- 1Research Institute for Marine Traditional Chinese Medicine (Qingdao Academy of Chinese Medical Sciences), Shandong University of Traditional Chinese Medicine, Qingdao, China
- 2Research Institute for Marine Traditional Chinese Medicine (Qingdao Academy of Chinese Medical Sciences), The SATCM’s Key Unit of Discovering and Developing New Marine TCM Drugs, Key Laboratory of Marine Traditional Chinese Medicine in Shandong Universities, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Shandong University of Traditional Chinese Medicine Qingdao Academy of Chinese Medical Sciences, Qingdao Key Laboratory of Research in Marine Traditional Chinese Medicine, Qingdao Key Technology Innovation Center of Marine Traditional Chinese Medicine’s Deep Development and Industrialization, Qingdao, China
- 4International Institute for Translational Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Octopus, an abundant marine species with diverse applications, is attracting growing attention for its unique biological characteristics and potential utilization in food science and biotechnology. Octopus contain amino acids, active peptides, fatty acids and trace elements and these bioactives exhibit a wide range of effects, including anti-bacterial, anti-tumor, antioxidant and anti-aging activities. The structure of octopus arm and suckers provides biomimetic design inspiration for tissue engineering and the regenerative ability of octopus provides reference for research in biomedical material. Therefore, this review systematically summarizes the research progress on octopus resource distribution, extraction methods, nutrients, bioactive compounds substances and research on regeneration material, aiming to promote the development and utilization of octopus resources. Firstly, the extractions methods of octopus were systematically reviewed. Secondly, seven kinds of nutrients concluded were introduced to reveal the nutritional components of octopus. Finally, the application of octopus in biotechnology and the research progress on their regenerative ability were summarized. This review provides a basis and reference for octopus functional nutrients and application of octopus biomedical materials.
1 Introduction
The ocean, occupying over 70% of the Earth’s surface, harbors complex ecosystems and rich biodiversity, thereby serving as a critical reservoir of biologically active compounds with pharmaceutical potential (Rigogliuso et al., 2023). Marine natural products, including proteins, peptides, and polysaccharides, have demonstrated significant utility in promoting cutaneous wound healing, bone/cartilage tissue regeneration, and the development of advanced biomaterials for healthcare applications (Lim et al., 2019; Salvatore et al., 2020; Tajbakhsh et al., 2021; Elkhenany et al., 2025).
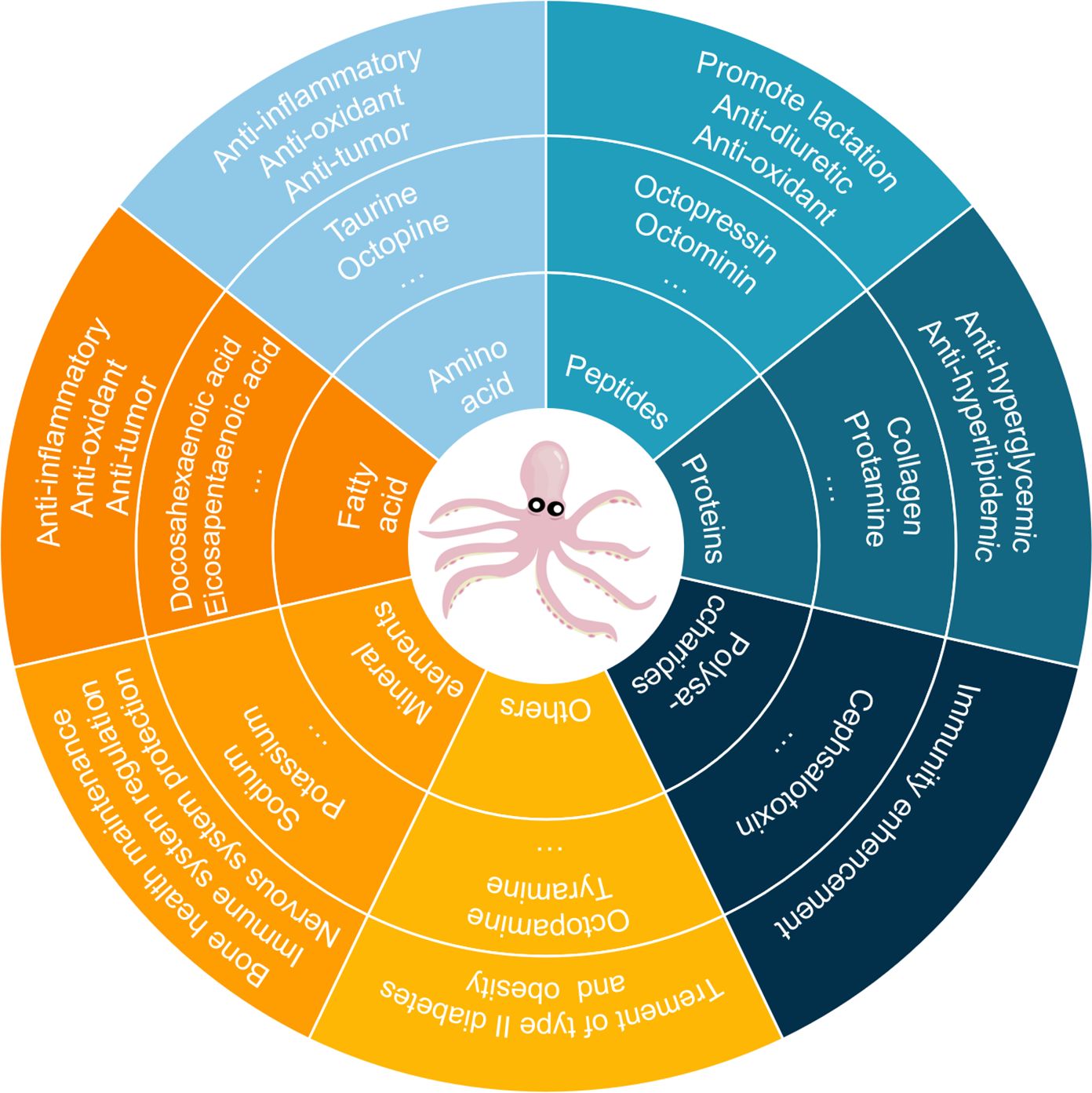
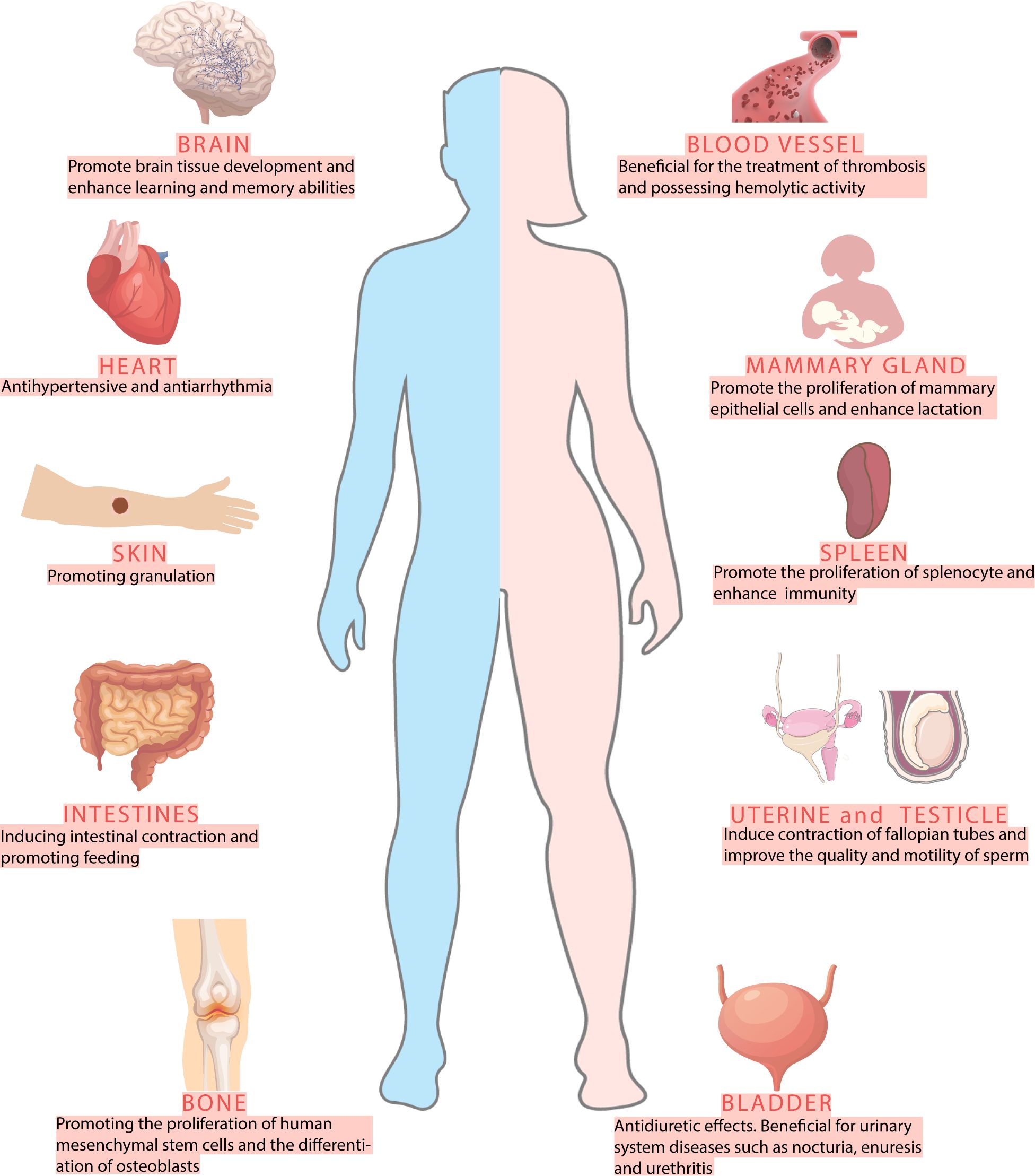
Recent scientific attention has increasingly focused on cephalopod-derived compounds, particularly from members of the order Octopoda known for their exceptional bioactive properties. Octopus is the taxonomic term for marine mollusks within the class Cephalopoda and family Octopusidae. Modern phylogenetic research has classified these organisms into five distinct subfamilies based on morphological and genetic evidence: Octopodinae, Eledoninae, Graneledoninae, Megaleledoninae and Bathypolypodinae. Biochemical analyses have identified amino acids, fatty acids, proteins, and peptides as the primary bioactive constituents in octopus, with glycosaminoglycans and specific peptide fractions demonstrating multifunctional therapeutic effects including immunomodulatory responses, lactogenic support, broad-spectrum antibacterial activity, and potent free radical scavenging capacity (Figure 1) (Lei et al., 2007; Oliveira et al., 2019; Cai et al., 2020). Octopus-derived peptides have garnered significant attention in recent years due to their potential for anti-microbial, anti-oxidant, anti-hypertensive and anti-tumoral properties (Ben Slama-Ben Salem et al., 2017; Sudhakar and Nazeer, 2017; Imran et al., 2023a). The suckers structure on the octopus arm provides ideas for the design of microneedles and robotic arms (Xie et al., 2023; Chen et al., 2024). The regenerative ability of octopuses may have potential for development in tissue repair and nerve regeneration (Imperadore and Fiorito, 2018).
This review systematically summarized octopus resource distribution, global trade volume, processing methods, nutrients, bioactive compounds and biomedical applications, and discussed the shortcomings of octopus peptides in quality control. While substantial advancements have been achieved in understanding octopus regeneration mechanisms, comprehensive investigations are essential to assess their translational potential in clinical contexts, particularly within regenerative medicine and wound healing applications.
2 Octopus global distribution and trade volume
At present, 136 species of octopus have been discovered worldwide, with a wide distribution across tropical and temperate waters, particularly along the Mediterranean, Pacific, and Atlantic coasts (Octopus Cuvier, 1798, n.d). In China, Octopus ocellatus, Octopus variabilis and Octopus vulgaris are recorded as marine traditional Chinese medicine, possessing properties that nourish blood and replenish qi, dredge collaterals to promote lactation, and detoxify and promote granulation. Beyond their medicinal value, the diversity of octopus species in Chinese waters has garnered significant attention. An analysis of octopus species diversity in the Bohai Sea, Yellow Sea, East China Sea, and South China Sea reveals that 37 species of octopus, classified into 10 genera within the Octopodidae family, are present in these waters, representing approximately 27% of the globally identified species (Figure 2) (Zheng et al., 2023).
