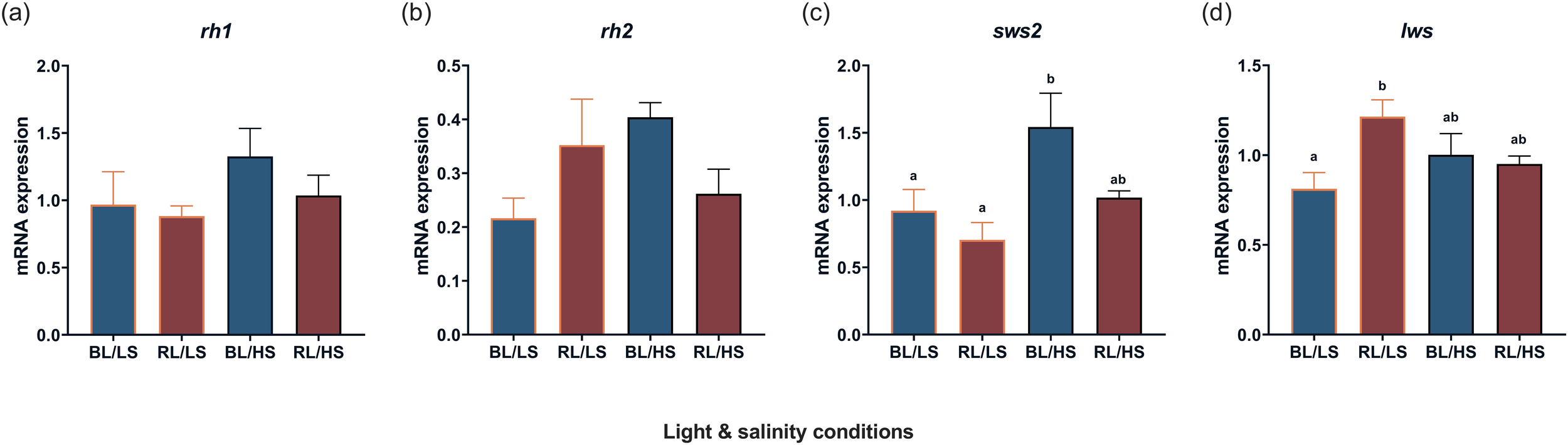
Transcript levels of visual opsin [rh1(a), rh2(b), sws2(c), lws(d)] in the diencephalon of Malabar grouper juveniles under varied conditions using LEDs (BL/LS; blue 463 nm - 11 psu, RL/LS; red 623 nm - 11 psu, BL/HS; blue 463 nm - 34 psu, and RL/HS; red 623 nm - 34 psu). Statistical significances for sws2 and for rh1 rh2 and lws were accepted at P < 0.05 by Kruskal-Wallis/Dunn’s test and by ANOVA/Tukey’s test, respectively. Each value represents mean value ± SEM. Different letters indicate statistically significant differences.
The transcript levels of acaca were significantly higher in the BL/LS group (P < 0.05) than in the RL/LS group (Figure 4a). Similar salinity effects were observed on the transcript levels of fas in the liver (Figure 4b).
Figure 4
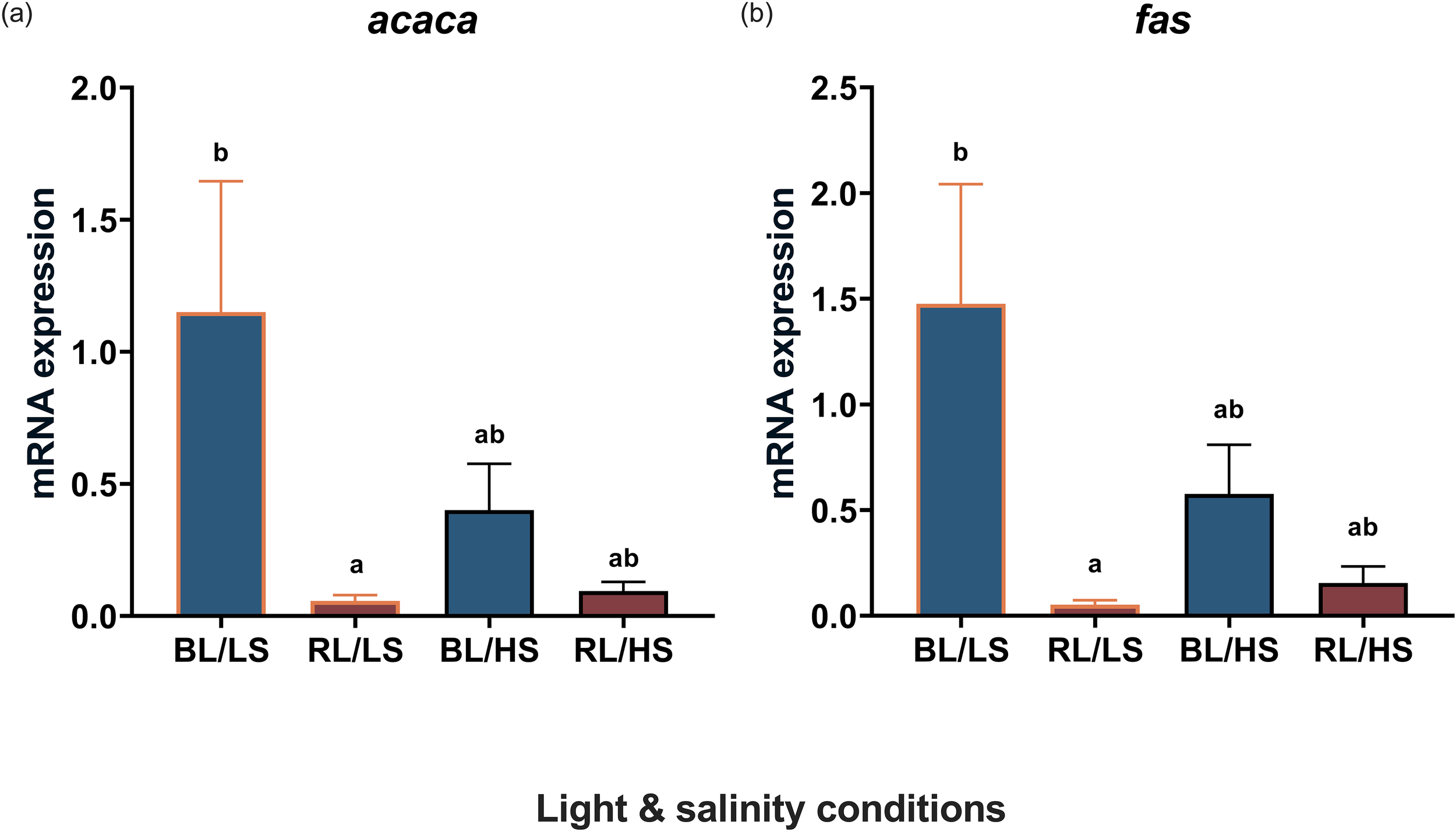
Transcript levels level of acaca(a) and fas(b) in the liver of Malabar grouper juveniles reared under varied conditions using LEDs (BL/LS; blue 463 nm - 11 psu, RL/LS; red 623 nm - 11 psu, BL/HS; blue 