- 1Subtropical Fisheries Research Institute, National Institute of Fisheries Science, Jeju, Republic of Korea
- 2Department of Fisheries Biomedical Sciences, Mokpo National University, Muan, Republic of Korea
Introduction: Chub mackerel (Scomber japonicus) is an economically important fishery resource in Northeast Asia. However, increasing consumer demand combined with declining wild stocks has highlighted the urgent need to develop aquaculture techniques for stable production. Currently, large-scale larval production is hindered by insufficient knowledge of broodstock reproductive biology under aquaculture conditions. Thus, identifying reproductive patterns of chub mackerel in land-based systems is essential to optimize breeding strategies and juvenile production. Methods: We monitored the reproductive characteristics of approximately one-year-old chub mackerel broodstock (initial weight ~250 g), maintained in a land-based tank under natural temperature and photoperiod conditions for 12 months. Monthly sampling included measuring gonadosomatic indices (GSI), histological analysis of gonadal developmental stages, plasma sex steroid hormones (testosterone [T], 11-ketotestosterone [11-KT], 17β-estradiol [E2]), and expression analysis of reproduction-related genes (fshβ and lhβ).
Results and Discussion: Male GSI markedly increased from March to June, peaking between April and June. Female GSI rose from March, reaching its peak in June. Histologically, gonads of both sexes showed four distinct developmental stages. Males entered late spermatogenesis in March, spermiation in April, and post-spawning by July. Females progressed through early vitellogenesis in March, late vitellogenesis by May, and post-spawning by July. Actual spawning events, confirmed by the presence of post-ovulatory follicles and egg release, occurred from late May to mid-July. This reproductive season, initiated by rising seawater temperatures, coincides with the natural spawning period reported for wild populations, suggesting that aquaculture conditions effectively replicated environmental spawning triggers. Plasma steroid hormones (T, 11-KT, and E2) peaked during key reproductive phases, closely mirroring gonadal development. Concurrently, expression of reproductive genes (fshβ, lhβ) correlated significantly with plasma hormone fluctuations, indicating regulatory roles of follicle-stimulating hormone and luteinizing hormone in gonadal maturation and spawning. These findings enhance our understanding of reproductive physiology in chub mackerel under controlled conditions, providing critical data for optimizing broodstock management and establishing reliable spawning strategies essential for sustainable aquaculture production.
1 Introduction
Understanding the reproductive biology of fish species is prioritized to provide basic knowledge to establish resource conservation plans and regulatory strategies for fishery production and for broodstock management, such as determining the spawning period and planning detailed breeding schemes in aquaculture (Grandcourt et al., 2009; Kujawa et al., 2019; Kucharczyk et al., 2023). The success of commercial-scale fish aquaculture predominantly depends on sufficient production of high-quality larvae from broodstock (Kujawa et al., 2019). Therefore, numerous studies have been conducted to identify the reproductive cycles of various fish species, including flatfish (Paralichthys dentatus, Pleuronectes americanus, and Tanakius kitaharai) (Morse, 1981; Harmin et al., 1995; Narimatsu et al., 2007), greater amberjack (Seriola dumerilii) (Micale et al., 1999), groupers (Epinephelus coioides, Epinephelus ongus, and Epinephelus marginatus) (Chai et al., 2013; Ohta and Ebisawa, 2015; Rodrigues-Filho et al., 2023), sea breams (Dentex tumifrons) (Tominaga et al., 2005), and tunas (Thunnus thynnus and Thunnus orientalis) (Medina, 2020; Park et al., 2023), during their early developmental stages of aquaculture. The reproductive cycle and gonadal development of fish are altered by the neuroendocrine reproductive axis, which is influenced by various internal (age and size) and external (temperature, photoperiod, and other physico-chemical parameters of water) factors (Taranger et al., 2010; Servili et al., 2020; Kumar et al., 2023). Hence, the ongoing and rapid alterations of marine environments caused by global warming and climate change further emphasize the importance of obtaining reliable data on the reproductive characteristics of fish under changing conditions (Servili et al., 2020). Understanding the effect of environmental shifts, particularly temperature variations, on the reproductive cycle and spawning timing is crucial for aquaculture systems. Therefore, clarifying the reproductive cycle of target fish species while accounting for the latest environmental conditions of a location is essential for managing successful spawning strategies.
Chub mackerel (Scomber japonicus), a migratory fish species widely distributed in the subtropical and temperate waters of the Pacific Ocean, is an economically important fishery resource in China, Japan, and the Republic of Korea (hereafter Korea). It is divided into two stocks, the Tsushima warm current (TWC) stock mainly fished by China and Korea (Wang et al., 2022) and the Pacific stock. Since 1996, during which the highest annual fishery production of chub mackerel in Korea reached approximately 400,000 metric tons, the production has fluctuated, reaching approximately 120,000 metric tons in 2023 (KOSIS, 2024). Liang et al. (2020) and Hong et al. (2022) assessed the status of chub mackerel resources caught in the Northwest Pacific Ocean and reported a pessimistic decline in recent years. To preserve chub mackerel resources, a Total Allowable Catch was established and implemented in Japan and Korea from 1997 and 1999, respectively. To meet the significant consumer demand, approximately 51,000 metric tons of Atlantic mackerel (Scomber scombrus), comprising 42% of the domestic fishery production of chub mackerel, have been imported from Norway to Korea in 2023 (KMI, 2024).
Chub mackerel aquaculture can be a suitable solution to ensure a stable supply for consumer demand while also contributing to fishery resource conservation. Over the last few decades, extensive research on this species in Japan has been conducted in various fields including ecology (Yukami et al., 2009; Wang et al., 2022), endocrinology (Matsuyama et al., 2005; Nyuji et al., 2012a; Selvaraj et al., 2015), ethology (Tsuda et al., 2013), and biology (Kobayashi et al., 2011; Shiraishi et al., 2005, 2008). Particularly, Shiraishi et al. (2008) monitored the seasonal changes in the gonadosomatic index (GSI) and ovarian stages of TWC stock in the waters off northern Kyushu and northern East China Sea and concluded that the TWC stock spawning season in this region extended from March to mid-May. Yukami et al. (2009) reported that the GSI of chub mackerel at 50% sexual maturity was 2.5% and identified sea surface temperatures of 15–22°C as suitable spawning conditions. These studies have provided significant insights into reproductive biology and sexual maturation; however, basic knowledge regarding the reproductive biology of chub mackerel under aquaculture conditions remains incomplete.
Previous studies have demonstrated significant variations in the reproductive season of chub mackerel depending on ocean geographical locations. For instance, the reproductive season of chub mackerel in the southern East China Sea typically begins approximately two months earlier than that in the northern East China Sea and four months earlier than that in the East Sea (Sea of Japan) (Yukami et al., 2009). Kim et al. (2020) reported that the reproductive season of wild chub mackerel in Korean coastal waters extended from March to June and peaked in May, based on the GSI and ovarian stages of fish. However, the findings of Kim et al. (2020) were obtained without considering environmental factors, and the reproductive characteristics between wild fish and captive fish could differ (Zupa et al., 2017). In Korea, chub mackerel has primarily been farmed along the southeastern coast and off the waters of Jeju Island since 2010; however, most farming still relied on feeding wild-caught juveniles. The waters surrounding Jeju Island are a major spawning ground for the TWC stock (Kim et al., 2020). Jeju Island boasts well-developed land-based aquaculture facilities and a sufficient supply of underground seawater, offering great potential for regulating environmental conditions throughout the year (Kim, 2022). These advantages provide desirable aquaculture conditions for stable growth and reproduction in this species. Identifying the reproductive characteristics of chub mackerel broodstock in land-based aquaculture systems in Jeju Island may provide foundational information for juvenile production and further spawning regulation.
Therefore, we hypothesized that differences could exist in the reproductive cycle of farmed fish in an aquaculture facility compared to wild chub mackerel, as observed in previous studies. The objective of this study was to identify the reproductive cycle of chub mackerel in an indoor land-based tank on Jeju Island. To the best of our knowledge, this is the first study to examine monthly changes in biological indices, histological gonadal development observations, plasma sex steroid hormone levels, and reproduction-related gene expression in captive male and female chub mackerel throughout the year. The results derived from this study provide crucial insights into the reproductive physiology of chub mackerel, focusing on gonadal development, hormone dynamics, and biological indices. This knowledge is essential for understanding the transition of chub mackerel from wild-caught to cultured species and will serve as a foundation for optimizing breeding schemes and achieving large-scale seed production in land-based tanks.
2 Materials and methods
2.1 Ethics statement
All experimental procedures were performed in adherence to ethical considerations and were approved by the Institutional Animal Care and Used Committee of the National Institute of Fisheries Science (NIFS), Korea (2023-NIFS-IACUC-17).
2.2 Broodstock management
Approximately 1,000 chub mackerel broodstock, aged approximately one year (average body weight of approximately 250 g) were captured via set nets off the waters of the Yokji Island (Tongyeong-si, Gyeongsangnam-do, Korea, 34°62′74″ N, 128°23′23″ E), transported to the Subtropical Fisheries Research Institute (Seogwipo-si, Jeju-do, Korea; 33°27′16″ N, 126°68′00″ E), and acclimated to an indoor circular concrete tank (15 m diameter and 6 m depth) in October 2022. The experiment began on January 1 and ended with the final sampling on December 18, 2023. During the acclimation and experimental periods, the fish were fed a commercial extruded pellet (52% crude protein and 12% crude lipid; Daebong LF, Jeju-do, Korea) until visually satiated, i.e., the point at which the fish ceased to exhibit active feeding behavior, characterized by reduced movement towards provided feed pellets and halted pellet consumption within a five-minute observation period, twice daily (09:00 and 15:00). Experimental conditions were maintained at the natural water temperature and photoperiod. The tank was continuously filled with sand-filtered natural seawater (200%/day), and sufficient aeration was provided. To maintain water quality, the bottom of the tank was cleaned weekly. Water temperature and dissolved oxygen were monitored daily using a YSI ProDSS (YSI Inc., OH, USA). Photoperiod data during the experimental period were provided by the Korea Astronomy Space Science Institute. To monitor the start and end of chub mackerel spawning, a passive egg collector was placed where the surface water overflowed. A passive egg collector was fabricated and installed taking the buoyancy characteristics of pelagic eggs into account, based on previous research demonstrating successful application in collecting eggs from pelagic spawners, particularly for mackerel and related pelagic fish species (Murata et al., 2005; Shiraishi et al., 2008). The passive egg collector was constructed using a stainless-steel frame and wrapped in a 400 μm micromesh net. The buoyancy rate of eggs was calculated as the proportion of buoyancy eggs to the total number of collected eggs, and the average egg diameter was measured through microscopic observation using a stereoscopic microscope (SMZ745T, Nikon, Tokyo, Japan).
2.3 Sampling procedure
After 24 h of starvation, 14 chub mackerel (7 males and 7 females) were randomly chosen and sampled on days 15–20 of each month from January to December 2023. In every sampling, experimental fish were captured until seven males and seven females were identified. The fish were anesthetized with 2-phenoxyethanol at 200 ppm in seawater. Then, the fish body weight and total length were measured to calculate the condition factor (CF). The liver and gonads were weighed to calculate the hepatosomatic index (HSI) and GSI as follows: CF (g/cm3) = 100 × body weight (g)/total length (cm)3; HSI (%) = 100 × liver weight (g)/body weight (g); GSI (%) = 100 × gonad weight (g)/body weight (g).
