Abstract
Microplastic pollution is ubiquitous in the oceans. However, little is known about the physiological impact of microplastics on corals, particularly under predicted future ocean conditions. This study investigated the individual impacts of microplastic exposure (MP) and predicted future ocean conditions [ocean acidification and warming (OAW)] as well as the combination of these stressors (OAW+MP) on the growth and physiology of Acropora cervicornis, a threatened Caribbean coral and its associated symbiont, Symbiodiniaceae. After 22 days, the OAW+MP treatment resulted in more pronounced physiological changes than either stressor individually or the control. OAW conditions alone had minimal impacts, despite A. cervicornis generally being sensitive to thermal stress. The OAW+MP treatment and the MP treatment also disrupted the host-symbiont relationship evidenced by the higher symbiont densities relative to the control and the OAW treatments. Additionally, the OAW+MP treatment resulted in lower chlorophyll a per symbiont cell. Microplastic handling is energetically costly, possibly leading to changes in host-symbiont signaling. Photosynthetic efficiency was only marginally lower in the OAW+MP treatment, and values did not indicate photosystem damage. Negative host health impacts were found with the OAW+MP treatment exhibiting lower skeletal growth compared to the control and lower host protein concentrations compared to the OAW treatment. These results indicate that although short term microplastic exposure alone may not pose a significant threat to coral health, when adding additional stressors, it can further threaten the health and recovery of this already vulnerable species.
1 Introduction
Coral reef ecosystems are extraordinarily biodiverse and are among the most economically valuable habitats on earth (Plaisance et al., 2011). In recent decades, disease outbreaks, invasive species, nutrient pollution, and climate change impacts such as ocean warming and ocean acidification have all led to unprecedented declines in coral cover (Hoegh-Guldberg et al., 2007; Alvarez-Filip et al., 2009; Anthony et al., 2011; Cramer et al., 2020). Ocean warming (OW) results in more extreme and frequent temperature anomalies, such as the Caribbean thermal event in 2023 (Neely et al., 2024), which are linked to massive bleaching events and significant loss of coral biomass (Brown, 1997; Hughes et al., 2007). Ocean acidification (OA) reduces relative carbonate ion availability, inhibiting the ability of calcifying organisms to create their limestone skeletons (Doney et al., 2008; Fabry et al., 2008; Erez et al., 2011). The combined impact of OA and OW (OAW) on corals is less studied, though these conditions are likely to be the reality for coral reef ecosystems in the near future. On a reef-wide scale, OAW is predicted to reduce reef accretion to the point of net erosion by 2100 (Cornwall et al., 2021). On a smaller scale, some evidence points to synergistic effects of OA and OW on coral colonies, such as increased bleaching (Erez et al., 2011), increased bioerosion (Reyes-Nivia et al., 2013), decreased calcification (Reynaud et al., 2003; Rodolfo-Metalpa et al., 2011), and lowered phase-shift thresholds (Anthony et al., 2011). Conversely, evidence has also been found indicating that lower pH can mitigate the harmful effects of higher temperature in coral larvae (Pitts et al., 2020), does not prolong recovery from bleaching in adults (Dobson et al., 2024), and that corals may be able to co-evolve tolerance to both OA and OW (Jury and Toonen, 2024).
An additional anthropogenic hazard to coral reefs that is not yet well understood is plastic pollution in the oceans, which has recently garnered attention as an emergent threat (Biswas et al., 2024; Huang et al., 2021; Soares et al., 2020; Zhang et al., 2023). Microplastics are generally defined as fragments of plastic between 1 and 1000 µm in diameter of many shapes and compositions (Hartmann et al., 2019) and carry harmful chemicals and pathogens in their surface biofilm (Tang et al., 2018; Caruso, 2019; Curren and Leong, 2019; Naik et al., 2019; Saliu et al., 2019). Microplastic concentrations vary greatly across the oceans, ranging from 0.002 to 66.50 items m-3 (Mutuku et al., 2024). Corals can passively accumulate microplastics in their surface mucous layer, incorporate them into their tissues, and deposit them into their skeletons, acting as a microplastic sink in the oceans (Martin et al., 2019; Reichert et al., 2022; Soares et al., 2023) which can negatively impact coral health (Allen et al., 2017; Hankins et al., 2018; Martin et al., 2019). Equally concerning impacts arise from active coral-microplastic interactions where the coral handles particles for extended periods of time and can ingest them; such interactions can be energetically costly and result in reduced growth and calcification (Chapron et al., 2018; Reichert et al., 2018; Mouchi et al., 2019; Rotjan et al., 2019; Hankins et al., 2021; Rades et al., 2024), bleaching and tissue necrosis (Reichert et al., 2018, 2019; Syakti et al., 2019), feeding impairment (Hankins et al., 2018; Reichert et al., 2018, 2019; Mouchi et al., 2019; Rotjan et al., 2019; Savinelli et al., 2020), and health impacts at the molecular level (Tang et al., 2018, 2021; Lanctôt et al., 2020). In addition to bleaching, studies have found other impacts to the coral symbionts (Symbiodiniaceae), with microplastic presence leading to changes in symbiont density, chlorophyll content, and photosynthetic activity (Tang et al., 2018; Mouchi et al., 2019; Syakti et al., 2019; Lanctôt et al., 2020; Gao et al., 2024; Rades et al., 2024; Liang et al., 2025). These impacts have been observed in wild corals across varying regions and depths, and in laboratory settings for a wide range of microplastic concentrations and exposure times. Additionally, responses are highly species specific, with different species exhibiting varying responses to the same treatments (Reichert et al., 2018, 2019; Mendrik et al., 2021; Tang et al., 2021) and some species experiencing little or no impact from microplastic exposure (Berry et al., 2019; Bejarano et al., 2022; Boodraj and Glassom, 2022; Zhou et al., 2023).
Few studies have investigated microplastic effects on Western Atlantic coral species, despite the Atlantic Ocean having the highest concentration of microplastics across the world’s oceans (Mutuku et al., 2024). The staghorn coral, Acropora cervicornis, a once-dominant reef-builder in the region, has suffered large-scale losses in the last 50 years (Carpenter, 1990; Bythell et al., 1993; Hughes, 1994; Aronson and Precht, 2001; Alvarez-Filip et al., 2009). It is now one of the six coral species listed as critically endangered on the IUCN Red List of endangered species (IUCN, 2021) and one of the seven coral species listed as threatened under the U.S. Endangered Species Act (NOAA Fisheries, 2014). As A. cervicornis has been a central focus of Caribbean restoration efforts, understanding the impacts that microplastics and OAW may have on this species is crucial for successful conservation and management efforts.
Although research on the impacts of microplastics to coral health is emerging, very few studies have examined how microplastics may affect corals under predicted future ocean conditions. One study found that previous exposure to thermal stress in certain corals resulted in lower successful prey capture of Artemia nauplii, with no significant decrease in microplastic consumption (Axworthy and Padilla-Gamiño, 2019), while other studies have reported no interactive effects of microplastic exposure and thermal stress (Mendrik et al., 2021; Plafcan and Stallings, 2022). It remains unknown, however, how microplastics may affect A. cervicornis under combined warming and OA. To address this knowledge gap, we exposed fragments of A. cervicornis to microplastics and OAW conditions over 22 days to test the impacts of these stressors both individually and combined. Previous research from this project demonstrated that microplastic exposure and OAW conditions stimulated an immune response in A. cervicornis corals (Bove et al., 2023). This study builds on that work by investigating the physiological impacts to the coral host and its symbionts. We hypothesized that both the OAW and microplastics treatments would have negative health impacts on the corals, and that the combined stressor treatment would have greater effects than either stressor alone.
2 Materials and methods
2.1 Coral collection
Nine putative genotypes of A. cervicornis coral were collected off the coast of Fort Lauderdale, Florida from the Nova Southeastern University coral nursery (Florida Fish and Wildlife Conservation Commission permit #SAL-21-2200-SRP). In a separate portion of this project, two genotypes of six tested were identified as clonemates using single nucleotide polymorphisms (SNPs) (Bove et al., 2023). As this was determined after all analyses had been conducted, the nine putative genotypes were treated as unique for statistical analyses within this study. After one week in an outdoor ex-situ nursery, donor colonies were transported to the University of North Carolina Wilmington Center for Marine Science (UNCW-CMS). Corals were acclimated to laboratory conditions in a recirculating holding tank for three months. During this acclimation period, the same parameters (i.e., pH, temperature, salinity, lighting, and feeding regime) to be used during experimentation were maintained at the ambient treatment levels; see “experimental design” section for details. Corals were suspended from monofilament and water flow was provided by powerheads circulating water within the tank. Two weeks before experimentation, four 5 cm fragments from each genotype’s donor colonies were clipped and glued to ceramic plugs. After a one-week recovery period in the holding tank following fragmentation, the fragments were placed in their individual experimental tanks (12 tanks per rack with one recirculating sump) for a one-week acclimation period prior to treatment. During this period, each rack recirculated through a sump and ambient water conditions were maintained.
2.2 Experimental design
Ambient water conditions (AMB) were set to 8.07 pH (total scale will be used for pH from here forward) and 28.6 °C, reflecting average oceanic pH and temperature in Fort Lauderdale at the start of the experiment. OAW water conditions were chosen to reflect intermediate predictions for 2075 (pH = 7.92 and temperature = 30.6 °C) (IPCC, 2019; Jiang et al., 2019). For the microplastic addition treatments (MP), two size classes of UV-fluorescent blue polyethylene microspheres (density: 1.13 g cc-1; Cospheric, LLC) were used. Cospheric polyethylene microbeads have been widely used in coral microplastic research, including studies by Hankins et al. (2018, 2021), Rotjan et al. (2019), and Corinaldesi et al. (2021). The blue fluorescence aids in identification, and polyethylene is the most prevalent polymer found in marine plastic pollution (Erni-Cassola et al., 2019). The smaller size class (180 –212 µm) approximates the diameter of the acclimated diet of powdered zooplankton (Polyp Lab Reef Roids: 150-200 μm) and the larger size class (325-425 μm) is similar to potential prey items. Acropora cervicornis corals can ingest particles of a wide range of sizes from approximately 100 μm (powdered plankton) (Towle et al., 2015) to over 1000 μm (microplastics) (Hankins et al., 2022). Nine fragments, one from each of the putative genotypes, were subjected to each treatment [(1) “control” (AMB water conditions without microplastics), (2) “MP” (AMB water conditions with microplastics), (3) “OAW” (OAW water conditions without microplastics), and (4) “OAW+MP” (OAW water conditions with microplastics)] for a total of 36 fragments. Coral fragments were exposed to the treatments, including the ramp-up periods, for 22 days.
The experimental system consisted of 36 10-gallon glass aquariums divided among three separate racks, holding twelve tanks each. Treatments were randomly assigned to each tank, with three tanks of each treatment per rack, and the 36 fragments were randomly assigned to tanks such that there was one fragment per tank. Neptune Systems Apex microcontrollers were used to control the pH of each OAW tank and temperature of all tanks individually. The microcontrollers maintained pH by modulating solenoids that bubbled CO2 into each OAW tank through airline tubing and controlled temperature by modulating aquarium heaters. The pH programming for every OAW tank was adjusted based on daily pH readings using an Orion Dual Star pH meter (Thermo Fisher Scientific). At the start of experimentation, temperature in the OAW tanks was increased from the 28.6 °C acclimation temperature in approximately 0.5 °C increments on days 1, 2, 4, 9, and 16 to the treatment conditions of 30.6 °C, to avoid acute thermal stress. The experimental degree heating weeks (eDHW) was calculated based on methods described in Leggat et al. (2022) using a local mean monthly maximum of 29.44 °C from the coral collection region (NOAA Coral Reef Watch, 2019), taking into account the ramp up period. The pH during acclimation and in all AMB tanks throughout the experiment was not manipulated. At the start of experimentation, pH in all OAW tanks was decreased from the starting value of 8.07 pH by 0.05 pH units each day over the first three days to the final value of 7.92 pH.
