- 1College of Fisheries, Guangdong Ocean University, Zhanjiang, Guangdong, China
- 2Guangdong Provincial Key Laboratory of Aquatic Animal Disease Control and Healthy Culture, Zhanjiang, Guangdong, China
- 3Guangdong Key Laboratory of Control for Diseases of Aquatic Economic Animals, Zhanjiang, Guangdong, China
- 4Key Laboratory of Marine Ecology and Aquaculture Environment of Zhanjiang, Zhanjiang, Guangdong, China
- 5Shenzhen Institute of Guangdong Ocean University, Shenzhen, Guangdong, China
- 6Southern Marine Science and Engineering Guangdong Laboratory, Zhanjiang, China
- 7Guangxi Key Laboratory of Aquatic Biotechnology and Modern Ecological Aquaculture, Guangxi Academy of Marine Sciences, Guangxi Academy of Sciences, Nanning, China
- 8Department of Fisheries and Watershed Management, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
The issue of antimicrobial resistance in farm-raised fish presents a significant challenge for aquaculture operations. Long-term antibiotic treatment of fish for bacterial infections has led to bacteria thriving in the aquatic ecosystem and developing resistance to antibiotics. On the other hand, increasing research suggests that probiotics and prebiotics may be viable alternatives to antibiotics in regulating the immune system. Probiotics and prebiotics interact with fish metabolism in complex ways. These interactions offer promising alternatives to reduce antibiotic use in aquaculture. Introducing live microorganisms, known as probiotics, into an organism’s system can help improve overall health by altering the microflora and boosting immunity. Acting as immunostimulants, prebiotics directly impact the fish’s innate immune system. When used together, probiotics and prebiotics enhance immunomodulatory activity, providing numerous health benefits to aquatic animals. However, successfully replacing antibiotics with probiotics and prebiotics requires a deep understanding of metabolic pathways, optimization strategies, and innovative approaches. There has been a lack of extensive research on how probiotics and prebiotics impact lipid metabolism in various types of fish. This review aims to explore the intricate relationship between probiotics, prebiotics, and fish metabolism, with a specific focus on how these beneficial microorganisms and dietary fibers interact with fish antioxidant systems. We have also discussed the challenges faced by farmers when using probiotics and prebiotics. This review analyzes metabolic and antioxidant interactions mediated by probiotics and prebiotics in cultured fish species. It synthesizes findings on histological effects, enzymatic activity, and microbial interactions, with emphasis on lipid metabolism and immune modulation, and also discusses the practical implications for sustainable aquaculture.
1 Introduction
The aquaculture industry is experiencing exponential growth and is known to support the livelihoods of around 1 billion individuals globally (Huynh et al., 2018). Aquaculture is reported to account for 46% of the world’s fish supply (Wee et al., 2024). Asia is the primary producer of aquatic animals, and China is the highest producer among countries (Chen and Gao, 2023). Nevertheless, serious health issues, such as diseases, have been created due to the intensive culturing and increased production of aquatic products over the years. In tackling such problems, aquaculture scientists have sought to use chemicals, antibiotics, and other chemotherapeutics that have been criticized due to their adverse effects on the environment. The widespread use of antibiotics and chemotherapeutics in aquaculture health management has led to an increase in antibiotic resistance among pathogenic bacteria at aquaculture sites, which can subsequently contaminate the food chain (Pepi and Focardi, 2021). Therefore, it is imperative to implement alternative methods for managing the health of aquaculture species. Modern aquaculture practices prioritize sustainability, environmental responsibility, and producing safe consumer products (Gall et al., 1995). In aquaculture, beneficial feed additives such as probiotics and prebiotics are utilized to stimulate growth, enhance immunity to combat diseases, and provide alternative antimicrobial solutions (Badguzar et al., 2024).
According to Wee et al. (2024), a prebiotic serves as nourishment for the beneficial bacteria in the host’s digestive tract, whether in the form of a substance, substrate, long-chain sugar, vitamin, or fiber. Furthermore, as stated by Davani-Davari et al. (2019) and Dhanasiri et al. (2023), a prebiotic is a substance that can withstand the harsh acidity of the stomach, is digested by intestinal microorganisms, and aids in enhancing host health by supporting the proliferation of beneficial gut bacteria. Xylooligosaccharides (XOS) have been shown to enhance mineral absorption, reduce glucose and lipid levels, improve antioxidant status, and specifically stimulate the growth of beneficial intestinal microflora. These microflora play various important roles, such as regulating metabolism and preventing illness (Chen et al., 2022). On the other hand, probiotics, which are live microorganisms that provide enormous host benefits when given in the right amount (Fachri et al., 2024), are known to work through multiple pathways to strengthen the intestinal mucosa and enhance gut barrier integrity (Rohani et al., 2022). Moreover, probiotics are essential for sustaining a harmonious microbial environment by favoring helpful bacteria and preventing harmful ones through competitive exclusion (Mishra et al., 2015). Furthermore, they defend against infections by generating antimicrobial metabolites, modifying toxins or pathogen receptors, and activating distinct immune responses to pathogens (Pardo-Esté et al., 2024). For example, the use of isolated probiotics Bacillus amyloliquefaciens AV5 was noted to enhance the growth conditions, antioxidant capacities, microbial composition, and intestinal structure of Nile tilapia (Oreochromis niloticus) (Shija et al., 2025). Researchers discovered that feeding probiotic live yeast to sea bass led to changes in the activities of antioxidant enzymes and gene expression (De et al., 2014). Probiotics have the potential to alter the metabolism of hindgut bacterial ecosystems, leading to an increase in short-chain fatty acids and other organic acids while decreasing the production of ammonia and isovaleric acid. This is likely achieved by improving the breakdown of complex carbohydrates, ultimately enhancing protein breakdown.
Despite the pressing need for research on the significance of prebiotics and probiotics in various fish species, a noticeable gap exists in comprehensive studies within aquaculture. Therefore, further research is needed to investigate the role of prebiotics and probiotics in various fish species, considering their significance in aquaculture. This review aims to systematically analyze the mechanisms of action of probiotics and prebiotics in fish, with a focus on their roles in antioxidant defense and metabolic regulation. To better understand their potential applications, following the discussion on antimicrobial resistance and the need for alternative strategies, the subsequent section examines the role of probiotics in aquaculture.
2 Probiotics in aquaculture
2.1 Background and histological development of probiotics
As elaborated earlier, probiotics are described as live microorganisms that help the host’s health when given in sufficient quantities. Recent studies have extended their uses to aquaculture, specifically fish farming, despite their lengthy history in human and animal health. The use of probiotics in fish aquaculture began much later, in the late 20th century, driven by the growing demand for sustainable fish farming practices. Élie Metchnikoff first proposed the concept of probiotics in the early 20th century, arguing that bacteria such as lactic acid bacteria might be beneficial for the human digestive system (Martínez Cruz et al., 2012). It is noteworthy that Metchnikoff was ahead of his time regarding the microbiome when he added, “Systematic investigations should be performed on the relation of intestinal bacteria to premature aging,” as well as the impact of diets that avoid intestinal putrefaction in extending life and preserving bodily functions (Metchnikoff, 1907).
Initially, the development of probiotics for aquaculture focused on enhancing feed efficiency and disease resistance; however, as time passed, their beneficial effects expanded to include immunological regulation, stress reduction, and overall health management (Behera et al., 2022). Histological investigations offer crucial information on the structural alterations that probiotic exposure causes in fish’s digestive tracts. A vital component of digestion, nutritional absorption, and immunological responses, the fish gut is a dynamic and extremely adaptive organ (Ntakirutimana et al., 2023).
Probiotics interact closely with the host’s immune system, gut microbiota, and gut epithelium. These interactions alter the gut’s histological structure and may explain some of the beneficial effects of probiotics observed in aquaculture (Auclert et al., 2024). According to studies, taking probiotics can increase the size and number of intestinal villi, which are finger-like projections in the gut that enhance the surface area available for nutrient absorption. By strengthening the gut’s absorptive capacity, this morphological alteration facilitates improved nutrient absorption (De Marco et al., 2023). Probiotics can also increase mucus production from goblet cells in the intestinal lining, creating a protective layer that protects the epithelial cells from pathogens and irritants.
Histological analysis of the intestines of fish supplemented with probiotics often reveals a thicker mucosal layer and a higher number of goblet cells, indicating enhanced gut health and protection in fish (Feng et al., 2025). Probiotics can affect the histological development of the gut-associated lymphoid tissue (GALT) by modifying the quantity and distribution of immune cells, including lymphocytes, macrophages, and dendritic cells. The GALT is a vital component of the immune system in fish and plays a crucial role in defending the fish against enteric pathogens (Picchietti et al., 2007). Probiotics have been shown in histological investigations to enhance the organization of GALT in fish and increase the number of lymphoid follicles. This improvement in GALT structures is associated with a stronger immune response, which increases resistance to infections. Additionally, it has been demonstrated that probiotics stimulate the release of antimicrobial peptides (like piscidins) and immunoglobulins (such as IgM and IgT), all of which are essential for the body’s defense against infections in fish (Nayak, 2010).
2.2 Sources of probiotics
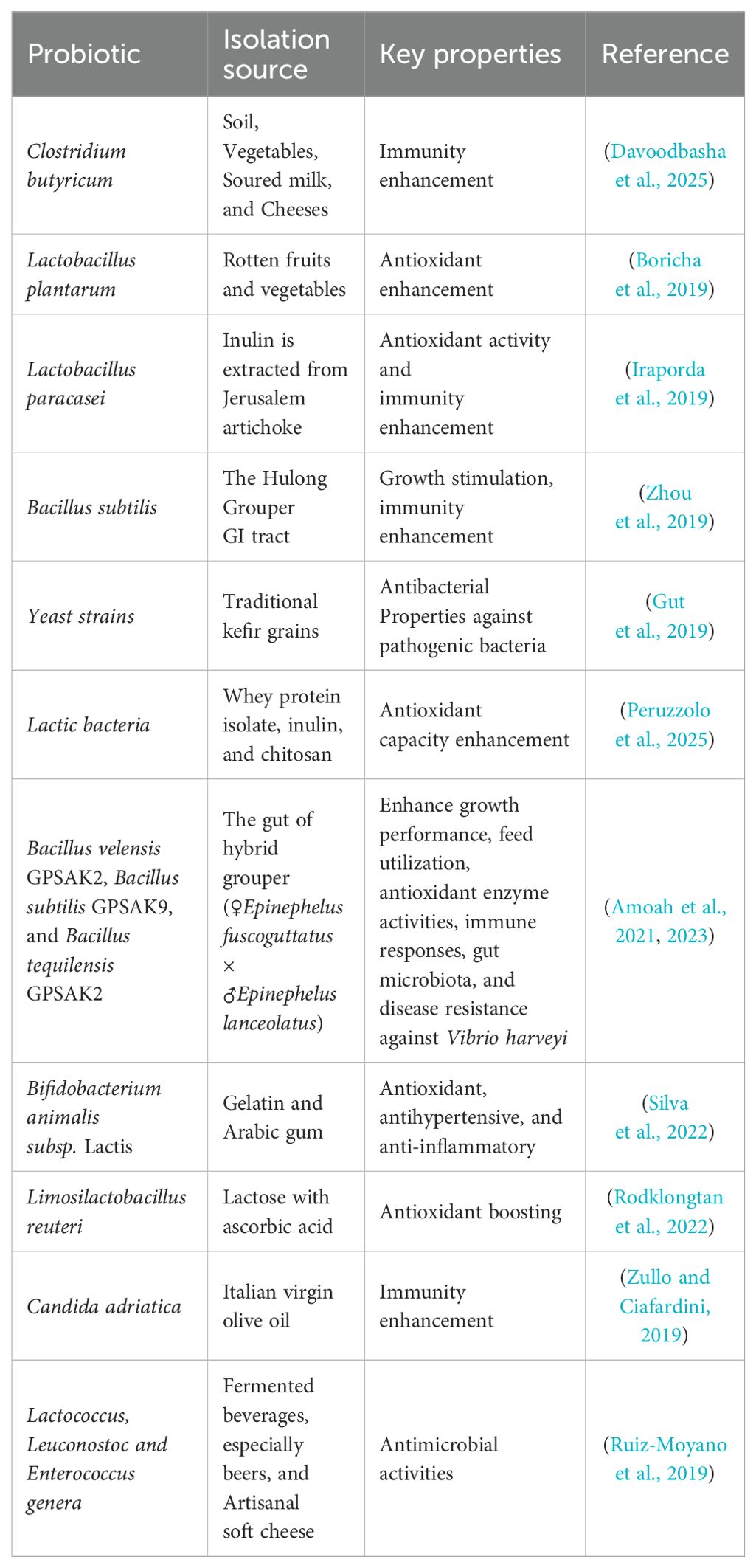
Numerous probiotic strains used in aquaculture originate from diverse ecological niches. Commonly utilized genera such as Bacillus, Lactobacillus, Bifidobacterium, and Pediococcus are frequently isolated from the gastrointestinal tracts of fish due to their natural adaptation to the host environment. Other strains, including Clostridium, Enterococcus, and Debaryomyces, have also shown probiotic potential and are sourced from aquatic sediments, rearing water, or fermented products (Table 1). For example, Bacillus species are often preferred for their spore-forming ability and resilience in harsh aquaculture conditions. At the same time, lactic acid bacteria such as Lactobacillus and Weissella contribute to gut microbiota balance and immune enhancement. Then, some studies have explored the application of cyanobacteria and Shewanella due to their unique metabolic capacities and symbiotic interactions within aquatic environments (Fachri et al., 2024; Merrifield and Carnevali, 2014). These diverse origins underscore the importance of selecting strains that are both host-adapted and environmentally compatible for optimal probiotic function (Table 1).
2.3 Screening criteria and security
For bacteria to be regarded as a probiotic, there should be no known side effects or health risks associated with using it; thus, its safety, which is a critical factor, must be guaranteed. As a result, when sourcing probiotic bacteria, one of the requirements must include identifying strains resistant to commonly used antibiotics, such as tetracyclines, quinolones, and macrolides, and ensuring the absence of drug-resistance genes or virulence plasmids (Amoah et al., 2021; Vulla, 2024). The composition of the final product is also important to consider, as any errors could have negative health consequences or negate the benefits of probiotics.
2.4 Application methods and actual results
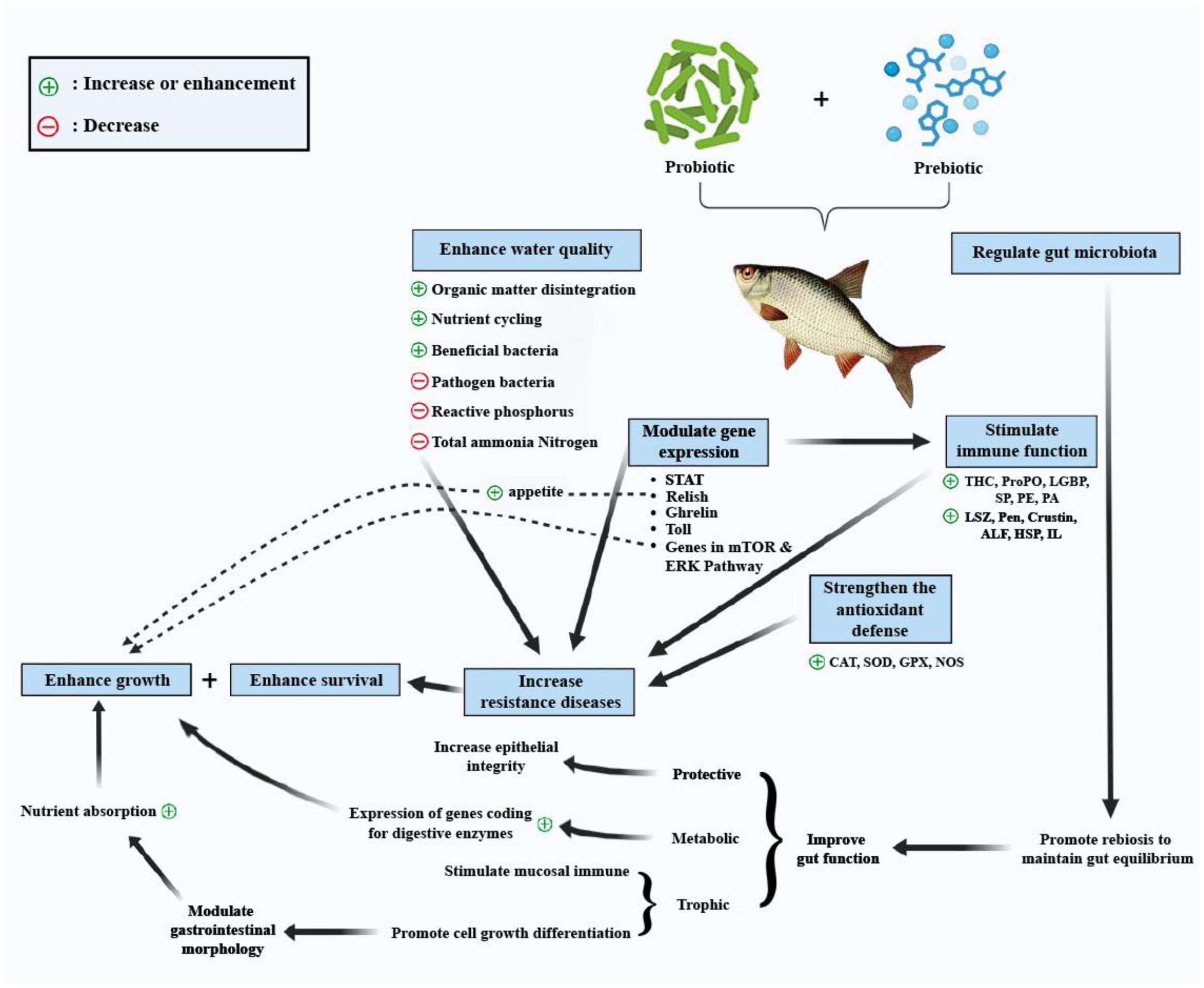
The positive impact of probiotics on bacteria is evident in their ability to suppress Vibrio spp. Populations (Moghadam et al., 2018). In the work of Hamdan et al. (2016), dietary supplementation with 0.5% marine probiotic bacterium Lactobacillus plantarum AH 78 was noted to improve growth performance in Nile tilapia significantly. Furthermore, after challenging fish with the pathogenic bacterium Aeromonas hydrophila, the survival rate of Nile tilapia fish, as well as their immunological responses and expression of cytokine genes, including IL-4, IL-12, and IFN-γ, were enhanced when fish were supplemented with 1.0% of L. plantarum strain AH78 (Hamdan et al., 2016). The importance of prebiotics and probiotics in fish farming is highlighted in Figure 1 below. These beneficial supplements play a crucial role in promoting the health and growth of fish, ultimately leading to improved productivity and sustainability in aquaculture operations.
