- 1Faculty of Fisheries and Protection of Waters, South Bohemian Research Center of Aquaculture and Biodiversity of Hydrocenoses, Research Institute of Fish Culture and Hydrobiology, University of South Bohemia in České Budějovice, Vodňany, Czechia
- 2Department of Agricultural, Environmental and Food Sciences, University of Molise, Campobasso, Italy
The current study aimed to compare the effects of decreasing osmolality in glucose-based extenders containing methanol alone versus a methanol-ethylene glycol combination on the post-thaw motility of Mediterranean brown trout (Salmo cettii) spermatozoa. Milt was collected from mature males in the Biferno River, Southern Italy, and cryopreserved using a control cryomedium (150 mM glucose with 7.5% methanol) and experimental variants with reduced glucose (100 mM, 50 mM) paired with methanol alone or combined with 2.5% ethylene glycol. Samples were cryopreserved in 0.25 mL straws with a final sperm concentration of 3.0 × 109 spermatozoa/mL, corresponding to 750 × 106 spermatozoa/straw. Sperm motility and kinematic parameters of Mediterranean brown trout were assessed for fresh sperm and post-thaw samples cryopreserved in various cryomedia. Fresh sperm exhibited robust motility (89.0 ± 8.4%) and curvilinear velocity (VCL, 121.2 ± 22.4 µm/s), while the control cryomedium preserved motility at 65.9 ± 12.9% and VCL at 117.6 ± 26.1 µm/s; the experimental 100 mM glucose with 7.5% methanol and 2.5% ethylene glycol treatment yielded comparable motility (65.6 ± 11.6%) but reduced VCL (81.5 ± 16.1 µm/s, p < 0.05). Other treatments showed diminished efficacy, particularly at 50 mM glucose. These findings reveal that a hypotonic extender with methanol-ethylene glycol synergy sustains motility after cryopreservation despite lower glucose levels, demonstrating that the osmotic threshold of extender can be lowered to 100 mOsm/kg without compromising salmonid sperm function. This approach offers a practical tool for conserving S. cettii, supporting broader restoration efforts amid escalating environmental pressures.
1 Introduction
The Mediterranean brown trout Salmo cettii (Osteichthyes: Salmonidae), an indigenous salmonid of southern Europe, represents a vital emblem of biodiversity within the Mediterranean ecosystems. Frequently included within the broader Salmo trutta complex, S. cettii faces escalating threats that have placed it among the most critically endangered freshwater fish in its native range (Rossi et al., 2019). According to recent genetic findings, the Italian peninsular Mediterranean brown trout belongs to a different taxon known as S. ghigii, therefore limiting the name S. cettii to Sicilian trout (Lorenzoni et al., 2019; D’Agaro et al., 2022). We still use the name S. cettii, because Mediterranean brown trout populations are still protected by the Habitat Directive and subsequent conservation status updates under this taxon.
In addition to human-induced pressures such as habitat degradation, water pollution, and poorly regulated fishing practices, one of the most serious threats is genetic introgression resulting from the widespread introduction of alien, interfertile trout genetic strains. In particular, stocking activities involving Atlantic lineages of Salmo trutta for recreational fishing purposes have caused extensive replacement and erosion of the native gene pools (Lorenzoni et al., 2019; Rossi et al., 2019; Splendiani et al., 2019; Carosi et al., 2020; D’Agaro et al., 2022). Salmo cettii is classified as “critically endangered” in the Italian IUCN Red List, as the few remaining genetically pure populations are now confined to highly fragmented river systems, particularly within the Apennine region (Lorenzoni et al., 2019; Splendiani et al., 2019; Talarico et al., 2023), including the Biferno and Volturno rivers in Southern Italy (Iaffaldano et al., 2016a, 2016; Di Iorio et al., 2023).
The vulnerability of the Mediterranean brown trout highlights the urgent need to protect its genetic integrity, which is crucial for sustaining ecological functions and preserving its evolutionary potential under ongoing environmental changes (Di Iorio et al., 2023; Talarico et al., 2023).
Cryopreservation of sperm has emerged as an especially effective technique for securing the genetic resources of fish, with demonstrated success across multiple salmonid species (Nynca et al., 2017; Di Iorio et al., 2019; Bozkurt, 2023; Mayer and Pšenička, 2024). Integrated within broader conservation frameworks – such as the LIFE Nat.Sal.Mo. project, aimed to restore native genetic diversity and habitats of S. cettii in Molise rivers (Southern Italy) (Di Iorio et al., 2023) – semen cryobanks provide a stable reservoir of genetic variability to support restoration programs and management actions through the application of controlled artificial reproduction, aimed at reinforcing the integrity of remnant wild populations.