Each tank contained an air stone bubbling oxygen to maintain adequate dissolved oxygen (DO) levels and a 75-watt aquarium heater. Lighting for each tank was provided by an individual LED fixture with a 12-hour photoperiod set at irradiance values of 195 µmol m-2 S-1. In each tank, the coral fragment was placed in a 400 mL plastic beaker with a 100 µm Nitex® mesh bottom suspended in the water using a plastic rack so that the tank water level was below the top of the beaker (Figure 1). This allowed for maintenance of the microplastic concentration while allowing adequate water flow from within the tank. Each tank contained a powerhead connected to an airline tube that pumped water from the tank at a constant rate from the top of the beaker (Figure 1) to create water flow for the coral and to aid in suspending the microplastics. Salinity, temperature, and dissolved oxygen were measured daily in every tank using a YSI ProDSS multiparameter probe (YSI Inc.). Ammonia, nitrate, and phosphate were measured every day in two new tanks chosen randomly (one AMB and one OAW) using a ReefBot Auto Titration System (Reef Kinetics). On days 1, 8, 15, and 23, a water sample was taken from each tank for alkalinity measurements as a proxy for the OA treatment. Samples were stored in the dark at 4 °C and were later analyzed with a Metrohm 848 Titrino Plus auto-titrator following SOP3b from the Guide to Best Practices for Ocean CO2 Measurements Manual (Dickson et al., 2007). Dickson Certified Reference Materials are analyzed regularly as part of the quality assurance protocols. Feedings throughout acclimation and the experiment occurred twice weekly via target feeding. For each event, 2 mL of a homogenous Reef Roids (Polyp Lab) suspension (prepared by dissolving 0.101 g of Reef Roids in 80 mL of filtered seawater) was pipetted directly into each 400 mL beaker, delivering approximately 2.53 mg of particulate food per feeding (equivalent to 6.33 mg L-1).
Figure 1

Setup for each individual tank. (A) A beaker suspended in a rack with tubing delivering a steady flow of water and (B) egg crate wedged into the bottom of a beaker with the fragment glued to the ceramic plug in the center.
For this study, a microplastic concentration of 1.25 x 10–4 g mL-1 was used. Although this concentration is higher than that of environmental ocean conditions in most locations in the Western Atlantic and Caribbean (Aranda et al., 2022; Orona-Návar et al., 2022; Wightman and Renegar, 2023), the aim of this study was to investigate separate and synergistic impacts of microplastics and OAW conditions on the health metrics of A. cervicornis. By choosing a higher concentration of microplastics, we sought to increase the resolution of our physiological data for understanding these coral-microplastic interactions under an additional environmental stressor.
To avoid excess fouling in the beakers while maintaining the microplastic concentration in the MP treatments, a rotation procedure was employed. Two days before water change days, a clean beaker was placed in each tank’s rack adjacent to the beaker with the coral. In the MP tanks, a mixture containing 0.025 g of each microplastic size class was deposited into the empty 400 mL beaker, thus creating the desired concentration of 1.25 x 10–4 g mL-1, and the microplastics were forced into suspension by expelling water from a baster into the cup two times. The microplastics were left in the beaker to acquire a natural biofilm over 48 h. Three times per week during acclimation and experimentation, on the day after corals were fed, 30% water changes were conducted and the corals were moved into the new beakers.
Biofilm acquisition caused the microplastics to become negatively buoyant. Despite a constant water flow from the top of the beaker, the microplastics tended to settle on the bottom – a typical result of closed-system hydrodynamics. So, the plastics were forced into suspension by gently expelling water from a baster into the beaker, thus creating intermittent, turbulent water movement; this process was also repeated in the non-MP beakers to avoid confounding factors. Microplastics were in suspension for approximately 10 minutes after each basting, allowing interaction between the coral and the microplastics. The basting procedure was conducted three times per day over the course of the 22-day experiment in every coral beaker.
2.3 Live measurements
Immediately before starting the treatments on day 1 of the experiment, the buoyant weight of each fragment was measured according to the buoyant wet weight method first described by Jokiel et al. (1978). Buoyant weight measurements were taken again immediately after the end of the experiment, on day 22. The skeletal mass of each sample was calculated based on the equations in Jokiel et al. (1978). The sample mass in water was measured with the buoyant weight technique, the seawater density was calculated using the buoyant weight, air weight, and volume of the mass standard, and the density of the sample was determined using a dried A. cervicornis skeletal fragment. The starting skeletal mass of each fragment was subtracted from the final skeletal mass to obtain the amount of skeletal growth per fragment.
Pulse amplitude modulated (PAM) fluorometry was employed to measure the symbiont photosynthetic efficiency in photosystem II of the symbiont chloroplasts five times throughout the experiment on days 7, 11, 14, 17, and 21. Decreases in the quantum yield ratio, generally falling below 0.6, correlate with damage to the photosynthetic machinery and often precede bleaching (Warner et al., 1999; Gorbunov et al., 2001). Measurements were taken with a Diving-PAM fluorometer (UWFA0204A - Walz GmbH) one hour after the artificial sunset, so that the corals were fully acclimated to the dark. To ensure consistent measuring distances, plastic tubing (7 mm) was affixed to the end of the probe and placed directly on the coral. Each fragment was measured in two places (near the top and bottom) and these measurements were averaged.
2.4 Physiological analyses
At the end of the 22-day experiment, the fragments were removed one at a time from their tanks and prepared for analysis. Each fragment was clipped from the ceramic plug 0.5 cm above the plug to provide a buffer from the glue and then divided into multiple pieces using bone cutters. Because the growing apical tips of acroporid branches are morphologically distinct from the rest of the colony (Wallace, 1999) and generally have lower concentrations of symbionts (Pearse and Muscatine, 1971), these segments were excluded from physiological analyses and were instead preserved and used for microplastic observations. As such, the top centimeter of the coral fragment and the tip of any branch was clipped and preserved in Z-Fix Concentrate diluted in a 1:4 ratio with seawater. The branch tip samples from the MP treatments were observed under a UV light microscope and any UV-fluorescent particles found on the coral surface were counted and characterized. The bottom centimeter from each experimental fragment was clipped, and that piece was clipped in half. Each of these pieces were placed in separate cryogenic vials, dipped in liquid nitrogen, and preserved at -80 °C. These samples were sent to collaborators for analysis in a separate study (Bove et al., 2023). The remaining 2–3 cm fragment was placed in a Whirl-Pak® bag, dipped in liquid nitrogen, and preserved at -80 °C for further analyses.
Physiological analyses were conducted following a protocol developed by Hall et al. (2018). Each fragment was removed from the freezer and weighed. All coral tissue was removed from the skeleton using an airbrush with 0.2 µm filtered seawater (FSW). The tissue was captured in a 50 mL Falcon tube and the liquid in each tube was normalized to 45 mL with FSW. The skeleton was allowed to air dry and weighed again. The surface area of the skeleton was determined using a Next Engine 3D scanner for normalization of the health parameters. Each tissue sample was vortexed and then homogenized with a Power Gen 125 homogenizer (Thermo Fisher Scientific) for 5 minutes. A 200 µL aliquot was removed from the homogenate, covered, and placed in a refrigerator for total protein analysis. The falcon tube was then centrifuged for 5 minutes at 3500 rpm at 4 °C using a Sorvall ST 40R centrifuge (Thermo Fisher Scientific) to separate the symbiont cells into a pellet and the host cells into the supernatant. Another 200 µL aliquot was extracted from the supernatant for host protein analysis. The remaining supernatant was then poured off and the algal pellet was resuspended with 2 mL FSW. This process of centrifuging, pouring off the supernatant, and resuspending the pellet was repeated once more in the Falcon tube with 1 mL FSW and then again in a 1.5 mL Eppendorf tube using an Eppendorf Centrifuge 5418 R. The sample was vortexed and a 100 µL aliquot was removed and placed in a new Eppendorf tube for symbiont density analysis. For chlorophyll analysis, 1 mL 90% acetone was added to the sample while working in total darkness, and the sample was vortexed. The symbiont density sample and chlorophyll sample were stored in the dark at 4 °C for approximately 24 hours.
Symbiont density was determined with eight replicate counts per sample using a hemocytometer under a compound microscope. The counts for each sample were averaged, converted to total number of cells per fragment, and divided by the surface area of the fragment to determine the number of symbiont cells per square centimeter of coral tissue.
After 24 h, the chlorophyll sample was centrifuged again to re-form the algal pellet. Working in total darkness, triplicates of 200 µL of each sample were placed in a 96-well flat-bottomed tissue culture plate along with two triplicates of 90% acetone blanks. The absorbance endpoints were read in a Molecular Devices FlexStation 3 microplate reader at 652 nm and 665 nm. However, a microplate reader error rendered all values from one of the three plates inaccurate. The excess of each sample, which had been stored at 4 °C, was centrifuged and reanalyzed as above with only one 200 µL sample per fragment. The plate was run three times at each wavelength, absorbances were corrected using the average of the 90% acetone blanks, and values were averaged per sample. Chlorophyll a concentrations were calculated from the 1 cm pathlength correction of these values and normalized using both surface area measurements and symbiont density. The initial chlorophyll values and re-run values from the two correct plates had a 97% correlation; to avoid bias in the results, only the re-run values were used in chlorophyll a analyses for all 36 samples.
For soluble protein analysis, a Bradford Protein Assay was conducted (Bradford, 1976). To establish a calibration curve for obtaining sample values, dilutions of bovine serum albumin at concentrations of 0.125, 0.25, 0.5, 0.75, 1.0, 1.5, and 2.0 mg mL-1 were created. Next, a 20 μL aliquot of each dilution was pipetted into an Eppendorf tube and combined with 1 mL of Bradford dye reagent (containing Coomassie Brilliant Blue G-250) in completely dark conditions. The samples were then incubated for a period of 20–40 minutes. Working again in total darkness, the standards were pipetted into a 96-well VMR Tissue Culture Plate. The first three wells were filled as blanks with 200 μL FSW, followed by three blanks of FSW + dye, then 200 μL triplicates of each dilution, and finally another three blanks of FSW + dye and three blanks of FSW. Optical densities were scanned three times at 590 nm using a Molecular Devices FlexStation 3 microplate reader. The entire process was then repeated using 20 μL of each total protein sample and each host protein sample from the experiment. Average absorbances of the samples were corrected using the average absorbances of the FSW and FSW + dye blanks. Concentration values were then calculated using the calibration curve line of best fit. Protein concentration values were multiplied by 45 to account for total tissue volume, normalized to fragment surface area, and averaged for each sample.
2.5 Data analysis
Statistical tests were conducted using R. For skeletal growth, symbiont density, chlorophyll a concentration per cm2, chlorophyll a concentration per symbiont cell, total protein, host protein, and the difference between total and host protein, generalized linear mixed effects models (GLMM) were used to determine significance among the four treatments. For each response variable, several candidate models were assessed, and the best model was selected according to information criteria, r squared values, and root square mean error using the R function “compare_performance” (Lüdecke et al., 2021). All models used experimental treatment as a categorical predictor variable. Candidate models included different combinations of error distributions and random effects. Because the response variables were all continuous, strictly positive, and moderately to highly right-skewed, the Gamma distribution and the Gaussian distribution (applied to both raw and log-transformed values) were tested. Random effects for experimental rack and coral putative genotype were tested. Standard model diagnostics (e.g. homogeneity of variance, influence of outliers, normality of random effects) were then verified for each selected model using the R function “check_model” (Lüdecke et al., 2021). Finally, to test for significant differences among all treatment combinations, each model was followed by a pairwise, post hoc comparison test using estimated marginal means with Tukey’s p-value adjustment for multiple comparisons. Additionally, a GLMM was run on the photosynthetic yield data across the five dates of measurement. Again, several candidate models were tested, however all models specifically included tank as a random effect to account for repeated measures on individual coral fragments.
