Introduction
The marine environment plays a crucial role as a primary food source and a vital driver of the global economy (Shenoi, 2024). However, industrialization and urbanization have had detrimental impacts on the marine ecosystem. The discharge of a burgeoning list of chemical pollutants from these sources leads to adverse alterations in the physicochemical and biological components of the ecosystem. These changes directly or indirectly impact the ecological equilibrium of the environment, ultimately affecting human beings (Batvari Dass et al., 2008). The escalating pollution levels, especially in nearshore coastal areas, have emerged as a critical global concern due to population growth and industrial activities near coastlines, oil spills, and excessive discharge of nutrients, which have continually led to the deterioration of water quality, significantly harming marine flora and fauna (Yuvaraj et al., 2018; Kumar et al., 2022; Jha et al., 2022).
Among innumerable pollutants arising from various anthropogenic activities, heavy metals, in particular, pose a significant threat to marine ecosystems because of their toxicity, persistence, bioaccumulation and biomagnification in the food chain, as extensively discussed in the recent scientific literature (Jha et al., 2019, 2021; Pandey et al., 2021, 2022; Koduvayur et al., 2022; Sathish Kumar et al., 2023; Verma et al., 2024; Pandey et al., 2024). These studies have thoroughly examined the sources, fate and transport, toxicity, mode of action, and bioaccumulation of metals in coastal environments. Metals have been found to adsorb from water columns onto particle surfaces and settle into sediment, accumulating in marine organisms’ tissues (Zaynab et al., 2022). Research has shown evidence of metal contamination in seawater, sediments, and their bioaccumulation in coastal and estuarine ecosystems, underscoring the widespread impacts of metal pollution in marine environments (Jayaprakash et al., 2015; Otchere, 2019; Pandey et al., 2021). Toxicological studies have revealed various harmful effects of metal contamination, including neurotoxicity, immunodeficiency, osteoporosis, kidney failure, and potential implications for reduced fertility (Rzymski et al., 2015).
Heavy metals typically enter fish through the gills and digestive system before spreading to other organs via the bloodstream (Chevreuil et al., 1995). Certain metals have a higher affinity for specific organs and accumulate in higher concentrations in those organs (Rainbow, 2002). For instance, Cd tends to accumulate in high levels in the liver and kidneys (Campenhout et al., 2004), Cu in the liver, and Zn has a higher accumulative affinity with fish muscles, skin, and bones (Olsson et al., 1998). Notwithstanding that, long-term exposure to metal pollution generally leads to their accumulation in the muscles, whereas during short-term exposure, muscles are not the primary site of accumulation (Shalini et al., 2021). Moreover, the bioavailability of these metals to fish depends on environmental conditions, types of pollutants, location, feeding patterns, age, size, sex, habitats, and trophic level (Asuquo et al., 2004). The presence of these metals in marine organisms and their subsequent bioaccumulation as they progress through the food chain poses a significant threat to the ecosystem and the animals that rely on them, including humans. In recent years, there has been a growing interest in the nutritional and medicinal benefits of marine-based food, mainly fish, leading to increased consumption (El-Moselhy et al., 2014). Fish is the most widely consumed type of seafood in India, and the accumulation of heavy metals in fish presents a significant risk to human health. Beyond being a vital source of protein, fish also harbor substantial amounts of essential minerals, vitamins, and fatty acids. Predators at the top of the aquatic food chain typically exhibit elevated levels of heavy metals due to their ability to accrue these substances from water, sediments, and food sources (Medeiros et al., 2012; Zhao et al., 2012). The widespread occurrence of these metal and the vital importance of fish in the global seafood market have generated considerable interest among scientists worldwide in examining the metal levels present in these aquatic species (Raja et al., 2009).
Ennore Port is situated at the northeastern part of Chennai City in Tamil Nadu on the Eastern Coastal Plains, along the Coromandel Coast in the Bay of Bengal, 2.6 km north of Ennore Creek. The port’s location on the thermal equator zone ensures minimal seasonal temperature fluctuations, with summer temperatures between 38–42 °C and winter temperatures dipping to 18–20 °C. This area is characterized by a hot and humid climate for most of the year, under the influence of a tropical wet and dry climate. The northeast monsoon significantly impacts the region’s weather patterns, bringing most of the annual rainfall of approximately 1400 mm between September and December and occasional cyclonic events. The last two decades have witnessed substantial development in Ennore and the wider Chennai metropolitan area, posing significant environmental threats to the local aquatic ecosystems. A major contributor to the pollution in Ennore Creek, which demarcates the port from Ennore town, is the discharge of wastewater effluents, leachates, and chemicals, primarily from petroleum refining industries in the city’s north. Additionally, a coal-fired thermal power station near the creek contributes to the pollution through its effluent discharge (Vasanthi et al., 2013). The environmental impact of such activities is further exacerbated by heavy metal pollutants in the Ennore fishing harbor, a hub for industrial activities, including thermal power generation, oil refining, and fertilizer production. The main environmental concerns for the Ennore coast include oil spillages and the accumulation of industrial sludge in the marine environment. The population of the Ennore region was recorded to be around 40,000, according to the 2011 census data, highlighting the human dimension of the environmental challenges faced by this area.