Extensive cryobiological research on sperm of Salmoniformes has focused on refining cryoprotective media and cooling/thawing protocols (Ciereszko et al., 2013; Nynca et al., 2014, 2015; Dietrich et al., 2016; Bozkurt et al., 2019). Cryoprotective media typically comprise an extender – containing salts, sugars, and organic compounds - and cryoprotective agents (CPAs). The extender’s composition is critical, as shifts in ionic and osmotic conditions profoundly affect sperm motility by altering cell volume regulation and ionic balance (Cosson, 2004). In freshwater-spawning species like trout, hypotonic conditions trigger sperm motility, complicating cryopreservation strategies. In early studies, extenders for sperm cryopreservation were designed to match seminal plasma osmolality (isotonic) or exceed it (hypertonic) (Erdhal and Graham, 1980; Stoss and Holtz, 1981; McNiven et al., 1993; Bozkurt et al., 2005; Bozkurt and Yavaş, 2024; Lahnsteiner et al., 2024). However, recent studies over the past decade have demonstrated that hypotonic glucose-based extenders enhance post-thaw outcomes for salmonid sperm (Ciereszko et al., 2014; Nynca et al., 2016, 2017; Judycka et al., 2018; Fujimoto et al., 2022). Research highlights their protective capacity during cooling without impairing fertilization potential. Notably, glucose concentrations of 130–170 mM (~130–170 mOsm/kg) extender have yielded high post-thaw motility in rainbow trout (Oncorhynchus mykiss) sperm (Judycka et al., 2020).
Furthermore, recent research demonstrates that hypotonic extender-based cryomedia, combining methanol and low doses of ethylene glycol, effectively cryopreserve sperm of freshwater-spawning species, including common carp Cyprinus carpio (Dokina et al., 2019, 2023; Krasilnikova et al., 2025a) and sturgeons (Krasilnikova et al., 2025b). This approach challenges the conventional use of isotonic or hypertonic extenders by leveraging cryoprotectant synergy. The synergistic interaction of methanol and ethylene glycol with a slow cooling rate enabled the use of hypotonic extender-based cryomedia for the effective cryopreservation of common carp sperm in larger sample volumes (4.5 ml cryotubes), achieving high post-thaw motility without causing larval malformations (Krasilnikova et al., 2025a). Similarly, a low-osmotic extender-based cryomedium supported embryo development and hatching rates in sturgeons (Krasilnikova et al., 2025b). These findings demonstrated the efficacy of this novel cryopreservation approach across cyprinid and chondrostean.
Building on these advances, this study aimed to evaluate the impact of reduced osmolality in glucose-based extenders, comparing methanol-only cryomedia with formulations including methanol and ethylene glycol, to determine if hypotonic conditions with synergistic cryoprotectants can preserve the post-thaw motility of Salmo cettii spermatozoa.
2 Materials and methods
2.1 Sperm collection
Native mature Mediterranean brown trout (Salmo cettii) were captured via electrofishing in the Biferno River, Molise region, Southern Italy, within the Adriatic basin. The sampling site was located at coordinates 41°28’52.4” North latitude, 14°28’51.4” East longitude. Sperm samples were collected during the middle phase of the spawning season, specifically in the morning hours between 10:00 and 12:00. The age of the fish ranged from 2 to 3 years. The average total length was 31.9 ± 3.2 cm (range = 25.5-36.0 cm). Regarding the phenotypic characteristics of the males selected for semen collection, the fish showed the typical features of the native Molise population of Salmo cettii. These included a blue-black mark near the preopercular area, the frequent presence of dark vertical bands along the sides, and a general absence of pronounced black and white borders on the dorsal and anal fins. The body displayed a combination of black and red spots, often irregular in shape, with no oval spots encircled by halos. When present, parr marks appeared in more than one row and were always numerous (more than nine). The adipose fin was consistently free of any spotting. The health status of the individuals was assessed through external observation and behavioral evaluation before sperm extraction. All selected fish showed no visible injury, malformation, or parasitic infection.
Milt was obtained by stripping individual males after meticulously drying the abdomen and urogenital papilla to prevent contamination with urine, mucus, or blood cells. Sperm was collected and analyzed from five mature males. The ejaculate’s color and volume are evaluated immediately after collection. All the samples used in this study had a 1.5–2 ml volume and were white-cream-colored. Immediately after collection in the field, the semen samples were transported to the laboratory on ice (0-4°C) in an insulated container under aerobic conditions. The time after collecting the sperm to transfer the sperm samples to the laboratory was approximately 2–3 hours. As previously shown (Rusco et al., 2021), this time is sufficient to prevent loss of Mediterranean brown trout sperm quality.
2.2 Recording of sperm motility and sperm analysis
Sperm motility assessments were conducted using a Computer-Assisted Sperm Analysis (CASA) system linked to a phase contrast microscope (Nikon, model Ci-L, Japan) with Sperm Class Analyzer (SCA) software (VET Edition, Barcelona, Spain). Prior to evaluation, spermatozoa were activated in an activation medium composed of 1 mM CaCl2, 20 mM Tris, 30 mM glycine, and 125 mM NaCl, adjusted to pH 9.0 and enriched with 0.5% bovine albumin (Billard, 1992; Judycka et al., 2018), using a dilution rate 1:300 or 1:30 for fresh or thawed semen, respectively. An aliquot (0.7 μL) of this solution was immediately placed into a well (diameter 5 mm) of a 12-well multitest glass slide (TEKDON Inc., Florida, USA), and a coverslip was applied. Sperm motility parameters were analyzed 10 s post-activation using a 25-fps rate during recording. Key kinematic characteristics measured included the percentage of progressive motile spermatozoa (%), the percentage of total motile spermatozoa (%), curvilinear velocity (VCL, µm/s), average path velocity (VAP, µm/s), straight-line velocity (VSL, µm/s), straightness (STR, %), linearity (LIN, %), oscillation of the track (WOB, %, VAP/VCL), and beat cross frequency (BCF, Hz).
