- 1Center for Global Discovery and Conservation Science, Arizona State University, Hilo, HI, United States
- 2School of Ocean Futures, Arizona State University, Hilo, HI, United States
Sewage pollution is a global threat to coastal ecosystems and amplifies the negative effects of climate change on coral reefs. Submarine groundwater discharge (SGD) is a major transport pathway for land-based pollution, but underlying drivers of SGD water quality are poorly understood, especially in nearshore coral reef ecosystems. We combined airborne mapping, field sampling, and statistical modeling to identify locations along the West Hawai‘i Island coastline where SGD is contaminated with sewage. Water samples collected from 47 distributed shoreline SGD locations were assayed for fecal indicator bacteria. A geostatistical model was used scale from field to regional levels at more than 1000 mapped SGD point locations to derive a geographic understanding of areas highly susceptible to contamination. We estimate that SGD delivers sewage-contaminated groundwater to at least 42% of reefs in West Hawaiʻi. Subsequent analyses indicate that contaminated points are associated with infrastructural build-up near the shoreline and an abundance of inland on-site sewage disposal systems. Mitigation of sewage pollution will require the prevention of numerous point sources from cesspools, septic leach fields, and similar sources.
1 Introduction
Coastal sewage pollution is broadly recognized as a global challenge, especially due to its negative impacts on coral reef ecosystems (Tuholske et al., 2021; Wear and Thurber, 2015). Wastewater effluent amplifies the negative outcomes of climate change on reefs and is highly associated with coral disease and mortality (Amato et al., 2016; Gove et al., 2023). On Hawai‘i Island, on-site sewage disposal systems (OSDS) release an estimated 55 million gallons of wastewater effluent into the ground per day (Mezzacapo et al., 2020). This material travels through highly permeable volcanic substrate and reaches the coast primarily through discrete point sources of submarine groundwater discharge (SGD) (Dimova et al., 2012; Knee et al., 2010), resulting in negative impacts to coral, fish, and other reef organisms (Foo and Asner, 2021; Gove et al., 2023).
Although SGD is broadly distributed, each point source of discharge is relatively difficult to measure (Sawyer et al., 2016), and consequently, the influence of this input is often overlooked in investigations of coastal processes (Santos et al., 2021). Recent technological advances have facilitated detecting and mapping the locations, magnitude, and spatio-temporal variability of SGD plumes (Asner et al., 2024; Kelly et al., 2013; McKenzie et al., 2021a). These studies have revealed that SGD is ubiquitous along the West Hawai‘i coastline, meaning the potential for wastewater effluent to be transported to coastal ecosystems is widespread.
SGD can play a major role in determining nearshore ecosystem processes and structure due to its effects on aquatic temperature, nutrients, and salinity levels (Santos et al., 2021). SGD variability drives the structure of benthic (La Valle et al., 2021) and phytoplankton (Adolf et al., 2019) communities, as well as ecosystem processes such as growth, net productivity, and carbon uptake (La Valle et al., 2019; La Valle et al., 2023; Richardson et al., 2017; Silbiger et al., 2020). The nature of this influence depends on the composition of the discharging water and includes non-linear and interactive effects. For example, SGD is often a highly disproportionate source of inorganic nitrogen to reefs (Paytan et al., 2006) relative to its volume (Knee et al., 2008). Native biota are adapted to the low salinity and naturally elevated nutrient levels in SGD, which can result in positive effects on coral growth rates (Lubarsky et al., 2018). However, eutrophication beyond the naturally elevated nutrient levels can allow invasive macroalgae to dominate over culturally valued native species (Dulai et al., 2023; Okuhata et al., 2023; Richards Donà et al., 2023), and can magnify the negative effects of ocean acidification on bioerosion and coral calcification rates (Prouty et al., 2017). Therefore, it is critical to identify not just where SGD occurs, but also where SGD is delivering land-based pollution to reefs.
Although progress has been made in quantifying the influence of land cover and watershed management on the magnitude of SGD in West Hawai‘i, especially in the context of hydrologic budgets and concern over projected declines in freshwater availability (e.g (Brauman et al., 2012; Dudley et al., 2020)), much less is known about the underlying drivers of SGD water quality. One investigation documented variability in nutrient fluxes over three orders of magnitude across eleven sites (Knee et al., 2010). These fluxes were not correlated with urban development and land cover variables that are strongly associated with groundwater quality in more densely populated areas on the windward side of the island (Saingam et al., 2021; Strauch et al., 2014) and throughout the Hawaiian archipelago (Bishop et al., 2017). Along the West Hawai‘i coastline, the presence and impact of sewage contamination in nearshore reefs are well documented in the northern region of Kohala (Abaya et al., 2018; Aguiar et al., 2023; Panelo et al., 2022; Wiegner et al., 2021). Modeling studies suggest there may be extensive contamination beyond this region (Delevaux et al., 2018; Gove et al., 2023; Okuhata et al., 2022a), but there is both a lack of empirical water quality data for a majority of the coastline, as well as a need for finer scale information to support coastal and watershed management and restoration activities. This lack of data also hinders efforts to validate hydrologic models of contaminant transport in Hawaiian aquifers (Mezzacapo et al., 2020).
Here we report on the results of a field sampling and modeling study to assess indicators of wastewater pollution. In doing so, we addressed the following: (1) Where SGD is contaminated by wastewater and posing a chronic threat to nearshore reefs; (2) How much variation in contamination can be explained by environmental factors; and (3) How water quality varies between contaminated and non-contaminated sites.
2 Methods
2.1 Study area and field sampling
Water samples were collected from 47 shoreline locations along the West Hawai‘i coastline at locations of previously mapped submarine groundwater discharge (Asner et al., 2024). Asner et al. (2024) identified 1058 SGD outfall locations in West Hawai‘i, which were subsequently used here to upscale results from our 47 field sampling sites to the regional level using a landscape statistical model. Samples were collected during early morning outgoing low tide to capture negative hydraulic gradient to the shoreline and minimize degradation of bacteria from solar radiation (Feng et al., 2013). Water was collected into sterilized 500 mL Nalgene bottles triple-rinsed with site water. Sampling was focused on embayments in northern and southern watersheds, where there is less frequent beach monitoring and potential for coral reef restoration. Samples were collected between August 2023 and February 2025. At 15 sites, multiple samples were collected throughout the sampling period resulting in a total of 117 observations.
2.2 Laboratory analysis
Fecal indicator bacteria levels were quantified as Enterococcus abundance measured after samples were incubated with Enterolert nutrient reagent (Sercu et al., 2011). Enterococcus was used as an indicator of fecal contamination because it is more persistent in marine environments compared to Escherichia coli, and levels can be reliably assessed with low cost, rapid turnaround testing procedures (Griffin et al., 2001; Vaccaro et al., 1950; Zhang et al., 2015). Results are reported as minimum probable number (MPN) per 100 mL and categorized using public health safety thresholds used by the Hawai‘i Department of Health. A sample was considered contaminated if levels exceeded 35 MPN, and for sites sampled multiple times, it was considered contaminated if any sample exceeded the 35 MPN contamination threshold. Each set of sample incubations also included a sample blank for quality control. Nitrate levels were quantified using a portable benchtop photometer (HI97115 Hanna Instruments, Woonsocket, RI, USA). Salinity, dissolved organic matter (DOM), chlorophyll, and turbidity levels were quantified in the laboratory using a multiparameter water quality sonde (YSI EXO1, Yellow Springs Instruments, Yellow Springs, OH, USA).
2.3 Environmental drivers
Land cover characteristics potentially contributing to or mitigating the prevalence of groundwater contamination were calculated for the land area immediately surrounding each sampling site and for the upstream contributing land area. Variables included: the percentages of five classes of land cover (Built-Up, Bare/Barren, Shrubland, Tree Cover, Grassland) (Homer et al., 2004; Zanaga et al., 2022), total impervious cover (USGS 2014); the total number of all OSDS (Whittier and El-Kadi, 2014) and the total number of all upstream OSDS classified as cesspools (i.e. Class IV OSDS). Cesspools are OSDS systems in which wastewater effluent receives no treatment prior to being released into the environment. Land cover variables are derived from the finer 10 m resolution WorldCover database (Zanaga et al., 2022) unless specified as National Land Cover Database (NLCD). Each variable was calculated at twelve spatial scales: (a) within a 100 m and 500 m radius of the site location; (b) within the entire adjacent upstream watershed as well as the area within 100 m, 500 m, 1 km, and 2 km of the coastline; and (c) within the adjacent upstream coastal catchment as well as the area within 100 m, 500 m, 1 km, and 2 km of the coastline. Watersheds were defined by the Division of Aquatic Resources delineations (State of Hawaii, 2005), and coastal catchments were defined from the USGS National Hydrography Dataset (Moore et al., 2019). Watersheds and catchments are both based on digital elevation models, however, the coastal catchments only include land area that drains directly to the coast and not to a stream channel, following the assumption Sawyer et al. (2016) use in their continental-scale analysis of submarine groundwater discharge. Daily rainfall data from the Hawai‘i Climate Data portal (Longman et al., 2024) were used to calculate the antecedent rainfall total for the two days prior to each sample collection for sample-level models.