For histological analysis, a middle section of the gonads of two males and two females whose GSI values were closest to the average values in each month was transversely sectioned and fixed in 10% neutral-buffered formalin (Sigma-Aldrich, MO, USA). Blood samples were collected from the caudal vein using heparinized syringes after measuring the fish body weight and total length. Samples were centrifuged at 1500 ×g and 4°C for 10 min. Plasma samples were isolated and stored at –80°C until plasma sex steroid hormone analysis was performed. The brain and pituitary gland samples were collected, placed in RNAlater (Thermo Fisher Scientific Inc., MA, USA), and stored at –80°C until reproduction-related gene expression analysis was performed.
2.4 Histological analysis
The fixed gonadal tissues were dehydrated using a TP1120 system (Leica Microsystems, Wetzlar, Germany) and embedded in paraffin with an EG1150 system (Leica Microsystems). Sections of 5–6 μm thickness were prepared using a microtome (RM2235, Leica Microsystems), stained with hematoxylin and eosin, and examined under a microscope (AXio Imager A2, Carl Zeiss, Oberkochen, Germany) using Zen imaging software v. 3.1 (Carl Zeiss). Male gonads were classified into four stages (immature, late spermatogenesis, spermiation, and post-spawning), and female gonads were classified into four stages (immature, early vitellogenesis, late vitellogenesis, and post-spawning) based on previous studies (Selvaraj et al., 2010, 2012).
2.5 Plasma sex steroid hormone analysis
To assess the monthly changes in plasma sex steroid hormone levels in male (testosterone [T] and 11-ketotestosterone [11-KT]) and female (T and 17β-estradiol [E2]) chub mackerel, plasma samples were collected monthly from January to December 2023. The plasma 11-KT level was measured using an enzyme-linked immunosorbent assay (ELISA) kit purchased from Cayman Chemical (MI, USA), with a sensitivity of 1.3 pg/mL. The plasma E2 and T levels were measured using an ELISA kit purchased from DRG International, Inc. (NJ, USA), with sensitivities of 0.083 ng/mL and 10.6 pg/mL, respectively. The assays were performed as per the manufacturer’s instructions, and absorbance was quantified using a microplate reader (Tecan, Mannedorf, Switzerland). Final concentrations were calculated using Magellan v17.2 software (Tecan). All samples were analyzed concurrently. The intra- and inter-assay coefficient variations were 3.6% and 7.1% for T (n=3), 9.0% and 10.9% for E2 (n=3), and 13.7% and 12.6% for 11-KT (n=8), respectively. The recovery data from the spiking experiment for T and E2 were 106.2% and 98.1%, respectively.
2.6 Reproduction-related gene expression analysis
To analyze the monthly changes in reproduction-related gene expression, total RNA was extracted from brain and pituitary samples as per the manufacturer’s instructions of RNAiso Plus (Takara Bio Inc., Shiga, Japan). The samples were transferred to a 2-mL microtube with 1 mL of RNAiso Plus and homogenized using a bead beater (Bead Ruptor 12, OMNI International, GA, USA). Total RNA was obtained after completely dissolving the RNA pellet by adding 50 μL of diethylpyrocarbonate-treated water (Thermo Fisher Scientific Inc.). The total RNA concentration was determined using a Quan-IT™ RiboGreen RNA assay kit (Invitrogen, CA, USA) as per the manufacturer’s instructions. Total RNA was diluted to 50 ng/μL with diethylpyrocarbonate-treated water.
Three target genes (gonadotropin-releasing hormone 1 [gnrh1], follicle-stimulating hormone beta subunit [fshβ], and luteinizing hormone beta subunit [lhβ]) and two reference genes (actin beta [β-actin] and eukaryotic translation elongation factor 1 alpha [ef1α]) were selected for reverse transcriptase real-time polymerase chain reaction (RT-qPCR) assay using an Applied Biosystems™ QuantStudio™ 5 (Thermo Fisher Scientific Inc.). Total RNA extracted from the brain was used for gnrh1 expression analysis, whereas that from the pituitary gland was employed for fshβ and lhβ expression analysis. The primers used for RT-qPCR amplification are presented in Table 1. Total RNA at a concentration of 50 ng/μL was used as the RT-qPCR template. An AccuPower® GreenStar™ RT-qPCR Master Mix kit (Bioneer, Daejeon-si, Korea) was used for the reaction as per the manufacturer’s instructions for an initial denaturation step of 5 min at 95°C. Subsequently, the mixture was subjected to 40 cycles at 95°C for 15 s and 60°C for 1 min, followed by a final dissociation step. Internal standards, β-actin and ef1α, of the chub mackerel were used for normalization to account for differences in the total RNA amount in the reaction, using the 2-ΔΔCT method. β-actin was confirmed as the most appropriate and was used as the reference gene for normalization across all tested samples. The relative mRNA expression of each targeted gene was then calculated by calibrating the normalized value to the value of the initial pooled whole fish sample, collected immediately before the experimental period.
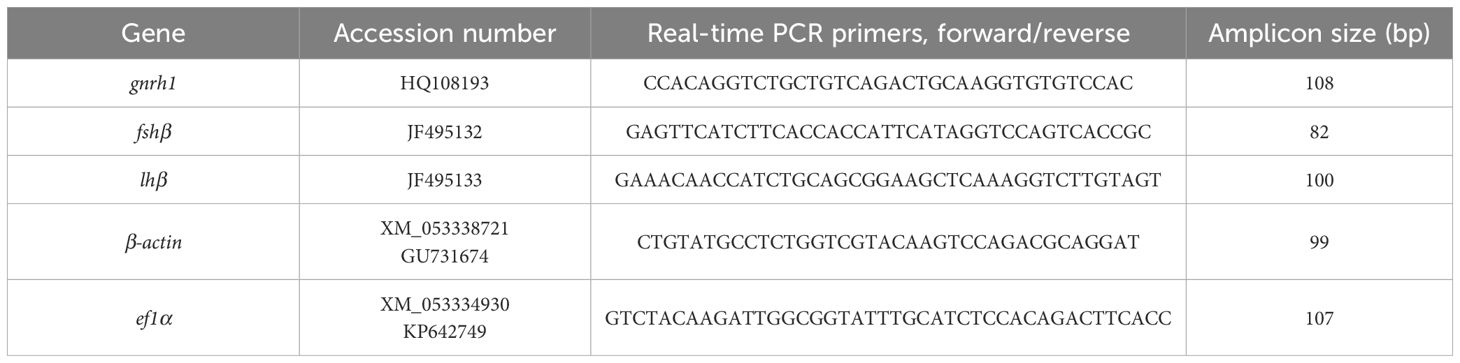
Table 1. Sequences of primers used in reproduction-related gene expression analysis and accession numbers.
2.7 Statistical analysis
All data are presented as the mean ± standard deviation (SD). Statistical analyses were performed using SPSS version 19.0 (SPSS Inc., IL, USA). Prior to analysis, assumptions including normality and homogeneity of variance were assessed using the Shapiro–Wilk and Levene tests, respectively. All dependent variables satisfied the assumption of normality (P > 0.05). The data were analyzed using one-way analysis of variance. When variances of the data were homogeneous, Tukey’s honestly significant differences post-hoc test was adopted. In case of no homogeneous variances, the Games–Howell post-hoc test was adopted. Statistical significance was set at α=0.05.
3 Result
3.1 Water temperature, photoperiod, and spawning of chub mackerel
Changes in the water temperature and photoperiod during the experimental period are presented in Figure 1. Water temperature was monitored twice daily (09:00 and 15:00), and average values were calculated. Day length was calculated as the time from sunrise to sunset as a measure of the photoperiod. The highest water temperature (28.1°C) was recorded on August 29 (average water temperature in August = 27.5 ± 0.30°C [mean ± SD]), whereas the lowest water temperature (13.3°C) was recorded on February 21 (average water temperature in February = 14.7 ± 0.56°C). The longest and shortest day lengths were observed on June 19 (14 h light: 10 h dark) and December 17 (10 h light: 14 h dark), respectively. During the experimental period, dissolved oxygen and salinity were recorded at 8.1 ± 0.87 mg/L and 32.0 ± 0.20 psu, respectively.
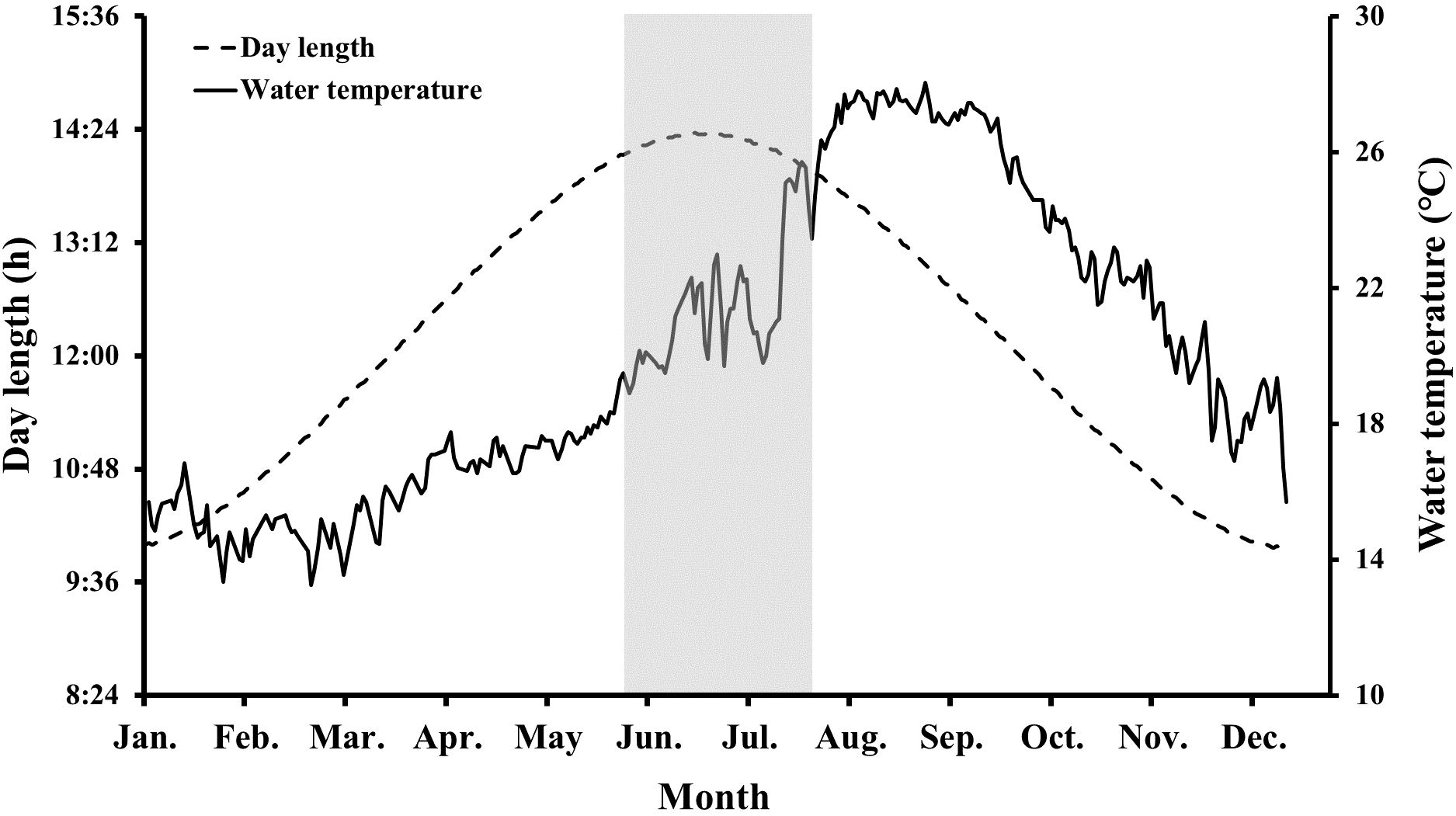
Figure 1. Changes in water temperature and photoperiod during the experimental period. The gray shaded part indicates the observed spawning period of chub mackerel (Scomber japonicus).
