- 1Department of Cell Biology, Physiology and Immunology, Faculty of Biology, University of Barcelona, Barcelona, Spain
- 2Department of Genetics, Microbiology and Statistics, Faculty of Biology, University of Barcelona, Barcelona, Spain
Biofilms, defined as aggregates of microorganisms embedded in a self-produced matrix of extracellular polymeric substances (EPS), are formed by most bacteria in both natural and pathogenic ecosystems. In aquaculture, biofilms pose a dual challenge: they confer recalcitrance to antimicrobials treatments and contribute to persistent infections by forming on facility surfaces such as tanks, nets, cages, and equipment. Tenacibaculum maritimum, the causative agent of tenacibaculosis, is responsible for significant economic losses in fish farming. Although the antibacterial activity of fish skin mucus against this pathogen has been evaluated in vitro, its effects on T. maritimum biofilms have not yet been determined. In this study, we provide a simple methodology for the in vitro formation and quantification of T. maritimum biofilms to monitor antibacterial properties of different compounds or substances, such as fish skin mucus. For this purpose, biofilm formation was assessed under varying culture volumes (200, 300, and 400 µL) and incubation times (24, 48, and 72 hours) in 48-well microplates. Then, the effects of gilthead seabream (Sparus aurata) skin mucus were evaluated on planktonic growth, biofilm formation, and biofilm dispersion, measuring both biomass and metabolic activity. Based on the tested volumes and incubation times, the optimal condition for biofilm formation was defined as 24 hours in MB at 25 ºC using 200 µL culture volume. These conditions supported the development of a biofilm (OD570>1.5 after crystal violet staining) while conserving time and mucus. Seabream mucus significantly impaired T. maritimum planktonic growth and biofilm formation in a concentration-dependent manner. Non-diluted mucus completely inhibited planktonic growth and biofilm metabolic activity, and reduced biofilm biomass by 81.16 ± 2.54%. In contrast, its effect on mature biofilms was limited, with reductions of approximately 50% in metabolic activity and 40% in biomass. This study provides a platform to assess how different fish culture conditions affect the host’s susceptibility to T. maritimum infections, which is crucial for preventing economic losses in fish farming. Additionally, it opens the door to studies analyzing the components of fish skin mucus responsible for its antibacterial activity, aiming to develop novel therapeutic compounds for targeting biofilms formed by this pathogen.
1 Introduction
Upon attached to a solid surface, numerous bacteria initiate a complex process of coordinate differentiation and production of a protective extracellular matrix that will lead to the formation of a biofilm (Manning, 2003). Biofilms can be defined as aggregates of microorganisms embedded in a self-produced matrix of extracellular polymeric substances (EPS) that adhere to each other and/or to a surface, where cells differ from their planktonic form in terms of growth rate and gene expression (Rather et al., 2021c). These structures are produced by the vast majority of bacteria in most natural and pathogenic ecosystems (Stoodley et al., 2002). In clinical and veterinary microbiology, the presence of biofilms supposes a great challenge since elicit recalcitrance to antimicrobial treatments (Flemming et al., 2016; Koo et al., 2017). In aquaculture, biofilms pose a significant challenge-not only because they make treating infected fish more difficult, but also because they form on tank surfaces, nets, cages, equipment, and culture sediments. These biofilms serve as reservoirs for pathogens, increasing the risk of disease outbreaks (Acosta et al., 2021). Besides, biofilms also represent a major concern in postharvest processes in the fish and seafood processing industries (Rather et al., 2021a). Different human pathogenic bacterial species have been identified in fish and seafood, and the biofilms formed on the skin of the animals or the transport boxes may contaminate the surfaces of the processing plants (Rajkowski, 2009).
Tenacibaculum maritimum (previously Flexibacter maritimum) is a Gram-negative filamentous bacteria that accounts for the majority of tenacibaculosis cases in marine fish species (Fernández-Álvarez and Santos, 2018; Mabrok et al., 2023). This disease affects mainly external tissues, causing gross lesions on the body surface, including ulcerative skin lesions, tissue necrosis, fin and tail rots, mouth erosion, and gill and eye necrosis (Avendaño-Herrera et al., 2006b). Early-stage ulcerations can rapidly evolve into severe tissue damage or necrosis and subsequently into septicemia (Bernardet et al., 1994; Mabrok et al., 2023). Since the first description of this disease in red seabream and gilthead fry cultures in Japan (Masumura and Wakabayashi, 1977), this bacterium has been identified and isolated in numerous outbreaks affecting both food and ornamental fish cultures worldwide. Its ability to grow in temperatures ranging from 15°C to 34°C, coupled to its extensive broad host range, explains its extensive geographical distribution and the (re)-emergence of this pathogen (Mabrok et al., 2023). Significant economic losses occur due to the high mortality rates and the external lesions that diminish the economic value of the fish (López et al., 2009; Lopez et al., 2022; Masumura and Wakabayashi, 1977; Handlinger et al., 1997).
Although the pathogenic mechanisms of Tenacibaculum maritimum are not yet fully understood, evidence shows that Tenacibaculum species can be found in tidal flats (Choi et al., 2006; Li et al., 2022; Shang et al., 2021) and survive for extended periods in seawater microcosms (Avendaño‐Herrera et al., 2006a), indicating that sediments may serve as natural reservoirs for these bacteria (Mabrok et al., 2023) and water being considered an important route of infection. Bacterial cells present in the water can adhere to and colonize the surfaces of fish, particularly targeting areas such as the skin and mucus layer. Fish rely on a continuous production and secretion of mucus on their skin that acts as the first line of defense against a variety of environmental conditions or stressors, including bacterial infections (Fernández-Alacid et al., 2018). Skin mucus is composed of a matrix of glycoproteins (mucins) that contains a wide range of molecules, some of them involved in protecting fish against pathogens. The presence of antimicrobial compounds in skin mucus, including immunoglobulins, lysozyme, lectins, secondary metabolites, and antimicrobial peptides, is well documented (Esteban, 2012). Consequently, fish mucus is a potential source of new therapeutic agents against bacterial infections (Díaz-Puertas et al., 2023; Tiralongo et al., 2020). Lacking flagella, pili, and fimbriae, T. maritimum adhesion is mediated by adhesins, exopolysaccharides and proteins displaying lectin or sugar-binding motifs (Pérez-Pascual et al., 2017). Binding to the carbohydrate residues in skin mucus may be the first step in T. maritimum infection, and its progress will depend on its ability to evade the immunological strategies and the antimicrobial compounds in skin mucus (Mabrok et al., 2023). It is well known that environmental and culture conditions can alter fish immunity (Bowden, 2008; Corbel, 1975; Magnadottir, 2010; Watts et al., 2001). The antibacterial activity of skin mucus suffers changes in front of different challenges, such as dietary conditions (Fernández-Alacid et al., 2021, 2022; Firmino et al., 2021; Gisbert et al., 2021; Sanahuja et al., 2023a), stress situations (Herrera et al., 2020; Tacchi et al., 2015), anaesthetic treatments (Soltanian et al., 2018), and pathogen challenges (Guardiola et al., 2019; Kumari et al., 2019). Hence, T. maritimum cells adhering to the skin mucus of carrier fish may initiate an infection process when environmental conditions become favorable or in immunosuppression conditions. Despite the described adherence of T. maritimum to different surfaces (Burchard et al., 1990; Magariños et al., 1995; Sorongon et al., 1991) and its ability to form cell aggregates when incubated in both static and slight shaking conditions (Mabrok et al., 2016; Wakabayashi et al., 1986; Yamamoto et al., 2010), scarce works studying T. maritimum biofilms are available (Escribano et al., 2023; Levipan et al., 2019). In addition, biofilms have not been usually included in studies assessing the antibacterial activity of fish skin mucus against this pathogen, although biofilm formation is presumably a necessary step in the establishment of the infection by Tenacibaculum (Avendaño-Herrera et al., 2006b; Levipan et al., 2019).
In this context, the present study aims to establish a simple and reproducible protocol for the formation and quantification of T. maritimum biofilms that will allow a more realistic approximation when studying the antibacterial properties of different compounds against this pathogen. For this purpose, a first attempt to elucidate the effects of gilthead seabream (Sparus aurata) skin mucus on T. maritimum planktonic and biofilm growth was included.
