Abstract
The escalating global demand for sustainable energy has stimulated research into renewable alternatives, with microalgae emerging as a promising feedstock for biodiesel production. This study evaluates the potential of two microalgal species, Tetradesmus obliquus and Monoraphidium sp., for efficient and sustainable biodiesel synthesis. These species were selected based on their high lipid content, favorable fatty acid profiles, and adaptability to diverse cultivation conditions. Growth optimization experiments under varying nitrogen-to-phosphorus (N:P) ratios and light intensities revealed that balanced N:P ratios (1:1) and moderate light intensity (2.6 klux) significantly enhanced biomass and lipid yields in both species. Monoraphidium sp. achieved higher dry biomass productivity (0.43 g L−¹) and lipid accumulation (50.6%) than T. obliquus (0.105 g L−¹ and 41.8%, respectively). Gas chromatography–mass spectrometry (GC–MS) analysis of fatty acid methyl esters (FAMEs) confirmed the presence of key biodiesel components, including methyl palmitate and methyl oleate, in both species. Biodiesel quality predictions using specialized software indicated that T. obliquus exhibited better oxidative stability and cold flow properties, making it more versatile for varying climates. Monoraphidium sp., however, had higher cetane numbers but poorer cold flow performance. Collectively, these findings highlight the potential of T. obliquus and Monoraphidium sp. as viable candidates for biodiesel production, emphasizing their distinct advantages depending on application requirements. Further research into scalable cultivation and cost-effective extraction methods is essential for industrial implementation.
1 Introduction
Microalgae, a diverse subset of photosynthetic organisms that play a crucial role in various ecological and industrial applications (Mofeed and Mosleh, 2013; Gurau et al., 2025). Ranging from microscopic microalgae to large macroalgae, they are fundamental to aquatic ecosystems, forming the base of food webs and contributing significantly to global oxygen production (Naselli-Flores and Padisák, 2023). Beyond their ecological importance, algae have garnered attention for their potential in biotechnology, including applications in pharmaceuticals, nutraceuticals, wastewater treatment, and notably, energy production (El Basuini et al., 2023; Samoraj et al., 2024). Their rapid growth rates, high biomass yields, and ability to produce valuable metabolites make them promising candidates for sustainable energy solutions (Bora et al., 2024).
The escalating global demand for energy, coupled with concerns over environmental degradation and climate change, has intensified the search for renewable and environmentally friendly energy sources (Sharif et al., 2024). Traditional fossil fuels are finite and their combustion releases significant amounts of greenhouse gases (Wang and Azam, 2024). In contrast, renewable energy sources such as solar, wind, and biofuels offer sustainable alternatives that can mitigate environmental impacts (Karlilar Pata and Pata, 2025). Biofuels, particularly biodiesel, have attracted interest due to their compatibility with existing diesel engines and infrastructure, as well as their potential to reduce carbon emissions (Wan Osman et al., 2024). However, the sustainability of biodiesel production depends largely on the choice of feedstock.
Microalgae are superior feedstocks for biodiesel due to their high lipid content and rapid growth rates (Abdel-Aal and Mofeed, 2015; Sharma et al., 2025). Among the various microalgal species, Tetradesmus obliquus and Monoraphidium sp. have shown particular promise. Tetradesmus obliquus is a unicellular green alga known for its adaptability to diverse environmental conditions and its capacity for substantial lipid accumulation (He et al., 2017). Studies have demonstrated that under nitrogen-deficient conditions, T. obliquus can achieve lipid contents up to 43% of its dry cell weight, with a lipid yield of 2.0 g/L, making it a viable candidate for biodiesel production (Mandal and Mallick, 2009). The fatty acid profile of T. obliquus is predominantly composed of C16 and C18 fatty acids, which are ideal for biodiesel synthesis (Bagchi and Mallick, 2016).
Complementarily, Monoraphidium sp., another microalga, has been identified as a potential feedstock for biodiesel (Khoo et al., 2023). Research indicates that this species possesses a favorable lipid profile, with a significant proportion of saturated fatty acids like palmitic acid (C16:0) and unsaturated fatty acids such as oleic acid (C18:1) and linolenic acid (C18:3). These fatty acids contribute to the production of biodiesel that meets established standards, highlighting the suitability of Monoraphidium sp. for biofuel applications (Díaz et al., 2015).
While numerous studies have characterized microalgae for biodiesel production, most have focused on either biomass productivity or lipid content alone, often neglecting a comprehensive evaluation of fuel quality parameters under uniform culture conditions. Moreover, cold flow properties such as cold filter plugging point (CFPP), which are critical for biodiesel usability in colder climates, remain underreported for these species. There is also limited comparative data systematically examines multiple microalgal strains under standardized protocols to draw meaningful conclusions about their suitability for scalable biofuel production.
The objective of this study is to address these gaps by evaluating and comparing the growth kinetics, lipid accumulation, and full fuel property profiles including cetane number, iodine value, and CFPP of Tetradesmus obliquus and Monoraphidium sp. cultivated under identical conditions. By integrating both physiological and biodiesel quality assessments, this research contributes to a more holistic understanding of microalgal biofuel potential and provides valuable insights for future biorefinery development and strain selection.
2 Materials and methods
2.1 Microalgae culture conditions
Pure strains of Tetradesmus obliquus and Monoraphidium sp. were isolated from two different locations in the Damietta branch of Nile River, Mansoura, Egypt (31°2′25″N 31°22′58″E). Tetradesmus obliquus was cultured in Bold’s Basal Medium (BBM), while Monoraphidium sp. was cultivated in Mannose Binding Lectin (MBL) medium. The selection of BBM and MBL media was based on preliminary experiments conducted to determine optimal growth and lipid accumulation conditions for each strain. BBM supported higher lipid yields in T. obliquus, whereas MBL was more effective for Monoraphidium sp. Both media were prepared using distilled water and sterilized by autoclaving at 121°C for 15 minutes. Cultures were maintained in 5-liter flasks under standard conditions: 25 ± 1°C, a 16:08 h light-dark photoperiod, and illumination of approximately 1.2 klux at the flask surface, provided by cool white fluorescent lamps, over a 28-day cultivation period. To promote homogeneity and prevent sedimentation, cultures received continuous filtered aeration and were manually mixed twice daily. Growth was monitored by daily measurements of optical density at 640 nm using a microplate spectrophotometer (Infinite 200 PRO series, TECAN). The pH of the culture medium was continuously monitored using a pH sensor (DFRobot Gravity) throughout the entire cultivation period.
2.2 Morphological and molecular identification
Morphological identification of the selected microalgal species was performed using both light microscopy and scanning electron microscopy (SEM) at magnifications of 1000×. Light microscopy (ZEISS PRIMO STAR BINOCULAR) was conducted to examine the general cellular morphology, including cell shape, size, and arrangement. For more detailed surface and structural analysis, scanning electron microscopy was employed using a JEOL JSM 6510 system. Sample preparation for SEM followed the protocol described by Echlin (2009). Algal cells were initially fixed in 2.5% glutaraldehyde in phosphate buffer (pH 7.2) and stored at 4°C overnight. Following fixation, samples were rinsed three times with the same buffer to remove excess fixative. A graded ethanol dehydration series was then applied, involving successive treatments with 50%, 70%, 80%, 90%, and 100% ethanol, each for 15–20 minutes. The final dehydration step used absolute ethanol to ensure complete removal of water. A drop of the dehydrated sample was placed on a carbon-coated copper grid and air-dried using a Sputter Coating Evaporator (SPI Module – Sputter Carbon/Gold Coater) to create a conductive coating. The prepared specimens were then examined under the SEM to obtain high-resolution images for taxonomic comparison with established morphological descriptions.
For molecular identification, genomic DNA was extracted from the algal biomass using a modified CTAB protocol (Hossen et al., 2025). Amplification of the 18S rRNA gene was carried out using PCR with universal eukaryotic primers: the forward primer (5′-AACCTGGTTGATCCTGCCAGT-3′) and the reverse primer (5′-CCTTGTTACGACTTCACCTTCC-3′). The PCR mixture (25 µL final volume) consisted of 2.5 µL of 10× Taq polymerase buffer containing 1 mM MgCl2, 2 µL of 2.5 mM dNTPs, 1 µL of each primer (10 pmol µL−¹), 0.2 µL of Taq DNA polymerase (5 U µL−¹), and ultra-pure water. Amplification was performed using a PTC-100 thermal cycler under the following conditions: initial denaturation at 94°C for 5 minutes, followed by 35 cycles of 94°C for 30 seconds, 52°C for 30 seconds, and 72°C for 1 minute, with a final extension at 72°C for 10 minutes. PCR products were resolved on a 1.5% agarose gel in 0.5× TBE buffer at 120 V for 30 minutes, stained with 0.5 µg cm−³ ethidium bromide, and visualized using a Syngene gel documentation system. Fragment sizes were assessed using a DNA molecular weight marker ranging from 100 to 5000 bp (Fisher, Canada). Amplified DNA fragments were purified using a PCR clean-up column kit (Maxim Biotech Inc., USA) according to the manufacturer’s instructions, involving membrane binding, ethanol-based washing, and elution. The purified PCR products were stored at –20°C until further analysis.
Sequencing of the purified products was conducted using the forward primer and the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) on a 3130xl Genetic Analyzer (Applied Biosystems). The resulting nucleotide sequences were subjected to Basic Local Alignment Search Tool (BLAST) analysis against the NCBI GenBank database to confirm the identity of the isolates. Multiple sequence alignments were carried out using CLUSTALW version 1.83 (Chenna et al., 2003), and phylogenetic relationships were inferred using the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) implemented in MEGA version 12 (Kumar et al., 2018), allowing for the placement of the studied isolates within a broader evolutionary context.
2.3 Growth optimization and biomass determination
To determine the optimal conditions for biomass and lipid production, the growth performance of Tetradesmus obliquus and Monoraphidium sp. was evaluated under different nitrogen-to-phosphorus (N:P) mass ratios (1:1, 1:4, 1:0.5, and 4:1[control]) and varying light intensities (1.2 [control], 2.6, 3.0, and 4.2 klux). The microalgae were cultivated in 250 mL Erlenmeyer flasks containing 100 mL of Bold’s Basal Medium (BBM) for T obliquus and Mannose Binding Lectin (MBL) medium for Monoraphidium sp., respectively. Each flask was inoculated with 20 mL of a two-week-old algal culture with an initial dry biomass of approximately 0.05 g L−¹. The cultures were maintained under controlled conditions (25 ± 1°C) with a 16:8 h light: dark photoperiod for a total duration of seven weeks. Growth was monitored daily through direct cell counting using a ZEISS PRIMO STAR binocular light microscope and by measuring optical density at 640 nm using a microplate spectrophotometer (Infinite® 200 PRO series, TECAN Group Ltd., Switzerland).
Biomass yield was quantified at the end of the cultivation period by centrifuging the cultures at 4000 rpm for 10 minutes, followed by drying the algal pellets at 60°C until a constant weight was achieved. Biomass was expressed as dry weight (mg DW L−¹), and the specific growth rate (µ, d−¹) was calculated using the formula:
Where W0 is the initial biomass, Wt is the final biomass, and d is the incubation duration in days.
To determine the effect of nutrient availability, different N:P mass ratios (1:4, 1:1, 1:0.5) were tested against a control ratio of 4:1. Cultures were incubated for four weeks under these conditions, and biomass concentration and lipid content were evaluated. Additionally, the impact of light intensity on algal growth was investigated using a range of illumination levels: 1.2 ± 0.2 klux, 2.6 ± 0.2 klux (control), 3.0 ± 0.2 klux, and 4.2 ± 0.2 klux, provided by cool white fluorescent tubes. Following four weeks of cultivation, dry weight biomass and lipid productivity were measured to assess the influence of illumination. All growth experiments were conducted in triplicate to ensure reproducibility and statistical reliability.
2.4 Lipid content, extraction, and GC–MS analysis
Total lipid content was determined using the Soxhlet solvent extraction method. Dried algal biomass (0.5 g) was mixed with a chloroform–methanol (2:1 v/v) solution and sonicated at 40 kHz for 10 minutes to disrupt cell walls. The resulting mixture was centrifuged at 6,000 rpm for 10 minutes, and the organic phase containing lipids was carefully collected. Extracted lipids were weighed to calculate lipid yield as a percentage of dry biomass (Dayananda et al., 2005). For fatty acid analysis, lipid extracts were subjected to gas chromatography–mass spectrometry (GC–MS) to identify and quantify the fatty acid profile. Analysis was carried out using an Agilent GC–MS system equipped with a capillary column (e.g., HP-88), following standard temperature programming. Identification was based on retention times and mass spectra compared to FAME standards.
2.5 Saponification and esterification of lipid extracts
The extracted lipids were converted to fatty acid methyl esters (FAMEs) using a two-step transesterification process (Hartman and Lago, 1973). First, saponification was performed by heating 50 mg of lipid with 2 mL of 0.5 M KOH in methanol at 60°C for 1 hour. After cooling, esterification was carried out by adding 2 mL of 14% boron trifluoride in methanol and heating the mixture again at 60°C for 15 minutes. After the reaction, FAMEs were extracted using hexane, dried over anhydrous sodium sulfate, and stored at −20°C until analysis.
