Abstract
Introduction:
Fish oil is a key component in aquatic feeds due to its high energy content, essential omega-3 fatty acids, and rich nutrient profile. However, its high cost necessitates sustainable alternatives. This study investigated the effects of dietary lysophospholipids (LPLs) on growth performance, digestive enzyme activity, and the expression of lipid metabolism-related genes in Pacific white shrimp (Litopenaeus vannamei).
Methods:
Juvenile shrimp (n = 400; initial weight: 1.55 ± 0.02 g) were randomly assigned to four dietary treatments in a 2 × 2 factorial design with four replicates per treatment: a positive control (PC) with 2% fish oil, a negative control (NC) with 1% fish oil, and two LPL-supplemented groups (NC+0.03% and NC+0.06% LPLs). After 8 weeks of feeding, growth performance, digestive enzyme activity, and gene expression related to lipid metabolism were evaluated.
Results:
LPL supplementation (0.03% and 0.06%) significantly improved almost growth parameters such as total production, weight gain rate, specific growth rate, feed conversion ratio and protein efficiency ratio compared with the NC group (P< 0.05). The PC group showed significant improvement only in specific growth rate, feed conversion ratio, and protein efficiency ratio. Lipase activity was significantly higher in both LPL groups and the PC group than in the NC group, while protease, trypsin, and chymotrypsin activities were highest in the NC+0.06% LPLs group (P< 0.05). Amylase activity was significantly higher in the PC and NC+0.06% groups compared to others. LPL supplementation also significantly upregulated lipid metabolism genes, lipase (Lip) and delta fatty acid desaturase 6 (dFAD-6), along with the antioxidative stress gene superoxide dismutase (SOD) compared to NC group. In contrast, the PC group showed significant upregulation only of SOD compared to the NC group.
Discussion:
Dietary inclusion of 0.03%–0.06% LPLs effectively enhanced growth performance, nutrient utilization, and lipid metabolism, offering potential to reduce fish oil use by 1% in shrimp diets. These findings provide a basis for improving feed efficiency and sustainability in shrimp aquaculture.
Introduction
Marine fish oil is rich in high-energy and essential omega-3 fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). These fatty acids play vital roles in cell membrane structure and function, which are essential for the growth, health, and reproduction of aquatic species (NRC, 2011). Fish oil also contains other essential nutrients, such as cholesterol and phospholipids, which improve lipid digestibility and contribute to superior growth performance and efficient nutrient utilization in aquatic animals, especially crustaceans. Cholesterol, a precursor of steroid hormones and bile acids, constitutes a key component of the cell membrane (Liang et al., 2023). Bile acids and phospholipids, key components of cell membranes, act as natural emulsifiers that enhance lipid digestion and absorption in aquatic animals (Lordan and Blesso, 2023; Lee et al., 2024). However, the availability of marine fish oil in the aquaculture industry is declining owing to increasing consumer demand. Moreover, the impacts of climate change have led to increases in the cost of fish oil, making it less economically viable (IFFO, 2024). Therefore, it is crucial to explore sustainable alternative oils for aquatic feed formulations.
Currently, alternative oils, such as soybean oil, are increasingly being utilized to partially replace fish oil in commercial aquatic feed, offering a sustainable and cost-effective solution (Hodar et al., 2020; Radhakrishnan et al., 2024). Previous studies have shown that combining dietary soybean oil with fish oil in freshwater fish feed can support optimal growth performance and feed utilization without adverse effects. For example, soybean oil can replace a significant portion of fish oil in the diets of various freshwater fish species, such as tilapia and carp, without compromising growth rate or feed efficiency (Xu et al., 2016; Oliva-Teles et al., 2022). However, several factors must be considered when using soybean oil in marine shrimp diet. Unlike freshwater fish, marine shrimp has specific nutritional requirements and physiological characteristics that make the effects of soybean oil less predictable and dependent on various conditions. For example, Pacific white shrimp (Litopenaeus vannamei) require specific long-chain polyunsaturated fatty acids, such as EPA and DHA, which are abundant in fish oil but not in soybean oil (Lima and Figueiredo-Lima, 2016). Additionally, marine shrimp has a unique digestive system with a short gut passage period and limited bile acid synthesis. Therefore, it is necessary to use fish oil because it is more easily digested and absorbed than soybean oil (Li et al., 2023). Moreover, fish oil provides a more comprehensive nutrient profile that supports optimal growth and health in marine shrimp than soybean oil (NRC, 2011). Although complete replacement of fish oil with vegetable oils reduced shrimp growth due to essential fatty acid (EFA) imbalances (Xie et al., 2020), partial substitution with soybean oil has shown no adverse effects (Chen et al., 2022; Xu et al., 2016). Diets containing soybean oil or soybean and lecithin oil combined with fish oil (2% total dietary lipid) showed higher survival rates than those fed 3% fish oil or 3% soybean oil without soy lecithin or phospholipids (Hu et al., 2011). Although combined administration of fish oil, soybean oil, and soy lecithin did not significantly affect growth performance, it improved survival rate, total cholesterol, and phospholipid concentrations in the hemolymph. Lecithin and phospholipids are natural emulsifiers generally used in animal feed, especially for shrimp. Phospholipids consist of two lipophilic fatty acid tails, which play key roles as efficient emulsifiers under water-in-oil conditions. However, phospholipids function as weak emulsifiers in oil-in-water emulsions, such as those in the digestive tract of shrimp (Lin et al., 2021).
In the animal feed industry, several emulsifiers are used to enhance lipid digestion and absorption of essential nutrients. Key emulsifiers, such as phospholipids, lysophospholipids (LPLs), bile salts, glycerol polyethylene glycol ricinoleate (GPEGR), and sodium steroyl-2-lactylate (SSL), help improve growth performance and feed efficiency, especially in young terrestrial animals and crustaceans with limited bile acid- and lipase-producing ability (Siyal et al., 2017; Saleh et al., 2020). LPLs are natural emulsifiers derived from the enzymatic hydrolysis of phospholipids, which removes one fatty acid tail. LPLs are more hydrophilic than phospholipids and exhibit enhanced emulsification under oil-in-water conditions for lipid digestion in the hepatopancreas and midgut of shrimp (Lin et al., 2021). Additionally, the key characteristics of effective emulsifiers are assessed based on the hydrophilic–lipophilic balance (HLB), critical micelle concentration (CMC), emulsion stability index (ESI), safety, non-cytotoxicity, and cost-effectiveness (Nayak et al., 2021; Ravera et al., 2021). In terms of emulsifying properties, higher HLB values indicate enhanced hydrophilicity, which facilitates the formation of micelles that are essential for emulsification and solubilization processes. Generally, LPLs, phospholipids, bile acids, GPEGR, and SSL have HLB values of approximately 10–20, 4–6, 18, 12–14, and 10–12, respectively, indicating the superior hydrophilicity of LPLs (Smejkal et al., 2024; Van Hoogevest, 2017). A comparison of CMC values, the concentration of each emulsifier at which micelles start to form, showed that LPLs have a lower CMC value (0.01–0.1 mM) (Alkademi, 2020) than phospholipids (0.3–2 mM) (Khan et al., 2018), bile acids (2–20 mM) (Pavlović et al., 2018), GPEGR (0.531 mM) (Held, 2014), and SSL (0.214 mM) (Wang and Marangoni, 2015). Additionally, ESI indicates the ability of the compound to maintain stable emulsions. A high ESI can promote digestive enzymes, especially lipase, to break down fats and other nutrients in the digesta by reducing the size of fat droplets and increasing their surface area. LPLs are key emulsifiers with high ESIs of approximately 200–300 min and strong emulsion stability (Calvano et al., 2020). In contrast, other emulsifiers, such as bile acids, GPEGR, and SSL, generally have lower ESIs of approximate 150–250 min (Shen et al., 2022), 150–250 min (Li et al., 2021), and 100–200 min (Li et al., 2019), respectively. Additionally, safety, cost effectiveness, and sustainability should be considered when selecting emulsifiers for shrimp feed.
LPLs have advantages, including cost effectiveness, safety, non-cytotoxicity, compatibility with various biological systems, and sustainability. LPLs have been studied for their numerous benefits in shrimp, such as increased nutrient digestion and absorption, improved feed utilization, and improved growth performance and feed efficiency. For example, the use of LPLs promoted emulsifying capacity, improves fatty acid digestibility, and increases productivity in tiger shrimp (Penaeus monodon) (Khan et al., 2018). Therefore, LPLs could replace dietary emulsifiers in the diet of Pacific white shrimp (Lin et al., 2021). For example, replacement of 1%–2% dietary soybean lecithin oil with 0.1% dietary LPLs improved survival rate, growth performance, and immunity in oriental river prawn (Macrobrachium nipponense) (Guoliang et al., 2023). Additionally, LPL supplementation enhanced the antioxidant system and color in shrimp, suggesting that LPLs could enhance emulsification and promote cholesterol transport and dietary astaxanthin efficiency, resulting in improved growth, survival rate, stress resistance, and pigmentation in juvenile Pacific white shrimp (Song et al., 2024a).
Despite the benefits of LPLs, the optimal supplementation level necessary to maximize growth performance and nutrient utilization in shrimp fed fish oil-reduced diets remains unknown. Additionally, the long-term effects of LPL supplementation on shrimp health and the overall sustainability of using LPLs as substitutes for fish oils are not fully understood. Therefore, the aim of this study was to investigate the effects of varying LPLs levels in partial fish oil reduction diet on shrimp growth and nutrient absorption, especially digestive enzyme activity and the expression of genes related to lipid metabolism. Overall, it is anticipated that this study will provide valuable insights on strategies to reduce fish oil dependence in aquafeeds.