463 nm - 34 psu, and RL/HS; red 623 nm - 34 psu). Statistical significance was accepted at P < 0.05 by Kruskal-Wallis/Dunn’s test. Each value represents mean value ± SEM. Different letters indicate statistically significant differences.
Figure 5 shows the transcript levels of pomc and npy in the diencephalons of fish under experimental conditions. The transcript levels of pomc were significantly higher (P < 0.05) in the RL/HS group than in the RL/LS group. The transcript levels of npy were significantly higher (P < 0.05) in the BL/HS group than in the RL/LS group.
Figure 5
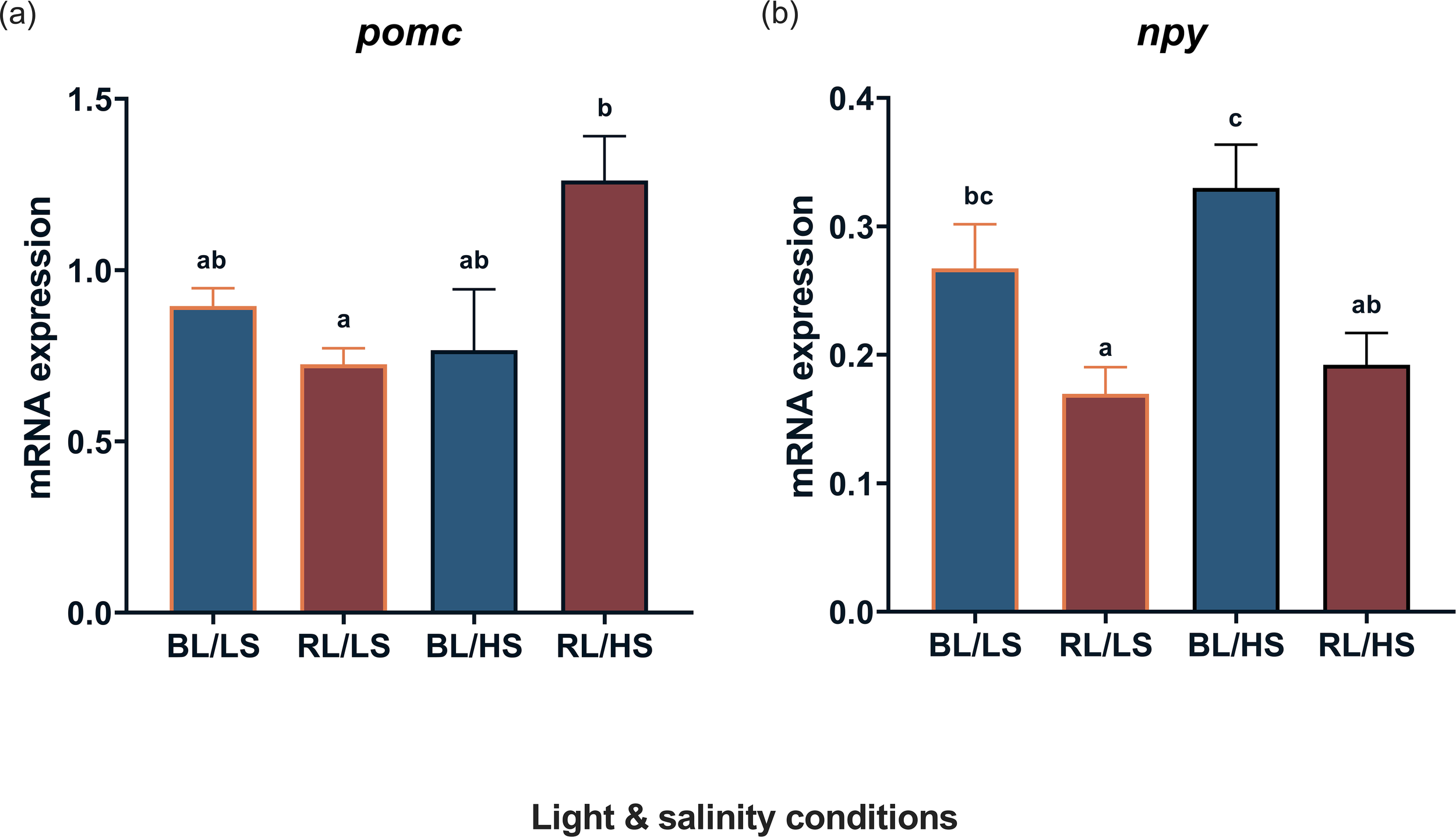
Transcript levels of pomc(a) and npy(b) in the diencephalon of Malabar grouper juveniles reared under varied conditions using LEDs (BL/LS; blue 463 nm - 11 psu, RL/LS; red 623 nm - 11 psu, BL/HS; blue 463 nm - 34 psu, and RL/HS; red 623 nm - 34 psu). Statistical significance was accepted at P < 0.05 by ANOVA/Tukey’s test. Each value represents mean value ± SEM. Different letters indicate statistically significant differences.
