Abstract
The coastal zone of the Gulf of Guinea holds significant natural potential for the production of the mangrove oyster Crassostrea tulipa. This study assessed the larval dispersal of C. tulipa in the Benin’s coastal lagoon. Oyster larvae were sampled monthly from January to December 2023 in three traditional oyster production areas (Ahouandji, Dégouè, and Djondji) using a 50 µm mesh plankton net. The larvae samples collected, were analyzed under a light microscope, and the different larval stages (D-, veliger, and pediveliger larvea) of C. tulipa were identified and counted. The results show a major larvae abundance period extending from November to April, with maximum density occurring in March and/or April, depending on the site. A secondary phase of larvae abundance production was observed from May to July, although it was characterized by a very low presence of advanced larval stages (pediveliger). A dominance of D- and veliger larvae was noted in the Djondji area, whereas pediveliger larvae were more prevalent in the Ahouandji production zone. The highest cumulative number of larvae (56,220.0) was recorded in the Djondji area, compared to 35,586.7 in Dégouè and 36,160.0 in Ahouandji. In terms of larval survival, the lowest rate (0.1%) was observed in Djondji, compared to 0.2% in Ahouandji and 0.4% in Dégouè. Therefore, the high larval abundance observed in the Djondji production area does not necessarily reflect favorable survival conditions for larvae. The production areas of Ahouandji and Dégouè may offer more favorable environments for larval development. These results provide valuable data that can be used to improve oyster farming and management in Benin’s coastal waters.
1 Introduction
Larval dispersal and availability, as well as recruitment, are fundamental processes in the dynamics of marine populations, particularly in bivalve molluscs such as the oysters (Theuerkauf et al., 2021). Ecologically adapted to dynamic coastal environments, these species play a key role in the functioning of these ecosystems, contributing significantly to global fisheries production (Toupoint et al., 2016; Androuin et al., 2021). Oysters are benthic macroinvertebrates belonging to the class of Bivalvia and exhibit a bentho-pelagic life cycle characterized by a relatively long larval phase (Thorson, 1950; Doinsing and Ransangan, 2022). Like annelids, polychaetes and crustaceans, bivalve molluscs constitute an important part of benthic macrofauna due to their diversity, abundance and the essential ecological functions, including bioturbation and their role as intermediate trophic links (Dimitriadis et al., 2014; Barbier, 2016; Politi et al., 2019).
The dynamic of the population of these organisms, particularly that of oysters, depends largely on the success of larval dispersal and the transition from the pelagic to the benthic phase (Olafsson et al., 1994; Pedersen et al., 2008; Pineda et al., 2010; Androuin et al., 2021). This process ensures population renewal (Cowen and Sponaugle, 2009) and contributes to spatial structuring by enabling colonization of diverse habitats (Adams et al., 2014). The connectivity established through larval dispersal between different habitats is crucial for the survival of metapopulations, as it maintains genetic diversity and supports low-performing patches that cannot produce sufficient larvae (Powers et al., 2023). Green et al. (2015) demonstrated that this demographic connectivity across habitats is also a critical parameter in the design of marine reserves due to its implications for the resilience and persistence of populations following disturbance.
The dispersal of larvae is influenced by various abiotic factors, such as temperature, salinity, hydrodynamics and substrate type and availability, biotic factors including competition and predation, as well as intrinsic variables related to the larvae themselves (Androuin et al., 2021). Manuel et al. (2023) demonstrated that salinity and larval age influence the swimming behavior of Crassostrea gigas, while Marcelino et al. (2023) revealed the combined effect of temperature and salinity on the survival of Saccostrea cucullata larvae. Hydrodynamic conditions (Tedford-Callahan et al., 2025) and substrate quality also play a significant role in the success of metamorphosis (Chuku et al., 2020). The viability, abundance and cohort structure of the larvae influence their capacity to disperse, settle and metamorphose on favorable substrates (Androuin et al., 2021).
Oysters (Crassostrea tulipa) have significant socio-economic value for local populations in the Gulf of Guinea region. Chuku et al. (2022) estimated that shellfish fisheries, which are largely dominated by mangrove oyster harvesting, generate over 300 million USD in annual revenue in Gulf of Guinea countries, with more than 570,000 households directly dependent on this fishery. However, despite the socio-economic importance of oyster fisheries for local populations in the Gulf of Guinea region and the favorable ecological potential of the area, oyster farming remains predominantly traditional and relies primarily on hand-harvesting. Due to the lack of a sub-regional management policy for oyster fishing, oysters are subject to intensive and unregulated harvesting in estuaries and lagoons (Adite et al., 2013). As a result, the natural stock is exposed to the risks of overexploitation. Additionally, pollution and climate change also impact oyster populations (Mahu et al., 2022). It is therefore necessary to sustain the natural oyster stocks through management measures, primarily by promoting C. tulipa farming in the Gulf of Guinea region. In recent years, research efforts have been made to improve understanding of this species ecology, focusing on substrate selection for spat collection, demographic aspects and growth experiments (Adite et al., 2013; Chuku et al., 2020; Chuku and Osei, 2020; Chuku et al., 2022; Mahu et al., 2022; Chuku et al., 2023; Bunting et al., 2024). Unfortunately, data on the reproduction of C. tulipa in its natural habitat remains poorly documented.
Assessing the spatial and temporal variability of oyster reproduction in the natural environment requires data on spat collection, spawning, and larval dispersal in the environment (Bernard, 2011). Spat recruitment tends to be high in areas where larvae are abundant (Christo et al., 2021). Data on larval distribution are therefore crucial to manage efficiently the spat collection and the oyster farming (Pineda et al., 2010; Ubertini et al., 2017; Oliveira et al., 2024). The present study provides the first data on the availability of C. tulipa larvae in the coastal waters of the Gulf of Guinea. It aimed to assess the spawning dynamics and larval distribution of C. tulipa in the coastal lagoon of Benin with regard to environmental conditions.
2 Materials and methods
2.1 Study area
This study was conducted in the coastal lagoon of Benin. The lagoon is located between meridians 1°48′ and 2°16′ East, and parallels 6°16′ and 6°20′ North and covers an area of 55 km². It stretches almost parallel to the Atlantic Ocean for approximately 60 km, from Grand-Popo to Togbin. The Grand-Popo coastal lagoon receives water from the Mono River and Lake Ahémé, and discharges into the sea through the “BOUCHE DU ROY” outlet near the village of Avlo-Plage, and more recently through Djondji mouth (Viaho, 2014).
The region has a subequatorial climate, with two rainy seasons (April to July; mid-September to October) and two dry seasons (December to March; mid-August to mid-September). Annual average rainfall is approximately 1385.3 mm (Oyede, 2023), and ambient temperatures range from 25 °C to 27.7 °C. The vegetation is highly diverse but dominated by mangrove species such as Rhizophora racemosa and Avicennia africana. These mangrove ecosystems serve as spawning and refuge areas for many aquatic species and are therefore characterized by high biodiversity (Sinsin et al., 2018). With their dense and submerged root networks, they provide extensive surfaces for the settlement of C. tulipa oyster. Fishing is the primary livelihood for communities along the lagoon. These activities include mainly oyster harvesting and traditional oyster farming, primarily carried out by women.
2.2 Sampling design
As part of this study, the coastal lagoon was divided into three sectors, each representing a major oyster (C. tulipa) production area. This subdivision was based on traditional oyster production areas in the region, specifically the villages of Ahouandji, Dégouè, and Djondji. In each production area, three sampling stations were established (Figure 1), taking into account parameters such as biotope characteristics, presence or absence of mangroves, and land use along the lagoon banks.
