Introduction
Plastic debris in the marine environment is comprised of various synthetic organic polymers, including polypropylene, polystyrene, polyamide, polyvinyl chloride, and polyethylene (PE). Among these, polyethylene (PE) is the most abundant type of plastic debris (Tao et al., 2023). PE is the most prevalent form of plastics due to high production rate. The presence of PE-MP in marine environment significantly alters the potential toxicity to marine invertebrates and vertebrates through rapid ingestion post weathering (Gewert et al., 2021). Research has shown that exposure to polyethylene can lead to increased mortality rates in various vertebrate and invertebrate species (Kühn and van Franeker, 2018; Ziajahromi et al., 2018; El-Sherif et al., 2022);. MPs by themselves and also due to leaching of various toxic chemicals into the marine environment has adverse effects on organisms including stunted growth, decreased reproductive success (Rochman et al., 2013), histopathological changes, aberrant offspring development (Browne et al., 2013; Hariharan et al., 2022), intestinal blockages, altered behavior, decreased feeding, and elevated mortality (Browne et al., 2013; Lusher et al., 2013).PE is inert and capable of adsorbing pollutants in environment causing immense toxic effects to nontarget organism (Teuten et al., 2009). Their presence could alter the entire food web leading to deleterious impacts on human health.
In this study we used woven sac bags which are prevalent across various industries and exhibit high reusability due to their malleability, structural integrity, and resistance to corrosion. In the context of eliminating plastics, High-Density Polyethylene (HDPE) emerges as one of the most versatile polymers, characterized by its linear chain structure, which imparts increased density and strength. The processes of weathering, photodegradation, and biofouling contribute to an elevation in HDPE density, causing it to settle at the substrate and become more accessible to filter feeders (Ali Chamas et al., 2020). Weathering further exacerbates the fragmentation of microplastics (MPs), resulting in an augmented surface area that enhances their reactivity and susceptibility to degradation (Klein et al., 2018).
Modulation of antioxidants leads to oxidative stress that can damage DNA, cell components, and inactivate enzymes. Antioxidants are essential for preserving homeostasis and aquatic organisms have been demonstrated to upregulate their antioxidant systems including glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD), as well as non-enzymatic free radical scavengers, such as reduced glutathione (GSH) and biotransformation enzymes, such as glutathione-S-transferase (GST). Changes in these antioxidants’ levels can therefore be used as markers of xenobiotic exposure (Singaram et al., 2013). It is helpful to identify possible exposure to hazardous chemicals by using a battery of biomarkers because many of the pollutants to which crustaceans may be exposed are frequently unknown (Gopalakrishnan et al., 2011).
Over the past ten years, marine shrimp farming has grown significantly in India due to rising domestic shrimp consumption and overseas exports, particularly in South India, which has the most shrimp farms on the country’s east coast (Krishnan and Babu, 2022). Furthermore, the region’s mariculture has become contaminated as a result of the significant industrial expansion and urbanization that have occurred in this same area. Shrimp can accumulate contaminants, microplastics which can have a variety of negative physiological and metabolic impacts. In a prior investigation, commercial fish collected from south India’s coastal region were found to contain microplastics (Janardhanam et al., 2022; Frank et al., 2023; Harikrishnan et al., 2023).
Only a few studies have examined the bioaccumulation of MPs/MFs in crab larvae, where these authors have investigated effects of MPs on survival, feeding and swimming behavior (Wang et al., 2020; Woods et al., 2020; Hodkovicova et al., 2022; Timilsina et al., 2023). Our previous studies show that high density poly ethylene induce proximate imbalance in the post larval stages of Litopenaeus vannamei (Leela et al., 2025). Until now to our knowledge there are no studies on the effects of weathered MPs concerning the changes in antioxidant level during the early growth. The current investigation was undertaken to evaluate the interactions between environmentally weathered high-density polyethylene microplastics (wHDPE-MPs) in L. vannamei under controlled laboratory conditions. L. vannamei was selected due to its increasing commercial significance, ecological relevance, and the substantial environmental variability it encounters. The primary objectives of this study were to (i) assess the accumulation of small-sized wHDPE-MPs in L. vannamei and (ii) examine the modulation of antioxidant responses in post larval stage of L. vannamei under chronic exposure to wHDPE-MPs. This research aims to enhance our understanding of the detrimental effects of wHDPE-MP particles on the developmental stage of invertebrates under coastal and aquaculture ecosystems.
Methodology
Post-larvae (PL-10) white leg shrimp (Litopennaeus vannamei) were obtained from commercial hatchery in Marakanam (Tamil Nadu, India) and transported to the laboratory and acclimated for a period of 5 days under laboratory condition in 100 L glass tank with continuous aeration, 20 PSU of salinity, and a 12:12 h light/dark photoperiod (800–1200 lx). The water was changed every day. Until the necessary salinity was reached, the original salinity of 25 PSU was steadily increased by 2 PSU every 24 hours (Hariharan et al., 2022). The shrimps were fed daily with micro particulated PL-shrimp feed considering the 10% of the biomass. Using pre-calibrated Hydro-Lab, USA-multi parameter water quality probes, physiochemical parameters such as pH, temperature, and salinity were frequently monitored.