With that background, this study focuses on analyzing the concentration of heavy metals such as Copper (Cu), Chromium (Cr), Aluminum (Al), Zinc (Zn), Cobalt (Co), Mercury (Hg), Lead (Pb), Nickel (Ni), Iron (Fe), Cadmium (Cd) and Manganese (Mn) in different organs of commonly consumed fish species - Indian Oil Sardine (Sardinella longiceps), Indian Mackerel (Rastrelliger kanagurta), and Red Snapper (Lutjanus campechanus) from the Ennore fishing port, Chennai. The selected species were identified due to their ecological significance and economic relevance. Fish can bioaccumulate high concentrations of contaminants, occasionally surpassing established regulatory thresholds. This bioaccumulation phenomenon is intricately linked to the trophic positioning of particular fish species at the apex of the aquatic food web (Chahid et al., 2014). Consequently, assessing the chemical quality of marine organisms, particularly regarding their heavy metal concentrations, is crucial for safeguarding human health (Tuzen, 2009). These metals encompass both essential trace elements and non-essential toxicants, providing a balanced assessment of the nutritional and contamination status of commercially important marine fish species. Furthermore, their inclusion allows for comprehensive monitoring of anthropogenic impacts on marine ecosystems, particularly in Indian coastal waters with significant industrial and urban influences. The objectives addressed here are (1) to assess the metal concentration in different organs, such as gills, liver, intestine, and muscle, and (2) to compare the metal concentration in organs vis-à-vis fish species. These fish species have diverse feeding habits, and their consumption of organisms in which heavy metals can accumulate warrants attention to potential human health risks (Shah et al., 2019; Hakimelahi et al., 2020; Switzer et al., 2015).
Materials and methods
A total of 15 fish specimens (15×4 organs = 60 samples) belonging to three species—Sardinella longiceps, Rastrelliger kanagurta, and Lutjanus campechanus were collected from the Ennore landing center (Latitude: 13°13’55.84”N; Longitude: 80°19’42.49”E). According to standard protocols, the samples were immediately transported to the laboratory in an icebox for further processing and species verification. In the laboratory, each specimen’s total length and body weight were recorded. Subsequently, the fish were dissected through the anal opening using sterile dissection tools (knife and forceps) to extract selected tissues, namely: (1) muscle, (2) liver, (3) intestine, and (4) gills. The dissected tissues were placed in pre-labelled, pre-acid-washed Falcon tubes and stored at −20 °C until further analysis, following the procedure outlined by Koduvayur et al. (2022). All reagents used were of analytical grade, and solutions were prepared using Milli-Q water (Elix UV5 and Milli-Q system, Millipore, USA). Teflon and polypropylene containers were pre-cleaned by soaking in 5% nitric acid (HNO3) for 24 hours, then thoroughly rinsed with Milli-Q water and dried before use. For heavy metal analysis, 1 gram of dried tissue was digested at 140 °C with 5 mL of concentrated nitric acid (Suprapur®, Merck) in a microwave digestion system (Anton Paar), by the standard digestion procedure described by APHA (American Public Health Association) (2005). After digestion, the resulting clear solution was transferred into centrifuge tubes and diluted to a final volume of 10 mL with Milli-Q water (resistivity: 18.2 MΩ·cm), as per the method by Cortada and Collin (2013). Trace element concentrations were quantified using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500). To ensure data reliability, certified reference materials (CRM), procedural blanks, and sample replicates were included in the analysis, following the quality control measures described by Jha et al. (2019). The analytical accuracy and precision were validated by comparing the measured values against certified values for NIST CRM QC3163 (seawater), with mean ± standard deviation results. Metal concentrations in fish organs were expressed in ppm.
Data analysis
The data generated for metals from fish tissues were subjected to descriptive statistics such as mean and range to summarize the metal concentration in different fish species and their respective organs. The normality of the data was assessed using the Shapiro-Wilk test, and data transformation was applied where necessary to meet the assumptions of parametric tests. A one-way analysis of variance (ANOVA) test was conducted to assess the significant differences (α = 0.05) among various organs and species of fish. Box whisker plots were created, and the relationship between metal concentrations in different organs of the fish was analyzed. Multivariate statistical techniques were applied to explore further the metal distribution patterns and underlying factors influencing metal accumulation. Principal Component Analysis (PCA) was conducted on the normalized dataset to gain insights into the distribution of metals and the key components affecting the dataset. Before PCA, the Kaiser Meyer Olkin (KMO) criterion was used to establish sample adequacy, whereas Bartlett’s Test of Sphericity was used to check whether variables are significantly correlated. Hierarchical clustering was performed using Ward’s method, and squared Euclidean distances from the normalized dataset to measure the dissimilarity matrix.
Results and discussion
The overall metal concentration in fish samples revealed notably high levels of iron (Fe) and aluminum (Al) across all examined organs, with Fe ranging from 4.05 to 4717.2 ppm and Al from 0.52 to 2663.81 ppm. Among these, iron stands out due to its essential role in numerous physiological and metabolic functions. According to the World Health Organization (1989), adequate Fe intake is crucial for maintaining human health. However, excessive iron accumulation can pose health risks, particularly for individuals with conditions such as hemochromatosis or other iron overload disorders. In contrast, Al, despite being one of the most abundant metallic elements in the Earth’s crust, lacks a defined biological role (Zhong et al., 2018). Nevertheless, increasing evidence has linked Al exposure to neurotoxicity and its potential involvement in neurodegenerative diseases, including Alzheimer’s disease (Huat et al., 2019; Bryliński et al., 2023).