2 Materials and methods
2.1 Animal conditions, skin mucus sampling and processing
Gilthead seabream juveniles were obtained from a local fish farm and maintained in 400 L tanks (3 kg biomass·m-3) at the University of Barcelona indoor facilities at 22°C under a 12 h light: 12 h dark photoperiod. Fish were fed daily with a commercial standard diet (DIBAQ, Optimus) at a 3% body weight·day-1 rate. After one month of acclimation, skin mucus was sampled.
Seabream individuals (body weight, 172 ± 5 g) from six different tanks were caught and anesthetized (100 mg/L tricaine methanesulfonate, MS-222, Sigma-Aldrich). Then, skin mucus was collected following a previously described method (Fernández-Alacid et al., 2018). Briefly, mucus was gently removed using sterile glass slides, from the skin above the lateral line of the fish in a front-to-caudal direction and collected in sterile microtube. Ventral zones were excluded to avoid fecal and urogenital contamination. For each tank, the skin mucus of nine fish was randomly collected. The skin mucus samples were homogenized with a sterile Teflon sticker and centrifuged at 14,000 × g for 15 minutes at 4°C. The supernatants (soluble fractions of mucus) were retrieved and stored at -80°C. An amount of approximately 250–300 mg of crude mucus per fish could be obtained, and the soluble fraction represents approximately 60-80% of this weight. Thus, the total amount of soluble mucus obtained per fish ranged from 150 mg to 240 mg, corresponding approximately to 0.15-0.24 mL of mucus per fish. For the experiments conducted in this work, a total of 5.1 mL of soluble mucus per experiment (inhibition of planktonic growth, biofilm formation and dispersion) is required.
To ensure consistency across all assays, a pooled sample was prepared mixing the soluble fraction of the mucus of all the individuals sampled. This pooled sample was divided into different aliquots that were stored at -80°C until use. Also, its protein concentration was determined by the Bradford method (Bradford, 1976) using a bovine serum albumin (BSA, Sigma) standard curve. The Optical Density (OD) was determined at λ=596 nm with a microplate reader (Infinity Pro200 spectrophotometer, Tecan, Spain).
The experimental procedures in this study complied with the Guiding Principles for Biomedical Research Involving Animals (EU2010/63), as well as with the Spanish regulation (Law 32/2007 and RD 53/2013). The study was approved by the Ethics Committee of the University of Barcelona for the Use of Laboratory Animals and the Generalitat de Catalunya (DAAM 9383).
2.2 Tenacibaculum maritimum strain and growth conditions
Tenacibaculum maritimum CECT 4276 strain (Wakabayashi et al., 1986) from the Spanish Type Culture Collection (CECT, University of Valencia, Spain) was used. Following the CECT recommendations, T. maritimum was stored at -80°C in 20% glycerol and routinely streaked on Marine Agar (MA, BD Difco™) plates and incubated at 30°C for 48 h. Marine Broth (MB, BD Difco™) was used for liquid cultures, and was prepared at different concentrations: 1x, 2x and 3x.
2.3 Evaluation of skin mucus antibacterial activity on planktonic cultures
Overnight cultures (16 h, 25°C, shaking conditions) were used to inoculate 20 mL of fresh medium (MB) at an initial cell density of ~ 1x107 CFU·mL-1, which corresponds to an OD600 of 0.04, and incubated in the same conditions until reaching exponential growth phase (~ 7x107 CFU·mL-1, which corresponds to an OD600 of 0.1). The assay of antibacterial activity of skin mucus was performed by monitoring the absorbance of bacterial cultures grown in flat-bottomed 96-well plates (Biolite Thermo Scientific) as previously described (Sanahuja et al., 2019). Aliquots of 50 µL of cultured bacteria were mixed with 50 µL of concentrated fresh medium (MB 3x) and 100 µL of skin mucus sample at 9.66 mg prot·mL-1 (no diluted mucus), 4.83 mg prot·mL-1 (1:2 diluted mucus), and 1.93 mg prot·mL-1 (1:5 diluted mucus)). Several controls were included in the plate: untreated control, where 50 μL of the same cultured bacteria were mixed with 150 μL of fresh medium (MB 1x); negative control, where 100 µL of the skin mucus sample dilutions used were incubated with 100 µL of medium (MB 2x); and media negative control, with 200 µL of MB (1x) were incubated. Triplicates were used for statistical purposes.
Bacterial growth was measured by absorbance at λ=400 nm every 30 min for 14 h at 25°C with a microplate reader (Infinity Pro200 spectrophotometer, Tecan, Spain). A 30-second shaking was performed every 2 minutes.
Bacterial growth inhibition was calculated at even hours of culture, as:
2.4 Biofilm formation assay setup
Before assessing skin mucus effects on T. maritimum biofilm, the settlement of optimal conditions for in vitro biofilm formation was needed. These conditions were decided based on being less time-consuming and using the smallest amount of mucus (limited component). Therefore, an experiment evaluating biofilm formation in flat-bottomed 48-well-polystyrene plate at 25°C, using different inoculum volumes (200, 300, and 400 µL) and incubation times (24, 48, and 72 hours) was designed.
T. maritimum overnight culture was diluted with fresh medium (MB) to ~ 4.6x106 CFU·mL-1 (which correspond to an OD600 of 0.02) to use as the inoculum. The different inoculum volumes used were placed (sextuplicate) in three 48-well-polystyrene plates. The same volumes of MB were used as negative controls. The plates, placed inside plastic bags with wet cellulose paper to maintain the proper humidity levels, were incubated statically at 25°C for 24, 48, and 72 h. After the incubation time, all liquid culture was removed from the plate and half of the wells were gently washed twice with distilled water. Next, all the wells were stained with Cristal Violet (CV) (see below). The negative control values were subtracted from the experimental values.
Once the optimal conditions for the biofilm formation were settled (24 h, 25°C and 200 µL inoculum), the effect of the presence of skin mucus in biofilm formation and biofilm dispersal assays were performed, by adapting the previously described method (Paytubi et al., 2017).
2.4.1 Inhibition of biofilm -formation assay
An overnight culture of T. maritimum diluted to OD600 = 0.04 (~1·107 CFU·mL-1) in MB (2x) was used as inoculum. 100 µL of bacterial suspension were incubated with 100 µL of mucus at 9.66 mg prot·mL-1 (no diluted mucus), 4.83 mg prot·mL-1 (1:2 diluted mucus) or 1.93 mg prot·mL-1 (1:5 diluted mucus) in triplicate. Several controls were included: untreated control (100 µL of bacterial suspension were incubated with 100 µL sterile MilliQ water); negative control (100 µL of MB 2x were incubated with 100 µL of mucus sample); and media negative controls (100 µL of MB 2x were incubated with 100 µL of sterile MilliQ water), in triplicate as well. The same bacterial suspension volume was used to validate the procedure with a gradient of ampicillin (6.25 µg·mL-1 to 0.049 µg·mL-1 in a ½ serial dilution). Two replicas of the flat-bottomed 48-well plates were statically incubated at 25°C for 24 hours. After the incubation, the quantitative measurement of the biofilm biomass was determined by CV staining. For quantitative monitoring of living cells within the biofilm, resazurin (RZN) staining was performed.
2.4.2 Biofilm dispersal assay
Mature biofilms of T. maritimum were obtained as described in the biofilm formation assay setup. After the incubation, planktonic cells were removed and 100 µL of fresh media (MB 2x) were loaded to each well. 100 µL of mucus, sterile water, and antibiotic gradient concentrations were added as described for the biofilm-formation assay. Plates were incubated in the same conditions for another 24 hours. Biofilm biomass and living cells were determined by CV and RZN staining.
2.4.3 Crystal Violet staining
Crystal Violet (CV) is a protein-dye that binds to negatively charged molecules, such as the peptidoglycan and the extracellular matrix (Petrachi et al., 2017). Hence, its use to quantify biofilm biomass in microtiter-plates is widely established (Stepanović et al., 2000). CV staining protocol was adapted from Paytubi et al. (2017), with some modifications. After removing planktonic cells from the plates, no washes with distilled water were performed (except for the assay used to establish the optimal biofilm forming conditions). Biofilms were fixed by heating at 80°C for 30 minutes. Next, staining was performed by adding CV (1% w/v in MilliQ water) to the each well and incubating the plates for 15 minutes at room temperature. The CV volume added to each well was the double of the final volume used in the assays to ensure that all biofilm biomass was stained (400, 600, or 800 µL/well in the assay for the establishment of optimal conditions and 400 µL/well for biofilm formation and elimination assays). Then CV solution was discarded, wells were washed with distilled water and plates were air-dried. Finally, CV was solubilized by adding acetic acid 30% (v/v) (200, 300, or 400 µL/well in the assay for the establishment of procedural conditions and 200 µL/well in the biofilm inhibition formation and dispersion assays). Biofilm biomass was determined by measuring the OD570 of the resulting solution in a microplate reader (Infinity Pro200 spectrophotometer, Tecan, Spain), after subtracting OD570 of the media negative controls.