High density weathered Microplastics (wHDPE) were prepared following the methods of Hariharan et al. (2022). Briefly, plastic woven sacks that had partly weathered were retrieved from the beaches along the Chennai coast for the current study and the gathered plastic woven sacks were thoroughly cleaned with distilled water. The material was characterized using Fourier Transform Infrared Spectroscopy-FTIR (PerkinElmer Spectrum Version 10.5.4) to identify the polymer type. The characterized materials were then promptly pulverized to fine grains in a ball mill sediment grinder (Fritsch Industrie Str. 8, Idar-Oberstein, Germany) after being frozen at -80°C. Later, the fine particles were sieved through a sequence of test sieves less than 45 μm and the particles retained in 45 μm test sieve were taken for the investigation. Multiple measurements were used to determine the concentration per microgram of microplastics, the number of dissolved wHDPE particles, and the size ratio of the particles. To ensure there were enough clean particles, the wHDPE microplastics were carefully combined with 1 ml milli-Q water in sterile tubes. To induce equal mixing of the particles in the water, the tubes were vigorously swirled. The particles were then immediately transferred to a Sedgewick-Rafter counting slide and counted under a microscope. The tube was examined for particles that had attached to their surfaces in order to standardize the number of particles in the prepared concentration.
Toxicity procedures were adopted from the protocol of USEPA, (1998). In the present study, L. vannamei were exposed to two concentrations of wHDPE microplastics. Triplicate tanks were maintained for each concentration. The concentration used for the present study was chosen from the previous literature (Hariharan et al., 2022; Leela et al., 2025). The larvae in Group I was reared in normal seawater without any microplastics as control group. Group II larvae were exposed to 0.1 mg/L and Group III were exposed to 0.2mg/L wHDPE microplastic in seawater. The whole exposure experiment was conducted through static renewal test method for 45 days. 150 larvae were used per replicate per group for the chronic exposure. To avoid the settlement of suspended wHDPE-MP in the tanks mild aeration were provided. Seawater was changed once in two days. Following exposure the animals were maintained in the normal seawater for depuration for 15 days. The concentrations were selected based on environmental conditions and previous literature (Hariharan et al., 2022). The experimental shrimps were kept under a 12:12 h light-dark cycle. Moreover, physicochemical information including water temperature, salinity, pH, and dissolved oxygen was recorded twice daily using a multiparameter water quality probe (Hydrolab, USA). Dissolved oxygen (5± 2 mg/L), salinity (24 ± 1 ppt), temperature (28 ± 1 oC), and pH (8.1 ± 0.1) were maintained throughout the experimental period.
Sampling was done at every 15 days interval, so total 3 time points were fixed for exposure study and one time point was fixed for depuration study. At every time point 30 larvae from each tank were taken and homogenized in a buffer (pH 7.6) that contained 0.5 M sucrose, 20 mM Tris, 1 mM EDTA, 1 mM DTT, 0.15 M KCl, and 0.05 mM phenylmethylsulfonyl fluoride (PMSF). The samples were then centrifuged for 20 min at 4°C at 10,000×g, and the supernatants were utilized for testing the oxidative stress and antioxidant associated parameters. CAT activity was determined according to the method of Sinha (1972) and expressed as µmol of H2O2 consumed/min/mg protein. The Marklund and Marklund approach (1974) was used to measure the extent of inhibition of pyrogallol auto-oxidation at an alkaline pH in order to estimate SOD activity. GPx was calculated using the Rotruck et al. (1973) methodology, which involved measuring the amount of GSH consumed in the reaction mixture. According to Moron et al. (1979), GSH was determined by measuring the optical density of the yellow color produced when glutathione reduces 5,5’-dithio-bis-(2-nitrobenzoic acid) (DTNB) at 412 nm. Glutathione S-transferase (GST) activity was measured using the 1-chloro-2, 4-dinitrobenzene (CDNB) substrate following the conjugation of glutathione with the acceptor substrate (Habig et al., 1974). Malondialdehyde (MDA) level was expressed as nmol of MDA generated/mg protein, the color developed was measured at 532 nm, and LPO was computed using the Devasagayam and Tarachand (1987) methodology. According to Lushchak et al. (2005), the amount of protein carbonyl (CP) was determined by reacting with 2,4-dinitrophe-nylhydrazine (DNPH). CP was measured using spectrophotometry at 370 n, indicating a molar extinction coefficient of In the guanidine chloride solution, concentrations of CP were quantified as nanomoles of CP per milligram of protein (103 M-1 cm-1). Total ROS was measured following Driver et al. (2000).Statistical analysis was carried out using SPSS (version 20). The results of the measured parameters are presented as the mean ± SD after being examined for significance. The data was analyzed using one-way analysis of variance (ANOVA) and then post-hoc Tukey’s multiple-comparison test. To ascertain how the parameters interacted with one another, the Pearson correlation coefficient was employed.
