Introduction
Symbiotic associations between marine animals and sessile invertebrates are a remarkable feature of coral reef ecosystems. However, most studies often concentrate on free-living organisms, which are easier to collect and identify. In contrast, symbiotic animals are frequently overlooked or underestimated because of their small size, hidden lifestyles, and the broad distribution of sibling or cryptic species that are specialized to specific host species (Knowlton, 1993; Horka et al., 2016). Although the uniqueness of these symbiotic relationships is not fully understood, crustacean decapods associate with other macro invertebrates. In particular, various echinoderms are notably common and exhibit astonishing diversity in their morphology, ecology and sexual biology within the tropical Indo-Pacific region (Bruce, 1976; Williams, 1984; Ng and Jeng, 1999; Hayes, 2007; Hayes et al., 2016).
Among different classes in the phylum Echinodermata, Crinoids are a vital component of coral reef ecosystems, exhibiting notable diversity in the coral reef ecosystems of India (Sastry et al., 2019). Earlier studies on crinoids in India have been reported by Clark (1912) and Clark and Rowe (1971). About 25–30 species of crinoids have been hitherto recorded from the Indian waters. There are about 28 species reported in the Andaman and Nicobar Islands whereas 12 species in the Lakshadweep archipelago (Sastry et al., 2019). Recently, studies on crinoid-associated crustacean decapods have received further attention in Indian waters with special reference to the Lakshadweep archipelago (Prakash and Marimuthu, 2020, Prakash and Marimuthu, 2022, Prakash and Marimuthu, 2024). The present study aims to establish a baseline dataset spatially to understand the diversity of crustacean decapod communities that are associated with different crinoid species in Lakshadweep by using multivariate analyses.
Material and methods
During our regular faunal explorations under Zoological Survey of India In-house activities funded by the Ministry of Environment, Forest and Climate Change, Government of India entitled, “Fauna of protected areas of Lakshadweep: newly declared PAs (2020-24) and Fauna of Lakshadweep: Echinoderms (2017-2020)”, these Crinoidea and associated crustacean fauna were collected from 85 locations. These fauna were collected systematically from the sessile coral habitat using the line intercept transect method (English et al., 1997) followed parallel to the shore. The mean abundance data on the Crinoid host and their associated crustacean fauna based on their availability in the Islands were calculated and used as raw data for validation. Six surveys were conducted between February 2018 and February 2023 to assess these species distribution patterns along the Islands and Islet of Lakshadweep Archipelago.
The position of the Islands and Islet has been classified into three based on the proximity and geographical locations of the archipelago (Supplementary Figure 1). They were Amindivi Islands (Amini, Kadamat and Kilthan Islands), Laccadive Islands (Agatti, Kavaratti, Bangaram, Androth, Suheli, Kalpeni, Piramulpar Islands and Pitti Islet) and Minicoy Island. The collected crinoid (Sastry et al., 2019) and their associated crustacean fauna (Prakash and Marimuthu, 2020; Prakash and Marimuthu, 2022; Prakash and Marimuthu, 2024) were identified through standard methods and the taxonomical notes were published. The distribution pattern of the crinoid host was assessed with respect to the Islands and the associated faunal distribution was evaluated with respect to their host fauna. SCUBA diving was conducted for collecting these organisms at a depth between 10 and 40m. The spatial analyses such as principal component analysis (distribution variability of crinoid host and associated crustacean fauna), metric multidimensional scaling analysis (distance matrix of Islands based on the available crinoid host), Mondrian plot and indicator species analysis (probability of association within the community structure) were assessed through PRIMER 7 version 7.0.5 (Clarke and Gorley, 2015) and PAST, version 4.13 (Hammer et al., 2001; Hammer and Harper, 2024).