2.5 The mechanisms of probiotics
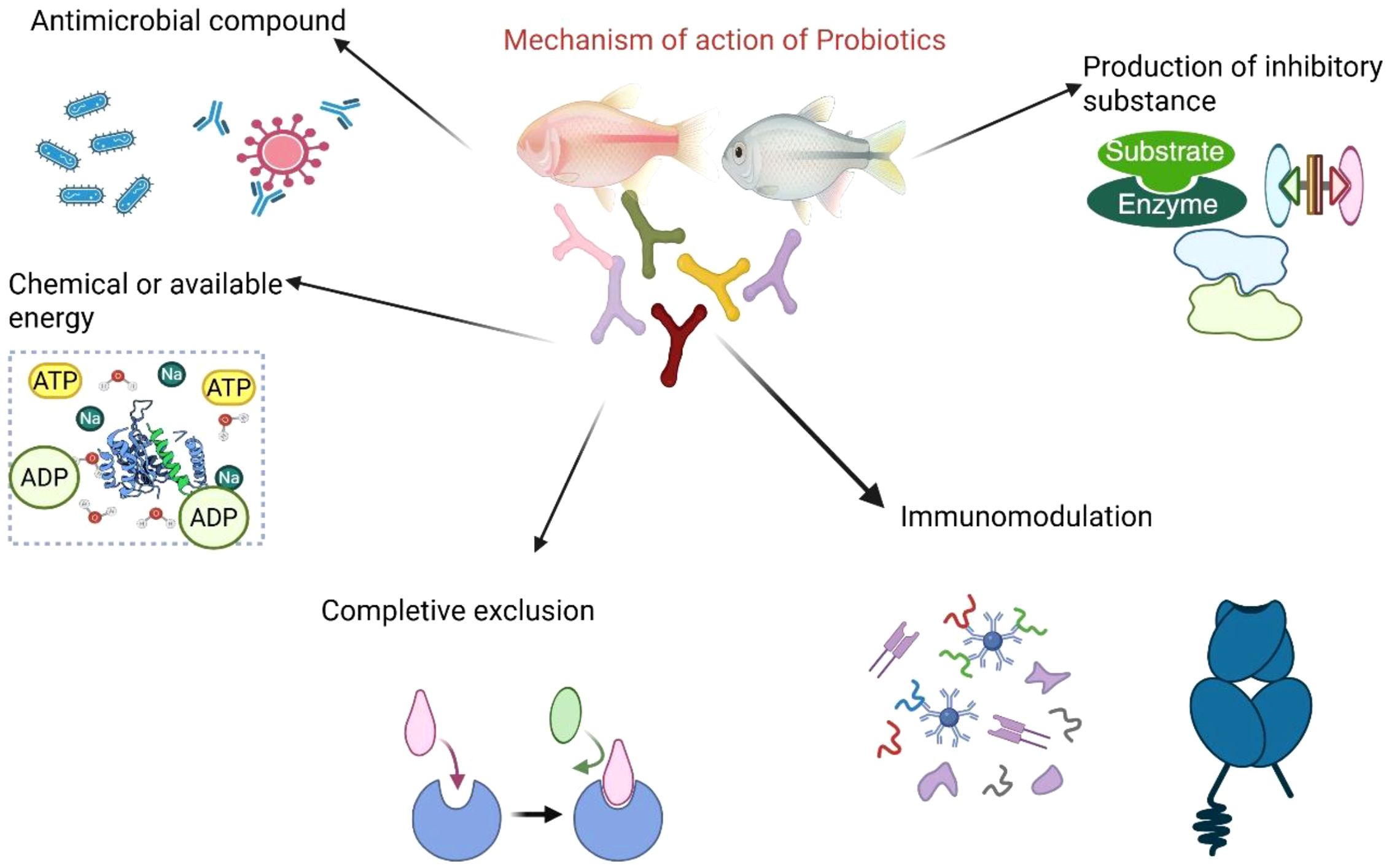
The role of probiotics extends far beyond merely modulating the immune system; they operate through various mechanisms within living organisms. In animals, probiotics play a crucial role in eliminating potential pathogens by producing inhibitory substances or competing directly for space, resources, and oxygen in the gut (Raheem et al., 2021; Guerreiro et al., 2024). By blocking pathogens’ access to vital nutrients and binding sites on the gut’s surface, probiotics significantly reduce the incidence of bacterial infections (El-Saadony et al., 2021). Beyond their role in infection prevention, probiotics also produce antimicrobial compounds, such as bacteriocins and organic acids, which further suppress the proliferation of harmful bacteria. Figure 2 below summarizes the mechanisms of probiotics.
3 Prebiotics in fish aquaculture
3.1 Background and histological development of prebiotics
In aquaculture, prebiotics are increasingly applied to enhance gut health, nutrient absorption, and disease resistance in farmed fish species. The concept was first introduced by Gibson and Roberfroid (1995), who defined prebiotics as fermented ingredients that cause specific and beneficial changes in the activity and/or composition of the intestinal microbiota, which subsequently enhances host health. This definition has been refined over the years, limiting prebiotic classification to a few carbohydrates such as lactulose, GOS, and short and long-chain β-fructans (FOS and inulin). According to the 2008 6th Meeting of the International Scientific Association of Probiotics and Prebiotics (ISAPP), led by Gibson et al. (2010), dietary prebiotics are ingredients that are selectively fermented, resulting in specific changes in the composition and activity of the gastrointestinal microbiota. This, in turn, benefits the host’s health. A compound is classified as a prebiotic if it meets certain criteria: it must be resistant to the acidic pH of the stomach, not be broken down by mammalian enzymes, not be absorbed in the gastrointestinal tract, ferment in the intestinal microbiota, and selectively stimulate the growth and activity of intestinal bacteria. These factors contribute to improving the host’s health, as outlined by Davani-Davari et al. (2019).
3.2 Sources of prebiotics
Prebiotics play a crucial role in maintaining animal health, with a wide range of foods naturally containing them. Some examples include asparagus, sugar beet, garlic, chicory, onion, Jerusalem artichoke, wheat, honey, banana, barley, tomato, rye, soybean, peas, and beans. More recently, seaweeds and microalgae have emerged as promising sources of prebiotics (Moreno-Garcia et al., 2017; Varzakas et al., 2018). Due to their low concentration in food sources, prebiotics are now being produced on a large industrial scale. Raw materials such as lactose, sucrose, and starch are commonly used in the production of prebiotics (Hijova and Chmelarova, 2007).
3.3 Mechanism of prebiotics and their effects on fish health and growth
3.3.1 Enzyme activity and digestion
The by-products produced through the fermentation of prebiotics by beneficial commensal bacteria have been shown to improve health significantly. Prebiotics, also known as functional saccharides, support the growth of beneficial gut microbiota that produce digestive enzymes such as protease, amylase, and lipase (Song et al., 2014). These enzymes enhance nutrient breakdown and absorption, thereby improving gut health and feed efficiency in fish (Ta’ati et al., 2011; Xu et al., 2022b). By encouraging the growth of beneficial gut bacteria and enhancing gut health, prebiotics facilitate improved nutrient absorption and digestion in fish. By fermenting prebiotics, beneficial bacteria can produce enzymes that aid in breaking down complex carbohydrates, proteins, and other food ingredients into forms that are easier to absorb. This enhanced nutrient uptake results in improved fish growth performance and feed efficiency (Rohani et al., 2022). However, it is important to note that increased digestive enzyme activity is not the sole factor contributing to improved growth performance. Other factors, such as alterations in gut morphology and the fermentation of prebiotic compounds by beneficial bacteria, including Bacillus and Lactobacillus, also play a significant role (Tran et al., 2023).
3.3.2 Nutrient bioavailability
Plants, algae, and yeasts are the sources of natural ingredients, including alginate, inulin, and various oligosaccharides (Su et al., 2020; Whisner and Castillo, 2018). Typically, these compounds consist of carbohydrate structures or soluble dietary fibers specifically broken down by microbes in and on the body. Prebiotics are crucial for promoting the growth and proliferation of beneficial bacteria within the gut, ultimately benefiting host health. In animal nutrition, prebiotics such as inulin, FOS, MOS, and IMO have been widely utilized, showing significant benefits in various farmed aquatic species (Bamigbade et al., 2022; Davani-Davari et al., 2019; Huynh et al., 2018). Prebiotics have gained widespread acceptance in aquaculture in recent years due to their capacity to enhance growth performance, balance gut microbial composition, improve enzymatic functions, improve water quality, and provide essential nutrients. They also help strengthen the immune system, allowing host organisms to fight off disease infections (Dobrogosz et al., 2010).
3.3.3 Microbiome modulation
Prebiotics lower the risk of inflammatory illnesses in fish and help prevent systemic infections by promoting the growth of beneficial bacteria that compete with harmful germs for resources and adhesion sites in the gut (Arif et al., 2024). They also help to maintain gut health by strengthening the intestinal epithelial barrier, which can reduce the amount of pathogens and inflammatory stimuli that enter the bloodstream. Prebiotics play a major role in regulating the composition of the fish gut microbiota. By serving as substrates for beneficial bacteria, prebiotics selectively promote the development and activity of specific microbial populations while inhibiting the growth of harmful ones. This regulation leads to a more diverse and balanced gut microbiota, which, in turn, enhances gut health, improves nutritional absorption, and promotes overall host well-being (Anguiano et al., 2013). Furthermore, by using competitive exclusion, prebiotics can eliminate viruses from fish guts. Prebiotics function by promoting the growth of beneficial bacteria, which reduces the conditions that allow pathogenic microorganisms to colonize and proliferate. This competitive exclusion mechanism boosts fish disease resistance overall and reduces the risk of infections by preventing pathogens from attaching to the gut epithelium (Nakhei Rad et al., 2023).
3.3.4 SCFA synthesis and immune modulation
When broken down by beneficial bacteria such as Lactobacillus and Bifidobacterium, prebiotics produce short-chain fatty acids (SCFAs), lactate, and other beneficial compounds and nutrients (Chen et al., 2017). These bioactive compounds, specifically carboxylic acids with fewer than six carbon atoms, can induce bacterial fermentation and have a positive impact on the digestive system and metabolism, including anti-inflammatory and immunostimulatory actions (Nawaz et al., 2018). Moreover, prebiotics provide energy to enterocytes for the repair and maintenance of gastrointestinal homeostasis, as demonstrated in a study by Liu et al. (2020) on the effects of prebiotic treatment in shrimps. Although several studies have been conducted to assess the prebiotic impact on fish, crustaceans have been at the forefront of numerous studies on this topic. For example, a study by Chen et al. (2017) found that the giant freshwater prawn (Macrobrachium rosenbergii) experienced significantly higher growth rates and increased acetate concentration in the gut after being fed 0.4% FOS. In another study, Tran et al. (2020) discovered that GOS and resistant starch (RS) led to an increase in the synthesis of short-chain fatty acids (SCFAs) in the gut microbiota of the mud crab (S. paramamosain) during an in vitro investigation. Similarly, research has identified the numerous commercial and economic benefits of using prebiotics in fish culture, including tilapia, salmonids, carp, and catfish, which collectively account for the largest proportion of global production in inland waters.
Over the last twenty years, aquaculture has experienced significant growth, with both marine and coastal environments making a substantial contribution to this increase (Amillano-Cisneros et al., 2023). The expansion has brought about challenges, including disease outbreaks and environmental stressors, which prebiotics can help alleviate. The economic and commercial implications of prebiotics in aquaculture are substantial, as they provide a sustainable alternative to antibiotics, enhancing fish health and lowering production costs. Moreover, the effect of prebiotics on stress resistance has been demonstrated in juvenile groupers. For example, a four-week study on the supplementation of MOS and XOS was noted to enhance growth performance, antioxidant capacity, nonspecific immunity, ammonia nitrogen stress resistance, and crowding stress resistance of juvenile hybrid grouper. However, while MOS and XOS showed similar anti-stress effects, the antioxidant and nonspecific immunity parameters they regulated differed, suggesting that the precise mechanisms of MOS and XOS’s anti-stress effects were likely distinct. Then, the four weeks of MOS supplementation significantly improved the disease resistance of hybrid grouper against V. harveyi (Zhu et al., 2023).
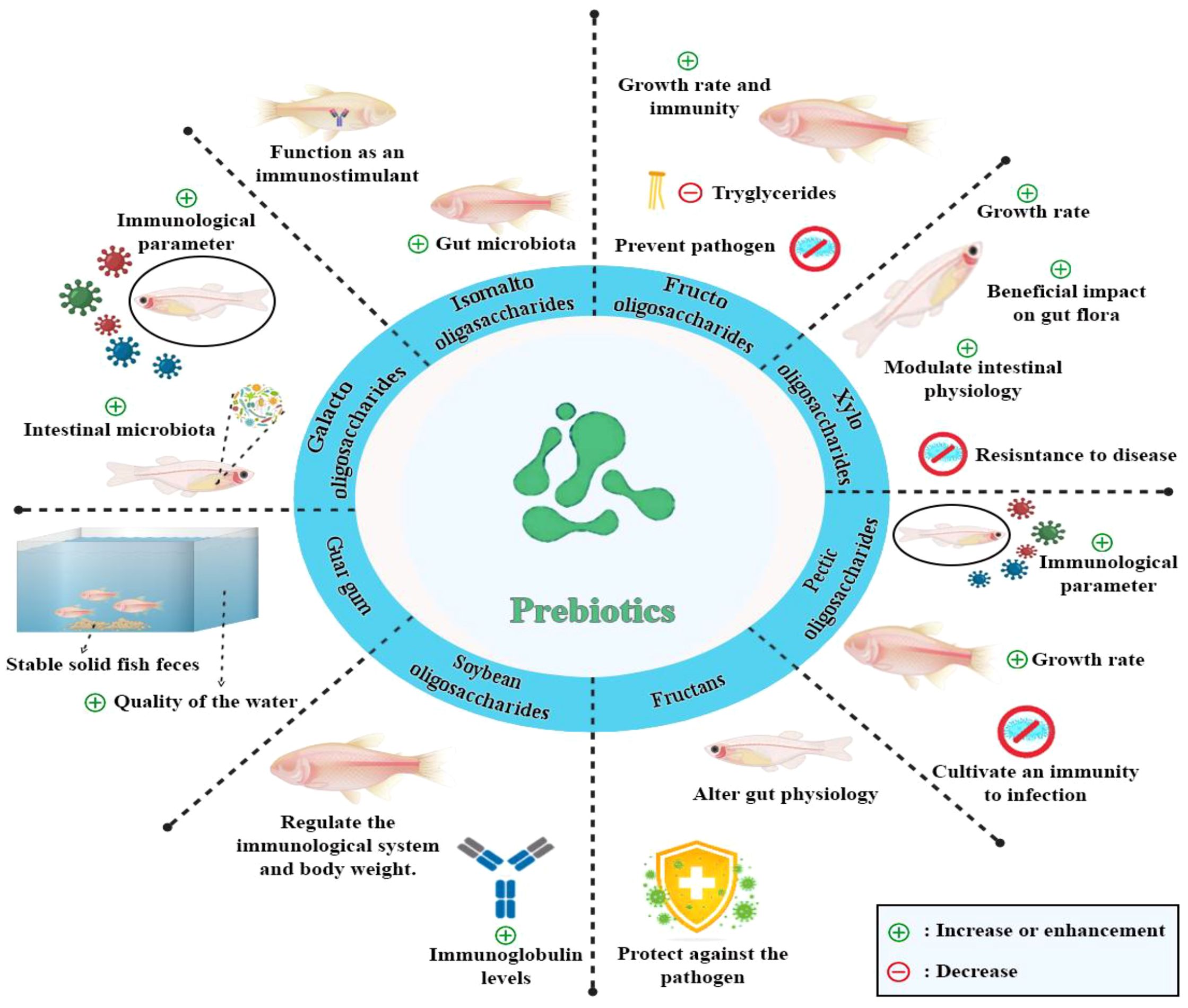
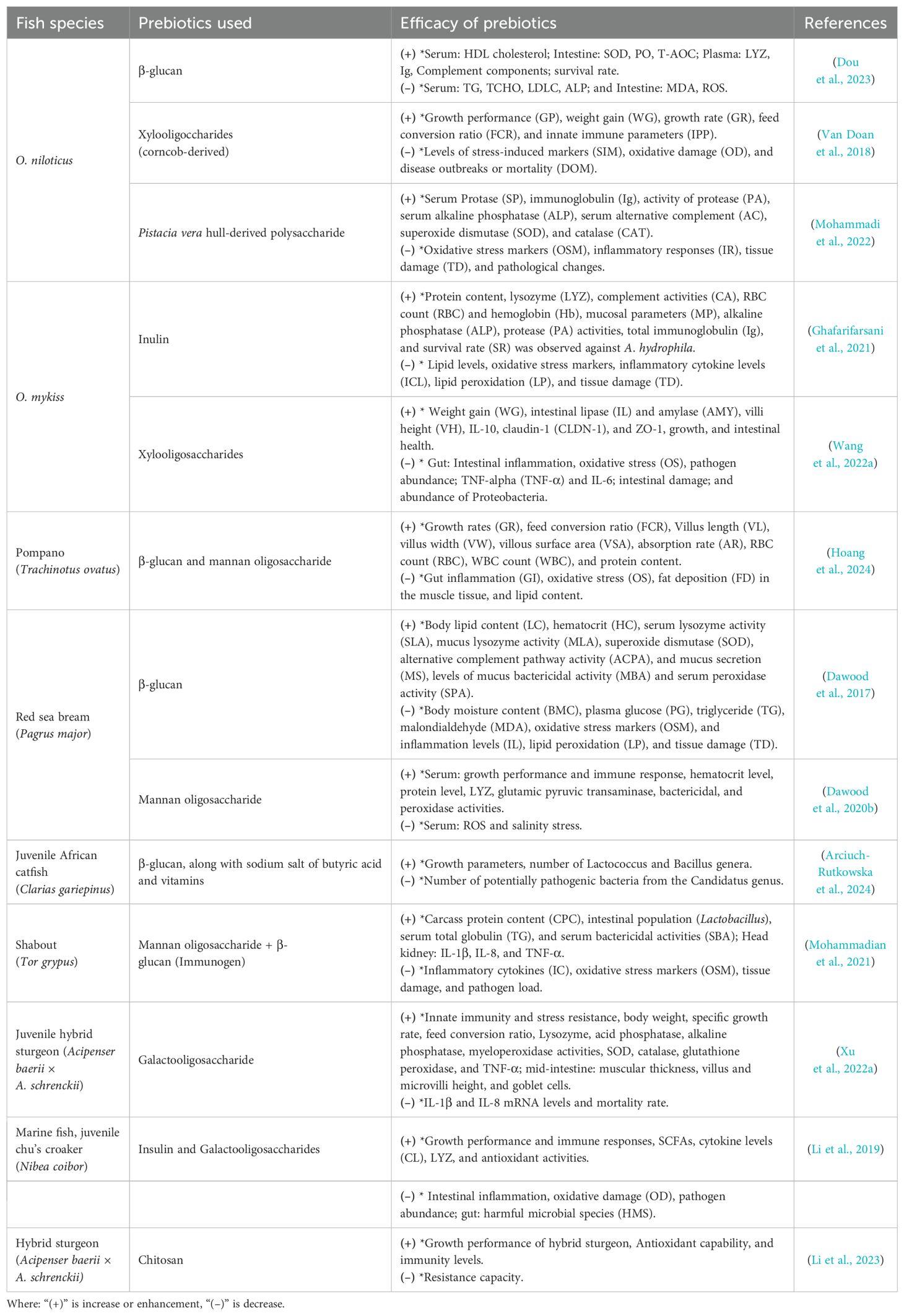
The roles and functions of various prebiotics commonly used in aquaculture are summarized in Table 2, providing insights into their sources and specific benefits for fish health and immunity. The key functions of prebiotics in enhancing fish health and aquaculture performance are summarized in Figure 3. A detailed summary of the efficacy of various prebiotics on a wide range of fish species, including growth, immunity, and antioxidant capacity parameters, is presented in Table 3.
