Abstract
Introduction:
Pesticides can be transported into estuaries via spray drift, ground water contamination and surface runoff. Increasing climatic variability and global pesticide use are likely to increase the exposure of harvested estuarine species, and therefore seafood consumers, to agrichemicals. Post-harvest processing strategies present opportunities to reduce pesticide residues in seafood and so mitigate consumer exposure.
Materials and methods:
We evaluated the efficacies of thermal processing (cooking) and depuration (holding individuals in clean flow-through seawater) for reducing pesticide residues in wild-caught giant mud crab (Scylla serrata) edible tissues (flesh and brown meat (i.e. hepatopancreas and gonads)). Pesticide residues were detected in 82% of assessed crabs, with five analytes quantified (cyprodinil, diuron, imidacloprid, propargite and triazophos).
Results:
Correlative analyses revealed cooking at ~82°C for 2 min 100-g–1 body mass reduced all pesticide concentrations (and the total residues) in both tissues by 7–99%—except for cyprodinil (51% increase). Imidacloprid residues in crab flesh were reduced (by 81%) after six days of depuration, with complete elimination after 12 days. While a human health-risk assessment identified that the concentrations of pesticide residues in crab tissues posed no negative health effects to seafood consumers, the data support post-harvest processing methods for mitigating consumer exposure.
Discussion:
These findings do not constitute health advice regarding post-harvest possessing methods for reducing contaminants in seafood, but rather highlight the potential utility of the methods assessed for achieving this objective and the broader need for ongoing pesticide surveillance of Australian seafood. Food safety regulation should consider establishing maximum residue limits for seafood, with consideration of consumption preferences.
Introduction
Pesticides (namely insecticides, herbicides and fungicides) are widely applied in modern agricultural practices to control pest species (Tudi et al., 2021). These chemicals can be introduced into aquatic environments through various pathways, including spray drift, groundwater contamination and surface runoff (Leadprathom et al., 2009; Lefrancq et al., 2017; Commelin et al., 2022). Pesticides are often deposited in estuaries, which can act as sinks for these contaminants (Cuevas et al., 2018; Ouyang et al., 2019). Due to their environmental persistence, high bioaccumulation potential and lipophilic nature (Ellgehausen et al., 1980; Hornsby et al., 2012), pesticides are readily uptaken by estuarine species, including those harvested for human consumption (Gray et al., 2025). The accumulation of agrichemicals in seafood species can compromise resource quality and safety, threatening fisheries sustainability and human health (Islam and Tanaka, 2004; Rodrigues et al., 2018; Gray et al., 2025). Climate-driven increases in the frequency and magnitude of heavy rainfall events (Milly et al., 2002; Wasko et al., 2021) and expanding global pesticide use (FAO, 2021) are likely to increase the exposure of estuarine seafood species and their consumers to agrichemicals.
The general population is exposed to pesticides indirectly through dietary intake (Lu et al., 2006). Long-term dietary exposure to pesticide residues has been linked to various chronic human health effects, such as birth defects, cancer, endocrine disruption, and immunological and neurodegenerative disorders (recent reviews by Asghar et al., 2016; Kim et al., 2017; Beyuo et al., 2024). As part of food safety and trade legislation, maximum residue limits (MRLs)—which are the upper permissible limits for pesticide residue(s) in food products—have been set by regulatory agencies globally (Handford et al., 2015). Residue limits are primarily established for agricultural commodities to which pesticides are purposely applied. Seafood is a key source of protein and micronutrients to billions of consumers globally (Béné et al., 2015; Hicks et al., 2019; Shalders et al., 2022). A growing body of research has highlighted the potential for seafood contamination by pesticides (e.g. Rodríguez-Hernández et al., 2017; Ernst et al., 2018; Azzouz et al., 2019; Hussein et al., 2022; Ivorra et al., 2023; Jamal et al., 2024). Despite the increasing potential for contamination, MRLs have not been established for seafoods in many nations (De Witte et al., 2022).
Appropriate post-harvest processing strategies have been shown to reduce pesticide residues in seafood to within the ranges for safe consumption. Doing so facilitates the continued operation and profitability of seafood industries in the short-term until mitigation efforts are undertaken at the point-source(s) of contamination. Specifically, two potentially viable adaptation options include thermal processing (i.e. cooking; Taylor et al., 2019) and depuration (i.e. holding individuals in clean water over short periods; O’Connor et al., 2018). Both processes are currently applied to a variable extent by seafood industries. Nevertheless, research testing the efficacies of cooking or depuration for reducing pesticide residues is limited, particularly on decapods, despite their economic value throughout global estuaries and coastal areas (Boenish et al., 2022).
Cooking evokes a range of chemical changes in foods. Specifically, heat treatment influences the nutritional components of ‘muscle’ foods (including seafoods) by denaturing protein and lipid profiles, resulting in the shrinkage and solubilisation of connective tissue, and water loss (Bayen et al., 2005; Momenzadeh et al., 2017; Sobral et al., 2018). These changes to the structure and proximate composition of seafoods, along with the direct effects of heat, can reduce pesticide residues through hydrolysis, volatilisation, thermal degradation (and transformation), and leaching into cooking media (reviewed by Kaushik et al., 2009; Bajwa and Sandhu, 2014; Sobral et al., 2018; Yigit and Velioglu, 2020). The relative importance of these mechanisms for eliminating residues depends on the physico-chemical properties of pesticides, which are highly variable between chemical classes (Huan et al., 2015; Yigit and Velioglu, 2020). Most work on seafood is limited to legacy organochlorine residues (e.g. Bayen et al., 2005; Arisekar et al., 2022; Sundhar et al., 2023), with less focus on current-use pesticides.
Further, research has also only examined the effects of cooking on pesticide residues in the muscle portion of seafoods. Crustacean brown meat, which constitutes the gonads and hepatopancreas (i.e. viscera or tomalley), is widely consumed as a flavour component of soups, stocks and sauces (Lordan and Zabetakis, 2022). The crustacean hepatopancreas is the primary organ responsible for the sequestration and metabolism of contaminants (Vogt, 2019). Hence, crustacean brown meat can pose an elevated health risk to consumers relative to the muscle portions (e.g. Noël et al., 2011; Maulvault et al., 2013; Bolam et al., 2016; Wiech et al., 2017). The effectiveness of thermal processing to reduce pesticide residues in crustacean brown meat remains untested.
Depuration—the process of holding live fishery resources in clean water for a sufficient time to reduce contaminants to acceptable levels for human consumption—has widely been utilised to eliminate microbial contaminants in bivalve culture (McLeod et al., 2017; Ayres, 2018). Recent studies have demonstrated that short-term depuration (days to weeks) can also effectively reduce tissue-pesticide concentrations in seafood species, and so mitigate consumer health risks (e.g. Ewere et al., 2019; Butcherine et al., 2021; Wu et al., 2023). Residue elimination may occur through various mechanisms, including biotransformation, excretion, or dissociation (Nyman et al., 2013; Butcherine et al., 2021; Wang et al., 2022). The feasibility of utilising depuration depends on the depuration kinetics of compounds (i.e. the rate of elimination), which can be species-, compound- and/or concentration-dependent (Serrano et al., 1997; Kwong et al., 2008; Wu et al., 2023). Due to the logistical and financial costs associated with holding live animals for protracted periods, depuration may be challenging to operationalise for large species, including the various swimming crabs comprising the family Portunidae.
Portunids are the most economically important group of crabs harvested from estuaries and coastal areas, and are favoured for their taste and nutritional qualities (Santhanam, 2018). In Australia, the giant mud crab (Scylla serrata) is a key commercial species harvested across multiple jurisdictions, with a national harvest exceeding 1000 t annum–1 (Kirke et al., 2023). Recently, concerns were raised by commercial fishers in New South Wales (NSW), Australia, about the potential exposure of this species to agricultural runoff during the 2020–2023 La Niña floods. Eastern Australian estuaries support various broadacre (e.g. sugarcane and livestock) and intensive (e.g. berry fruits and macadamia nuts) agricultural practices (Australian Bureau of Statistics (ABS), 2022; Harrison et al., 2023), with previous studies detecting a plethora of pesticide residues in adjacent environments (e.g. Hook et al., 2018; Conrad et al., 2021; Laicher et al., 2022; Taylor et al., 2022). Less is known about the potential for pesticide contamination of seafood species in this region (but see Jamal et al., 2024) and, to date, no studies have tested the efficacies of thermal processing or depuration for reducing pesticide residues in the edible portions of portunid crabs.
While many studies have documented pesticide residues in seafood, few have assessed dietary risk to seafood consumers after typical post-harvest practices. Here, we sourced giant mud crabs from an agriculturally intensive subtropical estuary to: (1) evaluate the efficacies of thermal processing and depuration for reducing pesticide residues in the edible tissues; and (2) undertake a human health-risk assessment to quantify any potential risks to seafood consumers based on pesticide concentrations in tissues pre- and post-processing. Given the popularity of giant mud crab among seafood consumers, this study provides important baseline data on the concentrations of current-use pesticide in their tissues.
Materials and methods
Site selection and crab collection
To quantify baseline pesticide concentrations and obtain experimental individuals, giant mud crabs were sampled from a site in the subtropical Clarence River (29.45°S; 153.19°E), eastern Australia. This site incurs considerable regional trapping effort and was previously established as being contaminated by pesticides. In collaboration with a commercial fisher, 71 intact legal-sized (≥ 85 mm carapace length) giant mud crabs (hereafter ‘crabs’) were randomly selected from the catches of 30 collapsible round traps (see Broadhurst et al., 2018 for a description of the traps and fishery). Crabs were collected over two nights (four days between collections) during March 2024. Traps were deployed overnight (~16-h soak) and baited with local-caught sea mullet (Mugil cephalus). Immediately following removal from traps (up to four ind. trap–1), crabs were bound with ~50 cm of 2-mm diameter polypropylene cord securing chelipeds to the carapace for safe handling. Bound crabs were placed into polystyrene boxes (max. 16 crabs box–1) and transported to the National Marine Science Centre (NMSC), Coffs Harbour, Australia (30.27°S, 153.14°E) within ~120 min of collection. All handling methods, including air exposure times, followed conventional practices.
Thermal processing experiment
At the NMSC, 40 randomly selected crabs were immersed in an iced seawater slurry for ~30 min before subsequent handling. Crabs were randomly partitioned between cooked and raw (i.e. control, uncooked) treatments (n = 20 for each), with an approximately equal sex ratio and range of body sizes. Crabs were allocated a unique identification number (‘crab ID’) and allowed to drain for ~1 min before recording biometric data (sex, weight (g), carapace length (CL) and width (CW) (nearest mm) and shell flex (as a proxy for carapace grade) according to industry standards (National Mud Crab Industry Reference Group, 2012)) and any pre-existing injuries or observed physiological damage. Individuals were euthanised by pithing both ganglia prior to experimental treatment.
The control crabs remained on ice, while the treatments were individually boiled in seawater (~36 PSU) at ~82°C (± 6°C SD) for 2-min 100-g–1 body weight, following recommended cooking practices (Calogeras et al., 2011). Four stainless-steel 7.6-L pots were filled with 4 to 6 L of seawater (depending on the size of the individual crab) and heated using four 2.34 kW single burner butane gas stoves until boiling. Water temperatures were recorded every 2 min using a Fluke 62 MAX handheld infrared thermometer (± 1.5°C precision). Once cooked, individual crabs were removed and left to cool at ambient temperature for ~15 min. In alternation, the pots were cleaned, refilled, boiled, and crabs cooked individually, as above. All control and treatment crabs were placed into individual polyethene bags and held at −20°C prior to dissection.
To provide a robust assessment of the health risks associated with consuming crabs, we sampled the entirety of the edible portions. Specifically, composite samples of both flesh (~1-g samples of cheliped, walking leg and abdominal muscle) and brown meat (hepatopancreas and available gonad tissue; < 5 g) were prepared from cooked and raw individuals for pesticide residue analyses (see below).