As we anticipated that chub mackerel would spontaneously begin spawning in May, a passive egg collector was placed on May 1. Fertilized eggs were first observed on June 15, and continuous spawning was observed until mid-July (Figure 1). After the last fertilized eggs were observed on July 20, further spawning was not recorded. The buoyancy rate and average diameter of eggs collected during the observed spawning period were calculated to be 78.7% and 1.07 ± 0.004 mm, respectively.
3.2 Biological indices of chub mackerel
The monthly changes in total length, body weight, CF, HSI, and GSI of male and female chub mackerel are presented in Table 2 and Figure 2. The CF of males in October was the highest, but not significantly (P>0.05) different from that of male fish in January, April, July, November, and December. The CF of females in October was significantly (P<0.05) higher than that of female fish during the other months, except in November. The HSI of males in January was significantly higher (P<0.05) than that of males in the other months, whereas that of females in August and September was significantly lower (P<0.05) than that of females in the other months, except for July. The GSI of males tended to increase from January to March and maintained significantly (P<0.05) higher levels from March to June than that of males in other months. From July, the GSI of males tended to decrease and maintained low levels until November. The GSI of females increased from January to June, reaching its peak in June, with values significantly higher (P<0.05) than those observed in other months, with the exception of April and May. The GSI of females tended to decrease from July and maintained low levels until November.
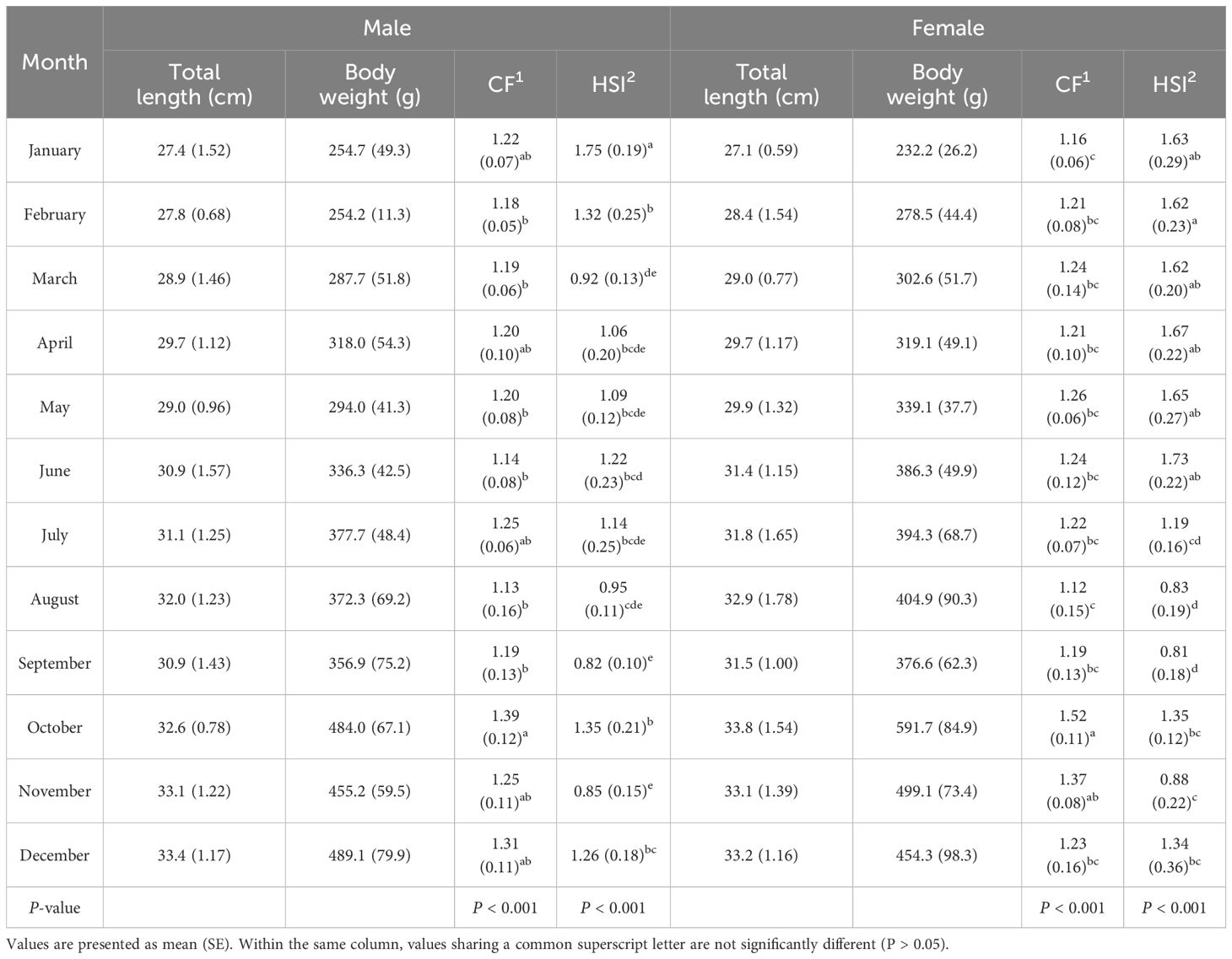
Table 2. Monthly changes in biological indices of chub mackerel (Scomber japonicus) during the experimental period.
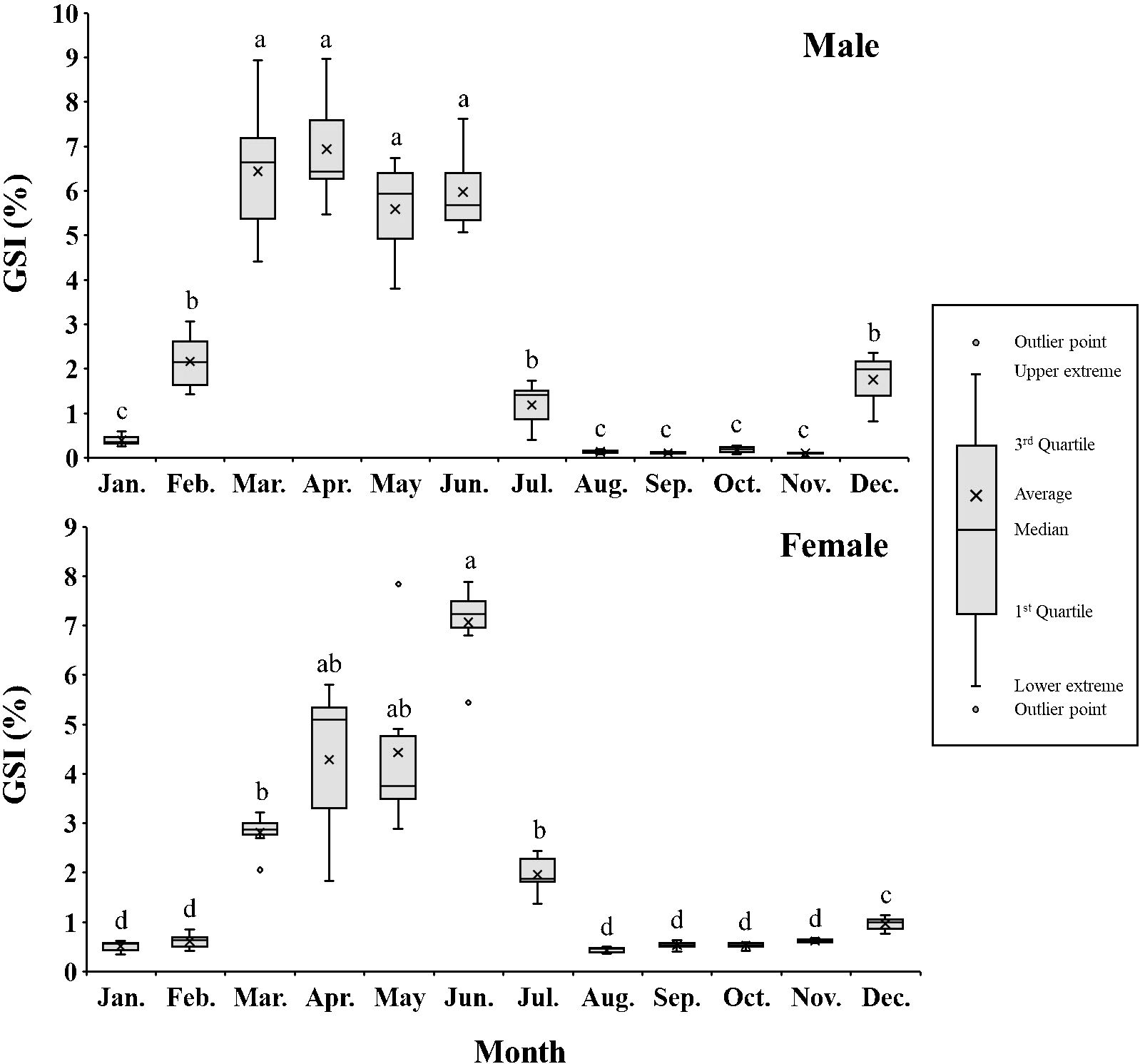
Figure 2. Monthly changes in gonadosomatic index (GSI, %) of male and female chub mackerel (Scomber japonicus) (Box-Whisker diagram). Significant differences between months were determined using the Games–Howell post hoc test (P < 0.0001 for both). Common letters indicate significant differences (P > 0.05).
3.3 Gonadal histology
Histological sections depicting the gonadal stages of male and female chub mackerel are shown in Figures 3 and 4, respectively. Male chub mackerel sampled in January and February were immature, characterized primarily by spermatogonia in the testicular lobules (Figure 3a). In March, the testes had progressed to the late spermatogenesis stage, with testicular lobules containing spermatogonia, spermatocytes, spermatids, and spermatozoa, whereas spermatocytes and spermatids were predominant (Figure 3b). In April, spermatozoa were predominant, whereas other germ cell stages were infrequent, indicating the spermiation stage (Figure 3c). In July, the testes were in the post-spawning stage, showing several residual spermatozoa, with no further spermatogenetic activity observed (Figure 3d).