Figure 6 shows the transcript levels of gh in the pituitary gland, as well as those of ghr and igf-1 in the liver. There was an effect of salinity on the transcript levels of gh and igf-1, but not ghr; fish reared in high salinity (BL/HS group) had significantly higher transcript levels of gh than fish reared in low salinity (BL/LS and RS/LS groups, Figure 6a), and the transcript levels of igf-1 were significantly higher in the BL/HS group than in the BL/LS group (Figure 6c).
Figure 6
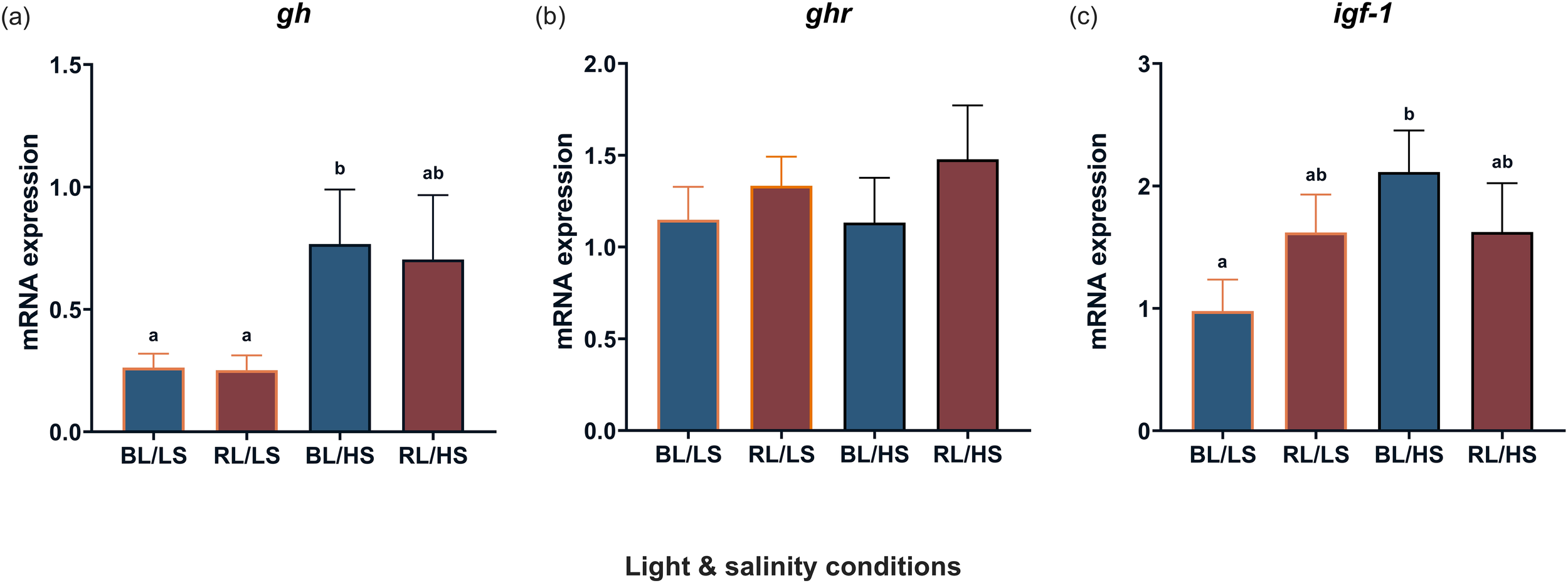
Transcript levels of gh(a) in the pituitary gland and ghr(b) and igf-1(c) in the liver of Malabar grouper juveniles under varied conditions using LEDs (BL/LS; blue 463 nm - 11 psu, RL/LS; red 623 nm - 11 psu, BL/HS; blue 463 nm - 34 psu, and RL/HS; red 623 nm - 34 psu). Statistical significance for gh and for ghr and igf-1 was accepted at P < 0.05 by Kruskal-Wallis/Dunn’s test and by ANOVA/Tukey’s test. Each value represents mean value ± SEM. Different letters indicate statistically significant differences.