2.3 Measurement of sperm concentration and osmolality of cryomedia
Sperm concentration was quantified using a photometric approach. Semen was diluted in 0.9% NaCl at a 1:200 (v:v) ratio, and optical density was measured at 530 nm with a portable DR 1900 photometer (HACH Company, Loveland, CO, USA). The concentration, expressed as ×109 spermatozoa/mL, was determined from a pre-established standard curve correlating optical density to sperm density, as detailed by (Nynca and Ciereszko, 2009).
The osmolality of cryomedia (extender + cryoprotective agents) was measured using a freezing-point osmometer OSMOMAT 3000 (Gonotec, Germany) and expressed as mOsm/kg. For each sample, 50 µL of the solution was used, and each measurement was performed in triplicate.
2.4 Cryopreservation
The cryopreservation protocol for semen followed established methods (Rusco et al., 2020; Di Iorio et al., 2023). The control cryomedium consisted of 150 mM glucose with 7.5% methanol at the final concentration (150 GM) (Ciereszko et al., 2014). Experimental treatments used a glucose extender at final concentrations of 100 mM and 50 mM. These were paired with either 7.5% methanol alone (100 GM, 50 GM) or a mixture of 7.5% methanol and 2.5% ethylene glycol (150 GMEg, 100 GMEg, 50 GMEg). Semen was mixed with cryomedia to achieve a final sperm density of 3.0 × 109 spermatozoa/mL, corresponding to 750 × 106 spermatozoa/straw. Semen was aspirated into 0.25 mL straws using a manual micro-aspirator (IMV-Technologies) and sealed with polyvinyl alcohol (PVA). Straws were equilibrated on ice for 15 minutes. Freezing occurred in liquid nitrogen vapor, 3 cm above the liquid, for 5 minutes with a rapid, uncontrolled cooling rate in a Styrofoam box measuring 630 mm (length) × 410 mm (width) × 260 mm (height). Frozen straws were then plunged into liquid nitrogen (-196°C) for storage. For analysis, straws were thawed in a 40°C water bath for 5 seconds. The study utilized five males. Each treatment included three replicates and two post-thaw sperm activation assessments per treatment (n=30).
2.5 Statistical analysis
Before statistical evaluation, the normality of the data was assessed with the Kolmogorov-Smirnov test, and variance homogeneity was examined using Levene’s test. Due to the non-normal distribution of the data, a Kruskal-Wallis nonparametric test was employed for analysis, with subsequent pairwise comparisons of mean ranks conducted for multiple independent groups. Data processing and visualization were carried out using Statistica software (TIBCO Statistica, version 14.0.0.15, USA) and Microsoft Excel, with all statistical tests evaluated at a significance threshold of p < 0.05.
3 Results
The results of measurement of osmolality (mean ± standard deviation) are as follows: 1) 150 mM glucose + 7.5% methanol = 2523 ± 9 mOsm/kg (osmolality counting via calculation method ~ 2150 mOsm/kg); 2) 100 mM glucose + 7.5% methanol = 2373 ± 27 mOsm/kg (osmolality counting via calculation method ~ 2100 mOsm/kg); 3) 50 mM glucose + 7.5% methanol = 2242 ± 35 mOsm/kg (osmolality counting via calculation method ~ 2050 mOsm/kg); 4) 150 mM glucose + 7.5% methanol + 2.5% ethylene glycol = 3193 ± 41 mOsm/kg (osmolality counting via calculation method ~ 2700 mOsm/kg); 5) 100 mM glucose + 7.5% methanol + 2.5% ethylene glycol = 3127 ± 22 mOsm/kg (osmolality counting via calculation method ~ 2650 mOsm/kg); 6) 50 mM glucose + 7.5% methanol + 2.5% ethylene glycol = 3064 ± 33 mOsm/kg (osmolality counting via calculation method ~ 2600 mOsm/kg).
The motility and kinematic parameters of Mediterranean brown trout (Salmo cettii) sperm were assessed post-thaw across fresh samples and various cryopreservation treatments (Figure 1).
