Abstract
Understanding premature senescence in macroalgae is essential for progress in marine biology, ecosystem management, and sustainable aquaculture. This mini-review addresses four key and interconnected dimensions: (i) the pivotal role of photosynthesis in mediating seaweed responses to environmental stress and its influence on delaying or inducing premature senescence; (ii) the taxon-specific yet environmentally modulated biochemical profiles that collectively shape seaweed lifespan; (iii) the dynamic interactions between seaweeds and their associated microbiomes, and how these holobiont relationships contribute to host resilience and longevity; and (iv) the importance of understanding how environmental factors trigger premature senescence, alongside the current state of research on the disciplines involved. Although studies remain limited -particularly regarding how macroalgal holobionts are reshaped in terms of stability and interaction with their environment-senescence has been documented in several macroalgal species. Nevertheless, it is essential to broaden the holobiont approach, particularly in long-lived taxa, such as large brown algae and coralline red algae. This mini-review advocates for a multidisciplinary approach to unravel the mechanisms governing macroalgal aging and premature senescence. This approach should integrate physiology, biochemistry, microbial ecology, and environmental science, while also accounting for factors such as genetic regulation and reproductive strategies, in order to better-understand seaweed premature senescence-whether for management, aquaculture, or fundamental research.
Graphical Abstract
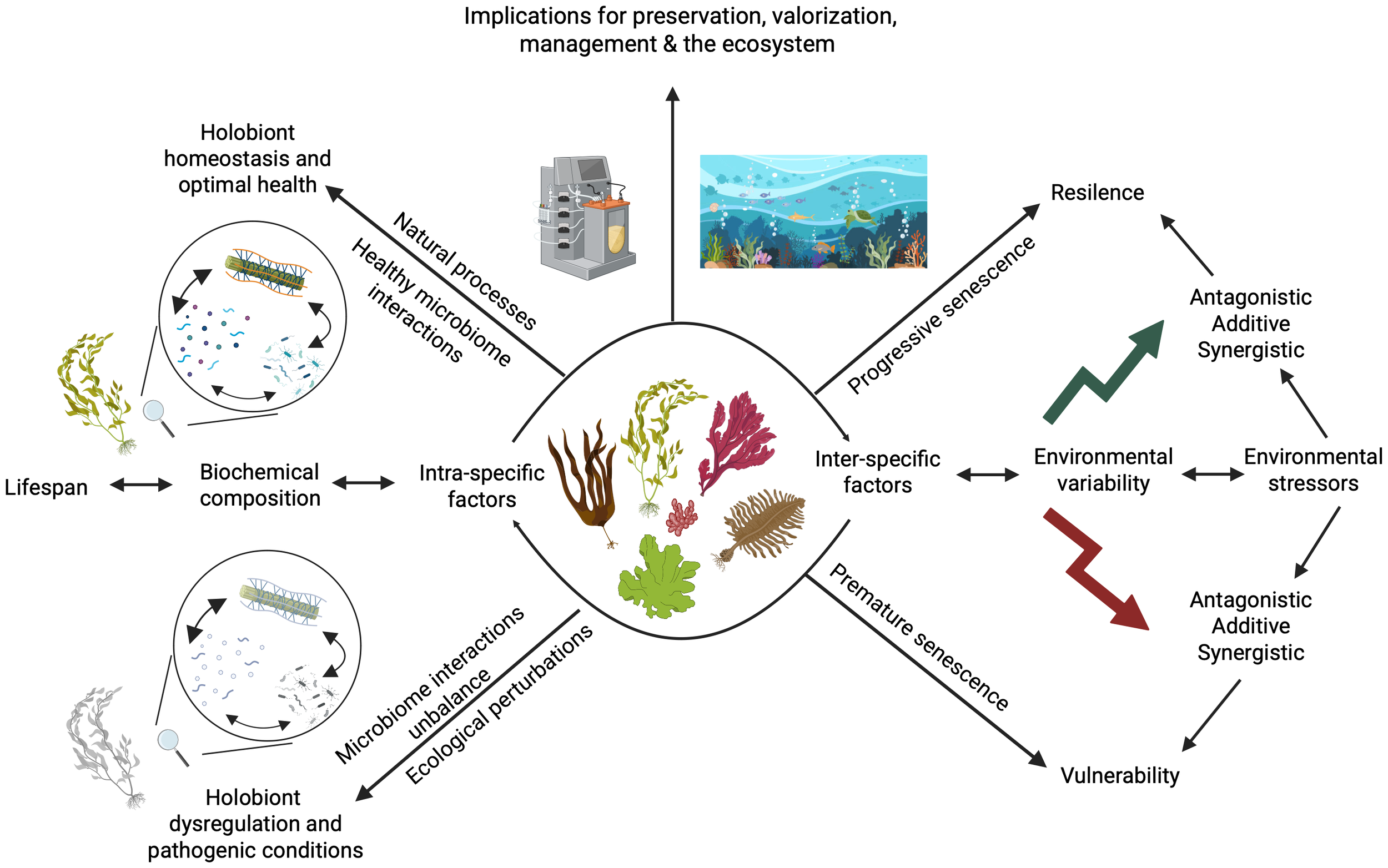
The high diversity among seaweed species necessitates consideration of intraspecific factors to understand how biochemical composition and lifespan are influenced by interactions within the holobiont. Similarly, interspecific factors—such as environmental variability and stressors (whether additive, synergistic, or antagonistic)—also shape holobiont dynamics. These interactions, in turn, determine the resilience or vulnerability of the seaweed holobiont, influencing whether it maintains optimal health or shifts toward dysregulation and pathogenic conditions, ultimately leading to progressive or premature senescence. These aspects have important implications for seaweed conservation, sustainable valorization, and ecosystem-based management. The figure was created with Biorender. Qui-Minet, (2025)https://BioRender.com/w1zjnkm.
1 Introduction
Seaweeds comprise a heterogeneous, polyphyletic assemblage of photosynthetic aquatic organisms that contribute significantly to ecosystem functioning and services in marine environments. They are divided into three main groups: green algae (Chlorophyta), red algae (Rhodophyta), and brown algae (Phaeophyceae). At present, an estimated 12,155 seaweed species have been described (Guiry, 2023), with red algae representing the most diverse clade. Seaweed lifespans exhibit considerable variability, ranging from ephemeral species lasting only a few weeks to long-lived perennials that persist for years or even centuries, as observed in some red coralline algae (Halfar et al., 2007; Liu et al., 2017). Interestingly, slower-growing macroalgae with lower photosynthetic rates tend to live longer than fast-growing species with higher photosynthetic activity (Reich et al., 1999; Migné et al., 2025). This trend is exemplified in maerl beds, where slow-growing coralline algae, capable of living for decades or even centuries, exhibit primary production rates per gram up to 100 times lower than those of their fast-growing, short-lived fleshy epiphytes (Qui-Minet et al., 2022). This contrast underscores a fundamental ecological trade-off: long-lived species prioritize structural robustness and persistence, while ephemerals and annuals favor rapid growth and reproduction, often at the expense of longevity (Liu et al., 2017). This pattern can be observed within the same species depending on the life-cycle stage; for example, in Gracilaria chilensis (previously known as Agarophyton chilense), haploids invest more in reproduction, whereas diploids grow larger and persist longer (Guillemin et al., 2013; Vieira et al., 2021).
Although intraspecific and geographic variation in seaweed lifespan has been documented across several taxa and environments, comprehensive cross-taxon datasets remain scarce (Table 1), leaving our understanding incomplete, notably when it comes to perennial seaweed species. For instance, the brown seaweed Laminaria hyperborea exhibit markedly greater longevity in UK waters compared to populations in Norway (Kain, 1979; Rinde and Sjøtun, 2005; de Bettignies et al., 2020), emphasizing the influence of local environmental conditions on seaweed lifespan. Persistence is determined not only by intrinsic biological traits but also by the surrounding environmental context. Similarly, the longevity of holopelagic Sargassum spp. remains largely uncertain (Magaña-Gallegos et al., 2023; Schell et al., 2024), although these species are consistently observed to degrade rapidly upon reaching Caribbean coastlines (Rodríguez-Martínez et al., 2019; Stiger-Pouvreau et al., 2023; Resiere et al., 2025). The mechanisms driving their rapid decay are not yet fully understood, but are thought to involve shifts in surface irradiance, nutrient availability, and hydrodynamic conditions, all of which also alter the composition of the thalli-associated microbiome (Theirlynck et al., 2023; Mendonça et al., 2024; Debue et al., 2025).
Table 1
| Longevity | Type | Species | Lifespan | Status | References |
|---|---|---|---|---|---|
| Perennial | Brown | Laminaria hyperborea | From 3–4 years (Ireland) up to 20 years (northern Europe) | Regional pressures | (Soler-Vila et al., 2022) |
| Fucus vesiculosus | 4–6 years | Regional pressures | (Hill, 2008) | ||
| Laminaria ochroleuca | No data | Declining due to trawling | (Barrientos et al., 2022) | ||
| Laminaria digitata | 4–6 years | Declining | (Smale et al., 2013) | ||
| Macrocystis pyrifera | 1–10 years | Not classified as threatened but affected by global change | (van Tussenbroek, 1989) | ||
| Ecklonia radiata | 2–10 years | Not threatened | (Novaczek, 1981) New Zealand Threat Classification System (NZTCS |
||
| Ascophyllum nodosum | Fronds:10–15 years. Hold-fast:40–60 years |
Not threatened | Marlin UK | ||
| Ecklonia cava | 3–5 years | Not classified as threatened but affected by global change | (Kim et al., 2018; Choi et al., 2024) | ||
| Red | Maerl spp. | >100 years | Threatened | (Foster, 2001) EU Habitats Directive (Annex V) and OSPAR Convention |
|
| Chondrus crispus | 2–6 years | Not threatened | Marlin UK | ||
| Furcellaria lumbricalis | Up to 10 years | Depends on location. Indicator of ecological status. |
Marlin UK | ||
| Ceramium virgatum | 3–5 years | Not threatened | Marlin UK | ||
| Gracilaria chilensis | 2–3 years | Threatened due to overexploitation | (Vieira et al., 2018) | ||
| Acanthophora spicifera | No data | Invasive | (Weijerman et al., 2008) | ||
| Sarcothalia radula* | No data | Not threatened | (Nelson et al., 2019) | ||
| Green | Codium fragile | 1–3 years | Invasive | (Garbary et al., 2004) | |
| Perennial/Annual | Brown | Saccharina latissima | Holdfast: 2–5 years Blade: 1 year |
Declining | (Smale et al., 2013) |
| Sargassum muticum | Holdfast and main axis: + 3–4 years Laterals: 1 year |
Invasive | (Wernberg et al., 2001) | ||
| Annual | Brown | Undaria pinnatifida | 1 year | Invasive | (Epstein and Smale, 2013) |
| Red | Asparagopsis armata | 1 year | Invasive | (Pinteus et al., 2021) | |
| Palmaria palmata | 1 year | Not threatened | Marlin UK | ||
| Green | Chaetomorpha spp. | 1 year | Invasive | (Vranken et al., 2023) | |
| Ephemeral | Brown | Ectocarpus siliculosus | 6 weeks | Not threatened | (Peters et al., 2004) |
| Red | Pyropia yezoensis | 1 year | Not threatened | (Chen et al., 2012) | |
| Porphyra spp. | 2–4 months | Opportunistic and resilient | (Tillin and Budd, 2016) | ||
| Ceramium spp. | No data | Varies across regions | (Bunker et al., 2017) | ||
| Green | Ulva spp. | Few months | Invasive | (Obolski et al., 2022) | |
| Bryopsis plumosa | 7 weeks | Non-invasive, not threatened | (Rietema, 1970) |
Representative examples of brown, red, and green macroalgae classified as perennial, annual, or ephemeral taxa, with their current ecological status.
Annual/perennial refers to species that persist through a perennial holdfast and main axis but regenerate a new laterals.
*Previously known as Sarcothalia lanceata.
To survive in such dynamic environments, marine macroalgae must constantly adapt to fluctuating abiotic conditions, such as light availability, salinity, wave exposure and temperature, as well as biotic stressors like herbivory, pathogenic attacks, and intra (Scrosati and DeWreede, 1998; Scrosati, 2005; Creed et al., 2019) and interspecific competition (Küpper et al., 2002; Lalegerie et al., 2020). These pressures interact with each species’ biochemical traits and defense mechanisms, ultimately shaping their physiological performance and lifespan. When stress thresholds are exceeded, premature senescence may be triggered, reducing individual longevity and affecting ecosystem-level processes. At high densities, intraspecific competition for limiting resources, such as light, nutrients and space does not exclusively involve adults; high macroalgal mats can inhibit the germination of sporelings and the growth of juveniles (Scrosati and DeWreede, 1998; Scrosati, 2005).
While some regional studies have documented seaweed lifespans (Parke, 1948; North, 1961; Kain, 1979; Novaczek, 1981; Chapman, 1993; Pedersen et al., 2012), a growing body of research highlights the promising role of the seaweed microbiome in modulating longevity, particularly through host–microbiome interactions that respond dynamically to environmental variability (Dittami et al., 2016; Ghaderiardakani et al., 2022). It is now widely recognized that macroalgal health, development, and stress resilience are closely tied to their microbiome (Egan et al., 2013; van der Loos et al., 2019). Consequently, the influence of microbial communities—and their potential impacts on seaweed biochemistry and lifespan is a topic that needs to be further explored.
To fully understand patterns of senescence and degradation kinetics, it is crucial to adopt a holobiont perspective, wherein the macroalgal host and its associated microbiome (including bacteria, fungi and epiphytic microalgae) are studied as an integrated ecological unit (Egan et al., 2013). For this purpose, we define senescence as the process in which seaweed’s health deteriorates, ultimately leading to its death, and degradation as the subsequent remineralization of seaweed detritus.
Global and local environmental changes are increasingly disrupting holobiont dynamics, altering senescence and degradation kinetics across habitats. These disruptions are contributing to the decline of perennial species, many of which function as foundation species and ecosystem engineers (Wahl et al., 2015), while also promoting mass blooms of opportunistic taxa.