4 Discussion
Light wavelength detection is mediated by visual opsins in vertebrates (Yokoyama, 2000). These visual opsins of the Malabar grouper were previously cloned and characterized, and the transcript levels of sws2 and lws increased in the eyes and/or diencephalon after exposure to blue light and red light, respectively (Zhu et al., 2022). Similar results were obtained in the present study when the transcript levels of sws2 and lws were measured in the diencephalons of the Malabar grouper. Interestingly, their expression appeared to be affected by a combination of light wavelength and salinity. The mRNA abundance of sws, which is sensitive to blue light (Nam et al., 2011; Valen et al., 2014; Patel et al., 2020), increased in the diencephalons of fish reared under the combined conditions of blue light and high salinity. In contrast, the mRNA abundance of lws, which is sensitive to red light (Smith et al., 2012; Shao et al., 2014), increased in the diencephalons of fish reared under the combined conditions of red light and low salinity. These results suggest that the transcription of these opsins in the brain is influenced by the alteration of environments in which fish are accommodated in their habitats. Because visual opsins are involved in the activation and/or inactivation of growth and appetite networks (Yamanome et al., 2009; Takeuchi et al., 2011; Byun et al., 2020; Zhu et al., 2022), it is likely that the synthesis and release of growth- and appetite-related hormones/peptides are altered by changes in light and salinity.
The present study investigated the combined effects of light wavelength and salinity on the growth performance of Malabar groupers and found that some combinations of these two factors could induce higher growth in juveniles and alter the mRNA abundance of appetite- and growth-related genes, as well as lipogenesis-related genes. It is possible that physiological actions stimulating growth performance synergically and simultaneously occurs in various tissues including the liver and muscle because the involvement in the endocrine control may be different among tissues. Under long-day conditions (LD = 14:10), the combination of two light wavelengths (463 and 623 nm) and two salinities (11 and 34 psu) was studied herein because the effectiveness of these light wavelengths and salinities has been clarified separately in previous studies (Zhu et al., 2022, 2023). When juvenile Malabar groupers were reared under four sets of conditions (BL/LS; blue 463 nm - 11 psu, RL/LS; red 623 nm - 11 psu, BL/HS; blue 463 nm - 34 psu, and RL/HS; red 623 nm - 34 psu), BW increased in the fish of all groups. High CF—a marker of healthier body condition— was recorded only in fish reared under blue light (BL/LS and BL/HS). In the case of Malabar grouper juveniles, rearing under a combination of shorter wavelengths of light and lower salinity is likely to stimulate lipid accumulation because the transcript levels of lipogenesis-related genes, including acaca and fas, were upregulated in the liver of juveniles reared under these conditions. Because ACACA and FAS are the major enzymes that convert citrate into fatty acids in the cytosol (Batchuluun et al., 2022), it is likely that lipogenesis is activated by the alternation of salinity and/or light wavelength. For example, fas was upregulated in the liver of the spotted scat Scatophagus argus, which was reared under low salinity (5 ppt) (Chen et al., 2023), whereas the lipid metabolism pathway, including acaca mRNA, was downregulated in the liver of the turbot Scophthalmus maximus, which was reared under hypoosmotic conditions (Liu et al., 2021). In general, rearing fish under salinity around isotonic conditions reduces the energy demand for osmoregulation and is expected to promote other physiological processes, including growth performance, using the saved energy (Vargas-Chacoff et al., 2015). A similar mechanism is probable in the Malabar grouper, because low oxygen consumption was recorded in fish reared at 11psu (Zhu et al., 2022). In addition to the juveniles of the Malabar grouper (Zhu et al., 2023), superior growth performance at isotonic salinity has been reported in other groupers, including the longtooth grouper Epinephelus bruneus and the hybrid grouper E. fuscoguttatus × E. lanceolattus (Inoue et al., 2015; Noor et al., 2019). As suggested in the present study, some of the energy saved may be related to the activation of metabolic processes, including lipogenesis. However, relatively few studies have shown the effectiveness of light wavelengths on lipogenesis in fish, although exposure to blue light promotes lipid accumulation in the liver of mice (Guan et al., 2022, 2024). The present study showed the effectiveness of blue light on lipogenesis in fish because the transcript levels of both fas and acaca increased under BL/LS and BL/HS conditions. Therefore, it is worth mentioning that, herein, there was a combined effect of light wavelength and salinity on lipogenesis in fish. A combined effect of these two factors was also observed on the SGR because the BL/LS group had the highest SGR. Conversely, a potent effect of salinity, rather than light wavelength, was observed in FCR, where a lower value indicates better feed efficiency, because there was little difference in FCR under both blue-light and red-light conditions.
The present study showed that the transcript levels of npy increased in the brain of the juveniles of the BL/LS and BL/HS groups, suggesting that, as expected from previous studies (Zhu et al., 2022, 2023), its transcription is affected by light wavelength but not by salinity. As this orexigenic neuropeptide is produced in the hypothalamus and affects the feeding behavior of fish (Assan et al., 2021), it is likely that the appetite of the Malabar grouper was promoted under blue-light conditions. In contrast, the transcript levels of pomc increased in the brain of the RL/HS group. This result was different from that of previous studies, in which rearing under growth-promoting light conditions resulted in the upregulation of pomc in the brain of the juveniles of the Maraber grouper (Zhu et al., 2022) and in the barfin flounder Verasper moseri (Takahashi et al., 2016). POMC is an anorexigenic neuropeptide produced in the hypothalamus. In this regard, the reduction in food intake of rainbow trout associated with increase in hypothalamic POMC levels (Pérez-Maceira et al., 2016). Therefore, it is likely that high salinity and red light combined exert an inhibitory effect on the upregulation of pomc in the fish brain because low growth performance in the fish of the RL/HS group may be attributable to limited feeding behavior caused by low appetite.