Materials and methods
Ethical approval
All animal experiments in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Kasetsart University, Thailand (ACKU 61-FIS-040).
Experimental diets
Four isonitrogenous diets were formulated to contain 2% dietary fish oil and 1.5% dietary soybean oil as positive control (PC), 1% dietary fish oil and 1.5% dietary soybean oil as negative control (NC), NC supplemented with 0.03% lysophospholipids (Lypotech EC., BIS Factory, Samut Sakhon, Thailand) as NC+0.03% LPLs, NC supplemented with 0.06% lysophospholipids (Lypotech EC., BIS Factory, Samut Sakhon, Thailand) as NC+0.06% LPLs. Briefly, the coarse ingredients, including fish meal, poultry by-product meal, squid meal, dehulled soybean meal, fermented soybean meal, rice bran, wheat flour, wheat gluten meal, synthetic amino acids, vitamins, minerals, premix-preservation, and other components, were ground using a hammer mill, passed through a 250 μm mesh, and mixed for 10 min. Tuna oil, soybean oil, lecithin, and water were gradually added until homogenous and then added lysophospholipids (Lypotech EC.) into the negative diets at the levels as shown in Table 1, using a mechanical mixer for 10 minutes (second mixing). Subsequently, 2.5% water was added to the mixture to form a paste, which was manually kneaded into a uniform dough. Thereafter, the dough was pelleted using a Mincer machine with a 1.5 mm die and steamed at 100°C for 15 min. After steaming, the experimental samples were dried at 80°C for 6 h in a hot air oven to maintain a moisture content<11%. Finally, the samples were cooled, sealed in plastic bags, and stored at room temperature (25°C) until further use. Additionally, the proximate compositions of the feeds were analyzed using the AOAC, 2023 method (AOAC, 2023), and the results are presented in Table 1.
Table 1
| Ingredient (%) | PC | NC1 | NC+0.03% LPLs | NC+0.06% LPLs |
|---|---|---|---|---|
| Plant-based protein | 41.00 | 41.00 | 41.00 | 41.00 |
| Animal protein by-product | 24.00 | 24.00 | 24.00 | 24.00 |
| Wheat flour | 24.00 | 24.00 | 24.00 | 24.00 |
| Broken rice | 2.33 | 3.33 | 3.30 | 3.27 |
| Fish oil | 2.00 | 1.00 | 1.00 | 1.00 |
| Soya oil | 1.50 | 1.50 | 1.50 | 1.50 |
| Soya lecithin | 1.00 | 1.00 | 1.00 | 1.00 |
| Total synthetic amino acids | 0.22 | 0.22 | 0.22 | 0.22 |
| Micronutrientsa | ||||
| (Vitamins & Minerals) | 3.65 | 3.65 | 3.65 | 3.65 |
| Lypotech ECb | 0.00 | 0.00 | 0.03 | 0.06 |
| Pellet binder | 0.20 | 0.20 | 0.20 | 0.20 |
| Antioxidant | 0.10 | 0.10 | 0.10 | 0.10 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Chemical composition | ||||
| (% dry matter) | ||||
| Moisture | 10.94 | 10.59 | 10.66 | 10.75 |
| Crude protein | 40.38 | 40.34 | 40.35 | 40.29 |
| Crude lipid | 6.93 | 5.91 | 5.96 | 5.93 |
| Gross energy (Kcal/kg) | 4,372.41 | 4,323.91 | 4,323.38 | 4,323.88 |
| Cholesterol analysis (ppm) | ||||
| Cholesterol concentration | 951.41 | 904.29 | 903.86 | 903.78 |
The experimental ingredients and proximate composition of shrimp diets.
Micronutrient premix-preservation are supplied by DSM Nutritional Products (Thailand) Ltd. and had the following component; Vitamin and mineral mix (supplements per kg of the mixed feed): vitamin A, 3,500 IU; vitamin D3, 1,500 IU; vitamin E, 75 mg; vitamin B1, 12.5 mg; vitamin B2, 10 mg; vitamin B6, 12.5 mg; vitamin B12, 10 µg; vitamin K3, 15 mg; ascorbic acid, 100 mg; Niacin, 50 mg; Pantothenic acid, 40 mg; folic acid, 5 mg; biotin, 500 µg; Fe, 15 mg; Mn, 15 mg; Zn, 50 mg; I, 0.5 mg; Cu, 12.5 mg; Se, 0.175 mg; Co, 0.1 mg.
Lysophospholipids (LPLs) used in this study were supplied by Bioscience Animal Health Public Company Limited (BIS Factory, Samut Sakhon, Thailand). The product contained 4% available lysophosphatidylcholine.
Shrimp and feeding trial
Briefly, the animal experiments were organized in a 2 × 2 factorial design consisting of four treatments and four replicates. Animal experiments were conducted at the Aquatic Animal Nutrition and Aquafeed Laboratory, Department of Aquaculture, Faculty of Fisheries, Kasetsart University, Thailand. Healthy juvenile (30 to 45 days post-larval stage) Pacific white shrimp (Litopenaeus vannamei) were obtained from commercial hatcheries in Samut Songkhram Province, Thailand, and acclimatized in a 1-cubic-ton fiberglass tank with commercial diets for 14 days. The juvenile shrimp were fasted for 8 h before the experiment began. F-test ANOVA power analysis using G*Power indicated that a minimum of 279 shrimp was required to detect a medium effect size (f = 0.25) at a significance level of α = 0.05 and a statistical power of 0.95. To account for expected variability and ensure robust statistical confidence, 400 healthy shrimp (initial weight, 1.55 ± 9.93 g per individual) were randomly distributed into four treatments with four replicates each (25 shrimp per replicate). Specifically, the shrimp were reared in 16 aquariums (0.53 m × 1.22 m × 0.37 m) at a density of 140 shrimp/m³ under a 12 h/12 h light/dark cycle and fed four times per day (08:00, 12:00, 16:00, and 20:00) to apparent satiation until the end of the experiment. Notably, the feeding rate started at 12% of the shrimp’s body weight and was monitored and adjusted every 2 weeks to optimize satiation feeding. Water quality was measured and monitored every 2 days, maintaining key parameters such as salinity at 12–13 ppt, dissolved oxygen concentration ≥5 mg/L, pH 7.5–8.5, temperature 28–30°C, and nitrite concentration< 0.5 mg/L. During the 8-week experimental trial, approximately 60% of the water was changed daily to maintain water quality. Every 2 weeks, three shrimp from each replicate were randomly weighed to evaluate growth performance. Additionally, the total amount of feed consumed by each replicate was recorded every 2 weeks to calculate some parameters at the end of the experiment. At the end of the 8-week period, all shrimp in each aquarium were pooled and weighed to record their performance and survival rates.
Sample collection
After the 8-week experiment, all the shrimp in each aquarium were fasted for 12 h to prepare them for sample collection. After anesthetizing in an ice bath for 10 min, all shrimp in each bucket were counted and weighed to determine growth parameters, feed utilization, and survival rates. Hemolymph samples were collected from six shrimp in each aquarium to analyze the cholesterol concentration. Hemolymph was collected from the ventral sinus using 1.0 mL syringes, with sodium citrate as an anticoagulant in a 1:1 ratio, and then pooled into an Eppendorf tube. Muscle samples were collected from the six shrimp to determine cholesterol concentration and nutrient profile. Muscle moisture, crude protein, and crude fat contents were determined using the AOAC, 2023 method (AOAC, 2023). Additionally, hepatopancreas samples were collected from the six shrimp and stored at −20°C for cholesterol concentration analysis. Another set of hepatopancreas samples from four shrimp were classified and sorted as pre-molt (D4) based on the appearance of the epidermis, pigmentation, formation of new setae, and the presence of matrix or internal cones in the setal lumen, as observed under a light microscope at ×400 magnification (Taneerat et al., 2023). Each hepatopancreas tissue was fixed in RNAlater (Qiagen, Hilden, Germany) and immediately preserved at −80°C for RNA extraction to analyze mRNA expression. Finally, hepatopancreas samples were collected from the remaining three shrimp in each aquarium and stored at −20°C in a refrigerator for analysis of digestive enzyme activity.
