- 1Department of Environmental Biology Fisheries Science, National Taiwan Ocean University, Keelung, Taiwan
- 2Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung, Taiwan
- 3Department of Aquatic Resources, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 4Coastal and Offshore Research Center, Taiwan Fisheries Research Institute, Kaohsiung, Taiwan
- 5Doctoral Degree Program in Ocean Resource and Environmental Changes, National Taiwan Ocean University, Keelung, Taiwan
Introduction: Comprehending species-specific reactions to climate variability is crucial for ecosystem-based fisheries management, especially for high-value multispecies fisheries. The waters of Taiwan harbour numerous commercially significant seabream species; nevertheless, the impact of large-scale climatic oscillations on their catch dynamics is inadequately characterised.
Methods: We examined catch trends of six principal seabream species (Acanthopagrus schlegelii, Dentex hypselosomus, Evynnis cardinalis, Pagrus major, Parargyrops edita, and Rhabdosargus sarba) employing Generalised Additive Models (GAM) to evaluate associations with significant climatic factors. Cross-spectrum analysis was utilised to identify predominant periodicities, whereas wavelet coherence analysis investigated temporal coherence and phase correlations between climate indices and catch rates.
Results: The Pacific Decadal Oscillation (PDO) was identified as the predominant predictor for all species, with explained deviance between 24.6% and 42.3%. Cross-spectrum analysis revealed notable low-frequency periodicities of around 2.4 years. Wavelet coherence demonstrated both immediate and lagged responses, exhibiting asynchronous species-specific patterns influenced by a shared PDO.
Discussion: These findings underscore the influence of decadal climate variability on the dynamics of multispecies fisheries in Taiwanese seas. Integrating species-specific and time-lagged responses into management techniques is essential for adaptive, climate-resilient fisheries governance. The results also offer evidence pertinent to international policy frameworks about the effects of climate change on marine ecosystems.
1 Introduction
Climatic variabilities are extensive patterns of climatic fluctuation that manifest throughout various time spans, ranging from a few months to many decades and these variabilities signify periodic alterations in atmospheric and oceanic conditions that may have a substantial impact on global and regional weather patterns (Bi et al., 2020). These impacts are evident in changes to temperature patterns, precipitation trends, and the frequency and severity of severe weather phenomena. For instance, during an El Niño event, the elevation of sea surface temperatures (SST) in the Pacific Ocean can cause a decline in trade winds and a change in atmospheric pressure systems. This leads to modified rainfall patterns, resulting in droughts in certain areas like Southeast Asia and Australia, and increased rainfall and flooding in other regions, such as the western coast of South America (Jiang et al., 2024). Therefore, the effect of climatic variabilities on atmospheric conditions has significant physical consequences on global and regional weather.
Fluctuations in weather patterns caused by climate variations have a significant effect on the state of the ocean, resulting in interconnected cycles that affect both systems. During El Niño occurrences, changes in atmospheric pressure patterns, such as the weakening of the Walker Circulation, cause warm water to be redistributed over the Pacific Ocean. This disrupts the usual wind patterns and ocean currents. This change not only increases SSTs but also decreases the strength of upwelling, which is crucial for transporting cold, nutrient-rich waters to the top. These changes in the atmosphere may also modify the intensity and course of significant ocean currents. This, in turn, affects the distribution of heat in the oceans and contributes to fluctuations in sea level and abnormal SSTs (Lian and Gao, 2024). In addition, changes in the atmospheric conditions caused by phenomena such as the Pacific Decadal Oscillation (PDO) has a significant impact on ocean conditions, particularly in the North Pacific, by altering SSTs, ocean currents, over decadal timescales. During the warm phase of the PDO, the western Pacific, encounters decreased SSTs. This might improve the upwelling mechanisms that send cold, nutrient-rich waters to the top. Conversely, during the cold phase of the PDO, higher SSTs in the western Pacific Ocean decline the upward movement of nutrient-rich water, which in turn reduces the biological productivity of the area (Zhang K. et al., 2022). Thus, interaction between atmospheric changes and oceanographic conditions during climatic variations leads to intricate and dynamic alterations in the oceanic environment.
The fluctuations in oceanic conditions caused by climatic variations, such as the PDO and El-Nino Southern Oscillation (ENSO), have significant effects on the biological aspects of the ocean modifying the distribution, productivity, and composition of marine species. For instance, during the warm phase of the PDO or an ENSO event, decreased SSTs and increased upwelling result in induced nutrient availability in the top layers of the ocean, which leads to an increase in primary production, especially in areas such as the western Pacific (Poupon et al., 2023). The increase in the number of phytoplankton, which serves as the basis of the marine food chain, has an important effect across the ecosystem, impacting zooplankton populations, fish stocks, and higher trophic levels, such as seabirds and marine mammals. In addition, modified ocean currents and temperature gradients may cause the displacement of diverse marine organisms, resulting in alterations to the species composition and richness in distinct places. Conversely, during the cold phase of the PDO or an ENSO occurrence, increased SSTs and decreased upward movement of water may decline the availability of nutrients, biological production in the areas of western Pacific (Lee et al., 2024). Thus, understanding the dynamics of marine ecosystems, the viability of fisheries, and the larger consequence on global biodiversity relies significantly on comprehending the climate-driven variations in ocean conditions.
The North-western Pacific fishery is a crucial component of worldwide marine resources, serving as a vital player in both local and global seafood markets. The region in question includes portions of the Exclusive Economic Zones (EEZs) of nations such as Japan, China, South Korea, Russia, and Taiwan. It is renowned for its exceptionally fertile waters, which are the result of the convergence of significant ocean currents like the Kuroshio and Oyashio. The presence of these currents generates habitats rich in nutrients, which sustain a wide range of marine organisms, including valuable fish (Hsu et al., 2024). Fisheries play a crucial role in the economy of these countries, offering job opportunities to millions in coastal areas and supporting related businesses such as fishing, processing, and exporting. Japan, for example, has a longstanding fishing heritage, where its seafood culture is profoundly ingrained in everyday life and the country’s cuisine (Syddall et al., 2021). Furthermore, the North-western Pacific region plays a crucial role in both satisfying local demand and facilitating global commerce. All the coastal countries of the North-western Pacific are significant seafood consumers, highly dependent on local fisheries to satisfy their needs. Simultaneously, the area plays a crucial role in exporting seafood goods, making significant contributions to worldwide markets, especially in North America and Europe (Simard et al., 2024). Therefore, the North-western Pacific fisheries may be regarded as a leading contributor to world food security and economic stability. The North-western Pacific Sea bream fishery is crucial for its economic value, cultural significance, and cultural significance. It supports local economies, provides jobs, and contributes to international trade.
The seabream fishery in the North-Western Pacific is understudied due to a lack of detailed research on its oceanography and the effects of climate variability on its biology and ecology. This lack of understanding has led to ambiguities in the implementation of sustainable fisheries management and conservation initiatives. The species’ ecological function and adaptability to environmental changes are currently lacking, emphasizing the need for targeted scientific investigation to address these knowledge deficiencies and ensure the sustainable management and conservation of this significant fishery. Therefore, this study sought to examine the influence of climatic variability on multiple seabream species in the North-western Pacific, addressing one of the existing research gaps. This study tests whether catch variability in the Northwestern Pacific seabream fishery is more strongly associated with a single dominant climate index or with multiple interacting climatic oscillations.
The proposed research has the potential to directly contribute to the improvement of sustainable fisheries management at both local and global levels. At the local level, it may provide valuable information on certain climatic elements that can be observed and controlled to maintain the long-term sustainability of the Sea Bream population, so helping the economic well-being of fishing communities (Cavole et al., 2020). On a global scale, these results can enhance overall comprehension of the influence of climatic variability on fisheries (Furuzono, 2024). They provide guidance for international policies and cooperative management techniques, particularly under frameworks such as the sustainable development goals (SDGs) and biodiversity beyond national jurisdiction (BBNJ). The research could potentially allow for the creation of adaptive management strategies that may be used in comparable fisheries globally, enhancing their ability to withstand the impacts of climate change and guaranteeing the sustainable utilization of marine resources.
2 Materials and methods
2.1 Data collection
Fishery data. The monthly logbook data for the Taiwanese fishery targeting seabreams in the Taiwan coastal waters, namely within the geographical area bounded by 22°N, 117°E and 27°N, 126°E, were obtained from the Fisheries Agency, Ministry of Agriculture, Taiwan. The duration of the research was from January 2014 to December 2019 with 0.1° spatial resolution. The dataset included information on the fishing date (year and month), fishing location (latitude and longitude), catch (in kilograms), fishing effort (in hours), total weight of the catch (without differentiating between dry and wet weights), fishing gear used, and vessel tonnage. The six seabream species considered in this study were as follows: (1) red seabream (Pagrus major), (2) black seabream (Spondyliosoma cantharus), (3) silver seabream (Sparus aurata), (4) yellowback seabream (Dentex tumifrons), (5) yellowfin seabream (Acanthopagrus latus), and (6) cardinal seabream (Evynnis cardinalis).