Results and discussion
The primary antioxidant and associated enzymes such as catalase, SOD, GPx, GST and GSH in the PL stage of L. vannamei were observed to be modulated as the concentration and the exposure periods increased (Figure 1). SOD activities increased as the exposure period and MP concentration increased. After 30 days of exposure the SOD values for control was 0.232 ± 0.014 and the same increased to 0.333 ± 0.040 when the PL were exposed to 0.2 mg/L. Similarly, during depuration the level of SOD in both exposed concentration was greater compared to respective controls however the lower concentration did not show significant difference. After 15 days of depuration period the SOD values in control group was 0.239 ± 0.021 and the same was 0.316 ± 0.032 at 0.2mg/L of MPs. The GSH and GPx activities decreased in the PL stage when exposed to MPs, however the highest concentration and the longest exposure periods showed significant decrease compared to the respective controls. After 45 days of exposure GSH value in control group was 32.36 ± 3.52 and the same decreased to 19.76 ± 2.82 at 0.2mg/L. Similarly after 45 days of exposure GPx value in control group was 93.97 ± 4.36 and the same decreased to 51.93 ± 8.93 at 0.2mg/L Moreover the PL stage did not show any recovery for these two antioxidants during the depuration period. GST activities were induced significantly in PL stage due to MP exposure and this was observed to be dose dependent. Following 45 days of exposure, GST activity in control group was 1.05 ± 0.33 and it increased to 5.33 ± 1.58 at 0.2mg/L. Similarly during the depuration period the level of GST were greater compared to the respective control groups.
Figure 1
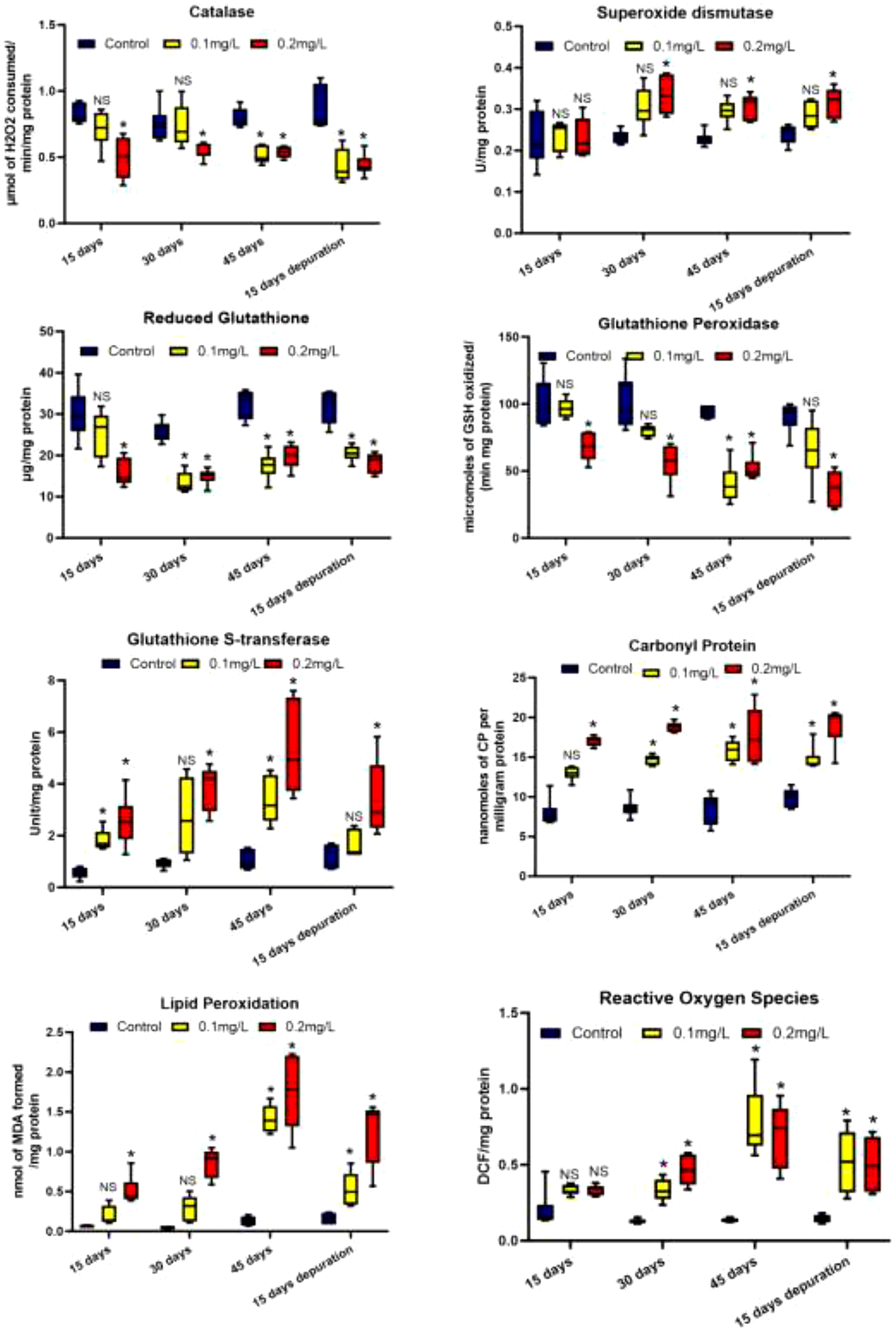
Antioxidant response and oxidative stress levels in post larval stages of Litopenaeus vannamei exposed to weathered high density polyethylene microplastics. The line in each box represent median and whiskers represents the upper and lower 95% confidence intervals of the mean of six determinations using samples from different preparations. One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used. The significant (P < 0.05) differences between control and exposure groups were indicated by the asterisks (*); NS indicate no significant difference with respective control group.