Cholesterol concentration analysis
Feed, hemolymph, hepatopancreas, and shrimp samples were extracted using chloroform/methanol, according to a modified method by Lee et al (Lee et al., 2014). Briefly, each sample was weighed into a 4-ml glass vial with a polytetrafluoroethylene (PTFE) screw cap, followed by the addition of 750 µl of absolute methanol. After vortexing for 1 min, 1500 µl of chloroform was added, followed by homogenization for 1 h using a multichannel stirrer. To partition the chloroform and methanol, 625 µl of deionized water was added, and the vial was centrifuged at 2500 × g for 10 min. Thereafter, the cholesterol content in the lower chloroform layer containing the lipid extract was analyzed using a gas chromatography-single quadrupole mass spectrometer (GCMS-QP2020 NX, Shimadzu, Japan) coupled with a PAL autosampler system (CTC Analytics AG, Switzerland), following previously described procedures (Chinarak et al., 2022). Specifically, the chloroform extract was transferred into a 2-ml glass vial, followed by the addition of 50 µl of myristic acid-d27 as an internal standard (IS). Thereafter, the vial containing the extracted sample and IS was dried under vacuum at 60°C for 30 min using a vacuum centrifuge (Concentrator plus, Eppendorf, USA) to remove the residual solvent. Pyridine (50 µl) and 50 µl of N-trimethyl-N-methyl trifluoroacetamide containing 1% trimethylchlorosilane (MSTFA + 1% TMCS) were added to the dried sample, followed by incubation at 70°C for 30 min to complete derivatization. After cooling to room temperature, 1 µl of the derivatized sample was injected in split mode with a 1:200 split ratio at 250°C into a DB-5MS + DG column (30 m, 0.25 mm i.d., Agilent J&W GC column). Helium was used as the carrier gas at a constant flow rate of 1 ml/min. The oven temperature program started at 60°C for 1 min, then ramped up to 325°C at 10°C/min and held for 1 min. The transfer line, ion source, and quadrupole temperatures were maintained at 290, 240, and 150°C, respectively. The mass spectrometer was operated in the selected ion monitoring (SIM) mode. The quantifier, qualifier, and retention times for myristic acid-d27-TMS and cholesterol-TMS derivatives were m/z 73.1, m/z 119.1, RT = 15.99 min, and m/z 128.9, m/z 328.9, RT = 26.78 min, respectively.
Digestive enzyme activity assays
Enzyme extracts were prepared to determine lipase, protease, trypsin, chymotrypsin, and amylase activities. Briefly, hepatopancreas samples from each group were weighed and homogenized on ice in 0.01 M Tris buffer (pH 7.5) at a tissue-to-buffer ratio of 1:3 (w/v) for enzyme extraction. Following homogenization, each sample was centrifuged twice at 12,000 × g for 15 min at 4°C. Thereafter, supernatants containing crude enzymes were collected and stored at −20°C for subsequent analysis of digestive enzyme activity. Total protein and digestive enzyme activities were measured using a spectrophotometer.
Briefly, the total protein concentration of the enzyme extract was determined via the Folin reaction using the Lowry assay. Protein quantification in the alkaline solution was performed at 750 nm using a spectrophotometer. Bovine serum albumin was used as the standard for analyzing protein levels in this study (Lowry et al., 1951).
Lipase activity (U mg−¹ protein) was determined following a previously described method (Markweg-Hanke et al., 1995). Briefly, a 20 µl aliquot of crude enzyme extract was mixed with 880 µl of 0.1 M p-nitrophenylpalmitate substrate in a pH buffer, followed by incubation at room temperature for 30 min. After stopping the reaction by adding 400 µl of 0.1 mM Na2CO3, the sample was centrifuged at 4,000 rpm for 10 min and the absorbance of the supernatant was measured at 420 nm using a spectrophotometer. Notably, one unit of lipase activity (U mg−¹ protein) was defined as the amount of enzyme that released 1 mM of p-nitrophenol per minute per milligram of protein.
Protease activity (U mg−¹ protein) was measured using a previously described modified casein method (Pan et al., 2005). A 20 µl aliquot of crude enzyme extract was mixed with 250 µl of 2% casein substrate dissolved in a pH buffer, followed by incubation at room temperature for 10 min. After stopping the reaction by adding 1 ml of 1.2 M trichloroacetic acid, the sample was centrifuged at 10,000 rpm for 5 min. Thereafter, the supernatant was mixed with 1 ml of 0.4 N NaOH, followed by incubation at 40°C for 10 min. After incubation, 200 µl of 50% Folin reagent was added to the mixture and incubated at room temperature for 10 min. Finally, protease activity was measured at 660 nm using a spectrophotometer. Tyrosine production was determined using Folin-hydroxybenzene. One unit of protease activity (U mg−¹ protein) was defined as the amount of enzyme that liberated 1 mM of tyrosine per minute through casein hydrolysis.
Additionally, trypsin activity (U mg−¹ protein) was evaluated using a previously described method (Sunde et al., 2001), with modifications (Torrissen et al., 1994). Briefly, a 5 µl aliquot of crude enzyme extract was mixed with 1 ml of trypsin substrate (1.25 mM benzoyl-L-arginine-p-nitroanilide in 5% dimethylformamide within pH buffer solution). The rate of p-nitroaniline production was measured from 0–15 s at an absorbance of 410 nm to determine trypsin activity using a spectrophotometer. One unit of trypsin activity (U mg−¹ protein) was defined as the amount of enzyme that produced 1 mM of p-nitroaniline per minute per milligram of protein.
Moreover, chymotrypsin activity (U mg−¹ protein) was measured using a reaction mixture containing 1,000 µl of chymotrypsin substrate (0.1 mM succinyl-(Ala)2-Pro-Phe-p-nitroanilide [SAPNA]), 5% dimethylformamide, and 50 mM Tris-HCl with 20 mM CaCl2 (pH 7.5), according to a previously described method (Garcia-carreno et al., 1994). Crude enzyme extract (5 µl) was added to the solution. Thereafter, the rate of p-nitroaniline production was measured from 0–15 s at an absorbance of 410 nm using a spectrophotometer. One unit of chymotrypsin activity (U mg−¹ protein) was defined as the amount of enzyme that produced 1 mM of p-nitroaniline per minute per milligram of protein.
Furthermore, amylase activity (U mg−¹ protein) was measured using starch as the substrate to evaluate the increase in reducing sugar (maltose) from the hydrolysis of α-D (1,4) glycosidic bonds in polysaccharides, stained with 3,5-dinitrosalicylic acid (DNS) according to a previous method (Bernfeld, 1955). Starch was prepared as a substrate by boiling a 1% soluble starch solution for 10 min in a pH buffer (2–12) with 60 µl of 6 mM NaCl. Thereafter, a 20 µl aliquot of crude enzyme extract was mixed with 250 µl of substrate solution and the mixture was incubated at room temperature for 5 min. After stopping the reaction by adding 250 µl of 1% DNS, the sample was heated in a boiling water bath for 5 min and allowed to cool. Distilled water (1.5 mL) was added to the final solution. Finally, amylase activity was measured at 550 nm using a spectrophotometer. One unit of amylase was defined as the amount of enzyme that produced 1 mM of maltose per minute per milligram of protein.
RNA extraction and mRNA expression analysis
Total RNA was extracted from hepatopancreas using the RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA quality and yield were determined using a 1.0% denaturing agarose gel and Nanodrop ND-1000 spectrophotometer (NanoDrop, Wilmington, Delaware), respectively. Thereafter, the RNA samples were treated with RNase-free DNase (Qiagen, Hilden, Germany) to remove contaminating DNA. Gene expression was analyzed using reverse transcription quantitative PCR (RT-qPCR) on the Eco™ Real-Time PCR System (Illumina, San Diego, CA, USA). The reactions were performed using the SuperScript™ III Platinum® SYBR® Green One-Step qRT-PCR Kit (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instructions. Both cDNA synthesis and PCR were performed in a single tube using gene-specific primers and either total RNA or mRNA. PCR was using 50 µl of reaction reagent containing 25 µl of 2X SYBR® Green Reaction Mix, 1 µl of SuperScript® III RT/Platinum® Taq Mix, 1 µl of each primer (forward and reverse primer,10 µmol), 20 µl DEPC-treated water, and 2 µl of template (200 µg total RNA). The PCR conditions were as follows: initial denaturation at 50°C for 3 min; 95°C for 5 min; followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 55°C for 30 s, and 40°C for 1 min; and melting curve analysis. Notably, the mRNA expression levels of lipid metabolism- and antioxidative stress-related genes were normalized to that of the housekeeping gene β-actin (Gao et al., 2023). Four replicates from each treatment were performed for each target gene. The real-time PCR primers used in this study are listed in Table 2. The mRNA expressions of the target genes were calculated using the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Table 2
| Primer | (5´–3´) | Nucleotide sequence | GenBank No. |
|---|---|---|---|
| β-actin | Forward | CCACGAGACCACCTACAAC | AF300705.2 |
| (Housekeeping) | Reverse | AGCGAGGGCAGTGATTTC | |
| Lip | Forward | GACGAGCACCCTTGTTCCTA | NW 020868542.1 |
| Reverse | AGGTGGATGATGCCAAACTC | ||
| dFAD-6 | Forward | TGTAGCTTCTCCAGCGTGTG | SRP048814 |
| Reverse | CTCTTCCCTTCCATCCATGA | ||
| HK | Forward | AATGCCACTGATGCTGACTG | NW 020871341.1 |
| Reverse | TTGTGGAAGTGTGGATGGAA | ||
| GPx | Forward | CCAAAGTGCATCATTTGGAC | NW 020870204.1 |
| Reverse | CAGCAAGTTTGCGATTTCAT | ||
| SOD | Forward | TTAGTGGGACCTCGTACGGT | NW 020870204.1 |
| Reverse | CTCAAGCGTGACCTATGACC |
Primers used for quantitative real-time PCR.
Growth performance analysis
The following growth performance and feed utilization parameters were measured in this study:
Statistical analysis
All data analyses, including tests of homogeneity and group comparisons, were performed using SPSS Statistics software (version 29.0; IBM, Armonk, NY, USA). Significant differences were determined using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test. Statistical significance was set at P< 0.05 and indicated in all tables and figures using alphabetical notations. Data are presented as mean ± standard deviation (SD).