2.6 Fatty acid profiling and biodiesel characterization
The composition of fatty acid methyl esters (FAMEs) derived from algal lipids was analyzed using GC–MS. Analysis was performed with a Thermo Scientific Trace GC Ultra system coupled with an ISQ Single Quadrupole Mass Spectrometer. Separation was achieved using a TG-5MS fused silica capillary column (30 m × 0.25 mm × 0.1 µm film thickness), with helium as the carrier gas at a constant flow rate of 1.0 mL/min. The GC injector and MS transfer line were maintained at 280°C, and electron ionization was carried out at 70 eV. The oven temperature program initiated at 150°C, held for 4 minutes, and ramped to 280°C at a rate of 5°C/min, followed by a final hold for 4 minutes. Samples (1 µL) were injected in split mode. The identification of individual FAMEs, ranging from C14 to C22, was based on comparison of retention times and mass spectral data with entries in the NIST and WILLY libraries integrated into instrument software. Quantification was performed using the relative peak area percentage of each compound.
Following fatty acid profiling, the physicochemical properties of the produced biodiesel were predicted using the Biodiesel Analyzer software, a specialized computational tool developed by Talebi et al. (2014) and validated for assessing biodiesel fuel quality based on fatty acid composition. This software estimated key fuel parameters including density, kinematic viscosity, cetane number, iodine value, acid value, flash point, and pour point. The predicted values were further compared against international biodiesel standards such as ASTM D6751 and EN 14214 to evaluate the suitability of the algal-derived biodiesel for practical fuel applications.
2.7 Statistical analysis
All experimental data were analyzed using one-way analysis of variance (ANOVA) to evaluate significant differences among treatments. Duncan’s multiple range test was employed to separate means at a confidence level of p< 0.05. Statistical analyses were conducted using SPSS software (version 20, IBM, USA).
3 Results
3.1 Morphological and molecular identification
Tetradesmus obliquus exhibited a typical colonial morphology under light microscopy, forming coenobia of four to eight elongated cells with straight margins and rounded apices. The cells were arranged linearly or slightly curved, and the cell wall appeared smooth without visible ornamentation. SEM analysis confirmed the linear arrangement and revealed more defined ultrastructural details, including the smooth surface and compact coenobial structure (Figure 1).
Figure 1
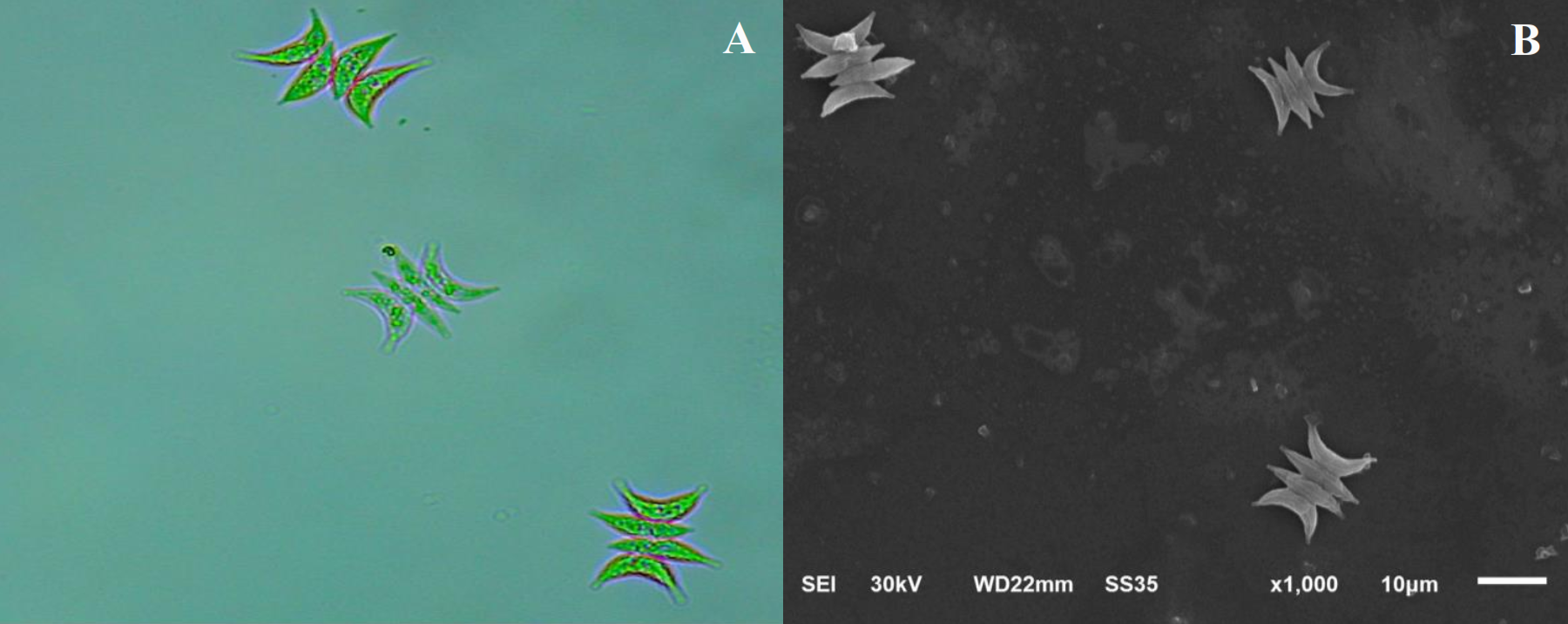
Morphological features of Tetradesmus obliquus at 1,000× magnification. (A) Light microscopy image. (B) Electron microscopy image.
Monoraphidium sp. displayed a unicellular form with a crescent-shaped cell morphology and tapering ends. Light microscopy revealed individual cells dispersed in the medium, while SEM provided further details on cell curvature and surface texture. The cells lacked visible spines or ornamentation and had a smooth exterior, indicative of the genus (Figure 2).
Figure 2
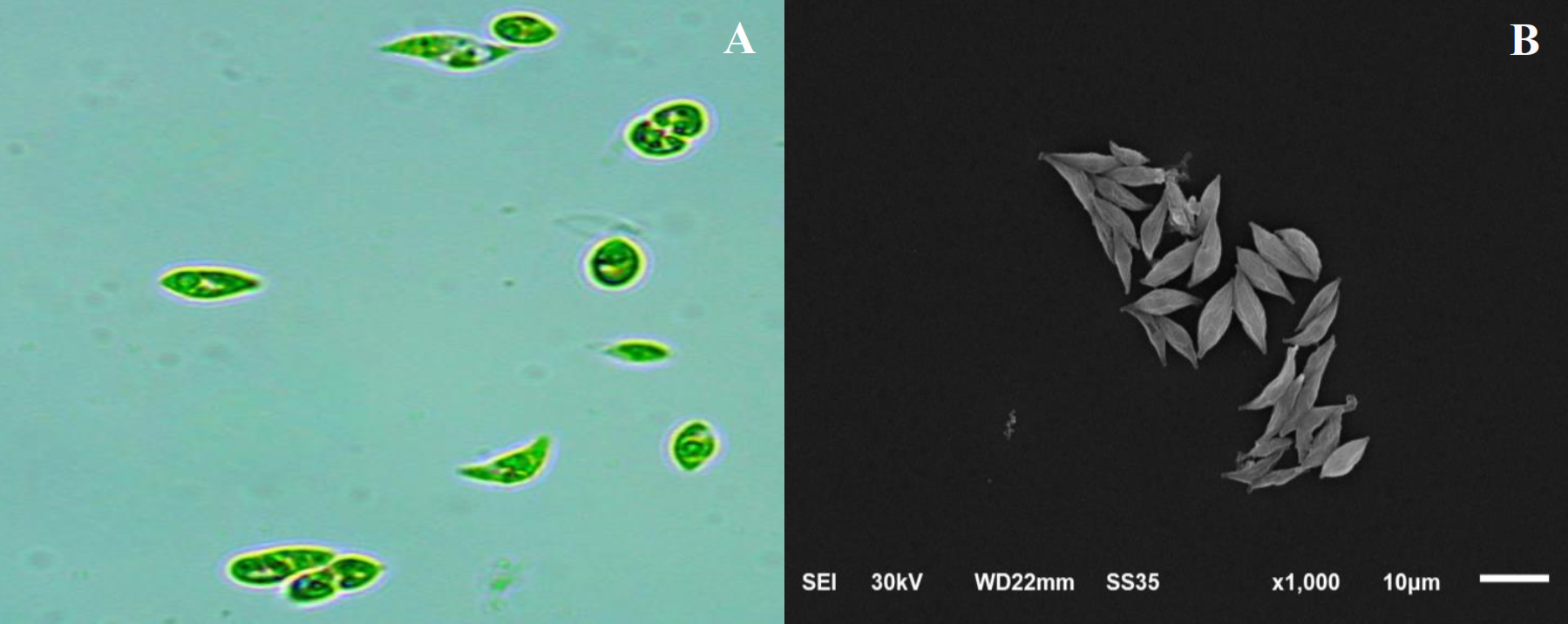
Morphological features of Monoraphidium sp. at 1,000× magnification. (A) Light microscopy image. (B) Electron microscopy image.
Molecular identification based on 18S rRNA gene sequencing confirmed the taxonomic status of both isolates. The nucleotide sequence products of algal DNA were analyzed using NCBI-BLAST for confirming the sequences. The obtained sequences of the two algal isolates were submitted to the GenBank and were given accession numbers, Tetradesmus obliquus (GenBank accession no. PV361776) and Monoraphidium sp. (GenBank accession no. PV300550). Phylogenetic analysis placed the isolates firmly within their respective clades, corroborating morphological findings (Figure 3).
Figure 3
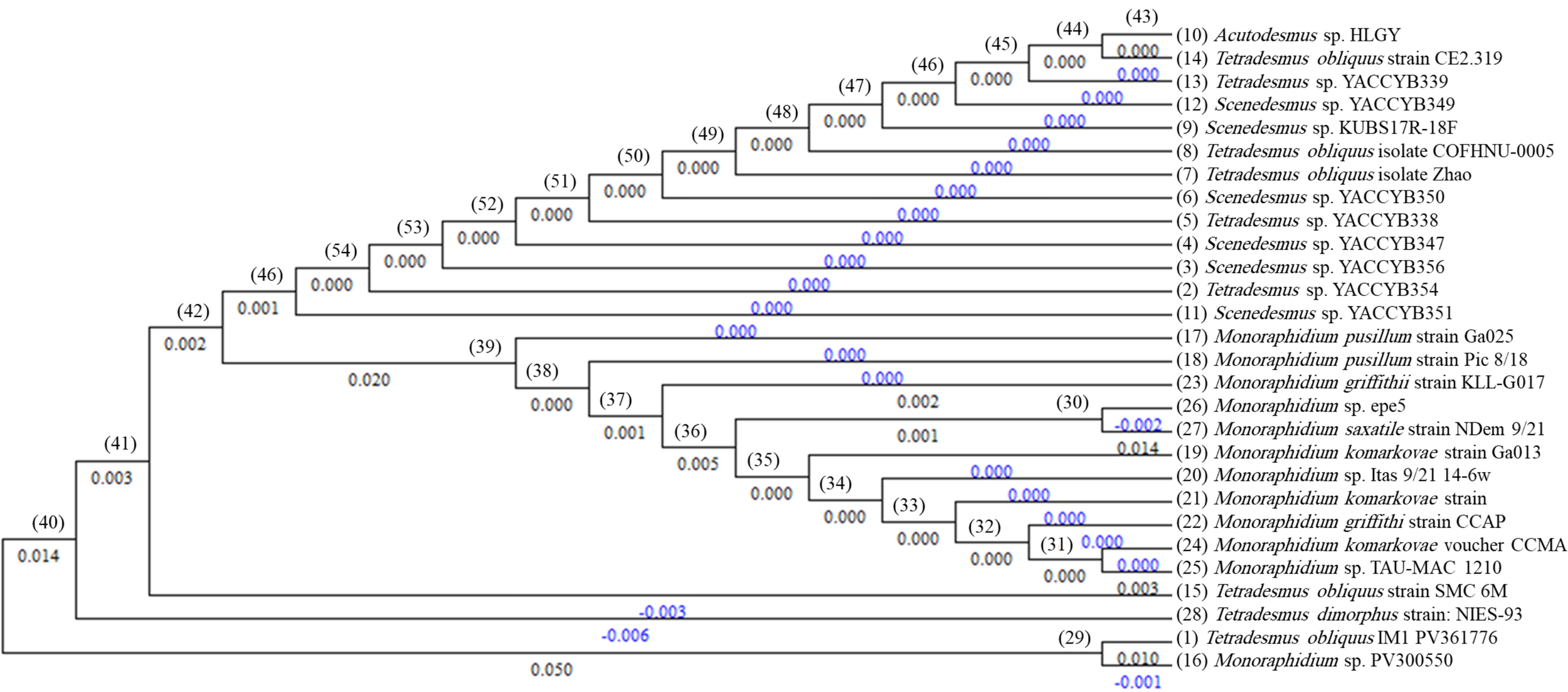
Phylogenetic tree of the two algal isolates identified using 18S rRNA gene universal primers, in comparison with related sequences retrieved from GenBank. The two characterized isolates were Tetradesmus obliquus (Accession No. PV361776) and Monoraphidium sp. (Accession No. PV300550).