Environmental data (temperature, pH, salinity, DO, alkalinity) were tested for differences between OAW and AMB treatments using the Anderson-Darling test for normality, the Levene and Bartlett tests for homogeneity of variances, and then a Kruskal-Wallis test, accordingly. An alpha-level of 0.05 was used to determine significance across all tests. All means and error bars are reported with standard error.
3 Results
3.1 Experimental conditions
Temperature, pH, salinity, and DO measurements were taken daily over the 22-day experiment. Outside of the intended lower pH and higher temperature treatment, DO and salinity were significantly different between the two treatments as well. However, these parameters still fell within the normal range of best practices for DO and salinity for coral husbandry. Ambient temperature was stable (28.6 ± 0.01 °C) across the experiment; OAW temperature was stable as well (30.1 ± 0.05 °C) after the initial ramp up during the first four days of the experiment and subsequent increases on days 9 and 16. The OAW treatment corresponded to approximately 2.3 eDHW across the study, taking into account the ramp up period. As intended, temperature was significantly higher in the OAW tanks (p < 0.0001; Figure 2), and pH was significantly lower in the OAW tanks (p < 0.0001; Figure 2.). The pH of both treatments was stable (AMB: 8.06 ± 0.01; OAW: 7.96 ± 0.01), though there were fluctuations throughout the study. DO was stable for both treatments (AMB: 6.42 ± 0.01 mg L-1; OAW: 6.28 ± 0.01 mg L-1) and was significantly lower in the OAW tanks (p < 0.0001; Figure 2). Salinity fluctuated throughout the experiment (AMB: 35.70 ± 0.26 ppt; OAW: 35.66 ± 0.30 ppt), and was significantly lower in the OAW treatments (p = 0.008; Figure 2). Alkalinity was averaged across the four measurements (AMB: 9.19 ± 0.05 dKH; OAW: 9.41 ± 0.07 dKH) and was significantly higher in the OAW tanks (p < 0.05). Throughout the experiment, the ReefBot auto-titrator reported 0 ppm for nitrate, phosphate, and ammonia.
Figure 2
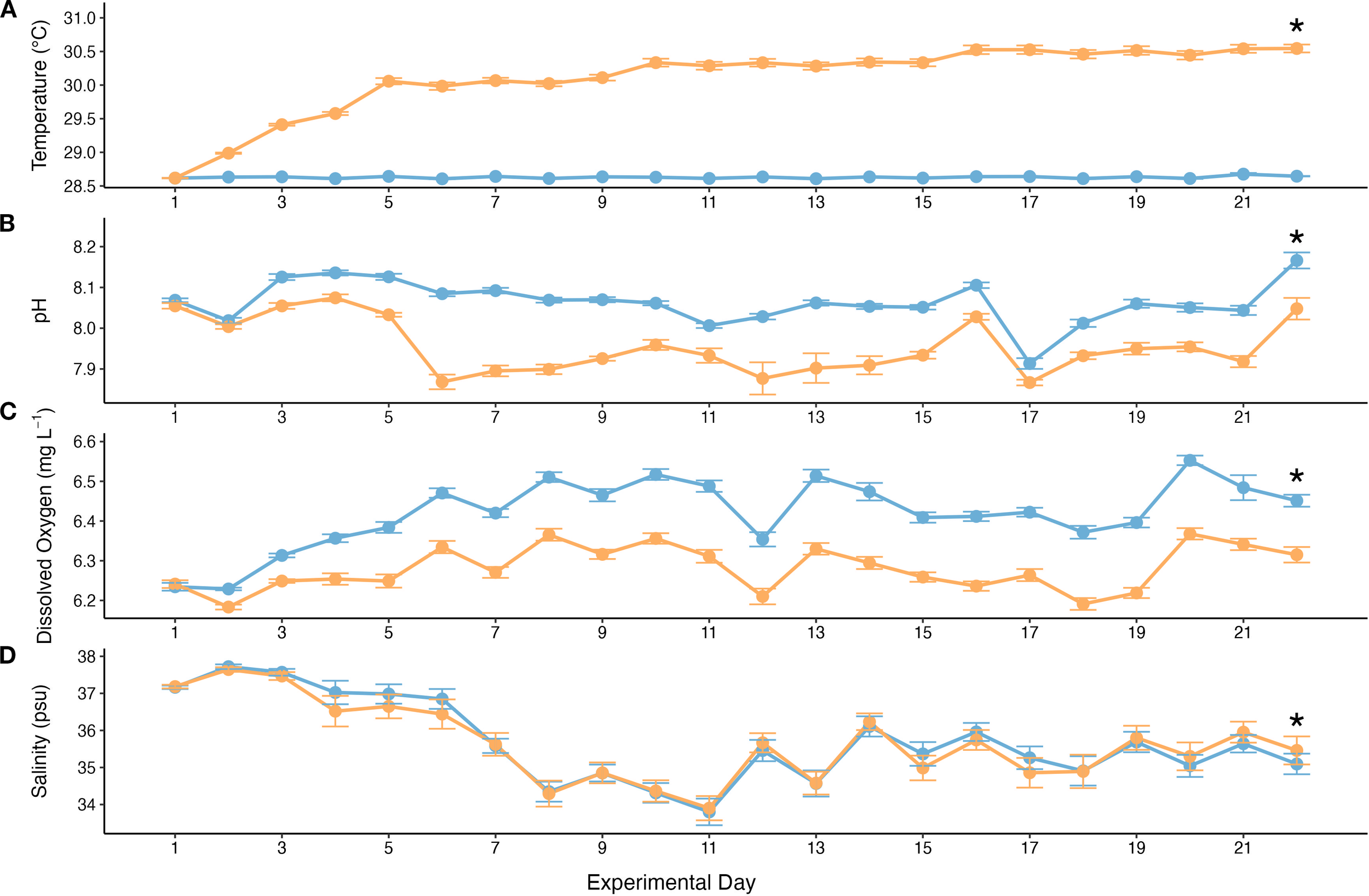
Water quality parameters throughout the experiment (means ± SE). Orange line denotes pooled OAW treatments (OAW and OAW+MP) and blue line denotes pooled AMB treatments (MP and Control). Asterisk above line indicates significance of p < 0.05. (A) Average daily temperature values taken from the Neptune temperature probes. Ramp up of approximately 0.5 °C occurred on days 1, 2, 4, 9, and 16 in OAW tanks. (B) Average daily pH values taken from YSI pH probe readings. Ramp down of approximately 0.05 pH on days 1, 2, and 3. (C) Average daily DO levels taken from YSI DO probe readings. (D) Average daily salinity values taken from YSI salinity probe readings.
3.2 Skeletal growth
Skeletal growth was measured as the difference between day 1 skeletal mass and day 22 skeletal mass. The selected GLMM for growth used a Gamma error distribution with a log link function and included random effects for both experimental rack and putative genotype. The GLMM identified a significant treatment effect and the post hoc test confirmed that growth was significantly lower in the combined stressor treatment compared to the control (p = 0.004; Figure 3). Growth was also noticeably lower in the OAW treatment compared to the control, although this effect was only marginally significant (p = 0.099; Figure 3).
Figure 3
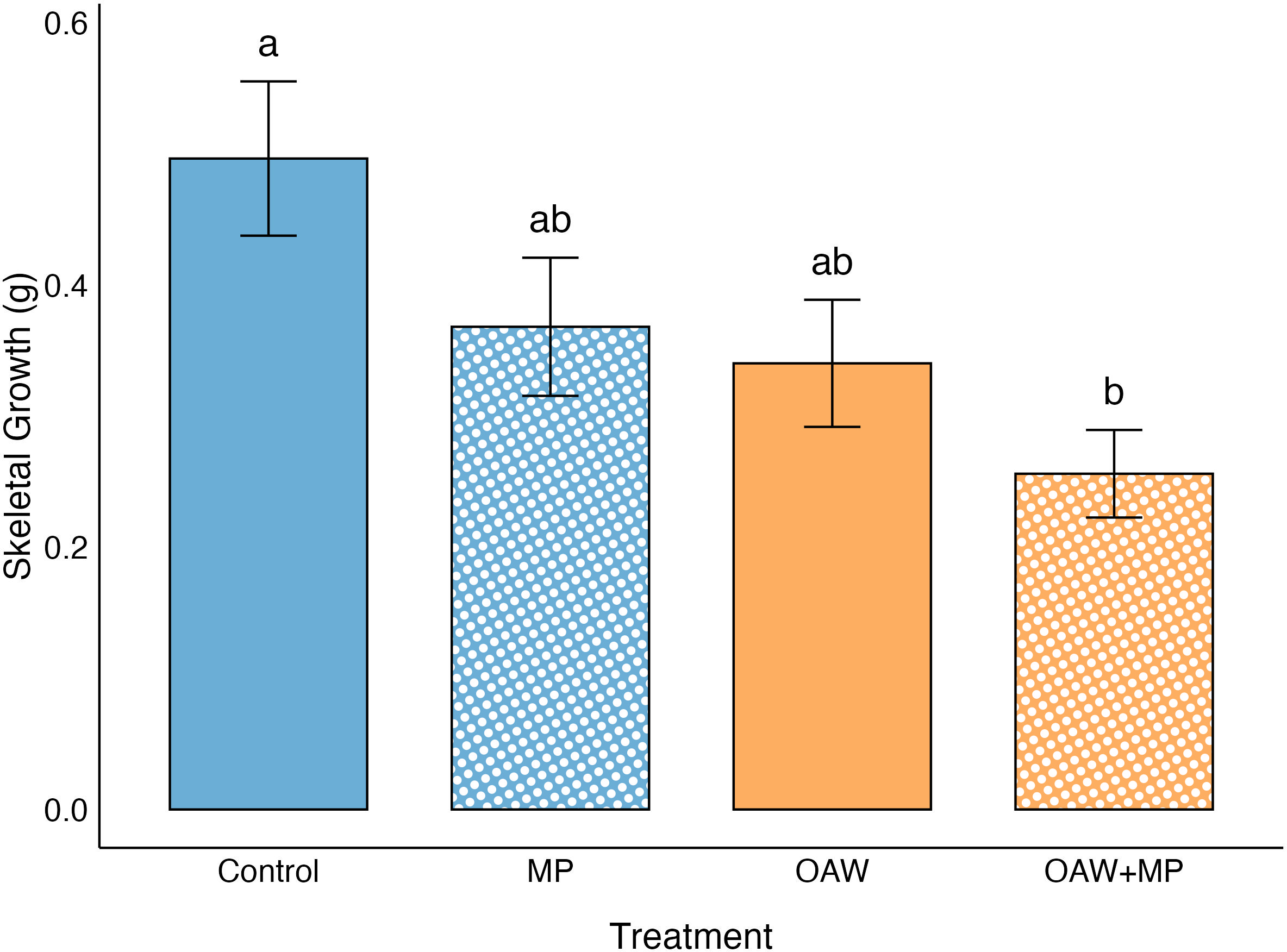
Effect of treatments on skeletal growth (means ± SE). Significance (p < 0.05) denoted by difference in letters above error bars.