Understanding longevity of seaweed holobionts in the face of rapid environmental change demands a closer examination of their biochemical composition, especially the molecular mechanisms involved in delaying or resisting premature senescence. While considerable research has explored the biotechnological potential of seaweed-derived compounds, leading to the discovery of numerous bioactive molecules with industrial, nutraceutical, and pharmaceutical value (Stengel and Connan, 2015), the taxonomic and functional diversity of seaweeds challenges the efforts to unravel the physiological and ecological drivers of metabolite production and release. Key challenges include identifying the specific nutritional requirements for metabolite synthesis and elucidating the potential role of the associated microbiome in supporting or enhancing these biochemical processes. In addition, many potentially valuable or ecologically important compounds remain uncharacterized, underscoring the need for integrative approaches to seaweed holobionts biology and metabolomics.
This mini-review addresses: (i) the central role of photosynthesis in mediating responses to environmental stress and premature senescence; (ii) the biochemical profiles of seaweeds, which are taxon-specific yet shaped by environmental conditions, collectively influencing lifespan; (iii) the dynamic interplay between seaweeds and their associated microbiomes, and how these interactions affect host physiology resilience and longevity; and (iv) the significance of understanding how the environment triggers macroalgal premature senescence. Given the limited scope of this minireview, we will only highlight the importance of further research across the three macroalgal groups, with particular emphasis on long-lived species, especially brown and red coralline algae.
2 Photosynthesis as a key to understanding seaweed senescence
Senescence in macroalgae remains poorly characterized, yet it is fundamentally linked to aging of organisms as an intrinsic biological process, marked by a progressive decline in physiological function and ultimately leading to mortality. It can be prematurely induced by excessive or insufficient incident irradiance, nutritional imbalances and a range of biotic and abiotic stressors (Smith and Berry, 1986; Zheng and Gao, 2009; Mayta et al., 2019). In vascular plants, chloroplast redox signaling plays a pivotal role in the regulation of cell death, with reactive oxygen species (ROS) both preceding and accompanying natural senescence (Thomas et al., 2009; Mayta et al., 2019). As primary sites of ROS production, chloroplasts are central to redox homeostasis, and shifts in their redox status have been shown to significantly modulate the timing and progression of senescence (Mayta et al., 2019).
In macroalgae, ROS at low concentrations function as essential signaling molecules in various physiological pathways. However, under environmental stress, the delicate balance between ROS production and scavenging systems can be disrupted, leading to oxidative damage and impaired cellular functions, particularly photosynthesis (Rezayian et al., 2019). Reduced photosynthetic efficiency directly diminishes macroalgal performance and alters biochemical composition (Raven and Hurd, 2012). For example, desiccation induces oxidative stress and biochemical responses in the intertidal red alga Gracilaria corticata (Kumar et al., 2014). Similarly, intertidal fronds of Gracilaria dura, which are periodically exposed to excessive temperature and UV radiation, accumulate significantly higher ROS levels, exhibit reduced growth, and attain lower biomass compared to fronds than remain permanently submerged (Vieira et al., 2024). Thus, stressors that impair photosynthesis (Hurd et al., 2014), including nutrient limitation (Neill et al., 2018), may accelerate senescence by mimicking aging processes and triggering premature tissue degradation. Progressive senescence appears irreversible; however, premature senescence has been shown to be reversible in certain Ulva species, as evidenced by tissue recovery after extended UV exposure, desiccation, and darkness (Choi et al., 2011; Del Olmo et al., 2025). They can fully recover from visible signs of aging after being kept in total darkness for up to 41 days (Markager and Sand-Jensen, 1990). To our knowledge, there is little direct evidence of premature senescence reversal in red or brown seaweeds, though its occurrence cannot be ruled out. Notably, some brown seaweeds, like Dictyota dichotoma, exhibit tissue regeneration and repair following damage (Tanaka et al., 2017). Additionally, de Bettignies et al (de Bettignies et al., 2020) reported ongoing photosynthetic activity and reproductive structures development in degrading thalli, indicating that some physiological functions may persist even during late stages of tissue degradation. In red seaweeds, examples of premature senescence reversal have been evidenced such as in Gracilaria chilensis after a salinity and/or light stress (Guillemin et al., 2013; Vieira et al., 2021).
Senescence encompasses a suite of biological processes that progressively impair cellular, tissue, and whole-organisms function. In macroalgae, these processes occur at multiple organizational levels and are likely to differ among the three macroalgal groups, due to divergent genetic, metabolic and ecological adaptations. Distinct pigment compositions and photosynthetic apparatus among these groups influence their respective capacities to cope with oxidative stress and shape broader physiological responses (Rezayian et al., 2019). These differences are especially evident in their tolerance to high irradiance and ultraviolet (UV) radiation, which can be further exacerbated by additive or synergistic environmental stressors such as nutrient deprivation and salinity changes (Pereira et al., 2017). For instance, nitrogen deprivation in Pyropia yezoensis has been shown to impair photosynthesis and accelerate pigment degradation, leading to tissue discoloration and early onset of senescence (Li et al., 2019).
Macroalgal thallus complexity influences senescence dynamics. The distinct evolutionary origins of brown, red, and green seaweeds lead to different strategies for resource allocation across life stages (Scrosati et al., 2020; Vieira et al., 2024), particularly under environmental stress and during senescence (Figure 1A). Brown seaweeds are among the largest macroalgal species that require substantial energy investment to maintain key thallus parts. They are the only group to have evolved a plant-like body plan and a specialized, phloem-like transport network (Drobnitch et al., 2005). Brown seaweeds also include a higher proportion of long-lived thalli compared with red and green seaweeds, exposing them repeatedly to herbivory, hydrodynamic forces, and biofouling by micro- and macroorganisms. As these structures age, maintenance costs increase while functional efficiency declines, rendering them progressively more vulnerable to environmental stressors (Rodriguez et al., 2013a; Cao et al., 2020).
Figure 1
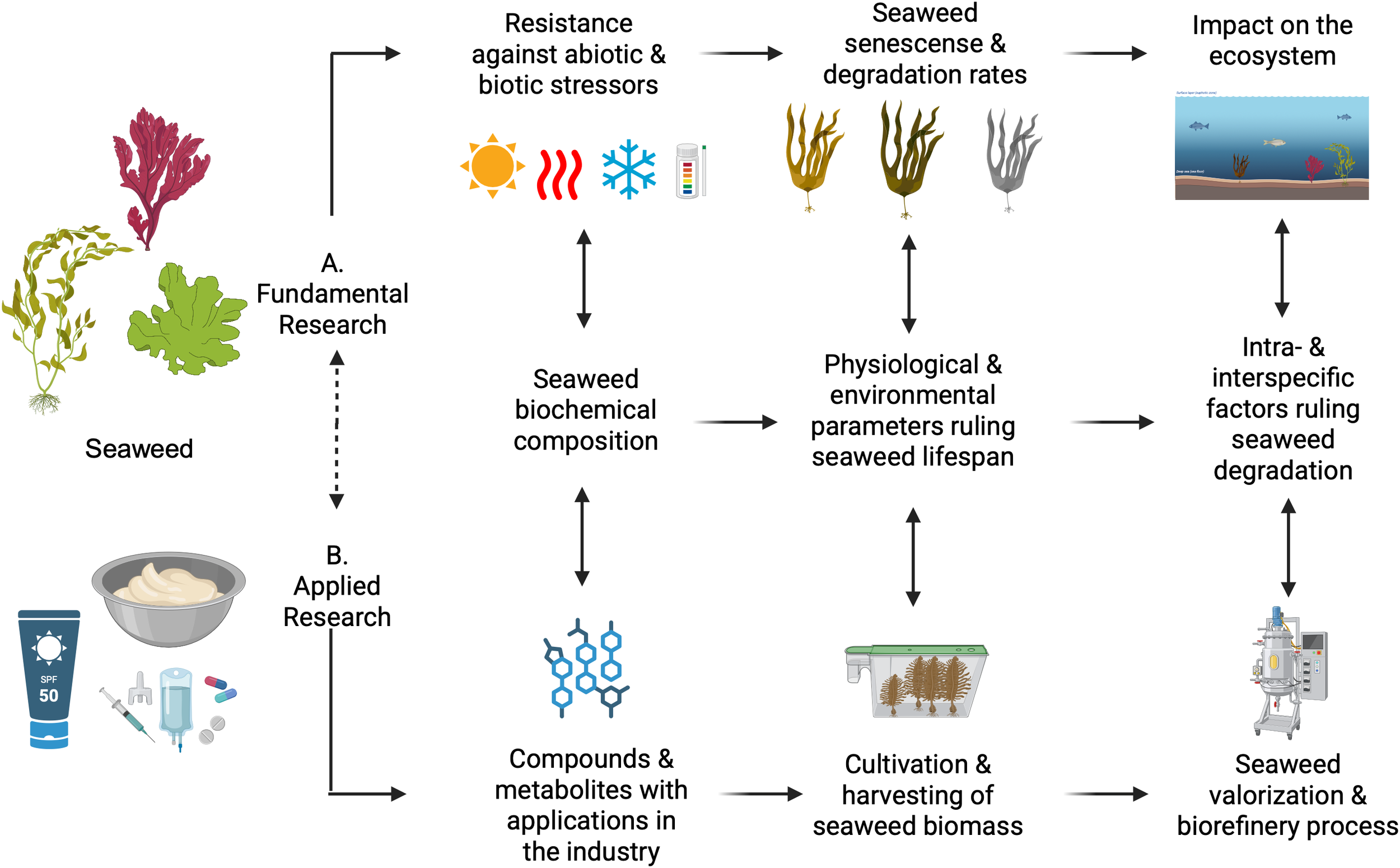
Fundamental (A) and applied (B) research are closely interconnected. First, understanding the biochemical composition of seaweeds is essential for characterizing taxa and for assessing how this composition influences the physiological and environmental factors that govern seaweed lifespan. These factors, in turn, shape the intra- and interspecific dynamics involved in seaweed degradation. Together, these three aspects provide a foundation for addressing key scientific questions from both fundamental and applied perspectives. The figure was created with Biorender. The following licences were obtained: Figure 1. Created in Biorender. Qui-Minet, (2025)https://BioRender.com/bblkl0q.
A central question in macroalgal ecology is the extent to which senescence is driven by endogenous factors (progressive senescence) versus exogenous environmental pressures (premature senescence), including interactions between the two, such as those mediated by the host’s metabolome and its associated microbiome. Progressive senescence likely predominates under relatively stable conditions, whereas environmental disturbances exceeding seaweed tolerance thresholds - such as extreme changes in certain physicochemical parameters - can accelerate senescence and markedly influence macroalgal population dynamics (Pommerville and Kochert, 1981; Mayta et al., 2019; Li et al., 2020). Furthermore, the lifespan of perennial species can vary considerably between locations, suggesting that non-extreme abiotic and biotic interactions modifying seaweed photosynthesis, modulate the holobiont compartment interactions, thereby affecting fitness and shaping longevity.
3 Biochemical composition of seaweed as protection against decay: cell wall and metabolites
Seaweed cell walls, which account for 30-70% of dry weight (DW), serve as the primary protective barrier against environmental stressors such as desiccation, high irradiance, hydrodynamic forces, and pathogenic attacks (Popper et al., 2011; Stengel et al., 2011; Vera et al., 2011; Synytsya et al., 2015; Stiger-Pouvreau et al., 2016; Lee and Ho, 2021). Each macroalgal group synthesizes distinct polysaccharides that contribute to both fibrillar components (e.g. cellulose) and matrix-associated polysaccharides of the cell wall, exhibiting marked structural and functional differences across phylogenetic lineages (Vera et al., 2011; Stiger-Pouvreau et al., 2016; Alzate-Gaviria et al., 2021). Polysaccharide composition is genus-dependent. Accordingly, depending on species and environmental conditions, brown seaweeds contain 17 to 45% DW of alginate and 5 to 20% DW of fucoidans (Kloareg and Quatrano, 1988). Red seaweeds typically contain 30-75% DW as either agars or carrageenans. Agar is mainly found in Gelidium, Gracilaria, Pterocladia and Gelidiella, while carrageenans are predominant in Kappaphycus, Eucheuma, Chondrus, Gigartina and Hypnea (Vera et al., 2011; Synytsya et al., 2015). In green algae, Ulva spp. contain 8-29% DW of ulvans, while Monostroma, Caulerpa and Codium contain variable percentages of rhamnans, galactans and arabinogalactans, respectively (Wang et al., 2014).
Cell wall composition is not static; it is highly dynamic and modulated by both intra-specific factors—such as life stage and physiological condition—and interspecific factors, including a range of abiotic and biotic environmental conditions (Kloareg and Quatrano, 1988; Popper et al., 2014; Stiger-Pouvreau et al., 2016; Lee and Ho, 2021). Key structural features-such as polysaccharide architecture, cross-linking density, cellulose content, and degree of sulfatation- govern flexibility and mechanical resistance of thalli (Mach et al., 2007a; Mach et al., 2007b; Vieira et al., 2021). These traits are essential for maintaining tissue integrity under stress and underpin species-specific resilience to desiccation, light exposure, salinity changes and pathogen pressure. In red seaweeds, carrageenans have a higher sulfate content when the seaweeds are grown in colder or cooler conditions (autumn, winter, or rainy season) compared to hotter conditions (summer or dry season) (Marinho-Soriano and Bourret, 2003). In the same manner, several correlations have been made between cell wall sulfatation level of brown macroalgae and the duration of emersion (Kloareg et al., 2021). Changes in cell wall composition may also reflect imbalances due to nutrient or light limitation. Therefore, comparative analyses of co-occurring species with distinct biochemical architectures have provided insights into intra-species differences in stress resilience. For example, the red alga Gracilaria vermiculophylla is more resistant to desiccation, burrowing and grazing than the green alga Codium fragile, largely due to its more fibrous and robust cell wall (Thomsen and McGlathery, 2007) and the production of isethionic acid (Surget et al., 2017).