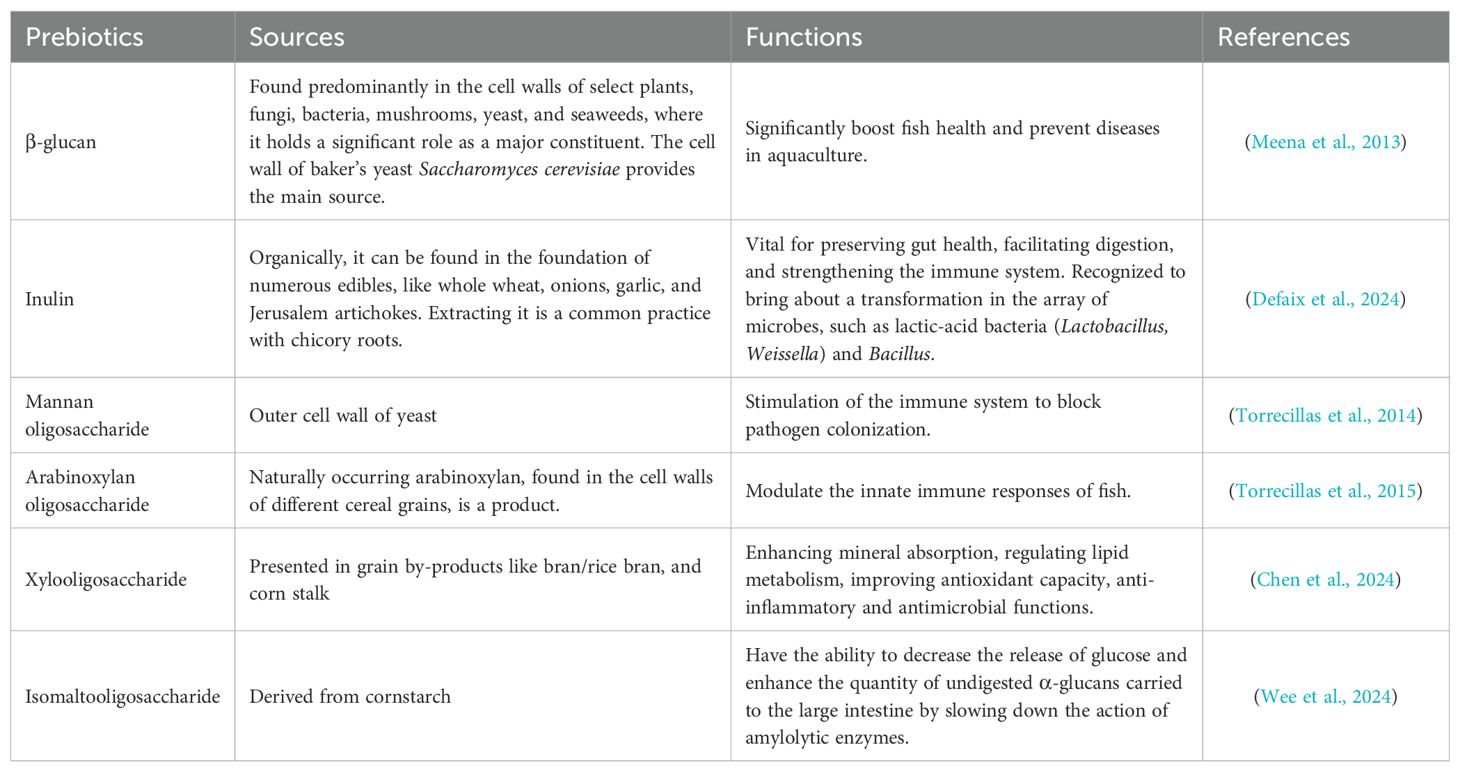
Table 2. The use of different prebiotics in aquaculture highlights their functions within the industry.
4 Probiotics and prebiotics metabolism in fish
4.1 Probiotics and fish metabolism
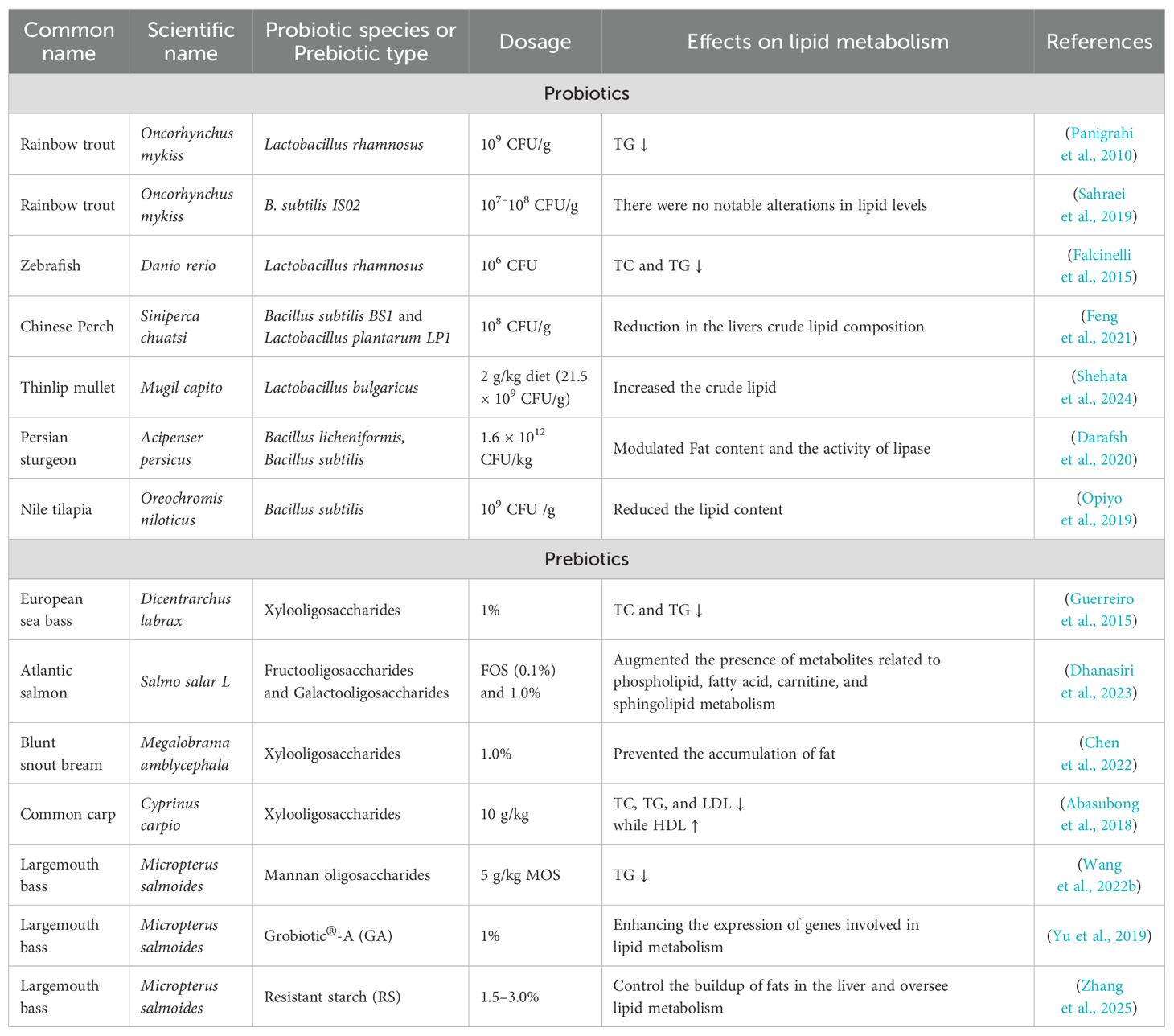
Probiotics have surged in popularity over the years due to mounting evidence suggesting their ability to impact host nutrient metabolism, energy balance, and gastrointestinal health by modifying the microbiota (Falcinelli et al., 2017; Liu et al., 2021a; Ringø et al., 2022). Various mechanisms make probiotics vital in managing lipid metabolism. Their production of digestive enzymes facilitates the absorption and utilization of nutrients, reduces cholesterol levels, and exhibits anti-inflammatory and immunological benefits (Liu et al., 2021b; Semova et al., 2012). Lye et al. (2010) identified five ways in which probiotics can influence lipid metabolism, including cholesterol absorption, binding, micelle destabilization, bile salt deconjugation, and bile salt hydrolysis. When examining probiotic products containing live LAB, Cho and Kim (2015) noted a decline in total cholesterol and LDL cholesterol, with no substantial differences in HDL cholesterol or triglycerides. Studies conducted on zebrafish larvae revealed that providing Lactobacillus rhamnosus IMC 501 resulted in reduced gene transcription related to cholesterol and triglyceride metabolism (Falcinelli et al., 2015). Furthermore, adult zebrafish exposed to varying lipid levels showed that high dietary lipids reduced gut microbiota diversity, impacting genes related to hunger regulation, while adding L. Rhamnosus reduced total body cholesterol (Falcinelli et al., 2017). Table 4 provides information on how probiotics impact lipid metabolism in fish.
4.2 Prebiotics and fish metabolism
Species, feeding patterns, gut microbiome composition, type of basal food consumed, and the specific prebiotic used all play a role in determining the effectiveness of aquatic animals (Lokesh et al., 2022). To date, current research on the modulation of carbohydrate metabolism by prebiotics remains limited to a few model species. Although there are advantages to consider, research has only focused on a restricted range of fish species to investigate the effects of prebiotics on the metabolism and utilization of carbohydrates. Investigations indicate that polysaccharides like inulin and mannan-oligosaccharides (MOS) might affect the gene expression linked to diverse metabolic pathways in rainbow trout’s liver and muscle tissues (Sharma and Puri, 2015; Lokesh et al., 2022). With abundant raw materials, cost-effectiveness, and sustainability, the nutraceutical industry can successfully produce xylooligosaccharides (XOS) from agricultural by-products. Research has demonstrated that XOS can elevate antioxidant status, enhance mineral absorption, decrease glucose and lipid levels, and stimulate the growth of beneficial gut flora, resulting in a range of health benefits, including improved metabolism and disease prevention (Chen et al., 2022). The supplementation of 10 g/kg and 20 g/kg XOS to the high-fat diets of fish resulted in decreased HIS, ADF, liver lipid, plasma TC, TG, and LDL levels while increasing plasma HDL concentrations (Abasubong et al., 2018). Moreover, research conducted by Torrecillas et al. (2015) demonstrated a reduction in levels of long-chain monoenoic fatty acids, including 20:1 and 22:1, in European sea bass that were fed MOS, as these acids are predominantly metabolized via β-oxidation. Contrarily, GOS could alter lipid transport and metabolism by directly influencing the gut microbiota (Dhanasiri et al., 2023). Elevated oxidative stress markers in giant freshwater prawns have been associated with high-concentration FOS treatment (Genc et al., 2007). The importance of carefully examining how different dietary supplements affect lipid metabolism in aquatic species should not be overlooked.
5 Mechanism of probiotics and prebiotics in lipid metabolism
5.1 Short-chain fatty acids & AMPK
Lipid metabolism involves a series of complex reactions, including digestion, absorption, synthesis, and breakdown of lipids, all of which are controlled by different enzymes. Genetic factors, environmental conditions, and other external factors influence these processes. The impact of probiotics on lipid metabolism is substantial, primarily due to the generation of two important metabolites: short-chain fatty acids (SCFAs) and bile acids (BAs). Moreover, regulating enzyme production and inhibitors can effectively reduce cholesterol synthesis (Song et al., 2023). The digestion of fats is greatly influenced by bile acids, which serve as essential signaling molecules (Wang et al., 2023). Derived from cholesterol produced in the liver, BA acts as messengers that trigger nuclear receptors involved in controlling metabolism and general well-being. Moreover, they serve as biological cleansers that aid in the uptake and delivery of fats, vitamins, and essential elements. SCFAs are the primary metabolites produced by beneficial gut bacteria, facilitating the energy metabolism of cells in the colon and liver. SCFAs offer numerous benefits to target tissues. For example, butyrate may enhance mucus layer thickness and strengthen the integrity of the gastrointestinal barrier by activating intestinal AMPK (Zhuge et al., 2024). AMPK, as described, acts as a cellular fuel gauge that regulates metabolic pathways involved in protein synthesis, glucose metabolism, and fatty acid metabolism. The activation of AMPK can be triggered by acetate through an increase in the liver AMP/ATP ratio, which consequently reduces the transcription of lipogenic genes (Liu et al., 2021a). Propionate is linked to gluconeogenesis, while the liver utilizes acetate for the synthesis of fatty acids and cholesterol. Acetate, the primary SCFA in mammals, is essential for controlling lipid metabolism and is present in various tissues and excreta as a free acid (Feng et al., 2021).
5.2 Bile acid
Bile acid is one of the key signaling molecules that play a significant role in the digestion of fat (Lin et al., 2020). These BAs can break down TAGs into fatty acids, most of which can be reabsorbed by the intestines and sent back to the liver. They can also emulsify fat into smaller fat particles when lipoprotein lipase is active. The term “bile acid hepatic and enteric circulation” refers to this type of circulation of BA between the colon and liver. Probiotics can accelerate this cycle to achieve the goal of reducing cholesterol. In other words, primary BAs, which are produced in the liver from cholesterol, can be transformed into secondary bile acids under the combined influence of probiotics (Song et al., 2023). They are typically eliminated with meal residue because they are less likely to be absorbed, which leads to the liver producing bile acids from scratch.