Results
Growth performance and nutrient utilization
Table 3 shows the growth performance and nutrient utilization parameters. Shrimp fed NC diet supplemented with 0.03% LPLs (NC+0.03%LPLs) and 0.06% LPLs (NC+0.06%LPLs) had significantly higher (P< 0.05) total production (Tprod), weight gain rate (WGR), specific growth rate (SGR), and protein efficiency ratio (PER) and significantly lower feed conversion ratio (FCR) than those in the NC group. However, there were no significant differences (P > 0.05) in all growth performance and nutrient utilization parameters between the PC, NC+0.03% LPLs, and NC+0.06% LPLs groups. Additionally, there was no significant difference (P > 0.05) in survival rate (SR) among the experimental groups.
Table 3
| Parameters | PC | NC | NC+0.03% LPLs | NC+0.06% LPLs |
|---|---|---|---|---|
| Tprod (g/aquarium) | 225.27 ± 11.44ab | 203.28 ± 25.43a | 239.15 ± 11.78b | 243.49 ± 13.13b |
| ADG (g/day) | 0.173 ± 0.013 | 0.158 ± 0.010 | 0.184 ± 0.010 | 0.184 ± 0.019 |
| WGR (%) | 480.86 ± 13.63ab | 423.89 ± 59.78a | 512.95 ± 24.10b | 535.46 ± 38.55b |
| SGR (%) | 3.05 ± 0.04b | 2.84 ± 0.22a | 3.14 ± 0.07b | 3.21 ± 0.11b |
| SR (%) | 81.33 ± 4.99 | 74.67 ± 4.99 | 82.67 ± 1.89 | 84.00 ± 5.66 |
| FCR | 1.44 ± 0.02a | 1.74 ± 0.24b | 1.33 ± 0.07a | 1.31 ± 0.08a |
| PER | 1.72 ± 0.02b | 1.45 ± 0.19a | 1.86 ± 0.09b | 1.90 ± 0.12b |
Growth performance and nutrient utilization of Pacific white shrimp (Litopenaeus vannamei) fed the experimental diets for 8 weeks.
Data was presented as mean ± standard deviation (SD). Means with different superscript letters in the same row are significantly different at P< 0.05.
Nutrient profile of shrimp muscle
As shown in Table 4, shrimp in the NC+0.06% LPLs group had the highest (P< 0.05) crude protein among the groups. Additionally, crude protein was significantly higher in the NC+0.03% LPLs group than in the PC and NC groups. In contrast, crude fat content was highest (P< 0.05) in the NC+0.03% LPLs group among the groups. Additionally, crude fat content was significantly higher in the NC+0.06% LPLs group than in the PC and NC groups (P< 0.05).
Table 4
| Parameters | PC | NC | NC+0.03% LPLs | NC+0.06% LPLs |
|---|---|---|---|---|
| Muscle composition (%) | ||||
| Crude protein | 20.88 ± 0.77a | 20.97 ± 0.57a | 22.33 ± 2.31b | 22.67 ± 2.31c |
| Crude fat | 0.825 ± 0.049b | 0.795 ± 0.021a | 0.850 ± 0.053d | 0.840 ± 0.071c |
| Moisture | 76.12 ± 0.02 | 76.67 ± 0.02 | 76.26 ± 0.01 | 76.18 ± 0.04 |
| Cholesterol concentration (ppm) | ||||
| Hepatopancreas | 1232.19 ± 145.78a | 1298.37 ± 128.86a | 1669.66 ± 98.74b | 1336.37 ± 65.30a |
| Hemolymph | 132.43 ± 4.73c | 79.91 ± 16.81a | 108.38 ± 7.77b | 124.74 ± 15.65bc |
| Muscle | 1662.32 ± 115.64 | 1725.07 ± 68.57 | 1571.52 ± 102.20 | 1703.46 ± 41.89 |
| Whole body | 2970.75 ± 110.82a | 3068.86 ± 158.76ab | 3316.60 ± 49.04c | 3174.61 ± 67.17bc |
Nutrient accumulation in muscle and cholesterol content of Pacific white shrimp (Litopenaeus vannamei) fed the experimental diets for 8 weeks.
Data were presented as mean ± standard deviation (SD). Means with different superscript letters in the same row are significantly different at P< 0.05.
Cholesterol content in hemolymph, hepatopancreas, and muscle
Table 4 shows the effects of dietary LPLs on cholesterol levels in the hemolymph, hepatopancreas, and muscle. Among the groups, the highest cholesterol content (P< 0.05) in the hepatopancreas was observed in the NC+0.03% LPLs group, whereas the lowest cholesterol content (P< 0.05) in the hemolymph was observed in the NC group. Additionally, shrimp in the PC, NC+0.03% LPLs, and NC+0.06% LPLs had significantly higher (P< 0.05) cholesterol levels in the hemolymph than those in the NC group. Moreover, shrimp in the NC+0.03% LPLs group had significantly higher cholesterol content in the whole body than those in the PC group (P< 0.05). Notably, there was no significant difference (P > 0.05) in muscle cholesterol content among the groups.
Digestive enzyme activity in the hepatopancreas
In this study, we examined the activities of digestive enzymes in the hepatopancreas (Figures 1). Lipase activity in the hepatopancreas was significantly higher (P< 0.05) in the PC, NC+0.03% LPLs, and NC+0.06% LPLs groups than in the NC group (Figure 1). Protease and trypsin activities in the hepatopancreas were significantly higher (P< 0.05) in the NC+0.06% LPLs group than in the other groups. Chymotrypsin activity was significantly higher (P< 0.05) in the NC+0.06% LPLs group than in the NC, NC+0.03% LPLs, and PC groups. Additionally, chymotrypsin activity was significantly higher (P< 0.05) in the NC group than in the NC+0.03% LPLs and PC groups. Moreover, amylase activity in the hepatopancreas was significantly higher (P< 0.05) in the PC and NC+0.06% LPLs groups than in the NC+0.03% LPLs and NC groups.
Figure 1
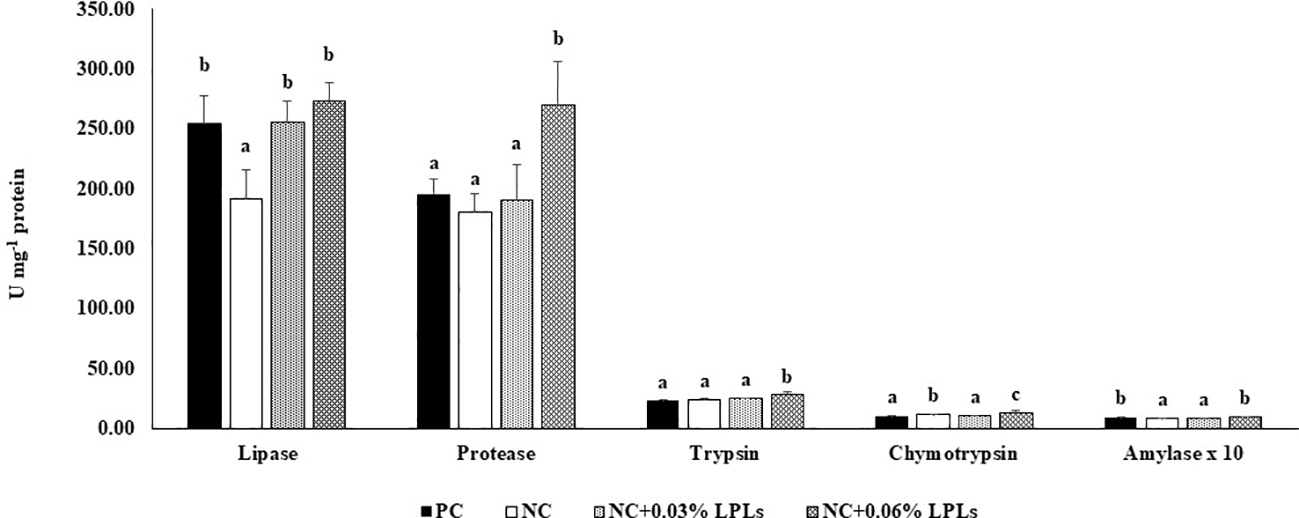
Activities of the digestive enzymes lipase, protease, trypsin, chymotrypsin, and amylase in the hepatopancreas of Pacific white shrimp fed the experimental diets for 8 weeks. Data are presented as mean ± standard deviation (SD). Means with different superscript alphabets are significantly different at P< 0.05.
mRNA expression of lipid metabolism- and antioxidative stress-related genes
Figure 2 shows the effects of dietary LPLs on the mRNA expression of lipid metabolism and antioxidative stress genes. Compared with those in the PC and NC groups, there was a significant increase in the mRNA expression of Lip and dFAD6 genes in the NC+0.03% LPLs and NC+0.06% LPLs groups (P< 0.05). However, there were no significant differences (P > 0.05) in the expression levels of these genes between the NC+0.03% LPLs and NC+0.06% LPLs groups. Additionally, there were no significant differences (P > 0.05) in the mRNA expression of the lipid metabolism gene hexokinase (HK) and the antioxidative stress-related gene glutathione peroxidase (GPx) among the groups. Importantly, LPL supplementation (NC+0.03% LPLs and NC+0.06% LPLs) and the positive control treatment (PC group) significantly upregulated (P< 0.05) the mRNA expression of the antioxidative stress enzyme superoxide dismutase (SOD) gene compared with that in the NC group. However, there was no significant difference in SOD gene expression between the LPL supplementation groups and the PC group (P > 0.05).