Depuration experiment
Following previous work by Taylor et al. (2017; 2021) on the depuration of poly- and per-fluorinated alkyl substances (PFASs) from giant mud crabs, we utilised a repeated sampling method to test the potential elimination of pesticides from individuals over 12 days. In arthropods, limb autonomy is a defence function whereby an injured limb is cast off to reduce trauma and improve an individual’s survival (Kennelly et al., 1990; Hopkins and Das, 2015). In adult giant mud crabs, these autotomic appendages contain a sufficient amount of tissue (>1 g) to undertake pesticide screening, presenting the opportunity to measure temporal changes in pesticide concentrations in individuals throughout the depuration period.
We sampled pereiopods (i.e. walking legs) through manual removal, because the physiological stress associated with excision was expected to be lower than for other more-specialised appendages (i.e. chelipeds and swimmerets)—owing to the relatively lower biomass and functionality (Reichman, 1984; Juanes and Smith, 1995). Further, compared to other appendages, missing or damaged pereiopods are less frequently observed for most brachyurans in the field (reviewed by Juanes and Smith, 1995). The loss and regeneration of appendages in brachyurans has been hypothesised to explain the observed variation in the biodistribution of contaminants across individuals’ body plans (see Taylor et al., 2021). Because pereiopods are less frequently autotomised or injured than other appendages, their sampling may reduce variation in contaminant concentrations among appendages.
Upon arrival at the NMSC, crabs (n =31) were held in a single aerated 1000-L tank for up to ~1 h. Crabs were individually removed, assigned an identification number (crab ID) and information on existing injuries and biometric data were recorded as described above. All crabs were subsequently evenly partitioned (6 to 7 ind. tank–1) among five aerated 1000-L outdoor experimental tanks. Tanks were half-filled with flow-through seawater pumped from the adjacent ocean at 150 L h–1 (~4-h turnover rate). Crabs remained bound and housed individually in plastic mesh cages (~24 × 24 × 11 cm; grid size 3 × 3 cm) throughout the experiment to prevent competitive interactions. Cages were negatively buoyant and allowed sufficient room for individuals to move for feeding, while enabling limbs to be easily identified and removed at the subsequent sampling points. Each cage was labelled with their respective crab's ID.
Each walking leg was allocated a number from 1 to 8, and a random number generator was used to select which legs were to be removed on days 0 (i.e. pre-depuration), 2, 6 and 12. The same walking leg was taken from each individual at each sampling point. At each of the sampling points, cages were retrieved, and the respective individuals' pereiopods (above) were obtained by making a small incision below the fracture plane at the base using a pair of sterilised (with 100% ethanol) poultry scissors following Uhlmann et al. (2009). In most cases, individuals autotomised the appendage upon incision. In the case where one individual was already missing the given pereiopod, an adjacent leg was removed with an attempt to maintain symmetry in limb loss to reduce potential impacts to locomotion (Smith, 1995). On day 12, all individuals were placed into an iced seawater slurry for ~30 min (and biological data were recorded) before being euthanised and dissected for analyses as described above.
Throughout the experiment, tanks were cleaned and checked for mortalities twice daily. Any morbid crabs were immediately removed from tanks, placed into individual polyethene bags and held at −20°C. Survivors were fed half a locally sourced pilchard (Sardinops sagax) every second day following leg excision, and any remaining food was removed the following morning. Total ammonia nitrogen and dissolved oxygen levels were recorded every second day following feeding (Supplementary Material S1).
Pesticide residue extraction and analyses
A QuEChERS (Q-Sep, LECO) protocol was used to extract pesticide residues from crab tissues, as described in Butcherine et al. (2020), with minor modifications. In summary, 1-g samples of each cooked and raw crabs’ flesh and brown meat were prepared and homogenised using a mortar and pestle. For crabs that survived the 12-d depuration period (n = 21; see Results), samples of flesh from each individuals’ pereopods at each sampling point were prepared as above. Composite samples of three local-caught sea mullet fillets (used to bait traps) and 10 pilchards (experimental food) were also prepared. Samples were transferred to 15-mL polypropylene centrifuge tubes and High Performance Liquid Chromatography (HPLC)-grade acetonitrile (MeCN; 99.9% purity; Sigma-Aldrich, Germany) was added (1 g sample: 1 mL MeCN) before samples were vortexed for 1-min. Batches of Q-sep™ salt were prepared following method EN 15662 and added to the samples (1 g sample: 0.65 g salt), which were agitated as before. All salt components were analytical grade (Sigma-Aldrich, Germany). Samples were centrifuged for 5 min at 4500 RPM and the resulting MeCN aliquots (~0.5–1 mL) were collected and transferred to 3-mL amber vials for liquid chromatography–mass spectrometry–mass spectrometry (LC-MS-MS) analysis. Each extract was analysed once due to limited sample volume and financial constraints. Samples were stored at −20°C prior to transport to ALS Environmental Laboratory, Sydney for analysis.
Preliminary samples of raw crab flesh and brown meat (n = 5) were submitted and screened for the extended multi-residue pesticide suite of 171 analytes (EP234 A-J ext.; Supplementary Material S2). Due to the high costs of this screening suite, all subsequent samples from the thermal processing and depuration experiments were screened for a reduced suite of 156 analytes (EP234 A-I ext.), which covered all compounds detected in the preliminary samples and in crabs previously collected from the Clarence River (Supplementary Material S2; Gray, et al., unpublished data). From the depuration experiment, only samples from individuals that survived the 12-d experimental period were processed and included in our analyses. Samples from the first and second sampling points (i.e., day 0 and day 2; n = 21) were initially submitted for analyses, whereas for all subsequent points, samples were only analysed from individuals associated with previously detected residues (n = 11).
Pesticide screening involved in-house methods developed by ALS (EP068-ES, EP234-1, EP234-1x, EP234–2 and EP234-2x) with dilution (10 × in MilliQ water) and filtration (20 µm) prior to direct injection. Method EP068-ES was referenced to the USEPA SW 846–8270 method compliant with NEPM Schedule B(3). All other methods employed electrospray ionisation (ESI) in positive or negative modes to accommodate the ionisation properties of different target analytes. Analysis was conducted using Shimadzu LCMS-8060 and LCMS-8060NX instruments (Japan) calibrated using custom reference standards from AccuStandard (ANLAB and ISO 17034 accredited). Quality control standards were compliant with the Australian National Environment Protection Measures, which included laboratory duplicates, spiked laboratory control samples, method blanks and matrix spikes for each work order. All methods were compliant with the International Standards ISO/IEC17025:2017. Only analytes detected above the LOR were included in our analyses, and values below the limit of detection (LOD) were considered to be equal to 1/10 of the LOR.
Moisture content and mass balance concentration calculations
Thermal processing methods can influence food moisture content and, therefore, the residual concentrations of pesticides (see Sundhar et al., 2023). Subsamples (~1 g) of replicate raw and cooked crab flesh and brown meat were weighed using an analytical balance (± 0.0001 g precision) and dried at 60°C to a constant weight. Samples were re-weighed to calculate moisture content (%). Moisture content was only determined for the brown meat of cooked individuals with sufficient tissue present (n = 10). The average moisture content of cooked and raw crab flesh was 81.7% (± 1.1 SD) and 84.7% (± 1.7), respectively (Supplementary Material S3). The moisture content of the brown meat was 86.3% (± 3.6) for raw crabs and 76.0% (± 5.3) for cooked crabs. To account for the effects of moisture error on pesticide residues (PRs) in cooked crab tissues, PR mass balanced concentrations and the PR reduction % were calculated for each detected compound following the below equations:
where and are the average pesticide residue concentrations in cooked and raw crabs, and and is the average moisture content in cooked and raw crabs, respectively.
Statistical analyses
Thermal processing experiment
Gaussian linear (LMs) and linear mixed models (LMMs) were used to assess for effects of treatment (i.e. cooked and raw) on the residual concentrations of different pesticide compounds in giant mud crab tissues. Despite the potential for toxicokinetic interactions between pesticides (see Hernández et al., 2017), all analytes were treated independently here. Separate models were fitted to evaluate the effects of treatment on each compound associated with a sufficient number of detections to meaningfully test for effects, as well as the total pesticide residual concentration (∑ all detected residues). For all initial (full) models fitted, ‘treatment’ (categorical: cooked and raw), crab ‘sex’ (categorical: male and female), ‘carapace grade’ (categorical: A, B and C), body size (continuous: ‘CL’ and ‘CW’ (cm), and ‘weight’ (g)) and average ‘cooking temperature’ (°C; continuous; set at zero for raw crabs) were included as fixed effects.
Several crabs were missing chelipeds, and weights for these individuals were back-calculated using the standard sex-specific growth function y = axb (see Froese, 2006) for crabs measured across the two collections. Collinear predictors were considered redundant. Thus, only CL (which correlated with CW; Pearson’s r > ± 0.95) was retained in all models, given the utility of this metric in the management of giant mud crabs off eastern Australia (Hewitt et al., 2023). 'Tissue' (flesh and brown meat; fixed) was also included as a predictor for compounds detected in both edible portions and crab ID was included in LMMs as a random effect to account for the correlation structure between residues detected in multiple tissues from the same individual (Zuur, 2009). All of the full models are presented in Supplementary Material S4.
To derive optimal models, a stepwise-backward selection process was used, where the least significant predictor was removed at each stage, and models refitted until all terms were significant (p< 0.05). The significance of predictors was determined using Type III F statistics for LMs and Wald chi-square statistics for LMMs using the ‘anova’ function from the ‘car’ package (Fox and Weisberg, 2018). For terms with more than two levels, significant differences were determined using pairwise comparisons adjusted for the false discovery rate (FDR; Benjamini and Hochberg, 1995). Terms with p-values 0.05–0.10 were initially excluded from the optimal models and refitted. Akaike information criterion (AIC) values were computed for both models, and these predictors were retained if their inclusion did not result in an increase in the AIC (>2 AIC units; Zuur, 2009).
Diagnostic plots, normal quantile-quantile plots and dispersion tests were performed to evaluate the distribution of residuals and check for normality using the ‘DHARMa’ package (Hartig, 2022). Responses were log-transformed to help validate these assumptions, which were met for all models except for propargite. All LMMs were fitted using the ‘lmer’ function in the ‘lme4’ package (Bates, 2015), and LMs fitted using the ‘lm’ function in base R version 4.3.1 (R Core Team, 2023).
Depuration experiment
Imidacloprid was the only pesticide detected in the crabs held for depuration (see Results), and only data for individuals that this compound was detected in on days zero or two (n = 11) were analysed. Because imidacloprid residues did not consistently decrease across individuals throughout depuration, a decay model was unable to be fitted (see Results). Instead, we tested for temporal changes in imidacloprid residues in individual crabs using a LMM.
The initial (full) depuration model utilised the same methodology as described above, but included depuration ‘time’ (categorical: 0, 2, 6 and 12) as a fixed effect. The optimal depuration model was produced using the same backward selection process. Due to low replication and statistical power, pairwise post-hoc t-tests using model estimated marginal means assessed for significant differences in imidacloprid residues between different time points using the ‘emmeans’ package (Lenth, 2024). The distribution of residuals and normality were checked, and imidacloprid residual concentrations were log-transformed to better validate these assumptions.