Figure 1
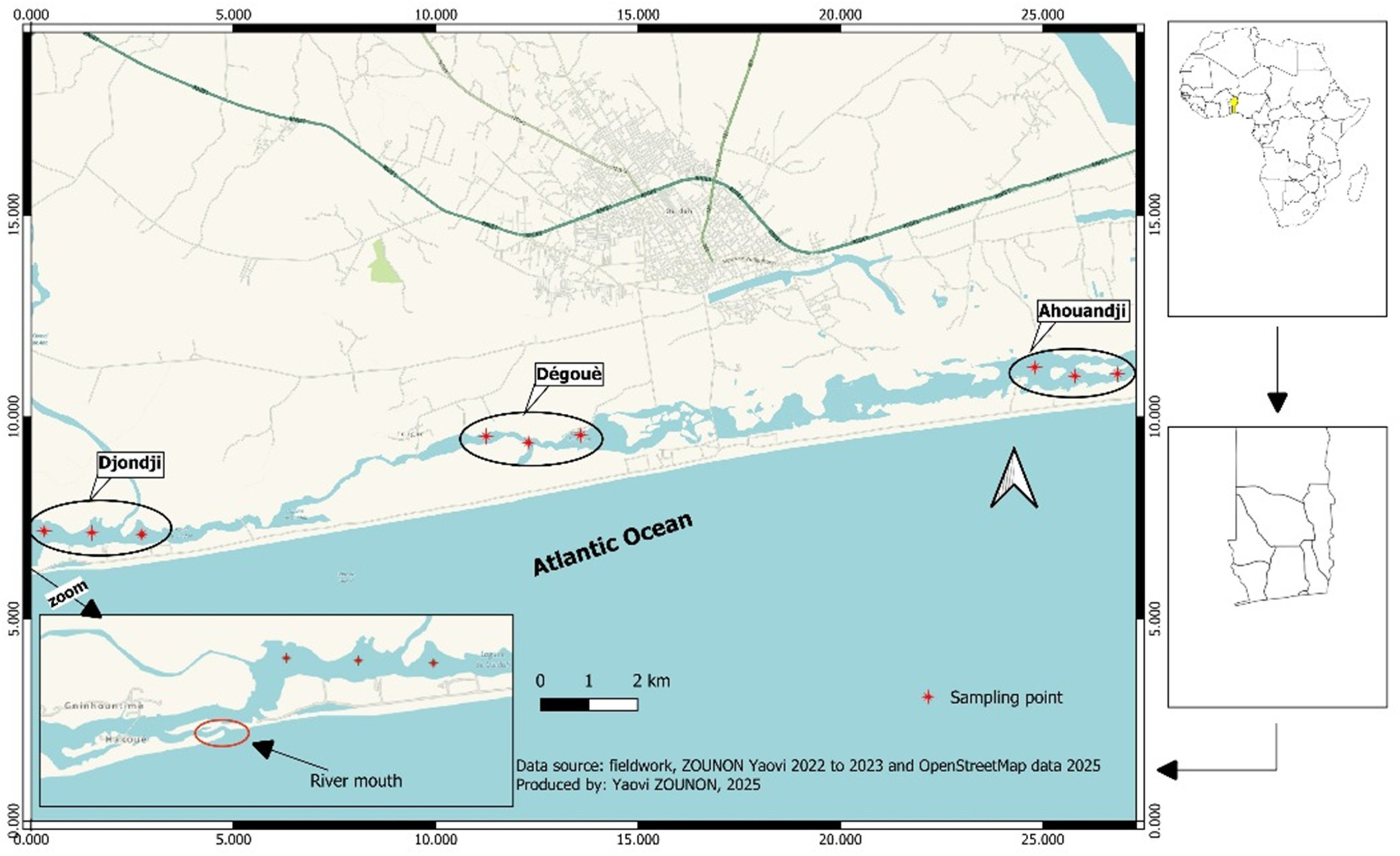
Map of the sampling stations.
Larval sampling was carried out monthly over a one-year period from January to December 2023. At each of the three sampling stations in each production area, 0.2 cubic meters (m³) of lagoon water were collected in a bucket and filtered through a 50 µm mesh plankton net with a collector at the end. After filtration, the plankton concentrate was reduced to a final volume of 0.00019 m³ and fully preserved in 4% formalin for subsequent analysis. Thus, three samples were collected per production area each month throughout the study period.
2.3 Identification and enumeration of C. tulipa larvae
In the laboratory, six “1 × 10-6 m3” samples were taken for independent observations under the microscope (KERN ODC Version 1.0 01/201). Before each was taken, the sample was mixed by hand to ensure its contents were homogeneous. Larvae and their developmental stages were identified and counted with the aid of the Sedgewick-Rafter cell Counts were recorded separately for each larval stage was counted in a total volume of 6 × 10-6 m3 (Vp), which was taken from the 0.00019 m³ (Vr) of water that was recovered and preserved in the field after filtering, 0.2 m³ (Vf).
The larval density (Ds) was calculated using the following formula:
n = Total number of larvae counted in the analyzed aliquots.
Vp = Total volume of the analyzed aliquots (m³).
Vr = Volume of the sample recovered after filtration (m³).
Vf = Total volume of water filtered (m³).
2.4 Descriptors of larval dynamics
To describe larval dynamics throughout the year 2023, five Descriptors were computed to characterize the larval cohort across the study sites. These Descriptors cover three main categories: abundance, survival and persistence. The Descriptors are summarized in Table 1.
Table 1
| Descriptor number | Definition | Equation | Unit | Remark |
|---|---|---|---|---|
| Descriptor 1 | Mean larval density by stage and production area | D̄s = (1/n) Σ Ds,i | ind.m-³ | Allows comparison of mean abundance levels between sites and larval stages |
| Descriptor 2 | Median larval density by stage and production area | Median(Ds) | ind.m-³ | Less sensitive to extreme values, summarizes distribution |
| Descriptor 3 | Total number of larvae counted | Ns = Σ (Ds,i *Ve) | ind | Reflects the relative contribution of each stage over the study period |
| Descriptor 4 | Survival index | SI = (Σ Pedi-veliger/Σ D-larvae) × 100 | % | A proxy of larval survival, to be interpreted with caution (not absolute survival) |
| Descriptor 5 | Duration of pediveliger presence | Duration = (nobs/ntotal) × 100 | % | Percentage of sampling campaigns in which pediveliger larvae were detected |
Summary of descriptors of larval dynamics used to characterize Crassostrea tulipa larval assemblages.
Ds,i: density of larval stages (D-larvae, umbo-veliger, or pediveliger) measured at sampling event i (ind.m-³).
D̄s: mean density of larval stages across all sampling dates.
Ns: Total number of larvae counted of stages across all sampling dates.
Ve: Volume of water filtered at each date and at each station.
SI (Survival Index): ratio of cumulative pediveliger density to cumulative D-larvae density.
Duration of pedi-veliger larvae: percentage of sampling campaigns in which pediveliger larvae were detected, with nobs = number of campaigns with pediveliger occurrence, and ntotal = total number of sampling campaigns.
2.4.1 Abundance
Four abundance-related Descriptors were calculated for each larval stage: the mean, median, maximum, and the total number of larvae (the product of density and filtered volume at each date and station), all expressed in individuals per cubic meter (ind.m⁻³), except for the last one (the total number of larvae), which is expressed as ind. These values provide an overview of larval presence and density throughout the sampling period.
2.4.2 Survival and persistence
A survival rate was computed as a proxy for larval survival, based on the ratio of cumulative densities of pediveliger larvae to D-larvae, following Bernard (2011). This ratio is expressed as a percentage (%). Additionally, the presence duration of pediveliger larvae was calculated as the proportion of sampling dates where they were detected, relative to the total number of sampling events. This provides an indication of larval persistence in the water column.1.5 Measurement of physico-chemical parameters of the coastal lagoon waters.
Environmental factors strongly influence the reproduction and development of oysters of (Hawkins et al. 2013; Paixao et al. 2013; Mahu et al., 2022). In this study, salinity (ppt), temperature (°C), pH, dissolved oxygen (mg/L), and water transparency (cm) were measured in situ at sampling stations during each field campaign. For each sampling event, these parameters were measured once at each station using a multi-parameter probe (Aquaread AP-700 & AP-800), and transparency was assessed with a Secchi disk.
2.5 Data analysis
Larval density data are presented as mean ± standard deviation. Non-parametric Kruskal-Wallis tests were used to compare mean larval density for each stage (Ds) between collection sectors and months, as the assumptions of homoscedasticity and normality were not met. Environmental variables are represented as boxplots. Multiple Factor Analysis (MFA) was performed to jointly analyze environmental variables (temperature, salinity, transparency) and a reduced set of larval indices (mean density, maximum density, survival index, duration of presence). This approach allowed us to visualize relationships between larval dynamics and environmental conditions across sampling dates and sites Quantitative variables are represented using a correlation circle. Individuals are displayed in plots with ellipses corresponding to the monitored months or sectors. R version 4.3.0 was used for all the analyses.
3 Results
3.1 Larval dynamics of the coastal lagoon waters
The evolution of the average density of each larval stage and the total density s is presented in Tables 2–4. The total density for each sampling site is summarized Figure 2.
Table 2
| Density(ind/m3) | Month | Larvae stage | Production area |
|---|---|---|---|
| 1323.3 c | Jan | D-larvae | AH |
| 1163.3 ab | Jan | veliger | AH |
| 0.0 c | Jan | pedi-veliger | AH |
| 1636.7 bc | Feb | D-larvae | AH |
| 1106.7 abc | Feb | veliger | AH |
| 106.7 bc | Feb | pedi-veliger | AH |
| 8076.7 a | March | D-larvae | AH |
| 4380.0 a | March | veliger | AH |
| 7126.7 a | March | pedi-veliger | AH |
| 2693.3 ab | April | D-larvae | AH |
| 846.7 abcd | April | veliger | AH |
| 213.3 ab | April | pedi-veliger | AH |
| 1216.7 c | May | D-larvae | AH |
| 373.3 bcde | May | veliger | AH |
| 53.3 bc | May | pedi-veliger | AH |
| 530.0 d | June | D-larvae | AH |
| 266.7 def | June | veliger | AH |
| 53.3 bc | June | pedi-veliger | AH |
| 1320.0 c | July | D-larvae | AH |
| 160.0 ef | July | veliger | AH |
| 160.0 abc | July | pedi-veliger | AH |
| 2056.7 ab | Aug | D-larvae | AH |
| 950.0 abcde | Aug | velyger | AH |
| 160.0 ab | Aug | pedi-veliger | AH |
| 583.3 d | Sept | D-larvae | AH |
| 213.3 def | Sept | veliger | AH |
| 0.0 c | Sept | pedi-veliger | AH |
| 476.7 d | Oct | D-larvae | AH |
| 320.0 cdef | Oct | veliger | AH |
| 0.0 c | Oct | pedi-veliger | AH |
| 423.3 d | Nov | D-larvae | AH |
| 213.3 def | Nov | veliger | AH |
| 370.0 abc | Nov | pedi-veliger | AH |
| 633.3 d | Dec | D-larvae | AH |
| 0.0 f | Dec | veliger | AH |
| 0.0 c | Dec | pedi-veliger | AH |
Evolution of larval densities by larval stage over time at Ahouandji.