The elevated concentrations of Fe and Al in fish tissues may stem from both natural geological sources and anthropogenic influences, such as industrial discharges, mining operations, and agricultural runoff, which can enhance the mobility and bioavailability of these metals in aquatic ecosystems. In contrast to these findings, mercury (Hg) was consistently below the limit of quantification (BLQ) across all sampled tissues. Mercury, particularly in its methylated form, is one of the most toxic heavy metals and poses severe risks to both human and ecological health (Compeau and Bartha, 1985; Pandey et al., 2024). The absence of detectable Hg concentrations in the samples could be interpreted as a positive indication for suitability of fish consumption by human. The overall mean concentrations of metals in the studied fish species were ranked as Fe (525.23 ppm) > Al (275.57 ppm) > Zn (29.12 ppm) > Mn (5.87 ppm) > Cu (3.92 ppm) > Cr (1.49 ppm) > Ni (0.99 ppm) > Co (0.25 ppm) (Supplementary Table S1). Toxic metals such as Cd, and Pb were present at very low concentrations. Interestingly, these metals were often below the limit of quantification in most of the samples. Cd and Pb reached maximum concentrations of 1.19 ppm and 2.95 ppm, respectively. The concentrations of heavy metals, including Pb and Cd, detected in the samples were evaluated against international safety guidelines for human consumption. According to the World Health Organization (WHO) and the Food and Agriculture Organization (FAO), the maximum permissible limits in edible tissues are as follows: Lead (Pb): 0.3 mg/kg, Cadmium (Cd): 0.05–0.1 mg/kg, and Mercury (Hg): 0.5 mg/kg for methylmercury in fish (Codex Alimentarius Commission, 2024). In our analysis, the concentration of Hg was found to be below the limit of quantification. Although the overall maximum concentrations of Cd and Pb may have exceeded permissible limits, the mean concentration of Cd and Pb in muscle tissue (edible part) was 0.047 ± 0.05 and 0.084 ± 0.04 ppm, respectively, which remained below the established limits. The observed pattern of metal concentration in fish samples is consistent with previous research along the southeast coast of India (Koduvayur et al., 2022; Samantara et al., 2023). The frequency of BLQ values further supports the idea that the contamination from these metals is relatively low in the study area, with Co being BLQ in 49 out of 60 samples, Pb in 34, and Cd in 32. The sporadic detection of these metals at low concentrations has significant implications for food safety and human health. It suggests that consuming these fish species does not pose a substantial risk of heavy metal toxicity.
Among the three Fishes, the relative concentration of all the metals, except Al, Fe, and Zn, was higher in Snapper. The Al, Fe, and Zn concentrations were higher in Sardines. In contrast, all metals’ concentrations were lower in Mackerel (Figure 1). The observed differences in metal accumulation could be attributed to species-specific factors such as habitat preference, feeding habits, metabolic rate, and detoxification mechanisms (Canli and Atli, 2003; Dural et al., 2006). The significantly higher Al, Fe, and Zn accumulation in Sardines may be linked to their feeding behavior and trophic position, as these metals are commonly associated with planktonic food sources and sediment exposure (Romeo et al., 1999). In contrast, Mackerel’s general lower metal concentrations suggest a reduced bioaccumulation capacity. The one-way ANOVA test showed that except for Ni and Co, all the metals showed significant variation between fish species – Cu (F(2,57) = 6.787, p = 0.002), Cd (F(2,57) = 7.435, p = 0.001), Pb (F(2,57) = 3.946, p = 0.025), Zn (F(2,57) = 8.107, p = 0.001), Mn (F(2,57) = 3.508, p = 0.037), Al (F(2,57) = 3.626, p = 0.033), Fe (F(2,57) = 3.537, p = 0.036), Cr (F(2,57) = 3.337, p = 0.043) - which further reinforce the influence of interspecific differences on metal uptake (Supplementary Table S2A). Despite collecting from the same environment, the significant differences in metal concentration between fish species can result from differences in gill morphology, metal-binding protein expression, and dietary intake (Romeo et al., 1999). The lack of significant variation in Ni and Co concentrations across species suggests a more uniform bioavailability or homeostatic regulation mechanisms that control their accumulation (Heath, 1995). Nickel and cobalt play physiological roles in fish, such as enzyme cofactor activity and vitamin B12 synthesis, which could explain their stable concentrations across species (Ragsdale, 2009; Bagheri et al., 2024).
Figure 1
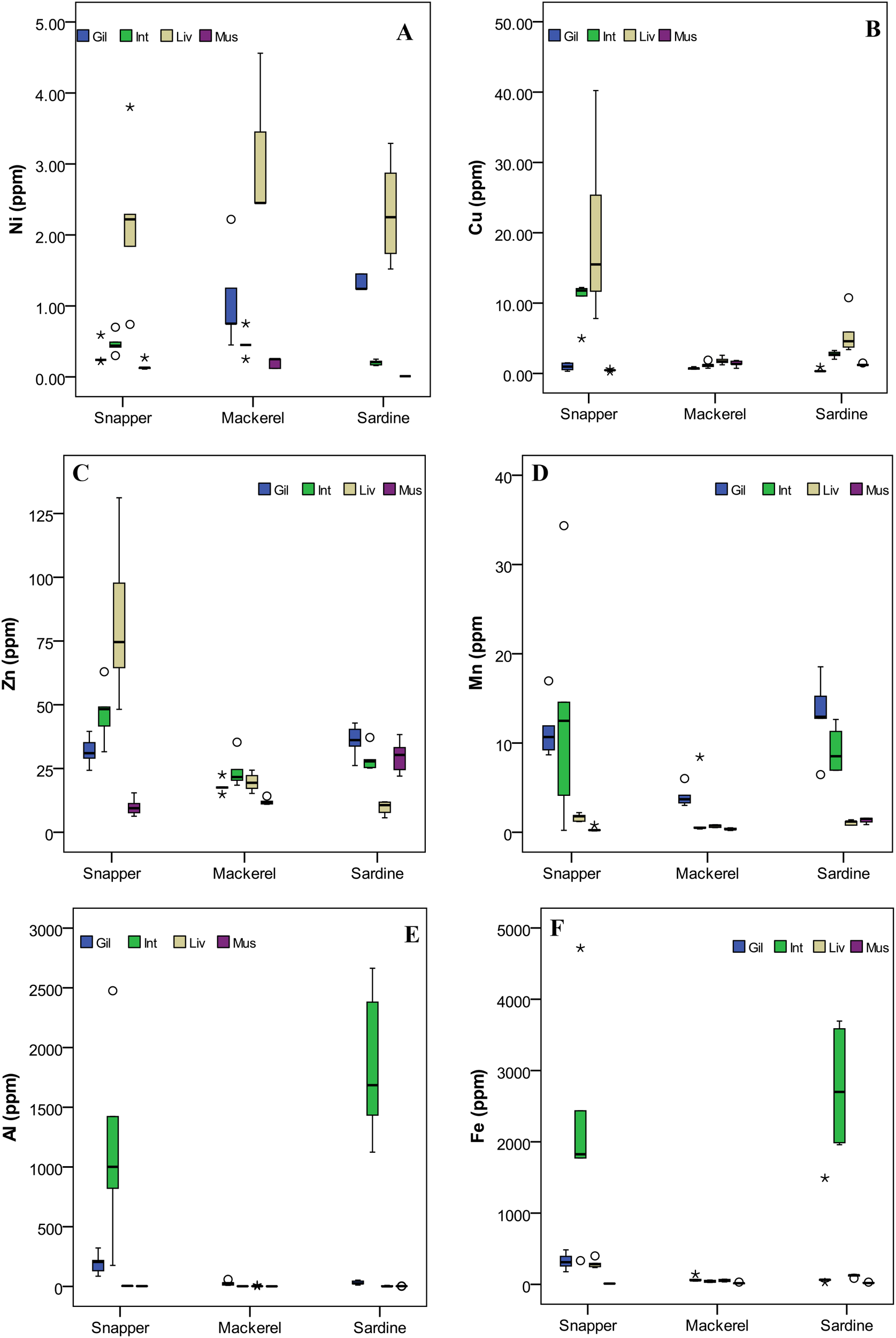
Box-whisker plots for selected metal variables in different fish samples (A) Ni, (B) Cu, (C) Zn, (D) Mn, (E) Al, and (F) Fe. In each plot, the median is represented by the central point, the interval is defined by the rectangle (i.e., 25% and 75% percentiles), and the range is indicated by the whisker (*: extreme outlier; ◦: mild outlier). Different fish organs (Gil, Gill; Int, Intestine; Liv, Liver; Mus, Muscle).