5.3 Lipid oxidation and synthesis regulation
However, many prebiotics share similar physiological characteristics with dietary fibers, leading researchers to focus on exploring their potential impact on lipid metabolism. This research initially began with animal studies and has since progressed to human studies. Certain prebiotics have been shown to influence triglyceride metabolism, resulting in varying effects on serum or hepatic triglyceride levels, depending on the specific experimental conditions (Cho and Kim, 2015). In animal studies, a decrease in triglyceride levels is often associated with a reduction in hepatic de novo lipogenesis rather than in adipose tissue cells (Delzenne and Kok, 2001). A decrease in hepatic lipogenic enzymes may be linked to lower expression of key genes, typically caused by the consumption of fructan or resistant starch. As prebiotics are broken down in the intestines, the digestive system generates a considerable amount of SCFAs like acetate, propionate, and butyrate. The liver receives acetate and propionate through the portal vein, while enterocytes primarily break down butyrate (Hijova and Chmelarova, 2007). Through the cholesterogenesis and lipogenesis pathways, acetate enters hepatocytes after being activated by cytosolic acetyl-CoA synthetase 2. This process has been implicated in the hypercholesterolemic effects of indigestible carbohydrates, such as lactulose, which increases acetate production during fermentation in the colon but not propionate. Interestingly, propionate competitively inhibits the protein responsible for acetate entry into liver cells (Cho and Kim, 2015). By investigating the effects of prebiotics and probiotics on lipid metabolism, scientists can gain valuable insights that could lead to the development of new treatments for metabolic disorders. The function of probiotics and prebiotics in fish lipid metabolism is depicted in Figure 4.
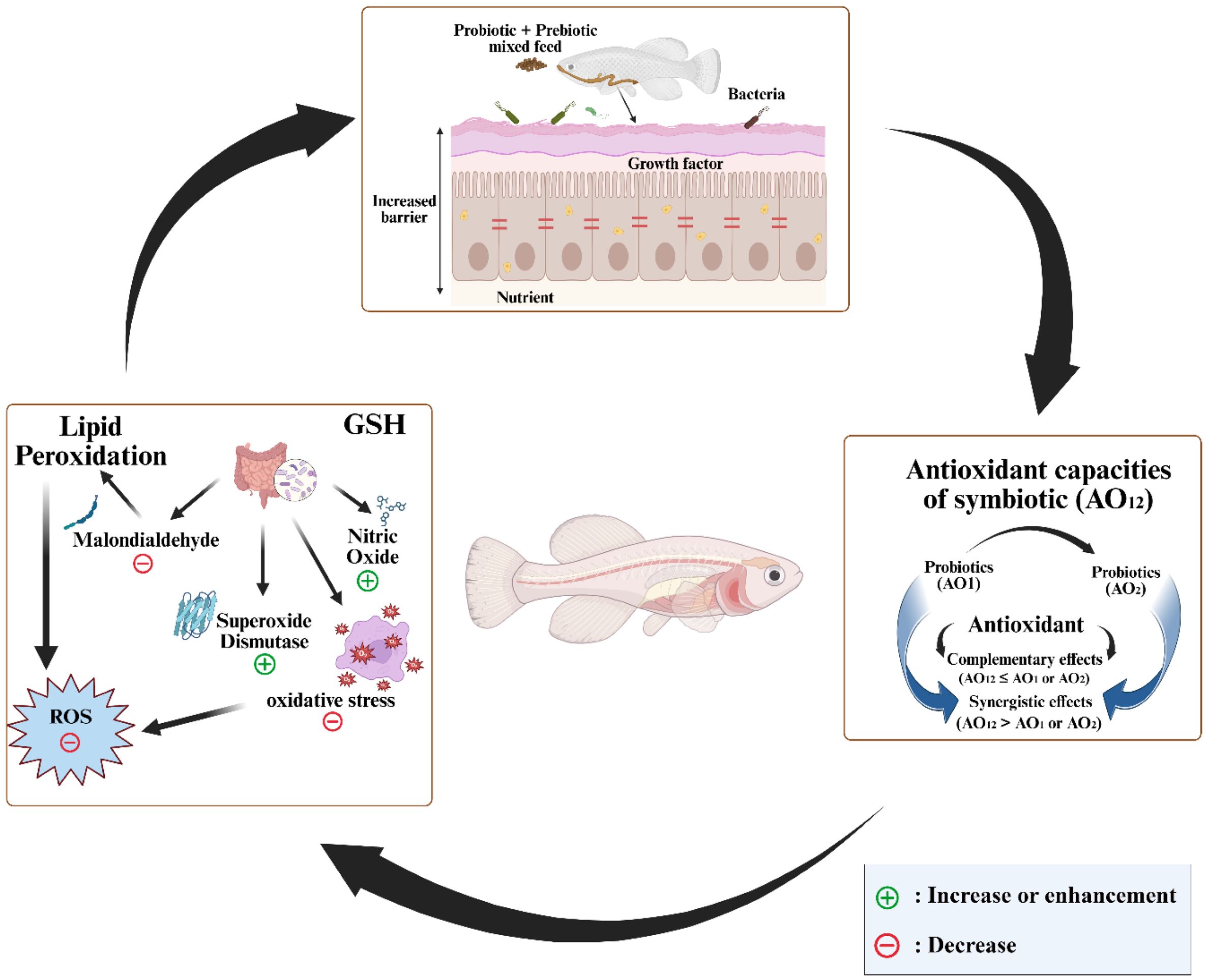
Figure 4. The function of probiotics and prebiotics in fish lipid metabolism. This diagram provides a visual representation of how these dietary supplements influence the way fish process lipids.
6 Mechanism of probiotics and prebiotics in carbohydrate metabolism
Carbohydrates play a crucial role as a non-protein energy source for aquatic species, helping to spare proteins and reduce nitrogen emissions into the water (Abasubong et al., 2019). However, unlike mammals, aquatic animals struggle to utilize dietary carbohydrates efficiently. Excessive carbohydrate intake can lead to metabolic stress, disrupt metabolic balance, and pose various health risks for fish, including hyperglycemia, liver damage, and histopathological issues (Siri and Krauss, 2005; Wang et al., 2021).
An excess of glucose is typically converted into glycogen and lipogenesis, which can be targeted to alleviate symptoms of hyperglycemia and hyperlipidemia resulting from a high-carbohydrate diet. Research has shown (Castro et al., 2016) that prolonged consumption of high-carbohydrate meals can increase the enzymatic activities of GS, G6PDH, and FAS in Sparus aurata, leading to increased fat and glycogen production. High-carbohydrate diets can also trigger fish lipid metabolism disorders, characterized by excessive fat accumulation in the liver and abdomen (Luo et al., 2020). This fat buildup can disrupt endocrine system activities, leading to elevated levels of pro-inflammatory cytokines and insulin resistance (Zhang et al., 2024).
Probiotics can potentially influence immunity, physiology, metabolism, and nutrition by modifying the gut microbiota. Research has shown that probiotics can have beneficial effects on metabolic inflammation and obesity resulting from a high-fat/carb diet by altering the gut microbiota and producing SCFAs (Xu et al., 2022c). Studies have indicated that SCFA butyrate can enhance the production of the peptide GLP-1, which plays a crucial role in regulating appetite, food intake, and glucose metabolism by increasing the expression of the insulin gene in intestinal L-cells (Kim et al., 2018; Yadav et al., 2013). Additionally, research has demonstrated that probiotic-treated larvae exhibit increased glp-1 gene expression, potentially due to the metabolic activity of lactic acid bacteria producing SCFAs (Falcinelli et al., 2016). Moreover, as highlighted by Delzenne et al. (2007), propionate has been found to stimulate the production of glucagon-like peptide-1 (GLP-1) in the intestine, leading to enhanced insulin secretion and increased glycogen synthesis in the liver. Furthermore, SCFAs can activate the AMPK/peroxisome proliferator-activated receptor-γ co-activator-1α/peroxisome proliferator-activated receptor α pathway, facilitating the transport of SCFAs to various tissues and promoting lipid oxidation. This process facilitates the proper metabolism and utilization of fat in various organs.
Research has demonstrated that XOS can improve the function of the intestinal barrier by selectively increasing the presence of beneficial microbes like Lactobacillus and Bifidobacterium, boosting the production of SCFAs, and enhancing the levels of tight junction proteins in the gut (Chen et al., 2024). In addition, compared to a high-carbohydrate diet, supplementing with 1.0% XOS resulted in a decrease in lipid accumulation in muscles and the liver, and an increase in glycogen deposition in the liver (Chen et al., 2022). European sea bass given 1% XOS also exhibited heightened glycolytic activity (Guerreiro et al., 2015). Moreover, the administration of MOS was found to reduce insulin resistance and glucose intolerance in mice fed a high-carb diet, potentially through the modulation of gut microbial composition (Wang et al., 2022c). It has been suggested that combining L. plantarum with a high-carb diet can elevate intestinal acetate levels, trigger uranosol synthesis in hepatocytes, and regulate nucleotide metabolism to enhance oxidative stress and reduce liver lipid deposition (Deng et al., 2024). The manipulation of gut microbiota by probiotics and the subsequent production of SCFAs have shown promising effects on various aspects of health and metabolism.
7 Mechanism of probiotics and prebiotics in protein metabolism
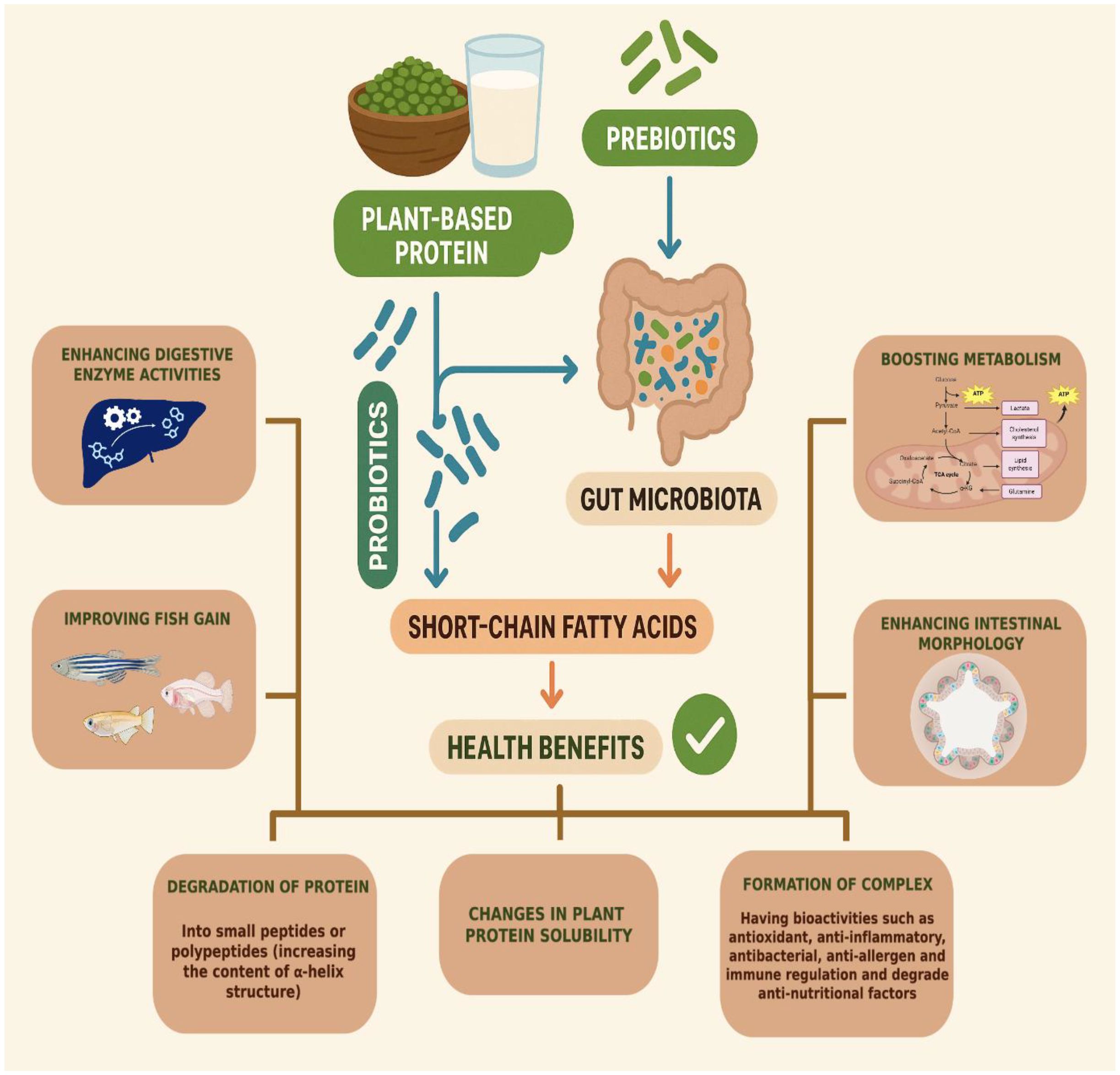
Probiotic and prebiotic supplementation has been shown to enhance fish weight gain by improving appetite, increasing digestive enzyme activity, enhancing intestinal morphology, and boosting metabolism (Midhun et al., 2019). These factors are crucial in improving nutrient absorption and digestion, leading to increased metabolism and accelerated growth (Zhang et al., 2022). Probiotics have the notable capacity to modify the structure and function of plant proteins through fermentation, producing bioactive compounds such as vitamins, antioxidants, and antimicrobial peptides. Additionally, probiotics aid in addressing protein energy deficiency by facilitating the absorption and utilization of proteins. They also influence the metabolic activity of gut microbiota, maintaining a balance between protein synthesis and breakdown (Rasika et al., 2021). Research by Wu et al. (2024) emphasizes the significance of probiotics in regulating the gut microbiota, which, in turn, influences gut bacteria involved in proteolysis. By breaking down complex plant proteins into simpler forms, probiotics promote the digestion and absorption of nutrients in the host body. This metabolic process also yields beneficial compounds, including SCFAs, exopolysaccharides, and vitamins. Furthermore, probiotics can break down plant-based proteins with anti-nutritional factors in feed into smaller peptides and amino acids, thereby enhancing the nutritional value and digestibility of these proteins. For instance, the addition of 1.5% XOS to rice protein meal has been shown to enhance the hepatic activity of Glutamate dehydrogenase, aspartate aminotransferase, and alanine transaminase in Megalobrama amblycephala (Abasubong et al., 2019).
In conclusion, incorporating probiotics and prebiotics into fish nutrition has significantly enhanced nutrient absorption and overall growth. Figure 5 illustrates the mechanism of probiotics and prebiotics in a plant-based protein diet.
8 The influence of probiotics and prebiotics on fish antioxidant capacity
The relationship between an organism’s antioxidant defense and physiological state is crucial, as higher levels and efficiency of antioxidant defense offer numerous advantages to the host. Fish have evolved sophisticated antioxidant defense mechanisms involving primary enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), alongside non-enzymatic antioxidants like glutathione, thioredoxin (Trx), and vitamins C and E (Shija et al., 2025; Słowińska et al., 2013; Hoseinifar et al., 2020). Thioredoxin (Trx) is one of the primary intracellular redox systems, and as such, it plays a crucial role in regulating reactive oxygen species (ROS) accumulation (Pacitti et al., 2014). Several studies have demonstrated that probiotics, such as S. cerevisiae and L. bulgaricus, significantly elevate SOD, CAT, and GSH-Px activities in Mugil capito (Shehata et al., 2024). Conversely, supplementation with Aspergillus oryzae enhances antioxidant enzymes and reduces stress markers in Nile tilapia during hypoxic conditions (Dawood et al., 2020a).
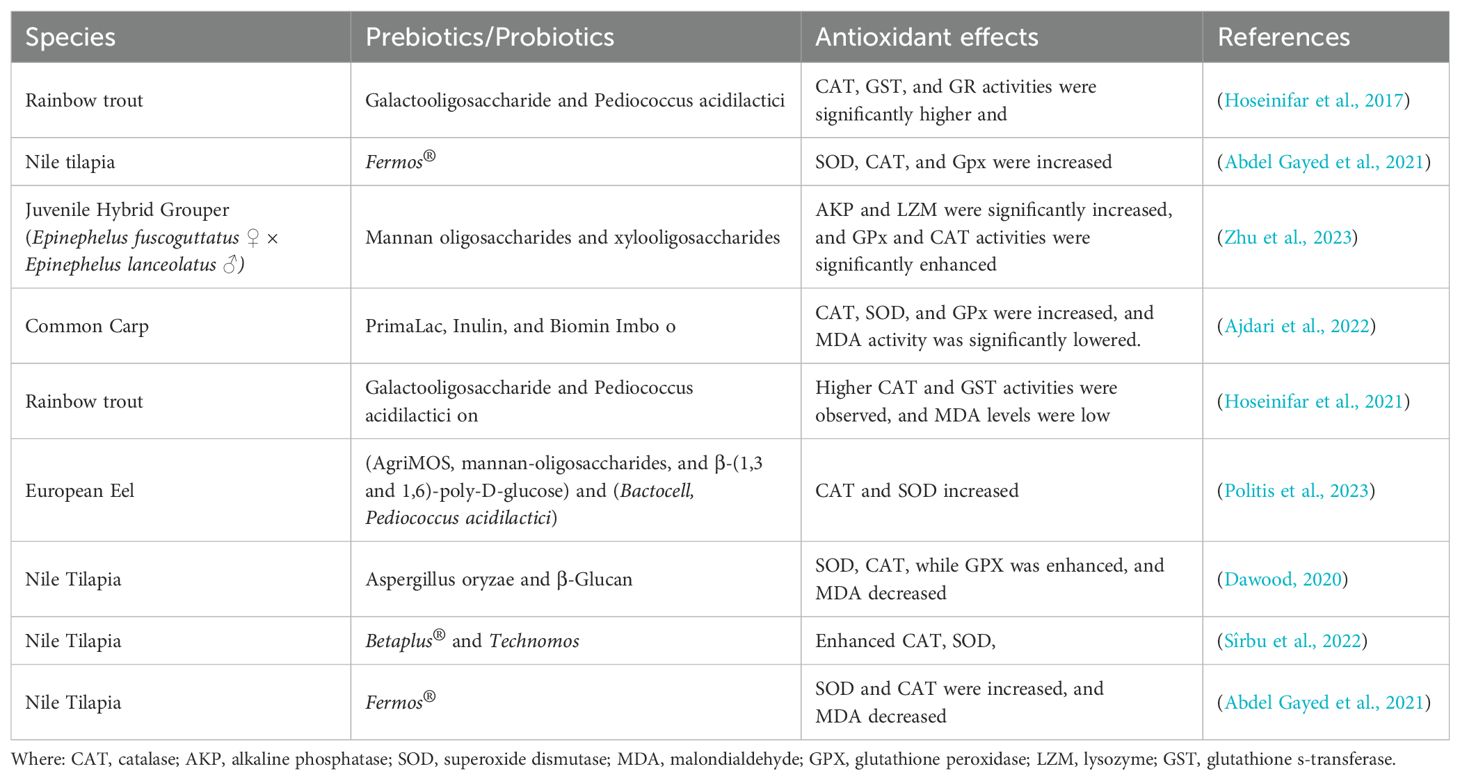
The research conducted by Yi et al. (2018) demonstrated a noticeable increase in GSH-Px activity in Carassius auratus when they were fed diets containing Bacillus velezensis JW. Ringø et al. (2022) found that dietary MOS and XOS had a notable impact on antioxidant levels, resulting in a significant decrease in MDA and a significant increase in CAT, GSH-PX, and SOD. The ability of chitosan to eliminate free radicals from the body’s cells gives it its strong antioxidant potential, helping to prevent oxidative damage. This is achieved through the chelation of metal ions and the provision of hydrogen or electron pairs (Yen et al., 2008). Jia et al. (2017) observed a notable increase in SOD and CAT activities in crabs treated with FOS, accompanied by decreased MDA activity. The utilization of XOS and GOS has been shown to enhance the enzymatic activity of GSH-Px and promote the synthesis of glutathione-related enzymes in fish (Xu et al., 2022c). The research suggests that prebiotics play a crucial role in enhancing the immune system through antioxidant pathways. An overview of the effects of various probiotics and prebiotics on antioxidant enzyme activities in different fish species is provided in Table 5.