The first antioxidant enzyme that scavenges superoxide radicals (O2_) is SOD, while CAT converts H2O2 that is produced as a result of the reaction that SOD catalyzes. The lower levels of SOD and CAT activity seen in this investigation are in line with ROS production occurring in response to MPs. Increased formation of the superoxide anion radical, which has been shown to impede CAT activity, may also be the cause of the decreased CAT activity (Snezhkina et al., 2019). It is possible that the lower levels of enzymatic and non-enzymatic antioxidant activity found in the tissues of L. vannamei exposed to MPs represent an adaptive response of prawns to oxidative stress due to wHDPE exposure.
CP was found in greater levels in the PL stage of L. vannamei subjected to the highest wHDPE concentration in all the exposure periods (Figure 1). After 15 days of exposure, the CP levels in the control group was 7.96 ± 1.56 and it increased to 17.04 ± 0.56.when the PL were exposed to highest concentration at 0.2mg/L, Similarly, when the PL stage was exposed for 30 and 45 days to both MPs concentrations, CP were greater compared with the respective control group and, all these values were statistically significant (p<0.01). Lipid peroxidation levels in the PL stage were measured in the form of MDA. MPs at higher concentration caused a significant increase in oxidative stress in PL stage after all the exposure periods (Figure 1). After 15 and 30 days of exposure the LPO levels in PL stage were 0.06 ± 0.007 and 0.046 ± 0.009 in control group and it increased to 0.509 ± 0.162 and 0.859 ± 0.167 at 0.2mg/L respectively, and these values are highly significant compared to the respective control group. ROS levels in PL stage increased significantly after 30 and 45 days of MP exposure in case of both the MP concentration, however after 15 days the change was insignificant with respective control group (Figure 1).
Although xenobiotics can cause adaptive changes, such as phase I enzymes that oxidize them to make them more polar so they can be conjugated and eliminated, this process involves redox reactions that can produce free radicals (Gu and Manautou, 2012; Banaee et al., 2025). The existence of several organic and inorganic pollutants might induce the generation of ROS. According to Almeida et al. (2007), ROS can cause oxidation of proteins, lipids, and DNA, and other biological components. Little evidence of differential ROS production was seen in the tissues of L. vannamei exposed to MPs in the PL stage in the current investigation. The findings indicating ROS damage was higher at the highest concentration tested for the PL stage are strikingly comparable to those obtained when fish fry were exposed to weathered MPs (Janardhanam et al., 2022; Páez-Osuna et al., 2024). Protein oxidation is the outcome of carbonylation and creation of irreversible protein carbonyls (Estévez et al., 2022), can lead to protein conformational changes, and this is one of the consequence of ROS production. As a result of the alteration, enzyme catalytic activity would decrease, which will ultimately lead to protein degradation (Zhang et al., 2008).
Induced carbonyl protein levels in PL stages exposed to MPs which may be a sign of exposure stress in PL stage of L. vannamei. A rise would probably be the more drastic of the two conceivable reactions. PL subjected to 0.2 mg of MPs had higher CP. Such levels, however, probably have no negative effects that could compromise the PL stages of L. vannamei. Numerous investigations have shown that aquatic organisms exposed in vivo to various pollutants exhibit increased levels of LPO in a variety of tissues (Almeida et al., 2007). GPx and GSH are antioxidants which can be depleted during ROS scavenging, which is supported by the current study’s findings that prawns exposed to MPs had greater MDA concentrations, reduced GPx activity, and lower GSH concentrations. Exposure to a variety of contaminants can deplete antioxidant defenses, increasing susceptibility to lipid peroxidation. Significant reduction in GPx activity and corresponding rise in MDA are in line with glutathione’s function in electrophile detoxification. Greater use of GSH by GPx to catalyze the reduction of H2O2 to H2O and O2 is consistent with lower GSH concentrations. Lower glutathione concentrations are the cause of GPx’s decreased action (Raffa et al., 2011). The increased GST activity after MP exposure indicates that detoxifying processes have been activated. These results imply that MPs interfere with at least one of the detoxification and lipid peroxidation processes, which are both impacted by GST. Since MPs have been demonstrated to induce oxidative stress in a number of species (Paul-Pont et al., 2016; Páez-Osuna et al., 2024), it is possible that the increased GST activity seen in prawns exposed to MPs is an adaptive reaction to oxidative stress.