Human health-risk assessment
The dietary exposure of pesticides through crab consumption was calculated using established hazard indices used by Food Safety Australia New Zealand (FSANZ) and the US Environmental Protection Agency. All hazard indices were calculated for crabs pre- (i.e. raw) and post-processing (i.e. cooked and post-depuration). Estimated daily intake (EDI; mg kg−1 body weight day−1) is a measure of the amount of a pesticide residue a person ingests over a day for a given food commodity, adjusted for body mass. Estimated daily intake was calculated for adults (ages 2+) and children (ages 2 to 6) for each residue detected in crab tissue(s). As a precautionary measure, EDIs were calculated using the maximum detected concentrations of pesticides, following:
where is the maximum pesticide residual concentration detected in the tissue (g kg−1), IR is the 90th percentile of the consumer’s crustacean ingestion rate (g day−1 for adults and children) derived from the Australian National Nutrition and Physical Activity Survey 2011 (Australian Bureau of Statistics (ABS), 2014), FI is the fraction of sample from a contaminated source (set as 1 for a conservative estimate) and BW is the consumer’s body weight (70 and 19 kg for adults and children, respectively) following FSANZ (2017). Because no Australian consumption data for crab brown meat are available, ingestion rates for white meat (flesh) were used.
The risk quotient (RQ) is a measure of the non-carcinogenic risk of a contaminant to consumers, quantified as the ratio of the EDI to a toxicity reference value (TRV). Risk quotients > 1 indicate that there may be negative health effects, whereas RQs< 1 suggest no negative health effects. Risk quotients were calculated for adults and children for each of the detected residues, following:
where TRV is the toxicity reference value, calculated by subtracting the background dietary intake (where available) from the acceptable daily intake (ADI) provided by the Australian Pesticides and Veterinary Medicines Authority (APVMA), (2024).
Results
Among the crabs collected for both the thermal processing and depuration experiments, five pesticide residues were detected in edible portions (Table 1). Specifically, pesticide residues were detected in 98% of the crabs collected for the thermal processing experiment, whereas imidacloprid was the only residue detected in 52% of crabs (pereopod flesh) in the depuration experiment. All compounds were less frequently detected in cooked individuals’ flesh and brown meat relative to raw crabs, with the exception of cyprodinil (flesh only). All five analytes were detected in raw crabs, while only cyprodinil, imidacloprid and propargite were detected in cooked crabs (Table 1). Propargite was also detected at low levels (<5 µg L−1) in composite samples of regional sea mullet used to bait traps, and in pilchards used for food in the depuration experiment (Supplementary Material S5).
Table 1
| Cyprodinil | Diuron | Imidacloprid | Propargite | Triazophos | ||
|---|---|---|---|---|---|---|
| Flesh | n | |||||
| Raw | 20 | 35% | 20% | 10% | 5% | ND |
| Cooked | 20 | 70% | ND | 5% | 5% | ND |
| Pre-depuration † | 21 | ND | ND | 52% | ND | ND |
| Brown meat | ||||||
| Raw | 20 | ND | ND | 20% | 100% | 5% |
| Cooked | 10 | ND | ND | ND | 70% | ND |
Percentage of wild-caught giant mud crabs (Scylla serrata) collected from the Clarence River, eastern Australia, with pesticide residues detected in their flesh and ‘brown meat’ (i.e. hepatopancreas and gonads).
Crabs used in the thermal processing and depuration experiments were from separate collections. ND, not detected.
†pereiopod only sampled from survivors of the 12-d depuration period; includes detections in individuals pooled across all time points.
Thermal processing experiment
On average, the concentrations of all individual pesticides and their combined total residues were higher in raw than cooked crab tissues, except for cyprodinil (Figure 1). Body weight, CL and average cooking temperature had no significant effects on any response variables (LM, p > 0.05; Supplementary Material S4). The optimal LM for cyprodinil residues in crab flesh contained two fixed factors: treatment and sex (Table 2). Significantly higher (by ~51%) cyprodinil concentrations were detected in cooked (mean: 0.54 µg kg−1) than raw crabs (0.25 µg kg−1; LM, p = 0.025; Figures 1a, 2). Further, significantly greater cyprodinil residues were detected in males (0.49 µg kg−1) than females (0.01 µg kg−1 (ND); LM, p < 0.001; Supplementary Material S6).
Figure 1
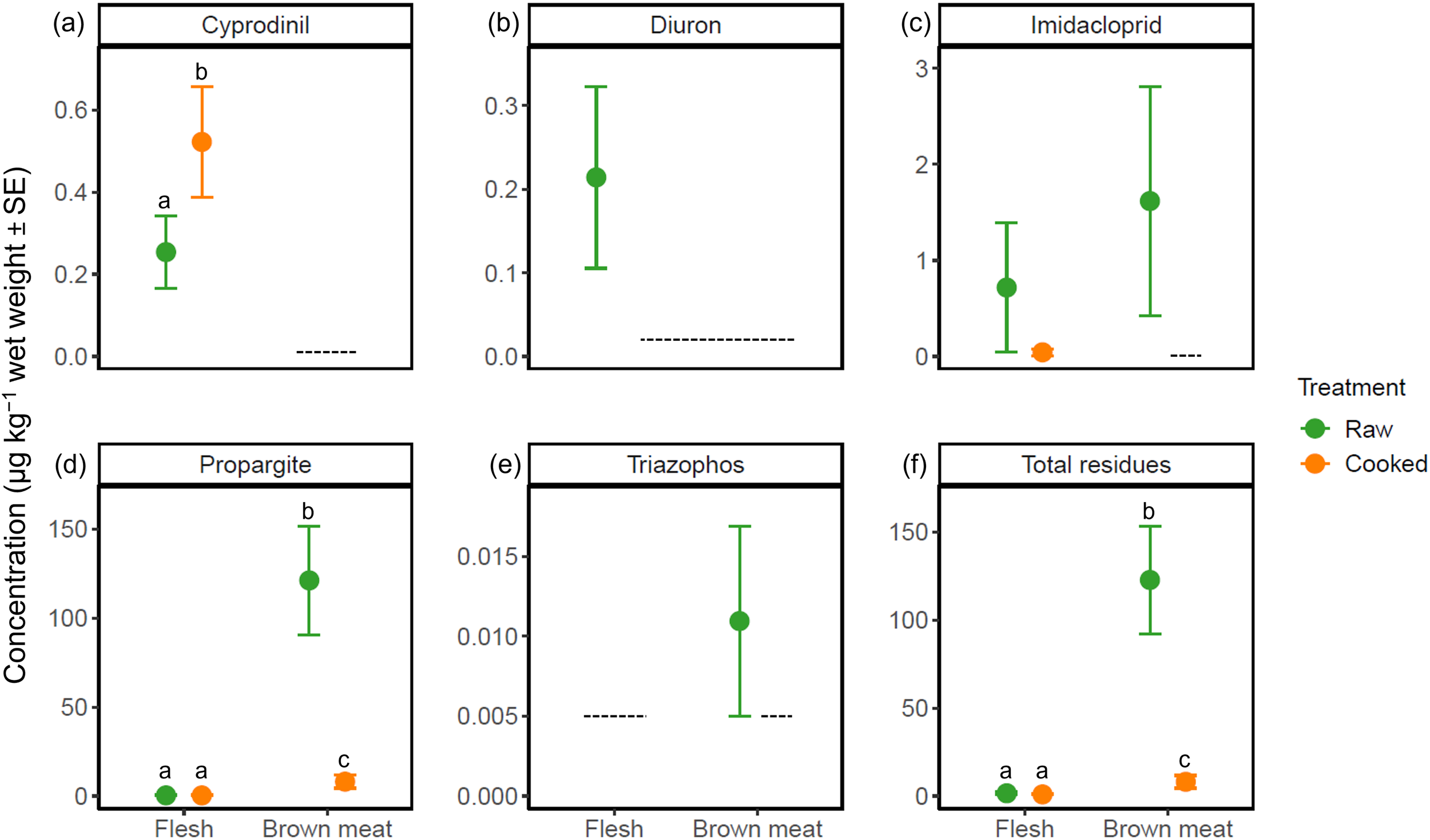
Average (µg kg−1 wet weight ± SE) concentrations of (a) cyprodinil, (b) diuron, (c) imidacloprid, (d) propargite, (e) triazophos and (f) total pesticide residues detected in raw and cooked giant mud crab (Scylla serrata) flesh (muscle) and brown meat (i.e. hepatopancreas and gonads). Data were mass-balanced to account for the reduction in moisture content following thermal processing. Dashed lines denote limits of detection. Letters denote statistical differences between treatments and tissues for compounds with sufficient numbers of detections based on the results of separate linear models (cyprodinil only) and linear mixed models. n = 10 for cooked brown meat and n = 20 for all other treatments.
Table 2
| Thermal processing | ||||||
|---|---|---|---|---|---|---|
| Compound | Treatment | Tissue | Treatment × tissue | Sex | Carapace grade | LMM or FDR separated means |
| Cyprodinil | 0.025 | NA | NA | <0.001 | – | • cooked > raw; • males > females |
| Propargite | – | <0.001 | <0.001 | – | 0.102 | • brown meat > flesh • raw brown meat > cooked brown meat > raw flesh = cooked flesh • Grade A = B = C |
| Total residues | – | 0.007 | <0.001 | 0.082 | – | • brown meat > flesh • males =(.) females • raw brown meat > cooked brown meat > raw flesh = cooked flesh |
| Depuration | ||||||
| Compound | Time | Comparison(s) | ||||
| Imidacloprid | 0.044 | day 0 = day 2 = day 6 > day 12 | ||||
Summary of fixed factors and their significance in optimal linear mixed models assessing variability among log-transformed concentrations of different pesticides and total residues detected in cooked and raw (treatment) giant mud crab (Scylla serrata) tissues, and the depuration of imidacloprid residues in crab pereiopods.
Relevant significant effects were separated via false discovery rate (FDR) or LMM pairwise comparisons (p< 0.05). ‘Crab ID’ was included as a random effect in thermal processing models for compounds detected across both crab tissues (flesh and brown meat) and in the depuration model. Significant terms are in bold (p< 0.05).
-: not significant, NA: not applicable, (.) denotes marginally significant difference between levels (0.05< p < 0.10).