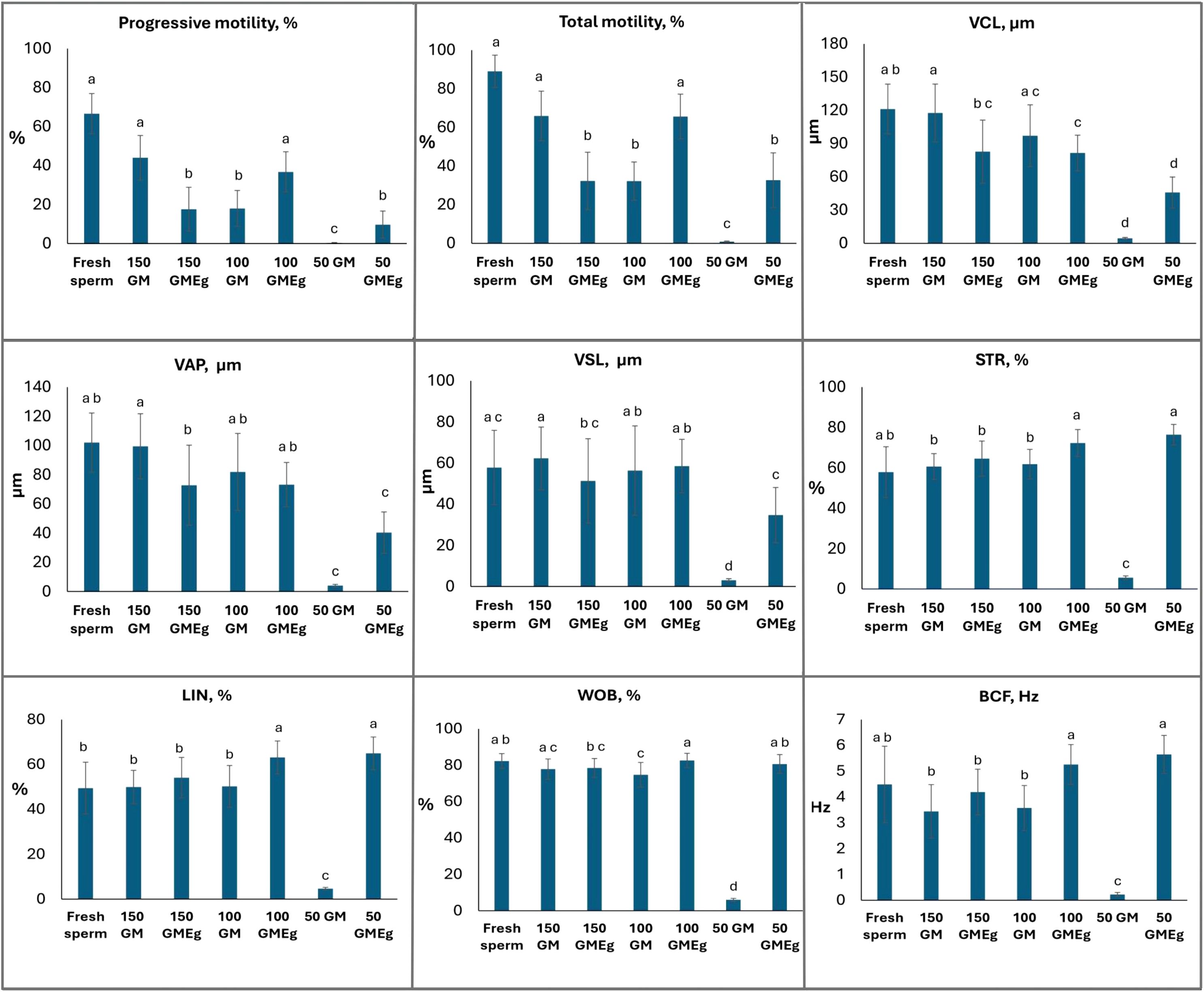
Figure 1. Post-thawed kinetic parameters spermatozoa at 10 s after activation. Data are expressed as mean ± SD. Different lowercase letters indicate a significant difference among the cryomedia (Kruskal–Wallis test followed by multiple comparisons of mean ranks for all groups, p < 0.05).
Fresh sperm exhibited robust motility and velocity, with progressive motility averaging 66.6 ± 10.3%, total motility at 89.0 ± 8.4%, and curvilinear velocity (VCL) at 121.2 ± 22.4 µm/s. Other kinematic parameters included average path velocity (VAP) at 102.0 ± 20.2 µm/s and straight-line velocity (VSL) at 57.9 ± 18.1 µm/s.
Among cryopreserved samples, the traditional cryomedium (150 mM glucose + 7.5% methanol, osmolality 2523 ± 9 mOsm/kg) preserved progressive motility at 44.0 ± 11.4%, total motility at 65.9 ± 12.9%, and VCL at 117.6 ± 26.1 µm/s. The one of experimental cryomedium (100 mM glucose + 7.5% methanol + 2.5% ethylene glycol, osmolality 3127 ± 22 mOsm/kg) yielded comparable motility, with progressive motility at 36.7 ± 10.3%, total motility at 65.6 ± 11.6%, but a significantly lower VCL of 81.5± 16.1 µm/s (p<0.05) (Figure 1).
Other experimental cryomedia showed variable efficacy. The 150 mM glucose + 7.5% methanol + 2.5% ethylene glycol treatment resulted in reduced motility (progressive motility: 17.6 ± 11.3%, total motility: 32.3 ± 14.9%) and VCL (82.8 ± 28.4 µm/s). The 100 mM glucose + 7.5% methanol cryomedium maintained moderate progressive motility (18.0 ± 9.2%) and total motility (32.2 ± 9.9%), with VCL at 96.1 ± 27.8 µm/s. Treatments with 50 mM glucose + 7.5% methanol was largely ineffective (Figure 1).
4 Discussion
The results obtained in this study suggest that the choice of cryomedia significantly impacts the quality of frozen semen and the preservation of sperm motility. The traditional cryomedium (150 mM glucose and 7.5% methanol) effectively preserved Mediterranean brown trout sperm quality during cryopreservation (Figure 1). These results are consistent with previous studies (Rusco et al., 2020, 2021; Di Iorio et al., 2023; Rusco et al., 2023), which have similarly underscored the effectiveness of this cryomedium in maintaining post-thaw semen quality.