The letter groupings (a, ab, cd…) indicate significance groups derived from the Kruskal-Wallis tests. Groups assigned different letters are significantly different.
Table 3
| Density (ind/m3) | Month | Larvae stage | Production area |
|---|---|---|---|
| 3746.7 abc | Jan | D-larvae | DG |
| 2110.0 a | Jan | veliger | DG |
| 0.0 c | Jan | pedi-veliger | DG |
| 8286.7 a | Feb | D-larvae | DG |
| 1270 ab | Feb | veliger | DG |
| 53.3 bc | Feb | pedi-veliger | DG |
| 3956.7 ab | March | D-larvae | DG |
| 1900.0 a | March | veliger | DG |
| 2480.0 a | March | pedi-veliger | DG |
| 2270.0 bcd | April | D-larvae | DG |
| 1000.0 ab | April | veliger | DG |
| 423.3 ab | April | pedi-veliger | DG |
| 1266.7 de | May | D-larvae | DG |
| 686.67 bc | May | veliger | DG |
| 0.0 c | May | pedi-veliger | DG |
| 686.7 ef | June | D-larvae | DG |
| 320.0 cd | June | veliger | DG |
| 106.7 abc | June | pedi-veliger | DG |
| 2113.3 cd | July | D-larvae | DG |
| 1316.7 ab | July | veliger | DG |
| 263.3 abc | July | pedi-veliger | DG |
| 1106.7 de | Aug | D-larvae | DG |
| 106.7 cd | Aug | veliger | DG |
| 106.7 bc | Aug | pedi-veliger | DG |
| 213.3 f | Sept | D-larvae | DG |
| 106.7 d | Sept | veliger | DG |
| 106.7 bc | Sept | pedi-veliger | DG |
| 266.7 f | Oct | D-larvae | DG |
| 160.0 d | Oct | veliger | DG |
| 0.0 c | Oct | pedi-veliger | DG |
| 316.7 f | Nov | D-larvae | DG |
| 266.7 cd | Nov | veliger | DG |
| 106.7 abc | Nov | pedi-veliger | DG |
| 2426.7 bcd | Dec | D-larvae | DG |
| 2426.7 bcd | Dec | D-larvae | DG |
| 106.7 abc | Dec | pedi-veliger | DG |
Evolution of larval densities by larval stage over time at Dégouè.
The letter groupings (a, ab, cd…) indicate significance groups derived from the Kruskal-Wallis tests. Groups assigned different letters are significantly different.
Table 4
| Density (ind/m3) | Month | Larvae stage | Production area |
|---|---|---|---|
| 7336.7 a | Jan | D-larvae | DJ |
| 2640.0 ab | Jan | veliger | DJ |
| 106.7 c | Jan | pedi-veliger | DJ |
| 8286.7 a | Feb | D-larvae | DJ |
| 1846.7 abc | Feb | veliger | DJ |
| 0.0 c | Feb | pedi-veliger | DJ |
| 6333.3 ab | March | D-larvae | DJ |
| 1900.0 abc | March | veliger | DJ |
| 2060.0 ab | March | pedi-veliger | DJ |
| 9920.0 a | April | D-larvae | DJ |
| 4643.3 a | April | veliger | DJ |
| 2850.0 a | April | pedi-veliger | DJ |
| 1263.3 c | May | D-larvae | DJ |
| 1056.7 bc | May | veliger | DJ |
| 106.7 bc | May | pedi-veliger | DJ |
| 106.7 d | June | D-larvae | DJ |
| 0.0 d | June | veliger | DJ |
| 0.0 c | June | pedi-veliger | DJ |
| 1266.7 c | July | D-larvae | DJ |
| 106.7 d | July | veliger | DJ |
| 0.0 c | July | pedi-veliger | DJ |
| 160.0 d | Aug | D-larvae | DJ |
| 106.7 d | Aug | veliger | DJ |
| 0.0 c | Aug | pedi-veliger | DJ |
| 160.0 d | Sept | D-larvae | DJ |
| 106.7 d | Sept | veliger | DJ |
| 106.7 c | Sept | pedi-veliger | DJ |
| 316.7 d | Oct | D-larvae | DJ |
| 53.3 d | Oct | veliger | DJ |
| 0.0 c | Oct | pedi-veliger | DJ |
| 213.3 d | Nov | D-larvae | DJ |
| 160.0 d | Nov | veliger | DJ |
| 0.0 c | Nov | pedi-veliger | DJ |
| 2113.3 bc | Dec | D-larvae | DJ |
| 1056.7 c | Dec | veliger | DJ |
| 106.7 c | Dec | pedi-veliger | DJ |
Evolution of larval densities by larval stage over time at Djondji.
The letter groupings (a, ab, cd…) indicate significance groups derived from the Kruskal-Wallis tests. Groups assigned different letters are significantly different.
Figure 2
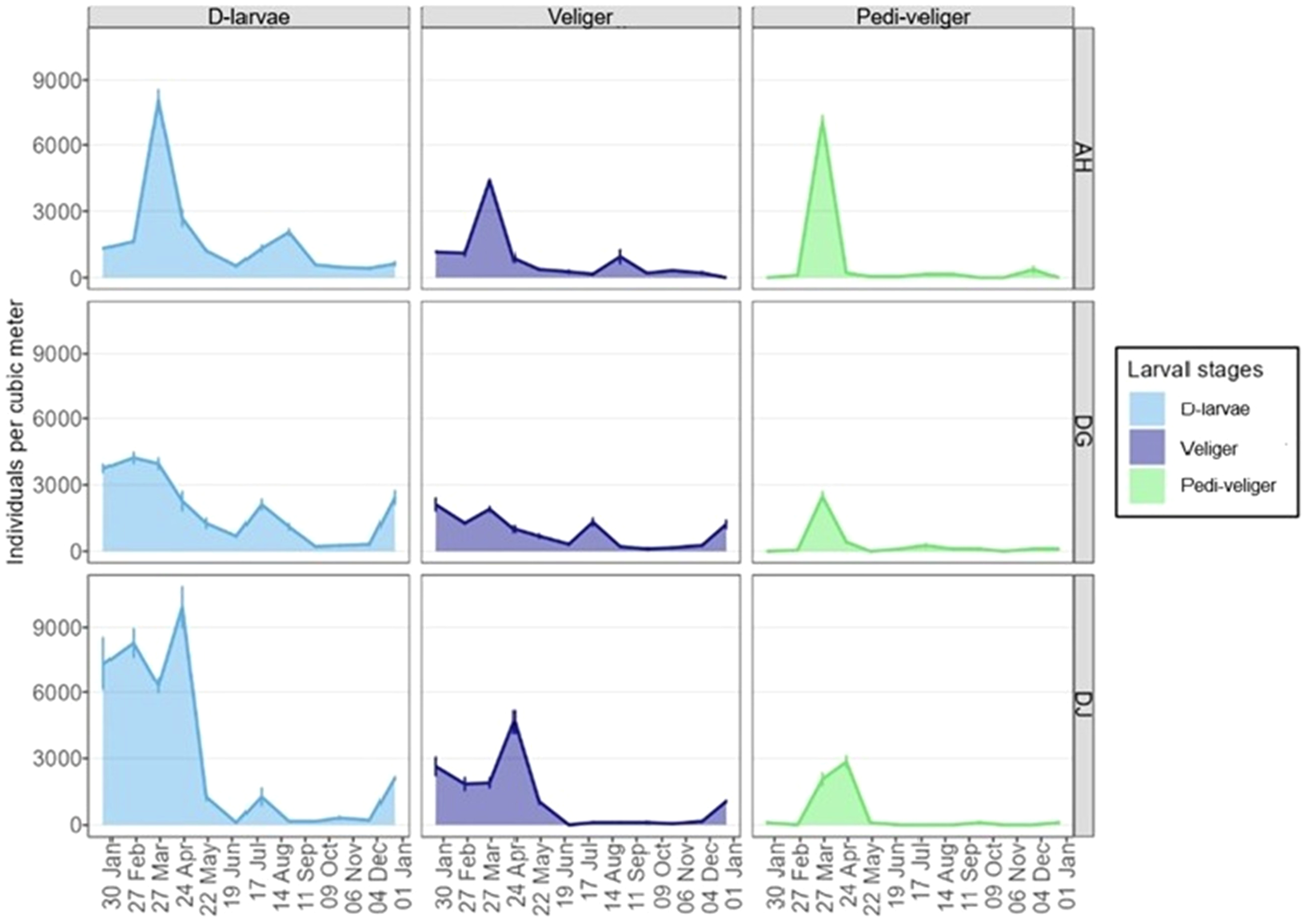
Larval dynamics by stage, over time and by oyster production areas (ind. m⁻³).
3.1.1 Case of the Ahouandji production area
Larval densities varied significantly from month to month for each larval stage at Ahouandji. D- larvae show an overall trend of two periods of abundance throughout the year. A large period of abundance covers the period from January to May, with peaks in March and April, followed by a smaller period of abundance from May to July, with a drop in density in June. The other months of the year are characterized by low abundance of D- larvae in the coastal lagoon waters at Ahouandji. November has the lowest larval density (423.3 ind/m³). Veliger larvae also showed a similar trend, with a large period of abundance from January to May, peaking only in March. From June to August, the density of veliger larvae in the lagoon waters at Ahouandji was less consistent and lower than in the beginning of the year, with a peak in August. The month with the lowest density was December, in which no veliger larvae were observed (0.0 ind/m³).