It was previously reported that the transcript levels of gh and igf-1 increased in the pituitary gland and liver of juvenile Malabar groupers reared under blue light (Zhu et al., 2022) or isotonic conditions at 11 psu (Zhu et al., 2023). These results suggested that the GH-IGF-1 axis is stimulated by suitable light wavelengths and/or salinity conditions. Regarding light wavelength, upregulation of IGF-1 was reported in the barfin flounder, when fish were irradiated with green LED light for four weeks under an average water temperature of 6.6°C (Takahashi et al., 2016). A similar effect of specific light wavelengths on IGF-1 mRNA abundance was also observed in the larvae of the turbot Scophthalmus maximus, which were exposed to orange light at 595 nm and blue light at 450 nm (Wu et al., 2020, 2021). In contrast, the transcript levels of igf-1 increased in the liver of the Mediterranean meager Argyrosomus regius under isotonic conditions at 12 psu (Mohammed-Geba et al., 2017). Unlike in previous studies, rearing fish under both conditions failed to enhance igf-1 expression in the liver. This contrasting result implies that there is little combined effect on the GH–IGF-1 axis. Alternatively, concomitant with an increase in gh transcripts in the pituitary gland of fish reared in seawater, igf-1 seemed to be upregulated. GH is involved in seawater acclimation (Varsamos et al., 2006; Tine et al., 2007) and enhances Na+,K+-ATPase activity in the gills (Mancera and McCormick, 1998). Therefore, it is possible that the expression profiles of the GH-IGF-1 pathway are affected by seawater adaptivity. Notably, the transcript levels of gh in the pituitary gland were enhanced at both 12 psu (isosmotic conditions) and 55 psu (extremely hyperosmotic conditions) (Mohammed-Geba et al., 2017). Our results indicated that there is an additional pathway that accelerates the combined effects of light wavelength and salinity. One possibility is that thyroid hormones (THs) play a role in controlling the synergic effect, because the transcript levels of iodothyronine deiodinase 2 (dio2), the rate-limiting enzyme of conversion from thyroxine (T4) to triiodothyronine (T3), increased during the photophase and treatment with T3 increased hepatic igf-1 levels in the liver of the sapphire devil Chrysiptera cyanea (Rizky et al., 2024). In addition, cortisol seems to lower the circulating IGF-1 and alter the transcript levels of IGF-1 binding proteins (igfbps) in the blue rockfish Sebastes mystinus (Mapes et al., 2025), suggesting that stress is involved in the growth performance in fish. Therefore, it is possible that multiple endocrine pathways exist in controlling the growth in fish (Mapes et al., 2025).
In conclusion, a high growth performance of Malabar grouper juveniles can be induced by selecting the optimal light wavelength (blue-light condition) and salinity (11psu). This combined effect on growth performance is related to the activation of appetite and lipogenic activity in the liver, although the involvement of the GH-IGF-1 axis in this combined effect remains unclear. Therefore, the adoption of simultaneous and multiple environmental controls provides new insights into aquaculture. Further studies are required to clarify whether the combined effects of certain environments promote growth performance in other fish species.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal study was approved by the Animal Care and Use Committee of the University of the Ryukyus. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YZ: Data curation, Methodology, Validation, Formal analysis, Conceptualization, Software, Investigation, Writing – original draft, Resources, Writing – review & editing, Funding acquisition, Visualization. KF: Methodology, Writing – review & editing, Conceptualization, Investigation. SU: Writing – review & editing, Methodology, Investigation. SL: Methodology, Investigation, Writing – review & editing. AS: Resources, Writing – review & editing. AT: Funding acquisition, Supervision, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partially funded by COI-NEXT (JPMJPF2012 to AT) and the University of the Ryukyus Foundation (Shohei Suzuki Research Foundation) awardee to YZ.
Acknowledgments
The authors extend their sincere gratitude to the dedicated staff of the Okinawa Prefectural Sea Farming Center and the Sesoko Station at the Tropical Biosphere Research Center, University of the Ryukyus, in Okinawa, Japan, for their invaluable assistance in providing fish specimens and access to research facilities. Special recognition is due to Dr. Yuki Takeuchi from the Developmental Neurobiology Unit at the Okinawa Institute of Science and Technology Graduate University, who generously provided the contig sequences of the Malabar grouper genome. The authors also wish to acknowledge Editage (www.editage.jp) for their professional English language editing services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1613939/full#supplementary-material
References
1
Assan D. Mustapha U. F. Chen H. Li Z. Peng Y. Li G. (2021). The roles of neuropeptide Y (Npy) and peptide YY (Pyy) in teleost food intake: A mini review. Life11, 1–15. doi: 10.3390/life11060547
2
Batchuluun B. Pinkosky S. L. Steinberg G. R. (2022). Lipogenesis inhibitors: therapeutic opportunities and challenges. Nat. Rev. Drug Discov.21, 283–305. doi: 10.1038/s41573-021-00367-2
3
Bernier N. Kraak G.V. D. Farrell A. Brauner C. (2009). Fish Neuroendochinology, Vol. 28. (Academic Press).
4
Boeuf G. (2001). “Photoperiod and growth in fish,” in HAL (Le Centre pour la Communication Scientifique Directe). Available at: https://www.academia.edu/107899405/Photoperiod_and_Growth_in_Fish.