Results
Among 12 crinoid species (including one identified up to the genus level) were documented in the Lakshadweep Archipelago (Supplementary Figure 2), only eight species served as hosts for symbiotic crustaceans. It includes three species of Phanogenia (Phanogenia gracilis, Phanogenia distincta and Phanogenia multibrachiata), three species of Stephanometra (Stephanometra indica, Stephanometra tenuipinna and Stephanometra sp.), Himerometra robustipinna and Comaster multifidus. The associated crustacean fauna observed were: seven palaemonid shrimps (Palaemonella pottsi, Pontoniopsis comanthi, Laomenes nudirostris, Laomenes tigris, Periclimenes pitti, Periclimenes affinis and Cuapetes seychellensis), three species of alphid shrimps (Synalpheus carinatus, Synalpheus comatularum and Synalpheus stimpsonii), two species squat lobsters (Allogalathea elegans and Allogalathea babai), one species of Brachyuran (Permanotus purpureus) and a porcelain crab (Aliaporcellana pygmaea).
Crinoidea diversity
Principal component analysis (Figure 1A) revealed the spatial variability of crinoid distribution along the Lakshadweep archipelago. There was a variability (PC1: 26.8% variance) between the Islands and the most influenced crinoid genus observed in this study was Stepanometra (Stephanometra tenuipinna and Stephanometra Indica) followed by Phanogenia distincta. Stepanometra spp. are more contributed towards Minicoy Island followed by Laccadive group of Islands led by Kavaratti Island. Phanogenia distincta followed as the second key contributor towards Laccadive group such as Bangaram and Agatti Islands. A little variability (PC2: 20.2% variance) was also observed due to the influence of Comaster multifidus around Kadamat Island of Amindivi group and Dichrometra palmata around Agatti Islands.
Figure 1
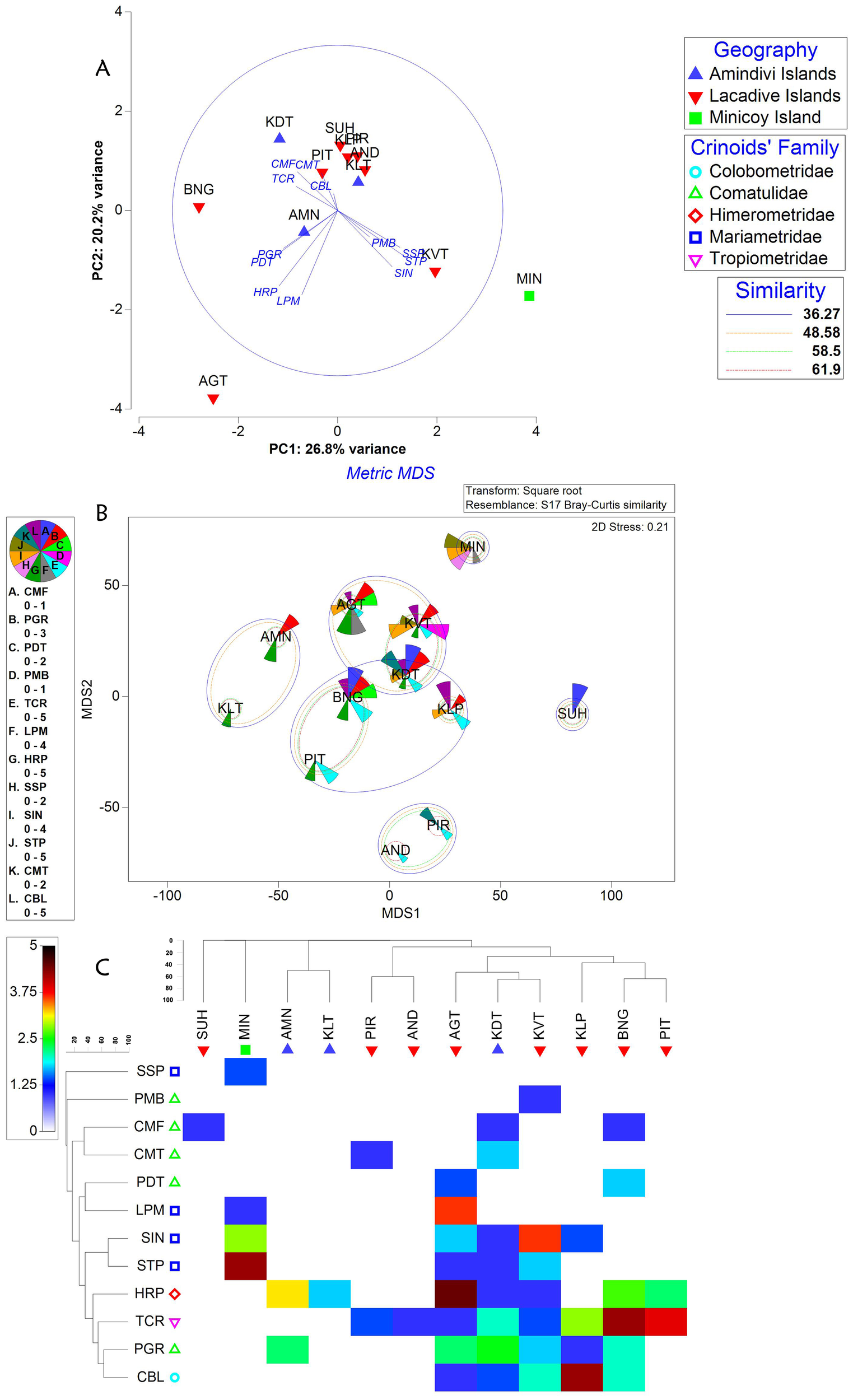