A decline in these structural properties is strongly associated with tissue aging and senescence. However, directly linking specific cell wall modifications to senescence patterns remains challenging due to the complexity of seaweed life-cycle in interaction with environmental heterogeneity and seasonal variability of coastal habitats. While cell wall architecture provides the first line of defense, long-term resilience in seaweeds also depends on a suite of dynamic biochemical mechanisms. These include both high-molecular-weight compounds embedded in the cell wall—such as structural proteins and certain lipids—as well as a diverse array of low-molecular-weight compounds (<1500 Da), including polyunsaturated fatty acids (PUFAs), phenolic compounds, organosulfur compounds, and mycosporine-like amino acids (Stengel et al., 2011). These molecules contribute significantly to tolerance against both environmental stress and senescence, and exhibit taxonomic specificity as well as spatio-temporal variation within species (Stengel et al., 2011). In macroalgae, senescence is tightly coupled to life-cycle dynamics, with growth rates and biochemical composition shifting markedly across stages and taxonomic groups (Valero et al., 1992; Liu et al., 2017; de Bettignies et al., 2018). Some brown and green species, like Fucus spiralis and Codium sp. present monogenetic life-cycles (Prince and Trowbridge, 2018; Hatchett et al., 2022). Otherwise, in other brown seaweeds, only the diploid sporophyte is macroscopic and long-lived, whereas in some green seaweeds both haploid (H) and diploid (D) stages are macroscopic and have comparable lifespans (De Reviers and De Reviers, 2022). Red seaweeds exhibit greater complexity, with biphasic cycles generally comprising macroscopic gametophytes (G), with some exceptions, and tetrasporophytes (T) of similar lifespans, or triphasic cycles that include a small carposporophyte stage on the female gametophyte (Thornber, 2006).
In some red seaweeds, life-cycle phases differ markedly in hydrocolloid composition: gametophytes mainly produce κ-carrageenans, while tetrasporophytes are rich in λ-carrageenans (McCandless et al., 1973). This has been mainly studied in Chondrus crispus, whose tetrasporophytes are especially valuable commercially because of the structural composition of their cell walls (Lipinska et al., 2020). Clearly, these biochemical traits not only enhance their economic significance but also support their survival and longevity in the ecosystems. Ecologically, differences in biochemical composition among life-stages manifest in nutrient content (and thus, nutrient uptake) to metabolite composition, such as halogenated compounds, with overall impacts on herbivore preferences (Vergés et al., 2008) and breakage under wave events (Mach et al., 2011; Vieira et al., 2021).
Beyond structural barriers, marine algal defense strategies rely heavily on shifts in the metabolome, particularly the activity of low-molecular-weight secondary metabolites or allelochemicals (Stengel and Connan, 2015; Ghaderiardakani et al., 2022). These compounds play essential roles in the organism survival, notably antifouling, herbivore deterrence, and antioxidant protection (Stengel et al., 2011; Vieira et al., 2024). For instance, proline, polyamines, small carbohydrates, polyols, oxylipins, and PUFAs can regulate ROS, and maintain redox homeostasis (Kumar et al., 2014; Vieira et al., 2018). The composition and diversity of these chemical defenses are shaped by both genetic background and environmental conditions, resulting in species- and habitat-specific metabolomic profiles (Sudatti et al., 2021). In Asparagopsis armata and Gracilaria chilensis, herbivore preference varies across life-cycle stages, with cystocarps on female thalli being least consumed because of their high concentration of deterrent secondary metabolites (Vergés et al., 2008; Vieira et al., 2018). By contrast, in green seaweeds, secreted metabolites are known to change over time and with developmental state (Grueneberg et al., 2016; Alsufyani et al., 2017; Alsufyani et al., 2020; Wichard, 2023). However, differences between haploid and diploid stages remain far less characterized than in red seaweeds. While proximal composition has been investigated in the context of aquaculture (Steinhagen et al., 2022), to our knowledge, differences in cell wall structure between life-cycle stages have not yet been characterized.
Although thousands of macroalgal compounds have been identified (Amsler et al., 2005; Maschek and Baker, 2007), the full extent of bioactive compounds remains to be verified. For instance, the relatively recent discovery of the presence of saponines in seaweeds -notably in green and red species- highlights the undiscovered potential (Abbott et al., 2020). In addition, our understanding of how seaweed metabolism responds to environmental change continues to be an evolving area of research. These biochemical defenses operate within a broader metabolic network that includes dynamic metabolomic shifts and complex interactions with associated microbial communities (Dittami et al., 2014). Notably, growing evidence points to a strong complementarity between the surface metabolome of macroalgae and their associated microbiome (Wichard and Beemelmanns, 2018; Paix et al., 2020; Paix et al., 2021), which plays a critical role in defense strategies and may influence aging trajectories. This underscores the need for integrated studies of host–microbe metabolic networks.
In parallel, dissolved organic matter (DOM) dynamics -particularly the release of dissolved organic carbon (DOC)- represent a key physiological process intimately linked with these metabolic interactions. Shifts in DOM exudation can trigger premature or progressive senescence and affect degradation rates. Abiotic stressors such as high irradiance, suboptimal temperatures, salinity fluctuations, desiccation, and elevated dissolved CO2 levels significantly influence DOC exudation patterns (Paine et al., 2021). Green algae generally exhibiting the highest release rates, and red algae the least (Paine et al., 2021). However, the distinction between green and brown macroalgae remains unclear. For instance, Hall et al. (2022) reported lower exudation rates in Ulva pertusa under dark conditions, but higher rates in Fucus vesiculosus under light. Nonetheless, these studies do not account for thallus age (a particularly relevant factor for longer-lived species), which may influence exudation dynamics and confound interspecific comparisons. Interestingly, a global assessment of C:N ratios, comparing the three seaweed groups, displayed the highest C:N ratio for brown seaweeds and the lowest for red seaweeds (Sheppard et al., 2023), which may partly explain differences in carbon exudation rates.
4 Seaweeds as holobionts: the interaction with their microbiome shapes their lifespan
The seaweed microbiome -comprising bacteria, protozoa, fungi, microalgae and microscopic animals inhabiting algal surfaces or tissues- is dominated by bacteria, which represent about 90% of the community (Egan et al., 2013; Lage and Graca, 2016). Research has largely focused on bacterial interactions within the holobiont. Some bacterial families are shared across green, brown and red seaweeds (Florez et al., 2017), suggesting convergent host traits that support similar microbial communities (Weigel and Pfister, 2019). Seaweed cell walls and extracellular matrices - rich in polysaccharides - provide favorable niches that facilitate microbial colonization (Domozych, 2019).
The holobiont comprises a host and its associated symbionts, while the hologenome refers to their combined genetic reportoire (Theis et al., 2016). The hologenome theory states that hosts and their microbiome are interconnected, multipartite entities shaped by ecological, evolutionary, and genetic processes at multiple levels (Theis et al., 2016). Unlike the relatively stable host genome, microbial genomes are highly dynamic, capable of rapid changes through shifts in microbial abundance, acquisition of new partners, horizontal gene transfer or mutations (Rosenberg and Zilber-Rosenberg, 2018). These microbial dynamics are fundamental in seaweed adaptation to changing environments (Dittami et al., 2016; Ghaderiardakani et al., 2020) and likely to play a fundamental role in determining macroalgal longevity, a role that remains to be fully elucidated. Advances in molecular biology and metadata analysis are shedding light into host-microbiome co-evolution, revealing distinct evolutionary patterns among ephemeral (Ectocarpus subulatus), annual (Nereocystis luetkeana), and perennial (Ascophyllum nodosum) brown seaweed species (Pfister et al., 2025). Notably, unlike N. luetkeana and A. nodosum, the ephemeral E. subulatus shows no evidence that its associated bacteria have evolved to complement host metabolism (Pfister et al., 2025). Research investigating how holobiont evolutionary trajectories vary with host lifespan is only beginning to emerge (Pfister et al., 2025).
Perennial seaweeds typically harbor greater bacterial diversity than annual or ephemeral species, likely because their extended lifespans, allow for the development of mature, stable microbiomes and require resilience to seasonal and interannual fluctuations (Allison and Martiny, 2008; Lemay et al., 2018; Weigel and Pfister, 2019). For instance, the perennial kelp Macrocystis pyrifera supports richer microbial communities than the annual Nereocystis luetkeana (Weigel and Pfister, 2019), supporting the notion that perennial species act as reservoirs for microbial colonization (Bengtsson et al., 2010; Weigel and Pfister, 2019). Red coralline macroalgae, given their longevity, are expected to host particularly diverse microbiomes, yet diverse comparisons with fleshy species remain limited. Reports of lower diversity in corallines than in turf algae (Hochart et al., 2024) may reflect methodological biases: DNA extractions based on equal dry biomass underestimate microbial content in calcified species due to high carbonate content and reduced extraction efficiency. Nevertheless, long-living corallines such as Porolithon onkodes are recognized for relatively high and stable microbial diversity over time, in contrast to short-lived fleshy macroalgae like Ulva spp. (Cavalcanti et al., 2018; Yang et al., 2021; van der Loos et al., 2025). Comparisons within macroalgal groups or tissue types suggest longevity remains a key factor shaping microbial diversity (Wichard and Beemelmanns, 2018; Paix et al., 2021; Hall et al., 2022).
Under optimal synergistic conditions, microbial communities enhance host fitness. For instance, in young algae, oxidative stress can trigger the release of protective compounds, such as mannitol in Phaeophyceae, floridoside and isofloridoside in Rhodophyta, and sorbitol in Chlorophyta, which in turn support the growth of specific microbial taxa (Karsten et al., 1993; Salaün et al., 2012; Gao et al., 2014), that protect their host. Indeed, epiphytic algae and bacteria enhance host defense against abiotic stressors, pathogens and fouling organisms via complementary metabolic pathways (Dahms and Dobretsov, 2017; Dittami et al., 2019; van der Loos et al., 2019; Wichard and Beemelmanns, 2008), producing bioactive molecules such as terpenoids and osmolytes (Burgunter-Delamare et al., 2020; Menaa et al., 2020; Ghaderiardakani et al., 2022). Although the roles of holobiont-derived metabolites remain underexplored, integrative metabolomic and (meta)genomic approaches are beginning to reveal the mechanisms of these synergistic interactions (Burgunter-Delamare et al., 2020; Reverter et al., 2020; Ghaderiardakani et al., 2022).
Beyond species and group-specific traits, environmental conditions are major drivers of seaweed microbiome composition, as evidenced by seasonal and geographic variation (Burgunter-Delamare et al., 2023). Abiotic factors such as temperature, irradiance, and photoperiod strongly influence algal physiology by modulating photosynthesis, respiration, overall metabolism, and the compounds released by the macroalgal host (Eggert, 2012; Heinrich et al., 2015), potentially selecting for specific microbial taxa and reshaping microbiome composition (Figure 2). For instance, heterotrophic microbial communities may be favored under short photoperiods and dim light conditions. Algal activity also alters the physico-chemical environment of the diffusive boundary layer (DBL): photosynthesis elevates oxygen and pH during light periods, while respiration reduces them in darkness, creating dynamic microhabitats for microbial communities (Figure 2). The microbiome reciprocally influence DBL chemistry through photosynthesis (e.g., cyanobacteria, epiphytic microalgae) or respiration (e.g., heterotrophic bacteria and fungi), forming a feedback loop (Barer, 2012; Bengtsson et al., 2018; Mancuso et al., 2023a; Qui-Minet et al., 2025). The broad microbial tolerance to physicochemical parameters (e.g., pH, salinity, temperature, and carbon sources), often accompanied by shifts in metabolomic expression, can further affect host physiology (Figure 2). Depending on the magnitude and direction of environmental variability, these interactions may reinforce mutualism or drive dysbiosis, impacting host health, performance and senescence (van der Loos et al., 2019; Abdul Malik et al., 2020; Ghaderiardakani et al., 2020). For instance, bacterial partners have been shown to play a fundamental role in the acclimation of Ectocarpus spp. to freshwater conditions (Dittami et al., 2016).
Figure 2
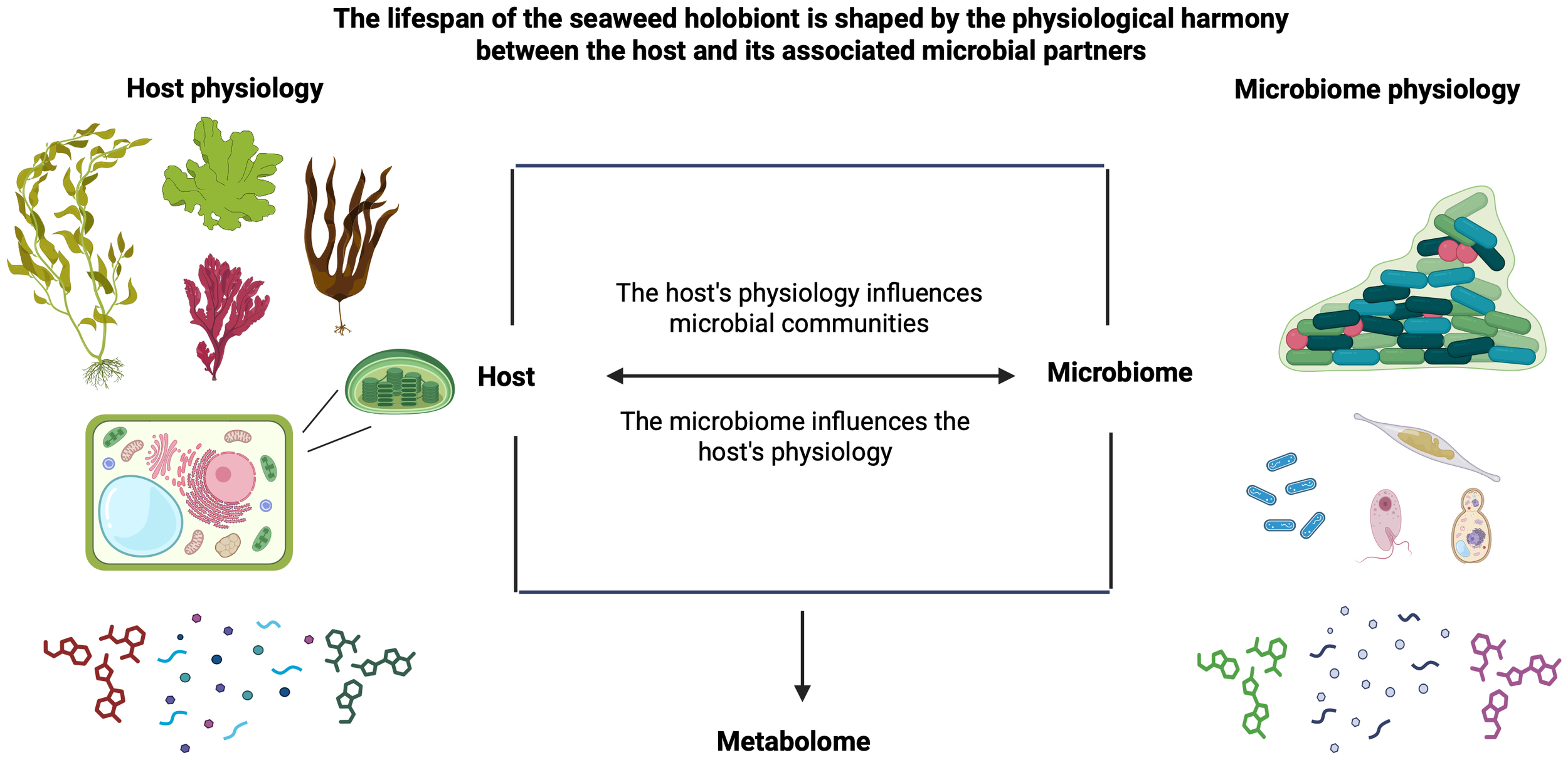
The lifespan of the seaweed holobiont depends on the physiological balance between the host and its associated microbial partners. The host’s physiological state shapes the composition and dynamics of the microbial community, while, in turn, the microbiome influences the host’s metabolism and overall health. The resulting holobiont metabolome emerges from this complex, bidirectional interaction. The figure was created with Biorender. Qui-Minet, (2025)https://BioRender.com/5d56rza.