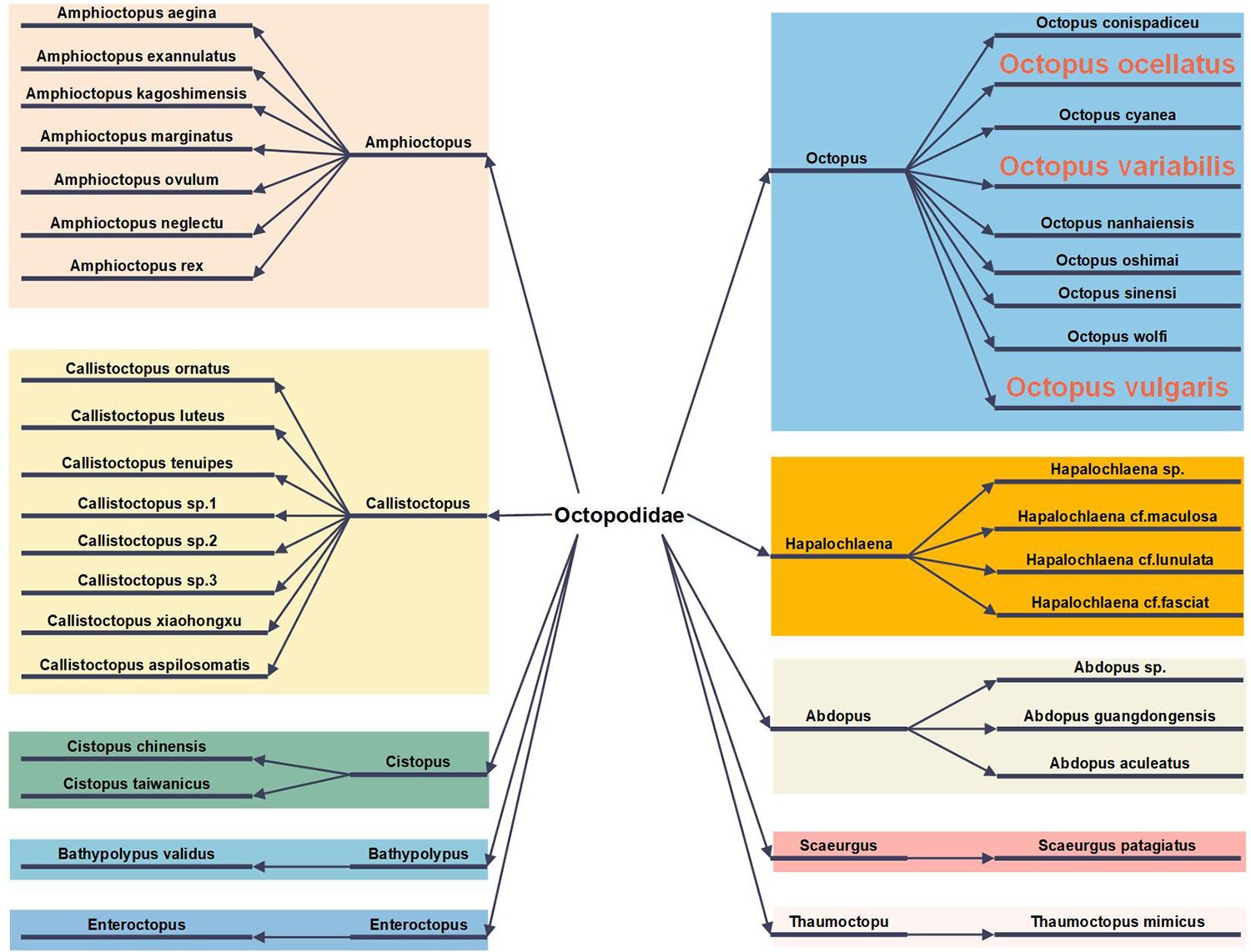
Figure 2. Species classification of 37 octopus taxa: Medicinal Octopus species are labeled in orange font, and different colors denote distinct genera.
This biodiversity, along with the species’ nutritional attributes, forms the basis of their commercial viability in food and pharmaceutical industries. Octopus is widely demanded in world import and export trade due to its high protein content and essential micronutrients composition. Since 2000, China has been a major exporter of octopus. In 2023, China, Spain, Vietnam and Morocco emerged as the dominant exporters of octopus (live, fresh, chilled and processed) globally, while South Korea, Japan, Italy and the United States were the main importers of octopus (Figure 3) (Ospina-Alvarez et al., 2022). Over the past two decades, the trade landscape has not evolved significantly. By 2023, the catch of octopus in China was 112100 tons, an increase of 1.96% compared to 2022 (Ministry Agriculture and Rural Affairs of the People‘s Republic of China et al., 2024). These sustained production capacities and trade volumes support the functional food development of octopus.
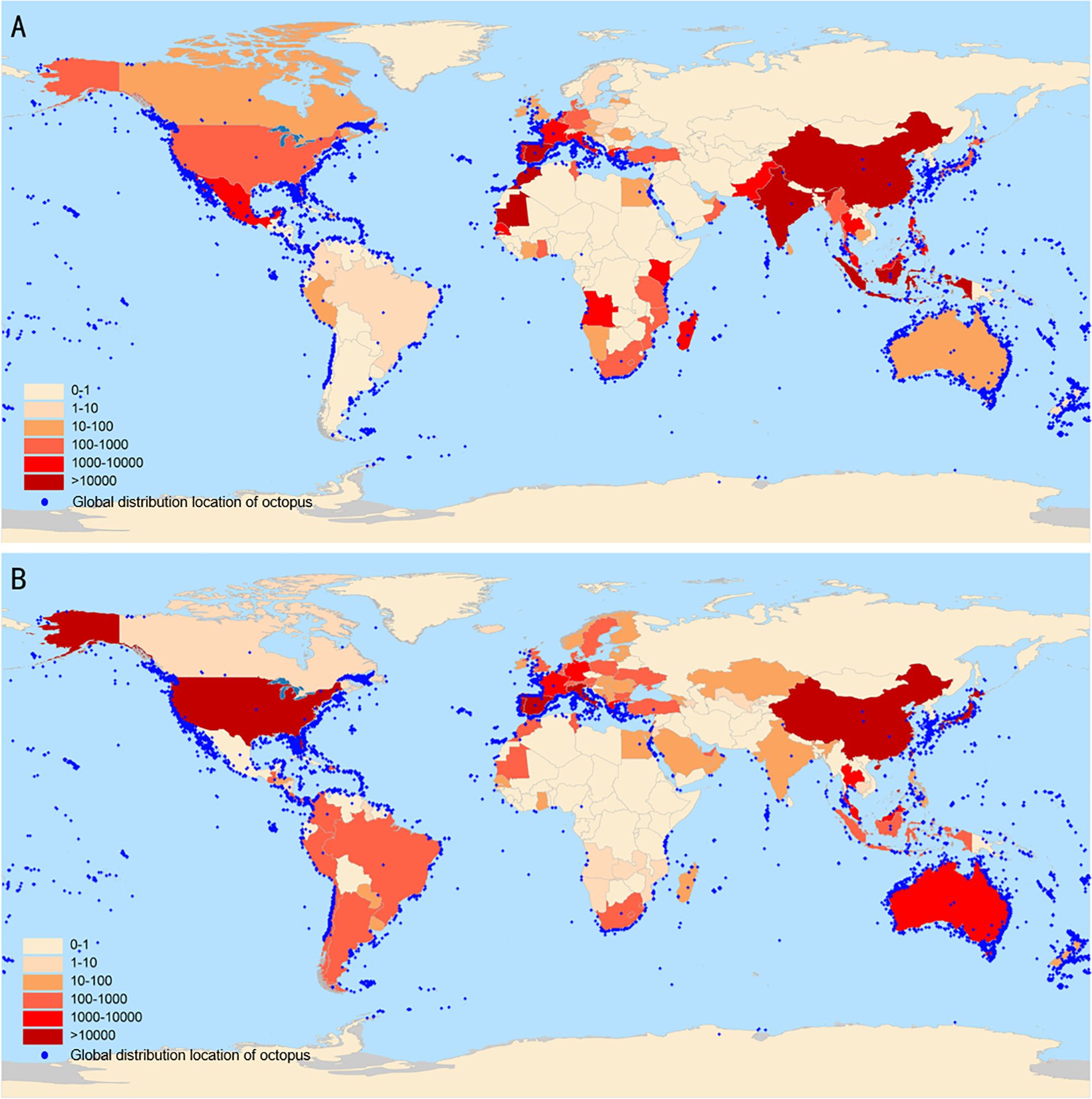
Figure 3. The distribution of octopus resources worldwide and global octopus (live, fresh, chilled and elaborated) import and export volume in 2023 (Ton). Import and export of octopus data obtained from UN Comtrade (02 January 2025). The global distribution data of octopus obtained from GBIF (02 January 2025) GBIF Occurrence Download https://doi.org/10.15468/dl.yy7vca. (A) Export volume, (B) Import volume.
3 Extraction methods of octopus
To optimize the recovery of these bioactive constituents, contemporary extraction methodologies have been systematically developed, primarily encompassing aqueous solvent extraction, ethanolic fractionation, enzymatic hydrolysis, and their combinatorial applications (Hernández-Zazueta et al., 2021). Different extraction portions of octopus demonstrated specific bioactivities. The bioactive compounds of octopus extracts are presented in Figure 4.
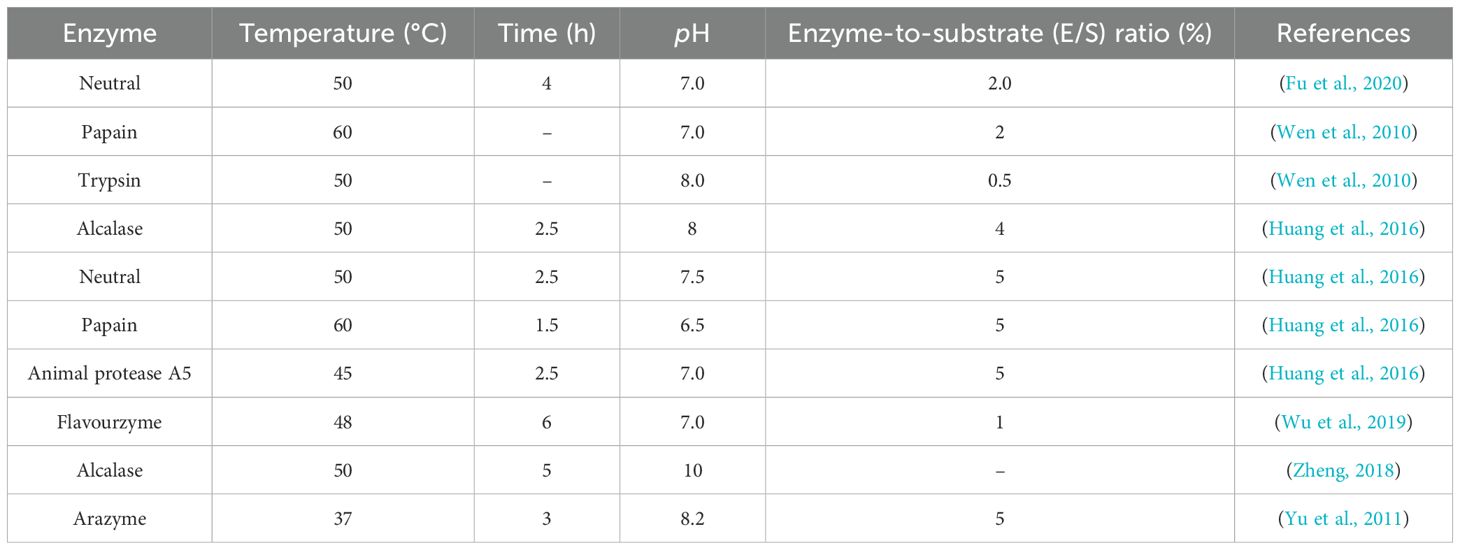
Enzymatic hydrolysis, leveraging protease cocktails, is a well- established method for isolating bioactive peptides from octopus, ensuring efficient cleavage of protein matrices under controlled conditions. Commonly selected enzymes include neutral protease, alcalase, trypsin, papain and pepsin (Table 1). Cai et al. utilized neutrase for the production of octopus peptides. After homogenizing octopus, the sample was mixed with twice its volume of distilled water. The process was initiated by incubating the mixture with 3000 U/g neutrase at pH 6.5 and 50°C for 5 hours. Then, heat inactivation was carried out at 100°C for 10 minutes. After that, the mixture was centrifuged to remove insoluble particles before filtration. Finally, it was further filtered using Vivaflow 10000 and 5000 molecular weight cutoff PES ultrafiltration membranes to obtain three fractions with molecular weights of >10 kDa, 5–10 kDa and <5 kDa for subsequent studies (Cai et al., 2020).
Fu et al. used neutral protease, alkaline protease, flavor protease, trypsin, and composite protease to extract fish oil from octopus’ viscera and optimized the sample processing. The optimized sample preparation conditions were determined as follows: application of neutral protease, enzymolysis at 50°C, a solid-to-liquid ratio of 1:0.5, an enzyme dosage of 3500 U/g, and a reaction duration of 4 hours. Under these optimized parameters, the oil extraction rate was determined to be 74.81%, demonstrating the efficiency of the established protocol (Fu et al., 2020).
Wen et al. used distilled water and enzymolysis with combination of papain and trypsin digestion to extract polysaccharides from the mantle and wrist of Octopus ocellatus (O. ocellatus) and found that the average molecular weights of these polysaccharides extracted from O. ocellatus ranged from 127.9 to 266.4 kDa. In terms of monosaccharide composition, the major component of the polysaccharides extracted from the mantle of O. ocellatus was glucose, along with traces of mannose, N-acetylglucosamine and glucuronic acid. In contrast, the monosaccharide compositions of the polysaccharides extracted from the wrist included mannose, N-acetylglucosamine, glucuronic acid and glucosamine, with small amounts of galactose, fucose and fructose (Wen et al., 2010).
In addition, after ultrasonic treatment of the edible portion of Amphioctopus marginatus with n-hexane for 3–4 h, the residue was extracted with ethyl acetate-methanol 1:1 v/v, 6 × 500 mL) for 6 h, and the supernatant was separated and purified to obtain Δ5 steroid analogues (Paulose and Chakraborty, 2022).