2.4.4 Resazurin assay
Resazurin (RZN) is a blue, water-soluble, and non-fluorescent dye that, when reduced by electron transfer reactions of cell respiration, turns into a pink, fluorescent product (resorufin) easily measured by fluorescence (O’Brien et al., 2000). Therefore, resazurin can be used to monitor biofilm cell viability or metabolic activity (Mariscal et al., 2009). After removing of planktonic cells from the plates, no washes with distilled water were performed and 200 µL of fresh culture medium (MB 1x) and 50 µL of RZN solution (resazurin sodium salt (Sigma) at 0.02% w/v in MilliQ water and sterilized by filtration) were added to each well. Plates were statically incubated in the darkness at 37°C for 20–45 minutes. Fluorescence was measured (excitation 570 nm, emission 615 nm) with a microplate reader (Infinity Pro200 spectrophotometer, Tecan, Spain).
2.4.5 Biofilm inhibition analysis
Biofilm inhibition by skin mucus was evaluated integrating biofilm biomass (CV) and biofilm metabolic activity (RZN) inhibitions. For this purpose, the different inhibitions indexes were calculated with the following equations:
Biofilm biomass inhibition
Biofilm viability inhibition
2.5 Statistical analysis
Statistical analysis was performed using one-way ANOVA test of SPSS software Version 22.0 (IBM Corp, Armonk, NY, USA), followed by post-hoc Bonferroni’s test (if equal variances were assumed by Levene’s test) or Dunnett’s test (if variances among groups were unbalanced). Differences between mucus dilutions were considered statistically significant at p<0.05.
3 Results
3.1 Evaluation of skin mucus antibacterial activity against T. maritimum
The antibacterial activity of seabream skin mucus was tested on T. maritimum planktonic growth. The results obtained (Figure 1) showed that gilthead seabream skin mucus has a high antibacterial activity against this pathogen in a concentration-dependent manner. Non-diluted mucus (9.66 mg of protein·mL-1) completely inhibited T. maritimum growth, whereas 1:2-diluted mucus initially caused high inhibition, but the antimicrobial effect was reduced during the later hours of culture. On the other hand, a dilution of the mucus to 1.93 mg of protein·mL-1 resulted in an almost complete loss of antibacterial activity against the pathogen.
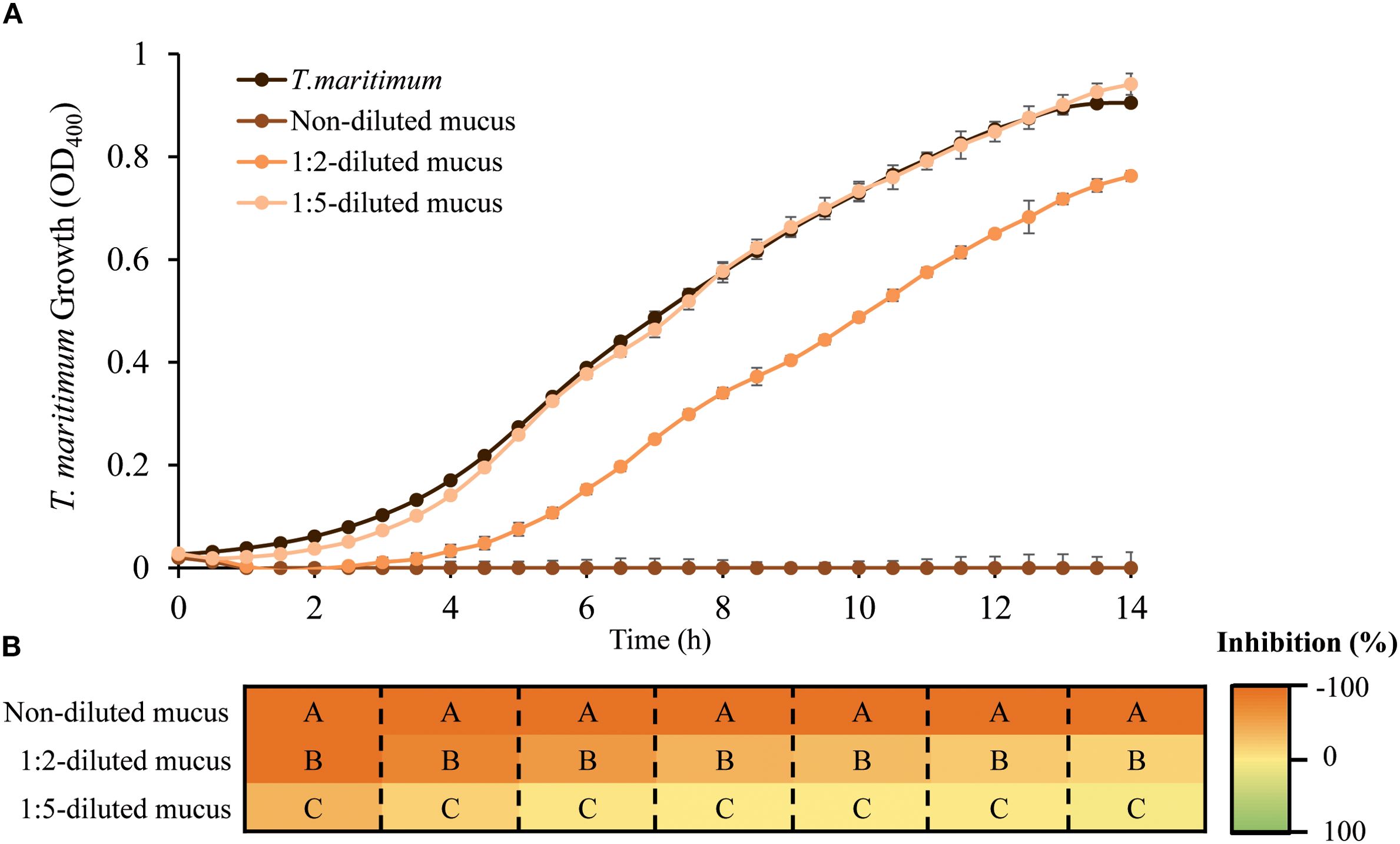
Figure 1. Skin mucus antibacterial activity against planktonic cultures of T. maritimum. (A) Growth of T. maritimum incubated without or with different dilutions of gilthead seabream skin mucus. Three different concentrations of mucus were used, corresponding to 9.66, 4.83 and 1.93 mg protein/mL (non-diluted, 1:2-diluted mucus and 1:5-diluted mucus respectively). Values are expressed as mean ± SEM (Standard Error of the Mean). (B) Significant differences in growth inhibition caused at even hours of culture between mucus dilutions are indicated by capital letters (one-way ANOVA, p<0.05).
3.2 Experimental setup conditions for T. maritimum biofilm formation
Biofilm formation assays were initially conducted in 96-well polystyrene plates, however, the data yielded was characterized by high variability and limited reproducibility. Furthermore, visual inspection of T. maritimum biofilms using crystal violet (CV) staining revealed notably irregular morphologies (data not shown). Due to the apparent weak adherence of the biofilm to the surface of the microplate wells, the T. maritimum biofilms could easily be disrupted by mechanical force and subsequently removed during washing steps (see Supplementary Material). The following biofilm assays were performed using 48-well plates. In addition to improve biofilm visualization, using 48-well plates offers several advantages over 96-well plates. The increased well size and culture volume in 48-well plates resulted in more consistent and reproducible biofilm formation data, as previously described (Gomes and Mergulhão, 2021). We evaluated different culture volumes and incubation periods, as well as non-washing or washing steps before fixing and CV staining, to determine the optimal experimental conditions for T. maritimum biofilm formation (Figure 2).