Among the organs, the muscles, which are the edible part of the fish, consistently showed lower concentration regardless of the fish species, which aligns with previous studies indicating that muscle tissues have limited metal-binding capacity due to lower metabolic activity and reduced affinity for metal storage (Canli and Atli, 2003). Meanwhile, the liver and intestine are organs with higher metal accumulation, which could be attributed to their crucial roles in metal metabolism, detoxification, and excretion (Romeo et al., 1999). The finding (Supplementary Table S1) aligns with the previous studies where the higher concentration of metals in the liver has been linked to its role in metabolism (Bawuro et al., 2018). One-way ANOVA results revealed significant organ-specific differences in the accumulation of metals in the sampled fish species. Statistically significant variation (p < 0.05) was observed for Ni (F(3,56) = 7.837, p = .000), Cu (F(3,56) = 6.861, p = .001), Cd (F(3,56) = 5.893, p = .001), Pb (F(3,56) = 3.502, p = .021), Mn (F(3,56) = 12.918, p = .000), Co (F(3,56) = 12.667, p = .000), Al (F(3,56) = 13.136, p = .000), Fe (F(3,56) = 14.060, p = .000), and Cr (F(3,56) = 14.774, p = .000), indicating strong organ-level differentiation in bioaccumulation patterns (Supplementary Table S2B). Zinc showed the lowest variability and did not reach statistical significance (F(3,56) = 2.553, p = .065), suggesting a more uniform distribution across organs. The organ-specific significant differences in metal concentration can be linked to the physiological role of each metal (El-Moselhy et al., 2014), with essential metals preferentially accumulating in metabolically active tissues such as the liver and gills. However, the absence of significant variation in Zn concentration across organs suggests a more uniform distribution, likely due to its essential role in various physiological functions, including enzyme activation, cellular signaling, and structural stability of proteins (Al-Yousuf et al., 2000).
Principal Component Analysis (PCA) was performed on the dataset to assess the distribution of metals in different fish organs (Figure 2A). The KMO test (0.73) and Bartlett’s Test of Sphericity (χ2 = 891.54, p = 0.001) revealed that the dataset is adequate and variables are significantly correlated, indicating that PCA results are reliable. The PCA showed two principal components with eigenvalues >1. The first principal component (PC1) accounted for 53.3% of the variance. The second principal component (PC2) explained 25.9%, with the first two PCs cumulatively capturing 79.2% of the dataset’s total variability. This high cumulative variance suggests that the majority of the data structure can be visualized effectively within a two-dimensional space, facilitating a clear interpretation of metal distribution patterns across the organs. Notably, the gills exhibited clustering near the origin, which implies a balanced metal composition, where no single metal overwhelmingly influences the overall metal profile in this organ. This could suggest that the gills may regulate a range of metals within the fish’s body without preferential accumulation of any particular element. The intestine, in contrast, demonstrated a broad distribution along PC1, reflecting a strong association with metals such as Fe, Al, Cr, Mn, and Ni. This widespread data indicates that the intestine is more variable in its metal content, likely due to its role in metal absorption and the influence of dietary intake or environmental exposure. The liver, central to metal detoxification and storage, was predominantly positioned along PC2, suggesting a significant correlation with Pb and Cu. The association of the liver with these metals may reflect its involvement in metabolic processes, where Pb is typically absorbed and stored. At the same time, Cu is integral to enzyme systems and metabolic functions (Bawuro et al., 2018). Muscles formed a compact cluster near the origin, suggesting minimal variability in metal accumulation. This indicates that the bioaccumulation of metals has less influence on muscle tissue due to the lower metabolic activity or reduced metal storage capacity than other organs, such as the liver or intestine (Zhang et al., 2017).