Figure 2
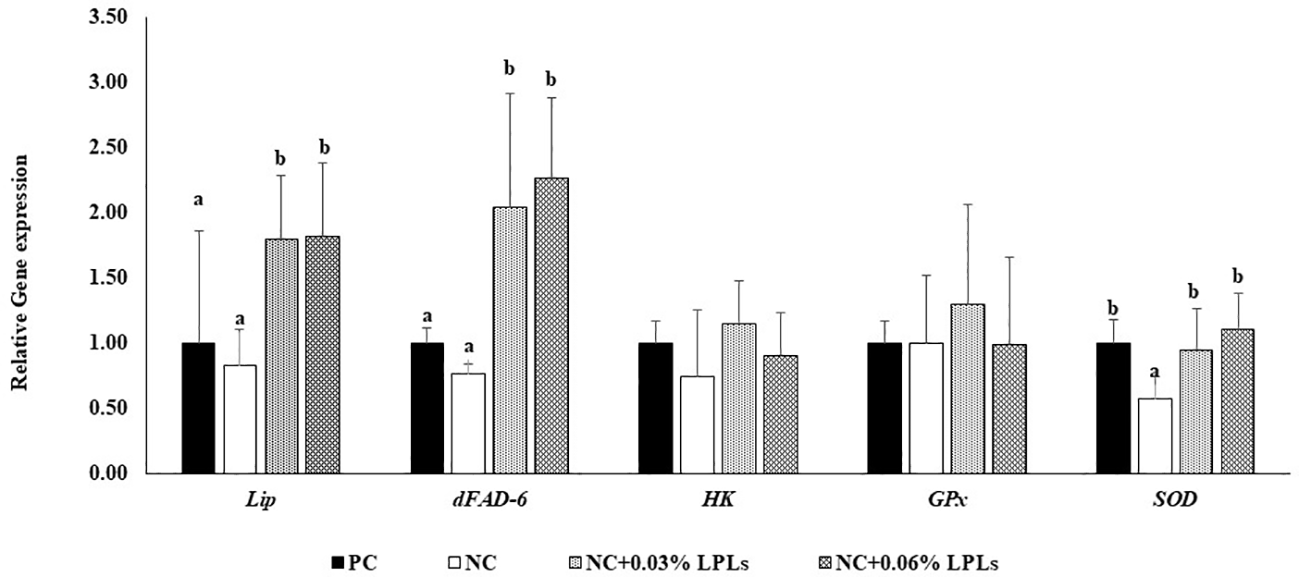
mRNA expression levels of genes involved in lipid metabolism and antioxidant response in the hepatopancreas of Pacific white shrimp fed the experimental diets for 8 weeks. Data are presented as mean ± standard deviation (SD). Means with different superscript alphabets are significantly different at P< 0.05. The relative expression levels of the target genes were normalized to that of β-actin. Lip, lipase; dFAD-6, delta 6-fatty acid desaturase; HK, hexokinase; GPx, glutathione peroxidase; SOD, superoxide dismutase.
Discussion
In this study, we hypothesized that supplementing an energy-low diet with LPLs would compensate for the optimal nutrients required for growth performance in shrimp. Shrimp fed diets supplemented with 0.03–0.06% LPLs showed comparable performance with those in the PC group (higher energy and higher fish oil diet). Shrimp have insufficient bile acids, which are essential for lipid digestion and absorption (Shi et al., 2023; Kumar et al., 2021). Therefore, the efficiency of the lipid digestion processes, including emulsification, lipase activation, and micelle formation, may be suboptimal in shrimp, indicating the need for diets with essential additives that enhance lipid digestion. LPLs, which are derived from the enzymatic hydrolysis of phospholipids, promote emulsification and enhance fat digestion in animal feed and nutrient absorption (Song et al., 2024a; Thornhill, 2020). Research findings indicate that dietary LPLs improve growth performance, health, and feed efficiency in various species, including largemouth bass (Lu et al., 2022), rainbow trout (Taghavizadeh et al., 2020), turbot (Li et al., 2019), tiger shrimp (Khan et al., 2018), and Pacific white shrimp (Wang et al., 2024). LPLs can promote growth and lipid metabolism, improve astaxanthin deposition and body coloration, and enhance lipid and cholesterol transportation, especially in aquatic animals such as Pacific white shrimp (Wang et al., 2024). Research on the substitution of dietary fish oil with LPLs in crustacean nutrition remains relatively limited. However, existing studies have reported various beneficial effects associated with LPL supplementation. For instance, Wang et al. (2024) demonstrated that dietary inclusion of LPLs significantly improved growth performance, specifically weight gain and SGR, as well as enhanced lipid metabolism, antioxidant enzyme activity, and immune responses in Pacific white shrimp (Wang et al., 2024). Furthermore, the inclusion of 0.1% LPLs was found to mitigate the negative effects of a 1% reduction in dietary phospholipids, and 0.2% LPLs was recommended to compensate for a 1% reduction in fish oil. In this experiment, dietary inclusion levels of 0.03% and 0.06% LPLs were selected based on prior research in aquatic species. For instance, Xu et al. (2022) reported that similar concentrations of lysophosphatidylcholine (LPC) in LPLs significantly enhanced growth performance and modulated the expression of lipid metabolism-related genes in juvenile turbot (Xu et al., 2022). Additionally, Song et al. (2024b) demonstrated that LPL supplementation may contribute to a 15% decrease in dietary lipid levels without adverse effects on growth performance in Pacific white shrimp, indicating the potential of LPLs to improve feed efficiency under lipid-restricted conditions (Song et al., 2024b). In the present study, supplementation with 0.03%–0.06% LPLs improved growth performance parameters, including Tprod, WGR, SGR, FCR, and PER, compared with those in the NC group. Notably, similar results were observed in shrimp in the PC group. Additionally, the growth-regulating effects of LPLs may be dose-dependent. Collectively, these findings suggest that supplementing a low-energy diet (50 kcal/kg; 1% fish oil) with 0.03%–0.06% LPLs can reduce dietary lipid requirements and enhance lipid digestibility and metabolic efficiency. Furthermore, the observed improvements in growth performance with LPL supplementation can be attributed to improved lipid digestion and enhanced energy utilization efficiency. However, further studies are necessary to investigate the optimal inclusion level of 0.06% LPLs to minimize the use of dietary fish oil, promote sustainability, and reduce production costs without affecting growth and production quality.
Dietary LPLs significantly increased the crude protein and lipid contents in shrimp muscle. Similarly, previous studies have shown that LPLs can enhance protein and lipid deposition in various species, including large yellow croaker (Yi et al., 2014), rainbow trout (Adhami et al., 2021), and yellow drum (Wang et al., 2016). LPLs play important roles in cellular functions, including increasing protein synthesis and cellular size and upregulating fatty acid synthetase (FAS) mRNA expression, thereby contributing to improved lipid metabolism and utilization (Li et al., 2019). Our findings indicate that LPLs can increase the permeability and fluidity of hepatopancreatic epithelial cells, improving nutrient digestion, transportation, and absorption in the midgut, particularly the utilization of essential nutrients such as lipids and proteins, and promoting nutrient deposition in shrimp muscle.
Although several studies have examined the effects of LPLs on lipid metabolism in aquatic animals, research on their effects on cholesterol levels in hemolymph, hepatopancreas, and shrimp muscle remains scarce. Song et al. (2024a) reported that dietary LPLs increased astaxanthin deposition, regulated cholesterol transportation, increased high-density lipoprotein cholesterol (HDL-C) levels, and decreased low-density lipoprotein cholesterol (LDL-C) levels in the hemolymph. LDL transports cholesterol from the hepatopancreas to peripheral tissues, whereas HDL removes cholesterol by transporting it back to the hepatopancreas. HDL-C plays a crucial role in lipid metabolism by mediating reverse cholesterol transport and conversion into bile acids in the hepatopancreas (Shi et al., 2023). A study showed that a 1% decrease in fish oil in the diet of Pacific white significantly decreased total cholesterol (T-CHO) level in the hemolymph and that LPL supplementation mitigated this reduction and enhanced lipid metabolism. Importantly, similar findings have been reported in turbot and rainbow trout (Taghavizadeh et al., 2020; Li et al., 2019; Wang et al., 2024). Cholesterol is a biosynthetic precursor of ecdysteroid hormones in crustaceans that supports molting and cellular functions (Su et al., 2022). Khan et al. (2018) reported that dietary LPL supplementation in a fishmeal- and fish oil-reduced diet significantly decreased cholesterol content in the tail muscle and non-muscle portions, promoting growth performance and carcass fatty acid composition in tiger shrimp. In the present study, LPL supplementation in energy-reduced diets increased cholesterol levels in the hemolymph and hepatopancreas, supporting growth, development, and molting by promoting lipid storage and utilization. Particularly, shrimp in the NC+0.03% LPLs group showed increased cholesterol levels in the hepatopancreas and hemolymph, which could be due to the ability of LPLs to promote lipid digestion, absorption, and transportation. Future studies should investigate the relationship between LPL supplementation and its benefits in lipid digestion and cholesterol utilization for growth and molting, with the aim of reducing cholesterol accumulation in shrimp meat. Although research on this function remains limited, the use of LPLs as valuable natural emulsifiers in aquaculture could help produce healthier and more nutritious shrimp for consumers.