9 The use of antibiotics in aquaculture
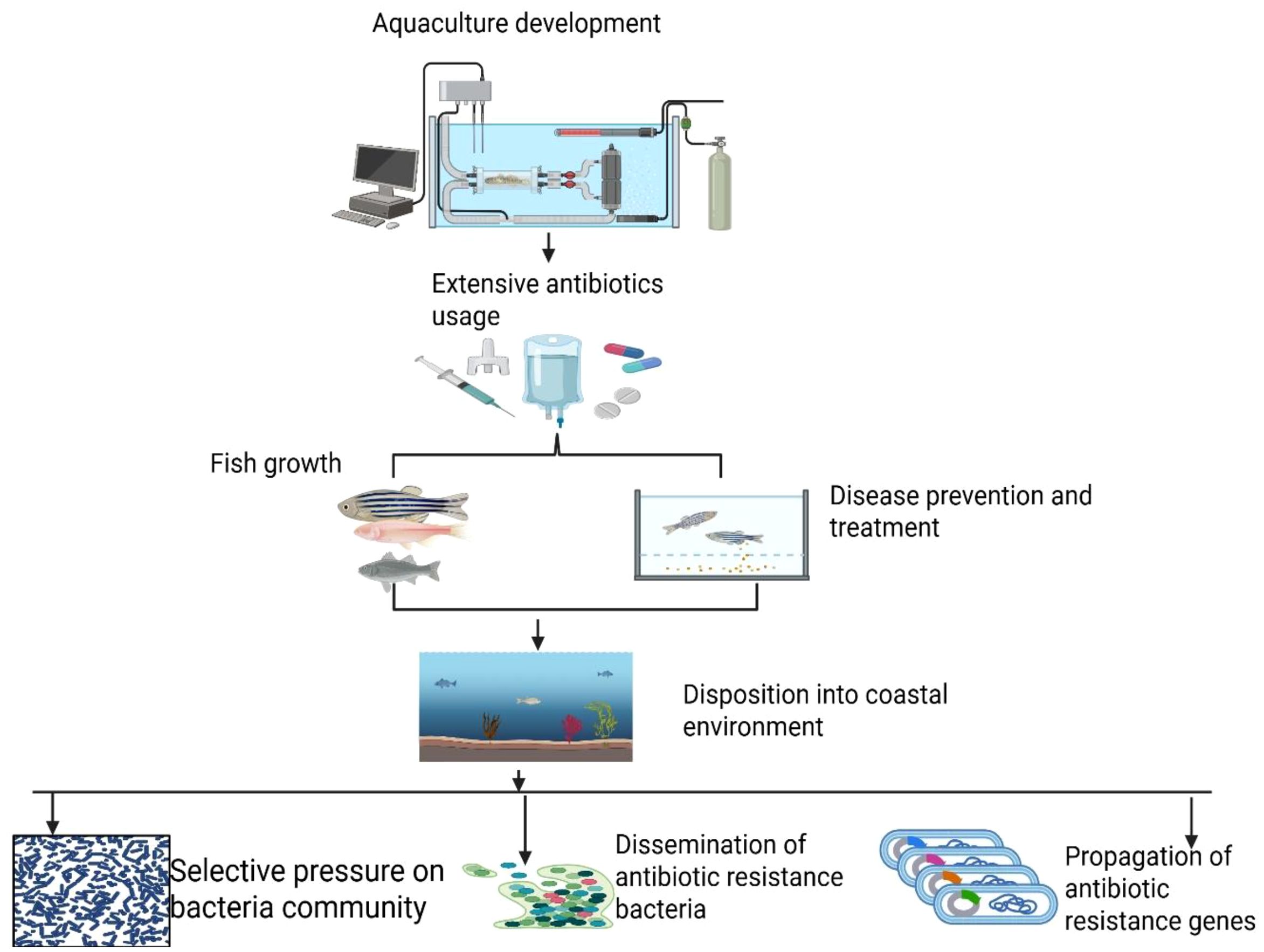
The increasing global demand for aquatic food has led to a significant rise in the use of antibiotics within the aquaculture industry. To enhance productivity, these antibiotics are utilized to promote the growth and health of fish stocks. Over the past few decades, the global use of antibiotics in aquaculture has increased significantly. In 2017, worldwide antibiotic consumption reached 93 million tons (Tiseo et al., 2020), and projections indicate that this figure could exceed 236 million tons by 2030, with aquaculture contributing approximately 5.7% of that total (Schar et al., 2020). A concerning aspect of this trend is that many antibiotics are applied directly to coastal habitats, often without effective measures to control their spread. In 2017 alone, over 10 million tons of antibiotic compounds were consumed in aquaculture, with an anticipated increase of 33% by 2030. The distribution of antibiotic use in aquaculture was notably concentrated, with China accounting for 58%, India for 11%, Indonesia for 9%, and Vietnam for 5% of global consumption (Schar et al., 2020). As the aquaculture sector continues to expand over the next decade, the risk of antibiotic resistance is expected to rise, posing a significant threat to ecological biodiversity and the proper functioning of ecosystems. Antibiotics are among the most prevalent chemical pollutants that enter the environment and subsequently infiltrate the food chain (Albarano et al., 2024). Antibiotics can lead to an imbalance in intestinal flora, which may adversely affect fish health, particularly in intensive rearing conditions characterized by high stocking densities that facilitate the spread of infectious diseases (Cox, 2016; Carlson et al., 2017). Studies have shown that the preventive use of antibiotics can reduce the symbiotic bacteria in aquatic animals, ultimately affecting host immunity (Schmidt et al., 2017; Milijašević at al., 2024). For instance, research on the fry of Oncorhynchus mykiss and Cyprinus carpio demonstrated that florfenicol suppressed their immunological responses (Mallik, 2023). Additionally, studies using zebrafish models have shown that antibiotics such as oxytetracycline and sulfamethoxazole can negatively affect gastrointestinal health when administered over extended periods, even at legally permissible dosages. These antibiotics may induce inflammation or disrupt gut flora (Jia, 2023). According to Manage (2018), the use of antibiotics for growth promotion can contribute to the development of antimicrobial-resistant bacteria in aquatic ecosystems. Furthermore, the accumulation of residues in fish tissues may stem from the subtherapeutic use of antibiotics. This practice can lead to the proliferation of antibiotic-resistant bacteria, which may subsequently be transferred to humans through environmental pathways or by consuming contaminated fish. Such transmission poses a significant risk, potentially resulting in diseases that are challenging to treat (Yuan et al., 2023). Moreover, antibiotic residues can persist in the environment and fish, raising critical concerns about their long-term toxicity, potential allergic reactions, and broader implications for human health (Albarano et al., 2024). Figure 6 below summarizes the benefits and drawbacks of antibiotic use, particularly when overprescribed.
10 Replacing antibiotics with probiotics and prebiotics in aquaculture
The mismanagement of antibiotics in aquaculture poses a grave concern with widespread impacts (Hemamalini et al., 2022). This practice has led to producers routinely administering antibiotics within aquaculture systems, creating a cycle of dependency. Unfortunately, the excessive use of antibiotics has triggered antimicrobial resistance in bacteria from aquaculture settings (Monteiro et al., 2016). As microorganisms evolve, they become immune to the effects of antibiotics that were originally effective against them, leading to antimicrobial resistance (Dcosta et al., 2011; Foster, 2017). The introduction of streptomycin, chloramphenicol, and tetracycline in the late 1940s also led to documented cases of bacterial resistance (Thuy et al., 2011). The continued and widespread use of antimicrobials in aquaculture systems creates a breeding ground for antimicrobial-resistant bacteria, as they face constant selective pressure (Gao et al., 2012). The World Health Organization (WHO) has pointed out the alarming threat of antibiotic resistance to global public health and the safety of aquatic food sources (Hong et al., 2018). Administering antimicrobials via water or medicated feed exacerbates the issue (Zainab et al., 2020). Most antibiotics are poorly absorbed by fish, leading to their release into the environment through waste. This issue is exacerbated because fish farm wastewater, containing runoff water, feces, and uneaten feed, is often discharged directly into natural aquatic environments (Henriksson et al., 2018). Therefore, a large number of bacteria are exposed to antibiotics within aquaculture production systems, such as tanks and ponds, creating ideal conditions for the evolution of antimicrobial resistance (Xu et al., 2017). The exchange of plasmids containing resistance traits and the merging of resistant bacterial populations with various bacterial communities are also part of antimicrobial resistance (Mathers et al., 2015). Resistance genes can spread between bacterial populations through the exchange of plasmids, enabling the formation of multidrug-resistant communities. Despite the increasing popularity of probiotics and prebiotics in aquaculture, a significant lack of understanding persists regarding their overall effectiveness and environmental benefits. Most of the current literature focuses on the effects of probiotics on specific species or environments, resulting in a limited understanding of their overall applicability in diverse aquaculture systems.
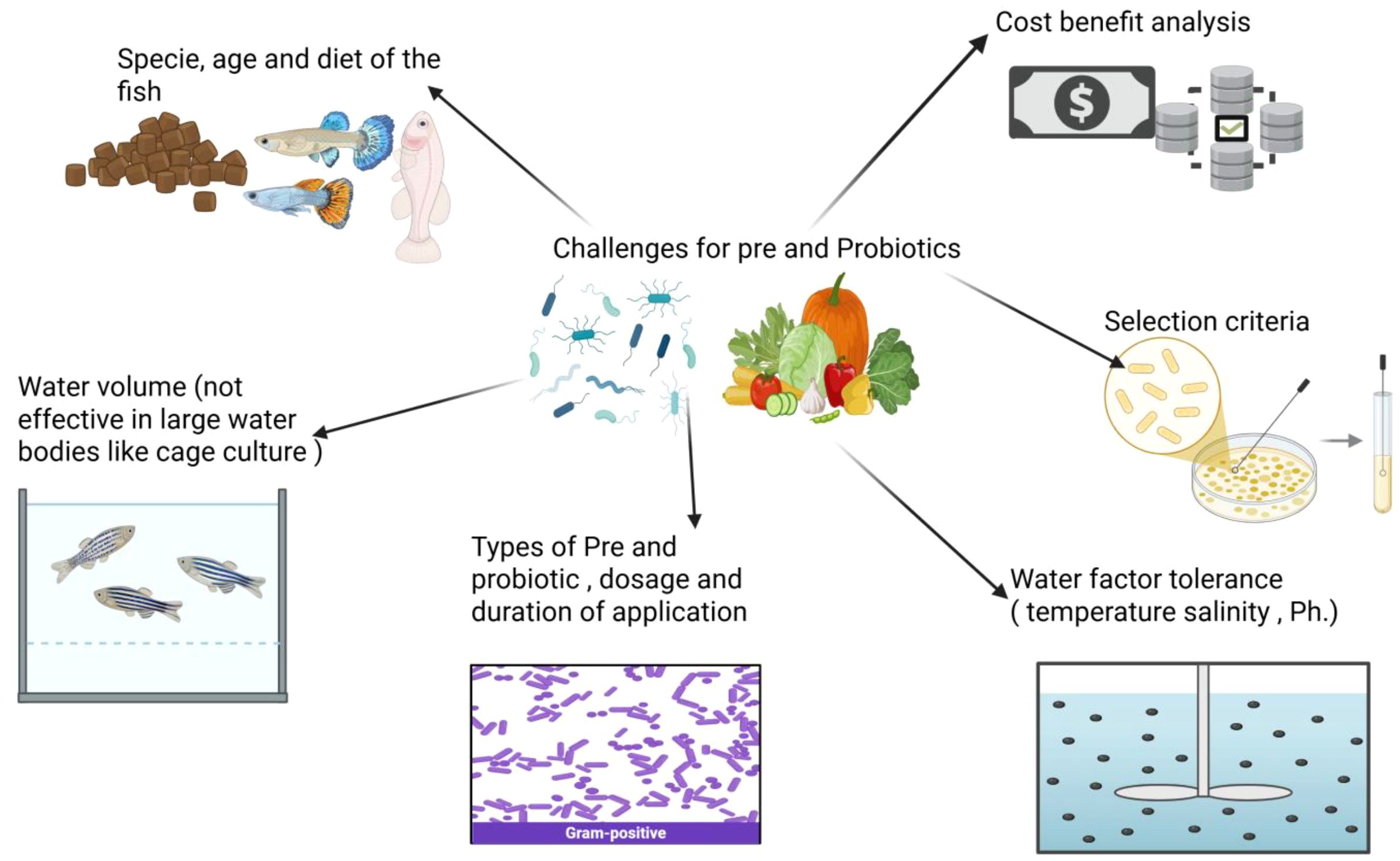
In addition to conducting comprehensive scientific research, selecting the appropriate probiotics relies on various technological considerations. Some of these considerations pertain to logistical challenges. Producing and distributing probiotics in tightly controlled laboratory environments poses unique challenges, as does ensuring their effectiveness on a large industrial scale (Todorov et al., 2024). Before incorporating these beneficial microbes into aquaculture practices, it is crucial to consider the key characteristics of probiotics, including their hydrophobicity, acid tolerance, and sensitivity to antibiotics. Probiotics are most effective when used as a preventative measure rather than a cure for illnesses. They are easily incorporated into low-water-level or stationary systems such as tanks and circulatory systems. However, in larger bodies of water, such as lakes used for cage cultures, probiotics may not be as effective. To prevent contamination, it is important to add probiotics immediately after sterilizing the water in the culture system, regardless of its size (Vulla, 2024). Probiotics have gained popularity as an eco-friendly alternative to antibiotics due to their ability to enhance host growth and immunity. The purpose of this study was to identify and isolate novel Bacillus species from the gut of hybrid groupers (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) that may be used as probiotics, as reports indicate that commercially available probiotics are ineffective because the majority come from non-fish sources (Amoah et al., 2024). Refusing other medications or chemicals for illness prevention or treatment is essential once probiotics are introduced into the system. This is because these substances may not be selective and could potentially destroy the beneficial bacteria (Lieke et al., 2020).
Current research findings show mixed results on the influence of prebiotics in fish farming. The effectiveness of prebiotics is influenced by various factors, including fish species, age, diet, environment, type of prebiotic used, dosage, and duration. Before introducing prebiotics, it’s essential to understand the specific nutritional needs of each type of fish, as improper dosages may cause harm or prove ineffective. Additionally, considering species with similar physiological traits to those that have responded positively to prebiotics in the past may be beneficial. There are scarce regulations governing the use of prebiotics in aquaculture feed, as the current regulations only apply to human consumption and vary between countries (Amillano-Cisneros et al., 2023). Like other costs incurred in aquaculture, probiotics and prebiotics come with associated expenses. Farmers can evaluate the financial viability of incorporating probiotic and prebiotic supplementation into their operations through cost-benefit analyses (CBAs).