2.4 Statistical analysis
We used multivariate Bayesian generalized logistic regression modeling (Bürkner, 2017; Carpenter et al., 2017) to identify which environmental factors were most associated with Enterococcus measurements. Models were fit at the level of sites and at the level of individual samples, in order to account for variability in outcomes at sites that were sampled multiple times. Site-level models were fit with environmental site-level variables as fixed effects, with contamination as a binary response variable. Informative predictors were identified as those that explained at least 10% of the variability in univariate models based on R2 values. Next, models were fit with all combinations of the identified informative predictors, while excluding combinations of highly collinear variables. Variables were considered collinear if Pearson correlations exceeded 0.7 (Dormann et al., 2013). Sample-level models were fit as mixed effect models using site as a random effect, and water quality measurements and the sum of antecedent rainfall in the two days prior to each sample collection as fixed effects. Models were run with uninformative priors, and all continuous variables were log-transformed and scaled before model fitting, except for count variables (i.e. total number of OSDS or cesspools) which were square root transformed. We compared model fits using the expected log pointwise predictive density (ELPD), pareto-smoothed leave-one-out cross validation adjusted R2, Bayes posterior distribution of R2 values, and both marginal and conditional R2 for mixed effect models (Vehtari et al., 2017). Marginal R2 values quantify the proportion of variance explained by fixed effects, whereas conditional R2 values include the variance associated with differences between sites. The relationships among water quality parameters were assessed using mixed effect generalized linear regression models with site as a random effect.
3 Results
3.1 Prevalence of contamination
Out of the 47 sampled SGD locations, 20 sites (42%) had elevated levels of Enterococcus (i.e. exceeding the recommended average level of 35 MPN), and 11 sites (23%) had concerning levels, which exceeded the 130 MPN public health risk threshold. Contaminated sites were distributed throughout the South Kona and South Kohala watersheds (Figure 1), including eight sites in the resort areas south of Puakō, three sites surrounding Hōnaunau Bay, and eight sites near Miloli‘i Beach Park. We found no evidence of contamination in the areas of Māhukona, Pauoa Bay, Ho‘okena, or Honomalino Bay. For the 15 sites in Hōnaunau and Miloli‘i with repeated sampling, Enterococcus levels typically varied above and below the contamination threshold across sampling dates. The exceptions were one site in Miloli‘i Beach Park where every sample far exceeded contamination thresholds and three sites north of Miloli‘i Beach Park that consistently measured below the contamination threshold.
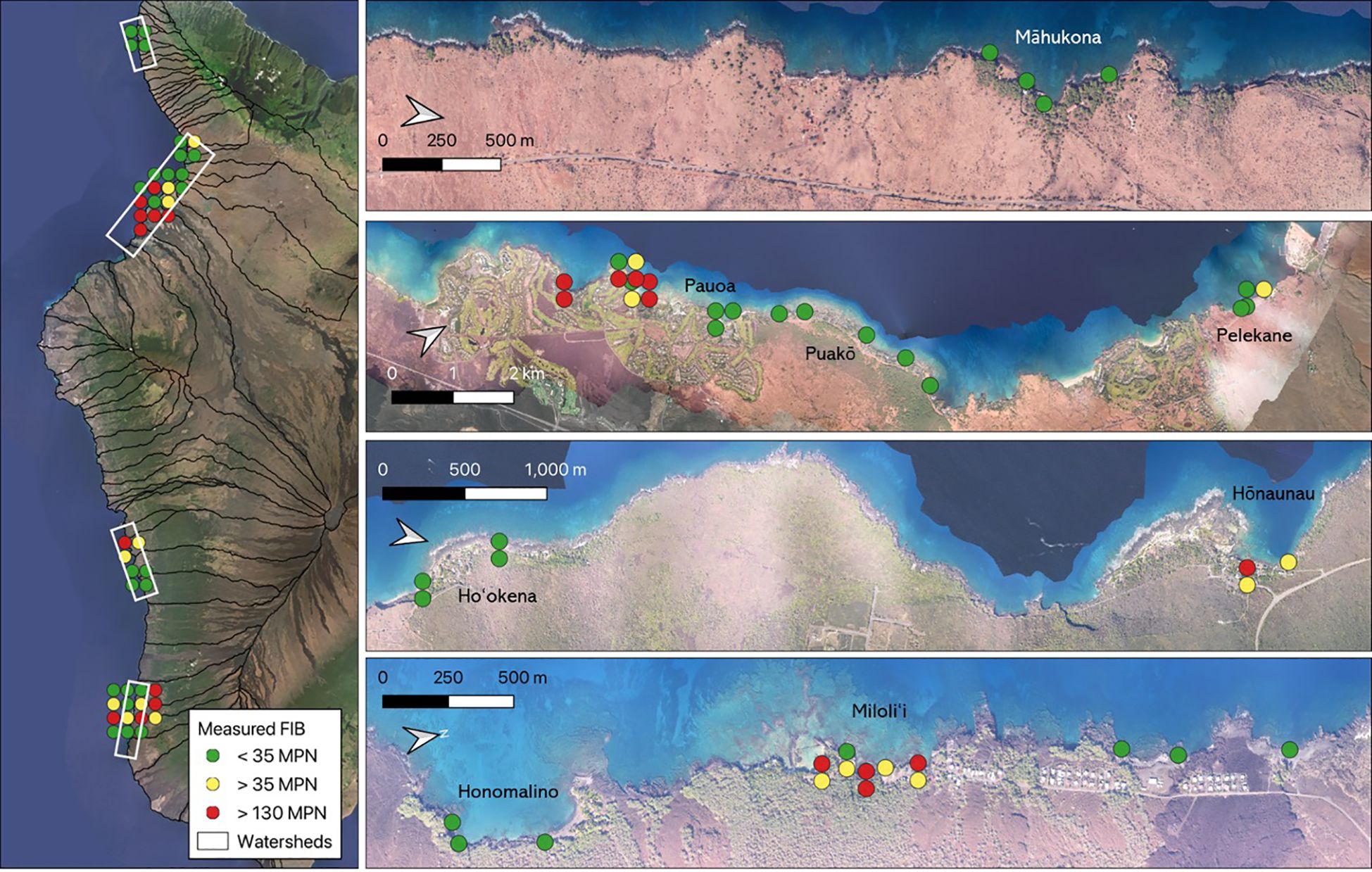
Figure 1. Measured levels of fecal indicator bacteria (Enterococcus spp.) in shoreline samples collected from submarine groundwater discharge locations along the west coast of Hawaiʻi Island displayed in terms of recommended thresholds for public health. Yellow points represent sites where levels exceeded 35 MPN and red points represent sites where levels exceeded 130 MPN. Background imagery is from the ASU Center for Global Discovery and Conservation Science, Global Airborne Observatory. Background imagery for the left panel is © 2025 Planet Labs.
3.2 Factors explaining site level variability in contamination
Sites were more likely to be contaminated if they were in watersheds with higher levels of built-up land cover near the coast, had more cesspools and OSDS in the upstream catchment, or were in watersheds with greater amounts of bare land (Figures 2, 3, Supplementary Table S1). Sites were also more likely to be contaminated if they had relatively high dissolved organic matter or nitrate levels, or more freshwater influence (i.e. lower salinity). Salinity (R2 = 0.35) was negatively correlated and dissolved organic matter (R2 = 0.20) was positively correlated with Enterococcus levels. The most influential land-cover variable was the percentage of built-up land cover within 500 m of the coast in the upstream watershed (R2 = 0.14). Land-cover variables describing development (built-up, bare, developed or impervious land cover) explained the most variability when calculated over watershed scales, whereas variables describing OSDS and shrubland land cover explained more variability when calculated at catchment scales. Many land-cover variables were significantly correlated when calculated over different spatial scales (Figure 4), but they explained the most variability in contamination outcomes when calculated over only the region of each watershed or catchment nearest the coast (e.g. within 500 m of the shoreline).