Figure 2
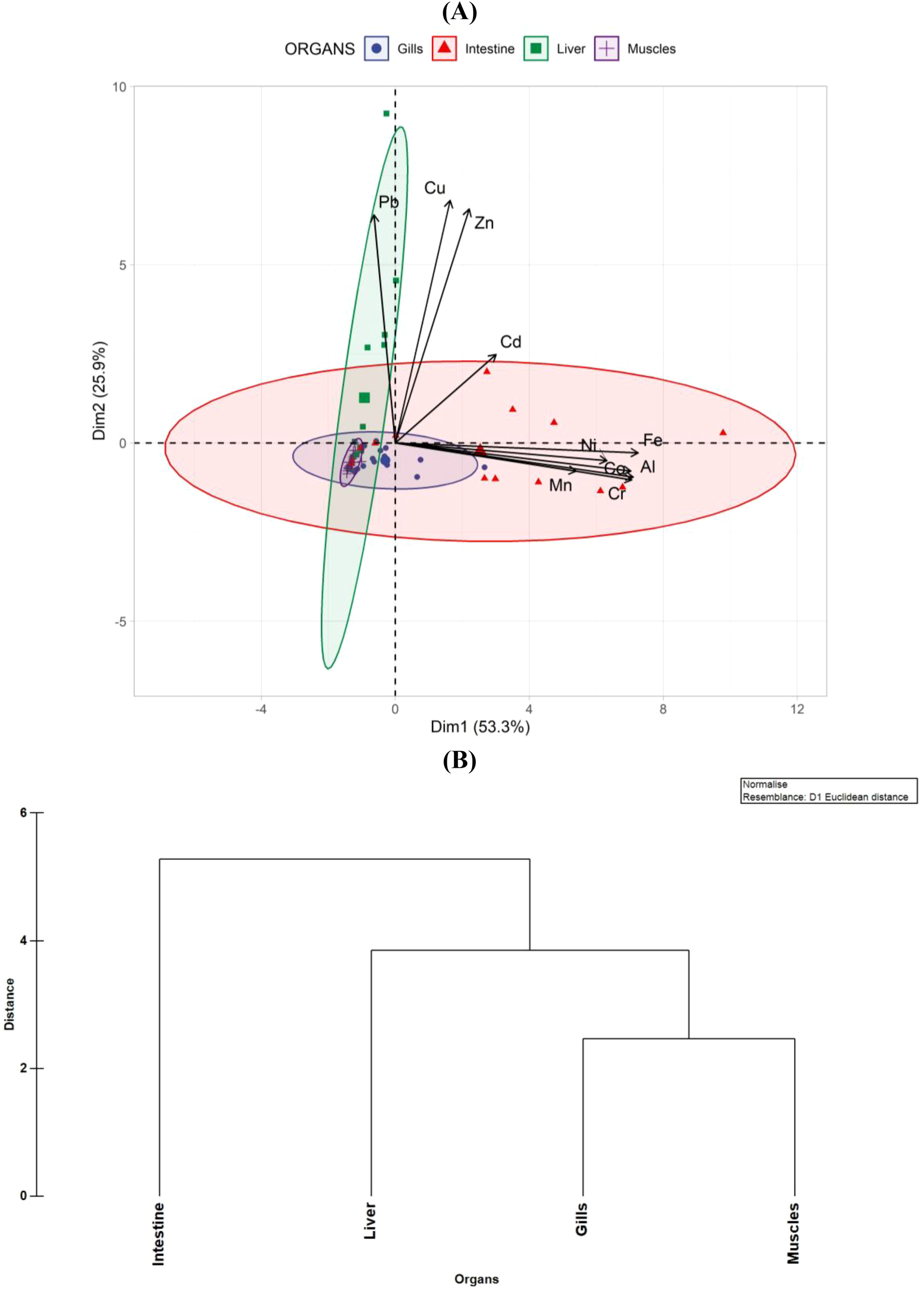
(A) PCA biplot with confidence ellipses showing metal concentration patterns across fish organs. (B) HCA dendrogram illustrating organ-wise clustering based on metal profiles.
The hierarchical cluster analysis of metals in different organs of fish revealed that gills and muscles exhibit the highest similarity in terms of metal accumulation (Figure 2B). The gills, as the primary site of respiration and direct interaction with the surrounding aquatic environment, are known to accumulate metals from waterborne exposure (Shah et al., 2020). The clustering of the liver with the gills-muscles group suggests a moderate level of similarity, likely due to its central role in metal detoxification and storage. The liver is well-recognized for its function in metal metabolism, including biotransformation, sequestration, and excretion. Metallothioneins and other detoxifying enzymes in hepatic tissues allow the liver to accumulate and regulate metal concentrations, leading to a distinct but related clustering pattern (Al-Yousuf et al., 2000).
In contrast, the intestine was identified as the most distinct organ in terms of metal accumulation, joining the cluster at the highest distance. This unique positioning suggests that the intestine accumulates metals in a manner substantially different from the other organs. One possible explanation is the direct ingestion and absorption of metal-contaminated food and sediments, leading to localized accumulation patterns that differ from those observed in gills, muscles, and liver. The role of the intestine in digestion and metal absorption, coupled with variations in metal-binding affinity and transport mechanisms, could contribute to its distinct clustering (Kraal et al., 1995). These findings align with previous studies indicating that metal accumulation patterns vary across organs due to exposure route, metabolic activity, and detoxification mechanisms (Dallinger et al., 1997; Romeo et al., 1999). Considering that this Ennore is a well-frequented fishing harbor, continuous monitoring is crucial to evaluate metal levels, develop effective strategies to protect marine ecosystems, and aid in coastal conservation and sustainable management of fisheries (Murugan et al., 2005; Vijayakumaran et al., 2005; Kumar et al., 2009; Jha et al., 2017). Future research should investigate the specific metal-binding mechanisms in each organ, the impact of environmental factors on metal bioaccumulation, and the potential health risks for aquatic organisms and their predators within the food chain.