Figure 2
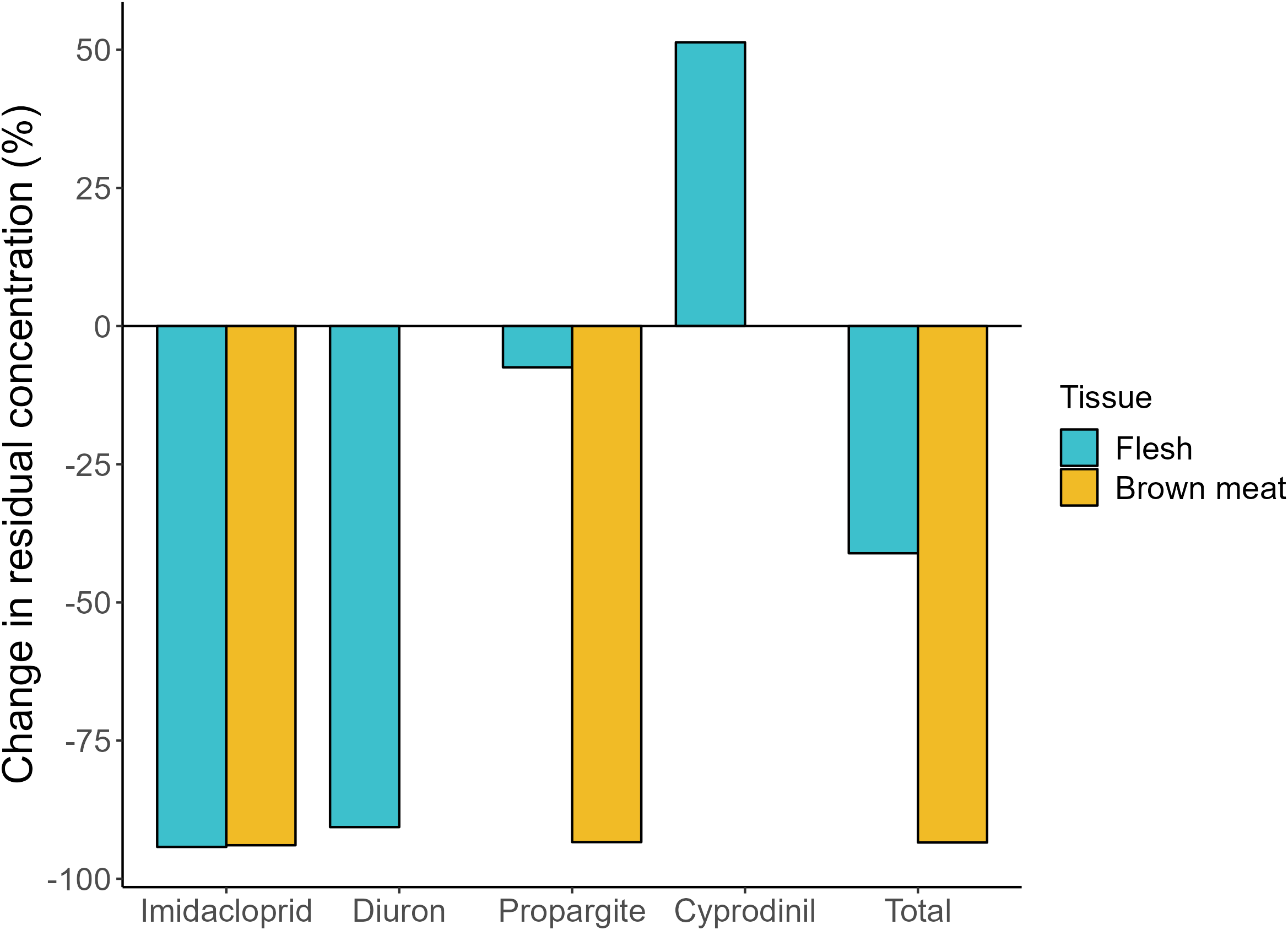
Percentage change in residual pesticide concentrations in giant mud crab (Scylla serrata) flesh and brown meat (i.e. hepatopancreas and gonads) following thermal processing (cooking). Data were mass-balanced to account for the reduction in moisture content following cooking. Negative values indicate the pesticide concentration was reduced by thermal processing, while positive values indicate the residual concentration was higher in cooked than raw crabs. n = 10 for cooked brown meat, n = 20 for all other tissues.
The optimal LMM for propargite comprised three predictors: carapace grade, tissue and its interactive effect with treatment, which accounted for ~84% of the variation in residues in tissues (Table 2; Supplementary Material S4). False discovery rate pairwise comparisons found no significant differences in propargite residues across carapace grades, although ‘A’-grade crabs tended to have greater concentrations (means across both tissues: 67.02 µg kg−1) than ‘B’-grade crabs (25.32 µg kg−1; p = 0.10; Table 2; Supplementary Material S6). Pairwise comparisons for the interactive term found that cooking significantly reduced propargite residues in brown meat (by ~93%; FDR, p < 0.001; Figures 1d, 2). Propargite residues in crab flesh (means: 0.34 and 0.32 µg kg−1 for raw and cooked, respectively) were significantly lower than in brown meat (121.21 and 9.06 µg kg−1, respectively; FDR, p < 0.001; Figure 1d; Table 2). However, treatment did not influence propargite concentrations in flesh (FDR, p > 0.05), which were on average ~7% lower in raw crabs (Figure 2).
Diuron residual concentrations averaged 0.21 µg kg−1 in raw crab flesh, which were ~91% higher than in cooked flesh (0.02 µg kg−1 (ND); Figures 1b, 2). Triazophos residues were not detected in the edible portions of any cooked crabs, but were in the brown meat of one raw individual (mean: 0.01 µg kg−1; Figure 1e). Imidacloprid residues in both raw crab flesh and brown meat (means: 0.72 and 1.62 µg kg−1, respectively) were ~94 and ~99% higher than concentrations in the respective cooked tissues (0.05 µg kg−1 and ND; Figures 1c, 2). Statistical differences were unable to be determined between treatments for any of these analytes, due to an insufficient number of detections for robust comparison (Table 1).
Three predictors were included in the optimal LMM for the total residues: sex, tissue and the latter’s interaction with treatment (Table 2). Although not significant, across treatments and tissues, total pesticide residues were higher in males (mean: 44.80 µg kg−1) than females (8.85 µg kg−1) (LMM, p = 0.08; Table 2). The interaction between tissue and treatment was significant (LMM, p < 0.001), and FDRs revealed significantly lower (by ~93%) total residues in cooked (9.07 µg kg−1) than raw brown meat (122.83 µg kg−1) (p< 0.01; Figure 3f, 4; Table 2). Thermal processing decreased the total residual concentrations of pesticides in flesh by 41% (Figure 2). However, due to the high variability in the data, the total residues in flesh did not vary between raw (1.53 µg kg−1) and cooked crabs (0.93 µg kg−1; FDR, p > 0.05), although these concentrations were significantly lower than in the brown meat (FDR, p < 0.01). These results were largely driven by propargite concentrations, which accounted for >90% of the residues detected across all samples.
Figure 3
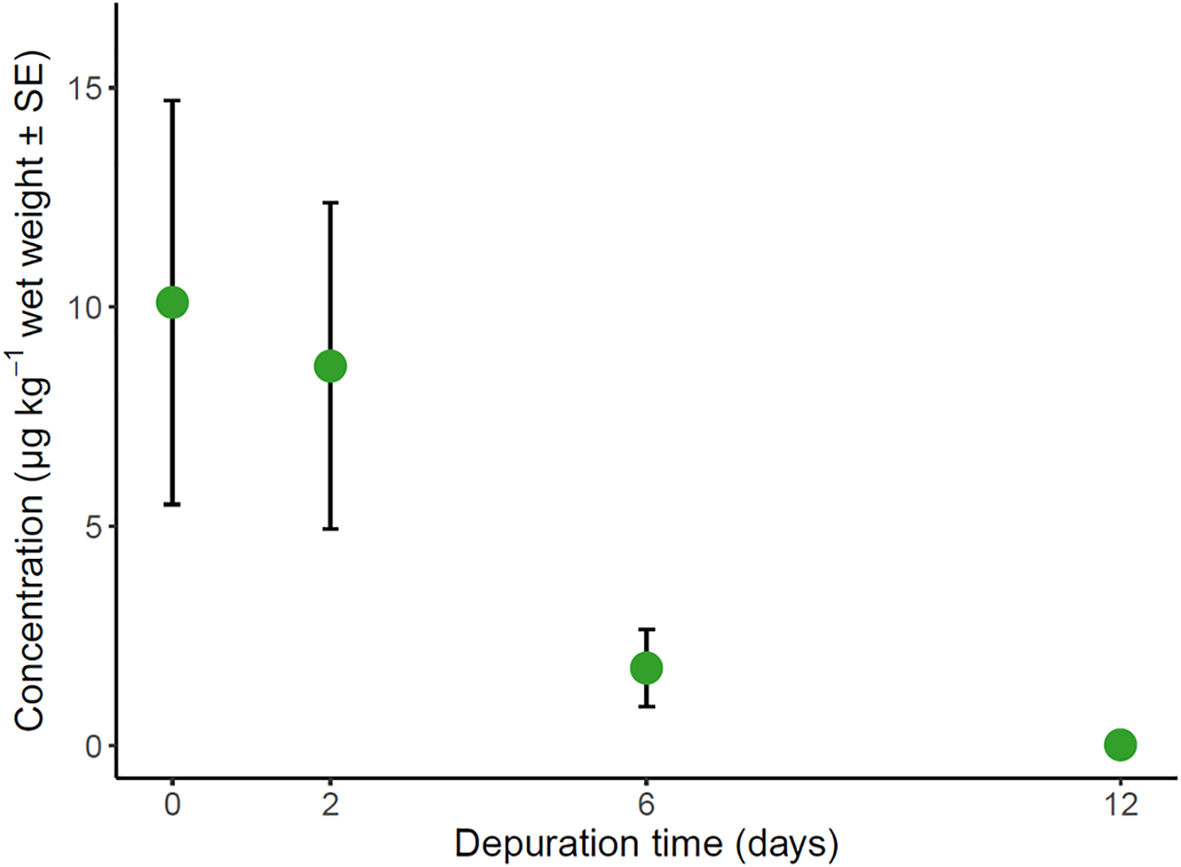
Average (µg kg−1 wet weight ± SE) concentrations of imidacloprid residues detected in giant mud crab (Scylla serrata) pereiopod flesh (walking legs) over the 12-day depuration experiment, n = 11 for each sampling point.
Depuration experiment
Of the 31 individuals collected for depuration, 21 (68%) survived the 12 days in holding (Supplementary Material S7). Mortalities occurred in five of the seven tanks, with 80% of deaths occurring within three-days post-collection. Total ammonia and dissolved oxygen levels remained stable in all tanks throughout the depuration period (Supplementary Material S1).
Imidacloprid residues in surviving crab pereiopods were highest pre-depuration, reaching 38.9 µg kg−1 (Figure 3). Crab biometrics had no significant effects on the depuration of imidacloprid residues from flesh (LMM, p > 0.05; Supplementary Material S4). Time was the only fixed predictor included in the optimal model (LMM, p = 0.04; Table 2), which accounted for ~16% of the variation in the concentration of imidacloprid residues (Supplementary Material S4). On average, imidacloprid residues decreased throughout the 12-day holding period, although starting concentrations and depuration trajectories varied among individuals (Figure 3; Supplementary Material S8). Notably, peaks in the residual concentrations of imidacloprid were recorded in several individuals after two- and six-days of depuration (n = 4 and 3, respectively; Supplementary Material S8). On average, imidacloprid concentrations were ~81% lower in crab muscle after six days in holding (1.95 µg kg−1) than for pre-depuration (day 0; 10.11 µg kg−1), although these differences were not significant (post-hoc test, p > 0.05). Pairwise comparisons found that imidacloprid residues were significantly lower in crabs sampled on day 12 (mean: 0.04 µg kg−1) than all other time points (post-hoc test, p < 0.05; Table 2).
Human health-risk assessment
Overall, EDI values for adults and children were low for all pesticides across all tissues and treatments, ranging from <0.00001–0.00058 mg kg−1 body weight day−1 (Table 3). Adult and child risk quotients (RQs) were also low (< 0.1) for all analytes detected in all tissues and treatment groups, except for propargite in raw brown meat—which was associated with relatively higher RQ values for adults (0.47) and children (0.58), but still posed no negative health effects to consumers (i.e. RQ < 1; Table 3). Furthermore, cooking markedly reduced the RQs for this analyte in brown meat to 0.03 and 0.04 for adults and children, respectively. Post-harvest processing reduced RQs for all detected analytes, except cyprodinil.
Table 3
| Flesh | ||||||
|---|---|---|---|---|---|---|
| Pesticide | Treatment | Max. concentration (mg kg−1) | EDI (adult) | EDI (child) | RQ (adult) | RQ (child) |
| Cyprodinil | Raw | 0.00131 | <0.00001 | <0.00001 | 0.00004 | 0.00005 |
| Cooked | 0.00226 | <0.00001 | <0.00001 | 0.00007 | 0.00008 | |
| Diuron | Raw | 0.00171 | <0.00001 | <0.00001 | 0.00022 | 0.00027 |
| Cooked | ND | – | – | – | – | |
| Imidacloprid | Raw | 0.03890 | 0.00004 | 0.00004 | 0.00058 | 0.00072 |
| Cooked | 0.00068 | <0.00001 | <0.00001 | 0.00001 | 0.00001 | |
| Depuration | 0.00015 | <0.00001 | <0.00001 | <0.00001 | <0.00001 | |
| Propargite | Raw | 0.00480 | <0.00001 | 0.00001 | 0.00432 | 0.00531 |
| Cooked | 0.00430 | <0.00001 | 0.00001 | 0.00387 | 0.00475 | |
| Brown meat | ||||||
| Imidacloprid | Raw | 0.02370 | 0.00002 | 0.00003 | 0.00036 | 0.00044 |
| Cooked | ND | – | – | – | – | |
| Propargite | Raw | 0.52300 | 0.00047 | 0.00058 | 0.47070 | 0.57805 |
| Cooked | 0.03740 | 0.00003 | 0.00004 | 0.03366 | 0.04134 | |
| Triazophos | Raw | 0.00012 | <0.00001 | <0.00001 | 0.00056 | 0.00069 |
| Cooked | ND | – | – | – | – | |
Estimated daily intake (EDI; mg kg−1 body weight day−1) and risk quotient (RQ) values of different pesticide residues for consumers (adults and children) of giant mud crab (Scylla serrata) flesh and brown meat in eastern Australia.