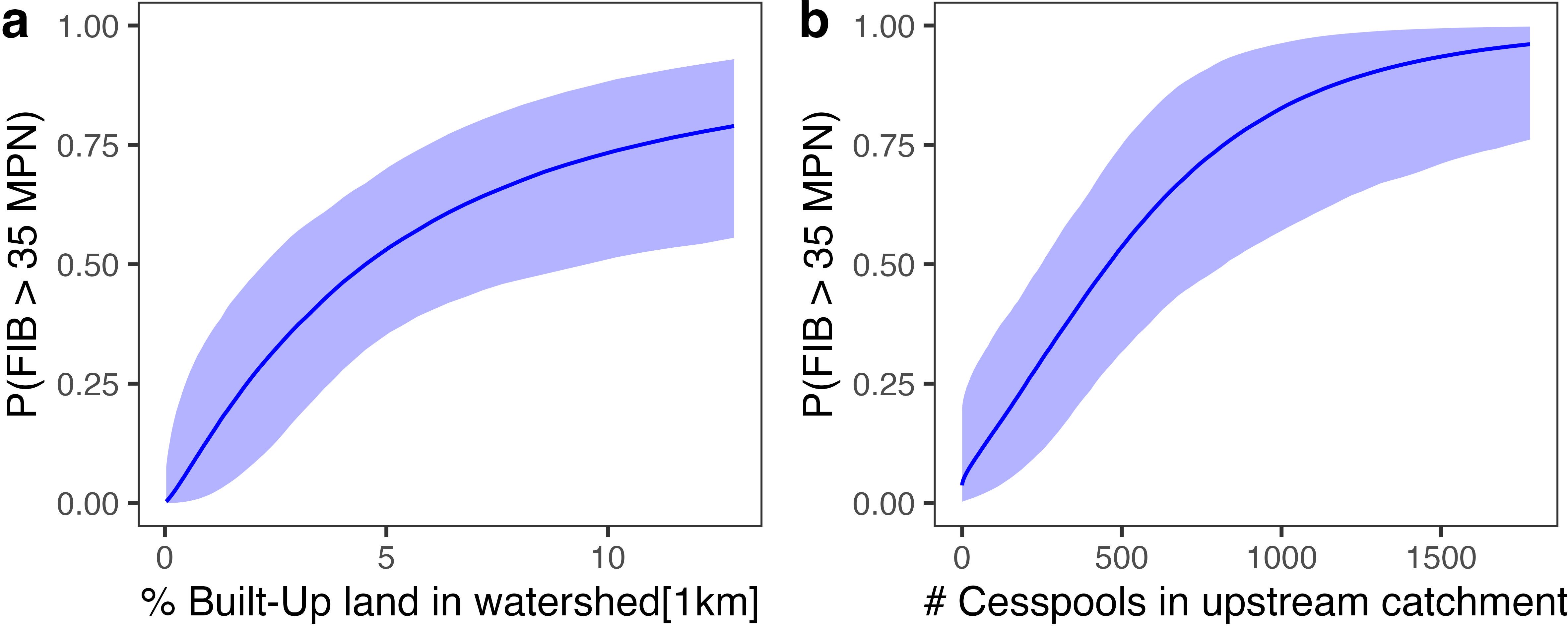
Figure 2. Predictive models of SGD contamination modeled as the probability of fecal indicator bacteria exceeding 35 minimum probable number [P(FIB > 35 MPN)]: (a) Percent of infrastructural build-up in the upstream watershed within 1 km of the coastline; (b) Number of cesspools (Class IV OSDS) in the upstream catchment. Lines show the predicted probability of contamination holding other variables constant. The shaded area is the 95% credible interval for the expected response. Predictor variables were log-transformed (% Built-Up) or square-root transformed (number of cesspools) and standardized prior to modeling, and axes have been back-transformed to the original scale for interpretability.
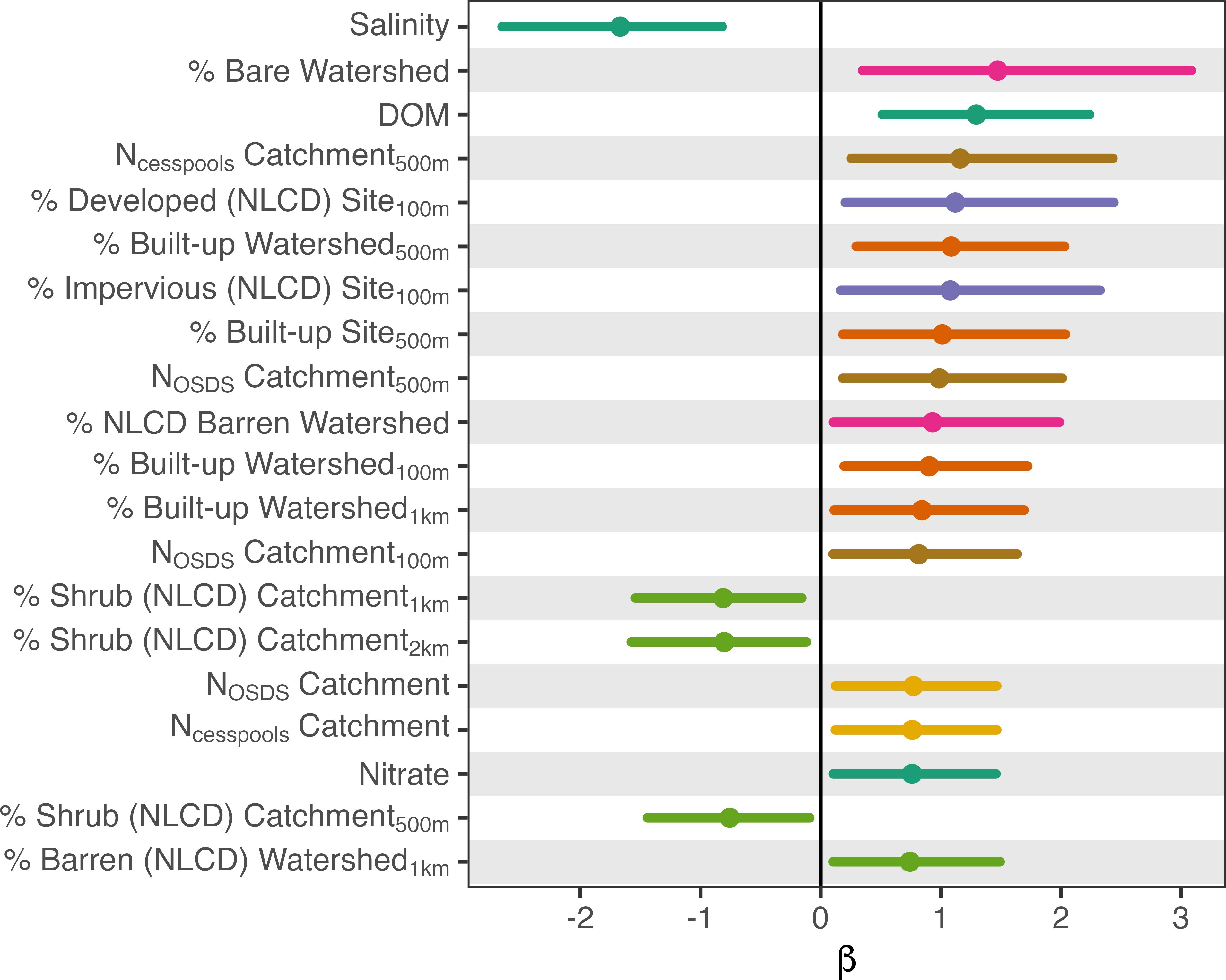
Figure 3. Effect sizes (β) and 95% credible intervals for the most explanatory fixed effects (all variables where R2 > 0.10) in univariate Bayesian logistic regression models to predict site-level contamination. Positive effects indicate that higher values of the variable are associated with a higher probability of contamination and negative effects indicate that lower values are associated with a higher probability of contamination. Colors indicate separate clusters of highly collinear variables.