This research only examined the fishing gears that had the most substantial impact in capturing seabream. The selection procedure was meticulously conducted by considering the percentage of catch contribution for each gear type, ensuring that only those with the highest contribution were selected (Lee et al., 2022). This methodology enables a focused analysis of the most efficient fishing techniques. The monthly catch rate was determined using the catch–effort relationship (Equation 1), as follows:
where ∑Catch and ∑Fishing effort refer to the total catch weight (per day) and total fishing effort (per day) made by fishing vessels, respectively.
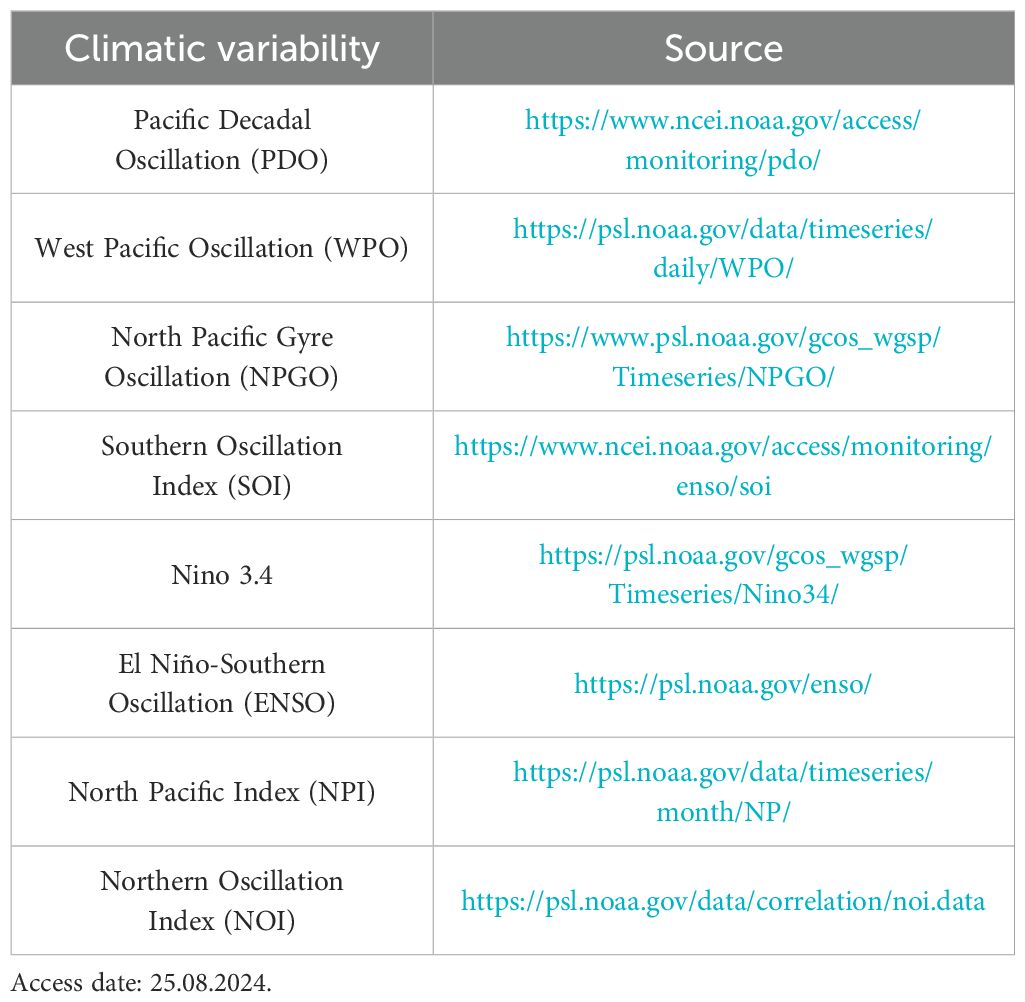
Climatic variability data. Monthly data on various climatic variability from the North-Western Pacific was collected from January 2014 to December 2019 and the sources of these climatic variability data are provided in Table 1.
2.2 Importance of climatic variabilities
The study used generalized additive models (GAM) to assess the significance of climate variability on the catch rates of each species. GAMs were generated (Equations 2–4) by using the “smoothing” function from the “mgcv” package using the “gam” method (Vahabnezhad et al., 2023).
The observed values, y, are assumed to be of some exponential family distribution, and µ is related to the model predictors via a link function. The linear predictor incorporates smooth functions f(x) of at least some (possibly all) covariates.
The general form of GAM used in this study is:
Where is the expected catch rate in year t, g(.) is the link function (e.g., identity distribution), is the intercept, and f(·) is a smoothing function applied to the climate index predictor variable. This model structure assumes that the response variable (catch rate) follows a distribution from the exponential family and is related to the explanatory predictor via a link function. The smooth term allows flexibility to capture non-linear relationships.
In each GAM (Equation 5), the response variable was the species-specific annual catch rate, while the explanatory variable was one climate index (e.g., PDO, ENSO, NPGO), tested independently. The relevance of climatic oscillation for each species was assessed by calculating three metrics: deviance explained (%), generalized cross validation (GCV) and Akaike information criterion (AIC). The climatic oscillation with the highest deviance explained, lowest GCV and AIC values was selected as the most important (Vahabnezhad et al., 2023) and only used in the next portion of the investigation.
2.3 Significance of climatic variabilities
Only the climatic oscillation that was specifically selected for each species from section 2.2 were included in this phase of analysis. The current study used cross-spectral analysis to examine the possible significance of correlation between two time series, namely climatic variability and catch rate, in the frequency domain (Contreras-Reyes and Hernández-Santoro, 2020). The “spectrum” function from the “stats” package was used to do cross-spectral analysis. This analysis aimed to investigate the cross-spectral density in order to detect common periodic patterns, assess the strength of relationships at different frequencies, and understand the significant lead-lag relationships between the catch rate of different species and specific climatic variability associated with those species in frequency domain (Contreras-Reyes and Hernández-Santoro, 2020). This analysis was conducted using R-studio version 4.2.3.
2.4 Temporal impact of climatic variabilities
Only climatic variability that were specifically selected for each species from section 2.2 were included in this phase of analysis. The present study utilized cross-wavelet analysis to investigate the temporal impact of the selected climatic variability on Seabream catch rates, in terms of lead-lag relationships, in the time-frequency domain (Raubenheimer and Phiri, 2023). The “xwt” function from the “biwavelet” package was used to do cross-wavelet analysis. The primary objective of this investigation was to investigate the cross-wavelet power spectrum and phase connection between the catch rate of various species and the unique climatic variability associated with those species (Raubenheimer and Phiri, 2023). This analysis was conducted using R-studio version 4.2.3.
3 Results
3.1 Seabream catch variation (Fishing gear specific fishery data selection)
To reduce bias arising from differences in fishing gear and effort, only the dominant gear type contributing the highest percentage of landings for each seabream species was selected for analysis (Table 2). For Red Seabream (12001), longline was the most frequently used gear, accounting for 59% of total catch. The remaining five species—Yellowback (12002), Cardinal (12003), Black (12004), Silver (12005), and Yellowfin Seabream (12006)—were all predominantly captured using trawl nets, contributing 40%, 71%, 66%, 55%, and 69% of their respective total catches. This targeted gear-based selection ensured consistency in catch rate estimation and allowed the study to focus on the most representative fishing activity for each species. It also reduced the influence of heterogeneous gear types on observed catch variability, thereby strengthening the reliability of ecological inferences in the subsequent analyses.