3.2 Growth performance
The growth kinetics of T. obliquus and Monoraphidium sp. were evaluated under standard conditions (25 ± 2°C, 1.2 klux, 16:8 h light/dark cycle) are presented in Table 1. The comparative analysis of Tetradesmus obliquus and Monoraphidium sp. over a 28-day cultivation period revealed marked differences in biomass accumulation, lipid productivity, and growth kinetics. Monoraphidium sp. demonstrated superior performance in dry biomass yield, reaching a maximum of 0.177 ± 0.0078 g L−¹ at day 28, compared to 0.047 ± 0.0025 g L−¹ recorded for T. obliquus at the same time point. Lipid content in Monoraphidium sp. also showed a significant increase over time, peaking at 0.368 ± 0.049 g g−¹ on day 28, while T. obliquus achieved a maximum of 0.213 ± 0.028 g g−¹.
Table 1
| Algae strain | Days | Biomass yield (g.L-1) | Lipid production (g.g-1) | µ | Dd-1 | G |
|---|---|---|---|---|---|---|
| Tetradesmus obliquus | 14 | 0.017 ± 0.0037 | 0.043 ± 0.008 | 0.113 ± 0.002 | 0.163 ± 0.003 | 6.15 ± 0.12 |
| 21 | 0.020 ± 0.008 | 0.122 ± 0.013 | 0.066 ± 0.009 | 0.095 ± 0.014 | 10.50 ± 1.75 | |
| 28 | 0.047 ± 0.0025 | 0.213 ± 0.028 | 0.085 ± 0.006 | 0.122 ± 0.008 | 8.17 ± 0.58 | |
| Monoraphidium sp. | 14 | 0.033 ± 0.0061 | 0.061 ± 0.007 | 0.127 ± 0.042 | 0.184 ± 0.061 | 5.44 ± 1.78 |
| 21 | 0.086 ± 0.0017 | 0.167 ± 0.024 | 0.132 ± 0.019 | 0.191 ± 0.027 | 5.23 ± 0.73 | |
| 28 | 0.177 ± 0.0078 | 0.368 ± 0.049 | 0.123 ± 0.015 | 0.177 ± 0.022 | 5.65 ± 0.71 |
Dry biomass yield(g/L), lipid production (g.g-1), specific growth rate (µ), growth doubling per day (Dd-1), and generation time (G) of Tetradesmus obliquus and Monoraphidium sp.
The specific growth rate (µ) was generally higher in Monoraphidium sp., with the highest rate observed on day 21 (0.132 ± 0.019 d−¹), slightly declining by day 28 (0.123 ± 0.015 d−¹), whereas T. obliquus exhibited its peak growth rate earlier, at day 14 (0.113 ± 0.002 d−¹). Correspondingly, the growth doubling per day (Dd−¹) followed a similar pattern, being higher in Monoraphidium sp. (0.191 ± 0.027 d−¹ on day 21) than in T. obliquus (0.163 ± 0.003 d−¹ on day 14). Generation time (G) was shortest in Monoraphidium sp., particularly on day 21 (5.23 ± 0.73 days), while T. obliquus required more time to double its population, especially on day 21 (10.50 ± 1.75 days).
3.3 Optimization of biomass yield and lipid accumulation
The impact of varying nitrogen to phosphorus (N:P) ratios on dry biomass and lipid content was evaluated for Tetradesmus obliquus and Monoraphidium sp., as illustrated in Figure 4. In Tetradesmus obliquus, the dry biomass yield was significantly influenced by the N:P ratio (Figure 4A). The highest dry biomass (0.065 g L−¹) was observed at an N:P ratio of 1:1, followed by 4:1 and 1:0.5, with the lowest biomass (0.038 g L−¹) recorded at the 1:4 ratio. Statistically, the 1:1 ratio was significantly higher than all other treatments (p< 0.05), except 4:1, which showed intermediate values. For Monoraphidium sp., dry biomass followed a different trend (Figure 4B). The 4:1 N:P ratio resulted in the highest biomass production (0.085 g L−¹), which was significantly greater than that of all other treatments (p< 0.05). The lowest biomass (0.054 g L−¹) was found at the 1:4 N:P ratio. In terms of lipid content, Tetradesmus obliquus showed a significant increase at the 1:1 N:P ratio, reaching 34.8% (Figure 4C). All other N:P ratios (1:4, 1:0.5, and 4:1) resulted in significantly lower lipid percentages (19.2–22.5%) and did not differ significantly among each other (p > 0.05). Similarly, Monoraphidium sp. exhibited the highest lipid content (47.3%) at the 1:1 N:P ratio (Figure 4D), which was significantly greater than all other treatments (p< 0.05). The other ratios showed moderate lipid contents ranging between 34.6% and 37.2%, without statistically significant differences between them. These results demonstrate that optimal N:P ratios differ between species for biomass productivity, while a balanced 1:1 N:P ratio generally favors lipid accumulation in both Tetradesmus obliquus and Monoraphidium sp.
Figure 4
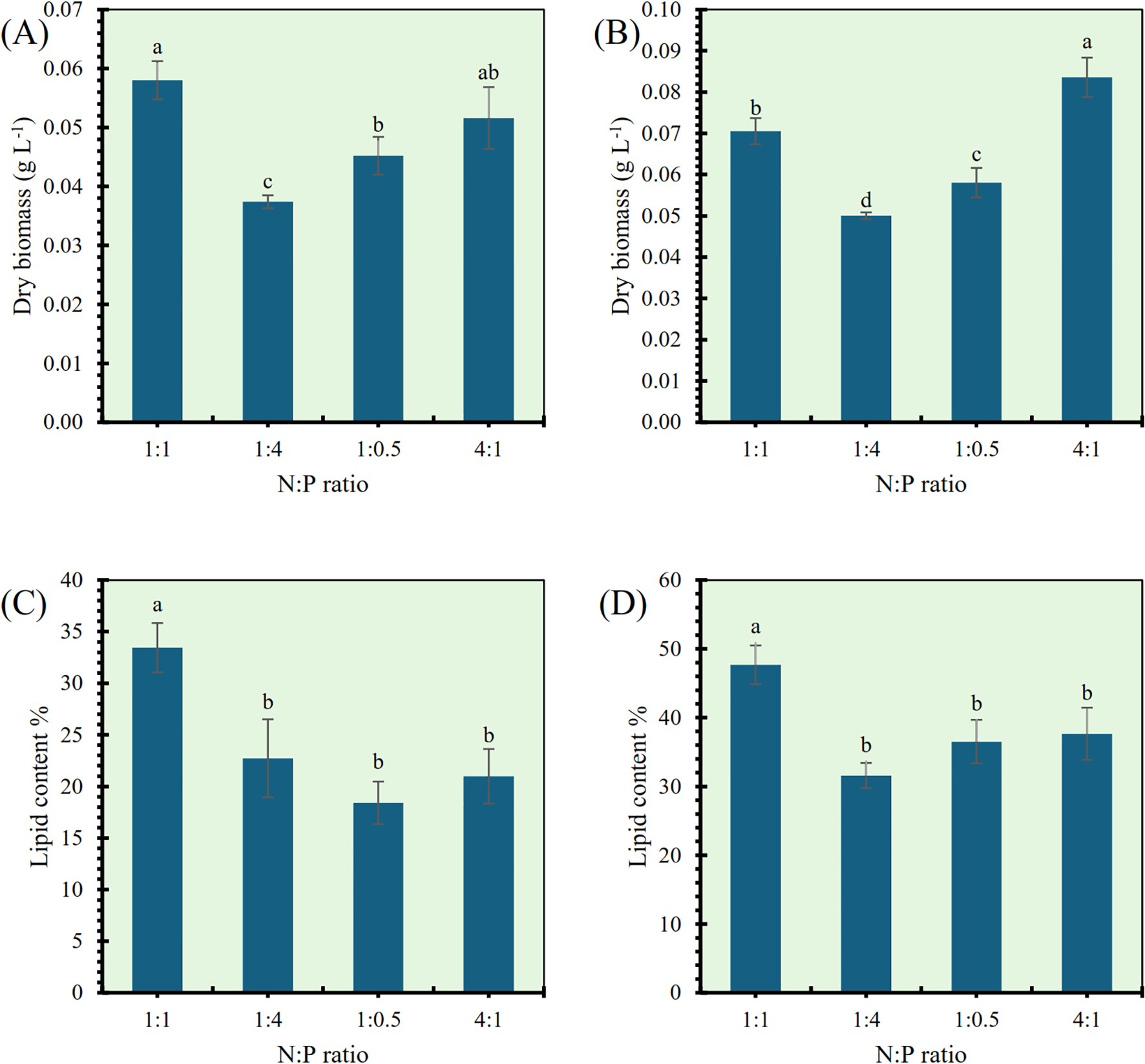
Effect of nitrogen to phosphorus (N:P) ratios on dry biomass and lipid content in Tetradesmus obliquus(A, C) and Monoraphidium sp. (B, D). Different letters indicate significant differences (p< 0.05).
The effects of different light intensities (1.2, 2.6, 3, and 4.2 klux) on dry biomass production and lipid content were examined in Tetradesmus obliquus and Monoraphidium sp., as shown in Figure 5. For Tetradesmus obliquus, dry biomass was significantly affected by light intensity (Figure 5A). The highest dry biomass yield (0.105 g L−¹) was recorded at 2.6 klux, followed by 3 and 4.2 klux, which showed moderate but significantly lower values. The lowest biomass (0.056 g L−¹) was observed at 1.2 klux. Statistical analysis indicated significant differences among all treatments (p< 0.05), with the exception of 3 and 4.2 klux, which were not significantly different from each other. In Monoraphidium sp., light intensity had a marked effect on biomass accumulation (Figure 5B). Maximum dry biomass (0.42–0.43 g L−¹) was observed at 3 and 4.2 klux, with no significant difference between these two intensities. A moderate biomass yield was recorded at 2.6 klux (0.32 g L−¹), while the lowest value (0.14 g L−¹) was at 1.2 klux, significantly different from all other treatments (p< 0.05). Lipid content in Tetradesmus obliquus also responded significantly to light intensity (Figure 5C). The highest lipid percentage (41.8%) was obtained at 2.6 klux, which was significantly higher than the 1.2 klux treatment (34.1%) and not significantly different from the 3 klux level. The lowest lipid content was observed at 1.2 klux, while 4.2 klux produced a moderate lipid content (38.5%), significantly different from both the highest and lowest values. Similarly, Monoraphidium sp. showed the greatest lipid content (50.6%) at 2.6 klux (Figure 5D), significantly higher than the 1.2 klux condition (44.2%) and comparable to the 3 klux treatment. The lowest lipid yield was recorded at 1.2 klux, while 4.2 klux showed intermediate values (46.8%). Overall, 2.6 klux light intensity provided optimal conditions for lipid accumulation in both species, while biomass production was maximized at higher intensities (2.6–4.2 klux), with species-specific responses.
Figure 5
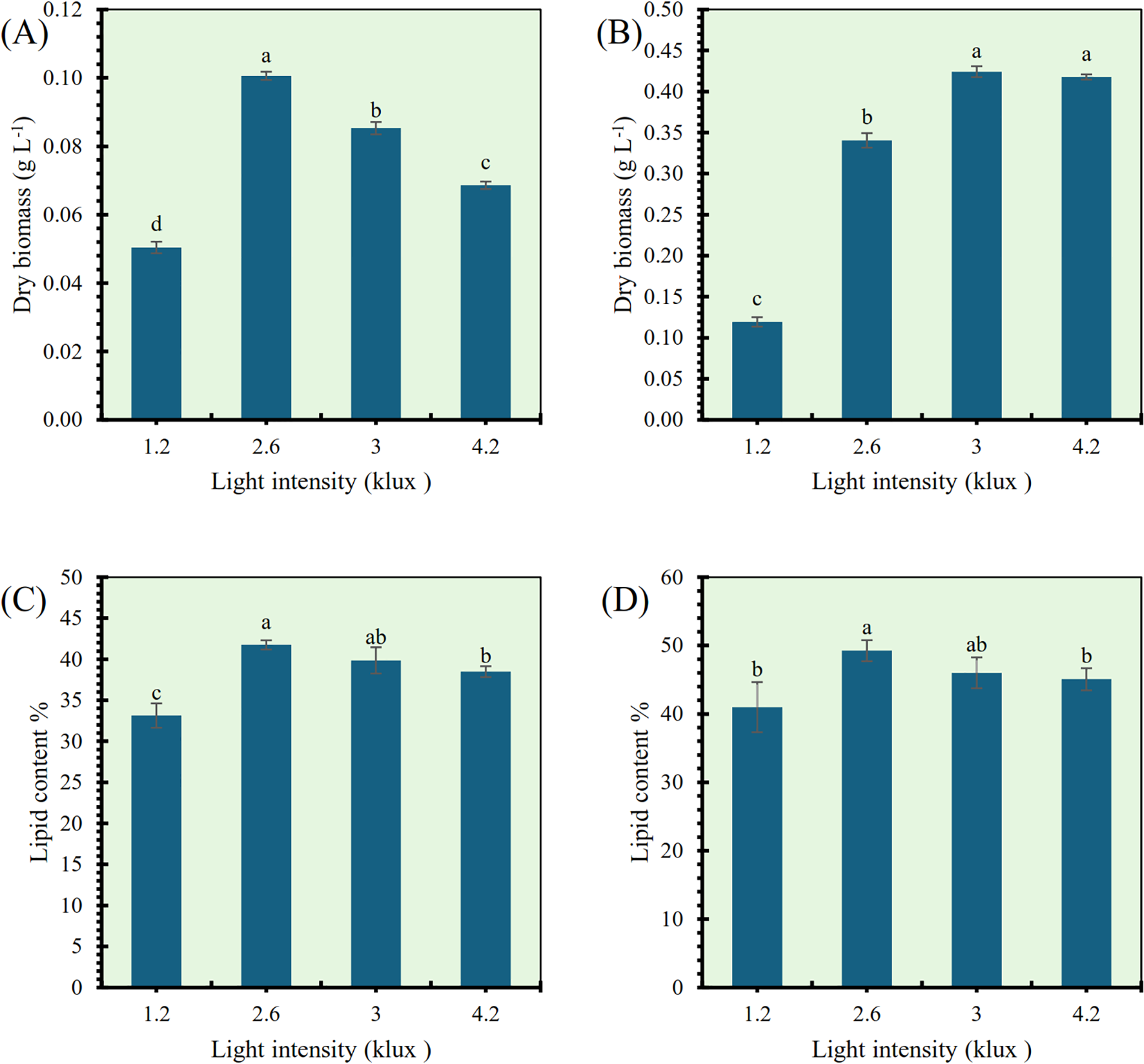
Effect of light intensity (klux) on dry biomass and lipid content in Tetradesmus obliquus(A, C) and Monoraphidium sp. (B, D). Different letters indicate significant differences (p< 0.05).