Values are provided for all analytes detected in crab tissues pre- (raw) and post-processing (cooked and subject to depuration). Calculations are based on the maximum detected concentrations of pesticides in crab edible portions. All RQs were < 1, indicating no negative impacts on health.
Discussion
This study further supports the utility of cooking and depuration for reducing pesticide residues in seafood species (e.g. Bayen et al., 2005; Kwong et al., 2008; Ewere et al., 2019; Butcherine et al., 2021; Arisekar et al., 2022; Wang et al., 2022; Sundhar et al., 2023), while providing essential baseline data on agrichemical accumulation by giant mud crabs in an agriculturally intensive eastern Australian estuary. Further, the detection of pesticide residues in some local-caught teleosts sourced for the experimental work also indicates some widespread regional seafood contamination by agrichemicals. While concentrations were below the thresholds for consumer health concerns, they were nevertheless reduced in giant mud crab tissues via the assessed post-harvesting procedures. The likely mechanisms underlying the reductions in pesticide residues are explored below, followed by a discussion of the utility and practicality of each method for future application. The findings here do not constitute post-harvest seafood handling recommendations for minimising human health risks associated with seafood contamination and consumers should follow formal advice from relevant food safety authorities (e.g. Food Safety Australia New Zealand).
Efficacy of thermal processing for reducing pesticide residues
Our study shows that standard cooking practices (boiling in seawater at ~82°C for 2-min 100-g–1 body weight) significantly reduced the concentrations of most pesticide residues detected in the edible portions of giant mud crabs. Changes in residual concentrations varied among compounds, ranging from 7 to 99% across tissues (excluding cyprodinil), likely due to differences in their physico-chemical properties (Table 4). Previous studies have shown thermal processing comparably reduced various organochlorine and pyrethroid residues in fishes (Bayen et al., 2005), seaweed (Sundhar et al., 2023) and prawns (Arisekar et al., 2022). Considered collectively, the available data imply that cooking (including prior to sale) is likely a viable precautionary measure for any consumers concerned about exposure to pesticides. Nevertheless, the added temporal costs and reduction in revenue return for cooked products are some drawbacks for operationalisation by seafood industries (Poole et al., 2008).
Table 4
| Pesticide | Chemical class | Log Kow | Solubility (mg L−1) | Approved uses for relevant agricultural activities in Clarence Valley |
|---|---|---|---|---|
| Cyprodinil | Anilinopyrimidine fungicide | 3.59 (high) |
13 (low) |
Cucumbers and berry fruits |
| Diuron | Phenylurea herbicide | 2.68 (moderate) |
42 (low) |
Beans, cotton, potatoes, pulses and legumes, sugarcane, and wheat |
| Imidacloprid | Neonicotinoid insecticide | 0.57 (low) |
610 (moderate) |
Beans, berry fruits, cotton, cucumbers, cut flowers, hay, potatoes, pulses and legumes, sorghum, sugarcane, tomatoes, and turf wheat |
| Propargite | Organosulfur acaricide | 5.7 (high) |
0.215 (very low) |
Beans, cotton, and tomatoes |
| Triazophos | Organophosphate insecticide | 3.34 (high) |
39 (low) |
– |
Summary of chemical properties and relevant agricultural uses for pesticides detected in the edible portions of wild-caught giant mud crabs (Scylla serrata) collected from the Clarence River, eastern Australia.
All Log Kow and solubility values were sourced from National Center for Biotechnology Information (NCBI) (2021). The approved uses for the detected pesticides were derived from Australian Pesticides and Veterinary Medicines Authority (Australian Pesticides and Veterinary Medicines Authority (APVMA), (2022) and relevant agricultural industries to the Clarence Valley Local Government Area, New South Wales (gross economic value: >$100,000) were sourced from the Australian Bureau of Statistics (ABS), (2022).
Propargite—an organosulfurus acaricide—constituted most of the residues and was detected in 98% of crabs from the first collection. This chemical was also detected at low levels in the muscle of regional teleosts sourced for the experimental work, and previously in Sydney rock oysters (Saccostrea glomerata) collected from a similar location (Jamal et al., 2024). Propargite residues were higher in the lipid-rich brown meat constituents (Maulvault et al., 2012), likely due to this compound’s lipophilic nature (Table 4). The higher accumulation of propargite residues in ‘A’-grade crabs, relative to ‘softer’, recently moulted ‘B’-grade individuals may also be due to differences in tissue-lipid concentrations between moult stages (Harrison, 1990; Sánchez-Paz et al., 2006).
Thermal processing effectively reduced propargite residues in the brown meat, whereas only a small reduction was observed for the muscle. The relatively greater reduction in propargite residues in brown meat may be attributed to the higher moisture loss during boiling (present study; Zabik et al., 1992), which may have facilitated greater leaching into cooking media. Although not quantified here, the loss or denaturing of lipids and other brown meat proximate components during cooking may have also contributed to the reduction in propargite (and other lipophilic) residues (e.g. Bayen et al., 2005). Future work should account for changes to the nutritional constituents of seafood and test cooking media for residues, as this may help to explain the mechanism(s) responsible for the elimination of pesticides.
Of the detected analytes, the neonicotinoid insecticide imidacloprid exhibited the greatest average post-cooking reduction in residues (>94% in both portions), although these changes were based on relatively few detections. Imidacloprid is moderately water-soluble (Table 4) and hydrolyses rapidly at ~100°C (Wei et al., 2023). Hence, the loss of imidacloprid is likely to have occurred through hydrolysis or solubilisation into cooking media. Further, diuron (phenylurea herbicide) and triazophos (organophosphate insecticide) were both only detected in raw crab tissues. Both analytes have low solubility, and thermal degradation is the most likely mechanism for their (potential) elimination. Although not accounted for in the analyses, the (known) degradation products of several of these pesticides are more toxic and environmentally persistent than the parent compound (e.g. diuron; Giacomazzi and Cochet, 2004). Given the potential human health implications, further research into the biotransformation of pesticides during thermal processing is warranted.
Contrary to the general reduction in residues post-cooking, concentrations of cyprodinil (aminopyrimidine fungicide) were higher in cooked than raw crab tissues. Similar post-cooking increases in PFASs residues in various seafoods have been widely reported (e.g. Bhavsar et al., 2014; Vassiliadou et al., 2015; Taylor et al., 2019) and were attributed to changes in mass (i.e. the concentration effect). However, our analyses used mass-balanced concentrations of pesticide residues, which accounted for the loss of moisture content during cooking. Instead, the increase in cyprodinil residues may reflect changes to the binding efficiency in crab muscle. For instance, Xie et al. (2023) showed that thermal processing affected the binding capacity of the pyrethroids cypermethrin and fenpropathrin to oyster tissue, which increased their recovery. Although speculative, similar extraction methods were used in both studies and hence, it is feasible to conject a similar mechanism drove the observed change in cyprodinil concentrations. Alternatively, changes to the proximate components of crab muscle due to matrix shrinkage during cooking (e.g. protein; Maulvault et al., 2012) may have contributed to the increase in cyprodinil concentrations.
Higher levels of cyprodinil (and total pesticide) residues were detected in male than female crabs. This variability may be attributed to differences in physiology (Hidir et al., 2021) or behaviour. Female giant mud crabs have been documented undertaking spawning migrations off northern NSW during the same time as the study, which may have influenced their potential for either exposure, catchability and/or their natural depuration away from contaminated areas (Hewitt et al., 2022). Such sex-effects (and consumer preferences) may be useful in refining exposure risk models (e.g. Madenjian et al., 2016) and could have implications for the species’ market price, given these can vary substantially between sexes (Brown, 2010). Contrastingly, crab size metrics had little influence on the concentrations of residues, which may indicate some natural capacity for depuration—as shown for imidacloprid (discussed below).
The potential for any spatial movement of crabs away from contaminated areas raises an important consideration in terms of the broader selectivity of the baited traps used to target giant mud crabs in NSW. These gears are passive, only selecting those crabs that are actively feeding (Bacheler, 2024). Hence, the level of pesticide contamination reported in the sampled specimens may not accurately represent the environmental condition of this estuary. Sampling giant mud crabs and other crustaceans using active methods (e.g. hand-gathering, trawls or seines) might better inform regional contaminant loads.
The utility and challenges of operationalising depuration for eliminating pesticide residues
Imidacloprid was completely eliminated from >90% of giant mud crabs after 12 days, which is consistent with previous experimental studies on Sydney rock oysters (Ewere et al., 2019) and juvenile black tiger prawns (Penaeus monodon; Butcherine et al., 2021), where depuration occurred within ~four days. The relatively slower rate of depuration reported here may reflect the higher (and considerably more variable) concentrations of imidacloprid in crab tissues pre-depuration (Wang et al., 2022), or toxicokinetic differences between species (Kwong et al., 2008). Notably, peaks in the trajectories of imidacloprid residues were recorded in several individuals up to six days post-harvest—an important consideration for selecting timeframes for effective depuration. The delayed peak in imidacloprid residues post-collection likely reflects the mobilisation of residues throughout the body plan following uptake (Beyer and Meador, 2011), potentially due to recent exposure following periodic rainfall events during the collection dates (Yadav and Watanabe, 2018).
Depurating giant mud crabs would incur additional economic and temporal costs. The necessary holding duration would also require MRLs for current-use pesticides in Australian seafoods to be set (discussed below). Several commercial crab fishers and fishing cooperatives in eastern Australia already use holding tanks for the short-term (< 5 days) containment of crabs between capture and sale (Gray, pers. obs.). In this region, short-term (36 h) depuration of oysters is a well-established practice for eliminating microbial contaminants (Eyles and Davey, 1984; Ayres, 2018), and may also be effective for reducing tissue-PFASs concentrations (see O’Connor et al., 2018). Although currently applied by some industries, large-scale depuration of giant mud crabs would be inherently more challenging, given the size of animals, potential for aggressive interactions between conspecifics (Romano and Zeng, 2017), and prospectively longer holding periods (> 6 days, based on the present findings).
There are also some logistical considerations for appropriate depuration systems and recontamination. Unlike our approach here, which involved high water flow-through aquaria, many commercial holding systems are recirculating, and there is the potential for recontamination with eliminated compounds (and metabolites), which may be detected in water post-depuration (e.g. Butcherine et al., 2021). Recontamination is likely to be a major drawback for operationalising depuration for reducing pesticide residues and warrants further research into the fate of pesticides following elimination and potential mitigation options (e.g. filtration or chemical remediation). Notwithstanding these considerations, depuration presents a promising means for increasing marketability for some seafood species (i.e. caught in fishing gears with low associated mortality) contaminated with agrichemicals, especially with increasing global pesticide use (FAO, 2021).