Seaweed-associated microbial shifts throughout the host’s life-cycle and in response to physiological states (Glasl et al., 2021). However, these dynamics have been mainly studied in short living and annual species of the three groups like Ulva spp., Delisea pulchra and Sargassum spp. (Lefèvre and Bellwood, 2010; Fernandes et al., 2012; Glasl et al., 2021; van der Loos et al., 2024), likely due to the logistical challenges of long-term monitoring in perennial species located in contrasting environments. As such, inter-annual and regional variation in microbiomes of long-lived perennial species remains largely uncharacterized. Bridging this knowledge gap is essential to understand how microbial communities evolve over time and under shifting environmental conditions -insights that are critical for unraveling the resilience of perennial species and the mechanisms driving both premature and progressive senescence.
Once senescence is established -whether premature or progressive- it is often characterized with an increased bacterial load coupled with reduced microbial diversity, creating conditions that favor disease (Lang et al., 2025). This shift can drive dysbiosis and promote the proliferation of opportunistic, polysaccharide-degrading taxa that accelerate host tissue decay and facilitate biomass recycling (Allison and Martiny, 2008; Egan et al., 2013; Ihua et al., 2019; Brunet et al., 2021). For instance, during algal green tides, senescence-associated release of organic molecules supports the growth of saprophytic microbes and detritivores, steering the microbiome toward a decomposition-oriented community (Van Alstyne et al., 2015). It is important to note that the microbiome itself can actively influence algal senescence: in some cases, it can induce premature senescence (Case et al., 2011; Kumar et al., 2016; de Oliveira et al., 2017), while in others it may delay it or even reverse it by preventing or mitigating diseases that trigger premature senescence (Case et al., 2011; Li et al., 2022).
5 Decay is only a matter of time: environmental stress and disease lead to the senescence of seaweeds
Regardless of their lifespan, senescence is a natural process in seaweeds. To discuss premature senescence it is important to understand senescence and what are the aspects involved. Despite their adaptive mechanisms, seaweeds often undergo premature senescence due to multiple environmental stressors that either (a) hinder their ability to activate defense responses, or (b) overwhelm these defenses. These stressors disrupt primary production and respiration rates, ultimately compromising seaweed fitness and their ability to resist disease, predators and other biotic and abiotic stressors (Campbell et al., 2011) (Figure 1A).
Stress tolerance in macroalgae varies widely among species (Lotze and Worm, 2000; Küpper et al., 2002; Navarro et al., 2016) and across life stages, with early developmental stages being particularly vulnerable (reviewed by Coelho et al., 2000). These differences indicate that resilience and lifespan depend not only on environmental and physiological factors but also on the presence of a monogenetic life-cycle or the dominance of particular life-cycle phases. In some green species and in red seaweeds, variation in haploid-to-diploid (H:D) or gametophyte-to-tetrasporophyte (G:T) ratios reflects shifts in resource allocation and efficiency, with implications for growth and stress tolerance (Destombe et al., 1989). Vieira et al. (2018). extended the Resource Limitation Hypothesis to red seaweeds and to resources beyond nutrients, and comparable dynamics are likely in green seaweeds. Therefore, looking at differences in H:D and G:T ratios among ecosystems is important to understand environmental adaptation (Thornber, 2006; da Silva Vieira VMN de and Santos, 2012). Nevertheless, in situ studies can be challenging because some macroscopic phases can only be recognized through microscope observation of reproductive cells or molecular techniques. Furthermore, to advance our understanding in this topic future research should go beyond biochemical differences, and examine how microbiome composition and holobiont-derived metabolites vary across life-cycle phases and how they are differently impacted by environmental stress.
Understanding the premature senescence of brown seaweed species is fundamental, given their important role as ecosystem engineers and the risk they face in the current context on global climate change (Wernberg et al., 2018; Filbee-Dexter et al., 2020; Wernberg, 2021; Arafeh-Dalmau et al., 2025). The effects of environmental stress depend not only on the intensity, but also on the duration and combination of stressors. When multiple stressors act additively or synergistically over time, they can surpass the physiological plasticity and defense mechanisms of seaweeds, ultimately reducing survival and resilience (Williams et al., 2013; Fernández et al., 2015). Light intensity determines seaweed’s capacity to uptake carbon and nutrients, and as we previously discussed, deficiency or excessive light is known to accelerate senescence in photosynthetic organisms (Hurd et al., 2014). Excessive incident irradiance generates ROS, which in turn alters the permeability of chloroplast membranes and causes their loss of electron transport capacity, disrupting carbon fixation and protein synthesis (Heinrich et al., 2015; Vieira et al., 2024; Xu et al., 2024). The latter leads to chronic photoinhibition and photodamage when photoprotection fails to mitigate photoinactivation (Mabin et al., 2019). Conversely, a reduction in incident bottom irradiance by turbidity may significantly reduce seaweeds’ photosynthetic rates. If low values of incident irradiance prevail, and seaweeds are not capable of photo-acclimation, their fitness and survival may be threatened (Hurd et al., 2014). Key environmental stressors include extreme fluctuations in light intensity, nutrient limitation or excesive enrichment, ocean warming and acidification, salinity changes, pollution and hydrodynamic forces, and sedimentation (Harley et al., 2012; Oppliger et al., 2012; Vieira et al., 2015).
Regardless of their longevity (perennial, annual or ephemeral), macroalgal resilience against premature senescence emerge from complex interactions between intrinsic traits and external pressures. In red coralline algae -the longest lived seaweeds- responses to ocean acidification are shaped by light, water motion, and epiphytic loads, with some maerl species able to tolerate pH and temperature stress under favorable irradiance and nutrient conditions (Qui-Minet et al., 2019; Cornwall et al., 2022). Yet, fleshy taxa such as Gelidium corneum and Delisea pulchra illustrate how multifactorial stressors, erode population stability, through reduced light, extreme wave events, or microbiome-mediated bleaching (Campbell et al., 2011; Borja et al., 2018). More broadly, while certain invasive species such as Gracilaria spp. (Rhodophyta), Ulva spp. (Chlorophyta) and Sargassum muticum (Phaeophyceae) display remarkable ecological plasticity (Shiu and Lee, 2005; Thomsen and McGlathery, 2007; Le Lann et al., 2012; Surget et al., 2017; He et al., 2018; Stiger-Pouvreau et al., 2023; Xu et al., 2024), stress tolerance is not universal across invasive taxa. Regardless of taxon-specific longevity, seaweed lifespan is closely shaped by environmental pressures that are closely interconnected. Physical stressors can reduce tissue toughness and mimic senescence (De Bettignies et al., 2012), while chemical defenses-particularly polyphenolic content in brown seaweeds- are linked to differential senescence rates among species like as Nereocystis luetkeana and Neoagarum fimbriatum (previously known as Agarum fimbriatum) (Dethier et al., 2014). Yet, the synthesis of protective compounds is environmentally regulated and can decline under suboptimal conditions (Parys et al., 2009; Almeida et al., 2021). Therefore, lifespan is regulated by the interaction between progressive aging, disturbance regimes (Rodriguez et al., 2013b), and defense capacity-the later determining how effectively macroalgal holobionts can buffer stress. This buffering depends on their ability to uptake and store nutrients under varying light and temperature conditions, with limitations potentially weakening defenses and accelerating senescence.
Seaweed resilience to environmental change is tightly coupled to photosynthetic capacity, which underpins the synthesis of defense-related metabolites (Duarte, 2014). Stressors like desiccation can impair photosynthesis (Contreras-Porcia et al., 2017), limiting the production of protective compounds and threatening survival. Perennial species must endure both seasonal and interannual variability, regenerate tissues following damage, and maintain functional immunity over extended lifespans (Ram et al., 2000). Yet, the thresholds for recovery from stress or disease remain poorly understood. Many bacterial diseases contribute to premature senescence by imparing photosynthesis. This has been particularly well-documented in brown and red species, where disease strongly influences the structure and dynamics of associated microbial communities (Kumar et al., 2014; Zozaya-Valdes et al., 2015; Ling et al., 2022; Mancuso et al., 2023b). In contrast, studies on green seaweeds have focused mainly on Ulva spp., but monitoring microbiome shifts as indicators of health is more challenging in these algae, since their associated microbial communities change rapidly (van der Loos et al., 2025) and they are ephemeral species. However, diseases caused by filamentous endophytes and viruses have been detected in Ulva spp. (Del Campo et al., 1998; van der Loos et al., 2023).
Overall it is more relevant to study premature senescence in perennial species. Intraspecific differences in longevity across geographical areas highlight the importance of local environmental conditions and metabolic flexibility. Ultimately, seaweed immunity depends not only on the activation of defense mechanisms but also on sustaining effective concentrations of signaling and protective metabolites under varying environmental pressures (Weinberger, 2007).
6 Perspectives for seaweeds preservation and management
Sustainable management and utilization of seaweeds hinge on understanding the physiological and environmental factors driving their senescence and decay (Figure 1B). Despite advances in metabolomics and cell wall studies (Liu et al., 2012; Stiger-Pouvreau and Guerard, 2018; Stiger-Pouvreau and Zubia, 2020; Gager et al., 2021), species-specific responses and complex environmental interactions make predictions difficult (Kloareg et al., 2021). Limited knowledge of seaweed ecophysiology constrains ecosystem conservation, aquaculture optimization, and biomass valorization (Harley et al., 2012). Addressing these challenges requires interdisciplinary, long-term research across life stages. Notably, we highlight the importance of including all the compartments of the seaweed holobiont into the understanding of these dynamics. Understanding seaweed ecophysiology is therefore both crucial and challenging, as it forms the foundation for effective conservation and sustainable exploitation (Figure 1B).
Beyond the intrinsic differences among the three macroalgal groups, progress in disciplines related to seaweed lifespan and premature senescence has evolved unevenly. Life-cycle dynamics and biochemical composition are comparatively well understood in seaweeds (Valero et al., 1992; Heesch et al., 2021). However, within this group, maerl algae—despite being among the longest-lived red species—remain poorly studied, likely due to the rarity of their reproductive organs (Birkett et al., 1998) and the limited research interest in their metabolome beyond their CaCO3 content. From a genomic perspective, Ectocarpus spp. and Saccharina spp. are established models in brown seaweeds, Ulva mutabilis in green macroalgae, and Pyropia spp. is evolving as a model in red seaweeds (Coelho and Cock, 2020; Blomme et al., 2023). Likewise, the holobiont perspective is advancing in several brown and green seaweed models (Dittami et al. 2016; Wichard et al., 2018), but remains underexplored in red algae (Marzinelli et al., 2024). Studies on environmental stress have been facilitated in brown species owing to their conspicuous macroscopic life-stage; while such research has also been led in commercially important red seaweeds despite the complexity of their life cycle. On the other hand work on green seaweeds has largely focused on Ulva spp., which are responsible for massive coastal blooms. This mini-review highlights the need to adopt a holobiont perspective across research questions in seaweed biology to better understand premature senescence, and thus improve ecosystem management.
Ultimately, improving seaweed cultivation is foundational for meeting the growing global demand for sustainable biomass. Cultivation offers the only reliable pathway to control both the quantity and quality of bioactive compounds (Duarte et al., 2021). Unlocking the full potential of seaweed-derived products will depend on overcoming existing cultivation bottlenecks and understanding the physiological mechanisms that regulate senescence and degradation.
7 Conclusions
Understanding seaweed premature senescence, requires improving our understanding of senescence. It is particularly relevant to emphasize research on ecosystem engineer species, notably on brown seaweeds and on red coralline seaweeds, the group containing the longest living species. A multidisciplinary approach is essential, starting with photosynthesis—the core physiological process sustaining seaweed life—but integrating microbial interactions, which shape host biochemistry and metabolomic trajectories throughout the organism’s lifespan. Although not the main focus here, genetic regulation and reproductive strategies do play crucial roles in the onset and progression of senescence and have been extensively studied in red seaweeds, but require further investigation in green species (Liu et al., 2017) Additionally, the role of sexual reproduction in shaping seaweed lifespan and resilience remains an open question, particularly how different life-stages and reproductive modes shape the holobiont. For instance, vegetative reproduction narrows the genetic pool of the host (Liu et al., 2017), but it is still unclear how reproductive patterns alter host–microbiome composition and interactions.
Advancing this field could illuminate the molecular and ecological foundations of longevity in marine photosynthetic organisms, with broader implications for photoautotrophs biology, aging research, marine ecosystem management and macroalgal biomass valorization.