Owing to their hydrophilic and hydrophobic properties, LPLs enhance lipid metabolism and provide significant benefits, especially under oil-in-water conditions, by increasing digestive enzyme activity (Rodríguez-Viera et al., 2022). Overall, these properties improve emulsification in the shrimp gut, stimulate bile secretion, facilitate micelle formation, and increase the contact area for efficient lipase activity (Li et al., 2018). LPL supplementation enhances lipid digestion as well as improves protein digestibility by increasing protein solubility and substrate concentration, thereby accelerating digestive enzyme activity (Lu et al., 2022). Consistent with findings in turbot (Li et al., 2019) and largemouth bass (Lu et al., 2022), 0.03 and 0.06% dietary LPLs significantly increased lipase activity in the present study. Che et al. (2023) reported that 0.1% dietary LPLs increased lipase, protease, and amylase activities, particularly glucose-6-phosphate dehydrogenase (G-6-PDH), enhancing energy supply in largemouth bass. Based on these findings, the reduction of fish oil in the NC group resulted in decreased levels of long-chain polyunsaturated fatty acids (LC-PUFAs), as well as led to a reduction in other essential components naturally present in fish oil, such as phospholipids and cholesterol. These components play a crucial role in micelle formation, which is necessary for lipid solubilization and absorption in the hepatopancreas, enhancing the activity of digestive enzymes. For example, the activities of lipase and amylase were lower in the NC group, potentially affecting the digestion of nutrients such as lipids and carbohydrates. In contrast, dietary supplementation with 0.06% LPLs in the NC group significantly enhanced the activities of lipase, protease, trypsin, chymotrypsin, and amylase, suggesting improved digestive capacity under conditions of limited dietary fish oil.
LPLs act as natural emulsifiers, promoting lipid digestion and the absorption of fatty acids and other lipid-soluble substances. Additionally, they facilitate micelle formation, lipid transportation, and the transfer of absorbed lipids from the hepatopancreas into the hemolymph. To elucidate the potential mechanisms of LPL supplementation in lipid metabolism and oxidative stress response, we examined the mRNA expression of genes involved in lipid metabolism and antioxidant response. Consistent with findings in juvenile turbot (Li et al., 2019) and Pacific white shrimp (Wang et al., 2024), supplementing energy-reduced diets with 0.03% and 0.06% LPLs upregulated the expression of the lipid metabolism genes Lip and dFAD-6. Lip is crucial for triglyceride hydrolysis in the hepatopancreas, whereas dFAD-6 is essential for synthesizing polyunsaturated fatty acids, including arachidonic acid and eicosatetraenoic acid. Arachidonic acid benefits shrimp by enhancing cellular health and immune response, and both arachidonic acid and eicosatetraenoic acid improve nutrient absorption and utilization. In contrast, the absence of significant changes in HK and GPx genes expression following LPL supplementation may be attributed to the lipid-specific functional role of LPLs and the lack of substantial metabolic or oxidative stress during the experimental period. HK gene encodes a key glycolytic enzyme that phosphorylates glucose to glucose-6-phosphate, initiating energy production, and its expression often reflects cellular energy demand (Cruz-Moreno et al., 2024). GPx gene encodes the glutathione peroxidase enzyme, a selenium-dependent antioxidative stress enzyme responsible for detoxifying peroxides and protecting cells from oxidative damage. The stable expression of both genes suggests that LPLs did not induce a level of physiological stress sufficient to trigger transcriptional responses in glycolytic or antioxidant pathways (Parida and Sahoo, 2023). The unchanged expression of HK and GPx genes following LPL supplementation, as well as in the positive and negative control groups, may reflect a metabolically balanced state in which energy demands were adequately met through lipid-derived pathways, thereby minimizing the reliance on glycolysis and limiting the activation of antioxidant defense mechanisms. This observation implies that the dietary energy levels provided in the present study were presumably sufficient to prevent the onset of energy deficiency or metabolic stress, which would otherwise trigger compensatory upregulation of glycolytic or redox-regulatory genes. Consequently, the absence of transcriptional changes in these pathways may not indicate a lack of LPL efficacy, but rather a reflection of adequate energy homeostasis under the experimental conditions. This outcome suggests LPLs effectively promoted lipid metabolism and enhanced digestive enzyme activity, their influence on glycolytic and redox-regulatory pathways appeared limited under stable, non-stressful conditions. Consistent with findings in Pacific white shrimp (Wang et al., 2024), the mRNA expression of the antioxidant gene SOD was significantly upregulated in the NC+0.03% LPLs and NC+0.06% LPLs groups, enhancing growth, antioxidative stress enzyme, and immune capacity. From a statistical perspective, some response parameters did not reach statistical significance, certain values showed trends that were close to the threshold, which may indicate potential biological relevance. Duncan’s multiple range test was selected in this study due to its higher sensitivity in detecting differences, despite being more permissive and carrying a greater risk of type I error. These near-significant results were interpreted with caution. To reduce the chance of type I error in future research, the use of more conservative post-hoc tests such as Tukey’s HSD, along with increasing the sample size and considering adjusted p-values, may improve the reliability of statistical outcomes. Collectively, our findings suggest that LPL supplementation enhances the utilization of energy (fish oil)-reduced diets, promoted lipid, protein, and glucose metabolism, improved vital nutrient transportation, and upregulated lipid metabolism and antioxidative stress gene in shrimp. LPL supplementation in energy-reduced diets might provide positive benefits without adverse effects on growth performance or feed utilization. However, it is important to acknowledge that some studies have reported neutral or context-dependent effects of LPLs. For example, Al-Jebory et al. (2023) observed limited improvements in growth performance during the finisher period when dietary lipid digestibility was already sufficient (Al-Jebory et al., 2023). Similarly, Lee and Stein (2022) reported that the positive effects of LPLs on nutrient digestibility and growth performance may be more pronounced in young or developing animals with relatively underdeveloped digestive systems than in fully developed adults (Lee and Stein, 2022). These findings underscore the importance of both dietary composition and physiological status in modulating the efficacy of LPLs. Therefore, although the positive effects of LPLs are evident under specific nutritional conditions, further research is required to more precisely define the conditions and interactions that influence their effectiveness across different species and dietary formulations. In summary, these findings highlight the potential of LPLs as functional feed additives to support shrimp growth and metabolic health, particularly under energy-reduced diets. Future studies should focus on dose optimization, long-term effects, and species-specific responses to maximize the practical applications of LPLs in aquaculture nutrition.
Conclusions
LPL supplementation in energy-reduced diets may contribute to improved growth performance and nutrient utilization in juvenile Pacific white shrimp, potentially through enhanced lipid digestion and increased absorption and transport of essential nutrients. LPL supplementation at inclusion levels of 0.03%–0.06% increased digestive enzyme activity, elevated cholesterol concentrations in the hepatopancreas and hemolymph, enhanced nutrient retention, and upregulated genes related to lipid metabolism and antioxidative stress responses. Collectively, these findings suggest that LPLs may play a role in mitigating some of the physiological impacts associated with 1% dietary fish oil or 50 kcal/kg energy reduction and may support shrimp health and development under such conditions. Although the current study demonstrated promising results under controlled experimental conditions, it is important to exercise caution when extrapolating these findings to commercial-scale shrimp farming. The long-term effects of LPL supplementation, as well as its cost-effectiveness and performance under different farming environments remain to be fully elucidated. Further research is recommended to investigate the mechanisms of LPLs on molting physiology. A more comprehensive understanding of these factors will be essential for the effective application and optimization of LPLs in practical aquafeed formulations.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Kasetsart University, Thailand (ACKU 61-FIS-040). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RL: Writing – review & editing, Formal Analysis, Software, Writing – original draft, Visualization, Data curation, Methodology, Conceptualization, Investigation. ST: Writing – review & editing, Formal Analysis, Supervision, Validation, Conceptualization, Investigation, Data curation. YZ: Methodology, Conceptualization, Investigation, Data curation, Writing – review & editing, Formal Analysis. OJ: Investigation, Conceptualization, Funding acquisition, Resources, Project administration, Writing – review & editing, Validation, Visualization, Supervision, Data curation, Methodology, Formal Analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Nutrition Improvement Company and the Aquatic Animal Nutrition and Aquafeed Laboratory, Department of Aquaculture, Faculty of Fisheries, Kasetsart University. Nutrition Improvement Company was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit this article for publication.
Acknowledgments
This study was supported by the Aquatic Animal Nutrition and Aquafeed Laboratory, Department of Aquaculture, Faculty of Fisheries, Kasetsart University, for providing experimental facilities. I would also like to express my gratitude to Professor Orapint Jintasaporn for her valuable advice and suggestions during my experiments. I am also thankful to all my colleagues for their assistance and cooperation during my PhD studies. Additionally, I gratefully acknowledge the Nutrition Improvement Company for providing the Lypotech EC and product information for this experiment. Lastly, I would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
Author RL was employed by Nutrition Improvement Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. In the preparation of this manuscript, generative AI tools were utilized to assist in creating and refining sentences. Specifically, Copilot was employed to enhance the clarity and coherence of the text.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Al-Jebory H. H. Qotbi A. A. A. Al-Saeedi M. K. I. Al-Khfaji F. R. Ajafar M. Safaei A. (2023). Biological activity of lysophospholipids in poultry and ruminants: A review. Int. J. Multidiscip. Res. Growth Evaluat.4, 504–511. Available online at: https://www.researchgate.net/publication/370963215 (Accessed February 2, 2025).
2
Alkademi Z. (2020). The influence of molecular structure of phospholipids on the transition from micelles to bilayers in bile salt surfactant/phospholipid mixtures. Uppsala University, Uppsala (Sweden.
3
AOAC (2023). “Official methods of analysis,” in Official Methods of Analysis of AOAC International. Ed. Latimer JRG. W. (Oxford University Press, Oxford).