Implications for regulation and seafood safety
In Australia, the potential for seafood contamination by current-use agrichemicals is not considered by legislation and MRLs are set at zero by default. Evidently, the detection of residues in giant mud crabs and other harvested species in eastern Australia (e.g. Jamal et al., 2024) suggests a range of MRLs for pesticides in seafoods should be developed. Further, crabs and seafoods, more generally, are consumed in various forms. For example, crustacean muscle is usually consumed cooked, whereas brown meat is consumed both raw and cooked (Maulvault et al., 2013; Madigan et al., 2018). Considering that thermal processing reduced the human health risks from pesticide residues in giant mud crab tissues, relevant consumption preferences should be accounted for in MRLs and future human risk exposure assessments.
The hepatopancreas functions as the primary site of contaminant accumulation in crustaceans (Vogt, 2019) and general food safety practice advises restricted consumption of this organ. However, some demographic groups are still likely to consume this tissue. A survey of recreational southern rock lobster (Jasus edwardsii) fishers in southern Australia showed that 15% of respondents consumed the hepatopancreas (mostly boiled) despite the heightened risk of exposure to paralytic shellfish toxins (Madigan et al., 2018). No Australian consumption studies have included portunid crab brown meat and the health risks from these tissues, which have relatively high residual concentrations of pesticides, are unclear. Since Australian brown meat consumption habits are unknown, our human health-risk assessment used established ingestion rates for crab muscle, which are likely higher than for these tissues (e.g. Madigan et al., 2018). Hence, our study likely provides a conservative overestimate of the risks of pesticide residues in crab brown meat.
It is well established that recreational fishers consume higher quantities of seafood than the general population, and may be at elevated risk of health effects from contaminated seafood (Picot et al., 2011). Restricted consumption advice and in extreme cases, fishing closures are implemented by agencies in response to public health concerns around seafood contamination (e.g. Manning et al., 2017). But, health concerns rarely play a role in setting recreational output controls (e.g. bag and size limits), particularly when managing consumer exposure to contamination from adjacent land uses. In Australia, recreational fisher bag and possession limits for giant mud crabs (both: five day–1 in NSW) are typically based on stock sustainability and household consumption habits (i.e. how many ‘fish can feed a family’). Based on the average meat yield of ~30% (Sreelakshmi et al., 2016), the average sized crab collected here (657 g wet weight) would yield 197 g of flesh—consumption of which, substantially exceeds the average daily crustacean ingestion rate (adult: 63 g) used in our health-risk assessment. Based on the low-level pesticide contamination reported here, current output controls for this species are likely to be protective of recreational fishers. However, our health-risk assessment neither accounts for exposure to multiple pesticides (Boobis et al., 2008), nor chronic health effects from total dietary intake (Nougadère et al., 2012). Adaptive changes to recreational fisher output controls could be refined in the future based on relevant exposure risk scenarios from seafood contamination, but this requires more comprehensive spatial and temporal monitoring of pesticides in seafood.
Pre-cooking processing methods, such as skinning, washing and dressing (i.e. removing viscera) have also been shown to reduce pesticide residues in meat and fish (reviewed by Sobral et al., 2018). Since the total residues were considerably higher in brown meat, removing the visceral mass prior to cooking may help to reduce the potential for pesticide leaching into abdominal muscle (Zabik et al., 1992). Further, cooking duration, temperature and the volume of cooking liquid have been shown to influence the elimination of PFASs from seafood (reviewed by Vendl et al., 2022), although these factors have not been considered for pesticides. Future studies could investigate the influence of dressing and cooking parameters to inform household culinary methods which maximise the reduction of pesticide residues for seafoods.
The high variation in pesticide profiles in crab muscle between collections may somewhat be an artefact of the different muscle portions sampled in the two experiments (see Materials and methods). Differences in metal profiles between abdominal and claw muscle have been established for this species (see Taylor, 2023) and other portunids (e.g. Yang et al., 2021), but biodistribution patterns are yet to be investigated for agrichemicals. Quantifying the distribution of pesticides across muscle portions would be useful for consumer exposure assessments and monitoring programs.
It is difficult to identify the potential point- and non-point sources of the pesticides detected in this study, given the high number of agricultural industries (as well as urban and industrial uses) these chemicals are registered for use (except for triazophos; Table 4). The Clarence Valley region from which crabs were sampled supports various broadacre (e.g. sugarcane, cereal crops and livestock) and intensive (e.g. berry fruits, cucumbers, macadamia nuts and turf farms) agriculture practices (Australian Bureau of Statistics (ABS), 2022). Comprehensive environmental and seafood monitoring in estuaries is required to link pesticide contamination to specific land uses.
Although the health risks to consumers from pesticide residues in these crabs appear low, sublethal impacts on crabs may have implications for future harvests (reviewed by Gray et al., 2025). A viable pathway for regulation to consider the increasing potential for seafood contamination with current-use agrichemicals is the establishment of reasonable MRLs, with consideration of the influence of typically used post-harvest processing methods. Exposure risk scenarios should reflect local seafood consumption habits and be supported by a comprehensive pesticide surveillance program in order to better safeguard consumer exposure.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because it is not required for work on invertebrates.
Author contributions
BG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft. CC: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. MB: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. MC: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. KB: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by a Southern Cross University Australian Research Training Program Scholarship to BG and funding from the Fisheries Research and Development Corporation (FRDC) on behalf of the Australian Government via projects 2022-010: Assessment of the interactive effects of climate change, floods and discard stress on the commercially important mud crab (Scylla serrata) and blue swimmer crab (Portunus armatus) and 2021-018: SafeFish (post-graduate student grant). Additional funding was provided by the NSW DPIRD Estuarine Asset Protection (NEAP) program.
Acknowledgments
We thank Dr. Laura Martín-Díaz for assisting with sample processing and commercial fisher Troy Billin for collecting crabs. We thank Dr. Rowan Chick (DPIRD fisheries) and the two reviewers for their time and comments that helped improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could beconstrued as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1624922/full#supplementary-material
References
1
Arisekar U. Shakila R. J. Shalini R. Jeyasekaran G. Padmavathy P. (2022). Effect of household culinary processes on organochlorine pesticide residues (OCPs) in the seafood (Penaeus vannamei) and its associated human health risk assessment: Our vision and future scope. Chemosphere297, 134075. doi: 10.1016/j.chemosphere.2022.134075
2
Asghar U. Malik M. F. Javed A. (2016). Pesticide exposure and human health: A review. J. Ecosyst. Ecogr.6, 5. doi: 10.4172/2157-7625.S5-005
3
Australian Bureau of Statistics (ABS) (2014). National nutrition and physical activity survey 2011–12.
4
Australian Bureau of Statistics (ABS) (2022). Value of agricultural commodities produced, Australia 2020–21.
5
Australian Pesticides and Veterinary Medicines Authority (APVMA) (2022). Public chemical registration information system search.
6
Australian Pesticides and Veterinary Medicines Authority (APVMA) (2024). Acceptable daily intakes (ADI) for agricultural and veterinary chemicals used in food producing crops or animals.
7
Ayres P. A. (2018). The status of shellfish depuration in Australia and South-East Asia (New York: CRC Press). doi: 10.1201/9781351074810-28
8
Azzouz A. Colón L. P. Souhail B. Ballesteros E. (2019). A multi-residue method for GC-MS determination of selected endocrine disrupting chemicals in fish and seafood from European and North African markets. Environ. Res.178, 108727. doi: 10.1016/j.envres.2019.108727
9
Bacheler N. M. (2024). A review and synthesis of the benefits, drawbacks, and considerations of using traps to survey fish and decapods. ICES J. Mar. Sci.81, 1–21. doi: 10.1093/icesjms/fsad20
10
Bajwa U. Sandhu K. S. (2014). Effect of handling and processing on pesticide residues in food—a review. J. Food Sci. Technol.51, 201–220. doi: 10.1007/s13197-011-0499-5
11
Bates D. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
12
Bayen S. Barlow P. Lee H. K. Obbard J. P. (2005). Effect of cooking on the loss of persistent organic pollutants from salmon. J. Toxicol. Environ. Health A68, 253–265. doi: 10.1080/15287390590887816
13
Béné C. Barange M. Subasinghe R. Pinstrup-Andersen P. Merino G. Hemre G.-I. et al . (2015). Feeding 9 billion by 2050 – Putting fish back on the menu. Food Secur.7, 261–274. doi: 10.1007/s12571-015-0427-z
14
Benjamini Y. Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc Ser. B Stat. Methodol.57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
15
Beyer W. N. Meador J. P. (2011). Environmental Contaminants in Biota: Interpreting Tissue Concentrations (New York: CRC Press).
16
Beyuo J. Sackey L. N. Yeboah C. Kayoung P. Y. Koudadje D. (2024). The implications of pesticide residue in food crops on human health: A critical review. Discov. Agric.2, 123. doi: 10.1007/s44279-024-00141-z
17
Bhavsar S. P. Zhang X. Guo R. Braekevelt E. Petro S. Gandhi N. et al . (2014). Cooking fish is not effective in reducing exposure to perfluoroalkyl and polyfluoroalkyl substances. Environ. Int.66, 107–114. doi: 10.1016/j.envint.2014.01.004
18
Boenish R. Kritzer J. P. Kleisner K. Steneck R. S. Werner K. M. Zhu W. et al . (2022). The global rise of crustacean fisheries. Front. Ecol. Environ.20, 102–110. doi: 10.1002/fee.2431
19
Bolam T. Bersuder P. Burden R. Shears G. Morris S. Warford L. et al . (2016). Cadmium levels in food containing crab brown meat: A brief survey from UK retailers. J. Food Compos. Anal.54, 63–69. doi: 10.1016/j.jfca.2016.01.005
20
Boobis A. R. Ossendorp B. C. Banasiak U. Hamey P. Y. Sebestyen I. Moretto A. (2008). Cumulative risk assessment of pesticide residues in food. Toxicol. Lett.180, 137–150. doi: 10.1016/j.toxlet.2008.06.012
21
Broadhurst M. K. Millar R. B. Hughes B. (2018). Utility of multiple escape gaps in Australian Scylla serrata traps. Fish. Res.204, 88–94. doi: 10.1016/j.fishres.2018.02.017
22
Brown I. W. (2010). Taking Female Mud Crabs (Scylla Serrata): Assessment of Risks and Benefits (Fisheries Research and Development Corporation).
23
Butcherine P. Kelaher B. P. Taylor M. D. Barkla B. J. Benkendorff K. (2020). Impact of imidacloprid on the nutritional quality of adult black tiger shrimp (Penaeus monodon). Ecotoxicol. Environ. Saf.198, 110682. doi: 10.1016/j.ecoenv.2020.110682
24
Butcherine P. Kelaher B. P. Taylor M. D. Lawson C. Benkendorff K. (2021). Acute toxicity, accumulation and sublethal effects of four neonicotinoids on juvenile black tiger shrimp (Penaeus monodon). Chemosphere275, 129918. doi: 10.1016/j.chemosphere.2021.129918
25
Calogeras C. Mayze J. Poole S. (2011). Using Industry Expertise to Build a National Scheme for Grading of Live Mud Crabs (Fisheries Research and Development Corporation).