Conclusion
The study highlights significant variations in metal concentrations across different fish species and organs, with Fe and Al being the most abundant metals detected. The lower concentration of toxic metals such as Pb, and Cd suggest minimal contamination, reinforcing the safety of consuming these fish species. Species-specific differences in metal accumulation indicate that Snapper accumulates higher concentrations of most metals, while Sardines show elevated Fe, Al, and Zn, and Mackerel exhibits the lowest metal levels. These variations are likely influenced by dietary habits, habitat, and metabolic factors. The organ-specific analysis further reveals that the intestine accumulates Fe, Al, and Cr, the liver is associated with Pb and Cu, and muscles show minimal metal variability, supporting their role in metal detoxification and storage. Statistical validation through ANOVA and PCA confirms significant variations in metal distribution, with PCA explaining 79.2% of the total variance, emphasizing the distinct roles of fish organs in metal metabolism. Overall, the findings suggest that while metal contamination in these fish species remains within safe limits, ongoing monitoring is essential to assess potential risks from environmental pollution and anthropogenic activities.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
RD: Formal analysis, Writing – original draft, Data curation, Methodology, Investigation. VP: Methodology, Investigation, Validation, Writing – original draft, Software, Formal analysis. DJ: Writing – original draft, Writing – review & editing, Validation, Supervision, Project administration, Conceptualization. SR: Validation, Writing – review & editing, Supervision. GD: Validation, Project administration, Writing – review & editing. BR: Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors are thankful to the Director, NIOT, Chennai, for allowing the student internship project at NIOT. First author thanks Bharathidasan University for allowing him to do an internship at NIOT. Further, the authors thank the scientific and supporting staff of MBT Group, Chennai, India, for their timely help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1634855/full#supplementary-material
References
1
Al-Yousuf M. H. El-Shahawi M. S. Al-Ghais S. M. (2000). Trace metals in liver, skin, and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci. Total Environ.256, 87–94. doi: 10.1016/S0048-9697(99)00363-0
2
APHA (American Public Health Association) . (2005). “Standard Methods for the Examination of Water and Wastewater,” in American Works Association and Water Environmental Federation, (APHA-AWWA-WEF) 21th Edition (Washington, D.C).
3
Asuquo F. E. Ewa-Oboho I. Asuquo E. F. Udo P. J. (2004). Fish species used as biomarker for heavy metal and hydrocarbon contamination for cross river, Nigeria. Environmentalist24, 29–37. doi: 10.1023/B:ENVR.0000046344.04734.39
4
Bagheri S. Gholamhosseini A. Hoseinifar S. H. Banaee M. (2024). Investigation of the effects of heavy metals (Copper, cobalt, manganese, selenium, and zinc) on fish immune systems – an overview. Ann. Anim. Sci.24, 1025–1035. doi: 10.2478/aoas-2024-0017
5
Batvari Dass B. P. Kamala-Kannan S. Shanthi K. Krishnamoorthy R. Lee K. J. Jayaprakash M. (2008). Heavy metals in two fish species (Carangoidel malabaricus and Belone stronglurus) from Pulicat Lake, North of Chennai, Southeast Coast of India. Environ. Monit Assess.145, 167 175. doi: 10.1007/s10661-007-0026-3
6
Bawuro A. A. Voegborlo R. B. Adimado A. A. (2018). Bioaccumulation of heavy metals in some tissues of fish in lake geriyo, adamawa state, Nigeria. J. Environ. Public Health, 854892. doi: 10.1155/2018/1854892
7
Bryliński Ł. Kostelecka K. Woliński F. Duda P. Góra J. Granat M. et al . (2023). Aluminum in the human brain: routes of penetration, toxicity, and resulting complications. Int. J. Mol. Sci.24, 7228. doi: 10.3390/ijms24087228
8
Campenhout K. V. Infante H. G. Adams F. Blust R. (2004). Induction and binding of cd, cu, and zn to metallothionein in carp (Cyprinus carpio) using HPLC-ICP-TOFMS. Toxicological Sci.80, 276–287. doi: 10.1093/toxsci/kfh149
9
Canli M. Atli G. (2003). The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. pollut.121, 129–136. doi: 10.1016/S0269-7491(02)00194-X
10
Chahid A. Hilali M. Benlhachimi A. Bouzid T. (2014). Contents of cadmium, mercury and lead in fish from the Atlantic sea (Morocco) determined by atomic absorption spectrometry. Food chemistry. 147, 357–360. doi: 10.1016/j.foodchem.2013.10.008
11
Chevreuil M. Carru A. M. Chesterikoff A. Boët P. Tales E. Allardi J. (1995). Contamination of fish from different areas of the river Seine (France) by organic (PCB and pesticides) and metallic (Cd, Cr, Cu, Fe, Mn, Pb and Zn) micropollutants. Sci. Total Environ.162, 31–42. doi: 10.1016/0048-9697(95)04335-X
12
Compeau G. C. Bartha R. (1985). Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl. Environ. Microbiol.50, 498–502. doi: 10.1128/aem.50.2.498-502.1985
13
Cortada U. Collin M . (2013). The nature and contamination of sediments in the Plentzia estuary (Biscay province, Spain). Geogaceta54, 147–150.
14
Codex Alimentarius Commission . (2024). General standard for contaminants and toxins infood and feed (CXS 193-1995, Rev. 2024). Food and Agriculture Organization of the UnitedNations / World Health Organization. Available online at: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?
15
Dallinger R. Egg M. Köck G. Hofer R. (1997). The role of metallothionein in cadmium accumulation of Arctic char (Salvelinus alpinus) from high alpine lakes. Aquat. Toxicol.38, 47–66. doi: 10.1016/S0166-445X(96)00840-5
16
Dural M. Lugal Göksu M. Z. Özak A. A. Derici B. (2006). Bioaccumulation of some heavy metals in different tissues of dicentrarchus labrax L 1758, sparus aurata L 1758 and mugil cephalus L 1758 from the çamlIk lagoon of the eastern cost of mediterranean (Turkey). Environ. Monit Assess.118, 65–74. doi: 10.1007/s10661-006-0987-7
17
El-Moselhy K. M. Othman A. I. Abd El-Azem H. El-Metwally M. E. A. (2014). Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egyptian J. Basic Appl. Sci.1, 97–105. doi: 10.1016/j.ejbas.2014.06.001
18
Hakimelahi M. Savari A. Doustshenas B. Ghodrati Shojaei M. Lewis K. A. (2020). Food and feeding habits of Indian mackerel (Rastrelliger kanagurta) in the southern part of Qeshm Island, Persian Gulf. Iranian J. Fisheries Sci.19, 563–573. doi: 10.22092/IJFS.2018.120058
19
Heath A. G . (1995). Water Pollution and Fish Physiology. 2nd Edition, Lewis Publisher. New York and London, 359.