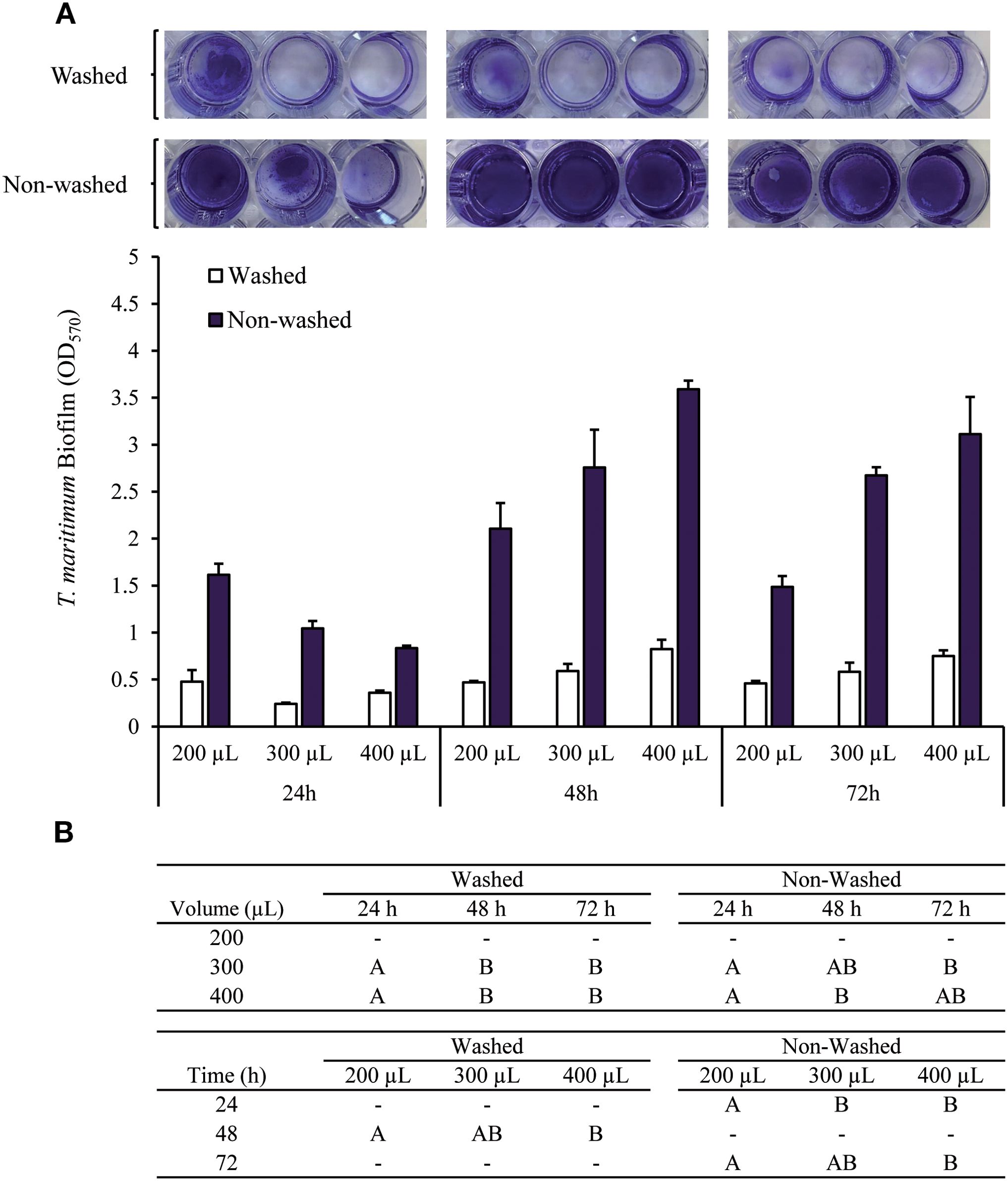
Figure 2. T. maritimum biofilm biomass determined by CV staining after 24, 48, or 72 hours of incubation at 25 °C. The values were corrected by subtracting the respective media negative control value. (A) Washing (twice) or not the microplate wells before fixing and staining the biofilm biomass, and using different volumes of bacterial suspension (200, 300, and 400 µL). (B) Capital letters indicate significant differences between bacterial suspension volumes used after the same incubation period, and differences between incubation time (one-way ANOVA, p<0.05).
T. maritimum produce a visible thick biofilm in all the conditions tested. The biofilm formed was almost completely lost during the two washing steps, as shown in Figure 2A. The biofilm loss during washing was not proportional to the preexisting biofilm formed and was highly variable among replicas. These results suggested that the differences observed for washed biofilms were related to manipulation differences during the washing steps.
Regarding the non-washed biofilms, the highest biofilm biomass was achieved when the higher culture volumes (300 and 400 µL) were used, at both 48 and 72 hours of incubation. After 24h of incubation, the highest biofilm biomass was achieved using 200 µL of bacterial inoculum (Figure 2).
Having in consideration that fish skin mucus volume could be a limited component, it was considered that the biofilm biomass achieved after 24 hours with a 200 µL inoculum was suitable to study the putative effects of mucus on T. maritimum biofilm formation. These conditions allowed saving both incubation time and most importantly fish skin mucus volume. Considering all these results, the conditions for the biofilm assays were settled as follows: 200 µL culture volume by well using 48-wells microplates, 24 h of incubation at 25°C, and no washing steps before biofilms fixation. However, it subsequently implied detecting some non-attached sedimented cells as if they were part of the biofilm structure, a limitation that should be taken into account when analyzing the results.
3.3 Skin mucus antimicrobial effect on T. maritimum biofilm formation
The effect of the skin mucus on the inhibition of biofilm formation and the dispersion of previously formed biofilm was evaluated by measuring the biofilm biomass and the cell viability of biofilm cells and comparing the results with those obtained in the absence of skin mucus (Figure 3). A gradient of ampicillin concentrations was used to validate the results (Figure 3A).
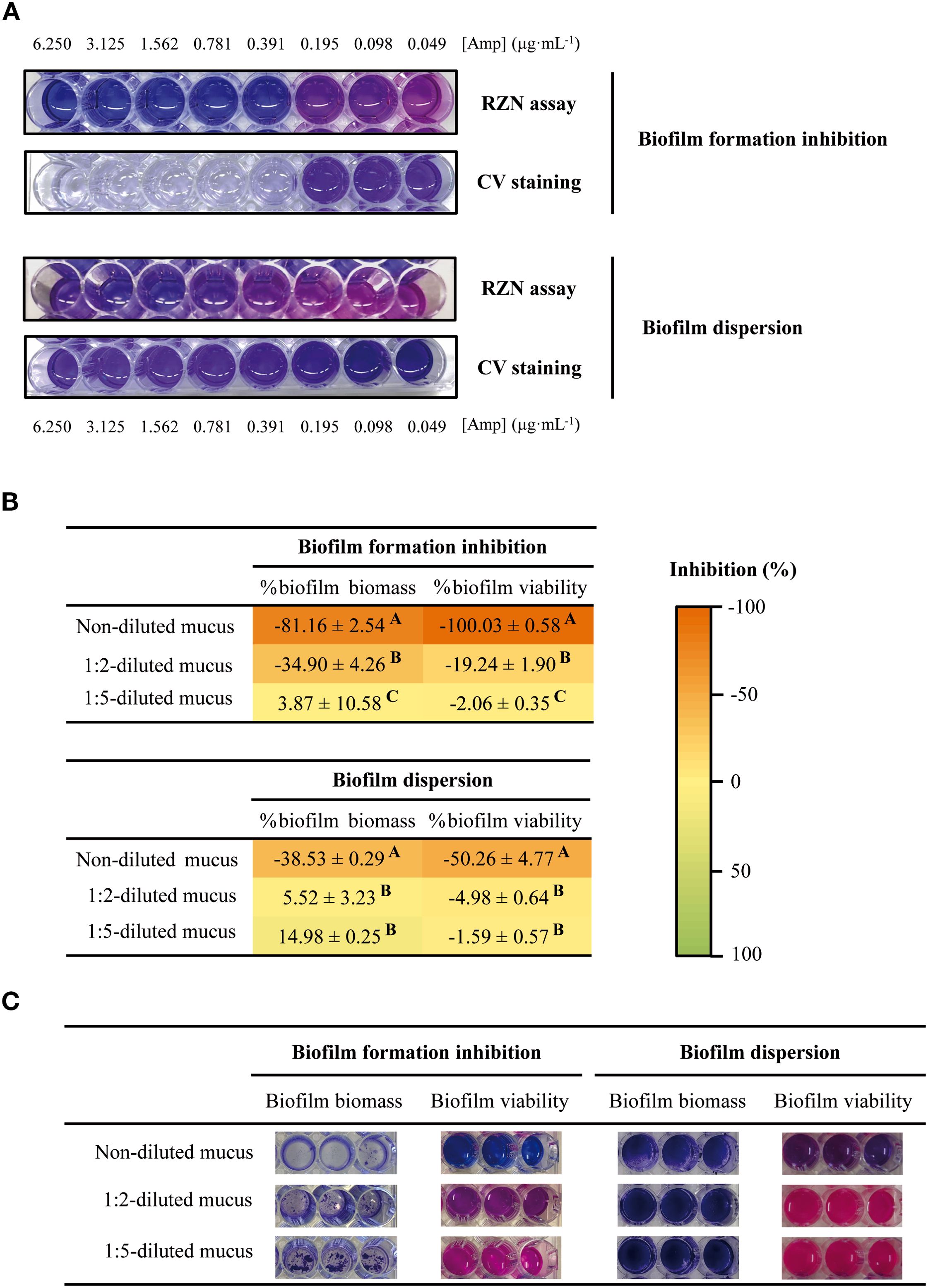
Figure 3. (A) Susceptibility dose-curves with 2-fold serial dilutions of ampicillin on inhibition of biofilm formation (upper panels) and dispersal of preformed biofilm (bottom panels) assays, by CV and RZN staining. (B, C) Effect of skin mucus dilutions on T. maritimum biofilm biomass and biofilm viability in the biofilm formation and dispersion assays. Three different concentrations of mucus were used, corresponding to 9.66, 4.83 and 1.93 mg protein/mL (non-diluted, 1:2-diluted mucus and 1:5-diluted mucus respectively). Differences between the effects of different skin mucus dilutions are indicated by capital letters (one-way ANOVA, p<0.05).