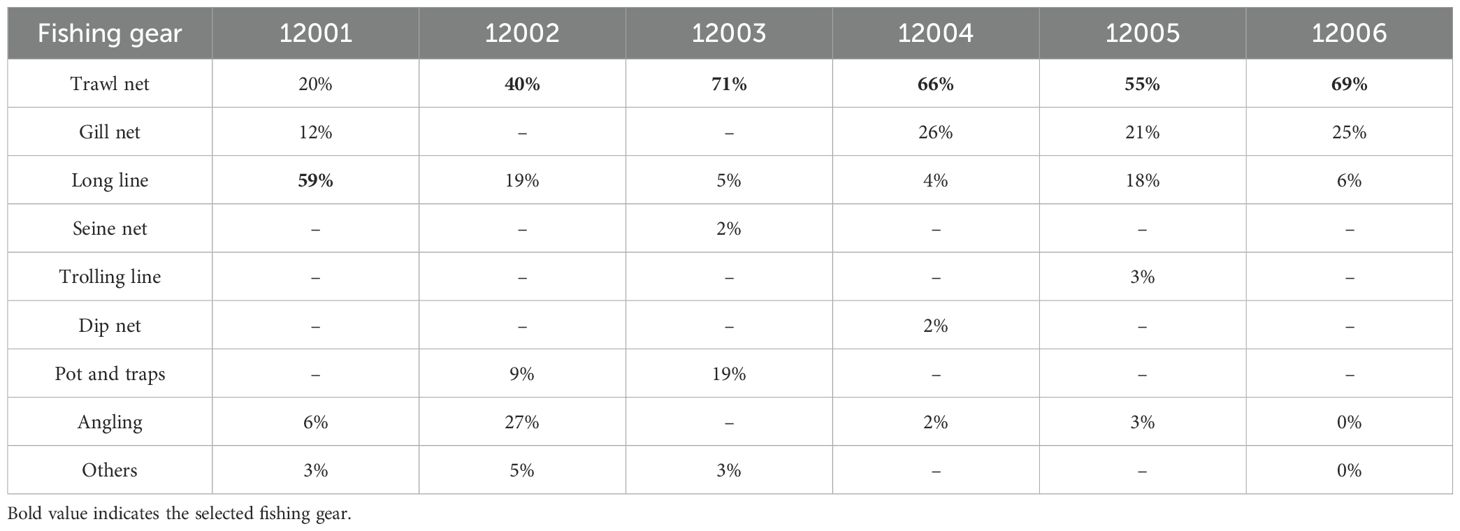
Table 2. Seabream species catch contribution (%) from various fishing gears during the study period.
3.2 Importance of climatic variabilities (GAM analysis)
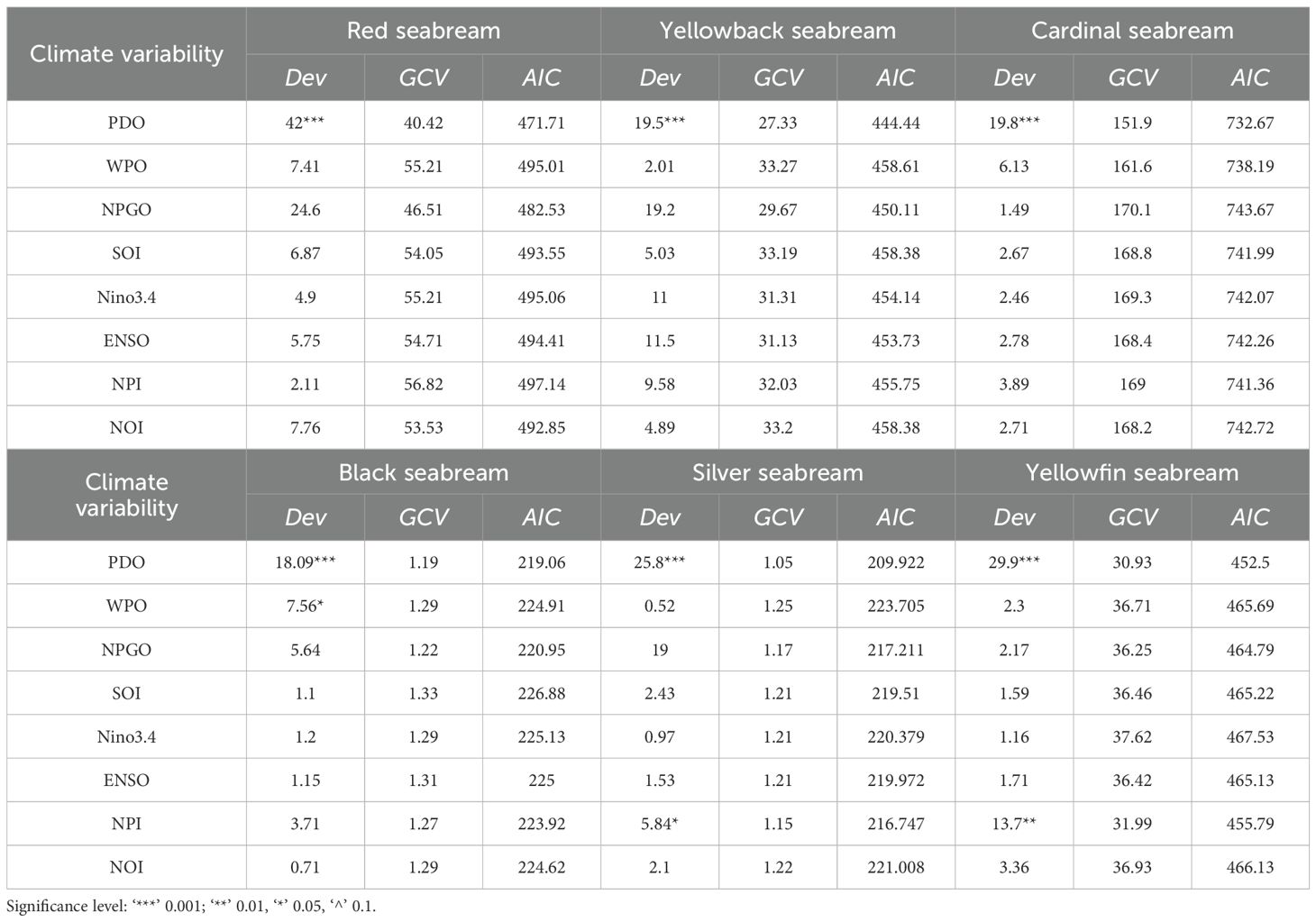
GAM results revealed that PDO exerted the strongest influence on catch rates across all six seabream species (Table 3; Figure 1). PDO consistently exhibited the highest deviance explained and the lowest GCV and AIC values, marking it as the dominant climatic predictor among the tested indices.
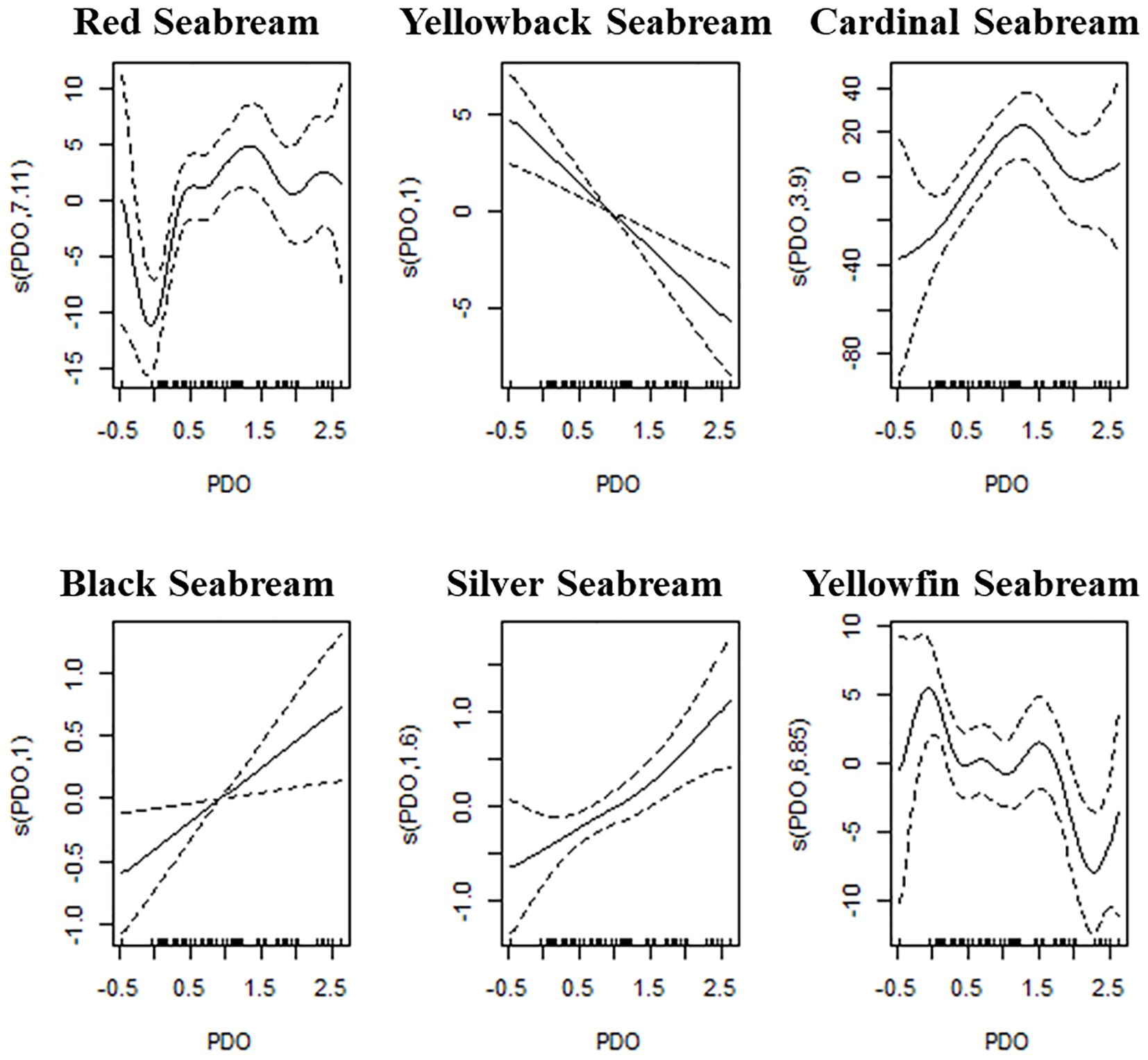
Figure 1. Generalized additive model (GAM) plots depicting the relationship between the Pacific Decadal Oscillation (PDO) and the catch rates of six seabream species. Each plot corresponds to a different species, showing the smooth function of PDO on the y-axis, with confidence intervals represented by dashed lines. The x-axis represents the PDO values, and the y-axis represents the estimated effect of PDO on catch rates, with different smooth functions applied across species. The tick marks along the x-axis indicate the distribution of PDO values in the dataset. The plots illustrate how the catch rates of the respective seabream species vary non-linearly with changes in PDO, with significant effects observed at specific PDO values, as indicated by deviations from the baseline and the accompanying confidence intervals.