As the popularity of probiotic and prebiotic products continues to rise due to their numerous health benefits, conducting a CBA becomes essential for understanding the financial implications of their introduction. However, performing a cost-benefit analysis for probiotics and prebiotics can be complex and requires meticulous attention to detail to yield reliable and accurate results. One of the primary challenges lies in assessing the efficacy of probiotics and prebiotics. While research suggests that these supplements can enhance immunity and promote gut health, their effects can vary significantly depending on the specific strain, dosage, and the medical conditions they aim to address. For a CBA to be effective, it is crucial to establish a clear cause-and-effect relationship between the consumption of probiotics and prebiotics and specific health outcomes. This can be particularly challenging due to individual variability and the presence of confounding factors. Additionally, the diverse range of health issues that probiotics target must be considered when evaluating their economic impact. The prevalence, severity, and financial burden of various conditions ranging from immune-related disorders to digestive problems can differ widely, necessitating comprehensive data and reliable algorithms to accurately estimate potential cost savings and benefits across this broad spectrum. Another significant challenge in estimating the financial advantages of probiotics is recognizing their benefits beyond immediate health effects. Furthermore, the cost component of a CBA encompasses not only the price of probiotic and prebiotic products but also expenses related to marketing, distribution, research, and development. Accurately estimating these costs can be particularly difficult, especially as the probiotic market continues to evolve and expand. The obstacles associated with using probiotics and prebiotics in fish are highlighted in the diagram shown in Figure 7.
11 Conclusion
Using probiotics and prebiotics in aquaculture can mitigate the harmful effects of pathogen outbreaks, decreasing economic losses from fish deaths, and reducing the need for antibiotics in controlling bacterial pathogens. This advancement is crucial for promoting the environmental sustainability of the fish farming industry. The health benefits of probiotics, prebiotics, or their combination are widely acknowledged, with strong evidence supporting their effectiveness against pathogenic or drug-resistant organisms. These probiotics and prebiotics offer a potential alternative approach to addressing the growing issue of antimicrobial resistance due to their unique antagonistic mechanisms against target microorganisms. Changing the makeup of gut microbes, boosting the host’s immune system, and improving the efficacy of the epithelial barrier are all essential steps in warding off pathogens by blocking their colonization and survival through exclusion and antimicrobial actions. The effectiveness of biologics as treatments is largely influenced by a combination of factors, including the disease stage, delivery method, and the host’s physiological condition. While probiotics and prebiotics hold great potential in aquaculture, current understanding of their mechanisms, strain-specific effects, and interactions with host metabolism remains limited. Thus, ongoing research and cautious application are essential.
12 Recommendations
Using probiotics and prebiotics demonstrates potential in minimizing antibiotic dependency. However, to establish them as a reliable treatment option, further well-planned studies are necessary to evaluate their efficacy against multidrug-resistant organisms in real-world disease scenarios. Estimating the true impact of probiotics can be a challenging task. The impact of different strains, dosages, and specific conditions on the efficacy of probiotics for gut health and immunity varies greatly. To conduct an accurate CBA, it is crucial to establish a direct connection between the use of probiotics and the resulting health benefits. Individual characteristics and other variables can influence the outcome and complicate this process. For a more comprehensive understanding of the relationship between lipid metabolism and antioxidants in aquatic species, future studies should focus on key aspects of this relationship. Understanding how hosts maintain a balance of beneficial microbial strains and lipid metabolism is crucial, despite obstacles such as pollution and climate change. Moreover, scientists should investigate the molecular mechanisms underlying the selection and preservation of bacterial types that facilitate specific lipid processing and overall well-being. Applying metabolomics methods to aquatic organisms will play a crucial role in connecting lipid metabolism pathways, microbial composition, and overall well-being. Future studies should investigate molecular mechanisms underlying gut microbiota modulation and lipid metabolism in fish.
Author contributions
LN: Data curation, Methodology, Conceptualization, Writing – original draft, Formal analysis. KA: Writing – original draft, Data curation, Funding acquisition, Methodology, Conceptualization, Supervision, Writing – review & editing. HC: Supervision, Methodology, Writing – review & editing. YH: Writing – review & editing, Methodology, Supervision. BW: Writing – review & editing, Formal analysis, Supervision. VMS: Writing – review & editing, Visualization. AM: Visualization, Writing – review & editing. MF: Writing – review & editing, Visualization. JC: Conceptualization, Writing – review & editing, Methodology, Supervision, Funding acquisition. DA: Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work is supported by the special project of Guangdong Province for the transformation of science and technology to promote the regional coordinated development of urban-rural (2025B0202010041), the Fund of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (ZJW-2024-14), Open Fund of Tianjin Key Lab of Aquatic Ecology and Aquaculture (TJAE201506), the Science and Technology Plan of Guangdong province (2023B0202010016), the Program for Scientific Research Start-up Funds of Guangdong Ocean University (060302022310), the Youth Science and Technology Innovation Talent of Guangdong TeZhi plan talent (2023TQ07A888), and the Science and Technology Plan of Zhanjiang City (2024E03007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abasubong K. P., Li X. F., Zhang D. D., Jia E. T., Xiang-Yang Y., Xu C., et al. (2018). Dietary supplementation of xylooligosaccharides benefits the growth performance and lipid metabolism of common carp (Cyprinus carpio) fed high-fat diets. Aquac. Nutr. 24, 1416–1424. doi: 10.1111/anu.12678
Abasubong K. P., Liu W.B., Adjoumani Y. J. J., Xia S. L., Xu C., and Li X. F. (2019). Xylooligosaccharides benefits the growth, digestive functions and TOR signaling in Megalobrama amblycephala fed diets with fish meal replaced by rice protein concentrate. Aquaculture 500, 417–428. doi: 10.1016/j.aquaculture.2018.10.048
Abdel Gayed M., Abbass A., Shehabeldin M., and Elabd H. (2021). Fermos® prebiotic dietary supplementation enhances immune, antioxidative responses and growth performance of Nile tilapia Oreochromis niloticus. Benha Vet. Med. J. 40, 135–140. doi: 10.21608/bvmj.2021.61282.1335
Ajdari A., Ghafarifarsani H., Hoseinifar S. H., Javahery S., Narimanizad F., Gatphayak K., et al. (2022). Effects of dietary supplementation of primaLac, inulin, and biomin imbo on growth performance, antioxidant, and innate immune responses of common carp (Cyprinus carpio). Aquac. Nutr. 2022, 1–13. doi: 10.1155/2022/8297479
Albarano L., Padilla Suarez E. G., Maggio C., La Marca A., Iovine R., Lofrano G., et al. (2024). Assessment of ecological risks posed by veterinary antibiotics in European aquatic environments: A comprehensive review and analysis. Sci. Total Environ. 954, 176280. doi: 10.1016/j.scitotenv.2024.176280
Amillano-Cisneros J. M., Fuentes-Valencia M. A., Leyva-Morales J. B., Davizón Y. A., Marquéz-Pacheco H., Valencia-Castañeda G., et al. (2023). Prebiotics in global and mexican fish aquaculture: A review. Animals 13, 1–21. doi: 10.3390/ani13233607
Amoah K., Cai J., Huang Y., Wang B., Shija V. M., Wang Z., et al. (2024). Identification and characterization of four Bacillus species from the intestine of hybrid grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂), their antagonistic role on common pathogenic bacteria, and effects on intestinal health. Fish Shellfish Immunol. 152, 109795. doi: 10.1016/j.fsi.2024.109795
Amoah K., Dong X., Tan B., Zhang S., Kuebutornye F. K. A., Chi S., et al. (2021). In vitro Assessment of the Safety and Potential Probiotic Characteristics of Three Bacillus Strains Isolated From the Intestine of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.675962
Amoah K., Tan B., Zhang S., Chi S., Yang Q., Liu H., et al. (2023). Host gut-derived Bacillus probiotics supplementation improves growth performance, serum and liver immunity, gut health, and resistive capacity against Vibrio harveyi infection in hybrid grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus). Anim. Nutr. 14, 163–184. doi: 10.1016/j.aninu.2023.05.005
Anguiano M., Pohlenz C., Buentello A., and Gatlin D. M. (2013). The effects of prebiotics on the digestive enzymes and gut histomorphology of red drum (Sciaenops ocellatus) and hybrid striped bass (Morone chrysops × M. saxatilis). Br. J. Nutr. 109, 623–629. doi: 10.1017/S0007114512001754
Arciuch-Rutkowska M., Nowosad J., Łuczyński M. K., Jasiński S., and Kucharczyk D. (2024). Effects of the diet enrichment with β-glucan, sodium salt of butyric acid and vitamins on growth parameters and the profile of the gut microbiome of juvenile African catfish (Clarias gariepinus). Anim. Feed Sci. Technol. 310, 1–14. doi: 10.1016/j.anifeedsci.2024.115941
Arif A., Kousar S., Nazir A., Umair K., Hafeez M., and Aslam S. (2024). Therapeutic potential of prebiotics in fish husbandry. Int. J. Vet. Sci. 3, 185–192. doi: 10.47278/book.cam/2024.029
Auclert L. Z., Chhanda M. S., and Derome N. (2024). Interwoven processes in fish development: microbial community succession and immune maturation. PeerJ 12, 1–56. doi: 10.7717/peerj.17051
Badguzar V. S., Satkar S. G., Kumar R., and A A. (2024). Comprehensive review of prebiotics and probiotics in aquaculture: Mechanisms and applications. Int. J. Vet. Sci. Anim. Husb. 9, 777–780. doi: 10.22271/veterinary.2024.v9.i1sk.1157
Bamigbade G. B., Subhash A. J., Kamal-Eldin A., Nyström L., and Ayyash M. (2022). An updated review on prebiotics: insights on potentials of food seeds waste as source of potential prebiotics. Molecules 27, 1–34. doi: 10.3390/molecules27185947
Behera K. K., Bist R., Mohanty S., and Bhattacharya M. (2022). Prebiotics, probiotics and nutraceuticals, prebiotics, probiotics and nutraceuticals. Probiotics and Nutraceuticals. 1–340. doi: 10.1007/978-981-16-8990-1
Boricha A. A., Shekh S. L., Pithva S. P., Ambalam P. S., and Manuel Vyas B. R. (2019). In vitro evaluation of probiotic properties of Lactobacillus species of food and human origin. Lwt 106, 201–208. doi: 10.1016/j.lwt.2019.02.021
Carlson J. M., Leonard A. B., Hyde E. R., Petrosino J. F., and Primm T. P. (2017). Microbiome disruption and recovery in the fish Gambusia affinis following exposure to broad-spectrum antibiotic. Infect. Drug Resist. 10, 143–154. doi: 10.2147/IDR.S129055
Castro C., Corraze G., Basto A., Larroquet L., Panserat S., and Oliva-Teles A. (2016). Dietary lipid and carbohydrate interactions: implications on lipid and glucose absorption, transport in gilthead sea bream (Sparus aurata) juveniles. Lipids 51, 743–755. doi: 10.1007/s11745-016-4140-2
Chen W. L., Dong Y. Z., Zhang L., Liu Z. S., He C. F., Liu W., et al. (2024). Xylooligosaccharides alleviate the carbohydrate-enriched diet-induced intestinal barrier dysfunction in carp Megalobrama amblycephala by promoting intestinal development, immunity and gut microbiota. Int. J. Biol. Macromol. 277, 134346. doi: 10.1016/j.ijbiomac.2024.134346
Chen W. and Gao S. (2023). Current status of industrialized aquaculture in China: a review. Environ. Sci. pollut. Res. 30, 32278–32287. doi: 10.1007/s11356-023-25601-9
Chen W. L., Ge Y. P., Sun M., He C. F., Zhang L., Liu W., et al. (2022). Insights into the correlations between prebiotics and carbohydrate metabolism in fish: Administration of xylooligosaccharides in Megalobrama amblycephala offered a carbohydrate-enriched diet. Aquaculture 561, 738684. doi: 10.1016/j.aquaculture.2022.738684
Chen W. W., Romano N., Ebrahimi M., and Natrah I. (2017). The effects of dietary fructooligosaccharide on growth, intestinal short chain fatty acids level and hepatopancreatic condition of the giant freshwater prawn (Macrobrachium rosenbergii) post-larvae. Aquaculture 469, 95–101. doi: 10.1016/j.aquaculture.2016.11.034
Cho Y. A. and Kim J. (2015). Effect of probiotics on blood lipid concentrations: A meta-analysis of randomized controlled trials. Med. (United States) 94, 1–10. doi: 10.1097/MD.0000000000001714
Cox L. M. (2016). Antibiotics shape microbiota and weight gain across the animal kingdom. Anim Front. 6 (3), 8–14. doi: 10.2527/af.2016-0028
Darafsh F., Soltani M., Abdolhay H. A., and Shamsaei Mehrejan M. (2020). Improvement of growth performance, digestive enzymes and body composition of Persian sturgeon (Acipenser persicus) following feeding on probiotics: Bacillus licheniformis, Bacillus subtilis and Saccharomyces cerevisiae. Aquac. Res. 51, 957–964. doi: 10.1111/are.14440
Davani-Davari D., Negahdaripour M., Karimzadeh I., Seifan M., Mohkam M., Masoumi S. J., et al. (2019). Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 8, 1–27. doi: 10.3390/foods8030092
Davoodbasha M. A., Mani A., Arunachalam K., Jagadeesan A., Kamli M. R., Kim J. W., et al. (2025). Isolation and characterization of probiotic bacteria from traditional foods. Appl. Biochem. Biotechnol. 197, 2197–2215. doi: 10.1007/s12010-024-05125-9
Dawood M. A. O., Eweedah N. M., Moustafa E. M., and Shahin M. G. (2020c) Synbiotic effects of Aspergillus oryzae and β-glucan on growth and oxidative and immune responses of Nile tilapia, Oreochromis niloticus. Probiotics Antimicrob. Proteins 12(1), 172–183. doi: 10.1007/s12602-018-9513-9
Dawood M. A. O., Eweedah N. M., Moustafa E. M., and Farahat E. M. (2020a). Probiotic effects of Aspergillus oryzae on the oxidative status, heat shock protein, and immune related gene expression of Nile tilapia (Oreochromis niloticus) under hypoxia challenge. Aquaculture 520, 734669. doi: 10.1016/j.aquaculture.2019.734669
Dawood M. A. O., Koshio S., El-Sabagh M., Billah M. M., Zaineldin A. I., Zayed M. M., et al. (2017). Changes in the growth, humoral and mucosal immune responses following β-glucan and vitamin C administration in red sea bream, Pagrus major. Aquaculture 470, 214–222. doi: 10.1016/j.aquaculture.2016.12.036
Dawood M. A. O., Koshio S., Fadl S. E., Ahmed H. A., El Asely A., Abdel-Daim M. M., et al. (2020b). The modulatory effect of mannanoligosaccharide on oxidative status, selected immune parameters and tolerance against low salinity stress in red sea bream (Pagrus major). Aquac. Rep. 16, 100278. doi: 10.1016/j.aqrep.2020.100278
Dcosta V. M., King C. E., Kalan L., Morar M., Sung W. W. L., Schwarz C., et al. (2011). Antibiotic resistance is ancient. Nature 477, 457–461. doi: 10.1038/nature10388
De B. C., Meena D. K., Behera B. K., Das P., Das Mohapatra P. K., and Sharma A. P. (2014). Probiotics in fish and shellfish culture: Immunomodulatory and ecophysiological responses. Fish Physiol. Biochem. 40, 921–971. doi: 10.1007/s10695-013-9897-0
Defaix R., Lokesh J., Frohn L., Le Bechec M., Pigot T., Véron V., et al. (2024). Exploring the effects of dietary inulin in rainbow trout fed a high-starch, 100% plant-based diet. J. Anim. Sci. Biotechnol. 15, 1–20. doi: 10.1186/s40104-023-00951-z
Delzenne N. M., Cani P. D., and Neyrinck A. M. (2007). Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: Experimental data. J. Nutr. 137, 2547S–2551S. doi: 10.1093/jn/137.11.2547s
Delzenne N. M. and Kok N. (2001). Effects of fructans-type prebiotics on lipid metabolism. Am. J. Clin. Nutr. 73, 456s–458s. doi: 10.1093/ajcn/73.2.456s
De Marco G., Cappello T., and Maisano M. (2023). Histomorphological changes in fish gut in response to prebiotics and probiotics treatment to improve their health status: A review. Animals 13 (18), 1–39. doi: 10.3390/ani13182860
Deng Y., Zhang W., Yang Z., Kong Q., Liu P., Liao H., et al. (2024). Dietary Lactobacillus plantarum can alleviate high starch diet-induced liver lipid deposition, tissue damage and oxidative stress in largemouth bass (Micropterus salmoides). Aquac. Rep. 35, 101955. doi: 10.1016/j.aqrep.2024.101955
Dhanasiri A. K. S., Jaramillo-Torres A., Chikwati E. M., Forberg T., Krogdahl Å., and Kortner T. M. (2023). Effects of dietary supplementation with prebiotics and Pediococcus acidilactici on gut health, transcriptome, microbiota, and metabolome in Atlantic salmon (Salmo salar L.) after seawater transfer. Anim. Microbiome 5, 1–22. doi: 10.1186/s42523-023-00228-w
Dobrogosz W. J., Peacock T. J., and Hassan H. M. (2010). “Evolution of the probiotic concept: From conception to validation and acceptance in medical science,” in Advances in Applied Microbiology, 1st ed (San Diego, CA, USA: Elsevier Inc). doi: 10.1016/S0065-2164(10)72001-3
Dou X., Huang H., Li Y., Deng J., and Tan B. (2023). Effects of dietary β-glucan on growth rate, antioxidant status, immune response, and resistance against Aeromonas hydrophila in genetic improvement of farmed tilapia (GIFT, Oreochromis niloticus). Aquac. Rep. 29, 101480. doi: 10.1016/j.aqrep.2023.101480
El-Saadony M. T., Alagawany M., Patra A. K., Kar I., Tiwari R., Dawood M. A. O., et al. (2021). The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 117, 36–52. doi: 10.1016/j.fsi.2021.07.007
Fachri M., Amoah K., Huang Y., Cai J., Alfatat A., Ndandala C. B., et al. (2024). Probiotics and paraprobiotics in aquaculture: a sustainable strategy for enhancing fish growth, health and disease prevention-a review. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1499228
Falcinelli S., Picchietti S., Rodiles A., Cossignani L., Merrifield D. L., Taddei A. R., et al. (2015). Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci. Rep. 5, 8–10. doi: 10.1038/srep09336
Falcinelli S., Rodiles A., Hatef A., Picchietti S., Cossignani L., Merrifield D. L., et al. (2017). Dietary lipid content reorganizes gut microbiota and probiotic L. rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Sci. Rep. 7, 1–15. doi: 10.1038/s41598-017-05147-w
Falcinelli S., Rodiles A., Unniappan S., Picchietti S., Gioacchini G., Merrifield D. L., et al. (2016). Probiotic treatment reduces appetite and glucose level in the zebrafish model. Sci. Rep. 6, 1–13. doi: 10.1038/srep18061
Feng H., Zhang Y., Liang X. F., He S., and Li L. (2021). Dietary supplementation of exogenous probiotics reduces excessive liver lipid deposition in Chinese perch (Siniperca chuatsi). Aquac. Res. 52, 5430–5440. doi: 10.1111/are.15413
Feng Y., Zuo Z., Xie G., Chen Y., Yin X., Lu B., et al. (2025). Combined analysis of 16S rRNA sequencing and metabolomics reveals the growth-promoting mechanism of compound probiotics in zig-zag eel (Mastacembelus armatus). Aquac. Rep. 40, 102571. doi: 10.1016/j.aqrep.2024.102571
Foster T. J. (2017). Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 41, 430–449. doi: 10.1093/femsre/fux007
Gall K., Specialist S., York N., and Grant S. (1995). Haccp guide for the aquaculture industry. NRAC Publ.