The antimicrobial effect of the fish skin mucus on the biofilm formation shows similar results to those obtained when the effect of skin mucus was assayed in planktonic cells (Figure 3B). Non-diluted mucus strongly inhibited biofilm formation: a reduction of 81.16 ± 2.54% in biofilm biomass was observed and, remarkably, no metabolic activity was detected. The inhibitory effect decreased when 1:2-diluted mucus was used (-34.90 ± 4.26% and -19.24 ± 1.90% of reduction in biofilm biomass and cell viability respectively).
Undiluted gilthead seabream skin mucus demonstrated significant effects on mature biofilms, promoting substantial biofilm biomass dispersion (-38.53 ± 0.29%) and reducing the metabolic activity by approximately 50% (Figure 3B). In contrast, diluted mucus appears not to affect biofilm biomass and viability in the biofilm dispersion assay.
There is a clear correlation between the quantity of skin mucus used in the assays and the effects caused in biofilm formation and dispersion of mature biofilm.
4 Discussion
Mucus plays an essential role in protecting fish against pathogens, not only by preventing bacterial contact with the underlying tissue (skin, gills, and intestine) but also by serving as a depository of many immunological molecules (Esteban, 2012). These protective properties have led to extensive research on the antibacterial activity of fish skin mucus (Díaz-Puertas et al., 2023; Tiralongo et al., 2020). The growing interest in this field is further supported by the fact that mucus can be collected using non-invasive methods (Sanahuja et al., 2023b), making it an attractive subject for study.
Different approaches to evaluate the effect of fish skin mucus on T. maritimum planktonic growth have been previously reported (Echeverría-Bugueño et al., 2023; Guardiola et al., 2019; Mabrok et al., 2016). The present study revealed that gilthead seabream skin mucus strongly inhibited T. maritimum planktonic growth (Figure 1). Our findings contradict those reported by Magariños et al. (1995), who observed resistance of several T. maritimum strains, including the one used in this study, to the antibacterial activity of seabream, turbot, and seabass skin mucus. The discrepancies between these results could arise from variations in methodologies employed for mucus processing and antibacterial activity testing. Firstly, the mucus processing reported by these authors did not include a homogenization and centrifugation process to remove the insoluble fraction of mucus. As a result, the soluble antimicrobial molecules could have remained trapped within the mucin matrix, thereby limiting their ability to interact with bacterial cells. Besides, before the antibacterial activity testing and probably to obtain a more homogenous sample to work with, a 1:20 dilution of the crude mucus was made in seawater. Regarding the antimicrobial testing, the referred work uses the disk diffusion method on agar plates. This method is widely established in clinical microbiology but, despite it has been reported for T. maritimum agar-supported antimicrobial susceptibility testing (Avendaño-Herrera et al., 2005), standard protocols have not been developed for MIC or disc diffusion assays for this species (Smith, 2019). The planktonic growth inhibition assay protocol used in the present study was adapted from a previously established methodology applied to other marine pathogens (Sanahuja et al., 2019) and it has been proven to be suitable for T. maritimum. Other approaches to evaluate the effect of fish skin mucus on T. maritimum growth in a liquid medium have been previously reported using Senegalese sole skin mucus (Solea senegalensis Kaup) (Guardiola et al., 2019; Mabrok et al., 2016). When using diluted mucus samples in TBS, low antibacterial activity was detected (Mabrok et al., 2016), whereas when using the soluble fraction of non-diluted skin mucus, higher inhibitions (20-90%), similarly to our results, were observed (Guardiola et al., 2019). It has been reported that Atlantic salmon skin mucus exhibits a strong antibacterial activity against T. dicentrarchi and T. maritimum, even though being previously diluted (Echeverría-Bugueño et al., 2023). The antibacterial activity of skin mucus against different pathogens has been described to be fish species-dependent (Sanahuja et al., 2019), outlining the importance of standardizing protocols for each fish species. The results obtained in the present study suggests that mucus sample dilution should be avoided when specifically studying the antibacterial activity of gilthead seabream skin mucus on T. maritimum planktonic growth.
Bacterial biofilms constitute significant challenges in medical and ecological contexts due to their remarkable ability to resist antibiotic treatments, leading to persistent infections (Flemming et al., 2016). Therefore, research efforts are being made to develop new strategies to control the formation of these structures, such as the phage therapy (Wroe et al., 2020), the search of new molecules derived from plant extracts (Rather et al., 2021b), or the use of nanoparticles for the delivery of antimicrobial compounds (Rather and Mandal, 2023). Biofilms have been associated with fish skin infections caused by different pathogens, such as Vibrio anguillarum (Croxatto et al., 2007), Piscirickettsia salmonis (Levipan et al., 2020), and T. maritimum (Nowlan et al., 2021). Unfortunately, few therapeutic options are currently available to control biofilm formation or to treat established biofilms (Koo et al., 2017). This study aimed to provide a platform for studying the potential antimicrobial effects of fish skin mucus on the formation and dispersion of biofilms of T. maritimum, an important pathogen in aquaculture.
The ability of T. maritimum to rapidly form a biofilm has been previously described (Levipan et al., 2019). Multilayered-like cell aggregates are already detected after 24 h incubation, and the biofilm biomass produced remains stable during the next four days. These results match with the profile observed in this report, as no differences were found whether cells were incubated for 24, 48, or 72 hours when using 200 µL of bacterial cultures (Figure 2). While the highest biofilm biomass was achieved using 400 µL of culture and 48–72-hour incubation periods, we used 200 µL inoculum with a 24-hour incubation time to investigate the effects of skin mucus on Tenacibaculum maritimum biofilm growth. This approach was chosen for two key reasons: i) it minimizes the overall experimental time and ii) it reduces the amount of fish skin mucus required for each assay, which is often a limited and valuable resource. Moreover, as microplates operate in batch mode, meaning nutrients are depleted and metabolic wastes accumulated, they are more suited for short-term experiments (Gomes and Mergulhão, 2021).
The biofilm generated under the experimental conditions used can be easily disturbed during conventional washing step prior biofilm formation. We demonstrate that eliminating the washing steps, a thick biofilm can be detected and quantified. While this step is typically used to remove planktonic and sedimented cells that are not part of the biofilm structure, it can cause unintended detachment of an unpredictable number of adherent microorganisms. This unintended detachment can lead to poor reproducibility in biofilm biomass measurements (Azeredo et al., 2017; Coenye and Nelis, 2010; Gomes and Mergulhão, 2021; Gómez-Suárez et al., 2001). Although it has been described that this pathogen strongly adheres to hydrophobic surfaces, such as polystyrene (Burchard et al., 1990; McEldowney and Fletcher, 1988; Sorongon et al., 1991), the results obtained after a two-washes step results in a high loss of T. maritimum biofilm biomass (Figure 2) (see Supplementary Material). Accordingly with our results, recent studies using microplates describe the ability of this bacteria to produce biofilms that were quantified by CV staining without previous washes (Escribano et al., 2023; Levipan et al., 2019). Nevertheless, it cannot be ruled out a different attachment of T. maritimum on different types of surfaces.