5
Boeuf G. Le Bail P. Y. (1999). Does light have an influence on fish growth? Aquaculture177, 129–152. doi: 10.1016/S0044-8486(99)00074-5
6
Bœuf G. Payan P. (2001). How should salinity influence fish growth? Comp. Biochem. Physiol. C Toxicol. Pharmacol.130, 411–423. doi: 10.1016/S1532-0456(01)00268-X
7
Brett J. R. (1979). Environmental factors and growth. Fish Physiol.8, 599–675. doi: 10.1016/S1546-5098(08)60033-3
8
Byun J.-H. Hyeon J.-Y. Kim E.-S. Kim S.-K. Hur S.-P. Kim S.-J. et al . (2020). Daily variation of D2 dopamine receptor transcription in the brain of the Japanese eel Anguilla japonica and its regulation with dopamine and melatonin. Comp. Biochem. Physiol. A Mol. Integr. Physiol.240, 110581. doi: 10.1016/j.cbpa.2019.110581
9
Chen J. Cai B. Tian C. Jiang D. Shi H. Huang Y. et al . (2023). RNA sequencing (RNA-seq) analysis reveals liver lipid metabolism divergent adaptive response to low- and high-salinity stress in spotted scat (Scatophagus argus). Animals13, 1503. doi: 10.3390/ANI13091503/S1
10
Delgado M. J. Cerdá-Reverter J. M. Soengas J. L. (2017). Hypothalamic integration of metabolic, endocrine, and circadian signals in fish: Involvement in the control of food intake. Front. Neurosci.11. doi: 10.3389/fnins.2017.00354
11
Fauconneau B. Alami-Durante H. Laroche M. Marcel J. Vallot D. (1995). Growth and meat quality relations in carp. Aquaculture129, 265–297. doi: 10.1016/0044-8486(94)00309-C
12
Garcia de Yebenes E. Li S. Fournier A. St-Pierre S. Pelletier G. (1995). Regulation of proopiomelanocortin gene expression by neuropeptide Y in the rat arcuate nucleus. Brain Res.674, 112–116. doi: 10.1016/0006-8993(94)01429-L
13
Guan Q. Wang Z. Cao J. Dong Y. Chen Y. (2022). Monochromatic blue light not green light exposure is associated with continuous light-induced hepatic steatosis in high fat diet fed-mice via oxidative stress. Ecotoxicol. Environ. Saf.239, 113625. doi: 10.1016/j.ecoenv.2022.113625
14
Guan Q. Wang Z. Cao J. Dong Y. Tang S. Chen Y. (2024). Melatonin restores hepatic lipid metabolic homeostasis disrupted by blue light at night in high-fat diet-fed mice. J. Pineal Res.76 (4), e12963. doi: 10.1111/jpi.12963
15
Hosomi N. Furutani T. Takahashi N. Masumoto T. Fukada H. (2014). Yellowtail neuropeptide Y: Molecular cloning, tissue distribution, and response to fasting. Fisheries Sci.80, 483–492. doi: 10.1007/S12562-014-0711-4/FIGURES/6
16
Huang Z. H. Ma A. J. Wang X. A. Lei J. L. (2014). The interaction of temperature, salinity and body weight on growth rate and feed conversion rate in turbot (Scophthalmus maximus). Aquaculture432, 237–242. doi: 10.1016/j.aquaculture.2014.04.013
17
Imamura S. Hur S. Takeuchi Y. Bouchekioua S. Takemura A. (2017). Comparative Biochemistry and Physiology, Part A Molecular cloning of kisspeptin receptor genes (gpr54–1 and gpr54-2) and their expression pro fi les in the brain of a tropical damsel fi sh during different gonadal stages. Comp. Biochem. Physiology A Mol. Integr. Physiol.203, 9–16. doi: 10.1016/j.cbpa.2016.07.015
18
Imsland A. K. Handeland S. O. Stefansson S. O. (2014). Photoperiod and temperature effects on growth and maturation of pre- and post-smolt Atlantic salmon. Aquaculture Int.22, 1331–1345. doi: 10.1007/S10499-014-9750-1/METRICS
19
Inoue N. Iwasaki T. Shimada Y. Satoh J. Nishioka T. (2015). Effect of salinity on the growth of juvenile longtooth grouper Epinephelus bruneusin tank culture. Nippon Suisan Gakkaishi81, 803–810. doi: 10.2331/suisan.81.803
20
Ji W. Ping H. C. Wei K. J. Zhang G. R. Shi Z. C. Yang R.B. et al . (2015). Ghrelin, neuropeptide Y (NPY) and cholecystokinin (CCK) in blunt snout bream (Megalobrama amblycephala): cDNA cloning, tissue distribution and mRNA expression changes responding to fasting and refeeding. Gen. Comp. Endocrinol.223, 108–119. doi: 10.1016/j.ygcen.2015.08.009
21
Kehoe A. S. Volkoff H. (2007). Cloning and characterization of neuropeptide Y (NPY) and cocaine and amphetamine regulated transcript (CART) in Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. A Mol. Integr. Physiol.146, 451–461. doi: 10.1016/J.CBPA.2006.12.026
22
Lin X. Volkoff H. Narnaware Y. Bernier N. J. Peyon P. Peter R. E. (2000). Brain regulation of feeding behavior and food intake in fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol.126, 415–434. doi: 10.1016/S1095-6433(00)00230-0
23
Liu Z. Ma A. Yuan C. Zhao T. Chang H. Zhang J. (2021). Transcriptome analysis of liver lipid metabolism disorders of the turbot Scophthalmus maximus in response to low salinity stress. Aquaculture534, 736273. doi: 10.1016/j.aquaculture.2020.736273