3.3 Symbiont photosynthetic efficiency
Photosynthetic yield (Fv Fm-1) was averaged for each time point across the two measurements taken for each fragment. The value was not normalized to surface area, as it is not dependent on fragment size. The selected GLMM for photosynthetic yield used a Gamma error distribution with a log link function and included random effects for tank and putative genotype. The GLMM identified a significant treatment effect; however, the post hoc test found that the combined stressor treatment was only marginally lower than the control (p = 0.092; Figure 4).
Figure 4
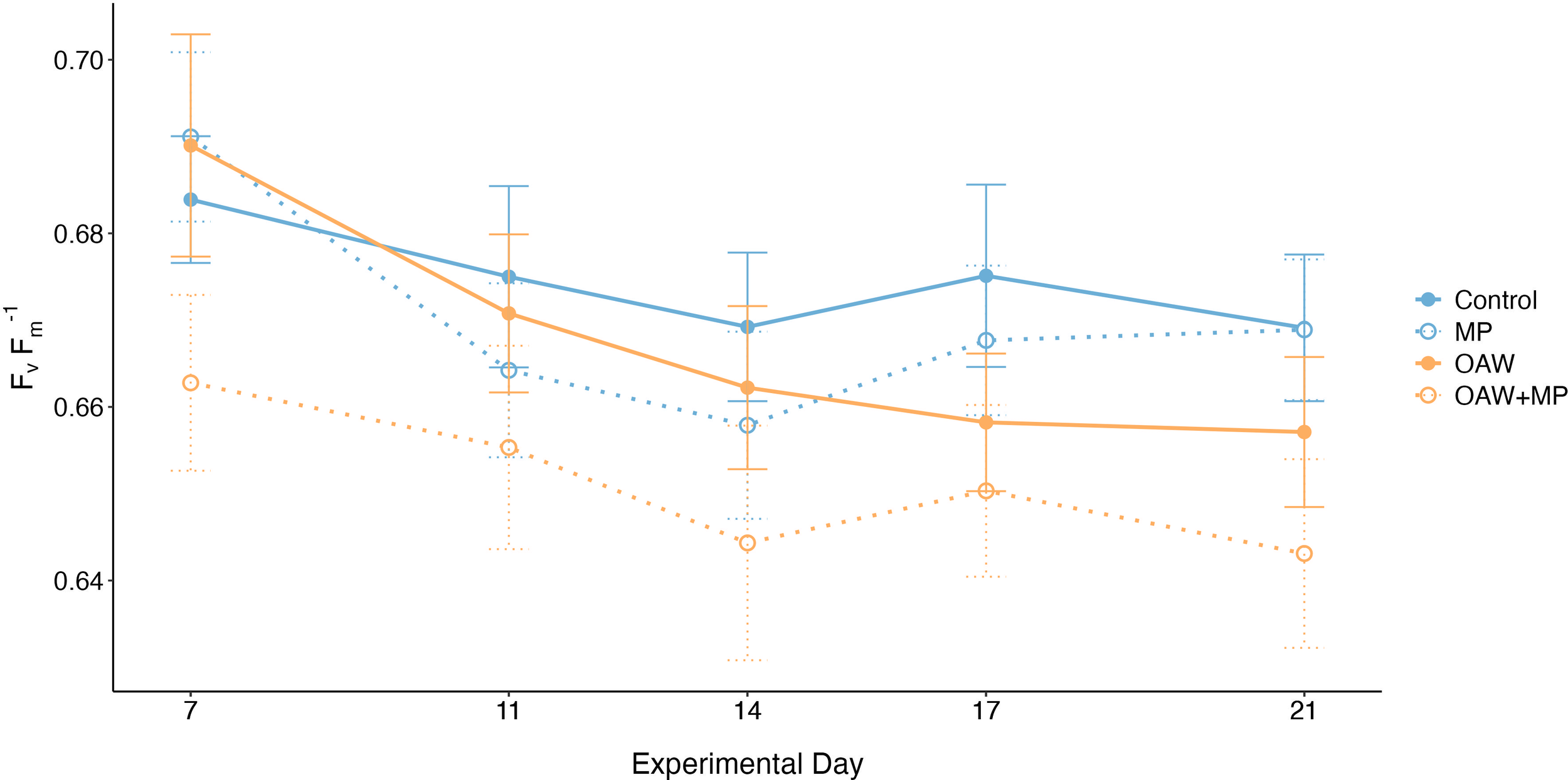
Average photosynthetic efficiency (means ± SE) across the five measured time points for each treatment.
3.4 Symbiont density and chlorophyll a content
Symbiont density was normalized to surface area and compared across treatments. The selected GLMM for symbiont density used a Gamma error distribution with a log link function and included no random effects. The GLMM identified a significant treatment effect and the post hoc test confirmed that symbiont density was significantly higher in both the MP treatment and the combined stressor treatment compared to the control (p = 0.0008; p = 0.0009, respectively; Figure 5). The symbiont densities in both the MP and OAW+MP treatments were also significantly higher than the OAW single stressor treatment (p = 0.001; p = 0.001, respectively; Figure 5).
Figure 5
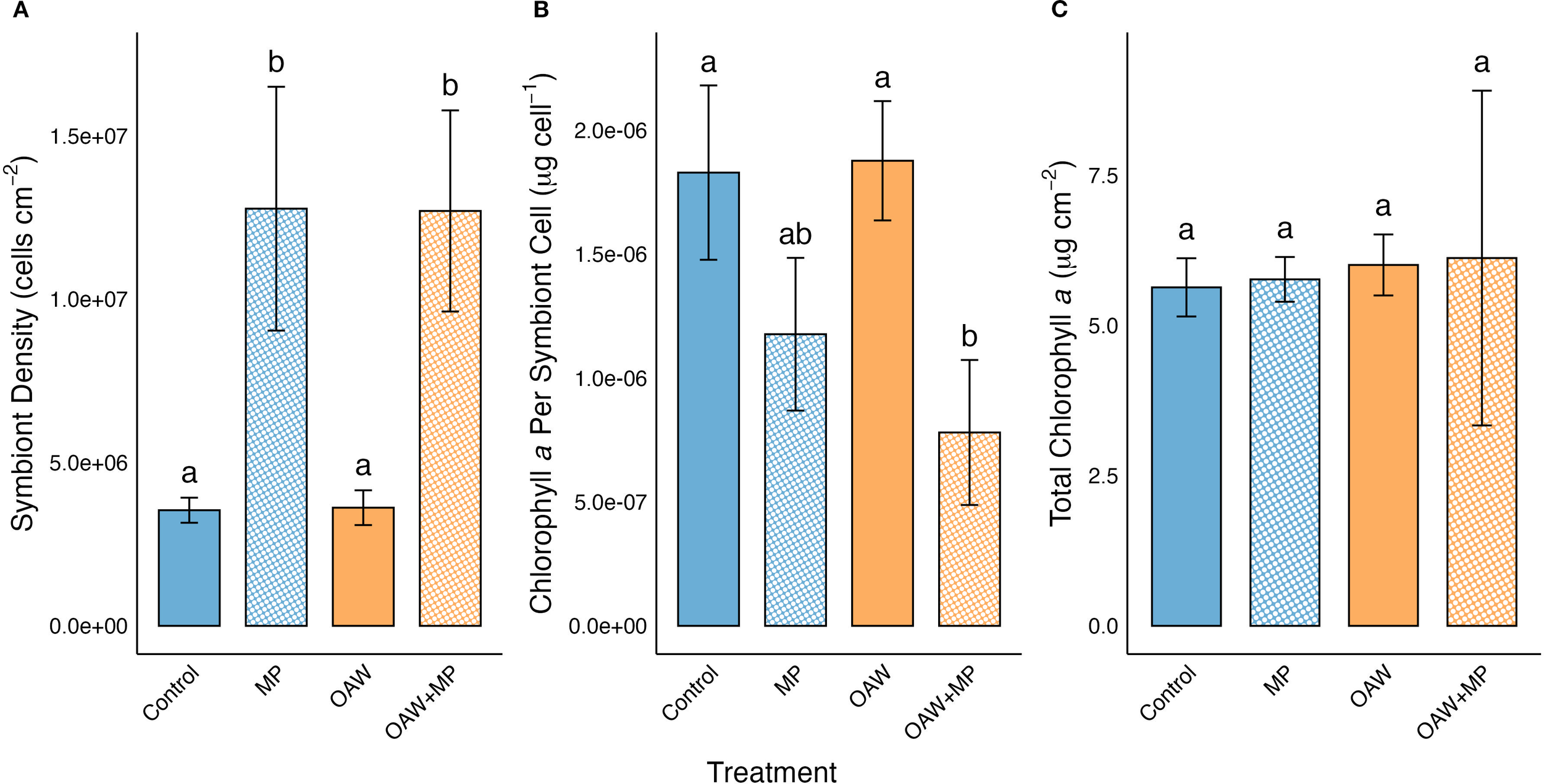
Effect of treatments on (A) symbiont density, (B) chlorophyll a concentration per symbiont cell, and (C) chlorophyll a concentration per cm2 (means ± SE). Significance (p < 0.05) denoted by difference in letters above error bars.
Chlorophyll a concentrations were normalized to both surface area and to symbiont density. The selected GLMMs for both surface area- and symbiont density-normalized chlorophyll a used a Gaussian error distribution applied to log-transformed values and included no random effects. When normalized to surface area, there was no significant difference between any of the treatment levels. However, when normalized to symbiont density, there was significantly higher chlorophyll a concentrations per symbiont cell in both the control treatment and the OAW treatment compared to the combined stressor treatment (p = 0.007; p = 0.005, respectively; Figure 5).
3.5 Protein
The total soluble protein (Symbiodiniaceae + coral host) concentration was normalized to surface area and compared across treatments. The selected GLMM for total soluble protein used a Gaussian error distribution and included random effects for experimental rack and putative genotype. Both the GLMM and post hoc test found that there were no significant differences between any of the four treatments.
The selected GLMM for host protein concentration, also normalized to surface area, used a Gamma error distribution and included random effects for experimental rack and putative genotype. The GLMM identified a significant treatment effect, and the post hoc test confirmed that the OAW treatment was significantly higher than the combined stressor treatment (p = 0.02; Figure 6).