Gao P., Mao D., Luo Y., Wang L., Xu B., and Xu L. (2012). Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 46, 2355–2364. doi: 10.1016/j.watres.2012.02.004
Genc M. A., Aktas M., Genc E., and Yilmaz E. (2007). Effects of dietary mannan oligosaccharide on growth, body composition and hepatopancreas histology of Penaeus semisulcatus (de Haan 1844). Aquac. Nutr. 13, 156–161. doi: 10.1111/j.1365-2095.2007.00469.x
Ghafarifarsani H., Rashidian G., Bagheri T., Hoseinifar S. H., and Van Doan H. (2021). Study on growth enhancement and the protective effects of dietary prebiotic inulin on immunity responses of rainbow trout (Oncorhynchus mykiss) fry infected with Aeromonas hydrophila. Ann. Anim. Sci. 21, 543–559. doi: 10.2478/aoas-2020-0074
Gibson G. R. and Roberfroid M. B. (1995). Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 125, 1401–1412. doi: 10.1093/jn/125.6.1401
Gibson G. R., Scott K. P., Rastall R. A., Tuohy K. M., Hotchkiss A., Dubert-Ferrandon A., et al. (2010). Dietary prebiotics: current status and new definition. Food Sci. Technol. Bull. Funct. Foods 7, 1–19. doi: 10.1616/1476-2137.15880
Guerreiro I., Oliva-Teles A., and Enes P. (2015). Improved glucose and lipid metabolism in European sea bass (Dicentrarchus labrax) fed short-chain fructooligosaccharides and xylooligosaccharides. Aquaculture 441, 57–63. doi: 10.1016/j.aquaculture.2015.02.015
Guerreiro I., Oliva-Teles A., and Enes P. (2024). The effect of probiotics and prebiotics on feed intake in cultured fish. Rev. Aquac. 17, 1–24. doi: 10.1111/raq.12982
Gut A. M., Vasiljevic T., Yeager T., and Donkor O. N. (2019). Characterization of yeasts isolated from traditional kefir grains for potential probiotic properties. J. Funct. Foods 58, 56–66. doi: 10.1016/j.jff.2019.04.046
Hamdan A. M., El-Sayed A. F. M., and Mahmoud M. M. (2016). Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus). J. Appl. Microbiol. 120, 1061–1073. doi: 10.1111/jam.13081
Hemamalini N., Shanmugam S. A., Kathirvelpandian A., Deepak A., Kaliyamurthi V., and Suresh E. (2022). A critical review on the antimicrobial resistance, antibiotic residue and metagenomics-assisted antimicrobial resistance gene detection in freshwater aquaculture environment. Aquac. Res. 53, 344–366. doi: 10.1111/are.15601
Henriksson P. J. G., Rico A., Troell M., Klinger D. H., Buschmann A. H., Saksida S., et al. (2018). Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: a review from a systems perspective. Sustain. Sci. 13, 1105–1120. doi: 10.1007/s11625-017-0511-8
Hijova E. and Chmelarova A. (2007). Short chain fatty acids and colonic health. Bratisl. Lek. Listy 108, 354–358.
Hoang D. H., Thi Thanh Thuy N., and Ky P. X. (2024). A synergistic effect of dietary β-glucan and mannan oligosaccharide on growth performance, haematology, body composition, nutrient utilisation, and intestinal morphology in pompano, Trachinotus ovatus. Reg. Stud. Mar. Sci. 73, 103494. doi: 10.1016/j.rsma.2024.103494
Hong B., Ba Y., Niu L., Lou F., Zhang Z., Liu H., et al. (2018). A comprehensive research on antibiotic resistance genes in microbiota of aquatic animals. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.01617
Hoseinifar S. H., Hoseini S. M., and Bagheri D. (2017). Effects of galactooligosaccharide and Pediococcus acidilactici on antioxidant defence and disease resistance of rainbow trout, Oncorhynchus mykiss. Ann. Anim. Sci. 17, 217–227. doi: 10.1515/aoas-2016-0024
Hoseinifar S. H., Yousefi S., Van Doan H., Ashouri G., Gioacchini G., Maradonna F., et al. (2021). Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Reviews in Fisheries Science & Aquaculture, 29 (2), 198–217. doi: 10.1080/23308249.2020.1795616
Hoseinifar S. H., Yousefi S., Van Doan H., Ashouri G., Gioacchini G., Maradonna F., et al. (2020). Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 29, 198–217. doi: 10.1080/23308249.2020.1795616
Huynh T. G., Chi C. C., Nguyen T. P., Tran T. T. T. H., Cheng A. C., and Liu C. H. (2018). Effects of synbiotic containing Lactobacillus plantarum 7–40 and galactooligosaccharide on the growth performance of white shrimp, Litopenaeus vannamei. Aquac. Res. 49, 2416–2428. doi: 10.1111/are.13701
Iraporda C., Rubel I. A., Manrique G. D., and Abraham A. G. (2019). Influence of inulin rich carbohydrates from Jerusalem artichoke (Helianthus tuberosus L.) tubers on probiotic properties of Lactobacillus strains. Lwt 101, 738–746. doi: 10.1016/j.lwt.2018.11.074
Jia P. (2023). Chronic exposure to environmentally relevant concentrations of Tetracycline perturbs gut homeostasis in Zebrafish. Environ Health. 1 (4), 258–269. doi: 10.1021/envhealth.3c00072
Jia E., Li Z., Xue Y., Jiang G., Li X., Liu W., et al. (2017). Effects of dietary fructooligosaccharide on the growth, antioxidants, immunity and disease resistance of Chinese mitten crab. Aquaculture 481, 154–161. doi: 10.1016/j.aquaculture.2017.08.033
Tiseo K., Huber L., Gilbert M., Robinson T. P., and Van Boeckel T. P. (2020). Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 9, 1–14. doi: 10.3390/antibiotics9120918
Kim Y. A., Keogh J. B., and Clifton P. M. (2018). Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 31, 35–51. doi: 10.1017/S095442241700018X
Li Z., Tran N. T., Ji P., Sun Z., Wen X., and Li S. (2019). Effects of prebiotic mixtures on growth performance, intestinal microbiota and immune response in juvenile chu’s croaker, Nibea coibor. Fish Shellfish Immunol. 89, 564–573. doi: 10.1016/j.fsi.2019.04.025
Li R., Wang X., Yu D., Liang Q., Liu F., Zhang L., et al. (2023). Dietary chitosan alleviates intestinal and liver injury of hybrid sturgeon (Acipenser baerii♀ × A. schrenckii♂) induced by Aeromonas hydrophila infection. Anim. Feed Sci. Technol. 299, 115624. doi: 10.1016/j.anifeedsci.2023.115624
Lieke T., Meinelt T., Hoseinifar S. H., Pan B., Straus D. L., and Steinberg C. E. W. (2020). Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev. Aquac. 12, 943–965. doi: 10.1111/raq.12365
Lin S., Yang X., Long Y., Zhong H., Wang P., Yuan P., et al. (2020). Dietary supplementation with Lactobacillus plantarum modified gut microbiota, bile acid profile and glucose homoeostasis in weaning piglets. Br. J. Nutr. 124, 797–808. doi: 10.1017/S0007114520001774
Liu L., Fu C., Liu Y., and Li F. (2021a). Acetate stimulates lipogenesis via AMPKα signaling in rabbit adipose-derived stem cells. Gen. Comp. Endocrinol. 303, 113715. doi: 10.1016/j.ygcen.2021.113715
Liu M., Tang L., Hu C., Huang Z., Sun B., Lam J. C. W., et al. (2021b). Antagonistic interaction between perfluorobutanesulfonate and probiotic on lipid and glucose metabolisms in the liver of zebrafish. Aquat. Toxicol. 237, 105897. doi: 10.1016/j.aquatox.2021.105897
Liu W. C., Zhou S. H., Balasubramanian B., Zeng F. Y., Sun C. B., and Pang H. Y. (2020). Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 104, 202–212. doi: 10.1016/j.fsi.2020.05.079
Lokesh J., Ghislain M., Reyrolle M., Bechec M., Pigot T., Terrier F., et al. (2022). Prebiotics modify host metabolism in rainbow trout (Oncorhynchus mykiss) fed with a total plant-based diet: Potential implications for microbiome-mediated diet optimization. Aquaculture 561, 1–35. doi: 10.1016/j.aquaculture.2022.738699
Luo Y., Hu C. T., Qiao F., Wang X. D., Qin J. G., Du Z. Y., et al. (2020). Gemfibrozil improves lipid metabolism in Nile tilapia Oreochromis niloticus fed a high-carbohydrate diet through peroxisome proliferator activated receptor-α activation. Gen. Comp. Endocrinol. 296, 113537. doi: 10.1016/j.ygcen.2020.113537
Lye H. S., Rahmat-Ali G. R., and Liong M. T. (2010). Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int. Dairy J. 20, 169–175. doi: 10.1016/j.idairyj.2009.10.003
Mallik S. K. (2023). Pharmacokinetics and biosafety evaluation of a veterinary drug florfenicol in rainbow trout, Oncorhynchus mykiss (Walbaum 1792) as a model cultivable fish species in temperate water. Front. Pharmacol. 14, 1–19. doi: 10.3389/fphar.2023.1033170
Manage P. M. (2018). Heavy use of antibiotics in aquaculture: Emerging human and animal health problems – A review. Sri Lanka J. Aquat. Sci. 23, 13. doi: 10.4038/sljas.v23i1.7543
Martínez Cruz P., Ibáñez A. L., Monroy Hermosillo O. A., and Ramírez Saad H. C. (2012). Use of probiotics in aquaculture. ISRN Microbiol. 2012, 1–13. doi: 10.5402/2012/916845
Mathers A. J., Peirano G., and Pitout J. D. D. (2015). The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin. Microbiol. Rev. 28, 565–591. doi: 10.1128/CMR.00116-14
Meena D. K., Das P., Kumar S., Mandal S. C., Prusty A. K., Singh S. K., et al. (2013). Beta-glucan: An ideal immunostimulant in aquaculture (a review). Fish Physiol. Biochem. 39, 431–457. doi: 10.1007/s10695-012-9710-5
Merrifield D. L. and Carnevali O. (2014). Probiotic modulation of the gut microbiota of fish. Aquac. Nutr. Gut Heal. Probiotics Prebiotics 3, 185–222. doi: 10.1002/9781118897263.ch8
Midhun S. J., Neethu S., Arun D., Vysakh A., Divya L., Radhakrishnan E. K., et al. (2019). Dietary supplementation of Bacillus licheniformis HGA8B improves growth parameters, enzymatic profile and gene expression of Oreochromis niloticus. Aquaculture 505, 289–296. doi: 10.1016/j.aquaculture.2019.02.064
Milijasevic M., Veskovic-Moracanin S., Babic Milijasevic J., Petrovic J., and Nastasijevic I (2024). Antimicrobial Resistance in Aquaculture: Risk Mitigation within the One Health Context. Foods 13, 1–30. doi: 10.3390/foods13152448
Mishra V., Shah C., Mokashe N., Chavan R., Yadav H., and Prajapati J. (2015). Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 63, 3615–3626. doi: 10.1021/jf506326t
Moghadam S. S., Khodaii Z., Zadeh S. F., Ghooshchian M., Aghmiyuni Z. F., and Shabestari T. M. (2018). Synergistic or antagonistic effects of probiotics and antibiotics-alone or in combination-on antimicrobial-resistant pseudomonas aeruginosa isolated from burn wounds. Arch. Clin. Infect. Dis. 13 (3), 1–5. doi: 10.5812/archcid.63121
Mohammadi G., Hafezieh M., Karimi A. A., Azra M. N., Van Doan H., Tapingkae W., et al. (2022). The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Fish Shellfish Immunol. 120, 304–313. doi: 10.1016/j.fsi.2021.11.028
Mohammadian T., Ghanei-Motlagh R., Molayemraftar T., Mesbah M., Zarea M., Mohtashamipour H., et al. (2021). Modulation of growth performance, gut microflora, non-specific immunity and gene expression of proinflammatory cytokines in shabout (Tor grypus) upon dietary prebiotic supplementation. Fish Shellfish Immunol. 112, 38–45. doi: 10.1016/j.fsi.2021.02.012
Monteiro S. H., Garcia F., Gozi K. S., Romera D. M., Francisco J. G., Moura-Andrade G. C. R., et al. (2016). Relationship between antibiotic residues and occurrence of resistant bacteria in Nile tilapia (Oreochromis niloticus) cultured in cage-farm. J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes 51, 817–823. doi: 10.1080/03601234.2016.1208457
Moreno-Garcia L., Adjallé K., Barnabé S., and Raghavan G. S. V (2017). Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renew. Sustain. Energy Rev. 76, 493–506. doi: 10.1016/j.rser.2017.03.024
Nakhei Rad M. H., Haghighi Khiabania Asl A., Azari Takami G. H., Razavillar V., and Tehrani Sharif M. (2023). The effects of probiotics and prebiotics on growth, stress responses, blood and immune parameters of rainbow trout. Iran. J. Fish. Sci. 22, 278–302. doi: 10.22092/ijfs.2023.128921
Nawaz A., Bakhsh javaid A., Irshad S., Hoseinifar S. H., and Xiong H. (2018). The functionality of prebiotics as immunostimulant: Evidences from trials on terrestrial and aquatic animals. Fish Shellfish Immunol. 76, 272–278. doi: 10.1016/j.fsi.2018.03.004
Nayak S. K. (2010). Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 29, 2–14. doi: 10.1016/j.fsi.2010.02.017
Ntakirutimana R., Syanya F. J., and Mwangi P. (2023). Exploring the impact of probiotics on the gut ecosystem and morpho-histology in fish: current knowledge of tilapia. Asian J. Fish. Aquat. Res. 25, 93–112. doi: 10.9734/ajfar/2023/v25i3670
Opiyo M. A., Jumbe J., Ngugi C. C., and Charo-Karisa H. (2019). Different levels of probiotics affect growth, survival and body composition of Nile tilapia (Oreochromis niloticus) cultured in low input ponds. Sci. Afr. 4, e00103. doi: 10.1016/j.sciaf.2019.e00103
Pacitti D., Wang T., Martin S. A. M., Sweetman J., and Secombes C. J. (2014). Insights into the fish thioredoxin system: Expression profile of thioredoxin and thioredoxin reductase in rainbow trout (Oncorhynchus mykiss) during infection and in vitro stimulation. Dev. Comp. Immunol. 42, 261–277. doi: 10.1016/j.dci.2013.09.013
Panigrahi A., Kiron V., Satoh S., and Watanabe T. (2010). Probiotic bacteria Lactobacillus rhamnosus influences the blood profile in rainbow trout Oncorhynchus mykiss (Walbaum). Fish Physiol. Biochem. 36, 969–977. doi: 10.1007/s10695-009-9375-x
Pardo-Esté C., Cortés J., Castro-Severyn J., Pérez V., Henriquez-Aedo K., Cuadros F., et al. (2024). Secondary metabolites with antimicrobial activity produced by thermophilic bacteria from a high-altitude hydrothermal system. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1477458
Pepi M. and Focardi S. (2021). Antibiotic-resistant bacteria in aquaculture and climate change: A challenge for health in the mediterranean area. Int. J. Environ. Res. Public Health 18 (11), 1–31. doi: 10.3390/ijerph18115723
Peruzzolo M., Ceni G. C., Junges A., Zeni J., Cansian R. L., and Backes G. T. (2025). Probiotics: Health benefits, microencapsulation, and viability, combination with natural compounds, and applications in foods. Food Biosci. 66, 106253. doi: 10.1016/j.fbio.2025.106253
Picchietti S., Mazzini M., Taddei A. R., Renna R., Fausto A. M., Mulero V., et al. (2007). Effects of administration of probiotic strains on GALT of larval gilthead seabream: Immunohistochemical and ultrastructural studies. Fish Shellfish Immunol. 22, 57–67. doi: 10.1016/j.fsi.2006.03.009
Politis S. N., Benini E., Miest J. J., Engrola S., Sørensen S. R., Syropoulou E., et al. (2023). First assessment of prebiotics, probiotics, and synbiotics affecting survival, growth, and gene expression of european eel (Anguilla Anguilla) larvae. Aquac. Res. 2023, 1–13. doi: 10.1155/2023/1260967
Raheem A., Liang L., Zhang G., and Cui S (2021). Modulatory Effects of Probiotics During Pathogenic Infections With Emphasis on Immune Regulation. Front. Immunol. 12, 1–32. doi: 10.3389/fimmu.2021.616713
Rasika D. M., Vidanarachchi J. K., Rocha R. S., Balthazar C. F., Cruz A. G., Sant’Ana A. S., et al. (2021). Plant-based milk substitutes as emerging probiotic carriers. Curr. Opin. Food Sci. 38, 8–20. doi: 10.1016/j.cofs.2020.10.025
Ringø E., Harikrishnan R., Soltani M., and Ghosh K. (2022). The effect of gut microbiota and probiotics on metabolism in fish and shrimp. Animals 12, 1–13. doi: 10.3390/ani12213016
Rodklongtan A., Nitisinprasert S., and Chitprasert P. (2022). Antioxidant activity and the survival-enhancing effect of ascorbic acid on Limosilactobacillus reuteri KUB-AC5 microencapsulated with lactose by spray drying. Lwt 164, 113645. doi: 10.1016/j.lwt.2022.113645
Rohani M. F., Islam S. M., Hossain M. K., Ferdous Z., Siddik M. A., Nuruzzaman M., et al. (2022). Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. 120, 569–589. doi: 10.1016/j.fsi.2021.12.037
Ruiz-Moyano S., Gonçalves dos Santos M. T. P., Galván A. I., Merchán A. V., González E., Córdoba M., et al. (2019). Screening of autochthonous lactic acid bacteria strains from artisanal soft cheese: probiotic characteristics and prebiotic metabolism. Lwt 114, 108388. doi: 10.1016/j.lwt.2019.108388
Sahraei F., Ahari H., and Kakoolaki S. (2019). Effect of Bacillus subtilis as a probiotic on protein, lipid content, and trypsin and chymotrypsin enzymes in rainbow trout biometry (Oncorhynchus mykiss). Aquac. Int. 27, 141–153. doi: 10.1007/s10499-018-0313-8
Schar D., Klein E. Y., Laxminarayan R., Gilbert M., and Van Boeckel T. P. (2020). Global trends in antimicrobial use in aquaculture. Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-78849-3
Schmidt V., Gomez-Chiarri M., Roy C., Smith K., and Amaral-Zettler L (2017). Subtle Microbiome Manipulation Using Probiotics Reduces Antibiotic-Associated Mortality in Fish. mSystems 2, 1–13. doi: 10.1128/msystems.00133-17
Semova I., Carten J. D., Stombaugh J., MacKey L. C., Knight R., Farber S. A., et al. (2012). Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 12, 277–288. doi: 10.1016/j.chom.2012.08.003
Sharma S. and Puri S. (2015). Prebiotics and lipid metabolism: A review. Altern. Ther. Health Med. 21, 34–42.