4
Bernfeld P. (1955). Amylases, α and β. Meth. Enzymol.1, 149–150. doi: 10.1016/0076-6879(55)01021-5
5
Calvano C. D. Bianco M. Ventura G. Losito I. Palmisano F. Cataldi T. R. (2020). Analysis of phospholipids, lysophospholipids, and their linked fatty acyl chains in yellow lupin seeds (Lupinus luteus L.) by liquid chromatography and tandem mass spectrometry. Molecules25, 805. doi: 10.3390/molecules25040805
6
Che M. Lu Z. Liu L. Li N. Ren L. Chi S. (2023). Dietary lysophospholipids improves growth performance and hepatic lipid metabolism of largemouth bass (Micropterus salmoides). Anim. Nutr.13, 426–434. doi: 10.1016/j.aninu.2023.04.004
7
Chen Y. Zheng C. Zhang X. Li X. Yao X. He G. et al . (2022). Evaluation of ratios of fish-to-soybean oils on growth, lipid and cholesterol metabolism and muscle metabolites of Pacific white shrimp (Litopenaeus vannamei) fed low fishmeal diets containing Clostridium autoethanogenum protein. Aquac. Rep.27, 101417. doi: 10.1016/j.aqrep.2022.101417
8
Chinarak K. Panpipat W. Panya A. Phonsatta N. Cheong L. Z. Chaijan M. (2022). Improved long-chain omega-3 polyunsaturated fatty acids in sago palm weevil (Rhynchophorus ferrugineus) larvae by dietary fish oil supplementation. Food Chem.393, 133354. doi: 10.1016/j.foodchem.2022.133354
9
Cruz-Moreno D. G. Hernández-Aguirre L. E. Peregrino-Uriarte A. B. Leyva-Carrillo L. Gómez-Jiménez S. Contreras-Vergara C. et al . (2024). Changes of glycolysis and gluconeogenesis key enzymes in the muscle of the shrimp Penaeus vannamei in response to hypoxia and reoxygenation. J. Exp. Mar. Biol. Ecol.580, 152052. doi: 10.1016/j.jembe.2024.152052
10
Gao Y. Yao Y. Huang J. Sun Y. Wu Q. Guo D. et al . (2023). Effect of dietary bile acids supplementation on growth performance, feed utilization, intestinal digestive enzyme activity and fatty acid transporters gene expression in juvenile leopard coral grouper (Plectropomus leopardus). Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1171344
11
Garcia-carreno F. L. Hernandez-cortes M. P. Haard N. F. (1994). Enzymes with peptidase and proteinase activity from the digestive systems of a freshwater and a marine decapod. J. Agric. Food. Chem.42, 1456–1461. doi: 10.1021/jf00043a013
12
Guoliang C. Zhengjun P. Chuankun Z. Haitao Z. Zhang Y. Rajput S. et al . (2023). Effect of lysolecithin replacement of soybean lecithin oil on the survival, growth performance and immunity of Macrobrachium nipponense juveniles. Pakistan J. Zool.56, 2901–2908. doi: 10.17582/journal.pjz/20220805060857
13
Held P. (2014). Rapid critical micelle concentration (CMC) determination using fluorescence polarization. Available online at: https://www.agilent.com/cs/library/applications/critical-micelle-concentration-CMC-determination-5994-3345EN-agilent.pdf (Accessed September 1, 2021).
14
Hodar A. Vasava R. Mahavadiya D. Joshi N. (2020). Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. J. Exp. Zoology India.23, 13–21.
15
Hu Y. Tan B. Mai K. Ai Q. Zhang L. Zheng S. (2011). Effects of dietary menhaden oil, soybean oil and soybean lecithin oil at different ratios on growth, body composition and blood chemistry of juvenile Litopenaeus vannamei. Aquac. Int.19, 459–473. doi: 10.1007/s10499-010-9361-4
16
IFFO (2024). IFFO’s estimates of the 2023 fishmeal and fish oil production and insights for 2024. Available online at: https://www.iffo.com/iffos-estimates-2023-fishmeal-and-fish-oil-production-and-insights-2024 (Accessed October 26, 2024).
17
Khan H. I. Dayal J. S. Ambasankar K. Madhubabu E. P. Jannathulla R. Rajaram V. (2018). Enhancing the dietary value of palm oil in the presence of lysolecithin in tiger shrimp, Penaeus monodon. Aquac. Int.26, 509–522. doi: 10.1007/s10499-017-0235-x
18
Kumar R. Tung T.-C. Ng T. H. Chang C.-C. Chen Y.-L. Chen Y.-M. et al . (2021). Metabolic alterations in shrimp stomach during acute hepatopancreatic necrosis disease and effects of taurocholate on Vibrio parahaemolyticus. Front. Microbiol.12, 631468. doi: 10.3389/fmicb.2021.631468
19
Lee S. Jo K. Jeong S. K. C. Choi Y. S. Jung S. (2024). Strategies for modulating the lipid digestion of emulsions in the gastrointestinal tract. Crit. Rev. Food Sci. Nutr.64, 9740–9755. doi: 10.1080/10408398.2023.2215873
20
Lee D. Y. Kind T. Yoon Y. R. Fiehn O. Liu K. H. (2014). Comparative evaluation of extraction methods for simultaneous mass-spectrometric analysis of complex lipids and primary metabolites from human blood plasma. Anal. Bioanal. Chem.406, 7275–7286. doi: 10.1007/s00216-014-8124-x
21
Lee S. A. Stein H. H. (2022). “Digestibility and availability of nutrients in feed ingredients” in Sustainable Swine Nutrition (2nd rev. ed.), Chap. 19. ed. ChibaA. I. doi: 10.1002/9781119583998.ch19
22
Li Z. Gu K. Zhang H. (2018). Progress in preparation, separation, determination and application of lysophospholipids. China Oils Fats43, 132–136. doi: 10.1007/s00343-022-2169-z
23
Li X. Li H. Qu K. Liu Y. Chi S. Yang Q. et al . (2023). Dietary bile acids promote sterol metabolism, bile acids enterohepatic circulation, and apoptosis in juvenile Pacific white shrimp (Litopenaeus vannamei). Anim. Feed Sci. Technol.303, 115710. doi: 10.1016/j.anifeedsci.2023.115710
24
Li M. Ritzoulis C. Du Q. Liu Y. Ding Y. Liu W. et al . (2021). Recent progress on protein-polyphenol complexes: Effect on stability and nutrients delivery of oil-in-water emulsion system. Front. Nutr.8. doi: 10.3389/fnut.2021.765589
25
Li B. Li Z. Sun Y. Wang S. Huang B. Wang J. (2019). Effects of dietary lysolecithin (LPC) on growth, apparent digestibility of nutrient and lipid metabolism in juvenile turbot Scophthalmus maximus L. Aquac. Fish.4, 61-66. doi: 10.1016/j.aaf.2018.12.002
26
Liang X. Luo X. Chang T. Han F. Xu C. Li E. (2023). Positive effects of optimal dietary cholesterol levels on the ovary development and health of female Pacific white shrimp, Litopenaeus vannamei brood stock. Aquaculture.577, 739987. doi: 10.1016/j.aquaculture.2023.739987
27
Lima R. B. Figueiredo-Lima D. F. (2016). Critical review: essential fatty acids on shrimp feeding. Sci. Agrar.15, 236–243. doi: 10.18188/1983-1471/sap.v15n3p236-243
28
Lin Y. H. Dehasque M. Nuez-Ortín W. G. (2021).Lyso-phospholipid supplementation to replace lecithin and improve growth performance in Pacific white shrimp. Available online at: https://www.adisseo.com/wp-content/uploads/2023/06/publication-2021-05-aqualyso-std-lyso-phospholipid-supplementation-to-replace-lecithin-in-shrimp-aap.pdf (Accessed June 2021).
29
Livak K. J. Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods25, 402–408. doi: 10.1006/meth.2001.1262
30
Lordan R. Blesso C. N. (2023). Phospholipids and sphingolipids in nutrition, metabolism, and health. Front. Nutr.10, 1153138. doi: 10.3389/fnut.2023.1153138
31
Lowry O. H. Rosebrough N. J. Farr A. Randall R. J. (1951). Protein measurement with the folin phenol reagent. J. Biol. Chem.193, 256–275. doi: 10.1016/S0021-9258(19)52451-6
32
Lu Z. Yao C. Tan B. Dong X. Yang Q. Liu H. et al . (2022). Effects of lysophospholipid supplementation in feed with low protein or lipid on growth performance, lipid metabolism, and intestinal flora of largemouth bass (Micropterus salmoides). Aquac. Nutr.2022, 1234567. doi: 10.1155/2022/1234567
33
Markweg-Hanke M. Lang S. Wagner F. (1995). Dodecanoic acid inhibition of a lipase from Acinetobacter sp. OPA 55. Enzyme Microb. Technol.17, 512–516. doi: 10.1016/0141-0229(94)00067-2
34
Nayak N. S. Purohit M. S. Pradhan R. R. Tipre D. R. Dave S. R. (2021). “Biosurfactant from the marine microorganisms potentials and future prospects,” in Microbial Surfactants: Volume I: Production and Applications, 1st edn. eds. SayyedR. Z.El‑EnshasyH. AHameedaB.. (Boca Raton, FL, USA; Oxford, UK: CRC Press). chapter 6, pp. 122–139. doi: 10.1201/9781003056638‑6
35
NRC (2011). Nutrient Requirements of Fish and Shrimp (Washington, DC: National academies press).