Red Seabream (Pagrus major, Code 12001): The GAM model explained 42% of the deviance, the highest among all species, with GCV = 40.42 and AIC = 471.71. The partial response plot exhibited a non-linear multi-peak pattern, indicating a complex response of red seabream catch rates to varying PDO phases. The response function s(PDO, 7.11) reflects moderate smoothness with clear confidence intervals that diverge significantly from the baseline.
Yellowback Seabream (Dentex tumifrons, Code 12002): PDO explained 19.5% deviance (GCV = 27.33, AIC = 444.44). The GAM response was negatively linear, indicating that catch rates declined steadily as PDO increased. The smooth function s(PDO, 0.1) indicates a simple model with minimal curvature and tightly constrained confidence bands.
Cardinal Seabream (Evynnis cardinalis, Code 12003): With 19.8% deviance explained, GCV = 151.9, and AIC = 732.67, PDO again emerged as the best predictor. The partial response curve showed a U-shaped pattern, with significant positive and negative excursions, represented by s(PDO, 0.39), suggesting alternating PDO phases influence catch differently over time.
Black Seabream (Acanthopagrus schlegelii, Code 12004): PDO accounted for 18.09% deviance, with GCV = 1.19 and AIC = 219.06. The partial effect showed a positive linear trend, where catch rates increased with higher PDO values. The smooth function s(PDO, 0.1) indicates a nearly linear fit, corroborated by narrow 95% confidence intervals.
Silver Seabream (Rhabdosargus sarba, Code 12005): The GAM explained 25.8% deviance, with GCV = 1.05 and AIC = 209.92, again selecting PDO as the dominant driver. The response curve was non-linear and positively curved, indicating that catch rates rose gradually with increasing PDO values. The smooth term s(PDO, 1.6) implies moderate flexibility with well-separated confidence bands.
Yellowfin Seabream (Acanthopagrus latus, Code 12006): With 29.9% deviance explained, GCV = 30.93, and AIC = 452.5, PDO exerted a strong influence. The GAM curve s(PDO, 6.85) showed pronounced undulations, suggesting dynamic, multi-phase responses of catch rates to PDO, likely reflecting the interplay between direct environmental changes and lagged biological effects.
Across all species, PDO was consistently identified as the top-performing predictor, as indicated by the deviance, GCV, and AIC metrics. These findings are visually represented in Figure 1, where the shape and confidence intervals of each species-specific smooth term demonstrate significant and biologically plausible non-linearities in response to PDO variation.
These results suggest that PDO-driven changes in ocean conditions, particularly SST and nutrient cycling, may affect key habitat parameters for seabream species. Warmer or cooler phases of PDO can influence the vertical mixing and upwelling of nutrient-rich waters, altering primary productivity and, in turn, prey availability for seabream. Species like Pagrus major, which rely on benthic invertebrates, may respond to such bottom-up ecological changes via shifts in foraging success, growth rates, or reproductive output. The species-specific nature of the GAM results supports the idea that each seabream species may interact with environmental drivers differently depending on their habitat preference, feeding ecology, and physiological thresholds.
3.3 Significance of climatic variabilities (cross-spectrum analysis)
Cross-spectrum analysis between PDO and seabream catch rates revealed statistically significant associations across multiple frequencies, particularly in the lower-frequency bands, indicating strong long-term periodicity in the climate–catch relationships. Notably, all species exhibited a consistent spectral peak around frequency 0.42 (cycles per year)—equivalent to a ~2.4-year periodicity, marked by a blue vertical line in each plot (Figure 2), suggesting a shared dominant periodicity in the PDO signal that synchronizes with catch fluctuations. This recurring frequency further establishes a shared underlying periodic climatic driver likely modulating ecological dynamics at multi-year scales. The black solid lines representing the cross-spectral magnitude exceeded the red dashed 95% confidence threshold in various low-to-mid frequency ranges, confirming the robustness of these associations. While species differed in spectral intensity, each displayed evidence of long-term, climate-linked periodic variation in catch rates, with Red and Cardinal Seabream exhibiting particularly strong spectral coherence.
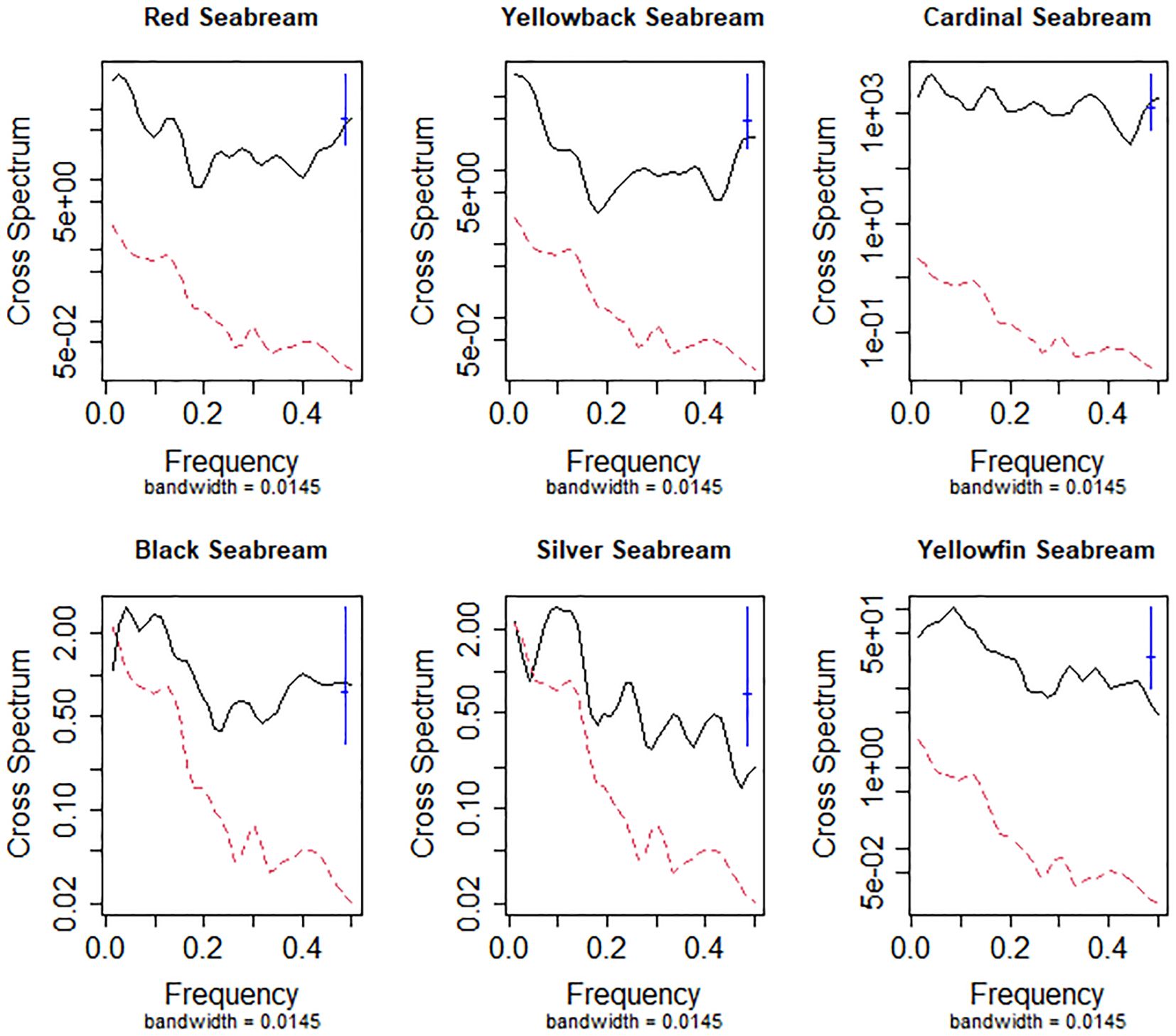
Figure 2. Cross-spectrum analysis between PDO and the catch rates of six seabream species. The x-axis represents the frequency (cycles per year), while the y-axis represents the spectral magnitude. The black solid line represents the observed cross-spectrum, and the red dashed line shows the 95% confidence limit. The presence of a blue vertical line around the frequency of 0.42 across all species highlights a specific frequency where a significant association is observed. The black line above the red line at various frequencies indicates statistically significant correlations between PDO and the catch rates of the respective seabream species. The bandwidth used for the analysis is 0.0145.