As for pedi-veliger larvae, they showed a single large period of abundance early in the year, in March and April, with a peak in March. For the rest of the year, their abundance in the lagoon waters at Ahouandji was relatively low. Pedi-veliger larvae were not observed in the coastal lagoon waters at Ahouandji in December, September, October, or January (0.0 ind/m³ for each of these months).
3.1.2 Case of the Dégouè production area
As at Ahouandji, densities vary significantly from month to month for each larval stage at Dégouè. An abundance of D-larvae was observed at Dégouè early in the year, covering the period from December to April, with peaks in February and March (8286.7 ind/m³ and 3956.7 ind/m³, respectively). Larval density then decreased in May and June, before another, but smaller, peak of abundance in July (2113.3 ind/m³). The larval density drastically dropped in the coastal lagoon waters from September to November.
A similar trend was observed for veliger larvae in this production area, which saw an increase in density starting in December, with abundance peaks in January (2110.0 ind/m³) and March (1900.0 ind/m³). Their density then decreased from April onwards, before rising again with a peak in July (1316.7 ind/m³), although still lower in amplitude than those observed in January and March. The months with the lowest larval abundance were August, September, and October (106.7 ind/m³/month).
As for pedi-veliger larvae, a similar pattern was observed, with a peak in March (2480.0 ind/m³). For the rest of the year, their density remained relatively low.
3.1.3 Case of the Djondji production area
The evolution of larval density at Djondji followed almost the same trend observed in the production areas of Ahouandji and Dégouè. D- and veliger larvae showed high abundance early in the year, with a peak in April (9920.0 ind/m³ for D-larvae and 4643.3 ind/m³ for veliger). Their densities relatively decreased for the rest of the year, with a complete absence of Umbo-veliger larvae in June.
Pedi-veliger larvae also showed a high abundance early in the year in the coastal lagoon waters at Djondji, with a peak in April (2850.0 ind/m³), before stabilizing for the remainder of the year with a declining trend. Pedi-veliger larvae were absent from the coastal lagoon waters at Djondji in February, June, July, August, October, and November.
3.2 Descriptors of larval dynamics
3.2.1 Mean density per larval stage
The average larval densities per stage and per production area in the coastal lagoon waters obtained during this study are presented in Table 5 as descriptor 1.
Table 5
| Sector | Larvae stage | Descriptor 1 | Descriptor 2 | Descriptor 3 | Descriptor 4 | Descriptor 5 | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mediane | SD | Sum | Survival (%) | Percentage of observations of pedi-velyger larvae | ||
| AH | D-larvae | 1747.5 | 58.7 | 1190.0 | 58.7 | 12582 | 0.4 | 66.7 |
| Veliger | 832.8 | 33.1 | 320.0 | 33.1 | 5996 | |||
| pedi-veliger | 686.9 | 55.2 | 0.0 | 55.2 | 4946 | |||
| Total_Larve | 3013.3 | 145.0 | 1190.0 | 145.0 | 23524 | |||
| DG | D-larvae | 1882.8 | 43.0 | 1425.0 | 43.0 | 13556 | 0.2 | 75.0 |
| velyger | 880.3 | 21.2 | 710.0 | 21.2 | 6338 | |||
| pedi-veliger | 312.8 | 19.5 | 0.0 | 19.5 | 2252 | |||
| Total_Larvae | 2965.6 | 74.5 | 2375.0 | 74.5 | 22146 | |||
| DJ | D-larvae | 3123.1 | 106.2 | 1105.0 | 106.2 | 20206 | 0.1 | 50.0 |
| Velyger | 1139.7 | 41.6 | 475.0 | 41.6 | 7826 | |||
| pedi-veliger | 444.7 | 27.1 | 0.0 | 27.1 | 3202 | |||
| Total_Larvae | 4685.0 | 162.5 | 2060.0 | 162.5 | 31234 | |||
| Lagoon | D-larvae | 2251.1 | 25.2 | 2251.1 | 25.2 | 46344 | 0.2 | 91.7 |
| velyger | 950.9 | 11.0 | 950.9 | 11.0 | 20160 | |||
| pedi-veliger | 481.5 | 12.4 | 481.5 | 12.4 | 10400 | |||
| Total_Larvae | 3554.6 | 44.5 | 3554.6 | 44.5 | 76904 | |||
Larval descriptors by stage and production area.
A total average of 2251 ± 25 D- larvae was obtained in the coastal lagoon waters during this study. The highest average value of D- larvae was recorded in the Djondji production area (3123 ± 106 larvae), followed by the Dégouè production area (1883 ± 43 larvae). The lowest average value of D- larvae was found in the Ahouandji production area (1748 ± 59 larvae).
For veliger larvae, a total average of 951 ± 11 larvae was recorded in the lagoon waters. As with the D- larvae, the same trend was observed. The highest average value (1140 ± 42 larvae) was found in the Djondji production area. It was followed by the Dégouè production area (880 ± 21 larvae). The lowest average value for veliger larvae was observed in the Ahouandji production area (833 ± 33 larvae).
For pedi-veliger larvae, a total average of 481 ± 12 larvae was recorded in the lagoon waters. The highest average value in the coastal lagoon was observed in the Ahouandji production area (687 ± 55 larvae), followed by the Djondji production area (445 ± larvae). The Dégouè production area had the lowest average value of pedi-veliger larvae (313 ± larvae).
Overall, a total average of 3555 ± 45 larvae was recorded in the coastal lagoon waters during this study. The Djondji production area presented the highest total average of C. tulipa oyster larvae (4685 ± 163 larvae), followed by the Ahouandji production area (3013 ± 145 larvae). The Dégouè production area, which also had a notable value, had the lowest total average of larvae (2966 ± 75 larvae).
3.2.2 Median density per larval stage
The median values of larval density by stage and production area in the coastal lagoon waters obtained during this study are presented in Table 5 as descriptor 2.
A total median value of 2251 ± 25 D- larvae was recorded in the coastal lagoon waters during this study. The highest median value of D- larvae was observed in the Dégouè production area (1425 ± 43 larvae), followed by the Ahouandji production area (1190 ± 59 larvae). The lowest median value of D- larvae was found in the Djondji production area (1105 ± 106 larvae). For veliger larvae, a total median value of 951 ± 11 larvae was recorded in the lagoon waters. The highest median value (710 ± 21 larvae) was found in the lagoon waters of the Dégouè production area. It was followed by the Djondji production area (475 ± 42 larvae). The lowest median value of veliger larvae was found in the Ahouandji production area (320 ± 33 larvae). For pedi-veliger larvae, a total median value of 481 ± 12 larvae was recorded in the lagoon waters. In the three production areas, the median value for pedi-veliger larvae in the coastal lagoon waters was zero.
Overall, a median value of 3555 ± 45 larvae was recorded in the coastal lagoon waters. The Dégouè production area had the highest total median value of C. tulipa oyster larvae (2375 ± 75 larvae), followed by the Djondji production area (2060 ± 163 larvae). The Ahouandji production area had the lowest total median value of larvae (1190 ± 145 larvae).
3.2.3 Total number of individuals counted by larval stage
Descriptor 3 (Table 5) provides information on the total number of larvae counted by larval stage in the coastal lagoon waters across the different production areas of the oyster C. tulipa. In the Ahouandji production area, a total of 23,524 larvae were counted, including 12,582 D- larvae, 5996 veliger larvae, and 4946 Pedi-veliger larvae during the study period. In the Dégouè production area, a total of 22,146 larvae were recorded, including 13,556 D-, 6338 veliger, and 2252 Pedi-veliger larvae. In the Djondji production area, a total of 31,234 larvae were counted, comprising 20,206 D-, 7826 veliger, and 3202 Pedi-veliger larvae. Considering the entire lagoon, a total of 76904 larvae were recorded, including 46,344 D-, 20,160veliger, and 10,400 Pedi-veliger larvae.
3.2.4 Larval survival = ratio of pedi-veliger to D-veliger densities (sum of pedi-veliger/sum of D-veliger)
Descriptor 4 (Table 5) provides an estimate of the survival rate of C. tulipa larvae in the coastal lagoon waters of Benin, expressed as the ratio between the number of pedi-veliger and D- larvae.
The larval survival rate, calculated as the density ratio between Pedi-veliger and D-veliger larvae, was 0.4 in the Ahouandji production area. In Dégouè, the ratio was 0.2. In the Djondji production area, a ratio of 0.1 was obtained. Considering the entire lagoon, the overall larval survival ratio was 0.2.
3.2.5 Presence duration of pedi-veliger larvae in the environment (percentage of sampling campaigns in which pedi-veliger larvae were recorded)
Descriptor 5 (Table 5) indicates the percentage of sampling campaigns during which Pedi-veliger larvae were observed in the coastal lagoon waters of Benin.
During the study period, the Dégouè production area exhibited the highest observation rate of pedi-veliger larvae, with 75% of sampling campaigns recording their presence. This was followed by the Ahouandji area, with a 66.67% observation rate. The Djondji production area showed the lowest rate, with only 50% of sampling campaigns detecting pedi-veliger larvae. At the scale of the entire lagoon, the observation rate of pedi-veliger larvae in the water column was 91.67%.
3.3 Influence of environmental parameters on larval abundance in the coastal lagoon waters
3.3.1 Physico-chemical parameters of coastal lagoon waters in oyster production areas
The variation in physico-chemical parameters of the coastal lagoon waters in the oyster production areas during the study period is shown in the graphs in Figure 3.