To study the antibacterial properties of various compounds against T. maritimum biofilms, this study proposes the use of uncoated 48-well polystyrene microplates and to omit washing steps prior to crystal violet (CV) and resazurin (RZN) staining. This approach streamlines the experimental process while maintaining accuracy in biofilm assessment.
Once stablished the conditions for biofilm formation, the effects of gilthead seabream skin mucus in T. maritimum biofilm formation and dispersion have been tested. The results show that the mucus inhibited T. maritimum CECT 4276 biofilm formation in a concentration-dependent manner (Figure 3B). Having in consideration the results of the effect of skin mucus on planktonic growth, the inhibition of biofilm formation observed could be a consequence of the inhibition of total bacterial growth in the presence of the skin mucus, as showed in the planktonic growth assays (Figure 1).
While a commercial vaccine exists to prevent T. maritimum infections in turbot, the control of tenacibaculosis in other cultured species primarily relies on curative treatments, particularly antibiotics. As the infection by this pathogen is characterized by the formation of a biofilm (Nowlan et al., 2021), research into novel antimicrobial agents should focus not only the ability to inhibit the biofilm formation, but also the ability to eliminate a pre-existing biofilm.
This dual approach is crucial because T. maritimum rapidly forms biofilms on various surfaces, and these biofilms enhance the pathogen’s environmental persistence and facilitate disease transmission. Effective control strategies must address both preventing new biofilm formation and eradicating existing biofilms to combat tenacibaculosis in aquaculture settings. Our results demonstrated that, in addition to inhibit biofilm formation, seabream skin mucus could alter previously formed T. maritimum biofilms, reducing both biofilm biomass and cell viability (Figure 3B). Similarly, it has been previously reported that the skin mucus of the freshwater fish Puntius sophore can distort the preformed biofilms as well as obstruct the adhesion property of different human bacterial pathogens (Patel et al., 2020). However, as expected, the impact of skin mucus on the disaggregation of T. maritimum pre-formed biofilms was found to be less pronounced than that observed in the biofilm formation assays, evidencing the high resistance that these structures confer to the bacterial cells. This limited dispersal effect represents a biological limitation of the mucus.
To our knowledge, this is the first study to provide a simple methodology for assessing the impact of skin mucus on both the formation and dispersion of T. maritimum biofilms.
5 Conclusions
T. maritimum infections are characterized by the formation of a biofilm on the fish skin. Hence, studies providing knowledge on its pathogenicity and possible treatments should include the formation of these structures in addition to working with its planktonic form. Here, we described a simple and rapid protocol for assessing the antimicrobial effects of fish skin mucus on T. maritimum biofilm growth in polystyrene microplates. The results showed that gilthead seabream mucus strongly inhibited T. maritimum biofilm formation, and it reduces the biomass and viability of previously formed biofilms. Therefore, the protocols employed could be used as a tool to study how different fish culture conditions affect fish susceptibility to T. maritimum infections. Moreover, it opens the door to investigate the components of fish skin mucus and the molecular mechanisms involved in its antibiofilm activity, with the aim of developing new strategies to control these structures.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was conducted in accordance with the Guiding Principles for Biomedical Research involving Animals (EU2010/63), the guidelines of Spanish legislation (Law 32/2007 and RD 53/2013) and approved by the Ethics Committee of the University of Barcelona for the Use of Laboratory Animals and the Generalitat de Catalunya (DAAM 9383). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MT: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. IS: Data curation, Formal analysis, Writing – review & editing. CB: Funding acquisition, Supervision, Validation, Writing – review & editing. AI: Funding acquisition, Supervision, Writing – review & editing. CM: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. LF-A: Conceptualization, Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was financially supported by project PID2023-147976OR-C22 (funded by MICIU/ AEI/10.13039/501100011033 and by FEDER, EU), PID2023-149851NB-I00 (funded by MICIU/AEI/10.13039/501100011033 and by FEDER, EU) and 2021SGR00646 (Generalitat de Catalunya). MT was granted a Spanish predoctoral fellowship (FI-SDUR 2022).

Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1631980/full#supplementary-material
Supplementary VIDEO 1 | Video showing the washing procedure of T. maritimum biofilms incubated during 24h at 25°C in 48-well polystyrene plates before biofilm fixing.
References
Acosta F., Montero D., Izquierdo M., and Galindo-Villegas J. (2021). High-level biocidal products effectively eradicate pathogenic γ-proteobacteria biofilms from aquaculture facilities. Aquaculture 532, 736004. doi: 10.1016/j.aquaculture.2020.736004, PMID: 39175494
Avendaño-Herrera R., Irgang R., Magariños B., Romalde J. L., and Toranzo A. E. (2006a). Use of microcosms to determine the survival of the fish pathogen Tenacibaculum maritimum in seawater. Environ. Microbiol. 8, 921–928. doi: 10.1111/j.1462-2920.2005.00981.x, PMID: 16623748
Avendaño-Herrera R., Irgang R., Núñez S., Romalde J. L., and Toranzo A. E. (2005). Recommendation of an appropriate medium for in vitro drug susceptibility testing of the fish pathogen tenacibaculum maritimum. Antimicrobial. Agents Chemother. 49, 82–87. doi: 10.1128/AAC.49.1.82-87.2005, PMID: 15616279
Avendaño-Herrera R., Toranzo A. E., and Magariños B. (2006b). Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: A review. Dis. Aquat. Organisms 71, 255–266. doi: 10.3354/dao071255, PMID: 17058606
Azeredo J., Azevedo N. F., Briandet R., Cerca N., Coenye T., Costa A. R., et al. (2017). Critical review on biofilm methods. Crit. Rev. Microbiol. 43, 313–351. doi: 10.1080/1040841X.2016.1208146, PMID: 27868469
Bernardet J.-F., Kerouault B., and Michel C. (1994). Comparative Study on Flexibacter maritimus Strains isolated from farmed sea bass (Dicentrarchus labrax) in France. Fish Pathol. 29, 105–111. doi: 10.3147/jsfp.29.105
Bowden T. J. (2008). Modulation of the immune system of fish by their environment. Fish Shellfish Immunol. 25, 373–383. doi: 10.1016/j.fsi.2008.03.017, PMID: 18562213
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3, PMID: 942051
Burchard R. P., Rittschof D., and Bonaventura J. (1990). Adhesion and motility of gliding bacteria on substrata with different surface free energies. Appl. Environ. Microbiol. 56, 2529–2534. doi: 10.1128/aem.56.8.2529-2534.1990, PMID: 2403259
Choi D. H., Kim Y.-G., Hwang C. Y., Yi H., Chun J., and Cho B. C. (2006). Tenacibaculum litoreum sp. Nov., isolated from tidal flat sediment. Int. J. Systematic Evolutionary Microbiol. 56, 635–640. doi: 10.1099/ijs.0.64044-0, PMID: 16514041
Coenye T. and Nelis H. J. (2010). In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiological Methods 83, 89–105. doi: 10.1016/j.mimet.2010.08.018, PMID: 20816706
Corbel M. J. (1975). The immune response in fish: A review. J. Fish Biol. 7, 539–563. doi: 10.1111/j.1095-8649.1975.tb04630.x