Octopus generates a large amount of waste during the sample preparation process, causing problems such as resource waste and environmental pollution. These wastes possess significant nutritional value; proper handling can improve the utilization rate of octopus wastes. The proportion of content of polyunsaturated fatty acids in total oil extracted from octopus viscera is 50.51%; with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) content reaching 37.30% (Fu et al., 2020). Zheng established the optimal conditions for extracting fish oil from the byproduct of O. ocellatus, the results showed that the extraction rate was 53.50% ± 1.04% under the conditions of alkaline protease, hydrolysis time 3 h and temperature 50 °C (Zheng, 2018). (Huang et al., 2016). investigated the effects of four common proteolytic enzymes—alkaline protease, neutral protease, papain, and animal protease A5—on octopus processing by-products, evaluating the amino acid composition and antioxidant properties of the resulting hydrolysates. Among the tested enzymes, animal protease A5 produced the highest mass fraction of complex amino acids (57.12%). This indicates that animal proteases may better hydrolyze most of the protein in octopus’ leftovers into amino acids. Additionally, compared with other enzymes, the papain-generated hydrolysate exhibited better hydroxyl radical (·OH) scavenging activity (IC50 value of 0.56 mg/mL), and the alkaline protease hydrolysate demonstrated superior Fe²+ chelating ability (IC50 of 0.46 mg/mL). Food-derived metal-chelating peptides (MCPs) may prevent metal deficiency by inhibiting metal precipitation induced by gastrointestinal conditions and exogenous compounds (Yu et al., 2024). Complementing these findings, Wu et al. developed a novel octopus processing by-products protein hydrolysate (OSPH) and identified that the amides and carboxylates in OSPH may be binding sites for chelation. OSPH Ca chelates exhibit good stability and high calcium absorption efficiency in simulated human gastrointestinal environments and improved the utilization rate of octopus by-products, which may help promote economic circulation and reduce environmental pollution (Wu et al., 2019). Yu et al. treated octopus by-products with Arazyme enzyme. They compared the enzyme dosage, enzymatic hydrolysis time and solid-liquid ratio through orthogonal experiments. The results showed that the optimal conditions were water-to-materials ratio as 1:15 (w/v), an enzyme dosage of 300 U/g and an enzymatic hydrolysis time of 3 hours, the content of peptides in this condition was 5.08 mg/mL (Yu et al., 2011). These findings establish theoretical frameworks for octopus byproduct utilization, offering both functional food development guidelines and resource optimization strategies.
Taurine (a sulfur-containing amino sulfonic acid) has multiple activities such as anti-inflammatory, anti-oxidant, maintaining homeostasis, inhibiting organ fibrosis and promoting brain tissue and intellectual development that provide developmental benefits for infants and young children (Demarçay, 1838; Qaradakhi et al., 2020; Beggan et al., 2023; Hu et al., 2024; Ju et al., 2024). Due to its significant biological activity, there is increasing interest in efficiently extracting taurine from natural sources. Jia and Chen determined the optimal process parameters for taurine extraction from octopus by-products through orthogonal experiments, which included: extraction temperature of 80 °C, ethanol concentration of 80%, solid-liquid ratio of 1:12, extraction time of 2 h, and extraction times of 3. Under these optimized conditions, the taurine extraction rate reached 0.93%, demonstrating the effectiveness of the established protocol (Jia and Chen, 2012).
4 Bioactive components of octopus
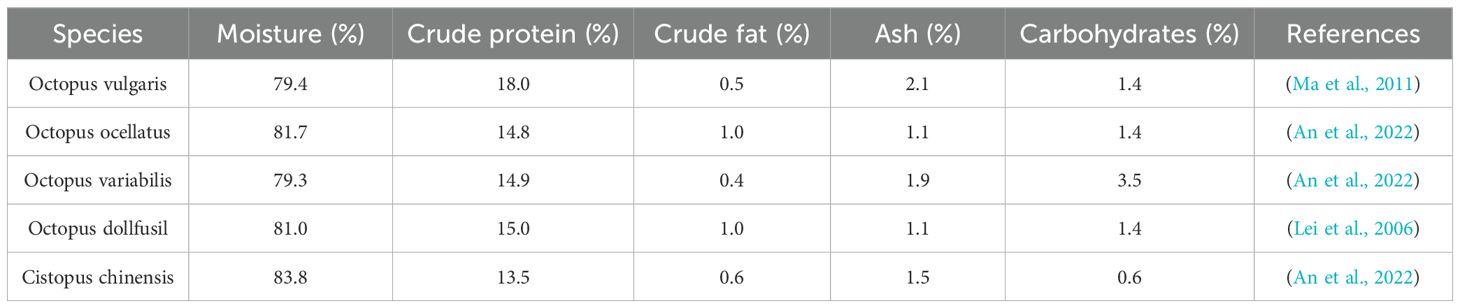
The variations in extraction techniques mentioned above influence the yield of bioactive components and further highlight the potential divergence in biological activities among different constituents. Building on this, further exploration of the pharmacological mechanisms of key nutrients in octopus extracts has become a pivotal step in advancing their translation from laboratory research to clinical applications. Modern studies have revealed that Octopus has various biological activities. Octopuses are a nutrient-rich food containing essential proteins and minerals and also acting as functional foods due to their bioactive components with health benefits (Li et al., 2023). The nutrients of octopus include moisture, protein, fat, ash, and carbohydrates (Table 2). Octopus is abundant in amino acids, fatty acids and mineral elements. Peptides, proteins, polysaccharides and bioactive biogenic amines were isolated from octopus through different methods (Supplementary Table S1). As illustrated, the biological activities of octopus were summarized in Figure 5. At present, extensive pharmacological studies have been carried out on the bioactive components of octopuses. However, quantitative analysis of these bioactive components remains scarce. In future research, advanced techniques such as metabolomics and pharmacokinetics should be employed to fill the existing knowledge gaps (Yu et al., 2022; Cheng et al., 2024).
4.1 Small molecule bioactives
The small molecule bioactive components of octopus mainly include various amino acids, fatty acids such as DHA and EPA and various mineral elements. Eating 150 grams of boiled octopus every day seems to provide sufficient beneficial nutrients (Oliveira et al., 2019). Hence, octopus is a high-protein and low-fat species with high nutritional value and medicinal potential (Ozogul et al., 2008).
4.1.1 Amino acid
Amino acids, as bioactive molecules, are the fundamental units for building the body (Church et al., 2020; Chandel, 2021). The human body requires continuous food supply to get the essential amino acids (EAAs), which include lysine, tryptophan, phenylalanine, methionine, threonine, leucine, isoleucine, valine and histidine. Octopus is a high-quality source of amino acids. Lei et al. detected 19 amino acids in the Octopus dollfusi (O. dollfusi), of which EAAs accounted for 41.2% of the total amino acids (Lei et al., 2006). In addition, 19 types of amino acids were also found in Octopus variabilis (O. variabilis) with EAAs accounting for 39.36% and glutamic acid having the highest content at 99.6 mg/g (Zheng et al., 2011).
In addition to the essential amino acids, octopus also contain various nonessential amino acids including taurine, glycine, arginine and glutamate (Zhang and Lei, 2006; An et al., 2022). The content of taurine in cooked octopus was 5.59 mmol/100 g. Taurine was the highest content amino acid in boiled octopus, account for 29.66% of the total free amino acid contents (Onozato et al., 2024). Taurine could inhibit mitochondrial dysfunction, alleviate inflammation and promote health by reducing cellular aging, lack of taurine may cause cardiomyocyte atrophy, mitochondrial and myofiber damage and cardiac dysfunction (Ito et al., 2008). The taurine content decreases gradually with age in different species (McGaunn and Baur, 2023). Researchers have found that the serum taurine content in 56-week-old mice decreased by about 70% compared to 4 weeks old, and the serum taurine concentration in 15-year-old monkeys was 85% lower than that at 5 years old, the serum taurine concentration in the 60-year-old population has decreased by more than 80%. Given taurine in the dietary supplementation intake would prolong median lifespan of elderly mice by increasing 10-12% (Singh et al., 2023). Taurine enhanced T cell proliferation in vitro by increasing PLCγ1-mediated calcium signaling, the MAPK signaling pathway (Ping et al., 2023). Taurine may enhance the anti-tumor activity of CD8+ T cells, while tumor cells compete with CD8+ T cells for taurine through high expression of SLC6A6. Research has found that SP1 activates the expression of SLC6A6, and supplementing taurine may participate in the regulation of Sp1-Slc6a6, CD8+ T cells activation, enhancement of the efficacy of cancer therapeutics and prevention of coronary heart disease (Wójcik et al., 2010; Bosevski et al., 2019; Cao et al., 2024). (Liu et al., 2022). found that taurine may improve the degree of cartilage tissue lesions in osteoarthritis rats by decreasing HMGB1 protein level, reducing the generation of free radicals and releasing inflammatory factors. Taurine may also inhibit apoptosis induced by myocardial cell ischemia through inactivating caspase-9 and increasing Akt activity (Takatani et al., 2004). In addition, taurine also plays an important role in treating psychiatric and neurodegenerative diseases (Aamer et al., 2024). Experimental evidence indicates that taurine could alleviate depression triggered by chronic social failure stress and chemically induced models (Zhu et al., 2023a; Li et al., 2024). Taurine has neuroprotective activities, such as therapeutic potential for treating Alzheimer’s disease (AD) by enhancing miRNA-181 and miRNA-21 gene levels, protecting against dopaminergic neuronal degeneration by mitigating 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced damage (Abuirmeileh et al., 2021; Cui et al., 2023; Almohaimeed et al., 2024). With the continuous advancement of modern nutrition research, taurine has gained increasingly widespread applications in the health sector. In infant nutrition, taurine has become a critical additive in infant formula, playing a pivotal role in promoting brain and retinal development in infants while regulating osmotic pressure balance in the body. In the functional beverage industry, sports drinks such as Red Bull utilize taurine as a core ingredient to rapidly replenish nutrients expended during physical activity, aiding in quick bodily recovery. Within the health product market, taurine not only enhances metabolic efficiency but also activates immune cell function, thereby strengthening the body’s resistance to infections (Froger et al., 2014; Jong et al., 2021; Bakshi et al., 2023; Cao et al., 2024). Given these significant health benefits, taurine is progressively emerging as an indispensable nutrient in the food and healthcare industries.
In addition, octopuses also contain other amino acids. Octopine is mainly found in marine mollusks such as octopuses in nature. It is formed by the condensation of arginine with alanine or pyruvate under the action of enzymes, and has osmotic regulation, pH stabilization and anti-tumor effects (Wang, 2012; Xie et al., 2016; Hakimelahi et al., 2022). Octopus side products contain 1% L-carnitine (Zhang, 2007). L-carnitine is an endogenous component involved in fatty acid metabolism that helps reduce oxidative stress and relieving heart failure, angina and fatigue (Pekala et al., 2011). In recent years, L-carnitine and its acetylated derivatives exhibit neuroprotective activities and may have therapeutic potential in diseases such as hypoxia ischemia, traumatic brain injury, AD and diseases related to central or peripheral nervous system damage (Ferreira and McKenna, 2017).
4.1.2 Fatty acids
Fatty acids (FAs) are important molecules as lipids and cell membrane constitutes, ensuring the normal functioning of cells while also serving as a source of energy for the body (Tvrzicka et al., 2011; Huang et al., 2025). In the W-Mediterranean Sea coastal areas, twenty-seven types of FAs were found in the O. vulgaris, including nine saturated fatty acids (SFA), eight monounsaturated fatty acids (MUFA) and ten polyunsaturated fatty acids (PUFA), the unsaturated fatty acids accounted for 69.98%, with eicosapentaenoic acid (EPA) having the highest content, accounting for 23.1%, followed by margaric acid (13.7%) and arachidonic acid (10.1%), the DHA accounting for only 0.74% (Arechavala-Lopez et al., 2019). By comparison, eighteen FAs detected in O. variabilis, the main ones were palmitic acid (17.02%), DHA (18.13%) and EPA (12.56%). PUFA accounted for 51.9% of the total fatty acids (Zheng et al., 2011). Interestingly, Octopus ochellatus (O. ochellatus) contains eighteen types of FAs, among which seven are PUFA, accounting for 39.54% of the total. Palmitic acid (25.33%), DHA (21.67%) and EPA (13.36%) are the main components (Xue et al., 2015).
DHA and EPA are two crucial omega-3 PUFA. Previous reports have indicated that they possess various beneficial effects, such as anti-arrhythmic properties, the ability to lower triglyceride levels and anti-depressant effects (Kaur et al., 2024; Marcus and Link, 2024; Serefko et al., 2024). EPA and DHA exhibit notable anti-inflammatory activity. They may inhibit the production of inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, in monocytes and endothelial cells (Calder et al., 2009, 2013). As dietary supplements, DHA and EPA have the potential to enhance the quality and motility of male sperm. This suggests that they could play a significant role in the treatment of male infertility (Hosseini et al., 2019). In the food industry, the application of DHA and EPA is hindered by their poor water solubility and high oxidative susceptibility. The implementation of food-grade delivery systems addresses this challenge. These systems could protect the fatty acids from environmental degradation, regulate their release sites, and thereby enhance their stability and bioavailability. In the future, clinical trials are still required to investigate how to achieve targeted release and elucidate their mechanism of action (Zhou and Wei, 2023; Alijani et al., 2025). Boiling octopus effectively preserved key nutrients. When the internal temperature of boiled octopus reaches the microbiological safety standard of 75 °C, the retention rates of EPA and DHA are 90.2% and 89.1%, respectively (Oliveira et al., 2019). Appropriate processing methods can enhance the absorption and utilization of nutritional value in octopuses.
4.1.3 Mineral elements
Mineral elements play a crucial role in maintaining various physiological and metabolic functions (Zemrani and Bines, 2020). Twenty-eight mineral elements were detected in O. vulgaris from the Mediterranean coast. These mainly included arsenic, zinc, copper, sodium, iron and potassium (Arechavala-Lopez et al., 2019). Fifteen mineral elements were detected in both O. variabilis and O. ochellatus. These elements included phosphorus, sodium, potassium, magnesium, zinc and calcium were present in relatively high amounts (Xue et al., 2015).