3.4 Biomass composition
GC-MS analysis of the chloroform–methanol extracts of Tetradesmus obliquus and Monoraphidium sp. revealed a diverse array of bioactive and lipid-derived compounds (Figure 6). In Tetradesmus obliquus, the dominant compounds included hexadecanoic acid, methyl ester (methyl palmitate), 9-octadecenoic acid, methyl ester (methyl oleate), and octadecanoic acid, methyl ester (methyl stearate). Additionally, notable quantities of 1,2-benzenedicarboxylic acid, diisooctyl ester, a phthalate derivative, and hydrocarbons such as 2,4-dimethylheptane were identified (Table 2). Similarly, Monoraphidium sp. exhibited a comparable compound profile, with methyl palmitate, methyl oleate, and methyl stearate again being the dominant constituents (Table 3). Other significant shared compounds included 1,2-benzenedicarboxylic acid, diisooctyl ester and branched alkanes like 2,6,10-trimethylpentadecane.
Figure 6
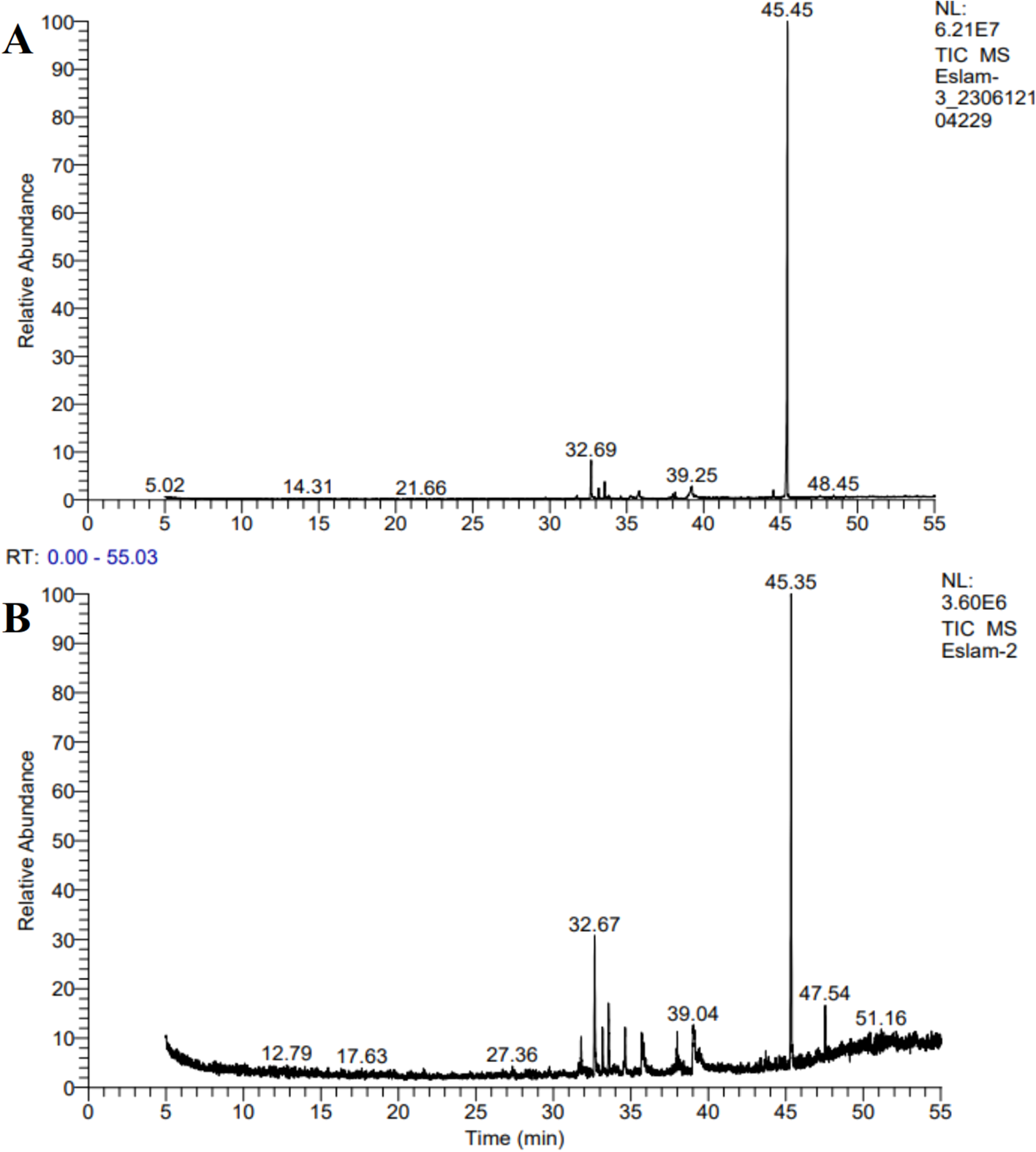
GC–MS chromatograms of the biomass extracts of (A)Tetradesmus obliquus and (B)Monoraphidium sp.
Table 2
| No | Chemical molecule | Chemical Formula | Retention Time (Min) | Peak Area % | Chemical group |
|---|---|---|---|---|---|
| 1 | 2,4,6,8,10-Tetradecapentaenoic acid, 9a-(acetyloxy)-1a,1b,4, 4a,5,7a,7b,8,9,9a-deca hydro-4a,7b-dihydroxy3-(hydroxymethyl)-1,1,6,8-tetramethyl-5-oxo-1 H-cyclopropa[3,4]benz[ 1,2-e]azulen-9-yl ester | C36H46O8 | 5. 47 | 0.12 | Carboxylic Acid Ester |
| 2 | (2,2-Dibenzyloxy-3-nit ro-5,10,15,20-tetraphen yl-2,3-dihydroporphyri nato)copper(II) | C58H40CuN5O2 | 5. 57 | 0.08 | Ether |
| 3 | Spherodenon | C41H58O2 | 6. 64 | 0.12 | Alcohol Keton |
| 4 | (2-Acetamido-3-nitro-5,10,15,20-tetraphenylpo | C46H30CuN6O3 | 7. 60 | 0.10 | Nitro Group |
| 5 | (2,3-Dihydro-5,10,15,2 0-tetraphenyl[2-(2)H1] prophyrinato)copper(II) | C44H29DCuN4 | 8. 76 | 0.10 | porphyrin ring |
| 6 | GLYCOCHOLIC ACID METHYL ESTER TMS | C36H69NO6Si3 | 8. 80 | 0.08 | Ester |
| 7 | 2-bis(ethoxycarbonyl)m ethyl-9(2,3,5-tri-O-(2-m ethylprop-2-yl)dimethyl silyloxy-á-D-ribofuran osyl)purine | C35H64N4O8Si3 | 19.57 | 0.08 | Ester |
| 8 | Lucenin 2 | C27H30O16 | 27.89 | 0.11 | Alcohol Keton |
| 9 | 5,5'-Bis(3,5-di-tert-buty l-4-oxo-2,5-cyclohexad ien-1-ylidene)-5,5'-dihy dro-2,2'-biselenophene | C36H44O2Se2 | 28.05 | 0.09 | Keton Hydrocarbon |
| 10 | Dimethyl 2,anti-4,anti-9,12,anti14-pentabromodecacyc lo[9.9.0.0(1,8).0(2,12). 0(6,10).0(11,18)0(13,1 7).0(16,20)] icosane-syn-4,syn-9-di carboxylate | C24H23Br5O4 | 28.95 | 0.11 | Ester |
| 11 | Docosane (CAS) | C22H46 | 29.73 | 0.31 | Hydrocarbon |
| 12 | 5-(Dibromomethyl)-1,3 -bis(tribromomethyl)benzene | C9H4Br8 | 30.18 | 0.08 | benzene ring |
| 13 | Dodecane, 2,2,4,9,11,11-hexamet hyl- (CAS) | C18H38 | 31.66 | 0.33 | Hydrocarbon |
| 14 | 9-Tricosene, (Z)- (CAS) | C23H46 | 31.78 | 0.67 | Hydrocarbon |
| 15 | 9-Octadecen-12-ynoic acid, methyl ester | C19H32O2 | 31.89 | 0.15 | Ester |
| 16 | 9-Methyl-Z-10-tetradec en-1-ol acetate | C17H32O2 | 32.47 | 0.13 | Ester |
| 17 | 9-Eicosyne | C20H38 | 32.69 | 6.09 | Hydrocarbon |
| 18 | Heneicosane, 11-(1-ethylpropyl)- | C26H54 | 32.90 | 0.15 | Hydrocarbon |
| 19 | 3,7,11,15-Tetramethyl-2 -hexadecen-1-ol | C20H40O | 33.18 | 1.54 | Alcohol |
| 20 | Hexadecanoic acid, methyl ester (CAS) | C17H34O2 | 34.62 | 0.43 | Ester |
| 21 | cis-5,8,11,14,17-Eicosa pentaenoic acid | C20H30O2 | 35.24 | 0.77 | Carboxylic Acid |
| 22 | 9-Octadecenoic acid (Z)- (CAS) | C18H34O2 | 35.36 | 0.35 | Carboxylic Acid |
| 23 | Hexadecanoic acid (CAS) | C16H32O2 | 35.81 | 1.75 | Carboxylic Acid |
| 24 | 10-Acetoxy-2-hydroxy1,2,6a,6b,9,9,12a-hepta methyl-1,3,4,5,6,6a,6b, 7,8,8a,9,10,11,12,12a,1 2b,13,14b-octadecahyd ro-2H-picene-4a-carbox ylic acid, methyl ester | C33H52O5 | 37.68 | 0.09 | Ester Alcohol |
| 25 | 6,9,12-Octadecatrienoic acid, methyl ester (CAS) | C19H32O2 | 38.00 | 0.68 | Ester |
| 26 | 2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-, [R-[R*,R*-(E)]]- (CAS) | C20H40O | 38.15 | 1.03 | Alcohol |
| 27 | Benzene, [3-(2-cyclohexylethyl)-6 -cyclopentylhexyl]- (CAS | C25H40 | 39.24 | 2.46 | benzene ring |
| 28 | Hexadecatrienoic acid, methyl ester (CAS) | C17H28O2 | 39.46 | 0.41 | Ester |
| 29 | 9-Octadecenoic acid, (2-phenyl-1,3-dioxolan -4-yl)methyl ester, cis | C28H44O4 | 39.69 | 0.08 | Ester |
| 30 | Cholestan-3-one, cyclic 1,2-ethanediyl aetal, (5á) | C29H50O2 | 39.83 | 0.08 | Ketone |
| 31 | Cyclohexane, 1,4-dimethyl-2-octadecy l | C26H52 | 39.91 | 0.08 | Hydrocarbon |
| 32 | 2,2-DIDEUTERO OCTADECANAL | C18H34D2O | 41.26 | 0.24 | Aldehyde |
| 33 | 2(1H)-Pyrimidinethione, | C8H16N2OS | 41.56 | 0.10 | Ketone |
| 34 | 9,12,15-Octadecatrienoic acid, 2,3-bis[(trimethylsilyl) oxy]propyl ester, (Z,Z,Z) | C27H52O4Si2 | 42.06 | 0.08 | Ester |
| 35 | 4H-Cyclopropa[5',6']ben z[1',2':7,8]azuleno[5,6- b]oxiren-4-one, 8-(acetyloxy)-1,1a,1b,1c,2a,3,3a,6a,6b,7,8,8a-d odecahydro-3a,6b,8a-tr ihydroxy-2a-(hydroxym ethyl)-1,1,5,7-tetrameth yl-, [1ar-(1aà,1bá,1cà,2aà,3 aá,6aà,6bà,7à,8á,8aà)]- | C22H30O8 | 42.92 | 0.19 | Ester Alcohol |
| 36 | 3,18-Epoxyandrosta-5, 7-dien-17-ol, 4,4-dimethyl-3-methoxy- (13á) | C22H32O3 | 43.64 | 0.16 | Alcohol Ether |
| 37 | Hexadecanoic acid, 2,3-dihydroxypropyl ester (CAS) | C19H38O4 | 44.30 | 0.26 | Ester |
| 38 | 10-Heneicosene (c,t) | C21H42 | 44.53 | 1.23 | Hydrocarbon |
| 39 | (22S)-21-Acetoxy-6à,1 1á-dihydroxy-16à,17à-p ropylmethylenedioxypre gna-1,4-diene-3,20-dio ne | C27H36O8 | 45.11 | 0.26 | Ester Alcohol |
| 40 | 1,2-Benzenedicarboxylic acid, isodecyl octyl ester | C26H42O4 | 45.44 | 74.11 | Ester |
| 41 | 4-(4'-pentylbicyclohexy l)-1-(propylcyclohexyl)b enzene | C32H52 | 46.16 | 0.12 | benzene ring |
| 42 | Milbemycin b, 13-chloro-5-demethoxy -28-deoxy-6,28-epoxy5-(hydroxyimino)-25-( 1-methylethyl)-, (6R,13R,25R)- | C33H46ClNO7 | 47.00 | 0.11 | Alcohol Ketone |
| 43 | Cyclohexane, 1,1'-dodecylidenebis[4-m ethyl | C26H50 | 47.53 | 0.18 | Hydrocarbon |
| 44 | Lucenin 2 | C27H30O16 | 48.45 | 0.44 | Alcohol Keton |
| 45 | 5,11,17 23-tetrakis(1,1-dimethy lethyl)-28-methoxypent acyclo[19.3.1.1(3,7).1(9,13).1(15,19)]octacosa1(25),3,5,7(28),9,11,13 (27),15,17,19(26),21,23 -dodecene-25,26,27-triol | C45H58O4 | 48.99 | 0.08 | Alcohol |
| 46 | cis-10-Heptadecenoic acid | C17H32O2 | 49.23 | 0.22 | Carboxylic Acid |
Chemical composition of the biomass extract of Tetradesmus obliquus.