Figure 3
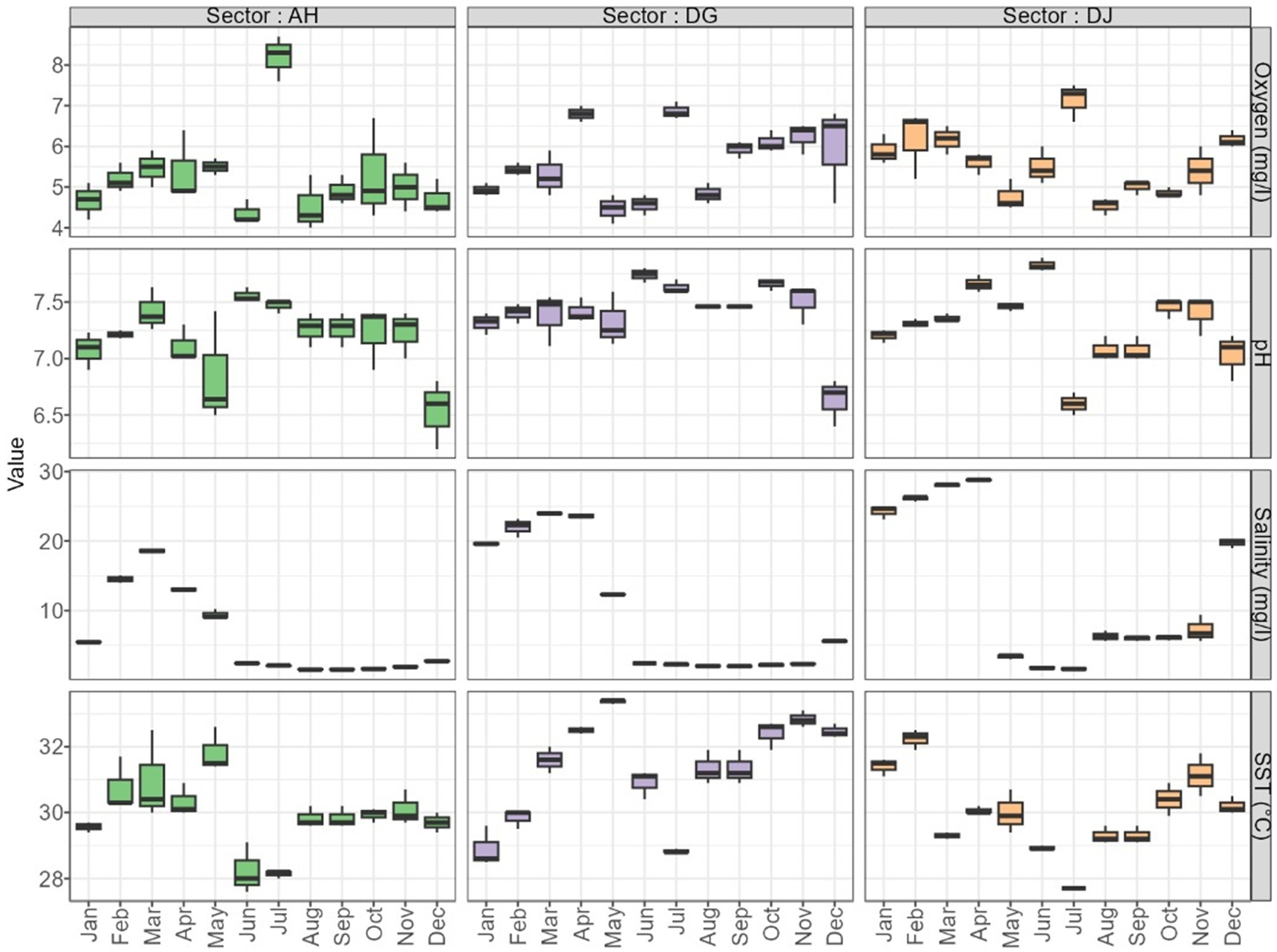
Evolution of the physio-chemical parameters of the coastal lagoon waters in the different oyster production areas.
The physicochemical parameters measured in the coastal lagoon waters during this study vary from one area to another and depending on the period.
Dissolved oxygen ranged from 4.37 mg/L to 8.2 mg/L, with an average of 5.27 ± 0.03 mg/L in the coastal lagoon waters at Ahouandji. In the Dégouè production area, dissolved oxygen fluctuated between 4.47 mg/L and 6.87 mg/L, with an average of 5.62 ± 0.02 mg/L. At Djondji, dissolved oxygen ranged from 4.53 mg/L to 7.13 mg/L, with an average of 5.6 ± 0.02 mg/L. The highest value (8.2 mg/L) was recorded in July (specify the zone), while the lowest value (4.37 mg/L) was measured in June.
The pH values measured ranged from 6.53 to 7.56, with an average of 7.18 ± 0.01 in the Ahouandji production area At Dégouè, the pH of the lagoon waters fluctuated between 6.63 and 7.74, with an average of 7.41 ± 0.01. In the Djondji production area, the pH values measured during this study ranged from 6.6 to 7.83, with an average of 7.29 ± 0.01. The lowest pH value (6.53) was observed in December, while the highest value (7.83) was recorded in June.
During the study period, the salinity of the coastal lagoon waters ranged from 1.53 to 14.53 g/L with an average of 6.25 ± 0.164 g/L in the Ahouandji production area. At Dégouè, it fluctuated between 2.2 g/L and 23.97 g/L, with an average of 10.02 ± 0.26 g/L. In the Djondji production area, the salinity values measured ranged from 1.6 g/L to 28.03 g/L, with an average of 13.27 ± 0.30 g/L. The highest value (28.03 g/L) was recorded in March, while the lowest value (1.53 g/L) was measured in August.
The temperature of the coastal lagoon waters was relatively homogeneous during the study period. It ranged from 28.23°C to 31.83°C, with an average of 29.94 ± 0.03°C in Ahouandji production area. In Dégouè production area, it ranged from 28.9°C to 33.37°C, with an average of 31.36 ± 0.04°C. In the Djondji production area, the temperature values measured in the coastal lagoon waters ranged from 27.7°C to 32.23°C, with an average of 29.99 ± 0.034°C. The lowest temperature value (27.7°C) was recorded in July, while the highest temperature value (33.37°C) was recorded in May.
During the study period, transparency values measured in the coastal lagoon waters in the Ahouandji production area ranged from 0.51 m to 1.71 m, with an average of 0.71 ± 0.35 m. In the Dégouè production zone, transparency ranged from 0.38 m to 2.08 m, with an average of 1.67 ± 0.62 m. In the Djondji production area, transparency values measured in the coastal lagoon waters ranged from 0.20 m to 1.92 m, with an average of 1.75 ± 0.93 m. The lowest transparency value (0.20 m) was recorded in May, while the highest value (2.08 m) was recorded in April.
3.3.2 Relationship between environmental parameters and the presence of different larval stages in the coastal lagoon waters of Benin
The results of the MFA (Multiple Factor Analysis) conducted on the quantitative data (larval density) and environmental variables (Figure 4) showed a positive correlation between the increase in salinity and the presence as well as the abundance of the three larval stages of the oyster C. tulipa in the coastal lagoon waters of Benin.
Figure 4
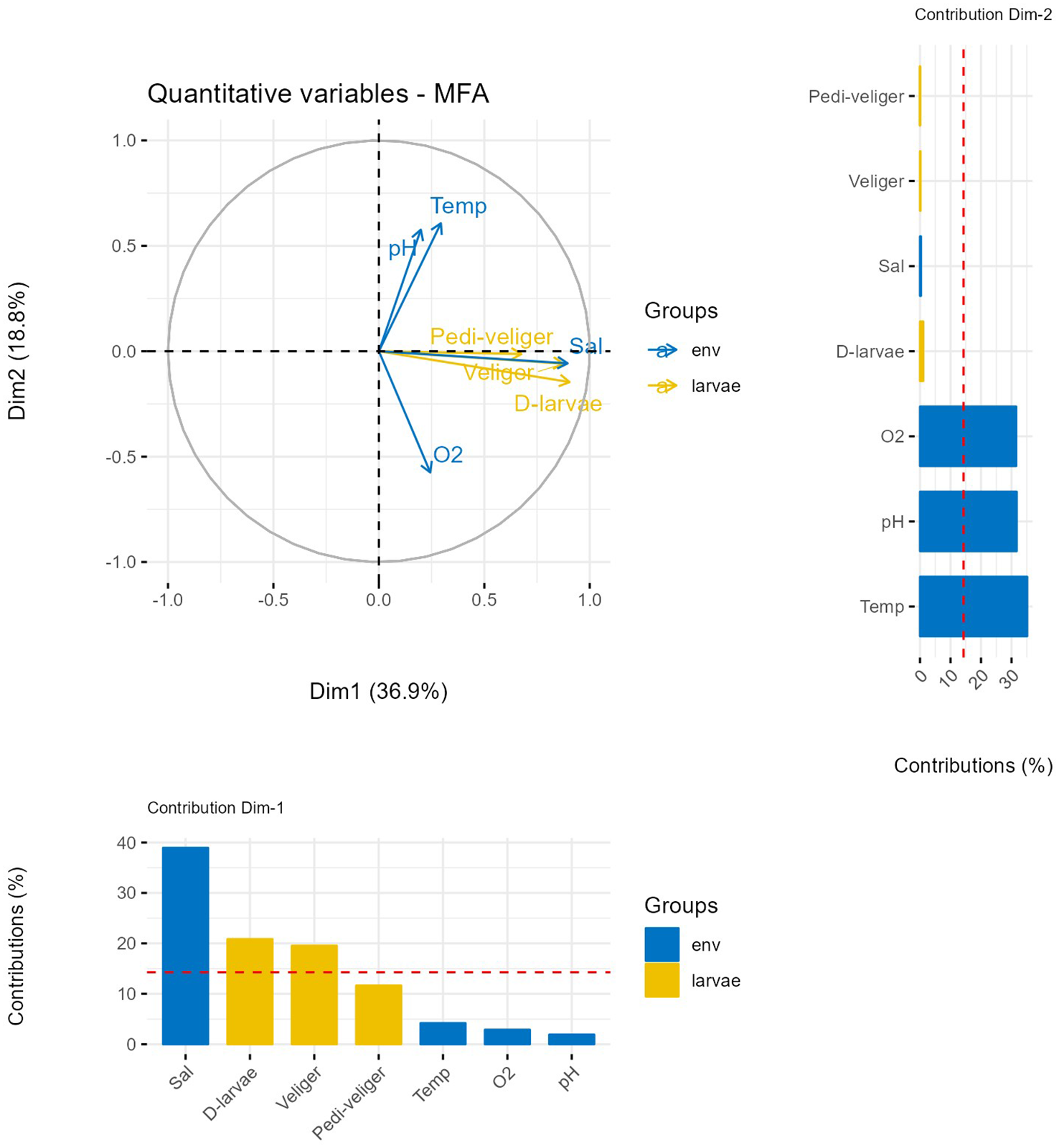
MFA (Multiple Factor Analysis) conducted on the quantitative data (larval density) and environmental variables.