20
Jayaprakash M. Kumar R. S. Giridharan L. Sujitha S. B. Sarkar S. K. Jonathan M. P. (2015). Bioaccumulation of metals in fish species from water and sediments in macrotidal Ennore creek, Chennai, SE coast of India: A metropolitan city effect. Ecotoxicology Environ. Saf.120, 243–255. doi: 10.1016/j.ecoenv.2015.05.042
21
Jha D. K. Dharani G. Verma P. Ratnam K. Senthil Kumar R. Rajaguru S. (2021). Evaluation of factors influencing the trace metals in Puducherry and Diu coasts of India through multivariate techniques. Mar. pollut. Bull.167, 2021. doi: 10.1016/j.marpolbul.2021.112342
22
Jha D. K. Rajaprabhu G. Kirubagaran R. Sendhil Kumar R. Dharani G. Das A. K. (2017). Estimation of potential zones for offshore mariculture in the Indian Sea using geographical information system as a management tool. J. Coast. Conserv.21, 893–902. doi: 10.1007/s11852-017-0556-y
23
Jha D. K. Pandey V. Muthukumar C. Sathish Kumar P. Venkatnarayanan S. Jebakumar J. P. P. et al . (2022) Investigation of Coastal Water Characteristics Along the Southeast Coast of India: A Multivariate Approach. Front. Mar. Sci. 9:945495. doi: 10.3389/fmars.2022.945495
24
Jha D. K. Ratnam K. Rajaguru S. Dharani G. Devi M. P. Kirubagaran R. (2019). Evaluation of trace metals in seawater, sediments, and bivalves of Nellore, southeast coast of India, by using multivariate and ecological tools. Mar. pollut. Bull.146, 1–10. doi: 10.1016/j.marpolbul.2019.05.044
25
Koduvayur M. V. Vasudevan S. Pandey V. Santhanakumar J. Jha D. K. Dharani G. (2022). Comparative evaluation of heavy metal concentration in different organs of the Asian seabass: a multivariate approach. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.1012541
26
Kraal M. H. Kraak M. H. De Groot C. J. Davids C. (1995). Uptake and tissue distribution of dietary and aqueous cadmium by carp (Cyprinus carpio). Ecotoxicology Environ. Saf.31, 179–183. doi: 10.1006/eesa.1995.1060
27
Kumar P. S. Venkatnarayanan S. Pandey V. Ratnam K. Kumar D. Jha S. et al . (2022) Atmanand, Multivariate approach to evaluate the factors controlling the phytoplankton abundance and diversity along the coastal waters of Diu, northeastern Arabian Sea, Oceanologia, 64 (2), 267–275, doi: 10.1016/j.oceano.2021.11.005
28
Kumar T. S. Vijayakumaran M. Murugan T. S. Jha D. K. Sreeraj G. Muthukumar S. (2009). Captive breeding and larval development of the scyllarine lobster petrarctusrugosus, New Zealand. J. Mar. Freshw. Res.43, 101–112. doi: 10.1080/00288330909509985
29
Medeiros R. J. dos Santos L. M. G. Freire A. S. Santelli R. E. Braga A. Krauss T. M. et al . (2012). Determination of inorganic trace elements in edible marine fish from Rio de Janeiro State, Brazil. Food Control23, 535–541. doi: 10.1016/j.foodcont.2011.08.027
30
Murugan T. S. Remany M. C. Mary Leema T. Jha D. K. Santhanakumar J. Vijayakumaran M. et al . (2005). Growth, repetitive breeding, and aquaculture potential of the spiny lobster, panulirus ornatus, New Zealand. J. Mar. Freshw.39, 311–315. doi: 10.1080/00288330.2005.9517311
31
Olsson P. E. Kling P. Hogstrand C. (1998). “Mechanisms of heavy metal accumulation and toxicity in fish,” in Metal Metabolism in Aquatic Environments. Eds. LangstonW. J.BebiannoM. J. (Springer, Boston, MA). doi: 10.1007/978-1-4757-2761-6_10
32
Otchere F. A. (2019). A 50-year review on heavy metal pollution in the environment: Bivalves as bio-monitors. Afr. J. Environ. Sci. Technol.13, 220–227. doi: 10.5897/ajest2018.2597
33
Pandey V. Jha D. K. Kirubagaran R. Dharani G. (2024). Assessment of metal-associated health risk in different trophic levels in a tropical estuary on the southeast coast of India. Mar. Environ. Res., 106772. doi: 10.1016/j.marenvres.2024.106772
34
Pandey V. Jha D. K. Kumar P. S. Santhanakumar J. Venkatnarayanan S. Prince Prakash Jebakumar J. et al . (2022). Effect of multiple stressors on the functional traits of sub-tidal macrobenthic fauna: a case study of the southeast coast of India. Mar. pollut. Bull.175, 113355. doi: 10.1016/j.marpolbul.2022.113355
35
Pandey V. Venkatnarayanan S. Sathish Kumar P. Ratnam K. Jha D. K. Rajaguru S. et al . (2021). Assessment of ecological health of Swarnamukhi river estuary, southeast coast of India, through AMBI indices and multivariate tools. Mar. pollut. Bull.164, 112031. doi: 10.1016/j.marpolbul.2021.112031
36
Ragsdale S. W. (2009). Nickel-based enzyme systems. J. Biol. Chem.284, 18571–18575. doi: 10.1074/jbc.R900020200
37
Rainbow P. S. (2002). Trace metal concentrations in aquatic invertebrates: Why and so what? Environ. pollut.120, 497–507. doi: 10.1016/S0269-7491(02)00238-5
38
Raja P. Veerasingam S. Suresh G. Marichamy G. Venkatachalapathy R. (2009). Heavy metals concentration in four commercially valuable marine edible fish species from Parangipettai coast, south-east coast of India. Int. J. Anim. Vet. Adv.1, 10–14.