Multivariate analyses on spatial distribution of Cinoidea fauna. (A) PCA, (B) MDS analysis and (C) Mondrian plot based on abundance of crinoidea faunal diversity along the Islands of Lakshadweep archipelago. Host Crinoids: PGR, Phonogenia gracilis; PMB, Phanogenia multibrachiata; PDT, Phanogenia distincta; SIN, Stephanometra indica; STP, Stephanometra tenuipinna; SSP, Staphanometra sp.; HRP, Himerometra robustipinna; CMF, Comaster multifidus; TCR, Tropiometra carinata; LPM, Dichrometra palmata; CMT, Capillaster multiradiatus; CBL, Cenometra bella; Amindivi Islands: AMN, Amini Island; KLT, Kilthan Island; KDT, Kadamat Island; Lacadive Islands: AGT, Agatti Island; BNG, Bangaram Island; PIT, Pitti Islet; SUH, Suheli Island; KLP, Kalpeni Island; PIR, Piramul Par; KVT, Kavaratti Island; AND, Androth Island; Minicoy Island (MIN).
Metric multidimensional scaling analysis (Figure 1B) showed the distance matrix of the Islands based on the intensity of multiple crinoidea species observed. It indicates the complete segregation of all classified Islands such as Minicoy Island, Amindivi and Laccadive group of Islands. Bray-curtis cluster analysis (Figure 1B) found four main clusters: two complete clusters in the Laccadive group with 58.5% and 36.27% similarity, one complete cluster in the Amindivi group with 48.58% similarity, one mixed cluster between Laccadive and Amindivi group of Islands with 48.58% similarity and one outlier, Minicoy Island. The rationale behind the two complete clusters formed in Laccadive group of Islands due to the contribution in highest intensity of multiple crinoidea species such as Tropiometra carinata and Cenometra bella followed by Himerometra robustipinna and Phanogenia gracilis. These species are also clustered together with 37.17% similarity based on their occurrence in higher intensity at the Laccadive group of Islands (Figure 1C). Similarly, Himerometra robustipinna and Phanogenia gracilis played a vital role in single complete cluster formed in Amindivi group. Minicoy Island was an outlier due to higher intensity of multiple crinoidea species from the Mariametridae family (Figure 1C).