A representation of the ellipses based on the months or sectors (Figure 5) showed that the months of January, February, March, and April were similar in terms of larval density, with a slight distinction in the month of March. These months were positioned opposite to the other months of the year, where the larval density appeared to be similar, except for the month of July, which stood out from the two groups. Considering the production areas (sectors), the evolution of larval density appears to be similar across the three production areas considered in this study, with minor specificities for each zone.
Figure 5
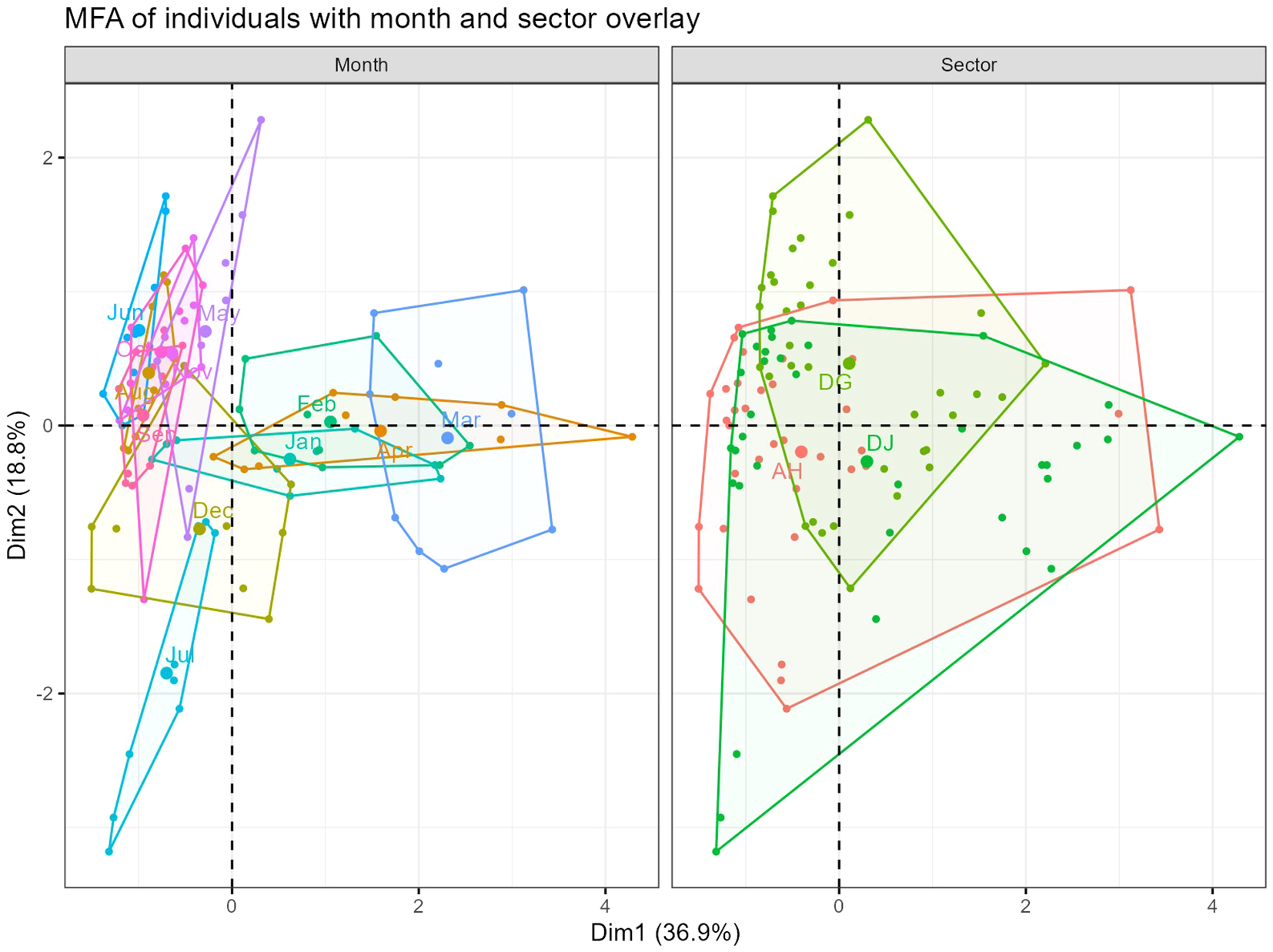
MFA: Representation of ellipses based on months or sectors.
Some photos of larvae observed during this study are presented in Figure 6.
Figure 6
Some photos of mangrove oyster larvae C. tulipa in the Coastal lagoon.
4 Discussion
4.1 Larval dynamics in the coastal lagoon waters
The density of C. tulipa oyster larvae showed significant variation over time during this study. The results show two periods of abundance for D- and veliger larvae across the three lagoon production areas. The first period of abundance occurs at the beginning of the year, starting as early as December and extending through April, with peak abundance observed in March or April, depending on the production area. This can be explained by the fact that this period coincides largely with the dry season in southern Benin. Chuku et al. (2023) noted a predominance of C. tulipa oyster spat in the coastal waters of the Gulf of Guinea along the Ghanaian coast during the dry season. During this period, environmental parameters recorded in the coastal lagoon waters show high salinity values in the three production areas, reaching up to 28.03 g/L in Djondji zone, 23.97 g/L in Dégouè area, and 14.53 g/L in Ahouandji area. Several studies have demonstrated the effect of salinity on oyster reproduction.
Indeed, a sudden change in the salinity or temperature of the water creates thermal or salinity stress, which acts as a trigger for reproduction in oysters (Bernard, 2011). This is exactly what was observed in the lagoon waters during this study, where an increase in salinity was noted during this period. From December to February, the salinity of the lagoon waters increased from 16.4 ± 0.90 to 26.1 ± 0.86 ‰; from 6.23± 0.63 to 22± 3.421 ‰, and from 5.03 ± 0.14 to 14.53 ‰ respectively at Djondji, Dégouè, and Ahouandji.
Regarding the pediveliger larvae, they were abundant in the lagoon waters only during the early months of the year, with a peak in March or April depending on the production area. Unlike the D- and veliger larvae, pediveliger larvae were relatively scarce during the second period of larval abundance. This suggests that the D-and veliger larvae from the second spawning event did not reach maturity. This could be explained by unfavorable environmental conditions, particularly related to the low salinity of lagoon waters during that period. Several studies have shown that Crassostrea larvae fail to reach maturity when water salinity is too low. McFarland et al. (2022) demonstrated that Crassostrea virginica larvae are vulnerable when exposed to salinities below 10 ppt. However, the survival of larvae of the same oyster species can be strongly influenced by the origin of the broodstock. Thus, the larval pool of a single estuary contains abundant genetic variation for survival at different salinities, partly due to functional genetic differences between source reefs (Eierman and Hare, 2013).
To ensure the sustainability of oyster farming, it is crucial to identify the zones and periods that are most favorable for oyster reproduction. This enables the establishment of strategic spat collection areas or integrated hatcheries in zones with optimal salinity conditions during critical reproductive periods (Phei Fang et al., 2016). Based on the larval dynamics of C. tulipa in the coastal lagoon waters of Benin, a spat collection calendar targeting the main dry season (December to April) as the key collection period would support the development of sustainable, productive oyster farming in these waters.
4.2 Larval descriptors
The highest overall mean density of C. tulipa larvae in the lagoon was recorded in Djondji production zone. This indicates a high intensity of oyster reproduction in this area, linked to the high salinities recorded there. This can be explained by the very close position of Djondji to the mouth of the “Bouche du Roi,” where the coastal lagoon is connected to the Atlantic Ocean.
The predominance of D- and veliger larvae in Djondji and Dégouè observed during this study is thus justified, as Dégouè is the second closest site to the estuary after Djondji. As observed in this study, during the salinization and low-water periods, a decreasing salinity gradient forms from the points closest to the mouths to those farther away in the lagoon systems of the Gulf of Guinea. This is due to the strong influx of marine waters during this season (Wognin et al., 2005; Honfo et al., 2024).