Statements
Author contributions
ZNQ-M: Writing – original draft, Writing – review & editing. SC: Writing – review & editing. VS-P: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 101154408.
Acknowledgments
This article is also based upon work from COST Action CA20106 “Tomorrow’s wheat of the sea’: Ulva, a model for an innovative mariculture”, supported by COST (European Cooperation in Science and Technology, www.cost.eu).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abbott D. W. Aasen I. M. Beauchemin K. A. Grondahl F. Gruninger R. Hayes M. et al . (2020). Seaweed and seaweed bioactives for mitigation of enteric methane: challenges and opportunities. Animals.10, 2432. doi: 10.3390/ani10122432
2
Abdul Malik S. A. Bedoux G. Garcia Maldonado J. Q. Freile-Pelegrín Y. Robledo D. Bourgougnon N. (2020). “ Chapter Ten - Defence on surface: macroalgae and their surface-associated microbiome,” in Advances in botanical research, vol. 95 . Ed. BourgougnonN. (London, England: Academic Press), 327–368. Seaweeds Around the World: State of Art and Perspectives. doi: 10.1016/bs.abr.2019.11.009
3
Allison S. D. Martiny J. B. H. (2008). Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA.105Suppl 1, 11512–11519. doi: 10.1073/pnas.0801925105
4
Almeida B. Barroso S. Ferreira A. S. D. Adão P. Mendes S. Gil M. M. (2021). Seasonal evaluation of phlorotannin-enriched extracts from brown macroalgae Fucus spiralis. Molecules26, 4287. doi: 10.3390/molecules26144287
5
Alsufyani T. Califano G. Deicke M. Grueneberg J. Weiss A. Engelen A. H. et al . (2020). Macroalgal–bacterial interactions: identification and role of thallusin in morphogenesis of the seaweed Ulva (Chlorophyta). J. Exp. Bot.71, 3340–3349. doi: 10.1093/jxb/eraa102
6
Alsufyani T. Weiss A. Wichard T. (2017). Time course exo-metabolomic profiling in the green marine macroalga ulva (Chlorophyta) for identification of growth phase-dependent biomarkers. Mar. Drugs15, 14. doi: 10.3390/md15010014
7
Alzate-Gaviria L. Domínguez-Maldonado J. Chablé-Villacís R. Olguin-Maciel E. Leal-Bautista R. M. Canché-Escamilla G. et al . (2021). Presence of polyphenols complex aromatic “lignin” in Sargassum spp. from Mexican Caribbean. J. Mar. Sci. Eng.9, 6. doi: 10.3390/jmse9010006
8
Amsler C. D. Iken K. McClintock J. B. Amsler M. O. Peters K. J. Hubbard J. M. et al . (2005). Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Mar. Ecol. Prog. Ser.294, 141–159. doi: 10.3354/meps
9
Arafeh-Dalmau N. Villaseñor-Derbez J. C. Schoeman D. S. Mora-Soto A. Bell T. W. Butler C. L. et al . (2025). Global floating kelp forests have limited protection despite intensifying marine heatwave threats. Nat. Commun.16, 3173. doi: 10.1038/s41467-025-58054-4
10
Barer M. R. (2012). “ Bacterial growth, physiology and death,” in Medical microbiology (Eighteenth edition). Eds. GreenwoodD.BarerM.SlackR.IrvingW. (Edinburgh, Scotland: Churchill Livingstone, Edinburgh), 39–53. doi: 10.1016/B978-0-7020-4089-4.00019-6
11
Barrientos S. Piñeiro-Corbeira C. Barreiro R. (2022). Temperate kelp forest collapse by fish herbivory: A detailed demographic study. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.817021
12
Bengtsson M. Sjøtun K. Øvreås L. (2010). Seasonal dynamics of bacterial biofilms on the kelp Laminaria hyperborea. Aquat. Microb. Ecol.60, 71–83. doi: 10.3354/ame01409
13
Bengtsson M. M. Wagner K. Schwab C. Urich T. Battin T. J. (2018). Light availability impacts structure and function of phototrophic stream biofilms across domains and trophic levels. Mol. Ecol.27, 2913–2925. doi: 10.1111/mec.14696
14
Birkett D. Maggs C. Dring M. Boaden P. (1998). An overview of dynamic and sensitivity characteristics for conservation management of marine SACs. Scott. Assoc. Mar. Sci. (SAMS)5, 116.
15
Blomme J. Wichard T. Jacobs T. B. De Clerck O. (2023). Ulva: An emerging green seaweed model for systems biology. J. Phycol.59, 433–440. doi: 10.1111/jpy.13119
16
Borja A. Chust G. Fontán A. Garmendia J. M. Uyarra M. C. (2018). Long-term decline of the canopy-forming algae Gelidium corneum, associated to extreme wave events and reduced sunlight hours, in the southeastern Bay of Biscay. Estuar. Coast. Shelf Sci.205, 152–160. doi: 10.1016/j.ecss.2018.03.016
17
Brunet M. de Bettignies F. Le Duff N. Tanguy G. Davoult D. Leblanc C. et al . (2021). Accumulation of detached kelp biomass in a subtidal temperate coastal ecosystem induces succession of epiphytic and sediment bacterial communities. Environ. Microbiol.23, 1638–1655. doi: 10.1111/1462-2920.15389
18
Bunker F. Brodie J. A. Maggs C. A. Bunker A. R. (2017). Seaweeds of britain and Ireland: second edition (Plymouth: Plymton St. Maurice), 312.
19
Burgunter-Delamare B. Kleinjan H. Frioux C. Fremy E. Wagner M. Corre E. et al . (2020). Metabolic complementarity between a brown alga and associated cultivable bacteria provide indications of beneficial interactions. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00085
20
Burgunter-Delamare B. Rousvoal S. Legeay E. Tanguy G. Fredriksen S. Boyen C. et al . (2023). The Saccharina latissima microbiome: Effects of region, season, and physiology. Front. Microbiol.13. doi: 10.3389/fmicb.2022.1050939
21
Campbell A. Harder T. Nielsen S. Kjelleberg S. Steinberg P. (2011). Climate change and disease: Bleaching of a chemically defended seaweed. Glob. Change Biol.17, 2958–2970. doi: 10.1111/j.1365-2486.2011.02456.x
22
Cao L. Lee S. Lim K. Kim H. R. (2020). Potential anti-aging substances derived from seaweeds. Mar. Drugs18, 564. doi: 10.3390/md18110564
23
Case R. J. Longford S. R. Campbell A. H. Low A. Tujula N. Steinberg P. D. et al . (2011). Temperature induced bacterial virulence and bleaching disease in a chemically defended marine macroalga. Environ. Microbiol.13, 529–537. doi: 10.1111/j.1462-2920.2010.02356.x
24
Cavalcanti G. S. Shukla P. Morris M. Ribeiro B. Foley M. Doane M. P. et al . (2018). Rhodoliths holobionts in a changing ocean: host-microbes interactions mediate coralline algae resilience under ocean acidification. BMC Genom.19, 701. doi: 10.1186/s12864-018-5064-4
25
Chapman A. R. O. (1993). ‘Hard’ data for matrix modelling of Laminaria digitata (Laminariales, Phaeophyta) populations. Hydrobiologia260, 263–267. doi: 10.1016/bs.abr.2019.11.007
26
Chen W. M. Sheu F. S. Sheu S. Y. (2012). Aquimarina salinaria sp. nov., a novel algicidal bacterium isolated from a saltpan. Arch. Microbiol.194, 103–112. doi: 10.1007/s00203-011-0730-9
27
Choi S. K. Kim T. Son Y. B. Park S. R. (2024). Threats to a Temperate Kelp Forest S pecies, Ecklonia cava, through Tropical Fish Herbivory Associated with Sea Surface Warming in the East China Sea. Diversity.16, 253. doi: 10.3390/d16050253
28
Choi E. M. Park J. J. Han T. (2011). Temporal changes in oxidative stress and antioxidant activities in Ulva pertusa Kjellman. Toxicol. Environ. Health Sci.3, 206–212. doi: 10.1007/s13530-011-0106-1
29
Coelho S. M. Cock J. M. (2020). Brown algal model organisms. Annu. Rev. Genet.54, 71–92. doi: 10.1146/annurev-genet-030620-093031
30
Coelho S. M. Rijstenbil J. W. Brown M. T. (2000). Impacts of anthropogenic stresses on the early development stages of seaweeds. J. Aquat. Ecosyst. Stress Recover.7, 317–333. doi: 10.1023/A:1009916129009
31
Contreras-Porcia L. López-Cristoffanini C. Meynard A. Kumar M. (2017). Tolerance pathways to desiccation stress in seaweeds. In. Syst. Biol. Mar. Ecosyst., 13–33. doi: 10.1007/978-3-319-62094-7_2
32
Cornwall C. E. Harvey B. P. Comeau S. Cornwall D. L. Hall-Spencer J. M. Peña V. et al . (2022). Understanding coralline algal responses to ocean acidification: Meta-analysis and synthesis. Glob. Change Biol.28, 362–374. doi: 10.1111/gcb.15899
33
Creed J. C. Vieira VMNCS Norton T. A. Caetano D. (2019). A meta-analysis shows that seaweeds surpass plants, setting life-on-Earth’s limit for biomass packing. BMC Ecology.19, 6. doi: 10.1186/s12898-019-0218-z
34
Dahms H. U. Dobretsov S. (2017). Antifouling compounds from marine macroalgae. Mar. Drugs15, 265. doi: 10.3390/md15090265
35
da Silva Vieira VMN de C. Santos R. O. P. (2012). Regulation of geographic variability in haploid:diploid ratios of biphasic seaweed life cycles (1). J. Phycol.48, 1012–1019. doi: 10.1111/j.1529-8817.2012.01183.x
36
de Bettignies F. Dauby P. Thomas F. Gobet A. Delage L. Bohner O. et al . (2020). Degradation dynamics and processes associated with the accumulation of Laminaria hyperborea (Phaeophyceae) kelp fragments: an in situ experimental approach. J. Phycol.56, 1481–1492. doi: 10.1111/jpy.13041
37
de Bettignies T. Thomsen M. Wernberg T. (2012). Wounded kelps: patterns and susceptibility to breakage. Aquat. Biol.17, 223–233. doi: 10.3354/ab
38
de Bettignies T. Wernberg T. Gurgel C. F. D. (2018). Exploring the influence of temperature on aspects of the reproductive phenology of temperate seaweeds. Front. Mar. Sci.5, 218. doi: 10.3389/fmars.2018.00218
39
Debue M. Guinaldo T. Jouanno J. Chami M. Barbier S. Berline L. et al . (2025). Understanding the Sargassum phenomenon in the Tropical Atlantic Ocean: From satellite monitoring to stranding forecast. Mar. pollut. Bull.216, 117923. doi: 10.1016/j.marpolbul.2025.117923
40
Del Campo E. García-Reina G. Correa J. A. (1998). Degradative disease in Ulva rigida (Chlorophyceae) associated with Acrochaete geniculata (Chlorophyceae). J. Phycol.34, 160–166. doi: 10.1046/j.1529-8817.1998.340160.x
41
Del Olmo G. Ruiz P. Nappi J. Thomas T. Egan S. Cremades J. (2025). Optimizing Ulva-Phaeobacter co-culture: A two-phase light intensity approach for integrated multi-trophic aquaculture applications. J. Appl. Phycol.37, 1227–1240. doi: 10.1007/s10811-025-03461-9
42
de Oliveira L. S. Tschoeke D. A. Magalhães Lopes A. C. R. Sudatti D. B. Meirelles P. M. Thompson C. C. et al . (2017). Molecular mechanisms for microbe recognition and defense by the red seaweed laurencia dendroidea. mSphere.2, e00094–e00017. doi: 10.1128/mSphere.00094-17
43
De Reviers B. D. De Reviers B. (2022). “ Biologie et phylogénie des algues,” in Cours: tome 1, vol. 352. ( Belin Sup Sciences-Biologie, Paris: Belin).