Figure 6
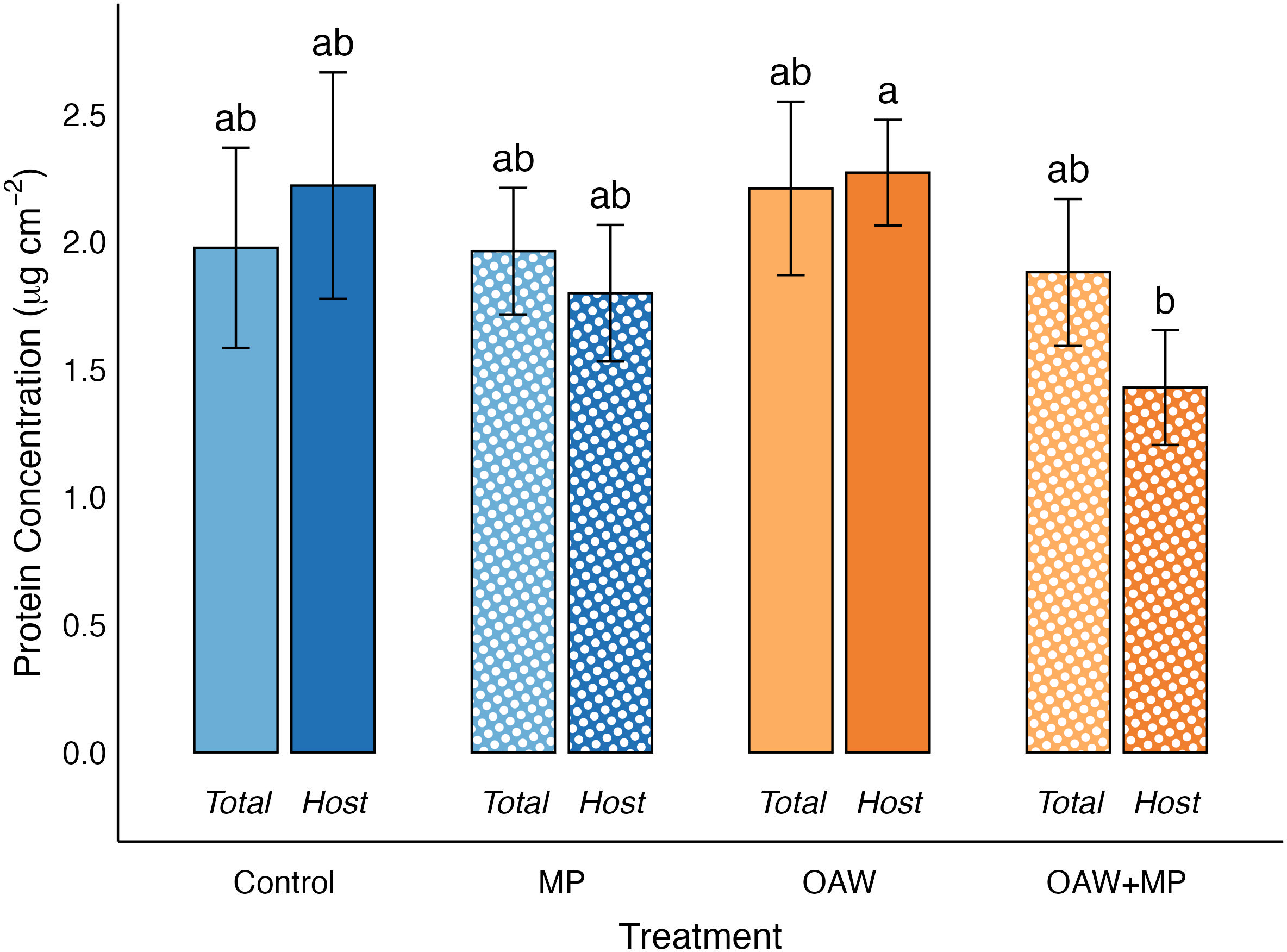
Effect of treatments on host protein concentration and total protein concentration (means ± SE). Significance (p < 0.05) denoted by difference in letters above error bars.
Lastly, a GLMM was used to compare whether total and host proteins differed among treatments. This model used a Gamma error distribution with random effects for rack and putative genotype and included an interaction between treatment and protein source (host or total). No significant difference was found between total and host proteins among the treatments.
3.6 Quantification of microplastics on coral surface
All branch tip samples from fragments in the MP and OAW+MP treatments were observed under a UV light microscope to determine presence of the UV-fluorescent blue microbeads on the surface of the coral. Of the 18 corals, seven were found to have at least one particle of UV-fluorescent blue plastic on the coral surface. Only one of these particles seemed to be a whole microbead of the same shape and size as the added microplastics. The remainder were smaller pieces of irregular shape that had the same fluorescence and color as the added microbeads.
4 Discussion
4.1 Acropora cervicornis shows resilience to the environmental stressor
Targeted environmental conditions were achieved, yet they had little effect on the corals and their symbionts. Both temperature and pH were significantly different between the AMB and OAW treatments, validating the treatment parameters. The salinity and DO levels were significantly lower and the alkalinity was significantly higher in the OAW tanks; however, these values are all within normal ranges and these differences are not ecologically significant. The lower oxygen saturation is due to the higher temperatures and is therefore linked to the treatment (Brierley and Kingsford, 2009). Furthermore, DO levels above 4 mg L-1 do not impact the health of Acropora spp. corals (Haas et al., 2014), so the slightly lower DO levels in the OAW tanks likely had no impacts relative to the AMB DO levels. Though significant, differences in salinity were also very slight and within the range of best practices in coral husbandry (O’Neil et al., 2021). Lastly, the average alkalinity measurements were also within normal ranges for coral reefs (O’Neil et al., 2021). Elevated temperatures and lowered pH, both individually and combined, are known to have negative impacts on coral health (Hoegh-Guldberg, 1999; Anthony et al., 2011). Acropora cervicornis is especially sensitive to temperature stress, showing decreases in growth, symbiont density, and photosynthetic efficiency and 100% mortality after 25 days (Langdon et al., 2018). In this study, A. cervicornis showed resilience to environmental parameters; the OAW single stressor treatment resulted in marginally lower growth than the control but there were no differences in symbiont density, chlorophyll content, photosynthetic efficiency, or protein content relative to the control treatment. While an eDHW of 2.3 can still elicit physiological responses, it generally correlates with moderate thermal stress (Leggat et al., 2022). Perhaps the environmental treatment parameters were not severe enough to significantly impact skeletal growth or the host-symbiont relationship as these corals came from a region already experiencing elevated temperatures and extreme thermal anomalies (Neely et al., 2024). Longer studies with varied temperatures are needed to determine realistic impacts under chronic exposure to elevated temperature.
4.2 Limited impact of microplastic exposure alone
The MP single stressor treatment did not significantly impact skeletal growth, photosynthetic efficiency, chlorophyll content, or protein content. We hypothesized negative impacts of microplastic exposure on skeletal growth, as that is consistent across several other studies (Chapron et al., 2018; Mouchi et al., 2019; Reichert et al., 2019), including a study that also used A. cervicornis (Hankins et al., 2021). The energetic costs of handling solid foreign entities like plastic particles might be insignificant in short time periods or at low concentrations, but could become detrimental to coral health over extended time periods; so, more study is needed. The MP treatment did, however, produce significantly higher symbiont densities compared to both the control and OAW treatment, as did the combined stressor treatment.
4.3 Combined stressor treatment results in lower growth
The combined stressor treatment elicited stronger responses than either single stressor, indicating that there may be interactive effects between OAW and MP. Corals exposed to the OAW+MP treatment had significantly lower skeletal growth compared to the control treatment, while the OAW treatment was only marginally significantly lower and the MP treatment effect was not significant. Lower growth due to OAW conditions is an expected result, but would likely occur over longer time periods. In this study, the impact on skeletal growth was significant in the OAW+MP treatment after only 22 days. This result is possibly due to the probable energetic cost associated with microplastic interaction. While the basting method resulted in higher concentrations of microplastics interacting with the coral at one time and is therefore not necessarily indicative of in situ conditions, the exaggerated active response of the corals to the microplastics warrants further investigation. Throughout the experiment, corals were observed interacting extensively with microplastics; corals quickly moved microplastic particles caught in the mucus layer to their mouths using tentacular movement and mucus secretion, where the particles were held from several seconds to well over 30 minutes. During this time, the coral manipulated the microplastic with its tentacles, often closing tightly and opening repeatedly (documented in Supplementary Video 1). While ingestion of the microplastics was not directly observed, it is possible that corals did ingest and egest some of the microplastics and hold them on their surface. This is supported by observations of the coral tissue after the experiment. Seven of the 18 fragments exposed to microplastics contained at least one fluorescent particle on the tissue surface of the observed samples. Most particles seemed to be smaller fragments of the microbeads as they were smaller than the 180–212 µm size class; likely, the microbeads were broken apart either from weathering in the water or potentially from mechanical breakdown by the coral’s manipulations. There is currently no evidence to suggest that corals can actively degrade or metabolize microplastics, but many plastic-degrading microbes have been identified across various environments (Gambarini et al., 2021; Zrimec et al., 2021; Ali et al., 2023) which raises the question of whether the coral holobiont could possess this capacity.
Anecdotally, the detritus layer that accumulated on the Nitex® mesh in the MP beakers was consistently much thicker than the layer in the non-MP beakers, possibly due to the excess mucus produced to remove microplastics. Indeed, the corals in the MP treatment were observed producing mucus both to remove microplastics and to bring them towards polyp mouths. Extensive manipulation and excess mucus production are mechanisms corals employ for removing non-nutritional items, such as sand particles, as well as assisting in food capture (Larsson and Purser, 2011; Allen et al., 2017; Duckworth et al., 2017). However, microplastics offer no nutritional value to offset the energy output and are novel items with different densities, microbial films, and chemical compositions than what corals have evolved to manage. Some coral species have shown no heterotrophic plasticity in response to microplastics (Rades et al., 2022), indicating that corals may not be able to adapt to the energetic costs of microplastic handling over extended periods of exposure time. It is possible that the extra energy expended due to microplastic encounters causes enough of a deficit to impact energy available for and allocated to processes like growth and reproduction. Corals in the combined stressor treatment suffered reduced growth perhaps for this reason; microplastic interaction seems to be energetically costly, thus worsening impacts from the OAW stressor.
4.4 Host-symbiont relationship impacted by microplastic exposure under climate change conditions
The combined stressor treatment elicited significantly higher symbiont densities than both the control and OAW treatments, as did the MP single stressor, indicating that microplastics were the main driver for this result. The increased symbiont density is intriguing because several other coral-microplastic exposure experiments have found decreases in symbiont densities (Reichert et al., 2018; Syakti et al., 2019; Su et al., 2020), or no impact (Tang et al., 2018; Reichert et al., 2019). One study also found a negative correlation between microplastic concentration and symbiont densities in wild corals (Tang et al., 2021). This would be the expected outcome based on the propensity for corals to expel their symbionts when stressed.
The symbionts in the coral tissue are not directly interacting with microplastic particles, therefore any changes seen in symbiont densities or chlorophyll concentrations are likely a result of changes at the host level. Evidence suggests that cnidarians such as corals use an array of signaling molecules and chemical stimuli known as host release factor (HRF) in several ways: to arrest dinoflagellates in their non-motile phase upon uptake into the gastrodermal cells (Koike et al., 2004; Stat et al., 2006), to force symbiont cells to release their photosynthetic products for host use (Grant et al., 1997), and to regulate symbiont density by manipulating algal production of various compounds (Grant et al., 2006). Another strategy corals employ for maintaining low reproduction rates in their symbionts is nutrient limitation. By maintaining a limitation in certain nutrients, like nitrogen, within the gastrodermal cells, the coral inhibits symbiont growth and maximizes photosynthesis (Cook et al., 1994). When encountering an energetically costly stressor, such as microplastic interaction and removal, it is possible that the host could employ HRF or remove nutrient limitations to increase symbiont densities and thus increase photosynthetic production. Alternatively, an external stressor could reduce the host’s ability to maintain nutrient limitations, resulting in an abnormally high rate of symbiont division.
The combined stressor treatment also resulted in significantly lower chlorophyll a per symbiont cell compared to both the control and OAW treatments. Previous studies have found no change in chlorophyll content (Reichert et al., 2018; Aminot et al., 2020), as well as increased chlorophyll concentrations as a function of surface area (Tang et al., 2018) and symbiont density (Su et al., 2020) after exposure to microplastics. So, this result was unexpected, as was the symbiont density result. However, the lowered chlorophyll a concentrations per symbiont cell supports the hypothesis of increased symbiont cell division. Chlorophyll a concentrations, when normalized to surface area, were almost identical among treatments, so there was no change in total chlorophyll content, but chlorophyll density was reduced in individual symbiont cells. Perhaps chlorophyll synthesis was outpaced by the rapid cell proliferation, thus diluting the overall chlorophyll content among more symbiont cells. Additionally, chlorophyll production may have decreased from the stress of the treatments and their impacts on the coral host, a result that has been seen in other microplastic studies (Gao et al., 2024; Isa et al., 2024).