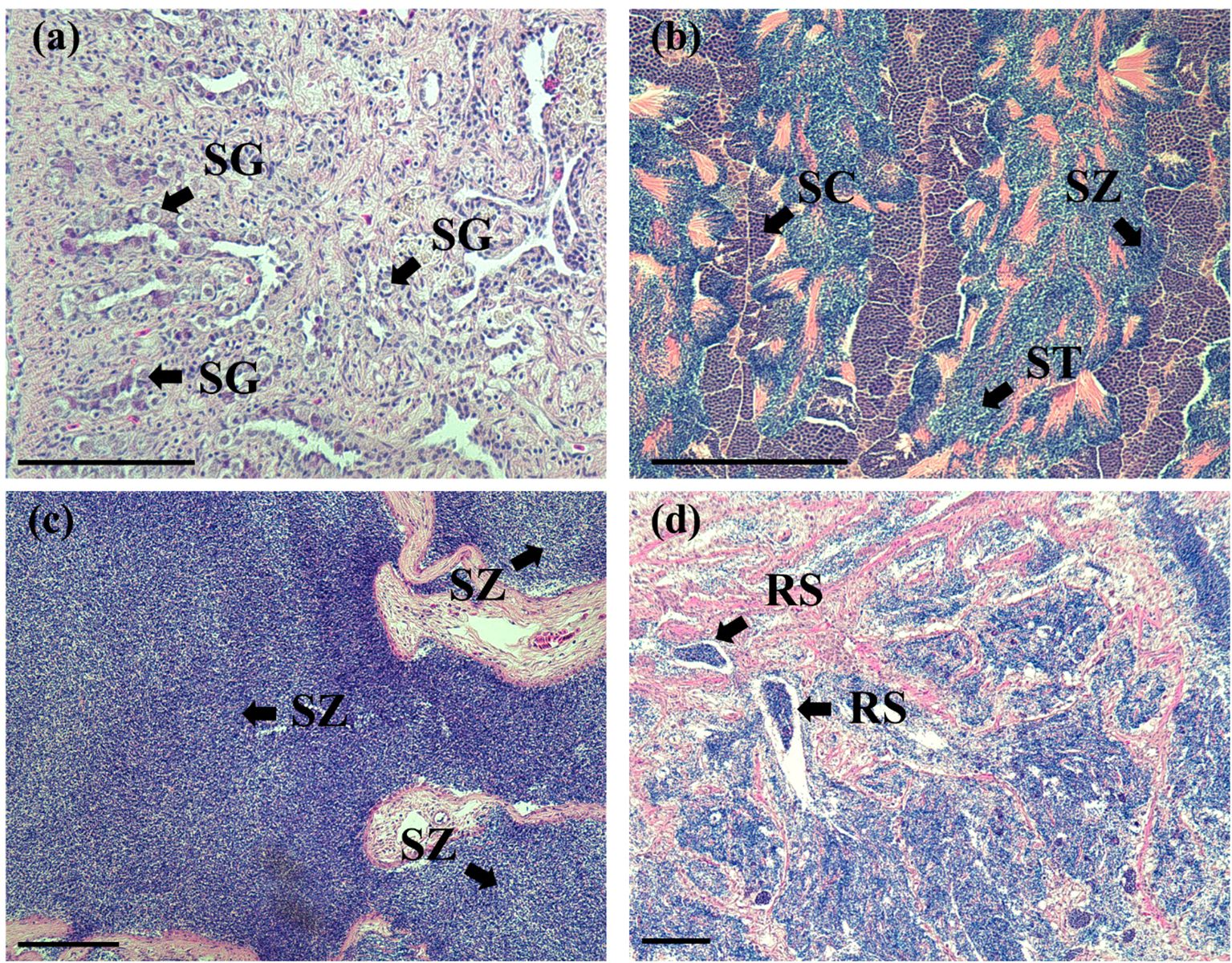
Figure 3. Histological sections of chub mackerel testes at different stages. (a) immature testis (January); (b) testis at late spermatogenesis (March); (c) testis at spermiation (April); (d) testis at post-spawning stage (July). RS, residual spermatozoa; SC, spermatocyte; SG, spermatogonia; ST, spermatids; SZ, spermatozoa; Scale bars = 100 μm.
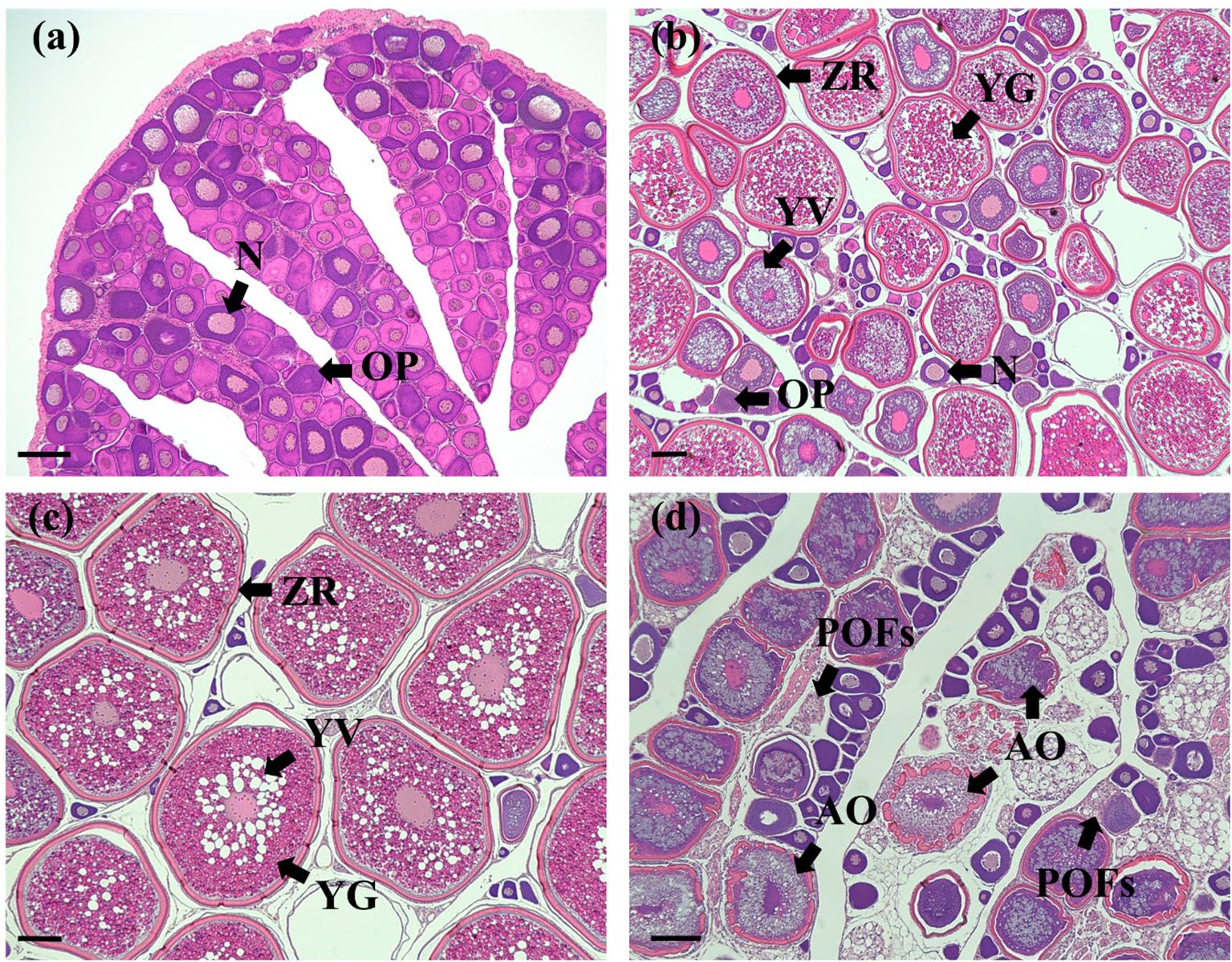
Figure 4. Histological sections of chub mackerel ovaries at different stages. (a) immature ovary (January); (b) ovary at early vitellogenesis (March); (c) ovary at late vitellogenesis (May); (d) ovary at post-spawning stage (July). N, nucleus; OP, ooplasm; ZR, zona radiate; YG, yolk granule; YV, yolk vesicle; POFs, post-ovulatory follicles; AO, atretic oocyte. Scale bars = 100 μm.
Female chub mackerel sampled in January and February were immature, characterized mainly by peri-nucleolar oocytes (Figure 4a). From March, the ovaries progressed to the early vitellogenesis stage, with yolk granules in the oocyte (Figure 4b). During this stage, the oocyte diameter was generally smaller than 300 μm. From May, the oocytes had progressed to the late vitellogenesis stage, with diameters exceeding 400 μm, and exhibited germinal vesicle migration (Figure 4c). Post-ovulatory follicles were scarcely observed in several individuals in May. In July, oocytes were categorized as being in the post-spawning stage. Atretic oocytes and post-ovulatory follicles were mainly observed during this stage (Figure 4d).
3.4 Plasma sex steroid hormones of chub mackerel
Monthly changes in plasma T and 11-KT levels in male and T and E2 levels in female chub mackerel are presented in Figures 5 and 6, respectively. Plasma T levels in males peaked twice in February (13.14 ng/mL) and June (12.97 ng/mL), significantly (P<0.05) higher than that in males in other months. From July, it decreased sharply and remained consistently low (<1.5 ng/mL) until December. The plasma 11-KT level in males peaked in February (50.81 ng/mL), significantly (P<0.05) higher than that of males in other months. Subsequently, it remained consistently low (<2.7 ng/mL) from April to December, except for a slight increment in June (7.45 ng/mL).
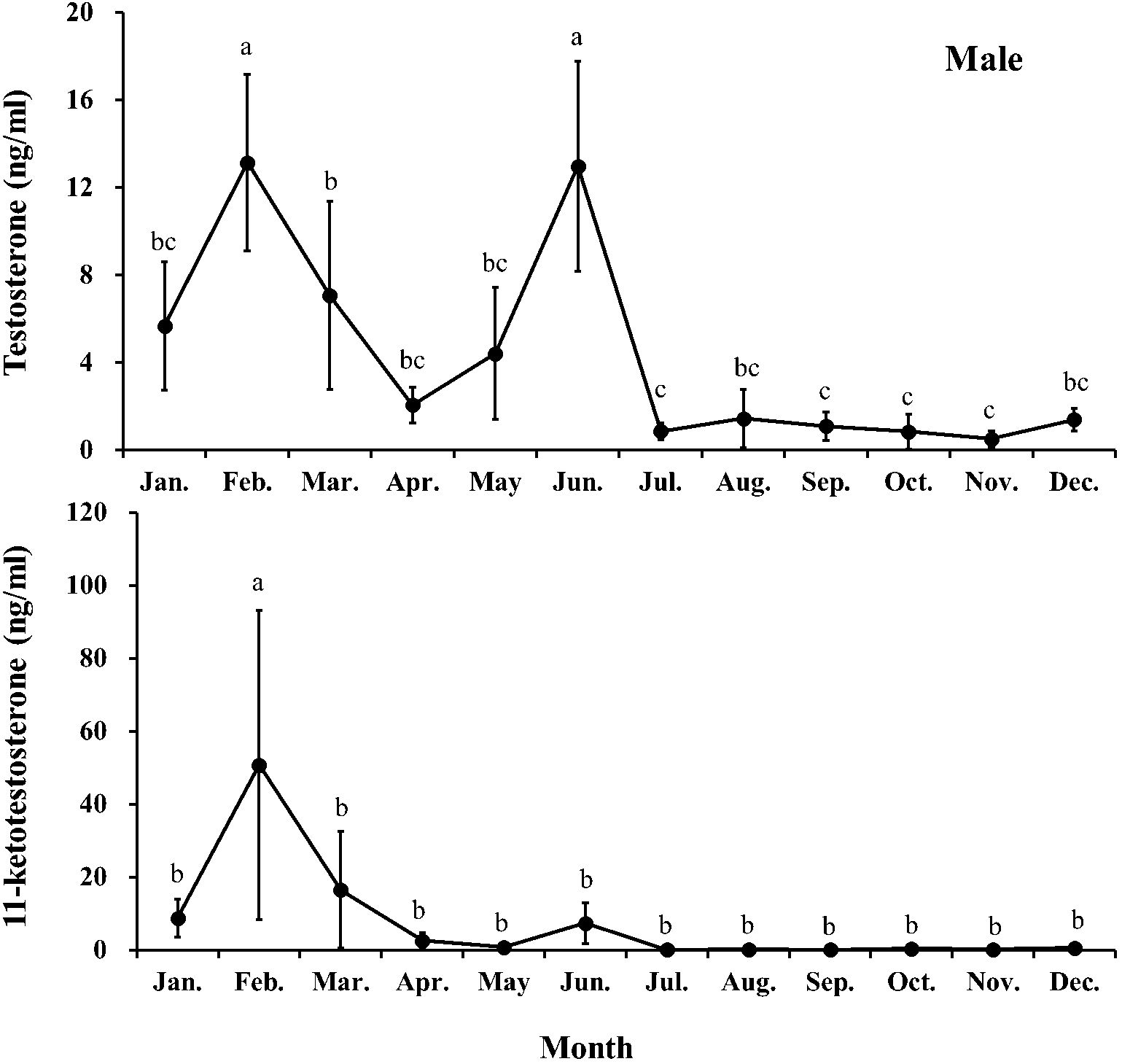
Figure 5. Monthly changes in testosterone and 11-ketotestosterone levels of male chub mackerel (Scomber japonicus). Significant differences between months (means of five replicates ± SD) were determined using Games-Howell test (P < 0.001 for both). Common letters indicate significant differences (P > 0.05).