Research on Salmoniformes spermatozoa has demonstrated that glucose exerts a cryoprotective effect within a narrow concentration range of 130–170 mM (Judycka et al., 2020). The present study suggests that incorporating a small amount of ethylene glycol into cryomedia may reduce this lower threshold to 100 mM. Furthermore, the cryoprotective synergy between methanol and ethylene glycol offsets the reduced glucose concentration, sustaining sperm viability post-thaw. This methanol-ethylene glycol combination has been investigated in other freshwater species, such as common carp (Cyprinus carpio) (Krasilnikova et al., 2025a), and was showed that it inhibits motility in hypotonic conditions by promoting cell shrinkage and preventing activation. Consequently, this cryomedium formulation synergistically averts premature activation, preserving sperm viability for post-thaw applications.
In salmonids, sperm motility is tightly regulated by osmolality and ionic composition (Alavi and Cosson, 2006). The suppression of sperm motility primarily results from K+ ions (Billard et al., 1995; Kho et al., 2001). However, research demonstrates that potassium ions are not essential in cryomedia for salmonid spermatozoa (Judycka et al., 2016), contrasting with findings for sturgeons (Krasilnikova et al., 2025b). In the current study, despite being hypotonic (~100–150 mOsm/kg post-dilution), the glucose-methanol extender does not trigger spermatozoal activation during cryopreservation preparation. It can be attributed to the glucose component, while contributing to hypotonicity, providing a non-ionic environment that prevents activation of potassium- or calcium-dependent activation pathways, critical for motility onset in salmonids (Alavi and Cosson, 2006). Additionally, studies also indicate that glucose, sucrose, or trehalose can be effectively substituted as cryoprotectants for brown trout, brook trout, and sex-reversed semen (Nynca et al., 2016). It underscores the efficacy of hypotonic, non-ionic cryomedia for salmonid species.
The reduced kinematic parameters in the 100 mM glucose with 7.5% methanol and 2.5% ethylene glycol treatment, which could link with osmotic stress in the presence of different cryoprotectants, may not critically impair fertilization success in controlled aquaculture settings, where sperm-to-egg ratios are high and diffusion distances are minimal. Decreasing VCL was also observed after using hypotonic extender-based cryomedium with methanol and ethylene glycol for sturgeon sperm (Krasilnikova et al., 2025b). It can explain the higher molecular weight and viscosity of ethylene glycol, which could influence the decreasing this parameter. Furthermore, ethylene glycol has demonstrated a non-toxic effect on spermatozoa, oocytes, and embryos, supporting successful fertilization of aquatic species (Sansone et al., 2002; Ieropoli et al., 2004; Le et al., 2011; Heres et al., 2019).
Treatment with lower glucose concentrations (50 mM) was particularly ineffective, resulting in very low motility, which indicates poor protection of the sperm during cryopreservation. This suggests that low glucose concentration does not offer adequate osmotic protection, leading to membrane destabilization and compromised semen quality during freezing. Evaluating the progeny derived from an alternative sperm cryopreservation method employing a low-ionic cryomedium is essential. Additionally, recent research has increasingly explored epigenetic alterations linked to cryopreservation. Studies indicate that cryopreservation does not significantly alter DNA methylation in rainbow trout spermatozoa (El Kamouh et al., 2023). Nevertheless, further investigation into epigenetic markers (e.g., DNA methylation, histone modifications) in spermatozoa post-cryopreservation and embryos produced using a hypotonic non-ionic cryomedium with methanol and ethylene glycol is warranted.
It is critical to note that the osmolality of cryomedia (extender + cryoprotective agents) was measured using freezing point osmometry. The observed discrepancies between the calculated and measured osmolality values can be attributed to several factors inherent to the non-ideal behavior of complex cryomedia. Theoretical osmolality calculations are based on the assumption of ideal solution behavior, where solutes are fully dissociated and additive in their osmotic contributions. However, in real systems - particularly those containing high glucose, methanol, and ethylene glycol - solute and solvent interactions can significantly alter water activity and freezing point depression, resulting in higher measured osmolality values. Moreover, the physical properties of alcohol-based cryoprotectants, such as their ability to alter water structure and freezing dynamics, further contribute to this discrepancy. As a result, measured osmolality values are consistently higher, highlighting the importance of choosing an osmolality estimation method and the limitations of theoretical approaches when applied to complex cryopreservation solutions.
5 Conclusion
This study demonstrates that the post-thaw motility of Mediterranean brown trout (Salmo cettii) spermatozoa can be effectively preserved using a hypotonic, glucose-based cryomedium supplemented with methanol and a low concentration of ethylene glycol. The combination of 100 mM glucose with 7.5% methanol, and 2.5% ethylene glycol sustained total motility at levels comparable to the control medium despite a significant reduction in glucose concentration.