Table 3
| No | Chemical molecule | Chemical Formula | Retention Time (Min) | Peak Area % | Chemical group |
|---|---|---|---|---|---|
| 1 | Astaxanthin | C40H52O4 | 8. 04 | 0.57 | Ketone |
| 2 | Docosane (CAS) | C22H46 | 8. 30 | 0.56 | Hydrocarbon |
| 3 | (2,3-Dihydro-2-nitro-5,1 0,15,20-tetraphenyl[3-( 2)H1]prophyrinato)copp er(II) | C44H28DCuN5O2 | 9. 86 | 0.42 | Nitro Group |
| 4 | (2,2-Dibenzyloxy-3-nit ro-5,10,15,20-tetraphen yl-2,3-dihydroporphyri nato)copper(II) | C58H40CuN5O2 | 10.06 | 0.42 | Ether |
| 5 | 2(4)-(1-Hydroxyethyl)- 4(2)-(1-isopropoxyethy l)deuteroporprophyrin Dimethyl Ester | C39H47DN4O6 | 11.25 | 0.42 | Ester |
| 6 | PENITREM A | C37H44ClNO6 | 11.69 | 0.57 | Alcohol Ketone |
| 7 | Cyclohexane, 1,1',1'',1'''-(1,6-hexanedi ylidene)tetrakis- (CAS) | C30H54 | 11.87 | 0.51 | Hydrocarbon |
| 8 | Nephthoside - 1,2',3',4'-Tetraacetate 4 3 | C40H56O10 | 11.94 | 0.47 | Ester |
| 9 | Russuphelol | C26H16Cl6O8 | 12.35 | 0.76 | Alcohol Ketone |
| 10 | Milbemycin B, 5-demethoxy-5-one-6,2 8-anhydro-25-ethyl-4- methyl-13-chloro-oxime | C32H44ClNO7 | 12.88 | 0.75 | Alcohol Ether |
| 11 | PENITREM A | C37H44ClNO6 | 13.33 | 0.56 | Alcohol Ketone |
| 12 | Tungsten, pentacarbonyl(4,5-dieth yl-2,2,3-trimethyl-1-phe nyl-1-phospha-2-sila-5- boracyclohex-3-ene-P1 )-, (oc-6-22)- | C21H26BO5PSiW | 16.32 | 0.44 | Organometallic |
| 13 | 2-(16-Acetoxy-11-hydro xy-4,8,10,14-tetramethy l-3-oxohexadecahydroc yclopenta[a]phenanthre n-17-ylidene)-6-methyl -hept-5-enoic acid, methyl ester | C32H48O6 | 24.63 | 0.57 | Ester |
| 14 | 2-OCTADEC-1''-ENY LOXY-1,1,2,2-TETRA DEUTERO ETHANOL | C20H36D4O2 | 27.36 | 0.77 | alcohol |
| 15 | 3-Pyridinecarboxylic acid, | C32H39NO10 | 28.68 | 0.63 | Carboxylic Acid |
| 16 | Docosane (CAS) | C22H46 | 29.75 | 0.89 | Hydrocarbon |
| 17 | ç-PICOLINE-à,ê-D5 | C6H2D5N | 31.67 | 0.83 | Amine |
| 18 | 1,1-dichloro-2-dodecanol | C12H24Cl2O | 31.79 | 2.72 | alcohol |
| 19 | 2,4(1H)-Cyclo-3,4-seco akuammilanium, 3,17-dihydroxy-16-(me thoxycarbonyl)-4-meth yl-, (3á,16R)- | C22H29N2O4 | 31.88 | 0.83 | Ester |
| 20 | Dodecachloroperylene | C20Cl12 | 32.04 | 0.54 | Hydrocarbon |
| 21 | (2-hydroxy-5,10,15,20- tetraphenylporphinato)c opper(II) | C44H28CuN4O | 32.42 | 0.47 | alcohol |
| 22 | 3,7,11,15-Tetramethyl-2 -hexadecen-1-ol | C20H40O | 32.67 | 9.34 | Alcohol Hydrocarbon |
| 23 | Phytol, acetate | C22H42O2 | 33.18 | 3.10 | Alcohol Ester |
| 24 | 9-Octadecenoic acid (Z)-, octadecyl ester | C36H70O2 | 33.32 | 0.42 | Ester |
| 25 | 2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-, [R-[R*,R*-(E)]]- (CAS) | C20H40O | 33.56 | 4.99 | Alcohol Hydrocarbon |
| 26 | 9-Octadecenoic acid (Z)-, 2-butoxyethyl ester | C24H46O3 | 34.55 | 0.53 | Ester |
| 27 | Pentadecanoic acid, 14-methyl-, methyl ester (CAS) | C17H34O2 | 34.63 | 3.04 | Ester |
| 28 | Octadecanoic acid (CAS) | C18H36O2 | 35.71 | 3.72 | Carboxylic Acid |
| 29 | 2,2-DIDEUTERO OCTADECANAL | C18H34D2O | 35.82 | 3.24 | Aldehyde |
| 30 | Ethyl iso-allocholate | C26H44O5 | 37.90 | 0.73 | Ester |
| 31 | 7,10,13-Hexadecatrienoic acid, methyl ester | C17H28O2 | 38.00 | 2.80 | Ester |
| 32 | (2-hydroxy-5,10,15,20- tetraphenylporphinato)c | C44H28CuN4O | 38.15 | 1.45 | alcohol |
| 33 | 9-Octadecenoic acid (Z)- (CAS) | C18H34O2 | 39.04 | 5.74 | Carboxylic Acid |
| 34 | Octadecanoic acid, 4-hydroxy-, methyl ester | C19H38O3 | 39.43 | 0.63 | Ester alcohol |
| 35 | Pregn-4-ene-3,20-dion e, 17,21-dihydroxy-, bis(O-methyloxime) | C23H36N2O4 | 43.38 | 0.46 | Ketone alcohol |
| 36 | Lycoxanthin | C40H56O | 43.70 | 0.66 | Alcohol Ketone |
| 37 | 1,2-Benzenedicarboxylic acid, dioctyl ester (CAS) | C24H38O4 | 45.35 | 32.62 | Ester |
| 38 | 2,4,6-Decatrienoic acid, 1a,2,5,5a,6,9,10,10a-oc tahydro-5,5a-dihydroxy -4-(hydroxymethyl)-1,7, 9-trimethyl-1-[[(2-meth yl-1-oxo-2-butenyl)oxy ]methyl]-11-oxo-1H-2, 8a-methanocyclopenta[a ]cyclopropa[e]cyclodece n-6-yl ester | C35H46O8 | 45.90 | 0.80 | Ester alcohol |
| 39 | Milbemycin B, 5-demethoxy-5-one-6,2 8-anhydro-25-ethyl-4- methyl-13-chloro-oxime | C32H44ClNO7 | 46.04 | 0.83 | Alcohol Ether |
| 40 | Glucobrassicin | C16H20N2O9S2 | 46.34 | 0.57 | – |
| 41 | TRISTRIMETHYLSILY L ETHER DERIVATIVE OF 1,25-DIHYDROXYVIT AMIN D2 | C37H68O3Si3 | 46.42 | 0.43 | Alcohol Ether |
| 42 | Perhydroindene-4-carbo xylic acid, 6-acetoxy-2,3-epoxy-1, 1-epoxymethyl-3a-hydro xy-5-isopropenyl-7a-m ethyl-7-oxo-, methyl ester | C18H22O8 | 46.98 | 0.69 | Ester |
| 43 | ZINC CHLORIDE OXIDIZED | C28H37ClN4OZn | 47.08 | 0.71 | – |
| 44 | Cyclohexane, 1,1'-dodecylidenebis[4-m ethyl | C26H50 | 47.54 | 3.66 | Hydrocarbon |
| 45 | Cinobufotalin | C26H34O7 | 48.01 | 0.40 | Alcohol Ketone |
| 46 | Methanesulfonic acid, 2-(3-hydroxy-4,4,10,13,14-pentamethyl-2,3,4,5,6,7,10,11,12,13,14,15, 16,17-tetradecahydro-1 H-cyclopenta[a]phenant hryl)- | C26H44O4S | 48.93 | 0.47 | Acid alcohol |
| 47 | 7,8-Epoxylanostan-11- ol, 3-acetoxy | C32H54O4 | 49.01 | 0.57 | Alcohol Ester |
| 48 | 2,5-Dibromo-1,4-di-n-h exadecylbenzene | C38H68Br2 | 49.10 | 0.54 | Hydrocarbon |
| 49 | Cyclohexane, 1,1',1'',1'''-(1,6-hexanedi | C30H54 | 49.21 | 1.22 | Hydrocarbon |
| 50 | Phenol, 2-methoxy-6-(3,7,11,1 5,19,23,27,31,35-nona methyl-2,6,10,14,18,22,26,30,34-hexatriacont anonaenyl)- (CAS) | C52H80O2 | 49.30 | 0.96 | Alcohol Ether |
Chemical composition of the biomass extract of Monoraphidium sp.
3.5 Biodiesel composition
GC-MS analyses of the biodiesel derived from Tetradesmus obliquus and Monoraphidium sp. revealed the presence of a range of fatty acid methyl esters (FAMEs), which are essential indicators of biodiesel quality (Figure 7).
Figure 7
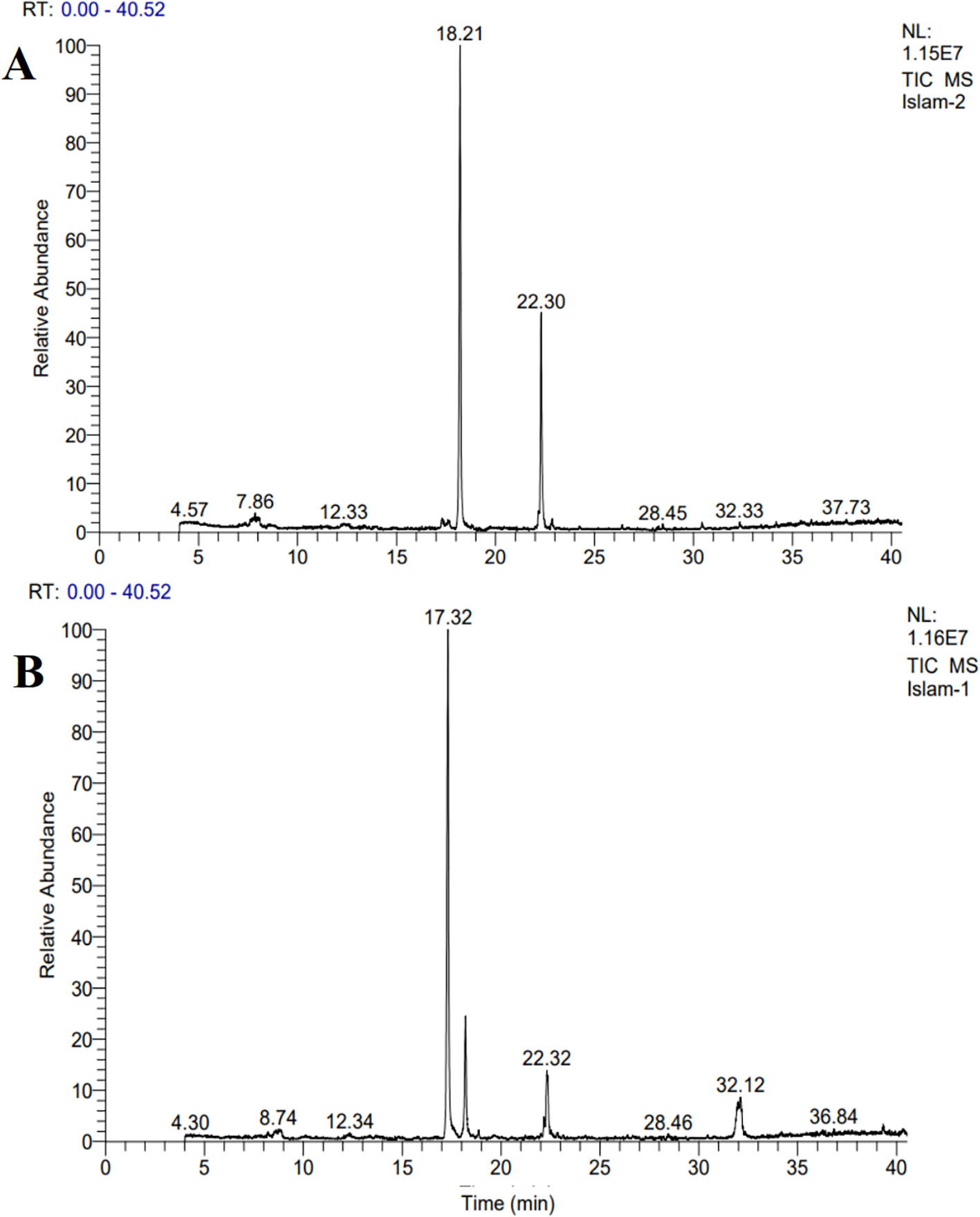
GC–MS chromatograms of the biodiesel profiles of (A)Tetradesmus obliquus and (B)Monoraphidium sp.