Croxatto A., Lauritz J., Chen C., and Milton D. L. (2007). Vibrio Anguillarum colonization of rainbow trout integument requires a DNA locus involved in exopolysaccharide transport and biosynthesis. Environ. Microbiol. 9, 370–382. doi: 10.1111/j.1462-2920.2006.01147.x, PMID: 17222135
Díaz-Puertas R., Adamek M., Mallavia R., and Falco A. (2023). Fish skin mucus extracts: an underexplored source of antimicrobial agents. Mar. Drugs 21, 350. doi: 10.3390/md21060350, PMID: 37367675
Echeverría-Bugueño M., Irgang R., Mancilla-Schulz J., and Avendaño-Herrera R. (2023). Healthy and infected Atlantic salmon (Salmo salar) skin-mucus response to Tenacibaculum dicentrarchi under in vitro conditions. Fish Shellfish Immunol. 136, 108747. doi: 10.1016/j.fsi.2023.108747, PMID: 37059254
Escribano M. P., Balado M., Toranzo A. E., Lemos M. L., and Magariños B. (2023). The secretome of the fish pathogen Tenacibaculum maritimum includes soluble virulence-related proteins and outer membrane vesicles. Front. Cell. Infection Microbiol. 13. doi: 10.3389/fcimb.2023.1197290, PMID: 37360528
Esteban M.Á. (2012). An overview of the immunological defenses in fish skin. ISRN Immunol. 1–29. doi: 10.5402/2012/8534
Fernández-Alacid L., Firmino J. P., Sanahuja I., Madrid C., Polo J., De Borba M. R., et al. (2021). Impact of dietary porcine blood by-products in meagre (Argyrosomus regius) physiology, evaluated by welfare biomarkers and the antibacterial properties of the skin mucus. Fish Shellfish Immunol. 118, 241–250. doi: 10.1016/j.fsi.2021.09.011, PMID: 34530078
Fernández-Alacid L., Sanahuja I., Madrid C., Polo J., Firmino J. P., Balsalobre C., et al. (2022). Evaluating the functional properties of spray-dried porcine plasma in gilthead seabream (Sparus aurata) fed low fish meal diets. Animals 12, 3297. doi: 10.3390/ani12233297, PMID: 36496818
Fernández-Alacid L., Sanahuja I., Ordóñez-Grande B., Sánchez-Nuño S., Viscor G., Gisbert E., et al. (2018). Skin mucus metabolites in response to physiological challenges: A valuable non-invasive method to study teleost marine species. Sci. Total Environ. 644, 1323–1335. doi: 10.1016/j.scitotenv.2018.07.083, PMID: 30743845
Fernández-Álvarez C. and Santos Y. (2018). Identification and typing of fish pathogenic species of the genus Tenacibaculum. Appl. Microbiol. Biotechnol. 102, 9973–9989. doi: 10.1007/s00253-018-9370-1, PMID: 30291367
Firmino J. P., Fernández-Alacid L., Vallejos-Vidal E., Salomón R., Sanahuja I., Tort L., et al. (2021). Carvacrol, thymol, and garlic essential oil promote skin innate immunity in gilthead seabream (Sparus aurata) through the multifactorial modulation of the secretory pathway and enhancement of mucus protective capacity. Front. Immunol. 12. doi: 10.3389/fimmu.2021.633621, PMID: 33777020
Flemming H.-C., Wingender J., Szewzyk U., Steinberg P., Rice S. A., and Kjelleberg S. (2016). Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575. doi: 10.1038/nrmicro.2016.94, PMID: 27510863
Gisbert E., Ibarz A., Firmino J. P., Fernández-Alacid L., Salomón R., Vallejos-Vidal E., et al. (2021). Porcine protein hydrolysates (PEPTEIVA®) promote growth and enhance systemic immunity in gilthead sea bream (Sparus aurata). Animals 11, 2122. doi: 10.3390/ani11072122, PMID: 34359250
Gomes L. C. and Mergulhão F. J. M. (2021). A selection of platforms to evaluate surface adhesion and biofilm formation in controlled hydrodynamic conditions. Microorganisms 9, 1993. doi: 10.3390/microorganisms9091993, PMID: 34576888
Gómez-Suárez C., Busscher H. J., and Van Der Mei H. C. (2001). Analysis of bacterial detachment from substratum surfaces by the passage of air-liquid interfaces. Appl. Environ. Microbiol. 67, 2531–2537. doi: 10.1128/AEM.67.6.2531-2537.2001, PMID: 11375160
Guardiola F. A., Mabrok M., MaChado M., Azeredo R., Afonso A., Esteban M. A., et al. (2019). Mucosal and systemic immune responses in Senegalese sole (Solea Senegalensis Kaup) bath challenged with Tenacibaculum maritimum: A time-course study. Fish Shellfish Immunol. 87, 744–754. doi: 10.1016/j.fsi.2019.02.015, PMID: 30763617
Handlinger J., Soltani M., and Percival S.. (1997). The pathology of Flexibacter maritimus in aquaculture species in Tasmania, Australia. Journal of Fish Diseases, 20: 159–168. doi: 10.1046/j.1365-2761.1997.00288.x
Herrera M., Fernández-Alacid L., Sanahuja I., Ibarz A., Salamanca N., Morales E., et al. (2020). Physiological and metabolic effects of a tryptophan-enriched diet to face up chronic stress in meagre (Argyrosomus regius). Aquaculture 522, 735102. doi: 10.1016/j.aquaculture.2020.735102
Koo H., Allan R. N., Howlin R. P., Stoodley P., and Hall-Stoodley L. (2017). Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740–755. doi: 10.1038/nrmicro.2017.99, PMID: 28944770
Kumari S., Tyor A. K., and Bhatnagar A. (2019). Evaluation of the antibacterial activity of skin mucus of three carp species. Int. Aquat. Res. 11, 225–239. doi: 10.1007/s40071-019-0231-z
Levipan H. A., Irgang R., Yáñez A., and Avendaño-Herrera R. (2020). Improved understanding of biofilm development by Piscirickettsia salmonis reveals potential risks for the persistence and dissemination of piscirickettsiosis. Sci. Rep. 10, 12224. doi: 10.1038/s41598-020-68990-4, PMID: 32699383
Levipan H. A., Tapia-Cammas D., Molina V., Irgang R., Toranzo A. E., Magariños B., et al. (2019). Biofilm development and cell viability: An undervalued mechanism in the persistence of the fish pathogen Tenacibaculum maritimum. Aquaculture 511, 734267. doi: 10.1016/j.aquaculture.2019.734267
Li T., Guo D., Shen Y., Bao J., and Jin L. (2022). Comparative analysis of bacterial communities in the sediment and seawater environments from marine large yellow croaker cages (Zhejiang coast, China). Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.963242
López J. R., Núñez S., Magariños B., Castro N., Navas J. I., de la Herran R., et al. (2009). First isolation of Tenacibaculum maritimum from wedge sole, Dicologoglossa cuneata (Moreau). J. Fish Dis. 32, 603–610. doi: 10.1111/j.1365-2761.2009.01029.x, PMID: 19486238
Lopez P., Saulnier D., Swarup-Gaucher S., David R., Lau C., Taputuarai R., et al. (2022). First Isolation of Virulent Tenacibaculum maritimum Isolates from Diseased Orbicular Batfish (Platax orbicularis) Farmed in Tahiti Island. Pathogens 11, 131. doi: 10.3390/pathogens11020131, PMID: 35215075
Mabrok M., Algammal A. M., Sivaramasamy E., Hetta H. F., Atwah B., Alghamdi S., et al. (2023). Tenacibaculosis caused by Tenacibaculum maritimum: Updated knowledge of this marine bacterial fish pathogen. Front. Cell. Infection Microbiol. 12. doi: 10.3389/fcimb.2022.1068000, PMID: 36683696
Mabrok M., MaChado M., Serra C. R., Afonso A., Valente L. M. P., and Costas B. (2016). Tenacibaculosis induction in the Senegalese sole (Solea Senegalensis) and studies of Tenacibaculum maritimum survival against host mucus and plasma. J. Fish Dis. 39, 1445–1455. doi: 10.1111/jfd.12483, PMID: 27134184
Magariños B., Pazos F., Santos Y., Romalde J., and Toranzo A. (1995). Response of Pasteurella piscicida and Flexibacter maritimus to skin mucus of marine fish. Dis. Aquat. Organisms 21, 103–108. doi: 10.3354/dao021103
Magnadottir B. (2010). Immunological control of fish diseases. Mar. Biotechnol. 12, 361–379. doi: 10.1007/s10126-010-9279-x, PMID: 20352271
Manning S. C. (2003). Basics of biofilm in clinical otolaryngology. Ear Nose Throat J. 82, 18–20. doi: 10.1177/014556130308208s07, PMID: 12974054
Mariscal A., Lopez-Gigosos R. M., Carnero-Varo M., and Fernandez-Crehuet J. (2009). Fluorescent assay based on resazurin for detection of activity of disinfectants against bacterial biofilm. Appl. Microbiol. Biotechnol. 82, 773–783. doi: 10.1007/s00253-009-1879-x, PMID: 19198831
Masumura K. and Wakabayashi H. (1977). An Outbreak of Gliding Bacterial Disease in Hatchery-born Red Seabream (Pagrus major) and Gilthead (Acanthopagrus schlegeli) Fry in Hiroshima. Fish Pathol. 12, 171–177. doi: 10.3147/jsfp.12.171