Red Seabream (Pagrus major, Code 12001): The black spectrum curve lies above the red 95% confidence threshold predominantly at low frequencies, indicating that long-term PDO variability exerts a statistically significant influence on catch rates. A pronounced peak around 0.42 frequency cycles per year implies a recurring climatic impact on Red Seabream availability.
Yellowback Seabream (Dentex tumifrons, Code 12002): A strong spectral signal is observed at both low frequencies and around 0.42, with statistically significant correlations marked by elevated black lines above the red threshold. This indicates a dual influence of both long-term PDO variability and medium-term oscillatory events on Yellowback Seabream catch dynamics.
Cardinal Seabream (Evynnis cardinalis, Code 12003): The spectral magnitude remains consistently high across most frequencies, with statistically significant regions at both low to mid frequencies and near 0.42. The consistent rise of the black line above the red dashed line suggests a persistent and broad-band PDO effect on this species’ catch variability.
Black Seabream (Acanthopagrus schlegelii, Code 12004): The spectrum shows elevated power in the low-frequency band with a visible peak at 0.42, pointing to a dominant long-period signal in PDO that correlates significantly with catch rate changes. This pattern supports the hypothesis that Black Seabream catch dynamics are sensitive to long-term PDO fluctuations.
Silver Seabream (Rhabdosargus sarba, Code 12005): The spectrum indicates statistically significant coherence with PDO at lower frequencies and again around the 0.42 mark. This result emphasizes the importance of both decadal and sub-decadal oscillatory regimes in shaping catch patterns of Silver Seabream.
Yellowfin Seabream (Acanthopagrus latus, Code 12006): A broad and significant spectral association is detected at low frequencies, with the black line surpassing the red confidence threshold across a wide range. A distinct rise around 0.42 suggests a recurring PDO signal, aligning with the patterns seen in the other species.
The cross-spectral plots revealed significant periodicity in the 2–4-year band for most seabream species, indicating synchronous fluctuations between PDO and catch rates. For instance, the red seabream exhibited persistent power in the ~2.4-year band across the time series, suggesting a possible entrainment of catch patterns to PDO cycles. The concentration of spectral power at low frequencies for all species supports the influence of long-term environmental cycles, consistent with the decadal periodicity of PDO. Biologically, such low-frequency climate forcing could modulate interannual reproductive success, larval transport patterns, or year-class strength through cumulative effects on temperature regimes, prey field dynamics, or ocean current structure. For example, prolonged favourable or unfavorable PDO phases may lead to strong or weak cohorts that emerge in the fishery over multiple years.
3.4 Temporal impact of climatic variabilities (wavelet analysis)
The wavelet coherence plots (Figure 3) further supported these associations, highlighting periods of significant synchrony (indicated by warm-colored regions enclosed by black contours). Wavelet coherence analysis revealed dynamic and statistically significant associations between PDO, and seabream catch rates, particularly between 2015 and 2019. High coherence zones, denoted by warm colors and thick black contours (p < 0.05), were predominantly concentrated in the 8- to 16-month period bands, suggesting interannual synchrony. Phase arrows indicated a mix of in-phase (synchronous) and lagged (PDO leading) relationships, varying by species and time. Red and Yellowback Seabream predominantly exhibited arrows pointing leftward or upward, indicating that catch rates lagged behind PDO variations—suggesting delayed ecological responses. Conversely, Cardinal, Black, Silver, and Yellowfin Seabream showed a mix of rightward (in-phase) and upward (PDO leads) arrows, implying both synchronous and lead-lag relationships. This variation in phase orientation and coherence timing reflects interspecific differences in ecological sensitivity, exposure timing, or life-history traits that modulate how climate variability influences catch dynamics. The phase relationships observed in the wavelet coherence plots suggest that PDO affects catch rates with both immediate and lagged biological consequences. Immediate responses may result from adult seabream altering their distribution or catchability in response to short-term SST anomalies or prey shifts. In contrast, lagged responses likely reflect population-level effects such as changes in larval survival, recruitment success, or growth conditions that manifest in catch rates after a delay of several months to a year. These mechanisms align with known life-history strategies of seabream, which are sensitive to early developmental conditions influenced by environmental variability.
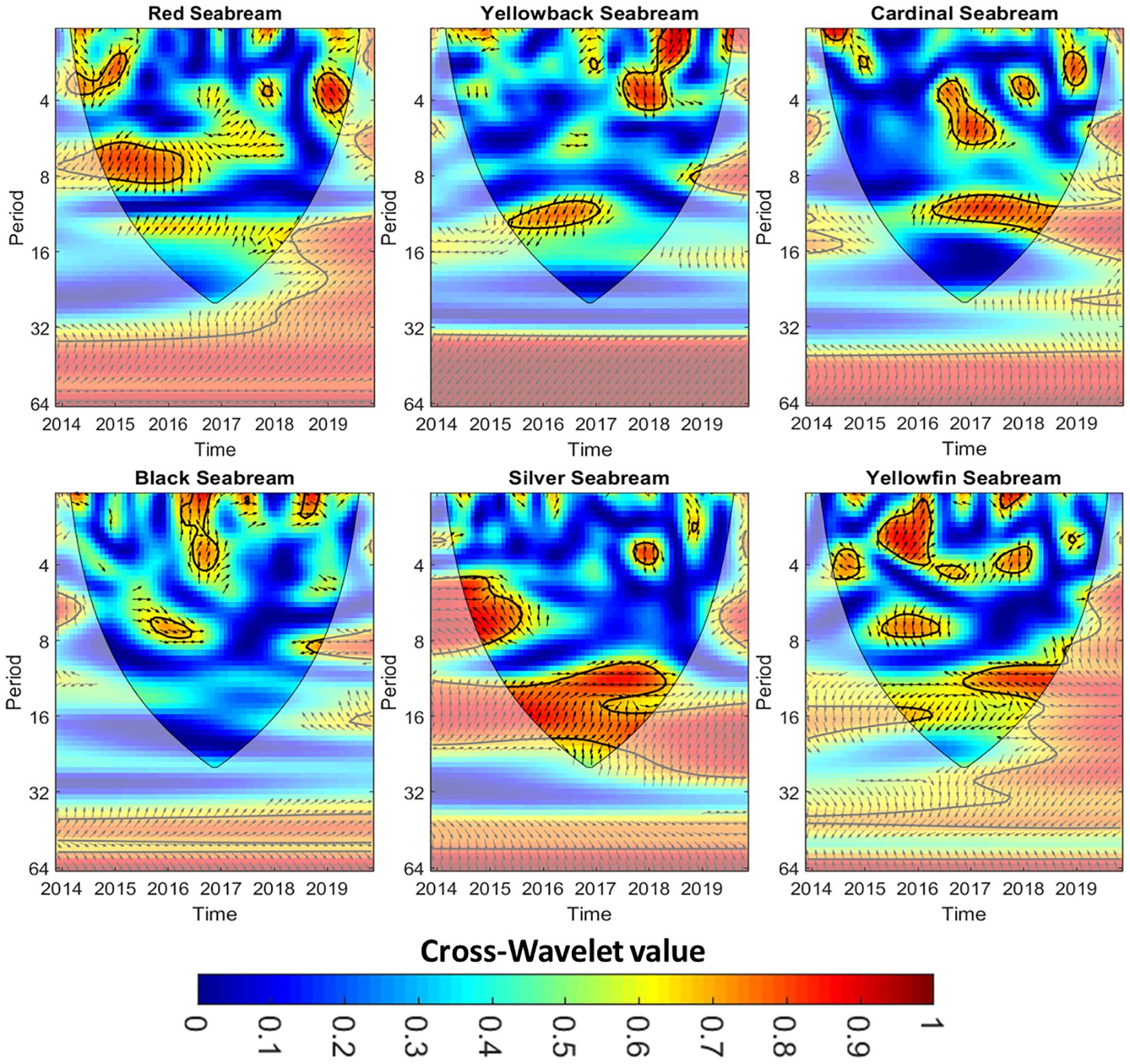
Figure 3. Wavelet coherence analysis between PDO and the catch rates of six seabream species from 2014 to 2019. High power regions are indicated by warmer colors (yellow/red), and significant coherence is outlined by thick black contours (p < 0.05). The y-axis indicates the period (in years), and the x-axis shows the time (in years). The black outlines identify areas of substantial coherence with a confidence level of 95%. Arrows indicate phase relationships: arrows pointing right = in-phase; left = anti-phase; up = PDO leads catch rate; down = catch rate leads PDO. Every figure is enclosed by a cone of influence (white region), which shows the places where edge effects might potentially impact the accuracy of the study. The color bar located at the bottom of the display indicates the coherence scale, which spans from 0 (indicating no coherence) to 1 (representing complete coherence).