The relationship between the measured antioxidant associated components in PL stage of L. vannamei exposed to wHDPE MPs for 45 d is depicted in Figure 2 by a correlation analysis; the majority of the measured components tested exhibit highly significant relation r values. After 45 d of exposure to wHDPE MPs, most of the parameters showed significant correlation with other antioxidant and oxidative stress associated parameters. Notably, the several antioxidant parameters in PL stage of L. vannamei exhibited a negative correlation with large r values (Figure 2). GST have a negative correlation with CAT, GPx and GSH and all these (r) values were highly significant. Similarly LPO have a positive correlation with CP and ROS generated in the tissues of PL stage of L. vannamei. In the majority of these cases, the correlation was very significant. The majority of oxidative stress related indicators exhibit a negative correlation with antioxidants. These findings unequivocally demonstrate that increased stress-related parameters directly affect antioxidant related parameters. There is a positive and negative relationship between the parameters under study as a result of the effect of wPE MPs on antioxidant-associated measures. The majority of the measured components examined had extremely significant relation r values, according to the relationship between the measured oxidative stress and antioxidant responses in PL stage of L. vannamei exposed to wHDPE MPs for 45 d, moreover illustration of the correlation results demonstrated that the correlation coefficients were more than 0.700 in most cases.
Figure 2
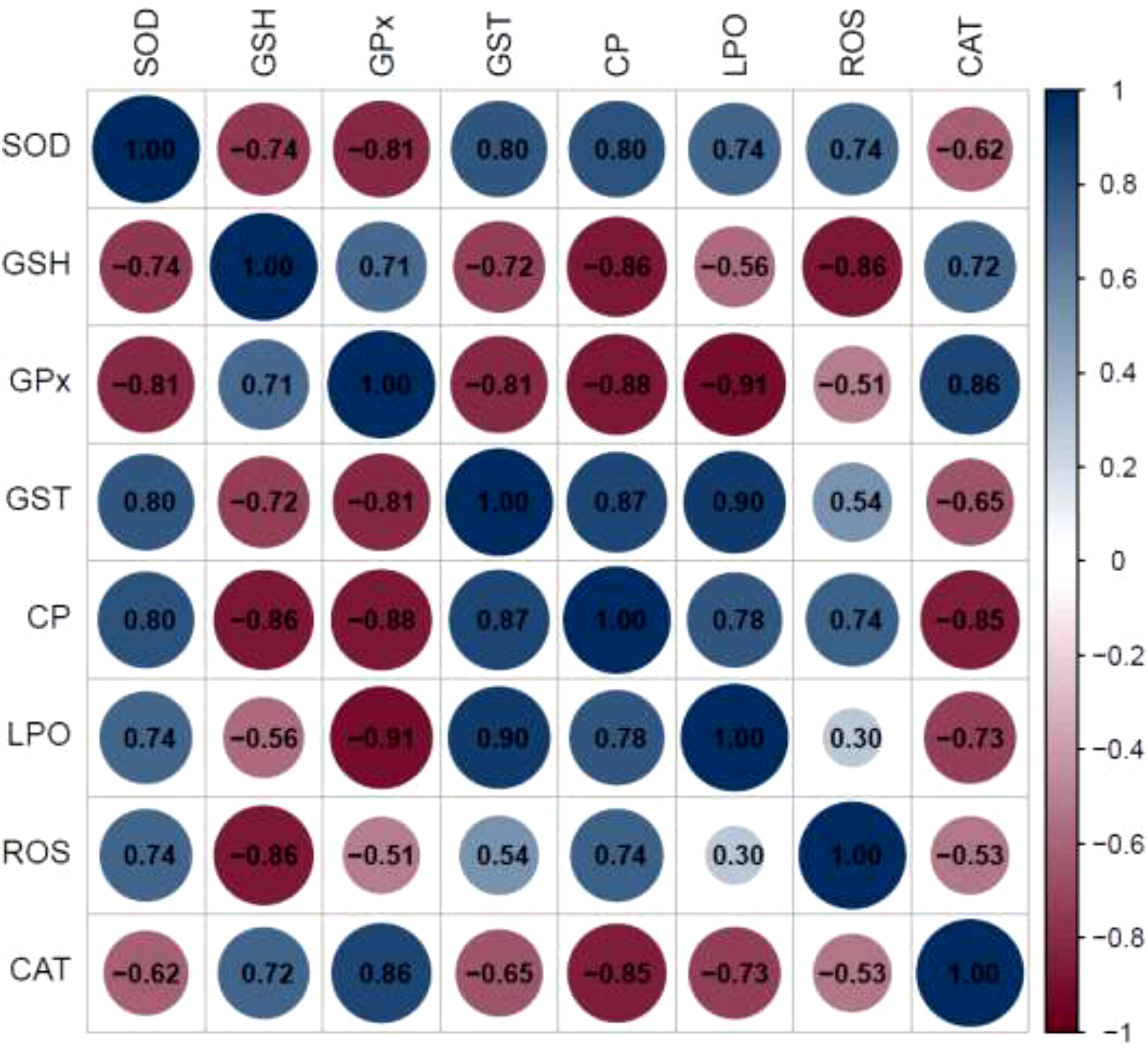
Depicts the correlation analysis between the oxidative stress and antioxidant response in post larval stages of Litopenaeus vannamei exposed to weathered high density polyethylene microplastics. The blue color represents the positive correlation and the pink tint the negative connection. The measured components’ higher relation is shown by the dark color, and their lesser relation is shown by the light hue.