Photosynthetic efficiency of photosystem II, measured via PAM fluorometry, was only marginally lower in the combined stressor comparted to the control, indicating the symbionts’ photosynthetic capabilities were largely unharmed due to the stressors. The values trended lower over time for all treatments, likely due to tank conditions, but the values remained within ranges for healthy corals (Ralph et al., 1999; Warner et al., 1999). Previous studies using photosynthetic efficiency as a health parameter have seen both higher (Reichert et al., 2019; Lanctôt et al., 2020) and lower (Mendrik et al., 2021) efficiencies as a result of microplastic exposure, as well as no change (Reichert et al., 2019; Su et al., 2020). The response of this more complex mechanism to microplastic impacts seems to be highly species-specific and is likely also dependent on symbiont species.
No significance for total proteins was found across treatments; however, there was a significantly lower mean host protein concentration in the OAW+MP treatment than the combined stressor treatment. It is possible that the lower host protein concentrations are a product of a protein catabolism stress response by the host coral. This finding is consistent with the results of the gene expression portion of this project that found enrichment of terms associated with amino acid catabolism in response to the OAW stressor (Bove et al., 2023). As a result of the catabolism of coral host tissue and amino acids, ammonium is released, in turn being utilized by the symbiont. In most circumstances, host-symbiont interactions in corals are nitrogen limited (Muscatine and Porter, 1977; Morris et al., 2019). With an influx in ammonium, the symbiont is no longer nutrient limited, and undergoes a population increase, thus recycling the host’s ammonium to synthesize its own cellular proteins (Baker et al., 2018; Rädecker et al., 2021). This disturbance to host-symbiont nitrogen balance has a positive feedback loop effect. When the symbionts are in the presence of excess nitrogen, photosynthates are used for growth and reproduction rather than reallocation to the coral host. As a response, the coral continues to break down its own protein stores, resulting in eventual starvation or bleaching in the host coral (Baker et al., 2018; Rädecker et al., 2021). It is possible that the efficient recycling of nitrogen between host and symbiont during host protein catabolism is responsible for the lower host protein concentrations found in the combined stressor treatment. If this experiment had a longer duration or reached more extreme temperatures for OAW treatments, a higher, more measurable incidence of protein catabolism may have occurred. Future work on coral catabolism responses to treatments like OAW and microplastics could measure coral lipid and carbohydrate energy reserves as higher sensitivity markers of health, and as potential precursors to catabolism of proteins in stressed corals.
5 Conclusions
The results of this study suggest that microplastics alone may not pose a significant threat to A. cervicornis coral health in short time periods, but there are interactive effects of microplastic exposure and OAW conditions that warrant further investigation. No other study has examined the combined effect of OAW and microplastics; however, several have examined thermal stress with microplastic exposure. One study found that thermal stress may not reduce microplastic ingestion in the same way it reduces natural feeding (Axworthy and Padilla-Gamiño, 2019). Two studies observed no interaction between OW and microplastic exposure (Mendrik et al., 2021; Plafcan and Stallings, 2022). The complimentary study of the current experiment found that the OAW+MP treatment elicited the strongest immune response compared to the single stressors (Bove et al., 2023), consistent with the findings of this study. Based on the high variability in species response seen in other microplastic studies, it is likely that differences in physiology between species and genotypes play a role in responses seen. Furthermore, the increase in symbiont density in response to microplastic interaction is a novel result and raises questions regarding the host-algal signaling pathways in relation to microplastics.
Health impacts and potential compensatory changes in the host-symbiont relationship were seen after only 22 days of exposure to experimental conditions with microplastic interactions generally occurring only thrice per day after the basting procedure. Given the high variability in coral reef ecosystem hydrodynamics, microplastic concentrations, topography, and species morphology, further study is needed to understand the frequency and duration of microplastic suspension and thus interactions in situ to determine how these results may be relevant to natural coral populations. As climate change progresses in the coming decades, the threats of ocean acidification and ocean warming will become more prevalent and corals will likely experience additional stress and negative health impacts (Hoegh-Guldberg et al., 2007). Furthermore, the rate of plastic entering the ocean will continue to increase (Jambeck et al., 2015; Borrelle et al., 2020). Even if action is taken to slow plastic pollution, microplastic concentrations reaching corals will still increase as larger plastics, already at sea, break down into smaller pieces, form aggregations with biogenic materials (Michels et al., 2018), sink into benthic environments (Wayman and Niemann, 2021), and become incorporated into coral tissues and skeletal structures (Reichert et al., 2022). As corals are already facing climate change stressors, the added impacts of microplastic pollution could critically impact coral reefs around the world.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
SS: Funding acquisition, Visualization, Project administration, Formal Analysis, Validation, Conceptualization, Data curation, Methodology, Investigation, Writing – original draft. CC: Methodology, Data curation, Investigation, Writing – original draft. EH: Methodology, Validation, Writing – review & editing. MM: Writing – review & editing, Validation, Formal Analysis. NF: Resources, Formal Analysis, Project administration, Funding acquisition, Validation, Supervision, Investigation, Methodology, Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding for this work was provided by the UNCW College of Arts and Sciences Research Initiative Award, UNCW’s Department of Biology and Marine Biology, UNCW’s Center for Marine Science (CMS), The Florida Department of Environmental Protection (B8E160), and an Explorer’s Club OceanX Grant.
Acknowledgments
We thank Dave Gilliam and his lab and Joana Figueiredo and her lab at Nova Southeastern University for collecting and quarantining the corals used in this experiment, and the Florida Fish and Wildlife Conservation Commission for permitting this collection (#SAL-21-2200-SRP). Thank you to Joe Pawlik and Alison Taylor for their guidance and feedback throughout the experiment. We thank Jimmy White, Ron Moore, Jennifer McCall, and Mike Duraco for their assistance with experimental logistics. Thank you to Rob Whitehead and the nutrient analysis core at UNCW-CMS for helping with alkalinity analyses. We thank Keri Dobson for her helpful review of the manuscript. Finally, thank you to the Coral REEF Lab members for their contributions to the experiment and analyses: Kory Enneking, Bryce Corbett, Reanna Jeanes, Carly Stines, Rachael Skerkis, Wesley Nelson, Michael Petrizzo, Callie Dalton, Connor Davis, Ryan Besemer, Pantho Tsali, Mia Lenzenweger, Natalie Snyder, Jenna Pullarkat, Juanita Gonzalez, Pheobe Whitbeck, Ryan Walsh, and Breck Collins.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1615308/full#supplementary-material
Supplementary Video 1Video showing clips of Acropora cervicornis corals interacting with UV-fluorescent blue microbeads. Coral polyps are seen manipulating and closing around the microbeads. Video was captured with a Samsung Galaxy Note 10+ through a microscope just after a basting event to showcase the extensive manipulation of the coral when initially interacting with the microplastics.
References
1
Ali S. Rehman A. Hussain S. Z. Bukhari D. A. (2023). Characterization of plastic degrading bacteria isolated from sewage wastewater. Saudi J. Biol. Sci.30, 103628. doi: 10.1016/j.sjbs.2023.103628
2
Allen A. S. Seymour A. C. Rittschof D. (2017). Chemoreception drives plastic consumption in a hard coral. Mar. pollut. Bull.124, 198–205. doi: 10.1016/j.marpolbul.2017.07.030
3
Alvarez-Filip L. Dulvy N. K. Gill J. A. Côté I. M. Watkinson A. R. (2009). Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc. R Soc. B Biol. Sci.276, 3019–3025. doi: 10.1098/rspb.2009.0339
4
Aminot Y. Lanctôt C. Bednarz V. Robson W. J. Taylor A. Ferrier-Pagès C. et al . (2020). Leaching of flame-retardants from polystyrene debris: Bioaccumulation and potential effects on coral. Mar. pollut. Bull.151, 110862. doi: 10.1016/j.marpolbul.2019.110862
5
Anthony K. R. N. Maynard J. A. Diaz-Pulido G. Mumby P. J. Marshall P. A. Cao L. et al . (2011). Ocean acidification and warming will lower coral reef resilience. Glob Chang Biol.17, 1798–1808. doi: 10.1111/j.1365-2486.2010.02364.x
6
Aranda D. A. Oxenford H. A. Medina J. Delgado G. Díaz M. E. Samano C. et al . (2022). Widespread microplastic pollution across the Caribbean Sea confirmed using queen conch. Mar. pollut. Bull.178, 113582. doi: 10.1016/j.marpolbul.2022.113582
7
Aronson R. B. Precht W. F. (2001). The ecology and etiology of newly emerging marine diseases. Ed. PorterJ. W. (Athens: Kluwer Academic Publishers). doi: 10.1007/978-94-017-3284-0
8
Axworthy J. B. Padilla-Gamiño J. L. (2019). Microplastics ingestion and heterotrophy in thermally stressed corals. Sci. Rep.9, 1–14. doi: 10.1038/s41598-019-54698-7
9
Baker D. M. Freeman C. J. Wong J. C. Y. Fogel M. L. Knowlton N. (2018). Climate change promotes parasitism in a coral symbiosis. ISME J.12, 921–930. doi: 10.1038/s41396-018-0046-8
10
Bejarano S. Diemel V. Feuring A. Ghilardi M. Harder T. (2022). No short-term effect of sinking microplastics on heterotrophy or sediment clearing in the tropical coral. Stylophora pistillata. Sci. Rep.12, 1–14. doi: 10.1038/s41598-022-05420-7
11
Berry K. L. E. Epstein H. E. Lewis P. J. Hall N. M. Negri A. P. (2019). Microplastic contamination has limited effects on coral fertilisation and larvae. Diversity (Basel)11, 1–13. doi: 10.3390/d11120228
12
Biswas T. Pal S. C. Saha A. Ruidas D. Shit M. Islam A. R. M. T. et al . (2024). Microplastics in the coral ecosystems: A threat which needs more global attention. Ocean Coast. Manag249, 1–11. doi: 10.1016/j.ocecoaman.2023.107012
13
Boodraj P. Glassom D. (2022). Experimental exposure to microplastics does not affect the physiology of healthy or moderately bleached Anomastraea irregularis and Pocillopora verrucosa corals. Mar. Biol.169. doi: 10.1007/s00227-022-04038-7
14
Borrelle S. B. Ringma J. Lavender Law K. Monnahan C. C. Lebreton L. McGivern A. et al . (2020). Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Sci. (1979)369, 1515–1518. doi: 10.1126/science.aba3656
15
Bove C. B. Greene K. Sugierski S. Kriefall N. G. Huzar A. K. Hughes A. M. et al . (2023). Exposure to global change and microplastics elicits an immune response in an endangered coral. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.1037130
16
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.72, 248–254. doi: 10.1016/0003-2697(76)90527-3
17
Brierley A. S. Kingsford M. J. (2009). Impacts of climate change on marine organisms and ecosystems. Curr. Biol.19, R602–R614. doi: 10.1016/j.cub.2009.05.046
18
Brown B. E. (1997). Coral bleaching: Causes and consequences. Coral Reefs16, S129–S138. doi: 10.1007/s003380050249
19
Bythell J. C. Gladfelter E. H. Bythell M. (1993). Chronic and catastrophic natural mortality of three common Caribbean reef corals. Coral Reefs12, 143–152. doi: 10.1007/bf00334474
20
Carpenter R. C. (1990). Mass mortality of Diadema antillarum: Long-term effects on sea urchin population-dynamics and coral reef algal communities. Mar. Biol.104, 67–77. doi: 10.1007/bf01313159
21
Caruso G . (2019). Microplastics as vectors of contaminants. Mar. Pollut. Bull.146, 921–4. doi: 10.1016/j.marpolbul.2019.07.052
22
Chapron L. Peru E. Engler A. Ghiglione J. F. Meistertzheim A. L. Pruski A. M. et al . (2018). Macro- and microplastics affect cold-water corals growth, feeding and behaviour. Sci. Rep.8, 1–8. doi: 10.1038/s41598-018-33683-6
23
Cook C. B. Muller-Parker G. Orlandini C. D. (1994). Ammonium enhancement of dark carbon fixation and nitrogen limitation in zooxanthellae symbiotic with the reef corals Madracis mirabilis and Montastrea annularis. Mar. Biol.118, 157–165. doi: 10.1007/bf00699230
24
Corinaldesi C. Canensi S. Dell’Anno A. Tangherlini M. Di Capua I. Varrella S. et al . (2021). Multiple impacts of microplastics can threaten marine habitat-forming species. Commun. Biol.4, 1–13. doi: 10.1038/s42003-021-01961-1
25
Cornwall C. E. Comeau S. Kornder N. A. Perry C. T. van Hooidonk R. DeCarlo T. M. et al . (2021). Global declines in coral reef calcium carbonate production under ocean acidification and warming. Proc. Natl. Acad. Sci. U.S.A.118, e2015265118. doi: 10.1073/pnas.2015265118
26
Cramer K. L. Jackson J. B. C. Donovan M. K. Greenstein B. J. Korpanty C. A. Cook G. M. et al . (2020). Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv.6, 1–10. doi: 10.1126/sciadv.aax9395
27
Curren E. Leong S. C. Y . (2019). Profiles of bacterial assemblages from microplastics of tropical coastal environments. Sci. Total Environ.655, 313–20. doi: 10.1016/j.scitotenv.2018.11.250
28
Dickson A. G. Sabine C. L. Christian J. R. (2007). Guide to best practices for ocean CO2 measurements. 3rd Edn. Eds. DicksonA. G.SabineC. L.RobertJ. (Christian: PICES Special Publication).