Crinoid associated crustacean diversity
Principal component analysis (Figure 2A) showed a strong variability (PC1: 52.1% variance) between the associated crustacean fauna with the crinoid hosts. The most influenced crustacean species observed were palaemonid shrimps (Periclimenes pitti, Periclimenes affinis and Cuapetes seychellensis), brachyuran (Permanotus purpureus) and porcelain crabs (Aliaporcellana pygmaea) followed by alpheid shrimps (Synalpheus carinatus and Synalpheus stimpsonii) which were totally dependent on Phanogenia gracilis. A little variability (PC2: 14.6% variance) was also observed between the crustacean fauna in choosing the Crinoid host. Similarly, Phanogenia gracilis acts as a host for Synalpheus comatularum.
Figure 2
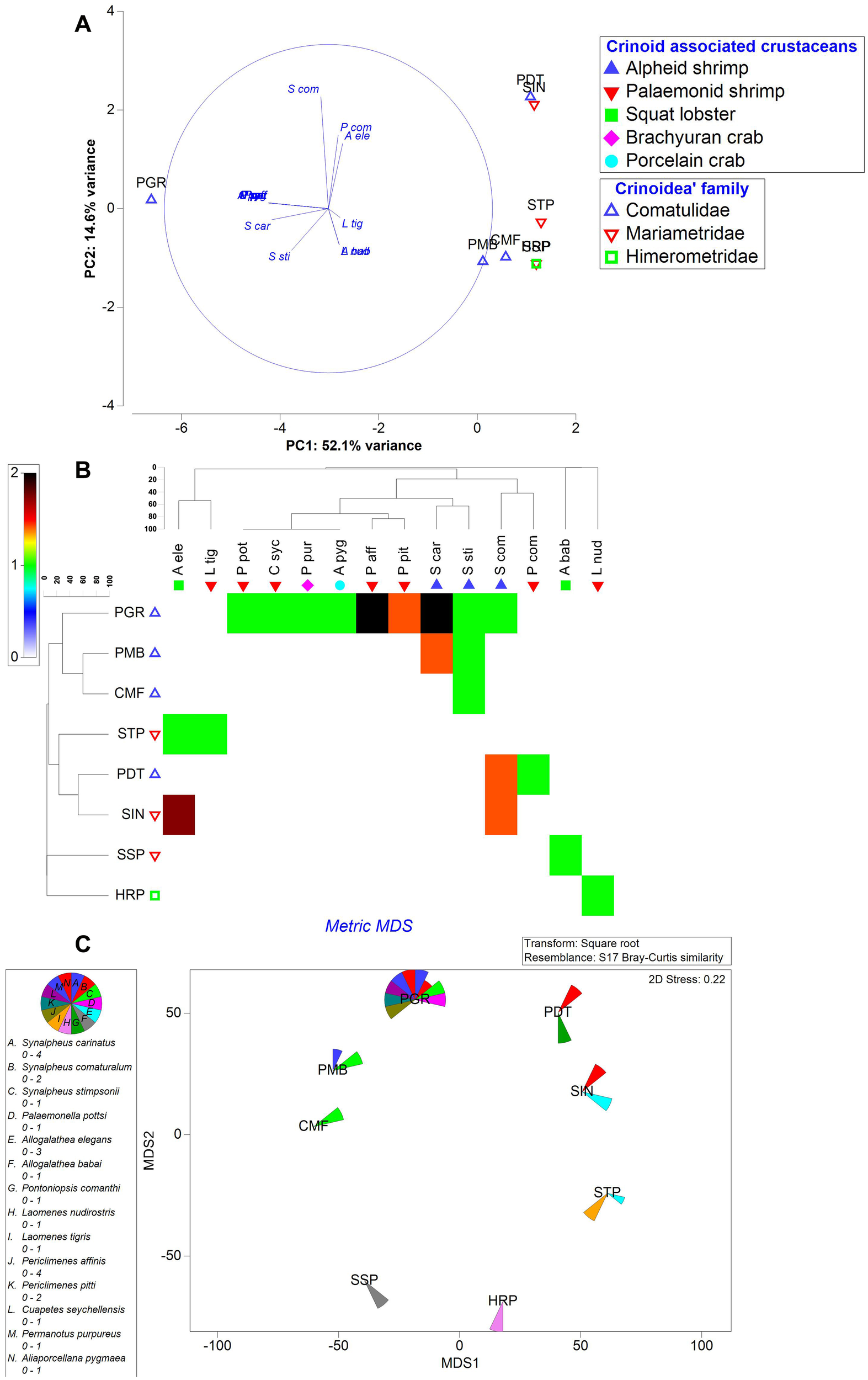
Multivariate analyses on spatial distribution of Crinoid associated crustaceans. (A) PCA (B) Mondrian plot (C) MDS analysis based on abundance of crustacean diversity with respect to host crinoids. Host Crinoids acronyms (Refer Figure 1). Crinoid associated crustaceans: S. car, Synalpheus carinatus; S. com, Synalpheus comatularum; S. sti, Synalpheus stimpsonii; P. pot, Palaemonella pottsi; A. ele, Allogalathea elegans; A. bab, Allogalathea babai; P. com, Pontoniopsis comanthi; L. nud, Laomenes nudirostris; L. tig, Laomenes tigris; P. aff, Periclimenes affinis; P. pit, Periclimenes pitti; C. syc, Cuapetes seychellensis; P. pur, Permanotus purpureus; A. pyg, Aliaporcellana pygmaea.