4.1.4 Steroid
Secondary metabolites are also an important direction for research. Steroids are a significant class of secondary metabolites that are extensively distributed among marine invertebrates. Dipeptidyl peptidase-4 (DPP-4) inhibitors are glucose-lowering drugs for type 2 diabetes mellitus (Pham et al., 2023). Three Δ5 steroid analogues were purified from the organic extract of Amphioctopus marginatus. The purified steroid displayed superior anti - hyperglycemic activity with DPP - 4 attenuation potential (IC50 3.49 µM) comparable activity with the standard DPP - 4 inhibitor (DPP - 4i) diprotin A (IC50 4.53 µM) (Paulose and Chakraborty, 2022).
4.2 Macromolecular bioactives
Macromolecules, including proteins, peptides and polysaccharides, are the foundation of life activities. Bioactive peptides and proteins originating from terrestrial mammals, marine animals, amphibians, and animal venoms may inhibit cell growth and induce apoptosis, endowing them with potential anti-cancer activity (Wang et al., 2017; Ejaz et al., 2018). Meanwhile, 200 mg/kg Misgurnus anguillicaudatus polysaccharide via oral gavage may increase the levels of superoxide dismutase and glutathione, counteract oxidative stress and inhibit the production of pro-inflammatory factors in streptozotocin-induced diabetic mice (Zhou et al., 2015).
4.2.1 Peptides
Marine invertebrates depend on their innate immune systems to ensure survival and reproduction. Their immune systems contain a variety of active peptide compounds that possess anti-bacterial, anti-aging and anti-cancer properties (Negi et al., 2017; Guryanova et al., 2023; Yang et al., 2023). Octopuses, as a type of invertebrate, also contain various proteins and peptide compounds. Peptide sequences were identified from different octopuses in Supplementary Table S1. and the activity in Figure 6.
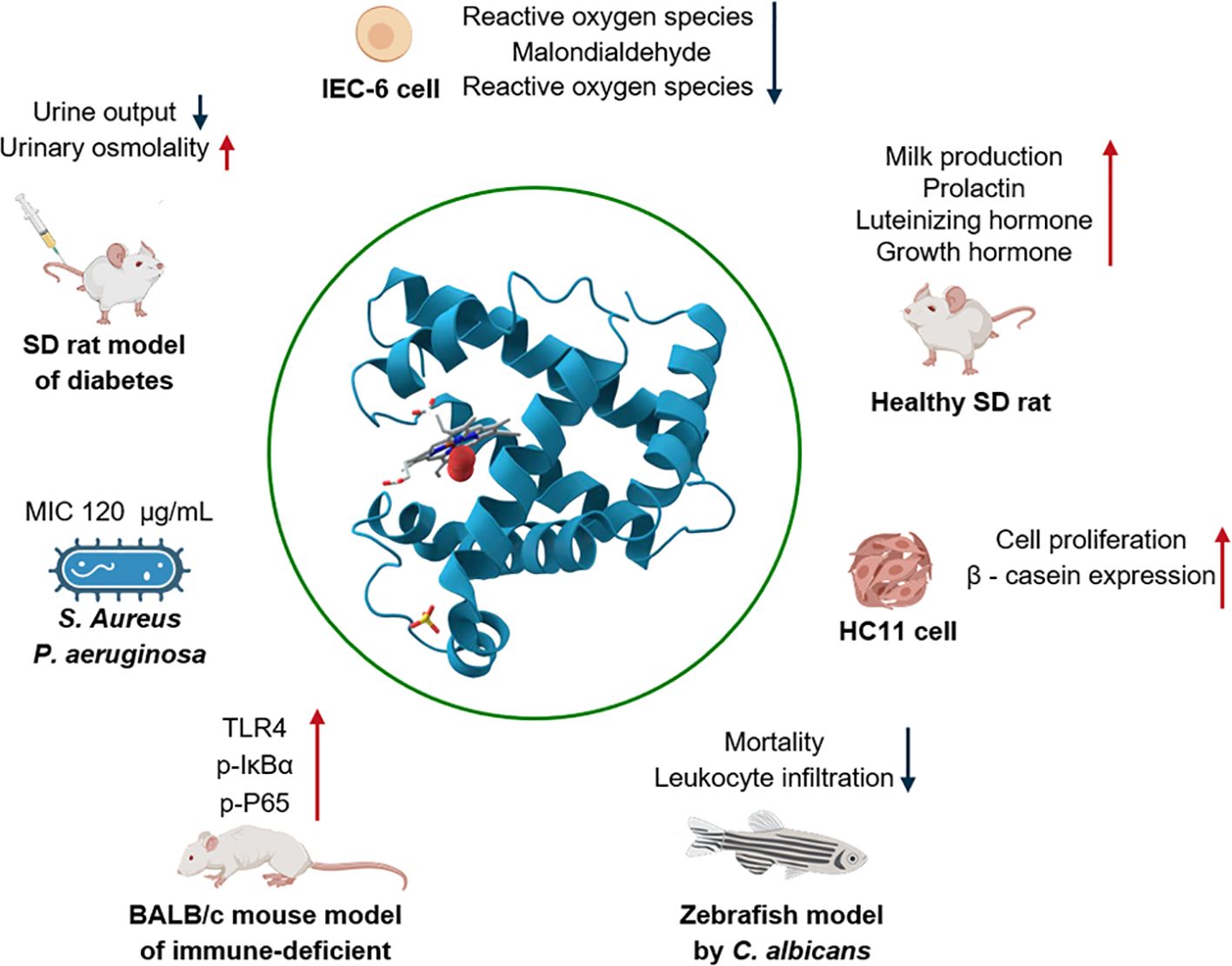
Octopressin (OP) and cephalotocin (CT) are two neuropeptides isolated from O. vulgaris, exhibiting oxytocin-like and arginine-vasopressin-like activities, respectively. RT-PCR/Southern blot analysis results showed that the expression of OP mRNA is detected in the brain and ganglia. CT mRNA was expressed only in the brain (Takuwa-Kuroda et al., 2003). CT may activate human V1b and V2 vasopressin receptors. Meanwhile, single intravenous injection of CT into the tail vein reduced Sprague-Dawley rats urine output and increased urinary osmolality. These studies indicate that CT may be beneficial for treating urinary system diseases such as nocturia, enuresis, and urethritis (Reich, 1992; Kim et al., 2022). Octopus peptides with a molecular weight of less than 5 kDa, which were extracted from O. vulgaris by using neutral protease, may promote the proliferation of mouse mammary epithelial cells. They are also capable of increasing the synthesis of β-casein, active the JAK2-STAT5 and mTOR signaling pathways. Additionally, these peptides can enhance the nutrient content and elevate the immunoglobulin concentration in the milk of lactating SD rats. The characteristic peptides potentially include MGLAGPR, MGDVLNF, EAPLMHV and TEAPLMHV. This study offered a valuable reference regarding the biological activity of octopus polypeptides during the lactation process (Figure 7) (Cai et al., 2015, 2020). In contrast to the lactation-related peptides, another peptide hydrolysate extracted from O. vulgaris using alkaline proteases can activate the NF-κB pathway to prevent cyclophosphamide-mediated disruption of the intestinal barrier and regulating the immune system (Ali et al., 2024). In addition, six peptides derived and purified from the protein hydrolyzates of O. vulgaris showed better oxygen radical absorbance capacity and 2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging capacity. IEC-6 cells were protected by GGAW from H2O2-induced oxidative damage by significantly reducing the generation of reactive oxygen species, malondialdehyde and lactate dehydrogenas, and increasing the activity of superoxide dismutase and glutathione peroxidase (Peng et al., 2022). In response to multidrug-resistant pathogens, Octominin (23 amino acids, GWLIRGAIHAGKAIHGLIHRRRH), a peptide derived from the cDNA sequence of Octopus minor, was designed, synthesized, and demonstrated to exhibit antibacterial activity against Candida albicans. This peptide exhibited a minimum inhibitory concentration (MIC) of 50 µg/mL and a minimum fungicidal concentration (MFC) of 200 µg/mL. After 72 h of treatment with octominin, the mortality rate of C. albicans infected zebrafish model by decreased from 84% to 25%, while leukocyte infiltration decreased (Nikapitiya et al., 2020). Moreover, a peptide named OctoPartenopin, which was extracted from the suckers of O. vulgaris, also exhibits remarkable anti-bacterial activities against Staphylococcus aureus and Pseudomonas aeruginosa. Based on the purified peptide AGTNK, four analogues with better antibacterial activity were further synthesized and QAGSNKGASQKGMS exhibited the best antibacterial and antimicrobial activity (Maselli et al., 2020). A specific peptide named eledoisin, which exhibits vasodilatory and hypotensive effects, was isolated from the posterior salivary glands of Eledone moschata and Eledone aldovandi (Anastasi and Erspamer, 1962). The water extract of octopus ink demonstrated stronger anti-mutagenic and protective effects on 22Rv1 cancer cells. In contrast, the dichloromethane extract of octopus ink had a lower semi-lethal concentration and a novel compound, N-(2-oxoazepan-3-yl)-pyrrolidine-2-carboxamide (OPC), was successfully purified (Hernández-Zazueta et al., 2021). In addition, Minakata et al. identified a peptide in the brain of O. vulgaris that exhibits structural similar features to vertebrate gonadotropin-releasing hormone (GnRH) and this peptide was named oct-GnRH. Oct-GnRH may potentially trigger gonadal maturation and stimulate egg-laying in the reproductive system of the octopus. In addition, Oct-GnRH may also stimulate neural regulation to affect feeding behavior, memory system, cardiac activity and movement (Minakata et al., 2009).
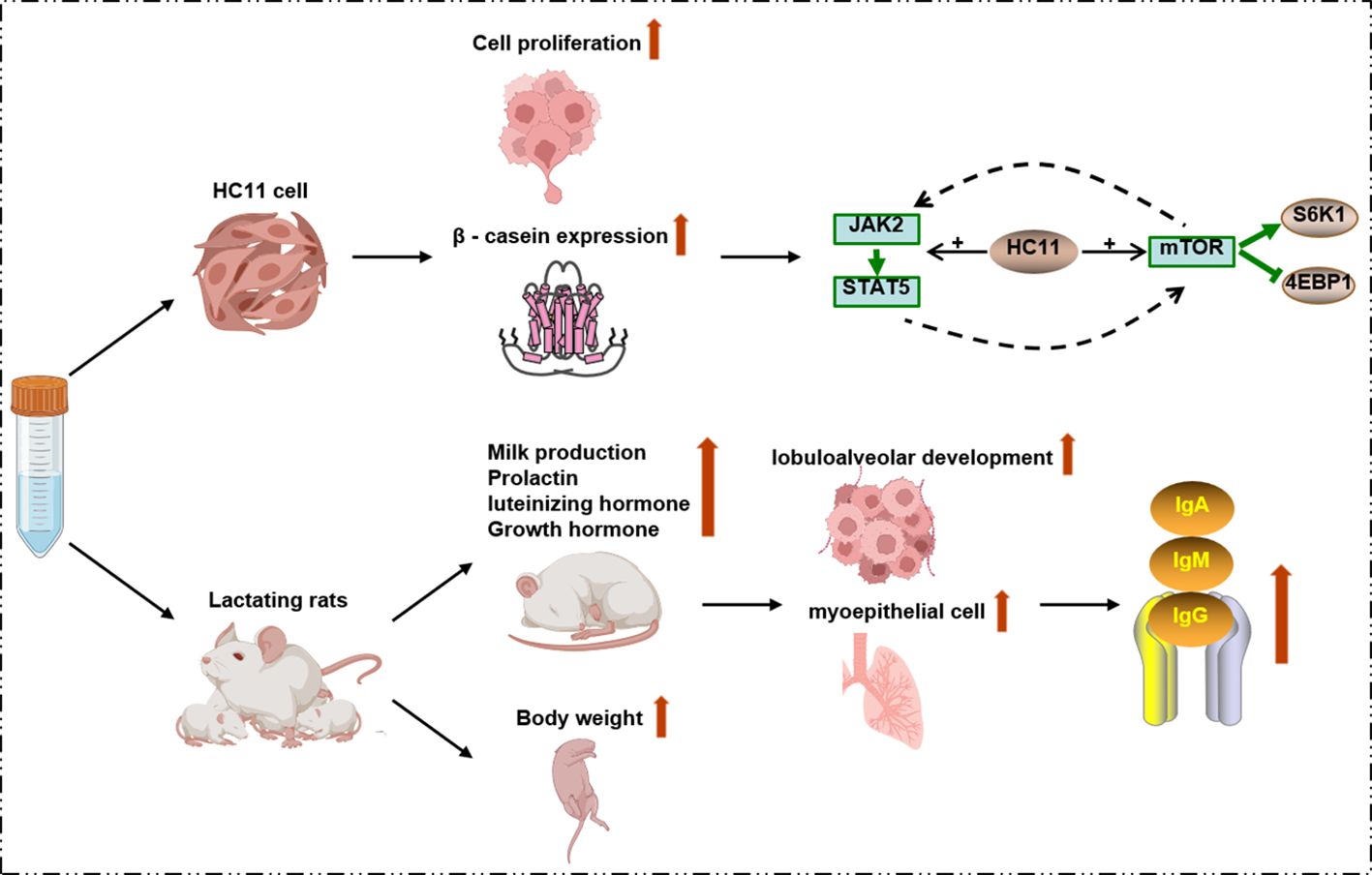
Figure 7. Octopus peptides (<5 kDa) from O. vulgaris promote lactation by activating JAK2-STAT5 and mTOR signaling pathways.