In this investigation, exposure to MPs clearly caused an increase in SOD and a decrease in GPx activities in the PL stage of L. vannamei. The activities remained elevated and never returned to normal during the depuration phase. According to these findings, MPs exposure might cause SOD and reduce GPx activities; however, at lower MPs exposure concentrations, the activities would considerably decline following a period of depuration. Prior research have shown that stored electrons might be used to generate ROS utilizing whatever oxygen was left, increasing the generation of ROS under stress conditions brought on by MPs exposure (Li et al., 2016; Páez-Osuna et al., 2024).
Biological macromolecules like proteins, lipids, and DNA would be harmed when ROS concentrations were higher than the levels of antioxidant (Páez-Osuna et al., 2024). MDA was produced oxidatively when free radicals and polyunsaturated fatty acids reacted, and its quantity may serve as a marker of oxidative stress. MDA concentrations in the PL stages subjected to the greatest concentration of MPs in this investigation were higher than the control, and following a period of depuration, the concentrations were unable to recover to normal. This suggested that MPs increased oxidative stress, which can be difficult to recover from.
In conclusion, our data suggests that the oxidative stress produced may reveal information about the overall health of an organism exposed to MPs, and more specifically to wHDPEs. These findings lead us to hypothesize that, like in higher vertebrates and other invertebrates, the CAT, SOD, and glutathione-mediated antioxidant may play a significant role in shrimp’s detoxification process. The increased SOD activity indicates that these processes are generating reactive oxygen species, which is supported by the rise in oxidative stress parameters, clearly states suggesting that the antioxidant system is modulated due to MPs exposure. Moreover when combined, these biomarkers may be essential instruments for determining the exposure and toxicity of MPs to marine life, and they can be applied in routine monitoring programs for coastal and marine environments.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
ML: Writing – original draft, Formal analysis. VP: Writing – original draft, Formal analysis. SS: Methodology, Writing – original draft. SA: Writing – review & editing, Project administration. SM: Methodology, Writing – original draft. SE: Formal analysis, Writing – original draft. HT: Writing – original draft, Validation, Supervision. RT: Validation, Writing – review & editing, Writing – original draft. RK: Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Head, PG and Research Department of Zoology and Aquaculture and also the Principal Government Arts College for Men Chennai-600 035.We acknowledge the Research department of Zoology Pachaiyappa’s College for Men, Chennai- 600–030 for their help during the experimental analysis. Staff from National Centre for Sustainable Coastal Management, Chennai- 600–025 are also acknowledged for the suggestions and help throughout the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Almeida E. A. Bainy A. C. D. deMeloLoureiro A. P. Martinez G. R. Miyamoto S. Onuki J. et al . (2007). Oxidative stress in Perna perna and other bivalves as indicators of environmental stress in the Brazilian marine environment:antioxidants,lipid peroxidation and DNA damage. Comp. Biochem. Physiol.A146, 588–600.
2
Banaee M. Multisanti C. R. Impellitteri F. Piccione G. Faggio C. (2025). Environmental toxicology of microplastic particles on fish: A review. Comp. Biochem. Physiol. C. Toxicol. Pharmacol.287, 110042. doi: 10.1016/j.cbpc
3
Browne M. A. Niven S. J. Galloway T. S. Rowland S. J. Thompson R. C. (2013). Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol.23, 2388–2392. doi: 10.1016/j.cub.2013.10.012
4
Chamas A. Moon H. Zheng J. Qiu Y. Tabassum T. Jang J. H. et al . (2020). Degradation Rates of Plastics in the Environment ACS Sustainable. Chem. Eng.8, 3494–3511.