44
Destombe C. Valero M. Vernet P. Couvet D. (1989). What controls haploid—diploid ratio in the red alga, Gracilaria verrucosa? J. Evol. Biol.2, 317–338. doi: 10.1046/j.1420-9101.1989.2050317
45
Dethier M. N. Brown A. S. Burgess S. Eisenlord M. E. Galloway A. W. E. Kimber J. et al . (2014). Degrading detritus: changes in food quality of aging kelp tissue varies with species. J. Exp. Mar. Biol. Ecol.460, 72–79. doi: 10.1016/j.jembe.2014.06.010
46
Dittami S. Arboleda E. Auguet J. C. Bigalke A. Briand E. Cárdenas P. et al . (2019). A community perspective on the concept of marine holobionts: state-of-the-art, challenges, and future directions. Peer J.7, e27519v1. doi: 10.7717/peerj.10911
47
Dittami S. M. Duboscq-Bidot L. Perennou M. Gobet A. Corre E. Boyen C. et al . (2016). Host-microbe interactions as a driver of acclimation to salinity gradients in brown algal cultures. ISME J.10, 51–63. doi: 10.1038/ismej.2015.104
48
Dittami S. M. Eveillard D. Tonon T. (2014). A metabolic approach to study algal-bacterial interactions in changing environments. Mol. Ecol.23, 1656–1660. doi: 10.1111/mec.12670
49
Domozych D. (2019). “ Algal cell walls,” (Chichester, UK: eLS. John Wiley & Sons, Ltd). doi: 10.1002/9780470015902.a0000315.pub4
50
Drobnitch S. T. Jensen K. H. Prentice P. Pittermann J. (2005). Convergent evolution of vascular optimization in kelp (Laminariales). Proc. R. Soc. B: Biol. Sci.282, 20151667. doi: 10.1098/rspb.2015.1667
51
Duarte C. (2014). Global change and the future ocean: a grand challenge for marine sciences. Front. Mar. Sci.1. doi: 10.3389/fmars.2014.00063
52
Duarte C. M. Bruhn A. Krause-Jensen D. (2021). A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain.5(3), 185–193. doi: 10.1038/s41893-021-00773-9
53
Egan S. Harder T. Burke C. Steinberg P. Kjelleberg S. Thomas T. (2013). The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiol. Rev.37, 462–476. doi: 10.1111/1574-6976.12011
54
Eggert A. (2012). “ Seaweed responses to temperature,” in Seaweed biology: novel insights into ecophysiology, ecology and utilization. Eds. WienckeC.BischofK.(Springer, Berlin, Heidelberg: Bischof), 47–66. doi: 10.1007/978-3-642-28451-9_3
55
Epstein G. Smale D. A. (2013). Undaria pinnatifida: A case study to highlight challenges in marine invasion ecology and management. Ecol. Evol.7), 8624–8642. doi: 10.1002/ece3.3430
56
Fernandes N. Steinberg P. Rusch D. Kjelleberg S. Thomas T. (2012). Community structure and functional gene profile of bacteria on healthy and diseased thalli of the red seaweed Delisea pulchra. PloS One7, e50854. doi: 10.1371/journal.pone.0050854
57
Fernández Á Arenas F. Trilla A. Rodríguez S. Rueda L. Martínez B. (2015). Additive effects of emersion stressors on the ecophysiological performance of two intertidal seaweeds. Mar. Ecol. Prog. Ser.536, 135–147. doi: 10.3354/meps11401
58
Filbee-Dexter K. Wernberg T. Grace S. P. Thormar J. Fredriksen S. Narvaez C. N. et al . (2020). Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Sci. Rep.10, 13388. doi: 10.1007/978-3-030-71330-0
59
Florez J. Camus C. Hengst M. Buschmann A. (2017). A functional perspective analysis of macroalgae and epiphytic bacterial community interaction. Front. Microbiol.8. doi: 10.3389/fmicb.2017.02561
60
Foster M. S. (2001). Rhodoliths: between rocks and soft places. J. Phycol.37, 659–667. doi: 10.1046/j.1529-8817.2001.00195.x
61
Gager L. Lalegerie F. Connan S. Stiger-Pouvreau V. (2021). Marine algal derived phenolic compounds and their biological activities for medicinal and cosmetic applications. In: Recent Adv. Micro Macroalgal John Wiley Sons Ltd;p, 278–334. doi: 10.1002/9781119542650.ch11
62
Gao S. Zheng Z. Gu W. Xie X. Huan L. Pan G. et al . (2014). Photosystem I shows a higher tolerance to sorbitol-induced osmotic stress than photosystem II in the intertidal macro-algae Ulva prolifera (Chlorophyta). Physiol. Plant152, 380–388. doi: 10.1111/ppl.12188
63
Garbary D. J. Fraser S. J. Hubbard C. Kim K. Y. (2004). Codium fragile: rhizomatous growth in the Zostera thief of eastern Canada. Helgol. Mar. Res.58, 141–146. doi: 10.1007/s10152-004-0173-7
64
Ghaderiardakani F. Langhans L. Kurbel V. B. Fenizia S. Wichard T. (2022). Metabolite profiling reveals insights into the species-dependent cold stress response of the green seaweed holobiont Ulva (Chlorophyta). Environ. Exp. Bot.200, 104913. doi: 10.1016/j.envexpbot.2022.104913
65
Ghaderiardakani F. Quartino M. L. Wichard T. (2020). Microbiome-dependent adaptation of seaweeds under environmental stresses: A perspective. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.575228
66
Glasl B. Haskell J. B. Aires T. Serrão E. A. Bourne D. G. Webster N. S. et al . (2021). Microbial surface biofilm responds to the growth-reproduction-senescence cycle of the dominant coral reef macroalgae Sargassum spp. Life (Basel).11, 1199. doi: 10.3390/life11111199
67
Grueneberg J. Engelen A. H. Costa R. Wichard T. (2016). Macroalgal morphogenesis induced by waterborne compounds and bacteria in coastal seawater. PloS One11, e0146307. doi: 10.1371/journal.pone.0146307
68
Guillemin M. L. Sepúlveda R. D. Correa J. A. Destombe C. (2013). Differential ecological responses to environmental stress in the life history phases of the isomorphic red alga Gracilaria Chilensis (Rhodophyta). J. Appl. Phycol.25, 215–224. doi: 10.1007/s10811-012-9855-8
69
Guiry M. D. (2023). How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing. J. Phycol.60, 214–228. doi: 10.1111/jpy.13431
70
Halfar J. Steneck R. Schone B. Moore G. W. K. Joachimski M. Kronz A. et al . (2007). Coralline alga reveals first marine record of subarctic North Pacific climate change. Geophysical Res. Lett.34, L07702. doi: 10.1029/2006GL028811
71
Hall J. R. Albert G. Twigg I. M. Baltar F. Hepburn C. D. Martin G. (2022). The production of dissolved organic carbon by macroalgae and its consumption by marine bacteria: Implications for coastal ecosystems. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.934229
72
Harley C. D. G. Anderson K. M. Demes K. W. Jorve J. P. Kordas R. L. Coyle T. A. et al . (2012). Effects of climate change on global seaweed communities. J. Phycol.48, 1064–1078. doi: 10.1111/j.1529-8817.2012.01224.x
73
Hatchett W. J. Coyer J. A. Sjøtun K. Jueterbock A. Hoarau G. (2022). A review of reproduction in the seaweed genus Fucus (Ochrophyta, Fucales): Background for renewed consideration as a model organism. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.1051838
74
He Y. Hu C. Wang Y. Cui D. Sun X. Li Y. et al . (2018). The metabolic survival strategy of marine macroalga Ulva prolifera under temperature stress. J. Appl. Phycol.30, 3611–3621. doi: 10.1007/s10811-018-1493-3
75
Heesch S. Serrano-Serrano M. Barrera-Redondo J. Luthringer R. Peters A. F. Destombe C. et al . (2021). Evolution of life cycles and reproductive traits: Insights from the brown algae. J. Evol. Biol.34, 992–1009. doi: 10.1111/jeb.13880
76
Heinrich S. Valentin K. Frickenhaus S. Wiencke C. (2015). Temperature and light interactively modulate gene expression in Saccharina latissima (Phaeophyceae). J. Phycol.51, 93–108. doi: 10.1111/jpy.12255
77
Hill J. M. (2008). Laminaria digitata (Oarweed Plymouth: Marine Biological Association of the United Kingdom). Available online at: https://plymsea.ac.uk/id/eprint/8365/ (Accessed September 10, 2025).
78
Hochart C. Rouzé H. Rivière B. Ruscheweyh H. J. Hédouin L. Pochon X. et al . (2024). High diversity of crustose coralline algae microbiomes across species and islands, and implications for coral recruits. Environ. Microbiome.19, 112. doi: 10.1186/s40793-024-00640-y
79
Hurd C. L. Harrison P. J. Bischof K. Lobban C. S. (2014). Seaweed ecology and physiology Vol. 567 (Cambridge, England: Cambridge University Press). doi: 10.1017/CBO9781139192637
80
Ihua M. W. Guihéneuf F. Mohammed H. Margassery L. M. Jackson S. A. Stengel D. B. et al . (2019). Microbial population changes in decaying Ascophyllum nodosum result in macroalgal-polysaccharide-degrading bacteria with potential applicability in enzyme-assisted extraction technologies. Mar. Drugs17, 200. doi: 10.3390/md17040200
81
Kain J. M. J. (1979). A view of the genus Laminaria. Oceanography Mar. Biology: Annu. Review.17, 101–161.
82
Karsten U. Barrow K. D. King R. J. (1993). Floridoside, L-Isofloridoside, and D-Isofloridoside in the red alga Porphyra columbina (seasonal and osmotic effects). Plant Physiol.103, 485–491. doi: 10.1104/pp.103.2.485
83
Kim S. Youn S. H. Oh H. J. Choi S. K. Kang Y. H. Kim T. H. et al . (2018). Stipe length as an indicator of reproductive maturity in the kelp ecklonia cava. Ocean Sci. J.53, 595–600. doi: 10.1007/s12936-018-0043-5
84
Kloareg B. Badis Y. Cock J. M. Michel G. (2021). Role and evolution of the extracellular matrix in the acquisition of complex multicellularity in eukaryotes: A macroalgal perspective. Genes.12, 1059. doi: 10.3390/genes12071059
85
Kloareg B. Quatrano R. S. (1988). Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. Annu. Rev.26, 259–314. doi: 10.3390/jmse9010006
86
Kumar M. Kumari P. Reddy C. R. K. Jha B. (2014). “ Chapter four - salinity and desiccation induced oxidative stress acclimation in seaweeds,” in Advances in botanical research, vol. 71 . Ed. BourgougnonN., Academic Press (Elsevier) 91–123. doi: 10.1016/B978-0-12-408062-1.00004-4
87
Kumar V. Zozaya-Valdes E. Kjelleberg S. Thomas T. Egan S. (2016). Multiple opportunistic pathogens can cause a bleaching disease in the red seaweed Delisea pulchra. Environ. Microbiol.18, 3962–3975. doi: 10.1111/1462-2920.13403
88
Küpper F. C. Müller D. G. Peters A. F. Kloareg B. Potin P. (2002). Oligoalginate recognition and oxidative burst play a key role in natural and induced resistance of sporophytes of laminariales. J. Chem. Ecol.28, 2057–2081. doi: 10.1023/a:1020706129624
89
Lage O. M. Graca A. P. (2016). “ Biofilms an extracoat on macroalgae,” in Algae: organisms for imminent biotechnology, BoD-Books on Demand1–16. BoD – Books on Demand. doi: 10.5772/63053
90
Lalegerie F. Gager L. Stiger-Pouvreau V. Connan S. (2020). “ Chapter Eight - The stressful life of red and brown seaweeds on the temperate intertidal zone: effect of abiotic and biotic parameters on the physiology of macroalgae and content variability of particular metabolites,” in Adv. Bot. Res, vol. 95 . Ed. BourgougnonN., Academic Press (Elsevier). 247–287.
91
Lang T. Cummins S. F. Paul N. A. Pascelli C. Campbell A. H. (2025). The meta-transcriptome of a seaweed holobiont in culture: Linking gene expression with growth and senescence. Algal Res.85, 103834. doi: 10.1016/j.algal.2024.103834
92
Lee W. K. Ho C. L. (2021). Ecological and evolutionary diversification of sulphated polysaccharides in diverse photosynthetic lineages: a review. Carbohydr. Polym.265, 118764. doi: 10.1016/j.carbpol.2021.118764
93
Lefèvre C. D. Bellwood D. R. (2010). Seasonality and dynamics in coral reef macroalgae: variation in condition and susceptibility to herbivory. Mar. Biol.157, 955–965. doi: 10.1007/s00227-009-1376-x
94
Le Lann K. Connan S. Stiger-Pouvreau V. (2012). Phenology, TPC and size-fractioning phenolics variability in temperate Sargassaceae (Phaeophyceae, Fucales) from Western Brittany: native versus introduced species. Mar. Environ. Res.80, 1–11. doi: 10.1016/j.marenvres.2012.05.011
95
Lemay M. A. Martone P. T. Keeling P. J. Burt J. M. Krumhansl K. A. Sanders R. D. et al . (2018). Sympatric kelp species share a large portion of their surface bacterial communities. Environ. Microbiol.20, 658–670. doi: 10.1111/1462-2920.13993
96
Li C. Ariga I. Mikami K. (2019). Difference in nitrogen starvation-inducible expression patterns among phylogenetically diverse ammonium transporter genes in the red seaweed Pyropia yezoensis. Am. J. Plant Sci.10, 1325–1349. doi: 10.4236/ajps.2019.108096
97
Li J. Majzoub M. E. Marzinelli E. M. Dai Z. Thomas T. Egan S. (2022). Bacterial controlled mitigation of dysbiosis in a seaweed disease. ISME J.16, 378–387. doi: 10.1038/s41396-021-01070-1
98
Li H. Scheschonk L. Heinrich S. Valentin K. Harms L. Glöckner G. et al . (2020). Transcriptomic responses to darkness and the survival strategy of the kelp Saccharina latissima in the early polar night. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.592033
99
Ling F. Egan S. Zhuang Y. Chang L. Xiao L. Yang Q. et al . (2022). Epimicrobiome shifts with bleaching disease progression in the brown seaweed saccharina japonica. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.865224
100
Lipinska A. P. Collén J. Krueger-Hadfield S. A. Mora T. Ficko-Blean E. (2020). To gel or not to gel: differential expression of carrageenan-related genes between the gametophyte and tetasporophyte life cycle stages of the red alga Chondrus crispus. Sci. Rep.10, 11498. doi: 10.1038/s41598-020-67728-6
101
Liu X. Bogaert K. Engelen A. H. Leliaert F. Roleda M. Y. Clerck O. D. (2017). Seaweed reproductive biology: environmental and genetic controls. Bot. Mar.60, 89–108. doi: 10.1515/bot-2016-0091
102
Liu L. Heinrich M. Myers S. Dworjanyn S. A. (2012). Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: a phytochemical and pharmacological review. J. Ethnopharmacol.142, 591–619. doi: 10.1016/j.jep.2012.05.046
103
Lotze H. Worm B. (2000). Variable and complementary effects of herbivores on different life stages of bloom-forming macroalgae. Mar. Ecol. Prog. Ser.200, 167–175. doi: 10.3354/meps200167
104
Mabin C. J. T. Johnson C. R. Wright J. T. (2019). Physiological response to temperature, light, and nitrates in the giant kelp Macrocystis pyrifera from Tasmania, Australia. Mar. Ecol. Prog. Ser.614, 1–9. doi: 10.3354/meps12900
105
Mach K. J. Hale B. B. Denny M. W. Nelson D. V. (2007a). Death by small forces: a fracture and fatigue analysis of wave-swept macroalgae. J. Exp. Biol.210, 2231–2243. doi: 10.1242/jeb.001578
106
Mach K. J. Nelson D. V. Denny M. W. (2007b). Techniques for predicting the lifetimes of wave-swept macroalgae: a primer on fracture mechanics and crack growth. J. Exp. Biol.210, 2213–2230. doi: 10.1242/jeb.001560
107
Mach K. J. Tepler S. K. Staaf A. V. Bohnhoff J. C. Denny M. W. (2011). Failure by fatigue in the field: a model of fatigue breakage for the macroalga Mazzaella, with validation. J. Exp. Biol.214, 1571–1585. doi: 10.1242/jeb.051623
108
Magaña-Gallegos E. Villegas-Muñoz E. Salas-Acosta E. R. Barba-Santos M. G. Silva R. van Tussenbroek B. I. (2023). The effect of temperature on the growth of holopelagic sargassum species. Phycol.3, 138–146. doi: 10.3390/phycology3010009
109
Mancuso F. P. Morrissey K. L. De Clerck O. Airoldi L. (2023). Warming and nutrient enrichment can trigger seaweed loss by dysregulation of the microbiome structure and predicted function. Sci. Total Environ.879, 162919. doi: 10.1016/j.scitotenv.2023.162919
110
Marinho-Soriano E. Bourret E. (2003). Effects of season on the yield and quality of agar from Gracilaria species (Gracilariaceae, Rhodophyta). Bioresour Technol.90, 329–333. doi: 10.1016/S0960-8524(03)00112-3
111
Markager S. Sand-Jensen K. (1990). Heterotrophic growth of Ulva lactuca (Chlorophyceae). J. Phycol.26, 670–673. doi: 10.1111/j.0022-3646.1990.00670.x
112
Marzinelli E. M. Thomas T. Vadillo Gonzalez S. Egan S. Steinberg P. D. (2024). Seaweeds as holobionts: Current state, challenges, and potential applications. J. Phycol.60, 785–796. doi: 10.1111/jpy.13485
113
Maschek J. Baker B. (2007). The chemistry of algal secondary metabolism. In. Algal Chem. Ecol., 1–24. doi: 10.1007/978-3-540-74181-7_1
114
Mayta M. L. Hajirezaei M. R. Carrillo N. Lodeyro A. F. (2019). Leaf senescence: the chloroplast connection comes of age. Plants (Basel)8, 495. doi: 10.1127/pip/2015/0019
115
McCandless E. L. Craigie J. S. Walter J. A. (1973). Carrageenans in the gametophytic and sporophytic stages of Chondrus crispus. Planta.112, 201–212. doi: 10.1007/BF00385324
116
Menaa F. Wijesinghe P. A. U. I. Thiripuranathar G. Uzair B. Iqbal H. Khan B. A. et al . (2020). Ecological and industrial implications of dynamic seaweed-associated microbiota interactions. Mar. Drugs18, 641. doi: 10.3390/md18120641
117
Mendonça I. R. W. Theirlynck T. Zettler E. R. Amaral-Zettler L. A. Oliveira M. C. (2024). Microbiome changes in a stranding simulation of the holopelagic macroalgae Sargassum natans and Sargassum fluitans. Ocean Coast. Res.72, e24037. doi: 10.1590/2675-2824072.23111
118
Migné A. Bordeyne F. Davoult D. (2025). Long-term survey of intertidal rocky shore macrobenthic community metabolism and structure after primary succession. Peer Community J.5, (10). doi: 10.24072/pcjournal.517
119
Navarro N. P. Huovinen P. Gómez I. (2016). Stress tolerance of Antarctic macroalgae in the early life stages. Rev. Chi.l Hist. Nat.89, 5. doi: 10.1186/s40693-016-0051-0
120
Neill K. Nelson W. Hurd C. Falshaw R. (2018). Growth and carrageenan composition of two populations of the New Zealand carrageenophyte Sarcothalia lanceata (Gigartinaceae, Rhodophyta). J. Appl. Phycol.30, 2485–2497. doi: 10.1007/s10811-018-1416-3
121
Nelson W. A. Neill K. D’Archino R. Rolfe J. R. (2019). “ Conservation status of New Zealand macroalgae,” in New zealand threat classification series 30, vol. 33. ( Department of Conservation, Wellington).