In Tetradesmus obliquus, hexadecanoic acid, methyl ester (methyl palmitate) was the most abundant component, accounting for 56.61% of the total peak area, followed by methyl oleate (9-octadecenoic acid, methyl ester) at 22.30%. Other notable compounds included methyl myristate and methyl linoleate, as well as lesser quantities of methyl linolenate and 2,3-dihydroxypropyl palmitate. Both saturated (C14:0, C16:0) and unsaturated (C18:1, C18:2, C18:3) FAMEs indicate a biodiesel profile with balanced oxidative stability and cold flow properties (Table 4).
Table 4
| No | Chemical molecule | Chemical Formula | Retention Time (Min) | Peak Area % | Chemical group | Carbon double bond |
|---|---|---|---|---|---|---|
| 1 | Acetic acid, 5-(2,2-dimethyl-6-oxocy clohexylidene)-3-methy l-pent-3-enyl ester | C16H24O3 | 7. 79 | 0.28 | methyl palmitate (palmitic acid) |
C16:0 |
| 2 | 2H-2,4a-Methanonapht halen-8(5H)-one, 1,3,4,6,7,8a-hexahydro -1,1,5,5-tetramethyl- (CAS) | C15H24O | 8. 07 | 1.15 | Methyl myristate (myristic acid) |
C14:0 |
| 3 | 9-Octadecenoic acid (Z)- (CAS) | C18H34O2 | 12.61 | 0.54 | oleic acid | C18:1 |
| 4 | 6-(1',1'-Dimethylethyl)- 2-methyl-2,4,5-decatrie n-1-ol | C15H26O | 13.90 | 0.23 | methyl tetradecanoate (myristic acid) |
C14:0 |
| 5 | 7-Oxabicyclo[4.1.0]hep tane-3-carboxylic acid, 7-oxabicyclo[4.1.0]hep t-3-ylmethyl ester | C14H20O4 | 17.30 | 1.31 | Methyl myristate (myristic acid) |
C14:0 |
| 6 | Hexadecatrienoic acid, methyl ester (CAS) | C17H28O2 | 17.64 | 0.51 | Methyl linolenate (linolenic acid) |
C18:3 |
| 7 | Hexadecanoic acid, methyl ester (CAS) | C17H34O2 | 18.21 | 56.61 | Methyl palmitate (palmitic acid) |
C16:0 |
| 8 | Hexadecanoic acid, 2,3-dihydroxypropyl ester (CAS) | C19H38O4 | 21.73 | 0.24 | 2,3-dihydroxypropyl palmitate (palmitic acid) |
C16:0 |
| 9 | 8,11-Octadecadienoic acid, methyl ester (CAS) | C19H34O2 | 22.16 | 0.50 | methyl linoleate (linoleic acid) |
C18:2 |
| 10 | 9-Octadecenoic acid (Z)-, methyl ester (CAS) | C19H36O2 | 22.30 | 22.30 | methyl oleate (oleic acid) |
C18:1 |
| 11 | Cyclopentanetridecanoic acid, methyl ester (CAS) | C19H36O2 | 22.86 | 1.37 | methyl 16-cyclopentylpalmitate (palmitic acid) |
C16:0 |
Chemical composition of biodiesel derived from Tetradesmus obliquus.
Similarly, Monoraphidium sp. biodiesel was dominated by 1-hexadecanol, a fatty alcohol derived from palmitic acid, with a peak area of 54.75%, and methyl palmitate at 12.09%. Other major constituents included methyl oleate (7.69%), methyl octacosanoate (4.39%), and methyl lignocerate (4.77%) (Table 5). Both species shared several key compounds, particularly methyl palmitate and methyl oleate.
Table 5
| No | Chemical molecule | Chemical Formula | Retention Time (Min) | Peak Area % | Chemical group | Carbon double bond |
|---|---|---|---|---|---|---|
| 1 | Dodecanoic acid, methyl ester (CAS) | C13H26O2 | 8.19 | 0.39 | Methyl laurate (lauric acid) |
C12:0 |
| 2 | Perhydroindene-4-carbo xylic acid, 6-acetoxy-2,3-epoxy-1, 1-epoxymethyl-3a-hydro xy-5-isopropenyl-7a-m ethyl-7-oxo-, methyl ester | C18H22O8 | 8. 54 | 0.39 | Methyl ester of 9,10-dihydroxy-12-octadecenoic acid, or Methyl 9,10-dihydroxy-octadecenoate. | ------ |
| 3 | 2-Propenoic acid, 2-methyl-, dodecyl ester (CAS) | C16H30O2 | 8. 75 | 0.22 | Palmitic acid | C16:0 |
| 4 | 1-Hexadecanol (CAS) | C16H34O | 17.31 | 54.75 | palmitic acid | C16:0 |
| 5 | 2,15-Heptadecadiene, 9-(ethoxymethyl)- | C20H38O | 17.60 | 0.22 | arachidate methyl ester arachidic acid |
C20:0 |
| 6 | Hexadecanoic acid, methyl ester (CAS) | C17H34O2 | 18.20 | 12.09 | Methyl Palmitate palmitic acid |
C16:0 |
| 7 | 7-Methyl-Z-tetradecene1-ol ace | C17H32O2 | 22.16 | 0.94 | margaric acid (or heptadecanoic acid) | C17:0 |
| 8 | 16-Octadecenoic acid, methyl ester | C19H36O2 | 22.32 | 7.69 | methyl oleate (oleic acid) |
C18:1 |
| 9 | Pentadecanoic acid, 14-methyl-, methyl ester (CAS) | C17H34O2 | 22.86 | 0.58 | Iso-pentadecanoic acid methyl ester or 14-methylpentadecanoic acid methyl ester. | C15:0 |
| 10 | Docosane (CAS) | C22H46 | 23.13 | 0.38 | behenic acid methyl ester (Behenic acid) |
C22:0 |
| 11 | Docosane (CAS) | C22H46 | 30.44 | 0.31 | behenic acid methyl ester (Behenic acid) |
C22:0 |
| 12 | Octacosanoic acid, methyl ester (CAS) | C29H58O2 | 31.98 | 4.39 | Methyl octacosanoate. octacosanoic acid |
------ |
| 13 | (rac)-4,4',7,7'-Tetramet hoxy-5,5'-dimethyl-2H,2 'H-6,6'-bichromen-2,2'- | C24H22O8 | 32.09 | 4.77 | Lignoceric acid methyl ester | C24:0 |
Chemical composition of biodiesel derived from Monoraphidium sp.
3.6 Biodiesel quality estimation
The predicted biodiesel properties of Tetradesmus obliquus and Monoraphidium sp., as estimated using Biodiesel Analyzer software are displayed in Table 6. Monoraphidium sp. showed a higher proportion of saturated fatty acids (SFA) at 74.65%, compared to 61.19% in T. obliquus, which contributed to its elevated cetane number (CN = 75.96) and reduced degree of unsaturation (DU = 7.69). Conversely, T. obliquus presented a more balanced profile with 22.84% monounsaturated fatty acids (MUFA) and 1.01% polyunsaturated fatty acids (PUFA), leading to a higher DU (24.86) and improved oxidative stability (OS = 119.35 hours), whereas Monoraphidium sp. recorded zero oxidative stability. The iodine value (IV), an indicator of unsaturation, was notably higher in T. obliquus (22.84) than in Monoraphidium sp. (6.92), correlating with its enhanced oxidative resistance. Despite the superior CN and lower IV, Monoraphidium sp. exhibited significantly higher cold flow properties, including a long-chain saturated factor (LCSF = 17.50), cloud point (30.28°C), and cold filter plugging point (CFPP = 38.50°C), suggesting lower suitability for colder climates compared to T. obliquus, which recorded lower values in these parameters (LCSF = 5.85, CP = 25.78°C, CFPP = 1.90°C). Both species exhibited similar kinematic viscosities (1.15–1.16 mm²/s) and densities (0.71–0.74 g/cm³), with T. obliquus also displaying a slightly higher heating value (HHV = 33.41 MJ/kg). Collectively, these findings indicate that Tetradesmus obliquus offers a more favorable balance between fuel stability, cold flow properties, and energy content, making it a more versatile and climate-resilient biodiesel feedstock. While both microalgae strains are viable candidates for biodiesel production, T. obliquus is characterized by a higher content of conventional biodiesel esters, contributing to better oxidative stability and combustion quality, whereas Monoraphidium sp. is distinguished by a higher proportion of longer-chain saturated and alcohol-based lipids, which may enhance specific fuel attributes such as lubricity and viscosity but potentially limit cold flow performance.
Table 6
| Item | Tetradesmus obliquus | Monoraphidium sp. |
|---|---|---|
| Saturated Fatty Acids (SFA) | 61.19 | 74.65 |
| Monounsaturated Fatty Adds (MUFA) | 22.84 | 7.69 |
| Polyunsaturated Fatty Acids (PUFA) | 1.01 | 0.00 |
| Degree of Unsaturation (DU) | 24.86 | 7.69 |
| Saponification Value (SV) | 181.66 | 174.86 |
| Iodine Value (IV) | 22.84 | 6.92 |
| Cetan Number (CN) | 71.21 | 75.96 |
| Long-Chain Saturated Factor (LCSF) | 5.85 | 17.50 |
| Cold Filter Plugging Point (CFPP) | 1.90 | 38.50 |
| Cloud point (CP) | 25.78 | 30.28 |
| Allylic Position Equivalents (APE) | 24.86 | 7.69 |
| Bis -Allylie Position Equivalents (BAPE) | 1.52 | 0.44 |
| Oxidation Stability (OS) | 119.35 | 0 |
| Higher Heating Value (HHV) | 33.41 | 32.41 |
| Kinematic Viscosity (u) | 1.15 | 1.16 |
| Density (p) | 0.74 | 0.71 |
Biodiesel properties of Tetradesmus obliquus and Monoraphidium sp. predicted using Biodiesel Analyzer software.
4 Discussion
In recent years, extensive research has focused on exploring the biodiesel potential of microalgae, yet significant knowledge gaps remain regarding the comparative performance of different strains under standardized conditions. This study addresses these gaps by providing a comprehensive evaluation of Tetradesmus obliquus and Monoraphidium sp., incorporating morphological, molecular, physiological, biochemical, and fuel property analyses. Unlike most previous studies, which often examine single strains or focus on limited parameters, our work offers an integrated assessment, including the rarely reported cold flow properties of biodiesel critical for determining real-world applicability. Furthermore, the investigation of species-specific responses to nutrient ratios and light intensities highlights the complex trade-offs between maximizing biomass and enhancing fuel quality.
Algae have emerged as a promising renewable resource for biodiesel production due to their high lipid content and rapid growth rates (Gaurav et al., 2024; Sharma et al., 2025). Among various species, Tetradesmus obliquus and Monoraphidium sp. have demonstrated significant potential (Holbrook et al., 2014; Bibi et al., 2022; Falfushynska, 2024). T. obliquus has been shown to accumulate substantial lipid content under various stress conditions, including heavy metal exposure, which enhances its suitability for biodiesel applications (Alwaleed et al., 2025). Additionally, the application of p-coumaric acid has been reported to significantly increase lipid accumulation in T. obliquus, further improving its biodiesel yield (Esakkimuthu et al., 2020). In Egypt, studies have identified T. obliquus as a high biomass-producing species, while Monoraphidium minutum exhibited the highest lipid productivity among tested microalgae, indicating their complementary roles in biodiesel feedstock development (Mohamed et al., 2022). These findings underscore the importance of selecting and optimizing specific algal strains to advance sustainable biodiesel technologies.