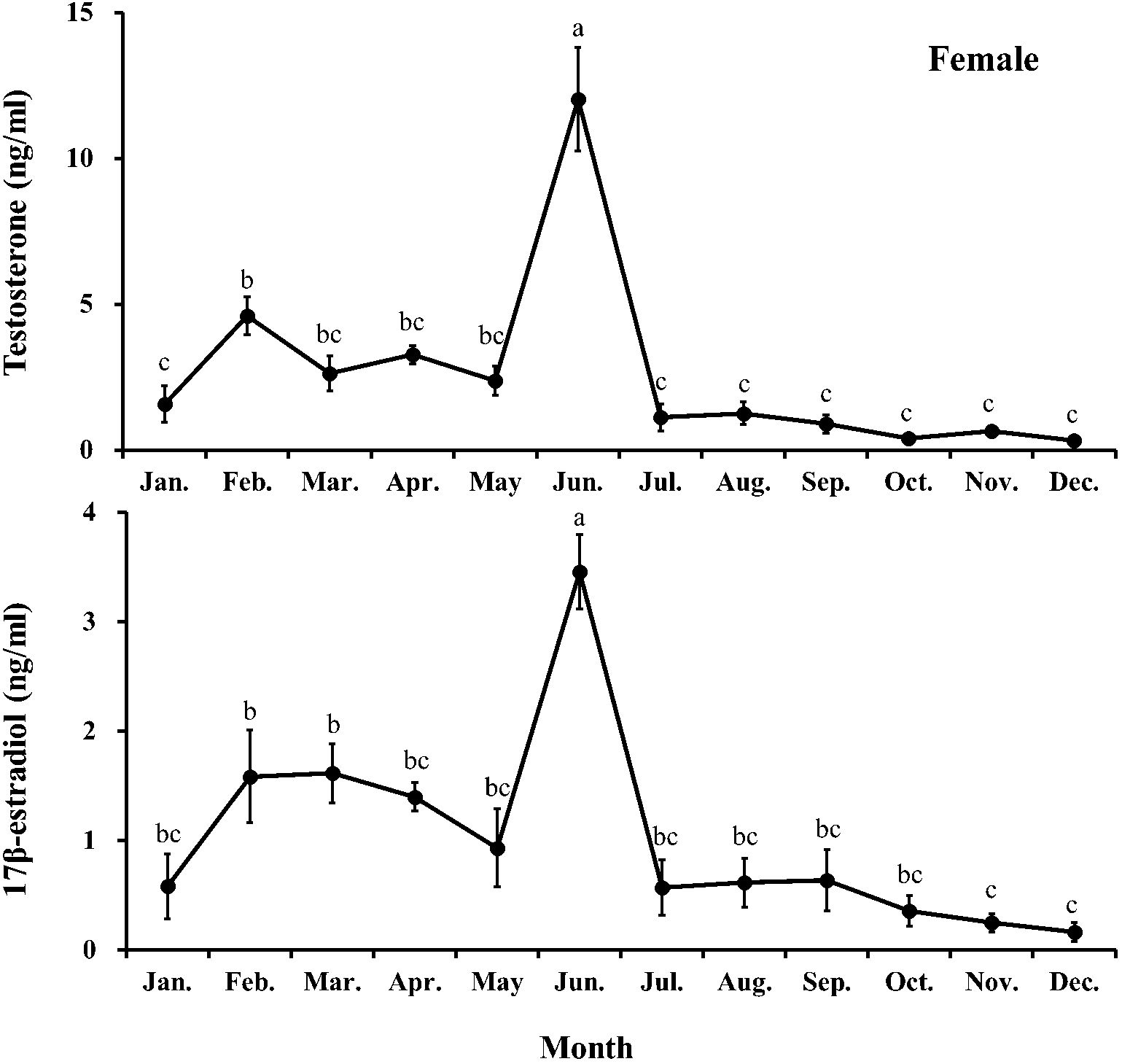
Figure 6. Monthly changes in testosterone and 17β-estradiol levels of female chub mackerel (Scomber japonicus). Significant differences between months (means of five replicates ± SD) were determined using Tukey’s honestly significant difference post hoc test (P < 0.001 for both). Common letters indicate significant differences (P > 0.05).
The plasma T level in females fluctuated from January to May and peaked in June (12.02 ng/mL), significantly (P<0.05) higher than that in females in other months. After that, it decreased sharply in July and remained consistently low (<1.3 ng/ml) until December. The plasma E2 level in females increased first and then decreased from January to May (0.58–1.62 pg/mL). In June, it increased sharply and peaked (3.46 pg/mL), significantly (P<0.05) higher than that in females in other months. From July, it decreased and remained low (<0.65 pg/mL) until December.
3.5 Reproduction-related gene expression of chub mackerel
Changes in the relative expression of reproduction-related genes (gnrh1, fshβ, and lhβ) in both male and female chub mackerel are presented in Figures 7 and 8, respectively. The expression level of brain gnrh1 in males showed no significant (P>0.05) changes from January to May, followed by a rapid increase in June, significantly higher (P<0.05) than that in January and July. The pituitary fshβ expression in males increased sharply from January, peaked in February, and then significantly (P<0.05) decreased from March to May. However, it increased again in June, comparable to that in males in February. From July, its expression level remained low until December. The expression level of pituitary lhβ in males increased sharply from January, was the highest in February, and maintained comparable levels until June, except for March. From July, its expression level remained low until December.
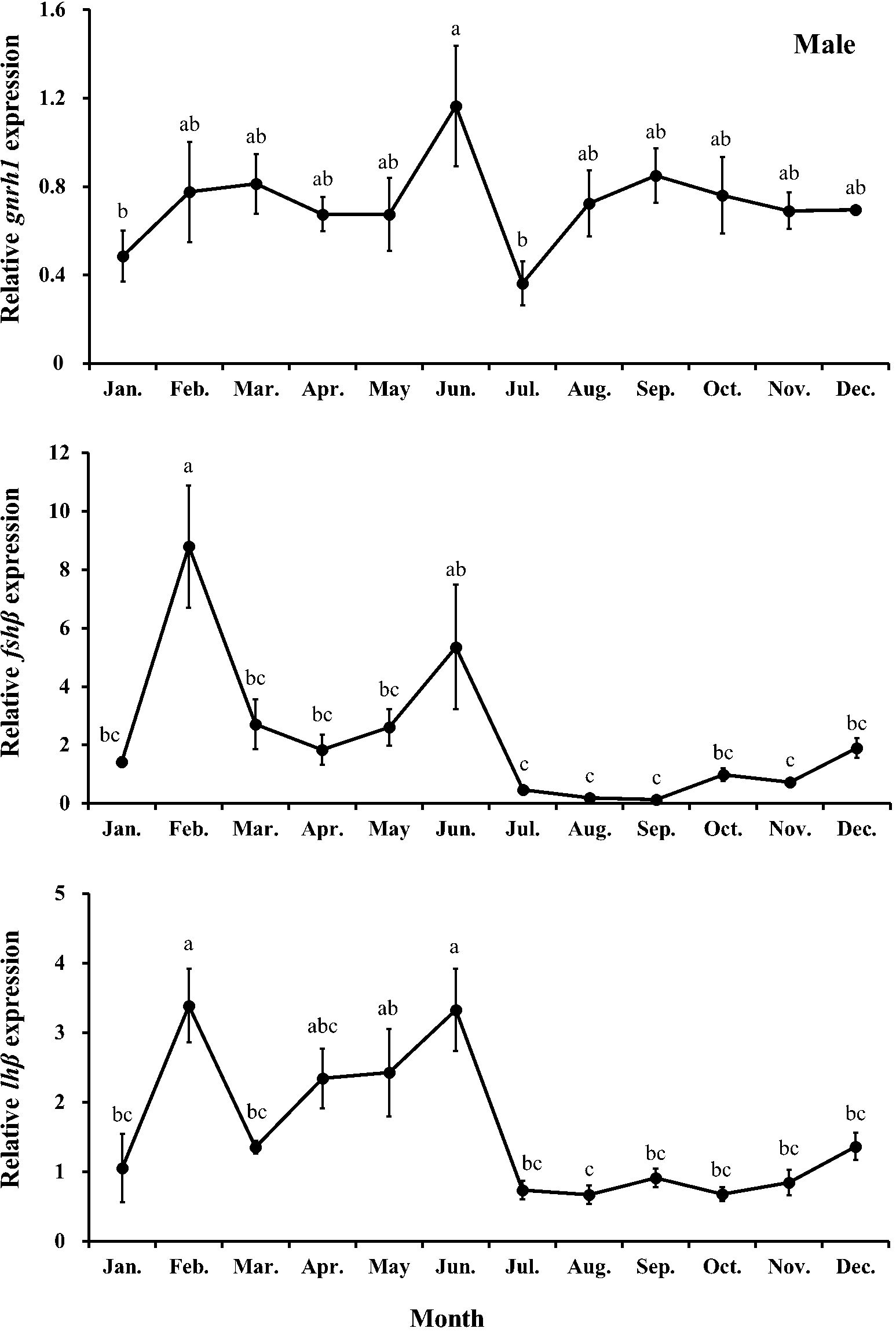
Figure 7. Relative expression of gnrh1, fshβ, and lhβ in male chub mackerel (Scomber japonicus). Significant differences between months (means of five replicates ± SD) were determined using Tukey’s honestly significant difference post hoc test (P < 0.001 for all). Common letters indicate significant differences (P > 0.05).