This advancement enhances the flexibility of cryopreservation protocols for salmonids. It reinforces the potential of hypotonic, non-ionic cryomedia to maintain post-thaw sperm quality, offering a practical solution for genetic resource preservation and supporting restoration initiatives for this critically endangered species. However, these promising results will need to be further validated through fertilization trials to confirm their effectiveness in reproductive and conservation contexts.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The experiments were conducted in accordance with the Code of Ethics of the EU Directive 2010/63/EU for animal experiments. The sampling and handling of fish followed animal welfare practices as reported in the Ministerial Protocol (ISPRA). All experiments were carried out with the appropriate authorizations from the Molise Region–Dipartimento Governo del Territorio, Mobilità e Risorse Naturali cod. DP.A4.02.4N.01 (protocol number 3969, 3 August 2018), according to the current regulations on the protection of the species, biosecurity, protocols of sampling of fresh water and animal welfare. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AK: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Investigation. MD: Methodology, Writing – review & editing. EA: Methodology, Writing – review & editing. SE: Resources, Writing – review & editing. NI: Writing – review & editing. MP: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was supported by the Ministry of Education, Youth and Sports of the Czech Republic - project Biodiversity (CZ.02.1.01/0.0/0.0/16_025/0007370), and the Czech Science Foundation (grant number 22-31141J).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alavi S. M. and Cosson J. (2006). Sperm motility in fishes. (II) Effects of ions and osmolality: a review. Cell Biol. Int. 30, 1–14. doi: 10.1016/j.cellbi.2005.06.004
Billard R. (1992). Reproduction in rainbow trout: sex differentiation, dynamics of gametogenesis, biology and preservation of gametes. Aquaculture 100, 263–298. doi: 10.1016/0044-8486(92)90385-X
Billard R., Cosson J., Crim L. W., and Suquet M. (1995). Sperm physiology and quality. In: Bromage N. and Roberts R. (Eds.), Broodstock management and egg and larval quality (Oxford, Reino Unido: Blackwell), 25–52.
Bozkurt Y. (2023). Conservation strategies for the aquatic genetic resources: Opportunities and challenges. Bozkurt Y. editor. Modern Reproductive Biotechnologies in the Conservation of Aquatic Genetic Resources. 1st ed. (Ankara: Türkiye Klinikleri). 1–5.
Bozkurt Y., Akcay E., Tekin N., and Secer S. (2005). Effect of freezing techniques, extenders and cryoprotectants on the fertilization rate of frozen rainbow trout (Oncorhynchus mykiss) sperm. Israeli J. aquaculture = Bamidgeh 57, 125–130. doi: 10.46989/001c.20398
Bozkurt Y., Yavas I., Gul A., Bucak M. N., Yeni D., and Avdatek F. (2019). Effect of Extender Supplemented with Boron on Post-Thaw Motility, Viability, DNA Damage and Fertilization Ability of Cryopreserved Brown Trout (Salmo trutta macrostigma) Spermatozoa. Cryo Lett. 40, 275–283.
Bozkurt Y. and Yavaş İ. (2024). Comparison of different freezing techniques, extenders, and cryoprotectants on quality and fertility of cryopreserved Salmo trutta f. fario sperm. Acta Scientiarum. Technol. 46(1), e64924. doi: 10.4025/actascitechnol.v46i1.64924
Carosi A., Bonomo G., and Lorenzoni M. (2020). Effectiveness of alien brown trout Salmo trutta L. removal activities for the native trout conservation in Mediterranean streams. J. Appl. Ichthyology 36, 461–471. doi: 10.1111/jai.14063
Ciereszko A., Dietrich G. J., Nynca J., Dobosz S., and Zalewski T. (2014). Cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture 420-421, 275–281. doi: 10.1016/j.aquaculture.2013.11.014
Ciereszko A., Dietrich G. J., Nynca J., Liszewska E., Karol H., and Dobosz S. (2013). The use of concentrated extenders to improve the efficacy of cryopreservation in whitefish spermatozoa. Aquaculture 408-409, 30–33. doi: 10.1016/j.aquaculture.2013.05.016
Cosson J. (2004). The ionic and osmotic factors controlling motility of fish spermatozoa. Aquac. Int. 12, 69–85. doi 10.1023/B:AQUI.0000017189.44263.bc
D’Agaro E., Gibertoni P., Marroni F., Messina M., Tibaldi E., and Esposito S. (2022). Genetic and phenotypic characteristics of the salmo trutta complex in Italy. Appl. Sci. 12, 3219. doi: 10.3390/app12073219
Dietrich G. J., Nynca J., Szczepkowski M., Dobosz S., Szczepkowska B., and Ciereszko A. (2016). The effect of cryopreservation of semen from whitefish (Coregonus lavaretus) and northern pike (Esox lucius) using a glucose-methanol extender on sperm motility parameters and fertilizing ability. Aquaculture 464, 60–64. doi: 10.1016/j.aquaculture.2016.06.015
Di Iorio M., Esposito S., Rusco G., Roncarati A., Miranda M., Gibertoni P. P., et al. (2019). Semen cryopreservation for the Mediterranean brown trout of the Biferno River (Molise-Italy): comparative study on the effects of basic extenders and cryoprotectants. Sci. Rep. 9, 9703. doi: 10.1038/s41598-019-45006-4
Di Iorio M., Rusco G., Esposito S., D’andrea M., Roncarati A., and Iaffaldano N. (2023). The role of semen cryobanks for protecting endangered native salmonids: Advantages and perspectives as outlined by the LIFE Nat.Sal.Mo. project on Mediterranean brown trout (Molise region – Italy). Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1075498
Dokina O., Kovalev K., and Pronina N. (2023). Effective technology of carp (Cyprinus carpio) sperm cryopreservation for use in a large-scale cryobank. Genet. Aquat. Organisms 7, GA715. doi: 10.4194/2459-1831
Dokina O. B., Pronina N. D., Kovalev K. V., Milenko B. A., and Tcvetkova L. I. (2019). Advanced technology of cryopreservation of carp sperm in a large-scale cryobank. Fisheries 5, 99–107.