Phanogenia gracilis dependent crustacean fauna got clustered together (Figure 2B) with a similarity of 49.92% in connection with their selection of this crinoid as a host. Among the crustacean fauna, Periclimenes affinis, Synalpheus carinatus and Periclimenes pitti were observed as dominant taxa in selecting Phanogenia gracilis as host (Figure 2B). Alpheid shrimps preferred to associate only with the Comatulidae family of crinoids (Phanogenia gracilis, Phanogenia multibrachiata and Comaster multifidus) with a lower similarity of 25.51% (Figure 2B). Metric multidimensional scaling analysis (Figure 2C) and Indicator species analysis (Supplementary Figure 3) showed that the highest diversity of crustacean fauna associated with Phanogenia gracilis with the high Shannon-Weiner diversity index of 1.993, while squat lobsters preferred Mariametridae family of Crinoids (Stephanometra indica, Stephanometra tenuipinna) as host (Figures 2B, C).
Discussion
The distribution pattern of faunal resources spatially by using statistical methods helps to understand the relationship of species richness along the study sites/regions. It would also be helpful to express the variability in the temporal assessment when the present assessment will be compared in the future. The present status of both the crinoid host and their associated crustacean fauna along the Islands of Lakshadweep archipelago was presented by using multivariate approach. The live specimens documentation of host crinoids (Supplementary Figure 2) and their associated decapod crustaceans (Prakash and Marimuthu, 2020; Prakash and Marimuthu, 2022; Prakash and Marimuthu, 2024) were presented based on the systematic identification on the duplicate specimens in the laboratory (Sastry et al., 2019; Prakash and Marimuthu, 2020; Prakash and Marimuthu, 2022; Prakash and Marimuthu, 2024).