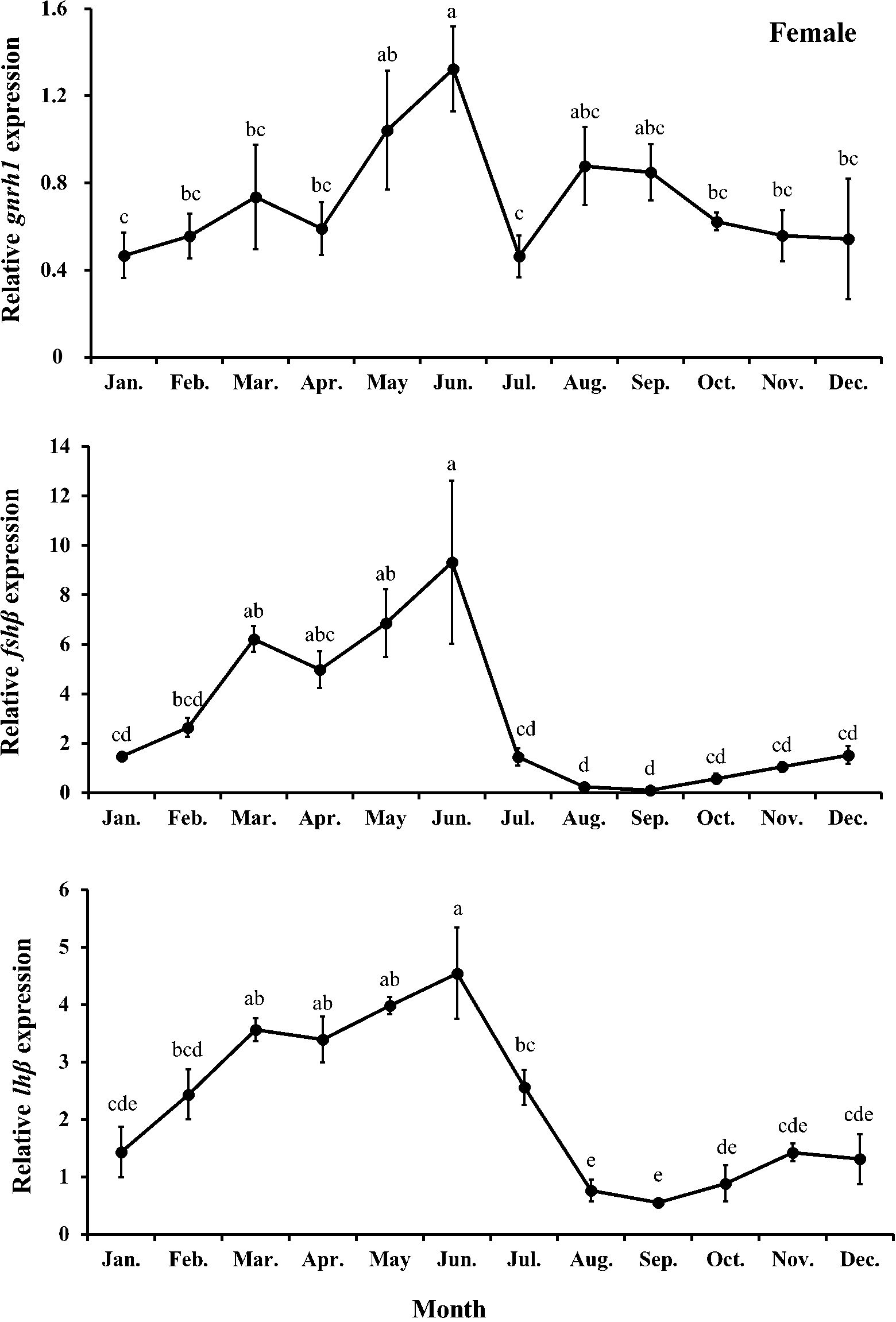
Figure 8. Relative expression of gnrh1, fshβ, and lhβ in female chub mackerel (Scomber japonicus). Significant differences between months (means of five replicates ± SD) were determined using Tukey’s honestly significant difference post hoc test (P < 0.001 for all). Common letters indicate significant differences (P > 0.05).
The expression level of brain gnrh1 in females showed no significant (P>0.05) changes from January to April, followed by a significant (P<0.05) increase in June. However, its expression sharply decreased in July, increased again in August, and gradually decreased thereafter until December. The expression levels of brain fshβ and lhβ in females tended to increase from April to June and peaked in June, significantly (P<0.05) higher than those in females in the other months except for March, April, and May. Their expression then sharply decreased and remained low until December.
4 Discussion
This study determined the reproductive characteristics of chub mackerel cultured in an indoor land-based tank on Jeju Island based on its biological, histological, endocrinological, and genetic evidence. In this study, one-year-old fish were used, indicating it is first spawning for them. Even if Yoneda et al. (2022) emphasized that 2- or 3-year-old chub mackerel (repeat spawners) could produce a greater number of more nutrient-rich eggs compared to one-year-old fish (first-time spawners), several studies have reported no significant differences in the timing of gonadal development between first-time spawners and repeat spawners (Nyuji et al., 2012a, 2012, 2014). Therefore, comparing the gonadal development of fish in this study and with that of wild fish in past studies seemed to be feasible. In addition, although it is difficult to determine the maturity rates of fish and their participation in spawning from the data obtained in this study, considering that the 50% total length and GSI for mature female chub mackerel were calculated to be 31.4 cm and 2.5%, respectively (Yukami et al., 2009; Kim et al., 2020), and that the total length and GSI of females in May and June ranged from 29.0–30.9 cm and exceeded 4%, respectively, it can be inferred that most individuals likely participated in maturation and spawning. On the basis of the presence of post-ovulatory follicles observed in the histological analysis, spawning event occurrence, and GSI variation, we ascertained that the species spawning period extended from end-May to mid-July and occurred primarily in June. The findings partially align with those of Murata et al. (2005), who reported that captive chub mackerel reared in land-based tanks in Shirahama spawned between May and June at water temperatures of 20.8–24.2°C. However, in contrast to these results, previous studies have documented spawning-capable chub mackerel from March to June in northern Kyushu, the northeastern East China Sea (Shiraishi et al., 2008; Yukami et al., 2009), and the coastal waters of Korea (Kim et al., 2020). These comparisons suggest that chub mackerel reared in indoor land-based tanks on Jeju Island exhibit a spawning period that begins approximately 2 months later than that of their wild counterparts in regions of comparable latitude. This delay highlights the potential influence of controlled rearing conditions on the timing of spawning events. Specifically, potential factors such as stress from confinement, differences in diet, altered photoperiod and temperature regimes, and limited environmental stimuli compared to wild habitats are likely contributors to delayed spawning. In particular, chronic stress has been extensively documented to cause reproductive delays in fish (Campbell et al., 1994; Pankhurst, 2016). Additionally, aquaculture conditions, wherein complex environmental cues are lacking, might affect hormone regulation involved in reproductive readiness, thereby influencing the timing of spawning events (Okuzawa and Gen, 2013; Zupa et al., 2017).
The relatively higher HSI levels (1.62–1.73%) observed in females from January to June compared with those in other months (0.81–1.35%) may be indicative of physiological processes associated with reproduction, though the exact mechanisms remain unclear, as vitellogenin analysis was not carried out in this study. However, the relatively low plasma E2 levels observed in females from January to May do not strongly support the occurrence of active vitellogenesis during this period. Notably, previous studies have shown that both HSI and GSI in fish tend to increase prior to the spawning period and decrease after spawning (Lim et al., 2010; Hismayasari et al., 2015). In this study, the highest plasma levels of E2 and T in June were consistent with histological observations of fully vitellogenic oocytes and post-ovulatory follicles. These findings suggest that vitellogenesis predominantly occurred in June, coinciding with the observed peak in HSI and GSI. This delay in vitellogenesis likely influenced the spawning period, as supported by GSI trends and histological observations (Shiraishi et al., 2008; Mañanós et al., 2009; Kim et al., 2020).
Rearing fish in captivity often causes reproductive dysfunction, particularly in females, characterized by lower GSI levels due to incomplete vitellogenesis. Though reproductive dysfunctions in males are less severe, they often fail to produce a sufficient amount of high-quality sperm despite completing spermatogenesis and spermiation. Lower GSI levels have often been observed in migratory fish, such as the Pacific bluefin tuna (Thunnus orientalis), Atlantic bluefin tuna (Thunnus thynnus), and greater amberjack in captivity (Chen et al., 2006; Corriero et al., 2007; Masuma et al., 2011; Zupa et al., 2017). Lower GSI levels and the presence of fewer vitellogenic oocytes strongly indicate that rearing fish in captivity can negatively affect vitellogenesis (Corriero et al., 2007; Pousis et al., 2011). In this study, the GSI levels of both males (5.59–6.94%) and females (4.29–7.06%) during the observed spawning period were comparable to those of wild fish (6.63–8.33% and 4.70–7.74%, respectively) (Shiraishi et al., 2008; Kim et al., 2020). Conversely, we observed relatively delayed gonadal development in females and a later spawning period compared with the results of previous studies (Shiraishi et al., 2008; Yukami et al., 2009; Kim et al., 2020). It is difficult to clearly explain specific factors causing delayed gonadal development, but a possible hypothesis is that undesirable conditions of rearing in land-based tanks may have caused significant stress to the chub mackerel, which could have delayed fish spawning. Accordingly, Pankhurst (2016) reviewed the consistent inhibitory effect of artificial stress (capture, confinement, and husbandry) on the reproductive performance of fish. Similarly, delayed ovulation, reduced egg weight, and egg production were observed in rainbow trout (Oncorhynchus mykiss) exposed to disturbance stress over nine months (Campbell et al., 1992). Significantly elevated plasma cortisol levels but reduced plasma T and E2 levels were observed in rainbow trout exposed to chronic confinement stress for two weeks (Campbell et al., 1994). The effects of stress hormones on the reproductive performance of chub mackerel should be investigated in future studies.
The higher levels of T and 11-KT observed in plasma indicated that male germ cells underwent active spermatogenesis and spermiation (Weltzien et al., 2002; Lintelmann et al., 2003). Sundaray et al. (2021) reported that the pre-spawning peak of T stimulated gonadal growth, increased pituitary gonadotropin hormone levels, and was a precursor of 11-KT. In this study, significantly higher levels of plasma T and 11-KT were observed in males in February; these increases were associated with strong spermatogenesis in the testes. A distinct peak of plasma T was observed in June. Both T and 11-KT are major androgens that play crucial roles in male reproductive performance, of which 11-KT is recognized as the most effective stimulator of spermatogenesis (Cavaco et al., 2001), whereas T also plays a significant role by stimulating hypothalamic and pituitary actions that regulate the testes (Montero et al., 1995). Therefore, the secondary peak of plasma T levels in June could be associated with sexual behavior regulation during the spawning season. Similar to the results of this study, multiple peaks in plasma T levels have been observed during the reproductive cycle in several male fish species (Barannikova et al., 2004; Gerriero et al., 2005). Both T and E2 are produced in teleost fish ovaries. In addition to serving as a precursor for E2, T can enhance oocyte growth by initiating germinal vesicle breakdown during final oocyte maturation (Sang et al., 2019). E2 is secreted by the ovarian follicle and acts on the liver to produce vitellogenin. The highest levels of plasma T and E2 in females in June indicate that the ovaries underwent vitellogenesis and attained maturity. Similar trends have been observed in E2 levels in other fish species, where they remained high in females during the late vitellogenesis stage and rapidly decreased in the post-spawning stage (Mylonas et al., 1998; Kumar et al., 2015).