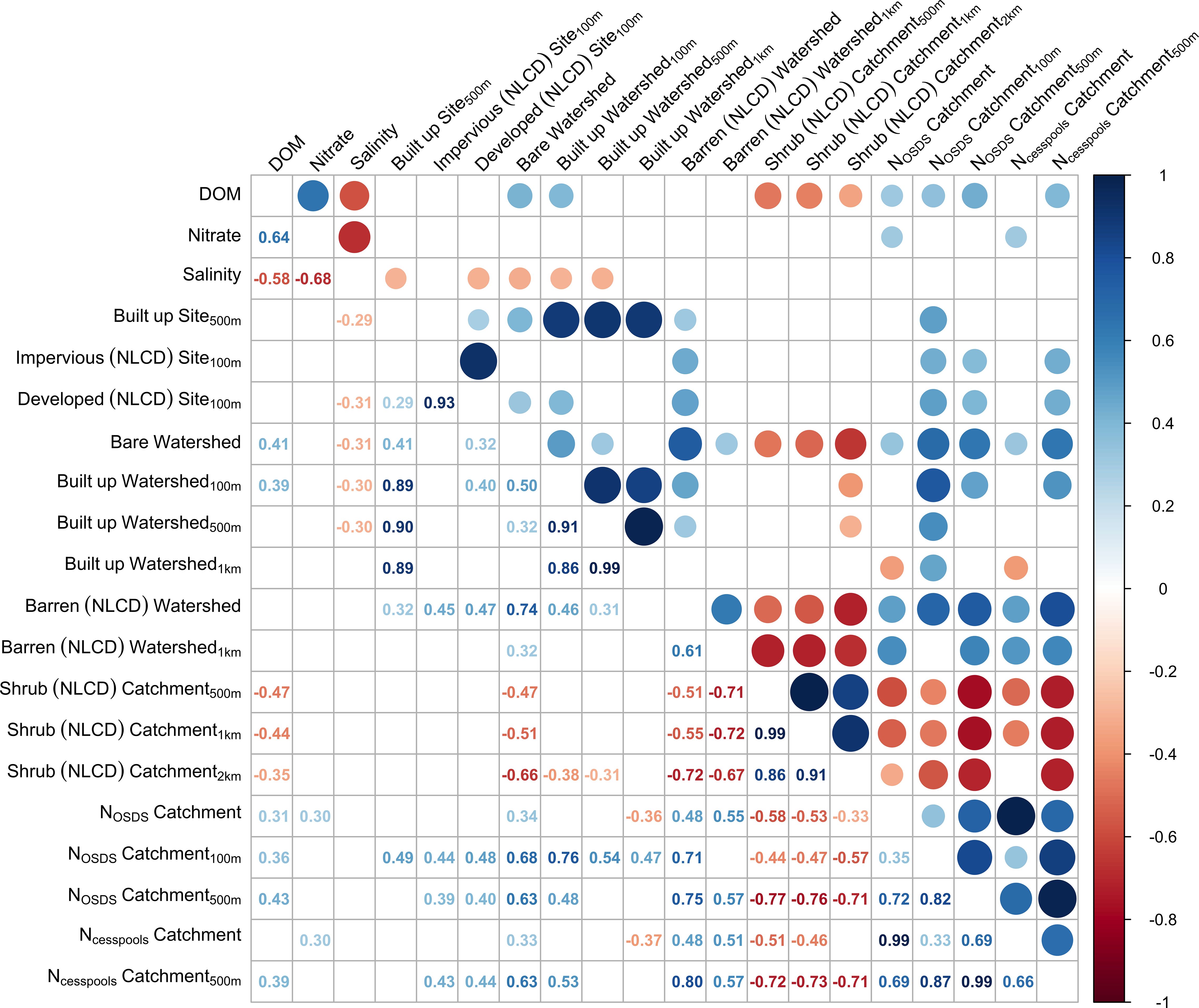
Figure 4. Correlation matrix showing Pearson correlation coefficients between site level predictors used in regression models. Blue indicates variables that are positively correlated and red indicates variables that are negatively correlated. Only significant correlations at α = 0.05 are shown.
The multivariate model that best predicted site-level Enterococcus contamination explained 51% of the variability (Bayes R2 = 0.51 ± 0.05) and included three fixed effects: number of cesspools in the upstream catchment (β = 1.74 ± 0.69 standard error), percent of built-up land cover within 1 km of the coast in the upstream watershed (β = 1.69 ± 0.83), and salinity (β = −1.62 ± 0.60). Only considering land-cover variables, the most predictive model explained 35% of Enterococcus variability (Bayes R2 = 0.35 ± 0.07) and included two fixed effects: percent built-up land cover within 1 km of the coast in the upstream watershed (β = 2.00 ± 0.74) and the number of cesspools in the upstream catchment (β = 1.86 ± 0.62). The model including salinity was more predictive (ELPD = −23.8 ± 6.8) than the model only including land-cover variables (ELPD = −27.1 ± 6.0), but there was no statistically significant difference in predictive performance between these models or similar combinations of the influential fixed effects (Supplementary Table S2).
3.3 Factors explaining sample level variability in contamination
Sample-level models, that included time-varying factors as fixed effects, indicated that water samples were more likely to be contaminated alongside higher levels of dissolved organic matter (DOM; R2 = 0.23), higher nitrate concentrations (R2 = 0.21), and lower salinity levels (R2 = 0.27) (Figure 5; Supplementary Table S3). Turbidity levels and antecedent rainfall amounts were not correlated with Enterococcus presence when considered independently (Supplementary Table S3). Although DOM had the strongest association with Enterococcus based on its effect size, salinity had higher ELPD and LOO-adjusted R2, indicating a more consistent and generalizable relationship with Enterococcus compared to DOM. Site-level factors (i.e. random effects in the mixed effect model) explained as much or more variability in Enterococcus levels compared to sample-level fixed effects. Salinity, DOM, and nitrate were also strongly related to each other. DOM (Marginal R2 = 0.55; p < 0.001) and nitrate (Marginal R2 = 0.64; p < 0.001) both had significant negative relationships with salinity, and there was a significant positive relationship between nitrate and DOM (Marginal R2 = 0.36, p < 0.001).
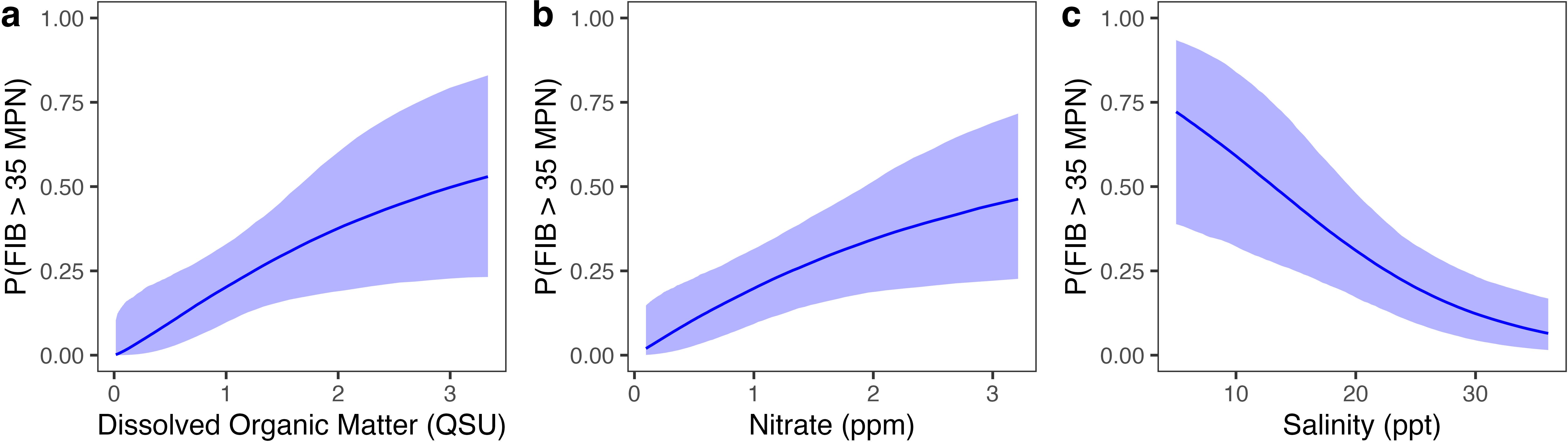
Figure 5. Individual conditional effects of water quality parameters to predict SGD contamination for a given sampling event, showing the marginal effect of each predictor on the probability that fecal indicator bacteria (FIB) in that sample exceeded 35 MPN: (a) dissolved organic matter (DOM), (b) nitrate, and (c) salinity. The shaded area is the 95% credible interval for the expected response. All variables were standardized prior to modeling, and DOM and nitrate were log-transformed. Axes have been back-transformed to the original scale for interpretability.
3.4 Coast-wide model predictions
We calculated the probability of Enterococcus contamination for each mapped SGD point source location along the West Hawai‘i coastline using an ensemble prediction from the highest performing models, with each model prediction weighted by its out-of-sample predictive accuracy (Yao et al., 2018). Results suggest that 52% (545 out of 1058) of the SGD locations along the coastline are highly susceptible to contamination based on upstream land cover. If the locations with land cover conditions beyond the range of our sampling sites are excluded, model results suggest 70% (358 out of 515) of sites are highly susceptible to contamination (Supplementary Figure S1). Watersheds with high numbers of sites predicted to be contaminated are distributed throughout South Kohala (‘Anaeho‘omalu), North Kona (Ka‘ūpūlehu, Pu‘uwa‘awa‘a), and South Kona (Ho‘ōpūloa, Keauhou) and include culturally significant places and regions with historically high live coral cover (Figure 6; Supplementary Table S4).