The principal component analysis helps to observe the influencing crinoid species and their crustacean association between the study sites/cluster of study sites/regions recorded during the study period. In the present study, Stephanometra spp., Phanogenia distincta, Comaster multifidus and Dichrometra palmata acted as principal components of crinoid hosts with 20.2 to 26.8% variability. Based on the proximity of Islands in the archipelago, the crinoid species diversity gradually decreased from the north (Amindivi group) and central (Laccadive group) Islands to southern Minicoy Island based on the contribution of most influenced (PC1) species; Stephanometra spp. (Stephanometra tenuipinna and Stephanometra indica). Among the crustacean associates, palaemonid shrimps, brachyuran and porcelain crabs followed by alpheid shrimps acted as principal components associated exclusively with the crinoid, Phanogenia gracilis with 52.1% variability. Such kind of expression was made for the taxa (coral and fish communities) based on proximity of pollution/non-pollution and on/offshore as factors in the Red Sea ecosystem (Sawall et al., 2014). In India, the variability of bio-physical parameters (Jha et al., 2013; Manikandan et al., 2014; Riyas et al., 2020) pertaining to coral community structure, Drupella cornus on coral colonies (Marimuthu et al., 2022), Holothuroidea (Gole et al., 2022) and phytoplankton diversity (Sachithanandam et al., 2022) based on the proximity of Island/mainland were practiced by using different multivariate analyses. But, the influencing fauna of the host and their associated crustaceans were expressed here individually to understand both the proximity of the Islands as well as species-specific association. Further, few species of crustacean decapods were associated with more than one crinoid species. Horka et al. (2016) reported such multiple host switching phenomenon particularly on palaemonid shrimps. Similarly, such associations in particularly, squat lobsters (Allogalathea elegans) and alphaeid shrimps (Synalpheus stimpsonii, Synalpheus carinatus, and Synalpheus comatularum) were found associated with multiple hosts in the present study. Interestingly, certain crinoid species attract specific crustacean associates due to differences in crinoid morphology, habitat complexity, and chemical cues, which provide suitable microhabitats and protection for particular decapods (Prakash and Marimuthu, 2020; Korzhavina et al., 2024). Further, the decline in crinoid diversity from northern Amindivi Islands to southern Minicoy Island may stem from environmental gradients similar to Red Sea coral-reef fish dynamics (Ellis et al., 2017; Lucia, 2024). In the Red Sea, coral diversity and symbiotic relationships weaken near polluted or nearshore areas due to factors like nutrient runoff, sedimentation, and human activity (Hoegh-Guldberg et al., 2017; Lucia, 2024). Similarly, the Lakshadweep archipelago’s northern and central islands (Amindivi and Laccadive) likely offer more stable habitats for healthier crinoid populations, supporting diverse decapods like Synalpheus shrimps and Allogalathea squat lobsters (Prakash and Marimuthu, 2020). Hence, the monitoring of such environmental variables is need of the hour to interpret the diversity of crinoids and their associates. Nowshad et al. (2020) also expressed about the less diversity of shallow water crinoids in Lakshadweep Island compared to the nearest Maldive Islands.
The resulted baseline information would be helpful to track future changes in crinoid associated symbiotic communities. By including their distribution pattern in the current management plan of State Forest Department of UT Lakshadweep, the dataset can strengthen the local conservation efforts and act as indicators of the richness of such lesser-known taxa for supporting better biodiversity protection in the Lakshadweep archipelago.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
NM: Data curation, Validation, Writing – original draft, Investigation, Supervision, Visualization, Methodology, Project administration, Conceptualization, Funding acquisition, Software, Resources, Formal Analysis, Writing – review & editing. SP: Formal Analysis, Writing – review & editing, Methodology, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
NM thank the Ministry of Environment, Forest and Climate Change, Government of India and the Director, Zoological Survey of India for the financial support to collect the specimens through ZSI in-house programs, “Fauna of Lakshadweep: Echinodermata and Fauna of Protected Areas of Lakshadweep”. NM is eternally grateful to the late Dr. D.R.K. Sastry who identified the Crinoid specimens. An eternal gratitude is also owed to the late Dr. Charles Messing of Nova Southeastern University for suggesting the key for crinoid identification. He would also like to thank the CF, Environment and Forest Department, UT Lakshadweep, Shri. Abdul Raheem and Dr. Syed Ali, RFOs, Environment and Forest Department, UT Lakshadweep for the permit and logistic support to carry out the study in the inhabited Islands and uninhabited protected environment. SP thank the authorities of Sathyabama Institute of Science and Management, Chennai for the research facilities to identify the Crustacean fauna. Authors thank three reviewers whose constructive comments have greatly improved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1614247/full#supplementary-material
References
1
Bruce A. J. (1976). Coral reef caridea and “commensalism. Micronesica.12, 83–98.
2
Clark A. H. (1912). “The crinoidea of the Indian ocean,” in Echinoderma of the Indian museum. PartVII (Indian Museum, Calcutta), 1–325.