The observed morphological characteristics of Tetradesmus obliquus align well with previous descriptions of this species, which typically forms coenobia composed of four to eight elongated cells with smooth walls and rounded apices arranged in a linear or slightly curved pattern (Cho and Lee, 2024). The presence of these compact coenobial structures under both light and scanning electron microscopy (SEM) reflects the adaptive colonial nature of T. obliquus, which may contribute to its resilience and high biomass yield in mass culture systems (Lürling and Van Donk, 2000; Cardon et al., 2018). The smooth cell wall surface observed under SEM supports its taxonomic classification and indicates minimal extracellular ornamentation, a feature commonly reported for this genus (do Carmo Cesário et al., 2022). In contrast, Monoraphidium sp. exhibited a distinctive unicellular, crescent-shaped morphology with tapering ends, as consistently reported in the literature for members of this genus (Fawley et al., 2006). The solitary cell arrangement and absence of spines or visible wall ornamentation under SEM further validate its identity and suggest potential advantages for ease of harvesting and processing in biotechnological applications.
Molecular identification through 18S rRNA gene sequencing effectively validated the taxonomic classification of the isolated microalgae strains, supporting their identification as Tetradesmus obliquus and Monoraphidium sp. The observed sequences displayed high similarity with GenBank reference sequences indicating a high level of genetic conservation within these taxa. This degree of similarity is consistent with previous studies that highlight the robustness of 18S rRNA markers for accurate phylogenetic placement and species-level discrimination among green microalgae (Hanan et al., 2022; Lortou et al., 2022). The phylogenetic analysis further reinforced these findings, as the isolates clustered within their respective clades with strong bootstrap support, aligning with morphological observations and demonstrating congruence between molecular and classical taxonomic approaches.
The comparative analysis of growth kinetics and lipid productivity between Tetradesmus obliquus and Monoraphidium sp. under standard cultivation conditions (25 ± 2 °C, 1.2 klux, 16:8 h light/dark cycle) revealed significant differences in their biofuel potential. Monoraphidium sp. exhibited a markedly higher dry biomass yield, reaching 0.177 ± 0.0078 g L−¹ by day 28, compared to 0.047 ± 0.0025 g L−¹ for T. obliquus. This superior biomass accumulation aligns with previous findings where Monoraphidium sp. achieved biomass levels up to 1.18 g L−¹ under optimized conditions (Dong et al., 2019). In terms of lipid content, Monoraphidium sp. peaked at 0.368 ± 0.049 g g−¹ on day 28, surpassing T. obliquus, which reached 0.213 ± 0.028 g g−¹. Such lipid accumulation in Monoraphidium sp. is consistent with reported ranges of 19–35% of dry weight (Hawrot-Paw et al., 2020). The specific growth rate (µ) was generally higher in Monoraphidium sp., with a peak of 0.132 ± 0.019 d−¹ on day 21, while T. obliquus reached its maximum µ of 0.113 ± 0.002 d−¹ earlier, on day 14. Correspondingly, the growth doubling per day (Dd−¹) and generation time (G) metrics favored Monoraphidium sp., indicating a more efficient growth profile. These findings suggest that Monoraphidium sp. holds greater promise for biodiesel production under the tested conditions, although further optimization could enhance the performance of T. obliquus, as studies have demonstrated improved biomass and lipid yields under modified cultivation strategies (Jin et al., 2024).
The optimization of biomass yield and lipid accumulation in Tetradesmus obliquus and Monoraphidium sp. under varying nitrogen to phosphorus (N:P) ratios and light intensities reveals species-specific responses that are crucial for biodiesel production. In T. obliquus, the highest dry biomass yield (0.065 g L−¹) was observed at an N:P ratio of 1:1, indicating that a balanced nutrient supply supports optimal growth. This aligns with previous findings that suggest balanced N:P ratios enhance biomass productivity in green microalgae (Dhup and Dhawan, 2014). Conversely, Monoraphidium sp. achieved its maximum biomass (0.085 g L−¹) at a 4:1 N:P ratio, suggesting a preference for nitrogen-rich conditions, which corroborates studies highlighting the influence of nitrogen availability on microalgal growth and lipid productivity (Dhup and Dhawan, 2014).
Regarding lipid accumulation, both species exhibited the highest lipid content at the 1:1 N:P ratio, with T. obliquus reaching 34.8% and Monoraphidium sp. attaining 47.3%. These results are consistent with literature indicating that balanced nutrient conditions favor lipid biosynthesis in microalgae (Morales et al., 2021).
Light intensity also significantly impacted biomass and lipid production. For T. obliquus, the optimal biomass yield (0.105 g L−¹) and lipid content (41.8%) were achieved at 2.6 klux, suggesting that moderate light intensities promote both growth and lipid accumulation. This observation is supported by studies demonstrating that specific light intensities can enhance lipid synthesis in microalgae (Mulgund, 2022). Similarly, Monoraphidium sp. showed maximum biomass (0.42–0.43 g L−¹) at higher light intensities (3 and 4.2 klux), while the highest lipid content (50.6%) was recorded at 2.6 klux, indicating that optimal light conditions for lipid accumulation may differ from those for biomass production. These findings align with research highlighting the species-specific responses of microalgae to light intensity variations (Maltsev et al., 2021).
The GC-MS analysis of chloroform–methanol extracts from Tetradesmus obliquus and Monoraphidium sp. revealed a diverse array of bioactive and lipid-derived compounds, underscoring their potential in biodiesel production and biotechnological applications. In T. obliquus, the predominant fatty acid methyl esters (FAMEs) identified were methyl palmitate (hexadecanoic acid, methyl ester), methyl oleate (9-octadecenoic acid, methyl ester), and methyl stearate (octadecanoic acid, methyl ester). These saturated and monounsaturated FAMEs are known to enhance biodiesel quality by improving oxidative stability and cetane number, aligning with previous findings that highlight T. obliquus as a favorable feedstock for biodiesel due to its high palmitic acid content (Ahiahonu et al., 2022). Additionally, the presence of hydrocarbons such as 2,4-dimethylheptane and phthalate derivatives like 1,2-benzenedicarboxylic acid, diisooctyl ester, suggests potential applications in bioplastics and industrial solvents, although the latter’s origin warrants further investigation to rule out contamination (Vladić et al., 2023).
Similarly, Monoraphidium sp. exhibited a comparable compound profile, with methyl palmitate, methyl oleate, and methyl stearate as dominant constituents. The detection of branched alkanes such as 2,6,10-trimethylpentadecane further indicates the species’ capacity to produce hydrocarbons suitable for biofuel applications. The shared presence of these compounds in both microalgae underscores their potential as sustainable sources for biodiesel production. Moreover, the identification of bioactive compounds with known antioxidant and antimicrobial properties, such as certain fatty acid methyl esters, aligns with reports that microalgal metabolites can serve as functional food ingredients and therapeutic agents (Eze et al., 2023). These findings highlight the multifaceted applications of T. obliquus and Monoraphidium sp., extending beyond energy production to include roles in health and industrial sectors.
The biodiesel profiles of Tetradesmus obliquus and Monoraphidium sp. reveal distinct compositions and properties, influencing their suitability for biodiesel applications (Ahiahonu et al., 2022). In T. obliquus, the predominant fatty acid methyl ester (FAME) is methyl palmitate (hexadecanoic acid methyl ester), comprising 56.61% of the total peak area. This aligns with findings by Ahiahonu et al. (2022), who reported palmitic acid as the most abundant fatty acid in T. obliquus, accounting for 34.26% of the total composition. The presence of both saturated (C14:0, C16:0) and unsaturated (C18:1, C18:2, C18:3) FAMEs suggest a biodiesel profile with balanced oxidative stability and cold flow properties. This balance is crucial, as higher unsaturation levels can lead to increased oxidative instability and lower cetane numbers. The calculated cetane number (CN) of 73 for T. obliquus biodiesel falls within acceptable standards, indicating reliable ignition quality. Moreover, the iodine value (IV) of 22.84 reflects a moderate degree of unsaturation, correlating with enhanced oxidative stability. These characteristics position T. obliquus as a favorable feedstock for biodiesel, offering a balance between performance and stability. The higher heating value (HHV) of Tetradesmus obliquus biodiesel was 33.41 MJ/kg, which is notably lower than that of conventional petrodiesel (~45 MJ/kg). This difference is expected, as biodiesels generally have lower energy content due to their oxygenated nature. However, the HHV of T. obliquus falls within the typical range reported for microalgal biodiesels, which often varies between 30 and 40 MJ/kg, depending on lipid composition and fatty acid profile. Although the energy density is lower than petrodiesel, T. obliquus still presents a viable alternative biofuel source, particularly when considering its renewable origin and the potential for optimizing cultivation and extraction conditions to improve fuel quality. This makes T. obliquus a viable candidate for biodiesel production, especially in regions where optimization of cultivation processes can lead to enhanced biofuel quality. Additionally, blending T. obliquus biodiesel with other biodiesels or petrodiesel could mitigate the lower HHV and improve its overall fuel performance.
Conversely, Monoraphidium sp. biodiesel is characterized by a higher proportion of saturated fatty acids (74.65%), leading to a higher CN of 75.96. However, this high saturation correlates with elevated cold flow properties, such as a cloud point of 30.28°C and a cold filter plugging point (CFPP) of 38.50°C, suggesting reduced suitability for colder climates. The absence of oxidative stability in Monoraphidium sp. biodiesel may be attributed to its high saturated and low unsaturated fatty acid content, which typically enhances oxidative stability. This finding contrasts with studies on other microalgae, such as Desmodesmus sp. (I-AU1), which, despite a high saturated fatty acid content, exhibited favorable oxidative stability, possibly due to different lipid compositions or cultivation conditions (Arguelles et al., 2018). The presence of 1-hexadecanol (a fatty alcohol derived from palmitic acid) as a major component in Monoraphidium sp. biodiesel is noteworthy. Fatty alcohols can influence biodiesel properties, potentially enhancing lubricity and viscosity, which are beneficial for engine performance. However, excessive concentrations may adversely affect cold flow properties (Gurau et al., 2025).
Both species share key compounds, notably methyl palmitate and methyl oleate, highlighting their potential as biodiesel feedstocks. The distinct differences in their fatty acid compositions underscore the importance of strain selection and cultivation conditions in optimizing biodiesel quality. T. obliquus offers a more balanced biodiesel profile, suitable for diverse climatic conditions, while Monoraphidium sp. may be more appropriate for applications where high cetane numbers and specific fuel properties are desired, despite potential challenges with cold flow performance.
5 Conclusion
This study evaluated Tetradesmus obliquus and Monoraphidium sp. as potential feedstocks for sustainable biodiesel production. Both microalgae demonstrated high lipid content and favorable fatty acid profiles, with Monoraphidium sp. showing superior biomass productivity (0.43 g L−¹) and lipid accumulation (50.6%) compared to T. obliquus (0.105 g L−¹ and 41.8%, respectively). GC–MS analysis confirmed key biodiesel components like methyl palmitate and methyl oleate in both species. Predictive assessments indicated that T. obliquus offers better oxidative stability and cold flow properties, making it versatile for varying climates, while Monoraphidium sp. exhibited higher cetane numbers but limited cold flow performance. However, Monoraphidium sp. biodiesel may still be suitable for use in warmer regions where cold flow properties are less critical. Additionally, blending Monoraphidium biodiesel with other biodiesels, particularly those with better cold flow properties, could mitigate its performance limitations in cooler climates.
These findings highlight the distinct advantages of each species depending on application requirements. However, further research into scalable cultivation techniques and cost-effective extraction methods is essential for industrial implementation. By advancing these areas, we can harness the full potential of microalgae for sustainable energy solutions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
JM: Visualization, Data curation, Resources, Writing – original draft, Conceptualization, Formal Analysis, Validation, Methodology, Investigation, Supervision, Writing – review & editing, Software. IM: Software, Resources, Formal Analysis, Data curation, Investigation, Writing – original draft, Writing – review & editing, Methodology, Validation. EE: Supervision, Validation, Conceptualization, Writing – original draft, Writing – review & editing, Methodology, Data curation, Investigation, Software. AS: Writing – review & editing, Data curation, Software, Investigation, Resources, Validation, Formal Analysis, Methodology, Writing – original draft. AE: Investigation, Writing – original draft, Writing – review & editing, Methodology, Software, Formal Analysis, Resources, Data curation, Validation. IT: Formal Analysis, Writing – original draft, Data curation, Software, Visualization, Resources, Supervision, Conceptualization, Investigation, Writing – review & editing, Methodology. EE: Conceptualization, Visualization, Validation, Resources, Methodology, Data curation, Writing – review & editing, Writing – original draft, Investigation, Formal Analysis, Supervision, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was received from the Academy of Scientific Research and Technology (ASRT), Egypt.
Acknowledgments
This research was conducted as part of a Home aquaculture system: Sustainable opportunities in South Sinai project/ASRT-APPLE: Blue Economy supported by the Academy of Scientific Research and Technology ASRT Egypt.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdel-Aal E. I. Mofeed J. (2015). Optimization of medium components for high biomass and lipid production of the freshwater diatom tryblionella hungarica niof-dm-017 by using plackett-burman design. Egypt. J. Exp. Biol.11, 41–50.