Shehata A. I., Soliman A. A., Ahmed H. A., Gewaily M. S., Amer A. A., Shukry M., et al. (2024). Evaluation of different probiotics on growth, body composition, antioxidant capacity, and histoarchitecture of Mugil capito. Sci. Rep. 14, 1–10. doi: 10.1038/s41598-024-57489-x
Shija V. M., Chen H., Li Y., Ng’onga L., Amoah K., Yong Z., et al. (2025). Effects of dietary supplementation with fish-derived Bacillus amyloliquefaciens AV5 on growth status, immune response, microbiota, and intestinal health of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 41, 1–12. doi: 10.1016/j.aqrep.2025.102658
Silva M. P., Farsoni E. G., Gobato C. F., Thomazini M., and Favaro-Trindade C. S. (2022). Simultaneous encapsulation of probiotic and guaraná peel extract for development of functional peanut butter. Food Control 138, 109050. doi: 10.1016/j.foodcont.2022.109050
Sîrbu E., Dima M. F., Tenciu M., Cretu M., Coadă M. T., Țoțoiu A., et al. (2022). Effects of dietary supplementation with probiotics and prebiotics on growth, physiological condition, and resistance to pathogens challenge in nile tilapia (Oreochromis niloticus). Fishes 7 (5), 1–21. doi: 10.3390/fishes7050273
Siri P. W. and Krauss R. M. (2005). Influence of dietary carbohydrate and fat on LDL and HDL particle distributions. Curr. Atheroscler. Rep. 7, 455–459. doi: 10.1007/s11883-005-0062-9
Słowińska M., Nynca J., Cejko B. I., Dietrich M. A., Horváth Á., Urbányi B., et al. (2013). Total antioxidant capacity of fish seminal plasma. Aquaculture 400–401, 101–104. doi: 10.1016/j.aquaculture.2013.03.010
Song S. K., Beck B. R., Kim D., Park J., Kim J., Kim H. D., et al. (2014). Prebiotics as immunostimulants in aquaculture: A review. Fish Shellfish Immunol. 40, 40–48. doi: 10.1016/j.fsi.2014.06.016
Song X., Liu Y., Zhang X., Weng P., Zhang R., and Wu Z. (2023). Role of intestinal probiotics in the modulation of lipid metabolism: implications for therapeutic treatments. Food Sci. Hum. Wellness 12, 1439–1449. doi: 10.1016/j.fshw.2023.02.005
Su C., Fan D., Pan L., Lu Y., Wang Y., and Zhang M. (2020). Effects of Yu-Ping-Feng polysaccharides (YPS) on the immune response, intestinal microbiota, disease resistance and growth performance of Litopenaeus vannamei. Fish Shellfish Immunol. 105, 104–116. doi: 10.1016/j.fsi.2020.07.003
Ta’ati R., Soltani M., Bahmani M., and Zamini A. A. (2011). Effects of the prebiotics Immunoster and Immunowall on growth performance of juvenile beluga (Huso huso). J. Appl. Ichthyol. 27, 796–798. doi: 10.1111/j.1439-0426.2010.01664.x
Thuy H. T. T., Nga L. P., and Loan T. T. C. (2011). Antibiotic contaminants in coastal wetlands from Vietnamese shrimp farming. Environ. Sci. pollut. Res. 18, 835–841. doi: 10.1007/s11356-011-0475-7
Todorov S. D., Lima J. M. S., Bucheli J. E. V., Popov I. V., Tiwari S. K., and Chikindas M. L. (2024). Probiotics for aquaculture: hope, truth, and reality. Probiotics Antimicrob. Proteins 16, 2007–2020. doi: 10.1007/s12602-024-10290-8
Torrecillas S., Montero D., Caballero M. J., Robaina L., Zamorano M. J., Sweetman J., et al. (2015). Effects of dietary concentrated mannan oligosaccharides supplementation on growth, gut mucosal immune system and liver lipid metabolism of European sea bass (Dicentrarchus labrax) juveniles. Fish Shellfish Immunol. 42, 508–516. doi: 10.1016/j.fsi.2014.11.033
Torrecillas S., Montero D., and Izquierdo M. (2014). Improved health and growth of fish fed mannan oligosaccharides: Potential mode of action. Fish Shellfish Immunol. 36, 525–544. doi: 10.1016/j.fsi.2013.12.029
Tran N. T., Liang H., Li J., Deng T., Zhang M., and Li S. (2023). Health benefits of butyrate and its producing bacterium, Clostridium butyricum, on aquatic animals. Fish Shellfish Immunol. Rep. 4, 100088. doi: 10.1016/j.fsirep.2023.100088
Tran N. T., Tang Y., Li Z., Zhang M., Wen X., Ma H., et al. (2020). Galactooligosaccharides and Resistant Starch Altered Microbiota and Short-Chain Fatty Acids in an in vitro Fermentation Study Using Gut Contents of Mud Crab (Scylla paramamosain). Front. Microbiol. 11. doi: 10.3389/fmicb.2020.01352
Van Doan H., Hoseinifar S. H., Faggio C., Chitmanat C., Mai N. T., Jaturasitha S., et al. (2018). Effects of corncob derived xylooligosaccharide on innate immune response, disease resistance, and growth performance in Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 495, 786–793. doi: 10.1016/j.aquaculture.2018.06.068
Varzakas T., Kandylis P., Dimitrellou D., Salamoura C., Zakynthinos G., and Proestos C. (2018). Innovative and fortified food: Probiotics, prebiotics, GMOs, and superfood, Preparation and Processing of Religious and Cultural Foods (Oxford, UK: Elsevier Ltd). doi: 10.1016/B978-0-08-101892-7.00006-7
Vulla K. E. (2024). Unlocking potential benefits on applications of probiotics in inland aquaculture industry : A review. Aquaculture, Fish and Fisheries. 1–20. doi: 10.1002/aff2.70027
Wang L., Sagada G., Wang C., Liu R., Li Q., Zhang C., et al. (2023). Exogenous bile acids regulate energy metabolism and improve the health condition of farmed fish. Aquaculture 562, 738852. doi: 10.1016/j.aquaculture.2022.738852
Wang C., Xu Z., Lu S., Jiang H., Li J., Wang L., et al. (2022a). Effects of dietary xylooligosaccharide on growth, digestive enzymes activity, intestinal morphology, and the expression of inflammatory cytokines and tight junctions genes in triploid Oncorhynchus mykiss fed a low fishmeal diet. Aquac. Rep. 22, 1–10. doi: 10.1016/j.aqrep.2021.100941
Wang T., Xu R., Qiao F., Du Z. Y., and Zhang M. L. (2022b). Effects of mannan oligosaccharides (MOS) on glucose and lipid metabolism of largemouth bass (Micropterus salmoides) fed with high carbohydrate diet. Anim. Feed Sci. Technol. 292, 115449. doi: 10.1016/j.anifeedsci.2022.115449
Wang T., Wu H. X., Li W.j., Xu R., Qiao F., Du Z. Y., et al. (2022c). Effects of dietary mannan oligosaccharides (MOS) supplementation on metabolism, inflammatory response and gut microbiota of juvenile Nile tilapia (Oreochromis niloticus) fed with high carbohydrate diet. Fish Shellfish Immunol. 130, 550–559. doi: 10.1016/j.fsi.2022.09.052
Wang T., Zhang N., Yu X. B., Qiao F., Chen L. Q., Du Z. Y., et al. (2021). Inulin alleviates adverse metabolic syndrome and regulates intestinal microbiota composition in Nile tilapia (Oreochromis niloticus) fed with high-carbohydrate diet. Br. J. Nutr. 126, 161–171. doi: 10.1017/S000711452000402X
Wee W., Abdul Hamid N. K., Mat K., Khalif R. I. A. R., Rusli N. D., Rahman M. M., et al. (2024). The effects of mixed prebiotics in aquaculture: A review. Aquac. Fish. 9, 28–34. doi: 10.1016/j.aaf.2022.02.005
Whisner C. M. and Castillo L. F. (2018). Prebiotics, bone and mineral metabolism. Calcif. Tissue Int. 102, 443–479. doi: 10.1007/s00223-017-0339-3
Wu Q., Kan J., Cui Z., Ma Y., Liu X., Dong R., et al. (2024). Understanding the nutritional benefits through plant proteins-probiotics interactions: mechanisms, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 0, 1–19. doi: 10.1080/10408398.2024.2369694
Xu R., Ding F. F., Zhou N. N., Wang T., Wu H. X., Qiao F., et al. (2022b). Bacillus amyloliquefaciens protects Nile tilapia against Aeromonas hydrophila infection and alleviates liver inflammation induced by high-carbohydrate diet. Fish Shellfish Immunol. 127, 836–842. doi: 10.1016/j.fsi.2022.07.033
Xu W., Lutz C. G., Taylor C. M., and Ortega M. C. (2022c). Improvement of fish growth and metabolism by Oligosaccharide prebiotic supplement. Aquac. Nutr 2022, 5715649. doi: 10.1155/2022/5715649
Xu H., Su Y., Zhang L., Tian T., Xu R., Sun H., et al. (2022a). Effects of dietary galactooligosaccharide on growth, antioxidants, immunity, intestinal morphology and disease resistance against Aeromons hydrophila in juvenile hybrid sturgeon (Acipenser baerii♀ × A. schrenckii♂). Aquac. Rep. 23, 1–11. doi: 10.1016/j.aqrep.2022.101097
Xu Y., Wang C., Zhang G., Tian J., Liu Y., Shen X., et al. (2017). ISCR2 is associated with the dissemination of multiple resistance genes among Vibrio spp. and Pseudoalteromonas spp. isolated from farmed fish. Arch. Microbiol. 199, 891–896. doi: 10.1007/s00203-017-1365-2
Yadav H., Lee J. H., Lloyd J., Walter P., and Rane S. G. (2013). Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 288, 25088–25097. doi: 10.1074/jbc.M113.452516
Yen M. T., Yang J. H., and Mau J. L. (2008). Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 74, 840–844. doi: 10.1016/j.carbpol.2008.05.003
Yi Y., Zhang Z., Zhao F., Liu H., Yu L., Zha J., et al. (2018). Probiotic potential of Bacillus velezensis JW: Antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish Shellfish Immunol. 78, 322–330. doi: 10.1016/j.fsi.2018.04.055
Yu H. H., Liang X. F., Chen P., Wu X. F., Zheng Y. H., Luo L., et al. (2019). Dietary supplementation of Grobiotic®-A increases short-term inflammatory responses and improves long-term growth performance and liver health in largemouth bass (Micropterus salmoides). Aquaculture 500, 327–337. doi: 10.1016/j.aquaculture.2018.10.033
Yuan X., Lv Z., Zhang Z., Han Y., Liu Z., and Zhang H. (2023). A review of antibiotics, antibiotic resistant bacteria, and resistance genes in aquaculture: occurrence, contamination, and transmission. Toxics 11 (5), 1–14. doi: 10.3390/toxics11050420
Zainab S. M., Junaid M., Xu N., and Malik R. N. (2020). Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 187, 116455. doi: 10.1016/j.watres.2020.116455
Zhang B. Y., Cai G. H., Yang H. L., Nie Q. J., Liu Z. Y., and Sun Y. Z. (2024). New insights on intestinal microorganisms and carbohydrate metabolism in fish. Aquac. Int. 32, 2151–2170. doi: 10.1007/s10499-023-01262-w
Zhang X., Jiang A., An S., Guo C., You F., Huang Z., et al. (2025). Dietary resistant starch supplementation improves the fish growth, lipid metabolism and intestinal barrier in largemouth bass (Micropterus salmoides) fed high-fat diets. Int. J. Biol. Macromol. 306, 141356. doi: 10.1016/j.ijbiomac.2025.141356
Zhang Y., Liang X. F., He S., Feng H., and Li L. (2022). Dietary supplementation of exogenous probiotics affects growth performance and gut health by regulating gut microbiota in Chinese Perch (Siniperca chuatsi). Aquaculture 547, 737405. doi: 10.1016/j.aquaculture.2021.737405
Zhou S., Song D., Zhou X., Mao X., Zhou X., Wang S., et al. (2019). Characterization of Bacillus subtilis from gastrointestinal tract of hybrid Hulong grouper (Epinephelus fuscoguttatus × E. lanceolatus) and its effects as probiotic additives. Fish Shellfish Immunol. 84, 1115–1124. doi: 10.1016/j.fsi.2018.10.058
Zhu L., Wang S., Cai Y., Shi H., Zhou Y., Zhang D., et al. (2023). Effects of Five Prebiotics on Growth, Antioxidant Capacity, Non-Specific Immunity, Stress Resistance, and Disease Resistance of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Animals 13 (4), 1–17. doi: 10.3390/ani13040754
Zhuge A., Li S., Han S., Yuan Y., Shen J., Wu W., et al. (2024). Akkermansia muciniphila-derived acetate activates the hepatic AMPK/SIRT1/PGC-1α axis to alleviate ferroptosis in metabolic-associated fatty liver disease. Acta Pharm. Sin. B 15, 151–167. doi: 10.1016/j.apsb.2024.10.010
Keywords: antioxidant capacity, antibiotics, disease resistance, prebiotics, probiotics, metabolism
Citation: Ng’onga L, Amoah K, Chen H, Huang Y, Wang B, Shija VM, Mpwaga AY, Fachri M, Cai J and Adjei-Boateng D (2025) The metabolism and antioxidant properties of probiotics and prebiotics in fish: a review. Front. Mar. Sci. 12:1622474. doi: 10.3389/fmars.2025.1622474
Received: 03 May 2025; Accepted: 13 August 2025;
Published: 04 September 2025.
Edited by:
Luodong Huang, Guangxi University, ChinaReviewed by:
Sofia Priyadarsani Das, National Taiwan Ocean University, TaiwanAmit Ranjan, Tamil Nadu Fisheries University, India
Copyright © 2025 Ng’onga, Amoah, Chen, Huang, Wang, Shija, Mpwaga, Fachri, Cai and Adjei-Boateng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kwaku Amoah, YW1vYWhrMjAxMEB5YWhvby5jb20=; Jia Cai, bWF0cml4OTI0QGZveG1haWwuY29t
 Lishuko Ng’onga
Lishuko Ng’onga Kwaku Amoah
Kwaku Amoah Huapu Chen1,2,3,4,5,6
Huapu Chen1,2,3,4,5,6 Yu Huang
Yu Huang Bei Wang
Bei Wang Vicent Michael Shija
Vicent Michael Shija Alatwinusa Yohana Mpwaga
Alatwinusa Yohana Mpwaga Muhammad Fachri
Muhammad Fachri Jia Cai
Jia Cai