3
Clark A. M. Rowe F. W. E. (1971). “Crinoidea,” in Monograph of Shallow-Water Indo-West Pacific Echinoderms: i–vii. (London: Trustees of the British Museum), 238.
4
Clarke K. R. Gorley R. N. (2015). PRIMER V7. PRIMER-E. Ltd., devon PL21 9RH, UK. Available online at: http://updates.primer-e.com/primer7/manuals/User_anual_v7a.pdf (Accessed January 15, 2025).
5
Ellis J. Anlauf H. Kürten S. (2017). Cross shelf benthic biodiversity patterns in the Southern Red Sea. Sci. Rep.7, 437. doi: 10.1038/s41598-017-00507-y
6
English S. Wilkinson C. Baker V. (1997). Survey manual for tropical marine resource (Townsville: Australian institute of marine science). Available online at: https://www.aims.gov.au/sites/default/files/Survey%20Manual-sm01.pdf (Accessed September 20, 2017).
7
Gole S. Mohammed P. I. Apte D. Marimuthu N. (2022). Holothurian spatial variability and substratum preference in the intertidal habitats of the Andaman Sea. Regional Stud. Marine Science.56, 102633. doi: 10.1016/j.rsma.2022.102633
8
Hammer Ø. Harper D. A. T. (2024). Paleontological data analysis. 2nd ed (USA: John Wiley & Sons Ltd.), 400.
9
Hammer Ø. Harper D. A. T. Ryan P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica.4, 9pp.
10
Hayes F. E. (2007). Decapod crustaceans associating with the sea urchin Diadema antillarum in the Virgin Islands. Nauplius.15, 81–85.
11
Hayes F. E. Holthouse M. C. Turner D. G. Baumbach D. S. Holloway S. (2016). Decapod crustaceans associating with echinoids in Roatán. Honduras Crustacean Res.45, 37–47. doi: 10.18353/crustacea.45.0_37
12
Hoegh-Guldberg O. Poloczanska E. S. Skirving W. Dove S. (2017). Coral reef ecosystems under climate change and ocean acidification. Front. Mar. Sci.4. doi: 10.3389/fmars.2017.00158
13
Horka I. De Grave S. Fransen C. H. J. M. Petrusek A. Duris Z. (2016). Multiple host switching events shape the evolution of symbiotic palaemonid shrimps (Crustacea: Decapoda). Sci. Reports.6, 26486. doi: 10.1038/srep26486
14
Jha D. K. Vinithkumar N. V. Marimuthu N. Baskar B. Sahu B. K. Das A. K. et al . (2013). Field and GIS based post-tsunami assessment of Scleractinian coral cover in the Aerial Bay group of Islands, North Andaman, India. J. Coast. Conserv.17, 671–677. doi: 10.1007/s11852-013-0266-z
15
Knowlton N. (1993). Sibling species in the sea. Annu. Rev. Ecology Evol. Systematics.24, 189–216. doi: 10.1146/annurev.es.24.110193.001201
16
Korzhavina O. A. Gubareva N. V. Kitashov A. V. Britayev T. A. Ivanenko V. N. (2024). From Microscale Interactions to Macroscale Patterns in Copepod-Crinoid Symbiosis. Anim. (Basel)14, 877. doi: 10.3390/ani14060877
17
Lucia G. M. (2024). Red sea coral reefs. Available online at: https://www.ebsco.com/research-starters/environmental-sciences/red-sea-coral-reefs (Accessed 07.05.2025).
18
Manikandan B. Ravindran J. Shrinivaasu S. Marimuthu N. Paramasivam K. (2014). Community structure and coral status across reef fishing intensity gradients in Palk Bay reef, southeast coast of India. Environ. Monit. Assess.186, 5989–6002. doi: 10.1007/s10661-014-3835-1
19
Marimuthu N. Wilson J. J. Al-Sofyani A. A. Kumaraguru A. K. (2022). Coral reef health status versus muricid bioindicator in the Lakshadweep archipelago – A multivariate approach. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.914240
20
Ng P. K. L. Jeng M. S. (1999). The brachyuran crabs (Crustacea: Decapoda: Eumedonidae and Portunidae). Zoological Stud.38, 268–274.