4.2.2 Proteins
Cephalopods possess a clever strategy for paralyzing their prey. Since they lack sharp teeth and large bodies, they achieve this by releasing toxins from their salivary glands (Ueda et al., 2008). Proteins hydrolysates derived from O. vulgaris exhibit significant inhibitory activity and dose-dependent effects on α-amylase in vitro. This protein hydrolysate may play a role in preventing diabetes by regulating the content of total hemoglobin and glycated hemoglobin. After treating diabetic Wistar rats by gastric gavage at 400 mg/kg body weight for 30 days, the activity of α-amylase in rat plasma, pancreas and intestine decreased by 34.03%, 53.24% and 46.70%, respectively, glucose levels returned to normal, while plasma insulin levels and liver glycogen content significantly increased (Ben Slama-Ben Salem et al., 2018). A toxin purified from the salivary glands of O. vulgaris may be capable of killing crabs and this toxin can be inactivated by trypsin (Ghiretti, 1960). Afterwards, another toxin with a molecular weight of 23 ± 1 kDa was discovered in the salivary gland extract of O. dofleini (Songdahl and Shapiro, 1974). Alkaline proteins with a molecular weight below 70 kDa from Eledone cirrhosa were found to have the activity of blocking nerve conduction. Additionally, it was discovered that the saliva of Eledone cirrhosa has hemolytic effects (McDonald and Cottrell, 1972; Key et al., 2002). This research indicated that the proper use of octopus toxins may be beneficial in the treatment of certain neurological disorders and thrombotic diseases.
Octopuses contain hemocyanin, which exhibits hemolytic activity, anti-bacterial activity and anti-tumor activity (Zhang et al., 2009; Qin et al., 2018; Mora Román et al., 2019). Furthermore, hemocyanin not only plays a role in regulating the respiratory system but also promotes the proliferation of human mesenchymal stem cells and the differentiation of osteoblasts (Costa-Paiva et al., 2018; Kruppke et al., 2020a, 2020b).Furthermore, collagen and protamine were found from the mantle and sperm for octopus, respectively (Morales et al., 2000; Giménez-Bonafé et al., 2004). Collagen has been widely applied in the food, cosmetics, pharmaceutical and biomedical industries. It contributes to the stability of tissues and organs, maintaining their structural integrity. Additionally, it displays biological activity by enhancing the capacity of bone tissue. It also interacts with the extracellular matrix, thereby influencing the activity of cancer cells (Gelse et al., 2003; Ferreira et al., 2012; Sorushanova et al., 2019; Xu et al., 2019). Protamine, a valuable substance, possesses remarkable anti-bacterial and anti-thrombotic properties. These characteristics make it an important component in various medical and biological applications. Its anti-bacterial effect helps in combating harmful bacteria, while the anti-thrombotic property plays a crucial role in preventing the formation of blood clots, which is essential for maintaining proper blood circulation and overall health (Kim et al., 2015; Chandiramani et al., 2022). Clinically, it is significant to note that the administration of protamine through the ascending aorta has the potential to maintain the stability of cardiopulmonary function in patients who are undergoing cardiac surgery, which is crucial for successful surgical procedures and patient recovery (Chaney et al., 2016). Octopus ink is secreted from ink sacs and it has a protein content ranging from 5% to 8%. A totally of 1432 different peptides and 361 non-redundant proteins have been identified in it. These components may potentially be utilized in the future for their anti-microbial, anti-viral and anti-cancer properties (Imran et al., 2023a).
4.2.3 Polysaccharides
Marine-derived polysaccharides have been demonstrated to possess inhibitory effects against cancer and viral infections, as well as anti-inflammatory and anti-oxidant properties (Lee et al., 2017; Zhong et al., 2019). Due to their remarkable biocompatibility, biodegradability and low toxicity, marine-derived polysaccharides have exhibited substantial therapeutic promise in wound healing, presenting great potential for the creation of innovative wound care solutions (Kumar et al., 2023). The polysaccharides extracted from O. dollfusi significantly increase the proliferation of spleen cells in immunosuppressed mice induced by cyclophosphamide at doses of 12.5–50 mg/L, promote the proliferation of mouse splenocytes and possess the ability to enhance immunity (Lei et al., 2007) (Wen et al., 2010).
4.2.4 Biogenic amines
Octopamine (OA), first isolated from octopus’ salivary glands. Moreover, it exhibits potential pharmacological activities in the management of type II diabetes and obesity. A study demonstrated that following four weeks of continuous octopamine gavage, obese mice exhibited significant reductions in fat wet weight, fat coefficient, as well as serum total cholesterol and triglyceride levels (Qiu et al., 2009). This implies that OA may play a role in regulating metabolic processes related to these conditions (Qu, 2016). Specifically, as an endogenous β3-adrenergic receptor agonist structurally analogous to norepinephrine, octopamine has been shown to modulate energy expenditure and glucose metabolism through activation of thermogenic pathways in adipocytes. Further research into its specific mechanisms of action could lead to the development of more effective therapeutic strategies for type II diabetes and obesity, opening up new possibilities in the field of pharmacology (Erspamer and Boretti, 1951). In addition, tyramine, histamine, serotonin (enteramine is more commonly known as serotonin) and acetylcholine have also been isolated from the posterior salivary gland of octopuses (Songdahl and Shapiro, 1974).
5 Biomedical applications of octopus
The octopus, with its highly sophisticated and adaptable biology, is an ideal model for biomedical application research. Octopus arms are distributed with suckers and the special structure and micro-serrations of these suckers give octopuses great potential in biomaterial applications, its regenerative capabilities are particularly relevant to the field of regenerative medicine (Figure 8) (Baik et al., 2017; Hwang et al., 2022; Lee et al., 2024b).
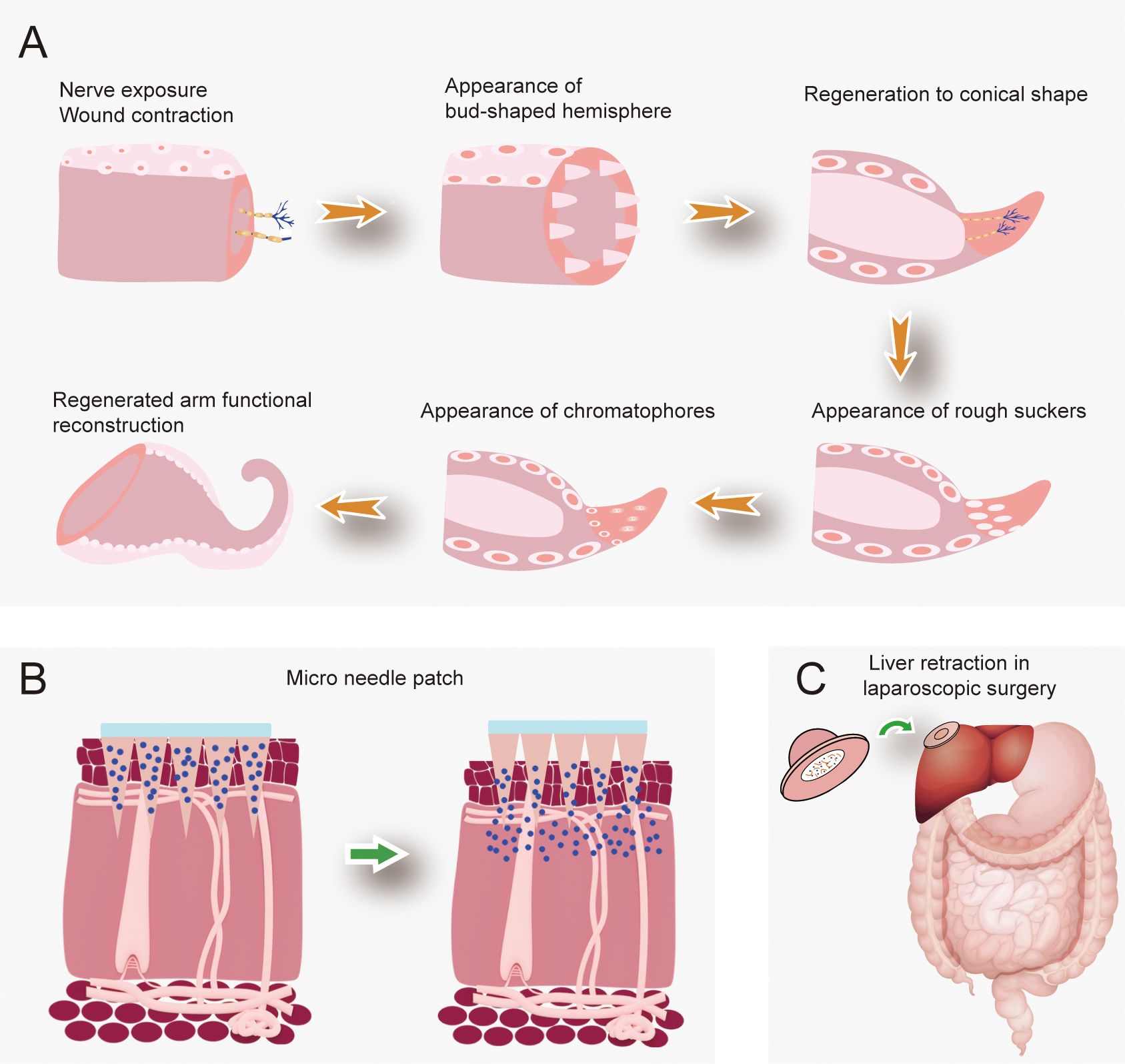
Figure 8. Regenerative capacity of octopuses and their biomedical applications. (A) Schematic illustration of octopus arm regeneration process; (B, C) Octopus-derived microneedle patches engineered for diverse medical scenarios.
A novel biomimetic material, developed using microstructural features inspired by octopus tentacles and suckers, has been designed as a controlled-release nitric oxide (NO) carrier for biomedical applications. This carrier significantly reduces inflammation in diabetic skin injuries in type I diabetic rats, promote the production of vascular endothelial growth factor, thereby stimulating blood vessel regeneration (Chen et al., 2024). In addition to structural applications, the biomimetic properties of octopuses have inspired the development of novel drug delivery systems. Luo et al. designed two types of suction patches based on the structure of octopus suction cups, a simple suction cup (SC) and the other is a suction cup orifice design (SCOD). Pharmacokinetic experiments in beagle dogs showed that the SCOD patch significantly improved the bioavailability of desmopressin compared with SC. In addition, participants in human studies demonstrated high patient compliance (Luo et al., 2023). Wu et al. designed an adhesive patch with excellent mechanical properties, adhesion and biocompatibility based on octopus suction cups. In the adhesion to the liver surface in laboratory rabbits for 24 h, histological analysis revealed no apparent tissue damage, lesions, inflammation, or noticeable abnormality observed in the major organs of all the laboratory rabbits, and may be developed as a new apparatus for liver traction in laparoscopic surgery (Wu et al., 2024). Zhu et al. engineered a bioinspired patch that emulates the tentacle adhesion and venom-delivery mechanisms of the blue-ring octopus. This innovative device enables precisely controlled, on-demand drug release and demonstrates robust adhesion to tissue surfaces. Experiments have shown that the patch significantly accelerates ulcer healing, inhibits tumor growth, and has good biocompatibility and potential for smart wearables (Zhu et al., 2023b). An octopus-inspired flexible multivalent penetrating system was designed as a non-viral vector. In human corneal epithelial cells and human conjunctival epithelial cells have observed high fluorescence intensity and low toxicity. In a retinoblastoma-bearing mice model, local infusion can effectively inhibit the expression of intraocular tumor proteins. This study provides a new method for gene therapy (Jiang et al., 2019). Another type of transdermal delivery patch features a double-layer suction cup cluster inspired by octopus tentacles. The upper layer mimics the central protrusion of octopus suckers to enhance negative pressure adsorption, while the lower layer incorporates a flexible cup-shaped structure that conforms to skin texture, expands the contact area, and generates negative pressure via finger pressing to enable non-invasive transdermal drug delivery. In an atopic dermatitis model, this design demonstrated a 44% reduction in serum IgE, a 56% decrease in IL-4, and a 45% reduction in epidermal thickness, alongside high biocompatibility and low irritation, providing an efficient and safe new strategy for transdermal drug delivery (Lee et al., 2024a).
The arm regeneration process in octopuses follows a well-defined sequence: the protrusion of the central nervous axis and contraction of the wound’s edge, the appearance of a bud-shaped hemisphere, the development of the regenerating tissue into a conical shape, the appearance of rough suckers, the appearance of chromatophores and regenerated arm functional reconstruction (Imperadore and Fiorito, 2018). This morphological progression correlates with specific cellular events observed in recent studies. Molecular analyses reveal blood cells and connective tissue cells may play a major role in the neural regeneration ability of O. vulgaris. After 30–45 days of nerve resection, axons passed through the injury gap into the stellate ganglion and formed a network. By 5 months post-injury, angiogenesis occurs alongside nerve fibers regeneration to re-establish the functional connection between the brain and the periphery (Imperadore et al., 2017, 2019). Histological evidence demonstrates O. vulgaris may rapidly repair wounds within 24 hours after the arm is amputated through various mechanisms such as tissue contraction, epithelial cell migration and the coalescing of cells at the site of injury (Shaw et al., 2016). Imperadore et al. used label free multiphoton microscopy to study the regeneration process of O. vulgaris and found that dermal contraction and blood cell debris clearance were involved in wound healing. After healing, new tissues, cells and nerve fibers were generated with proliferative characteristics (Imperadore et al., 2022). Translating this biological phenomenon to human medicine could transform treatments for traumatic injuries, neurodegenerative diseases, and organ loss. Neuron regeneration and synaptic regeneration provide ideas for the treatment of AD and PD. The neural regeneration ability of octopus may not only inform stem cell therapies but also establish novel targets for neurodegenerative disease research (Qian et al., 2020; Qu et al., 2021).
6 Conclusions
This review presents a summary of the distribution, nutrients, bioactive compounds properties, regenerative ability and processing methods of marine octopus. Octopuses are mainly distributed in tropical and temperate waters. They have abundant resources and are widely distributed around the world, demonstrating significant research value. China, Spain, Vietnam and Morocco are among the main export countries of octopuses, while South Korea, Japan, Italy and the United States are the primary import countries.