2
Ahiahonu E. K. Anku W. W. Roopnarain A. Green E. Govender P. P. Serepa-Dlamini M. H. (2022). Bioresource potential of Tetradesmus obliquus UJEA_AD : critical evaluation of biosequestration rate, biochemical and fatty acid composition in BG11 media. J. Chem. Technol. Biotechnol.97, 689–697. doi: 10.1002/jctb.6951
3
Alwaleed E. A. Galal H. R. M. Aboueldahab M. Saber H. (2025). Maximizing lipid accumulation in Tetradesmus obliquus under heavy metal stress for sustainable biodiesel innovation. BMC Biotechnol.25, 20. doi: 10.1186/s12896-025-00951-z
4
Arguelles E. D. L. R. Laurena A. C. Monsalud R. G. Martinez-Goss M. R. (2018). Fatty acid profile and fuel-derived physico-chemical properties of biodiesel obtained from an indigenous green microalga, Desmodesmus sp. (I-AU1), as potential source of renewable lipid and high quality biodiesel. J. Appl. Phycol.30, 411–419. doi: 10.1007/s10811-017-1264-6
5
Bagchi S. K. Mallick N. (2016). Carbon dioxide biofixation and lipid accumulation potential of an indigenous microalga Scenedesmus obliquus (Turpin) Kützing GA 45 for biodiesel production. RSC Adv.6, 29889–29898. doi: 10.1039/C6RA02811J
6
Bibi F. Ishtiaq Ali M. Ahmad M. Bokhari A. Shiong Khoo K. Zafar M. et al . (2022). Production of lipids biosynthesis from Tetradesmus nygaardii microalgae as a feedstock for biodiesel production. Fuel326, 124985. doi: 10.1016/j.fuel.2022.124985
7
Bora A. Thondi Rajan A. S. Ponnuchamy K. Muthusamy G. Alagarsamy A. (2024). Microalgae to bioenergy production: Recent advances, influencing parameters, utilization of wastewater – A critical review. Sci. Total Environ.946, 174230. doi: 10.1016/j.scitotenv.2024.174230
8
Cardon Z. G. Peredo E. L. Dohnalkova A. C. Gershone H. L. Bezanilla M. (2018). A model suite of green algae within the Scenedesmaceae for investigating contrasting desiccation tolerance and morphology. J. Cell Sci.131 (7). doi: 10.1242/jcs.212233
9
Chenna R. Sugawara H. Koike T. Lopez R. Gibson T. J. Higgins D. G. et al . (2003). Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res.31, 3497–3500. doi: 10.1093/nar/gkg500
10
Cho H. S. Lee J. (2024). Taxonomic reinvestigation of the genus Tetradesmus (Scenedesmaceae; Sphaeropleales) based on morphological characteristics and chloroplast genomes. Front. Plant Sci.15. doi: 10.3389/fpls.2024.1303175
11
Dayananda C. Sarada R. Bhattacharya S. Ravishankar G. A. (2005). Effect of media and culture conditions on growth and hydrocarbon production by Botryococcus braunii. Process Biochem.40, 3125–3131. doi: 10.1016/j.procbio.2005.03.006
12
Dhup S. Dhawan V. (2014). Effect of nitrogen concentration on lipid productivity and fatty acid composition of Monoraphidium sp. Bioresour. Technol.152, 572–575. doi: 10.1016/j.biortech.2013.11.068
13
Díaz G. C. Cruz Y. R. Carliz R. G. de Paula R.C.V. Aranda D. A. G. Dario M. A. G. et al . (2015). Cultivation of microalgae monoraphidium sp., in the plant pilot the grand valle bio energy, for biodiesel production. Nat. Sci.07, 370–378. doi: 10.4236/ns.2015.77040
14
do Carmo Cesário C. Soares J. Cossolin J. F. S. Almeida A. V. M. Bermudez Sierra J. J. de Oliveira Leite M. et al . (2022). Biochemical and morphological characterization of freshwater microalga Tetradesmus obliquus (Chlorophyta: Chlorophyceae). Protoplasma259, 937–948. doi: 10.1007/s00709-021-01712-3
15
Dong X. Han B. Zhao Y. Ding W. Yu X. (2019). Enhancing biomass, lipid production, and nutrient utilization of the microalga Monoraphidium sp. QLZ-3 in walnut shell extracts supplemented with carbon dioxide. Bioresour. Technol.287, 121419. doi: 10.1016/j.biortech.2019.121419
16
Echlin P. (2009). Handbook of Sample Preparation for Scanning Electron Microscopy and X-Ray Microanalysis (Boston, MA: Springer US). doi: 10.1007/978-0-387-85731-2
17
El Basuini M. F. Khattab A. A. A. Hafsa S. H. A. Teiba I. I. Elkassas N. E. M. El-Bilawy E. H. et al . (2023). Impacts of algae supplements (Arthrospira & Chlorella) on growth, nutrient variables, intestinal efficacy, and antioxidants in New Zealand white rabbits. Sci. Rep.13, 7891. doi: 10.1038/s41598-023-34914-1
18
Esakkimuthu S. Krishnamurthy V. Wang S. Hu X., K, S. Abomohra A. E.-F. (2020). Application of p-coumaric acid for extraordinary lipid production in Tetradesmus obliquus: A sustainable approach towards enhanced biodiesel production. Renew. Energy157, 368–376. doi: 10.1016/j.renene.2020.05.005
19
Eze C. N. Onyejiaka C. K. Ihim S. A. Ayoka T. O. Aduba C. C. Ndukwe J. K. et al . (2023). Bioactive compounds by microalgae and potentials for the management of some human disease conditions. AIMS Microbiol.9, 55–74. doi: 10.3934/microbiol.2023004
20
Falfushynska H. (2024). Advancements and prospects in algal biofuel production: A comprehensive review. Phycology4, 548–575. doi: 10.3390/phycology4040030
21
Fawley M. W. Dean M. L. Dimmer S. K. Fawley K. P. (2006). Evaluating the morphospecies concept in the selenastraceae (chlorophyceae, chlorophyta) 1. J. Phycol.42, 142–154. doi: 10.1111/j.1529-8817.2006.00169.x
22
Gaurav K. Neeti K. Singh R. (2024). Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technol. Sustain.2, 100060. doi: 10.1016/j.grets.2023.100060
23
Gurau S. Imran M. Ray R. L. (2025). Algae: A cutting-edge solution for enhancing soil health and accelerating carbon sequestration – A review. Environ. Technol. Innov.37, 103980. doi: 10.1016/j.eti.2024.103980
24
Hanan M. Y. Yahya A. Jaafar M. Z. Amatul-Samahah M. A. (2022). A dataset representing the identification of three microalgae species isolated from freshwater areas at Glami Lemi River, Malaysia. Data Br.45, 108761. doi: 10.1016/j.dib.2022.108761
25
Hartman L. Lago R. C. (1973). Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract.22, 475–476. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4727126.
26
Hawrot-Paw M. Koniuszy A. Gałczyńska M. (2020). Sustainable production of monoraphidium microalgae biomass as a source of bioenergy. Energies13, 5975. doi: 10.3390/en13225975
27
He M. Yan Y. Pei F. Wu M. Gebreluel T. Zou S. et al . (2017). Improvement on lipid production by Scenedesmus obliquus triggered by low dose exposure to nanoparticles. Sci. Rep.7, 15526. doi: 10.1038/s41598-017-15667-0
28
Holbrook G. P. Davidson Z. Tatara R. A. Ziemer N. L. Rosentrater K. A. Scott Grayburn W. (2014). Use of the microalga Monoraphidium sp. grown in wastewater as a feedstock for biodiesel: Cultivation and fuel characteristics. Appl. Energy131, 386–393. doi: 10.1016/j.apenergy.2014.06.043
29
Hossen R. Courtney M. Sim A. Khan M. A. A. K. Verbruggen H. Bringloe T. (2025). An optimized CTAB method for genomic DNA extraction from green seaweeds (Ulvophyceae). Appl. Plant Sci.13, e11625. doi: 10.1002/aps3.11625
30
Jin Y. Li Y. Qi Y. Wei Q. Yang G. Ma X. (2024). A modified cultivation strategy to enhance biomass production and lipid accumulation of Tetradesmus obliquus FACHB-14 with copper stress and light quality induction. Bioresour. Technol.400, 130677. doi: 10.1016/j.biortech.2024.130677
31
Karlilar Pata S. Pata U. K. (2025). Comparative analysis of the impacts of solar, wind, biofuels and hydropower on load capacity factor and sustainable development index. Energy319, 134991. doi: 10.1016/j.energy.2025.134991
32
Khoo K. S. Ahmad I. Chew K. W. Iwamoto K. Bhatnagar A. Show P. L. (2023). Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: A review. Prog. Energy Combust. Sci.96, 101071. doi: 10.1016/j.pecs.2023.101071
33
Kumar S. Stecher G. Li M. Knyaz C. Tamura K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol.35, 1547–1549. doi: 10.1093/molbev/msy096
34
Lortou U. Panteris E. Gkelis S. (2022). Uncovering new diversity of photosynthetic microorganisms from the mediterranean region. Microorganisms10, 1571. doi: 10.3390/microorganisms10081571
35
Lürling M. Van Donk E. (2000). Grazer-induced colony formation in Scenedesmus : are there costs to being colonial? Oikos88, 111–118. doi: 10.1034/j.1600-0706.2000.880113.x
36
Maltsev Y. Maltseva K. Kulikovskiy M. Maltseva S. (2021). Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biol. (Basel).10 (10), 1060. doi: 10.3390/biology10101060
37
Mandal S. Mallick N. (2009). Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol.84, 281–291. doi: 10.1007/s00253-009-1935-6
38
Mofeed J. Mosleh Y. Y. (2013). Toxic responses and antioxidative enzymes activity of Scenedesmus obliquus exposed to fenhexamid and atrazine, alone and in mixture. Ecotoxicol. Environ. Saf.95, 234–240. doi: 10.1016/j.ecoenv.2013.05.023
39
Mohamed S. Eladel H. Battah M. Ibrahim Y. (2022). Screening of the potentiality of four green microalgae to be used as feedstock for biodiesel and nutraceutical production. Egypt. J. Phycol.23, 1–33. doi: 10.21608/egyjs.2022.132130.1011
40
Morales M. Aflalo C. Bernard O. (2021). Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy150, 106108. doi: 10.1016/j.biombioe.2021.106108
41
Mulgund A. (2022). Increasing lipid accumulation in microalgae through environmental manipulation, metabolic and genetic engineering: a review in the energy NEXUS framework. Energy Nexus5, 100054. doi: 10.1016/j.nexus.2022.100054
42
Naselli-Flores L. Padisák J. (2023). Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia850, 2691–2706. doi: 10.1007/s10750-022-04795-y
43
Samoraj M. Çalış D. Trzaska K. Mironiuk M. Chojnacka K. (2024). Advancements in algal biorefineries for sustainable agriculture: Biofuels, high-value products, and environmental solutions. Biocatal. Agric. Biotechnol.58, 103224. doi: 10.1016/j.bcab.2024.103224
44
Sharif A. Bashir U. Mehmood S. Cheong C. W. H. Bashir M. F. (2024). Exploring the impact of green technology, renewable energy and globalization towards environmental sustainability in the top ecological impacted countries. Geosci. Front.15, 101895. doi: 10.1016/j.gsf.2024.101895
45
Sharma A. K. Jaryal S. Sharma S. Dhyani A. Tewari B. S. Mahato N. (2025). Biofuels from microalgae: A review on microalgae cultivation, biodiesel production techniques and storage stability. Processes13, 488. doi: 10.3390/pr13020488
46
Talebi A. F. Tabatabaei M. Chisti Y. (2014). BiodieselAnalyzer: a user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res. J.1, 55–57. doi: 10.18331/BRJ2015.1.2.4
47
Vladić J. Jazić J. M. Ferreira A. Maletić S. Cvetković D. Agbaba J. et al . (2023). Application of green technology to extract clean and safe bioactive compounds from tetradesmus obliquus biomass grown in poultry wastewater. Molecules28 (5) 2397. doi: 10.3390/molecules28052397
48
Wang J. Azam W. (2024). Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geosci. Front.15, 101757. doi: 10.1016/j.gsf.2023.101757
49
Wan Osman W. N. A. Rosli M. H. Mazli W. N. A. Samsuri S. (2024). Comparative review of biodiesel production and purification. Carbon Capture Sci. Technol.13, 100264. doi: 10.1016/j.ccst.2024.100264
Summary
Keywords
biodiesel production, fatty acid methyl esters (FAMEs), microalgae, Monoraphidium sp., Tetradesmus obliquus
Citation
Mofeed J, Mamdouh I, Eltanahy E, Shehata AI, Elzaawely AA, Teiba II and El-Bilawy EH (2025) Evaluation of Tetradesmus obliquus and Monoraphidium sp. as potential feedstocks for sustainable biodiesel production. Front. Mar. Sci. 12:1616335. doi: 10.3389/fmars.2025.1616335
Received
23 April 2025
Accepted
20 May 2025
Published
06 June 2025
Volume
12 - 2025
Edited by
Santhiyagu Prakash, Tamil Nadu Fisheries University, India
Reviewed by
Shankha Koley, Indian Institute of Technology Kharagpur, India
Manikandan Gurusamy, Tshwane University of Technology, South Africa
Updates
Copyright
© 2025 Mofeed, Mamdouh, Eltanahy, Shehata, Elzaawely, Teiba and El-Bilawy.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Islam I. Teiba, islam.tayba@agr.tanta.edu.eg; Akram Ismael Shehata, akramismael2@alexu.edu.eg; akramismael2@gmail.com
†These authors share first authorship
‡These authors share last authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.