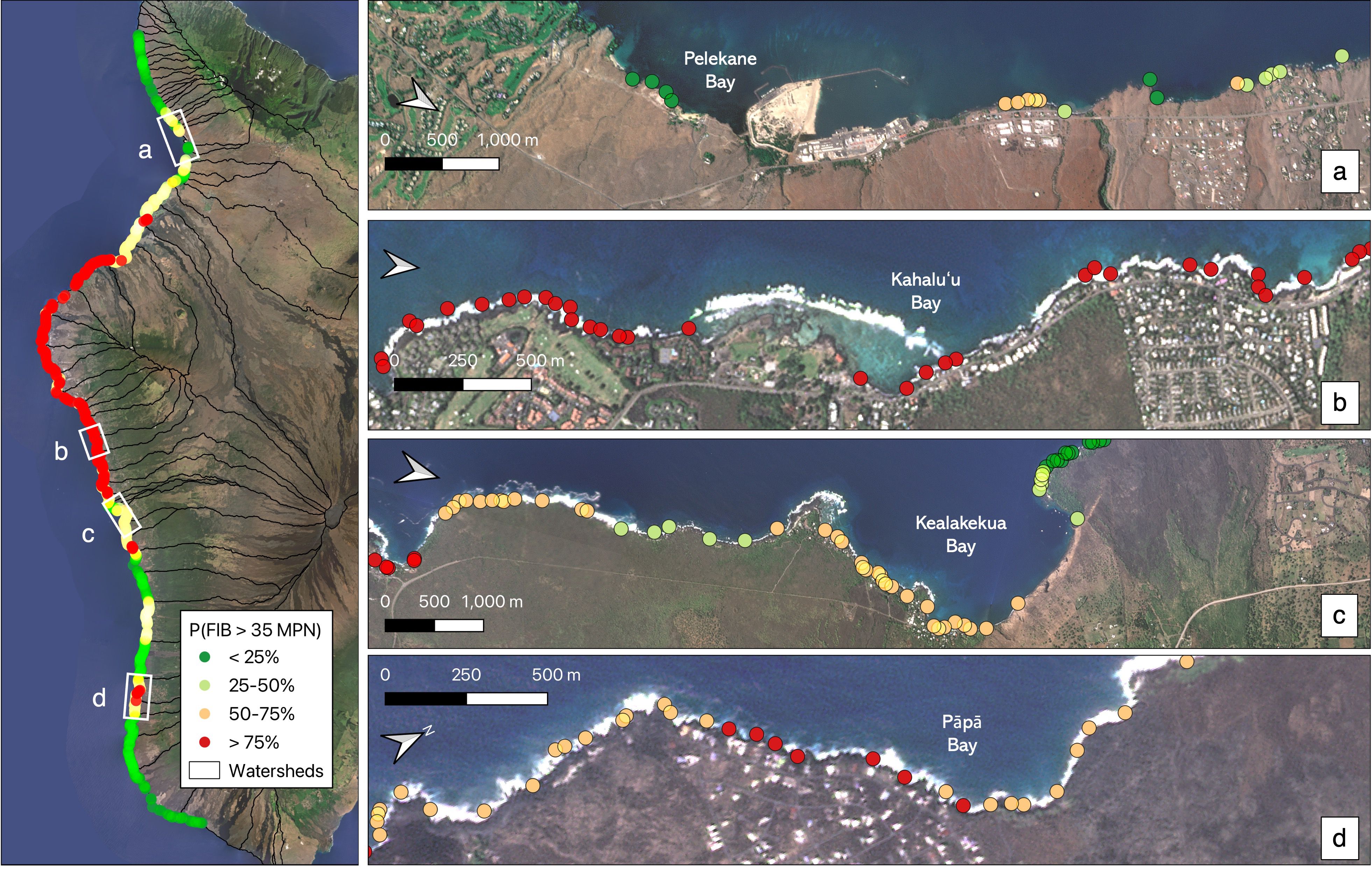
Figure 6. Point locations of submarine groundwater discharge along the West Hawaiʻi coast, colored based on the modeled susceptibility to wastewater contamination at each site. Contamination is modeled as the probability of elevated fecal indicator bacteria (FIB) based on upstream landscape characteristics, calculated using a weighted average of predictions from the 10 highest performing multivariate logistic regression models. Background imagery is © 2025 Planet Labs.
4 Discussion
Field sampling and mixed effects modeling showed that SGD drives widespread but spatially variable contamination of submarine groundwater discharge along the West Hawai‘i coastline. Contamination was more likely at sites impacted by higher numbers of cesspools and higher levels of coastal development. These findings agree with and confirm more localized studies establishing the presence of sewage in coastal waters along the northern portion of our study region (Abaya et al., 2018; Aguiar et al., 2023; Panelo et al., 2022; Wiegner et al., 2021), and provide new evidence of groundwater contaminated by fecal bacteria in several watersheds in the southern portion of our study (Figure 1). Modeling suggested that groundwater discharge at the coast is more susceptible to contamination in areas where there are 450 or more cesspools in the upstream catchment, and greater than 4.5% of the land within 1 km of the coastline is built up. While the South Kona region currently has a low level of development compared to the population centers and resort areas farther north, our data suggests that even small increases in built-up land near the coastline increase the likelihood of groundwater contamination.
The southern coast of our study region, known as South Kona, not only contains several rapidly expanding coastal developments, it is also underlain by some of the youngest and most porous volcanic substrate in the archipelago, with little soil development and a high degree of hydrologic connectivity between point sources of pollution and coastal waters (Perez-Fodich et al., 2024). Importantly, this region also provides critical ecosystem functions to the larger reef system because its bays still contain high levels of live coral cover (Asner et al., 2022; Gove et al., 2023). These corals are critical nurseries for spawning coral species and also serve as habitat for fish populations (Carlson et al., 2024). Local regulations and design standards for OSDS in new construction do not account for leaching of permitted septic systems in rock substrate that lacks soil. The cumulative impacts of OSDS at the watershed level are also not taken into account despite extensive evidence linking wastewater to negative outcomes in West Hawai‘i reefs (Aguiar et al., 2023; Gove et al., 2023; Yoshioka et al., 2016). Conversely, we found no evidence of wastewater contamination at multiple bays, such as Māhukona, Ho‘okena, and Honomalino, which suggests these areas are not facing the chronic pressure from land-based wastewater pollution observed elsewhere, and may therefore be promising locations to target for coral restoration activities (Foo and Asner, 2019; Schill et al., 2021).
The spatial patterns of Enterococcus detections indicate there is highly localized control of SGD contamination. Although land cover in upstream watersheds (i.e. coastal development and number of wastewater point sources) was strongly associated with contamination outcomes, site level factors only explained up to half of the variability in our data. Previous studies in the region have found that watershed characteristics were not strong predictors of SGD composition (Knee et al., 2010), and this was attributed to the relatively low levels of urban development and agriculture. Studies from other regions have found high spatial variability in SGD contamination on the scale of meters (Stieglitz et al., 2008), and a significant role of beach geomorphology and coastal drainage geometry (Donahue et al., 2017; Sawyer et al., 2016). We found stronger relationships between land cover and contamination outcomes when variables were calculated over the region closest to the coastline, based on a presumed larger degree of hydrologic connectivity to the coast (Abaya et al., 2018; Okuhata et al., 2022b). This underscores previous research demonstrating that small portions of the landscape can have an outsized effect on pollution into adjacent reefs (Carlson et al., 2019). Highly localized control of groundwater quality is also consistent with resistivity surveys that have highlighted the role of preferential flow through subsurface features, such as 10–15 m diameter lava tubes, that can act as a direct conduit for groundwater and contribute additional spatial heterogeneity in hydrologic flow paths (Dimova et al., 2012; Kreyns et al., 2020).
The most predictive statistical model for contamination outcomes included both water quality and land cover variables, indicating that DOM and salinity levels are informative indicators of wastewater contamination. These parameters, which can potentially be measured remotely from UAVs and aircraft (Harringmeyer et al., 2021) may be used for rapid screening over large areas and complement more labor-intensive laboratory analyses and incubations. Although benthic reef organisms can exude substantial amounts of DOM (Quinlan et al., 2018), terrestrially-derived organic matter typically has optical properties that can readily distinguish it from the surrounding water column (Nelson et al., 2015). Satellite-derived estimates of DOM have been used to track large plumes of wastewater effluent off the coast of southern California (Nezlin et al., 2020); however, more work is required to develop reliable algorithms to estimate DOM abundance in optically shallow reef areas surrounding tropical islands (Aurin and Dierssen, 2012; Hondula et al., 2024) and to investigate the relative importance of dissolved versus particulate matter in transporting pollutants from land (Wiegner et al., 2013).
Although this study provides a synoptic view of contamination along the West Hawai‘i coastline, it is important to note a few caveats and limitations. As in other studies, we use Enterococcus as an indicator of wastewater contamination; however, this bacterium is not exclusive to humans and occurs naturally in tropical soils and survives in sediment and wrack (Boehm et al., 2004; Miller-Pierce and Rhoads, 2019). Microbial source tracking methods using host-specific markers such as cross-assembly phages (crAssphage) could be carried out to confirm that detected bacteria originate from human sources (Sala-Comorera et al., 2021; Vanderzalm et al., 2024). However, multiple studies in Hawai‘i have shown strong positive correlations between Enterococcus and Clostridium perfringens, which is less likely to naturally occur (Gerken et al., 2022; Viau et al., 2011), as well as other pathogens (Steadmon et al., 2024). Enterococcus is also a more conservative indicator of sewage presence compared to isotopic measurements (Aguiar et al., 2023). Another caveat is that we only conducted repeated sampling at a subset of the sites, and for the remainder, our inference is limited to only chronic and persistent contamination issues. Elsewhere, studies have demonstrated seasonal tourism-driven increases in groundwater contamination (Alorda-Kleinglass et al., 2024) as well as significant relationships with rainfall, temperature, tidal activity, and currents (Jennings et al., 2018). Additionally, even if persistent SGD contamination can be excluded as a site-level stressor for reef organisms, resource managers and restoration practitioners also should consider potential exposure to other adverse and acute conditions, such as high sediment loading and marine heat waves, when making management decisions (Foo and Asner, 2021; Schill et al., 2021).