122
North W. J. (1961). Life-span of the fronds of the giant kelp, Macrocystis pyrifera. Nature190, 1214–1215. doi: 10.1038/1901214a0
123
Novaczek I. (1981). Stipe growth rings in Ecklonia radiata (C.Ag.) J.Ag. (Laminariales). Br. Phycol J.16, 363–371. doi: 10.1080/00071618100650361
124
Obolski U. Wichard T. Israel A. Golberg A. Liberzon A. (2022). Modelling the growth and sporulation dynamics of the macroalga Ulva in mixed-age populations in cultivation and the formation of green tides. Biogeosciences19, 2263–2271. doi: 10.5194/bg-19-2263-2022
125
Oppliger L. V. Correa J. A. Engelen A. H. Tellier F. Vieira V. Faugeron S. et al . (2012). Temperature effects on gametophyte life-history traits and geographic distribution of two cryptic kelp species. PloS One7, e39289. doi: 10.1371/journal.pone.0039289
126
Paine E. R. Schmid M. Boyd P. W. Diaz-Pulido G. Hurd C. L. (2021). Rate and fate of dissolved organic carbon release by seaweeds: a missing link in the coastal ocean carbon cycle. J. Phycol.57, 1375–1391. doi: 10.1111/jpy.13198
127
Paix B. Carriot N. Barry-Martinet R. Greff S. Misson B. Briand J. F. et al . (2020). A multi-omics analysis suggests links between the differentiated surface metabolome and epiphytic microbiota along the thallus of a Mediterranean seaweed holobiont. Front. Microbiol.11. doi: 10.3389/fmicb.2020.00494
128
Paix B. Layglon N. Le Poupon C. D’Onofrio S. Misson B. Garnier C. et al . (2021). Integration of spatio-temporal variations of surface metabolomes and epibacterial communities highlights the importance of copper stress as a major factor shaping host-microbiota interactions within a Mediterranean seaweed holobiont. Microbiome9, 201. doi: 10.1186/s40168-021-01124-8
129
Parke M. (1948). Studies on British Laminariaceae. I. Growth in laminaria saccharina (L.) lamour. J. Mar. Biol. Assoc. U. K.27 (3), 651–709. doi: 10.1017/S0025315400056071
130
Parys S. Kehraus S. Pete R. Küpper F. C. Glombitza K. W. König G. M. (2009). Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophyceae). Eur. J. Phycol.44, 331–338. doi: 10.1080/09670260802578542
131
Pedersen M. F. Nejrup L. B. Fredriksen S. Christie H. Norderhaug K. M. (2012). Effects of wave exposure on population structure, demography, biomass and productivity of the kelp Laminaria hyperborea. Mar. Ecol. Prog. Ser.451, 45–60. doi: 10.3354/meps
132
Pereira D. T. Simioni C. Filipin E. P. Bouvie F. Ramlov F. Maraschin M. et al . (2017). Effects of salinity on the physiology of the red macroalga, Acanthophora spicifera (Rhodophyta, Ceramiales). Acta Bot. Bras.31, 555–565. doi: 10.1590/0102-33062017abb0059
133
Peters A. F. Marie D. Scornet D. Kloareg B. Cock J. M. (2004). Proposal of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) as a model organism for brown algal genetics and genomics. J. Phycol.40, 1079–1088. doi: 10.1111/j.1529-8817.2004.04058.x
134
Pfister C. A. Berlinghof J. Bogan M. Cardini U. Gobet A. Hamon-Giraud P. et al . (2025). Evolutionary history and association with seaweeds shape the genomes and metabolisms of marine bacteria. mSphere.10, e00996–e00924. doi: 10.1128/msphere.00996-24
135
Pinteus S. Lemos M. F. L. Alves C. Silva J. Pedrosa R. (2021). The marine invasive seaweeds Asparagopsis armata and Sargassum muticum as targets for greener antifouling solutions. Sci. Total Environ.750, 141372. doi: 10.1016/j.scitotenv.2020.141372
136
Pommerville J. C. Kochert G. D. (1981). Changes in somatic cell structure during senescence of Volvox carteri. Eur. J. Cell Biol.24, 236–243. doi: 10.1016/00144827(82)901537
137
Popper Z. A. Michel G. Hervé C. Domozych D. S. Willats W. G. T. Tuohy M. G. et al . (2011). Evolution and diversity of plant cell walls: from algae to flowering plants. Annu. Rev. Plant Biol.62, 567–590. doi: 10.1146/annurev-arplant-042110-103809
138
Popper Z. A. Ralet M. C. Domozych D. S. (2014). Plant and algal cell walls: diversity and functionality. Ann. Bot.114, 1043–1048. doi: 10.1093/aob/mcu214
139
Prince J. S. Trowbridge C. D. (2018). Reproduction in the green macroalga Codium (Chlorophyta): characterization of gametes. Bot. Mar.47, 461–470. doi: 10.1515/BOT.2004.062
140
Qui-Minet Z. N. Coudret J. Davoult D. Grall J. Mendez-Sandin M. Cariou T. et al . (2019). Combined effects of global climate change and nutrient enrichment on the physiology of three temperate maerl species. Ecol. Evol.9, 13787–13807. doi: 10.1002/ece3.5802
141
Qui-Minet Z. N. Davoult D. Grall J. Martin S. (2022). The relative contribution of fleshy epiphytic macroalgae to the production of temperate maerl (rhodolith) beds. Mar. Ecol. Prog. Ser.693, 69–82. doi: 10.3354/meps
142
Qui-Minet Z. N. Wichard T. Del Olmo G. Pereira M. Holbl H. Ruiz P. et al . (2025). Light-regulated interactions between Phaeobacter sp. and Ulva ohnoi (Chlorophyta): Effects on microbiome dynamics, metabolome composition, and tropodithietic acid production. Environ. Exp. Bot.202:106093. doi: 10.1016/j.envexpbot.2025.106093
143
Ram M. Vijayaraghavan M. R. Babbar S. (2000). Wound response and regeneration in Coelarthrum opuntia. Aquat. Bot.68, 345–351. doi: 10.1016/S0304-3770(00)00117-0
144
Raven J. A. Hurd C. L. (2012). Ecophysiology of photosynthesis in macroalgae. Photosynth. Res.113, 105–125. doi: 10.1007/s11120-012-9768-z
145
Reich P. B. Ellsworth D. S. Walters M. B. Vose J. M. Gresham C. Volin J. C. et al . (1999). Generality of leaf trait relationships: A test across six biomes. Ecol.80, 1955–1969. doi: 10.2307/176671
146
Resiere D. Kallel H. Florentin J. Banydeen R. Compton K. Gueye P. et al . (2025). Sargassum seaweed in the Caribbean: A major public health problem still unsolved. J. Global Health. 13, 03017. doi: 10.7189/jogh.13.03017
147
Reverter M. Rohde S. Parchemin C. Tapissier-Bontemps N. Schupp P. J. (2020). Metabolomics and marine biotechnology: coupling metabolite profiling and organism biology for the discovery of new compounds. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.613471
148
Rezayian M. Niknam V. Ebrahimzadeh H. (2019). Oxidative damage and antioxidative system in algae. Toxicol. Rep.6, 1309–1313. doi: 10.1016/j.toxrep.2019.10.001
149
Rietema H. (1970). Life-histories of Bryopsis plumosa (Chlorophyceae, Caulerpales) from European coasts. Acta Bot. Neerl.19, 859–866. doi: 10.1111/j.1438-8677.1970.tb00189.x
150
Rinde E. Sjøtun K. (2005). Demographic variation in the kelp Laminaria hyperborea along a latitudinal gradient. Mar. Biol.146, 1051–1062. doi: 10.1007/s00227-004-1513-5
151
Rodriguez G. E. Rassweiler A. Reed D. C. Holbrook S. J. (2013a). The importance of progressive senescence in the biomass dynamics of giant kelp (Macrocystis pyrifera). J. Ecol.94, 1848–1858. doi: 10.1890/12-1340.1
152
Rodriguez G. E. Rassweiler A. Reed D. C. Holbrook S. J. (2013b). The importance of progressive senescence in the biomass dynamics of giant kelp (Macrocystis pyrifera). Ecology94, 1848–1858. doi: 10.1890/12-1340.1
153
Rodríguez-Martínez R. E. Medina-Valmaseda A. E. Blanchon P. Monroy-Velázquez L. V. Almazán-Becerril A. Delgado-Pech B. et al . (2019). Faunal mortality associated with massive beaching and decomposition of pelagic Sargassum. Mar. pollut. Bull.146, 201–205. doi: 10.1016/j.marpolbul.2019.06.015
154
Rosenberg E. Zilber-Rosenberg I. (2018). The hologenome concept of evolution after 10 years. Microbiome6, 78. doi: 10.1186/s40168-018-0457-9
155
Salaün S. La Barre S. Dos Santos-Goncalvez M. Potin P. Haras D. Bazire A. (2012). Influence of exudates of the kelp Laminaria digitata on biofilm formation of associated and exogenous bacterial epiphytes. Microb. Ecol.64, 359–369. doi: 10.1007/s00248-012-0048-4
156
Schell J. M. Goodwin D. S. Volk R. H. Siuda A. N. S. (2024). Preliminary explorations of environmental tolerances and growth rates of holopelagic Sargassum morphotypes. Aquat. Bot.190, 103723. doi: 10.1016/j.aquabot.2023.103723
157
Scrosati R. (2005). Review of studies on biomass-density relationships (including self-thinning lines) in seaweeds: Main contributions and persisting misconceptions. Phycological Res.53, 224–233. doi: 10.1111/j.1440-1835.2005.tb00375.x
158
Scrosati R. DeWreede R. E. (1998). The impact of frond crowding on frond bleaching in the clonal intertidal alga mazzaella cornucopiae (rhodophyta, gigartinaceae) from british columbia, Canada. J. Phycology34, 228–232. doi: 10.1046/j.1529-8817.1998.340228.x
159
Scrosati R. A. MacDonald H. L. Córdova C. A. Casas G. N. (2020). Length and biomass data for atlantic and pacific seaweeds from both hemispheres. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.592675
160
Sheppard E. J. Hurd C. L. Britton D. D. Reed D. C. Bach L. T. (2023). Seaweed biogeochemistry: Global assessment of C:N and C:P ratios and implications for ocean afforestation. J. Phycol.59, 879–892. doi: 10.1111/jpy.13381
161
Shiu C. T. Lee T. M. (2005). Ultraviolet-B-induced oxidative stress and responses of the ascorbate–glutathione cycle in a marine macroalga Ulva fasciata. J. Exp. Bot.56, 2851–2865. doi: 10.1093/jxb/eri277
162
Smale D. A. Burrows M. T. Moore P. O’Connor N. Hawkins S. J. (2013). Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol. Evol.3, 4016–4038. doi: 10.1002/ece3.774
163
Smith C. M. Berry J. A. (1986). Recovery of photosynthesis after exposure of intertidal algae to osmotic and temperature stresses: comparative studies of species with differing distributional limits. Oecologia.70, 6–12. doi: 10.1007/BF00377105
164
Soler-Vila A. Edwards M. Whelan S. Guiry M. (2022). Macroalgae Fact sheet. 2nd (Galway, Ireland: Irish Seaweed Research Group). Available online at: https://www.seaweed.ie/pdf/Fact_Sheets_2022.pdf (Accessed September 10, 2025).