El Kamouh M., Brionne A., Sayyari A., Laurent A., and Labbé C. (2023). Cryopreservation effect on DNA methylation profile in rainbow trout spermatozoa. Sci. Rep. 13, 19029. doi: 10.1038/s41598-023-44803-2
Erdhal D. and Graham E. (1980). Preservation of spermatozoa of brook trout and rainbow trout. Cryo-Letters 1, 203–208.
Fujimoto T., Kaneyasu T., Endoh M., Kogame Y., Nynca J., Ciereszko A., et al. (2022). Cryopreservation of masu salmon sperm using glucose-methanol extender and seminal plasma biomarkers related to post-thaw sperm motility. Aquaculture 557, 738305. doi: 10.1016/j.aquaculture.2022.738305
Heres P., Rodriguez-Riveiro R., Troncoso J., and Paredes E. (2019). Toxicity tests of cryoprotecting agents for Mytilus galloprovincialis (Lamark 1819) early developmental stages. Cryobiology 86, 40–46. doi: 10.1016/j.cryobiol.2019.01.001
Iaffaldano N., Di Iorio M., Manchisi A., Esposito S., and Gibertoni P. P. (2016a). Effective freezing rate for semen cryopreservation in endangered Mediterranean brown trout (Salmo trutta macrostigma) inhabiting the Biferno river (South Italy). Zygote 24, 668–675. doi: 10.1017/S0967199415000647
Iaffaldano N., Di Iorio M., Manchisi A., Gibertoni P., and Esposito S. (2016b). Semen quality of threatened native population of Mediterranean brown trout (Salmo cettii, Rafinesque 1810) in the Biferno River (Molise Region-South Italy). Turkish J. fisheries Aquat. Sci. 16, 259–266. doi: 10.4194/1303-2712-v16_2_05
Ieropoli S., Masullo P., Santo M. D. E., and Sansone G. (2004). Effects of extender composition, cooling rate and freezing on the fertilisation viability of spermatozoa of the Pacific oyster (Crassostrea gigas). Cryobiology 49, 250–257. doi: 10.1016/j.cryobiol.2004.08.005
Judycka S., Nynca J., Liszewska E., Dobosz S., Grudniewska J., and Ciereszko A. (2018). Optimal sperm concentration in straws and final glucose concentration in extender are crucial for improving the cryopreservation protocol of salmonid spermatozoa. Aquaculture 486, 90–97. doi: 10.1016/j.aquaculture.2017.12.019
Judycka S., Nynca J., Liszewska E., Dobosz S., Zalewski T., and Ciereszko A. (2016). Potassium ions in extender differentially influence post-thaw sperm motility of Salmonidae fish. Anim. Reprod. Sci. 169, 106. doi: 10.1016/j.anireprosci.2016.03.033
Judycka S., Słowińska M., Nynca J., Liszewska E., Dobosz S., and Ciereszko A. (2020). Effects of glucose, methanol concentration, and time of equilibration on post-thaw sperm motility of rainbow trout semen. Aquaculture 520, 734996. doi: 10.1016/j.aquaculture.2020.734996
Kho K. H., Tanimoto S., Inaba K., Oka Y., and Morisawa M. (2001). Transmembrane cell signaling for the initiation of trout sperm motility: roles of ion channels and membrane hyperpolarization for cyclic AMP synthesis. Zoological Sci. 18, 919–928. doi: 10.2108/zsj.18.919
Krasilnikova A., Rodina M., Gela D., Sotnikov A., and Pšenička M. (2025a). Cryopreservation of common carp (Cyprinus carpio L.) sperm: Insights into low-ionic cryoprotective medium mechanism and efficacy. Aquaculture 596, 741807. doi: 10.1016/j.aquaculture.2024.741807
Krasilnikova A., Rodina M., Gela D., Sotnikov A., and Pšenička M. (2025b). Exploring cryomedium with hypotonic extender for sturgeon sperm cryopreservation. Aquaculture 605, 742497. doi: 10.1016/j.aquaculture.2025.742497
Lahnsteiner F., Berger B., Mansour N., and Kunz F. (2024). Quality of cryopreserved salmonid semen is not affected by a 25 years storage period. Aquaculture 578, 740100. doi: 10.1016/j.aquaculture.2023.740100
Le M. H., Lim H. K., Min B. H., Park M. W., and Chang Y. J. (2011). Semen cryopreservation of yellow croaker Larimichthys polyactis. Rev. Fish Biol. Fisheries 21, 789–797. doi: 10.1007/s11160-011-9209-7
Lorenzoni M., Carosi A., Giovannotti M., La Porta G., Splendiani A., and Barucchi V. C. (2019). Ecology and conservation of the Mediterranean trout in the central Apennines (Italy). J. limnology 78. doi: 10.4081/jlimnol.2018.1806
Mayer I. and Pšenička M. (2024). Conservation of teleost fishes: Application of reproductive technologies. Theriogenology Wild 4, 100078. doi: 10.1016/j.therwi.2024.100078
McNiven M. A., Gallant R. K., and Richardson G. F. (1993). Dimethyl-acetamide as a cryoprotectant for rainbow trout spermatozoa. Theriogenology 40, 943–948. doi: 10.1016/0093-691X(93)90362-9