Our findings indicate that SGD delivers sewage-contaminated groundwater to multiple embayments and nearshore regions along the West Hawai‘i coast, and that contaminated sites are associated with both built-up land cover near the shoreline and the abundance of upstream OSDS. This problem is particularly acute in South Kona, where young volcanic substrate rapidly flushes point source contamination into coastal bays that are critical areas for reef-wide coral population dynamics (Carlson et al., 2024). Although there are recent commitments to upgrade a centrally located wastewater treatment plant, this upgrade is only anticipated to improve water quality conditions in the immediate vicinity of the city of Kailua-Kona because only a small fraction of the coastal population is sewered (Wada et al., 2021), and point-source OSDS discharge can have higher bacteria and nutrient loads than that of outfall plumes from wastewater treatment plants (Waiki et al., 2025). Implementing specific types of green infrastructure practices, such as bioretention cells (Hayes et al., 2023; Lancaster et al., 2024), into development practices may also help mitigate negative impacts of untreated runoff and SGD on water quality (Jennings et al., 2018; Reeves et al., 2004). Additionally, although projected climate and land cover changes are anticipated to result in a drier climate and reduction in groundwater recharge and discharge for this region (Okuhata et al., 2023), such changes will not eliminate wastewater pollution to coastal ecosystems because hydrologic connectivity is a bi-directional process; inundation of wastewater infrastructure due to rising sea levels can also transport contaminated groundwater to nearshore ecosystems (McKenzie et al., 2021b).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
KH: Methodology, Visualization, Data curation, Conceptualization, Formal Analysis, Investigation, Writing – review & editing, Writing – original draft. RM: Conceptualization, Writing – review & editing, Methodology, Investigation. GA: Project administration, Methodology, Conceptualization, Investigation, Writing – review & editing, Funding acquisition, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Dorrance Family Foundation grant G-10759-300 to G. Asner.
Acknowledgments
We thank members of the ʻĀkoʻakoʻa cultural advisory board, who were critical to design and execution of field sampling. We thank Dominica Harrison, Taha‘a Kahele, and Caleb Labo for valuable help with field sampling and laboratory analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1634234/full#supplementary-material
References
Abaya L. M., Wiegner T. N., Beets J. P., Colbert S. L., Carlson K. M., and Kramer K. L. (2018). Spatial distribution of sewage pollution on a Hawaiian coral reef. Mar. pollut. Bull. 130, 335–347. doi: 10.1016/j.marpolbul.2018.03.028
Adolf J. E., Burns J., Walker J. K., and Gamiao S. (2019). Near shore distributions of phytoplankton and bacteria in relation to submarine groundwater discharge-fed fishponds, Kona coast, Hawai’i, USA. Estuarine Coast. Shelf Sci. 219, 341–353. doi: 10.1016/j.ecss.2019.01.021
Aguiar D. K., Wiegner T. N., Colbert S. L., Burns J., Abaya L., Beets J., et al. (2023). Detection and impact of sewage pollution on South Kohala’s coral reefs, Hawai’i. Mar. pollut. Bull. 188, 114662. doi: 10.1016/j.marpolbul.2023.114662
Alorda-Kleinglass A., Rodellas V., Diego-Feliu M., Marbà N., Morell C., and Garcia-Orellana J. (2024). The connection between Submarine Groundwater Discharge and seawater quality: The threat of treated wastewater injected into coastal aquifers. Sci. Total Environ. 922, 170940. doi: 10.1016/j.scitotenv.2024.170940
Amato D. W., Bishop J. M., Glenn C. R., Dulai H., and Smith C. M. (2016). Impact of submarine groundwater discharge on marine water quality and reef biota of Maui. PloS One 11, e0165825. doi: 10.1371/journal.pone.0165825
Asner G. P., Vaughn N. R., and Heckler J. (2024). Operational mapping of submarine groundwater discharge into coral reefs: application to West Hawai’i island. Oceans 5, 547–559. doi: 10.3390/oceans5030031
Asner G. P., Vaughn N. R., Martin R. E., Foo S. A., Heckler J., Neilson B. J., et al. (2022). Mapped coral mortality and refugia in an archipelago-scale marine heat wave. Proc. Natl. Acad. Sci. 119 (19), e2123331119. doi: 10.1073/pnas.2123331119
Aurin D. A. and Dierssen H. M. (2012). Advantages and limitations of ocean color remote sensing in CDOM-dominated, mineral-rich coastal and estuarine waters. Remote Sens. Environ. 125, 181–197. doi: 10.1016/j.rse.2012.07.001
Bishop J. M., Glenn C. R., Amato D. W., and Dulai H. (2017). Effect of land use and groundwater flow path on submarine groundwater discharge nutrient flux. J. Hydrol.: Regional Stud. 11, 194–218. doi: 10.1016/j.ejrh.2015.10.008
Boehm A. B., Shellenbarger G. G., and Paytan A. (2004). Groundwater discharge: potential association with fecal indicator bacteria in the surf zone. Environ. Sci. Technol. 38, 3558–3566. doi: 10.1021/es035385a
Brauman K. A., Freyberg D. L., and Daily G. C. (2012). Land cover effects on groundwater recharge in the tropics: Ecohydrologic mechanisms. Ecohydrology 5, 435–444. doi: 10.1002/eco.236
Bürkner P.-C. (2017). brms: An R package for Bayesian multilevel models using Stan. J. Stat. Software 80, 1–28. doi: 10.18637/jss.v080.i01
Carlson R. R., Crowder L. B., Martin R. E., and Asner G. P. (2024). The effect of reef morphology on coral recruitment at multiple spatial scales. Proc. Natl. Acad. Sci. 121 (4), e2311661121. doi: 10.1073/pnas.2311661121
Carlson R. R., Foo S. A., and Asner G. P. (2019). Land use impacts on coral reef health: A ridge-to-reef perspective. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00562
Carpenter B., Gelman A., Hoffman M. D., Lee D., Goodrich B., Betancourt M., et al. (2017). Stan: A probabilistic programming language. J. Stat. Software 76 (1), 1–32. doi: 10.18637/jss.v076.i01
Delevaux J. M. S., Whittier R., Stamoulis K. A., Bremer L. L., Jupiter S., Friedlander A. M., et al. (2018). A linked land-sea modeling framework to inform ridge-to-reef management in high oceanic islands. PloS One 13, e0193230. doi: 10.1371/journal.pone.0193230
Dimova N. T., Swarzenski P. W., Dulaiova H., and Glenn C. R. (2012). Utilizing multichannel electrical resistivity methods to examine the dynamics of the fresh water–seawater interface in two Hawaiian groundwater systems. J. Geophysical Research: Oceans 117, 2011JC007509. doi: 10.1029/2011JC007509
Donahue A., Feng Z., Kelly E., Reniers A., and Solo-Gabriele H. M. (2017). Significance of beach geomorphology on fecal indicator bacteria levels. Mar. pollut. Bull. 121, 160–167. doi: 10.1016/j.marpolbul.2017.05.024
Dormann C. F., Elith J., Bacher S., Buchmann C., Carl G., Carré G., et al. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. doi: 10.1111/j.1600-0587.2012.07348.x
Dudley B. D., Hughes R. F., Asner G. P., Baldwin J. A., Miyazawa Y., Dulai H., et al. (2020). Hydrological effects of tree invasion on a dry coastal Hawaiian ecosystem. For. Ecol. Manage. 458, 117653. doi: 10.1016/j.foreco.2019.117653
Dulai H., Smith C. M., Amato D. W., Gibson V., and Bremer L. L. (2023). Risk to native marine macroalgae from land-use and climate change-related modifications to groundwater discharge in Hawai‘i. Limnol. Oceanogr. Lett. 8, 141–153. doi: 10.1002/lol2.10232
Feng Z., Reniers A., Haus B. K., and Solo-Gabriele H. M. (2013). Modeling sediment-related enterococci loading, transport, and inactivation at an embayed nonpoint source beach. Water Resour. Res. 49, 693–712. doi: 10.1029/2012WR012432
Foo S. A. and Asner G. P. (2019). Scaling up coral reef restoration using remote sensing technology. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00079
Foo S. A. and Asner G. P. (2021). Impacts of remotely sensed environmental drivers on coral outplant survival. Restor. Ecol. 29, e13309. doi: 10.1111/rec.13309