165
Steinhagen S. Larsson K. Olsson J. Albers E. Undeland I. Pavia H. et al . (2022). Closed life-cycle aquaculture of sea lettuce (Ulva fenestrata): performance and biochemical profile differ in early developmental stages. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.879238
166
Stengel D. B. Connan S. (2015). Natural products from marine algae: methods and protocols Vol. 1308 (New York, NY: Springer). Methods in Molecular Biology. doi: 10.1007/978-1-4939-2684-8
167
Stengel D. B. Connan S. Popper Z. A. (2011). Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol. Adv.29, 483–501. doi: 10.1016/j.biotechadv.2011.05.016
168
Stiger-Pouvreau V. Bourgougnon N. Deslandes E. (2016). “ Chapter 8 – carbohydrates from seaweeds,” in Seaweed in health and disease prevention. Eds. FleurenceJ.LevineI. ( Academic Press, San Diego), 223–274. doi: 10.1016/B978-0-12-802772-1.00008-7
169
Stiger-Pouvreau V. Guerard F. (2018). Bio-inspired molecules extracted from marine macroalgae: a new generation of active ingredients for cosmetics and human health. In: Blue Biotechnol. John Wiley Sons Ltd;p, 709–746. doi: 10.1002/9783527801718.ch22
170
Stiger-Pouvreau V. Mattio L. De Ramon N’Yeurt A. Uwai S. Dominguez H. Flórez-Fernández N. et al . (2023). A concise review of the highly diverse genus Sargassum C. Agardh with wide industrial potential. J. Appl. Phycol.35, 1453–1483. doi: 10.1007/s10811-023-02959-4
171
Stiger-Pouvreau V. Zubia M. (2020). Macroalgal diversity for sustainable biotechnological development in French tropical overseas territories. Bot. Mar.63, 17–41. doi: 10.1515/bot-2019-0032
172
Sudatti D. B. Oliveira A. S. da Gama B. A. P. Fujii M. T. Rodrigues S. V. Pereira R. C. (2021). Variability in seaweed chemical defense and growth under common garden conditions. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.720711
173
Surget G. Le Lann K. Delebecq G. Kervarec N. Donval A. Poullaouec M. A. et al . (2017). Seasonal phenology and metabolomics of the introduced red macroalga Gracilaria vermiculophylla, monitored in the Bay of Brest (France). J. Appl. Phycol.29, 2651–2666. doi: 10.1007/s10811-017-1060-3
174
Synytsya A. Čopíková J. Kim W. J. Park Y. I. (2015). Cell wall polysaccharides of marine algae. In Springer Handb. Mar. Biotechnol. Berlin Heidelberg: Springer, 543–590. doi: 10.1007/978-3-642-53971-8_22
175
Tanaka A. Hoshino Y. Nagasato C. Motomura T. (2017). Branch regeneration induced by sever damage in the brown alga Dictyota dichotoma (dictyotales, phaeophyceae). Protoplasma.254, 1341–1351. doi: 10.1007/s00709-016-1025-4
176
Theirlynck T. Mendonça I. R. W. Engelen A. H. Bolhuis H. Collado-Vides L. van Tussenbroek B. I. et al . (2023). Diversity of the holopelagic Sargassum microbiome from the Great Atlantic Sargassum Belt to coastal stranding locations. Harmful Algae.122, 102369. doi: 10.1016/j.hal.2022.102369
177
Theis K. R. Dheilly N. M. Klassen J. L. Brucker R. M. Baines J. F. Bosch T. C. G. et al . (2016). Getting the hologenome concept right: an eco-evolutionary framework for hosts and their microbiomes. mSystems.1, e00028–e00016. doi: 10.1128/mSystems.00028-16
178
Thomas H. Huang L. Young M. Ougham H. (2009). Evolution of plant senescence. BMC. Evol. Biol.9, 16. doi: 10.1186/1471-2148-9-163
179
Thomsen M. McGlathery K. (2007). Stress tolerance of the invasive macroalgae Codium fragile and Gracilaria vermiculophylla in a soft-bottom turbid lagoon. ECU Publ.9, 499–513. doi: 10.1007/s10530-006-9043-3
180
Thornber C. S. (2006). Functional properties of the isomorphic biphasic algal life cycle. Integr. Comp. Biol.46, 605–614. doi: 10.1093/icb/icj067
181
Tillin H. Budd G. (2016). Porphyra purpurea and Ulva spp. on sand-scoured mid or lower eulittoral rock. In: Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Ed. Tyler-WaltersH. (Plymouth: Marine Biological Association of the United Kingdom).
182
Valero M. Richerd S. Perrot V. Destombe C. (1992). Evolution of alternation of haploid and diploid phases in life cycles. Trends Ecol. Evol.7, 25–29. doi: 10.1016/0169-5347(92)90195-H
183
Van Alstyne K. L. Nelson T. A. Ridgway R. L. (2015). Environmental chemistry and chemical ecology of “Green tide. Seaweed Blooms Integr. Comp. Biol.55, 518–532. doi: 10.1093/icb/icv035
184
van der Loos L. M. De Coninck L. Zell R. Lequime S. Willems A. De Clerck O. et al . (2023). Highly divergent CRESS DNA and picorna-like viruses associated with bleached thalli of the green seaweed Ulva. Microbiol. Spectr.11, e00255–e00223. doi: 10.1128/spectrum.00255-23
185
van der Loos L. M. De Wilde C. Willems A. De Clerck O. Steinhagen S. (2024). The cultivated sea lettuce (Ulva) microbiome: Successional and seasonal dynamics. Aquaculture.585, 740692. doi: 10.1016/j.aquaculture.2024.740692
186
van der Loos L. M. Eriksson B. K. Salles J. F. (2019). The macroalgal holobiont in a changing sea. Trends Microbiol.27, 635–650. doi: 10.1016/j.tim.2019.03.002
187
van der Loos L. M. Steinhagen S. Stock W. Weinberger F. D’hondt S. Willems A. et al . (2025). Low functional change despite high taxonomic turnover characterizes the Ulva microbiome across a 2000-km salinity gradient. Sci. Adv.11, eadr6070. doi: 10.1126/sciadv.adr6070
188
van Tussenbroek B. I. (1989). The life-span and survival of fronds of Macrocystis pyrifera (Laminariales, Phaeophyta) in the Falkland Islands. Br. Phycol. J.24, 137–141. doi: 10.1080/00071618900650131
189
Vera J. Castro J. Gonzalez A. Moenne A. (2011). Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs9, 2514–2525. doi: 10.3390/md9122514
190
Vergés A. Paul N. A. Steinberg P. D. (2008). Sex and life-history stage alter herbivore responses to a chemically defended red alga. Ecol.89, 1334–1343. doi: 10.1890/07-0248.1
191
Vieira VMNCS Dawange P. S. Jaiswar S. Sardinha J. P. Mantri V. A. (2024). Adaptation of functional traits in Gracilaria dura with the local environment: implications for resource management and exploitation. Front. Mar. Sci.11. doi: 10.3389/fmars.2024.1397379
192
Vieira VMNCS Engelen A. H. Huanel O. R. Guillemin M. L. (2018). Haploid females in the isomorphic biphasic life-cycle of Gracilaria Chilensis excel in survival. BMC Evol. Biol.18, 174. doi: 10.1186/s12862-018-1284-4
193
Vieira VMNCS Engelen A. H. Huanel O. R. Guillemin M. L. (2021). Differential frond growth in the isomorphic haploid-diploid red seaweed agarophyton Chilense by long-term in situ monitoring. J. Phycol.57, 592–605. doi: 10.1111/jpy.13110
194
Vieira VMNCS Oppliger L. V. Engelen A. H. Correa J. A. (2015). A new method to quantify and compare the multiple components of fitness—A study case with kelp niche partition by divergent microstage adaptations to temperature. PloS One10, e0119670. doi: 10.1371/journal.pone.0119670
195
Vranken S. Robuchon M. Dekeyzer S. Bárbara I. Bartsch I. Blanfuné A. et al . (2023). AlgaeTraits: a trait database for (European) seaweeds. Earth Syst. Sci. Data.15, 2711–2754. doi: 10.5194/essd-15-2711-2023
196
Wahl M. Molis M. Hobday A. Dudgeon S. Neumann R. Steinberg P. et al . (2015). The responses of brown macroalgae to environmental change from local to global scales: direct versus ecologically mediated effects. Perspect. Phycol.2, 11–30. doi: 10.1127/pip/2015/0019
197
Wang L. Wang X. Wu H. Liu R. (2014). Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs12, 4984–5020. doi: 10.3390/md12094984
198
Weigel B. L. Pfister C. A. (2019). Successional Dynamics and Seascape-Level Patterns of Microbial Communities on the Canopy-Forming Kelps Nereocystis luetkeana and Macrocystis pyrifera. Front. Microbiol.10. doi: 10.3389/fmicb.2019.00346
199
Weijerman M. Most R. Wong K. Beavers S. (2008). Attempt to control the invasive red alga acanthophora spicifera (Rhodophyta: ceramiales) in a hawaiian fishpond: an assessment of removal techniques and management options1. pasc.62, 517–532. doi: 10.1046/j.1529-8817.2001.00195.x
200
Weinberger F. (2007). Pathogen-induced defense and innate immunity in macroalgae. Biol. Bull.213, 290–302. doi: 10.2307/25066646
201
Wernberg T. (2021). “ Marine heatwave drives collapse of kelp forests in western Australia,” in Ecosystem collapse and climate change. Ecological studies, vol. 241 . Eds. CanadellJ. G.JacksonR. B. ( Springer, Cham). doi: 10.1007/978-3-030-71330-0_12
202
Wernberg T. Coleman M. A. Bennett S. Thomsen M. S. Tuya F. Kelaher B. P. (2018). Genetic diversity and kelp forest vulnerability to climatic stress. Sci. Rep.8, 1851. doi: 10.1038/s41598-018-20009-9
203
Wernberg T. Thomsen M. S. Stæhr P. A. Pedersen M. F. (2001). Comparative Phenology of Sargassum muticum and Halidrys siliquosa (Phaeophyceae: Fucales) in Limfjorden, Denmark, Vol. 44. 31–39. doi: 10.1515/BOT.2001.005
204
Wichard T. (2023). From model organism to application: Bacteria-induced growth and development of the green seaweed Ulva and the potential of microbe leveraging in algal aquaculture. Semin. Cell Dev. Biol.134, 69–78. doi: 10.1016/j.semcdb.2022.04.00
205
Wichard T. Beemelmanns C. (2018). Role of chemical mediators in aquatic interactions across the prokaryote–eukaryote boundary. J. Chem. Ecol.44, 1008–1021. doi: 10.1007/s10886-018-1004-7
206
Wichard T. Charrier B. Mineur F. Bothwell J. H. Clerck O. D. Coates J. C. (2018). The green seaweed Ulva: a model system to study morphogenesis. Front Plant Sci.6:72. doi: 10.3389/fpls.2015.00072
207
Williams S. Bracken M. Jones E. (2013). Additive effects of physical stress and herbivores on intertidal seaweed biodiversity. Ecology.94, 1089–1101. doi: 10.1890/12-0401.1
208
Xu J. Zhao X. Zhong Y. Qu T. Sun B. Zhang H. et al . (2024). Acclimation of intertidal macroalgae Ulva prolifera to UVB radiation: the important role of alternative oxidase. BMC Plant Biol.24, 143. doi: 10.1186/s12870-024-04762-w
209
Yang F. Xiao Z. Wei Z. Long L. (2021). Bacterial communities associated with healthy and bleached crustose coralline alga porolithon onkodes. Front. Microbiol.12. doi: 10.3389/fmicb.2021.646143
210
Zheng Y. Gao K. (2009). Impacts of solar UV radiation on the photosynthesis, growth, and UV-absorbing compounds in gracilaria lemaneiformis (rhodophyta) grown at different nitrate concentrations. J. Phycol45, 314–323. doi: 10.1111/j.1529-8817.2009.00654.x
211
Zozaya-Valdes E. Egan S. Thomas T. (2015). A comprehensive analysis of the microbial communities of healthy and diseased marine macroalgae and the detection of known and potential bacterial pathogens. Front. Microbiol.6. doi: 10.3389/fmicb.2015.00146
Summary
Keywords
holobiont, lifespan, longevity, metabolome, premature senescence, seaweed
Citation
Qui-Minet ZN, Connan S and Stiger-Pouvreau V (2025) To die or not to die: how seaweed holobionts chemistry influences lifespan and stress resilience. Front. Mar. Sci. 12:1635698. doi: 10.3389/fmars.2025.1635698
Received
26 May 2025
Accepted
13 October 2025
Published
18 November 2025
Volume
12 - 2025
Edited by
Lior Guttman, University of Haifa, Israel
Reviewed by
Vasco Manuel Nobre de Carvalho da Silva Vieira, University of Lisbon, Portugal
Vanessa Urrea-Victoria, National University of Colombia, Colombia
Updates
Copyright
© 2025 Qui-Minet, Connan and Stiger-Pouvreau.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zujaila Nohemy Qui-Minet, quiminet@univ-brest.fr
ORCID: Zujaila Nohemy Qui-Minet, orcid.org/0000-0001-6002-5240; Solène Connan, orcid.org/0000-0002-8280-1041; Valérie Stiger-Pouvreau, orcid.org/0000-0003-3041-0468
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.