Gonadotropin-releasing hormone stimulates the pituitary gland to produce and secrete the gonadotropic hormones, follicle-stimulating and luteinizing hormones (Pierce and Parsons, 1981). Follicle-stimulating hormone is involved in initiating gametogenesis and regulating gonadal growth, whereas luteinizing hormone mainly regulates gonadal maturation and spermiation/ovulation by stimulating sex steroid hormone release (Schulz et al., 2001; Mateos et al., 2002). In this study, we found that monthly trends of relative expression levels of pituitary fshβ and lhβ in both males and females had positive correlations with their gonadal developmental stages and plasma sex steroid levels. Supporting this result, Nyuji et al. (2012a) reported that the relative expression level of pituitary fshβ in female chub mackerel gradually increased during ovarian development and peaked during the late vitellogenesis stage. The relative expression level of lhβ in the late vitellogenesis stage was remarkably higher than those in immature and early vitellogenesis stages and that of fshβ and lhβ sharply decreased in the post-spawning stage. Compared with the relative pituitary expression levels of fshβ and lhβ in males from a previous study (Nyuji et al., 2012b), a distinct peak was found in the pituitary fshβ level in the immature stage of males in this study. The significant increase in the pituitary fshβ level in February directly stimulated the testes to upregulate the release of T and 11-KT, causing a transition from the immature stage to the spermatogenesis stage.
In this study, the observed spawning period of chub mackerel extended until mid-July, with plasma sex steroid hormones (T, 11-KT, and E2) and reproduction-related gene expression levels (gnrh1, fshβ, and lhβ) significantly decreasing in July. While a rapid increase in water temperature above 25°C occurred from June to September, coinciding with the observed end of the spawning period, it is important to note that a relationship between high water temperatures and the termination of spawning was not established in this study. Previous research has suggested that water temperature and photoperiod are critical regulators of the reproductive cycle in fish, with moderate increases in temperature generally enhancing reproductive processes within an optimal range (Taranger et al., 2010; Wang et al., 2010). However, exposure to abnormally high temperatures has been associated with gonadal regression and reduced reproductive function, as confirmed in red sea bream (Pagrus major) at 24°C, where suppression of gnrh1, fshβ, and lhβ expression, along with lower serum E2 levels, was reported (Okuzawa and Gen, 2013). While wild chub mackerel typically avoid water temperatures exceeding 25°C (Yasuda et al., 2018, 2023), the specific thermal range for optimal reproductive performance in this species remains unclear. Given these findings, it is plausible that chronic exposure to temperatures above 25°C could negatively impact reproductive function, potentially contributing to the cessation of spawning. However, further studies are needed to confirm the precise thermal thresholds and mechanisms involved.
5 Conclusion
Based on GSI and gonad histology, we observed fully developed testes and ovaries from April to June and from May to June, respectively. The spawning was extended from end-May to mid-July. We confirmed that gonadal developments were likely mediated by sex steroid hormones, which were regulated by gonadotropic hormones such as follicle-stimulating and luteinizing hormones. The findings of this study provide a better understanding of the reproductive characteristics of chub mackerel in a land-based aquaculture system in Jeju Island and offer useful data for establishing detailed breeding schemes for aquaculture purposes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of the National Institute of Fisheries Science (NIFS), Korea (2023-NIFS-IACUC-17). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SIB: Visualization, Writing – original draft, Data curation. SCJ: Conceptualization, Data curation, Project administration, Writing – original draft. J-HC: Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Institute of Fisheries Science, Ministry of Oceans and Fisheries, South Korea (R2025016 and R2025030).
Acknowledgments
We would like to thank J.H. Kim, K.H. Lee, H.G. Yang, K.H. Kim, and D.H. Jung at the National Institute of Fisheries Science for their assistance in the feeding trial.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barannikova I. A., Bayunova L. V., and Semenkova T. B. (2004). Serum levels of testosterone, 11-ketotestosterone and oestradiol-17β in three species of sturgeon during gonadal development and final maturation induced by hormonal treatment. J. Fish. Biol. 64, 1330–1338. doi: 10.1111/j.0022-1112.2004.00395.x
Campbell P. M., Pottinger T. G., and Sumpter J. P. (1992). Stress reduces the quality of gametes produced by rainbow trout. Biol. Reprod. 47, 1140–1150. doi: 10.1095/biolreprod47.6.1140
Campbell P. M., Pottinger T. G., and Sumpter J. P. (1994). Preliminary evidence that chronic confinement stress reduces the quality of gametes produced by brown and rainbow trout. Aquaculture 120, 151–169. doi: 10.1016/0044-8486(94)90230-5
Cavaco J. E. B., Bogerd J., Goos H., and Schulz R. W. (2001). Testosterone inhibits 11-ketotestosterone-induced spermatogenesis in African catfish (Clarias gariepinus). Biol. Reprod. 65, 1807–1812. doi: 10.1095/biolreprod65.6.1807
Chai K. E., Liu X., Zhang Y., and Lin H. (2013). Day-night and reproductive cycle profiles of melatonin receptor, kiss, and gnrh expression in orange-spotted grouper (Epinephelus coioides). Mol. Reprod. Dev. 80, 535–548. doi: 10.1002/mrd.22191
Chen K. S., Crone P., and Hsu C. C. (2006). Reproductive biology of female Pacific bluefin tuna Thunnus orientalis from South-Western North Pacific Ocean. Fish. Sci. 72, 985–994. doi: 10.1111/j.1444-2906.2006.01247.x
Corriero A., Medina A., Mylonas C. C., Abascal F. J., Deflorio M., Aragón L., et al. (2007). Histological study of the effects of treatment with gonadotropin-releasing hormone agonist (GnRHa) on the reproductive maturation of captive-reared Atlantic bluefin tuna (Thunnus thynnus L.). Aquaculture 272, 675–686. doi: 10.1016/j.aquaculture.2007.08.030
Gerriero G., Ferro R., and Ciarcia G. (2005). Correlations between plasma levels of sex steroids and spermatogenesis during the sexual cycle of the chub, Leuciscus cephalus L. (Pisces: Cyprinidae). Zool. Stud. 44, 228.
Grandcourt E. M., Al Abdessalaam T. Z., Francis F., Al Shamsi A. T., and Hartmann S. A. (2009). Reproductive biology and implications for management of the orange-spotted grouper Epinephelus coioides in the southern Arabian Gulf. J. Fish. Biol. 74, 820–841. doi: 10.1111/j.1095-8649.2008.02163.x
Harmin S. A., Crim L. W., and Wiegand M. D. (1995). Plasma sex steroid profiles and the seasonal reproductive cycle in male and female winter flounder, Pleuronectes americanus. Mar. Biol. 121, 601–610. doi: 10.1007/BF00349295
Hismayasari I. B., Marhendra A. P. W., Rahayu S., Saidin S. D., and Supriyadi D. S. (2015). Gonadosomatic index (GSI) hepatosomatic index (HSI), and proportion of oocytes stadia as an indicator of rainbow fish Melanotaenia boesemani spawning season. Int. J. Fish. Aquat. Stud. 2, 359–362.
Hong J. B., Kim D. Y., and Kim D. H. (2022). Stock assessment of chub mackerel (Scomber japonicus) in the northwest Pacific Ocean based on catch and resilience data. Sustainability. 15, 358. doi: 10.3390/su15010358
Kim Y. (2022). Investigation of water qualities and microbials on the flow-through olive flounder, Paralichthys olivaceus farms using coastal seawater and underground seawater in Jeju. J. Korean Soc Fish. Technol. 58, 59–67. doi: 10.3796/KSFOT.2022.58.1.059
Kim S. R., Kim J. J., Park H. W., Kang S., Cha H. K., and Baek H. J. (2020). Maturity and spawning of the chub mackerel Scomber japonicus in the Korean waters. Korean. J. Fish. Aquat. Sci. 53, 9–18. doi: 10.5657/KFAS.2020.0009
KMI (2024). Korea maritime institute. Available online at: http://kfishinfo.co.kr/kor/view.do/ (Accessed 21 Febr 2024).
Kobayashi T., Ishibashi R., Yamamoto S., Otani S., Ueno K., and Murata O. (2011). Gonadal morphogenesis and sex differentiation in cultured chub mackerel, Scomber japonicus. Aquacult. Res. 42, 230–239. doi: 10.1111/j.1365-2109.2010.02616.x
KOSIS (2024). Korean statistical information service. Available online at: http://kosis.kr/statisticsList.statisticsListIndex.do/ (Accessed 21 Febr 2024).
Kucharczyk D., Jaczewski J., Nowosad J., Łuczyński M. K., Piech P., Dietrich G., et al. (2023). Artificial reproduction of the indoor-cultured brackish form of maraena whitefish (Coregonus maraena) under recirculated aquaculture system (RAS) conditions. Anim. Reprod. Sci. 257, 107329. doi: 10.1016/j.anireprosci.2023.107329
Kujawa R., Nowosad J., Biegaj M., Cejko B. I., and Kucharczyk D. (2019). Use of ultrasonography to determine sex in sexually immature European river lamprey Lampetra fluviatilis (L.). Anim. Reprod. Sci. 204, 95–100. doi: 10.1016/j.anireprosci.2019.03.009
Kumar P., Arasu A. R. T., Kailasam M., Sukumarran K., Subburj R., Tyagraj G., et al. (2015). Gonadal development and steroid hormone profile of wild caught grey mullet (Mugil cephalus). Biol. Rhythm. Res. 46, 601–610. doi: 10.1080/09291016.2015.1034974
Kumar P., Babita M., Kailasam M., Muralidhar M., Hussain T., Behera A., et al. (2023). Effect of changing environmental factors on reproductive cycle and endocrinology of fishes. Outlook Clim. In Change Fish. Nutr. pp. doi: 10.1007/978-981-19-5500-6_25
Liang C., Xian W., and Pauly D. (2020). Assessments of 15 exploited fish stocks in Chinese, South Korean and Japanese waters using the CMSY and BSM methods. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00623
Lim H. K., Le M. H., An C. M., Kim S. Y., Park M. S., and Chang Y. J. (2010). Reproductive cycle of yellow croaker Larimichthys polyactis in southern waters off Korea. Fish. Sci. 76, 971–980. doi: 10.1007/s12562-010-0288-5
Lintelmann J., Katayama A., Kurihara N., Shore L., and Wenzel A. (2003). Endocrine disruptors in the environment (IUPAC Technical Report). Pure Appl. Chem. 75, 631–681. doi: 10.1351/pac200375050631
Mañanós E., Duncan N., and Mylonas C. C. (2009). “Reproduction and control of ovulation, spermiation and spawning in cultured fish,” in Methods in reproductive aquaculture, vol. 2 . Eds. Ration B., Cabrita E., Robles V., and Herraez P. (Boca Raton, FL: CRC Press Taylor & Francis Group), 3–80.