5
Devasagayam T. P. Tarachand U. (1987). Decreased lipid peroxidation in rat kidneys during gestation. Biochem. Biophys. Res. Commun.145, 134–138. doi: 10.1016/0006-291X(87)91297-6
6
Driver A. S. Kodavanti P. R. Mundy W. R. (2000). Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol. Teratol.22, 175–181. doi: 10.1016/S0892-0362(99)00069-0
7
El-Sherif D. M. Eloffy M. G. Elmesery A. Abouzid M. Gad M. El-Seedi H. R. et al . (2022). Environmental risk, toxicity, and biodegradation of polyethylene: a review. Environ. Sci. Pollut. Res.29, 81166–81182. doi: 10.1007/s11356-022-23382-1
8
Estévez M. Díaz-Velasco S. Martínez R. (2022). Protein carbonylation in food and nutrition: a concise update. Amino Acids Apr.54, 559–573. doi: 10.1007/s00726-021-03085-6
9
Frank Y. A. Interesova E. A. Solovyev M. M. Xu J. Vorobiev D. S. (2023). Effect of Microplastics on the Activity of Digestive and Oxidative-Stress-Related Enzymes in Peled Whitefish (Coregonus peled Gmelin) Larvae. Int. J. Mol. Sci.24, 10998. doi: 10.3390/ijms241310998
10
Gewert B. MacLeod M. Breitholtz M. (2021). Variability in Toxicity of Plastic Leachates as a Function of Weathering and Polymer Type: A Screening Study with the Copepod Nitocra spinipes. Biol. Bull.240, 191–199. doi: 10.1086/714506
11
Gopalakrishnan S. Chen F. Y. Thilagam H. Qiao K. Xu W. F. Wang K. J. (2011). Modulation and Interaction of Immune-Associated Parameters with Antioxidant in the Immunocytes of Crab Scylla paramamosain Challenged with Lipopolysaccharides. Evid. Based Complement Alternat. Med., 824962. doi: 10.1155/2011/824962
12
Gu X. Manautou J. E. (2012). Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med.14, e4. doi: 10.1017/S1462399411002110
13
Habig W. H. Pabst M. J. Jakoby W. B. (1974). Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.249, 7130–7139. doi: 10.1016/S0021-9258(19)42083-8
14
Hariharan G. Purvaja R. Anandavelu I. Robin R. S. Ramesh R. (2022). Ingestion and toxic impacts of weathered polyethylene (wPE) microplastics and stress defensive responses in whiteleg shrimp (Penaeus vannamei). Chemosphere.300, 134487. doi: 10.1016/j.chemosphere.2022.134487
15
Harikrishnan T. Janardhanam M. Sivakumar P. Sivakumar R. Rajamanickam K. Raman T. et al . (2023). Microplastic contamination in commercial fish species in southern coastal region of India. Chemosphere.313, 137486. doi: 10.1016/j.chemosphere.2022.137486
16
Hodkovicova N. Hollerova A. Svobodova Z. Faldyna M. Faggio C. (2022). Effects of plastic particles on aquatic invertebrates and fish - A review. Environ. Toxicol. Pharmacol.96, 104013. doi: 10.1016/j.etap.2022.104013
17
Janardhanam M. Sivakumar P. Srinivasan G. Sivakumar R. Marcus P. N. Balasubramaniam S. et al . (2022). Microplastics in Demersal Sharks From the Southeast Indian Coastal Region. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.914391
18
Klein S. Dimzon I. K. Eubeler J. Knepper T. P. (2018). “Analysis, Occurrence, and Degradation of Microplastics in the Aqueous Environment,” in Freshwater Microplastics. The Handbook of Environmental Chemistry, vol. 58 . Eds. WagnerM.LambertS. (Springer, Cham). doi: 10.1007/978-3-319-61615-5_3
19
Krishnan M. Babu S. C. (2022). Covid-19 Opens up domestic market for Indian shrimp. Aquaculture550, 737818. doi: 10.1016/j.aquaculture.2021.737818
20
Kühn S. van Franeker J. A. (2018). Quantitative overview of marine debris ingested by marine megafauna. Mar. Pollut. Bull.151, 110858. doi: 10.1016/j.marpolbul.2019.110858
21
Leela M. Pushpalatha V. Swetha S. Anusuya S. Madhavan S. Eswari S. et al . (2025). Weathered High Density Polyethylene Microplastics Induce Proximate Imbalance in the Post Larval Stages of Litopenaeus Vannamei. Uttar Pradesh J. Zool.46, 388–399. doi: 10.56557/upjoz/2025/v46i94951
22
Li Y. Wei L. Cao J. Qiu L. Jiang X. Li P. et al . (2016). Oxidative stress, DNA damage and antioxidant enzyme activities in the pacific white shrimp (Litopenaeus vannamei) when exposed to hypoxia and reoxygenation. Chemosphere.144, 234–240. doi: 10.1016/j.chemosphere.2015.08.051
23
Lushchak V. I. Bagnyukova T. V. Husak V. V. Luzhna L. I. Lushchak O. V. Storey K. B. (2005). Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in gold fish tissues. Int. J. Biochem. Cell Biol.37, 1670–1680. doi: 10.1016/j.biocel.2005.02.024
24
Lusher A. L. McHugh M. Thompson R. C. (2013). Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull.67, 94–99. doi: 10.1016/j.marpolbul.2012.11.028
25
Marklund S. Marklund G. (1974). Involvement of superoxide radicals in the auto oxidation of pyrogallol and a convenient assay for superoxide pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.47, 469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x
26