24
Mancera J. M. McCormick S. D. (1998). Evidence for growth hormone/insulin-like growth factor I axis regulation of seawater acclimation in the euryhaline teleost Fundulus heteroclitus. Gen. Comp. Endocrinol.111, 103–112. doi: 10.1006/gcen.1998.7086
25
Mapes H. M. Shew J. E. Marden H. M. Journey M. L. Beckman B. R. Lema S. C. (2025). Cortisol reduces insulin-like growth factor-1 (Igf1) and alters liver Igf binding protein (Igfbp) and muscle myogenic gene expression in blue rockfish (Sebastes mystinus). Gen. Comp. Endocrinol.361, 114659. doi: 10.1016/J.YGCEN.2024.114659
26
Mohammed-Geba K. González A. A. Suárez R. A. Galal-Khallaf A. Martos-Sitcha J. A. Ibrahim H. M. et al . (2017). Molecular performance of Prl and Gh/Igf1 axis in the Mediterranean meager, Argyrosomus regius, acclimated to different rearing salinities. Fish Physiol. Biochem.43, 203–216. doi: 10.1007/s10695-016-0280-9
27
Moriyama S. Swanson P. Nishii M. Takahashi A. Kawauchi H. Diekhoff W. W. et al . (1994). Development of a homologous radioimmunoassay for coho salmon insulin-like growth factor-I. Gen. Comp. Endocrinol.96, 149–161. doi: 10.1006/gcen.1994.1167
28
Nam B.-H. Moon J.-Y. Kim Y.-O. Kong H. J. Kim W.-J. Kim K.-K. et al . (2011). Molecular and functional analyses of growth hormone-releasing hormone (GHRH) from olive flounder (Paralichthys olivaceus). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 159 (2), 84–91. doi: 10.1016/j.cbpb.2011.02.006
29
Noor N. M. Cob Z. C. Ghaffar M. A. Das S. K. (2019). An evaluation of the effect of salinities on oxygen consumption and wellbeing in the hybrid grouper Epinephelus fuscoguttatus × E. Lanceolatus. Turk J. Fish Aquat Sci.19, 1017–1023. doi: 10.4194/1303-2712-v19_12_04
30
Patel D. Barnes J. E. Davies W. I. L. Stenkamp D. L. Patel J. S. (2020). Short-wavelength-sensitive 2 (Sws2) visual photopigment models combined with atomistic molecular simulations to predict spectral peaks of absorbance. PloS Comput. Biol.16, 1–20. doi: 10.1371/journal.pcbi.1008212
31
Pérez-Maceira J. J. Otero-Rodiño C. Mancebo M. J. Soengas J. L. Aldegunde M. (2016). Food intake inhibition in rainbow trout induced by activation of serotonin 5-HT2C receptors is associated with increases in POMC, CART and CRF mRNA abundance in hypothalamus. J. Comp. Physiol. B186, 313–321. doi: 10.1007/S00360-016-0961-9/FIGURES/5
32
Pierce A. L. Dickey J. T. Larsen D. A. Fukada H. Swanson P. Dickhoff W. W. (2004). A quantitative real-time RT-PCR assay for salmon IGF-I mRNA, and its application in the study of GH regulation of IGF-I gene expression in primary culture of salmon hepatocytes. Gen. Comp. Endocrinol.135, 401–411. doi: 10.1016/j.ygcen.2003.10.010
33
Reinecke M. Björnsson B. T. Dickhoff W. W. McCormick S. D. Navarro I. Power D. M. et al . (2005). Growth hormone and insulin-like growth factors in fish: Where we are and where to go. Gen. Comp. Endocrinol.142, 20–24. doi: 10.1016/j.ygcen.2005.01.016
34
Rizky D. Byun J. H. Mahardini A. Fukunaga K. Udagawa S. Pringgenies D. et al . (2024). Two pathways regulate insulin-like growth factor genes in the brain and liver of the tropical damselfish Chrysiptera cyanea: A possible role for melatonin in the actions of growth and thyroid hormones. Comp. Biochem. Physiol. A Mol. Integr. Physiol.296, 111679. doi: 10.1016/J.CBPA.2024.111679
35
Ruchin A. B. (2004). Influence of colored light on growth rate of juveniles of fish. Fish Physiol. Biochem.30, 175–178. doi: 10.1007/s10695-005-1263-4
36
Shao Y. T. Wang F. Y. Fu W. C. Yan H. Y. Anraku K. Chen I. S. et al . (2014). Androgens increase lws opsin expression and red sensitivity in male three-spined sticklebacks. PloS One9, e100330. doi: 10.1371/JOURNAL.PONE.0100330
37
Sierra-Flores R. Davie A. Grant B. Carboni S. Atack T. Migaud H. (2016). Effects of light spectrum and tank background colour on Atlantic cod (Gadus morhua) and turbot (Scophthalmus maximus) larvae performances. Aquaculture450, 6–13. doi: 10.1016/j.aquaculture.2015.06.041
38
Smith A. R. Ma K. Soares D. Carleton K. L. (2012). Relative LWS cone opsin expression determines optomotor thresholds in Malawi cichlid fish. Genes Brain Behav.11, 185–192. doi: 10.1111/J.1601-183X.2011.00739.X
39
Takahashi A. Kasagi S. Murakami N. Furufuji S. Kikuchi S. Mizusawa K. et al . (2016). Chronic effects of light irradiated from LED on the growth performance and endocrine properties of barfin flounder Verasper moseri. Gen. Comp. Endocrinol.232, 101–108. doi: 10.1016/j.ygcen.2016.01.008
40