21
Nowshad M. B. Babu I. K. K. Parameswaran U. V. Messing C. G. Sureshkumar S. (2020). The shallow-water crinoid fauna of lakshadweep atolls, north-western Indian ocean. Zootaxa.4789, 247–265. doi: 10.11646/zootaxa.4789.1.9
22
Prakash S. Marimuthu N. (2020). Notes on some crinoid associated decapod crustaceans (Crustacea: Decapoda) of Lakshadweep Archipelago, Central Indian Ocean. Zootaxa.4766, 086–100. doi: 10.11646/zootaxa.4766.1.4
23
Prakash S. Marimuthu N. (2022). First record of some crinoid and sponge associated crustacean decapods (Crustacea: Decapoda) from Lakshadweep Archipelago. Thalassas: Int. J. Marine Sci.38, 879–884. doi: 10.1007/s41208-022-00434-4
24
Prakash S. Marimuthu N. (2024). Palaemonid shrimps from lakshadweep archipelago, India with a new species of periclimenes costa. Thalassas: Int. J. Marine Sci.40, 1351–1361. doi: 10.1007/s41208-024-00735-w
25
Riyas C. A. Idreesbabu K. K. Marimuthu N. Sureshkumar S. (2020). Impact of the tropical cyclone Ockhi on ecological and geomorphological structures of the small low-lying Islands in the Central Indian Ocean. Regional Stud. Marine Sci.33, 100963. doi: 10.1016/j.rsma.2019.100963
26
Sachithanandam V. Mohan P. M. Karthik R. Elangovan S. S. Mageswaran T. Marimuthu N. (2022). A biogeographic assessment of phytoplankton richness and composition in the Andaman Archipelago and the potential links to anthropogenic and environmental impacts: A multivariate approach. Regional Stud. Marine Sci.52, 102248. doi: 10.1016/j.rsma.2022.102248
27
Sastry D. R. K. Marimuthu N. Rajan R. (2019). Echinodermata of Lakshadweep, Arabian Sea with the description of a new genus and a species. Rec. Zool. Surv. India.119, 348–372. doi: 10.26515/rzsi/v119/i4/2019/144963
28
Sawall Y. Al-Sofyani A. Banguera-Hinestroza E. Voolstra C. R. (2014). Spatio-Temporal Analyses of Symbiodinium Physiology of the Coral Pocillopora verrucosa along Large-Scale Nutrient and Temperature Gradients in the Red Sea. PloS One9, e103179. doi: 10.1371/journal.pone.0103179
29
Williams A. B. (1984). Shrimps, lobsters, and crabs of the atlantic coast of the eastern United States, maine to florida (Washington, D.C.: Smithsonian Institution Press), 550.
Summary
Keywords
crinoid associated crustaceans, multivariate analyses, Lakshadweep archipelago, oligotrophic waters, echinoderms
Citation
Marimuthu N and Prakash S (2025) Beneath the waves: a multivariate approach on species-specific crinoid-crustacean decapod association in the Lakshadweep archipelago. Front. Mar. Sci. 12:1614247. doi: 10.3389/fmars.2025.1614247
Received
18 April 2025
Accepted
13 May 2025
Published
06 June 2025
Volume
12 - 2025
Edited by
Josephine Anthony, Meenakshi Academy of Higher Education and Research, India
Reviewed by
Sivakumar Kuppusamy, Pondicherry University, India
Hoang Xuan Ben, Institute of Oceanography in Nhatrang, Vietnam
Selvin Kani, National Centre for Sustainable Coastal Management, India
Updates
Copyright
© 2025 Marimuthu and Prakash.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nithyanandam Marimuthu, marinemari@hotmail.com; Sanjeevi Prakash, prakash.cccs@sathyabama.ac.in
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.