The main nutrients in octopuses include small molecular components such as amino acids, fatty acids and trace elements, as well as both small and large molecular components like proteins, peptides, biogenic amines and polysaccharides. Octopus-derived peptides exhibit diverse biological activities. These include promoting lactation in SD rats, reducing urine output in diabetic rats, enhancing urinary osmolality, and decreasing mortality in Candida albicans-infected zebrafish, among others. Octopus peptide-based products exhibit significant commercialization potential. If further researched and developed, these bioactive components could demonstrate enormous potential in functional foods, nutritional supplements, clinical translation, and other domains. However, the current quality evaluation system for these peptides still requires substantial improvement. During octopus processing, substantial by-products is generated. Proper extraction of these can obtain valuable ingredients such as taurine, fish oil, and octopamine. These methods not only enhance the utilization efficiency of octopus resources but also effectively mitigate resource waste and environmental pollution.
Moreover, the unique structural characteristics and self-repair capabilities of octopuses suggest their potential positive applications in tissue engineering, nerve repair, and the treatment of neurodegenerative diseases. The advancement of microneedle technology serves as a promising demonstration, while micro-serrated structures have been mimicked, manufacturing these textures at a clinical grade—with consistent adhesion strength and sterility—remains continuous validation. Regulatory approval for such novel devices would require rigorous testing on parameters like biocompatibility and shelf life. In addition, the application of octopus neuron regeneration and wound healing faces significant challenges. Simulating the dynamic regulatory mechanisms of octopus stem cells also presents a significant challenge. Octopus neural regeneration highly relies on the rapid activation and differentiation of local stem cell niches. In contrast, the nervous systems of mammals (including humans) lack comparable stem cell reserves or exhibit lower activation efficiency. Additionally, OA levels in mammals are extremely low. A key challenge in clinical translation is how to mimic octopamine’s neuroprotective effects through pharmacological interventions or gene editing while avoiding interference with existing metabolic network diseases. In the future, interdisciplinary collaboration may emerge as a critical breakthrough. Developing mammalian-specific regeneration signaling pathway activators for precise intervention in nerve injury sites appears feasible. For instance, constructing vascularized organoids and AI-driven dynamic pathological models could overcome the limitations of existing animal models and in vitro systems, authentically replicating the multicellular interaction mechanisms within the human neural regeneration microenvironment. Additionally, the advancement of ethical frameworks must be prioritized, particularly in establishing long-term risk monitoring systems for stem cell activation and chimeric brain technology applications. Future research should further dissect molecular details such as lactate metabolism reprogramming and epigenetic regulation in octopus’ regeneration and uncover their conserved associations with the human nervous system through multi-omics techniques, thereby accelerating the transition from basic mechanism analysis to clinical therapy development and ultimately achieving revolutionary breakthroughs.
Author contributions
ML: Methodology, Writing – original draft, Visualization. ZD: Writing – original draft, Visualization. KC: Writing – original draft, Validation. XF: Writing – review & editing, Funding acquisition. XL: Funding acquisition, Methodology, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. NATCM’s Project of High-level Construction of Key TCM Disciplines (Marine Traditional Chinese Medicine; No. zyyzdxk-2023124), Fundamental Research Funds for the Central Universities (No. 202241008) Taishan Scholars Program of Shandong Province, China (NO. tstp20240825).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1615994/full#supplementary-material
References
Aamer H., Tariq M., Yawar M., Shaheryar M., Khan A. A., Iftikhar S., et al. (2024). Exploring taurine’s potential in alzheimer’s treatment: A comprehensive review. Cureus 16, e60997. doi: 10.7759/cureus.60997
Abuirmeileh A. N., Abuhamdah S. M., Ashraf A., and Alzoubi K. H. (2021). Protective effect of caffeine and/or taurine on the 6-hydroxydopamine-induced rat model of Parkinson’s disease: Behavioral and neurochemical evidence. Restor. Neurol. Neurosci. 39, 149–157. doi: 10.3233/RNN-201131
Ali M., Ullah H., Farooqui N. A., Deng T., Siddiqui N. Z., Ilyas M., et al. (2024). NF-κB pathway activation by Octopus peptide hydrolysate ameliorates gut dysbiosis and enhances immune response in cyclophosphamide-induced mice. Heliyon 10, e38370. doi: 10.1016/j.heliyon.2024.e38370
Alijani S., Hahn A., Harris W. S., and Schuchardt J. P. (2025). Bioavailability of EPA and DHA in humans - a comprehensive review. Prog. Lipid Res. 97, 101318. doi: 10.1016/j.plipres.2024.101318
Almohaimeed H. M., Almars A. I., Alsulaimani F., Basri A. M., Althobaiti N. A., Albalaw A. E., et al. (2024). Investigating the potential neuroprotective benefits of taurine and Dihydrotestosterone and Hydroxyprogesterone levels in SH-SY5Y cells. Front. Aging Neurosci. 16. doi: 10.3389/fnagi.2024.1379431
An J., Ge J., Bian L., Liu C., and Chen S. (2022). Analysis and evaluation of the nutritional composition of Cistopus chinensis muscles. Marine Sci. 46, 103–110. doi: 10.11759/hykx20210506002
Anastasi A. and Erspamer V. (1962). Occurrence and some properties of eledoisin in extracts of posterior salivary glands of Eledone. Br. J. Pharmacol. Chemother. 19, 326–336. doi: 10.1111/j.1476-5381.1962.tb01197.x
Arechavala-Lopez P., Capó X., Oliver-Codorniú M., Sillero-Rios J., Busquets-Cortés C., Sanchez-Jerez P., et al. (2019). Fatty acids and elemental composition as biomarkers of Octopus vulgaris populations: Does origin matter? Mar Pollut. Bull. 139, 299–310. doi: 10.1016/j.marpolbul.2018.12.048
Octopus Cuvier 1798. Available online at: https://www.gbif.org/species/2289226 (Accessed December 2, 2024).
Baik S., Kim D. W., Park Y., Lee T.-J., Ho Bhang S., and Pang C. (2017). A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 546, 396–400. doi: 10.1038/nature22382
Bakshi S., Paswan V. K., Yadav S. P., Bhinchhar B. K., Kharkwal S., Rose H., et al. (2023). A comprehensive review on infant formula: nutritional and functional constituents, recent trends in processing and its impact on infants’ gut microbiota. Front. Nutr. 10. doi: 10.3389/fnut.2023.1194679
Beggan L. A., Mulhern M. S., Mæhre H. K., McSorley E. M., Yeates A. J., Zavez A., et al. (2023). Associations between serum taurine concentrations in mothers and neonates and the children’s anthropometrics and early neurodevelopment: Results from the Seychelles Child Development Study, Nutrition Cohort 2. Neurotoxicology 99, 43–49. doi: 10.1016/j.neuro.2023.08.004
Ben Slama-Ben Salem R., Bkhairia I., Abdelhedi O., and Nasri M. (2017). Octopus vulgaris protein hydrolysates: characterization, antioxidant and functional properties. J. Food Sci. Technol. 54, 1442–1454. doi: 10.1007/s13197-017-2567-y
Ben Slama-Ben Salem R., Ktari N., Bkhairia I., Nasri R., Mora L., Kallel R., et al. (2018). In vitro and in vivo anti-diabetic and anti-hyperlipidemic effects of protein hydrolysates from Octopus vulgaris in alloxanic rats. Food Res. Int. 106, 952–963. doi: 10.1016/j.foodres.2018.01.068
Bosevski M., Nikolovski P., Stojanovska L., and Apostolopoulos V. (2019). Progression of carotid artery disease could stratify a risk of coronary artery disease patients with type 2 diabetes. Acta Biochim. Biophys. Sin. (Shanghai) 51, 120–122. doi: 10.1093/abbs/gmy149
Cai B., Chen H., Sun H., Sun H., Wan P., Chen D., et al. (2015). Lactogenic Activity of an Enzymatic Hydrolysate from Octopus vulgaris and Carica papaya in SD Rats. J. Med. Food 18, 1262–1269. doi: 10.1089/jmf.2014.3355
Cai B., Wan P., Chen H., Chen X., Sun H., and Pan J. (2020). Identification of octopus peptide and its promotion of β-casein synthesis in a mouse mammary epithelial cell line. J. Food Biochem. 44, e13467. doi: 10.1111/jfbc.13467
Calder P. C., Ahluwalia N., Albers R., Bosco N., Bourdet-Sicard R., Haller D., et al. (2013). A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 109 Suppl 1, S1–34. doi: 10.1017/S0007114512005119
Calder P. C., Albers R., Antoine J.-M., Blum S., Bourdet-Sicard R., Ferns G. A., et al. (2009). Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 101 Suppl 1, S1–45. doi: 10.1017/S0007114509377867
Cao T., Zhang W., Wang Q., Wang C., Ma W., Zhang C., et al. (2024). Cancer SLC6A6-mediated taurine uptake transactivates immune checkpoint genes and induces exhaustion in CD8+ T cells. Cell 187, 2288–2304.e27. doi: 10.1016/j.cell.2024.03.011
Chandel N. S. (2021). Amino acid metabolism. Cold Spring Harb Perspect. Biol. 13, a040584. doi: 10.1101/cshperspect.a040584
Chandiramani A. S., Jenkin I., Botezatu B., and Harky A. (2022). Protamine-induced coronary graft thrombosis: A review. J. Cardiothorac Vasc. Anesth 36, 2679–2684. doi: 10.1053/j.jvca.2021.10.008
Chaney M. A., Devin Roberts J., Wroblewski K., Shahul S., Gaudet R., and Jeevanandam V. (2016). Protamine administration via the ascending aorta may prevent cardiopulmonary instability. J. Cardiothorac Vasc. Anesth 30, 647–655. doi: 10.1053/j.jvca.2015.11.014
Chen Z., Zhang H., Lyu Y., Lv K., Xing H., Shen P., et al. (2024). Octopus-inspired adaptive molecular motion for synergistic photothermal and nitric oxide antibacterial therapy in diabetic wound repair. Advanced Funct. Materials 34, 2402591. doi: 10.1002/adfm.202402591
Cheng C., Ren C., Li M.-Z., Liu Y.-H., Yao R.-Q., Yu Y., et al. (2024). Pharmacologically significant constituents collectively responsible for anti-sepsis action of XueBiJing, a Chinese herb-based intravenous formulation. Acta Pharmacol. Sin. 45, 1077–1092. doi: 10.1038/s41401-023-01224-1
Church D. D., Hirsch K. R., Park S., Kim I.-Y., Gwin J. A., Pasiakos S. M., et al. (2020). Essential amino acids and protein synthesis: insights into maximizing the muscle and whole-body response to feeding. Nutrients 12, 3717. doi: 10.3390/nu12123717
Costa-Paiva E. M., Schrago C. G., Coates C. J., and Halanych K. M. (2018). Discovery of novel hemocyanin-like genes in metazoans. Biol. Bull. 235, 134–151. doi: 10.1086/700181
Cui C., Song H., Han Y., Yu H., Li H., Yang Y., et al. (2023). Gut microbiota-associated taurine metabolism dysregulation in a mouse model of Parkinson’s disease. mSphere 8, e0043123. doi: 10.1128/msphere.00431-23
Demarçay H. (1838). Ueber die natur der galle. Ann. Pharm. 27, 270–291. doi: 10.1002/jlac.18380270304
Ejaz S., Hashmi F. B., Malik W. N., Ashraf M., Nasim F. U.-H., and Iqbal M. (2018). Applications of venom proteins as potential anticancer agents. Protein Pept. Lett. 25, 688–701. doi: 10.2174/0929866524666180614102104
Elkhenany H., Soliman M. W., Atta D., and El-Badri N. (2025). Innovative marine-sourced hydroxyapatite, chitosan, collagen, and gelatin for eco-friendly bone and cartilage regeneration. J. BioMed. Mater Res. A 113, e37833. doi: 10.1002/jbm.a.37833
Erspamer V. and Boretti G. (1951). Substances of a phenolic and indolic nature present in acetone extracts of the posterior salivary glands of Octopoda (Octopus vulgaris, Octopus macropus and Eledone moschata). Experientia 7, 271–273. doi: 10.1007/BF02154550
Ferreira A. M., Gentile P., Chiono V., and Ciardelli G. (2012). Collagen for bone tissue regeneration. Acta Biomater 8, 3191–3200. doi: 10.1016/j.actbio.2012.06.014
Ferreira G. C. and McKenna M. C. (2017). L-carnitine and acetyl-L-carnitine roles and neuroprotection in developing brain. Neurochem. Res. 42, 1661–1675. doi: 10.1007/s11064-017-2288-7
Froger N., Moutsimilli L., Cadetti L., Jammoul F., Wang Q.-P., Fan Y., et al. (2014). Taurine: the comeback of a neutraceutical in the prevention of retinal degenerations. Prog. Retin Eye Res. 41, 44–63. doi: 10.1016/j.preteyeres.2014.03.001
Fu X., Zhong H., Song W., and Li B. (2020). Extraction and quality analysis of octopus viscera fish oil. China Oils Fats 45, 17–22. doi: 10.12166/j.zgyz.1003-7969/2020.05.004
Gelse K., Pöschl E., and Aigner T. (2003). Collagens–structure, function, and biosynthesis. Advanced Drug delivery Rev. 55 (12), 1531–1546. doi: 10.1016/j.addr.2003.08.002
Ghiretti F. (1960). Toxicity of octopus saliva against crustaces. Ann. N Y Acad. Sci. 90, 726–741. doi: 10.1111/j.1749-6632.1960.tb26417.x
Giménez-Bonafé P., Soler F. M., Buesa C., Sautière P.-E., Ausió J., Kouach M., et al. (2004). Chromatin organization during spermiogenesis in Octopus vulgaris. II: DNA-interacting proteins. Mol. Reprod. Dev. 68, 232–239. doi: 10.1002/mrd.20068
Guryanova S. V., Balandin S. V., Belogurova-Ovchinnikova O. Y., and Ovchinnikova T. V. (2023). Marine invertebrate antimicrobial peptides and their potential as novel peptide antibiotics. Mar Drugs 21, 503. doi: 10.3390/md21100503
Hakimelahi A., Sharifi R., Mahmoodi M., and Kassaee S. M. (2022). The effect of opine on matrix metalloproteinase expression in mice with breast cancer. Arch. Physiol. Biochem. 128, 501–506. doi: 10.1080/13813455.2019.1696367
Hernández-Zazueta M. S., García-Romo J. S., Noguera-Artiaga L., Luzardo-Ocampo I., Carbonell-BarraChina Á.A., Taboada-Antelo P., et al. (2021). Octopus vulgaris ink extracts exhibit antioxidant, antimutagenic, cytoprotective, antiproliferative, and proapoptotic effects in selected human cancer cell lines. J. Food Sci. 86, 587–601. doi: 10.1111/1750-3841.15591
Hosseini B., Nourmohamadi M., Hajipour S., Taghizadeh M., Asemi Z., Keshavarz S. A., et al. (2019). The effect of omega-3 fatty acids, EPA, and/or DHA on male infertility: A systematic review and meta-analysis. J. Diet Suppl. 16, 245–256. doi: 10.1080/19390211.2018.1431753
Hu L., Hu J., Wei Y., Qi M., He Z., and Zhao D. (2024). The physiological functions and applications of taurine. FEED INDUSTRY MAGAZINE 45 (18), 49–54. doi: 10.13302/j.cnki.fi.2024.18.008
Huang H., Wang C., Chang S., Cui T., Xu Y., Huang M., et al. (2025). Structure and catalytic mechanism of exogenous fatty acid recycling by AasS, a versatile acyl-ACP synthetase. Nat. Struct. Mol. Biol. 32 (5), 802–817. doi: 10.1038/s41594-024-01464-7
Huang H., Zhang S., Zhang Y., Zhang L., and Wang K. (2016). Product analysis of protein hydrolysate from octopus leftovers. J. Huaqiao University(Natural Science) 37, 330–335. doi: 10.11830/ISSN.1000-5013.2016.03.0330
Hwang G. W., Lee H. J., Kim D. W., Yang T.-H., and Pang C. (2022). Soft microdenticles on artificial octopus sucker enable extraordinary adaptability and wet adhesion on diverse nonflat surfaces. Adv. Sci. (Weinh) 9, e2202978. doi: 10.1002/advs.202202978
Imperadore P. and Fiorito G. (2018). Cephalopod tissue regeneration: consolidating over a century of knowledge. Front. Physiol. 9. doi: 10.3389/fphys.2018.00593
Imperadore P., Galli R., Winterhalder M. J., Zumbusch A., and Uckermann O. (2022). Imaging arm regeneration: label-free multiphoton microscopy to dissect the process in octopus vulgaris. Front. Cell Dev. Biol. 10. doi: 10.3389/fcell.2022.814746
Imperadore P., Parazzoli D., Oldani A., Duebbert M., Büschges A., and Fiorito G. (2019). From injury to full repair: nerve regeneration and functional recovery in the common octopus, Octopus vulgaris. J. Exp. Biol. 222, jeb209965. doi: 10.1242/jeb.209965
Imperadore P., Shah S. B., Makarenkova H. P., and Fiorito G. (2017). Nerve degeneration and regeneration in the cephalopod mollusk Octopus vulgaris: the case of the pallial nerve. Sci. Rep. 7, 46564. doi: 10.1038/srep46564
Imran M. A. S., Carrera M., Pérez-Polo S., Pérez J., Barros L., Dios S., et al. (2023a). Insights into Common Octopus (Octopus vulgaris) Ink Proteome and Bioactive Peptides Using Proteomic Approaches. Mar Drugs 21, 206. doi: 10.3390/md21040206
Ito T., Kimura Y., Uozumi Y., Takai M., Muraoka S., Matsuda T., et al. (2008). Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J. Mol. Cell Cardiol. 44, 927–937. doi: 10.1016/j.yjmcc.2008.03.001
Jia R. and Chen J. (2012). Research on extraction of taurine from octopus waste. Food Industry 33, 9–11.