McEldowney S. and Fletcher M. (1988). Effect of pH, temperature, and growth conditions on the adhesion of a gliding bacterium and three nongliding bacteria to polystyrene. Microbial. Ecol. 16, 183–195. doi: 10.1007/BF02018913, PMID: 24201571
Nowlan J. P., Britney S. R., Lumsden J. S., and Russell S. (2021). Experimental Induction of Tenacibaculosis in Atlantic Salmon (Salmo salar L.) Using Tenacibaculum maritimum, T. dicentrarchi, and T. finnmarkense. Pathogens 10, 1439. doi: 10.3390/pathogens10111439, PMID: 34832595
O’Brien J., Wilson I., Orton T., and Pognan F. (2000). Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267, 5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x, PMID: 10951200
Patel M., Ashraf M. S., Siddiqui A. J., Ashraf S. A., Sachidanandan M., Snoussi M., et al. (2020). Profiling and role of bioactive molecules from puntius sophore (Freshwater/brackish fish) skin mucus with its potent antibacterial, antiadhesion, and antibiofilm activities. Biomolecules 10, 920. doi: 10.3390/biom10060920, PMID: 32560562
Paytubi S., de la Cruz M., Tormo J. R., Martín J., González I., González-Menendez V., et al. (2017). A high-throughput screening platform of microbial natural products for the discovery of molecules with antibiofilm properties against salmonella. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00326, PMID: 28303128
Pérez-Pascual D., Lunazzi A., Magdelenat G., Rouy Z., Roulet A., Lopez-Roques C., et al. (2017). The Complete Genome Sequence of the Fish Pathogen Tenacibaculum maritimum Provides Insights into Virulence Mechanisms. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.01542, PMID: 28861057
Petrachi T., Resca E., Piccinno M., Biagi F., Strusi V., Dominici M., et al. (2017). An alternative approach to investigate biofilm in medical devices: A feasibility study. Int. J. Environ. Res. Public Health 14, 1587. doi: 10.3390/ijerph14121587, PMID: 29258219
Rajkowski K. T. (2009). “Biofilms in fish processin,” in Biofilms in the Food and Beverage Industries. Eds. Fratamico P. M., Annous B. A., and Gunther &N.W. (Abington Hall, Granta Park, Great Abington, Cambridge CB21 6AH, UK: Woodhead Publishing), 499–516. doi: 10.1533/9781845697167.4.499
Rather M. A., Gupta K., Bardhan P., Borah M., Sarkar A., Eldiehy K. S. H., et al. (2021a). Microbial biofilm: A matter of grave concern for human health and food industry. J. Basic Microbiol. 61, 380–395. doi: 10.1002/jobm.202000678, PMID: 33615511
Rather M. A., Gupta K., and Mandal M. (2021b). Inhibition of biofilm and quorum sensing-regulated virulence factors in Pseudomonas aeruginosa by Cuphea carthagenensis (Jacq.) J. F. Macbr. Leaf extract: An in vitro study. J. Ethnopharmacol. 269, 113699. doi: 10.1016/j.jep.2020.113699, PMID: 33340600
Rather M. A., Gupta K., and Mandal M. (2021c). Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 52, 1701–1718. doi: 10.1007/s42770-021-00624-x, PMID: 34558029
Rather M. A. and Mandal M. (2023). Attenuation of biofilm and quorum sensing regulated virulence factors of an opportunistic pathogen Pseudomonas aeruginosa by phytofabricated silver nanoparticles. Microbial. Pathogenesis 185, 106433. doi: 10.1016/j.micpath.2023.106433, PMID: 37913826
Sanahuja I., Fernández-Alacid L., Ordóñez-Grande B., Sánchez-Nuño S., Ramos A., Araujo R. M., et al. (2019). Comparison of several non-specific skin mucus immune defences in three piscine species of aquaculture interest. Fish Shellfish Immunol. 89, 428–436. doi: 10.1016/j.fsi.2019.04.008, PMID: 30978446
Sanahuja I., Fernandez-Alacid L., Torrecillas S., Ruiz A., Vallejos-Vidal E., Firmino J. P., et al. (2023a). Dietary Debaryomyces hansenii promotes skin and skin mucus defensive capacities in a marine fish model. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1247199, PMID: 37711618
Sanahuja I., Guerreiro P. M., Girons A., Fernandez-Alacid L., and Ibarz A. (2023b). Evaluating the repetitive mucus extraction effects on mucus biomarkers, mucous cells, and the skin-barrier status in a marine fish model. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1095246
Shang D.-D., Lun H.-Y., Zhu K.-L., Chen G.-J., and Du Z.-J. (2021). Tenacibaculum pelagium sp. Nov., isolated from marine sediment. Arch. Microbiol. 203, 2229–2236. doi: 10.1007/s00203-021-02208-7, PMID: 33629140
Smith P. (2019). The performance of antimicrobial susceptibility testing programmes relevant to aquaculture and aquaculture products (FAO Fisheries and Aquaculture Circular No. 1191) (Rome, Italy: FAO). Available online at: https://www.fao.org/documents/card/es/c/ca6028en/ (Accessed September 9, 2025).
Soltanian S., Hoseinifar S. H., and Gholamhosseini A. (2018). Modulation of rainbow trout (Oncorhynchus mykiss) cutaneous mucosal immune responses following anesthesia: A comparative study on different anesthetic agents. Fish Shellfish Immunol. 80, 319–324. doi: 10.1016/j.fsi.2018.06.032, PMID: 29920385
Sorongon M. L., Bloodgood R. A., and Burchard R. P. (1991). Hydrophobicity, adhesion, and surface-exposed proteins of gliding bacteria. Appl. Environ. Microbiol. 57, 3193–3199. doi: 10.1128/aem.57.11.3193-3199.1991, PMID: 16348583
Stepanović S., Vuković D., Dakić I., Savić B., and Švabić-Vlahović M. (2000). A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiological Methods 40, 175–179. doi: 10.1016/S0167-7012(00)00122-6, PMID: 10699673
Stoodley P., Sauer K., Davies D. G., and Costerton J. W. (2002). Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56, 187–209. doi: 10.1146/annurev.micro.56.012302.160705, PMID: 12142477
Tacchi L., Lowrey L., Musharrafieh R., Crossey K., Larragoite E. T., and Salinas I. (2015). Effects of transportation stress and addition of salt to transport water on the skin mucosal homeostasis of rainbow trout (Oncorhynchus mykiss). Aquaculture 435, 120–127. doi: 10.1016/j.aquaculture.2014.09.027, PMID: 25705060
Tiralongo F., Messina G., Lombardo B. M., Longhitano L., Li Volti G., and Tibullo D. (2020). Skin mucus of marine fish as a source for the development of antimicrobial agents. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.541853
Wakabayashi H., Hikida M., and Masumura K. (1986). Flexibacter maritimus sp. Nov., a Pathogen of Marine Fishes. Int. J. Systematic Evolutionary Microbiol. 36, 396–398. doi: 10.1099/00207713-36-3-396
Watts M., Munday B., and Burke C. (2001). Immune responses of teleost fish. Aust. Veterinary J. 79, 570–574. doi: 10.1111/j.1751-0813.2001.tb10753.x, PMID: 11599820
Wroe J. A., Johnson C. T., and García A. J. (2020). Bacteriophage delivering hydrogels reduce biofilm formation in vitro and infection in vivo. J. Biomed. Materials Res. Part A 108, 39–49. doi: 10.1002/jbm.a.36790, PMID: 31443115
Keywords: Tenacibaculum maritimum, fish skin mucus, Sparus aurata, antibacterial, antibiofilm
Citation: Tejero M, Sanahuja I, Balsalobre C, Ibarz A, Madrid C and Fernandez-Alacid L (2025) Biofilm formation of Tenacibaculum maritimum, a fish pathogenic bacteria, to evaluate the antimicrobial activity of fish skin mucus. Front. Mar. Sci. 12:1631980. doi: 10.3389/fmars.2025.1631980
Received: 20 May 2025; Accepted: 27 August 2025;
Published: 13 October 2025.
Edited by:
Jorge T Antunes, University of Porto, PortugalReviewed by:
Lillian G. Acuña, Universidad Andres Bello, ChileMuzamil Ahmad Rather, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, India
Copyright © 2025 Tejero, Sanahuja, Balsalobre, Ibarz, Madrid and Fernandez-Alacid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Fernandez-Alacid, ZmVybmFuZGV6X2FsYWNpZEB1Yi5lZHU=
 Marc Tejero
Marc Tejero Ignasi Sanahuja
Ignasi Sanahuja Carlos Balsalobre
Carlos Balsalobre Antoni Ibarz
Antoni Ibarz Cristina Madrid
Cristina Madrid Laura Fernandez-Alacid
Laura Fernandez-Alacid