Unlike D and veliger larvae, pedi-veliger larvae were more predominant in Ahouandji production area. This predominance of Pedi-veliger larvae in Ahouandji may be explained by optimal environmental conditions for oyster larval development, particularly water salinity that is neither too high nor too low during this period (Phei Fang et al., 2016). This predominance may also be due to the lower anthropogenic pressure observed in the Ahouandji area during the study period, compared to Djondji and Dégouè, which are within the perimeter of major road construction and sand dredging operations undertaken by the Beninese government. Indeed, human activities can affect water quality and larval distribution (Wall et al., 2005; Williams et al., 2024).
Descriptors 3 and 4, which concern respectively the total number of larvae counted by stage and larval survival in the coastal waters, confirm the spatial heterogeneity in distribution and highlight the survival of C. tulipa larvae in the lagoon. The Djondji area, despite showing the highest larval abundance (56,220 larvae), is characterized by a low survival rate (0.14), indicating a predominance of early larval stages in this area, which may serve as a temporary aggregation area without providing favorable conditions for further larval development (Cowen and Sponaugle, 2009). In contrast, the combination of relatively high larval abundance and the highest survival rate (0.4) observed at Ahouandji suggests a potentially more stable or less disturbed environment for larval development (Williams et al., 2024), thus supporting the predominance of Pedi-veliger larvae observed in this area, as previously discussed.
The results of Descriptor 5, which corresponds to the percentage of observations of Pedi-veliger larvae, indicate that the Dégouè area, despite having a lower total abundance compared to Djondji, shows a higher observation percentage of pedi-veliger larvae (75%). This suggests that these larvae, although fewer in number, are more consistently present over time, indicating stable environmental conditions that favor their development. Ahouandji follows with 66.7%, while Djondji has the lowest frequency (50%), supporting the hypothesis of a transit area or an area where larvae face developmental constraints. The regular presence of pedi-veliger larvae throughout the sampling campaigns can be explained by a prolonged larval residence time, which is an indicator of stable environmental conditions that support development (Pouvreau, 2009).
These calculated Descriptors reveal that the Djondji production area is a “source area” that plays an important role in maintaining larval flow to other areas (Ahouandji and Dègouè), where the conditions are favorable for the larvae to mature. In the context of implementing a policy for the sustainable management of the natural stock of C. tulipa oysters and promoting oyster farming in the waters of Benin’s coastal lagoon, it could thus serve as a buffer zone. However, this would require strict regulation of human activities during breeding periods (Smyth et al., 2023; Pouvreau, 2009). The results of the MFA (Multi-Factor Analysis) conducted on larval densities and environmental variables show a positive correlation between increasing salinity and the presence as well as the abundance of the three larval stages of the oyster C. tulipa in the coastal waters of Benin. This confirms that salinity is a key environmental factor influencing the emission and distribution of C. Tulipa larvae in the coastal waters of Benin. Several studies have already reported the strong recruitment of C. Tulipa oyster spat in the coastal waters of the Gulf of Guinea (Diadhiou, 1995; Chuku et al., 2023).
5 Conclusion
The objective of this study was to assess the larval dispersal dynamics of the oyster C. tulipa in the coastal waters of Benin and to describe the associated environmental conditions. The study indicates that a significant spawning event with high larval production episode occurred from December to April in the coastal waters of Benin. Although Djondji area stands out as the best area in terms of larval abundance, it was observed that the Dégouè and Ahouandji areas offer better conditions for larval maturation, with a predominance and regular observation of advanced larval stages (Pedi-veliger larvae) in these zones. Djondji area, due to its position relative to the mouth, could serve as a larval transfer area towards optimal areas for larval maturation and development. A second spawning event episode occurred in July, but it was short-lived, and the environmental conditions (low salinity) likely did not allow for larval development, as evidenced by the near absence of Pedi-veliger larvae in the lagoon waters during this period. Salinity appears to be a major trigger for oyster reproduction in these waters.
This study therefore provides detailed information on the seasonal and spatial patterns of larval abundance in the coastal lagoon waters of Benin. This information can be used to plan spat collection and grow-out periods in order to optimize natural recruitment as part of a sustainable management policy for the natural stocks of C. tulipa, as well as to encourage oyster farming in Beninese waters.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Ethics statement
The animal study protocol was approved by the Ethics Committee of the Ministry of Higher Education and Scientific Research and the Ministry of Agriculture, Livestock, and Fisheries of the Republic of Benin (Protocol code: law N◦ 2014-19, approved on 2014 Aug 7), and complied with Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YZ: Investigation, Methodology, Software, Conceptualization, Writing – review & editing, Formal Analysis, Writing – original draft. DA: Conceptualization, Writing – review & editing, Investigation, Methodology. PB: Writing – review & editing, Methodology, Formal Analysis, Conceptualization. SW: Writing – review & editing, Methodology, Investigation, Conceptualization. TG: Methodology, Investigation, Conceptualization, Writing – review & editing. FM-R: Writing – review & editing, Supervision, Conceptualization, Methodology. MV-Y: Methodology, Supervision, Conceptualization, Writing – review & editing. MG: Methodology, Supervision, Conceptualization, Writing – review & editing. ZS: Methodology, Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This publication is funded by CSIC CALL I-COOP 2023 (Grant Number: CSIC/I-COOP2023/COOPB23085).
Acknowledgments
The authors thank the editor-in-chief and the peer reviewers for their constructive comments and suggestions. They also wish to express their gratitude to the African Union Commission through the GMES & Africa project, and to the Spanish National Research Council (CSIC) through the I-COOP program, for their financial support which facilitated data collection and processing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adams T. P. Aleynik D. Burrows M. T. (2014). Larval dispersal of intertidal organisms and the influence of coastline geography. Ecography37, 698–710. doi: 10.1111/j.1600-0587.2013.00259.x
2
Adite A. Sonon S. P. Gbedjissi G. L. (2013). Feeding ecology of the mangrove oyster, Crassostrea gasar (Dautzenberg 1891) in traditional farming at the coastal zone of Benin, West Africa. Natural Science.5, 1238–1248. doi: 10.4236/ns.2013.512151
3
Androuin T. Barbier P. Forêt M. Meziane T. Thomas M. Archambault P. et al . (2021). Pull the trigger: interplay between benthic and pelagic cues driving the early recruitment of a naturalbivalve assemblages. Ecosphere.13, e03672. doi: 10.1002/ecs2.3672
4
Barbier P. (2016). Déterminisme du recrutement des bivalves sous contraintes environnementales et anthropiques. Biodiversité et Ecologie (Français HAL: Muséum National d’Histoire Naturelle). Available online at: https://hal.science/tel-01297996 (Accessed March 13, 2025).
5
Bernard I. (2011). Ecology of Cupped Oyster Reproduction and Recruitment Variability on French Atlantic Coasts, TEL - Thèses en ligne (France). Available online at: https://coilink.org/20.500.12592/1mwfrdy (Accessed November 21, 2024).
6
Bunting S. W. Thiao D. Ahern M. Ansah Y. B. Ward A. Wesana J. et al . (2024). Evaluating rational and healthy use options for small pelagic fish species in sub-Saharan Africa. Food Sec.16, 1459–1477. doi: 10.1007/s12571-024-01491-8
7
Christo S. W. Ferreira A. L. Absher T. M. Kaled A. C. D. C. (2021). Spatio-temporal distribution pattern of oyster larvae in guaratuba bay, paraná State-Brazil. Braz. Arch. Biol. Technol.64, e21180617. doi: 10.1590/1678-4324-2021180617
8
Chuku E. O. Effah E. Adotey J. Abrokwah S. Adade R. Okyere I. et al . (2022). Spotlighting women-led fisheries livelihoods toward sustainable coastal governance: the estuarine and mangrove ecosystem shellfisheries of west africa. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.8847
9
Chuku E. O. Osei I. (2020). Estimating accurate rope length to minimize wastagein cultch construction for mangrove oyster farming. J. Fish. Coast. Manage.2, 34–40. doi: 10.5455/jfcom.20200407063121
10
Chuku E. O. Yankson K. Obodai E. A. Acheampong E. Aheto D. W. (2023). Spatiotemporal spatfall dynamics and prevailing estuarine conditions for optimal oyster (Crassostrea tulipa) spat availability in selected Gulf of Guinea brackish systems. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1075313
11
Chuku E. O. Yankson K. Obodai E. A. Acheampong E. Boahemaa-Kobil E. E. (2020). Effectiveness of different substrates for collecting wild spat of the oyster Crassostrea tulipa along the coast of Ghana. Aquaculture Rep.18, 100493. doi: 10.1016/j.aqrep.2020.100493
12
Cowen R. K. Sponaugle S. (2009). Larval dispersal and marine population connectivity. Annu. Rev. Mar. Science.1, 443–466. doi: 10.1146/annurev.marine.010908.163757
13
Diadhiou H. D. (1995). Biologie de l’huître de palétuvier Crassostrea gasar (Dautzenberg) dans l’estuaire de la Casamance (Sénégal): Reproduction, larves et captage du naissain (Brest (France: Thèse de doctorat de l’Université de Bretagne Occidentale).