Jiang K., Hu Y., Gao X., Zhan C., Zhang Y., Yao S., et al. (2019). Octopus-like flexible vector for noninvasive intraocular delivery of short interfering nucleic acids. Nano Lett. 19, 6410–6417. doi: 10.1021/acs.nanolett.9b02596
Jong C. J., Sandal P., and Schaffer S. W. (2021). The role of taurine in mitochondria health: more than just an antioxidant. Mol. (basel Switz.) 26, 4913. doi: 10.3390/molecules26164913
Ju M., Qiao C., Xu Y., Li F., Liu N., Liu Y., et al. (2024). Inhibitory effect of taurine on myocardial fibrosis. J. Beihua University(Natural Science) 25, 615–619. doi: 10.11713/j.issn.1009-4822.2024.05.009
Kaur G., Mason R. P., Steg P. G., and Bhatt D. L. (2024). Omega-3 fatty acids for cardiovascular event lowering. Eur. J. preventive Cardiol. 31 (8), 1005–1014. doi: 10.1093/eurjpc/zwae003
Key L. N., Boyle P. R., and Jaspars M. (2002). Novel activities of saliva from the octopus Eledone cirrhosa (Mollusca; Cephalopoda). Toxicon 40, 677–683. doi: 10.1016/s0041-0101(01)00254-9
Kim Y.-H., Kim S. M., and Lee S. Y. (2015). Antimicrobial activity of protamine against oral microorganisms. Biocontrol Sci. 20, 275–280. doi: 10.4265/bio.20.275
Kim Y.-J., Lee J. H., Jung S.-H., Kim K. H., Choi C.-H., Jo S., et al. (2022). An octopus-derived peptide with antidiuretic activity in rats. Mar Drugs 20, 328. doi: 10.3390/md20050328
Kruppke B., Farack J., Weil S., Aflalo E. D., Poláková D., Sagi A., et al. (2020a). Crayfish hemocyanin on chitin bone substitute scaffolds promotes the proliferation and osteogenic differentiation of human mesenchymal stem cells. J. BioMed. Mater Res. A 108, 694–708. doi: 10.1002/jbm.a.36849
Kruppke B., Heinemann C., Farack J., Weil S., Aflalo E. D., Sagi A., et al. (2020b). Hemocyanin modification of chitosan scaffolds with calcium phosphate phases increase the osteoblast/osteoclast activity ratio-A co-culture study. Molecules 25, 4580. doi: 10.3390/molecules25194580
Kumar M., Kumar D., Garg Y., Mahmood S., Chopra S., and Bhatia A. (2023). Marine-derived polysaccharides and their therapeutic potential in wound healing application - A review. Int. J. Biol. Macromol 253, 127331. doi: 10.1016/j.ijbiomac.2023.127331
Lee J., Hwang G. W., Lee B. S., Park N.-J., Kim S.-N., Lim D., et al. (2024a). Artificial octopus-limb-like adhesive patches for cupping-driven transdermal delivery with nanoscale control of stratum corneum. ACS Nano. 18, 5311–5321. doi: 10.1021/acsnano.3c09304
Lee Y.-E., Kim H., Seo C., Park T., Lee K. B., Yoo S.-Y., et al. (2017). Marine polysaccharides: therapeutic efficacy and biomedical applications. Arch. Pharm. Res. 40, 1006–1020. doi: 10.1007/s12272-017-0958-2
Lee J., Park H.-K., Hwang G. W., Kang G. R., Choi Y. S., and Pang C. (2024b). Highly adaptive kirigami-metastructure adhesive with vertically self-aligning octopus-like 3D suction cups for efficient wet adhesion to complexly curved surfaces. ACS Appl. Mater Interfaces 16, 37147–37156. doi: 10.1021/acsami.4c03363
Lei X., Zhao S., Wu H., Peng X., and Yang Z. (2007). Purification of glycosaminoglycan extracted from octopus dollfusi and effect on proliferation of mouse splenocytes. J. South China Univ. Technology(Natural Sci. Edition) (11), 120–124. doi: 10.3321/j.issn:1000-565x.2007.11.025
Lei X., Zhao S., Yang Z., Fan X., and Wu H. (2006). The nutrient analysis and evaluation of octopus dollfusi in south China sea. Acta Nutrimenta Sin. (01), 58–61. doi: 10.3321/j.issn:0512-7955.2006.01.016
Li Y., Li L., Wei S., Yao J., Liang B., Chu X., et al. (2024). Integrating transcriptomics and metabolomics to elucidate the mechanism by which taurine protects against DOX-induced depression. Sci. Rep. 14, 2686. doi: 10.1038/s41598-023-51138-5
Li R., Zhou Y., Zhang X., Yang L., Liu J., Wightman S. M., et al. (2023). Identification of marine natural product Pretrichodermamide B as a STAT3 inhibitor for efficient anticancer therapy. Mar Life Sci. Technol. 5, 94–101. doi: 10.1007/s42995-022-00162-x
Lim Y.-S., Ok Y.-J., Hwang S.-Y., Kwak J.-Y., and Yoon S. (2019). Marine collagen as A promising biomaterial for biomedical applications. Mar Drugs 17, 467. doi: 10.3390/md17080467
Liu H., Ding N., Tian L., Song X., Li X., and Zhou N. (2022). Therapeutic effect and possible mechanism of taurine on osteoarthritis rats. Prog. Anatomical Sci. 28, 471–474. doi: 10.16695/j.cnki.1006-2947.2022.04.024
Luo Z., Klein Cerrejon D., Römer S., Zoratto N., and Leroux J.-C. (2023). Boosting systemic absorption of peptides with a bioinspired buccal-stretching patch. Sci. Transl. Med. 15, eabq1887. doi: 10.1126/scitranslmed.abq1887
Ma Z., Xu S., Chen Y., Jia X., and Wu W. (2011). Analysis of nutritive composition and amino acids contents in muscle of octopus vulgaris. J. Anhui Agric. Sci. 39, 1059–1061. doi: 10.13989/j.cnki.0517-6611.2011.02.166
Marcus M. D. and Link M. S. (2024). Omega-3 fatty acids and arrhythmias. Circulation 150, 488–503. doi: 10.1161/CIRCULATIONAHA.123.065769
Maselli V., Galdiero E., Salzano A. M., Scaloni A., Maione A., Falanga A., et al. (2020). OctoPartenopin: identification and preliminary characterization of a novel antimicrobial peptide from the suckers of octopus vulgaris. Marine Drugs 18, 380. doi: 10.3390/md18080380
McDonald N. M. and Cottrell G. A. (1972). Purification and mode of action of toxin from Eledone cirrosa. Comp. Gen. Pharmacol. 3, 244–248. doi: 10.1016/0010-4035(72)90033-x
McGaunn J. and Baur J. A. (2023). Taurine linked with healthy aging. Science 380, 1010–1011. doi: 10.1126/science.adi3025
Minakata H., Shigeno S., Kano N., Haraguchi S., Osugi T., and Tsutsui K. (2009). Octopus gonadotrophin-releasing hormone: a multifunctional peptide in the endocrine and nervous systems of the cephalopod. J. Neuroendocrinol 21, 322–326. doi: 10.1111/j.1365-2826.2009.01852.x
Ministry Agriculture and Rural Affairs of the People‘s Republic of China, National Fisheries Technology Extension Center, and China Society of Fisheries (2024). 2024 China Fishery Statistical Yearbook (Beijing: China Agriculture Press).