39
Romeo M. Siau Y. Sidoumou Z. Gnassia-Barelli M. (1999). Heavy metal distribution in different fish species from the Mauritania coast. Sci. Total Environ.232, 169–175. doi: 10.1016/S0048-9697(99)00099-6
40
Rzymski P. Tomczyk K. Rzymski P. Poniedziałek B. Opala T. Wilczak M. (2015). Impact of heavy metals on the female reproductive system. Ann. Agric. Environ. Med.22, 259–264. doi: 10.5604/12321966.1152077
41
Samantara M. K. Panigrahi S. Mohanty A. K. Sahu G. Mishra S. S. Palaniswami K. et al . (2023). Heavy metal concentration in marine edible fishes and associated health risks: an assessment from Tamil Nadu coast, Bay of Bengal. Environ. Chem. Ecotoxicology5, 193–204. doi: 10.1016/j.enceco.2023.09.002
42
Sathish Kumar P. Dharani G. Santhanakumar J. Jha D. K. Pandey V. Venkatnarayanan S. et al . (2023). Assessment of phytoplankton diversity, distribution, and environmental variables along the southeast coast of India. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1215627
43
Shah T. H. Chakraborty S. K. Kumar T. Sadawarte R. K. Sandhya K. M. (2019). Food and feeding habits of oil sardine Sardinella longiceps from Ratnagiri coast off Maharashtra India. Indian J. Geo Mar. Sci.48, 309–318.
44
Shah N. Khan A. Ali R. Marimuthu K. Uddin M. N. Rizwan M. et al . (2020). Monitoring bioaccumulation (in gills and muscle tissues), hematology, and genotoxic alteration in ctenopharyngodon idella exposed to selected heavy metals. BioMed. Res. Int.2020, 6185231. doi: 10.1155/2020/6185231
45
Shalini R. Jeyasekaran G. Shakila R. J. Arisekar U. (2021). Trace element concentrations in the organs of fish along the southeast coast of India. Mar. pollut. Bull.162, 111817. doi: 10.1016/J.MARPOLBUL.2020.111817
46
Shenoi S. S. C. (2024). Nurturing the blue economy: a call for sustainable ocean utilization. Curr. Sci.126, 119–120.
47
Switzer T. S. Chesney E. J. Baltz D. M. (2015). Habitat use by juvenile red snapper in the northern gulf of Mexico: ontogeny, seasonality, and the effects of hypoxia. Trans. Am. Fisheries Soc.144, 300–314. doi: 10.1080/00028487.2014.991447
48
Tuzen M. (2009). Toxic and essential trace elemental contents in fish species from the Black Sea, Turkey. Food Chem. Toxicol.47, 1785–1790. doi: 10.1016/j.fct.2009.04.029
49
Vasanthi L. A. Revathi P. Mini J. Munuswamy N. (2013). Integrated use of histological and ultrastructural biomarkers in Mugil cephalus for assessing heavy metal pollution in Ennore estuary, Chennai. Chemosphere91, 1156–1164. doi: 10.1016/j.chemosphere.2013.01.021
50
Verma P. Pandey V. Seleyi S. C. Alagarsamy A. Dharani G. (2024). Exploring the hidden treasures: Deep-sea bacterial community structure in the Bay of Bengal and their metabolic profile. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1308953
51
Vijayakumaran M. Murugan T. S. Remany M. C. Mary Leema T. Jha D. K. Santhanakumar J. et al . (2005). Captive breeding of the spiny lobster, panulirus homarus, New Zealand. J. Mar. Freshw. Res.39, 325–334. doi: 10.1080/00288330.2005.9517313
52
World Health Organization (1989). Heavy metals Environmental Aspects (Geneva, Switzerland: Environ. Health Criteria), 85.
53
Yuvaraj P. Satheeswaran T. Damotharan P. Karthikeyan V. Jha D. K. Dharani G. et al . (2018) Evaluation of the environmental quality of Parangipettai, Southeast Coast of India, by using multivariate and geospatial tool. Marine Pollution Bulletin, 131, 239–247. doi: 10.1016/j.marpolbul.2018.04.022
54
Zaynab M. Al-Yahyai R. Ameen A. Sharif Y. Ali L. Fatima M. et al . (2022). Health and environmental effects of heavy metals. J. King Saud University-Science34, 101653. doi: 10.1016/j.jksus.2021.101653
55
Zhang J. Zhu L. Li F. Liu C. Yang Z. Qiu Z. et al . (2017). Heavy metals and metalloid distribution in different organs and health risk assessment for edible tissues of fish captured from Honghu Lake. Oncotarget8, 101672–101685. doi: 10.18632/oncotarget.21901
56
Zhao S. Feng C. Quan W. Chen X. Niu J. Shen Z. (2012). Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Mar. pollut. Bull.64, 1163–1171. doi: 10.1016/j.marpolbul.2012.03.023
57
Zhong W. Zhang Y. Wu Z. Yang R. Chen X. Yang J. et al . (2018). Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicol. Environ. Saf.157, 343–349. doi: 10.1016/j.ecoenv.2018.03.048
Summary
Keywords
heavy metals, bioaccumulation, fish organs, metal toxicity, public health
Citation
Dinesh Kumar R, Pandey V, Jha DK, Rajakumar S, Dharani G and Ramakrishnan B (2025) Comparative evaluation of heavy metal concentration in three commercially important fish: insights from organ-specific and interspecies variability. Front. Mar. Sci. 12:1634855. doi: 10.3389/fmars.2025.1634855
Received
25 May 2025
Accepted
15 August 2025
Published
11 September 2025
Volume
12 - 2025
Edited by
Edison Barbieri, Agência de Agronegócio e Tecnologia de São Paulo (APTA), Brazil
Reviewed by
Ganesh Thiruchitrambalam, Pondicherry University, India
Sharali Sharma, Chandigarh University, India
Updates
Copyright
© 2025 Dinesh Kumar, Pandey, Jha, Rajakumar, Dharani and Ramakrishnan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dilip Kumar Jha, dilipjhaniot@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.