Red Seabream (Pagrus major, Code 12001): Significant coherence was observed between 2015 and 2017, particularly in the 8- to 16-month band. Most phase arrows pointed leftward, suggesting a lagged response, where PDO changes preceded fluctuations in catch rates. Coherence diminished after 2018, indicating a weakening of PDO influence.
Yellowback Seabream (Dentex tumifrons, Code 12002): Strong coherence was identified during 2015–2017, with dominant peaks at ~8 months (2017) and 12–16 months (2018). Arrows commonly pointed 45°–90°, implying a 1–3-month lag between PDO and catch responses.
Cardinal Seabream (Evynnis cardinalis, Code 12003): A consistent coherence signal emerged between 2016–2018 in the 8–16-month range, with phase arrows mostly rightward or right-downward, indicating a blend of synchronous and slightly lagged responses. This implies that PDO fluctuations were closely aligned with catch variability, with some temporal lead by PDO.
Black Seabream (Acanthopagrus schlegelii, Code 12004): Coherence zones were detected from 2016–2018, predominantly in the 4–8 and 8–12-month periods. Most arrows pointed right, suggesting in-phase relationships, though downward-pointing arrows in 2017–2018 hinted at possible short lags or bidirectional feedback.
Silver Seabream (Rhabdosargus sarba, Code 12005): Significant coherence emerged between 2015–2018, mainly at 4–8 and 12–18-month periods. Phase arrows were mostly rightward or right-downward, indicating both synchronous behavior and periods where PDO led catch rates, particularly in the 8–16-month band.
Yellowfin Seabream (Acanthopagrus latus, Code 12006): The coherence spectrum showed strong signals across 4–8 and 8–16-month bands, with persistent coherence from 2016 to 2019. Rightward arrows dominated, implying synchrony, while upward arrows in 2018–2019 indicated PDO-leading dynamics, where changes in PDO preceded catch fluctuations.
These results reinforce the temporal complexity of PDO’s influence across seabream species, with dominant sub-annual to interannual periodicities and both synchronous and delayed responses, underscoring species-specific lags and sensitivities to climate variability.
4 Discussion
4.1 Importance of fishing gears
The present study determined that long-line fishing (passive fishing method) was the primary method of capturing red seabreams, while the remaining seabream species were primarily captured using trawl nets (active fishing method). Red seabreams are oval and deep-bodied, which, while providing stability, generates more drag than more streamlined species. Additionally, this species is generally bulkier and larger than other seabream species, which can impede its swimming pace. The resistance encountered while traveling through water is further exacerbated by the deeper body (Gutarra et al., 2019). The body morphology of the remaining seabream species of the study ranges from moderately elongated to elongated, moderately deep-bodied to round, and moderately elliptical to round. Additionally, these species are smaller and less bulky than the red seabream species, which results in a slightly more dexterous and quicker swimming ability than the red seabream (Howe et al., 2021). Passive fishing gear, like longlines, is more effective for slow-swimming fish due to their natural, languid movement patterns. This method captures fish without disrupting their environment, unlike active gear that requires pursuing, thereby increasing the chances of successful capture (Ward and Myers, 2005). On the other hand, active fishing gear, like trawl nets, is more effective for fast-swimming fish due to its ability to use their speed and movement patterns to corral or chase them into the gear. In contrast, passive gear, which relies on fish coming to it, is less effective for fast swimmers that move quickly through wide areas (Ward and Myers, 2005). Therefore, the body structure of the seabream species may be an important contributing factor to the fact that trawl nets were predominant for all seabream species except for red seabream, which was primarily captured using longlines.
4.2 Importance of PDO
The most influential climatic variability for all sea bream species in the North-Western Pacific was determined to be PDO. The PDO is a climate variability pattern that affects fisheries over extended periods due to persistent changes in ocean conditions and large-scale modulation of key oceanographic processes. It operates on a multi-decadal scale, with phases lasting 20–30 years, while short-term climate variability patterns like ENSO, and others, last months to a few years (Moon et al., 2015). During the warm phase of the PDO, SST in the western Pacific tends to decrease, which may enhance coastal upwelling and vertical mixing (Zhang Y. et al., 2022). These processes increase the transport of cold, nutrient-rich (such as nitrate and phosphate) waters to surface layers, stimulating primary productivity and supporting zooplankton and benthic prey availability (Poupon et al., 2023). Seabreams, being benthopelagic feeders, benefit from such productivity surges that improve their foraging success, growth rates, and reproductive output (Poupon et al., 2023). Changes in primary productivity affect the entire food web, affecting fish species’ abundance and catch rates, affecting fish population dynamics (Huang et al., 2024). In contrast, the cold phase is characterized by warmer surface temperatures and reduced upwelling in the western Pacific, which can diminish nutrient availability, lower chlorophyll concentrations, and reduce the abundance of seabream prey items such as small crustaceans and benthic invertebrates (Lee et al., 2024). These trophic constraints may directly impact seabream catchability and abundance. Additionally, PDO-induced shifts in ocean currents (e.g., Kuroshio extension variability) may influence larval transport, spawning success, and spatial overlap between seabreams and fishing grounds. Collectively, these ecological and physical mechanisms explain why the PDO exerts a stronger, persistent influence on catch dynamics than shorter-term climatic fluctuations like ENSO or Southern Oscillation Index (SOI). Long-term PDO leads to significant changes in the overall composition and structure of marine ecosystems, alter dominant species, and disrupt fisheries, while short-term impacts can cause rapid fluctuations in yields, posing challenges for fishery managers and economic disruption for communities (Zhang et al., 2022).
4.3 Catch response to PDO
The temporal patterns observed—where some seabream species respond immediately while others show delayed responses—are likely linked to the species-specific ecological traits and life-history strategies. Immediate responses may result from adult seabreams reacting to short-term environmental cues such as temperature changes or food pulses triggered by PDO-driven upwelling (Ma et al., 2020). Conversely, lagged responses could stem from bottom-up processes where the PDO influences phytoplankton and zooplankton productivity, which then affects seabream growth and recruitment with a time delay (Mondal et al., 2024). For example, enhanced primary productivity may boost juvenile survival, but these cohorts would only become catchable to fisheries after months or years (Mondal et al., 2024). This ecological lag also reflects life-history characteristics: species with longer maturation periods or slower growth may show delayed recruitment into the fishery. Hence, the observed lead-lag structure in wavelet coherence plots aligns with biologically plausible pathways. PDO’s decadal-scale persistence thus provides a more stable framework for anticipating long-term population trends and fishery outcomes compared to interannual oscillations such as ENSO or NPGO, which often cause year-to-year unpredictability in stock conditions (Pepin et al., 2022). Recognizing these mechanisms allows for improved ecosystem-based fishery management that is responsive not only to statistical associations but also to the biological and physical processes driving seabream population dynamics.