Morales J., Montero P., and Moral A. (2000). Isolation and partial characterization of two types of muscle collagen in some cephalopods. J. Agric. Food Chem. 48, 2142–2148. doi: 10.1021/jf990711k
Mora Román J. J., Del Campo M., Villar J., Paolini F., Curzio G., Venuti A., et al. (2019). Immunotherapeutic potential of mollusk hemocyanins in combination with human vaccine adjuvants in murine models of oral cancer. J. Immunol. Res. 2019, 7076942. doi: 10.1155/2019/7076942
Negi B., Kumar D., and Rawat D. S. (2017). Marine peptides as anticancer agents: A remedy to mankind by nature. Curr. Protein Pept. Sci. 18, 885–904. doi: 10.2174/1389203717666160724200849
Nikapitiya C., Dananjaya S. H. S., Chandrarathna H. P. S. U., De Zoysa M., and Whang I. (2020). Octominin: A novel synthetic anticandidal peptide derived from defense protein of octopus minor. Mar Drugs 18, 56. doi: 10.3390/md18010056
Oliveira H., Muniz J. A., Bandarra N. M., Castanheira I., Coelho I. R., Delgado I., et al. (2019). Effects of industrial boiling on the nutritional profile of common octopus (Octopus vulgaris). Foods 8, 411. doi: 10.3390/foods8090411
Onozato M., Horinouchi M., Yoshiba Y., Sakamoto T., Sugasawa H., and Fukushima T. (2024). Determination of imidazole dipeptides and related amino acids in natural seafoods by liquid chromatography-tandem mass spectrometry using a pre-column derivatization reagent. Foods 13, 1951. doi: 10.3390/foods13121951
Ospina-Alvarez A., de Juan S., Pita P., Ainsworth G. B., Matos F. L., Pita C., et al. (2022). A network analysis of global cephalopod trade. Sci. Rep. 12, 322. doi: 10.1038/s41598-021-03777-9
Ozogul Y., Duysak O., Ozogul F., Özkütük A. S., and Türeli C. (2008). Seasonal effects in the nutritional quality of the body structural tissue of cephalopods. Food Chem. 108, 847–852. doi: 10.1016/j.foodchem.2007.11.048
Paulose S. K. and Chakraborty K. (2022). Anti-hyperglycemic Δ5 steroids, marginoids A-C from marine veined octopus Amphioctopus marginatus (Octopodidae): Prospective natural leads inhibit serineexopeptidase dipeptidyl peptidase-4. Steroids 186, 109090. doi: 10.1016/j.steroids.2022.109090
Pekala J., Patkowska-Sokoła B., Bodkowski R., Jamroz D., Nowakowski P., Lochyński S., et al. (2011). L-carnitine–metabolic functions and meaning in humans life. Curr. Drug Metab. 12, 667–678. doi: 10.2174/138920011796504536
Peng B., Cai B., and Pan J. (2022). Octopus-derived antioxidant peptide protects against hydrogen peroxide-induced oxidative stress in IEC-6 cells. Food Sci. Nutr. 10, 4049–4058. doi: 10.1002/fsn3.3000
Pham T. K., Nguyen T. H. T., Yi J. M., Kim G. S., Yun H. R., Kim H. K., et al. (2023). Evogliptin, a DPP-4 inhibitor, prevents diabetic cardiomyopathy by alleviating cardiac lipotoxicity in db/db mice. Exp. Mol. Med. 55, 767–778. doi: 10.1038/s12276-023-00958-6
Ping Y., Shan J., Liu Y., Liu F., Wang L., Liu Z., et al. (2023). Taurine enhances the antitumor efficacy of PD-1 antibody by boosting CD8+ T cell function. Cancer Immunol. Immunother 72, 1015–1027. doi: 10.1007/s00262-022-03308-z
Qaradakhi T., Gadanec L. K., McSweeney K. R., Abraham J. R., Apostolopoulos V., and Zulli A. (2020). The anti-inflammatory effect of taurine on cardiovascular disease. Nutrients 12, E2847. doi: 10.3390/nu12092847
Qian H., Kang X., Hu J., Zhang D., Liang Z., Meng F., et al. (2020). Reversing a model of parkinson’s disease with in situ converted nigral neurons. Nature 582, 550–556. doi: 10.1038/s41586-020-2388-4
Qin Z., Babu V. S., Wan Q., Muhammad A., Li J., Lan J., et al. (2018). Antibacterial activity of hemocyanin from red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 75, 391–399. doi: 10.1016/j.fsi.2018.02.010
Qiu W., Chen S., Wang J., and Qu Y. (2009). Weight reducing effect of octopamine on nutritional obesity mice. Hunan Agricultural Sciences. 2009 (12), 15–17. doi: 10.16498/j.cnki.hnnykx.2009.12.035
Qu Y. (2016). Research progress on octopamine. Shandong Chemcal Industry 45, 51–52. doi: 10.19319/j.cnki.issn.1008-021x.2016.12.019
Qu A., Sun M., Kim J.-Y., Xu L., Hao C., Ma W., et al. (2021). Stimulation of neural stem cell differentiation by circularly polarized light transduced by chiral nanoassemblies. Nat. Biomed. Eng. 5, 103–113. doi: 10.1038/s41551-020-00634-4
Reich G. (1992). A new peptide of the oxytocin/vasopressin family isolated from nerves of the cephalopod Octopus vulgaris. Neurosci. Lett. 134, 191–194. doi: 10.1016/0304-3940(92)90514-8
Rigogliuso S., Campora S., Notarbartolo M., and Ghersi G. (2023). Recovery of bioactive compounds from marine organisms: focus on the future perspectives for pharmacological, biomedical and regenerative medicine applications of marine collagen. Molecules 28, 1152. doi: 10.3390/molecules28031152
Salvatore L., Gallo N., Natali M. L., Campa L., Lunetti P., Madaghiele M., et al. (2020). Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater Sci. Eng C Mater Biol. Appl. 113, 110963. doi: 10.1016/j.msec.2020.110963
Serefko A., Jach M. E., Pietraszuk M., Świąder M., Świąder K., and Szopa A. (2024). Omega-3 polyunsaturated fatty acids in depression. Int. J. Mol. Sci. 25, 8675. doi: 10.3390/ijms25168675
Shaw T. J., Osborne M., Ponte G., Fiorito G., and Andrews P. L. R. (2016). Mechanisms of wound closure following acute arm injury in Octopus vulgaris. Zoological Lett. 2, 8. doi: 10.1186/s40851-016-0044-5
Singh P., Gollapalli K., Mangiola S., Schranner D., Yusuf M. A., Chamoli M., et al. (2023). Taurine deficiency as a driver of aging. Science 380, eabn9257. doi: 10.1126/science.abn9257
Songdahl J. H. and Shapiro B. I. (1974). Purification and composition of a toxin from the posterior salivary gland of Octopus dofleini. Toxicon 12, 109–115. doi: 10.1016/0041-0101(74)90234-7
Sorushanova A., Delgado L. M., Wu Z., Shologu N., Kshirsagar A., Raghunath R., et al. (2019). The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv. Mater 31, e1801651. doi: 10.1002/adma.201801651
Sudhakar S. and Nazeer R. A. (2017). In vitro preparation and assessment of radical reducing peptide from Octopus aegina using digestive proteases. J. Biosci Bioeng 124, 36–42. doi: 10.1016/j.jbiosc.2017.02.014
Tajbakhsh E., Khamesipour A., Hosseini S. R., Kosari N., Shantiae S., and Khamesipour F. (2021). The effects of medicinal herbs and marine natural products on wound healing of cutaneous leishmaniasis: A systematic review. Microb Pathog 161, 105235. doi: 10.1016/j.micpath.2021.105235
Takatani T., Takahashi K., Uozumi Y., Matsuda T., Ito T., Schaffer S. W., et al. (2004). Taurine prevents the ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes through Akt/caspase-9 pathway. Biochem. Biophys. Res. Commun. 316, 484–489. doi: 10.1016/j.bbrc.2004.02.066
Takuwa-Kuroda K., Iwakoshi-Ukena E., Kanda A., and Minakata H. (2003). Octopus, which owns the most advanced brain in invertebrates, has two members of vasopressin/oxytocin superfamily as in vertebrates. Regul. Pept. 115, 139–149. doi: 10.1016/s0167-0115(03)00151-4
Tvrzicka E., Kremmyda L.-S., Stankova B., and Zak A. (2011). Fatty acids as biocompounds: their role in human metabolism, health and disease–a review. Part 1: classification, dietary sources and biological functions. BioMed. Pap Med. Fac Univ Palacky Olomouc Czech Repub 155, 117–130. doi: 10.5507/bp.2011.038
Ueda A., Nagai H., Ishida M., Nagashima Y., and Shiomi K. (2008). Purification and molecular cloning of SE-cephalotoxin, a novel proteinaceous toxin from the posterior salivary gland of cuttlefish Sepia esculenta. Toxicon 52, 574–581. doi: 10.1016/j.toxicon.2008.07.007
Wang C. (2012). Study on the determination of Cartinine, Octopine in octopus (Qingdao: Ocean University of China).
Wang L., Dong C., Li X., Han W., and Su X. (2017). Anticancer potential of bioactive peptides from animal sources (Review). Oncol. Rep. 38, 637–651. doi: 10.3892/or.2017.5778
Wen S., Zhao X., Gao Y., and Yu G. (2010). Extraction,separation and physicochemical properties analyses of Octopus ocellatus polysaccharides. Chin. J. Marine Drugs 29, 24–31. doi: 10.13400/j.cnki.cjmd.2010.01.009
Wójcik O. P., Koenig K. L., Zeleniuch-Jacquotte A., Costa M., and Chen Y. (2010). The potential protective effects of taurine on coronary heart disease. Atherosclerosis 208, 19–25. doi: 10.1016/j.atherosclerosis.2009.06.002
Wu J., Cai X., Tang M., and Wang S. (2019). Novel calcium-chelating peptides from octopus scraps and their corresponding calcium bioavailability. J. Sci. Food Agric. 99, 536–545. doi: 10.1002/jsfa.9212
Wu X., Deng J., Jian W., Yang Y., Shao H., Zhou X., et al. (2024). A bioinspired switchable adhesive patch with adhesion and suction mechanisms for laparoscopic surgeries. Mater Today Bio 27, 101142. doi: 10.1016/j.mtbio.2024.101142
Xie Z., Yuan F., Liu J., Tian L., Chen B., Fu Z., et al. (2023). Octopus-inspired sensorized soft arm for environmental interaction. Sci. Rob. 8, eadh7852. doi: 10.1126/scirobotics.adh7852
Xie Q., Zhang Y., Chen H., Hong Z., Chen W., and Fang H. (2016). Development of octopine certified reference material. Chem. Anal. Meterage 25, 29. doi: 10.3969/j.issn.1008–6145.2016.03.001
Xu S., Xu H., Wang W., Li S., Li H., Li T., et al. (2019). The role of collagen in cancer: from bench to bedside. J. Transl. Med. 17, 309. doi: 10.1186/s12967-019-2058-1
Xue J., Ma J., Zhang X., Wang H., Chen S., and Dai Z. (2015). Analysis and quality evaluation of nutritional components in the muscle of two kinds of octopus. J. Chin. Institute Of Food Sci. Technol. 15, 203–211. doi: 10.16429/j.1009-7848.2015.12.028
Yang H., Zhang Q., Zhang B., Zhao Y., and Wang N. (2023). Potential active marine peptides as anti-aging drugs or drug candidates. Mar Drugs 21, 144. doi: 10.3390/md21030144
Yu X., Liu X., and Zhou D. (2024). A critical review of a typical research system for food-derived metal-chelating peptides: production, characterization, identification, digestion, and absorption. Compr. Rev. Food Sci. Food Saf. 23, e13277. doi: 10.1111/1541-4337.13277
Yu M., Ning J., and Pu M. (2011). Optimization technology of the enzymatic octopus by-product. Acad. Periodical Farm Products Process. (06), 52–54. doi: 10.3969/j.issn.1671-9646(X).2011.06.014
Yu X., Niu W., Wang Y.-Y., Olaleye O. E., Wang J.-N., Duan M.-Y., et al. (2022). Novel assays for quality evaluation of XueBiJing: Quality variability of a Chinese herbal injection for sepsis management. J. Pharm. Anal. 12, 664–682. doi: 10.1016/j.jpha.2022.01.001
Zemrani B. and Bines J. E. (2020). Recent insights into trace element deficiencies: causes, recognition and correction. Curr. Opin. Gastroenterol. 36, 110–117. doi: 10.1097/MOG.0000000000000612
Zhang Y. (2007). Research on resource development of octopus processing leftovers. China Sci. Technol. Inf. (24), 64–65. doi: 10.3969/j.issn.1001-8972.2007.24.034
Zhang W. and Lei X. (2006). Analysis and evaluation of nutritional components in different tissues of Octopus ocellatus. J. Zhanjiang Ocean Univ. (04), 91–93. doi: 10.3969/j.issn.1673-9159.2006.04.020
Zhang Y., Yan F., Hu Z., Zhao X., Min S., Du Z., et al. (2009). Hemocyanin from shrimp Litopenaeus vannamei shows hemolytic activity. Fish Shellfish Immunol. 27, 330–335. doi: 10.1016/j.fsi.2009.05.017
Zheng X. (2018). Study on Fish Oil Extraction Technology of OctopusBy-product (Baoding: Hebei Agricultural University).
Zheng W., Cheng H., Meng X., and Li S. (2011). Analysis and evaluation of nutrient composition of Octopus variabilis in inshore waters of Haizhou Bay. Prog. Fishery Sci. 32, 57–62. doi: 10.3969/j.issn.1000-7075.2011.03.009
Zheng X., Lv Y., and Lu C. (2023). Species diversity of cephalopods in the China seas. Periodical Ocean Univ. China 53, 1–18. doi: 10.16441/j.cnki.hdxb.20220247
Zhong Q., Wei B., Wang S., Ke S., Chen J., Zhang H., et al. (2019). The antioxidant activity of polysaccharides derived from marine organisms: an overview. Mar Drugs 17, 674. doi: 10.3390/md17120674
Zhou Q. and Wei Z. (2023). Food-grade systems for delivery of DHA and EPA: opportunities, fabrication, characterization and future perspectives. Crit. Rev. Food Sci. Nutr. 63, 2348–2365. doi: 10.1080/10408398.2021.1974337
Zhou J., Yan J., Bai Z., Li K., and Huang K. (2015). Hypoglycemic activity and potential mechanism of a polysaccharide from the loach in streptozotocin-induced diabetic mice. Carbohydr Polym 121, 199–206. doi: 10.1016/j.carbpol.2014.12.037
Zhu Y., Wang R., Fan Z., Luo D., Cai G., Li X., et al. (2023a). Taurine alleviates chronic social defeat stress-induced depression by protecting cortical neurons from dendritic spine loss. Cell Mol. Neurobiol. 43, 827–840. doi: 10.1007/s10571-022-01218-3
Keywords: octopus, global distribution, extraction methods, bioactive biomaterials, biomedical applications
Citation: Li M, Deng Z, Cheng K, Fu X and Li X (2025) Marine octopus: global distribution, extraction methods, bioactive biomaterials, and biomedical applications. Front. Mar. Sci. 12:1615994. doi: 10.3389/fmars.2025.1615994
Received: 23 April 2025; Accepted: 19 May 2025;
Published: 03 June 2025.
Edited by:
Santhiyagu Prakash, Tamil Nadu Fisheries University, IndiaReviewed by:
Rajesh Rajaian Pushpabai, Chettinad University, IndiaRanjithkumar Murugesan, Syed Ammal Arts And Science College, India
Manikandan Gurusamy, Tshwane University of Technology, South Africa
Copyright © 2025 Li, Deng, Cheng, Fu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianjun Fu, eGlhbnhpdUBob3RtYWlsLmNvbQ==; Xiuxue Li, bGl4aXV4dWVAc2R1dGNtLmVkdS5jbg==
 Mengfei Li1,2,3
Mengfei Li1,2,3 Xianjun Fu
Xianjun Fu Xiuxue Li
Xiuxue Li