14
Dimitriadis C. Koutsoubas D. Garyfalou Z. Tselepides A. (2014). Benthic molluscan macrofauna structure in heavily trawled sediments (Thermaikos Gulf, North Aegean Sea): spatiotemporal patterns. . J. Biol. Research-Thessaloniki21, 10. doi: 10.1186/2241-5793-21-10
15
Doinsing J. W. Ransangan J. (2022). An overview of the life cycle, reproduction and factors influencing recruitment success of tropical oysters. Egyptian J. Aquat. Biol. Fisheries26, 1–29. doi: 10.21608/ejabf.2022.234926
16
Eierman L. E. Hare M. P. (2013). Survival of oyster larvae in different salinities depends on source population within an estuary. J. Exp. Mar. Biol. Ecology.449, 61–68. doi: 10.1016/j.jembe.2013.08.015
17
Green A. L. Maypa A. P. Almany G. R. Rhodes K. L. Weeks R. Abesamis R. A. et al . (2015). Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biol. Rev.90, 1215–1247. doi: 10.1111/brv.12155
18
Hawkins A. J. S. Pascoe P. L. Parry H. Brinsley M. Black K. D. McGonigle C. et al (2013). Shellsim: a generic model of growth and environmental effects validated across contrasting habitats in bivalve shellfish. J. Shellfish Res.32(2), 237–253.
19
Honfo K. J. Chaigneau A. Morel Y. Duhaut T. Marsaleix P. Okpeitcha O. V. et al . (2024). Water mass circulation and residence time using Eulerian approach in a large coastal lagoon (Nokoué Lagoon, Benin, West Africa). Ocean Modelling.190, 102388, 1–43. doi: 10.1016/j.ocemod.2024.102388
20
Mahu E. Sanko S. Allieubakarr K. Chuku E. O. Effah E. Sohou Z. et al . (2022). Climate resilience and adaptation in west african oyster fisheries: an expert-based assessment of the vulnerability of the oyster crassostrea tulipa to climate change. Fishes.7, 205. doi: 10.3390/fishes7040205
21
Manuel E. C. Caracappa J. Munroe D. (2023). Changes in larval oyster swimming behavior with salinity and larval age. Biol. Bull.244, 94–102. doi: 10.1086/725418
22
Marcelino J. A. Macia A. Mafambissa M. J. Castejón D. Andrade C. (2023). Combined effects of salinity and temperature on survival and growth during the early life cycle of the rock oyster Saccostrea cuccullata (Born 1778). Western Indian Ocean J. Mar. Sci.22, 95–102. doi: 10.4314/wiojms.v22i1.10
23
McFarland K. Vignier J. Standen E. Volety A. K. (2022). Synergistic effects of salinity and temperature on the eastern oyster Crassostrea virginica throughout the lifespan. Mar. Ecol. Prog. Ser.700, 111–124. doi: 10.3354/meps14178
24
Olafsson E. B. Peterson C. Ambrose W. G. Jr. (1994). Does recruitment limitation structure populationsn and communities of macro-invertebrates in marine soft sediments: the relative significance of pre- and post-settlement processes. Oceanography Mar. Biology: an Annu. Review.32, 65–109.
25
Oliveira G. F. Pimentel-Santos J. Gomes I. Albuquerque R. Queiroga H. Peteiro L. G. (2024). Seasonal and synoptic spatio-temporal variability on larval delivery mechanisms inferred from mussel settlement patterns in the Portuguese coast. Estuarine Coast. Shelf Sci.305, 108875. doi: 10.1016/j.ecss.2024.108875
26
Oyede M. I. (2023). Direction de la Climatologie et de l’Agrometeorologie: Etat du climat de l’année 2023 au Bénin. Available online at: https://www.meteoBenin.bj/documents/1069/revue6_2023_lWZmabP.pdf (Accessed April 17, 2025).
27
Paixao L. Ferreira M. A. Nunes Z. Fonseca-Sizo F. Rocha R . (2013). Effects of salinity and rainfall on the reproductive biology of the mangrove oyster (Crassostrea gasar): Implications for the collection of broodstock oysters. Aquaculture, 380, 6–12.
28
Pedersen T. M. Hansen J. L. S. Josefson A. B. Hansen B. W. (2008). Mortality through ontogeny of soft-bottom marine invertebrates with planktonic larvae. J. Mar. Systems.73, 185–207. doi: 10.1016/j.jmarsys.2007.10.008
29
Phei Fang A. N. Chiew Peng T. Khoy Yen P. Hwai A. T. S. (2016). Effect of salinity on embryo and larval development of oyster Crassostrea iredalei. Trop. Life Sci. Res.27, 23–29. doi: 10.21315/tlsr2016.27.3.4
30
Pineda J. Porri F. Starczak V. Blythe J. (2010). Causes of decoupling between larval supply and settlement and consequences for understanding recruitment and population connectivity. J. Exp. Mar. Biol. Ecology.392, 9–21. doi: 10.1016/j.jembe.2010.04.008
31
Politi T. Zilius M. Castaldelli G. Bartoli M. Daunys D. (2019). Estuarine macrofauna affects benthic biogeochemistry in a hypertrophic lagoon. Water11, 1186. doi: 10.3390/w11061186
32
Pouvreau S. (2009). “ Observer, Analyser et Gérer la variabilité de la reproduction et du recrutement de l’huître creuse en France: Le Réseau Velyger,” in Rapport annuel 2009. (Brest, Ifremer). Available online at: https://wwz.ifremer.fr/velyger/Rapports-Annuels/Annee-2009 (Accessed April 21, 2025).
33
Powers S. P. Roman H. Meixner J. Wirasaet D. Brus S. Fricano G. et al . (2023). Establishing connectivity patterns of eastern oysters (Crassostrea virginica) on regional oceanographic scales. Ecosphere14, e4337. doi: 10.1002/ecs2.4337
34
Sinsin B. Tente B. Tiémoko Y. O. Adanguidi J. Ahouansou S. Padonou E. Agbani (2018). Inventaire floristique et faunique des écosystèmes de mangroves et des zones humides côtières du Bénin. (Cotonou, Bénin: FAO).
35
Smyth D. Millar R. Clements A. McIvenny H. Hayden-Hughes M. (2023). Population dynamics of the European native oyster in a Marine Conservation Zone exposed to unregulated harvesting. Aquat. Living Resour.36, 3. doi: 10.1051/alr/2022023
36
Tedford-Callahan K. N. Smith R. S. Lusk B. W. Castorani M. C. (2025). Hydrodynamics, elevation, and restoration history structure intertidal oyster recruitment. Landscape Ecol.40, 114. doi: 10.1007/s10980-025-02126-9
37
Theuerkauf S. J. Puckett B. J. Eggleston D. B. (2021). Metapopulation dynamics of oysters: sources, sinks, and implications for conservation and restoration. Ecosphere12, e03573. doi: 10.1002/ecs2.3573
38
Thorson G . (1950). Reproduction and larval ecology of marine bottom invertebrates. Biol. Rev.25, 1–45.
39
Toupoint N. Barbier P. Tremblay R. Archambault P. McKindsey C. W. Winkler G. et al . (2016). Influence of intertidal recreational fisheries and ‘bouchot’mussel culture on bivalve recruitment. Mar. Environ. Res.117, 1–12. doi: 10.1016/j.marenvres.2016.03.006
40
Ubertini M. Lagarde F. Mortreux S. Le Gall P. Chiantella C. Fiandrino A. et al . (2017). Gametogenesis, spawning behavior and larval abundance of the Pacific oyster Crassostrea gigas in the Thau lagoon: Evidence of an environment-dependent strategy. Aquaculture473, 51–61. doi: 10.1016/j.aquaculture.2017.01.025
41
Viaho C. (2014). Inventaire de la faune ichtyologique du lac Ahémé et ses chenaux et quelques aspects bioécologiques des espèces dominantes’’ (Université d’Abomey-Calavi, Bénin: Mémoire de Master).
42
Wall L. M. Walters L. J. Grizzle R. Sacks P. (2005). Recreational boating activity and its impact on the recruitment and survival of the oyster Crassostrea virginica on intertidal reefs in Mosquito lagoon. Florida. J. @ Shellfish Res.24, 965–973. doi: 10.2983/0730-8000(2005)24[965:RBAAII]2.0.CO;2
43
Williams B. R. McAfee D. Connell S. D. (2024). Anthropogenic noise disrupts acoustic cues for recruitment. Proc. R. Soc B.291, 20240741. doi: 10.1098/rspb.2024.0741
44
Wognin V. Monde S. Coulibaly A. Kouassi K. L. Aka K. (2005). Waters model circulation in the estuary of bandama. Rivers flows and tide condition’s incidence. Editorial Advisory Board.19, 304–314.
Summary
Keywords
coastal lagoon, larval dynamics, oysters, Crassostrea tulipa , oyster farming
Citation
Zounon Y, Adjahouinou DC, Barbier P, Wanou SE, Godome T, Moya-Ruiz F, Vargas-Yáñez M, García-Martínez MC and Sohou Z (2025) Unveiling seasonal variability in Crassostrea tulipa larval dispersal in Gulf of Guinea coastal waters: foundations for sustainable shellfish farming. Front. Mar. Sci. 12:1634636. doi: 10.3389/fmars.2025.1634636
Received
04 June 2025
Accepted
13 October 2025
Published
06 November 2025
Volume
12 - 2025
Edited by
Roger A. Rulifson, East Carolina University, United States
Reviewed by
Monica Cristina Rodriguez Palacio, Metropolitan Autonomous University, Mexico; Hamet Diaw Diadhiou, BERGRN DR Sarl, Senegal
Updates
Copyright
© 2025 Zounon, Adjahouinou, Barbier, Wanou, Godome, Moya-Ruiz, Vargas-Yáñez, García-Martínez and Sohou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaovi Zounon, zounonyaovi@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.