Gerken T., Wiegner T. N., and Economy L. M. (2022). A comparison of soil Staphylococcus aureus and fecal indicator bacteria concentrations across land uses in a Hawaiian watershed. J. Environ. Qual. 51, 916–929. doi: 10.1002/jeq2.20380
Gove J. M., Williams G. J., Lecky J., Brown E., Conklin E., Counsell C., et al. (2023). Coral reefs benefit from reduced land–sea impacts under ocean warming. Nature 621, 536–542. doi: 10.1038/s41586-023-06394-w
Griffin D. W., Lipp E. K., McLAUGHLIN M. R., and Rose J. B. (2001). Marine recreation and public health microbiology: quest for the ideal indicator. BioScience 51, 817. doi: 10.1641/0006-3568(2001)051[0817:MRAPHM]2.0.CO;2
Harringmeyer J. P., Kaiser K., Thompson D. R., Gierach M. M., Cash C. L., and Fichot C. G. (2021). Detection and sourcing of CDOM in urban coastal waters with UV-visible imaging spectroscopy. Front. Environ. Sci. 9. doi: 10.3389/fenvs.2021.647966
Hayes G. M., Burgis C., Zhang W., Henderson D., and Smith J. A. (2023). Evaluation of the export of fecal contamination from roadside green infrastructure. J. Sustain. Water Built Environ. 9, 04022016. doi: 10.1061/JSWBAY.0001002
Homer C., Huang C., Yang L., Wylie B., and Coan M. (2004). Development of a 2001 national land-cover database for the United States. Photogrammetric Eng. Remote Sens. 70, 829–840. doi: 10.14358/PERS.70.7.829
Hondula K. L., König M., Grunert B. K., Vaughn N. R., Martin R. E., Dai J., et al. (2024). Mapping water quality in nearshore reef environments using airborne imaging spectroscopy. Remote Sens. 16, 1845. doi: 10.3390/rs16111845
Jennings W. C., Chern E. C., O’Donohue D., Kellogg M. G., and Boehm A. B. (2018). Frequent detection of a human fecal indicator in the urban ocean: Environmental drivers and covariation with enterococci. Environ. Science: Processes Impacts 20, 480–492. doi: 10.1039/C7EM00594F
Kelly J. L., Glenn C. R., and Lucey P. G. (2013). High-resolution aerial infrared mapping of groundwater discharge to the coastal ocean. Limnol. Oceanogr.: Methods 11, 262–277. doi: 10.4319/lom.2013.11.262
Knee K. L., Layton B. A., Street J. H., Boehm A. B., and Paytan A. (2008). Sources of nutrients and fecal indicator bacteria to nearshore waters on the North shore of Kaua`i (Hawai`i, USA). Estuaries Coasts 31, 607–622. doi: 10.1007/s12237-008-9055-6
Knee K. L., Street J. H., Grossman> E. E., Boehm A. B., and Paytan A. (2010). Nutrient inputs to the coastal ocean from submarine groundwater discharge in a groundwater-dominated system: Relation to land use (Kona coast, Hawaii, U.S.A.). Limnol. Oceanogr. 55, 1105–1122. doi: 10.4319/lo.2010.55.3.1105
Kreyns P., Geng X., and Michael H. A. (2020). The influence of connected heterogeneity on groundwater flow and salinity distributions in coastal volcanic aquifers. J. Hydrol. 586, 124863. doi: 10.1016/j.jhydrol.2020.124863
Lancaster E., Winston R., Martin J., and Lee J. (2024). Urban stormwater green infrastructure: Evaluating the public health service role of bioretention using microbial source tracking and bacterial community analyses. Water Res. 259, 121818. doi: 10.1016/j.watres.2024.121818
La Valle F. F., Jacobs J. M., Thomas F. I., and Nelson C. E. (2023). Nutrient-rich submarine groundwater discharge increases algal carbon uptake in a tropical reef ecosystem. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1178550
La Valle F. F., Kantar M. B., and Nelson C. E. (2021). Coral reef benthic community structure is associated with the spatiotemporal dynamics of submarine groundwater discharge chemistry. Limnol. Oceanogr. 66, 188–200. doi: 10.1002/lno.11596
La Valle F., Thomas F., and Nelson C. (2019). Macroalgal biomass, growth rates, and diversity are influenced by submarine groundwater discharge and local hydrodynamics in tropical reefs. Mar. Ecol. Prog. Ser. 621, 51–67. doi: 10.3354/meps12992
Longman R. J., Lucas M. P., Mclean J., Cleveland S. B., Kodama K., Frazier A. G., et al. (2024). The Hawai ‘i climate data portal (HCDP). Bull. Am. Meteorological Soc. 105, E1074–E1083. doi: 10.1175/BAMS-D-23-0188.1
Lubarsky K. A., Silbiger N. J., and Donahue M. J. (2018). Effects of submarine groundwater discharge on coral accretion and bioerosion on two shallow reef flats. Limnol. Oceanogr. 63, 1660–1676. doi: 10.1002/lno.10799
McKenzie T., Dulai H., and Fuleky P. (2021a). Traditional and novel time-series approaches reveal submarine groundwater discharge dynamics under baseline and extreme event conditions. Sci. Rep. 11, 22570. doi: 10.1038/s41598-021-01920-0
McKenzie T., Habel S., and Dulai H. (2021b). Sea-level rise drives wastewater leakage to coastal waters and storm drains. Limnol. Oceanogr. Lett. 6, 154–163. doi: 10.1002/lol2.10186
Mezzacapo M., Donohue M. J., Smith C., El-Kadi A., Falinski K., and Lerner D. T. (2020). Review article: Hawai’i’s cesspool problem: review and recommendations for water resources and human health. J. Contemp. Water Res. Educ. 170, 35–75. doi: 10.1111/j.1936-704X.2020.03339.x
Miller-Pierce M. R. and Rhoads N. A. (2019). Clostridium perfringens testing improves the reliability of detecting non-point source sewage contamination in Hawaiian coastal waters compared to using Enterococci alone. Mar. pollut. Bull. 144, 36–47. doi: 10.1016/j.marpolbul.2019.04.053
Moore R. B., McKay L. D., Rea A. H., Bondelid T. R., Price C. V., Dewald T. G., et al. (2019). User’s guide for the national hydrography dataset plus (NHDPlus) high resolution: U.S. Geological Survey Open-File Report 2019–1096. Reston, Virginia: U.S. Geological Survey, 66. doi: 10.3133/ofr20191096
Nelson C. E., Donahue M. J., Dulaiova H., Goldberg S. J., La Valle F. F., Lubarsky K., et al. (2015). Fluorescent dissolved organic matter as a multivariate biogeochemical tracer of submarine groundwater discharge in coral reef ecosystems. Mar. Chem. 177, 232–243. doi: 10.1016/j.marchem.2015.06.026
Nezlin N. P., Beegan C., Feit A., Gully J. R., Latker A., McLaughlin K., et al. (2020). Colored Dissolved Organic Matter (CDOM) as a tracer of effluent plumes in the coastal ocean. Regional Stud. Mar. Sci. 35, 101163. doi: 10.1016/j.rsma.2020.101163
Okuhata B. K., Delevaux J. M. S., Richards Donà A., Smith C. M., Gibson V. L., Dulai H., et al. (2023). Effects of multiple drivers of environmental change on native and invasive macroalgae in nearshore groundwater dependent ecosystems. Water Resour. Res. 59, e2023WR034593. doi: 10.1029/2023WR034593
Okuhata B. K., El-Kadi A. I., Dulai H., Lee J., Wada C. A., Bremer L. L., et al. (2022a). A density-dependent multi-species model to assess groundwater flow and nutrient transport in the coastal Keauhou aquifer, Hawai’i, USA. Hydrogeol. J. 30, 231–250. doi: 10.1007/s10040-021-02407-y
Okuhata B. K., Thomas D. M., Dulai H., Popp B. N., Lee J., and El-Kadi A. I. (2022b). Inference of young groundwater ages and modern groundwater proportions using chlorofluorocarbon and tritium/helium-3 tracers from West Hawai’i Island. J. Hydrol. 609, 127755. doi: 10.1016/j.jhydrol.2022.127755
Panelo J., Wiegner T. N., Colbert S. L., Goldberg S., Abaya L. M., Conklin E., et al. (2022). Spatial distribution and sources of nutrients at two coastal developments in South Kohala, Hawai’i. Mar. pollut. Bull. 174, 113143. doi: 10.1016/j.marpolbul.2021.113143
Paytan A., Shellenbarger G. G., Street J. H., Gonneea M. E., Davis K., Young M. B., et al. (2006). Submarine groundwater discharge: An important source of new inorganic nitrogen to coral reef ecosystems. Limnol. Oceanogr. 51, 343–348. doi: 10.4319/lo.2006.51.1.0343
Perez-Fodich A., Derry L. A., Marçais J., and Walter M. T. (2024). The effect of weathering in runoff-to-groundwater partitioning in the Island of Hawai’i: Perspectives for landscape evolution. Earth Planetary Sci. Lett. 635, 118687. doi: 10.1016/j.epsl.2024.118687