26
Commelin M. C. Baartman J. E. Zomer P. Riksen M. Geissen V. (2022). Pesticides are substantially transported in particulate phase, driven by land use, rainfall event and pesticide characteristics—a runoff and erosion study in a small agricultural catchment. Front. Environ. Sci.10. doi: 10.3389/fenvs.2022.830589
27
Conrad S. R. White S. A. Santos I. R. Sanders C. J. (2021). Assessing pesticide, trace metal, and arsenic contamination in soils and dam sediments in a rapidly expanding horticultural area in Australia. Environ. Geochem. Health43, 3189–3211. doi: 10.1007/s10653-020-00770-1
28
Cuevas N. Martins M. Costa P. M. (2018). Risk assessment of pesticides in estuaries: A review addressing the persistence of an old problem in complex environments. Ecotoxicology27, 1008–1018. doi: 10.1007/s10646-018-1947-8
29
De Witte B. Coleman B. Bekaert K. Boitsov S. Botelho M. J. Castro-Jimenez J. et al . (2022). Threshold values on environmental chemical contaminants in seafood in the European Economic Area. Food Control138, 108978. doi: 10.1016/j.foodcont.2022.108978
30
Ellgehausen H. Guth J. A. Esser H. O. (1980). Factors determining the bioaccumulation potential of pesticides in the individual compartments of aquatic food chains. Ecotoxicol. Environ. Saf.4, 134–157. doi: 10.1016/0147-6513(80)90053-0
31
Ernst F. Alonso B. Colazzo M. Pareja L. Cesio V. Pereira A. et al . (2018). Occurrence of pesticide residues in fish from South American rainfed agroecosystems. Sci. Total Environ.631, 169–179. doi: 10.1016/j.scitotenv.2018.03.002
32
Ewere E. E. Powell D. Rudd D. Reichelt-Brushett A. Mouatt P. Voelcker N. H. et al . (2019). Uptake, depuration and sublethal effects of the neonicotinoid, imidacloprid, exposure in Sydney rock oysters. Chemosphere230, 1–13. doi: 10.1016/j.chemosphere.2019.05.001
33
Eyles M. J. Davey G. R. (1984). Microbiology of commercial depuration of the Sydney rock oyster, Crassostrea commercialis. J. Food Prot.47, 703–707. doi: 10.4315/0362-028X-47.9.703
34
FAO (2021). “Pesticides use, pesticides trade and pesticides indicators 1990–2019,” in FAOSTAT Analytical Brief 46 (FAO, Rome).
35
Fox J. Weisberg S. (2018). An R companion to applied regression. Sage publications.
36
Froese R. (2006). Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol.22, 241–253. doi: 10.1111/j.1439-0426.2006.00805.x
37
FSANZ (2017). Assessment of potential dietary exposure to perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and perfluorohexane sulfonate (PFHxS) occurring in foods sampled from contaminated sites (FSANZ).
38
Giacomazzi S. Cochet N. (2004). Environmental impact of diuron transformation: A review. Chemosphere56, 1021–1032. doi: 10.1016/j.chemosphere.2004.05.001
39
Gray B. C. T. Champion C. Broadhurst M. K. Coleman M. A. Benkendorff K. (2025). Effects of contaminants and flooding on the physiology of harvested estuarine decapod crustaceans: A global review and meta-analysis. Environ. Pollut.364, 125347. doi: 10.1016/j.envpol.2024.125347
40
Handford C. E. Elliott C. T. Campbell K. (2015). A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manage.11, 525–536. doi: 10.1002/ieam.1635
41
Harrison K. E. (1990). The role of nutrition in maturation, reproduction and embryonic development of decapod crustaceans: A review. J. Shellfish Res.9, 1–28.
42
Harrison A. J. Rayner D. S. Tucker T. A. Lumiatti G. Rahman P. F. Gilbert D. M. et al . (2023). Clarence river floodplain prioritisation study.
43
Hartig F. (2022). DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.6. Available online at: https://CRAN.R-project.org/package=DHARMa.
44
Hernández A. F. Gil F. Lacasaña M. (2017). Toxicological interactions of pesticide mixtures: An update. Arch. Toxicol.91, 3211–3223. doi: 10.1007/s00204-017-2043-5
45
Hewitt D. E. Niella Y. Johnson D. D. Suthers I. M. Taylor M. D. (2022). Crabs go with the flow: Declining conductivity and cooler temperatures trigger spawning migrations for female giant mud crabs (Scylla serrata) in subtropical estuaries. Estuaries Coasts45, 2166–2180. doi: 10.1007/s12237-022-01066-6
46
Hewitt D. E. Taylor M. D. Suthers I. M. Johnson D. D. (2023). Environmental drivers of variation in southeast Australian giant mud crab (Scylla serrata) harvest rates. Fish. Res.268, 106850. doi: 10.1016/j.fishres.2023.106850
47
Hicks C. C. Cohen P. J. Graham N. A. J. Nash K. L. Allison E. H. D’lima C. et al . (2019). Harnessing global fisheries to tackle micronutrient deficiencies. Nature574, 95–98. doi: 10.1038/s41586-019-1592-6
48
Hidir A. Aaqillah-Amr M. A. Azra M. N. Shahreza M. S. Abualreesh M. H. Peng T. H. et al . (2021). Sexual dimorphism of mud crab, genus Scylla between sexes based on morphological and physiological characteristics. Aquac. Res.52, 5943–5961. doi: 10.1111/are.15467
49
Hook S. E. Doan H. Gonzago D. Musson D. Du J. Kookana R. et al . (2018). The impacts of modern-use pesticides on shrimp aquaculture: An assessment for north eastern Australia. Ecotoxicol. Environ. Saf.148, 770–780. doi: 10.1016/j.ecoenv.2017.11.011
50
Hopkins P. M. Das S. (2015). Regeneration in crustaceans. Natural history Crustacea4, 168–198. (UK: Oxford University Press).
51
Hornsby A. G. Wauchope R. D. Herner A. (2012). Pesticide properties in the environment (New York: Springer Science & Business Media).
52
Huan Z. Xu Z. Jiang W. Chen Z. Luo J. (2015). Effect of Chinese traditional cooking on eight pesticides residue during cowpea processing. Food Chem.170, 118–122. doi: 10.1016/j.foodchem.2014.08.032
53
Hussein M. A. Hammad O. S. Tharwat A. E. Darwish W. S. Sayed-Ahmed A. Zigo F. et al . (2022). Health risk assessment of organochlorine pesticide residues in edible tissue of seafood. Front. Vet. Sci.9. doi: 10.3389/fvets.2022.1042956
54
Islam M. S. Tanaka M. (2004). Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: A review and synthesis. Mar. pollut. Bull.48, 624–649. doi: 10.1016/j.marpolbul.2003.12.004
55
Ivorra L. Cardoso P. G. Chan S. K. Cruzeiro C. Tagulao K. (2023). Quantification of insecticides in commercial seafood sold in East Asian markets: Risk assessment for consumers. Environ. Sci. pollut. Res.30, 34585–34597. doi: 10.1007/s11356-022-25517-z
56
Jamal E. Reichelt-Brushett A. Gillmore M. Pearson B. Benkendorff K. (2024). Pesticide occurrence in a subtropical estuary, Australia: Complementary sampling methods. Environ. pollut.342, 123084. doi: 10.1016/j.envpol.2023.123084
57
Juanes F. Smith L. D. (1995). The ecological consequences of limb damage and loss in decapod crustaceans: A review and prospectus. J. Exp. Mar. Biol. Ecol.193, 197–223. doi: 10.1016/0022-0981(95)00101-8
58
Kaushik G. Satya S. Naik S. (2009). Food processing a tool to pesticide residue dissipation–A review. Food Res. Int.42, 26–40. doi: 10.1016/j.foodres.2008.09.009
59
Kennelly S. Watkins D. Craig J. (1990). Mortality of discarded spanner crabs Ranina ranina (Linnaeus) in a tangle-net fishery—Laboratory and field experiments. J. Exp. Mar. Biol. Ecol.140, 39–48. doi: 10.1016/0022-0981(90)90058-E
60
Kim K.-H. Kabir E. Jahan S. A. (2017). Exposure to pesticides and the associated human health effects. Sci. Total Environ.575, 525–535. doi: 10.1016/j.scitotenv.2016.09.009
61
Kirke A. Johnson D. Johnston D. Robins J. (2023). “Mud crabs, (2023),” in State of Australian Fish Stocks Report.
62
Kwong R. W. M. Yu P. K. N. Lam P. K. S. Wang W. X. (2008). Uptake, elimination, and biotransformation of aqueous and dietary DDT in marine fish. Environ. Toxicol. Chem.27, 2053–2063. doi: 10.1897/07-508.1
63
Laicher D. Benkendorff K. White S. Conrad S. Woodrow R. L. Butcherine P. et al . (2022). Pesticide occurrence in an agriculturally intensive and ecologically important coastal aquatic system in Australia. Mar. pollut. Bull.180, 113675. doi: 10.1016/j.marpolbul.2022.113675
64
Leadprathom N. Parkpian P. Satayavivad J. Delaune R. Jugsujinda A. (2009). Transport and deposition of organochlorine pesticides from farmland to estuary under tropical regime and their potential risk to aquatic biota. J. Environ. Sci. Health Part B44, 249–261. doi: 10.1080/03601230902728955
65
Lefrancq M. Jadas-Hécart A. La Jeunesse I. Landry D. Payraudeau S. (2017). High frequency monitoring of pesticides in runoff water to improve understanding of their transport and environmental impacts. Sci. Total Environ.587–588, 75–86. doi: 10.1016/j.scitotenv.2017.02.022
66
Lenth R. V. (2024). emmeans: estimated marginal means, aka least-squares means. R package version 1.10.2. https://CRAN.R-project.org/package=emmeans.
67
Lordan R. Zabetakis I. (2022). Cadmium: A focus on the brown crab (Cancer pagurus) industry and potential human health risks. Toxics10, 591. doi: 10.3390/toxics10100591
68
Lu C. Toepel K. Irish R. Fenske R. A. Barr D. B. Bravo R. (2006). Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ. Health Perspect.114, 260–263. doi: 10.1289/ehp.8418
69
Madenjian C. P. Rediske R. R. Krabbenhoft D. P. Stapanian M. A. Chernyak S. M. O’Keefe J. P. (2016). Sex differences in contaminant concentrations of fish: A synthesis. Biol. Sex Differ.7, 42. doi: 10.1186/s13293-016-0096-4
70
Madigan T. Turnbull A. Tan J. Pearn R. McLeod C. (2018). Rock lobster hepatopancreas consumption data for dietary exposure assessment among recreational harvesters in Tasmania and South Australia. Hum. Ecol. Risk Assess.24, 1565–1578. doi: 10.1080/10807039.2018.1438171
71
Manning T. M. Roach A. C. Edge K. J. Ferrell D. J. (2017). Levels of PCDD/Fs and dioxin-like PCBs in seafood from Sydney Harbour, Australia. Environ. pollut.224, 590–596. doi: 10.1016/j.envpol.2017.02.051
72
Maulvault A. L. Anacleto P. Lourenço H. M. Carvalho M. L. Nunes M. L. Marques A. (2012). Nutritional quality and safety of cooked edible crab (Cancer pagurus). Food Chem.133, 277–283. doi: 10.1016/j.foodchem.2012.01.019
73
Maulvault A. L. Cardoso C. Nunes M. L. Marques A. (2013). Risk–benefit assessment of cooked seafood: Black scabbard fish (Aphanopus carbo) and edible crab (Cancer pagurus) as case studies. Food Control32, 518–524. doi: 10.1016/j.foodcont.2013.01.045
74
McLeod C. Polo D. Le Saux J. C. Le Guyader F. S. (2017). Depuration and relaying: A review on potential removal of norovirus from oysters. Compr. Rev. Food Sci. Food Saf.16, 692–706. doi: 10.1111/1541-4337.12273
75
Milly P. C. D. Wetherald R. T. Dunne K. Delworth T. L. (2002). Increasing risk of great floods in a changing climate. Nature415, 514–517. doi: 10.1038/415514a
76
Momenzadeh Z. Khodanazary A. Ghanemi K. (2017). Effect of different cooking methods on vitamins, minerals and nutritional quality indices of orange-spotted grouper (Epinephelus coioides). J. Food Meas. Charact.11, 434–441. doi: 10.1007/s11694-016-9421-6
77
National Center for Biotechnology Information (NCBI) (2021). PubChem compound summary.
78
National Mud Crab Industry Reference Group (2012). Guide to using the Australian industry live mud crab grading scheme.