29
Dobson K. L. Jury C. P. Toonen R. J. McLachlan R. H. Williams J. C. Grottoli A. G. (2024). Ocean acidification does not prolong recovery of coral holobionts from natural thermal stress in two consecutive years. Commun. Earth Environ.5, 515. doi: 10.1038/s43247-024-01672-5
30
Doney S. C. Fabry V. J. Feely R. A. Kleypas J. A. (2008). Ocean acidification: the other CO 2 problem. Ann. Rev. Mar. Sci.1, 169–92. doi: 10.1146/annurev.marine.010908.163834
31
Duckworth A. Giofre N. Jones R. (2017). Coral morphology and sedimentation. Mar. pollut. Bull.125, 289–300. doi: 10.1016/j.marpolbul.2017.08.036
32
Erez J. Reynaud S. Silverman J. Schneider K. Allemand D. (2011). “Coral calcification under ocean acidification and global change,” in Coral Reefs: An Ecosystem in Transition (Dordrecht:: Springer) 151–176. doi: 10.1007/978-94-007-0114-4_10
33
Erni-Cassola G. Zadjelovic V. Gibson M. I. Christie-Oleza J. A. (2019). Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard Mater369, 691–698. doi: 10.1016/j.jhazmat.2019.02.067
34
Fabry V. J. Seibel B. A. Feely R. A. Orr J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci.65, 414–432. doi: 10.1093/icesjms/fsn048
35
Gambarini V. Pantos O. Kingsbury J. M. Weaver L. Handley K. M. Lear G. (2021). Phylogenetic distribution of plastic-degrading microorganisms. mSystems6, 1–13. doi: 10.1128/msystems.01112-20
36
Gao B. Wang Y. Long C. Long L. Yang F. (2024). Microplastics inhibit the growth of endosymbiotic Symbiodinium tridacnidorum by altering photosynthesis and bacterial community. Environ. pollut.346. doi: 10.1016/j.envpol.2024.123603
37
Gorbunov M. Y. Kolber Z. S. Lesser M. P. Falkowski P. G. (2001). Photosynthesis and photoprotection in symbiotic corals. Limnol Oceanogr46, 75–85. doi: 10.4319/lo.2001.46.1.0075
38
Grant A. J. Rémond M. People J. Hinde R. (1997). Effects of host-tissue homogenate of the scleractinian coral Plesiastrea versipora on glycerol metabolism in isolated symbiotic dinoflagellates. Mar. Biol.128, 665–670. doi: 10.1007/s002270050133
39
Grant A. J. Rémond M. Starke-Peterkovic T. Hinde R. (2006). A cell signal from the coral Plesiastrea versipora reduces starch synthesis in its symbiotic alga, Symbiodinium sp. Comp. Biochem. Physiol. A Mol. Integr. Physiol.144, 458–463. doi: 10.1016/j.cbpa.2006.04.012
40
Haas A. F. Smith J. E. Thompson M. Deheyn D. D . (2014). Effects of reduced dissolved oxygen concentrations on physiology and fluorescence of hermatypic corals and benthic algae. PeerJ2014, e235. doi: 10.7717/peerj.235/supp-4
41
Hall E. R. Muller E. M. Goulet T. Bellworthy J. Ritchie K. B. Fine M. (2018). Eutrophication may compromise the resilience of the Red Sea coral Stylophora pistillata to global change. Mar. Pollut. Bull.131, 701–11. doi: 10.1016/j.marpolbul.2018.04.067
42
Hankins C. Duffy A. Drisco K. (2018). Scleractinian coral microplastic ingestion: Potential calcification effects, size limits, and retention. Mar. pollut. Bull.135, 587–593. doi: 10.1016/j.marpolbul.2018.07.067
43
Hankins C. Moso E. Lasseigne D. (2021). Microplastics impair growth in two atlantic scleractinian coral species, Pseudodiploria clivosa and Acropora cervicornis. Environ. pollut.275. doi: 10.1016/j.envpol.2021.116649
44
Hankins C. Raimondo S. Lasseigne D. (2022). Microplastic ingestion by coral as a function of the interaction between calyx and microplastic size. Sci. Total Environ.810. doi: 10.1016/j.scitotenv.2021.152333
45
Hartmann N. B. Hüffer T. Thompson R. C. Hassellöv M. Verschoor A. Daugaard A. E. et al . (2019). Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol.53, 1039–1047. doi: 10.1021/acs.est.8b05297
46
Hoegh-Guldberg O. (1999). Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res.50, 839–866. doi: 10.1071/mf99078
47
Hoegh-Guldberg O. Mumby P. J. Hooten A. J. Steneck R. S. Greenfield P. Gomez E. et al . (2007). Coral reefs under rapid climate change and ocean acidification. Science31 (5857), 1737–42. doi: 10.1126/science.1152509
48
Huang W. Chen M. Song B. Deng J. Shen M. Chen Q. et al . (2021). Microplastics in the coral reefs and their potential impacts on corals: A mini-review. Sci. Total Environ.762. doi: 10.1016/j.scitotenv.2020.143112
49
Hughes T. P. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Sci. (1979)265, 1547–1551. doi: 10.1126/science.265.5178.1547
50
Hughes T. P. Rodrigues M. J. Bellwood D. R. Ceccarelli D. Hoegh-Guldberg O. McCook L. et al . (2007). Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol.17, 360–365. doi: 10.1016/j.cub.2006.12.049
51
IPCC (2019). “Summary for policymakers,” in Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems.
52
Isa V. Seveso D. Diamante L. Montalbetti E. Montano S. Gobbato J. et al . (2024). Physical and cellular impact of environmentally relevant microplastic exposure on thermally challenged Pocillopora damicornis (Cnidaria, Scleractinia). Sci. Total Environ.918, 1–14. doi: 10.1016/j.scitotenv.2024.170651
53
IUCN (2021). Acropora cervicornis (Staghorn Coral). Available online at: https://www.iucnredlist.org/species/133381/165860142 (Accessed April 11, 2025).
54
Jambeck J. R. Geyer R. Wilcox C. Siegler T. R. Perryman M. Andrady A. et al . (2015). Plastic waste inputs from land into the ocean. Sci. (1979)347, 768–771. doi: 10.1126/science.1260352
55
Jiang L. Q. Carter B. R. Feely R. A. Lauvset S. K. Olsen A. (2019). Surface ocean pH and buffer capacity: past, present and future. Sci. Rep.9, 1–11. doi: 10.1038/s41598-019-55039-4
56
Jokiel P. Maragos J. Franzisket L. (1978). “Coral growth: buoyant weight technique,” in Coral reefs: research methods. Eds. StoddartD.JohannesR. (UNESCO, Paris), 529–541.
57
Jury C. P. Toonen R. J. (2024). Widespread scope for coral adaptation under combined ocean warming and acidification. Proc. R. Soc. B291, 1–12. doi: 10.1098/rspb.2024.1161
58
Koike K. Jimbo M. Sakai R. Kaeriyama M. Muramoto K. Ogata T. et al . (2004). Octocoral chemical signaling selects and controls dinoflagellate symbionts. Biol. Bull.207, 80–86. doi: 10.2307/1543582
59
Lanctôt C. M. Bednarz V. N. Melvin S. Jacob H. Oberhaensli F. Swarzenski P. W. et al . (2020). Physiological stress response of the scleractinian coral Stylophora pistillata exposed to polyethylene microplastics. Environ. pollut.263. doi: 10.1016/j.envpol.2020.114559
60
Langdon C. Albright R. Baker A. C. Jones P. (2018). Two threatened Caribbean coral species have contrasting responses to combined temperature and acidification stress. Limnol Oceanogr63, 2450–2464. doi: 10.1002/lno.10952
61
Larsson A. I. Purser A. (2011). Sedimentation on the cold-water coral Lophelia pertusa: Cleaning efficiency from natural sediments and drill cuttings. Mar. pollut. Bull.62, 1159–1168. doi: 10.1016/j.marpolbul.2011.03.041
62
Leggat W. Heron S. F. Fordyce A. Suggett D. J. Ainsworth T. D. (2022). Experiment Degree Heating Week (eDHW) as a novel metric to reconcile and validate past and future global coral bleaching studies. J. Environ. Manage301, 113919. doi: 10.1016/j.jenvman.2021.113919
63
Liang J. Niu T. Zhang L. Yang Y. Li Z. Liang Z. et al . (2025). Polystyrene microplastics exhibit toxic effects on the widespread coral symbiotic Cladocopium goreaui. Environ. Res.268, 1–12. doi: 10.1016/j.envres.2025.120750
64
Lüdecke D. Ben-Shachar M. Patil I. Waggoner P. Makowski D. (2021). performance: An r package for assessment, comparison and testing of statistical models. J. Open Source Softw6, 3139. doi: 10.21105/joss.03139
65
Martin C. Corona E. Mahadik G. A. Duarte C. M. (2019). Adhesion to coral surface as a potential sink for marine microplastics. Environ. pollut.255, 1–8. doi: 10.1016/j.envpol.2019.113281
66
Mendrik F. M. Henry T. B. Burdett H. Hackney C. R. Waller C. Parsons D. R. et al . (2021). Species-specific impact of microplastics on coral physiology. Environ. pollut.269, 116238. doi: 10.1016/j.envpol.2020.116238
67
Michels J. Stippkugel A. Lenz M. Wirtz K. Engel A. (2018). Rapid aggregation of biofilm-covered microplastics with marine biogenic particles. Proc. R. Soc. B285, 1–9. doi: 10.1098/rspb.2018.1203
68
Morris L. A. Voolstra C. R. Quigley K. M. Bourne D. G. Bay L. K. (2019). Nutrient availability and metabolism affect the stability of coral–Symbiodiniaceae symbioses. Trends Microbiol.27, 678–689. doi: 10.1016/j.tim.2019.03.004
69
Mouchi V. Chapron L. Peru E. Pruski A. M. Meistertzheim A. L. Vétion G. et al . (2019). Long-term aquaria study suggests species-specific responses of two cold-water corals to macro-and microplastics exposure. Environ. Pollut.253, 322–329. doi: 10.1016/j.envpol.2019.07.024
70
Muscatine L. Porter J. W. (1977). Reef corals: Mutualistic symbioses adapted to nutrient-poor environments. BioScience27 (7), 454–60. doi: 10.2307/1297526
71
Mutuku J. Yanotti M. Tocock M. Hatton MacDonald D. (2024). The abundance of microplastics in the world’s oceans: A systematic review. Oceans5, 398–428. doi: 10.3390/oceans5030024/S1
72
Naik R. K. Naik M. M. D’Costa P. M. Shaikh F . (2019). Microplastics in ballast water as an emerging source and vector for harmful chemicals, antibiotics, metals, bacterial pathogens and HAB species: A potential risk to the marine environment and human health. Mar. Pollut. Bull.149, 110525. doi: 10.1016/j.marpolbul.2019.110525
73
Neely K. L. Nowicki R. J. Dobler M. A. Chaparro A. A. Miller S. M. Toth K. A. (2024). Too hot to handle? The impact of the 2023 marine heatwave on Florida Keys coral. Front. Mar. Sci.11. doi: 10.3389/fmars.2024.1489273
74
NOAA Coral Reef Watch (2019). NOAA Coral Reef Watch version 3.1 daily 5km satellite regional virtual station time series data for southeast Florida (Maryland, USA: College Park).