Moron M. S. Fierre J. Mannerwick B. (1979). Levels of glutathione, glutathione reductase and GST activities in rat lung and liver. Biochem. Biophys. Acta582, 67–78. doi: 10.1016/0304-4165(79)90289-7
27
Páez-Osuna F. Valencia-Castañeda G. Bernot-Simon D. Arreguin-Rebolledo U. (2024). A critical review of microplastics in the shrimp farming environment: Incidence, characteristics, effects, and a first mass balance model. Sci. Total Environ.955, 176976. doi: 10.1016/j.scitotenv.2024.176976
28
Paul-Pont I. Lacroix C. González Fernández C. Hégaret H. Lambert C. Le Goïc N. et al . (2016). Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut., 2016, 724–737. doi: 10.1016/j.envpol.2016.06.039
29
Raffa M. Atig F. Mhalla A. Kerkeni A. Mechri A. (2011). Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry11, 124. doi: 10.1186/1471-244X-11-124
30
Rochman C. Hoh E. Kurobe T. Swee J. T. (2013). Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep.3, 3263. doi: 10.1038/srep03263
31
Rotruck J. T. Pope A. L. Gather H. E. Swanson A. B. Hafeman D. G. Hoekstra W. G. (1973). Selenium biochemical roleas a component of glutathione peroxidase. Science179, 588–590. doi: 10.1126/science.179.4073.588
32
Singaram G. Harikrishnan T. Chen F. Y. Bo J. Giesy J. P. (2013). Modulation of immune-associated parameters and antioxidant responses in the crab (Scylla serrata) exposed to mercury. Chemosphere.90, 917–928. doi: 10.1016/j.chemosphere.2012.06.031
33
Sinha A. K. (1972). Colorimetric assay of catalase. Anal.Biochem47, 389–394. doi: 10.1016/0003-2697(72)90132-7
34
Snezhkina A. V. Kudryavtseva A. V. Kardymon O. L. Savvateeva M. V. Melnikova N. V. Krasnov G. S. et al . (2019). ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell Longev.2019, 6175804. doi: 10.1155/2019/6175804
35
Tao X. Ouyang H. Zhou A. Wang D. Matlock H. Morgan J. S. et al . (2023). Polyethylene Degradation by a Rhodococcous Strain Isolated from Naturally Weathered Plastic Waste Enrichment. Environ. Sci. Technol.57, 13901–13911. doi: 10.1021/acs.est.3c03778
36
Teuten E. L. Saquing J. M. Knappe D. R. Barlaz M. A. Jonsson S. Björn A. et al . (2009). Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. Lond. B. Biol. Sci.364, 2027–2045. doi: 10.1098/rstb.2008.0284
37
Timilsina A. Adhikari K. Yadav A. K. Joshi P. Ramena G. Bohara K. (2023). Effects of microplastics and nanoplastics in shrimp: Mechanisms of plastic particle and contaminant distribution and subsequent effects after uptake. Sci. Total Environ.894, 164999. doi: 10.1016/j.scitotenv.2023.164999
38
USEPA . (1998). Guidelines for Ecological risk assessment. 63(93), 26846–26924. doi: 10.1016/j.marpolbul.2019.110660
39
Wang X. Liu L. Zheng H. Wang M. Fu Y. Luo X. et al . (2020). Polystyrene microplastics impaired the feeding and swimming behavior of mysid shrimp Neomysis japonica. Mar. Pollut. Bull.150, 110660. doi: 10.1016/j.marpolbul.2019.110660
40
Woods M. N. Hong T. J. Baughman D. Andrews G. Fields D. M. Matrai P. A. (2020). Accumulation and effects of microplastic fibers in American lobster larvae (Homarus americanus). Mar. Pollut. Bull.157, 111280. doi: 10.1016/j.marpolbul.2020.111280
41
Zhang X. Yang F. Zhang X. Xu Y. Liao T. Song S. et al . (2008). Induction of Hepatic enzymes and oxidative stress in Chinese rare minnow (Gobiocypris rarus) exposed to water borne hexa bromo cyclodo decane (HBCDD). Aquat. Toxicol.86, 4–11. doi: 10.1016/j.aquatox.2007.07.002
42
Ziajahromi S. Kumar A. Neale P. A. Leusch F. D. L. (2018). Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ. Pollut.236, 425–431. doi: 10.1016/j.envpol.2018.01.094
Summary
Keywords
weathered microplastics, oxidative stress, antioxidant, post larval stages, Litopenaeus vannamei
Citation
Leela M, Pushpalatha V, Swetha S, Anusuya S, Madhavan S, Eswari S, Thilagam H, Thiagarajan R and Krishnamurthy R (2025) Ingestion of weathered high density polyethylene microplastics-induced oxidative stress and modulation of antioxidant responses in post larval stages of Litopenaeus vannamei. Front. Mar. Sci. 12:1617070. doi: 10.3389/fmars.2025.1617070
Received
24 April 2025
Accepted
26 May 2025
Published
10 June 2025
Volume
12 - 2025
Edited by
Ashok Kumar Sekar, Anna University, India
Reviewed by
Chezhian Eswer, Dharmapuram Gnanambigai Government Arts College for Women, India
Jayaprakashvel Mani, Amet University, India
Meiyalagan Velayutham, Kongunadu Arts and Science College, India
Updates
Copyright
© 2025 Leela, Pushpalatha, Swetha, Anusuya, Madhavan, Eswari, Thilagam, Thiagarajan and Krishnamurthy.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Krishnamurthy, professorkrishnamurthy@gmail.com
†These authors contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.