79
Noël L. Chafey C. Testu C. Pinte J. Velge P. Guérin T. (2011). Contamination levels of lead, cadmium and mercury in imported and domestic lobsters and large crab species consumed in France: Differences between white and brown meat. J. Food Compos. Anal.24, 368–375. doi: 10.1016/j.jfca.2010.12.004
80
Nougadère A. Sirot V. Kadar A. Fastier A. Truchot E. Vergnet C. et al . (2012). Total diet study on pesticide residues in France: Levels in food as consumed and chronic dietary risk to consumers. Environ. Int.45, 135–150. doi: 10.1016/j.envint.2012.03.001
81
Nyman A.-M. Hintermeister A. Schirmer K. Ashauer R. (2013). The insecticide imidacloprid causes mortality of the freshwater amphipod Gammarus pulex by interfering with feeding behavior. PloS One8, e62472. doi: 10.1371/journal.pone.0062472
82
O’Connor W. A. Zammit A. Dove M. C. Stevenson G. Taylor M. D. (2018). First observations of perfluorooctane sulfonate occurrence and depuration from Sydney Rock Oysters, Saccostrea glomerata, in Port Stephens NSW Australia. Mar. pollut. Bull.127, 207–210. doi: 10.1016/j.marpolbul.2017.11.069
83
Ouyang W. Zhang Y. Gu X. Tysklind M. Lin C. Wang B. et al . (2019). Occurrence, transportation, and distribution difference of typical herbicides from estuary to bay. Environ. Int.130, 104858. doi: 10.1016/j.envint.2019.03.066
84
Picot C. Nguyen T. A. Carpentier F. G. Roudot A. C. Parent-Massin D. (2011). Relevant shellfish consumption data for dietary exposure assessment among high shellfish consumers, Western Brittany, France. Int. J. Environ. Health Res.21, 86–105. doi: 10.1080/09603123.2010.533486
85
Poole S. Mayze J. Exley P. Paulo C. (2008). Maximising revenue within the NT mud crab fishery by enhancing post-harvest survival of mud crabs.
86
R Core Team (2023). R: A language and environment for statistical computing, R Foundation for Statistical. Computing.
87
Reichman O. (1984). Evolution of regeneration capabilities. Am. Nat.123, 752–763. doi: 10.1086/284236
88
Rodrigues E. T. Alpendurada M. F. Ramos F. Pardal M.Â. (2018). Environmental and human health risk indicators for agricultural pesticides in estuaries. Ecotoxicol. Environ. Saf.150, 224–231. doi: 10.1016/j.ecoenv.2017.12.010
89
Rodríguez-Hernández Á. Camacho M. Henríquez-Hernández L. A. Boada L. D. Valerón P. F. Zaccaroni A. et al . (2017). Comparative study of the intake of toxic persistent and semi persistent pollutants through the consumption of fish and seafood from two modes of production (wild-caught and farmed). Sci. Total Environ.575, 919–931. doi: 10.1016/j.scitotenv.2016.09.182
90
Romano N. Zeng C. (2017). Cannibalism of decapod crustaceans and implications for their aquaculture: a review of its prevalence, influencing factors, and mitigating methods. Rev. Fish. Sci. Aquac.25, 42–69. doi: 10.1080/23308249.2016.1231708
91
Sánchez-Paz A. García-Carreño F. Muhlia-Almazán A. Peregrino-Uriarte A. B. Hernández-López J. Yepiz-Plascencia G. (2006). Usage of energy reserves in crustaceans during starvation: status and future directions. Insect Biochem. Molec. Biol.36, 241–249. doi: 10.1016/j.ibmb.2006.01.005
92
Santhanam R. (2018). Biology and culture of portunid crabs of world seas (Florida: Apple Academic Press).
93
Serrano R. Hernández F. López F. Pena J. (1997). Bioconcentration and depuration of chlorpyrifos in the marine mollusc Mytilus edulis. Arch. Environ. Contam. Toxicol.33, 47–52. doi: 10.1007/s002449900228
94
Shalders T. C. Champion C. Coleman M. A. Benkendorff K. (2022). The nutritional and sensory quality of seafood in a changing climate. Mar. Environ. Res.176, 105590. doi: 10.1016/j.marenvres.2022.105590
95
Smith L. D. (1995). Effects of limb autotomy and tethering on juvenile blue crab survival from cannibalism. Mar. Ecol. Prog. Ser.116, 65–74. doi: 10.3354/meps116065
96
Sobral M. M. C. Cunha S. C. Faria M. A. Ferreira I. M. (2018). Domestic cooking of muscle foods: Impact on composition of nutrients and contaminants. Compr. Rev. Food Sci. Food Saf.17, 309–333. doi: 10.1111/1541-4337.12330
97
Sreelakshmi K. Manjusha L. Vartak V. Venkateshwarlu G. (2016). Variation in proximate composition and fatty acid profiles of mud crab meat with regard to sex and body parts. Indian J. Fish.63, 147–150. doi: 10.21077/ijf.2016.63.2.34511-23
98
Sundhar S. Shakila R. J. Shalini R. Aanand S. Jayakumar N. (2023). Impact of thermal cooking processes on organochlorine pesticide residues (OCPs) in the edible green seaweed, Ulva lactuca, and associated human health risk assessment. J. Food Compos. Anal.121, 105370. doi: 10.1016/j.jfca.2023.105370
99
Taylor M. D. (2023). Metal concentrations in the edible claw and body muscle of a popular recreational crab species (Scylla serrata). Mar. pollut. Bull.197, 115703. doi: 10.1016/j.marpolbul.2023.115703
100
Taylor M. D. Bowles K. C. Johnson D. D. Moltschaniwskyj N. A. (2017). Depuration of perfluoroalkyl substances from the edible tissues of wild-caught invertebrate species. Sci. Total Environ.581, 258–267. doi: 10.1016/j.scitotenv.2016.12.120
101
Taylor M. D. Johnson D. D. Nilsson S. Lin C. Y. Braeunig J. Mueller J. et al . (2021). Trial of a novel experimental design to test depuration of PFASs from the edible tissues of Giant Mud Crab following exposure under natural conditions in the wild. Sci. Total Environ.758, 143650. doi: 10.1016/j.scitotenv.2020.143650
102
Taylor M. Laicher-Edwards D. White S. Woodrow R. Passos T. Sanders C. (2022). Investigating pesticide and heavy metal distribution from water and sediments near expanding horticulture activities in the Coffs Harbour NSW region. National Marine Science Centre, Southern Cross University, Coffs Harbour. NSW.
103
Taylor M. D. Nilsson S. Bräunig J. Bowles K. C. Cole V. Moltschaniwskyj N. A. et al . (2019). Do conventional cooking methods alter concentrations of per-and polyfluoroalkyl substances (PFASs) in seafood? Food Chem. Toxicol.127, 280–287. doi: 10.1016/j.fct.2019.03.042
104
Tudi M. Daniel Ruan H. Wang L. Lyu J. Sadler R. Connell D. et al . (2021). Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health18, 1112. doi: 10.3390/ijerph18031112
105
Uhlmann S. S. Broadhurst M. K. Paterson B. D. Mayer D. G. Butcher P. Brand C. P. (2009). Mortality and blood loss by blue swimmer crabs (Portunus pelagicus) after simulated capture and discarding from gillnets. ICES J. Mar. Sci.66, 455–461. doi: 10.1093/icesjms/fsn199
106
Vassiliadou I. Costopoulou D. Kalogeropoulos N. Karavoltsos S. Sakellari A. Zafeiraki E. et al . (2015). Levels of perfluorinated compounds in raw and cooked Mediterranean finfish and shellfish. Chemosphere127, 117–126. doi: 10.1016/j.chemosphere.2015.01.020
107
Vendl C. Pottier P. Taylor M. D. Bräunig J. Gibson M. J. Hesselson D. et al . (2022). Thermal processing reduces PFAS concentrations in blue food–A systematic review and meta-analysis. Environ. pollut.304, 119081. doi: 10.1016/j.envpol.2022.119081
108
Vogt G. (2019). Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphol.280, 1405–1444. doi: 10.1002/jmor.21035
109
Wang J. X. Cheng Y. F. Pan X. H. Luo P. (2022). Tissue-specific accumulation, transformation, and depuration of fipronil in adult crucian carp (Carassius auratus). Exotoxicol. Environ. Saf.232, 113234. doi: 10.1016/j.ecoenv.2022.113234
110
Wasko C. Nathan R. Stein L. O’shea D. (2021). Evidence of shorter more extreme rainfalls and increased flood variability under climate change. J. Hydrol.603, 126994. doi: 10.1016/j.jhydrol.2021.126994
111
Wei Z. Zhang B. Li X. Gao Y. He Y. Xue J. et al . (2023). Changing on the concentrations of neonicotinoids in rice and drinking water through heat treatment process. Molecules28, 4194. doi: 10.3390/molecules28094194
112
Wiech M. Vik E. Duinker A. Frantzen S. Bakke S. Maage A. (2017). Effects of cooking and freezing practices on the distribution of cadmium in different tissues of the brown crab (Cancer pagurus). Food Control75, 14–20. doi: 10.1016/j.foodcont.2016.11.014
113
Wu M. Tang X. Sun C. Miao J. Wang Q. Pan L. (2023). Kinetics of uptake and depuration of synthetic pyrethroid insecticides in manila clam (Ruditapes philippinarum). Environ. Sci. pollut. Res.30, 76246–76252. doi: 10.1007/s11356-023-28637-2
114
Xie H. Jiao Y. Li T. Zhang T. Zheng Y. Luo Y. et al . (2023). Isomerization and bioaccessibility of cypermethrin and fenpropathrin in Pacific oyster during simulated digestion as influenced by domestic cooking methods. Food Innov. Adv.2, 9–17. doi: 10.1016/j.fia.2023.04.002
115
Yadav I. C. Watanabe H. (2018). Soil erosion and transport of Imidacloprid and Clothianidin in the upland field under simulated rainfall condition. Sci. Total Environ.640, 1354–1364. doi: 10.1016/j.scitotenv.2018.05.379
116
Yang L. Wang D. Xin C. Wang L. Ren X. Guo M. et al . (2021). An analysis of the heavy element distribution in edible tissues of the swimming crab (Portunus trituberculatus) from Shandong Province, China and its human consumption risk. Mar. pollut. Bull.169, 112473. doi: 10.1016/j.marpolbul.2021.112473
117
Yigit N. Velioglu Y. S. (2020). Effects of processing and storage on pesticide residues in foods. Crit. Rev. Food Sci. Nutr.60, 3622–3641. doi: 10.1080/10408398.2019.1709802
118
Zabik M. E. Harte J. B. Zabik M. J. Dickmann G. (1992). Effect of preparation and cooking on contaminant distributions in crustaceans: PCBs in blue crab. J. Agric. Food Chem.40, 1197–1203. doi: 10.1021/jf00018a010
119
Zuur A. (2009). Mixed effects models and extensions in Ecology with R (New York: Springer).
Summary
Keywords
contaminants, fisheries adaptation, insecticide, herbicide, fungicide, portunid
Citation
Gray BCT, Champion C, Broadhurst MK, Coleman MA and Benkendorff K (2025) Efficacies of cooking and depuration for reducing current-use pesticide residues in wild-caught giant mud crabs (Scylla serrata). Front. Mar. Sci. 12:1624922. doi: 10.3389/fmars.2025.1624922
Received
08 May 2025
Accepted
21 July 2025
Published
28 August 2025
Volume
12 - 2025
Edited by
Bernardo Duarte, Center for Marine and Environmental Sciences (MARE), Portugal
Reviewed by
Fatih Ozogul, Çukurova University, Türkiye
Chao Shen, Fujian Academy of Agricultural Sciences, China
Updates
Copyright
© 2025 Gray, Champion, Broadhurst, Coleman and Benkendorff.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin C. T. Gray, b.gray.21@student.scu.edu.au
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.