75
NOAA Fisheries (2014). Endangered and threatened wildlife and plants; Final listing determinations on proposal to list 66 reef-building coral species and reclassification of Acropora species. Available online at: https://www.fisheries.noaa.gov/species/staghorn-coral (Accessed April 11, 2025).
76
O’Neil K. L. Serafin R. M. Patterson J. T. Craggs J. R. K. (2021). Repeated ex situ spawning in two highly disease susceptible corals in the family Meandrinidae. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.669976
77
Orona-Návar C. García-Morales R. Loge F. J. Mahlknecht J. Aguilar-Hernández I. Ornelas-Soto N. (2022). Microplastics in Latin America and the Caribbean: A review on current status and perspectives. J. Environ. Manage309, 114698. doi: 10.1016/j.jenvman.2022.114698
78
Pearse V. B. Muscatine L. (1971). Role of symbiotic algae (zooxanthellae) in coral calcification. The Biological Bulletin141, 350–363. doi: 10.2307/1540123
79
Pitts K. A. Campbell J. E. Figueiredo J. Fogarty N. D. (2020). Ocean acidification partially mitigates the negative effects of warming on the recruitment of the coral, Orbicella faveolata. Coral Reefs39, 281–292. doi: 10.1007/S00338-019-01888-4
80
Plafcan M. M. Stallings C. D. (2022). Microplastics do not affect bleaching of Acropora cervicornis at ambient or elevated temperatures. PeerJ10, 1–16. doi: 10.7717/peerj.13578
81
Plaisance L. Caley M. J. Brainard R. E. Knowlton N. (2011). The diversity of coral reefs: What are we missing? PLoS One6, 1–7. doi: 10.1371/journal.pone.0025026
82
Rädecker N. Pogoreutz C. Gegner H. M. Cárdenas A. Roth F. Bougoure J. et al . (2021). Heat stress destabilizes symbiotic nutrient cycling in corals. Proc. Natl. Acad. Sci. U.S.A.118, e2022653118. doi: 10.1073/pnas.202265311
83
Rades M. Poschet G. Gegner H. Wilke T. Reichert J. (2024). Chronic effects of exposure to polyethylene microplastics may be mitigated at the expense of growth and photosynthesis in reef-building corals. Mar. pollut. Bull.205, 1–10. doi: 10.1016/j.marpolbul.2024.116631
84
Rades M. Schubert P. Wilke T. Reichert J. (2022). Reef-building corals do not develop adaptive mechanisms to better cope with microplastics. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.863187
85
Ralph P. J. Gademann R. Larkum A. W. D. Schreiber U. (1999). In situ underwater measurements of photosynthetic activity of coral zooxanthellae and other reef-dwelling dinoflagellate endosymbionts. Mar. Ecol. Prog. Ser.180, 139–147. doi: 10.3354/meps180139
86
Reichert J. Arnold A. L. Hammer N. Miller I. B. Rades M. Schubert P. et al . (2022). Reef-building corals act as long-term sink for microplastic. Glob. Chang. Biol.28, 33–45. doi: 10.1111/gcb.15920
87
Reichert J. Arnold A. L. Hoogenboom M. O. Schubert P. Wilke T. (2019). Impacts of microplastics on growth and health of hermatypic corals are species-specific. Environ. pollut.254, 113074. doi: 10.1016/j.envpol.2019.113074
88
Reichert J. Schellenberg J. Schubert P. Wilke T. (2018). Responses of reef building corals to microplastic exposure. Environ. pollut.237, 955–960. doi: 10.1016/j.envpol.2017.11.006
89
Reyes-Nivia C. Diaz-Pulido G. Kline D. Guldberg O. H. Dove S. (2013). Ocean acidification and warming scenarios increase microbioerosion of coral skeletons. Glob Chang Biol.19, 1919–1929. doi: 10.1111/gcb.12158
90
Reynaud S. Leclercq N. Romaine-Lioud S. Ferrier-Pagès C. Jaubert J. Gattuso J. P. (2003). Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob Chang Biol.9, 1660–1668. doi: 10.1046/J.1365-2486.2003.00678.x
91
Rodolfo-Metalpa R. Houlbrèque F. Tambutté É. Boisson F. Baggini C. Patti F. P. et al . (2011). Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Climate Change6, 308–312. doi: 10.1038/nclimate1200
92
Rotjan R. D. Sharp K. H. Gauthier A. E. Yelton R. Baron Lopez E. M. Carilli J. et al . (2019). Patterns, dynamics and consequences of microplastic ingestion by the temperate coral, Astrangia poculata. Proc. R. Soc. B286, 1–9. doi: 10.1098/rspb.2019.0726
93
Saliu F. Montano S. Leoni B. Lasagni M. Galli P . (2019). Microplastics as a threat to coral reef environments: Detection of phthalate esters in neuston and scleractinian corals from the Faafu Atoll, Maldives. Mar. Pollut. Bull.142, 234–41. doi: 10.1016/j.marpolbul.2019.03.043
94
Savinelli B. Vega Fernández T. Galasso N. M. D’Anna G. Pipitone C. Prada F. et al . (2020). Microplastics impair the feeding performance of a Mediterranean habitat-forming coral. Mar. Environ. Res.155, 1–6. doi: 10.1016/j.marenvres.2020.104887
95
Soares M. de O. Matos E. Lucas C. Rizzo L. Allcock L. et al . (2020). Microplastics in corals: An emergent threat. Mar. pollut. Bull.161. doi: 10.1016/j.marpolbul.2020.111810
96
Soares M. O. Rizzo L. Ximenes Neto A. R. Barros Y. Martinelli Filho J. E. Giarrizzo T. et al . (2023). Do coral reefs act as sinks for microplastics? Environ. pollut.337. doi: 10.1016/j.envpol.2023.122509
97
Stat M. Carter D. Hoegh-Guldberg O. (2006). The evolutionary history of Symbiodinium and scleractinian hosts—Symbiosis, diversity, and the effect of climate change. Perspect. Plant Ecol. Evol. Syst.8, 23–43. doi: 10.1016/j.ppees.2006.04.001
98
Su Y. Zhang K. Zhou Z. Wang J. Yang X. Tang J. et al . (2020). Microplastic exposure represses the growth of endosymbiotic dinoflagellate Cladocopium goreaui in culture through affecting its apoptosis and metabolism. Chemosphere244, 125485. doi: 10.1016/j.chemosphere.2019.125485
99
Syakti A. D. Jaya J. V. Rahman A. Hidayati N. V. Raza’i T. S. Idris F. et al . (2019). Bleaching and necrosis of staghorn coral (Acropora formosa) in laboratory assays: Immediate impact of LDPE microplastics. Chemosphere228, 528–535. doi: 10.1016/j.chemosphere.2019.04.156
100
Tang J. Ni X. Zhou Z. Wang L. Lin S. (2018). Acute microplastic exposure raises stress response and suppresses detoxification and immune capacities in the scleractinian coral Pocillopora damicornis. Environ. pollut.243, 66–74. doi: 10.1016/j.envpol.2018.08.045
101
Tang J. Wu Z. Wan L. Cai W. Chen S. Wang X. et al . (2021). Differential enrichment and physiological impacts of ingested microplastics in scleractinian corals in situ. J. Hazard Mater404, 124205. doi: 10.1016/j.jhazmat.2020.124205
102
Towle E. K. Enochs I. C. Langdon C. (2015). Threatened Caribbean coral is able to mitigate the adverse effects of ocean acidification on calcification by increasing feeding rate. PLoS One10, e0123394. doi: 10.1371/journal.pone.0123394
103
Wallace C. (1999). Staghorn corals of the world (Collingwood, Australia: CSIRO). doi: 10.1071/9780643101388
104
Warner M. E. Fitt W. K. Schmidt G. W. (1999). Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc. Natl. Acad. Sci. U.S.A.96, 8007–8012. doi: 10.1073/pnas.96.14.8007
105
Wayman C. Niemann H. (2021). The fate of plastic in the ocean environment – a minireview. Environ. Sci. Process Impacts23, 198–212. doi: 10.1039/d0em00446d
106
Wightman E. Renegar D. A. (2023). The microscopic threat with a macroscopic impact: Microplastics along the southeast Florida reef tract. Mar. pollut. Bull.191, 114917. doi: 10.1016/J.MARPOLBUL.2023.114917
107
Zhang W. Sik Ok Y. Bank M. S. Sonne C. (2023). Macro- and microplastics as complex threats to coral reef ecosystems. Environ. Int.174, 1–5. doi: 10.1016/j.envint.2023.107914
108
Zhou Z. Tang J. Cao X. Wu C. Cai W. Lin S. (2023). High heterotrophic plasticity of massive coral Porites pukoensis contributes to its tolerance to bioaccumulated microplastics. Environ. Sci. Technol.57, 3391–3401. doi: 10.1021/acs.est.2c08188
109
Zrimec J. Kokina M. Jonasson S. Zorrilla F. Zelezniak A. (2021). Plastic-degrading potential across the global microbiome correlates with recent pollution trends. mBio12. doi: 10.1128/mbio.02155-21
Summary
Keywords
Acropora cervicornis , ocean warming, microplastics, ocean acidification, corals, plastic pollution, global change
Citation
Sugierski S, Campbell C, Hall ER, McLean M and Fogarty ND (2025) Microplastic exposure under future oceanic conditions further threatens an endangered coral, Acropora cervicornis. Front. Mar. Sci. 12:1615308. doi: 10.3389/fmars.2025.1615308
Received
21 April 2025
Accepted
20 August 2025
Published
18 September 2025
Volume
12 - 2025
Edited by
Jessica Reichert, University of Hawaii at Manoa, United States
Reviewed by
Hailong Zhou, Hainan University, China
Prishani Boodraj, University of KwaZulu-Natal, South Africa
Updates
Copyright
© 2025 Sugierski, Campbell, Hall, McLean and Fogarty.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharla Sugierski, sugierski@gmail.com
†Present address: Sharla Sugierski, Florida Fish and Wildlife Research Institute, Florida Fish and Wildlife Conservation Commission, Marathon, FL, United States
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.