Species-Specific Responses. The observed variability in seabream responses to PDO phases can be further understood through species-specific ecological traits. For instance, Pagrus major (red seabream) exhibits well-defined seasonal spawning, typically peaking from March to June in the coastal waters off Taiwan and southern Japan (Blanco Gonzalez et al., 2015). This species prefers relatively warm SST between 17–23°C during its reproductive season and relies on nutrient-enriched nearshore habitats for larval and juvenile development (Takahashi and Masuda, 2019). During warm PDO phases, the enhancement of upwelling and increased productivity in the western Pacific may create favourable pre-spawning and spawning conditions for Pagrus major, resulting in improved recruitment success in subsequent months. This could explain the observed delayed response in catch rate, where enhanced cohort strength becomes detectable in the fishery following the spawning season.
Moreover, Pagrus major displays moderate migratory behavior, often undertaking seasonal inshore–offshore migrations associated with temperature shifts and spawning cues (Tanaka et al., 2020). These behavioral shifts are potentially modulated by PDO-linked SST changes, influencing both spatial overlap with fishing effort and vulnerability to gear types such as longlines.
In contrast, species such as Dentex tumifrons (yellowback seabream) and Evynnis cardinalis (cardinal seabream), which have more localized movement patterns and broader thermal tolerance (16–26 °C), may respond more immediately to PDO-induced changes in prey distribution or habitat suitability (Ma et al., 2020). Their shorter maturation periods and rapid response to environmental cues may account for the near-synchronous or short-lag responses observed in wavelet coherence analyses.
These ecological distinctions provide a clearer biological rationale for the heterogeneous temporal catch responses observed across seabream species in relation to the PDO. The integration of species-specific traits with climatic variability improves the predictive power of fishery-environment models and strengthens the development of adaptive management strategies.
4.4 Implementation of the study
The PDO functions over a period of 20–30 years, offering a more extended pattern of oceanic and atmospheric conditions. This enables more consistent and foreseeable planning for fisheries management in contrast to transient climate fluctuations. This may be advantageous in attaining certain sustainable development goals (SDGs) framework (Figure 4).
1. The enduring characteristic of the PDO can enable the formulation of strategies to enhance the ability of ecosystems to withstand and recover from disturbances. Management methods may be intentionally developed to progressively adjust to different phases of the PDO, so promoting ecosystems that are more resilient to shorter-term disturbances (Zhang et al., 2018).
2. The cumulative effect of the PDO on seabream occurs over a period. This can facilitate more precise stock estimates and enhance comprehension of seabream fisheries interaction to climatic variabilities. Conversely, the other short-time climatic variabilities might result in fluctuations from one year to another, which can confound evaluations and choices about management (Jiao and Chen, 2020).
3. The extended length of PDO phases can facilitate the constant application of seabream fisheries policy and international agreements. Policy adjustments may be customized to align with the current prevalent phase of the PDO cycle, hence assuring their continued relevance over extended periods of time. The fast swings of ENSO might complicate the task of maintaining consistent policy responses (Pinsky and Mantua, 2014).
4. PDO may provide a more comprehensive framework for comprehending and addressing the effects of climate change on marine ecosystems. Short-time occurrences of climatic variabilities may either worsen or temporarily counteract the impacts of climate change. However, the PDO provides valuable information about long-term patterns that are crucial for creating effective climate adaptation plans (Di Lorenzo and Mantua, 2016).

Figure 4. Relevance of studying PDO to multiple Sustainable Development Goals (SDGs). Understanding how large-scale climate oscillations like PDO affect marine ecosystems and fisheries supports progress toward SDG 14 (Life Below Water), particularly targets 14.2 to 14.7, by enhancing sustainable fisheries management and marine biodiversity conservation. Additionally, this knowledge can inform climate action (SDG 13), food security (SDG 2), sustainable economic growth (SDG 8), and ecosystem-based policies that contribute to broader sustainability, health, and resilience goals (SDGs 1, 3, 12, 15, and 17).
4.5 Limitations of fishery-dependent data
A key limitation of this study is the exclusive reliance on fishery-dependent data, which is inherently shaped by both ecological and human-driven processes. While CPUE is commonly used as a proxy for fish abundance, it may also reflect variations in fishing effort allocation, changes in gear efficiency, shifts in fisher behavior, market demand, or management regulations (Robinson et al., 2014). These non-ecological drivers can introduce potential biases in interpreting catch trends. To mitigate some of these biases, we selected only the dominant fishing gear for each seabream species based on cumulative catch contribution, ensuring that the analysis was focused on the most consistent and representative datasets.
Additionally, effort was standardized in terms of fishing hours to enable comparable temporal catch trends. However, unrecorded factors such as evolving vessel technology, variable catchability, or socio-economic pressures (e.g., fuel costs or market closures) may still affect catch rate trends in ways that are difficult to retroactively correct (Eigaard et al., 2014).
Moreover, catch rates were standardized by effort (fishing hours), allowing for relative comparisons across time. Nonetheless, we acknowledge that gear selectivity, unrecorded changes in vessel operation, or socio-economic pressures may still influence catch variability in ways that are difficult to control retrospectively. Given these considerations, the associations observed between climatic indices (e.g., PDO) and seabream catch rate should be interpreted with caution. While the consistency of PDO’s influence across all six species suggests a strong climate signal, we do not claim direct ecological causality. Future studies should incorporate fishery-independent datasets such as acoustic surveys, larval sampling, or tagging studies to validate the observed patterns and better disentangle environmental signals from anthropogenic variability.
Furthermore, this study does not include other potential environmental confounders such as bottom temperature, oxygen concentration, or habitat modification, which may also mediate species distributions and availability to gear (McLaverty et al., 2024). Although the consistent influence of PDO across all six species supports the presence of a strong climate signal, we do not claim a direct causal relationship. Future studies incorporating fishery-independent datasets (e.g., scientific trawl surveys, acoustic biomass indices, larval distribution maps) and broader environmental datasets (e.g., oxygen minimum zones, ocean acidification) would help disentangle climate-driven effects from human-mediated pressures and enhance attribution accuracy.
5 Conclusion
The study’s findings indicate that the North-Western Pacific Sea Bream Fishery is notably impacted by the PDO rather than by isolated or shorter-term climate fluctuations. The PDO, which occurs over a longer period, has a significant influence on the abundance of different seabream species. The response to these oscillations may be either immediate or delayed, depending on the seabream species and the time periods involved. This emphasizes the need to incorporate long-term climate trends such as the PDO into seabream fisheries management to guarantee sustainable practices and successful response to climate-induced alterations in marine ecosystems. The results highlight the need for focused and species-specific management techniques that take into consideration the lasting impact of PDO on seabream populations in this critical fishery. We recommend future integration of fishery-independent data to validate these relationships and improve ecological inference for climate-informed management.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SM: Conceptualization, Validation, Software, Writing – original draft, Formal Analysis, Methodology. AG: Writing – review & editing, Validation, Visualization, Methodology. AP: Writing – review & editing, Software, Methodology, Visualization. AR: Writing – review & editing. IB: Supervision, Data curation, Writing – review & editing, Software. J-SW: Validation, Visualization, Writing – review & editing, Supervision, Investigation. ML: Resources, Funding acquisition, Project administration, Conceptualization, Investigation, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study received funding from the National Science & Technology Council of Taiwan (NSTC), under grant number NSTC 113-2611-M-019-007.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bi R., Jiao Y., Bakka H., and Browder J. A. (2020). Long-term climate ocean oscillations inform seabird bycatch from pelagic longline fishery. ICES J. Mar. Sci. 77, 668–679. doi: 10.1093/icesjms/fsz255
Blanco Gonzalez E., Aritaki M., Knutsen H., and Taniguchi N. (2015). Effects of large-scale releases on the genetic structure of red sea bream (Pagrus major, Temminck et Schlegel) populations in Japan. PloS One 10, e0125743. doi: 10.1371/journal.pone.0125743
Cavole L. M., Andrade-Vera S., Jarrin J. R. M., Dias D. F., Aburto-Oropeza O., and Barrágan-Paladines M. J. (2020). Using local ecological knowledge of Fishers to infer the impact of climate variability in Galápagos’ small-scale fisheries. Mar. Policy 121, 104195. doi: 10.1016/j.marpol.2020.104195
Contreras-Reyes J. E. and Hernández-Santoro C. (2020). Assessing Granger-causality in the southern Humboldt current ecosystem using cross-spectral methods. Entropy 22, 1071. doi: 10.3390/e22101071
Di Lorenzo E. and Mantua N. (2016). Multi-year persistence of the 2014/15 North Pacific marine heatwave. Nat. Climate Change 6, 1042–1047. doi: 10.1038/nclimate3082
Eigaard O. R., Marchal P., Gislason H., and Rijnsdorp A. D. (2014). Technological development and fisheries management. Rev. Fisheries Sci. Aquaculture. 22, 156–174. doi: 10.1080/23308249.2014.899557
Furuzono Y. (2024). Climate Adaptation in Japanese Fisheries: A Policy Analysis. Seattle, USA: University of Washington.