Takeuchi Y. Bapary M. A. J. Igarashi S. Imamura S. Sawada Y. Matsumoto M. et al . (2011). Molecular cloning and expression of long-wavelength-sensitive cone opsin in the brain of a tropical damselfish. Comp. Biochem. Physiol. A Mol. Integr. Physiol.160, 486–492. doi: 10.1016/j.cbpa.2011.08.007
41
Tian H. Y. Zhang D. D. Xu C. Wang F. Liu W.B. (2015). Effects of light intensity on growth, immune responses, antioxidant capability and disease resistance of juvenile blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol.47, 674–680. doi: 10.1016/J.FSI.2015.08.022
42
Tine M. de Lorgeril J. Panfili J. Diop K. Bonhomme F. Durand J. D. (2007). Growth hormone and Prolactin-1 gene transcription in natural populations of the black-chinned tilapia Sarotherodon melanotheron acclimatised to different salinities. Comp. Biochem. Physiol. B Biochem. Mol. Biol.147, 541–549. doi: 10.1016/J.CBPB.2007.03.010
43
Valen R. Edvardsen R. B. Søviknes A. M. Drivenes Ø. Helvik J. V. (2014). Molecular evidence that only two opsin subfamilies, the blue light- (SWS2) and green light-sensitive (RH2), drive color vision in Atlantic Cod (Gadus morhua). PloS One9, 1–24. doi: 10.1371/journal.pone.0115436
44
Vargas-Chacoff L. Saavedra E. Oyarzún R. Martínez-Montaño E. Pontigo J. P. Yáñez A. et al . (2015). Effects on the metabolism, growth, digestive capacity and osmoregulation of juvenile of Sub-Antarctic Notothenioid fish Eleginops maclovinus acclimated at different salinities. Fish Physiol. Biochem.41, 1369–1381. doi: 10.1007/s10695-015-0092-3
45
Varsamos S. Xuereb B. Commes T. Flik G. Spanings-Pierrot C. (2006). Pituitary hormone mRNA expression in European sea bass Dicentrarchus labrax in seawater and following acclimation to fresh water. J. Endocrinol.191, 473–480. doi: 10.1677/JOE.1.06847
46
Wu L. Wang Y. Han M. Song Z. Song C. Xu S. et al . (2020). Growth, stress and non-specific immune responses of turbot (Scophthalmus maximus) larvae exposed to different light spectra. Aquaculture520, 734950. doi: 10.1016/j.aquaculture.2020.734950
47
Wu L. Wang Y. Li J. Song Z. Xu S. Song C. et al . (2021). Influence of light spectra on the performance of juvenile turbot (Scophthalmus maximus). Aquaculture533, 736191. doi: 10.1016/j.aquaculture.2020.736191
48
Yamanome T. Mizusawa K. Hasegawa E. I. Takahashi A. (2009). Green light stimulates somatic growth in the barfin flounder Verasper moseri. J. Exp. Zool. A Ecol. Genet. Physiol.311, 73–79. doi: 10.1002/jez.497
49
Yamashina F. Takeuchi Y. Fukunaga K. Udagawa S. Tan E. S. Byun J. et al . (2019). Daily expression of a clock gene in the brain and pituitary of the Malabar grouper (Epinephelus malabaricus). Gen. Comp. Endocrinol.280, 9–14. doi: 10.1016/j.ygcen.2019.03.019
50
Yokoyama S. (2000). Molecular evolution of vertebrate visual pigments. Prog. Retin Eye Res.19, 385–419. doi: 10.1016/S1350-9462(00)00002-1
51
Zhu Y. Fukunaga K. Udagawa S. Shimabukuro A. Takemura A. (2022). Effects of selected light wavelengths on the transcript levels of photoreceptors and growth-related hormones and peptides in the Malabar grouper Epinephelus malabaricus. Aquac Rep.27, 101393. doi: 10.1016/j.aqrep.2022.101393
52
Zhu Y. Negishi R. Fukunaga K. Udagawa S. Shimabukuro A. Takemura A. (2023). Activation of the growth–IGF-1 axis, but not appetite, is related to high growth performance in juveniles of the Malabar grouper, Epinephelus malabaricus, under isosmotic condition. Comp. Biochem. Physiol. A Mol. Integr. Physiol.283, 111456. doi: 10.1016/j.cbpa.2023.111456
53
Zou Y. Peng Z. Wang W. Liang S. Song C. Wang L. et al . (2022). The stimulation effects of green light on the growth, testicular development and stress of olive flounder Paralichthys olivaceus. Aquaculture546, 737275. doi: 10.1016/j.aquaculture.2021.737275
Summary
Keywords
appetite, aquaculture, grouper, growth, light wavelength, lipogenesis, salinity
Citation
Zhu Y, Fukunaga K, Udagawa S, Lu S, Shimabukuro A and Takemura A (2025) Combined effects of light and salinity on the growth performance in juveniles of the Malabar grouper, Epinephelus malabaricus. Front. Mar. Sci. 12:1613939. doi: 10.3389/fmars.2025.1613939
Received
18 April 2025
Accepted
13 May 2025
Published
13 June 2025
Volume
12 - 2025
Edited by
Yafei Duan, South China Sea Fisheries Research Institute, China
Reviewed by
Sofia Priyadarsani Das, National Taiwan Ocean University, Taiwan
Li Xin, Dalian Ocean University, China
Updates
Copyright
© 2025 Zhu, Fukunaga, Udagawa, Lu, Shimabukuro and Takemura.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akihiro Takemura, takemura@sci.u-ryukyu.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.