36
Oliva-Teles A. Enes P. Couto A. Peres H. (2022). “Replacing fish meal and fish oil in industrial fish feeds,” in Feed and Feeding Practices in Aquaculture, 2nd edn. ed. Allen DavisD.. (Sawton, UK:Woodhead Publishing (Elsevier)), 231–268. doi: 10.1016/B978-0-12-823488-7.00009-3
37
Pan L. Q. Xiao G. Q. Zhang H. X. Luan Z. H. (2005). Effects of different dietary protein content on growth and protease activity of Eriocheir sinensis larvae. Aquaculture.246, 313–319. doi: 10.1016/j.aquaculture.2004.12.023
38
Parida S. Sahoo P. K. (2023). Antioxidant defence in Labeo rohita to biotic and abiotic stress: insight from mRNA expression, molecular characterization and recombinant protein-based ELISA of catalase, glutathione peroxidase, CuZn superoxide dismutase, and glutathione S-transferase. Antioxidants.13, 18. doi: 10.3390/antiox13010018
39
Pavlović N. Goločorbin-Kon S. Ðanić M. Stanimirov B. Al-Salami H. Stankov K. et al . (2018). Bile acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front. Pharmacol.9. doi: 10.3389/fphar.2018.01283
40
Radhakrishnan D. K. Akbarali I. Velayudhannair K. Kari Z. A. Liew H. J. (2024). Exploring the role of plant oils in aquaculture practices: An overview. Aquac. Int.32, 7719–7745. doi: 10.1007/s10499-024-01538-9
41
Ravera F. Dziza K. Santini E. Cristofolini L. Liggieri L. (2021). Emulsification and emulsion stability: The role of the interfacial properties. Adv. Coll. Int. Sci.288, 102344. doi: 10.1016/j.cis.2020.102344
42
Rodríguez-Viera L. Perera E. Pila I. A. Moyano F. J. Mancera J. M. Díaz M. (2022). True lipase activity and in vitro digestibility of potential lipid sources for the spiny lobster Panulirus argus feeds. Aquaculture555, 738191. doi: 10.1016/j.aquaculture.2022.738191
43
Saleh A. A. Amber K. A. Mousa M. M. Nada A. L. Awad W. Dawood M. A. O. et al . (2020). A mixture of exogenous emulsifiers increased the acceptance of broilers to low energy diets: Growth performance, blood chemistry, and fatty acids traits. Animals.10, 437. doi: 10.3390/ani10030437.10
44
Shen Y. Liu K. Luo X. Guan Q. Cheng L. (2022). A simple and reliable bile acid assay in human serum by LC-MS/MS. J. Clin. Lab. Anal.36, e24279. doi: 10.1002/jcla.24279
45
Shi M. Zheng C. Sun Y. Li X. He G. Cao J. et al . (2023). Effects of dietary chenodeoxycholic acid supplementation in a low fishmeal diet containing Clostridium autoethanogenum protein on growth, lipid and cholesterol metabolism, and hepatopancreas health of Litopenaeus vannamei. Animals13, 2109. doi: 10.3390/ani13132109
46
Siyal F. A. Babazadeh D. Wang C. Arain M. A. Saeed M. Ayasan T. et al . (2017). Emulsifiers in the poultry industry. World’s Poult. Sci. J.73, 611–620. doi: 10.1017/S0043933917000502
47
Smejkal G. Gross V. Lazarev A. (2024). Theoretical and experimental determinations of the hydrophilic–lipophilic balance (HLB) of representative oils and lecithins. Colloids Interfaces.8, 21. doi: 10.3390/colloids8020021
48
Song Z. Liu Y. Liu H. Ye Z. Ma Q. Wei Y. et al . (2024a). Dietary lysophosphatidylcholine improves the uptake of astaxanthin and modulates cholesterol transport in pacific white shrimp Litopenaeus vannamei. Antioxidants.13, 505. doi: 10.3390/antiox13050505
49
Song Z. Liu H. Liu Y. Ye Z. Ma Q. Wei Y. et al . (2024b). Effects of the supplementation of lysophospholipid in low-lipid diets on juvenile Pacific white shrimp. Aquac. Res.2024, 9594116. doi: 10.1155/2024/9594116
50
Su C. Li J. Lu Y. Wang Y. Ding Y. Pan L. Zhang M. (2022). Interactive effects of dietary cholesterol and bile acids on the growth, lipid metabolism, immune response and intestinal microbiota of Litopenaeus vannamei: Sparing effect of bile acids on cholesterol in shrimp diets. Aquaculture547, 737412. doi: 10.1016/j.aquaculture.2021.737412
51
Sunde J. Taranger G. Rungruangsak-Torrissen K. (2001). Digestive protease activities and free amino acids in white muscle as indicators for feed conversion efficiency and growth rate in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem.25, 335–345. doi: 10.1023/A:1023233024001
52
Taghavizadeh M. Hosseini Shekarabi S. P. Mehrgan M. S. Islami H. R. (2020). Efficacy of dietary lysophospholipids (Lipidol™) on growth performance, serum immuno-biochemical parameters, and the expression of immune and antioxidant-related genes in rainbow trout (Oncorhynchus mykiss). Aquaculture525, 735315. doi: 10.1016/j.aquaculture.2020.735315
53
Taneerat C. Olasard P. Suksri P. Whankaew S. Sathapondecha P. (2023). Identification and profiling of long non-coding RNAs during molt cycle: An involvement of Lnc1182 in the molt of white shrimp, Litopenaeus vannamei. Aquac. Rep.30, 101611. doi: 10.1016/j.aqrep.2023.101611
54
Thornhill A. W. (2020). Evaluation of a lysophospholipid using two oils on performance, carcass composition and organ characteristics of broilers. Thesis, (Stellenbosch, South Africa: Stellenbosch University).
55
Torrissen K. Lied E. Espe M. (1994). Differences in digestion and absorption of dietary protein in Atlantic salmon (Salmo salar) with genetically different trypsin isozymes. J. Fish Biol.45, 1087–1104. doi: 10.1111/j.1095-8649.1994.tb01075.x
56
Van Hoogevest P. (2017). Review – An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci.108, 1–12. doi: 10.1016/j.ejps.2017.07.008
57
Wang F. C. Marangoni A. G. (2015). pH and stability of the α-gel phase in glycerol monostearate–water systems using sodium stearoyl lactylate and sodium stearate as the co-emulsifier. RSC Adv.5, 96746–96749. doi: 10.1039/C5RA16457E
58
Wang J. Peng H. Jin M. Li M. He Y. Li S. et al . (2024). Dietary lysophospholipids supplementation promotes growth performance, enhanced antioxidant capacity, and improved lipid metabolism of Litopenaeus vannamei. Aquac. Rep. 39, 102476. doi: 10.1016/j.aqrep.2023.102476
59
Wang L. Lu Q. Luo S. Zhan W. Chen R. Lou B. Xu D. (2016). Effect of dietary lipid on growth performance, body composition, plasma biochemical parameters and liver fatty acids content of juvenile yellow drum Nibea albiflora. Aquac. Rep.4, 10–16. doi: 10.1016/j.aqrep.2016.07.002
60
Xie S. Liu Y. Tian L. Niu J. Tan B. (2020). Low dietary fish meal induced endoplasmic reticulum stress and impaired phospholipids metabolism in juvenile pacific white shrimp, Litopenaeus vannamei. Front. Physiol.11. doi: 10.3389/fphys.2020.01024
61
Xu H. Luo X. Bi Q. Wang Z. Meng X. Liu J. et al . (2022). Effects of dietary lysophosphatidylcholine on growth performance and lipid metabolism of juvenile turbot. Aquac. Nutr.2022, 3515101. doi: 10.1155/2022/3515101
62
Xu Z. Wang A. Wang H. Zhang H (2016). The effect of replacement of fish oil by soybean oil in practical diets, on tissue fatty acid and expression of related genes in Pacific white shrimp Litopenaeus vannamei. Isr. J. Aquac. - Bamidgeh.68. doi: 10.46989/001c.20838
63
Yi X. Zhang F. Xu W. Li J. Zhang W. Mai K. (2014). Effects of dietary lipid content on growth, body composition and pigmentation of large yellow croaker Larimichthys croceus. Aquaculture434, 355–361. doi: 10.1016/j.aquaculture.2014.09.007
Summary
Keywords
energy reduction, fish oil reduction, growth performance, lysophospholipids, lipid metabolism, gene expression, Pacific white shrimp
Citation
Limwachirakhom R, Triwutanon S, Zhang Y and Jintasataporn O (2025) Effects of dietary lysophospholipids on the performance of Pacific white shrimp (Litopenaeus vannamei) fed fish oil- and energy-reduced diets. Front. Mar. Sci. 12:1624057. doi: 10.3389/fmars.2025.1624057
Received
06 May 2025
Accepted
16 June 2025
Published
02 July 2025
Volume
12 - 2025
Edited by
Seyyed Morteza Hoseini, Iranian Fisheries Science Research Institute (IFSRI), Iran
Reviewed by
Ercüment Genç, Ankara University, Türkiye
Yafei Duan, South China Sea Fisheries Research Institute, China
Updates
Copyright
© 2025 Limwachirakhom, Triwutanon, Zhang and Jintasataporn.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rawiwan Limwachirakhom, rawiwan.nic@gmail.com; Orapint Jintasataporn, ffisora@ku.ac.th
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.