Gutarra S., Moon B. C., Rahman I. A., Palmer C., Lautenschlager S., Brimacombe A. J., et al. (2019). Effects of body plan evolution on the hydrodynamic drag and energy requirements of swimming in ichthyosaurs. Proc. R. Soc. B 286, 20182786. doi: 10.1098/rspb.2018.2786
Howe S., Bryant K., Duff A., and Astley H. (2021). Testing the effects of body depth on fish maneuverability via robophysical models. Bioinspiration Biomimetics 17, 016002. doi: 10.1088/1748-3190/ac33c1
Hsu J., Chang Y. J., Brodziak J., Kai M., and Punt A. E. (2024). On the probable distribution of stock-recruitment resilience of Pacific saury (Cololabis saira) in the Northwest Pacific Ocean. ICES J. Mar. Sci. 81, 748–759. doi: 10.1093/icesjms/fsae030
Huang C., Liu H., Li H., Zuo J., and Wang R. (2024). Combined effects of ENSO and PDO on activity of major hurricanes in the eastern North Pacific. Climate Dynamics 62, 1467–1486. doi: 10.1007/s00382-023-06973-7
Jiang N., Zhu C., Hu Z. Z., McPhaden M. J., Chen D., Liu B., et al. (2024). Enhanced risk of record-breaking regional temperatures during the 2023–24 El Niño. Sci. Rep. 14, 2521. doi: 10.1038/s41598-024-52846-2
Jiao Y. and Chen Y. (2020). The impact of climate variability on fish stock assessments and management in the context of climate change. Fish Fisheries 21, 271–291.
Lee M. A., Mondal S., Wu J. H., Huang Y. H., and Boas M. (2022). Cyclic variation in fishing catch rates-influenced by climatic variability in the waters around Taiwan. J. Taiwan Fisheries Soc. 49, 113–125. doi: 10.29822/JFST.202206_49(2).0005
Lee S., Chae J. Y., Park J. H., Kim Y. T., Kang B., Shin C. W., et al. (2024). Remote impacts of low-latitude oceanic climate on coastal upwelling in a marginal sea of the Northwestern Pacific. Regional Stud. Mar. Sci. 69, 103344. doi: 10.1016/j.rsma.2023.103344
Lian P. and Gao L. (2024). Impacts of central-Pacific El Niño and physical drivers on eastern Pacific bigeye tuna. J. Oceanology Limnology 42, 972–987. doi: 10.1007/s00343-023-3051-3
Ma S., Tian Y., Li J., Yu H., Cheng J., Sun P., et al. (2020). Climate variability patterns and their ecological effects on ecosystems in the northwestern North Pacific. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.546882
McLaverty C., Beukhof E. D., Bromhall K., Dinesen G. E., Erichsen A. C., and Eigaard O. R. (2024). The relative effects of bottom trawling, organic enrichment, and natural environmental factors on coastal seabed communities. Mar. pollut. Bulletin. 209, 117169. doi: 10.1016/j.marpolbul.2024.117169
Mondal S., Ray A., Boas M., Navus S., Lee M. A., Dey S., et al. (2024). Can the delayed effects of climatic oscillations have a greater influence on global fisheries compared to their immediate effects? PloS One 19, e0307644. doi: 10.1371/journal.pone.0307644
Moon J. H., Song Y. T., and Lee H. (2015). PDO and ENSO modulations intensified decadal sea level variability in the tropical Pacific. J. Geophysical Research: Oceans 120, 8229–8237. doi: 10.1002/2015JC011139
Pepin P., King J., Holt C., Gurney-Smith H., Shackell N., Hedges K., et al. (2022). Incorporating knowledge of changes in climatic, oceanographic and ecological conditions in Canadian stock assessments. Fish Fisheries 23, 1332–1346. doi: 10.1111/faf.12692
Pinsky M. L. and Mantua N. J. (2014). Emerging adaptation approaches for climate-ready fisheries management. Oceanography 27, 146–159. doi: 10.5670/oceanog.2014.93
Poupon M. A., Resplandy L., Lévy M., and Bopp L. (2023). Pacific decadal oscillation influences tropical oxygen minimum zone extent and obscures anthropogenic changes. Geophysical Res. Lett. 50, e2022GL102123. doi: 10.1029/2022GL102123
Raubenheimer C. and Phiri A. (2023). The impact of climate change and economic development on fisheries in South Africa: a wavelet-based spectral analysis. Humanities Soc. Sci. Commun. 10, 1–11. doi: 10.1057/s41599-023-02408-0
Robinson J., Cinner J. E., and Graham N. A. (2014). The influence of fisher knowledge on the susceptibility of reef fish aggregations to fishing. PloS One 9, e91296. doi: 10.1371/journal.pone.0091296
Simard N. S., Militz T. A., Kinch J., Nunn P. D., and Southgate P. C. (2024). Social-ecological factors, stock status, and governance relating to a shell craft fishery in the indo-pacific region. J. Ethnobiology 44, 2780771241261223. doi: 10.1177/02780771241261223
Syddall V., Thrush S., and Fisher K. (2021). Transdisciplinary analysis of Pacific tuna fisheries: A research framework for understanding and governing oceans as social-ecological systems. Mar. Policy 134, 104783. doi: 10.1016/j.marpol.2021.104783
Takahashi K. and Masuda R. (2019). Nurture is above nature: nursery experience determines habitat preference of red sea bream Pagrus major juveniles. J. Ethology. 37, 317–323. doi: 10.1007/s10164-019-00605-6
Tanaka H., Kodama T., Suzuki N., Mochizuki Y., Ashida H., Sato T., et al. (2020). The distribution and early growth of juvenile Pacific bluefin tuna Thunnus orientalis around Sado Island in the eastern Sea of Japan. Fisheries Science, 86(6), pp.1019–1028.
Vahabnezhad A., Taghavimotlagh S. A., Salarpouri A., and Mirzaei M. (2023). Identifying the ecologically significant habitats of Yellow-fin tuna (Thunnus albacares, Bonnaterre 1788) of Iranian purse seine fishery in the Gulf of Oman and Indian Ocean: An approach using satellite imagery and fishery data. Regional Stud. Mar. Sci. 68, 103257. doi: 10.1016/j.rsma.2023.103257
Ward P. and Myers R. A. (2005). Shifts in open-ocean fish communities coinciding with the commencement of commercial fishing. Ecology 86, 835–847. doi: 10.1890/03-0746
Zhang L., Delworth T. L., and Zeng F. (2018). The impact of the Pacific Decadal Oscillation on simulated North Pacific climate and ocean circulation. Climate Dynamics 50, 4175–4194.
Zhang K., Li M., Li J., Sun M., Xu Y., Cai Y., et al. (2022). Climate-induced small pelagic fish blooms in an overexploited marine ecosystem of the South China Sea. Ecol. Indic. 145, 109598. doi: 10.1016/j.ecolind.2022.109598
Keywords: climate variability, cross-spectral analysis, GAM, North-western Pacific, sea bream fishery, wavelet
Citation: Mondal S, Ghosh A, Pradhan A, Ray A, Biswas I, Weng J-S and Lee M-A (2025) Single or multiple climate oscillations? Impacts on the Northwestern Pacific seabream fishery. Front. Mar. Sci. 12:1628259. doi: 10.3389/fmars.2025.1628259
Received: 14 May 2025; Accepted: 29 July 2025;
Published: 22 August 2025.
Edited by:
Pranaya Kumar Parida, Central Inland Fisheries Research Institute (ICAR), IndiaReviewed by:
Muhammed Duman, Bursa Uludağ University, TürkiyeKarankumar Kishorkumar Ramteke, Central Institute of Fisheries Education (ICAR), India
Copyright © 2025 Mondal, Ghosh, Pradhan, Ray, Biswas, Weng and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-An Lee, bWFsZWVAbWFpbC5udG91LmVkdS50dw==
 Sandipan Mondal1,2
Sandipan Mondal1,2 Jinn-Shing Weng
Jinn-Shing Weng Ming-An Lee
Ming-An Lee