Prouty N. G., Cohen A., Yates K. K., Storlazzi C. D., Swarzenski P. W., and White D. (2017). Vulnerability of coral reefs to bioerosion from land-based sources of pollution. J. Geophysical Research: Oceans 122, 9319–9331. doi: 10.1002/2017JC013264
Quinlan Z. A., Remple K., Fox M. D., Silbiger N. J., Oliver T. A., Putnam H. M., et al. (2018). Fluorescent organic exudates of corals and algae in tropical reefs are compositionally distinct and increase with nutrient enrichment. Limnol. Oceanogr. Lett. 3, 331–340. doi: 10.1002/lol2.10074
Reeves R. L., Grant S. B., Mrse R. D., Copil Oancea C. M., Sanders B. F., and Boehm A. B. (2004). Scaling and management of fecal indicator bacteria in runoff from a coastal urban watershed in Southern California. Environ. Sci. Technol. 38, 2637–2648. doi: 10.1021/es034797g
Richards Donà A., Smith C. M., and Bremer L. L. (2023). Divergent responses of native and invasive macroalgae to submarine groundwater discharge. Sci. Rep. 13, 13984. doi: 10.1038/s41598-023-40854-7
Richardson C. M., Dulai H., Popp B. N., Ruttenberg K., and Fackrell J. K. (2017). Submarine groundwater discharge drives biogeochemistry in two Hawaiian reefs. Limnol. Oceanogr. 62 (S1), S348–S363. doi: 10.1002/lno.10654
Saingam P., Li B., Sung S., and Yan T. (2021). Immediate impact of hurricane lane on microbiological quality of coastal water in Hilo bay, Hawaii. Environ. Sci. Technol. 55, 2960–2967. doi: 10.1021/acs.est.0c07082
Sala-Comorera L., Reynolds L. J., Martin N. A., Pascual-Benito M., Stephens J. H., Nolan T. M., et al. (2021). crAssphage as a human molecular marker to evaluate temporal and spatial variability in faecal contamination of urban marine bathing waters. Sci. Total Environ. 789, 147828. doi: 10.1016/j.scitotenv.2021.147828
Santos I. R., Chen X., Lecher A. L., Sawyer A. H., Moosdorf N., Rodellas V., et al. (2021). Submarine groundwater discharge impacts on coastal nutrient biogeochemistry. Nat. Rev. Earth Environ. 2, 307–323. doi: 10.1038/s43017-021-00152-0
Sawyer A. H., David C. H., and Famiglietti J. S. (2016). Continental patterns of submarine groundwater discharge reveal coastal vulnerabilities. Science 353, 705–707. doi: 10.1126/science.aag1058
Schill S. R., Asner G. P., McNulty V. P., Pollock F. J., Croquer A., Vaughn N. R., et al. (2021). Site selection for coral reef restoration using airborne imaging spectroscopy. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.698004
Sercu B., Van De Werfhorst L. C., Murray J. L., and Holden P. A. (2011). Cultivation-independent analysis of bacteria in IDEXX Quanti-Tray/2000 fecal indicator assays. Appl. Environ. Microbiol. 77, 627–633. doi: 10.1128/AEM.01113-10
Silbiger N. J., Donahue M. J., and Lubarsky K. (2020). Submarine groundwater discharge alters coral reef ecosystem metabolism. Proc. R. Soc. B: Biol. Sci. 287, 20202743. doi: 10.1098/rspb.2020.2743
State of Hawaii (2005). Watersheds (DAR Version). Available online at: https://planning.hawaii.gov/gis/download-gis-data-expanded/ (Accessed April 1, 2025).
Steadmon M., Takakusagi M., Wiegner T. N., Jones M., Economy L. M., Panelo J., et al. (2024). Detection and modeling of Staphylococcus aureusand fecal bacteria in Hawaiian coastal waters and sands. Water Environ. Res. 96, e11037. doi: 10.1002/wer.11037
Stieglitz T., Taniguchi M., and Neylon S. (2008). Spatial variability of submarine groundwater discharge, Ubatuba, Brazil. Estuarine Coast. Shelf Sci. 76, 493–500. doi: 10.1016/j.ecss.2007.07.038
Strauch A. M., Mackenzie R. A., Bruland G. L., Tingley R., and Giardina C. P. (2014). Climate change and land use drivers of fecal bacteria in tropical Hawaiian rivers. J. Environ. Qual. 43, 1475–1483. doi: 10.2134/jeq2014.01.0025
Tuholske C., Halpern B. S., Blasco G., Villasenor J. C., Frazier M., and Caylor K. (2021). Mapping global inputs and impacts from of human sewage in coastal ecosystems. PloS One 16, e0258898. doi: 10.1371/journal.pone.0258898
Vaccaro R. F., Briggs M. P., Carey C. L., and Ketchum B. H. (1950). Viability of escherichia coli in sea water. Am. J. Public Health Nations Health 40, 1257–1266. doi: 10.2105/AJPH.40.10.1257
Vanderzalm J., Currie S., Smith W., Metcalfe S., Taylor N., and Ahmed W. (2024). Microbial source tracking of fecal pollution to coral reef lagoons of Norfolk Island, Australia. Sci. Total Environ. 912, 168906. doi: 10.1016/j.scitotenv.2023.168906
Vehtari A., Gelman A., and Gabry J. (2017). Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Computing 27, 1413–1432. doi: 10.1007/s11222-016-9696-4
Viau E. J., Goodwin K. D., Yamahara K. M., Layton B. A., Sassoubre L. M., Burns S. L., et al. (2011). Bacterial pathogens in Hawaiian coastal streams—Associations with fecal indicators, land cover, and water quality. Water Res. 45, 3279–3290. doi: 10.1016/j.watres.2011.03.033
Wada C. A., Burnett K. M., Okuhata B. K., Delevaux J. M. S., Dulai H., El-Kadi A. I., et al. (2021). Identifying wastewater management tradeoffs: Costs, nearshore water quality, and implications for marine coastal ecosystems in Kona, Hawai’i. PloS One 16, e0257125. doi: 10.1371/journal.pone.0257125
Waiki S. M. P., Colbert S. L., Wiegner T. N., Puniwai N., Nakoa III, J. W. P., Storie N. M., et al. (2025). Sewage pollution from onsite sewage disposal systems and an offshore wastewater treatment plant outfall in coastal waters of Keaukaha, Hawai‘i Island. J. Hydrol.: Regional Stud. 57, 102122. doi: 10.1016/j.ejrh.2024.102122
Wear S. L. and Thurber R. V. (2015). Sewage pollution: Mitigation is key for coral reef stewardship. Ann. New York Acad. Sci. 1355, 15–30. doi: 10.1111/nyas.12785
Whittier R. and El-Kadi A. (2014). Human and environmental risk ranking of onsite sewage disposal systems for the Hawaiian Islands of Kauai, Molokai, Maui, and Hawaii - Final. Prepared by the University of Hawaii, Dept. of Geology and Geophysics for the State of Hawai`i Department of Health, Safe Drinking Water Branch. September 2014. Available online at: https://health.hawaii.gov/wastewater/files/2015/09/OSDS_NI.pdf (Accessed April 1, 2025).
Wiegner T. N., Colbert S. L., Abaya L. M., Panelo J., Remple K., and Nelson C. E. (2021). Identifying locations of sewage pollution within a Hawaiian watershed for coastal water quality management actions. J. Hydrol.: Regional Stud. 38, 100947. doi: 10.1016/j.ejrh.2021.100947
Wiegner T. N., Mead L. H., and Molloy S. L. (2013). A comparison of water quality between low- and high-flow river conditions in a tropical estuary, Hilo bay, Hawaii. Estuaries Coasts 36, 319–333. doi: 10.1007/s12237-012-9576-x
Yao Y., Vehtari A., Simpson D., and Gelman A. (2018). Using stacking to average bayesian predictive distributions (with discussion). Bayesian Anal. 13 (3), 917–1007. doi: 10.1214/17-BA1091
Yoshioka R. M., Kim C. J. S., Tracy A. M., Most R., and Harvell C. D. (2016). Linking sewage pollution and water quality to spatial patterns of Porites lobata growth anomalies in Puako, Hawaii. Mar. pollut. Bull. 104, 313–321. doi: 10.1016/j.marpolbul.2016.01.002
Zanaga D., Van De Kerchove R., Daems D., De Keersmaecker W., Brockmann C., Kirches G., et al. (2022). ESA WorldCover 10 m 2021 v200. doi: 10.5281/zenodo.7254221
Keywords: fecal indicator bacteria, submarine groundwater discharge, sewage, Hawai‘i, coastal development, coral reef
Citation: Hondula KL, Martin RE and Asner GP (2025) Variability in contamination of submarine groundwater discharge into West Hawai‘i coral reefs. Front. Mar. Sci. 12:1634234. doi: 10.3389/fmars.2025.1634234
Received: 23 May 2025; Accepted: 31 July 2025;
Published: 26 August 2025.
Edited by:
Christian Joshua Sanders, Southern Cross University, AustraliaReviewed by:
Michael G. LaMontagne, University of Houston–Clear Lake, United StatesShênia Patricia Corrêa Novo, Oswaldo Cruz Foundation (Fiocruz), Brazil
Copyright © 2025 Hondula, Martin and Asner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelly L. Hondula, a2hvbmR1bGFAYXN1LmVkdQ==
 Kelly L. Hondula
Kelly L. Hondula Roberta E. Martin
Roberta E. Martin Gregory P. Asner
Gregory P. Asner