Nynca J. and Ciereszko A. (2009). Measurement of concentration and viability of brook trout (Salvelinus fontinalis)spermatozoa using computer-aided fluorescent microscopy. Aquaculture 292, 256–258. doi: 10.1016/j.aquaculture.2009.04.020
Nynca J., Dietrich G. J., Dobosz S., Grudniewska J., and Ciereszko A. (2014). Effect of cryopreservation on sperm motility parameters and fertilizing ability of brown trout semen. Aquaculture 433, 62–65. doi: 10.1016/j.aquaculture.2014.05.037
Nynca J., Dietrich G. J., Grudniewska J., Dobosz S., Liszewska E., Krzyś M., et al. (2015). Efficient method for cryopreservation of European huchen (Hucho hucho L.) and grayling (Thymallus thymallus L.) semen. Aquaculture 435, 146–151. doi: 10.1016/j.aquaculture.2014.09.031
Nynca J., Judycka S., Liszewska E., Dobosz S., and Ciereszko A. (2017). Standardization of spermatozoa concentration for cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture 477, 23–27. doi: 10.1016/j.aquaculture.2017.04.036
Nynca J., Judycka S., Liszewska E., Dobosz S., Grudniewska J., Arai K., et al. (2016). Utility of different sugar extenders for cryopreservation and post-thaw storage of sperm from Salmonidae species. Aquaculture 464, 340–348. doi: 10.1016/j.aquaculture.2016.07.014
Rossi A., Petrosino G., Milana V., Martinoli M., Rakaj A., and Tancioni L. (2019). Genetic identification of native populations of Mediterranean brown trout Salmo trutta L. complex (Osteichthyes: Salmonidae) in central Italy. Eur. Zoological J. 86, 424–431. doi: 10.1080/24750263.2019.1686077
Rusco G., Di Iorio M., Esposito S., Antenucci E., Roncarati A., and Iaffaldano N. (2023). The use of ovarian fluid as natural fertilization medium for cryopreserved semen in mediterranean brown trout: the effects on sperm swimming performance. Vet. Sci. 10, 219. doi: 10.3390/vetsci10030219
Rusco G., Di Iorio M., Iampietro R., Esposito S., Gibertoni P. P., Penserini M., et al. (2020). A simple and efficient semen cryopreservation method to increase the genetic variability of endangered mediterranean brown trout inhabiting molise rivers. Animals 10, 403. doi: 10.3390/ani10030403
Rusco G., Di Iorio M., Iampietro R., Roncarati A., Esposito S., and Iaffaldano N. (2021). Cryobank of mediterranean brown trout semen: evaluation of the use of frozen semen up to six hours post-collection. Fishes 6, 26. doi: 10.3390/fishes6030026
Sansone G., Fabbrocini A., Ieropoli S., Langellotti A. L., Occidente M., and Matassino D. (2002). Effects of extender composition, cooling rate, and freezing on the motility of sea bass (Dicentrarchus labrax, L.) spermatozoa after thawing. Cryobiology 44, 229–239. doi: 10.1016/S0011-2240(02)00026-3
Splendiani A., Fioravanti T., Ruggeri P., Giovannotti M., Carosi A., Marconi M., et al. (2019). Life history and genetic characterization of sea trout Salmo trutta in the Adriatic Sea. Freshw. Biol. 65, 460–473. doi: 10.1111/fwb.13441
Stoss J. and Holtz W. (1981). Cryopreservation of rainbow trout (Salmo gairdneri) sperm: I. Effect of thawing solution, sperm density and interval between thawing and insemination. Aquaculture 22, 97–104. doi: 10.1016/0044-8486(81)90136-8
Talarico L., Caniglia R., Carosi A., Lorenzoni M., Greco C., Padula A., et al. (2023). Population structure, genetic diversity and demographic patterns unveil massive Mediterranean brown trout manipulations in a protected area of the northern Apennines (Italy). Eur. Zoological J. 90, 470–486. doi: 10.1080/24750263.2023.2223222
Keywords: cryopreservation, sperm, cryomedium, Mediterranean brown trout, motility
Citation: Krasilnikova A, Di Iorio M, Antenucci E, Esposito S, Iaffaldano N and Pšenička M (2025) Effect of reduced osmolality in glucose-based extenders on the post-thaw motility of Mediterranean brown trout (Salmo cettii) spermatozoa. Front. Mar. Sci. 12:1621496. doi: 10.3389/fmars.2025.1621496
Received: 01 May 2025; Accepted: 23 June 2025;
Published: 28 July 2025.
Edited by:
Yusuf Bozkurt, Mersin University, TürkiyeReviewed by:
Faruk Aral, Independent Researcher, Konya, TürkiyeTakafumi Fujimoto, Hokkaido University, Japan
Copyright © 2025 Krasilnikova, Di Iorio, Antenucci, Esposito, Iaffaldano and Pšenička. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksandra Krasilnikova, a3Jhc2lsbmlrb3ZhQGZyb3YuamN1LmN6; YWxlLmtyYXNpbG5pa292YUBnbWFpbC5jb20=
 Aleksandra Krasilnikova
Aleksandra Krasilnikova Michele Di Iorio
Michele Di Iorio Emanuele Antenucci
Emanuele Antenucci Stefano Esposito2
Stefano Esposito2 Nicolaia Iaffaldano
Nicolaia Iaffaldano Martin Pšenička
Martin Pšenička