Masuma S., Takebe T., and Sakakura Y. (2011). A review of the broodstock management and larviculture of the Pacific northern bluefin tuna in Japan. Aquaculture 315, 2–8. doi: 10.1016/j.aquaculture.2010.05.030
Mateos J., Mañanos E., Carrillo M., and Zanuy S. (2002). Regulation of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) gene expression by gonadotropin-releasing hormone (GnRH) and sexual steroids in the Mediterranean Sea bass. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 132, 75–86. doi: 10.1016/S1096-4959(01)00535-8
Matsuyama M., Shiraishi T., Sundaray J. K., Rahman A., Ohta K., and Yamaguchi A. (2005). Steroidogenesis in ovarian follicles of chub mackerel, Scomber japonicus. Zool. Sci. 22, 101–110. doi: 10.2108/zsj.22.101
Medina A. (2020). Reproduction of Atlantic bluefin tuna. Fish. Fish. 21, 1109–1119. doi: 10.1111/faf.12489
Micale V., Maricchiolo G., and Genovese L. (1999). The reproductive biology of the amberjack, Seriola dumerilii (Risso 1810). I. Oocyte development in captivity. Aquacult. Res. 30, 349–355. doi: 10.1046/j.1365-2109.1999.00336.x
Montero M., Le Belle N., King J. A., Millar R. P., and Dufour S. (1995). Differential regulation of the two forms of gonadotropin-releasing hormone (mGnRH and cGnRH-II) by sex steroids in the European female silver eel (Anguilla Anguilla). Neuroendocrinology 61, 525–535. doi: 10.1159/000126876
Morse W. W. (1981). Reproduction of the summer flounder, Paralichthys dentatus (L.). J. Fish. Biol. 19, 189–203. doi: 10.1111/j.1095-8649.1981.tb05823.x
Murata O., Yamamoto S., Ishibashi R., Oka Y., Yoneshima H., Kato K., et al. (2005). Egg development and growth of larval and juvenile cultured chub mackerel Scomber japonicus (Perciformes: Scombridae) in a captive spawning experiment. Aquacult. Sci. 53, 319–324. doi: 10.11233/aquaculturesci1953.53.319
Mylonas C. C., Woods L. C. III, Thomas P., and Zohar Y. (1998). Endocrine profiles of female striped bass (Morone saxatilis) in captivity, during postvitellogenesis and induction of final oocyte maturation via controlled-release GnRHa-delivery systems. Gen. Comp. Endocrinol. 110, 276–289. doi: 10.1006/gcen.1998.7073
Narimatsu Y., Yamanobe A., and Takahashi M. (2007). Reproductive cycle, age, and body size at maturity and fecundity of female willowy flounder Tanakius kitaharai. Fish. Sci. 73, 55–62. doi: 10.1111/j.1444-2906.2007.01301.x
Nyuji M., Kodama R., Kato K., Yamamoto S., Yamaguchi A., and Matsuyama M. (2014). Gonadal development and gonadotropin gene expression during puberty in cultured chub mackerel (Scomber japonicus). Zool. Sci. 31, 398–406. doi: 10.2108/zs130254
Nyuji M., Selvaraj S., Kitano H., Ohga H., Yoneda M., Shimizu A., et al. (2012a). Changes in the expression of pituitary gonadotropin subunits during reproductive cycle of multiple spawning female chub mackerel Scomber japonicus. Fish. Physiol. Biochem. 38, 883–897. doi: 10.1007/s10695-011-9576-y
Nyuji M., Selvaraj S., Kitano H., Shiraishi T., Yamaguchi A., Shimizu A., et al. (2012b). Immunoreactivity of gonadotrophs (FSH and LH cells) and gonadotropin subunit gene expression in the male chub mackerel Scomber japonicus pituitary during the reproductive cycle. Zool. Sci. 29, 623–629. doi: 10.2108/zsj.29.623
Ohta I. and Ebisawa A. (2015). Reproductive biology and spawning aggregation fishing of the white-streaked grouper, Epinephelus ongus, associated with seasonal and lunar cycles. Environ. Biol. Fishes. 98, 1555–1570. doi: 10.1007/s10641-015-0382-8
Okuzawa K. and Gen K. (2013). High water temperature impairs ovarian activity and gene expression in the brain-pituitary-gonadal axis in female red seabream during the spawning season. Gen. Comp. Endocrinol. 194, 24–30. doi: 10.1016/j.ygcen.2013.08.015
Pankhurst N. W. (2016). “Reproduction and development,” in Biology of stress in fish. Fish physiology, vol. 35 . Eds. Schreck C. B., Tort L., Farrell A. P., and Brauner C. J. (San Diego, CA, USA: Academic Press), 295–331. doi: 10.1016/B978-0-12-802728-8.00008-4
Park J. W., Kim J. H., Ji S. C., Ryu Y. W., and Cho J. H. (2023). The reproductive potential of Pacific bluefin tuna (Thunnus orientalis) farmed in sea cages in South Korea. J. World Aquacult. Soc 54, 1497–1512. doi: 10.1111/jwas.13026
Pierce J. G. and Parsons T. F. (1981). Glycoprotein hormones: Structure and function. Annu. Rev. Biochem. 50, 465–495. doi: 10.1146/annurev.bi.50.070181.002341
Pousis C., De Giorgi C., Mylonas C. C., Bridges C. R., Zupa R., Vassallo-Agius R., et al. (2011). Comparative study of liver vitellogenin gene expression and oocyte yolk accumulation in wild and captive Atlantic bluefin tuna (Thunnus thynnus L.). Anim. Reprod. Sci. 123, 98–105. doi: 10.1016/j.anireprosci.2010.10.005
Rodrigues-Filho J. A., Araújo B. C., Mello P. H., Garcia C. E. O., Silva V. F. D., Li W., et al. (2023). Hormonal profile during the reproductive cycle and induced breeding of the dusky grouper Epinephelus marginatus (Teleostei: Serranidae) in captivity. Aquaculture. 566, 739150. doi: 10.1016/j.aquaculture.2022.739150
Sang H. M., Lam H. S., Hy L. H. K., Ky P. X., and Minh-Thu P. (2019). Changes in plasma and ovarian steroid hormone level in wild female blue tang fish Paracanthurus hepatus during a reproductive cycle. Animals 9, 889. doi: 10.3390/ani9110889
Schulz R. W., Vischer H. F., Cavaco J. E. B., Santos E. M., Tyler C. R., Goos H. J., et al. (2001). Gonadotropins, their receptors, and the regulation of testicular functions in fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 129, 407–417. doi: 10.1016/S1096-4959(01)00339-6
Selvaraj S., Kitano H., Amano M., Nyuji M., Kaneko K., Yamaguchi A., et al. (2012). Molecular characterization and expression profiles of three GnRH forms in the brain and pituitary of adult chub mackerel (Scomber japonicus) maintained in captivity. Aquaculture. 356–357, 200–210. doi: 10.1016/j.aquaculture.2012.05.015
Selvaraj S., Kitano H., Fujinaga Y., Ohga H., Yoneda M., Yamaguchi A., et al. (2010). Molecular characterization, tissue distribution, and mRNA expression profiles of two Kiss genes in the adult male and female chub mackerel (Scomber japonicus) during different gonadal stages. Gen. Comp. Endocrinol. 169, 28–38. doi: 10.1016/j.ygcen.2010.07.011
Selvaraj S., Ohga H., Nyuji M., Kitano H., Nagano N., Yamaguchi A., et al. (2015). Effects of synthetic kisspeptin peptides and GnRH analogue on oocyte growth and circulating sex steroids in prepubertal female chub mackerel (Scomber japonicus). Aquacult. Res. 46, 1866–1877. doi: 10.1111/are.12342
Servili A., Canario A. V. M., Mouchel O., and Muñoz-Cueto J. A. (2020). Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 291, 113439. doi: 10.1016/j.ygcen.2020.113439
Shiraishi T., Ohta K., Yamaguchi A., Yoda M., Chuda H., and Matsuyama M. (2005). Reproductive parameters of the chub mackerel Scomber japonicus estimated from human chorionic gonadotropin-induced final oocyte maturation and ovulation in captivity. Fish. Sci. 71, 531–542. doi: 10.1111/j.1444-2906.2005.00997.x
Shiraishi T., Okamoto K., Yoneda M., Sakai T., Ohshimo S., Onoe S., et al. (2008). Age validation, growth and annual reproductive cycle of chub mackerel Scomber japonicus off the waters of northern Kyushu and in the East China Sea. Fish. Sci. 74, 947–954. doi: 10.1111/j.1444-2906.2008.01612.x
Sundaray J. K., Rather M. A., Kumar S., and Agarwal D. (2021). “Recent updates in molecular endocrinology and reproductive physiology of fish,” in In an imperative step in aquaculture, recent updates in molecular endocrinology and reproductive physiology of fish (Singapore: Springer Nature Singapore Pte. Ltd). doi: 10.1007/978-981-15-8369-8
Taranger G. L., Carrillo M., Schulz R. W., Fontaine P., Zanuy S., Felip A., et al. (2010). Control of puberty in farmed fish. Gen. Comp. Endocrinol. 165, 483–515. doi: 10.1016/j.ygcen.2009.05.004
Tominaga O., Inoue M., Kamata M., and Seikai T. (2005). Reproductive cycle of yellow sea bream Dentex tumifrons in Wakasa Bay, the Sea of Japan off central Honshu. Fish. Sci. 71, 1069–1077. doi: 10.1111/j.1444-2906.2005.01065.x
Tsuda Y., Yamamoto S., Yamaguchi H., Ohnishi T., Sakamoto W., and Murata O. (2013). Vertical movement of spawning cultured chub mackerel (Scomber japonicus) in a net-cage. Aquaculture. 422–423, 136–140. doi: 10.1016/j.aquaculture.2013.12.008
Wang L., Ma S., Liu Y., Li J., Sun D., and Tian Y. (2022). Climate-induced variation in a temperature suitability index of chub mackerel in the spawning season and its effect on the abundance. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.996626
Wang N., Teletchea F., Kestemont P., Milla S., and Fontaine P. (2010). Photothermal control of the reproductive cycle in temperate fishes. Rev. Aquacult. 2, 209–222. doi: 10.1111/j.1753-5131.2010.01037.x
Weltzien F. A., Taranger G. L., Karlsen Ø., and Norberg B. (2002). Spermatogenesis and related plasma androgen levels in Atlantic halibut (Hippoglossus hippoglossus L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 132, 567–575. doi: 10.1016/S1095-6433(02)00092-2
Yasuda T., Kinoshita J., Niino Y., and Okuyama J. (2023). Vertical migration patterns linked to body and environmental temperatures in chub mackerel. Prog. Oceanogr. 213, 103017. doi: 10.1016/j.pocean.2023.103017
Yasuda T., Nagano N., and Kitano H. (2018). Diel vertical migration of chub mackerel: Preliminary evidence from a biologging study. Mar. Ecol. Prog. Ser. 598, 147–151. doi: 10.3354/meps12636
Yoneda M., Kitano H., Nyuji M., Nakamura M., Takahashi M., Kawabata A., et al. (2022). Maternal spawning experience and thermal effects on offspring viability of chub mackerel and their influence on reproductive success. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1063468
Yukami R., Ohshimo S., Yoda M., and Hiyama Y. (2009). Estimation of the spawning grounds of chub mackerel Scomber japonicus and spotted mackerel Scomber australasicus in the East China Sea based on catch statistics and biometric data. Fish. Sci. 75, 167–174. doi: 10.1007/s12562-008-0015-7
Keywords: chub mackerel, reproductive cycle, gonadal development, sex steroid hormone, reproduction-related gene expression
Citation: Baek SI, Ji SC and Cho J-H (2025) Reproductive cycle of cultured chub mackerel (Scomber japonicus), in a land-based tank system in Jeju Island, Korea. Front. Mar. Sci. 12:1617181. doi: 10.3389/fmars.2025.1617181
Received: 24 April 2025; Accepted: 24 June 2025;
Published: 09 July 2025.
Edited by:
Yang Gao, Zhejiang Ocean University, ChinaReviewed by:
Ronghua Li, Ningbo University, ChinaJie Yu, Chinese Academy of Fishery Sciences (CAFS), China
Copyright © 2025 Baek, Ji and Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeong-Hyeon Cho, Y2poLmphbjIzQGdtYWlsLmNvbQ==
 Seong Il Baek
Seong Il Baek Seung Cheol Ji1
Seung Cheol Ji1 Jeong-Hyeon Cho
Jeong-Hyeon Cho